Abstract
Background
Mold-active primary antifungal prophylaxis (PAP) is routinely recommended in neutropenic patients with newly diagnosed acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome (MDS) undergoing remission-induction chemotherapy (RIC). Isavuconazole (ISAV) is an extended spectrum mold-active triazole and has superior tolerability and fewer significant drug–drug interactions compared with other triazoles.
Methods
In our investigator-initiated, phase 2 trial, treatment-naive adult patients with AML or MDS starting RIC received ISAV per the dosing recommendations in the US label until neutrophil recovery (absolute neutrophil count [ANC] ≥ 0.5 × 109/L) and attainment of complete remission, occurrence of invasive fungal infection (IFI), or for a maximum of 12 weeks. The primary endpoint was the incidence of proven/probable IFI during ISAV PAP and up to 30 days after the last dose.
Results
Sixty-five of 75 enrolled patients received ISAV PAP (median age, 67 years, median ANC at enrollment, 0.72 × 109/L). Thirty-two patients (49%) received oral targeted leukemia treatments (venetoclax, FTL3 inhibitors). Including the 30-day follow-up period, probable/proven and possible IFIs were encountered in 4 (6%) and 8 patients (12%), respectively. ISAV trough serum concentrations were consistently > 1 µg/mL, showed low intraindividual variation, and were not significantly influenced by chemotherapy regimen. Tolerability of ISAV was excellent, with only 3 cases (5%) of mild to moderate elevations of liver function tests and no QTc prolongations.
Conclusions
ISAV is a safe and effective alternative for PAP in patients with newly diagnosed AML/MDS undergoing RIC in the era of recently approved or emerging small-molecule antileukemia therapies.
Clinical Trials Registration
Keywords: isavuconazole, chemotherapy, invasive fungal infection, antifungal prophylaxis, leukemia
In this prospective study, isavuconazole prophylaxis was safe in 65 neutropenic patients with newly diagnosed acute myeloid leukemia/myelodysplastic syndrome undergoing remission-induction chemotherapy, including 32 who received venetoclax and/or FLT3 inhibitors. Four patients (6%) had probable/proven breakthrough fungal infections. Survival rate was 92%.
(See the Editorial Commentary by Young on pages 1764–6.)
Invasive fungal infections (IFIs), especially those caused by molds, remain an important concern during treatment of acute myeloid leukemia (AML) [1]. Mold-active primary antifungal prophylaxis (PAP) is widely recommended in neutropenic patients with newly diagnosed AML or high-risk myelodysplastic syndrome (MDS) who undergo curative-intent chemotherapy [2]. Posaconazole prophylaxis has been shown to result in fewer IFIs than fluconazole and was associated with a survival advantage in this population [3]. Posaconazole is, therefore, widely endorsed as the preferred drug for PAP in patients with AML/MDS [2]. However, this agent can lead to significant drug–drug interactions (DDIs) through inhibition of cytochrome P450 3A4 (CYP3A4) and p-glycoprotein as well as prolongation of the QTc interval [4].
Currently available targeted antileukemic agents such as the BCL-2 antagonist venetoclax and fms3-like tyrosine kinase 3 (FLT3) inhibitors midostaurin, gilteritinib, and sorafenib have demonstrated high rates of complete remission (CR) and decreased rates of relapse when incorporated as part of remission-induction chemotherapy (RIC) in AML [5, 6]. Coadministration of strong CYP3A4 inhibitors, such as posaconazole, is commonly prohibited in clinical trial settings due to concerns of DDIs, especially QTc prolongation. Therefore, pharmacokinetic data for these combinations are limited [7]. As achievement of CR is the single most important early determinant of outcome in AML [8, 9], there is an urgent need for discovery of alternative PAP agents that are devoid of the DDI issues of posaconazole in the growing context of new targeted antileukemia treatments [10]. Consequently, there has been increased interest in the use of isavuconazole (ISAV) as an alternative triazole for PAP during RIC in patients with AML/MDS. ISAV is an extended-spectrum triazole with superior tolerability, reliability of absorption, and fewer significant DDIs. Importantly, this triazole does not cause QTc prolongation and, while optimal therapeutic concentrations and a potential need for therapeutic drug monitoring remain to be defined, routine monitoring of serum concentrations is not recommended [11]. ISAV is approved for the treatment of invasive aspergillosis and mucormycosis, the 2 most common mold infections in patients with hematological malignancies [11]. To that end, we conducted a single-institution, investigator-initiated, prospective, phase 2 trial of ISAV as PAP in patents with AML/MDS during RIC.
PATIENTS AND METHODS
Study Design
The full protocol (NCT03019939) that details assessment, enrollment procedures, administration of the study drug, monitoring, and treatment of enrolled patients is available at https://clinicaltrials.gov/ct2/show/NCT03019939. In brief, untreated adult (≥ 18 years old) patients who were or were anticipated to become neutropenic as a result of their first RIC for AML/MDS were eligible. In patients who had already begun antileukemic treatment, ISAV had to be initiated within 4 days. Use of systemic antifungals for > 72 hours during the week prior to ISAV initiation was not permitted. ISAV was administered orally as its pro-drug, isavuconazonium sulfate, and dosed per the US label [12]: Patients received 2 capsules of isavuconazonium sulfate (2 × 186 mg, equivalent to 2 × 100 mg ISAV) every 8 hours for 6 doses (48 hours) and, thereafter, 2 capsules once daily. ISAV PAP was administered until recovery from neutropenia (absolute neutrophil count [ANC] ≥ 0.5 × 109/L) and attainment of complete remission with (CR) or without (CRi) complete count recovery [13], development of proven, probable, or possible IFI as per European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria [14], development of unacceptable toxicity as graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [15], patient withdrawal or death, or for a maximum of 12 weeks. For patients who received multiple cycles of antileukemic therapy within 12 weeks without attainment of CR/CRi and an ANC ≥ 0.5 × 109/L, ISAV prophylaxis continued in between cycles. The primary endpoint was the occurrence of proven/probable IFI during the study period (up to 30 days from the last ISAV dose). Clinical and laboratory markers of toxicity (complete blood count, biochemical tests) were evaluated at baseline, weekly while patients were on ISAV PAP, and following the cessation of ISAV in all enrolled patients. Electrocardiography was performed at baseline and after 10 days of ISAV. This study was conducted at MD Anderson Cancer Center, a tertiary care cancer hospital. The protocol was approved by the institutional review board and all patients signed informed consent prior to enrollment.
Pharmacokinetic Analyses
ISAV plasma concentrations were determined immediately before dosing on days 8 and 15 using a validated, ultraperformance liquid chromatography, single quadrupole mass spectrometry analytical assay [16]. In brief, a standard curve was prepared by spiking blank human plasma with ISAV (BAL4815), followed by the addition of an internal standard (valethamate bromide) to each sample. All samples were buffered and loaded onto conditioned solid phase extraction columns, which were then washed with 5% ammonia solution (NH4OH) and 15% methanolic water. The samples were eluted with 1 mL of methanol and 1 mL of an acidic methanolic mixture (2% formic acid in methanol), and the combined eluates were dried under a stream of nitrogen. The dried residues were reconstituted with 60:40 acetonitrile/water and analyzed using a mass to charge ratio (m/z) for ISAV of 438.2. The lowest limit of quantitation was 0.25 µg/mL of ISAV.
Statistical Analysis
Two-group comparisons of continuous variables were performed using the 2-sided Mann-Whitney U test (unpaired analysis) or Wilcoxon signed-rank test (paired analysis). A P value of < .05 was considered significant. GraphPad Prism 8 and Microsoft Excel 2013 were used for data tabulation, statistical analyses, and compilation of diagrams.
RESULTS
Seventy-five patients were enrolled between 28 April 2017 and 26 July 2019, but only 65 patents received ISAV PAP (Figure 1). Ten patients did not receive the study drug due to insurance issues (n = 4), investigator decision (n = 3), incomplete screening tests (n = 2), or caspofungin use within the past week (n = 1). The median age of the 65 evaluable patients was 67 years (range, 21–86 years), with a median absolute neutrophil count of 0.72 × 109/L (range, 0.00–23.18) at enrollment (Table 1). Ninety-five percent of evaluable patients had AML and 5% MDS. Thirty patients (46%) received high-intensity RIC (Table 1), defined as regimens containing high-dose cytarabine (>1 g/m2/day) or those with cytarabine administered continuously in combination with an anthracycline. Thirty-two of 65 (49%) patients received venetoclax and/or a FLT3 tyrosine kinase inhibitor (TKI) as a component of RIC; the numbers of patients who received venetoclax, FLT3 TKIs, or both venetoclax and FLT3 TKIs were 25, 5, and 2 respectively (Table 1). The median age of patients who received venetoclax and/or a FLT3 TKI was 69 years (range, 31–79 years).
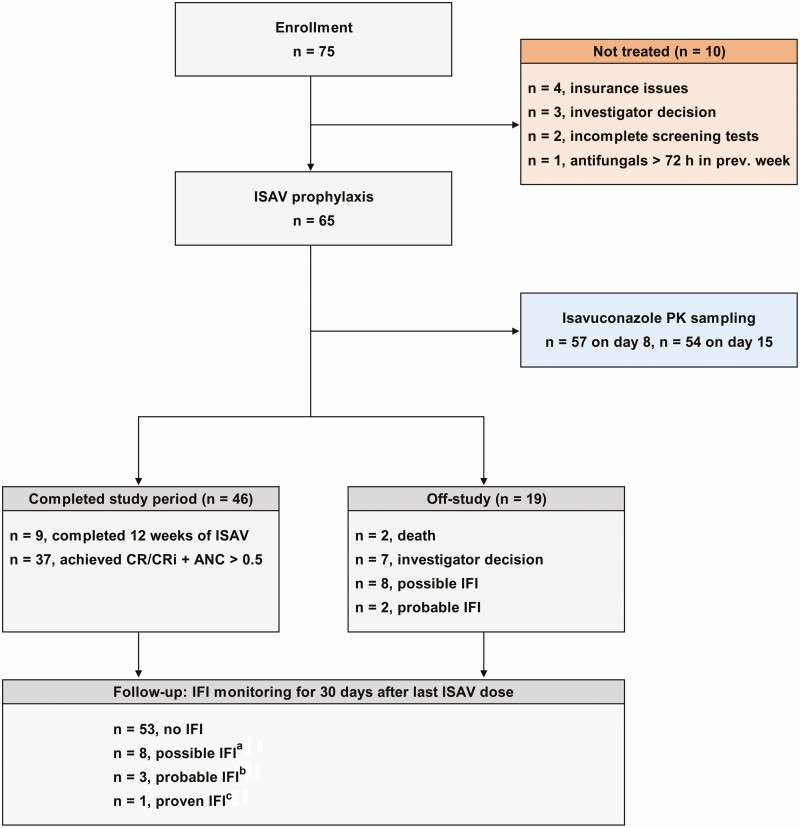
Figure 1.
Trial flowchart summarizing the reasons for discontinuation of isavuconazole (ISAV) prophylaxis as well as the incidence of possible, probable, or proven invasive fungal infection (IFI) per 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria [14] at the time of ISAV discontinuation and at the end of the follow-up period (30 days after the last dose of ISAV). The IFI classification of 3 patients changed during the follow-up period: a1 patient who had stopped ISAV upon investigator decision had follow-up chest computed tomography consistent with fungal lung infection during the follow-up period; b1 patient was upgraded from possible to probable IFI during the follow-up period due to a positive serum Aspergillus antigen assay; cCandida glabrata was isolated from a gluteal abscess in a patient who had stopped ISAV upon achievement of complete remission. Abbreviations: ANC, absolute neutrophil count; CR/CRi, complete remission with or without complete count recovery; PK, pharmacokinetics.
Table 1.
Demographics of the 65 Evaluable Patients
| Characteristic | No. (%) |
|---|---|
| Age, y, median (range) | 67 (21–86) |
| Sex, No. (%) | |
| Male | 36 (55) |
| Female | 29 (45) |
| Diagnosis, No. (%) | |
| AML | 62 (95) |
| MDS | 3 (5) |
| Absolute neutrophil count at enrollment, median (range) | 0.72 (0.00–23.18) |
| RIC received | |
| High-intensity RIC,a no. (%) | 30 (46%) |
| Without FLT3 TKI or venetoclax | 22 |
| With FLT3 TKI | 5 |
| With venetoclax | 2 |
| With FLT3 TKI + venetoclax | 1 |
| Hypomethylating agents,b no. (%) | 25 (38) |
| Without FLT3 TKI or venetoclax | 1 |
| With venetoclax | 23 |
| With FLT3 TKI + venetoclax | 1 |
| Low-intensity chemotherapy,c no. (%) | 10 (15) |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; RIC, remission-induction chemotherapy; TKI, tyrosine kinase inhibitor.
aFifteen CLIA (cladribine, idarubicin, and cytarabine); 5 CLIA + sorafenib; 1 CLIA + venetoclax; 1 CLIA + midostaurin + venetoclax; 3 FLAG (fludarabine, cytarabine, and granulocyte colony-stimulating factor) + idarubicin; 1 FLAG + idarubicin + GO (gemtuzumab-ozogamicin); 1 FLAG + GO; 1 fludarabine, cytarabine, and idarubicin; 1 CPX-351 (liposomal cytarabine-daunorubicin); 1 CPX-351 + venetoclax.
bOne decitabine; 23 decitabine + venetoclax; 1 decitabine + venetoclax + sorafenib.
cOne low-dose cytarabine; 9 cladribine + low-dose cytarabine.
The reasons for discontinuation of ISAV PAP were achievement of CR with neutrophil recovery (n = 37), completion of 12 weeks of PAP (n = 9), possible IFI (n = 8), probable IFI (n = 2), investigator decision (n = 7), and death (n = 2, 1 due to leukemia progression, 1 due to cardiac arrest) (Figure 1 and Table 2). Among the 7 patients who discontinued ISAV due to an investigator decision, 2 patients (3%) had abnormal computed tomographic (CT) scans, not consistent with a fungal infection, but were switched to other antifungals at the treating physician’s discretion. Three patients (5%) had mild to moderate elevations of aminotransferases or total bilirubin as detailed below and were switched to caspofungin. Two patients (3%) were transitioned to alternative azole prophylaxis at the discretion of the treating physician due to greater clinical experience and comfort with other commercially available azole antifungals.
Table 2.
Summary of Reasons for Isavuconazole Discontinuation and Invasive Fungal Infection Outcomes After the 30-Day Follow-up Period
| Reason for Discontinuation of ISAV | Total | IFI Outcome 30 d After the Last Dose of ISAV | |||
|---|---|---|---|---|---|
| No. (%) | No IFI | Possible IFI | Probable IFI | Proven IFI | |
| Completed 12 wk of ISAV | 9 (14) | 9 | 0 | 0 | 0 |
| Achieved CR/CRi + ANC > 0.5 | 37 (57) | 36 | 0 | 0 | 1 |
| Investigator decision | 7 (11) | 6 | 1 | 0 | 0 |
| Death, not IFI-related | 2 (3) | NA (2) | NA | NA | NA |
| Possible IFI | 8 (12) | 0 | 7 | 1 | 0 |
| Probable IFI | 2 (3) | 0 | 0 | 2 | 0 |
| All patients, no. (%) | … | 53 (82) | 8 (12) | 3 (5) | 1 (2) |
Patients with a change in their IFI classification during the follow-up period are highlighted in bold.
Abbreviations: ANC, absolute neutrophil count; CR/CRi, complete remission with our without complete count recovery; IFI, invasive fungal infection; ISAV, isavuconazole; NA, not applicable.
The CR rate in the entire cohort was 57% (37/65 patients), and similar (56%) in the 32 patients who received venetoclax, FLT3 TKIs, or both. The survival rate during the study period was high (92% [60/65 patients]). The median duration of severe neutropenia (ANC < 0.5 × 109/L) and ISAV PAP was 23 (range, 3–86; mean, 32) days and 33 (range, 8–87; mean, 42) days, respectively.
The incidence of probable and possible IFIs during ISAV PAP was 15% (n = 10). All patients were profoundly neutropenic (ANC, 0.00–0.13) and not in CR when they developed ISAV-breakthrough IFI (b-IFI; Table 3). Two patients (3%) developed probable pulmonary aspergillosis (1 patient with focal mass–like opacity with ground glass halo on CT and elevated Aspergillus galactomannan [GM] antigen in bronchoalveolar lavage [BAL] fluid; the other patient with bilateral lower lobe opacities on CT and elevated Aspergillus GM antigen in BAL). Another 8 patients (12%) had possible fungal pneumonia based on pulmonary radiologic findings alone (Figure 1 and Table 3); lower respiratory fungal cultures remained negative at 4 weeks and GM was not detected in serum or BAL fluid except in 1 patient, who was upgraded to probable b-IFI during the follow-up period (see below).
Table 3.
Characteristics of the 10 Patients Who Developed Breakthrough Invasive Fungal Infections While on Isavuconazole Prophylaxis
| Age/Sex | Diagnosis | Remission-induction Chemotherapy | IFIa (EORTC/MSG Criteria) | ISAV Serum Concentration Day 8/15, µg/mL | Days on ISAV Prophylaxis | Initial Antifungal Treatment of b-IFI | ANC at the Time of IFI Diagnosis | CR Status at the Time of IFI Diagnosis | CR Status/Alive at 42 d After IFI Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 44/M | AML | CLIA | Possible | 4.49/6.96 | 19 | LipoAMB (7 d) + PCZ → PCZ | 0.01 | Not in CR | CR/Yes |
| 63/M | AML | CLIA | Possible | 4.60/6.99 | 15 | LipoAMB (7 d) + VRC → VRC | 0.00 | Not in CR | Deceased |
| 42/F | AML | CLIA | Possible | NA/NA | 53 | LipoAMB (21 d) + PCZ → PCZ | 0.13 | Not in CR | Not in CR/Yes |
| 67/M | AML | Decitabine + venetoclax | Possible | 5.77/3.67 | 23 | LipoAMB (4 d) + PCZ → PCZ | 0.00 | Not in CR | CR/Yes |
| 72/F | AML | Decitabine + venetoclax | Possible | 3.81/5.52 | 21 | LipoAMB (3 d) + PCZ → PCZ | 0.00 | Not in CR | CR/Yes |
| 64/M | AML | Decitabine + venetoclax | Probable BAL GM 1.14 | 4.01/4.53 | 29 | LipoAMB (14 d) + PCZ (16 d) → VRC | 0.00 | Not in CR | CR/Yes |
| 43/M | MDS | CLIA | Possible | 2.90/Discontinued | 10 | LipoAMB (4 d) + PCZ → PCZ | 0.03 | Not in CR | Not in CR/Yes |
| 71/M | AML | Decitabine + venetoclax | Possible | 3.27/4.83 | 57 | PCZ + CAS → PCZ + CAS + LipoAMB | 0.00 | Not in CR | Deceased |
| 66/M | AML | FLAG + GO | Probable BAL GM 0.81 | 2.37/Discontinued | 8 | PCZ | 0.00 | Not in CR | CR/Yes |
| 79/F | AML | Decitabine + venetoclax | Possibleb | 5.62/5.36 | 26 | PCZ + CAS → PCZ | 0.00 | Not in CR | CR/Yes |
Abbreviations: AML, acute myeloid leukemia; ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; b-IFI, breakthrough fungal infection; CAS, caspofungin; CLIA, cladribine, idarubicin, cytarabine; CR, complete remission; EORTC/MSG, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group–National Institute of Allergy and Infectious Diseases Mycoses Study Group; FLAG + GO, fludarabine, cytarabine, granulocyte colony-stimulating factor + gemtuzumab-ozogamicin; GM, Aspergillus galactomannan; IFI, invasive fungal infection; ISAV, isavuconazole; LipoAMB, liposomal formulation of amphotericin B; MDS, myelodysplastic syndrome; NA, not available; PCZ, posaconazole; VRC, voriconazole.
aAll b-IFIs during ISAV primary antifungal prophylaxis were lung infections.
bPatient was upgraded from possible to probable IFI during the follow-up period due to a positive Aspergillus serum antigen assay.
The median time to occurrence of b-IFI from initiation of ISAV prophylaxis was 22 days (range, 8–57 days). All 10 patients with b-IFIs received antifungal treatment (liposomal amphotericin B with another triazole in 7, caspofungin plus posaconazole in 2, posaconazole alone in 1; Table 3) and 8 of 10 were alive 42 days after the diagnosis of b-IFI (Table 3). Nine of 10 patients with b-IFIs received venetoclax-based RIC (5 patients) or high-intensity regimens (cladribine, cytarabine plus idarubicin [CLIA] in 4 patients; Table 3). In comparison, 38 of 55 patients who did not develop b-IFIs received venetoclax-based regimens or the CLIA regimen as RIC. None of the 7 patients receiving FTL3 TKIs developed a b-IFI and all achieved CR. Neither of the 2 patients who died within 42 days of the diagnosis of b-IFI had achieved CR, in contrast to 6 of 8 patients who were alive at day 42 (Table 3).
Two additional patients developed b-IFIs within 30 days following the discontinuation of ISAV PAP. One patient who had stopped ISAV upon investigator decision had a subsequent chest CT consistent with fungal lung infection during the follow-up period (possible IFI). A second patient developed a fungal gluteal abscess due to Candida glabrata. The isolate was susceptible to echinocandins (minimum inhibitory concentration [MIC] of micafungin, 0.008 µg/mL) and amphotericin B (1 µg/mL) and had dose-dependent susceptibility to fluconazole (4 µg/mL), whereas the MICs of itraconazole, posaconazole, voriconazole, and ISAV were 0.25 µg/mL, 0.5 µg/mL, 0.12 µg/mL, and 1 µg/mL, respectively (Clinical and Laboratory Standards Institute M27, fourth edition). The IFI classification of another patient was upgraded from possible (while on ISAV) to probable IFI during the 30-day follow-up period due to a positive Aspergillus serum antigen assay. Collectively, 4 probable or proven (6%) and 8 possible (12%) IFI cases were seen during ISAV PAP and the 30-day follow-up period (Figure 1 and Table 2).
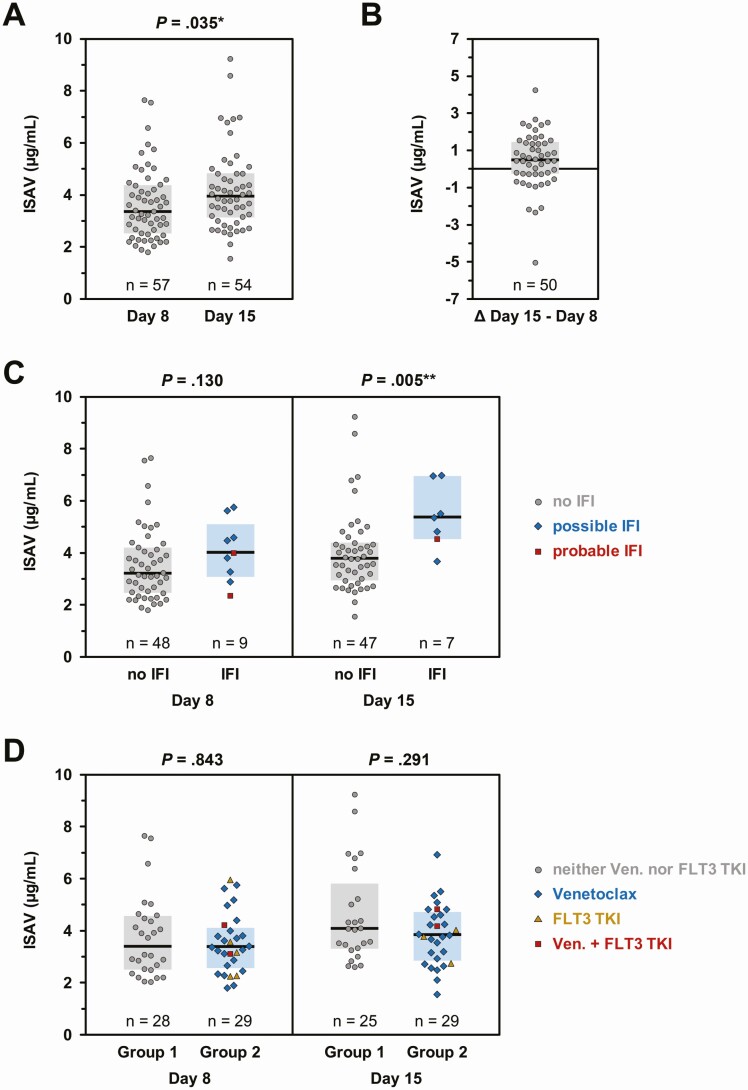
ISAV trough serum concentrations were available in 57 patients on day 8 (median, 3.37 µg/mL [range, 1.81–7.65]) and 54 patients on day 15 (median, 3.95 µg/mL [range, 1.56–9.25]). Day 15 serum ISAV levels were significantly higher (P = .035; Figure 2A). ISAV concentrations had very low intrapatient variability, with only 2 patients showing a ≥ 3 µg/mL change in serum concentrations between day 8 and day 15 (Figure 2B). Among patients who had their ISAV levels checked, no difference in day 8 ISAV trough concentrations were seen between those who developed b-IFIs (possible/probable) while on ISAV and those without b-IFIs, whereas, unexpectedly, day 15 concentrations tended to be higher in patients with b-IFIs (Figure 2C). Importantly, there were no differences in ISAV levels between patients who received venetoclax, FLT3 TKIs, or combinations thereof and those who did not (Figure 2D).
Figure 2.
Isavuconazole (ISAV) trough concentrations on day 8 and day 15. ISAV trough concentrations were measured on days 8 and 15 using a validated liquid chromatography–tandem mass spectrometry assay [16]. A, Trough concentrations were available from 57 patients on day 8 and 54 patients on day 15. B, Matched pairs of day 8 and day 15 ISAV trough concentrations were available in 50 patients. The difference between serum concentrations on day 15 minus day 8 is plotted to document the intraindividual stability of ISAV serum levels. C, Comparison of ISAV trough concentrations on days 8 and 15 relative to the invasive fungal infection status at the time of ISAV discontinuation. D, Comparison of ISAV trough concentrations depending on the patients’ chemotherapy regimen. Group 1: patients receiving neither venetoclax nor FLT3 tyrosine kinase inhibitors (gray circles). Group 2: patients receiving venetoclax (blue diamonds), FLT3 tyrosine kinase inhibitors (golden triangles), or both (red squares). For all panels, individual values, median (horizontal bars), and interquartile range (colored boxes) are shown. A, C, and D, Mann-Whitney U test. Significance levels are denoted by asterisks: *P < .05, **P < .01. Abbreviations: IFI, invasive fungal infection; TKI, tyrosine kinase inhibitor; Ven., venetoclax.
Finally, tolerability of ISAV was excellent, with grade 1 transaminitis, possibly attributable to ISAV, reported in only 2 patients (alanine aminotransferase [ALT] 85 U/L, aspartate aminotransferase 64 U/L in 1 patient, ALT 100 U/L in another one) who had normal liver function tests at baseline. In addition, 1 possibly ISAV-related case of elevated total bilirubin (2.9 mg/dL, grade 2) was seen. No patient experienced QTc prolongation while on ISAV. The median QTc times at baseline and on day 10 of ISAV were 404 and 402 ms, respectively.
DISCUSSION
This is the first prospective study of ISAV as PAP in patients with newly diagnosed AML/MDS undergoing RIC. Overall, we found that ISAV PAP is a safe and effective alternative in this patient cohort. ISAV absorption was excellent, and ISAV levels were higher on day 15, consistent with the fact that it takes 10–14 days for ISAV to reach steady-state levels [17]. However, on both day 8 and day 15, serum ISAV levels in all patients were above the proposed breakpoints (1 µg/mL) for Aspergillus [18], the most common causative agent of IFI in patients with hematological malignancies [19].
The (proven/probable) breakthrough IFI rate was 3% (15% if adding possible cases) during ISAV PAP and 6% (18% including possible cases) by the end of the 30-day follow-up period. Although comparable, the incidence of b-IFIs appears to be slightly higher in the present study than in randomized controlled [3] and real-life [20] studies of posaconazole as PAP as well as our own historical data [21]. One possible explanation could be that our patients were older, with a median age of 67 years vs 49 years in the randomized registration trial of posaconazole [3]. Older age (> 60 years) is a well-established risk factor for severe infections including IFI and inferior outcome during RIC in AML [22, 23]. Our patients also had a longer mean duration of severe neutropenia than in the posaconazole registration trial (32 days vs 25 days). In prior reports, ISAV has been associated with b-IFIs, mostly in patients with refractory leukemia and prolonged cytopenia [24]. There is a possibility that ISAV might be inferior to other triazoles in the setting of protracted neutropenia and lack of neutrophil recovery [25] and therefore may be prudent to avoid ISAV in heavily pretreated patients where marrow recovery might be delayed.
Among the patients who developed b-IFIs, nearly all received either venetoclax-based regimens or CLIA, agents/regimens associated with high efficacy but severe immunosuppression in patients with AML [26, 27]. Specifically, venectoclax is associated with prolonged and profound neutropenia [27, 28], a risk factor for IFI, as mature neutrophils depend on the antiapoptotic function of BCL-2 for survival. A recent study found prolonged neutropenia and thrombocytopenia in patients receiving venetoclax in combination with triazoles [29]. Although ISAV is a less potent CYP3A4 inhibitor compared with posaconazole [11] and venetoclax was dose-reduced by 50% in patients receiving ISAV following the recommendations in the venetoclax US label [30], ISAV may still lead to increased venetoclax serum concentrations, thereby potentiating the myelosuppressive effect [28, 29, 31]. However, therapeutic drug monitoring is not currently available for this newly approved BCL-2 antagonist to test this hypothesis. Therefore, further studies would be warranted to define the comparative impact of different triazoles on the duration and intensity of chemotherapy-induced cytopenia, especially in combination with venetoclax or other CYP3A4 substrates.
Importantly, the short-term survival of patients with b-IFIs was excellent in our study, and associated with subsequent achievement of CR. This, along with the fact that b-IFIs were not associated with low serum ISAV levels, underscores the importance of host-related factors to both the risk and outcomes of b-IFIs during RIC in the era of potent mold active triazoles such as posaconazole, voriconazole, and ISAV. Interestingly, outcomes were good in patients who developed b-IFIs while on ISAV prophylaxis and received treatment with other triazoles, consistent with anecdotal published experience [32].
ISAV toxicity was minimal and much less compared to our historical controls with posaconazole used as PAP [21]. Although the incorporation of “real-world” data in the study by Tverdek et al [21] could explain the excess toxicities of posaconazole when used as PAP, ISAV appears to be less toxic [33] and a good alternative in patients with posaconazole-associated toxicities [34]. In particular, no increased incidence of ISAV-associated side effects was seen and ISAV serum levels were adequate in patents given venetoclax and/or FLT3 TKIs as part of their RIC. Despite the inclusion of older subjects in our study, patients in general and specifically those who received either venetoclax, a FTL3 TKI, or both, achieved high CR rates and had excellent survival. This underscores the importance of the ability to incorporate the most potent molecularly targeted agents into RIC regimens, which is facilitated by the use of ISAV as PAP. As more novel targeted antileukemia agents (eg, mutant isocitrate dehydrogenase inhibitors and newer-generation, potent FLT3 TKIs [gilteritinib, quizartinib, and crenolanib]) continue to be studied as frontline AML therapies with promising results [35], the strategy of personalizing PAP—for example, studying these agents in combination with ISAV—has merit and deserves further evaluation.
Despite its strength as a prospective and detailed study of a relatively homogeneous neutropenic patient cohort naive to prior antifungals, there are limitations of our single-institution, unblinded, nonrandomized study. Importantly, our experience cannot be extrapolated to patients receiving secondary antifungal prophylaxis (ie, patients who had received prior posaconazole or voriconazole or those with prior IFI), or to patients with relapsed or refractory leukemia. The choice of PAP in the frontline setting might be different compared to PAP in the setting of salvage chemotherapy, and factors such as prolonged cumulative immunosuppression, comorbidities, use of QTc-prolonging agents, local epidemiology, and sequential exposures to antifungals (and hence, concerns of selection of antifungal resistance) need to be considered. In addition, trials of ISAV PAP would be warranted during RIC in acute lymphocytic leukemia, a disease with a high background rate of IFIs [36]. As echinocandins have been shown to be inferior to triazoles as PAP in AML in some [37] but not all studies [38], the role of echinocandins as PAP in patients receiving molecularly targeted therapies in acute leukemia also needs to be revisited. Finally, as b-IFIs have been reported in high-risk hematology patients on prophylaxis with all the major triazoles (voriconazole, posaconazole, ISAV) [39], it would be important to study the comparative effectiveness of these agents and their long-term tolerability in the context of the changing landscape of acute leukemia treatment [40]. Well-conducted prospective comparative trials of each triazole used as PAP or, more realistically, well-constructed multi-institutional registries would be important.
Notes
Financial support. Astellas Pharma Global Development provided the study drug and funding for this investigator-initiated trial (to D. P. K.). D. P. K. acknowledges the Texas 4000 Distinguished Professorship for Cancer Research and the National Institutes of Health/National Cancer Institute Cancer Center CORE Support grant (number 16672).
Potential conflicts of interest. P. B. has received grants (research support) and personal fees from Incyte, Celgene, CTI Biopharma, Kartos Therapeutics, and BluePrint Medicines, and grants from Astellas, Constellation Pharmaceuticals, NS Pharma, Promedior, and Pfizer. N. P. W. has received grants from Astellas Pharma, bioMérieux, Cidara, Covance, F2G, and Viamet; has served on the advisory board of Mayne Pharma; and has served on the speaker’s bureau for Gilead. T. M. K. has received grants and personal fees from Pfizer, AbbVie, Genetech, and Jazz Pharma, as well as grants from BMS and Celgene; and has served as a consultant for Novartis and Agios. N. P. has received research funding from Samus, Cellectis, Plexxikon, and Daiichi-Sankyo; has received grants from the Sager Strong Foundation and Affymetrix; has received research support and personal fees from AbbVie, Stemline, and Novartis; has received personal fees from the Blueprint Medicines Corporation, Celgene, Incyte, MustangBio, Roche Diagnostics, and FLB; has served on the Board of Directors of Dan’s House of Hope; and has been a Board Member of HemOnc Times/Oncology Times. H. M. K. has received grants and honoraria from AbbVie, Agios, Amgen, Cyclacel, Immunogen, and Pfizer; grants from Ariad, Astex, BMS, Daiichi-Sankyo, Jazz Pharma, and Novartis; and honoraria from Takeda; and has also served on the advisory board of Actinium. D. P. K. has received research support from Astellas Pharma, Merck, Pfizer, and T2 Biosystems; has received honoraria for lectures from Merck & Co, Gilead, F2G Inc, Pfizer, and United Medical; has served as a consultant for Astellas Pharma, Cidara, Amplyx, Astellas, Pulmocide, and Mayne Pharma; and has served on the advisory board of Merck & Co. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Leventakos K, Lewis RE, Kontoyiannis DP. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis 2010; 50:405–15. [DOI] [PubMed] [Google Scholar]
- 2. Halpern AB, Lyman GH, Walsh TJ, Kontoyiannis DP, Walter RB. Primary antifungal prophylaxis during curative-intent therapy for acute myeloid leukemia. Blood 2015; 126:2790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–59. [DOI] [PubMed] [Google Scholar]
- 4. Lipp HP. Posaconazole: clinical pharmacokinetics and drug interactions. Mycoses 2011; 54(Suppl 1):32–8. [DOI] [PubMed] [Google Scholar]
- 5. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 2017; 377:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Röllig C, Serve H, Hüttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol 2015; 16:1691–9. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther 2017; 39:359–67. [DOI] [PubMed] [Google Scholar]
- 8. Ravandi F, Cortes J, Faderl S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010; 116:5818–23; quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol 2010; 28:1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindsay J, Teh BW, Micklethwaite K, Slavin M. Azole antifungals and new targeted therapies for hematological malignancy. Curr Opin Infect Dis 2019; 32:538–45. [DOI] [PubMed] [Google Scholar]
- 11. Ananda-Rajah MR, Kontoyiannis D. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol 2015; 10:693–708. [DOI] [PubMed] [Google Scholar]
- 12. Astellas Pharma. Cresemba: prescribing information. Available at: https://www.astellas.us/docs/cresemba.pdf. Accessed 4 February 2020.
- 13. Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia . Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21:4642–9. [DOI] [PubMed] [Google Scholar]
- 14. De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed 4 February 2020.
- 16. Gebremariam T, Wiederhold NP, Alqarihi A, et al. Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis. J Antimicrob Chemother 2017; 72:462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitt-Hoffmann AH, Kato K, Townsend R, et al. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 2017; 61:e01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andes DR, Ghannoum MA, Mukherjee PK, et al. Outcomes by MIC values for patients treated with isavuconazole or voriconazole for invasive aspergillosis in the phase 3 SECURE and VITAL trials. Antimicrob Agents Chemother 2018; 63:e01634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mulanovich VE, Kontoyiannis DP. Fungal pneumonia in patients with hematologic malignancies: current approach and management. Curr Opin Infect Dis 2011; 24:323–32. [DOI] [PubMed] [Google Scholar]
- 20. Pagano L, Caira M, Candoni A, et al. SEIFEM Group . Evaluation of the practice of antifungal prophylaxis use in patients with newly diagnosed acute myeloid leukemia: results from the SEIFEM 2010-B registry. Clin Infect Dis 2012; 55:1515–21. [DOI] [PubMed] [Google Scholar]
- 21. Tverdek FP, Heo ST, Aitken SL, Granwehr B, Kontoyiannis DP. Real-life assessment of the safety and effectiveness of the new tablet and intravenous formulations of posaconazole in the prophylaxis of invasive fungal infections via analysis of 343 courses. Antimicrob Agents Chemother 2017; 61:e00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klepin HD. Elderly acute myeloid leukemia: assessing risk. Curr Hematol Malig Rep 2015; 10:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neofytos D, Lu K, Hatfield-Seung A, et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis 2013; 75:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rausch CR, DiPippo AJ, Bose P, Kontoyiannis DP. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin Infect Dis 2018; 67:1610–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kontoyiannis DP, Selleslag D, Mullane K, et al. Impact of unresolved neutropenia in patients with neutropenia and invasive aspergillosis: a post hoc analysis of the SECURE trial. J Antimicrob Chemother 2018; 73:757–63. [DOI] [PubMed] [Google Scholar]
- 26. Kadia T, Cortes J, Jabbour E, et al. Phase II study of cladribine, idarubicin, and cytarabine (araC) in patients with acute myeloid leukemia (AML). Blood 2015; 126:2541.26500341 [Google Scholar]
- 27. DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019; 133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Q, Cheng L, Shen K, et al. Efficacy and safety of Bcl-2 inhibitor venetoclax in hematological malignancy: a systematic review and meta-analysis of clinical trials. Front Pharmacol 2019; 10:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rausch CR, DiNardo CD, Maiti A, et al. Venetoclax dosing in combination with antifungal agents: real world experience in patients with acute myeloid leukemia. In: 61st American Society of Hematology Annual Meeting and Exposition, Orlando, FL, 2019.. Abstract 2640. [Google Scholar]
- 30. US Food and Drug Administration. Venclexta: prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Accessed 3 March 2020. [Google Scholar]
- 31. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect 2018; 24(Suppl 2):53–70. [DOI] [PubMed] [Google Scholar]
- 32. Cheng MP, Orejas JL, Arbona-Haddad E, et al. Use of triazoles for the treatment of invasive aspergillosis: a three-year cohort analysis. Mycoses 2020; 63:58–64. [DOI] [PubMed] [Google Scholar]
- 33. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387:760–9. [DOI] [PubMed] [Google Scholar]
- 34. DiPippo AJ, Rausch CR, Kontoyiannis DP. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses 2019; 62:81–6. [DOI] [PubMed] [Google Scholar]
- 35. Short NJ, Kantarjian H, Ravandi F, Daver N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther Adv Hematol 2019; 10:2040620719827310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornely OA, Kontoyiannis DP. How to prophylax against invasive fungal infections in adult ALL? An unmet need. Mycoses 2018; 61:646–9. [DOI] [PubMed] [Google Scholar]
- 37. Gomes MZ, Jiang Y, Mulanovich VE, Lewis RE, Kontoyiannis DP. Effectiveness of primary anti-Aspergillus prophylaxis during remission induction chemotherapy of acute myeloid leukemia. Antimicrob Agents Chemother 2014; 58:2775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park H, Youk J, Shin DY, et al. Micafungin prophylaxis for acute leukemia patients undergoing induction chemotherapy. BMC Cancer 2019; 19:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lionakis MS, Lewis RE, Kontoyiannis DP. Breakthrough invasive mold infections in the hematology patient: current concepts and future directions. Clin Infect Dis 2018; 67:1621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DiPippo AJ, Kontoyiannis DP. Lack of toxicity with long-term isavuconazole use in patients with hematologic malignancy. Clin Infect Dis 2019; 69:1624–7. [DOI] [PubMed] [Google Scholar]