Abstract
Solitary organ autoimmune disorders, formerly known as autoimmune pancreatitis (AIP), autoimmune sialadenitis, and autoimmune sclerosing cholangitis, are now considered organ-specific manifestations of systemic immunoglobulin G4-related disease (IgG4-RD). AIP and IgG4-RD are characterized by elevated serum concentration of IgG4 antibody (Ab), accumulation of IgG4-expressing plasmacytes in the affected organs, and involvement of multiple organs. It is well established that enhanced IgG4 Ab responses are a hallmark of AIP and IgG4-RD for diagnosis and monitoring disease activity. However, a significant fraction of patients with AIP and IgG4-RD who develop chronic fibroinflammatory responses have normal serum concentrations of this IgG subtype. In addition, disease flare-up is sometimes seen even in the presence of normalized serum concentrations of IgG4 Ab after successful induction of remission by prednisolone. Therefore, it is necessary to identify new biomarkers based on the understanding of the pathophysiology of AIP and IgG4-RD. Recently, we found that activation of plasmacytoid dendritic cells producing both interferon-α (IFN-α) and interleukin-33 (IL-33) mediate murine AIP and human IgG4-RD. More importantly, we provided evidence that serum concentrations of IFN-α and IL-33 could be useful biomarkers for the diagnosis and monitoring of AIP and IgG4-RD activity after induction of remission in these autoimmune disorders. In this Frontier article, we have summarized and discussed biomarkers of AIP and IgG4-RD, including Igs, autoAbs, and cytokines to provide useful information not only for clinicians but also for researchers.
Keywords: Biomarker, Autoimmune pancreatitis, Immunoglobulin G4-related disease, Plasmacytoid dendritic cells, Cytokine, Chemokine
Core Tip: Autoimmune pancreatitis (AIP) and immunoglobulin G4-related disease (IgG4-RD) are new disease entities characterized by enhanced IgG4 antibody responses. Serum concentration of IgG4 antibody is widely used as a useful biomarker for diagnosis and disease activity monitoring in AIP and IgG4-RD. Recent studies have highlighted the importance of cytokine responses in the immunopathogenesis of these disorders. In this Frontier article, we have summarized our knowledge regarding cytokine responses in AIP and IgG4-RD and then discussed the utility of serum concentrations of cytokines as possible biomarkers.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a unique form of the chronic fibroinflammatory disorder of the pancreas, which is driven by autoimmune responses[1]. AIP is classified into type 1 and type 2, and more than 95% of AIP cases represent the former, which is a pancreatic manifestation of systemic immunoglobulin G4-related disease (IgG4-RD)[2-4]. In this article, type 1 AIP is hereafter referred to as AIP. AIP and IgG4-RD are recently established disease entities proposed by rheumatologists and gastroenterologists[2-4]. As awareness and recognition of these disorders by physicians increase, the number of patients diagnosed with AIP and IgG4-RD is growing. Thus, the clinical manifestations and immunopathogenesis of AIP and IgG4-RD are attracting much attention from physicians and researchers.
IgG4-RD occurs most commonly in elderly men; it is characterized by a marked elevation of serum IgG4 antibody (Ab) and accumulation of plasma cells secreting IgG4 Ab into injured organs[2-4]. Another important feature of IgG4-RD is multiple organ involvement: this disorder preferentially affects the pancreas, bile duct, lung, salivary glands, and kidney. AIP is a pancreatic manifestation of IgG4-RD. The elevated concentration of serum IgG4 Ab is widely used as a diagnostic marker for AIP and IgG4-RD[5,6]. In addition, patients with IgG4-RD exhibiting multiple organ involvement display higher concentrations of serum IgG4 Ab[7,8], suggesting that measurement of serum IgG4 concentration is useful not only for the diagnosis but also for the evaluation of disease activity. It should be noted, however, that the concentration of this IgG subtype is not always regarded as a perfect biomarker for the diagnosis or evaluation of disease activity in AIP and IgG4-RD. In fact, serum concentration of IgG4 Ab is elevated in a significant fraction of patients with pancreatic cancer[9] and about 20% of patients with AIP display normal serum concentration of IgG4 Ab[10]. Furthermore, patients with AIP sometimes relapse even if they have normal serum concentration of IgG4 Ab[11]. Therefore, it is necessary to identify other biomarkers that could be useful for the diagnosis and evaluation of disease activity in AIP and IgG4-RD.
Remarkable progress has been made in understanding the immunopathogenesis of AIP and IgG4-RD. Elucidation of immune networks associated with the development of these autoimmune disorders has led us to identify candidate biomarkers other than IgG4 Ab. In this Frontier article, we summarize recent progress in the biomarkers of AIP and IgG4-RD based on the knowledge of abnormal immune microenvironments.
IMMUNOPATHOGENESIS OF AUTOIMMUNE PANCREATITIS AND IGG4-RD
Adaptive immunity
AIP and IgG4-RD are characterized by enhanced IgG4 Ab responses; thus, immune microenvironments leading to IgG4 Ab production are likely to be involved in the development of these disorders[2-4]. Various types of differentiated T cell subpopulations are involved in the enhanced IgG4 Ab response (Figure 1). These effector T cells include T helper type 2 (Th2) cells, regulatory T cells (Tregs), follicular helper T (Tfh) cells, and cytotoxic CD4+ T cells (CD4+ CTLs)[4]. Cytokines produced by effector T cell subpopulations promote IgG4 Ab production by B cells.
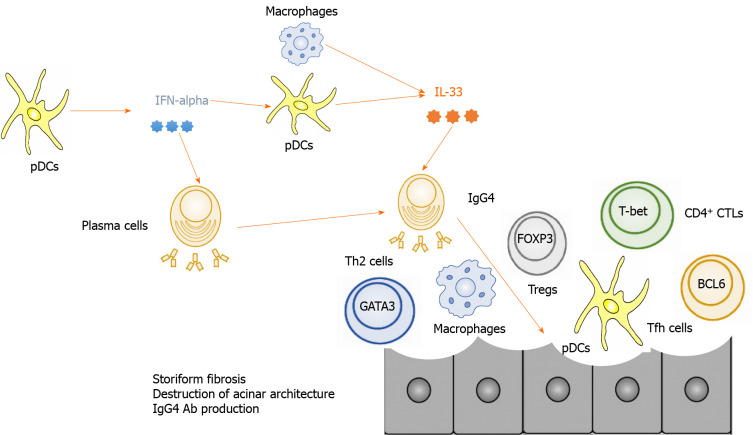
Figure 1.
Immunopathogenesis of autoimmune pancreatitis and immunoglobulin G4-related disease. Plasmacytoid dendritic cells produce interferon-α and interleukin-33 and thereby mediate chronic fibroinflammatory responses in the pancreas. T helper type 2 cells expressing GATA-binding protein 3, regulatory T cells expressing forkhead box P3, follicular helper T cells expressing B cell lymphoma 6, and CD4+ cytotoxic T cells expressing T-box-expressed-in-T-cells are involved in the development of autoimmune pancreatitis and immunoglobulin G4-related disease. IgG4: Immunoglobulin G4; pDCs: Plasmacytoid dendritic cells; IFN: Interferon; IL: Interleukin; Th2: T helper type 2 cells; Tregs: Regulatory T cells; GATA3: GATA-binding protein 3; FOXP3: Forkhead box P3; Tfh: Follicular helper T; BCL6: B cell lymphoma 6; T-bet: T-box-expressed-in-T-cells.
Interleukin-4 (IL-4), IL-10, and IL-13 secreted by Th2 cells and/or Tregs promoted IgG4 Ab production by healthy control B cells in vitro[12]. In fact, expression of IL-10, IL-13, and transforming growth factor-β1 (TGF-β1), also produced by Th2 and/or Tregs, was found to be higher in the livers of patients with IgG4-RD than in the livers of patients with other autoimmune biliary diseases[13]. Moreover, the cytokine responses seen in IgG4-RD were accompanied by the enhanced expression of forkhead box P3 (FOXP3), a critical transcription factor for Tregs[13]. Koyabu et al[14] reported that the number of Tregs correlated with that of IgG4+ cells in the livers of IgG4-RD patients. In line with the enhanced expression of Th2 and Treg-associated cytokines in the liver, patients with IgG4-RD displayed higher expression of Th2 cytokines and chemokines such as IL-4, IL-5, IL-10, C-C motif chemokine ligand 17 (CCL17), and CCL22 in the salivary glands as compared with the levels of these molecules in healthy controls and individuals with the Sjogren syndrome[15]. Such enhanced Th2 responses in the salivary glands were accompanied by TGF-β1 production and accumulation of FOXP3+ Tregs[15]. These data support the idea that Th2 cells and Tregs are involved in the development of AIP and IgG4-RD. Given that Tregs are potent negative regulators of autoimmune reactions, it might be possible that activation of Tregs is an epiphenomenon of persistent strong inflammation rather than a component of inflammation in AIP and IgG4-RD.
Ectopic germinal center formation is observed in the salivary glands of IgG4-RD patients[15]. Tfh cells, which express B cell lymphoma 6 (BCL6) and C-X-C chemokine receptor type 5 (CXCR5), and produce IL-21, play critical roles in germinal center reactions[4]. Expression levels of BCL6, CXCR5, and IL-21 in the ectopic germinal centers in the salivary glands were significantly higher in patients with IgG4-RD than in those with Sjogren syndrome[16]. Tfh cells isolated from the salivary glands and peripheral blood of patients with IgG4-RD had a greater capacity to stimulate IgG4 Ab production by B cells than tonsillar Tfh cells[17,18]. The percentage of circulating Tfh2 cells, defined as CXCR5+ CXCR3− C-C chemokine receptor type 6− cells, positively correlated with serum IgG4 concentration in patients with IgG4-RD[19]. Thus, these data support the idea that Tfh cells are involved in the immunopathogenesis of IgG4-RD through the induction of germinal center reaction. However, the roles played by Tfh cells in AIP have not been clarified.
CD4+ CTLs are a unique population of effector T cells that are often seen in patients with chronic viral infections. CD4+ CTLs are localized in the salivary glands of IgG4-RD patients[20,21]. These cells express T-box-expressed-in-T-cells (T-bet) and produce interferon-g (IFN-γ)[4]. Although both Th1 cells and CD4+ CTLs express T-bet and IFN-γ, they differ in expression levels of myeloid cell markers (CD11b) and in the ability to produce CCL4 and IL-1β[20,21]. In addition, these cells produce several cytotoxic proteins, including perforin and granzymes[20,21]. More importantly, these cells secrete TGF-β1, one of the prototypical pro-fibrogenic factors. Thus, CD4+ CTLs are involved in chronic fibroinflammatory responses associated with IgG4-RD. However, accumulation of CD4+ CTLs has not been verified in the pancreas of patients with AIP.
Innate immunity
Innate immunity is one of the major host defense mechanisms against microbial infections[22,23]. Recognition of microbial components by Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) induces pro-inflammatory cytokine responses to eradicate microbial infections[22,23]. It is well established that excessive innate immune responses underlie various types of autoimmune disorders[4,24]. Recent studies have highlighted the importance of innate immunity in AIP and IgG4-RD (Figure 1)[4].
We were the first to address the role of innate immunity in the development of AIP and IgG4-RD. We initially examined whether peripheral blood mononuclear cells (PBMCs) isolated from IgG4-RD patients produced pro-inflammatory cytokines upon exposure to TLR ligands and found that they secreted more IgG4 Ab and Th2 cytokines than PBMCs from healthy controls[25]. In the subsequent studies, we utilized a co-culture system composed of peripheral blood CD14+ monocytes, CD19+ B cells, and CD3+ T cells isolated from patients with AIP and IgG4-RD. This co-culture system allowed us to show that B cells produced a large amount of IgG4 Ab in the presence of NLR and TLR ligands upon co-culture with monocytes isolated from patients with AIP and IgG4-RD, but not with monocytes from healthy controls[26]. Interestingly, peripheral blood monocytes isolated from patients with AIP and IgG4-RD efficiently induced IgG4 Ab production by B cells from healthy controls in a T cell-independent manner. Stimulation of TLRs and NLRs led to the production of B cell-activating factor (BAFF), which induced IgG4 Ab responses[26]. In addition to monocytes, peripheral blood basophils isolated from patients with AIP and IgG4-RD also promoted IgG4 Ab production by B cells from healthy controls in a T cell-independent and BAFF-dependent manner[27]. These pioneering studies fully support the concept that excessive innate immune responses are involved in the development of AIP and IgG4-RD. Indeed, the expression of TLRs was verified in the salivary glands and pancreas of patients with AIP and IgG4-RD[28-30].
It remains uncertain whether innate immune responses are shared by peripheral blood and affected organs in AIP and IgG4-RD. To identify innate immune cells responsible for the development of AIP and IgG4-RD, we utilized a murine experimental model of AIP and IgG4-RD. Repeated intraperitoneal injections of polyinosinic-polycytidylic acid [poly (I:C)] into MRL/MpJ mice led to the development of AIP characterized by the destruction of the pancreatic acinar architecture, immune cell infiltration, and fibrosis[31,32]. Thus, this murine experimental AIP model recapitulated pathological findings observed in human AIP. Extensive flow cytometry analyses revealed massive accumulation of plasmacytoid dendritic cells (pDCs), defined as PDCA-1+B220low, in the pancreas[31]. pDCs are a specialized DC population that produce type I IFNs (IFN-α) upon recognition of TLR7 and TLR9 ligands[33]. Indeed, activation and accumulation of pDCs in the pancreas mediated experimental AIP through the production of IFN-α, because the depletion of pDCs or neutralization of type I IFN by Abs efficiently prevented the development of AIP[31]. Furthermore, pDCs expressing IFN-α and BAFF were found in the pancreas of patients with AIP and IgG4-RD, and peripheral blood pDCs isolated from these patients promoted IgG4 Ab production by healthy control B cells in a type I IFN dependent manner[31]. Thus, these results strongly suggest that activation of pDCs and type I IFN production are prominent features of murine experimental and human AIP.
Although a unique form of fibrosis, called storiform fibrosis, is one of the characteristic findings in human AIP[2-4], molecular mechanisms accounting for the induction and generation of this fibrogenic response have been poorly understood. We recently discovered that the type I IFN-IL-33 axis plays a pro-inflammatory and pro-fibrogenic role in chronic alcoholic pancreatitis[34]. Type I IFN production by pancreatic acinar cells acts in concert with TNF-a produced by pancreatic macrophages to induce a robust production of IL-33 by the former cells[34]. Given that type I IFN produced by pDCs mediates experimental AIP, we hypothesized that IL-33 is involved in the generation of chronic fibroinflammatory responses in the pancreas. pDCs, which accumulate in the pancreas after repeated injections of poly (I:C), produced IL-33 in a type I IFN-dependent manner[32]. Importantly, the blockade of IL-33-mediated signaling pathways by an Ab against the IL-33 receptor attenuated chronic fibroinflammatory responses of the pancreas, which was accompanied by a marked reduction in pro-fibrogenic cytokines such as IL-13 and TGF-β1[32]. Immunofluorescence studies of pancreatic specimens from patients with AIP and IgG4-RD confirmed pancreatic localization of pDCs expressing IL-33[32]. Taken together, these results support the idea that activation of pDCs followed by the production of IFN-α and IL-33 mediates both experimental and human AIP. However, it should be noted that pDCs are not the only cellular source of IL-33. For example, M2 macrophages have been shown to co-localize with IL-33 in the salivary glands of IgG4-RD patients [30,35].
IMMUNOGLOBULINS AS BIOMARKERS IN AUTOIMMUNE PANCREATITIS AND IGG4-RD
The diagnosis of AIP and IgG4-RD relies on the detection of elevated serum concentration of IgG4 Ab as well as on the characteristic pathological findings, including abundant infiltration of IgG4-expressing plasma cells, storiform fibrosis, and obliterative phlebitis[2-4]. Thus, serum level of IgG4 Ab is widely used as an established biomarker for the diagnosis of AIP and IgG4-RD. Moreover, serum concentration of IgG4 declines rapidly after the induction of remission by prednisolone[11]. Indeed, serum concentration of IgG4 Ab was much higher in patients with AIP and IgG4-RD than in individuals with chronic alcoholic pancreatitis and in healthy controls in our previous study[36]. Therefore, there is no doubt that measurement of serum IgG4 Ab concentration in clinical practice is necessary not only for the diagnosis of AIP and IgG4-RD but also for the assessment of disease activity. However, IgG4 Ab level is not always informative for the diagnosis or assessment of disease activity in such patients. Around 20% of patients with AIP have normal serum concentration of IgG4 Ab[10]. Furthermore, a significant fraction of patients with pancreatic cancer also exhibit elevated IgG4 Ab concentration[9]. Moreover, disease flare-up is sometimes seen in patients with AIP and IgG4-RD, even on the background of normalized serum concentrations of IgG4 Ab[11].
IgG4 Ab is unique in that it has a limited ability to activate Fcγ receptors and complements[37]. Thus, IgG4 Ab is considered to play non-pathogenic rather than pathogenic roles in the development of AIP and IgG4-RD. Shiokawa et al[38] directly addressed this issue by utilizing a passive transfer of patient IgG subtypes into neonatal mice. They found that pancreatic injury was successfully induced by a passive transfer of total IgG isolated from patients with AIP, but not by total IgG from healthy controls. The degree of pancreatic injury was much greater in neonatal mice treated with the IgG1 Ab from AIP patients than in those that received the IgG4 Ab from the same patients. Moreover, pancreatic injury induced by a passive transfer of IgG1 Ab was efficiently inhibited by a co-transfer of IgG4 Ab[38]. These studies conducted by Shiokawa et al[38] strongly suggest that IgG1 Ab rather than IgG4 Ab contributes to the immunopathogenesis of AIP and IgG4-RD. Consistent with this idea, serum concentrations of both IgG1 and IgG4 Abs were significantly higher in patients with AIP and IgG4-RD than in individuals with chronic alcoholic pancreatitis or in healthy controls (Table 1)[36].
Table 1.
Possible biomarkers in autoimmune pancreatitis and immunoglobulin G4-related disease
| Biomarkers | Ref. | |
| Immunoglobulins | IgG1 | Minaga et al[36] |
| IgG2 | Chan et al[39] | |
| IgE | Minaga et al[36], Culver et al[41] | |
| IgM | Taguchi et al[40] | |
| Cytokines | IFN-α | Arai et al[31], Minaga et al[36], Minaga et al[53] |
| IL-5 | Yamamoto et al[49] | |
| IL-6 | Tsukuda et al[60] | |
| IL-33 | Furukawa et al[35], Minaga et al[36], Minaga et al[53] | |
| BAFF | Arai et al[31], Kiyama et al[58] | |
| Chemokines | CCL17 | Umeda et al[63] |
| Autoantibodies | Laminin 511 | Shiokawa et al[42] |
| Annexin A11 | Hubers et al[43] | |
| Galectin-3 | Perugino et al[44] | |
BAFF: B cell activating factor; CCL17: C-C motif chemokine ligand 17.
As for the other IgG subtypes, no significant differences were observed in serum concentrations of IgG3 Ab in patients with AIP and IgG4-RD in comparison with those in patients with chronic alcoholic pancreatitis or in healthy controls[36]. Serum concentration of IgG2 Ab was significantly lower in patients with AIP and IgG4-RD than in patients with chronic alcoholic pancreatitis (Table 1)[36]. In contrast, another report showed that serum concentration of IgG2 Ab was elevated in patients suffering from IgG4-RD[39]. This discrepancy can be explained by the difference in the organ distribution of IgG4-RD. Serum concentration of this IgG subtype is preferentially elevated in patients with orbital IgG4-RD, but not in those with pancreatic IgG4-RD[36,39].
Serum total IgG concentration is also elevated in patients with AIP and IgG4-RD[2-4]. Thus, elevations in serum concentrations of both total IgG and IgG4 are prominent features of patients with AIP and IgG4-RD. In contrast to augmented IgG and IgG4 levels, serum concentrations of IgM and IgA are decreased in patients with AIP and IgG4-RD[40]. Furthermore, serum concentration of IgM inversely correlated with those of IgG and IgG4 (Table 1)[40]. The diagnostic value of reduced serum concentrations of IgA and IgM needs to be determined in future studies.
Co-occurrence of AIP/IgG4-RD and allergic disorders is often observed[2-4]. In fact, serum concentration of IgE is significantly higher in patients with AIP and IgG4-RD than in those with chronic alcoholic pancreatitis and in healthy controls[36]. This raises the possibility that serum concentration of IgE can be used as a biomarker for AIP and IgG4-RD. In line with this idea, approximately 50% of patients with AIP and IgG4-RD exhibit elevated serum concentration of IgE[41]. Moreover, changes in serum concentration of IgE are associated with the relapse of these disorders[41]. Therefore, serum concentration of IgE can be used as a biomarker for the diagnosis and prediction of relapse in AIP and IgG4-RD[41].
AUTOANTIBODIES AS BIOMARKERS IN AUTOIMMUNE PANCREATITIS AND IGG4-RD
Although AIP and IgG4-RD are considered to be caused by autoimmune reactions, autoAbs responsible for the development of autoimmunity have not been identified. Recently, three different types of autoAbs have been identified[42-44]. These autoAbs recognize laminin 511, annexin A11, and galectin-3 (Table 1). Fifty-one percent of AIP patients were positive for autoAb against laminin 511-E8, a truncated variant of the extracellular matrix protein laminin 511. Furthermore, serum IgG1 purified from AIP patients co-localized with laminin 511 in the pancreas of neonatal mice upon passive transfer[42]. Huber et al[43] identified annexin A11, a calcium-dependent phospholipid-binding protein, as a candidate autoantigen in AIP. Interestingly, annexin A11-specific IgG4 and IgG1 Abs purified from patients with AIP shared antigenic epitopes and IgG4 autoAbs inhibited pathogenic binding of IgG1 Ab to the shared epitopes[43]. These data suggest that IgG1 autoAbs rather than IgG4 autoAbs play pathogenic roles in the development of AIP and IgG4-RD. Confirmation of these results awaits future studies that should address the diagnostic utility of these autoAbs in a large number of patients with AIP and IgG4-RD. However, identification of autoAbs associated with AIP and IgG4-RD strongly supports the idea that these disorders arise from autoimmune reactions.
ADAPTIVE IMMUNITY CYTOKINES AS BIOMARKERS IN AUTOIMMUNE PANCREATITIS AND IGG4-RD
Effector CD4+ T cell subpopulations, including Th2 cells, Tregs, Tfh cells, and CD4+ CTLs, are involved in the immunopathogenesis of AIP and IgG4-RD, as shown by the localization of these T cells in the affected organs. Th2 cells, Tregs, Tfh cells, and CD4+ CTLs cells were detected in the peripheral blood of patients with AIP or IgG4-RD[17,19,20,45-48] and found to be markedly decreased after induction of remission[20,46], raising the possibility that serum concentrations of cytokines derived from effector T cells can be useful biomarkers (Table 1 and Figure 2). Yamamoto et al[49] found that serum concentration of IL-5 was elevated in patients with IgG4-RD, whereas serum concentrations of IL-10, IL-13, IL-21, and TGF-β1 were comparable in patients and healthy controls. Given that the major cellular source of IL-5 is Th2 cells, these data support the idea that the activation status of Th2 cells might be a surrogate marker for IgG4-RD and AIP. In line with this notion, bile concentrations of Th2 cytokines such as IL-4, IL-5, and IL-13 were significantly higher in the patients with IgG4-related sclerosing cholangitis than in those with primary sclerosing cholangitis[50]. Mechanistically, these Th2 cytokines induce bile leakage due to the impairment of the tight junction-associated biliary epithelial cell barrier, thereby causing chronic biliary inflammation[50]. Moreover, two cases of IgG4-RD successfully treated with dupilumab that neutralizes IL-4 receptor α have been reported[51,52]. Thus, Th2 responses may underlie the immunopathogenesis of AIP and IgG4-RD. Therefore, Th2 cytokines, especially IL-5, can be used as biomarkers of AIP and IgG4-RD.
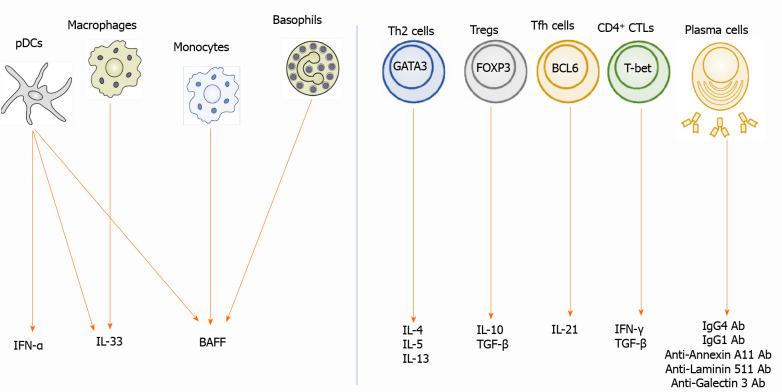
Figure 2.
Biomarkers in autoimmune pancreatitis and immunoglobulin G4-related disease. Left panel: Interferon-a (IFN-α) is produced by plasmacytoid dendritic cells (pDCs). Interleukin-33 (IL-33) is produced by pDCs and macrophages. B cell-activating factor (BAFF) is produced by pDCs, monocytes, and basophils; Right panel: IL-4, IL-5, and IL-13 are produced by T helper type 2 cells expressing GATA-binding protein 3 (GATA3). IL-10 and transforming growth factor-β1 (TGF-β) are produced by regulatory T cells (Tregs) expressing forkhead box P3. IL-21 is produced by follicular helper T cells expressing B cell lymphoma 6. TGF-β is produced by Tregs and CD4+ cytotoxic T cells (CTLs) expressing T-box-expressed-in-T-cells. IFN-γ is produced by CD4+ CTLs. Plasma cells produce immunoglobulin G1 (IgG1) and IgG4 Ab. These cytokines are possible biomarkers for autoimmune pancreatitis and IgG4-related disease. IgG: Immunoglobulin G; pDCs: Plasmacytoid dendritic cells; IFN: Interferon; IL: Interleukin; BAFF: B cell-activating factor; Th2: T helper type 2 cells; Tregs: Regulatory T cells; GATA3: GATA-binding protein 3; FOXP3: Forkhead box P3; Tfh: Follicular helper T; BCL6: B cell lymphoma 6; T-bet: T-box-expressed-in-T-cells; CTLs: CD4+ cytotoxic T cells.
A positive correlation between serum concentration of IgG4 Ab and circulating numbers of Tfh cells has been demonstrated in patients with IgG4-RD[17,19,46]. However, the utility of serum concentration of IL-21, a prototypical cytokine produced by Tfh cells, for IgG4-RD diagnosis has not been verified. Similarly, the usefulness of serum concentrations of IFN-γ and TGF-β1 produced by CD4+ CTLs as biomarkers of AIP and IgG4-RD has not been reported either.
Therefore, at present, the utility of adaptive immunity cytokines as biomarkers for AIP and IgG4-RD is limited. The reason why previous studies did not successfully identify adaptive immunity cytokines as biomarkers might be partially explained by a broad range of affected organs in AIP and IgG4-RD or by complex effector T cell responses. Thus, the classification of IgG4-RD into subtypes by affected organ distribution might lead to the identification of biomarkers specific for each such subtype.
INNATE IMMUNITY CYTOKINES AS BIOMARKERS IN AUTOIMMUNE PANCREATITIS AND IGG4-RD
Activation of pDCs and the subsequent robust production of IFN-α and IL-33 are characteristic pathogenic immune responses in experimental AIP and human IgG4-RD[31,32]. These findings led us to examine whether serum concentrations of IFN-α and IL-33 could be useful biomarkers for AIP and IgG4-RD (Table 1 and Figure 2). For this purpose, we measured serum concentrations of these cytokines in patients with AIP and IgG4-RD who met the well-established diagnostic criteria[5,6,36]. In comparison with the patients with chronic alcoholic pancreatitis and healthy controls, the patients with AIP and IgG4-RD displayed markedly elevated serum concentrations of IFN-α and IL-33[36]. In contrast, serum levels of the prototypical proinflammatory cytokines IL-1β and IL-6 were comparable in the patients with AIP/IgG4-RD and those with chronic alcoholic pancreatitis[36]. Serum concentrations of IFN-α and IL-33 positively correlated with those of IgG4 Ab[36]. Thus, measurements of serum concentrations of IFN-α and IL-33 may be very useful for the diagnosis of AIP and IgG4-RD[36].
We then evaluated the diagnostic performance of serum IFN-α and IL-33 concentrations for AIP and IgG4-RD. Surprisingly, the diagnostic performance of serum IFN-α and IL-33 concentrations as diagnostic markers for AIP and IgG4-RD was comparable to that of serum IgG4 Ab, as calculated by the receiver operating characteristic curve analysis[36]. Moreover, the induction of remission by prednisolone markedly reduced serum concentrations of IFN-α and IL-33. Thus, serum IFN-α and IL-33 concentrations can be biomarkers that are useful not only for the diagnosis but also for the assessment of disease activity. Taken together, our data strongly suggested that serum concentrations of IFN-α and IL-33 might serve as novel biomarkers in AIP and IgG4-RD. This idea is fully supported by an observation of an AIP/IgG4-RD case in which serum concentrations of IFN-α and IL-33 were markedly reduced soon after the induction of remission, whereas those of IgG4 remained unchanged even after the successful induction of remission[53]. Identification of the IFN-α-IL-33 axis as a crucial pathogenic pathway as well as a biomarker leads us to speculate that patients with AIP and IgG4-RD can be treated with biologics targeting IFN-α as in the case of systemic lupus erythematosus[54,55].
BAFF and a proliferation-inducing ligand (APRIL) are cytokines produced by antigen-presenting cells[56]. BAFF and APRIL are crucial factors for B cell survival and thus, they promote Ig production[56]. Given that AIP and IgG4-RD are characterized by elevated concentrations of serum total IgG and IgG4, in particular, it is likely that BAFF and APRIL are involved in the immunopathogenesis of these disorders. Indeed, pDCs producing BAFF have been demonstrated in the pancreas of patients with AIP and IgG4-RD[31]. Moreover, T cell-independent class switch recombination of IgG4 Ab requires BAFF production by monocytes[26]. The involvement of B cell survival factors in the development of AIP and IgG4-RD is further supported by the high probability of successful remission induction by rituximab in patients with IgG4-RD[57]. As for the utility of BAFF and APRIL as biomarkers for AIP and IgG4-RD, serum concentrations of BAFF and APRIL were significantly higher in patients with these disorders than in healthy controls[31,58]. In addition, induction of remission by prednisolone markedly reduced serum concentrations of BAFF[58]. Therefore, measurements of serum concentrations of BAFF and APRIL might be very useful not only for the diagnosis but also for monitoring disease activity.
IL-6 is a pleiotropic cytokine associated with autoimmune responses[59]. Although serum concentrations of this cytokine were comparable in patients with chronic pancreatitis, patients with AIP/IgG4-RD, and healthy controls in our study, elevated serum IL-6 level might help to discriminate a specific type of patients with AIP and IgG4-RD. Tsukuda et al[60] compared clinical manifestations of patients with AIP and IgG4-RD in relation to serum concentration of IL-6. They found that hepatosplenomegaly and biliary tract involvement tended to be more prevalent in patients with high IL-6 serum level than in those with low IL-6 concentration[60]. However, we need to be cautious regarding the interpretation of these data, because hepatosplenomegaly is often seen in patients with multicentric Castleman disease, an IL-6-driven systemic autoimmune disorder[61]. Therefore, some patients with AIP and IgG4-RD might exhibit clinical manifestations similar to those of Castleman disease.
These previous studies on innate immunity cytokines have opened up new research vistas that can facilitate identification of novel biomarkers in AIP and IgG4-RD. In particular, serum concentrations of IFN-α and IL-33, which faithfully reflect disease activity, may be informative diagnostic examinations in AIP and IgG4-RD[36].
CHEMOKINES AS BIOMARKERS IN AUTOIMMUNE PANCREATITIS AND IGG4-RD
AIP and IgG4-RD are characterized by Th2 responses[2-4]. The prototypical Th2 chemokine, thymus and activation-regulated chemokine, also known as CCL17, is a well-established biomarker for atopic dermatitis[62]. Given that atopic dermatitis and IgG4-RD share Th2 responses, it is likely that serum concentration of CCL17 could be a useful biomarker for IgG4-RD and AIP. Umeda et al[63] explored the utility of serum concentration of CCL17 as a possible biomarker for IgG4-RD (Table 1). They found that serum concentration of CCL17 was significantly higher in patients with IgG4-RD than in those with Sjogren syndrome or in healthy controls[63]. Although no association between serum concentrations of CCL17 and IgG4 Ab have been observed, those of CCL17 positively correlated with the number of affected organs. However, the utility of this chemokine as a biomarker for AIP has not been examined.
As mentioned above, production of IFN-α by pDCs is a prominent feature of AIP and IgG4-RD. Excessive IFN-α responses result in the robust production of chemokines such as C-X-C motif chemokine ligand 9 (CXCL9) and CXCL10[31]. In fact, the development of experimental AIP is accompanied by the enhanced expression of CXCL9 and CXCL10 in the pancreas[31]. The utility of CXCL9 and CXCL10 as biomarkers for AIP and IgG4-RD awaits future studies with the use of samples from patients with AIP and IgG4-RD.
CONCLUSION
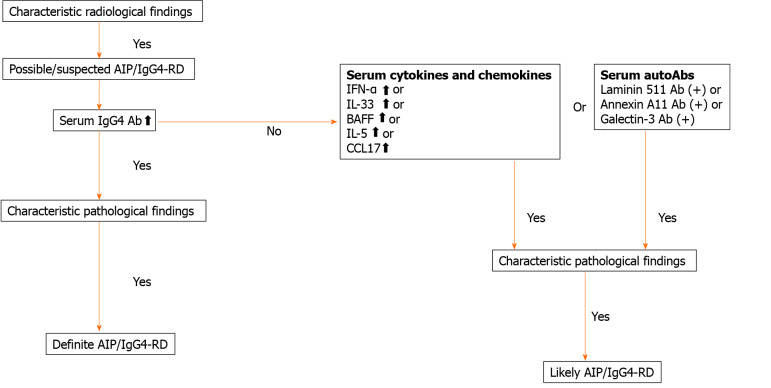
AIP and IgG4-RD are newly established disease entities[2-4]. Both disorders are characterized by elevated serum concentration of IgG4 Ab and accumulation of IgG4-expressing plasma cells in the affected organs[2-4]. Moreover, the induction of remission by prednisolone is accompanied by a marked decrease in serum concentration of IgG4 Ab in patients with AIP and IgG4-RD[2-4]. Therefore, serum concentration of this IgG subtype is undoubtedly a useful biomarker for both the diagnosis and assessment of disease activity. However, a significant fraction of patients with AIP display active disease even at normal serum IgG4 Ab concentration. Recent elegant studies have shown that IgG1 rather than IgG4 plays the main pathogenic role in the development of AIP and IgG4-RD[38]. Thus, elevated IgG4 Ab responses seen in AIP and IgG4-RD are an epiphenomenon associated with chronic inflammatory reactions. Therefore, novel biomarkers based on the understanding of immunopathogenesis need to be established. We have recently found that pDCs producing IFN-α and IL-33 mediate experimental AIP and human IgG4-RD[31,32]. Interestingly, serum concentrations of IFN-α and IL-33 have been identified as potent biomarkers for the diagnosis and assessment of disease activity in AIP and IgG4-RD[36,53]. Based on recently identified biomarkers of these disorders, we propose diagnostic algorithm for patients with AIP and IgG4-RD exhibiting normal or slightly elevated concentrations of serum IgG4 Ab (Figure 3). As shown in Figure 3, measurement of serum concentrations of cytokines and autoAbs in combination with serum IgG4 Ab might be useful for the diagnosis of AIP and IgG4-RD affluent in diversity.
Figure 3.
Diagnostic algorithm for autoimmune pancreatitis and immunoglobulin G4-related disease. Serum concentration of cytokines and chemokines or the presence of serum auto-antibodies may be useful for diagnosis of autoimmune pancreatitis and immunoglobulin G4 (IgG4)-related disease displaying normal or slightly elevated concentrations of IgG4. AIP: Autoimmune pancreatitis; IgG4-RD: Immunoglobulin G4-related disease; IFN: Interferon; IL: Interleukin; BAFF: B cell-activating factor; CCL17: C-C motif chemokine ligand 17.
As our knowledge of the immunopathogenesis of AIP and IgG4-RD increases, many candidate biomarkers will likely be identified in the future. The discovery of such biomarkers will contribute to the clinical practice and advance further our understanding of AIP and IgG4-RD immunopathogenesis.
ACKNOWLEDGEMENTS
We would like to thank Ms. Yukiko Ueno for her secretarial assistance.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicts of interest to disclose.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, No. 034410.
Peer-review started: February 11, 2021
First decision: March 14, 2021
Article in press: April 26, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Strainiene S, Tabibian JH S-Editor: Liu M L-Editor: A P-Editor: Ma YJ
Contributor Information
Akane Hara, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama 589-8511, Japan.
Tomohiro Watanabe, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama 589-8511, Japan. tomohiro@med.kindai.ac.jp.
Kosuke Minaga, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama 589-8511, Japan.
Tomoe Yoshikawa, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama 589-8511, Japan.
Ken Kamata, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama 589-8511, Japan.
Masatoshi Kudo, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama 589-8511, Japan.
References
- 1.Kamisawa T, Chari ST, Lerch MM, Kim MH, Gress TM, Shimosegawa T. Recent advances in autoimmune pancreatitis: type 1 and type 2. Gut. 2013;62:1373–1380. doi: 10.1136/gutjnl-2012-304224. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Minaga K, Kamata K, Kudo M, Strober W. Mechanistic Insights into Autoimmune Pancreatitis and IgG4-Related Disease. Trends Immunol. 2018;39:874–889. doi: 10.1016/j.it.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Kawa S, Kamisawa T, Notohara K, Fujinaga Y, Inoue D, Koyama T, Okazaki K. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011. Pancreas. 2020;49:e13–e14. doi: 10.1097/MPA.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umehara H, Okazaki K, Nakamura T, Satoh-Nakamura T, Nakajima A, Kawano M, Mimori T, Chiba T. Current approach to the diagnosis of IgG4-related disease - Combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol. 2017;27:381–391. doi: 10.1080/14397595.2017.1290911. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Cai S, Ye C, Dong L. Biomarkers in IgG4-related disease: A systematic review. Semin Arthritis Rheum. 2020;50:354–359. doi: 10.1016/j.semarthrit.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Culver EL, Sadler R, Simpson D, Cargill T, Makuch M, Bateman AC, Ellis AJ, Collier J, Chapman RW, Klenerman P, Barnes E, Ferry B. Elevated Serum IgG4 Levels in Diagnosis, Treatment Response, Organ Involvement, and Relapse in a Prospective IgG4-Related Disease UK Cohort. Am J Gastroenterol. 2016;111:733–743. doi: 10.1038/ajg.2016.40. [DOI] [PubMed] [Google Scholar]
- 9.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 10.Kamisawa T, Takuma K, Tabata T, Inaba Y, Egawa N, Tsuruta K, Hishima T, Sasaki T, Itoi T. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol. 2011;46:108–116. doi: 10.1007/s00535-010-0317-2. [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T, Takatori H, Yamakita K, Kubota K, Hamano H, Okamura K, Hirano K, Ito T, Ko SB, Omata M. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–1507. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 12.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–3561. [PubMed] [Google Scholar]
- 13.Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 14.Koyabu M, Uchida K, Miyoshi H, Sakaguchi Y, Fukui T, Ikeda H, Takaoka M, Hirohara J, Nishio A, Uemura Y, Uemoto S, Okazaki K. Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol. 2010;45:732–741. doi: 10.1007/s00535-010-0199-3. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, Shinozaki S, Kubo Y, Nakamura S. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64:254–263. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 16.Maehara T, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Tanaka A, Shinozaki S, Kubo Y, Nakamura S. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Ann Rheum Dis. 2012;71:2011–2019. doi: 10.1136/annrheumdis-2012-201477. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Lin W, Yang H, Wang M, Zhang P, Feng R, Chen H, Peng L, Zhang X, Zhao Y, Zeng X, Zhang F, Zhang W, Lipsky PE. Aberrant Expansion and Function of Follicular Helper T Cell Subsets in IgG4-Related Disease. Arthritis Rheumatol. 2018;70:1853–1865. doi: 10.1002/art.40556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamekura R, Takano K, Yamamoto M, Kawata K, Shigehara K, Jitsukawa S, Nagaya T, Ito F, Sato A, Ogasawara N, Tsubomatsu C, Takahashi H, Nakase H, Himi T, Ichimiya S. Cutting Edge: A Critical Role of Lesional T Follicular Helper Cells in the Pathogenesis of IgG4-Related Disease. J Immunol. 2017;199:2624–2629. doi: 10.4049/jimmunol.1601507. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama M, Suzuki K, Yamaoka K, Yasuoka H, Takeshita M, Kaneko Y, Kondo H, Kassai Y, Miyazaki T, Morita R, Yoshimura A, Takeuchi T. Number of Circulating Follicular Helper 2 T Cells Correlates With IgG4 and Interleukin-4 Levels and Plasmablast Numbers in IgG4-Related Disease. Arthritis Rheumatol. 2015;67:2476–2481. doi: 10.1002/art.39209. [DOI] [PubMed] [Google Scholar]
- 20.Mattoo H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E, Wallace ZS, Kulikova M, Drijvers JM, Daccache J, Carruthers MN, Castelino FV, Stone JR, Stone JH, Pillai S. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016;138:825–838. doi: 10.1016/j.jaci.2015.12.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maehara T, Mattoo H, Ohta M, Mahajan VS, Moriyama M, Yamauchi M, Drijvers J, Nakamura S, Stone JH, Pillai SS. Lesional CD4+ IFN-γ+ cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis. 2017;76:377–385. doi: 10.1136/annrheumdis-2016-209139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 23.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 24.Ganguly D. Do Type I Interferons Link Systemic Autoimmunities and Metabolic Syndrome in a Pathogenetic Continuum? Trends Immunol. 2018;39:28–43. doi: 10.1016/j.it.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Akitake R, Watanabe T, Zaima C, Uza N, Ida H, Tada S, Nishida N, Chiba T. Possible involvement of T helper type 2 responses to Toll-like receptor ligands in IgG4-related sclerosing disease. Gut. 2010;59:542–545. doi: 10.1136/gut.2009.200972. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Yamashita K, Fujikawa S, Sakurai T, Kudo M, Shiokawa M, Kodama Y, Uchida K, Okazaki K, Chiba T. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012;64:914–924. doi: 10.1002/art.33386. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Yamashita K, Sakurai T, Kudo M, Shiokawa M, Uza N, Kodama Y, Uchida K, Okazaki K, Chiba T. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J Gastroenterol. 2013;48:247–253. doi: 10.1007/s00535-012-0626-8. [DOI] [PubMed] [Google Scholar]
- 28.Fukui Y, Uchida K, Sakaguchi Y, Fukui T, Nishio A, Shikata N, Sakaida N, Uemura Y, Satoi S, Okazaki K. Possible involvement of Toll-like receptor 7 in the development of type 1 autoimmune pancreatitis. J Gastroenterol. 2015;50:435–444. doi: 10.1007/s00535-014-0977-4. [DOI] [PubMed] [Google Scholar]
- 29.Yanagawa M, Uchida K, Ando Y, Tomiyama T, Yamaguchi T, Ikeura T, Fukui T, Nishio A, Uemura Y, Miyara T, Okamoto H, Satoi S, Okazaki K. Basophils activated via TLR signaling may contribute to pathophysiology of type 1 autoimmune pancreatitis. J Gastroenterol. 2018;53:449–460. doi: 10.1007/s00535-017-1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro N, Moriyama M, Furusho K, Furukawa S, Shibata T, Murakami Y, Chinju A, Haque ASMR, Gion Y, Ohta M, Maehara T, Tanaka A, Yamauchi M, Sakamoto M, Mochizuki K, Ono Y, Hayashida JN, Sato Y, Kiyoshima T, Yamamoto H, Miyake K, Nakamura S. Activated M2 Macrophages Contribute to the Pathogenesis of IgG4-Related Disease via Toll-like Receptor 7/Interleukin-33 Signaling. Arthritis Rheumatol. 2020;72:166–178. doi: 10.1002/art.41052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arai Y, Yamashita K, Kuriyama K, Shiokawa M, Kodama Y, Sakurai T, Mizugishi K, Uchida K, Kadowaki N, Takaori-Kondo A, Kudo M, Okazaki K, Strober W, Chiba T, Watanabe T. Plasmacytoid Dendritic Cell Activation and IFN-α Production Are Prominent Features of Murine Autoimmune Pancreatitis and Human IgG4-Related Autoimmune Pancreatitis. J Immunol. 2015;195:3033–3044. doi: 10.4049/jimmunol.1500971. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Yamashita K, Arai Y, Minaga K, Kamata K, Nagai T, Komeda Y, Takenaka M, Hagiwara S, Ida H, Sakurai T, Nishida N, Strober W, Kudo M. Chronic Fibro-Inflammatory Responses in Autoimmune Pancreatitis Depend on IFN-α and IL-33 Produced by Plasmacytoid Dendritic Cells. J Immunol. 2017;198:3886–3896. doi: 10.4049/jimmunol.1700060. [DOI] [PubMed] [Google Scholar]
- 33.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe T, Sadakane Y, Yagama N, Sakurai T, Ezoe H, Kudo M, Chiba T, Strober W. Nucleotide-binding oligomerization domain 1 acts in concert with the cholecystokinin receptor agonist, cerulein, to induce IL-33-dependent chronic pancreatitis. Mucosal Immunol. 2016;9:1234–1249. doi: 10.1038/mi.2015.144. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa S, Moriyama M, Miyake K, Nakashima H, Tanaka A, Maehara T, Iizuka-Koga M, Tsuboi H, Hayashida JN, Ishiguro N, Yamauchi M, Sumida T, Nakamura S. Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Sci Rep. 2017;7:42413. doi: 10.1038/srep42413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minaga K, Watanabe T, Hara A, Kamata K, Omoto S, Nakai A, Otsuka Y, Sekai I, Yoshikawa T, Yamao K, Takenaka M, Chiba Y, Kudo M. Identification of serum IFN-α and IL-33 as novel biomarkers for type 1 autoimmune pancreatitis and IgG4-related disease. Sci Rep. 2020;10:14879. doi: 10.1038/s41598-020-71848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–477. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 38.Shiokawa M, Kodama Y, Kuriyama K, Yoshimura K, Tomono T, Morita T, Kakiuchi N, Matsumori T, Mima A, Nishikawa Y, Ueda T, Tsuda M, Yamauchi Y, Minami R, Sakuma Y, Ota Y, Maruno T, Kurita A, Sawai Y, Tsuji Y, Uza N, Matsumura K, Watanabe T, Notohara K, Tsuruyama T, Seno H, Chiba T. Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016;65:1322–1332. doi: 10.1136/gutjnl-2015-310336. [DOI] [PubMed] [Google Scholar]
- 39.Chan ASY, Mudhar H, Shen SY, Lang SS, Fernando M, Hilmy MH, Guppy NJ, Rennie I, Dunkley L, Al Jajeh I. Serum IgG2 and tissue IgG2 plasma cell elevation in orbital IgG4-related disease (IgG4-RD): Potential use in IgG4-RD assessment. Br J Ophthalmol. 2017;101:1576–1582. doi: 10.1136/bjophthalmol-2017-310148. [DOI] [PubMed] [Google Scholar]
- 40.Taguchi M, Kihara Y, Nagashio Y, Yamamoto M, Otsuki M, Harada M. Decreased production of immunoglobulin M and A in autoimmune pancreatitis. J Gastroenterol. 2009;44:1133–1139. doi: 10.1007/s00535-009-0106-y. [DOI] [PubMed] [Google Scholar]
- 41.Culver EL, Sadler R, Bateman AC, Makuch M, Cargill T, Ferry B, Aalberse R, Barnes E, Rispens T. Increases in IgE, Eosinophils, and Mast Cells Can be Used in Diagnosis and to Predict Relapse of IgG4-Related Disease. Clin Gastroenterol Hepatol 2017; 15: 1444-1452. :e6. doi: 10.1016/j.cgh.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, Yamazaki H, Morita T, Marui S, Sogabe Y, Kakiuchi N, Matsumori T, Mima A, Nishikawa Y, Ueda T, Tsuda M, Yamauchi Y, Sakuma Y, Maruno T, Uza N, Tsuruyama T, Mimori T, Seno H, Chiba T. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med. 2018;10:eaaq0997. doi: 10.1126/scitranslmed.aaq0997. [DOI] [PubMed] [Google Scholar]
- 43.Hubers LM, Vos H, Schuurman AR, Erken R, Oude Elferink RP, Burgering B, van de Graaf SFJ, Beuers U. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut. 2018;67:728–735. doi: 10.1136/gutjnl-2017-314548. [DOI] [PubMed] [Google Scholar]
- 44.Perugino CA, AlSalem SB, Mattoo H, Della-Torre E, Mahajan V, Ganesh G, Allard-Chamard H, Wallace Z, Montesi SB, Kreuzer J, Haas W, Stone JH, Pillai S. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol 2019; 143: 736-745. :e6. doi: 10.1016/j.jaci.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto M, Takano KI, Kamekura R, Aochi S, Suzuki C, Ichimiya S, Nakase H, Himi T, Takahashi H. Interleukin 5-producing ST2+ memory Th2 cells in IgG4-related dacryoadenitis and sialadenitis. Mod Rheumatol. 2019;29:856–860. doi: 10.1080/14397595.2018.1526357. [DOI] [PubMed] [Google Scholar]
- 46.Kubo S, Nakayamada S, Zhao J, Yoshikawa M, Miyazaki Y, Nawata A, Hirata S, Nakano K, Saito K, Tanaka Y. Correlation of T follicular helper cells and plasmablasts with the development of organ involvement in patients with IgG4-related disease. Rheumatology (Oxford) 2018;57:514–524. doi: 10.1093/rheumatology/kex455. [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi H, Uchida K, Taniguchi T, Yazumi S, Matsushita M, Takaoka M, Okazaki K. Circulating naïve and CD4+CD25high regulatory T cells in patients with autoimmune pancreatitis. Pancreas. 2008;36:133–140. doi: 10.1097/MPA.0b013e3181577553. [DOI] [PubMed] [Google Scholar]
- 48.Mattoo H, Della-Torre E, Mahajan VS, Stone JH, Pillai S. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy. 2014;69:399–402. doi: 10.1111/all.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto M, Takano K, Kamekura R, Suzuki C, Ichimiya S, Himi T, Nakase H, Takahashi H. Stage classification of IgG4-related dacryoadenitis and sialadenitis by the serum cytokine environment. Mod Rheumatol. 2018;28:1004–1008. doi: 10.1080/14397595.2018.1436029. [DOI] [PubMed] [Google Scholar]
- 50.Müller T, Beutler C, Picó AH, Otten M, Dürr A, Al-Abadi H, Guckelberger O, Meyer Zum Büschenfelde D, Jöhrens K, Volkmann M, Lankisch T, Voigtländer T, Anders M, Shibolet O, Jefferson DM, Podolsky DK, Fischer A, Veltzke-Schlieker W, Adler A, Baumgart DC, Sturm A, Wiedenmann B, Schott E, Berg T. Increased T-helper 2 cytokines in bile from patients with IgG4-related cholangitis disrupt the tight junction-associated biliary epithelial cell barrier. Gastroenterology. 2013;144:1116–1128. doi: 10.1053/j.gastro.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 51.Ebbo M, De Sainte-Marie B, Muller R, Piperoglou C, Grados A, Vély F, Schleinitz N. Comment on article: 'Dupilumab as a novel steroid-sparing treatment for IgG4-related disease' by Simpson et al. Ann Rheum Dis. 2020:epub ahead of print. doi: 10.1136/annrheumdis-2020-217010. [DOI] [PubMed] [Google Scholar]
- 52.Simpson RS, Lau SKC, Lee JK. Dupilumab as a novel steroid-sparing treatment for IgG4-related disease. Ann Rheum Dis. 2020;79:549–550. doi: 10.1136/annrheumdis-2019-216368. [DOI] [PubMed] [Google Scholar]
- 53.Minaga K, Watanabe T, Kamata K, Takenaka M, Yasukawa S, Kudo M. The IFN-α-IL-33 Axis as Possible Biomarkers in IgG4-Related Disease. Am J Gastroenterol. 2019;114:1002–1003. doi: 10.14309/ajg.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 54.Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, Illei GG, Drappa J, Wang L, Yoo S CD1013 Study Investigators. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69:376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, Drappa J, Wang L, Greth W CD1067 study investigators. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 57.Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, Deshpande V, Smyrk TC, Chari S, Stone JH. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–1177. doi: 10.1136/annrheumdis-2014-206605. [DOI] [PubMed] [Google Scholar]
- 58.Kiyama K, Kawabata D, Hosono Y, Kitagori K, Yukawa N, Yoshifuji H, Omura K, Fujii T, Mimori T. Serum BAFF and APRIL levels in patients with IgG4-related disease and their clinical significance. Arthritis Res Ther. 2012;14:R86. doi: 10.1186/ar3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 60.Tsukuda S, Ikeura T, Ito T, Nakamaru K, Masuda M, Hori Y, Ikemune M, Yanagawa M, Tanaka T, Tomiyama T, Yamaguchi T, Ando Y, Uchida K, Fukui T, Nishio A, Terasawa R, Tanigawa N, Okazaki K. Clinical implications of elevated serum interleukin-6 in IgG4-related disease. PLoS One. 2020;15:e0227479. doi: 10.1371/journal.pone.0227479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato Y, Kojima M, Takata K, Morito T, Asaoku H, Takeuchi T, Mizobuchi K, Fujihara M, Kuraoka K, Nakai T, Ichimura K, Tanaka T, Tamura M, Nishikawa Y, Yoshino T. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Mod Pathol. 2009;22:589–599. doi: 10.1038/modpathol.2009.17. [DOI] [PubMed] [Google Scholar]
- 62.Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K, Tamaki K. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 63.Umeda M, Origuchi T, Kawashiri SY, Koga T, Ichinose K, Furukawa K, Sato T, Tsuji S, Endo Y, Takatani A, Shimizu T, Fukui S, Iwamoto N, Igawa T, Tamai M, Nakamura H, Kawakami A. Thymus and Activation-regulated Chemokine as a Biomarker for IgG4-related Disease. Sci Rep. 2020;10:6010. doi: 10.1038/s41598-020-62941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]