Abstract
Compelling evidence supports the crucial role of the receptor for advanced glycation end-products (RAGE) axis activation in many clinical entities. Since the beginning of the coronavirus disease 2019 pandemic, there is an increasing concern about the risk and handling of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in inflammatory gastrointestinal disorders, such as inflammatory bowel diseases (IBD). However, clinical data raised during pandemic suggests that IBD patients do not have an increased risk of contracting SARS-CoV-2 infection or develop a more severe course of infection. In the present review, we intend to highlight how two potentially important contributors to the inflammatory response to SARS-CoV-2 infection in IBD patients, the RAGE axis activation as well as the cross-talk with the renin-angiotensin system, are dampened by the high expression of soluble forms of both RAGE and the angiotensin-converting enzyme (ACE) 2. The soluble form of RAGE functions as a decoy for its ligands, and soluble ACE2 seems to be an additionally attenuating contributor to RAGE axis activation, particularly by avoiding the transactivation of the RAGE axis that can be produced by the virus-mediated imbalance of the ACE/angiotensin II/angiotensin II receptor type 1 pathway.
Keywords: COVID-19, Inflammatory bowel diseases, Advanced glycation, Angiotensin-converting enzyme 2, Alarmins, Receptor for advanced glycation end-products, Receptor for advanced glycation end-products axis, Inflammation
Core Tip: Data raised during the pandemic suggest that inflammatory bowel diseases do not have an increased risk of contracting severe acute respiratory syndrome coronavirus 2 infection or develop a more severe course of infection. These findings are in some way unexpected considering that inflammatory bowel disease is a chronic inflammatory state of the gastrointestinal tract. We herein discuss how the receptor for advanced glycation end-products axis activation as well as the cross-talk with the renin-angiotensin system are dampened by the high expression of soluble forms of both receptor for advanced glycation end-products and angiotensin-converting enzyme 2.
INTRODUCTION
At the end of 2019, China reported several cases of severe pneumonia of unknown cause; the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was subsequently identified as the etiological agent[1]. Due to its rapid spread all over the world, the World Health Organization defined coronavirus disease 2019 (COVID-19) as a pandemic on January 30, 2020.
The main symptoms of COVID-19 affect the lower respiratory tract, causing high mortality-rate complications such as acute distress respiratory syndrome[2-6]. However, recent reports reveal that gastrointestinal (GI) manifestations of SARS-CoV-2 infection are common clinical symptoms among patients who develop COVID-19[7-11].
The SARS-CoV-2 uses the cellular transmembrane angiotensin-converting enzyme 2 (ACE2) molecule as the receptor for viral cell entry. Under physiological conditions, epithelial ACE2 is widely expressed in several tissues. However, the expression of epithelial ACE2 in the terminal ileum and colon are amongst the highest in the body, which could explain why COVID-19 patients experience several GI symptoms[12-16].
Consequently, there is an increasing concern about the risk and handling of SARS-CoV-2 infection in inflammatory GI disorders, such as inflammatory bowel disease (IBD). The IBDs are chronic intestinal diseases that comprise Crohn´s disease (CD) and ulcerative colitis, which are characterized by chronic and relapsing intestinal inflammation[17,18]. Thus, since the beginning of the SARS-CoV-2 pandemic, IBD patients were considered a high-risk group for increased severity and adverse outcomes in SARS-CoV-2 infection[19,20].
However, clinical data raised during pandemic suggest that IBD patients do not have an increased risk of contracting SARS-CoV-2 infection or develop a more severe course of infection[21-25]. A compelling body of both clinical and experimental evidence has shed light on the crucial role of the receptor of advanced glycation end-products (RAGE) activation in many chronic inflammatory diseases[26-31]. More recently, the role of RAGE axis activation as a key contributor in the clinical course of SARS-CoV-2 infection has been documented[32].
In the present review, we intend to highlight the role of the RAGE axis activation in the context of SARS-CoV-2 infection and the clinical evolution of the IBD patient.
RAGE AXIS
Firstly described in 1992, the RAGE is a type I single-pass transmembrane protein that can bind advanced glycation-end products (AGEs). This molecule belongs to the immunoglobulin superfamily of cell surface receptors, which is now considered as a pattern recognition receptor and is regarded as a central mediator in chronic inflammatory and immune responses[33-35].
RAGE is usually expressed at low levels in many cell types and tissues, except for the lungs. However, this expression is noticeably increased under inflammatory conditions[36-38].
Besides the transmembrane form of RAGE, several soluble isoforms of this receptor (sRAGE) are generated either by alternative splicing or by the action of membrane associated-proteases, such matrix metalloproteinase-9 (MMP-9), a disintegrin metalloproteases (ADAM)-10, and ADAM-17[39-42]. These soluble variants may function as a decoy receptor for ligands and thus prevent the interaction with the membrane-anchored full-length RAGE. In consequence, a high bioavailability of sRAGE will decreases the inflammatory responses driven by full-length RAGE activation [35,43,44]. Besides AGEs, RAGE can recognize many other ligands including the alarmin high-mobility group box 1 (HMGB1), members of the S100 protein family, glycosaminoglycans, and amyloid β peptides, among many others[35,45].
As a consequence of RAGE engagement by its ligands, multiple signaling pathways are triggered, including reactive oxygen species, p21ras, extracellular signal-regulated protein kinase 1/2 (p44/p42) mitogen-activated protein (MAP) kinases, p38 and stress-activated protein kinases/c-Jun N-terminal kinase mitogen-activated protein kinases, rhoGTPases, phosphoinositol-3 kinase, and the janus kinase/signal transducer and activator of transcription pathway, having crucial downstream inflammatory consequences such as activation of nuclear factor-kappaB (NF-κB), AP-1, and signal transducer and activator of transcription-3[35].
Indeed, the RAGE axis signaling not only triggers pro-inflammatory gene expression but also a positive feed-forward loop, in which the inflammatory stimuli activate NF-κB, which induces RAGE expression, following an enhanced and sustained inflammatory response[35,46-48].
RAGE AXIS ACTIVATION IN IBD
Initially, RAGE axis activation was linked to the complications of diabetes such as macro-and microvascular complications[49,50]. However, a growing body of evidence indicates RAGE as a key molecule involved in many chronic inflammatory diseases[28-30,51].
Many underlying molecular mechanisms are involved in the onset and perpetuation of the disease, particularly those fueling the robust pro-inflammatory signals found in IBD patients[26,52]. Noteworthy, some pieces of evidence reveal an increased expression of RAGE and its ligands on intestinal cells in IBD patients, especially in inflamed areas[53-55]. In this context, it is important to highlight that the release of the RAGE ligand HMGB1 and members of the S100 protein family is increased under inflammation conditions[54-57]. Thus, the engagement of RAGE may play an important role in the maintenance of intestinal injury and inflammatory environment [53-57].
Strikingly, increased levels of both MMP-9 and ADAM17 have been reported in IBD patients[58,59], and both metalloproteases are involved in RAGE shedding, thus increasing the levels of sRAGE, which in turn can modulate the inflammatory responses driven by RAGE axis activation in IBD patients[58]. At present, a compelling body of evidence supports the fact that increased sRAGE levels correlate with a decrease in the RAGE activation-mediated inflammatory responses in many clinical entities[60-63]. In this context, it is important to highlight that CD147 significantly contributes to epithelial inflammation in many clinical entities including IBD[64,65], and it has been recently shown to act as a receptor for SARS-CoV-2[66]. Noteworthy, the inhibition of RAGE activation-mediated inflammatory response leads to a reduced expression of CD147[67].
THE RENIN-ANGIOTENSIN SYSTEM
The renin-angiotensin system (RAS) is a hormonal system regulated by two complementary pathways that mediate opposing effects on inflammation, fibrosis, and cell proliferation[68-70]. Thus, the balance of both pathways determines pro-inflammatory or anti-inflammatory conditions among several systems such as cardiovascular, renal, and respiratory systems[71-74].
The classical pathway mediated via ACE, angiotensin II (Ang II) and its receptor Ang II receptor type 1 (AT1R), triggers activation of pro-inflammatory signals such as oxidative and nitrosative stresses, the induction of cytokines and cell adhesion molecules, as well as the activation of transcription factors such NF-κB[75-78]. On the contrary, the alternative pathway predominantly mediated by ACE2, Ang (1-7) and its receptor Mas (MasR), induces the opposite effects of AT1R activation, being an anti-inflammatory and anti-fibrotic counter regulator of the effects of ACE/Ang II/AT1R[71,75,79,80]. ACE and ACE2 are highly expressed in several tissues such as the lungs, kidneys, and blood vessels. However, the brush border of the ileum and the colon are among the tissues with the highest expression of both enzymes[13-16,81]. Both enzymes can cleave angiotensin, generating different sub-products and regulating the balance between both pathways of the RAS system[79,82,83].
RAS IMBALANCE IN IBD
Recent studies suggest high expression of the major components of both RAS pathways across the ileum and colon[81]. In this sense, the gut could be an especially susceptible organ for the imbalance of RAS pathways. Thus, the dysregulation of these components could have potential implications for inflammation and fibrosis for IBD patients[84,85]. Strikingly, several studies have revealed that the intestinal expression of ACE2 is inversely correlated with fibrosis in IBD patients[81,86].
Additionally, Ang (1-7) ameliorates colonic myofibroblast collagen secretion via MasR[81]. Furthermore, angiotensin receptor blockers and ACE inhibitors are reported to decrease mucosal pro-inflammatory cytokines, ameliorate colitis, and were associated with lower rates of complications, surgery, and hospitalization in patients with IBD[87-89].
Normally, ACE2 breaks down Ang II to Ang 1–7 peptide and thus avoiding the activation of the pro-inflammatory pathways of RAS. However, SARS-CoV-2 can hijack ACE2 and use it to gain entry into host cells[12,90]. Noteworthy, high bioavailability of soluble ACE2 has been reported in IBD patients[81,84], mainly ascribed to the increased level of ADAM17 observed in these patients[58,91-93], which in turn may function as a decoy receptor for SARS-CoV-2 and thus avoiding the hijacking of the counterbalancing enzyme.
This is particularly important considering that a novel ligand-independent mechanism for RAGE transactivation has been recently reported to occur following activation of the AT1R by Ang-II, thus leading to NF-κB dependent expression of pro-inflammatory mediators[48].
RAGE AXIS ACTIVATION AND RAS IMBALANCE IN IBD PATIENTS INFECTED WITH SARS-COV-2
Contrary to what is expected, considering the pathophysiology of IBD, there is currently no evidence for an increased risk of worse clinical outcomes in patients with IBD in the context of COVID-19[21-25]. The role of the RAGE axis in the pathophysiology of IBD has been suggested by different reports[53-57]. The colonic expression of RAGE and some RAGE ligands, such as HMGB1 and some members of the S100 protein family, are significantly higher in IBD patients[54-56]. Besides, this receptor has been also considered a key contributor to the dysregulated and misdirected COVID-19 inflammatory response[32,94].
However, a counterbalancing element must be added to this scenario: The soluble RAGE. This molecule is generated by alternative splicing or by cleavage of the ectodomain of the membrane-anchored RAGE by the action of both MMP-9 and ADAM17, which are highly expressed in IBD patients[58,59]. Therefore, the high bioavailability of soluble RAGE may dampen RAGE activation, despite the abundance of both receptor and ligands in the inflamed intestinal mucosa of IBD patients.
On the other hand, the high expression of ACE2 in GI tract, especially among IBD patients, makes this tissue a particularly trophic niche for infection with SARS-CoV-2. Furthermore, the ACE2 exhaustion mediated by the entry of SARS-CoV-2 may then induce a robust RAS imbalance in favor of the pro-inflammatory ACE/Ang II/AT1R pathway[95]. These observations suggest that the inflamed gut of IBD patients represents an optimal doorway for SARS-CoV-2 entry, driving poor clinical outcomes in IBD patients who develop COVID-19.
However, this hypothetical scenario also has an important counterbalancing actor, the soluble form of ACE2, which is also increased in patients with IBD due to the shedding of the membrane-anchored ACE2 by ADAM17[58-59]. This is particularly important considering the non-cognate transactivation mechanism described for RAGE because of AT1R activation by Ang II[48], which is dampened by the preservation of membrane-associated ACE2 exhaustion by its soluble form.
A growing body of evidence demonstrates that in IBD patients the use of systemic immunosuppression is not associated with an increased risk of COVID-19 patients with IBD[96-100]. Furthermore, we must also keep in mind that the main objective of pharmacological treatments in IBD is to reduce inflammation levels. In this sense, in addition to interfering with signaling pathways, many drugs used in the current treatments also decrease the expression of RAGE and the bioavailability of some RAGE ligands, particularly the alarmins HMGB1 and S100 protein family members[96,97]. Indeed, several authors remark the possible protective role of IBD therapy against SARS-CoV-2 infection, especially through interfering with cytokine activity observed in the clinical course of COVID-19[98-100].
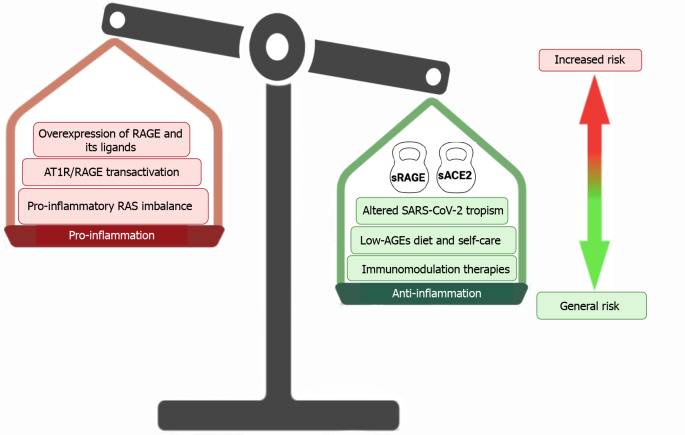
Additionally, the IBD patients have a high self-care standard and follow diets that help them to maintain good nutritional levels and the disease under control[101]. Some of these nutritional regimens are associated with a low-AGE diet, which may contribute to reducing the proinflammatory intestinal milieu mediated by RAGE activation[102] (Figure 1).
Figure 1.
In inflammatory bowel diseases patients, different inflammation–prone mechanisms are known to be activated. Among them, the overexpression of receptor for advanced glycation end-products (RAGE) and the abundance of its ligands may produce a sustained activation of the axis, which can be also fueled by a non-cognate mechanism due to the pro-inflammatory rat sarcoma imbalance. These elements seem to be crucial contributors to the worsening course of inflammatory bowel diseases (IBD) patients with coronavirus disease 2019. However, other elements may dampen these inflammatory contributions, such as the high bioavailability of the soluble forms of both RAGE and angiotensin-converting enzyme 2. Soluble angiotensin-converting enzyme 2 may even interfere with severe acute respiratory syndrome coronavirus 2 entry to epithelial cells. Additionally, most if not all IBD patients are under pharmacological treatments directed to control inflammation. IBD patients deserve special attention to their diets, and as consequence, it is likely the ingestion of dietary advanced glycation-end products is also limited. RAGE: Receptor for advanced glycation end-products; RAS: Renin-angiotensin; ACE2: Angiotensin-converting enzyme 2; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; AT1R: Angiotensin II receptor type 1; AGEs: Advanced glycation-end products; sRAGE: Several soluble isoforms of this receptor.
CONCLUSION
The COVID-19 pandemics represent the worst challenge for a century for health systems all over the world. Severity and mortality have been highest in people with underlying morbidities. Therefore, special efforts have been done to understand how SARS-CoV-2 may particularly fuel inflammation in many clinical entities where the chronicity of an inflammatory environment is a relevant part of the pathogenesis of diseases. Based on a particularly inflamed landscape depicted in IBD patients, the activation of the RAGE axis as well the RAS imbalance seem to be crucial contributors to worsen inflammation in the gut. However, data raised during the pandemic suggests that IBD patients have neither an increased risk of contracting SARS-CoV-2 infection nor developing a more severe course of infection.
RAGE axis activation seems to be dampened by the high bioavailability of soluble receptors functioning as a decoy for its ligands. Additionally, soluble ACE2 seems to be another attenuating contributor to RAGE axis activation, particularly by avoiding the transactivation of RAGE axis that can be produced by the virus-mediated imbalance of the ACE/Ang II/ AT1R pathway. Thus, RAGE axis activation in COVID-19 IBD patients does not seem to be a dangerous liaison.
Footnotes
Conflict-of-interest statement: The authors declare that there is no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: January 24, 2021
First decision: February 22, 2021
Article in press: April 13, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maric I S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Armando Rojas, Biomedical Research Labs, Medicine Faculty, Catholic University of Maule, Talca 3634000, Chile. arojasr@ucm.cl.
Iván Schneider, Biomedical Research Labs, Medicine Faculty, Catholic University of Maule, Talca 3634000, Chile.
Cristian Lindner, Biomedical Research Labs, Medicine Faculty, Catholic University of Maule, Talca 3634000, Chile.
Ileana Gonzàlez, Biomedical Research Labs, Medicine Faculty, Catholic University of Maule, Talca 3634000, Chile.
Miguel Angel Morales, Department of Molecular and Clinical Pharmacology Program, Institute of Biomedical Sciences, University of Chile, Santiago 8320000, Chile.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui KPY, Cheung MC, Perera RAPM, Ng KC, Bui CHT, Ho JCW, Ng MMT, Kuok DIT, Shih KC, Tsao SW, Poon LLM, Peiris M, Nicholls JM, Chan MCW. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020;8:687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831-1833. :e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg M, Christensen B, Lubel JS. Gastrointestinal ACE2, COVID-19 and IBD: Opportunity in the Face of Tragedy? Gastroenterology. 2020;159:1623–1624.e3. doi: 10.1053/j.gastro.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassia R, Testa S, Pan A, Conti CB. SARS-CoV-2 and gastrointestinal tract: The dark side of the pandemic. Dig Liver Dis. 2020;52:700–701. doi: 10.1016/j.dld.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GTEx Consortium . The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 16.Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21:827–835. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 17.Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578:527–539. doi: 10.1038/s41586-020-2025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An P, Ji M, Ren H, Su J, Ding NS, Kang J, Yin A, Zhou Q, Shen L, Zhao L, Jiang X, Xiao Y, Tan W, Lv X, Li J, Liu S, Zhou J, Chen H, Xu Y, Liu J, Chen M, Cao J, Zhou Z, Tan S, Yu H, Dong W, Ding Y. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;5:525–527. doi: 10.1016/S2468-1253(20)30121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, Bloom S, Brooks AJ, Cooney R, Dart RJ, Edwards C, Fraser A, Gaya DR, Ghosh S, Greveson K, Hansen R, Hart A, Hawthorne AB, Hayee B, Limdi JK, Murray CD, Parkes GC, Parkes M, Patel K, Pollok RC, Powell N, Probert CS, Raine T, Sebastian S, Selinger C, Smith PJ, Stansfield C, Younge L, Lindsay JO, Irving PM, Lees CW. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984–990. doi: 10.1136/gutjnl-2020-321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macaluso FS, Orlando A. COVID-19 in patients with inflammatory bowel disease: A systematic review of clinical data. Dig Liver Dis. 2020;52:1222–1227. doi: 10.1016/j.dld.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteleone G, Ardizzone S. Are Patients with Inflammatory Bowel Disease at Increased Risk for Covid-19 Infection? J Crohns Colitis. 2020;14:1334–1336. doi: 10.1093/ecco-jcc/jjaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The Incidence and Outcomes of COVID-19 in IBD Patients: A Rapid Review and Meta-analysis. Inflamm Bowel Dis. 2020;26:e132–e133. doi: 10.1093/ibd/izaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, Danese S, Peyrin-Biroulet L. Incidence and Patterns of COVID-19 Among Inflammatory Bowel Disease Patients From the Nancy and Milan Cohorts. Clin Gastroenterol Hepatol. 2020;18:2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moura FA, Goulart MOF, Campos SBG, da Paz Martins AS. The Close Interplay of Nitro-Oxidative Stress, Advanced Glycation end Products and Inflammation in Inflammatory Bowel Diseases. Curr Med Chem. 2020;27:2059–2076. doi: 10.2174/0929867325666180904115633. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr Opin Investig Drugs. 2006;7:985–991. [PubMed] [Google Scholar]
- 29.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam. 2013;2013:403460. doi: 10.1155/2013/403460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6:1219–1225. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Yalcin Kehribar D, Cihangiroglu M, Sehmen E, Avci B, Capraz A, Yildirim Bilgin A, Gunaydin C, Ozgen M. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomarkers. 2021;26:114–118. doi: 10.1080/1354750X.2020.1861099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 34.Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 35.Rojas A, Delgado-López F, González I, Pérez-Castro R, Romero J, Rojas I. The receptor for advanced glycation end-products: a complex signaling scenario for a promiscuous receptor. Cell Signal. 2013;25:609–614. doi: 10.1016/j.cellsig.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Zen K, Chen CX, Chen YT, Wilton R, Liu Y. Receptor for advanced glycation endproducts mediates neutrophil migration across intestinal epithelium. J Immunol. 2007;178:2483–2490. doi: 10.4049/jimmunol.178.4.2483. [DOI] [PubMed] [Google Scholar]
- 37.González I, Romero J, Rodríguez BL, Pérez-Castro R, Rojas A. The immunobiology of the receptor of advanced glycation end-products: trends and challenges. Immunobiology. 2013;218:790–797. doi: 10.1016/j.imbio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Oczypok EA, Perkins TN, Oury TD. All the "RAGE" in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Bukulin M, Kojro E, Roth A, Metz VV, Fahrenholz F, Nawroth PP, Bierhaus A, Postina R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283:35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 41.Deuss M, Reiss K, Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- 42.Metz VV, Kojro E, Rat D, Postina R. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS One. 2012;7:e41823. doi: 10.1371/journal.pone.0041823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grauen Larsen H, Marinkovic G, Nilsson PM, Nilsson J, Engström G, Melander O, Orho-Melander M, Schiopu A. High Plasma sRAGE (Soluble Receptor for Advanced Glycation End Products) Is Associated With Slower Carotid Intima-Media Thickness Progression and Lower Risk for First-Time Coronary Events and Mortality. Arterioscler Thromb Vasc Biol. 2019;39:925–933. doi: 10.1161/ATVBAHA.118.312319. [DOI] [PubMed] [Google Scholar]
- 44.Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971–1978. doi: 10.2174/092986706777585013. [DOI] [PubMed] [Google Scholar]
- 45.Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Häring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 47.Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349–364. doi: 10.1146/annurev-med-041316-085215. [DOI] [PubMed] [Google Scholar]
- 48.Pickering RJ, Tikellis C, Rosado CJ, Tsorotes D, Dimitropoulos A, Smith M, Huet O, Seeber RM, Abhayawardana R, Johnstone EK, Golledge J, Wang Y, Jandeleit-Dahm KA, Cooper ME, Pfleger KD, Thomas MC. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J Clin Invest. 2019;129:406–421. doi: 10.1172/JCI99987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramasamy R, Yan SF, Schmidt AM. The RAGE axis and endothelial dysfunction: maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc Med. 2005;15:237–243. doi: 10.1016/j.tcm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 52.Yadav V, Varum F, Bravo R, Furrer E, Bojic D, Basit AW. Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Transl Res. 2016;176:38–68. doi: 10.1016/j.trsl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Ciccocioppo R, Vanoli A, Klersy C, Imbesi V, Boccaccio V, Manca R, Betti E, Cangemi GC, Strada E, Besio R, Rossi A, Falcone C, Ardizzone S, Fociani P, Danelli P, Corazza GR. Role of the advanced glycation end products receptor in Crohn's disease inflammation. World J Gastroenterol. 2013;19:8269–8281. doi: 10.3748/wjg.v19.i45.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Z, Wang X, Gong L, Wu G, Peng X, Tang X. Role of high-mobility group box 1 protein in inflammatory bowel disease. Inflamm Res. 2015;64:557–563. doi: 10.1007/s00011-015-0841-x. [DOI] [PubMed] [Google Scholar]
- 55.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamasaki H, Mitsuyama K, Masuda J, Kuwaki K, Takedatsu H, Sugiyama G, Yamada S, Sata M. Roles of high-mobility group box 1 in murine experimental colitis. Mol Med Rep. 2009;2:23–27. doi: 10.3892/mmr_00000056. [DOI] [PubMed] [Google Scholar]
- 57.Manolakis AC, Kapsoritakis AN, Tiaka EK, Potamianos SP. Calprotectin, calgranulin C, and other members of the s100 protein family in inflammatory bowel disease. Dig Dis Sci. 2011;56:1601–1611. doi: 10.1007/s10620-010-1494-9. [DOI] [PubMed] [Google Scholar]
- 58.Cesaro A, Abakar-Mahamat A, Brest P, Lassalle S, Selva E, Filippi J, Hébuterne X, Hugot JP, Doglio A, Galland F, Naquet P, Vouret-Craviari V, Mograbi B, Hofman PM. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1332–G1343. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 59.Meijer MJ, Mieremet-Ooms MA, van der Zon AM, van Duijn W, van Hogezand RA, Sier CF, Hommes DW, Lamers CB, Verspaget HW. Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with Crohn's disease phenotype. Dig Liver Dis. 2007;39:733–739. doi: 10.1016/j.dld.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 61.Lalla E, Lamster IB, Feit M, Huang L, Spessot A, Qu W, Kislinger T, Lu Y, Stern DM, Schmidt AM. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105:1117–1124. doi: 10.1172/JCI8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, Jenkins DG, Stein G, Schmidt AM, Yan SF. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–77. doi: 10.1016/j.atherosclerosis.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 64.Wang H, Ye J, Liu R, Chen G, Zhao J, Huang L, Yang F, Li M, Zhang S, Jingxie , Xiong L, Chen H, Xu Y, Su M, Xie Y, Zheng F, Geng L, Xu W, Gong S. Clinical Significance of CD147 in Children with Inflammatory Bowel Disease. Biomed Res Int. 2020;2020:7647181. doi: 10.1155/2020/7647181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Z, Liu R, Huang L, Xu Y, Su M, Chen J, Geng L, Xu W, Gong S. CD147 Aggravated Inflammatory Bowel Disease by Triggering NF-kB-Mediated Pyroptosis. Biomed Res Int . 2020;2020:5341247. doi: 10.1155/2020/5341247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, Wei D, Zhang Y, Sun XX, Gong L, Yang X, He L, Zhang L, Yang Z, Geng JJ, Chen R, Zhang H, Wang B, Zhu YM, Nan G, Jiang JL, Li L, Wu J, Lin P, Huang W, Xie L, Zheng ZH, Zhang K, Miao JL, Cui HY, Huang M, Zhang J, Fu L, Yang XM, Zhao Z, Sun S, Gu H, Wang Z, Wang CF, Lu Y, Liu YY, Wang QY, Bian H, Zhu P, Chen ZN. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao W, Min D, Twigg SM, Shackel NA, Warner FJ, Yue DK, McLennan SV. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications. Am J Physiol Cell Physiol. 2010;299:C1212–C1219. doi: 10.1152/ajpcell.00228.2010. [DOI] [PubMed] [Google Scholar]
- 68.Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012;35:414–428. doi: 10.1111/j.1365-2036.2011.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-Inflammatory Action of Angiotensin 1-7 in Experimental Colitis. PLoS One. 2016;11:e0150861. doi: 10.1371/journal.pone.0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos RAS, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. The renin-angiotensin system: going beyond the classical paradigms. Am J Physiol Heart Circ Physiol. 2019;316:H958–H970. doi: 10.1152/ajpheart.00723.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014;13:224–234. doi: 10.2174/1871528113666140713164506. [DOI] [PubMed] [Google Scholar]
- 72.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 73.Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res. 2016;107:154–162. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Tan WSD, Liao W, Zhou S, Mei D, Wong WF. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9–17. doi: 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224–236. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 77.Husain K, Hernandez W, Ansari RA, Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem. 2015;6:209–217. doi: 10.4331/wjbc.v6.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18:963–970. doi: 10.2174/138161212799436593. [DOI] [PubMed] [Google Scholar]
- 79.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 80.Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Egido J. Modulation of angiotensin II effects, A potential novel approach to inflammatory and immune diseases. Curr Med Chem. 2003;2:379–394. [Google Scholar]
- 81.Garg M, Royce SG, Tikellis C, Shallue C, Batu D, Velkoska E, Burrell LM, Patel SK, Beswick L, Jackson A, Britto K, Lukies M, Sluka P, Wardan H, Hirokawa Y, Tan CW, Faux M, Burgess AW, Hosking P, Monagle S, Thomas M, Gibson PR, Lubel J. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69:841–851. doi: 10.1136/gutjnl-2019-318512. [DOI] [PubMed] [Google Scholar]
- 82.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7) Physiol Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 84.Garg M, Burrell LM, Velkoska E, Griggs K, Angus PW, Gibson PR, Lubel JS. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J Renin Angiotensin Aldosterone Syst. 2015;16:559–569. doi: 10.1177/1470320314521086. [DOI] [PubMed] [Google Scholar]
- 85.Hirasawa K, Sato Y, Hosoda Y, Yamamoto T, Hanai H. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem. 2002;50:275–282. doi: 10.1177/002215540205000215. [DOI] [PubMed] [Google Scholar]
- 86.Ferreira-Duarte M, Estevinho MM, Duarte-Araújo M, Magro F, Morato M. Unraveling the Role of ACE2, the Binding Receptor for SARS-CoV-2, in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1787–1795. doi: 10.1093/ibd/izaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs JD, Wagner T, Gulotta G, Liao C, Li YC, Bissonnette M, Pekow J. Impact of Angiotensin II Signaling Blockade on Clinical Outcomes in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2019;64:1938–1944. doi: 10.1007/s10620-019-5474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wengrower D, Zanninelli G, Pappo O, Latella G, Sestieri M, Villanova A, Faitelson Y, Pines M, Goldin E. Prevention of fibrosis in experimental colitis by captopril: the role of tgf-beta1. Inflamm Bowel Dis. 2004;10:536–545. doi: 10.1097/00054725-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 89.Mantaka A, Tsoukali E, Fragkaki M, Karmiris K, Viazis N, Mantzaris GJ, Koutroubakis IE. Is there any role of renin-angiotensin system inhibitors in modulating inflammatory bowel disease outcome? Eur J Gastroenterol Hepatol. 2021;33:364–371. doi: 10.1097/MEG.0000000000001912. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020; 181: 894-904. :e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colón AL, Menchén LA, Hurtado O, De Cristóbal J, Lizasoain I, Leza JC, Lorenzo P, Moro MA. Implication of TNF-alpha convertase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine. 2001;16:220–226. doi: 10.1006/cyto.2001.0969. [DOI] [PubMed] [Google Scholar]
- 92.Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut. 2002;51:37–43. doi: 10.1136/gut.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He L, Du J, Chen Y, Liu C, Zhou M, Adhikari S, Rubin DT, Pekow J, Li YC. Renin-angiotensin system promotes colonic inflammation by inducing TH17 activation via JAK2/STAT pathway. Am J Physiol Gastrointest Liver Physiol. 2019;316:G774–G784. doi: 10.1152/ajpgi.00053.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Francesco EM, Vella V, Belfiore A. COVID-19 and Diabetes: The Importance of Controlling RAGE. Front Endocrinol (Lausanne) 2020;11:526. doi: 10.3389/fendo.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rojas A, Gonzalez I, Morales MA. SARS-CoV-2-mediated inflammatory response in lungs: should we look at RAGE? Inflamm Res. 2020;69:641–643. doi: 10.1007/s00011-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, Casini V, Ricci C, Zingone F, Amato A, Caprioli F, Lenti MV, Viganò C, Ascolani M, Bossa F, Castiglione F, Cortelezzi C, Grossi L, Milla M, Morganti D, Pastorelli L, Ribaldone DG, Sartini A, Soriano A, Manes G, Danese S, Fantini MC, Armuzzi A, Daperno M, Fiorino G Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 97.Burke KE, Kochar B, Allegretti JR, Winter RW, Lochhead P, Khalili H, Colizzo FP, Hamilton MJ, Chan WW, Ananthakrishnan AN. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients With Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2021;27:155–161. doi: 10.1093/ibd/izaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Occhipinti V, Pastorelli L. Challenges in the Care of IBD Patients During the CoViD-19 Pandemic: Report From a "Red Zone" Area in Northern Italy. Inflamm Bowel Dis. 2020;26:793–796. doi: 10.1093/ibd/izaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tursi A, Angarano G, Monno L, Saracino A, Signorile F, Ricciardi A, Papa A. COVID-19 infection in Crohn's disease under treatment with adalimumab. Gut. 2020;69:1364–1365. doi: 10.1136/gutjnl-2020-321240. [DOI] [PubMed] [Google Scholar]
- 100.Bezzio C, Pellegrini L, Manes G, Arena I, Picascia D, Della Corte C, Devani M, Schettino M, Saibeni S. Biologic Therapies May Reduce the Risk of COVID-19 in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:e107–e109. doi: 10.1093/ibd/izaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sigall-Boneh R, Levine A, Lomer M, Wierdsma N, Allan P, Fiorino G, Gatti S, Jonkers D, Kierkus J, Katsanos KH, Melgar S, Yuksel ES, Whelan K, Wine E, Gerasimidis K. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO] J Crohns Colitis. 2017;11:1407–1419. doi: 10.1093/ecco-jcc/jjx109. [DOI] [PubMed] [Google Scholar]
- 102.Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, Portero-Otin M, Rojas A, Sampaio GR, Wrobel K, Garay-Sevilla ME. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6:461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]