Abstract
Objective
African ancestry seems to be a risk factor for hypertension; however, few genetic studies have addressed this issue. This study aimed to investigate the prevalence of polymorphisms NOS3; rs1799983, IGFBP3; rs11977526 and TCF7L2; rs7903146 in Brazilian women of African descent and their association with hypertension.
Results
The prevalences of the less frequent genotypes were 26.5% TT genotype of NOS3; rs1799983, 16.7% AA genotype of IGFBP3; rs11977526, and 18.3% TT genotype of TCF7L2; rs7903146. For these conditions, the prevalence of hypertension and PR (adjusted) relatively to the ancestral genotype were, respectively: 52.0% vs 24.5% (PR = 1.54; p < 0.001), 62.0% vs 24.1% (PR = 1.59; p < 0.001), and 38.9% vs 27.9% (PR = 0.86; p = 0.166). Associations with hypertension were statistically significant, except for the TCF7L2; rs7903146 polymorphism, after adjusted analysis. Brazilian Afro-descendant women with the TT genotype for the NOS3 gene and the AA genotype for the IGFBP3 gene are more susceptible to hypertension. The understanding of underlying mechanisms involving the pathogenesis of hypertension can motivate research for the development of new therapeutic targets related to nitric oxide metabolism and the management of oxidative stress.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-021-05598-5.
Keywords: Nitric oxide synthase, Hypertension, IGFBP3 human protein, Oxidative stress, African Continental Ancestry Group
Objective /Introduction
Considered a disease of multifactorial etiology, hypertension is more prevalent among people of African descent [1]. Although there is a higher prevalence of hypertension in Afro-descendant populations in comparison with other ethnicities, studies involving the association of single nucleotide polymorphism (SNP) with this pathology have mostly been conducted with people of European ancestry, and few studies are dealing with populations of African origin [2, 3].
The endothelial dysfunction of hypertension is mainly characterized by a non-relaxation of blood vessels caused by lower bioactivity of nitric oxide (NO) in the vascular wall, due to oxidative stress, causing an imbalance between the antioxidant and pro-oxidant systems, and leading to the prevalence of deleterious actions of reactive oxygen species on cells, tissues and organs [4, 5].
IGFBP3 is a protein with the function of regulating the bioavailability of IGF-1 [6]. In vitro experiments indicate that IGFBP3 regulates IGF-1 by reducing vascular resistance when stimulating the synthesis of NO in endothelial cells [7]. Therefore, IGFBP3 serum levels are closely related to the production of endothelial NO and, consequently, to oxidative stress and hypertension [8].
Sedentary lifestyle, visceral adiposity, and insulin resistance are important risk factors for both hypertension and diabetes mellitus (DM). However, although several studies have demonstrated a relationship between the TCF7L2 gene and DM, its relationship with the prevalence of arterial hypertension and oxidative stress has not been investigated [9, 10].
The SNPs in the NOS3 rs1799983, IGFBP3 rs11977526, and TCF7L2 rs7903146 genes can directly influence the protein expression in the respective genes, making them important biomarkers for the development of hypertension. However, no studies addressing this association in Afro-descendant populations were found [11, 12].
Given the above, this study aimed to verify the prevalence of SNPs in the NOS3 rs1799983, IGFBP3 rs11977526, and TCF7L2 rs7903146 genes, as well as to investigate the possible association of SNPs occurrence with arterial hypertension in Afro-descendant women, in quilombola communities in the state of Alagoas, northeastern Brazil.
Main text
Materials and methods
This is a household cross-sectional population-based survey, whose data were collected in remaining quilombola communities, in the state of Alagoas, Brazil. In the sample size calculation, hypertension was the was the variable of interest, whose prevalence in women of African descent was estimated at 35.8% [13]. The calculations were performed using the StatCalc software (Epi Info, version 3.5.4). For a sampling error of 3.0%, a 95% confidence interval, and adding 10% (852 + 85), to compensate for possible sample losses, 937 women would be needed.
Hypertension was the dependent variable, defined by systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg and/or when the participant reported regular use of antihypertensive drugs [14].
The NOS3; rs1799983, IGFBP3; rs11977526 and TCF7L2; rs7903146 polymorphisms were the independent variables. For DNA extraction and polymorphisms testing, cell samples were collected from the women’s oral mucosa. The samples were stored in a refrigerator for subsequent DNA extraction using the salting-out method [15].
The NOS3, rs1799983, IGFBP3, rs11977526, and TCF7L2, rs7903146 SNPs were chosen for this study after bibliographic research in a complete genome database (Genome-Wide Association Studies—GWAS) [16, 17]. Genotyping was performed using the Step One Plus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), based on a previously standardized protocol [15].
The following covariables were used to control possible confounding factors and characterize the sample:
Demographic variables: age (19–30, 30.1–40, and 40.1–59 years).
Socioeconomic variables: unemployment (yes or no); per capita family income (≥ 1 minimum wage and < 1 minimum wage); “Bolsa Família” Program (yes or no); single register for social programs (yes or no); schooling level (≤ 4 years, > 4 years); self-reported race/skin color (Black/Brown; others: white, yellow or indigenous). Although the investigated population belongs to remaining quilombo communities (scenarios that, at the time of slavery in Brazil, were used as a refuge for fugitive African slaves), due to the miscegenation process, there are also people of other races/colors, although to a lesser extent than that of blacks and browns.
The food and nutrition security (FNS) or food and nutrition insecurity (FNI) was measured based on the Brazilian Food Insecurity Scale (EBIA) [18, 19].
Variables related to health and lifestyle: Alcoholism (yes or no); smoking (yes or no); physical activity level (PAL) measured based on the results obtained by applying the International Physical Activity Questionnaire (IPAQ) [20].
Anthropometric indicators: Body mass index (BMI; kg/m2) and waist circumference (WC) were used. The cut-off point proposed by the World Health Organization were used, obtaining the following categories: eutrophy (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obesity (≥ 30.0 kg/m2). The WC was measured with the woman standing. A cut-off point ≥ 80 cm was used to identify high cardiovascular risk or metabolic complications associated with obesity [21].
Biochemical variables: Total cholesterol and fractions, triglycerides, and diabetes mellitus were determined without mandatory fasting. The lipid profile was determined in an Alere Cholestech LDX® System (Abbott, USA). HbA1c was determined using a NycoCard Reader II® device (Abbott, USA) [22].
Data processing and analysis: Double independent data entry was performed using the Epi-Infotm 3.5.4 software. The obtained database was exported to the Stata/SE 12.1 software for Windows (StataCorp LP, College Station, TX, USA), through which all analyses were performed.
According to the chi square test, all genotypes behaved according to the balance as Hardy–Weinberg equilibrium (HWE) [23].
The distribution adherence to parametric assumptions was verified using the Kolmogorov–Smirnov test. Thus, the means were subjected to analysis of variance (ANOVA), and the medians were tested using the Kruskal–Wallis test. Bonferroni and Dunnet post-hoc tests were used, respectively.
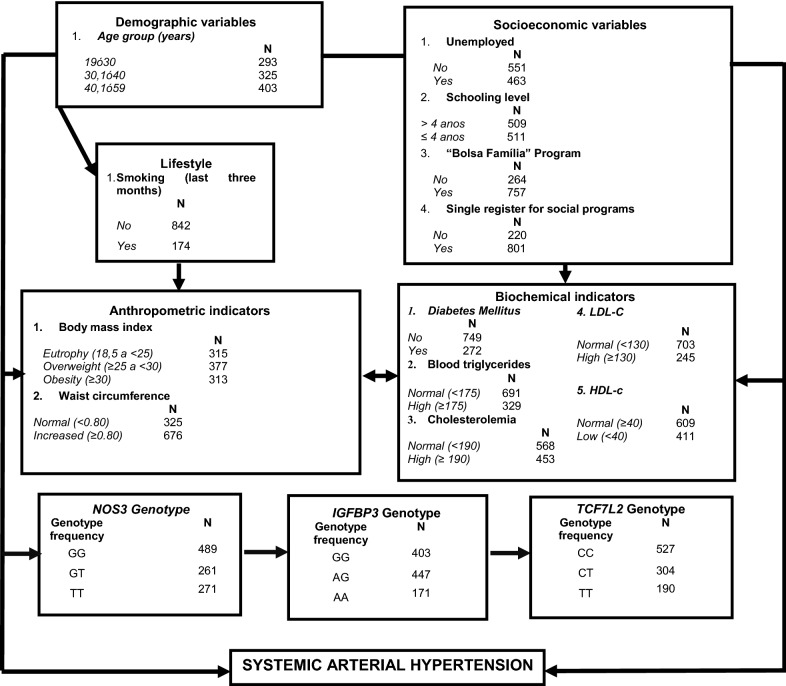
Multiple analysis was performed according to an adapted four levels hierarchical theoretical model. (NETO et al. [15]) (Fig. 1). To identify an association between hypertension and the polymorphism genotypes, prevalence ratio (PR), and respective confidence interval (95% CI), were used, which were calculated using Poisson regression with robust variance.
Fig. 1.
Hierarchical conceptual model explaining systemic arterial hypertension (
Adapted from Neto et al. [15])
Results
The sample was composed of 1021 women (37.9 ± 10.9 years old), most of them self-declared as African/hispanic (91.1%). The prevalence of hypertension among them was 31.4% the sample characterization according to socioeconomic, demographic, lifestyle, anthropometric, biochemical, and genetic variables are shown in Additional file 1: Table S1.
The prevalences of genotypes for NOS3; rs1799983 SNP were GG = 47.9%, GT = 25.6% and TT = 26.5%, which was the least frequent genotype. The prevalences for TCF7L2; rs7903146 SNP was CC = 51.6%, CT = 29.8% and TT = 18.6%. The IGFBP3; rs11977526 SNP had the following prevalences: GG = 39.5%, GA = 43.8% and AA = 16.7%. There was a statistically significant difference between blood pressure levels and the NOS3, rs1799983 and IGFBP3 rs11977526, SNPs, with emphasis on the genotypes: TT NOS3; rs1799983, SNP and AA IGFBP3; rs11977526 SNP.
The less frequent genotypes of the NOS3, rs1799983, TCF7L2 rs7903146, and IGFBP3 rs11977526, SNPs were associated with a higher prevalence of hypertension in comparison with the ancestral and heterozygous genotypes. The distribution of polymorphisms in accordance with the Hardy–Weinberg equilibrium (Additional file 1: Table S2).
Results similar to those recorded for distribution of prevalence were found when analyzing the measures of central tendency (mean and median) related to systolic blood pressure (Table 2). The values found for genotypes of NOS3, rs1799983 and IGFBP3 rs11977526 were significantly higher than those obtained for the other genes, both in the ANOVA, in the Kruskal–Wallis nonparametric test. For the TCF7L2, rs7903146 SNP, PAS levels were considered statistically similar (p > 0.05 in both analyses).
Table 2.
Dominant and recessive models for determining systemic arterial hypertension in Brazilian women of African descent
| Models | Hypertension (%) | *PR (95% CI) | P |
|---|---|---|---|
| Dominant | |||
| (NOS3 GG) + (IGFBP3 GG) | 21.3 | 1 | – |
| (NOS3 TT + GT) + (IGFBP3 AG + AA) | 34.7 | 1.42 (1.12–1.79) | 0.003 |
| Recessive | |||
| (NOS3 GG + GT) + ( IGFBP3 GG + AG) | 26.5 | 1 | – |
| (NOS3 TT) + (IGFBP3 AA) | 77.0 | 2.07 (1.78–2.42) | < 0.001 |
Prevalence ratios (PR) and respective 95% confidence intervals (95% CI) adjusted by Poisson regression using the risk factors: age group (30.1–40.0), age group (40.1–59, 0), income supplementation program, overweight (BMI ≥ 25– < 30), obesity (BMI ≥ 30), HDL-C and diabetes mellitus; *hypertension: systemic arterial hypertension
For the NOS3, rs1799983 SNP, the prevalences of hypertension among women with GG, GT and TT genotypes were 24.5%, 23.0% and 52.0%, respectively, with a statistically significant difference between TT and GG (PR = 1.54; 95% CI 1.27–1.86; p < 0.001). Regarding IGFBP3; rs11977526 SNP, the prevalences of hypertension among women with GG, GA and AA genotypes were 24.1%, 26.4% and 62.0%, respectively, with a statistically significant difference between AA and GG (PR = 1.59; 95% CI 1.27–1.98; p < 0.001). For TCF7L2; rs7903146 SNP, hypertension among women with CC, CT and TT genotypes had prevalences of 27.9%, 32.9% and 38.9%, respectively, with no statistical difference between TT and CC (PR = 0.86; 95% CI 0.69–1.06; p = 0.166) (Table 1).
Table 1.
Prevalence ratios (PR) and respective 95% confidence intervals (95% CI) obtained by multivariable Poisson regression, according to the hierarchical theoretical model for determining arterial hypertension
| Variables | Level 1 PR (95% CI) | P | Level 2 *PR (95% CI) | P | Level 3 *PR (95% CI) | P | Level 4 PR (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| Level 1 | ||||||||
| Age group: 30.1–40 | 3.95 (2.46–6.34) | < 0.001 | 3.95 (2.46–6.34) | < 0.001 | 3.54 (2.20–5.69) | < 0.01 | 3.36 (2.10–5.36) | < 0.01 |
| Age group: 40.1–50 | 8.06 (5.16–12.60) | < 0.001 | 8.06 (5.16–12.60) | < 0.001 | 7.33 (4.69–11.48) | < 0.01 | 6.78 (4.35–10.59) | < 0.01 |
| Insertion in the formal labor market | 1.18 (0.99–1.41) | 0.050 | 1.18 (0.99–1.41) | 0.051 | 1.17 (0.99–1.40) | 0.06 | * | * |
| Schooling level: ≤ 4 years | 1.21 ( 0.99–1.48) | 0.060 | 1.21 ( 0.99–1.48) | 0.056 | * | * | * | * |
| “Bolsa Família” Program: yes | 1.20 (1.01–1.41) | 0.037 | 1.20 (1.01–1.41) | 0.037 | 1.15 (0.98–1.36) | 0.094 | 1.12 (0.96–1.31) | 0.156 |
| Single register for social programs: yes | 1.06 (0.78–1.44) | 0.680 | * | * | * | * | * | * |
| Level 2 | ||||||||
| Smoking | – | – | 0.99 (0.77–1.13) | 0.48 | * | * | * | * |
| Level 3 | ||||||||
| BMI Overweight (≥ 25– < 30) | 1.26 (0.98–1.61) | 0.067 | 1.29 (1.02–1.64) | 0.033 | ||||
| BMI Obesity (≥ 30) | – | – | – | – | 1.63 (1.29–2.06) | < 0.001 | 1.63 (1.29–2.06) | < 0.001 |
| Waist circumference ≥ 80 cm | – | – | – | – | 1.02 (0.72–1.43) | 0.091 | * | |
| Triglycerides ≥ 175 mg/dL | – | – | – | – | 1.05 (0.88–1.26) | 0.551 | * | * |
| Total cholesterol ≥ 190 mg/dL | – | – | – | – | 1.20 (0.96–1.49) | 0.101 | * | * |
| *LDL-C (mg/dL) ≥ 160 mg/dL | – | – | – | – | 0.90 ( 0.73–1.12) | 0.384 | * | * |
| *HDL-C (mg/dL) > 50 mg/dL | – | – | – | – | 1.27 (1.08–1.50) | 0.003 | 1.24 (1.06–1.45) | 0.007 |
| Diabetes mellitus | – | – | – | – | 1.28 (1.09–1.51) | 0.003 | 1.16 (0.99–1.37) | 0.060 |
| Level 4 | ||||||||
| TCF7L2 | ||||||||
| CC | – | – | – | – | – | – | 1 | – |
| CT | – | – | – | – | – | – | 1.04 (0.78–1.19) | 0.623 |
| TT | – | – | – | – | – | – | 0.86 (0.69–1.06) | 0.166 |
| NOS3 | ||||||||
| GG | – | – | – | – | – | – | 1 | – |
| GT | – | – | – | – | – | – | 0.95 (0.74–1.23) | 0.755 |
| TT | – | – | – | – | – | – | 1.54 (1.27–1.86) | < 0.001 |
| IGFBP3 | ||||||||
| GG | – | – | – | – | – | – | 1 | – |
| AG | – | – | – | – | – | – | 0.96 (0.78–1.19) | 0.751 |
| AA | – | – | – | – | – | – | 1.59 (1.27–1.98) | < 0.001 |
LDL, low-density lipoprotein; HDL, high density lipoprotein; Prevalence ratios and respective 95% confidence intervals (95% CI)
After the adjusted analysis according to four levels hierarchical conceptual model, older age group, family beneficiary of the “Bolsa Família” Program, obesity (BMI ≥ 30 kg/m2), low HDL-C level, and diabetes mellitus were significantly associated with hypertension.
The dominant and recessive model analysis was performed using the following risk factors: age group (30.1–40.0), age group (40.1–59.0), “Bolsa Família” Program, overweight (BMI ≥ 25), HDL-C, and glycated hemoglobin. In the dominant model of oxidative stress (NOS3: GG + IGFBP3: GG vs NOS3 GT + TT; IGFBP3: AG + AA), women had a prevalence of 34.7% hypertension (PR = 1.42; 95% CI 1.12–1.79; p = 0.003). However, in the recessive model of oxidative stress (NOS3: TT + IGFBP3: AA vs NOS3: GG + GT IGFBP3: GG + AG), they had a prevalence of 77.0% hypertension (PR = 2.07; 95% CI1.78–2.42 p < 0.001). There was a statistical significance even after adjusting for all risk factors, in the dominant and recessive models, referring to the NOS3 and IGFBP3 genes (Table 2).
Discussion
This study provides evidence that, in Afro-descendant women from northeastern Brazil, the NOS3, rs1799983 and IGFBP3, rs11977526, SNPs gene are associated with higher blood pressure levels and, consequently with a higher prevalence of arterial hypertension, except for the TCF7L2; rs7903146 polymorphism.
A study in an African population investigated whether biomarkers of endothelial function were related to the bioavailability of IGF-1 (IGF-1, IGFBP3, or IGF-1/IGFBP3M ratio) and showed that the bioavailable IGF-1, measured by the IGF1/IGFBP3 ratio, is beneficially associated with CAV-1, which is a biomarker of endothelial activation [24]. Also, bioavailable IGF-1 tended to be inversely associated with ICAM-1, another marker of endothelial activation, thereby increasing the expression of CAV-1 and ICAM-1 [8, 25].
Previous studies indicated that some SNPs in the IGFBP3 and NOS3 genes are associated with decreased serum levels of these proteins [26–28]. Research on SNPs of the IGFBP3 rs11977526 gene and hypertension indicated an association of these factors, as found in a study involving East African people, was associated with the risk of such disease [29, 30].
In this study, there was a higher predisposition for hypertension in the presence of TT and AA genotypes for the NOS3 and IGFBP3 genes, respectively. In the case of heterozygous Afro-descendant women (GT of the NOS3 gene and AG of the IGFBP3 gene), no statistically significant difference was found between the prevalence of hypertension. However, when analyzing the dominant and recessive models for oxidative stress, even after adjusting the risk factors, a significance was found for NOS3 and IGFBP3 models. Therefore, these data show that the presence of the TT genotype of the NOS3 gene and the AA genotype of the IGFBP3 gene constitutes an important risk factor for arterial hypertension.
The SNP rs1799983, variant of the NOS3 gene causes a change in which the amino acid Asp is replaced by Glu at position 298. This substitution is associated with a decrease in protein stability [12, 31]. The AA genotype of the IGFBP3 gene has been shown to regulate protein expression through miRNAs by destabilizing the mRNA, which is associated with a decrease in IGFBP3 serum levels [30, 32, 33].
In our analysis, the dominant and recessive showed a strong association of TT and AA genotypes of the NOS3 and IGFBP3 genes, respectively, with hypertension. CAV-1 is the main link between the NOS3 gene and IGFBP3 because it physically interacts with these gene regions, making possible a co-expression between the two proteins [34–36]. Recently, some studies have shown that CAV-1, which is a protein responsible for regulating eNOS function, is closely linked to IGF-1 and IGFBP3, regulating endothelial cell proliferation, vascular development, and oxidative stress [37–42].
Conclusion
The TT genotype of the NOS3 rs1799983 gene and the AA genotype of the IGFBP3 rs11977526 gene are associated with a higher prevalence of arterial hypertension, except for the TCF7L2; rs7903146 polymorphism, after adjusted analysis. Considering that the mechanism of action, responsible for higher blood pressure levels in women with the TT (NOS3) and AA (IGFBP3) genotypes, involves less metabolic production of NO and, consequently, an increase in oxidative stress, the results presented here suggest that these SNPs are directly related to blood pressure regulation.
Future molecular studies are needed to reveal the important roles of eNOS and IGFBP3 when they are related to hypertension. Association studies such as the one presented here are of great relevance for motivating research aimed to elucidate the molecular pathways involved in the etiology of hypertension and, consequently, in the development of new drugs related to these pathways.
Limitations
Thus, it did not provide for the inclusion of men and elderly people. Therefore, the absence of men is a limitation of this research. Due to the differences in the occurrence of hypertension by gender, further studies including male participants should be conducted.
Supplementary Information
Additional file 1: Table S1. Distribution of systemic arterial hypertension the according to socioeconomic, demographic, lifestyle, anthropometric, and biochemical variables in women of African descent in the state of Alagoas (n = 1021). Table S2. Prevalence of arterial hypertension (%) and systolic blood pressure (mean ± SD; median and interquartile range), according to the genotypic frequencies for NOS3 rs1799983, TCF7L2 rs7903146, and IGFBP3 rs11977526 genes. Brazilian women descended from African descent, 2018.
Acknowledgements
For their support, National Council for Scientific and Technological Development (CNPq), National Council for the Improvement of Higher Education (CAPES), Foundation for Research Support of Alagoas state (FAPEAL), Federal University of Alagoas.
Abbreviations
- Asp
Aspartic acid
- BMI
Body mass index
- CI
Confidence interval
- CAV-1
Caveolin-1
- DNA
Deoxyribonucleic acid
- DM
Diabetes mellitus
- DBP
Diastolic blood pressure
- EBIA
Brazilian Food Insecurity Scale
- eNOS
Nitric oxide synthase
- FNS
Food and nutrition security
- FNI
Food and nutrition insecurity
- Glu
Glutamic acid
- GWAS
Genome-Wide Association Studies
- HbA1c
Glycated hemoglobin
- HDL
High density lipoprotei
- HWE
Hardy–Weinberg equilibrium
- ICAM-1
Intercellular Adhesion Molecule 1
- IGF-1
Insulin-like growth factor-1
- IGFBP3
Insulin-like growth factor binding protein -3
- IPAQ
Physical Activity Questionnaire
- LDL
Low-density lipoprotein
- NO
Nitric oxide
- PAL
Physical activity level
- PR
Prevalence ratio
- SBP
Systolic blood pressure
- SNP
Single nucleotide polymorphism
- WC
Waist circumference
Authors' contributions
ABLN participated in designing the study, analysis and interpretation of data and drafting the article. NBRV, TRS, LECD, CSM and MLA took part in the acquisition of data, data entry, analysis and interpretation and writing. HSF took part in the project’s conception and obtained the respective financial support, coordinating all implementation steps and realized the final review of the article. All authors read and approved the final manuscript.
Funding
This study was funded by the Brazilian National Council of Technological and Scientific Development—CNPq (Grant No. 466718/2014-4) and the Foundation for Research Support of the State of Alagoas—Fapeal (Grant No. 60030.000849/2016). The views expressed in the present article are those of the authors and not necessarily those of any funding agencies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval for this study was obtained from the Ethics Committee of Federal University of Alagoas, Brazil (No: 33527214.9.0000.5013). The study conformed to the principles of the Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Munroe PB, Barnes MR, Caulfield MJ. Advances in blood pressure genomics. Circ Res. 2013;112(10):1365–1379. doi: 10.1161/CIRCRESAHA.112.300387. [DOI] [PubMed] [Google Scholar]
- 2.Doris PA. Hypertension genetics, single nucleotide polymorphisms, and the common disease: common variant hypothesis. Hypertension. 2002;39(2):323–331. doi: 10.1161/hy0202.104087. [DOI] [PubMed] [Google Scholar]
- 3.Franceschini N, Le TH. Genetics of hypertension: discoveries from the bench to human populations. Am J Physiol Renal Physiol. 2014;306(1):F1–F11. doi: 10.1152/ajprenal.00334.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzoni D. Endothelial dysfunction in hypertension: fact or fantasy? J Hypertens. 2002;20(8):1479–1481. doi: 10.1097/00004872-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ferroni P, Basili S, Paoletti V, Davi G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis. 2006;16(3):222–233. doi: 10.1016/j.numecd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Curhan GC, Forman JP. Plasma insulin-like growth factor-1 level and risk of incident hypertension in non-diabetic women. J Hypertens. 2011;29(2):229. doi: 10.1097/HJH.0b013e32834103bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers JR. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension. 1997;29(3):691–699. doi: 10.1161/01.HYP.29.3.691. [DOI] [PubMed] [Google Scholar]
- 8.Schutte AE, Volpe M, Tocci G, Conti E. Revisiting the relationship between blood pressure and insulin-like growth factor-1. Hypertension. 2014;63(5):1070–1077. doi: 10.1161/HYPERTENSIONAHA.113.03057. [DOI] [PubMed] [Google Scholar]
- 9.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J. 2007;28(23):2937–2943. doi: 10.1093/eurheartj/ehm400. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380(9841):601–610. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]
- 11.Yan Y, Klein R, Heiss G, Girman CJ, Lange EM, Klein BE, et al. The transcription factor 7-like 2 (TCF7L2) polymorphism may be associated with focal arteriolar narrowing in Caucasians with hypertension or without diabetes: the ARIC Study. BMC Endocr Disord. 2010;10(1):9. doi: 10.1186/1472-6823-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senthil D, Raveendran M, Shen YH, Utama B, Dudley D, Wang J, et al. Genotype-dependent expression of endothelial nitric oxide synthase (eNOS) and its regulatory proteins in cultured endothelial cells. DNA Cell Biol. 2005;24(4):218–224. doi: 10.1089/dna.2005.24.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilha BM, da Silva DA, da Silva FH, Tomiya MTO, Cabral PC. Anthropometric predictors of hypertension in afro-descendant women. Scientia Medica. 2017;27(3):27527. doi: 10.15448/1980-6108.2017.3.27527. [DOI] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Neto ABL, Farias MC, Vasconcelos NB, Xavier JA, Assunção ML, Ferreira HS. Prevalence of endothelial nitric oxide synthase (ENOS) gene G894T polymorphism and its association with hypertension: a population-based study with Brazilian women. Arch Med Sci Atherosclerotic Dis. 2019;4:e63–e73. doi: 10.5114/amsad.2019.84539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush WS, Moore JH. Genome-wide association studies. PLoS Comput Biol. 2012;8(12):e1002822. doi: 10.1371/journal.pcbi.1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6(2):95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 18.Kepple AW, Segall-Corrêa AM. Conceituando e medindo segurança alimentar e nutricional. Cien Saude Colet. 2011;16:187–199. doi: 10.1590/S1413-81232011000100022. [DOI] [PubMed] [Google Scholar]
- 19.Segall-Corrêa AM, Marin-León L, Melgar-Quiñonez H, Pérez-Escamilla R. Refinement of the Brazilian household food insecurity measurement scale: Recommendation for a 14-item EBIA. Rev Nutr. 2014;27(2):241–251. doi: 10.1590/1415-52732014000200010. [DOI] [Google Scholar]
- 20.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8(1):115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caminha TC, Ferreira HS, Costa NS, Nakano RP, Carvalho RES, Xavier AF, Jr, et al. Waist-to-height ratio is the best anthropometric predictor of hypertension: a population-based study with women from a state of northeast of Brazil. Medicine. 2017;96(2):e58574. doi: 10.1097/MD.0000000000005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scartezini M, Ferreira CES, Izar MCO, Bertoluci M, Vencio S, Campana GA, et al. Positioning about the flexibility of fasting for lipid profiling. Arq Bras Cardiol. 2017;108:195–197. doi: 10.5935/abc.20170039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genetics. 2005;76(5):887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnard SA, Smith W, Mels CM, Botha S, Schutte AE. Bioavailable IGF-1 is beneficially associated with biomarkers of endothelial function in young healthy adults: the African-PREDICT study. Growth Hormon IGF Res. 2018;41:28–33. doi: 10.1016/j.ghir.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Balaram SK, Agrawal DK, Allen RT, Kuszynski CA, Edwards JD. Cell adhesion molecules and insulin-like growth factor-1 in vascular disease. J Vasc Surg. 1997;25(5):866–876. doi: 10.1016/S0741-5214(97)70216-7. [DOI] [PubMed] [Google Scholar]
- 26.Al-Zahrani A, Sandhu MS, Luben RN, Thompson D, Baynes C, Pooley KA, et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006;15(1):1–10. doi: 10.1093/hmg/ddi398. [DOI] [PubMed] [Google Scholar]
- 27.Cheng I, DeLellis HK, Haiman CA, Kolonel LN, Henderson BE, Freedman ML, et al. Genetic determinants of circulating insulin-like growth factor (IGF)-I, IGF binding protein (BP)-1, and IGFBP-3 levels in a multiethnic population. J Clin Endocrinol Metab. 2007;92(9):3660–3666. doi: 10.1210/jc.2007-0790. [DOI] [PubMed] [Google Scholar]
- 28.Sakar MN, Atay AE, Demir S, Bakir VL, Demir B, Balsak D, et al. Association of endothelial nitric oxide synthase gene G894T polymorphism and serum nitric oxide levels in patients with preeclampsia and gestational hypertension. J Matern Fetal Neonatal Med. 2015;28(16):1907–1911. doi: 10.3109/14767058.2014.971748. [DOI] [PubMed] [Google Scholar]
- 29.Kayima J, Liang J, Natanzon Y, Nankabirwa J, Ssinabulya I, Nakibuuka J, et al. Association of genetic variation with blood pressure traits among East Africans. Clin Genet. 2017;92(5):487–494. doi: 10.1111/cge.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang J, Le TH, Edwards DRV, Tayo BO, Gaulton KJ, Smith JA, et al. Single-trait and multi-trait genome-wide association analyses identify novel loci for blood pressure in African-ancestry populations. PLoS genetics. 2017;13(5):e1006728. doi: 10.1371/journal.pgen.1006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip I, Plantefeve G, Vuillaumier-Barrot S, Vicaut E, LeMarie C, Henrion D, et al. G894T polymorphism in the endothelial nitric oxide synthase gene is associated with an enhanced vascular responsiveness to phenylephrine. Circulation. 1999;99(24):3096–3098. doi: 10.1161/01.CIR.99.24.3096. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan RC, Petersen A-K, Chen M-H, Teumer A, Glazer NL, Döring A, et al. A genome-wide association study identifies novel loci associated with circulating IGF-I and IGFBP-3. Hum Mol Genet. 2011;20(6):1241–1251. doi: 10.1093/hmg/ddq560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Z, Yang S, Zhu L, Li Y, Chen Y, Jin Y, et al. Association study of IGFBP1 and IGFBP3 polymorphisms with hypertension and cardio-cerebral vascular diseases in a Chinese Han population. Oncotarget. 2017;8(44):77836. doi: 10.18632/oncotarget.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto M, Hatakeyama S, Oyamada K, Oda Y, Nishimura T, Nakayama KI. Large-scale analysis of the human ubiquitin-related proteome. Proteomics. 2005;5(16):4145–4151. doi: 10.1002/pmic.200401280. [DOI] [PubMed] [Google Scholar]
- 35.Singh G, Kucukural A, Cenik C, Leszyk JD, Shaffer SA, Weng Z, et al. The cellular EJC interactome reveals higher-order mRNP structure and an EJC-SR protein nexus. Cell. 2012;151(4):750–764. doi: 10.1016/j.cell.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petschnigg J, Groisman B, Kotlyar M, Taipale M, Zheng Y, Kurat CF, et al. The mammalian-membrane two-hybrid assay (MaMTH) for probing membrane-protein interactions in human cells. Nat Methods. 2014;11(5):585. doi: 10.1038/nmeth.2895. [DOI] [PubMed] [Google Scholar]
- 37.Bahr TM, Hughes GJ, Armstrong M, Reisdorph R, Coldren CD, Edwards MG, et al. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49(2):316–323. doi: 10.1165/rcmb.2012-0230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shyu H-Y, Chen M-H, Hsieh Y-H, Shieh J-C, Yen L-R, Wang H-W, et al. Association of eNOS and Cav-1 gene polymorphisms with susceptibility risk of large artery atherosclerotic stroke. PloS one. 2017;12(3):e0174110. doi: 10.1371/journal.pone.0174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarajapu YP, Cai J, Yan Y, Calzi SL, Kielczewski JL, Hu P, et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PLoS One. 2012;7(7):e39398. doi: 10.1371/journal.pone.0039398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyfuss K, Hood DA. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018;23(1):100–117. doi: 10.1080/13510002.2017.1416773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aharinejad S, Salama M, Rödler S, Ehrlich M, Zuckermann A, Laufer G. Low serum IGF-1 is a risk factor for cardiac allograft vasculopathy in cardiac transplant recipients. Transplantation. 2012;93(3):309–313. doi: 10.1097/TP.0b013e31823ec10d. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi Y, Yasuoka H, Stolz DB, Feghali-Bostwick CA. Decreased caveolin-1 levels contribute to fibrosis and deposition of extracellular IGFBP-5. J Cell Mol Med. 2011;15(4):957–969. doi: 10.1111/j.1582-4934.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Distribution of systemic arterial hypertension the according to socioeconomic, demographic, lifestyle, anthropometric, and biochemical variables in women of African descent in the state of Alagoas (n = 1021). Table S2. Prevalence of arterial hypertension (%) and systolic blood pressure (mean ± SD; median and interquartile range), according to the genotypic frequencies for NOS3 rs1799983, TCF7L2 rs7903146, and IGFBP3 rs11977526 genes. Brazilian women descended from African descent, 2018.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.