To the Editor,
Eosinophilic esophagitis (EoE) is a chronic inflammatory disease characterized by an eosinophil-predominant inflammation of the esophageal mucosa.1 We have previously characterized bacterial communities of the human esophagus in healthy children and adults.2 Our data and work from others suggest that the normal esophageal bacterial microbiota is dominated by Firmicutes species.2–4 Active inflammation in EoE is associated with bacterial dysbiosis, specifically with an increase in members of the Proteobacteria.2,3 However, while some have described the role of bacteria in atopic gastrointestinal diseases,5 little is known about the role of fungi in allergic esophageal inflammation or the role of topical swallowed steroids (TSS) in disease-associated dysbiosis. We aimed to characterize the bacterial and fungal communities in the esophageal mucosa of children with EoE and non-EoE controls and examine the effects of TSS on the microbiota in children with EoE. We additionally provide a longitudinal analysis of the EoE fungal and bacterial communities before and after TSS.
The esophageal bacterial and fungal microbiota was analyzed in 69 subjects with EoE and 10 non-EoE controls by 16S rRNA marker gene sequencing and internal transcribed spacer (ITS) sequencing, respectively (Appendix S1). Subjects’ demographics, clinical phenotypes, and therapies are indicated in Table S1. Subjects were 1.7 to 19.7 years of age. A total of 33 subjects were classified as active EoE (≥15 eos/hpf), of which 17 were steroid-naïve, and 16 were on TSS. Of the 36 subjects with inactive EoE (<15 eos/hpf), 18 were steroid-naïve and 18 were on TSS. A group of 9 steroid-naïve subjects who later responded to TSS was studied longitudinally (Figure S1).
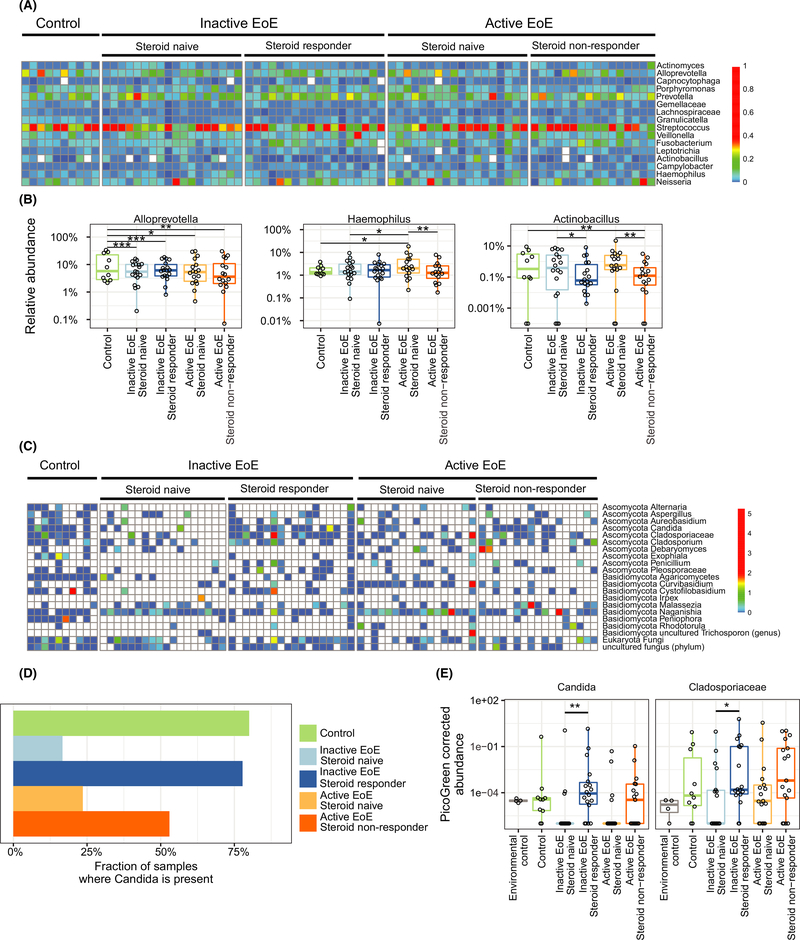
Characterization of the esophageal microbiota in EoE subjects relative to non-EoE controls was performed by comparison of steroid-naïve subjects with active or inactive EoE to non-EoE controls. Streptococcus, Prevotella, and Alloprevotella were prominent in esophageal biopsies from all three groups (Figure 1A), consistent with our previous findings.2 Bacterial community composition based on PERMANOVA test on Bray-Curtis distances was not different between groups; however, differences in individual taxon abundance were observed based on linear mixed-effects models. Alloprevotella was significantly decreased in both active (q = 0.02) and inactive EoE (q = 0.001) compared to non-EoE controls (Figure 1B). Conversely, the abundance of Haemophilus increased in a stepwise manner from inactive to active EoE subjects, relative to non-EoE controls (q = 0.02, Figure 1B), as seen by Harris et al.3
FIGURE 1.
Alterations in bacterial and fungal communities in esophageal samples of patients with EoE and non-EoE controls. A, Heatmap of the bacterial composition. Colors represent the relative abundance of a taxon. B, Relative abundance of Alloprevotella, Haemophilus, and Actinobacillus. Haemophilus showed significant differences between control and EoE steroid-naïve subjects. C, Heatmap of the fungal composition. Colors represent PicoGreen-corrected operational taxonomic unit (OTU) abundances. D, Fraction of samples where the fungal genus Candida is present. E, PicoGreen-corrected OTU abundances of Candida and Cladospiraceae
We then compared steroid-naïve EoE subjects to steroid responders and non-responders to investigate the effect of TSS on bacterial communities (Figure 1A,B). No significant differences associated with TSS or disease activity were seen based on weighted and unweighted UniFrac. However, the relative abundance of Actinobacillus was lower in the presence of TSS, relative to the steroid-naïve populations (P = .01, Figure 1B). The relative abundance of Haemophilus was lower in active steroid non-responders relative to active steroid-naïve subjects (P = .004, Figure 1B), suggesting that the Haemophilus signature in active EoE may be diminished by TSS regardless of response to therapy.
Fungal taxa commonly present in esophageal samples included Candida, Cladosporiaceae, and Malassezia (Figure 1C–E). Agaricomycetes, Candida, Cladosporiaceae, and Peniophora were seen most often in control samples. A negative correlation with total fungal abundance and eosinophil count in steroid-naïve subjects was found (P = .03); however, this was not seen in the presence of TSS (P = .3, Figure S2).Candida was present in a greater proportion of non-EoE controls compared to steroid-naïve EoE subjects (P = .002, Figure 1D); however, the prevalence of Candida was not different between active and inactive steroid-naïve subjects. TSS were associated with perturbations in the fungal community. In inactive EoE subjects, Candida was significantly increased in TSS-treated vs untreated subjects (P = .007, Figure 1E). These results are concordant with clinical observations of higher Candida infection rate during TSS therapy.6,7 Additionally, the family Cladosporiaceae was significantly increased in inactive steroid responders compared to inactive steroid-naïve subjects (P = .022, Figure 1E).
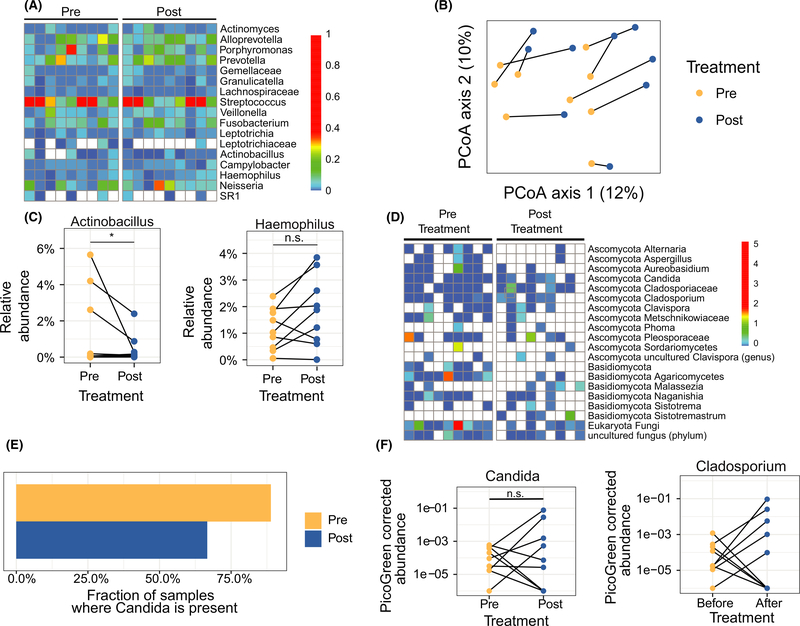
Longitudinal changes were assessed in EoE subjects following TSS therapy (Figure 2). Nine subjects from the cross-sectional analysis who were steroid-naïve at Timepoint 1 and achieved remission on TSS at Timepoint 2 were analyzed. Bacterial community composition was altered between timepoints (Figure 2B). The abundance of Actinobacillus was decreased, consistent with the cross-sectional comparison (q = 0.09), while the relative abundance of Haemophilus was unchanged (Figure 2C). Longitudinal changes in fungal composition were also assessed (Figure 2D). Interestingly, Candida was not detected more frequently following initiation of TSS (P = .6, Figure 2E), and neither was the relative abundance significantly different (P = .73, Figure 2F). The relative abundance of Cladosporium was also not significantly different following TSS (P = .11, Figure 2F).
FIGURE 2.
Alterations in bacterial and fungal communities of active steroid-naïve subjects achieving remission after topical swallowed steroid (TSS) therapy. A, Heatmap of bacterial composition. The colors represent the relative abundance of a taxon. B, Principle coordinate analysis of unweighted UniFrac distances between samples. The lines connect the pre- and post-TSS samples from the same subject. C, Relative abundances of Actinobacillus and Haemophilus. D, Heatmap of fungal composition. The colors represent PicoGreen-corrected operational taxonomic unit (OTU) abundances. E, Fraction of samples where fungal genus Candida is present. F, PicoGreen-corrected OTU abundances of Candida and Cladosporium. Lines connect pre- and post-TSS samples from the same subjects
For the first time, we characterize the fungal population of the EoE esophagus and provide a longitudinal assessment of the microbiota in EoE. Similar to others, we found that Haemophilus had higher abundance in active EoE subjects. Despite these differences, a lower abundance was not seen longitudinally in steroid responders. Environmental factors could have contributed to the lower abundance of Haemophilus in responders prior to therapy. Similarly, the lack of longitudinal changes in the abundance of Candida in the presence of TSS suggests that factors such as non-eosinophilic inflammation or disease chronicity could have impacted the establishment of fungal communities after resolution of eosinophilic inflammation.
Even though sample size and the heterogeneous indications of the patients were limiting factors, we have found significant alterations in bacterial and fungal communities in patients with EoE during TSS therapy. New studies should take into account the effect of proton pump inhibitor (PPI) therapy when comparing results to these findings as PPI use may vary with revised guidelines. This study highlights the importance of mapping the esophageal microbiota to potentially identify how environmental factors influence responses to therapies. Additionally, proliferation or reduction of some species could indicate an evolving inflammatory process or the resolution of it. Our findings provide the foundation for future studies investigating the effect of TSS or other EoE therapies on esophageal microbial communities. Further studies are needed to identify associations between the resident microbiota, mucosal markers, and risk factors, to determine cause and effect relationships in inflammatory disorders.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the members of the GI Endoscopy Suite in the Division of Gastroenterology, Hepatology and Nutrition, members of the Division of Allergy and Immunology and member of the PennCHOP Microbiome Center at Children’s Hospital of Philadelphia (CHOP). We would like to specially thank Babette Zemel, PhD, Stephen Budhu, MPH, and Ariel Myatt for their editing and technical support. We are immensely grateful to the patients and families who participated in this study.
FUNDING INFORMATION
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001879 (AJB and MAR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the following NIH Grants: K08DK106444, R03DK118310 01A1, and 1R01DK12426601 (ABM), CEGIR (U54 AI117804) (ABM, JMS). CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Diseases (CURED), and Eosinophilic Family Coalition (EFC).This work was also supported by the Penn/CHOP Microbiome Pilot Grant (ABM) and the Suzi and Scott Lustgarten Center for GI Motility at CHOP (AJB).
Footnotes
CONFLICTS OF INTEREST
Dr Benitez, Dr Tanes, Dr Mattei, Mr Hofstaedter, Ms Kim, Mr Gross, Dr Ruffner, Dr Albenberg, Dr Bittinger, and Dr Muir have nothing to disclose. Dr Spergel reports grants from Regeneron, grants from NIH, personal fees from DBV Technology, personal fees from Uptodate, personal fees from ACAAI, and grants from Aimmune Therapeutics, outside the submitted work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Liacouras CA, Markowitz JE. Eosinophilic esophagitis: a subset of eosinophilic gastroenteritis. Curr Gastroenterol Rep. 1999;1(3):253–258. [DOI] [PubMed] [Google Scholar]
- 2.Benitez AJ, Hoffmann C, Muir AB, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. 2015;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris JK, Fang R, Wagner BD, et al. Esophageal microbiome in eosinophilic esophagitis. PLoS One. 2015;10(5):e0128346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137(2):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir AB, Benitez AJ, Dods K, Spergel JM, Fillon SA. Microbiome and its impact on gastrointestinal atopy. Allergy. 2016;71(9):1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aceves SS, Dohil R, Newbury RO, Bastian JF. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116(3):705–706. [DOI] [PubMed] [Google Scholar]
- 7.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.