Abstract
Background
Neurogenesis in the neonatal period involves the proliferation and differentiation of neuronal stem/progenitor cells and the establishment of synaptic connections. This process plays a critical role in determining the normal development and maturation of the brain throughout life. Exposure to certain physical or chemical factors during the perinatal period can lead to many neuropathological defects that cause high cognitive dysfunction and are accompanied by abnormal hippocampal neurogenesis and plasticity. As an endocrine disruptor, gossypol is generally known to exert detrimental effects in animals exposed under experimental conditions. However, it is unclear whether gossypol affects neurogenesis in the hippocampal dentate gyrus during early developmental stages.
Methods
Pregnant Institute of Cancer Research mice were treated with gossypol at a daily dose of 0, 20, and 50 mg/kg body weight from embryonic day 6.5 to postnatal day (P) 21. The changes of hippocampal neurogenesis as well as potential mechanisms were investigated by 5-bromo-2-deoxyuridine labeling, behavioral tests, immunofluorescence, quantitative reverse transcription-polymerase chain reaction, and western-blot analyses.
Results
At P8, maternal gossypol exposure impaired neural stem cell proliferation in the dentate gyrus and decreased the number of newborn cells as a result of reduced proliferation of BLBP+ radial glial cells and Tbr2+ intermediate progenitor cells. At P21, the numbers of NeuN+ neurons and parvalbumin+ γ-aminobutyric acid-ergic interneurons were increased following 50 mg/kg gossypol exposure. In addition, gossypol induced hippocampal neuroinflammation, which may contribute to behavioral abnormalities and cognitive deficits and decrease synaptic plasticity.
Conclusions
Our findings suggest that developmental gossypol exposure affects hippocampal neurogenesis by targeting the proliferation and differentiation of neuronal stem/progenitor cells, cognitive functions, and neuroinflammation. The present data provide novel insights into the neurotoxic effects of gossypol on offspring.
Keywords: Brain development, cognitive deficits, gossypol, hippocampal neurogenesis, neuroinflammation
Significance Statement.
As an endocrine disruptor, gossypol is generally known to exert detrimental effects in animals and humans. However, the mechanisms underlying the neurotoxic effects of gossypol on the central nervous system are still largely unknown. Thus, a greater understanding of the neurotoxicity of gossypol on hippocampal development is required, as it could lead to neuropathological defects and because hippocampal neurogenesis is essential for regulating the normal development and maturation of the brain throughout life. In this study, we explored the effects of gossypol exposure on hippocampal neurogenesis as well as related possible mechanisms. Our results showed that developmental gossypol exposure affects hippocampal neurogenesis by targeting the proliferation and differentiation of neuronal stem/progenitor cells, cognitive functions, along with neuroinflammation. This finding allowed us to answer the important question of whether gossypol affects early postnatal hippocampal neurogenesis, which provides novel insights into understanding the gossypol neurotoxicity on offspring.
Introduction
The hippocampus is a component of the limbic system of the brain and is generally accepted as an important center controlling behavioral and cognitive functions. As part of the hippocampus, the dentate gyrus (DG) is a primary site of neurogenesis during embryonic development, a process that continues postnatally and throughout adulthood (Green and Nolan, 2014; Scharfman, 2016). Importantly, it has been reported that neurogenesis in the DG is related to the behavioral performance of animals and is conducive to hippocampus-dependent learning and memory (Kempermann et al., 1997; van Praag et al., 1999).
In the developing brain, hippocampal neurogenesis in the DG consists of multistep processes involved in the proliferation and asymmetric division of neural stem cells (NSCs) to give rise to neural progenitor cells (NPCs) that differentiate into neurons, astrocytes, and oligodendrocytes. Following migration and loss of cells via programmed cell death, NPCs then become fully functional neurons that are finally integrated into the neural networks and brain circuitry (Green and Nolan, 2014; Abbott and Nigussie, 2020). NSCs migrate toward the hilus in the DG and form the subgranular zone (SGZ) located in the hippocampus along the granule cell layer (GCL) where it abuts the hilus (Bond et al., 2015). Radial glial cells (RGCs) are NSCs during embryonic development and are still abundant in the first postnatal week. RGCs in the SGZ give rise to intermediate progenitor cells (IPCs) and become migrating neuroblasts, which migrate and settle in the GCL and differentiate into fully functional granule cells (GCs) around postnatal day (P) 21 (Seri et al., 2001; Hochgerner et al., 2018). Over recent years, strong evidence has demonstrated that neurogenesis can be altered adversely by many conditions, including stress, physical or chemical factors, and toxins (Anacker et al., 2018). The influences are associated with modifications of the formation of DG structure and brain plasticity, such as an impaired neurogenesis and a reduced number of GCs in the DG of the hippocampus, which are manifested as impaired behavioral functions.
Gossypol is a yellow natural toxic polyphenol compound extracted from the roots, stem, and seeds of cotton. Gossypol could enter the human body through the food chain with the consumption of agricultural by-products such as cottonseed oil, milk, and meat from the affected animals. The pathological signs of gossypol toxicity include reduced growth rate, anorexia, liver damage, lymphocyte cytotoxicity leading to immunodeficiency, decreased reproduction capacity, and neurological disorders (Fombad and Bryant, 2004; Fonseca et al., 2013). The most widely studied effect of gossypol is the toxicity of male reproduction mediated by inhibition of spermatogenesis and testicular steroidogenesis, mitochondrial DNA damage, and germinal epithelium injury (Donaldson et al., 1985; Pearce et al., 1986; Gadelha et al., 2014). The negative effects of gossypol on female fertility, which have also been described, are associated with interference with the estrous cycle, affecting follicular maturation, and disturbance of early embryonic development (Gadelha et al., 2014; Luz et al., 2018). Gossypol also exerts immunosuppressive effects by inhibition of lymphocyte proliferation and induction of cell apoptosis, resulting in increased incidence of some infectious diseases (Xu et al., 2009; Gadelha et al., 2014). Moreover, previous studies showed that gossypol caused hippocampal hemorrhage, membrane permeability alteration, and microtubule assembly interference, which may result in the formation of obvious neurofibrillary tangles in rodents (Sharma et al., 1966; Semon, 2012). However, the mechanisms underlying the neurotoxic effects of gossypol on the central nervous system (CNS) are still largely unknown. Our recent study found that prenatal exposure to gossypol disrupted neurogenesis in the developing neocortex, suggesting the potentially harmful impact of gossypol on the cerebral cortex development of humans and livestock (Zhu et al., 2020). Hippocampal neurogenesis is active during developmental stage, and developmental exposure to chemicals risks strong impacts on neurogenesis (Ogawa et al., 2012). Thus, a greater understanding of the neurotoxicity of gossypol on hippocampal development is required, as it could lead to neurological disorders and because hippocampal neurogenesis is essential for regulating the normal development and maturation of the brain throughout life.
The purpose of the present study was to investigate the neurotoxicity of gossypol in hippocampal neurogenesis. In this study, we found that DG neurogenesis was impaired by gossypol exposure, with impaired proliferation of RGCs and IPCs, decreased newborn cells in DG, and inhibited maturation of NSCs. Following exposure to gossypol, microglial modulation in the hippocampus, behavioral abnormalities, learning and memory impairment, and decreased expressions of synapse-associated proteins were also observed. This study could provide a reference for the connection of gossypol with neurogenesis in the early DG development.
Materials and Methods
Reagents and Chemicals
Gossypol (Sigma-Aldrich, St. Louis, MO) was originally dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich). This solution was further diluted in 0.9% saline and stored at −20°C. Storing solutions (10 mg/mL) were dissolved in DMSO (500 μL) and 0.9% saline (49.5 mL). The working gossypol solutions of experimental groups (2.0 and 5.0 mg/mL) were prepared from the storing solutions. To eliminate the possible effects of DMSO, control group (0 mg/mL) were also added DMSO (10 μL). To ensure freshness, the working gossypol solutions (0, 2.0, and 5.0 mg/mL) were prepared daily. 4’, 6’-Diamidino-2-phenylindole was purchased from Invitrogen (Carlsbad, CA, USA), and propidium iodide (PI) and 5-bromo-2’-deoxyuridine (BrdU) were purchased from Sigma-Aldrich. Other reagents used in the present study are indicated below.
Ethics Statement and Animals
The use of mice in this experiment was approved by the Animal Care Commission of the College of Veterinary Medicine, Northwest A&F University, China (certificate no. SCXK [SHAAN] 2017-003). All experimental procedures involving animal care and handling were conducted according to the Guidelines for Care and Experimental Use of Laboratory Animals of Northwest A&F University and the ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines). Also, this study was not pre-registered, and all experiments were performed between 8:00 am to 10:00 pm.
Institute of Cancer Research mice (aged 3 months) were obtained from Xi’an Jiaotong University and adaptively bred in a laboratory environment for 7 days. Animals were maintained under a 12-hour-light/-dark schedule from 8:00 am to 10:00 pm at 22°C–26°C with access to food and water ad libitum. Male mice were mated with 2 female mice, and the time of vaginal plug appearance in the female mice was classified as embryonic day (E) 0.5. To avoid interference between mice, each dam was housed individually following pregnancy classification.
Experimental Design
Pregnant animals were randomly and blindly assigned to 3 different groups (n = 5 per group), and gossypol was administered daily at 20 and 50 mg per kg body weight daily via the oral route at 9:00 am from E6.5; the control group received 0.9% saline. The doses used in the present study were determined according to previous studies (Hahn et al., 1981; Li et al., 1989; Zhu et al., 2020). Up to 10 newborn pups from each mother who had more than 10 pups were culled at P3. In the experiment, offspring from litters containing 6 to 10 pups with similar numbers of males and females were used. In each group, 1 pup per litter was randomly selected for immunofluorescence, western-blot, and quantitative reverse transcription-polymerase chain reaction (RT-PCR) analyses, and 10 offspring from 5 dams in each group were subjected to behavioral tests. At P8 and P21, the offspring were killed by an overdose of ether anesthetic to minimize suffering, subjected to autopsy, and the brain removed (Figure 1a). There were no predetermined exclusion criteria (no exclusions were made), and this study was exploratory in nature.
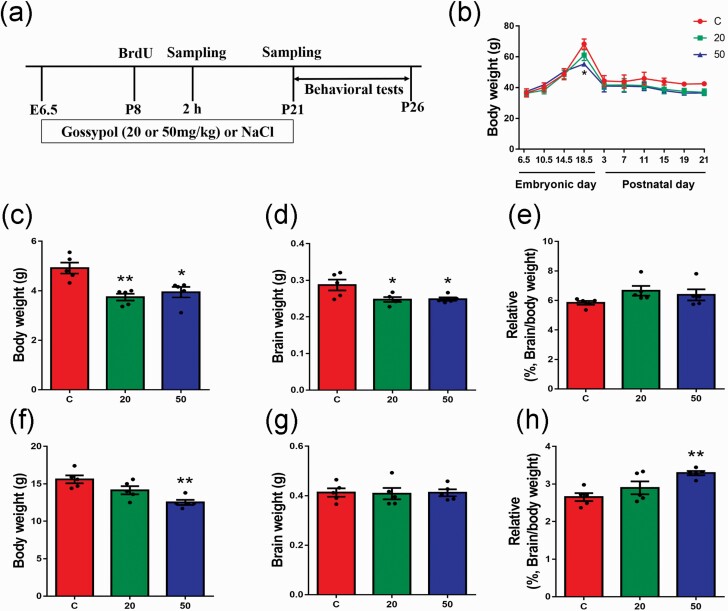
Figure 1.
Effects of gossypol exposure on the body weight of dams, and the body weight and brain weight of their offspring. (a) Timeline of the experiment. Two groups received 20 or 50 mg/kg body weight/d of gossypol from embryonic day (E) 6.5 to the time of sample harvest. The control group received the vehicle (0.9% NaCl). (b) Body weights of dams from E6.5 to postnatal day (P) 21. (c), Body weights of offspring at P8. (d) Brain weights of offspring at P8. (e) The ratio of brain weight to body weight of offspring at P8. (f) Body weights of offspring at P21. (g) Brain weights of offspring at P21. (h) The ratio of brain weight to body weight of offspring at P21. (b–h), n = 5 per group. Differences in body weights of offspring were found between the gossypol exposure and control group. Data represent the mean ± SEM. *P < .05 and **P < .01 compared with the controls.
Behavioral Tests
Elevated Plus Maze Tests
The elevated plus maze (EPM) measures exploration ability and anxiety levels using the conflict state formed by the animal’s exploration of new and different environments and the fear of an elevated field (Hei et al., 2019). The apparatus consists of 2 open arms (50 × 10 cm), crossed by 2 closed arms (50 × 10 × 40 cm), and a central region (10 × 10 cm). The apparatus was placed 70 cm above the ground. At P21, the mice were placed in the open box for 5 minutes to prevent hiding in the closed arm and then were placed in the central area facing the open arms. The mice were then allowed to freely explore the maze for 5 minutes. After the mice were put in the apparatus, the time and the times and distance traveled in open arms were measured using the ANY-maze video tracking/computer-digitizing system. The apparatus was wiped with ethanol after each trial to eliminate the interference by the residual odor. The whole experiment was performed under dim red light indirectly (2 × 60 W) to illuminate the EPM apparatus, and all the arms were similarly illuminated without shadows, as previously described (Holmes et al., 1999).
Morris Water Maze Tests
Morris water maze (MWM) tests were carried out to examine learning and memory impairment. Briefly, at P21, the mice underwent training and learning for 5 consecutive days, with 4 trials per day from 4 quadrants. The mice were allowed to find a hidden platform (10-cm diameter) located 1.0 cm below the water surface in a circular pool (120-cm diameter, 45-cm height) filled with water (23°C–25°C) rendered opaque with nontoxic black-paint. Escape latency (the time that the mice found and climbed onto the platform) was recorded for each training session lasting for up to 60 seconds. If a mouse did not find the platform in the allotted time, it was guided to the platform for 15 seconds. On day 6, the platform was removed from the pool, and each mouse was allowed to explore from the starting point opposite the platform for 60 seconds. Times of crossing the platform, distance and time traveled in the target quadrant, the distance and time traveled in the position of the platform, and the total distance and average velocity were recorded using the ANY-maze system. After each trial, each mouse was dried with a towel and placed in a cage under a heat lamp for 2 minutes until dry before returning to its regular cage. During the experiment, dim red light indirectly (2 × 60 W) was used to illuminate the MWM apparatus, and the surface of circular pool was similarly illuminated without reflecting the light.
BrdU Labeling
BrdU has been described as a principal marker of neurogenesis and mitotic labeling through its incorporation into the DNA of dividing cells during S-phase (Kee et al., 2002). BrdU was dissolved in 0.9% saline, and each pup was injected i.p. with a dose of 50 mg/kg body weight. To assess the effects of gossypol on NPC proliferation in the DG, the P8 pups in each group were treated with BrdU 2 hours prior to tissue harvesting. Pups were deeply anesthetized with 0.56% sodium pentobarbital at the different stages indicated and subjected to successive cardiac perfusion with 0.9% saline and 4% paraformaldehyde (pH 7.4). The brains were harvested and post-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) at 4°C.
Immunofluorescence Analysis
Coronal sections (thickness 60 μm) were prepared from the fixed brain tissues of the offspring using a vibratome (VT 1000S, Leica, Germany). Immunofluorescence analysis was carried out as described previously (Zhu et al., 2020). For immunofluorescent detection of BrdU, brain sections were denatured for 30 minutes in 2 M HCl at 37°C, rinsed 3 times (8 minutes per wash) in 0.1 M PB, followed by a 30-minute wash in 0.1 M borate buffer (pH 8.5), and then rinsed with 0.1 M PB before incubation with the primary detection antibodies.
The following primary detection antibodies were used in this study: rat anti-BrdU (1:500, Millipore, Temecula, CA); rabbit anti-Prox1 (1:500, Abcam, Cambridge, UK); rabbit anti-Tbr2 (1:500, Invitrogen); rabbit anti-BLBP (1:500, Millipore); mouse anti-NeuN (1:500, Millipore); rabbit anti-Reelin (1:500, Invitrogen); rabbit anti-parvalbumin (PV; 1:500, Invitrogen); rabbit anti-GAD (1:500, Invitrogen); and rabbit anti-Iba1 (1:500, Abcam). The secondary detection antibodies were as follows: Alexa Fluor 488 goat anti-mouse IgG (1:500, Abcam); Alexa Fluor 488 donkey anti-rabbit IgG (1:500, Invitrogen); Alexa Fluor 568 donkey anti-rabbit IgG (1:500, Cell Signaling Technology, Boston, MA); Alexa Fluor 647 goat anti-rat IgG (1:500, Chemicon, Nürnberg, Germany); and Alexa Fluor 647 donkey anti-rabbit IgG (1:500, Invitrogen).
Imaging Acquired and Cell Counting
Immunofluorescence images were captured using a structured illumination microscope (Zeiss Axio Observer Z1). An observer who was blinded to the group assignment was responsible for counting the number of positive cells. Cell counts were performed on 5 mice in each group using Zeiss Axio Observer Z1 software as well. Two slices in each offspring were acquired and 10 images were analyzed in each group.
Western-Blot Analysis
At P21, the hippocampus of mice was isolated and stored at −80°C. Western-blot analysis was carried out as described previously (Li et al., 2018; Hei et al., 2019). In brief, the hippocampus was homogenized in ice-cold RIPA buffer (Solarbio, Beijing, China) containing 1 mM phenylmethanesulfonyl fluoride (Solarbio) and a PhosSTOP EASYpack (Roche, Basle, Switzerland). Equal amounts of proteins (20 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride (0.45-μm) membranes (IPVH00010, Millipore). After blocking with 5% non-fat milk, membranes were incubated overnight at 4°C with specific primary detection antibodies against Iba1, spinophilin, postsynaptic density (PSD95), synaptophysin (1:500, Millipore), toll-like receptor 4 (TLR4) and GAPDH (1:1,000, Abcam), nuclear transcription factor-kappa B (NF-κB), p38, phospho-p38, and β-actin (1:1000, Cell Signaling Technology, Danvers, MA). After rinsing, membranes were incubated for 2 hours at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody or goat anti-mouse IgG antibody (1:5,000, Cell Signaling Technology). The protein bands were visualized using an enhanced chemiluminescence detection kit (CW0048M, Cowin) and imaged using the GelDoc XR Gel Documentation System (BioRad). Intensities of the bands were measured and quantified by ImageJ analysis software.
Quantitative RT-PCR Analysis
For quantitative RT-PCR analysis, hippocampus was removed from offspring mice at P21. Isolation of total RNA, reverse transcription, and quantitative PCR were performed as previously described (Zhu et al., 2017). Primers were designed using Primer Express version 5.0 (Applied Biosystems, Foster City, CA). The forward (F) and reverse (R) primers were as follows: IL -1β, F: 5’-TGACGGACCCCAAAAGATGA-3’, R: 5’-TCTCCACA GCCACAATGAGT-3’; TNFα, F: 5’-AGTCCGGGCAGGTCTACTTT-3’, R: 5’-GTCACTGTCCCAGCATCTTGT-3’; IL -1ra, F: 5’-ACTCATCC CTGTGACTTTGG-3’, R: 5’-GTGGCTCATTTGCTATCTTT-3’; BDN F, F: 5’-TTACAGGTCCAAGGTCAACG-3’, R: 5’-CTCAGGAG GATGCCCAAA-3’; NGF, F: 5’-CGACTCCAAACACTGGAACTC A-3’, R: 5’-GCCTGCTTCTCATCTGTTGTCA-3’; mPPARγ, F: 5’-GT CTTGGATGTCCTCGATGGG-3’, R: 5’-TTATGGAGCCTAAGTTTGAGTTTGC-3’; GAPDH, F: 5’-AGGTTGTCTCCTGCGACTGCA-3’, R: 5’-GTGGTCCAGGGTTTCTTACTCC-3’. The real-time quantitative PCR was conducted using the NovoStart SYBR qPCR SuperMix Plus (Novoprotein Scientific Inc., China) with the ABI Prism 7300 sequence detection system (Applied Biosystems). The relative mRNA expression was determined using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Statistical Analysis
Sample sizes for the study were calculated using free software at http://www.gpower.hhu.de/. The following parameters were set: test family (T tests), statistical test (mean: difference between 2 independent means), type of power analysis (a prior: compute required sample size-given α, power, and effect size). Based on the experience of our team and in particular on the results already obtained in our previous study (Zhu et al., 2020), we calculated effect size of 1.8, power (1-βerr prob) of 0.2, αerr prob of 0.05, and two tails were used to arrive at the calculated sample size of each group was 6. Data were expressed as mean ± SEM, and n referred to the number of animals in each group. All statistical analysis was performed using GraphPad Prism 7.0 (GraphPad Software Inc.). Statistical comparisons were performed by 1-way ANOVA followed by Tukey’s multiple comparisons tests between groups. Normality testing was not performed for all analyses since the sample size was low (<30). Furthermore, in the present study, no tests for outliers (no exclusion of data points) were performed. A value of P < .05 was considered statistically significant.
Results
Effects of Gossypol on the Body Weight of Dams and Offspring
During the embryonic period from E6.5 to E17.5, the weight of dams treated with gossypol was indistinguishable from that of the control mice, while we found a striking difference in body weight at E18.5. During the postnatal period, there was no significant difference between the control and gossypol-treated groups (Figure 1b). For the offspring, body weights and brain weights of offspring at P8 were significantly decreased in the gossypol-treated group compared with those in the control group (Figure 1c–d), while there were no significant differences in the ratio of brain weight to body weight of the offspring among the groups (Figure 1e). Additionally, at P21, gossypol suppressed the body weights of offspring, especially at the dose of 50 mg/kg gossypol (Figure 1f). There were no significant differences in the brain weights of offspring among the groups (Figure 1g), while the ration of the brain weight to the body weight of the gossypol-treated group was significantly higher than that of the control group (Figure 1h).
Gossypol Inhibited the Proliferation of NSCs in the DG and Decreased the Newborn GCs
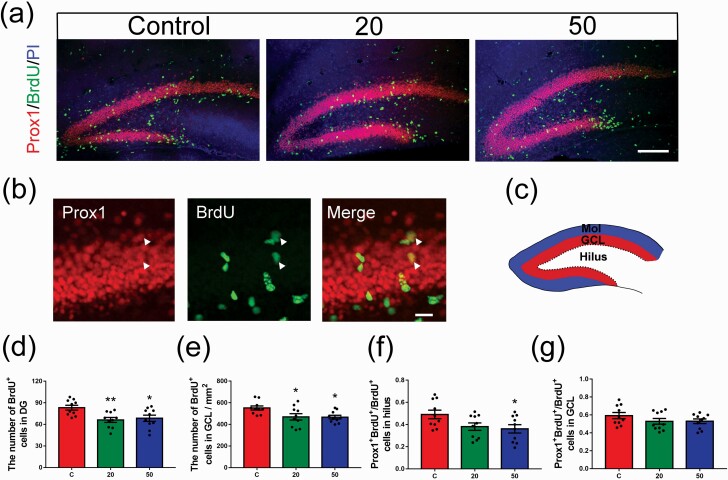
To study the effect of gossypol administration on NSC proliferation in the DG, the P8 pups in each group were injected with BrdU 2 hours prior to harvesting. A schematic diagram of the DG is shown in Figure 2c. The DG can be roughly divided into 3 layers: the hilus, the molecular layer (Mol), and the GCL including the SGZ. The number of BrdU+ cells in the DG and GCL decreased remarkably in the gossypol-treated mice compared with the controls (Figure 2a, d, e). To determine whether the generation of neurons was affected by gossypol, we examined the expression of prospero-related homeobox 1 (Prox1), which is widely used as a specific marker of mature GCs and is required for survival and maturation of newborn cells in the hippocampus (Figure 2a–b). Analysis of cell proliferation in the DG by co-labeling with Prox1 and BrdU showed an obvious reduction in the ratio of BrdU+ Prox1+ cells to total BrdU+ cells in the hilus in the gossypol group (Figure 2f), whereas there were no significant differences in the number of BrdU+ Prox1+ double-labeled cells in the GCL among the groups (Figure 2g). These results suggested that gossypol exposure impaired not only NSC proliferation in the DG but also the generation of newborn cells.
Figure 2.
Effects of gossypol exposure on neural stem cell (NSC) proliferation in the dentate gyrus (DG) and newborn granule cells (GCs). (a) Representative images of BrdU (green) and Prox1 (red) labeling of newly generated cells in the DG at 2 hours after BrdU injection at postnatal day (P) 8. Brain sections were counterstained with PI (blue). (b) Higher magnification of newly generated cells (white arrows). (c) Schematic diagram of the DG. The DG could be roughly divided into 3 layers: the hilus, the molecular layer (Mol), and the GC layer (GCL), including the subgranular zone (SGZ). (d–g) Quantification of the number of BrdU-labeled newly generated cells in the DG (d) and GCL (e), BrdU+ Prox1+ cells in the hilus (f) and GCL (g). There were significant differences in the number of BrdU cells and the ratio of BrdU+ Prox1+ cells to the total BrdU+ cells among the 3 groups of offspring. Data represent the mean ± SEM (n = 10 per group, 10 sections from 5 offspring in each group). Scale bars = 100 μm. *P < .05 and **P < .01 compared with the controls.
Inhibition of Proliferation of RGCs and IPCs in the DG in Gossypol-Treated Offspring
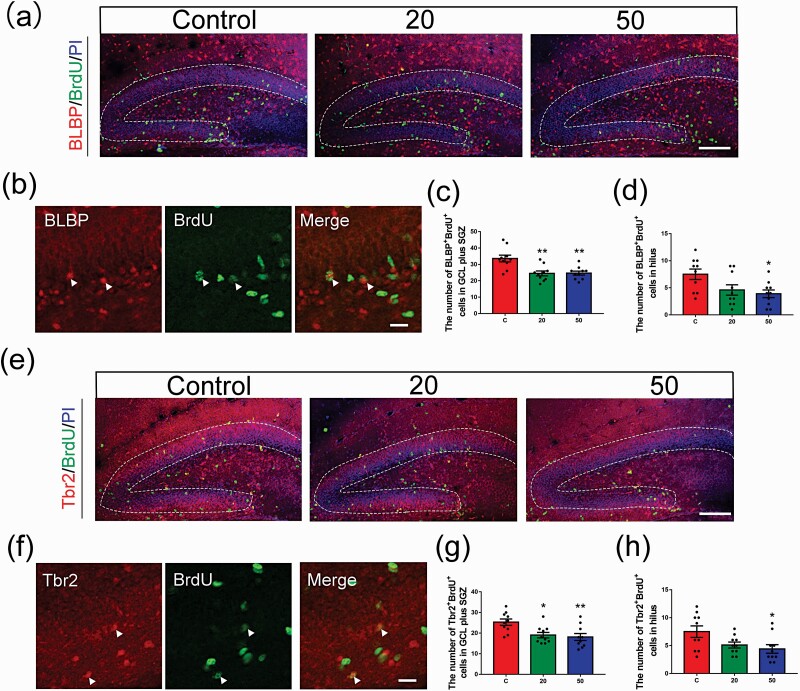
Neurons are generated from NSCs and IPCs in the DG during hippocampal neurogenesis in the developing brain (Chen et al., 2015). To examine the mechanism by which gossypol treatment decreased the number of newborn cells, sections were immunofluorescently stained for brain lipid binding protein (BLBP) and T-box transcription factor (Tbr2) to detect the proliferating RGCs and IPCs, respectively (Figure 3a, b, e, f). BLBP is a specific marker of RGCs, and IPCs are highly proliferative and express Tbr2. Our data showed that the number of BLBP+ BrdU+ double-labeled cells in the region of the GCL plus the SGZ at P8 following gossypol treatment was remarkably lower than that in the control mice (Figure 3c). The same result was also observed in hilus (Figure 3d). In addition, fewer Tbr2+ BrdU+ cells were observed in a pattern similar to that of BLBP+ BrdU+ cells in the region of GCL plus the SGZ and in hilus at P8 in the gossypol-treated groups compared with that in the control offspring (Figure 3g–h). These data indicated that the decreased generation of newborn cells in gossypol-treated animals results from a reduction in the number of proliferating BLBP+ and Tbr2+ cells.
Figure 3.
Effects of gossypol exposure on the proliferation of RGCs and IPCs in the DG. (a) Representative images of proliferating RGCs labeled with BrdU (green) and BLBP (red) in the DG at 2 hours after BrdU injection at P8. Brain sections were counterstained with PI (blue). (b) Higher magnification of proliferating RGCs (white arrows). (c) Quantification of the number of BLBP+ BrdU+ cells in the GCL plus SGZ. (d) Quantification of the number of BLBP+ BrdU+ cells in the hilus. Gossypol decreased the number of BLBP+ BrdU+ cells. (e) Representative images of proliferating IPCs labeled with BrdU (green) and Tbr2 (red) in the DG at 2 hours after BrdU injection at P8. Brain sections were counterstained with PI (blue). (f) Higher magnification of proliferating IPCs (white arrows). (g) Quantification of the number of Tbr2+ BrdU+ cells in the GCL plus SGZ. (h) Quantification of the number of Tbr2+ BrdU+ cells in the hilus. Gossypol decreased the number of Tbr2+ BrdU+ cells. Data represent the mean ± SEM (n = 10 per group, 10 sections from 5 offspring in each group). Scale bars = 100 μm. *P < .05 and **P < .01 compared with the controls.
Gossypol Affected NeuN+ Mature Neurons and Interneuron Subpopulations in the DG
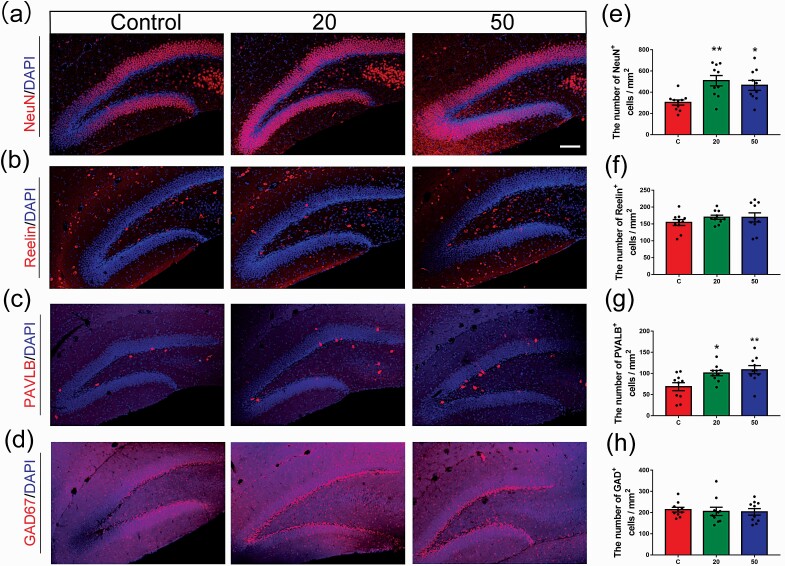
To examine whether gossypol affects the maturation of neurons, we performed immunofluorescent staining of neuronal nuclei (NeuN), which is an extensively used marker of mature neurons (Figure 4a). We found that at P21, the number of NeuN+ cells in the hilus sharply increased in the gossypol-treated mice compared with that in the controls (Figure 4e). Based on our detection of NeuN+, we tested reelin expression in the mouse DG during development (Figure 4b). There was no significant increase in the number of reelin+ cells following gossypol exposure compared with that in the control group (Figure 4f). In addition, γ-aminobutyric acid (GABA) is a major inhibitory neurotransmitter in mature neurons, and L-glutamic acid decarboxylase (GAD) isoform GAD67 is predominantly expressed by inhibitory GABAergic neurons. The majority of GABAergic interneurons produce PV to regulate the migration, proliferation, and differentiation of NPCs as well as neurotransmitter release between neurons. Based on this information, we assessed the expression of these 2 interneuron-specific immunocytochemical markers in the hippocampus at P21 (Figure 4c–d). The number of PV+ interneurons was remarkably increased in the hippocampus of gossypol-treated mice compared with the controls, especially at the dose of 50.0 mg/kg gossypol (Figure 4g). However, there was no significant difference in the number of GAD67+ interneurons between the gossypol-treated and control mice (Figure 4h). These results suggested that gossypol exposure inhibits the maturation of NSCs in the hippocampal DG of mice and ultimately leads to a reduction in the number of neurons.
Figure 4.
Effects of gossypol exposure on NSC maturation in the DG at P21. (a–d) Representative images of NeuN, Reelin, PV, or GAD67 (red) and 4’, 6’-diamidino-2-phenylindole (blue) at P21. (e–h) Quantification of the number of NeuN+ (e), Reelin+ (f), PV+ (g), and GAD67+ (h) cells in the hilus. Gossypol increased the number of NeuN+ and PV+ cells. Data represent the mean ± SEM (n = 10 per group, 10 sections from 5 offspring in each group). Scale bars = 100 μm. *P < .05 and **P < .01 compared with the controls.
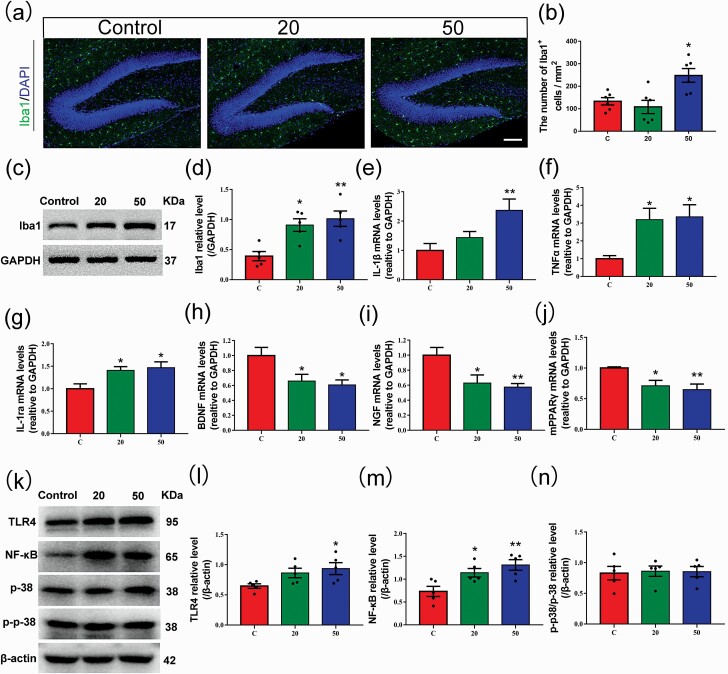
Gossypol Modulated the Inflammatory Response in the Hippocampus of Gossypol-Treated Offspring
The impact of inflammation on hippocampal neurogenesis has been widely investigated. Hippocampal neurogenesis is continuously maintained by the proliferation of NSCs located in the SGZ of the DG (Bond et al., 2015). Microglial cells, the resident immune cells of the CNS, are involved in several components of the neuroinflammatory response and play an important part in regulating hippocampal neurogenesis (Sierra et al., 2014). To further investigate whether the influence of gossypol on hippocampal neurogenesis is the result of neuroinflammation, we performed immunofluorescence analysis of ionized calcium binding adaptor molecule 1 (Iba1), which is a marker of microglial cells and macrophages (Figure 5a). The density of Iba1+ microglial cells in the hilus was higher in the gossypol exposure offspring than that in the control group (Figure 5b). In accordance with this result, we also found higher levels of Iba1 protein in gossypol-treated mice (Figure 5c–d). Studies have shown that activated microglia respond to some environmental toxins that modulate the neuronal microenvironment by changing the expression of cytokines, leading to neurotoxicity (Block et al., 2007; Patterson, 2015). Our results showed a remarkable increase in the levels of the proinflammatory cytokines IL-1β and TNF α, and IL-1ra, which is an anti-inflammatory cytokine, in the gossypol-treated group (Figure 5e–g). The expression of BNDF and NGF was lower in the gossypol-treated groups (Figure 5h–i). In addition, our results showed the mRNA expression of mPPARγ in hippocampus was significantly decreased in gossypol-treated mice than in controls (Figure 5j). Given the evidence that the TLR4, NF-κB, and mitogen-activated protein kinase signaling pathways play crucial roles after inflammation of the CNS (Okun et al., 2011), their protein levels were investigated (Figure 5k). The results showed that the protein levels of TLR4 and NF-κB significantly increased following gossypol exposure (Figure 5l–m). However, there were no significant differences in p38 (Figure 5n). These results indicated that the disruption of hippocampal neurogenesis following gossypol treatment is mediated by the induction of neuroinflammation and was possibly related to mPPAR-γ/TLR4/NF-κB signaling.
Figure 5.
Effects of gossypol exposure on microglia and inflammation-related indicators in the hippocampus at P21. (a) Representative immunofluorescence analysis of microglia using Iba1 as a marker in the hippocampus at P21. (b) Quantification of the number of Iba1+ cells in the hilus. Gossypol increased the density of activated microglia. (c), Representative western blots of Iba1 and GAPDH and densitometric quantification of protein levels in the hippocampus at P21. (d) Relative Iba1 protein levels. Iba1 expression was increased following gossypol exposure. IL-1β (e), TNFα (f), IL-1ra (g), BDNF (h), NGF (i), and mPPARγ (j) mRNA levels in the hippocampus. Gossypol increased pro-inflammatory cytokine expressions, while the expressions of mPPARγand trophic factors were decreased in mouse offspring. (k) Representative western blots of TLR4, NF-κB, p-38, p-p-38, andβ-actin and densitometric quantification of protein levels in the hippocampus at P21. Relative protein levels of TLR4 (l), NF-κB (m), and the ratio of phosphorylation of p38 and p38 (n). Expressions of TLR4 and NF-κB were increased following gossypol exposure. Data represent the mean ± SEM. (b) n = 10 per group, 10 sections from 5 offspring in each group. (d, l–n) n = 5 per group, 5 offspring from 5 dams in each group. (e–j) n = 10 per group, 10 offspring from 5 dams in each group. *P < .05 and **P < .01 compared with the controls.
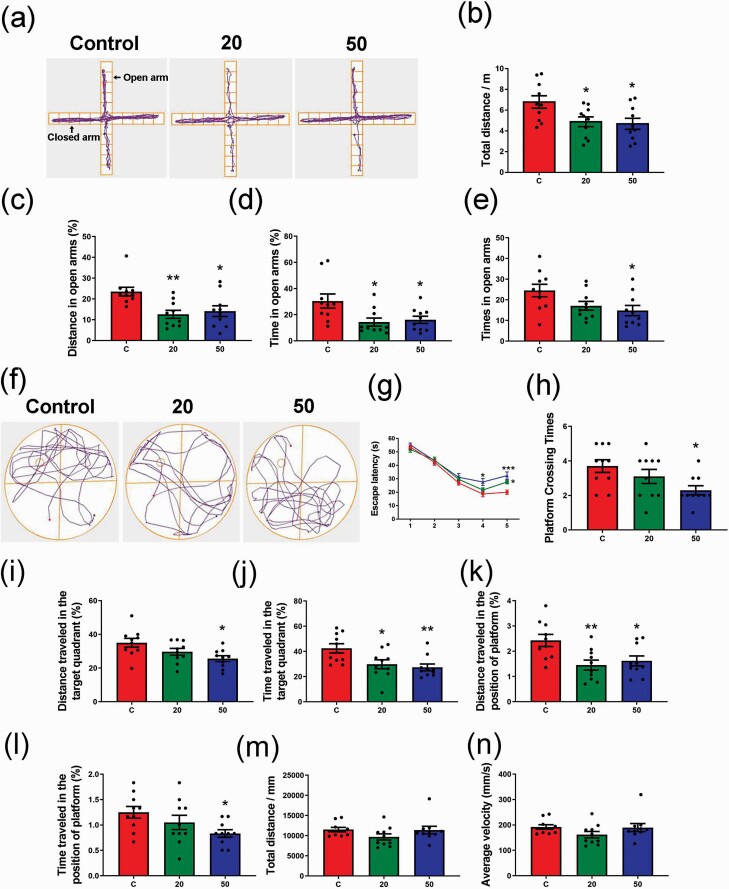
Gossypol Induced Anxiety-Like Behavior and Impaired Spatial Learning and Memory in Offspring Mice
Numerous studies have provided evidence that inflammation can induce behavioral and cognitive changes (Cunningham et al., 2009; Yirmiya and Goshen, 2011; Patterson, 2015). To further investigate the effect of gossypol on hippocampal neurogenesis, we evaluated anxiety-like behavior, learning, and memory in offspring through EPM and MWM tests. In EPM tests (Figure 6a), the gossypol-treated offspring exhibited a remarkable decline in the total distance (Figure 6b), distance (Figure 6c), time (Figure 6d), and times (Figure 6e) in open arms compared with the control group. MWM tests were performed to investigate the effects of gossypol on learning and memory in offspring (Figure 6f). We found that the escape latency of gossypol-treated offspring was significantly longer than that of the controls at days 4 and 5 of the training period (Figure 6g). During the test, mice treated with 50 mg/kg gossypol exhibited a decrease in the platform crossing times (Figure 6h). In addition, the distance and time traveled in the target quadrant in gossypol-treated mice were shorter than those of the controls (Figure 6i–j). Similar results were also observed in the distance and time traveled in the position of the platform, with a smaller proportion in the gossypol-treated groups (Figure 6k–l). However, there were no significant differences in total distance and average velocity among 3 groups (Figure 6m–n). These results indicated the reduction of the exploratory behavior, induction of the anxiety-like behavior, and impairment of learning and memory of offspring following gossypol exposure.
Figure 6.
Effects of gossypol exposure on anxiety-like behaviors, memory, and learning. (a) Trace chart of elevated plus maze tests of offspring at P21. The blue point represents the original position, and the red point represents the end position. Quantitative analysis of total distance (b), distance (c), time (d), and time traveled (e) in the open arms. There were differences between the gossypol exposure and control groups. (f) Trace chart of Morris water maze tests of offspring at P21. The blue point represents the original position, and the red point represents the end position. Quantitative analysis of escape latency (g), times of crossing the platform (h), distance and time traveled in the target quadrant (i–j), distance and time traveled in the position of platform (k–l), total distance (m), and average velocity (n). There were differences between the gossypol exposure and control groups. Data represent the mean ± SEM (n = 10 per group, 10 offspring from 5 dams in each group). *P < .05 and **P < .01 compared with the controls.
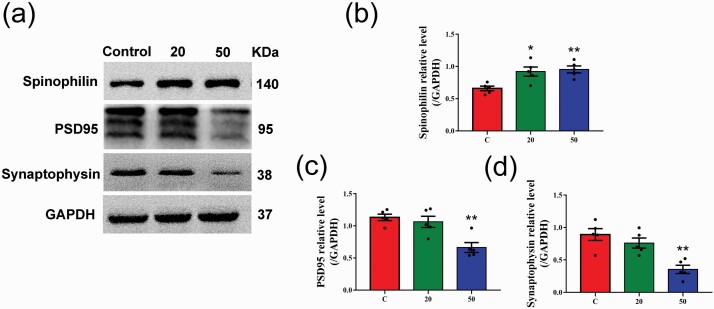
Gossypol Affected the Expression of Synaptic Proteins in the Hippocampus
Synapses, which mediate neural information transfer, are composed of a presynaptic element, a synaptic cleft, and a postsynaptic element. Synaptic proteins are directly or indirectly involved in learning and memory. Therefore, we performed western-blot analysis of the expression of synaptic proteins, and the optical density ratios of the band intensities of proteins normalized to GAPDH were expressed as fold changes in mice (Figure 7a). The expression of spinophilin, which is highly enriched in dendritic spines, was significantly increased in gossypol-treated mice compared with the controls (Figure 7b). PSD95 is a typical excitatory synaptic protein in the hippocampus. Western-blot analysis showed that the levels of PSD95 protein in the hippocampus of the mice dosed with gossypol at 50 mg/kg were significantly decreased at P21 compared with the control mice (Figure 7c). Similarly, the expression of synaptophysin, which is a specific marker of synaptic vesicles in the presynaptic element, was also markedly decreased in gossypol-treated mice (Figure 7d). These results suggested that gossypol affected the expression of synaptic proteins in the hippocampus.
Figure 7.
Effects of gossypol exposure on the expression of synaptic proteins in the hippocampus. (a) Representative western blots of spinophilin, PSD95, synaptophysin, and GAPDH and densitometric quantification of the protein levels in the hippocampus at P21. Relative protein levels of spinophilin (b), PSD95 (c), and synaptophysin (d). Gossypol increased the levels of spinophilin in the hippocampus, while the levels of PSD95 and synaptophysin were decreased. Data represent the mean ± SEM (n = 5 per group, 5 offspring from 5 dams in each group). *P < .05 and **P < .01 compared with the controls.
Discussion
It is well known that the high gossypol concentration in cottonseed and its by-products can lead to impairment of reproductive performance, including reduced rates of pregnancy in livestock (Santos et al., 2003) and fertility problems in humans (Gadelha et al., 2014). Considering the potential of gossypol to transfer transplacentally and lactationally to offspring as well as to permeate the blood brain barrier, investigation of offspring hippocampal neurogenesis can be helpful to understand the neurotoxicity of gossypol in the brain. In this study, we exposed pregnant mice to gossypol to investigate the neurotoxic effects on the development of hippocampal DG in offspring as well as the potential mechanisms. Specifically, we showed that gossypol affected neuronal proliferation and maturation, and observed behavioral abnormalities and cognitive deficits, all of which may be associated with neuroinflammation. These findings provide important evidence that gossypol affects the early postnatal hippocampal neurogenesis.
As a common sign of gossypol toxicity, impaired body weight gain was observed in many studies. As reported by our recent study (Zhu et al., 2020), the weight of the gossypol-treated dams showed a striking decrease at E18.5. Importantly, decreased weight gain was also observed in offspring treated with gossypol at P8 and P21 in the current study. This finding is in agreement with a previous study indicating that gossypol significantly reduced fetal body weight in pregnant mice (Sein et al., 1986). However, during the postnatal period, there was no significant difference between the control and gossypol-treated groups in dams. This suggests that fetuses and pregnant individuals are more sensitive to gossypol.
Postnatal hippocampal neurogenesis is a particularly interesting target to investigate in neurotoxicity, since it is one of few developmental stages in which this process occurs at high levels after birth (Kang et al., 2017). Postnatal hippocampal neurogenesis comprises a series of consecutive developmental events and is critically essential for brain function. This process participates in learning and memory, and its disruption may be connected with psychiatric disease (Kang et al., 2016; de Miranda et al., 2017). Importantly, NSCs reside primarily in the DG and participate in these brain functions. We examined the influence of gossypol exposure on NSC proliferation by quantifying BrdU+ cells in the DG after injection of BrdU. The results showed 21% and 16% decreases in the groups treated with gossypol at 20 and 50 kg/mg, respectively, indicating that a decrease in NSC proliferation in the DG is a negative effect of gossypol on neurogenesis. Most GCs are generated perinatally, predominantly around P10 (Hochgerner et al., 2018). Increasing evidence suggests that newly generated neurons are of importance in spatial learning and memory and are involved in hippocampal functions that are particularly dependent on the DG (Kee et al., 2007; Nakashiba et al., 2012). Newly generated cells are affected by multiple factors and undergo certain complex processes of morphological and functional maturation (Knowles et al., 1999). In the current study, we found that the number of Prox1+ BrdU+ cells was significantly decreased in the hilus but did not change in the GCL following gossypol treatment, which is in agreement with our previous study (Liu et al., 2019). Since GCs are produced from stem cells located in the hilus during development of DG, our study indicated that gossypol exposure is associated with a reduction in GC generation.
Development of the DG is a highly sophisticated process requiring the coordination of multiple steps, such as cell proliferation, migration, differentiation, and maturation. RGCs and IPCs, which are located and proliferate in the hilus, play key roles in hippocampal neurogenesis and are responsible for the evolutional process of the brain. RGCs provide the “scaffolding” that guides the migration of neurons, forming the layered structure of the normal hippocampus. As a type of NPC, RGCs are the source of neurons and glial cells in the hippocampus. IPCs are derived from asymmetric division of RGCs. The stunted development of RGCs and IPCs has a definite effect on the formation and function of the normal hippocampal structure. To investigate the potential association of the reduction in new GCs with the impairment of neuronal proliferation, we analyzed the immunofluorescent staining of the cells expressing BLBP and Tbr2. Results showed that with gossypol exposure, the number of proliferating RCGs and INPs was reduced in both the hilus and GCL. These data implied that gossypol caused the decreased generation of newborn cells resulting from the proliferative capacity of RGCs and INPs. Interestingly, gossypol treatment decreased the number of RCGs and INPs in the hilus and GCL and decreased newborn GCs in the hilus, but not in GCL. This observation may be explained by the view that gossypol affects the neuronal migration process, although the mechanism is not well understood. However, it is certain that in the gossypol-treated model, the inhibition of RGCs and IPCs resulted in defective neurogenesis. Thus, it can be speculated that the detection of RGC and IPC defects is essential for assessment of the risks of chemical exposure in brain development in livestock and humans.
In the developing brain, GABA can act to modulate the proliferation and differentiation of NSCs to form newly generated neurons in the DG (Kang et al., 2017). GABAergic interneurons in the hilus of the DG innervate GC lineage populations to regulate neurogenesis processes in the SGZ. PV, which is expressed in GABAergic interneurons, regulates neurogenesis in the SGZ and is responsible for the cognitive functional capacities of the brain (Bartos et al., 2007). Reelin, which is synthesized and secreted by early Cajal-Retzius cells in the marginal zone, plays a critical role in neuronal fate and migration during the development of the CNS, especially during morphogenesis of the DG (Förster et al., 2010; Caruncho et al., 2016; Chai et al., 2016; Ampuero et al., 2017; Abbott and Nigussie, 2020). GAD67 is contained within the large majority of interneurons, specifically those that employ GABA as a neurotransmitter (Freund and Buzsáki, 1996). In the present study, significantly increased densities of PV+ interneurons were observed in the DG in offspring following exposure to 50 mg/kg gossypol. However, there were no fluctuations in the number of reelin and GAD67 cells. There are 2 possible explanations for this phenomenon. On the one hand, since gossypol decreased the number of GCs in the present study, a compensatory mechanism might be responsible for the gossypol-induced aberration in neurogenesis, causing upregulation of PV in interneurons. Considering the function of PV in the brain, gossypol may result in disruption of the cognitive function of offspring in later life. On the other hand, GAD-positive cells in interneurons are relatively resistant to the effects of some toxins (Kohler, 1984). The lack of changes in reeling and GAD67 indicates that these cells are more resistant to the toxicity of gossypol. Importantly, we found that gossypol resulted in an increase in NeuN+ at P21. This result suggests that developmental gossypol exposure causes impairment of hippocampal neurogenesis and neuronal mismigration.
The interaction between neuroinflammation and neurogenesis has been widely reported (Na et al., 2014; Sierra et al., 2014). The mechanisms underlying the neurogenesis-impairing effect of gossypol could be due to direct or indirect induction of neuroinflammation. To further investigate the potential mechanism underlying the neurotoxicity of gossypol on hippocampal neurogenesis, we performed immunofluorescence and western-blotting analyses, which suggested that gossypol induced microglia activation. The microglia can be neuroprotective against detrimental effects on neurogenesis, progenitor proliferation, migration, and differentiation, depending on their state of activation (Ekdahl et al., 2009; Kohman and Rhodes, 2013). Our results showed that gossypol inhibited progenitor proliferation. This is consistent with the previous report that microglial cells express an inflammatory phenotype that generally inhibits cell proliferation (Kohman and Rhodes, 2013). This demonstrates that hippocampal neurogenesis is regulated by microglial activation and inflammation after gossypol exposure. Importantly, activation of the microglia can be reflected by the expression of proinflammatory cytokines as well as trophic factors (Zhao et al., 2014; Minnone et al., 2017). Regarding the functions of the microglia and neurons, it should be noted that activated microglia as well as the elevated production of proinflammatory cytokines could change normal neuronal functions and impact neurogenesis. In the present study, activation of the microglia was accompanied by the release of proinflammatory cytokines, including IL-1β and TNFα, and inhibition of mPPARγ and trophic factors, including BDNF and NGF. This demonstrates that activation of the microglia and changes in the inflammatory cytokines resulting from gossypol exposure can influence neurogenesis and may further impact brain development, leading to developmental disorders. However, expression of the anti-inflammatory cytokine IL-1ra was also increased following exposure to gossypol. The possible reasonable explanation for this is that a dynamic balance of pro- and anti-inflammatory cytokines is needed when potentially toxic exposures occur, although whether this phenomenon is actually responsible for the observed changes in impairment of hippocampal neurogenesis remains to be determined.
TLR4 is highly expressed on microglia after inflammation of the CNS (Okun et al., 2011). Stimulation of TLR4 can lead to the activation of NF-κB and mitogen-activated protein kinase and the production of inflammatory mediators, including IL-1β and TNFα (Lehnardt et al., 2008; Hanke et al., 2011). Our previous study found that gossypol exposure significantly increased the protein levels of NF-κB, suggesting that prenatal gossypol-increased progenitor apoptotic cell death may related to NF-κB (Zhu et al., 2020). A number of studies suggest that, under pathological state, TLR4/NF-κB signaling can be activated, followed by promoting the release of inflammatory factors. PPARγ regulates the alternative activation of immune cells by increasing anti-inflammatory–related gene expression and mediates downregulation of pro-inflammatory genes (Villapol, 2018). In the current study, we found that gossypol increased the expressions of TLR4 and NF-κB and increased the proinflammatory cytokines in the hippocampus. To provide additional support, we detected the expression of mPPARγ and found that gossypol decreased the mRNA expression of mPPARγ in the hippocampus. Thus, our results showing increased expression of TLR4 following gossypol exposure may be responsible for the production of proinflammatory genes via the mPPARγ/TLR4/NF-κB signaling.
Various studies have revealed that inflammatory changes in the hippocampus profoundly affect behavior (Lynch, 2010; Rooney et al., 2020). Extensive studies of IL-1β have revealed multiple effects on behavior, including modulation of exploratory behavior, social exploration, and learning and memory (Goshen and Yirmiya, 2009; Lynch, 2010). McKim and colleagues (2018) reported that the development of anxiety during stress was caused by microglial recruitment of inflammatory monocytes that upregulated IL-1β and stimulated brain endothelial IL-1R1. Moreover, the changes of hippocampal neurogenesis can affect behaviors related to anxiety and depression (Sah et al., 2012; Hill et al., 2015; Snyder and Drew, 2020). In the present study, we have demonstrated that gossypol affects the early postnatal hippocampal neurogenesis. Also, we observed that gossypol-treated mice spent less time in the open arms by EPM tests, indicating a reduced exploratory ability in unfamiliar environments and an increased level of anxiety-like behavior. Generally, anxiety is accompanied by the impairment of learning and memory. Indeed, in the MWM test, gossypol-treated offspring required significantly more time to find the hidden platform, indicating impairment of learning and memory. The above results indicate that impaired hippocampal neurogenesis following developmental gossypol exposure is associated with increased anxiety and impairment of learning and memory. Furthermore, PSD95, a synaptic vesicle glycoprotein involved in synaptic transmission, was remarkably decreased in the hippocampus of gossypol-treated mice. This finding is consistent with previous studies, which demonstrated that developmental exposure to toxins induced neurotoxicity by decreasing the PSD95 expression in the hippocampus (Wang et al., 2013; Zheng et al., 2013). The similar result was also found in the expression of synaptophysin, which is involved in the neuronal damage in hippocampus (Zhang et al., 2014). As one of the typical synaptic markers, spinophilin is responsible for spine growth, which may be associated with health behaviors. Previous study showed that spinophilin was significantly decreased in prenatal stress mice (Dong et al., 2018). However, in the present study, the expression of spinophilin was increased in gossypol-treated mice. This discrepancy could possibly attribute to a compensatory mechanism to overcome the damage of neuronal function. Importantly, previous studies showed that the changes of synaptic proteins are responsible for the impairment of learning and memory (Sultana et al., 2010). Thus, an exaggerated brain inflammatory response arising from gossypol exposure is a critical factor in the changes in neurobehavioral performance observed in mice offspring.
In the present study, we showed that the influence of gossypol on hippocampal neurogenesis was related to microglial activation and inflammatory cytokines response events. However, the influence of neuroinflammation on neurogenesis is not mediated simply via effects on proliferation, differentiation, and cell death, but also through modulation of the cellular processes and cellular properties of the new neurons (Kohman and Rhodes, 2013). The mechanisms by which neuroinflammation influences hippocampal neurogenesis are still not fully understood. Defective neurogenesis may depend on many factors, such as gene-environment interactions, which could modify hippocampal neurogenesis (Levone et al., 2015). Therefore, further neurological studies of neuroinflammation and neurogenesis in the presence of maternal stimulation are required at both the biochemical and molecular levels to assess the pathology of the developing brain.
In conclusion, the present study provides the first evidence, to our knowledge, of the effects of developmental gossypol exposure on hippocampal neurogenesis, apart from its function in steroidogenesis. The results showed that developmental gossypol exposure impaired hippocampal neurogenesis and induced neuroinflammation in offspring. These findings demonstrate that gossypol is potentially hazardous to fetal neurodevelopment. This study may shed light on a previously little-explored aspect of gossypol neurotoxicity and elucidate the potential related mechanism.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 31802154 to X.Z.), the China Postdoctoral Science Foundation funded project (no. 2019T120957 to X.Z.), Key Research and Development Project of Shaan’xi Province (no. 2019ZDXM3-02 to X.Z., and 2018ZDXM2 to S.Z.), and Foundation for top talent recruitment of Xi’an Medical College (no. 2018RCYJ04 to X.C.).
Statement of Interest
None.
References
- Abbott LC, Nigussie F (2020) Adult neurogenesis in the mammalian dentate gyrus. Anat Histol Embryol 49:3–16. [DOI] [PubMed] [Google Scholar]
- Ampuero E, Jury N, Härtel S, Marzolo MP, van Zundert B (2017) Interfering of the Reelin/ApoER2/PSD95 signaling axis reactivates dendritogenesis of mature hippocampal neurons. J Cell Physiol 232:1187–1199. [DOI] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R (2018) Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. [DOI] [PubMed] [Google Scholar]
- Bond AM, Ming GL, Song H (2015) Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 17:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruncho HJ, Brymer K, Romay-Tallón R, Mitchell MA, Rivera-Baltanás T, Botterill J, Olivares JM, Kalynchuk LE (2016) Reelin-related disturbances in depression: implications for translational studies. Front Cell Neurosci 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Zhao S, Fan L, Zhang W, Lu X, Shao H, Wang S, Song L, Failla AV, Zobiak B, Mannherz HG, Frotscher M (2016) Reelin and cofilin cooperate during the migration of cortical neurons: a quantitative morphological analysis. Development 143:1029–1040. [DOI] [PubMed] [Google Scholar]
- Chen C, Shen FY, Zhao X, Zhou T, Xu DJ, Wang ZR, Wang YW (2015) Low-dose sevoflurane promotes hippocampal neurogenesis and facilitates the development of dentate gyrus-dependent learning in neonatal rats. ASN Neuro 7:1759091415575845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH (2009) Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry 65:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda AS, Zhang CJ, Katsumoto A, Teixeira AL (2017) Hippocampal adult neurogenesis: does the immune system matter? J Neurol Sci 372:482–495. [DOI] [PubMed] [Google Scholar]
- Donaldson A, Sufi SB, Jeffcoate SL (1985) Inhibition by gossypol of testosterone production by mouse Leydig cells in vitro. Contraception 31:165–171. [DOI] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Zhang H, Pandey SC (2018) Prenatal stress leads to chromatin and synaptic remodeling and excessive alcohol intake comorbid with anxiety-like behaviors in adult offspring. Neuropharmacology 140:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158:1021–1029. [DOI] [PubMed] [Google Scholar]
- Fombad RB, Bryant MJ (2004) An evaluation of the use of cottonseed cake in the diet of growing pigs. Trop Anim Health Prod 36:295–305. [DOI] [PubMed] [Google Scholar]
- Fonseca NB, Gadelha IC, Oloris SC, Soto-Blanco B (2013) Effectiveness of albumin-conjugated gossypol as an immunogen to prevent gossypol-associated acute hepatotoxicity in rats. Food Chem Toxicol 56:149–153. [DOI] [PubMed] [Google Scholar]
- Förster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S (2010) Emerging topics in Reelin function. Eur J Neurosci 31:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470. [DOI] [PubMed] [Google Scholar]
- Gadelha IC, Fonseca NB, Oloris SC, Melo MM, Soto-Blanco B (2014) Gossypol toxicity from cottonseed products. ScientificWorldJournal 2014:231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R (2009) Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30:30–45. [DOI] [PubMed] [Google Scholar]
- Green HF, Nolan YM (2014) Inflammation and the developing brain: consequences for hippocampal neurogenesis and behavior. Neurosci Biobehav Rev 40:20–34. [DOI] [PubMed] [Google Scholar]
- Hahn DW, Rusticus C, Probst A, Homm R, Johnson AN (1981) Antifertility and endocrine activities of gossypol in rodents. Contraception 24:97–105. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Kielian T (2011) Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 121:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei M, Chen P, Wang S, Li X, Xu M, Zhu X, Wang Y, Duan J, Huang Y, Zhao S (2019) Effects of chronic mild stress induced depression on synaptic plasticity in mouse hippocampus. Behav Brain Res 365:26–35. [DOI] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R (2015) Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 40:2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgerner H, Zeisel A, Lönnerberg P, Linnarsson S (2018) Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat Neurosci 21:290–299. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ (1999) Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev 23:971–980. [DOI] [PubMed] [Google Scholar]
- Kang E, Wen Z, Song H, Christian KM, Ming GL (2016) Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Bio 8:a019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Berg DA, Furmanski O, Jackson WM, Ryu YK, Gray CD, Mintz CD (2017) Neurogenesis and developmental anesthetic neurotoxicity. Neurotoxicol Teratol 60:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002) The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115:97–105. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT (1999) Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer’s disease. Proc Natl Acad Sci U S A 96:5274–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C (1984) Neuronal degeneration after intercerebral injections of excitotoxins. A histological analysis of kainic acid, ibotenic acid and quinolinic acid lesions in the rat brain. In: Excitotoxins (Fuxe K, Robert P, Schwarcz R, eds), pp 99–111. New York: Plenum Press. [Google Scholar]
- Kohman RA, Rhodes JS (2013) Neurogenesis, inflammation and behavior. Brain Behav Immun 27:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR (2008) A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci 28:2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Cryan JF, O’Leary OF (2015) Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress 1:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xie J, Hei M, Tang J, Wang Y, Förster E, Zhao S (2018) High level of CTP synthase induces formation of cytoophidia in cortical neurons and impairs corticogenesis. Histochem Cell Biol 149:61–73. [DOI] [PubMed] [Google Scholar]
- Li YF, Booth GM, Seegmiller RE (1989) Evidence for embryotoxicity of gossypol in mice and chicks with no evidence of mutagenic activity in the Ames test. Reprod Toxicol 3:59–62. [DOI] [PubMed] [Google Scholar]
- Liu M, Xu M, Wang M, Wang S, Li K, Cheng X, Wu Y, Wang Y, Zhu X, Zhao S (2019) Maternal exposure to swainsonine impaired the early postnatal development of mouse dentate gyrus of offspring. Neurochem Int 129:104511. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Luz VB, Gadelha ICN, Cordeiro LAV, Melo MM, Soto-Blanco B (2018) In vitro study of gossypol’s ovarian toxicity to rodents and goats. Toxicon 145:56–60. [DOI] [PubMed] [Google Scholar]
- Lynch MA (2010) Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP (2018) Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry 23:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnone G, De Benedetti F, Bracci-Laudiero L (2017) NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci 18:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK (2014) The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48:277–286. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S (2012) Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa B, Wang L, Ohishi T, Taniai E, Akane H, Suzuki K, Mitsumori K, Shibutani M (2012) Reversible aberration of neurogenesis targeting late-stage progenitor cells in the hippocampal dentate gyrus of rat offspring after maternal exposure to acrylamide. Arch Toxicol 86:779–790. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP (2011) Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL (2015) Immune dysregulation and cognitive vulnerability in the aging brain: interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology 96:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Sufi SB, O’Shaughnessy PJ, Donaldson A, Jeffcoate SL (1986) Site of gossypol inhibition of steroidogenesis in purified mouse Leydig cells. J Steroid Biochem 25:683–687. [DOI] [PubMed] [Google Scholar]
- Rooney S, Sah A, Unger MS, Kharitonova M, Sartori SB, Schwarzer C, Aigner L, Kettenmann H, Wolf SA, Singewald N (2020) Neuroinflammatory alterations in trait anxiety: modulatory effects of minocycline. Transl Psychiatry 10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah A, Schmuckermair C, Sartori SB, Gaburro S, Kandasamy M, Irschick R, Klimaschewski L, Landgraf R, Aigner L, Singewald N (2012) Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl Psychiatry 2:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JE, Villasenor M, Robinson PH, DePeters EJ, Holmberg CA (2003) Type of cottonseed and level of gossypol in diets of lactating dairy cows: plasma gossypol, health, and reproductive performance. J Dairy Sci 86:892–905. [DOI] [PubMed] [Google Scholar]
- Scharfman HE (2016) The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci 17:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sein GM (1986) The embryotoxic and immunodepressive effects of gossypol. Am J Chin Med 14:110–115. [DOI] [PubMed] [Google Scholar]
- Semon B (2012) Dietary intake of cottonseed toxins is hypothesized to be a partial cause of Alzheimer’s disorder. Med Hypotheses 78:293–298. [DOI] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MP, Smith FH, Clawson AJ (1966) Effects of levels of protein and gossypol, and length of feeding period on the accumulation of gossypol in tissues of swine. J Nutr 88:434–438. [DOI] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay MÈ (2014) Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast 2014:610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Drew MR (2020) Functional neurogenesis over the years. Behav Brain Res 382:112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Banks WA, Butterfield DA (2010) Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J Neurosci Res 88:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S (2018) Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol 38:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Fang F, Xue ZG, Cang J, Zhang XG (2013) Neonatal sevoflurane anesthesia induces long-term memory impairment and decreases hippocampal PSD-95 expression without neuronal loss. Eur Rev Med Pharmacol Sci 17:941–950. [PubMed] [Google Scholar]
- Xu WB, Xu LH, Lu HS, Ou-Yang DY, Shi HJ, Di JF, He XH (2009) The immunosuppressive effect of gossypol in mice is mediated by inhibition of lymphocyte proliferation and by induction of cell apoptosis. Acta Pharmacol Sin 30:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25:181–213. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao Q, Chen CH, Qin QZ, Zhou Z, Yu ZP (2014) Synaptophysin and the dopaminergic system in hippocampus are involved in the protective effect of rutin against trimethyltin-induced learning and memory impairment. Nutr Neurosci 17:222–229. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Peng C, Wu X, Chen Y, Wang C, You Z (2014) Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiol Dis 68:57–65. [DOI] [PubMed] [Google Scholar]
- Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, Xie Z (2013) Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology 118:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Fréchou M, Liere P, Zhang S, Pianos A, Fernandez N, Denier C, Mattern C, Schumacher M, Guennoun R (2017) A role of endogenous progesterone in stroke cerebroprotection revealed by the neural-specific deletion of its intracellular receptors. J Neurosci 37:10998–11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wu Y, Li C, Yan W, Pan J, Wang S, Zhao S (2020) Prenatal exposure to gossypol impairs corticogenesis of mouse. Front Neurosci 14:318. [DOI] [PMC free article] [PubMed] [Google Scholar]