Abstract
The mortality rate of patients with schizophrenia is high, and life expectancy is shorter by 10 to 20 years. Metabolic abnormalities including type 2 diabetes mellitus (T2DM) are among the main reasons. The prevalence of T2DM in patients with schizophrenia may be epidemiologically frequent because antipsychotics induce weight gain as a side effect and the cognitive dysfunction of patients with schizophrenia relates to a disordered lifestyle, poor diet, and low socioeconomic status. Apart from these common risk factors and risk factors unique to schizophrenia, accumulating evidence suggests the existence of common susceptibility genes between schizophrenia and T2DM. Functional proteins translated from common genetic susceptibility genes are known to regulate neuronal development in the brain and insulin in the pancreas through several common cascades. In this review, we discuss common susceptibility genes, functional cascades, and the relationship between schizophrenia and T2DM. Many genetic and epidemiological studies have reliably associated the comorbidity of schizophrenia and T2DM, and it is probably safe to think that common cascades and mechanisms suspected from common genes’ functions are related to the onset of both schizophrenia and T2DM. On the other hand, even when genetic analyses are performed on a relatively large number of comorbid patients, the results are sometimes inconsistent, and susceptibility genes may carry only a low or moderate risk. We anticipate future directions in this field.
Keywords: DISC1, kalirin, ARHGEF11, Akt/GSK3β, Wnt/β-catenin
Introduction
Schizophrenia is found in all cultures and appears to affect 0.5% to 1.5% of people during their lifetime (Pedersen et al., 2014). Due to its early age of onset and subsequent tendency to persist chronically, it produces great suffering for patients and their family members (Weinberger and Harrison, 2011). The mortality rate of patients with schizophrenia is twice as high as that of the general population, and their life expectancy is 10–20 years shorter (Crump et al., 2013; Lawrence et al., 2013). Although suicide and other unnatural causes account for more than 10% of the excess mortality, a substantial proportion of this excess mortality is due to the increased comorbidity of various medical illnesses in patients with schizophrenia (Crump et al., 2013; Lawrence et al., 2013; Olfson et al., 2015). Patients with schizophrenia have an increased risk for development of type 2 diabetes mellitus (T2DM). The prevalence of T2DM in patients with schizophrenia is approximately 6% to 21%, 2 to 3 times higher than in the general population (Mitchell et al., 2013; Stubbs et al., 2015). T2DM manifests as persistent hyperglycemia due to pancreatic beta-cell dysfunction, which leads to long-term complications. T2DM is a major risk factor for cardiovascular disease, and cardiovascular disease is the main cause of a substantial proportion of excess deaths of patients with schizophrenia (Murray et al., 2012; Lawrence et al., 2013; Olfson et al., 2015).
The mechanisms of the increasing prevalence of T2DM in patients with schizophrenia are multifactorial. T2DM and schizophrenia are caused by shared etiological factors (Ward and Druss, 2015). Traditional risk factors include a sedentary lifestyle and poor diet (Ward and Druss, 2015). Risk factors unique to schizophrenia include low socioeconomic status, cognitive dysfunction, and iatrogenic risk during treatment with antipsychotics (Ward and Druss, 2015). Some evidence suggests that a longer duration of schizophrenia increases the risk for diabetes (Philippe et al., 2005; Nuevo et al., 2011). Patients who have had schizophrenia for more than 25 years have nearly twice the risk for diabetes as those with less than 25 years since the first admission to hospital (Philippe et al., 2005). It has been found that impaired hormonal regulation of appetite, in terms of low leptin and high insulin levels, often occurs in early psychosis before antipsychotic treatment (Misiak et al., 2019; Lis et al., 2020a, 2020b). Hence, schizophrenia itself is a risk for increased onset of diabetes. Apart from these traditional risk factors and risk factors unique to schizophrenia, recent studies show an elevated risk of T2DM among drug-naïve or first-episode patients with schizophrenia and their relatives (Perry et al., 2016; Pillinger et al., 2017; Rajkumar et al., 2017). A recent systematic review and meta-analysis of glucose homeostasis in unaffected first-degree relatives of schizophrenia patients suggested impaired glucose tolerance in this population as well (Misiak et al., 2020). Thus, previous evidence has strongly suggested that schizophrenia and T2DM are caused by multiple genetic variants (Gough and O’Donovan, 2005). Multiple twin and family studies and heritability of intermediate phenotypes provide convincing evidence for an important role of the genetic etiologies, respectively (Das and Elbein, 2006; Demjaha et al., 2012). The risk of T2DM in patients with psychoses such as schizophrenia is elevated two- to fourfold in association with a positive family history of diabetes (Foley et al., 2014; Chung and Miller, 2020). Further, one-half of patients with schizophrenia are reported to have a family history of T2DM compared with 4.6% of healthy adult controls (Bushe and Holt, 2004). Interestingly, the polygenic risk score related to the onset of schizophrenia is also associated with insulin resistance in first-episode and antipsychotic-naive patients with schizophrenia (Tomasik et al., 2019). Thus, schizophrenia and T2DM may influence each other and share susceptibility gene variants.
Accumulating evidence indicates that potential environmental risk factors affecting both the premorbid phase and after the onset of schizophrenia include exposure to stress in early life, poor dietary habits, and a sedentary life style, as noted above. Stress leads to the alteration of several biological mechanisms that has been termed “allostasis” (Misiak et al., 2014). These processes enable adaptation to novel situations. However, their prolonged and cumulative activation exerts systemic and detrimental effects called the allostatic load (AL) (Juster et al., 2016; Misiak, 2019). The AL concept can be a useful framework for apprehending biological dysregulations related to chronic stress. Biological alterations associated with AL (AL mediators) in schizophrenia include a subclinical inflammatory state, enhanced oxidative stress levels, decreased level of neurotrophins, and impaired hypothalamic-pituitary-adrenal (HPA) axis response (Misiak et al., 2014). There are also markers that enable the measurement of AL (AL index). A higher AL index has been associated with a higher severity of positive and depressive symptoms, working memory impairments, lower general functioning, and health outcomes, including all-cause mortality (Misiak, 2019; Piotrowski et al., 2019).
In this review, we describe the comorbidity of schizophrenia and T2DM in genetic and functional pathways. First, we describe susceptibility genes common to schizophrenia and T2DM (Table 1). Second, we discuss molecular mechanisms that might explain a common functional cascade in schizophrenia and T2DM. Third, we describe common mechanisms in schizophrenia and T2DM, such as inflammation, oxidative stress, and HPA axis dysfunction.
Table 1 1.
Common susceptibility genes in schizophrenia (SCZ) and type 2 diabetes mellitus (T2DM)
| Candidate genes | Location | Official full name | Functions | Method | Samples | References |
|---|---|---|---|---|---|---|
| Common cascade | ||||||
| ACE (Upstream of Akt/GSK3) | 17q23.3 | angiotensin I converting enzyme | Hydrolyze angiotensin I into angiotensin 2 | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| ARHGEF11 (Rho GTPase) | 1q23.1 | Rho guanine nucleotide exchange factor 11 | Numerous cellular process, synaptic plasticity | Association study | T2DM (n = 685) vs. Con (n = 461) | Böttcher et al. (2008) |
| Association study | SCZ (n = 490) vs. Con (n = 500) | Mizuki et al. (2014) | ||||
| BCL9 (Wnt/β-catenin) | 1q21.1 | B-cell CLL/lymphoma 9 | Wnt signaling pathway | Association study | SCZ (n = 4,187) vs. Con (n = 5,772) | Li et al. (2011) |
| CNV analysis | Cumulative scoring evidence,based on 32 CNV studies in SCZ | Luo et al. (2014) | ||||
| GWAS | T2DM (n = 402) vs. Con (n = 1,092) | Anderson et al. (2015) | ||||
| COMT (Upstream of Akt/GSK3) | 22q11.21 | catechol-O-methyltransferase | Degradation of catecholamines | CNV analysis | Cumulative scoring evidence,based on 32 CNV studies in SCZ | Luo et al. (2014) |
| Association study | T2DM (n = 595) vs. Con (n = 725) | Xiu et al. (2015) | ||||
| TCF7L2 (Wnt/β-catenin) | 10q25.2-3 | transcription factor 7 like 2 | Participates in the Wnt signaling pathway | Association study | T2DM (n = 2,201) | Lyssenko et al. (2008) |
| GWAS | SCZ (n = 924), T2D (n = 822), comorbid (n = 505); controls (n = 1,125) | Hackinger et al. (2018) | ||||
| Inflammation | ||||||
| ANXA1 | 9q21.13 | annexin A1 | Anti-inflammatory activity, endogenous regulator of RhoA | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| APOE | 19q13.32 | apolipoprotein E | Lipid homeostasis and inflammation | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| C4 | 6p21.33 | complement C4 | Encodes complement factor 4, classical activation pathway | Association study | SCZ (n = 28,799) vs.. Con (n = 35,986) | Sekar et al. (2016) |
| IL10 | 1q32.1 | interleukin 10 | Cytokine produced by monocytes and lymphocytes | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| Inflammation & HPA axis | ||||||
| IL1B | 2q14.1 | interleukin 1 beta | Potent proinflammatory cytokine | Association study | T2DM (n = 200) vs. Con (n = 223) | Achyut et al. (2007) |
| Association study | SCZ (n = 621) vs. Con (n= 531) | Kapelski et al. (2015) | ||||
| Meta-analysis | SCZ (n = 20,185) vs. Con (n = 20,542) | Hudson and Miller (2018) | ||||
| IL6 | 7p15.3 | interleukin 6 | Inflammation and the maturation of B cells | Association study | T2DM Twins (n = 6,720) | Arora et al. (2011) |
| Association study | SCZ (n = 621) vs. Con (n= 531) | Kapelski et al. (2015) | ||||
| Meta-analysis | SCZ (n = 19,022) vs. Con (n = 19,378) | Hudson and Miller (2018) | ||||
| TNF | 6p21.33 | tumor necrosis factor | Encodes a multifunctional proinflammatory cytokine | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| Oxidative stress | ||||||
| GSTM1 | 1p13.3 | glutathione S-transferase mu 1 | Conjugattes reduced glutathione to hydrophobic electrophiles Detoxification of electrophilic compounds | Association study | SCZ (n = 111) vs. Con (n = 130) | Pae et al. (2004) |
| Association study | SCZ (n = 138) vs. Con (n = 133) | Gravina et al. (2011) | ||||
| GWAS | SCZ and T2DM database | Liu et al. (2013) | ||||
| Meta-analysis | T2DM (n = 1,354) vs. Con (n = 1,666) | Tang et al. (2013) | ||||
| Meta-analysis | T2DM (n = 2,577) vs. Con (n = 4,572) | Zhang et al. (2013) | ||||
| GSTT1 | 22q11.23 | glutathione S-transferase theta 1 | Conjugates reduced glutathione to hydrophobic electrophiles Detoxification of electrophilic compounds | Association study | SCZ (n = 138) vs. Con (n = 133) | Gravina et al. (2011) |
| Meta-analysis | T2DM (n = 1,271) vs. Con (n = 1,470) | Tang et al. (2013) | ||||
| Meta-analysis | T2DM (n = 2,577) vs. Con (n = 4,572) | Zhang et al. (2013) | ||||
| MTHFR | 1p36.22 | methylenetetrahydrofolate reductase | Catalyzes conversion of methylenetetrahydrofolate | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| PON1 | 7q21.3 | paraoxonase 1 | Hydrolyzes toxic metabolites | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| SOD2 | 6q25.3 | superoxide dismutase 2 | Destroys superoxide anion radicals | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| TXNRD2 | 22q11.21 | thioredoxin reductase 2 | Regulation of mitochondrial redox homeostasis | CNV analysis | Cumulative scoring evidence,based on 32 CNV studies in SCZ | Luo et al. (2014) |
| Association study | T2DM patients with myocardial infarction (n = 166) vs. Con (n = 811) | Kariž et al. (2015) | ||||
| UCP2 | 11q13.4 | uncoupling protein 2 | Mitochondrial transporter protein | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| Prolactin pathway | ||||||
| NPY | 7p15.3 | neuropeptide Y | Control of feeding, secretion of gonadotrophin-release hormone | Association study | SCZ (n = 212) vs. Con (n = 199) | Itokawa et al. (2003) |
| Association study | T2DM (n = 263) vs. Con (n = 469) | Nordman et al. (2005) | ||||
| GWAS | SCZ and T2DM database | Liu et al. (2013) | ||||
| PRL | 6p22.3 | prolactin | Encodes anterior pituitary horomone prolactin | Association study | SCZ (n = 403) vs. Con (n = 653) | Rybakowski et al. (2012) |
| Other | ||||||
| DLGAP1 | 18p11.31 | DLG associated protein 1 | Scaffold protein in postsynaptic density, glutamate neurotransmission | CNV analysis | SCZ (n = 662) vs. Con (n = 2,623) | Kirov et al. (2012) |
| CNV analysis | T2DM (n = 1,715) | Prabhanjan et al. (2016) | ||||
| Herv K-18 | 1q23.3 | endogenous retrovirus group K member 18 | CD48 signaling lymphocyte activating (SLAM) gene | Association study | SCZ with T2DM (n = 29) vs. SCZ (n = 200) | Dickerson et al. (2008) |
| IGF2BP2 | 3q27.2 | insulin-like growth factor 2 mRNA binding protein 2 | Embryonic growth and development, decrease insulin secretion | Association study | T2DM (n = 2,201) | Lyssenko et al. (2008) |
| Association study | SCZ (n = 790) vs. Con (n = 1,083) | Zhang et al. (2013b) | ||||
| PACRG | 6q26 | parkin coregulated | Molecular chaperone/chaperonin-binding | GWAS | SCZ (n = 924), T2D (n = 822), comorbid (n = 505); controls (n = 1,125) | Hackinger et al. (2018) |
| PSMD9 | 12q24.31 | proteasome 26S subunit, non-ATPase 9 | Chaperone of 26S proteasome complex assembly Insulin gene transcription coactivator |
Association study | T2DM affected siblings/families (n = 201) | Gragnoli (2010) |
| Association study | SCZ (n = 1,351) vs. Con (n = 1,378) | Lee et al. (2013) | ||||
| SRR | 17p13.3 | serine racemase | Catalyzes synthesis of D-serine | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| SYN2 | 3p25.2 | synapsin Ⅱ | Synaptogenesis, modulation of neurotransmitter release | GWAS | SCZ and T2DM database | Liu et al. (2013) |
| TSPAN18 | 11p11.2 | tetraspanin 18 | Tetraspanin families which regulate cell development | GWAS | SCZ and T2DM database | Liu et al. (2013) |
Common Susceptibility Genes in Schizophrenia and T2DM
Previous family-based genome-wide linkage studies show that schizophrenia and T2DM have a number of overlapping risk loci, including chromosomes 1p13, 1p36, 1q21–24, 1q25, 2q14, 2q33, 2q36, 3p22, 3q29, 4q25, 5q13, 6p21, 6q25, 7p15, 7p21, 7q21, 7q31, and 9p24 (Lin and Shuldiner, 2010). These loci include gene-rich regions that will harbor multiple common candidate genes for susceptibility to schizophrenia and T2DM. Although many of these loci cover large distances in genomic DNA, chromosome 1q was reported to have a linkage to T2DM by several previous studies (Das and Elbein, 2007; Tziastoudi et al., 2019). This location has also been implicated as a schizophrenia susceptibility locus (Brzustowicz et al., 2000). Therefore, these findings have suggested that chromosome 1q may be remarkably rich in linkage findings for co-occurrence of schizophrenia and T2DM. Within this region of linkage, susceptibility genes for T2DM such as endogenous retrovirus group K member 18 (Herv K-18) and Rho guanine-nucleotide exchange factor 11 (ARHGEF11) (Böttcher et al., 2008) have been found to be associated with schizophrenia among sampled populations (Dickerson et al., 2008; Mizuki et al., 2014).
The most straightforward method to identify the genetic risk for comorbidity of schizophrenia and T2DM is searching for overlapped candidate genes or regions of these 2 individual diseases (Lin and Shuldiner, 2010). Common candidate genes shared by association studies focused on each individual disease are glutathione S-transferase mu 1 (GSTM1), glutathione S-transferase theta 1 (GSTT1), neuropeptide Y (NPY), and proteasome 26S subunit, non-ATPase 9 (PSMD9) (Itokawa et al., 2003; Pae et al., 2004; Nordman et al., 2005; Gragnoli, 2010; Lin and Shuldiner, 2010; Lee et al., 2013; Tang et al., 2013; Zhang et al., 2013a). According to data from the Genetic Association Database (http://geneticassociationdb.nih.gov/) (Becker et al., 2004), Catalog of Published Genome-Wide Association Studies (GWAS) (http://www.genome.gov/gwastudies/) (Hindorff et al., 2009), and Type 2 Diabetes Genetic Association Database (http://t2db.khu.ac.kr:8080/) (Lim et al., 2010), there are 196 schizophrenia susceptibility genes and 200 T2DM susceptibility genes. Among them, 14 genes (annexin A1 [ANXA1], apolipoprotein E [APOE], angiotensin I converting enzyme [ACE], GSTM1, interleukin 10 [IL10], methylenetetrahydrofolate reductase [MTHFR], NPY, paraoxonase 1 [PON1], superoxide dismutase 2 [SOD2], synapsin II [SYN2], tumor necrosis factor [TNF], uncoupling protein 2 [UCP2], serine racemase [SRR], and tetraspanin 18 [TSPAN18]) are common to both diseases (Liu et al., 2013). These genes could be divided into 2 functional categories. One category is inflammation-associated genes (APOE, IL10, TNF), and the other is genes that are involved in oxidative stress (GSTM1, MTHFR, PON1, SOD2, UCP2). Currently, the National Human Genome Research Institute–European Bioinformatics Institute catalog of published GWASs lists 402 schizophrenia-susceptibility genes and 890 genes associated with T2DM (Nagalski et al., 2016). They found 26 candidate genes that are shared by schizophrenia and T2DM. Functional analysis of the 79 candidate genes shared by T2DM and any of the severe mental illnesses (schizophrenia, bipolar disorder, and major depression) revealed several clusters of common candidate risk genes.
Other candidate genes, such as insulin like growth factor 2 mRNA binding protein 2 (IGF2BP2) or transcription factor 7 like 2 (TCF7L2), may also contribute to the genetic basis of the co-occurrence of schizophrenia and T2DM. The IGF2BP2 polymorphisms are associated with vulnerability to schizophrenia in a Han Chinese population (Zhang et al., 2013b) and with impaired pancreatic β-cell function, including lower fasting insulin levels, which reduced glucose-stimulated insulin secretion (Lyssenko et al., 2008). The TCF7L2 polymorphisms have been detected in consistent association with T2DM in multiple ethnic populations, including Japanese, Chinese, Americans, and Asian Indians (Wang et al., 2013). A GWAS in a Greek population identified genomic regions with evidence of colocalizing schizophrenia and T2DM. In this study, the most strongly associated variant resides within an intron of the Parkin coregulated (PACRG) gene in schizophrenia patients with T2DM vs controls, and another variant that reached genome-wide significance resides within the intron of the TCF7L2 gene (Hackinger et al., 2018). However, the findings were negative in a GWAS performed in a Japanese population (Kajio et al., 2014).
Though common variants of SNP polymorphisms have only small effects, large, rare chromosomal copy number variants (CNVs) identified by comparative genomic analyses are known to increase the risk for schizophrenia and have relatively larger effects (Chen et al., 2015; Bray and O’Donovan, 2018). Of more than 20,000 schizophrenia patients and controls, 8 CNVs (on chromosomes 1q21.1, 2p16.3, 3q29, 7q11.2, 15q13.3, distal 16p11.2, proximal 16p11.2, and the velocardiofacial syndrome region on chromosome 22q11.2) are associated with the onset of schizophrenia at a significant genome-wide threshold (CNV and Schizophrenia Working Groups of the Psychiatric Genomics Consortium) (Marshall et al., 2017; Bray and O’Donovan, 2018). It is well known that 20%–30% of people with a 22q11.2 deletion have schizophrenia (Murphy et al., 1999), and the prevalence of obesity and T2DM is reported to be greater than normal in 22q11.2 deletion syndrome (Voll et al., 2017). A partial 22q11.2 deletion that includes several genes related to the neuropsychiatric phenotype, catechol-O-methyltransferase (COMT) (Xiu et al., 2015) and thioredoxin reductase 2 (TXNRD2) (Kariž et al., 2015), is associated with the onset of T2DM. Of 8 top candidate genes for schizophrenia affected by CNVs (Luo et al., 2014), only B-cell CLL/lymphoma 9 (BCL9) (1q21.1), which is required in the Wnt signaling pathway, was reported to be associated with T2DM (Anderson et al., 2015) or schizophrenia (Li et al., 2011), although there are not enough studies on the relationship between CNVs and T2DM, unlike schizophrenia. Prabhanjan et al. (2016) reported 24 CNV genes in patients with T2DM, and DLG associated protein 1 (DLGAP1), a scaffold protein of the postsynaptic density that is related to post-synapse neurotransmission of glutamate, is suspected to be genetically and functionally related to schizophrenia (Kirov et al. 2012; Rasmussen et al. 2017).
Common Functional Cascade in Schizophrenia and T2DM
Rho GTPase—
Disturbances in synaptic connectivity during perinatal and adolescent periods underlie the pathophysiology of schizophrenia (McGlashan and Hoffman, 2000). Postmortem brain studies of individuals with schizophrenia have reported reduced dendritic spine density in the cerebral neocortex (Glantz and Lewis, 2000; Konopaske et al., 2014). These dendritic spine abnormalities are likely the result of disturbances in the molecular mechanisms that contribute to spine formation, pruning, and/or maintenance (Glausier and Lewis, 2013). Dendritic spine morphogenesis is regulated through cytoskeletal actin, which is concentrated highly in the spines (Fischer et al., 1998).
The Rho family of small GTPases (Rho GTPases), which includes Cdc42, Rac1, and RhoA, is a critical regulator of actin cytoskeleton dynamics and organization in the spines (Hall, 1998). The activation of Rho GTPases is mediated by specific guanine-nucleotide exchange factors (GEFs) that catalyze the exchange of bound GDP (inactive state) for bound GTP (active state) (Van Aelst and D’Souza-Schorey, 1997). Several Rho GEFs that localize to dendritic spines play important roles in dendritic spine morphogenesis by modulating the activity of Rho GTPases (Xie et al., 2007).
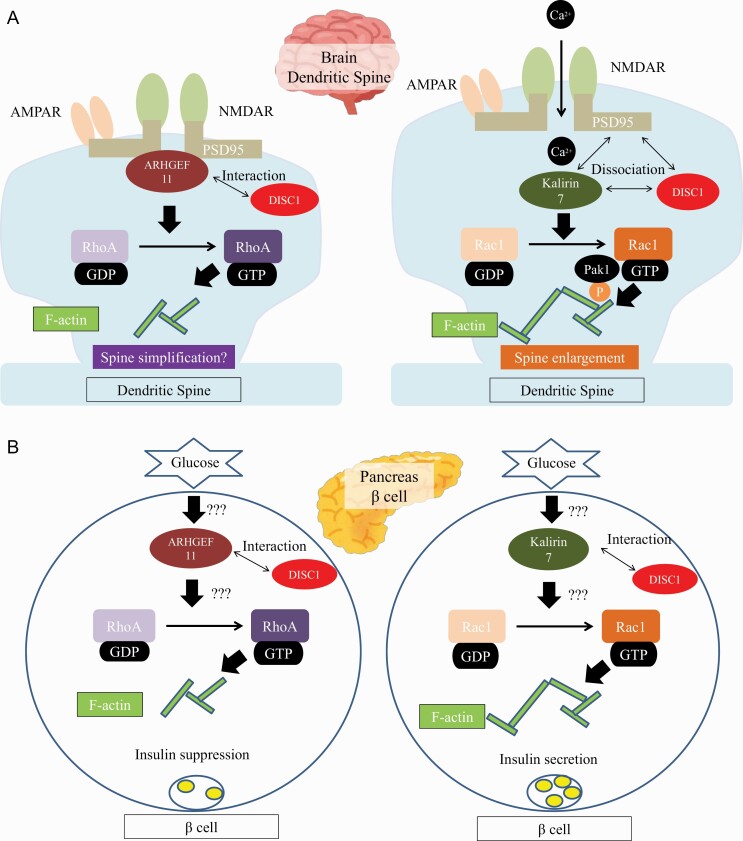
ARHGEF11 is a specific GEF for RhoA (Rumenapp et al., 1999). ARHGEF11 is expressed in the pancreas, liver, adipose tissue, and highly in the brain (Jackson et al., 2001). ARHGEF11 variants are associated with a higher risk for the onset of schizophrenia in a Japanese population (Mizuki et al., 2014). ARHGEF11 interacts and colocalizes with synapse marker postsynaptic density protein 95 (PSD-95) at synapse sites and negatively regulates the formation of dendritic spines in cortical primary neurons (Mizuki et al., 2016). In a yeast 2-hybrid screen, ARHGEF11 interacts with disrupted-in-schizophrenia 1 (DISC1) (Millar et al., 2003). DISC1 directly interacts with PSD-95 and kalirin-7, a GEF for Rac1, and blocks access of kalirin-7 to Rac1. This binding is released by N-methyl-D-aspartate (NMDA) receptor activation, allowing free access of kalirin-7 to Rac1 and leading to the resultant activation of Rac1 and spine enlargement (Hayashi-Takagi et al., 2010). On the other hand, platelet-activating factor acetylhydrolase 1B1 (LIS1), one of the major binding partners of DISC1, is associated with RhoA activity (Kholmanskikh et al., 2003). Haploinsufficiency in LIS1 has also been shown to reduce spine density, while downregulation of RhoA rescued spine motility (Sudarov et al., 2013). DISC1 may also regulate the access of ARHGEF11 to RhoA, resulting in spine shrinkage. Regulation of Rho GTPases by DISC1 may be crucial for proper maintenance of the dendritic spine (Tropea et al., 2018). DISC1 (Ma et al., 2018; Xu et al., 2018) and kalirin (Kushima et al., 2012) are also reported to be associated with schizophrenia. Though DISC1 is not considered a common risk gene for schizophrenia by GWAS, DISC1 may play critical roles as a pathological mediator in a wide range of psychiatric disorders (Niwa et al., 2016).
Dysfunction of insulin release from pancreatic islet β-cells is considered to be one of the causal factors in the etiology of T2DM. Rac1 is particularly important for glucose-stimulated insulin secretion (Wang and Thurmond, 2009). In contrast, RhoA expression is increased in β-cells under diabetic conditions, and Rho/Rho-kinase activation is involved in the suppression of insulin biosynthesis (Nakamura et al., 2006). Thus, insulin release from pancreatic islet β-cells could be determined by the resulting balance between RhoA and Rac1 activities. These findings suggest that Rho GTPase signaling affects not only the dendritic spine structure but also a number of cellular processes, including insulin release from pancreatic islet β-cells, and that aberrations in Rho GTPase signaling, including its activation by GEFs, could therefore contribute to the comorbidity of schizophrenia and T2DM (Figure 1).
Figure 1.
Summary of plausible shared mechanisms for the pathogenetic association between schizophrenia and type 2 diabetes mellitus (T2DM). (A) Schematic representation of Rho family of small GTPases (Rho GTPases) signaling cascades involved in synaptic plasticity. Rho guanine-nucleotide exchange factor 11 (ARHGEF11) interacts and colocalizes with synapse marker postsynaptic density protein 95 (PSD-95) at synapse sites and negatively regulated the formation of dendritic spines in cortical primary neurons (Mizuki et al., 2016). Disrupted-in-schizophrenia 1 (DISC1) directly interacts with PSD-95 and kalirin-7, a GEF for Rac1, and blocks access of kalirin-7 to Rac1. This binding is released by N-methyl-D-aspartate (NMDA) receptor activation, allowing free access of kalirin-7 to Rac1 and leading to the resultant activation of Rac1 and spine enlargement (Hayashi-Takagi et al., 2010). (B) The role of Rho GTPase in pancreatic β cells. Rac1 is particularly important for glucose-stimulated insulin secretion (Wang and Thurmond, 2009). In contrast, RhoA expression is increased in β-cells under diabetic conditions, and Rho/Rho-kinase activation is involved in the suppression of insulin biosynthesis (Nakamura et al., 2006). Insulin release from pancreatic islet β-cells could be determined by the resulting balance of Rho GTPase signaling. Illustrating this schematic figure, we referenced figures of Hayashi-Takagi et al., 2010 and Wang et al., 2009.
Wnt/β-Catenin
Wnts are secreted glycoproteins known as extracellular ligands. Wnt/β-catenin signaling (canonical pathway) is a critical and well-studied pathway (MacDonald et al., 2009). In the absence of Wnt ligands, cytoplasmic β-catenin protein is tightly regulated at a low level by casein kinase 1 (CK1)-mediated phosphorylation and the regulatory adenomatous polyposis coli (APC)/axin/glycogen synthase kinase-3 (GSK-3β) complex, leading to its ubiquitination and subsequent proteasomal degradation (Gao et al., 2014). In an activated state, Wnt signaling promotes destruction of APC/axin/GSK-3β complex components and inhibition of β-catenin degradation. β-Catenin accumulates in the cytoplasm and eventually translocates into the nucleus, where it binds with T-cell factor/lymphoid enhancer factor (TCF/LEF) family members and induces the transcription of target genes (Shang et al., 2017). The Wnt signaling pathway is crucial for regulating diverse biological processes such as embryonic development, organ formation, and cell proliferation. This pathway plays an important role in the pathophysiology of T2DM and has been shown to be critical for the development of the pancreas and islets during embryonic growth (Papadopoulou and Edlund, 2005). The Wnt/β-catenin pathway also plays a role in neuronal development (Brafman and Willert, 2017).
TCF7L2, also called TCF4, is one of the TCF/LEF family members. The TCF7L2 gene encodes a high mobility (HMG) box that plays an important role in the downstream Wnt/β-catenin signal pathway (Hansson et al., 2010). Functionally, TCF7L2 is critical for β-cell proliferation and survival as well as insulin production and secretion (Liu and Habener, 2010) and is a key regulator of insulin and proinsulin synthesis and processing (Zhou et al., 2014). Although studies of TCF7L2 in brain development and pathologies have been relatively scarce, evidence from animal studies strongly implicates TCF7L2-dependent transcription in the development of changes in the volume of cortical areas, thalamo-cortical dysconnectivity, and white matter microstructural alterations (Bem et al., 2019). TCF7L2 also regulates synaptic plasticity (Kennedy et al., 2016). Therefore, TCF7L2 may contribute to the comorbidity between schizophrenia and T2DM.
DISC1 also regulates the stability of Wnt/β-catenin signaling by an interaction with GSK3β and acts through this pathway to regulate neural progenitor proliferation and modulate mental homeostasis (Mao et al., 2009). Disheveled-axin domain-containing 1 (DIXDC1), a direct interacting partner of DISC1, contributes to psychiatric pathogenesis by regulating dendritic spine and glutamatergic synapse density downstream of Wnt/β-catenin signaling (Martin et al., 2018).
Akt/GSK3
GSK3 plays several roles in differentiation and development, intracellular trafficking, apoptosis, and regulation of gene transcription (Emamian, 2012). Some studies suggest that GSK3 in the brain could modulate synaptic plasticity (Emamian, 2012). GSK-3β is a key player of Wnt signaling pathways (Freyberg et al., 2010). GSK3 is a molecule immediately downstream to Akt, a serine/threonine kinase (Freyberg et al., 2010). GSK3 and Akt are serine threonine kinases that were initially identified as playing a role in regulating the activity of glycogen synthesis in response to insulin receptor stimulation (Beaulieu, 2012). Insulin signals through the tyrosine kinase activity of its receptor to activate Akt through the phosphatidylinositol 3-kinase pathway. Akt phosphorylates GSK-3 and inactivates it (Lovestone et al., 2007). Of interest, DISC1 regulates pancreatic beta-cell function, decreases beta-cell proliferation, and promotes apoptosis and glucose intolerance in transgenic mice via regulation of GSK3β (Jurczyk et al., 2016).
GSK3 has also been implicated in the pathogenesis of schizophrenia and the actions of neurotransmission of dopamine (Kaidanovich-Beilin et al., 2012). GSK3 is a major downstream regulator of dopamine receptor D2 (DRD2), which is targeted by most antipsychotics. Besides the canonical G protein-dependent cAMP-protein kinase A signaling pathway, the non-canonical DRD2 transduction pathway is the G protein-independent Akt/GSK3 pathway (Beaulieu et al., 2011). Activation of DRD2 by dopamine facilitates the arrestin 2/protein phosphatase 2A/Akt complex and dephosphorylates and inactivates Akt, followed by dephosphorylation (activation) of GSK3 (Beaulieu et al., 2011). Chronic administration of a dopamine agonist, such as amphetamine or apomorphine, also leads to increased inhibitory phosphorylation of Akt and increased activation of GSK3β (Beaulieu et al., 2011). On the other hand, antipsychotics are able to increase Akt activation (Emamian et al., 2004; Takaki et al., 2018). These data establish a strong relationship between dopamine levels and Akt/GSK3β signaling (Singh, 2013). Akt and GSK-3 may be modulated by DISC1 with indirect and direct interactions, respectively (Dahoun et al., 2017). The Akt/GSK-3 pathway may be responsible for the co-occurrence of T2DM and schizophrenia (Lin and Shuldiner, 2010).
Multiple genetic, functional, and animal studies have shown that COMT is significantly associated with schizophrenia (Luo et al., 2014). COMT is a prime candidate for ameliorating the cognitive dysfunction of schizophrenia (Tunbridge et al., 2006). COMT polymorphism is also associated with hyperglycemia and hemoglobin A1C in T2DM (Hall et al., 2016). Furthermore, COMT is related to the Akt/GSK3 cascade. The COMT Val108/158Met genotype is related to Akt phosphorylation, and information on functional interactions between COMT and AKt may provide novel insights into the pathogenesis of schizophrenia (Sei et al., 2010).
Increasing evidence reveals regulatory interactions between dopamine and the central renin-angiotensin system (Oh and Fan, 2019). ACE catalyzes the conversion of angiotensin I to the active hypertensive peptide angiotensin II. Angiotensin II induces dopamine release in mesolimbic dopaminergic neurons (Rodriguez et al., 2020). Although several studies reported that ACE activity is inconsistent in patients with schizophrenia, higher ACE activity is associated with cognitive dysfunction in patients with schizophrenia (Rodriguez et al., 2020). Because angiotensin II increases hepatic glucose production and decreases insulin sensitivity, ACE inhibitor and angiotensin receptor blockers are reported to reduce the occurrence of T2DM (Gillespie et al., 2005).
Common Mechanisms in Schizophrenia and T2DM
Inflammation Abnormality—Inflammation is a necessary response to infection, harmful chemicals, and tissue damage (Muller, 2018). Inflammation comes at the cost of a transient decline in tissue function, which in turn contributes to altering the homeostasis and becomes the pathogenesis of diseases (Medzhitov, 2010). The long-term effects of inflammatory mediators induce neuroinflammatory disease in the brain as well as metabolic disease in the pancreas (Bauer and Teixeira, 2019).
The origins of inflammatory and immune activation in schizophrenia include (1) genetic predisposition; (2) prenatal exposure to infections (Brown and Derkits, 2010), maternal inflammation during pregnancy (Canetta et al., 2014), and obstetric complications (Cannon et al., 2002); (3) gastrointestinal permeability and the gut microbiome (Severance et al., 2016); (4) psychological trauma (Popovic et al., 2019; Stilo and Murray, 2019) and other environmental exposures such as a low level of serum vitamin D (Davis et al., 2016) and substance use (Miller et al., 2018); and (5) abnormalities in brain insulin action (Agarwal et al., 2020). These inflammations affect the development and activity of microglial cells, which work in the primary inflammations in the central nervous system (Howes and McCutcheon, 2017). Findings from the current meta-analysis seem to support elevated levels of pro-inflammatory marker cytokines, such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor A (TNF-A), in the blood and cerebrospinal fluid of patients with schizophrenia (Goldsmith et al., 2016; Capuzzi et al., 2017). Inflammatory markers have been found to be increased in the first episode of schizophrenia, and manifested alterations in the severity and resistance to treatment in various stages of the illness (Fineberg and Ellman, 2013; Upthegrove et al., 2014; Capuzzi et al., 2017). Furthermore, these inflammatory markers are associated with negative symptoms, indicating loss of brain matter and cognitive impairment in patients with schizophrenia (Garcia-Rizo et al., 2012; Fillman et al., 2013; Meyer, 2013).
Low-grade inflammation has also been described as a risk factor for future development of T2DM. Many studies reported increased levels of pro-inflammatory markers, such as IL-6, IL-1β, and TNF-A, which are predictive components in patients with T2DM (Rehman and Akash, 2016). Pediatric studies, which have the advantage of not being influenced by other diseases, medications, or active tobacco smoking, have demonstrated that IL-1β, IL-6, TNF-A, and other markers are increased in insulin resistance (Reinehr, 2019). Thus, pro-inflammatory mediators could promote insulin resistance and β-cell failure, ultimately resulting in the development of T2DM. Psychological stress can be considered to have a significant role in the onset and progression of diabetes (Afrisham et al., 2019). Although the liver and adipose tissue are important sites for the activation of inflammation pathways, chronic stress directly activates the innate immune system, which, in turn, activates the production of IL-6 and other cytokines (Pickup, 2004). With a genetic predisposition, subsequent stress contributes a vulnerability factor for inflammation-associated schizophrenia. Multiple genome-wide association studies have shown that the major histocompatibility complex on chromosome 6p, which is known to play a key role in the immune system, is an important region for allelic association in schizophrenia (Ripke, 2014). In particular, alleles of complement C4 within the human leukocyte antigen were found to be associated with schizophrenia, and C4 promotes synaptic elimination (Sekar et al., 2016). C4 levels also correlate with body mass index (Copenhaver et al., 2019). IL-1B, IL6, and IL6R genes were associated with schizophrenia in a meta-analysis (Hudson and Miller, 2018) and an association study (Kapelski et al., 2015). Variants in IL1B, IL6, and other cytokine genes were associated with T2DM in multiple studies (Achyut et al., 2007; Arora et al., 2011; Banerjee and Saxena, 2014). An altered immune system and inflammatory components induced by chronic stress were associated with the molecular mechanisms of diabetes in schizophrenia (van Beveren et al., 2014; Ward and Druss, 2015).
Thus, inflammation may be a common underlying mechanism for schizophrenia and diabetes mellitus, which are highly comorbid with each other (Khandaker et al., 2017).
Oxidative Stress
Oxidative stress is defined as an imbalance between the production of reactive oxygen species and their elimination by a protective mechanism (antioxidant system), which can lead to chronic inflammation (Hussain et al., 2016). Oxidative stress is harmful because excess reactive oxygen species attacks biological molecules such as proteins and DNA (Yoshikawa and Naito, 2002). An accumulation of oxidative damage to biological molecules is involved in the pathogenesis of various diseases, including metabolic diseases, diabetes complications, and neurodegenerative disorders (Emiliani et al., 2014). Multiple lines of evidence have identified increased oxidative stress in patients with schizophrenia (Koga et al., 2016). Most studies examined markers of oxidative status in the blood, such as endogenous antioxidants glutathione (GSH) (Barron et al., 2017). A recent extensive review found that peripheral markers of GSH were consistently decreased but found equivocal results for other antioxidants such as superoxide dismutase and catalase (Koga et al., 2016). Genetic studies have shown associations between oxidative stress gene polymorphisms and schizophrenia, including genetic variations in a subunit or GSH cysteine ligase, the enzyme responsible for GSH synthesis, and several glutathione S-transferases (GST), utilizing GSH as a co-factor (Gysin et al., 2007; Gravina et al., 2011). Meta-analysis studies have found the association of the most important genes of the GST family, GSTM1 and GSTT1 variants, with T2DM (Tang et al., 2013; Zhang et al., 2013a). However, unlike genetic association studies, the available GWASs have not provided convincing evidence for an oxidative stress–related genetic predisposition to schizophrenia (Maas et al., 2017).
PON1 is a candidate for a gene that overlaps schizophrenia and T2DM. PON1 enzyme is known to have a protective effect against oxidative stress (Menini and Gugliucci, 2014; Bigagli and Lodovici, 2019). In this context, PON1 activity is inversely associated with inflammatory responses. Drug-naïve first-episode patients with schizophrenia show an inverse relationship between decreased activity of the enzyme PON1 and increased cytokine levels, including IL-6, IL-4, and IL-10 (Brinholi et al., 2015). PON1 activity is also decreased in T2DM and related to β-cell function (Meneses et al., 2019).
MTHFR is a key enzyme for 1-carbon metabolism and DNA methylation. MTHFR polymorphisms (C677T and A1298C) are related to enzymatic activity, and an approximately 20% reduction of MTHFR enzyme activity is shown in patients with schizophrenia (Wan et al., 2018). Interestingly, these polymorphisms are frequently reported in the onset of T2DM and diabetic nephropathy (Mtiraoui et al., 2007).
HPA Axis Dysfunction
The HPA axis, a neuroendocrine system, plays a fundamental role in the maintenance of reactions to stress and affects the physiologic adaptive reactions of the organism to stressors (Nicolaides et al., 2015). HPA is involved in the homeostasis of metabolic, cardiovascular, and reproductive systems, as well as the immune system (van den Brink et al., 2018). The central stress system triggers the synthesis and secretion of corticotropin-releasing hormone (CRH) in the paraventricular nuclei of the hypothalamus (Chrousos, 1995). Through the hypophysial portal system, CRH reaches the anterior pituitary gland and releases adrenocorticotropic hormone (ACTH) into the systemic circulation. On binding to the glucocorticoid receptors of the adrenocortical cells, ACTH subsequently induces glucocorticoid synthesis and secretion, which control CRH and ACTH release via a negative feedback loop (van den Brink et al., 2018). Consequently, the blood glucocorticoid concentration is increased by the stress reaction. Glucocorticoids are steroid hormones that regulate multiple aspects of glucose homeostasis. Glucocorticoids promote gluconeogenesis in the liver by induction of gluconeogenesis enzymes and decrease glucose uptake and utilization by antagonizing the insulin response in skeletal muscle and adipose tissue (Chiodini et al., 2007). Therefore, excess or long-lasting (chronic) glucocorticoid exposure causes hyperglycemia and insulin resistance. In patients with T2DM, glucocorticoid secretion has been suggested to be a possible link between insulin resistance and the features of metabolic syndrome (Chiodini et al., 2007).
Epidemiological studies have revealed that HPA activity plays a role in the pathophysiology of schizophrenia. Pro-inflammatory cytokines such as IL-1, IL-6, or TNF are also involved in activating the HPA axis (Chrousos, 1995; Meyer, 2013). Although there are contradictory reports (Ciufolini et al., 2014), schizophrenia is associated with elevated baseline and challenge-induced HPA activity (Walker et al., 2008). Furthermore, control of the HPA axis was also impaired in drug-naïve and first-episode patients with schizophrenia (Ryan et al., 2004), and baseline cortisol levels are higher in prodromal (clinically high risk) patients (Walker et al., 2013). However, these findings are not universal, and there is limited agreement about elevations in glucocorticoids (Bradley and Dinan, 2010).
Other Endocrine Systems (Prolactin)
The prolactin (PRL) pathway may contribute to the comorbidity of schizophrenia and T2DM (Gragnoli et al., 2016). PRL lies on locus 6p22.3, which is strongly associated with T2DM in the GWAS replication study (Lu et al., 2012). PRL plays a role in regulation of beta-cell mass (Nielsen et al., 2001), islet regeneration and proliferation (Nyblom et al., 2009), and insulin secretion (Sorenson and Brelje, 2009). Low PRL levels are related to a higher T2DM risk in both sexes (Balbach et al., 2013). On the other hand, higher PRL levels were associated with lower glucose levels and higher insulin sensitivity (Wagner et al., 2014).
PRL levels are also associated with schizophrenia. First-episode drug-naïve male schizophrenia patients have serum PRL levels 3 times higher than healthy male controls (Albayrak et al., 2014). It has also been shown that the PRL level is negatively associated with the severity of positive psychosis symptoms in drug-naïve male patients with schizophrenia (Ramsey et al., 2013). Furthermore, PRL polymorphism is associated with schizophrenia, especially in male patients (Rybakowski et al., 2012). Although increased or decreased PRL levels have not been found consistently across studies or by gender difference (Rajkumar, 2014), PRL dysfunction may sustain disrupted mental development and T2DM-related metabolism.
Other candidate genes, PRL-releasing hormone receptor (PRLRH), PRL receptor (PRLR), oxytocin (OXT), oxytocin receptor (OXTR), and NPY, may also correlate with the PRL pathway and contribute to schizophrenia and T2DM. However, genetic data on PRLRH, PRLR, OXT, OXTR, and NPY in human T2DM and schizophrenia patients are scarce (Postolache et al., 2019).
CONCLUSION
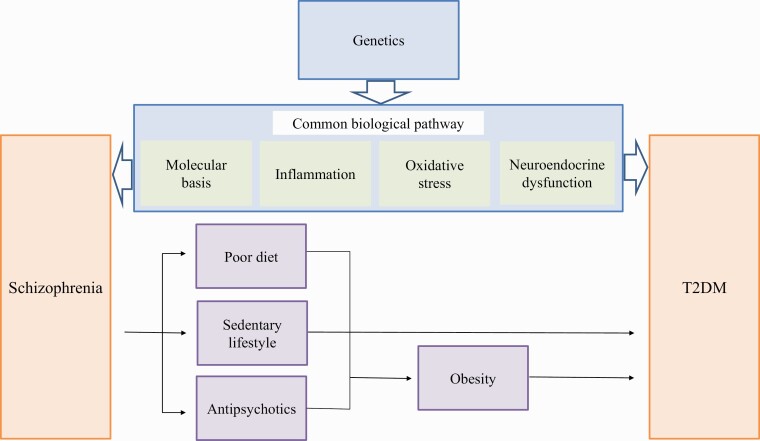
We summarized the genetics and functional mechanisms underlying the comorbidity of schizophrenia and T2DM (Figure 2). Even when genetic analyses are performed on a relatively large number of comorbid patients, the results are sometimes inconsistent, and susceptibility genes may also have only a low or moderate risk to the onset of both. Genetic association studies have revealed the number of common risk variants underlying diseases, but these variants explain only a proportion of heritability. Among the reasons for the complexity in this field are suspected to be the following: (1) the heterogeneity of schizophrenia; (2) many environmental factors, such as lifestyle and vulnerability to life events, which are related to genetic factors; and (3) both genetic and environmental factors that affect common mechanisms in schizophrenia and T2DM, such as abnormal inflammation, oxidative stress, and HPA axis dysfunction. It is very difficult to distinguish purely environmental factors from purely genetic factors. A new approach is to estimate environmental factors statistically compensated by digitized intermediate phenotypes directly related to genetic factors, such as cognitive function, structural or functional magnetic resonance imaging analysis, and cerebral blood flow.
Figure 2.
Mechanisms that underlie the association between schizophrenia and type 2 diabetes mellitus (T2DM). The mechanisms of the increasing prevalence of T2DM in patients with schizophrenia are multifactorial. Poor diet and sedentary lifestyle are included in the traditional risk factors. Iatrogenic risk during treatment with antipsychotics is included in risk factors unique to schizophrenia (Ward and Druss, 2015). Accumulating evidence suggests shared genetic susceptibility and biological common pathway of both schizophrenia and T2DM.
On the other hand, based on many genetic and epidemiological studies, the comorbidity of schizophrenia and T2DM is established, and it is probably safe to assume that common cascades and mechanisms suspected from common genes’ functions in the brain or pancreas are related to the onset of schizophrenia and T2DM. At the point of preemptive medicine, genetic and epidemiological information will be used in making decisions for the prevention and treatment of schizophrenia and T2DM. Though introduction of new medications or supplements that are effective in these cascades and mechanisms may be expected, 1 target point may not be adequate because each common cascade and mechanism is also closely linked. In the future, in addition to more comprehensive whole genome and epigenome analyses of schizophrenia and T2DM, a more precise approach such as familial and rare gene analyses of CNVs of comorbid patients, further subdivision of the diagnoses of schizophrenia, and the following basic functional research may eliminate these difficulties and clarify the treatment target for patients with the same phenotype but different causes.
Acknowledgments
The authors thank the Zikei Institute of Psychiatry (Okayama, Japan).
Interest Statement
Y.Y. has received honoraria for speaking at educational events sponsored by Novartis and Dainippon Sumitomo. N.Y. has received unrestricted research funding from Daiichi Sankyo, Eisai, Pfizer, Otsuka, Astellas, and Merck Sharp & Dohme, which was deposited into research accounts at Okayama University. N.Y. has also received honoraria for his participation as a speaker at educational events from UCB Japan, Tsumura, Pfizer, Dainippon-Sumitomo, Daiichi-Sankyo, Merck Sharp & Dohme, Pfizer, Eisai, Meiji-Seika, and Mochida. M.T. has received honoraria for his participation as a speaker at educational events sponsored by Daiichi Sankyo, Takeda, Tsumura, Otsuka, and Dainippon Sumitomo. S.S. has received unrestricted research funding from Eli Lilly, which was deposited into research accounts at Okayama University Hospital. S.S. has received honoraria for his participation as a speaker at an educational event sponsored by Otsuka and Meiji-Seika. Y.M. has received honoraria for his participation as a speaker at educational events sponsored by Otsuka and Dainippon Sumitomo. N.H. received honoraria for his participation as a speaker at educational events sponsored by Otsuka, Eisai, and Nippon Shinyaku. Y.O. reports no additional financial or other relationship relevant to this article.
References
- Achyut BR, Srivastava A, Bhattacharya S, Mittal B (2007) Genetic association of interleukin-1beta (-511C/T) and interleukin-1 receptor antagonist (86 bp repeat) polymorphisms with Type 2 diabetes mellitus in North Indians. Clin Chim Acta 377:163–169. [DOI] [PubMed] [Google Scholar]
- Afrisham R, Paknejad M, Soliemanifar O, Sadegh-Nejadi S, Meshkani R, Ashtary-Larky D (2019) The influence of psychological stress on the initiation and progression of diabetes and cancer. Int J Endocrinol Metab 17:e67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal SM, Caravaggio F, Costa-Dookhan KA, Castellani L, Kowalchuk C, Asgariroozbehani R, Graff-Guerrero A, Hahn M (2020) Brain insulin action in schizophrenia: something borrowed and something new. Neuropharmacology 163:107633. [DOI] [PubMed] [Google Scholar]
- Albayrak Y, Beyazyüz M, Beyazyüz E, Kuloğlu M (2014) Increased serum prolactin levels in drug-naive first-episode male patients with schizophrenia. Nord J Psychiatry 68:341–346. [DOI] [PubMed] [Google Scholar]
- Anderson D, Cordell HJ, Fakiola M, Francis RW, Syn G, Scaman ES, Davis E, Miles SJ, McLeay T, Jamieson SE, Blackwell JM (2015) First genome-wide association study in an Australian aboriginal population provides insights into genetic risk factors for body mass index and type 2 diabetes. PLoS One 10:e0119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P, Garcia-Bailo B, Dastani Z, Brenner D, Villegas A, Malik S, Spector TD, Richards B, El-Sohemy A, Karmali M, Badawi A (2011) Genetic polymorphisms of innate immunity-related inflammatory pathways and their association with factors related to type 2 diabetes. BMC Med Genet 12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbach L, Wallaschofski H, Völzke H, Nauck M, Dörr M, Haring R (2013) Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes? BMC Endocr Disord 13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Saxena M (2014) Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World J Diabetes 5:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron H, Hafizi S, Andreazza AC, Mizrahi R (2017) Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci 18:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Teixeira AL (2019) Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci 1437:57–67. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM (2012) A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci 37:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR (2011) Beyond cAMP: the regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KG, Barnes KC, Bright TJ, Wang SA (2004) The genetic association database. Nat Genet 36:431–432. [DOI] [PubMed] [Google Scholar]
- Bem J, Brożko N, Chakraborty C, Lipiec MA, Koziński K, Nagalski A, Szewczyk ŁM, Wiśniewska MB (2019) Wnt/β-catenin signaling in brain development and mental disorders: keeping TCF7L2 in mind. FEBS Lett 593:1654–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigagli E, Lodovici M (2019) Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid Med Cell Longev 2019:5953685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher Y, Schleinitz D, Tönjes A, Blüher M, Stumvoll M, Kovacs P (2008) R1467H variant in the rho guanine nucleotide exchange factor 11 (ARHGEF11) is associated with impaired glucose tolerance and type 2 diabetes in German Caucasians. J Hum Genet 53:365–367. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Dinan TG (2010) A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol 24:91–118. [DOI] [PubMed] [Google Scholar]
- Brafman D, Willert K (2017) Wnt/β-catenin signaling during early vertebrate neural development. Dev Neurobiol 77: 1239–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NJ, O’Donovan MC (2018) The genetics of neuropsychiatric disorders. Brain Neurosci Adv 2:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinholi FF, Noto C, Maes M, Bonifácio KL, Brietzke E, Ota VK, Gadelha A, Cordeiro Q, Belangero SI, Bressan RA, Vargas HO, Higachi L, de Farias CC, Moreira EG, Barbosa DS (2015) Lowered paraoxonase 1 (PON1) activity is associated with increased cytokine levels in drug naïve first episode psychosis. Schizophr Res 166:225–230. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EW, Honer WG, Bassett AS (2000) Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science 288:678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushe C, Holt R (2004) Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl 47:S67–S71. [DOI] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, McKeague IW, Brown AS (2014) Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry 171:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM (2002) Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 159:1080–1092. [DOI] [PubMed] [Google Scholar]
- Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà G (2017) Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: a meta-analysis. Neurosci Biobehav Rev 77:122–128. [DOI] [PubMed] [Google Scholar]
- Chen J, Cao F, Liu L, Wang L, Chen X (2015) Genetic studies of schizophrenia: an update. Neurosci Bull 31:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, Epaminonda P, Masserini B, Beck-Peccoz P, Orsi E, Ambrosi B, Arosio M (2007) Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 30:83–88. [DOI] [PubMed] [Google Scholar]
- Chrousos GP (1995) The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332:1351–1362. [DOI] [PubMed] [Google Scholar]
- Chung J, Miller BJ (2020) Meta-analysis of comorbid diabetes and family history of diabetes in non-affective psychosis. Schizophr Res 216:41–47. [DOI] [PubMed] [Google Scholar]
- Ciufolini S, Dazzan P, Kempton MJ, Pariante C, Mondelli V (2014) HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies. Neurosci Biobehav Rev 47:359–368. [DOI] [PubMed] [Google Scholar]
- Copenhaver M, Yu CY, Hoffman RP (2019) Complement components, C3 and C4, and the metabolic syndrome. Curr Diabetes Rev 15:44–48. [DOI] [PubMed] [Google Scholar]
- Crump C, Winkleby MA, Sundquist K, Sundquist J (2013) Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 170:324–333. [DOI] [PubMed] [Google Scholar]
- Dahoun T, Trossbach SV, Brandon NJ, Korth C, Howes OD (2017) The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: a systematic review. Transl Psychiatry 7:e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Elbein SC (2006) The genetic basis of type 2 diabetes. Cellscience 2:100–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Elbein SC (2007) The search for type 2 diabetes susceptibility loci: the chromosome 1q story. Curr Diab Rep 7:154–164. [DOI] [PubMed] [Google Scholar]
- Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, Debnath M, McGrath J, Maes M, Amminger P, McGorry PD, Pantelis C, Berk M (2016) A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci Biobehav Rev 65:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demjaha A, MacCabe JH, Murray RM (2012) How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr Bull 38:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Rubalcaba E, Viscidi R, Yang S, Stallings C, Sullens A, Origoni A, Leister F, Yolken R (2008) Polymorphisms in human endogenous retrovirus K-18 and risk of type 2 diabetes in individuals with schizophrenia. Schizophr Res 104:121–126. [DOI] [PubMed] [Google Scholar]
- Emamian ES (2012) AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA (2004) Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36:131–137. [DOI] [PubMed] [Google Scholar]
- Emiliani FE, Sedlak TW, Sawa A (2014) Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr Opin Psychiatry 27:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18:206–214. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM (2013) Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry 73:951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A (1998) Rapid actin-based plasticity in dendritic spines. Neuron 20:847–854. [DOI] [PubMed] [Google Scholar]
- Foley DL, Mackinnon A, Morgan VA, Watts GF, McGrath JJ, Castle DJ, Waterreus A, Galletly CA (2014) Predictors of type 2 diabetes in a nationally representative sample of adults with psychosis. World Psychiatry 13:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA (2010) Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Xiao G, Hu J (2014) Regulation of Wnt/β-catenin signaling by posttranslational modifications. Cell Biosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Bernardo M, Kirkpatrick B (2012) Inflammatory markers in antipsychotic-naïve patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatry Res 198:212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI (2005) The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 28:2261–2266. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA (2000) Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57:65–73. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA (2013) Dendritic spine pathology in schizophrenia. Neuroscience 251:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough SC, O’Donovan MC (2005) Clustering of metabolic comorbidity in schizophrenia: a genetic contribution? J Psychopharmacol 19:47–55. [DOI] [PubMed] [Google Scholar]
- Gragnoli C (2010) PSMD9 gene in the NIDDM2 locus is linked to type 2 diabetes in Italians. J Cell Physiol 222:265–267. [DOI] [PubMed] [Google Scholar]
- Gragnoli C, Reeves GM, Reazer J, Postolache TT (2016) Dopamine-prolactin pathway potentially contributes to the schizophrenia and type 2 diabetes comorbidity. Transl Psychiatry 6:e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravina P, Spoletini I, Masini S, Valentini A, Vanni D, Paladini E, Bossù P, Caltagirone C, Federici G, Spalletta G, Bernardini S (2011) Genetic polymorphisms of glutathione S-transferases GSTM1, GSTT1, GSTP1 and GSTA1 as risk factors for schizophrenia. Psychiatry Res 187:454–456. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuénod M, Do KQ (2007) Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A 104:16621–16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackinger S, Prins B, Mamakou V, Zengini E, Marouli E, Brčić L, Serafetinidis I, Lamnissou K, Kontaxakis V, Dedoussis G, Gonidakis F, Thanopoulou A, Tentolouris N, Tsezou A, Zeggini E (2018) Evidence for genetic contribution to the increased risk of type 2 diabetes in schizophrenia. Transl Psychiatry 8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279:509–514. [DOI] [PubMed] [Google Scholar]
- Hall KT, Jablonski KA, Chen L, Harden M, Tolkin BR, Kaptchuk TJ, Bray GA, Ridker PM, Florez JC, Mukamal KJ, Chasman DI; Diabetes Prevention Program Research Group (2016) Catechol-O-methyltransferase association with hemoglobin A1c. Metabolism 65:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zhou Y, Renström E, Osmark P (2010) Molecular function of TCF7L2: Consequences of TCF7L2 splicing for molecular function and risk for type 2 diabetes. Curr Diab Rep 10:444–451. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A (2010) Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci 13:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106:9362–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R (2017) Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 7:e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ZD, Miller BJ (2018) Meta-analysis of cytokine and chemokine genes in schizophrenia. Clin Schizophr Relat Psychoses 12:121–129B. [DOI] [PubMed] [Google Scholar]
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N (2016) Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev 2016:7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokawa M, Arai M, Kato S, Ogata Y, Furukawa A, Haga S, Ujike H, Sora I, Ikeda K, Yoshikawa T (2003) Association between a novel polymorphism in the promoter region of the neuropeptide Y gene and schizophrenia in humans. Neurosci Lett 347:202–204. [DOI] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, Bowers WJ, Federoff HJ, Sternweis PC, Rothstein JD (2001) Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 410:89–93. [DOI] [PubMed] [Google Scholar]
- Jurczyk A, Nowosielska A, Przewozniak N, Aryee KE, DiIorio P, Blodgett D, Yang C, Campbell-Thompson M, Atkinson M, Shultz L, Rittenhouse A, Harlan D, Greiner D, Bortell R (2016) Beyond the brain: disrupted in schizophrenia 1 regulates pancreatic β-cell function via glycogen synthase kinase-3β. FASEB J 30:983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Russell JJ, Almeida D, Picard M (2016) Allostatic load and comorbidities: a mitochondrial, epigenetic, and evolutionary perspective. Dev Psychopathol 28:1117–1146. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Beaulieu JM, Jope RS, Woodgett JR (2012) Neurological functions of the masterswitch protein kinase - gsk-3. Front Mol Neurosci 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajio Y, Kondo K, Saito T, Iwayama Y, Aleksic B, Yamada K, Toyota T, Hattori E, Ujike H, Inada T, Kunugi H, Kato T, Yoshikawa T, Ozaki N, Ikeda M, Iwata N (2014) Genetic association study between the detected risk variants based upon type II diabetes GWAS and psychotic disorders in the Japanese population. J Hum Genet 59:54–56. [DOI] [PubMed] [Google Scholar]
- Kapelski P, Skibinska M, Maciukiewicz M, Wilkosc M, Frydecka D, Groszewska A, Narozna B, Dmitrzak-Weglarz M, Czerski P, Pawlak J, Rajewska-Rager A, Leszczynska-Rodziewicz A, Slopien A, Zaremba D, Twarowska-Hauser J (2015) Association study of functional polymorphisms in interleukins and interleukin receptors genes: IL1A, IL1B, IL1RN, IL6, IL6R, IL10, IL10RA and TGFB1 in schizophrenia in Polish population. Schizophr Res 169:1–9. [DOI] [PubMed] [Google Scholar]
- Kariž S, Mankoč S, Petrovič D (2015) Association of thioredoxin reductase 2 (TXNRD2) gene polymorphisms with myocardial infarction in Slovene patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 108:323–328. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Rahn EJ, Paulukaitis BS, Savell KE, Kordasiewicz HB, Wang J, Lewis JW, Posey J, Strange SK, Guzman-Karlsson MC, Phillips SE, Decker K, Motley ST, Swayze EE, Ecker DJ, Michael TP, Day JJ, Sweatt JD (2016) Tcf4 regulates synaptic plasticity, DNA methylation, and memory function. Cell Rep 16:2666–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Dantzer R, Jones PB (2017) Immunopsychiatry: important facts. Psychol Med 47:2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholmanskikh SS, Dobrin JS, Wynshaw-Boris A, Letourneau PC, Ross ME (2003) Disregulated RhoGTPases and actin cytoskeleton contribute to the migration defect in Lis1-deficient neurons. J Neurosci 23:8673–8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, et al. (2012) De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 17:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M, Serritella AV, Sawa A, Sedlak TW (2016) Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr Res 176:52–71. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM (2014) Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 71:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushima I, Nakamura Y, Aleksic B, Ikeda M, Ito Y, Shiino T, Okochi T, Fukuo Y, Ujike H, Suzuki M, Inada T, Hashimoto R, Takeda M, Kaibuchi K, Iwata N, Ozaki N (2012) Resequencing and association analysis of the KALRN and EPHB1 genes and their contribution to schizophrenia susceptibility. Schizophr Bull 38:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Hancock KJ, Kisely S (2013) The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ 346:f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Kim JH, Song GG (2013) Pathway analysis of a genome-wide association study in schizophrenia. Gene 525:107–115. [DOI] [PubMed] [Google Scholar]
- Li J, Zhou G, Ji W, Feng G, Zhao Q, Liu J, Li T, Li Y, Chen P, Zeng Z, Wang T, Hu Z, Zheng L, Wang Y, Shen Y, He L, Shi Y (2011) Common variants in the BCL9 gene conferring risk of schizophrenia. Arch Gen Psychiatry 68:232–240. [DOI] [PubMed] [Google Scholar]
- Lim JE, Hong KW, Jin HS, Kim YS, Park HK, Oh B (2010) Type 2 diabetes genetic association database manually curated for the study design and odds ratio. BMC Med Inform Decis Mak 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PI, Shuldiner AR (2010) Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr Res 123:234–243. [DOI] [PubMed] [Google Scholar]
- Lis M, Stańczykiewicz B, Liśkiewicz P, Misiak B (2020a) Impaired hormonal regulation of appetite in schizophrenia: a narrative review dissecting intrinsic mechanisms and the effects of antipsychotics. Psychoneuroendocrinology 119:104744. [DOI] [PubMed] [Google Scholar]
- Lis M, Stańczykiewicz B, Pawlik-Sobecka L, Samochowiec A, Reginia A, Misiak B (2020b) Assessment of appetite-regulating hormones provides further evidence of altered adipoinsular axis in early psychosis. Front Psychiatry 11:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li Z, Zhang M, Deng Y, Yi Z, Shi T (2013) Exploring the pathogenetic association between schizophrenia and type 2 diabetes mellitus diseases based on pathway analysis. BMC Med Genomics 6(Suppl 1):S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Habener JF (2010) Wnt signaling in pancreatic islets. Adv Exp Med Biol 654:391–419. [DOI] [PubMed] [Google Scholar]
- Lovestone S, Killick R, Di Forti M, Murray R (2007) Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci 30:142–149. [DOI] [PubMed] [Google Scholar]
- Lu F, Qian Y, Li H, Dong M, Lin Y, Du J, Lin Y, Chen J, Shen C, Jin G, Dai J, Hu Z, Shen H (2012) Genetic variants on chromosome 6p21.1 and 6p22.3 are associated with type 2 diabetes risk: a case-control study in Han Chinese. J Hum Genet 57:320–325. [DOI] [PubMed] [Google Scholar]
- Luo X, Huang L, Han L, Luo Z, Hu F, Tieu R, Gan L (2014) Systematic prioritization and integrative analysis of copy number variations in schizophrenia reveal key schizophrenia susceptibility genes. Schizophr Bull 40:1285–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L (2008) Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 359:2220–2232. [DOI] [PubMed] [Google Scholar]
- Ma JH, Sun XY, Guo TJ, Barot E, Wang DF, Yan LL, Ni DW, Huang NH, Xie Q, Zeng J, Ou-Yang L, Liu YQ, Lu QB (2018) Association on DISC1 SNPs with schizophrenia risk: a meta-analysis. Psychiatry Res 270:306–309. [DOI] [PubMed] [Google Scholar]
- Maas DA, Vallès A, Martens GJM (2017) Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry 7:e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH (2009) Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136:1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, et al. ; Psychosis Endophenotypes International Consortium; CNV and Schizophrenia Working Groups of the Psychiatric Genomics Consortium (2017) Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet 49:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Stanley RE, Ross AP, Freitas AE, Moyer CE, Brumback AC, Iafrati J, Stapornwongkul KS, Dominguez S, Kivimäe S, Mulligan KA, Pirooznia M, McCombie WR, Potash JB, Zandi PP, Purcell SM, Sanders SJ, Zuo Y, Sohal VS, Cheyette BNR (2018) DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/β-catenin signaling. Mol Psychiatry 23:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE (2000) Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 57:637–648. [DOI] [PubMed] [Google Scholar]
- Medzhitov R (2010) Inflammation 2010: new adventures of an old flame. Cell 140:771–776. [DOI] [PubMed] [Google Scholar]
- Meneses MJ, Silvestre R, Sousa-Lima I, Macedo MP (2019) Paraoxonase-1 as a regulator of glucose and lipid homeostasis: impact on the onset and progression of metabolic disorders. Int J Mol Sci 20:4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini T, Gugliucci A (2014) Paraoxonase 1 in neurological disorders. Redox Rep 19:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U (2013) Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 42:20–34. [DOI] [PubMed] [Google Scholar]
- Millar JK, Christie S, Porteous DJ (2003) Yeast two-hybrid screens implicate DISC1 in brain development and function. Biochem Biophys Res Commun 311:1019–1025. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley PF, McEvoy JP (2018) Inflammation, substance use, psychopathology, and cognition in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Schizophr Res 195:275–282. [DOI] [PubMed] [Google Scholar]
- Misiak B (2019) Stress, allostatic load, and psychosis: one step forward in research but where to go next? Front Psychiatry 10:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B, Frydecka D, Zawadzki M, Krefft M, Kiejna A (2014) Refining and integrating schizophrenia pathophysiology - relevance of the allostatic load concept. Neurosci Biobehav Rev 45:183–201. [DOI] [PubMed] [Google Scholar]
- Misiak B, Bartoli F, Stramecki F, Samochowiec J, Lis M, Kasznia J, Jarosz K, Stańczykiewicz B (2019) Appetite regulating hormones in first-episode psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev 102:362–370. [DOI] [PubMed] [Google Scholar]
- Misiak B, Wisniewski M, Lis M, Samochowiec J, Stanczykiewicz B (2020) Glucose homeostasis in unaffected first-degree relatives of schizophrenia patients: a systematic review and meta-analysis. Schizophr Res 223:2–8. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M (2013) Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull 39:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki Y, Takaki M, Okahisa Y, Sakamoto S, Kodama M, Ujike H, Uchitomi Y (2014) Human Rho guanine nucleotide exchange factor 11 gene is associated with schizophrenia in a Japanese population. Hum Psychopharmacol 29:552–558. [DOI] [PubMed] [Google Scholar]
- Mizuki Y, Takaki M, Sakamoto S, Okamoto S, Kishimoto M, Okahisa Y, Itoh M, Yamada N (2016) Human rho guanine nucleotide exchange factor 11 (ARHGEF11) regulates dendritic morphogenesis. Int J Mol Sci 18:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtiraoui N, Ezzidi I, Chaieb M, Marmouche H, Aouni Z, Chaieb A, Mahjoub T, Vaxillaire M, Almawi WY (2007) MTHFR C677T and A1298C gene polymorphisms and hyperhomocysteinemia as risk factors of diabetic nephropathy in type 2 diabetes patients. Diabetes Res Clin Pract 75:99–106. [DOI] [PubMed] [Google Scholar]
- Müller N (2018) Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull 44:973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ (1999) High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56:940–945. [DOI] [PubMed] [Google Scholar]
- Murray CJ, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. [DOI] [PubMed] [Google Scholar]
- Nagalski A, Kozinski K, Wisniewska MB (2016) Metabolic pathways in the periphery and brain: contribution to mental disorders? Int J Biochem Cell Biol 80:19–30. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kaneto H, Miyatsuka T, Matsuoka TA, Matsuhisa M, Node K, Hori M, Yamasaki Y (2006) Marked increase of insulin gene transcription by suppression of the Rho/Rho-kinase pathway. Biochem Biophys Res Commun 350:68–73. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E (2015) Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 22:6–19. [DOI] [PubMed] [Google Scholar]
- Nielsen JH, Galsgaard ED, Møldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C (2001) Regulation of beta-cell mass by hormones and growth factors. Diabetes 50(Suppl 1):S25–S29. [DOI] [PubMed] [Google Scholar]
- Niwa M, Cash-Padgett T, Kubo KI, Saito A, Ishii K, Sumitomo A, Taniguchi Y, Ishizuka K, Jaaro-Peled H, Tomoda T, Nakajima K, Sawa A, Kamiya A (2016) DISC1 a key molecular lead in psychiatry and neurodevelopment: No-More Disrupted-in-Schizophrenia 1. Mol Psychiatry 21:1488–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman S, Ding B, Ostenson CG, Kärvestedt L, Brismar K, Efendic S, Gu HF (2005) Leu7Pro polymorphism in the neuropeptide Y (NPY) gene is associated with impaired glucose tolerance and type 2 diabetes in Swedish men. Exp Clin Endocrinol Diabetes 113:282–287. [DOI] [PubMed] [Google Scholar]
- Nuevo R, Chatterji S, Fraguas D, Verdes E, Naidoo N, Arango C, Ayuso-Mateos JL (2011) Increased risk of diabetes mellitus among persons with psychotic symptoms: results from the WHO World Health Survey. J Clin Psychiatry 72:1592–1599. [DOI] [PubMed] [Google Scholar]
- Nyblom HK, Bugliani M, Fung E, Boggi U, Zubarev R, Marchetti P, Bergsten P (2009) Apoptotic, regenerative, and immune-related signaling in human islets from type 2 diabetes individuals. J Proteome Res 8:5650–5656. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Fan X (2019) The possible role of the angiotensin system in the pathophysiology of schizophrenia: implications for pharmacotherapy. CNS Drugs 33:539–547. [DOI] [PubMed] [Google Scholar]
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS (2015) Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 72:1172–1181. [DOI] [PubMed] [Google Scholar]
- Pae CU, Yu HS, Kim JJ, Kim W, Lee CU, Lee SJ, Jun TY, Lee C, Paik IH, Serretti A (2004) Glutathione S-transferase M1 polymorphism may contribute to schizophrenia in the Korean population. Psychiatr Genet 14:147–150. [DOI] [PubMed] [Google Scholar]
- Papadopoulou S, Edlund H (2005) Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes 54:2844–2851. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, Mortensen PB, Eaton WW (2014) A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 71:573–581. [DOI] [PubMed] [Google Scholar]
- Perry BI, McIntosh G, Weich S, Singh S, Rees K (2016) The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry 3:1049–1058. [DOI] [PubMed] [Google Scholar]
- Philippe A, Vaiva G, Casadebaig F (2005) Data on diabetes from the French cohort study in schizophrenia. Eur Psychiatry 20(Suppl 4):S340–S344. [DOI] [PubMed] [Google Scholar]
- Pickup JC (2004) Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27:813–823. [DOI] [PubMed] [Google Scholar]
- Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD (2017) Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski P, Kotowicz K, Rymaszewska J, Beszłej JA, Plichta P, Samochowiec J, Kalinowska S, Trześniowska-Drukała B, Misiak B (2019) Allostatic load index and its clinical correlates at various stages of psychosis. Schizophr Res 210:73–80. [DOI] [PubMed] [Google Scholar]
- Popovic D, Schmitt A, Kaurani L, Senner F, Papiol S, Malchow B, Fischer A, Schulze TG, Koutsouleris N, Falkai P (2019) Childhood trauma in schizophrenia: current findings and research perspectives. Front Neurosci 13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postolache TT, Del Bosque-Plata L, Jabbour S, Vergare M, Wu R, Gragnoli C (2019) Co-shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am J Med Genet B Neuropsychiatr Genet 180:186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhanjan M, Suresh RV, Murthy MN, Ramachandra NB (2016) Type 2 diabetes mellitus disease risk genes identified by genome wide copy number variation scan in normal populations. Diabetes Res Clin Pract 113:160–170. [DOI] [PubMed] [Google Scholar]
- Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Børglum AD, Gasse C (2017) Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a Danish population-based cohort study. Am J Psychiatry 174:686–694. [DOI] [PubMed] [Google Scholar]
- Rajkumar RP (2014) Prolactin and psychopathology in schizophrenia: a literature review and reappraisal. Schizophr Res Treatment 2014:175360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JM, Schwarz E, Guest PC, van Beveren NJ, Leweke FM, Rothermundt M, Bogerts B, Steiner J, Bahn S (2013) Distinct molecular phenotypes in male and female schizophrenia patients. PLoS One 8:e78729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen AH, Rasmussen HB, Silahtaroglu A (2017) The DLGAP family: neuronal expression, function and role in brain disorders. Mol Brain 10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Akash MS (2016) Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci 23:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T (2019) Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin Chim Acta 496:100–107. [DOI] [PubMed] [Google Scholar]
- Rodríguez B, Nani JV, Almeida PGC, Brietzke E, Lee RS, Hayashi MAF (2020) Neuropeptides and oligopeptidases in schizophrenia. Neurosci Biobehav Rev 108:679–693. [DOI] [PubMed] [Google Scholar]
- Rümenapp U, Blomquist A, Schwörer G, Schablowski H, Psoma A, Jakobs KH (1999) Rho-specific binding and guanine nucleotide exchange catalysis by KIAA0380, a dbl family member. FEBS Lett 459:313–318. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sharifi N, Condren R, Thakore JH (2004) Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology 29:1065–1070. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Dmitrzak-Weglarz M, Kapelski P, Hauser J (2012) Functional -1149 g/t polymorphism of the prolactin gene in schizophrenia. Neuropsychobiology 65:41–44. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei Y, Li Z, Song J, Ren-Patterson R, Tunbridge EM, Iizuka Y, Inoue M, Alfonso BT, Beltaifa S, Nakai Y, Kolachana BS, Chen J, Weinberger DR (2010) Epistatic and functional interactions of catechol-o-methyltransferase (COMT) and AKT1 on neuregulin1-ErbB signaling in cell models. PLoS One 5:e10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA; Schizophrenia Working Group of the Psychiatric Genomics Consortium (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Yolken RH, Eaton WW (2016) Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res 176:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang S, Hua F, Hu ZW (2017) The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8:33972–33989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK (2013) An emerging role for Wnt and GSK3 signaling pathways in schizophrenia. Clin Genet 83:511–517. [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC (2009) Prolactin receptors are critical to the adaptation of islets to pregnancy. Endocrinology 150:1566–1569. [DOI] [PubMed] [Google Scholar]
- Stilo SA, Murray RM (2019) Non-genetic factors in schizophrenia. Curr Psychiatry Rep 21:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B, Vancampfort D, De Hert M, Mitchell AJ (2015) The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr Scand 132:144–157. [DOI] [PubMed] [Google Scholar]
- Sudarov A, Gooden F, Tseng D, Gan WB, Ross ME (2013) Lis1 controls dynamics of neuronal filopodia and spines to impact synaptogenesis and social behaviour. EMBO Mol Med 5:591–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Kodama M, Mizuki Y, Kawai H, Yoshimura B, Kishimoto M, Sakamoto S, Okahisa Y, Yamada N (2018) Effects of the antipsychotics haloperidol, clozapine, and aripiprazole on the dendritic spine. Eur Neuropsychopharmacol 28:610–619. [DOI] [PubMed] [Google Scholar]
- Tang ST, Wang CJ, Tang HQ, Zhang Q, Wang Y (2013) Evaluation of glutathione S-transferase genetic variants affecting type 2 diabetes susceptibility: a meta-analysis. Gene 530:301–308. [DOI] [PubMed] [Google Scholar]
- Tomasik J, Lago SG, Vázquez-Bourgon J, Papiol S, Suárez-Pinilla P, Crespo-Facorro B, Bahn S (2019) Association of insulin resistance with schizophrenia polygenic risk score and response to antipsychotic treatment. JAMA Psychiatry 76:864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Hardingham N, Millar K, Fox K (2018) Mechanisms underlying the role of DISC1 in synaptic plasticity. J Physiol 596:2747–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR (2006) Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 60:141–151. [DOI] [PubMed] [Google Scholar]
- Tziastoudi M, Stefanidis I, Stravodimos K, Zintzaras E (2019) Identification of chromosomal regions linked to diabetic nephropathy: a meta-analysis of genome-wide linkage scans. Genet Test Mol Biomarkers 23:105–117. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM (2014) Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 155:101–108. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes Dev 11:2295–2322. [DOI] [PubMed] [Google Scholar]
- van Beveren NJ, Schwarz E, Noll R, Guest PC, Meijer C, de Haan L, Bahn S (2014) Evidence for disturbed insulin and growth hormone signaling as potential risk factors in the development of schizophrenia. Transl Psychiatry 4:e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink WJ, Palic S, Köhler I, de Lange ECM (2018) Access to the CNS: biomarker strategies for dopaminergic treatments. Pharm Res 35:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll SL, Boot E, Butcher NJ, Cooper S, Heung T, Chow EW, Silversides CK, Bassett AS (2017) Obesity in adults with 22q11.2 deletion syndrome. Genet Med 19:204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Heni M, Linder K, Ketterer C, Peter A, Böhm A, Hatziagelaki E, Stefan N, Staiger H, Häring HU, Fritsche A (2014) Age-dependent association of serum prolactin with glycaemia and insulin sensitivity in humans. Acta Diabetol 51:71–78. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K (2008) Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4:189–216. [DOI] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ, Tsuang MT, Cannon TD, McGlashan TH, Woods SW (2013) Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry 74:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Li Y, Zhang Z, Sun Z, He Y, Li R (2018) Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry 8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang J, Li L, Wang Y, Wang Q, Zhai Y, You H, Hu D (2013) Association of rs12255372 in the TCF7L2 gene with type 2 diabetes mellitus: a meta-analysis. Braz J Med Biol Res 46:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Thurmond DC (2009) Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M, Druss B (2015) The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry 2:431–451. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Harrison P (2011) Schizophrenia. 3rd ed. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56:640–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu L, Lin M, Liu W, Kong D, Liu Z, Zhang Y, Ouyang P, Liang Y, Zhong S, Chen C, Jin X, Fan X, Qin J, Zhao X, Rao S, Ding Y (2015) Association of DRD3, COMT, and SLC6A4 gene polymorphisms with type 2 diabetes in southern Chinese: a hospital-based case-control study. Diabetes Technol Ther 17:580–586. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ren J, Ye H (2018) Association between variations in the disrupted in schizophrenia 1 gene and schizophrenia: a meta-analysis. Gene 651:94–99. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Naito Y (2002) What is oxidative stress? JMAJ 45:271–276. [Google Scholar]
- Zhang J, Liu H, Yan H, Huang G, Wang B (2013a) Null genotypes of GSTM1 and GSTT1 contribute to increased risk of diabetes mellitus: a meta-analysis. Gene 518:405–411. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hui L, Liu Y, Wang ZQ, You Y, Miao LN, Sun SL, Guan SL, Xiang Y, Kosten TR, Zhang XY (2013b) The type 2 diabetes mellitus susceptibility gene IGF2BP2 is associated with schizophrenia in a Han Chinese population. J Clin Psychiatry 74:e287–e292. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Park SY, Su J, Bailey K, Ottosson-Laakso E, Shcherbina L, Oskolkov N, Zhang E, Thevenin T, Fadista J, Bennet H, Vikman P, Wierup N, Fex M, Rung J, Wollheim C, Nobrega M, Renström E, Groop L, Hansson O (2014) TCF7L2 is a master regulator of insulin production and processing. Hum Mol Genet 23:6419–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]