Abstract
Acute myeloid leukemia (AML) is a hematologic malignancy with an unfavorable prognosis. A better understanding of AML pathogenesis and chemotherapy resistance at the molecular level is essential for the development of new therapeutic strategies. Apart from DNA methylation and histone modification, RNA epigenetic modification, another layer of epigenetic modification, also plays a critical role in gene expression regulation. Among the more than 150 kinds of RNA epigenetic modifications, N6-methyladenosine (m6A) is the most prevalent internal mRNA modification in eukaryotes and is involved in various biological processes, such as circadian rhythms, adipogenesis, T cell homeostasis, spermatogenesis, and the heat shock response. As a reversible and dynamic modification, m6A is deposited on specific target RNA molecules by methyltransferases and is removed by demethylases. Moreover, m6A binding proteins recognize m6A modifications, influencing RNA splicing, stability, translation, nuclear export, and localization at the posttranscriptional level. Emerging evidence suggests that dysregulation of m6A modification is involved in tumorigenesis, including that of AML. In this review, we summarize the most recent advances regarding the biological functions and molecular mechanisms of m6A RNA methylation in normal hematopoiesis, leukemia cell proliferation, apoptosis, differentiation, therapeutic resistance, and leukemia stem cell/leukemia initiating cell (LSC/LIC) self-renewal. In addition, we discuss how m6A regulators are closely correlated with the clinical features of AML patients and may serve as new biomarkers and therapeutic targets for AML.
Keywords: N6-methyladenosine (m6A), Epigenetics, RNA methylation, Acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults and is characterized by infiltration of malignant myeloid progenitor cells into the bone marrow, peripheral blood and other tissues, which causes uncontrolled proliferation, poor differentiation and abnormal hematopoiesis [1, 2]. Despite advances in medical treatment, less than 40% of AML patients treated with the current standard chemotherapies survive for over 5 years after diagnosis [2, 3]. Thus, a better understanding of AML pathogenesis and chemotherapy resistance at the molecular level is needed so that new therapeutic strategies can be developed. Apart from abnormal genetic changes, epigenetic regulation also plays a critical role in AML [4, 5]. Epigenetic modification, which has become a rapidly evolving field in cancer biology, involves dynamic and reversible regulation of gene expression that does not alter the DNA sequence [4, 6]. The main types of epigenetic modification are DNA methylation and histone modification [4, 6]. Importantly, some epigenetic drugs that target these modifications, such as decitabine, azacitidine and sedamine, have been approved for the treatment of hematopoietic malignancies, particularly AML, lymphoma and myelodysplastic syndromes [4, 5, 7].
Similar to DNA methylation and histone modification, RNA epigenetic modification has recently been implicated in AML initiation and progression [8]. To date, more than 150 kinds of RNA epigenetic modifications have been discovered in cellular RNA types, including messenger RNA (mRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), and long noncoding RNA (lncRNA) [9, 10]. Among them, the N6-methyladenosine (m6A) RNA modification, which refers to methylation at the N6 position of adenosine, is the most prevalent internal mRNA modification in eukaryotes [11, 12]. Recent transcriptome-wide mapping has revealed that m6A sites are enriched mainly at the motif RRACH (R = G or A, H = A, C, or U) in the 3′ untranslated region (3′ UTR), in the long internal exon, and near the stop codon in mRNA [13–16]. m6A can be deposited on specific target RNA molecules by methyltransferases and removed by demethylases [17–20]. Moreover, m6A-specific binding proteins recognize and bind to the m6A motif of RNA, influencing RNA splicing, stability, translation, nuclear export, and localization at the posttranscriptional level [21–26]. Furthermore, reversible mRNA m6A modification is involved in various biological processes, such as circadian rhythms, adipogenesis, T cell homeostasis, spermatogenesis, and the heat shock response, and its dysregulation causes abnormal gene expression, leading to type 2 diabetes, cancers and other diseases [20, 27–33].
In this review, we summarize the most recent information on m6A RNA methylation in AML, including the biological functions and molecular mechanisms. In addition, we discuss m6A regulators that are closely correlated with the clinical features of AML patients and can be used as new biomarkers and therapeutic targets for AML.
The process of m6A RNA modification
Methyltransferases (writers)
Methyltransferases, also called writers, mainly include methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), and Wilm’s tumor 1-associated protein (WTAP), which are categorized as the core components of the m6A methyltransferase complex [17, 34]. METTL3, which binds to the methyl donor S-adenosylmethionine (SAM), was the first writer of the methyltransferase complex to be identified [35]. METTL3 and METTL14 localize to nuclear speckles and form a stable heterodimer to synergistically induce m6A methylation [34]. In this complex, METTL3 acts as the only catalytic subunit, and most studies have indicated that METTL14 provides structural support for METTL3 and contributes to recognition of RNA substrates without possessing independent catalytic activity [18, 36–38]. WTAP, a regulatory component that enhances the catalytic activity of the m6A methyltransferase complex, can interact with the METTL3-METTL14 heterodimer to support localization to nuclear speckles and recruit the complex to target mRNA [17, 38]. Since WTAP itself has no methyltransferase activity, its effect depends strictly on a functional METTL3-METTL14 heterodimer [34, 39].
Additional adaptor proteins, such as RNA-binding motif protein 15 (RBM15), vir-like m6A methyltransferase-associated (VIRMA, also known as KIAA1429), and zinc finger CCCH-type containing 13 (ZC3H13), have since been identified as subunits of the writer complex and contribute to catalyzing m6A methylation [40–42]. RBM15 and its paralog RBM15B bind the methyltransferase complex and recruit WTAP and METTL3 to their target sites in cellular mRNA and the lncRNA X-inactive specific transcript (XIST), promoting m6A formation and XIST-mediated gene transcriptional repression [40]. Recently, the methyltransferase subunit VIRMA was found to recruit the core components METTL3/METTL14/WTAP and mediate their preferential m6A deposition in the 3′ UTR and near the stop codon [43]. The methyltransferase subunit ZC3H13 plays a critical role in anchoring the complex in the nucleus to improve its catalytic activity [44].
Methyltransferase-like 16 (METTL16) is a newly discovered m6A methyltransferase for U6 spliceosomal small nuclear RNA [45]. It can catalyze m6A modification in introns or intron-exon boundaries rather than the common m6A sites of the METTL3-METTL14 methyltransferase complex, suggesting that METTL16 functions independently of the complex [46]. METTL16 also participates in pre-messenger RNA (pre-mRNA) splicing and maintains SAM homeostasis [45–47].
Demethylases (erasers)
Demethylases, also known as erasers, are proteins that remove m6A methylation modifications to enable reversible and dynamic regulation; these erasers include fat mass and obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5) [19, 20]. FTO was the first identified m6A demethylase and plays an important role in energy homeostasis and obesity development, while the m6A demethylase ALKBH5 is involved in spermatogenesis [19, 20, 48, 49]. The two proteins belong to the AlkB family and predominantly localize with nuclear speckles, catalyzing m6A demethylation on nuclear RNA in an iron(II)/α-ketoglutarate-dependent manner [19, 20].
Unlike ALKBH5, which specifically demethylates m6A, FTO displays demethylase activity towards both internal m6A and 5′ cap N6, 2-O-dimethyladenosine (m6Am) modifications on mRNA [50, 51]. It remains controversial whether m6A or m6Am is the primary substrate of FTO. Some studies have reported that FTO has a greater impact on the expression of mRNA with m6A modifications than that with m6Am modifications, while others have shown that FTO preferentially demethylates m6Am rather than m6A [50–52]. However, Wei et al. found that FTO catalyzes m6A and m6Am demethylation in mRNA with differential substrate preferences in the nucleus and cytoplasm [51]. They further demonstrated that FTO preferentially demethylates m6A in the nucleus while targeting both m6A and m6Am in the cytoplasm [51]. Because the abundance of m6A is considerably higher than that of m6Am in mRNA, FTO is still thought to have a more obvious impact on m6A demethylationthan on m6Am demethylation even in the cytoplasm [51]. Furthermore, Su et al. confirmed that FTO mainly affects internal m6A demethylation rather than m6Am demethylation in human AML cells [53].
AlkB homolog 3 (ALKBH3) is a novel m6A demethylase that preferentially targets m6A in tRNA rather than in mRNA or rRNA [54]. In addition, ALKBH3 mediates m6A demethylation in tRNA, facilitating efficient protein translation in vitro [54].
m6A binding proteins (readers)
The last group of m6A regulatory proteins, known as readers, can specifically recognize and bind to the m6A sites on RNA to regulate RNA fate and have diverse effects on gene expression [55]. The members of the YT521-B homology (YTH) domain family, including YTH domain family protein 1/2/3 (YTHDF1/2/3) and YTH domain-containing protein 1/2 (YTHDC1/2), are the most important m6A reader proteins and have conserved YTH domains that can recognize m6A-modified RNA [56]. Among them, YTHDC1 is the only m6A binding protein in the nucleus, while YTHDC2 and YTHDF1–3 localize to the cytoplasm [56, 57]. Functionally, YTHDC1 regulates splicing events and promotes the nuclear export of m6A-methylated mRNA to the cytoplasm by interacting with the splicing factor SRSF3 and the mRNA export receptor NXF1 [25, 26, 58]. YTHDC1 can also recognize m6A sites on the lncRNA XIST, which induces XIST-mediated gene transcriptional repression [40]. YTHDC2 is another reader protein with RNA helicase activity and has complex effects on m6A-modified transcripts [59]. YTHDC2 enhances the translation efficiency of its target RNA but then reduces target mRNA stability, ultimately decreasing target mRNA abundance [60, 61]. Among the readers in this family, YTHDF1 promotes translation and protein synthesis of its target mRNA by interacting with the translation initiation factor eIF3 and ribosomes [24]. YTHDF2 facilitates m6A-containing mRNA decay by selectively binding and transporting them from the translatable pool to RNA decay sites [62]. In addition, YTHDF2 recruits the CCR4-NOT deadenylase complex to promote deadenylation and degradation of methylated mRNA [63]. YTHDF3, the last cytoplasmic reader protein of the YTH domain family, can enhance mRNA translation in synergy with YTHDF1 and promote m6A-modified mRNA degradation in cooperation with YTHDF2 [23, 64]. Thus, YTHDF3 plays a synergistic role with YTHDF1 and YTHDF2 in RNA metabolism in the cytoplasm.
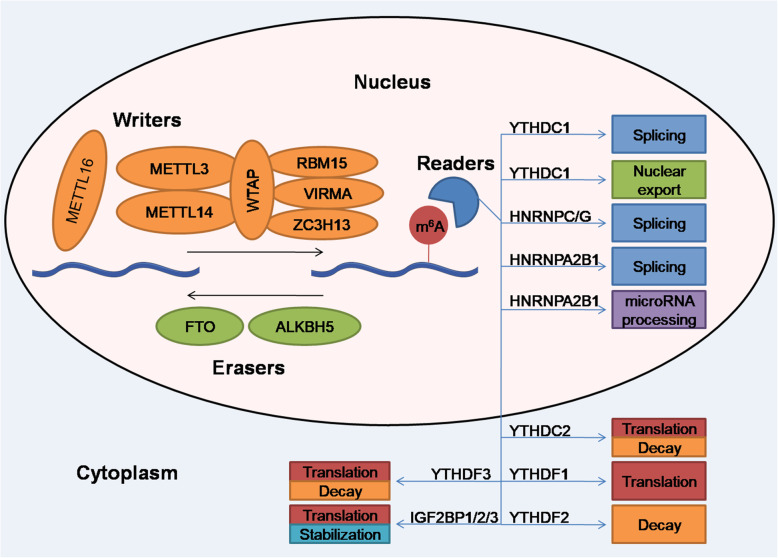
Apart from the YTH domain family, the heterogeneous nuclear ribonucleoprotein (HNRNP) family and insulin-like growth factor 2 mRNA-binding protein (IGF2BP) family have also been identified as m6A reader families [21, 65]. In the HNRNP family, m6A-dependent local structural alterations in RNA can increase the accessibility of methylated mRNA to HNRNPC and HNRNPG, affecting alternative splicing of target mRNA, while HNRNPA2B1 recognizes m6A-modified transcripts and promotes primary microRNA processing and alternative splicing [21, 22, 66]. Moreover, IGF2BP family proteins, including IGF2BP1/2/3, are a group of cytoplasmic m6A readers with K homology domains that recognize m6A-containing transcripts, thus enhancing mRNA stability and translation [65]. The functions of the major writers, erasers, and readers are summarized in Fig. 1.
Fig. 1.
The process of m6A RNA modification
m6A is deposited on specific target RNA molecules by the methyltransferase complex, which includes primarily METTL3, METTL14, WTAP, RBM15, VIRMA, and ZC3H13. m6A can also be reversibly removed by demethylases, including FTO and ALKBH5. Moreover, m6A-specific binding proteins, known as readers, can specifically recognize and bind to m6A sites on RNA, influencing RNA splicing, stability, translation, and nuclear export at the posttranscriptional level.
The role of m6A RNA methylation in normal hematopoiesis and leukemogenesis
Recent studies have suggested that m6A RNA methylation is closely associated with normal and malignant hematopoiesis and related processes including leukemia cell proliferation, apoptosis, differentiation, therapeutic resistance, and leukemia stem cell/leukemia initiating cell (LSC/LIC) self-renewal. Herein, we summarize recent findings on the biological functions and molecular mechanisms of m6A regulators during normal hematopoiesis and leukemogenesis (Table 1).
Table 1.
The roles of m6A regulators in normal hematopoiesis and leukemogenesis
| Type | m6A regulators | Role in cancer | Target genes | Biological functions | Mechanism | Upstream | Reader | Refs |
|---|---|---|---|---|---|---|---|---|
| Leukemogenesis | METTL3 | Oncogene | MYC, BCL2, PTEN | Promotes cell proliferation and colony formation, as well as inhibits differentiation and apotosis | Promotes translation of MYC, BCL2, PTEN and inhibits pAKT pathway | No study | No study | [67] |
| METTL3 | Oncogene | SP1 | Promotes cell proliferation and inhibits differentiation | Promotes translation of SP1 | CEBPZ | No study | [68] | |
| METTL14 | Oncogene | MYB, MYC | Promotes cell proliferation, as well as inhibits differentiation and apotosis | Enhances mRNA stability and promotes translation of MYB and MYC | SPI1 | No study | [69] | |
| WTAP | Oncogene | MYC | Promotes cell proliferation, colony formation and chemoresistance, as well as inhibits differentiation | Suppresses MYC mRNA stability | No study | No study | [70] | |
| WTAP | Oncogene | No study | Promotes cell proliferation, colony formation and chemoresistance, as well as inhibits differentiation | The molecular chaperone Hsp90 maintains the protein stability of WTAP | No study | No study | [71] | |
| FTO | Oncogene | MYC, CEBPA | Promotes cell proliferation | Enhances mRNA stability of MYC and CEBPA | No study | YTHDF2 | [53] | |
| FTO | Oncogene | ASB2, RARA | Promotes cell proliferation and colony formation, as well as inhibits differentiation and apotosis | Suppresses mRNA stability of ASB2 and RARA | No study | No study | [72] | |
| FTO | Oncogene | PFKP, LDHB | Promotes aerobic glycolysis | Enhances mRNA stability of PFKP and LDHB | No study | YTHDF2 | [73] | |
| FTO | Oncogene | MERTK, BCL2 | Promotes cell proliferation and drug resistance | Enhances mRNA stability of MERTK and BCL2 | No study | YTHDF2 | [74] | |
| FTO | Oncogene | LILRB4 | Promotes decitabine-induced immune evasion and makes AML cells more resistant to T cell cytotoxicity | Enhances LILRB4 mRNA stability | No study | YTHDF2 | [75] | |
| ALKBH5 | Oncogene | AXL | Promotes cell proliferation and colony formation, as well as inhibits differentiation and apotosis | Enhances AXL mRNA stability | KDM4C, MYB, Pol II | YTHDF2 | [76] | |
| ALKBH5 | Oncogene | TACC3 | Promotes cell proliferation and colony formation, as well as inhibits apoptosis | Enhances TACC3 mRNA stability | No study | No study | [77] | |
| YTHDF2 | Oncogene | TNFR2 | Promotes cell proliferation and inhibits apotosis | Suppresses TNFR2 mRNA stability | No study | / | [78] | |
| IGF2BP1 | Oncogene | ALDH1A1, HOXB4, MYB | Promotes cell proliferation, colony formation and chemoresistance, as well as inhibits differentiation | Enhances expression of ALDH1A1, HOXB4, and MYB through posttranscriptional regulation | No study | / | [79] | |
| Normal hematopoiesis | METTL3 | / | notch1a | Promotes HSPCs generation through the endothelial-to-haematopoietic transition (EHT) | Suppresses notch1a mRNA stability and inhibits Notch signaling pathway | No study | YTHDF2 | [80] |
| METTL3 | / | No study | Maintains HSCs in a quiescent state | No study | No study | No study | [81] | |
| YTHDF2 | / | Wnt target genes | Maintains HSCs in a quiescent state | Facilitates mRNAs decay of Wnt target genes and represses Wnt signaling | No study | / | [82] | |
| YTHDF2 | / | Tal1 | Maintains HSCs in a quiescent state | Facilitates mRNAs degradation of multiple key transcription factors (e.g., Tal1) | No study | / | [83] | |
| YTHDF2 | / | Inflammation related genes | Sustains HSC integrity and reconstitutes multilineage hematopoiesis upon aging | Facilitates mRNAs degradation of inflammation related genes | No study | / | [84] |
“/” indicates that m6A regulators in normal hematopoiesis did not identify a role in cancer or m6A binding proteins did not identify readers in their molecular mechanisms
Refs references
The role of m6A RNA methylation in normal hematopoiesis
Normal hematopoiesis depends on hematopoietic stem cells (HSCs), which are characterized by self-renewal and multilineage differentiation capacities, as they are able to generate all blood cell types [85]. It has been reported that epigenetic regulation, including DNA methylation and histone acetylation, is involved in HSC function [86]. m6A RNA methylation has also been suggested to play an important role in the function of HSCs.
A recent study found that forced expression of METTL3 significantly promoted proliferation, increased colony numbers and inhibited myeloid differentiation in human hematopoietic stem/progenitor cells (HSPCs) [67]. Additionally, METTL3 mRNA is highly expressed in HSPCs but downregulated in mature differentiated myeloid cells [67]. Mechanistically, METTL3 maintains the undifferentiated state of HSPCs by increasing m6A modification of MYC, BCL2, and PTEN to activate translation of these proteins [67]. Another study has revealed that METTL3 maintains HSCs in a quiescent state and that conditional deletion of METTL3 in mice increases HSC numbers and initiates the cell cycle, but the mechanism still needs to be further studied [81]. It has also been reported that HSPC generation through endothelial-to-hematopoietic transition (EHT) in METTL3-deficient embryos is blocked [80]. METTL3 promotes EHT and the expansion of HSPCs in embryos by regulating different targets and pathways; for example, it decreases notch receptor 1a (notch1a) mRNA stability via YTHDF2-mediated degradation and continuous suppression of the Notch signaling pathway [80]. Similar to METTL3 expression, METTL14 expression is significantly increased in HSPCs and downregulated during myeloid differentiation [69]. Knockdown of METTL14 in normal CD34+ HSPCs substantially promotes differentiation and inhibits colony formation, but only has slight impacts on cell growth and apoptosis, implying that METTL14 plays an important role in inhibiting normal myeloid differentiation [69]. In addition, METTL14 is critical for normal hematopoiesis, as it enhances the mRNA stability and translation of the oncogenic transcription factors MYB and MYC, while METTL14 itself is negatively regulated by SPI1 [69]. However, FTO is dispensable for normal HSPCs [73]. In addition, ALKBH5 does not affect normal hematopoietic function in the steady state and plays only a very minor role in maintaining HSC self-renewal and differentiation under competitive repopulation stress [76, 77]. Similar to METTL3, many studies have shown that YTHDF2 also plays an important role in maintaining adult HSC quiescence, and YTHDF2 deletion facilitates HSC expansion without any noticeable lineage bias or leukemic potential [78, 82, 83]. Mechanistically, YTHDF2 deficiency prevents the degradation of m6A-modified mRNA associated with Wnt target genes and survival-related genes, and abnormal activation of Wnt signaling results in enhancement of the regenerative capacity of HSCs [82]. Another study has found that YTHDF2 suppresses HSC self-renewal by facilitating the degradation of mRNA encoding key transcription factors (e.g., Tal1) that are critical for stem cell self-renewal [83]. In addition, Mapperley and colleagues found that YTHDF2 expression is induced by inflammation and that YTHDF2 functions to downregulate multiple m6A-modified inflammation-related transcripts, sustain long-term HSC integrity and reconstitute multilineage hematopoiesis upon aging [84]. These findings emphasize that some m6A regulators may be essential for normal hematopoiesis. However, whether WTAP is involved in normal hematopoiesis remains elusive and needs further study.
The role of m6A RNA methylation in LSC/LIC self-renewal
LSCs/LICs, which are characterized by properties of self-renewal and chemotherapy resistance, initiate and maintain AML and are responsible for treatment failure and disease relapse [87–89]. Therefore, novel therapeutic targets that can be exploited to selectively eliminate LSCs/LICs are needed. A recent study revealed that METTL14 is essential for the self-renewal of LSCs/LICs, as METTL14 enhances mRNA stability and promotes the translation of the oncogenic transcription factors MYB and MYC, but it is negatively regulated by SPI1 [69]. Pharmacological inhibition or knockdown of FTO also impairs LSC/LIC self-renewal capacity and decreases LSC/LIC frequency by downregulating the expression of MYC and CEBPA in an FTO/m6A-dependent manner [75]. In addition, ALKBH5 depletion in AML patient-derived LSCs significantly inhibits proliferation, reduces colony formation, induces apoptosis, and impairs the leukemogenic potential of the cells in immunodeficient mice [76]. ALKBH5 plays a critical role in the development and functional maintenance of LSCs by promoting the expression of the receptor tyrosine kinase (RTK) AXL via prevention of m6A-YTHDF2-dependent mRNA degradation [76]. Research has also shown that ALKBH5 facilitates LSC/LIC self-renewal and increases LSC/LIC frequency by stabilizing the transcript of TACC3, a prognosis-associated oncogene in multiple tumor types [77]. Furthermore, YTHDF2 promotes the development and maintenance of LSCs by downregulating tumor necrosis factor (TNF) receptor 2 (TNFR2) through an m6A-dependent pathway, and TNFR2 downregulation can protect LSCs from apoptosis [78]. The above results suggest that some of the m6A regulators have critical roles in promoting LSC stemness. Whether METTL3 or WTAP regulates LSC self-renewal deserves to be further studied.
The role of m6A RNA methylation in leukemogenesis
A recent study showed that METTL3 depletion blocked leukemia cell growth, induced differentiation and apoptosis, and delayed leukemia development in immunodeficient recipient mice [67]. Currently, m6A-specific methylated RNA immunoprecipitation sequencing (MeRIP-seq or m6A-seq) is the most widely used method for identifying transcriptome-wide m6A sites [13, 14]. This method relies on m6A-specific antibodies to pull down m6A-containing RNA fragments, which are subsequently identified by high-throughput sequencing. However, its resolution is limited by the RNA fragment size (approximately 200 nucleotides). Another new method called m6A individual nucleotide resolution crosslinking and immunoprecipitation (miCLIP) can detect the mutations generated by UV-induced crosslinking of m6A-specific antibodies with methylated RNA, which can accurately identify m6A sites at a single-nucleotide resolution [16]. In this study, miCLIP mapping of m6A sites coupled with ribosome profiling indicated that METTL3 facilitates the translation of downstream targets, including MYC, BCL2, and PTEN mRNA molecules, by increasing their m6A levels [67]. Moreover, METTL3 leads to decreased expression of pAKT, which contributes to blockade of myeloid differentiation [67]. Another study also reported a similar phenotype and confirmed that METTL3 is critical for maintaining the undifferentiated leukemic state [68]. METTL3 is recruited by the transcription factor CEBPZ to the promoter region of its target gene, SP1, an oncogene in several cancers [68]. Binding of METTL3 to the promoter increases the m6A methylation level of SP1 mRNA and promotes its protein translation by relieving ribosome stalling [68]. In addition, METTL14 knockdown promotes myeloid differentiation, inhibits cell growth, induces apoptosis in vitro, significantly delays leukemia onset, and prolongs survival in immunodeficient recipient mice in vivo [69]. In addition, METTL14 is downregulated during myeloid differentiation in AML cells [69]. METTL14 promotes AML progression by positively regulating the expression of its targets, such as MYB and MYC, through enhancement of their m6A modification, and METTL14 transcription is negatively regulated by SPI1 [69]. Furthermore, it hasbeen reported that WTAP depletion in human AML cells induces differentiation and inhibits cell proliferation and colony formation by preventing MYC mRNA degradation through m6A-based posttranscriptional regulation [70]. Bansal et al. also suggested that WTAP plays an oncogenic role in AML by binding to the molecular chaperone Hsp90, which helps maintain the protein stability of WTAP by preventing its degradation via the ubiquitin-proteasome pathway [71].
Similar to writers, m6A erasers are also involved in leukemogenesis. Overexpression of FTO significantly enhances AML cell proliferation, increases colony numbers, and suppresses apoptosis and differentiation in vitro [72]. In addition, forced expression of FTO significantly promotes leukemic oncogene-mediated leukemogenesis in mice [72]. Collectively, these data indicate that FTO plays a critical oncogenic role by posttranscriptionally promoting the mRNA degradation of the tumor suppressors ASB2 and RARA, resulting in decreased expression of these targets [72]. In addition, ALKBH5 knockdown significantly promotes differentiation, blocks cell growth, induces apoptosis, reduces clonogenic ability in vitro and delays leukemia progression in vivo [76]. ALKBH5 knockdown promotes m6A methylation on the downstream target AXL, which therefore decreases AXL mRNA stability through an m6A-YTHDF2-dependent mechanism [76]. Moreover, downregulation of AXL caused by ALKBH5 knockdown reduces the activation of downstream kinase signaling pathways, such as the PI3K, MAPK, JAK/STAT, and NF-kB pathways, which are linked to AML chemoresistance [76, 90, 91]. ALKBH5 expression is also positively affected by KDM4C, which increases chromatin accessibility and promotes the recruitment of MYB and Pol II to the ALKBH5 promoter; this mechanism indicates the existence of a KDM4C-ALKBH5-AXL signaling axis in AML [76]. Another study has shown that ALKBH5 promotes AML progression by decreasing m6A modification of the target TACC3, resulting in an increase in mRNA stability rather than translation [77].
Like erasers, m6A readers also play essential roles in leukemogenesis. YTHDF2 knockdown in human AML cell lines decreases their proliferative capacity, increases their apoptosis rate, and impairs their engraftment ability in immunodeficient mice [78]. Mechanistically, YTHDF2 loss extends the half-life of m6A-modified transcripts, including those of TNFR2, whose upregulation increases the sensitivity of leukemia cells to TNF-induced apoptosis [78]. Knockdown of another m6A binding protein, IGF2BP1, significantly inhibits colony formation, reduces leukemia cell proliferation, enhances myeloid differentiation and delays leukemia development in immunodeficient recipient mice [79]. IGF2BP1 maintains tumorigenicity by enhancing the expression of critical transcriptional and metabolic regulators, including ALDH1A1, HOXB4, and MYB, through posttranscriptional regulation [79].
Collectively, these findings suggest that m6A RNA methylation is required for the development of AML, as it influences the stability and translation of different target mRNA molecules and regulates the expression of components of several pathways.
The role of m6A RNA methylation in therapeutic resistance
Dysregulated expression of m6A regulators has been associated with therapeutic resistance. Naren et al. found that WTAP knockdown in leukemia cell lines enhances the sensitivity of the cells to the common chemotherapy drug daunorubicin by preventing MYC mRNA degradation in an m6A-dependent manner [70]. Another study also showed that upregulation of WTAP makes AML cells more resistant to etoposide treatment [71]. Mechanistically, the binding of WTAP to Hsp90 stabilizes the WTAP protein by preventing polyubiquitination and proteasomal degradation [71]. Moreover, FTO overexpression upregulates the expression of survival and proliferation genes in an m6A-dependent manner, which contributes to the induction of tyrosine kinase inhibitor (TKI) resistance in leukemia cells [74]. Upregulation of IGF2BP1 increases the resistance of leukemia cells to chemotherapeutic drugs by enhancing the expression of ALDH1A1, HOXB4, and MYB through posttranscriptional regulation [79]. These findings highlight the potential therapeutic value of targeting m6A regulators to combat drug resistance in AML.
Potential clinical application of targeting m6A RNA methylation in AML
m6A RNA methylation as a biomarker in AML
Methyltransferase complex components (METTL3, METTL14, WTAP), demethylases (FTO, ALKBH5), and the common m6A binding protein YTHDF2 are all highly expressed in patients with various subtypes of AML [67, 69–72, 76–78]. Among them, METTL14 is significantly overexpressed in AML patients carrying common chromosomal translocations such as t(11q23), t(15;17), and t(8;21) [69]. Naren et al. found that there were more patients with FLT3-ITD than without FLT3-ITD in a WTAP high-expression group, while the frequency of patients with t(15;17) was reduced in the WTAP high-expression group [70]. In another study, NPM1 and FLT3-ITD mutations were also found to be closely correlated with WTAP levels [71]. In addition, overexpression of FTO has been found in AML patients with t(11q23), t(15;17), FLT3-ITD and/or NPM1 mutations [72]. ALKBH5, another m6A demethylase, is significantly upregulated in AML patients with a normal karyotype, inv.(16), t(11q23), and t(8;21) [76]. Moreover, high expression of WTAP, ALKBH5, or IGF2BP1 predicts a poor prognosis in AML patients [70, 76, 77, 79], while the expression of METTL3, METTL14, FTO, and YTHDF2 is not correlated with prognosis. Furthermore, WTAP protein levels are decreased in AML patients with complete remission [70]. In addition, high ALKBH5 expression is related to a significantly elevated relapse rate and is negatively associated with the duration until relapse in AML patients [76]. The above findings demonstrate that the expression levels of m6A regulators may be promising biomarkers for diagnosis, prognostic prediction and therapeutic response evaluation in AML patients.
m6A RNA methylation as a potential therapeutic target in AML
The above results demonstrate that knockdown of METTL14, FTO, ALKBH5 or YTHDF2 exerts a weaker inhibitory effect on normal hematopoiesis than on leukemogenesis and that m6A RNA methylation is important in the initiation and progression of AML, suggesting that m6A regulators may be potential therapeutic targets for selective eradication of leukemia cells. Currently, no specific inhibitors targeting m6A regulators other than FTO have been identified for the treatment of AML. R-2-hydroxyglutarate (R-2HG) is the major metabolic product of mutant isocitrate dehydrogenase 1/2 (IDH1/2) enzymes. It has been shown to suppress FTO activity, increase the global m6A modification level and exert antitumor effects in AML without IDH1/2 mutations by decreasing the mRNA stability of MYC/CEBPA, leading to the suppression of related pathways [53]. R-2HG also effectively suppresses aerobic glycolysis in IDH-wild-type leukemia cells by targeting the FTO/m6A/YTHDF2 signaling pathway to downregulate the expression of two critical glycolytic genes, PFKP and LDHB, which contributes to its antitumor effects [73]. Meclofenamic acid (MA), a nonsteroidal anti-inflammatory drug, has been identified as a highly selective inhibitor of FTO over ALKBH5 [92]. The ethyl ester form of MA (MA2) is also a specific FTO inhibitor that can inhibit tumorigenesis mediated by glioblastoma stem cells [93]. Recently, the MA derivatives FB23 and FB23–2 have been identified as two novel small molecule inhibitors of FTO that are highly selective in inhibiting FTO-mediated demethylation [52]. FB23–2 has been found to exhibit antileukemia effects in a patient-derived xenograft (PDX) AML mouse model [52]. Moreover, two potent small molecule inhibitors against FTO, CS1 and CS2, exert more effective antileukemic effects than the above reported FTO inhibitors, with minimal side effects on healthy control cells [75]. Mechanistically, targeting FTO can suppress the expression of immune checkpoint genes, especially LILRB4, in an m6A-dependent manner, substantially increasing AML cell sensitivity to T cell cytotoxicity and overcoming decitabine-induced immune evasion [75]. Therefore, a combination of FTO inhibitors and hypomethylating agents might exert synergistic effects in the treatment of AML. In addition, combined treatment with FTO inhibitors and nilotinib eradicates the TKI resistance phenotype and impairs leukemia cell propagation both in vitro and in vivo [74]. Importantly, combining FTO inhibitor treatment with existing therapeutic agents, such as standard chemotherapeutic agents or RTK inhibitors, enhances antitumor efficacy in leukemia patients, especially those with high FTO expression and relapsed/refractory AML [53, 74]. Thus, there is an increasing need to develop more selective and effective small molecule inhibitors targeting m6A regulators to treat AML.
Conclusions
In summary, emerging research has revealed that m6A RNA modification influences normal hematopoiesis and leukemogenesis via regulation of the expression of different targets or pathways at the posttranscriptional level, primarily by influencing mRNA stability and translation efficiency. It is becoming clear that m6A modifications have broad influences on normal hematopoiesis; leukemia cell proliferation, apoptosis, differentiation, and therapeutic resistance; and LSC/LIC self-renewal. In addition, m6A regulators are highly expressed in AML and are related to patient survival as well as relapse rates, indicating that m6A regulators can serve as promising biomarkers for diagnosis, prognostic prediction and therapeutic response evaluation in AML patients. However, more large-scale and multicenter studies are needed to identify the specificity and sensitivity of m6A regulators as biomarkers that can contribute to individualized precision treatment of AML. Deregulation of m6A regulators has also been associated with drug resistance, suggesting the potential therapeutic value of targeting m6A regulators to combat drug resistance in AML. Moreover, FTO inhibitors such as R-2HG, FB23, FB23–2, CS1 and CS2 can suppress the progression of AML in vitro and in vivo, further indicating that m6A regulators may be promising therapeutic targets for AML.
One may expect that m6A writer and eraser genes can play opposite roles in the same cancer. However, the m6A regulators METTL3, METTL14, WTAP, FTO, ALKBH5, YTHDF2, and IGF2BP1 are all oncogenic in AML. Based on the current understanding of m6A modification in cancers, m6A itself is not pro-oncogenic or anti-oncogenic, but deregulation of m6A regulators may promote or inhibit the malignant process of AML by regulating the expression of related oncogenes or tumor suppressor genes with aberrant m6A levels on their transcripts. A similar phenomenon is observed for DNA methylation. For example, double knockout of DNMT3A (a DNA methyltransferase) and TET2 (a DNA demethylase) in mice can cooperatively accelerate malignancy development [94].
Compared to research on DNA methylation and histone modification, research on the role of m6A RNA methylation in AML is still in a relatively early stage. No specific inhibitors targeting m6A regulators are currently available in clinical practice. Therefore, more studies are required to further explore the mechanism and reveal the relationship between RNA m6A modification and AML pathogenesis. Development of more selective and effective small molecule inhibitors targeting m6A regulators through structural studies and large-scale chemical screening may provide not only tool compounds for future research but also novel therapeutic strategies for AML. In addition, combinations of such inhibitors and existing therapeutic agents may lead to major innovations in AML treatment in the future.
Acknowledgements
We thank a professional language editing by American Journal Experts.
Abbreviations
- 3′ UTR
3′ untranslated region
- ALDH1A1
Aldehyde dehydrogenase 1 family member A1
- ALKBH3
AlkB homolog 3
- ALKBH5
AlkB homolog 5
- AML
Acute myeloid leukemia
- ASB2
Ankyrin repeat and SOCS box containing 2
- AXL
AXL receptor tyrosine kinase
- BCL2
BCL2 apoptosis regulator
- CCR4-NOT
Glucose-repressible alcohol dehydrogenase transcriptional effector
- CEBPA
CCAAT enhancer binding protein alpha
- CEBPZ
CCAAT enhancer binding protein zeta
- DNMT3A
DNA methyltransferase 3 alpha
- EHT
Endothelial-to-hematopoietic transition
- eIF3
Eukaryotic translation initiation factor 3
- FLT3-ITD
Fms related receptor tyrosine kinase 3-internal tandem duplication
- FTO
Fat mass and obesity-associated protein
- HNRNP
Heterogeneous nuclear ribonucleoprotein
- HOXB4
Homeobox B4
- HSC
Hematopoietic stem cell
- HSPC
Hematopoietic stem/progenitor cell
- IDH1/2
Isocitrate dehydrogenase 1/2
- IGF2BP
Insulin-like growth factor 2 mRNA-binding protein
- KDM4C
Lysine demethylase 4C
- LDHB
Lactate dehydrogenase B
- LILRB4
Leukocyte immunoglobulin like receptor B4
- lncRNA
Long noncoding RNA
- LSC/LIC
Leukemia stem/initiating cell
- m6A
N6-methyladenosine
- m6Am
N6, 2-O-dimethyladenosine
- MA
Meclofenamic acid
- MA2
The ethyl ester form of MA
- METTL3
Methyltransferase-like 3
- METTL14
Methyltransferase-like 14
- METTL16
Methyltransferase-like 16
- mRNA
Messenger RNA
- MYB
MYB proto-oncogene, transcription factor
- MYC
MYC proto-oncogene, bHLH transcription factor
- notch1a
Notch receptor 1a
- NPM1
Nucleophosmin 1
- NXF1
Nuclear RNA export factor 1
- PDX
Patient-derived xenotransplantation
- PFKP
Phosphofructokinase platelet
- pre-mRNA
Pre-messenger RNA
- PTEN
Phosphatase and tensin homolog
- RARA
Retinoic acid receptor alpha
- RBM15
RNA-binding motif protein 15
- rRNA
Ribosomal RNA
- RTK
Receptor tyrosine kinase
- R-2HG
R-2-hydroxyglutarate
- SAM
S-adenosylmethionine
- SP1
Sp1 transcription factor
- SPI1
Spi-1 proto-oncogene
- SRSF3
Serine and arginine rich splicing factor 3
- TACC3
Transforming acidic coiled-coil containing protein 3
- Tal1
T cell acute lymphocytic leukemia 1
- TET2
Tet methylcytosine dioxygenase 2
- TKI
Tyrosine kinase inhibitor
- TNF
Tumor necrosis factor
- TNFR2
TNF receptor superfamily member 1B
- tRNA
Transfer RNA
- VIRMA
Vir-like m6A methyltransferase-associated
- WTAP
Wilm’s tumor 1-associated protein
- XIST
X-inactive specific transcript
- YTH
YT521-B homology
- YTHDC1/2
YTH domain-containing protein 1/2
- YTHDF1/2/3
YTH domain family protein 1/2/3
- ZC3H13
Zinc finger CCCH-type containing 13
Authors’ contributions
Both authors have contributed to writing and revising the manuscript. Both authors have read and approved the final version.
Funding
The study was supported by the Foundation of the Science and Technology Department of Sichuan Province (No. 2019YFS0026).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 3.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ning B, Li W, Zhao W, Wang R. Targeting epigenetic regulations in cancer. Acta Biochim Biophys Sin Shanghai. 2016;48:97–109. doi: 10.1093/abbs/gmv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127(1):42–52. doi: 10.1182/blood-2015-07-604512. [DOI] [PubMed] [Google Scholar]
- 6.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17(8):487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 7.Ahuja N, Sharma AR, Baylin SB. Epigenetic therapeutics: a new weapon in the war against cancer. Annu Rev Med. 2016;67(1):73–89. doi: 10.1146/annurev-med-111314-035900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, Ma P, Liu Y, Li W, Shu Y. Multiple functions of m(6)A RNA methylation in cancer. J Hematol Oncol. 2018;11(1):48. doi: 10.1186/s13045-018-0590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46(D1):D303–D3d7. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N, Pan T. N6-methyladenosine–encoded epitranscriptomics. Nat Struct Mol Biol. 2016;23(2):98–102. doi: 10.1038/nsmb.3162. [DOI] [PubMed] [Google Scholar]
- 11.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149(7):1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Wu K, Quan W, Yu L, Chen S, Cheng C, Wu Q, Zhao S, Zhang Y, Zhou L. The dynamics of FTO binding and demethylation from the m(6)A motifs. RNA Biol. 2019;16(9):1179–1189. doi: 10.1080/15476286.2019.1621120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12(8):767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, Zhao X, Li A, Yang Y, Dahal U, Lou XM, Liu X, Huang J, Yuan WP, Zhu XF, Cheng T, Zhao YL, Wang X, Danielsen JMR, Liu F, Yang YG. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 19.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RPG, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162(6):1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45(10):6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, Wang X, Ma HL, Huang CM, Yang Y, Huang N, Jiang GB, Wang HL, Zhou Q, Wang XJ, Zhao YL, Yang YG. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, He E, Shen B, He C. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017;6:e31311. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155(4):793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, Jin KX, Wang X, Huang CM, Fu Y, Ge XM, Song SH, Jeong HS, Yanagisawa H, Niu Y, Jia GF, Wu W, Tong WM, Okamoto A, He C, Danielsen JMR, Wang XJ, Yang YG. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24(12):1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, Wang G, Broughton JP, Chen YG, Kluger Y, Simon MD, Chang HY, Yin Z, Flavell RA. M(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548(7667):338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526(7574):591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Shen F, Huang W, Qin S, Huang JT, Sergi C, Yuan BF, Liu SM. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(3):665–673. doi: 10.1210/jc.2018-00619. [DOI] [PubMed] [Google Scholar]
- 32.De Jesus DF, Zhang Z, Kahraman S, Brown NK, Chen M, Hu J, et al. m(6)A mRNA methylation regulates human β-cell biology in physiological states and in type 2 diabetes. Nat Metab. 2019;1(8):765–774. doi: 10.1038/s42255-019-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo J, Liu H, Luan S, He C, Li Z. Aberrant regulation of mRNA m6A modification in cancer development. Int J Mol Sci. 2018;19(9):2515. doi: 10.3390/ijms19092515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna. 1997;3(11):1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 36.Śledź P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016;5:e18434. doi: 10.7554/eLife.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63(2):306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schöller E, Weichmann F, Treiber T, Ringle S, Treiber N, Flatley A, Feederle R, Bruckmann A, Meister G. Interactions, localization, and phosphorylation of the m(6)A generating METTL3-METTL14-WTAP complex. Rna. 2018;24(4):499–512. doi: 10.1261/rna.064063.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorci M, Ianniello Z, Cruciani S, Larivera S, Ginistrelli LC, Capuano E, Marchioni M, Fazi F, Fatica A. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9(8):796. doi: 10.1038/s41419-018-0843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, ter-Ovanesyan D, Habib N, Cacchiarelli D, Sanjana NE, Freinkman E, Pacold ME, Satija R, Mikkelsen TS, Hacohen N, Zhang F, Carr SA, Lander ES, Regev A. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8(1):284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D, Andrade-Navarro MA, Biggiogera M, Helm M, Soller M, Bühler M, Roignant JY. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32(5-6):415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, Wang F, Wang X, Shen B, Wang Y, Feng X, He C, Liu J. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4(1):10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen J, Lv R, Ma H, Shen H, He C, Wang J, et al. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–38.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA m(6)A Methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824–35.e14. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Höbartner C, Sloan KE, Bohnsack MT. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18(11):2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doxtader KA, Wang P, Scarborough AM, Seo D, Conrad NK, Nam Y. Structural basis for regulation of METTL16, an S-Adenosylmethionine homeostasis factor. Mol Cell. 2018;71:1001–11.e4. doi: 10.1016/j.molcel.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458(7240):894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 49.Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Brüning JC, Nolan PM, Ashcroft FM, Cox RD. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42(12):1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, Gross SS, Elemento O, Debart F, Kiledjian M, Jaffrey SR. Reversible methylation of m(6)A(m) in the 5′ cap controls mRNA stability. Nature. 2017;541(7637):371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, et al. Differential m(6)A, m(6)A(m), and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71:973–85.e5. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677–91.e10. doi: 10.1016/j.ccell.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172:90–105.e23. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueda Y, Ooshio I, Fusamae Y, Kitae K, Kawaguchi M, Jingushi K, Hase H, Harada K, Hirata K, Tsujikawa K. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7(1):42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20(10):608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 56.Liao S, Sun H, Xu C. YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genomics Proteomics Bioinformatics. 2018;16(2):99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain D, Puno MR, Meydan C, Lailler N, Mason CE, Lima CD, et al. ketu mutant mice uncover an essential meiotic function for the ancient RNA helicase YTHDC2. Elife. 2018;7:e30919. doi: 10.7554/eLife.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, Gregory BD, Schultz RM, Wang PJ. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14(5):e1007412. doi: 10.1371/journal.pgen.1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m(6)A Transcripts by the 3′→5′ RNA Helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68:374–87.e12. doi: 10.1016/j.molcel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Kretschmer J, Rao H, Hackert P, Sloan KE, Höbartner C, Bohnsack MT. The m(6)A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5′-3′ exoribonuclease XRN1. Rna. 2018;24(10):1339–1350. doi: 10.1261/rna.064238.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J, Cheng Y, Luo G, Dai Q, Liu M, Guo X, Sha J, Shen B, He C. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27(9):1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7(1):12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, Bhattarai DP, Zhao YL, Sun BF, Yang YG. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, Hu YC, Hüttelmaier S, Skibbe JR, Su R, Deng X, Dong L, Sun M, Li C, Nachtergaele S, Wang Y, Hu C, Ferchen K, Greis KD, Jiang X, Wei M, Qu L, Guan JL, He C, Yang J, Chen J. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, Schulman J, Famulare C, Patel M, Klimek VM, Garrett-Bakelman FE, Melnick A, Carroll M, Mason CE, Jaffrey SR, Kharas MG. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23(11):1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, de Braekeleer E, Ponstingl H, Hendrick A, Vakoc CR, Vassiliou GS, Kouzarides T. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature. 2017;552(7683):126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22:191–205.e9. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naren D, Yan T, Gong Y, Huang J, Zhang D, Sang L, Zheng X, Li Y. High Wilms’ tumor 1 associating protein expression predicts poor prognosis in acute myeloid leukemia and regulates m(6)A methylation of MYC mRNA. J Cancer Res Clin Oncol. 2021;147(1):33–47. doi: 10.1007/s00432-020-03373-w. [DOI] [PubMed] [Google Scholar]
- 71.Bansal H, Yihua Q, Iyer SP, Ganapathy S, Proia DA, Penalva LO, Uren PJ, Suresh U, Carew JS, Karnad AB, Weitman S, Tomlinson GE, Rao MK, Kornblau SM, Bansal S. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28(5):1171–1174. doi: 10.1038/leu.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, Qin X, Tang L, Wang Y, Hong GM, Huang H, Wang X, Chen P, Gurbuxani S, Arnovitz S, Li Y, Li S, Strong J, Neilly MB, Larson RA, Jiang X, Zhang P, Jin J, He C, Chen J. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017;31(1):127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qing Y, Dong L, Gao L, Li C, Li Y, Han L, et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m(6)A/PFKP/LDHB axis. Mol Cell. 2021;81:922–39.e9. doi: 10.1016/j.molcel.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan F, Al-Kali A, Zhang Z, Liu J, Pang J, Zhao N, et al. A dynamic N(6)-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res. 2018;28(11):1062–1076. doi: 10.1038/s41422-018-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11. doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Li Y, Wang P, Han G, Zhang T, Chang J, et al. Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling axis. Cell Stem Cell. 2020;27:81–97.e8. doi: 10.1016/j.stem.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Shen C, Sheng Y, Zhu AC, Robinson S, Jiang X, Dong L, et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell. 2020;27:64–80.e9. doi: 10.1016/j.stem.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A, Ivanova I, et al. Targeting the RNA m(6)A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell. 2019;25:137–48.e6. doi: 10.1016/j.stem.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elcheva IA, Wood T, Chiarolanzio K, Chim B, Wong M, Singh V, Gowda CP, Lu Q, Hafner M, Dovat S, Liu Z, Muljo SA, Spiegelman VS. RNA-binding protein IGF2BP1 maintains leukemia stem cell properties by regulating HOXB4, MYB, and ALDH1A1. Leukemia. 2020;34(5):1354–1363. doi: 10.1038/s41375-019-0656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, Lv J, Heng J, Ding Y, Xue Y, Lu X, Xiao W, Yang YG, Liu F. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549(7671):273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 81.Yao QJ, Sang L, Lin M, Yin X, Dong W, Gong Y, Zhou BO. Mettl3-Mettl14 methyltransferase complex regulates the quiescence of adult hematopoietic stem cells. Cell Res. 2018;28(9):952–954. doi: 10.1038/s41422-018-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, Zuo H, Liu J, Wen F, Gao Y, Zhu X, Liu B, Xiao F, Wang W, Huang G, Shen B, Ju Z. Loss of YTHDF2-mediated m(6)A-dependent mRNA clearance facilitates hematopoietic stem cell regeneration. Cell Res. 2018;28(10):1035–1038. doi: 10.1038/s41422-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Z, Qian P, Shao W, Shi H, He XC, Gogol M, Yu Z, Wang Y, Qi M, Zhu Y, Perry JM, Zhang K, Tao F, Zhou K, Hu D, Han Y, Zhao C, Alexander R, Xu H, Chen S, Peak A, Hall K, Peterson M, Perera A, Haug JS, Parmely T, Li H, Shen B, Zeitlinger J, He C, Li L. Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 2018;28(9):904–917. doi: 10.1038/s41422-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mapperley C, van de Lagemaat LN, Lawson H, Tavosanis A, Paris J, Campos J, Wotherspoon D, Durko J, Sarapuu A, Choe J, Ivanova I, Krause DS, von Kriegsheim A, Much C, Morgan M, Gregory RI, Mead AJ, O’Carroll D, Kranc KR. The mRNA m6A reader YTHDF2 suppresses proinflammatory pathways and sustains hematopoietic stem cell function. J Exp Med. 2021;218(3):e20200829. doi: 10.1084/jem.20200829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avgustinova A, Benitah SA. Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol. 2016;17(10):643–658. doi: 10.1038/nrm.2016.76. [DOI] [PubMed] [Google Scholar]
- 87.Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129(12):1577–1585. doi: 10.1182/blood-2016-10-696054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krause DS, Van Etten RA. Right on target: eradicating leukemic stem cells. Trends Mol Med. 2007;13(11):470–481. doi: 10.1016/j.molmed.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, Medeiros JJF, Rao-Bhatia A, Jaciw-Zurakowsky I, Marke R, McLeod JL, Doedens M, Bader G, Voisin V, Xu CJ, McPherson JD, Hudson TJ, Wang JCY, Minden MD, Dick JE. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547(7661):104–108. doi: 10.1038/nature22993. [DOI] [PubMed] [Google Scholar]
- 90.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14(12):769–785. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 91.Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, Lai GM, Chuang SE. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008;268(2):314–324. doi: 10.1016/j.canlet.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, Yang CG. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43(1):373–384. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, Riggs AD, He C, Shi Y. m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18(11):2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang X, Su J, Jeong M, Ko M, Huang Y, Park HJ, Guzman A, Lei Y, Huang YH, Rao A, Li W, Goodell MA. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet. 2016;48(9):1014–1023. doi: 10.1038/ng.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.