Abstract
Humanity has regularly faced the threat of epidemics and pandemics over the course of history. Successful attempts to protect populations were initially made with the development of new vaccines, such as those against plague and cholera, under the leadership of the bacteriologist Waldemar Haffkine. Vaccines have led to a complete eradication of smallpox and bovine plague and a major reduction in other infectious diseases including diphtheria, typhoid fever, poliomyelitis, and Haemophilus influenzae type B meningitis. While a few coronaviruses have been identified that seasonally infect humans causing mild symptoms, the emergence of a new zoonotic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly triggered the ongoing coronavirus disease 2019 (COVID-19) as a global pandemic responsible for widespread mortality. The severe phenotypes of COVID-19 resemble a previous infectious threat that was initially designated as hospital fever and puerperal fever, presently known as sepsis. A SARS-CoV-2 infection has frequently been considered as a form of viral sepsis (owing to common features with bacterial sepsis) but is also associated with an array of specific and unique symptoms. Rapid progress in anti-SARS-CoV-2 vaccine development, in particular, the design of efficient messenger RNA (mRNA) and recombinant adenovirus vaccines, is crucial for curbing the pandemic.
Keywords: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus, Sepsis, Cytokines, Hydroxychloroquine, Vaccines, Pandemics
Introduction
A twenty-first century historic pandemic following many others in the chronology of humanity
The word “pandemic” was used as early as 1674 by the Dutch physician Gideon Harvey (1636–1702) in his work entitled “Morbus anglicus or a theoretick and practical discourse of consumptions and hipochondriack melancholy.” In 1828, Noah Webster included the term in his “American spelling book,” currently known as the Webster dictionary. In 1800, Webster published a book entitled “A brief history of epidemic and pestilential diseases.” Associations between epidemics and pandemics and the countries from which they were alleged to have emerged have been regularly reported, some of which are false. For example, the 1889 “Chinese influenza” was not of Chinese origin but emerged from the Kirghiz steppes of Russia [1], and the famous 1918 “Spanish flu” originated from Kansas, USA (and not from Spain). The first well-described epidemic/pandemic was the Athens plague (430 BCE) recorded by Thucydides, an Athenian historian, who also observed that individuals who survived an earlier plaque during the Peloponnesian war between Sparta and Athens were protected from the subsequent disease, specifically “the same man was never attacked twice — never at least fatally.” The nature of that pandemic is disputed but historians attribute it to typhus. Galen (129-c. CE 216), a Greek physician, described the symptoms and the course of the Antonin plague (166–189) as caused by smallpox. The Justinian plague encompassing the entire Mediterranean Basin (541–542) was the first known pandemic, referred to as bubonic plague. The most lethal plague pandemic was the Black Plague (also known as Pestilence, Black Death; 1331–1352), which led to the deaths of 75–200 million people throughout Europe, Asia, and Africa. On June 20, 1894, Alexandre Yersin (1863–1943), sent by the Institut Pasteur to study a plague epidemic in Hong Kong, identified Yersinia pestis as the causative bacillus.
Cholera also constitutes a historical threat for major cities. John Snow (1813–1858), a British physician, performed the original epidemiological study that led to the identification of a specific water pump in London responsible for the local epidemic. The bacillus Vibrio cholerae was originally discovered in Italy in 1854 by Filippo Pacini (1812–1883), and later it was re-identified in 1884 by Robert Koch (1843–1910) after his travel to Alexandria (Egypt) and during his stay in Calcutta (India) to study local cholera epidemics. Of note, Louis Thuillier (1856–1883), one of the members of the Institut Pasteur research team sent to Alexandria with Émile Roux, Isidore Strauss, and Edmond Nocard to investigate cholera, succumbed to the disease.
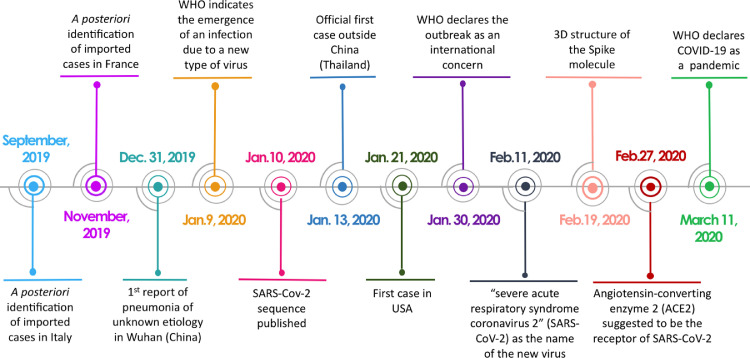
Even though numerous epidemics/pandemics have left a deadly imprint on the history of humankind, the advent of the coronavirus disease 2019 (COVID-19) pandemic was wholly unexpected. Officially, COVID-19 was reported as an outbreak in Wuhan (China) for the first time in December 2019. However, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was apparently circulating in the human population earlier than this time, since imported cases were identified a posteriori in Italy and France in September and November, respectively [Fig. 1] [2,3]. The first case outside China was reported in Thailand (January 13, 2020) and the first case in the USA was reported on January 21, 2020. The genetic sequence of this new SARS-CoV-2 was released early [Fig. 1]. Based on its homology with previously identified SARS-CoV and existing knowledge on SARS-CoV-1, the nature of the SARS-CoV-2 receptor [angiotensin-converting enzyme 2 (ACE2)] was rapidly determined.
Fig. 1.
Timeline of some key events associated with the early period of the COVID-19 pandemic. ACE2: Angiotensin-converting enzyme 2; COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
While an estimated 3204 coronaviruses exist in bats [4], only six have been identified in humans to date, including two alpha coronaviruses (229E and NL63) and two beta coronaviruses (OC43 and HKU1) that trigger common colds. In 2003, a different coronavirus epidemic caused by the SARS-CoV-1 virus emerged in Guangdong, China. Symptoms analogous to the common cold, such as low-grade fever, muscle aches, and dry cough, were the first indicators of infection, frequently followed by unilateral or bilateral pneumonia. A total of 8096 people in 29 countries got SARS and 774 of them died [5]. In 2012, another coronavirus was identified in Saudi Arabia as the cause of the so-called “Middle East respiratory syndrome” (MERS-CoV) associated with a wide range of symptoms, such as mild fever and respiratory signs, upper respiratory tract infection, diarrhea, and weakness to lethargy. Severe cases showed progressive infection of the lungs and respiratory failure, renal failure, and multiple organ failure. A total of 2442 cases were reported, leading to 842 deaths [6].
Sepsis: a recurring disease
The word “sepsis” means putrefaction in Greek. The term septicemia was coined by Pierre Piorry in 1837, and present-day sepsis is recognized as the consequence of dysregulated host response to infection associated with organ failure [7]. In the past, sepsis was mainly reported within two populations: (1) hospitalized pregnant women who frequently died after delivery of so-called puerperal fever [a term coined by Edward Strother (1675–1737) in 1718] and (2) wounded soldiers admitted to field hospitals. These patients would acquire nosocomial fever, nosocomial typhus, or nosocomial gangrene (nosocomial is a Latin word meaning “from the hospital”), later also referred to as putrid fever, hospital gangrene, or hospital fever. Appropriate hygiene as a countermeasure was soon advocated by Ignaz Semmelweis (1818–1865) in 1847 in Vienna and Florence Nightingale (1820–1910) in her hospital ward in the barracks of Scutari (Turkey) in 1854 [8]. In 1850 and 1858, respectively, two physicians were the first to consider puerperal fever and hospital fever as similar entities: James Simpson (1811–1870), President of the Royal College of Physicians of Edinburgh, known for his demonstration of the usefulness of chloroform as an anesthetic, and Armand Trousseau (1801–1867), a member of the French Academy of Medicine, who as a young doctor was confronted with the yellow fever epidemic in Southern France and Gibraltar as well as to a Parisian cholera epidemic. Most of these cases of sepsis were of bacterial origin. Interestingly, the word “virus” was used in early works, given its Latin meaning of poison or venom, and thus by extension, an organic substance capable of transmitting a disease. For example, in 1575, Ambroise Paré (1510–1590), a royal surgeon at the French court, wrote: “The virus will swarm and travel through the veins, artery and nerves to the noble parts as we see the fire along a harquebus rope”. Claude Pouteau (1724–1775), a French surgeon, wrote in 1775 about hospital gangrene: “This irritating fever ignited by a foreign virus.” In 1839, Sir Henry Holland (1788–1873), Queen Victoria's physician (and cousin of Charles Darwin), wrote: “We can consider the animalcules brought by the air or by humans as the source of the disease, in a form non-recognized by our senses, or by any other direct means of research, but nonetheless subject to certain laws of propagation and dissemination similar to those of other species and producing the virus that acts deleterious to the human body.” [9], [10], [11]
Through the centuries, sepsis has killed many famous people, including the daughter of a future pope (Lucrece Borgia), musicians (Lully, Rossini, Manet and Mahler), scientists and physicians (Hertz and Semmelweis), kings and state leaders (Alexander I of Greece, Rainier III of Monaco and Pope John Paul II), sportsmen (Socrates and Mohammad Ali), actors (Christopher Reeve), and explorers (Lord Carnarvon). Unfortunately, even in the twenty-first century, sepsis remains a serious health threat worldwide. In 2017, WHO recognized sepsis as a global health priority [12].
Puerperal fever has been regularly reported as an epidemic. The first officially recorded outbreak occurred at the Hôtel Dieu Hospital in Paris in 1646. In 1795, during an epidemic of puerperal fever in Aberdeen (Scotland), and later in 1842 in Birmingham, Alexander Gordon (1752–1799) and John Thomas Ingleby (1794–1845) independently recognized that the hands of physicians were the origin of contamination, an observation further validated in Boston in 1843 by Oliver W. Holmes (1809–1894). The COVID-19 pandemic shares a common feature with earlier sepsis epidemics/pandemics, in particular, in-hospital spread and acquisition, especially in the early phase. Among the early cases in Wuhan (China), 44% of 179 SARS-CoV-2 infections were hospital-acquired [13]. However, the contamination primarily threatened the attending medical personnel rather than patients. Nevertheless, in a major teaching hospital in London, 15% of COVID-19 inpatient cases between March 2 and April 12, 2020, were either definitely or probably hospital-acquired [14]. In contrast, between March 7 and May 30, 2020, at a large US academic medical center with rigorous infection control measures, nosocomial COVID-19 was very rare during the height of the pandemic in the region [15]. Even after the extreme contagiousness of SARS-CoV-2 was established and protective equipment widely introduced (e.g., face masks), findings by Santé Publique France on 2328 clusters during September 14–20, 2020, revealed that 9.8% of COVID-19 infections were acquired in health institutions and 13.0% in nursing homes [16].
Severe COVID-19: a viral sepsis?
Similarities and differences between COVID-19 and bacterial sepsis
A direct inference between sepsis and severe COVID-19 has been made, although limited information is available about this new global disease. This emerging concept advocates that the most severe cases of SARS-CoV-2 infection could be considered as a form of viral sepsis [17], [18], [19]. However, this alleged similarity appears to be too hastily made, and, in many instances, unjustified. Based on present reports, one of the most striking incongruities is an association of SARS-CoV-2 infection with a cytokine storm. Comprehensive analysis of the reported absolute values of circulating cytokines in COVID-19 patients and their comparison with other critical care conditions revealed a discrepancy in the cytokine storm theory [20], [21], [22], [23]. Several comparative studies have focused on this issue. The groups of Pickkers and Deutschman compared circulating cytokines in COVID-19 vs. acute respiratory distress syndrome (ARDS) patients, sepsis patients with or without ARDS, trauma patients, and those with out-of-hospital cardiac arrest [24,25]. Considering the bloodstream readouts, a “cytokine drizzle” rather than the storm was characteristic in the most severe COVID-19 cases. In contrast, a major cytokine storm appeared to occur within the lungs. A transcriptome of bronchoalveolar lavage cells suggested a robust innate immune response, chemokine-dominant hypercytokinemia, and interferon-inducible gene (ISG) expression, exhibiting pathogenic potential with overrepresentation of genes involved in inflammation [26,27]. The above results are suggestive of the so-called compartmentalization [28] of the cytokine expression profile, in turn, implying that excessive pro-inflammatory cytokine release, albeit at defined locations and not necessarily systemic, may be a hallmark of severe COVID-19. Notably, major coagulopathy/endotheliopathy featuring disseminated intravascular coagulation and thrombocytopenia have been frequently associated with the most severe cases of sepsis [29] and the lungs are the most common organ to fail first in sepsis patients [30]. Interestingly, acute lung injury (ALI) and ARDS constitute common complications of sepsis [31]. In severe COVID-19 cases, lung disease is specifically characterized by diffuse alveolar damage with perivascular T-cell infiltration, severe pulmonary vascular endothelialitis, widespread thrombosis with microangiopathy, and peculiar intussusceptive angiogenesis [32]. Other similarities between bacterial sepsis and COVID-19 include the occurrence of severe lymphopenia [33] and apoptosis of splenic and lymph node cells [34]. While major reduction of Human Leukocyte Antigen – DR (HLA-DR) expression in circulating monocytes is detected in COVID-19 patients similar to bacterial sepsis [35], the reduced capacity of monocytes to produce inflammatory cytokines (after ex vivo stimulation) typically observed in sepsis patients [36] does not seem to occur in COVID-19 infection [37,38]. However, ex vivo cytokine production by activated T-cells is strongly reduced in COVID-19 patients [23].
Another common feature is the ambiguous role of type I interferon. The yin-yang property of the type I interferon cytokines is a signaling hallmark in most bacterial infections [39] and their detrimental role is widely recognized [40]. A study by Wang et al. [41] elegantly demonstrated that in COVID-19 cases, an early administration of interferon-α2b was associated with a reduction in hospital mortality. Conversely, late interferon treatment increased mortality and delayed recovery, suggesting that the timing of therapeutic interferon application is crucial. The difficulty in addressing the role of interferon in COVID-19 is a consequence of the capacity of the causative virus to prevent its induction and effects in the infected host.
In the context of characterizing disease-specific long-term effects, the occurrence of post-intensive care syndrome (PICS) in both sepsis and COVID-19 survivors presents a significant confounding factor. While new data regarding COVID-19 patients are emerging, the existing evidence reveals numerous respiratory, muscular, neurologic, and intellectual disorders associated with fatigue and pulmonary fibrosis [42], the majority of which have also been reported in recovering sepsis patients. Moreover, due to the COVID-19-induced lockdowns introduced in numerous countries, psychiatric disorders with wide-ranging effects have become an increasing concern in patients post-infection and, in many cases, non-infected individuals subjected to lockdown conditions.
From pathophysiology to novel treatment options
Despite exhaustive efforts to develop effective treatments against bacterial sepsis based on evolving understanding of the pathophysiology of the syndromes, no new strategies (excluding antimicrobials) have emerged over the last 30 years. Although numerous positive preclinical assays have been published, it appears increasingly clear that the hunt for the “magic bullet” is futile [43]. Surprisingly, observations in the field of sepsis over 30 years have been practically reproduced within a single year for COVID-19; in particular, an emergence of numerous attempts at rapid treatments, which, in many instances have been poorly (and often only theoretically) justified and follow the same over-simplistic paradigms that ultimately failed to develop effective treatments against bacterial sepsis. A key explanation could be strong multitier synergy existing between multiple individual molecular and cellular factors that leave limited room for a drug/intervention that would induce benefits by targeting only a single selected element of the complex synergistic puzzle. Recently, researchers investigating COVID-19 have rediscovered some elements of that synergy (known for almost three decades), re-publishing virtually the same phenomena in prestigious journals (and without referencing the original seminal findings). For example, a key role of anaphylatoxin C5a as an amplifier of the septic inflammatory response [[44] has been reported in COVID-19 [45]. Similarly, a deleterious synergy between tumor necrosis factor (TNF) and interferon (IFN)-γ , originally reported to explain lethality in response to endotoxin [46], was also shown in SARS-CoV-2 infection [47].
Clearly, for bacterial sepsis originating from virtually any pathogen, the idea of vaccination as a remedy could be perceived as utopian. However, attempts have been made to develop an anti-endotoxin vaccine targeting the common structure shared by Gram-negative bacteria [48]. A recombinant human antibody binding the common lipid A moiety of endotoxin failed to neutralize endotoxin [49]. In contrast, the development of vaccines against Neisseria meningitidis, one of the most common causes of meningococcal sepsis, resulted in a significant reduction of meningococcal serogroup C disease and its spread across Europe. Since vaccines are now available for five out of six meningococcal disease-causing serogroups (A, B, C, W, and Y), vaccination programs will induce broad protection leading to effective control of future meningococcal outbreaks [50]. Similarly, after the introduction of the Haemophilus influenzae type B meningitis vaccine, a significant decrease in the incidence and mortality rates of the disease across all age groups was evident [51].
COVID-19 vaccine development: new and old approaches
Historical success of vaccines
On May 14, 1796, Edward Jenner vaccinated an 8-year-old boy, James Phipps, against smallpox, unknowingly initiating the most successful quest in combating infectious diseases. Taking the pus from a sore on the hand of Sarah Nelmes, a dairymaid, he applied it to the arm of the boy using two superficial incisions. Jenner submitted a manuscript describing the procedure and its justification to the Royal Society in London, which was returned without presentation. This was the fate of the first report on the attempt to prevent the occurrence of smallpox, which finally led to complete eradication of the disease as announced by WHO on October 26, 1979. Another vaccination success of equal proportions was that for cattle rinderpest, which was officially declared eradicated by the Food and Agriculture Organization (FAO) in 2011. On August 25, 2020, WHO reported that poliomyelitis has been eliminated from the African continent and currently remains prevalent only in three Asian countries. Between 1880 and 1885, Louis Pasteur developed four vaccines against fowl cholera, anthrax, swine erysipelas, and rabies in France. At that time, different approaches were proposed for vaccine preparation. For example, Henri Toussaint (1847–1890) suggested antiseptic-treated vaccines based on phenol, while Louis Pasteur (1822–1895) favored air-attenuated vaccines. In fact, without purposely disclosing it (to avoid officially endorsing Toussaint's approach), Pasteur used a similar chemical method (potassium dichromate) during a public demonstration of the efficiency of his vaccine in preventing anthrax. In 1881, at the International Medical Congress of London, Pasteur declared: “I have given the term vaccination a broad meaning. I hope science will dedicate it as a tribute to the merit and vast favor rendered by one of England's greatest men, your Jenner. What a joy for me to glorify this immortal name on the very ground of the noble and hospitable city of London.” [52] The marked reduction in the incidence of many prevalent diseases during this time, such as typhoid fever and diphtheria, further illustrated the success of vaccination.
Vaccines have been extensively investigated as a means to prevent several epidemics/pandemics. Jaume Ferran (1852–1929), a Spanish physician, claimed to have developed a vaccine against cholera, but an investigation team sent to Spain headed by Dr. Paul Brouardel (1837–1906) returned with a negative conclusion of its efficacy. The group stated “The more closely the problems concern human life, the more the scientific method must be perfect, the more the scientist must be armed. M. Ferran seems not to have understood the importance of these truths, and to have abandoned the field of scientific experiments and studies to enter too early into what he calls ‘practice’.” [53]. Unfortunately, this conclusion appears pertinent to the current pandemic, as evident from the irresponsible behavior of some medical professionals and politicians in recommending the use of certain drugs without reliable evidence of their efficacy and safety. Astonishingly, the paper alleging the benefits of hydroxychloroquine (HCQ) in COVID-19 patients submitted on March 16, 2020, was accepted only 1 day later by the International Journal of Antimicrobial Agents (IJAA), whose Editor-in-Chief works at the same institution and has a collaborative relationship with the senior author (D. Raoult). Subsequently, the publisher requested an a posteriori review of the manuscript (published in July 2020) that was conducted by Professor Frits Rosendaal, chairman of the Department of Clinical Epidemiology at Leiden University [54]. The evaluation identified 10 major flaws in the study. A retraction was subsequently requested by the publisher but declined by the senior author. In the same July IJAA issue, a similar independent criticism was published [55]. Since this time, many well-powered clinical trials (e.g., RECOVERY) and meta-analyses have objectively demonstrated the absence of any HCQ benefits [56], [57] and emerging findings further revealed that the combination of HCQ and azithromycin significantly increased mortality. Interestingly, quinine, another anti-malarial drug, was also proposed as a putative cure for the 1918 Spanish flu pandemic.
A key figure in the development of vaccines against nineteenth - to twentieth-century epidemics/pandemics was Waldemar Haffkine (1860–1930; Fig. 2) [58], a Russian physician who joined Elie Metchnikoff's team at Institut Pasteur in 1890. Two years later, Haffkine developed a vaccine that he tested on himself and three other volunteers. Ernest H. Hankin (1865–1939) [59], a British bacteriologist who would later discover the bactericidal properties of Ganges water on cholera bacillus (initiating the discovery of bacteriophages), attested to the efficacy of vaccination. After having developed a vaccine against cholera, Haffkine was sent to Calcutta and initiated a vaccination campaign (1893–1894) in several locations (Danapur, Lucknow, Agra, and Bombay). By July 1895, he had vaccinated >42,000 people, including British and Indian troops as well as civilians. In Germany, Drs. Koch, Pfeiffer and Kolle demonstrated the bactericidal power of serum collected from subjects inoculated with the Haffkine vaccine. An epidemic of the plague emerged in 1896 in Bombay, resulting in the deaths of 170,000 people by 1906. Haffkine [60] went back to India in 1897 and offered his anti-plague vaccine that was produced in the Plague Research Laboratory in Bombay. Unfortunately, in 1902, the vaccine program underwent a serious setback in the Mulkowal disaster (attributed to a tetanus infection) that led to an inquiry after the death of 19 vaccinated people. Haffkine was dismissed until an investigation published in The Lancet on February 2, 1907, cleared him of any responsibility and error. The tetanus infections were linked to a vial that had been dropped on the soil containing spores of Clostridium tetani and improperly disinfected before further use. The above event clearly illustrates the importance of sustained rigorous practices in a pandemic environment despite the urgency of required action, since any consequent errors are typically widely disseminated and markedly erode public confidence.
Fig. 2.

Waldemar Haffkine (1860–1930). Physician and scientist born in Odessa who studied zoology at the University of Odessa under the supervision of Elie Metchnikoff. Haffkine joined the Parisian laboratory of Metchnikoff at Institut Pasteur in 1890 and developed the first effective cholera vaccine. In 1893–1894, he was offered the possibility to organize a cholera vaccination campaign in various regions of India where epidemics of cholera were prevalent by Lord Dufferin (Viceroy of India from 1884 to 1888 and Ambassador of Great Britain in Paris). Back in India in 1897, Haffkine produced a plague vaccine composed of killed germs to combat the bubonic plague epidemic. Institut Pasteur ©.
Antibodies: friends or foes?
The aim of vaccines is to induce adaptive immunity, which involves (1) a cellular arm: antigen-presenting cells T-lymphocytes (Th1, Th2, and T-cytotoxic) and B-lymphocytes and (2) production of antibodies that neutralize the invading agent and facilitate its elimination. Accordingly, natural antibodies found in post-infectious convalescent patients could be used to protect newly infected patients. A study performed on 39 patients with severe and potentially lethal COVID-19 reported a significant protective effect (25% vs. 60% mortality in the placebo group) [61]. Similarly, in a study of 160 patients who underwent randomization, early administration of high-titer convalescent plasma against SARS-CoV-2 to infected older adults within 72 h after the onset of mild COVID-19 symptoms reduced disease progression [62]. However, an investigation on 464 adults with moderate COVID-19 showed no benefits of convalescent plasma [63]. Another study including hospitalized adult patients with severe COVID-19-induced pneumonia assigned to receive convalescent plasma (n = 208) or placebo (n = 105) also revealed no differences in clinical status or overall mortality between the groups [64]. The most recent finding from the multicenter randomized trials (RECOVERY) re-confirmed its inefficacy [118]. Convalescent plasma should be hypothetically standardized based not on titer but its virus neutralization capacity; in other words, qualitative not quantitative standardization should be advocated for any possible future testing. In the above context, patients with the most severe infection groups were shown to have anti-type I interferon antibodies [65]. The presence of such detrimental antibodies in transferred plasma should be avoided. Following treatment with a recombinant human antibody (Bamlanivimab®; LY-CoV555), a lower number of patients required hospitalization, specifically (5/30; 1.6%), compared with placebo (9/143; 6.3%) [66]. However, the monoclonal antibody coadministered with remdesivir did not demonstrate efficacy in 162 hospitalized patients who had COVID-19 without end-organ failure compared with placebo (n = 151) [67]. The issue of whether the increasing frequency of neutralization-resistant variants could have undermined the efficacy of Bamlanivimab® in this trial remains to be established. Interestingly, two agammaglobulinemia patients who developed COVID-19-associated interstitial pneumonia did not require oxygen ventilation or intensive care. Another study suggested that humoral immunity may not be a prerequisite to combat COVID-19. No serious complications were observed in 60 SARS-CoV-2-positive patients undergoing anti-CD20 antibody treatment [68]. These results suggest that T-cell-dependent immunity may suffice in the absence of anti-SARS-CoV-2 antibodies.
The use of poly-specific antibodies present in intravenous immunoglobulin (IVIG) preparations has also been proposed to treat bacterial sepsis. A recent meta-analysis suggested that IVIG treatment reduces the all-cause mortality of patients with sepsis, but the study showed significant heterogeneity [69]. Enriched preparations with IgM have also been investigated but the available evidence is too ambiguous to support the widespread use of intravenous IgM for the treatment of sepsis [70]. Furthermore, IVIG with enhanced polyspecificity may have clinical potential in sepsis and related systemic inflammatory syndromes [71].
Several other questions regarding antibodies need to be addressed. (1) How long does humoral immunity last? Owing to the limited observation period for SARS-CoV-2 infections, this question cannot as yet be precisely answered. (2) Are antibodies fully protective or could they exert adverse effects? The phenomenon, known as “antibody-dependent enhancement,” could contribute to further dissemination of the virus by favoring its entry into cells devoid of ACE2 but harboring Fcγ receptors (e.g., macrophages) [72]. Notably, this type of antibody-dependent infection mediated by antibodies against spike proteins has been reported for SARS-CoV-1 [73]. This finding is particularly prevalent in dengue virus infection and has confounded vaccine development research in this area. Another potentially detrimental effect is the capacity of antibodies to activate the release of pro-inflammatory cytokines. The non-neutralizing antibodies form immune complexes with viral antigens, resulting in the release of pro-inflammatory cytokines, immune cell recruitment, and activation of the complement within tissues [74]. Additionally, patients with severe COVID-19 produce a unique serologic signature, including an increase in IgG1 with afucosylated Fc glycans (on asparagine #297). Afucosylated IgG responses, in turn, enhance the production of inflammatory cytokines by monocytes, including interleukin (IL)−1β, IL-6, and TNF [75].
Long-lasting and cross-reactive T-cell immunity
Robust T-cell immunity has been reported in convalescent individuals with asymptomatic and mild COVID-19 [76]. Interestingly, this T-cell reactivity has also been detected in some healthy blood donors, suggesting the existence of some cross-reactivity with previous ordinary circulating coronavirus. In an earlier study, circulating SARS-CoV-2-specific CD8+ and CD4+ T-cells were identified in approximately 70% and 100% COVID-19 convalescent patients, respectively, and CD4+ T-cell responses to spike, the main vaccine-related target, were robust and correlated with the magnitude of anti-SARS-CoV-2 antibody levels [77]. Interestingly, the frequency of SARS-CoV-2-specific T-cells was similar between asymptomatic and symptomatic individuals but the former showed increased IFN-γ and IL-2 production. Thus, asymptomatic SARS-CoV-2-infected individuals were not characterized by weak antiviral immunity, and in fact, mounted a highly functional virus-specific T-cell immune response [78]. T-cell responses recognize at least 30–40 epitopes in each donor and immunodominant regions for CD4+ T-cells have minimal overlap with antibody epitopes [79]. Owing to the broad T-cell repertoire, viral escape of T-cell immunity should be unlikely, which is an important assumption, particularly in view of the rapid emergence of multiple SARS-CoV-2 variants. Unfortunately, a recent investigation on the South African B.1.351 variant failed to show protection by the recombinant adenovirus AstraZeneca vaccine against mild to moderate COVID-19. However, vaccine efficacy against severe COVID-19 was not determined [80]. While the contribution and induction of specific Th1 cells and cytotoxic T-cells in response to the vaccines are highly expected, induction of Th2 cells that could favor a Th2-type immunopathology with prominent eosinophil infiltration should be avoided [81].
Challenges
As of April 2021, 311 vaccine candidates had been developed against SARS-CoV-2 (84 tested in clinical trials and 227 under preclinical investigation) [82]. Multiple approaches for vaccine mass production rely on existing developmental blueprints using inactivated full virus, attenuated virus, virus-like particles, and protein subunits. New methodologies with non-commercialized vaccines employing DNA, viral vectors, and messenger RNA (mRNA) encapsulated in lipid nanoparticles have led to outstanding results in humans and animal models [117], suggesting that the different vaccine types can facilitate the effective generation of protective antibodies and T-cell-dependent immunity [83,84]. mRNA vaccines have been investigated for >30 years [85]. In vivo induction of virus-specific cytotoxic T lymphocytes by liposome-entrapped mRNA was achieved in 1993 [86]. Katalin Kariko, a Hungarian-born researcher working at the University of Pennsylvania, is considered by some as Nobel Prize-worthy for rendering this approach feasible. Dr. Kariko proposed the use of 1-methylpseudouridine to avoid activation of Toll-like receptors (TLR)7 and TLR8 by uridine [87,88]. Furthermore, uridine-containing mRNA induced interferon, which is counterproductive during vaccination, preventing the selection of high-affinity antibodies. Dr. Kariko also purified the mRNA to remove dsRNA that activates TLR3, retaining the lipid nanoparticles with an adjuvant effect. Incorporation of non-inflammatory, modified nucleosides in mRNA is required for the production of large amounts of antigen and robust immune responses following the activation of T follicular helper cells, germinal center B-cells [89], and appropriate activation of dendritic cells [86]. mRNA coding for spike protein of SARS-CoV-2 has been designed for COVID-19 vaccines and preclinical and phase III studies have confirmed the significant efficiency of this approach within a very short period [90], [91], [92], [93], [94], [95]. Other efficient vaccines (developed in <1 year) have been highlighted, such as the use of recombinant adenovirus coding for spike protein [96], [97], [98], [99], [100], [101], [102], whole virion vaccine [103], purified spike protein [104], and recombinant measles virus expressing the SARS-CoV-2 spike protein [105].
Currently, vaccines are administered through the classical intramuscular route, but an intranasal route would be of significant interest given the ease of application and significant role of IgA and mucosal immunity. Indeed, IgAs are predominantly involved in the early neutralizing antibody response to SARS-CoV-2 [106]. Intranasal vaccination with a lentiviral vector has been shown to confer strong protection against SARS-CoV-2 in mouse and golden hamsters [107].
The bacilli Calmette–Guérin (BCG) vaccine, set up by the two Pasteurian researchers working in Lille in 1921–1924 to prevent tuberculosis, has been recommended to boost the immune status of the population during the COVID-19 pandemic based on epidemiological analyses of COVID-19 propensity. Upon examination of different countries with varying vaccination policies, it was initially proposed that BCG exposure could contribute to attenuation of the spread and severity of the COVID-19 pandemic [108]. Further investigations suggested that BCG vaccination is associated with a decrease in the incidence of sickness induced by COVID-19 [109]. However, other groups reported no protective effect in middle-aged individuals administered the BCG vaccine at birth against SARS-CoV-2 [110] and the most recent epidemiological findings questioned the claim of BCG-induced protection, demonstrating a possible arbitrary selection bias in the analyses [111,112]. At least 11 trials including over 16,000 patients have been registered to examine the efficacy of BCG-dependent protection against SARS-CoV-2 infection. While non-specific boosting of the immune system to combat viral infection (associated with the concept of trained innate immunity) remains an interesting theory, this approach needs reliable validation.
As in the case of anti-COVID-19 therapeutics, the development, registration, and commercialization/distribution processes of anti-SARS-CoV-2 vaccines are not free from political pressures, international turf wars, and various conspiracy theories. While these issues may never be fully eliminated, the best available remedy to overcome and dispel such impediments lies in maximal reliance on high-quality evidence-based science and transparency of all proceedings.
Conclusions
The SARS-CoV-2 pandemic emerged in late 2019. No effective drugs are immediately available, resulting in various non-pharmaceutical protective intervention attempts. Face masks are currently advocated worldwide as a protective approach while waiting for herd immunity to emerge. Charles Delorme (1584–1678), a graduate from the University of Montpellier, moved to Paris to practice medicine and became the chief physician to three consecutive French kings (Henri IV, Louis XIII and Louis XIV). In 1619, following the eruption of the bubonic plague in Paris, Delorme designed a “plague preventive costume” featuring a famous beak-like-shaped nose filled with perfume and herbs and eyes protected by spectacles. This protection was worn by doctors in France and Italy until the Great Plague of Marseille in 1720. In 1870, John Tyndall (1820–1893) [113], an Irish physician and great supporter of the germ theory of Louis Pasteur, proposed breathing through cotton wool to filter the air and protect against infections. Subsequently, the use of surgical masks was recommended in 1897 by Paul Berger (1845–1908), a French surgeon, and the following year by Jan Mikulicz-Radecki (1850–1905), a Polish surgeon. In the current day, community-wide mask usage contributes to the control of COVID-19 by reducing the emission level of infected respiratory droplets from individuals with subclinical and mild COVID-19 [114]. Despite the well-recognized protective role of face masks, both doubts fueled by deniers and their transient shortage have delayed the early wide implementation of this effective tool, particularly in many Western countries. For example, in France, the failure by the Director of Health to renew supplies of masks following their expiration resulted in a major country-wide shortage. This led to an ambiguous and misleading informational policy by the French authorities regarding the usefulness of wearing masks concurrent with a frantic hunt for personal protective equipment by medical staff during the first wave of the pandemic.
Virtually all countries have collectively devoted vast financial resources to promote research aimed at elucidating the characteristics of this deadly new disease, identifying new anti-viral drugs, and developing innovative vaccine approaches. The scientific community has robustly, and in many instances, cooperatively risen to these challenges. Open access to all COVID-19-related publications and mostly unimpeded data sharing among research centers attest to the global cooperative efforts. While several errors have occurred in the process, the speed and the overall quality of research advances are unprecedented.
In contrast to the other fields, research on vaccines is essentially performed by pharmaceutical companies, either alone (66%) or in collaboration with academic centers (21%) [115]. A number of the proposed approaches are classical vaccine strategies that are already employed for many anti-viral vaccines. Others are new approaches with no commercialized examples among human vaccines, although some already exist in veterinary medicine. The lack of data may have an impact on the attitude of the general population. Reluctance toward the use of anti-SARS-CoV-2 vaccines appears to be on the rise [116] but strongly varies across countries. For example, the Chinese population has a very high level of confidence while in France, the motherland of Louis Pasteur, acceptance of anti-SARS-CoV-2 vaccination is relatively low. Given the considerable existing political influence of various governments and/or public officials, often reverting to nationalistic overtones for self-serving goals, it is appropriate to recall the famous statement by Louis Pasteur: “Science has no homeland, because knowledge belongs to humanity, the torch that lights the world.” The current and future epidemics will be more effectively and rapidly overcome with smaller human and economic losses if the above counsel is followed worldwide.
Conflicts of Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Managing Editor: Jingling Bao
References
- 1.Clemow F. The recent pandemic of influenza: its place of origin and mode of spread. Lancet. 1894;143:329–331. doi: 10.1016/S0140-6736(01)66317-7. [DOI] [Google Scholar]
- 2.Apolone G., Montomoli E., Manenti A., Boeri M., Sabia F., Hyseni I., et al. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy. Tumori. 2020 doi: 10.1177/0300891620974755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrat F., Figoni J., Henny J., Desenclos J.C., Kab S., de Lamballerie X., et al. Evidence of early circulation of SARS-CoV-2 in France: findings from the population-based “CONSTANCES” cohort. Eur J Epidemiol. 2021;36(2):219–222. doi: 10.1007/s10654-020-00716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H., et al. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1) doi: 10.1093/ve/vex012. vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. SARS (10 Years After). Available from: https://www.cdc.gov/dotw/sars/index.html.
- 6.Donnelly C.A., Malik M.R., Elkholy A., Cauchemez S., Van Kerkhove M.D. Worldwide reduction in MERS cases and deaths since 2016. Emerg Infect Dis. 2019;25(9):1758–1760. doi: 10.3201/eid2509.190143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavaillon J.M., Chrétien F. From septicemia to sepsis 3.0 – from Ignaz Semmelweis to Louis Pasteur. Microbes Infect. 2019;21(5–6):213–221. doi: 10.1016/j.micinf.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Paré A. Gabriel Buon; Paris: 1585. Le 19e livre traitant de la grosse vérole. in Œuvres (4e éd. [Google Scholar]
- 10.Pouteau C. Oeuvres posthumes. Paris: imprimerie Ph.D. Pierres; 1783.
- 11.Holland H. Medical Notes and Reflections. 1839 [Google Scholar]
- 12.Reinhart K., Daniels R., Kissoon N., Machado F.R., Schachter R.D., Finfer S. Recognizing sepsis as a global health priority – a WHO resolution. N Engl J Med. 2017;377(5):414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X., et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8(10):629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickman H.M., Rampling T., Shaw K., Martinez-Garcia G., Hail L., Coen P., et al. Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2021;72(4):690–693. doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C., Baker M., Vaidya V., Tucker R., Resnick A., Morris C.A., et al. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santé Publique France. COVID-19 Point épidémiologique hebdomadaire du 24 Septembre 2020. Available from: https://www.google.fr/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiE_Oqg36XwAhVFyxoKHcEmBlUQFjABegQIAxAD&url=https%3A%2F%2Fwww.santepubliquefrance.fr%2Fcontent%2Fdownload%2F283689%2F2742445&usg=AOvVaw33F4jcy1ytXwk0LsIcnt9M.
- 17.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H.Y. The severe COVID-19: a sepsis induced by viral infection? And its immunomodulatory therapy. Chin J Traumatol. 2020;23:190–195. doi: 10.1016/j.cjtee.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colantuoni A., Martini R., Caprari P., Ballestri M., Capecchi P.L., Gnasso A., et al. COVID-19 sepsis and microcirculation dysfunction. Front Physiol. 2020;11:747. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osuchowski M.F., Aletti F., Cavaillon J.M., Flohé S.B., Giamarellos-Bourboulis E.J., Huber-Lang M., et al. SARS-CoV-2/COVID-19: evolving reality, global response, knowledge gaps, and opportunities. Shock. 2020;54(4):416–437. doi: 10.1097/SHK.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osuchowski M.F., Winkler M.S., Skirecki T., Cajander S., Shankar-Hari M., Lachmann G., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha P., Matthay M.A., Calfee C.S. Is a "Cytokine Storm" relevant to COVID-19. JAMA Intern Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 23.Remy K.E., Mazer M., Striker D.A., Ellebedy A.H., Walton A.H., Unsinger J., et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kox M., Waalders N., Kooistra E.J., Gerretsen J., Pickkers P. Cytokine levels in critically Ill patients with COVID-19 and other conditions. JAMA. 2020;324(15):1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890. doi: 10.1016/j.chom.2020.04.017. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavaillon J.M., Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12(3):151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 29.Iba T., Levi M., Levy J.H. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Semin Thromb Hemost. 2020;46(1):89–95. doi: 10.1055/s-0039-1694995. [DOI] [PubMed] [Google Scholar]
- 30.Bastarache J.A., Ware L.B., Bernard G.R. The role of the coagulation cascade in the continuum of sepsis and acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):365–376. doi: 10.1055/s-2006-948290. [DOI] [PubMed] [Google Scholar]
- 31.Sadowitz B., Roy S., Gatto L.A., Habashi N., Nieman G. Lung injury induced by sepsis: lessons learned from large animal models and future directions for treatment. Expert Rev Anti Infect Ther. 2011;9(12):1169–1178. doi: 10.1586/eri.11.141. [DOI] [PubMed] [Google Scholar]
- 32.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) directly decimates human spleens and lymph nodes. MedRxiv. 2021 Available from: https://www.medrxiv.org/content/10.1101/2020.03.27.20045427v1. [Google Scholar]
- 35.Monneret G., Finck M.E., Venet F., Debard A.L., Bohé J., Bienvenu J., et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95(2):193–198. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Munoz C., Carlet J., Fitting C., Misset B., Blériot J.P., Cavaillon J.M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88(5):1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benlyamani I., Venet F., Coudereau R., Gossez M., Monneret G. Monocyte HLA-DR measurement by flow cytometry in COVID-19 patients: an interim review. Cytometry A. 2020;97(12):1217–1221. doi: 10.1002/cyto.a.24249. [DOI] [PubMed] [Google Scholar]
- 38.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decker T., Müller M., Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5(9):675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 40.Stifter S.A., Feng C.G. Interfering with immunity: detrimental role of type I IFNs during infection. J Immunol. 2015;194(6):2455–2465. doi: 10.4049/jimmunol.1402794. [DOI] [PubMed] [Google Scholar]
- 41.Wang N., Zhan Y., Zhu L., Hou Z., Liu F., Song P., et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28(3):455–464. doi: 10.1016/j.chom.2020.07.005. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamal M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2020:e13746. doi: 10.1111/ijcp.13746. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavaillon J.M., Singer M., Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. 2020;12(4):e10128. doi: 10.15252/emmm.201810128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czermak B.J., Sarma V., Pierson C.L., Warner R.L., Huber-Lang M., Bless N.M., et al. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5(7):788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 45.Carvelli J., Demaria O., Vély F., Batista L., Chouaki Benmansour N., Fares J., et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588(7836):146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doherty G.M., Lange J.R., Langstein H.N., HR Alexander, Buresh C.M., Norton J.A. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J Immunol. 1992;149(5):1666–1670. [PubMed] [Google Scholar]
- 47.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149–168. doi: 10.1016/j.cell.2020.11.025. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cross A.S., Opal S., Cook P., Drabick J., Bhattacharjee A. Development of an anti-core lipopolysaccharide vaccine for the prevention and treatment of sepsis. Vaccine. 2004;22(7):812–817. doi: 10.1016/j.vaccine.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Warren H.S., Amato S.F., Fitting C., Black K.M., Loiselle P.M., Pasternack M.S., et al. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med. 1993;177(1):89–97. doi: 10.1084/jem.177.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pizza M., Bekkat-Berkani R., Rappuoli R. Vaccines against Meningococcal Diseases. Microorganisms. 2020;8(10):1521. doi: 10.3390/microorganisms8101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peltola H. Worldwide Haemophilus influenzae type B disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13(2):302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oeuvres de Pasteur. (Tome 6) réunies par Pasteur Vallery-Radot. Paris: Masson et Cie; 1933.
- 53.Brouardel P., Charrin A., Albarran J. Masson Ed; Paris: 1885. Rapport sur les essais de vaccination cholérique entrepris en espagne par M. le docteur ferran. [Google Scholar]
- 54.Rosendaal F.R. Review of: “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Gautret et al. 2010. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. 10.1016/j.ijantimicag.2020.106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machiels J.D., Bleeker-Rovers C.P., Ter Heine R., Rahamat-Langendoen J., de Mast Q., Ten Oever J., et al. Reply to Gautret et al.: hydroxychloroquine sulfate and azithromycin for COVID-19: what is the evidence and what are the risks. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(1):19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebina-Shibuya R., Namkoong H., Horita N., Kato H., Hara Y., Kobayashi N., et al. Hydroxychloroquine and chloroquine for treatment of coronavirus disease 19 (COVID-19): a systematic review and meta-analysis of randomized and non-randomized controlled trials. J Thorac Dis. 2021;13(1):202–212. doi: 10.21037/jtd-20-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haffkine W.M. Inoculation de vaccins anticholériques à l'homme. CR Soc Biol. 1892;4:740–741. [Google Scholar]
- 59.Hankin E.H. Remarks on Haffkine's method of protective inoculation against cholera. Br Med J. 1892;2:569–571. doi: 10.1136/bmj.2.1654.569. 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haffkine W.M. Remarks on the plague prophylactic fluid. Br Med J. 1897;1:1461–1462. 1902. [Google Scholar]
- 61.Liu S., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F., et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 62.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., et al. Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lundgren J.D., Grund B., Barkauskas C.E., Holland T.L., Gottlieb R.L., Sandkovsky U., et al. A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2021;384(10):905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montero-Escribano P., Matías-Guiu J., Gómez-Iglesias P., Porta-Etessam J., Pytel V., Matias-Guiu J.A. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord. 2020;42 doi: 10.1016/j.msard.2020.102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y., Yu X., Zhang F., Xia Y. Evaluation of the effect of intravenous immunoglobulin dosing on mortality in patients with sepsis: a network meta-analysis. Clin Ther. 2019;41(9):1823–1838. doi: 10.1016/j.clinthera.2019.06.010. e4. [DOI] [PubMed] [Google Scholar]
- 70.Cui J., Wei X., Lv H., Li Y., Li P., Chen Z., et al. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: a meta-analysis with trial sequential analysis. Ann Intensive Care. 2019;9(1):27. doi: 10.1186/s13613-019-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Djoumerska-Alexieva I., Roumenina L., Pashov A., Dimitrov J., Hadzhieva M., Lindig S., et al. Intravenous immunoglobulin with enhanced polyspecificity improves survival in experimental sepsis and aseptic systemic inflammatory response syndromes. Mol Med. 2016;21(1):1002–1010. doi: 10.2119/molmed.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20(6):339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang S.F., Tseng S.P., Yen C.H., Yang J.Y., Tsao C.H., Shen C.W., et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PubMed] [Google Scholar]
- 75.Chakraborty S., Gonzalez J., Edwards K., Mallajosyula V., Buzzanco A.S., Sherwood R., et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021;22(1):67–73. doi: 10.1038/s41590-020-00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Bert N., Clapham H.E., Tan A.T., Chia W.N., Tham C., Lim J.M., et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218(5) doi: 10.1084/jem.20202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tarke A., Sidney J., Kidd C.K., Dan J.M., Ramirez S.I., Yu E.D., et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep Med. 2021;2(2) doi: 10.1016/j.xcrm.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2102214. NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7(4):e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Available from: https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/. Accessed on.
- 83.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 84.Liu X., Liu C., Liu G., Luo W., Xia N. COVID-19: progress in diagnostics, therapy and vaccination. Theranostics. 2020;10(17):7821–7835. doi: 10.7150/thno.47987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics – Developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 86.Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.G., et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23(7):1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 87.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni H., Capodici J., Cannon G., Communi D., Boeynaems J.M., Karikó K., et al. Extracellular mRNA induces dendritic cell activation by stimulating tumor necrosis factor-alpha secretion and signaling through a nucleotide receptor. J Biol Chem. 2002;277(15):12689–12696. doi: 10.1074/jbc.M110729200. [DOI] [PubMed] [Google Scholar]
- 91.Laczkó D., Hogan M.J., Toulmin S.A., Hicks P., Lederer K., Gaudette B.T., et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity. 2020;53(4):724–732. doi: 10.1016/j.immuni.2020.07.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 93.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Voysey M., Clemens S., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):47988. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J., Wang W., Chen Z., Lu S., Yang F., Bi Z., et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586(7830):572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 105.Hörner C., Schürmann C., Auste A., Ebenig A., Muraleedharan S., Dinnon K.H., 3rd, et al. A highly immunogenic and effective measles virus-based Th1-biased COVID-19 vaccine. Proc Natl Acad Sci U S A. 2020;117(51):32657–32666. doi: 10.1073/pnas.2014468117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ku M.W., Bourgine M., Authié P., Lopez J., Nemirov K., Moncoq F., et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29(2):236–249. doi: 10.1016/j.chom.2020.12.010. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klinger D., Blass I., Rappoport N., Linial M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: a multivariable analysis. Vaccines (Basel) 2020;8(3):378. doi: 10.3390/vaccines8030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moorlag S., van Deuren R.C., van Werkhoven C.H., Jaeger M., Debisarun P., Taks E., et al. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: a retrospective cohort study. Cell Rep Med. 2020;1(5) doi: 10.1016/j.xcrm.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Chaisemartin C., de Chaisemartin L. BCG vaccination in infancy does not protect against COVID-19. Evidence from a natural experiment in Sweden. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hensel J., McAndrews K.M., McGrail D.J., Dowlatshahi D.P., LeBleu V.S., Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep. 2020;10(1):18377. doi: 10.1038/s41598-020-75491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szigeti R., Kellermayer D., Trakimas G., Kellermayer R. BCG epidemiology supports its protection against COVID-19? A word of caution. PLoS ONE. 2020;15(10) doi: 10.1371/journal.pone.0240203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tyndall J. On haze and dust. Nature. 1870;1:339–342. [Google Scholar]
- 114.Cheng V.C., Wong S.C., Chuang V.W., So S.Y., Chen J.H., Sridhar S., et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020;81(1):107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DeFrancesco L. Whither COVID-19 vaccines. Nat Biotechnol. 2020;38(10):1132–1145. doi: 10.1038/s41587-020-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.IPSOS, 2020. Available from: https://www.ipsos.com/en-us/news-polls/WEF-covid-vaccine-global.
- 117.Winkler Martin, S, Skirecki Tomasz, Brunkhorst Frank, M, Cajander Sara, Cavaillon Jean-Marc, Ferrer Ricard, et al. Bridging animal and clinical research during SARS-CoV-2 pandemic: A new-old challenge. EBioMedicine. 2021;66:103291. doi: 10.1016/j.ebiom.2021.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. The Lancet. 2021 doi: 10.1016/S0140-6736(21)00897-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]