Abstract
Tauopathies are a group of neurodegenerative diseases characterized by the alteration/aggregation of TAU protein, for which there is still no effective treatment. Therefore, new pharmacological targets are being sought, such as elements of the endocannabinoid system (ECS). We analysed the occurrence of changes in the ECS in tauopathies and their implication in the pathogenesis. By integrating gene expression analysis, immunofluorescence, genetic and adeno-associated virus expressing TAU mouse models, we found a TAU-dependent increase in CB2 receptor expression in hippocampal neurons, that occurs as an early event in the pathology and was maintained until late stages. These changes were accompanied by alterations in the endocannabinoid metabolism. Remarkably, CB2 ablation in mice protects from neurodegeneration induced by hTAUP301L overexpression, corroborated at the level of cognitive behaviour, synaptic plasticity, and aggregates of insoluble TAU. At the level of neuroinflammation, the absence of CB2 did not produce significant changes in concordance with a possible neuronal location rather than its classic glial expression in these models. These findings were corroborated in post-mortem samples of patients with Alzheimer’s disease, the most common tauopathy. Our results show that neurons with accumulated TAU induce the expression of the CB2 receptor, which enhances neurodegeneration. These results are important for our understanding of disease mechanisms, providing a novel therapeutic strategy to be investigated in tauopathies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40478-021-01196-5.
Keywords: TAU, Cannabinoid receptor, CB2, Alzheimer’s disease, Neurodegeneration, Neuroinflammation
Background
TAU protein is the major component of the intracellular filamentous deposits that characterize several neurodegenerative diseases termed tauopathies, which include Alzheimer's disease (AD), frontotemporal lobar degeneration (FTLD-TAU), progressive supranuclear palsy, corticobasal degeneration, among others [42]. In general, alterations in synaptic plasticity, cell death, proteinopathy, and neuroinflammation are common features in tauopathies. Pathogenic mutations in the TAU-encoding MAPT gene underlying familial frontotemporal dementia (FTD), such as TAUP301L or TAUP301S, have supported the generation of multiple mouse models that recapitulate pathological and/or behavioural aspects of this disease. These TAU mutations reduce the ability of the protein to interact with microtubules and increase its propensity to assemble into abnormal filaments. On the other hand, there are other tauopathies where the dysregulation of TAU protein derives from different posttranslational changes, e.g. hyperphosphorylation, inducing the formation of neurofibrillary tangles, harmful to the neuron, as in the case of AD. Despite the huge efforts made to find a therapy, there is still no effective treatment for tauopathies, so finding neuroprotective treatments for these incapacitating diseases have become a priority. Actually, the failure of different clinical trials with drugs targeting TAU protein has pointed out the need for finding innovative therapeutic approaches [18].
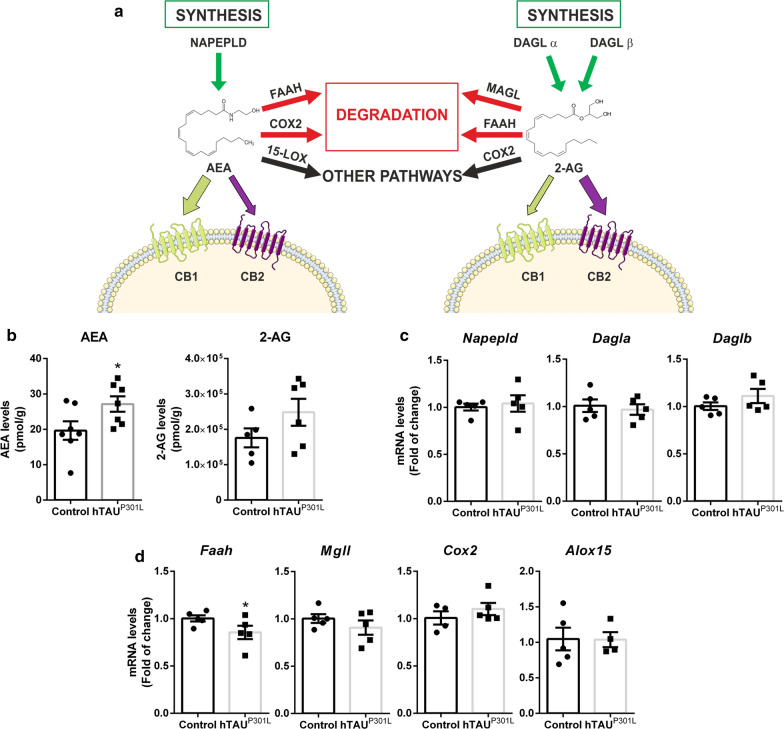
Over the last decades, the endocannabinoid system (ECS), in particular some of its receptors and hydrolysing enzymes, has emerged as a new promising target for neurodegenerative diseases [10, 29, 57]. Recent studies have shown that the ECS modulates synaptic plasticity, as well as the neuronal homeostasis, integrity, and survival, which may be of great interest in disorders involving neurodegeneration and neuroinflammation [1]. The ECS is formed by two G protein-coupled receptors named cannabinoid receptor type-1 (CB1) and type-2 (CB2), their ligands and the enzymes responsible for their synthesis and degradation. Endocannabinoids (ECs) are amides, esters, and ethers of long-chain polyunsaturated fatty acids that act as lipid mediators [9], with N-arachidonoyl ethanolamine (AEA, or anandamide) and 2-arachidonoylglycerol (2-AG) being the main endogenous ligands of cannabinoid receptors. AEA is synthesized from N-arachidonoyl phosphatidylethanolamine (NAPE) due to the action of a specific NAPE-phospholipase D (NAPE-PLD), whereas diacylglycerol lipases α and β (DAGL α/β) are responsible for the synthesis of 2-AG from diacylglycerol (DAG) substrates (Fig. 3a). Intracellular degradation of both ECs is triggered by many different enzymes, with fatty acid amide hydrolase (FAAH), which acts on 2-AG and, in particular, on AEA, and monoacylglycerol lipase (MAGL), which act specifically on 2-AG, as the most active [9] (Fig. 3a). Also, ECs may serve as precursors for the synthesis of novel signaling lipids (e.g. prostamides, prostaglandin-glyceryl esters) when they behave as substrates for arachidonate-related enzymes as cyclooxygenase-2 (COX-2) and 15-lipoxygenase (15-LOX) (Fig. 3a).
Fig. 3.
hTAUP301L overexpression increased AEA levels in the hippocampus. a Scheme of the ECS: main receptors, ligands, and enzymes involved in the endocannabinoid signalling pathway. b Analysis of AEA and 2-AG levels (pmol/g) measured by LC–MS on the ipsilateral and contralateral hippocampi from wild type mice injected with the AAV-hTAUP301L, n = 8 samples ± SEM. c qRT-PCR determination of mRNA levels of Napepld, Dagla, and Dglb, involved in the synthesis of AEA and 2-AG, and d Faah and Mgll, involved in the degradation of AEA and 2-AG. Cox2 and Alox15, involved in other pathways. All measures were normalized by Tbp mRNA levels. n = 5 samples ± SEM. Asterisks denote significant differences *p < 0.05, according to Student's t-test
Cannabinoid receptors have been identified as possible therapeutic targets against different neurodegenerative disorders with very positive results in preclinical studies [37, 38, 40, 46, 60]. This has included the activation of both CB1 and CB2 receptors, although the overactivation of CB1 receptors can lead to detrimental psychotropic effects. Moreover, it has been described a dual neuroprotective/neurotoxic profile of cannabinoid drugs, for example with Δ9‐tetrahydrocannabinol [15, 61]. By contrast, activation of CB2 receptors does not appear to produce these serious adverse effects, although most of compounds with CB2 affinity also share affinity for CB1 subtype [63]. Therefore, selective modulation of CB2 receptors has aroused great interest to exert those beneficial effects without alterations in mood or perception [13].
CB2 cannabinoid receptors have been traditionally found in higher levels in cells from the immune system and microglia [30], where they participate in the modulation of inflammation [16, 48]. However, recent publications have pointed their expression in neurons of the central nervous system, despite the problems of specificity shown by the different antibodies developed so far (review [8]). Using additional methodological tools (e.g. fluorescence in situ hybridization; proximity ligand assays), a neuronal location for the CB2 receptor has been much more proved in some specific neuronal subpopulations [64]. This recent presence in neurons has facilitated the involvement of CB2 receptors in the modulation of different neurobiological processes, for example, neuroplasticity and memory [44], processes that are also impaired in tauopathies. Interestingly, it has been found that CB2 receptors are selectively overexpressed in cells associated with amyloid-β (Aβ) enriched neuritic plaques in AD samples from postmortem human brains [11]. Nevertheless, the specific effects that TAU protein dysregulation may have on CB2 expression and activities have not been well established yet.
In this work, we focused on the relation between dysregulated TAU and CB2 receptors in different tauopathy mouse models. We also wanted to explore this relation in post-mortem human brain samples of tauopathies, in particular from TAU-dependent FTD, but samples from this specific pathology are difficult to obtain due to its low incidence, so we have to concentrate this study in AD, whose samples are easier to be collected. First, we analysed whether TAU overexpression modulates CB2 receptor expression in early and late-stage tauopathy mouse models and determined whether this effect is TAU-dependent. Secondly, we studied whether hTAUP301L overexpression could alter ECS signalling. Due to the possible role that CB2 may have in memory [65] and the potential of this receptor and other elements of the ECS to exert neuroprotection by blocking of microglial activation [56], we elucidated whether the induction of CB2 receptors and their activation could have a positive or negative effect on the progression of tauopathy-associated neurodegeneration. For this purpose, we analysed the involvement of the CB2 receptor in the neurodegeneration induced by hTAUP301L using CB2-deficient mice. We compared the cognitive impairment induced by hTAUP301L between wild type (Cnr2+/+) and CB2-knockout (Cnr2−/−) mice and the results were correlated with the phosphorylation and aggregation status of TAU. Furthermore, we studied the implication of CB2 in the neuroinflammatory process in this model, given the classic role assigned to this receptor concerning the control of glial activation and reactivity [48]. Finally, we wanted to translate the issue to the human pathology scenario by determining whether CB2 levels could be altered in postmortem samples from the hippocampus of AD patients, the most common tauopathy.
Overall, our work offers timely insight into the role of CB2 receptor in tauopathies and highlights pharmacological modulation of CB2 receptor as a potential therapy in TAU-associated diseases.
Methods
Animals and stereotaxic injections
7 and 12-month-old transgenic mice overexpressing hTAUP301S protein (B6;C3-Tg(Prnp-MAPT*P301S)PS19Vle/J, The Jackson Laboratory) and 10-month-old TAU-knockout mice (B6.129X1-Mapttm1Hnd/J, The Jackson Laboratory) were used. Tg- hTAUP301S carried a mutant (P301S) human microtubule-associated protein tau (MAPT) gene driven by the mouse prion-protein promoter (Prnp). These animals showed progressively accumulated TAU in association with striking neuron loss as well as hippocampal and entorhinal cortical atrophy by 9–12 months of age [69]. Each experimental group comprised 4–5 mice. Regarding the experiments with CB2-knockout mice [45], each experimental group comprised 22–30 wild type mice (C57BL/6 J, The Jackson Laboratory) and 19–21 CB2-knockout mice of 6 months of age. Recombinant adeno-associated viral vectors of serotype 6, which express hTAUP301L under control of the human synapsin 1 gene promoter (AAV-hTAUP301L), were injected in the right hippocampus (ipsilateral side) as described elsewhere [21]. In brief, 2 μL of viral suspension containing 2.1 × 10E11 GC/ml were injected at the stereotaxic coordinates − 1.94 mm posterior, − 1.4 mm lateral, and − 1.8 mm ventral relative to bregma. Three weeks after injection, mice were sacrificed, and the left side (contralateral side) was used as a control. All experiments were performed in a P2 biosafety facility and by certified researchers according to regional, national, and European regulations concerning animal welfare and animal experimentation, and were authorized by the Ethics Committee for Research of the Universidad Autónoma de Madrid and the Comunidad Autónoma de Madrid, Spain, with Ref PROEX 279/14, following institutional, Spanish and European guidelines (Boletín Oficial del Estado (BOE) of 18 March 1988 and 86/609/EEC, 2003/65/EC European Council Directives).
Randomization and blinding
Animals were randomized for treatment. Data collection and evaluation of all experiments were performed blindly of the group identity. The data and statistical analysis with the recommendations on experimental design and analysis in pharmacology [23].
Analysis of mRNA levels by quantitative real-time PCR
Total RNA extraction, reverse transcription, and quantitative polymerase chain reaction (qRT-PCR) was done as detailed in previous articles [21, 39]. Briefly, total RNA was extracted using TRIzol® reagent according to the manufacturer’s instructions (Invitrogen). One microgram of RNA from each experimental condition was treated with DNase (Invitrogen) and reverse-transcribed using 11 µl high capacity RNA-to-cDNA Master Mix (Applied Biosystem). Primer sequences are shown in Additional file 4: Table S1. Data analysis was based on the ΔΔCT method with normalization of the raw data to housekeeping genes (Applied Biosystems). All PCRs were performed in triplicates.
Quantification of EC levels by LC–MS
In brief, frozen ipsilateral and contralateral hippocampi from wild type mice injected unilaterally with AAV-hTAUP301L were weighed and homogenized in methanol containing 5 μl of N-arachidonoyl ethanolamine-d8 (AEA-d8), 2-arachidonoylglycerol-d8 (2-AG-d8) and 1-arachidonoylglycerol-d8 (1-AG-d8) (Cayman Chemical) as internals standards. Lipids were extracted with chloroform: H2O 0.1% Formic Acid 2:1. The organic phase was collected and dried in SpeedVac at 60 °C and samples were reconstituted in methanol for Liquid Chromatography-Mass Spectrometry (LC–MS) analyses. Samples were analysed by LC–MS using an Acquity H class (UPLC H-Class, Waters) online QTrap 4500 system (Sciex), and Acquity HSS T3 column (1.2 × 100 mm and 1.8 µm), as described elsewhere [53]. Briefly, a total of 5 μl of the stock solution containing extracted EC was injected and separated using precolumn and column with mobile phase A (0.1% formic acid in double distilled water Milli-Q, Millipore System) and mobile phase B (acetonitrile, Merck). The mass spectrometer was operated in positive ionization mode. For the quantification of EC, two calibration curves were realized using commercial EC standards. Individual signals were normalized based on total weight to account for sample variability and normalized peak areas for internal standard (MultiQuant software, Sciex).
Behavioural test
The novel object recognition (NOR) test was used to assess recognition memory and was performed as described [43]. Briefly, the first day mice were placed in the empty open field and were allowed to explore the open field for 5 min. as short habituation. 24 h after, mice were placed in the open field with two identical objects for 7 min, as familiarization session. Finally, the third day mice were placed in the open field test where one of the objects has been replaced by a novel object for 7 min, as test session. The amount of time spent exploring the novel (TN) or familiar (TF) object was recorded and the differences were represented as Discrimination Index (DI). DI allows discrimination between the novel and familiar objects: [DI = (TN − TF)/(TN + TF)]. Each experimental group comprised 8–15 animals and the test was performed once two days before sacrifice.
Immunofluorescence on mouse tissues
Mouse tissue was sectioned at 30 µm on a cryostat and stained as free floating sections using Netwell baskets [3]. Briefly, sections were washed on TBS (Tris Buffered Saline) and followed by permeabilization in TBS supplemented with 0.05% Triton X-100 (TBS-T). After washing, citric acid-based antigen retrieval for 20 min at 94°C was performed. Following antigen retrieval, sections were cooled to room temperature (RT), washed 3 times in TBS-T, and incubated in blocking buffer (TBS-T supplemented with 5% normal donkey serum and 1% bovine serum albumin [BSA]) at RT for 2 h. Sections were then incubated in primary antibody solutions diluted in blocking buffer for 48 h at 4 °C. After 48 h, sections were washed 3 times in TBS-T and incubated with fluorescent secondary antibodies diluted 1:500 in blocking buffer for 2 h at RT. After incubation in secondary antibodies, the sections were washed 2 times in TBS-T, followed by a 10 min incubation in DAPI nuclear stain (1:5.000 in TBS-T), and 2 final washes in TBS. Sections were then mounted on microscope slides, Primary antibodies are described in Additional file 5: Table S2 and secondary antibodies were: Alexa Fluor 546 donkey anti-mouse and Alexa Fluor 488 donkey anti-rabbit (1:500, Life technologies). Confocal microscope Espectral Leica TCS SP5 was used to take the images. To quantify the percent of CB2-TAU+ neurons, neuron counts were performed using Fiji Software (http://fiji.sc/Fiji) in aprox. 30 neurons/animal (n = 4 animals) of the hippocampus sections. To determine the area of the dendate gyrus, a total of 3 images per condition was analysed as follows. The images are transformed into 16 bits with the Image J program. Then, with the "Free Hand Selection" tool of the Imagen J program, we manually selected only the dentate gyrus area of each image stained with DAPI. The dimension of the dentate gyrus inside the selected area was quantified using the "Measure" tool in Image J program and the raw results measured in inches were represented.
Sarkosyl-soluble and -insoluble fractions of mouse hippocampi
Ipsilateral hippocampi were homogenized in Buffer A (0.1 M Buffer MES pH 7, 1 mM EDTA, 0.5 mM MgSO4, 1 M sucrose, 1 mM NaF, 1 mM Na3VO4, 10 µg/ml leupeptin and phenylmethylsulfonyl fluoride (PMSF)). Homogenates were centrifuged at 20.000 rpm for 20 min at 4 °C. To obtain the sarkosyl-insoluble fraction (SI), the pellets were resuspended in RAB buffer described in [58], vortexed for 1 min at room temperature, incubated at 4 °C overnight and then centrifuged at 69.000 rpm for 30 min at 4 °C. The supernatants were collected as sarkosyl-soluble fractions (SS) and the pellets, SI fractions, were resuspended in RAB buffer with 1 × SDS protein loading buffer and incubated at 95 °C for 5 min.
Protein extracts of mouse hippocampi
Protein lysates from ipsilateral and contralateral hippocampi were homogenized in RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1 μg/ml leupeptine). Homogenates were centrifuged at 13.000 rpm for 15 min at 4 °C.
Immunoblotting
25 μg of SS, SI, and protein extracts from mouse hippocampi were resolved in SDS-PAGE and transferred to Immobilon-P membranes (Millipore). These membranes were analysed by using the following primary antibodies (Additional file 5: Table S2), and appropriate peroxidase-conjugated secondary antibodies (Amersham). Proteins were detected by enhanced chemiluminescence (ECL). Images of the immunoblotting were analyzed using ImageJ, and the lane profiles were obtained in grayscale and uncalibrated optical density.
Human tissues
Samples and data from patients included in this study were provided by the Biobank Banco de Tejidos CIEN (PT17/0015/0014), integrated in the Spanish National Biobanks Network and they were processed following standard operating procedures with the appropriate approval of the Ethics and Scientific Committees. Immediately after brain extraction, midsagittal sectioning was performed to separate the right and left hemispheres of the brain. The left hemisphere was fixed in 10% buffered formalin for at least three weeks, and the hemisphere right were sliced and these slices were quick frozen fresh at − 50 °C (in NOVEC) and were immediately placed in at − 80 °C, where they were stored.
The frozen postmortem hippocampal tissues were obtained from four control (age 43, 58, 74 and 83 years) and four AD patients (age 80, 85, 86 and 88 years, Braak stages III-IV) within less than 6 h postmortem interval, according to the standardized procedures of Banco de Tejidos de la Fundación CIEN (Madrid, Spain). These frozen samples were used for RNA and qRT-PCR analysis. The protocol used was similar to the one described in [39].
From the same patients, we obtained 5 μm paraffinized sections from the hippocampus to perform immunohistochemistry analysis. Briefly, human tissues were deparaffinized before antigen retrieval (citric acid and sodium citrate 0.1 M). Tissues were left in a blocking solution for 1 h at room temperature and incubated with primary antibodies for 48 h. Primary antibodies were prepared in Dako REAL antibody diluent (Dako Diagnostics) and are described in Additional file 5: Table S2. Samples were incubated for 2 h at room temperature with the secondary antibodies Alexa Fluor 555 donkey anti-rabbit and Alexa Fluor 488 donkey anti-mouse (1:500, Life technologies). Finally, tissues were incubated with Sudan Black (Sigma-Aldrich) to quench endogenous autofluorescence, rinsed 2 × in 70% ethanol and stained with DAPI for 15 min.
Statistical analysis
Data are presented as mean ± SEM (Standard Error of the Mean). To determine the statistical test to be used, we employed GraphPad Instat 3, which includes the analysis of the data to normal distribution via the Kolmogorov–Smirnov test. Besides, statistical assessments of differences between groups were analysed (GraphPad Prism 5, San Diego, CA) by unpaired Student's t-tests when normal distribution and equal variances were fulfilled. Two-way or one-way ANOVA with posthoc Bonferroni or Tukey tests were also used, as appropriate.
Results
Overexpression of hTAUP301S induces specific changes in CB2 expression in a mouse model of late stage tauopathy.
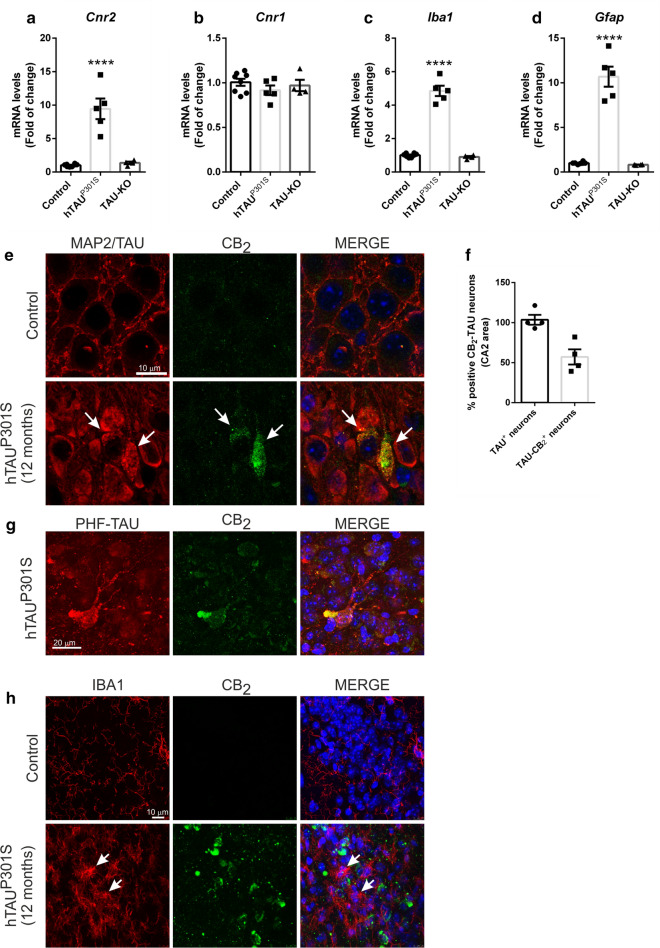
First, we determined if the neurodegeneration induced by TAU overexpression could produce alterations in the expression levels of the CB2 receptor. To do this, we analysed CB2 mRNA levels by qRT-PCR in the hippocampus of transgenic mice that overexpressed the hTAUP301S protein at 12 months of age. At this age, mice present an exacerbated neurodegenerative picture with loss of neurons in the hippocampus and neuroinflammation [68], indicating that it is a late model of tauopathy. Our results indicated that hTAUP301S overexpression induced significantly Cnr2 mRNA expression in late stages of the pathology (9.44 ± 1.54) (Fig. 1a) and this change appears to be dependent on the presence of this mutant TAU form and its potential to aggregate. In support of this dependence, TAU-deficient mice, which are perfectly viable [27, 35], did not show any alterations regarding CB2 receptor. Moreover, this effect was specific towards the CB2 receptor, as mRNA levels for Cnr1 did not change significantly among mice from the three different genotypes (Fig. 1b). Furthermore, previous evidence from our laboratory indicated that overexpression of hTAUP301L induced a neuroinflammatory process [17, 21, 39], which is a key hallmark in the neuronal degeneration in tauopathies [41]. Analysis of mRNA expression levels of Iba1 and Gfap, microglial and astrocytic markers respectively, also confirmed that gliosis induced by hTAUP301S overexpression (4.85 ± 0.31 and 10.68 ± 1.12, respectively) was absent in TAU-knockout mice (Fig. 1c and d). In relation with neuroinflammation, we also detected that hTAUP301S overexpression significantly induced the mRNA levels of the transcription factor NF-κB (Rela) (1.98 ± 0.09), the master regulator of inflammation as well as the proinflammatory cytokines Il-1β (10.22 ± 0.64) and Tnf (12.98 ± 1.29) (Additional file 1: Figure S1). These results suggested that changes observed on CB2 expression and inflammation were directly related to hTAUP301S overexpression.
Fig. 1.
Enhanced neuronal CB2 expression due to hTAUP301S overexpression in a late stage tauopathy transgenic mouse model. Quantitative real-time PCR determination of mRNA levels of a Cnr2, b Cnr1, c Iba1, d Gfap. All genes were normalized by Tbp (TATA-box binding protein) mRNA levels, n = 4–5 samples ± SEM. Asterisks denote significant differences ****p < 0.0001, comparing the indicated groups with the wild type mice according to one-way ANOVA followed by Tukey post-test. e Neuronal localization of the CB2 receptor in the hippocampus (CA2) of 12 months old hTAUP301S transgenic mice. Immunofluorescence with anti-MAP2 (wild mice) or anti-TAU (transgenic mice) (red), anti-CB2 (green), and nuclear staining with DAPI (blue). Arrows point to hTAUP301S protein aggregates. f Quantification of CB2-TAU positive neurons in the CA2 hippocampal area. Number of TAU+ or CB2-TAU+ neurons (n = 4 animals/experimental group) g Co-localization of neuronal CB2 receptor with aggregated TAU in the hippocampus (CA2). Immunofluorescence with anti-PHF-TAU (red), anti-CB2 (green), and nuclear staining with DAPI (blue) in the transgenic mice. h Immunofluorescence with anti-IBA1 (red), anti-CB2 (green), and nuclear staining with DAPI (blue)
Given that the elevation in the expression of the CB2 receptor in neurodegenerative disorders has been frequently associated with its overexpression in glial elements (e.g. reactive microglia) elicited by the local inflammatory effects [7, 14, 15], we next investigated whether the increase found in hTAUP301S transgenic mice is a general effect or whether it is specific of any cell type. Therefore, we analysed specifically in which cell type CB2 was overexpressed using co-immunofluorescence procedures. Our data indicated that around 50% of the neurons that overexpressed hTAUP301S are those that expressed the CB2 receptor (Fig. 1e–f). CB2 receptor was preferentially expressed in neurons in which the formation of aggregates of TAU protein is observed (as detected with an antibody that identifies paired helical filaments (PHF) of TAU (Thr212/Ser214) (Fig. 1g). As a control, MAP2 protein was used as a neuronal marker in the wild type mice. The finding of overexpression of the CB2 receptor in neurons in a neurodegenerative pathology is not frequent [59], but it is not unexpected, given the incipient evidence supporting that CB2 receptors may be also present in neurons in the healthy brain [44, 65]. To confirm whether this overexpression was specific or whether it also occurred in glial elements, we conducted a similar double-staining analysis, using IBA1, a marker of microglial cells. In this case, it was again observed that CB2 was expressed only at the neuronal level (CA2, CA3, and dentate gyrus), with no co-localization in the microglia (Fig. 1h). In turn, the difference in microglial morphology between wild-type mice (quiescent-non-reactive form) and transgenic mice (ameboid-reactive form) was strongly evident, confirming the presence of a reactive microgliosis. These data clearly indicated that hTAUP301S induced selective neuronal CB2 expression in a late-stage tauopathy mouse model.
Increased neuronal CB2 receptor expression is an early event in tauopathies.
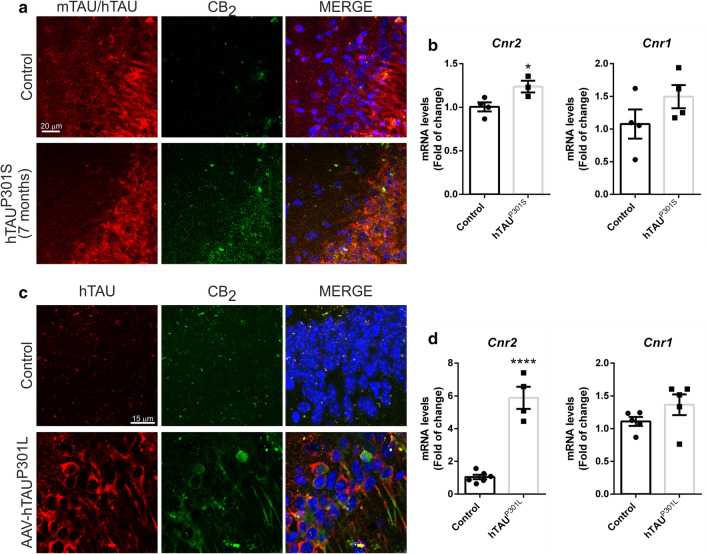
Our next objective was to determine if the increase in the expression of CB2 receptors was also seen at earlier stages in the pathology, so that it may be considered as an early event possibly contributing to the pathogenesis. In 7 months old hTAUP301S transgenic mice, we detected a significant slight increased expression of CB2 receptors, at mRNA (1.24 ± 0.06) and protein level (Fig. 2a and b), without alterations in the mRNA expression of Cnr1, RelA, Il-1β and Tnf (Additional file 1: Figure S1). To eliminate the possibility that these changes are due to adaptations during development, we confirmed these results in another early model of tauopathy consisting of mice stereotaxically injected into the right hippocampus with an AAV-hTAUP301L vector for 21 days [17, 21, 39] (Fig. 2c and d). Analysis of the samples by qRT-PCR showed that overexpression of hTAUP301L induced a very significant increase in Cnr2 mRNA levels (5.88 ± 0.67) (Fig. 2d). As in the transgenic mouse model, this effect was specific for CB2, since the expression of Cnr1 did not show significant changes. These results reproduced what was observed in the hippocampus of the 12-month-old transgenic mice that overexpressed the hTAUP301S protein (Figs. 1 and 2a and b), confirming that these are events that occur at an early stage of the pathology, possibly contributing to the pathogenesis. Finally, we confirmed that CB2 expression also takes place at the neuronal level in this additional tauopathy mouse model, using immunofluorescence for CB2 and TAU in hippocampus sections (Fig. 2c). As seen in Fig. 1e, there was co-localization between the CB2 receptor and neurons that overexpress the hTAUP301S or hTAUP301L protein, confirming again its expression at the neuronal level.
Fig. 2.
Increased CB2 expression is an early event in tauopathies. Analysis of 7 months old hTAUP301S transgenic mice. a Neuronal location of the CB2 receptor in the hippocampus (CA2) of 7 months old hTAUP301S transgenic mice. Immunofluorescence with anti-mTAU (wild type mice) or anti-hTAU (transgenic mice) (red), anti-CB2 (green), and nuclear staining with DAPI (blue). b Quantification of Cnr2 and Cnr1 mRNA levels, n = 4 samples ± SEM. The data has been processed with Student's t-test analysis to determine the significance of the changes. The asterisks represent the difference in significance * p < 0.05. c Neuronal location of the CB2 receptor in the hippocampus (CA2) in the AAV-hTAUP301L mouse model. Immunofluorescence with anti-hTAU (red), anti-CB2 (green), and nuclear staining with DAPI (blue). d Analysis of wild type mice injected into the ipsilateral hippocampus with the AAV-hTAUP301L vector for 3 weeks. Quantification of Cnr2 and Cnr1 mRNA levels, n = 4–6 samples ± SEM. The data has been processed with Student's t-test analysis to determine the significance of the changes. The asterisks represent the difference in significance **** p < 0.0001
AEA levels are induced by overexpression of hTAUP301L in the hippocampus.
Besides cannabinoid receptors, we must take into consideration that TAU overexpression may also induce changes in other elements of the endocannabinoid system. Therefore, we analysed the levels of ECs and the enzymes involved in their synthesis/inactivation in hippocampal samples of mice injected stereotaxically into the right hippocampus with an AAV-hTAUP301L vector (ipsilateral). As we have previously mentioned, AEA and 2-AG are the two main ECs, with different affinities for CB1 and CB2 receptors [51] (Fig. 3a). LC–MS analyses showed that hTAUP301L overexpression increased significantly the levels of AEA (Control: 19.64 ± 2.60; hTAUP301L: 27.13 ± 2.18) and to a lesser extent (only as a trend), those of 2-AG (Control: 175,773 ± 26,855; hTAUP301L: 248,234 ± 38,184) (Fig. 3b). In order to check whether these results could be due to alterations in the expression of the enzymes involved in their synthesis, we measured mRNA levels of Napepld, Dagla, and Daglb, but we did not observe any changes (Fig. 3c). Then, we analysed mRNA levels of Faah, Mgll, Cox2, and Alox15 (Fig. 3d), enzymes involved in the degradation of ECs and other associated pathways. In this case, we found a statistically significant decrease in the expression of Faah (0.854 ± 0.070) that could be responsible for the increase in AEA levels, whereas a numerical trend towards a decrease could be appreciated for Mgll that could be related to the trend found for 2-AG levels.
CB2 receptor deficiency ameliorated cognitive impairment and reduced degeneration of the granular cell layer of the dentate gyrus induced by hTAUP301L overexpression.
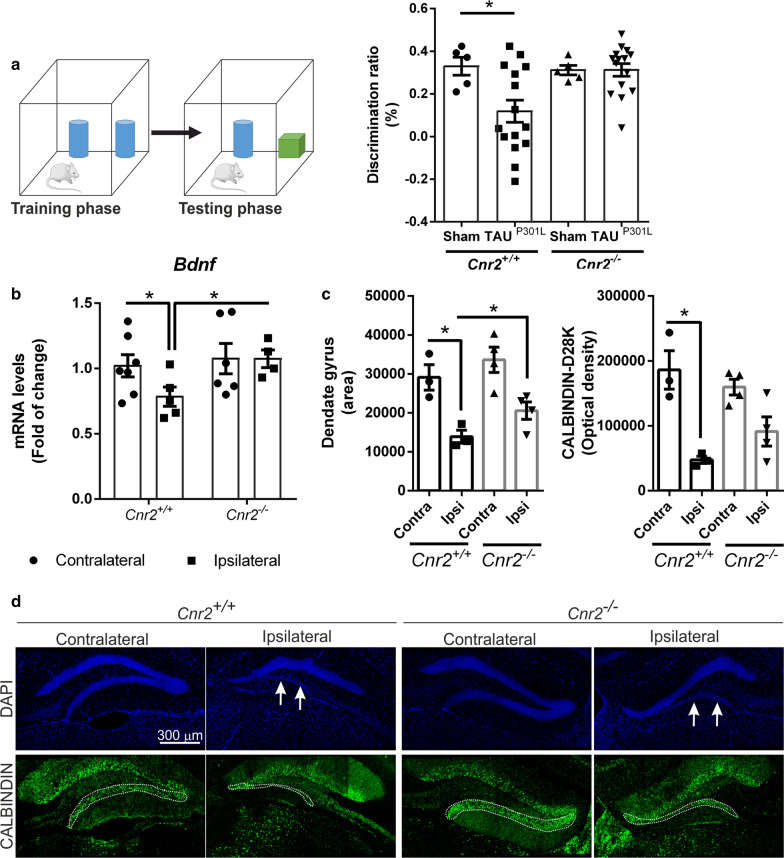
All our results indicated that the overexpression of TAU increases the neuronal levels of CB2 in different tauopathy models. To determine if this increase of CB2 is a mechanism of the brain to fight against neurodegeneration or, on the contrary, is contributing to the pathogenesis, we overexpressed hTAUP301L in CB2-deficient mice (AAV-hTAUP301L model) and wild type animals. Then, to assess whether the CB2 expression affects the recognition memory alterations induced by TAU overexpression in the hippocampus, we performed the NOR test [43] two days before sacrifice. As specific controls for both genotypes, we used wild type or CB2-deficient mice from the same age, without hTAUP301L overexpression (Sham). Cnr2+/+ mice injected with the AAV-hTAUP301L in the hippocampus showed the expected significant decrease in the discrimination index compared to sham animals (Cnr2+/+ Sham: 0.329 ± 0.042; Cnr2+/+ -AAV-hTAUP301L: 0.118 ± 0.051; Cnr2−/− Sham: 0.311 ± 0.022; Cnr2−/− -AAV-hTAUP301L: 0.312 ± 0.029). However, the lack of CB2 receptors in Cnr2−/− mice avoided this cognitive impairment caused by hTAUP301L overexpression (Fig. 4a). On the other hand, the recognition memory was similar in Cnr2−/−-sham mice compared to Cnr2+/+-sham animals. These results suggested that the expression of CB2 could be involved in mechanisms of neuronal plasticity associated with recognition memory. We already have described that this tauopathy mouse model has alterations in synaptic plasticity [17, 21]. To assess the implication of CB2 in this mechanism, we determined the expression levels of Brain-Derived Neurotrophic Factor (BDNF), involved in the acquisition and consolidation of overlapping spatial memories in the dentate gyrus [49]. In Cnr2+/+ mice, hTAUP301L overexpression significantly decreased Bdnf mRNA levels (0.784 ± 0.073) on the ipsilateral hippocampus (Fig. 4b). However, Bdnf expression levels did not change due to hTAUP301L overexpression in Cnr2−/− mice and remained the same as in Cnr2+/+-contralateral samples (Fig. 4b). These results inversely correlated with CB2 receptor expression levels, measured by qRT-PCR and immunofluorescence (Additional file 2: Figures S2), where higher levels of Cnr2 (1.714 ± 0.104) correlated with lower Bdnf expression. In the Additional file 2: Figures S2 it can be observed that the overexpression of hTAUP301L induced CB2 at the neuronal level, corroborating previous data (Figs. 1e and 2c), and this increase is specific since it did not occur in CB2-deficient mice. Thus, it seems that the increase in CB2 expression induced by hTAUP301L overexpression in neurons is detrimental for the cognitive status.
Fig. 4.
Cognitive impairment induced by hTAUP301L overexpression is prevented in Cnr2−/− mice. a Recognition memory was tested by NOR test in control mice (Sham), and Cnr2+/+ and Cnr2−/− mice overexpressing hTAUP301L (n = 8–15 per experimental group). b qRT-PCR determination of mRNA levels of Bdnf, normalized by Tbp mRNA levels, n = 5–7 samples ± SEM. Asterisks denote significant differences with *p < 0.05, comparing each group with the contralateral hippocampi from Cnr2+/+ mice or the indicated groups, according to two-way ANOVA followed by Bonferroni post-test. Reduction in the granular cell layer from the dentate gyrus due to hTAUP301L overexpression is attenuated in Cnr2−/− mice. c Quantification of the area stained with DAPI (arbitrary units) in the dentate gyrus from Cnr2+/+ and Cnr2−/− mice injected with AAV-hTAUP301L. n = 3–4 samples ± SEM. Asterisks denote significant differences with *p < 0.05 comparing the ipsilateral side with the contralateral side of the indicated groups, according to two-way ANOVA followed by Bonferroni post-test. d Immunofluorescence staining in hippocampal sections from Cnr2+/+ and Cnr2−/− mice injected with AAV-hTAUP301L (n = 3–4 per experimental group). Blue, DAPI. Green, anti-CALBINDIN-D28K
Within the hippocampus, the area of the dentate gyrus is thought to contribute to the formation of new episodic memories [26] and the spontaneous exploration of novel environments, among other functions. Therefore, we determined if there was a correlation between the data we obtained in the NOR (Fig. 4a) and alterations in the structure of the dentate gyrus. Staining of the dentate gyrus area with DAPI, a fluorescent staining that binds strongly to adenine–thymine rich regions in DNA, indicated that hTAUP301L overexpression induced the loss of part of the granular cell layer in Cnr2+/+ mice (Fig. 4d). Nevertheless, this loss was partially attenuated in Cnr2−/− mice injected with AAV-hTAUP301L, as can be observed in the quantification of the area (Cnr2+/+-contra: 29,108 ± 3266; Cnr2+/+-ipsi: 13,876 ± 1670; Cnr2−/−-contra: 33,628 ± 3246; Cnr2−/−-ipsi: 20,565 ± 2222 (Fig. 4c). Concerning synaptic plasticity, we analysed the expression levels of CALBINDIN-D28K, a member of the calcium-binding protein superfamily whose expression strongly correlates with protection against TAU neurodegeneration [21]. Immunofluorescence analysis of the dentate gyrus showed that hTAUP301L overexpression reduced CALBINDIN-D28K expression levels in the granular layer in Cnr2+/+ mice (Fig. 4d) and to a lesser extent in Cnr2−/− mice (Cnr2+/+-contra: 186,160 ± 29,602; Cnr2+/+-ipsi: 47,521 ± 5462; Cnr2−/−-contra: 159,640 ± 12,124; Cnr2−/−-ipsi: 91,311 ± 22,406). Taken together, these results suggest that the lack of CB2 receptor protects against TAU induced neurodegeneration.
p-TAU insolubility is reduced in CB2-deficient mice
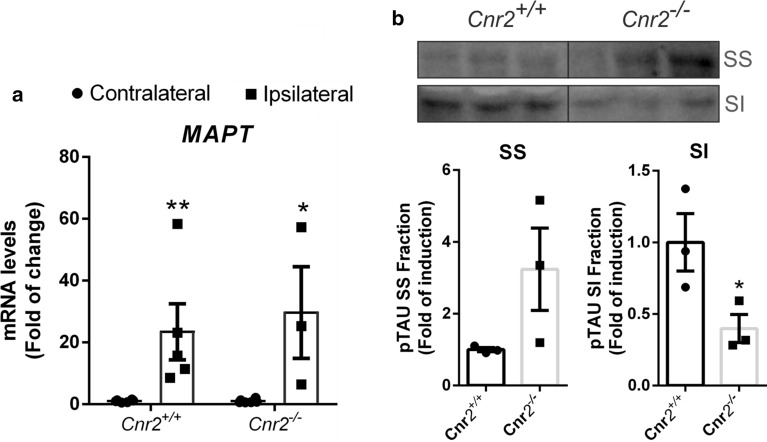
In tauopathies, abnormal metabolism of TAU protein leads to its intracellular accumulation, hyper- or aberrant phosphorylation and formation of neurofibrillary tangles, which can lead to protein toxicity, cell death and neurodegeneration [33]. We analysed if the levels of soluble (SS) and insoluble (SI) TAU fractions depend on CB2 expression, using sarkosyl extraction as a standard protocol for investigating insoluble TAU aggregates in the brain [58].
First, we determined that both genotypes expressed similar levels of MAPT mRNA in the injected side (Fig. 5a). It has been described that sarkosyl-insoluble (SI) TAU correlates with the pathological features of tauopathy. In the ipsilateral hippocampi, the Cnr2−/− mice showed decreased hyperphosphorylated TAU at Ser202/Thr205 in the sarkosyl-insoluble fraction (SI) in comparison to Cnr2+/+ mice (Fig. 5b). Regarding the soluble fraction SS, we observed the opposite effect. Our data suggest that the absence of CB2 receptor modulates TAU levels by increasing the soluble fraction (SS), indicating a reduction of p-TAU aggregates.
Fig. 5.
TAU-SI levels are reduced in Cnr2−/− mice. a qRT-PCR determination of mRNA levels of MAPT. b Ipsilateral hippocampal tissue obtained from Cnr2+/+ and Cnr2−/− AAV-hTAUP301L injected mice were separated into SS and SI fractions. Levels of p-TAU-AT8 were analysed in by immunoblotting and their respective protein quantifications. n = 3 samples per experimental group ± SEM. Asterisks show significant differences with **p < 0.01 comparing each group according to a Student’s t-test
The absence of CB2 receptor does not change the inflammation status when overexpressing hTAUP301L
As we mentioned before, along with alterations in neuronal plasticity and proteostasis, neuroinflammation is a key element in neurodegenerative processes [41]. Concerning CB2, previous evidence from other laboratories pointed out at the possible role of CB2 in modulating microglial activation in different neurodegenerative disorders such as AD [12, 16], so we explored whether the up-regulatory response experienced by CB2 receptors in our tauopathy model could have any relation with the inflammatory response. Our results indicated that overexpression of hTAUP301L was followed by the expected proinflammatory scenario reflected by increased expression of markers of astrogliosis (Gfap), microgliosis (Iba1) and proinflammatory cytokines (Il-1β and Tnf), but this was seen at the same extent in both genotypes (Additional file 3: Figures S3). Therefore, our data support that the CB2 receptor does not appear to play a relevant role at the level of neuroinflammation induced by hTAUP301L overexpression.
Increased expression of neuronal CB2 in post-mortem tissues from AD patients
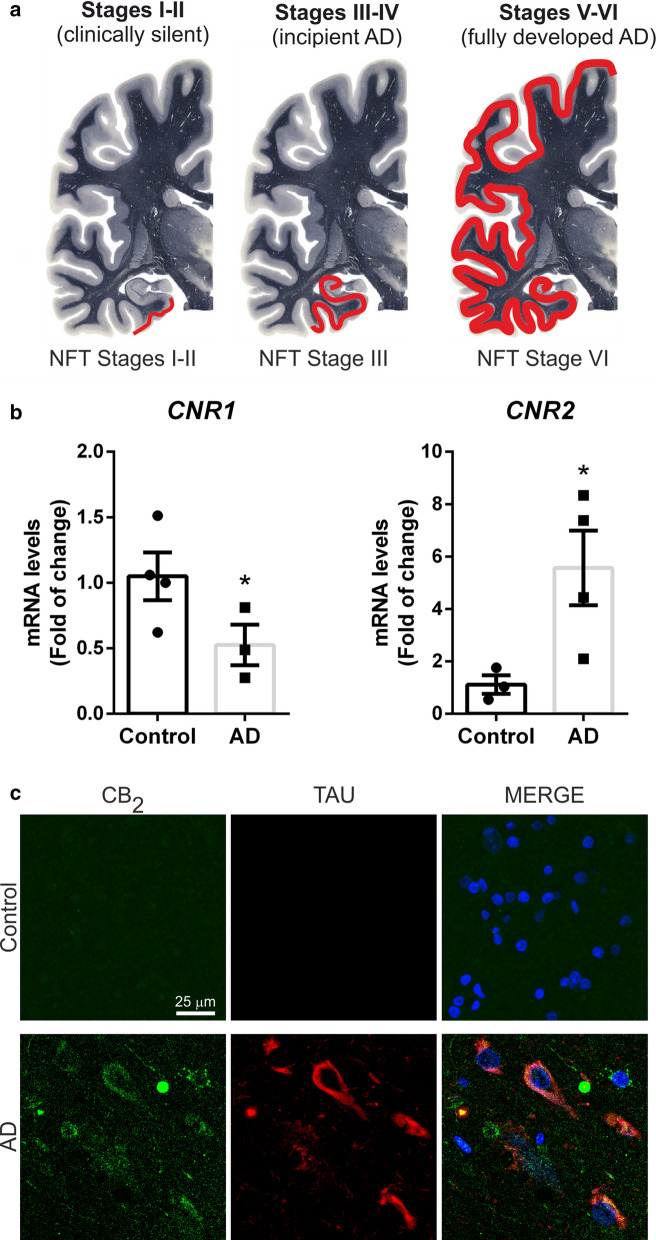
Our preclinical data indicated that overexpression of TAUP301L induces an increase in the expression of neuronal CB2 and that this induction was toxic to the neuron in animal models. To determine that aberrant TAU aggregation (formation of neurofibrillary tangles (NFT)) in patients lead to similar results, in our last objective, we wanted to work with tissues from patients with tauopathies. We assume that tauopathies are an heterogenous group of pathologies and that including TAU-dependent cases of the other pathologies (e.g. TAU-dependent frontotemporal dementia) would have been desirable, but, due to its low incidence, it is difficult to collect a sufficient number of cases of this tauopathy, so we finally worked with AD, the more frequent tauopathy. We used samples from AD patients with Braak stages III-IV, when they present symptoms of incipient AD and the entorhinal and transentorhinal layers are affected (Fig. 6a) to determine CB2 status. Analysis of mRNA levels from both cannabinoid receptors by qRT-PCR showed that CNR2 expression was significantly increased in AD patients (5.567 ± 1.422) compared to controls whereas CNR1 levels were 50% decreased (0.525 ± 0.155) (Fig. 6b), indicating that the ECS is deregulated in AD, as described before [20].
Fig. 6.
CB2 is increased in neurofibrillary tangle TAU positive neurons from AD patients. a Scheme of the different Braak stages of AD progression in the human brain: Stages I-II (transenthorinal), stages III-IV (limbic), and stages V-VI (neocortical) (Modified from http://www.thehumanbrain.info/ at the hippocampus level). b qRT-PCR determination of mRNA levels of CNR1 and CNR2. Both measures were normalized by TBP mRNA levels. n = 3–4 ± SEM. Asterisks denote significant differences *p < 0.05, comparing the AD patients with the control condition according to Student's t-test. c Double immunofluorescence staining of 15 μm-thick sections of hippocampal tissue from control (n = 4) and AD (n = 4) patients. Green, anti-CB2. Red, anti-TAU-HT7. Blue, DAPI
Next, we explored the cell substrates in which the up-regulatory response of CB2 receptors takes place, so we analysed the location of CB2 receptors in AD, using immunofluorescence assays. Interestingly, we observed overexpression of CB2 receptors in those neurons that presented TAU neurofibrillary tangles (Fig. 6c), corroborating the results obtained in the murine models. These data confirmed that aberrant TAU accumulation induces CB2 receptor expression in human patients as in tauopathy mouse models.
Discussion
Aberrant TAU plays a role in the pathogenesis of a variety of neurodegenerative diseases, and the study of the implication of TAU in those pathologies suggested the involvement of different molecular mechanisms. Among them, our results demonstrate for the first time that TAU overexpression increased levels of CB2 receptors at neuronal level and that CB2-deficiency protects against TAU harmful mechanism.
Although there is no specific study regarding the interaction between CB2 and TAU, several studies in AD models indicate a controversial role of CB2 in the progression of the disease. In a triple transgenic mouse model (mutations in the APP, presenilin 1 (PSEN1) and TAU genes), the deletion of CB2 induces AD-like TAU pathology and memory impairment [67]. Similar results were observed in another transgenic AD APP-based mouse model, in which CB2 receptor deficiency increased amyloid pathology and altered TAU processing [36]. Other studies indicate that in the APP/PSEN1 AD mouse model, the lack of CB2 receptor intensifies cortical Aβ deposition and rises the levels of soluble Aβ40 [5]. However, CB2 receptor ablation does not affect the survival of APP/PSEN1 mice and has no impact on memory impairment or TAU hyperphosphorylation. In this AD mouse model, it has been shown that the treatment with JWH-133, a specific CB2 cannabinoid receptor agonist, induced cognitive improvement due to decreased microglial reactivity and reduced expression of pro-inflammatory cytokines IL-1β, IL-6, TNFα, and IFNγ [7]. However, it has been also demonstrated that CB2 deletion also improves cognitive and learning deficits in another transgenic APP/PSEN1/CB2−/− mice [62], in which reduced neuronal loss and decreased plaque levels were observed. Interestingly, in this study, authors demonstrated that the microglia surrounding plaques showed a less activated morphology in the absence of CB2 receptor, being the plaques smaller and more condensed than in APP/PSEN1 mice. Altogether, these data indicate divergent effects of CB2 related to APP or TAU. To date, about AD, in studies relating to the CB2 receptor with pathology, only models based exclusively on overexpression of the APP protein have been used, and no studies have been carried out on models based on the TAU protein, so our results are extremely novel and innovative. In this context, our results regarding the involvement of CB2 receptor in neurodegenerative processes that only implicate TAU protein shed light on the molecular mechanisms involved. Besides, it must be taken into account that the possible beneficial effects that have been seen with the activation of the CB2 receptor in these AD models are mainly based on its anti-inflammatory effects related to its expression in the microglia [4, 66], which may indicate that the direction in which the CB2 receptor should be modulated may depend on the type of neurotoxic event to be controlled: activation against inflammatory events in APP-based models and inhibition against neuronal deterioration in TAU-dependent models. If this is so, this should be necessarily taken into consideration when these treatments progress towards the clinical scenario given the complexity of neurodegenerative events in AD. In relation to this personalized medicine, it is also interesting to highlight the implication that modulation of the CB2 receptor would have in the development of disease-modifying drugs for frontotemporal dementia (FTD) spectrum disorders, although to date no change in CB2 levels (neither in microglia nor in neurons) has been described in this pathology, so it would be interesting to determine the status of CB2 and its cellular location. FTLD are classified according to the predominant protein that accumulates abnormally in cells and 40% of all FTLD are associated with aberrant TAU accumulation [52]. Therefore, the pharmacological modulation of CB2 as a therapeutic strategy for FTLD should be personalized, based on the molecular alterations displayed by the different type of patients.
In our study, we have shown that the neuronal induction of CB2 levels by overexpression of TAU is an event that is maintained throughout the neurodegenerative process until late stages of the disease and that it is dependent on TAU aggregation, since it is not altered in TAU-knockout mice. P301L or P301S mutations in the TAU protein prevent the anchoring of TAU to the microtubules and favor the aggregation of the protein, which happens, although by other mechanisms, in AD [19]. Thus, in both scenarios we have a neurotoxic form of the TAU protein, and in both cases the expression of CB2 was induced at the neuronal level.
Our results indicated that overexpression of hTAUP301L increases AEA levels possibly by decreasing the expression of FAAH, an enzyme involved in its degradation. It has been reported that pharmacological elevation of anandamide impairs short-term memory in the hippocampus [31], indicating that in our AAV-hTAUP301L mouse model the increase in AEA is implicated in cognitive impairment. These results are the first evidence of alterations in the ECS in a tauopathy mouse model. Nevertheless, it remains to be determined if variations in the levels of ECs would also be related to changes in the protein levels of these enzymes and their enzymatic activity, something already described for other neurodegenerative disorders [24].
To determine whether the increase in CB2 levels is beneficial or toxic to the neuron, we analysed the effect of hTAUP301L overexpression in CB2-deficient mice. The results clearly indicate that the absence of CB2 improves the cognitive impairment and the synaptic plasticity (Fig. 4) induced by hTAUP301L overexpression. Similar observations were found in a study of CB2 receptors in cerebral malaria, where the benefits were reached by blocking the receptor or ablating its gene expression [2]. However, this contrasts with most of the literature on CB2 receptor in other neurodegenerative/neuroinflammatory disorders, in which the benefits were reached after the activation of this receptor [6, 14, 25, 28, 32, 50]. The difference compared with our current study may be in the cell type where the CB2 receptor is located: glial versus neuronal location. Although classical expression of CB2 has always been related to microglia and neuroinflammatory processes [16], our results suggest that in relation to TAU, CB2 does not play an essential role in inflammation. Moreover, we observed that in all the tauopathy mouse models used and in AD postmortem brain samples increased CB2 expression occurs at neuronal levels. This agrees with recent evidence that indicates that the CB2 receptor can also be expressed at the neuronal level [44, 65], being involved in neuroplasticity processes like learning and memory [34], by being able to induce hyperpolarization in hippocampal neurons [55]. In line with our results, it has been demonstrated that CB2 disruption enhanced spatial working memory, while their overexpression reduced anxiety levels [44]. Related to the participation of the CB2 receptor in regulating memory, one of the main characteristics presented by patients with Alzheimer's disease (AD) is memory loss. In this work, we have confirmed an increase in CNR2 expression in samples from AD patients. Increased CB2 protein expression is present in the TAU damaged neuron, with total co-localization. These results indicate that CB2 overexpression could be detrimental for neuroplasticity and neuronal survival and be associated with disease progression. All these pieces of evidence indicate that CB2 expression has different roles depending on the cell-type where it is expressed in the mature hippocampus and is important in regulating memory. Taken together, it could be speculated that when the CB2 receptor is expressed in the microglia, its activation has anti-inflammatory and beneficial effects against neurodegeneration, but when CB2 receptor is overexpressed at the neuronal level, its functionality changes radically, having a detrimental effect against neurodegeneration.
Regarding the mechanism by which the absence of CB2 prevents TAU toxicity, our data suggest that it could be due to an improvement in the solubility of TAU (CB2 deficiency led to impairment of TAU aggregate formation). It is commonly accepted that pathological features of tauopathy correlate with the levels of insoluble TAU aggregates (SI) in the brain. We found that Cnr2−/− mice had less hyperphosphorylated TAU aggregates than Cnr2+/+ mice in the ipsilateral side of hTAUP301L overexpression in the hippocampus, although they had similar MAPT expression levels. It has been described that CB2 receptors have been shown to regulate a plethora of kinases, including PI3K/AKT/GSK-3, JNK and p38 [22], which are linked to TAU phosphorylation [47, 54, 70]. These results suggested that the lack of CB2 could be implicated in reducing TAU phosphorylation. Further experiments will be needed to determine the involvement of these kinases in the removal of TAU aggregates and the involvement of the CB2 receptor in these processes.
Conclusions
This study describes for the first time how TAU overexpression increases CB2 receptor expression at the neuronal level in the hippocampus, being an early event in tauopathies. Unlike CB2 induction in microglia, this neuronal CB2 overexpression enhances the neurodegenerative process associated with the TAU protein. This study paves the way to propose CB2 antagonists (or negative allosteric modulators) as an entirely novel therapeutic approach in tauopathies.
Supplementary Information
Additional file 1. Fig. S1: hTAUP301S overexpression in 12 months old transgenic mice induced inflammatory response in the hippocampus. (A) Analysis of mRNA levels of inflammatory genes RelA, Tnf and Il-1β in 12 months old transgenic mice. (B) Analysis of mRNA levels of inflammatory genes RelA, Tnf and Il-1β in 7 months old transgenic mice. All genes were normalized by Tbp (TATA-box binding protein) mRNA levels, n=4-5 samples ± SEM. The data has been processed with Student's t-test analysis to determine the significance of the changes. The asterisks represent the difference in significance **** p <0.0001
Additional file 2. Fig. S2: (A) qRT-PCR determination of mRNA levels of Cnr2. Measures were normalized by Tbp mRNA levels. n=5-7 samples ± SEM. Asterisks denote significant differences with ***p<0.001, comparing each group with the contralateral hippocampi from Cnr2+/+ mice or the indicated groups, according to two-way ANOVA followed by Bonferroni post-test. (B) Double immunofluorescence staining of 30 μm-thick sections of contralateral and ipsilateral hippocampus from Cnr2+/+ mice injected with AAV-hTAUP301L (n=3). The ipsilateral side from Cnr2-/- was used as a control (n=1). Green, anti-CB2. Red, anti-TAU-HT7. Blue, DAPI.
Additional file 3. Fig. S3: The deficiency in CB2 does not produce changes in the neuroinflammation associated with hTAUP301L. qRT-PCR determination of mRNA levels of (A) Gfap, (B) Iba1, (C) Il-1β, and (D) Tnf. All genes were normalized by Tbp mRNA levels. n=5-7 samples ± SEM. Asterisks denote significant differences with *p<0.05, **p<0.01, and ***p<0.001, comparing each group with the contralateral hippocampi from Cnr2+/+ mice or the indicated groups, according to two-way ANOVA followed by Bonferroni post-test.
Acknowledgements
We want to particularly acknowledge the patients and the Biobank Banco de Tejidos CIEN (PT17/0015/0014) integrated into the Spanish National Biobanks Network for their collaboration. The authors are grateful to Dr. Julián Romero (Universidad Francisco de Vitoria, Madrid, Spain) and Dr. Cecilia J. Hillard (Medical College of Wisconsin, USA) for providing us with CB2-knockout mice. The authors want to acknowledge the laboratory of “Microscopía Confocal (SIdI-UAM)” and the “Instituto de Investigaciones Biomédicas” Confocal service, for their help with the Confocal microscope.
Abbreviations
- AAV vector
Adeno-associated viral
- Aβ
Amyloid-β
- AD
Alzheimer’s disease
- AEA, or anandamide
N-arachidonoyl ethanolamine
- 2-AG
2-Arachidonoylglycerol
- BDNF
Brain-Derived Neurotrophic Factor
- COX-2
Cyclooxygenase-2
- DAG
Diacylglycerol
- DAGL α/β
Diacylglycerol lipases α and β
- ECs
Endocannabinoids
- ECS
Endocannabinoid system
- FAAH
Fatty acid amide hydrolase
- FTD
Frontotemporal dementia
- FTLD-TAU
Frontotemporal lobar degeneration
- MAGL
Monoacylglycerol lipase
- NAPE
N-arachidonoyl phosphatidylethanolamine
- NOR
Novel object recognition test
- 15-LOX
15-Lipoxygenase
- SI
Sarkosyl-insoluble fraction
- SS
Sarkosyl-soluble fractions
- PMSF
Phenylmethylsulfonyl fluoride
- PSEN1
Presenilin 1
Authors' contributions
ILB contributed to conception and design of the study. ILB, MGG, JMR, FH and JA were implicated in the work with the TAUP301S transgenic mice. ILB, MGG and MPA were implicated in the analysis of the ECS levels. JLL generated the AAV-TAUP301L. EL, ILB and MGL were implicated in the novel recognition analysis. ILB and MGG acquisition and analysis of data. CRC, ILB and JFR were implicated in the work with CB2-deficient mice. AR was involved in the human sample analysis. ILB and JFR contributed to drafting a significant portion of the manuscript and figures. All authors read and approved the final manuscript.
Funding
This work was supported by a Spanish Ministry of Economy and Competitiveness Grants refs. SAF2016-76520-R and PID2019-105600RB-I00 to ILB; Ref. RTI2018-095793-B-I00 to MGL. General Council for Research and Innovation of the Community of Madrid and European Structural Funds Ref. S2017/BMD-3813-ELA_Madrid to ILB and B2017/BMD-3827 – NRF24ADCM to MGL. Fundación Tatiana Pérez de Guzmán el Bueno P-043-FTPGB 2020 to ILB.
Availability of data and materials
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All experiments were performed by certified researchers according to regional, national, and European regulations concerning animal welfare and animal experimentation and were authorized by the Ethics Committee for Research of the Universidad Autónoma de Madrid and the Comunidad Autónoma de Madrid, Spain, with Ref PROEX 279/14, following institutional, Spanish and European guidelines (Boletín Oficial del Estado (BOE) of 18 March 1988 and 86/609/EEC, 2003/65/EC European Council Directives).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M. Galán-Ganga, Email: marcos.galanganga@gmail.com
C. Rodríguez-Cueto, Email: carc@med.ucm.es
J. Merchán-Rubira, Email: jesus.merchan.rubira@gmail.com
F. Hernández, Email: fhernandez@cbm.csic.es
J. Ávila, Email: jesus.avila@csic.es
M. Posada-Ayala, Email: m.posada.prof@ufv.es
J. L. Lanciego, Email: jlanciego@unav.es
E. Luengo, Email: enrique.luengo9292@gmail.com
M. G. Lopez, Email: manuela.garcia@uam.es
A. Rábano, Email: arabano@fundacioncien.es
J. Fernández-Ruiz, Email: jjfr@med.ucm.es
I. Lastres-Becker, Email: ilbecker@iib.uam.es
References
- 1.Aizpurua-Olaizola O, Elezgarai I, Rico-Barrio I, Zarandona I, Etxebarria N, Usobiaga A. Targeting the endocannabinoid system: future therapeutic strategies. Drug Discovery Today. 2017;22:105–110. doi: 10.1016/j.drudis.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Alferink J, Specht S, Arends H, Schumak B, Schmidt K, Ruland C, Lundt R, Kemter A, Dlugos A, Kuepper JM, et al. Cannabinoid receptor 2 modulates susceptibility to experimental cerebral malaria through a CCL17-dependent mechanism. J Biol Chem. 2016;291:19517–19531. doi: 10.1074/jbc.M116.746594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apicco DJ, Ash PEA, Maziuk B, LeBlang C, Medalla M, Al Abdullatif A, Ferragud A, Botelho E, Ballance HI, Dhawan U, et al. Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat Neurosci. 2018;21:72–80. doi: 10.1038/s41593-017-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aso E, Andres-Benito P, Carmona M, Maldonado R, Ferrer I. Cannabinoid Receptor 2 participates in Amyloid-beta processing in a mouse model of Alzheimer's disease but plays a minor role in the therapeutic properties of a cannabis-based medicine. J Alzheimer's Dis JAD. 2016;51:489–500. doi: 10.3233/jad-150913. [DOI] [PubMed] [Google Scholar]
- 6.Aso E, Ferrer I. CB2 Cannabinoid Receptor As Potential Target against Alzheimer's disease. Front Neurosci. 2016;10:243. doi: 10.3389/fnins.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aso E, Juves S, Maldonado R, Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AbetaPP/PS1 mice. J Alzheimer's Dis JAD. 2013;35:847–858. doi: 10.3233/jad-130137. [DOI] [PubMed] [Google Scholar]
- 8.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem. 2006;6:257–268. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 10.Basavarajappa BS, Shivakumar M, Joshi V, Subbanna S. Endocannabinoid system in neurodegenerative disorders. J Neurochem. 2017;142:624–648. doi: 10.1111/jnc.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedse G, Romano A, Lavecchia AM, Cassano T, Gaetani S. The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer's disease. J Alzheimer's Dis JAD. 2015;43:1115–1136. doi: 10.3233/jad-141635. [DOI] [PubMed] [Google Scholar]
- 12.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci Off J Soc Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisogno T, Oddi S, Piccoli A, Fazio D, Maccarrone M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol Res. 2016;111:721–730. doi: 10.1016/j.phrs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Bu W, Ren H, Deng Y, Del Mar N, Guley NM, Moore BM, Honig MG, Reiner A. Mild traumatic brain injury produces neuron loss that can be rescued by modulating microglial activation using a CB2 receptor inverse agonist. Front Neurosci. 2016;10:449. doi: 10.3389/fnins.2016.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrese EJ, Rubio-Casillas A. Biphasic effects of THC in memory and cognition. Eur J Clin Invest. 2018;48:e12920. doi: 10.1111/eci.12920. [DOI] [PubMed] [Google Scholar]
- 16.Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017 doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Sánchez S, García-Yagüe ÁJ, Kügler S, Lastres-Becker I. CX3CR1-deficient microglia shows impaired signaling of the transcription factoR NRF2: implications in tauopathies. Redox Biol. 2019 doi: 10.1016/j.redox.2019.101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosacak MI, Bhattarai P, Bocova L, Dzewas T, Mashkaryan V, Papadimitriou C, Brandt K, Hollak H, Antos CL, Kizil C. Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain. Sci Rep. 2017;7:12959. doi: 10.1038/s41598-017-13311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29. doi: 10.1038/s41582-019-0284-z. [DOI] [PubMed] [Google Scholar]
- 21.Cuadrado A, Kugler S, Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. doi: 10.1016/j.redox.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cudaback E, Marrs W, Moeller T, Stella N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS ONE. 2010;5:e8702. doi: 10.1371/journal.pone.0008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, et al. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol. 2018;175:987–993. doi: 10.1111/bph.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiurchiu V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, Maccarrone M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol. 2013;73:626–636. doi: 10.1002/ana.23875. [DOI] [PubMed] [Google Scholar]
- 25.Chung YC, Shin WH, Baek JY, Cho EJ, Baik HH, Kim SR, Won SY, Jin BK. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson's disease. Exp Mol Med. 2016;48:e205. doi: 10.1038/emm.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denk F, Wade-Martins R. Knock-out and transgenic mouse models of tauopathies. Neurobiol Aging. 2009;30:1–13. doi: 10.1016/j.neurobiolaging.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espejo-Porras F, Garcia-Toscano L, Rodriguez-Cueto C, Santos-Garcia I, de Lago E, Fernandez-Ruiz J. Targeting glial cannabinoid CB2 receptors to delay the progression of the pathological phenotype in TDP-43 (A315T) transgenic mice, a model of amyotrophic lateral sclerosis. Br J Pharmacol. 2019;176:1585–1600. doi: 10.1111/bph.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Ruiz J, Moro MA, Martinez-Orgado J. Cannabinoids in neurodegenerative disorders and stroke/brain trauma: from preclinical models to clinical applications. Neurotherapeutics J Am Soc Exp NeuroTherapeutics. 2015;12:793–806. doi: 10.1007/s13311-015-0381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galan-Ganga M, Del Rio R, Jimenez-Moreno N, Diaz-Guerra M, Lastres-Becker I (2019) Cannabinoid CB2 receptor modulation by the transcription factor NRF2 is specific in microglial cells. Cellul Molecul Neurobiol 10.1007/s10571-019-00719-y [DOI] [PMC free article] [PubMed]
- 31.Goonawardena AV, Sesay J, Sexton CA, Riedel G, Hampson RE. Pharmacological elevation of anandamide impairs short-term memory by altering the neurophysiology in the hippocampus. Neuropharmacology. 2011;61:1016–1025. doi: 10.1016/j.neuropharm.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Yang L, Huang R, Lin L, Shen Y, Cheng L, Jin L, Wang S, Zhu R (2020) Activation of CB2R with AM1241 ameliorates neurodegeneration via the Xist/miR-133b-3p/Pitx3 axis. J Cellul Physiol 10.1002/jcp.29530 [DOI] [PubMed]
- 33.Jadhav S, Avila J, Schöll M, Kovacs GG, Kövari E, Skrabana R, Evans LD, Kontsekova E, Malawska B, de Silva R, et al. A walk through tau therapeutic strategies. Acta Neuropathol Commun. 2019;7:22–22. doi: 10.1186/s40478-019-0664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan CJ, Xi ZX. Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev. 2019;98:208–220. doi: 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, Ittner LM (2012) Lessons from Tau-Deficient Mice. Int J Alzheimer’s Disease 2012: 873270 10.1155/2012/873270 [DOI] [PMC free article] [PubMed]
- 36.Koppel J, Vingtdeux V, Marambaud P, d'Abramo C, Jimenez H, Stauber M, Friedman R, Davies P. CB2 receptor deficiency increases amyloid pathology and alters tau processing in a transgenic mouse model of Alzheimer's disease. Mol Med (Cambridge, Mass) 2014;20:29–36. doi: 10.2119/molmed.2013.00140.revised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lastres-Becker I, De Miguel R, Fernandez-Ruiz JJ. The endocannabinoid system and Huntington's disease. Curr Drug Targets CNS Neurol Disord. 2003;2:335–347. doi: 10.2174/1568007033482751. [DOI] [PubMed] [Google Scholar]
- 38.Lastres-Becker I, Fernandez-Ruiz J. An overview of Parkinson's disease and the cannabinoid system and possible benefits of cannabinoid-based treatments. Curr Med Chem. 2006;13:3705–3718. doi: 10.2174/092986706779026156. [DOI] [PubMed] [Google Scholar]
- 39.Lastres-Becker I, Innamorato NG, Jaworski T, Rabano A, Kugler S, Van Leuven F, Cuadrado A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain. 2014;137:78–91. doi: 10.1093/brain/awt323. [DOI] [PubMed] [Google Scholar]
- 40.Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Laurent C, Buée L, Blum D. Tau and neuroinflammation: What impact for Alzheimer's Disease and Tauopathies? Biomedical Journal. 2018;41:21–33. doi: 10.1016/j.bj.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee G, Leugers CJ. Tau and tauopathies. Prog Mol Biol Transl Sci. 2012;107:263–293. doi: 10.1016/b978-0-12-385883-2.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protocols. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Kim J. Distinct roles of neuronal and microglial CB2 cannabinoid receptors in the mouse hippocampus. Neuroscience. 2017;363:11–25. doi: 10.1016/j.neuroscience.2017.08.053. [DOI] [PubMed] [Google Scholar]
- 45.López A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vázquez C, Amores M, Ruiz-Pérez G, García-García E, et al. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation. 2018;15:158. doi: 10.1186/s12974-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maccarrone M, Maldonado R, Casas M, Henze T, Centonze D. Cannabinoids therapeutic use: what is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert Rev Clin Pharmacol. 2017;10:443–455. doi: 10.1080/17512433.2017.1292849. [DOI] [PubMed] [Google Scholar]
- 47.Maphis N, Jiang S, Xu G, Kokiko-Cochran ON, Roy SM, Van Eldik LJ, Watterson DM, Lamb BT, Bhaskar K. Selective suppression of the α isoform of p38 MAPK rescues late-stage tau pathology. Alzheimer's Res Therapy. 2016;8:54. doi: 10.1186/s13195-016-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mecha M, Feliu A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutierrez S, de Sola RG, Guaza C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233–245. doi: 10.1016/j.bbi.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Miranda M, Kent BA, Morici JF, Gallo F, Saksida LM, Bussey TJ, Weisstaub N, Bekinschtein P. NMDA receptors and BDNF are necessary for discrimination of overlapping spatial and non-spatial memories in perirhinal cortex and hippocampus. Neurobiol Learn Mem. 2018;155:337–343. doi: 10.1016/j.nlm.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Navarro G, Morales P, Rodriguez-Cueto C, Fernandez-Ruiz J, Jagerovic N, Franco R (2016) Targeting Cannabinoid CB2 receptors in the central nervous system. medicinal chemistry approaches with focus on neurodegenerative disorders. Front Neurosci 10: 406. 10.3389/fnins.2016.00406 [DOI] [PMC free article] [PubMed]
- 51.Paloczi J, Varga ZV, Hasko G, Pacher P. Neuroprotection in oxidative stress-related neurodegenerative diseases: role of Endocannabinoid system modulation. Antioxid Redox Signal. 2018;29:75–108. doi: 10.1089/ars.2017.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panza F, Lozupone M, Seripa D, Daniele A, Watling M, Giannelli G, Imbimbo BP. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat Rev Neurol. 2020;16:213–228. doi: 10.1038/s41582-020-0330-x. [DOI] [PubMed] [Google Scholar]
- 53.Piyanova A, Lomazzo E, Bindila L, Lerner R, Albayram O, Ruhl T, Lutz B, Zimmer A, Bilkei-Gorzo A. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech Ageing Dev. 2015;150:55–64. doi: 10.1016/j.mad.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Ploia C, Antoniou X, Sclip A, Grande V, Cardinetti D, Colombo A, Canu N, Benussi L, Ghidoni R, Forloni G, et al. JNK plays a key role in tau hyperphosphorylation in Alzheimer's disease models. J Alzheimer's Dis JAD. 2011;26:315–329. doi: 10.3233/jad-2011-110320. [DOI] [PubMed] [Google Scholar]
- 55.Quraishi SA, Paladini CA. A central move for CB2 receptors. Neuron. 2016;90:670–671. doi: 10.1016/j.neuron.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci Offic J Soc Neurosci. 2005;25:1904–1913. doi: 10.1523/jneurosci.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren S-y, Wang Z-z, Zhang Y, Chen N-h (2020) Potential application of endocannabinoid system agents in neuropsychiatric and neurodegenerative diseases—focusing on FAAH/MAGL inhibitors. Acta Pharmacologica Sinica: 10.1038/s41401-020-0385-7 [DOI] [PMC free article] [PubMed]
- 58.Ren Y, Sahara N. Characteristics of Tau Oligomers. Front Neurol. 2013 doi: 10.3389/fneur.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Cueto C, Benito C, Fernandez-Ruiz J, Romero J, Hernandez-Galvez M, Gomez-Ruiz M. Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. Br J Pharmacol. 2014;171:1472–1489. doi: 10.1111/bph.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sagredo O, Gonzalez S, Aroyo I, Pazos MR, Benito C, Lastres-Becker I, Romero JP, Tolon RM, Mechoulam R, Brouillet E, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington's disease. Glia. 2009;57:1154–1167. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarne Y, Asaf F, Fishbein M, Gafni M, Keren O. The dual neuroprotective-neurotoxic profile of cannabinoid drugs. Br J Pharmacol. 2011;163:1391–1401. doi: 10.1111/j.1476-5381.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmole AC, Lundt R, Toporowski G, Hansen JN, Beins E, Halle A, Zimmer A. Cannabinoid receptor 2-deficiency ameliorates disease symptoms in a mouse model with Alzheimer's disease-like pathology. Journal of Alzheimer's disease : JAD. 2018;64:379–392. doi: 10.3233/jad-180230. [DOI] [PubMed] [Google Scholar]
- 63.Shahbazi F, Grandi V, Banerjee A, Trant JF (2020) Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 23: 101301 10.1016/j.isci.2020.101301 [DOI] [PMC free article] [PubMed]
- 64.Sierra S, Luquin N, Rico AJ, Gómez-Bautista V, Roda E, Dopeso-Reyes IG, Vázquez A, Martínez-Pinilla E, Labandeira-García JL, Franco R, et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct. 2015;220:2721–2738. doi: 10.1007/s00429-014-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, et al. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turcotte C, Blanchet M-R, Laviolette M, Flamand N. The CB(2) receptor and its role as a regulator of inflammation. Cell Mol Life Sci. 2016;73:4449–4470. doi: 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, Liu BJ, Cao Y, Xu WQ, Sun DS, Li MZ, Shi FX, Li M, Tian Q, Wang JZ, et al. Deletion of Type-2 Cannabinoid Receptor induces Alzheimer's disease-like Tau pathology and memory impairment through AMPK/GSK3beta Pathway. Mol Neurobiol. 2018;55:4731–4744. doi: 10.1007/s12035-017-0676-2. [DOI] [PubMed] [Google Scholar]
- 68.Yoshiyama Y, Higuchi M, Zhang B, Huang S-M, Iwata N, Saido Takaomi C, Maeda J, Suhara T, Trojanowski JQ, Lee VMY. Synapse loss and microglial activation precede tangles in a p301s tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig. S1: hTAUP301S overexpression in 12 months old transgenic mice induced inflammatory response in the hippocampus. (A) Analysis of mRNA levels of inflammatory genes RelA, Tnf and Il-1β in 12 months old transgenic mice. (B) Analysis of mRNA levels of inflammatory genes RelA, Tnf and Il-1β in 7 months old transgenic mice. All genes were normalized by Tbp (TATA-box binding protein) mRNA levels, n=4-5 samples ± SEM. The data has been processed with Student's t-test analysis to determine the significance of the changes. The asterisks represent the difference in significance **** p <0.0001
Additional file 2. Fig. S2: (A) qRT-PCR determination of mRNA levels of Cnr2. Measures were normalized by Tbp mRNA levels. n=5-7 samples ± SEM. Asterisks denote significant differences with ***p<0.001, comparing each group with the contralateral hippocampi from Cnr2+/+ mice or the indicated groups, according to two-way ANOVA followed by Bonferroni post-test. (B) Double immunofluorescence staining of 30 μm-thick sections of contralateral and ipsilateral hippocampus from Cnr2+/+ mice injected with AAV-hTAUP301L (n=3). The ipsilateral side from Cnr2-/- was used as a control (n=1). Green, anti-CB2. Red, anti-TAU-HT7. Blue, DAPI.
Additional file 3. Fig. S3: The deficiency in CB2 does not produce changes in the neuroinflammation associated with hTAUP301L. qRT-PCR determination of mRNA levels of (A) Gfap, (B) Iba1, (C) Il-1β, and (D) Tnf. All genes were normalized by Tbp mRNA levels. n=5-7 samples ± SEM. Asterisks denote significant differences with *p<0.05, **p<0.01, and ***p<0.001, comparing each group with the contralateral hippocampi from Cnr2+/+ mice or the indicated groups, according to two-way ANOVA followed by Bonferroni post-test.
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author on reasonable request.