INTRODUCTION
The prevalence of peripheral artery disease (PAD) is increasing worldwide and it is estimated to affect about 360 million patients by 2030[1, 2]. PAD contributes significantly towards patient’s disability and adversely affect their health status, including their symptoms, functioning, and quality of life[3–5]. In patients with PAD, mental health concerns particularly self-perceived stress are highly prevalent[6]. It has been shown that about 30% of the patients with PAD, have high stress at presentation[6]. Stress is associated with adverse health behaviors such as smoking, decreased sleep, decreased physical activity and various pathophysiological effects resulting in hypertension, hyperglycemia and endothelial dysfunction[7]. Moreover, stress is also associated with decreased compliance and delays in seeking care[8]. Through an interplay of these complex mechanisms stress is a potent cardiovascular risk factor [7] and the latest American College of Cardiology guidelines on prevention lay emphasis on addressing psychosocial stressors in patients with cardiovascular disease[9]. In our previous work we have shown that higher level of chronic stress is associated incrementally with higher mortality in patients with PAD [10].
Beyond mortality, patients with PAD are equally or more concerned about their health status outcomes[11]. Some studies have examined the association of stress and distressed personality traits with disease-specific health status in patients with PAD[12, 13]. However, these studies looked at either stable personality constructs or examined cross-sectional associations and did not account for changes in stress levels over time. There exists a gap in understanding the association of chronic stress with health status in patients presenting with new symptoms of PAD. A deeper understanding of the potential impact of chronic self-perceived stress on patient’s health status over time, could inform the design of chronic disease management programs for PAD as stress is a modifiable risk factor for which evidence-based treatments exist[14, 15].
METHODS
Study Population
The PORTRAIT registry enrolled patients who presented with symptoms of PAD to 16 sub-specialty clinics across USA, Netherlands, and Australia from June 2011 to December 2015. The detailed methodology and study protocol have been described previously[16]. Briefly, patients presenting to a specialty clinic with symptoms of PAD and an ankle brachial index (ABI) ≤ 0.90 or a significant drop in post-exercise ankle pressure (≥20mm of Hg) were enrolled. Patients with non-compressible ABI ≥ 1.30, critical limb ischemia, lower-limb revascularization in the 12 months prior to the PAD visit and those who were incarcerated, hard of hearing or unable to provide informed consent were excluded.
Patient demographics, health status, psychosocial characteristics, socioeconomic variables and cardiovascular lifestyle factors were obtained through interviews at the initial visit. Patients’ symptoms, medical history, comorbidities, and PAD diagnostic information were abstracted from medical records. Serial information about health status was collected at baseline, 3-, 6- and 12-month follow-up through centralized follow-up. All study participants provided either written or telephonic informed consent. The study protocol was approved by the Institution Review Boards of all participating sites.
Assessment of Self-Perceived Stress
Patients’ level of perceived stress was assessed at enrollment and follow-up visits with the 4-item perceived stress scale (PSS-4). The PSS-4 is a reliable and valid measure (Cronbach’s Alpha 0.67–0.79) of an individual’s self-evaluation of control and confidence in handling the stressful situations over the past month[17]. Cronbach’s Alpha for PSS-4 in our study was 0.67. Scores range from 0–16, with higher scores indicating stress exceeding an individual’s ability to cope. As the PSS-4 is a non-diagnostic instrument, there are no established thresholds, and scores are compared to a normative value[18]. Hence, to describe the impact of chronic stress with health status outcomes, we examined the association of PSS-4 as a continuous variable with health status outcomes.
For purposes of comparing patient characteristics by low vs. high levels of stress, we used a score of ≥ 6 to describe patients with high levels of self-perceived stress. This threshold is in accordance with prior global normative data described in an English population and other countries. This threshold is in accordance with prior global normative data described in an English population and other countries [18–21].
We wanted to examine high overall stress levels in the immediate management period following a recent diagnosis of new or worsening PAD, and how these are related to patient’s health status outcomes. The period of stress levels assessed over time coincided with the PAD treatment pathway, with the underlying thought that future interventions could support individuals who are vulnerable to high stress experiences during this time that they are receiving PAD care and as ways to potentially optimize the PAD rehabilitation process and patients’ subsequent health status outcomes. Hence, to capture a patient’s stress levels during this period, we calculated the PSS-4 score at baseline, 3- and 6-month follow-up and averaged them across these time points. This was our main exposure variable.
Outcome Assessment
Our primary outcome was recovery in disease-specific and generic health status at 12-months. This was defined as change in health status at 12-month follow-up compared to baseline.
Disease specific health status was assessed using the PAD Questionnaire (PAQ). PAQ is a 20-item multidimensional instrument that is a valid, reliable and responsive disease specific measure of patients with PAD[22, 23]. A single item identifies the more symptomatic limb and remaining 19 are answered according to variable Likert response scales to assess 6 domains, physical limitation, symptoms, symptom stability, social limitation, treatment satisfaction and quality of life. The PAQ summary score integrates all domains except symptom stability and treatment satisfaction.[22] Scores range from 0–100 with higher scores indicating less functional limitation, fewer symptoms and better quality of life. A change ≥ 8-points on the PAQ summary score was found to be a clinically meaningful difference. The PAQ has been translated and validated in the Dutch population[24].
Generic health status was assessed using the EuroQoL Visual Analog Scale (VAS).[25] The VAS is a measure of perceived general health that consists of a single item “feeling thermometer”, on which the patients rate their general health state from 0 (worst imaginable) to 100 (best imaginable)[25].
Other Variables
Depressive symptoms were assessed using the eight-item patient health questionnaire (PHQ-8)[26]. Scores range from 0–24 with higher score indicating greater depressive symptoms. Score ≥ 10 are considered to imply clinically significant symptoms[26]. PAD disease severity was assessed using ABI at baseline and Rutherford classification of symptoms. Treatment strategy at 3-months following patient’s PAD work-up was categorized as invasive (percutaneous or surgical intervention) or non-invasive (medical therapy only). Socioeconomic status was assessed using patient responses to questions regarding their highest level of education (high school/college/post graduate), avoidance of care due to costs (yes/no) and financial resources left at the end of the month (some/just enough/not enough).
Statistical Analysis
For descriptive analyses, we dichotomized our analytical cohort based on patients averaged stress scores (from enrollment to 6-month follow up) as high (average PSS-4 ≥ 6) vs. low (average PSS-4 <6). Patient demographics, PAD severity, treatment strategy and comorbidities at baseline were compared in patients with high and low chronic stress. Continuous variables were presented as means ± standard deviations and were compared across groups using independent Student t-tests for independent samples or Mann-Whitney U tests for non-normally distributed variables. Categorical variables were presented as frequencies with percentages and were compared using Chi-square tests.
To examine the association of chronic stress with patients’ health status trajectories, we plotted mean PAQ scores at each follow-up assessment in patients with high and low levels of chronic stress. To examine the association of averaged stress scores with change (baseline to 12-months) in generic and disease specific health status at 12-months, we used hierarchical multivariable regression models, with a fixed effect for country and a random effect for site to account for clustering at the site level. To understand the impact of factors that could explain the relationship of chronic stress with PAD outcomes we defined several models a priori and used a step-wise modelling approach. In Model 1, we assessed the unadjusted association of average PSS-4 score with change in health status at 12-months, except for adjusting for baseline health status. In Model 2, we additionally adjusted for patient demographics (age, sex and race). As stress has been associated development of diabetes[27], hypertension [28], myocardial infarction [29], heart failure[30] and smoking[27] we adjusted for these covariates as well as PAD severity (assessed with baseline ABI) and treatment type (invasive/medical) in Model 3. This was to assess if there remained an independent association after accounting for these factors; which could potentially have either a confounding or mediating effect, or both. Additionally, socioeconomic status has been associated with adverse outcomes in patients with PAD[31]. Hence in Model 4, we additionally adjusted for socioeconomic status. To understand the association of stress with recovery in health status independent of depressive symptoms we did the following exploratory analysis. In our model 4, we adjusted for averaged (across all time points) PHQ-8 score to examine if there remained a significant association between chronic exposure to stress and recovery in health status, outside the context of depressive symptoms.
As a sensitivity analysis we quantified each patient’s average stress over 12-months of the study, by taking the average of PSS-4 scores at baseline, 3-, 6- and 12-month follow-up and repeated the above analysis, to examine the impact on recovery in health status at 12-months.
All our models included restricted cubic splines for estimating effects of continuous variables to accommodate for non-linear relationships. In cases where no significant evidence of non-linearity was found, associations were re-estimated using linear effects to simplify interpretation. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). All statistical tests were 2-tailed, and significance was determined using α = 0.05.
Missing Data
Patients with missing baseline PSS-4 scores and missing health status assessments at either baseline or 12-months were excluded. For follow-up PSS-4 assessments, 244 (23.02%) had 1 and 302 (28.49%) had 2 missing assessments respectively. The average score for all available PSS-4 measurements was calculated for each patient as the main exposure variable. The rate of missing data at the covariate level was minimal (<1% for all covariates), and therefore, no imputation was pursued.
RESULTS
After excluding patients who had missing baseline PSS-4 assessment (n=21), and patients who had missing health status assessment at 12-months (n=194), our final study cohort included 1060 patients. Figure 1 shows the STROBE diagram describing the derivation of the analytical cohort. Supplementary Table 1 compares the differences in the patients who were excluded and those who were not. Patients who were excluded had a higher average PSS-4 and PHQ-8 scores and were less likely to have at least high school level of education and have hypertension. There were no significant differences in demographics or prevalence of other comorbidities except hypertension which was less prevalent in patients who were excluded.
Figure 1.
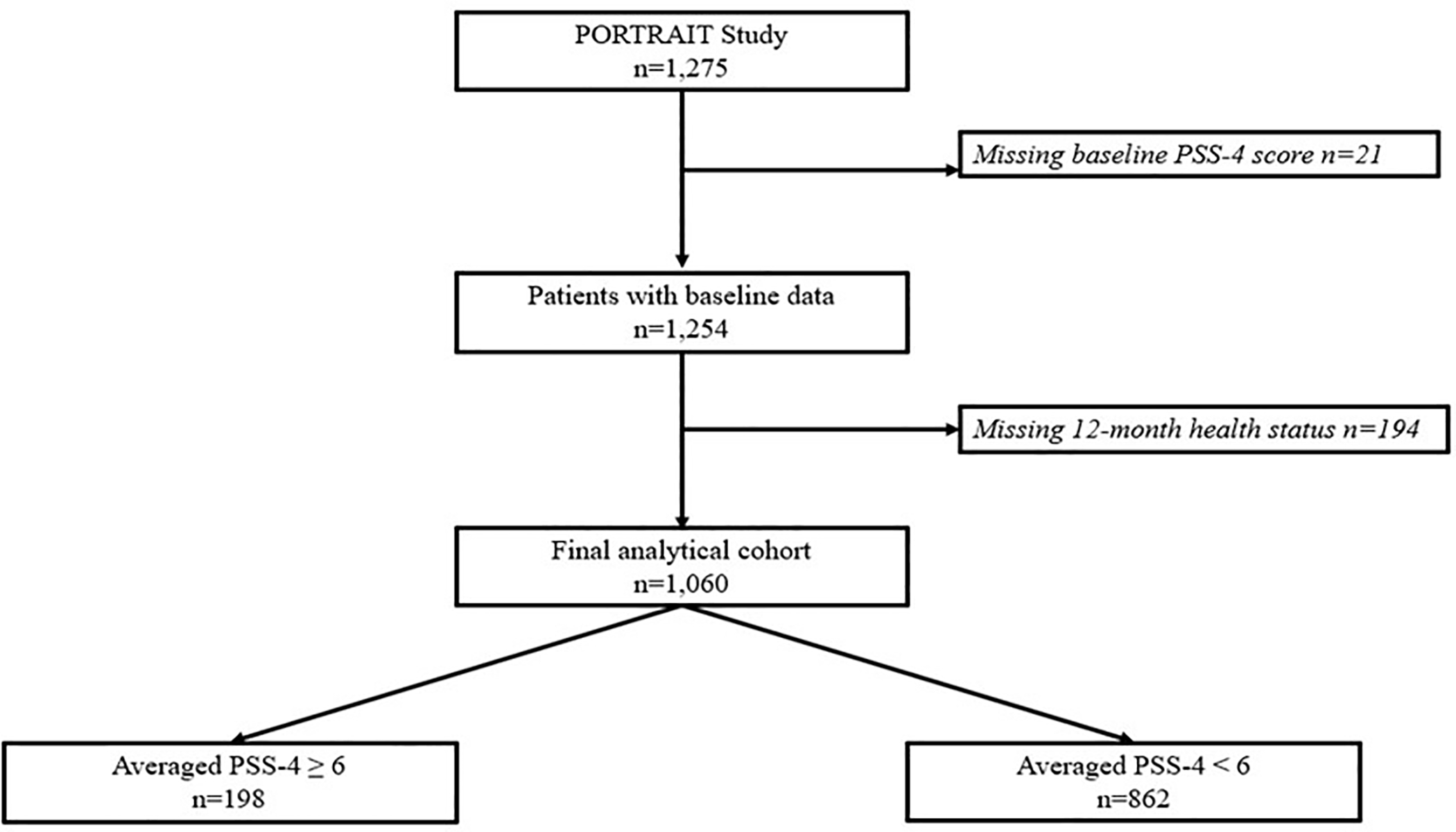
STROBE diagram showing selection process for final study cohort.
In the final study cohort, mean age was 67.7 ± 9.3 years, 37.1% (n=393) of the patients were females and 82.3% (n=872) were white. Comorbidities were highly prevalent with 80.9% (n=858) patients having hypertension, 19.6% (n=208) having history of myocardial infarction, 32.5% (n=345) having diabetes, 9.7% (n=103) having congestive heart failure, 36.4% (n=386) being active smokers and 14.1% (n=149) having clinically relevant depressive symptoms (PHQ-8 ≥10). About 10% (n=103) of the patients reported not having enough finances left at the end of the month to make ends meet, and 70.1% (n=743) reported having at least high school education.
Table 1 describes the baseline demographic and clinical characteristics of patients stratified by high (≥6) and low (<6) average PSS-4 score. Patients with high average stress were younger, more likely to be females and less likely to be white. Additionally, patients with high average stress had greater prevalence of diabetes, heart failure, sleep apnea, coronary artery disease and hypertension, and were less likely to receive an invasive treatment strategy. There were no significant differences in baseline ABI and prevalence of other comorbid conditions. More patients with high chronic stress reported not having enough finances left at the end of the month and avoiding care due to costs. Moreover, depressive symptoms were significantly more common in patients with higher stress.
Table 1.
Patient demographics, comorbidities and treatment and socioeconomic factors stratified by patients with high vs low chronic stress level.
| PSS-4 Score ≥ 6 n=198 | PSS-4 Score <6 n=862 | P value | |
|---|---|---|---|
| Demographics | |||
| Age (Mean ± SD) | 65.2 ± 9.5 | 68.2 ± 9.2 | < 0.001 |
| Female Sex (%) | 44.9 | 35.3 | 0.01 |
| White (%) | 70.7 | 84.9 | < 0.001 |
| Comorbidities and treatment (%) | |||
| Current smoker | 40.9 | 35.4 | 0.32 |
| Diabetes | 41.9 | 30.4 | 0.001 |
| Hypertension | 85.4 | 79.1 | 0.02 |
| Congestive Heart Failure | 14.6 | 8.6 | 0.009 |
| Chronic Kidney Disease | 11.6 | 10.6 | 0.67 |
| Cancer | 11.6 | 9.6 | 0.40 |
| Osteoarthritis | 8.6 | 9.2 | 0.78 |
| Sleep Apnea | 11.6 | 7.8 | 0.08 |
| Chronic Back Pain | 13.6 | 14.0 | 0.88 |
| Coronary Artery Disease | 51.0 | 42.7 | 0.03 |
| History of MI | 23.7 | 18.7 | 0.11 |
| History of stroke | 11.6 | 11.6 | 0.81 |
| Invasive Treatment Strategy | 18.3 | 28.2 | 0.004 |
| ABI (Mean ± SD) | 0.65 ± 0.20 | 0.67 ± 0.18 | 0.20 |
| Rutherford Classification of Symptoms (%) | |||
| Mild Claudication | 16.8 | 23.9 | <0.001 |
| Moderate Claudication | 45.7 | 50.0 | |
| Severe Claudication | 37.6 | 26.1 | |
| Socioeconomic Status (%) | |||
| High school education | 73.1 | 69.9 | 0.37 |
| Not enough money left at month end | 24.4 | 6.7 | <0.001 |
| Avoiding care due to cost | 25.0 | 11.4 | <0.001 |
| Work for pay | 46.2 | 36.4 | 0.25 |
| Depressive Symptoms at Baseline | |||
| PHQ-8 ≥ 10 (%) | 41.0 | 8.2 | < 0.001 |
| PHQ-8 (Mean ± SD) | 8.5 ± 6.4 | 3.6± 3.8 | < 0.001 |
Ankle Brachial Index (ABI), Myocardial Infarction (MI), 8-point patient health questionnaire (PHQ-8),
Overall in the study cohort the mean PAQ summary score at baseline and 12-month follow-up was 49.6 ± 21.6 and 70.1 ± 25.5 respectively. Mean VAS was 66.6 ± 19.0 at baseline and 70.2 ± 17.8 at 12-month follow-up. Figure 2 describes the trajectories of disease-specific and generic health status in patients with high and low levels of chronic stress over the 12-months of the study. Patients who were exposed to high levels of chronic stress had poorer disease-specific health status (across all PAQ domains) as well as generic health status (VAS) at baseline and all follow-up assessments.
Figure 2.
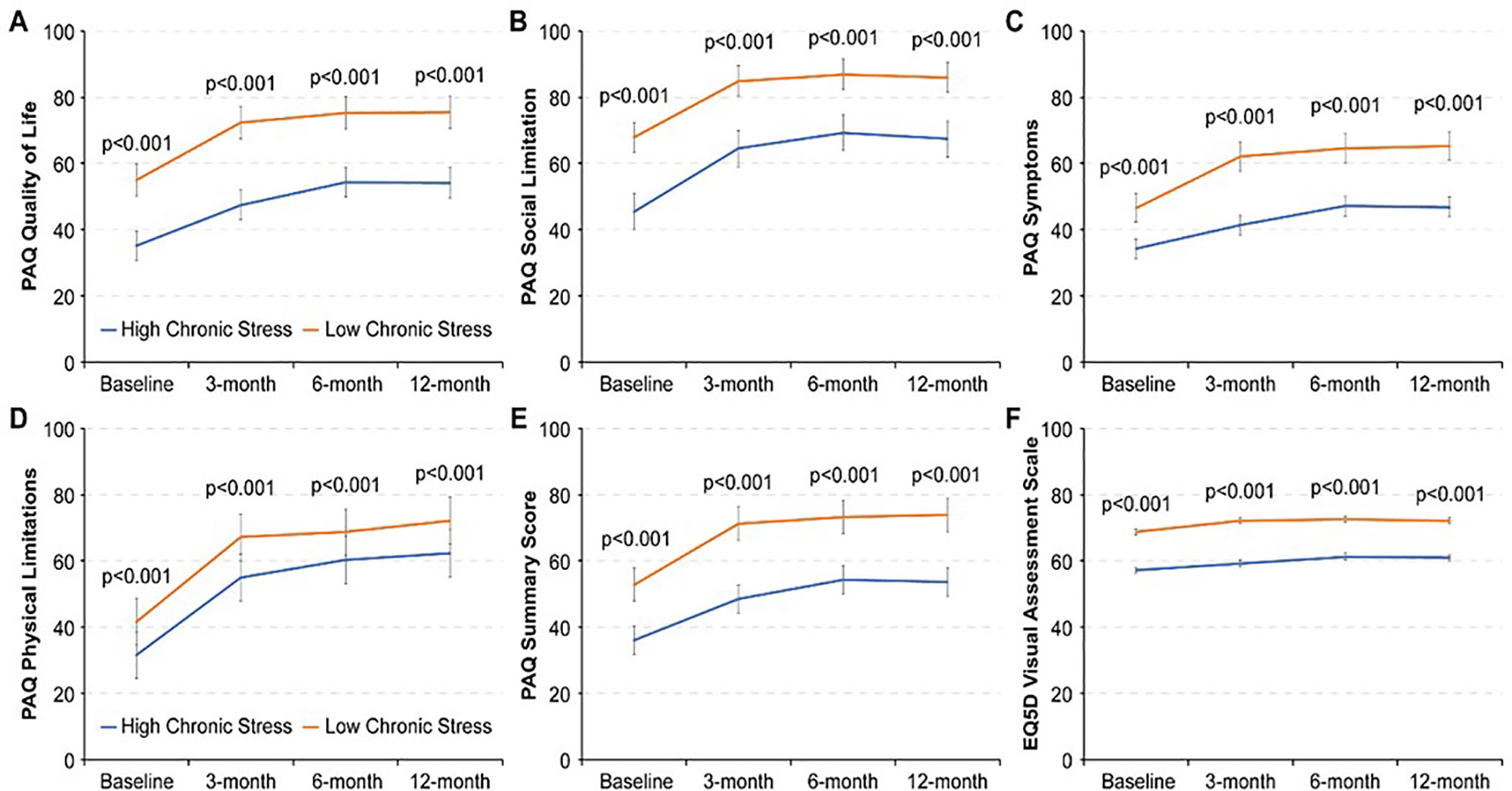
Generic and disease-specific health status at baseline, 3-, 6- and 12-month follow-up in patients with high and low chronic stress. (disease specific health status assessed by Peripheral Artery Disease Questionairre Summary Score and generic health status examined by Euro-Quality of Life Visual Analog Scale)
[PAQ= Peripheral Artery Disease Questionnaire]
Figure 3 and 4 show the association of average PSS-4 scores with recovery (from baseline) in PAQ summary score and VAS at 12-months. Results for Model 1 are unadjusted analysis, and Model 2–4 are results after sequentially adjusting for patient demographics in Model 2, comorbidities, disease severity and treatment in Model 3, and socioeconomic status in Model 4. In adjusted models accounting for potential confounding factors including patient demographics, comorbidities, disease severity, treatment type and socioeconomic status, higher stress scores were associated with lower PAQ SS (−1.4 points per +1-point increase in PSS-4 95% CI −2.0, −0.9 p <0.001) and VAS (−1.2 points per +1-point increase in PSS-4 95% CI −1.6, −0.8 p <0.001) at 12-months. After accounting for depressive symptoms (by adjusting for averaged PHQ-8 score), the association between chronic stress and 12-month change in PAQ summary score attenuated (−0.10 points per +1-point increase in PSS-4 95% CI −0.7, 0.5 p=0.75). However there remained a significant association of chronic stress with 12-month change in VAS (−0.5 points per +1-point increase in PSS-4 95% CI −0.9, 0.00 p=0.05).
Figure 3.
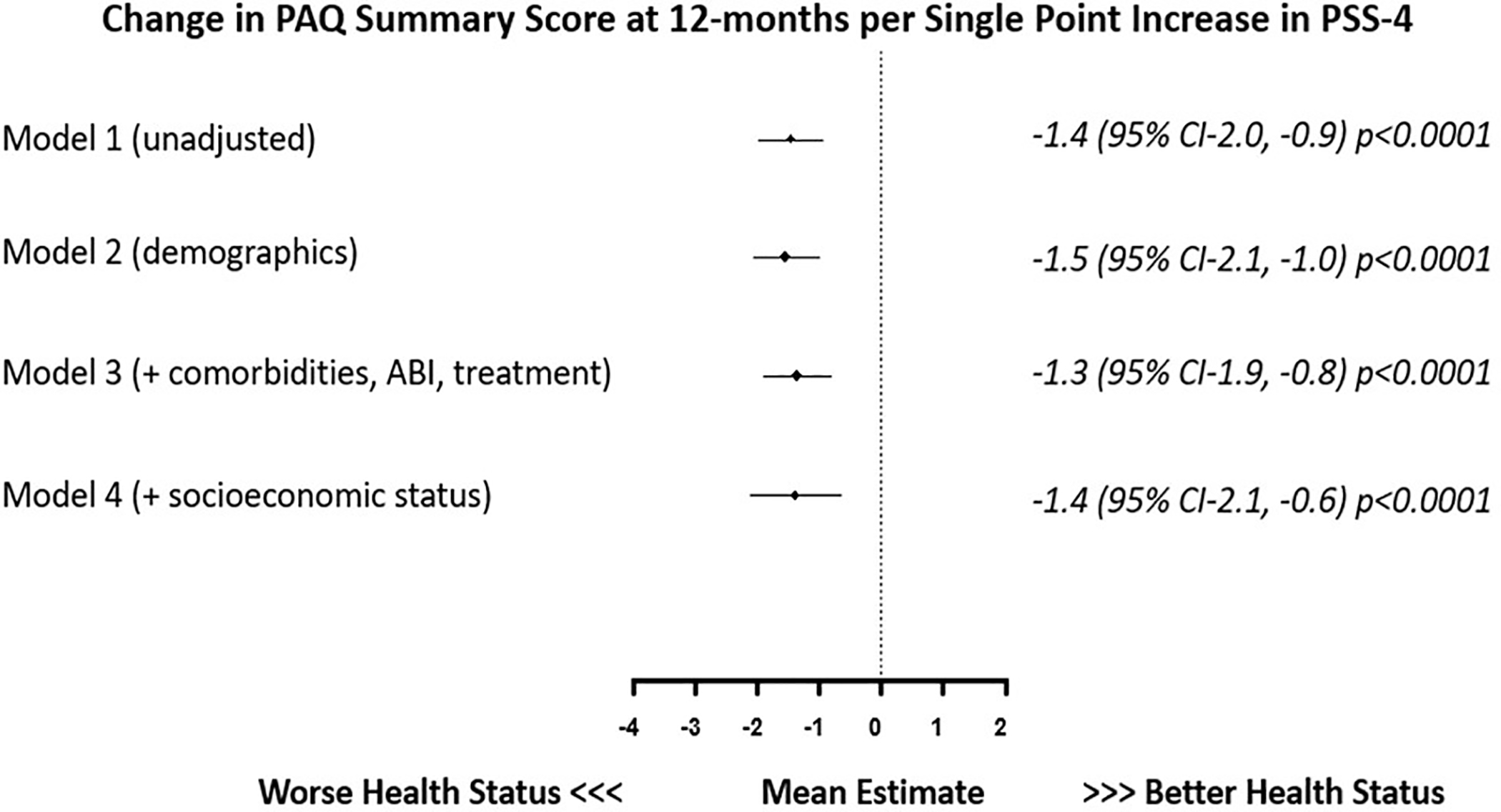
Association of a single point increase in averaged PSS-4 with corresponding change in PAQ SS (baseline to 12-months) in patients with peripheral artery disease.
[PSS-4= 4-point Perceived Stress Scale, PAQ= Peripheral Artery Disease Questionnaire]
Figure 4.
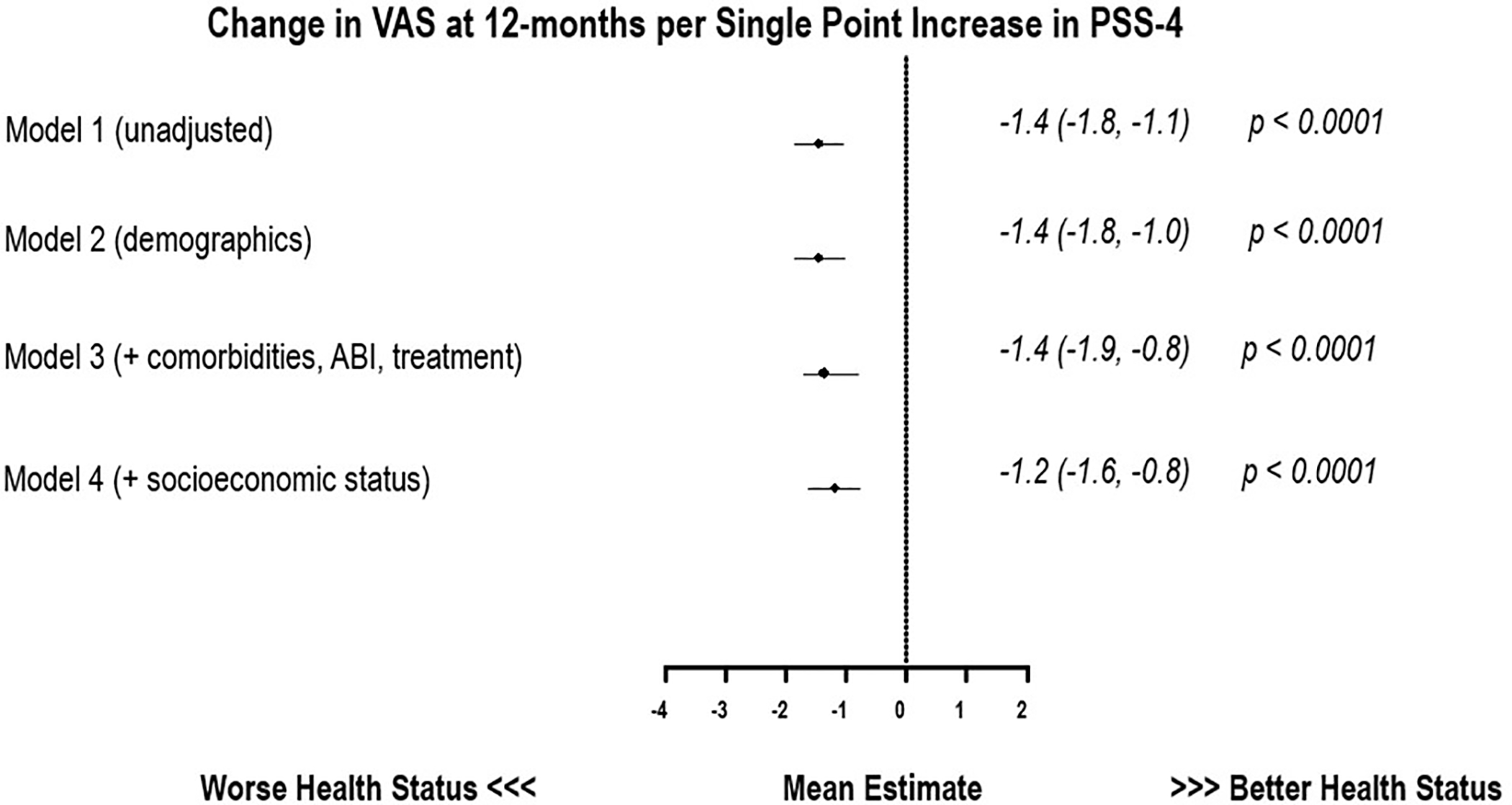
Association of a single point increase in averaged PSS-4 with corresponding change in (baseline to 12-months) EuroQoL Visual Analog Scale in patients with PAD.
[PSS-4= 4-point Perceived Stress Scale]
In our sensitivity analysis, including the 12-month PSS-4 assessment in the exposure variable, there remained a substantial association of chronic stress over 12-month follow-up with poorer recovery in health status at 12-months. Supplementary Figure 1 and 2, show these results. In adjusted models accounting for potential confounding factors including patient demographics, comorbidities, disease severity, treatment type and socioeconomic status, higher stress scores were associated with lower PAQ SS (−2.2 points per +1-point increase in PSS-4 95% CI −2.9, −1.6 p <0.001) and VAS (−2.0 points per +1-point increase in PSS-4 95% CI −2.4, −1.5 p <0.001) at 12-months.
DISCUSSION
With a growing population of PAD patients, it is critical to examine factors associated with worse outcomes in patients with PAD as this could identify potential novel therapeutic strategies and treatment plans. Chronic stress is a debilitating condition that is common, adversely impacts people’s lives and exerts a significant burden on health care systems globally[32, 33]. In a multi-national contemporary registry of patients with PAD we found that about 19% of the patients with PAD continue to have high stress levels after their diagnosis. Moreover, higher chronic self-perceived stress was strongly associated with worse symptoms, function and quality of life. These associations were independent of patients’ demographics, baseline ABI, major comorbidities, treatment type, and socioeconomic indicators.
We have previously described the association of chronic stress with an incremental risk of mortality in patients with PAD [10]. This study significantly extends the understanding of the adverse outcomes associated with chronic stress in patients with PAD, by including patient’s health status. Previous studies have shown the prevalence of mental stress and anxious personality traits is high in patients with PAD and is associated with poorer health status[12, 13]. However these studies have only assessed generic measures of health status[12] and only examined select populations without specifically quantifying patients’ chronic mental stress[13]. Our results substantially add to current literature by using data for patients enrolled at similar time point for progression of PAD (development of new symptoms), use of validated instrument (PSS-4) to assess patients chronic self-perceived stress at multiple assessments over a 6-month follow-up period and looking at longitudinal association with health status.
Chronic mental stress is associated with development and progression of cardiovascular disease. The mechanisms underlying this association are complex and multi-faceted. These include development of adverse health behaviors such as physical inactivity[34], obesity[27] and smoking[35]. Stress is also associated with pathophysiological pathways leading to development of higher blood pressure[28], insulin resistance[27], enhanced activity of hypothalamic-pituitary axis [36], platelet reactivity[37] and inflammation[38]. Moreover, patients with higher stress are less likely to be compliant with treatment and may present with vague symptoms causing treatment delays[8]. Through interplay of these complex processes, stress has been recognized as a strong independent determinant of outcomes in patients with atherosclerotic vascular disease[7]. However, stress is a modifiable risk factor and the impact of stress on adverse outcomes can be attenuated. In randomized studies, interventions to improve coping skills for stress such as cognitive behavioral therapy and transcendental meditation in addition to standard therapy were associated with decreased risk of mortality, recurrent myocardial infarction and stroke in patients with cardiovascular disease [14, 15]. Additionally it has also been shown that in a heterogenous patient population group mindfulness meditation training programs to help patients cope with chronic stress improved patients functional status and physical symptoms[39]. Optimizing health status and disease-specific quality of life have been recognized as important goals of treatment in patients with cardiovascular disease [40]. Given that, we found a strong independent association of stress with worse health status outcomes, our findings highlight potential for targeted interventions such as transcendental meditation, cognitive behavioral therapy and group meditation therapy in addition to standard therapy to directly improve health status outcomes of patients with PAD.
Our findings should be interpreted in the context of the following potential limitations. Firstly, the observational design of this study precludes any inferences about causation. More research is needed to test the efficacy of interventions to mitigate the impact of chronic stress on outcomes in PAD. Furthermore, other unmeasured factors could have influenced health status outcomes and could have biased our results. However, even after extensively adjusting for factors that could be along the causal pathway between exposure to stress and patient’s health status, a significant and strong association with poorer health status with higher exposure to stress remained. Secondly, the sites that recruited patients for the PORTRAIT study may not be representative of other vascular clinics that were not represented in this study. Third, we had a large proportion of patients with missing data for stress and health status assessment and this could have biased our results. Finally, we quantified stress using the PSS-4 which is a generic and brief instrument to assess perceived stress levels in communities and this measurement may not necessarily extend to other measures of stress or other domains of mental health functioning, nor should it be used for diagnosing purposes, as stress reactions are universal responses.
Across a broad spectrum of PAD-specific health status assessments quantifying the impact on patient’s symptoms, function and quality of life as well as measures of generic health status, we found that recovery in health status in patients with PAD was adversely impacted by higher chronic self-perceived stress. Moreover, about 1 in 5 patients diagnosed with PAD continue to have high stress level following their diagnosis. This highlights the potential of using a holistic treatment approach that includes addressing patients’ psychosocial issues, as a strategy that could improve outcomes in patients with PAD.
Supplementary Material
Acknowledgement
Conflict of Interest:
Dr. Malik, Dr. Peri-Okonny and Dr. Thomas are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Mena is a paid consultant for Abbott, Cook, Cardinal Health, Bard, Boston Scientific, Gore, and Medtronic. Dr. Spertus owns the copyright for the KCCQ; has equity interest in Health Outcomes Sciences; has received consulting income from Novartis, Bayer, AstraZeneca, V-wave, Corvia, and Janssen; has served on the Advisory Board for United Healthcare; and has served on the Board of Directors for Blue Cross Blue Shield of Kansas City. Dr. Smolderen reports support through unrestricted research grants from Abbott Vascular, Boston Scientific, and Terumo.
Sources of Funding:
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CE-1304-6677). The views, statements, and opinions in this report are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute its Board of Governors or Methodology Committee.
Abbreviations
- ABI
Ankle Brachial Index
- VAS
EuroQoL Visual Analog Scale
- PAD
Peripheral Arterial Disease
- PAQ
Peripheral Arterial Disease Questionnaire
- PORTRAIT
Patient-Centered Outcomes Related to Treatment Practices in Peripheral Artery Disease: Investigating Trajectories
- PSS-4
4-item perceived stress scale
- PHQ-8
8-item patient health questionnaire
REFERENCES
- [1].Shu J, Santulli G, Update on peripheral artery disease: Epidemiology and evidence-based facts, Atherosclerosis 275 (2018) 379–381. 10.1016/j.atherosclerosis.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thiruvoipati T, Kielhorn CE, Armstrong EJ, Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes, World journal of diabetes 6(7) (2015) 961–9. 10.4239/wjd.v6.i7.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Breek JC, Hamming JF, De Vries J, van Berge Henegouwen DP, van Heck GL, The impact of walking impairment, cardiovascular risk factors, and comorbidity on quality of life in patients with intermittent claudication, J Vasc Surg 36(1) (2002) 94–9 [DOI] [PubMed] [Google Scholar]
- [4].Chetter IC, Dolan P, Spark JI, Scott DJ, Kester RC, Correlating clinical indicators of lower-limb ischaemia with quality of life, Cardiovasc Surg 5(4) (1997) 361–6 [DOI] [PubMed] [Google Scholar]
- [5].Breek JC, Hamming JF, De Vries J, Aquarius AE, van Berge Henegouwen DP, Quality of life in patients with intermittent claudication using the World Health Organisation (WHO) questionnaire, Eur J Vasc Endovasc Surg 21(2) (2001) 118–22. 10.1053/ejvs.2001.1305 [DOI] [PubMed] [Google Scholar]
- [6].Thomas M, Patel KK, Gosch K, Labrosciano C, Mena-Hurtado C, Fitridge R, Spertus JA, Smolderen KG, Mental health concerns in patients with symptomatic peripheral artery disease: Insights from the PORTRAIT registry, Journal of psychosomatic research 131 (2020) 109963–109963. 10.1016/j.jpsychores.2020.109963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kivimaki M, Steptoe A, Effects of stress on the development and progression of cardiovascular disease, Nature reviews. Cardiology 15(4) (2018) 215–229. 10.1038/nrcardio.2017.189 [DOI] [PubMed] [Google Scholar]
- [8].Hamer M, Molloy GJ, Stamatakis E, Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms, J Am Coll Cardiol 52(25) (2008) 2156–62. 10.1016/j.jacc.2008.08.057 [DOI] [PubMed] [Google Scholar]
- [9].Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J, Ziaeian B, 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Journal of the American College of Cardiology 74(10) (2019) 1376–1414. 10.1016/j.jacc.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Malik AO, Peri-Okonny P, Gosch K, Thomas M, Mena C, Hiatt WR, Jones PG, Provance JB, Labrosciano C, Jelani QU, Spertus JA, Smolderen KG, Association of Perceived Stress Levels With Long-term Mortality in Patients With Peripheral Artery Disease, JAMA network open 3(6) (2020) e208741. 10.1001/jamanetworkopen.2020.8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Provance JB SJ., Decker C, Smolderen KG, Abstract 16038: How Patients Value Different Treatment Goals and Outcomes in Peripheral Artery Disease Outcomes: Insights From the PORTRAIT Registry, Circulation 138 (2018) [Google Scholar]
- [12].Aquarius AE, De Vries J, Henegouwen DP, Hamming JF, Clinical indicators and psychosocial aspects in peripheral arterial disease, Archives of surgery (Chicago, Ill. : 1960) 141(2) (2006) 161–6; discussion 166. 10.1001/archsurg.141.2.161 [DOI] [PubMed] [Google Scholar]
- [13].Aquarius AE, Denollet J, Hamming JF, Van Berge Henegouwen DP, De Vries J, Type-D personality and ankle brachial index as predictors of impaired quality of life and depressive symptoms in peripheral arterial disease, Archives of surgery (Chicago, Ill. : 1960) 142(7) (2007) 662–7. 10.1001/archsurg.142.7.662 [DOI] [PubMed] [Google Scholar]
- [14].Schneider RH, Grim CE, Rainforth MV, Kotchen T, Nidich SI, Gaylord-King C, Salerno JW, Kotchen JM, Alexander CN, Stress reduction in the secondary prevention of cardiovascular disease: randomized, controlled trial of transcendental meditation and health education in Blacks, Circulation. Cardiovascular quality and outcomes 5(6) (2012) 750–8. 10.1161/circoutcomes.112.967406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svardsudd K, Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent recurrent cardiovascular events in patients with coronary heart disease: Secondary Prevention in Uppsala Primary Health Care project (SUPRIM), Archives of internal medicine 171(2) (2011) 134–40. 10.1001/archinternmed.2010.510 [DOI] [PubMed] [Google Scholar]
- [16].Smolderen KG, Gosch K, Patel M, Jones WS, Hirsch AT, Beltrame J, Fitridge R, Shishehbor MH, Denollet J, Vriens P, Heyligers J, Stone MN, Aronow H, Abbott JD, Labrosciano C, Tutein-Nolthenius R, J AS, PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): Overview of Design and Rationale of an International Prospective Peripheral Arterial Disease Study, Circ Cardiovasc Qual Outcomes 11(2) (2018) e003860. 10.1161/circoutcomes.117.003860 [DOI] [PubMed] [Google Scholar]
- [17].Cohen S, Kamarck T, Mermelstein R, A global measure of perceived stress, J Health Soc Behav 24(4) (1983) 385–96 [PubMed] [Google Scholar]
- [18].Warttig SL, Forshaw MJ, South J, White AK, New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4), J Health Psychol 18(12) (2013) 1617–28. 10.1177/1359105313508346 [DOI] [PubMed] [Google Scholar]
- [19].Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA, Perceived stress in myocardial infarction: long-term mortality and health status outcomes, J Am Coll Cardiol 60(18) (2012) 1756–63. 10.1016/j.jacc.2012.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lesage FX, Berjot S, Deschamps F, Psychometric properties of the French versions of the Perceived Stress Scale, International journal of occupational medicine and environmental health 25(2) (2012) 178–84. 10.2478/s13382-012-0024-8 [DOI] [PubMed] [Google Scholar]
- [21].Vallejo MA, Vallejo-Slocker L, Fernández-Abascal EG, Mañanes G, Determining Factors for Stress Perception Assessed with the Perceived Stress Scale (PSS-4) in Spanish and Other European Samples, Frontiers in psychology 9 (2018) 37. 10.3389/fpsyg.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spertus J, Jones P, Poler S, Rocha-Singh K, The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease, Am Heart J 147(2) (2004) 301–8. 10.1016/j.ahj.2003.08.001 [DOI] [PubMed] [Google Scholar]
- [23].Hoeks SE, Smolderen KG, Scholte Op Reimer WJ, Verhagen HJ, Spertus JA, Poldermans D, Clinical validity of a disease-specific health status questionnaire: the peripheral artery questionnaire, J Vasc Surg 49(2) (2009) 371–7. 10.1016/j.jvs.2008.08.089 [DOI] [PubMed] [Google Scholar]
- [24].Smolderen KG, Hoeks SE, Aquarius AE, Scholte op Reimer WJ, Spertus JA, van Urk H, Denollet J, Poldermans D, Further validation of the peripheral artery questionnaire: results from a peripheral vascular surgery survey in the Netherlands, Eur J Vasc Endovasc Surg 36(5) (2008) 582–91. 10.1016/j.ejvs.2008.07.015 [DOI] [PubMed] [Google Scholar]
- [25].EuroQol--a new facility for the measurement of health-related quality of life, Health Policy 16(3) (1990) 199–208 [DOI] [PubMed] [Google Scholar]
- [26].Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH, The PHQ-8 as a measure of current depression in the general population, J Affect Disord 114(1–3) (2009) 163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- [27].Rod NH, Gronbaek M, Schnohr P, Prescott E, Kristensen TS, Perceived stress as a risk factor for changes in health behaviour and cardiac risk profile: a longitudinal study, J Intern Med 266(5) (2009) 467–75. 10.1111/j.1365-2796.2009.02124.x [DOI] [PubMed] [Google Scholar]
- [28].Steptoe A, Roy MP, Evans O, Snashall D, Cardiovascular stress reactivity and job strain as determinants of ambulatory blood pressure at work, J Hypertens 13(2) (1995) 201–10 [PubMed] [Google Scholar]
- [29].Iso H, Date C, Yamamoto A, Toyoshima H, Tanabe N, Kikuchi S, Kondo T, Watanabe Y, Wada Y, Ishibashi T, Suzuki H, Koizumi A, Inaba Y, Tamakoshi A, Ohno Y, Perceived mental stress and mortality from cardiovascular disease among Japanese men and women: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC Study), Circulation 106(10) (2002) 1229–36 [DOI] [PubMed] [Google Scholar]
- [30].York KM, Hassan M, Sheps DS, Psychobiology of depression/distress in congestive heart failure, Heart Fail Rev 14(1) (2009) 35–50. 10.1007/s10741-008-9091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vart P, Coresh J, Kwak L, Ballew SH, Heiss G, Matsushita K, Socioeconomic Status and Incidence of Hospitalization With Lower-Extremity Peripheral Artery Disease: Atherosclerosis Risk in Communities Study, J Am Heart Assoc 6(8) (2017). 10.1161/jaha.116.004995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A, No health without mental health, Lancet 370(9590) (2007) 859–77. 10.1016/s0140-6736(07)61238-0 [DOI] [PubMed] [Google Scholar]
- [33].Whiteford H, Ferrari A, Degenhardt L, Global Burden Of Disease Studies: Implications For Mental And Substance Use Disorders, Health affairs (Project Hope) 35(6) (2016) 1114–20. 10.1377/hlthaff.2016.0082 [DOI] [PubMed] [Google Scholar]
- [34].Stults-Kolehmainen MA, Sinha R, The effects of stress on physical activity and exercise, Sports Med 44(1) (2014) 81–121. 10.1007/s40279-013-0090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M, Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation, Jama 311(2) (2014) 172–82. 10.1001/jama.2013.284985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS, Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder, Biol Psychiatry 50(12) (2001) 965–77 [DOI] [PubMed] [Google Scholar]
- [37].Matsuhisa F, Kitamura N, Satoh E, Effects of acute and chronic psychological stress on platelet aggregation in mice, Stress 17(2) (2014) 186–92. 10.3109/10253890.2014.888548 [DOI] [PubMed] [Google Scholar]
- [38].Jain S, Mills PJ, von Kanel R, Hong S, Dimsdale JE, Effects of perceived stress and uplifts on inflammation and coagulability, Psychophysiology 44(1) (2007) 154–60. 10.1111/j.1469-8986.2006.00480.x [DOI] [PubMed] [Google Scholar]
- [39].Reibel DK, Greeson JM, Brainard GC, Rosenzweig S, Mindfulness-based stress reduction and health-related quality of life in a heterogeneous patient population, Gen Hosp Psychiatry 23(4) (2001) 183–92. 10.1016/s0163-8343(01)00149-9 [DOI] [PubMed] [Google Scholar]
- [40].Rumsfeld JS, Alexander KP, Goff DC Jr., Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ, Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association, Circulation 127(22) (2013) 2233–49. 10.1161/CIR.0b013e3182949a2e [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


