Abstract
Epstein-Barr virus (EBV)–associated posttransplant lymphoproliferative disorder (EBV-PTLD) is a serious complication in lung transplant recipients (LTRs) associated with significant mortality. We performed a single-center retrospective study to evaluate the risks for PTLD in LTRs over a 7-year period. Of 611 evaluable LTRs, we identified 28 cases of PTLD, with an incidence of 4.6%. Kaplan-Meier analysis showed a decreased freedom from PTLD in idiopathic pulmonary fibrosis (IPF)-LTRs (P < .02). Using a multivariable Cox proportional hazards model, we found IPF (hazard ratio [HR] 3.51, 95% confidence interval [CI] 1.33–8.21, P = .01) and alemtuzumab induction therapy (HR 2.73, 95% CI 1.10–6.74, P = .03) as risk factors for PTLD, compared to EBV mismatch (HR: 34.43, 95% CI 15.57–76.09, P < .0001). Early PTLD (first year) was associated with alemtuzumab use (P = .04), whereas IPF was a predictor for late PTLD (after first year) (P = .002), after controlling for age and sex. Kaplan-Meier analysis revealed a shorter time to death from PTLD in IPF LTRs compared to other patients (P = .04). The use of alemtuzumab in EBV mismatch was found to particularly increase PTLD risk. Together, our findings identify IPF LTRs as a susceptible population for PTLD. Further studies are required to understand the mechanisms driving PTLD in IPF LTRs and develop strategies to mitigate risk.
Keywords: clinical research/practice, hematology/oncology, immunosuppression/immune modulation, immunosuppressive regimens – induction, infection and infectious agents – viral: Epstein-Barr virus (EBV), lung disease, lung transplantation/pulmonology, posttransplant lymphoproliferative disorder (PTLD)
1 |. INTRODUCTION
Epstein-Barr virus (EBV), a member of the herpesvirus family, is a double-stranded DNA virus and following acquisition, establishes lifelong infection.1 Chronic EBV infection is one of the most common viral infections in humans, with >90% of adults in the United States being EBV seropositive.2 While the adaptive immune system plays a critical role in maintaining EBV latency in the immunocompetent host, solid organ transplant recipients (SOTRs) on maintenance immunosuppression therapy (IST) are at increased risk for EBV reactivation.3 The most serious manifestation of EBV reactivation, a B cell–trophic virus, is the development of posttransplant lymphoproliferative disorder (PTLD). Lung transplant recipients (LTRs) are particularly susceptible to PTLD, given their overall higher degree of IST compared to other SOTRs, and younger LTRs who are EBV-seronegative and at risk for EBV transmission via the allograft from a seropositive donor (ie, EBV mismatch).3 Though the rates of PTLD vary across lung transplant centers, the majority of studies suggest an incidence of ≈2%−9% among LTRs, with >70% manifesting thoracic disease.3–6 While the majority of PTLD cases are EBV-associated monomorphic B cell lymphomas, EBV-associated T cell lymphoproliferative disorders also occur, as well as natural killer cell lymphomas and smooth muscle neoplasias in more rare instances. Reduction of IST is central to the treatment of PTLD, along with targeted anti-CD20 monoclonal antibody therapy (rituximab) for B cell lymphomas and/or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for more recalcitrant or widespread disease.7–9 The reported median survival among LTRs with PTLD is 10–18 months, underscoring how devastating a posttransplant complication it can be.10–12
Idiopathic pulmonary fibrosis (IPF) is a leading indication for lung transplantation in North America.13 In recent years it has become clear that the IPF population is enriched for short telomeres in >50% of patients, with ≈12% having germline mutations in telomerase and the telomere maintenance genes.14–16 We recently have shown that IPF LTRs with short telomeres have increased susceptibility to reactivation of the β-herpesvirus, cytomegalovirus (CMV), with impaired CMV-specific T cell immunity compared to age-matched non-IPF LTRs.14 Based on these findings, we hypothesized that IPF LTRs would have increased susceptibility to EBV-associated PTLD in the setting of IST.
2 |. METHODS
This single-center retrospective cohort study was approved by the local institutional review board. Adult LTRs from January 1, 2010 until December 31, 2016 were included. Patients were excluded from analysis if they died within the first 3 months posttransplant or if they transferred care to a different center. Follow-up data were collected through April 1, 2018, with censoring at the last known follow-up before this date. Collected patient characteristics including sex, age, EBV serostatus, induction agent, indication for transplant, and survival were collected via retrospective chart review.
Our center uses alemtuzumab for induction, except in those with a history of malignancy, human immunodeficiency virus, hepatitis C virus, CMV mismatch, or history of stem cell transplant. In these patients, basiliximab is preferred. Within the study period, the induction protocol was changed, and EBV mismatch was added to the list of exceptions where basiliximab is preferred. Our standard maintenance immunosuppressive regimen consists of tacrolimus, mycophenolate mofetil, and prednisone. Changes to immunosuppressive regimens were made at the discretion of the treating physician.
The primary outcome was development of PTLD. Cases of PTLD were identified and characterized via chart review. Subgroup evaluation of early and late PTLD was also conducted. Early PTLD was defined as PTLD occurring in the first year posttransplant.17 Late PTLD was defined as PTLD occurring after the first year posttransplant. Survival after PTLD was assessed as a secondary outcome.
2.1 |. Statistical analysis
Baseline characteristics were compared between the IPF and non-IPF cohorts using the Mann-Whitney U test for continuous variables and χ2 or Fisher exact tests for categorical variables. Time to PTLD was assessed using univariable and multivariable Cox Proportional Hazards models for the primary outcome. Variables with P < .20 in the univariable models were included in the multivariable model. Additionally, age at transplant and sex were forced into the final model due to their known differences in IPF compared to other transplant diagnoses. Proportional hazard assumption was checked in the final model and violation of the proportional hazards assumption was accounted for by using a 2 time-window Cox proportional hazards model to reduce the influence of variable-time interactions. Kaplan-Meier methods were used to generate time-to-event curves for PTLD and survival. For all time-to-event analyses, patients were censored at date of last follow-up. Analysis was completed using Stata version 15 (StataCorp, College Station, TX).
3 |. RESULTS
We identified 663 adult patients who underwent lung transplantation over a 7-year period. As summarized in the consort diagram (Figure 1), 44 patients were excluded due to early death within the first 3 months posttransplant and 8 were excluded due to transfer of care or lost to follow-up. Data for the remaining 611 patients were analyzed. Baseline patient characteristics are summarized in Table 1. Of the 611 patients, 140 (22.9%) were IPF LTRs compared to 471 (77.1%) non-IPF LTRs. The induction protocol for EBV mismatch patients changed during the study period; 27 LTRs were EBV mismatched, with 15 of these transplanted prior to the protocol change and 12 transplanted after the protocol change. Within the EBV mismatch LTRs, 13 received basiliximab induction and 14 received alemtuzumab induction. There were no differences in EBV serostatus or transplant induction regimen between IPF and non-IPF LTRs. Unsurprisingly, IPF LTRs were significantly older and had a higher proportion of men compared to non-IPF LTRs, consistent with disease demographics. The median duration of follow-up was 1381 (IQR: 621–2020) days.
FIGURE 1.
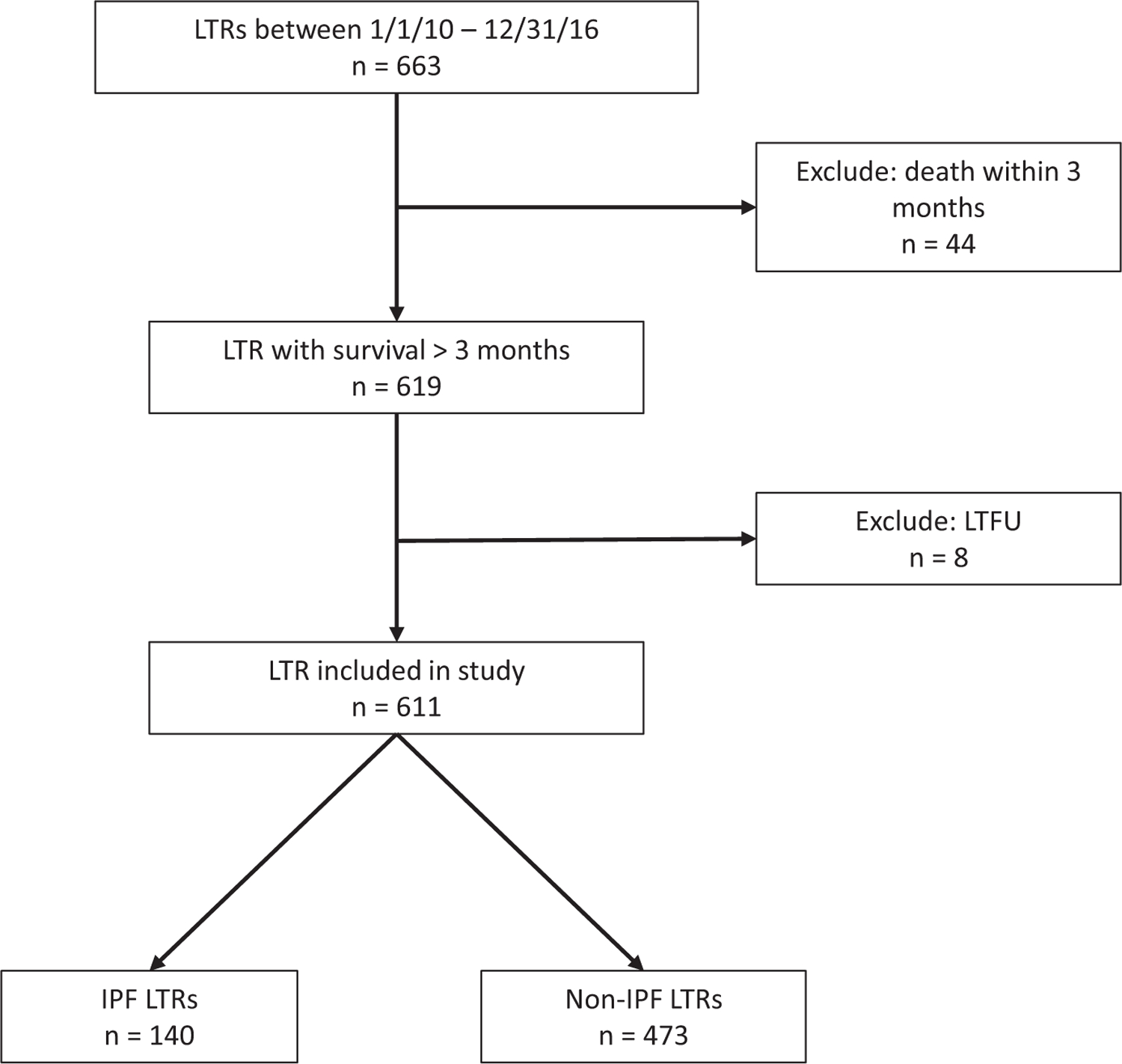
Study design flow diagram. IPF, idiopathic pulmonary fibrosis; LTFU, lost to follow-up; LTR, lung transplant recipient
TABLE 1.
Patient characteristics
| IPF LTRs (n = 140) | Non-IPF LTRs (n = 471) | P value | |
|---|---|---|---|
| Age at transplant (median, IQR) | 65 (61–69) | 58 (44–65) | <.001 |
| Male sex, n (%) | 113 (80.7%) | 244 (51.8%) | <.001 |
| Induction, n (%) | |||
| Alemtuzumab | 90 (64.3%) | 303 (64.3%) | .9 |
| Basiliximab | 50 (35.7%) | 168 (35.7%) | |
| EBV serostatus, n (%) | |||
| D+/R+ | 126 (90.0%) | 412 (87.47%) | .8 |
| D+/R− | 6 (4.29%) | 21 (4.46%) | |
| D−/R+ | 8 (5.71%) | 37 (7.86%) | |
| D−/R− | 0 (0%) | 1 (0.21%) |
Abbreviations: D, donor; EBV, Epstein-Barr virus; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; LTR, lung transplant recipient; R, recipient.
We identified a total of 28/611 (4.6%) patients who developed EBV-associated neoplasia during the study period. Individual patient characteristics are described in Table 2. Twelve (42.9%) of these were IPF LTRs. Of the 28 cases, 27 were classified as PTLD while 1 case manifested as an EBV-related smooth muscle tumor in the allograft lung. Notably, 1 patient developed simultaneous, yet histologically distinct, neoplasms (B cell PTLD of the small bowel and an EBV-associated sarcoma of the liver). A plurality of cases (n = 12) were identified as having their primary site in the lung allograft. All patients who had EBV polymerase chain reaction tested at the time of diagnosis had detectable EBV viremia. No clear patterns were observed in location or histology between early and late PTLD.
TABLE 2.
Posttransplant lymphoproliferative disorder case characteristics
| Transplant diagnosis | Age (y) | UIP on explant | EBV Serostatus | Immunosuppression at diagnosis | Time to diagnosis (mo) | Location | Histology | Peak EBV VL (copies/mL) | Treatment | Survival after diagnosis (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| IPF | 52 | Yes | D+/R+ | Tacro, Pred | 39 | Allograft lung | Monomorphic B cell, EBER+ | 100 000 | None | 0.4 |
| IPF | 51 | Yes | D+/R+ | Tacro, Evero, Hydro | 64 | Allograft lung | Monomorphic B cell, EBER+ | 3 850 000 | Rituximab | 3.7 |
| Cystic fibrosis | 23 | No | D+/R− | Tacro, Myco, Pred | 5 | Small bowel | Monomorphic B cell, EBER+ | 30 000 | Rituximab | 16.4 |
| Cystic fibrosis | 24 | No | D+/R− | Tacro, Pred | 6 | Allograft lung | Polymorphic B cell, EBER+ | 23 000 | Rituximab | 8.4 |
| IPF | 69 | Yes | D+/R+ | Tacro, Aza, Pred | 35 | Allograft lung | Polymorphic B cell, EBER+ | 380 | Rituximab | 26.2 |
| IPF | 65 | Yes | D+/R+ | Tacro, Hydro | 57 | Allograft lung | Smooth muscle tumor, EBER+ | 16 000 | Lobectomy | 21.2 |
| COPD | 67 | No | D+/R+ | Tacro, Evero, Hydro | 9 | Allograft lung | Monomorphic B cell, EBER+ | 1000 | Rituximab | 7.5 |
| Scleroderma | 50 | Yes | D+/R− | Tacro, Myco, Pred | 9 | Cerebellum | Monomorphic B cell, EBER+ | 580 | Methotrexate/Rituximab | 20.4 |
| Cystic fibrosis | 51 | No | D+/R− | Tacro, Myco, Pred | 4 | Small bowel, nasopharynx, liver | Monomorphic B cell, EBER+; Liver sarcoma, EBER+ | 9410 | Rituximab | 35.6 |
| IPF | 65 | Yes | D+/R− | Tacro, Myco, Pred | 57 | Bone marrow, allograft lung | Monomorphic, peripheral T cell, EBER− | 1000 | None | 0.9 |
| Cystic fibrosis | 37 | No | D+/R− | Tacro, Myco, Pred | 4 | Tonsils | Polymorphic B cell, EBER+ | 8800 | R-CHOP | 14.8 |
| LAM | 40 | No | D+/R+ | Cyclo, Hydro, Aza | 6 | Allograft lung | Monomorphic B cell, EBER+ | Not tested | Rituximab | 56.9 |
| IPF | 73 | Yes | D+/R+ | Tacro, Hydro, Aza | 40 | Native lung, kidney, spleen, liver, bone marrow | Polymorphic B cell, EBER+ | 110 000 | None | 0 |
| IPF | 57 | Yes | D+/R+ | Tacro, Myco, Pred | 14 | Lung | Monomorphic B cell, EBER+ | 53 000 | R-CHOP | 6.6 |
| COPD | 67 | No | D+/R+ | Tacro, Myco, Pred, Evero | 24 | Brain, lungs, bone marrow, kidney, liver, spleen | Monomorphic B cell, EBER+ | 800 000 | None | 1.4 |
| COPD | 70 | No | D+/R+ | Tacro, Myco, Pred | 2 | Liver | Monomorphic B cell, EBER+ | 3300 | R-CHOP | 78.8 |
| COPD | 66 | No | D+/R+ | Belat, Myco, Pred | 15 | Liver | Monomorphic B cell, EBER+ | 12 000 | None | 9.6 |
| IPF | 62 | Yes | D+/R− | Tacro, Evero, Pred | 8 | Allograft lung | Monomorphic B cell, EBER+ | 100 000 | R-CHOP | 2 |
| IPF | 61 | Yes | D+/R− | Cyclo, Pred | 9 | Cerebrospinal fluid | Monomorphic B cell, EBER+ | 107 750 | Rituximab | 1.1 |
| Cystic fibrosis | 20 | No | D+/R− | Tacro, Myco, Pred | 3 | Small bowel | Mixed poly/monomorphic B cell, EBER+ | 96 000 | None | 0.1 |
| IPF | 65 | Yes | D+/R− | Tacro, Pred | 3 | Liver | Monomorphic DLBCL, EBER+ | 660 000 | Rituximab | 0.7 |
| IPF | 69 | Yes | D+/R− | Tacro, Pred | 4 | Allograft lung | Monomorphic B cell, EBER+ | 5 340 000 | Rituximab | 1.2 |
| COPD | 67 | No | D−/R+ | Cyclo, Pred | 7 | Allograft lung | Monomorphic B cell, EBER+ | 44000 | R-CHOP | 2.7 |
| Cystic fibrosis | 22 | No | D+/R− | Tacro, Myco, Pred | 2 | Allograft lung | Monomorphic B cell, EBER+ | 45000 | Rituximab | 10.2 |
| IPF | 51 | Yes | D+/R− | Tacro, Evero, Pred | 52 | Allograft lung | Monomorphic B cell, EBER+ | 190000 | Rituximab | 6.2 |
| Langerhans cell histiocytosis | 62 | No | D+/R− | Tacro, Evero, Pred | 23 | Tongue | Monomorphic B cell, EBER+ | 5200 | Rituximab | 6.3 |
| COPD | 51 | No | D−/R+ | Tacro, Aza, Pred | 41 | Colon | Monomorphic DLBCL, EBER+ | 13000 | Rituximab | 35.6 |
| COPD | 67 | No | D−/R+ | Tacro, Myco, Pred | 85 | Bone | Monomorphic DLBCL, EBER− | Not tested | R-CHOP | 7.6 |
All 28 cases of posttransplant lymphoproliferative disorder in the cohort are described.
Abbreviations: Aza, azathioprine; Belat, belatacept; COPD, chronic obstructive pulmonary disease; Cyclo, cyclosporine; DLBCL, diffuse large B cell lymphoma; D, donor; EBV, Epstein-Barr virus; EBER, EBV-encoded RNA; Evero, everolimus; Hydro, hydrocortisone; IPF, idiopathic pulmonary fibrosis; LAM, lymphangiomyomatosis; Myco, mycophenolic acid; Pred, prednisone; R, recipient; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; Tacro, tacrolimus; UIP, usual interstitial pneumonia.
Figure 2 depicts Kaplan-Meier curves evaluating freedom from PTLD between non-IPF LTRs and IPF LTRs (Figure 2A) and EBV mismatch vs nonmismatch as a control analysis for a known risk factor (Figure 2B). IPF LTRs had significantly shorter time to PTLD than non-IPF LTRs (P < .01). As anticipated, EBV mismatch was associated with shorter time to PTLD vs nonmismatch (P < .001). When EBV mismatch was stratified by induction agent, a significant difference in overall time to PTLD was observed (P < .001) as shown in Figure 2C. EBV mismatch LTRs who received alemtuzumab had the shortest time to PTLD of the 4 groups. A greater degree of separation for induction agent was observed in the EBV mismatch LTRs compared to the EBV nonmismatch LTRs, whose event curves for each induction agent overlapped. Taken together, these data suggest a significant impact of induction agent on the EBV mismatch population. When IPF and non-IPF LTRs were stratified by induction agent (Figure 2D), a significant difference in time to PTLD was observed overall (P = .02). This difference appeared to be driven by the IPF LTRs, who had the shortest time to PTLD regardless of induction agent. For patients with IPF, the survival functions for those who received alemtuzumab and those who received basiliximab were overlapping.
FIGURE 2.
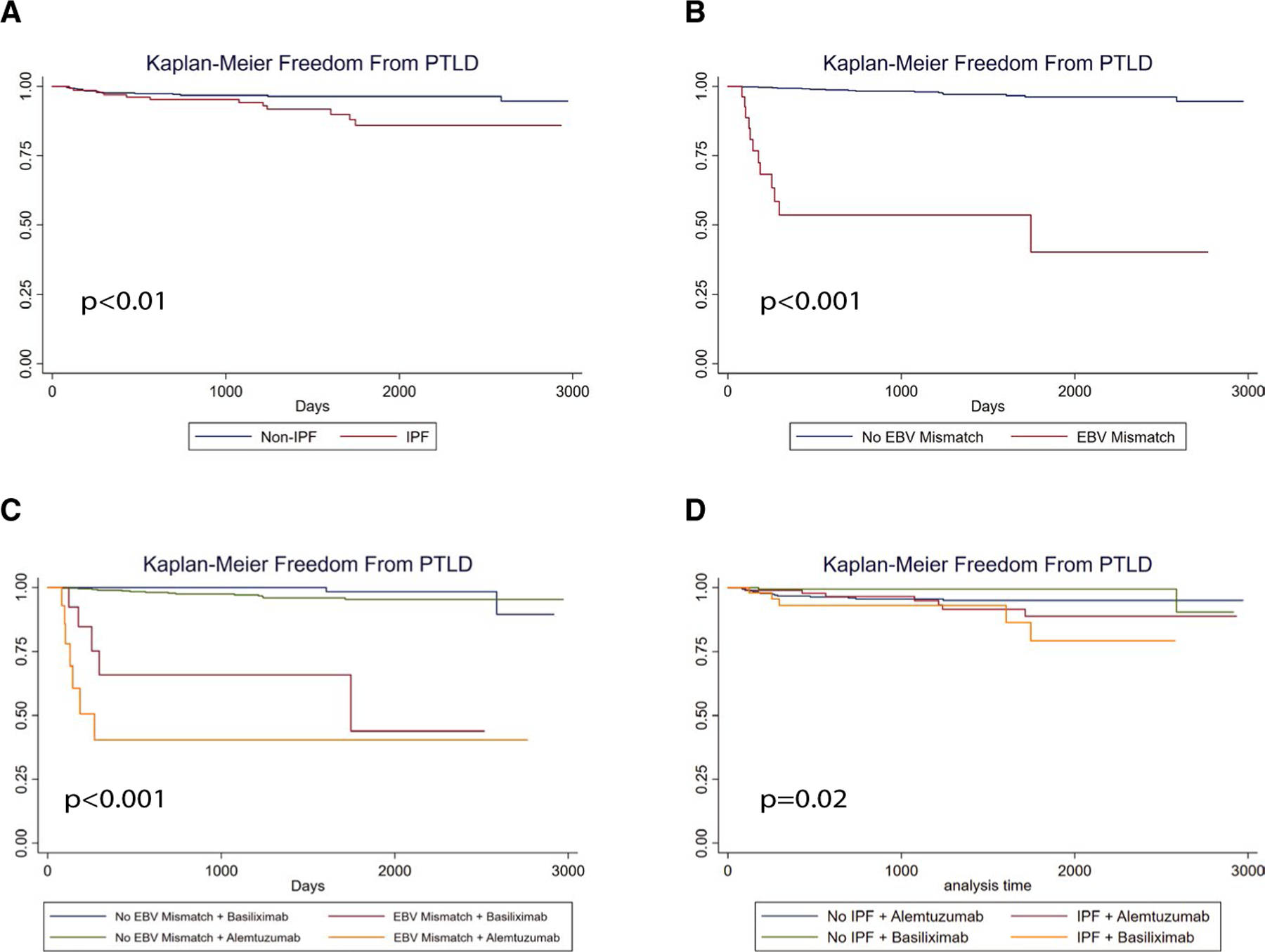
IPF, alemtuzumab induction therapy, and EBV mismatch are associated with PTLD after lung transplant. Kaplan-Meier methods with log-rank conversion were used to evaluate time to PTLD in the study cohort. A, Depicts cohort freedom-from-event curves for PTLD for IPF vs non-IPF transplant indications. B, Shows cohort freedom-from-event curves for PTLD for EBV mismatch vs all other LTRs. C, Shows cohort freedom-from-event curves for PTLD for EBV mismatch, stratified for induction agent (alemtuzumab or basiliximab). D, Shows cohort freedom-from-event curves for PTLD for IPF, stratified by induction agent. EBV, Epstein-Barr virus; IPF, idiopathic pulmonary fibrosis; PTLD, posttransplant lymphoproliferative disorder
A multivariable Cox proportional hazards model was constructed to identify independent predictors of PTLD. IPF (hazard ratio [HR]: 3.51, 95% confidence interval [CI]: 1.33–8.21, P = .01), EBV mismatch (HR: 34.43, 95% CI: 15.57–76.09, P < .0001), and alemtuzumab (HR: 2.73, 95% CI: 1.10–6.74, P = .03) were all significantly associated with an increased hazard of PTLD, whereas age and sex did not affect the rate of PTLD (Table 3).
TABLE 3.
Multivariable Cox proportional hazards model for the development of PTLD
| Variable | n (% of total PTLD events)a | Hazard ratio | Confidence interval | P value |
|---|---|---|---|---|
| IPF | 12 (43%) | 3.51 | [1.33–9.21] | .01 |
| EBV mismatch | 12 (43%) | 34.43 | [15.57–76.09] | <.001 |
| Alemtuzumab | 21 (75%) | 2.73 | [1.10–6.74] | .03 |
| Male sex | 17 (61%) | 0.99 | [0.44–2.23] | .99 |
| Age at transplant (y) | — | 0.98 | [0.95–1.02] | 34 |
Abbreviations: EBV, Epstein-Barr virus; IPF, idiopathic pulmonary fibrosis; PTLD, posttransplant lymphoproliferative disorder.
Out of 28 PTLD events.
Significant interactions between time and EBV mismatch as well as time and alemtuzumab were identified, where the hazard of PTLD associated with EBV mismatch and alemtuzumab changed over time.A 2 time-window Cox proportional hazards model was constructed to control for this, using a cut-point time of 365 days, which aligned with the definition of early and late PTLD (Table 4). Out of 28 cases of PTLD, 15 met the definition of early PTLD (≤365 days), while 13 were classified as late PTLD (>365 days). Controlling for age and sex, EBV mismatch (P < .0001) and alemtuzumab induction (P = .04) were associated with early PTLD, or PTLD occurring within the first year posttransplant. IPF diagnosis was not significantly associated with PTLD in the first year. In contrast, IPF diagnosis (P = .02) was the only significant predictor in the Cox proportional hazards model for late PTLD, controlling for age and sex. EBV mismatch (HR: 4.01, 95% CI: 0.51–31.69) and alemtuzumab induction (HR: 1.24, 95% CI: 0.33–4.71) did not reach significance in the model for late PTLD.
TABLE 4.
Two time-window Cox proportional hazards model (early and late PTLD)
| Hazard ratio | Confidence interval | P value | |
|---|---|---|---|
| ≤365 d | |||
| IPF | 2.14 | [0.98–9.77] | .327 |
| EBV mismatch | 102.30 | [30.37–344.49] | <.0001 |
| Alemtuzumab | 3.39 | [1.03–11.14] | .044 |
| >365 d | |||
| IPF | 4.56 | [1.29–16.16] | .019 |
| EBV mismatch | 4.01 | [0.51–31.69] | .187 |
| Alemtuzumab | 1.24 | [0.33–4.71] | .326 |
Abbreviations: EBV, Epstein-Barr virus; IPF, idiopathic pulmonary fibrosis; PTLD, posttransplant lymphoproliferative disorder.
The majority of LTRs who developed PTLD received anti-CD 20 therapy with rituximab ± other chemotherapeutic agents (Table 2). Survival after diagnosis was compared between IPF LTRs and non-IPF LTRs. As shown in the Kaplan-Meier curves in Figure 3, IPF LTRs had a shorter time to death compared to non-IPF LTRs (P = .042). Median survival after PTLD diagnosis for non-IPF LTRs was 446 days compared to just 38 days in the IPF LTR group. Additional analysis of survival after PTLD diagnosis using regression methods was not attempted due to the small sample size of LTRs who developed PTLD (n = 28).
FIGURE 3.
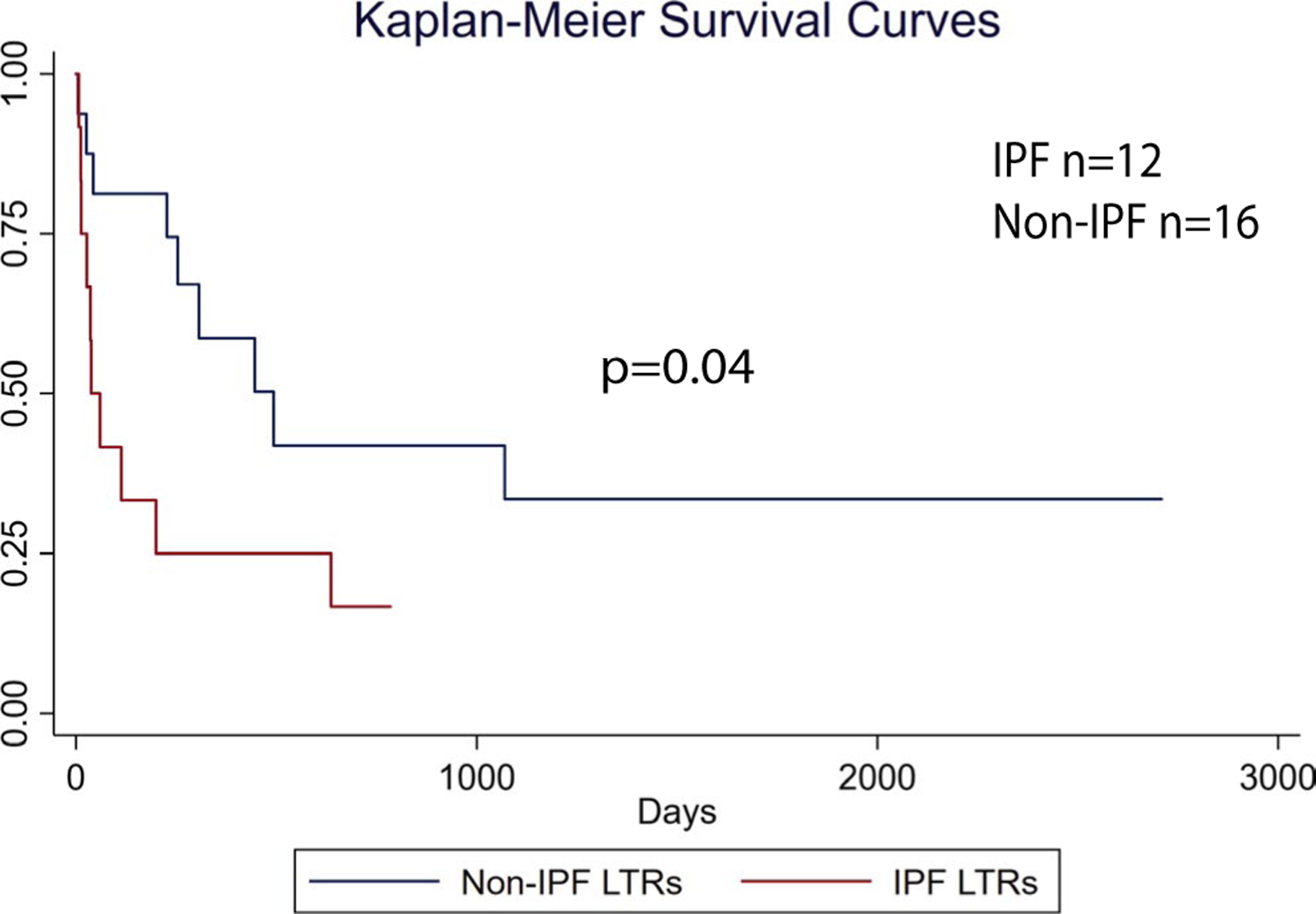
IPF LTRs have worse survival with PTLD. PTLD cases (n = 28) from the cohort were analyzed by Kaplan-Meier methods for survival comparing IPF to non-IPF LTRs using the log-rank test. IPF, idiopathic pulmonary fibrosis; LTRs, lung transplant recipients; PTLD, posttransplant lymphoproliferative disorder [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
To our knowledge, this is the first report identifying IPF as a risk factor for PTLD after lung transplantation. Existing literature surrounding risk factors for PTLD in LTRs is limited. While EBV mismatch has been well established, retrospective study and meta-analysis findings suggesting risk in association with age, cystic fibrosis, and race have demonstrated conflicting results.18–21 Importantly, IPF was the only end-stage lung diagnosis found to be a risk factor for the development of PTLD irrespective of age. Additionally, IPF was an important predictor identified for the development of late PTLD. One possible explanation for these findings is an impaired ability of IPF patients on IST to control EBV chronic infection. We previously demonstrated an increased susceptibility to CMV, another herpesvirus infection in patients with IPF and short telomeres in association with impaired viral immunity posttransplant, possibly related to changes in T cell immunity.22,23 In this historical cohort, blood samples were not available for all patients to assess telomere length in those who developed PTLD.
Unexpectedly, IPF LTRs who developed PTLD had significantly shortened survival after diagnosis compared to non-IPF LTRs with PTLD. This finding may be due to reduced immunologic and hematologic reserve in IPF LTRs on IST, leading to increased susceptibility to PTLD and poor survival. Regardless, these data suggest enhanced and, given the increased risk for late PTLD, extended monitoring of EBV in this higher risk population is warranted for early detection of EBV viremia to stratify for PTLD risk.24 The detection of rising EBV viral loads in this population would merit reduction of IST to possibly mitigate risk for PTLD.
Alemtuzumab (anti-CD52) is a longer-term lymphodepleting agent and the predominant induction agent used at our center. While previous work has not identified risk associated with alemtuzumab induction, in this study, it was independently associated with PTLD, particularly early PTLD.25 This early time-frame corresponds with the prolonged lymphodepletion associated with alemtuzumab. Additionally, this risk appears to be magnified in EBV mismatch patients, supporting the practice of avoiding alemtuzumab in EBV mismatched LTRs. While we did not observe an interaction between IPF and alemtuzumab induction, this could be a result of lack of power. Nevertheless, the additive risk of IPF and alemtuzumab induction cannot be excluded and warrants caution in this patient population.
This study has several strengths. Notably, it has a large sample size compared to other retrospective studies in lung transplantation. Additionally, the median follow-up time was long at over 3.7 years. Using single-center data helps to ensure a more consistent approach to immunosuppression and other programmatic factors that could impact rates of PTLD. Limitations include the relatively low event rate of PTLD, which impacts power and resulted in particularly wide confidence intervals for the early and late PTLD analyses. Additionally, because some of the LTRs who developed PTLD are deceased, our ability to obtain samples to further characterize the underlying immune mechanisms of disease in these patients was limited. Finally, this study is subject to the inherent limitations of retrospective research that restrain our ability to establish causal relationships, so future studies to confirm these findings should be pursued.
In summary, we have identified IPF and alemtuzumab, in addition to EBV mismatch as risk factors for EBV-related PTLD after lung transplantation. We demonstrated that IPF is associated with late PTLD and poorer survival after PTLD diagnosis. In addition, we identify induction with alemtuzumab as an independent risk factor for PTLD that is synergistic with EBV mismatch. Further studies are needed to genetically characterize and stratify risk in the IPF LTR population at increased risk for EBV-PTLD and to identify the underlying immunologic mechanisms of this serious transplant complication.
Abbreviations:
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- IPF
idiopathic pulmonary fibrosis
- IST
immunosuppression therapy
- LTR
lung transplant recipient
- PTLD
posttransplant lymphoproliferative disorder
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Odumade OA, Hogquist KA, Balfour HH. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011;24(1):193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunmire SK, Verghese PS, Balfour HH. Primary Epstein-Barr virus infection. J Clin Virol. 2018;102:84–92. [DOI] [PubMed] [Google Scholar]
- 3.Green M, Michaels MG. Epstein-Barr virus infection and post-transplant lymphoproliferative disorder. Am J Transplant. 2013;13(s3):41–54. [DOI] [PubMed] [Google Scholar]
- 4.Armitage JM, Kormos RL, Stuart RS, et al. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10(6):877–886. [PubMed] [Google Scholar]
- 5.Levine SM, Angel L, Anzueto A, et al. A low incidence of posttransplant lymphoproliferative disorder in 109 lung transplant recipients. Chest. 1999;116(5):1273–1277. [DOI] [PubMed] [Google Scholar]
- 6.Walker RC, Paya CV, Marshall WF, et al. Pretransplantation seronegative Epstein-Barr virus status is the primary risk factor for posttransplantation lymphoproliferative disorder in adult heart, lung, and other solid organ transplantations. J Heart Lung Transplant. 1995;14(2):214–221. [PubMed] [Google Scholar]
- 7.Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8(3):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elstrom RL, Andreadis C, Aqui NA, et al. Treatment of PTLD with Rituximab or Chemotherapy. Am J Transplant. 2006;6(3):569–576. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Calle N, Alfonso A, Rifón J, et al. First-line use of rituximab correlates with increased overall survival in late post-transplant lymphoproliferative disorders: retrospective, single-centre study. Eur J Haematol. 2017;98(1):38–43. [DOI] [PubMed] [Google Scholar]
- 10.Wudhikarn K, Holman CJ, Linan M, et al. Post-transplant lymphoproliferative disorders in lung transplant recipients: 20-yr experience at the University of Minnesota. Clin Transplant. 2011;25(5):705–713. [DOI] [PubMed] [Google Scholar]
- 11.Kremer BE, Reshef R, Misleh JG, et al. Post-transplant lymphoproliferative disorder after lung transplantation: a review of 35 cases. J Heart Lung Transplant. 2012;31(3):296–304. [DOI] [PubMed] [Google Scholar]
- 12.Kumarasinghe G, Lavee O, Parker A, et al. Post-transplant lymphoproliferative disease in heart and lung transplantation: defining risk and prognostic factors. J Heart Lung Transplant. 2015;34(11):1406–1414. [DOI] [PubMed] [Google Scholar]
- 13.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37(10):1169–1183. [DOI] [PubMed] [Google Scholar]
- 14.Popescu I, Mannem H, Winters SA, et al. Impaired cytomegalovirus immunity in idiopathic pulmonary fibrosis lung transplant recipients with short telomeres. Am J Respir Crit Care Med. 2019;199(3):362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alder JK, Chen JJ-L, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105(35):13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muchtar E, Kramer MR, Vidal L, et al. Posttransplantation lymphoproliferative disorder in lung transplant recipients. Transplant J. 2013;96(7):657–663. [DOI] [PubMed] [Google Scholar]
- 18.Lowery EM, Adams W, Grim SA, Clark NM, Edwards L, Layden JE. Increased risk of PTLD in lung transplant recipients with cystic fibrosis. J Cyst Fibros. 2017;16(6):727–734. [DOI] [PubMed] [Google Scholar]
- 19.Courtwright AM, Burkett P, Divo M, et al. Posttransplant lymphoproliferative disorders in Epstein-Barr virus donor positive/ recipient negative lung transplant recipients. Ann Thorac Surg. 2018;105(2):441–447. [DOI] [PubMed] [Google Scholar]
- 20.Metcalfe MJ, Kutsogiannis DJ, Jackson K, et al. Risk factors and outcomes for the development of malignancy in lung and heart-lung transplant recipients. Can Respir J. 2010;17(1):e7–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng J, Moore CA, Iasella CJ, et al. Systematic review and meta-analysis of post-transplant lymphoproliferative disorder in lung transplant recipients. Clin Transplant. 2018;32(5):e13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popescu I, Mannem H, Winters SA, et al. Impaired CMV immunity in idiopathic pulmonary fibrosis lung transplant recipients with short telomeres. Am J Respir Crit Care Med. 2018:rccm.201805–0825OC. 10.1164/rccm.201805-0825OC. [DOI] [PMC free article] [PubMed]
- 23.Wagner CL, Hanumanthu VS, Talbot CC, et al. Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest. 2018;128(12):5222–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8(5):1016–1024. [DOI] [PubMed] [Google Scholar]
- 25.Shyu S, Dew MA, Pilewski JM, et al. Five-year outcomes with alemtuzumab induction after lung transplantation. J Heart Lung Transplant. 2011;30(7):743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


