Abstract
Trichomonas vaginalis is a protozoan with an extracellular obligatory parasitic lifestyle exclusively adapted to the human urogenital tract and responsible for nearly a quarter billion sexually transmitted infections worldwide each year. This review focuses on symbiotic Trichomonasvirus and mycoplasmas carried by the protozoan, their molecular features and their role in altering the human vaginal microbiome and the immunopathogenicity of the parasite. Improved diagnostics and larger clinical interventional studies are needed to confirm the causative role of protozoan symbionts in the variable clinical presentation of trichomoniasis and its morbid sequelae, including adverse reproductive outcome, susceptibility to viral infections and cancer.
Keywords: Trichomoniasis, Endosymbionts, dsRNA viruses, Mycoplasma hominis, Candidatus Mycoplasma girerdii, Microbiome
1. Introduction
Protozoan genera known to carry endobiont viruses that can only propagate within the protozoan host include Babesia, Cryptosporidum, Eimeria, Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium and Trichomonas [1]. While these viruses are presumably non-infectious to the human/animal host, recent evidence suggests some may significantly influence the outcome of parasitic disease by modifying immune responses to the protozoan parasite [2–4]. Protozoan parasites that carry symbiont microorganisms that are capable of multiplying in the vertebrate host and that cause an infectious disease are more rarely described. Trichomonas vaginalis falls within both categories of protozoan pathogens. It has adapted to symbiosis with double-stranded RNA (dsRNA) endobiont viruses, recently classified by the International Committee on Taxonomy of Viruses as Trichomonasvirus genus within the Family Totiviridae, as well as with eubacterial Mycoplasma species, with Mycoplasma hominis as the best studied representative. This review will focus on T. vaginalis and its toolbox of symbionts as emerging key players in human disease. We believe that understanding the molecular features of the symbiont infections and their interactions with the human host is essential for improvement of the diagnostics and therapeutics of this parasitic disease.
T. vaginalis is an extracellular, obligatory sexually transmitted parasite, exclusively adapted to the epithelial lining of the human vagina, the uterine cervix and the male and female urethra. It causes over 220 million cases of trichomoniasis each year, which is more than the most prevalent bacterial sexually transmitted infections taken together [5]. The infection is often asymptomatic and, when present, symptoms range widely from itching to burning, dyspareunia and malodorous discharge [6]. Trichomoniasis is associated with persistence of most carcinogenic HPV types, cervical and prostate cancer and higher risk of HIV and HPV infection, and is especially damaging to reproductive health and pregnancy (reviewed in [6]).
In culture supernatant, the parasite is pear-shaped, measuring 7–23 × 5–10 microns, and in the trophozoite state it can be almost half the size of the host epithelial cells (Fig. 1). It swims using 5 flagella − four anterior and one embraced by an undulating membrane across its antero–posterior axis. It can survive on wet surfaces outside the human body for only a limited amount of time.
Fig. 1.
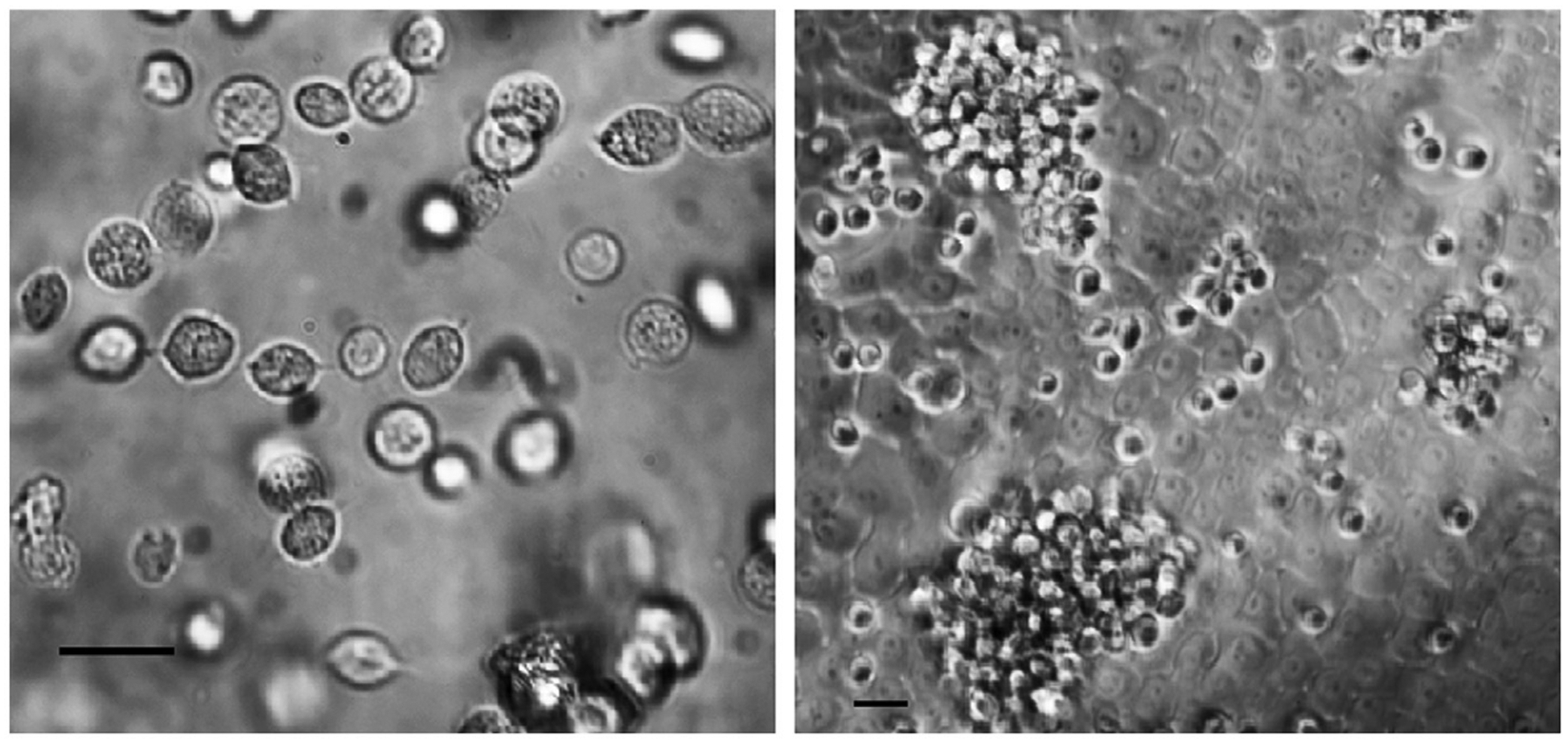
Light microscopic images of T. vaginalis taken by phase invert microscopy. Left image depicts free-swimming parasites isolated from a vaginal swab and placed into culture medium. Right image depicts in vitro infection of human vaginal epithelial cells with T. vaginalis. The epithelial cells are grown in a monolayer. The parasites appear over the vaginal epithelial surface as single organisms or in swarms of many closely assembled bodies. Size bars in each image represent 15 microns.
2. Symbiotic species prevalent among T. vaginalis isolates across the globe
2.1. Trichomonasvirus
The presence of long, linear dsRNA molecules in many strains of T. vaginalis was first reported in 1985, followed shortly by evidence of their association with virus-like particles and their recognition as T. vaginalis viruses (TVVs) [7]. Significant progress has been made since then, especially in establishing the molecular and structural characteristics of the virus. The genome is monosegmented, with plus-strand RNA (viral mRNA) containing two open reading frames encoding the coat protein (CP) and the RNA-dependent RNA polymerase (RdRp) [8]. Like most other members of Totiviridae, TVV is believed to lack virion-associated machinery for active cell entry and is transmitted from one parasite to another during cytokinesis and possibly sexual reproduction inferred from genetic evidence (reviewed in [9] and [10]). Thus far, four different TVV species (TVV1, 2, 3 and 4) have been identified in the genus Trichomonasvirus by phylogenetic and genomic sequence analysis, and complete genome sequences of all four species have been deposited in GenBank and assigned accession numbers as published [8,11,12]. Recently, Parent et al. described the 3D-structure of TVV-1, making it the fourth member of the Family Totiviridae with reported crystallographic structure, next to the prototype Totivirus Saccharomyces cerevisiae virus L-A (ScV-L-A), which encodes toxins in the killer yeast S. cervevisiae, the Helminthosporium victoriae virus-190S (HvV190S), which inhabits a number of pathogenic fungi and protozoa in plants, and the infectious myonecrosis virus (IMNV), which inhabits the penaeid scrimp [9]. One of the most interesting features determined by cryo-transmission electron microscopy in this study was that the TVV-1 capsid has unusually large channels that may allow the dsRNA genome to escape the virions and interact directly with the human host as an immunity modifier once released from the protozoan host and taken up by the human cells, where they engage pathogen recognition receptors without causing a productive infection [3].
The reported infection rate of TVVs in different clinical isolates of T. vaginalis varies depending mostly on detection methods and limitations of small sample size (Table 1). A few studies reported low prevalence of T. vaginalis dsRNA virus (14–20%) in isolates from Korea, Iran, Egypt and the Philippines [13–16]; however, most other studies reported high infection rates of 40–100% around the globe [3,17–24], suggesting that variations among studies may be driven by technical factors and/or clinical and socio-economic covariates, rather than by geographic and racial/ethnic differences. In each of the listed studies in Table 1, TVV genomic RNA was detected by gel electrophoresis of total nucleic acid extracts, electrophoresis of RNA or dsRNA extracts and RTPCR. Other methods of TVV detection, still pending a broader validation, include immunodetection with TVV-specific antibodies [25] and nucleic acid microarrays to detect TVV RNAs [26]. None of these methods has yet been adapted as a standard clinical diagnostic test. Over 100 strains representing the four TVV species have been described to date, although not always fully characterized (Table 1). TVV purification techniques vary significantly between studies, and include filtration, CsCl density-gradient centrifugation, sucrose cushion and ultracentrifugation, which may contribute to variations in the identification of TVV-positive clinical isolates of T. vaginalis. Methods for TVV species identification also vary, and may account for failure to detect multiple species within a single isolate (Table 1). Those commonly used are reverse transcription-PCR (RT-PCR) with species-specific primers, analysis of the coding sequences of the capsid protein, viral RNA-dependent RNA polymerase (RdRp) or full-length sequencing of the dsRNA genome (Table 1). Early electron microscopy studies suggested that different types of virus-like particles can be concurrently detected in T. vaginalis [27]. Goodman et al. [12] reported for the first time that individual parasites can carry infection with all four known TVV species simultaneously. In the global characterization of over 100 strains of T. vaginalis from Brazil, China, Cuba, Iran, Korea, Philippines and the USA by multiple studies, the majority were infected with a single TVV species (Table 1). Among the isolates/strains that were characterized for mixed infections, 29.7% were positive for multiple TVV species. It is possible that, in some instances, the presence of multiple TVVs in a single ‘isolate’ of T. vaginalis was due to a mixture of parasites, each infected with a different TVV species; however, the presence of a stable co-infection with all four TVV species has been confirmed by cloning of the protozoan host [12]. TVV1 (44.5%) and TVV2 (31.4%) were the most prevalent viral species identified in T. vaginalis isolates, followed by TVV3 (13.1%) and, finally, TVV4 (10.9%), which may be least commonly detected because of its later discovery [12]. Future studies including isolates from different geographic origins with robust methods for TVV detection and identification are needed to determine the true prevalence of the Trichomonasvirus and the individual TVV species around the globe.
Table 1.
Summary of studies on detection and identification of TVV species in T. vaginalis.
| Study reference | Number of T. vaginalis isolates | Country | Detection rate of TVVs | TVV detection method | TVV species: number of isolates | Typing method |
|---|---|---|---|---|---|---|
| [18] | 16 | Czech Republic and Austria | 50.0% | Electrophoresis of a total nucleic acid extract | nd | nd |
| [22] | 28 | Austria, Czech Republic and USA | 50.0% | Electrophoresis of RNA | nd | nd |
| [77,78] | 4 | China | nd | nd | TVV1: 2 TVV2: 1 TVV3: 1 |
Sequencing, TVV viral genome and phylogenetic analyses |
| [38,39] | 20 | Austria, Brazil, China, Czech Republic, Estonia, Slovakia, Sweden and USA | 44.0% | Electrophoresis of a total nucleic acid extract | nd | nd |
| [40] | 109 | USA | 50.0% | Electrophoresis of a total nucleic acid extract | nd | nd |
| [24] | 28 | USA | 72.0% | Electrophoresis of a total nucleic acid extract | nd | nd |
| [23] | 72 | South Africa | 81.9% | Electrophoresis of a total nucleic acid extract | nd | nd |
| [19,20] | 40 | Cuba | 55.0% | Electrophoresis of a total nucleic acid extract | TVV1: 9 TVV2: 16 co-infection: 3 |
RT-PCR and sequencing, TVV viral genome and phylogenetic analysis |
| [13] | 22 | Korea | 14.0% | Electrophoresis of RNA | TVV1: 1 | Sequencing, TVV viral genome and phylogenetic analyses |
| [21] | 30 | India | 100% | Electrophoresis of a total nucleic acid extracts | nd | nd |
| [12] | 5 | USA | nd | Electrophoresis of dsRNA | TVV1: 1 TVV1 & 4: 1 TVV1, 2 & 3: 1 TVV1, 2, 3 & 4: 2 |
RT-PCR and sequencing, TVV viral genome and phylogenetic analyses |
| [3] | 16 | USA | 81.3% | Electrophoresis of dsRNA | Co-infections: 11 | RT-PCR and sequencing, TVV viral genome and phylogenetic analysis |
| [14] | 46 | Iran | 17.4% | Electrophoresis of RNA | TVV1: 8 | RT-PCR for TVV1 |
| [15] | 96 | Philippines | 19.0% | Electrophoresis of dsRNA | TVV1: 6 co-infection: 6 |
RT-PCR and sequencing, TVV viral genome and phylogenetic analysis |
| [17] | 26 | Brazil | 90.0% | Electrophoresis of dsRNA | TVV1: 13 TVV2: 2 TVV3: 2 co-infections: 10 |
RT-PCR |
| [16] | 40 | Egypt | 20.0% | Electrophoresis of dsRNA | TVV2: 5 TVV4: 3 |
RT-PCR |
nd: not determined; dsRNA: double stranded RNA; TVV: Trichomonasvirus; RT-PCR: reverse transcriptase PCR.
2.2. Mycoplasma
In 1975, Nielsen [28] showed, by transmission electron microscopy, the presence of apparently intact Mollicutes within the cytoplasm of T. vaginalis cells after 6 weeks of in vitro cultivation. In 1985, Scholtyseck et al. identified Mycoplasma fermentans in T. vaginalis based on morphologic criteria [29]. Twelve years later, a large clinical study showed an association of T. vaginalis infection with M. hominis [30]. That same year, Rappelli et al. isolated viable M. hominis identified by PCR from long-term in-vitro-cultivated T. vaginalis isolates [31].
M. hominis lives in the human urogenital tract and is like the other mycoplasmas, characterized by a small size (0.2–0.3 μm) and by the absence of a cell wall. Mycoplasmas are considered the smallest organisms capable of independent replication, with a small genome of about 650 kb. The small genome renders these bacteria strongly dependent on host cell metabolism. The energy metabolism of M. hominis is strictly dependent upon the fermentative degradation of free arginine. M. hominis has the ability to enter trichomonad cells by endocytosis and to multiply in coordination with the protozoan host.
Since the observations of Rappelli et al. [32,33], several groups used PCR to demonstrate the presence of M. hominis in trichomonad isolates of different geographic origin, with infection rates ranging from 5% to over 89% (Table 2). In addition, the intracytoplasmic location of M. hominis in T. vaginalis cells has been demonstrated by gentamicin protection assays and by confocal and electron microscopy [32,33].
Table 2.
Summary of studies on detection of M. hominis in T. vaginalis.
| Study reference | Number of T. vaginalis isolates | Geographic origin | Detection rate of M. hominis (%) | Detection method |
|---|---|---|---|---|
| [31] | 37 | Italy, Mozambique, Angola | 89.1 | PCR, cultivation |
| [17] | 30 | Brazil | 56.7 | PCR |
| [75] | 40 | Cuba | 5 | PCR |
| [74] | 55 | USA | 20 | PCR |
| [73] | 28 | China | 50 | PCR |
| [79] | 59 | The Netherlands | 69 | PCR |
| [38] | 20 | Czech Republic, Estonia, Sweden, Austria, China, USA, Brazil | 25 | PCR |
Very recently, next-generation sequencing of the vaginal microbiome identified a new Mycoplasma species, initially named Mnol and later renamed Candidatus Mycoplasma girerdii. C. M. girerdii, which has been found almost exclusively in women positive for T. vaginalis with 63% prevalence of T. vaginalis and C. M. girerdii co-infections [34,35]. The bacterium is uncultivable in common mycoplasma media and thus had not been identified in past culture-based studies of the vaginal microbiota. Considering the strict association between C. M. girerdii and T. vaginalis, it may be that the bacterium is an obligate symbiont of T. vaginalis. M. girerdii shows a very small genome of 619 kb (with 28.6% GC content) and lacks gluconeogenesis, the tricarboxylic acid cycle (Krebs cycle) and enzymes for purine, pyrimidine and amino acid synthesis. In addition, arginine dihydrolase pathway (ADH) genes, essential for mycoplasma energy metabolism, are absent, which may explain the dependence on T. vaginalis. Interestingly, M. girerdii shows genes encoding proteins homologous to microbial virulence factors, such as collagenase, hemolysin and endopeptidase [34].
Further studies will better characterize the role of this new bacterial species in modulating the virulence of, and the host immune response to, T. vaginalis infection.
3. Molecular characteristics of symbiont infections associated with protozoan genomic features and virulence factors
3.1. Trichomonasvirus
Cytoadherence to vaginal epithelial cells is a critical step in pathogenesis and is essential for the colonization and the persistence of T. vaginalis infection [36]. The adhesion level is higher in virus-infected compared to non-infected parasites, and higher among TVV-2-infected versus TVV-1-infected parasites [37]. Given the advantage provided by the TVV infection, it can be hypothesized that the viral infection may upregulate virulence genes of the parasite and that, vice-versa, evolutionary preserved characteristics of the protozoan genome may favor the viral infection.
To identify genes and proteins involved in the parasite predisposition to symbiotic infection and its virulence to the human host, a correlation between TVV infection and the genetic polymorphism of the parasite has been investigated using a number of molecular tools. In contrast to a few small studies [38,39], larger studies using the RAPD or microsatellite techniques reported correlation a between the presence of TVV and protozoan genetic polymorphism. The correlation was shown in 109 isolates from the United States [40], in 37 Cuban isolates [19] and in 235 isolates from 10 regions in Mexico, Chile, India, Australia, Papua New Guinea, Italy, Africa and the United States [41]. These findings support the notion of a genetic protozoan predisposition to entry and/or survival of the virus in the protozoan host.
Fraga et al. [42] found a specific RAPD marker of 490 bp in all symptomatic, but not in asymptomatic, isolates using primer Tv-5. This genetic virulence marker exhibited significant sequence similarity to the T. vaginalis hypothetical G3 leucine-rich repeat (LRR) protein family and to Giardia lamblia LRR protein 1, which could mediate viral entry. Further studies should be conducted to confirm the precise role of this gene in protozoan susceptibility/resistance to TVV and in overall clinical phenotypes.
Several studies suggested a possible role of TVV in the virulence of the parasite via expression of protozoan immunogenic proteins and modulating the human host immune responses. The TVV infection upregulates synthesis and surface expression of a highly immunogenic protein, P270 [43]. Parasites infected with TVVs alternate expression of P270 to cytoplasmatic expression. This depends on protein phosphorylation, but also, iron plays a role in the modulation of the P270 localization in virus-harboring parasites [44], though the precise cellular function of P270 is still unknown.
Viral infection of trichomonads is also associated with differential qualitative and quantitative expression of cysteine proteinases (CPs) [45], which are important virulence factors linking cytoadherence to host vaginal cells and subsequent cytotoxity, and to degradation of basement membrane components [46]. Several CPs that are released from the parasite are implicated in pathogenesis, although their specific functions and targets remain unknown [47].
3.2. Mycoplasma
The infection of T. vaginalis by M. hominis has important implications for the biochemistry and physiopathology of the protozoon. Interestingly, T. vaginalis and M. hominis share a common biochemical pathway, i.e., the arginine dihydrolase (ADH) pathway [48]. The ADH pathway represents a major energetic source for mycoplasmas [49] and, under anaerobic conditions, T. vaginalis can exploit the ADH pathway to obtain up to 10% of its energy requirements [48]. In this pathway, arginine is converted to ornithine and ammonia through arginine deiminase (ADI), catabolic ornithine carbamyltransferase (OCT) and carbamate kinase (CK) resulting in ATP production. Details of these metabolic pathways were published by Margarita et al. [50]. This energy-producing pathway is particularly important under glucose restriction in that, in this condition, OCT and CK are both upregulated in the log growth phase of T. vaginalis and the ADH pathway provides an alternative energy source [51]. The protozoan and bacterial pathways compete for the same biochemical substrate (i.e. environmental free arginine) and, as a consequence, the T. vaginalis-Mycoplasma consortium consumes larger amounts of free arginine compared to the mycoplasma-free protozoa.
In addition, M. hominis-infected protozoa are able to produce a ~16-fold increase in intracellular ornithine and a threefold increase in putrescine [52]. Since M. hominis cannot synthesize putrescine [49], the additional supply of putrescine represents an important benefit for symbiotic bacteria.
In several bacterial species [53] and in the intestinal mucosal protist Giardia intestinalis [54], ADI is implicated in microbial mechanisms of pathogenicity. M. hominis-infected T. vaginalis shows higher production of ATP per cell as a consequence of the redundancy of the protozoan and bacterial ADH pathways [50]. The increase in ATP production is likely delivered by the Mycoplasma ADH pathway, since protozoan ADH genes are not upregulated by the symbionts [52]. Increasing the free arginine levels caused the T. vaginalis-M. hominis consortium to further increase ATP production per cell. Free arginine levels are high in the vaginal environment during infections, thus facilitating energy metabolism of both T. vaginalis and M. hominis.
It has been demonstrated that a higher intracellular ATP concentration is correlated with an increased growth rate of several parasites and, in fact, M. hominis-infected T. vaginalis are characterized by a ~20% faster growth rate than mycoplasma-free parasites, leading to ~40% higher cell densities during stationary phase [50].
While the intracellular location and the support of putrescine represent a major advantage for M. hominis, the faster growth rate, higher production of intracellular ATP, rapid depletion of free environmental arginine and subsequent reduced production of free NO by activated macrophages are important physiological benefits for T. vaginalis.
All of these biochemical aspects of symbiosis might be fundamental for the establishment and maintenance of the strict microbial association between T. vaginalis and M. hominis, and thus become an essential part of parasite physiopathology and the dynamics of host-microbe interactions.
4. Impact of Trichomonas symbionts on human host immunity and disease – therapeutic challenges
Several protozoan virulence factors have been identified, but it is unclear as to whether they can be therapeutic targets on their own without considering the pathogenic implications of the host immune response. For example, targeting T. vaginalis with antibiotics has negative implications for the human inflammatory response which, unless addressed by anti-inflammatory treatment, may compromise the therapeutic outcome. As discussed below, both Trichomonasvirus and Mycoplasma symbionts can, when released by antibiotic-stressed parasites, induce antiviral and antibacterial types of innate immunity and inflammatory responses. These responses are unlikely to contribute to self-clearance of the protozoan infection, but rather, cause damage driven by heavy leukocyte infiltration and by high local concentrations of pro inflammatory cytokines and chemokines, which are frequently detected in trichomoniasis [6] and by the same mechanism of excessive inflammation, increasing the risk of invasive cervical cancer [6], prostate cancer [55] and HIV acquisition and shedding [56].
4.1. Trichomonasvirus
Studies meant to correlate Trichomonasvirus with severity of human disease have been hampered by the limited knowledge of TVV genetics, and hence, limited diagnostic and analytic tools [3]. Wendel et al. [24] were the first to focus on the prevalence and clinical features associated with infection by either dsRNA virus-negative or dsRNA virus-positive trichomonads. The study observed that patients with TVV infected isolates reported more genital irritation, odor, genital pruritus and discharge, and less dysuria, than patients with uninfected isolates; however, statistical significance could not be reached due to the small sample size (28 isolates). A Cuban study by Fraga et al. [37] reported the association of TVV infection with vaginal discharge, dysuria, dyspareunia and cervical, but not vaginal or vulvar erythema, and not with pruritus. A study in Egypt reported similar positive associations [16] and no association with burning and vaginal edema. Moreover, Fraga et al. [37] showed that the viral symbiont species can influence the severity of signs and symptoms, as the parasites isolated from patients with mild symptoms were only infected with TVV-1 whereas TVV-2 was present in isolates of patients with moderate or severe symptoms. It should be noted that TVV-3 and TVV-4 could not be evaluated, since only TVV-1 and −2 were detected in the Cuban isolates studied. Larger studies with improved diagnostics should be conducted in order to corroborate these results.
The association between TVV and more severe clinical features of trichomoniasis is indicative of a possible role of the virus in the pathogenesis of human trichomoniasis. Since observational clinical studies can only demonstrate associations, in order to study the causative effects of TVV on the human host responses, Fichorova et al. established an experimental in vitro cell culture model of the human vaginal mucosa to study T. vaginalis pathogenesis at the cellular and molecular level [2,3,57]. They showed for the first time that TVV dramatically upregulates human host pro-inflammatory responses, which are mediated by toll-like receptor (TLR)-3 and interferon regulatory factor (IRF)-3 signaling [3]. The findings corroborated the results from a mouse model of mucocutaneous leishmaniasis in which another protozoan virus, Leishmania RNA virus, controls the severity of that parasitic disease [4]. The TVV virus was even more pro-inflammatory when the epithelial cells were colonized by pathogenic vaginal bacteria instead of with Lactobacillus species characteristic of the healthy vaginal environment [2]. Furthermore, Fichorova et al. [3] were the first to show that conventional antiparasitic drugs such as metronidazole can aggravate TV-associated inflammatory pathology by causing stressed or dying parasites to release TVV virions, which then utilize the endosomal signaling pathway to amplify the inflammatory response to the parasitic infection, including increased expression of interleukin (IL)-8, macrophage inflammatory protein (MIP)-3α, inter-cellular adhesion molecule (ICAM)-1, IL-1β, IL-6, interferon (IFN)-β and regulated on activation, normal T cell expressed and secreted (RANTES) and reduced levels of anti-inflammatory IL-1 receptor antagonist (RA) [3]. Such increased levels of inflammatory cytokines and chemokines have prognostic value in women with preterm delivery [6]. These findings implicate TVV in a mechanism that can explain why current antibiotic therapy fails to prevent preterm birth even when it achieves parasitic clearance, and can even worsen the inflammatory complications associated with TV infection [58].
The involvement of Trichomonasvirus in prostate and cervical cancer and the delayed clearance of oncogenic HPV infection associated with trichomoniasis are yet to be elucidated.
4.2. Mycoplasma
The association between T. vaginalis and M. hominis is the first symbiosis described, involving two obligate human mucosal pathogens, able to invade and infect the same anatomical region, with both agents capable of producing independent diseases as well as converging syndromes such as pelvic inflammatory disease and bacterial vaginosis [59]. Both infectious agents are associated with pregnancy and post-partum complications, including premature rupture of the placental membranes, preterm delivery and low-birth-weight infants [6,60].
T. vaginalis isolates naturally harboring M. hominis are able to transmit the bacterium to a mycoplasma-free recipient T. vaginalis. Moreover, infected protozoa can transmit M. hominis to human epithelial cells in vitro, suggesting a potential role of the protozoon in transmitting the bacterial infection to the human host [61]. These in vitro findings are supported by clinical findings in symptomatic women. In a study conducted in The Netherlands [62], M. hominis could be detected in 79% of all samples positive for T. vaginalis, as compared to only 6% in negative samples. Similarly, in Italy, M. hominis was detected in 78.6% of samples from women positive for T. vaginalis and in only 4.8% of samples from T. vaginalis-negative patients [61].
T. vaginalis naturally infected by M. hominis shows a higher level of cytopathogenicity in vitro [33]. Margarita et al. [50] confirmed that mycoplasmas enhance the parasite’s cytopathogenicity by showing that the hemolytic properties of M. hominis–infected protists were nearly double those of mycoplasma-free T. vaginalis.
It is well known that some surface M. hominis molecules are very efficient ligands for TLRs, and are able to stimulate a massive immune response mediated by production of proinflammatory cytokines. Mercer et al. [63] showed that the intracellular presence of M. hominis in T. vaginalis increased IL-1β, IL-6 and IL-8 production by peripheral human macrophages. Fiori et al. [64] demonstrated that M. hominis-associated T. vaginalis upregulated IL-1β, IL-8, IL-23 and tumor necrosis factor (TNF)-α secretion by human macrophage cell lines more than mycoplasma-free T. vaginalis. Interestingly, IL-23, which skews the adaptive immunity to a Th17 response, was secreted only by macrophages stimulated with M. hominis-infected T. vaginalis, but not with the protozoon alone, suggesting the ability of M. hominis symbionts to influence the fate of the infection. NF-κB activation was also synergistically upregulated by the symbiotic association between T. vaginalis and M. hominis.
Depletion of free environmental arginine is considered to be an important microbial virulence strategy to escape from the toxic effects which follow from NO production by macrophages [53]. Since arginine is the exclusive substrate for the synthesis of NO by macrophages, M. hominis can be protective for T. vaginalis through the depletion of arginine by its ADH pathway enzymes, which leads to reduced NO production by macrophages [50].
T. vaginalis seems to be associated with malignant transformation and cancer maintenance and should be added to the list of potentially carcinogenic agents. T. vaginalis infection has, in fact, been implicated as a risk factor for cervical cancer [65], benign prostate hyperplasia [66] and also for aggressive prostate cancer on the basis of serological data from large caseecontrol studies [67,68]. Very recently, a direct molecular mechanism for potential T. vaginalis involvement in prostate tumor transformation and progression has been proposed, implicating a protozoan homologue of human macrophage migration inhibitory factor (MIF), a pleiotropic cytokine that mediates inflammation and promotes oncogenesis and prostate cancer [69].
In addition to massive local inflammation synergistically upregulated by Trichomonasvirus and M. hominis symbiosis, a possible direct role of M. hominis infection in prostate cancer has been proposed. M. hominis infection of the prostate is associated with malignant transformation and genomic instability [70]. A computational prediction has generated a list of M. hominis proteins putatively implicated in prostate cancer, able to interfere with growth of human host cells [71]. Additional experiments are needed to confirm the role of M. hominis in tumor transformation and development, and to better understand whether these symbiotic bacteria can modulate the cancer-promoting activities of protozoa.
5. Impact of the protozoan symbionts on the urogenital microbiome
Accumulating evidence points to polymicrobial T. vaginalis virulence factors as the cause of vaginal microbiome disturbance. T. vaginalis is a frequent companion of bacterial vaginosis (BV), which is the most common morbid micro-biological syndrome among women of childbearing age, characterized by a shift from a Lactobacillus-dominated bacteriome to more diverse polymicrobial states, with abundant Prevotella, Atopobium, Gardnerella and other anaerobes [72]. Fichorova et al. showed that TVV-infected T. vaginalis can substantially reduce vaginal epithelial cell colonization by lactobacilli, including the pillars of the healthy vaginal microbiome L. crispatus, L. jensenii and L. gasseri, while at the same time favoring BV-associated bacteria such as P. bivia and A. vaginae. Moreover, TVV increased the epithelial colonization by the major BV biofilm forming pathogen G. vaginalis [2]. A genomic study by Martin et al. [35] showed that there are two entirely unique vaginal microbiome clusters that are exclusively associated with T. vaginalis infection. One of these had a high abundance of the uncultured C. M. girerdii and the other had a high abundance of M. hominis. Both of these microbiomes also had a high abundance of Prevotella spp. and a low abundance of Lactobacillus and were associated with severe inflammation, including vaginal erythema and cervical petechiae [35]. Despite the clinical significance of these findings, the knowledge and understanding of the role of the T. vaginalis symbionts in modifying the vaginal microbiome is lagging behind. The data presented above on the ability of both Trichomonas and Mycoplasma to change metabolic factors that may affect growth of other bacteria as well as host cells depicts a very complicated scenario, involving a number of multiple actors (cells and molecules) based on a fine-tuned equilibrium among host defenses, a range of microbial pathogens and the resident commensal microflora. A better understanding of all these complex interactions can help in designing effective pharmacological therapies to prevent and control acute disease and complications, avoiding tissue damage due to inflammatory processes following T. vaginalis infections.
6. Relevance to therapeutic challenges
6.1. Impact on protozoan resistance to antibiotics
The 5-nitroimidazole derivatives (metronidazole and tinidazole) are the only class of drugs known to be effective against T. vaginalis infections. The emergence of nitroimidazole-resistant trichomoniasis is of concern, because effective alternative therapies are not yet available.
A correlation between metronidazole resistance and M. hominis infection of T. vaginalis has been debated. In 2006, Xiao et al. reported a positive association between infection by M. hominis and resistance to metronidazole in T. vaginalis [73], but their results are in contrast with those described by other authors. Butler et al. analyzed 55 T. vaginalis isolates (51% were metronidazole-resistant) collected in the USA; 18% of the metronidazole-resistant and 22% of the metronidazole-susceptible T. vaginalis isolates were PCR-positive for M. hominis, suggesting no significant association (P = 0.746) [74]. Moreover, metronidazole sensitivity of two infected T. vaginalis isolates did not change after they were cleared of their M. hominis infection. Results obtained with Cuban and Brazilian strains seem to confirm the absence of a relationship between metronidazole susceptibility and M. hominis infection of the protozoon [17,75].
Although it has been argued that M. hominis parasitism may confer T. vaginalis drug resistance, we conclude that most data do not confirm such an association.
6.2. Futility of current antiparasitic therapy to prevent adverse reproductive outcome
The well-described intracellular localization of mycoplasmas can also explain some paradoxical data reporting the failure of metronidazole treatment of subclinical T. vaginalis infections in pregnancy [76]: it can be hypothesized that the administration of the drug, when effective against the protozoan infection, allows the massive release of M. hominis, that can subsequently invade membranes and amniotic fluid. Experimental in vitro evidence shows that the release of TVV by dying or stressed parasites can complicate the outcome in metronidazole-treated women, and may be harmful during pregnancy or when women are at increased risk of viral sexually transmitted infections, e.g. HIV which is facilitated by vaginal inflammation [3].
7. Outlook – future research and novel therapeutic strategies
The symbiotic relationship between T. vaginalis and its endobiont viruses and intracellular mycoplasmas could represent an interesting model to study basic biological mechanisms both of the evolutionary origin of intracellular organelles and the role of protozoa as reservoirs or vectors in the transmission of infections to human hosts. The ability of Mycoplasma species to invade, resist and multiply in the T. vaginalis cytoplasm demonstrates that the bacteria have evolved effective strategies to resist and adapt to intracellular hostile environments. The intracellular location of endosymbiotic bacteria can explain their ability to cope with the adverse environment of the vaginal tract and to resist clearance by the host and antimicrobial therapies. TVV infection, on the other hand, appears to aid the parasite by making it more competitive against bacterial inhabitants of the vaginal environment and by generating a peculiar mimicry by diverting the host towards an antiviral inflammatory response that is incapable of clearing protozoan infection. The inflammatory response to both TVV and Mycoplasma can harm vulnerable pregnant women; thus, new therapeutic strategies may employ a combination of anti-inflammatory modalities and target both the parasite and its symbionts.
Acknowledgments
Dr. Raina Fichorova received grant support from the National Institutes of Health to study T. vaginalis infection (NIAID R01AI079085, RC1AI086788, R56AI091889 and NICHD R21HD054451). Dr. Fiori received grant support from the Ministero dell’Istruzione, dell’Università e della Ricerca—PRIN 2012, Grant number 2012WJSX8K_004.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References
- [1].Wang AL, Wang CC. Viruses of the protozoa. Annu Rev Microbiol 1991; 45:251–63. [DOI] [PubMed] [Google Scholar]
- [2].Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, et al. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 2013;89:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, et al. Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PLoS One 2012;7:e48418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 2011;331:775–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].WHO. Global incidence and prevalence of selected curable sexually transmitted infections 2008. Geneva, Switzerland: WHO Press; 2012. p. 20. [Google Scholar]
- [6].Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol 2009;83:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang AL, Wang CC. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci USA 1986;83:7956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goodman RP, Ghabrial SA, Fichorova RN, Nibert ML. Trichomonasvirus: a new genus of protozoan viruses in the family Totiviridae. Arch Virol 2011;156:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parent KN, Takagi Y, Cardone G, Olson NH, Ericsson M, et al. Structure of a protozoan virus from the human genitourinary parasite Trichomonas vaginalis. MBio 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weedall GD, Hall N. Sexual reproduction and genetic exchange in parasitic protists. Parasitology 2015;142(Suppl 1):S120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bessarab IN, Nakajima R, Liu HW, Tai JH. Identification and characterization of a type III Trichomonas vaginalis virus in the protozoan pathogen Trichomonas vaginalis. Arch Virol 2011;156:285–94. [DOI] [PubMed] [Google Scholar]
- [12].Goodman RP, Freret TS, Kula T, Geller AM, Talkington MW, et al. Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (Family Totiviridae). J Virol 2011;85:4258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim JW, Chung PR, Hwang MK, Choi EY. Double-stranded RNA virus in Korean isolate IH-2 of Trichomonas vaginalis. Korean J Parasitol 2007;45:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heidary S, Bandehpour M, Valadkhani Z, Seyyed-Tabaee S, Haghighi A, et al. Double-stranded RNA viral infection in Tehran Trichomonas vaginalis isolates. Iran J Parasitol 2013;8:60–4. [PMC free article] [PubMed] [Google Scholar]
- [15].Rivera WL, Justo CA, Relucio-San Diego MA, Loyola LM. Detection and molecular characterization of double-stranded RNA viruses in Philippine Trichomonas vaginalis isolates. J Microbiol Immunol Infect 2015. pii: S1684–1182(15)00853–1. [DOI] [PubMed] [Google Scholar]
- [16].El-Gayar EK, Mokhtar AB, Hassan WA. Molecular characterization of double-stranded RNA virus in Trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol Res 2016;115(10): 4027–36. [DOI] [PubMed] [Google Scholar]
- [17].da Luz Becker D, dos Santos O, Frasson AP, de Vargas Rigo G, Macedo AJ, et al. High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect Genet Evol 2015;34:181–7. [DOI] [PubMed] [Google Scholar]
- [18].Flegr J, Cerkasov J, Kulda J, Cerkasovoca A, Stokrova J. Double stranded RNA in Trichomonas vaginalis. Acta Uni Car Biol 1986;30: 281–6. [Google Scholar]
- [19].Fraga J, Rojas L, Sariego I, Fernandez-Calienes A. Double-stranded RNA viral infection in Cuban Trichomonas vaginalis isolates. Braz J Infect Dis 2005;9:521–4. [DOI] [PubMed] [Google Scholar]
- [20].Fraga J, Rojas L, Sariego I, Fernandez-Calienes A. Genetic characterization of three Cuban Trichomonas vaginalis virus. Phylogeny of Totiviridae family. Infect Genet Evol 2012;12:113–20. [DOI] [PubMed] [Google Scholar]
- [21].Malla N, Kaul P, Sehgal R, Gupta I. The presence of dsRNA virus in Trichomonas vaginalis isolates from symptomatic and asymptomatic Indian women and its correlation with in vitro metronidazole sensitivity. Indian J Med Microbiol 2011;29:152–7. [DOI] [PubMed] [Google Scholar]
- [22].Wang A, Wang CC, Alderete JF. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med 1987;166:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weber B, Mapeka TM, Maahlo MA, Hoosen AA. Double stranded RNA virus in South African Trichomonas vaginalis isolates. J Clin Pathol 2003;56:542–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wendel KA, Rompalo AM, Erbelding EJ, Chang TH, Alderete JF. Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. J Infect Dis 2002;186:558–61. [DOI] [PubMed] [Google Scholar]
- [25].Alderete JF, Wendel KA, Rompalo AM, Erbelding EJ, Benchimol M, et al. Trichomonas vaginalis: evaluating capsid proteins of dsRNA viruses and the dsRNA virus within patients attending a sexually transmitted disease clinic. Exp Parasitol 2003;103:44–50. [DOI] [PubMed] [Google Scholar]
- [26].Baptista CS, Wu X, Munroa DJ. Viral nucleic acid microarray and method use. Geneva, Switzerland: World Intellectual Property Organization; 2007. [Google Scholar]
- [27].Benchimol M, Monteiro S, Chang TH, Alderete JF. Virus in Trichomonas–an ultrastructural study. Parasitol Int 2002;51:293–8. [DOI] [PubMed] [Google Scholar]
- [28].Nielsen MH. The ultrastructure of Trichomonas vaginalis donne before and after transfer from vaginal secretion to Diamonds medium. Acta Pathol Microbiol Scand Suppl 1975;83:581–9. [DOI] [PubMed] [Google Scholar]
- [29].Scholtyseck E, Teras J, Kasakova I, Sethi KK. Electron microscope observations on the interaction of Mycoplasma fermentans with Trichomonas vaginalis. Z Parasitenkd 1985;71:435–42. [DOI] [PubMed] [Google Scholar]
- [30].Koch A, Bilina A, Teodorowicz L, Stary A. Mycoplasma hominis and Ureaplasma urealyticum in patients with sexually transmitted diseases. Wien Klin Wochenschr 1997;109:584–9. [PubMed] [Google Scholar]
- [31].Rappelli P, Addis MF, Carta F, Fiori PL. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet 1998;352:1286. [DOI] [PubMed] [Google Scholar]
- [32].Dessi D, Delogu G, Emonte E, Catania MR, Fiori PL, et al. Long-term survival and intracellular replication of Mycoplasma hominis in Trichomonas vaginalis cells: potential role of the protozoon in transmitting bacterial infection. Infect Immun 2005;73:1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vancini RG, Benchimol M. Entry and intracellular location of Mycoplasma hominis in Trichomonas vaginalis. Arch Microbiol 2008;189: 7–18. [DOI] [PubMed] [Google Scholar]
- [34].Fettweis JM, Serrano MG, Huang B, Brooks JP, Glascock AL, et al. An emerging mycoplasma associated with trichomoniasis, vaginal infection and disease. PLoS One 2014;9:e110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martin DH, Zozaya M, Lillis RA, Myers L, Nsuami MJ, et al. Unique vaginal microbiota that includes an unknown Mycoplasma-like organism is associated with Trichomonas vaginalis infection. J Infect Dis 2013; 207:1922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Singh BN, Hayes GR, Lucas JJ, Sommer U, Viseux N, et al. Structural details and composition of Trichomonas vaginalis lipophosphoglycan in relevance to the epithelial immune function. Glycoconj J 2009;26:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fraga J, Rojas L, Sariego I, Fernández-Calienes A, Nuñez FA. Species typing of Cuban Trichomonas vaginalis virus by RT-PCR and association of TVV-2 with high parasite adhesion levels and high pathogenicity in patients. Arch Virol 2012;157:1789–95. [DOI] [PubMed] [Google Scholar]
- [38].Hampl V, Vanacova S, Kulda J, Flegr J. Concordance between genetic relatedness and phenotypic similarities of Trichomonas vaginalis strains. BMC Evol Biol 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vanacova S, Tachezy J, Kulda J, Flegr J. Characterization of trichomonad species and strains by PCR fingerprinting. J Eukaryot Microbiol 1997;44: 545–52. [DOI] [PubMed] [Google Scholar]
- [40].Snipes LJ, Gamard PM, Narcisi EM, Beard CB, Lehmann T, et al. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J Clin Microbiol 2000;38: 3004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Conrad MD, Gorman AW, Schillinger JA, Fiori PL, Arroyo R, et al. Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis 2012;6:e1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fraga J, Rojas L, Sariego I, Fernandez-Calienes A. Characterization of specific RAPD markers of virulence in Trichomonas vaginalis isolates. Iran J Parasitol 2015;10:448–56. [PMC free article] [PubMed] [Google Scholar]
- [43].Khoshnan A, Alderete JF. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J Virol 1994;68:4035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alderete JF. Iron modulates phenotypic variation and phosphorylation of P270 in double-stranded RNA virus-infected Trichomonas vaginalis. Infect Immun 1999;67:4298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Provenzano D, Khoshnan A, Alderete JF. Involvement of dsRNA virus in the protein composition and growth kinetics of host Trichomonas vaginalis. Arch Virol 1997;142:939–52. [DOI] [PubMed] [Google Scholar]
- [46].Arroyo R, Alderete JF. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect Immun 1989; 57:2991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ryan CM, de Miguel N, Johnson PJ. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem 2011;51:161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yarlett N, Martinez MP, Moharrami MA, Tachezy J. The contribution of the arginine dihydrolase pathway to energy metabolism by Trichomonas vaginalis. Mol Biochem Parasitol 1996;78:117–25. [DOI] [PubMed] [Google Scholar]
- [49].Pereyre S, Sirand-Pugnet P, Beven L, Charron A, Renaudin H, et al. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet 2009;5:e1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Margarita V, Rappelli P, Dessi D, Pintus G, Hirt RP, et al. Symbiotic association with Mycoplasma hominis can influence growth rate, ATP production, cytolysis and inflammatory response of Trichomonas vaginalis. Front Microbiol 2016;7:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Huang KY, Chen YY, Fang YK, Cheng WH, Cheng CC, et al. Adaptive responses to glucose restriction enhance cell survival, antioxidant capability and autophagy of the protozoan parasite Trichomonas vaginalis. Biochim Biophys Acta 2014;1840:53–64. [DOI] [PubMed] [Google Scholar]
- [52].Morada M, Manzur M, Lam B, Tan C, Tachezy J, et al. Arginine metabolism in Trichomonas vaginalis infected with Mycoplasma hominis. Microbiology 2010;156:3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ryan S, Begley M, Gahan CG, Hill C. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol 2009;11:432–45. [DOI] [PubMed] [Google Scholar]
- [54].Touz MC, Ropolo AS, Rivero MR, Vranych CV, Conrad JT, et al. Arginine deiminase has multiple regulatory roles in the biology of Giardia lamblia. J Cell Sci 2008;121:2930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sutcliffe S, Neace C, Magnuson NS, Reeves R, Alderete JF. Trichomonosis, a common curable STI and prostate carcinogenesis–a proposed molecular mechanism. PLoS Pathog 2012;8:e1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect 2013;89:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, et al. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun 2006;74:5773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gulmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev 2011:CD000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dessi D, Rappelli P, Diaz N, Cappuccinelli P, Fiori PL. Mycoplasma hominis and Trichomonas vaginalis: a unique case of symbiotic relationship between two obligate human parasites. Front Biosci 2006;11:2028–34. [DOI] [PubMed] [Google Scholar]
- [60].Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis 2006;25:562–9. [DOI] [PubMed] [Google Scholar]
- [61].Rappelli P, Carta F, Delogu G, Addis MF, Dessi D, et al. Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility of M. hominis to human cells. Arch Microbiol 2001;175:70–4. [DOI] [PubMed] [Google Scholar]
- [62].van Belkum A, van der Schee C, van der Meijden WI, Verbrugh HA, Sluiters HJ. A clinical study on the association of Trichomonas vaginalis and Mycoplasma hominis infections in women attending a sexually transmitted disease (STD) outpatient clinic. FEMS Immunol Med Microbiol 2001;32:27–32. [DOI] [PubMed] [Google Scholar]
- [63].Mercer F, Diala FG, Chen YP, Molgora BM, Ng SH, et al. Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis 2016;10:e0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fiori PL, Diaz N, Cocco AR, Rappelli P, Dessi D. Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex Transm Infect 2013;89:449–54. [DOI] [PubMed] [Google Scholar]
- [65].Tao L, Han L, Li X, Gao Q, Pan L, et al. Prevalence and risk factors for cervical neoplasia: a cervical cancer screening program in Beijing. BMC Public Health 2014;14:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mitteregger D, Aberle SW, Makristathis A, Walochnik J, Brozek W, et al. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Med Microbiol Immunol 2012;201:113–6. [DOI] [PubMed] [Google Scholar]
- [67].Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: physicians’ Health Study. J Natl Cancer Inst 2009;101:1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15:939–45. [DOI] [PubMed] [Google Scholar]
- [69].Twu O, Dessi D, Vu A, Mercer F, Stevens GC, et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness and inflammatory responses. Proc Natl Acad Sci USA 2014;111:8179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Namiki K, Goodison S, Porvasnik S, Allan RW, Iczkowski KA, et al. Persistent exposure to Mycoplasma induces malignant transformation of human prostate cells. PLoS One 2009;4:e6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Khan S, Zakariah M, Palaniappan S. Computational prediction of Mycoplasma hominis proteins targeting in nucleus of host cell and their implication in prostate cancer etiology. Tumour Biol 2016;37:10805–13. [DOI] [PubMed] [Google Scholar]
- [72].Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev 2016;29:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Xiao JC, Xie LF, Fang SL, Gao MY, Zhu Y, et al. Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol Res 2006;100:123–30. [DOI] [PubMed] [Google Scholar]
- [74].Butler SE, Augostini P, Secor WE. Mycoplasma hominis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States. Parasitol Res 2010;107:1023–7. [DOI] [PubMed] [Google Scholar]
- [75].Fraga J, Rodriguez N, Fernandez C, Mondeja B, Sariego I, et al. Mycoplasma hominis in Cuban Trichomonas vaginalis isolates: association with parasite genetic polymorphism. Exp Parasitol 2012;131:393–8. [DOI] [PubMed] [Google Scholar]
- [76].Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001; 345:487–93. [DOI] [PubMed] [Google Scholar]
- [77].Tai JH, Ip CF. The cDNA sequence of Trichomonas vaginalis virus-T1 double-stranded RNA. Virology 1995;206:773–6. [DOI] [PubMed] [Google Scholar]
- [78].Su HM, Tai JH. Genomic organization and sequence conservation in type I Trichomonas vaginalis viruses. Virology 1996;222:470–3. [DOI] [PubMed] [Google Scholar]
- [79].van der Schee C, Sluiters HJ, van der Meijden WI, van Beek P, Peerbooms P, et al. Host and pathogen interaction during vaginal infection by Trichomonas vaginalis and Mycoplasma hominis or Ureaplasma urealyticum. J Microbiol Methods 2001;45:61–7. [DOI] [PubMed] [Google Scholar]


