Abstract
A systematic review and meta-analysis of the entire COVID-19 Tracheostomy cohort was conducted to determine the cumulative incidence of complications, mortality, time to decannulation and ventilatory weaning. Outcomes of surgical versus percutaneous and outcomes relative to tracheostomy timing were also analysed. Studies reporting outcome data on patients with COVID-19 undergoing tracheostomy were identified and screened by 2 independent reviewers. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed. Outcome data were analysed using a random-effects model. From 1016 unique studies, 39 articles reporting outcomes for a total of 3929 patients were included for meta-analysis. Weighted mean follow-up time was 42.03 ± 26 days post-tracheostomy. Meta-analysis showed that 61.2% of patients were weaned from mechanical ventilation [95%CI 52.6%–69.5%], 44.2% of patients were decannulated [95%CI 33.96%–54.67%], and cumulative mortality was found to be 19.23% [95%CI 15.2%–23.6%] across the entire tracheostomy cohort. The cumulative incidence of complications was 14.24% [95%CI 9.6%–19.6%], with bleeding accounting for 52% of all complications. No difference was found in incidence of mortality (RR1.96; p = 0.34), decannulation (RR1.35, p = 0.27), complications (RR0.75, p = 0.09) and time to decannulation (SMD 0.46, p = 0.68) between percutaneous and surgical tracheostomy. Moreover, no difference was found in mortality (RR1.57, p = 0.43) between early and late tracheostomy, and timing of tracheostomy did not predict time to decannulation. Ten confirmed nosocomial staff infections were reported from 1398 tracheostomies. This study provides an overview of outcomes of tracheostomy in COVID-19 patients, and contributes to our understanding of tracheostomy decisions in this patient cohort.
Keywords: Tracheostomy, COVID-19, Surgical tracheostomy, Percutaneous tracheostomy, Decannulation, Ventilatory weaning
Introduction
The outbreak and global propagation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), led the World Health Organization to declare a pandemic in March 2020. An extraordinary burden has been placed on healthcare systems as a consequence of the respiratory and other end-organ sequelae of COVID-19, with many patients requiring intensive care unit (ICU) admission for invasive mechanical ventilation and other organ support.1, 2, 3
Tracheostomies may be undertaken where prolonged mechanical ventilation is anticipated in order to facilitate weaning from both mechanical ventilation and sedation, as well as to try and reduce the length of stay and ventilator-associated morbidity.4, 5 Furthermore, tracheostomies may help to ameliorate resource constraints by increasing tube tolerance, which can reduce the incidence of accidental extubations and thus mitigate the strain on critical care services inherent to the COVID-19 pandemic.2, 6, 7
To date, several studies have been published pertaining to tracheostomies in patients with COVID-19. Nevertheless, there is no consensus with regards to tracheostomy strategy and this is exacerbated by limited data, patient heterogeneity and the dynamic nature of the pandemic.8, 9, 10, 11 This study therefore had several aims. First, to synthesise the current outcome data on all COVID-19 patients who have undergone tracheostomy; secondly, to compare outcomes and patient demographics between those patients that have had surgical or percutaneous tracheostomy; thirdly, to determine whether the timing of tracheostomy post-intubation influences outcomes and, finally, to provide recommendations to improve the quality of reporting on COVID-19 outcome data as the pandemic progresses.
Methods
This study was completed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Search strategy
A systematic literature search was performed using Scopus, MEDLINE, PubMed Central, Embase, the Web of Science Core Collection and the Cochrane Library for all articles published between 1st December 2019 through 27th January 2021. The following search terms were used: ((“tracheostom*” OR “tracheotom*”) AND (“COVID*” OR “coronavirus” OR “SARS-COV-2” OR “severe acute respiratory syndrome coronavirus 2”)). References of identified records were iteratively searched for further suitable records.
Selection criteria
Studies were considered for inclusion based on the following criteria: comprised patients diagnosed with COVID-19 who had undergone tracheostomy; contained outcome data specific to tracheostomy on mortality, decannulation, mechanical weaning, and complications. Exclusion criteria included: studies lacking outcome data specific to tracheostomy, full texts not available in English, and studies reporting case series of fewer than 10 patients. Moreover, guidelines, recommendations and expert opinions were excluded if they did not conform to the inclusion criteria above.
Titles, abstracts and full texts were screened independently by 2 authors according to the inclusion and exclusion criteria. Discrepancies were resolved by consensus following discussion between reviewers to minimise selection bias.
Data extraction
Study characteristics were independently extracted from selected articles by 2 independent investigators. The following baseline characteristics were extracted: total number of tracheostomies; patient age; body mass index (BMI); comorbidities; smoking status; proportion of female vs male; tracheostomy type; patient ethnicity; preoperative C-reactive protein (CRP); preoperative respiratory indices (including fraction of inspired oxygen (FiO2); mean partial pressure of oxygen (PaO2) and positive end-expiratory pressure (PEEP)); Acute Physiology and Chronic Health Evaluation II (APACHE II) score; and time from intubation to tracheostomy. Collected outcome criteria include number of patients decannulated; number of patients successfully weaned off mechanical ventilation; the time from tracheostomy to decannulation and successful weaning; the number of patients who died during follow-up and the mean follow-up period. The number of staff members directly involved in tracheostomies who received a positive COVID-19 result or who became symptomatic during the follow up period was also recorded. Outcome data were also collected for surgical versus percutaneous tracheostomy subgroups independently, and for those studies that reported outcomes for early versus late tracheostomy.
Assessment of bias
The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias of included studies; this scoring system has been validated for assessing risk of bias for non-randomised cohort studies and is recommended by the Cochrane Collaboration.12 Disagreement in the NOS score was resolved through consensus. No studies were excluded from meta-analysis on the grounds of bias. Studies were classified as high risk of bias (0–3), moderate risk of bias (4–6 points) and low risk of bias (≥7 points).13
Statistical analysis
Continuous outcomes and baseline characteristics were calculated and reported as weighted combined means using formulae provided by the Cochrane Collaboration.12 Where means and standard deviations were not provided, they were approximated according to Wan et al 2014.14
A meta-analysis of proportions was used to calculate the cumulative incidence of dichotomous outcome variables across all studies: mortality, weaning off mechanical ventilation, decannulation, and complications. The Freeman-Tukey double arcsine transformation was used to obtain weighted summary proportions as part of a random-effects model to account for sample heterogeneity. Results were presented as forest plots, including 95% confidence intervals of proportions. For a meta-analysis of binary outcomes between groups (surgical versus percutaneous or early versus late) the Mantel-Haenszel approach was used as part of a random-effects model. Results are reported as risk ratios (RR) with 95% confidence intervals. Hedges g was used as the measure of standardised mean difference (SMD) between groups where continuous outcome data (such as time to decannulation) were analysed, and a random-effects model for continuous outcome data was performed with the Hartung-Knapp adjustment.
Study heterogeneity was assessed using tau,2 and the proportion of true variance of a weighted outcome was estimated using Higgins I 2. I 2 was interpreted according to Borenstein et al15 though 0–40% was considered as low heterogeneity, 30–60% was considered as moderate heterogeneity, 50–90% as substantial heterogeneity and >75% as considerable heterogeneity.12 A Cochrane Q statistic p-value <0.10 was considered as significant.
Univariate linear regression was used to determine the influence of timing of tracheostomy post-intubation on timing of decannulation. Influential outliers were identified according to leverage, standardised residuals and Cooke's distance. Statistical analysis was performed using R Statistical Software version 4.0.0. All scripts for meta-analysis are available upon reasonable request to the corresponding author.
Results
Study selection
The initial literature search identified 2429 records from the 6 databases and through reference screening; 1016 unique records remained following duplicate removal (Fig. 1 ). Of these, 46 studies met inclusion criteria. Seven further studies were removed with shared data, leaving 39 studies for meta-analysis.16, 17, 18, 19, 20, 21, 22
Fig. 1.
PRISMA flowchart of the study selection process.
The majority of studies were performed in the United States (US), accounting for 33.3% of all included studies. The United Kingdom (UK) provided 9 studies,23, 24, 25, 26, 27, 28, 29, 30, 31 Spain 7 studies,32, 33, 34, 35, 36, 37, 38 Italy 6 studies,39, 40, 41, 42, 43, 44 China 3 studies,45, 46, 47 and India 1 study.48
Assessment of study quality
The Newcastle-Ottawa Scale (NOS) was used to assess bias across the 39 included studies. The median total score was 7 (out of a possible maximum of 9), with a range of 4–8 (Supplementary figure 1). A total of 23 studies scored as low risk of bias; 16 studies scored as moderate risk of bias.
Baseline characteristics
Baseline characteristics of included patients were variably reported throughout the 39 studies. Available preoperative characteristics are summarised in Table 1 .
Table 1.
baseline characteristics of included patients. “References” refers to those studies that reported the characteristic. “Count” refers to the number of patients across reporting studies to which the characteristic pertains. Mean and standard deviation, where provided, are combined weighted estimates. SD, standard deviation; BMI, body-mass index; PEEP, positive end-expiratory pressure; APACHE II, Acute Physiology and Chronic Health Evaluation II.
| Characteristic | Value | References | Count No. (%) |
|---|---|---|---|
| Total patients | 3929 | 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 | 3929 (100) |
| Sex n (%) | |||
| Female | 437 (27.01) | 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 36, 38, 39, 40, 41, 42, 43, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 59, 60, 61 | 1618 (41.18) |
| Male | 1181 (72.99) | ||
| Ethnicity n (%) | |||
| Asian | 111 (12.61) | 25, 26, 27, 30, 38, 49, 51, 52, 54, 55, 56, 60 | 880 (22.40) |
| Hispanic | 179 (20.34) | ||
| Mixed | 14 (1.59) | ||
| White | 298 (33.86) | ||
| Black | 158 (17.95) | ||
| Other | 83 (9.43) | ||
| Unknown | 37 (4.20) | ||
| Age (years); Mean (SD) | 59.86 (12.77) | 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 36, 38, 39, 40, 41, 42, 43, 45, 46, 47, 48, 49, 50, 51, 52, 54, 55, 56, 57, 59, 60, 61 | 1721 (43.80) |
| BMI; Mean (SD) | 30.35 (7.49) | 23, 26, 27, 31, 33, 41, 49, 50, 51, 55, 56, 57, 59, 60 | 1026 (26.11) |
| Tracheostomy type No. (%) | 23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 62 | 3744 (95.29) | |
| Percutaneous | 1457 (38.92) | ||
| Surgical | 2179 (58.20 | ||
| Novel/Hybrid | 108 (2.88) | ||
| PEEP (mmHg); Mean (SD) | 24, 25, 26,30,33,36,39,40,49,50,54,60 | 674 (17.15) | |
| 8.94 (3.60) | |||
| PaO2/FiO2 ratio | 772 (19.65) | ||
| Mean (SD) | 206.74 (122.89) | 25,27,30,32,33,36,40,41,47,49,50,60 | |
| APACHE II | 13.85 (3.89) | 26,27,30,33,36,47 | 488 (12.42) |
| Mean (SD) | |||
| Time from intubation to tracheostomy (days); Mean (SD) | 16.75 (7.06) | 23, 25, 26, 27, 28, 30, 31, 32, 33, 35, 36, 38, 40, 41, 43, 45, 46, 47, 49, 50, 51, 52, 53, 54, 55, 57, 59, 61 | 3478 (88.55) |
Mortality
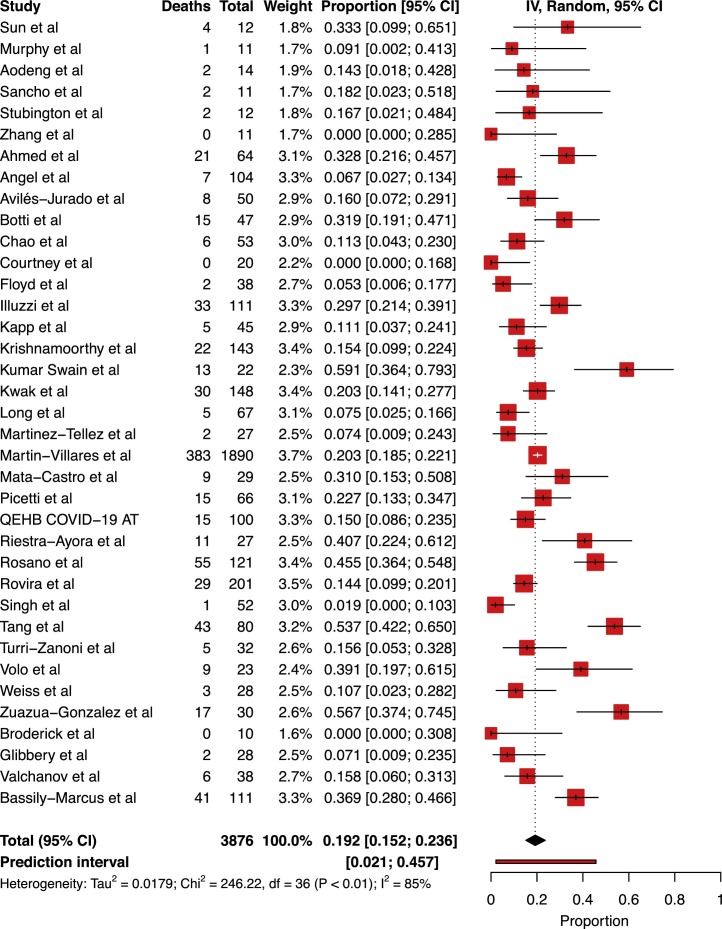
A total of 37 studies included mortality data, irrespective of subgrouping. Of the 3876 patients included across these 37 studies, 824 patients had died by the time of follow-up.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 34, 61 Only 14 studies explicitly reported a mean follow-up duration, giving a combined weighted mean of 42.03 days (SD 26.43).24, 26, 27, 30, 33, 37, 42, 43, 48, 50, 52, 56, 59, 60 Pooled tracheostomy mortality was calculated at 19.23% [95% CI 15.2%–23.6%]. Study heterogeneity was found to be substantial (I 2 = 85.5% [95% CI 80.4%–99.9%], p < 0.0001; Fig. 2 ). Overall weighted mean time to death from tracheostomy was 17.64 days (SD 13.25).24, 30, 39, 42, 43, 48, 51
Fig. 2.
Forest plot of cumulative mortality across all tracheostomised patients with COVID-19.
Decannulation
A total of 28 studies reported outcomes related to decannulation. Of a total of 3326 tracheostomies performed across these 28 studies, 1416 patients were successfully decannulated at the time of follow-up.24, 25, 26, 27, 28, 29, 31, 32, 33, 35, 39, 40, 41, 42, 43, 44, 45, 46, 48, 49, 50, 51, 52, 55, 56, 57, 59, 60, 34 Analysis of pooled data identified a cumulative incidence of decannulation of 44.2% [95% CI 33.96%–54.67%]. Heterogeneity was found to be substantial, with I 2 95% [95%CI 94.4%–96.4%] (p < 0.0001; Supplementary figure 2). Mean time to decannulation was reported in 19 of the 39 studies, identifying an overall weighted mean time from intubation to decannulation of 17.70 days (SD 11.31).25, 26, 27, 28, 30, 31, 32, 33, 38, 39, 41, 43, 46, 48, 49, 51, 52, 55, 56, 57, 60
Ventilator weaning
A total of 32 studies encompassing a total of 3510 tracheostomy patients reported the number of patients weaned off mechanical ventilation at the time of follow-up.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 35, 36, 39, 40, 42, 43, 45, 46, 47, 48, 49, 50, 51, 52, 55, 56, 57, 58, 59, 60, 34, 61 The pooled incidence of ventilatory weaning was 61.20% [95%CI 52.6%–69.5%]. I 2 was 94.2% [95%CI 92.7%–95.4%] (p < 0.0001), indicating substantial heterogeneity. Time from tracheostomy to weaning from mechanical ventilation was reported in only 8 studies.27, 28, 30, 33, 36, 38, 49, 51 The overall weighted mean time from tracheostomy to mechanical ventilatory weaning was 24.14 days (SD 10.19 days).
Complications
Specific complications in the perioperative period of tracheostomy were reported in 31 of the 39 studies, encompassing a total of 3479 tracheostomies.23, 24, 25, 26, 27, 28, 29, 30, 31, 33, 35, 38, 39, 40, 42, 43, 45, 46, 47, 48, 49, 50, 51, 52, 54, 55, 56, 57, 59, 60, 34 305 complications were recorded. Table 2 provides an overview of overall complications by proportion of total complications.
Table 2.
Overview of Complications. S/C, subcutaneous.
| Complication | Frequency (No.) | Proportion of total (%) |
|---|---|---|
| Tube replacement <24 hours | 2 | 0.66 |
| Tracheal injury | 3 | 0.98 |
| Hypoxia <80% | 7 | 2.30 |
| Conversion to surgical | 2 | 0.66 |
| False passage | 2 | 0.66 |
| Vocal cord palsy | 2 | 0.66 |
| Cardiac arrest | 1 | 0.33 |
| Cuff deflation | 5 | 1.64 |
| Mucous plugging | 5 | 1.64 |
| Tube obstruction | 4 | 1.31 |
| Tube dislodgement | 26 | 8.52 |
| Pneumothorax/mediastinum | 7 | 2.30 |
| S/C emphysema | 7 | 2.30 |
| Peristomal skin ulceration | 6 | 1.97 |
| Cuff leak | 44 | 14.43 |
| Bleeding | 160 | 52.46 |
| Local infection | 34 | 11.15 |
| Total | 305 | 100 |
Analysis of pooled data across the 31 records reporting complete complication outcomes identified a cumulative incidence of complications of 14.24% [95%CI 9.6%–19.6%] (Supplementary figure 3). Similar to previous, substantial heterogeneity was noted (I 2 = 90.3% [95%CI 87.4%–92.6%], p < 0.0001).
Percutaneous versus surgical tracheostomy
A total of 36 studies reported on the distribution of tracheostomy performed across the studied population, accounting for a total of 3744 patients.23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 54, 56, 57, 58, 59, 60, 34, 61 Of 3744 patients, 2179 were performed as surgical procedures, 1457 as percutaneous and 108 as novel/hybrid procedures (Table 3 ).
Table 3.
Baseline characteristics of patients according to tracheostomy type. Mean and standard deviation, where provided, are combined weighted estimates. Count refers to the number of patients to which the characteristic pertains. SD, standard deviation; BMI, body-mass index. p Values relate to Welch's unpaired t-test for continuous data and χ2 test for categorical data.
| Characteristic | Tracheostomy type |
Reference | Count |
p-Value | ||
|---|---|---|---|---|---|---|
| Percutaneous | Surgical | Percutaneous | Open | |||
| Total patients | 1457 | 2179 | 23, 24, 25, 26, 27, 29, 30, 31, 32, 33, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 54, 56, 57, 58, 59, 60, 61, 62 | 1457 | 2179 | NA |
| Age (years); mean (SD) | 58.86 (19.54) | 59.60 (19.68) | 26,27,37,46,54,56 | 400 | 207 | 0.585 |
| BMI; mean (SD) | 29.69 (5.88) | 32.10 (13.48) | 26,27,56 | 234 | 134 | 0.589 |
| Ethnicity n (%) | 26,27,54,56 | 350 | 192 | 0.242 | ||
| Hispanic | 57 (16.29) | 43 (22.40) | ||||
| Mixed | 5 (1.43) | 2 (1.04) | ||||
| White | 131 (37.43) | 56 (29.17) | ||||
| Black | 61 (17.43) | 39 (20.31) | ||||
| Other | 24 (6.86) | 24 (12.5) | ||||
| Unknown | 2 (0.57) | 2 (1.04) | ||||
| Time from intubation to tracheostomy (days); mean (SD) | 19.71 (8.03) | 22.36 (6.82) | 26,27,46,54 | 290 | 165 | 0.783 |
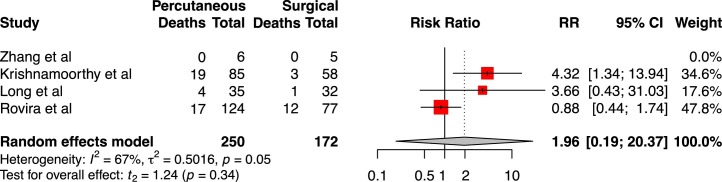
Four studies provided mortality data on surgical versus percutaneous tracheostomy, for a total of 250 percutaneous tracheostomies and 172 surgical tracheostomies.27, 46, 54, 56 Forty patients who underwent percutaneous tracheostomy died during the follow-up period, whilst 16 surgical tracheostomy patients died. Analysis of pooled mortality between groups identified no difference in cumulative mortality between percutaneous and surgical tracheostomy groups (RR 1.96 [95%CI 0.19–20.37], t = 1.24, p = 0.34; Fig. 3). Heterogeneity was moderate, as indicated by I 2 of 67% and tau2 of 0.5016. Zhang et al reported zero deaths during the follow-up period, and so was not weighted in meta-analysis.
Fig. 3.
Forest plot of relative risk of mortality between percutaneous tracheostomy and surgical tracheostomy groups.
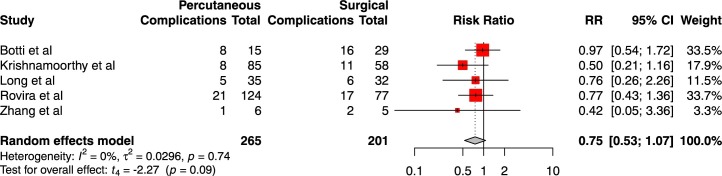
Five studies reported outcome data on overall complication rate between surgical and percutaneous patients, for a total of 265 percutaneous tracheostomy patients and 201 surgical tracheostomy patients.27, 39, 46, 54, 56 A total of 43 complications were reported in the percutaneous group compared to 52 complications in the surgical tracheostomy group. No difference in relative risk of complications was determined through meta-analysis of complications (RR 0.751 [95%CI 0.53–1.07], t = −2.27, p = 0.09; Fig. 4 ). I 2 was 0%, indicating probable homogeneity.
Fig. 4.
Forest plot of relative risk of complications between percutaneous tracheostomy and surgical tracheostomy groups.
Only 2 studies reported outcomes on both decannulation and time to decannulation between patients undergoing surgical or percutaneous tracheostomy, encompassing 82 open tracheostomy patients and 130 percutaneous tracheostomy patients.27, 46 Over the follow up period, a total of 122 percutaneous patients were decannulated and a total of 54 surgical tracheostomy patients were decannulated. The overall weighted mean time to decannulation was 27.63 days (SD 15.42 days) for surgical tracheostomy compared to 26.26 days (SD 12.02 days) for percutaneous tracheostomy. No difference was identified in both cumulative decannulation rate (RR 1.35 [95%CI 0.24–7.68], t = 2.21, p = 0.27, I 2 = 18%] and time to decannulation (SMD 0.46 [95%CI −9.91 to 10.83], p = 0.68, I 2 = 75.2%) between percutaneous and surgical tracheostomy. Insufficient data were available to justify an analysis on either sedation weaning or weaning from mechanical ventilation.
Early versus late tracheostomy
Six studies reported group-specific outcomes related to the timing of tracheostomy (early versus late), encompassing a total of 191 patients who underwent early tracheostomy and 238 patients who underwent late trachestomy.26, 30, 33, 43, 47, 55 However, whereas Aviles-Jurado et al and Kwak et al defined early tracheostomy as <10 days from intubation, the remaining 4 studies defined early as under 14 days from intubation. Given the overlap of patients between 10–14 days, outcome data were pooled only for those studies defining early tracheostomy as <14 days post-intubation. Three studies (within the definition of <14 days as early) reported specific mortality data on early vs late tracheostomy, encompassing 98 early tracheostomy patients and 105 late tracheostomy patients.26, 43, 47 Analysis of pooled mortality between groups using a random-effects model identified no difference in cumulative mortality between early and late tracheostomy (RR 1.57 [95%CI 0.21–11.80], p = 0.4343, I 2 = 67.5%).
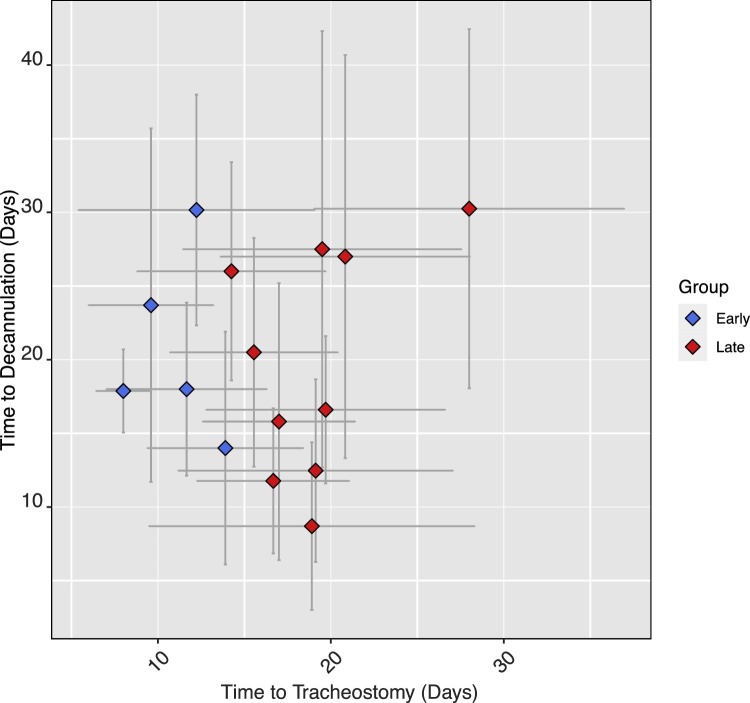
To overcome the limited sample size on early versus late studies, a linear regression model was fit to determine the influence of mean time to tracheostomy from intubation on time to decannulation. Sixteen studies reported on both time from intubation to tracheostomy and on time to decannulation, including a total of 585 decannulation events.25, 26, 27, 28, 30, 31, 32, 33, 41, 43, 46, 48, 49, 51, 55, 57 Linear regression confirmed that time to tracheostomy did not significantly predict time to decannulation (B = 0.196, SE 0.382, t = 0.514, p = 0.616, Fig. 5 ) and that model fit was poor (F(1,13) = 0.264, p = 0.616; R 2 = 0.020).
Fig. 5.
mean time to tracheostomy from intubation against time to decannulation from tracheostomy, grouped according to ‘early’ and ‘late’ tracheostomy (defined here as <14 or >14 days post-intubation).25, 30, 31, 32, 33, 41, 43, 46, 48, 49, 51, 55, 57.
Staff nosocomial infections
Rates of staff infection were poorly reported throughout the identified studies. Twenty-six of the 39 included studies did report on staff infection, though few studies provided an estimate of the number of staff involved in insertion of tracheostomies through the follow up period, such that an estimate of staff infection rate cannot be reliably determined.23, 25, 26, 29, 32, 33, 37, 39, 40, 41, 42, 45, 46, 48, 50, 51, 52, 54, 55, 57, 58, 59, 60, 34, 61 Twenty-two studies reported 0 staff infections across a total of 1055 tracheostomies.23, 25, 26, 29, 32, 33, 37, 39, 40, 42, 45, 46, 50, 51, 52, 54, 57, 58, 59, 60, 34, 61 Three studies reported 1 staff infection across a total of 222 tracheostomies,28, 48, 55 and 1 study reported 7 cases of staff infection across 121 tracheostomies.41
Discussion
The rapidly evolving nature of the COVID-19 pandemic means that providing tracheostomy-specific guidelines in this cohort of patients is inherently challenging due to a high degree of uncertainty with regards to indication, technique and timing.62 It should be emphasised that due to exceptional pressures placed on healthcare systems globally, treatment pathways have been influenced by factors other than purely patient-related outcomes. In some instances, the choice of tracheostomy technique may instead be dictated by resource allocation.22, 63
As with pre-COVID-19, the predominant indication for tracheostomy remains the facilitation of weaning with prolonged mechanical ventilation.64 Indeed, this was the case in 31 of the 34 included studies that reported on tracheostomy indications. The impact of tracheostomy timing remains uncertain, even before the COVID-19 pandemic, with some studies suggesting that early tracheostomy might improve outcomes in specific patients groups.65 The debate on tracheostomy timing in COVID-19 has focused less on patient outcomes and more on the risk benefit in relation to aerosol-generating procedures and nosocomial viral transmission to healthcare professionals. Delaying tracheostomy potentially exposes patients to the complications of protracted endotracheal intubation.64 In a virological assessment of 9 cases of COVID-19, seroconversion early in the second week post-infection was accompanied by steady decline in viral load, and it was suggested that there is little residual risk of infectivity beyond day 10.66 Given that tracheostomy timing after intubation throughout most studies extends beyond day 10 the risk of nosocomial viral transmission may in fact not be as high as originally anticipated.
Moreover, the optimal tracheostomy procedure is uncertain, and McGrath et al recommend that institutions continue to adopt an approach according to expertise and familiarity.64 Meta-analyses, encompassing 1212 patients from randomised controlled trials, on surgical versus percutaneous tracheostomy pre-COVID-19 suggest that percutaneous dilatational tracheostomy may reduce the overall incidence of wound infection and mortality, and should be considered ‘the procedure of choice for performing elective tracheostomises in critically ill adult patients’.67 There are currently insufficient data on COVID-19-specific tracheostomy outcomes to recommend one technique over the other.
A previous meta-analysis on tracheostomy outcomes in COVID-19 patients by Benito et al identified the cumulative incidence of mortality, mechanical weaning and decannulation across 18 studies.68 Although this study provided useful insight into overall mortality, weaning and decannulation in the COVID-19 patient population, estimates given were limited by the comparatively short follow up durations reported at the time of meta-analysis. Insufficient data were available to allow a comparison of outcomes between surgical versus percutaneous procedures and between early versus late tracheostomy. The current study, including 3929 tracheostomy patients across 39 studies, aimed to summarise currently the outcome data across all eligible reported COVID-19 tracheostomy patients, and to compare outcomes between tracheostomy procedures and timings.
The current study identified a cumulative mortality of 19.23% over a follow up period of approximately 42 days. This appears less favourable than the 13.1% reported by Benito et al and most likely reflects the relative completeness of the current dataset as the pandemic has progressed (Supplementary figure 4). Yet, ICU and hospital mortality of invasively-ventilated COVID-19 patients across a number of national audit programs, including the most comprehensive and up to date UK Intensive Care National Audit & Research Centre (ICNARC) reporting system, show hospital mortality rates of over 40% in ventilated patients and throughout both phases of the Pandemic.69 This is most likely a reflection of age and procedure bias towards only tracheostomising patients who are more likely to survive. The cumulative incidences of decannulation and weaning from mechanical ventilation were 44.2% and 61.2% at the time of follow-up, respectively, which are broadly in keeping with previous literature.70, 71 Overall complication rate was 14.2% across 3479 patients, which is considerably lower than that reported in the literature, perhaps reflecting careful patient selection during the current COVID-19 pandemic and improved familiarity with procedures in light of the high patient demand.72, 73, 74 Moreover, that the majority of included studies were retrospective and observational may well have contributed to under-reporting of minor complications.
Importantly, no difference was identified in mortality, decannulation and complications between percutaneous and surgical tracheostomy, and baseline characteristics between patients were similar (Table 4 ), suggesting that these results are not confounded by the baseline status of either population. Comparison of early versus late tracheostomy groups yielded similar findings, with no difference identified in cumulative mortality between the two groups, and no convincing relationship between the timing of tracheostomy and time to decannulation. Unfortunately, incomplete data on staff infection precluded any formal comparison of infection rates between early and late tracheostomy groups, and so a recommendation cannot be made on the appropriate timing of tracheostomy with regard to staff risk. It is telling, however, that of the 1398 tracheostomies from studies which reported viral transmission data, only 10 confirmed healthcare worker infections were reported. This equates to an incidence rate of 7‰, assuming all these transmissions were procedure related.
Table 4.
Recommendations for minimum reporting in future studies on tracheostomy outcomes in COVID-19 patients.
| Baseline characteristics | Outcomes |
|---|---|
| Patient age (years) | Specific peri-procedure complications |
| Sex | Severity of complication (minor vs major) |
| Comorbidities | Number of patients sedation weaned |
| Smoking status | Time from tracheostomy to sedation wean |
| Ethnicity | Number of patients weaned from mechanical ventilation. |
| Tracheostomy type (surgical versus perc) | Time from tracheostomy to weaning from mechanical ventilation. |
| Presence or absence (and duration) of steroid | Number of patients decannulated |
| Preoperative CRP (mg/L) | Time from tracheostomy to decannulation |
| Preoperative PEEP (mmHg) | Number of patients died |
| Preoperative PaO2/FiO2 ratio | Time from tracheostomy to death |
| APACHE II (or equivalent) | Time from tracheostomy to ICU discharge |
| Time between intubation and tracheostomy (days) | Mean follow up period |
| Time from symptoms onset to tracheostomy | Staff infection rate (including estimated total staff involved in tracheostomy insertion) |
An appreciation of true outcomes across studies is complicated by both incomplete baseline characteristic and outcome data. To facilitate reliable reporting with a view to optimising the reliability of meta-analyses, we have suggested recommendations for minimal reporting. Each baseline characteristic and outcome should be reported for both the total studied population and any subgroups (Table 4).
Collaborative projects are currently underway that will contribute to our understanding of tracheostomy outcomes. The COVIDTrach national tracheostomy evaluation database was established in the UK by the Federation of Surgical Speciality Associations (FSSA), in conjunction with the British Association of Oral and Maxillofacial Surgeons (BAOMS) and ENT UK, to evaluate the effectiveness of tracheostomies in COVID-19 patients. At the time of analysis, only the interim report had been published, providing outcome data on 563 patients.16 Its exclusion from the current study was justified given its comparative sparsity of outcome data compared to those studies that contributed data. The full report is now available, encompassing 1605 tracheostomies from 126 UK hospitals.75 The results of this study corroborate those of the current meta-analysis. Overall mortality was determined at 18% (285), and median time from intubation to tracheostomy was 15 days (IQR11-21). Moreover, this multicentre study also found no difference in outcomes between percutaneous versus surgical tracheostomy. Interestingly, in contrast to our meta-analysis where no difference in mortality was identified between early and late tracheostomy (defined here as <14 days post-intubation), the COVIDTrach collaborative found that tracheostomy under 7 days post-intubation did portend a poorer prognosis. The reasons for this are unclear. The authors have quite sensibly concluded that this may not be a causal relationship and that the question of timing is best addressed through prospective randomised controlled trials.
There are several limitations of the current study that are related to the nature of the COVID-19 pandemic. Although the weighted mean follow-up period in this study was 42 days, included studies, by necessity, contain incomplete data. The priority early in the course of the pandemic was rapid collection and dissemination of data, to potentially inform treatment decisions on COVID-19 patients. Thus, the pooled outcomes reported here will likely be underestimated. Moreover, overall weighted means reported in this study are estimates, borne from differences in reporting between individual studies. As highlighted by Benito et al this likely gives rise to selection bias.68 Notwithstanding, the current study provides the largest meta-analysis on tracheostomy outcomes in COVID-19 patients to date, and includes an overview of key tracheostomy outcomes, as well as comparisons between clinically relevant patient cohorts.
Conclusions
Our findings suggest that there is no difference in mortality, complications, and time to decannulation between surgical and percutaneous tracheostomy in COVID-19 patients. Moreover, no difference in mortality was identified between early and late tracheostomy (where early was defined as <14 days post-intubation), and time to tracheostomy did not influence time to decannulation. We share the recommendation that tracheostomy timing should be decided on a case-by-case basis under the guidance of a multidisciplinary team. Currently, there is insufficient evidence to suggest that early tracheostomy increases risk of viral transmission to staff, particularly with existing robust guidelines on personal protective equipment, and insufficient evidence to suggest that delayed tracheostomy influences outcomes in COVID-19 patients. Furthermore, the type of tracheostomy should be performed according to preferences, equipment familiarity, availability, and expertise.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Ethics statement/confirmation of patients’ permission
Ethics approval was not required. Written consent was not required.
Financial disclosure statement
The authors report no commercial or financial associations that might pose or create conflict with information presented in the manuscript.
Declaration of competing interest
The authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.bjoms.2021.05.011.
Appendix A. Supplementary data
The following are supplementary data to this article:
References
- 1.Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations – The Lancet Respiratory Medicine. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30161-2/fulltext. [DOI] [PMC free article] [PubMed]
- 2.Huang C., Wang W., Xingwang L., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Pierson D.J. Tracheostomy and weaning. Respir Care. 2005;50:526–533. [PubMed] [Google Scholar]
- 5.Hosokawa K., Nishimura M., Egi M., Vincent J.-L. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19 doi: 10.1186/s13054-015-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Chiesa-Estomba C.M., Lechien J., Calvo-Henriquez C., et al. Systematic review of international guidelines for tracheostomy in COVID-19 patients. Oral Oncol. 2020;108:104844. doi: 10.1016/j.oraloncology.2020.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szakmany T., Russell P., Wilkes A.R., Hall J.E. Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: meta-analysis of randomized controlled trials. Br J Anaesth. 2015;114:396–405. doi: 10.1093/bja/aeu440. [DOI] [PubMed] [Google Scholar]
- 9.Siempos I.I., Ntaidou T.K., Filippidis F.T., Choi A.M.K. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:150–158. doi: 10.1016/S2213-2600(15)00007-7. [DOI] [PubMed] [Google Scholar]
- 10.Young D., Harrison D.A., Cuthbertson B.H., Rowan K., TracMan Collaborators Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309:2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths J., Barber V.S., Morgan L., Young J.D. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330:1243. doi: 10.1136/bmj.38467.485671.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochrane Handbook for Systematic Reviews of Interventions. /handbook/current.
- 13.Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein M., Higgins J.P.T., Hedges L.V., Rothstein H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 16.COVIDTrach The outcomes of mechanically ventilated COVID-19 patients undergoing tracheostomy in the UK: interim report. Br J Surg. 2020;107:e583–e584. doi: 10.1002/bjs.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botti C., Lusetti F., Neri T., et al. Comparison of percutaneous dilatational tracheotomy versus open surgical technique in severe COVID-19: complication rates, relative risks and benefits. Auris Nasus Larynx. 2020 doi: 10.1016/j.anl.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takhar A., Tornari C., Wyncoll D., et al. Safety and outcomes of percutaneous tracheostomy in coronavirus disease 2019 pneumonitis patients requiring prolonged mechanical ventilation. J Laryngol Otol. 2020:1–10. doi: 10.1017/S0022215120002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takhar A., Surda P., Ahmed I., et al. Timing of tracheostomy for prolonged respiratory wean in critically ill coronavirus disease 2019 patients: a machine learning approach. Crit Care Explor. 2020;2:e0279. doi: 10.1097/CCE.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tornari C., Surda P., Takhar A., et al. Tracheostomy, ventilatory wean, and decannulation in COVID-19 patients. Eur Arch Otorhinolaryngol. 2020:1–10. doi: 10.1007/s00405-020-06187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith T.G., Ahmad I., Takhar A., Surda P., El-Boghdadly K. Unconventional multidisciplinary team strategy for tracheostomy in COVID-19. Anaesth Rep. 2020;8:178–182. doi: 10.1002/anr3.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung E., Hopkins P., Auzinger G., Fan K. Challenges of tracheostomy in COVID-19 patients in a tertiary centre in inner city London. Int J Oral Maxillofac Surg. 2020;49:1385–1391. doi: 10.1016/j.ijom.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson A., Roberts M.T., Phillips J., Saha R. Early percutaneous tracheostomy for patients with COVID-19. Anaesthesia. 2021;76:138–139. doi: 10.1111/anae.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stubington T.J., Mallick A., Garas G., et al. Tracheotomy in COVID-19 patients: optimizing patient selection and identifying prognostic indicators. Head Neck. 2020;42:1386–1391. doi: 10.1002/hed.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtney A., Lignos L., Ward P.A., Vizcaychipi M.P. Surgical tracheostomy outcomes in COVID-19-positive patients. OTO Open. 2021;5 doi: 10.1177/2473974X20984998. 2473974X20984998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Queen Elizabeth Hospital Birmingham COVID-19 airway team Safety and 30-day outcomes of tracheostomy for COVID-19: a prospective observational cohort study. Br J Anaesth. 2020;125:872–879. doi: 10.1016/j.bja.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovira A., Tricklebank S., Surda P., et al. Open versus percutaneous tracheostomy in COVID-19: a multicentre comparison and recommendation for future resource utilisation. Eur Arch Otorhinolaryngol. 2021 doi: 10.1007/s00405-020-06597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S., Hind M., Jordan S., et al. Weaning by surgical tracheostomy and portable ventilators released ICU ventilators during coronavirus disease 2019 surge in London. Crit Care Explor. 2020;2:e0193. doi: 10.1097/CCE.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broderick D., Kyzas P., Baldwin A., et al. Surgical tracheostomies in COVID-19 patients: a multidisciplinary approach and lessons learned. Oral Oncol. 2020;106:104767. doi: 10.1016/j.oraloncology.2020.104767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glibbery N., Karamali K., Walker C., et al. Tracheostomy in the coronavirus disease 2019 patient: evaluating feasibility, challenges and early outcomes of the 14-day guidance. J Laryngol Otol. 2020;134:688–695. doi: 10.1017/S0022215120001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valchanov K., Salaunkey K., Parmar J. Percutaneous dilatational tracheostomy in coronavirus disease 2019 extracorporeal membrane oxygenation patients: a case series. J Cardiothorac Vasc Anesth. 2021;35:348–350. doi: 10.1053/j.jvca.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sancho J., Ferrer S., Lahosa C., et al. Tracheostomy in patients with COVID-19: predictors and clinical features. Eur Arch Otorhinolaryngol. 2021 doi: 10.1007/s00405-020-06555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avilés-Jurado F.X., Prieto-Alhambra D., Gonzalez-Sanchez N., et al. Timing, complications, and safety of tracheotomy in critically ill patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Téllez E., Dotu C., Trujillo-Reyes J., et al. Tracheotomy in patients COVID-19: a necessary high risk procedure. Two center experience. Arch Bronconeumol. 2020;56:673–674. doi: 10.1016/j.arbres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Villares C., Perez Molina-Ramirez C., Bartolome-Benito M., et al. Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain. Eur Arch Otorhinolaryngol. 2020;1–8 doi: 10.1007/s00405-020-06220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mata-Castro N., Sanz-Lopez L., Pinacho-Martinez P., et al. Tracheostomy in patients with SARS-CoV-2 reduces time on mechanical ventilation but not intensive care unit stay. Am J Otolaryngol. 2021;42:102867. doi: 10.1016/j.amjoto.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riestra-Ayora J., Yanes-Diaz J., Penuelas O., et al. Safety and prognosis in percutaneous vs surgical tracheostomy in 27 patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:462–464. doi: 10.1177/0194599820931801. [DOI] [PubMed] [Google Scholar]
- 38.Zuazua-Gonzalez A., Collazo-Lorduy T., Coello-Casariago G., et al. Surgical tracheostomies in COVID-19 patients: indications, technique, and results in a second-level Spanish hospital. OTO Open. 2020;4 doi: 10.1177/2473974X20957636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Botti C., Lusetti F., Peroni S., et al. The role of tracheotomy and timing of weaning and decannulation in patients affected by severe COVID-19. Ear Nose Throat J. 2020 doi: 10.1177/0145561320965196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picetti E., Fornaciari A., Taccone F., et al. Safety of bedside surgical tracheostomy during COVID-19 pandemic: a retrospective observational study. PLOS ONE. 2020;15:e0240014. doi: 10.1371/journal.pone.0240014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosano A., Martinelli E., Fusina F., et al. Early percutaneous tracheostomy in coronavirus disease 2019: association with hospital mortality and factors associated with removal of tracheostomy tube at ICU discharge. A cohort study on 121 patients. Crit Care Med. 2021;49:261–270. doi: 10.1097/CCM.0000000000004752. [DOI] [PubMed] [Google Scholar]
- 42.Turri-Zanoni M., Battaglia P., Czaczkes C., et al. Elective tracheostomy during mechanical ventilation in patients affected by COVID-19: preliminary case series from Lombardy, Italy. Otolaryngol Head Neck Surg. 2020;163:135–137. doi: 10.1177/0194599820928963. [DOI] [PubMed] [Google Scholar]
- 43.Volo T., Stritoni P., Battel I., et al. Elective tracheostomy during COVID-19 outbreak: to whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol. 2020;1–9 doi: 10.1007/s00405-020-06190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piccin O., Albertini R., Caliceti U., et al. Early experience in tracheostomy and tracheostomy tube management in Covid-19 patients. Am J Otolaryngol. 2020;41:102535. doi: 10.1016/j.amjoto.2020.102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aodeng S., Wang W., Chen Y., et al. Safety and efficacy of tracheotomy for critically ill patients with coronavirus disease 2019 (COVID-19) in Wuhan: a case series of 14 patients. Eur J Cardiothorac Surg. 2020;58:745–751. doi: 10.1093/ejcts/ezaa312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X., Huang Q., Niu X., et al. Safe and effective management of tracheostomy in COVID-19 patients. Head Neck. 2020;42:1374–1381. doi: 10.1002/hed.26261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y., Wu Y., Zhu F., et al. Tracheostomy in 80 COVID-19 patients: a multicenter, retrospective, observational study. Front Med. 2020;7 doi: 10.3389/fmed.2020.615845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahu A., Swain S.K., Das S.R. Performing bedside surgical tracheostomy on COVID-19 patients at intensive care unit-our experiences at a tertiary care Indian teaching hospital. Eur J Mol Clin Med. 2020;7:1208–1217. [Google Scholar]
- 49.Ahmed Y., Cao A., Thal A., et al. Tracheotomy outcomes in 64 ventilated COVID-19 patients at a high-volume center in Bronx, NY. Laryngoscope. 2021 doi: 10.1002/lary.29391. [DOI] [PubMed] [Google Scholar]
- 50.Angel L., Kon Z., Change S., et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg. 2020;110:1006–1011. doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chao T.N., Harbinson S., Braslow B., et al. Outcomes after tracheostomy in COVID-19 patients. Ann Surg. 2020;272:e181–e186. doi: 10.1097/SLA.0000000000004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Floyd E., Harris S., Lim J., et al. Early data from case series of tracheostomy in patients with SARS-CoV-2. Otolaryngol Head Neck Surg. 2020;163:1150–1152. doi: 10.1177/0194599820940655. [DOI] [PubMed] [Google Scholar]
- 53.Kapp C., Esther O., Thiboutot J., et al. Tracheostomy and COVID-19 ARDS: one academic center’s experience. Chest. 2020;158:A1954–A1956. [Google Scholar]
- 54.Krishnamoorthy S., Polanco A., Coleman N., et al. The safety and efficacy of tracheostomy in patients diagnosed with COVID-19: an analysis of 143 patients at a major NYC medical center. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004612. [DOI] [PubMed] [Google Scholar]
- 55.Kwak P.E., Connors J., Benedict P., et al. Early outcomes from early tracheostomy for patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long S.M., Chern A., Feit N., et al. Percutaneous and open tracheostomy in patients with COVID-19: comparison and outcomes of an institutional series in New York City. Ann Surg. 2021;273:403–409. doi: 10.1097/SLA.0000000000004428. [DOI] [PubMed] [Google Scholar]
- 57.Weiss K.D., Coppolino A., Wiener D., et al. Controlled apneic tracheostomy in patients with coronavirus disease 2019 (COVID-19) JTCVS Tech. 2020 doi: 10.1016/j.xjtc.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bassily-Marcus A., Leibner E.S., Kohli-Seth R. Tracheostomy for coronavirus disease 2019 patients: maintaining the standard of care. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun B.J., Wolff C., Bechtold H., et al. Modified percutaneous tracheostomy in patients with COVID-19. Trauma Surg Acute Care Open. 2020;5 doi: 10.1136/tsaco-2020-000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy P., Holler E., Lindroth H., et al. Short-term outcomes for patients and providers after elective tracheostomy in COVID-19-positive patients. J Surg Res. 2020;260:38–45. doi: 10.1016/j.jss.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Illuzzi E., Bassily-Marcus A., Kohli-Seth R., Leibner E., Mohammed A. Tracheostomy timing and outcomes in patients with COVID-19 pneumonia. Chest. 2020;158:A598. [Google Scholar]
- 62.Auzinger G. Early percutaneous tracheostomy during the pandemic ‘As Good as It Gets’. Crit Care Med. 2021;49:361–364. doi: 10.1097/CCM.0000000000004759. [DOI] [PubMed] [Google Scholar]
- 63.Mattioli F., Fermi M., Ghirelli M., et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2133–2135. doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGrath B.A., Brenner M., Warrillow S., et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elkbuli A., Narvel R., Spano P., et al. Early versus late tracheostomy: is there an outcome difference? Am Surg. 2019;85:370–375. [PubMed] [Google Scholar]
- 66.Wölfel R., Corman V., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 67.Delaney A., Bagshaw S.M., Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care. 2006;10:R55. doi: 10.1186/cc4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benito D.A., Bestourous D.E., Tong J.Y., Pasick L.J., Sataloff R.T. Tracheotomy in COVID-19 patients: a systematic review and meta-analysis of weaning, decannulation, and survival. Otolaryngol Head Neck Surg. 2021 doi: 10.1177/0194599820984780. 0194599820984780. [DOI] [PubMed] [Google Scholar]
- 69.ICNARC – Reports. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports.
- 70.Guia M., Ciobanu L., Sreedharan J., et al. The role of non-invasive ventilation in weaning and decannulating critically ill patients with tracheostomy: a narrative review of the literature. Pulmonology. 2021;27:43–51. doi: 10.1016/j.pulmoe.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Farrell M.S., Gillin T., Emberger J., et al. Improving tracheostomy decannulation rate in trauma patients. Crit Care Explor. 2019;1 doi: 10.1097/CCE.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahla A.S., Mallat J., Zoumot Z., et al. Complications of surgical and percutaneous tracheostomies, and factors leading to decannulation success in a unique Middle Eastern population. PLOS ONE. 2020;15:e0236093. doi: 10.1371/journal.pone.0236093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson-Obaseki S., Veljkovic A., Javidnia H. Complication rates of open surgical versus percutaneous tracheostomy in critically ill patients. Laryngoscope. 2016;126:2459–2467. doi: 10.1002/lary.26019. [DOI] [PubMed] [Google Scholar]
- 74.Glossop A., Meekings T.C., Hutchinson S.P., Webber S.J. Complications following tracheostomy insertion in critically ill patients – experience from a large teaching hospital. Journal of the Intensive Care Society. 2011;12:301–306. [Google Scholar]
- 75.COVIDTrach collaborative COVIDTrach; a prospective cohort study of mechanically ventilated COVID-19 patients undergoing tracheostomy in the UK. medRxiv. 2020 doi: 10.1101/2020.10.20.20216085. 2020.10.20.20216085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.