Abstract
Objective
The purpose of this study is to evaluate chest CT imaging features, clinical characteristics, laboratory values of COVID-19 patients who underwent CTA for suspected pulmonary embolism. We also examined whether clinical, laboratory or radiological characteristics could be associated with a higher rate of PE.
Materials and methods
This retrospective study included 84 consecutive patients with laboratory-confirmed SARS-CoV-2 who underwent CTA for suspected PE. The presence and localization of PE as well as the type and extent of pulmonary opacities on chest CT exams were examined and correlated with the information on comorbidities and laboratory values for all patients.
Results
Of the 84 patients, pulmonary embolism was discovered in 24 patients. We observed that 87% of PE was found to be in lung parenchyma affected by COVID-19 pneumonia. Compared with no-PE patients, PE patients showed an overall greater lung involvement by consolidation (p = 0.02) and GGO (p < 0.01) and a higher level of D-Dimer (p < 0,01). Moreover, the PE group showed a lower level of saturation (p = 0,01) and required more hospitalization (p < 0,01).
Conclusion
Our study showed a high incidence of PE in COVID-19 pneumonia. In 87% of patients, PE was found in lung parenchyma affected by COVID-19 pneumonia with a worse CT severity score and a greater number of lung lobar involvement compared with non-PE patients. CT severity, lower level of saturation, and a rise in D-dimer levels could be an indication for a CTPA.
Advances in knowledge
Certain findings of non-contrast chest CT could be an indication for a CTPA.
Abbreviations: SARS-COV-2, severe acute respiratory syndrome coronavirus 2; CT, computed tomography; CTPA, computed tomography pulmonary angiography; ARDS, acute respiratory distress syndrome; DVT, deep venous thrombosis; COVID-19, Coronavirus disease 2019; PE, pulmonary embolism; GGO, ground glass opacities; ICU, intensive care unit; RT-PCR, reverse-transcription–polymerase-chain-reaction
Keywords: Covid-19, CT, Pulmonary embolism, D-dimer, CTPA
1. Introduction
In December 2019, different pneumonia cases of unknown etiology and with severe acute respiratory syndrome (SARS), occurred in Wuhan, Hubei Province, China.1 , 2 In the following weeks, the virus spread quickly all over the world with dramatic consequences for global health. Several reports describe clinical signs associated with COVID-19, with disease expression ranging from mild infection to severe acute respiratory distress.2 , 3 In most patients, symptoms are mild.4 However, certain patients, most notably elderly and those with comorbidities, can develop acute respiratory distress syndrome (ARDS) and other complications.5
Several studies suggest that COVID-19 may predispose patients to thrombotic diseases, such as deep venous thrombosis (DVT) and pulmonary embolism (PE).6 , 7 Despite the quick rate at which new information is being published, many questions remain regarding the pathogenesis.
Infection and sepsis lead to a hypercoagulable state because of excessive inflammation, platelet activation, endothelial dysfunction, and stasis.6 Moreover, an altered coagulation parameter correlates with mortality in patients with COVID-19 pneumonia.8 , 9 In order to improve the patient outcome, an early diagnosis is necessary.
Current guidelines support the use of non-contrast chest CT as an important tool to assess the severity and monitoring COVID-19 infection and in places with limited access to RT-PCR for the initial diagnosis, with potential limits in the early stage of the disease.10., 11., 12. Computed tomography pulmonary angiography (CTPA) in patients with COVID-19 pneumonia is performed when there is a suspicion of PE or an unexplained worsening of clinical conditions with hemodynamic or cardio-respiratory decompensation.13 , 14 The objective of our study was to evaluate baseline characteristics, laboratory values, chest CT imaging features of COVID-19 patients on CTPA.
2. Material and methods
2.1. Study population
In this retrospective study approved by Institutional Review Board, we have included 84 consecutive patients (57 males; 27 females; 60,4 ± 16,4 mean age years) with laboratory-confirmed PCR of COVID-19 studied from March 1st to May 25th, 2020 in three hospitals Massachusetts General Hospital (Site A): 50 patients, Ospedale San Giovanni Bosco, Torino, Italy (Site B): 28 patients, Ospedale di Sassari, Italy (Site C): 6 patients for suspected PE. All studies were clinically indicated and performed according to the established standard of care CT protocols. No patients were excluded. CTPA in patients with COVID-19 pneumonia was performed when there is a suspicion of PE with a physical exam and laboratory data revealed suggestive features.
Patient characteristics including age, gender, clinical symptoms, time-course of the symptoms, medical history, and outcomes were recorded. Patients were divided into two groups: a PE group for patients with an acute pulmonary embolism and a non-PE group for patients without acute pulmonary embolism.
2.2. CT technique
All chest CT scans were performed during a single full inspiratory breath-hold in a supine position on a slice multidetector-row CT scanner.
Patients from Site A underwent dual-energy CTPA on either a 192-slice third-generation, dual-source CT (Siemens Definition Force, Siemens Healthineers) (n = 27) or 64-slice, single-source, multidetector-row CT (GE Discovery 750HD, GE Healthcare) (n = 23 patients). The scan and reconstruction parameters for dual-source DECT-PA were 80/150 kV with tin filter, automatic exposure control (CareDose 4D, Siemens Healthineers) at quality reference mAs of 180, 0.55:1 pitch, 0.28-second rotation time, 192 ∗ 0.6 mm detector configuration with double z-sampling, 1 mm section thickness at 0.5 mm section interval, soft tissue reconstruction kernel (Br40s) with Admire strength of 2 (advanced model-based iterative reconstruction). The single-source dual energy CTPAs were performed with 80/140 kV fixed tube current of 280–360 mA, 0.5-second rotation time, 1.375:1 pitch, 64 ∗ 0.625 mm detector configuration, 1.25 mm section thickness at 0.625 mm section interval, soft tissue reconstruction kernel (Standard kernel) with ASIRv strength of 40% (advanced statistical iterative reconstruction-v). All CTPA exams were performed with suspension of breathing in inspiration with the patient in supine position.
Patients from Site B were all scanned on a 64-slice multidetector-row CT (GE Discovery 750 HD, GE Healthcare). Single energy scans (24 patients) were acquired with 120 kV, 150–600 mA with automatic exposure control (Smart mA, Noise Index 18–22), pitch 1.375:1, 0.4-second gantry rotation time, 64 ∗ 0.625 mm detector configuration. Dual energy scans (2 patients) were performed at 80/140 kV with rapid kV switching, tube current 150–600 mA, pitch 1.531:1, 0.5 second gantry rotation, 64 ∗ 0.625 detector configuration. Thin-section images were reconstructed at 1.25 mm thickness using a soft tissue kernel and 0.625 mm-thick images were displayed using a sharp kernel for lung parenchyma. All images were reconstructed with ASIR technique.
At Site C, all patients were all scanned on a 128-slice multidetector-row CT (Philips Brilliance iCT, Philips Healthcare). Exams were acquired with 120 kV, 150–600 mA with automatic exposure control (Smart mA, Dose Right, Z-DOM), pitch 1.11, 0.75-second gantry rotation time, 64 ∗ 0.625 mm detector configuration. Thin-section images were reconstructed at 1 mm thickness using a soft tissue kernel and 0.75 mm-thick images were displayed using a sharp kernel for lung parenchyma.
All CTPA exams were performed using either 65–80 ml of Iopamidol 370 mg (Site A) or 60 ml of Iomeprol 400 (Sites B and C) injected at 4–5 cm3/s followed by 40 cm3 of saline injection. Tracking region of interest was drawn in the main pulmonary artery with 100 HU as the trigger threshold.
2.3. Imaging feature analysis
Two radiologists (AC with 14 years and LS with 9 years of experience in chest radiology) assessed chest CT images. Imaging was reviewed independently, and a final decision reached by consensus is reported. No negative control cases were examined. All 84 CT examinations were evaluated for the presence or absence of intraluminal filling defects in the central, proximal, or distal (segmental or subsegmental) pulmonary arteries, pulmonary vascular dilatation, and pulmonary infarcts (wedge-shaped opacities with broad pleural abutment). The degree of arterial obstruction was assessed by using Qanadli obstruction index, including 10 lung segments for each lung.53
In addition, we assessed the presence of ground-glass opacity (GGO), consolidation, mixed GGO and consolidation, crazy paving (opacities with ground-glass opacities and interlobular septal thickening), reverse halo or atoll sign (central ground-glass with surrounding consolidation), bilateral lung involvement, emphysema and lymph node enlargement (lymphadenopathy). Radiological lesions were categorized based on the Fleischner Society's glossary of terms.16
The severity of CT was estimated by visual assessment based on the areas of pulmonary opacities.15 The distribution of lung changes was also classified based on the number of lobes involved from 0 (no lobes) to 5 (all lobes). Mediastinal and hilar lymph nodes were considered enlarged when their short-axis dimension was greater than 1.0 cm.
2.4. Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (with interquartile range) values. Categorical variables were described as frequency rates and percentages, comparisons of continuous data were performed using the independent samples test or Mann-Whitney U test. Kolmogorov-Smirnov tests were used to check continuous variables for normal distribution. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. A receiver-operating characteristic (ROC) analysis was performed to calculate optimal thresholds and areas under the curves (AUCs). The Youden index was used to depict the optimal cut-off values from the ROC curves. Sensitivities and specificities were calculated for these cut-off values with 95% confidence intervals. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics version 24 (SPSS Inc., Chicago, IL, Us; 27 females).
3. Results
The most common symptoms were dyspnoea (n = 67/84; 78%), followed by cough (n = 64/84; 75%). Among the comorbidities, hypertension was the most common (n = 45/84; 52%), followed by diabetes (n = 25/84; 29%) and coronary heart disease (n = 14/84; 16%). Sixty-seven patients had fever on admission. There was no significant difference between the two groups (p > 0.05).
The D-Dimer level was statistically significant in the PE group compared to the no-PE group (3725 ng/mL vs 1754 ng/mL, p < 0,01). ROC analysis on D-dimer identified that a value greater than 1929 ng/mL predicted PE with an area under the curve (AUC) = of 0,728 with 67% sensitivity and 70% specificity (95% CI 0,620–0,840) (Fig. 1 ).
Fig. 1.
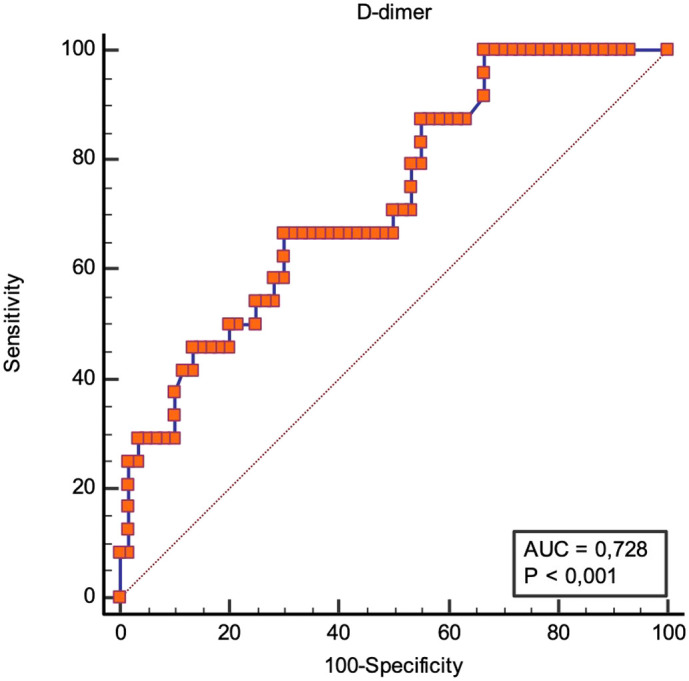
ROC curves for D-dimer level to identify patients with pulmonary embolism.
Baseline characteristics and laboratory findings are reported in Table 1 .
Table 1.
Clinical and laboratory characteristic of COVID-19 patients who had diagnostic CTPA to assess the presence of pulmonary embolism, and comparison between PE group and no-PE group.
| PE | Non-PE | Overall patients | p | |
|---|---|---|---|---|
| Age | 63,6 ± 16,6 | 59,1 ± 16 | 60,4 ± 16 | 0,35 |
| Male | 20/24, 83% | 37/60, 61% | 57/84. 68% | 0,7 |
| Hypertension | 10/24, 42% | 35/60, 58% | 45/84, 53% | 0,9 |
| Diabetes mellitus | 4/24, 16% | 21/60, 35% | 25/84, 30% | 0,1 |
| CAD | 4/24, 16% | 10/60, 17% | 14/84, 16% | 0,4 |
| Cancer | 4/24, 16% | 4/60, 6% | 8/84, 9% | 0,5 |
| Smoke | 3/24, 12% | 6/60, 10% | 9/84, 10% | 0,6 |
| D-dimer | 3725 ng/ml | 1754 ng/ml | 2324 ng/ml | <0,01* |
| Temperatures | 37,66 (36,66–38,66) | 37,56 (36,52–38,60) | 37,6 (36,5–38,5) | 0,4 |
| Cough | 16/24, 66% | 48/60, 80% | 64/84, 76% | 0,3 |
| Sore throat | 2/24, 8% | 2/60, 3% | 4/84, 5% | 0,2* |
| Dyspnoea | 16/24, 66% | 51/60, 85% | 67/84, 80 | 0,1 |
| Sp02 | 86,9% | 90% | 89% | 0,01* |
| ICU | 9/24, 37% | 26/60, 43% | 35/84, 42% | 0,5 |
| CPAP | 1/24, 4% | 28/60, 46% | 29/84, 35% | 0,6 |
| Intubation | 7/24, 29% | 22/60, 36% | 29/84, 35% | 0,4 |
| Death | 1/24, 4% | 2/60, 3% | 3/84, 4% | 0,5 |
ICU: intensive care unit; CAD: coronary artery disease; PE: pulmonary embolism.
Mean ± DS. Data are n (%), or median (interquartile range).
3.1. Imaging findings
More than a quarter of patients (28%, 24/84) had pulmonary embolism on CTPA. Among these, the location of the PE is as follows: 3 (3/84, 0,3%) central emboli, 12 (12/84, 14%) segmental embolus and 20 (20/84, 24%) subsegmental embolus. Eight (8/84, 10%) patients had a single embolus, while 16 (16/84, 19%) had multiple emboli. Moreover, there was a predilection for the right lower lobe (16/24, 66%). The mean obstruction index was 19 ± 15%.
Most PE (19/24, 80%) were present in regions with pulmonary opacities related to COVID-19 pneumonia. The most common chest CT features of patients with and without PE were GGO (79/84, 93%), consolidation (71/84, 83%), and mixed GGO and consolidation (67/84, 79%). The less frequent CT findings were crazy paving pattern (22/84, 26%), reversed halo sign (2/84, 0,2%). In addition, 40% of patients had hilar and/or mediastinal lymphadenopathy. Both PE patients and non-PE patients showed similar proportions of chest CT anomalies, such as GGO (100% vs 92%, p = n.s.), consolidation (79% vs 83%, p = n.s.), mixed lesions (79% vs 78%, p = n.s.) and crazy paving (37% vs 18%, p = n.s.). Moreover, we did not find any significant difference in chest CT pneumonia findings in the overall cohort of the two countries under analysis. On the other hands, the Italian cohort showed a higher incidence of PE compared to the American cohort (44% vs 18%, p = 0,01).
Imaging findings are reported in Table 2 .
Table 2.
Distribution of pulmonary embolism.
| Central | 3/24, 12% |
| Segmental | 12/24, 50% |
| Subsegmental | 20/24, 83% |
| One | 8/24, 33% |
| Multiple | 16/24, 66% |
| Coexistence lung opacities | 21/24, 87% |
| Left upper lobe | 2/24, 8% |
| Left lower lobe | 5/24, 20% |
| Right upper lobe | 7/24, 29% |
| Right middle lobe | 7/24, 29% |
| Right lower lobe involvement | 16/24, 66% |
| Mean obstruction index | 19 ± 15% |
GGO: ground-glass opacities; PE: pulmonary embolism.
Data are n (%).
We compared PE positive and PE negative groups and noted that there was a statistical difference regarding CT severity with a greater lung involvement by consolidation (p = 0.02) and GGO (p < 0.01), with the type of pulmonary involvement. Moreover, the PE group showed a lower level of peripheral oxygen saturation (86,8% vs 88,6% p = 0,016) and required longer time of hospitalization (p < 0,01) compared with the no-PE group.
Comparison between the PE group and no-PE group are summarized in Table 3 .
Table 3.
Chest CT imaging findings of COVID-19 patients who had diagnostic CTPA to assess the presence of pulmonary embolism, and comparison between PE group and non-PE group.
| PE | No-PE | Overall patients | p | |
|---|---|---|---|---|
| GGO | 24/24, 100% | 55/60, 92% | 78/84, 93% | 0,5 |
| Consolidation | 19/24, 79% | 50/60, 83% | 69/84, 82% | 0,15 |
| GGO (lobes involved) | 3,83 ± 1,30 | 2,75 ± 1,65 | 3,07 ± 1,62 | 0,019* |
| Consolidation (lobes involved) | 2,67 ± 1,82 | 1,93 ± 1,53 | 2,14 ± 1,65 | 0,01* |
| Mixed GGO and consolidation | 19/24, 79% | 47/60, 78% | 66/84, 78% | 0,2 |
| Nodes involvement | 6/24, 25% | 28/60, 46% | 34/84, 40% | 0,69 |
| Crazy paving | 9/24, 37% | 11/60, 18% | 20/84, 24% | 0,19 |
| Reversed halo sign | 1/24, 0,4% | 1/60, 0,2% | 2/84, 0,2% | 0,5 |
GGO: ground-glass opacities; PE: pulmonary embolism.
Mean ± DS. Data are n (%).
4. Discussion
Several reports described the association between COVID-19 pneumonia and PE.6 , 7 , [16], [17], [18] In our study of COVID-19, of the patients who had CTPA, 28% of exams were positive for pulmonary embolism. Our rate is similar to a recently published study that reported an incidence of PE in COVID-19 patients between 22 and 30%.18 These results confirm the association between COVID-19 and PE.14 The proportion of PE is higher in the Italian cohort than what is observed in the American cohort. This could be explained, as suggested by Grasselli et al, by a demographic difference between the populations in two countries.46
Other viral pneumonia has also been related to procoagulant change. However, several researches did not show a higher prevalence of vascular complications. van Wissen et al assessed the frequency of influenza A and B in a cohort of patients with a suspicion of PE. They showed that influenza A is rare among patients with PE.47
Similar results were obtained by Bunce et al that evaluated the prevalence of vascular complications among patients with pH1N1 influenza. They reported that infection with the pH1N1 did not appear to be correlated with a higher risk of vascular complications.48
In this respect, it is important to recognize whether clinical, laboratory or radiological characteristics could be associated with a higher rate of PE in COVID-19 patients.
Current guidelines suggested non-contrast chest CT assesses COVID-19 pneumonia and extension11 , 12 and outcome prediction.41 , 54 Our findings suggested a higher prevalence of subsegmental thrombosis with a particular tendency for PE lesion in the right lower lobe, according to recently published studies.33 , 42
Although the CT pattern of COVID-19 pneumonia does not allow a selection of patients at risk of pulmonary embolism, patients who developed PE showed a worse CT severity score and a greater number of lung lobar involvement by consolidation and GGO compared with non-PE patients, and the majority of embolus was found in lung parenchyma affected by COVID-19 pneumonia (see Fig. 2 ). This is consistent with recent literature in relationship with prothrombotic change in COVID-19 patients.[49], [50], [51], [52]
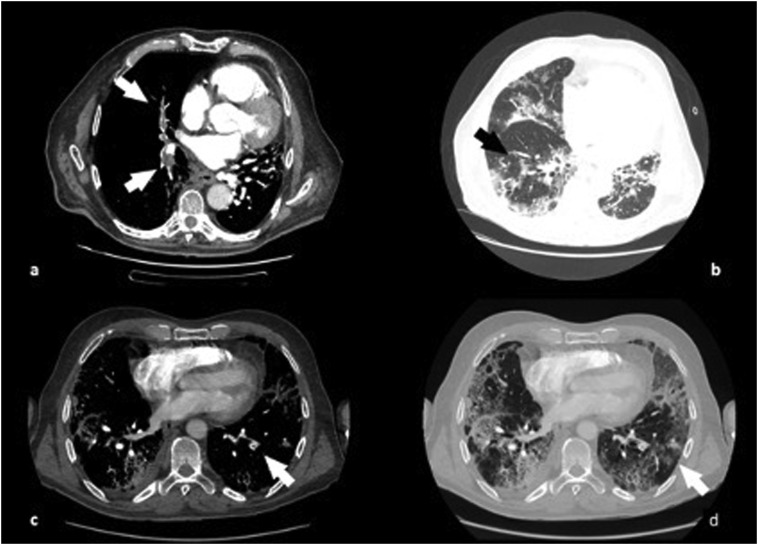
Fig. 2.
68 years old patients, chest CT images showed pulmonary embolism in the lobar arteries for the right upper and inferior lobes (a) and in the segmental arteries for the left lower lobe (c). Lung parenchyma is characterized by ground glass opacities and consolidation (b–d).
The pathogenesis of COVID-19 associated with PE is unclear. But it has become clear that some COVID-19 patients showed pulmonary vascular involvement.43 Therefore, some imaging studies reported a vascular involvement in areas of lung opacities,[31], 32., [33] which could indicate an inflammatory response with vascular involvement leading to thrombosis.31 Bösmüller and al. described vascular changes in different stages of COVID-19 pneumonia, such as endotheliitis with microthrombus formation and macrothrombosis of pulmonary arteries.34 As also reported by other autopsy studies, the inflammatory process may cause a localized immunothrombosis,[35], 36., 37. as an alternative to the pathogenesis of thromboembolic PE.38 , 39
We speculate this close association between the location of thromboembolic phenomenon and areas of lung opacities may confirm in situ immunothrombosis pathomechanism of PE in COVID-19 patients. This mechanism may be related to local inflammatory response around areas of lung affected due to an inflammatory infiltration with endothelial damage.
Moreover, CT severity and overall lung lobar involvement are worse in those patients who developed PE. These findings may indicate that greater extent of lung parenchymal lesion could be in relation to a more severe inflammatory response leading to an alveolar injury and to an alteration of the vascular endothelial cells.35 , 36 , 44 , 45
Our results highlight the relevance of the integration of CT extent and severity in COVID-19 pneumonia and a possible correlation with patients who develop pulmonary embolism.
In our cases of SARS-CoV-2 infection, dyspnoea was the most common presenting symptom followed by cough.19 Although 40% of patients had a fever, there were no statistically significant differences in the presence of high temperatures in the two groups under analysis; this suggests that the fever was not a useful criterion to determine the severity of illness.20 PE patients showed a lower level of oxygen saturation compared with non-PE group and required more hospitalization. These results are in line with previous reports in which a worsening of clinical respiratory symptoms could suggest a pulmonary embolism.13 , 14 , [21], [22], [23] We did not find a significant difference in ICU admission, requirements for CPAP, and intubation between PE group compared to non-PE group. These results seem to suggest that even non-severe patients can develop acute PE.
We found a higher D-dimer level in the PE group compared with the non-PE group. Elevated D-dimer levels have been reported to be associated with an increase in risk of developing PE,[24], [25], [26], [27] and may have a predictive value in detecting PE.28 , 29 However, high D-dimer levels are not an ultimate diagnostic criterion for PE because elevated D-dimer could be seen in other clinical conditions. In fact, a rise in D-dimer values is frequent in COVID-19 patients, even in the absence of acute pulmonary embolism.22
In our study, a D-dimer threshold of 1929 ng/mL had a sensibility of 67% sensitivity and 70% specificity in detecting patients with PE on chest CT. Therefore, the increase in D-Dimer values might be related to the inflammatory state or directly to the action of the COVID-19.18 IFCC guidelines regarding COVID-19 pneumonia suggest D-dimer testing in patient with COVID-19.30 As reported by Ghuysen et al in the diagnosis of pulmonary embolism the preferred method is CTPA.40
Our results suggested that an integrated approach on basis of non-contrast CT combined with clinical and laboratory data can help clinicians in detection PE and improve patient outcomes.
This study has some limitations. Firstly, the data collection was retrospective. Secondly, the number of patients in our study was small. Other large-scale studies are needed to confirm our findings. Third, the quantitative method for measuring the pulmonary involvement may have subjectivity and we cannot exclude the possibility of superinfection. Our cases are few because we restricted the study to patients who underwent CTPA within one week of the onset of symptoms, as CTPA is the gold standard test to confirm or eliminate the diagnosis of PE. Although restriction to CTPA ensured optimal quality for evaluation of PE, this likely excluded patients with unsuspected PE which would have been otherwise evident on post-contrast chest CT performed without CTPA protocol. Another caveat of our study is that given the preponderance of pulmonary emboli, several hospitals including site A institute prophylactic anticoagulation in high-risk or sicker patients without CTPA. Thus, the actual prevalence of PE might be higher than the one reported in our study. Finally, although CTPA is the preferred method of diagnosis of PE, it is limited by its spatial and contrast resolution to detection of macro-emboli or -thrombi and cannot detect microvascular inflammation or obstruction.
5. Conclusion
The results of this study confirmed the high incidence of PE in COVID-19 patients. Chest radiographs and, if required an unenhanced CT thorax is the recommended standard of care in cases of COVID 19 infection. CTPA should be used to in COVID-19 patients presenting a major risk for developing PE based on serial clinical, laboratory, and radiological characteristics. Specifically, CT severity, lower level of saturation, and increased D-dimer levels could lead to a CTPA. Additional studies with the enrollment of a higher number and with external validation are necessary to provide clinicians the usefulness of these findings.
Declaration of competing interest
The authors state that this work has not received any funding. One study coauthor has received research grant from Siemens Healthineers for unrelated projects.
The scientific guarantor of this publication is the corresponding author.
Institutional Review Board approval was obtained for this study. Written informed consent was waived for all subjects (patients) in this retrospective study.
This is a multicenter retrospective study.
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020 Apr;92(4):401–402. doi: 10.1002/jmv.25678. 31950516 Epub 2020 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. Feb 7 2020 doi: 10.1001/jama.2020.1585. [Epub ahead of print, PubMed PMID: 32031570; PubMed Central PMCID: PMC7042881] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cau R., Bassareo P.P., Mannelli L., et al. Imaging in COVID-19-related myocardial injury. Int J Cardiovasc Imaging. 2020 doi: 10.1007/s10554-020-02089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. Feb 24 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print, PubMed PMID: 32091533] [DOI] [PubMed] [Google Scholar]
- 5.Suri J.S., Puvvula A., Biswas M., et al. COVID-19 pathways for brain and heart injury in comorbidity patients: a role of medical imaging and artificial intelligence-based COVID severity classification: a review. Comput Biol Med. Sep 2020;124 doi: 10.1016/j.compbiomed.2020.103960. [Epub 2020 Aug 14. PMID: 32919186; PMCID: PMC7426723] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faggiano P., Bonelli A., Paris S., et al. Acute pulmonary embolism in COVID-19 disease: preliminary report on seven patients. Int J Cardiol. 2020;313:129–131. doi: 10.1016/j.ijcard.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Tang N, Li D, Wang X, Sun Z J Thromb Haemost. 2020 Apr; 18(4):844–847. [DOI] [PMC free article] [PubMed]
- 9.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost doi: 10.1111/jth.14817. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 10.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing [published online ahead of print, 2020 Feb 12]. Radiology. 2020;200343. doi: 10.1148/radiol.2020200343 ). [DOI] [PMC free article] [PubMed]
- 11.Imaging profile of the COVID-19 infection: radiologic findings and literature Review Ming-Yen Ng, Elaine YP Lee, Jin Yang, Fangfang Yang, Xia Li, Hongxia Wang, Macy Mei-sze Lui, Christine Shing-Yen Lo, Barry Leung, Pek-Lan Khong, Christopher Kim-Ming Hui, Kwok-yung Yuen, and Michael David Kuo Radiology: Cardiothoracic Imaging 2020 2:1. [DOI] [PMC free article] [PubMed]
- 12.ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 13.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 14.Gervaise A., Bouzad C., Peroux E., et al. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020 doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020 doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 17.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard-Lorant I., Delabranche X., Severac F., et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [published online ahead of print, 2020 Feb 24] [DOI] [PubMed] [Google Scholar]
- 20.Bhatraju Pavan K., Ghassemieh Bijan J., Nichols Michelle, et al. Covid-19 in critically ill patients in the Seattle Region — case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cellina M., Oliva G. Acute pulmonary embolism in a patient with COVID-19 pneumonia. Diagn Interv Imaging. 2020;101(5):325–326. doi: 10.1016/j.diii.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Wang X., Zhang S. Findings of acute pulmonary embolism in COVID-19 patients. Lancet Infect Dis. 2020 doi: 10.2139/ssrn.3548771. [DOI] [Google Scholar]
- 23.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., Qanadli S.D. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Olivé I., Sintes H., Radua J., Abad Capa J., Rosell A. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med. 2020;169:106023. doi: 10.1016/j.rmed.2020.106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui S., Chen S., Li X., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klok F.A., Kruip M.J.H.A. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oudkerk M., Büller H.R., Kuijpers D., et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology. 2020 doi: 10.1148/radiol.2020201629. [Advance online publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.J.L. Bosson, C. Barro, B. Satger, P.H.Carpentier, B. Polack, G. Pernod Quantitative high D-dimer value is predictive of pulmonary embolism occurrence independently of clinical score in a well-defined low risk factor population J Thromb Haemost, 3 (1) (2005), pp. 93–99. [DOI] [PubMed]
- 29.Ghanima W., Abdelnoor M., Holmen L.O., Nielssen B.E., Ross S., Sandset P.M. D- dimer level is associated with the extent of pulmonary embolism. Thromb Res. 2007;120:281–288. doi: 10.1016/j.thromres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.IFCC information guide on COVID-19. 2020. https://www.ifcc.org/ifcc-news/2020-03-26-ifcc-information-guide-on-covid-19/ Available on: [Internet]
- 31.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/ajr.20.22976. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol 2020:1–7. doi: 10.2214/AJR.20.22954. [DOI] [PubMed]
- 33.Saba L., Sverzellati N. Is COVID evolution due to occurrence of pulmonary vascular thrombosis? J Thorac Imaging. 2020 doi: 10.1097/RTI.0000000000000530. [Advance online publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bösmüller H., Traxler S., Bitzer M., et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020 doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.S.E. Fox, A. Akmatbekov, J.L. Harbert, G. Li, J.Q. Brown, R.S. Vander Heide Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans (2020)(2020.04.06.20050575). [DOI] [PMC free article] [PubMed]
- 37.L.F. van Dam, L.J.M. Kroft, L.I. van der Wal, S.C. Cannegieter, J. Eikenboom, E. de Jonge, M.V. Huisman, F.A. Klok, Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb. Res. 193 (2020) 86–89, doi:10.1016/j. thromres.2020.06.010 [ 10.1016/j.thromres.2020.05.049 [doi] S0049-3848(20) 30252-8 [pii]]. [DOI] [PMC free article] [PubMed]
- 38.Whyte M.B., Kelly P.A., Gonzalez E., Arya R., Roberts L.N. Pulmonary embolism in hospitalised patients with COVID-19 [published online ahead of print, 2020 Jul 10] Thromb Res. 2020;195:95–99. doi: 10.1016/j.thromres.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huisman M.V., Barco S., Cannegieter S.C., et al. Pulmonary embolism. Nat Rev Dis Primers. 2018;4:18028. doi: 10.1038/nrdp.2018.28. [DOI] [PubMed] [Google Scholar]
- 40.Ghuysen A., Ghaye B., Willems V., et al. Computed tomographic pulmonary angiography and prognostic signicance in patients with acute pulmonary embolism. Thorax. 2005;60(11):956–961. doi: 10.1136/thx.2005.040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao, H., Fang, X., Zhang, J., Homayounieh, F., Arru, C. D., Digumarthy, S. R., Babaei, R., Mobin, H. K., Mohseni, I., Saba, L., Carriero, A., Falaschi, Z., Pasche, A., Wang, G., Kalra, M. K., & Yan, P. (2020). 1. ArXiv, arXiv:2007.10416v1.
- 42.Lu T., Pu H. Computed tomography manifestations of 5 cases of the Novel Coronavirus Disease 2019 (COVID-19) pneumonia from patients outside Wuhan. J Thorac Imaging. 2020;35:W90–W93. doi: 10.1097/RTI.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song B.G., Hong J., Kim S.H., et al. Clinical features in patients with acute pulmonary edema with confirmed coronavirus disease 2019 (COVID-19): comparison with those without acute pulmonary edema. Ann Clin Case Rep. 2020;5:1842. [Google Scholar]
- 45.Mahammedi A., Saba L., Vagal A., et al. Imaging in neurological disease of hospitalized COVID-19 patients: an Italian multicenter retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020201933. [Advance online publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 47.van Wissen M., Keller T.T., Ronkes B., et al. Influenza infection and risk of acute pulmonary embolism. Thromb J. 2007;5(16) doi: 10.1186/1477-9560-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bunce Paul E., High Sasha M., Nadjafi Maral, Stanley Katherine, Liles W. Conrad, Christian Michael D. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis. January 15 2011;52(2):e14–e17. doi: 10.1093/cid/ciq125. [DOI] [PubMed] [Google Scholar]
- 49.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooi M.W.X., Rajai A., Patel R., Gerova N., Godhamgaonkar V., Liong S.Y. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography - prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol. 2020;132:109336. doi: 10.1016/j.ejrad.2020.109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Chen F., Bai L., Yi Q., Peng Y. In situ pulmonary thrombosis in patients with COVID-19 pneumonia: different phenotypes may exist [published online ahead of print, 2020 Oct 23] Thromb Res. 2020;196:541–542. doi: 10.1016/j.thromres.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dam L.F., Kroft L.J.M., van der Wal L.I., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qanadli S.D., El Hajjam M., Vieillard-Baron A., et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415–1420. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 54.Cau R., Falaschi Z., Paschè A., et al. Computed tomography findings of COVID-19 pneumonia in Intensive Care Unit-patients. J Public Health Res. 2021 doi: 10.4081/jphr.2021.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]