Abstract
Objective
To determine characteristics, outcomes, and clinical factors associated with death in patients with COVID-19 requiring extracorporeal membrane oxygenation (ECMO) support.
Methods
A multicenter, retrospective cohort study was conducted. The cohort consisted of adult patients (18 years of age and older) requiring ECMO in the period from March 1, 2020, to September 30, 2020. The primary outcome was in-hospital mortality after ECMO initiation assessed with a time to event analysis at 90 days. Multivariable Cox proportional regression was used to determine factors associated with in-hospital mortality.
Results
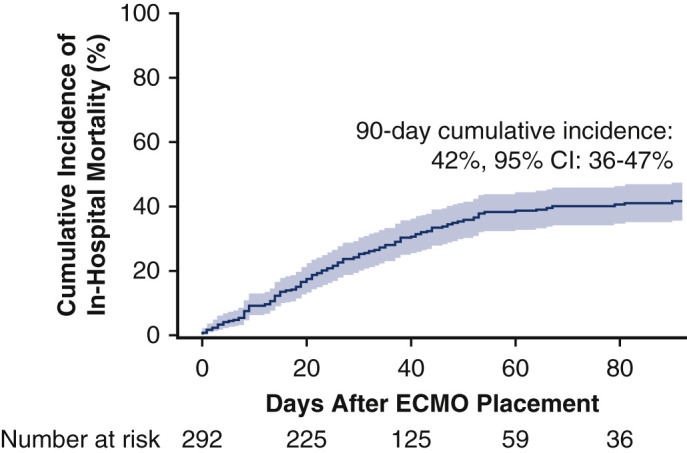
Overall, 292 patients from 17 centers comprised the study cohort. Patients were 49 (interquartile range, 39-57) years old and 81 (28%) were female. At the end of the follow-up period, 19 (6%) patients were still receiving ECMO, 25 (9%) were discontinued from ECMO but remained hospitalized, 135 (46%) were discharged or transferred alive, and 113 (39%) died during the hospitalization. The cumulative in-hospital mortality at 90 days was 42% (95% confidence interval [CI], 36%-47%). Factors associated with in-hospital mortality were age (adjusted hazard ratio [aHR], 1.31; 95% CI, 1.06-1.61 per 10 years), renal dysfunction measured according to serum creatinine level (aHR, 1.21; 95% CI, 1.01-1.45), and cardiopulmonary resuscitation before ECMO placement (aHR, 1.87; 95% CI, 1.01-3.46).
Conclusions
In patients with severe COVID-19 necessitating ECMO support, in-hospital mortality occurred in fewer than half of the cases. ECMO might serve as a viable modality for terminally ill patients with refractory COVID-19.
Key Words: COVID-19, ECMO, ARDS, mortality
Graphical abstract
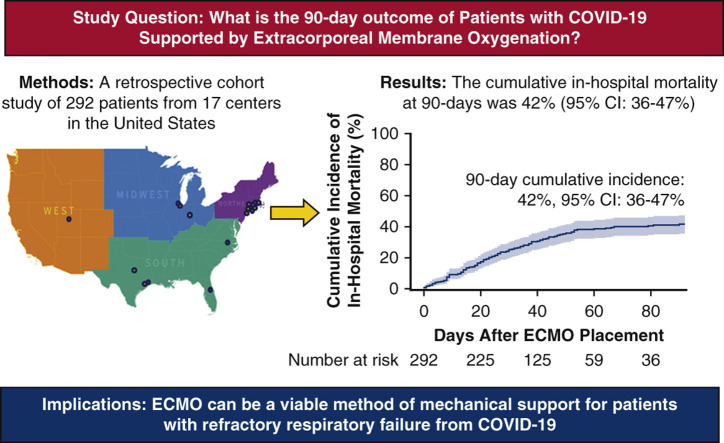
A multicenter, retrospective cohort study of 292 patients with COVID-19 given extracorporeal membrane oxygenation (ECMO) in 17 centers across the United States from March 1, 2020 to September 30, 2020. Clinical characteristics and outcomes were entered into a Research Electronic Data Capture (REDCap) database. The primary outcome of cumulative in-hospital mortality at 90 days was 42% (95% confidence interval [CI], 36%-47%).
More than half of the patients survived after ECMO support for COVID-19.
Central Message.
ECMO can be a suitable method of mechanical support for patients with refractory respiratory failure from COVID-19.
Perspective.
In this retrospective multicenter study, the cumulative incidence of in-hospital mortality for patients with COVID-19 who received ECMO was 42%. Older age, renal dysfunction, and cardiopulmonary resuscitation before ECMO placement were associated with death during hospitalization.
Mortality with COVID-19 is related to progressive respiratory failure leading to acute respiratory distress syndrome (ARDS) with eventual cardiopulmonary collapse.1, 2, 3 Institution of mechanical ventilation support in these patients during the early pandemic period was associated with a disturbingly high mortality nearing 90%.2 Although extracorporeal membrane oxygenation (ECMO) has been used during ARDS in non-COVID-19 patients with variable success,4 its role remains undetermined in those afflicted with severe respiratory failure from COVID-19. Although ECMO can often lead to normalization of gas exchange and acid-base status and it might provide time for resolution of the pulmonary insult, its use is associated with major complications including bleeding, thrombosis, infection, and stroke, which collectively occur in most cases.5 Moreover, ECMO is highly resource-intensive and most implanting centers can only offer such mechanical support to a limited number of patients.
In the early COVID-19 experience in 2020, scant reports and single-center series of outcomes with ECMO showed variability in mortality ranging from 25% to 90%.6, 7, 8, 9, 10, 11 Despite the absence of rigorous and adjusted outcomes data, ECMO was suggested for appropriate patients with COVID-19 and was commonly used by experienced centers in the United States.12 , 13 For optimal usage of this limited yet potentially life-saving modality, we sought to determine the characteristics, outcomes, and clinical factors associated with death during hospitalization in patients with COVID-19 supported with ECMO (Video 1).
Methods
Study Population
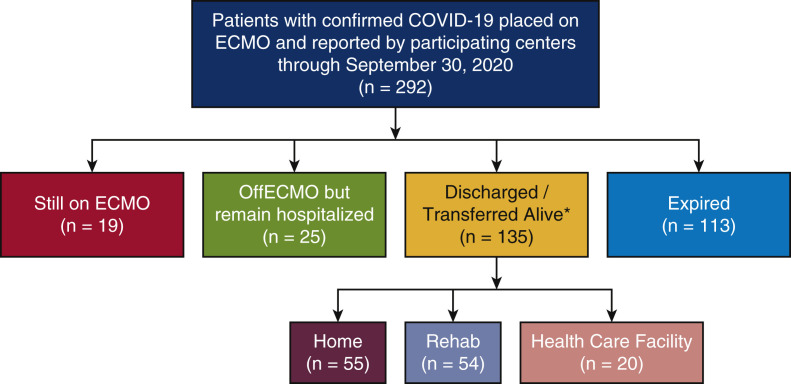
Our study was a multicenter, retrospective cohort study of patients aged 18 years and older, with COVID-19 confirmed with a positive real-time reverse transcriptase polymerase chain reaction assay, who received ECMO support anytime between March 1, 2020, and September 30, 2020 (Figure 1 ). Investigators at the data coordination site at Montefiore Medical Center invited centers for participation by directly contacting surgical directors of mechanical circulatory programs. A data use agreement was mutually agreed upon between every participating center and the data coordinating institution at Montefiore Medical Center, Albert Einstein College of Medicine. The study was approved by the institutional review board at all the participating centers and informed consent was waived. Institutional review board approval was granted on April 5, 2020, under protocol number 2020-11375.
Figure 1.
A multicenter, retrospective cohort study of 292 patients with COVID-19 given extracorporeal membrane oxygenation (ECMO) in 17 centers across the United States from March 1, 2020, to September 30, 2020. Clinical characteristics and outcomes were entered into a Research Electronic Data Capture (REDCap) database. The primary outcome of cumulative in-hospital mortality at 90 days was 42% (95% confidence interval [CI], 36%-47%).
Data Source
A data capture tool was created using Research Electronic Data Capture for record entry by the participating centers. Data fields included demographic characteristics, laboratory parameters, ECMO characteristics, and patient outcomes. All data were anonymized. Before data entry, sites were individually familiarized with the data capture tool and consistency was ensured by continuous technical support provided by the corresponding author at the data coordination center throughout the data collection period. To maintain accuracy, the data capture fields contained checks for validity such as input masks and range rules for date fields and branching logic. Data consistency was maintained through built-in drop boxes with standardized responses. Records were manually inspected for data entry errors, such as those in date temporality, by the data coordination center and rectified by sites before analysis. All of the captured data fields are listed in Table E1.
Outcomes
The primary outcome was in-hospital mortality after ECMO placement assessed with a time to event analysis at 90 days. We used competing risk analysis to calculate the cumulative incidence of in-hospital mortality.14 Discharge to home and transfer to a rehabilitation facility were treated as competing events. Cases transferred to another heath care facility or other inpatient settings were censored at the time of transfer. Patients who remained hospitalized at the ECMO center as of the data update through September 30, 2020, were censored with their final status as still receiving ECMO or discontinued ECMO but still hospitalized. Cumulative incidence was administratively censored at 90 days after ECMO placement. Additional outcomes that were reported include the proportion of patients with secondary infections that occurred after ECMO placement, deep venous thrombosis, stroke, limb ischemia, changes in ECMO configuration, circuit exchange, and renal failure requiring dialysis. Causes of death during hospitalization were also reported.
Statistical Analysis
Continuous data are displayed as mean ± SD or median quartile 1-quartile 3 interquartile range (IQR) and categorical data are shown as proportions. Comparisons between survival curves are on the basis of Fine and Gray's method.15 A multivariable Cox regression analysis using Fine and Gray's subdistribution model to accommodate competing risks was used to determine factors associated with in-hospital mortality. Variables included in the model were those known to have an association with mortality during COVID-19 on the basis of existing literature, captured for >80% of cases or with a univariate association with mortality at a P < .2. The model included the following variables: age, sex, body mass index, race/ethnicity, presence of comorbidities, being transferred from another center, cardiopulmonary resuscitation before ECMO, usage of vasopressors, PaO2 to FiO2 ratio, serum creatinine and lactate dehydrogenase levels before ECMO, time from intubation to ECMO, and intravenous steroid use. Presence of comorbidities was treated as a single binary variable and was marked as yes if the patient had either a history of hypertension and/or diabetes mellitus. A supplemental model was made in which hypertension and diabetes mellitus were present as separate covariates (Figure E1). The number of observations for each covariate are listed in Table E2. Because lactate dehydrogenase was missing in >10% of the patients, we created a categorical variable using tertile cut points with an additional category for missing values and added this variable to the multivariable model. No data were imputed and the Cox multivariable model contained 255 (87%) of the possible 292 cases. Stata version 16 (Stata Corp, LLC, College Station, Tex) and SAS software version 9.4 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
Figure E1.
A multivariable Cox proportional hazards model of factors associated with in-hospital mortality in patients given extracorporeal membrane oxygenation (ECMO) for COVID-19. Other race/ethnicity includes Asian, Pacific Islander, American Indian or other. Hypertension and diabetes mellitus are shown as separate covariates to distinguish from Figure 6, in which they are combined. aHR, Adjusted hazard ratio; CI, confidence interval; CPR, cardiopulmonary resuscitation; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen.
Results
Patient Characteristics
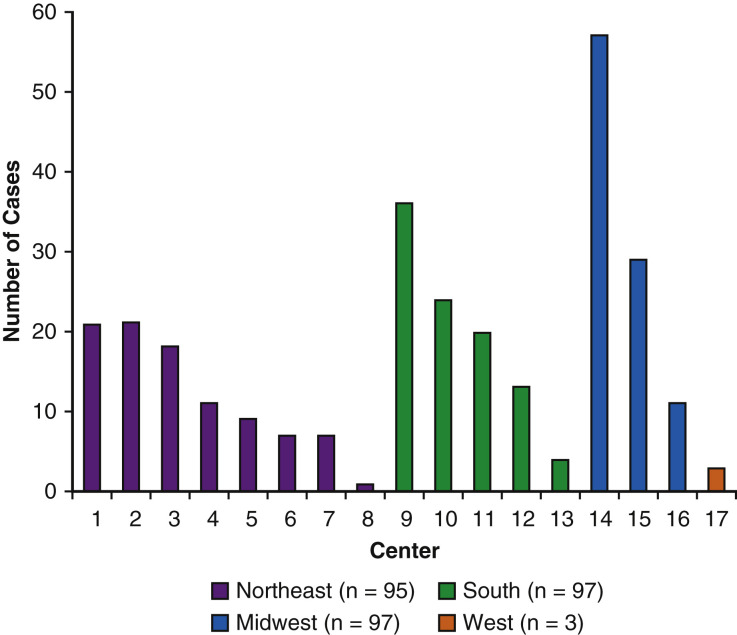
Overall, 292 patients with COVID-19 from 17 centers (Figure 2 ) were supported by ECMO during the study period and comprised the study cohort. They were 49 (IQR, 39-57) years old, 81 (28%) were female, and 131 (45%) were classified as Hispanic. Within the entire cohort, 179 (62%) had preexisting comorbidities, including 119 (41%) with hypertension and 90 (31%) with diabetes mellitus. One hundred sixty-four (56%) were transferred from another center for ECMO placement and 34 (12%) were given ECMO after having received cardiopulmonary resuscitation previously during admission. Patients presented 6 (IQR, 4-8) days after symptom onset and were given ECMO 3 (IQR, 1-6) days after intubation. Inflammatory markers including ferritin (1187; IQR, 638-1905 ng/mL), C-reactive protein (21; IQR, 9-45 mg/dL), d-dimer (8.6; IQR, 2.6-963 μg/mL), and lactate dehydrogenase (593; IQR, 429-844 U/L) levels were elevated before ECMO placement.
Figure 2.
Number of reported COVID-19 patients given extracorporeal membrane oxygenation by the 17 participating centers stratified according to region in the United States.
By the end of the follow-up period, 113 (39%) had died in the hospital, 135 (46%) were discharged or transferred alive, 19 (6%) continued with ECMO, and 25 (9%) discontinued ECMO but remained hospitalized (Figure 3 ). Table 1 shows a comparison of the differences in baseline demographic characteristics and laboratory parameters of patients who had died and those who were discharge or transferred, continued with ECMO and/or were hospitalized by the end of the follow-up period. Most notably, patients who died were older compared with those who were discharged or transferred alive (52 [IQR, 43-59] vs 44 [IQR, 34-54] years; P < .01).
Figure 3.
Consort diagram showing the study population and their clinical outcomes. ∗Discharge/transfer location not available for 6 patients. ECMO, Extracorporeal membrane oxygenation.
Table 1.
Baseline characteristics before ECMO placement
| All patients (N = 292) | Still receiving ECMO (n = 19) | No ECMO but remain hospitalized (n = 25) | Discharged or transferred alive (n = 135) | Deceased (n = 113) | |
|---|---|---|---|---|---|
| Age, years | 49 (39-57) | 51 (44-57) | 49 (41-59) | 44 (34-54) | 52 (43-59)∗ |
| Sex, n (%) | |||||
| Female | 81 (28) | 1 (5) | 4 (16) | 42 (31) | 34 (30) |
| Male | 211 (72) | 18 (95) | 21 (84) | 93 (69) | 79 (70) |
| BMI | 32 (29-37) | 30 (25-36) | 32 (27-38) | 33 (30-39) | 32 (29-36) |
| Race/ethnicity, n (%) | |||||
| Asian | 11 (4) | 1 (5) | 2 (8) | 4 (3) | 4 (4) |
| Hispanic | 131 (45) | 14 (74) | 9 (36) | 56 (42) | 52 (46) |
| Non-Hispanic black | 59 (20) | 3 (16) | 5 (20) | 28 (21) | 23 (20) |
| Non-Hispanic white | 66 (23) | 0 (0) | 9 (36) | 35 (26) | 22 (19) |
| Other/unknown | 25 (8) | 1 (5) | 0 (0) | 12 (9) | 12 (11) |
| Preexisting comorbidities, n (%) | 179 (62) | 9 (50) | 14 (56) | 81 (60) | 75 (67) |
| Hypertension | 119 (41) | 8 (42) | 9 (36) | 53 (39) | 49 (43) |
| Diabetes mellitus | 90 (31) | 2 (16) | 8 (32) | 49 (36) | 30 (27) |
| Chronic respiratory disease | 8 (3) | 0 (0) | 2 (8) | 5 (4) | 2 (2) |
| Malignant neoplasm | 4 (1) | 0 (0) | 1 (4) | 1 (1) | 2 (2) |
| Coronary artery disease | 12 (4) | 0 (0) | 1 (5) | 5 (4) | 5 (4) |
| CPR before ECMO, n (%) | 34 (12) | 2 (11) | 1 (4) | 8 (6) | 16 (14) |
| Transferred to ECMO hospital, n (%) | 164 (56) | 14 (74) | 10 (40) | 77 (57) | 63 (56) |
| Prone positioning, n (%) | 220 (77) | 16 (84) | 18 (72) | 94 (73) | 91 (81) |
| Time from symptom onset to admission, days | 6 (4-8) | 7 (6-10) | 6 (4-7) | 6 (4-8) | 6 (3-8) |
| Time from admission to intubation, days | 2 (1-7) | 6 (0-10) | 3 (0-9) | 1 (1-5) | 4 (1-10)∗ |
| Time from intubation to ECMO, days | 3 (1-6) | 4 (1-8) | 2 (0-5) | 3 (1-5) | 4 (1-6) |
| Systolic blood pressure, mm Hg | 111 (100-125) | 116 (99-125) | 113 (109-120) | 116 (101-130) | 106 (98-122) |
| Diastolic blood pressure, mm Hg | 62 (55-71) | 63 (55-72) | 65 (56-75) | 62 (55-70) | 61 (54-70) |
| Vasopressors, % | 176 (64) | 6 (43) | 17 (68) | 75 (58) | 78 (73)∗ |
| Blood gas parameters | |||||
| pH | 7.31 (7.21-7.38) | 7.25 (7.21-7.36) | 7.33 (7.27-7.40) | 7.32 (7.22-7.38) | 7.29 (7.18-7.37) |
| PaO2/FiO2 | 77 (63-101) | 64 (55-80) | 77 (57-114) | 76 (64-117) | 80 (66-95) |
| PaCO2, mm Hg | 56 (45-71) | 65 (58-78) | 56 (38-68) | 55 (44-69) | 56 (45-72) |
| Laboratory parameters | |||||
| White blood cells, ×103/μL | 14 (10-19) | 17 (12-22) | 12 (10-22) | 12 (9-17) | 14 (12-20) |
| Platelet count, ×103/μL | 252 (184-341) | 323 (211-369) | 188 (164-278) | 262 (184-343) | 248 (191-324) |
| Lactic acid, mmol/L | 1.7 (1.3-2.5) | 1.5 (1.1-2.1) | 2 (1.6-2.8) | 1.7 (1.1-2.2) | 1.7 (1.3-2.6) |
| Creatinine, mg/dL | 0.9 (0.7-1.4) | 0.7 (0.6-1.2) | 0.8 (0.7-1.3) | 0.9 (0.7-1.4) | 1.0 (0.7-2.0)∗ |
| International normalized ratio | 1.2 (1.1-1.3) | 1.1 (1.1-1.3) | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) |
| Total bilirubin, mg/dL | 0.6 (0.4-0.9) | 0.5 (0.3-1.3) | 0.5 (0.4-0.6) | 0.6 (0.4-1.0) | 0.6 (0.4-0.8) |
| Ferritin, ng/mL | 1187 (638-1905) | 1398 (858-2775) | 1089 (692-1809) | 1131 (517-1822) | 1255 (745-1968) |
| C-reactive protein, mg/dL | 21 (9-45) | 14 (2-78) | 16 (8-24) | 22 (9-39) | 24 (9-89)∗ |
| D-Dimer, μg/mL | 8.6 (2.6-963) | 7.2 (3.8-575) | 20 (3.4-7424) | 5.1 (2.0-762) | 9.9 (3.2-1093) |
| Fibrinogen, mg/dL | 640 (487-789) | 715 (637-885) | 587 (417-699) | 663 (514-793) | 614 (457-779) |
| Lactate dehydrogenase, U/L | 593 (429-844) | 510 (427-722) | 688 (572-972) | 556 (421-779) | 624 (429-913)∗ |
| Procalcitonin, ng/mL | 0.70 (0.3-1.9) | 0.4 (0.3-0.9) | 0.90 (0.3-2.2) | 0.6 (0.30-1.60) | 0.70 (0.3-2.1) |
Number observations for each variable are listed in Table E2. Percentages represent the proportion of reported observations. Continuous variables are displayed as median (quartile 1-quartile 3).
ECMO, Extracorporeal membrane oxygenation; BMI, body mass index; CPR, cardiopulmonary resuscitation; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen; PaCO2, partial pressure of carbon dioxide.
P < .05. Blood gas parameters were measured before ECMO placement.
ECMO Characteristics and Course
Venovenous (VV) was the predominant type of initial ECMO support provided to 280 (96%) patients, whereas venoarterial (VA) was used in 10 (3%) and 2 (1%) received VA venous. Most of the patients with VV ECMO (129; 47%) underwent dual cannulation in the femoral and jugular veins, whereas only 54 (19%) were cannulated in the bilateral femoral veins. Cannulas with dual lumens placed only in the internal jugular vein were used in the remainder of patients with VV ECMO as noted in Table 2 . The most common location in the hospital for cannulation was at bedside or in the intensive care unit procedure room (186; 66%), followed by the operating room (74; 27%). Heparin was used for anticoagulation in 198 (71%), argatroban in 87 (32%), and bivalirudin in 28 (10%) cases. Changes in ECMO configuration (from VV to VA or from VA to VA venous) were infrequent, occurring in 19 (7%) of the patients.
Table 2.
ECMO characteristics and outcomes (all patients, N = 292)
| Value | |
|---|---|
| Type of initial ECMO support, n (%) | |
| Venovenous | 280 (96) |
| Femoral vein–femoral vein | 54 (19) |
| Femoral vein–right internal jugular vein | 129 (47) |
| Femoral vein–left internal jugular vein | 4 (1) |
| Protek Duo | 59 (21) |
| Single right internal jugular vein | 31 (11) |
| Venoarterial | 10 (3) |
| Venoarterial venous | 2 (1) |
| Hospital location for ECMO initiation, n (%) | |
| Bedside or ICU procedure room | 186 (66) |
| Operating room | 74 (27) |
| Other | 16 (6) |
| Intravenous anticoagulation, n (%) | |
| Heparin | 198 (71) |
| Bivalirudin | 28 (10) |
| Argatroban | 87 (32) |
| Complications, n (%) | |
| Secondary infection | 153 (55) |
| Bacterial pneumonia | 91 (31) |
| Bacteremia | 92 (32) |
| Central line infection | 8 (3) |
| Urinary tract infection | 31 (11) |
| Deep vein thrombosis | 42 (15) |
| Hemorrhagic stroke | 17 (6) |
| Ischemic stroke | 4 (1) |
| Limb ischemia | 7 (3) |
| Bleeding requiring transfusion | 145 (74) |
| Change in ECMO configuration | 19 (7) |
| Circuit exchange | 26 (13) |
| Renal replacement therapy | 93 (46) |
| Died during ECMO | 79 (27) |
| Cause of death, n (%) | |
| Cardiac failure | 18 (16) |
| Hemorrhagic shock | 3 (3) |
| Liver failure | 1 (1) |
| Multiorgan failure | 39 (34) |
| Respiratory failure | 15 (13) |
| Septic shock | 9 (8) |
| Stroke | 11 (10) |
| Other | 17 (16) |
| Discharge location, n (%) | |
| Home | 55 (43) |
| Rehabilitation facility | 54 (42) |
| Other health care facility | 20 (15) |
The Protek Duo is from TandemLife (Pittsburgh, Pa).
Number of observations reported when missing values: venovenous type, 289; hospital location for ECMO cannulation, 284; heparin, 280; bivalirudin, 273; argatroban, 273; deep vein thrombosis, 278; bleeding requiring transfusion, 197; change in ECMO configuration, 276; circuit exchange, 193; renal replacement therapy, 183; discharge location, 286. Percentages represent the proportion of reported observation. ECMO, Extracorporeal membrane oxygenation; ICU, intensive care unit.
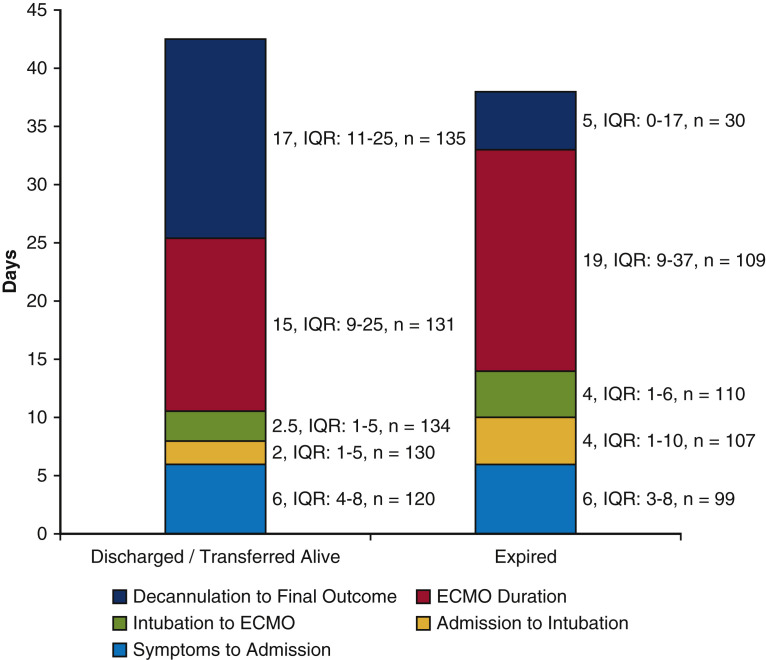
Patients discharged or transferred alive received ECMO nearly 4 days earlier from the time of admission compared with those who died (Figure 4 ). However, the duration of ECMO support was longer in patients who died compared with those discharged or transferred alive (19 [IQR, 9-37] vs 15 [IQR, 9-25] days; P < .01). Secondary infections were common during ECMO support and occurred in more than half of the patients (55%). Of these infections, bacteremia (92; 32%) and bacterial pneumonia (91; 31%) occurred most often, followed by urinary tract infections (31; 11%). Neither location of cannulation (P = .35) nor whether a patient was transferred (P = .35) were associated with bacteremia. Deep venous thrombosis was noted in 42 (15%) of the patients. Hemorrhagic stroke occurred in 17 (6%) and ischemic stroke was noted in 4 (1%) of the patients. After ECMO decannulation, patients remained in the hospital for 17 (IQR, 11-25) days before discharge or transfer.
Figure 4.
A comparison of the duration of hospitalization phases showing that extracorporeal membrane oxygenation (ECMO) was initiated earlier after admission in patients who were discharged/transferred alive compared with those who died. IQR, Interquartile range.
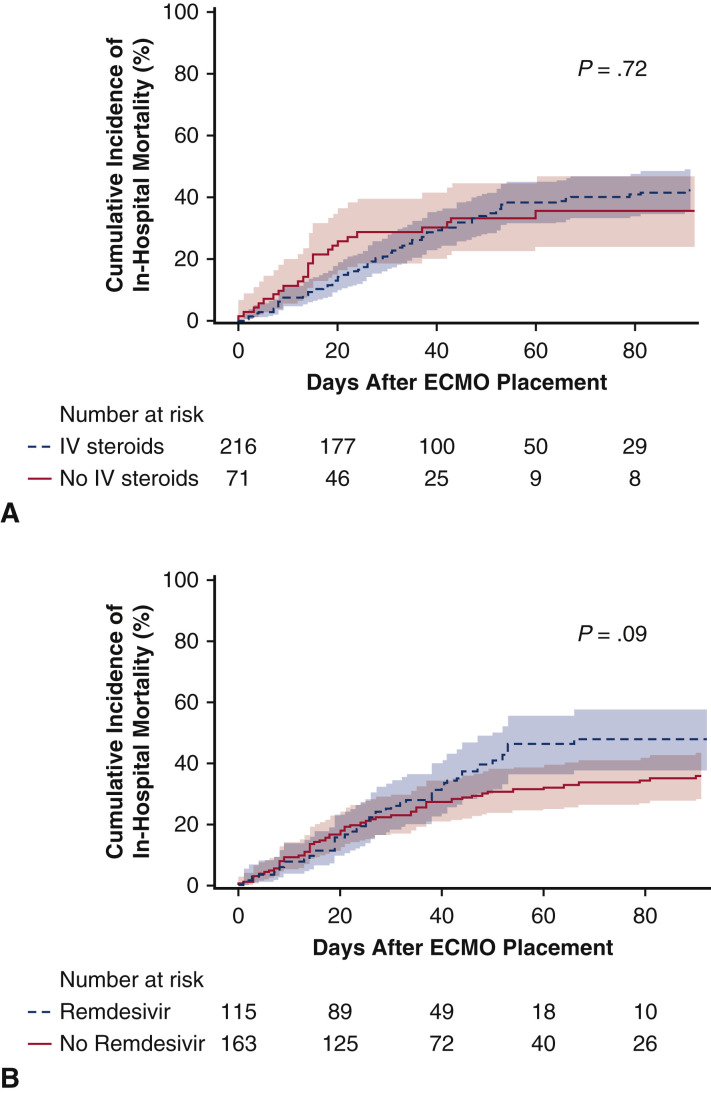
A broad spectrum of adjunctive COVID-19 medical therapies were used during ECMO support as shown in Table E3. None of the reported medical therapies were associated with reduced in-hospital mortality, including intravenous steroids and remdesivir (Figure E2).
Figure E2.
Estimated incidence of in-hospital mortality and usage of intravenous (IV) steroids (A) and remdesivir (B) after initiation of extracorporeal membrane oxygenation (ECMO) support for COVID-19 patients. Administration of IV steroids or remdesivir was not associated with in-hospital mortality.
Outcomes
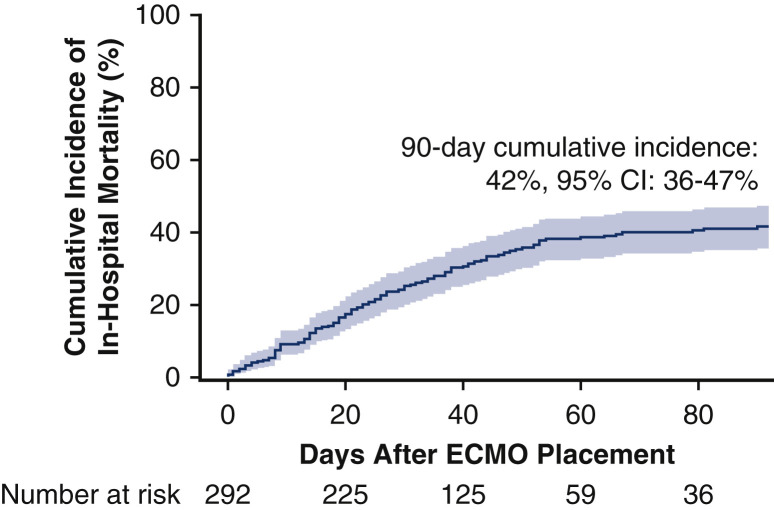
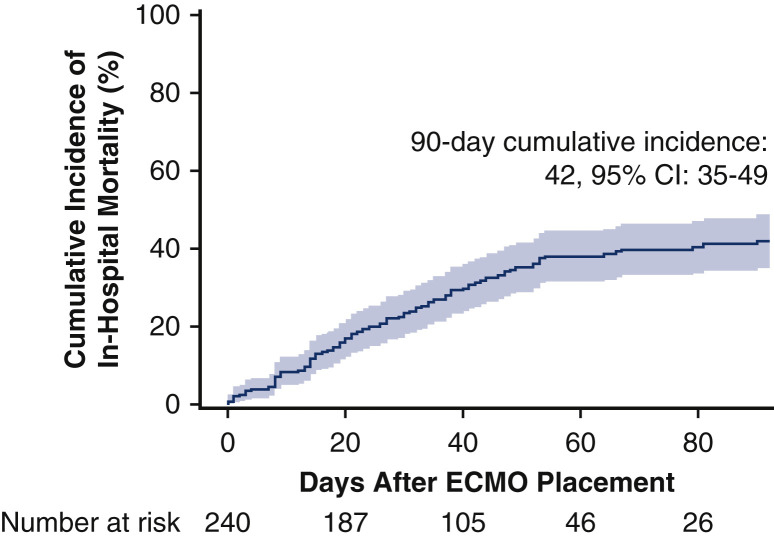
The cumulative incidence of in-hospital mortality at 90 days after ECMO initiation was 42% (95% confidence interval [CI], 36%-47%; Figure 5 ). This incidence of in-hospital death remained similar at 42% (95% CI, 35%-49%; Figure E3) after exclusion of 52 patients from centers overlapping with the recently published report from the Extracorporeal Life Support Organization (ELSO) registry.16 Within the subset of 248 (85%) patients who died or were discharged or transferred alive, in-hospital mortality occurred in 114 (46%) of cases. The most common causes of death were multiorgan failure (39; 34%), cardiac failure (18; 16%), and respiratory failure (15; 13%). For patients who were discharged or transferred alive, post-hospital disposition was reported in all but 6 cases with 55 (43%) discharged to home, 54 (42%) transported to a rehabilitation facility, and 20 (15%) transferred to another health care facility such as long-term acute care or a lower-acuity hospital.
Figure 5.
The estimated cumulative incidence of in-hospital mortality after initiation of extracorporeal membrane oxygenation (ECMO) for COVID-19 at 90 days was 42% (95% confidence interval [CI], 36-47). The solid line shows the estimated cumulative incidence of in-hospital mortality and the shaded region represents the 95% CI.
Figure E3.
The estimated cumulative incidence of in-hospital mortality after initiation of extracorporeal membrane oxygenation (ECMO) only in patients from centers not included in the Extracorporeal Life Support Organization report. In this subset of patients, the 90-day cumulative incidence of death in the hospital was 42% (95% confidence interval [CI], 35-49). The solid line shows the estimated cumulative incidence of in-hospital mortality and the shaded region represents the 95% CI.
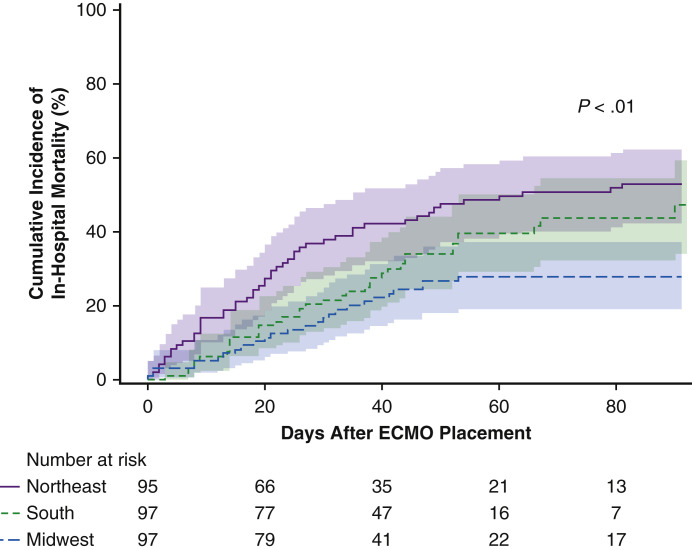
In an exploratory analysis, we grouped centers according to geographical region within the United States and noted variation in hospital mortality (Figure E4). In compared with the Northeast, patients in the South incurred a numerically lower proportion of deaths (hazard ratio, 0.69; 95% CI, 0.43-1.04; P = .08) and those in the Midwest experienced significantly reduced mortality (hazard ratio, 0.43; 95% CI, 0.27-0.69; P < .01).
Figure E4.
A comparison of the estimated cumulative incidence of in-hospital mortality after initiation of extracorporeal membrane oxygenation (ECMO) for COVID-19 for centers from the Northeast, South, and Midwest regions of the United States showed variation in survival.
Clinical Factors Associated With In-Hospital Mortality
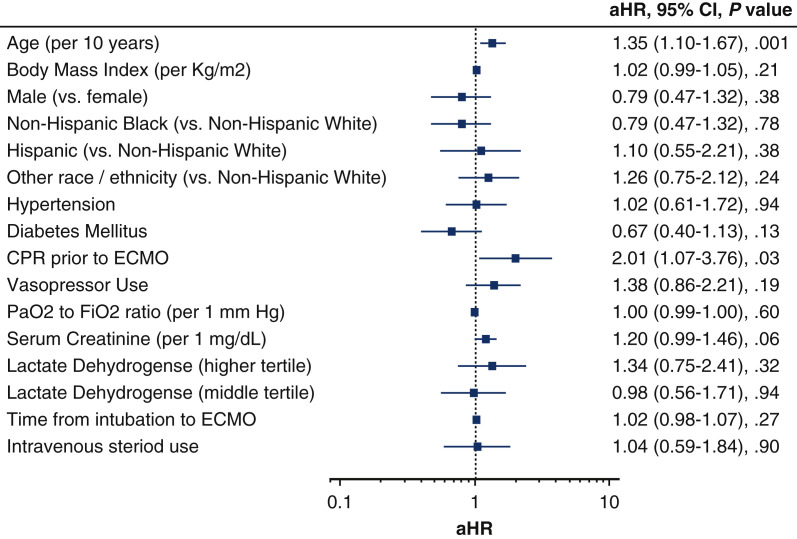
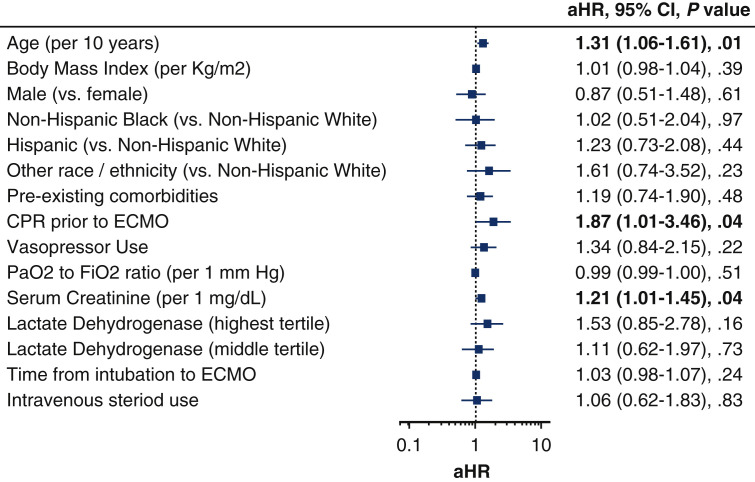
Multivariable adjustment analysis of baseline characteristics and laboratory variables revealed several factors related to in-hospital mortality. As shown in Figure 6 , older age (adjusted hazard ratio [aHR], 1.26; 95% CI, 1.02-1.57 per 10 years), renal dysfunction measured according to serum creatinine level (aHR, 1.24; 95% CI, 1.01-1.53), and receiving cardiopulmonary resuscitation (CPR) before ECMO placement (aHR, 1.87; 95% CI, 1.01-3.46) were associated with death during hospitalization. Notably, sex, preexisting comorbidities, and length of intubation time before ECMO placement were not associated with death.
Figure 6.
A multivariable Cox proportional hazards model of factors associated with in-hospital mortality in patients given extracorporeal membrane oxygenation (ECMO) for COVID-19. Older age, renal dysfunction, and cardiopulmonary resuscitation before ECMO placement were associated with in-hospital mortality. Preexisting comorbidities include hypertension and/or diabetes mellitus. Other race/ethnicity includes Asian, Pacific Islander, American Indian or other. aHR, Adjusted hazard ratio; CI, confidence interval; CPR, cardiopulmonary resuscitation; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen.
Discussion
The principal findings of this, to our knowledge, largest to date US experience with ECMO use during COVID-19 are as follows: (1) death during hospitalization occurred in less than half of the patients, (2) patients discharged or transferred alive were given ECMO sooner after admission than those who died, (3) advancing age, renal injury, and cardiopulmonary arrest requiring cardiopulmonary resuscitation during admission before ECMO placement forecasted reduced survival, (4) secondary infections occurred most of the patients, and (5) more than 80% of the patients discharged or transferred alive were either sent home or to a rehabilitation facility. Regional variation in hospital mortality is likely multifactorial and might be related to the initial burden of the pandemic in the United States, which was greatest in the Northeast. The lack of association between potential COVID-19 therapeutics and survival, in particular steroids, which have been shown to reduce mortality in hospitalized patients17 could be related to the extreme severity of illness in patients who underwent ECMO support; however, the efficacy of such regimens cannot be determined using our registry-based study design and with concurrent administration of multiple therapies.
Our findings showed a similar cumulative incidence of in-hospital mortality at 90 days for patients with COVID-19 requiring ECMO compared with the worldwide experience in the ELSO registry, reported as 37%,16 which persisted after exclusion of overlapping centers. Although both studies contain patients with a similar age distribution, burden of comorbidities, and levels of disease severity as evidenced by comparable PaO2/FiO2 ratios, small differences in outcome might be related to local unmeasured variations in patient selection and practices. Similar to the worldwide ELSO experience, we also noted that stroke occurred in 7% of the cases, with the bleeding subtype as most common, as is also apparent in non-COVID-19 patients.5 , 18 As an external validation for the international ELSO study, this US-based experience provides corroborative evidence that ECMO support might lead to the survival for most patients afflicted by COVID-19-related ARDS.
Patients in our cohort met typical hypoxemia criteria during severe pneumonia and ARDS of a PaO2 to FiO2 ratio of <80 mm Hg despite standard ventilator management, which has been proposed as an indication for VV ECMO placement.19 Upon comparing the clinical characteristics of our cohort with those from a large ECMO registry of non-COVID-19 patients (n = 2355), it is notable that patients included in our study were older (49 [IQR, 39-57] vs 41 [IQR, 28-34] years), but similar in proportion of cardiac arrest before ECMO (12% vs 9%) and had a higher PaO2 to FiO2 ratio (77 [IQR, 63-101] vs 59 [IQR 48-75]).20 Few randomized trials have assessed the clinical utility of ECMO in patients with COVID-19. The Conventional ventilation or ECMO for Severe Adult Respiratory failure (CESAR) trial showed a reduction in death or severe disability at 60 days or before hospital discharge with ECMO (relative risk, 0.69; 95% CI, 0.05-0.97; P = .03).21 More recently, the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial showed that 60-day mortality was 35% with immediate ECMO compared with 46% with continued conventional treatment (relative risk, 0.76; 95% CI, 0.55-1.04; P = .09).22 Additional meta-analyses also show an improvement in survival with ECMO for patients with severe and refractory respiratory failure.23 Our cohort of patients with COVID-19 were similar to those in the EOLIA trial in age, PaO2/FiO2 ratio, and presence of comorbidities, thus providing a rationale to extrapolate that ECMO might indeed offer an effective treatment platform. However, such comparisons are limited because our patients received VV and VA ECMO and definitive measurements of efficacy can only be done in a randomized controlled trial.
Because nearly half of the patients did not survive to 90 days after ECMO placement, our findings do raise caution and point to judicious use of this treatment modality. To potentially optimize survival outcomes and manage expectations, we suggest that a treatment algorithm for patients with refractory respiratory failure from COVID-19 should take into consideration factors such as advanced age, renal failure, and pre-ECMO cardiopulmonary arrest when deciding whether to initiate ECMO. For patients with the preceding risk factors, consideration of comfort care measures might be appropriate as an alternative to ECMO, if consistent with their goals of care.
Survivors received ECMO sooner during admission than patients who expired. We speculate that this finding might relate to relatively more rapid restoration of oxygenation and limitation of irreversible end organ damage earlier during illness by ECMO placement. Thus, in appropriate candidates with refractory respiratory failure from COVID-19, early ECMO might potentially improve outcomes. Beyond survival, and in light of the elevated burden of adverse events including infection and renal replacement therapy during ECMO support, it remains essential to determine the long-term functional and readmission outcomes of patients who were discharged or transferred alive. In our study, 54 (40%) and 20 (15%) of the patients who were discharged or transferred alive were sent to a rehabilitation facility or another health care facility, respectively. Further follow-up of these patients is warranted and will inform if ECMO is indeed an effective mode of therapy to reach meaningful recovery and quality of life.
Our study has several limitations. Because of the retrospective study design and lack of a non-ECMO control group, we cannot determine the efficacy of ECMO for patients with severe respiratory failure from COVID-19. In addition, a deeper characterization of the study cohort and outcomes is limited by lack of data availability for all collected variables. Outcomes from participating centers might not be reflective of those from institutions with lesser experience and different resource availability. There were no standardized criteria for patient selection or management among the participating centers. Because 15% of the patients were still hospitalized, the cumulative incidence of in-hospital mortality might change when these patients reach a final outcome. ARDS was not formally defined in our data collection tool. Notwithstanding the aforementioned limitations, we surmise that in light of the pandemic nature of COVID-19, our data provide important knowledge that can meaningfully affect de novo and ongoing use of this resource-intensive therapy. We advocate for the development of a comprehensive and centralized prospective database to capture granular clinical information and show outcomes in real time. The database and network of centers formed through this collaborative analysis could serve as an expandable platform to address gaps in knowledge noted by our data and listed in Table 3 to pave the path toward improving outcomes.
Table 3.
Proposed areas of intervention and investigation for patients with COVID-19 requiring extracorporeal membrane oxygenation
Patient selection
|
Circuit deployment
|
Patient management
|
Follow-up care
|
Network level
|
ECMO, Extracorporeal membrane oxygenation; PaO2/FiO2, partial presure of oxygen/fraction of inspired oxygen; RVAD, right ventricular assist device.
In summary, our findings indicate that ECMO might serve as a useful method of advanced pulmonary support in patients with refractory respiratory failure from COVID-19. These data provide further credence for usage of ECMO in appropriate patients with severe COVID-19 and reinforce resource allocation toward this beneficial modality.
Conflict of Interest Statement
A.J.T and G.S are consultants for Abbott Laboratories outside of the submitted work. S.S is a consultant for Abbott Laboratories, Medtronic, Syncardia, and Abiomed outside o the submitted work. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors acknowledge the centers and collaborators:
Advent Health Center: Callie Arnold, BS, CCRC; Delores Barnes, MBA, BSN; Nadiye Dehnert, BS; Ginnette Guzman, CMA; Crystal Labozzetta; Angela Martinez, BSN, RN; Courtney Morrison, BSN, RN; Theresa Munro, BSN, RN, PCCN
Ascension, St Vincent Medical Center: Regina Margiotti, CRC
Cardiothoracic and Vascular Surgical Associates at Advocate Christ Medical Center: Colleen Gallagher, BSN
Baylor University Medical Center: Omar Hernandez, BSN, RN, CCRN-CSC
Duke University Medical Center: Jacob N. Schroder, MD
Hartford HealthCare Heart and Vascular Institute: Ethan Kurtzman, MBA, RRT-NPS
Maimonides Medical Center: Ellen Reeves, RN, BSN
Massachusetts General Hospital: Kamila Drezek, BS, Annika Gallandt
Montefiore Medical Center: Dominica Marino, BS
Northwell Health: Efstathia Mihelis, RPA-C
Northwestern University Medical Center: Anna Huskin, RN, BSN, CCRC; Randy McGregor, MS, CCP, LP; Kaleigh Powers, MSN, RN, CNL, RN-BC
New York University, Winthrop: Elizabeth Rerisi, MS, RN
St Francis Medical Center
University of Texas Medical Branch at Galveston: Sean Yates, MD
University of Utah Medical Center: Chloe Skidmore, MS
University of Texas Health Science Center at Houston: Katrina Atencio; Francine Cummings; Lisa Janowiak; Rene Michelle Sauer Gehring, PhD
Westchester Medical Center
COVID-19 ECMO Working Group: Chikezie Alvarez, MD, Division of Cardiology, Department of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY; Abe DeAnda Jr, MD, Department of Cardiovascular and Thoracic Surgery, University of Texas Medical Branch, Galveston, Tex; Jason Gluck, DO, Division of Cardiology, Department of Medicine, Hartford HealthCare Heart and Vascular Institute, Hartford, Conn; Rita Jermyn, MD, Division of Cardiology, Department of Medicine, Saint Francis Medical Center, Roslyn, NY; Matthew Kuntzman, BS, Division of Cardiology, Department of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY; Stephen McKellar, MD, MSc, Division of Cardiothoracic Surgery, Department of Surgery, University of Utah, Salt Lake City, Utah; Michael K. Parides, PhD, Division of Biostatistics, Department of Cardiothoracic and Vascular Surgery, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY; and Paul Saunders, MD, Division of Cardiothoracic Surgery, Department of Surgery, Maimonides Medical Center, New York, NY.
Footnotes
O.S. is supported by the National Institutes of Health/National Heart, Lung and Blood Institute (K23HL145140). The research described was supported by a National Institutes of Health/National Center for Advancing Translational Science Clinical and Translational Science Award grant to Einstein-Montefiore (UL1TR002556).
Omar Saeed and Daniel J. Goldstein contributed equally to this work.
Contributor Information
COVID-19 ECMO Working Group:
Chikezie Alvarez, Abe DeAnda, Jr., Jason Gluck, Rita Jermyn, Matthew Kuntzman, Stephen McKellar, Michael K. Parides, and Paul Saunders
Supplementary Data
Oral slide presentation of the study given by the lead author, Dr Omar Saeed. Video available at: https://www.jtcvs.org/article/S0022-5223(21)00801-1/fulltext.
Appendix E1
Table E1.
List of collected variables in the REDCap database
| Center ID |
| Covid 19 confirmed (Y/N) |
| Age |
| Sex |
| Weight, Kg |
| Body mass index |
| Race (drop box) |
| Ethnicity (drop box) |
| Preexisting conditions (drop box) |
| Other significant medical history |
| Date of symptoms onset |
| Date of presentation to hospital |
| Date of intubation |
| Time of intubation |
| Prone position prior to ECMO (Y/N) |
| Transferred (Y/N) |
| If transferred, date of transfer? |
| CPR before ECMO (Y/N) |
| Glasgow Coma Scale∗ |
| White blood cell count, 103/μL |
| Systolic blood pressure, mm Hg |
| Diastolic blood pressure, mm Hg |
| Vasopressor use (Y/N) |
| Platelet count, 103/μL |
| Serum creatinine, mg/dL |
| Serum total bilirubin, mg/dL |
| Ferritin, ng/mL |
| C-reactive protein, mg/dL |
| D-dimer, μg/mL |
| International normalized ratio |
| Fibrinogen, mg/dL |
| Lactate dehydrogenase, U/L |
| High-sensitivity troponin, ng/mL |
| Troponin I, ng/mL |
| Troponin T, ng/mL |
| pO2 |
| FiO2 |
| pH |
| P/F ratio (calculated) |
| pCO2 |
| Lactic acid, mmol/L |
| Procalcitonin, ng/mL |
| Chloroquine (Y/N) |
| Hydroxychloroquine (Y/N) |
| Azithromycin (Y/N) |
| IL-6 inhibitor (Y/N) |
| IL-1 inhibitor (Y/N) |
| CCR5 inhibitor (Y/N) |
| Intravenous steroids (Y/N) |
| Remdesivir (Y/N) |
| Lopinavir/ritonavir (Y/N) |
| Convalescent plasma (Y/N) |
| Intravenous heparin (Y/N) |
| Intravenous bivalirudin (Y/N) |
| Intravenous argatroban (Y/N) |
| Left ventricular ejection fraction, %∗ |
| Date of ECMO placement |
| Time of ECMO placement |
| Initial ECMO configuration (VV, VA, VAV) |
| Cannulation type (drop box) |
| Location of cannulation in the hospital (drop box) |
| Complications (Y/N) |
| Circuit exchange (Y/N) |
| Bleeding requiring transfusion (Y/N) |
| Renal failure requiring renal replacement therapy (Y/N) |
| Secondary infection (Y/N) |
| Which secondary infection (drop box) |
| Date of secondary infection |
| Deep vein thrombosis (Y/N), if yes then date of diagnosis |
| Hemorrhagic stroke during ECMO (Y/N), if yes then date of diagnosis |
| Ischemic stroke during ECMO, if yes then date of diagnosis |
| Change in ECMO configuration (drop box) |
| Continues receiving ECMO (Y/N) |
| Died during ECMO (Y/N), if yes, date of death |
| Cause of death (drop box) |
| Decannulated (Y/N), if yes, date of decannulation |
| Died after ECMO decannulation, if yes date of death |
| Discharged (Y/N), if yes date of discharge |
| 90-Day outcome after ECMO placement (drop box) |
Troponin was not reported due to variations in assay type.
REDCap, Research Electronic Data Capture; Y, yes; N, no; ECMO, extracorporeal membrane oxygenation; CPR, cardiopulmonary resuscitation; pO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; P/F, partial pressure of oxygen to fraction of inspired oxygen ratio; pCO2, partial pressure of carbon dioxide; IL, interleukin; CCR5, C-C chemokine receptor type 5; VV, venovenous; VA, venoarterial; VAV, venoarterial venous.
Not reported since missing for >80% of cases.
Table E2.
Univariable associations between baseline demographic characteristics, laboratory parameters, and in-hospital mortality
| Available observations | HR (95% CI) | P value | |
|---|---|---|---|
| Age, y | 292 | 1.03 (1.01-1.04) | <.01 |
| Sex, n (%) | |||
| Male sex (vs female sex) | 292 | 0.85 (0.57-1.25) | .40 |
| BMI | 288 | 0.99 (0.97-1.01) | .43 |
| Race/ethnicity, n (%) | 285 | ||
| Non-Hispanic black (vs non-Hispanic white) | 1.23 (0.67-2.23) | .51 | |
| Hispanic (vs non-Hispanic white) | 1.11 (0.68-1.80) | .69 | |
| Other (vs non-Hispanic white) | 1.42 (0.69-2.95) | .34 | |
| Preexisting comorbidities, n (%) | 290 | 1.35 (0.91-1.99) | .14 |
| Hypertension | 292 | 1.11 (0.78-1.62) | .55 |
| Diabetes mellitus | 292 | 0.77 (0.51-1.16) | .21 |
| COPD | 292 | 0.54 (0.13-2.17) | .39 |
| Malignant neoplasm | 292 | 1.53 (0.31-7.51) | .41 |
| Coronary artery disease | 292 | 1.07 (0.45-2.57) | .87 |
| Cardiopulmonary resuscitation, n (%) | 292 | 1.66 (0.99-2.84) | .06 |
| Transferred to ECMO hospital, n (%) | 292 | 0.98 (0.68-1.42) | .93 |
| Prone positioning, n (%) | 287 | 1.28 (0.81-2.20) | .30 |
| Time from symptom onset to admission, days | 261 | 0.97 (0.92-1.02) | .19 |
| Time from admission to intubation, days | 279 | 1.04 (1.01-1.07) | .01 |
| Time from intubation to ECMO, days | 288 | 1.02 (0.99-1.06) | .21 |
| Systolic blood pressure, mm Hg per 10 units | 269 | 0.93 (0.85-1.02) | .13 |
| Diastolic blood pressure, mm Hg per 10 units | 269 | 0.93 (0.81-1.07) | .30 |
| Vasopressors, % | 275 | 1.71 (1.12-2.62) | .01 |
| Blood gas parameters | |||
| pH | 279 | 0.42 (0.10-1.74) | .23 |
| PaO2/FiO2, per 10 units | 278 | 0.98 (0.95-1.02) | .33 |
| PaCO2, mm Hg per 10 units | 277 | 0.99 (0.95-1.04) | .81 |
| Laboratory parameters | |||
| White blood cells, ×103/μL | 278 | 1.01 (0.99-1.03) | .22 |
| Platelet count, ×103/μL per 100 units | 271 | 0.91 (0.78-1.07) | .25 |
| Lactic acid, mmol/L | 256 | 0.98 (0.95-1.02) | .40 |
| Creatinine, mg/dL | 278 | 1.03 (1.00-1.06) | .02 |
| INR | 237 | 1.00 (0.86-1.16) | .96 |
| Total bilirubin, mg/dL | 266 | 1.02 (0.80-1.30) | .22 |
| Ferritin, ng/mL per 100 units | 233 | 1.00 (0.99-1.01) | .96 |
| C-reactive protein, mg/dL per 10 units | 210 | 1.01 (1.00-1.01) | .02 |
| D-dimer, μg/mL | 261 | 1.00 (0.99-1.00) | .80 |
| Fibrinogen, mg/dL per 100 units | 162 | 0.96 (0.88-1.04) | .27 |
| Lactate dehydrogenase, U/L per 100 units | 241 | 1.02 (1.05-1.03) | <.01 |
| Highest tertile | 80 | 1.25 (0.76-2.04) | .37∗ |
| Middle tertile | 80 | 0.99 (0.60-1.63) | .96∗ |
| Lowest tertile | 81 | ||
| Missing | 51 | ||
| Procalcitonin, ng/mL | 217 | 1.03 (0.98-1.03) | .82 |
HR, Hazard ratio; CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen; PaCO2, partial pressure of carbon dioxide; INR, international normalized ratio.
Compared with lowest tertile.
Table E3.
Usage of potential COVID-19 therapeutics and univariate association with in-hospital mortality
| All patients (N = 292) | Still receiving ECMO (n = 19) | No ECMO but remain hospitalized (n = 25) | Discharged/transferred alive (n = 135) | Died (n = 113) | Available observations | HR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| Chloroquine | 3 (1) | 0 (0) | 0 (0) | 1 (1) | 2 (2) | 277 | 3.49 (0.50-24.39) | .21 |
| Hydroxychloroquine | 137 (49) | 7 (37) | 11 (46) | 75 (56) | 44 (44) | 277 | 0.79 (0.52-1.14) | .19 |
| Azithromycin | 182 (64) | 10 (56) | 17 (68) | 94 (70) | 61 (57) | 284 | 0.73 (0.50-1.06) | .10 |
| Interleukin 1 inhibitor | 12 (4) | 0 (0) | 1 (4) | 5 (4) | 6 (6) | 272 | 1.57 (0.66-3.76) | .31 |
| Interleukin 6 inhibitor | 171 (61) | 13 (72) | 15 (63) | 81 (60) | 62 (59) | 281 | 0.89 (0.61-1.31) | .56 |
| CCR5 inhibitor | 4 (2) | 0 (0) | 0 (0) | 3 (2) | 1 (1) | 273 | 0.61 (0.13-3.02) | .55 |
| Intravenous steroids | 216 (75) | 16 (84) | 19 (76) | 97 (72) | 84 (78) | 287 | 1.07 (0.67-1.72) | .78 |
| Remdesivir | 115 (41) | 10 (53) | 10 (42) | 46 (35) | 49 (47) | 278 | 1.36 (0.93-1.99) | .11 |
| Lopinavir/ritonavir | 6 (2) | 0 (0) | 0 (0) | 3 (2) | 3 (3) | 273 | 1.49 (0.48-4.66) | .49 |
| Convalescent plasma | 122 (43) | 10 (53) | 8 (32) | 50 (37) | 54 (50) | 285 | 1.43 (0.98-2.07) | .07 |
Data are presented as n (%); percentages represent the proportion of reported observations.
ECMO, Extracorporeal membrane oxygenation; HR, hazard ratio; CI, confidence interval; CCR5, C-C chemokine receptor type 5.
References
- 1.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potere N., Valeriani E., Candeloro M., Tana M., Porreca E., Abbate A., et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24:1–12. doi: 10.1186/s13054-020-03022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams D., Brodie D. Extracorporeal membrane oxygenation for adult respiratory failure: 2017 update. Chest. 2017;152:639–649. doi: 10.1016/j.chest.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Vaquer S., de Haro C., Peruga P., Oliva J.C., Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care. 2017;7:51. doi: 10.1186/s13613-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs J.P., Stammers A.H., Louis J.S., Awori Hayanga J.W., Firstenberg M, Mongero L.B., et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultan I., Habertheuer A., Usman A.A., Kilic A., Gnall E., Friscia M.E., et al. The role of extracorporeal life support for patients with COVID-19: Preliminary results from a statewide experience. J Card Surg. 2020;35:1410–1413. doi: 10.1111/jocs.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry B.M., Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M., Hajage D., Lebreton G., Monsel A., Voiriot G., Levy D., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miike S., Sakamoto N., Washino T., Kosaka A., Kuwahara Y., Ishida T., et al. Critically ill patients with COVID-19 in Tokyo, Japan: a single-center case series. J Infect Chemother. 2020;27:291–295. doi: 10.1016/j.jiac.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustafa A.K., Alexander P.J., Joshi D.J., Tabachnick D.R., Cross C.A., Pappas P.S., et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. 2020;155:990–992. doi: 10.1001/jamasurg.2020.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett R.H., Ogino M.T., Brodie D., McMullan D.M., Lorusso R., MacLaren G., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ñamendys-Silva S.A. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–349. doi: 10.1016/j.hrtlng.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satagopan J., Ben-Porat L., Berwick M., Robson M., Kutler D., Auerbach A. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zangrillo A., Landoni G., Biondi-Zoccai G., Greco M., Greco T., Frato G., et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 19.Brodie D., Slutsky A.S., Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M., Bailey M., Sheldrake J., Hodgson C., Aubron C., Rycus P.T., et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 21.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 22.Combes A., Hajage D., Capellier G., Demoule A., Lavoue S., Guervilly C., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 23.Munshi L., Walkey A., Goligher E., Pham T., Uleryk E.M., Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oral slide presentation of the study given by the lead author, Dr Omar Saeed. Video available at: https://www.jtcvs.org/article/S0022-5223(21)00801-1/fulltext.