Abstract
The iodide recycling enzyme, iodotyrosine deiodinase (IYD), is a largely unstudied molecular mechanism through which environmental chemicals can potentially cause thyroid disruption. This highly conserved enzyme plays an essential role in maintaining adequate levels of free iodide for thyroid hormone synthesis. Thyroid disruption following in vivo IYD inhibition has been documented in mammalian and amphibian models; however, few chemicals have been tested for IYD inhibition in either in vivo or in vitro assays. Presented here are the development and application of a screening assay to assess susceptibility of IYD to chemical inhibition. With recombinant human IYD enzyme, a 96-well plate in vitro assay was developed and then used to screen over 1,800 unique substances from the U.S. EPA ToxCast screening library. Through a tiered screening approach, 194 IYD inhibitors were identified (inhibited IYD enzyme activity by 20% or greater at target concentration of 200 μM). 154 chemicals were further tested in concentration-response (0.032 – 200 μM) to determine IC50 and rank-order potency. This work broadens the coverage of thyroid-relevant molecular targets for chemical screening, provides the largest set of chemicals tested for IYD inhibition, and aids in prioritizing chemicals for targeted in vivo testing to evaluate thyroid-related adverse outcomes.
Keywords: thyroid, deiodinase, DEHAL1, high-throughput, screening
1. Introduction
Environmental contaminants have been shown to disrupt thyroid function through a variety of molecular mechanisms, including those involved with thyroid hormone (TH) synthesis, transport, elimination, metabolic activation and inactivation, as well as feedback mechanisms (Boas et al., 2012; Brucker-Davis, 1998; DeVito et al., 1999; Noyes et al., 2019). Given the complexity of these coordinated events, there are multiple potential molecular targets for chemical disruption. Many targets, however, have little known of their capacity for disruption by xenobiotics or linkages to thyroid-relevant endpoints (DeVito et al., 1999; EU, 2017; Noyes et al., 2019). This challenge has been addressed by several efforts to define critical elements of TH regulation or action, and recommendations of priority molecular targets for assay development and testing (Murk et al., 2013; OECD, 2014; Noyes et al., 2019; Thomas et al., 2019).
These recommendations led to focused efforts to develop and/or optimize in vitro assays for rapidly screening chemical disruption of molecular targets related to TH synthesis [thyroperoxidase (TPO; Paul et al., 2014; Paul Friedman et al., 2016; Dong et al., 2020) and sodium-iodide symporter (NIS; Hallinger et al., 2017; Lecat-Guillet et al., 2007)], TH transport [transthyretin (TTR; Montano et al., 2012); monocarboxylate transporter 8 (MCT8; Dong and Wade, 2017; Jayarama-Naidu et al., 2015)], and TH metabolic activation/inactivation [iodothyronine deiodinases (DIOs; Hornung et al., 2018; Renko et al., 2012, 2015)]. Several of these assays have already screened chemicals in the U.S. EPA’s Toxicity Forecaster (ToxCast) Program (U.S. EPA, 2015; Paul Friedman et al., 2016; Hornung et al., 2018; Olker et al., 2019; Wang et al., 2018, Wang et al., 2019a; Buckalew et al., 2020). Other molecular targets, however, still have little known of their toxicological relevance. The iodine recycling enzyme, iodotyrosine deiodinase (IYD), is one such molecular target that has an important role in maintaining adequate levels of TH; yet is not well-characterized with regard to its susceptibility to chemical inhibition and has been largely omitted from identification of thyroid endpoints for screening (Murk et al., 2013; Noyes et al., 2019).
IYD is a reductive dehalogenation enzyme that works in concert with NIS to maintain adequate iodide concentrations for TH synthesis (Friedman et al., 2006; Rokita et al., 2010; Rousset et al., 2015; Thomas et al., 2009). IYD promotes iodide retention in the thyroid gland by catalyzing iodide recycling from byproducts of TH synthesis: monoiodotyrosine (MIT) and diiodotyrosine (DIT). This membrane-bound protein, located at the apical pole of the thyroid follicular cell, rapidly deiodinates MIT and DIT released from thyroglobulin, providing free iodide for TH production (Gnidehou et al., 2004; Rokita et al., 2010). IYD has also been identified in the kidney and liver of mammals (Gnidehou et al., 2006; Sun et al. 2015) and amphibians (Olker et al., 2018), where IYD is thought to prevent loss of iodide through metabolism and excretion in waste.
The biological importance of IYD has been documented in humans, rodents, and amphibians. First documented in humans, failure of IYD (named DEHAL1) due to genetic mutations resulted in hypothyroidism, goiter, and mental retardation (Moreno et al., 2008; Moreno and Visser, 2010). Subsequent in vivo testing of IYD inhibition by nitrotyrosines in rodents showed reduced circulating TH levels, increased thyroid gland size, and increased thyroid-stimulating hormone (TSH) levels (Green, 1968, 1971; Meinhold and Buchholz, 1983). Recently, effects of IYD inhibition were demonstrated in the amphibian Xenopus laevis, where in vivo exposure to the known inhibitor 3-nitro-L-tyrosine (MNT) resulted in decreased TH synthesis and circulating TH, glandular histopathological changes, and, ultimately, altered metamorphic development (Olker et al., 2018). These adverse effects are dependent on available dietary and/or environmental iodine, as evidenced by mild phenotypes in humans with iodine-rich diets (Moreno et al., 2008; Moreno and Visser, 2010) and prevention or reversal of negative effects in chemical IYD inhibition studies with rats (Green 1971) and amphibians (Olker et al., 2018). Thus, IYD is a potential thyroid-relevant target for chemical disruption, which may be particularly critical for low iodine diets and environments.
Presented here are the development and application of a screening assay to assess susceptibility of IYD to chemical inhibition. The aims of this study were: 1) develop a robust 96-well plate-based in vitro assay amenable for screening chemicals for IYD inhibition, and 2) screen over 1,800 ToxCast chemicals to identify inhibitors of IYD. The IYD inhibition assay described herein provides an additional tool to screen chemicals for potential thyroid-disrupting activity and the screening results greatly expand the number of chemicals tested for IYD inhibition, with most chemicals (89%) producing little to no inhibition.
2. Materials and methods
Development of this IYD enzyme inhibition assay was based on a variety of previously published methods to detect IYD activity (Rosenberg, 1970; Rosenberg and Goswami, 1984; Renko et al., 2016). The assay was optimized for non-radioactive methods in a 96-well plate with a colorimetric readout that measures release of iodide from the substrate. The final assay used recombinant human IYD enzyme produced in a baculovirus system with insect cells, MIT as the substrate, NADPH as the reducing agent, and 3-nitro-L-tyrosine (MNT, CASRN: 621-44-3, purity >98%, Alfa Aesar, Tewksbury, Massachusetts, USA) as the model inhibitor (positive control). A set of pilot chemicals was used for assay development, with details and results included in Table 1. Screening of chemicals from the ToxCast libraries closely followed the tiered strategy described in Hornung et al. (2018) and Olker et al. (2019), with initial testing at a single concentration (200 μM) followed by concentration-response screening (0.032 – 200 μM) with a subset of chemicals. Single-concentration and concentration-response assay plate layouts are included in Supplementary Fig. 1.
Table 1.
Pilot Set of Chemicals used in Assay Development and Initial Testing for Inhibition of Iodotyrosine Deiodinase (IYD) Activity in Concentration-Response Mode, with Chemical Source, Reported Purity, Maximum Tested Concentration, Median Percent Inhibition (n=3) Produced at Maximum Concentration, and Absolute IC20 and IC50.
| Information from the Literature | Chemical | Sourceg | CASRN | Chemicalh Purity, % | Max Tested Conc., μM | Median % Inhibition at Max Conc. | IC20 (μM) | IC50 (μM) |
|---|---|---|---|---|---|---|---|---|
| Known or suspected inhibitors | 3-Nitro-L-tyrosine (MNT) a, b, c | AA | 621-44-3 | 99.2 | 200 | 100 | 0.01 | 0.04 |
| 3,5-L-Dibromotyrosine (DBT) a, d,e | TCI | 300-38-9 | 96.9 | 200 | 93 | 6.72 | 15.58 | |
| 3,5-L-Dinitrotyrosine (DNT) a, d | TCI | 502481-30-3 | 99.8 | 200 | 82 | 8.73 | 26.44 | |
| Phloxine B f | SA | 18472-87-2 | NA | 200 | 88 | 17.27 | 28.70 | |
| Triclosan f | SA | 3380-34-5 | 99.8 | 200 | 64 | 68.36 | 133.27 | |
| Bromoxynil f | ToxCast | 1689-84-5 | 98.2 | 200 | −5 | --i | -- | |
| Non-inhibitors | Bisphenol A e | SA | 80-05-7 | 99.9 | 200 | 4 | -- | -- |
| Dibutyl phthalate e | SA | 84-74-2 | 99.6 | 200 | −6 | -- | -- | |
| Genistein e | RPI | 446-72-0 | NA | 200 | −12 | -- | -- | |
Chemicals used for development of the screening assay from stocks on hand at EPA/ORD/CCTE/GLTED, sources: AA = Alfa Aesar, TCI=TCI America, SA = Sigma-Aldrich, RPI=Research Products International, ToxCast = received on previous chemical source plate of ToxCast chemicals;
Purity of chemical as reported by the vendor. Not available (NA) for phloxine B or genistein.
--The chemical did not produce an inhibition curve, or it was not possible to calculate an IC20 or IC50 because the curve did not reach 20% or 50% inhibition.
2.1. Chemicals
Test chemicals from the ToxCast chemical libraries (Richard et al., 2016) were obtained via the ToxCast program on 26 chemical source plates containing 1,951 samples with a total of 1,837 unique chemicals. This set included 293 ToxCast Phase 1_v2 (ph1v2), 750 ToxCast Phase 2 (ph2), and 776 ToxCast e1k chemicals, as well as 18 ToxCast Phase 3 (ph3) chemicals that were requested as additional compounds to include as space allowed on the chemical source plates. These test chemicals were supplied in 96-well plates with one chemical sample per well, each at a target stock concentration of 20 mM in dimethyl sulfoxide (DMSO). Quality control of the plated chemicals relied on requirements (e.g., identification, purity, analytical verification) of the ToxCast library (Richard et al., 2016). Plates were received with chemical identities masked until completion of the single-concentration screening. Actual plated concentrations were also provided at that point and, in some cases, these differed from 20 mM target. These differences were due to lower solubility of a chemical in DMSO or samples that were an oil or mixture (for which concentrations were provided in mg/ml). For 92% of the chemicals the concentration on the chemical source plates received was within 1 mM of the target 20 mM; however, 3 chemicals were provided at much higher concentration (100 mM), and 8 chemicals were provided at less than 1 mM. As described below under Assay quality/performance, 12 chemicals were identified with data flags as interfering with the assay; these compounds are listed in Supplementary Table 1 and were not included in data summaries or analyses. Thus, the final set of test chemicals included 1,825 unique chemicals tested in the initial single-concentration screening (listed in Supplementary Table 2, with maximum concentration tested).
Multiple control compounds were included on each test plate, including a known IYD inhibitor as the positive control, solvent controls as the negative control, and several chemicals that had previously been tested for IYD inhibition (see next paragraph on replicated chemicals). When chemical source plates were received, column 1 and several random wells had been left empty for the addition of positive and negative controls. MNT was selected for the positive control in this assay. MNT is a tyrosine derivative that has historically been used in in vivo and in vitro studies of IYD inhibition and produced the highest potency of known IYD inhibitors tested with this assay (see Initial Assay Development). MNT, however, is not a chemical of concern for human or environmental exposure. A MNT concentration-response curve was added to column 1 of each plate using a stock solution of 20 mM MNT that was prepared in 0.05 M NaOH and diluted to yield final test concentrations of 200, 1.0, 0.16, 0.075, 0.025, 0.005, and 0.0005 μM MNT, with 200 μM MNT serving as the completely inhibited control. These concentrations were selected to match the target of 200 μM for test chemicals while also obtaining a full concentration-response for MNT. Both 0.05 M NaOH and DMSO were used as solvent controls and were considered negative controls that reflected maximum IYD activity (no inhibition). Each plate contained the MNT concentration-response curve, 7 high concentration (200 μM final) MNT wells, 4 0.05 M NaOH solvent control wells, and 3 DMSO solvent control wells (see Supplementary Fig. 1 for example locations in 96-well format).
As a measure of intra-assay reproducibility, 10 chemicals were selected for replication across chemical source plates in single-concentration screening (Supplementary Fig. 2). These chemicals were identified from the published IYD studies and selected based on availability for plating on the chemical source plates. Tribromsalan (CASRN: 87-10-5), bithionol (CASRN: 97-18-7), triclosan (CASRN: 3380-34-5), and bromoxynil (CASRN: 1689-84-5) were selected as known or suspected IYD inhibitors based on results in Shimizu et al. (2013). Bisphenol A (CASRN: 80-05-7), dibutyl phthalate (CASRN: 84-74-2), acetochlor (CASRN: 34256-82-1), genistein (CASRN: 446-72-0), 4-nonylphenol (CASRN: 104-40-5), 2,2’,4,4’-tetrahydroxybenzophenone (CASRN: 131-55-5) selected as likely ‘inactive’ for inhibition of IYD enzyme activity based on producing no inhibition in Renko et al. (2016). These chemicals were included on the chemical source plates when received from the supplier, with three to seven of these chemicals per plate and identities masked.
Initial assay development and preliminary experiments were performed with a set of pilot chemicals identified from the literature (Table 1). This set included three nitrotyrosines with documented IYD inhibition in vitro (Green, 1968; Greer and Grimm, 1968; Renko et al., 2016; Solis-S et al., 2004) and experimental in vivo effects on the thyroid system consistent with IYD inhibition (Green, 1968, 1971; Meinhold and Buchholz, 1983; Olker et al., 2018). Additional pilot chemicals included three suspected inhibitors previously tested in Shimizu et al. (2013) and three suspected non-inhibitors that did not produce IYD inhibition in Renko et al. (2016). Nominal concentrations were used for these pilot chemicals and for MNT added to the test plates. Chemical reagents, other than pilot chemicals described here and the chemical test set described above, were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
2.2. Baculovirus Expression of Human Iodotyrosine Deiodinase
Human Iodotyrosine Deiodinase, based on GenBank BC056253.1 open reading frame, was synthesized (GenScript, Piscataway, New Jersey, USA). This sequence encodes the full length IYD gene and was optimized for synthesis and expression in insect cells using GenScript proprietary software. It was directionally cloned into the shuttle vector pVL1392 using Eco RI (5’) and Bam H1 (3’) linkers and sequenced. SF21 cells were propagated in shaker cultures in TNM-FH medium with 10% fetal bovine serum. Culture methods, conditions and pVL1392 are described in O’Reilly et al. (1992). IYD expression was controlled by the polyhedron promoter and recombinant baculovirus produced using AcVEPA parental DNA as described previously (Hartig et al., 1991, 1992). Stationary cultures produced the maximum protein yield. Therefore, 150 mM dishes were plated with 4.5 × 10E7 SF21 cells, allowed to attach for 2 h, medium replaced with 2.5 ml virus (3.8 X10E9 plaque forming units per ml), incubated 1 h and fed with 18 ml medium, incubated 48 h, scraped free, centrifuged (700xg 5 min), suspended in Hanks buffered salt solution and 7.5% DMSO to 5 X 10E6 cells/ml, and gently frozen with cell freezer. Cell lysates were kept at –80°C until use in the assay. This approach consistently produced sufficient yield and activity for a screening assay using cells that are readily available, adapted well to shaking water bath cultures, and contained no detectable endogenous IYD activity (data not shown).
Several batches of these IYD-containing cells were prepared, with some variability in protein concentration and specific activity between batches. A typical preparation contained about 1.8 mg/ml total protein, as determined with the Bradford assay using bovine serum albumin (BSA) as a standard (Sigma-Aldrich). In the screening of the first four plates, preps were diluted 2 to 3-fold with 0.1% BSA. The BSA was later found to be unnecessary, so dilutions were subsequently made with water instead. After dilution, the cell suspensions were briefly homogenized by sonication. The preparations were diluted to contain an amount of activity that would produce the optimum iodide release for a 15-min Sandell-Kolthoff (SK) reaction (see below) to result net change in absorbance of about 0.6 absorbance units after a 3-h chemical inhibition assay incubation. This was similar to a typical response generated from 60 pmoles iodide standards under similar conditions. Dilutions were adjusted for each batch to maintain similar uninhibited enzyme activity across all assay plates. A typical assay contained about 25 μg cell lysate protein per well. Under these conditions the specific activity was determined to be about 3 pmoles iodide per hour per μg of protein.
2.3. Assay for Inhibition of Iodotyrosine Deiodinase
2.3.1. Initial Assay Development.
Assay development was based on the work of Rosenberg and Goswami (1984), Renko et al. (2016), and our own experience with assays for DIO Types 1, 2, and 3 (Hornung et al., 2018; Olker et al., 2019). Initially, this work used a truncated form of purified IYD received as a gift from Steven Rokita (Johns Hopkins University). Ultimately, it was determined that this assay needed to use the native full-length form of the enzyme. In part, this was based on the fact that two well-known IYD inhibitors, mono- and dinitrotyrosine (MNT and DNT), could not be assayed in the presence of dithionite, which is a necessary reductant when using the truncated form of the enzyme (Watson, 2006). Numerous attempts at producing a purified soluble form of full-length IYD yielded enzyme that was not active in the presence of NADPH as the reductant. The native form of the enzyme is believed to require a separate reductase to be able to utilize NADPH as a reductant (Goswami and Rosenberg, 1977). Transient expression of full-length IYD into CHO and HEK293 cells produced the protein, but IYD activity could only be shown in HEK293 cells, which were thought to be able to express the reductase (Gnidehou et al., 2004). Fortunately, the preparations described here, from the insect cell line, seem to contain a satisfactory amount of reductase as it was possible to use NADPH as the reductant. Preliminary work showed that using MIT as the substrate produced a stronger signal than DIT. This preference is likely due to reported substrate inhibition observed with concentrations of DIT above 5 μM and a higher turnover number with MIT versus DIT (Rosenberg and Goswami, 1984).
The pilot plate of chemicals tested before screening ToxCast test chemicals included 9 chemicals [Table 1; di-bromotyrosine (DBT), bisphenol A, MNT, bromoxynil, DNT, genistein, dibutyl phthalate, phloxine-B, and triclosan] at final concentrations of 200, 100, 20, 4, 0.8, 0.16 and 0.032 μM. DBT, MNT, and DNT were dissolved in 0.05 M NaOH, with the rest of the compounds dissolved in DMSO. These seven concentrations were selected to match the target 200 μM for test chemicals on the high end, with serial dilution to obtain concentrations expected to produce full inhibition curves. Pilot chemicals were tested on three separate assay plates, with two sets of wells containing MNT and DBT on each plate, for n = 6 for each concentration of MNT and DBT and n = 3 for each concentration the other seven chemicals. The pilot plate showed that MNT was a much more effective inhibitor than DBT, which had previously been used as a model IYD inhibitor. Based on these results, the concentrations of MNT were adjusted downward to obtain full concentration-response curves in all subsequent testing. Consistent with the screening of the test plates, the plate with pilot chemicals showed little difference in the solvent controls.
2.3.2. Inhibition assay and iodide extraction
The assay development (described above) resulted in an IYD inhibition assay that was optimized for measuring IYD-liberated iodide with the SK reaction in a 96-well plate format using the native full length form of IYD, MIT as the substrate, NADPH as the reducing agent, and MNT as the model inhibitor (positive control). The cell lysate with expressed IYD was thawed, mixed, sonicated, and diluted in pH 7.4 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer. For convenience, the enzyme preparation and master mix were combined in amounts large enough for a full plate and then 111.25 μl of this combination was added with a multichannel pipet to each well of the 96-well assay plate (untreated polystyrene, 360 μl well volume, Corning, Corning, New York, USA). Each well contained 72.5 μl of diluted enzyme preparation (about 25 μg protein) and 38.75 μl of a master mix containing 0.323 M HEPES (pH 7.4), 0.645 M KCl, 96.8 μM FAD (flavin adenine dinucleotide), 32.3 μM MIT, and 0.161 M DTT (dithiothreitol), which resulted in 100 mM HEPES (pH 7.4), 200 mM KCl, 50 mM DTT, 30 μM FAD, and 10 μM MIT in the final assay conditions Then, 1.25 μl from the chemical source plate was added using a Liquidator 96–20 pipettor (Mettler-Toledo Rainin, LLC, Oakland, California, USA). Finally, the assay was initiated by adding 12.5 μl of 1 mM NADPH in 1% NaHCO3 with the liquidator pipet for a final assay volume of 125 μl in each well. The final target concentration of each chemical was 200 μM and 1.65% DMSO (1% DMSO from the chemical source plate plus 0.65% from the cell suspensions in enzyme production). This was the maximum testable target concentration while maintaining the chemical source plate contribution at 1% DMSO (given the target concentration of 20 mM on the chemical source plates). In the assay, the wells with 1% DMSO added did not produce any inhibition of enzyme activity. The assay plate was sealed with an adhesive cover sheet (Thermo Fisher Scientific, Waltham, Massachusetts, USA), mixed in a plate shaker, and incubated for 3 h at 37° C. Following the incubation, 75 μl were transferred to a 96-well, 2 ml polypropylene filtration plate (Biotage USA, Charlotte, North Carolina, USA) containing Dowex 50WX2 (Sigma-Aldrich) and the free iodide was eluted into a 96-well collection plate (Biotage USA) with application of 100 μl of 10% acetic acid.
The remaining steps through the SK reaction are the same as those described in Hornung et al. (2018) and Olker at al. (2019), except the 15-min reading during the SK reaction gave the best result for determining the change in absorbance at 420 nm (compared to the 10-min reading used in the DIO assays). Briefly, the SK reaction provides a colorimetric readout in which the rate of change from the yellow-colored cerium IV (Ce+4) to the colorless cerium III (Ce+3) in presence of arsenic (As+3) is dependent on the concentration of free iodide (Sandell and Kolthoff, 1937). The eluent (75 μl) from the wells of the collection plate was transferred to a new untreated polystyrene 96-well plate, where 75 μl of arsenic reagent [25 mM NaAsO2, 0.8 M NaCl, 0.5 M H2SO4] was added with the Liquidator 96–200 pipet and mixed well. Then, 75 μl of Ce+4 reagent [20 mM (NH4)4Ce(SO4)4.2H20, 0.44 M H2SO4] was added and the plate was immediately placed on the plate reader, where it was mixed on the fast setting for three seconds, and absorbance at 420 nm was read every minute at room temperature. Nearly all assay plates were processed with a Synergy 4 plate reader (BioTek Instruments, Inc., Winooski, Vermont, USA); however, this instrument had to be replaced and a Synergy Neo2 (BioTek Instruments, Inc.) was used for the final 3 concentration-response plates. No difference was found in the time course of the absorbance for the control chemicals on the plates processed with the new instrument.
2.4. Chemical screening with a tiered approach
Screening of chemicals followed the tiered strategy previously used for the iodothyronine deiodinases (Hornung et al., 2018; Olker et al., 2019). All chemicals were tested at a single target concentration of 200 μM. A subset of chemicals was further tested at seven concentrations (0.032 – 200 μM) to obtain a concentration-response curve and metrics of potency. Chemical source plates were thawed and test chemical mixed before use in the single-concentration screening. The model inhibitor (MNT), DMSO, and 0.05 N NaOH were added to empty wells on each plate, as described in Chemicals above. Each chemical source plate was tested on three individual assay plates on the same day, for n = 3 data points for each chemical. Data were normalized to percent of control, with solvents (DMSO, NaOH) representing maximal enzyme activity (0% inhibition) and 200 μM MNT as no enzyme activity (100% inhibition) as described in detail below in Data processing and analysis section. Calculations were based on this full range of inhibition, with % inhibition and absolute IC50s reported. In the single-concentration screening, chemicals that produced median inhibition of less than 20% were considered ‘inactive’. Chemicals that produced inhibition of 20% or greater were considered potential IYD inhibitors (hereafter called ‘IYD inhibitors’). The background variability of the maximal activity (in the solvent controls: DMSO, NaOH) supported the use of this 20% threshold; using a calculation similar to that in the ToxCast Analysis Pipeline (tcpl), the background variability was based on three times the median absolute deviation (MAD) of the solvent control(s), which was 17.8 across all replicates of all tested plates.
A subset of 154 chemicals were included in concentration-response screening. This set included 80 ToxCast test chemicals that produced 50% median inhibition or greater and all 10 of the replicated chemicals previous tested for IYD inhibition (6 ‘inactive’ and 4 that produced inhibition of 50% or greater). The 50% level of inhibition was selected for greater separation from the background variability and biological relevance. In addition, the following were included in concentration-response screening: 31 chemicals with high variability across replicates in single-concentration screening, 14 chemicals that produced less than 20% inhibition but were structurally similar to a compound producing greater than 30% inhibition, and 19 chemicals previously demonstrated to inhibit DIO Type 1, 2, or 3. For concentration-response screening, chemicals were removed from the original chemical plate and added to new 96-well polypropylene plates (Corning). Dilutions in DMSO were made in this new plate so that they could be tested at final target concentrations of 200, 100, 20, 4.0, 0.8, 0.16, and 0.032 μM. Each of the concentration-response chemical plates were also tested in three separate assay plates for n = 3 data points for each concentration of each chemical (see Supplementary Fig. 1 for example locations).
2.5. Data processing and analysis
Data were processed and analyzed using R (version 3.6.1; R Core Team 2019) with data from each plate processed through an automated pipeline to normalize data, calculate plate diagnostics, and assign assay-specific flags. The plate-wise normalization used the solvent controls (DMSO, NaOH) and the high concentration of the model inhibitor (MNT). First, change in absorbance between the 1- and 15-min readings was calculated for each well. Then net change in absorbance was calculated for each well by subtracting the background change in absorbance (mean of the seven completely inhibited reactions from the 200 μM MNT wells). The mean net change in absorbance of the solvent control reactions (4 from 0.05 M NaOH and 3 from DMSO) was used to represent the uninhibited reaction and used to normalize all data to percent of control. The difference between the 0.05 M NaOH and DMSO solvent controls was insignificant. For single-concentration screening, the median of the three replicates was calculated. Results are reported as percent inhibition (calculated as 100 minus the percent of control). For the pilot chemicals, model fitting of the concentration-response data was completed with the Analysis of Dose-Response Curves (drc) package version 3.0 (Ritz et al., 2015), using the three replicates for each concentration of a chemical, the 4-parameter log-logistic model, and calculation of absolute IC20, absolute IC50, and Hill slope, where appropriate.
Data from concentration-response screening of ToxCast test chemicals were analyzed with the ToxCast Analysis Pipeline (tcpl) package version 2.0.2 (Filer et al., 2017; Filer, 2019) using the tcplLite option to work with stand-alone csv files. For this analysis, percent inhibition was used as the response value, 20% inhibition as the threshold cutoff, and all replicates for each concentration of a chemical were included to fit dose-response curves based on three models (constant, constrained Hill, and constrained gain-loss model). The best model is identified based on lowest Akaike Information Criterion (AIC) value, and absolute IC20, absolute IC50, and Hill slope were calculated from the model fit parameters from the tcpl package for those chemicals that fit the robust Hill model. Concentration-response results are displayed as inhibition from maximum response, with chemical concentration (log-10 scale) on the x-axis and percent of control on the y-axis.
For the ToxCast ph1v2 and ph2 chemicals, a single predominant descriptive use category for each chemical was assigned following previously published approaches (Strickland et al., 2018; Iyer et al., 2019), which were based on the general use categories in the Chemical Products Categories (CPCat) database (Dionisio et al., 2015) that are available in the EPA’s CompTox Chemicals Dashboard (https:\\comptox.epa.gov/dashboard). Categories with less than 6 chemicals tested were combined into an ‘Other’ category. These categories were used for describing general patterns in chemicals tested, ‘inactive’ chemicals (producing less than 20% inhibition), and chemicals producing inhibition of IYD enzyme activity of 50% or greater.
2.5.1. Assay quality/performance
Quality control measures were calculated for each assay plate, closely following that described in Olker et al. (2019). Variability and separation between the positive control (200 μM MNT) and solvent controls (DMSO, NaOH) were evaluated with plate-wise median absolute deviations and plate-wise Z’ factors. The Z’ factor is typically used as an indication of assay quality, with values above 0.5 indicating good separation between the positive and negative controls (Zhang et al., 1999). Assay plates were typically re-run if quality criteria were not met (e.g., low Z’ factor, high variability of controls, unusual MNT standard curve). In several instances, plates with minor deviations (e.g., Z’ factor = 0.4) from acceptable quality were not re-run. For chemicals replicated across multiple single-concentration screening plates, median percent inhibition was compared across plates. The median values from the ToxCast ph2 plates were used in summaries except for dibutyl phthalate (not tested on ToxCast ph2 plates) for which the median values from the ToxCast e1k plates were used.
Three types of flags were applied to single data points and test chemical wells that fell outside of acceptable parameters. These flags included those described in Olker et al. (2019), with: 1) High variability flagged when the absolute difference between the mean and median of the three replicate runs was greater than 10%, or the range of the three replicate runs was greater than 30%); 2) Extreme values flagged when median percent of control was greater than 190% or less than –20%. 3) Potential issues with reagents or assay interference (four sub-types), flagged when the time course of absorbance was outside of that produced by the solvent and positive controls based on (a) change in absorbance was less than 0.1 absorbance units; (b) absorbance at one min was less than 85% of the solvent controls; (c) absorbance at 15 min was either less than 50% of the DMSO control or greater than 115% of the positive control; or (d) no change in absorbance (typically due to a well not receiving one or more reagent). Wells and chemicals with flags were manually reviewed and excluded, re-tested, or determined to be marginally acceptable (just outside of acceptability values, but usable for screening purposes). Chemicals with multiple replicates flagged based on the criteria in 2) and 3) above were considered potentially interfering with the assay. The 12 chemicals with evidence of assay interference based on these flags were excluded from summaries and analyses and are listed in Supplementary Table 1 with reason for exclusion. Further investigation of assay interference for each of these chemicals was beyond the scope of this study.
3. Results
3.1. Assay development, performance, and quality control
The development and optimization efforts resulted in an assay amenable for screening large chemical libraries for inhibition of the native full-length form of the human IYD enzyme using the reductant NADPH and the most potent documented inhibitor (MNT). To ensure the quality of the assay data, model inhibitor inhibition curves, Z’ factor, and variability of the positive and solvent controls were evaluated for each assay plate and summarized across all plates. The control chemicals and quality control metrics were consistent during multiple months of data collection across the triplicate testing of 42 chemical plates (one plate in assay development, 26 single-concentration plates, and 15 concentration-response plates). Inhibition curves for the positive control chemical (MNT) were consistent with mean IC50 of 0.035 (± 0.016 SD) and mean Hill slope of −1.089 (± 0.145 SD) (Supplementary Fig. 3). The assay had good dynamic range and acceptable variability in positive control and solvent controls, as indicated by Z’ factor that was generally 0.5–0.9 and mean Z’ factor above 0.5 for all assay plates, with the exception of three plates each with mean Z’ factor = 0.4. The plate-wise MADs for the solvent controls and model inhibitor (MNT) were 12% or less for most (93%) of the assay plates. In general, there was higher variability in the uninhibited wells (both DMSO and NaOH solvent controls, median MAD 3–13%) than in the fully inhibited wells (200 μM MNT, median MAD 2–10%).
The set of ten replicated chemicals used for intra-assay reproducibility consistently identified inhibitors of IYD across multiple plates (Supplementary Fig. 2). Six of the chemicals were considered ‘inactive’ producing IYD inhibition of <20% in all instances except for genistein, which produced median inhibition of 22% on one of the five plates that it was included on. The other four chemicals inhibited IYD enzyme activity by 40–100% with consistent categorization of chemicals as IYD inhibitors, but with some variable amount of inhibition. The largest variability in these replicated chemicals was in triclosan and tribromsalan, which may have been due to the different lots of chemicals used in testing on the ph1v2 plates (for triclosan) or the steep Hill curve when tested in concentration-response screening (tribromsalan).
3.2. Single-concentration screening
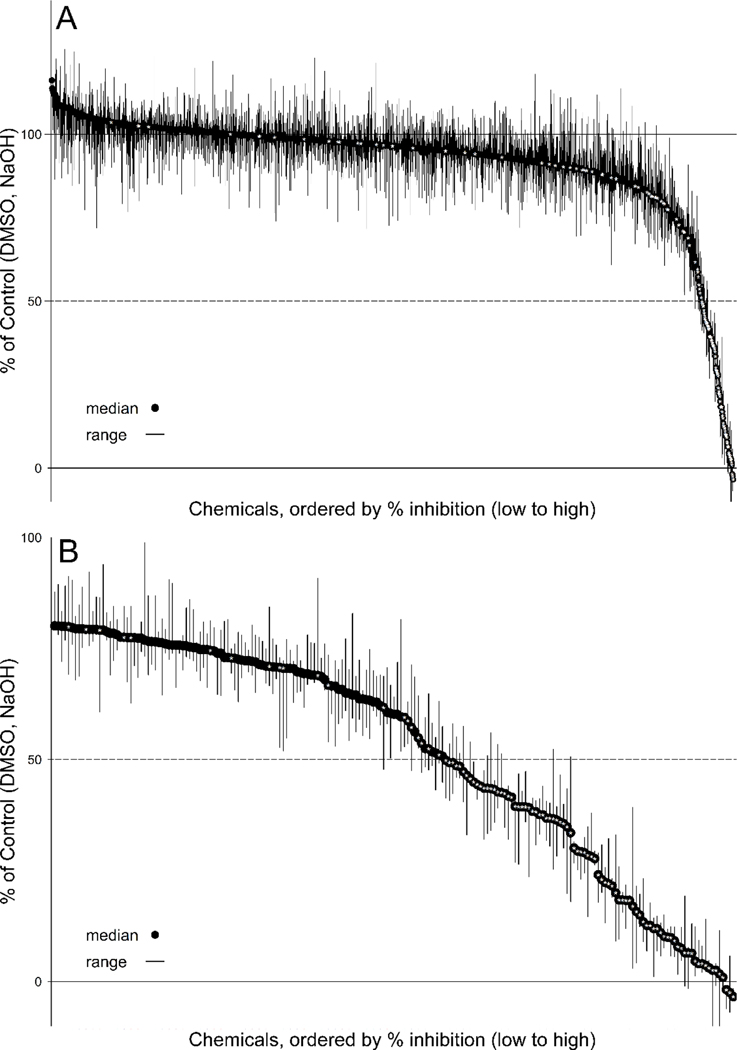
Reported here are the single-concentration screening results for a total of 1,825 chemicals at a single target concentration of 200 μM (as permissible with solubility). As described above in Assay quality/performance, 12 of the 1,837 chemicals received had evidence of assay interference and were excluded from the analysis and summaries (listed in Supplementary Table 1). The tested ToxCast chemicals produced a range of responses; however, most produced little to no inhibition of IYD enzyme activity (Table 2, Fig. 1), with 1,631 chemicals producing median inhibition of less than 20% compared with activity of the negative (solvent) controls. There were 194 chemicals (10.6%) that produced greater than 20% inhibition of IYD enzyme activity, of which 84 chemicals (4.6%) produced greater than 50% inhibition.
Table 2.
Single-Concentration Screening at Target Concentration of 200 μM, with Number and Percent of Chemicals in Each Set that Produced ≥ 20% Inhibition and ≥ 50% Inhibition.
| Chemicals producing ≥ 20%
inhibition |
Chemicals producing ≥ 50%
inhibition |
||||
|---|---|---|---|---|---|
| Chemical Set | No. chemicals tested a | No. | % | No. | % |
| Replicated Test Set b | 10 | 4 | 40.0% | 4 | 40.0% |
| ToxCast Phase 1_v2 | 286 | 24 | 8.4% | 1 | 0.3% |
| ToxCast Phase 2 | 760 | 68 | 8.9% | 29 | 3.8% |
| ToxCast e1k | 769 | 98 | 12.7% | 50 | 6.5% |
| Total | 1,825 | 194 | 10.6% | 84 | 4.6% |
Chemical source plates received from ToxCast included 1,837 unique chemicals, however 12 chemicals were excluded from results and summaries based on evidence of assay interference. Numbers of chemicals represent those included on plates for each requested chemical set, with the replicated test set chemicals excluded from the count. Note that the ToxCast Phase 2 (ph2) set included 16 chemicals from ToxCast Phase 3 (ph3); these additions were to replace chemicals no longer available.
Identified from the literature based on previous testing for IYD inhibition, including chemicals from ToxCast Phase 1_v2 (triclosan, dibutyl phthalate, bromoxynil, bisphenol A, acetochlor), ToxCast ph2 (genistein, 4-nonylphenol, 2,2’,4,4’-Tetrahydroxybenzophenone), and ToxCast ph3 (tribromsalan, bithionol).
Figure 1.
Inhibition of iodotyrosine deiodinase (IYD) enzyme activity produced by chemicals in single-concentration screening (ToxCast Phase 1_v2, Phase 2, and e1k chemical libraries plus the 10 chemicals in the replicated test set) at target concentration of 200 μM, displayed as % of control with median (black circle) and range (line) of three biological replicates. [A] All chemicals, [B] Expanded plot of chemicals that produced ≥20% inhibition. Chemicals are plotted by rank order based on median % inhibition in single-concentration screening, with those producing the greatest inhibition to the right. Chemicals further tested in concentration-response screening are indicated with white asterisk (*). The top 25 ranked chemicals based on absolute IC50 are shown in Table 3, with tested concentrations and median % inhibition values for each chemical in Supplementary Table 2.
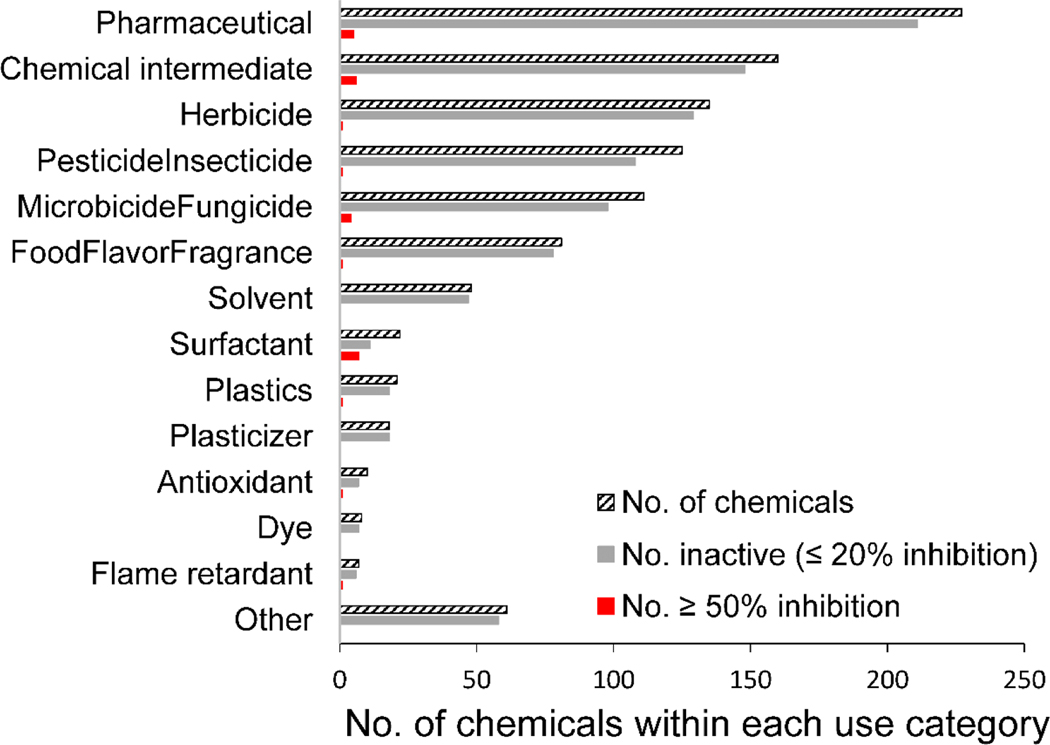
Of the tested chemicals, 1,034 had chemical use previously summarized to approximately 50 primary uses (Strickland et al., 2018). In order of number of chemicals tested (from largest to smallest), these primary uses include pharmaceutical, chemical intermediate, herbicide, pesticide/insecticide, microbicide/fungicide, food/flavor/fragrance, solvent, surfactant, plastics, plasticizers, antioxidant, dye, flame retardants, and a variety of other uses that each included 4 or fewer compounds (Fig. 2). Chemicals producing greater than 50% inhibition were found across multiple use categories, including the following with examples: pharmaceutical (troglitazone, UK-337312), chemical intermediate (1,2-dinitrobenzene, 4-heptylphenol), herbicide (diquat dibromide monohydrate), pesticide/insecticide (kepone), microbicide/fungicide (triclosan, fenaminosulf), food flavor/fragrance (tannic acid), surfactant (sodium dodecyl sulfate, dodecylbenzenesulfonic acid), plastics (4-octylphenol), antioxidant (2,4-di-tertbutylphenol), and flame retardant (3,3’,5,5’-tetrabromobisphenol A).
Figure 2.
Distribution of chemicals from ToxCast Phase 1_v2 and Phase 2 libraries screened in the iodotyrosine deiodinase enzyme assay by general use categories (diagonal stripes, n = 1,034) with ‘inactive’ chemicals from this set (≤ 20% inhibition, grey, n = 994) and chemicals that produced 50% inhibition or greater (red, n = 28) for each use category.
Single-concentration screening results are listed for all tested chemicals in Supplementary Table 2, including chemical name, DSSTox Substance ID, CASRN, maximum concentration tested, and the median percent inhibition.
3.3. Concentration-response screening
Concentration-response screening included a total of 154 chemicals, including the 84 chemicals that produced inhibition of 50% or greater as well as another 70 chemicals selected to check intra-assay consistency, verify results for chemicals that produced variable inhibition or other issues in single-concentration screening, and further investigate chemicals of interest with structural similarity to known inhibitors or activity in other assays (e.g., DIO assays). Concentration-response screening results are listed in Supplementary Table 3, including chemical name, DSSTox Substance ID, CASRN, maximum concentration tested, median percent inhibition produced at the maximum tested concentration, and Hill model fit parameters (Hill slope, absolute IC20, and absolute IC50, when applicable), with inhibition curves shown in Supplementary Fig. 4. A constant model (no change in activity across chemical concentration) was the best model fit in the ToxCast pipeline for 43 chemicals tested in concentration-response screening. The Hill model was identified as an acceptable model fit with tcpl for the other 111 chemicals, with the Hill model as the best fit for nearly all (based on AIC, with 2 chemicals for which the gain-loss model had a lower AIC score). Thus, Hill model parameters are reported for these chemicals.
The concentration-response and single-concentration results were compared for consistency in inhibition produced at the highest tested concentration and classification of a chemical as ‘inactive’ versus IYD inhibitor. For 96% (148/154) of the chemicals, the concentration-response confirmed the single-concentration screening results, with no change in classification. There were six chemicals for which the results differed by more than 25% with a change in classification from inhibitor to ‘inactive’ (3 chemicals: acid orange 156, linolenic acid, FR167356) or from ‘inactive’ to inhibitor (3 chemicals: allura red CI1603, 4,5-dichloro-2-octyl-3(2H)-isothiazolone, and 4-dodecylmorpholine). These few chemicals represent the possibility of false positives and false negatives; hence, it is worth noting that they produced moderate inhibition at most (no more than 51%) with IC50 values of greater than 200 μM in concentration-response screening. These chemicals also highlight the variability observed with no enzyme inhibition (as seen in the solvent controls) and low-moderate inhibition in this assay. This variability could be due to heterogeneity of the enzyme preparations and/or variability in enzyme activity within the IYD-containing cells used as the enzyme source. There were other instances where the single-concentration and concentration-response screening results differed by greater than 25%; however, these differences did not change the classification of each chemical and, in general, the concentration-response screening was more sensitive (greater inhibition at the same maximum concentration).
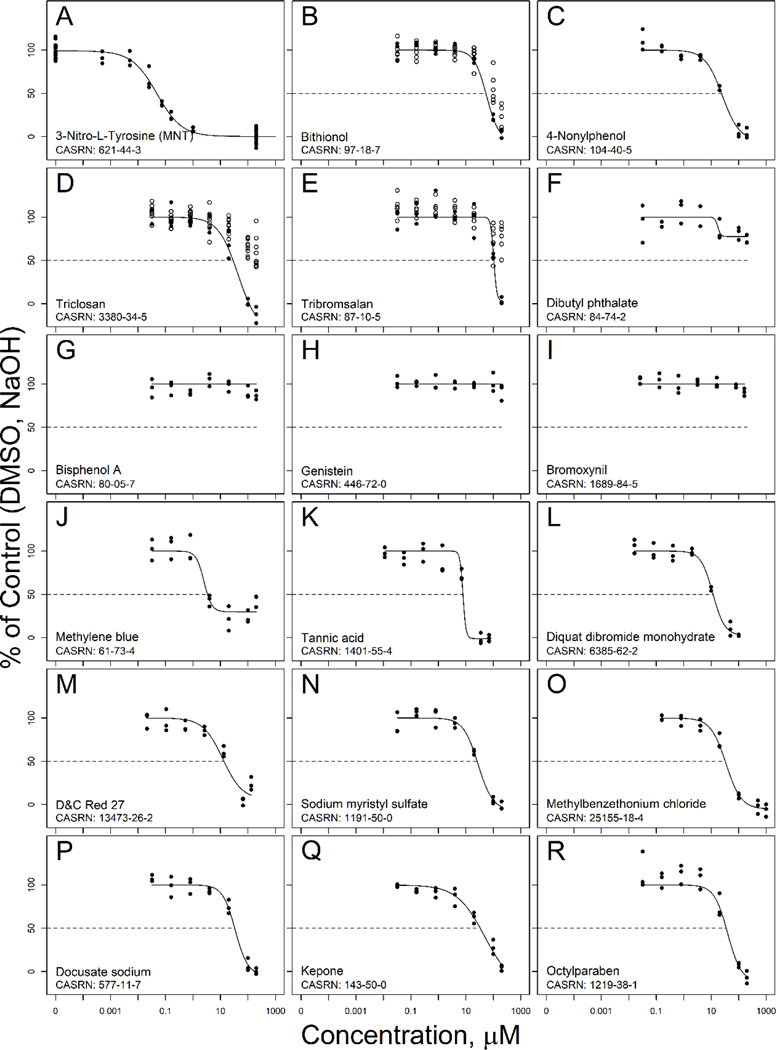
The 25 most potent ranked chemicals, based on absolute IC50, are included in Table 3 along with the mean values for the model inhibitor MNT, with inhibition curves for 10 compounds shown in Fig. 3. None of the tested chemicals inhibited IYD enzyme activity with greater potency than MNT. The previously documented inhibitors of IYD (methylene blue, triclosan, bithionol, and tribromsalan) had IC50 values of 3.4, 28.7, 53.8, and 105.0 μM, respectively; which are two or more orders of magnitude higher than the IC50 of 0.035 μM for MNT. Several of the ToxCast test chemicals had similar or higher potency than these previously documented IYD inhibitors, with tannic acid and diquat dibromide monohydrate producing IC50 values of 7.8 and 11.7 μM, respectively, which are still 200 to 300 times higher than the IC50 for MNT.
Table 3.
The Top 25 Ranked ToxCast Chemicals for Inhibition of Iodotyrosine Deiodinase (IYD), Based on Absolute IC50.
| Single-Conc. |
Concentration-Response |
|||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Chemical | CASRN | Max Tested Conc., μM | Median % Inhibition a | % Inhibition at Max Conc. b | IC20 (μM) | IC50 (μM) | |
| - | 3-Nitro-L-tyrosine c | 621-44-3 | 200 | NA d | 100 | 0.01 | 0.04 | |
| 1 | Methylene blue | 61-73-4 | 200 | 54 | 53 | 1.9 | 3.4 | |
| 2 | Tannic acid | 1401-55-4 | 70 | 92 | 101 | 6.6 | 7.8 | |
| 3 | Diquat dibromide monohydrate | 6385-62-2 | 100 | 90 | 98 | 5.8 | 11.7 | |
| 4 | D&C Red 27 | 13473-26-2 | 132 | 78 | 78 | 4.1 | 13.4 | |
| 5 | Dodecylphenol | 27193-86-8 | 200 | 97 | 100 | 11.6 | 20.1 | |
| 6 | Lauryl gallate | 1166-52-5 | 200 | 87 | 107 | 11.9 | 22.3 | |
| 7 | 4-Dodecylphenol | 104-43-8 | 200 | 90 | 99 | 6.9 | 23.1 | |
| 8 | 4-Nonylphenol | 104-40-5 | 200 | 80 | 98 | 8.9 | 23.6 | |
| 9 | Dinocap | 39300-45-3 | 200 | 88 | 98 | 19.8 | 23.8 | |
| 10 | Hexadecyltrimethylammonium bromide | 57-09-0 | 200 | 97 | 104 | 20.7 | 24.7 | |
| 11 | Sodium myristyl sulfate | 1191-50-0 | 200 | 82 | 104 | 11.3 | 25.7 | |
| 12 | Calcium dodecylbenzene sulfonate | 26264-06-2 | 200 | 102 | 105 | 17.1 | 26.0 | |
| 13 | Methyltrioctylammonium chloride | 5137-55-3 | 200 | 96 | 101 | 7.7 | 26.1 | |
| 14 | Triclosan | 3380-34-5 | 200 | 79 | 112 | 10.4 | 28.7 | |
| 15 | 2,2’-Methylenebis(4methyl-6-tertbutylphenol) | 119-47-1 | 200 | 72 | 92 | 7.7 | 28.9 | |
| 16 | Sodium hexyldecyl sulfate | 1120-01-0 | 100 | 58 | 102 | 14.5 | 29.5 | |
| 17 | Sodium tridecyl sulfate | 3026-63-9 | 200 | 95 | 107 | 16.1 | 31.7 | |
| 18 | Sodium dodecylbenzenesulfonate | 25155-30-0 | 200 | 90 | 107 | 17.8 | 32.1 | |
| 19 | Methylbenzethonium chloride | 25155-18-4 | 1000 | 103 | 105 | 13.6 | 32.2 | |
| 20 | Docusate sodium | 577-11-7 | 200 | 94 | 100 | 16.2 | 33.0 | |
| 21 | Kepone | 143-50-0 | 200 | 56 | 95 | 7.6 | 33.4 | |
| 22 | Oleic acid | 112-80-1 | 200 | 87 | 89 | 21.3 | 34.1 | |
| 23 | Dodecylbenzenesulfonic acid | 27176-87-0 | 200 | 88 | 102 | 20.1 | 34.5 | |
| 24 | Octylparaben | 1219-38-1 | 200 | 82 | 107 | 16.8 | 35.2 | |
| 25 | C.I. Acid Red 114 | 6459-94-5 | 140 | 99 | 103 | 18.0 | 36.9 | |
Median of three replicates in single concentration screening (at maximum concentration)
Median of three replicates at maximum concentration of chemical in concentration-response screening; in three chemicals (Methylene blue, Tannic Acid, and D&C Red 27) concentrations below the maximum tested concentration produced greater inhibition (see Fig. 3).
MNT, known IYD inhibitor included as positive control in concentrationresponse on every assay plate, with 41 plates each tested in triplicate. Mean IC20 and IC50 values across all plate reported in table. IC50 values ranged from 0.012 to 0.076 μM with mean = 0.035 μM and SD = 0.016.
NA, not applicable
Figure 3.
Concentration-response curves for inhibition of iodotyrosine deiodinase enzyme activity by 18 compounds, tested at seven concentrations with three replicates at each concentration (black circles), Hill model fit (black line), and testing on additional plates for some compounds (open circles); examples include the model inhibitor (A), 6 of the replicated test chemicals (B-I), and 9 of the top 25 ranked inhibitors (J-R), selected from Table 3 to represent a variety across types of compounds and inhibition curves.
3.4. Chemicals flagged for potential assay interference
There were 12 chemicals that were flagged as potentially interfering based on assay results falling outside of acceptable parameters as described above (see Materials and Methods: Assay quality/performance). These chemicals are listed in Supplementary Table 1 and include several chemicals known to interfere with the SK reaction (e.g., thiocyanate, Sandell and Kolthoff, 1937) as well as multiple iodine-containing chemicals that may contain free iodide in the chemical solution or may be substrates for deiodinases (e.g., iopanoic acid, Renko et al., 2012). All remaining iodinated chemicals were manually reviewed (even in the absence of automatic flags used to indicate assay interference) and were found to meet acceptable parameters with time course of absorbance data within the expected ranges of the positive and negative controls. Potentially interfering chemicals were considered unsuitable for testing with this assay and excluded from summaries.
4. Discussion
Presented here is the development and application of a new screening assay for inhibition of the iodine recycling enzyme, IYD, which has an important role in maintaining sufficient free iodide for TH synthesis. Through a tiered approach to screen 1,825 chemicals from the ToxCast chemical libraries, 194 IYD inhibitors were identified, of which 84 chemicals produced inhibition of 50% or greater. These 84 inhibitors include 4 chemicals that had previously been documented to inhibit IYD enzyme activity (triclosan, bithionol, tribromsalan, methylene blue), with the rest not previously reported as IYD inhibitors, to the best of our knowledge.
Recombinant human IYD was successfully produced in the native full-length form using the baculovirus system with insect (SF21) cells. This approach produced sufficient enzyme yield and activity for development of a screening assay that uses small volumes and a colorimetric readout. Using the full-length form of the enzyme allowed use of the native reducing agent NADPH rather than the artificial reductant dithionite, which has been shown to react with the nitro groups with several known IYD inhibitors (MNT, DNT) and may interfere with assaying test chemicals. With this assay, chemicals were screened for direct inhibition of IYD as well as interaction with the required separate reductase responsible for transferring reducing equivalents from NADPH to IYD, thus identifying chemicals that reduced IYD activity through effects on this unknown reductase that would be missed when using dithionite to drive dehalogenation.
Across an extensive screening effort, this assay produced reliable responses in positive and negative controls, consistent inhibition curves for the model inhibitor, and high Z’ factors, indicating suitability for high-throughput screening. There is limited research on IYD, with few chemicals previously tested and no established reference chemicals. Previous studies were limited to three nitrotyrosines studied with in vivo rodent experiments as well as mammalian thyroid gland slices and tissue homogenates (Green, 1968, 1971; Greer and Grimm, 1968; Meinhold and Buchholz, 1983; Solis-S et al., 2004), and recent in vitro assays that tested 12 suspected endocrine disrupting compounds (Renko et al., 2016) and 44 halogenated compounds (Shimizu et al., 2013).
Overall, the assay results presented here are consistent with previous reports IYD inhibition. The three nitrotyrosines (MNT, DBT, and DNT) were strong IYD inhibitors, with MNT more potent than DBT (see Initial Assay Development). The five chemicals that were also tested by Shimizu et al. (2013) matched rank order potency, with phloxine B as the most potent, followed by triclosan, bithionol, tribromsalan, and bromoxynil. In fact, IC20 values were very close for triclosan (10 versus 19 μM), bithionol (27 μM in both studies), and tribromsalan (88 versus 66 μM) on the chemical source plates. Bromoxynil differed, with less than 8% inhibition produced across multiple plates at 160 μM (Supplementary Fig. 2) compared to moderate inhibition in Shimizu et al. (2013) when tested at a higher concentration (600 μM). Results can also be compared for six chemicals tested in both this assay and Renko et al. (2016). Dibutyl phthalate, bisphenol A, genistein, acetochlor, and 2,2’,4,4’-tetrahydroxybenzophenone matched results from Renko et al. (2016), with little to no IYD inhibition. Unexpectedly, 4-nonylphenol produced 60–90% inhibition across multiple plates while it was documented as ‘inactive’ by Renko et al. (2016). Closer comparison, however, revealed no contradiction between studies. In Renko et al. (2016), chemicals were tested only at 10 μM and, based on concentration-response testing reported here, 4-nonylphenol at a comparable concentration would produce enzyme inhibition of 20% or less (Supplementary Fig. 4).
The screening of 1,825 chemicals reported here is the most comprehensive testing for inhibition of IYD to date, greatly expanding the chemical space screened for IYD inhibition. The ToxCast chemical libraries screened here include ph1v2 with over 200 pesticides and several other compounds of regulatory interest (e.g., PFOS), ph2 with nearly 750 environmental, industrial, and pharmaceutical compounds, and e1k with over 750 compounds primarily selected based on their known or suspected activity towards estrogen or androgen receptors (Richard et al., 2016). This diverse set of compounds includes hundreds of chemical use categories, for which the ToxCast ph1v2 and ph2 compounds were previously summarized to approximately 50 primary uses (Fig. 2) (Strickland et al., 2018). Most chemicals in each of these primary use categories were considered inactive. Chemicals producing IYD inhibition of 50% or greater were in multiple use categories, suggesting a range of possible chemical types that could perturb this molecular target. Thus, further research could investigate the physiochemical properties and structural features associated with IYD inhibition.
Interpretation of all in vitro screening assays requires acknowledgement of assay limitations and uncertainties, as previously described in detail (Judson et al., 2013, 2016; Thorne et al., 2010; McGovern et al., 2002, 2003; Shoichet, 2006). Here, potential assay interference was identified by flagging data points that failed to meet quality control parameters; however, some sources of interference and nonspecific chemical activity could have been missed. For example, surfactants may disrupt the test system or produce nonspecific enzyme inhibition via protein denaturation. Surfactants had a higher relative frequency (17/32) of producing greater than 50% inhibition than other primary use categories (Fig. 2). In addition, 7 of the top 25 ranked inhibitors were identified as surfactants, suggesting that surfactants may be inaccurately identified as IYD inhibitors due to nonspecific enzyme inhibition. However, many other surfactants did not produce inhibition greater than 20%, and one that produced IYD inhibition (4-nonylphenol) has published in vivo studies suggesting thyroid disruption (Christensen et al., 2005; He et al., 2019; Shirdel and Kalbassi, 2016; Wang et al., 2019b; Xi et al., 2013). It must be recognized that some chemicals (e.g., surfactants, redox active substances) could inhibit the reduction of IYD, which is not distinguished in this assay from those that directly inhibit IYD enzyme activity. This could be further explored for chemicals of interest through comparing results with NADPH and dithionite each used as the reducing agent for the full-length native enzyme.
Caution must also be used when interpreting in vitro results to evaluate potential in vivo effects. Pathway-based predictive models are necessary to link molecular targets on the HPT axis to downstream biochemical responses and, ultimately, adverse outcomes for organisms (Noyes et al., 2019). While recent progress has been made in this regard for TPO in mammalian and amphibian models (Haselman et al., 2020; Hassan et al., 2020), data are limited for IYD. Adverse organismal outcomes have only been evaluated with human congenital hypothyroidism due to IYD mutation (Medeiros-Neto and Stanbury, 1994; Moreno et al., 2008; Afink et al., 2008; Moreno and Visser 2010) and in a few IYD inhibition studies with nitrotyrosines (Green 1971; Olker et al., 2018).
Chemicals producing the maximum inhibition and highest potency are of most interest for potential thyroid effects in vivo. For the top 25 ranked chemicals, very little information on thyroid-related endpoints could be found in the literature, and only methylene blue and triclosan were previously reported as IYD inhibitors. Methylene blue, the most potent tested ToxCast chemical, was shown in Bastomsky and Rosenberg (1966) to inhibit deiodination of DIT in calf thyroid slices and rat thyroid homogenates. In addition, several mammalian studies have shown methylene blue to interfere with binding to the TH nuclear receptors and alter circulating THs in rats after in vivo dietary exposure (Brtko et al., 1997; Nedvidkova et al., 1995) and interfere with the actions of TSH in isolated mouse thyroid lobes (Hashizume et al., 1975). Disruption of thyroid endpoints by triclosan has been studied in multiple in vivo and in vitro studies (as reviewed in Dann and Hontela, 2011; Johnson et al., 2016; Mihaich et al., 2017), with evidence of thyroid disruption reported in some species. For example, in vivo exposure to triclosan has been shown to reduce circulating THs in rodents (Crofton et al., 2007; Stoker et al., 2010; Zhang et al., 2018). Using in vitro studies, triclosan was reported as active against several thyroid-relevant targets, including inhibition of IYD enzyme activity (Shimizu et al., 2013), binding to TTR (Cavanaugh et al., 2018), inhibition of NIS-mediated iodide uptake (Wu et al., 2016), and weak inhibition of TPO enzyme activity (Butt et al., 2011; Paul et al., 2014; Wu et al., 2016). Another compound, D&C Red 27 is closely related to the food colorant phloxine B (D&C Red 28) that was a potent IYD inhibitor in Shimizu et al. (2013), yet no other published literature indicating thyroid disruption was found for either of these colorants.
Publications related to thyroid disruption were found for three other compounds in the top 25 ranked IYD inhibitors: 4-nonylpenol, kepone, and oleic acid. For 4-nonylphenol, a surfactant documented to bind to the estrogen receptor (Routledge and Sumpter, 1997), in vivo exposure has resulted in thyroid effects including altered levels of circulating THs in rodents (He et al., 2019; Wang et al., 2019b; Xi et al., 2013) and fish (Naderi et al., 2015; Shirdel and Kalbassi, 2016). There are a few studies in the literature on thyroid disruption associated with exposure to kepone, including associations between TH and peri- and post-natal exposure in humans (Cordier et al., 2015) and altered circulating THs in adult rare minnow (Gobiocypris rarus) after long-term exposure (Yang et al., 2020). Oleic acid, a naturally occurring fatty acid, has been studied for a variety of thyroid effects with evidence of interference with TH binding to transport proteins (Lim et al., 1995; Herrmann et al., 1985) and nuclear receptors (Fiona et al., 1991; Inoue et al., 1989; Romo et al., 1997). No other reported in vivo thyroid effects were found for the other 20 compounds in the top 25 ranked IYD inhibitors, despite significant effort put into a systematic search through multiple literature search engines (PubMed, Web of Science, Science Direct, ECOTOX).
In summary, the development and optimization of this 96-well plate screening assay for inhibition of IYD enzyme activity has been successfully demonstrated and is a significant contribution to the expansion of available thyroid-relevant in vitro assays for identification and prioritization of chemicals that could negatively affect TH synthesis and signaling. The application of this assay to screen large chemical libraries greatly expands the understanding of susceptibility of IYD to chemical inhibition and provides relevant data for development and evaluation of structure-activity relationships. Importantly, most of the tested ToxCast chemicals (89%) were classified as ‘inactive’, producing less than 20% inhibition at the highest tested concentration. Thus, there is a much smaller list of chemicals for which further testing is warranted to understand potential effects on THs and translation to adverse in vivo effects.
Supplementary Material
Acknowledgements:
The authors thank Joseph O’Flanagan (ORAU Student Services Contractor to the U.S. EPA) and Phillip Degoey (U.S. EPA, Center for Computational Toxicology and Exposure) for laboratory technical support for screening chemicals. We also thank Dr. Steve Rokita (Johns Hopkins University) for sharing his expertise on IYD and the gift of purified IYD enzyme for initial assay development; Dr. Ann Richard, Katherine Coutros, and Dr. Chris Grulke (U.S. EPA, Center for Computational Toxicology and Exposure) for their valuable role in obtaining the ToxCast chemical libraries for screening; and Dr. Katie Paul Friedman (U.S. EPA, Center for Computational Toxicology and Exposure) for guidance on ToxCast pipeline data analysis. Additionally, we acknowledge Dr. Paul Friedman and Drs. Tammy Stoker, Susan Laws, and Jun Wang (U.S. EPA Center for Public Health and Environmental Assessment) for providing comments and suggestions on an earlier draft of the manuscript. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency, nor does the mention of trade names or commercial products indicate endorsement by the federal government.
Funding Information: This work was supported by the U.S. Environmental Protection Agency.
Abbreviations:
- BSA
bovine serum albumin
- DBT
3,5-L-dibromotyrosine
- DIO
iodothyronine deiodinase
- DIT
diiodotyrosine
- DMSO
dimethyl sulfoxide
- DNT
3,5-L-dinitrotyrosine
- DTT
dithiothreitol
- e1k
ToxCast e1k chemical library
- FAD
flavin adenine dinucleotide
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IYD
iodotyrosine deiodinase
- MAD
median absolute deviation
- MCT8
monocarboxylate transporter 8
- MIT
monoiodotyrosine
- MNT
3-nitro-L-tyrosine
- NADPH
β-nicotinamide adenine dinucleotide 2′-phosphate (reduced form)
- NIS
sodium-iodide symporter
- ph1v2
ToxCast Phase 1_v2 chemical library
- ph2
ToxCast Phase 2 chemical library
- ph3
ToxCast Phase 3 chemical library
- SK
Sandell-Kolthoff
- T4
thyroxine tcpl, ToxCast Analysis Pipeline (R package)
- TH
thyroid hormone
- TPO
thyroperoxidase
- TSH
thyroid-stimulating hormone
- TTR
transthyretin
Footnotes
Supplementary Data: Appendix A includes Supplementary Materials in the form of supplementary tables and figures with results for all tested chemicals.
Data available at: https://edg.epa.gov/metadata/catalog/main/home.page
Conflict of Interest: The authors claim no conflicts of interest.
Disclaimer: The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References.
- Afink G, Kulik W, Overmars H de Randamie J, Veenboer T, van Cruchten A, Craen M, Ris-Stalpers C, 2008. Molecular characterization of iodotyrosine dehalogenase deficiency in patients with hypothyroidism. J. Clin. Endocrinol. Meta 93, 4894–4901. 10.1210/jc.2008-0865. [DOI] [PubMed] [Google Scholar]
- Bastomsky CH, Rosenberg IN, 1966. Inhibition of thyroidal deiodination of diiodotyrosine by compounds which enhance NADPH oxidation. Endocrinol. 79:505–510. 10.1210/endo-79-3-505. [DOI] [PubMed] [Google Scholar]
- Boas M, Felt-Fasmussenn U, Main KM, 2012. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol 355, 240–248. 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Brtko J, Nedvidková J, Haluzik M, Schreiber V, 1997. Comparison of in vivo long - term treatment of rats by methylene blue with its in vitro effects on thyroid hormone - nuclear receptor complex formation in liver. Endocr. Res, 23, 157–165. 10.3109/07435809709031850. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, 1998. Effects of environmental synthetic chemicals on thyroid function. Thyroid 8, 827–856. 10.1089/thy.1998.8.827. [DOI] [PubMed] [Google Scholar]
- Buckalew AR, Wang J, Murr AS, Deisenroth C, Stewart VM, Stoker TE, Laws SC, 2020. Evaluation of potential sodium-iodide symporter (NIS) inhibitors using a secondary Fischer rat thyroid follicular cell (FRTL-5) radioactive iodide uptake (RAIU) assay. Arch. Toxicol 94, 873–885. 10.1007/s00204-020-02664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Wang D, Stapleton HM, 2011. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol. Sci 124, 339–347. 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JE, Trought K, Mitchell C, Northcott G, Tremblay LA, 2018. Assessment of endocrine disruption and oxidative potential of bisphenol-A, triclosan, nonylphenol, diethylhexyl phthalate, galaxolide, and carbamazepine, common contaminants of municipal biosolids. Toxiocol. In Vitro 48, 342–349. 10.1016/j.tiv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Christensen JR, Richardson JS, Bishop CA, Pauli B, Elliott J, 2005. Effects of nonylphenol on rates of tail resorption and metamorphosis in Rana catesbeiana tadpoles. J. Toxicol. Environ. Health, Part A 68, 557–572. 10.1080/15287390590909698. [DOI] [PubMed] [Google Scholar]
- Cordier S, Bouquet E, Warembourg C, Massart C, Rouget F, Kadhel P, Bataille H, Monfort C, Boucher O, Muckle G, Multigner L, 2015. Perinatal exposure to chlordecone, thyroid hormone status and neurodevelopment in infants: the Timoun cohort study in Guadeloupe (French West Indies). Environ Res. 138, 271–278. 10.1016/j.envres.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Paul KB, DeVito MJ, Hedge JM, 2007. Short-term in vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol 24, 194–197. 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Dann AB, Hontela A, 2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol 31, 285–311. 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- DeVito M, Biegel L, Brouwer A, Brown S, Brucker-Davis F, Oliver Cheek A, Christensen R, Colborn T, Cooke P, Crissman J, et al. , 1999. Screening methods for thyroid hormone disruptors. Environ. Health Persp 107, 407–415. 10.1289/ehp.99107407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Phillips K, Price PS, Grulke CM, Williams A, Biryol D, Hong T, Isaacs KK, 2015. The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 5:180125. 10.1038/sdata.2018.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wade MG, 2017. Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8. Toxicol. In Vitro 40, 234–242. 10.1016/j.tiv.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Dong H, Godlewska M, Wade MG, 2020. A rapid assay of human thyroid peroxidase activity. Toxicol. In Vitro 62, 104662. 10.1016/j.tiv.2019.104662. [DOI] [PubMed] [Google Scholar]
- EU (European Union)., 2017. Supporting the Organisation of a Workshop on Thyroid Disruption – Final Report. European Commission, Framework Contract ENV.A.3/FRA/2014/0029 on implementation of the Community strategy on Endocrine Disrupters. https://op.europa.eu/en/publication-detail/-/publication/472d2c88-a8b1-11e7-837e-01aa75ed71a1 (accessed 29 June 2020).
- Filer DL, 2019. tcpl: ToxCast Data Analysis Pipeline. R package version 2.0.2. https://CRAN.R-project.org/package=tcpl. (accessed 1 April 2020).
- Filer DL, Kothiya P, Setzer RW, Judson RS, Martin MT, 2017. tcpl: the ToxCast pipeline for high-throughput screening data. Bioinformatics 33, 618–620. 10.1093/bioinformatics/btw680. [DOI] [PubMed] [Google Scholar]
- Fiona RM van der Klis, Schmidt EDL, van Beeren HC, Wiersinga WM, 1991. Competitive inhibition of T3 binding to αl and β1 thyroid hormone receptors by fatty acids. Biochem. Biophys. Res. Comm 179, 1011–1016. 10.1016/0006-291x(91)91919-4. [DOI] [PubMed] [Google Scholar]
- Friedman JE, Watson JA, Lam DW-H, Rokita SE, 2006. Iodotyrosine deiodinase in the first mammalian member of the NADH oxidase/Flavin reductase superfamily. J. Biol. Chem 281, 2812–2819. 10.1074/jbc.m510365200. [DOI] [PubMed] [Google Scholar]
- Gnidehou S, Caillou B, Talbot M, Ohayon R, Kaniewski J, Noel-Hudson M-S, Morand S, Agnangji D, Sezan A, Courtin F, Virion A, Dupuy C, 2004. Iodotyrosine dehalogenase 1 (DEHAL1) is a transmembrane protein involved in the recycling of iodide close to the thyroglobulin iodination site. FASEB J. 18, 1574–1576. 10.1096/fj.04-2023fje. [DOI] [PubMed] [Google Scholar]
- Gnidehou S, Lacroix L, Sezan A, Ohayon R, Noel-Hudson M-S, Morand S, Francon J, Courtin F, Virion A, Dupuy C, 2006. Cloning and characterization of a novel isoform of iodotyrosine deiodinase 1 (DEHAL1) DEHAL1C from human thyroid: comparisons with DEHAL1 and DEHAL1B. Thyroid 16, 715–724. 10.1089/thy.2006.16.715. [DOI] [PubMed] [Google Scholar]
- Goswami A, Rosenberg IN, 1977. Studies on a Soluble Thyroid Iodotyrosine Deiodinase: activation by NADPH and Electron Carriers. Endocrinol. 101: 331–341. 10.1210/endo-101-2-331. [DOI] [PubMed] [Google Scholar]
- Green WL, 1968. Inhibition of thyroidal iodotyrosine deiodination by tyrosine analogues. Endocrinol. 83, 336–347. 10.1210/endo-83-2-336. [DOI] [PubMed] [Google Scholar]
- Green WL, 1971. Effects of 3-nitro-L-tyrosine on thyroid function in the rat: an experimental model for the dehalogenase defect. J. Clin. Invest 50, 2472–2484. 10.1172/JCI106748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer MA, Grimm Y, 1968. Changes in thyroid secretion produced by inhibition of iodotyrosine deiodinase. Endocrinol. 83, 405–410. 10.1210/endo-83-3-405. [DOI] [PubMed] [Google Scholar]
- Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Stoker TE, Laws SC, 2017. Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS). Toxicol. In Vitro 40, 66–78. 10.1016/j.tiv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Hartig PC, Cardon MC, 1992. Rapid efficient production of baculovirus expression vectors. J. of Virol. Meth 38:61–70. 10.1016/0166-0934(92)90169-E. [DOI] [PubMed] [Google Scholar]
- Hartig PC, Cardon MC, Kawanishi CY, 1991. Generation of recombinant baculovirus via liposome mediated transfection. BioTechniques 11: 310–313. [PubMed] [Google Scholar]
- Haselman JT, Olker JH, Kosian PA, Korte JJ, Swintek JA, Denny JS, Nichols JW, Tietge JE, Hornung MW, Degitz SJ, 2020. Targeted pathway-based in vivo testing using thyroperoxidase inhibition to evaluate plasma thyroxine as a surrogate metric of metamorphic success in model amphibian Xenopus laevis. Toxicol. Sci 175:236–250. 10.1093/toxsci/kfaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Onaya T, Sato A, 1975. The role of the pentose phosphate shunt in thyrotropin-induced thyroid hormone secretion: in vivo and in vitro studies with 6-aminonicotinamide in mouse thyroids. Endocrinol. 97, 962–968. 10.1210/endo-97-4-962. [DOI] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Ford J, Brennan A, Handa S, Paul Friedman K, and Gilbert ME, 2020. Extrapolating in vitro screening assay data for thyroperoxidase inhibition to predict serum thyroid hormones in the rat. Toxicol. Sci 173, 280–292. 10.1093/toxsci/kfz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yang J, Huang S, Liu R, Liu H, Zheng D, Huang Q,Yang Y, Liua C, 2019. Protective effect of mulberry crude extract against nonylphenol-induced thyroid disruption by inhibiting the activity of deiodinase in rats. Gen. Comp. Endocrinol. 270, 90–95. 10.1016/j.ygcen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Herrmann J, Alasso G, Beyer M, Heinen E, Romisch J,and Weyer P, 1985. Thyroid hormone binding inhibitor (THBI) mainly associated with serum oleic acid concentration. Horm. Metabol. Res 17, 426–427. 10.1055/s-2007-1013565. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Korte JJ, Olker JH, Denny JS, Knutsen C, Hartig PC, Cardon MC, Degitz SJ, 2018. Screening the ToxCast phase 1 chemical library for inhibition of deiodinase type 1 activity. Toxicol. Sci 162, 570–581. 10.1093/toxsci/kfx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Yamamoto N, Morisawa Y, Uchimoto T, Yukioka M, Morisawa S, 1989. Unesterified long-chain fatty acids inhibit thyroid hormone binding to the nuclear receptor: solubilized receptor and the receptor in cultured cells. Eur. J. Biochem 183, 565–572. 10.1111/j.1432-1033.1989.tb21085.x. [DOI] [PubMed] [Google Scholar]
- Iyer S, Pham N, Marty M, Sandy M, Solomon G, Zeise L, 2019. An integrated approach using publicly available resources for identifying and characterizing chemicals of potential toxicity concern: proof-of-concept with chemicals that affect cancer pathways. Toxicol. Sci 169, 14–24. 10.1093/toxsci/kfz017. [DOI] [PubMed] [Google Scholar]
- Jayarama-Naidu R, Johannes J, Meyer F, Wirth EK, Schomburg L, Köhrle J, Renko K, 2015. A nonradioactive uptake assay for rapid analysis of thyroid hormone transporter function. Endocrinology 156, 2739–2745. 10.1210/en.2015-1016. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutto P, Atchley DS, Kim AN, Campbell M, Donald JM, Sen S, Bero L, Zeise L, Woodruff TJ, 2016. Application of the navigation guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environment Intl. 92–93, 716–728. 10.1016/j.envint.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R, Houck K, Martin M, Richard AM, Knudsen TB, Shah I, Little S, Wambaugh J, Setzer RW, Kothiya P, et al. , 2016. Analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space. Toxicol. Sci 152, 323–339. 10.1093/toxsci/kfw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R, Kavlock R, Martin M, Reif D, Houck K, Knudsen T, Richard A, Tice RR, Whelan M, Xia M, et al. , 2013. Perspectives on validation of high-throughput assays supporting 21st century toxicity testing. ALTEX 30, 51–56. 10.14573/altex.2013.1.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecat-Guillet N, Merer G, Lopez R, Pourcher T, Rousseau B, Ambroise Y, 2007. A 96-well automated radioiodide uptake assay for sodium/iodide symporter inhibitors. Assay Drug Devel. Tech 5, 535–540. 10.1089/adt.2007.068. [DOI] [PubMed] [Google Scholar]
- Lim CF, Munro SLA, Wynne KN, Topliss DJ, Stockigt JR, 1995. Influence of nonesterified fatty acids and lysolecithins on thyroxine binding to thyroxine-binding globulin and transthyretin. Thyroid 5, 319–324. 10.1089/thy.1995.5.319. [DOI] [PubMed] [Google Scholar]
- McGovern SL, Caselli E, Grigorieff N, Shoichet BK, 2002. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem 45, 1712–1722. 10.1021/jm010533y. [DOI] [PubMed] [Google Scholar]
- McGovern SL, Helfand BT, Feng B, Shoichet BK, 2003. A specific mechanism of nonspecific inhibition. J. Med. Chem 46, 4265–4272. 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- Medeiros-Neto G, Stanbury JB, 1994. The Iodotyrosine Deiodinase Defect. Inherited Disorders of the Thyroid System CRC Press, Boca Raton, FL, pp. 139–159. [Google Scholar]
- Meinhold H, Buchholz R, 1983. Effects of iodotyrosine deiodinase inhibition on serum concentrations and turnover of diiodotyrosine (DIT) and thyroxine (T4) in the rat. Acta Endocrinol. 103, 521–527. 10.1530/acta.0.1030521. [DOI] [PubMed] [Google Scholar]
- Mihaich E, Capdevielle M, Urbach-Ross D, 2017. Hypothesis-driven weight-of-evidence analysis of endocrine disruption potential: a case study with triclosan. Crit. Rev. Toxicol 47, 263–385. 10.1080/10408444.2016.1269722. [DOI] [PubMed] [Google Scholar]
- Montano M, Cocco E, Guignard C, Marsh G, Hoffman L, Bergman A, Gutleb AC, Murk AJ, 2012. New approaches to assess the transthyretin binding capacity of bioactivated thyroid hormone disruptors. Toxicol. Sci 130, 94–105. 10.1093/toxsci/kfs228. [DOI] [PubMed] [Google Scholar]
- Moreno JC, Visser TJ, 2010. Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations. Mol. Cell. Endocrinol 322, 91–98. 10.1016/j.mce.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Moreno JC, Klootwijk W, van Toor H, Pinto G, D’Alessandro M, Leger A, Goudie D, Polak M, Gruters A Visser TJ, 2008. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N. Engl. J. Med 358, 1811–1818. 10.1056/nejmoa0706819. [DOI] [PubMed] [Google Scholar]
- Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MM, Furlow JD, Kavlock R, Köhrle J, Opitz R, et al. , 2013. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. In Vitro 27, 1320–1346. 10.1016/j.tiv.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Naderi M, Zargham D, Asadj A, Bashti T, Kamayi K, 2015. Short-term responses of selected endocrine parameters in juvenile rainbow trout (Oncorhynchus mykiss) exposed to 4-nonylphenol. Toxicol. Ind. Health 12, 1218–1228. 10.1177/0748233713491806. [DOI] [PubMed] [Google Scholar]
- Nedvidková J, Šterzl I, Haluzik M, Schreiber V, 1995. An increase in the blood thyroxine level after methylene blue in rats: the interaction with carbimazole. Endocr. Res 21, 709–717. 10.3109/07435809509030485. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Friedman KP, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S Jr., Crofton KM, Laws SC, Stoker TE, et al. , 2019. Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ. Health Persp 127, 095001. 10.1289/EHP5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co-operation and Development)., 2014. New Scoping Document on In Vitro and Ex Vivo Assays for the Identification of Modulators of Thyroid Hormone Signaling. In OECD Environment, Health and Safety Publications, Series on Testing and Assessment, No. 207. Paris, France. ENV/JM/MONO; (2014)23. 10.1787/9789264274716-en. (accessed 15 April 2020). [DOI] [Google Scholar]
- Olker JH, Haselman JT, Kosian PA, Donnay KG, Korte JJ, Blanksma C, Hornung MW, Degitz SJ, 2018. Evaluating iodide recycling inhibition as a novel molecular initiating event for thyroid axis disruption in amphibians. Toxicol. Sci 166, 318–331. 10.1093/toxsci/kfy203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olker JH, Korte JJ, Denny JS, Hartig PC, Cardon MC, Knutsen CN, Kent PM, Christensen JP, Degitz SJ, Hornung MW, 2019. Screening the ToxCast phase 1, phase 2, and e1k chemical libraries for inhibitors of iodothyronine deiodinases. Toxicol. Sci 168, 430–442. 10.1093/toxsci/kfy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly DR, Miller LK, and Luckow VA, 1992. Baculovirus Expression Vectors: A Laboratory Manual. New York, W. H. Freeman and Company. 347 pp. [Google Scholar]
- Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO, 2016. Tiered high-throughput screening approach to identify thyroperoxidase inhibitors within the ToxCast phase I and II chemical libraries. Toxicol. Sci 151, 160–180. 10.1093/toxsci/kfw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Rotroff DM, Hornung MW, Crofton KM, Simmons SO, 2014. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem. Res. Toxicol 27, 387–399. 10.1021/tx400310w. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (accessed 15 April 2020). [Google Scholar]
- Renko K, Hoefig CS, Dupuy C, Harder L, Schwiebert C, Kohrle J, Schomburg L, 2016. A non-radioactive DEHAL assay for testing substrates, inhibitors and monitoring endogenous activity. Endocrinol. 157, 4516–4525. 10.1210/en.2016-1549. [DOI] [PubMed] [Google Scholar]
- Renko K, Hoefig CS, Hiller F, Schomburg L, Köhrle J, 2012. Identification of iopanoic acid as substrate of type 1 deiodinase by a novel nonradioactive iodide-release assay. Endocrinol. 153, 2506–2513. 10.1210/en.2011-1863. [DOI] [PubMed] [Google Scholar]
- Renko K, Schäche S, Hoefig CS, Welsink T, Schwiebert C, Braun D, Becker NP, Köhrle J, Schomburg L, 2015. An improved nonradioactive screening method identifies genistein and xanthohumol as potent inhibitors of iodothyronine deiodinases. Thyroid 25, 962–968. 10.1089/thy.2015.0058. [DOI] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, Yang C, Rathman J, Martin M, Wambaugh JF, et al. , 2016. ToxCast chemical landscape: Paving the road to 21st century toxicology. Chem. Res. Toxicol 29, 1225–1251. 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- Ritz C, Baty F, Streibig JC, Gerhard D, 2015. Dose-response analysis using R. PLOS ONE, 10, e0146021. 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokita SE, Adler JM, McTamney PM, Watson JA Jr., 2010. Efficient use and recycling of the micronutrient iodide in mammals. Biochimie 92, 1227–1235. 10.1016/j.biochi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo GA, Elsasser TH, Kahl S, Erdman RA, Casper DP, 1997. Dietary fatty acids modulate hormone responses in lactating cows: mechanistic role for 5’-deiodinase activity in tissue. Domest. Anim. Endocrin 14, 409–420. 10.1016/s0739-7240(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg I, 1970. Purification of iodotyrosine deiodinase from bovine thyroid. Metabolism 19, 785–798. 10.1016/0026-0495(70)90076-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg I, Goswami A, 1984. Iodotyrosine deiodinase from bovine thyroid. Method. Enzymol 107, 488–500. 10.1016/0076-6879(84)07033-6. [DOI] [PubMed] [Google Scholar]
- Rousset B, Dupuy C, Miot F, Dumont J, 2015. Thyroid hormone synthesis and secretion. [Updated 2015 Sep 2] In: De Groot LJ, Chrousos G, Dungan K, et al. , editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–2015. https://www.ncbi.nlm.nih.gov/books/NBK285550/ (accessed on 12 May 2020). [Google Scholar]
- Routledge EJ, Sumpter JP, 1997. Structural features of alkylphenolic chemicals associated with estrogenic activity. J. Biol. Chem 272, 3280–3288. 10.1074/jbc.272.6.3280. [DOI] [PubMed] [Google Scholar]
- Sandell EB, Kolthoff IM, 1937. Micro determination of iodine by a catalytic method. Michrochim. Acta, 1, 9–25. 10.1007/BF01476194. [DOI] [Google Scholar]
- Shimizu R, Yamaguchi M, Uramaru N, Kuroki H, Ohta S, Kitamura S, Sugihara K, 2013. Structure-activity relationships of 44 halogenated compounds for iodotyrosine deiodinase-inhibitory activity. Toxicology 314, 22–29. 10.1016/j.tox.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Shirdel I, Kalbassi MR, 2016. Effects of nonylphenol on key hormonal balances and histopathology of the endangered Caspian brown trout (Salmo trutta caspius) Comp. Biochem. Physiol. Part C 183–184, 28–35. 10.1016/j.cbpc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Shoichet BK, 2006. Interpreting steep dose-response curves in early inhibitor discovery. J. Med. Chem 49, 7274–7277. 10.1021/jm061103g. [DOI] [PubMed] [Google Scholar]
- Solis-S JC, Villalobos P, Orozco A, and Valverde-R C, 2004. Comparative kinetic characterization of rat thyroid iodotyrosine dehalogenase and iodothyronine deiodinase type 1. J. Endocrinol 181, 385–392. 10.1677/joe.0.1810385. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, Zorrilla LM, 2010. Triclosan exposure modulate estrogen-dependent responses in the female wistar rat. Toxicol. Sci 117, 45–53. 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- Strickland JD, Martin MT, Richard AM, Houck KA, Shafer TJ, 2018. Screening the ToxCast phase II libraries for alterations in network function using cortical neurons grown on multi-well microelectrode array (mwMEA) plates. Arch. Toxicol 92:487–500. 10.1007/s00204-017-2035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X Zhang X, Jiang Y, Bao S, Shan Z, Teng W, 2015. Expression of iodotyrosine deiodinase in thyroid and other organs in iodine-deficient and iodine-excess rats. Biol. Trace Elem. Res 167, 272–279. 10.1007/s12011-015-0328-1. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Bahadori T, Buckley TJ, Cowden J, Deisenroth C, Dionisio KL, Frithsen JB, Grulke CM, Gwinn MR, Harrill JA, et al. , 2019. The next generation blueprint of computational toxicology at the U.S. Environmental Protection Agency. Toxicol. Sci 169, 317–332. 10.1093/toxsci/kfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SR, McTamney PM, Adler JM, LaRonde-LeBlanc N, Rokita SE, 2009. Crystal structure of iodotyrosine deiodinase, a novel flavoprotein responsible for iodide salvage in thyroid glands. J. Biol. Chem 284, 19659–19667. 10.1074/jbc.m109.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Auld DS, Inglese J, 2010. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr. Opin. Chem. Biol 14, 315–324. 10.1016/j.cbpa.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA), 2015. ToxCast Assay Summary Information. Data released October 2015. U.S. Environmental Protection Agency. https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data/ (accessed 12 May 2020). [Google Scholar]
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Lougee RR, Richard AM, Laws SC, Stoker TE, 2019a. High-throughput screening and chemotype-enrichment analysis of ToxCast phase II chemicals evaluated for human sodium-iodide symporter (NIS) inhibition. Environ. Int 126:377–386. 10.1016/j.envint.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Laws SC, Stoker TE, 2018. High-throughput screening and quantitative chemical ranking for sodium-iodide symporter inhibitors in ToxCast phase I chemical library. Environ. Sci. Technol 52, 5417–5426. 10.1021/acs.est.7b06145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xu J, Zeng F, Fu X, Xu W, Yu J, 2019b. Influence of nonylphenol exposure on basic growth, development, and thyroid tissue structure in F1 male rats. Peer J 7, e7039. 10.7717/peerj.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JA, 2006. Insight into the structure and mechanism of iodotyrosine deiodinase, the first mammalian member of the NADH Oxidase/Flavin reductase superfamily. PhD thesis, Univ Maryland, 109pp. [DOI] [PubMed] [Google Scholar]
- Wu Y, Beland FA, Fang JL, 2016. Effect of triclosan, triclocarban, 2,2’,4,4’-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol. In Vitro 32, 310–319. 10.1016/j.tiv.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Xi Y, Li DH, San W, 2013. Exposure to the endocrine disruptor nonylphenol alters structure and function of thyroid gland in rats. Regul. Peptides 185, 52–56. 10.1016/j.regpep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Yang L, Zha J, Guo Y, Zhou B, 2020. Evaluation and mechanistic study of chlordecone-induced thyroid disruption: based on in vivo, in vitro and in silico assays. Sci. Total Environ 716, 136987. 10.1016/j.scitotenv.2020.136987. [DOI] [PubMed] [Google Scholar]
- Zhang J-H, Chung TDY, Oldenburg KR, 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomolec. Screening 4, 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang P, Yang M, Zeng L, Liu C, 2018. P38/TRHr-dependent regulation of TPO in thyroid cells contributes to the hypothyroidism of triclosan-treated rats. Cell. Physiol. Biochem 45, 1303–1315. 10.1159/000487558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.