Abstract
There is evidence that use of drugs with anticholinergic properties increases the risk of cognitive impairment, and increased exposure to these drugs potentiates this risk. Anticholinergic drugs are commonly used even with associated risk of adverse events. Aging, sex, and genetic polymorphisms of cytochrome P450 (CYP) enzymes are associated with alterations in pharmacokinetic processes, which increase drug exposure and may further increase the risk of adverse drug events. Due to the increasing burden of cognitive impairment in our aging population and the future of personalized medicine, the objective of this review was to provide a critical clinical perspective on age, sex, and CYP genetic polymorphisms and their role in the metabolism and exposure to anticholinergic drugs. Age‐related changes that may increase anticholinergic drug exposure include pseudocapillarization of liver sinusoidal endothelial cells, an approximate 3.5% decline in CYP content for each decade of life, and a reduction in kidney function. Sex‐related differences that may be influenced by anticholinergic drug exposure include women having delayed gastric and colonic emptying, higher gastric pH, reduced catechol‐O‐methyl transferase activity, reduced glucuronidation, and reduced renal clearance and men having larger stomachs which may affect medication absorption. The overlay of poor metabolism phenotypes for CYP2D6 and CYP2C19 may further modify anticholinergic drug exposure in a significant proportion of the population. These factors help explain findings of clinical trials that show older adults and specifically older women achieve higher plasma concentrations of anticholinergic drugs and that poor metabolizers of CYP2D6 experience increased drug exposure. Despite this knowledge neither age, sex nor CYP phenotype are routinely considered when making decisions about the use or dosing of anticholinergic medications. Future study of anticholinergic medication needs to account for age, sex and CYP polymorphisms so that we may better approach personalized medicine for optimal outcomes and avoidance of medication‐related cognitive impairment.
Keywords: aging, anticholinergics, pharmacokinetics, sex differences
This review examines the effects of age, sex and genetic polymorphisms on the pharmacokinetics (absorption, distribution, metabolism, excretion) of anticholinergic medications. Increased drug exposure increases risk of adverse drug events; including cognitive impairment which is associated with anticholinergic medication use.

Abbreviations
- 5‐HMT
5‐hydroxymethyl tolterodine
- AUC
area under the curve
- CYP
cytochrome P450
- EM
extensive metabolizers
- GPCR
G‐protein coupled receptor
- IM
intermediate metabolizers
- IV
intravenous
- M
muscarinic
- PM
poor metabolizers
- UM
ultra‐rapid metabolizers
1. INTRODUCTION
Anticholinergic medications are potentially inappropriate for older adults. 1 , 2 Further to general prescribing guidelines 1 , 2 which caution against anticholinergic medication use in older adults, two academic groups (5th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 3 and the Lancet Commission 4 ) have recently identified anticholinergic medications among potential risk factors for developing dementia. Subsequent to the publication of these guidelines, several new studies have identified exposure to anticholinergic agents as risk factors for mild cognitive impairment and dementia. 5 , 6 , 7 Clinical experience and research demonstrate an increased risk of adverse drug events, 8 , 9 , 10 , 11 , 12 cognitive impairment, 13 , 14 , 15 , 16 , 17 , 18 and mortality 12 related to the use of anticholinergic drugs in older adults. These adverse events can result in emergency department visits, 19 hospital admission, 20 or death 21 with older adults being at increased risk of these sequelae. 22 , 23 , 24 Due to variability in the anticholinergic activity of individual medications, one agent in isolation may fail to cause any noticeable effect but when two or three anticholinergic agents are combined the total anticholinergic burden can result in adverse events. 10 , 17 , 20 , 21 , 25 , 26 , 27 , 28 Total medication exposure or anticholinergic burden depends upon the pharmacokinetic factors in the subject relating to the particular medication(s) consumed.
Age, sex, and genetic variation in cytochrome P450 (CYP) enzymes 29 , 30 , 31 , 32 , 33 are important factors leading to variability in drug metabolism and disposition. As a result, these factors may also contribute to variation in systemic drug exposure, response including resultant adverse events 22 , 23 , 24 and toxicity to a variety of drugs including anticholinergic medications. This review supports clinical decision‐making surrounding anticholinergic medication use as we come to understand their potential risk for causing cognitive impairment and dementia, particularly in older female patients. Our analysis examines the effects of age, sex, and genetic polymorphisms of CYP2D6, CYP2C19, and CYP3A4 on the pharmacokinetics (absorption, distribution, metabolism, excretion), subsequent exposure, and pharmacologic response to anticholinergic medications. This review will provide clinicians with the pharmacokinetic considerations required when using anticholinergic medications.
2. METHODS
2.1. Data sources for review
The PubMed database was searched across all available dates (1950–January 2020) with the initial search terms age, sex, anticholinergic agent, and pharmacokinetics. Anticholinergic agents were considered any drugs appearing on the anticholinergic cognitive burden scale 34 as these were medications used commonly, and this anticholinergic scale is freely available for consultation. The preliminary search lacked recent studies including human subjects. A second search included limits of human subjects, English language, and clinical trials. In this directed search each term; sex, age, CYP2C19, CYP2D6, and CYP3A4 (the most common CYP enzymes involved in the metabolism of anticholinergic drugs) were searched in combination with anticholinergic and pharmacokinetics. Further searches were completed using the specific pharmacokinetic parameter of interest (absorption, distribution, metabolism, excretion) with each of the search terms sex, age, CYP2C19, CYP2D6, or CYP3A4. The Web of Science database was consulted to find citing articles. Further articles were taken from review articles examined during the literature searches. Details of the three searches are shown in Figure 1. This review was not meant to be an exhaustive summary of all available literature on the topic but instead a review of the literature to inform clinical decision‐making about anticholinergic drugs when used by older adults.
FIGURE 1.
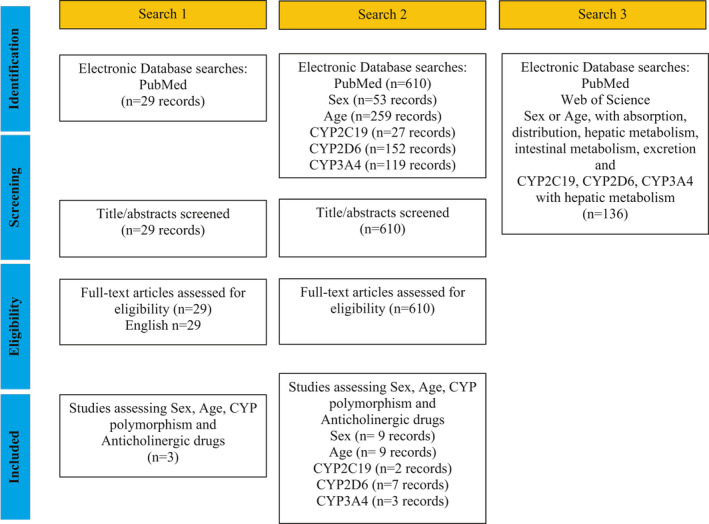
PRISMA style flow diagram
3. RESULTS
3.1. Anticholinergic receptors and signaling
The term anticholinergic agent refers to those drugs that antagonize the muscarinic acetylcholine (M) receptor. The M receptor is a G‐protein‐coupled receptor (GPCR) that resides on the cell membrane. It is comprised of seven alpha helices that span the cell membrane and an extracellular binding domain. When activated, the GPCR undergoes a conformational change that induces dissociation of the trimeric G protein‐complex into the free and active Gα and Gβγ subunits. The Gα and Gβγ subunits activate enzyme effectors or ion channels which regulate intracellular concentrations of secondary messengers such as cyclic adenosine monophosphate, guanosine 3′,5′‐cyclic monophosphate, diacylglycerol, inositol trisphosphate, diacylglycerol, arachidonic acid, sodium, potassium, or calcium depending on the receptor subtype. 35 Gα and Gβγ activity is terminated by activation of an endogenous high‐affinity GTPase located in the Gα subunit, which hydrolyzes the terminal γ‐phosphate of Gα‐GTP to Gα‐GDP which then binds Gβγ to reform the trimeric G protein‐ complex. 36 , 37 In response to prolonged signaling, receptors can be internalized by separation from the effector and binding to small endosomes. This desensitizes the receptor by reducing the number of receptors on the cell surface. This occurs in response to receptor phosphorylation which is often related to a hormone response. 37 , 38 The five M receptor subtypes and their associated functional response to agonism and antagonism are described in Table 1. M1, M3, and M5 receptors all couple with Gq/11 and lead to release of calcium from the sarcoplasmic reticulum. M2 and M4 receptors are coupled to Gi proteins and their activation leads to inhibition of adenylyl cyclase. 39 , 40
TABLE 1.
Description of the five muscarinic receptor subtypes, their distribution throughout the body, and effect of agonism or antagonism at each muscarinic receptor subtype
| Receptor | Most common locations | Functional response (agonism) | Anticholinergic side effect (antagonism) |
|---|---|---|---|
| M1 | Cerebral cortex, hippocampus and striatum, autonomic ganglia, gastric and salivary glands, enteric nerves |
Increase cognitive function—learning and memory Increase seizure activity |
Delirium, sedation, confusion |
| M2 | CNS, heart, smooth muscle, autonomic nerve terminals |
Heart—SA node: slowed spontaneous depolarization, hyperpolarization, decrease HR AV node: decrease conduction velocity Atrium: decrease refractory period, decrease contraction Ventricle: slight decrease in contraction |
Increased heart rate, arrhythmia |
| M3 | CNS, smooth muscle, and glands |
Increase contraction (predominantly in bladder smooth muscle) Increase secretion (predominant in salivary glands) Increase tremor |
Urinary retention, decreased salivation |
| M4 | CNS forebrain | Inhibition of neurotransmitter release | Delirium, sedation, confusion |
| M5 | Rare—CNS and periphery |
Facilitates dopamine release Involved with drug seeking behavior |
Reduced drug seeking |
3.2. Serum anticholinergic activity and anticholinergic burden
M receptor antagonists have limited therapeutic use and are represented predominantly by bladder antispasmodics used to treat urinary incontinence. Many other medications have anticholinergic properties despite the M receptor not being the intended receptor for effect. 41 , 42 Such agents tend to have a lower level of anticholinergic activity. However, when multiple drugs with low levels of anticholinergic activity are combined the cumulative anticholinergic activity and anticholinergic burden increases. 8 , 41 , 43 , 44
Anticholinergic activity is dependent upon many factors, including the drug's binding to the M receptor, its absorption and distribution to tissues (including the brain), its concentration in circulation, intestinal and hepatic CYP metabolism and drug transport, the presence of any active metabolites that are produced, and the rate of elimination of the parent drug and active metabolites from the body. As pharmacokinetics can be affected by sex, age, or genetic polymorphisms (CYP enzymes), all these must be understood to quantify the total anticholinergic activity and rationalize the use of anticholinergic medications in clinical practice. Our findings are shared below and summarized in a table format in Appendix.
3.3. Sex
3.3.1. Role of sex on the absorption of anticholinergic medications
Some, but not all, studies showed that gastric and colonic emptying was slowed in women, potentially increasing the oral bioavailability of some drugs. 45 , 46 , 47 , 48 , 49 , 50 , 51 When stratified by age, the rate of gastric emptying for postmenopausal women and men was similar 52 and significantly faster than premenopausal (younger) women. 50 Gastric pH was higher in females 53 which may increase absorption of basic medications such as tricyclic antidepressants, many of which are quite potently anticholinergic. This difference in gastric pH was quantified by Feldman and Barnett in 1991 as a mean pH of 2.79 for women and 2.16 for men, which was due to reduced acid secretion in women. 54 The greater stomach size in men allowed for more fluid to be contained therein which may have improved both the rate and extent of dissolution of introduced oral dosage forms for men in comparison with women. By contrast, intestinal pH was not found to differ by sex. 55 , 56 CYP enzymes exist in intestinal enterocytes, where they contribute to the first‐pass metabolism of orally administered drugs. Intestinal CYP3A4 metabolism inconsistently exhibited sex differences. Early reports suggested that the CYP3A4 substrates verapamil and midazolam had increased bioavailability in women. 57 , 58 , 59 However, in 2005 a detailed analysis of duodenal punch biopsies from 48 men and 45 women found no clinically meaningful sex difference in intestinal CYP3A4 content. 60 Krecic‐Shepard et al. observed that oral verapamil was cleared more quickly in men with no significant difference after intravenous (IV) administration, suggesting some differences in intestinal metabolism exist 61 which could affect those anticholinergic medications that are substrates of CYP3A4. In females, the CYP3A4 content in the intestine was shown to decrease by approximately 20% after menopause 60 which may reduce CYP3A4 metabolism and affect the sex‐difference in CYP3A4 pharmacokinetics in older women. This decrease in intestinal CYP3A4 in postmenopausal women has not been shown to be clinically meaningful to date. Similarly, male versus female differences in the drug efflux pump ABCB1 (p‐glycoprotein) in the intestinal lumen was hypothesized as a contributor to differences in drug absorption between sexes, 60 but this too has not been demonstrated to be clinically meaningful in studies to date.
3.3.2. Role of sex on the distribution of anticholinergic medications
In general, males are larger than females across the lifespan, with increased height, body mass index, and waist circumference. 62 Compared with men, women have increased adiposity. This difference in body composition has failed to show much difference in actual drug distribution and any differences attributable to body composition can largely be explained by differences in total body mass. 63 Distribution of drugs to the brain was dependent upon the lipophilic nature of the blood‐brain barrier which favored passage and accumulation of lipophilic drugs. At this time, no statistically significant difference has been found between similarly aged women and men with respect to albumin permeability of the blood brain barrier 64 which likely can be extrapolated to at least some medications. The brain is also protected by p‐glycoprotein, which prevents drugs from accumulating in the brain by pumping them from brain capillary endothelial cells to the blood. 65 These mechanisms have not demonstrated any sex difference to date.
3.3.3. Role of sex on the metabolism and transport of anticholinergic medications
Several studies supported that hepatic CYP metabolism varied between men and women although the clinical significance was a challenge to understand. The most abundant hepatic CYP enzyme, CYP3A4, was involved in the metabolism of some anticholinergic medications. In humans CYP3A4 had a higher level of protein expression in the female liver. 66 Consistent with the expression data, CYP3A4 oxidation was reported to be more efficient in women 57 , 67 with a two‐fold higher CYP3A4 hepatic content and 50% increase in the metabolizing capacity 68 but this finding has not been replicated in other scientific investigations. 69 , 70 An in vitro study from samples of 43 healthy livers in subjects between the ages of 27 and 83 showed a 24% increase in CYP3A4 activity identified by erythromycin N‐demethylation in females. 71 Women had an increase in CYP3A4 activity measured as a greater clearance of CYP3A4 substrates such as the weakly anticholinergic antihypertensive medication nifedipine 72 and the weakly anticholinergic sedative alprazolam. 73 On average, the weight‐normalized clearance of alprazolam to active metabolites 74 and nifedipine to inactive metabolites 75 was mainly due to CYP3A4 and was 20%–30% higher in young women than in young men. This difference applied to both parenteral and oral administration and was not explained away by p‐glycoprotein activity. 76 For context, CYP3A4 activity was studied in relation to metabolism of some non‐anticholinergic agents, such as midazolam to its active metabolites 77 and clindamycin. Meta‐analysis suggested that women exhibited a 16% higher weight‐corrected oral clearance of midazolam (p < .001) and 20% higher systemic clearance (p = .002) than men. No significant difference in the area under the curve (AUC) after oral dosing of midazolam was found but after IV administration women showed lower AUC than men (p = .02). No sex‐dependent differences were observed in midazolam bioavailability. 78 Clindamycin did not show any sex difference in its oral pharmacokinetics. 79 The study of midazolam and clindamycin confirmed sex variability in CYP3A4 metabolism, but failed to demonstrate any consistent sex‐differences.
There was less study of sex differences in CYP2D6 and 2C19 metabolism identified in the literature search. Investigations of sex‐differences in CYP2C19 activity included 4‐hydroxymephenytoin, the active metabolite of the anticonvulsant mephenytoin, and zonisamide metabolism to its inactive metabolites 80 which failed to show any sex‐differences. 81 , 82 A Spanish study examining caffeine metabolism found higher CYP2D6 activity in women. 83
Sex differences were demonstrated in the glucuronidation of some medications (acetaminophen) but not others (zidovudine), 84 , 85 , 86 suggesting that sex differences in drug conjugation exist and are drug‐dependent. To date, no anticholinergic agents have been explored with respect to glucuronidation. Clearance of some non‐anticholinergic drugs by glucuronidation were shown to be increased in men in comparison with women including oxazepam, 67 temazepam, 87 and acetaminophen. 88 With regard to catechol‐O‐methyltransferase activity, liver tissue from female subjects exhibited approximately 25% lower activity than samples from male subjects. 89 There was a two‐fold greater expression of hepatic p‐glycoprotein in men compared with women 90 with unclear clinical relevance.
3.3.4. Role of sex on the renal elimination of anticholinergic medications
Glomerular filtration is related to body mass. Males typically have a greater body weight than females, 62 so generally glomerular filtration is greater in males than females. This likely explains most sex‐differences in renal drug clearance, though this was not observed for all drugs. Sex was found to be a significant factor in methotrexate clearance, with a 17% reduction in females after standardizing doses for body weight. 91 Some authors reasoned that for narrow therapeutic index drugs, the sex‐related effect on kidney function may be clinically relevant. 91 , 92 Pharmacokinetic studies confirmed sex‐differences in renal clearance for many drugs including the weakly anticholinergic drug digoxin, which had slower clearance in females 93 and the moderately anticholinergic drug amantadine, which had been shown to have significantly higher renal clearance in men due to putative sex differences in renal tubule secretion by organic cation transporters. 94
Sex differences in pharmacokinetics have been explored with respect to some anticholinergic medications. Results of studies that examined sex differences in anticholinergic drug pharmacokinetics as their primary objective are listed in Table 2.
TABLE 2.
Details of study population, study objectives, methodology, and results of trials identified to have a primary objective of exploring sex‐differences in pharmacokinetic parameters for anticholinergic medications
| Study author & design | Study population | Study objective | Methodology | Results |
|---|---|---|---|---|
|
Vicente et al. 97 Randomized single‐lind controlled trial |
24 healthy non‐smoking volunteers (12 women and 12 men), 18–35 years old | To determine if quinidine induced prolongation of the time from the peak to the end of the T‐wave is greater in women than men | Subjects received either 4 mg/kg of quinidine IV or a matching placebo solution over 20 min with 28 blood samples and simultaneous ECGs collected after drug/placebo infusion for each subject at predetermined time points over the following 12 h |
Quinidine causes QTc prolongation and T‐wave morphology changes in both women and men Quinidine‐induced maximum QTc (541 ± 40 ms vs. 510 ± 38 ms; p = .07) or maximum T peak–T end (216 ± 60 ms vs. 222 ± 37 ms; p = .76) was similar for men and women There was a trend toward a lower maximum serum quinidine concentration in women compared with men (2.9 ± 0.7 μg/ml vs. 3.7 ± 1.2 μg/ml; p = .07) The slope describing serum quinidine concentration versus QTc prolongation was greater in women than in men (38 ± 10 ms/μg/ml vs. 28 ± 9 ms/μg/ml; p = .02) Differences between women and men occurred primarily in the first 20 min after quinidine infusion, when serum quinidine concentrations were higher in men than women |
|
Benton et al. 95 Randomized single‐blinded controlled trial |
24 healthy non‐smoking volunteers (12 women and 12 men), 18–35 years old | To determine if women have larger increases in QT interval than men at equivalent serum concentrations of quinidine after intravenous administration | Subjects received either 4 mg/kg of quinidine IV or a matching placebo solution over 20 min. 28 blood samples and simultaneous ECGs were collected after drug/placebo infusion for each subject at predetermined time points over the following 48 h |
There was a trend to greater weight‐adjusted clearance of quinidine in women than in men (5.2 ± 1.1 ml/min/kg vs. 4.3 ± 1.6 ml/min/kg) There was also a trend to a higher maximal plasma concentration of quinidine in men than in women (3.67 ± 0.13 μg/ml vs. 2.78 ± 0.87 μg/ml; p = .07) There were no sex‐related differences in the ratio of the AUC∞ of 3‐hydroxyquinidine to the AUC∞ of quinidine The estimated volume of distribution (V d) at steady state was not different between the men and women There was no difference in the free fraction of quinidine in serum between men and women The free fraction of 3‐hydroxyquinidine was slightly higher in women than in men (0.53 ± 0.05 μg/ml vs. 0.47 ± 0.05 μg/ml; p < .01) |
|
Winchell et al. 98 A series of open‐label, three‐period, randomized, crossover studies |
1. 24 healthy young subjects (mean age: 25.5 years; range: 19–39 years; 16 males and 8 females 2. 18 healthy subjects (mean age: 28.7 years; range: 22–40 years; 8 males, 10 females) 3. 12 elderly subjects (mean age: 71.3 years; range: 65–79 years; 6 males, 6 females |
To investigate the pharmacokinetics and bioavailability of cyclobenzaprine, including the effects of sex and age |
1. Bioavailability: Subjects received 5 mg orally or 1.25 mg IV cyclobenzaprine 2. Pharmacokinetics: Subjects received a single oral dose of 2.5, 5, or 10 mg cyclobenzaprine on Day 1 then every 8 h from Days 8 through 14 with final dose on Day 15 3. Pharmacokinetics in aging: Subjects received 5 mg cyclobenzaprine orally three times daily for 7 days and a final dose on Day 8 |
1. Plasma concentrations increased initially, peaking at 4 h post dose, and then declined slowly Mean plasma clearance was 689 ± 216 ml/min—Mean oral bioavailability 5 mg tablet formulations were 0.55 (90% CI [0.51, 0.60]) 2. There were no statistically significant differences between males and females for any of the pharmacokinetic parameters—AUC(0–8 h) and C Max after the last dose were marginally significantly different between sexes 3. The population‐by‐sex effect was marginally significant for AUC(0–8 h) (p = .056) but not for C Max |
|
El‐Eraky et al. 96 Open trial |
48 healthy volunteers (27 men, 21 women) aged 18–64 years | To determine why women are more susceptible to QT interval prolongation and torsade de pointes after administration of drugs that delay cardiac repolarization | All subjects took quinidine sulfate capsules 3 mg/kg orally then ECGs and blood samples for quinidine concentrations were taken over 24 h following drug administration |
There were no significant differences in quinidine concentrations between men and women or in any of the pharmacokinetic variables measured The QTa, and QTc intervals were larger in females than in males Quinidine did not affect QRS duration in women but reduced QRS duration in men |
|
Koren et al. 122 Single‐center, single dose open‐label, reference replicate bioavailability study |
12 healthy males and 12 healthy females, 18–45 years with a body mass index between 19–30 kg/m2 | To determine the effect of sex on the pharmacokinetics of doxylamine–pyridoxine 10–10 mg delayed‐release tablets | Participants were given doxylamine–pyridoxine 20–20 mg delayed‐release tablets with 240 ml water on an empty stomach with blood sampling starting 1 h pre‐dose with samples analyzed using high performance liquid chromatography‐ tandem mass spectrometry |
Females had significantly larger AUC0– t for doxylamine compared with males A higher C Max for doxylamine was observed in females compared with males |
|
Malhotra et al. 118 Two randomized double‐blind placebo‐controlled trials |
1. 32 healthy males aged 18–45 years 2. 16 young men, 16 older men and 16 older women |
To examine the effect of age, sex and race on the pharmacokinetics, pharmaco‐dynamics and safety profiles of fesoterodine | Subjects received either 8 mg of fesoterodine extended release or placebo with blood samples drawn over 36 h after drug administration and saliva samples on cotton wool collected over 24 h after drug administration |
No apparent differences in C Max, AUC0–∞, t max, or mean residual time between males and females Total plasma clearance was highest in young men and lowest in older women Elderly women experienced a 1 g decrease in salivary volume and elderly men did not 5 h after dose Elderly men experienced the greatest residual urinary volume increase 8 h after dose |
|
Ebert et al. 123 Open label crossover study |
7 men and 7 women of mean age 23 years and in good health | To identify any pharmacokinetic differences between male and female volunteers in the metabolism of scopolamine when given with grapefruit juice | Each subject received at random scopolamine 0.5 mg IV, scopolamine 0.5 mg orally, or scopolamine 0.5 mg orally mixed with 150 ml fresh grapefruit juice and blood sampling occurred over the 24 h following drug administration |
C Max was significantly higher in males than females (6.61 ng/ml vs. 3.93 ng/ml) after IV infusion All other parameters were similar |
|
Macleod et al. 99 Open label study |
4 men and 5 women aged 21–30 years, and 5 older men and 5 older women aged 70–88 years | To identify age and gender differences in diazepam pharmacokinetics | 10 ml blood samples were taken over 1 week after receiving 0.125 mg/kg diazepam IV over 10 min |
There was a significant difference in plasma clearance between men and women (male: 33.2 ml/min and women: 18.1 ml/min) The half‐life in men (32 h) was significantly shorter than in women (46.2 h) V d was not significantly different between sexes |
|
Bigos et al. 100 Naturalized prospective study |
332 men and 191 women who were using olanzapine for AD or schizophrenia | To evaluate population pharmacokinetics of olanzapine and factors that contribute to variability in exposure including sex, race and smoking status | Plasma levels of olanzapine were determined and then used to calculate non‐linear mixed effects modelling for pharmacokinetic analysis | Men cleared olanzapine 38% faster than women (p < .0001, unpaired t test) |
|
Hartter et al. 105 Prospective study |
15 male and female participants with major depression | To assess sex differences in fluvoxamine serum concentration at two different fixed dosing regimens (50 twice daily and 100 mg twice daily) | Drug monitoring after 14 days of either treatment | There was a significantly greater increase in fluvoxamine serum concentration in men than in women when the dose doubled (4.6‐fold vs. 2.4‐fold increase) |
Abbreviations: AUC, area under the curve; IV, intravenous.
3.3.5. Summary of studies showing sex‐differences in pharmacokinetics: Quinidine
The most commonly reported anticholinergic medication with a focus on sex‐related differences was quinidine, exploring drug‐induced QT interval prolongation (Table 2). 95 , 96 , 97 The findings of both Benton and Vicente 95 , 97 suggested that women cleared quinidine at a faster rate than men. Unexpectedly, women had a more rapid onset of ECG changes in response to drug activity than men, which was not entirely explained by increased quinidine clearance. These studies demonstrated sex‐differences in quinidine pharmacokinetics; however, the mechanism of this difference was not clear. 95 , 96 , 97 It was possible hormonal influences or rapid distribution after IV infusion contributed to the faster onset of activity in women which normalized over time to reach equilibrium between the sexes.
3.3.6. Summary of studies showing sex‐differences in pharmacokinetics: Psychoactive medications
Many anticholinergic psychoactive medications were investigated for sex‐differences in absorption, distribution, metabolism, and excretion. A study of cyclobenzaprine examined sex‐differences using a series of open‐label, three‐period, randomized, crossover studies. The first study included 24 healthy young subjects (mean age: 25.5 years), the second 18 healthy subjects (mean age: 28.7 years), and the third 12 older subjects (mean age: 71.3 years). The primary objective was to investigate the bioavailability and pharmacokinetics of cyclobenzaprine with attention to the effects of sex, age, and hepatic insufficiency (Table 2). There were small significant differences in the AUC and C Max for cyclobenzaprine between sexes in the older group. 98 This is most likely due to accumulation of drug in the group of older females. A study of the benzodiazepine diazepam demonstrated a shorter t 1/2 and a greater plasma clearance in men in comparison with women (Table 2). 99 In a population of men and women receiving olanzapine for Alzheimer's disease or schizophrenia, between one and six samples were analyzed from each individual to determine sex‐differences in olanzapine clearance. Sex was found to be responsible for 12% of variability in olanzapine elimination. Men cleared olanzapine 38% faster than women. 100 A natural pharmacokinetic study of anticholinergic antidepressants in older adults looked for sex‐differences in serum concentrations. The ratio of absolute serum concentration in comparison with the dose‐adjusted serum concentration was 1.1‐ to 1.5‐fold higher in women than in men for clomipramine and trimipramine. This was despite a dose reduction in females who received 10%–30% lower dose but still achieved serum levels equivalent to male participants. 101 Findings of Mundo and Unterecker et al. suggested that clomipramine levels were not related to sex, 102 , 103 but rather the metabolites of clomipramine accumulated contributing to the higher plasma levels seen in women. A second naturalistic study of antidepressants that examined 19,870 blood samples failed to show a difference for the tricyclic antidepressants clomipramine or fluvoxamine 104 which is in keeping with findings of Mundo and Unterecker. 102 , 103 However, in a study that examined dose regimens of fluvoxamine separately, a dose‐dependent sex difference in serum fluvoxamine concentration was observed. At a 100 mg daily oral dose, women achieved higher serum fluvoxamine concentrations than men, but with a 200 mg daily oral dose the serum concentrations were no longer statistically significantly different. 105 This may relate to a saturable metabolizing enzyme that was in a greater concentration or more active in men. Sex was correlated to paroxetine plasma concentrations in three studies that examined the effect of sex on paroxetine pharmacokinetics. In a study of 171 subjects aged ≥70 years, men had a higher paroxetine V d (461 ± 260 L) compared with women (346 ± 256 L). 106 In a study of 1677 older men and women, the serum concentration of paroxetine was 32% higher in women (86 nmol/L vs. 65 nmol/L, p < .001). 104 In a third study of 70 patients, the plasma concentration of paroxetine was higher in women across age groups (28 ng/ml vs. 16 ng/ml; p = .001). 107 The mean AUC and C Max for bupropion, a mildly anticholinergic antidepressant, were higher in women than men; however, once these parameters were standardized for body weight the statistical significance was lost. 108 For bupropion, older women had a larger V d and longer t 1/2 than young men. This does make it challenging to know how much of the effect was attributable to sex versus age. 109 Amitriptyline plasma levels were higher in women in a study of 110 inpatients receiving routine doses of amitriptyline, 110 but no significant sex‐difference in serum concentration of amitriptyline was noted in the study by Reis et al. 104 Nortriptyline plasma levels were affected by sex with females experiencing higher plasma levels. 111 Desipramine was shown to have a longer elimination t 1/2 and a faster oral clearance in older men than in older women. 112 When examining risperidone plasma concentrations, the only parameter to exhibit a statistically significant difference between males and females was the plasma concentration/dose ratio. When weight was used to adjust the plasma concentration, any difference was lost. 113 Many of these psychoactive medications are metabolized by CYP2D6, and a sex‐related difference in CYP2D6 activity has not consistently been identified in the literature, 114 which means there are likely other sex‐dependent mechanisms contributing to these pharmacokinetics differences. In summary, while many sex‐differences exist in the pharmacokinetics of psychoactive anticholinergic medications, no consistent patterns were identified. The small increases in drug exposure that were identified (most often by women) may help explain the increased experience of adverse events by women. 115 , 116
3.3.7. Summary of studies showing sex‐differences in pharmacokinetics: Bladder anticholinergics
Oxybutynin, the prototype bladder anticholinergic, is metabolized by CYP3A4 to N‐desmethyloxybutynin. This metabolite of oxybutynin is considered to cause many of the adverse events related to oxybutynin treatment, so understanding any role of sex in the metabolism of oxybutynin is important. Increased CYP3A4 activity and slowed renal elimination in women may increase exposure to the metabolite and increase the likelihood of adverse drug effects. However, an older study of oxybutynin pharmacokinetics failed to show any sex differences in the pharmacokinetics of oxybutynin or its active metabolite. 117
Two randomized double‐blind placebo‐controlled trials assessed the effects of age, sex, and race on the pharmacokinetics and safety profiles of fesoterodine in 32 healthy males aged 18–45 years (16 white and 16 black men) and 16 young men, 16 older men and 16 older women (Table 2). Total plasma clearance of fesoterodine was highest in young men and lowest in older women, but there were no apparent sex differences in C Max, AUC0–∞, or t Max. Interestingly, 5 h after the dose was given, older women experienced a 1 g decrease in salivary volume whereas older men did not, which provided some evidence that women were more likely to experience adverse effects (e.g. dry mouth) from this anticholinergic medication use. There was no clinically meaningful difference in any of the pharmacokinetic parameters studied based on race (mean AUC0–tz was 70.7 ng/ml × h in white and 64.1 ng/ml × h in black men, and mean C Max was 6.1 ng/ml in white and 5.5 ng/ml in black men). 118 Similarly, in a study of 337 individuals, darifenacin clearance was about 30% lower in females. 119 No sex differences in pharmacokinetics had been identified for solifenacin 120 or tolterodine. 118 Trospium demonstrated an unexplained prolonged t 1/2 in women compared with men. 121 This collection of studies demonstrates the complex influence of sex on pharmacokinetics of bladder anticholinergics which are frequently used by older adults.
3.3.8. Summary of studies showing sex‐differences in pharmacokinetics: Antihistamines
A single‐center, single‐dose, open‐label, reference replicate, bioavailability study in 12 healthy males and 12 healthy females aged 18–45 years with a body mass index between 19 and 30 kg/m2 was completed to determine the effect of sex on the pharmacokinetics of doxylamine–pyridoxine 10–10 mg delayed‐release tablets. Females had significantly larger AUC0– t and a higher C Max, for doxylamine compared with males. 122
3.3.9. Summary of studies showing sex‐differences in pharmacokinetics: Scopolamine
An open‐label crossover study of seven men and seven women of mean age 23 years and in good health was completed to identify any sex differences in pharmacokinetics in the metabolism of 0.5 mg scopolamine when given IV or orally with or without grapefruit juice. The C Max was significantly higher in males than females (6.61 ng/ml vs. 3.93 ng/ml) after IV infusion with all other parameters being similar. 123 No sex differences were found in urinary elimination of scopolamine for any of the three different routes of administration.
3.4. Age
3.4.1. Role of age on the absorption of anticholinergic medications
Gastric and colonic transit was significantly faster in postmenopausal women in comparison with premenopausal women, 50 which suggested altered absorption. In a study of 16 healthy adults average age 81 years and 16 healthy adults average age 24 years, advanced age did not influence gastric emptying or small intestinal transit but older individuals had a slower colonic transit. 46
3.4.2. Role of age on the metabolism and transport of anticholinergic medications
In humans, it was well established that total hepatic CYP enzyme levels decline from about age 40 onwards. This had been quantified as about a 3.5% decline in CYP enzyme content for each decade of life potentially influencing the elimination of anticholinergic drugs undergoing metabolism by the CYP enzyme system, resulting in greater exposure to these agents. 69 , 124 An older study investigating the metabolic ability of CYP enzymes across a variety of ages revealed that CYP3A4 activity was reduced in older adults. The microsomal content of CYP3A4 was found to decrease by approximately 8% per decade of life. 69 This trial failed to show a difference in CYP1A2, or CYP2C based on age. An in vitro study of healthy human liver samples obtained during surgical procedures from 43 subjects between the ages of 27 and 83 showed no variation in CYP3A4 activity in relation to age. In this study, CYP3A4 activity was quantified by measuring erythromycin N‐demethylation. While erythromycin N‐demethylation had been shown to decline with age, the results of this study suggested that the age‐related decline in enzyme activity was not due to declining CYP3A4 activity. Rather, other patient factors such as renal blood flow, renal filtration, or body composition were likely contributing. 71 In females, intestinal CYP3A4 content had been shown to decrease by approximately 20% after menopause, 60 which may have reduced intestinal CYP3A4 metabolism and contributed to an age‐dependent difference in CYP3A4 metabolism. Possibly due to a lack of studies, this decrease in intestinal CYP3A4 in postmenopausal women has not been shown to be clinically meaningful to date. Decreases in the clearance of CYP3A4 substrate drugs suggested that older people may experience increased adverse effects due to reduction in clearance of drugs that rely on CYP3A4 for metabolism prior to elimination. 125
Drug conjugation was shown in several studies to remain fairly constant with respect to age. 126 Undeniably, numerous factors such as genetics, medication use, and frailty 127 , 128 can influence glucuronidation and sulfonation, but in younger and older healthy people glucuronidation and sulfonation were not statistically significantly different. In aging rat models, liver sinusoidal endothelial cells undergo pseudocapillarization, 129 , 130 a process characterized by loss of sinusoidal fenestrations, thickening of the endothelium, perisinusoidal collagen deposition, and basal lamina formation. 131 This process suggested that drug passages through the liver were reduced in size which, in theory, could prevent large molecules, in particular protein therapeutics and extensively protein‐bound drugs, from travelling through the liver and being cleared; this was shown for liposomal doxorubicin in aged rats compared to young rats. 132 The relevance of these changes to anticholinergic drug pharmacokinetics in humans remains to be determined.
3.4.3. Role of age on the renal elimination of anticholinergic medications
Renal elimination declines with age by all renal routes (glomerular filtration, tubular secretion, and passive reabsorption). 133 , 134 Any anticholinergic agent that is renally eliminated or has renally eliminated active metabolites is likely to accumulate in older adults in comparison to younger adults.
3.4.4. Role of age on blood–brain barrier function
In men the V d of (R)‐[11C] verapamil, a known p‐glycoprotein substrate, increased with age in several cortical brain regions, strongly suggesting a progressive decrease in blood brain–barrier p‐glycoprotein function with age. 135 This could affect drug introduction to the brain which may affect efficacy or toxicity depending upon the anticholinergic agent used.
Studies with a primary objective of identifying age‐related differences in drug pharmacokinetics were listed in Table 3.
TABLE 3.
Details of study population, study objectives, methodology, and results of trials identified to have a primary objective of exploring age‐related differences in pharmacokinetic parameters for anticholinergic medications
| Study author & study design | Study population | Study objective | Methodology | Results |
|---|---|---|---|---|
|
Winchell et al. 98 A series of open‐label, three‐period, randomized, crossover studies |
1. 24 healthy young subjects (mean age: 25.5 years; range: 19–39 years; 16 males and 8 females) 2. 18 healthy subjects (mean age: 28.7 years; range: 22–40 years; 8 males, 10 females) 3. 12 older subjects (mean age: 71.3 years; range: 65–79 years; 6 males, 6 females) |
To investigate the pharmacokinetics and bioavailability of cyclobenzaprine, including the effects of age and hepatic insufficiency |
1. Subjects received 5 mg orally or 1.25 mg IV cyclobenzaprine 2. Subjects received a single oral dose of 2.5, 5, or 10 mg cyclobenzaprine on Day 1 then every 8 h from Days 8 through 14 and a final dose on Day 15 3. Subjects received 5 mg cyclobenzaprine orally three times daily for 7 days and a final dose on the 8th day |
Cyclobenzaprine plasma concentrations after multiple dosing were significantly higher for the older compared with young subjects After the first dose, plasma concentration profiles were similar in older and young subjects Mean accumulation ratio was 7.9 for older subjects compared with 4.3 for young subjects, and mean effective t 1/2 was 33.4 h (range: 20.0–53.4 h) in older subjects compared with 18.4 h (range: 9.3–41.3 h) in young subjects |
|
Malhotra et al. 118 Two randomized double‐blind placebo‐controlled trials |
1. 32 healthy males aged 18–45 years 2. 16 young men, 16 older men and 16 older women |
To examine the effect of age, sex, and race on the pharmacokinetics, pharmacodynamics and safety profiles of fesoterodine | Subjects received either 8 mg of fesoterodine extended release or matching placebo with blood samples drawn over 36 h after drug administration | Renal clearance was 28% lower in older men and women than younger men |
3.4.5. Summary of studies showing age‐differences in pharmacokinetics: Psychoactive medications
Risperidone and its 9‐hydroxyrisperidone metabolite are active and have anticholinergic properties. In a study of 129 adults on risperidone maintenance therapy grouped by age (<45, 45–60, and >60 years), the risperidone maintenance dose was lowest in the oldest age group, but the unadjusted plasma risperidone concentrations did not differ significantly across age groups. However, when adjusted for subject body weight or maintenance dose, the plasma risperidone concentration was significantly higher in the older group. The concentration of active drug comprised both the 9‐hydroxyrisperidone metabolite and risperidone parent drug, with the difference driven by the 9‐hydroxyrisperidone concentration. 136 This supported the use of the lowest dose possible of risperidone in older adults and provided support for the “start low and go slow” approach to antipsychotic dosing in geriatric populations. In comparison, the clearance of the sedative diazepam was not found to be affected by age in a study of young (21–30 years) males and females in comparison to older males and females (70–88 years). 99 A naturalized study of multiple anticholinergic antidepressants showed an increase in the absolute serum concentrations to dose‐adjusted serum concentrations for fluvoxamine (2‐fold), amitriptyline, and clomipramine (1.5‐fold) in the oldest age group (those more than 65 years of age) in comparison to controls <40 years. No significant age difference was observed for the dose‐adjusted fluoxetine and trimipramine serum concentrations. For fluoxetine and trimipramine users, older adults were using 10%–30% lower total daily doses. The concentration to dose ratio of nortriptyline was two‐fold higher in adults over 65 in comparison with the controls <40 years old 101 ; clearance was correlated with age with faster clearance at younger ages. No significant difference was found between patients younger than or older than 60 years in the mean dose‐corrected serum concentration of clomipramine and N‐clomipramine, which contradicted the findings of Waade. 103 However amitriptyline plasma levels were higher in older adults than younger subjects, 110 which was consistent with findings of Waade et al. and Dawling et al. who showed that both amitriptyline and nortriptyline levels were higher in older adults, with older women experiencing a more exaggerated effect than their male comparators. 137 At daily oral doses of 100 or 200 mg, fluvoxamine serum concentration did not correlate with age. 105 There was a trend to higher serum concentrations in older female patients with the lower dosage of fluvoxamine, but this diminished when the dosage was doubled and suggested there was an interaction between age and sex on fluvoxamine pharmacokinetics. Older subjects taking oral paroxetine had higher plasma concentrations than younger subjects. 138 In a study that examined bupropion kinetics in older adults with depression (mean age 71.5 years), clearance was 80% of that seen in younger adults 109 and t 1/2 was 34 h in comparison to most sources which report 11–14 h. 109 , 139 Among females, there was no significant difference between young and older groups in any of the pharmacokinetic variables for triazolam. Among males, the t 1/2 of triazolam increased. Furthermore, when age was evaluated as a continuous variable, AUC for triazolam increased significantly with age (p = .02) and clearance decreased with age (p = .02). Further examination of cyclobenzaprine pharmacokinetics showed increased t 1/2 in older versus younger adults. 98
3.4.6. Summary of studies showing age‐differences in pharmacokinetics: Bladder anticholinergics
The potently anticholinergic drug oxybutynin followed the trend of increasing peak plasma levels and bioavailability with increasing age and frailty. 140 This effect was so significant that study authors suggested halving the dose of oxybutynin for older adults to achieve the same plasma levels as younger adults. AUC and C Max were increased 20% and 16% respectively when an older population was given the same dose of oxybutynin as a younger population. Moreover, solifenacin, a newer bladder anticholinergic, had a longer t 1/2 due to slower elimination and longer time to reach C Max in older adults. This could be explained by the slowed absorption of solifenacin in older adults which increased their exposure to solifenacin by about 1.2‐fold. 120 In a study of 16 young men, 16 older men and 16 older women, receiving either 8 mg of fesoterodine extended release or matching placebo, the renal clearance of fesoterodine was 28% lower in older men and women than younger men 118 (Table 3). This increased exposure to fesoterodine in older adults may predict increased exposure of tolterodine in older adults as well, as fesoterodine and tolterodine are related compounds, with both being metabolized to the same active ingredient.
3.4.7. Summary of studies showing age‐differences in pharmacokinetics: Scopolamine
Healthy adult subjects were given scopolamine hydrobromide 0.5 mg IV if they were under 65 years of age and 0.3 mg if older than 65 years. These subjects then received a battery of tests of cognitive function in addition to measurement of pharmacokinetic variables. Older age was associated with slowed clearance and increased exposure to scopolamine. Age‐related increases in scopolamine exposure was likely the greatest contributor to the increased sensitivity to cognitive adverse effects in older adults. The study authors hypothesized that age‐related changes in CYP3A4 activity or content may have been responsible for the increased scopolamine exposure in older adults. 141
3.5. Genetics
In addition to age and sex, it is important that we understand how genetic variation in CYP activity could influence clinical effect or toxicity as drugs that are substrates for these enzymes are frequently used by older adults. 29
3.5.1. CYP2D6
Genetic variation in the CYP2D6 gene has been well characterized and identified 120 CYP2D6 variants (alleles) that have altered levels of CYP2D6 enzyme activity. These alleles result from point mutations, deletions or additions, gene rearrangements, and deletion or duplication/multiplication of the entire gene and have different distributions among various ethnic groups. Phenotypically, individuals with two normal CYP2D6 alleles are extensive metabolizers (EMs), those with one normal and one poor metabolism allele are intermediate metabolizers (IMs) and those with two reduced metabolism alleles are poor metabolizers (PMs). For CYP2D6, there is a fourth phenotype, the ultra‐rapid metabolizers (UMs) which have at least one active CYP2D6 gene duplication. Of interest, PM variants are common in East Asian populations and exist across the world. Understanding the effect of these CYP2D6 variants on pharmacokinetics is important for predicting drug effect and adverse effect.
The effect of CYP2D6 phenotype on anticholinergic medication exposure had been investigated in older adults. CYP2D6 phenotypes had been well characterized with respect to codeine pharmacokinetics. Limited activation and effect of codeine occurred in CYP2D6 PMs, and increased metabolism and toxicity was reported in UMs. 142 Nortriptyline plasma levels were mostly correlated to CYP2D6 genotype and sex. 111 In nursing home patients exposed to anticholinergic drugs, the highest serum anticholinergic activity was found in groups of CYP2D6 PMs. 143 Analysis of risperidone metabolism in 70 healthy volunteers (of whom 82.9% were either IM or EM) revealed that polymorphisms of the CYP2D6 enzyme were much more responsible than sex for variation in risperidone metabolism. CYP2D6 phenotype explained 52% of interindividual variability in risperidone pharmacokinetics. The AUC of the active moiety was found to be 28% higher in CYP2D6 PM compared with IM, EM, and UM. No other genetic markers were found to significantly affect risperidone concentrations. 144 This genetic variation in the metabolism of risperidone was of such magnitude that it could alter results when conducting bioequivalence studies. 145 Differences in dose responses should be considered as clinically relevant for any person initiated on risperidone, further supporting using the lowest possible doses at all times.
The bladder anticholinergic tolterodine is metabolized to a similarly active 5‐hydroxymethyl tolterodine (5‐HMT) by CYP2D6. The bioavailability of tolterodine was strictly related to the genetic polymorphism of CYP2D6 and it ranged from 10% to 74%. 146 Byeon et al. investigated the relationship between CYP2D6 phenotypes and tolterodine pharmacokinetics in 46 Korean subjects. The single dose and multiple dose C Max and AUC0–24 of tolterodine, respectively, were significantly higher in the PM groups than in the EMs. The ratio of clearance to bioavailability of tolterodine in the EMs was 5‐ to 18‐fold higher than PM (variant dependent) in multiple dosing studies. 147 A Swedish study also found a difference in the absorption t 1/2 of tolterodine between EM (0.41 h) and PM (0.53 h), and EM were found to have a slight increase in heart rate at steady state in comparison with baseline, which was thought to be related to drug exposure. 148 Interest in understanding drug‐induced QT interval prolongation led to study of the effect of CYP2D6 polymorphism on ECG changes in the use of tolterodine and its active metabolite 5‐HMT. In CYP2D6 PM, the systemic exposure to tolterodine was higher than in EM (t 1/2 of tolterodine immediate release was 10 h in PM vs. 2 to 3 h in EM), which may have contributed to differences in ECG changes. 148 However, the total concentration of active moieties (tolterodine plus 5‐HMT) was similar for PM and EM, which makes dose adjustment unhelpful for equalizing drug exposure. Interestingly, 5‐HMT and tolterodine may have contributed differently to QT interval prolongation risk and so this was studied as well. QT interval prolongation in CYP2D6 PM was only slightly greater for PM likely due to differences in protein binding between the two active components. 149 As a further illustration of the impact of CYP2D6 genetic variation on anticholinergic pharmacokinetics, 4 mg daily dosing of fesoterodine produced a C Max of 3.45 ng/ml in CYP2D6 PM versus 1.89 ng/ml in CYP2D6 EM. A similar proportional result was also observed for 8 mg daily dosing of fesoterodine in PM (C Max of 6.40 ng/ml) versus EM (C Max 3.98 ng/ml). Fesoterodine equally followed CYP2D6 and CYP3A4 metabolism which should lessen susceptibility to the effects of CYP2D6 reduced metabolism, but this was not been clearly demonstrated. 150 The oral antimuscarinic agent darifenacin was metabolized by CYP3A4 and CYP2D6 with the main metabolite being inactive. 151 The oral bioavailability of darifenacin was significantly altered by the CYP2D6 genotype in a dose‐dependent fashion. In EM, the bioavailability of 7.5, 15, and 30 mg CR oral doses of darifenacin were 15%, 19%, and 25%, respectively. In IM and PM, this bioavailability became 40%–90% higher. There was less impact of the CYP2D6 variants on the systemic elimination of darifenacin. In UM, the t 1/2 of darifenacin was 3.12 h, while in PM it was 3.83 h. 119
All told, CYP2D6 was an important contributor to variation in the pharmacokinetics of its substrates. In a study of patients with schizophrenia, Jürgens et al. reported that PM and UM did receive higher doses of medication, including CYP2D6‐dependent antipsychotics, than EM and IM. UM would likely need higher doses to compensate for their increased metabolism, so it was reassuring to see this in practice. However higher doses being used by PM may reflect adverse drug events being misinterpreted as psychotic symptoms leading to inappropriate and potentially harmful dose increases. 152
3.5.2. CYP2C19 and CYP3A4
Genetic polymorphisms in the CYP2C19 gene also result in PM, IM, and EM phenotypes. To date, no studies have demonstrated a role of CYP2C19 genetic variation in anticholinergic medication pharmacokinetics. Previous research has failed to identify individuals with no CYP3A4 activity. Due to the lack of genetic PM of CYP3A4, other factors such as exposure to drug inducers and inhibitors, liver function, blood flow, and possibly age and sex were the biggest considerations for variation in CYP3A4 activity. 66 , 71
4. DISCUSSION
Anticholinergic medications pose serious risks to older adults that include increased risk of cognitive impairment (including dementia). We know that adverse drug reactions are often proportional to plasma drug concentrations or for anticholinergic medications the total serum anticholinergic activity 14 , 18 , 153 which makes the effects of sex, age, and CYP polymorphisms on drug disposition relevant for clinical decision‐making. While most of the studies described were small in size and short in duration, there are findings supportive that for certain anticholinergic medications in certain settings (most often increased age or female sex) there is a risk of increased anticholinergic medication exposure. Most notably that older adults experience increased exposure to bladder anticholinergics. Being aware of the potential for increased drug exposure and the potential associated risks should help clinical decision‐making regarding use of anticholinergic medications.
This review on the role of sex, age, and CYP polymorphism on anticholinergic medications confirmed that lower doses are preferable for some individuals. First, women often experience increased drug exposure 49 , 122 which likely contributes to their experience of more adverse drug reactions than men. 95 , 97 , 115 , 116 , 118 Women can have other modifying factors such as increased age or CYP polymorphisms which can further potentiate their increased exposure to anticholinergic medications. While the tenants of Geriatric medicine have been relatively effective in communicating the importance of lower doses in older adults, the importance of sex in dosing has been poorly translated into clinical practice. Monographs frequently provide advice for dosing in the oldest users but rarely offer advice for dosing in women. Second, older age leads to alterations in drug metabolism and elimination that can also increase drug exposure. And third, clinical testing of CYP2D6 polymorphisms and adoption of peer‐reviewed published clinical practice guidelines for prescribing based on genotype 154 , 155 , 156 where strong evidence exists may also help reduce the burden of adverse drug responses in older people. With increased risk of hospitalization, cognitive impairment, and mortality as risks from anticholinergic drug use, improved understanding of sex, age, and genomic testing of CYP isozymes may be indicated to reduce serious anticholinergic adverse events. Rigorous pharmacokinetic analysis is a much needed and important next step to allow us to understand how dosing recommendations can be modified to treat older men and women most safely and effectively. Studies done in the past often examined age, sex, or CYP polymorphisms alone and future work needs to account for all these factors in combination so that we may better approach personalized medicine for optimal outcomes.
DISCLOSURE
There are no conflicts of interest to disclose for any author.
AUTHOR CONTRIBUTION
Shanna Trenaman chose the topic of the review, developed the search strategy, completed the search, chose studies to include, drafted the document and finalized revisions, Kerry Goralski supported during the search and manuscript drafting and then revised the manuscript, Susan Bowles and Melissa Andrew revised the final draft supporting the clinical context in the review.
1.
TABLE A1.
Comprehensive table of anticholinergic drugs with pharmacokinetic considerations for age, sex, and CYP polymorphism
| Generic drug name | ACB 1 | ARS 2 | ADME 3 | Effect of | ||
|---|---|---|---|---|---|---|
| Sex on ADME | Age on ADME | Genetics on ADME | ||||
| Alprazolam | 1 |
F: approximately 90% Distribution: 80%, mostly to albumin Metabolism: Liver, extensive via CYP3A Renal clearance: 371 ml/h Renal excretion: 80% Fecal excretion: 7% TBC: 76 ml/min T 1/2: After oral administration to healthy adults 11.2 h |
The weight‐normalized clearance of alprazolam is 20%–30% higher in young women than in young men | Renal clearance is significantly decreased in elderly men | ||
| Amantadine | 2 | 2 |
F: 86%–94% Distribution: 59%–67% bound to serum proteins V d: 404 L or 4.9 L/kg Metabolism: Liver, extensive via CYP3A Renal clearance: 371 ml/h Renal excretion: 80% Fecal excretion: 0.6% TBC: 0.2–0.3 L/h/kg T 1/2: 17 (±4) h |
Amantadine has significantly higher renal clearance in men | Reduced clearance in elderly patients and reduced renal function: 22.6–45 h | |
| Amitriptyline | 3 | 3 |
F: high Metabolism: Liver, CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 T 1/2: 15 h (range: 9–25 h) |
Amitriptyline plasma levels were higher in women than in men | 1.5‐fold higher ratio of absolute serum concentration to dose adjusted serum concentration in the oldest age group in comparison to controls <40 years of age | |
| Atenolol | 1 |
F: 46%–60% Distribution: <5% bound to serum proteins, brain tissue:blood concentration ratio of 0.2:1 V d: 50–75 L Metabolism: No liver metabolism and no active metabolites Renal excretion: 40%–50% Fecal excretion: 50% T 1/2: 6–7 h |
||||
| Atropine | 3 | 3 |
F: high Distribution: Serum protein binding is highly variable by age: 22.5% ±20.6% (<16 years), 14% ±9.1% (16–58 years), 22.2% ±16.7% (65–75 years) V d: 3.3–3.9 L/kg T 1/2: 4 h (adults), 6.5 h (children) |
Protein binding is highly variable upon age, t 1/2 varies by age | ||
| Baclofen | 2 |
F: 100% V d: 59.1 L Metabolism: Liver, limited Renal clearance: 103 ml/min Renal excretion: 69%–85% of oral dose Fecal excretion: 10% TBC: 180 ml/min T 1/2: 3–6.8 h |
||||
| Benztropine | 3 | 3 | F: poor | |||
| Brompheniramine | 3 |
V d: 11.7 L/kg Metabolism: Liver, extensive Renal excretion: 17% T 1/2: 25 h |
||||
| Bupropion | 1 |
Distribution: 84% bound to serum proteins, CSF concentration 10–25 fold higher than plasma V d: 19–21 L/kg Metabolism: Liver, extensive, primarily CYP2B6 Renal excretion: 87% Fecal excretion: 10% TBC: 160 ml/h (±23%) T 1/2: 14–21 h |
Mean AUC and C Max for bupropion are higher in women than men however once these parameters are standardized for body weight the statistical significance is lost | In older adults (mean age 71.5 years) the clearance was 80% that seen in younger adults and the elimination t 1/2 was extended to 34 h compared with most sources which report 11–14 h | ||
| Captopril | 1 |
F: 70%–75% Distribution: 25%–30% bound to serum proteins V d: 0.7 L/kg Metabolism: Liver, 50% Renal clearance: 0.4 L/kg/h Renal excretion: 95% TBC: 0.8 L/kg/h T 1/2: 1.9 h |
||||
| Carbamazepine | 2 |
F: 70%–79% Distribution: 76% bound to serum proteins, the CSF/serum ratio 0.22 V d: 0.8–2 L/kg Metabolism: Liver, 98%, extensive via CYP3A4, inducer of CYP3A4 and CYP1A2 Renal excretion: 72% Fecal excretion: 28% TBC: 80 ml/min T 1/2: 12–17 h |
Patients 70 years and older had a decreased clearance by approximately 70% | |||
| Cetirizine | 1 | 2 |
F: rapid and complete Distribution: 93% bound to serum proteins V d: 0.5–0.8 L/kg Metabolism: Liver, minimal Renal excretion: 60% Fecal excretion: 10% TBC: 53 ml/min T 1/2: 7.4–9 h |
The t 1/2 is prolonged by 50% in older adults and in patients with chronic liver disease as compared with normal healthy adults | ||
| Chlorpheniramine | 3 | 3 |
F: good V d: 3.2 L/kg Metabolism: Liver, extensive Renal excretion: 50% Fecal excretion: <1% TBC: 234–470 ml/h/kg T 1/2: 20 h |
|||
| Chlorpromazine | 3 | 3 |
F: 32% Distribution: 90%–99% bound to serum proteins, CSF concentration 5 times the plasma concentration V d: 8–160 L/kg Metabolism: Liver, large extent Renal excretion: 23% T 1/2: 6 h |
|||
| Cimetidine | 1 | 2 | ||||
| Clomipramine | 3 |
F: 20%–78% Distribution: 97% bound to serum proteins, mostly albumin, CSF:plasma ratio is 2.6 V d: 7–20 L/kg Metabolism: Liver, extensive Renal excretion: 51%–60% Fecal excretion: 24%–32% TBC: 12.7–56.5 L/h T 1/2: 19–37 h |
The ratio of absolute serum concentration in comparison with the dose‐adjusted serum concentration is 1.1‐ to 1.5‐fold higher in women than in men, which suggests a dose reduction of 10%–30% for females | There is a 1.5‐fold higher ratio of absolute serum concentration to dose adjusted serum concentration in the oldest age group in comparison to controls <40 years of age | ||
| Clozapine | 3 |
F: 50%–60% Distribution: 97% bound to serum proteins V d: 6 L/kg Metabolism: Liver, extensive via CYP2D6, CYP1A2 and CYP3A4 Renal excretion: 50% Fecal excretion: 30% T 1/2: 8–12 h |
TBC differs between men and women: Men—36.7 L/h; Women—27 L/h | TBC differs by age at 39 years of age or older clearance is decreased by 0.219 L/h | ||
| Codeine | 1 |
Distribution: 7%–25% bound to serum proteins V d: 3–6 L/kg Metabolism: Liver, extensive by CYP2D6, CYP3A4 and UDP‐glucuronosyltransferases Renal excretion: 90% T 1/2: 3 h |
A specific CYP2D6 genotype are ultra‐rapid metabolizers (UM) of codeine who convert codeine into morphine, more rapidly and completely which may lead to higher than expected serum morphine levels, increasing the risk of overdose symptoms even at labeled doses | |||
| Colchicine | 1 |
F: approximately 45% Distribution: 39% bound to albumin V d: 5–8 L/kg Metabolism: Liver, partial via CYP3A and p‐glycoprotein substrate Renal clearance: 0.727 L/h/kg Renal excretion: 40%–65% Fecal excretion: extensive TBC: 30.3 L/h T 1/2: 26.6–31.2 |
In a single dose study, the plasma t 1/2 in elderly males was 30 and 34 h in elderly females | Following a single oral dose of colchicine 0.6 mg, the mean apparent t 1/2 was 24.92 ± 5.34 h for subjects age 18–30 years (n = 21) and 30.06 ± 10.78 h for subjects of mean age 62.83 years (n = 18) | ||
| Cyclobenzaprine | 2 | 2 |
F: 33%–55% Distribution: 93% bound to serum proteins Metabolism: Liver, extensive via P450 CYP3A4, CYP1A2, CYP2D6 Renal excretion: 51% TBC: 0.7 L/min T 1/2: 18 h |
In those >65 years of age receiving cyclobenzaprine hydrochloride extended release 30 mg capsules, the plasma t 1/2 was prolonged (50 h) compared to younger subjects (32 h) | ||
| Cyproheptadine | 2 | 3 |
Metabolism: Liver 57% Renal excretion: 40% Fecal excretion: 2%–20% T 1/2: 16 h |
|||
| Darifenacin | 3 |
F: 15%–25% Distribution: 98% bound to serum proteins, mostly alpha‐1‐acid glycoprotein V d: 163 L Metabolism: Liver, extensive via CYP3A, CYP2D6 Renal excretion: 60% Fecal excretion: 40% TBC: 32–40 L/h T 1/2: 13–19 h |
Total body clearance is 31.1% lower in females than males | Approximately 7% of Caucasians and 2% of African Americans are poor metabolizers (PM) of CYP2D6 metabolized drugs which shunts its metabolism to CYP3A4, C Max/AUC for oral darifenacin 15 mg once daily at steady state was 1.9 for PM and 1.7 for extensive metabolizers (EM) | ||
| Desipramine | 3 | 2 |
V d: 33–42 L/kg Metabolism: Liver, extensive Renal excretion: 70% T 1/2: 14.3–24.7 h |
Faster oral clearance in older men than older women | T 1/2 is prolonged in older adults (t 1/2 30 h) | "Slow" metabolizers have a t 1/2 77 h |
| Desloratadine | 1 |
Distribution: 82%–87% bound to serum proteins Metabolism: Liver, extensive via CYP2C8 Renal excretion: 40.6% Fecal excretion: 46.5% TBC: 150 L/h T 1/2: 19–40 h |
||||
| Diazepam | 1 |
F: ~98% Distribution: 95%–99.3% bound to serum proteins, CSF concentration is 1.6% of the total plasma concentration V d: 0.8–1 L/kg Metabolism: Liver, extensive Renal excretion:75% T 1/2: up to 48 h |
Protein binding is significantly greater in males than in females (1.87 L/kg in young females vs. 1.34 L/kg in young males) Greater clearance in women than men based on CYP3A4 clearance Shorter t 1/2 in men compared to women (32 h vs. 46.2 h) |
Protein binding is significantly greater in older females than younger females (2.46 L/kg in older females vs. 1.38 L/kg in younger females), the V d is larger for older males than younger males (1.65 L/kg for older males vs. 1.19 L/kg for younger males), t 1/2 increases by about 1 h for each year beginning with a t 1/2 of 20 h at 20 years, the mean t 1/2 increased with age to 79 h (range, 37–169 h) | ||
| Dicyclomine | 3 | 3 |
F: rapidly absorbed V d: 3.65 L/kg Metabolism: Liver, extensive via CYP3A Renal excretion: 79.5% Fecal excretion: 8.4% T 1/2: 1.8 h |
|||
| Digoxin | 1 |
F: 60%–80% Distribution: 25% bound to serum proteins, does cross the blood brain barrier V d: 475–500 L Metabolism: Liver 13%, substrate of p‐glycoprotein Renal excretion: 50%–70% Fecal excretion: 3%–5% T 1/2: 36–48 h |
Slower digoxin clearance in females | In the elderly, the V d may be reduced, which could increase serum concentrations, elimination may occur more slowly in older adults, due to age‐related decline in renal function | ||
| Dimenhydrinate | 3 |
F: well absorbed Metabolism: Liver, extensive |
||||
| Diphenhydramine | 3 | 3 |
F: 65%–100% Distribution: 76%–85% bound to serum proteins V d: 480–292 L/70 kg Metabolism: Liver 50% TBC: 11.7–49.2 ml/min/kg T 1/2: 4–8 h |
|||
| Doxepin | 3 |
Distribution: 80% bound to serum proteins V d: 11,930 L Metabolism: Liver, extensive via CYP2D6, CYP2C19 Renal Excretion: <3% T 1/2: 15.3 h |
Females had significantly higher dose‐corrected serum concentration doxepine/N‐doxepine (29%) | Patients older than 60 years had significantly higher dose corrected serum concentration of doxepin and N‐doxepin (48%), than patients up to 60 years | ||
| Doxylamine | 3 |
F: good T 1/2: 10.1–13.11 h |
T 1/2 in older men (mean age, 66 years) is 15.5 ± 2.1 h, in older women (mean age, 73 years), the t 1/2 was longer than in young women, but the difference was not statistically significant (12.2 h vs. 10.1 h) | |||
| Fentanyl | 1 |
Distribution: 80%–86% bound to serum proteins V d: 4–6 L/kg Renal excretion: <7% Fecal excretion: 1%–9% TBC: 42–53 L/h T 1/2: 3–27 h (depending on dosage form) |
||||
| Fesoterodine | 3 |
F: 52% Distribution: 50% bound to serum proteins V d: 169 L Metabolism: Liver, extensive via CYP2D6, CYP3A Renal excretion: 70% Fecal excretion: 7% |
In older adults, renal clearance of fesoterodine is reduced | |||
| Fluvoxamine | 1 |
F: 53% Distribution: 80% bound to serum proteins, mostly albumin V d: 25 L/kg Metabolism: Liver, extensive, Inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 Renal excretion: 94% Fecal excretion: 7% T 1/2: 15.6–16.3 h |
Higher serum concentration in women than men at 100 mg orally | In older patients the clearance of fluvoxamine was reduced by 50% | ||
| Furosemide | 1 |
F: 47%–70% Distribution: 91%–99% bound to serum proteins, mostly albumin V d: 0.2 L/kg Metabolism: Liver 10% Renal clearance: 2 ml/min/kg Renal excretion: 60%–90% Fecal excretion: 7%–9% TBC: 76 ml/min T 1/2: 30–120 min |
T 1/2 is prolonged in older adults | |||
| Haloperidol | 1 | 1 |
F: 60%–70% Distribution: >90% bound to serum proteins V d: 9.5–21.7 L/kg Metabolism: Liver, extensive via CYP3A Renal excretion: 33%–40% Fecal excretion: 15% |
|||
| Hydralazine | 1 |
F: 38%–50% Distribution: 88%–90% bound to serum proteins V d: 0.3–8.2 L/kg Metabolism: Liver, extensive Renal excretion: 3%–14% Fecal excretion: 3–12% T 1/2: 3–5 h |
||||
| Hydrocortisone | 1 |
F: 96% Distribution: 90% bound to serum proteins, mostly corticosteroid‐binding globuli V d: 34 L Metabolism: Liver, extensive via CYP3A Renal excretion: extensive TBC: 18 L/h T 1/2: 1–2 h |
||||
| Hydroxyzine | 3 | 3 |
V d: 16 L/kg Metabolism: Liver T 1/2: 3–20 h |
A mean t 1/2 of 29.3 h was reported after administration of 0.7 mg/kg hydroxyzine syrup to 9 healthy, fasting adults mean age 69.5 years | ||
| Hyoscyamine | 3 | 3 |
F: complete Renal excretion: majority unchanged T 1/2: 7.47 h |
|||
| Imipramine | 3 | 3 |
F: 94%–96% Distribution: 89% bound to serum proteins V d: 10–20 L/kg Metabolism: Liver, extensive via CYP2C19 T 1/2: 6–18 h |
In older adults t 1/2 ranges from 25–30 h | ||
| Isosorbide | 1 |
F: approximately 100% Distribution: <5%% bound to serum proteins V d: 0.6–0.7 L/kg Metabolism: Liver 98% Renal clearance: 371 ml/h Renal excretion: 93% Fecal excretion: 1% TBC: 115–140 ml/min T 1/2: 5 h |
||||
| Loperamide | 1 | 2 |
F: 0.3% Renal excretion: 1% Fecal excretion: 25%–40% T 1/2: 7–15 h |
|||
| Loratadine | 1 | 2 |
Distribution: 97% bound to serum proteins Metabolism: Liver, extensive via CYP3A, CYP2D6 T 1/2: 12–15 h |
Older adults (n = 12) reported to have a t 1/2 of 17.5 h (range of 11–38 h) | ||
| Loxapine | 2 |
F: complete Distribution: 96.6% bound to serum proteins Metabolism: Liver, extensive via CYP1A2, CYP3A4, CYP 2D6, p‐glycoprotein inhibitor T 1/2: 17.6 h |
||||
| Meperidine | 2 |
Distribution: 65%–80% bound to serum proteins, mostly albumin and alpha‐1‐acid glycoprotein V d: 3.1–5 L/kg Metabolism: Liver, extensive T 1/2: 3.2–3.7 h |
In older adults, meperidine is less protein bound; however, the clearance rate is unchanged, therefore the V d may be greater with more available free drug, and in older adults the t 1/2 is extended | |||
| Methocarbamol | 3 | 1 |
F: completely Metabolism: Liver, extensive Renal excretion: 10%–15% Fecal excretion: small amount T 1/2: 0.9–2 h |
|||
| Metho‐trimeprazine | 2 |
V d: 29.8 L/kg Metabolism: Liver Fecal excretion: small amount T 1/2: 15 h |
||||
| Metoprolol | 1 |
F: 50% Distribution: 10% bound to serum albumin, CSF concentration close to the plasma concentration V d: 3.2–5.6 L/kg Metabolism: Liver, extensive via CYP2D6 Renal excretion: 95% T 1/2: 3–4 h |
In CYP2D6 PM the mean t 1/2 of metoprolol is 7–9 h | |||
| Morphine | 1 |
F: 20%–40% Distribution: 20%–36% bound to serum proteins V d: 1–6 L/kg Metabolism: Liver Renal excretion: 90% Fecal excretion: 7–10% TBC: 20–30 ml/min/kg T 1/2: 1.5–4.5 h |
||||
| Nifedipine | 1 |
F: complete Distribution: 92%–98% bound to serum proteins Metabolism: Liver, extensive via CYP3A4 Renal excretion: 80% Fecal excretion: 20% TBC: 4.3 ml/min/kg T 1/2: 2 h |
Greater clearance in women, mainly due to CYP3A4 and is 20%–30% higher in young women than young men, women reach higher plasma levels at same dose | Clearance is significantly reduced in older subjects (unrelated to renal function) compared to younger subjects, following IV administration clearance in older subjects was 348 ml/min compared with 519 ml/min in young subjects | ||
| Nortriptyline | 3 | 2 |
F: 60% Distribution: 86%–95% bound to serum proteins V d: 15–27 L/kg Metabolism: Liver, extensive via CYP2D6 Renal excretion: 2% T 1/2: 15–39 h |
Plasma levels are mostly affected by CYP2D6 genotype and sex with females experiencing higher plasma levels | The t 1/2 may be >90 h in older adults | Nortriptyline plasma levels are mostly affected by CYP2D6 genotype and sex with females experiencing higher plasma levels |
| Olanzapine | 3 | 2 |
F: well absorbed Distribution: 93% bound to serum proteins, mostly albumin and alpha‐1‐acid glycoprotein V d: 1,000 L Metabolism: Liver, extensive via CYP1A2, CYP2D6 Renal excretion: 57% Fecal excretion: 30% TBC: 26.1 L/h T 1/2: 21–54 h |
Men from a population including individuals with Alzheimer's disease or schizophrenia cleared olanzapine 38% faster than women | The mean t 1/2 was 1.5 times greater in healthy patients aged ≥65 years compared with younger patients age <65 years, according to a study of 24 healthy subjects | |
| Orphenadrine | 3 |
F: 95% Renal excretion: 60% T 1/2: 13.2–20.1 h |
||||
| Oxcarbazepine | 2 |
Distribution: 40% bound to serum proteins V d: 49 L Metabolism: Liver, extensive Renal excretion: >95% Fecal excretion: <4% T 1/2: 2 h |
||||
| Oxybutynin | 3 | 3 |
F: 6% Distribution: >99% bound to serum proteins, mostly alpha‐1‐acid glycoprotein Metabolism: Liver, extensive via CYP3A4 Renal excretion: <0.1% T 1/2: 2–3 h |
Oxybutynin was not shown to have any differences in AUC and C Max for men or women | Oxybutynin follows the trend of increasing peak plasma levels and bioavailability with increasing age and frailty | |
| Paroxetine | 3 | 1 |
F: complete Distribution: 93%–95% bound to serum proteins Metabolism: Liver, extensive via CYP2D6, also an inhibitor of CYP2D6 Renal excretion: 64% Fecal excretion: 36% TBC: 76 ml/min T 1/2: 15–21 h |
Sex is correlated to paroxetine plasma concentration, estimates of V 2 in male subjects were 461.30 ± 259.75 and in female subjects were 346.41 ± 255.81 | A naturalized study of paroxetine showed a 2‐fold higher ratio of absolute serum concentration to dose adjusted serum concentrations in the oldest age group in comparison to controls <40 years of age | |
| Perphenazine | 3 | 3 |
F: 20% V d: 10–34 L/kg Metabolism: Liver, extensive via CYP2D6 Renal excretion: 80% TBC: 100 L/h T 1/2: 8.4–12.3 h |
|||
| Prednisone | 1 |
F: 92% Distribution: 70% bound to serum proteins, mostly albumin and corticosteroid‐binding globuli V d: 0.4–1 L/kg Metabolism: Liver, extensive T 1/2: 2–3 h |
||||
| Quetiapine | 3 | 1 |
F: 100% Distribution: 83% bound to serum proteins V d: 10 L/kg Metabolism: Liver, extensive via CYP3A4 Renal excretion: 73% Fecal excretion: 20% T 1/2: 6–7 h |
Sex was not shown to effect pharmacokinetics of quetiapine | In a pharmacokinetic study, quetiapine clearance was reduced by 40% in patients ≥65 years (n = 9) compared with young patients (n = 12) | |
| Quinidine | 1 |
F: 70%–80% Distribution: 80%–88% bound to serum proteins, mostly albumin and alpha‐1‐acid glycoprotein V d: 2–3 L/kg Metabolism: Liver, extensive via CYP3A4 Renal clearance: 1 ml/min/kg Renal excretion: 5%–20% Fecal excretion: 1%–3% TBC: 3–5 ml/min/kg T 1/2: 6–8 h |
Women clear quinidine at a faster rate than men and women have ECG changes in response to drug activity much quicker than men which is not explained by quinidine clearance | |||
| Ranitidine | 1 | 1 |
F: 50% Distribution: 15% bound to serum proteins V d: 1.04–4.09 L/kg Metabolism: Liver, minor Renal clearance: 24.6–31.8 L/h Renal excretion: 3%–70% Fecal excretion: 3.1 ml/min/kg TBC: 1.29–1.44 L/h/kg T 1/2: 1.9–3 h |
The t 1/2 is 3–4 h in older adults after oral administration likely due to a decrease in renal function | ||
| Risperidone | 1 | 1 |
F: 70% Distribution: 90% bound to serum proteins V d: 1–2 L/kg Metabolism: Liver, extensive via CYP 2D6 Renal clearance: 0.96 L/h Renal excretion: 70% Fecal excretion: 14% TBC: 3.2–13.7 L/h T 1/2: 3–20 h |
Sex‐related differences in risperidone metabolism are unlikely to be significant | When the plasma concentration was adjusted for subject body weight or maintenance dose there were still significant differences between groups with the oldest group having the highest adjusted concentration | Polymorphisms of CYP2D6 are more responsible for variation in risperidone metabolism than sex |
| Scopolamine | 3 |
Metabolism: extensive Renal excretion: <10% T 1/2: 9.5 h |
||||
| Solifenacin | 3 |
F: approximately 90% Distribution: 98% bound to plasma proteins, primarily alpha‐1‐acid glycoprotein V d: 599–671 L Metabolism: Liver, extensively via CYP3A4 Renal clearance: 0.67–0.76 L/h Renal excretion: 3%–6% Fecal excretion: 22.5% TBC: 9.4 L/h T 1/2: 40–68 h |
Solifenacin has a longer t 1/2 due to slower elimination and to longer time to reach C Max in older adults, this can be explained by the reduced absorption of solifenacin in older adults. Exposure to solifenacin is increased about 1.2‐fold in older subjects | |||
| Theophylline | 1 |
F: well absorbed Distribution: 40% bound to serum proteins V d: 450 ml/kg Metabolism: Liver, extensive via CYP1A2 Renal excretion: 10%–13% Fecal excretion: 7% TBC: 76 ml/min T 1/2: 8.7 h |
Protein binding is reduced in older adults, older adults had reduced clearance 0.59 ± 0.07 ml/kg/min, and increased mean t 1/2 of 9.8 h (1.6–18 h) this was in healthy older non‐smokers and was not significantly different from clearance values in otherwise healthy non‐smoking younger asthmatics | |||
| Thioridazine | 3 | 3 |
V d: 17.8 L/kg Metabolism: Liver, extensive Renal excretion: small amounts T 1/2: 21–24 h |
|||
| Tolterodine | 3 | 2 |
F: 77% V d: 113 L Metabolism: Liver, extensive via CYP2D6 Renal excretion: 77% Fecal excretion: 17% T 1/2: 1.9–3.7 h |
Metabolism is slowed in individuals who are CYP2D6 PM as metabolism is shunted to CYP3A4, t 1/2 is prolonged to 6.5 h with single doses and 9.6 h with multiple doses | ||
| Trazodone | 1 | 1 |
F: 65% Distribution: 89%–95% bound to serum proteins V d: 0.47–0.84 L/kg Metabolism: Liver, extensive Renal clearance: 3–5.3 L/h Renal excretion: 70%–75% Fecal excretion: 21% TBC: 5.3 L/h T 1/2: 7 h |
|||
| Triamterene | 1 |
F: 30%–70% Distribution: 55%–67% bound to serum proteins Metabolism: Liver 80% Renal excretion: 21% T 1/2: 1.5–2.5 h |
T 1/2 was 4.3 h in young adults, and was prolonged to 6.5 h in an older patient | |||
| Trifluoperazine | 3 | 3 |
F: readily absorbed Distribution: 90%–99% bound to serum proteins Metabolism: Liver T 1/2: 24 h |
|||
| Trospium | 3 |
F: 9.6% Distribution: 50%–85% bound to serum proteins V d: 395 L Metabolism: Liver Renal clearance: 29.07 L/h Renal excretion: 5.8% Fecal excretion: 85.2% T 1/2: 20 h |
||||
| Venlafaxine | 1 |
Distribution: 27%–30% bound to serum proteins V d: 7.5 L/kg Metabolism: Liver, extensive via CYP2D6 Renal clearance: 0.074–0.079 L/h/kg Renal excretion: 87% Fecal excretion: 2% TBC: 1.3 L/h/kg T 1/2: 5 h |
Venlafaxine serum concentrations differed in men and women with higher concentrations achieved by women (215 and 151 nmol/L), the ratio of absolute serum concentration in comparison to the dose‐adjusted serum concentration is 1‐ to 1.5‐fold higher in women than in men | The concentration to dose ratio of venlafaxine was 1.5‐fold higher in adults over 65 in comparison with controls <40 years old | The serum concentration of N‐desmethyl‐venlafaxine was 5.5‐fold higher in a subset of CYP2D6 PMs (p <.01) and 22‐fold higher in second subset of CYP2D6 PMs (p <.001) than in EM | |
| Warfarin | 1 |
F: completely absorbed Distribution: 99% bound to serum proteins V d: 0.14 L/kg Metabolism: Liver, extensive via CYP2C9, CYP2C19, CYP2C8, CYP2C18, CYP1A2, CYP3A4 Renal excretion: 92% TBC: dependent on CYP2C19 genotype T 1/2: 1 week |
||||
Abbreviations: ACB, Anticholinergic Cognitive Burden Scale; ADME, Absorption, Distribution, Metabolism and Excretion; ARS, Anticholinergic Risk Scale; AUC, area under the curve; CYP, cytochrome P450; F, Bioavailability; IV, intravenous; TBC, Total Body Clearance.
Funding information
Funding for this review was received from the Dalhousie Pharmacy Endowment Fund, the Nova Scotia Health Research Foundation (MED‐Research Programs‐2016‐37), and the Canadian Consortium on Neurodegeneration in Aging (CCNA) under Team 14 (PI: Melissa Andrew), which investigates how multi‐morbidity modifies the risk of dementia and the patterns of disease expression. The CCNA receives funding from the Canadian Institutes of Health Research (CNA‐137794) and partner organizations (www.ccna‐ccnv.ca).
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. O’Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(3):213‐218. 10.1093/ageing/afx178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2019;67(4):674–694. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 3. Ismail Z, Black SE, Camicioli R, et al. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimer's & Dementia. 2020;16(8):1182–1195. 10.1002/alz.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Lond Engl. 2020;396(10248):413–446. 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weigand AJ, Bondi MW, Thomas LR, et al. Association of anticholinergic medications and AD biomarkers with incidence of MCI among cognitively normal older adults. Neurology. 2020;95:e2295‐e2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chatterjee S, Bali V, Carnahan RM, Chen H, Johnson ML, Aparasu RR. Anticholinergic burden and risk of cognitive impairment in elderly nursing home residents with depression. Res Soc Adm Pharm. 2020;16(3):329–335. 10.1016/j.sapharm.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 7. Pieper NT, Grossi CM, Chan WY, et al. Anticholinergic drugs and incident dementia, mild cognitive impairment and cognitive decline: a meta‐analysis. Age Ageing. 2020;49:939‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hilmer MD, Simonsick E. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781. [DOI] [PubMed] [Google Scholar]
- 9. Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well‐tolerated once‐daily treatment for overactive bladder. Eur Urol. 2004;45:420‐429; discussion 429. [DOI] [PubMed] [Google Scholar]
- 10. Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non‐degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochs KL, Zell‐Kanter M, Mycyk MB, TOXIKON Consortium . Hot, blind, and mad: avoidable geriatric anticholinergic delirium. Am J Emerg Med. 2012;30(514):e1‐e3. [DOI] [PubMed] [Google Scholar]
- 12. Myint PK, Fox C, Kwok CS, Luben RN, Wareham NJ, Khaw KT. Total anticholinergic burden and risk of mortality and cardiovascular disease over 10 years in 21,636 middle‐aged and older men and women of EPIC‐Norfolk prospective population study. Age Ageing. 2015;44:219‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case‐control study. BMJ. 2018;361:k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chew ML, Mulsant BH, Pollock BG. Serum anticholinergic activity and cognition in patients with moderate‐to‐severe dementia. Am J Geriatr Psychiatry. 2005;13:535‐538. [DOI] [PubMed] [Google Scholar]
- 15. Mulsant B, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum Anticholinergic Activity in a Community‐Based Sample of Older Adults. Archives of General Psychiatry. 2003;60(2):198. 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 16. Lechevallier‐Michel N, Molimard M, Dartigues J‐F, Fabrigoule C, Fourrier‐Réglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005;59:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han L, McCusker J, Cole M, Abrahanowicz M, Primeau F, Elie M. Use of Medications With Anticholinergic Effect Predicts Clinical Severity of Delirium Symptoms in Older Medical Inpatients. Arch. Intern. Med. 2001;161(8):1099‐1105. 10.1001/archinte.161.8.1099 [DOI] [PubMed] [Google Scholar]
- 18. Golinger R, Peet T, Tune L. Association of Elevated Plasma Anticholinergic activity with delirium in surgical patients. Am J Psychiatry. 1987;144:1218. [DOI] [PubMed] [Google Scholar]
- 19. Trenaman S. Risk Factors for Drug‐Related Problems Causing Emergency Department Visits in Older Adults. Halifax, NS: Dalhousie University; 2014. [Google Scholar]
- 20. Kalisch Ellett LM, Pratt NL, Ramsay EN, Barratt JD, Roughead EE. Multiple anticholinergic medication use and risk of hospital admission for confusion or dementia. J Am Geriatr Soc. 2014;62:1916. [DOI] [PubMed] [Google Scholar]
- 21. Myint PK, Fox C, Kwok CS, Luben RN, Wareham NJ, Khaw K‐T. Total anticholinergic burden and risk of mortality and cardiovascular disease over 10 years in 21,636 middle‐aged and older men and women of EPIC‐Norfolk prospective population study. Age Ageing. 2015;44:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Risacher SL, McDonald BC, Tallman EF, et al. Association Between Anticholinergic Medication Use and Cognition, Brain Metabolism, and Brain Atrophy in Cognitively Normal Older Adults. JAMA Neurology. 2016;73(6):721. 10.1001/jamaneurol.2016.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe S, Fukatsu T, Kanemoto K. Risk of hospitalization associated with anticholinergic medication for patients with dementia. Psychogeriatrics. 2018;18:57‐63. [DOI] [PubMed] [Google Scholar]
- 24. Wauters M, Klamer T, Elseviers M, et al. Anticholinergic Exposure in a Cohort of Adults Aged 80 years and Over. Basic & Clinical Pharmacology & Toxicology. 2017;120(6):591–600. 10.1111/bcpt.12744 [DOI] [PubMed] [Google Scholar]
- 25. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477. [DOI] [PubMed] [Google Scholar]
- 26. Hilmer SN, Mager DE, Simonsick EM, et al. Drug Burden Index Score and functional decline in older people. Am J Med. 2009;122:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koyama A, Steinman M, Ensrud K, Hillier T, Yaffe K. Long‐term cognitive and functional effects of potentially inappropriate medications in older women. J Gerontol Biol Sci Med Sci. 2014;69:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lechevallier‐Michel N, Molimard M, Dartigues J, Fabrigoule C, Fourrier‐Reglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID study. Br J Clin Pharmacol. 2005;59(143):2005592143‐2005592151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabrera MA, Dip RM, Furlan MO, Rodrigues SL. Use of drugs that act on the cytochrome P450 system in the elderly. Clin Sao Paulo Braz. 2009;64:273‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naujokaitis D, Asmoniene V, Kadusevicius E. Cytochrome P450 2C19 enzyme, cytochrome P450 2C9 enzyme, and cytochrome P450 2D6 enzyme allelic variants and its possible effect on drug metabolism: a retrospective study. Medicine. 2021;100:e24545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103‐141. [DOI] [PubMed] [Google Scholar]
- 32. McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol. 2012;8:371‐382. [DOI] [PubMed] [Google Scholar]
- 33. Zhang HF, Zhu LL, Yang XB, Gao N, Fang Y, Wen Q, Qiao HL. Variation in the expression of cytochrome P450‐related miRNAs and transcriptional factors in human livers: correlation with cytochrome P450 gene phenotypes. Toxicol Appl Pharmacol. 2021;412:115389. [DOI] [PubMed] [Google Scholar]
- 34. Buostani M. Anticholinergic cognitive burden scale. Harvard Health; 2009:https://www.health.harvard.edu/newsletter_article/anticholinergic‐cognitive‐burden‐scale. Accessed July 30, 2020. [Google Scholar]
- 35. Goodman L, Gilman A, Brunton L, Lazo J, Parker K. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw‐Hill; 2006. [Google Scholar]
- 36. Stryer L. Visual excitation and recovery. J Biol Chem. 1991;266:10711. [PubMed] [Google Scholar]
- 37. Svoboda P, Teisinger J, Novotny J, et al. Biochemistry of transmembrane signaling mediated by trimeric G proteins. Physiol Res. 2004;53(Suppl 1):S141. [PubMed] [Google Scholar]
- 38. Svoboda P, Milligan G. Agonist‐induced transfer of the α subunits of the guanine‐nucleotide‐binding regulatory proteins Gq and G11 and of muscarinic m1 acetylcholine receptors from plasma membranes to a light‐vesicular membrane fraction. Eur J Biochem. 1994;224:455. [DOI] [PubMed] [Google Scholar]
- 39. Khan KM, Drescher MJ, Hatfield JS, Khan A‐M, Drescher DG. Muscarinic receptor subtypes are differentially distributed in the rat cochlea. Neuroscience. 2002;111:291‐302. [DOI] [PubMed] [Google Scholar]
- 40. Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent m2‐mediated inhibition via Galphai3 and m3‐mediated stimulation via Gbetagammaq. J Biol Chem. 1997;272:21317‐21324. [DOI] [PubMed] [Google Scholar]
- 41. Rudolph J, Salow M, Angelini M, McGlinchey R. The anticholinergic risk scale and anticholinergic adverse effects in older person. Arch Intern Med. 2008;168:508. [DOI] [PubMed] [Google Scholar]
- 42. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium. Am J Psychiatry. 1992;149:1393. [DOI] [PubMed] [Google Scholar]
- 43. Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4:311. [Google Scholar]
- 44. Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug‐related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481. [DOI] [PubMed] [Google Scholar]
- 45. Madsen JL. Effects of gender, age, and body mass index on gastrointestinal transit times. Dig Dis Sci. 1992;37:1548. [DOI] [PubMed] [Google Scholar]
- 46. Madsen JL, Graff J. Effects of ageing on gastrointestinal motor function. Age Ageing. 2004;33:154‐159. [DOI] [PubMed] [Google Scholar]
- 47. Bennett EJ, Evans P, Scott AM, et al. Psychological and sex features of delayed gut transit in functional gastrointestinal disorders. Gut. 2000;46:83‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Córdova‐Fraga T, De la Roca‐Chiapas JM, Solis S, Sosa M, Bernal‐Alvardo J, Hernandez E, Hernandez‐Gonzalez MH. Gender difference in the gastric emptying measured by magnetogastrography using a semi‐solid test meal. Acta Gastroenterol Latinoam. 2008;38:240. [PubMed] [Google Scholar]
- 49. Degen J, Phillips S. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36‐42. [DOI] [PubMed] [Google Scholar]
- 51. Stephen AM, Wiggins HS, Englyst HN, Cole TJ, Wayman JH. The effect of age, sex and level of intake of dietary fibre from wheat on large‐bowel function in thirty healthy subjects. Br J Nutr. 1986;56:349‐361. [DOI] [PubMed] [Google Scholar]
- 52. Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11‐17. [DOI] [PubMed] [Google Scholar]
- 53. Grossman M, Kirsner J, Gillespie I. Basal and histalogstimulated gastric secretion in control subjects and in patients with peptic ulcer or gastric cancer. Gastroenterology. 1963;45:14. [PubMed] [Google Scholar]
- 54. Feldman M, Barnett C. Fasting gastric pH and its relationship to true hypochlorhydria in humans. Dig Dis Sci. 1991;36:866‐869. [DOI] [PubMed] [Google Scholar]
- 55. Lindahl A, Ungell AL, Knutson L, Lennernas H. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm Res. 1997;14:497‐502. [DOI] [PubMed] [Google Scholar]
- 56. Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J, Augustijns P. Characterization of fasted‐state human intestinal fluids collected from duodenum and jejunum. J Pharm Pharmacol. 2006;58:1079‐1089. [DOI] [PubMed] [Google Scholar]
- 57. Gorski JC, Jones DR, Haehner‐Daniels BD, Hamman MA, O'Mara EM, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133. [DOI] [PubMed] [Google Scholar]
- 58. Kates RE, Keefe DLD, Schwartz J, Harapat S, Kirsten EB, Harrison DC. Verapamil disposition kinetics in chronic atrial fibrillation. Clin Pharmacol Ther. 1981;30:44. [DOI] [PubMed] [Google Scholar]
- 59. Krecic‐Shepard ME, Barnas CR, Slimko J, Jones MP, Schwartz JB. Gender‐specific effects on verapamil pharmacokinetics and pharmacodynamics in humans. J Clin Pharmacol. 2000;40:219. [DOI] [PubMed] [Google Scholar]
- 60. Paine MF, Ludington SS, Chen M‐L, Stewart PW, Huang S‐M, Watkins PB. Do men and women differ in proximal small intestinal cyp3a or P‐glycoprotein expression? Drug Metab Dispos. 2005;33:426. [DOI] [PubMed] [Google Scholar]
- 61. Kreci‐Schepard ME, Barnas CR, Slimko J, Schwartz JB. Faster clearance of sustained release verapamil in men versus women: continuing observations on sex‐specific differences after oral administration of verapamil. Clin Pharmacol Ther. 2000;68:286‐292. [DOI] [PubMed] [Google Scholar]
- 62. López‐Ortega M, Arroyo P. Anthropometric characteristics and body composition in Mexican older adults: age and sex differences. Br J Nutr. 2016;115:490. [DOI] [PubMed] [Google Scholar]
- 63. Schwartz J. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107. [DOI] [PubMed] [Google Scholar]
- 64. Pakulski C, Drobnik L, Millo B. Age and sex as factors modifying the function of the blood‐cerebrospinal fluid barrier. Med Sci Monit. 2000;6:314. [PubMed] [Google Scholar]
- 65. Roberts DJ, Goralski KB. A critical overview of the influence of inflammation and infection on P‐glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol. 2008;4:1245‐1264. [DOI] [PubMed] [Google Scholar]
- 66. Parkinson A, Mudra D, Johnson C, Dwyer A, Carroll K. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199:193. [DOI] [PubMed] [Google Scholar]
- 67. Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Oxazepam kinetics: effects of age and sex. J Pharmacol Exp Ther. 1980;215:86‐91. [PubMed] [Google Scholar]
- 68. Wolbold R, Klein K, Burk O, et al. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003;38:978‐988. [DOI] [PubMed] [Google Scholar]
- 69. George J, Byth K, Farrell G. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem Pharmacol. 1995;50:727. [DOI] [PubMed] [Google Scholar]
- 70. Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James OF, McManus M, Kremers P. Effects of age and gender on in vitro properties of human liver microsomal monooxygenases. Clinical Pharmacology and Therapeutics. 1990;48(4):365–374. 10.1038/clpt.1990.164 [DOI] [PubMed] [Google Scholar]
- 71. Hunt C, Westerkam W, Stave G. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275. [DOI] [PubMed] [Google Scholar]
- 72. Krecic‐Shepard ME, Park K, Barnas C, Slimko J, Kerwin DR, Schwartz JB. Race and sex influence clearance of nifedipine: Results of a population study. Clinical Pharmacology & Therapeutics. 2000;68(2):130–142. 10.1067/mcp.2000.108678 [DOI] [PubMed] [Google Scholar]
- 73. Greenblatt DJ, Allen MD, Harmatz JS, Shader RI. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27:301‐312. [DOI] [PubMed] [Google Scholar]
- 74. Pai HV, Upadhya SC, Chinta SJ, Hegde SN, Ravindranath V. Differential metabolism of alprazolam by liver and brain cytochrome (P4503A) to pharmacologically active metabolite. Pharmacogenomics J. 2002;2:243‐258. [DOI] [PubMed] [Google Scholar]
- 75. Chung M, Reitberg DP, Gaffney M, Singleton W. Clinical pharmacokinetics of nifedipine gastrointestinal therapeutic system: a controlled‐release formulation of nifedipine. Am J Med. 1987;83:10‐14. [DOI] [PubMed] [Google Scholar]
- 76. Greenblatt DJ, von Moltke LL. Gender has a small but statistically significant effect on clearance of CYP3A substrate drugs. J Clin Pharmacol. 2008;48:1350‐1355. [DOI] [PubMed] [Google Scholar]
- 77. Boulieu R, Lehmann B, Salord F, Fisher C, Morlet D. Pharmacokinetics of midazolam and its main metabolite 1‐hydroxymidazolam in intensive care patients. Eur J Drug Metab Pharmacokinet. 1998;23:255‐258. [DOI] [PubMed] [Google Scholar]
- 78. Hu ZY, Zhao YS. Sex‐dependent differences in cytochrome P450 3A activity as assessed by midazolam disposition in humans: a meta‐analysis. Drug Metab Dispos Biol Fate Chem. 2010;38:817‐823. [DOI] [PubMed] [Google Scholar]
- 79. del Carmen Carrasco‐Portugal M, Lujan M, Flores‐Murrieta FJ. Evaluation of gender in the oral pharmacokinetics of clindamycin in humans. Biopharm Drug Dispos. 2008;29:427‐430. [DOI] [PubMed] [Google Scholar]
- 80. Sills GJ, Brodie MJ. Pharmacokinetics and drug interactions with zonisamide. Epilepsia. 2007;48:435‐441. [DOI] [PubMed] [Google Scholar]
- 81. Bebia Z, Buch SC, Wilson JW, et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther. 2004;76:618‐627. [DOI] [PubMed] [Google Scholar]
- 82. Okada Y, Seo T, Wanibuchi A, Hashimoto N, Higa Y, Nakagawa K. Population estimation regarding the effects of cytochrome P450 2C19 and 3A5 polymorphisms on zonisamide clearance. Ther Drug Monit. 2008;30(4):540‐543 [DOI] [PubMed] [Google Scholar]
- 83. Sinues B, Fanlo A, Mayayo E, Carcas C, Vicente J, Arenaz I, Cebollada A. CYP2A6 activity in a healthy Spanish population: effect of age, sex, smoking, and oral contraceptives. Human & Experimental Toxicology. 2008;27(5):367–372. 10.1177/0960327107082224 [DOI] [PubMed] [Google Scholar]
- 84. Bock KW, Schrenk D, Forster A, Griese EU, Morike K, Eichelbaum M. The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP‐glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharmacogenetics. 1994;4:209‐218. [DOI] [PubMed] [Google Scholar]
- 85. Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI, et al. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP‐glucuronosyltransferase isoforms. J Pharmacol Exp Ther. 2001;299:998‐1006. [PubMed] [Google Scholar]
- 86. Pacifici GM, Evangelisti L, Giuliani L, Metelli RM, Giordani R. Zidovudine glucuronidation in human liver: interindividual variability. Int J Clin Pharmacol Ther. 1996;34:329‐334. [PubMed] [Google Scholar]
- 87. Divoll M, Greenblatt DJ, Harmatz JS, Shader RI. Effect of age and gender on disposition of temazepam. J Pharm Sci. 1981;70:1104‐1107. [DOI] [PubMed] [Google Scholar]
- 88. Miners JO, Attwood J, Birkett DJ. Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol. 1983;16:503‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol‐O‐methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381‐389. [DOI] [PubMed] [Google Scholar]
- 90. Schuetz E, Furuya K, Schuetz J. Interindividual variation in expression of P‐glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther. 1995;275:1011. [PubMed] [Google Scholar]
- 91. Godfrey C, Sweeney K, Miller K, Hamilton R, Kremer J. The population pharmacokinetics of long‐term methotrexate in rheumatoid arthritis. Br J Clin Pharmacol. 1998;46:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81‐95. [DOI] [PubMed] [Google Scholar]
- 93. Yukawa E, Mine H, Higuchi S. Digoxin population pharmacokinetics from routine clinical data: role of patient characteristics for estimating dosing regimens. J Pharm Pharmacol. 1992;44:761‐765. [DOI] [PubMed] [Google Scholar]
- 94. Gaudry SE, Sitar DS, Smyth DD, McKenzie JK, Aoki FY. Gender and age as factors in the inhibition of renal clearance of amantadine by quinine and quinidine. Clin Pharmacol Ther. 1993;54:23‐27. [DOI] [PubMed] [Google Scholar]
- 95. Benton R, Sale M, Flockhart D, Woosley R. Greater quinidine induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67:413. [DOI] [PubMed] [Google Scholar]
- 96. El‐Eraky H, Thomas S. Effects of sex on the pharmacokinetic and pharmacodynamics properties of quinidine. Br J Clin Pharmacol. 2003;56:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vicente J, Simlund J, Johannesen L, et al. Investigation of potential mechanisms of sex differences in quinidine‐induced torsade de pointes risk. Journal of Electrocardiology. 2015;48(4):533–538. 10.1016/j.jelectrocard.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 98. Winchell G, King J, Chavez‐Eng C, Constanzer M, Korn S. Cyclobenzaprine pharmacokinetics, including the effects of age, gender, and hepatic insufficiency. J Clin Pharmacol. 2002;42:61. [DOI] [PubMed] [Google Scholar]
- 99. MacLeod SM, Giles HG, Bengert B, Liu FF, Sellers EM. Age‐ and gender‐related differences in diazepam pharmacokinetics. J Clin Pharmacol. 1979;19:15‐19. [DOI] [PubMed] [Google Scholar]
- 100. Bigos KL, Pollock BG, Coley KC, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48:157‐165. [DOI] [PubMed] [Google Scholar]
- 101. Waade RB, Molden E, Refsum H, Hermann M. Serum concentrations of antidepressants in the elderly. Ther Drug Monit. 2012;34:25‐30. [DOI] [PubMed] [Google Scholar]
- 102. Mundo E, Pirola R, Bellodi L, Smeraldi E, Bareggi SR. Are gender differences in antiobsessional response related to different clomipramine metabolism? J Clin Psychopharmacol. 2002;22:341‐342. [DOI] [PubMed] [Google Scholar]
- 103. Unterecker S, Riederer P, Proft F, Maloney J, Deckert J, Pfuhlmann B. Effects of gender and age on serum concentrations of antidepressants under naturalistic conditions. J Neural Transm. 2013;1996(120):1237‐1246. [DOI] [PubMed] [Google Scholar]
- 104. Reis M, Aamo T, Spigset O, Ahlner J. Serum concentrations of antidepressant drugs in a naturalistic setting: compilation based on a large therapeutic drug monitoring database. Ther Drug Monit. 2009;31:42‐56. [DOI] [PubMed] [Google Scholar]
- 105. Hartter S, Wetzel H, Hammes E, Torkzadeh M, Hiemke C. Nonlinear pharmacokinetics of fluvoxamine and gender differences. Ther Drug Monit. 1998;20:446‐449. [DOI] [PubMed] [Google Scholar]
- 106. Feng Y, Pollock BG, Ferrell RE, Kimak MA, Reynolds CF, Bies RR. Paroxetine: population pharmacokinetic analysis in late‐life depression using sparse concentration sampling. British Journal of Clinical Pharmacology. 2006;61(5):558–569. 10.1111/j.1365-2125.2006.02629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gex‐Fabry M, Eap CB, Oneda B, Gervasoni N, Aubry JM, Bondolfi G, Bertschy G. CYP2D6 and ABCB1 Genetic Variability: Influence on Paroxetine Plasma Level and Therapeutic Response. Therapeutic Drug Monitoring. 2008;30(4):474–482. 10.1097/ftd.0b013e31817d6f5d [DOI] [PubMed] [Google Scholar]
- 108. Findlay JW, Van Wyck Fleet J, Smith PG, Butz RF, Hinton ML, Blum MR, Schroeder DH. Pharmacokinetics of bupropion, a novel antidepressant agent, following oral administration to healthy subjects. European Journal of Clinical Pharmacology. 1981;21(2):127–135. 10.1007/bf00637513 [DOI] [PubMed] [Google Scholar]
- 109. Sweet RA, Pollock BG, Kirshner M, Wright B, Altieri LP, DeVane CL. Pharmacokinetics of Single‐ and Multiple‐Dose Bupropion in Elderly Patients with Depression. The Journal of Clinical Pharmacology. 1995;35(9):876–884. 10.1002/j.1552-4604.1995.tb04132.x [DOI] [PubMed] [Google Scholar]
- 110. Preskorn SH, Mac DS. Plasma levels of amitriptyline: effect of age and sex. J Clin Psychiatry. 1985;46:276‐277. [PubMed] [Google Scholar]
- 111. Dahl ML, Bertilsson L, Nordin C. Steady‐state plasma levels of nortriptyline and its 10‐hydroxy metabolite: relationship to the CYP2D6 genotype. Psychopharmacology. 1996;123:315‐319. [DOI] [PubMed] [Google Scholar]
- 112. Abernethy DR, Greenblatt DJ, Shader RI. Imipramine and desipramine disposition in the elderly. J Pharmacol Exp Ther. 1985;232:183‐188. [PubMed] [Google Scholar]
- 113. Aichhorn W, Weiss U, Marksteiner J, et al. Influence of age and gender on risperidone plasma concentrations. J Psychopharmacol. 2005;19:395. [DOI] [PubMed] [Google Scholar]
- 114. McCune JS, Lindley C, Decker JL, et al. Lack of gender differences and large intrasubject variability in cytochrome P450 activity measured by phenotyping with dextromethorphan. J Clin Pharmacol. 2001;41:723. [DOI] [PubMed] [Google Scholar]
- 115. Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol. 2001;2:349‐351. [DOI] [PubMed] [Google Scholar]
- 116. Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17:100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lukkari E, Hakonen T, Neuvonen PJ. The pharmacokinetics of oxybutynin is unaffected by gender and contraceptive steroids. Eur J Clin Pharmacol. 1998;53:351‐354. [DOI] [PubMed] [Google Scholar]
- 118. Malhotra B, Wood N, Sachse R. Influence of age gender and race on pharmacokinetics, pharmacodynamics and safety of fesoterodine. Int J Clin Pharmacol Ther. 2009;47:570. [DOI] [PubMed] [Google Scholar]
- 119. Kerbusch T, Wahlby U, Milligan PA, Karlsson MO. Population pharmacokinetic modelling of darifenacin and its hydroxylated metabolite using pooled data, incorporating saturable first‐pass metabolism, CYP2D6 genotype and formulation‐dependent bioavailability. Br J Clin Pharmacol. 2003;56:639‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Doroshyenko O, Fuhr U. Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet. 2009;48:281‐302. [DOI] [PubMed] [Google Scholar]
- 121. Diefenbach K, Donath F, Maurer A, et al. Randomised, Double‐Blind Study??? of the Effects of Oxybutynin, Tolterodine, Trospium Chloride and Placebo on Sleep in Healthy Young Volunteers. Clinical Drug Investigation. 2003;23(6):395–404. 10.2165/00044011-200323060-00003 [DOI] [PubMed] [Google Scholar]
- 122. Koren G, Vranderick M, Gill S, Macleod S. Combination of doxylamine succinate‐pyridoxine hydrochloride; implications for pharmacotherapy in pregnancy. J Clin Pharmacol. 2013;53:1268. [DOI] [PubMed] [Google Scholar]
- 123. Ebert U, Oertel R, Kirch W. Influence of grapefruit juice on scopolamine pharmacokinetics and pharmacodynamics in healthy male and female subjects. Int J Clin Pharmacol Ther. 2000;38:523‐531. [DOI] [PubMed] [Google Scholar]
- 124. Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450‐linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61:331‐339. [DOI] [PubMed] [Google Scholar]
- 125. Greenblatt DJ, Harmatz JS, von Moltke LL, Wright CE, Shader RI. Age and gender effects on the pharmacokinetics and pharmacodynamics of triazolam, a cytochrome P450 3A substrate. Clin Pharmacol Ther. 2004;76:467‐479. [DOI] [PubMed] [Google Scholar]
- 126. Court MH. Interindividual variability in hepatic drug glucuronidation: studies into the role of age, sex, enzyme inducers, and genetic polymorphism using the human liver bank as a model system. Drug Metab Rev. 2010;42:209‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wynne HA, Cope LH, Herd B, Rawlins MD, James OF, Woodhouse KW. The association of age and frailty with paracetamol conjugation in man. Age Ageing. 1990;19:419‐424. [DOI] [PubMed] [Google Scholar]
- 128. Wynne HA, Yelland C, Cope LH, Boddy A, Woodhouse KW, Bateman DN. The association of age and frailty with the pharmacokinetics and pharmacodynamics of metoclopramide. Age Ageing. 1993;22:354‐359. [DOI] [PubMed] [Google Scholar]
- 129. Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age‐related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42(6):1349–1354. 10.1002/hep.20937 [DOI] [PubMed] [Google Scholar]
- 130. Le Couteur DG, Fraser R, Hilmer S, Rivory LP, McLean AJ. The hepatic sinusoid in aging and cirrhosis: effects on hepatic substrate disposition and drug clearance. Clin Pharmacokinet. 2005;44:187‐200. [DOI] [PubMed] [Google Scholar]
- 131. Le Couteur DG, Cogger VC, Markus AM, Harvey PJ, Yin ZL, Ansselin AD, McLean AJ. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33(3):537–543. 10.1053/jhep.2001.22754 [DOI] [PubMed] [Google Scholar]
- 132. Hilmer SN, Cogger VC, Muller M, Le Couteur DG. The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin. Drug Metab Dispos Biol Fate Chem. 2004;32:794‐799. [DOI] [PubMed] [Google Scholar]
- 133. Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31. [DOI] [PubMed] [Google Scholar]
- 134. Isah A, Rawlins M, Bateman D. The pharmacokinetics and effects of prochlorperazine in elderly female volunteers. Age Aging. 1992;21:27. [DOI] [PubMed] [Google Scholar]
- 135. van Assema D, Lubberink M, Boellaard R, et al. P‐glycoprotein function at the blood‐brain barrier: effects of age and gender. Mol Imaging Biol. 2012;14:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP 2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive‐related gender differences. Eur J CLin Pharmacol. 1999;55:177. [DOI] [PubMed] [Google Scholar]
- 137. Dawling S. Monitoring of tricyclic antidepressant therapy. Clin Biochem. 1982;15:56‐61. [DOI] [PubMed] [Google Scholar]
- 138. Sawamura K, Suzuki Y, Someya T. Effects of dosage and CYP2D6‐mutated allele on plasma concentration of paroxetine. Eur J Clin Pharmacol. 2004;60:553‐557. [DOI] [PubMed] [Google Scholar]
- 139. Goodnick PJ. Pharmacokinetics of second generation antidepressants: bupropion. Psychopharmacol Bull. 1991;27:513‐519. [PubMed] [Google Scholar]
- 140. Hughes KM, Lang JC, Lazare R, Gordon D, Stanton SL, Malone‐Lee J, Geraint M. Measurement of oxybutynin and its N‐desethyl metabolite in plasma, and its application to pharmacokinetic studies in young, elderly and frail elderly volunteers. Xenobiotica Fate Foreign Compd Biol Syst. 1992;22:859‐869. [DOI] [PubMed] [Google Scholar]
- 141. Alvarez‐Jimenez R, Groeneveld GJ, van Gerven JMA, Goulooze SC, Baakman AC, Hay JL, Stevens J. Model‐based exposure‐response analysis to quantify age related differences in the response to scopolamine in healthy subjects. Br J Clin Pharmacol. 2016;82:1011‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lopes GS, Bielinski SJ, Moyer AM, et al. Sex Differences in Associations Between CYP2D6 Phenotypes and Response to Opioid Analgesics. Pharmacogenomics and Personalized Medicine. 2020;13 71–79. 10.2147/pgpm.s239222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kersten H, Wyller T, Molden E. Association between inherited CYP2D6/2C19 phenotypes and anticholinergic measures in elderly patients using anticholinergic drugs. Ther Drug Monit. 2014;36:125. [DOI] [PubMed] [Google Scholar]
- 144. Vandenberghe F, Guidi M, Choong E, von Gunten A, Conus P, Csajka C, Eap CB. Genetics‐Based Population Pharmacokinetics and Pharmacodynamics of Risperidone in a Psychiatric Cohort. Clinical Pharmacokinetics. 2015;54(12):1259–1272. 10.1007/s40262-015-0289-8 [DOI] [PubMed] [Google Scholar]
- 145. Cabaleiro T, Ochoa D, Roman M, Moreno I, Lopez‐Rodriguez R, Noalbos J, Abad‐Santos F. Polymorphisms in CYP2D6 have a greater effect on variability of risperidone pharmacokinetics than gender. Basic Clin Pharmacol Toxicol. 2015;116(2):124‐128. 10.1111/bcpt.12286 [DOI] [PubMed] [Google Scholar]
- 146. Brynne N, Stahl MM, Hallen B, Edlund PO, Palmer L, Hoglund P, Gabrielsson J. Pharmacokinetics and pharmacodynamics of tolterodine in man: a new drug for the treatment of urinary bladder overactivity. Int J Clin Pharmacol Ther. 1997;35:287‐295. [PubMed] [Google Scholar]
- 147. Byeon J‐Y, Lee CM, Lee YJ, et al. Influence of CYP2D6 genetic polymorphism on pharmacokinetics of active moiety of tolterodine. Arch Pharm Res. 2019;42(2):182–190. 10.1007/s12272-018-1099-y [DOI] [PubMed] [Google Scholar]
- 148. Brynne N, Dalén P, Alván G, Bertilsson L, Gabrielsson J. Influence of CYP2D6 polymorphism on the pharmacokinetics and pharmacodynamic of tolterodine. Clin Pharmacol Ther. 1998;63:529‐539. [DOI] [PubMed] [Google Scholar]
- 149. Patel N, Wisniowska B, Polak S. Virtual thorough QT (TQT) trial‐extrapolation of in vitro cardiac safety data to in vivo situation using multi‐scale physiologically based ventricular cell‐wall model exemplified with tolterodine and fesoterodine. AAPS J. 2018;20:83. [DOI] [PubMed] [Google Scholar]
- 150. Nilvebrant L, Hallén B, Larsson G. Tolterodine–a new bladder selective muscarinic receptor antagonist: preclinical pharmacological and clinical data. Life Sci. 1997;60:1129‐1136. [DOI] [PubMed] [Google Scholar]
- 151. Beaumont KC, Cussans NJ, Nichols DJ, Smith DA. Pharmacokinetics and metabolism of darifenacin in the mouse, rat, dog and man. Xenobiotica Fate Foreign Compd Biol Syst. 1998;28:63‐75. [DOI] [PubMed] [Google Scholar]
- 152. Jürgens G, Rasmussen HB, Werge T, Dalhoff K, Nordentoft M, Andersen SE. Does the Medication Pattern Reflect the CYP2D6 Genotype in Patients With Diagnoses Within the Schizophrenic Spectrum?. Journal of Clinical Psychopharmacology. 2012;32(1):100–105. 10.1097/jcp.0b013e31823f6b6a [DOI] [PubMed] [Google Scholar]
- 153. Mulsant B, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community based sample of older adults. Arch Gen Psychiatry. 2003;60:198. [DOI] [PubMed] [Google Scholar]
- 154. Nassan M, Nicholson WT, Elliott ME, Rohrer Vitek CR, Black JL, Frye MA. Pharmacokinetic Pharmacogenetic Prescribing Guidelines for Antidepressants: A Template for Psychiatric Precision Medicine. Mayo Clinic Proceedings. 2016;91(7):897–907. 10.1016/j.mayocp.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 155. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm. 2015;122:5‐28. [DOI] [PubMed] [Google Scholar]
- 156. Guidelines. https://cpicpgx.org/guidelines/. Accessed July 30, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


