Abstract
Despite multiple attempts to develop a unifying hypothesis that explains the pathophysiology of heart failure with a reduced ejection fraction (HFrEF), no single conceptual model has withstood the test of time. In the present review we discuss how the results of recent successful phase III clinical development programs in HFrEF are built upon existing conceptual models for drug development. We will also discuss where recent successes in clinical trials do not fit existing models, in order to identify areas where further refinement of current paradigms may be needed. To provide the necessary structure for this review, we will begin with a brief overview of the pathophysiology of HFrEF, followed by an overview of the current conceptual models for HFrEF, and end with an analysis of the scientific rationale and clinical development programs for four new therapeutic classes of drugs that have improved clinical outcomes in HFrEF. The four new therapeutic classes that discussed are angiotensin receptor neprilysin inhibitors (ARNIs), sodium-glucose co-transoporter-2 inhibitors (SGLT2i), soluble guanylate cyclase stimulators and myosin activators. With the exception of SGLT2 inhibitors, each of these therapeutic advances were informed by the insights provided by existing conceptual models of heart failure. Although the quest to determine the mechanism of action of SGLT2i’s is ongoing, this therapeutic class of drugs may represent the most important advance in cardiovascular therapeutics of recent decades, and may lead to rethinking or expanding our current conceptual models for HFrEF.
Keywords: heart failure, HFrEF, translational medicine, therapeutic advances, clinical trial, drug therapy, Heart Failure, Translational Studies, Treatment
Despite repeated attempts to develop a unifying hypothesis that explains the pathophysiology of heart failure with a reduced ejection fraction (HFrEF), no single conceptual model has withstood the test of time. One reason for this shortcoming is that the development of clinical heart failure in patients with a reduced ejection fraction represents the complex interplay between structural and functional biological changes that are occurring in the heart, the autonomic nervous system, the kidney, the peripheral vasculature, and skeletal muscle. These biological changes are influenced by aging, genetic background, co-morbidities, and nutrition, as well as non-biological environmental factors, all of which add to the complexity of understanding the pathophysiology of heart failure.
The current review will discuss the translational framework that has provided a platform for developing drugs to treat patients with HFrEF for the past 30 years. Here we will depart from the traditional approach that places an emphasis on molecular signaling pathways as a means to identify new drug targets, in favor of discussing how the results of four recent successful phase III clinical development programs in HFrEF fit within our existing models for drug development, with the hope that the recent past will provide prologue for future drug discoveries. More importantly, we will discuss where recent successes in clinical trials do not fit existing models, in order to identify areas where further refinement of current paradigms may be needed. To provide the structure for this type of review, we will begin with a brief overview of the pathophysiology of HFrEF. This subject has been the topic of extensive reviews,1, 2 and will be discussed here briefly to provide context for understanding the clinical models for therapeutic drug development. Following, the review of the different clinical models, we will conclude with a discussion of what we have learned from recent successful phase III clinical trials.
Pathophysiology of Heart Failure with a Reduced Ejection Fraction
HFrEF arises secondary to a series of complex changes in the molecular and cellular composition of the heart (reviewed in 2, 3). These changes lead to the phenotypic changes in the size, shape and function of the failing heart that ultimately result in a decreased pumping capacity of the heart with a subsequent decrease in cardiac output (Figure 1). The molecular and cellular changes may occur suddenly (e.g., following a myocardial infarction), or may arise more gradually (e.g. following exposure to toxic chemotherapies or sustained hemodynamic overload), or may develop secondary to inherited mutations of genes that affect sarcomere function. The initial decline in cardiac output is perceived as “arterial underfilling” by the peripheral arterial baroreceptors that regulate autonomic nervous system parasympathetic and sympathetic signaling. When the baroreceptor mechanoreceptors detect a decrease in arterial filling, they trigger a series of homeostatic reflexes that are initiated by a withdrawal of parasympathetic tone that is followed by a reciprocal increase in sympathetic (adrenergic) nervous system signaling (reviewed in 4, 5). The baroreceptor-mediated increase in sympathetic nervous system signaling (SNS) triggers increased renin production by the kidneys, with resultant activation of the renin-angiotensin-aldosterone system (RAAS). Because SNS and RAAS signaling is highly synergistic they are referred to as the neurohumoral axis.6
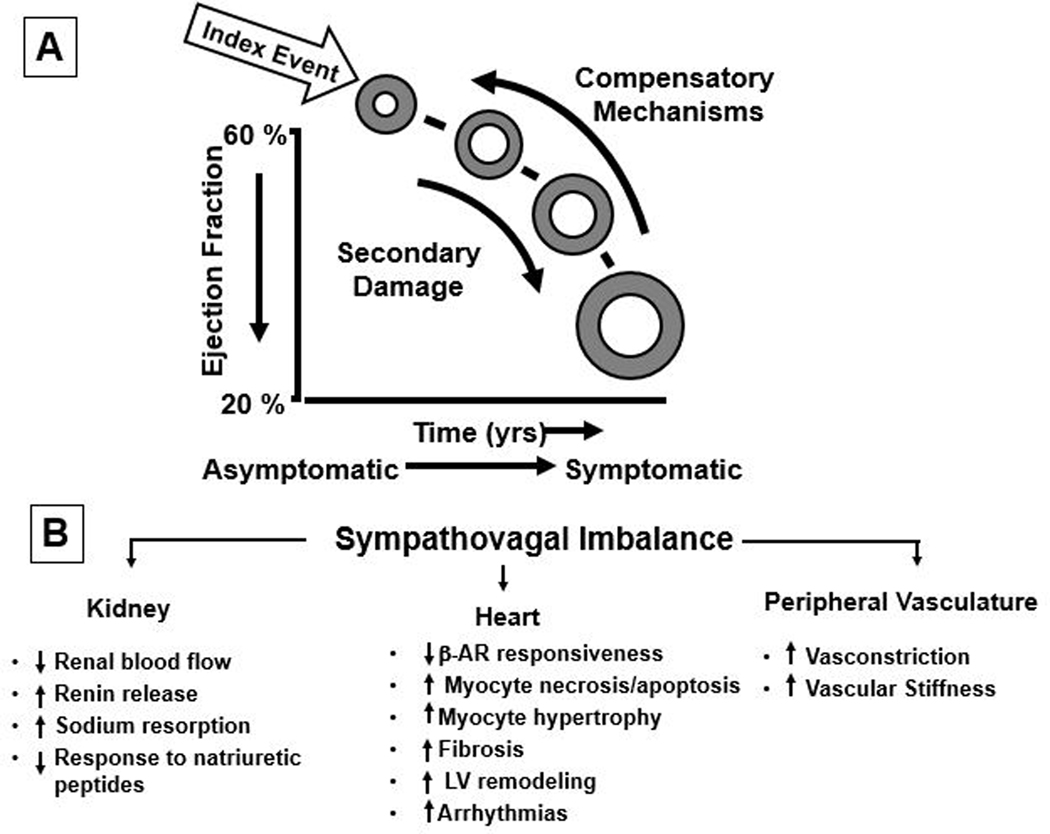
Figure 1. Pathogenesis of heart failure with a reduced ejection fraction.
A, heart failure begins after a so-called index event produces an initial decline in pumping capacity of the heart. B, The initial decline in cardiac output is perceived as “arterial underfilling” by peripheral arterial baroreceptors, which leads to a withdrawal of parasympathetic tone that is accompanied by a reciprocal increase in sympathetic (adrenergic) nervous activity (sympthovagal imbalance). The increase in sympathetic nervous signaling leads to activation of the renin angiotensin aldosterone system in the kidney, increased contractile force of the heart, and peripheral arterial vasoconstriction. In the short term, these changes restore cardiovascular function to a normal homeostatic range, with the result that the patient remains asymptomatic. With time, however, the sustained activation of these systems can lead to secondary end-organ damage within the ventricle, with worsening LV remodeling and subsequent cardiac decompensation. (From Mann DL: Mechanisms and models in HF: a combinatorial approach. Circulation 199;100:99)
The initial activation of the SNS and RAAS restores circulatory homeostasis by increasing contractility, increasing retention of sodium and water by the kidney, and by increasing peripheral arterial vasoconstriction.1, 6 In some patients pumping capacity of the heart will return to normal once the tissue injury is resolved or the inciting stress is removed. In this setting, the normalization of LV function results in restoration of circulatory homeostasis. However, if left ventricular function remains depressed, the SNS and RAAS remain persistently activated in an ongoing attempt to maintain circulatory homeostasis. Some patients with LV dysfunction will remain asymptomatic despite RAAS-induced expansion of the circulatory blood volume. The precise mechanism(s) that explains why certain patients remain asymptomatic is not known. One plausible explanation is that the compensatory mechanisms that become activated are sufficient to modulate cardiovascular and renal function within a physiologic/homeostatic range, such that the patient’s exercise capacity is preserved or is depressed only minimally.1
A number of counter regulatory biological systems are upregulated in the setting of heart failure to offset the deleterious effects of SNS and RAAS signaling on the cardiovascular system. Principal among these, are the natriuretic peptides, including atrial natriuretic peptide (ANP) and brain (B-type) natriuretic peptide (BNP).7 Under physiologic conditions, ANP and BNP function as natriuretic hormones that are released in response to increases in atrial and myocardial stretch. Once released, these cardiac derived peptides act on the kidney and peripheral circulation to unload the heart, by increasing renal excretion of sodium and water and arterial vasodilation, as well as inhibiting the release of renin and aldosterone by the kidney. 7
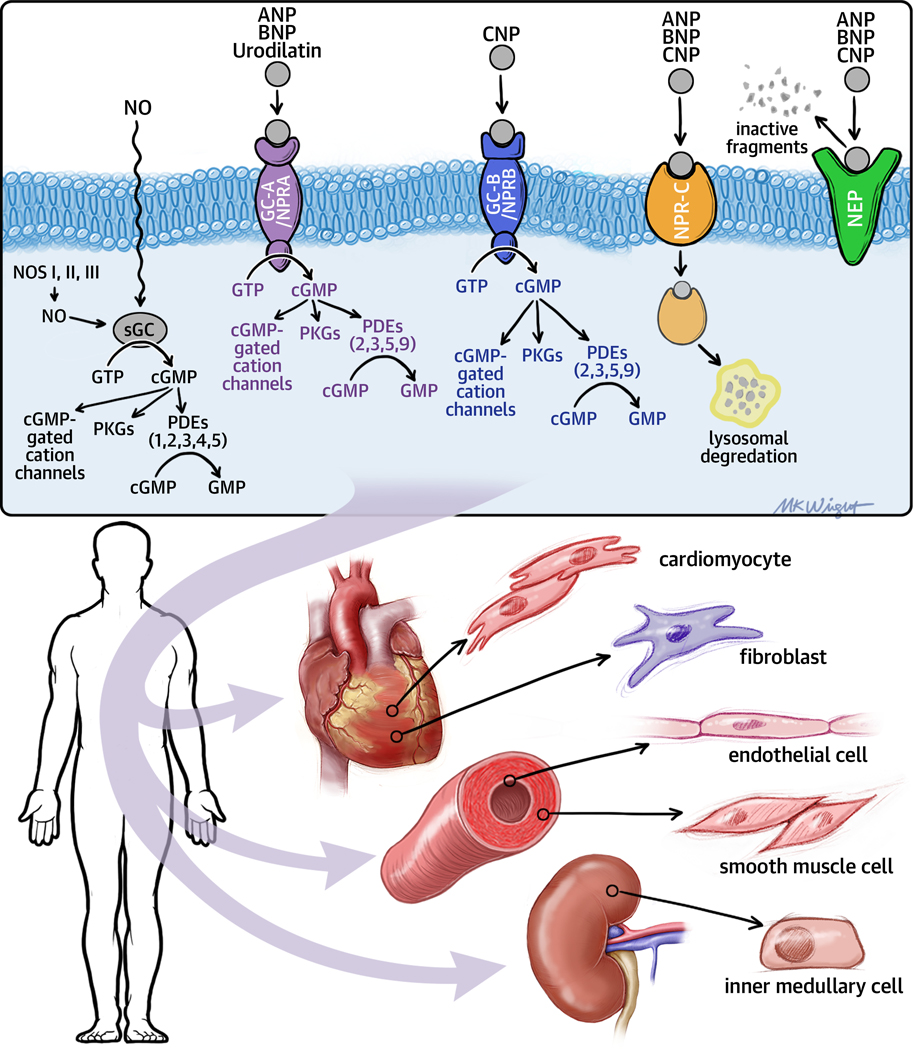
Figure 2 illustrates the signaling pathway of the natriuretic peptide system. As shown, natriuretic peptides stimulate the production of the intracellular second-messenger cyclic guanosine monophosphate (cGMP), via binding to the natriuretic peptide A receptor (NPR-A), which binds ANP and BNP preferentially, and the natriuretic peptide B receptor (NPR-B), which binds C-type natriuretic peptide preferentially. Both NPR-A and NPR-B are coupled to membrane bound (particulate) guanylate cyclase (GC), which activates the downstream signaling pathways of cyclic guanosine monophosphate (cGMP), including cGMP-dependent protein kinases I and II, cGMP-gated ion channels and cGMP-regulated phosphodiesterases.8 Natriuretic peptides are degraded by neutral endopeptidase (NEP) 24.11 (neprilysin), or clearance via natriuretic peptide receptor-C mediated internalization into cells for lysosomal degradation. 8,9 The biologic importance of the natriuretic peptides in renal sodium handling has been demonstrated in multiple studies employing NPR antagonists, as well as overexpression of ANP or BNP. Moreover, the concentrations of natriuretic peptides that are detected in the circulation have provided important diagnostic and prognostic information in heart failure.7 For reasons that are not entirely clear, the effects of the natriuretic peptides on teh kidney are blunted with advancing heart failure, leaving SNS and RAAS signaling unopposed.10–12 The release of nitric oxide (NO) by endothelial cells is a second biological system that plays a central role in mediating vascular tone in heart failure. NO elicits physiological effects in cells by binding to cytosolic GC (soluble GC [sGC]), leading to cGMP accumulation (Figure 2).
Figure 2. Natriuretic peptides and particulate (pGC) and soluble (sGC) guanylyl cyclase signaling pathways.
The natriuretic peptide system consists of five structurally similar peptides: ANP, urodilatin (an isoform of ANP), BNP, C-type natriuretic peptide (CNP), and dendroaspis natriuretic peptide (DNP). Natriuretic peptides bind to GC-A/NPRA and/or GC-B/NPRB (membrane-bound/particulate guanylate cyclases [pGCs]) and activate the signaling pathways of cyclic guanosine monophosphate (cGMP). The GC-A/NPRA receptor preferentially binds ANP and BNP, and the GC-B/NPRB receptor preferentially binds CNP. Both NPR-A and NPR-B are coupled to particulate particulate guanylate cyclase. Nitric oxide (NO) binds to soluble guanylate cyclase (sGC) in the cytoplasm, inducing cGMP production and activation of cGMP signaling pathways. Once the intracellular concentration of cGMP increases, cGMP-gated cation channels, cGMP-dependent protein kinases generate important biological responses in different tissues. Cyclic nucleotide phosphodiesterases (PDEs) hydrolyze cGMP thus inhibiting signal transduction. The PDEs are comprised of a superfamily of 11 diverse isozymes (numbered PDE1, PDE2, etc) that are compartmentalized within the cell. PDE5 is primarily expressed in the vascular smooth muscle cells and catabolizes cGMP generated by soluble guanylate cyclase. PDE9 also catabolizes cGMP, but is primarily expressed in the gastrointestinal tract, kidney and brain and catabolizes cGMP generated by particulate guanylate cyclase. The natriuretic peptides are degraded by two major mechanisms: natriuretic peptide C receptor (NPR-C) –mediated internalization, followed by lysosomal degradation and enzymatic degradation by neutral endopeptidase (NEP) 24.11 (neprilysin), which is widely expressed in which is widely expressed in multiple tissues, where it often is co-localized with angiotensin converting enzyme. (Other abbreviations: ANP- atrial natriuretic peptide, BNP- brain (B-type) natriuretic peptide, CNP –C type natriuretic peptide, GC-A, particulate guanylyl cyclase A; GC-B, particulate guanylyl cyclase B; GTP, guanosine triphosphate; NOS, nitric oxide synthase; NPRA – natriuretic peptide receptor A, NPRB – natriuretic peptide receptor B, NPRC – natriuretic peptide receptor C)
Sustained SNS and RAAS signaling leads, respectively, to increased circulating levels of norepinephrine and angiotensin II. These molecules have been referred to as neurohormones in the literature, which is an historical term that reflects the observation that that many of the molecules detected in the circulation of patients with HFrEF are produced by the neuroendocrine system, and thus act on the heart in an endocrine manner. However, studies have shown that many neurohormones (e.g., angiotensin II) are synthesized by cell types residing within the myocardium, and thus act in an autocrine and paracrine manner. Nonetheless, the important conceptual theme of the neurohormonal model is that the overexpression of portfolios of biologically active molecules can contribute to the pathogenesis of HFrEF because of the toxic effects of these molecules on the heart and circulation (reviewed in 1). At some point, patients with LV dysfunction will develop classic signs and symptoms of heart failure. The transition to symptomatic heart failure is accompanied by further activation of the neurohormonal and inflammatory signaling pathways, as well as a series of adaptive changes within the myocardium referred to as LV remodeling (reviewed in references 2, 3). Medical and device therapies that lead to improved clinical outcomes in HFrEF patients almost always lead to decreased LV volume and mass, and restore a more normal elliptical shape to the ventricle. These beneficial changes represent the summation of biologic changes in cardiac myocyte size and function, as well as modifications in LV structure and organization that are accompanied by shifts of the LV end-diastolic pressure-volume relationship toward normal. Collectively, these changes are referred to “reverse LV remodeling.” 13, 14 Relevant to this discussion, the assessment of LV remodeling is a potential surrogate end point for drug or device effects on heart failure outcomes.15, 16
Conceptual Therapeutic Models for Heart Failure with a Reduced Ejection Fraction
Investigators and clinicians have used different conceptual therapeutic models to develop strategies for treating heart HFrEF. These models are described in detail elsewhere,17, 18 and will be discussed here in brief to provide context for understanding more contemporary heart failure therapies.
Cardiorenal model
The first conceptual therapeutic model was derived from clinical observations dating as far back as 1680, that linked injury of the heart to the development of peripheral and/or pulmonary edema, which was described historically as “hydrops” or “dropsy.” The cardiorenal model posited that the formation of peripheral or pulmonary edema was the result of an injury to the heart that either reduced the ability of the heart to eject blood (“forward failure”), or impaired the ability of the heart to receive blood from peripheral organs (“backward failure”).17 Increases in left and right-sided venous pressures led, respectively, to pulmonary edema and/or peripheral edema by altering outward starling forces thereby increasing the flow of fluid from the microvasculature into the interstitium. As shown in Table 1, both interpretations of the cardiorenal model have merit, insofar as injury to the heart often leads to inappropriate retention sodium and water by the kidney, with resulting expansion of intravascular volume and interstitial edema. However, as discussed above studies have shown that the inappropriate retention sodium and water by the kidney is mediated by SNS and RAAS signaling, rather than decreased cardiac output, per se. One of the major therapeutic advances of the cardiorenal model is that it led to the use of diuretics to manage the volume overload in heart failure, which remains a mainstay of therapy in the current era (Table 1)19.
Table 1:
Conceptual Therapeutic Models for Heart Failure with a Reduced Ejection Fraction
| Heart Failure Models | Conceptual Advances | Conceptual Disadvantages | Therapeutic Advances |
|---|---|---|---|
| Cardiorenal | Recognized the contribution of cardiac injury to the pathogenesis of heart failure | Did not explain disease progression | Led to the use of diuretics |
| Recognized the importance of inappropriate sodium retention by the kidney | Impeded progress with vasodilators | ||
| Impeded progress with β-blockers | |||
| Cardiocirculatory Model (Hemodynamic) | Recognized the importance of the peripheral circulation | Did not explain disease progression | Led to the use of vasodilators |
| Fostered widespread use of inotropes | |||
| Impeded progress with β-blockers | |||
| Neurohormonal | Heart failure viewed as a biological problem, not as a hemodynamic problem | Does not explain disease progression completely | |
| Does not predict success for all “neurohormonal” antagonists | Led to the use of ACEI, β-blockers, Aldosterone antagonists and ARBs | ||
| Does not explain the benefit of emerging device therapies |
(Modified from Mann DL. The evolution of modern theory and therapy for heart failure. Prog Ped Cardiol. 2014;37:9–12)
Cardiocirculatory model
The second conceptual therapeutic model for treating heart failure was based on physiological concepts derived from studies of ventricular mechanics, which demonstrated that cardiac function was regulated by inotropy, as well as cardiac preload and afterload. The “cardiocirculatory” or “hemodynamic” model proposed that heart failure was the result of abnormal pumping capacity of the heart that was exacerbated by the increased afterload imposed by peripheral arterial vasoconstriction.17 Although peripheral vasoconstriction maintained perfusion to vital organs, it also increased the afterload on the heart, decreased renal blood flow which lead to increased sodium retention by the kidney, and reduced blood flow to exercising skeletal muscle, which was believed to contribute to exercise intolerance. The cardiocirculatory model led to the use of orally active vasodilators to unload the failing heart, and provided the scientific rationale for the first large scale morbidity mortality trial in heart failure.20 Additionally, the cardiocirculatory model also led to the use of inotropic agents to improve pumping capacity of the heart to stabilize hemodynamics and improve symptoms. Although inotropes produced dramatic immediate short-term hemodynamic effects, the long-term use of inotropes was associated with a dramatic and unexpected increase in patient morbidity and mortality.18, 21 As shown in Table 1 the cardiocirculatory model forms the basis for the current treatment of acute decompensated heart failure, wherein diuretics, vasodilators and intravenous inotropic agents remain the primary available therapies, despite multiple attempts to further expand the armamentarium.22–25 Unfortunately, the cardiocirculatory model impeded progress in the field of heart failure conceptually, insofar as it prohibited the use of beta-adrenergic blocking agents, which appeared counterintuitive because of their negative inotropic effects. Moreover, the cardiocirculatory model did not recognize the importance of cardiac remodeling as a mechanism for disease progression in heart failure.
Neurohormonal model
The neurohormonal model was based on the observation that many of the biologically active molecules elaborated by the SNS and RAAS (e.g. norepinephrine, angiotensin II, aldosterone) were overtly toxic to the heart and circulation when expressed at levels that were observed in the failing heart.26 The important conceptual advance of the neurohormonal model is that it focused on heart failure fundamentally as a biological problem rather than as a hemodynamic problem. The neurohormonal model led to the use of beta-adrenergic blocking agents to block the deleterious effects of the sympathetic nervous system in the heart, and angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists to block the deleterious effects of RAAS. These therapies were shown to clearly improve long term morbidity and mortality in a broad population of patients with HFrEF, and collectively they represent the cornerstone of modern guideline directed medical (GDMT) therapy for heart failure (Table 1). 27,28 Although the neurohormonal model has provided a number of important insights with respect to explaining disease progression, as well as provided important insights in terms of drug development for heart failure, many HFrEF patients continue to have progressive disease despite optimal GDMT. Moreover, as heart failure progresses, many HFrEF patients become refractory or intolerant to conventional medical therapy, often requiring the withdrawal of conventional medical therapies.29 Thus, there remains a clear need to develop additional treatments for this population, in particular treatments that do not have overlapping side effects or intolerances (hypotension, renal dysfunction, hyperkalemia) with existing treatments.
Recent therapeutic advances in heart failure with a reduced ejection fraction
In the preceding section we have described three conceptual models that were used to develop heart failure therapeutics (see Table 1). In the section that follows, we will discuss four new classes of heart failure therapeutics that have improved clinical outcomes in large phase III trials in patients with HFrEF (see data summarized in Table 2). As will be discussed three of these new therapeutic classes were developed based on the insights provided by existing conceptual heart failure models, whereas one therapeutic class, the SGLT2-inhibitors, has less well understood mechanisms of action, and may require refining, expanding or developing new conceptual models of heart failure.
Table 2.
Summary of Benefits of Novel Therapies for HF with reduced ejection fraction on clinical endpoints
| Sacubitril/valsartan | SGLT2 inhibitors | Vericiguat | Omecamtiv mecarbil | |
|---|---|---|---|---|
| Improves LV remodeling | Yes | Yes | No | Yes |
| Decreases natriuretic peptides | Yes | Yes | Yes | Yes |
| Improves symptoms/quality of life | Yes | Yes | ? | ? |
| CV death or HF hospitalization | Yes | Yes | Yes | Yes |
| Improves CV mortality | Yes | Yes | No | No |
| Improves HF hospitalizations | Yes | Yes | Yes | Yes |
Angiotensin–Neprilysin Inhibition
Mechanism of Action.
Angiotensin receptor-neprilysin inhibitors (ARNI) represent a novel “second generation” neurohormonal antagonist that combines a RAAS antagonist with a NEP inhibitor. NEP inhibitors attenuate RAAS signaling by preventing degradation of peptides that serve as natural counter-regulatory antagonists of RAAS signaling. NEP is expressed in multiple tissues, including vascular endothelium, smooth muscle cells, myocytes, fibroblasts, kidney tubule cells, and nerve cells. NEP degrades multiple peptides, including natriuretic peptides, angiotensin I, angiotensin II, endothelin-I, adrenomedullin, opioids, bradykinin, chemotactic peptides, enkephalins, and a30myloid-β peptide (Aβ). NEP-mediated inhibition of the degradation of natriuretic peptides leads to vasorelaxation and vasodilation of vascular arteries, natriuresis, inhibition of hypertrophy, and fibrosis. On the other hand, inhibition of the degradation of other vasoactive peptides, such as angiotensin II, angiotensin 1–7, and endothelin-I, opposes the vasodilatory effects of natriuretic peptides. Accordingly, NEP inhibition has variable effects on blood pressure.
Clinical Trials.
Because of the salutary effects of natriuretic peptides in heart failure, NEP inhibition has been pursued as a therapeutic strategy. The early use of omapatrilat, a dual vasopeptidase inhibitor that inhibits both ACE and NEP, was not shown to be more effective than ACE inhibition alone in heart failure patients.30 In the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE), which was conducted in 5770 chronic heart failure patients, omapatrilat reduced the composite of cardiovascular death or hospitalization (HR 0.91, 95% CI 0.84–0.99, P = 0.024); however, there was no difference between the therapies for the primary endpoint of death or heart failure hospitalization (P = 0.187).31 Parallel studies with omapatrilat in patients with hypertension demonstrated an increased risk for serious angioedema, presumably due to increased bradykinin levels secondary to combined ACE inhibition and NEP inhibition. The clinical development of omapatrilat was halted because of safety concerns.32
The next therapeutic attempt at NEP inhibition combined sacubitril, a prodrug neprilysin inhibitor, with an angiotensin receptor blocker (ARB). This therapeutic class is referred to as ARNIs (angiotensin receptor neprilysin inhibitors). The rationale for ARNIs was that the risk of angioedema secondary to elevated bradykinin levels would be reduced by combing a NEP inhibitor with an ARB rather than an ACE inhibitor. Given that valsartan dosing in heart failure was relatively well understood,33 and leveraging the experience with omapatrilat, sacubitril/valsartan was developed as a novel therapeutic agent for HFrEF without going through traditional testing in a phase II trial. An initial trial of sacubitril/valsartan compared to valsartan alone in 1328 patients with mild-moderate hypertension provide evidence of the magnitude of blood pressure lowering (2–4 mm difference in systolic blood pressure compared to valsartan), as well as initial evidence of safety.34 The effect of sacubitril/valsartan on morbidity and mortality in heart failure with a reduced ejection fraction was evaluated in a large phase III outcomes study, the Prospective Comparison of ARNI with ACE Inhibition to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial.35 PARADIGM-HF was a double-blind randomized controlled trial of enalapril versus sacubitril/valsartan in 8442 patients with NYHA class II-IV symptoms and an LVEF ≤ 40%. The primary endpoint of PARADIGM was the composite of time cardiovascular death or first heart failure hospitalization. For inclusion in the trial, participants had to be taking a stable dose of ACE or ARB equivalent to at least 10 mg of enalapril daily for at least 4 weeks. In addition, prior to randomization, patients had to tolerate a single-blind run-in period of enalapril 10 mg twice-daily followed by sacubitril/valsartan up-titrated to 97/103 twice-daily. The trial was stopped early by the Data Safety Monitoring Board, given the overwhelming evidence for a clinical important benefit of sacubitril/valsartan on the primary endpoint of cardiovascular death or heart failure hospitalization with sacubitril/valsartan compared with enalapril (HR 0.80; 95% CI 0.73 to 0.87; P<0.001). The benefits with sacubitril/valsartan were consistent across study endpoints including all-cause mortality (HR 0.84, 95% CI 0.76–0.93, P <0.001), cardiovascular mortality (HR 0.80, 95% CI 0.71–0.89, P <0.001), and hospitalization for heart failure (HR 0.79, 95% CI 0.71–0.89, P <0.001). These benefits were consistent across a broad array of prespecified subgroups, and resulted in an estimated increase in survival of 1.4 years for a 55 year old patient treated with sacubitril/valsartan compared to enalapril.36 Patients receiving sacubitril/valsartan had more symptomatic hypotension and non-serious angioedema, but less renal impairment, hyperkalemia, and cough than the enalapril group. The findings from PARADIGM-HF supported the role of sacubitril/valsartan as superior to ACE inhibition in the management of chronic heart failure with reduced ejection fraction, leading to a class IA indication for patients with chronic symptomatic heart failure with reduced ejection fraction (NYHA class II or III) to further reduce morbidity and mortality.37, 38 Sacubitril/valsartan also has favorable effects on symptom burden in heart failure with reduce ejection fraction. In PARAGIDM-HF, sacubitril/valsartan treatment led to clinically important benefits on health-related quality of life as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) compared with enalapril,39 as well as improvements in functional status as estimated by NYHA class. Subsequent randomized clinical trials and additional analyses of completed studies have added to the overall picture of both the clinical benefits and the safety of sacubitril/valsartan. The PIONEER study established the safety and efficacy of early initiation (in hospital) of sacubitril/valsartan in patients hospitalized for heart failure.40
Effects on LV Remodeling.
Given the atypical sequence of clinical development, there was substantial evidence for improvement in clinical outcomes with sacubitril/valsartan prior to understanding whether there were beneficial effects on reverse LV remodeling, which had been observed with prior neurohormonal therapies that improve outcomes. Subsequent studies have confirmed the substantial favorable effects on LV remodeling with sacubitril/valsartan. PROVE-HF, a single arm multi-center study, demonstrated significant improvements in cardiac remodeling with sacubitril/valsartan.41 As expected, the magnitude of the changes in LV remodeling after initiation of sacubitril/valsartan was related to the risk of clinical outcomes, with those patients having more favorable remodeling demonstrating improved prognosis.42 Similar findings on cardiac remodeling have been confirmed in the double blind EVALUATE trial, which showed decreased systolic and diastolic ventricular volumes, as well as atrial volumes with sacubitril/valsartan compared to enalapril.43
Sodium-glucose co-transoporter-2 (SGLT2) inhibitors
In contrast to the therapeutic development of ARNIs, which leveraged insights provided by the neurohormonal model, the development sodium-glucose co-transoporter-2 inhibitors (SGLT2i) as a therapy for heart failure with a reduced ejection was the result of FDA policies that required that all new anti-diabetic drugs should be evaluated in large cardiovascular outcome trials to establish the cardiovascular safety profile of new anti-diabetic therapies. As will be discussed below, subsequent studies demonstrated a clear benefit for this therapeutic class on important cardiovascular and renal outcomes. Although data continues to accumulate, it appears that SGLT2i’s will be among the most important advances in cardiovascular therapeutics of recent decades.
Mechanism of Action.
Since glucose is a polar molecule, it cannot be transported across the lipid cell membrane and requires carrier proteins, referred to as a glucose transporter, in order to enter the cell. Two families of glucose transporters have been identified, including facilitative diffusion glucose transporters (GLUTs) and Na+/glucose cotransporters (SGLTs). There are two major SGLT2 isoforms in human tissues: SGLT1 and SGLT2.44 SGLT1 serves as a high-affinity, low-capacity transporter that is able to transport glucose against a concentration gradient, whereas SGLT-2 is low affinity, high-capacity transporter. Quantitative studies of SGLT1 and SGLT2 gene expression in human tissue have shown that SGLT1 is highly expressed in the small intestine, whereas SGLT2 is very highly expressed in the kidney.44 In human tissue, SGLT2 mRNA is expressed ubiquitously (including the heart) and is generally 10–100 higher fold than the expression of SGLT1 in the same tissues. One notable exception is that SGLT1 mRNA is more highly expressed in the heart than SGLT2. Relevant to this discussion, SGLT1 mRNA and protein have been detected in cardiac myocytes,44, 45 whereas (at the time of the writing) there have been no comparable studies for SGLT2 in cardiac myocytes. Studies using Mendelian randomization have estimated that lower SGLT1 expression would be associated with lower rates of obesity, diabetes, heart failure, and mortality.46
In the kidney, SGLT-2 is located in the S1 and S2 segments of the proximal tubule in the kidneys and accounts for 90% of glucose reabsorption by the kidney. SGLT-1 is located in the S3 segment of the proximal tubules, and accounts for the remaining 10% of glucose absorption. SGLT-2 is also responsible for proximal tubular reabsorption of sodium, and the passive absorption of chloride that is driven by the resulting electrochemical gradient in the proximal tubule lumen. The increased absorption of sodium and chloride in the proximal tubule results in lower chloride concentration delivered to the macula densa, which in turn results in dilation of the afferent arteriole and increase glomerular filtration through “tubulo-glomerular feedback,” which preserves renal blood flow and glomerular filtration rate. Inhibition of SGLT2 results in a 1:1 stoichiometric inhibition of sodium and glucose uptake in the proximal tubule of the kidney. This leads to contraction of the plasma volume and modest lowering of blood pressure, without activation of the sympathetic nervous system. The contraction of plasma volume may contribute to changes in markers of hemoconcentration with SGLT2 inhibitors, including increases in blood urea nitrogen and hematocrit, although the latter may also be on the basis of increased erythropoiesis. The proximal natriuresis that occurs with SGLT2 inhibition results in afferent arteriole vasoconstriction through tubulo-glomerular feedback, thereby reducing glomerular hyperfiltration. Experimental studies showed that SGLT2 inhibitors reduce hyperfiltration and decrease inflammatory and fibrotic responses of proximal tubular cells.47 Beyond effects on traditional cardiovascular risk factors such as HbA1c and weight, SGLT2 inhibition also reduces plasma uric acid levels by 10% to 15% by increasing uricosuria via exchange of filtered glucose.
Clinical Trials.
Although SGLT2i’s were developed originally as a glucose lowering anti-diabetic therapy, SGLT2i’s demonstrated significant benefits on cardiovascular mortality, MI, and stroke in cardiovascular outcome trials.48–50 Somewhat unexpectedly, there was also a consistent signal in the trials of heart failure prevention in the SGLT2i treatment arm as compared to placebo. The landmark EMPA-REG-OUTCOME study demonstrated a significant reduction in the composite of CV death, non-fatal MI, or stroke (hazard ratio in the empagliflozin group, 0.86; 95.02% confidence interval, 0.74 to 0.99)50. More striking was the 34% reduction in heart failure hospitalization, that was consistently observed in patients with and without a prior history of heart failure (10% of the EMPA-REG population).51 These findings on CV outcomes were subsequently replicated for other SGLT2i’s, including dapagliflozin (in the DECLARE-TIMI58 study)49 and canagliflozin (in the CANVAS and CREDENCE studies).48, 52 These findings led to large programs designed to assess the benefit of these agents in patients across the spectrum of heart failure patients, including those with and without diabetes.
The first of these studies to present the results was the DAPA-HF study of dapagliflozin in patients with heart failure with reduced ejection fraction. DAPA-HF randomized 4744 patients with NYHA class II-IV heart failure and EF <= 40% to either dapagliflozin or placebo. This study demonstrated a significant reduction in both the combined endpoint of CV death + heart failure hospitalization (hazard ratio, 0.74; 95% confidence interval [CI], 0.65 to 0.85; P<0.001) as well as on CV death (hazard ratio, 0.82; 95% CI, 0.69 to 0.98).53 Notably, these findings were completely consistent whether or not patients had diabetes at baseline (42% of DAPA-HF population), showing for the first time important clinical benefits in patients without diabetes. Dapagliflozin also improved quality of life in this study assessed by the KCCQ, making this one of the few classes to improve morbidity, mortality, and quality of life in heart failure with reduced ejection fraction.54 More recently, the EMPEROR-Reduced study with empagliflozin also reported similar beneficial effects in heart failure patients with reduced ejection fraction. In a similar heart failure with reduced ejection fraction population to the DAPA-HF study (NYHA Class II-IV, EF <=40%), 10 mg of empagliflozin daily reduced heart failure hospitalization (hazard ratio 0.75; 95% CI, 0.65 to 0.86; P<0.001) compared to placebo55. In this study, however, the reduction in CV death with empagliflozin did not reach statistical significance (Hazard ratio = 0.92, 95% CI, 0.75 to 1.12). Quality of life was improved with empagliflozin treatment compared to placebo, similar to prior results with dapagliflozin.56 The combined data from the DAPA-HF and EMPEROR-Reduced trials have been recently summarized in a meta-analysis.57
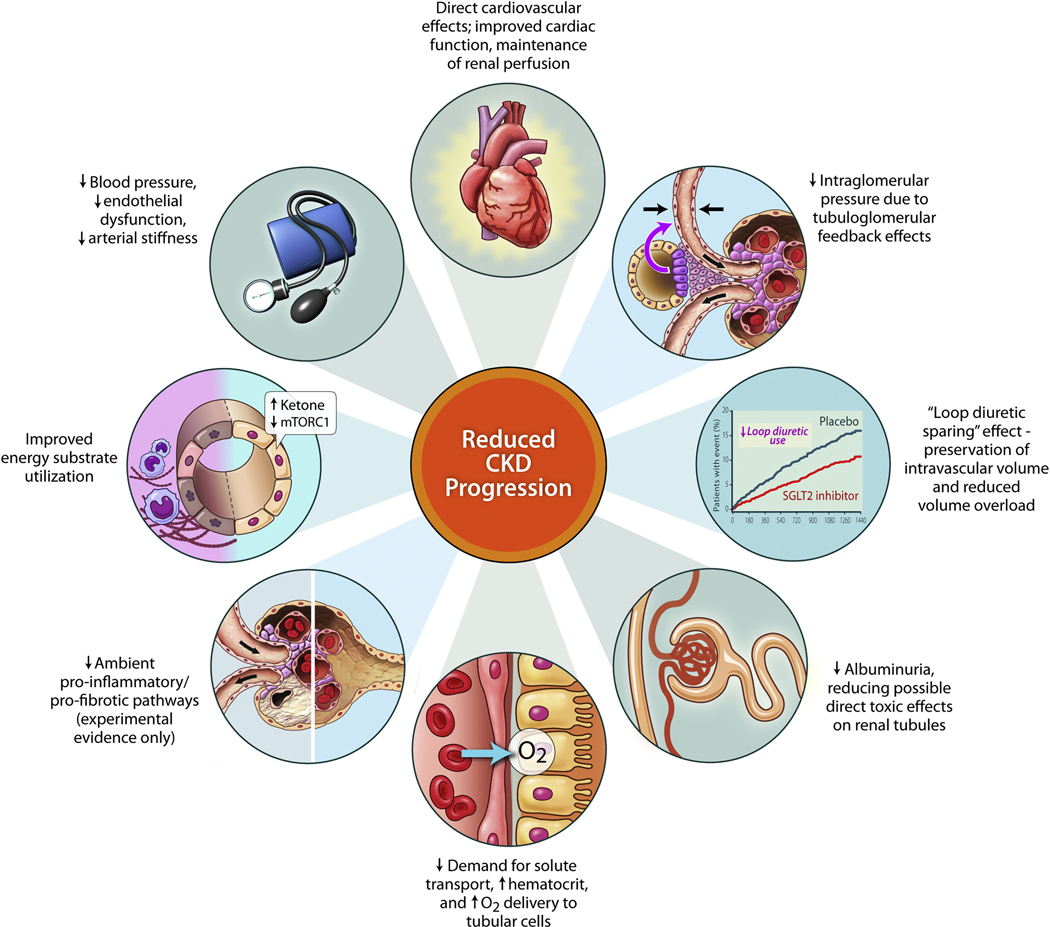
Another recent study, the SOLOIST-WHF trial, focused on a different population and clinical scenario. This trial randomized 1222 patients with diabetes regardless of ejection fraction to either sotagliflozin or placebo.58 Sotagliflozin differs from other available SGLT2i’s in that it inhibits both SGLT1 and SGLT2. Patients were enrolled at the time of a discharge from a hospitalization for heart failure or soon thereafter. The primary endpoint was CV death and total heart failure hospitalization (including recurrent heart failure hospitalizations). This study showed a dramatic treatment effect for sotagliflozin in this population, with a hazard ratio of 0.67 (95% confidence interval [CI], 0.52 to 0.85; P<0.001). This was consistent regardless of ejection fraction at study entry, including for patients with EF > 50%. Importantly, the study was discontinued early by the sponsor for financial reasons and thus was underpowered to show more definitive effects on CV mortality, but was broadly consistent with the findings from other HFrEF trials. This is notable as the first study to show benefit on clinical outcomes in patients with heart failure with preserved ejection fraction using a specific drug therapy. An additional notable feature of the long term SGLT2i trials has been their impact on slowing of the progression of chronic kidney disease (Figure 3), which has been shown with dapagliflozin,59 empagliflozin,60 and canagliflozin.52 SGLT2i in heart failure patients are generally very well tolerated, with very low risk of hypoglycemia, volume depletion, or hypotension similar to placebo.53, 55
Figure 3. Summary of Potential Renal Protective Mechanisms of SGLT2 Inhibitors.
(Reproduced from: Cherney DZI and Verma S. DAPA-CKD: The Beginning of a New Era in Renal Protection. JACC Basic Transl Sci. 2021;6:74–77.)
Effects on LV Remodeling.
Four studies have evaluated the effect of SGLT2i’s on LV remodeling in heart failure patients with a reduced ejection fraction. 61–64 Three studies demonstrated a significant decrease in LV volumes after a minimum of 3 months treatment with empagliflozin as compared to placebo.61, 62, 64 In contrast 1 year of treatment with dapagliflozin in diabetic heart failure patients did show beneficial effect of LV volumes by cardiac magnetic resonance imaging.63 Given that 100% of the patients in the dapagliflozin LV remodeling study had diabetes, whereas the number of diabetics varied widely (0–78%) in the empagliflozin trials, it is difficult to comment on whether the effects of SGLT2i’s on reverse LV remodeling should or should not be viewed as a class effect.
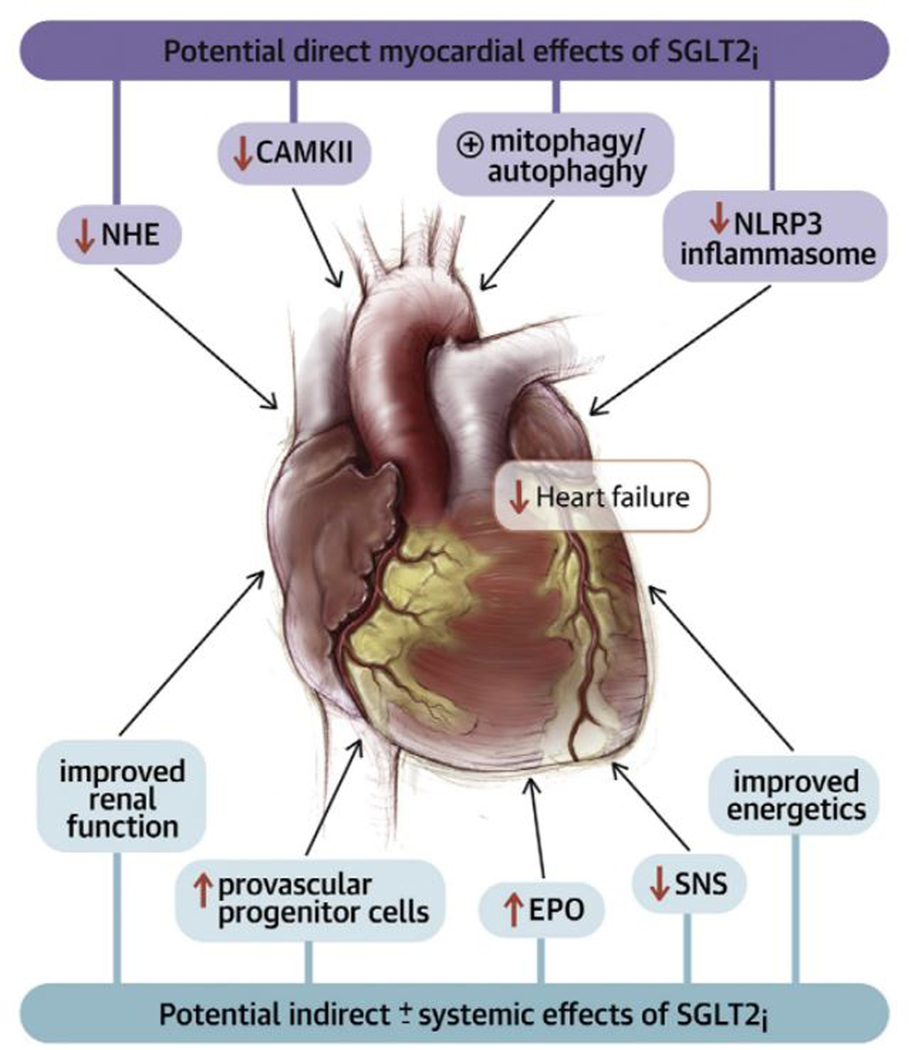
The striking results with SGLT2i in patients with HFrEF represent an exciting example of “reverse translation (bedside to bench),” whereby the unexpected results of a clinical trial serve as the stimulus to explore novel actions for a class of drugs that had demonstrated marked effects on clinically meaningful heart failure outcomes. However, what is most provocative about the reverse translation in this instance, is that it suggests that there are unknown mechanisms of action for a “diabetic drug,” that are operative even in the absence of diabetes. A number of cardiac and extra-cardiac mechanisms have been proposed, increased natriuresis and diuresis, renal protective effects, enhanced cardiac substrate metabolism, improved vascular stiffness, reduced LV mass, direct inhibitory effects on the cardiac sodium-hydrogen exchanger, decreased inflammation, stimulation of cardiac autophagy and mitophagy, reduction in adipokines, stimulation of erythropoietin (EPO) production, and attenuation or renal afferent sympathetic nervous system activity (Figures 4).65–68 As summarized in Table 3, many of these mechanisms of action do not fit neatly into one of the three aforementioned conceptual models for heart failure, rather the pleiotropic mechanisms of action of SGLT2i’s place them at the intersection of metabolic, hemodynamic, neurohumoral, and vascular endothelial pathways that impact the heart and the kidney, all of which are important in the pathogenesis of heart failure regardless of the LV ejection fraction. The precise mechanisms underlying the observed cardio-renal benefits of SGLT2i’s is an area of active research that is likely to evolve rapidly.
Figure 4. Potential Direct Myocardial and Indirect ± Systemic Effects of SGLT2 inhibitors.
(Key: CAMKII = calmodulin-dependent protein kinase II; EPO = erythropoietin; NHE = sodium/hydrogen exchanger; NLRP3 = nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3; SGLT2i = sodium glucose co-transporter 1(2) inhibitor; SNS = sympathetic nervous system. (Reproduced from Lopaschuk GD and Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632–644).
Table 3:
Overview of Potential Mechanism of Beneficial Cardiovascular Effects of SGLT2 Inhibitors
| Conceptual Model of Heart Failure | Mechanism of action |
|---|---|
| Cardiorenal | Stimulation of natriuresis |
| Stimulation of osmotic diuresis | |
| Decreased tubulo-glomerular feedback | |
| Cardiocirculatory | Improved systolic and diastolic function |
| Improved cardiac filling conditions secondary to reductions in preload and afterload | |
| Inhibition of cardiac fibrosis | |
| Increased cardiac output, increased coronary blood flow mediated by increased levels of circulating glucagon |
|
| Improved myocardial energetics | |
| Neurohormonal | Decreased central nervous system sympathetic nervous activity |
| Other | Reduction in myocardial CaM kinase II activity |
| Increased erythropoietin | |
| Increased circulating proangiogenic progenitor cells | |
| Inhibition of cardiac myocyte Na+/H exchanger | |
| Improved endothelial function | |
| Increased mitophagy/autophagy |
(Modified from Lam CSP et al. SGLT2 Inhibitors in heart failure: Current management, unmet needs, and therapeutic prospects. Journal of the American Heart Association. 2019;8:e013389).
Soluble guanylate cyclase stimulators
Mechanism of Action.
As noted above, NO is a free radical gas that serves as a key signaling molecule in the vasculature and the heart. NO is produced by three different isoforms of NO synthase (NOS), all of which are present in the heart, and include NOS1 (neuronal NOS [nNOS]), NOS2 (inducible NOS [iNOS]) and NOS3 (so-called endothelial-constitutive NOS [eNOS]). NO exerts it effects, at least in part, by binding to the soluble guanylate cyclase receptor (sGC), which consists of a larger α-subunit and a smaller heme-binding β-subunit, which is essential for detecting NO in the cytoplasm (Figure 2).69 Activation of sGC by NO requires the presence of a reduced Fe2+ heme moiety on the β-subunit. Oxidation of the heme moiety (Fe3+) abolishes endogenous NO-induced activation of sGG signaling. 70 NO-induced activation of sGC leads to the production of cGMP, which mediates three intracellular effector pathways, including cGMP-dependent protein kinases I and II, cGMP-gated ion channels and cGMP-regulated phosphodiesterases.69 The NO–sGC–cGMP pathway is crucial for the control of vascular homeostasis. In healthy subjects, NO is released continuously by vascular endothelial cells, thereby promoting vascular smooth muscle cell relaxation and vasodilation. Endothelium-dependent NO-mediated dilation of the peripheral vasculature is attenuated in heart failure patients, which has been attributed to decreased NO bioavailability secondary to NOS3 uncoupling.71 The loss of NO bioavailability impairs endothelium-dependent vasomotor regulation, and results in peripheral arterial vasoconstriction and increased ventricular afterload. Thus, the identification of impaired NO–sGC–cGMP signaling and the identification of sGC as a therapeutic target in heart failure is rooted in biological and physiological insights provided by the cardiocirculatory and neurohormonal heart failure models.
The relatively recent discovery of compounds that activate sGC in an NO-independent manner led to the therapeutic development of sGC stimulators and sGC activators.72 Soluble GC stimulators directly stimulate the reduced form of sGC and enhance the sensitivity of the reduced enzyme to low levels of bioavailable NO. In contrast sGC activators activate the NO-unresponsive heme oxidized enzyme.69 The NO-independent stimulators of sGC have been evaluated in several different heart failure trials, as will be discussed below.
Clinical Trials.
Vericiguat (BAY 1021189) is a novel oral soluble guanylate cyclase stimulator that has undergone extensive clinical testing. Initial studies of vericiguat in heart failure included a phase II program composed of 2 studies, SOCRATES-Reduced and SOCRATES-Preserved. SOCRATES-Reduced randomized patients with LVEF ≤ 45% within 4 weeks of a worsening heart failure event (hospitalization for heart failure or treatment of worsening heart failure with IV diuretics in the ambulatory setting) to either placebo or 1 of 4 different dosing regimens (from 1.5 mg daily to 10 mg daily).73 The primary endpoint of this phase 2 trial was change in log plasma NT-proBNP concentrations from baseline to 12 weeks. Although the study did not meet its primary endpoint, there was evidence of greater natriuretic peptide lowering in the higher dose arms, particularly 10 mg daily. The higher dose of 10 mg daily was also associated with a reduction in the estimated risk of rehospitalization of CV death, although this was not statistically significant in this phase 2 study (hazard ratio 0.53 (95%CI, 0.25–1.16). In contrast to SOCRATES-Reduced, the SOCRATES-Preserved study with a similar design in patients with EF > 45%, did not show significant improvement on NT-proBNP concentrations.74
The VICTORIA study randomized 5050 patients with NYHA class II-IV heart failure and EF ≤ 45% to verciguat 10 mg daily or placebo. Patients were selected to be a higher risk cohort, requiring a heart failure hospitalization within the prior 6 months or outpatient IV diuretic therapy without hospitalization within the prior 3 months, as well as elevate natriuretic peptide concentrations. Patients were followed for a median of 10.8 months, and the primary endpoint was the composite of cardiovascular death or first heart failure hospitalization. There was a significant treatment benefit for patients randomized to verciguat with a hazard ratio 0.90 (95% CI 0.81–1.00).75 Deaths from cardiovascular causes were non-significantly reduced with a hazard ratio of 0.93 (95% CI, 0.81 to 1.06). Of note, the overall risk profile of patients enrolled in VICTORIA was significantly higher than patients enrolled in many other heart failure with reduce ejection fraction outcome trials, with a 1 year control group event rate of 34% (compared to 14% in PARADIGM-HF, 16% in DAPA-HF, and 28% for GALACTIC-HF), suggesting that the relatively modest relative risk reduction of 10% in the primary endpoint was associated with a clinically important absolute risk reduction, given the high baseline risk of the study population.76 Although subgroup analyses generally showed a consistent benefit of verciguat across subgroups, there was notable heterogeneity with regard to baseline natriuretic peptide concentrations, with patients with higher natriuretic peptide levels deriving less benefit.77 The clinical interpretation of this finding remains uncertain, given the VICTORIA trial focused on higher risk patients overall but the primary benefit seemed to be in the lower risk cohorts within the trial. Verciguat was generally well tolerated, with verciguat treatment associated with more symptomatic hypotension (9.1% vs. 7.9%) and anemia (7.6% vs. 5.7%). Based on the results of the VICTORIA trial, the FDA has approved vericiguat to lower the risk of heart failure in high-risk patients who have symptomatic, chronic heart failure with reduced ejection fraction < 45%.
Effects on LV Remodeling
In an echocardiographic sub-study of the VICTORIA trial, patients were studied at baseline and after 8 months of therapy (n = 211 in each arm). The primary endpoint was a change in LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI). The VICTORIA echocardiographic sub-study showed that both LV ejection fraction and LVESVI significantly improved from baseline in both arms through 8 months of treatment. However, treatment with vericiguat had no additional significant effect on LV ejection fraction or LVESVI as compared to placebo.78
Cardiac Myosin Activators
Because decreased cardiac output is regarded as a cardinal feature of heart failure with a reduced ejection fraction, there have been many attempts to develop inotropic agents to improve cardiac output by increasing the force of cardiac contraction (cardiocirculatory model). However, despite three decades of intensive efforts, no positive inotrope is currently approved for long-term use in chronic heart failure with a reduced ejection fraction. 797979Several reasons have been proposed for the negative outcomes of clinical trials with positive inotropic agents, including patient selection, trial design and trial end points. 79 Moreover, it should also be recognized that many of the compensatory mechanisms that are activated in HFrEF restore cardiac output to normal or near normal levels in the early stages of the disease,80 albeit at the expense of inappropriate salt and water retention and excessive activation of the SNS and RAAS.
Omecamtiv mecarbil is a first-in-class small-molecule activator of cardiac myosin ATPase that increases the proportion of myosin heads that are tightly bound to actin. Omecamtiv mecarbil increases force generation of the heart by prolonging myocardial contraction. To differentiate myosin activators from classic inotropes, they have been referred to as “myotropes”.81 Whereas inotropic agents increase cardiac output by increasing intracellular calcium levels in the cell, which can lead to myocardial ischemia and cardiac arrhythmias, omecamtiv mecarbil does not alter intracellular calcium concentrations and should presumably have a more favorable safety profile. However, given that omecamtiv mecarbil increases systolic ejection time, there is at least theoretical concern that this agent could adversely affect diastolic coronary filling and potential myocardial ischemia. Early data in patients with known ischemic cardiomyopathy did not suggest an increase in ischemia with exercise treadmill testing during OM treatment.82 Clinical trials have consistently demonstrated a small increase in circulating cardiac troponin concentrations in patients treated with omecamtiv mecarbil compared to placebo (on the order of 1–4 ng/L), which resolves on discontinuation of omecamtiv mecarbil. Early studies also found that supra-therapeutic plasma concentrations (> 1200 ng/mL) of were associated with adverse ischemic events. Subsequent implementation of a therapeutic drug monitoring protocol targeting plasma concentrations < 300 pg/mL was successful at avoiding supratherapeutic drug levels in subsequent studies.83 Omecamtiv mecarbil prolonged systolic ejection time in a dose dependent manner and thereby improves stroke volume and systolic cardiac performance in both normal healthy volunteers84 and in patients with heart failure with a reduced ejection fraction.85
Clinical Trials.
The ATOMIC-HF study was a randomized dose finding clinical trial of short term (48 hour) infusion of omecamtiv mecarbil in patients with acute decompensated heart failure, ATOMIC-HF study, showed improvement in systolic ejection time and suggested of improvement in dyspnea in the higher dose group.86 Subsequently in a larger phase 2 clinical trial in heart failure with a reduced ejection fraction, the COSMIC study, administration of omecamtiv mecarbil for 20 weeks increased left ventricular systolic ejection time and stroke volume, decreased left ventricular systolic and diastolic volumes suggesting beneficial reverse cardiac remodeling, and reduced plasma natriuretic peptide concentrations and heart rate.87 These Phase II trial findings were notable in that interventions that lower natriuretic peptides and induce favorable LV remodeling have generally been shown to improve heart failure outcomes in cardiovascular death in larger trials. Subsequently analyses of these data also suggested improvements in health related quality of life.88
The Global Approach to Lowering Adverse Cardiac Outcomes through Improving Contractility in Heart Failure (GALACTIC-HF) study was a global, phase 3, double blind, placebo controlled randomized clinical trial evaluating the efficacy and safety of omecamtiv mecarbil compared to placebo in 8256 patients with symptomatic (NYHA class II-IV) heart failure with an ejection faction ≤ 35%. 89, 90 Enrolled patients were required to be currently hospitalized for heart failure (inpatients) or had an urgent visit to the emergency department or been hospitalized for heart failure within 1 year before screening (outpatients). All the patients had elevated natriuretic peptides (N-terminal pro–B-type natriuretic peptide (NT-proBNP) level of ≥ 400 pg/mL (1200 pg/mL for patients in atrial fibrillation) or B-type natriuretic peptide (BNP) ≥ 125 pg/mL (375 pg/mL for patients in atrial fibrillation). GALACTIC demonstrated a significant improvement in the primary endpoint, a composite of time to first heart failure event or death from cardiovascular causes (hazard ratio, 0.92; 95% confidence interval [CI], 0.86 to 0.99; P = 0.03).91 This finding was driven primarily by improvement in heart failure hospitalization events, as there was no difference in cardiovascular mortality ((hazard ratio, 1.01; 95% CI, 0.92 to 1.11). Subgroup analyses suggested that patients with lower ejection fractions and in normal sinus rhythm were more likely to have a favorable treatment effect with omecamtiv mecarbil. There did not appear to be a significant improvement in quality of life in the study population overall, although there were potentially important differences noted between those enrolled as inpatients vs. those enrolled as outpatients in the effect of omecamtiv mecarbil on quality of life. Although modestly elevated cardiac troponin was observed in omecamtiv mecarbil treated patients in GALCTIC consistent with prior studies, there was no increase in myocardial ischemia events, ventricular arrythmias, or mortality compared to placebo. An additional Phase III trial called METEORIC-HF (NCT03759392) is currently enrolling focused on the effect of omecamtiv mecarbil on exercise capacity as measured by cardiopulmonary exercise testing and actigraphy. The results of the subgroup analyses of GALACTIC-HF which suggested that patients with lower ejection fractions and in normal sinus rhythm were more likely to have a favorable treatment effect with omecamtiv mecarbil suggests that depressed contractile function is both necessary and sufficient to explain pathogenesis of heart failure in patients with more advanced disease. Multiple ongoing analyses will provide additional data to clarify the potential role of omecamtiv in heart failure care.
Summary
In the foregoing review we have discussed the results of recent heart failure clinical trials that have employed novel therapeutic classes of pharmacologic agents. With the exception of SGLT2 inhibitors, each of these therapeutic advances were informed by the insights provided by existing conceptual models of heart failure. For example, ARNIs and soluble guanylate cyclase stimulators attenuate the deleterious effects of SNS and RAAS signaling by upregulating cGMP-mediated signaling pathways that directly counteract the deleterious effects these signaling pathways (neurohormonal model). The early success with this therapeutic strategy has given rise to other approaches to augment endogenous natriuretic peptide and nitric oxide mediated signaling, using phosphodiesterase 9 inhibitors to delay cGMP degradation.92 Omecamtiv mecarbil improves the hemodynamic profile of patients with advanced heart by directly targeting sarcomere function rather than by altering calcium handling, suggesting that small molecules that allosterically modulate troponin or myosin might be also be developed in the future (hemodynamic model).93 While the mechanisms of action of SGLT2i’s are unknown and represent an area of active investigation, this therapeutic class of drugs has stimulated considerable interest in additional strategies to improve cardiac energy production (hemodynamic model) as well as improve renal function (cardiorenal model). The recent observation that SGLT2i’s may also attenuate excessive SNS signaling (neurohormonal model),94 raises the intriguing possibility that a single therapeutic class of drug may favorably impact all aspects of the pathogenesis of heart failure. In addition to ongoing efforts to develop new therapies, how best to combine available therapies in an era of multiple effective drugs remains a major focus of clinical research.95
ACKNOWLEDGEMENTS
This study was supported by: R01HL107594 (DLM) and U10 HL110309 (DLM), AN # 4345132 (DLM), AHA SFRN 30180010 (GMF), R01 HL144928–03 (GMF), R21 HL152148–01 GMF)
DISCLOSURES
Dr. Mann serves on the steering committee for the PARADISE-MI trial (Novartis) and the ANTHEM-HF trial( LivaNova) and is a member of the Scientific Advisory Board for MyoKardia, Tenaya, Cardurion and Cardior, and serves as a consultant for Novo Nordisk, Dr Felker recieves research support from, Amgen, Bayer Merck, Cytokinetics, Myokardia; he acts as a consultant to Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, Innolife, Boehringer-Ingelheim, American Regent, Abbott, Astra-Zeneca, Eidos Therapeutics, Reprieve, and Sequana, and has serves on clinical endpoint committees/data safety monitoring boards for Amgen, Merck, Medtronic, EBR Systems, V-Wave, LivaNova, Siemens, and Rocket Pharma.
Non-standard Abbreviations and Acronyms
- HFrEF
Heart failure with reduced ejection fraction
- ARNI
angiotensin receptor neprilysin inhibitors
- SGLT2i
sodium-glucose co-transoporter-2 inhibitors
- SNS
sympathetic nervous system
- RAAS
renin-angiotensin aldosterone system
- LV
Left ventricular
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- NPR A
natriuretic peptide A receptor
- NPR-B
natriuretic peptide B receptor
- GC
guanylate cyclase
- NEP
neutral endopeptidase
- NO
nitric oxide
- sGC
soluble guanylate cyclase
- GDMT
guideline directed medical therapy
- ACE
angiotensin converting enzyme
- OVERTURE
Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events
- HR
Hazard ratio
- CI
confidence interval
- ARB
angiotensin receptor blocker
- PARADIGM-HF
Prospective Comparison of ARNI with ACE Inhibition to Determine Impact on Global Mortality and Morbidity in Heart Failure
- PROVE-HF
Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure
- DECLARE–TIMI 58
Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction-58
- EMPEROR-Reduced
Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- CANVAS
Canagliflozin Cardiovascular Assessment Study
- CREDENCE
Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation
- CV
Cardiovascular
- NOS
Nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- eNOS
endothelial-constitutive nitric oxide synthase
- SOCRATES-REDUCED
Soluble Guanylate Cyclase Stimulator in Heart Failure with Reduced Ejection Fraction
- NT-proBNP
Amino-terminal b-type natriuretic peptide
- LVESVI
LV end-systolic volume index
- ATOMIC-HF
Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure
- COSMIC-HF
The Chronic Oral Study of Myosin activation to Increase Contractility in Heart Failure
- GALACTIC-HF
Global Approach to Lowering Adverse Cardiac Outcomes through Improving Contractility in Heart Failure
References
- 1.Hartupee J and Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann DL, Barger PM and Burkhoff D. Myocardial recovery: myth, magic or molecular target? J Amer Coll Cardiol. 2012;60:2465–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann DL and Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. [DOI] [PubMed] [Google Scholar]
- 4.Floras JS and Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36:1974–82b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Bilsen M, Patel HC, Bauersachs J, Bohm M, Borggrefe M, Brutsaert D, Coats AJS, de Boer RA, de Keulenaer GW, Filippatos GS, Floras J, Grassi G, Jankowska EA, Kornet L, Lunde IG, Maack C, Mahfoud F, Pollesello P, Ponikowski P, Ruschitzka F, Sabbah HN, Schultz HD, Seferovic P, Slart R, Taggart P, Tocchetti CG, Van Laake LW, Zannad F, Heymans S and Lyon AR. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2017. [DOI] [PubMed] [Google Scholar]
- 6.Schrier RW. Decreased Effective Blood Volume in Edematous Disorders: What Does This Mean? Journal of the American Society of Nephrology. 2007;18:2028–2031. [DOI] [PubMed] [Google Scholar]
- 7.Volpe M, Rubattu S and Burnett J Jr., Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buglioni A and Burnett JC Jr., New Pharmacological Strategies to Increase cGMP. Annu Rev Med. 2016;67:229–43. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y and Burnett JC Jr., Biochemistry, Therapeutics, and Biomarker Implications of Neprilysin in Cardiorenal Disease. Clin Chem. 2017;63:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpe M, Tritto C, De Luca N, Mele AF, Lembo G, Rubattu S, Romano M, De Campora P, Enea I, Ricciardelli B and et al. Failure of atrial natriuretic factor to increase with saline load in patients with dilated cardiomyopathy and mild heart failure. J Clin Invest. 1991;88:1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibebuogu UN, Gladysheva IP, Houng AK and Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CY and Burnett JC Jr., Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. [DOI] [PubMed] [Google Scholar]
- 13.Kass DA, Baughman KL, Pak PH, Cho PW, Levin HR, Gardner TJ, Halperin HR, Tsitlik JE and Acker MA. Reverse remodeling from cardiomyoplasty in human heart failure. External constraint versus active assist. Circulation. 1995;91:2314–2318. [DOI] [PubMed] [Google Scholar]
- 14.Levin HR, Oz MC, Chen JM, Packer M, Rose EA and Burkhoff D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation. 1995;91:2717–2720. [DOI] [PubMed] [Google Scholar]
- 15.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA and Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstam MA, Udelson JE, Anand IS and Cohn JN. Ventricular remodeling in heart failure: a credible surrogate endpoint. J Card Fail. 2003;9:350–353. [DOI] [PubMed] [Google Scholar]
- 17.Packer M. How Should Physicians View Heart Failure? - The Philosophical and Physiological Evolution of Three Conceptual Models of the Disease. Am J Cardiol. 1993;71:C3–C11. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL. The evolution of modern theory and therapy for heart failure. Prog Ped Cardiol. 2014;37:9–12. [Google Scholar]
- 19.Ellison DH and Felker GM. Diuretic Treatment in Heart Failure. N Engl J Med. 2018;378:684–685. [DOI] [PubMed] [Google Scholar]
- 20.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Hartson WE, Tristani EF, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC and Baker B. Effect of vasodilating therapy on mortality in chronic congestive heart failure: results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBiano R, Zeldis SM, Hendrix GH, Bommer WJ, Ulkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK and DeMets DL. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468–1475. [DOI] [PubMed] [Google Scholar]
- 22.Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias-Mendoza A, Avendano P, Bacal F, Bohm M, Bortman G, Cleland JGF, Cohen-Solal A, Crespo-Leiro MG, Dorobantu M, Echeverria LE, Ferrari R, Goland S, Goncalvesova E, Goudev A, Kober L, Lema-Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva-Cardoso J, Spinar J, Squire I, Stepinska J, Van Mieghem W, von Lewinski D, Wikstrom G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsody P, Gimpelewicz C and RELAX-AHF-2 Committees and Investigators. Effects of Serelaxin in Patients with Acute Heart Failure. N Engl J Med. 2019;381:716–726.31433919 [Google Scholar]
- 23.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr., Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M and Investigators REiAHF. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J and Investigators T-A. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N Engl J Med. 2017;376:1956–1964. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F and Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 26.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100:999–1008. [DOI] [PubMed] [Google Scholar]
- 27.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M and Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 29.Sucharov CC, Mariner P, Long C, Bristow M and Leinwand L. Yin Yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem. 2003;278:31233–31239. [DOI] [PubMed] [Google Scholar]
- 30.Volpe M, Carnovali M and Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond). 2016;130:57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau J-L and Swedberg K. Comparison of Omapatrilat and Enalapril in Patients With Chronic Heart Failure. Circulation. 2002;106:920–926. [DOI] [PubMed] [Google Scholar]
- 32.Kostis JB, Packer M, Black HR, Schmieder R, Henry D and Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–11. [DOI] [PubMed] [Google Scholar]
- 33.Cohn JN and Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 34.Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J and Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–66. [DOI] [PubMed] [Google Scholar]
- 35.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 36.Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, Jhund P, Lefkowitz M, Shi V, Solomon SD and Investigators P-H. Estimating the Long-Term Treatment Benefits of Sacubitril-Valsartan. N Engl J Med. 2015;373:2289–90. [DOI] [PubMed] [Google Scholar]
- 37.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P and Group ESD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 38.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC and Givertz MM. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of the American College of Cardiology. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 39.Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD and Swedberg K. Health-Related Quality of Life Outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10. [DOI] [PubMed] [Google Scholar]
- 40.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E and Investigators P-H. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 41.Januzzi JL Jr., Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD and Investigators P-H. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Januzzi JL, Camacho A, Piña IL, Rocha R, Williamson KM, Maisel AS, Felker GM, Prescott MF, Butler J and Solomon SD. Reverse Cardiac Remodeling and Outcome After Initiation of Sacubitril/Valsartan. Circulation Heart failure. 2020;13:e006946. [DOI] [PubMed] [Google Scholar]
- 43.Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF and Investigators ftE-H. Effect of Sacubitril-Valsartan vs Enalapril on Aortic Stiffness in Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2019;322:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Cryan EV, D’Andrea MR, Belkowski S, Conway BR and Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem. 2003;90:339–46. [DOI] [PubMed] [Google Scholar]
- 45.Banerjee SK, McGaffin KR, Pastor-Soler NM and Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seidelmann SB, Feofanova E, Yu B, Franceschini N, Claggett B, Kuokkanen M, Puolijoki H, Ebeling T, Perola M, Salomaa V, Shah A, Coresh J, Selvin E, MacRae CA, Cheng S, Boerwinkle E and Solomon SD. Genetic Variants in SGLT1, Glucose Tolerance, and Cardiometabolic Risk. Journal of the American College of Cardiology. 2018;72:1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cherney DZ, Odutayo A, Aronson R, Ezekowitz J and Parker JD. Sodium Glucose Cotransporter-2 Inhibition and Cardiorenal Protection. J Amer Coll Cardiol. 2019;74:2511–2524. [DOI] [PubMed] [Google Scholar]
- 48.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M and Matthews DR. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 49.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde A-M and Sabatine MS. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. New England Journal of Medicine. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 50.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE and Investigators E-RO. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 51.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC and Inzucchi SE. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME®trial. Eur Heart J. 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P-L, De Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM and Mahaffey KW. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 53.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D-HT and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019. [DOI] [PubMed] [Google Scholar]
- 54.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Kober L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjostrand M, Langkilde AM and McMurray JJV. Effects of Dapagliflozin on Symptoms, Function, and Quality of Life in Patients With Heart Failure and Reduced Ejection Fraction: Results From the DAPA-HF Trial. Circulation. 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C and Zannad F. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 56.Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, Giannetti N, Januzzi JL, Piña IL, Lam CSP, Ponikowski P, Sattar N, Verma S, Brueckmann M, Jamal W, Vedin O, Peil B, Zeller C, Zannad F and Packer M. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W and Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. The Lancet. 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 58.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P and Pitt B. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. New England Journal of Medicine. 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 59.Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, De Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O’Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M and McMurray JJV. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction. Circulation. 2021;143:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, Schnee J, Wanner C and Packer M. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function. Circulation. 2021;143:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Tuxen CD, Möller S, Gustafsson F, Køber L, Schou M and Møller JE. Associations of Empagliflozin With Left Ventricular Volumes, Mass, and Function in Patients With Heart Failure and Reduced Ejection Fraction: A Substudy of the Empire HF Randomized Clinical Trial. JAMA Cardiology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC and Sattar N. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021;143:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, Choy AMJ, Gandy S, George J, Khan F, Pearson ER, Houston JG, Struthers AD and Lang CC. Dapagliflozin Versus Placebo on Left Ventricular Remodeling in Patients With Diabetes and Heart Failure: The REFORM Trial. Diabetes Care. 2020;43:1356–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, Rodriguez-Cordero A, Zafar MU, Fergus I, Atallah-Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V and Badimon JJ. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. Journal of the American College of Cardiology. 2021;77:243–255. [DOI] [PubMed] [Google Scholar]
- 65.Lam CSP, Chandramouli C, Ahooja V and Verma S. SGLT2 Inhibitors in heart failure: Current management, unmet needs, and therapeutic prospects. Journal of the American Heart Association. 2019;8:e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, Lehrke M, Marx N and Lopaschuk GD. Empagliflozin Increases Cardiac Energy Production in Diabetes. JACC: Basic to Translational Science. 2018;3:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, Arcambal A, Kiuchi MG, Head GA, Schlaich MP and Matthews VB. SGLT2 Inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC: Basic to Translational Science. 2020;5:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopaschuk GD and Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HHHW and Stasch J-P. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nature Reviews Drug Discovery. 2006;5:755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foerster J, Harteneck C, Malkewitz J, Schultz G and Koesling D. A Functional Heme-Binding Site of Soluble Guanylyl Cyclase Requires Intact N-Termini of α1 and β1 Subunits. European Journal of Biochemistry. 1996;240:380–386. [DOI] [PubMed] [Google Scholar]
- 71.Farah C, Michel LYM and Balligand J-L. Nitric oxide signalling in cardiovascular health and disease. Nature Reviews Cardiology. 2018;15:292–316. [DOI] [PubMed] [Google Scholar]
- 72.Boerrigter G and Burnett JC Jr., Soluble guanylate cyclase: not a dull enzyme. Circulation. 2009;119:2752–4. [DOI] [PubMed] [Google Scholar]
- 73.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Muller K, Roessig L, Pieske B, Investigators S-R and Coordinators. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251–62. [DOI] [PubMed] [Google Scholar]
- 74.Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L and Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O’Connor CM and Group VS. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 76.Butler J, Anstrom KJ and Armstrong PW. Comparing the Benefit of Novel Therapies Across Clinical Trials: Insights From the VICTORIA Trial. Circulation. 2020;142:717–719. [DOI] [PubMed] [Google Scholar]
- 77.Ezekowitz JA, O’Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, Butler J, Lam CSP, Ponikowski P, Emdin M, Patel MJ, Pieske B, Roessig L, Hernandez AF and Armstrong PW. N-Terminal Pro-B-Type Natriuretic Peptide and Clinical Outcomes: Vericiguat Heart Failure With Reduced Ejection Fraction Study. JACC Heart failure. 2020;8:931–939. [DOI] [PubMed] [Google Scholar]
- 78.Stiles S. Discordant VICTORIA results deepen mystery of vericiguat in low-EF heart failure. 2020:https://www.medscape.com/viewarticle/931976 (accessed 3/21/21) [Google Scholar]
- 79.Ahmad T, Miller PE, McCullough M, Desai NR, Riello R, Psotka M, Böhm M, Allen LA, Teerlink JR, Rosano GMC and Lindenfeld J. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. European journal of heart failure. 2019;21:1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlsson M, Andersson R, Bloch KM, Steding-Ehrenborg K, Mosén H, Stahlberg F, Ekmehag B and Arheden H. Cardiac output and cardiac index measured with cardiovascular magnetic resonance in healthy subjects, elite athletes and patients with congestive heart failure. Journal of Cardiovascular Magnetic Resonance. 2012;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF Jr., Rosano GMC and Lancellotti P. Cardiac Calcitropes, Myotropes, and Mitotropes. Journal of the American College of Cardiology. 2019;73:2345–2353. [DOI] [PubMed] [Google Scholar]
- 82.Greenberg BH, Chou W, Saikali KG, Escandon R, Lee JH, Chen MM, Treshkur T, Megreladze I, Wasserman SM, Eisenberg P, Malik FI, Wolff AA and Shaburishvili T. Safety and tolerability of omecamtiv mecarbil during exercise in patients with ischemic cardiomyopathy and angina. JACC Heart failure. 2015;3:22–29. [DOI] [PubMed] [Google Scholar]
- 83.Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF Jr., Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsanyi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N and Investigators C-H. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388:2895–2903. [DOI] [PubMed] [Google Scholar]
- 84.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI and Wolff AA. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–75. [DOI] [PubMed] [Google Scholar]
- 85.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA and Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–83. [DOI] [PubMed] [Google Scholar]
- 86.Teerlink JR, Felker GM, McMurray JJ, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JG, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM and Investigators A-A. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study. J Am Coll Cardiol. 2016;67:1444–55. [DOI] [PubMed] [Google Scholar]
- 87.Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF Jr., Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsanyi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N and COSMIC-HF Investigators. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388:2895–2903. [DOI] [PubMed] [Google Scholar]
- 88.Felker GM, Solomon SD, McMurray JJV, Cleland JGF, Abbasi SA, Malik FI, Zhang H, Globe G and Teerlink JR. Effects of Omecamtiv Mecarbil on Symptoms and Health-Related Quality of Life in Patients with Chronic Heart Failure: Results from the COSMIC-HF Study. Circ Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Legg JC, Buchele G, Varin C, Kurtz CE, Malik FI and Honarpour N. Omecamtiv Mecarbil in Chronic Heart Failure With Reduced Ejection Fraction: Rationale and Design of GALACTIC-HF. JACC Heart failure. 2020;8:329–340. [DOI] [PubMed] [Google Scholar]
- 90.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sorensen T, Bohm M, Bonderman D, Cleland JGF, Corbalan R, Crespo-Leiro MG, Dahlstrom U, Diaz R, Echeverria Correa LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Lund M, Macdonald P, Mareev V, Momomura SI, O’Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Abbasi SA, Varin C, Malik FI, Kurtz CE and Investigators G-H. Omecamtiv Mecarbil in Chronic Heart Failure with Reduced Ejection Fraction, GALACTIC-HF: Baseline Characteristics and Comparison with Contemporary Clinical Trials. Eur J Heart Fail. 2020:doi: 10.1002/ejhf.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sorensen T, Bohm M, Bonderman D, Cleland JGF, Corbalan R, Crespo-Leiro MG, Dahlstrom U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O’Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE and Investigators G-H. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 92.Richards DA, Aronovitz MJ, Liu P, Martin GL, Tam K, Pande S, Karas RH, Bloomfield DM, Mendelsohn ME and Blanton RM. CRD-733, a Novel PDE9 (Phosphodiesterase 9) Inhibitor, Reverses Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2021;14:e007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alsulami K and Marston S. Small Molecules acting on Myofilaments as Treatments for Heart and Skeletal Muscle Diseases. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verma S. Are the Cardiorenal Benefits of SGLT2 Inhibitors Due to Inhibition of the Sympathetic Nervous System? JACC Basic Transl Sci. 2020;5:180–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhatt AS, Abraham WT, Lindenfeld J, Bristow M, Carson PE, Felker GM, Fonarow GC, Greene SJ, Psotka MA, Solomon SD, Stockbridge N, Teerlink JR, Vaduganathan M, Wittes J, Fiuzat M, O’Connor CM and Butler J. Treatment of HF in an Era of Multiple Therapies. JACC: Heart Failure. 2021;9:1–12. [DOI] [PubMed] [Google Scholar]