Abstract
Purpose
Many brain tumor patients suffer from radiation-induced toxicities. Chronotherapy is a treatment modality that utilizes circadian rhythms to optimize the effect on tumor while minimizing negative outcomes on healthy tissue. This review aims to systematically examine the literature on the application of a radiation chronotherapeutic for all cancers and determine the possible advantages of incorporating a circadian-based fixed time-of-day for radiotherapy into CNS cancers.
Methods
A systematic review of the literature was conducted in two electronic databases from inception to February 1, 2019. Primary research manuscripts were screened for those related to adult human subjects exposed to ionizing radiation using the chronotherapy technique.
Results
Nine manuscripts were included in the review from 79 eligible articles. Three were prospective randomized trails and 6 were retrospective reviews. This survey revealed that overall survival and tumor control do not have consistent effects with only 60% and 55.5% of paper which included the variables having some significance, respectively. Treatment symptoms were the primary endpoint for both the prospective trials and were examined in 3 of the retrospective reviews; effects were observed in sensitive tissue for all 5 studies including mucosal linings and skin basal layer.
Conclusions
Existing literature suggests that the application of radiation chronotherapy may reduce negative symptom outcome within highly proliferative tissues. Further examination of radiation chronotherapy in well-designed prospective trials and studies in brain tumor patients are merited.
Keywords: Chronotherapy, Radiation, Radiotherapy, Outcomes, Symptom burden
Introduction
Chronotherapy is a technique used to optimize treatment efficiency by precisely scheduling time of drug/therapy administration to enhance treatment effects on the disease while reducing negative outcomes in healthy tissue [1, 2].
These windows of optimal treatment time are regulated by the body’s endogenous circadian rhythms, which controls cellular mechanisms important for treatment sensitivity, e.g. cell cycle and DNA repair mechanisms, through the daily patterns of clock gene expression [3]. Clock genes regulate the expression of 24-h rhythms present across most tissue types within the body [4, 5] via an autoregulated transcription-translation feedback loop of the core clock genes; BMAL1, CLOCK/NPAS, PER, and CRY [6, 7]. Clock genes are also present in diseased tissue, several different cancer cell lines have been shown to have circadian rhythms in vitro [8–10] and in vivo [11, 12]. The differences in the phase and amplitude of clock gene rhythms and cellular mechanisms controlled by the circadian system can vary between different tissue types, importantly suggesting that cancer cells and healthy tissue may respond differently to treatment. The most explored application of chronotherapy in cancer has been the timed release of chemotherapeutic agents [13, 14], however, scheduling of other important therapeutic procedures, such as radiation, have been examined but not systematically reviewed.
Ionizing radiation (IR) can induce cell death by causing damage to DNA via double stranded breaks [15]. Circadian rhythms regulate several mechanisms that are crucial for facilitating cell death within tumors in response to radiotherapy [16]. Specifically, clock genes are involved in generating a rhythmicity of these IR-induced mechanisms across 24 h, which predispose cells to be more sensitive to treatment at specific times-of-day [17]. Clock genes have been shown to regulate DNA damage checkpoint response [18–20], DNA repair mechanisms [21–23], and apoptosis [21] in response to IR. Time-of-day for the exposure to both radiation [12, 24, 25] and DNA damaging agent, such as temozolomide [9], have a time specific impact on the survival of tumor cells in culture. However, the current focus of the chronotherapeutic field is moving away from the tumor as primary target and is now examining how time of administration can be used to minimize negative side effects of treatment [2]. Radiation induces many short-term and long-term side effects [26], particularly when given to the brain radiation immediately impacts the survival of proliferative tissues like neural stem cells in experimental models but can also cause lasting sleep disruptions and cognitive defects in patients undergoing treatment [27, 28].
We are primarily interested in understanding the feasibility of using chronomodulated administration of radiation in brain tumor patients to reduce treatment-related symptoms, such as fatigue. Radiation is the standard treatment for the majority of brain tumor patients and for the most malignant primary brain tumor, glioblastoma (GBM), radiation is combined with temozolomide [29]. Therefore, the goal of implementing chronotherapy is to improve symptom outcomes following treatment. These symptoms impact quality of life; specifically, in brain tumor patients, fatigue and sleep problems are the most prevalent symptoms impacting daytime functioning [30]. Additionally, patients with brain tumors have been shown to differentially develop sleep problems after radiotherapy that has been correlated with polymorphisms in two clock genes, PER2 and ARNTL2 [31]. This finding is supported by data demonstrating that polymorphisms found in PER2 are associated with sleep problems in other populations [32–34] and are also linked to sleep disorders [35, 36]. Deletion of the PER2 gene in mice induced a shorter circadian period, loss of rhythmicity in extended periods of constant darkness, and dampened expression of other clock genes within the suprachiasmatic nucleus [37, 38] which all can directly impact sleep behavior. The PER2 variant (rs934945) associated with negative outcomes has also been correlated with activity preference time in human carriers [39], suggesting differences in the period of the endogenous rhythms like those observed in mice which may predispose carriers to chronodisruption or expose carriers to treatment at a different and more detrimental circadian time.
We postulate that a relationship exists between circadian rhythms, radiation and quality of life is present within the primary brain tumor population and exploration of the mechanisms to guide clinical trials are warranted. A review of the impact of radiation chronotherapy in broader cancer literature would support examining and potentially testing this approach in patients with CNS tumors. The aim of this current review is to systematically examine the literature on the application of chronotherapeutic techniques to radiation therapy for all cancers and determine the possible advantages of incorporating circadian-based fixed time-of-day for radiotherapy. Specifically, we will separately investigate the findings in clinical settings while summarizing the effects observed in both disease progression and other quality of life factors, such as treatment-related symptoms and healthy tissue pathology.
Methods
The authors reviewed the literature to identify research on the importance and impact of time-of-day on radiation therapy effectiveness and toxicity. A systematic review of the literature was conducted on February 1st, 2019 using the PubMed (1946–Febuary 2019) and EMBASE (1947—February 2019) databases. Table 1 lists the MESH and key words used as the search criteria for both databases. The search encompassed all publication types and was filtered for articles only written in English. The inclusion criteria consisted of primary research articles conducted in adult human subjects exposed to ionized radiation, and these subjects could be healthy or cancer bearing. However, studies were excluded if the subject had non-solid tumors or were of pediatric age. A total of 79 studies met the eligibility criteria based on preliminary screening using title and abstract by one reviewer (DSM). Of those articles, nine included all required criteria and were further analyzed by both reviewers (DSM; see Fig. 1).
Table 1.
Search strategy for review: keywords and MESH terms
| Concepts | Keywords | MESH | |
|---|---|---|---|
| PubMed database | 1. Circadian rhythms | “Biological Clock” OR “Biological Clocks” OR “Biological Rhythm” OR “Biological Rhythms” OR Chronobiology OR Chronotherapy OR Chronotoxicity OR “Circadian Clock” OR “Circadian Clocks” OR “Circadian Rhythm” OR “Circadian Rhythms” OR “Circadian Timing” OR “Clock Gene” OR “Clock Genes” OR “Diurnal Rhythm” OR “Diurnal Rhythms” OR Periodicity OR “Time of Day” OR “Twenty-four Hour Rhythm” OR “Twenty-four Hour Rhythms” | Chronobiology phenomena OR Chronotherapy OR Circadian Rhythm |
| 2. Cancer therapy | Neoplasm OR Neoplasms OR Astrocytoma OR Astrocytomas OR Cancer OR Cancers OR Carcinoma OR Carcinomas OR Glioma OR Gliomas OR Radiotherapy OR Radiosurgery OR Radiosurgery OR Antineoplastic OR Chemotherapy OR Cisplatin | Neoplasms/Therapy OR Radiotherapy OR Antineoplastic Protocols | |
| EMBASE database | 1. Circadian Rhythms | ’Biological Clock’ OR ’Biological Clocks’ OR ’Biological Rhythm’ OR ’Biological Rhythms’ OR Chronobiology OR Chronotherapy OR Chronotoxicity OR ’Circadian Clock’ OR ’Circadian Clocks’ OR ’Circadian Rhythm’ OR ’Circadian Rhythms’ OR ’Circadian Timing’ OR ’Clock Gene’ OR ’Clock Genes’ OR ’Diurnal Rhythm’ OR ’Diurnal Rhythms’ OR Periodicity OR ’Time of Day’ OR ’Twenty-four Hour Rhythm’ OR ’Twenty-four Hour Rhythms’ | ’Chronobiology’ OR ’Chronotherapy’ OR ’Chronopharmacology’ OR ’Circadian Rhythm’ OR ’Biological Rhythm’ OR ’Periodicity’ |
| 2. Cancer therapy | Neoplasm OR Neoplasms OR Astrocytoma OR Astrocytomas OR Cancer OR Cancers OR Carcinoma OR Carcinomas OR Glioma OR Gliomas OR Radiotherapy OR Radiosurgery OR Antineoplastic OR Chemotherapy OR Cisplatin | ’Neoplasm’ OR ’Cancer Therapy’ OR ’Radiotherapy’ OR ’Radiosurgery’ OR ’Antineoplastic Agent’ | |
Fig. 1.
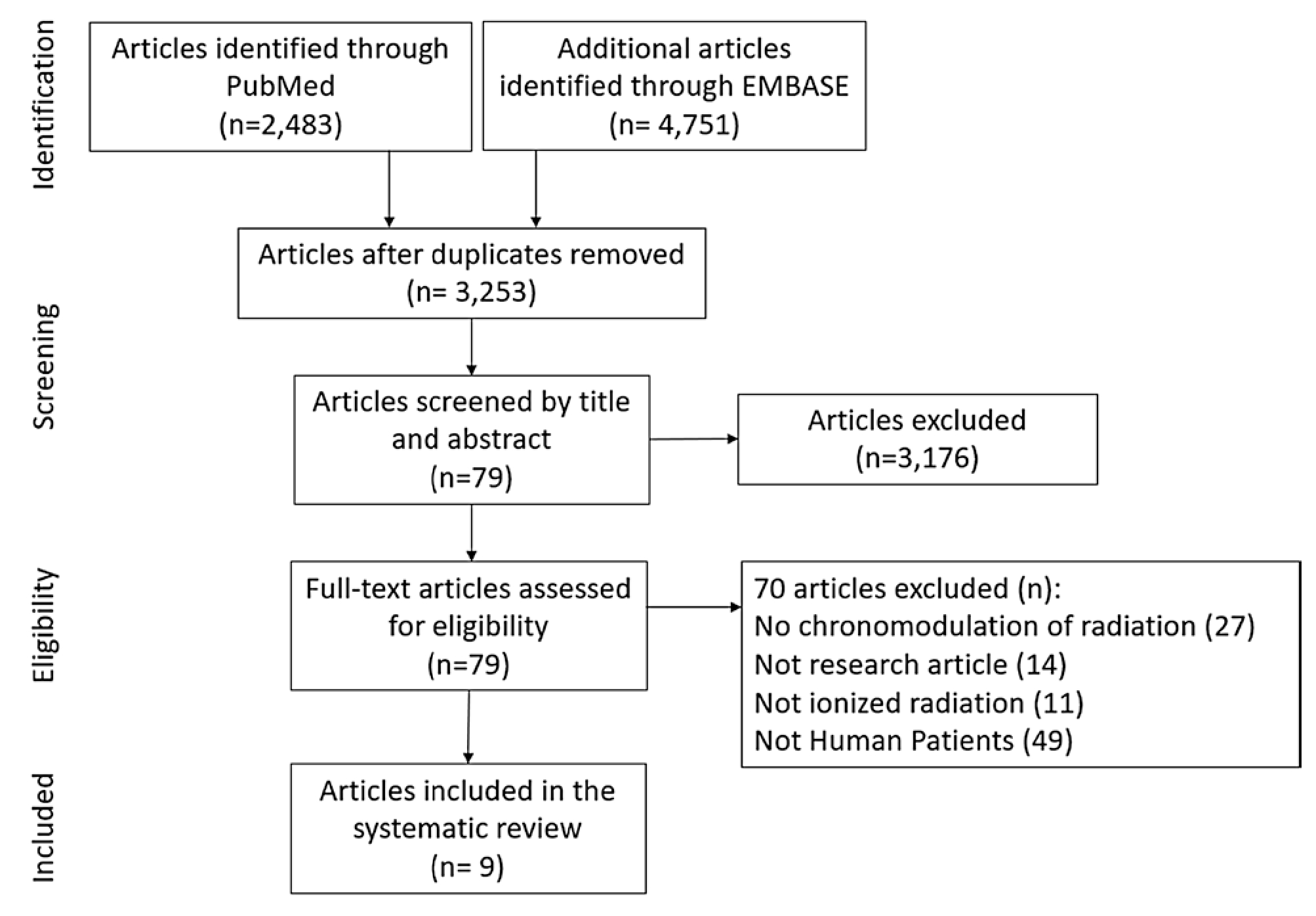
Flow diagram of search strategy
Results
Experimental groups and study design
The nine manuscripts included in the review were examined for demographic differences (Table 2 and described below, as well as study design and approach. These studies either conducted retrospective reviews (n = 6) or used a randomized trial design (n = 3). The three randomized trials divided arms into Morning and Afternoon groups but varied in the definition of their intervals and timing of each group. Bjarnson et al. [40] and Shukla et al. [ 41] used 2-h intervals with the Morning treat occurring between 8 and 10 AM. However, the Afternoon group were either treated from 4 to 6PM or 6 to 8 PM, thereby complicating the interpretation of the Afternoon treatment results. Goyal et al. [42], on the other hand, used 3-hour intervals from 8 to 11 AM and 3 to 6 PM. As anticipated, in the retrospective studies there was more variability for the divisions of radiation time and the size of the intervals (Fig. 2). Most studies had two groups, except Chan et al. [43], who had three time-divisions: morning (8–11 AM), midday (11–2 PM), and afternoon (2–5 PM). Interestingly, three studies had patients seen into the late evening; Shukla et al. [41] from 6 to 8 PM, Noh et al. [44] had a group irradiated from 3 to 10 PM and Hsu et al. [45] from 5 to 10 PM. These three studies were all conducted in Asian Institutes (Korea, India, and Taiwan) were radiation treatment centers continued service later into the evening.
Table 2.
Summary of radiation chronotherapy clinical studies in the present systematic review
| References | Population | N | Study design | Study hypotheses | Cancer type | Irradiation |
|---|---|---|---|---|---|---|
| Bjarnason et al. [40] | August 1999–November 2004 77% male Median age of 60 years |
205 | Randomized prospective study | Morning radiation will have less incidence of grade 3 oral mucositis Primary endpoint: grade 3 or greater RTOG oral mucositis |
Squamous cell carcinoma of the oral cavity, pharynx, or larynx | Irradiaton source not given 66–70 Gy (33–35 fractions) one fraction daily Monday–Friday Patients completed full course (93.3% morning vs. 96.1% evening) 40–50% of patients receiving one or two treatments < 1 h outside the allocated times |
| Badiyan et al. [46] | 1998–2011 51% male Median age 60 years |
437 | Retrospective review | Time of day of radiosurgery has an effect on local control and overall survival | Non-small cell lung cancer (NSCLC) brain metastases | Gamma Knife stereotactic radio surgery 18–20 Gy |
| Chan et al. [43] | January 2000–December 2010 62.4% male Median age of 68 years |
194 | Retrospective review | Time of day of radiation has an effect on metastases treatment response | Bone metastasis, primary cancer sites: prostate (30.4%), breast (25.3%), and lung (19.6%) | Irradiator Source Not Given 8 Gy in 1 fraction (57.2%), 20 Gy in 5 fraction (33.5%), 30 Gy in 10 fraction (6.19%) |
| Goyal et al. [42] | July 2006–June 2007 81% male Median age between 35–50 years |
177 | Randomized prospective study | Time of day of radiation has an effect on acute oral mucositis Primary endpoint: grade 3 or 4 ulceration in any location |
Squamous cell carcinoma (92%) of the oral cavity, pharynx, or larynx | Cobalt60 using parallel and opposite fields 60 Gy (2 Gy/fraction) with 5 fractions per week |
| Hsu et al. [45] | 2004–2010 100% Male Median age 74 years |
409 | Retrospective review | Time of day of radiation has an effect on disease control and normal mucosal tissue toxicity | Non-metastatic prostate adenocarcinoma | SS IMRTa or TomoTherapy® 78–74 Gy (337 fractions) |
| Noh et al. [44] | October 2001–December 2006 100% Females Median age of 47 years |
395 | Retrospective review | Time of day of radiation has an effect on disease control and acute skin reactions | Breast cancer | 4- or 6-MV photon beam linear accelerator (Varian Medical System) 50.4 Gy in 28 fractions or 50 Gy in 25 fractions |
| Rahn et al. [47] | 1989–2007 42% males Mean age of ∼60 years |
97 | Retrospective review | Time of day of radiation has an effect on overall survival and tumor control | Non-small cell lung cancer (NSCLC) brain metastases | Gamma Knife Model U (1989–2001) or Model C (2001–2007) 10–24.5 Gy (1.59–3.66 Gy/min or 1.98–3.88 Gy/min) |
| Shukla et al. [41] | July 2006–June 2008 100% Female Mean age of ∼49 years |
252 | Randomized prospective study | Time of day of radiation has an effect on acute Gastrointestinal (GI) mucositis Primary endpoint: GI mucositis and incidence of grade 3 or 4 diarrhea |
Cervical carcinoma | Cobalt60 using parallel and opposite fields 50 Gy (2 Gy/fraction) with 5 fractions per week |
| Squire et al. [49] | January 2010–November 2015 71% male Median age of 64 years |
155 | Retrospective review | Time of day of radiotherapy has an effect on local tumor control | Rectal adenocarcinoma | Cobalt60 using parallel and opposite fields 50 Gy (2 Gy/fraction) with 5 fractions per week |
Static step-and-shoot intensity-modulated radiation therapy
Fig. 2.

Distribution of time sampling groups within the randomized prospective studies and the retrospective reviews. Diagonal black stripes indicate the morning groups and solid black boxes indicate the afternoon groups, one study had a midday group indicated in vertical grey stripes
patient demographics
The average samples size of the studies was 258 (± 124 SD) patients however there was a wide range of N (97–437 patients, Table 2). While all patients were undergoing radiation treatment for cancer, the type varied and included skin cancer (squamous cell carcinoma, n = 2), lung cancer (NSCLC, n = 2), bone metastasis (n = 1), prostate cancer (prostate adenocarcinoma, n = 1), cervical cancer (cervical carcinoma, n = 1), and colorectal cancer (rectal adenocarcinoma, n = 1); none of the papers examined were in the primary brain tumor patient population. Most of these patients were also receiving other treatments including chemotherapy [44, 46–49], analgesics [42] and hormones [44, 45]; three studies excluded patients that receive chemotherapy prior to radiation [40–42]. Most of the studies had both male and female patients, except those that examined prostate cancer [45], which was 100% male patients, or breast [44] and cervical cancers [41], which had 100% female patients. The two all-female studies were skewed to younger subjects (breast cancer: median of 47; cervical carcinoma: mean of 49) as compared to the other studies that all had patients with medians/means ages equal to or greater than 60 years old. Treatment for the different cancers used varying types of irradiators and radiation regimens. Irradiator sources used to administer treatment included older cobalt-60 units (n = 3) and newer stereotactic radiosurgery approaches using True-beam (n = 1), TomoTherapy (n = 1) Gamma Knife (n = 2). Two studies did not specify the type of irradiator used for therapy [40, 46]. It should be noted that the cobalt systems were used in two of the three prospective studies and known to have increased toxicities due to the fixed energies of the beams. Radiation dosing and number of fractions ranged from 8 Gy in 1 fraction to 70 Gy in 35 fractions.
Impacts on cancer progression and tumor size
The direct impact of radiation chronotherapy on diseased tissue was examined by interrogating factors associated with the tumor, including overall survival (OS) and alterations in the tumor size or progression of disease over time (Table 3). Five studies [40, 44–47] presented overall survival data that was primarily analyzed with the Kaplan—Meier (KM) method although some studies [44, 46, 47] used Multivariate Cox regression analysis to interrogate time effects in relation to several other variables (such as KPS score, age, # targets, time to treatment, cancer stage, and molecular subtypes). Two studies found clear positive correlation with survival in patients treated in the morning for prostate cancer [45] and NSCLC brain metastases patients [47]. In the prostate cancer manuscript, the authors created matched groups attempted to reduce the influence of other possible variables and further used multivariate analysis to confirm the time effects in relation to these variables. Negative results were observed in two other studies: Bjarnason et al. [40] found that after 2 years there was similar survival between the two arms of their randomized trial of skin cancer patients (Morning: 61.1%; Afternoon: 64.1%), Noh et al. [44] also showed similar 7-year survival rates in breast cancer patients (Morning: 96.0%; Afternoon: 95.9%). Badiyan et al. [46] had mixed results in NSCLC patients; their KM analysis demonstrated a positive effect of morning treatment. However, multivariate analysis did not find a significant effect (p = 0.11) suggesting that the morning group disproportionally had received stereotactic radiosurgery closer to diagnosis which drove the effect in the KM analysis. The authors listed lack of statistical power necessary to identify significant changes in the multivariate analysis after accounting for imbalances between time groups as a major limitation of their study. They conclude that larger data sets are required to definitively confirm the impact of time in these patients. These studies demonstrate that the effects of radiation therapy on overall survival are not consistent and should not be the primary focus of the methodology.
Table 3.
Overall survival and tumor control measures in the present systematic review
| (a) Overall survival | ||||
| Reference | Duration | Effect | Statistical tests | Significance |
| Badiyan et al. [46] | 2 years | Mixed (morning better) | Kaplan–Meier (KM) method and Cox Regression Univariate and Multivariate |
KM: morning, 10 months versus afternoon, 8 months, p = 0.012 Univariate: HR 1.30(1.06–1.59) p = 0.11 Multivariate: N/A, p = 0.11 |
| Bjarnason et al. [40] | 2 years | No | Kaplan–Meier (KM) method | 61.1% for Morning and 64.1% for Afternoon (p = 0.42) |
| Hsu et al. [45] | 6 years | Yes (morning better) | Kaplan–Meier (KM) method with Matched Cohorts | Morning 92% vs. afternoon 75%, respectively, p = 0.09 |
| Noh et al. [44] | 7 years | No | Kaplan–Meier (KM) method and Cox Regression Multivariate | KM: morning 96.0% vs afternoon 95.9%, respectively, P = 0.8485 Multivariate: HR 0.867(0.33–2.275) p = 0.7714 |
| Rahn et al. [47] | 7 years | Yes (morning better) | Kaplan–Meier (KM) method and Cox Regression Multivariate |
KM: morning 9.5 months vs afternoon 5 months, respectively, P = 0.025 Multivariate: HR 2.00(1.25–3.17) p = 0.004 |
| (b) Tumor control | ||||
| References | Variables | Effect | Statistical test | Significance |
| Badiyan et al. [46] | Radiographic progression from MRI review | Mixed (morning better) | Kaplan–Meier (KM) method and Cox Regression Univariate and Multivariate | KM: morning 74% vs afternoon 54%, p
= 0.016 Univariate: HR 1.59(1.03–2.46) p = 0.035 Multivariate: N/A, p = 0.12 |
| Bjarnason et al. [40] | Time to Recurrence | No | Kaplan–Meier (KM) method | Morning 64% vs afternoon 60%, HR: 0.92(0.60–1.41) p = 0.70 |
| Chan et al. [43] | Treatment responsea | Mixed (midday better for ♀) | Kurskal-Wallis nonparametric -or- Fisher exact test | All groups: all treatment times, p = 0.3001; < 80% of treatment time, p = 0.8714 ♀: All treatment times, p = 0.0331; < 80% of treatment time, p = 0.02821; midday best results ♂: No significance reported |
| Goyal et al. [42] | Response rateb | No | Chi-squared test | Grade I–II: morning 90% and Afternoon 86%, p > 0.05 Grade III–IV: morning 10% and afternoon 14%, p > 0.05 |
| Hsu et al. [45] | Biochemical free survival | Yes (morning better) | Kaplan–Meier (KM) method with Matched Cohorts | HR: 1.95(1.00–3.81), p = 0.05 |
| Noh et al. [44] | Disease free survival, local recurrence, distant metastasis, contralateral | No | Kaplan–Meier (KM) method and Cox Regression Multivariate -or- Chi-squared test |
KM: morning 92.7% and afternoon 94.0%, p = 0.9742 Multivariate: HR: 1.073 (0.526–2.188) Local recurrence: p = 0.8936 Distant metastasis: p = 0.6733 Contralateral: p = 0.7254 |
| Rahn et al. [47] | Local control from MRI review | Yes (morning better) | Chi-squared test | Morning 97% and afternoon 67%, p = 0.014 |
| Shukla et al. [41] | Treatment responsec | No | t tests | Complete response: p = 0.11 Partial response: p = 0.10 Progression: p = 0.65 |
| Squire et al. [49] | Tumor size from MRI or CT and Lymph node pathology | Mixed (afternoon better) | Two-way Mann–Whitney U and Two-way ANOVA | Tumor size: group: p = 0.035; ♂: p < 0.003; ♀: p > 0.05 Lymph node: group: p > 0.05; ♂: p > 0.05; ♀: p > 0.05 |
Bolded values indicate papers that find some significant differences between times
International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastasis
Radiotherapy Therapy Oncology Group (RTOG) criteria
The RECIST (Response Evaluation Criteria in Solid Tumors) Criteria
Tumor size and disease progression were examined in all nine manuscripts, however, the variables used were dramatically different between the studies and only three manuscripts used consensus guidelines which are easily replicated or compile for meta-analysis (Table 3). Those three studies quantified treatment response using the International Consensus on Palliative Radiotherapy Endpoints for Future Clinical Trials in Bone Metastasis [48], Radiotherapy Therapy Oncology Group (RTOG) criteria, and The Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Interestingly, two of the three studies that used a standardized criterion were also the randomized prospective trials. Three other studies used imaging (MRI or CT) and their own defined criteria to determine the radiographic progression, local control or exact tumor size. Two studies examined time to recurrence but did not specify the exact methodology for identifying recurrence [40, 44]. Finally, one study used biochemical-free survival as defined by prostate specific antigen (PSA) in the blood [45]. Biochemical failure was defined as an elevated PSA that exceeded the nadir plus 2.0 ng/ml.
Four studies found no significant effects of radiation administration time to disease progression [40–42, 44]. Only two studies found definitive effects of radiation administration time, both with improvements in the morning group [45, 47]. Three studies found mixed results; Badiyan et al. [46] lost the Kaplan—Meier local control significance when using multivariate analysis, the other two studies [43, 49] only saw significance in one gender. Chan et al. [43] found that female patients with bone metastasis had better treatment response as defined by the International Consensus on Palliative Radiotherapy Endpoints for Future Clinical Trials in Bone Metastasis when given radiation during the midday. Squire et al. [49], on the other hand, observed a greater effect on tumor size in male patients when radiation was given during the afternoon. The differences in tumor size and disease progression effects between these studies also support using other non-tumor related variables to measure the impact of radiation chronotherapy.
Impacts on patient healthy tissue and outcomes
Beyond the control of disease, radiation can induce treatment side effects that can impact patient quality of life. Within the manuscripts reviewed, five measured other symptoms related to treatment (Table 4) but only one included a patient reported outcome, specifically a quality of life instrument [40]. Quality of life (QOL) was quantified in this study using the head-and-neck RT questionnaire (HNRQ) [50]; which examined patient oral, skin, throat energy, psychosocial, and digestive domains and demonstrated greater symptom burden with higher scores. Bjarnson et al. [40] found that patients had better QOL for the oral domain in week 1 and the throat domain in week 2 if treated in the morning. Four studies examined the effects of radiation on mucosal tissue, either in the oral cavity [40, 42] or the gastrointestinal (GI)/ Gastrourinary (GU) tracts [41, 45]. The remaining study [44] examined the effects of radiation on the skin. These regions, skin and mucosal tissue, share a high proliferative rate and are therefore more susceptible to acute toxicities related to radiation [51]. All five studies found at least one variable that was significantly different between the time groups examined and all but one significant variable, late GI toxicity [45], found that Morning radiation produced fewer side effects than Afternoon treatment. Hsu et al. [45] found that acute radiation effects demonstrated greater incidence of Grade 1 or higher GI and GU issues, however, when they followed up with patients after approximately 68 months, they saw lower toxicity in the evening for GI tract and no effects in GU tract. The other study that examined GI toxicity did not specify the length of time they monitored patients and only observed a positive effect of Morning treatment. It should also be noted that Hsu et al. (2016) [45] observed a significant effect of old age as a covariable for the late toxicity effect which may not be present in the Shukla et al. [41], as the population was much younger with a mean of 49 rather than median of 72 years old. One other study had interesting covariables, Bjarnason et al. [40] did not observe significant effects of time until they accounted for either smoking status or total radiation dose received.
Table 4.
Quality of life and treatment symptoms in the present systematic review
| References | Variables | Effect | Statistical tests | Significance |
|---|---|---|---|---|
| Bjarnason et al. [40] | Oral mucositis toxicitya | Mixed (morning better) | Cochran-Mantel–Haenszel test or Log-rank test | All groups: morning 52.9% vs. afternoon 62.4%, p = 0.17 High doses, grade 2 & 3: morning 44.6% vs. afternoon 67.3%, p = 0.022 Non-Smokers, grade 3 + : morning 42.9% vs. afternoon 76%, p = 0.025 |
| Quality of Lifeb | Yes (morning better) | Not specified |
Week 1 oral domain: Morning 0.00 vs afternoon 0.35, p = 0.046 Week 2 throat domain: Morning 0.26 vs afternoon 0.73, p = 0.021 |
|
| Hsu et al. [45] | Gastrointestinal toxicityc | Yes (acute morning better, late afternoon better) | Kaplan–Meier (KM) method and Log-rank test or Multivariate binary logistic regressions |
Acute: KM afternoon 56% vs. morning 42%, p = 0.01; multivariate HR 1.79(1.18–2.72), p = 0.01 Late: KM afternoon 81% vs. morning 93%, p = 0.001; multivariate HR 2.96(1.63–5.37), p < 0.001 (Age significant) |
| Gastrourinary toxicityc Gastrourinary toxicityc |
Mixed (only acute morning better) |
Acute: KM afternoon 52% vs. morning 32%, p < 0.001; multivariate HR 2.41 (1.49–3.88), p < 0.001 Late: Afternoon 88% vs. Morning 88%, p = 0.57 |
||
| Goyal et al. [42] | Oral Mucosa Ulcerationa | Mixed (morning better) | Mann–Whitney U (MWU) test and Chi-Squared (CS) test |
MWU: significant effects week 4 (p = 0.049) and week 7 (p = 0.041) CS: grade III and IV morning 26% vs. afternoon 38%, p = 0.08 |
| Shukla et al. [41] | Mucositis /diarrheaa | Yes (afternoon better) | t-tests | Morning 87.39% vs afternoon 68.18%, z = 3.36, p < 0.01 |
| Other toxicities | No | Skin reaction: z = 0.44, p > 0.05 Nausea/vomiting: z = 0.66, p > 0.05 Bladder toxicity: z = 0.65, p > 0.05 Hematological toxicity: z = 0.12, p > 0.05 |
||
| Noh et al. [44] | Acute skin reactiona | Yes (morning better) | Chi-squared test | Grade 2 + : morning 5.8% vs. afternoon 13.7%, p = 0.0088 |
Bolded values indicate papers that find some significant differences between times
Radiotherapy Therapy Oncology Group criteria
Head-and-neck RT questionnaire (HNRQ)
Common Toxicity Criteria for Adverse Events version 4.0
Discussion
The primary goal of this systematic review was to synthesize the radiation chronotherapy literature and determine the methods used to examine the time of day effects for radiation-induced symptom burden in patients anticipating the future application for brain tumor patients experiencing increased daytime sleep. Therefore, this systematic review examined the current landscape of radiation chronotherapy research in patients across different cancer types and compared the effects of treatment time within tumors and healthy tissue. A total of nine articles were reviewed, a majority of which were retrospective reviews and only three were randomized to treatment delivered either in the morning or afternoon. As expected, the three randomized trials had more consistent sample times and durations than the retrospective studies and defined clear primary and secondary endpoints. The primary endpoints for all three of these studies focused on treatment symptoms, specifically mucositis in the oral cavity or GI tract. One study found a significant difference between time groups, that was not seen in the two other studies. However, these studies either found a trend in their primary endpoint or significance after adjusting for other symptoms factors; both manuscripts recommended further investigations into radiation chronotherapy for their respective cancers. The main conclusions of the retrospective reviews were that chronotherapy had important therapeutic potential and recommended better sample selection and more randomized trials. These findings were recently echoed in a similar literature review of radiation chronotherapy [52], however, the authors encourage continued focus on circadian rhythms within tumors. Together both the retrospective and prospective studies reviewed here suggest that symptoms emanating from the effects of radiation on sensitive tissues, rather than tumor variables or overall survival, are the optimal primary endpoints for trials because symptomatology may be more affected by timing of treatment.
Symptoms as the primary endpoint
The clear focus of the radiation chronotherapy prospective trials on treatment related symptoms demonstrate that the field is moving toward optimizing treatment to healthy tissue. A systematic review of chronotherapy in colorectal cancer chemotherapeutics similarly found that overall survival and tumor response rate were less influenced than side effects by time of treatment [53]. All studies used the methods similar to that designed by Levi et al. [54] and later optimized [55], were 5-fluorouracil was given from 1:00 to 4:00 am and oxaliplatin was given at 1:00–4:00 pm via a pump system. Meta-analysis in this study demonstrated that chronomodulated drug administration improved mucositis when comparing five trials and neutropenia when comparing four trials. Treatment time can have different effects in different tissues because unique circadian rhythms are observed in many different cell types, both healthy and cancerous [4, 8, 10], and can vary in the phase of the rhythms expressed. These rhythms also control cell physiology based on the organ and cell type [5], suggesting that mechanisms like DNA repair could vary in peak expression between cell types thereby altering cell sensitivity to radiation at different times during the day. Additionally, highly replicating cells are disproportionally sensitive to radiation [51] as are tumor cells [56]. All the five studies that examine treatment related outcomes investigate cells that rapidly proliferate, mucosal lining and skin basal layer. Additionally, two studies not covered within the search criteria also showed heightened hematological toxicity [57] and Late Effects of Normal Tissue-Subject Objective Management Analytical values [58] for morning treatment in patients with cervical carcinoma and breast cancer, respectively. In in vivo rodent models, cell cycle and proliferative activity governs time-dependent radiation sensitivity [17, 59–61]. Within the brain, the primary focus of attention for radiation research has focused on cognition and the hippocampus [62]. However, little is known about how the circadian and sleep brain circuits are impacted by radiation and if these regions are predisposed to sensitivity at specific times. The mechanism which predisposes patients to radiation-induced hypersomnolence may be alleviated if treatment time reduces the injury caused by radiation to the brain. Further testing is merited in the use of radiation chronotherapy for cancers of the central nervous system.
Sample time and patient rhythms
Modern applications of chronotherapy have promoted the identification of each individual patient’s endogenous clock or chronotype to provide personalized chonomedicine, optimal treatment is based on the patient’s current circadian rhythms [63, 64]. None of the papers reviewed here, examined the patient’s circadian rhythm neither through surveys nor actigraphy. Within the retrospective reviews, patients selected the scheduled time for their respective treatment [46, 47]; therefore, diurnal preference (chronotype) distribution could vary dramatically between the Morning and Afternoon groups. Chronotype of an individual is a representation of the patient’s circadian rhythm and specifies the time when they optimally perform, generally people are either morning types (larks) or evening types (night owls). Identifying chronotypes can be achieved via survey, Morningness—Eveningness questionnaire (MEQ) [65] or Munich ChronoType Questionnaire (MCTQ) [66]. Diurnal preference is related to sleep, specifically evening types wake up latter [67], are less alert in the morning [68], have higher risk of poor sleep quality [69, 70], and more sleep debt during the work week [71]. Sleep dept caused by the misalignment between circadian rhythms and social pressures leads to negative work and health consequences [72, 73]. Mice with misaligned circadian rhythms have aggravated effects of radiation, with greater weight loss and lower survival [74, 75]. Therefore, understanding the patient’s endogenous circadian rhythms and possible chronodisruptions are important in interpreting the effects of radiation chronotherapy and should be considered in future studies.
Sample demographics: gender and age
There were several studies that only observed significance when the data were divided into subgroups. Two studies found that patient gender played a role in the effects observed on the tumor response. Chan et al. [43] found that female patients had a better response in bone metastasis, while Squire et al. [49] showed effects in male patients with Rectal Adenocarcinoma. In patients with whole brain radiation for metastasis in several different cancer types, overall survival was also significantly higher with morning treatment only in females [76]. Gender differences in circadian rhythms are well documented [77], these differences are observed within the central pacemaker of the brain (suprachiasmatic nucleus, SCN) and in downstream oscillators and their hormonal rhythms. Tumor cells between the gender will therefore be exposed to different hormonal profiles and have different expression of clock gene rhythms. In fact, colorectal cancer has been shown to have differing clock gene profiles between the genders and expression is related to estrogen receptors profiles of the cells [78]. A systematic review of the chronomodulated administration of chemotherapeutics in colorectal cancer found that males benefited more than females [79], these findings are similar to the results we report here in Squire et al. [49]. Another important variable is age, Hsu et al. [45] found an interaction between older age and later radiation time with worse GI toxicity (HR: 1.02 (1.00–1.03), p = 0.04). Age differences in circadian rhythms, like gender, have been well documented [80]. There is an overall dampening of physiological rhythms in older subjects with weaker SCN rhythms for glucose metabolism [81] and electrical activity [82, 83]. Dampened circadian rhythms leads to a more vulnerable circadian system which can have detrimental effect, including reducing survival [84]. Both gender and age are important factors that appear to play a major role in the effects observed in with chronotherapy. Future trials should ensure that arms are matched to address the effects of these covariates. Additionally, further investigation into the underlying mechanisms that predispose these groups and not others is critical in developing optimal treatment plans for all patients.
Limitations
The literature describing radiation chronotherapy is limited by several factors. Primarily, there are very few prospective randomized trials which makes definitive recommendations difficult. The majority of the articles in this systematic review (6/9) are based on retrospective studies. These studies have many more possible biases, the most precarious of which is the self-selection of treatment time by patients in some studies. As outlined above, we are unclear of the underlying circadian rhythms between groups that selected treatment in the morning vs afternoon and if preference may lead to different sensitivity to radiotherapy regardless of treatment time. Therefore, it would be difficult to make claims about the treatment time-of-day effects between these groups. Another major flaw within the retrospective reviews, is the extreme variability in the division of time groups as outlined in Fig. 2. The sizes (number of hours), start/end times, and more importantly consistence of treatment time within patient across treatment were all differently defined and could have impacted the findings. Finally, unlike the prospective trials clear endpoints were not defined prior to the analysis of the data in the retrospective reviews. These studies however highlight similar more prevalent effects in symptom variables rather than tumor control. The reviews, therefore, provide guidance for selecting variables for future trails.
Conclusion
Circadian rhythms drive the 24-h undulation of behavior and physiology into defined peaks and nadir. The expression of these rhythms is dependent on tissue type, so that different cells have heightened sensitivity to treatment at different times of day. This review highlights the importance of examining not only the direct effects of treatment time on the tumor, as measured by overall survival and progression, but for examining other symptoms caused by radiation damage in adjacent healthy tissues. The tumor data reviewed here is evenly divided into studies that find chronobiologic effects and those that do not. However, the data in healthy mucosal tissue and skin all demonstrate that radiation chronotherapy significantly reduce symptom burden in patients, with a general improvement when given in the morning. There are several distinct limitations to these studies, specifically a majority are retrospective reviews and vary wildly in the definition of time groups. Multivariate analysis also demonstrated the importance of balanced groups, as there were several subgroups (age, gender and disease status) which were found to interact with treatment time effect. The findings of this review underscore the need to further examine the effects of radiation chronotherapy on both tumor response and toxicities in a well-designed prospective manner, and to conduct studies in brain tumor patients based on the possibility of significant toxicities associated with this therapy.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Halberg F, Haus E, Cardoso SS, Scheving LE, Kühl JFW, Shiotsuka R et al. (1973) Toward a chronotherapy of neoplasia: tolerance of treatment depends upon host rhythms. Experientia 29(8):909–934 [DOI] [PubMed] [Google Scholar]
- 2.Ballesta A, Innominato PF, Dallmann R, Rand DA, Levi FA (2017) Systems chronotherapeutics. Pharmacol Rev 69(2):161–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Y, Xiang Y, Ozguc FM, Kim Y, Liu CJ, Park PK et al. (2018) The genomic landscape and pharmacogenomic interactions of clock genes in cancer chronotherapy. Cell Syst 6(3):314–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED et al. (2004) PERIOD2: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101(15):5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359(6381): eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley JM, Loros JJ, Dunlap JC (2016) Circadian oscillators: Around the transcription-translation feedback loop and on to output. Trends Biochem Sci 41(10):834–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossetti S, Esposito J, Corlazzoli F, Gregorski A, Sacchi N (2012) Entrainment of breast (cancer) epithelial cells detects distinct circadian oscillation patterns for clock and hormone receptor genes. Cell Cycle 11(2):350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slat EA, Sponagel J, Marpegan L, Simon T, Kfoury N, Kim A et al. (2017) Cell-intrinsic, Bmall-dependent circadian regulation of temozolomide sensitivity in glioblastoma. J Biol Rhythms 32(2):121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broadberry E, McConnell J, Williams J, Yang N, Zindy E, Leek A et al. (2018) Disrupted circadian clocks and altered tissue mechanics in primary human breast tumours. Breast Cancer Res 20(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You S, Wood PA, Xiong Y, Kobayashi M, Du-Quiton J, Hrushesky WJ (2005) Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Res Treat 91(1):47–60 [DOI] [PubMed] [Google Scholar]
- 12.Zhanfeng N, Yanhui L, Zhou F, Shaocai H, Guangxing L, Hechun X (2015) Circadian genes Per1 and Per2 increase radiosensitivity of glioma in vivo. Oncotarget 6(12):9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi F (2001) Circadian chronotherapy for human cancers. Lancet Oncol 2(5):307–315 [DOI] [PubMed] [Google Scholar]
- 14.Ozturk N, Ozturk D, Kavakli IH, Okyar A (2017) Molecular aspects of circadian pharmacology and relevance for cancer chronotherapy. Int J Mol Sci 18(10):2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskar R, Lee KA, Yeo R, Yeoh KW (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9(3):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N (2010) Circadian clock control of the cellular response to DNA damage. FEBS Lett 584(12):2618–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palombo P, Moreno-Villanueva M, Mangerich A (2015) Day and night variations in the repair of ionizing-radiation-induced DNA damage in mouse splenocytes. DNA Repair 28:37–7 [DOI] [PubMed] [Google Scholar]
- 18.Ünsal-Kaçmaz K, Mullen TE, Kaufmann WK, Sancar A (2005) Coupling of human circadian and cell cycles by the timeless protein. Mol Cell Biol 25(8):3109–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotta-Ramusino C, McDonald ER, Hurov K, Sowa ME, Harper JW, Elledge SJ (2011) A DNA damage response screen identifies RHINO, a 9–1-1 and TopBP1 interacting protein required for ATR signaling. Science 332(6035):1313–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang TH, Leem SH (2014) Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res 42(7):4427–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L, Pelicano H, Liu J, Huang P, Lee CC (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111(1):41–50 [DOI] [PubMed] [Google Scholar]
- 22.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP (2006) The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22(3):375–382 [DOI] [PubMed] [Google Scholar]
- 23.Corrà S, Salvadori R, Bee L, Barbieri V, Mognato M (2017) Analysis of DNA-damage response to ionizing radiation in serum-shock synchronized human fibroblasts. Cell Biol Toxicol 33(4):373–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia HC, Niu ZF, Ma H, Cao SZ, Hao SC, Liu ZT, Wang F (2010) Deregulated expression of the Per1 and Per2 in human gliomas. Can J Neurol Sci 37(3):365–370 [DOI] [PubMed] [Google Scholar]
- 25.Xia HC, Wang F, Li YH, Li ZK, Cao SZ, Li CY, Niu ZF (2012) The circadian gene expression of Per1 and Per2 and their influence on radiotherapeutic sensitivity of glioma in vitro. Future Neurol 7(3):341–348 [Google Scholar]
- 26.Bentzen SM (2006) Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 6(9):702. [DOI] [PubMed] [Google Scholar]
- 27.Donahue B (1992) Short-and long-term complications of radiation therapy for pediatric brain tumors. Pediatr Neurosurg 18(4):207–217 [DOI] [PubMed] [Google Scholar]
- 28.Prager I, Patties I, Himmelbach K, Kendzia E, Merz F, Müller K et al. (2016) Dose-dependent short-and long-term effects of ionizing irradiation on neural stem cells in murine hippocampal tissue cultures: neuroprotective potential of resveratrol. Brain Behav 6(10):e00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T (2016) The symptom burden of primary brain tumors: evidence for a core set of tumor-and treatment-related symptoms. Neuro-Oncology 18(2):252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong TS, Vera E, Zhou R, Acquaye AA, Sullaway CM, Berger AM et al. (2017) Association of genetic variants with fatigue in patients with malignant glioma. Neuro-Oncol Pract 5(2):122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comasco E, Nordquist N, Göktürk C, Åslund C, Hallman J, Oreland L, Nilsson KW (2010) The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Upsala J Med Sci 115(1):41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KA, Gay C, Byun E, Lerdal A, Pullinger CR, Aouizerat BE (2015) Circadian regulation gene polymorphisms are associated with sleep disruption and duration, and circadian phase and rhythm in adults with HIV. Chronobiol Int 32(9):1278–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gafarov V, Gromova E, Gagulin I, Panov D, Gafarova A, Krymov E (2019) Association of polymorphism RS934945 Gene PER2 with sleep disoders in the male population 25–44 years in Novosibirsk. Eur Neuropsychopharmacol 29:S923 [Google Scholar]
- 35.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM et al. (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291(5506):1040–1043 [DOI] [PubMed] [Google Scholar]
- 36.Satoh K, Mishima K, Inoue Y, Ebisawa T, Shimizu T (2003) Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep 26(4):416–417 [DOI] [PubMed] [Google Scholar]
- 37.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G et al. (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400(6740):169. [DOI] [PubMed] [Google Scholar]
- 38.Ikegami K, Iigo M, Yoshimura T (2013) Circadian clock gene Per2 is not necessary for the photoperiodic response in mice. PLoS ONE 8(3):e58482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ, Kim L, Kang SG, Yoon HK, Choi JE, Park YM et al. (2011) PER2 variation is associated with diurnal preference in a Korean young population. Behav Genet 41(2):273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjarnason GA, MacKenzie RG, Nabid A, Hodson ID, El-Sayed S, Grimard L et al. (2008) Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). Int J Radiat Oncol* Biol* Phys 73(1):166–172 [DOI] [PubMed] [Google Scholar]
- 41.Shukla P, Gupta D, Bisht SS, Pant MC, Bhatt ML, Gupta R et al. (2010) Circadian variation in radiation-induced intestinal mucositis in patients with cervical carcinoma. Cancer 116(8):2031–2035 [DOI] [PubMed] [Google Scholar]
- 42.Goyal M, Shukla P, Gupta D, Bisht SS, Dhawan A, Gupta S, et al. (2009) Oral mucositis in morning vs. evening irradiated patients: a randomised prospective study. Int J Radiat Biol 85(6):504–509 [DOI] [PubMed] [Google Scholar]
- 43.Chan S, Zhang L, Rowbottom L, McDonald R, Bjarnason GA, Tsao M et al. (2016) Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann Palliat Med 6(1):14–25 [DOI] [PubMed] [Google Scholar]
- 44.Noh JM, Choi DH, Park H, Huh SJ, Park W, Seol SW et al. (2014) Comparison of acute skin reaction following morning versus late afternoon radiotherapy in patients with breast cancer who have undergone curative surgical resection. J Radiat Res 55(3):553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu FM, Hou WH, Huang CY, Wang CC, Tsai CL, Tsai YC et al. (2016) Differences in toxicity and outcome associated with circadian variations between patients undergoing daytime and evening radiotherapy for prostate adenocarcinoma. Chronobiol Int 33(2):210–219 [DOI] [PubMed] [Google Scholar]
- 46.Badiyan SN, Ferraro DJ, Yaddanapudi S, Drzymala RE, Lee AY, Silver SA et al. (2013) Impact of time of day on outcomes after stereotactic radiosurgery for non—small cell lung cancer brain metastases. Cancer 119(19):3563–3569 [DOI] [PubMed] [Google Scholar]
- 47.Rahn DA III, Ray DK, Schlesinger DJ, Steiner L, Sheehan JP, O’Quigley JM, Rich T (2011) Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment? Cancer 117(2):414–420 [DOI] [PubMed] [Google Scholar]
- 48.Chow E, Hoskin P, Mitera G, Zeng L, Lutz S, Roos D et al. (2012) Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol* Biol* Phys 82(5):1730–1737 [DOI] [PubMed] [Google Scholar]
- 49.Squire T, Buchanan G, Rangiah D, Davis I, Yip D, Chua YJ et al. (2017) Does chronomodulated radiotherapy improve pathological response in locally advanced rectal cancer? Chronobiol Int 34(4):492–503 [DOI] [PubMed] [Google Scholar]
- 50.Browman GP, Wong G, Hodson I et al. (1993) Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 328:159–163 [DOI] [PubMed] [Google Scholar]
- 51.Stone HB, Coleman CN, Anscher MS, McBride WH (2003) Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 4(9):529–536 [DOI] [PubMed] [Google Scholar]
- 52.Harper E, Talbot CJ (2019) Is it time to change radiotherapy: the dawning of chronoradiotherapy? Clin Oncol 31(5):326–335 [DOI] [PubMed] [Google Scholar]
- 53.Huang Y, Yu Q, Liu Y, Zhu Z, Wang L, Wang H, Li K (2017) Efficacy and safety of chronomodulated chemotherapy for patients with metastatic colorectal cancer: a systematic review and meta-analysis. Asia-Pac J Clin Oncol 13(2):e171–e178 [DOI] [PubMed] [Google Scholar]
- 54.Lévi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R et al. (1994) Chronomodulated versus fixed-infusion—rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (Leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. JNCI 86(21):1608–1617 [DOI] [PubMed] [Google Scholar]
- 55.Eriguchi M, Levi F, Hisa T, Yanagie H, Nonaka Y, Takeda Y (2003) Chronotherapy for cancer. Biomed Pharmacoth 57:92–95 [DOI] [PubMed] [Google Scholar]
- 56.Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ (2006) Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Can Res 66(17):8352–8355 [DOI] [PubMed] [Google Scholar]
- 57.Chang L, Li L, Li W, Jiang M, Jv Y, Wang L, Hou Y, Long Q, Yu S (2016) Research on radiotherapy at different times of the day for inoperable cervical cancer. Int J Clin Pharmacol Ther 54(11):856. [DOI] [PubMed] [Google Scholar]
- 58.Johnson K, Chang-Claude J, Critchley AM, Kyriacou C, Lavers S, Rattay T, Seibold P, Webb A, West C, Symonds RP, Talbot CJ (2019) Genetic variants predict optimal timing of radiotherapy to reduce side-effects in breast cancer patients. Clin Oncol 31(1):9–16 [DOI] [PubMed] [Google Scholar]
- 59.Ijiri K, Potten CS (1988) Circadian rhythms in the incidence of apoptotic cells and number of clonogenic cells in intestinal crypts after radiation using normal and reversed light conditions. Int J Radiat Biol 53(5):717–727 [DOI] [PubMed] [Google Scholar]
- 60.Ijiri K, Potten CS (1990) The circadian rhythm for the number and sensitivity of radiation-induced apoptosis in the crypts of mouse small intestine. Int J Radiat Biol 58(1):165–175 [DOI] [PubMed] [Google Scholar]
- 61.Bernabei PA, Balzi M, Saccardi R, Becciolini A, Ferrini PR (1992) Time-dependent sensitivity of rat CFU-GM to total body irradiation. Haematologica 77(1):21–24 [PubMed] [Google Scholar]
- 62.Robbins M, Greene-Schloesser D, Peiffer AM, Shaw E, Chan MD, Wheeler KT (2012) Radiation-induced brain injury: a review. Front Oncol 2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill RJ, Innominato PF, Lévi F, Ballesta A (2019) Optimizing drug infusion schedules towards personalized cancer chronotherapy. BioRes 10.1101/688606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Münch M, Kramer A (2019) Timing matters: new tools for personalized chronomedicine and circadian health. Acta Physiol 227:e13300. [DOI] [PubMed] [Google Scholar]
- 65.Horne JA, Östberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4:97–110 [PubMed] [Google Scholar]
- 66.Roenneberg T, Wirz-Justice A, Merrow M (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18(1):80–90 [DOI] [PubMed] [Google Scholar]
- 67.Duffy JF, Dijk DJ, Hall EF, Czeisler CA (1999) Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med 47(3):141–150 [PMC free article] [PubMed] [Google Scholar]
- 68.Clodore M, Benoit O, Foret J (1986) Diurnal variation in subjective and objective measures of sleepiness: the effects of sleep reduction and circadian type. Chronobiol Int 3(4):255–263 [DOI] [PubMed] [Google Scholar]
- 69.Chung MH, Chang FM, Yang CC, Kuo TB, Hsu N (2009) Sleep quality and morningness—eveningness of shift nurses. J Clin Nurs 18(2):279–284 [DOI] [PubMed] [Google Scholar]
- 70.Vitale JA, Roveda E, Montaruli A, Galasso L, Weydahl A, Caumo A, Carandente F (2015) Chronotype influences activity circadian rhythm and sleep: differences in sleep quality between weekdays and weekend. Chronobiol Int 32(3):405–415 [DOI] [PubMed] [Google Scholar]
- 71.Taillard J, Philip P, Bioulac B (1999) Morningness/eveningness and the need for sleep. J Sleep Res 8(4):291–295 [DOI] [PubMed] [Google Scholar]
- 72.Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, Caspi A (2015) Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes 39(5):842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yong M, Fischer D, Germann C, Lang S, Vetter C, Oberlinner C (2016) Are chronotype, social jetlag and sleep duration associated with health measured by Work Ability Index? Chronobiol Int 33(6):721–729 [DOI] [PubMed] [Google Scholar]
- 74.Gerbes AL, Arbogast B, Schick P, Messerschmidt O (1984) Acute radiation injury of mice and the influence of sudden time shift. Radiat Res 99(2):285–293 [PubMed] [Google Scholar]
- 75.Cui M, Xiao H, Luo D, Zhang X, Zhao S, Zheng Q et al. (2016) Circadian rhythm shapes the gut microbiota affecting host radiosensitivity. Int J Mol Sci 17(11):1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan S, Rowbottom L, McDonald R, Zhang L, Bjarnason GA, Tsao M, Danjoux C, Barnes E, Lam H, Popovic M, DeAngelis C (2016) Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann Palliat Med 5(4):267–279 [DOI] [PubMed] [Google Scholar]
- 77.Bailey M, Silver R (2014) Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol 35(1):111–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giacchetti S, Dugué PA, Innominato PF, Bjarnason GA, Focan C, Garufi C et al. (2012) Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Ann Oncol 23(12):3110–3116 [DOI] [PubMed] [Google Scholar]
- 79.Mostafaie N, Kállay E, Sauerzapf E, Bonner E, Kriwanek S, Cross HS et al. (2009) Correlated downregulation of estrogen receptor beta and the circadian clock gene Per1 in human colorectal cancer. Mol Carcinog 48(7):642–647 [DOI] [PubMed] [Google Scholar]
- 80.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA (1999) Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol 516(2):611–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wise PM, Cohen IR, Weiland NG, London ED (1988) Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus. Proc Natl Acad Sci USA 85(14):5305–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Satinoff E, Li H, Tcheng TK, Liu CHEN, McArthur AJ, Medanic M, Gillette MU (1993) Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol-Regul Integr Comp Physiol 265(5):R1216–R1222 [DOI] [PubMed] [Google Scholar]
- 83.Aujard F, Herzog ED, Block GD (2001) Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106(2):255–261 [DOI] [PubMed] [Google Scholar]
- 84.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD (2006) Chronic jet-lag increases mortality in aged mice. Curr Biol 16(21):R914–R916 [DOI] [PMC free article] [PubMed] [Google Scholar]


