Abstract
Purpose:
To report the clinical features, severity, and management of ocular immune-related adverse events (irAEs) in the setting of immune checkpoint inhibitor therapy for metastatic malignancies.
Methods:
Retrospective chart review at three tertiary ophthalmology clinics. Electronic medical records were reviewed between 2000 and 2017 for patients with new ocular symptoms while undergoing checkpoint inhibition therapy.
Results:
Eleven patients were identified. Ocular irAEs ranged from keratoconjunctivitis sicca to Vogt-Koyanagi -Harada-like findings. Average timing of irAEs from starting checkpoint inhibitor therapy was 15.7 weeks. Ocular inflammation was successfully controlled with corticosteroids in most cases, however three patients discontinue treatment as a result of ocular inflammation with decreased visual acuity, two discontinued due to progression of metastatic disease, and one discontinued due to severe systemic irAEs.
Conclusion:
We found a wide spectrum of ocular irAEs associated with immune checkpoint inhibitors. In most cases, ocular AEs did not limit ongoing cancer treatment.
Keywords: Adverse, checkpoint, events, inflammation, inhibitor
INTRODUCTION
Immune checkpoint inhibition is an emerging therapeutic approach for metastatic or recurrent cancer with promising clinical efficacy. Checkpoint proteins are expressed on activated T, B, and NK cells and serve to down-regulate cellular apoptosis and inflammation. Therapeutics targeting this pathway act through antibody blockade and can be categorized by their inhibitory targets: programmed cell death protein 1 (PD-1) inhibitors nivolumab and pembrolizumab are FDA approved for melanoma of the skin, non-small cell lung cancer, kidney cancer, bladder cancer, head and neck cancers, and Hodgkin lymphoma; programmed cell death ligand 1 (PD-L1) inhibitors atezolizumab, avelumab, and durvalumab are used to treat bladder cancer, non-small cell lung cancer, and Merkel cell carcinoma; and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor Ipilimumab is used in the treatment of malignant melanoma.1
Blockade of these receptors upregulates the body’s immune system against these malignant processes. However, these checkpoint inhibitors are associated with a unique set of side effects through unbridled inflammation termed immune-related adverse events (irAEs).2–5
The newness of these medications and limited reports of their ocular complications pose challenges amongst ophthalmologists and oncologists as to the appropriateness of referrals and management regimens for these patients. The aim of this case series is to describe the spectrum and severity of ocular irAEs seen at three tertiary ophthalmology centers in the United States (US) and to highlight management approaches.
METHODS
Cases were identified by retrospective chart review at three tertiary ophthalmology clinics in the US (National Eye Institute, The Washington D.C. Veterans Hospital, and Massachusetts Eye and Ear Institute). Institutional Review Board and Ethics Committee approval was obtained at each of the individual centers in compliance with the Declaration of Helsinki. Electronic medical records were reviewed between 2000 and 2017 using search terms “atezolizumab, avelumab, durvalumab nivolumab, pembrolizumab or immune check point inhibitors” (ICI) in order to identify patients seen in the eye clinics while undergoing checkpoint inhibition therapy for metastatic or recurrent malignancies with particular attention to consults initiated by oncology services at each institution. All patients were referred to the eye clinic by the oncology teams when they experienced eye-related symptoms. All patients underwent standard comprehensive ophthalmic examination and were treated according to standard of care practices at those clinics. Ophthalmology clinical reports and imaging were reviewed to identify those patients who experienced irAEs. Subjects were excluded if there was a preexisting uveitis and if their inflammation was not worse or more frequent than prior to initiation of checkpoint inhibitor and was therefore deemed likely unrelated to the medication.
The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5 grades adverse events from medications based on their severity into five categories. According to this classification, uveitis irAEs are specified as: grade 1, mild and asymptomatic; grade 2, moderate presenting as anterior uveitis; grade 3, severe with posterior or panuveitis; grade 4, life threatening; which in ophthalmology would be blindness (VA of 20/200 or worse) in the affected eye.6
RESULTS
A total of 11 patients were identified with ocular irAEs while being treated with a checkpoint inhibitor. Table 1 displays patient’s age at presentation, gender, ethnicity, primary malignancy being treated, type of checkpoint inhibitor at time of AE, timing of the event in weeks after starting medication, initial presenting ocular symptoms, ocular diagnosis, treatment course, and checkpoint inhibitor discontinuation status. Each AE per eye was grouped based on location of ocular inflammation, using standardization of uveitis nomenclature criteria where applicable.7
TABLE 1.
Characteristics of patients in our report.
| Patient | Age | Gender | Ethnicity | Reason for Treatment | Checkpoint Inhibitor | Timing weeks | Initial presentation | Eye involvement | Ocular treatment | ICI held/terminated/reason |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | Caucasian | Metastatic Colon Cancer | Pembrolizumab | 9 | Redness, small amount of discharge OU | Dry Eyes and Blepharitis | Artificial tears | Continued |
| 2 | 53 | M | African American | Metastatic Prostate Cancer | Durvalumab | 1 | Redness, sensitivity to light, tearing, eye redness and mucus discharge OU | NGAU: 1 + cell, 0 flare OU | Topical corticosteroids | Continued |
| 3 | 61 | M | Caucasian | Metastatic Renal Cell Carcinoma | Nivolumab | 52 | Floaters and blurry vision OS | NGAU OS: 0.5 + cell/2 + flare, CME OS with recurrent CME 2 years after discontinuation of ICI | Topical corticosteroids | Yes - ocular toxicity |
| 4 | 42 | F | African American | Metastatic Renal Cell Carcinoma | Ipilimumab and Nivolumab | 1 | Difficulty reading | NGAU OU: 1 + cell/1 + flare. CN VI palsy | Topical and IV corticosteroid bolus | Yes - ocular toxicity |
| 5 | 63 | M | African American | Metastatic Renal Cell Carcinoma | Nivolumab | 20 | Significantly reduced vision, eye pain, head ache, back pain and light sensitivity for 1 day | NGAU OU: 3 + cell/2 + flare. 360 iridolenticular synechia | Topical corticosteroids | Yes - progression of metastases |
| 6 | 40 | M | Asian | Metastatic Melanoma | Pembrolizumab | 24 | Floaters and irritation OD | Intermediate Uveitis: Trace vitreous cell with no evidence of vasculitis or CME | Topical corticosteroids | Yes - progression of metastases |
| 7 | 56 | M | Caucasian | Metastatic Melanoma | Ipilimumab and Nivolumab | 20 | Pain and blurry vision OD, blurry vision OS | NGAU OS. Panuveitis OD: CME OD. Recurrence 18 months after termination of ICI | Topical and local corticosteroids | Yes - ocular toxicity |
| 8 | 62 | F | Caucasian | Metastatic Choroidal Melanoma | Ipilimumab and Nivolumab | 4 | Central scotoma OS with poliosis and vitÍligo | OS: VKH like reaction | Oral corticosteroids | Continued |
| 9 | 63 | M | Caucasian | Metastatic Melanoma | Pembrolizumab | 8 | Decrease vision, light sensitivity, redness OS > OD | OS: VKH like reaction | Topical corticosteroids | Continued |
| 10 | 30 | F | Caucasian | Metastatic Melanoma | Ipilimumab | 18 | Blurry vision and injection OU | VKH-like reaction OU: recurrent bilateral anterior uveitis | Topical and oral corticosteroids | Yes - severe systemic toxicity |
| 11 | 65 | M | Caucasian | Metastatic Prostate Cancer | Durvalumab | unknown | Left inferior scotoma with mild discomfort with extraocular movements | Optic neuropathy OS: 4 + disc edema OS. HVF 30–2: near complete central sparing inferior defect OS | IV corticosteroid bolus | Continued |
Abbreviations: ICI (Immune Checkpoint inhibitor), OD (right), OS (left), F (female), M (male), CN (cranial nerve), NGAU (non-granulomatous anterior uveitis), VKH (Vogt-Koyanagi- Harada). Patients one was status post-enucleation OD
Nine of the 11 patients presented with intraocular inflammation (anterior, intermediate, posterior, or panuveitis) and the others with keratoconjunctivitis sicca (dry eye syndrome) and optic neuropathy.
One patient (two eyes) in our cohort experienced new onset dry eye syndrome. A 34-year-old female with metastatic colon cancer presented with foreign body sensation, eye redness, and blurry vision. On exam she was noted to have inflammation of the lid margins and superficial punctate keratitis and diagnosed with blepharitis and dry eye syndrome 9 weeks after starting pembrolizumab therapy. The patient’s symptoms were managed with topical artificial tears and conservative measures and she was able to continue treatment with pembrolizumab.
Nongranulomatous anterior uveitis (NGAU) was the most common type of ocular inflammation seen in our cohort with four patients (seven eyes) who presented with typical complaints of eye redness, blurry vision, light sensitivity or ocular discomfort and who were found to have anterior chamber cell ranging from +0.5 to 3+ grade. The average age of the patients with NGAU was 55 years (range 42–63 years), with ocular inflammation occurring on average 18.5 weeks (range 1–52 weeks) after the initiation of ICI treatment. The most common checkpoint inhibitor used in this category was nivolumab (noted in 75% of patients receiving either monotherapy or in combination with ipilimumab). All eyes with anterior uveitis were managed with topical corticosteroid therapy, however one patient also received intravenous corticosteroids following the advent of cranial nerve VI palsy (in addition to her anterior uveitis). One patient had low-grade inflammation that completely resolved with topical corticosteroids and was continued on checkpoint inhibitor therapy. Two patients had their treatment discontinued due to ocular inflammation, one in the setting of decreased visual acuity and cranial nerve VI palsy while the other experienced marked decrease in visual acuity related to cystoid macular edema, lastly one patient had their treatment stopped due to poor response with progression of metastatic disease.
Intermediate uveitis occurred in one patient, a 40-year-old male who experienced mild unilateral intermediate uveitis with no associated vasculitis or cystoid macular edema 24 weeks after starting pembrolizumab. The patient responded to topical corticosteroid treatment; however, checkpoint inhibitor therapy was stopped due to ineffectiveness with progression of metastatic disease.
Panuveitis occurred in one patient in our cohort. A 56-year-old male with biopsy-proven pulmonary sarcoidosis with no previous ocular involvement, presented with panuveitis and cystoid macular edema in the right eye; and NGAU in the left eye that started 20 weeks after initiation of nivolumab and ipilimumab therapy for metastatic melanoma. The patient’s inflammation and cystoid macular edema resolved with topical and sub-tenons corticosteroids and cessation of nivolumab and ipilimumab therapy due to ocular toxicity. However, 18 months later the patient presented with subacute vision loss in his right eye with vitritis and recurrent cystoid macular edema on exam and OCT imaging despite remaining off checkpoint inhibitor.
Choroidal inflammation was noted in three patients (four eyes) in a pattern often referred to as a Vogt-Koyanagi-Harada (VKH)-like reaction (Figure 1). The average age at presentation in this category was 52 years (range 30–63 years), and average timing of adverse event was 10 weeks (range 4–18 weeks) following initiation of treatment. All patients in this category were being treated for malignant melanoma and all three patients had resolution of choroidal inflammation with oral and topical corticosteroids. Two patients were able to continue checkpoint inhibitor therapy while one patient had treatment stopped due to severe systemic adverse events to include rash, myalgias, diarrhea, and transaminitis.
FIGURE 1.
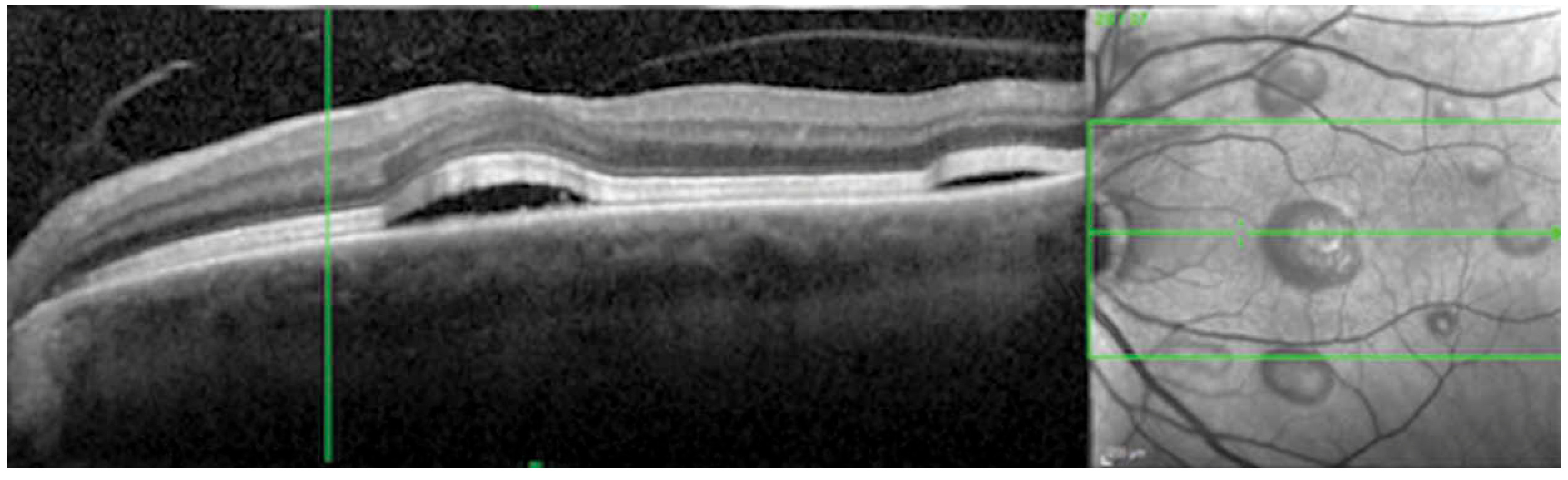
VKH-like syndrome associated with Ipilimumab and Nivolumab. - Optical Coherence Tomography of the left eye shows pockets of central subretinal fluid with multiple areas of localized retinal elevation correlating to the subretinal fluid on infrared image in patient 8.
One patient in our cohort experienced unilateral optic neuropathy. A 65-year-old male being treated with durvalumab for metastatic prostate cancer developed acute scotoma in the left eye with demonstration of grade 4 optic nerve edema and near complete inferior altitudinal defect on Humphrey Visual Field 30–2 testing. The patient had a normal brain MRI with and without contrast as part of his work up, and was treated with intravenous high dose corticosteroids with significant improvement in his subjective visual complaints and visual field testing and was able to continue treatment with durvalumab. Duration of durvalumab therapy prior to the incident was not documented.
DISCUSSION
Cancer immunotherapy when successful helps prolong life expectancy of patients with metastatic malignancy. Ipilimumab was the first checkpoint inhibitor to demonstrate improved overall survival rate from 5% to 18% for malignant melanoma when compared to controls in 2010.8 In 2015, the KEYNOTE-002 trial showed that pembrolizumab when compared to ipilimumab improved overall survival rate at 33 months from 39% to 50%.9 More recently in 2017, the Checkmate 067 trial demonstrated improved survival at 3 years for advanced melanoma with combination nivolumab and ipilimumab compared to nivolumab alone (58% versus 52%).10
Targeting CTLA-4 and PD-1 are used in combination in investigational trials as they have distinct targets with potential synergistic anti-tumor effects. However, there does appear to be increased risk of severe systemic irAEs when these agents are used in combination.10–12 Systemic irAEs are reported in up to 96% of patients while on combination therapy of Nivolumab and Ipilimumab with 59% consisting of grade 3 or 4 inflammation. Single therapy is only marginally safer with 28% of patients experiencing a grade 3 or 4 inflammation. The most common systemic irAEs are fatigue, infusion reaction, diarrhea, colitis, and skin rashes, however severe and life-threatening AEs like fatal myocarditis have been reported as well.10,13 In our study 55% of patients had reported non-ocular systemic irAEs, with only one experiencing severe grade 3 irAE. Since patients with severe systemic irAEs are taken off the medication, it is likely that they were not referred to us for ocular symptoms that began after cessation of the drug. Additionally, this patient cohort has a high mortality and morbidity rate off chemotherapy; thus, it is likely that ocular symptoms may have gone unreported or uninvestigated.
The incidence of ocular irAEs is rare, and ranges from 0.3% to 6% for uveitis and 1.2% to 24.2% for dry eye syndrome.14 Currently, recommended guidelines for oncologists include transient stopping of therapy for any grade 3 AE (anterior uveitis with 3+ or greater cells; intermediate, posterior, or pan-uveitis) until resolved and permanent discontinuation for grade 4 events which are defined as event resulting in visual acuity of 20/200 or worse in the affected eye.15,16
In our study, the most common ocular finding was anterior uveitis. Due to limited sample size, we were unable to draw any conclusion regarding specific ocular irAEs being associated with any specific drug or drug combination. We also were not able to identify demographic risk factors or associations between time of onset of inflammation with relation to immunotherapy infusions. However, we noted that many patients with ocular irAEs responded well to topical and oral corticosteroids, and continuing checkpoint therapy with close monitoring and treatment by ophthalmology was possible. Most of the patients in our cohort discontinued treatment due to progression of metastases or systemic adverse events, however there were a few that were discontinued due to duration and severity of ocular events, per previously specified clinical trial protocol safety criteria. We presume that these protocols were written by grouping ocular adverse events and systemic adverse events as having equal morbidity. We argue that ocular inflammation typically does not resolve immediately and low grades of ocular inflammation can be managed without discontinuing immune checkpoint inhibition.
Our cohort included patients that had recurrence of ocular inflammation despite being off ICI. It is unclear if this finding is incidental or if these patients warrant long-term surveillance. We also saw a patient with unilateral optic neuropathy, that may have been an incidental finding, however given the prior reports of optic neuritis with ICI17 we included the patient in this study. VKH-like reactions represent the more severe end of the spectrum of ocular AEs with checkpoint inhibitors. Our sample size is limited, however it is interesting that all three of the patients were being treated for malignant melanoma, with no clear association with a specific checkpoint medication or subclass. This association between patients treated for malignant melanoma and VKH like events has anecdotally been noted in previous published case reports.18–22 The association could stem from the higher numbers of patients with skin melanoma being treated with ICI as compared to other malignancies, however it is interesting to hypothesize a relationship between the two disease processes. While the precise mechanism of inflammation in VKH is still unknown, it is thought to involve CD4+ and CD8 + T cell targeting melanocytic antigens. Clinically it is characterized by bilateral serous detachments, choroiditis, sensorineural hearing loss, and vitiligo. It is possible that with checkpoint inhibition, the unbridled T-cell activation against melanoma-related antigens may contribute to the VKH-like clinical picture in a genetically predisposed patient. There is a known association between VKH and HLA-DR4 and HLA-A2. Indeed, one of these three patients (the only patient who had HLA typing) was HLA-DR4 positive. Itoh et al. demonstrated unique ability of T cells from HLA-A2 positive VKH patients to lyse melanoma cells and Yano et al. demonstrated a possible activation of melanoma cytotoxic T lymphocytes by peripheral blood leucocytes from patients with HLA-DR4 VKH phenotypic disease. Further research linking the VKH-like inflammatory reactions in the setting of checkpoint inhibitors with these HLA markers could provide greater understanding of the mechanism of VKH.23–25 Systemic corticosteroids, which are the standard of care for VKH would diminish checkpoint inhibitor, effectively negating the anti-tumor activity, and hence has to be used with caution. Oftentimes, we were able to manage our patients’ flares with topical or local corticosteroid injections as needed.
Our study recognizes its limitations. It is a retrospective review of a small unique cohort of patients. These patients were all seen in tertiary ophthalmology clinics and likely only represent a subset of this patient population. Current ocular literature is likely skewed due to the publication bias at reporting only more rare or severe events. While the exact causal relationship of checkpoint inhibitor therapy and ocular inflammatory events is outside the scope of this paper, our aim is to demonstrate the various ocular irAEs that can present during the course of treatment with ICI. We were able to demonstrate resolution of ocular inflammation with local (topical and periocular) corticosteroid injections, which permitted some of our patients who have limited treatment options to continue to receive immunotherapy.
It is increasingly important for ophthalmologists to be aware of this medication class and their potential ocular complications. Ophthalmologists, whenever possible, should be involved as these irAEs can be managed with local or oral corticosteroids while continuing life-saving checkpoint inhibitor therapy. A multidisciplinary approach between ophthalmologist, oncologist, and internist alongside the patient and family will improve decision-making by focusing on what is most likely to enhance the patients’ health and quality of life.
FUNDING
This work was supported by the NEI intramural research support.
Footnotes
DECLARATION OF INTEREST
No conflicting relationship exists for any author.
REFERENCES
- 1.Voena C, Chiarle R. Advances in cancer immunology and cancer immunotherapy. Discov Med. 2016;21:125–133. [PubMed] [Google Scholar]
- 2.Huillard O, Bakalian S, Levy C, et al. Ocular adverse events of molecularly targeted agents approved in solid tumours: a systematic review. Eur J Cancer. 2014;50 (3):638–648. doi: 10.1016/j.ejca.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 4.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27(7):1362. doi: 10.1093/annonc/mdw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Common terminology criteria for adverse events (CTCAE), version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Updated 2017.
- 7.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. https://www.sciencedirect.com/science/article/pii/S0002939405004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NE-JMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35 (7):785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 13.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Rahman O, Oweira H, Petrausch U, et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. 2017;17(4):387–394. doi: 10.1080/14737140.2017.1296765. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;JCO2017776385. doi: 10.1200/JCO.2017.77.6385. [DOI] [PubMed] [Google Scholar]
- 16.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5(1):z. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori S, Kurimoto T, Ueda K, et al. Optic neuritis possibly induced by Anti-PD-L1 antibody treatment in a patient with non-small cell lung carcinoma. Case Rep Ophthalmol. 2018; 9(2):348–356. Published Jul 20, 2018. doi: 10.1159/000491075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo T, Yamasaki O. Vogt-koyanagi-harada disease-like posterior uveitis in the course of nivolumab (anti-PD-1 antibody), interposed by vemurafenib (BRAF inhibitor), for metastatic cutaneous malignant melanoma. Clin Case Rep. 2017;5(5):694–700. doi: 10.1002/ccr3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28(4):288–294. doi: 10.1097/CCO.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 20.Wong RK, Lee JK, Huang JJ. Bilateral drug (ipilimumab)-induced vitritis, choroiditis, and serous retinal detachments suggestive of vogt-koyanagi-harada syndrome. Retin Cases Brief Rep. 2012;6 (4):423–426. doi: 10.1097/ICB.0b013e31824f7130. [DOI] [PubMed] [Google Scholar]
- 21.Mantopoulos D, Kendra KL, Letson AD, Cebulla CM. Bilateral choroidopathy and serous retinal detachments during ipilimumab treatment for cutaneous melanoma. JAMA Ophthalmol. 2015;133(8):965–967. doi: 10.1001/jamaophthalmol.2015.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crews J, Agarwal A, Jack L, Xu D, Do DV, Nguyen QD. Ipilimumab-associated retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):658–660. doi: 10.3928/23258160-20150610-10. [DOI] [PubMed] [Google Scholar]
- 23.Moorthy RS, Inomata H, Rao NA. Vogt-koyanagi-harada syndrome. Surv Ophthalmol. 1995;39(4):265–292. [pii]. doi: 10.1016/S0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]
- 24.Norose K, Yano A. Melanoma specific Th1 cytotoxic T lymphocyte lines in vogt-koyanagi-harada disease. Br J Ophthalmol. 1996;80:1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugita S, Sagawa K, Mochizuki M, Shichijo S, Itoh K. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with vogt-koyanagi-harada disease. Int Immunol. 1996;8:799–803. [DOI] [PubMed] [Google Scholar]


