Abstract
There is a growing need for a better understanding of sex differences in animal models of psychiatric disorders. The elevated plus-maze (EPM) test and large open field (LOF) test are widely used to study anxiety-like behavior in rodents. Our studies explored sex differences in anxiety and activity parameters in the LOF and EPM and determined whether these parameters correlate within and between tests. Drug naïve adult male and female Wistar rats (n = 47/sex) were used for the studies, and the rats were tested for 5 min in the EPM and 10 min in the LOF. The females spent more time on the open arms of the EPM and made more open arms entries than the males. The females also spent more time in the center zone of the LOF and made more center zone entries. The females traveled a greater distance in the LOF and EPM. There was a moderate positive correlation between time on the open arms of the EPM and time in the center zone of the LOF. There was also a moderate positive correlation between open arms entries in the EPM and center zone entries in the LOF. A hierarchical cluster analysis revealed one cluster with LOF parameters, one cluster with EPM parameters, and one cluster with parameters related to the avoidance of open spaces. In conclusion, these findings indicate that female rats display less anxiety-like behavior in the EPM and LOF. Furthermore, there are sex differences for almost all behavioral parameters in these anxiety tests.
Keywords: Anxiety, sex, elevated plus-maze, open field, hierarchical cluster analysis, dendrogram
1. Introduction
Anxiety disorders are the most common mental illness in the US (Kessler et al., 1994). It has been estimated that about 40 million adults in the US suffer from an anxiety disorder (Kessler et al., 1994). Clinical studies show that women have a higher prevalence of anxiety and fear-related disorders than men (Maeng and Milad, 2015). Women are more likely to suffer from a generalized anxiety disorder, panic disorder, agoraphobia, specific phobia, and post-traumatic stress disorder (Goodwin et al., 2005; McLean et al., 2011). Even though anxiety disorders are more common in women, most animal studies have been conducted with males. Furthermore, anti-anxiety drugs have been mainly evaluated in male rats. New findings indicate that there are dramatic differences between the male and female brain (Cahill, 2006; Lenroot and Giedd, 2010), and sexually dimorphic brain areas play a role in psychiatric disorders (Asami et al., 2009; Liu et al., 2020; Turano et al., 2018).
The elevated plus maze (EPM) test and the large open field (LOF) test are some of the most widely used tests to investigate anxiety-like behavior in rats (Hogg, 1996; Prut and Belzung, 2003). The benzodiazepines chlordiazepoxide and diazepam decrease anxiety-like behavior in the EPM (Pellow et al., 1985). In contrast, exposure to stressors increases anxiety-like behavior in the EPM (Korte and De Boer, 2003). Stressors have a complex effect on open-field behavior, with physical stressors decreasing activity and emotional stressors increasing activity (Meerlo et al., 1996; Pijlman et al., 2003). The benzodiazepine chlordiazepoxide increases activity in the LOF test and time in the center zone of the LOF (Gentsch et al., 1987).
Most prior studies have been conducted with male rats, and it is not well known if there are sex differences in anxiety parameters in the EPM and the LOF (Beery and Zucker, 2011; Shansky, 2019). Many older studies that were conducted in the 1970s evaluated locomotor activity in the LOF and counted fecal boli (Blizard et al., 1975; Gray and Lalljee, 1974; Harrington, 1972). However, entries into the center zone of the LOF or time in the center zone were often not determined. Furthermore, conflicting findings have been reported regarding sex differences in anxiety-like behavior in the EPM and the LOF (Blizard et al., 1975; Dichter et al., 1996; Elliott et al., 2004; Genn et al., 2003; Gray and Lalljee, 1974; Harrington, 1972; Johnston, Amanda L and File, Sandra E, 1991; Mansouri et al., 2019; Sakhaie et al., 2020; Scholl et al., 2019; Steenbergen et al., 1991; Winther et al., 2018; Yang et al., 2019). There is a lot of variability between animals in the LOF and EPM, and sex differences in anxiety-like behavior are often nonexistent or small. Because most studies used relatively small numbers of animals, these studies might not have had the statistical power to conclude that there are sex differences in the LOF and EPM test. In addition, it is not well known which behavioral parameters correlate within the LOF and EPM test, and which parameters correlate between these tests. In a recent study, the correlation between behavioral tests was investigated in adolescent heterogeneous stock rats (NMcwi: HS)(Wang et al., 2018). However, the Wistar strain is one of the most widely used strains (Johnson, 2012), and therefore it is also important to investigate the correlation between behavioral tests in Wistar rats. Furthermore, it is not well known if the EPM and LOF measure similar aspects of anxiety-like behavior (Ramos et al., 2008). The goal of these studies was to determine sex differences in anxiety-like behavior in the EPM and LOF and determine if behavioral parameters in the EPM correlate with those in the LOF. A hierarchical clustering method was used to determine which behaviors in each test and across the tests showed similarities. Hierarchical clustering is a method that can be used to group similar behaviors into clusters (Fraley and Raftery, 1998). The behavioral parameters within each cluster are more similar to each other than to behavioral parameters in the other clusters. The present studies indicate that under baseline conditions (no stress or drug treatments), there are large sex differences in virtually all behavioral parameters in adult Wistar rats in the EPM and LOF, with the females displaying less anxiety-like behavior than the males. Both the correlational analysis and the hierarchical clustering analysis indicated that the EPM and LOF measure similar aspects of anxiety-like behavior in adult Wistar rats.
2. Material and Methods
2.1. Animals
Wistar rats (males 200–225 g, females 175–200 g, Charles River, Raleigh, NC) were housed socially in a climate-controlled vivarium on a reversed 12 h light-dark cycle (light off at 7 AM). Food and water were available ad libitum. All animal procedures were performed in accordance with the University of Florida Institutional Animal Care and Use Committee as well as the National Institutes of Health guidelines.
2.2. Experimental design
Upon arrival in the vivarium, the rats (males n=47, females n=47) were left undisturbed for one week. They were then gently handled on three consecutive days. The rats were tested in the EPM for 5 min, and the following day they were tested in the LOF for 10 min.
2.3. Elevated plus maze test
The elevated plus-maze test is used to assess anxiety-like behavior and was conducted as described previously (Bruijnzeel et al., 2019; Qi et al., 2016; Rylkova et al., 2009). The test apparatus consisted of four black polypropylene arms (Coulbourn Instruments, Whitehall, PA). The two “open” arms had 0.5 cm ledges, and the two “closed” arms had 30 cm walls. The open arms were placed opposite of each other. The arms were 10 cm wide, 50 cm long, and were placed on 55 cm tall acrylic legs. Testing occurred in a quiet, dimly lit (75 lux) room. At the beginning of each test, the rats were placed in the center of the apparatus facing an open arm. The rats were allowed to explore the apparatus for 5 min, and their behavior was recorded with a camera mounted above the maze. The EPM was divided into five zones (two open arms, two closed arms, and a center zone). Behavior was analyzed automatically (center-point detection) using EthoVision XT 11.5 software (Noldus Information Technology, Leesburg, VA). The following behavioral parameters were automatically determined: duration and entries into the open arms, closed arms, and center zone, and total distance traveled. The percentage of open arm entries (open arm entries/total arm entries) and percentage of time on the open arms (time on open arms/total time on the arms) were calculated. EPM heatmaps were produced with the EthoVision heatmap generator. The apparatus was cleaned with a Nolvasan solution between rats.
2.4. Large open field test
The LOF test is used to assess locomotor activity and anxiety-like behavior (Liebsch et al., 1998). The test was conducted for 10 min in a dimly lit room (75 lux), as described previously (Bruijnzeel et al., 2016; Qi et al., 2016; Tan et al., 2019). The LOF apparatus consisted of a large arena measuring 120 × 120 × 60 cm (L × W × H). The arena was made of black high-density polyethylene panels that were fastened together and placed on a plastic bottom plate (Faulkner Plastics, Miami, FL). The rats’ behavior was recorded with a camera mounted above the arena and analyzed with EthoVision XT 11.5 software (Noldus Information Technology, Leesburg, VA). The LOF was divided into three zones: an outside zone (20 cm wide), a middle zone (20 cm wide), and a center zone (40 × 40 cm; L × W). The following behaviors were analyzed: total distance traveled, distance traveled in each zone (outside, middle, and center), number of entries into each zone, and latency to enter the middle and center zone. LOF heatmaps were produced with the EthoVision heatmap generator. The LOF was cleaned with a Nolvasan solution between rats.
2.5. COLORcation
The LOF heatmaps were produced with the COLORcation tool (Dagan et al., 2016). COLORcation is a MATLAB based analysis tool that reads individual EthoVision tracking files and then assembles a database of the experiment. COLORcation matches the rat’s coordinates to a corresponding spatial bin and calculates the total time spent in each bin. The average time spent in each bin is used to generate the COLORcation heatmap.
2.6. Data Analysis
Behavioral parameters in the EPM and the LOF were analyzed with a one-way ANOVA with sex as a between-subjects factor. Pearson’s correlation coefficients (r) were calculated to determine the correlation within and between tests. P values (two-sided t-test) and Holm-adjusted p values (−log10) were calculated to determine if the correlation coefficients were significant. A distance matrix of the behaviors was computed with Euclidian distance between correlation coefficients as the distance measure. Ward’s method was used for the hierarchical clustering analysis (Ward Jr, 1963). The data were analyzed with GraphPad Prism version 8.4.3, IBM SPSS Statistics version 27, and R version 3.6.3. The R packages corrplot, dplyr, ggplot2, lazyeval, pheatmap, factoextra, and dendextend were used to analyze and visualize the data (Galili, 2015; Kassambara and Mundt, 2017; Kolde, 2012; Mirzasoleiman et al., 2015; Ward Jr, 1963; Wei and Simko, 2017; Wickham, 2016; Wickham et al., 2015).
3. Results
3.1. Elevated Plus Maze Test
There were sex differences in all behavioral parameters (except center duration) in the EPM test (Figures 1–3). The females made more open arm entries (F1,92 = 31.16, p < 0.0001), had a higher percentage of open arm entries (F1,92 = 6.36, p < 0.05), spent more time on the open arms (F1,92 = 20.86, p < 0.0001), spent a greater percentage of time on the open arms (F1,92 = 22.18, p < 0.0001), and made more closed arm entries (F1,92 = 15.88, p < 0.0001). Furthermore, the females traveled a greater distance on the EPM (F1,92 = 83.15, p < 0.0001). Females made more entries into the center of the EPM (F1,92 = 24.92, p < 0.0001), but there was no sex difference in the amount of time spent in the center area (F1,92 = 0.45, n.s.). The males spent more time in the closed arms compared to the females (F1,92 = 21.42, p < 0.0001). Effect sizes (Cohen’s d), means, and standard deviations (SD) for behavioral parameters in the EPM are reported in Table S1.
Figure 1. Decreased anxiety-like behavior in female rats compared to male rats in the elevated plus-maze test.
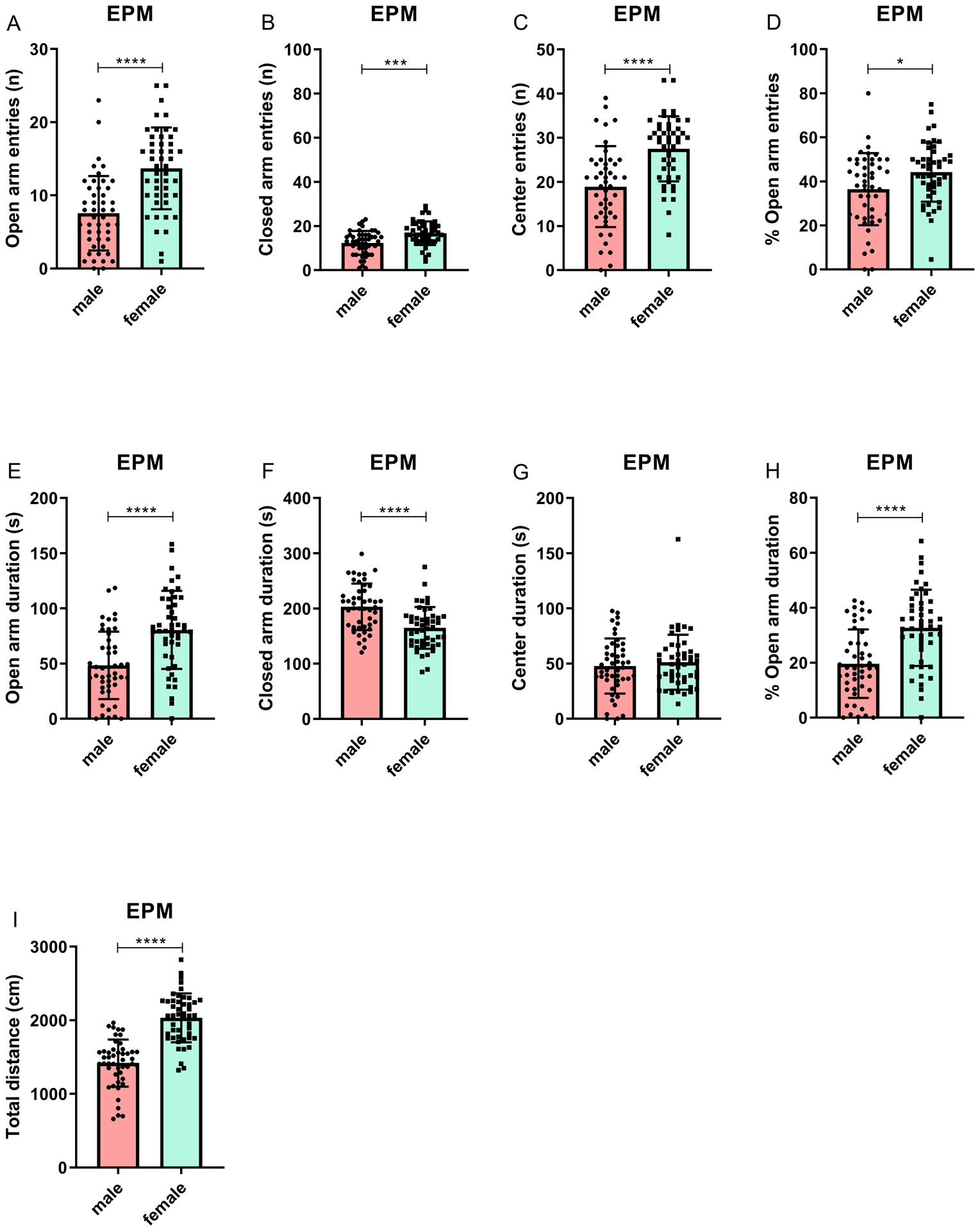
The figure shows open arm entries (A), closed arm entries (B), center entries (C), percentage open arm entries (D), open arm duration (E), closed arm duration (F), center duration (G), percentage open arm duration (H), and total distance traveled (I). The females were more active and displayed less anxiety-like behavior. N=47/group. *p<0.05, *** p<0.001, **** p<0.0001. Data are expressed as means ± SEM.
Figure 3. Sex differences in exploratory behavior in the large open field test and the elevated plus-maze test.
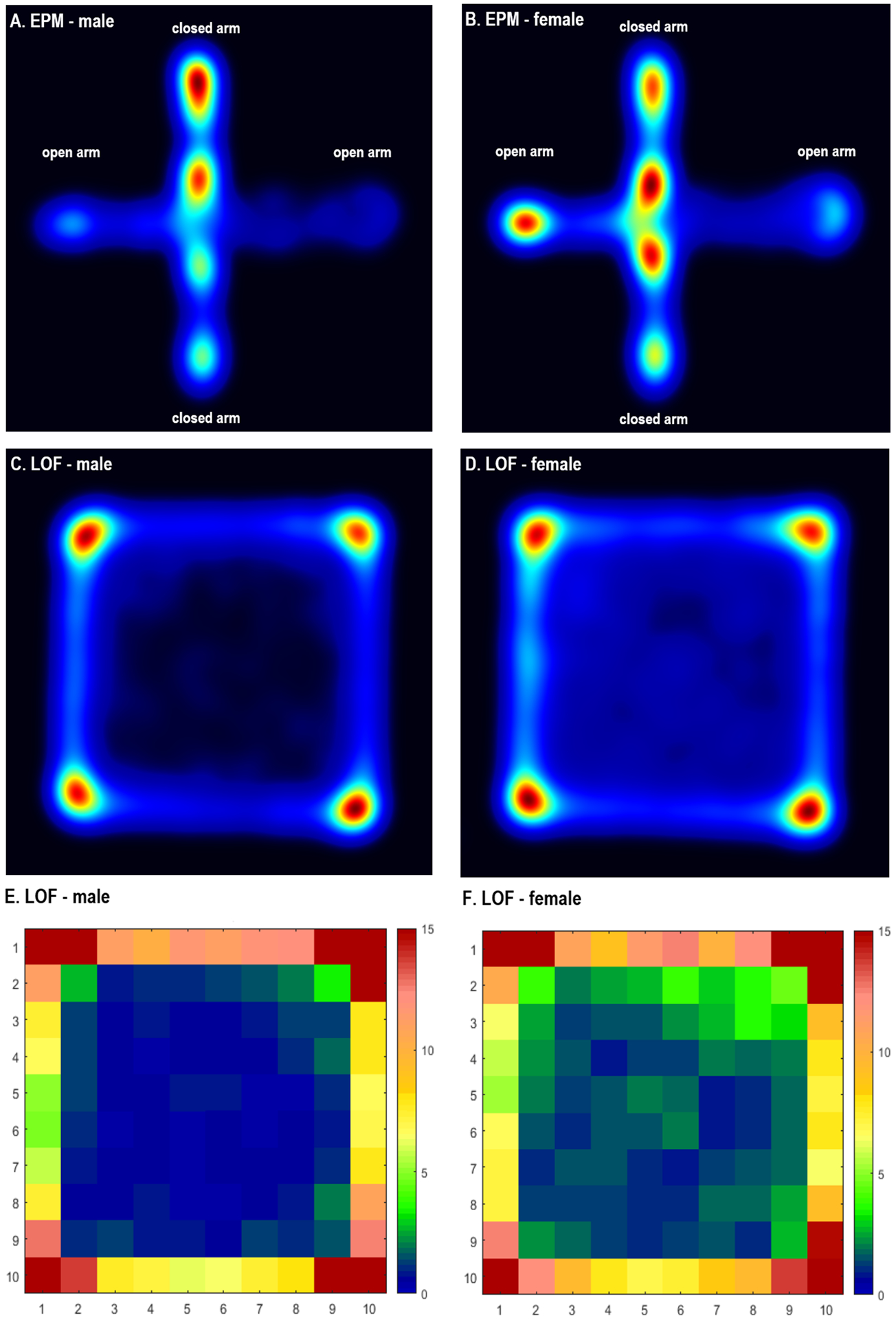
The figures show that the males (A) spent less time in the open arms of the EPM and more time in the closed arms of the EPM compared to the females (B). The males (C, E) spent less time in the middle/center zone than the females (D, F) in the LOF. Figs. 3A–D were generated with EthoVision software, and 3E and F were generated with COLORcation software. All track data were obtained with the EthoVision system. Warmer colors indicate more time in spent in a specific area. A and B, N=12/group; C–F, N=47/group.
3.2. Open Field Test
There were sex differences for all behavioral parameters in the LOF test (Figure 2–4). The females made more entries into the outside zone (F1,92 = 53.25, p<0.0001), middle zone (F1,92 = 49.62, p<0.0001), and center zone (F1,92 = 26.95, p<0.0001), and spent more time in the middle (F1,92 = 61.71, p<0.0001) and center zone (F1,92 = 22.84, p<0.0001). The males spent more time in the outside zone (F1,92 = 54.78, p<0.0001). The females had a shorter latency to enter the middle zone (F1,92 = 13.47, p<0.0001) and center zone (F1,92 = 31.44, p<0.0001). The females traveled a greater distance than the males in the LOF (F1,92 = 62.18, p<0.0001). Cohen’s d effect sizes, means, and the standard deviations (SDs) are reported in Table S1.
Figure 2. Automated tracking in the large open field and the elevated plus-maze test.
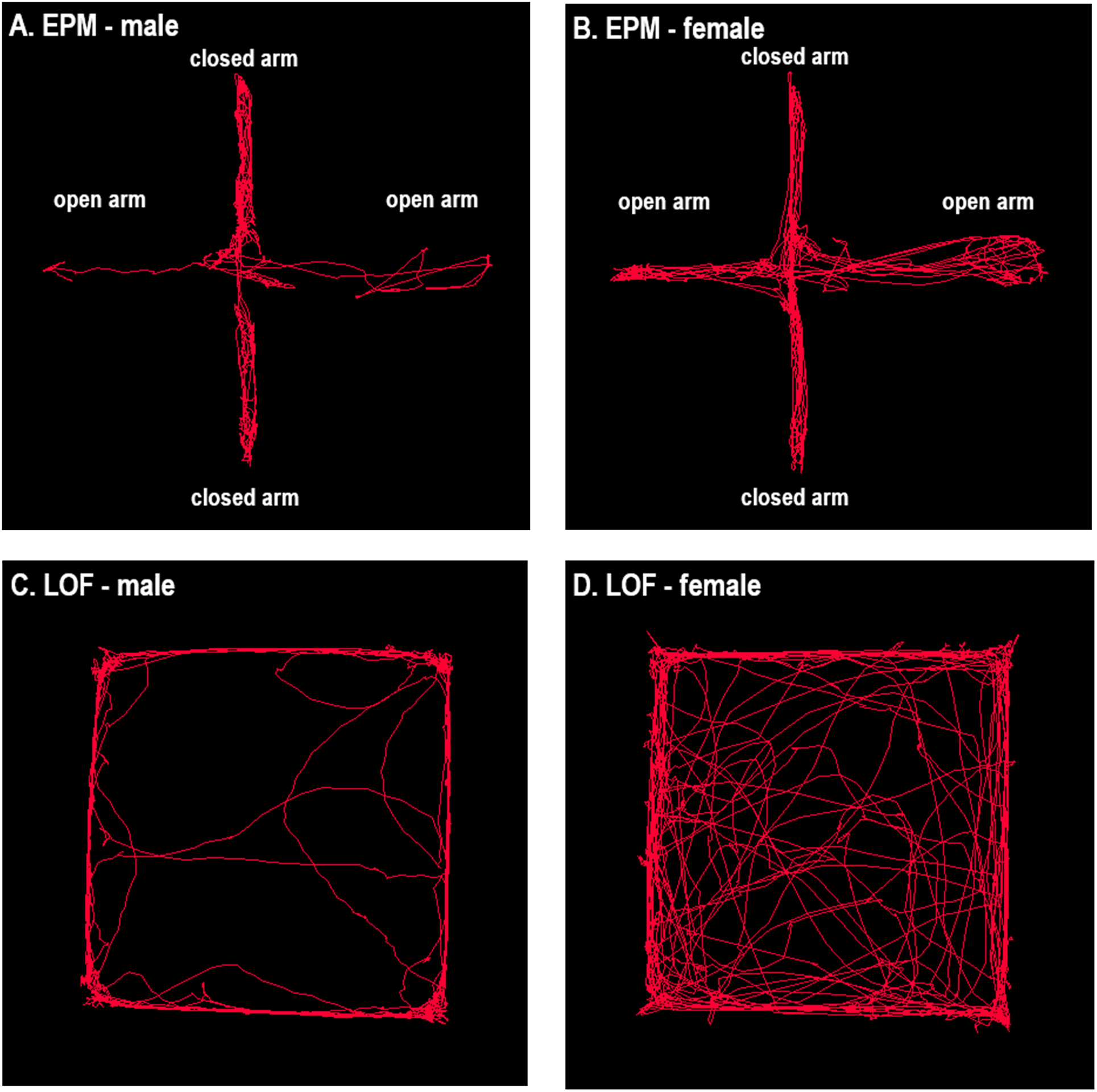
Representative figures of one male (A) and one female (B) rat in the EPM, and one male (C) and one female (D) rat in the LOF. The figures were generated with the EthoVision video tracking system. A–D, N=1.
Figure 4. Decreased anxiety-like behavior in female rats compared to male rats in the large open field test.
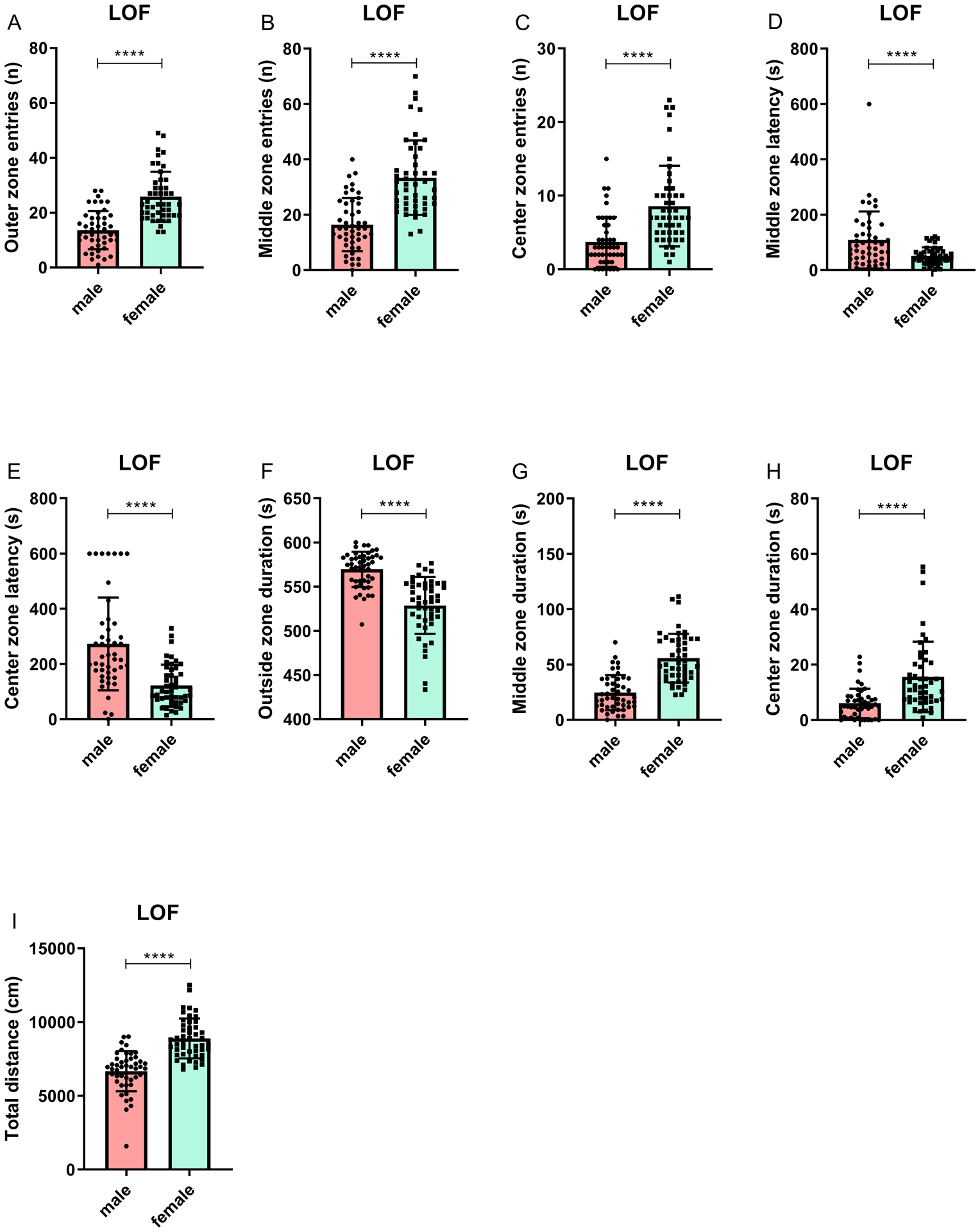
The figure shows entries into the outer (A), middle (B), and center (C) zone, latency to enter the middle (D) and center zone (E), duration in the outside (F), middle (G), and center zone (H), and total distance traveled. The females were more active and displayed less anxiety-like behavior. N=47/group. **** p<0.0001. Data are expressed as means ± SEM.
3.3. Correlation between behavioral parameters in the large open field and elevated plus-maze test
To provide insight into within and between test correlations, a correlation heatmap of the behavioral parameters is shown in Figure 5 (Pearson’s correlation coefficients and p-values are shown in Table 1 and S2). Separate heatmaps for the males and the females are shown in Figures S1A and B, and a heatmap with correlations (r) and Holm-adjusted p-values(−log 10) is shown in Figure S2. Selected scatterplots show the relationship between behavioral parameters (Figure 6). The heatmap shows a very strong correlation between behavioral parameters within the LOF and within the EPM. There was also a moderate cross-test correlation. A detailed hierarchical clustering dendrogram is shown in which similar behavioral parameters are placed in three clusters (Figure 7). Most EPM behavioral parameters are in cluster 1 (purple), most LOF behavioral parameters in cluster 2 (orange), and a third cluster contains a combination of EPM and LOF parameters (green). A tanglegram with a male and a female dendrogram is shown in Figure S3. The tanglegram shows that some clusters are the same for the males and the females. Baker’s Gamma correlation coefficient was calculated to determine how similar the male and female dendrograms were. The correlation coefficient was very high (value = 0.77), which indicates that the male and female dendrograms are similar.
Figure 5: Correlation heatmap of behavioral parameters in the large open field and elevated plus-maze test.
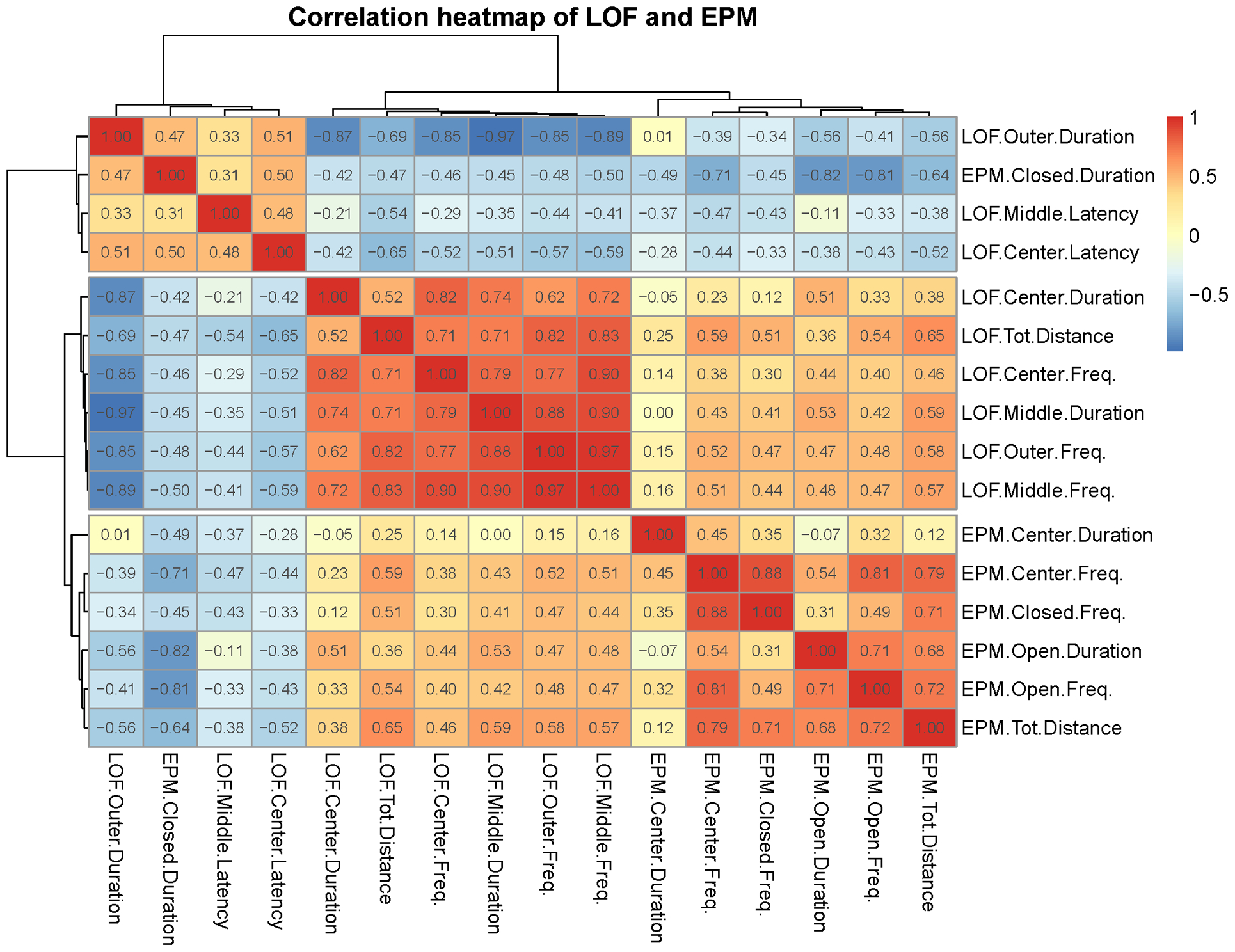
The heatmap shows the three clusters. One cluster contains LOF parameters, one cluster contains EPM parameters, and one cluster contains a mixture of LOF and EPM parameters. N=47/group. The degree of correlation is indicated by the intensity of the colors (red is indicative of a positive correlation and blue of a negative correlation). The Pearson correlation values are shown in the heatmap. N=47/group.
Table 1.
Parameters with highest correlation in the large open field and elevated plus maze test.
| Test | Correlation | P-value |
|---|---|---|
| Large open field (LOF) | ||
| LOF.Middle.Freq. - LOF.Outer.Freq. | 0.975 | 1.49E-61 |
| LOF.Center.Freq. - LOF.Middle.Freq. | 0.896 | 3.40E-34 |
| LOF.Middle.Duration - LOF.Middle.Freq. | 0.896 | 3.35E-34 |
| LOF.Middle.Duration - LOF.Outer.Freq. | 0.88 | 1.85E-31 |
| LOF.Tot.Distance - LOF.Middle.Freq. | 0.826 | 1.14E-24 |
| Elevated plus maze (EPM) | ||
| EPM.Closed.Freq. - EPM.Center.Freq. | 0.878 | 3.88E-31 |
| EPM.Center.Freq. - EPM.Open.Freq. | 0.812 | 2.89E-23 |
| EPM.Tot.Distance - EPM.Center.Freq. | 0.79 | 2.82E-21 |
| EPM.Tot.Distance - EPM.Open.Freq. | 0.725 | 1.45E-16 |
| EPM.Open.Duration - EPM.Open.Freq. | 0.713 | 7.47E-16 |
| LOF and EPM | ||
| LOF.Tot.Distance - EPM.Tot.Distance | 0.654 | 8.70E-13 |
| LOF.Middle.Duration - EPM.Tot.Distance | 0.595 | 2.58E-10 |
| LOF.Tot.Distance - EPM.Center.Freq. | 0.594 | 2.88E-10 |
| LOF.Outer.Freq. - EPM.Tot.Distance | 0.584 | 6.51E-10 |
| LOF.Middle.Freq. - EPM.Tot.Distance | 0.574 | 1.47E-09 |
Abbreviations: LOF, large open field; EPM, elevated plus maze; Freq., frequency; Tot, total.
Figure 6. Relationship between behavioral parameters in the large open field test and the elevated plus-maze test.
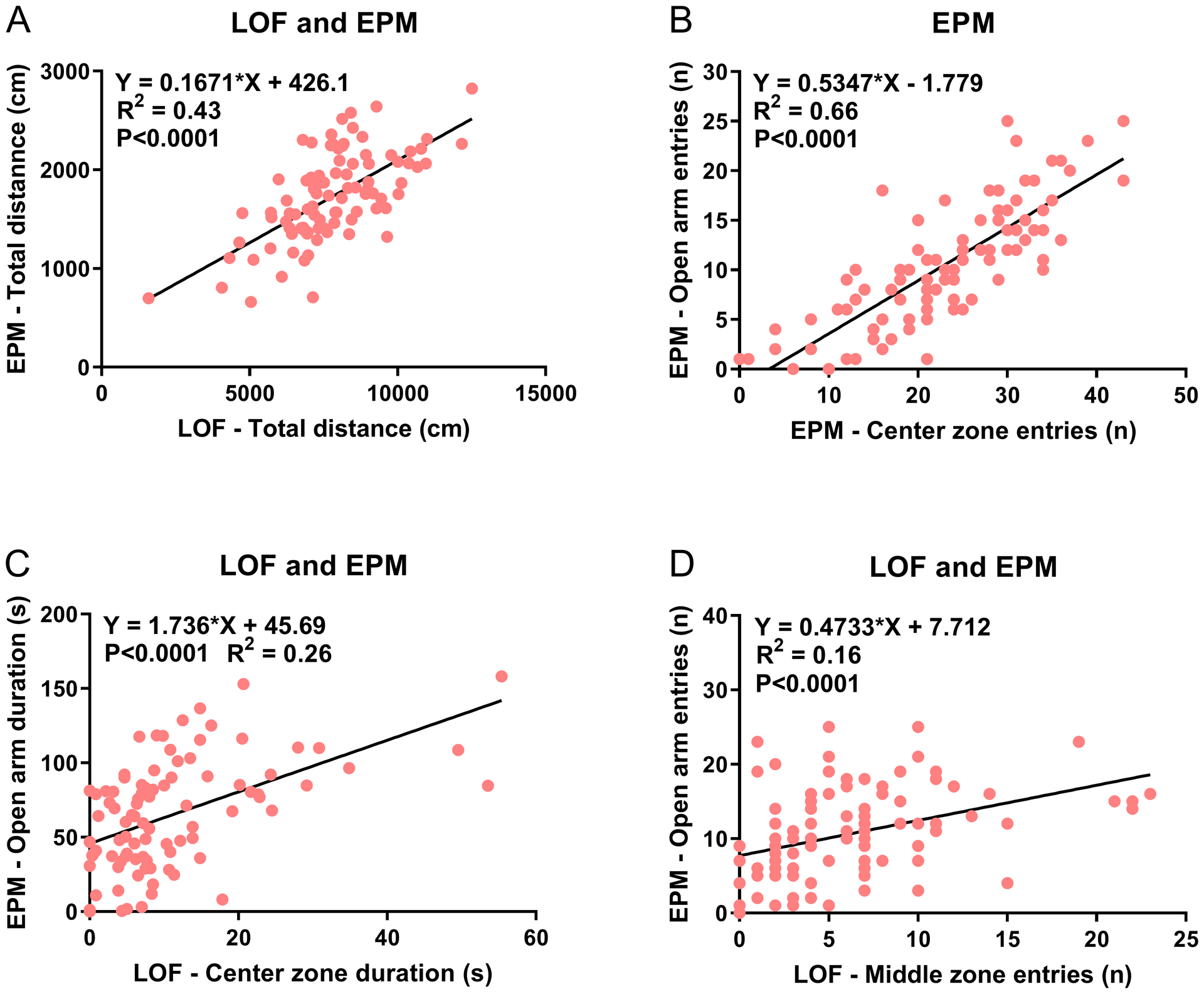
The linear regression analysis shows the relationship between various behavioral parameters in the LOF and EPM. Relationship between total distance in the LOF and EPM (A), entries into the center zone of the EPM and the open arms of the EPM (B), time in the center zone of the LOF and open arms of the EPM (C), and entries into the middle zone of the EPM and open arms of the EPM (D). N=47/group.
Figure 7. Detailed hierarchical clustering dendrogram of behavioral parameters in the large open field test and the elevated plus-maze test.
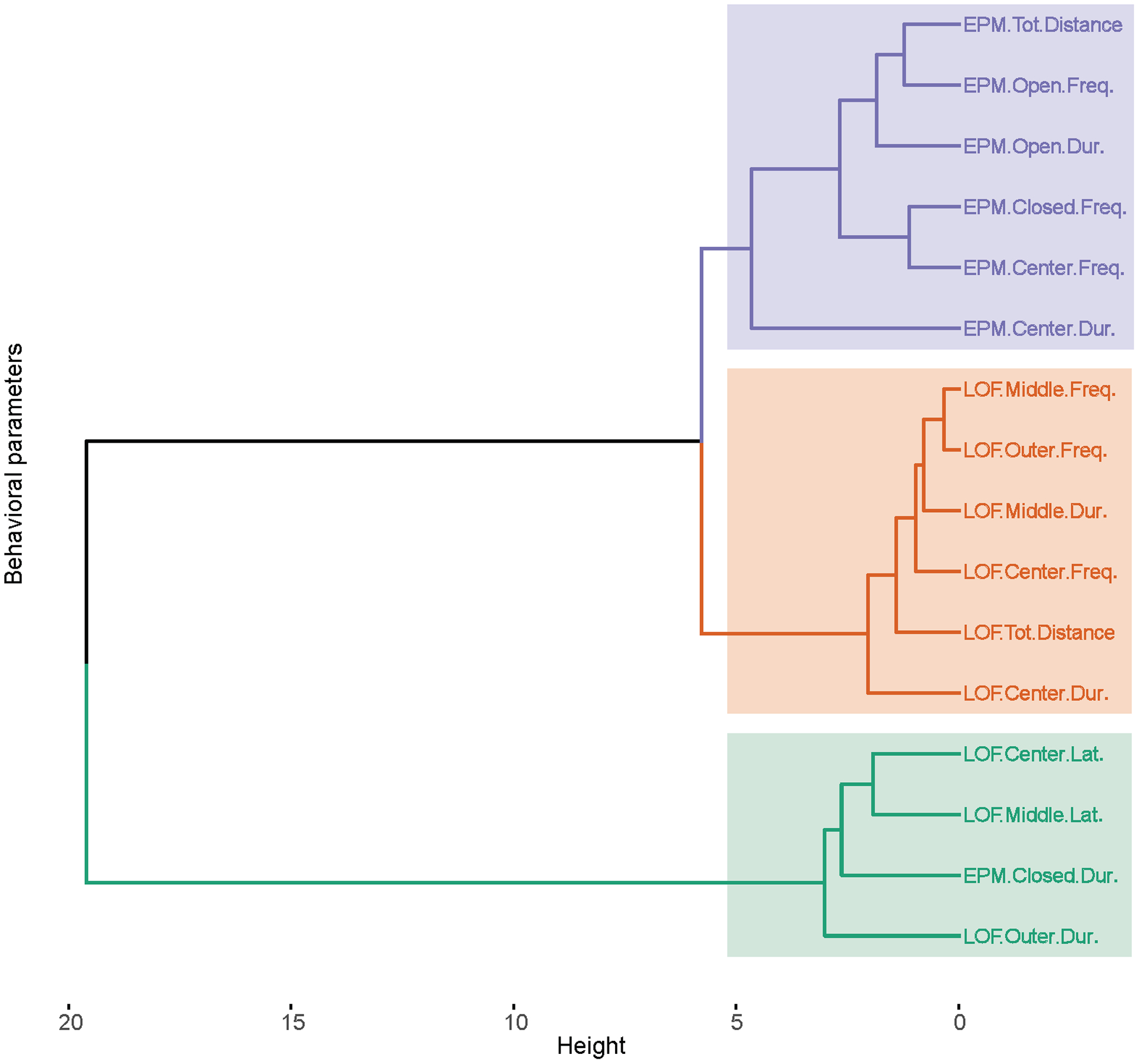
The length of the vertical lines reflects the similarity between the groups (short lines are indicative of greater similarity). N=47/group.
4. Discussion
The main goal of this study was to determine if there are sex differences in behavioral parameters in the EPM and the LOF. In both tests, the females displayed less anxiety-like behavior than males. Sex differences were observed for all behavioral parameters in the LOF and EPM, except for center duration in the EPM. The present study also showed that there was a strong correlation between behavioral parameters in the LOF test and between behavioral parameters in the EPM test. Furthermore, there was a modest cross-test correlation. The hierarchical cluster analysis indicated that there is a greater similarity in behavioral parameters within tests than between tests. Overall, these findings indicate that females are less anxious than males in the LOF and EPM test, and there are significant sex differences for almost all behavioral parameters in these anxiety tests.
In the present study, we conducted a thorough evaluation of sex differences in adult Wistar rats in the EPM test. We found that female rats were less anxious than male rats. The females spent more time on the open arms, spent a greater percentage of time on the open arms, made more open arms entries, and had a higher percentage of open arm entries in the EPM. The present finding is in line with studies that reported less anxiety-like behavior in female rats in the EPM (Domonkos et al., 2017; Imhof et al., 1993; Johnston, Amanda L and File, Sandra E, 1991; Ramos et al., 2002; Scholl et al., 2019). However, it should be noted that many studies did not find sex differences in behavioral parameters in the EPM in drug-naïve adult rats (Albrechet-Souza et al., 2020; Dichter et al., 1996; Elliott et al., 2004; Pohl et al., 2007; Renard et al., 2005; Soares-Cunha et al., 2018; Yang et al., 2019). Based on the current studies, it cannot be concluded that the strain of the rat plays a role in the expression of sex differences in the EPM. We conducted our studies with Wistar rats and found that female rats are less anxious in the EPM than male rats. This is in line with studies that reported that female Wistar rats, Sprague Dawley rats, Lister Hooded rats, and Lewis rats, display less anxiety-like behavior than males in the EPM (Domonkos et al., 2017; Imhof et al., 1993; Johnston, A. L. and File, S. E., 1991; Ramos et al., 2002; Scholl et al., 2019). In contrast, other studies with Wistar rats, Sprague Dawley rats, Long Evans rats, N:NIH rats, and Lister Hooded rats found no sex differences in the EPM (Dichter et al., 1996; Doremus-Fitzwater et al., 2009; Genn et al., 2003; Pavlova et al., 2020; Pohl et al., 2007; Sakhaie et al., 2020; Steenbergen et al., 1991; Winther et al., 2018). This suggests that there is no clear relationship between the strain of the rats and the expression of sex differences in the EPM. It is interesting to note that in humans, anxiety disorders are more common in females than males, but in the present studies, the female rats displayed less anxiety-like behavior. One possible explanation for this discrepancy is that our rats were not exposed to stressors prior to testing. Exposure to stressors plays an important role in the development of anxiety disorders, and studies with rodents show that males and females are differently affected by stressors (Dalla et al., 2010; Gué et al., 2004; Nugent et al., 2011).
Conflicting findings have been reported regarding sex differences in LOF tests. Many studies have shown that females are more active in the LOF than males, and they often, but not always, produce fewer fecal boli (Blizard et al., 1975; Gray and Lalljee, 1974; Harrington, 1972). This is often interpreted as less anxiety-like behavior or emotionality in females compared to males. More recent studies have compared time in the center and center entries between males and females in the LOF test. One study reported that female Wistar rats made more entries into the center zone of a LOF than males (Pavlova et al., 2020). However, in another study with Wistar rats, there was no sex difference in LOF center zone entries and duration (Sakhaie et al., 2020). Studies with Sprague-Dawley, Wistar-Kyoto rats, and Lewis rats reported that females spent more time in the center zone of the LOF than males (Burke et al., 2016; Domonkos et al., 2017). Several studies with Sprague Dawley rats showed that there are no sex differences in time spent in the center zone of the LOF (Scholl et al., 2019; Yang et al., 2019). It has also been reported that there is no sex difference in the amount of time spent in the outer zone (avoidance of center zone) of the LOF in Sprague Dawley Rats (Winther et al., 2018). Furthermore, there is no sex difference in center zone crossings in Sprague Dawley rats (Mansouri et al., 2019). It is interesting to note that we found robust and highly significant sex differences for all behavioral parameters in the LOF, while most studies found only differences in locomotor activity. There are several possible explanations for this discrepancy. The expression of sex differences in the LOF might be dependent on the strain of the rats and lighting condition. A study with Lewis rats showed that females spent more time in the LOF center zone than males under dim light conditions, but no sex difference was observed under bright light conditions (Ramos et al., 2002). We conducted the LOF under dim light conditions and found sex differences in anxiety-like behaviors. Most studies that reported no sex differences in time spent in the center zone of the LOF were conducted with Sprague Dawley rats, while our study was conducted with Wistar rats. Therefore, it is possible that sex differences in the LOF test are expressed somewhat more clearly in Wistar rats than in Sprague-Dawley rats. Another major difference between our study and the other studies is that we had more animals per group. In our study, we had 47 animals per group, while most other studies had 5–10 animals per group. The large group size leads to very high statistical power. An advantage of high statistical power is that the risk for type 2 errors (a false null hypothesis is not rejected) is very low. There is a large variability between animals in the LOF and EPM. For example, based on our data, we estimated that 17 animals per group are needed to compare sex differences in time spent in the center of the LOF (Difference in means: 9.6 s, common SD: 9.7 s) with 80% percent power. In contrast, only 7 animals are needed to compare sex differences in total locomotor activity in the LOF (Difference in means: 2188.7 cm, common SD: 1362.5 cm). It should be noted that these estimates are based on our very large group sizes. Large group sizes lead to small standard errors, and therefore we might underestimate the number of animals per group that is needed to detect sex differences (Whitley and Ball, 2002). Overall, it might be possible that some prior studies failed to conclude that there are sex differences in LOF parameters because of the relatively small group sizes. On a similar note, in the EPM, there is considerable variability between the animals. Because most EPM tests have been conducted with small groups of animals, they may also have been underpowered to detect sex differences.
In the present studies, we found that the females are less anxious in the LOF and EPM test, but the direction of the sex differences might depend on the anxiety test. Female rats also display less anxiety-like behavior in the light-dark transition test (Ramos et al., 2002). However, female rats display more anxiety-like behavior in the social interaction test and the Vogel conflict test (Genn et al., 2003; Johnston, Amanda L and File, Sandra E, 1991; Stack et al., 2010). Furthermore, female rats display more anxiety-like behavior in the fear-potentiated startle (FPS) paradigm (de Jongh et al., 2005), and it has been reported that light enhances the startle response in female, but not in male rats (Walker et al., 2003). In contrast, it has been reported that there are no sex differences in anxiety-like behavior in rats in the marble burying test (Burke et al., 2016; Zanni et al., 2020). These findings indicate that there are robust sex differences in behavioral parameters in most anxiety tests, but the direction of the effect depends on the anxiety test. An overview of sex differences in anxiety-like behavior in the EPM, LOF, marble burying test, and light-dark transition test is provided in Table S3.
The time on the open arms of the EPM and time in the center of the LOF are widely used parameters to determine the effects of environmental manipulations and drugs on anxiety-like behavior. In the present study, we found that there is a moderate correlation between the time on the open arms of the EPM and time in the center of the LOF (r = 0.512). Furthermore, there was a moderate correlation between the number of open arm entries in the EPM and the number of entries into the middle zone of the LOF (r = 0.473). There was a moderate negative correlation between time spent on the open arms of the EPM and time in the outer zone of the LOF (r = −0.557). These findings suggest that the EPM and the LOF measure similar components of anxiety-like behavior in rats. There was a high level of correlation between activity measures on the LOF and EPM. The highest correlation was between the total distance traveled in the LOF and EPM (r = 0.654). There was also a moderate correlation between other activity parameters such as total distance traveled in the EPM and entries into the outer (r = 0.584) and middle zone (r = 0.574) in the LOF. Thus, indicating a moderate to a high correlation between anxiety and activity parameters in the LOF and EPM.
In the present study, we also determined within-test correlations. The findings show a very strong correlation between entries into the outer zone and middle zone (r = 0.975) and between entries into the center zone and middle zone (r = 0.896) of the LOF. There was also a strong correlation between the total distance traveled and entries into the middle zone (r = 0.826) of the LOF. In the EPM, entries in the center zone was strongly correlated with entries in the open arms (r = 0.812) and closed arms (r = 0.878). Furthermore, there was a strong correlation between the total distance traveled and the number of entries in the open arms (r = 0.725). There was a moderate correlation between the total distance traveled on the EPM and the open arm duration (r = 0.675). Interestingly, in the LOF, there was also a correlation between the total distance traveled and center zone duration (r = 0.521) and center zone entries (r = 0.594). These findings suggest that animals with a high level of activity in the EPM and LOF also spent more time in the exposed areas (open arms of EPM and center zone of LOF) and make more entries into the exposed areas. In our study, we found a high correlation within tests and a moderate correlation between tests. This is in line with a previous study that investigated the correlation within and between five behavioral tests in adolescent heterogeneous stock rats (NMcwi: HS)(Wang et al., 2018). Furthermore, as in our study, they found that total distance traveled had the highest correlation between tests (Wang et al., 2018).
It is interesting to note that in the present studies, we found that the female rats displayed less anxiety-like behavior and were more active. In the EPM, the females made more open arm entries than the males, but they also made more closed arm entries. However, overall the females had a higher percentage of open arm entries and a higher percentage time on the open arms. On a similar note, in the LOF, the females made more entries into the center zone and had a shorter latency to enter the center zone than the male rats. Overall, these findings led us to conclude that females are less anxious than males. However, because the females travel a greater distance in the EPM and the LOF, it cannot be ruled out that the females’ higher level of activity contributes somewhat to the sex difference in anxiety-like behavior in adult Wistar rats.
The hierarchical clustering analysis was conducted to determine which behaviors are most similar to each other. The behavioral parameters were divided into three clusters. The first cluster solely consisted of EPM behavioral parameters, the second cluster only contained LOF behavioral parameters, and the third cluster contained a combination of LOF and EPM behavioral parameters. This indicates that EPM parameters are somewhat similar (cluster 1) and that LOF parameters are somewhat similar (cluster 2). The third cluster contained behavioral parameters that were related to the avoidance of open spaces (latencies to enter the middle and center zone of the LOF, and time in the outer zone of the LOF and closed arms of the EPM). A close look at the dendrogram indicates that entries into the middle zone and outer zone of the LOF were very similar and that these two behaviors were closely related to time in the middle zone of the LOF and entries into the center zone of the LOF. Entries into the open arms of the EPM and total distance traveled on the EPM entries were also very similar. Interestingly, time in the closed arms of the EPM was most similar to the time in the outside zone of the LOF. This underscores that these two parameters measure a similar type of behavior. Overall, these findings indicate that behaviors from the same test are mostly organized in the same cluster. Furthermore, behaviors that are related to the exploration of open spaces are similar, and behaviors that are related to the avoidance of open spaces are similar.
The tanglegram showed that there is a strong overlap in hierarchical clustering in the males and the females. For both the males and females, the total distance traveled on the EPM was similar to closed arm entries and center entries in the EPM. The only parameter that was very different between the males and the females was center duration in the EPM. In the males, this parameter was similar to parameters that were related to activity in the EPM (open and closed arm entries, center entries, and total distance traveled). In the females, this parameter was similar to the time in the outer zone of the LOF and latencies to enter the middle and center zone in the LOF, and duration in the closed arms in the EPM. This indicates that in the males, EPM center duration was closely related to EPM exploration parameters, and in the females, it was closely related to the avoidance of open spaces. Therefore, this analysis indicates that one specific behavioral parameter might be similar to different behavioral parameters in the males (exploration of the EPM) and females (avoidance of open spaces in EPM and LOF).
Overall, the present findings indicate that the female rats are less anxious than males in the EPM and LOF. There is also a strong correlation between behavioral parameters within the anxiety tests. Furthermore, there is some correlation between behaviors in the LOF and EPM. In general, rats that tend to avoid the open arms of the EPM also avoid the center of the LOF. Our findings suggest that the EPM and LOF investigate similar aspects of anxiety-like behavior. This work on sex differences in anxiety tests has important implications for drug development. The Wistar strain is widely used to test novel anxiolytics, and so far, mostly males have been used to evaluate the effects of drugs. The work presented here underscores that it is critical to test new psychotropic drugs in male and female Wistar rats.
Supplementary Material
Funding
AB was supported by a NIDA/NIH and FDA Center for Tobacco Products (CTP) grant (DA042530) and NIDA grant (DA046411) when working on this project.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Albrechet-Souza L, Schratz CL, Gilpin NW, 2020. Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol Sex Differ 11(1), 27–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Yamasue H, Hayano F, Nakamura M, Uehara K, Otsuka T, Roppongi T, Nihashi N, Inoue T, Hirayasu Y, 2009. Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Research: Neuroimaging 173(2), 128–134. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews 35(3), 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Lippman HR, Chen JJ, 1975. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiology & behavior 14(5), 601–608. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Knight P, Panunzio S, Xue S, Bruner MM, Wall SC, Pompilus M, Febo M, Setlow B, 2019. Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology 236, 2773–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Qi X, Guzhva LV, Wall S, Deng JV, Gold MS, Febo M, Setlow B, 2016. Behavioral Characterization of the Effects of Cannabis Smoke and Anandamide in Rats. PloS one 11(4), e0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke NN, Coppinger J, Deaver DR, Roche M, Finn DP, Kelly J, 2016. Sex differences and similarities in depressive-and anxiety-like behaviour in the Wistar-Kyoto rat. Physiology & behavior 167, 28–34. [DOI] [PubMed] [Google Scholar]
- Cahill L, 2006. Why sex matters for neuroscience. Nature reviews neuroscience 7(6), 477. [DOI] [PubMed] [Google Scholar]
- Dagan SY, Tsoory MM, Fainzilber M, Panayotis N, 2016. COLORcation: a new application to phenotype exploratory behavior models of anxiety in mice. Journal of neuroscience methods 270, 9–16. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z, 2010. Sex differences in response to stress and expression of depressive-like behaviours in the rat, Biological basis of sex differences in psychopharmacology. Springer, pp. 97–118. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Geyer MA, Olivier B, Groenink L, 2005. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behavioural brain research 161(2), 190–196. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Brunelli SA, Hofer MA, 1996. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiology & Behavior 60(1), 299–304. [DOI] [PubMed] [Google Scholar]
- Domonkos E, Borbélyová V, Csongová M, Bosý M, Kačmárová M, Ostatníková D, Hodosy J, Celec P, 2017. Sex differences and sex hormones in anxiety-like behavior of aging rats. Horm Behav 93, 159–165. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP, 2009. Effects of pretest manipulation on elevated plus-maze behavior in adolescent and adult male and female Sprague-Dawley rats. Pharmacology, biochemistry, and behavior 92(3), 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE, 2004. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol.Biochem.Behav 77(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Fraley C, Raftery AE, 1998. How many clusters? Which clustering method? Answers via model-based cluster analysis. The computer journal 41(8), 578–588. [Google Scholar]
- Galili T, 2015. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31(22), 3718–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genn RF, Tucci SA, Thomas A, Edwards JE, File SE, 2003. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neuroscience & Biobehavioral Reviews 27(1–2), 155–161. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H, 1987. Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behavioural brain research 25(2), 101–107. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Faravelli C, Rosi S, Cosci F, Truglia E, de Graaf R, Wittchen H-U, 2005. The epidemiology of panic disorder and agoraphobia in Europe. European Neuropsychopharmacology 15(4), 435–443. [DOI] [PubMed] [Google Scholar]
- Gray JA, Lalljee B, 1974. Sex differences in emotional behaviour in the rat: correlation between open-field defecation and active avoidance. Animal Behaviour 22, 856–861. [DOI] [PubMed] [Google Scholar]
- Gué M, Bravard A, Meunier J, Veyrier R, Gaillet S, Recasens M, Maurice T, 2004. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behavioural brain research 150(1–2), 149–157. [DOI] [PubMed] [Google Scholar]
- Harrington GM, 1972. Strain differences in open-field behavior of the rat. Psychonomic Science 27(1), 51–53. [Google Scholar]
- Hogg S, 1996. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacology, biochemistry, and behavior 54(1), 21–30. [DOI] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP, 1993. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behavioural brain research 56(2), 177–180. [DOI] [PubMed] [Google Scholar]
- Johnson M, 2012. Laboratory mice and rats. Mater Methods 2(10.13070). [Google Scholar]
- Johnston AL, File SE, 1991. Sex differences in animal tests of anxiety. Physiology & behavior 49(2), 245–250. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE, 1991. Sex differences in animal tests of anxiety. Physiol Behav 49(2), 245–250. [DOI] [PubMed] [Google Scholar]
- Kassambara A, Mundt F, 2017. Package ‘factoextra’. Extract and visualize the results of multivariate data analyses 76. [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS, 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Archives of general psychiatry 51(1), 8–19. [DOI] [PubMed] [Google Scholar]
- Kolde R, 2012. Pheatmap: pretty heatmaps. R package version 1(2). [Google Scholar]
- Korte SM, De Boer SF, 2003. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. European journal of pharmacology 463(1–3), 163–175. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN, 2010. Sex differences in the adolescent brain. Brain and cognition 72(1), 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Montkowski A, Holsboer F, Landgraf R, 1998. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behavioural brain research 94(2), 301–310. [DOI] [PubMed] [Google Scholar]
- Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A, 2020. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proceedings of the National Academy of Sciences 117(31), 18788–18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Milad MR, 2015. Sex differences in anxiety disorders: interactions between fear, stress, and gonadal hormones. Hormones and behavior 76, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri MT, Fidler JA, Meng QC, Eckenhoff RG, Garcia PS, 2019. Sex effects on behavioral markers of emergence from propofol and isoflurane anesthesia in rats. Behavioural brain research 367, 59–67. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG, 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of psychiatric research 45(8), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Overkamp G, Benning M, Koolhaas J, Van den Hoofdakker R, 1996. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiology & behavior 60(1), 115–119. [DOI] [PubMed] [Google Scholar]
- Mirzasoleiman B, Badanidiyuru A, Karbasi A, Vondrák J, Krause A, 2015. Lazier than lazy greedy, Twenty-Ninth AAAI Conference on Artificial Intelligence. [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH, 2011. Gene–environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology 214(1), 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova IV, Broshevitskaya ND, Onufriev MV, Moiseeva YV, 2020. Sex-Related Differences in Anxious-Depressive and Defensive Behavior in Wistar Rats. Neuroscience and Behavioral Physiology 50(9), 1163–1175. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J.Neurosci.Methods 14(3), 149–167. [DOI] [PubMed] [Google Scholar]
- Pijlman FT, Wolterink G, Van Ree JM, 2003. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behavioural brain research 139(1–2), 131–138. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL, 2007. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci 121(3), 462–474. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology 463(1–3), 3–33. [DOI] [PubMed] [Google Scholar]
- Qi X, Guzhva L, Yang Z, Febo M, Shan Z, Wang KK, Bruijnzeel AW, 2016. Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Eur Neuropsychopharmacol 26, 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Kangerski AL, Basso PF, Da Silva Santos JE, Assreuy J, Vendruscolo LF, Takahashi RN, 2002. Evaluation of Lewis and SHR rat strains as a genetic model for the study of anxiety and pain. Behavioural brain research 129(1–2), 113–123. [DOI] [PubMed] [Google Scholar]
- Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izídio GS, 2008. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behavioural brain research 193(2), 277–288. [DOI] [PubMed] [Google Scholar]
- Renard GM, Suárez MM, Levin GM, Rivarola MA, 2005. Sex differences in rats: effects of chronic stress on sympathetic system and anxiety. Physiol Behav 85(3), 363–369. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Shah HP, Small E, Bruijnzeel AW, 2009. Deficit in brain reward function and acute and protracted anxiety-like behavior after discontinuation of a chronic alcohol liquid diet in rats. Psychopharmacology (Berl) 203(3), 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhaie N, Sadegzadeh F, Mohammadnia A, Dadkhah M, Saadati H, 2020. Sex‐dependent effects of post‐weaning exposure to an enriched environment on novel objective recognition memory and anxiety‐like behaviors: the role of hippocampal BDNF level. International Journal of Developmental Neuroscience. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Afzal A, Fox LC, Watt MJ, Forster GL, 2019. Sex differences in anxiety-like behaviors in rats. Physiology & behavior 211, 112670. [DOI] [PubMed] [Google Scholar]
- Shansky RM, 2019. Are hormones a “female problem” for animal research? Science 364(6443), 825–826. [DOI] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Borges S, Domingues AV, Silva D, Sousa N, Rodrigues AJ, 2018. Mild Prenatal Stress Causes Emotional and Brain Structural Modifications in Rats of Both Sexes. Frontiers in behavioral neuroscience 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M, 2010. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology 35(2), 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen HL, Farabollini F, Heinsbroek RP, 1991. Sex-dependent effects of aversive stimulation on holeboard and elevated plus-maze behavior. Behavioural brain research 43(2), 159–165. [DOI] [PubMed] [Google Scholar]
- Tan S, Xue S, Behnood-Rod A, Chellian R, Wilson R, Knight P, Panunzio S, Lyons H, Febo M, Bruijnzeel AW, 2019. Sex differences in the reward deficit and somatic signs associated with precipitated nicotine withdrawal in rats. Neuropharmacology 160, 107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano A, Osborne BF, Schwarz JM, 2018. Sexual differentiation and sex differences in neural development. [DOI] [PubMed]
- Walker DL, Toufexis DJ, Davis M, 2003. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur.J.Pharmacol 463, 199–216. [DOI] [PubMed] [Google Scholar]
- Wang T, Han W, Chitre AS, Polesskaya O, Woods LCS, Palmer AA, Chen H, 2018. Social and anxiety-like behaviors contribute to nicotine self-administration in adolescent outbred rats. Scientific reports 8(1), 18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH Jr, 1963. Hierarchical grouping to optimize an objective function. Journal of the American statistical association 58(301), 236–244. [Google Scholar]
- Wei T, Simko V, 2017. R package “corrplot”: Visualization of a Correlation Matrix (Version 0.84). Retrieved from, https://github.com/taiyun/corrplot.
- Whitley E, Ball J, 2002. Statistics review 2: Samples and populations. Critical Care 6(2), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2016. ggplot2: Elegant graphics for data analysis, 2 ed. Springer International Publishing [Google Scholar]
- Wickham H, Francois R, Henry L, Müller K, 2015. dplyr: A grammar of data manipulation.
- Winther G, Elfving B, Müller HK, Lund S, Wegener G, 2018. Maternal High-fat Diet Programs Offspring Emotional Behavior in Adulthood. Neuroscience 388, 87–101. [DOI] [PubMed] [Google Scholar]
- Yang R, Sun H, Wu Y, Lu G, Wang Y, Li Q, Zhou J, Sun H, Sun L, 2019. Long-lasting sex-specific effects based on emotion-and cognition-related behavioral assessment of adult rats after post-traumatic stress disorder from different lengths of maternal separation. Frontiers in Psychiatry 10, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni G, DeSalle MJ, Deutsch HM, Barr GA, Eisch AJ, 2020. Female and male rats readily consume and prefer oxycodone to water in a chronic, continuous access, two-bottle oral voluntary paradigm. Neuropharmacology 167, 107978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


