Abstract
Non-heme iron complexes with cis-FeIII(OH)(SAr/OAr) coordination were isolated and examined for their reactivity with a tertiary carbon radical. The sulfur-ligated complex shows a temperature dependence on ·OH versus ArS transfer, whereas the oxygen-ligated complex does not. These results provide the first working model for C−S bond formation in isopenicillin N synthase and indicate that kinetic control may be a key factor in the selectivity of non-heme iron “rebound” processes.
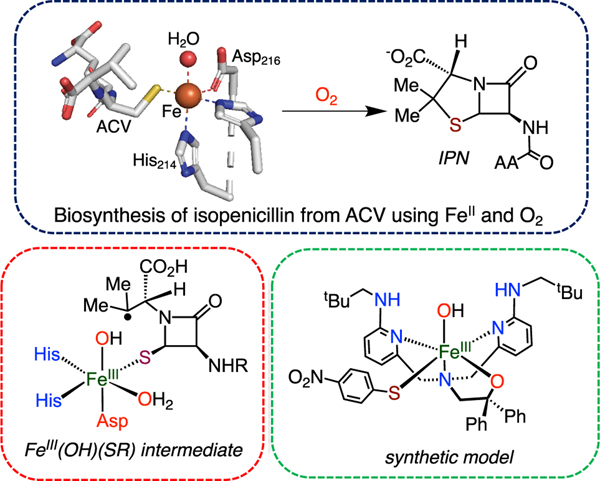
Isopenicillin N synthase (IPNS) belongs to a class of nonheme iron enzymes that utilize FeII/O2 in the absence of a cosubstrate to catalyze the oxidation of L-Δ-(α-aminoadipoyl)-L-cysteinyl-D-valine (ACV) tripeptide to isopenicillin (IPN) (Scheme 1).1−3 IPN gets further processed to form antibiotics such as penicillin and cephalosporins.4−6 The biosynthesis of IPN is divided into two major steps: (a) formation of the β-lactam ring via FeIII−OO•− and FeIII−OOH intermediates and (b) closure of the thiazolidine ring involving C−S bond formation from the reaction of a tertiary carbon radical (R·) with an FeIII(OH)(SR) intermediate.7−11 Substrate probes have shown that other products besides the native thiazolidine compound can be formed (e.g., S-oxygenates,10 thioacids,12 and ring-expanded13 or hydroxylated14 products). These studies suggest that the substrate structure and orientation are important in determining the outcome of the reaction between R· and FeIII(OH)(SR). Computational studies showed that sulfur transfer is kinetically favored over hydroxylation, supporting the observed selectivity of this step with the native substrate.11,15 However, experimental studies that directly examine the C−S bond formation step are absent.
Scheme 1.
IPNS Reactivity and Model
The inherent factors that may contribute to the selectivity of sulfuryl versus hydroxyl transfer in the absence of a protein pocket have not been examined previously. Similar selectivity arises for halogen versus hydroxyl transfer in the non-heme iron halogenases, and the properties that control the selectivity for halogenation are still under debate.16−26 A few synthetic non-heme iron catalysts have shown some promise toward selective halogenation and related processes,27−34 but the principles for designing a selective halogenation catalyst are not well-understood. Similarly, there are no reports describing the analogous FeIII(OH)(SR) species, and no studies to date have shown selective sulfuryl over hydroxyl transfer mediated by a non-heme iron complex.
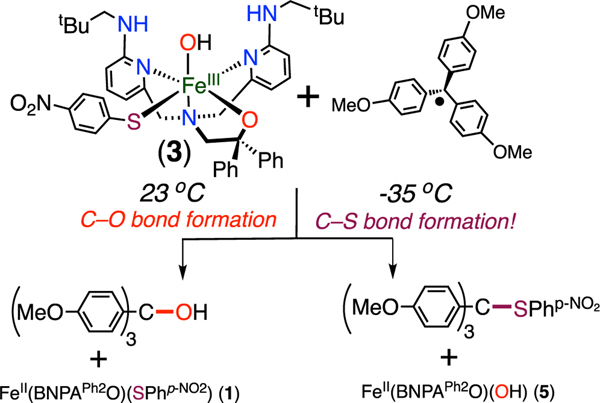
We previously developed a ligand with H-bonding groups (BNPAPh2O−) that allowed us to isolate FeIII(OH)(X) (X = OTf, Cl, Br) complexes and examine their reactivity toward carbon radicals.35,36 Herein we report the first structurally characterized FeIII(OH)(SAr) complex, prepared from BNPAPh2O−, and describe its reactivity toward a tertiary carbon radical. The phenolate analogue, FeIII(OH)(OAr), was also prepared and examined for comparative reactivity. A temperature-dependent switch in hydroxyl versus sulfur transfer is seen for the arylthiolate analogue.
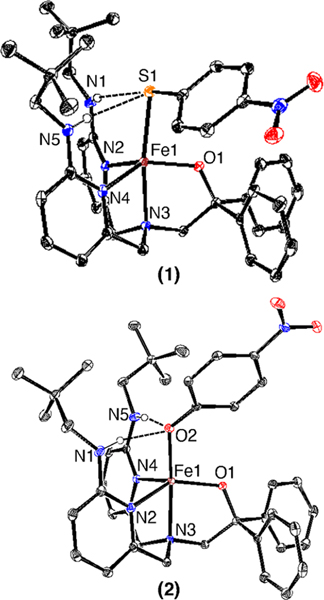
Complexes FeII(BNPAPh2O)(SPhp-NO2) (1) and FeII(BNPAPh2O)(OPhp-NO2) (2) were synthesized by the addition of the appropriate sodium thiophenolate or phenolate salt to FeII(BNPAPh2O)(OTf).36 The crystal structures (Figure 1) revealed five-coordinate iron(II) complexes with the thiophenolate/phenolate ligand bound in place of OTf−. Bond distances are typical for high-spin (hs) iron(II).37−39
Figure 1.
Displacement ellipsoid plots (50% probability level) for 1 and 2 at 110(2) K. Hydrogen atoms (except for N−H) have been omitted for clarity.
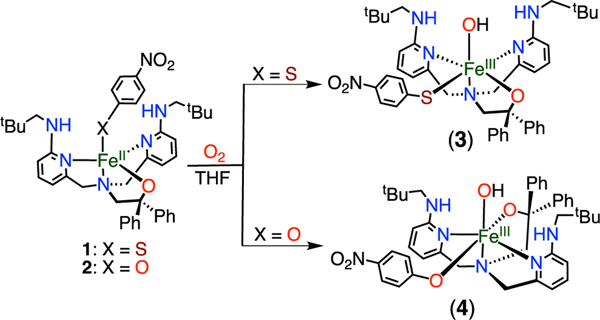
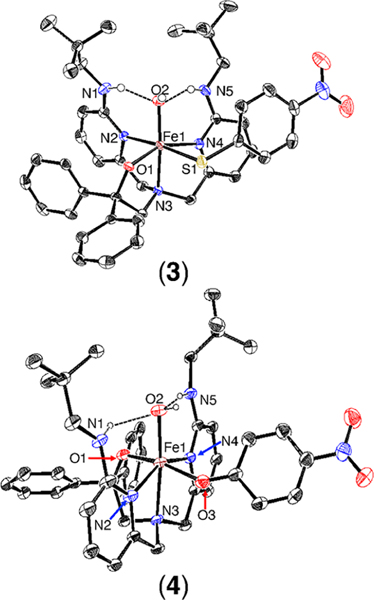
The reactions of 1 and 2 with dry excess O2 in THF at 23 °C (Scheme 2) led to the ferric complexes FeIII(BNPAPh2O)- (OH)(SPhp-NO2) (3) and FeIII(BNPAPh2O)(OH)(OPhp-NO2) (4), respectively, which were characterized by single-crystal X-ray diffraction (XRD). The crystal structures (Figure 2) reveal six-coordinate complexes with the thiophenolate or phenolate ligand bound in an equatorial position and a terminal hydroxide ligand occupying the axial H-bonded site, as observed previously.35,36 The FeIII−OH distances of 1.9034(18) and 1.908(2) Å in 3 and 4, respectively, are similar to those in other terminal FeIII(OH) complexes.38,40−48 In contrast, the FeIII−S bond length of 2.4483(8) Å in 3 is longer than the few other non-heme high-spin FeIII−SAr distances previously reported (2.35(1)−2.41(2) Å).49−52 However, the analogous FeIII−OAr distance of 2.000(3) Å in 4 is within the typical range (1.93−2.00 Å).53−55
Scheme 2.
Synthesis of 3 and 4
Figure 2.
Displacement ellipsoid plots (50% probability level) for 3 and 4 at 110(2) K. Hydrogen atoms (except for N−H and O−H) have been omitted for clarity.
Comparison of 3 and 4 shows that the phenolate group is trans to the alkoxide in 4, whereas the thiophenolate group is trans to a pyridine donor in 3. In addition, there is no π−π stacking between the phenolate ring and any of the pyridine rings in 4, while there is π−π stacking56 between the thiophenolate group and the pyridine ring containing N(4) in 3. This interaction is characterized by a centroid-to-centroid distance of 3.6 Å and an angle of 9.3° between the least-squares planes of the two aromatic rings.57
A density functional theory (DFT) calculation gave Fe−S = 2.484 Å for 3, reproducing the elongated Fe−S bond length observed in the crystal structure. A comparison of the structure of 3 with an optimized geometry (QM/MM) for the proposed cis-FeIII(OH)(SR) intermediate in IPNS15 reveals a resemblance between the FeIII−S and FeIII−OH bond lengths of 2.37 and 1.87 Å, respectively, for IPNS, and those of 3. Thus, complex 3 is, to our knowledge, the first synthetic model of the proposed cis-FeIII(OH)(SR) intermediate in IPNS.
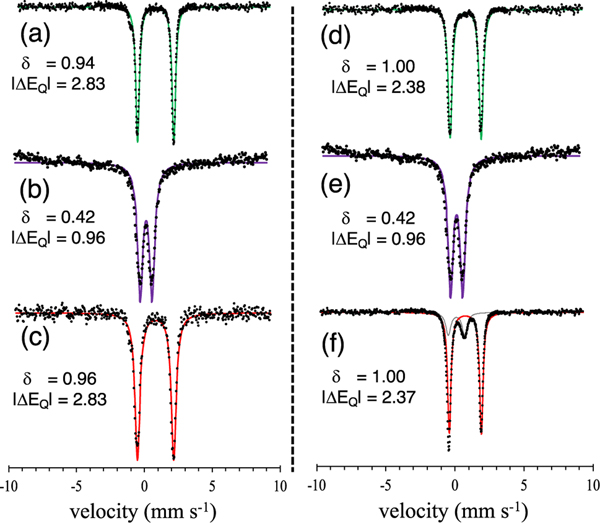
The 1H NMR spectra of 1 and 2 show relatively sharp paramagnetically shifted peaks from 90 to −10 ppm indicative of hs (S = 2) iron(II). In comparison, the spectra of 3 and 4 exhibit broad resonances from 80 to 10 ppm, as expected for a hs (S = 5/2) FeIII species. Zero-field Mössbauer spectroscopy of 57Fe-enriched 1 and 2 shows sharp quadrupole doublets with parameters Δ = 0.94 mm s−1 and |ΔEQ| = 2.87 mm s−1 for 1 and Δ = 1.03 mm s−1 and |ΔEQ| = 2.76 mm s−1 for 2. Mössbauer analysis of 57Fe-enriched 3 and 4 revealed broad quadrupole doublets with parameters Δ = 0.42 mm s−1 and |ΔEQ| = 0.96 mm s−1 for 3 and Δ = 0.47 mm s−1 and |ΔEQ| = 1.01 mm s−1 for 4. Such broadening for similar ferric complexes is known and can be explained by the population of an intermediate relaxation regime.35,36,58,59
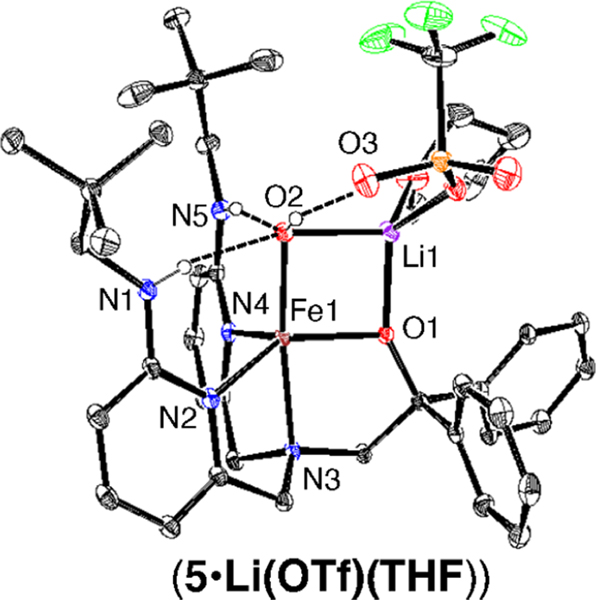
The complex FeII(BNPAPh2O)(OH) (5) was also prepared for comparison by adding OH− to FeII(BNPAPh2O)(OTf). Crystallization of 5 as orange blocks came from a reaction with LiOH and gave the structure shown in Figure 3. There is a lithium ion bound between OH− and O1 and coordinated by OTf− and THF, leading to the formula 5·Li(OTf)(THF). There is also an additional H-bond between OH− and OTf− that stabilizes the structure.
Figure 3.
Displacement ellipsoid plot (50% probability level) for 5· Li(OTf)(THF) at 110(2) K. Hydrogen atoms (except for N−H and O−H) are omitted for clarity.
1H NMR spectroscopy of 5 prepared in situ from nBu4NOH or from crystals of 5·Li(OTf)(THF) gave nearly identical spectra (Figures S9 and S10). Mössbauer spectroscopy on 57Fe-5 synthesized from either nBu4NOH or LiOH in 2-MeTHF showed identical isomer shifts but different quadrupole splittings (Table S4). These trends for 5 versus the lithium adduct are reproduced by DFT calculations and support the coordination of Li+ in solution.
The reaction of 3 with the substituted triphenylmethyl radical (p-OMe-C6H4)3C· in toluene/THF at 23 °C was then examined. Triarylmethyl radicals are relatively stable and have been used recently by us and others to examine their reactivity with M−X (X = O, N, halide) bonds.43,60−66,70 Analysis by 1H NMR spectroscopy showed the complete conversion of the ferric complex 3 into the ferrous thiolate complex 1, consistent with selective hydroxyl transfer over sulfur transfer from the iron complex to the carbon radical (Scheme 3). The alcohol product (p-OMe-C6H4)3COH was also identified in the 1H NMR spectrum, and no evidence for formation of the thioether (p-OMe-C6H4)3CSAr was detected. Mössbauer spectroscopy provided additional corroborating data for the selectivity of ·OH transfer. The reaction of isotopically enriched 57Fe-3 revealed a sharp quadrupole doublet with Δ = 0.96 mm s−1 and |ΔEQ| = 2.83 mm s−1, corresponding to the FeII(thiolate) complex 1 (Figure 4).
Scheme 3.
Reactions of 3 with Tertiary Carbon Radical
Figure 4.
Zero-field 57Fe Mössbauer spectra (80 K) of (a) 1, (b) 3, and (c) 3 + (p-OMe-C6H4)3C· at 23 °C and (d) 5, (e) 3, and (f) 3 + (p-OMe-C6H4)3C· at −35 °C. Isomer shift (Δ) and quadrupole splitting (|ΔEQ|) values are given in mm s−1.
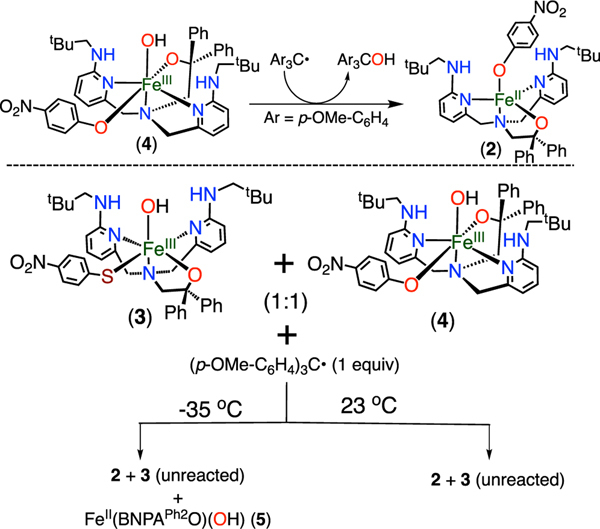
However, lowering the reaction temperature causes a dramatic shift in the product distribution. Addition of 1 equiv of (p-OMe-C6H4)3C· to 3 at −35 °C for 1 h in THF/ toluene leads to the formation of FeII(OH) complex 5 instead of FeII(SPhp-NO2) complex 1, as observed by 1H NMR spectroscopy. Corresponding analysis by Mössbauer spectroscopy (Figure 4) reveals a sharp quadrupole doublet with Δ = 1.00 mm s−1 and |ΔEQ| = 2.38 mm s−1, which is a close match to the spectrum of 5. Taken together, the data show that sulfur transfer preferentially occurs over hydroxyl transfer at −35 °C (Scheme 3).
To examine the generality of these reactions, the p-CF3-substituted complexes FeII(BNPAPh2O)(SArp-CF3) (6) and FeIII(BNPAPh2O)(OH)(SArp-CF3) (7) were prepared (see the Supporting Information). Reaction of 7 with (p-OMe-C6H4)3C· at −35 °C leads to formation of the FeII(OH) product 5, the same selectivity as seen for 3. The formation of (p-OMe-C6H4)3CSPhp-CF3 (80%) was also confirmed by 1H NMR and 19F NMR spectroscopy.
To examine the influence of temperature in more detail, the reaction of 3 and (p-OMe-C6H4)3C· was carried out between 23 and −35 °C at intervals of 10 °C. The 1H NMR spectrum for the reaction at 23 °C (Figure S45) shows only the presence of the FeII(SAr) complex, the product expected from selective · OH transfer. A second product begins to appear at −5 °C, as evidenced by new peaks at 84.8, 63.8, and 56.1 ppm. These peaks correspond to the FeII(OH) complex produced from ArS· transfer to the carbon radical. As the reaction temperature is further lowered, the ArS· transfer pathway becomes more favorable and is the dominant pathway by −25 °C. These data are consistent with a switch in mechanism that is dependent on the reaction temperature.
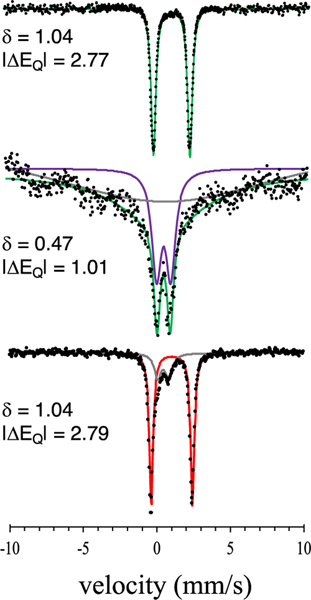
The phenoxide analogue FeIII(OH)(OPhp-NO2) (4) was reacted with the same tertiary carbon radical (p-OMe-C6H4)3C· to examine the reactivity of an O donor versus an S donor. In contrast to the sulfur analogue, only ·OH transfer was seen by Mössbauer spectroscopy (Figure 5) and NMR spectroscopy at both 23 and −35 °C. The relative reaction rates of 3 and 4 with (p-OMe-C6H4)3C· were assessed through competition experiments in which a 1:1 mixture of 3 and 4 was reacted with (p-OMe-C6H4)3C· (1 equiv) at either 23 or −35 °C. The reaction at 23 °C led to the formation of only the FeII(OAr) product 2 along with unreacted 3, showing that · OH transfer is significantly faster from 4 than from 3.
Figure 5.
Zero-field 57Fe Mössbauer spectra (80 K) of (top) 2, (middle) 4, and (bottom) 4 + (p-OMe-C6H4)3C· at 23 °C. Isomer shift (Δ) and quadrupole splitting (|ΔEQ|) values are given in mm s−1.
However, reaction at −35 °C led to a small amount of (p-OMe-C6H4)3C−SPhp-NO2 (by TLC) and 5 (by 1H NMR), together with the major product 2. These results show that ArS· transfer from 3 to the carbon radical has become competitive with ·OH transfer from 4 but still remains slower overall. We conclude that the relative rates of rebound follow the trend kOH(4) > kOH(3) at 23 °C and kOH(4) > kSAr(3) at −35 °C. The results from the reactions with (p-OMe-C6H4)3C· are summarized in Scheme 4.
Scheme 4.
Reaction of 4 with Tertiary Carbon Radical
In summary, a new series of iron(II) and iron(III) complexes are described. The iron(III) complexes provide a platform to examine the competition between cis-ligated OH versus SAr/OAr groups in reactions with carbon radicals. Reaction of FeIII(OH)(SAr) with (p-OMe-C6H4)3C· at 23 °C gives (p-OMe-C6H4)3COH. However, the same reaction at −35 °C leads to the thioether (p-OMe-C6H4)3CSAr. In contrast, FeIII(OH)(OAr) produces only (p-OMe-C6H4)3COH independent of temperature.
Transfer of ·OH from either 3 or 4 is likely thermodynamically favored because of the relative strength of the C−OH bond being formed, compared with the alternative C−OAr or C−SAr bonds.67 Consistent with the expected thermodynamic trend in bond strengths, complexes 3 and 4 produce only (p-OMe-C6H4)3COH at 23 °C. However, the same reaction for 3 at −35 °C preferentially produces (p-OMe-C6H4)3CSAr. The equatorial Fe−S bond in 3 is significantly elongated and therefore weakened, resulting in a lower kinetic barrier for sulfur transfer.
The kinetic versus thermodynamic pathways are illustrated in the qualitative reaction coordinate diagram in Figure S46. This analysis implies that formation of the thioether is reversible at 23 °C, which is supported by the reductive cleavage of thioether bonds.68,69 The FeIII−OAr bond in 4, on the other hand, does not show any significant elongation, which is consistent with the lack of phenoxyl transfer.
Complex 3 is, to our knowledge, the first synthetic model of the ferric hydroxothiolate intermediate in IPNS. The overall reactivity of 3 is similar to that revealed by calculations on IPNS, which indicate that sulfur transfer is kinetically favored whereas hydroxylation is thermodynamically controlled.15 These results show that the inherent electronic and structural features of a non-heme Fe center can significantly influence the outcome of the rebound step without contributions from an enzyme pocket or substrate orientation effects.
Supplementary Material
ACKNOWLEDGMENTS
The NIH (GM119374 to D.P.G.) is gratefully acknowledged for financial support. We thank Dr. Pierre LeMagueres (Rigaku Oxford Diffraction) for his help in obtaining the crystal structure of 4. Computer time was provided by the Maryland Advanced Research Computing Center (MARCC).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c09688.
X-ray crystallographic data for 1 (CIF)
X-ray crystallographic data for 2 (CIF)
X-ray crystallographic data for 3 (CIF)
X-ray crystallographic data for 4 (CIF)
X-ray crystallographic data for 6 (CIF)
X-ray crystallographic data for 5·Li(OTf)(THF) (CIF)
Syntheses, 1H and 19F NMR spectra, Mössbauer data, UV−vis spectra, and computational details (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.0c09688
The authors declare no competing financial interest.
Contributor Information
Vishal Yadav, Department of Chemistry, The Johns Hopkins University, Baltimore, Maryland 21218, United States.
Maxime A. Siegler, Department of Chemistry, The Johns Hopkins University, Baltimore, Maryland 21218, United States.
David P. Goldberg, Department of Chemistry, The Johns Hopkins University, Baltimore, Maryland 21218, United States.
REFERENCES
- (1).Roach PL; Clifton IJ; Hensgens CMH; Shibata N; Schofield CJ; Hajdu J; Baldwin JE Structure of isopenicillinN synthase complexed with substrate and the mechanism of penicillin formation. Nature 1997, 387, 827. [DOI] [PubMed] [Google Scholar]
- (2).Cohen G; Shiffman D; Mevarech M; Aharonowitz Y. Microbial isopenicillin N synthase genes: structure, function, diversity and evolution. Trends Biotechnol. 1990, 8, 105. [DOI] [PubMed] [Google Scholar]
- (3).Gordon JB; Goldberg DP Sulfur-Ligated, Oxidative Nonheme Iron Enzymes and Related Complexes. In Comprehensive Coordination Chemistry III; Liu Y, Que L, Eds.; Elsevier: London, 2020. [Google Scholar]
- (4).Baldwin JE; Bradley M. Isopenicillin N synthase: mechanistic studies. Chem. Rev. 1990, 90, 1079. [Google Scholar]
- (5).Blackburn JM; Sutherland JD; Baldwin JE A heuristic approach to the analysis of enzymic catalysis: Reaction of.delta.-(L-.alpha.-aminoadipoyl)-L-cysteinyl-D-.alpha.-aminobutyrate and.-delta.-(L-.alpha.-aminoadipoyl)-L-cysteinyl-D-allylglycine catalyzed by isopenicillin N synthase isoenzymes. Biochemistry 1995, 34, 7548. [DOI] [PubMed] [Google Scholar]
- (6).Baldwin JE; Abraham E. The biosynthesis of penicillins and cephalosporins. Nat. Prod. Rep. 1988, 5, 129. [DOI] [PubMed] [Google Scholar]
- (7).Tamanaha E; Zhang B; Guo Y; Chang WC; Barr EW; Xing G; St. Clair J; Ye S; Neese F; Bollinger JM Jr.; Krebs C. Spectroscopic Evidence for the Two C-H-Cleaving Intermediates of Aspergillus nidulans Isopenicillin N Synthase. J. Am. Chem. Soc. 2016, 138, 8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Solomon EI; Brunold TC; Davis MI; Kemsley JN; Lee S-K; Lehnert N; Neese F; Skulan AJ; Yang Y-S; Zhou J. Geometric and Electronic Structure/Function Correlations in Non-Heme Iron Enzymes. Chem. Rev. 2000, 100, 235. [DOI] [PubMed] [Google Scholar]
- (9).Roach PL; Clifton IJ; Fülöp V; Harlos K; Barton GJ; Hajdu J; Andersson I; Schofield CJ; Baldwin JE Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature 1995, 375, 700. [DOI] [PubMed] [Google Scholar]
- (10).Burzlaff NI; Rutledge PJ; Clifton IJ; Hensgens CM; Pickford M; Adlington RM; Roach PL; Baldwin JE The reaction cycle of isopenicillin N synthase observed by X-ray diffraction. Nature 1999, 401, 721. [DOI] [PubMed] [Google Scholar]
- (11).Lundberg M; Kawatsu T; Vreven T; Frisch MJ; Morokuma K. Transition States in a Protein Environment - ONIOM QM:MM Modeling of Isopenicillin N Synthesis. J. Chem. Theory Comput. 2009, 5, 222. [DOI] [PubMed] [Google Scholar]
- (12).Ogle JM; Clifton IJ; Rutledge PJ; Elkins JM; Burzlaff NI; Adlington RM; Roach PL; Baldwin JE Alternative oxidation by isopenicillin N synthase observed by X-ray diffraction. Chem. Biol. 2001, 8, 1231. [DOI] [PubMed] [Google Scholar]
- (13).Howard-Jones AR; Elkins JM; Clifton IJ; Roach PL; Adlington RM; Baldwin JE; Rutledge PJ Interactions of Isopenicillin N Synthase with Cyclopropyl-Containing Substrate Analogues Reveal New Mechanistic Insight. Biochemistry 2007, 46, 4755. [DOI] [PubMed] [Google Scholar]
- (14).Daruzzaman A; Clifton IJ; Adlington RM; Baldwin JE; Rutledge PJ Unexpected Oxidation of a Depsipeptide Substrate Analogue in Crystalline Isopenicillin N Synthase. ChemBioChem 2006, 7, 351. [DOI] [PubMed] [Google Scholar]
- (15).Lundberg M; Siegbahn PEM; Morokuma K. The Mechanism for Isopenicillin N Synthase from Density-Functional Modeling Highlights the Similarities with Other Enzymes in the 2-His-1-carboxylate Family. Biochemistry 2008, 47, 1031. [DOI] [PubMed] [Google Scholar]
- (16).Srnec M; Solomon EI Frontier Molecular Orbital Contributions to Chlorination versus Hydroxylation Selectivity in the Non-Heme Iron Halogenase SyrB2. J. Am. Chem. Soc. 2017, 139, 2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Huang J; Li C; Wang B; Sharon DA; Wu W; Shaik S. Selective Chlorination of Substrates by the Halogenase SyrB2 Is Controlled by the Protein According to a Combined Quantum Mechanics/Molecular Mechanics and Molecular Dynamics Study. ACS Catal. 2016, 6, 2694. [Google Scholar]
- (18).Quesne MG; de Visser SP Regioselectivity of substrate hydroxylation versus halogenation by a nonheme iron(IV)-oxo complex: possibility of rearrangement pathways. JBIC, J. Biol. Inorg. Chem. 2012, 17, 841. [DOI] [PubMed] [Google Scholar]
- (19).Timmins A; Fowler NJ; Warwicker J; Straganz GD; de Visser SP Does Substrate Positioning Affect the Selectivity and Reactivity in the Hectochlorin Biosynthesis Halogenase? Front. Chem. 2018, 6, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Timmins A; Quesne MG; Borowski T; de Visser SP Group Transfer to an Aliphatic Bond: A Biomimetic Study Inspired by Nonheme Iron Halogenases. ACS Catal. 2018, 8, 8685. [Google Scholar]
- (21).Timmins A; de Visser SP A Comparative Review on the Catalytic Mechanism of Nonheme Iron Hydroxylases and Halogenases. Catalysts 2018, 8, 314. [Google Scholar]
- (22).Mitchell AJ; Zhu Q; Maggiolo AO; Ananth NR; Hillwig ML; Liu X; Boal AK Structural basis for halogenation by iron- and 2-oxo-glutarate-dependent enzyme WelO5. Nat. Chem. Biol. 2016, 12, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Matthews ML; Neumann CS; Miles LA; Grove TL; Booker SJ; Krebs C; Walsh CT; Bollinger JM Substrate positioning controls the partition between halogenation and hydroxylation in the aliphatic halogenase, SyrB2. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Martinie RJ; Livada J; Chang W.-c.; Green MT; Krebs C; Bollinger JM; Silakov A. Experimental Correlation of Substrate Position with Reaction Outcome in the Aliphatic Halogenase, SyrB2. J. Am. Chem. Soc. 2015, 137, 6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Borowski T; Noack H; Radon M; Zych K; Siegbahn PEM Mechanism of Selective Halogenation by SyrB2: A Computational Study. J. Am. Chem. Soc. 2010, 132, 12887. [DOI] [PubMed] [Google Scholar]
- (26).Pandian S; Vincent MA; Hillier IH; Burton NA Why does the enzyme SyrB2 chlorinate, but does not hydroxylate, saturated hydrocarbons? A density functional theory (DFT) study. Dalton Trans. 2009, 6201. [DOI] [PubMed] [Google Scholar]
- (27).Puri M; Biswas AN; Fan R; Guo Y; Que L Jr. Modeling Non-Heme Iron Halogenases: High-Spin Oxoiron(IV)-Halide Complexes That Halogenate C-H Bonds. J. Am. Chem. Soc. 2016, 138, 2484. [DOI] [PubMed] [Google Scholar]
- (28).Rana S; Biswas JP; Sen A; Clemancey M; Blondin G; Latour J-M; Rajaraman G; Maiti D. Selective C-H halogenation over hydroxylation by non-heme iron(iv)-oxo. Chem. Sci. 2018, 9 (40), 7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Chatterjee S; Paine TK Hydroxylation versus Halogenation of Aliphatic C-H Bonds by a Dioxygen-Derived Iron-Oxygen Oxidant: Functional Mimicking of Iron Halogenases. Angew. Chem., Int. Ed. 2016, 55, 7717. [DOI] [PubMed] [Google Scholar]
- (30).Comba P; Wunderlich S. Iron-Catalyzed Halogenation of Alkanes: Modeling of Nonheme Halogenases by Experiment and DFT Calculations. Chem. - Eur. J. 2010, 16, 7293. [DOI] [PubMed] [Google Scholar]
- (31).Coin G; Patra R; Rana S; Biswas JP; Dubourdeaux P; Clemancey M; de Visser SP; Maiti D; Maldivi P; Latour J-M Fe-Catalyzed Aziridination Is Governed by the Electron Affinity of the Active Imido-Iron Species. ACS Catal. 2020, 10, 10010. [Google Scholar]
- (32).Sasmal S; Rana S; Lahiri GK; Maiti D. Manganese-salen catalyzed oxidative benzylic chlorination. J. Chem. Sci. 2018, 130, 88. [Google Scholar]
- (33).Rana S; Dey A; Maiti D. Mechanistic elucidation of C-H oxidation by electron rich non-heme iron(iv)-oxo at room temperature. Chem. Commun. 2015, 51, 14469. [DOI] [PubMed] [Google Scholar]
- (34).Rana S; Bag S; Patra T; Maiti D. Catalytic Electrophilic Halogenations and Haloalkoxylations by Non-Heme Iron Halides. Adv. Synth. Catal. 2014, 356, 2453. [Google Scholar]
- (35).Yadav V; Rodriguez RJ; Siegler MA; Goldberg DP Determining the Inherent Selectivity for Carbon Radical Hydroxylation versus Halogenation with FeIII(OH)(X) Complexes: Relevance to the Rebound Step in Non-heme Iron Halogenases. J. Am. Chem. Soc. 2020, 142, 7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Yadav V; Gordon JB; Siegler MA; Goldberg DP Dioxygen-Derived Nonheme Mononuclear FeIII(OH) Complex and Its Reactivity with Carbon Radicals. J. Am. Chem. Soc. 2019, 141, 10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Gordon Z; Drummond MJ; Matson EM; Bogart JA; Schelter EJ; Lord RL; Fout AR Tuning the Fe(II/III) Redox Potential in Nonheme Fe(II)-Hydroxo Complexes through Primary and Secondary Coordination Sphere Modifications. Inorg. Chem. 2017, 56, 4852. [DOI] [PubMed] [Google Scholar]
- (38).Cook SA; Ziller JW; Borovik AS Iron(II) Complexes Supported by Sulfonamido Tripodal Ligands: Endogenous versus Exogenous Substrate Oxidation. Inorg. Chem. 2014, 53, 11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).MacBeth CE; Gupta R; Mitchell-Koch KR; Young VG; Lushington GH; Thompson WH; Hendrich MP; Borovik AS Utilization of Hydrogen Bonds To Stabilize M-O(H) Units: Synthesis and Properties of Monomeric Iron and Manganese Complexes with Terminal Oxo and Hydroxo Ligands. J. Am. Chem. Soc. 2004, 126, 2556. [DOI] [PubMed] [Google Scholar]
- (40).Mukherjee J; Lucas RL; Zart MK; Powell DR; Day VW; Borovik AS Synthesis, Structure, and Physical Properties for a Series of Monomeric Iron(III) Hydroxo Complexes with Varying Hydrogen-Bond Networks. Inorg. Chem. 2008, 47, 5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).MacBeth CE; Hammes BS; Young VG; Borovik AS Hydrogen-Bonding Cavities about Metal Ions: Synthesis, Structure, and Physical Properties for a Series of Monomeric M-OH Complexes Derived from Water. Inorg. Chem. 2001, 40, 4733. [PubMed] [Google Scholar]
- (42).MacBeth CE; Golombek AP; Young VG; Yang C; Kuczera K; Hendrich MP; Borovik AS O2 Activation by Nonheme Iron Complexes: A Monomeric Fe(III)-Oxo Complex Derived From O2. Science 2000, 289, 938. [DOI] [PubMed] [Google Scholar]
- (43).Drummond MJ; Ford CL; Gray DL; Popescu CV; Fout AR Radical Rebound Hydroxylation Versus H-Atom Transfer in Non-Heme Iron(III)-Hydroxo Complexes: Reactivity and Structural Differentiation. J. Am. Chem. Soc. 2019, 141, 6639. [DOI] [PubMed] [Google Scholar]
- (44).Gordon Z; Miller TJ; Leahy CA; Matson EM; Burgess M; Drummond MJ; Popescu CV; Smith CM; Lord RL; Rodríguez-Lopez J; Fout AR Characterization of Terminal Iron(III)-Oxo and Iron(III)-Hydroxo Complexes Derived from O2 Activation. Inorg. Chem. 2019, 58, 15801. [DOI] [PubMed] [Google Scholar]
- (45).Ford CL; Park YJ; Matson EM; Gordon Z; Fout AR A bioinspired iron catalyst for nitrate and perchlorate reduction. Science 2016, 354, 741. [DOI] [PubMed] [Google Scholar]
- (46).Soo HS; Komor AC; Iavarone AT; Chang CJ A Hydrogen-Bond Facilitated Cycle for Oxygen Reduction by an Acidand Base-Compatible Iron Platform. Inorg. Chem. 2009, 48, 10024. [DOI] [PubMed] [Google Scholar]
- (47).Ogo S; Yamahara R; Roach M; Suenobu T; Aki M; Ogura T; Kitagawa T; Masuda H; Fukuzumi S; Watanabe Y. Structural and Spectroscopic Features of a cis (Hydroxo)-FeIII-(Carboxylato) Configuration as an Active Site Model for Lipoxygenases. Inorg. Chem. 2002, 41, 5513. [DOI] [PubMed] [Google Scholar]
- (48).Ogo S; Wada S; Watanabe Y; Iwase M; Wada A; Harata M; Jitsukawa K; Masuda H; Einaga H. Synthesis, Structure, and Spectroscopic Properties of [FeIII(tnpa)(OH)(PhCOO)]ClO4: A Model Complex for an Active Form of Soybean Lipoxygenase-1. Angew. Chem., Int. Ed. 1998, 37, 2102. [DOI] [PubMed] [Google Scholar]
- (49).Zhang S; Wang Q; Thierer LM; Weberg AB; Gau MR; Carroll PJ; Tomson NC Tuning Metal-Metal Interactions through Reversible Ligand Folding in a Series of Dinuclear Iron Complexes. Inorg. Chem. 2019, 58, 12234. [DOI] [PubMed] [Google Scholar]
- (50).Li M; Bonnet D; Bill E; Neese F; Weyhermüller T; Blum N; Sellmann D; Wieghardt K. Tuning the Electronic Structure of Octahedral Iron Complexes [FeL(X)] (L = 1-Alkyl-4,7-bis(4-tert-butyl-2-mercaptobenzyl)-1,4,7-triazacyclononane, X = Cl, CH3O, CN, NO). The S = 1/2 ⇌ S = 3/2 Spin Equilibrium of [FeLPr(NO)]. Inorg. Chem. 2002, 41, 3444. [DOI] [PubMed] [Google Scholar]
- (51).Noveron JC; Olmstead MM; Mascharak PK A Synthetic Analogue of the Active Site of Fe-Containing Nitrile Hydratase with Carboxamido N and Thiolato S as Donors: Synthesis, Structure, and Reactivities. J. Am. Chem. Soc. 2001, 123, 3247. [DOI] [PubMed] [Google Scholar]
- (52).Gordon JB; Vilbert AC; DiMucci IM; MacMillan SN; Lancaster KM; Moenne-Loccoz P; Goldberg DP̈. Activation of Dioxygen by a Mononuclear Nonheme Iron Complex: Sequential Peroxo, Oxo, and Hydroxo Intermediates. J. Am. Chem. Soc. 2019, 141, 17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Bhowmik S; Dey S; Sahoo D; Rath SP Unusual Stabilization of an Intermediate Spin State of Iron upon the Axial Phenoxide Coordination of a Diiron(III)-Bisporphyrin: Effect of Heme-Heme Interactions. Chem. - Eur. J 2013, 19, 13732. [DOI] [PubMed] [Google Scholar]
- (54).Velusamy M; Mayilmurugan R; Palaniandavar M. Iron(III) Complexes of Sterically Hindered Tetradentate Monophenolate Ligands as Functional Models for Catechol 1,2-Dioxygenases: The Role of Ligand Stereoelectronic Properties. Inorg. Chem. 2004, 43, 6284. [DOI] [PubMed] [Google Scholar]
- (55).Hartley CL; DiRisio RJ; Chang TY; Zhang W; McNamara WR Electrocatalytic hydrogen evolution by an iron complex containing a nitro-functionalized polypyridyl ligand. Polyhedron 2016, 114, 133. [Google Scholar]
- (56).Thakuria R; Nath NK; Saha BK The Nature and Applications of π-π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523. [Google Scholar]
- (57).Hunter CA; Sanders JKM The nature of HHH interactions. J. Am. Chem. Soc. 1990, 112, 5525. [Google Scholar]
- (58).Mørup S. Magnetic Relaxation Phenomena. In Mössbauer Spectroscopy and Transition Metal Chemistry: Fundamentals and Applications; Gütlich P, Bill E, Trautwein AX, Eds.; Springer: Berlin, 2011; pp 201–234. [Google Scholar]
- (59).Gupta R; Lacy DC; Bominaar EL; Borovik AS; Hendrich MP Electron Paramagnetic Resonance and Mössbauer Spectroscopy and Density Functional Theory Analysis of a High-Spin FeIV-Oxo Complex. J. Am. Chem. Soc. 2012, 134, 9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zaragoza JPT; Yosca TH; Siegler MA; Moenne-Loccoz P; Green MT; Goldberg DP Direct Observation of Oxygen Rebound with an Iron-Hydroxide Complex. J. Am. Chem. Soc. 2017, 139, 13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Pangia TM; Yadav V; Gerard EF; Lin Y-T; de Visser SP; Jameson GNL; Goldberg DP Mechanistic Investigation of Oxygen Rebound in a Mononuclear Nonheme Iron Complex. Inorg. Chem. 2019, 58, 9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Pangia TM; Davies CG; Prendergast JR; Gordon JB; Siegler MA; Jameson GNL; Goldberg DP Observation of Radical Rebound in a Mononuclear Nonheme Iron Model Complex. J. Am. Chem. Soc. 2018, 140, 4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jang ES; McMullin CL; Kaß M; Meyer K; Cundari TR¨; Warren TH Copper(II) Anilides in sp3 C-H Amination. J. Am. Chem. Soc. 2014, 136, 10930. [DOI] [PubMed] [Google Scholar]
- (64).Lu X; Li X-X; Seo MS; Lee Y-M; Clémancey M; Maldivi P; Latour J-M; Sarangi R; Fukuzumi S; Nam W. A Mononuclear Nonheme Iron(IV)-Amido Complex Relevant for the Compound II Chemistry of Cytochrome P450. J. Am. Chem. Soc. 2019, 141, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Iovan DA; Betley TA Characterization of Iron-Imido Species Relevant for N-Group Transfer Chemistry. J. Am. Chem. Soc. 2016, 138, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Bower JK; Cypcar AD; Henriquez B; Stieber SCE; Zhang S. C(sp3)-H Fluorination with a Copper(II)/(III) Redox Couple. J. Am. Chem. Soc. 2020, 142, 8514. [DOI] [PubMed] [Google Scholar]
- (67).Blanksby SJ; Ellison GB Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255. [DOI] [PubMed] [Google Scholar]
- (68).Xiao J; Deng L. Synthesis, Structure, and Reactivity Study of Iron(II) Complexes with Bulky Bis(anilido)thioether Ligation. Organometallics 2012, 31, 428. [Google Scholar]
- (69).Wang L; He W; Yu Z. Transition-metal mediated carbon-sulfur bond activation and transformations. Chem. Soc. Rev. 2013, 42, 599. [DOI] [PubMed] [Google Scholar]
- (70).Cummins DC; Alvarado JG; Zaragoza JPT; Effendy Mubarak MQ; Lin Y-T; de Visser SP; Goldberg DP Inorg. Chem. 2020, 59, 16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.