Abstract
Objectives: The Lower Anogenital Squamous Terminology (LAST) recommendations classify human papillomavirus–associated squamous lesions into low- and high-grade squamous intraepithelial lesions (LSILs/HSILs). Our study aimed to assess interobserver agreement among 6 experienced pathologists in assigning 40 anal lesions previously diagnosed as anal intraepithelial neoplasia 2 (AIN 2) to either HSIL or non-HSIL categories.
Methods: Agreement based on photomicrographs of H&E alone or H&E plus p16 immunohistochemistry was calculated using κ coefficients.
Results: Agreement was fair based on H&E alone (κ = 0.42; 95% confidence interval [CI], 0.34-0.52). Adding p16 improved agreement to moderate (κ = 0.55; 95% CI, 0.54-0.62). On final diagnosis, 21 cases (53%) had unanimous diagnoses, and 19 (47%) were divided. When designating p16 results as positive or negative, agreement was excellent (κ = 0.92; 95% CI, 0.83-0.95). Among variables (staining location, extent, and intensity), staining of the basal/parabasal layers was a consistent feature in cases with consensus for positive results (20/20). Of the 67 H&E diagnoses with conflicting p16 results, participants modified 32 (48%), downgrading 23 HSILs and upgrading 9 non-HSILs.
Conclusions: Although p16 increased interobserver agreement, disagreement remained considerable regarding intermediate lesions. p16 expression, particularly if negative, can reduce unwarranted HSIL diagnoses and unnecessary treatment.
Keywords: Interobserver agreement, Human papillomavirus, Anal intraepithelial neoplasia 2, p16 Immunohistochemistry
Key Points.
Substantial disagreement exists among experienced pathologists in diagnosing anal intraepithelial neoplasia 2.
The addition of p16 immunohistochemistry increased interobserver agreement.
Negative p16 results can reduce unwarranted diagnoses of high-grade squamous intraepithelial lesions and unnecessary treatment.
The Lower Anogenital Squamous Terminology (LAST) recommendations provide updated diagnostic guidelines for human papillomavirus (HPV)–associated lesions of the lower anogenital tract.1 The 2-tiered classification represents our current understanding of HPV-associated carcinogenesis: low-grade squamous intraepithelial lesions (LSILs) indicate productive infection with a low risk of malignant transformation, whereas high-grade squamous intraepithelial lesions (HSILs) represent transforming infection and a potential cancer precursor.2 In 2014, the World Health Organization (WHO) Classification of Tumors adopted the terms LSIL and HSIL for preinvasive squamous lesions in the cervix, vagina, vulva, and anus.3
The LAST nomenclature largely parallels the former intraepithelial neoplasia (-IN) classifications: LSIL encompasses -IN 1, whereas HSIL encompasses -IN 3. The former intermediate -IN 2 category was an admixture of transient productive infections, cancer precursors, and morphologic mimics of precancer and has been eliminated.4-6 Pathologists must now translate former -IN 2 lesions into negative, LSIL, or HSIL—a challenging and subjective task.7-12 To facilitate and standardize this process, LAST recommends using biomarker p16 immunohistochemistry (IHC) as an adjunctive tool. Under the new classification, lesions staining block-positive p16 support the diagnosis of HSIL in the appropriate morphology context; if negative (including nonblock staining), they are generally designated as LSIL or benign. Block-positive p16 is defined as nuclear with or without cytoplasmic staining that extends from basal layers upward at least one-third of the epithelial thickness and laterally over a significant distance.
When a disease classification changes, it is critical to assess pathologists’ performance to ensure both the accuracy and reproducibility of diagnoses. In the diagnosis of HPV-associated squamous lesions, most interobserver agreement studies relate to the cervix; comparable studies with a focus on anal intraepithelial neoplasia (AIN) are scarce.13-17 In recent years, incidence and mortality of HPV-associated anal cancers have significantly increased worldwide, resulting in the implementation of screening and treatment programs in certain countries.18-20 As in cervical disease, anal HSIL is the primary screening target and threshold for active intervention. Despite strong similarities between anal and genital sites, anal lesions pose their own diagnostic challenges given the unique anatomy, microenvironment, and viral factors.21,22 Whether p16 improves agreement among pathologists in triaging lesions previously diagnosed as AIN 2 has yet to be determined.
We conducted an interobserver agreement study focusing on lesions previously diagnosed as AIN 2. Participants included 6 pathologists from the United States, the United Kingdom, and Australia who had experience with HPV-associated anogenital diseases. We assessed agreement on the assignment of AIN 2 into HSIL or non-HSIL categories based on photomicrographs of H&E alone or H&E plus p16 IHC. We further analyzed the impact of p16 results on final diagnoses.
Materials and Methods
High-Resolution Anoscopy and Biopsy
The Institutional Review Board of the Icahn School of Medicine at Mount Sinai approved this study. The Mount Sinai Anal Dysplasia Program specializes in the screening and treatment of HPV-associated anal precancer and cancer.23 The target population primarily consists of people living with HIV. Individuals with abnormal anal cytology (ie, atypical squamous cells of undetermined significance or worse) undergo high-resolution anoscopy and biopsy of lesions suspicious for precancer or cancer following previously described techniques.24 Anal biopsy specimens (3-6 mm) were fixed in formalin, processed, and embedded in paraffin wax. Consecutive serial sections were used for H&E staining and p16 IHC. A mouse monoclonal antibody E6H4 against p16 was used (catalog No. 725-4713; Roche) on a Ventana Benchmark LT automated immunostainer. Positive and negative controls were routinely included.
Case Selection
The pathology database at the Mount Sinai Hospital was searched from 2015 to 2018 for anal biopsies diagnosed as AIN 2. A total of 300 cases with corresponding p16 IHC were retrieved. Authors Y.L. (9 years of gynecologic specialty experience) and M.B. (fourth-year pathology resident) reviewed and selected 40 cases representing the most common morphologic features of AIN 2 for the interobserver study. Based on conventional morphologic criteria, AIN 2 lesions were defined as dysplastic squamous cells with nuclear enlargement, coarse chromatin, and irregular nuclear size and shape extending from the lower third to the middle third of the epithelium. In addition, the presence of mitotic figures (including abnormal form) in the middle third was a common feature in the selected cases.
Interobserver Agreement Study
Six pathologists, from the United States, the United Kingdom, and Australia, participated in the study. All practiced surgical pathology for more than 15 years and had significant expertise in HPV-associated anogenital diseases, as demonstrated by clinical focus, research, and publications. Participants independently reviewed photomicrographs of each lesion (H&E stain at ×200; p16 IHC at ×100) and reported their initial diagnoses based on H&E alone and their final diagnoses based on H&E plus p16 IHC. Initial diagnoses used LAST terminology with an -IN qualifier in parentheses or descriptive terms such as “immature squamous metaplasia,” “benign transitional mucosa,” and “reactive/inflammatory changes.” Final diagnoses used only LAST terminology of HSIL, LSIL, or benign processes. Participants did not discuss among themselves specific criteria for H&E interpretation or the assessment of p16 staining for this study. The p16 IHC results were reported as negative or positive or with descriptive terms such as “no staining,” “focal,” or “patchy staining.”
Statistical Analysis
For the purpose of this study, initial and final diagnoses were categorized as HSIL or non-HSIL. The latter group included negative assessment for dysplasia or LSIL (including condyloma) and descriptive terms such as “immature squamous metaplasia,” “benign transitional mucosa,” and “reactive/inflammatory changes.” The p16 IHC results were categorized as positive or negative. Negative p16 for the purpose of this study also included descriptive terms such as “no staining,” “focal,” or “patchy staining.” Interobserver agreement based on H&E alone or H&E plus p16 was evaluated using κ coefficients. For each κ estimate, the 95% CI was calculated using the binomial distribution.
Results
Diagnoses Based on H&E Histology Alone
For the 40 selected cases previously diagnosed as AIN 2, the 6 participants rendered 240 diagnoses based on photomicrographs of H&E histology: 143 were HSIL (60%) and 97 were non-HSIL (40%), as shown in Table 1. Interobserver agreement was fair (κ = 0.42; 95% CI, 0.34-0.52). Of the 40 cases, 18 (45%) received a unanimous diagnosis from all participants, including 13 HSILs and 5 non-HSILs. The remaining 22 cases (55%) received divided diagnoses: consensus HSIL by the majority of participants (7 cases), consensus non-HSIL by the majority of participants (7 cases), and tied (8 cases). Of the 40 cases, 16 lesions originated in the anal transitional zone, whereas 24 were from the squamous zone. Agreement was 43.8% (7/16) for transitional zone lesions and 50% (12/24) for squamous zone lesions. The difference was not statistically significant (P = .7).
Table 1.
Impact of p16 IHC Result on the Final Diagnosis
| Initial Diagnosis Based on H&E (n = 240) | p16 IHC | Final Diagnosis Based on H&E and p16 IHC | ||
|---|---|---|---|---|
| HSIL, No. (%) | Non-HSIL, No. (%) | P Value | ||
| HSIL (n = 143, 60%) | Positive (n = 98) | 98 (100) | <.001a | |
| Negative (n = 45) | 22 (49) | 23 (51) | ||
| Non-HSIL (n = 97, 40%) | Positive (n = 22) | 9 (41) | 13 (59) | <.001a |
| Negative (n = 75) | 75 (100) | |||
| Total | 129 (54) | 111 (46) |
HSIL, high-grade squamous intraepithelial lesion; IHC, immunohistochemistry.
Six participants diagnosing 40 cases yielded 240 diagnoses. The 4 rows represent the 4 scenarios in which participants modified their H&E diagnoses based on p16 results.
aThe proportion of HSIL final diagnoses differs significantly based on p16 results (assessed by χ 2 tests).
Diagnoses Based on H&E Plus p16 IHC
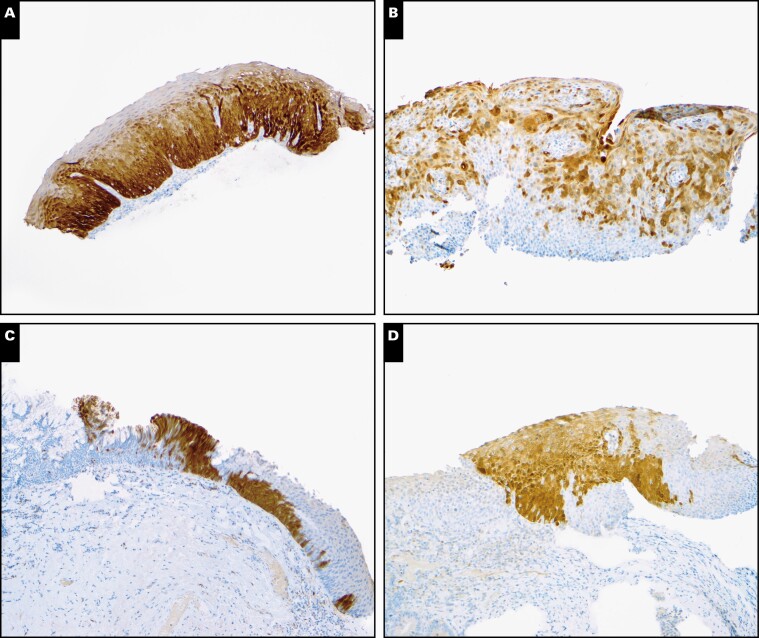
When designating p16 IHC results as positive or negative, participants showed excellent agreement (κ = 0.92; 95% CI, 0.83-0.95) Image 1A and Image 1B. Only 4 cases had divided interpretations: case 9 (2 participants interpreted it as positive, and 4 interpreted it as negative) Image 1C and cases 13, 14, and 26 (5 interpreted them as positive, and 1 interpreted them as negative) Image 1D.
Image 1.
Interpretation of p16 immunohistochemistry (IHC) results. A, Case 15 with diffuse, continuous staining of basal and parabasal layers with upward extension. All participants interpreted as positive. Positive to negative: 6:0. B, Case 7 with discontinuous staining throughout the epithelium but not basal and parabasal layers. All participants interpreted as negative. Positive to negative: 0:6. C, Case 9 with focal staining in the basal and parabasal layers. Two participants interpreted as positive, and 4 interpreted as negative. Positive to negative: 2:4. D, Case 13 with patchy staining of the epithelium, including limited basal and parabasal staining. Five participants interpreted as positive, and 1 interpreted as negative. Positive to negative: 5:1. (p16 IHC, original magnification ×100.)
After reviewing photomicrographs of p16 IHC, participants changed 32 initial H&E diagnoses from 21 cases, downgrading 23 HSILs to non-HSIL based on negative p16 results and upgrading 9 non-HSILs to HSIL based on positive p16 results (Table 1). Consequently, 129 final diagnoses were HSIL (54%) and 111 were non-HSIL (46%). Interobserver agreement on final diagnosis was moderate (κ = 0.55; 95% CI, 0.54-0.62). A unanimous final diagnosis among all participants was reached for 21 cases (53%), including 13 HSILs and 8 non-HSILs. The remaining 19 cases (48%) received divided diagnoses: consensus HSIL by the majority of participants (6 cases), consensus non-HSIL by the majority of participants (10 cases), and tied (3 cases).
Impact of p16 IHC on Final Diagnosis
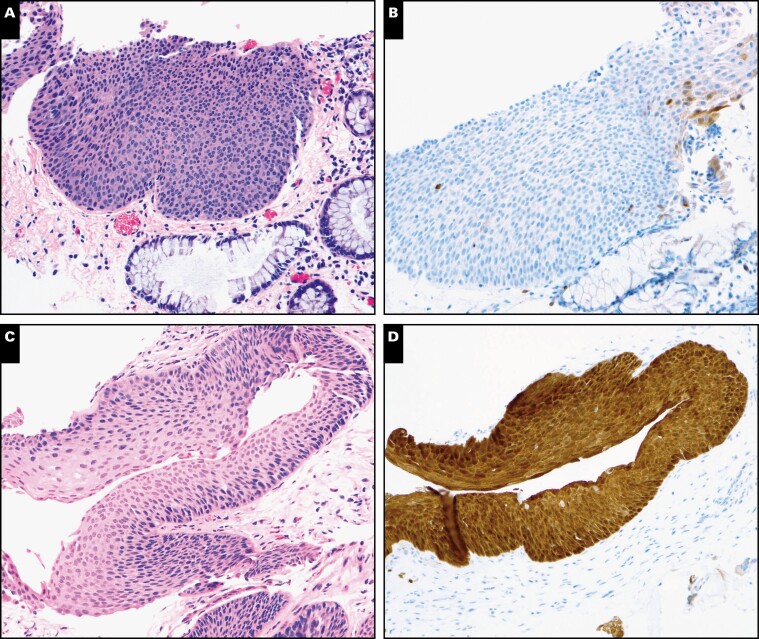
As shown in Table 1, p16 results were in line with 72% (173/240) of initial H&E diagnoses, whether an HSIL impression was supported by positive p16 (n = 98) or a non-HSIL impression was supported by negative p16 (n = 75). Changes in diagnosis occurred when the initial impression was HSIL but p16 was negative (n = 45). Of these cases, 23 HSIL diagnoses (51%) were downgraded to non-HSIL, whereas 22 (49%) were retained as HSIL. Most downgraded cases appeared to represent morphologic mimics of HSIL such as atypical squamous metaplasia (n = 3), inflammatory atypia (n = 2), and basal/parabasal expansion due to tangential sectioning (n = 5). For example, case 28 was diagnosed as HSIL by all participants based on the H&E image Image 2A and Image 2B. Given the negative p16 result, 4 participants downgraded the case to non-HSIL, whereas 2 participants retained an HSIL diagnosis with comments to explain their rationales: “unusual lesion, possible p16 gene mutation or deletion” and “morphology is the gold standard.”
Image 2.
Impact of p16 immunohistochemistry (IHC) results on final diagnosis. A, Case 28 was diagnosed as high-grade squamous intraepithelial lesion (HSIL) by all 6 participants based on H&E morphology. B, Given the negative p16 result, 4 participants downgraded it to non-HSIL, and 2 maintained the HSIL diagnosis. C, Case 11 was diagnosed as non-HSIL by all 6 participants based on H&E morphology. D, Given the positive p16 result, 3 participants upgraded it to HSIL, whereas 3 maintained the non-HSIL diagnosis. (A and C, H&E, ×200; B and D, p16 IHC, ×200.)
Conversely, in cases with an initial histologic impression of non-HSIL but positive p16 (n = 22), 9 (41%) were upgraded to HSIL on final diagnosis, whereas 13 (59%) were retained as non-HSIL (Table 1). For example, case 11 was diagnosed as non-HSIL by all participants based on H&E images Image 2C and Image 2D. Given the positive p16 result, 3 participants upgraded the case to HSIL, whereas 3 retained a non-HSIL diagnosis with comments: (1) “some LSILs express block-positive p16,” (2) “p16 is not required in this case,” and (3) “diagnosis should be based on morphology.”
Discussion
In this study, there was considerable disagreement among 6 experienced pathologists in the diagnosis of anal lesions previously designated as AIN 2. Agreement was only fair based on H&E alone (κ = 0.42; 95% CI, 0.34-0.52) and increased to moderate by adding p16 IHC (κ = 0.55; 95% CI, 0.54-0.62)—a limited improvement. Because the 2 confidence intervals did not overlap, the improvement was statistically significant. In nearly half (19/40) of the cases, even with p16 IHC, the participants did not achieve a unanimous diagnosis—namely, HSIL was diagnosed by at least one pathologist and non-HSIL by others. Because anal HSIL is the primary target for both screening and treatment, such high discordance in rendering pathologic diagnoses inevitably affects the efficacy of early anal cancer detection and prevention.
Compared with the -IN 1 and -IN 3 categories, -IN 2 is the least reproducible diagnosis owing to the equivocal morphologic features and heterogeneous biology.4,25 Our study focused on lesions previously diagnosed as AIN 2, for which the greatest interobserver variation is to be expected. Participants in our study composed a panel of experienced pathologists who have demonstrated expertise in this field through research and publications. If experts with a solid grasp of LAST significantly disagree, general pathologists likely face even greater challenges in diagnosing these intermediate lesions.
Maniar et al25 conducted the study most comparable to ours on a series of -IN 2 lesions from cervix (n = 168), vagina (n = 2), vulva (n = 2), and anus (n = 28). Using 2 categories (LSIL or less vs HSIL), the authors reported fair agreement among 3 pathologists based on H&E morphology (κ = 0.274). In conjunction with p16 IHC, agreement improved (κ = 0.397). Although the authors did not provide specific details regarding the agreement for AIN 2 cases, it is clear that -IN 2 lesions pose a significant diagnostic problem regardless of anogenital site. Although p16 improved agreement in both studies, it was unable to unify diagnoses in a significant number of cases.
The AIN 2 cases in our study displayed a spectrum of p16 expression. Their interpretation appeared to be more objective than H&E morphology (κ = 0.92); however, variations among participants remained. Among variables (staining location, extent, and intensity), continuous staining of the basal and parabasal layers appeared to be a consistent feature in cases with a “positive” p16 consensus. Only 4 of the 40 cases had discordant p16 interpretation. All were characterized by limited basal and parabasal staining, indicating that participants held a different minimum requirement for this criterion. Although LAST has been successful in defining the block-positive pattern, its relative ambiguity on lateral extent (ie, significant distance) may account for this disagreement. The British Association of Gynaecological Pathologists suggests “abnormal expression must extend for at least 6 cells across.” 26 This number requires further validation but at least provides a quantifiable measurement for this criterion.
Studying the impact of p16 results on final diagnosis, we found that p16 expression was in line with the majority of initial histologic impressions (72%, 173/240), whether an HSIL impression was supported by positive p16 or a non-HSIL impression was supported by negative p16. When p16 results and histologic impressions conflicted, it was predominately in the form of an HSIL H&E impression and negative p16, which led participants to downgrade 51% (23/45) of the diagnoses to non-HSIL. In a similar interobserver variability study, Krishnamurti et al17 reported that p16 IHC resulted in a change in diagnosis in 17 cases, of which 14 were downgraded from AIN 2 to AIN 1 based on negative p16.
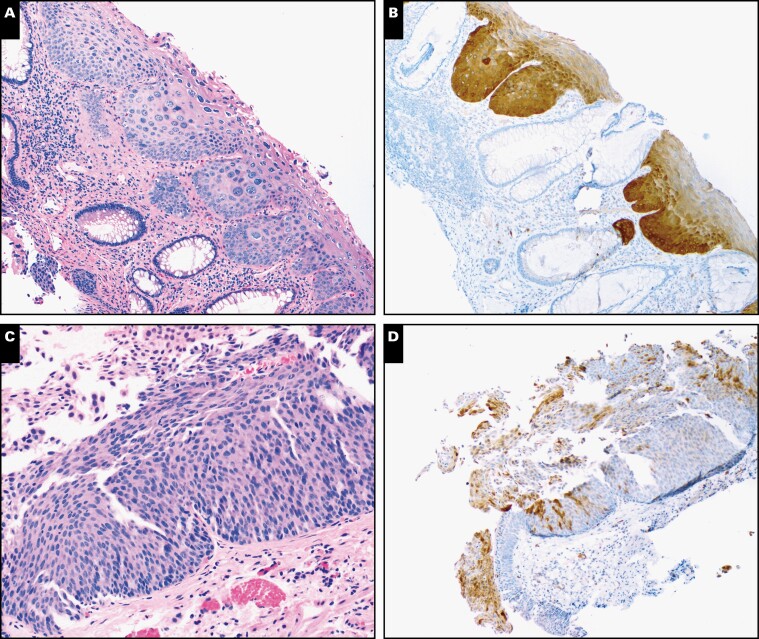
Most downgraded cases in our series were felt to represent morphologic mimics of HSIL such as atypical squamous metaplasia, inflammatory atypia, and tangential sectioning. In addition, the anal transitional zone, a unique anatomical region, was sometimes mistaken for HSIL because of its immature appearance Image 3.27 Our results indicate that even for experienced pathologists, these morphologic mimics are difficult to discern based on traditional morphologic criteria alone. Because p16 negative HSIL is exceedingly rare, the absence of p16 expression provides a reliable, more objective measure to distinguish morphologic mimics and prevent unwarranted HSIL diagnoses, reducing unnecessary treatment.
Image 3.
Lesions originating from anal transitional zone. A, Case 19 was diagnosed as high-grade squamous intraepithelial lesion (HSIL) by 3 participants and non-HSIL by 3 based on H&E morphology. B, Given the positive p16 result, 5 participants diagnosed it as HSIL, and 1 maintained a non-HSIL diagnosis. C, Case 3 was diagnosed as HSIL by 4 and non-HSIL by 2 based on H&E morphology. D, Given the negative p16 result, 5 participants diagnosed it as non-HSIL, whereas 1 maintained HSIL. (A and C, H&E, ×200; B and D, p16 immunohistochemistry, ×100.)
Notably, even when p16 was negative, 22 HSIL H&E impressions from 13 cases and 4 participants were retained as final diagnoses. Most of these cases revealed patchy or focal p16 staining rather than a complete absence of staining. Some participants considered patchy and focal p16 staining to be compatible with their histologic impression of HSIL, especially when they were confident in their morphologic assessment on H&E.
Our findings reflect a longstanding debate, predominantly in the context of cervical lesions, as to whether p16 results should override H&E diagnoses.28-33 Scant evidence suggests that p16-negative AIN 2 carries a low risk of progression and thus that downgrading such lesions to non- HSIL is justified. In the study by Maniar et al,25 one of 4 p16-negative AIN 2 lesions progressed to AIN 3. Albuquerque et al34 reported that none of 18 such lesions in their practice progressed to AIN 3 within a short follow-up of 9 months (±4 months). The behavior of anal lesions with patchy or focal p16 expression is even less clear. Liu et al35 reported that anal LSIL with patchy or focal p16 staining carried an intermediate risk of progression to HSIL (35%) compared to LSIL with block-positive or negative p16 (64% and 14%, respectively) within a median of 16 months of surveillance.
Our study has certain limitations. Participants based their diagnoses on a single H&E and p16 IHC photomicrograph, unlike routine practice in which pathologists review cases on multiple tissue sections under low and high magnifications. Participants were not queried as to whether p16 IHC was needed for diagnosis. In addition, because it was our intention to examine agreement among pathologists from different practice settings, we did not discuss specific criteria for p16 staining for the purpose of this study. As such, all interpretations reflect participants’ individual adaptation of the LAST recommendations in their respective practices.
In conclusion, our study highlights the challenges in classifying intermediate lesions previously diagnosed as AIN 2. In conjunction with p16 IHC, the expert panel assigned 55% of cases as HSIL and downgraded 45% as non-HSIL; this has significant implications for patient management because HSIL is the typical treatment threshold. However, even among the experts, diagnostic variation remained. Better understanding of the biology and prognosis of such intermediate lesions should lay the foundation for future diagnostic recommendations and guidelines.
Acknowledgments
Kay Park is supported by National Institutes of Health Grant (P30 CA008748). Keith Sigel receives funding through National Cancer Institute K07CA180782.
References
- 1. Darragh TM, Colgan TJ, Cox JT, et al. . Members of LAST Project Work Groups . The Lower Anogenital Squamous Terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297. [DOI] [PubMed] [Google Scholar]
- 2. Stoler MH. Human papillomaviruses and cervical neoplasia: a model for carcinogenesis. Int J Gynecol Pathol. 2000;19:16-28. [DOI] [PubMed] [Google Scholar]
- 3. WHO Classification of Tumours. Lyon, France: International Agency for Research on Cancer; 2019. [Google Scholar]
- 4. Castle PE, Stoler MH, Solomon D, et al. . The relationship of community biopsy-diagnosed cervical intraepithelial neoplasia grade 2 to the quality control pathology-reviewed diagnoses: an ALTS report. Am J Clin Pathol. 2007;127:805-815. [DOI] [PubMed] [Google Scholar]
- 5. Castle PE. A LASTing impression: incorporating p16 immunohistochemistry into routine diagnosis of cervical neoplasia. Pathol Case Rev. 2013;18:154-157. [Google Scholar]
- 6. Clinton LK, Miyazaki K, Ayabe A, et al. . The LAST guidelines in clinical practice: implementing recommendations for p16 use. Am J Clin Pathol. 2015;144:844-849. [DOI] [PubMed] [Google Scholar]
- 7. Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergeron C, Ordi J, Schmidt D, et al. ; European CINtec Histology Study Group . Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133:395-406. [DOI] [PubMed] [Google Scholar]
- 9. Galgano MT, Castle PE, Atkins KA, et al. . Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Bogaert LJ. P16INK4a immunocytochemistry/immunohistochemistry: need for scoring uniformization to be clinically useful in gynecological pathology. Ann Diagn Pathol. 2012;16:422-426. [DOI] [PubMed] [Google Scholar]
- 11. van Bogaert LJ. Cervical preneoplasia biomarkers: a conundrum for the community based gynecologic surgical pathologist. J Gynecol Oncol. 2014;25:3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark JL, Lu D, Kalir T, et al. . Overdiagnosis of HSIL on cervical biopsy: errors in p16 immunohistochemistry implementation. Hum Pathol. 2016;55:51-56. [DOI] [PubMed] [Google Scholar]
- 13. Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance–Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group . Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-1505. [DOI] [PubMed] [Google Scholar]
- 14. Walts AE, Lechago J, Hu B, et al. . P16 and Ki67 immunostains decrease intra and interobserver variability in the diagnosis and grading of anal intraepithelial neoplasia (AIN). Clin Med Pathol. 2008;1:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bean SM, Meara RS, Vollmer RT, et al. . p16 improves interobserver agreement in diagnosis of anal intraepithelial neoplasia. J Low Genit Tract Dis. 2009;13:145-153. [DOI] [PubMed] [Google Scholar]
- 16. Roberts JM, Jin F, Thurloe JK, et al. . High reproducibility of histological diagnosis of human papillomavirus-related intraepithelial lesions of the anal canal. Pathology. 2015;47:308-313. [DOI] [PubMed] [Google Scholar]
- 17. Krishnamurti U, Mohammad M, Monsrud A, et al. . Diagnosing anal squamous intraepithelial lesions with and without p16: an interobserver variability study. J Low Genit Tract Dis. 2020;24:69-74. [DOI] [PubMed] [Google Scholar]
- 18. Deshmukh AA, Suk R, Shiels MS, et al. . Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst. 2020;112:829-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palefsky JM. Human papillomavirus-associated anal and cervical cancers in HIV-infected individuals: incidence and prevention in the antiretroviral therapy era. Curr Opin HIV AIDS. 2017;12:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silverberg MJ, Lau B, Justice AC, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darragh TM, Winkler B. Anal cancer and cervical cancer screening: key differences. Cancer Cytopathol. 2011;119:5-19. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Gaisa MM, Wang X, et al. . Differences in the immune microenvironment of anal cancer precursors by HIV status and association with ablation outcomes. J Infect Dis. 2018;217:703-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaisa MM, Liu Y, Deshmukh AA, et al. . Electrocautery ablation of anal high-grade squamous intraepithelial lesions: effectiveness and key factors associated with outcomes. Cancer. 2020;126:1470-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jay N, Berry JM, Hogeboom CJ, et al. . Colposcopic appearance of anal squamous intraepithelial lesions: relationship to histopathology. Dis Colon Rectum. 1997;40:919-928. [DOI] [PubMed] [Google Scholar]
- 25. Maniar KP, Sanchez B, Paintal A, et al. . Role of the biomarker p16 in downgrading -IN 2 diagnoses and predicting higher-grade lesions. Am J Surg Pathol. 2015;39:1708-1718. [DOI] [PubMed] [Google Scholar]
- 26.Singh N, Gilks CB, Wong RW, et al. p16 IHC reporting in anogenital neoplasia version 1.0, August 2018. https://www.thebagp.org/download/bagp-ukneqas-c1qc-project-interpretation-guide-2018/. Accessed September 19, 2020.
- 27. Roberts JM, Cornall AM, Ekman D, et al. . Papillary immature metaplasia of the anal canal: a low-grade lesion that can mimic a high-grade lesion. Am J Surg Pathol. 2016;40:348-353. [DOI] [PubMed] [Google Scholar]
- 28. Thrall MJ. Effect of lower anogenital squamous terminology recommendations on the use of p16 immunohistochemistry and the proportion of high-grade diagnoses in cervical biopsy specimens. Am J Clin Pathol. 2016;145:524-530. [DOI] [PubMed] [Google Scholar]
- 29. Razmpoosh M, Sansregret A, Oligny LL, et al. . Assessment of correlation between p16INK4a staining, specific subtype of human papillomavirus, and progression of LSIL/CIN1 lesions: first comparative study. Am J Clin Pathol. 2014;142:104-110. [DOI] [PubMed] [Google Scholar]
- 30. Sagasta A, Castillo P, Saco A, et al. . p16 staining has limited value in predicting the outcome of histological low-grade squamous intraepithelial lesions of the cervix. Mod Pathol. 2016;29:51-59. [DOI] [PubMed] [Google Scholar]
- 31. Mills AM, Paquette C, Castle PE, et al. . Risk stratification by p16 immunostaining of CIN1 biopsies: a retrospective study of patients from the quadrivalent HPV vaccine trials. Am J Surg Pathol. 2015;39:611-617. [DOI] [PubMed] [Google Scholar]
- 32. Omori M, Hashi A, Nakazawa K, et al. . Estimation of prognoses for cervical intraepithelial neoplasia 2 by p16INK4a immunoexpression and high-risk HPV in situ hybridization signal types. Am J Clin Pathol. 2007;128:208-217. [DOI] [PubMed] [Google Scholar]
- 33. Kalof AN, Evans MF, Simmons-Arnold L, et al. . p16INK4A immunoexpression and HPV in situ hybridization signal patterns: potential markers of high-grade cervical intraepithelial neoplasia. Am J Surg Pathol. 2005;29:674-679. [DOI] [PubMed] [Google Scholar]
- 34. Albuquerque A, Rios E, Macedo G. The impact of P16 immunostaining in reducing anal squamous intraepithelial lesions indication for treatment. Am J Surg Pathol. 2017;41:1151-1152. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Blakely M, Sigel K, et al. . Biomarker P16 predicts progression risk of anal low-grade squamous intraepithelial lesions. AIDS. 2018;32:2309-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]