Abstract
Rationale
Nicotine acts as an agonist for nicotinic acetylcholine receptors (nAChRs), and mecamylamine, a nonselective nAChR antagonist, attenuates effects of nicotine on delay discounting in some rat strains; whether nicotine’s attenuation is specific to nAChR antagonism is unknown.
Objective
During experiment 1, we evaluated dose-dependent effects of nicotine on delay discounting of pair-housed Lewis (LEW) and Fischer 344 (F344) rats. During experiment 2, we examined the sensitivity of nicotine’s effects on delay discounting to pharmacological antagonism of nAChRs or muscarinic AChRs (mAChRs).
Materials and methods
Male LEW and F344 were trained to choose between one food pellet delivered immediately and three food pellets delivered after an increasing delay. During experiment 1, saline and nicotine (0.1–1.0 mg/kg) were tested acutely. During experiment 2, mecamylamine (0.25–1.0 mg/kg) or a nonselective mAChR antagonist, scopolamine (0.01–0.056 mg/kg), was administered prior to nicotine administration.
Results
Nicotine dose dependently reduced delay discounting for both rat strains, and no strain differences were observed (ΔAUC = + 107% for 1.0 mg/kg and + 69.6% for 0.3 mg/kg relative to saline). At some doses, pretreatment with mecamylamine (range ΔAUC = − 27.6 to − 7.3%) or scopolamine (range ΔAUC = − 0.74 to − 51.6%) significantly attenuated the nicotine-induced reduction in some measures of delay discounting for both strains.
Conclusions
Results from experiment 1 suggest that when LEW and F344 are pair housed, there are no strain differences in delay discounting in response to nicotine. Results from experiment 2 suggest that attenuation of nicotine’s effects on delay discounting may not be specific to nAChR antagonism.
Keywords: Scopolamine, Delay discounting, Nicotine, Mecamylamine
Introduction
Impulsive choice, which is operationally defined as choosing a smaller, more immediate reinforcer over a larger, delayed reinforcer, plays a role in many behavioral and psychological disorders, including addiction (see Hamilton and Potenza 2012 for a review). In general, impulsive choice is evaluated using delay-discounting procedures, which refer to a decline in the subjective value of a larger reinforcer as the delay to its receipt increases (Mazur 1987). Typically, delay-discounting procedures involve discrete-trial choices between a smaller, more immediate reinforcer and a larger, delayed reinforcer in which choice for the smaller, more immediate reinforcer is considered impulsive.
Nicotine (NIC), a nicotinic acetylcholine receptor (nAChR) agonist, is of continued interest due to its high rate of use throughout the human population (USDHHS 2014). In addition, greater delay discounting of several commodities is associated with cigarette smoking relative to nonsmoking (see Barlow et al. 2016 for a review). NIC binds to nAChRs (Benowitz 2009) and stimulates release of several neurotransmitters, including acetylcholine (ACh), dopamine (DA), and serotonin (5-HT) (Benowitz 2009; Summers and Giacobini 1995; Zhu et al. 2007). There is evidence that these neurotransmitter systems play a role in impulsive choice (e.g., Kolokotroni et al. 2011; Mendez et al. 2012; Mobini et al. 2000; Winstanley et al. 2003, 2006; Xie et al. 2012), although little is known about ACh’s direct role. In addition, it seems as though effects of NIC on delay discounting may depend, at least in part, upon subject biology.
To continue understanding biological influence on impulsive choice, Lewis (LEW) and Fischer 344 (F344) rat strains have been suggested as ideal animal models, particularly because LEW are considered “addiction-prone” while F344 are considered “addiction-resistant” (Cadoni 2016). Relative to F344, LEW have fewer DA transporters and receptors in the nucleus accumbens and striatum as well as less 5-HT binding in the hippocampus, prefrontal cortex, and nucleus accumbens (Flores et al. 1998; Selim and Bradberry 1996). In addition, choice is more impulsive for LEW across adjusting delay, concurrent chains, and Evenden and Ryan (1996; 1999) delay-discounting procedures when individually housed (Anderson and Diller 2010; Anderson and Woolverton 2005; Huskinson and Anderson 2012; Huskinson et al. 2012; Madden et al. 2008; Stein et al. 2012), giving support for a biological component in delay discounting. However, when LEW and F344 are pair housed, no strain differences are observed under the Evenden and Ryan (1996, 1999) delay-discounting procedure, which suggests that social enrichment interacts with biology to influence impulsive choice (Turturici et al. under review).
When administered acutely, NIC reduces delay discounting at a lower dose for LEW (0.3 mg/kg) relative to F344 (1.0 mg/kg) when rats are individually housed (Anderson and Diller 2010). When mecamylamine (MEC), a nonselective nAChR antagonist, was administered prior to NIC administration in male Lister-hooded rats, NIC’s effects were attenuated (Kolokotroni et al. 2011). However, when administered alone, MEC did not affect delay discounting of Lister-hooded (Kolokotroni et al. 2011) or Long-Evans rats (Mendez et al. 2012). Because nAChRs and muscarinic AChRs (mAChRs) are co-localized on dopaminergic and cholinergic neurons (van der Zee et al. 1991), it is unclear whether attenuation of NIC’s effects on delay discounting is specific to nAChR antagonism.
The present study was designed (1) to characterize dose-dependent effects of acute NIC on delay discounting in pair-housed LEW and F344 and (2) to examine whether effects of NIC on delay discounting in LEW and F344 are attenuated by general pharmacological antagonism of AChRs. Because strain differences in delay discounting at baseline are attenuated with pair housing relative to individual housing (Turturici et al. under review), the goal of experiment 1 was to evaluate whether differences in NIC’s effects on delay discounting of individually housed LEW and F344 (Anderson and Diller 2010) would also be attenuated by pair housing. In experiment 2, MEC (nAChR antagonist) or scopolamine (SCOP; mAChR antagonist) was administered prior to NIC administration to evaluate whether AChR antagonism would attenuate effects of the two highest NIC doses, which were determined to produce the largest effects on delay discounting during experiment 1.
Materials and methods
All procedures were approved by the Animal Care and Use Committee at West Virginia University and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Subjects
Male LEW (n = 6) and male F344 (n = 6; Envigo Inc., Indianapolis, IN) served as subjects. All rats were experimentally naïve and housed in littermate pairs in controlled environmental conditions (temperature, 24 °C; 12-h reverse light/dark cycle) with continuous access to water. Rats were fed ad libitum for 3 weeks following shipment, began food restriction at 9 weeks, and were 11 weeks old at the beginning of the experiment. Sessions were conducted during the dark phase at the same time each day, 5 days per week. Rats were fed approximately 30 min following each session, which was done by placing approximately 26–30 g of food in each shared cage. Rats were food restricted for approximately 22 h after-ward. At the start of the experiment, weights ranged from 263 to 317 g for LEW and 239 to 268 g for F344. Male rats were used as subjects to facilitate a direct comparison between the current study and prior studies with LEW and F344 (e.g., Anderson and Diller 2010; Huskinson et al. 2012).
Apparatus
Eight standard operant-conditioning chambers for rats were used, each enclosed in a melamine sound-attenuating cubicle (Med Associates, VT). Each chamber contained a working area of 30.5 cm by 24.5 cm by 21.0 cm, a grid floor, and a 45-mg pellet dispenser with a pellet receptacle centered between two retractable response levers. Levers were 11.5 cm apart from each other and required at least 0.25 N of force for a response to be recorded. Levers were 4.8 cm wide, protruded 1.9 cm into the chamber, and were elevated 8 cm from the grid floor. One 28-V stimulus lights, 2.5 cm in diameter, was placed approximately 7 cm above each lever. Each chamber had a 28 V houselight on the wall opposite to the working wall and a ventilation fan to circulate air and mask extraneous noise. Data collection and programmed consequences were controlled by a personal computer equipped with Med-PC software (Med Associates, VT).
Drugs
All drugs were purchased from Sigma-Aldrich (St. Louis, MO) and were diluted in 0.9% saline vehicle (SAL). Nicotine hydrogen tartrate (NIC) was delivered subcutaneously (SC) or intraperitoneally (IP), depending on the corresponding antagonist’s route of administration, in 0.1, 0.3, and 1.0 mg/mL concentrations. Mecamylamine hydrochloride (MEC) was delivered SC in 0.25, 0.5, 0.75, and 1.0 mg/mL concentrations. Scopolamine hydrobromide trihydrate (SCOP) was delivered IP in 0.01, 0.03, and 0.056 mg/mL concentrations. All doses were delivered in a volume of 1.0 mL/kg. MEC, SCOP, and SAL doses were administered approximately 15 min prior to the start of sessions. NIC and SAL doses were administered immediately prior to sessions. Doses and pretreatment durations are based on pharmacokinetic profiles of MEC, SCOP, and NIC and have been established as behaviorally relevant during prior studies (e.g., Anderson and Diller 2010; Kolokotroni et al. 2011; Mendez et al. 2012).
Delay-discounting task training
The procedure described below was modified from Evenden and Ryan (1996) and has been used previously to evaluate delay discounting of LEW and F344 (e.g., Anderson and Diller 2010; Anderson and Woolverton 2005). All sessions began with a 10-min blackout period, followed by five blocks of eight trials each (40 total trials). After the blackout, the houselight was illuminated and remained lit for the remainder of the session. Each block contained two forced-exposure trials, followed by six free-choice trials. During each forced-exposure trial within a block, one lever (randomly determined) was extended into the chamber and the cue light above it was illuminated. After one response, the lever retracted, and one or three food pellets were delivered either immediately or after a delay, dependent upon the magnitude of reinforcement and reinforcer delay associated with the lever. During free-choice trials, both levers were extended with corresponding cue lights, and choice for each lever was recorded. During both trial types, the houselight flashed for 0.1 s as each food pellet was delivered and each new trial began every 100 s, resulting in varying inter-trial intervals (ITIs). Groups were counterbalanced such that half of each group received the three-pellet option for presses on the right lever and levers correlated with each pellet option (one or three food pellets) remained constant throughout the experiment. If a response did not occur within 30 s of trial onset for either trial type, it was considered an omission. At this point, the cue light(s) turned off, lever(s) retracted, and a 70-s ITI began.
Initially, during each block, an FR 1 contingency was in effect for presses on both levers, with no delay associated with presses on either lever. After at least 80% of overall responses during free-choice trials was for the larger reinforcer for one session (adjustment criterion), the delay to the larger reinforcer was increased across blocks of trials according to the sequence: 0, 1, 2, 4, and 8 s. Again, after the adjustment criterion was met, the delay was increased to the sequence: 0, 2, 4, 8, and 16 s. This process was repeated until delay-discounting functions were obtained without floor (exclusive choice for the smaller, immediate reinforcer) or ceiling (exclusive choice for the larger, delayed reinforcer) effects. The sequence was increased to 0, 5, 10, 20, and 40 s as needed. A minimum of 10 sessions at the terminal delay series were conducted until stability met criteria across the last five baseline sessions, which included no increasing or decreasing trends in total percent choice for the larger reinforcer, mean percent larger reinforcer choice at least 80% for the 0-s delay block, and no more than 20% variation in total percent larger reinforcer choice. Probe sessions, in which all delays were set to 0 s across all blocks, were implemented as needed when percent larger reinforcer choice approached floor or ceiling, after which baseline was re-established according to the criteria listed above. Probe sessions were discontinued during testing. Three LEW and five F344 reached stability on the 16-s delay series, while three LEW and one F344 reached stability on the 40-s series.
Testing
Experiment 1
Before NIC administration, SC injections of SAL were given for at least two sessions to habituate rats to injection procedures. Drug administrations occurred on Tuesdays and Fridays of each week, given that larger reinforcer choice was at least 80% for the most recent free-choice 0-s block (control day; Mondays and Thursdays) and total percent larger reinforcer choice was within the range of the last five baseline sessions. All doses of NIC (0.0, 0.1, 0.3, 1.0 mg/kg) were administered SC in descending order, with each dose administered a minimum of twice. Additional doses were administered if substantial variability in choice occurred between the two administrations.
Experiment 2
MEC (0.00, 0.25, 0.50, 0.75, or 1.0 mg/kg) or SCOP (0.00, 0.01, 0.03, or 0.056 mg/kg) was administered 15 min prior to NIC (0.0, 0.3, or 1.0 mg/kg) administration using a within-subjects design. The two largest doses of NIC were chosen because they produced the largest effects on delay discounting during experiment 1. MEC was administered SC and was followed by SC NIC. SCOP was administered IP and was followed by IP NIC. In addition to SAL-SAL, each dose of MEC (0.25–1.0 mg/kg) and SCOP (0.01–0.056 mg/kg) was paired with SAL, 0.3 mg/kg NIC, and 1.0 mg/kg NIC. This resulted in a total of 24 drug dose combinations. The order of drug dose combinations was presented in a counterbalanced, pseudorandom order. Similar to experiment 1, each drug dose combination was administered at least twice per rat, and testing occurred on Tuesdays and Fridays if the most recent control session (Monday or Thursday) met stability criteria.
Data analysis
The average of all administrations at a particular dose or dose combination for individual rats was used for data analysis. Primary outcome measures included percent larger reinforcer choice, area under the discounting curve (AUC), and estimated slopes (k) and intercepts (A) of hyperbolic discounting functions. Percent larger reinforcer choice was calculated per block by dividing the number of free-choice responses on the lever associated with the larger, delayed reinforcer by the total number of free-choice responses made in each block. AUC was calculated according to the method proposed by Myerson et al. (2001), and Mazur’s hyperbolic discounting model (Mazur 1987), represented by V = A / (1 + kD), was fit to percent larger reinforcer choice data for individual rats. In the hyperbolic equation, V is the subjective value of the larger reinforcer, A is choice for the larger reinforcer when it is delivered immediately, D is the delay to the larger reinforcer, and k is the rate of discounting or slope of the delay-discounting function. Percent larger reinforcer choice during the 0-s delay block was used as an estimate of the A parameter, and estimates of k were interpolated from model fits. To control for any potential influence, delay series was included as a covariate in all statistical analyses. Total omitted trials per session were analyzed as a secondary outcome measure.
During both experiments, AUC, log-transformed k (log k), and total omitted trials per session were analyzed using rat strain (2 levels) by drug dose (4, 12, or 15 levels) mixed ANOVAs. Percent larger reinforcer choice was analyzed using rat strain by drug dose by block in session (five levels) mixed ANOVAs. Huynh-Feldt corrections were used to adjust for violations of the sphericity assumption, and Tukey’s honestly significant difference tests were used to make pairwise comparisons as needed. For all analyses, statistical significance was defined as p < 0.05. Nonparametric tests were performed when homogeneity of variance was violated.
Due to mortality issues toward the end of experiment 2 (n = 3 LEW; n = 4 F344), a multiple imputation was conducted. Following MEC pretreatment, 6.7% of sessions were imputed for LEW and 28.8% for F344. Following SCOP pretreatment, 13.9% of sessions were imputed for LEW and 8.3% for F344. Because drug dose combinations were administered pseudorandomly during experiment 2, risk of estimating data based upon other estimates for an individual rat was reduced. Briefly, multiple imputation predicts missing values for an individual subject based on other data that are available for that subject. This method gives unbiased results by taking uncertainty into account and minimally affecting standard error of the mean. Data were graphed before and after multiple imputations, and the imputation did not affect the overall pattern of results. If a single rat was missing more than three drug dose combinations with MEC or SCOP pretreatment, it was excluded from analyses during experiment 2. This resulted in one LEW and one F344 being excluded from MEC analyses and one LEWand three F344 being excluded from SCOP analyses.
Results
Experiment 1
Statistical results for all primary outcome measures are shown in Table 1.
Table 1.
Statistical analyses for primary outcomes during experiment 1
| Percent larger reinforcer choice | AUC | Log k | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Partial η2 | F | p | Partial η2 | F | p | Partial η2 | |
| Dosea | 21.242 | < 0.001 | 0.702 | 26.169 | < 0.001 | 0.744 | 4.094 | 0.016 | 0.313 |
| Rat strainb | 1.251 | 0.292 | 0.122 | 0.158 | 0.700 | 0.017 | 0.006 | 0.939 | 0.001 |
| Delay seriesb | 0.153 | 0.705 | 0.017 | 0.061 | 0.811 | 0.007 | 0.186 | 0.677 | 0.020 |
| Dose × strainb | 1.898 | 0.154 | 0.174 | 1.359 | 0.276 | 0.131 | 0.123 | 0.884 | 0.013 |
| Dose × delay seriesa | 3.806 | 0.021 | 0.297 | 5.898 | 0.003 | 0.396 | 4.892 | 0.008 | 0.352 |
| Blockc | 48.948 | < 0.001 | 0.845 | ||||||
| Block × delay seriesc | 3.574 | 0.035 | 0.284 | ||||||
| Block × strainc | 2.488 | 0.094 | 0.217 | ||||||
| Dose × blockc | 6.378 | < 0.001 | 0.415 | ||||||
| Dose × block × delay Seriesa | 2.713 | 0.016 | 0.232 | ||||||
| Dose × block × straina | 0.665 | 0.700 | 0.069 | ||||||
Italic values denote statistical significance, p < 0.05
df = (3, 27)
df = (1, 8)
df = (4, 32)
Percent larger reinforcer choice
Figure 1 shows percent larger reinforcer choice as a function of increasing delays to the larger reinforcer (i.e., delay-discounting curves) for SAL and each dose of acute NIC. NIC significantly increased larger reinforcer choice at the two highest doses of NIC (i.e., 0.3 and 1.0 mg/kg), and these increases occurred during the third, fourth, and fifth trial blocks in which delay durations were longest. Although larger reinforcer choice increased, delay discounting still occurred. Percent larger reinforcer choice during the 0-s block was not affected by any dose of acute NIC (A parameter), suggesting that NIC did not disrupt sensitivity to reinforcer magnitude.
Fig. 1.
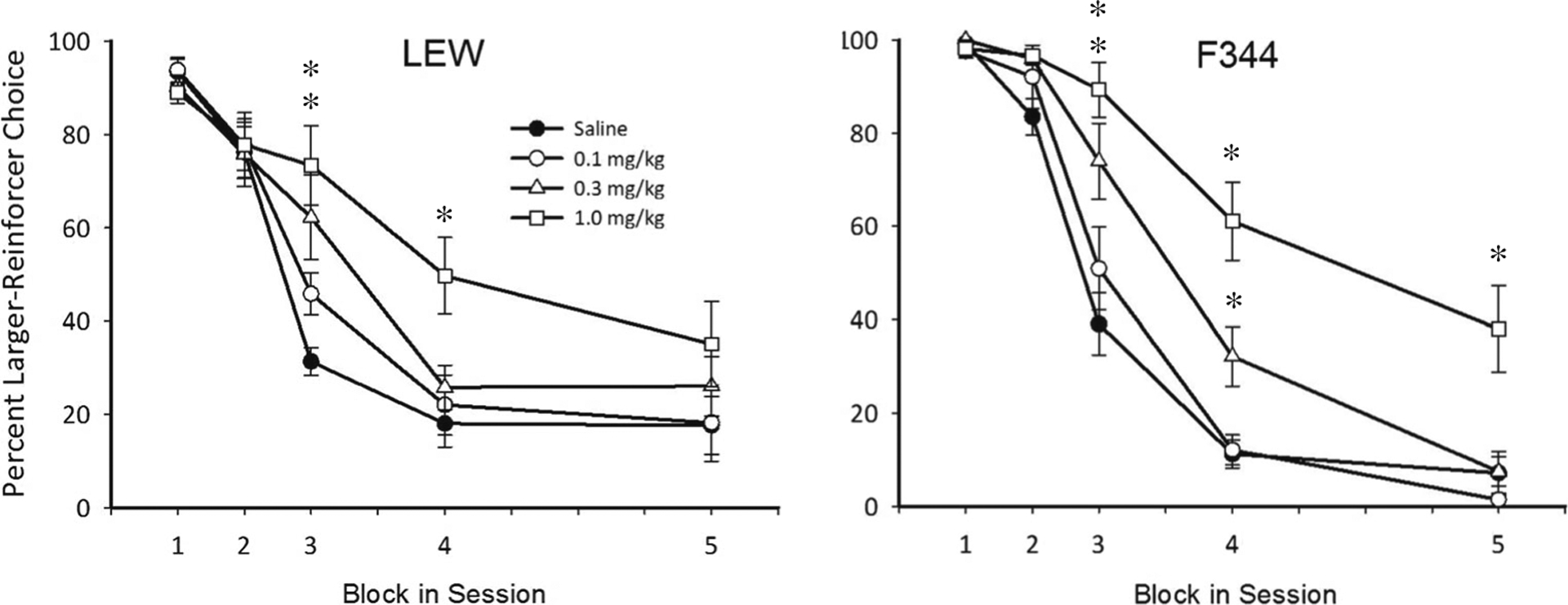
Percent larger reinforcer choice as a function of block in session (corresponding to the increasing delay to the larger reinforcer across successive blocks) across all doses of acute NIC during experiment 1 for LEW (left panel) and F344 (right panel) rats. Note that data for each rat strain are collapsed across delay series. Error bars represent standard error of the mean. Asterisks represent a statistically significant difference from SAL (p < 0.05)
AUC
Figure 2 shows AUC as a function of NIC dose. Collapsed across rat strain, post hoc tests revealed that mean AUC was significantly larger following 1.0 (M = 0.58, SEM = 0.04) and 0.3 mg/kg NIC (M = 0.46, SEM = 0.02) than following SAL (M = 0.29, SEM = 0.02). Percent change in AUC for individual rats are shown in Table 2. Percent change in AUC was significantly smaller for LEW responding on the 40-s delay series at 1.0 (M = 44, SEM = 08) and 0.3 mg/kg NIC doses (M = 21, SEM = 15) relative to LEW responding on the 16-s delay series (1.0 mg/kg: M = 120, SEM = 35; 0.3 mg/kg: M = 50, SEM = 19), suggesting that the longer delay series was associated with reduced effects of NIC.
Fig. 2.
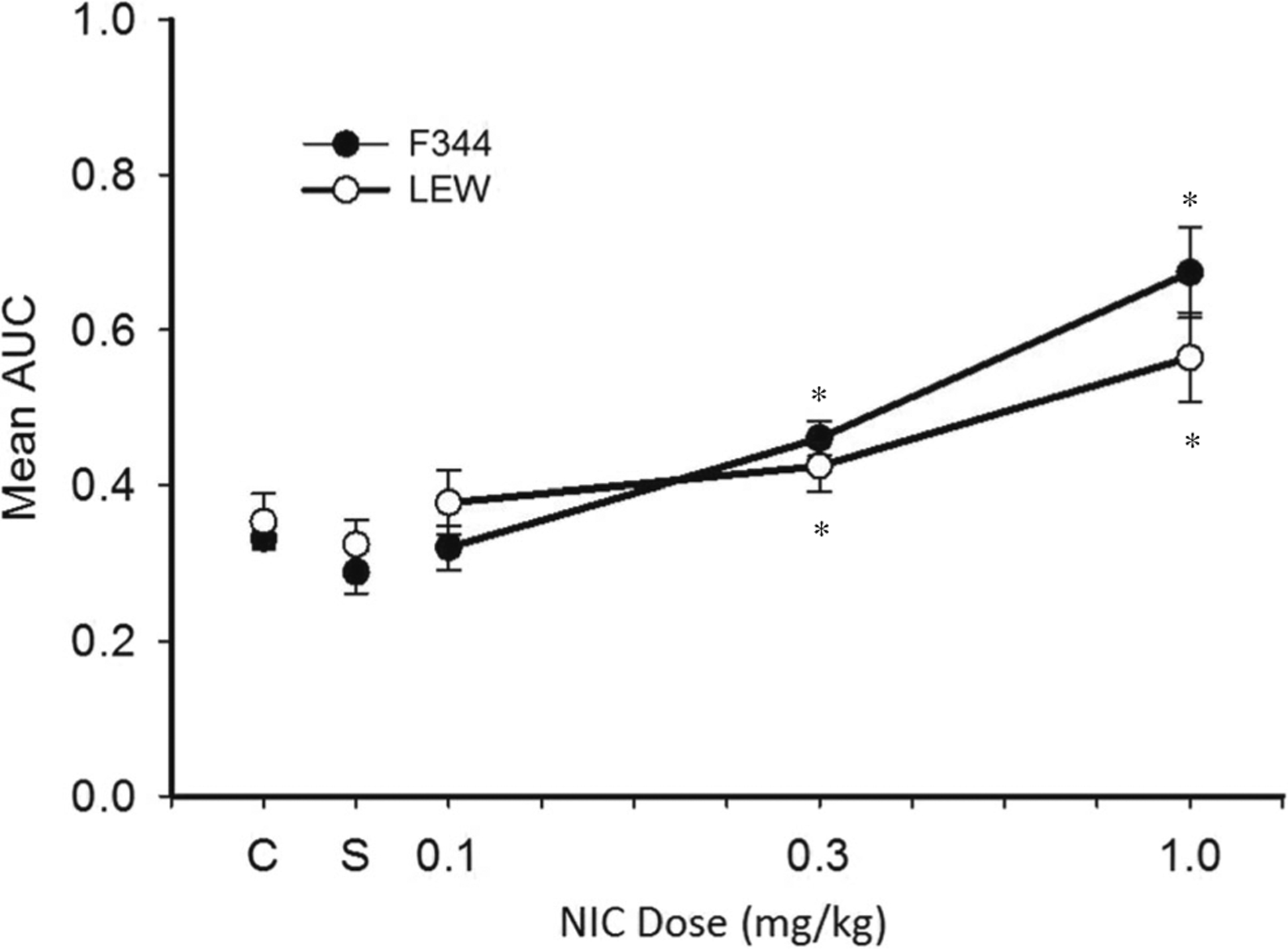
Mean AUC for LEW and F344 across all doses of acute NIC during experiment 1. “C” corresponds to control (no-drug) sessions, and “S” corresponds to sessions in which SAL was administered. An AUC value of 0.0 indicates exclusive choice for the smaller, immediate reinforce, and an AUC value of 1.0 indicates exclusive choice for the larger, delayed reinforcer. Note that data for each rat strain are collapsed across delay series. Error bars represent standard error of the mean. Asterisks represent a statistically significant difference from SAL (p < 0.05)
Table 2.
Slope estimates and percent change in AUC during experiment 1 for individual rats at each NIC dose
| Subject ID | SAL | 0.1 NIC | 0.3 NIC | 1.0 NIC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k | Log k | k | Log k | %ΔAUC | k | Log k | %ΔAUC | k | Log k | %ΔAUC | |
| JOL1 (16) | 0.12 | − 0.92 | 0.10 | − 1.00 | 16 | 0.09 | − 1.05 | 31 | 0.01 | − 2.00 | 144 |
| JOL2 (40) | 0.16 | − 0.80 | 0.12 | − 0.92 | 28 | 0.11 | − 0.96 | 07 | 0.07 | − 1.15 | 38 |
| JOL3 (16) | 0.16 | − 0.80 | 0.13 | − 0.89 | 09 | 0.05 | − 1.30 | 87 | 0.02 | − 1.70 | 165 |
| JOL4 (40) | 0.09 | − 1.05 | 0.08 | − 1.10 | 31 | 0.04 | − 1.40 | 51 | 0.04 | − 1.42 | 60 |
| JOL5 (16) | 0.14 | − 0.85 | 0.12 | − 0.92 | 04 | 0.07 | − 1.15 | 32 | 0.06 | − 1.22 | 50 |
| JOL6 (40) | 0.08 | − 1.10 | 0.03 | − 1.52 | 13 | 0.05 | − 1.30 | 04 | 0.03 | − 1.52 | 35 |
| Mean | 0.13 | − 0.92 | 0.10 | − 1.06 | 17 | 0.07 | − 1.19 | 35 | 0.04 | − 1.50 | 82 |
| SEM | 0.01 | 0.05 | 0.02 | 0.10 | 04 | 0.01 | 0.07 | 13 | 0.01 | 0.13 | 23 |
| JOF1 (16) | 0.21 | − 0.68 | 0.11 | − 0.96 | 47 | 0.06 | − 1.22 | 132 | 0.04 | − 1.40 | 205 |
| JOF2 (16) | 0.13 | − 0.89 | 0.17 | − 0.77 | − 10 | 0.07 | − 1.15 | 52 | 0.01 | − − 2.00 | 152 |
| JOF3 (16) | 0.09 | − 1.05 | 0.13 | − 0.89 | − 31 | 0.08 | − 1.10 | 06 | 0.04 | − 1.40 | 71 |
| JOF4 (16) | 0.11 | − 0.96 | 0.07 | − 1.15 | 20 | 0.06 | − 1.22 | 29 | 0.01 | − 2.00 | 123 |
| JOF5 (16) | 0.12 | − 0.92 | 0.09 | − 1.05 | 19 | 0.05 | − 1.30 | 58 | 0.01 | − 2.00 | 168 |
| JOF6 (40) | 0.19 | − 0.72 | 0.10 | − 1.00 | 43 | 0.06 | − 1.22 | 130 | 0.07 | −1.15 | 104 |
| Mean | 0.14 | − 0.87 | 0.11 | − 0.97 | 15 | 0.06 | − 1.20 | 68 | 0.03 | − 1.66 | 137 |
| SEM | 0.02 | 0.06 | 0.01 | 0.05 | 12 | 0.004 | 0.03 | 21 | 0.01 | 0.16 | 20 |
A positive value for percent change in AUC indicates an increase in AUC, and a negative value indicates a decrease in AUC. Values in parenthesis next to subject IDs refer to the delay series on which individual rats responded
Slope (k)
Raw and log k values for individual rats at each NIC dose are shown in Table 2. Collapsed across rat strain, log k values were significantly smaller following 1.0 (M = − 1.581, SEM = 0.09) and 0.3 mg/kg NIC (M = − 1.198, SEM = 0.04) relative to SAL (M = − 0.893, SEM = 0.04), suggesting that NIC reduced sensitivity to delayed reinforcement. Similar to AUC, LEW responding on the 40-s delay series experienced a reduced effect of acute NIC at 0.3 (M = − 1.05, SEM = 0.14) and 1.0 mg/kg (M = − 1.16, SEM = 0.17) doses relative to LEW responding on the 16-s delay series (0.3 mg/kg: M = − 1.15; SEM = 0.01; 1.0 mg/kg: M = − 1.94, SEM = 0.32).
Omissions
Few omitted trials occurred during experiment 1, and there were no statistically significant effects of any independent variable on omissions (data not shown).
Experiment 2
Statistical results for all primary outcome measures are shown in Table 3.
Table 3.
Statistical analyses for primary outcomes during experiment 2
| Percent larger reinforcer choice | AUC | Log k | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Partial η2 | F | p | Partial η2 | F | p | Partial η2 | |
| MEC pretreatment | |||||||||
| Rat straina | 3.184 | 0.080 | 0.060 | 0.022 | 0.882 | 0.000 | 0.250 | 0.635 | 0.040 |
| Delay seriesa | 34.663 | < 0.001 | 0.409 | 7.521 | 0.008 | 0.131 | 6.413 | 0.052 | 0.562 |
| Doseb | 12.119 | < 0.001 | 0.195 | 8.392 | < 0.001 | 0.144 | 2.526 | 0.012 | 0.336 |
| Dose × delay seriesb | 8.349 | < 0.001 | 0.143 | 6.831 | < 0.001 | 0.120 | 1.320 | 0.239 | 0.209 |
| Dose × strainb | 4.500 | < 0.001 | 0.083 | 2.060 | 0.036 | 0.040 | 0.381 | 0.958 | 0.071 |
| Trial blockc | 1008.66 | < 0.001 | 0.953 | ||||||
| Block × delay seriesc | 9.961 | < 0.001 | 0.166 | ||||||
| Block × strainc | 9.731 | < 0.001 | 0.163 | ||||||
| Dose × blockd | 11.569 | < 0.001 | 0.188 | ||||||
| Dose × block × delay seriesd | 6.494 | < 0.001 | 0.115 | ||||||
| Dose × block × straind | 4.950 | < 0.001 | 0.090 | ||||||
| SCOP pretreatment | |||||||||
| Rat straine | 6.945 | 0.012 | 0.151 | 5.148 | 0.029 | 0.112 | 0.903 | 0.396 | 0.184 |
| Delay seriese | 0.187 | 0.668 | 0.005 | 0.001 | 0.980 | 0.000 | 2.389 | 0.197 | 0.374 |
| Dosef | 32.581 | < 0.001 | 0.455 | 15.962 | < 0.001 | 0.280 | 2.369 | 0.021 | 0.372 |
| Dose × delay seriesf | 18.021 | < 0.001 | 0.316 | 9.328 | < 0.001 | 0.185 | 0.839 | 0.603 | 0.173 |
| Dose × strainf | 9.007 | < 0.001 | 0.188 | 8.173 | < 0.001 | 0.166 | 1.136 | 0.358 | 0.221 |
| Trial blockg | 1283.19 | < 0.001 | 0.971 | ||||||
| Block × delay seriesg | 6.375 | 0.004 | 0.140 | ||||||
| Block × straing | 1.832 | 0.173 | 0.045 | ||||||
| Dose × blockh | 21.880 | < 0.001 | 0.359 | ||||||
| Dose × block × delay seriesh | 8.096 | < 0.001 | 0.172 | ||||||
| Dose × block × strainh | 8.811 | < 0.001 | 0.184 | ||||||
Italic values denote statistical significance, p < 0.05
df = (1, 50)
df = (14, 700)
df = (4, 200)
df = (56, 2800)
df = (1, 39)
df = (11, 429)
df = (4, 156)
df = (44, 1716)
MEC pretreatment
Percent larger reinforcer choice
Figure 3 shows delay-discounting curves for each dose combination following MEC pretreatment. Relative to SAL alone, MEC did not affect percent larger reinforcer choice for either rat strain when administered in combination with SAL. For LEW, 1.0 mg/kg NIC significantly increased larger reinforcer choice during the third trial block relative to SAL only, and this increase was attenuated significantly by 0.75 mg/kg MEC. Although 0.3 mg/kg NIC increased larger reinforcer choice for LEW during experiment 1, this effect did not replicate during experiment 2. For F344, 1.0 mg/kg NIC significantly increased larger reinforcer choice during the third and fourth trial blocks relative to SAL only, and this increase was attenuated significantly by 0.25, 0.50, and 0.75 mg/kg MEC. In addition, 0.3 mg/kg NIC significantly increased larger reinforcer choice during the third and fourth trial blocks for F344 relative to SAL. The increase produced by 0.3 mg/kg NIC was attenuated significantly by all four doses of MEC, but only in the third block of trials. Percent larger reinforcer choice during the 0-s block was not affected by any dose combination following MEC pretreatment (A parameter), suggesting that sensitivity to reinforcer magnitude was not affected.
Fig. 3.
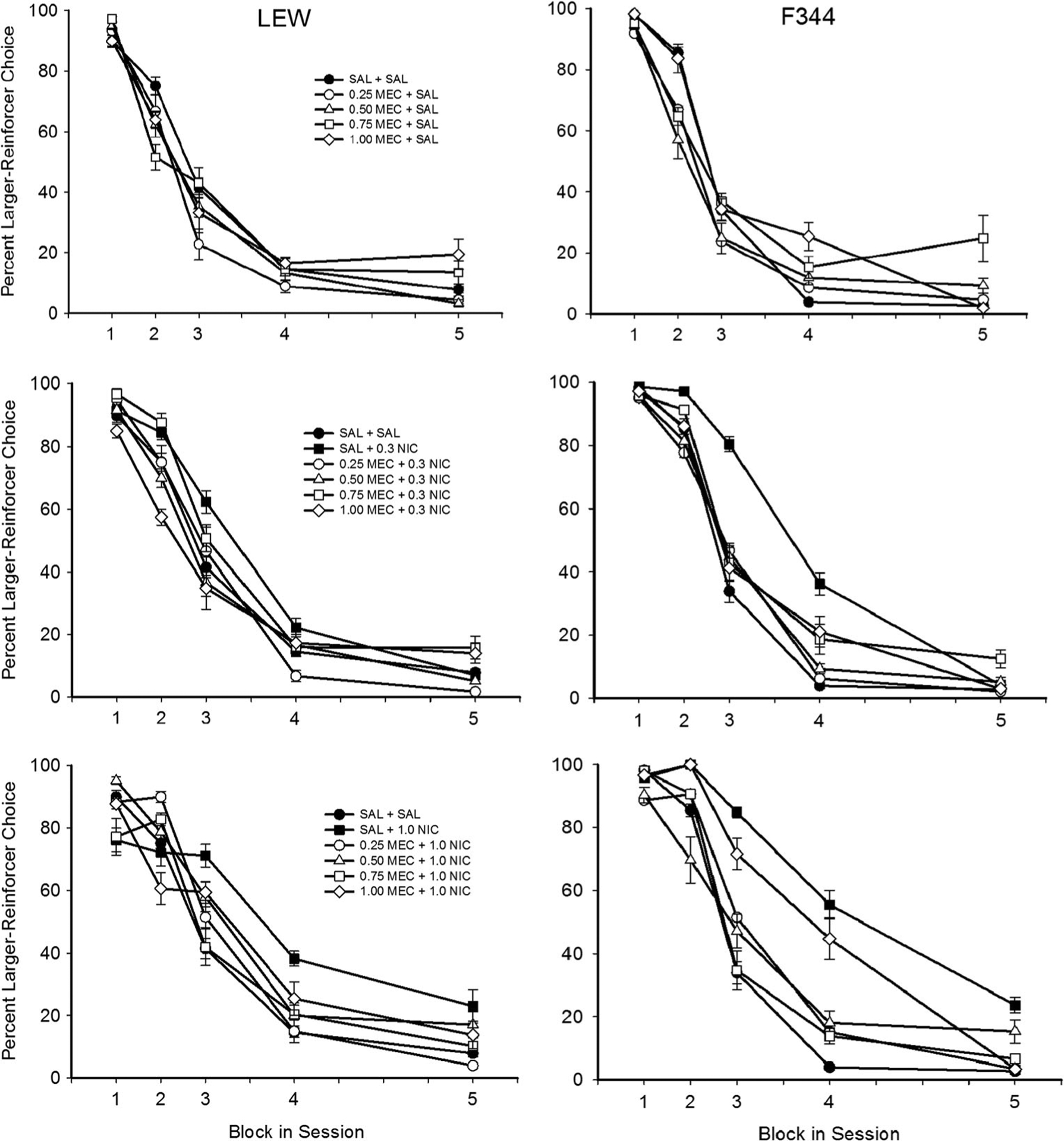
Percent larger reinforcer choice as a function of block in session (corresponding to the increasing delay to the larger reinforcer across successive blocks) across all MEC-NIC drug dose combinations during experiment 2 for LEW (left panel) and F344 (right panel) rats. Note that data for each rat strain are collapsed across delay series. Error bars represent standard error of the mean. Statistically significant differences are not indicated to facilitate clarity of the figure—see text for a description of significant pairwise comparisons
AUC
Figure 4 shows mean AUC for each dose combination following MEC pretreatment. Collapsed across rat strain, MEC did not affect AUC when administered in combination with SAL. NIC significantly increased AUC at the 1.0 mg/kg dose relative to SAL only, and this increase was attenuated significantly by 0.25, 0.50, and 0.75 mg/kg MEC. Similarly, 0.3 mg/kg NIC significantly increased AUC relative to SAL only, and this increase was attenuated significantly by 0.25 and 0.50 mg/kg MEC. Percent change in AUC at each dose combination following MEC pretreatment for individual subjects is shown in Table 4. In general, mean percent change in AUC for LEW responding on the 40-s delay series was greater than for LEW responding on the 16-s delay series regardless of dose combination.
Fig. 4.
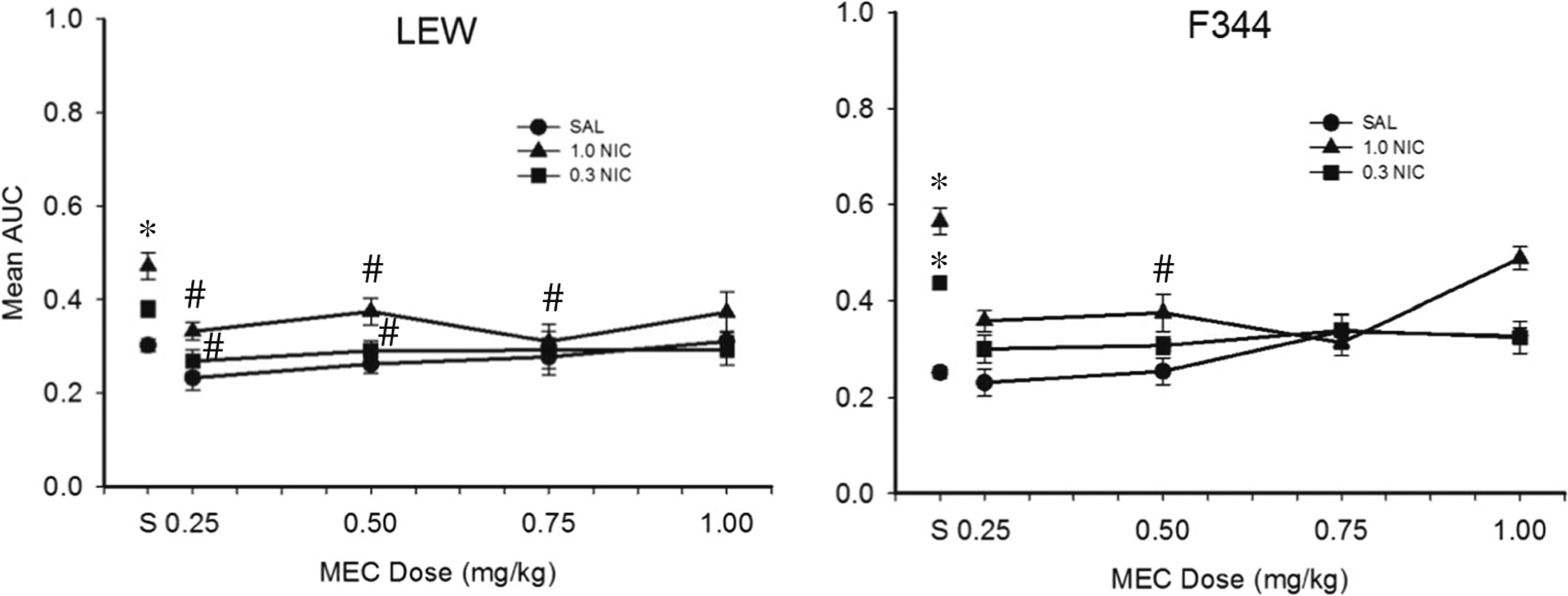
Mean AUC for LEW and F344 across all MEC-NIC drug dose combinations during experiment 2. “S” corresponds to sessions in which SAL-SAL was administered. Note that data for each rat strain are collapsed across delay series. Error bars represent standard error of the mean. Asterisks represent a statistically significant difference from SAL-SAL. Pound symbols represent a statistically significant difference from SAL-NIC (p < 0.05)
Table 4.
Percent change in AUC during MEC pretreatment for individual rats at each NIC dose
| SAL | 0.3 NIC | 1.0 NIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEC (mg/kg) | 0.25 | 0.50 | 0.75 | 1.0 | 0.25 | 0.50 | 0.75 | 1.0 | 0.25 | 0.50 | 0.75 | 1.0 |
| Subject ID | ||||||||||||
| JOL1 (16) | 13 | 17 | − 51 | 15 | 20 | 35 | 39 | − 17 | − 34 | − 25 | − 28 | − 04 |
| JOL2 (40) | 37 | 42 | 42 | 11 | − 61 | − 71 | − 66 | − 81 | − 39 | − 52 | − 55 | − 63 |
| JOL3 (16) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| JOL4 (40) | 62 | 42 | 40 | − 16 | 23 | 29 | − 100 | 09 | 50 | 72 | − 46 | 81 |
| JOL5 (16) | − 26 | − 20 | 27 | 52 | − 11 | − 36 | − 07 | 17 | − 25 | − 23 | − 06 | − 58 |
| JOL6 (40) | 53 | − 10 | 15 | − 112 | − 83 | − 46 | − 23 | − 33 | − 51 | − 18 | − 55 | 01 |
| Mean | 28 | 14 | 14 | − 10 | − 22 | − 18 | − 32 | − 21 | − 20 | − 12 | − 37 | − 09 |
| SEM | 14 | 12 | 20 | 25 | 19 | 19 | 24 | 18 | 16 | 30 | 11 | 26 |
| JOF1 (16) | − 12 | 73 | 19 | − 28 | − 22 | − 45 | − 38 | − 45 | − 95 | − 80 | − 46 | − 22 |
| JOF2 (16) | 05 | 16 | − 03 | 2 | − 38 | − 29 | − 17 | 58 | − 35 | − 29 | − 47 | 13 |
| JOF3 (16) | 33 | − 29 | 18 | − 31 | − 44 | − 19 | 23 | − 67 | − 31 | − 59 | − 50 | − 72 |
| JOF4 (16) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| JOF5 (16) | 04 | − 60 | − 208 | − 21 | 11 | − 02 | − 44 | − 28 | − 21 | 09 | − 23 | 02 |
| JOF6 (40) | − 26 | − 28 | − 22 | − 138 | − 47 | − 50 | − 41 | − 42 | − 02 | 48 | 23 | 55 |
| Mean | 01 | − 04 | − 55 | − 43 | − 28 | − 30 | − 24 | − 24 | − 33 | − 25 | − 39 | − 06 |
| SEM | 32 | 29 | 54 | 22 | 19 | 13 | 17 | 22 | 13 | 25 | 18 | 18 |
Percent change values at each dose of MEC pretreatment refers to change from the corresponding NIC-SAL dose combination. Values in parenthesis next to subject IDs refer to the delay series on which individual rats responded
Slope
Average log k values at each dose combination following MEC pretreatment are shown in Table 5. Post hoc tests revealed that 0.3 mg/kg NIC significantly reduced k relative to SAL only, suggesting that sensitivity to delayed reinforcement was reduced. No other pairwise comparisons were significant across doses of MEC within each NIC dose, suggesting that MEC did not attenuate effects of NIC on log k.
Table 5.
Mean slope estimates during experiment 2 at each drug dose combination
| Log k | ||
|---|---|---|
| Mean | SEM | |
| MEC pretreatment | ||
| SAL | ||
| SAL | − 0.883 | 0.05 |
| 0.25 | − 0.695 | 0.09 |
| 0.50 | − 0.769 | 0.09 |
| 0.75 | − 0.952 | 0.24 |
| 1.00 | − 0.880 | 0.09 |
| 0.3 NIC | ||
| SAL | − 1.169 | 0.05 |
| 0.25 | − 0.815 | 0.13 |
| 0.50 | − 0.877 | 0.07 |
| 0.75 | − 1.015 | 0.07 |
| 1.00 | − 0.894 | 0.11 |
| 1.0 NIC | ||
| SAL | − 1.522 | 0.12 |
| 0.25 | − 1.075 | 0.05 |
| 0.50 | − 1.098 | 0.05 |
| 0.75 | − 1.050 | 0.09 |
| 1.00 | − 1.177 | 0.11 |
| SCOP pretreatment | ||
| SAL | ||
| SAL | − 0.883 | 0.05 |
| 0.01 | − 0.673 | 0.04 |
| 0.03 | − 0.755 | 0.10 |
| 0.056 | − 0.944 | 0.13 |
| 0.3 NIC | ||
| SAL | − 1.169 | 0.05 |
| 0.01 | − 0.036 | 0.10 |
| 0.03 | − 1.017 | 0.14 |
| 0.056 | − 0.827 | 0.16 |
| 1.0 NIC | ||
| SAL | − 1.522 | 0.12 |
| 0.01 | − 1.476 | 0.16 |
| 0.03 | − 1.256 | 0.14 |
| 0.056 | − 0.996 | 0.09 |
Omissions
Average omitted trials per session and statistical results are shown in Table 6. A Friedman nonparametric test for within-subjects effects revealed a significant effect of drug dose on number of omitted trials per session, X2(14) = 280.53, p < 0.001. Wilcoxon signed-rank tests with Bonferroni corrections were applied, resulting in a significance level of p < 0.004. MEC produced a dose-dependent increase in number of omitted trials within each NIC dose. Significant Mann-Whitney U tests for between-subjects effects revealed that differences between LEW and F344 were rare, but when they did occur, F344 omitted a greater number of trials.
Table 6.
Average omitted trials per session during experiment 2 and statistical results from Wilcoxon signed-rank tests
| Mean | SEM | Z | p value | |
|---|---|---|---|---|
| MEC pretreatment | ||||
| SAL | ||||
| SAL | 0.472 | 0.131 | ||
| 0.25 | 1.044 | 0.176 | − 3.367 | < 0.001 |
| 0.50 | 1.857 | 0.278 | − 4.124 | 0.003 |
| 0.75 | 5.892 | 0.756 | − 6.341 | < 0.001 |
| 1.00 | 7.275 | 0.667 | − 3.274 | 0.001 |
| 0.3 NIC | ||||
| SAL | 0.333 | 0.056 | ||
| 0.25 | 0.626 | 0.050 | − 5.983 | < 0.001 |
| 0.50 | 1.403 | 0.284 | − 5.924 | < 0.001 |
| 0.75 | 3.703 | 1.004 | − 5.643 | < 0.001 |
| 1.00 | 3.567 | 0.691 | − 5.673 | < 0.001 |
| 1.0 NIC | ||||
| SAL | 0.042 | 0.016 | ||
| 0.25 | 2.859 | 0.509 | − 4.006 | < 0.001 |
| 0.50 | 5.393 | 0.811 | − 4.115 | < 0.001 |
| 0.75 | 4.627 | 1.027 | − 5.194 | < 0.001 |
| 1.00 | 6.250 | 0.833 | − 5.126 | < 0.001 |
| SCOP pretreatment | ||||
| SAL | ||||
| SAL | 0.472 | 0.131 | ||
| 0.01 | 1.997 | 0.312 | − 4.166 | < 0.001 |
| 0.03 | 3.501 | 0.488 | − 6.080 | < 0.001 |
| 0.056 | 6.936 | 0.547 | − 6.269 | < 0.001 |
| 0.3 NIC | ||||
| SAL | 0.333 | 0.056 | ||
| 0.01 | 4.807 | 0.579 | − 6.258 | < 0.001 |
| 0.03 | 6.428 | 0.729 | − 6.160 | < 0.001 |
| 0.056 | 7.609 | 0.768 | − 6.661 | < 0.001 |
| 1.0 NIC | ||||
| SAL | 0.042 | 0.016 | ||
| 0.01 | 3.367 | 0.499 | − 5.846 | < 0.001 |
| 0.03 | 6.771 | 0.835 | − 6.160 | < 0.001 |
| 0.056 | 5.461 | 0.545 | − 6.683 | < 0.001 |
Italic values denote a statistically significant difference from the corresponding NIC-SAL combination, p < 0.004 for MEC and p < 0.005 for SCOP
SCOP pretreatment
Percent larger reinforcer choice
Figure 5 shows delay-discounting curves for each dose combination following SCOP pretreatment. For LEW, SCOP reduced larger reinforcer choice at 0.01 and 0.056 mg/kg doses during the second, third, and fourth trial blocks relative to the SAL only. For F344, 0.03 mg/kg SCOP significantly reduced larger reinforcer choice during the second trial block and 0.056 mg/kg SCOP significantly reduced larger reinforcer choice during the first trial block (0-s delay), while also increasing larger reinforcer choice during the third, fourth, and fifth trial blocks relative to the SAL only. Effects of SCOP on choice during the 0-s delay block suggest that 0.056 mg/kg SCOP reduced sensitivity to reinforcer magnitude for F344. For LEW, increases in larger reinforcer choice produced by 1.0 NIC was attenuated significantly by 0.056 mg/kg SCOP. For F344, increases produced by 1.0 NIC were attenuated significantly by 0.01, 0.03, and 0.056 mg/kg SCOP. In addition, increases produced by 0.3 NIC for F344 were attenuated significantly by 0.03 and 0.056 mg/kg SCOP, but only in the third block of trials. In addition, percent larger reinforcer choice during the 0-s block was not affected by any other drug dose combination following SCOP pretreatment (A parameter), suggesting that reinforcer magnitude was not affected.
Fig. 5.
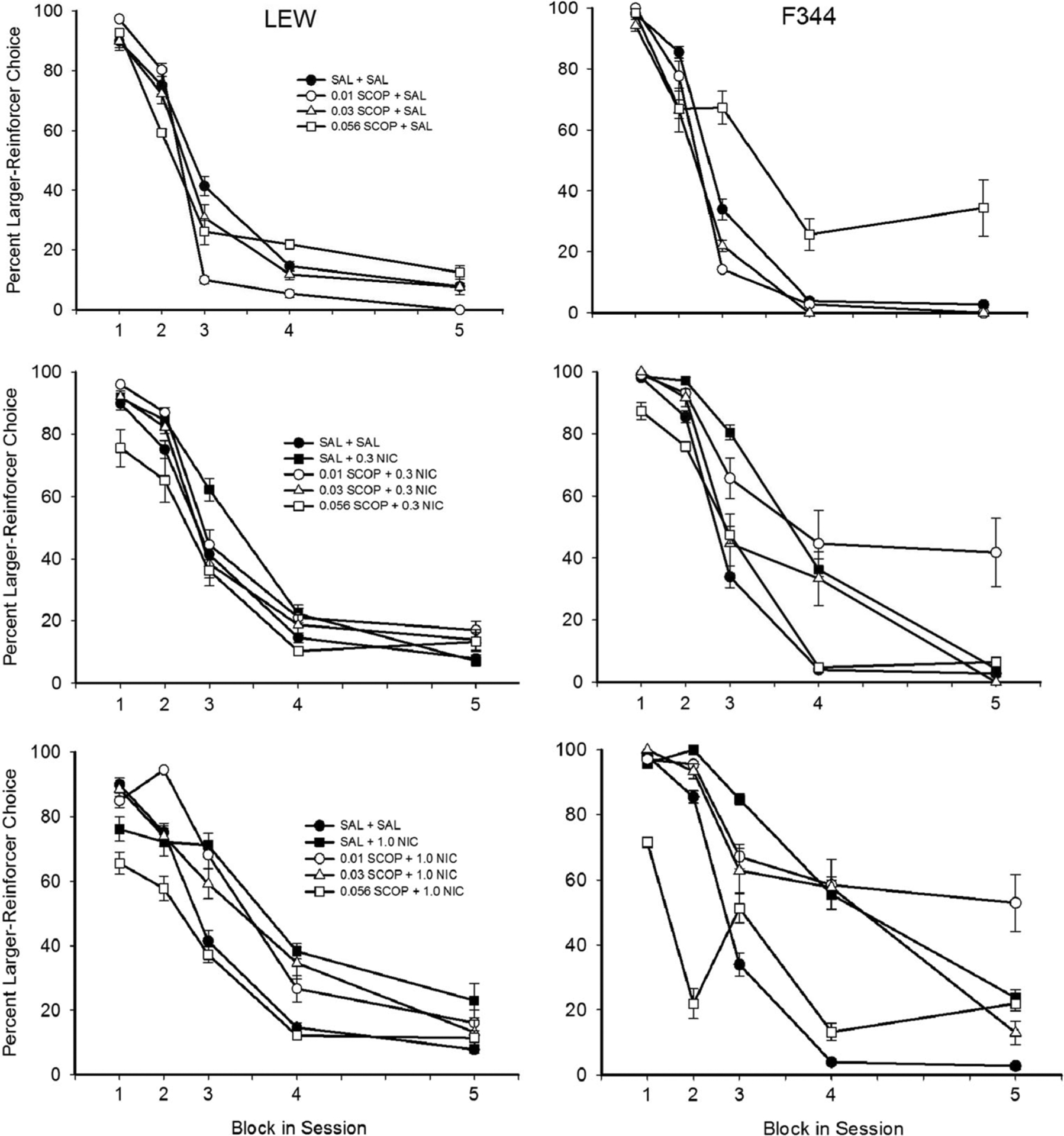
Percent larger reinforcer choice as a function of block in session (corresponding to the increasing delay to the larger reinforcer across successive blocks) across all SCOP-NIC drug dose combinations during experiment 2. Note that data for each rat strain are collapsed across delay series. Error bars represent standard error of the mean. Statistically significant differences are not indicated to facilitate clarity of the figure—see text for a description of significant pairwise comparisons
AUC
Figure 6 shows mean AUC for each dose combination following SCOP pretreatment. SCOP did not affect AUC for LEW relative to SAL only. In contrast, 0.056 SCOP significantly increased AUC for F344 relative to SAL. For LEW, increases produced by 1.0 NIC were attenuated by 0.03 and 0.056 mg/kg SCOP. For F344, increases produced by 1.0 NIC were attenuated by 0.056 mg/kg SCOP only. In addition, increases produced by 0.3 NIC for F344 were not attenuated by any dose of SCOP. Percent change in AUC at each dose combination following SCOP pretreatment for individual subjects is shown in Table 7. In general, mean percent change in AUC for LEW responding on the 40-s delay series was greater than for LEW responding on the 16-s delay series.
Fig. 6.
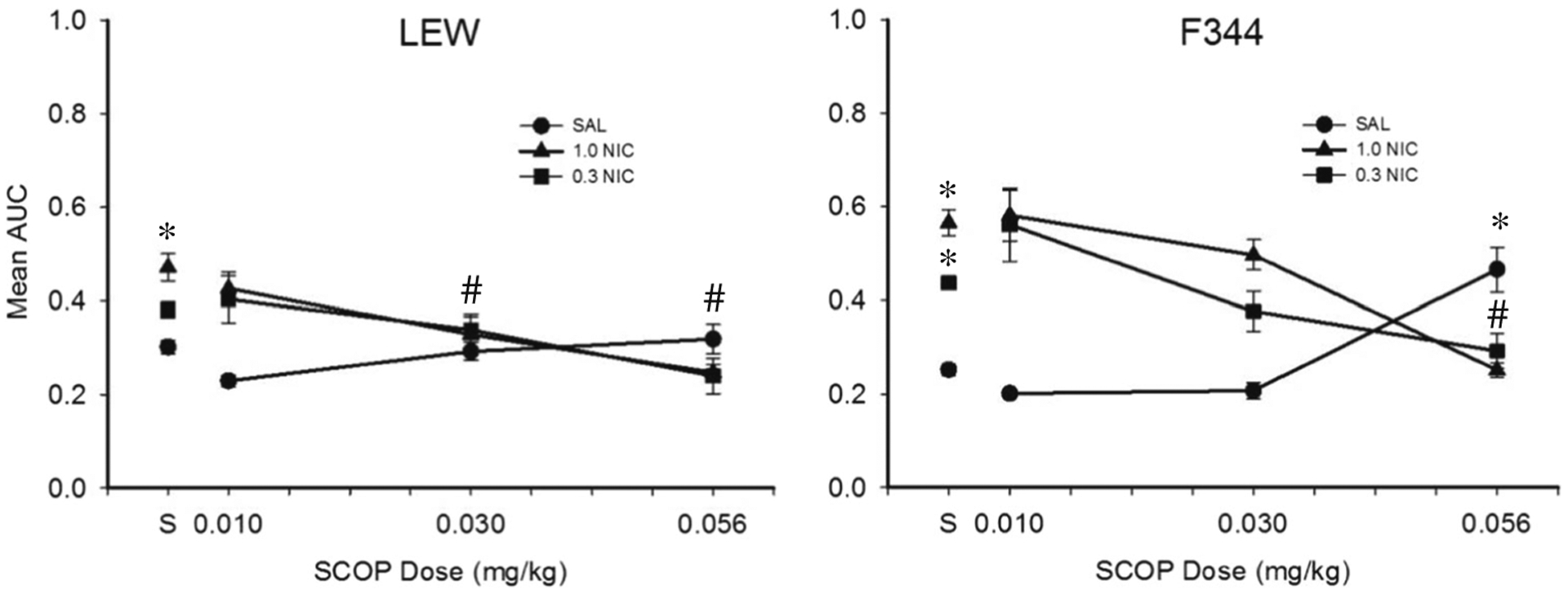
Mean AUC for LEW and F344 across all SCOP-NIC drug dose combinations during experiment 2. “S” corresponds to sessions in which SAL-SAL was administered. Note that data for each rat strain are collapsed across delay series. Error bars represent standard error of the mean. Asterisks represent a statistically significant difference from SAL-SAL. Pound symbols represent a statistically significant difference from SAL-NIC (p < 0.05)
Table 7.
Percent change in AUC during SCOP pretreatment for individual rats at each NIC dose
| SAL | 0.3 NIC | 1.0 NIC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SCOP (mg/kg) | 0.01 | 0.03 | 0.056 | 0.01 | 0.03 | 0.056 | 0.01 | 0.03 | 0.056 |
| Subject ID | |||||||||
| JOL1 (16) | 21 | 07 | 05 | 49 | 57 | 34 | − 07 | − 26 | − 57 |
| JOL2 (40) | − 20 | − 98 | 13 | − 41 | − 38 | − 91 | − 28 | − 47 | − 70 |
| JOL3 (16) | 26 | − 27 | − 40 | 52 | − 56 | − 20 | − 52 | − 100 | − 48 |
| JOL4 (40) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| JOL5 (16) | 55 | 39 | 29 | − 62 | − 27 | − 42 | − 19 | − 58 | − 41 |
| JOL6 (40) | − 05 | 41 | − 91 | 18 | − 27 | − 73 | 01 | 38 | − 54 |
| Mean | 15 | − 08 | − 17 | 03 | − 18 | − 38 | − 21 | − 39 | − 54 |
| SEM | 14 | 23 | 31 | 32 | 18 | 25 | 12 | 21 | 07 |
| JOF1 (16) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| JOF2 (16) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| JOF3 (16) | 40 | 37 | 12 | 37 | − 44 | − 26 | − 34 | − 16 | − 61 |
| JOF4 (16) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| JOF5 (16) | 01 | 35 | − 299 | − 23 | − 44 | − 16 | 13 | − 01 | − 41 |
| JOF6 (40) | − 17 | − 22 | − 157 | 78 | 22 | − 55 | 126 | 50 | − 38 |
| Mean | 08 | 17 | − 148 | 31 | − 22 | − 32 | 35 | 11 | − 47 |
| SEM | 14 | 16 | 90 | 33 | 18 | 19 | 41 | 17 | 10 |
Percent change values at each dose of SCOP pretreatment refers to change from the corresponding NIC-SAL dose combination. Values in parenthesis next to subject IDs refer to the delay series on which individual rats responded
Slope
Average log k values at each dose combination following SCOP pretreatment are shown in Table 5. Collapsed across rat strain, log k was significantly smaller following 1.0 mg/kg NIC (M = − 1.491; SEM = 0.18) than following 1.0 NIC with 0.056 mg/kg SCOP pretreatment (M = − 0.912; SEM = 0.104), suggesting that 0.056 mg/kg SCOP enhanced sensitivity to delay above and beyond that produced by 1.0 mg/kg NIC alone. No other pairwise comparisons were significant across SCOP doses within each NIC dose.
Omissions
Average omitted trials per session and statistical results are shown in Table 6. A Friedman nonparametric test for within-subjects effects revealed a significant effect of drug dose on number of omitted trials per session, X2(11) = 323.59, p < 0.001. Post hoc analyses using Wilcoxon signed-rank tests with Bonferroni corrections were applied, resulting in a significance level set at p < 0.005. In general, SCOP produced a dose-dependent increase in the number of omitted trials within each NIC dose. Significant Mann-Whitney U tests for between-subjects effects revealed that differences between LEW and F344 were rare, but when they did occur, F344 omitted a greater number of trials.
Discussion
The current study examined dose-dependent effects of NIC on delay discounting in pair-housed LEWand F344, as well as its attenuation by AChR antagonists—MEC and SCOP. When NIC was given alone during experiment 1 or in combination with SAL during experiment 2, it dose dependently reduced impulsive choice, which was indicated by a significant increase in percent larger reinforcer choice and AUC, as well as a significant reduction in log k values for both rat strains. Pretreatment with MEC (i.e., a nonselective nAChR antagonist) or SCOP (i.e., a nonselective mAChR antagonist) attenuated increases in percent larger reinforcer choice and AUC at some doses for both rat strains, although attenuation was not observed when analyzing k values. In addition, attenuation of NIC’s effects on percent larger reinforcer choice and AUC by SCOP differed between LEW and F344. Thus, the present study provides evidence that attenuation of NIC’s effects on some measures of delay discounting may not be specific to nAChR antagonism, and attenuation may depend upon biology and/or social enrichment.
The finding that NIC significantly reduced delay discounting during the present study is consistent with a previous report using individually housed LEW and F344 (Anderson and Diller 2010). Effects of NIC on k values in the present study and the absence of an effect on A parameters suggest that NIC affected sensitivity to delayed reinforcement selectively without affecting sensitivity to reinforcer magnitude. However, others have reported that acute NIC administration affects magnitude sensitivity and not delay sensitivity (Locey and Dallery 2009). It is possible that effects of NIC on A parameters were not observed in the present study due to ceiling effects of percent larger reinforcer choice during the 0-s delay block of trials, and cannot be excluded without further research.
Unlike Anderson and Diller (2010), which used singly housed rats, no strain differences were observed with pair-housed LEW and F344 following acute NIC administration. NIC significantly reduced delay discounting at 0.3 and 1.0 mg/kg doses regardless of rat strain during experiment 1, although effects of 0.3 mg/kg NIC on delay discounting of LEW were not replicated during experiment 2. This supports previous observations from our laboratory, in which strain differences in delay discounting at baseline between LEW and F344 are attenuated with paired housing (Turturici et al. under review). Because differences in response to other drugs have been observed between individually housed LEW and F344 (e.g., d-amphetamine; Huskinson et al. 2012), future research should be designed to evaluate whether strain differences in delay discounting in response to drugs other than NIC are also attenuated with paired housing.
In general, pretreatment with MEC (0.25–0.75 mg/kg) significantly attenuated NIC’s effects on percent larger reinforcer choice and AUC during the present study, but did not affect delay discounting when administered in combination with SAL. In addition, MEC did not affect reinforcer magnitude sensitivity, indicated by no significant change in percent larger reinforcer choice during the 0-s delay (A parameter). These results are consistent with previous reports of MEC’s effects on delay discounting of other rat strains (Kolokotroni et al. 2011; Mendez et al. 2012). The highest dose of MEC (1.0 mg/kg) did not attenuate effects of NIC on delay discounting in the current study, which is consistent with prior research (Kolokotroni et al. 2011). It is likely that peripheral effects of MEC on ganglion cell blockade produced impairments in general locomotor activity at this dose (e.g., Varanda et al. 1985). In support of this hypothesis, MEC produced a significant dose-dependent increase in the number of omitted trials during the present study for both rat strains.
Pretreatment with SCOP (0.03–0.056 mg/kg) also significantly attenuated effects of NIC on percent larger reinforcer choice and AUC during the present study, but did so at different doses for LEW and F344, and only affected percent larger reinforcer choice and AUC of F344 at the 0.056 mg/kg dose when administered in combination with SAL. However, effects of 0.056 mg/kg SCOP-SAL on delay discounting of F344 must be interpreted with caution, given that SCOP administration produced dose-dependent increases in omitted trials and SCOP results included three F344. The increase in omitted trials observed following SCOP administration is consistent with previous reports (Mendez et al. 2012), and the finding that SCOP reduced percent larger reinforcer choice during the 0-s delay block for F344 at 0.056 mg/kg suggests an impairment of sensitivity to reinforcer magnitude.
In terms of SCOP’s effects on attenuating the NIC-produced increases in percent larger reinforcer choice and AUC, it is possible that the higher doses of SCOP used in the present study were sufficient to produce nAChR antagonism (Falsafi et al. 2012; Schmeller et al. 1995). Alternatively, because mAChRs and nAChRs are co-localized on post-synaptic membranes of ACh and DA neurons (van der Zee et al. 1991), it is possible that SCOP antagonism at mAChRs prevented the depolarization of DA and/or ACh neurons initiated by NIC. To isolate the pharmacological mechanisms by which MEC or SCOP produced its attenuation of NIC effects on these measures of delay discounting, further research is needed. Specifically, it is unclear whether certain subtypes of nAChRs or mAChRs are involved in effects of NIC on delay discounting and/or effects of MEC or SCOP on attenuating NIC’s effects (e.g., Liu et al. 2007). In addition, differences in SCOP’s attenuation of NIC’s effects across LEW and F344 suggests that these two rat strains may differ in mAChR densities, and further study is needed to evaluate these potential neurobiological differences.
In summary, the current study suggests that attenuation of NIC’s effects on delay discounting may not be specific to nAChR antagonism and that mAChR antagonism may also attenuate effects of NIC on delay discounting. To our knowledge, this is the first study to suggest that NIC’s effects on some measures of delay discounting can be attenuated indirectly by targeting receptors other than nAChRs.
Acknowledgements
The authors thank Marissa Turturici, Matthew Eckard, Devin Galdieri, Joshua Tost, Marissa Hovey, and Christopher Iames for their diligent efforts with data collection.
Funding information
The stipend for author JEO was provided by NIGMS T32 GM081741, and this research was supported in part by the Master’s Thesis Grant (Basic Research) from the Society for the Advancement of Behavior Analysis, as well as funding from the West Virginia University Department of Psychology.
References
- Anderson KG, Diller JW (2010) Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol 21:754–764. 10.1097/FBP.0b013e328340a050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL (2005) Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav 80:387–393. 10.1016/j.pbb.2004.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P, McKee M, Reeves A, Galea G, Stuckler D (2016) Time-discounting and tobacco smoking: a systematic review and network analysis. Int J Epidemiol 10.1093/ije/dyw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49:57–71. 10.1146/annurev.pharmtox.48.113006.094742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C (2016) Fischer 344 and Lewis rat strains as a model of genetic vulnerability to drug addiction. Front Neurosci 10:13. 10.3389/fnins.2016.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128:161–170 [DOI] [PubMed] [Google Scholar]
- Falsafi SK, Deli A, Hoger H, Pollak A, Lubec G (2012) Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS One 7(2):e32082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK (1998) Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res 814:34–40 [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Potenza MN (2012) Relations among delay discounting, addictions, and money mismanagement: implications and future directions. Am J Drug Alcohol Abuse 38:30–42. 10.3109/00952990.2011.643978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG (2012) Effects of acute and chronic administration of diazepam on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol 23:315–330. 10.1097/FBP.0b013e3283564da4 [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Krebs CA, Anderson KG (2012) Strain differences in delay discounting between Lewis and Fischer 344 rats as baseline and following acute and chronic administration of d-amphetamine. Pharmacol Biochem Behav 101:403–416. 10.1016/j.pbb.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA (2011) Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology 217:455–473. 10.1007/s00213-011-2296-2 [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology 194:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locey ML, Dallery J (2009) Isolating behavioral mechanisms of intertemporal choice: nicotine effects on delay discounting and amount sensitivity. J Exp Anal Behav 91:213–223. 10.1901/jeab.2009.91-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS (2008) Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J Exp Anal Behav 90(3):333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987) An adjusting amount procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H (eds) Quantitative analysis of behavior: the effects of delay and of intervening events on reinforcement value. Erlbaum, Hillsdale, pp 55–73 [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, Setlow B (2012) Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology 224:489–499. 10.1007/s00213-012-2777-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E (2000) Effect of central 5-hydroxytryptamine depletion on intertemporal choice: a quantitative analysis. Psychopharmacology 149: 313–318 [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M (2001) Area under the curve as a measure of discounting. J Exp Anal Behav 76:235–243. 10.1901/jeab.2001.76-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeller T, Sporer F, Sauerwein M, Wink M (1995) Binding of tropane alkaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie 50:493–195 [PubMed] [Google Scholar]
- Selim M, Bradberry CW (1996) Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res 716:157–164. 10.1016/0006-8993(95)01385-7 [DOI] [PubMed] [Google Scholar]
- Stein JS, Pinkston JW, Brewer AT, Francisco MT, Madden GJ (2012) Delay discounting in Lewis and Fischer 344 rats: steady-state and rapid-determination adjusting-amount procedures. J Exp Anal Behav 97(3):305–321. 10.1901/jeab.2012.97-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KL, Giacobini E (1995) Effects of local and repeated systemic administration of (−)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res 20:753–759 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014) The health consequences of smoking—50 years of progress: a report of the Surgeon General. Altanta, GA: U.S. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health [Google Scholar]
- van der Zee EA, Streefland C, Strosberg AD, Schroder H, Luiten PG (1991) Colocalization of muscarinic and nicotinic receptors in cholinoceptive neurons of the suprachiasmatic region in young and aged rats. Brain Res 542:348–352 [DOI] [PubMed] [Google Scholar]
- Varanda WA, Aracava Y, Sherby SM et al. (1985) The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol 28: 128–137 [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2003) Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on delay-discounting task in r ats. Psychopharmacology 170:320–331. 10.1007/s00213-003-1546-3 [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW (2006) Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 16:106–114. 10.1093/cercor/bhi088 [DOI] [PubMed] [Google Scholar]
- Xie X, Arguello AA, Reittinger AM, Wells AM, Fuchs RA (2012) Role of nicotinic acetylcholine receptors in the effects of cocaine-paired contextual stimuli on impulsive decision making in rats. Psychopharmacology 223:271–279. 10.1007/s00213-012-2715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Green TA, Wedlund PJ, Dwoskin LP (2007) Nicotine increases dopamine clearance in medial prefrontal cortex in rats raised in an enriched environment. J Neurochem 103:2575–2588. 10.1111/j.1471-4159.2007.04951.x [DOI] [PubMed] [Google Scholar]


