Abstract
Despite advanced implant sterilization and aseptic surgical techniques, periprosthetic bacterial infection remains a major challenge for orthopedic and dental implants. Bacterial colonization/biofilm formation around implants and their invasion into the dense skeletal tissue matrices are difficult to treat and could lead to implant failure and osteomyelitis. These complications require major revision surgeries and extended antibiotic therapies that are associated with high treatment cost, morbidity and even mortality. Effective preventative measures mitigating risks for implant related infections are thus in dire need. This review focuses on recent developments of anti-periprosthetic infection strategies aimed at either reducing bacterial adhesion, colonization and biofilm formation or killing bacteria directly in contact with and/or in the vicinity of implants. These goals are accomplished through anti-fouling, quorum sensing-interfering or bactericidal implant surface topographical engineering or surface coatings through chemical modifications. Surface topographical engineering of lotus-leaf mimicking superhydrophobic anti-fouling features and cicada wing-mimicking, bacterium-piercing nanopillars are both presented. Conventional physical coating/passive release of bactericidal agents is contrasted with their covalent tethering to implant surfaces through either stable linkages or linkages labile to bacterial enzyme cleavage or environmental perturbations. Pros and cons of these emerging anti-periprosthetic infection approaches are discussed in terms of their safety, efficacy and translational potentials.
Keywords: Implant surface modifications, implant-related infections, surface topography, anti-fouling coatings, bactericidal coatings
Graphical Abstract
1. Introduction
Any orthopedic surgical interventions involving implantation of biomaterials and application of fixation devices run the risk of implant related infections. Despite advancement in sterilization techniques and implementation of rigorous operating room protocols, the probability of periprosthetic joint infections (PJI), for instance, remains over 1% for primary arthroplasty and up to 30% in revision arthroplasty.1–4 The elderly and immunocompromised patients are at a higher risk for acquiring implant related infections.5 Once infected, treatment for PJI could require multi-stage revision surgeries involving the removal of the infected implant and extensive surgical debridement of the surrounding tissues prior to the insertion of a replacement implant. These revisions, even in combination with prolonged high-dose systemic antibiotic therapies, could still fail to rectify the problem and result in amputations or even deaths.6–11 To date, no treatment strategies can guarantee the complete clearance of bacteria following PJI or prevent its recurrence.
Orthopedic implant related infections can be divided into three distinct categories based on the timing of infections with respect to implantation: early, delayed and late.12 Risk of bacterial infection is typically highest immediately following implant placement, with early infections generally detected within 3 months of implantation and often associated with bacteria such as S. aureus.9 This is due to the high tendency of implanted biomaterials, whether metallic alloys, polymers, ceramics or their combinations, to promote bacterial adhesion, colonization and the formation of biofilm, the dense extracellular matrices secreted by the colonized bacteria that are rich in viscous polysaccharides and proteins. The biofilm protects bacteria from host immune cells and is difficult to penetrate by conventionally administered antibiotics; it also prevents proper integration of the implant with surrounding tissues.13, 14 Delayed infections are manifested between 3 and up to 24 months post implantation12, 15 while late infection could occur >2 years post implantation. Delayed and late infections could result from either low-virulence bacteria like coagulase-negative Staphylococci9, 12 or due to bacteria invasion of the canalicular network of surrounding bone, a safe haven protecting them from immune cells until their eventual release upon the destruction of the invaded bone matrices. This later scenario has been recognized as a major cause for the difficulty in effectively treating osteomyelitis caused by S. aureus.16 Thus, development of implants with anti-fouling and/or bactericidal properties that minimize bacterial colonization and biofilm formation, timely eradicate them on implant surface and prevent their invasion and harboring within the periprosthetic tissue environment remains a top priority in combating periprosthetic infections.
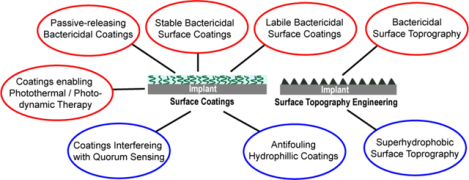
Since the early 1970s, antibiotics have been incorporated into bone cement as local antibiotic prophylaxis in cemented total joint arthroplasty.17 However, the high doses of antibiotics incorporated, coupled with their suboptimal release kinetics, present both safety and efficacy issues, with rapid burst releases risking local tissue toxicity while inadequate releases risking the development of antibiotic resistance.18, 19 In addition, this strategy is not applicable to cementless orthopedics implants.20 Considerable progress has been made in developing anti-periprosthetic infection surface modification strategies in the past 20 years.21–25 Conceptually, these surface modifications can be divided into two main categories: anti-fouling/anti-quorum sensing (QS) modifications designed to discourage bacterial adhesion/colonization/biofilm formation on surfaces in the first place (Figure 1A), and bactericidal modifications designed to kill bacteria in direct contact with the surface or in its vicinity (Figure 1B). They can be achieved by either implant surface topography engineering or chemical manipulations of implant surface coatings.26, 27 In this review, we highlight key anti-fouling, QS-interfering and bactericidal implant surface modification strategies, used alone or in synergy, with an emphasis on those demonstrating in vivo promises for preventing or mitigating the severity of periprosthetic infections. An electronic search of Google Scholar and PubMed for literature describing implant surface modifications, implant related infection, anti-fouling surfaces, bactericidal surfaces, and smart drug delivery, published in English between 2000 and 2020, was performed. The results were then screened by title and abstract to only include those evaluating in vivo or in vitro efficacy of the surface modifications. Implant surface coatings of bactericidal metal ions, antibiotics and antimicrobial peptides (AMPs) via both stable covalent linkages and labile linkers cleavable by environmental triggers are presented. Pros and cons of these strategies, relative to conventional local and systemic antimicrobial deliveries, are discussed from both safety and efficacy perspectives. Strategies for regulating bacterial adhesion or viability through the alteration of mechanical properties (e.g. stiffness28) or chemical compositions (e.g. Zn and copper alloys29) of the bulk implant are not the subject of this review. Readers are also encouraged to refer to other recent reviews emphasizing the pathogenic mechanisms underlying implant-related infections30, biofilm dispersal strategies,31 antimicrobial biomolecules,32 or surface modification strategies specific to metallic implants33, 34 including those focusing on mechanical/physical modification pf the implants.35
Figure 1.
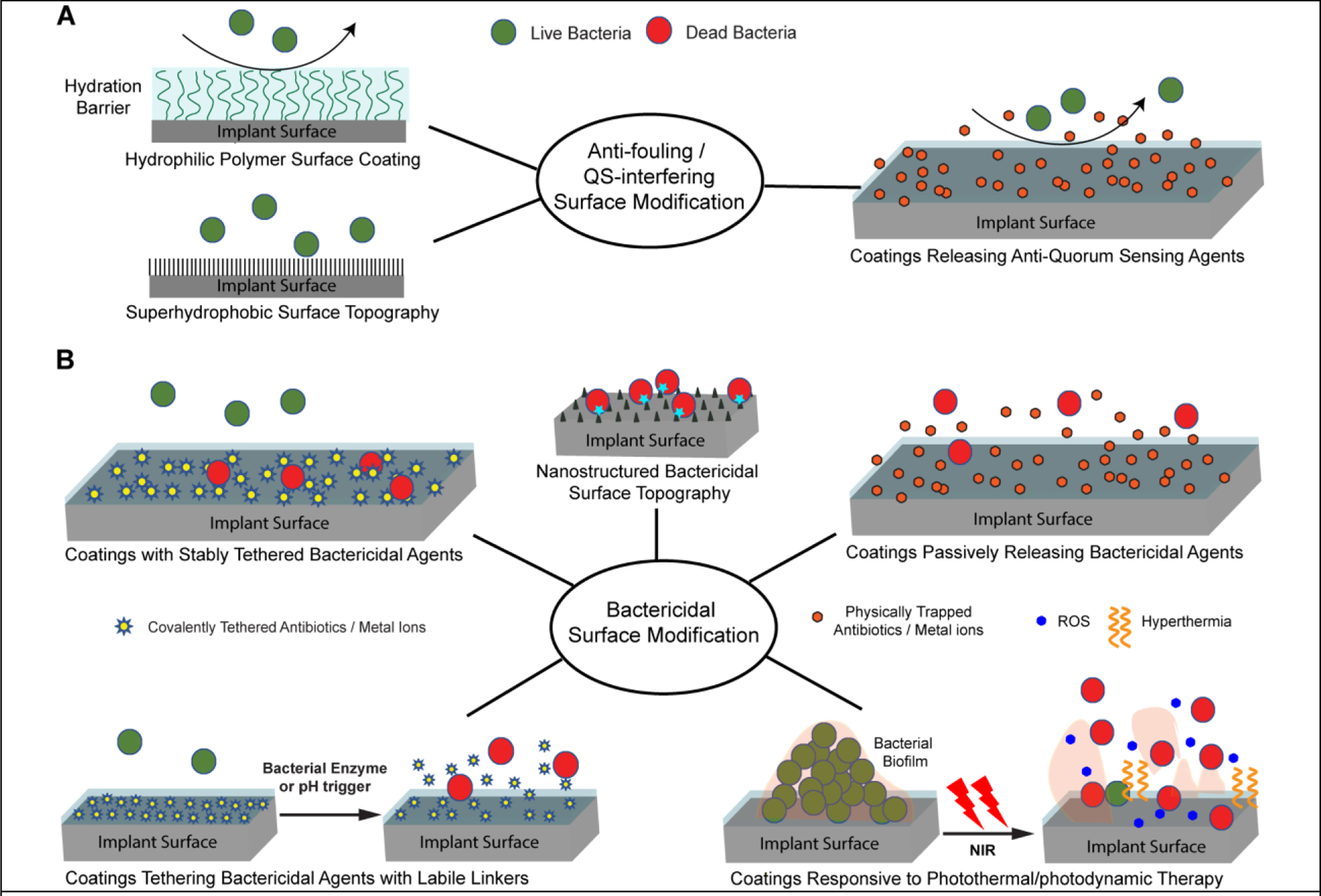
(A) Anti-fouling or QS-interfering surface modification strategies designed to inhibit bacterial adhesion, colonization and biofilm formation. These strategies involve coating surfaces with hydrophilic polymers, engineering superhydrophobic nanostructured surface topography to emulates the lotus leaf effect or applying coatings that releases agents interfering QS. (B) Bactericidal surface modification strategies designed to kill bacteria in direct contact with the surface and/or those in its vicinity. These strategies involve engineering nanostructured surface topography capable of physically rupturing bacteria, covalently tethering bactericidal agents to the surface with stable linkages or linkages labile to bacterial enzyme cleavage or pH perturbations, physically encapsulating bactericidal agents in surface coatings for passive releases, or applying photothermal/photodynamic responsive coatings (e.g. in response to near-infrared, or NIR, irradiation) designed to destruct established biofilms.
2. Anti-fouling Implant Surface Modifications
Since adhesion is the first step of biofilm formation, thus understanding bacteria-surface interactions is essential for designing anti-fouling surfaces. Bacterial cells approach implant surfaces by different means, including Brownian motion, sedimentation, movement with liquid flow, bacterial motility with cell surface appendages, and interaction with other cells to form aggregates.36 Bacterial attachment to the surface occurs through several mechanisms, among which hydrophobic and electrostatic interactions being the most common.37 The type of predominant interaction varies from one bacteria to another and may even change upon mutations. Although no single theoretical model alone can accurately predict bacterial adhesion on different surfaces due to the complex nature of bacteria-surface interactions, altering surface topographical and physiochemical properties have been pursued extensively in designing anti-fouling surfaces.38–42 Surface topography, roughness, hydrophilicity/hydrophobicity, and surface energy have all been recognized to play crucial roles in the initial adhesion of bacteria, non-specific adsorption of proteins and subsequent bacterial colonization and biofilm formation.43, 44 Some of the anti-fouling strategies reviewed here draw their inspirations from nature, such as the self-cleaning texture in lotus leaf, the superhydrophobic butterfly wings, mosquito eyes and shark skins.45 The anti-fouling surface topographical modifications discussed here involve changes in surface structures at atomic, molecular or textural levels.26, 46–48 The anti-fouling surface coatings refer to the spreading and formation of an additional layer on the implant surface achieved physically, chemically or by a combination of both.49–51 QS interference will also be discussed as they constitute an alternative means of mitigating biofilm formation on implant surfaces.
2.1. Superhydrophobic Anti-fouling Surface Topography
Surface topography and chemistry are known to alter microbial attachment to substrates.39 Generally, surface roughness promotes bacterial adhesion and subsequent biofilm formation due to increased total surface areas.52 Theoretically, a bacterium will have a maximum number of attachment points when it fits perfectly within a micro-structured depression on a rough surface, which in turn may attract more cells to colonize around it, facilitating biofilm formation.53,54
However, surface topography changes may also be accompanied with changes in surface hydrophobicity, a phenomenon first observed with natural biological surfaces. For example, lotus leaves always appear clean despite their mire environment. The self-cleaning behavior of the lotus leaf was later attributed to its hierarchical micro-nanostructures and hydrophobic wax crystalloids on its surface.55, 56 With air trapped within the rough surface topography, lotus leaf is superhydrophobic, significantly reducing the contact between water and the leaf surface as well as dirt particles and the leaf surface. When such a superhydrophobic surface tilts with a small sliding angle, water droplets roll off the surface with minimal spherical distortion, picking up loosely adhered foreign dirt particles as they roll.56 Typically, when the sliding angle (beyond which water droplets freely roll) of superhydrophobic surface is lower than 10°, the surface is considered to possess self-cleaning property.57 By contrast, a more spread water droplet on the normal surface just passes over the dust upon surface tilting.
Mimicking the lotus leaf self-clearing effect, superhydrophobic surfaces have been designed to reduce bacterial adhesion.58 Surface hydrophilicity or hydrophobicity is generally measured as liquid contact angle (θ) (equation 1)
| (1) |
where θ is Young’s contact angle, γ is the surface tension and s, l, and ν represent solid, liquid and vapor, respectively. A water contact angle of 0° means complete wetting while a contact angle of 180° means that water does not wet the substrate surface. In general, a surface with a water contact angle of >150° is considered superhydrophobic.5, 45 These surfaces exhibit significantly low adhesion forces to hydrophilic liquids like water, causing droplets to roll over with minimal distortions when tilted. During this process, bacteria and other particles on surface will presumably be removed along with the rolling liquid droplet much like dusts on the lotus leaf. To test this hypothesis, superhydrophobic surfaces were designed and tested for their anti-fouling capacity.56
Tang et al.59 studied the adhesion of S. aureus on medical grade titanium surface-engineered with varying wettability through the generation of nanotube structured TiO2 surface films using electrochemical oxidation and self-assembly techniques. Specifically, the titanium were treated by surface anodic oxidation only (NT), perfluorooctyltriethoxysilane (PTES) only (TiS), or PTES following the anodic oxidation (NTS), resulting in hydrophilic (water contact angle of 54°), hydrophobic (water contact angle of 133°), and superhydrophobic (water contact angle of 156°) surfaces, respectively. Figure 2 shows that although S. aureus adhered on all three surfaces after 2–4 h in vitro incubation, they were far more scattered on the hydrophobic and superhydrophobic surfaces, especially on the latter.
Figure 2.
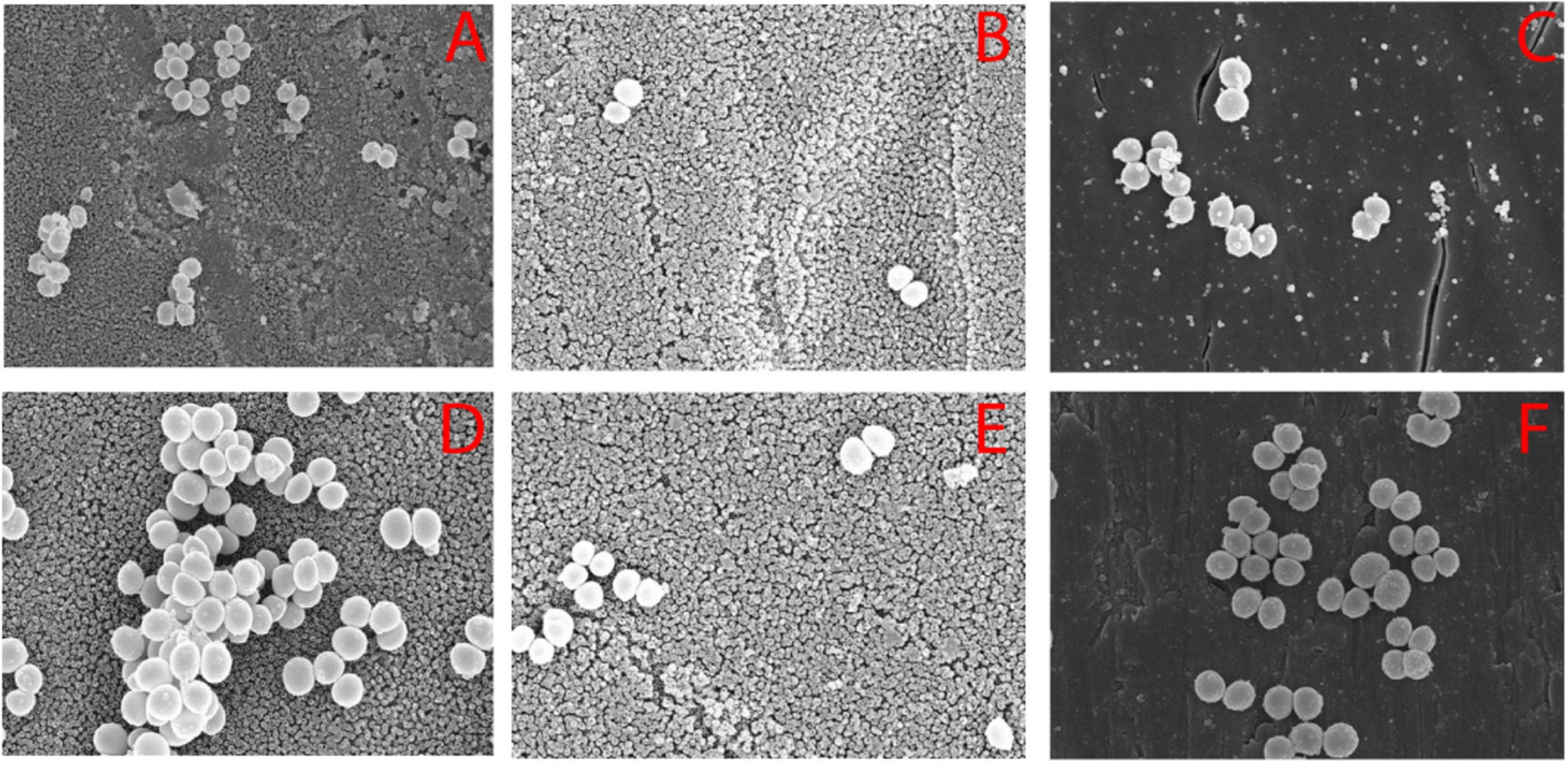
SEM images of bacteria colonies after 2h on (A) NT, (B) NTS, and (C) TiS, and after 4h (D) NT, (E) NTS, and (F) TiS (×10K). Reproduced with the permission from ref 59. Copyright 2011 Hindawi.
Besides the superhydrophobic micro- and nano-scaled surface topographical features, bacterial surface adhesions are also found to be affected by bacterial shape. For example, Fadeeva et al.58 used femtosecond laser ablation technique to produce superhydrophobic self-organized micro- and nano-structures mimicking those of the lotus leaf on titanium surfaces. The water contact angle was increased up to 166° upon the surface laser treatment (vs. 73° of the polished surface). Whereas the biomimetic superhydrophobic surface resisted the adhesion of rod-like P. aeruginosa, spherical S. aureus cells were found to still colonize on the superhydrophobic surface. The authors suggested that the spherical S. aureus may have required less attachment points to adhere on the surface compared to P.aeruginosa. Biophysical mechanisms underlying this phenomenon will need to be further investigated.
It should be noted that the air trapped within the periodical, lotus leaf-like micro/nano-structured topographical features plays a significant role in affording these surfaces anti-fouling properties. Huang et al.60 showed increased non-specific protein adsorption on superhydrophobic surface TiO2 nanotubes in the absence of the trapped air. Thus, the duration/longevity of the anti-fouling capabilities of such topographically engineered superhydrophobic surfaces in an aqueous physiological environment is a legitimate concern for their in vivo applications as the trapped air will eventually get excluded from the engineered surface over time.
2.2. Hydrophilic Anti-fouling Surface Coatings
Applying hydrophilic antifouling coatings to implants may also reduce bacterial attachment and biofilm formation by enabling the formation of a surface hydration shield37, 61–65 (Figure 1A, top left). This is in contrast to the superhydrophobic surfaces that prevent biofouling by facilitating the ready detachment/cleaning of loosely adsorbed proteins or bacteria. Although the hydrophilic surface coatings are not bactericidal in nature, they are particularly effective in minimizing the initial bacteria-implant interactions. Non-ionic poly(ethylene glycol) (PEG), amphiphilic fluoropolymers, and zwitterionic polymers are among the most common hydrophilic polymers explored as anti-fouling surface coatings.56, 66–68 Negatively charged polymers such as heparin, on the other hand, was shown to be able to promote biofilm formation by substituting extracellular DNA secreted by S. aureus,69 thus should be used with caution.
2.2.1. PEG Coating
PEG has long been appreciated for its anti-fouling properties and has been reviewed extensevely.40, 70, 71 Each ethylene glycol repeating unit in PEG can strongly bind to one water molecule via H-bonding, with structural water molecules bridging the ether oxygens along the helical PEG chain.72–74 This results in a highly hydrated layer that serves as a steric barrier to approaching biofoulants such as proteins or bacteria.75 The high mobility and large exclusion volume of PEG chains further contribute towards the anti-fouling nature of PEG-coated surfaces.40 Khoo et al.76 used a high affinity titanium-binding 22-mer peptide containing three repeats of HKH to graft PEG on a titanium surface. This peptide selectively binds titanium with sub-micromolar binding affinities over stainless steel, gold, polystyrene, or SiO2. The PEGylated-peptide coating on titanium surface efficiently blocked the adsorption of fibronectin and significantly reduced the extent of S. aureus attachment in vitro. Buxadera-Palomero et al.77 compared the anti-fouling properties of PEG-coated titanium surface prepared using three different techniques, plasma polymerization, electrodeposition and silanization. All three coatings significantly reduced non-specific protein adsorption (bovine serum albumin) and bacterial (S. sanguinis and Lactobacillus salivarius) adhesion. The tendency of PEG to be oxidized under sterilization conditions or in vivo78, 79 in the presence of oxygen and transition metal ions,80, 81 however, could lead to its eventual fouling and compromise their long-term anti-fouling performances.
2.2.2. Zwitterionic Coating
Zwitterionic polymers have been explored as alternative anti-fouling surface coatings due to their improved oxidative stability over PEG. Zwitterionic materials are characterized with an equal number of positively and negatively charged residues that maintain an overall electrical neutrality. The strong dipoles and electrostatic interactions of these charged residues facilitate the formation of a tightly bound hydration layer resisting biofoulant deposition. Naturally occurring zwitterionic lipid phosphatidylcholine that constitutes a significant fraction of the outer membrane of many cell types has long been recognized for its anti-fouling properties (e.g. anti-thrombogenic properties of the outer membrane of erythrocyte82, 83). Inspired by the zwitterionic membrane lipid, researchers have explored phosphorylcholine-based polymers84 and synthetic zwitterionic polymers to create anti-fouling surfaces for resisting bacterial adhesion.85–89 For example, Cheng et al.89 showed that zwitterionic poly(sulfobetadine methacrylate) (pSBMA) coated on gold or glass surfaces inhibited both Gram-positive (S. epidermis) and Gram-negative (P. aeruginosa) bacteria adhesion and biofilm formation in vitro. For orthopedic applications, another potential advantage of applying zwitterionic coatings as opposed to their non-ionic counterparts (e.g. PEG) to implant surfaces is the unique ability of zwitterions to attract oppositely charged precursor ions under pro-mineralization conditions, thereby promoting templated biomineralization and osteointegration as we demonstrated on both 2D surfaces90 and within 3D matrices.91, 92
2.3. Synergy of Anti-fouling Coating and Systemic Delivery of Antibiotics
One limitation of anti-fouling coatings is that they are not bactericidal, thus cannot eradicate infections in the periprosthetic tissue environment. We recently demonstrated that zwitterionic anti-fouling implant coatings, however, can significantly enhance the efficacy of systemic antibiotic injections in combating periprosthetic infections.93 We first confirmed that zwitterionic polymer brush pSBMA surface-grafted from Ti6Al4V (Ti-pSBMA) significantly reduced S. aureus adhesion both in vitro and in vivo. However, Ti-pSBMA intramedullary (IM) pin inserted in S. aureus-inoculated murine femoral canal expectedly did not kill the bacteria to prevent bone infections, as evidenced by the significant increase in cortical thickness (Ch. T.) and reductions in bone volume fraction (BVF) and bone mineral density (BMD) compared with uninfected control femurs (Figure 3). Meanwhile, a single systemic vancomycin injection 7 days after inserting an uncoated IM pin into the S. aureus-inoculated femoral canal (Ti6Al4V+VAN) also failed to prevent bone infections. However, when the pSBMA coating was combined with the single systemic vancomycin injection (Ti-pSBMA+VAN), it significantly inhibited both S. aureus colonization on implant surface and periprosthetic infections in the mouse femur (vs. Ti-pSBMA or Ti6Al4V+VAN, Figure 3). This study showed that the anti-fouling coating, by suppressing bacterial colonization/biofilm formation on implant surfaces, could make the planktonic bacteria within the periprosthetic tissue environment more susceptible to conventional systemic antibiotic treatment, thereby improving the efficacy and safety (reduced dose and frequency) of such treatments.
Figure 3.
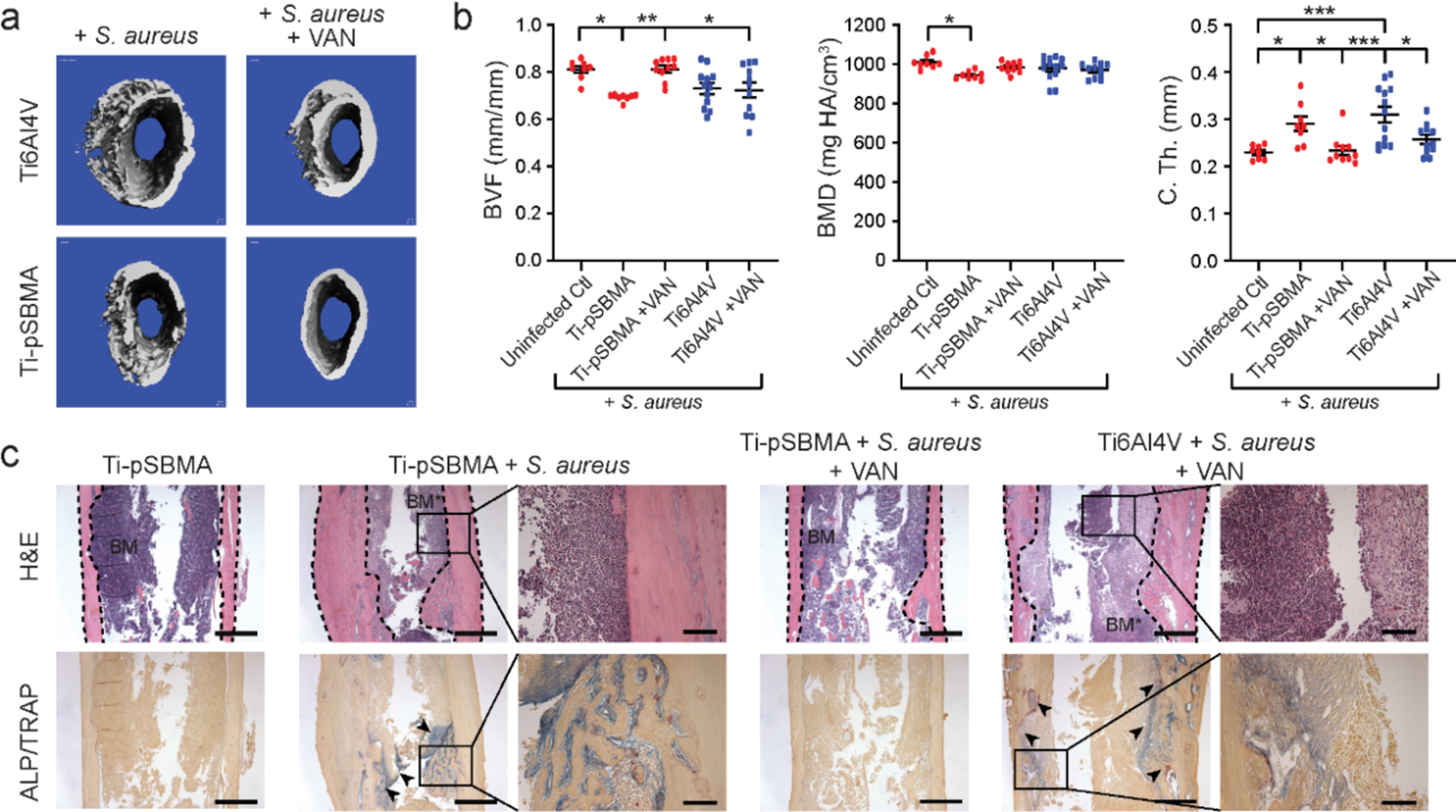
pSBMA grafted from the surface of Ti6Al4V IM pins combined with a single vancomycin injection (Ti-pSBMA+VAN) more effectively suppressed S. aureus periprosthetic infection in mouse femoral canals than pSMBA coating alone (Ti-pSBMA) or the single systemic vancomycin injection alone (Ti6Al4V+VAN). (a) μ-CT axial view of the femur at day 21 (pins contoured out). (b) μ-CT quantitation of bone volume fraction (BVF), bone mineral density (BMD) and cortical thickness (C. Th) at day 21. *P<0.05, **P<0.01, ***P<0.001. All femurs were inoculated with 40-CFU Xen 29 prior to pin insertion. Reproduced with the permission from ref 93. Copyright 2020 American Chemical Society.
2.4. Coatings Interfering with Quorum Sensing (QS)
QS is a population density dependent cell-cell signaling that triggers changes in behavior when the population reaches a critical density.94, 95 In QS, bacteria produce and release chemicals termed autoinducers as a common signal whose external concentration increases with bacteria population density. Bacterial cells detect these changes and when minimal threshold stimulatory concentration of these autoinducers are reached, they alter gene expressions and behavior in response.95 In recent years, various QS inhibitors such as cinnamaldehyde,96, 97 hamamelitannin,98 baicalin,99 silver nitrate, furanone C-30100, 101 have been used to interfere and disrupt the signaling processes in bacterial growth and subsequent biofilm formation. Kang et al.101 used poly(butyl methacrylate-co-methacryloyloxyethyl phosphate) (PBMP)/PLGA microparticles encapsulating furanone C-30 on hydroxyapatite (HA) surfaces to prevent biofilm formation. PBMA contains Ca2+-binding phosphomonoester groups to ensure good adherence to the HA surface and the PLGA-interacting butyl groups to encapsulate furanone C-30. The C-30-bearing PLGA/PBMP microparticles effectively inhibited the growth of Streptococcus mutans and its ability to form biofilms on HA surface for up to 100 h, which was much longer than either furanone C-30 in its free form or when encapsulated in PLGA microparticles.
However, the use of QS interference as a potential therapeutic approach against biofilm formation has its own challenges. Despite the shared QS mechanism among various bacteria, it is often a species-specific process.102 The primary translational challenge will be to design and synthesize agents that interfere and disrupt QS across a diverse range of bacterial species.
3. Bactericidal Implant Surface Modifications
The interest in biomaterials exerting bactericidal activities has seen an upsurge in recent decades. The bactericidal activities can be endowed via surface topography engineering and/or bactericidal agents covalently tethered to stable coatings or released from labile coatings.
3.1. Bactericidal Surface Topography
Micro/nano scale surface topography can not only be engineered to inhibit bacterial adhesion and growth but also to physically lyse bacterial cells. The initial concept of nanostructured bactericidal topography originated from the unique nano pillar patterns of Cicada (Psaltoda claripennis) wings that were postulated by Ivanova et al.103 to mechanically rupture bacterial cell walls. Later, Kelleher et al.104 studied three subspecies of cicada wings, Megapomponia intermedia, Ayuthia spectable, and Cryptotympana agulia, and found strong correlations between their bactericidal properties and their nanoscale surface topographies. Sharper and denser nanopillars on the Megapomponia intermedia wings were found to kill bacteria more efficiently, probably by inducing greater strains on the bacterial cell wall. Since this discovery, biomimetic bactericidal nanostructured surface architectures have been engineered with various biomaterials including titanium and its alloys.105–112
For example, Diu et al.107 investigated bactericidal properties of nanowires grown on titanium surfaces using an alkaline hydrothermal method. It was found that motile and Gram-negative bacteria are more susceptible to the killing by these surfaces compared to nonmotile and Gram-positive ones. The lack of mobility and the thicker cell wall found in Gram-positive bacteria have likely made them more resistant to being mechanically ruptured. Similar observations were reported by Sengstock et al.111 on titanium nanocolumnar surface structures produced using a glancing angle sputter deposition technique. It was observed that the Gram-negative, rod-shaped E. coli were killed more readily compared to the Gram-positive, sphere-shaped S. aureus. Apart from the cell wall difference, E. coli multiplies by elongation which requires in-plane movement of the bacterium attached to the surface nanostructures. The frictional force during cell division could result in damage or disruption of the cell wall. S. aureus, on the other hand, divides in three dimensions, leading to grape-like clusters or out of plane growth. This resulted in fewer daughter S. aureus cells in direct contact with the nanocolumnar titanium surface during the cell division, thus fewer cell death. Hasan et al.105 used a chlorine based reactive ion etching process to generate titanium nanopillars or black titanium. Within 4 hours of contact with the black titanium surface, 95±5% of E. coli, 98±2% of P. aeruginosa, 92±5% of M. smegmatis and only 22±8% of S. aureus attached were killed. The killing efficiency of the black titanium for S. aureus increased to 76±4% when the bacteria were allowed to adhere for 24 h (Figure 4).
Figure 4.
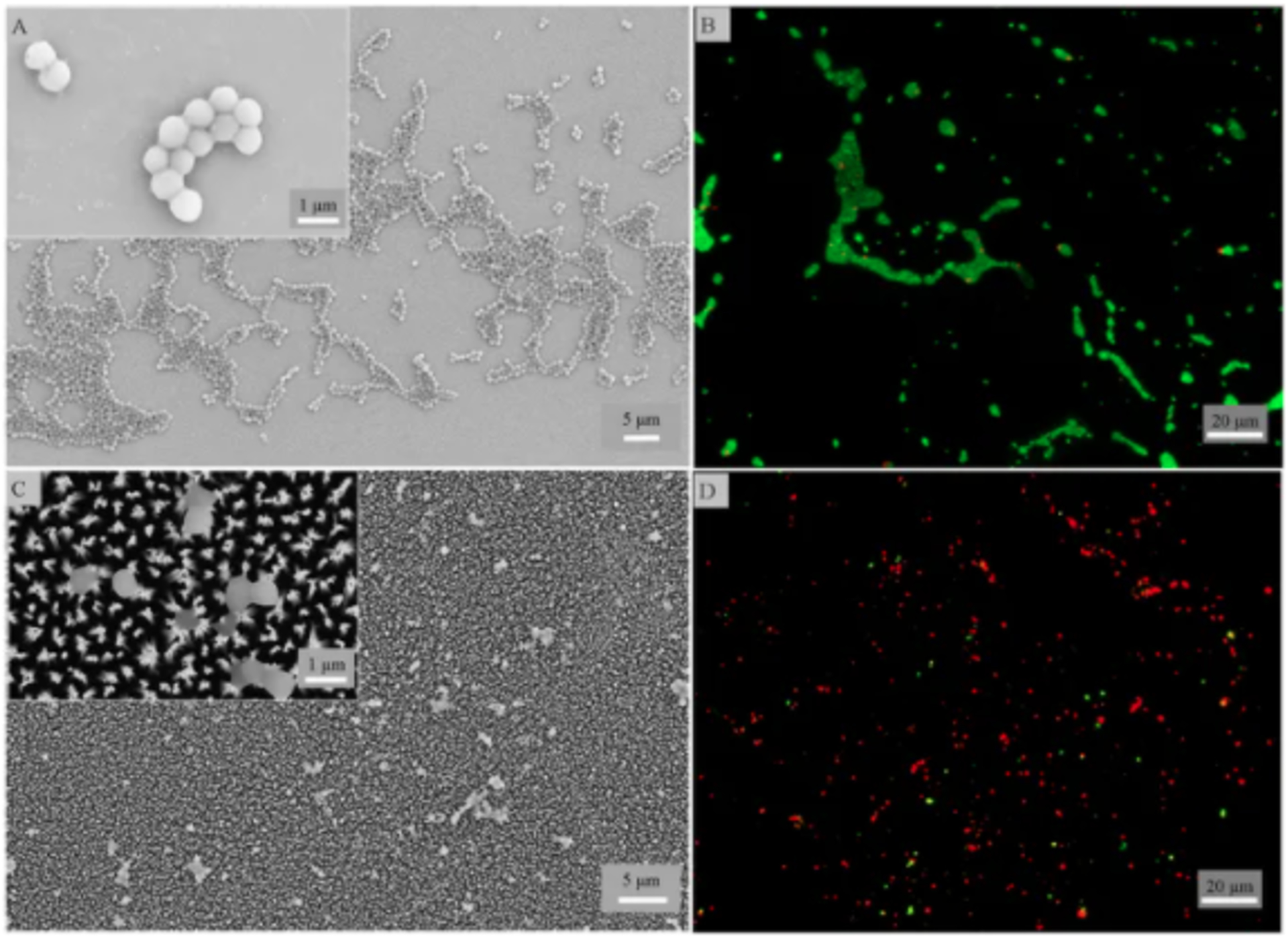
(A-D) SEM and fluorescence images of S. aureus attached for 24 h on the control (top) and black titanium (bottom) surfaces. Insets in panels A and C: enlarged views of live and dead bacteria on the respective surfaces. Reproduced with the permission from ref 105. Copyright 2017 Springer Nature.
The exact mechanisms of the bactericidal effect of these nanostructures are still under investigation. Besides the widely used explanation of physical deformation or rupturing of bacterial cell wall by the sharp nanostructures,113 physical entrapment of bacteria in-between the nanostructures may have also contributed to impeding their proliferation.109 The frictional forces exerted on the cell wall of Gram-negative bacteria during the cell division is another contributing factor to the bacterial cell death.111 A unique challenge for such bactericidal surface topographies to be implemented in vivo is to ensure their abilities to still support osteointegration. A typical reduction in bone cells proliferation on surfaces engineered with bactericidal topographies with high aspect ratio compared to unmodified implant surfaces has been reported.105, 107 A potential solution is to further functionalize these higher aspect ratio bactericidal topographies with cell receptor binding molecules. For example, Fraioli et al.114 used integrin-binding peptides to functionalize the bactericidal topographies to improve the adhesion of mesenchymal stem cells (MSCs) while preserving its bactericidal properties against P. aeruginosa. The in vivo efficacy of this approach as well as the universal adaptability (against different types of bacteria as well as “protection” against various host cells) remain established.
3.2. Stable Bactericidal Surface Coatings
Small molecule bactericidal agents and AMPs have been covalently attached to implant surfaces to enable contact killing of bacteria.71, 115 In this approach, bactericidal agents are often tethered to the surface vis either a flexible linker or a polymer for contact killing, with the latter ensuring its penetration through the thick bacterial cell wall to reach and disrupt the cytoplastic membrane (e.g. by polycations).116
Vancomycin, for instance, has been covalently tethered to surfaces for contact killing by inhibiting cell wall synthesis via direct binding to the peptidoglycan stem unit within the cell wall. For instance, Edupuganti et al.117 demonstrated that a monolayer of vancomycin covalently attached to Ti6Al4V pin surfaces via a bis(ethylene glycol) linker was effective in reducing S. aureus colonization in vitro. Similarly, Antoci et al.118 showed that a monolayer of vancomycin covalently attached to Ti6Al4V IM pin surfaces via an ethylene glycol linker was effective in reducing the bacterial colonization of S. aureus in vitro and in vivo and mitigated the severity of osteolysis of the surrounding bone in the short term. The rodent femur receiving the bacterial inoculation and unmodified titanium pin implant showed extensive periosteal reactions, enlargement of the IM canal, compromised cortical bone integrity, and extensive abscess formation after 14 days that are characteristic of periprosthetic infections/osteomyelitis. In contrast, the femur inoculated with the bacteria but inserted with the vancomycin modified IM pin showed minimal disruption of the surrounding bone by 14 days. However, the monolayer of tethered antibiotics is limited by the achievable drug density and was only able to provide limited short-term protection.118
To overcome the limited surface density of bactericidal agents achievable by monolayer functionalization, we grafted polymers with vancomycin-terminated sidechains from Ti4Al4V implant22 to significantly reduce the colonization/growth of S. aureus on the implant surface. Alkynylated vancomycin was covalently coupled through copper-catalyzed azide–alkyne cycloaddition to the azide-terminated sidechains of polymethacrylates grafted from Ti6AlV by surface-initiated atom transfer radical polymerization. Using a mouse femoral canal S. aureus infection model, we showed that with the vancomycin-bearing polymer coating, the IM pins inserted in the femoral canal significantly suppressed S. aureus colonization on the implant surface (68 CFU/pin) compared to unmodified pins (~1500 CFU/pin), >20-fold reduction, by 3 weeks. Furthermore, we showed that the bacterial counts from the retrieved pins remained low after 4 months, with 3 out of the 4 pins evaluated showing 0 CFU while one showing low counts (50 CFU/pin). However, this surface treatment did not prevent the infection from being developed in the surrounding bony tissue, underscoring the short-range protection by the vancomycin covalently tethered to surface polymer coating.
Zeng et al.119 designed an antibacterial and anti-fouling polymeric coating via a one-pot ring-opening reaction of antibiotic gentamycin and ethylene glycol species. The PEG moieties provided anti-fouling properties whereas the gentamycin exhibited antibacterial activities against both S. aureus and E. coli. However, this bifunctional surface coating still faced the same limitation of restricting the antibacterial activities to the immediate surface of the implant.
AMPs have been explored as a promising alternative to antibiotics in combating implant related infections because of its high potency against a broad spectrum of bacteria and lower propensity to develop antibacterial resistance.71, 120 Chen et al.121 silanized titanium surfaces with an alkyne-terminated linker, which was then conjugated with azido-functionalized cationic AMP (N3-PEG12-KRWWKWWRR) by copper catalyzed alkyne-azide cycloaddition (CuAAC). The AMP-modified surface exhibited 88–90% inhibition against S. aureus and E. coli within 2.5-h in vitro incubation, and the bactericidal activity declined over longer incubations. The modified surfaces exerted some degree of cytotoxicity against murine MSCs in an AMP-dose dependent manner over 24-h culture. Using a rabbit tibial metaphysis drill-hole (3-mm) model infected with S. aureus (5×106 CFU inoculation), the AMP-coated implant was shown to mitigate (although not eradicate) the periprosthetic infection after 7 days compared to that treated with the uncoated control implant. Longer-term bactericidal activity as well as potential cytotoxicity of the AMP coating against host cells need to be further evaluated. Godoy-Gallardo et al.122 used atom transfer radical polymerization to covalently attach a human lactoferrin protein-derived peptide hLF1–11, where the cationic AMP was functionalized with three 6-aminohexanoic acid (Ahx) residues as the spacer and a 3-mercaptopropionic acid (MPA) as the anchoring group [MPA-Ahx-Ahx-Ahx-GRRRRSVQWCA-NH2], to titanium surfaces. These AMP-modified surfaces exhibited good inhibition of oral bacteria S. sanguinis and L. salivarius attachment and biofilm progression in vitro. However, the scope (human foreskin fibroblasts used) and duration of the cytocompatibility evaluation (24 h) of the coating was quite limited, and the in vivo bactericidal properties are yet to be evaluated. Given the potential interaction/disruption of cationic AMPs with/to the negatively charged primary cell membranes, rigorous long-term cytocompatibility evaluations with a broad range of host cells surrounding the implant and in vivo biocompatibility evaluations of such coatings would be essential for their safe translational applications.
3.3. Labile Bactericidal Surface Coatings
An alternative approach for reducing implant related infections is through implant coatings that can release bactericidal agents.123, 124 The greatest benefits of such local release of bactericidal agents are the enhanced efficacy due to higher achievable local concentrations,125, 126 minimized systemic side effects, and the ability of the released bactericidal agents to diffuse into the periprosthetic tissue environment, thereby killing bacteria on both the implant surface and within the surrounding tissue environment. The effectiveness of these coatings is strongly dependent on the release kinetics of the bactericidal agents.
3.3.1. Metal ion Releasing Coatings
Silver/silver nitrate has been used to treat burns and chronic wounds (e.g. ulcers) for centuries.127, 128 Although silver-based treatment of bacterial infections declined after penicillin was introduced in the 1940s,129, 130 Moyer et al.131 reintroduced 0.5% silver nitrate solution to treat burn wounds in the 1960s as it was found to exert broad antibacterial property against S. aureus, P. aeruginosa, and E. coli without interfering epidermal cell proliferation. Indeed, silver/silver ion-bearing coatings have continued to attract attentions due to their broad-spectrum antimicrobial effects on both Gram-positive and Gram-negative bacteria. Although silver is chemically stable, silver ion is highly reactive and can incapacitate bacteria by binding to proteins (e.g. thiol-containing enzymes), permeating bacterial cell wall, and causing DNA condensation.132 Yavari et. al. showed that silver nitrate loaded on porous titanium implant surfaces (TiO2 nanotubes) effectively prevented S. aureus biofilm formation and decreased the number of planktonic bacteria in vitro, especially at adequate loading concentrations.133 Li et al. also showed that porous titanium loaded with silver (Ag0)-bearing gelatin microspheres inhibited bacterial growth of both S. aureus and E. coli.134
Copper/copper (II) ions also exhibit broad spectrum antibacterial activities against microorganisms such as S. aureus, E. coli, S. enterica, C. jejuni, and M. tuberculosis.135–137 In addition to the bactericidal effect, an appropriate dose of Cu2+ could also promote angiogenesis and osteogenesis.137, 138 Wang et al.139 studied the bactericidal activity of Cu2+-deposited titanium prepared using a polydopamine (PDA)-assisted surface modification technique. The obtained Ti-PDA-Cu substrate exhibited excellent antibacterial activity against S. aureus and promoted osseointegration in a rat tibia infection model.
Zinc/zinc (II) ions have also been exploited to combat orthopedic/dental implant related infections. Li et al.25 modified titanium surfaces with ZnO nanorods followed by polydopamine (PDA) and cell adhesive RGDC peptide. Using a S. aureus-infected rabbit femoral unicortical drill hole model, the Ti/ZnO/RGDC hybrid implant coating was shown to improve antibacterial efficacy, resulting in far less neutrophils and adherent bacteria in the surrounding tissue. The coating also accelerated new bone formation and implant osteointegration compared to unmodified titanium implant. Without additional controls, however, it is difficult to delineate the bactericidal contribution due to the physical puncturing of the bacteria by ZnO nanorods from that of the released Zn2+ or the osteogenic role of Zn2+ from that of PDA/RGDC.
Different mechanisms have been proposed to explain the bactericidal activity of metal ions. It has been suggested that the binding of metal ions to bacterial cell membrane, the primary site of action,140, 141 creates pits and holes.142 The increased bacterial membrane permeability results in leakage of intracellular material, shrinkage of cell membrane, and ultimately cellular lysis.143 The metal ions could also interfere with the bacterial gene replication and promote the generation of bacteria-killing reactive oxygen species (ROS).144–146 For example, Li et al.146 investigated the mechanism of S. aureus inactivation by Cu2+ ions released from Ti6Al4V5Cu alloy. In addition to the disruption in bacterial cell membrane permeability, they observed an increase in ROS production and the interference to the replication of nuc (species-specific) and 16SrRNA genes (Figure 5). Finally, microbial susceptibility to Zn2+ toxicity is shown to be mediated by extracellular cation competitions that result in the inhibition of the bacterial acquisition of essential metal ions such as Mn2+ by competing for binding to solute binding proteins.147
Figure 5.
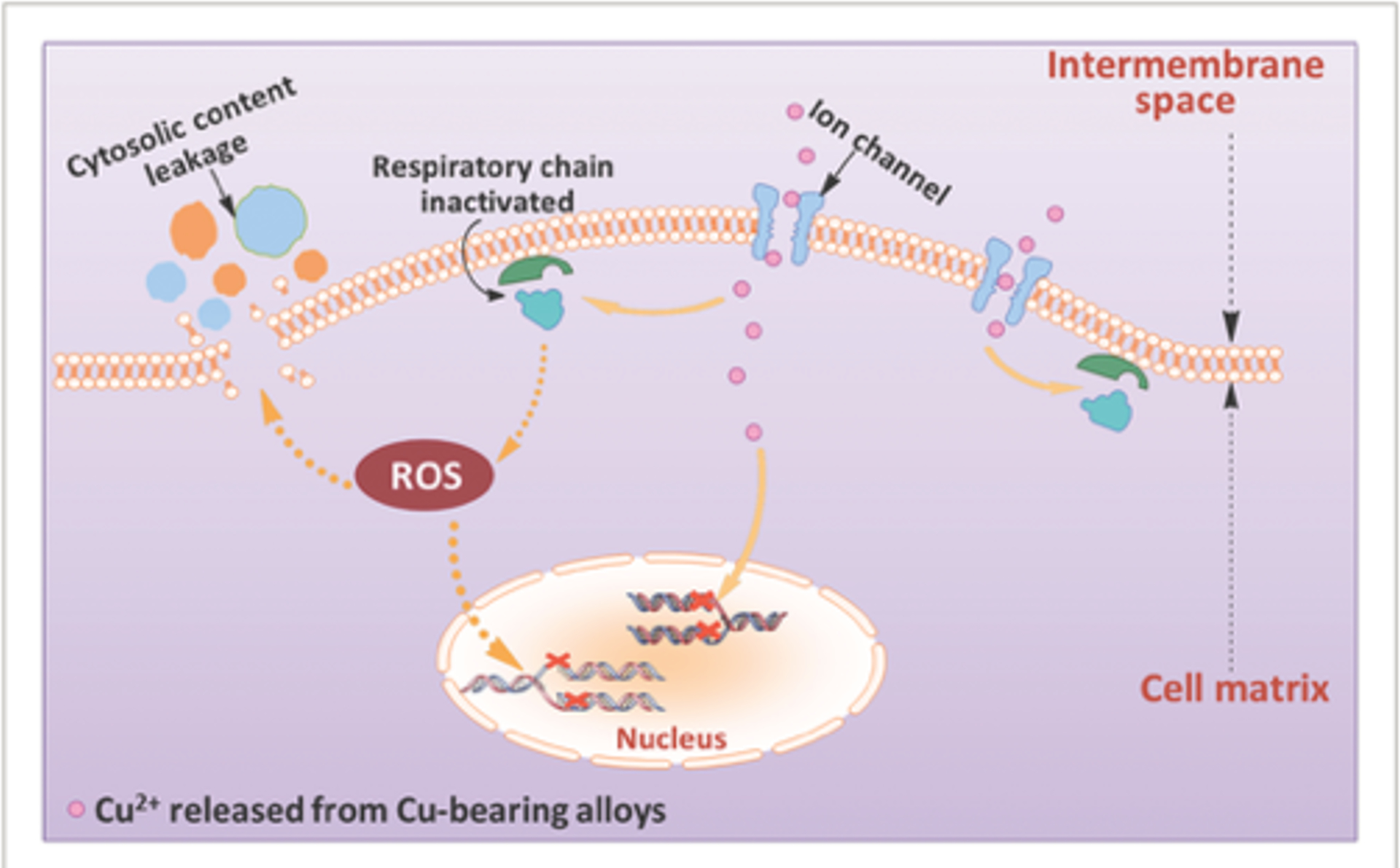
Schematic illustration of possible antibacterial mechanisms on the surface of Ti6Al4V5Cu implants. Ti6Al4V5Cu releases Cu ions. The released Cu ions could accumulate in the cell membrane affecting membrane permeability, disrupt the activity of respiratory chain, enter bacterial cells to generate ROS, and disrupt the gene replication of S. aureus. Reproduced with the permission from ref 146. Copyright 2015 Wiley Publication.
Despite the promises of these metal ion-based coatings for combating implant related infections, it remains controversial whether metal ion-mediated antibacterial activity may be inactivated in physiological fluids148 and whether a sublethal concentration of ions like silver is a cause of concern for the development of drug resistance. Another important translational consideration for this strategy is the potential cytotoxicity of the metal ions exerted to host periprosthetic tissues/cells.149,150
3.3.2. Antibiotics Releasing Coatings
Local release of antibiotics,151 if accomplished timely and in adequate yet not excessive concentrations, could directly target peri-implantitis bacteria without significant systemic side effects.152 A broad range of synthetic polymers such as PLGA,153 α-ω-functionalized PEG nanoparticles and hydrogels,154, 155 poly(2-hydroxyethyl methacrylate) (pHEMA),156 and heparin-dopamine157 have been used for localized antibiotics delivery. The effectiveness of an antibiotics-releasing system depends strongly on the rate and manner in which the drug is released, which in turn is dictated by the degradative properties of the polymeric coating or the responsiveness of the labile linkage tethering the antibiotics to its environmental trigger. Both hydrolytic degradation of polymer/hydrogel coatings and the cleavage of liable linkers sensitive to stimuli like pH or host/bacterial enzymes (e.g. nuclease and/or hyaluronidase) have been utilized.21, 158–160 Here we review a few bactericidal implant coatings based on passive, physical stimuli triggered, or enzyme triggered release of antibiotics.
3.3.2.1. Passive Release
Passive drug release systems utilize diffusion, osmotic potential, or concentration gradients as the driving forces for drug release from the coating material. For example, antibiotics release from polymethylmethacrylate bone cements is mainly achieved by diffusions through surface roughness, superficial pores, and surface erosion.161, 162 Stighter et al.163 studied the release of carbenicillin, amoxicillin, cefamandole, tobramycin, gentamicin and vancomycin from carbonated hydroxyapatite coatings on titanium surfaces, with all showing inhibition of S. aureus growth. PLGA is also widely used as an antibiotic carrier because of its excellent biocompatibility and hydrolytic degradability in vivo.164 Antibiotics embedded in PLGA coatings can be released by both diffusion and through the hydrolytic degradation of the polymer. For example, Yeh et al.153 used plasma spraying to coat PLGA loaded with vancomycin and cefuroxime on Ti6Al4V surfaces. At an optimized loading dose, the PLGA coating released 8% of the cefuroxime in the first 24 h followed by a slower, continuous release over a period of 17 days. The initial burst release was due to diffusion while the subsequent slower release was due to diffusion under the influence of PLGA degradation. However, such passive bactericidal agent releasing systems may not be adequate for achieving rapid clearance of active infections. Furthermore, the slow, continued release of antibiotics from these coatings may also increase the risk for developing bacteria resistance,18, 19, 165–167 which is a huge concern for both Gram-positive and Gram-negative bacteria.
3.3.2.2. Physical Stimuli Triggered Release
Stimuli-responsive materials have been investigated in the biomedical field for several decades including as drug release systems.168 Polymers or polymeric hydrogels can undergo volume changes, structural transformations or covalent bond cleavages in response to certain stimuli, causing subsequent release of encapsulated/tethered antibiotics.168 Physical stimuli such as pH, temperature, magnetic field, and ultrasound have all been used to trigger the release of bactericidal agents.169–175.
As bacterial metabolism produces lactic acid and acetic acid, the pH drops in the vicinity of bacterial infections can be used to trigger the release of bactericidal agents. Chemical bonds such as Schiff base,176 acetal linkage177 and metal ion coordination bonds159, 174, 178 that are stable under neutral conditions but labile at lower pH are often utilized to realize the pH-triggered release. Wang et al.159 designed a pH-responsive coordinated polymer (CP) / amine-functionalized titania nanotubes (TNTs-NH2) coating for titanium implants. The nanotubes were first filled with drugs like ibuprofen, vancomycin or Ag and then capped by CPs formed by 1,4-bis (imidazole-1-ylmethyl) benzene and Zn2+ or Ag+, where the metallic ions acted as coordination bonds between the polymer and the amino groups of the TNTs-NH2. Under acidic conditions, the coating released Zn2+ or Ag+ as a result of the protonation of the amino groups and the weakening of the coordination bonds, exerting local antibacterial activities. Tao et al.179 deposited levofloxacin (Levo)-loaded zeolitic imidazolate framework-8 (ZIF-8@Levo) nanoparticles onto collagen-modified titanium substrates by cathode electrophoresis deposition. ZIF-8, a metal organic framework based on Zn2+-imidazole coordination, can fall apart under acidic pH to release its drug contents as well as the bactericidal Zn2+. Gelatin and chitosan multilayers were spin-coated layer by layer (ZIF-8@Levo/LBL) to reduce the hydrolysis rate of ZIF-8@Levo. ZIF-8, ZIF-8@Levo and ZIF-8@Levo/LBL coatings all exhibited bactericidal properties against E. coli and S. aureus in vitro. Using a rat femoral canal S. aureus infection model, metal pins with these coatings were also shown to significantly reduce bacterial counts on both the implant and in surrounding bone compared to uncoated implants or those only coated with type I collagen. The benefit of the LBL coating on the drug release control, however, was not adequately reflected in this study.
Unlike the background leaching often observed with pH-triggered releases,180 ultrasound triggered release could lead to more rapid release far above the background when the coating is designed to minimize passive release. Noble et al.181 achieved low background leaching of ciprofloxacin from pHEMA by co-polymerizing it with hydrophobic monomer hydroxylpropyl methacrylate and further coating it with self-assembled multilayers of C12–C18 methylene chains that were predominantly crystalline and relatively impermeable. These added layers acted as a barrier for both water diffusion to and antibiotic release from the hydrogel. Upon ultrasound triggering, the ciprofloxacin release 14-fold more intense than background level was achieved.
3.3.2.3. Bacterial Enzyme Triggered Release
On demand release of antibiotics has the potential to provide timely protection of the periprosthetic tissue environment from infection while minimizing the risk for developing bacterial resistance by avoiding unwanted release of antibiotics. Proteases, lipases and nucleases have all been explored for the triggered release of antibiotics and antimicrobial agents. Johnson et al.182 engineered a PEG hydrogel for protease-triggered delivery of lysostaphin, an antimicrobial enzyme targeting the peptidoglycan of staphylococci and a known inhibitor of the growth of methicillin-resistant S. aureus (MRSA), to treat S. aureus infected, implant-fixed mouse femoral fractures. The hydrogels were prepared by mixing four-arm PEG macromers functionalized with terminal maleimide groups (PEG-4MAL), protease‐degradable 16-mer peptide crosslinker with terminal cysteines, and thiolated cell adhesive peptide RGD or GFOGER, with or without lysostaphin. The lysostaphin encapsulated in the hydrogel was shown to outperform prophylactic antibiotic or soluble lysostaphin therapy in reducing the infection in this model. However, the protease-triggered release of lysostaphin was not dependent on the presence of bacteria; its cleavage by host proteases would not have prevented undesired releases of the antimicrobial agent.
Wang et al.160 designed vertically aligned mesoporous silica coating on the surface of stainless steel for pH- and bacterial lipase-triggered antibiotic release. Alkyne-terminated surface linkers were conjugated to the mesoporous silica coating via sequential amidation and esterification. After loading antibiotics cinnamaldehyde and ampicillin into the perpendicular mesochannels, β-cyclodextrins functionalized with monopyridine and azides were covalently tethered to the alkyne-terminated surface linkers using azide-alkyne cycloaddition “click” chemistry, resulting in the “capping” of antibiotic-loaded mesochannels by the monopyridine-functionalzied cyclodextrin. It was demonstrated that the lowering of pH triggered the opening of the cyclodetrin “valve” by reorienting the monopyridine from the interior of the cyclodextrin to the exterior, enabling the release of the smaller antibiotic cargo cinnamaldehyde. Meanwhile, lipase was shown to cause the irreversible cleavage of the functionalized cyclodextrin, leading to the release of both antibiotic cargos. This dual release system was shown to inhibit the growth of S. aureus, E. coli and MRSA in vitro. It is unclear, however, whether the lipase-triggered cleavage may be confounded by the presence of host lipase activities in vivo due to the lack of specificity in the labile linker design.
Yuan et al.158 applied catechol-functionalized multilayer coatings composed of dopamine-modified hyaluronic acid and 3,4-dihydroxyhydrocinnamic acid-modified chitosan to titanium surfaces modified with TiO2 nanotubes (TNT) filled with vancomycin. The coating was designed to release vancomycin upon cleavage of hyaluronic acid by elevated hyaluronidase activities in the presence of bacterial infections. Using a rat femoral canal S. aureus infection model, the titanium IM implant applied with such coating was shown to mitigate the infections. Subsequently, this group183 also applied hyaluronic acid-gentamicin conjugates and chitosan polyelectrolyte multilayer coatings on deferoxamine (DFO) loaded TNT substrates. The coating was designed to release both the tethered gentamicin and the DFO encapsulated within the TNT upon cleavage by hyaluronidase, with the latter promoting osteogenesis and angiogenesis around the implant. In vitro cultures supported the antibacterial properties of the coating against E. coli and S. aureus, as well as the ability of the DFO-bearing coating to enhance the gene expression of osteogenic and angiogenic markers of rat bone marrow derived stromal cells. The in vivo performance of this coating in combating periprosthetic infections, however, was not evaluated. How hyaluronidases present in the host skeletal tissue environment may complicate the triggered release of antibiotics in these designs remains unaddressed.
We recently modified titanium surface using poly(ethylene glycol) dimethacrylate (PEGDMA) hydrogel to covalently attach vancomycin via an oligonucleotide linker sensitive to micrococcal nuclease (MN) of S. aureus. This design enabled the timely release of vancomycin in the presence of S. aureus to kill the bacteria both on the implant surface and within the periprosthetic tissue environment (Figure 6).21 By inoculating 40-CFU bioluminescent Xen-29 S. aureus in murine femoral canals prior to the insertion of Ti6Al4V IM pins coated with PEGDMA-Oligo control hydrogel (without vancomycin), we showed that the infection was established at 2 days post-operation and sustained over the course of 21 days as shown by bioluminescence imaging (Figure 6a, bottom panel). By contrast, no obvious bioluminescence was seen from the femurs inserted with the IM pins coated with PEGDMA-Oligo-Vanco at any time point during the 21 day follow-up (Figure 6a, top panel), and the quantification of bioluminescent signals confirmed significant reduction in intensity by >95% at 2 days post-operation compared to the control groups (Figure 6b). Consistent with the longitudinal imaging data, no bacteria were recovered from the retrieved IM pins with the PEGDMA-Oligo-Vanco coating, supporting complete eradication of surface-bound bacteria, while >500 CFU S. aureus were recovered from the retrieved IM pins with the PEGDMA-Oligo control coating (Figure 6c). Finally, μCT imaging of femurs treated with IM pins coated with the PEGDMA-Oligo control hydrogel showed clear signs of osteolysis at 3 weeks post-operation while those receiving PEGDMA-Oligo-Vanco coated IM pins exhibited completely normal cortical bone morphology (Figure 6d). This promising bacterial enzyme-triggered release strategy is being further optimized to further enhance the stability of the nucleotide linker to mammalian nucleases.
Figure 6.
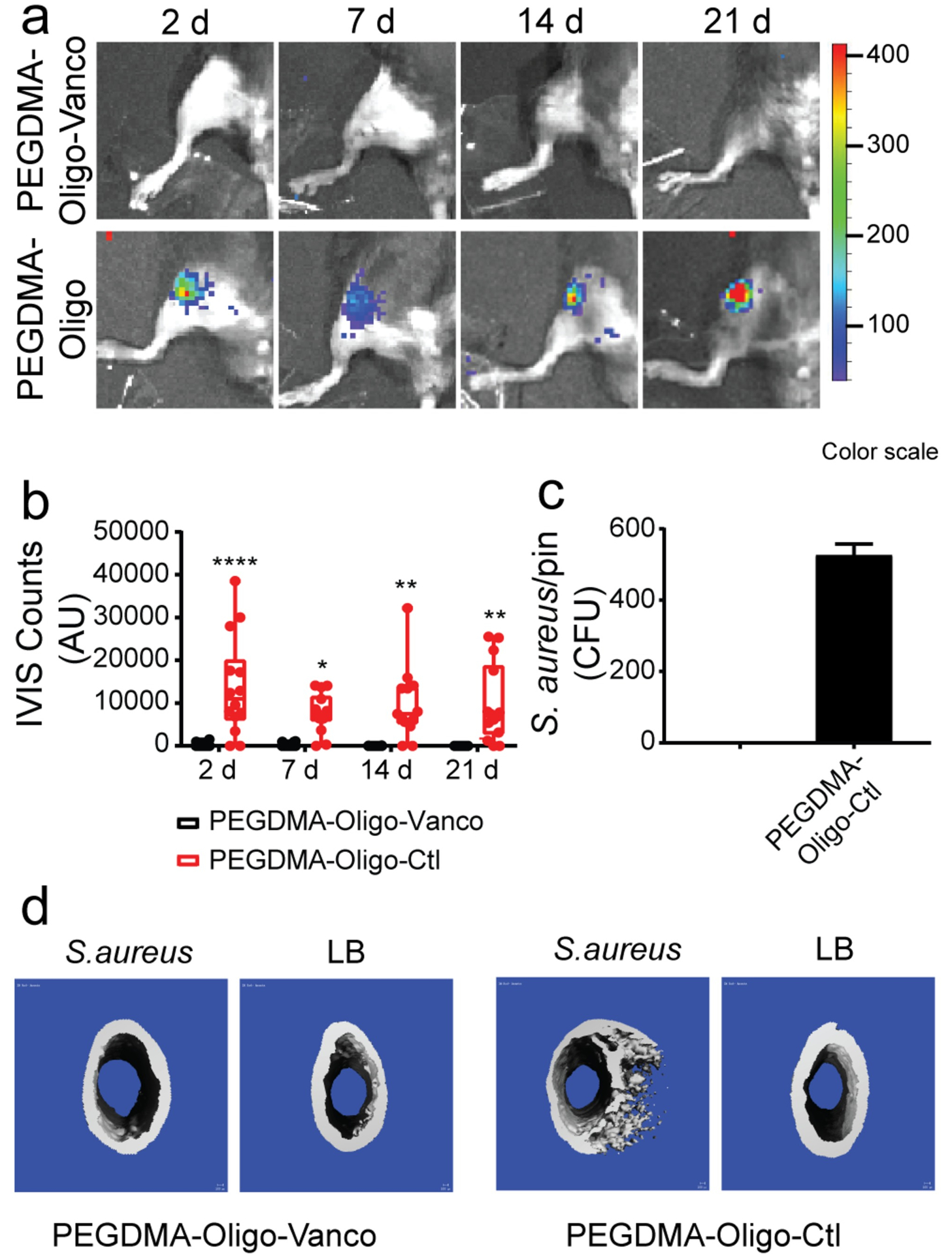
(a) IVIS images of mouse femurs injected with 40 CFU Xen-29 S. aureus and inserted with IM pins with PEGDMA-Oligo-Vanco or PEGDMA-Oligo coatings at 2, 7, 14, and 21 days. (b) Quantification of longitudinal bioluminescence signals of mouse femurs injected with 40 CFU Xen-29 S. aureus and inserted with the different hydrogel-coated pins at 2, 7, 14, and 21 days (n = 14). (c) S. aureus recovery from explanted pins at 21 days (n = 11). (d) 3D μCT axial images of the distal femoral region 21 days after the insertion of Ti6Al4V IM pins (pins excluded during contouring) with different hydrogel coatings, with or without the inoculation of 40-CFU Xen-29 S. aureus. Error bars represent standard deviations. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001 (two-way ANOVA for part b; Student’s t-test for part c). Reproduced with the permission from ref 21. Copyright 2019 American Chemical Society.
3.3.3. AMP releasing coatings
AMPs have also been released from implant coatings as free-diffusing bactericidal agents. For example, Kazemzadeh-Narbat et al.184 developed a multilayered coating consisting of TiO2 nanotubes (TNTs), a thin layer of calcium phosphate, and a phospholipid film for sustained delivery of broad-spectrum AMP HHC-36 (KRWWKWWRR-NH2). The AMP was impregnated in each layer while the lipid layer was designed as a membrane-mimicking barrier to prevent burst release of AMP while ensuring good cytocompatibility of the coating. The coating showed controlled and sustained release of the AMP over the course of a few days and was shown to inhibit the growth of both Gram-positive (S. aureus) and Gram-negative (P. aeruginosa) bacteria in vitro. Short-duration cell culture experiments (a few hours) showed good attachment of osteoblast-like cells MG-63, moderate platelet activation and adhesion, and low red blood cell lysis on the coated implant surface. Similarly, Shen et al.185 used TNTs on titanium substrates to load AMP cecropin B and sealed them with multilayer coatings of chitosan/sodium hyaluronic-cecropin B. The in vitro release of the AMP from the coating could be triggered by exogenous hyaluronidase or the hyaluronidase secreted by S. aureus. The coating exhibited good bactericidal capacity against S. aureus and S. epidermidis over 72 h. For both coating systems and other similar strategies, however, more rigorous longer-term cytocompatibility evaluations with relevant primary cells (instead of immortalized cell lines), particularly as a function of the AMP release, and appropriate in vivo bactericidal efficacy and biocompatibility evaluations of the coating would be necessary to establish their translational potentials.
Overall, despite AMPs’ bactericidal activities against both Gram-positive and Gram-negative bacteria, their applications have been limited by their in vivo instability and cytotoxicity.186, 187 Several approaches have been introduced in the AMP therapeutics design and delivery to address these limitations without compromising their bactericidal potencies. For examples, Carmona et al.188 used D-amino acids to synthesize AMPs which showed improved stability against trypsin digestion compared to those based on L-amino acids. Ron-Doitch et al.189 used liposomes to encapsulate AMPs to reduce their cytotoxicity. Finally, AMPs have also been conjugated with antibodies for more targeted delivery to bacteria of interest to reduce their systemic toxicity.190 Successful outcomes of these approaches may inspire their implementations in the design of more effective and safer AMP-releasing implant coatings.
3.4. Coatings Responsive to Photothermal/Photodynamic Therapy
The photothermal therapy (PTT) and photodynamic therapy (PDT) based on near infrared (NIR) irradiation have gained increasing attention for a range of medical interventions from cancer therapy to infection mitigation because of their minimal invasiveness and regional selectivity. PTT utilizes photothermal agents to transform optical energy into local heat (hyperthermia) while PDT delivers treatment through photochemical reactions (e.g. generation of ROS) triggered by photosensitizers. PTT has also been explored to destroy bacteria or biofilm via local hyperthermia.191–194 However, for the bactericidal efficacy to reach over 90%, local hyperthermia around 85 °C is required, which could cause significant tissue necrosis.195, 196 Combining NIR-based PTT and PDT, moderate local hyperthermia and the in situ generated ROS known for destructive actions on bacterial proteins or DNA197, 198 can be exploited to synergistically kill bacteria with reduced risk for tissue necrosis.
For example, Yuan et al.194 developed a multifunctional hybrid coating on titanium for combating established biofilms by PTT/PDT with NIR activation. Specifically, mesoporous polydopamine nanoparticles were immobilized onto amino modified titanium surface, functionalized with integrin-binding peptide RGDC to improve cytocompatibility and then loaded with photosensitizer Indocyanine Green (ICG) via π-π stacking. The functionalized surface reached a local temperature of ~50 °C after 300 s of NIR (808 nm) irradiation. Prior studies showed that local transient temperature increases should be kept below 70 °C to avoid potential skeletal tissue necrosis.199 The combination of the moderate PTT at 50 °C with the ICG-based PDT improved the in vitro inhibition of initial S. aureus adhesion on the substrate to nearly 99.7% from the 63% achieved by the moderate PTT alone. Importantly, the combinatorial PTT/PDT therapy eradicated S. aureus biofilm pre-formed on the implant in vivo with an efficiency of 95%, with the NIR treated implant still exhibiting good osteointegration, supporting mitigated negative impact on surrounding tissue. Similarly, Li et al.200 used red phosphorous (RP) and in situ generated peroxynitrite (•ONOO−), an oxidizing agent lethal towards pathogens, to eradicate MRSA biofilm on titanium surfaces via PTT/PDT. Poly(vinyl alcohol), chitosan and polydopamine and nitric oxide (NO)-releasing S-nitrosuccinic acid (RSNO)-based hydrogel was used to coat and stabilize RP deposited on the titanium surface. Under NIR irradiation, the ROS reacted with the NO released from RSNO to generate peroxynitrite. The synergistic effects of hyperthermia, ROS and peroxynitrite led to the elimination of MRSA biofilm with a 93% efficiency in vitro. In addition, the in vivo results validated excellent biofilm eradication from the titanium surfaces under NIR irradiation without compromising osteogenesis, supporting the safety of the employed PTT/PDT strategy.
4. Conclusions and Future Direction
The number of bactericidal and anti-fouling surfaces that has been designed and explored for anti-infection applications is quickly expanding. This review provides an overview of the technical developments in the past 2 decades for these translational efforts. Anti-fouling and QS-interfering surfaces have been designed to reduce/inhibit bacterial adhesion, colonization and biofilm formation in the first place. Bactericidal surfaces engineered with topographical features or stably tethered antibiotics or AMPs have been shown to disrupt bacterial cells that come in direct contact with the surface. These strategies, however, do not protect surrounding tissues from infections. The combination of anti-fouling/bactericidal surface modification with on-demand release of freely diffusing antibacterial agents, triggered by the presence of bacteria within the periprosthetic tissue environment, is highly desirable as it may provide timely bacterial clearance without suboptimal release of local bactericidal agents that could cause drug resistance. For such a design to successfully translate to practical uses, however, greater consideration should be given to designing scalable coating systems that can efficiently sense and respond to the bacterial presence and release bactericidal agents in response to enzymatic activities unique to the pathogen while remain stable against non-specific cleavages by host enzymes. Meanwhile, combining surface modifications that discourages bacterial colonization/biofilm formation with appropriately timed systemic injections of conventional antibiotics has the potential to improve both the safety (due to reduced injection dose and/or duration) and efficacy (due to greater susceptibility of planktonic bacteria to antibiotics) of the latter. Finally, strategies for combating established biofilms, such as photodynamic therapy and local delivery of biofilm matrix-degrading enzymes or surface tension-reducing surfactants that induce biofilm dispersal,31 may also find synergy with the anti-fouling/bactericidal surface modifications in discouraging the recolonization and survival of the remainder/dispersed bacteria. Overall, the need for multi-modality strategies32, 201, 202 that take into account of various stages of pathogenesis and translational considerations (cost and scalability) while minimizing negative impact on periprosthetic host tissues or encouraging implant-tissue integrations are increasingly recognized.
To expedite clinical translations, novel implant surface modification strategies should be rigorously examined for both short-term and long-term efficacies in preventing or eradicating periprosthetic infections using suitable animal infection models that replicate clinically relevant scenarios, from the type and inoculation doses of bacteria, design and anatomical location of the implant, choice of animals, to the duration and outcome measures of the studies. A number of reviews over the past 2 decades on the pros and cons of common small (e.g. rodents) and large animal (e.g. rabbits, dogs) models and prosthesis designs for evaluating therapeutic strategies against periprosthetic orthopedic infections203–207 will be of great values to such study designs. Other considerations include ensuring the cytocompatibility and biocompatibility of the implant surface modifications and the safety of the physical triggering methods (e.g. ultrasound, photo irradiation, hyperthermia) applied. These critical pre-clinical assessments will expedite the successful translation of these emerging implant surface modification strategies to combat PJI, not only significantly improving patients’ well-being but making a positive social and economic impact.
Acknowledgement
This work was supported by National Institutes of Health grants R01AR068418 and R01AR078044.
References
- 1.Lynch AS; Robertson GT Bacterial and Fungal Biofilm Infections. In Annu. Rev. Med 2008; 415–428. [DOI] [PubMed] [Google Scholar]
- 2.Donlan RM Biofilm formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis 2001, 33 (8), 1387–1392. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med 2004, 350 (14), 1422–1429. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz EM; Parvizi J; Gehrke T; Aiyer A; Battenberg A; Brown SA; Callaghan JJ; Citak M; Egol K; Garrigues GE; Ghert M; Goswami K; Green A; Hammound S; Kates SL; McLaren AC; Mont MA; Namdari S; Obremskey WT; O’Toole R; Raikin S; Restrepo C; Ricciardi B; Saeed K; Sanchez-Sotelo J; Shohat N; Tan T; Thirukumaran CP; Winters B, 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. J. Orthop. Res 2019, 37 (5), 997–1006. [DOI] [PubMed] [Google Scholar]
- 5.Francolini I; Vuotto C; Piozzi A; Donelli G Antifouling and Antimicrobial Biomaterials: an Overview. APMIS 2017, 125 (4), 392–417. [DOI] [PubMed] [Google Scholar]
- 6.Tande AJ; Patel R Prosthetic Joint Infection. Clin. Microbiol. Rev 2014, 27 (2), 302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z; Borgwardt L; Høiby N; Wu H; Sørensen TS; Borgwardt A Prosthesis Infections after Orthopedic Joint Replacement: the Possible Role of Bacterial Biofilms. Orthop. Rev. (Pavia) 2013, 5 (2), 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokogawa N; Ishikawa M; Nishitani K; Beck CA; Tsuchiya H; Mesfin A; Kates SL; Daiss JL; Xie C; Schwarz EM Immunotherapy Synergizes with Debridement and Antibiotic Therapy in a Murine 1-stage Exchange Model of MRSA Implant-associated Osteomyelitis. J Orthop. Res 2018, 36 (6), 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trampuz A; Widmer AF Infections Associated with Orthopedic Implants. Curr. Opin. Infect. Dis 2006, 19 (4), 349–356. [DOI] [PubMed] [Google Scholar]
- 10.Trampuz A; Zimmerli W Antimicrobial Agents in Orthopaedic Surgery: Prophylaxis and Treatment. Drugs 2006, 66 (8), 1089–1105.. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerli W; Widmer AF; Blatter M; Frei R; Ochsner PE Role of Rifampin for Treatment of Orthopedic Implant-related Staphylococcal Infections: a Randomized Controlled Trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998, 279 (19), 1537–1541. [DOI] [PubMed] [Google Scholar]
- 12.Anagnostakos K; Schmid NV; Kelm J; Grün U; Jung J Classification of Hip Joint Infections. Int. J. Med. Sci 2009, 6 (5), 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orapiriyakul W; Young PS; Damiati L; Tsimbouri PM Antibacterial Surface Modification of Titanium Implants in Orthopaedics. In J. Tissue Eng 2018, 9, 2041731418789838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puckett SD; Taylor E; Raimondo T; Webster TJ The Relationship Between the Nanostructure of Titanium Surfaces and Bacterial Attachment. Biomaterials 2010, 31 (4), 706–713. [DOI] [PubMed] [Google Scholar]
- 15.Moran E; Byren I; Atkins BL The Diagnosis and Management of Prosthetic Joint Infections. J. Antimicrob. Chemother 2010, 65, iii45–iii54. [DOI] [PubMed] [Google Scholar]
- 16.de Mesy Bentley KL; Trombetta R; Nishitani K; Bello-Irizarry SN; Ninomiya M; Zhang L; Chung HL; McGrath JL; Daiss JL; Awad HA; Kates SL; Schwarz EM Evidence of Staphylococcus aureus Deformation, Proliferation and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J. Bone Miner. Res 2017, 32 (5), 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchholz HW; Engelbrecht H [Depot Effects of Various Antibiotics Mixed with Palacos Resins]. Chirurg 1970, 41 (11), 511–515. [PubMed] [Google Scholar]
- 18.Jose B; Antoci V; Zeiger AR; Wickstrom E; Hickok NJ Vancomycin Covalently Bonded to Titanium Beads Kills Staphylococcus aureus. Chem. Biol 2005, 12 (9), 1041–1048. [DOI] [PubMed] [Google Scholar]
- 19.Gerits E; Kucharikova S; Van Dijck P; Erdtmann M; Krona A; Lovenklev M; Frohlich M; Dovgan B; Impellizzeri F; Braem A; Vleugels J; Robijns SC; Steenackers HP; Vanderleyden J; De Brucker K; Thevissen K; Cammue BP; Fauvart M; Verstraeten N; Michiels J Antibacterial Activity of a New Broad-spectrum Antibiotic Covalently Bound to Titanium Surfaces. J. Orthop. Res 2016, 34 (12), 2191–2198. [DOI] [PubMed] [Google Scholar]
- 20.Berger RA; Jacobs JJ; Quigley LR; Rosenberg AG; Galante JO Primary Cementless Acetabular Reconstruction in Patients Younger than 50 Years Old. 7- to 11-year results. Clin. Orthop. Relat. Res 1997, 344, 216–226. [PubMed] [Google Scholar]
- 21.Ghimire A; Skelly JD; Song J, Micrococcal-Nuclease-Triggered On-Demand Release of Vancomycin from Intramedullary Implant Coating Eradicates Staphylococcus aureus Infection in Mouse Femoral Canals. ACS Cent. Sci 2019, 5 (12), 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B; Braun BM; Skelly JD; Ayers DC; Song J, Significant Suppression of Staphylococcus aureus Colonization on Intramedullary Ti6Al4V Implants Surface-Grafted with Vancomycin-Bearing Polymer Brushes. ACS Appl. Mater. Interfaces 2019, 11 (32), 28641–28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivapooja P; Wang Q; Orihuela B; Rittschof D; López GP; Zhao X, Bioinspired Surfaces with Dynamic Topography for Active Control of Biofouling. Adv. Mater 2013, 25 (10), 1430–1434. [DOI] [PubMed] [Google Scholar]
- 24.Su Y; Zhi Z; Gao Q; Xie M; Yu M; Lei B; Li P; Ma PX, Autoclaving-Derived Surface Coating with In Vitro and In Vivo Antimicrobial and Antibiofilm Efficacies. Adv. Healthcare Mater 2017, 6 (6), 1601173. [DOI] [PubMed] [Google Scholar]
- 25.Li J; Tan L; Liu X; Cui Z; Yang X; Yeung KWK; Chu PK; Wu S, Balancing Bacteria–Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11 (11), 11250–11263. [DOI] [PubMed] [Google Scholar]
- 26.Ivanova EP; Truong VK; Webb HK; Baulin VA; Wang JY; Mohammodi N; Wang F; Fluke C; Crawford RJ Differential Attraction and Repulsion of Staphylococcus aureus and Pseudomonas aeruginosa on Molecularly Smooth Titanium Films. Sci. Rep 2011, 1 (1), 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mrabet B; Nguyen MN; Majbri A; Mahouche S; Turmine M; Bakhrouf A; Chehimi MM Anti-fouling Poly(2-hydoxyethyl methacrylate) Surface Coatings with Specific Bacteria Recognition Capabilities. Surf. Sci 2009, 603 (16), 2422–2429. [Google Scholar]
- 28.Lichter JA; Thompson MT; Delgadillo M; Nishikawa T; Rubner MF; Van Vliet KJ, Substrata Mechanical Stiffness Can Regulate Adhesion of Viable Bacteria. Biomacromolecules 2008, 9 (6), 1571–1578. [DOI] [PubMed] [Google Scholar]
- 29.Yang H; Jia B; Zhang Z; Qu X; Li G; Lin W; Zhu D; Dai K; Zheng Y Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-bearing Applications. Nat. Commun 2020, 11 (1), 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arciola CR; Campoccia D; Montanaro L, Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol 2018, 16 (7), 397–409. [DOI] [PubMed] [Google Scholar]
- 31.Guilhen C; Forestier C; Balestrino D, Biofilm Dispersal: Multiple Elaborate Strategies for Dissemination of Dacteria with Unique Properties. Mol. Microbiol 2017, 105 (2), 188–210. [DOI] [PubMed] [Google Scholar]
- 32.Fischer NG; Munchow EA; Tamerler C; Bottino MC; Aparicio C, Harnessing Biomolecules for Bioinspired Dental Biomaterials. J. Mater. Chem. B 2020, 8 (38), 8713–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orapiriyakul W; Young PS; Damiati L; Tsimbouri PM, Antibacterial Surface Modification of Titanium Implants in Orthopaedics. J. Tissue. Eng 2018, 9, 2041731418789838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chouirfa H; Bouloussa H; Migonney V; Falentin-Daudre C, Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta. Biomater 2019, 83, 37–54. [DOI] [PubMed] [Google Scholar]
- 35.Quinn J; McFadden R; Chan CW; Carson L, Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23 (11), 101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teughels W; Van Assche N; Sliepen I; Quirynen M Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implants Res 2006, 17 Suppl 2, 68–81. [DOI] [PubMed] [Google Scholar]
- 37.Park KD; Kim YS; Han DK; Kim YH; Lee EHB; Suh H; Choi KS Bacterial Adhesion on PEG Modified Polyurethane Surfaces. Biomaterials 1998, 19 (7), 851–859. [DOI] [PubMed] [Google Scholar]
- 38.Albrektsson T; Wennerberg A Oral implant surfaces: Part 1--Review Focusing on Topographic and Chemical Properties of Different Surfaces and In Vivo Responses to Them. Int. J. Prosthodont 2004, 17 (5), 536–543. [PubMed] [Google Scholar]
- 39.Scheuerman TR; Camper AK; Hamilton MA Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci 1998, 208 (1), 23–33. [DOI] [PubMed] [Google Scholar]
- 40.Damodaran VB; Murthy NS, Bio-Inspired Strategies for Designing Antifouling Biomaterials. Biomater. Res 2016, 20 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie M; Wang Y; Zhao W, Design Novel Three-dimensional Network Nanostructure for Lubricant Infused on Titanium Alloys Towards Long-term Anti-fouling. Colloids Surf. B: Biointerfaces 2021, 197, 111375. [DOI] [PubMed] [Google Scholar]
- 42.Hizal F; Rungraeng N; Lee J; Jun S; Busscher HJ; van der Mei HC; Choi C-H, Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9 (13), 12118–12129. [DOI] [PubMed] [Google Scholar]
- 43.Gallardo-Moreno AM; Pacha-Olivenza MA; Saldaña L; Pérez-Giraldo C; Bruque JM; Vilaboa N; González-Martín ML In Vitro Biocompatibility and Bacterial Adhesion of Physico-chemically Modified Ti6Al4V Surface by Means of UV Irradiation. Acta Biomater 2009, 5 (1), 181–192. [DOI] [PubMed] [Google Scholar]
- 44.Gottenbos B; van der Mei HC; Klatter F; Grijpma DW; Feijen J; Nieuwenhuis P; Busscher HJ Positively Charged Biomaterials Exert Antimicrobial Effects on Gram-negative Bacilli in Rats. Biomaterials 2003, 24 (16), 2707–2710. [DOI] [PubMed] [Google Scholar]
- 45.Li Z; Guo Z Bioinspired Surfaces with Wettability for Antifouling Application. Nanoscale 2019, 11 (47), 22636–22663. [DOI] [PubMed] [Google Scholar]
- 46.Nouri A; Wen C 1 - Introduction to Surface Coating and Modification for Metallic Biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen C, Ed.; Woodhead Publishing: 2015; pp 3–60. [Google Scholar]
- 47.Ludecke C; Roth M; Yu W; Horn U; Bossert J; Jandt KD Nanorough Titanium Surfaces Reduce Adhesion of Escherichia coli and Staphylococcus aureus Via Nano Adhesion Points. Colloids Surf. B Biointerfaces 2016, 145, 617–625. [DOI] [PubMed] [Google Scholar]
- 48.Song R; Zhang Y; Huang Q; Yang Y; Lin L; Liang J; Hu R; Rui G; Lin C Facile Construction of Structural Gradient of TiO2 Nanotube Arrays on Medical Titanium for High Throughput Evaluation of Biocompatibility and Antibacterial Property. ACS Appl. Bio Mater 2018, 1 (4), 1056–1065. [DOI] [PubMed] [Google Scholar]
- 49.Cheng YF; Mei YH; Sathishkumar G; Lu ZS; Li CM; Wang F; Xia QY; Xu LQ Tannic Acid-assisted Deposition of Silk Sericin on the Titanium Surfaces for Antifouling Application. Colloid Interface Sci. Commun 2020, 35, 100241. [Google Scholar]
- 50.Francolini I; Donelli G; Vuotto C; Baroncini FA; Stoodley P; Taresco V; Martinelli A; D’Ilario L; Piozzi A Antifouling Polyurethanes to Fight Device-related Staphylococcal Infections: Synthesis, Characterization, and Antibiofilm Efficacy. Pathog. Dis 2014, 70 (3), 401–407. [DOI] [PubMed] [Google Scholar]
- 51.Francolini I; Crisante F; Martinelli A; D’Ilario L; Piozzi A Synthesis of Biomimetic Segmented Polyurethanes as Antifouling Biomaterials. Acta Biomater 2012, 8 (2), 549–558. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X; Wang L; Levänen E Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion. RSC Adv 2013, 3 (30), 12003–12020. [Google Scholar]
- 53.Braem A; Van Mellaert L; Hofmans D; De Waelheyns E; Anné J; Schrooten J; Vleugels J Bacterial Colonisation of Porous Titanium Coatings for Orthopaedic Implant Applications – Effect of Surface Roughness and Porosity. Powder Metall 2013, 56 (4), 267–271. [Google Scholar]
- 54.Scardino AJ; Guenther J; de Nys R Attachment Point Theory Revisited: the Fouling Response to a Microtextured Matrix. Biofouling 2008, 24 (1), 45–53. [DOI] [PubMed] [Google Scholar]
- 55.Barthlott W; Neinhuis C Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202 (1), 1–8. [Google Scholar]
- 56.Zhang Y-L; Xia H; Kim E; Sun H-B Recent Developments in Superhydrophobic Surfaces with Unique Structural and Functional Properties. Soft Matter 2012, 8 (44), 11217–11231. [Google Scholar]
- 57.Zhang X; Shi F; Niu J; Jiang Y; Wang Z Superhydrophobic Surfaces: From Structural Control to Functional Application. J. Mater. Chem 2008, 18 (6), 621–633. [Google Scholar]
- 58.Fadeeva E; Truong VK; Stiesch M; Chichkov BN; Crawford RJ; Wang J; Ivanova EP Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27 (6), 3012–3019. [DOI] [PubMed] [Google Scholar]
- 59.Tang Peifu, Z. W, Wang Yan, Zhang Boxun, Wang Hao,; Lin Changjian, Z. a. L. Effect of Superhydrophobic Surface of Titanium on Staphylococcus aureus Adhesion. J. Nanomater 2011, 2011. [Google Scholar]
- 60.Huang Q; Lin L; Yang Y; Hu R; Vogler EA; Lin C Role of Trapped air in the Formation of Cell-and-Protein Micropatterns on Superhydrophobic/Superhydrophilic Microtemplated Surfaces. Biomaterials 2012, 33 (33), 8213–8220. [DOI] [PubMed] [Google Scholar]
- 61.Ostuni E; Chapman RG; Liang MN; Meluleni G; Pier G; Ingber DE; Whitesides GM Self-Assembled Monolayers That Resist the Adsorption of Proteins and the Adhesion of Bacterial and Mammalian Cells. Langmuir 2001, 17 (20), 6336–6343. [Google Scholar]
- 62.Kingshott P; Wei J; Bagge-Ravn D; Gadegaard N; Gram L Covalent Attachment of Poly(ethylene glycol) to Surfaces, Critical for Reducing Bacterial Adhesion. Langmuir 2003, 19 (17), 6912–6921. [Google Scholar]
- 63.Tedjo C; Neoh KG; Kang ET; Fang N; Chan V Bacteria–Surface Interaction in the Presence of Proteins and Surface Attached Poly(ethylene glycol) Methacrylate Chains. J. Biomed. Mater. Res. A 2007, 82 (2), 479–491. [DOI] [PubMed] [Google Scholar]
- 64.Lee HJ; Park KD; Park HD; Lee WK; Han DK; Kim SH; Kim YH Platelet and Bacterial Repellence on Sulfonated Poly(ethylene glycol)-acrylate Copolymer Surfaces. Colloids Surf. B: Biointerfaces 2000, 18 (3), 355–370. [DOI] [PubMed] [Google Scholar]
- 65.Saldarriaga Fernández IC; van der Mei HC; Lochhead MJ; Grainger DW; Busscher HJ The Inhibition of the Adhesion of Clinically Isolated Bacterial Strains on Multi-Component Cross-Linked Poly(ethylene glycol)-based Polymer Coatings. Biomaterials 2007, 28 (28), 4105–4112. [DOI] [PubMed] [Google Scholar]
- 66.Zoulalian V; Zürcher S; Tosatti S; Textor M; Monge S; Robin J-J, Self-Assembly of Poly(ethylene glycol)−Poly(alkyl phosphonate) Terpolymers on Titanium Oxide Surfaces: Synthesis, Interface Characterization, Investigation of Nonfouling Properties, and Long-Term Stability. Langmuir 2010, 26 (1), 74–82. [DOI] [PubMed] [Google Scholar]
- 67.Schlenoff JB, Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30 (32), 9625–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnan S; Weinman CJ; Ober CK Advances in Polymers for Anti-Biofouling Surfaces. J. Mater. Chem 2008, 18 (29), 3405–3413. [Google Scholar]
- 69.Mishra S; Horswill AR, Heparin Mimics Extracellular DNA in Binding to Cell Surface-Localized Proteins and Promoting Staphylococcus aureus Biofilm Formation. mSphere 2017, 2 (3), e00135–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maan AMC; Hofman AH; de Vos WM; Kamperman M, Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater 2020, 30 (32), 2000936. [Google Scholar]
- 71.Mas-Moruno C; Su B; Dalby MJ, Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Healthcare Mater 2019, 8 (1), 1801103. [DOI] [PubMed] [Google Scholar]
- 72.Lüsse S; Arnold K, The Interaction of Poly(ethylene glycol) with Water Studied by 1H and 2H NMR Relaxation Time Measurements. Macromolecules 1996, 29 (12), 4251–4257. [Google Scholar]
- 73.Oesterhelt F; Rief M; Gaub HE Single Molecule Force Spectroscopy by AFM Indicates Helical Structure of Poly(ethylene-glycol) in Water. New J. Phys 1999, 1, 6–6. [Google Scholar]
- 74.Aray Y; Marquez M; Rodríguez J; Vega D; Simón-Manso Y; Coll S; Gonzalez C; Weitz DA, Electrostatics for Exploring the Nature of the Hydrogen Bonding in Polyethylene Oxide Hydration. J. Phys. Chem. B 2004, 108 (7), 2418–2424. [Google Scholar]
- 75.Chen S; Li L; Zhao C; Zheng J Surface Hydration: Principles and Applications Toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51 (23), 5283–5293. [Google Scholar]
- 76.Khoo X; Hamilton P; O’Toole GA; Snyder BD; Kenan DJ; Grinstaff MW, Directed Assembly of PEGylated-Peptide Coatings for Infection-Resistant Titanium Metal. J. Am. Chem. Soc 2009, 131 (31), 10992–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buxadera-Palomero J; Calvo C; Torrent-Camarero S; Gil FJ; Mas-Moruno C; Canal C; Rodríguez D Biofunctional Polyethylene Glycol Coatings on Titanium: An In Vitro-Based Comparison of Functionalization Methods. Colloids Surf. B: Biointerfaces 2017, 152, 367–375. [DOI] [PubMed] [Google Scholar]
- 78.Herold DA; Keil K; Bruns DE Oxidation of Polyethylene Glycols by Alcohol Dehydrogenase. Biochem. Pharmacol 1989, 38 (1), 73–76. [DOI] [PubMed] [Google Scholar]
- 79.Ratner BD Reducing Capsular Thickness and Enhancing Angiogenesis around Implant Drug Release Systems. J. Control. Release 2002, 78 (1), 211–218. [DOI] [PubMed] [Google Scholar]
- 80.Crouzet C; Decker C; Marchal J Caractérisation de Réactions Primaires de Dégradation Oxydante au Cours de L’autoxydation des Poly(oxyéthylène)s à 25°C: Étude en Solution Aqueuse avec Amorçage par Radiolyse du Solvant, 8. Étude cinétique en fonction du ph compris entre 1 et 13. Die Makromolekulare Chemie 1976, 177 (1), 145–157. [Google Scholar]
- 81.Gerhardt W; Martens C, Zur Oxydation von Polyethylenoxiden und Polyethylenoxidethern; Die Bildung von Acetaldehyd bei der Oxydation von Diethylenglycol mit Sauerstoff. Zeitschrift für Chemie 1985, 25 (4), 143–143. [Google Scholar]
- 82.Zwaal RFA; Comfurius P; Van Deenen LLM Membrane Asymmetry and Blood Coagulation. Nature 1977, 268 (5618), 358–360. [DOI] [PubMed] [Google Scholar]
- 83.Zwaal RFA; Schroit AJ, Pathophysiologic Implications of Membrane Phospholipid Asymmetry in Blood Cells. Blood 1997, 89 (4), 1121–1132. [PubMed] [Google Scholar]
- 84.Lewis AL Phosphorylcholine-Based Polymers and Their use in the Prevention of Biofouling. Colloids Surf. B: Biointerfaces 2000, 18 (3), 261–275. [DOI] [PubMed] [Google Scholar]
- 85.Hall B; le E Bird R; Kojima M; Chapman D Biomembranes as Models for Polymer Surfaces: V. Thrombelastographic Studies of Polymeric Lipids and Polyesters. Biomaterials 1989, 10 (4), 219–224. [DOI] [PubMed] [Google Scholar]
- 86.Hayward JA; Durrani AA; Shelton CJ; Lee DC; Chapman D Biomembpanes as Models for Polymer Surfaces: III. Characterization of a Phosphorylcholine Surface Covalenay Bound to Glass. Biomaterials 1986, 7 (2), 126–131. [DOI] [PubMed] [Google Scholar]
- 87.Ishihara K; Fukumoto K; Iwasaki Y; Nakabayashi N Modification of Polysulfone with Phospholipid Polymer for Improvement of the Blood Compatibility. Part 1. Surface Characterization. Biomaterials 1999, 20 (17), 1545–1551. [DOI] [PubMed] [Google Scholar]
- 88.Liu H; Liu L; Jiang X; Fan J; Peng W; Liu P; Yang T; Chen H; Jiang W; Yin G; Liu P; Shen J Rational Design of a Zwitterionic–Phosphonic Copolymer for the Surface Antifouling Modification of Multiple Biomedical Metals. J. Mater. Chem. B 2019, 7 (25), 4055–4065. [Google Scholar]
- 89.Cheng G; Zhang Z; Chen S; Bryers JD; Jiang S Inhibition of Bacterial Adhesion and Biofilm Formation on Zwitterionic Surfaces. Biomaterials 2007, 28 (29), 4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu PS; Domingue E; Ayers DC; Song J, Modification of Ti6Al4V Substrates with Well-defined Zwitterionic Polysulfobetaine Brushes for Improved Surface Mineralization. ACS Appl. Mater. Interfaces 2014, 6 (10), 7141–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu P; Emmons E; Song J, A Comparative Study of Zwitterionic Ligands-mediated Biomineralization and the Potential of Mineralized Zwitterionic Matrices for Bone Tissue Engineering. J. Mater. Chem. B 2014, 2 (43), 7524–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu P; Song J, Sulfobetaine as a Zwitterionic Mediator for 3D Hydroxyapatite Mineralization. Biomaterials 2013, 34 (10), 2442–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang B; Skelly JD; Braun BM; Ayers DC; Song J Surface-Grafted Zwitterionic Polymers Improve the Efficacy of a Single Antibiotic Injection in Suppressing Staphylococcus aureus Periprosthetic Infections. ACS Appl. Bio Mater 2020, 3, 5896–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fuqua WC; Winans SC; Greenberg EP Quorum Sensing in Bacteria: The LuxR-LuxI Family of Cell Density-Responsive Transcriptional Regulators. J. Bacteriol 1994, 176 (2), 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waters CM; Bassler BL QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu Rev. Cell Dev. Biol 2005, 21 (1), 319–346. [DOI] [PubMed] [Google Scholar]
- 96.Niu C; Afre S; Gilbert ES Subinhibitory Concentrations of Cinnamaldehyde Interfere with Quorum Sensing. Lett. Appl. Microbiol 2006, 43 (5), 489–494. [DOI] [PubMed] [Google Scholar]
- 97.Topa SH; Palombo EA; Kingshott P; Blackall LL, Activity of Cinnamaldehyde on Quorum Sensing and Biofilm Susceptibility to Antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8 (3), 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brackman G; Breyne K; De Rycke R; Vermote A; Van Nieuwerburgh F; Meyer E; Van Calenbergh S; Coenye T, The Quorum Sensing Inhibitor Hamamelitannin Increases Antibiotic Susceptibility of Staphylococcus aureus Biofilms by Affecting Peptidoglycan Biosynthesis and eDNA Release. Sci. Rep 2016, 6 (1), 20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luo J; Dong B; Wang K; Cai S; Liu T; Cheng X; Lei D; Chen Y; Li Y; Kong J; Chen Y Baicalin Inhibits Biofilm Formation, Attenuates the Quorum Sensing-Controlled Virulence and Enhances Pseudomonas aeruginosa Clearance in a Mouse Peritoneal Implant Infection Model. PLoS One 2017, 12 (4), e0176883–e0176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Z; Wang Q; Hu Y; Liang J; Jiang Y; Ma R; Tang Z; Huang Z Use of the Quorum Sensing Inhibitor Furanone C-30 to Interfere with Biofilm Formation by Streptococcus Mutans and its luxS Mutant Strain. Int. J. Antimicrob. Agents 2012, 40 (1), 30–35. [DOI] [PubMed] [Google Scholar]
- 101.Kang M; Kim S; Kim H; Song Y; Jung D; Kang S; Seo J-H; Nam S; Lee Y, Calcium-Binding Polymer-Coated Poly(lactide-co-glycolide) Microparticles for Sustained Release of Quorum Sensing Inhibitors to Prevent Biofilm Formation on Hydroxyapatite Surfaces. ACS Appl. Mater. Interfaces 2019, 11 (8), 7686–7694. [DOI] [PubMed] [Google Scholar]
- 102.Mooney JA; Pridgen EM; Manasherob R; Suh G; Blackwell HE; Barron AE; Bollyky PL; Goodman SB; Amanatullah DF Periprosthetic Bacterial Biofilm and Quorum Sensing. J. Orthop. Res 2018, 36 (9), 2331–2339. [DOI] [PubMed] [Google Scholar]
- 103.Ivanova EP; Hasan J; Webb HK; Truong VK; Watson GS; Watson JA; Baulin VA; Pogodin S; Wang JY; Tobin MJ; Löbbe C; Crawford RJ, Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas aeruginosa Cells by Cicada Wings. Small 2012, 8 (16), 2489–2494. [DOI] [PubMed] [Google Scholar]
- 104.Kelleher SM; Habimana O; Lawler J; O’ Reilly B; Daniels S; Casey E; Cowley A Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8 (24), 14966–14974. [DOI] [PubMed] [Google Scholar]
- 105.Hasan J; Jain S; Chatterjee K Nanoscale Topography on Black Titanium Imparts Multi-biofunctional Properties for Orthopedic Applications. Sci. Rep 2017, 7 (1), 41118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wandiyanto JV; Cheeseman S; Truong VK; Kobaisi MA; Bizet C; Juodkazis S; Thissen H; Crawford RJ; Ivanova EP Outsmarting superbugs: Bactericidal Activity of Nanostructured Titanium Surfaces Against Methicillin- and Gentamicin-resistant Staphylococcus aureus ATCC 33592. J. Mater. Chem. B 2019, 7 (28), 4424–4431. [Google Scholar]
- 107.Diu T, Faruqi N; Sjostrom T; Lamarre B; Jenkison HF; Su B; Ryadnov MG Cicada-Inspired Cell-instructive Nanopatterned Arrays. Sci. Rep 2014, 4, 7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhadra CM; Khanh Truong V; Pham VTH; Al Kobaisi M; Seniutinas G; Wang JY; Juodkazis S; Crawford RJ; Ivanova EP Antibacterial Titanium Nano-patterned Arrays Inspired by Dragonfly Wings. Sci. Rep 2015, 5 (1), 16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao Y; Su B; Chinnaraj S; Jana S; Bowen L; Charlton S; Duan P; Jakubovics NS; Chen J Nanostructured Titanium Surfaces Exhibit Recalcitrance Towards Staphylococcus epidermidis Biofilm Formation. Sci. Rep 2018, 8 (1), 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jaggessar A; Mathew A; Wang H; Tesfamichael T; Yan C; Yarlagadda PKDV Mechanical, Bactericidal and Osteogenic Behaviours of Hydrothermally Synthesised TiO2 Nanowire Arrays. J. Mech. Behav. Biomed. Mater 2018, 80, 311–319. [DOI] [PubMed] [Google Scholar]
- 111.Sengstock C; Lopain M; Motemani Y; Borgmann A; Khare C; Buenconsejo PJS; Schildhauer TA; Ludwig A; Koller M Structure-Related Antibacterial Activity of a Titanium Nanostructured Surface Fabricated by Glancing Angle Sputter Deposition. Nanotechnol 2014, 25. [DOI] [PubMed] [Google Scholar]
- 112.Sjöström T; Nobbs AH; Su B Bactericidal Nanospike Surfaces via Thermal Oxidation of Ti Alloy Substrates. Mater. Lett 2016, 167, 22–26. [Google Scholar]
- 113.Pogodin S; Hasan J; Baulin VA; Webb HK; Truong VK; Nguyen HP; Boshkoviki V; Fluke CJ; Watson GS; Watson JA; Crawford RJ; Ivanova EP Biophysical Model of Bacterial Cell Interactions with Nanopatterned Cicada wing Surfaces. Biophys. J 2013, 104, 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fraioli R; Tsimbouri PM; Fisher LE; Nobbs AH; Su B; Neubauer S; Rechenmacher F; Kessler H; Ginebra M-P; Dalby MJ; Manero JM; Mas-Moruno C, Towards the Cell-instructive Bactericidal Substrate: Exploring the Combination of Nanotopographical Features and Integrin Selective Synthetic Ligands. Sci. Rep 2017, 7 (1), 16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tiller JC; Liao C-J; Lewis K; Klibanov AM Designing Surfaces that kill Bacteria on Contact. PNAS 2001, 98 (11), 5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis K; Klibanov AM Surpassing Nature: Rational Design of Sterile-Surface Materials. Trends Biotechnol 2005, 23 (7), 343–348. [DOI] [PubMed] [Google Scholar]
- 117.Edupuganti OP; Antoci V Jr.; King SB; Jose B; Adams CS; Parvizi J; Shapiro IM; Zeiger AR; Hickok NJ; Wickstrom E Covalent Bonding of Vancomycin to Ti6Al4V Alloy Pins Provides long-term Inhibition of Staphylococcus aureus Colonization. Bioorg. Med. Chem. Lett 2007, 17 (10), 2692–2696. [DOI] [PubMed] [Google Scholar]
- 118.Antoci V Jr.; Adams CS; Hickok NJ; Shapiro IM; Parvizi J Vancomycin Bound to Ti Rods Reduces Periprosthetic Infection: Preliminary Study. Clin. Orthop. Relat. Res 2007, 461, 88–95. [DOI] [PubMed] [Google Scholar]
- 119.Zeng Q; Zhu Y; Yu B; Sun Y; Ding X; Xu C; Wu Y-W; Tang Z; Xu F-J Antimicrobial and Antifouling Polymeric Agents for Surface Functionalization of Medical Implants. Biomacromolecules 2018, 19 (7), 2805–2811. [DOI] [PubMed] [Google Scholar]
- 120.Ageitos JM; Sánchez-Pérez A; Calo-Mata P; Villa TG, Antimicrobial Peptides (AMPs): Ancient Compounds that Represent Novel Weapons in the Fight Against Bacteria. Biochem.l Pharmacol 2017, 133, 117–138. [DOI] [PubMed] [Google Scholar]
- 121.Chen J; Zhu Y; Xiong M; Hu G; Zhan J; Li T; Wang L; Wang Y, Antimicrobial Titanium Surface via Click-Immobilization of Peptide and Its in Vitro/Vivo Activity. ACS Biomater. Sci. Eng 2019, 5 (2), 1034–1044. [DOI] [PubMed] [Google Scholar]
- 122.Godoy-Gallardo M; Mas-Moruno C; Yu K; Manero JM; Gil FJ; Kizhakkedathu JN; Rodriguez D, Antibacterial Properties of hLf1–11 Peptide onto Titanium Surfaces: A Comparison Study Between Silanization and Surface Initiated Polymerization. Biomacromolecules 2015, 16 (2), 483–496. [DOI] [PubMed] [Google Scholar]
- 123.Langer R, New Methods of Drug Delivery. Science 1990, 249 (4976), 1527–1533. [DOI] [PubMed] [Google Scholar]
- 124.Dash A; Cudworth G Therapeutic Applications of Implantable Drug Delivery Systems. J. Pharmacol. Toxicol. Methods 1998, 40 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- 125.Kumar A; Pillai J Chapter 13 - Implantable Drug Delivery Systems: An Overview. In Nanostructures for the Engineering of Cells, Tissues and Organs; Grumezescu AM, Ed.; William Andrew Publishing: 2018; pp 473–511. [Google Scholar]
- 126.Simchi A; Tamjid E; Pishbin F; Boccaccini AR Recent Progress in Inorganic and Composite Coatings with Bactericidal Capability for Orthopaedic Applications. Nanomedicine, 2011, 7 (1), 22–39. [DOI] [PubMed] [Google Scholar]
- 127.Lansdown AB Silver. I: Its Antibacterial Properties and Mechanism of Action. J. Wound Care 2002, 11 (4), 125–130. [DOI] [PubMed] [Google Scholar]
- 128.Klasen HJ Historical Review of the Use of Silver in the Treatment of Burns. I. Early Uses. Burns 2000, 26 (2), 117–130. [DOI] [PubMed] [Google Scholar]
- 129.Chopra I The Increasing Use of Silver-based Products as Antimicrobial Agents: a Useful Development or a Cause for Concern? J. Antimicrob. Chemother 2007, 59 (4), 587–590. [DOI] [PubMed] [Google Scholar]
- 130.Moore SL; Payne DN Types of Antimicrobial Agents. In Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization, 2004, pp 8–97. [Google Scholar]
- 131.Moyer CA; Brebtano L; Gravens DL; Margraf HW; Monafo WW Jr. Treatment of Large Human Burns With 0.5% Silver Nitrate Solution. Arch. Surg 1965, 90 (6), 812–867. [DOI] [PubMed] [Google Scholar]
- 132.Castellano JJ; Shafii SM; Ko F; Donate G; Wright TE; Mannari RJ; Payne WG; Smith DJ; Robson MC Comparative Evaluation of Silver-Containing Antimicrobial Dressings and Drugs. Int. Wound J 2007, 4 (2), 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amin Yavari S; Loozen L; Paganelli FL; Bakhshandeh S; Lietaert K; Groot JA; Fluit AC; Boel CHE; Alblas J; Vogely HC; Weinans H; Zadpoor AA Antibacterial Behavior of Additively Manufactured Porous Titanium with Nanotubular Surfaces Releasing Silver Ions. ACS Appl. Mater. Interfaces 2016, 8 (27), 17080–17089. [DOI] [PubMed] [Google Scholar]
- 134.Li M; Wang Y; Gao L; Sun Y; Wang J; Qu S; Duan K; Weng J; Feng B Porous Titanium Scaffold Surfaces Modified with Silver Loaded Gelatin Microspheres and Their Antibacterial Behavior. Surf. Coat. Technol 2016, 286, 140–147. [Google Scholar]
- 135.Dalecki AG; Haeili M; Shah S; Speer A; Niederweis M; Kutsch O; Wolschendorf F, Disulfiram and Copper Ions Kill Mycobacterium tuberculosis in a Synergistic Manner. Antimicrob. Agents Chemother 2015, 59 (8), 4835–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Faúndez G; Troncoso M; Navarrete P; Figueroa G, Antimicrobial Activity of Copper Surfaces Against Suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol 2004, 4 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu C; Zhou Y; Xu M; Han P; Chen L; Chang J; Xiao Y, Copper-Containing Mesoporous Bioactive Glass Scaffolds with Multifunctional Properties of Angiogenesis Capacity, Osteostimulation and Antibacterial Activity. Biomaterials 2013, 34 (2), 422–433. [DOI] [PubMed] [Google Scholar]
- 138.Gérard C; Bordeleau L-J; Barralet J; Doillon CJ, The Stimulation of Angiogenesis and Collagen Deposition by Copper. Biomaterials 2010, 31 (5), 824–831. [DOI] [PubMed] [Google Scholar]
- 139.Wang L; Yang X; Cao W; Shi C; Zhou P; Li Q; Han F; Sun J; Xing X; Li B, Mussel-Inspired Deposition of Copper on Titanium for Bacterial Inhibition and Enhanced Osseointegration in a Periprosthetic Infection Model. RSC Adv 2017, 7 (81), 51593–51604. [Google Scholar]
- 140.Hong R; Kang TY; Michels CA; Gadura N Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol 2012, 78 (6), 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.San K; Long J; Michels CA; Gadura N Antimicrobial Copper Alloy Surfaces are Effective Against Vegetative but not Sporulated Cells of Gram-positive Bacillus subtilis. MicrobiologyOpen 2015, 4 (5), 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y-M; Rock CO Membrane Lipid Homeostasis in Bacteria. Nat. Rev. Microbiol 2008, 6 (3), 222–233. [DOI] [PubMed] [Google Scholar]
- 143.Studer AM; Limbach LK; Van Duc L; Krumeich F; Athanassiou EK; Gerber LC; Moch H; Stark WJ Nanoparticle Cytotoxicity Depends on Intracellular Solubility: Comparison of Stabilized Copper Metal and Degradable Copper Oxide Nanoparticles. Toxicol. Lett 2010, 197 (3), 169–174. [DOI] [PubMed] [Google Scholar]
- 144.Stohs SJ; Bagchi D Oxidative Mechanisms in the Toxicity of Metal Ions. Free Radic. Biol. Med 1995, 18 (2), 321–336. [DOI] [PubMed] [Google Scholar]
- 145.Yamanaka M; Hara K; Kudo J Bactericidal Actions of a Silver ion Solution on Escherichia coli, Studied by Energy-filtering Transmission Electron Microscopy and Proteomic Analysis. Appl. Environ. Microbiol 2005, 71 (11), 7589–7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li M; Ma Z; Zhu Y; Xia H; Yao M; Chu X; Wang X; Yang K; Yang M; Zhang Y; Mao C Toward a Molecular Understanding of the Antibacterial Mechanism of Copper-Bearing Titanium Alloys against Staphylococcus aureus. Adv. Healthcare Mater 2016, 5 (5), 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McDevitt CA; Ogunniyi AD; Valkov E; Lawrence MC; Kobe B; McEwan AG; Paton JC A Molecular Mechanism for Bacterial Susceptibility to Zinc. PLOS Pathog 2011, 7 (11), e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Campoccia D; Montanaro L; Arciola CR A Review of the Biomaterials Technologies for Infection-Resistant Surfaces. Biomaterials 2013, 34 (34), 8533–8554. [DOI] [PubMed] [Google Scholar]
- 149.Bondarenko O; Juganson K; Ivask A; Kasemets K; Mortimer M; Kahru A, Toxicity of Ag, CuO and ZnO Nanoparticles to Selected Environmentally Relevant test Organisms and Mammalian Cells In Vitro: a Critical Review. Arch. Toxicol 2013, 87 (7), 1181–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao A; Hang R; Huang X; Zhao L; Zhang X; Wang L; Tang B; Ma S; Chu PK, The Effects of Titania Nanotubes with Embedded Silver Oxide Nanoparticles on Bacteria and Osteoblasts. Biomaterials 2014, 35 (13), 4223–4235. [DOI] [PubMed] [Google Scholar]
- 151.C Chouirfa H; Bouloussa H; Migonney V; Falentin-Daudré C Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater 2019, 83, 37–54. [DOI] [PubMed] [Google Scholar]
- 152.Lichter JA; Van Vliet KJ; Rubner MF, Design of Antibacterial Surfaces and Interfaces: Polyelectrolyte Multilayers as a Multifunctional Platform. Macromolecules 2009, 42 (22), 8573–8586. [Google Scholar]
- 153.Yeh M-L; Chen K-H; Chang N-J; Chen H-D; Lai K-A Prolonged Antibiotic Release by PLGA Encapsulation on Titanium Alloy. J. Med. Biol. Eng 2013, 33, 17–22. [Google Scholar]
- 154.Pichavant L; Amador G; Jacqueline C; Brouillaud B; Heroguez V; Durrieu MC pH-Controlled Delivery of Gentamicin Sulfate from Orthopedic Devices Preventing Nosocomial Infections. J. Control. Release 2012, 162 (2), 373–81. [DOI] [PubMed] [Google Scholar]
- 155.Lawson MC; Bowman CN; Anseth KS Vancomycin Derivative Photopolymerized to Titanium Kills S. epidermidis. Clin. Orthop. Relat. Res 2007, 461. [DOI] [PubMed] [Google Scholar]
- 156.Zhang F; Shi ZL; Chua PH; Kang ET; Neoh KG Functionalization of Titanium Surfaces via Controlled Living Radical Polymerization: From Antibacterial Surface to Surface for Osteoblast Adhesion. Ind. Eng. Chem. Res 2007, 46 (26), 9077–9086. [Google Scholar]
- 157.Lee D-W; Yun Y-P; Park K; Kim SE Gentamicin and Bone Morphogenic Protein-2 (BMP-2)-delivering Heparinized-titanium Implant with Enhanced Antibacterial Activity and Osteointegration. Bone 2012, 50 (4), 974–982. [DOI] [PubMed] [Google Scholar]
- 158.Yuan Z; Huang S; Lan S; Xiong H; Tao B; Ding Y; Liu Y; Liu P; Cai K Surface Engineering of Titanium Implants with Enzyme-triggered Antibacterial Properties and Enhanced Osseointegration in vivo. J. Mater. Chem. B 2018, 6 (48), 8090–8104. [DOI] [PubMed] [Google Scholar]
- 159.Wang T; Liu X; Zhu Y; Cui ZD; Yang XJ; Pan H; Yeung KWK; Wu S Metal Ion Coordination Polymer-Capped pH-Triggered Drug Release System on Titania Nanotubes for Enhancing Self-antibacterial Capability of Ti Implants. ACS Biomater. Sci. Eng 2017, 3 (5), 816–825. [DOI] [PubMed] [Google Scholar]
- 160.Wang T; Wang C; Zhou S; Xu J; Jiang W; Tan L; Fu J Nanovalves-Based Bacteria-Triggered, Self-Defensive Antibacterial Coating: Using Combination Therapy, Dual Stimuli-Responsiveness, and Multiple Release Modes for Treatment of Implant-Associated Infections. Chem. Mater 2017, 29 (19), 8325–8337. [Google Scholar]
- 161.Wu T-Y; Zhou Z-B; He Z-W; Ren W-P; Yu X-W; Huang Y Reinforcement of a New Calcium Phosphate Cement with RGD-chitosan-fiber. J. Biomed. Mater. Res. A 2014, 102 (1), 68–75. [DOI] [PubMed] [Google Scholar]
- 162.Wu T; Zhang Q; Ren W; Yi X; Zhou Z; Peng X; Yu X; Lang M Controlled Release of Gentamicin from Gelatin/Genipin Reinforced Beta-tricalcium Phosphate Scaffold for the Treatment of Osteomyelitis. J. Mater. Chem. B 2013, 1 (26), 3304–3313. [DOI] [PubMed] [Google Scholar]
- 163.Stigter M; Bezemer J; de Groot K; Layrolle P Incorporation of Different Antibiotics into Carbonated Hydroxyapatite Coatings on Titanium Implants, Release and Antibiotic Efficacy. J. Control. Release 2004, 99 (1), 127–137. [DOI] [PubMed] [Google Scholar]
- 164.Zhang L; Ning C; Zhou T; Liu X; Yeung KW; Zhang T; Xu Z; Wang X; Wu S; Chu PK Polymeric Nanoarchitectures on Ti-basedIimplants for Antibacterial Applications. ACS Appl. Mater. Interfaces 2014, 6 (20), 17323–17345. [DOI] [PubMed] [Google Scholar]
- 165.Zilberman M; Elsner JJ Antibiotic-eluting Medical Devices for Various Applications. J. Control. Release 2008, 130 (3), 202–215. [DOI] [PubMed] [Google Scholar]
- 166.Zhou Z; Seta J; Markel DC; Song W; Yurgelevic SM; Yu XW; Ren W Release of Vancomycin and Tobramycin from Polymethylmethacrylate Cements Impregnated with Calcium Polyphosphate Hydrogel. J. Biomed. Mater. Res. B, Appl. Biomater 2018, 106 (8), 2827–2840. [DOI] [PubMed] [Google Scholar]
- 167.Poole K Mechanisms of Bacterial Biocide and Antibiotic Resistance. J. Appl. Microbiol 2002, 92 Suppl, 55s–64s. [PubMed] [Google Scholar]
- 168.Alarcón C. d. l. H.; Pennadam S; Alexander C Stimuli Responsive Polymers for Biomedical Applications. Chem. Soc. Rev 2005, 34 (3), 276–285. [DOI] [PubMed] [Google Scholar]
- 169.Tang F; Li L; Chen D Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater 2012, 24 (12), 1504–1534. [DOI] [PubMed] [Google Scholar]
- 170.Kumar C; Chaudhari A Efficient Renaturation of Immobilized Met-hemoglobin at the Galleries of α-zirconium Phosphonate. Chem. Mater 2001, 13 (2), 238–240. [Google Scholar]
- 171.Kumar CV; Chaudhari A Proteins Immobilized at the Galleries of Layered α-zirconium Phosphate: Structure and Activity Studies. J. Am. Chem. Soc 2000, 122 (5), 830–837. [Google Scholar]
- 172.Li Z; Yuan D; Jin G; Tan BH; He C Facile Layer-by-Layer Self-Assembly toward Enantiomeric Poly(lactide) Stereocomplex Coated Magnetite Nanocarrier for Highly Tunable Drug Deliveries. ACS Appl. Mater. Interfaces 2016, 8 (3), 1842–1853. [DOI] [PubMed] [Google Scholar]
- 173.Zhou J; Horev B; Hwang G; Klein MI; Koo H; Benoit DS Characterization and Optimization of pH-responsive Polymer Nanoparticles for Drug Delivery to Oral Biofilms. J. Mater. Chem. B 2016, 4 (18), 3075–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xiang Y; Liu X; Mao C; Liu X; Cui Z; Yang X; Yeung KWK; Zheng Y; Wu S Infection-Prevention on Ti Implants by Controlled Drug Release from Folic acid/ZnO Quantum dots Sealed Titania Nanotubes. Mater. Sci. Eng. C 2018, 85, 214–224. [DOI] [PubMed] [Google Scholar]
- 175.Zhao J; Xu J; Jian X; Xu J; Gao Z; Song Y-Y NIR Light-Driven Photocatalysis on Amphiphilic TiO2 Nanotubes for Controllable Drug Release. ACS Appl. Mater. & Interfaces 2020, 12 (20), 23606–23616. [DOI] [PubMed] [Google Scholar]
- 176.Tao B; Deng Y; Song L; Ma W; Qian Y; Lin C; Yuan Z; Lu L; Chen M; Yang X; Cai K BMP2-loaded Titania Nanotubes Coating with pH-responsive Multilayers for Bacterial Infections Inhibition and Osteogenic Activity Improvement. Colloids Surf. B Biointerfaces 2019, 177, 242–252. [DOI] [PubMed] [Google Scholar]
- 177.Dong Y; Ye H; Liu Y; Xu L; Wu Z; Hu X; Ma J; Pathak JL; Liu J; Wu G pH Dependent Silver Nanoparticles Releasing Titanium Implant: A Novel Therapeutic Approach to ControlPperi-implant Infection. Colloids Surf. B Biointerfaces 2017, 158, 127–136. [DOI] [PubMed] [Google Scholar]
- 178.Zhang T; Xie C; Liu Y; Zhang F; Xiao X pH-responsive Drug Release System of Cu2+-Modified Ammoniated TiO2 Nanotube Arrays. Mater. Lett 2018, 215, 95–98. [Google Scholar]
- 179.Tao B; Zhao W; Lin C; Yuan Z; He Y; Lu L; Chen M; Ding Y; Yang Y; Xia Z; Cai K Surface Modification of Titanium Implants by ZIF-8@Levo/LBL Coating for Inhibition of Bacterial-associated Infection and Enhancement of In Vivo Osseointegration. Chem. Eng. J 2020, 390, 124621. [Google Scholar]
- 180.Cloutier M; Mantovani D; Rosei F Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol 2015, 33 (11), 637–652. [DOI] [PubMed] [Google Scholar]
- 181.Noble ML; Mourad PD; Ratner BD Digital Drug Delivery: on–off Ultrasound Controlled Antibiotic Release from Coated Matrices with Negligible Background Leaching. Biomater. Sci 2014, 2 (6), 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Johnson CT; Wroe JA; Agarwal R; Martin KE; Guldberg RE; Donlan RM; Westblade LF; García AJ Hydrogel Delivery of Lysostaphin Eliminates Orthopedic Implant Infection by Staphylococcus aureus and Supports Fracture Healing. PNAS 2018, 115 (22), E4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu Y; Ran Q; Shen X; Zheng H; Cai K Enzyme Responsive Titanium Substrates with Antibacterial Property and Osteo/Angio-genic Differentiation Potentials. Colloids Surf. B Biointerfaces 2020, 185, 110592. [DOI] [PubMed] [Google Scholar]
- 184.Kazemzadeh-Narbat M; Lai BF; Ding C; Kizhakkedathu JN; Hancock RE; Wang R, Multilayered Coating on Titanium for Controlled Release of Antimicrobial Peptides for the Prevention of Implant-associated Infections. Biomaterials 2013, 34 (24), 5969–77. [DOI] [PubMed] [Google Scholar]
- 185.Shen X; Zhang F; Li K; Qin C; Ma P; Dai L; Cai K, Cecropin B Loaded TiO2 Nanotubes Coated with Hyaluronidase Sensitive Multilayers for Reducing Bacterial Adhesion. Mater. Des 2016, 92, 1007–1017. [Google Scholar]
- 186.Ramesh S; Govender T; Kruger HG; de la Torre BG; Albericio F, Short AntiMicrobial Peptides (SAMPs) as a Class of Extraordinary Promising Therapeutic Agents. J. Pept. Sci 2016, 22 (7), 438–451. [DOI] [PubMed] [Google Scholar]
- 187.Chen CH; Lu TK, Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics (Basel) 2020, 9 (1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carmona G; Rodriguez A; Juarez D; Corzo G; Villegas E, Improved Protease Stability of the Antimicrobial Peptide Pin2 Substituted with d-Amino Acids. Protein J 2013, 32 (6), 456–466. [DOI] [PubMed] [Google Scholar]
- 189.Ron-Doitch S; Sawodny B; Kühbacher A; David MMN; Samanta A; Phopase J; Burger-Kentischer A; Griffith M; Golomb G; Rupp S, Reduced Cytotoxicity and Enhanced Bioactivity of Cationic Antimicrobial Peptides Liposomes in Cell Cultures and 3D Epidermis Model Against HSV. J. Control. Release 2016, 229, 163–171. [DOI] [PubMed] [Google Scholar]
- 190.Touti F; Lautrette G; Johnson KD; Delaney JC; Wollacott A; Tissire H; Viswanathan K; Shriver Z; Mong SK; Mijalis AJ; Plante OJ; Pentelute BL, Antibody-Bactericidal Macrocyclic Peptide Conjugates To Target Gram-Negative Bacteria. ChemBioChem 2018, 19 (19), 2039–2044. [DOI] [PubMed] [Google Scholar]
- 191.Wang C; Wang Y; Zhang L; Miron RJ; Liang J; Shi M; Mo W; Zheng S; Zhao Y; Zhang Y Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater 2018, 30 (46), 1804023. [DOI] [PubMed] [Google Scholar]
- 192.Hu D; Li H; Wang B; Ye Z; Lei W; Jia F; Jin Q; Ren K-F; Ji J Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano 2017, 11 (9), 9330–9339. [DOI] [PubMed] [Google Scholar]
- 193.Yin W; Yu J; Lv F; Yan L; Zheng LR; Gu Z; Zhao Y Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10 (12), 11000–11011. [DOI] [PubMed] [Google Scholar]
- 194.Yuan Z; Tao B; He Y; Mu C; Liu G; Zhang J; Liao Q; Liu P; Cai K Remote Eradication of Biofilm on Titanium Implant via Near-Infrared Light Triggered Photothermal/Photodynamic Therapy Strategy. Biomaterials 2019, 223, 119479. [DOI] [PubMed] [Google Scholar]
- 195.He C; Duan X; Guo N; Chan C; Poon C; Weichselbaum RR; Lin W Core-shell Nanoscale Coordination Polymers Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. Nat. Commun 2016, 7 (1), 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mao C; Xiang Y; Liu X; Cui Z; Yang X; Li Z; Zhu S; Zheng Y; Yeung KWK; Wu S Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation To Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12 (2), 1747–1759. [DOI] [PubMed] [Google Scholar]
- 197.Li X; Lee D; Huang J-D; Yoon J Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem. Int. Ed 2018, 57 (31), 9885–9890. [DOI] [PubMed] [Google Scholar]
- 198.Feng Z; Liu X; Tan L; Cui Z; Yang X; Li Z; Zheng Y; Yeung KWK; Wu S Electrophoretic Deposited Stable Chitosan@MoS2 Coating with Rapid In Situ Bacteria-Killing Ability under Dual-Light Irradiation. Small 2018, 14 (21), 1704347. [DOI] [PubMed] [Google Scholar]
- 199.Berman AT; Reid JS; Yanicko DR Jr.; Sih GC; Zimmerman MR, Thermally Induced Bone Necrosis in Rabbits. Relation to Implant Failure in Humans. Clin. Orthop. Relat. Res 1984, (186), 284–92. [PubMed] [Google Scholar]
- 200.Li Y; Liu X; Li B; Zheng Y; Han Y; Chen D.-f.; Yeung KWK; Cui Z; Liang Y; Li Z; Zhu S; Wang X; Wu S Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14 (7), 8157–8170. [DOI] [PubMed] [Google Scholar]
- 201.Raphel J; Holodniy M; Goodman SB; Heilshorn SC, Multifunctional Coatings to Simultaneously Promote Osseointegration and Prevent Infection of Orthopaedic Implants. Biomaterials 2016, 84, 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mas-Moruno C; Su B; Dalby MJ, Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Healthc. Mater 2019, 8 (1), e1801103. [DOI] [PubMed] [Google Scholar]
- 203.An YH; Friedman RJ, Animal Models of Orthopedic Implant Infection. J. Invest. Surg 1998, 11 (2), 139–46. [DOI] [PubMed] [Google Scholar]
- 204.An YH; Kang QK; Arciola CR, Animal Models of Osteomyelitis. Int. J. Artif. Organs 2006, 29 (4), 407–20. [DOI] [PubMed] [Google Scholar]
- 205.Gatin L; Saleh-Mghir A; Massin P; Cremieux AC, Critical analysis of experimental models of periprosthetic joint infection. Orthop Traumatol Surg Res 2015, 101 (7), 851–5. [DOI] [PubMed] [Google Scholar]
- 206.Bottagisio M; Coman C; Lovati AB, Animal Models of Orthopaedic Infections. A Review of Rabbit Models used to Induce Long Bone Bacterial Infections. J. Med. Microbiol 2019, 68, 506–537. [DOI] [PubMed] [Google Scholar]
- 207.Jie K; Deng P; Cao H; Feng W; Chen J; Zeng Y, Prosthesis Design of Animal Models of Periprosthetic Joint infection Following Total Knee Arthroplasty: A Systematic Review. PLoS One 2019, 14 (10), e0223402. [DOI] [PMC free article] [PubMed] [Google Scholar]


