Introduction
Trauma is the leading cause of death in children older than 1 year, accounting for 30 to 50% of deaths (1). Four percent of injured children receive a blood transfusion within the first 24 hours after hospital admission, and the mortality for children who receive any blood transfusion is 13.6% (2). Research studies from combat regions in the last decade and recent randomized controlled trials have advanced the science of adult trauma resuscitation (3–6). This has led to the generation of evidence-based clinical practice guidelines for transfusion in adult trauma patients (7, 8).
Evidence-based guidelines for transfusion in injured children, however, are lacking. Existing pediatric transfusion guidelines have been developed by extrapolation of recommendations for adult trauma patients (8, 9). However, these extrapolated guidelines do not account for the unique mechanistic and physiologic differences in pediatric trauma patients (10). Unfortunately, the lack of evidence for pediatric transfusion after trauma, specifically on indications and thresholds to initiate transfusion and the ratios of blood product components (red blood cells, plasma, and platelets), has prevented the creation of evidence-based guidelines for children.
Protocol development for the Traumatic Injury Clinical Trial Evaluating Tranexamic Acid in Injured Children (TIC-TOC) (ClinicalTrials.gov NCT02840097) created a need to standardize transfusion practices across study sites given the lack of pediatric-specific evidence-based guidelines. Given this need, we sought to use the Delphi method to build a comprehensive consensus-based guideline for transfusion of blood products in injured children.
The Delphi method was developed in the 1950’s by the Rand corporation to obtain reliable consensus statements regarding highly complex military questions from a panel of experts (11). Since then, the Delphi method has become a well-recognized process to generate consensus statements in medicine using iterative surveys (11). The fundamental Delphi tenets include: 1) anonymity of responses amongst Delphi panelists, 2) iterative surveys that converge over time towards consensus, 3) controlled feedback in between survey rounds, and 4) statistical “group response,” where feedback in between survey rounds includes quantitative data allowing non-emotional re-evaluation of previous responses in comparison to the whole panel (11). We hypothesized that we would be able to generate consensus regarding transfusion practices in pediatric trauma using the above principles in a modified Delphi process.
Methods
Panel Formation
We included Delphi panelists with extensive experience in pediatric trauma care from the four hospitals of the TIC-TOC trial (Table 1). Fifty panelists were invited from the four institutions across multiple specialties (pediatric emergency medicine, pediatric/trauma surgery, anesthesia, pediatric intensive care, and transfusion medicine) to meet our pre-specified goal of at least 23 panelists. A threshold of 23 panelists has been shown to produce statistically reliable results over multiple rounds in prior Delphi studies (12). Two authors (AFT/KMT) acted as study facilitators, did not participate on the panel, and were responsible for data analysis after each survey round and the construction of subsequent surveys.
Table 1.
Panel Composition
| Survey Site | Number (%) |
|---|---|
| Children’s Hospital of Philadelphia/University of Pennsylvania | 8 (23) |
| Nationwide Children’s Hospital/Ohio State University | 9 (26) |
| Primary Children’s/University of Utah | 8 (23) |
| University of California, Davis and UC Davis Health | 10 (28) |
| Total | 35 |
| Specialty | Number (%) |
| Pediatric Surgery/Trauma Surgery | 8 (23) |
| Emergency Medicine | 11 (31) |
| Pediatric Intensive Care | 6 (17) |
| Anesthesia | 5 (14) |
| Transfusion Medicine | 5 (14) |
| Total | 35 |
Delphi Survey Development
Electronic surveys were created in SurveyMonkey (www.surveymonkey.com) for distribution to the panelists (Appendix). The surveys were designed to be completed anonymously. Three rounds of surveys were planned with the option for a fourth round to address outstanding issues.
The first-round survey was developed based on a review of prior literature and pilot tested and refined based on group consensus of the authors prior to distribution to the Delphi panelists. The survey aimed to generate consensus across 25 clinical transfusion categories. Examples of clinical categories included transfusion triggers in hemodynamically stable children, volumes and order of intravenous (IV) fluids and blood products, massive transfusion definition, triggers, and blood product ratios, and laboratory measurements. Anonymous comments by panelists to the study facilitators were allowed and encouraged to ensure validity of the survey content. The 2nd through 4th survey rounds were distributed after data analysis of prior rounds and included a summary of the data analysis as feedback to the panelists to inform subsequent responses.
Feedback included the results of the most recently completed round as expressed in percent agreement and disagreement by the panelists on any one statement, interquartile ranges of responses, and the coefficients of variation for responses. Feedback to the panelists also included the results of the responses stratified by specialty. Comments by panelists from previous survey rounds were also selectively included in the feedback.
Definition of Consensus
Statements were scored on a 5-point Likert scale (1- Strongly agree, 2-Agree, 3-Unsure, 4-Disagree, and 5-This intervention may be harmful). Statements were accepted by the panel if 80% or more of panelists rated a statement as either “Strongly agree” or “Agree.” Panelists were allowed a response of “Unsure,” which did not affect acceptance or rejection of a statement. A statement was rejected if 80% or more panelists rated a statement “Disagree” or “This intervention may be harmful.” Statements that were neither accepted nor rejected were reconsidered in subsequent survey rounds. Remaining statements that did not reach consensus by the end of the third round were rejected. A limited fourth round was necessary to clarify several points of the final algorithm. Accepted statements were incorporated into a final transfusion algorithm. This was vetted by the panelists on a final teleconference and accepted with some caveats given.
Analysis
Percent agreement and disagreement by the panelists, interquartile ranges of responses, and the coefficients of variation were all tabulated for every statement during the Delphi process. Descriptive statistics were used to compare proportions between rounds for overall rate of statement acceptance. Differences in consensus by panelists’ specialty and site were evaluated within each round and between rounds with a Chi-square test. A p-value less than or equal to 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Statement Evaluation
Thirty-five of the 50 (70%) invited panelists participated in the entire modified Delphi process, exceeding our pre-specified goal of greater than 23 panelists (Table 1). A total of 176 statements were graded during the first-round survey across the 25 clinical categories. A flow chart of accepted and rejected statements is demonstrated in Figure 1. At the conclusion of the first-round survey, 12 (6.8%) statements were accepted as clinical recommendations by our established criteria and 31 (16.5%) statements were rejected (Table 2). An additional two statements which met criteria for acceptance in the first-round survey were re-rated in the second-round survey due to comments by panelists.
Figure 1.
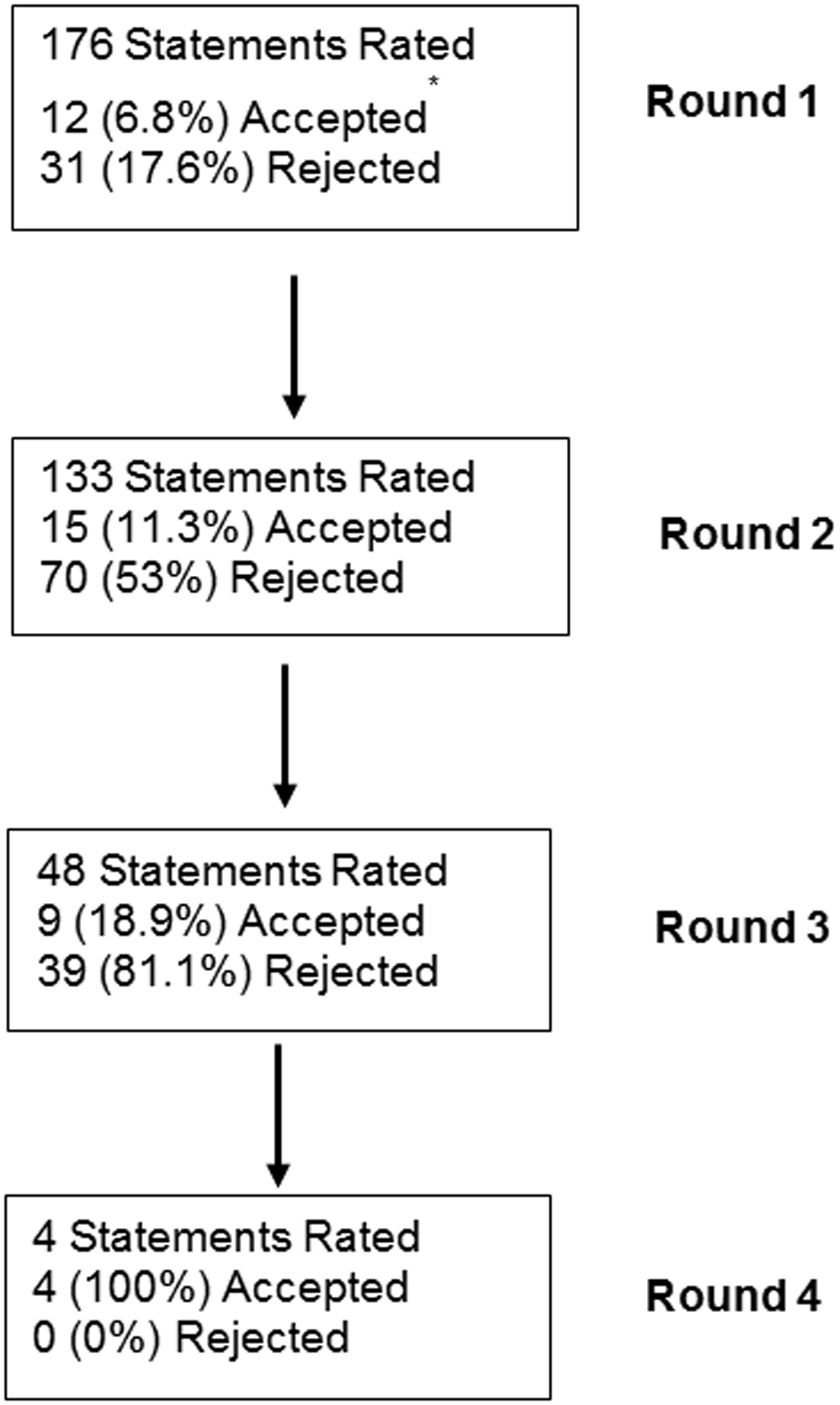
Flow chart of accepted statements by Delphi survey round.
*14 statements were accepted in round one, but two statements were re-rated based on participant comments.
Table 2.
List of statements accepted by Delphi survey round. (R1 = Round 1, R2 = Round 2, R3 = Round 3, R4 = Round 4)
| Accepted Statements by Round | % of panelists who accept per round | |
|---|---|---|
| Round 1 | ||
| The optimal resuscitative strategy in a hypotensive child with signs of head injury who arrives to the trauma room with potential ongoing bleeding is: | Crystalloid administration to attempt restoration of normotension while awaiting blood products. | 87.9% |
| During massive transfusion, the following laboratory values should be performed at baseline: | CBC | 94.1% |
| Chem 7 | 82.1% | |
| Type and Screen | 100% | |
| PT/PTT/INR | 94.1% | |
| The following laboratory values should be monitored periodically during the course of ongoing massive transfusion: | CBC | 94.1% |
| PT/PTT/INR | 94.1% | |
| Fibrinogen Level | 91.2% | |
| The following laboratory values should be monitored at the conclusion of a massive transfusion: | CBC | 94.1% |
| Chem 7 | 82.4% | |
| PT/PTT/INR | 88.2% | |
| Fibrinogen | 91.2% | |
| Round 2 | ||
| In pediatric trauma, a hemodynamically stable child without symptoms of anemia who is at low risk for bleeding should receive blood transfusion to have their hemoglobin maintained at, or above: | 6 g/dL | R1 – 71.4% R2 – 80.0% |
| In pediatric trauma, a hemodynamically stable child with symptoms of anemia who is at low risk for bleeding should receive blood transfusion to have their hemoglobin maintained at, or above1: | 7 g/dL | R1 – 94.3% R2 – 80.0% |
| A hemodynamically stable child at high risk for bleeding should have their platelet count maintained at or above: | 50,000/microL | R1 – 77.1% R2 – 88.6% |
| A hemodynamically stable child with an average risk of bleeding should have their platelet count maintained at or above: | 20,000/microL | R1 – 74.3% R2 – 85.7% |
| A hemodynamically stable child at high risk for bleeding should have their INR maintained at or below: | 2.0 | R1 – 64.7% R2 – 80.0% |
| A hemodynamically stable child at average risk of bleeding should have their INR maintained at or below: | 2.0 | R1 – 61.8% R2 – 90.9% |
| What defines “massive transfusion” in children? | The administration of blood products equaling one or more blood volume in 24 hours or one half a blood volume in 12 hours. | R1 – 70.6% R2 – 85.7% |
| During massive transfusion, the following laboratory values should be performed at baseline: | Lactate | R1 – 70.6% R2 – 82.4% |
| Fibrinogen | R1 – 73.5% R2 – 82.4% |
|
| Point of care testing ABG or VBG + Expanded lab assessment including chemistry, hemoglobin/hematocrit and lactate | R1 – 64.7% R2 – 88.2% |
|
| The following laboratory values should be monitored periodically during the course of ongoing massive transfusion: | Chem 7 | R1 – 79.4% R2 – 85.3% |
| Lactate | R1 – 67.6% R2 – 85.3% |
|
| Point of care testing ABG or VBG + Expanded lab assessment including chemistry, hemoglobin/hematocrit and lactate | R1 – 61.8% R2 – 94.1% |
|
| The following laboratory values should be monitored at the conclusion of a massive transfusion: | Lactate | R1 – 67.6% R2 – 85.3% |
| Point of care testing ABG or VBG + Expanded lab assessment including chemistry, hemoglobin/hematocrit and lactate | R1 – 50.0% R2 – 88.2% |
|
| Round 3 | ||
| The optimal resuscitative strategy in a hypotensive child without signs of head injury who arrives to the trauma room with potential ongoing bleeding is: | Crystalloid administration to attempt restoration of normotension while awaiting blood products. | R1 – 57.6% R2 – 68.6% R3 – 94.3% |
| In a hemodynamically unstable child with potential ongoing bleeding, the most appropriate first step in resuscitation is2: | 20mL/kg crystalloid solution | R1 – 55.9% R2 – 77.1% R3 – 97.1% |
| Under what circumstances should the pediatric massive transfusion protocol be activated? | Anticipation of transfusion of > 70mL/kg any blood product | R1 – 58.8% R2 – 61.8% R3 – 80.0% |
| Anticipation of transfusion of a blood volume in 24 hours. | R1 – 67.6% R2 – 70.6% R3 – 88.6% |
|
| During massive transfusion of a bleeding child, packed red blood cells (PRBC) should be given in the following ratio in relation to fresh frozen plasma (FFP): | 2:1 ratio by volume to simulate blood product activity/ratios found in whole blood | R1 – 55.9% R2 – 64.7% R3 – 80.0% |
| During massive transfusion of a bleeding child, platelets should be administered: | After every round of PRBC and FFP | R1 – 47.1% R2 – 67.6% R3 – 80.0% |
| During massive transfusion of a bleeding child, platelets should be given in the following ratio with PRBC and FFP3: | 1:1:1 ratio in an attempt to simulate the activity/ratios present in whole blood – lower platelet volume. | R1 – 50.0% R2 – 70.6% R3 – 82.9% |
| During massive transfusion of a bleeding child, cryoprecipitate should be administered: | When the fibrinogen level is < 100mg/dL | R1 – 47.1% R2 – 73.5% R3 – 88.6% |
| At a minimum, point of care testing (Ideally ABG or VBG/Chemistry/Hemoglobin/Hematocrit/Lactate) during massive transfusion should be performed every: | 1 hour | R1 – 47.1% R2 – 76.5% R3 – 94.3% |
| Round 4 | ||
| Under what circumstances should the pediatric massive transfusion protocol be activated?: | Anticipation of emergency transfusion of > 40mL/kg of any blood product. | R1 – 52.9% R2 – 70.6% R3 – 74.3% R4 – 97.1% |
| Under what circumstances should the pediatric massive transfusion protocol be activated?: | After emergency transfusion of > 40mL/kg of any blood product. | R1 – 64.7% R2 – 61.8% R3 – 51.4% R4 – 100% |
| Your patient remains hypotensive after your initial intervention. In a hemodynamically unstable child with potential ongoing bleeding, the most appropriate second step in resuscitation is: # | 20mL/kg PRBC | R1 – 32.4% R2 – 42.9% R3 – 71.4% R4 – 94.3% |
This statement was re-rated in round 2 – see text.
This statement was accepted with caveats given on the end of Delphi teleconference. Rejecting the concept of permissive hypotension for children, hypotensive children should receive resuscitation with whatever fluid is immediately available. If blood is immediately available, then it should be given first (also see round 4 statement marked with #).
This is rectified with the statement above in the discussion section. A 1:1:1 ratio of PRBC:FFP:Platelets to simulate the activity found in whole blood was determined to be 2:1:0.3–0.5 by volume.
In the second round, 133 statements were considered and rated and 15 (11.3%) were accepted, while 70 (53%) statements were rejected. Forty-eight statements were rated in the third round, with 9 (18.8%) statements accepted and 39 (81.3%) rejected.
Round four was a limited round to add clarity to previously accepted statements. The full list of accepted statements demonstrated in Table 2 was then used to create a clinically-useful transfusion algorithm (Figure 2). The ideal transfusion ratio was used to extrapolate ideal transfusion volumes by Broselow color (Figure 3).
Figure 2.
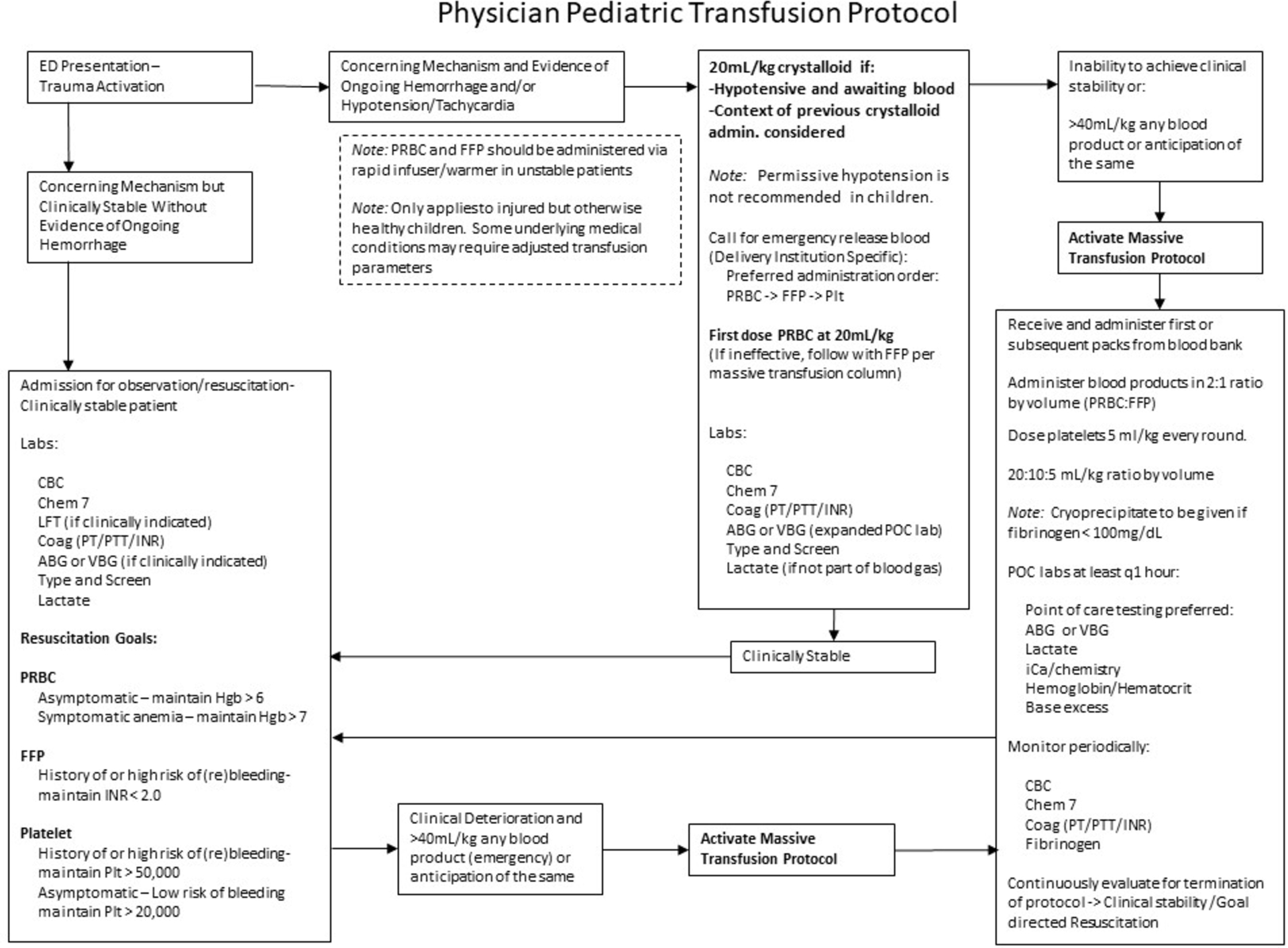
Transfusion Algorithm.
Figure 3.
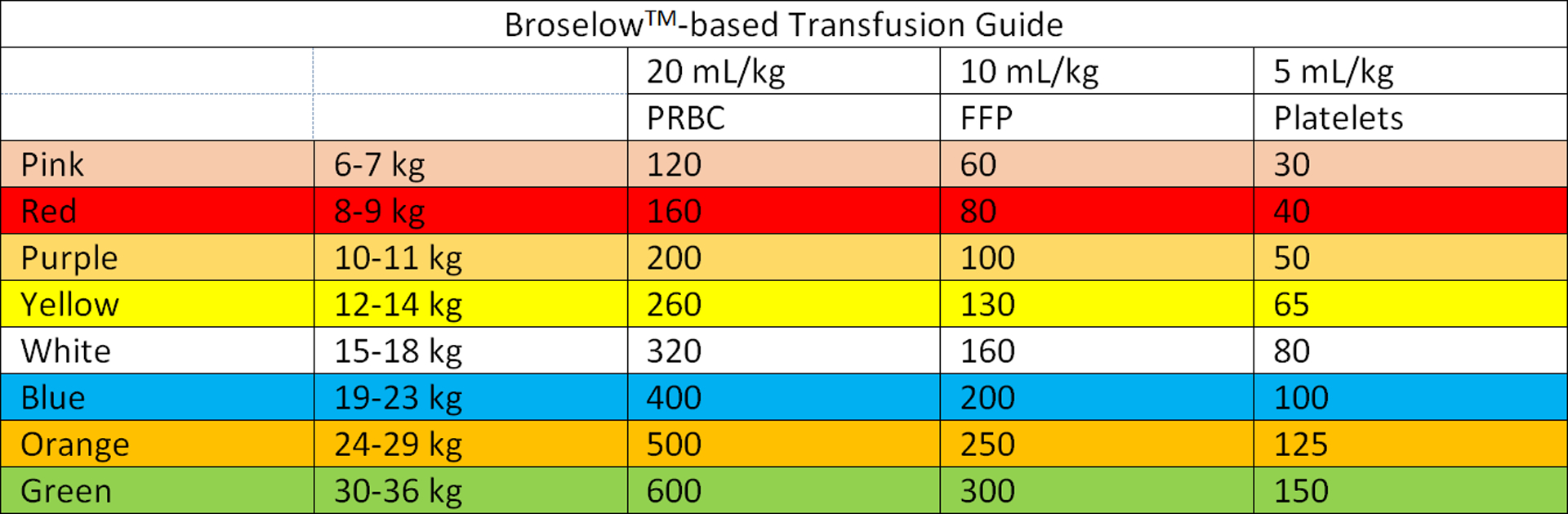
Broselow™-Based Transfusion Guide.
These interpretations of the results were vetted by the panel on a last teleconference and were agreed upon after discussion, with some caveats. One statement that was accepted in the third round was given a strong caveat during the end of study teleconference: “In a hemodynamically unstable child with potential ongoing bleeding, the most appropriate first step in resuscitation is: 20mL/kg of crystalloid.” The panel stated that this was intended to represent a rejection of the concept of permissive hypotension in children, and that if blood is immediately available (not the case in some of the participating institutions), it should be given first (20mL/kg). If further resuscitation is required, the panel stated that PRBC administration should be followed immediately by FFP (10mL/kg)
The proportion of statement acceptance between rounds was significantly different (p = 0.03) (Table 3). The trend was towards an increased proportion of statement acceptance throughout the process.
Table 3.
Statement acceptance by subgroups.
| Subgroup Consensus | Round | Between-Round Comparisonb | ||
|---|---|---|---|---|
| 1 | 2 | 3 | p value | |
| 80+% Agree (%) | 80+% Agree (%) | 80+% Agree (%) | ||
| Overall Percent Statement Acceptance | 6.8 | 11.3 | 18.8 | 0.0330 |
| Specialty | ||||
| Anesthesia | 3.4 | 4.5 | 20.8 | <0.0001 |
| Emergency Medicine | 14.2 | 19.5 | 25.0 | 0.1693 |
| Pediatric Intensive Care | 16.5 | 22.6 | 27.1 | 0.1840 |
| Pediatric Surgery/Trauma Surgery | 11.9 | 7.5 | 12.5 | 0.3945 |
| Transfusion Medicine | 4.0 | 10.5 | 18.8 | 0.0026 |
| Within-round comparisona | p<0.0001 | p<0.0001 | p=0.4105 | |
| Site | ||||
| A | 10.2 | 9.8 | 8.3 | 0.9263 |
| B | 12.5 | 17.3 | 18.8 | 0.3823 |
| C | 7.4 | 16.5 | 20.8 | 0.0102 |
| D | 9.7 | 4.5 | 29.2 | <0.0001 |
| Within-round comparisona | p=0.4572 | p=0.0032 | p=0.0787 | |
represent within-round comparisons between specialties and sites
represent between-round comparisons stratified by specialty and site except for the row demonstrating overall percent statement acceptance.
Responses by Site and Specialty
There was an association between percent statement acceptance by panelists and their institutional affiliations in the second-round survey (p=0.003) that was not identified in the first and third round surveys (p=0.46 and p=0.08, respectively) (Table 3). There was an association between percent statement acceptance based on specialty in the first- and second-round surveys, but this was not identified in the third round (p < 0.01 first and second round, and p=0.41 third round). In all rounds, the pediatric intensivists were the most likely subgroup to agree. The percentage of statements for which > 80% of subgroups (site and specialty) rated a statement either “Agree” or “Strongly agree” (in the “agree” range) are demonstrated in Table 3.
Discussion
Using a modified Delphi method, we achieved consensus regarding blood product transfusion practices for pediatric trauma patients across twenty-three clinical categories, including indicators, quantities and transfusion ratios. We were able to adapt these consensus statements into a comprehensive, consensus-based and sensible transfusion guideline, including a transfusion algorithm (including massive transfusion ratios/volumes) for injured children.
One of the strengths of this study was the multi-disciplinary, multi-institution panel. The panel’s recommendations represented a wide array of non-controversial and controversial topics. We interpreted those topics where our panel reached early consensus in the survey process as the least controversial, while those that reached consensus in the last rounds or required clarification on the end of study teleconference as the most controversial. Generally, those topics that reached early consensus (first and second rounds) are those that had more support by guidelines or evidence or had greater face-validity.
Topics that reached consensus in the first round: baseline labs for injured children requiring massive transfusion and the recommendation against permissive hypotension in head injured patients. The acquisition of baseline labs at the time of establishment of IV access for guiding resuscitation and aiding in prognosis are intuitive (13). Likewise, the recommendation against permissive hypotension in children with head injuries is supported by adult trauma (US Army Institute of Surgical Research [USAISR]) guidelines and adult studies that suggest that a strategy that purposefully decreases cerebral perfusion pressure is not advised and may lead to worse outcomes (8, 14).
The recommendations given in round two of the survey are supported by some published guidelines and data. These recommendations expanded on the recommended laboratory monitoring surrounding massive transfusion, and included recommendations for transfusion triggers for hemodynamically stable children. While data to support transfusion thresholds in these children are lacking, there is a trend towards restrictive transfusion strategies with all blood products, which is supported by guidelines from the British Standards in Hematology Guidelines, guidelines published by the American Association of Blood Banks (AABB), consensus recommendations from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (not available during this study), and some small trials (9, 15–18). Similarly, the recommendation for defining massive transfusion as “the administration of blood products equaling one or more blood volumes in 24 hours, or one half a blood volume in 12 hours” is supported by recent literature (19). In children, one-half of a blood volume is approximately 40mL/kg (20). In one study, resuscitation beyond this threshold was independently associated with an increased 24-hour mortality (19).
Topics that didn’t reach consensus until the third or fourth round of surveys are the most controversial topics pertaining to transfusion in pediatric trauma and panelist opinion represents the highest level of evidence available. These topics include a rejection of the concept of permissive hypotension in children with torso injuries (in addition to the rejection of the concept in head injury discussed above) and massive transfusion ratios and triggers. Regarding permissive hypotension in torso injury, the concept is part of adult trauma guidelines and is an accepted part of damage control resuscitative strategy (8, 14). Given the common observation that pediatric trauma patients may be able to maintain normal blood pressures until sudden circulatory collapse, the panel recommended immediate resuscitation in hypotensive pediatric patients with whatever resources are available, stating that, “In a hemodynamically unstable child with potential ongoing bleeding, the most appropriate first step in resuscitation is 20mL/kg of crystalloid.” Upon review of accepted statements at the end of study teleconference, the caveat was given that blood should be given first if immediately available (which is a challenge at some of the participating institutions).
Furthermore, in the third survey round, the panelists recommended that during massive transfusion of a bleeding child, packed red blood cells (PRBC) should be given in the following ratio in relation to fresh frozen plasma (FFP): 2:1 ratio by volume to simulate blood product activity/ratios found in whole blood. While the 2:1 ratio of PRBC to FFP is contrary to the findings of the PROPPR trial (4), we point out that these data were not generated in children and that data validating transfusion ratios in massive transfusion for injured children is lacking.
From a theoretical standpoint, a 2:1 ratio of PRBC to FFP by volume has been proposed, in order to maintain adequate coagulation factor activity as well as oxygen carrying capacity (21). The theoretical risk of improperly proportioned ratios (by volume) in children is magnified as the total circulating blood volume decreases. Moreover, an analysis of the Department of Defense Trauma Registry demonstrated that a low ratio of PRBC to FFP (approaching 1:1 by volume) increased the odds of mortality of pediatric patients receiving any blood product within the first 24 hours of trauma admission (22). A subsequent study of the same database by Cannon et al, revealed no difference in hospital mortality for “balanced resuscitation” using a 1:1 ratio by volume of PRBC to FFP (23). The study demonstrated that as the proportion of plasma increased in relation to PRBC, hospital length of stay significantly increased. Hence, the 2:1 ratio of PRBC/FFP proposed by the panel. Further study will be needed to clarify the appropriate ratio of PRBC/FFP for children during the empirical phase of massive transfusion.
Regarding platelet administration, the following statement was accepted by the panel: “During massive transfusion of a bleeding child, platelets should be given in the following ratio with PRBC and FFP: 1:1:1 ratio in an attempt to simulate the activity/ratios present in whole blood – lower platelet volume.” In order to rectify this statement with the 2:1 ratio by volume of PRBC to FFP stated above, the transfusion medicine specialists were asked to clarify that in order to attempt to simulate the activity of whole blood, platelets should be administered in a 2:1:0.3–0.5 (PRBC:FFP:Platelet) ratio by volume. For simplicity, this can be represented as 20:10:5 mL/kg per round of transfusion with the respective blood products.
By identifying a by-weight-based volume dosing of PRBC, FFP, and platelets (made simple by the proposed Broselow™-based guide) we are hoping to avoid over-resuscitation of children (especially the practice of using whole units of PRBC where it may not be appropriate). Additionally, by stating that this first dose of PRBC should be immediately followed by FFP and platelets, the panel recognizes the need to mitigate the development of trauma-induced coagulopathy.
While we think the recommendations of the panel represent a rational framework for resuscitation of injured children, our overall goal in its development was to attempt to standardize transfusion practices amongst the sites participating in the TIC-TOC trial. Given that the primary outcome for the trial is volume of blood product, standardization is of the utmost importance. Adoption of these transfusion practices by the participating sites is pending.
Limitations
Our results should be interpreted in the context of some limitations. While developed rigorously using a modified Delphi method, these recommendations still represent panelist opinion. Our goal was to have an equal number of panelists between specialties and sites, however some groups were represented somewhat more than others. To counter this, we performed analyses grouped by specialty and site which demonstrated no difference in statement acceptance based on these factors towards the last survey round. Furthermore, it is possible that the results could differ if we surveyed participants from other hospitals, however, we had representation from five different specialty groups from 4 geographically distinct trauma centers (including 3 freestanding children’s hospitals and an academic general hospital), making this unlikely.
Conclusion
Using a modified Delphi process, our multi-disciplinary panel achieved consensus on statements in pediatric trauma regarding transfusion triggers in hemodynamically stable and unstable patients, volumes and order of IV fluids and blood products, massive transfusion definition and triggers, blood product ratios, and laboratory measurements. These accepted statements were developed into a transfusion algorithm for severely injured children.
Funding source
This study was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R34HL135214. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project was also supported in part by the Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB), Emergency Medical Services for Children (EMSC) Network Development Demonstration Program under cooperative agreements U03MC00008, U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC22684, and U03MC22685. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government.
References
- 1.Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death in Selected Age Groups, by Race and Sex: United States, 1999–2015. In: System CfDCaP-NVS, editor. https://www.cdc.gov/nchs/nvss/mortality/lcwk3.htm2017.Accessed Nov 02, 2017. [Google Scholar]
- 2.Shroyer MC, Griffin RL, Mortellaro VE, Russell RT. Massive transfusion in pediatric trauma: analysis of the National Trauma Databank. J Surg Res. 2017;208:166–72. [DOI] [PubMed] [Google Scholar]
- 3.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. [DOI] [PubMed] [Google Scholar]
- 6.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. [DOI] [PubMed] [Google Scholar]
- 7.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–17. [DOI] [PubMed] [Google Scholar]
- 8.USAISR. Clinical Practice Guideline - Damage Control Resuscitation. Defense of Defense. http://www.usaisr.amedd.army.mil/cpgs.html2017. Accessed August 7, 2017. [Google Scholar]
- 9.New HV, Berryman J, Bolton-Maggs PH, Cantwell C, Chalmers EA, Davies T, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol. 2016;175(5):784–828. [DOI] [PubMed] [Google Scholar]
- 10.Inaba AS, Seward PN. An approach to pediatric trauma. Unique anatomic and pathophysiologic aspects of the pediatric patient. Emerg Med Clin North Am. 1991;9(3):523–48. [PubMed] [Google Scholar]
- 11.von der Gracht H Consensus measurement in Delphi studies: Review and implications for future quality assurance. Technol Forecast Soc Change. 2012;79(8). [Google Scholar]
- 12.Akins RB, Tolson H, Cole BR. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol. 2005;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalkwarf KJ, Jensen SD, Allukian M 3rd, Harting MT, Cox CS, Fox EE, et al. Can we identify futility in kids? An evaluation of admission parameters predicting 100% mortality in 1,292 severely injured children. J Am Coll Surg. 2018;226(4):662–7. [DOI] [PubMed] [Google Scholar]
- 14.Duchesne JC, McSwain NE Jr., Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, et al. Damage control resuscitation: the new face of damage control. J Trauma 2010;69(4):976–90. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–19. [DOI] [PubMed] [Google Scholar]
- 16.Nellis ME, Karam O, Mauer E, Cushing MM, Davis PJ, Steiner ME, et al. Platelet transfusion practices in critically ill children. Crit Care Med. 2018;46(8):1309–1317.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentine SL, Bembea MM, Muszynski JA, Cholette JM, Doctor A, Spinella PC, et al. Consensus recommendations for RBC transfusion practice in critically ill children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Ped Crit Care Med. 2018;19(9):884–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karam O, Russell RT, Stricker P, Vogel AM, Bateman ST, Valentine SL, et al. Recommendations on RBC transfusion in critically ill children with nonlife-threatening bleeding or hemorrhagic shock from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Ped Crit Care Med. 2018;19(9S Suppl 1):S127–s32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neff LP, Cannon JW, Morrison JJ, Edwards MJ, Spinella PC, Borgman MA. Clearly defining pediatric massive transfusion: cutting through the fog and friction with combat data. J Trauma Acute Care Surg. 2015;78(1):22–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 20.Chidester SJ, Williams N, Wang W, Groner JI. A pediatric massive transfusion protocol. J Trauma Acute Care Surg. 2012;73(5):1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory JA, Huitron SS, George AA, Simon CD. Optimizing transfusion ratios in massive transfusion protocols: an argument against the 1:1:1 dogma and approach to trauma resuscitation. Lab Med. 2015;46(2):e46–52. [DOI] [PubMed] [Google Scholar]
- 22.Edwards MJ, Lustik MB, Clark ME, Creamer KM, Tuggle D. The effects of balanced blood component resuscitation and crystalloid administration in pediatric trauma patients requiring transfusion in Afghanistan and Iraq 2002 to 2012. J Trauma Acute Care Surg. 2015;78(2):330–5. [DOI] [PubMed] [Google Scholar]
- 23.Cannon JW, Johnson MA, Caskey RC, Borgman MA, Neff LP. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J Trauma Acute Care Surg. 2017;83(2):211–7. [DOI] [PubMed] [Google Scholar]


