Abstract
Pollution by microplastics is of increasing concern due to their ubiquitous presence in most biological and environmental media, their potential toxicity and their ability to carry other contaminants. Knowledge on microplastics in freshwaters is still in its infancy. Here we reviewed 150 investigations to identify the common methods and tools for sampling microplastics, waters and sediments in freshwater ecosystems. Manta trawls are the main sampling tool for microplastic separation from surface water, whereas shovel, trowel, spade, scoop and spatula are the most frequently used devices in microplastic studies of sediments. Van Veen grab is common for deep sediment sampling. There is a need to develop optimal methods for reducing identification time and effort and to detect smaller-sized plastic particles.
Keywords: Microplastic pollution, Freshwater systems, Polymer, Sampling, Water, Sediment
Introduction
Ecosystems and their different organisms have been widely impacted by anthropogenic activities such as the discharge of pollutants (Hamidian et al. 2019; Mirzajani et al. 2016, 2015; Padash Barmchi et al. 2015; Rezaei Kalvani et al. 2019), including emerging contaminants (Jafari Ozumchelouei et al. 2020) such as microplastics. Endurance, flexibility, lightweight, being low cost and being waterproof allows for plastic use in different applications, leading to their accumulation in the environment (Pellini et al. 2018; Razeghi et al. 2020). Plastic materials are used in a wide variety of markets and industries, including packaging, building and construction, electrical, agriculture, consumer and household appliances such as toothpaste and facial scrubbers, etc. (Wu et al. 2018). According to data from Plastics Europe, world production of plastics reached 335 million tons in 2016 (Plastics Europe 2018). It is estimated that by 2050, this may increase to 33 billion tons (Horton et al. 2017). By then, 12,000 million metric tons (Mt) of plastic waste will have been accumulated in landfills and natural environments (Geyer et al. 2017). Recently, a sharp increase is induced in plastic waste production such as masks, gloves, and plastic shopping bags by the coronavirus disease (COVID-19) pandemic (Gorrasi et al. 2020). Once plastics are discharged into aquatic environments, they can persist for up to 50 years. Complete plastic mineralization may take hundreds or thousands of years (Holland et al. 2016).
Microplastics are generally defined as plastic particles smaller than a specified upper size limit (< 5 mm). However, sometimes smaller size limits have also been proposed. Currently, there is no specific lower size cutoff for this definition (Connors et al. 2017). Since the 1970s when the first reports of micro-sized particles were published, marine plastic pollution has been of concern (Carpenter and Smith 1972; Carpenter et al. 1972; Colton et al. 1974). Plastic debris in the ocean was recognized by the United Nations Environment Program (UNEP) as an emerging global environmental issue (Kershaw et al. 2011). However, “microplastics” were first described by Thompson and colleagues in 2004. They reported the occurrence and presence of plastics around 50 µm in size on shorelines and in water column (Thompson et al. 2004). Microplastics are commonly defined as plastic particles with sizes below 5 mm (Hidalgo-Ruz et al. 2012).
Depending on the way in which microplastics are produced, they can be classified into two classes as primary or secondary. Primary microplastics are small plastic particles released directly into the environment by domestic and industrial effluents, spills and sewage discharge or indirectly by runoff. Secondary microplastics are formed as a result of fragmentation of larger plastic particles already present in the environment. Fragmentation takes place due to UV radiation (photo-oxidation), mechanical transformation (e.g., via waves abrasion) and biological degradation by microorganisms (de Sá et al. 2018; Thompson et al. 2009).
There are hundreds of commercially available plastic materials. Polypropylene and low- and high-density polyethylene are the three most common used plastic polymers in packaging. Polyvinyl chloride, polyurethanes, polyethylene terephthalate, polystyrene and polyester are also widely used due to their various applications (Plastics Europe 2018).
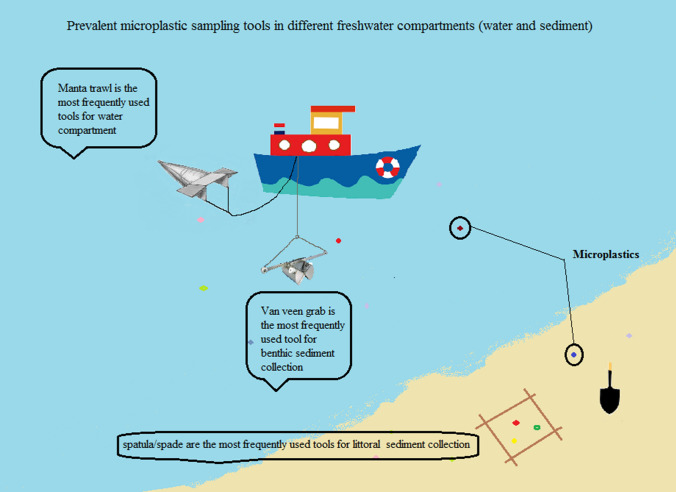
In terms of shape, microplastics fall into five main groups. Fragments are three-dimensional and hard jagged-edged particles. Pellets have hard rounded shape. Fibers are fibrous or thin uniform plastic strands, and films are thin, two-dimensional plastic pieces. Foam is a mass of tiny bubbles (i.e., styrofoam-type material) (Anderson et al. 2017; Rezania et al. 2018). Using different measuring instruments, different results may be obtained in terms of shape, size and type of microplastics. The major sampling techniques are shown in Fig. 1.
Fig. 1.
Common techniques to sample microplastics
Despite extensive research on microplastics in marine environments, less effort has been made to monitor microplastics in freshwaters. Freshwaters include water in ice sheets, ice caps, glaciers, icebergs, bogs, ponds, lakes, rivers, streams, marshlands, wetlands and groundwater. Freshwaters are generally characterized as having low concentrations (less than 1000 mg L−1) of dissolved salts and other total dissolved solids (American Meteorological Society 2012). Although the ocean floor is considered to be the ultimate fate of marine microplastics, inland water bodies might also be a terminal or transient sink for microplastics (Z. F. Wang et al. 2018b). Freshwater bodies can have comparable plastic concentrations to marine waters.
Microplastics can cause several harmful physical effects on humans and living organisms through such mechanisms as entanglement and ingestion. They can cause blockage of the gastrointestinal tract or inflammatory responses and consequently a range of adverse effects. Some effects include lower energy reserves, reduced reproduction/growth, oxidative damage, metabolism disruption, cellular lesions, starvation and even death (Ding et al. 2018; Ogonowski et al. 2016). Exposure of microplastics to a cohort of human adults (hand-face skin, head hair and saliva) has been reported (Abbasi and Turner 2021).
The trophic transfer of microplastics in the aquatic food web has been demonstrated by researchers (Farrell and Nelson 2013; Setälä et al. 2014). Microplastics’ large surface area to volume ratio provides a high association potential for environmental contaminants. Microplastics have an affinity for certain hazardous hydrophobic organic chemicals, non-essential trace elements and persistent organic pollutants. Some examples include polychlorinated biphenyls, dichlorodiphenyltrichloroethane, additives, plasticizers and heavy metals (Brennecke et al. 2016; Hartmann et al. 2017; Holland et al. 2016; Koelmans et al. 2016; Naqash et al. 2020).
Wastewater treatment plants receive large amounts of microplastics among other pollutants. However, efforts of treatment (Mojoudi et al. 2018, 2019) including biological methods (Alavian et al. 2018; Hamidian et al. 2016; Mansouri et al. 2013; Mirzajani et al. 2017) remove most of these emerging pollutants.
Different microplastics treatment methods include sorption and filtration, biological removal and ingestion, and chemical treatments. Sorption of microplastics on green algae is based on charged microplastics. Membrane technology regarding durability, influent flux, size and concentration of the microplastics in water and wastewater have shown good efficiency. Coagulation and agglomeration processes, using Fe-based and Al-based salts, are also reported. Electrocoagulation technique and photocatalytic degradation using TiO2 and ZnO semiconductors are used as robust and environmentally friendly techniques. Microplastics ingestion by organisms is also discussed as a removal strategy. However, sorption and filtration processes coupled with membrane bioreactors lead to higher microplastics removal compared to other methods (Hamidian et al. 2021; Padervand et al. 2020). Othman and coworkers reviewed microplastics degradation through enzymatic processes (Othman et al. 2021). ZnO nanorod photocatalysts excited by visible light were used to degrade low density polyethylene film in water (Tofa et al. 2019).
Inland waters and marine environments are facing similar issues related to microplastics presence. However, some differences like physical and chemical characteristics of water cannot be ignored. Here we review techniques for sampling microplastics in waters and sediments with focus of the following issues:
What is the evolution of the number of scientific studies on microplastics in freshwater and sediment?
Which freshwater compartments are more commonly investigated for microplastics?
Which sampling matrix, water or sediment, is most frequently studied?
What are the most common sampling methods in water and sediment studies?
What are the advantages and disadvantages of sampling methods?
Data acquisition
Literature was gathered through online search in the ISI Website of Knowledge, Science Direct and Google Scholar using keywords and phrases including “microplastic” OR AND “freshwater”, OR AND “plastic particle”, OR AND “plastic fragment”, OR AND “pellets” OR AND “river” OR AND “estuary” OR AND “lake”. The retrieved articles were then screened by study area, of which studies in water and sediment of inland water systems were selected including rivers, estuaries, lakes, reservoirs, estuaries, etc. After identifying candidate research, the abstracts of all studies reporting microplastics in freshwater ecosystems were studied. It is worthy noticing that microplastic research solely on microplastics in freshwater species was excluded. However, a combination of water or sediment studies with biota or all three (water, sediment and biota) were included simultaneously. A total of 150 published pieces of research between 2010 and 2020 were retrieved and evaluated. Details of each study were recorded in an EXCEL spreadsheet for subsequent analysis. This information was used to determine the extent and depth of current microplastic research and to identify important data gaps.
Microplastics presence in freshwater environments
Research papers with an emphasis on microplastics in inland water bodies are mostly published in the last ten years. Microplastics have been recorded along shorelines of the Tamar estuary, UK (Browne et al. 2010). In two urban rivers (the Los Angeles River and the San Gabriel River), Southern California, microplastics were found 16 times more abundant than macroplastics and three times heavier than the bigger particles (Moore et al. 2011). Zbyszewski and Corcoran (2011) scrutinized the distribution and degradation of plastic particles along the beaches of Lake Huron, Canada (Zbyszewski and Corcoran 2011). The first ecosystemic review, assessing microplastics in different compartments, including water, sediment and biota, was reported by Faure et al. in Lake Geneva, Switzerland. Plastics were found on every beach and in the surface layer of Lake Geneva. However, no plastics were observed in biota in this study (Faure et al. 2012). Studies detecting microplastics in different freshwater compartments across continents and even in remote areas (Free et al. 2014; Zhang et al. 2016) are summarized in Tables 1–4.
Table 2.
Microplastic studies in sediment of inland water bodies around the world
| Study compartment | Number | Study area | Sampling tool | Dominant microplastic characteristics (shape, polymer type, size) | Reference |
|---|---|---|---|---|---|
| River–estuary | 1 | Rivers and tidal flat of urban districts, Shanghai, China | Shovel (0.5 m × 0.5 m quadrat, depth of 5 cm) | Spheres, PP, | Peng et al. (2018) |
| Littoral sediment | 100 -500 µm | ||||
| 2 | Changjiang Estuary, China | Box corer (depth of 5–10 cm) | Fibers, rayon, 0–100 µm, 100 -500 µm, | Peng et al. (2017) | |
| Benthic sediment | 500–1000 µm (SMP) | ||||
| 3 | Beijiang River littoral zone, China | Stainless steel shovel (0.2 m × 0.2 m quadrate, depth of 2 cm) | PE | J.D. Wang et al. (2017) | |
| Littoral sediment | |||||
| 4 | St. Lawrence River, Canada | Petite Ponar grab (225 cm2 area), and Peterson Grab (930 cm2 area, depth of 10–15 cm) | Microbeads, PE | Castañeda et al. (2014) | |
| Benthic sediment | |||||
| 5 | Tamar Estuary, UK | – | Fibers, PES, | Browne et al. (2010) | |
| Littoral sediment | < 1 mm | ||||
| 6 | Rivers Rhine and Main, Germany | Stainless steel spoon (30 cm2 area) | Fragments, PS, | Klein et al. (2015) | |
| Littoral sediment | 63–200 µm | ||||
| 7 | Thames River Basin, UK | Stainless steel Scoop (depth of 10 cm) | Fragments, fiber, PET, | Horton et al. (2017a) | |
| Littoral sediment | 1–2 mm | ||||
| 8 | Gulf ofMexico estuaries (Mobile Bay, AL), USA | (0.25 m × 0.25 m quadrate, depth of 3–6 cm) | Hard plastics, PE, | Wessel et al. (2016) | |
| Littoral sediment | 0.2–1 mm | ||||
| 9 | 10 rivers, northwest UK | Cylinder resuspension technique | Microbeads | Hurley et al. (2018) | |
| Benthic sediment | |||||
| 10 | Two sandy beaches (Santubong and Trombol) in Kuching, Sarawak, Malaysia | Stainless steel scoop (0.2 m × 0.2 m quadrate, depth of 2 cm) | PP, PE | Noik and Tuah (2015) | |
| Littoral sediment | |||||
| 11 | Atoyac River Basin, Central Mexico | Van Veen grab sampler, trowel | Films | Shruti et al. (2019) | |
| Benthic sediment | |||||
| 12 | Urban river in Scotland (River Kelvin), UK | A spade (depth of 8–10 cm) | Fibers | Blair et al. (2019 | |
| Littoral sediment | |||||
| 13 | Derwent Estuary, Tasmania, Australia | Sediment corer (depth of 104 cm) | Fibers, < 63- > 100 µm | Willis et al. (2017) | |
| Benthic sediment | |||||
| 14 | Rhine River, Germany | Steel spade, buckets of a chain dredging (depth of 52 cm and 111 cm) | APV, 62–125 µm | Mani et al. (2019b) | |
| Benthic sediment | |||||
| 15 | Vitória bay estuarine system (SVB), Brazil | Van Veen grab sampler | Fibers | Neto et al. (2019 | |
| Benthic sediment | |||||
| 16 | River Tame and four of its tributaries, Birmingham, UK | Stainless steel scoop (depth of 5–10 cm) | FragmentS, PE, 63– < 250 µm, 250– < 1000 µm | Tibbetts et al. (2018) | |
| Benthic sediment | |||||
| 17 | BrisbaneRiver, Australia | Ponar stainless steel grab sampler (depth of 0–3 cm) | Films,, PE, 3–4 mm | He et al. (2020 | |
| Benthic sediment | |||||
| 18 | Liaohe estuary, Daliao River and Shuangtaizi River | Steel grab sampler | Films, PE | Xu et al. (2020) | |
| Benthic sediment | |||||
| 19 | Thames River, Ontario, Canada | Stainless steel petite ponar grab sampler (depth of 90–100 cm) | Pellets, PE, 1–5 cm | Corcoran et al. (2019) | |
| Benthic sediment | |||||
| 20 | Warnow estuarine, Germany | Van Veen grab, Sediment trap | PS | Enders et al. (2019) | |
| Benthic sediment | |||||
| 21 | River Yongfeng, China | Peterson Gravity Sampler | Films, PE, 200–500 μm, 500–1000 μm | Rao et al. (2020) | |
| Benthic sediment | |||||
| 22 | Jagir Estuary, Surabaya City, Indonesia | Ekman dredge sampler | Lines/fibers, PES, small MP (1 µm-1 mm) | Firdaus et al. (2020) | |
| Benthic sediment | |||||
| Stream | 1 | Seven water streamssurrounding the lagoon of Bizerte, Northern Tunisia | Stainless steel spatula (0.25 m × 0.25 m quadrate, depth of 2–3 cm) | Fibers, PP | Toumi et al. (2019) |
| Littoral sediment | |||||
| Lake–reservoir | 1 | Subalpine lake Garda, Italy | – | PE | Imhof et al. (2013) |
| Littoral sediment | |||||
| 2 | Lake Ontario, Canada | Glew gravity corer, shipek grab, ponar grab, split spoon corer | Fragments, PE | Ballent et al. (2016) | |
| Littoral sediment and Benthic sediment | |||||
| 3 | Lake Ontario, Canada | Mini box corer (depth of 30 cm) | Pellets, PE, 1–5 cm | Corcoran et al. (2015) | |
| Littoral sediment and Benthic sediment | |||||
| 4 | Remote lakes in Tibet plateau, China | Shovel (20 cm × 20 cm quadrate, depth of 2 cm) | PP, 1–5 mm | Zhang et al. (2016) | |
| Littoral sediment | |||||
| 5 | Beaches of Lake Huron, Canada | Stainless steel trowel | Pellets, PE, < 5 mm | Zbyszewski and Corcoran (2011) | |
| Littoral sediment | |||||
| 6 | Great Lakes, North America Lake Erie and St. Clair), USA | Stainless steel trowel | Fragments, PE | Zbyszewski et al. (2014) | |
| Littoral sediment | |||||
| 7 | Edgbaston Pool, Birmingham, UK | HTH gravity corer (depth of 10 cm) | Fibers, Films | Vaughan et al. (2017) | |
| Littoral sediment and Benthic sediment | |||||
| 8 | Setúbal Lake, Portugal | Stainless steel shovel (0.25 m × 0.25 m quadrate, 3 cm depth) | Fragments | Blettler et al. (2017) | |
| Littoral sediment | |||||
| 9 | Beaches of Lake Garda, Italy | Sediment cores (depth of 10 cm) | 1–50 μm | Imhof et al. (2018) | |
| Littoral sediment | |||||
| 10 | Lake Erie, Canada | Shipek sediment grab sampler and passive sediment trap, split spoon sampler, Petite Ponar grab sampler | Fibers, PE | Dean et al. (2018) | |
| Littoral sediment and Benthic sediment | |||||
| 11 | Subalpine Lake Garda, Italy | Sediment cores (depth of 5 cm) | – | Imhof et al. (2016) | |
| Littoral sediment | |||||
| 12 | Three Gorges Reservoir, China | Stainless steel Trowel (20 cm × 20 cm, depth of 2 cm) | Sheet,PP, 1—5 mm | Zhang et al. (2019 | |
| Littoral sediment | |||||
| 13 | Hampstead Pond (Lake), UK | Piston corer (depth of 212 cm) | Fibers | Turner et al. (2019) | |
| Benthic sediment | |||||
| 14 | Lake Mjøsa and Lake Femunden, Norway | Kajak-Brinkhurst sediment corer (depth of 3 cm), Van Veen grab (depth of 10–15 cm) | Fibers, PS, Small microplastics < 1 mm | Lusher et al. (2018) | |
| Benthic sediment | |||||
| 15 | Lake Victoria, Uganda, Africa | Stainless steel trowel (0.5 cm × 0.5 cm quadrat, depth of 5 cm), Ponar grab | Films (shoreline), filaments (lake), PE, 0.3–1 mm (lake) 1–2 (shoreline) | Egessa et al. (2020) | |
| Littoral sediment and Benthic sediment | |||||
| 16 | Donghu Lake, Wuhan, china | Piston gravity sampler (depth of 57 cm) | Fibers, PET, < 0.5 mm | Dong et al. (2020) | |
| Benthic sediment | |||||
| 17 | Lake Ziway, Ethiopia | Ekman grab sampler (depth of 0–2 cm) | Fragments, PE, 0.15–5 mm | Merga et al. (2020) | |
| Benthic sediment | |||||
| 18 | Donting Lake, China | Stainless steel shovel (0.25 m2 area, depth of 2 cm), grab sampler | Fibers, PET, PE, < 0.5 mm | Yin et al. (2020) | |
| Littoral sediment and Benthic sediment | |||||
| River–estuary–lake | 1 | Vembanad Lake, Kerala, India | Van Veen grab (25 cm2 area) | Films, Foams, LDPE | Sruthy and Ramasamy (2017) |
| Benthic sediment | |||||
| 2 | Urban water areas inChangsha, China | Shovel (depth of 5 cm) | Fragments, PS, < 0.5 mm | Wen et al. (2018) | |
| Littoral sediment | |||||
| 3 | Coastal plain river network (Wen-Rui Tang River watershed) in eastern China | Peterson grab (32 cm × 20 cm, depth of 0–15 cm) | Figments, PE, 20–100 µm | Z.F.Wang et al. (2018) | |
| Benthic sediment | |||||
| 4 | Skudai and Tebrau river, Malaysia | Box corer | 1001–5000 μm | Sarijan et al. (2018) | |
| Benthic sediment | |||||
| 5 | Cecina river estuary, Tuscany, Italy | Wide mouth glass jars 1 L by scientific scuba divers (depth of 5 cm) | Fragments, > 500 µm | Blašković et al. (2018) | |
| Littoral sediment andBenthic sediment | |||||
| Lagoon | 1 | Lagoon of Venice, Italy | Box corer (depth of 0–5 cm) | Fragments, PE, < 100 µm | Vianello et al. (2013) |
| Benthic sediment | |||||
| 2 | Complex Lagoon-Channel of Bizerte, Northern Tunisia | Stainless steel spatula (0.25 m × 0.25 m quadrats, depth of 2–3 cm) | Fibers | Abidli et al. (2016) | |
| Littoral sediment |
Table 3.
Microplastic studies in water and sediment of inland water bodies around the world
| Study compartment | Number | Study area | Sampling tools | Dominant microplastic characteristics (shape, polymer type, size) | Reference |
|---|---|---|---|---|---|
| River–estuary | 1 | Three GorgesReservoir, China | 12 V DC Teflon pump (25 L), passed through 48-μm stainless steel sieve (water) and Van Veen grab (0.25 m2 area) (sediment) | Fibers, PS, < 0.5 mm | Di and Wang (2018) |
| 2 | Five urban estuaries of KwaZulu-Natal, South Africa | Conical zooplankton net (300 µm mesh size) (water) and sediment corer (depth of 10 cm) (sediment) | Fragments | Naidoo et al. (2015) | |
| 3 | Pearl River along Guangzhou City, China | Water sampler (5 L) ( water), Van Veen grab (depth of 5 cm) (sediment) | Fibers, PP, 0.02–0.5 mm, 0.5–1 mm | Lin et al. (2018) | |
| 4 | Antuã River, Portugal | Water pump (55 µm mesh size (water), Van Veen grab (depth of 12 cm) (sediment) | Foams, PE, PP | Rodrigues et al. (2018) | |
| 5 | Ottawa River, Canada | Bottle sampling and Manta trawls (100 µm mesh size) (water), Ekman bottom grab sampler (sediment) | Fibers | Vermaire et al. (2017) | |
| 6 | Charleston Harbor and Winyah Bay, two developed estuaries in US | Sea surface microlayer collection apparatus (4L) (water) and stainless steel trowel (0.25 m × 0.25 m transect) (sediment) | Fragments, 150–499 µm | Gray et al. (2018) | |
| 7 | Slum and industrial area of Ciwalengke River, Majalaya, Indonesia | Grab samples with glass container (1L) (water), Ekman grab sampler and shovel (sediment) | – | Alam et al. (2019) | |
| 8 | Pearl River catchment, China | Plankton net (160 μm mesh size (water), grasp bucket and gravity corer (depth of 54 cm) (sediment) | Sheets, PP, LDPE, < 0.25 mm | Fan et al. (2019) | |
| 9 | Wei River, China | Bulk sampling using clean pump (5L) passed through 75 µm mesh size (water), grab (sediment) | Fibers, < 0.5 mm | Ding et al. (2019) | |
| 10 | Tibet Plateau Rivers, China (Buqu River (the source of the Yangtze | Large flow sampler (water) and a stainless steel shovel (depth of 2 cm) (sediment) | Fibers, PET (sediment samples), PE (water samples), < 0.5 mm | Jiang et al. (2019) | |
| 11 | Middle and lower reaches of the Yangtze River, China | A fishery administration vessel (AVANI trawl net 333 μm mesh size), A plankton net (64 µm mesh size) (water) and grab sampler (sediment) | Sheets, PP, 0.3–0.5 mm, 0.5–1 mm | Xiong et al. (2019) | |
| 12 | Nakdong River, South Korea | Stainless steel beaker, submersible pump (water), Van Veen grab (depth of 2 cm) (sediment) | Fragments, PP | Eo et al. (2019) | |
| 13 | Mohawk River, USA | Manta trawl (333 μm mesh size (water) and Ekman grab sampler was (sediment) | Fibers, fragments | Smith et al. (2017) | |
| 14 | Ebro River Delta, Northeastern Iberian Peninsula, Spain | Neuston net (5 µm mesh size) (water), stainless steel spoon (0.2 m × 0.2 m quadrant, depth of 2.5 cm) and van Veen grab sampler (sediments) | Fibers, PE, 200–500 µm | Simon-Sánchez et al. (2019) | |
| 15 | Yongjiang River, Nanning City, South China | 12 V DC Teflon pump (10 L) passed through 50-µm-mesh size sieve (water) and Van Veen grab (sediment) | Fibers, PE, 330–1000 μm and 1–3 mm | Zhang et al. (2020) | |
| 16 | Maozhou River, China | Stainless steel bucket (5L), depth of 50 cm (water) box corer, depth of 20 cm (sediment) | Fragments, 10 μm-0.1 mm | Wu et al. (2020) | |
| 17 | Chao Phraya River, Bangkok, Thailand | Manta trawl 2 m long, width of 50 cm, and a height of 20 cm, 300 µm mesh size (water), Van Veen grab sampler (sediment) | Fragments, PP, 0.5–1.0 (water samples) 0.053–0.5 mm (sediment samples) | Ta et al. (2020) | |
| 18 | Ravi River in urban center ( predominant drains and canals of Lahore district), Lahore, Pakistan | Stainless steel spatula, 0.3 m × 0.3 m quadrate, depth of 1 cm (sediment) | Fragments, PE, 150–300 μm (sediment samples), and large size MPs 300 μm–5 mm (water samples) | Irfan et al. (2020) | |
| 19 | Magdalena River, Colombia | Neuston net 20 µm mesh size (water), metal shovel, depth of 5 cm (sediment) | Fibers, PP | Martínez Silva and Nanny (2020) | |
| Lake–reservoir | 1 | Lake Bolsena and Lake Chiusi, Italy | Manta trawl 300 µm mesh size 60 cm × 18.5 cm (water), 0.25 m2 area, depth of 3 cm (sediment) | Fibers, < 0.3 mm, 0.3–0.5 mm | Fischer et al. (2016) |
| 2 | Dongting Lake, China | Flow sampler (30L), passed through 45 µm mesh size (water) and Stainless shovel 0.3 m × 0..2 m quadrat, depth of 0–2 cm (sediment) | Fibers, PET (sediment sediment), PE (water sediment), < 0.5 mm | Jiang et al. (2018) | |
| 3 | Six dams near Ithaca, USA | Grab sample 1 L plastic bottles (water), plastic scoop (sediment samples) | Fibers | Watkins et al. (2019a) | |
| 4 | Danjiangkou Reservoir, China | 12 V DC Teflon pump (20L), depth of 0–20 cm (water), grab (sediment) | Fibers, PP, group 1 (48 μm − 0.5 mm), group 2 (0.5–1 mm), group 3 (1–2 mm), | Di et al. (2019) | |
| 5 | Lakes along the middle and lower reaches of Yangtze River Basin, China | Neuston plankton net 74 µm mesh size (water), Van Veen grab (sediment) | Fibers, PET, 20–50 µm | Li et al. (2019) | |
| Stram | 1 | 18 streams in and around the city of Auckland, New Zealand | Phytoplankton net 63 µm mesh size (water) and scooped with container, depth of 5 cm (sediment) | Fragments, fibers (water samples), fragments (sediment samples), poly(hexadecyl) methacrylate (PHM), ethylene/ethyl acrylate copolymer (EEAC), 63–500 µm | Dikareva and Simon (2019) |
| Fish ponds | 1 | Central and Eastern European region | Jet pumps, passed through 2 mm mesh size strainer, depth of 10–20 cm (water), Veen grab sampler and a hand spade (sediment samples) | PP | Bordós et al. (2019 |
| River–estuary–lake–WWTPs | 1 | River Barrow, River, Nore, Lough, Lurgan (Cushina, Co. Offaly) and River Liffey (Newbridge, Co. Kildare), Ireland | - | PS | Cedro and Cleary 2015 |
Table 1.
Microplastic studies in water of inland water bodies around the world
| Study compartment | Number | Study area | Sampling tools | Dominant microplastic characteristics (shape, polymer type, size) | Reference |
|---|---|---|---|---|---|
| River–estuary | 1 | Three Gorges Dam—China | Trawl with a rectangular opening 50 cm high by 100 cm wide, and1.5 m long, 112-mm-mesh size nylon net with a 500-mL polyethylene collecting bottle at the end | Sheets, PP, 500 µm–1.6 mm | Zhang et al. (2015) |
| 2 | The North Shore Channel (NSC) in Chicago, Illinois (IL), USA | Two neuston nets (0.92 × 0.42 m and 0.36 × 0.41 m), 333 μm mesh size | Fiber | McCormick et al. (2014) | |
| 3 | 29 Great Lakes tributaries, USA | Neuston net 1.5-m-long net with an opening 100 cm wide by 40 cm high, 333 μm mesh size | Fibers, 0.355–0.999 mm | Baldwin et al. (2016) | |
| 4 | Inflow (Red and Assiniboine rivers) and outflow (Nelson River) of LakeWinnipeg, Canada | Manta trawl 295 cm long, an aperture width of 61 cm, and a heightof 18 cm, 333 µm mesh size | Fibers | Warrack et al. (2018) | |
| 5 | Tamar Estuary, UK | Manta net 0.50 m by 0.15 m, 300 µm mesh size | Fragments, PE, 1–3 mm | Sadri and Thompson (2014) | |
| 6 | Los Angeles and San Gabriel Rivers, USA | Manta trawl 0.9 m × 0.15 m, 333 µm mesh size, hand nets 0.46 m × 0.25 m, 800 µm mesh size and 0.43 m × 0.22 m, 500 µm mesh size, streambed 0.15 m × 0.15 m, 333 mesh size, rectangular net 0.45 m × 0.25 m, 333 mesh size | Foams, PS, ≥ 1 mm and < 4.75 mm | Moore et al. (2011) | |
| 7 | Danube River, Austria | Stationary conical drift nets 0.5 m diameter, 1.5 m long, 500 µm mesh size | – | Lechner et al. (2014) | |
| 8 | Four Estuarine Rivers in the Chesapeake Bay ( Patapsco, Magothy, Rhode, and Corsica rivers), USA | Manta net 70 cm wide, 330 µm mesh size | – | Yonkos et al. (2014) | |
| 9 | Yangtze Estuary and East China Sea, China | Teflon pump passed through a 32-µm steel sieve, neuston trawl 30 × 40 cm2 opening, 333 µm mesh size | Fibers, > 0.5–1 mm | Zhao et al. (2014) | |
| 10 | Rhine River—Switzerland | Manta trawl with rectangular opening of 60 cm × 18 cm, mesh 300 µm mesh size | Opaque spherules, PS | Mani et al. (2015) | |
| 11 | Solent estuarine complex ( Hamble, Itchen and Test rivers), UK | Plankton net trawl, 300 µm mesh size | Fibers, blue, black, clear, white | Gallagher et al. (2016) | |
| 12 | Three urban estuaries ( Jiaojiang, Minjiang and Oujiang Estuaries), China | Teflon pump passed through a 333-µm steel sieve | Fibers, PP, < 0.5–1 mm, < 1–2 mm | Zhao et al. (2015) | |
| 13 | Pearl River along Guangzhou city and Pearl River estuary, China | Water sampler passed through a 50-µm stainless steel sieve | Films, PA, < 0.5 mm | Yan et al. (2019) | |
| 14 | Saigon River, Vietnam | A bucket and plankton net, 300 µm mesh size | Fibers, PES | Lahens et al. (2018) | |
| 15 | Hudson River, USA | Grab sample, metal bucket (3L) | Fibers, PET | Miller et al. (2017) | |
| 16 | Raritan River, New Jersey, USA | Plankton nets 0.2 m diameter, 0.51 m long, 153 mm mesh size | – | Estahbanati and Fahrenfeld (2016) | |
| 17 | Rivers State, Nigeria | Plankton net | – | Briggs et al. (2019) | |
| 18 | Meuse, Rhine, Europe | – | Fibers | Brandsma et al. (2013) | |
| 19 | Goose Creek, Little Kickapoo Creek, and East Branch of the DuPage River, USA | Neuston nets 0.52 m × 0.36 m, 333 µm mesh size | Pellets, PE | McCormick et al. (2016) | |
| 20 | Rhine, Dalålven, Danube and Po Rivers, Europe | Manta net internal width 60 cm, 330 μm mesh size, Pump, waste free water sampler mesh size 3.2 mm | Fragments, pellets, PE | van der Wal et al. (2015) | |
| 21 | Jade system, south North Sea, Germany | Grab sample, PE bottle (100 ml) passed through 1.2-μm cellulose nitrate filters | Fibers | Dubaish and Liebezeit (2013) | |
| 22 | Snake River and Palisades Reservoir, USA | – | Fibers | McDevitt and Perez (2016) | |
| 23 | 29 Rivers, Japan | Plankton net 30 cm × 75 cm, 335 µm mesh size | – | Kataoka et al. (2019) | |
| 24 | Snake River and Columbia River, USA | Grab sample, glass jars, mean volume 1.85 L, plankton net, 100 μm mesh size | Fibers, 100–333 µm | Kapp and Yeatman (2018) | |
| 25 | Rhine River, Germany | Manta trawl 60 cm × 18 cm rectangular aperture, 300 µm mesh size | PS-DVB ion-exchange resins | Mani et al. (2019a) | |
| 26 | Gallatin River watershed, USA | Grab sample, stainless steel bottles (1L) | Fibers, Semi-synthetic cellulose, PES, 0.1–1.5 mm | Barrows et al. (2018) | |
| 27 | Changjiang Estuary, China | A screw pump (100 L) passed through a stainless steel sieve 60 μm pore size | Fiber, PE | Zhao et al. (2019) | |
| 28 | Changjiang Estuary, China | A pump (100 L) passed through stainless steel sieve 70 μm mesh size | Fibers, PE0.07–1.0 mm | Xu et al. (2018) | |
| 29 | Pasig River, Philippines | Two Manta trawl 25.7 cm diameter openingand 10.4 cm diameter opening, 355 µm mesh size | Fragments, 1.16 ± 0.42 mm | Deocaris et al. (2019) | |
| 30 | Danube River, Austria | Net 600 × 600 mm opening, 500, 250, 41 µm mesh size, BFG basket sampler 300 × 600 mm opening, 500 µm mesh size | – | Liedermann et al. (2018 | |
| 31 | Muskegon River, Milwaukee River, and St. Joseph River, USA | Grab samples, 2-L glass bottle passed through a 0.363 μm mesh (surfaces water), Wading seine nets (biota) | Fibers, < 1.5 mm | McNeish et al. (2018) | |
| 32 | Clyde, Bega and Hunter estuaries, Australia | Horizontal surface tows using 45 μm mesh size (biota), vertical towl 37 μm net (biota) | Fragments, 45–100 µm | Hitchcock and Mitrovic (2019) | |
| 33 | Douro estuary, Portugal | A conical 1 m diameter, 4 m long, 500 μm mesh size (biota) | – | Rodrigues et al. (2019) | |
| 34 | Ofanto River, Italy | Plankton nets 2.5 m long with an opening of 55 cm × 55 cm, 333 μm mesh size | Fragments, flakes, PE, 500–1000 μm, 1000–2000 μm | Campanale et al. (2020) | |
| 35 | Yellow River, China | Stainless steel bucket (5 L) | Fibers, 50–100 µm | Han et al. (2020) | |
| 36 | Swiss Rhine River catchment at Brugg and the downstream German-Dutch border at Rees (Germany and Switzerland) | Manta trawl 60 cm × 18 cm, 300 µm mesh size | Fragments, PE, 0.3–1 mm | Mani and Burkhardt-Holm (2020) | |
| 37 | Urban waters of seven cities in the Tuojiang River basin, China | Steel sampler (25 L) passed through 50 μm mesh size sieve | Fibers, PP, 0.5–1 mm | Zhou et al. (2020) | |
| 38 | Manas River, China | Stainless steel drum (2.5 L) | Fibers, PP, 0.3–1.0 mm | G. Wang et al. (2020) | |
| 39 | Minjiang River watershed, Southeast China | Metal pail passed through 300 µm mesh size | Fibers, PET, 1–2 mm | Huang et al. (2020) | |
| 40 | Meuse river and in Netherlands and the Dommel, Germany | A centrifugal water pump passed through of 300, 100, and 20 µm mesh size sieve | PE, 0–1000 µm | Mintenig et al. (2020) | |
| 41 | Cherating river and mangrove, Malaysia | Conical nylon plankton net 0.3 m x 1 m, 100 µm mesh size | Fragments, 0.5–1.0 mm | Pariatamby et al. (2020) | |
| 42 | Yulin River, China | Teflon pump ( 0.05 m3) passed through 64 µm stainless steel sieve mesh size | Lines/fibers, PE, 64–100 µm | Y. Mao et al. (2020) | |
| 43 | Qing River, Beijing, China | Stainless steel bucket (20 L) passed through stainless steel 5000 µm mesh size | Fragments, PE, EPR | C. Wang et al. (2020) | |
| Lake–reservoir | 1 | 3 connected urban lakes and drainage playa wetlands, Lubbock, Texas, USA | Grab sample (3.50 L) passed through sieve (> 300, 250–299, 180–249, 106–179, and 53–105 µm mesh size | 53–105 µm | Lasee et al. (2017) |
| 2 | Lake Hovsgol (mountain remote lake), Mongolia | Manta trawl 16 cm high × 61 cm wide and a 3 m long, 333 µm mesh size | Lines/fibers, 0.355–0.999 mm, 1.00–4.749 mm | Free et al. (2014) | |
| 3 | Laurentian Great Lakes ( Lakes Superior, Huron and Erie), USA | Manta trawl with a rectangular opening 16 cm high × 61 cm wide, and a 3 m long, 333 µm mesh size | Pellets, 0.355–0.999 mm | Eriksen et al. (2013) | |
| 4 | Lake Winnipeg, Canada | Manta 61 cm wide × 18 cm high and a 3 long, 333 µm mesh size | Fibers | Anderson et al. (2017) | |
| 5 | Dongting Lake and Hong Lake, China | 12 V DC Teflon pump (20 L) passed through 50 µm mesh size | Fibers, PP, PE, 50–330 μm, 330–1000 μm | W.F. Wang et al. (2018) | |
| 6 | Western Lake Superior, USA | Manta net 85 cm wide × 14 cm high and a 3 m long, 333 μm mesh size | Fibers, PVC | Hendrickson et al. (2018) | |
| 7 | Lake Michigan, USA | Manta trawl 61 cm wide × 16 cm high and a 3 m long, 333 μm mesh size | Fragments, PE, 0.355– .999 mm | Mason et al. (2016) | |
| 8 | Lake Maggiore, Iseo and Garda, Italy | Manta trawl 60 × 20 cm, 300 µm mesh size | Fragments, PE | Sighicelli et al. (2018) | |
| 9 | Urban Lakes in Changsha, China | 40 L water passed through 45 µm mesh size | Lines, 50–500 µm | Yin et al. (2019) | |
| 10 | Feilaixia Reservoir in the Beijiang River, China | Conical plankton 20 cm diameter, 112 µm mesh size | Films, PP, 0.6–2 mm | Tan et al. (2019) | |
| 11 | Lake Ulansuhai, China | 12-V DC Teflon pump passed through 48 µm mesh size | Fibers, PE, < 0.5 mm | Wang et al. (2019 | |
| 12 | Mecklenburg Lake District in Mecklenburg-Western Pomerania, Germany | Pump water samples, Manta trawl | Irregular particles, PE, PET, 0–1000 µm | Tamminga et al. 2020 | |
| 13 | Wuliangsuhai Lake, northern China | Stainless steel buckets (20L) passed through 75 µm mesh size sieve | Fibers, PS, < 0.5 mm | R. Mao et al. (2020) | |
| Stream | 1 | Six Mile Creek and Fall Creek streams, USA | Neuston net 1 × 0.5 m,335 µm mesh size | Fibers | Watkins et al. (2019b) |
| Stream–lake | 1 | Streams and wetlands, Victoria, Australia | Grab surface, polypropylene jars (5L) (water), dip nets (biota) | Fibers, PES, rayon, 0–1 mm | Nan et al. (2020) |
| Pond | 1 | North of Jutland, Denmark | A positive displacement pump passed through 10 µm stainless steel mesh size | PP | Liu et al. (2019) |
| River–estuary–lake | 1 | Urban lakes and urban reaches of the Hanjiang River and YangtzeRiver, Wuhan, China | 12 V DC Teflon pump (20 L) passed through 50 μm stainless steel sieve | Fibers, PET, 50–500 μm (or < 0.5 mm) | W.F. Wang et al. (2017) |
| City creeks–rivers–estuary and coastal waters | 1 | City creeks (Shanghai), rivers (Suzhou River and Huangpu River), an estuary (Yangtze Estuary) and coastal waters (East China Sea), Yangtze Delta area, China | Metal pail (5 L) passed through 20 µm mesh size filter, air lift pump | Fibers, PES, 0.1–1.0 mm | Luo et al. (2019) |
| River water–wastewater–total atmospheric fallout | 1 | Greater Paris–Seine River, France | Manta trawl 330 µm mesh size, Plankton net 80 µm mesh size | Fibers, 1001–5000 µm | Dris et al. (2015) |
| River–atmospheric fallout–urban runoff–WWTP effluents-CSOs | 1 | River Marne, France | Manta trawl 80 and 300 µm mesh size | Fibers | Dris et al. (2018) |
| Surface water, storm water runoff, agricultural runoff, and treated wastewater effluent | 1 | Lake Ontario of the Laurentian Great Lakes in Canada | Stainless steel bucket (4 L) passed through 10 µm mesh size filter | Fibers | Grbić et al. (2020) |
| Urban prairie creek | 1 | Wascana Creek, northern outskirts of Regina, Canada | Conical net (water), seine nets, gill nets, conventional tackle, and minnow traps (biota) | Fibers | Campbell et al. (2017) |
Table 4.
Microplastic studies in water, sediment and biota of inland water bodies around the world
| Water compartment | Number | Study area | Sampling tools | Dominant microplastic characteristics (shape, polymer type, size) | Reference |
|---|---|---|---|---|---|
| River–estuary–lake | 1 | Xiangxi Bay of Three Gorges Reservoir, China | Surface trawl 50 × 100 × 150 cm, 112 µm mesh size (water) and Petersen grab (sediment) | Sheets, PP, 1–5 mm | Zhang et al. (2017 |
| 2 | Qinghai Lake, China | Trawl net 50 × 100 × 150 cm, 112 µm mesh size (water) and stainless steel shovel, 0.2 m × 0.2 m quadrate, depth of 0–2 cm (sediment) | Sheets, PP,PE, 0.112–0.5 mm | Xiong et al. (2018 | |
| 3 | Taihu Lake, China | Nylon plankton net 333 µm mesh size and steel sampler (5L) (water), Peterson sampler (sediment) and a bottom fauna trawl (biota) | Fibers, 100–333 µm, 333–1000 µm | Su et al., 2016 | |
| 4 | Lake Geneva, Switzerland | Manta trawl 300 µm mesh size (water), fishes and birds were collected by a fisherman | < 5 mm, PS | Faure et al. (2012 | |
| 5 | Poyang Lake, China | Steel sampler (20 L), passed through 50 µm mesh size (water), Van Veen grab (sediment), fish samples were obtained from an aquatic product market | Fibers, PP, 0.1–0.5 mm | Yuan et al. (2019 | |
| River–estuary–lake | 1 | Six of the largest Swiss lakes and some rivers-Switzerland | Manta trawl 300 µm mesh size (water), multi-mesh gillnets and vertical benthic and pelagic nets (biota) | Fragments, PE, > 300 µm | Faure et al. (2015 |
| 2 | Middle-Lower Yangtze River Basin, China | Steel bucket (5 L), depth of 0.12 cm (water), Peterson sampler, depth of 10 cm (sediment), bottom fauna trawls (biota) | Fibers, PES, 0.25–1 mm | Su et al. (2018 | |
| Pond | 1 | Storm water pond, Viborg, Denmark | Bulk samples (10 L), glass bottles, depth of 10 cm (water), Sediment corer, depth of 5–8 cm (sediment), gill net and fishing net (biota) | PP | Olesen et al. (2019 |
| Small water bodies | 1 | YangtzeRiver Delta, China | Steel bucket (water), stainless steel spatula, depth of 0–5 cm (sediment) | Fibers, PES, < 0.5 mm | Hu et al. (2018 |
| River, Canal, WWTPs, Sea | 1 | Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota, Netherland and Germany | Bulk sample, glass bottles (2L) (water) and grab samples and Van Veen grab (sediment) | Fibers, 10–300 µm | Leslie et al. (2017 |
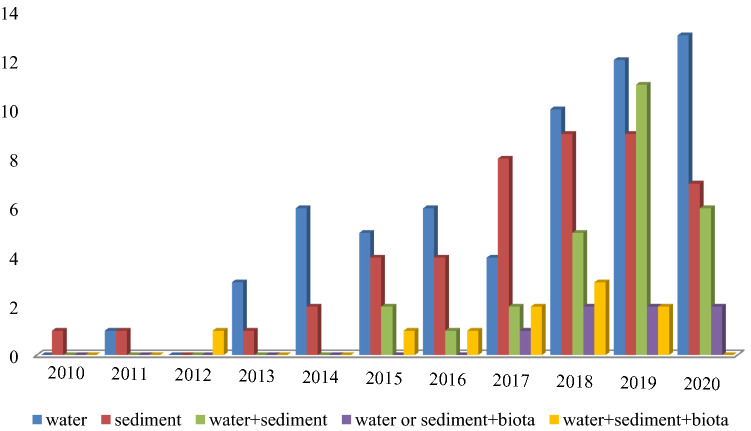
The numbers of microplastic studies in freshwater environments increased rapidly from four in 2013 to 37 studies in 2019 and 27 papers as of September 2020 (Fig. 2).
Fig. 2.
Frequency and trend of studies (n = 150) on the presence of microplastic particles in freshwater environment in different matrix including water, sediment, water + sediment, water or sediment + biota and water + sediment + biota
In the early stages, it was suggested that the chemical types of microplastics in freshwater seem to be less diverse compared to those collected from the marine salty environment. This observation was attributed to the higher density of seawater, which enables more types of plastic materials with different densities to float on the surface of the water (Zhang et al. 2015). However, this is not always the case, because the processes of controlling distribution and exposure to plastics particles are not necessarily restricted to a specific environmental compartment. Polymers with higher density (density > 1.0 g mL−1) were observed in freshwater environments (Moore et al. 2011; Zhou et al. 2020). Negatively buoyant particles (e.g., polyester, rayon, nylon and cellulose acetate) may remain suspended in water (Baldwin et al. 2016). There may be differing degrees of physical and chemical characteristics, such as storms and wave action and saline water in marine systems. But plastics in freshwater systems still experience physical and chemical degradation (Andrady et al. 2011). It was suggested that polymer density alone is not the most significant control on microplastic particle fate within the aquatic environment. Microplastic morphology, incorporation into copolymeric materials during manufacturing and inclusion within aggregates of varying overall densities may play major roles in microplastics distribution (Hendrickson et al. 2018).
The ability to capture plastic particles from water or sediment matrix and separating them from organic and mineral material are challenging. Identifying types of plastics in the samples and on different surfaces is also important (Costa et al. 2021). It is suggested that microplastics in freshwater systems are similar to those in marine environments, and they are exposed to similar threats (Holland et al. 2016). Therefore, microplastic characteristics, detection methods, methods of analysis and impacts on biota are suggested to be similar.
Sampling procedure and tools
Choice of preservation techniques in different stages of microplastic studies largely depend on the research question (Lusher et al. 2017), economic proportionality of the methods and also the study compartment. Microplastics have now been reported in a range of freshwater environments, including surface water, water column, benthic sediments, littoral sediments and aquatic biota. Three main strategies are identified for sampling. They include selective sampling, volume-reduced sampling and bulk sampling. Different sampling strategies may be selected when the type of matrix to be examined for microplastics (water or sediment or biota) has been taken into account. Selective sampling in field consists of direct collection of items from the environment which are recognizable by the naked eye. This method is usually used on the surface of shore sediments and is more practical for large microplastics (1–5 mm). Bulk samples refer to samples where the whole volume of the sample is taken without reducing it during the sampling process. Volume-reduced samples in both sediment and water samples refer to samples where the volume of the bulk sample is usually reduced during sampling. Only a portion of the sample is preserved in this method, and it is mostly used for water samples (Hidalgo-Ruz et al. 2012).
Water compartment
In water samples, microplastic burden in the measuring units is much lower compared to that in sediment samples. Consequently, analysis of water samples requires higher sampling volumes (Huppertsberg and Knepper 2018). Volume-reduced methods are on-site filtration by nets or sieving. They are more suitable for water samples as they give a promising specimen volume without the need to transfer the whole to the laboratory. Therefore, resulting in a relatively small concentrated final sample. Here we discus three main water sampling methods, including trawls, pump samplers and grab samples.
Trawls and nets
Different types of trawls and nets like manta, neuston or plankton nets and bongo net are used (Fig. 3). Trawl is usually deployed off of a boat, submerged and towed on a linear course at a low speed for a set time or distance (Hidalgo-Ruz et al. 2012; Sighicelli et al. 2018). The area of each sampling is calculated by multiplying the towing distance with the width of the trawl. The volume of water through the net uses either a flow meter or calculations based on the distance traveled by boat at a constant speed (Sadri and Thompson 2014). However, because the net's immersion depth changes constantly with waves, wind and boat movement, it is difficult to estimate the exact volume of water being filtered.
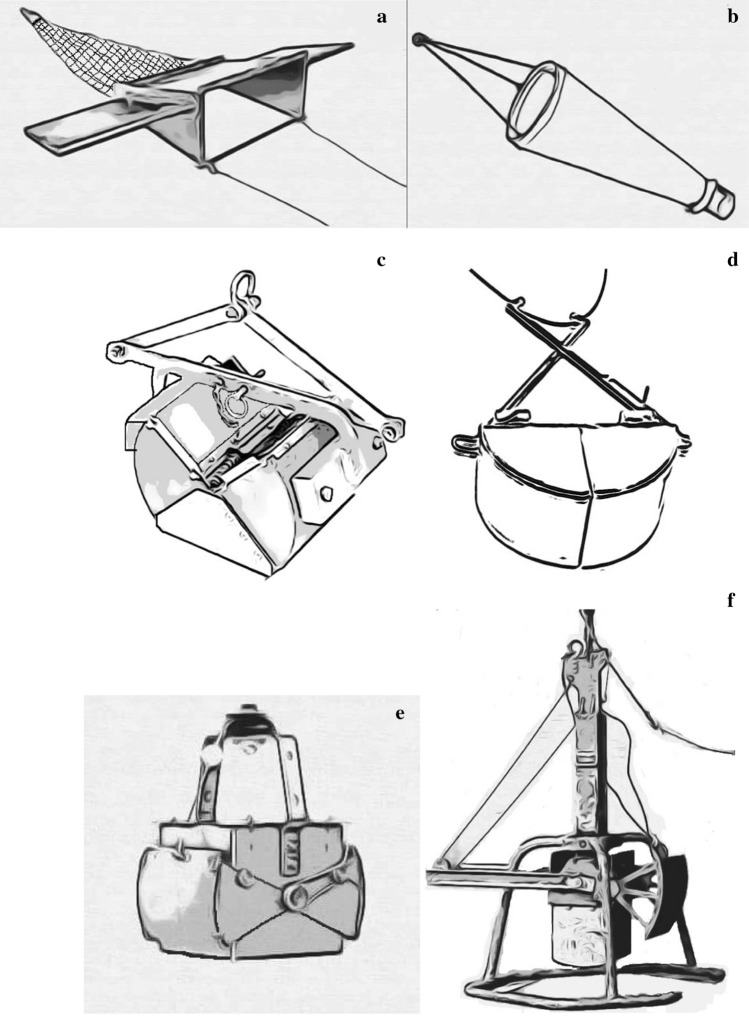
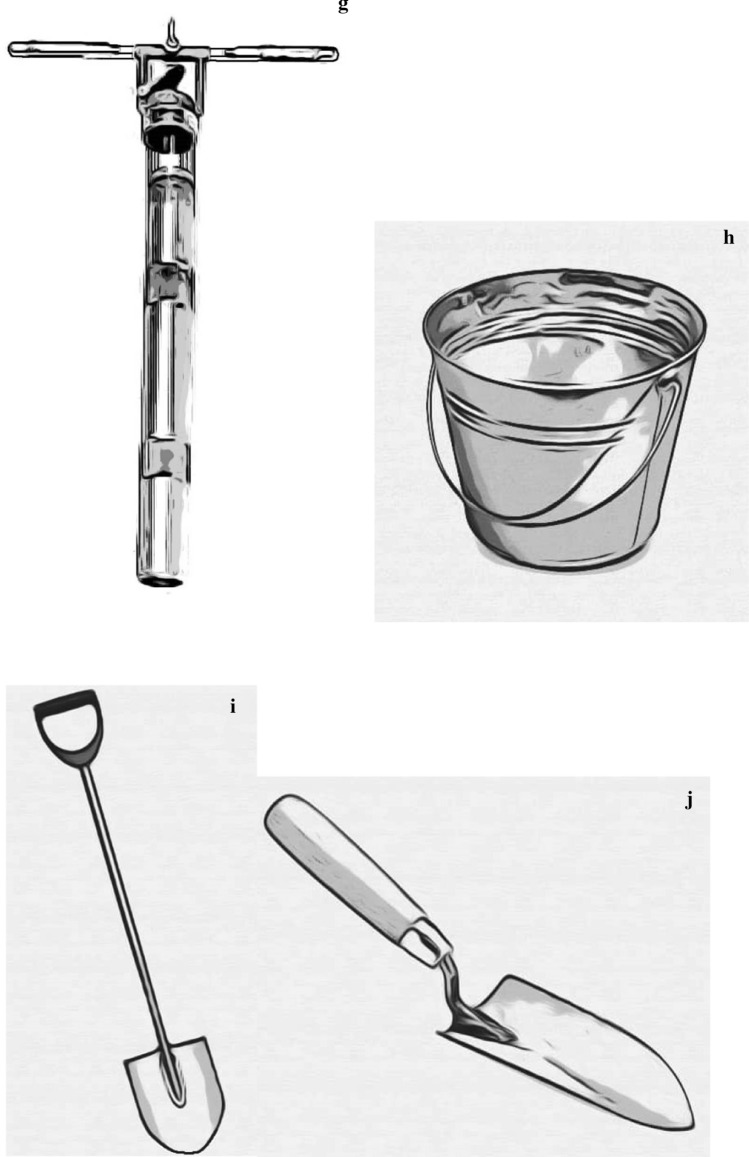
Fig. 3.
Microplastics sampling tools in freshwater studies; a manta trawl, b plankton net, c Petite ponar grab d Van Veen garb, e Ekman grab sampler, f box corer, g sediment corer, h metal pail, i showel, j trowel
Campanale et al. (2020) collected microplastics by three surface plankton nets fixed in the middle of Ofanto River, in order to reduce the spatial and temporal variability (Campanale et al. 2020). In order to ensure that the most representative body of water is being sampled, factors such as time, location and length of trawls in relation to the strength of tides should be carefully considered. Furthermore, the trawling distance using nets varies depending on the abundance of floating microplastics. It should further consider whether the trawl direction with reference to the prevailing wind direction could have an effect on the abundance and size of particles captured within the trawl (Zhang et al. 2018). In these methods, nets are limited by a single mesh size that is sometimes clogged by suspended material (e.g., organic matter or phytoplankton) (Liedermann et al. 2018; Sadri and Thompson 2014).
Types of microplastics are closely related to the mesh size of tools used for specimen collection. For instance, smaller-sized mesh used in some studies could increase plastic particles of certain shapes (e.g., fibers) to a concentration several orders of magnitude higher than those collected using nets with a larger mesh size. On that account, the abundance of microplastics is largely underestimated by researchers who used a trawl for sample collection (Z.F.Wang et al. 2018b). By using manta trawl, a significant fraction of actual small microplastic particles is very likely to be underestimated because they might pass through the net.
Regarding the limitations of obtaining sufficient water volumes while avoiding net clogging, it was strongly recommended to use tandem nets with different mesh sizes. This helps to better characterize smaller microplastics (Anderson et al. 2017). Dris et al. (2018) had a 250-times higher probability of sampling fibers when using an 80-µm mesh compared to a 330-µm mesh (Dris et al. 2018). Double neuston net trawl (500 µm mesh size) was used as sampling tool in assessing microplastics in surface waters of Lake Superior. No difference was detected between the paired net samples, suggesting that single net sampling produces a representative estimate of microplastic particle condition within a body of water (Cox 2018). A comparison study was conducted between a manta net and a neuston net for microplastics in ocean surface water. Results showed that the manta net tended to have slightly higher densities of microplastics than those of the neuston net. However, no statistical difference was observed. Neuston net is relatively stable in rough water although efforts are needed to maintain the net in submerged depth. Manta net tends to jump in rough water (Michida et al. 2019). Sampling with the manta trawl to function properly requires relatively calm conditions (Anderson et al. 2017). Modified BfG basket sampler used for the Austrian Danube River, clearly showed the necessity of a strong and stable equipment carrier. The nets were positioned on the surface, in the middle of the water column, and at the bottom of the river and with different mesh sizes (Liedermann et al. 2018).
Nets alone may fail to deliver the overall pattern of microplastic pollution in an area, because there does not seem to be sufficiently retaining fibers and small microplastics.
Pump samplers and grab samples
Volume reduction pump sampler and grab samples are also used in some of the research papers. Pump sampling consist of pumping water manually or using a motor through an inline filter. Grab sampling method includes using a bucket to collect water and sieve the water in the field (Han et al. 2020; Miller et al. 2017; Y. Mao et al. 2020a). A fixed amount of bottle is also submerged, filling with surface water for laboratory analysis (Barrows et al. 2018; Dubaish and Liebezeit 2013). Water collected using pumps or bulk samplers is taken from different depths with different volumes. Due to the high variability of microplastic spatial distribution, the sampling area covered is limited and using a pump or bulk sampler may not be representative. Therefore, taking multiple replicates is suggested (Zhang et al. 2018). However, pumps can be used to collect large volumes of water, which may be advantageous in areas where the density of microplastics is suspected to be low (Crawford and Quinn 2017). Water volume could be variable from 5 mL to 500 L (Braun et al. 2018). In addition, they do not possess the limitation caused by pacific sampling mesh tool. Based on literature reviews, significantly more microplastic particles are present in smaller size ranges. A combination of volume-reduced net-based sampling and bulk sampling seems to be very helpful in estimating the missing fractions and enables a greater spatial resolution (Fischer et al. 2016).
Comparison between different water sampling methods
In the comparison of manta trawling and pump sampling methods in microplastic sampling from water of Lake Tollense, Germany, different results were observed in the abundance of microplastics, microplastics shape and size. It was suggested that manta trawl is not sufficient in retaining fibers and small microplastics from water samples. Therefore, the pump sampling approach with the filtration of large water volumes is necessary to generate reliable results. However, the pump sampling covers small microplastics. Small plastic particles are greater in number. Volume-reduced sampling covers large microplastics, being less abundant but still important. Fibers detected in the manta samples were unevenly spread across the whole size range. Fibers found in the pump samples showed distinct positively skewed distribution peaking at > 500–600 µm in length. The most abundant polymer composition in manta trawl samples was polyethylene and polyethylene terephthalate for the pump sampling method (Tamminga et al. 2020).
Lahens and colleagues utilized a bucket and 300-µm plankton net. Bulk water sampling was used for anthropogenic fiber analysis and 300-µm-mesh size plankton net exposition for fragment analysis (Lahens et al. 2018). In the study of Su and colleagues, the average abundance of microplastics was found to be higher in plankton net samples rather than bulk surface water samples (Su et al. 2016). In general, water sampling volumes depend on the solid richness and the target microplastic size range. Barrows et al. (2017) compared grab samples to the conventional neuston net approach. Grab samples collected three orders of magnitude more microplastics than the net approach (Barrows et al. 2017).
In comparison between manta trawl and in situ pump filtration methods, it was found that the pump sampling method is more accurate in volume measurement and versatile for point sampling and filter size choice. However, due to the lower sampling volume, it might be more suitable for sampling in areas with a higher level of contamination. On the other hand, the trawling method has the ability to cover and sample a larger area and therefore overcomes some of the problems related to patchiness (Karlsson et al. 2020). A combination of volume-reduced net-based sampling and bulk sampling seems to be very effective in comprehensive monitoring of microplastic in aquatic environment.
Sediment compartment
Because of such characteristics as buoyancy and extreme durability, synthetic polymers are present in rivers, lakes and oceans and accumulate in sediments all over the world. Microplastic durability makes it highly resistant to degradation from decades to millennia in its polymer forms (Mathalon and Hill 2014). Small plastic particles are easily accessible to a wide range of aquatic organisms, accumulating in their cells and tissues and ultimately transferred through the food web. Most plastics are extremely durable and persistent (Sharma and Chatterjee 2017).
Microplastics in water compartment may be diluted due to seasonal variation in water volume and water dynamic behavior. For sediment compartments, with a static environment, dilution barely happens, and sediments can easily act as accumulation environments. Sediments are a site of microplastics accumulation and the habitat of benthic organisms, which are key components of food webs. In microplastic scientific assessment, sediment samples are taken from both the subtidal and benthic part of freshwater bodies. This issue affects the life quality of the organisms in ecosystems, both in benthic and littoral sediment zone. For example, microplastics were recorded in fecal samples and feathers of waterbirds from contaminated wetlands in South Africa. Plastic particles can fill the gizzard and possibly block the pyloric valve leading into the intestine (Reynolds and Ryan 2018). Microplastics were present in the benthic fish species and benthic organisms of the Caspian Sea, and the abundance of plastic particles in animals near the shore was greater than in the central part (Bagheri et al. 2020). High doses of microplastics led to fewer species and fewer juvenile isopods and periwinkles in European flat oysters and their associated benthic communities (Green 2016).
Sampling tools are selected with regard to sampling places. Sampling is performed in various directives with respect to the analysis of nutrients or pollutants, such as metal ions or persistent organic substances (Braun et al. 2018).
Manual grab samplers
Sediment manual grab methods utilize tools such as hand spades and stainless steel spoon for littoral and beach environments (Fig. 3). As sampling of sediments is facilitated compared to that of the water column, monitoring shore sediments appears to be advantageous. Moreover, non-buoyant particles can be analyzed in sediment samples rather than in water surface samples (Klein et al. 2015). Therefore, sampling from different compartments can give a comprehensive outlook of microplastic pollution problem.
Deep sediment samplers
Different kinds of grabs and corers are suggested to be suitable for deeper sediment sampling. Ekman and Van Veen grab samplers are deployed to study benthic sediment (Merga et al. 2020; Neto et al. 2019; Sruthy and Ramasamy 2017). Deep sediment sampler can provide a look at the changing abundance and microplastic debris in lake sediments that span a century to present day. These particle can contribute to the discussion on plastic wastes as stratigraphic markers for the Anthropocene (Turner et al. 2019; Vaughan et al. 2017). For specimen transition, the use of glass bottle is recommended. However, plastic or aluminum foil bags can also be used. In the case of plastic container utilization, blank control should be included to prevent bias in the study results (Zhang et al. 2018). Care must also be taken to homogenize the sample during further processing.
Comparison between different sediment sampling methods
Surface sediment in shore zone could reflect long-term interfacial interaction between waters and terrestrial environment (Yu et al. 2016). Shore sediment sampling consists of multiple transects at a right angle from the water line and the placement of quadrats along the transects. Transects may be visually scanned for bigger microplastics in the field. The surface layer can be removed to a proximate depth and sieved or transferred to the laboratory for separation steps (Ballent et al. 2016; Egessa et al. 2020). Variation in plastic abundance at different natural beach zones (water line, drift line and high-water line) in Lake Garda was investigated. Results showed that the water line contained the lowest level of plastic particles, whereas the highest proportion of plastic debris was observed in the drift line and high-water line (Imhof et al. 2018). Core samples have the advantage of being able to see depth profile and to study potential microplastic trends in considerable time periods. Surface analysis of these microplastics may show higher degradation effects due to longer time period. However, surface particles may be more exposed to degradation factors.
Sediment sampling tools are selected with regard to sampling places and sampling purposes, as they may show different aspects.
Sample preservation
Samples were are usually preserved with 5% methyl aldehyde and stored at 4 °C before analysis (Zhang et al. 2015) or fixed in 2.5% formalin (Zhao et al. 2014) or submerged in ~ 40% ethanol (EtOH) (Mani and Burkhardt-Holm 2020).
Discussion
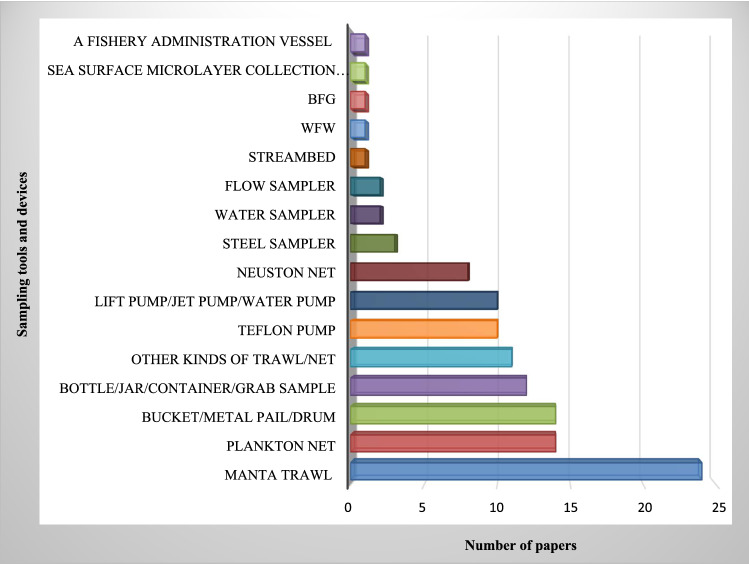
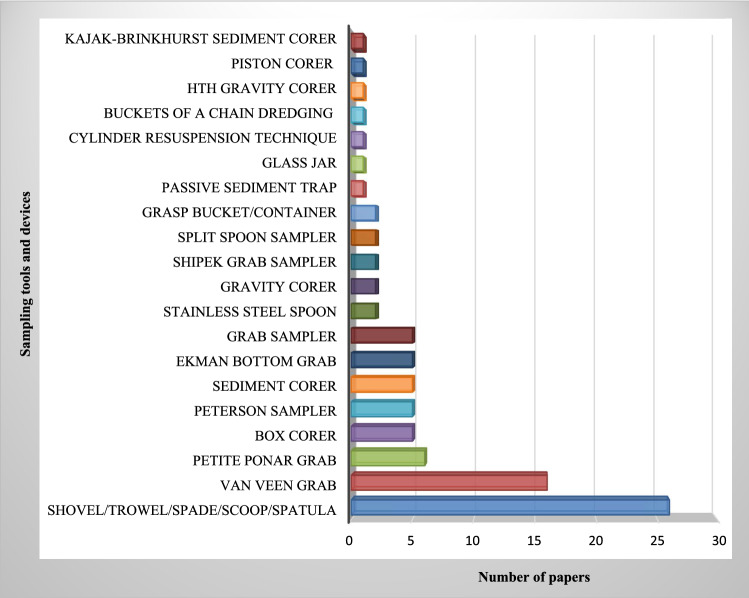
No standardized methods exist for selecting mesh size, sampling, clean up, enrichment and detection, making the comparison of different studies complicated. Improving methods is needed to save time and effort in identifying microplastics in different compartments. To the best of our knowledge, water compartments are the most investigated matrix, assessed for microplastics in freshwater environments (102/150). Rivers and estuarine systems are the most frequently studied compartments for microplastic detection, both in water and in sediment (85/150). This may be due to the reported importance of rivers and estuaries as a vector for microplastics transfer to seas and oceans. Littoral or shore sediments and bottom sediment are frequently assessed for microplastics in reviewed studies. Many microplastic studies used Manta nets to collect surface water samples (Fig. 4). Van Veen grabs and simple hand tools like trowels and stainless steel spoons were the most frequently used tools for the bottom and littoral sediments, respectively (Fig. 5). They are also the most common sediment sampling tools from benthic and beach zones and seem to be appropriate for microplastic studies. Regarding sampling tool characteristics, both false positive and false negative results in analyses of small microplastics occur. In recent studies, there is a tendency to detect microplastics in both water and sediment at the same time. The simultaneous detection of microplastics in the water and sediment compartment gives a better perspective of the situation in the ecosystem. The lack of uniformity in reporting the numbers of microplastics, mostly due to employing different units, is considerably noticeable in reviewing papers. This makes the comparison of results difficult and challenging. Some studies have reported microplastic numbers or weights per volume of sampled water or per total dry matter for sampled sediments (particles/kg); the latter is highly recommended.
Fig. 4.
Frequency of water sampling tools, used in microplastic freshwater studies
Fig. 5.
Frequency of sediment sampling tools, used in microplastic freshwater studies
Conclusion
Employing suitable and reliable sampling, treatment and identification methods is crucial to evaluate microplastic pollution. Sampling and experimental techniques should be standardized to more effectively assess microplastics. Although a smaller mesh size is more appropriate, the choice of trawl or sieve mesh size depends greatly on the study purpose. The kind of environment being studied, e.g., a dynamic river with high water velocity or a calm eutrophic lake or wetland, is also of importance. The exact sampling volume, place and depth must be chosen carefully to ensure that samples represent water body characteristics. Sample volumes should be large enough to minimize overestimation induced by scaling up results, especially for water samples. The pump sampling approach with the filtration of large water volumes is necessary to generate reliable results in the spatial association between microplastic pollution in the surface waters and sediments. The trawling method has the ability to cover a larger area during sampling. To cover different microplastic size and shape, it is advantageous to combine both volume reduction and bulk sampling methods for surface water. More research is required to extend the understanding of representative in the study of microplastics as a key factor for the potential development of reliable data.
Authors' contributions
Razeghi and Hamidian had the idea for the article, Razeghi performed the literature search and data analysis, Razeghi and Hamidian drafted, and Wu, Zhang and Yang critically revised the work.
Funding
This manuscript was supported by Iran National Science Foundation (INSF) under the contract No. 97002416 and CHINESE ACADEMY OF SCI CAS PRESIDENT’S INTERNATIONAL FELLOWSHIP INITIATIVE Grant No. 2021VEA0004.
Declarations
Conflicts of interest
The authors have no conflict of interests to disclose.
Consent for publication
All authors agreed with the content and all gave explicit consent to submit, and they obtained consent from the responsible authorities.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/31/2021
A Correction to this paper has been published: 10.1007/s10311-021-01303-x
References
- Abbasi S, Turner A. Human exposure to microplastics: a study in Iran. J Hazard Mater. 2021;403:123799. doi: 10.1016/j.jhazmat.2020.123799. [DOI] [PubMed] [Google Scholar]
- Abidli S, Toumi H, Lahbib Y, El Menif NT. The first evaluation of microplastics in sediments from the complex lagoon-channel of Bizerte (Northern Tunisia) Water Air Soil Pollut. 2017;228(7):262. doi: 10.1007/s11270-017-3439-9. [DOI] [Google Scholar]
- Alam FC, Sembiring E, Suendo MBS, V, Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia) Chemosphere. 2019;224:637–645. doi: 10.1016/j.chemosphere.2019.02.188. [DOI] [PubMed] [Google Scholar]
- Alavian SS, Hamidian AH, Ashrafi S, Eagderi S, Khazaei M (2018) Study on age-related bioaccumulation of some heavy metals in the soft tissue of rock oyster (Saccostrea cucullata) from Laft Port – Qeshm Island. Iran J Fish Sci 16(3): 897–906. http://aquaticcommons.org/id/eprint/23127
- American Meteorological Society (2012) Freshwater, Glossary of Meteorology. Available online http://glossary.ametsoc.org/wiki/Freshwater. Accessed 18 Aug 2020
- Anderson PJ, Warrack S, Langen V, Challis JK, Hanson ML, Rennie MD. Microplastic contamination in lake Winnipeg, Canada. Environ Pollut. 2017;225:223–231. doi: 10.1016/j.envpol.2017.02.072. [DOI] [PubMed] [Google Scholar]
- Andrady AL. Microplastics in the marine environment. Mar Pollut Bul. 2011;62(8):1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Bagheri T, Gholizadeh M, Abarghouei S, et al. Microplastics distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay. Caspian sea Chemosphere. 2020;257:127201. doi: 10.1016/j.chemosphere.2020.127201. [DOI] [PubMed] [Google Scholar]
- Baldwin AK, Corsi SR, Mason SA. Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology. Environ Sci Technol. 2016;50(19):10377–10385. doi: 10.1021/acs.est.6b02917. [DOI] [PubMed] [Google Scholar]
- Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe FJ. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull. 2016;110(1):383–395. doi: 10.1016/j.marpolbul.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Barrows AP, Christiansen KS, Bode ET, Hoellein TJ. A watershed-scale, citizen science approach to quantifying microplastic concentration in a mixed land-use river. Water Res. 2018;147:382–392. doi: 10.1016/j.watres.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Barrows AP, Neumann CA, Berger ML, Shaw SD. Grab vs. neuston tow net: a microplastic sampling performance comparison and possible advances in the field. Anal Methods. 2017;9(9):1446–1453. doi: 10.1039/C6AY02387H. [DOI] [Google Scholar]
- Blair RM, Waldron S, Phoenix VR, Gauchotte-Lindsay C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland. UK Environ Sci Pollut Res. 2019;26(12):12491–12504. doi: 10.1007/s11356-019-04678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blašković A, Guerranti C, Fastelli P, Anselmi S, Renzi M. Plastic levels in sediments closed to Cecina river estuary (Tuscany, Italy) Mar Pollut Bull. 2018;135:105–109. doi: 10.1016/j.marpolbul.2018.07.021. [DOI] [PubMed] [Google Scholar]
- Blettler MC, Ulla MA, Rabuffetti AP, Garello N. Plastic pollution in freshwater ecosystems: macro-, meso-, and microplastic debris in a floodplain lake. Environ Monit Assess. 2017;189(11):1–13. doi: 10.1007/s10661-017-6305-8. [DOI] [PubMed] [Google Scholar]
- Bordós G, Urbányi B, Micsinai A, et al. Identification of microplastics in fish ponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere. 2019;216:110–116. doi: 10.1016/j.chemosphere.2018.10.110. [DOI] [PubMed] [Google Scholar]
- Brandsma SH, Nijssen P, Van Velzen MJM., Leslie HA (2013) Microplastics in river suspended particulate matter and sewage treatment plants. Report R14/02, Institute for environmental studies. University Amsterdam, Netherland. Available online https://puc.overheid.nl/rijkswaterstaat/doc/PUC_147662_31/. Accessed 14 Feb 2020
- Braun U, Jekel M, Gerdts G, Ivleva N, Reiber J (2018) Microplastics Analytics: Sampling, Preparation and Detection Methods. Discussion Paper within the scope of the research focus. Plastics in the Environment Sources Sinks Solutions. Discussion paper. German Federal Ministry of Education and Research, Germany. Available online https://bmbf-plastik.de/sites/default/files/2018-12/Discussion%20Paper%20Mikroplastics%20Analytics%20Nov%202018.pdf. Accessed 26 November 2020
- Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuarine Coastal Shelf Sci. 2016;178:189–195. doi: 10.1016/j.ecss.2015.12.003. [DOI] [Google Scholar]
- Briggs E, de Moura EAB, Furusawa HA, Cotrim MEB, Oguzie EE, Lugao AB, et al. Microplastics: a novel method for surface wate sampling ad sample extraction in Elechi Creek, Rivers State, Nigeria. In: Li B, et al., editors. Characterization of minerals, metals, and materials the minerals, metals and materials 2019 series. Cham: Springer; 2019. [Google Scholar]
- Browne MA, Galloway TS, Thompson RC. Spatial patterns of plastic debris along estuarine shorelines. Environ Sci Technol. 2010;44(9):3404–3409. doi: 10.1021/es903784e. [DOI] [PubMed] [Google Scholar]
- Campanale C, Stock F, Massarelli C, et al. Microplastics and their possible sources: the example of Ofanto river in Southeast Italy. Environ Pollut. 2020;258:113284. doi: 10.1016/j.envpol.2019.113284. [DOI] [PubMed] [Google Scholar]
- Campbell SH, Williamson PR, Hall BD. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets. 2017;2(1):395–409. doi: 10.1139/facets-2017-0008. [DOI] [Google Scholar]
- Carpenter EJ, Anderson SJ, Harvey GR, Miklas HP, Peck BB. Polystyrene spherules in coastal waters. Science. 1972;178(4062):749–750. doi: 10.1126/science.178.4062.749. [DOI] [PubMed] [Google Scholar]
- Carpenter EJ, Smith KL. Plastics on the Sargasso Sea surface. Sci. 1972;175(4027):1240–1241. doi: 10.1126/science.175.4027.1240. [DOI] [PubMed] [Google Scholar]
- Castañeda RA, Avlijas S, Simard MA, Ricciardi A. Microplastic pollution in St. Lawrence river sediments. Can J Fish Aquat Science. 2014;71(12):1767–1771. doi: 10.1139/cjfas-2014-0281. [DOI] [Google Scholar]
- Cedro A, Cleary J (2015) Microplastics in Irish freshwaters: a preliminary study. In Lekkas TD (ed) Proceedings of the 14th International Conference on Environmental Science and Technology, Rhodes, Greece 3:1666–1669. Available online https://www.researchgate.net/profile/John-Cleary-9/publication/281619904_Microplastics_in_Irish_freshwaters_A_preliminary_study/links/57750b1e08aead7ba06fbfef/Microplastics-in-Irish-freshwaters-A-preliminary-study.pdf. Accessed 13 Jan 2020.
- Colton JB, Knapp FD, Burns BR (1974) Plastic particles in surface waters of the northwestern Atlantic. Sci 185(4150):491–497. http://www.jstor.org/stable/1738284 [DOI] [PubMed]
- Connors KA, Dyer SD, Belanger SE. Advancing the quality of environmental microplastic research. Environ Toxicol Chem. 2017;36(7):1697–1703. doi: 10.1002/etc.3829. [DOI] [PubMed] [Google Scholar]
- Corcoran PL, Belontz SL, Ryan K, Walzak MJ. Factors controlling the distribution of microplastic particles in Benthic sediment of the Thames River. Canada Environ Sci Technol. 2019;54(2):818–825. doi: 10.1021/acs.est.9b04896. [DOI] [PubMed] [Google Scholar]
- Corcoran PL, Norris T, Ceccanese T, et al. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ Pollut. 2015;204:17–25. doi: 10.1016/j.envpol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Costa CQ, Cruz J, Martins J, Teodósio MAA, Jockusch S, Ramamurthy V, Da Silva JP. Fluorescence sensing of microplastics on surfaces. Environ Chem Lett. 2021 doi: 10.1007/s10311-020-01136-0. [DOI] [Google Scholar]
- Cox K (2018) Distribution, Abundance, and Spatial Variability of Microplastic Pollution in Surface Waters of Lake Superior. Dissertation, University of Waterloo
- Crawford C, Quinn B. Microplastic collection techniques. In: Crawford B, Quinn B, editors. Microplastic pollutants. Amsterdam: Elsevier Inc; 2017. [Google Scholar]
- de Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN. Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci Total Environ. 2018;645:1029–1039. doi: 10.1016/j.scitotenv.2018.07.207. [DOI] [PubMed] [Google Scholar]
- Dean BY, Corcoran PL, Helm PA. Factors influencing microplastic abundances in nearshore, tributary and beach sediments along the Ontario shoreline of Lake Erie. J Great Lakes Res. 2018;44(5):1002–1009. doi: 10.1016/j.jglr.2018.07.014. [DOI] [Google Scholar]
- Deocaris CC, Allosada JO, Ardiente LT, et al. Occurrence of microplastic fragments in the Pasig River. H2Open J. 2019;2(1):92–100. doi: 10.2166/h2oj.2019.001. [DOI] [Google Scholar]
- Di M, Liu X, Wang W, Wang J. Manuscript prepared for submission to environmental toxicology and pharmacology pollution in drinking water source areas: Microplastics in the Danjiangkou Reservoir, China. Environ Toxicol Pharmacol. 2019;65:82–89. doi: 10.1016/j.etap.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Di M, Wang J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci Total Environ. 2018;616:1620–1627. doi: 10.1016/j.scitotenv.2017.10.150. [DOI] [PubMed] [Google Scholar]
- Dikareva N, Simon KS. Microplastic pollution in streams spanning an urbanisation gradient. Environ Pollut. 2019;250:292–299. doi: 10.1016/j.envpol.2019.03.105. [DOI] [PubMed] [Google Scholar]
- Ding J, Zhang S, Razanajatovo RM, Zou H, Zhu W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus) Environ Pollut. 2018;238:1–9. doi: 10.1016/j.envpol.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Ding L, et al. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci Total Environ. 2019;667:427–434. doi: 10.1016/j.scitotenv.2019.02.332. [DOI] [PubMed] [Google Scholar]
- Dong M, Luo Z, Jiang Q, Xing X, Zhang Q, Sun Y. The rapid increases in microplastics in urban lake sediments. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-57933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B. Microplastic contamination in an urban area: a case study in Greater Paris. Environ Chem. 2015;12(5):592–599. doi: 10.1071/EN14167. [DOI] [Google Scholar]
- Dris R, Gasperi J, Rocher V, Tassin B. Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: Sampling methodological aspects and flux estimations. Sci Total Environ. 2018;618:157–164. doi: 10.1016/j.scitotenv.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Dubaish F, Liebezeit G. Suspended microplastics and black carbon particles in the Jade system, southern North Sea. Water Air Soil Pollut. 2013;224(2):1352. doi: 10.1007/s11270-012-1352-9. [DOI] [Google Scholar]
- Egessa R, Nankabirwa A, Basooma R, Nabwire R. Occurrence, distribution and size relationships of plastic debris along shores and sediment of northern Lake Victoria. Environ Pollut. 2020;257:113442. doi: 10.1016/j.envpol.2019.113442. [DOI] [PubMed] [Google Scholar]
- Enders K, Käppler A, Biniasch O, et al. Tracing microplastics in aquatic environments based on sediment analogies. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-50508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo S, Hong SH, Song YK, Han GM, Shim WJ. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Res. 2019;160:228–237. doi: 10.1016/j.watres.2019.05.053. [DOI] [PubMed] [Google Scholar]
- Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Amato S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull. 2013;77(1–2):177–182. doi: 10.1016/j.marpolbul.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Estahbanati S, Fahrenfeld NL. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere. 2016;162:277–284. doi: 10.1016/j.chemosphere.2016.07.083. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zheng K, Zhu Z, Chen G, Peng X. Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China. Environ Pollut. 2019;251:862–870. doi: 10.1016/j.envpol.2019.05.056. [DOI] [PubMed] [Google Scholar]
- Farrell P, Nelson K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.) Environ Pollut. 2013;177:1–3. doi: 10.1016/j.envpol.2013.01.046. [DOI] [PubMed] [Google Scholar]
- Faure F, Corbaz M, Baecher H, de Alencastro L. Pollution due to plastics and microplastics in Lake Geneva and in the Mediterranean Sea. Arch des Sci. 2012;65:157–164. [Google Scholar]
- Faure F, Demars C, Wieser O, Kunz M, De Alencastro LF. Plastic pollution in Swiss surface waters: nature and concentrations, interaction with pollutants. Environ Chem. 2015;12(5):582–591. doi: 10.1071/EN14218. [DOI] [Google Scholar]
- Firdaus M, Trihadiningrum Y, Lestari P. Microplastic pollution in the sediment of Jagir Estuary, Surabaya City. Indonesia Mar Pollut Bull. 2020;150:110790. doi: 10.1016/j.marpolbul.2019.110790. [DOI] [PubMed] [Google Scholar]
- Fischer EK, Paglialonga L, Czech E, Tamminga M. Microplastic pollution in lakes and lake shoreline sediments–a case study on Lake Bolsena and Lake Chiusi (central Italy) Environ Pollut. 2016;213:648–657. doi: 10.1016/j.envpol.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull. 2014;85(1):156–163. doi: 10.1016/j.marpolbul.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Gallagher A, Rees A, Rowe R, Stevens J, Wright P. Microplastics in the Solent estuarine complex, UK: an initial assessment. Mar Pollut Bull. 2016;102(2):243–249. doi: 10.1016/j.marpolbul.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrasi G, Sorrentino A, Lichtfouse E. Back to plastic pollution in COVID times. Environ Chem Lett. 2020;19(1):1–4. doi: 10.1007/s10311-020-01129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AD, Wertz H, Leads RR, Weinstein JE. Microplastic in two South Carolina Estuaries: occurrence, distribution, and composition. Mar Pollut Bull. 2018;128:223–233. doi: 10.1016/j.marpolbul.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Grbić J, Helm P, Athey S, Rochman CM. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020;174:115623. doi: 10.1016/j.watres.2020.115623. [DOI] [PubMed] [Google Scholar]
- Green DS. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ Pollut. 2016;216:95–103. doi: 10.1016/j.envpol.2016.05.043. [DOI] [PubMed] [Google Scholar]
- Hamidian AH, Jafari Ozumchelouei E, Feizi F, Wu C, Zhang Y, Yang M. A review on the characteristics of microplastics in wastewater treatment plants: a source for toxic chemicals. J Clean Prod. 2021;295:126480. doi: 10.1016/j.jclepro.2021.126480. [DOI] [Google Scholar]
- Hamidian AH, Razeghi N, Zhang Y, Yang M. Spatial distribution of arsenic in groundwater of Iran, a review. J Geochem Explor. 2019;201:88–98. doi: 10.1016/j.gexplo.2019.03.014. [DOI] [Google Scholar]
- Hamidian AH, Zareh Reshqueih M, Poorbagher H, Ashrafi VL, S, Heavy metal bioaccumulation in sediment, common reed, algae and blood worm from the Shoor River. Iran J Toxicol Environ. 2016;32(3):398–409. doi: 10.1177/0748233713500835. [DOI] [PubMed] [Google Scholar]
- Han M, Niu X, Tang M, Zhang BT, Wang G, Yue W, Zhu J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci Total Environ. 2020;707:135601. doi: 10.1016/j.scitotenv.2019.135601. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Rist S, Bodin J, Jensen LH, Schmidt SN, Mayer P, Baun A. Microplastics as vectors for environmental contaminants: exploring sorption, desorption, and transfer to biota. Integr Environ Assess Manage. 2017;13(3):488–493. doi: 10.1002/ieam.1904. [DOI] [PubMed] [Google Scholar]
- He B, Goonetilleke A, Ayoko GA, Rintoul L. Abundance, distribution patterns, and identification of microplastics in Brisbane river sediments. Australia Sci Total Environ. 2020;700:134467. doi: 10.1016/j.scitotenv.2019.134467. [DOI] [PubMed] [Google Scholar]
- Hendrickson E, Minor EC, Schreiner K. Microplastic abundance and composition in western Lake superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ Sci Technol. 2018;52(4):1787–1796. doi: 10.1021/acs.est.7b05829. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol. 2012;46(6):3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- Hitchcock JN, Mitrovic SM. Microplastic pollution in estuaries across a gradient of human impact. Environ Pollut. 2019;247:457–466. doi: 10.1016/j.envpol.2019.01.069. [DOI] [PubMed] [Google Scholar]
- Holland ER, Mallory ML, Shutler D. Plastics and other anthropogenic debris in freshwater birds from Canada. Sci Total Environ. 2016;571:251–258. doi: 10.1016/j.scitotenv.2016.07.158. [DOI] [PubMed] [Google Scholar]
- Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Hu L, Chernick M, Hinton DE, Shi H. Microplastics in small waterbodies and tadpoles from Yangtze River Delta. China Environ Sci Technol. 2018;52(15):8885–8893. doi: 10.1021/acs.est.8b02279. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tian M, Jin F, Chen M, Liu Z, Mu HS. Coupled effects of urbanization level and dam on microplastics in surface waters in a coastal watershed of Southeast China. Mar Pollut Bul. 2020;154:111089. doi: 10.1016/j.marpolbul.2020.111089. [DOI] [PubMed] [Google Scholar]
- Huppertsberg S, Knepper TP. Instrumental analysis of microplastics—benefits and challenges. Anal Bioanal Chem. 2018;410(25):6343–6352. doi: 10.1007/s00216-018-1210-8. [DOI] [PubMed] [Google Scholar]
- Hurley R, Woodward J, Rothwell JJ. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat Geosci. 2018;11(4):251–257. doi: 10.1038/s41561-018-0080-1. [DOI] [Google Scholar]
- Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol. 2013;23(19):R867–R868. doi: 10.1016/j.cub.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Imhof HK, Laforsch C, Wiesheu AC, et al. Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res. 2016;98:64–74. doi: 10.1016/j.watres.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Imhof HK, Wiesheu AC, Anger PM, Niessner R, Ivleva NP, Laforsch C. Variation in plastic abundance at different lake beach zones-a case study. Sci Total Environ. 2018;613:30–537. doi: 10.1016/j.scitotenv.2017.08.300. [DOI] [PubMed] [Google Scholar]
- Irfan M, Qadir A, Mumtaz M, Ahmad SR. An unintended challenge of microplastic pollution in the urban surface water system of Lahore. Pakistan Environ Sci Pollut Res. 2020;27(14):16718–16730. doi: 10.1007/s11356-020-08114-7. [DOI] [PubMed] [Google Scholar]
- Jafari Ozumchelouei E, Hamidian AH, Zhang Y, Yang M. Physicochemical properties of antibiotics: a review with an emphasis on detection in the aquatic environment. Water Environ Res. 2020;92:177–188. doi: 10.1002/wer.1237. [DOI] [PubMed] [Google Scholar]
- Wang JD, Peng J, Tan Z, Gao Y, Zhan Z, Chen Q, Cai L. Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere. 2017;171:248–258. doi: 10.1016/j.chemosphere.2016.12.074. [DOI] [PubMed] [Google Scholar]
- Jiang C, Yin L, Li Z, Wen X, Luo X, Hu S, Liu Y. Microplastic pollution in the rivers of the Tibet Plateau. Environ Pollut. 2019;249:91–98. doi: 10.1016/j.envpol.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Jiang C, Yin L, Wen X, Du C, Wu L, Long Y, Pan H. Microplastics in sediment and surface water of west dongting lake and south dongting lake: abundance, source and composition. Int J Environ Res Public Health. 2018;15(10):2164. doi: 10.3390/ijerph15102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp KJ, Yeatman E. Microplastic hotspots in the Snake and Lower Columbia rivers: a journey from the greater yellowstone ecosystem to the Pacific ocean. Environ Pollut. 2018;241:1082–1090. doi: 10.1016/j.envpol.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson TM, Kärrman A, Rotander A, Hassellöv M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ Sci Pollut Res. 2020;27(5):5559–5571. doi: 10.1007/s11356-019-07274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Nihei Y, Kudou K, Hinata H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ Pollut. 2019;244:958–965. doi: 10.1016/j.envpol.2018.10.111. [DOI] [PubMed] [Google Scholar]
- Kershaw P, Katsuhiko S, Lee S, Samseth J, Woodring D (2011) Plastic debris in the ocean. In: UNEP Year Book 2011: Emerging Issues in our Global Environment, Nairobi, Kenya, pp 21
- Klein S, Worch E, Knepper TP. Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany. Environ Sci Technol. 2015;49(10):6070–6076. doi: 10.1021/acs.est.5b00492. [DOI] [PubMed] [Google Scholar]
- Koelmans AA, Bakir A, Burton GA, Janssen CR. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ Sci Technol. 2016;50(7):3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahens L, Strady E, Kieu-Le TC, Drisn R, Boukerma K, Rinnert E, Tassin B. Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity. Environ Pollut. 2018;236:661–671. doi: 10.1016/j.envpol.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Lasee S, Mauricio J, Thompson WA, et al. Microplastics in a freshwater environment receiving treated wastewater effluent. Integr Environ Assess Manage. 2017;13(3):528–532. doi: 10.1002/ieam.1915. [DOI] [PubMed] [Google Scholar]
- Lechner A, Keckeis H, Lumesberger-Loisl F, et al. The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe's second largest river. Environ Pollut. 2014;188:177–181. doi: 10.1016/j.envpol.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie HA, Brandsma SH, Van Velzen MJM, Vethaak AD. Microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ Int. 2017;101:133–142. doi: 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Li L, Geng S, Wu C, Song K, Sun F, Visvanathan C, Wang Q. Microplastics contamination in different trophic state lakes along the middle and lower reaches of Yangtze River Basin. Environ Pollut. 2019;254:112951. doi: 10.1016/j.envpol.2019.07.119. [DOI] [PubMed] [Google Scholar]
- Liedermann M, Gmeiner P, Pessenlehner S, Haimann M, Hohenblum P, Habersack H. A methodology for measuring microplastic transport in large or medium rivers. Water. 2018;10(4):414. doi: 10.3390/w10040414. [DOI] [Google Scholar]
- Lin L, Zuo LZ, Peng JP, Cai LQ, et al. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci Total Environ. 2018;644:375–381. doi: 10.1016/j.scitotenv.2018.06.327. [DOI] [PubMed] [Google Scholar]
- Liu F, Olesen KB, Borregaard AR, Vollertsen J. Microplastics in urban and highway stormwater retention ponds. Sci Total Environ. 2019;671:992–1000. doi: 10.1016/j.scitotenv.2019.03.416. [DOI] [PubMed] [Google Scholar]
- Luo W, Su L, Craig NJ, Du F, Wu C, Shi H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ Pollut. 2019;246:174–182. doi: 10.1016/j.envpol.2018.11.081. [DOI] [PubMed] [Google Scholar]
- Lusher A, Buenaventura NT, Eidsvoll D, Thrane JE, Økelsrud A, Jartun M (2018) Freshwater microplastics in Norway: A first look at sediment, biota and historical plankton samples from Lake Mjøsa and Lake Femunden. Norwegian Insititute for Water Research. Available online http://hdl.handle.net/11250/2588713. Accessed 20 Apr 2020
- Lusher AL, Welden NA, Sobral P, Cole M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal Methods. 2017;9(9):1346–1360. doi: 10.1039/C6AY02415G. [DOI] [Google Scholar]
- Mani T, Blarer P, Storck FR, Pittroff M, Wernicke T, Burkhardt-Holm P. Repeated detection of polystyrene microbeads in the lower Rhine River. Environ Pollut. 2019;245:634–641. doi: 10.1016/j.envpol.2018.11.036. [DOI] [PubMed] [Google Scholar]
- Mani T, Burkhardt-Holm P. Seasonal microplastics variation in nival and pluvial stretches of the Rhine River-From the Swiss catchment towards the North Sea. Sci Total Environ. 2020;707:135579. doi: 10.1016/j.scitotenv.2019.135579. [DOI] [PubMed] [Google Scholar]
- Mani T, Hauk A, Walter U, Burkhardt-Holm P. Microplastics profile along the Rhine River. Sci Rep. 2015;5(1):1–7. doi: 10.1038/srep17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani T, Primpke S, Lorenz C, Gerdts G, Burkhardt-Holm P. Microplastic pollution in benthic midstream sediments of the Rhine River. Environ Sci Technol. 2019;53(10):6053–6062. doi: 10.1021/acs.est.9b01363. [DOI] [PubMed] [Google Scholar]
- Mansouri B, Pourkhabbaz A, Ebrahimpour M, Hamidian BHAH. Bioaccumulation and elimination rate of cobalt in Capoeta fusca under controlled conditions. Chem Speciat Bioavailab. 2013;25(1):52–56. doi: 10.3184/095422913X13581898658634. [DOI] [Google Scholar]
- Mao Y, Li H, Gu W, Yang G, Liu Y, He Q. Distribution and characteristics of microplastics in the Yulin River, China: Role of environmental and spatial factors. Environ Pollut. 2020;265:115033. doi: 10.1016/j.envpol.2020.115033. [DOI] [PubMed] [Google Scholar]
- Mao R, Hu Y, Zhang S, Wu R, Guo X. Microplastics in the surface water of Wuliangsuhai Lake, northern China. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.137820. [DOI] [PubMed] [Google Scholar]
- Martínez Silva P, Nanny MA. Impact of microplastic fibers from the degradation of nonwoven synthetic textiles to the Magdalena river water column and river sediments by the city of Neiva, Huila (Colombia) Water. 2020;12(4):1210. doi: 10.3390/w12041210. [DOI] [Google Scholar]
- Mason SA, Kammin L, Eriksen M, Aleid G, Wilson S, Riley BCA. Pelagic plastic pollution within the surface waters of Lake Michigan, USA. J Great Lakes Res. 2016;42(4):753–759. doi: 10.1016/j.jglr.2016.05.009. [DOI] [Google Scholar]
- Mathalon A, Hill P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor. Nova Scotia Mar Pollut Bull. 2014;81(1):69–79. doi: 10.1016/j.marpolbul.2014.02.018. [DOI] [PubMed] [Google Scholar]
- McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol. 2014;48(20):11863–11871. doi: 10.1021/es503610r. [DOI] [PubMed] [Google Scholar]
- McCormick AR, Hoellein TJ, London MG, Hittie J, Scott JW, Kelly JJ. Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere. 2016;7(11):e01556. doi: 10.1002/ecs2.1556. [DOI] [Google Scholar]
- McDevitt C, Perez L, Kapp K (2016) The presence of microplastic in freshwater systems: Snake river and Palisades Reservoir. Department of Health and Science, Central Wyoming University. Available https://mountainscholar.org/handle/20.500.11919/2598/. Accessed 3 October 2019
- McNeish RE, Kim LH, Barrett HA, Mason SA, Kelly JJ, Hoellein TJ. Microplastic in riverine fish is connected to species traits. Sci Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-29980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merga LB, Redondo-Hasselerharm PE, Van den Brink PJ, Koelmans AA. Distribution of microplastic and small macroplastic particles across four fish species and sediment in an African lake. Sci Total Environ. 2020;741:140527. doi: 10.1016/j.scitotenv.2020.140527. [DOI] [PubMed] [Google Scholar]
- Michida Y, Chavanich S, Chiba S, Cordova MR, Cozsar Cabanas A, Glagani F Kozlovskii N (2019) Guidelines for Harmonizing Ocean Surface Microplastic Monitoring Methods. Version 1.1. Ministry of the Environment, JAPAN. Available online https://repository.oceanbestpractices.net/bitstream/handle/11329/1361/Guildlines_ver.1.1_v28_200618.pdf?sequence=1&isAllowed=y. Accessed Feb 2021
- Miller RZ, Watts AJ, Winslow BO, Galloway TS, Barrows AP. Mountains to the sea: river study of plastic and non-plastic microfiber pollution in the northeast USA. Mar Pollut Bull. 2017;124(1):245–251. doi: 10.1016/j.marpolbul.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Mintenig SM, Kooi M, Erich MW, Primpke S, Redondo-Hasselerharm PE, Dekker SC, van Wezel AP. A systems approach to understand microplastic occurrence and variability in Dutch riverine surface waters. Water Res. 2020;176:115723. doi: 10.1016/j.watres.2020.115723. [DOI] [PubMed] [Google Scholar]
- Mirzajani A, Hamidian AH, Karami M. Distribution and abundance of fish in the southwest of Caspian Sea coastal waters. Russ J Mar Bio. 2016;42(2):178–189. doi: 10.1134/S1063074016020073. [DOI] [Google Scholar]
- Mirzajani A, Hamidian AH, Karami M (2017) Metal bioaccumulation in representative organisms from different trophic levels of the Caspian Sea. Iran J Fish Sci 15(3):1027–1043. http://aquaticcommons.org/id/eprint/22930
- Mirzajani A, Hamidian AH, Bagheri S, Karami M. Possible effect of Balanus improvisus on Cerastoderma glaucum distribution in the south-western Caspian Sea. J Mar Biol Assoc U K. 2015;96(5):1031–1040. doi: 10.1017/S0025315415000788. [DOI] [Google Scholar]
- Mojoudi F, Hamidian AH, Goodarzian N, Eagderi S. Effective removal of heavy metals from aqueous solution by porous activated carbon/thiol functionalized graphene oxide composite. Desalin Water Treat. 2018;124:106–116. doi: 10.5004/dwt.2018.22695. [DOI] [Google Scholar]
- Mojoudi F, Hamidian AH, Zhang Y, Yang M. Synthesis and evaluation of Activated Carbon/Nanoclay/ Thiolated Graphene Oxide Nanocomposite for Lead (II) Removal from Aqueous Solution. Water Sci Technol. 2019;79(3):466–479. doi: 10.2166/wst.2019.071. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Lattin GL, Zellers AF (2011) Quantity and type of plastic debris flowing from two urban rivers to coastal waters and beaches of Southern California. J Coas Zone Manag 11(1):65–73. https://www.redalyc.org/pdf/3883/388340132008.pdf
- Naidoo T, Glassom D, Smit AJ. Plastic pollution in five urban estuaries of KwaZulu-Natal. South Africa Mar Pollut Bull. 2015;101(1):473–480. doi: 10.1016/j.marpolbul.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Nan B, Su L, Kellar C, Craig NJ, Keough MJ, Pettigrove V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria. Australia Environ Pollut. 2020;259:113865. doi: 10.1016/j.envpol.2019.113865. [DOI] [PubMed] [Google Scholar]
- Naqash N, Prakash S, Kapoor D, Singh R. Interaction of freshwater microplastics with biota and heavy metals: a review. Environ Chem Lett. 2020;18(6):1813–1824. doi: 10.1007/s10311-020-01044-3. [DOI] [Google Scholar]
- Neto JAB, Gaylarde C, Beech I, Bastos AC, da Silva QV, de Carvalho DG. Microplastics and attached microorganisms in sediments of the Vitória bay estuarine system in SE Brazil. Ocean Coast Manage. 2019;169:247–253. doi: 10.1016/j.ocecoaman.2018.12.030. [DOI] [Google Scholar]
- Noik VJ, Tuah PM. A first survey on the abundance of plastics fragments and particles on two sandy beaches in Kuching, Sarawak. Malaysia Conf Ser Mater Sci Eng. 2015;78(1):012035. doi: 10.1088/1757-899X/78/1/012035. [DOI] [Google Scholar]
- Ogonowski M, Schür C, Gorokhova JÅ. The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS ONE. 2016;11(5):e0155063. doi: 10.1371/journal.pone.0155063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KB, Stephansen DA, van Alst N, Vollertsen J. Microplastics in a stormwater pond. Water. 2019;11(7):1466. doi: 10.3390/w11071466. [DOI] [Google Scholar]
- Othman AR, Hasan HA, Muhamad MH, et al. Microbial degradation of microplastics by enzymatic processes: a review. Environ Chem Lett. 2021 doi: 10.1007/s10311-021-01197-9. [DOI] [Google Scholar]
- Padash Barmchi Z, Hamidian AH, Khorasani N, Kazemzad M, McCabe A, Halog A. Environmental life cycle assessments of emerging anode materials for Li-Ion batteries-metal oxide NPs. Environ Prog Sustain Energy. 2015;34(6):1740–1747. doi: 10.1002/ep.12148. [DOI] [Google Scholar]
- Padervand M, Lichtfouse E, Robert D, Wang C. Removal of microplastics from the environment. A Rev Environ Chem Lett. 2020;18(3):807–828. doi: 10.1007/s10311-020-00983-1. [DOI] [Google Scholar]
- Pariatamby A, Hamid FS, Bhatti MS, Anuar N, Anuar N. Status of microplastic pollution in aquatic ecosystem with a case study on Cherating River, Malaysia. J Eng Technol Sci. 2020 doi: 10.5614/j.eng.technol.sci.2020.52.2.7. [DOI] [Google Scholar]
- Pellini G, Gomiero A, Fortibuoni T, Ferrà C, Grati F, Tassetti AN, Scarcella G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ Pollut. 2018;234:943–952. doi: 10.1016/j.envpol.2017.12.038. [DOI] [PubMed] [Google Scholar]
- Peng G, Xu P, Zhu B, Bai M, Li D. Microplastics in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ Pollut. 2018;234:448–456. doi: 10.1016/j.envpol.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Peng G, Zhu B, Yang D, Su L, Shi H, Li D. Microplastics in sediments of the Changjiang Estuary, China. Environ Pollut. 2017;225:283–290. doi: 10.1016/j.envpol.2016.12.064. [DOI] [PubMed] [Google Scholar]
- Plastics Europe (2018). Plastics-The facts 2018. An analysis of European plastics production, demand and waste data. Available online https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf. Accessed 18 Aug 2020
- Rao Z, Niu S, Zhan N, Wang X, Song X. Microplastics in sediments of River Yongfeng from Maanshan city, Anhui Province. China Bull Environ Contam Toxicol. 2020;104(2):166–172. doi: 10.1007/s00128-019-02771-2. [DOI] [PubMed] [Google Scholar]
- Razeghi N, Hamidian AH, Wu C, Zhang Y, Yang M. Scientific studies on microplastics pollution in Iran: an in-depth review of the published articles. Mar Pollut Bull. 2020;162:111901. doi: 10.1016/j.marpolbul.2020.111901. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ryan PG. Micro-plastic ingestion by waterbirds from contaminated wetlands in South Africa. Mar Pollut Bul. 2018;126:330–333. doi: 10.1016/j.marpolbul.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Rezaei Kalvani S, Sharaai AH, Abd Manaf L, Hamidian AH. Assessing ground and surface water scarcity indices using ground and surface water footprints in the Tehran province of Iran. Appl Ecol Env Res. 2019;17(2):4985–4997. doi: 10.15666/aeer/1702_49854997. [DOI] [Google Scholar]
- Rezania S, Park J, Din MFM, Taib SM, Talaiekhozani A, Yadav KK, Kamyab H. Microplastics pollution in different aquatic environments and biota: a review of recent studies. Mar Pollut Bull. 2018;133:191–208. doi: 10.1016/j.marpolbul.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Rodrigues MO, Abrantes N, Gonçalves FJM, Nogueira H, Marques JC, Gonçalves AMM. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal) Sci Total Environ. 2018;633:1549–1559. doi: 10.1016/j.scitotenv.2018.03.233. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Almeida CMR, Silva D, Cunha J, Antunes C, Freitas V, Ramos S. Microplastic contamination in an urban estuary: abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci Total Environ. 2019;659:1071–1081. doi: 10.1016/j.scitotenv.2018.12.273. [DOI] [PubMed] [Google Scholar]
- Sadri SS, Thompson RC. On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary. Southwest England Mar Pollut Bull. 2014;81(1):55–60. doi: 10.1016/j.marpolbul.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Sarijan S, Azman S, Said MIM, Andu Y, Zon NF. Microplastics in sediment from Skudai and Tebrau river, Malaysia: a preliminary study. MATEC Web of Conf. 2018;250:06012. doi: 10.1051/matecconf/201825006012. [DOI] [Google Scholar]
- Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut. 2014;185:77–83. doi: 10.1016/j.envpol.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut Res. 2017;24(27):21530–21547. doi: 10.1007/s11356-017-9910-8. [DOI] [PubMed] [Google Scholar]
- Shruti VC, Jonathan MP, Rodriguez-Espinosa PF, Rodríguez-González F. Microplastics in freshwater sediments of atoyac river basin, puebla city, Mexico. Environ Sci Pollut Res. 2019;654:154–163. doi: 10.1016/j.scitotenv.2018.11.054. [DOI] [PubMed] [Google Scholar]
- Sighicelli M, Pietrelli L, Lecce F, Iannilli V, Falconieri M, Coscia L, Zampetti G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ Pollut. 2018;236:645–651. doi: 10.1016/j.envpol.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Simon-Sánchez L, Grelaud M, Garcia-Orellana J, Ziveri P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean) Sci Total Environ. 2019;687:1186–1196. doi: 10.1016/j.scitotenv.2019.06.168. [DOI] [PubMed] [Google Scholar]
- Smith JA, Hodge JL, Kurtz BH, Garver JI (2017) The distribution of microplastic pollution in the Mohawk River. Mohawk Watershed Symposium. Available online https://www.researchgate.net/profile/John-Garver/publication/314543747_Proceedings_of_the_2017_Mohawk_Watershed_Symposium_Union_College_Schenectady_NY_17_Mb/links/58c339a8aca272e36dd0467c/Proceedings-of-the-2017-Mohawk-Watershed-Symposium-Union-College-Schenectady-NY-17-Mb.pdf#page=78. Accessed 20 May 2020
- Sruthy S, Ramasamy EV. Microplastic pollution in Vembanad Lake, Kerala, India: the first report of microplastics in lake and estuarine sediments in India. Environ Pollut. 2017;222:315–322. doi: 10.1016/j.envpol.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Su L, Cai H, Kolandhasamy P, Wu C, Rochman CM, Shi H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ Pollut. 2018;234:347–355. doi: 10.1016/j.envpol.2017.11.075. [DOI] [PubMed] [Google Scholar]
- Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H. Microplastics in Taihu lake, China. Environ Pollut. 2016;216:711–719. doi: 10.1016/j.envpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Ta AT, Babel S, Haarstrick A (2020) Microplastics contamination in a high population density area of the Chao Phraya River ,Bangkok. J Eng Technol. 10.5614/j.eng.technol.sci.2020.52.4.6
- Tamminga M, Stoewer SC, Fischer EK. On the representativeness of pump water samples versus manta sampling in microplastic analysis. Environ Pollut. 2019;254:112970. doi: 10.1016/j.envpol.2019.112970. [DOI] [PubMed] [Google Scholar]
- Tan X, Yu X, Cai L, Wang J, Peng J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere. 2019;221:834–840. doi: 10.1016/j.chemosphere.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Moore CJ, Vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc. 2009;364(1526):2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, Russell AE. Lost at sea: Where is all the plastic? Science. 2004;304(5672):838–838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- Tibbetts J, Krause S, Lynch I, Sambrook Smith GH. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water. 2018;10(11):1597. doi: 10.3390/w10111597. [DOI] [Google Scholar]
- Tofa TS, Kunjali KL, Paul S, Dutta J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ Chem Lett. 2019;17(3):1341–1346. doi: 10.1007/s10311-019-00859-z. [DOI] [Google Scholar]
- Toumi H, Abidli S, Bejaoui M. Microplastics in freshwater environment: the first evaluation in sediments from seven water streams surrounding the lagoon of Bizerte (Northern Tunisia) Environ Sci Pollut Res. 2019;26(14):14673–14682. doi: 10.1007/s11356-019-04695-0. [DOI] [PubMed] [Google Scholar]
- Turner S, Horton AA, Rose NL, Hall C. A temporal sediment record of microplastics in an urban lake, London. UK J Paleolimnol. 2019;61(4):449–462. doi: 10.1007/s10933-019-00071-7. [DOI] [Google Scholar]
- van der Wal M, van der Meulen M, Tweehuijsen G, Peterlin M, Palatinus A, Kovac Viršek M (2015) SFRA0025: Identification and Assessment of Riverine Input of (Marine) Litter. Report for Michail Papadoyannakis, DG Environment, United Kingdom, pp 186. Available online https://portal.helcom.fi/meetings/PRESSURE%203-2015-278/MeetingDocuments/5-8_att%20Final%20Report%20for%20EC%20DG%20ENV%20-Identification%20and%20Assessment%20of%20Riverine%20Input%20of%20Marine%20Litter.pdf. Accessed 20 Feb 2020
- Vaughan R, Turner SD, Rose NL. Microplastics in the sediments of a UK urban lake. Environ Pollut. 2017;229:10–18. doi: 10.1016/j.envpol.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Vermaire JC, Pomeroy C, Herczegh SM, Haggart O, Murphy M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets. 2017;2(1):301–314. doi: 10.1139/facets-2016-0070. [DOI] [Google Scholar]
- Vianello A, Boldrin A, Guerriero P, Moschino V, Rella R, Sturaro A, Da Ros L. Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuarine Coastal Shelf Sci. 2013;130:54–61. doi: 10.1016/j.ecss.2013.03.022. [DOI] [Google Scholar]
- Wang C, Xing R, Sun M, Ling W, Shi W, Cui S, An L. Microplastics profile in a typical urban river in Beijing. Sci Total Environ. 2020;743:140708. doi: 10.1016/j.scitotenv.2020.140708. [DOI] [PubMed] [Google Scholar]
- Wang G, Lu J, Tong Y, Liu Z, Zhou H, Xiayihazi N. Occurrence and pollution characteristics of microplastics in surface water of the Manas River Basin. China Sci Total Environ. 2020;710:136099. doi: 10.1016/j.scitotenv.2019.136099. [DOI] [PubMed] [Google Scholar]
- Wang Z, Qin Y, Li W, Yang W, Meng Q, Yang J. Microplastic contamination in freshwater: first observation in Lake Ulansuhai, Yellow River Basin. China Environ Chem Lett. 2019;17(4):1821–1830. doi: 10.1007/s10311-019-00888-8. [DOI] [Google Scholar]
- Warrack S, Challis JK, Hanson ML, Rennie MD. Microplastics flowing into Lake Winnipeg: densities, sources, flux, and fish exposures. Proc Manit Undergrad Sci Eng Res. 2017;3:5–15. doi: 10.5203/pmuser.201730578. [DOI] [Google Scholar]
- Watkins L, McGrattan S, Sullivan PJ, Walter MT. The effect of dams on river transport of microplastic pollution. Sci Total Environ. 2019;664:834–840. doi: 10.1016/j.scitotenv.2019.02.028. [DOI] [PubMed] [Google Scholar]
- Watkins L, Sullivan PJ, Walter MT. A case study investigating temporal factors that influence microplastic concentration in streams under different treatment regimes. Environ Sci Pollut Res. 2019;26(21):21797–21807. doi: 10.1007/s11356-019-04663-8. [DOI] [PubMed] [Google Scholar]
- Wen X, Du C, Xu P, Zeng G, Huang D, Yin L, Tan S. Microplastic pollution in surface sediments of urban water areas in Changsha, China: abundance, composition, surface textures. Mar Pollut Bull. 2018;136:414–423. doi: 10.1016/j.marpolbul.2018.09.043. [DOI] [PubMed] [Google Scholar]
- Wessel CC, Lockridge GR, Battiste D, Cebrian J. Abundance and characteristics of microplastics in beach sediments: insights into microplastic accumulation in northern Gulf of Mexico estuaries. Mar Pollut Bull. 2016;109(1):178–183. doi: 10.1016/j.marpolbul.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Wang WF, Ndungu AW, Li Z, Wang J. Microplastics pollution in inland freshwaters of China: a case study in urban surface waters of Wuhan, China. Sci Total Environ. 2017;575:1369–1374. doi: 10.1016/j.scitotenv.2016.09.213. [DOI] [PubMed] [Google Scholar]
- Wang WF, Yuan W, Chen Y, Wang J. Microplastics in surface waters of dongting lake and hong lake. Sci Total Environ. 2018;633:539–545. doi: 10.1016/j.scitotenv.2018.03.211. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Su B, Xu X, Di D, Huang H, Mei K, Shang X. Preferential accumulation of small (< 300 μm) microplastics in the sediments of a coastal plain river network in eastern China. Water Res. 2018;144:393–401. doi: 10.1016/j.watres.2018.07.050. [DOI] [PubMed] [Google Scholar]
- Willis KA, Eriksen R, Wilcox C, Hardesty BD. Microplastic distribution at different sediment depths in an urban estuary. Front Mar Sci. 2017;4:419. doi: 10.3389/fmars.2017.00419. [DOI] [Google Scholar]
- Wu C, Zhang K, Xiong X. Microplastic pollution in inland waters focusing on Asia. In: Wagner M, Lambert S, editors. Freshwater Microplastics: emerging environmental contaminants? Heidelberg: Springer; 2018. pp. 85–99. [Google Scholar]
- Wu P, Tang Y, Dang M, Wang S, Jin H, Liu Y, Cai Z. Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Sci Total Environ. 2020;717:135187. doi: 10.1016/j.scitotenv.2019.135187. [DOI] [PubMed] [Google Scholar]
- Xiong X, Wu C, Elser JJ, Mei Z, Hao Y. Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River–from inland to the sea. Sci Total Environ. 2019;659:66–73. doi: 10.1016/j.scitotenv.2018.12.313. [DOI] [PubMed] [Google Scholar]
- Xiong X, Zhang K, Chen X, Shi H, Luo Z, Wu C. Sources and distribution of microplastics in China's largest inland lake–Qinghai Lake. Environ Pollut. 2018;235:899–906. doi: 10.1016/j.envpol.2017.12.081. [DOI] [PubMed] [Google Scholar]
- Xu P, Peng G, Su L, Gao Y, Gao L, Li D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar Pollut Bull. 2018;133:647–654. doi: 10.1016/j.marpolbul.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Xu Q, Xing R, Sun M, Gao Y, An L. Microplastics in sediments from an interconnected river-estuary region. Sci Total Environ. 2020;729:139025. doi: 10.1016/j.scitotenv.2020.139025. [DOI] [PubMed] [Google Scholar]
- Yan M, Nie H, Xu K, He Y, Hu Y, Huang Y. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere. 2019;217:879–886. doi: 10.1016/j.chemosphere.2018.11.093. [DOI] [PubMed] [Google Scholar]
- Yin L, Jiang C, Wen X, Du C, Zhong W, Feng Z, Ma Y. Microplastic pollution in surface water of urban lakes in Changsha, China. Int J Environ Res Public Health. 2019;16(9):1650. doi: 10.3390/ijerph16091650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wen X, Du C, Jiang J, Wu L, Long ZY. Comparison of the abundance of microplastics between rural and urban areas: a case study from East Dongting Lake. Chemosphere. 2020;244:125486. doi: 10.1016/j.chemosphere.2019.125486. [DOI] [PubMed] [Google Scholar]
- Yonkos LT, Friedel EA, Perez-Reyes AC, Ghosal S, Arthur CD. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environ Sci Technol. 2014;48(24):14195–14202. doi: 10.1021/es5036317. [DOI] [PubMed] [Google Scholar]
- Yu X, Peng J, Wang J, Wang K, Bao S. Occurrence of microplastics in the beach sand of the Chinese inner sea: the Bohai Sea. Environ Pollut. 2016;214:722–730. doi: 10.1016/j.envpol.2016.04.080. [DOI] [PubMed] [Google Scholar]
- Yuan W, Liu X, Wang W, Di M, Wang J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol Environ Saf. 2019;170:180–187. doi: 10.1016/j.ecoenv.2018.11.126. [DOI] [PubMed] [Google Scholar]
- Zbyszewski M, Corcoran PL. Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron. Can Water Air Soil Pollut. 2011;220(1–4):365–372. doi: 10.1007/s11270-011-0760-6. [DOI] [Google Scholar]
- Zbyszewski M, Corcoran PL, Hockin A. Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes North America. J Great Lakes Res. 2014;40(2):288–299. doi: 10.1016/j.jglr.2014.02.012. [DOI] [Google Scholar]
- Zhang K, Chen X, Xiong X, Ruan Y, Zhou H, Wu C, Lam PK. The hydro-fluctuation belt of the three Gorges reservoir: source or sink of microplastics in the water? Environ Pollu. 2019;248:279–285. doi: 10.1016/j.envpol.2019.02.043. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gong W, Lv J, Xiong X, Wu C. Accumulation of floating microplastics behind the three Gorges Dam. Environ Pollut. 2015;204:117–123. doi: 10.1016/j.envpol.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Zhang K, Shi H, Peng J, Wang Y, Xiong X, Wu C, Lam PK. Microplastic pollution in China's inland water systems: A review of findings, methods, characteristics, effects, and management. Sci Total Environ. 2018;630:1641–1653. doi: 10.1016/j.scitotenv.2018.02.300. [DOI] [PubMed] [Google Scholar]
- Zhang K, Su J, Xiong X, Wu X, Wu C, Liu J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ Pollut. 2016;219:450–455. doi: 10.1016/j.envpol.2016.05.048. [DOI] [PubMed] [Google Scholar]
- Zhang K, Xiong X, Hu H, Wu C, Bi Y, Wu Y, Liu J. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir. China Environ Sci Technol. 2017;51(7):3794–3801. doi: 10.1021/acs.est.7b00369. [DOI] [PubMed] [Google Scholar]
- Zhang X, Leng Y, Liu X, Huang K, Wang J. Microplastics’ pollution and risk assessment in an urban river: a case study in the Yongjiang River, Nanning City. South China Exposure Health. 2020;12(2):141–151. doi: 10.1007/s12403-018-00296-3. [DOI] [Google Scholar]
- Zhao S, Wang T, Zhu L, Xu P, Wang X, Gao L, Li D. Analysis of suspended microplastics in the Changjiang Estuary: Implications for riverine plastic load to the ocean. Water Res. 2019;161:560–569. doi: 10.1016/j.watres.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu L, Li D. Microplastic in three urban estuaries, China. Environ Pollut. 2015;206:597–604. doi: 10.1016/j.envpol.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu L, Wang T, Li D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Mar Pollut Bull. 2014;86(1–2):562–568. doi: 10.1016/j.marpolbul.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Zhou G, Wang Q, Zhang J, Li Q, Wang Y, Wang M, Huang X. Distribution and characteristics of microplastics in urban waters of seven cities in the Tuojiang River basin. China Environ Res. 2020;189:109893. doi: 10.1016/j.envres.2020.109893. [DOI] [PubMed] [Google Scholar]