Abstract
Background
Bacterial folliculitis and boils are globally prevalent bacterial infections involving inflammation of the hair follicle and the perifollicular tissue. Some folliculitis may resolve spontaneously, but others may progress to boils without treatment. Boils, also known as furuncles, involve adjacent tissue and may progress to cellulitis or lymphadenitis. A systematic review of the best evidence on the available treatments was needed.
Objectives
To assess the effects of interventions (such as topical antibiotics, topical antiseptic agents, systemic antibiotics, phototherapy, and incision and drainage) for people with bacterial folliculitis and boils.
Search methods
We searched the following databases up to June 2020: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, and Embase. We also searched five trials registers up to June 2020. We checked the reference lists of included studies and relevant reviews for further relevant trials.
Selection criteria
We included randomised controlled trials (RCTs) that assessed systemic antibiotics; topical antibiotics; topical antiseptics, such as topical benzoyl peroxide; phototherapy; and surgical interventions in participants with bacterial folliculitis or boils. Eligible comparators were active intervention, placebo, or no treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcomes were 'clinical cure' and 'severe adverse events leading to withdrawal of treatment'; secondary outcomes were 'quality of life', 'recurrence of folliculitis or boil following completion of treatment', and 'minor adverse events not leading to withdrawal of treatment'. We used GRADE to assess the certainty of the evidence.
Main results
We included 18 RCTs (1300 participants). The studies included more males (332) than females (221), although not all studies reported these data. Seventeen trials were conducted in hospitals, and one was conducted in clinics. The participants included both children and adults (0 to 99 years). The studies did not describe severity in detail; of the 232 participants with folliculitis, 36% were chronic. At least 61% of participants had furuncles or boils, of which at least 47% were incised. Duration of oral and topical treatments ranged from 3 days to 6 weeks, with duration of follow‐up ranging from 3 days to 6 months. The study sites included Asia, Europe, and America. Only three trials reported funding, with two funded by industry.
Ten studies were at high risk of 'performance bias', five at high risk of 'reporting bias', and three at high risk of 'detection bias'.
We did not identify any RCTs comparing topical antibiotics against topical antiseptics, topical antibiotics against systemic antibiotics, or phototherapy against sham light. Eleven trials compared different oral antibiotics.
We are uncertain as to whether cefadroxil compared to flucloxacillin (17/21 versus 18/20, risk ratio (RR) 0.90, 95% confidence interval (CI) 0.70 to 1.16; 41 participants; 1 study; 10 days of treatment) or azithromycin compared to cefaclor (8/15 versus 10/16, RR 1.01, 95% CI 0.72 to 1.40; 31 participants; 2 studies; 7 days of treatment) differed in clinical cure (both very low‐certainty evidence). There may be little to no difference in clinical cure rate between cefdinir and cefalexin after 17 to 24 days (25/32 versus 32/42, RR 1.00, 95% CI 0.73 to 1.38; 74 participants; 1 study; low‐certainty evidence), and there probably is little to no difference in clinical cure rate between cefditoren pivoxil and cefaclor after 7 days (24/46 versus 21/47, RR 1.17, 95% CI 0.77 to 1.78; 93 participants; 1 study; moderate‐certainty evidence).
For risk of severe adverse events leading to treatment withdrawal, there may be little to no difference between cefdinir versus cefalexin after 17 to 24 days (1/191 versus 1/200, RR 1.05, 95% CI 0.07 to 16.62; 391 participants; 1 study; low‐certainty evidence). There may be an increased risk with cefadroxil compared with flucloxacillin after 10 days (6/327 versus 2/324, RR 2.97, 95% CI 0.60 to 14.62; 651 participants; 1 study; low‐certainty evidence) and cefditoren pivoxil compared with cefaclor after 7 days (2/77 versus 0/73, RR 4.74, 95% CI 0.23 to 97.17; 150 participants; 1 study; low‐certainty evidence). However, for these three comparisons the 95% CI is very wide and includes the possibility of both increased and reduced risk of events. We are uncertain whether azithromycin affects the risk of severe adverse events leading to withdrawal of treatment compared to cefaclor (274 participants; 2 studies; very low‐certainty evidence) as no events occurred in either group after seven days.
For risk of minor adverse events, there is probably little to no difference between the following comparisons: cefadroxil versus flucloxacillin after 10 days (91/327 versus 116/324, RR 0.78, 95% CI 0.62 to 0.98; 651 participants; 1 study; moderate‐certainty evidence) or cefditoren pivoxil versus cefaclor after 7 days (8/77 versus 5/73, RR 1.52, 95% CI 0.52 to 4.42; 150 participants; 1 study; moderate‐certainty evidence). We are uncertain of the effect of azithromycin versus cefaclor after seven days due to very low‐certainty evidence (7/148 versus 4/126, RR 1.26, 95% CI 0.38 to 4.17; 274 participants; 2 studies). The study comparing cefdinir versus cefalexin did not report data for total minor adverse events, but both groups experienced diarrhoea, nausea, and vaginal mycosis during 17 to 24 days of treatment. Additional adverse events reported in the other included studies were vomiting, rashes, and gastrointestinal symptoms such as stomach ache, with some events leading to study withdrawal.
Three included studies assessed recurrence following completion of treatment, none of which evaluated our key comparisons, and no studies assessed quality of life.
Authors' conclusions
We found no RCTs regarding the efficacy and safety of topical antibiotics versus antiseptics, topical versus systemic antibiotics, or phototherapy versus sham light for treating bacterial folliculitis or boils. Comparative trials have not identified important differences in efficacy or safety outcomes between different oral antibiotics for treating bacterial folliculitis or boils.
Most of the included studies assessed participants with skin and soft tissue infection which included many disease types, whilst others focused specifically on folliculitis or boils. Antibiotic sensitivity data for causative organisms were often not reported. Future trials should incorporate culture and sensitivity information and consider comparing topical antibiotic with antiseptic, and topical versus systemic antibiotics or phototherapy.
Plain language summary
What are the benefits and risks of different treatments for bacterial folliculitis and boils (inflammation of the skin around hairs)?
Why is this question important?
Bacterial folliculitis is an inflammation of the tiny pockets in our skin from which hairs grow (hair follicles). It occurs when bacteria (tiny organisms not visible with the naked eye) infect hair follicles. Bacterial folliculitis typically causes red swelling, with or without a small blister that contains pus.
Without treatment, bacterial folliculitis may progress to hard and painful lumps filled with pus, known as boils. These cover several hair follicles, and affect the skin around them.
Bacterial folliculitis and boils affect people worldwide, and have an important negative impact on quality of life. Infections typically:
‐ cause unsightly infections on parts of the body visible to others (such as the face and neck); or
‐ develop where skin rubs, causing discomfort and pain (such as armpits and buttocks).
A range of treatment options for bacterial folliculitis and boils is available. These include:
‐ antibiotics (medicines that fight bacterial infections). These can be applied to part of the body (locally) in the form of creams (topical antibiotics); or they can be taken by mouth (orally) or given as injections, to treat the whole body (systemic antibiotics);
‐ antiseptics (chemicals applied to the skin to fight infections caused by micro‐organisms, such as bacteria);
‐ light therapy; and
‐ surgery, for example, doctors may make a small cut (incision) in the skin to allow pus to drain out.
To find out which treatments work best for bacterial folliculitis and boils, we reviewed the evidence from research studies.
How did we identify and evaluate the evidence?
First, we searched for randomised controlled studies, in which people were randomly put into one of two or more treatment groups. This makes it less likely that any differences between treatments were actually due to differences in the people who received them (rather than the treatments themselves, which is what we wanted to find out).
We then compared the results, and summarised the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found 18 studies that involved a total of 1300 people. People were followed‐up for between one week and three months. Studies were set in Asia, Europe and America. Only three studies reported information about funding: non‐profit organisations funded one study, and pharmaceutical companies funded two studies.
The studies compared:
‐ different oral antibiotics (11 studies);
‐ different topical antibiotics (2 studies);
‐ different treatments for wound care after boil incision (2 studies);
‐ different traditional Chinese medicines (1 study);
‐ co‐trimoxazole (antibiotics) with, and without, 8‐methoxypsoralen (a light‐sensitising treatment) followed by exposure to sunlight (1 study); and
‐ penicillin (an antibiotic) with, and without, fire cupping (a form of traditional Chinese medicine) after surgery (1 study).
We found no studies that evaluated antiseptics or investigated quality of life or recurrence of bacterial folliculitis or boils.
Here we report the findings from four comparisons of different oral antibiotics.
Cure
The evidence from studies that investigated how successfully different oral antibiotics cured bacterial folliculitis and boils suggests that:
‐ there is probably little to no difference between cefditoren pivoxil and cefaclor (1 study, 93 people);
‐ there may be little to no difference between cefdinir and cephalexin (1 study, 74 people).
The few studies available did not provide sufficiently robust information to determine if:
‐ cefadroxil is better or worse than flucloxacillin (1 study, 41 people); or
‐ azithromycin is better or worse than cefaclor (2 studies, 31 people).
Severe adverse events (such as fever or vomiting)
The evidence from studies that compared frequencies of severe adverse events suggests there may be little to no difference between:
‐ cefadroxil and flucloxacillin (1 study, 651 people);
‐ cefdinir and cephalexin (1 study, 391 people); and
‐ cefditoren pivoxil and cefaclor (1 study, 150 people).
We do not know if azithromycin is associated with more, or fewer, severe adverse events than cefaclor. This is because studies provided insufficiently robust information (2 studies, 274 people).
Minor adverse events (such as feeling thirsty or dizzy)
The evidence from studies that compared frequencies of minor adverse events suggests there is probably little to no difference between:
‐ cefadroxil and flucloxacillin (1 study, 651 people); and
‐ cefditoren pivoxil and cefaclor (1 study, 150 people).
We do not know whether there are more, or fewer, minor adverse events associated with:
‐ cefdinir or cephalexin (1 study, 391 people); or
‐ azithromycin or cefaclor (2 studies, 274 people).
This is because studies reported insufficiently robust information.
What does this mean?
The limited evidence available does not suggest that any one oral antibiotic is better than another for treating bacterial folliculitis and boils.
The comparative benefits and risks of other treatments such as antiseptics or light therapy are unclear, because too few studies have investigated this.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to June 2020.
Summary of findings
Background
Description of the condition
See Table 8 for explanations of specific terms used in this review.
1. Glossary.
| Clinical term | Explanation |
| Anterior nares | External portion of the nostrils, which opens anteriorly into the nasal cavity and allows air inhalation and exhalation |
| Antipseudomonal | Agents used as drugs to destroy bacteria of the genus Pseudomonas |
| Axilla (pl. axillae) | Also known as the armpit, underarm, or oxter; the area directly under the joint where the human arm connects to the shoulder |
| Cellulitis | Term commonly used to indicate non‐necrotising inflammation of the skin and subcutaneous tissues, a process usually related to acute infection that does not involve the fascia or muscles |
| Endogenous chromophobes | A chemical group (such as an azo group) that absorbs light at a specific frequency and so imparts colour to a molecule that originates from within an organism, tissue, or cell |
| Epidermis | One or more layers of cells forming the outermost portion of the skin or integument |
| Fluctuant | Being movable or compressible; often used to describe a tumour or abscess |
| Gram‐negative bacteria | Bacteria that contain an additional outer membrane composed of phospholipids and lipopolysaccharides that do not retain the crystal violet dye in the Gram stain protocol |
| Immunomodulatory | Substance that affects the functioning of the immune system |
| Keratolytic | Causing the horny outer layer of skin to soften and shed |
| Lymphadenitis | Associated with the lymph nodes, which are responsible for fighting off infections of the body; refers to the condition by which lymph nodes become inflamed, swell, and become tender during an infection |
| Monochromatic | Existing in only one colour or particular wavelength |
| Perifollicular tissue | Tissue surrounding a hair follicle; usually used to describe the histopathological appearance of the infiltrate surrounding a hair follicle |
| Pathogen | Any small organism, such as a virus or a bacterium, that can cause disease |
| Pseudomonal | Of or related to the Pseudomonas species, which is a ubiquitous strictly aerobic gram‐negative bacterium with a predilection to moist environments and is a clinically significant opportunistic pathogen, often causing nosocomial infections |
| Purulent | Full of pus or like pus |
| Superficial dermis | Middle layer of skin, deep to the epidermis and superficial to the subcutaneous layer |
| Dieda Xiaoyan Gao | A traditional Chinese medicine ointment with anti‐inflammatory effects |
| STSG | Split‐thickness skin graft, refers to a graft that contains the epidermis and a portion of the dermis |
| ASAT | Aspartate amino transferase, a blood test that checks for liver damage |
| ASLT | Alanine amino transferase, a blood test that checks for liver damage |
| SSTI | Skin and soft tissue infections, bacterial infections of the skin, muscles, and connective tissue such as ligaments and tendons |
| USSSI | Uncomplicated skin and skin structure infections, simple abscesses, impetiginous lesions, furuncles, and cellulitis |
Folliculitis is inflammation of the hair follicle caused by infection, chemical stimulation, or physical injury (Pasternack 2015). The aetiology of folliculitis is diverse, including occlusion folliculitis resulting from blockages caused by exposure to topical products that block the opening of the hair follicle, leading to inflammation, and Malassezia folliculitis, which is caused by Malassezia furfur (also known as Pityrosporum ovale) and presents as itching red papules over the chest, shoulders, or back (Gunatheesan 2018). In this review we were interested in bacterial folliculitis, a bacterial infection within the hair follicle that typically presents as a red swelling with or without a pustule over the follicular opening (Craft 2012). Without treatment, bacterial folliculitis may resolve in 7 to 10 days or may progress to boils.
A boil, also known as a furuncle, is a bacterial infection involving the perifollicular tissue that usually originates from pre‐existing folliculitis (Lopez 2006). A boil appears as a painful red swelling around the follicular opening and may progress to form an abscess (Craft 2012). Some boils may be treated with moist heat application; others with surrounding cellulitis or fever may require treatment with systemic antibiotics (Pasternack 2015). Systemic antibiotics should be continued until the lesion resolves (Pasternack 2015). Carbuncles are large painful swellings with multiple pus‐discharging openings and constitutional symptoms including fever and malaise (Craft 2012). They affect the deeper layers of soft tissue and can lead to scarring. Without control, boils may occasionally be complicated by severe skin infections such as cellulitis or lymphadenitis combined with constitutional symptoms such as fever, fatigue, and chills.
Bacterial folliculitis and boils are prone to occur in areas of the skin subject to rubbing, occlusion, and sweating, such as the neck, face, axillae, and buttocks (Craft 2012). Clinicians usually diagnose bacterial folliculitis and boils based on physical examination findings (Craft 2012).
Bacterial folliculitis and boils are bacterial infections with a worldwide prevalence, but their exact prevalence and incidence are unclear. One study reported a prevalence of around 1.3% in schoolchildren (Al‐Saeed 2006). Another study found that 27% of immunosuppressed organ transplant recipients presented with persistent folliculitis (Lally 2011). In 2010, at least 280,000 boil episodes were reported, and hospital admissions for abscesses, carbuncles, boils, and cellulitis almost doubled in the UK ‐ from 123 admissions per 100,000 in 1998/1999 to 236 admissions per 100,000 in 2010/2011 (Shallcross 2015). This rise might have occurred because staphylococcal strains have become more severe or difficult to treat and may cause recurrent infection, as seen with the increased virulence of community‐onset methicillin‐resistant Staphylococcus aureus (MRSA) produced by toxins such as Panton‐Valentine leukocidin (PVL) (Dufour 2002).
S aureus is the most common pathogen of folliculitis and boils. However, gram‐negative pathogens including Klebsiella, Enterobacter, and Proteus species may replace the gram‐positive flora on facial skin, nasal mucous membranes, and neighbouring areas, causing gram‐negative folliculitis and boils (Böni 2003). 'Hot tub' folliculitis is caused by Pseudomonas aeruginosa contamination of undertreated water in saunas or whirlpools (Zacherle 1982).
Certain people are affected by recurrent furunculosis (i.e. boils that have a propensity to recur and may spread amongst family members) (Ibler 2014). Recurrent boils are a bothersome disorder that may affect patients' quality of life (Ibler 2014). Colonisation of S aureus in the anterior nares plays an important role in the origin of chronic or recurrent furunculosis (Ibler 2014).
Description of the intervention
Various interventions have been suggested for treating folliculitis (Craft 2012; O'Dell 1998), including local application of moist heat, phototherapy, antiseptic agents, antibiotics alone, or combination therapy. Treatment of fluctuating boils often requires drainage of the lesion, and for severe infections systemic antibiotics should be given until signs of inflammation have regressed.
Local moist heat around 38 °C to 40 °C applied for 15 to 20 minutes may increase local blood flow, may establish drainage, and has proved helpful in the treatment of newly emerged folliculitis or boils (Pasternack 2015). No adverse effects of local moist heat are known (Petrofsky 2009).
Topical antibiotics may be used in treating folliculitis and boils when the number of lesions is limited, or they may be used in combination with other interventions, for example incision and drainage (Laureano 2014). Available preparations include fusidic acid 2% cream twice daily (Frosini 2017; Koning 2002), clindamycin 2% gel twice daily, and mupirocin 2% ointment applied two to three times daily (Micromedex 2018). These drugs are topically applied over the lesion. Topical antibiotics may cause contact dermatitis, dryness, or pruritus over the applied area. However, these adverse events are usually minor (Tran 2017). No major drug‐drug interactions between these topical antibiotics and other medications are known (Micromedex 2018).
Topical antiseptic agents may be manufactured as gel (such as benzoyl peroxide 2% to 10% twice daily), cream, soap, or solution (e.g. hypochlorite 3% to 5% solution) (Micromedex 2018). These antiseptics may be used alone or in combination with antibiotics for treating folliculitis and boils, especially in recurrent furunculosis (Davido 2013). The adverse events of benzoyl peroxide are usually mild and mainly include skin irritation over the application site (Kawashima 2017). No drug interactions of topical antiseptics are known (Micromedex 2018).
Some Chinese herbal compounds may be used in folliculitis and boils treatment, for example Dieda Xiaoyan Gao ointment containing baizaoxiu, danshen, huangyaopian, zhizi, dahuang, baizhi, shengbanxia, shengnanxing, narukawa, caowu, and camphor, have been given to boils patients (Xu 1992).
Systemic antibiotics may be used for treating folliculitis and boils, especially when systemic symptoms such as fever, lymphadenitis, or cellulitis appear (Pereira 1996). Regimens and common drug‐drug interactions of systemic antibiotics are listed in Table 9. First‐line oral antibiotics including dicloxacillin (250 mg four times daily) and cephalosporins (such as cefadroxil 500 mg twice daily) are commonly used. For antibiotic‐resistant S aureus that has emerged in the community, clindamycin, tetracyclines, trimethoprim‐sulfamethoxazole, linezolid, or glycopeptide (e.g. parenteral vancomycin) may be used (Laureano 2014; Nagaraju 2004). Oral or parenteral ciprofloxacin 400 to 500 mg twice daily with antipseudomonal activity may be administered for gram‐negative folliculitis such as 'hot tub' folliculitis (Craft 2012). Potential adverse events of systemic antibiotics include allergic reactions, neurological or psychiatric disturbances, and diarrhoea (Shehab 2008). Systemic antibiotics may be used in combination with topical antiseptics for treating folliculitis and boils (Pasternack 2015). For some cases of folliculitis, especially those caused by S aureus, a course of oral antibiotics may be administered over 7 to 10 days (Laureano 2014).
2. Regimens and drug‐drug interactions of systemic antibiotics.
| Drug | Dose/regimen | Drug‐drug interaction (Gilbert 2018;Micromedex 2018) |
| Cefadroxil |
|
|
| Ciprofloxacin |
|
|
| Clindamycin |
|
|
| Tetracyclines |
|
|
| Trimethoprim‐sulfamethoxazole |
|
|
| Linezolid |
|
|
| Glycopeptide (as vancomycin) | Adult: 30 mg/kg/d IV in 2 divided doses or 40 mg/kg/d IV in 4 divided doses |
|
Al: aluminium; Ca: calcium; CNS: central nervous system; Fe: iron; IM: intramuscular; IV: intravenous; Mg: magnesium; NSAIDs: non‐steroidal anti‐inflammatory drugs; Zn: zinc.
Surgical interventions, such as incision and drainage, are likely to be adequate for simple fluctuant folliculitis or boils (Ibler 2014). Incision may cause scarring at the incised site (Ahmad 2017). Combined topical or systemic antibiotics is often employed, especially when there is a lack of response to incision and drainage alone, or when the lesion is in an area where complete drainage is difficult (e.g. face, hands, genitalia) (Ibler 2014).
Phototherapy by monochromatic excimer light (308 nm) with 0.5 to 2 minimal erythema dose (MED) has been used as treatment for superficial folliculitis. Nisticò 2009 reported only mild adverse events such as local erythema.
How the intervention might work
As mentioned above, bacterial folliculitis and boils occur as inflammation of the follicle and perifollicular tissue caused by bacterial infection. Antibacterial, antiseptic, and anti‐inflammatory interventions may therefore be used for treatment.
Topical antibiotics such as clindamycin, aminoglycosides, and fusidic acid directly kill or inhibit pathogenic bacteria within the follicle, avoiding further tissue damage by these pathogens (Frosini 2017).
The therapeutic effects of antiseptic agents are attributed to the killing of bacteria that cause folliculitis and boils, such as S aureus (Fisher 2008). Benzoyl peroxide is an antiseptic that confers not only antibacterial effects but also keratolytic effects, which cause the skin to dry and peel (Kawashima 2017).
Systemic antibiotics can directly inhibit or kill the pathogenic bacteria causing folliculitis and boils. When bacterial cultures are available, systemic antibiotics may be administered according to the pathogen identified (Ibler 2014).
Some medications such as Dieda Xiaoyan Gao ointment have anti‐inflammatory effects and may be helpful in the treatment of folliculitis or boils. Pentoxifylline, a methylxanthine derivative with diverse pharmacological properties, may have a synergic effect in anti‐inflammation by inhibiting tumour necrosis factor alpha (TNF‐α) when combined with ciprofloxacin (Wahba‐Yahav 1992).
Ultraviolet‐B radiation, which primarily affects the epidermis and the superficial dermis, is absorbed by endogenous chromophobes, such as nuclear DNA, which initiates a cascade of immunomodulatory effects (Bulat 2011). Phototherapy has been proposed as a treatment option for folliculitis for its anti‐inflammatory effects (Nisticò 2009).
Given that pus, or even an abscess, may be present with fluctuant folliculitis and boils, incision and drainage may be used to remove toxic purulent material, decompress the tissues, and support better blood perfusion, which increases drug concentration in an affected area and improves local immune response and tissue repair (Ibler 2014).
Why it is important to do this review
Cochrane Skin undertook an extensive prioritisation exercise to identify a core portfolio of the most clinically important titles. Interventions for bacterial folliculitis and boils was identified as a clinically important priority by a panel of international editors. As aforementioned, folliculitis and boils are worldwide prevalent diseases that cause a great burden on the quality of life of individuals, with an estimated 1,944,776 DALYs (disability‐adjusted life years) worldwide in 2016 (range 1,249,848 to 2,603,083) (Global Burden of Disease).
To the best of our knowledge, no systematic reviews to date have examined interventions for folliculitis and boils. Our goal with this systematic review was to find and evaluate the best available evidence on the effects of interventions for folliculitis and boils.
Objectives
To assess the effects of interventions, such as topical antibiotics, topical antiseptic agents, systemic antibiotics, phototherapy, and incision and drainage, for people with bacterial folliculitis and boils.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including parallel, cluster, cross‐over, and split‐body within‐participant RCTs.
Types of participants
People with bacterial folliculitis or boils diagnosed by a healthcare professional or a trained researcher based on clinical presentation or bacterial culture. We excluded participants with non‐bacterial folliculitis, such as Pityrosporum folliculitis and mite folliculitis. We included RCTs conducted in any setting and placed no restrictions on demographic factors such as age and sex.
When a study included participants with various superficial bacterial infections of the skin, we included the study only if the authors reported separate data for those with bacterial folliculitis or boils. When the publication did not provide separate data, we contacted study authors and requested separate data for bacterial folliculitis and boils.
Types of interventions
Interventions included systemic antibiotics, topical antibiotics, topical antiseptics such as topical benzoyl peroxide, phototherapy, and surgical interventions (e.g. incision and drainage). Participants received a single intervention or a combination of interventions.
Comparators included another active intervention, placebo, or no treatment.
Types of outcome measures
We considered outcome data measured at ≤ 1 month and > 1 month as short‐ and long‐term outcomes, respectively. If a trial reported data at multiple time points within the short‐ or long‐term timeframe, we chose the longest time point.
Primary outcomes
Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment).
Severe adverse events leading to withdrawal of treatment.
Secondary outcomes
Quality of life: as measured by validated tools, including Dermatology Life Quality Index (DLQI), 36‐item Short Form Health Survey (SF‐36), Skindex 29, Skindex 17, or Dermatology Quality of Life Scale (DQOLS). We considered a DLQI score change of at least 5 as a minimally important difference (Khilji 2002).
Recurrence of folliculitis or boil following completion of treatment.
Minor adverse events not leading to withdrawal of treatment.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 11 June 2020 using strategies based on the draft strategy for MEDLINE in our published protocol (Lin 2018):
the Cochrane Skin Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2020, Issue 6, in the Cochrane Library, using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3; and
Embase via Ovid (from 1974) using the strategy in Appendix 4.
Trials registers
Two review authors (HL and YT) searched the following trials registers up to 18 June 2020 using the terms 'boil/s', 'furuncle/s', 'furunculosis', 'folliculitis', 'carbuncle', 'sycosis', and 'sycoses':
ISRCTN registry (www.isrctn.com);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/); and
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
Searching reference lists
We checked the bibliographies of included studies and related systematic reviews for further references to relevant trials.
Unpublished literature
We contacted the authors of reports of relevant RCTs published within the last three years to ask if they were aware of any relevant unpublished data.
Adverse effects
We did not perform a separate search for adverse effects of interventions used for the treatment of folliculitis and boils. We only considered adverse events described in the included RCTs.
Data collection and analysis
Some parts of this section use text that was originally published in another Cochrane protocol or in the Cochrane Handbook for Systematic Reviews of Interventions (Chi 2015; Higgins 2011).
Selection of studies
Two review authors (HL and PL) independently checked the titles and abstracts derived from the searches. Review authors were not blinded to the names of trialists or their institutions. If it was judged from the title and abstract that a study did not relate to an RCT on interventions for treating folliculitis and boils, it was excluded straight away. The same two review authors independently examined the full text of each remaining study and judged whether it met the inclusion criteria of the review. In case of disagreement between review authors on whether or not to include a study, unanimity was achieved through discussion with a third review author (CC). Studies excluded at full‐text review and the reasons for their exclusion are provided in the Characteristics of excluded studies tables. Covidence was used for selection of studies (Covidence).
Data extraction and management
Using a pilot‐tested data extraction form, two review authors (HL and PL) independently extracted the following data from the included RCTs: study methods, participants, interventions, outcomes, country, setting, and funding source (see Appendix 5). We used WebPlotDigitizer to extract data from figures and graphs (WebPlotDigitizer 2017). We used these extracted data to create the Characteristics of included studies tables. In case of disagreement regarding the extracted data, the two review authors consulted with a third review author (CC) to achieve unanimity. One review author (PL) entered the data into Review Manager 5 (Review Manager 2014), and another review author (HL) checked the entered data.
Assessment of risk of bias in included studies
We used Cochrane's tool for assessing risk of bias in RCTs, evaluating the following 'Risk of bias' domains (Higgins 2017).
Random sequence generation (selection bias): adequacy of the method of random sequence generation to produce comparable groups in every aspect except for the intervention.
Allocation concealment (selection bias): adequacy of the method used to conceal the allocation sequence to prevent anyone from foreseeing the allocation sequence in advance of, or during, enrolment.
Blinding of participants and personnel (performance bias): adequacy of blinding participants and investigators from knowledge of which intervention a participant received.
Blinding of outcome assessment (detection bias): adequacy of blinding outcome assessors from knowledge of which intervention a participant received.
Incomplete outcome data (attrition bias): completeness of outcome data for each main outcome, including attrition and exclusions from analysis, whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition or exclusions when reported, and any re‐inclusions in our analyses.
Selective reporting (reporting bias): when the trial protocol was available, we determined whether all prespecified outcomes were reported. When the study protocol was unavailable, we identified whether published reports included all expected outcomes, including those that were prespecified.
Other bias: any important concerns about bias not addressed in the other domains, e.g. design‐specific risk of bias and baseline imbalance.
Two review authors (HL and PL) independently assessed the risk of bias of included RCTs; a third review author (CC) was consulted in case of disagreement.
Measures of treatment effect
Dichotomous data
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). When the RR was statistically significant, we also presented the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs (Higgins 2011).
Continuous data
We expressed continuous data as mean differences (MDs) with 95% CIs. When different outcome scales were pooled, we would expressed continuous data as standardised mean differences (SMDs) with 95% CIs (Higgins 2011).
Time‐to‐event data
We planned to express time‐to‐event data as hazard ratios (HRs) with 95% CIs. We would extract HRs as presented in the included study report. When HRs were not reported, we would use the methods described in Tierney 2007 to estimate the HRs if sufficient data were provided.
Unit of analysis issues
We planned to separately analyse studies with the following designs using appropriate techniques as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); however, none of the included studies adopted these designs.
Cluster‐randomised trials
For cluster‐randomised trials that did not adjust for clusters in their analysis, we would employ the Rao methods described in Section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Rao 1992), and planned to estimate the intervention effect assuming an intracluster correlation coefficient (ICC) of 0.05.
Cross‐over trials
For cross‐over trials, we would only include data from the first period for analysis. When these data were not available, we would employ the statistical methods described in Section 16.4.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), undertaking paired analyses by imputing missing standard deviations.
Studies with multiple treatment groups
For studies with multiple intervention groups, we would make separate pairwise comparisons of one intervention versus another. For example, if an RCT included three interventions groups ‐ Group A (placebo or the most frequently used intervention), Group B, and Group C ‐ we would make separate pairwise comparisons of B versus A and C versus A.
Split‐body trials
For split‐body trials, we would conduct paired analyses using data from one side of the body versus the other side of the body. We would analyse continuous and dichotomous data by using the paired t‐test and McNemar's test, respectively.
Dealing with missing data
We contacted the authors of studies less than 10 years old to ask for missing data. Where data were unavailable, we conducted an intention‐to‐treat (ITT) analysis to recalculate the intervention effect estimates, included all randomised participants in the analysis, and assumed that those with missing dichotomous outcome data experienced treatment failure. If the ITT data were unavailable, we carefully evaluated other important numerical data for randomised participants as well as per‐protocol population (PP) and as‐treated (AT) and described this in the 'Risk of bias' assessment. For missing continuous outcome data, we planned to attempt to adopt the 'last observation carried forward' (LOCF) approach in analysis when the trials provided relevant original data, that is replacing a missing value with the participant's last observed value. We would furthermore conduct a sensitivity analysis by assuming that those participants with missing dichotomous outcome data experienced treatment success.
Assessment of heterogeneity
We calculated the I2 statistic to assess statistical heterogeneity across the included trials. The importance of the observed value of the I2 statistic depends on (1) the magnitude and direction of effects, and (2) the strength of evidence for heterogeneity (e.g. P value from Chi2 test, CI for I2 statistic) (Higgins 2011). We considered an I2 of ≥ 50% as representing at least moderate heterogeneity, and planned to follow the guidance in the Cochrane Handbook for Systematic Reviews of Interventions by exploring subgroups to explain the heterogeneity.
We also assessed statistical heterogeneity via forest plot inspection, as in some analyses a high I2 might not be a serious issue, especially if the estimates were all on the same side of the forest plot. We would examine whether statistical heterogeneity suggested a dose‐response relationship or the presence of minimum therapeutic dose by conducting a subgroup analysis based on different dosages of the intervention.
Assessment of reporting biases
We planned that when at least 10 trials were included in a meta‐analysis on primary outcomes for an intervention, we would use a funnel plot to assess publication bias (Higgins 2011).
Data synthesis
We provided a narrative description of all outcomes when data were available. For trials that were sufficiently similar in terms of participants, interventions, and outcomes, we performed a random‐effects model meta‐analysis to obtain a pooled intervention effect. When a meta‐analysis was not feasible, we summarised the data narratively instead.
When results were estimated for individual studies with low numbers of outcomes (fewer than 10 in total), or when the total sample size was less than 30 participants and an RR was used, we would report the proportion of outcomes in each group together with a P value based on Fisher's exact test.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses when relevant data were available.
Paediatric versus adult participants (further divided into bacterial culture‐proven or clinical diagnosis only).
Immunocompetent versus immunosuppressed participants (further divided into bacterial culture‐proven or clinical diagnosis).
Methicillin‐sensitive S aureus (MSSA) versus MRSA (including PVL gene type).
Different dosages of an intervention.
To test for subgroup differences, we would employ random‐effects model meta‐analysis and use the methods developed by Borenstein 2008, which have been implemented in Review Manager 5 software (Review Manager 2014).
Sensitivity analysis
We would conduct a sensitivity analysis to examine intervention effects after excluding trials with high risk of bias for one or more domains for a given outcome. We would also conduct a sensitivity analysis assuming that those with missing dichotomous outcome data experienced treatment success.
Summary of findings and assessment of the certainty of the evidence
We have presented 'Summary of findings' tables in order to summarise data on our primary outcomes (clinical cure and severe adverse events leading to withdrawal of treatment) and secondary outcomes (quality of life, recurrence, and minor adverse events not leading to withdrawal of treatment) for the most important comparisons: topical antibiotics versus topical antiseptics, topical antibiotics versus systemic antibiotics, and phototherapy versus sham light (see Types of outcome measures). When several major comparisons were reported, or when outcomes needed to be summarised for different populations, we produced additional 'Summary of findings' tables.
Two review authors (HL and PL) assessed the quality of the body of evidence using the five GRADE considerations: study limitations, consistency of effect, imprecision, indirectness, and publication bias (Schünemann 2013). We downgraded the certainty of the evidence from high to moderate, low, or very low based on these five considerations. Any disagreements were resolved by discussion with a third review author (CC). We used GRADEpro GDT, GRADEpro GDT, to prepare the 'Summary of findings' tables and to assess the certainty of the evidence (Atkins 2004; Schunemann 2011).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
The searches undertaken by the Cochrane Skin Information Specialist of the four databases retrieved 936 records (see Electronic searches). Our searches of the trials registers identified 650 further records. Our screening of the reference lists of the included studies and related systematic reviews did not reveal any additional RCTs. This resulted in a total of 1586 records. After removal of duplicates, we had 1510 records.
We excluded 1442 records based on scanning of titles and abstracts and obtained the full texts of the remaining 68 records. We excluded 31 studies reported in 29 papers (Narayanan 2014a includes three trials) (see Characteristics of excluded studies). We assessed 16 studies as awaiting classification and five studies as ongoing (see Characteristics of studies awaiting classification and Characteristics of ongoing studies).
We included 18 studies in the review (see Characteristics of included studies). For a further description of our screening process, see the study flow diagram (Figure 1).
1.
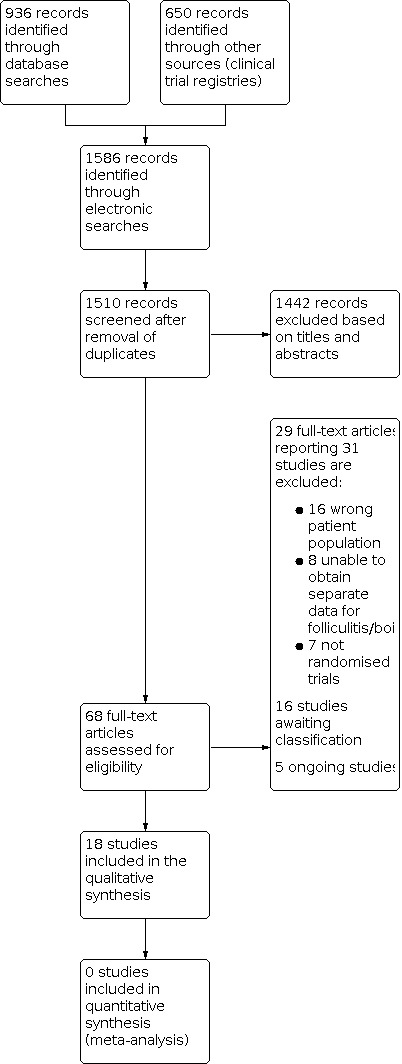
Study flow diagram.
Included studies
We included 18 trials with a total of 1300 participants, covering 30 treatments. Details of the included studies are described in the Characteristics of included studies tables.
Design
All 18 included studies were two‐arm parallel RCTs assessing the effects of interventions for bacterial folliculitis and boils.
Sample size
The number of participants in the included studies ranged from 7 to 260. Three included trials had a small sample size of less than 30 participants (Arata 1995a; Montero 1996; Tassler 1993).
Setting
Seventeen trials were conducted at hospitals, whilst the remaining trial was conducted in clinics (Baig 1988). Twelve trials were multicentre (Arata 1988; Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997; Baig 1988; Beitner 1996; Giordano 2006; Jin 1995; Montero 1996; Tassler 1993), and six trials were conducted at single centres (Iyer 2013; Kessler 2012; Parsad 1997; Shenoy 1990; Xu 1992; Xu 1999). The included trials were conducted in a total of 18 countries (Japan, Sweden, the UK, China, Colombia, Guatemala, Panama, South Africa, India, Germany, Argentina, Austria, Brazil, Belgium, Finland, France, Italy, and the USA).
Participants
The included studies involved at least 232 folliculitis patients (including 83 with chronic folliculitis) and at least 795 participants with furuncles or boils (at least 376 of them received incision). However, most studies did not report the duration of disease, and only one trial mentioned that the duration was more than four weeks (Parsad 1997).
In many of the included trials, participants with folliculitis and boils were only a subgroup without detailed age information, and we could not calculate the interquartile range (IQR) in these participants. The studies that provided the sex of their participants enrolled a total of 332 males and 221 females, with an age range from 0 to 99 years old.
Two trials did not report the age of participants (Shenoy 1990; Xu 1992). One trial enrolled only children (between 6 months to 12 years) (Montero 1996). Three trials included adults aged 18 years or older (Iyer 2013; Parsad 1997; Tassler 1993), and five trials included participants aged 16 years or older (Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997). Two trials included participants aged at least 10 years, Baig 1988, or 13 years old (Giordano 2006). Five trials included both paediatric and adult participants: aged 0 to over 70 years (Arata 1988), 1 to 25 years (Kessler 2012), 3 to 81 years (Beitner 1996), 3 to 65 years (Xu 1999), and 6 to 65 years (Jin 1995).
Interventions
The included studies assessed six topical treatments, 16 oral treatments, and eight other treatments, as either interventions or comparators.
Topical treatments
Ofloxacin (Jin 1995)
Norfloxacin (Jin 1995)
Sisomicin (Arata 1988)
Gentamicin (Arata 1988)
Dieda Xiaoyan Gao ointment (Xu 1992)
Ichthammol ointment (Xu 1992)
Oral treatments
Cefaclor (Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Montero 1996)
Flucloxacillin (Baig 1988; Beitner 1996)
Cefadroxil (Beitner 1996)
Cefdinir (Giordano 2006)
Cefalexin (Giordano 2006)
Cefditoren pivoxil (Arata 1993)
Fleroxacin (Tassler 1993)
Amoxicillin/clavulanate (Tassler 1993)
Erythromycin (Baig 1988)
Azithromycin (Arata 1995a; Montero 1996)
Grepafloxacin (Arata 1997)
Ofloxacin (Arata 1997)
Ciprofloxacin (Parsad 1997)
Pentoxifylline plus ciprofloxacin (Parsad 1997)
S‐1108 (Arata 1994a)
SY 5555 (Arata 1994b)
Other treatments
Co‐trimoxazole plus 8‐methoxypsoralen and sunlight (Shenoy 1990)
Co‐trimoxazole plus placebo and sunlight (Shenoy 1990)
Fire cupping plus penicillin intramuscular injection (Xu 1999)
Incision for pus plus penicillin intramuscular injection (Xu 1999)
Wound packing following incision and drainage (Kessler 2012)
Incision and drainage without wound packing (Kessler 2012)
Excision of carbuncle with primary split thickness skin grafting (STSG) (Iyer 2013)
Excision of carbuncle with delayed STSG (Iyer 2013)
The 14 trials that compared oral or topical treatments reported a treatment duration of between three days and six weeks (Arata 1988; Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997; Baig 1988; Beitner 1996; Giordano 2006; Jin 1995; Montero 1996; Parsad 1997; Tassler 1993; Xu 1992). Iyer 2013 measured outcomes seven days postoperatively; similarly, Xu 1999 measured clinical cure seven days after the cupping procedure. Kessler 2012 assessed failure 48 hours after the procedures, and healing at 1 week and 1 month afterwards. Shenoy 1990 measured outcomes 15, 45, and 90 days after the procedure. To monitor the relapse of the lesions, the Parsad 1997 trial followed up the participants for 6 months.
There were five trials with co‐interventions, including excision with primary or delayed STSG (Iyer 2013); incision and drainage with or without wound packing (Kessler 2012); oral ciprofloxacin with or without oral pentoxifylline (Parsad 1997); oral co‐trimoxazole with or without oral 8‐methoxypsoralen followed by sunlight exposure (Shenoy 1990); and penicillin intramuscular injection combined with lesion incision with or without fire cupping (Xu 1999).
Comparators
Most trials compared the efficacy between different medications for folliculitis or boils: three compared different topical drugs (Arata 1988; Jin 1995; Xu 1992), and 11 compared different oral drugs (Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997; Baig 1988; Beitner 1996; Giordano 2006; Montero 1996; Parsad 1997; Tassler 1993). Iyer 2013 assessed primary versus delayed STSG after boils incision and drainage. Kessler 2012 analysed the efficacy of wound packing after boils incision. Xu 1999 analysed the efficacy of fire cupping after boils incision and drainage. Shenoy 1990 assessed co‐trimoxazole (an antibiotic) with and without 8‐methoxypsoralen followed by exposure to sunlight.
Outcomes
Fifteen trials measured our primary outcome of clinical cure; 12 trials severe adverse events or safety; 13 studies minor adverse events or safety; and three trials recorded recurrence (Kessler 2012; Parsad 1997; Shenoy 1990). Although no trials assessed quality of life, one trial assessed wound healing and pain (Kessler 2012). With regard to safety, data were not always reported per diagnosis. The follow‐up duration in these trials ranged from three days to six months from start of treatment.
Funding sources
Of the 18 included trials, two were industry supported (Beitner 1996; Giordano 2006), and one was supported by nonprofit organisations (such as government or academic institutions) (Kessler 2012). The remaining 15 trials did not report funding sources.
Excluded studies
We excluded 31 articles because they did not report respective data for bacterial folliculitis and boils; were not a randomised trial; or were a prevention study. The reasons for exclusion are listed in Characteristics of excluded studies.
Studies awaiting classification
A total of 16 trials are awaiting classification. For five trials, only the study title was available, and we were only able to obtain the abstracts of the other 11 trials rather than full texts. Of the 11 trials, one trial included participants with chronic folliculitis (Balachandran 1995); one trial included participants with superficial pyoderma (Bernard 1997); six trials included participants with skin and soft tissue infections (Bilen 1998; Carr 1994; Chen 2011; Fujita 1982; Macedo De Souza 1995; Welsh 1987); and three trials included participants with folliculitis, furunculosis, and pyodermitis (cellulitis, erysipelas) (Lobo 1995; NCT01032499; Pereira 1996).
As for the interventions assessed, nine trials compared different oral antibiotics (Bernard 1997; Bilen 1998; Carr 1994; Chen 2011; Fujita 1982; Lobo 1995; Macedo De Souza 1995; NCT01032499; Pereira 1996); one trial compared oral antibiotics with placebo (Balachandran 1995); and one trial compared oral antibiotics with topical antibiotics (Welsh 1987).
Details of these studies are provided in Characteristics of studies awaiting classification.
Ongoing studies
Five clinical trials have not yet been completed, including two in Clinical Trials Registry‐India (CTRI/2015/01/005361; CTRI/2018/03/012411); two in the EU Clinical Trials Register (EUCTR 2008‐006151‐42; EUCTR 2016‐005105‐39); and one in ClinicalTrials.gov (NCT01281930). Three of these studies include participants with uncomplicated skin and soft tissue infections (CTRI/2015/01/005361; CTRI/2018/03/012411; EUCTR 2008‐006151‐42); one trial includes participants with folliculitis (EUCTR 2016‐005105‐39); and the remaining trial includes participants with boils (NCT01281930).
Two trials compare different oral antibiotics in adolescents and adults (CTRI/2015/01/005361; EUCTR 2008‐006151‐42). One trial compares different topical antibiotics (CTRI/2018/03/012411), and another compares antibiotics and antiseptic medications (EUCTR 2016‐005105‐39). One trial compares different wound packing after furunculosis incision and drainage in children (NCT01281930).
The protocols of the trials are listed in Characteristics of ongoing studies.
We attempted to contact the authors of the studies awaiting classification and ongoing studies if email addresses were provided (see Appendix 6).
Risk of bias in included studies
Our judgements about each 'Risk of bias' item presented as percentages across all of the included trials are shown in Figure 2, and we summarise our judgements about each 'Risk of bias' item for each included trial in Figure 3. Further details regarding risk of bias are provided in the 'Risk of bias' tables in the Characteristics of included studies section.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials used an adequate method of generation of the randomisation sequence (Giordano 2006; Kessler 2012). The remaining 16 trials did not describe the process of randomisation and were thus rated as unclear risk of bias.
Allocation was concealed in six trials (Arata 1988; Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997), whilst it was unclear if allocation was concealed in the other 12 trials.
Blinding
We rated eight trials as at low risk of performance bias because both the investigators and participants were blinded (Arata 1988; Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997; Parsad 1997; Shenoy 1990). We judged 10 RCTs as at high risk of performance bias because the participants were not blinded (Baig 1988; Beitner 1996; Giordano 2006; Iyer 2013; Jin 1995; Kessler 2012; Montero 1996; Tassler 1993; Xu 1992; Xu 1999).
In five trials, the blinded physicians assessed the outcomes (Arata 1993; Arata 1994a; Arata 1995a; Arata 1997; Shenoy 1990). Also, we judged the Giordano 2006 and Kessler 2012 trials as at low risk of detection bias because a third person was assigned to assess clinical response. We rated three open‐label trials as at high risk of detection bias because unblinded physicians assessed outcomes (Baig 1988; Montero 1996; Tassler 1993).
We considered the other eight trials as having an unclear risk of detection bias because it was not reported whether the outcome assessors were blinded (Arata 1988; Arata 1994b; Beitner 1996; Iyer 2013; Jin 1995; Parsad 1997; Xu 1992; Xu 1999).
Incomplete outcome data
The risk of attrition bias was low in six trials because of a low or null dropout rate (Baig 1988; Giordano 2006; Iyer 2013; Jin 1995; Kessler 2012; Xu 1992). The risk of attrition bias was high in one trial due to a high dropout rate (Shenoy 1990). We rated 10 trials as at unclear risk of attrition bias because ITT data were unavailable, and the outcome efficacy analysis was based on the PP data (Arata 1988; Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997; Beitner 1996; Montero 1996; Parsad 1997; Tassler 1993). No dropouts or withdrawals were mentioned in the Xu 1999 trial.
Selective reporting
Thirteen trials reported both the prespecified primary efficacy and adverse outcomes and were judged to be at a low risk of reporting bias (Arata 1988; Arata 1993; Arata 1994a; Arata 1994b; Arata 1995a; Arata 1997; Baig 1988; Beitner 1996; Giordano 2006; Jin 1995; Montero 1996; Parsad 1997; Tassler 1993). The other five trials did not report the adverse events and were considered to be at a high risk of reporting bias (Iyer 2013; Kessler 2012; Shenoy 1990; Xu 1992; Xu 1999).
Other potential sources of bias
The risk of other sources of bias was unclear in all studies because there was insufficient information to assess whether another important risk of bias existed.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings 1. Topical antibiotics compared to topical antiseptics for bacterial folliculitis and boils (furuncles and carbuncles).
| Topical antibiotics compared to topical antiseptics for bacterial folliculitis and boils (furuncles and carbuncles) | |||||
| Patient or population: bacterial folliculitis and boils (furuncles and carbuncles) Setting: no trials were identified Intervention: topical antibiotics Comparison: topical antiseptics | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with topical antiseptics | Risk with topical antibiotics | ||||
| Clinical cure | No trials were identified. | ||||
| Severe adverse events leading to withdrawal of treatment | No trials were identified. | ||||
| Quality of life | No trials were identified. | ||||
| Recurrence of folliculitis or boil following completion of treatment | No trials were identified. | ||||
| Minor adverse events not leading to withdrawal of treatment | No trials were identified. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 2. Topical antibiotics compared to systemic antibiotics for bacterial folliculitis and boils (furuncles and carbuncles).
| Topical antibiotics compared to systemic antibiotics for bacterial folliculitis and boils (furuncles and carbuncles) | |||||
| Patient or population: bacterial folliculitis and boils (furuncles and carbuncles) Setting: no trials were identified Intervention: topical antibiotics Comparison: systemic antibiotics | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with systemic antibiotics | Risk with topical antibiotics | ||||
| Clinical cure | No trials were identified. | ||||
| Severe adverse events leading to withdrawal of treatment | No trials were identified. | ||||
| Quality of life | No trials were identified. | ||||
| Recurrence of folliculitis or boil following completion of treatment | No trials were identified. | ||||
| Minor adverse events not leading to withdrawal of treatment | No trials were identified. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 3. Phototherapy compared to sham light for bacterial folliculitis and boils (furuncles and carbuncles).
| Phototherapy compared to sham light for bacterial folliculitis and boils (furuncles and carbuncles) | |||||
| Patient or population: bacterial folliculitis and boils (furuncles and carbuncles) Setting: no trials were identified Intervention: phototherapy Comparison: sham light | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with sham light | Risk with phototherapy | ||||
| Clinical cure | No trials were identified. | ||||
| Severe adverse events leading to withdrawal of treatment | No trials were identified. | ||||
| Quality of life | No trials were identified. | ||||
| Recurrence of folliculitis or boil following completion of treatment | No trials were identified. | ||||
| Minor adverse events not leading to withdrawal of treatment | No trials were identified. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 4. Cefadroxil compared to flucloxacillin for bacterial furunculosis.
| Cefadroxil compared to flucloxacillin for bacterial furunculosis | |||||
| Patient or population: bacterial furunculosis Setting: clinics Intervention: cefadroxil Comparison: flucloxacillin | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with flucloxacillin | Risk with cefadroxil | ||||
| Clinical cure (measured after 10 days of treatment) |
Study population | RR 0.90 (0.70 to 1.16) | 41 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 900 per 1000 | 810 per 1000 (630 to 1000) | ||||
| Severe adverse events leading to withdrawal of treatment (reported during 10 days of treatment) |
Study population | RR 2.97 (0.60 to 14.62) | 6514 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | |
| 6 per 1000 | 18 per 1000 (4 to 90) | ||||
| Quality of life | Not measured | ||||
| Recurrence of folliculitis or boil following completion of treatment | Not measured | ||||
| Minor adverse events not leading to withdrawal of treatment (reported during 10 days of treatment) |
Study population | RR 0.78 (0.62 to 0.98) | 6514 (1 RCT) | ⊕⊕⊕⊝ MODERATE3 | |
| 358 per 1000 | 279 per 1000 (222 to 351) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level due to high risk of performance bias and two levels for serious imprecision (not meeting optimal information size (total number of participants n = 70; 35 in each group), and the confidence interval included 1.0). 2Downgraded one level due to high risk of performance bias and one level for imprecision (the confidence of intervals included 1.0). 3Downgraded one level due to high risk of performance bias. 4The complete study participants were included in adverse event analysis.
Summary of findings 5. Cefdinir compared to cefalexin for bacterial folliculitis and boils (furuncles and carbuncles).
| Cefdinir compared to cefalexin for bacterial folliculitis and boils (furuncles and carbuncles) | |||||
| Patient or population: bacterial folliculitis and boils (furuncles and carbuncles) Setting: hospital Intervention: cefdinir Comparison: cefalexin | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with cefalexin | Risk with cefdinir | ||||
| Clinical cure (measured 17 to 24 days after treatment) |
Study population | RR 1.00 (0.73 to 1.38) | 74 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| 760 per 1000 | 770 per 1000 (670 to 876) | ||||
| Severe adverse events leading to withdrawal of treatment (reported during 17 to 24 days of treatment) |
Study population | RR 1.05 (0.07 to 16.62) | 3912 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| 5 per 1000 | 5 per 1000 (0 to 83) | ||||
| Quality of life | Not measured | ||||
| Recurrence of folliculitis or boil following completion of treatment | Not measured | ||||
| Minor adverse events not leading to withdrawal of treatment | Not reported. But the authors do state that of the 391 participants who received study medications, 10% in the cefdinir group and 4% in the cefalexin group experienced diarrhoea (P = 0.017), 3% and 6% nausea, respectively (P = 0.203), and 3% and 6% of females experienced vaginal mycosis (P = 0.500) during therapy. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level due to high risk of performance bias and one level for imprecision (confidence interval included 1.0). 2The complete study participants were included in adverse event analysis.
Summary of findings 6. Azithromycin compared to cefaclor for bacterial boils (furuncles and carbuncles).
| Azithromycin compared to cefaclor for bacterial boils (furuncles and carbuncles) | |||||
| Patient or population: bacterial boils (furuncles and carbuncles) Setting: hospitals and clinics (multicentre) Intervention: azithromycin Comparison: cefaclor | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with cefaclor | Risk with azithromycin | ||||
| Clinical cure (measured 7 days after treatment) |
Study population | RR 1.01 (0.72 to 1.40) | 31 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 625 per 1000 | 631 per 1000 (450 to 875) | ||||
| Severe adverse events leading to withdrawal of treatment (reported during 7 days of treatment) |
No severe adverse events leading to withdrawal of treatment occurred in either the azithromycin or cefaclor group. | ‐ | 2744 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | |
| Quality of life | Not measured | ||||
| Recurrence of folliculitis or boil following completion of treatment | Not measured | ||||
| Minor adverse events not leading to withdrawal of treatment (reported during 7 days of treatment) |
Study population | RR 1.26 (0.38 to 4.17) | 2744 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 | |
| 40 per 1000 | 51 per 1000 (15 to 166) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded two levels due to high risk of performance bias and detection bias, and one level due to imprecision (not meeting optimal information size of 70, with 35 in each group). 2Downgraded two levels due to high risk of performance bias and detection bias, and one level due to imprecision (few events). 3Downgraded two levels due to high risk of performance bias and detection bias, and one level due to imprecision (the confidence interval included 1). 4The complete study participants were included in adverse event analysis.
Summary of findings 7. Cefditoren pivoxil compared to cefaclor for bacterial boils (furuncles and carbuncles).
| Cefditoren pivoxil compared to cefaclor for bacterial boils (furuncles and carbuncles) | |||||
| Patient or population: bacterial boils (furuncles and carbuncles) Setting: hospitals and clinics (multicentre) Intervention: cefditoren pivoxil Comparison: cefaclor | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with cefaclor | Risk with cefditoren pivoxil | ||||
| Clinical cure (measured after 7 days of treatment) |
Study population | RR 1.17 (0.77 to 1.78) | 93 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 | |
| 447 per 1000 | 523 per 1000 (344 to 795) | ||||
| Severe adverse events leading to withdrawal of treatment (reported during 7 days of treatment) |
No participants taking cefaclor withdrew from treatment due to severe adverse events, whilst 2 participants in the cefditoren pivoxil group withdrew due to adverse events (nausea and heavy feeling in stomach). | RR 4.74 (0.23 to 97.17) | 1504 (1 RCT) | ⊕⊕⊝⊝ LOW2 | |
| Quality of life | Not measured | ||||
| Recurrence of folliculitis or boil following completion of treatment | Not measured | ||||
| Minor adverse events not leading to withdrawal of treatment (reported during 7 days of treatment) |
Study population | RR 1.52 (0.52 to 4.42) | 1504 (1 RCT) | ⊕⊕⊕⊝ MODERATE3 | |
| 68 per 1000 | 104 per 1000 (36 to 303) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level due to imprecision (just one modest‐size trial). 2Downgraded two levels due to serious imprecision (few events and the confidence of intervals included 1.0). 3Downgraded one level due to imprecision (the confidence of intervals included 1.0). 4The complete study participants were included in adverse event analysis.
No trials compared topical antibiotics versus topical antiseptics (Table 1), topical antibiotics versus systemic antibiotics (Table 2), or phototherapy versus sham light for bacterial folliculitis and boils (Table 3).
We could not undertake the following planned subgroup analyses due to the low number of studies included: paediatric versus adult participants, immunocompetent versus immunosuppressed participants, MSSA versus MRSA (including PVL gene type), and different dosages of an intervention.
Most comparisons included only one RCT, therefore we were unable to perform meta‐analyses for these comparisons.
Topical interventions
Ofloxacin gel versus norfloxacin cream
One RCT compared the efficacy of 0.5% ofloxacin gel with 1.0% norfloxacin gel applied over the lesions twice daily (Jin 1995).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The ofloxacin and ofloxacin groups did not differ in cure (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.94 to 1.07; participants = 60; studies = 1, see Analysis 1.1).
1.1. Analysis.

Comparison 1: Ofloxacin gel versus norfloxacin gel, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
No serious adverse events occurred in either group.
Secondary outcome 1. Quality of life: as measured by validated tools, including Dermatology Life Quality Index (DLQI), 36‐item Short Form Health Survey (SF‐36), Skindex 29, Skindex 17, or Dermatology Quality of Life Scale (DQOLS)
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
No adverse events occurred in either group.
Sisomicin ointment versus gentamicin ointment
One study compared the clinical response between sisomicin 1% ointment and gentamicin 1% ointment applied over folliculitis lesions two to three times daily for seven days (Arata 1988).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The trial detected no difference in clinical cure between the two study groups (RR 1.20, 95% CI 0.55 to 2.63, P = 0.24; participants = 38; studies = 1, see Analysis 2.1).
2.1. Analysis.

Comparison 2: Sisomicin ointment versus gentamicin ointment, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
No serious adverse events occurred in either group.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
The safety analysis enrolled 151 participants (75 in the sisomicin group and 76 in the gentamicin group). One participant that received gentamicin had adverse event (irritable sensation) (RR 0.34, 95% CI 0.01 to 8.16, P = 0.50; participants = 151; studies = 1, see Analysis 2.2).
2.2. Analysis.

Comparison 2: Sisomicin ointment versus gentamicin ointment, Outcome 2: Minor adverse events not leading to withdrawal of treatment
Dieda Xiaoyan Gao ointment versus ichthammol ointment
One trial compared the therapeutic efficacy between Dieda Xiaoyan Gao ointment and ichthammol ointment applied over the boils once daily for 10 days (Xu 1992).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The cure rate may be better in the Dieda Xiaoyan Gao group (83.3%; 25/30) than in the ichthammol group (33.3%; 10/30) (RR 2.50, 95% CI 1.47 to 4.25; participants = 60; studies = 1, see Analysis 3.1).
3.1. Analysis.

Comparison 3: Dieda Xiaoyan Gao ointment versus ichthammol ointment, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Serious adverse events were not reported.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
Minor adverse events were not reported.
Systemic drug interventions
Erythromycin versus flucloxacillin
One trial including 86 participants compared the clinical efficacy between erythromycin 500 mg oral twice daily and flucloxacillin 250 mg oral four times daily for 10 days (Baig 1988).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
Clinical cure data were not reported.
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Severe adverse events leading to withdrawal of treatment were not reported.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
In this boils study, there were nine adverse events: three in the erythromycin group (abdominal pain; nausea/vomiting; diarrhoea) and six in the flucloxacillin group (two with nausea/vomiting; dyspepsia; diarrhoea; flatulence; dizziness) (RR 0.48, 95% CI 0.13 to 1.79, P = 0.15; participants = 86; studies = 1, see Analysis 4.1).
4.1. Analysis.

Comparison 4: Erythromycin versus flucloxacillin, Outcome 1: Minor adverse events not leading to withdrawal of treatment
Cefadroxil versus flucloxacillin
One trial compared the efficacy between oral cefadroxil 40 mg/kg to a maximum dose of 1 g once daily for 10 days and oral flucloxacillin 750 mg tablets twice daily or suspension 30 to 50 mg/kg administered in two or three daily doses to a maximum dose of 1.5 g for 10 days (Beitner 1996). Of 41 participants with boils, 21 received cefadroxil and 20 received flucloxacillin.
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The two groups did not differ in clinical cure (RR 0.90, 95% CI 0.70 to 1.16; participants = 41; studies = 1, see Analysis 5.1).
5.1. Analysis.

Comparison 5: Cefadroxil versus flucloxacillin, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
A total of 651 participants were included in the safety analysis, of whom 327 received cefadroxil and 324 received flucloxacillin. There were no respective safety data for participants with boils. Eight participants had severe adverse events: six in the cefadroxil group (stomachache, rash, fever, or vomiting) and two in the flucloxacillin group (severe diarrhoea) (RR 2.97, 95% CI 0.60 to 14.62, P = 0.11; participants = 651; studies = 1, see Analysis 5.2).
5.2. Analysis.

Comparison 5: Cefadroxil versus flucloxacillin, Outcome 2: Severe adverse events leading to withdrawal of treatment
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
A total of 215 participants, including 97 in the cefadroxil group and 118 in the flucloxacillin group, reported minor adverse events not leading to withdrawal of treatment (RR 0.78, 95% CI 0.62 to 0.98; participants = 651; studies = 1, number needed to treat for an additional harmful outcome (NNTH) = 13 (95% CI 7 to 100), see Analysis 5.3).
5.3. Analysis.

Comparison 5: Cefadroxil versus flucloxacillin, Outcome 3: Minor adverse events not leading to withdrawal of treatment
Cefdinir versus cefalexin
One trial compared the efficacy between oral cefdinir capsules 300 mg twice daily and cefalexin capsules 250 mg four times daily for 10 days (Giordano 2006). A total of 391 participants received medical treatment, 44 of them with folliculitis and 30 with furunculosis.
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The two groups did not differ in the clinical cure of folliculitis (RR 1.17, 95% CI 0.84 to 1.63; participants = 44; studies = 1, see Analysis 6.1) and furunculosis (RR 0.85, 95% CI 0.59 to 1.22; participants = 30; studies = 1, see Analysis 6.1). When all participants were included, the groups also did not differ (RR 1.00, 95% CI 0.73 to 1.38; participants = 74; studies = 1, see Analysis 6.1).
6.1. Analysis.

Comparison 6: Cefdinir versus cefalexin, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Respective safety data for participants with folliculitis and boils were not provided. Of 391 participants who received study medications, 2 participants (1 in the cefdinir group (diarrhoea) and 1 in the cefalexin group (gastroenteritis)) had a treatment‐related adverse event leading to premature discontinuation of the study drug (RR 1.05, 95% CI 0.07 to 16.62, P = 0.50; participants = 391; studies = 1; see Analysis 6.2).
6.2. Analysis.

Comparison 6: Cefdinir versus cefalexin, Outcome 2: Severe adverse events leading to withdrawal of treatment
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
Of 391 participants who received study medications, the following minor adverse events not leading to withdrawal of treatment were experienced during therapy: diarrhoea (10% cefdinir, 4% cefalexin, P = 0.017); nausea (3% cefdinir, 6% cefalexin, P = 0.203); and vaginal mycosis (3% and 6% of females in cefdinir and cefalexin groups, respectively, P = 0.500).
Azithromycin versus cefaclor
Two trials compared the effects of oral azithromycin and cefaclor (Arata 1995a; Montero 1996). In the Arata 1995a trial, participants received azithromycin (AZT) 250 mg once daily (group L), azithromycin 500 mg once daily (group H), or cefaclor 250 mg three times per day (group C). In the Montero 1996 trial, children received azithromycin 10 mg/kg once daily for three days or cefaclor 20 mg/kg/day in three divided doses for 10 days.
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The azithromycin and cefaclor groups did not differ in clinical cure with different doses (AZT 250 mg daily for 3 days: RR 0.86, 95% CI 0.19 to 3.81, P = 0.40, participants = 16, studies = 1; AZT 500 mg daily for 3 days: RR 1.50, 95% CI 0.39 to 5.77, P = 0.39, participants = 13, studies = 1; AZT 10 mg/kg daily for 3 days: RR 1.00, 95% CI 0.71 to 1.41, P = 1.00, participants = 11, studies = 1; see Analysis 7.1). After pooling of these trials, the clinical cure rate was similar between the two groups (RR 1.01, 95% CI 0.72 to 1.40, P = 0.25; participants = 31; studies = 2, I2 = 0%, see Analysis 7.2).
7.1. Analysis.

Comparison 7: Azithromycin versus cefaclor, Outcome 1: Clinical cure subgroup
7.2. Analysis.

Comparison 7: Azithromycin versus cefaclor, Outcome 2: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
No severe adverse events leading to withdrawal of treatment occurred in either the azithromycin or cefaclor groups.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
The azithromycin and cefaclor groups did not differ in minor adverse events not leading to withdrawal of treatment (RR 1.26, 95% CI 0.38 to 4.17, P = 0.20; participants = 274; studies = 2, I2 = 0%, see Analysis 7.3).
7.3. Analysis.

Comparison 7: Azithromycin versus cefaclor, Outcome 3: Minor adverse events not leading to withdrawal of treatment
Ciprofloxacin versus ciprofloxacin plus pentoxifylline
One trial compared the effects of ciprofloxacin twice daily and placebo three times daily for two weeks followed by placebo for another four weeks versus ciprofloxacin twice daily and pentoxifylline 400 mg three times daily for two weeks followed by pentoxifylline 400mg three times daily for another four weeks in treating chronic folliculitis of legs (Parsad 1997).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The two groups did not differ in clinical cure (RR 0.76, 95% CI 0.52 to 1.09; participants = 35; studies = 1, see Analysis 8.1).
8.1. Analysis.

Comparison 8: Ciprofloxacin versus pentoxifylline plus ciprofloxacin, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Only one participant in the ciprofloxacin plus pentoxifylline group withdrew from this trial due to severe adverse events (dyspepsia and nausea).
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
The recurrence rate appeared to be higher in the ciprofloxacin group compared to the ciprofloxacin plus pentoxifylline group (RR 4.72, 95% CI 1.66 to 13.46, P < 0.01; participants = 35; studies = 1, number needed to treat for an additional beneficial outcome (NNTB) = 2 (95% CI 2 to 3), see Analysis 8.2).
8.2. Analysis.

Comparison 8: Ciprofloxacin versus pentoxifylline plus ciprofloxacin, Outcome 2: Recurrence of folliculitis or boil following completion of treatment
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
No minor adverse events not leading to withdrawal of treatment were reported in either study group.
Fleroxacin versus amoxicillin/clavulanate
One trial compared the effects of oral fleroxacin 200 mg once daily versus amoxicillin/clavulanate (500 mg/125 mg) three times daily for 7 to 21 days in treating folliculitis (Tassler 1993).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
A total of seven participants with folliculitis received study medications in this trial, five participants receiving fleroxacin and two amoxicillin/clavulanate. Three participants in the fleroxacin group and one in the amoxicillin/clavulanate group achieved clinical cure (RR 1.20, 95% CI 0.25 to 5.71, P = 0.57; participants = 7; studies = 1, see Analysis 9.1).
9.1. Analysis.

Comparison 9: Fleroxacin versus amoxicillin/clavulanate, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
There were no safety data for participants with folliculitis. A total of 15 of 189 participants receiving fleroxacin and 4 of 95 receiving amoxicillin/clavulanate withdrew due to adverse events. More participants in the fleroxacin group had a digestive reaction (RR 1.88, 95% CI 0.64 to 5.52, P = 0.11; participants = 284; studies = 1, see Analysis 9.2).
9.2. Analysis.

Comparison 9: Fleroxacin versus amoxicillin/clavulanate, Outcome 2: Severe adverse events leading to withdrawal of treatment
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
There were no safety data for participants with folliculitis. Mild adverse events occurred in 25 of 189 participants receiving fleroxacin and 12 of 95 receiving amoxicillin/clavulanate. Most participants with mild adverse events had digestive symptoms (nausea, vomiting, diarrhoea) and central nervous system symptoms (dizziness, insomnia, and somnolence) in the fleroxacin group; and digestive symptoms (diarrhoea) in the amoxicillin/clavulanate group (RR 1.05, 95% CI 0.55 to 1.99; participants = 284; studies = 1, see Analysis 9.3).
9.3. Analysis.

Comparison 9: Fleroxacin versus amoxicillin/clavulanate, Outcome 3: Minor adverse events not leading to withdrawal of treatment
Cefditoren pivoxil versus cefaclor
One trial compared the efficacy of cefditoren pivoxil 200 mg three times daily and cefaclor 250 mg three times daily for seven days (Arata 1993).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The two groups did not differ in clinical cure (RR 1.17, 95% CI 0.77 to 1.78; participants = 93; studies = 1, see Analysis 10.1).
10.1. Analysis.

Comparison 10: Cefditoren pivoxil versus cefaclor, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
There were no safety data for participants with folliculitis and boils. Of 77 participants taking cefditoren pivoxil, 2 withdrew from the trial due to adverse events (nausea and heavy feeling in stomach), whilst none of 73 participants taking cefaclor withdrew from treatment due to severe adverse events (RR 4.74, 95% CI 0.23 to 97.17, P = 0.26; participants = 150; studies = 1, see Analysis 10.2).
10.2. Analysis.

Comparison 10: Cefditoren pivoxil versus cefaclor, Outcome 2: Severe adverse events leading to withdrawal of treatment
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
There were no specific safety data for participants with folliculitis and boils. A total of 13 participants had mild adverse events (8 in the cefditoren pivoxil group and 5 in the cefaclor group), with one feeling thirsty and the others having gastrointestinal symptoms (RR 1.52, 95% CI 0.52 to 4.42, P = 0.17; participants = 150; studies = 1, see Analysis 10.3).
10.3. Analysis.

Comparison 10: Cefditoren pivoxil versus cefaclor, Outcome 3: Minor adverse events not leading to withdrawal of treatment
S‐1108 versus cefaclor
One trial compared the effects of oral S‐1108 (an oral cephem antibiotic), Totsuka 1992, 150 mg and cefaclor 250 mg three times daily (Arata 1994a).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
Both S‐1108 and cefaclor were effective in treating folliculitis or boils; the two groups did not differ in clinical cure (RR 0.88, 95% CI 0.62 to 1.26; participants = 132; studies = 1, Analysis 11.1).
11.1. Analysis.

Comparison 11: S‐1108 versus cefaclor , Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
No severe adverse events leading to withdrawal of treatment were reported for either study group.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
There were no specific safety data for participants with folliculitis and boils. Minor adverse events occurred in two participants in the S‐1108 group (diarrhoea and loose stools) and one participant in the cefaclor group (epigastric pain) (RR 1.94, 95% CI 0.18 to 21.01, P = 0.38; participants = 189; studies = 1, see Analysis 11.2).
11.2. Analysis.

Comparison 11: S‐1108 versus cefaclor , Outcome 2: Minor adverse events not leading to withdrawal of treatment
SY 5555 versus cefaclor group
One trial compared the therapeutic efficacy between oral SY 5555 (an oral penem antibiotic), Inoue 1994, 200 mg and cefaclor 250 mg three times per day for seven days (Arata 1994b).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The clinical cure rate did not differ between groups (RR 1.08, 95% CI 0.69 to 1.70; participants = 81; studies = 1, see Analysis 12.1).
12.1. Analysis.

Comparison 12: SY 5555 versus cefaclor, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
There were no specific safety data for participants with folliculitis and boils. Of 303 participants, 12 withdrew due to adverse events, 8 in the SY 5555 group (4 diarrhoea, 1 nausea, 1 facial swelling, 1 stomachache, and 1 abdominal fullness) and 4 in the cefaclor group (diarrhoea, nausea, stomachache, and weakness) (RR 2.04, 95% CI 0.63 to 6.63, P = 0.12; participants = 303; studies = 1, see Analysis 12.2).
12.2. Analysis.

Comparison 12: SY 5555 versus cefaclor, Outcome 2: Severe adverse events leading to withdrawal of treatment
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
There were no specific safety data for participants with folliculitis and boils. Of 303 participants, 11 experienced mild adverse events, 7 in the SY 5555 group (4 diarrhoea, 2 loose stools, and 1 oedema over lower extremities) and 4 in the cefaclor group (2 loose stools, 1 diarrhoea, and 1 fatigability) (RR 1.78, 95% CI 0.53 to 5.97, P = 0.16; participants = 303; studies = 1, see Analysis 12.3).
12.3. Analysis.

Comparison 12: SY 5555 versus cefaclor, Outcome 3: Minor adverse events not leading to withdrawal of treatment
Grepafloxacin versus ofloxacin
One trial compared the effects of grepafloxacin 200 mg once daily and ofloxacin 200 mg twice per day in treating folliculitis and boils (Arata 1997).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
The grepafloxacin and ofloxacin groups did not differ in clinical cure (RR 1.28, 95% CI 0.90 to 1.82; participants = 138; studies = 1, see Analysis 13.1). The efficacy rate (which included participants with excellent or good clinical efficacy) was similar between groups (92.75% versus 85.51%; RR 1.08, 95% CI 0.96 to 1.22).
13.1. Analysis.

Comparison 13: Grepafloxacin versus ofloxacin, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
No severe adverse events leading to withdrawal of treatment occurred in either group.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
There were no specific safety data for participants with folliculitis and boils. Of 219 participants (109 in the grepafloxacin group and 110 in the ofloxacin group) included in the safety analysis, 17 reported minor adverse events (7 in the grepafloxacin group and 10 in the ofloxacin group) including insomnia (2), nausea (2), sleepiness (1), stomachache (1), stomach heaviness (1), stomach discomfort (1), upper abdomen dull pain (1), vomiting (1), nausea with vomiting (1), diarrhoea (1), urticaria (1), pruritus and generalised erythema (1), erythema over limbs and trunk (1), and palpitations (1) (RR 0.71, 95% CI 0.28 to 1.79, P = 0.15; participants = 219; studies = 1, see Analysis 13.2).
13.2. Analysis.

Comparison 13: Grepafloxacin versus ofloxacin, Outcome 2: Minor adverse events not leading to withdrawal of treatment
Other interventions
Co‐trimoxazole plus 8‐methoxypsoralen and sunlight versus co‐trimoxazole plus placebo and sunlight
One trial compared the effects of co‐trimoxazole (sulfamethoxazole 800 mg and trimethoprim 160 mg) twice daily then 20 mg of 8‐methoxypsoralen at 8 AM followed by exposure to sunlight from 10 AM to 10:15 AM versus co‐trimoxazole twice daily and placebo at 8 AM followed by exposure to sunlight from 10 AM to 10:15 AM in chronic leg folliculitis therapy (Shenoy 1990).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
All participants were lesion‐free on day 15.
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Serious adverse events leading to withdrawal of treatment were not reported.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Due to very low‐certainty evidence, we are uncertain as to whether 8‐methoxypsoralen improved lesion‐free rate on day 45 (RR 1.38, 95% CI 0.88 to 2.17; participants = 45; studies = 1) and day 90 (RR 2.08, 95% CI 0.75 to 5.78; participants = 26; studies = 1, see Analysis 14.1).
14.1. Analysis.

Comparison 14: Co‐trimoxazole plus 8‐MOP and sunlight versus co‐trimoxazole plus placebo and sunlight, Outcome 1: Lesion‐free rate
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
Minor adverse events not leading to withdrawal of treatment were not reported.
Fire cupping plus penicillin intramuscular injection versus incision for pus plus penicillin intramuscular injection
One study compared the effects of fire cupping after boil incision plus penicillin 800,000 U intramuscular injection twice a day (group A) versus boil incision plus penicillin 800,000 U intramuscular injection twice per day (group B) (Xu 1999).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
Fire cupping might improve the clinical cure rate after boils incision on day 7 (RR 1.33, 95% CI 1.13 to 1.56; participants = 260; studies = 1, see Analysis 15.1).
15.1. Analysis.

Comparison 15: Fire cupping plus penicillin versus penicillin, Outcome 1: Clinical cure
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Serious adverse events leading to withdrawal of treatment were not reported.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
Minor adverse events not leading to withdrawal of treatment were not reported.
Wound packing versus no wound packing following incision and drainage
One study compared the efficacy (including Clinical Anger Scale (CAS) pain scale) and recurrence rate of boils receiving incision and drainage with or without wound packing (Kessler 2012).
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
Clinical cure rate data were not reported.
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Serious adverse events leading to withdrawal of treatment were not reported.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Although this trial did not report on quality of life, the pain scores (CAS 0 to 100) did not differ between groups (mean difference −1.00, 95% CI −13.95 to 11.95; participants = 49; studies = 1, see Analysis 16.1).
16.1. Analysis.

Comparison 16: Wound packing versus no wound packing following incision and drainage , Outcome 1: Pain score (48 h post‐incision and drainage)
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence rates did not differ between groups (RR 0.21, 95% CI 0.01 to 4.27, P = 0.39; participants = 56; studies = 1, see Analysis 16.2).
16.2. Analysis.

Comparison 16: Wound packing versus no wound packing following incision and drainage , Outcome 2: Recurrence rate (1 month)
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
Minor adverse events not leading to withdrawal of treatment were not reported.
Excision of carbuncle with primary split thickness skin grafting (STSG) versus delayed STSG
One study compared the efficacy (survival rate of STSG and duration of stay in ward) of primary STSG post‐carbuncle excision versus delayed STSG (Iyer 2013). Graft survival rate was higher in the primary STSG group than in the delayed STSG group (RR 1.48, 95% CI 1.15 to 1.92; participants = 56; studies = 1, NNTB = 3 (95% CI 2 to 7), see Analysis 17.1). Duration of stay in ward was shorter in the primary STSG group than in the delayed STSG group (mean 10.07 versus 21.08 days; P < 0.001).
17.1. Analysis.

Comparison 17: Primary STSG versus delay STSG, Outcome 1: Survival of STSG
Primary outcome 1. Clinical cure: clearance of all visible lesions of folliculitis or boils (i.e. disappearance of all papular or pustular lesions of folliculitis or boils at the end of treatment)
Clinical cure rate data were not reported.
Primary outcome 2. Severe adverse events leading to withdrawal of treatment
Serious adverse events leading to withdrawal of treatment were not reported.
Secondary outcome 1. Quality of life: as measured by validated tools, including DLQI, SF‐36, Skindex 29, Skindex 17, or DQOLS
Quality of life data were not reported.
Secondary outcome 2. Recurrence of folliculitis or boil following completion of treatment
Recurrence data were not reported.
Secondary outcome 3. Minor adverse events not leading to withdrawal of treatment
Minor adverse events not leading to withdrawal of treatment were not reported.
Discussion
Summary of main results
None of the studies included in this review assessed what we classed as the most important comparisons: topical antibiotics versus topical antiseptics, topical antibiotics versus systemic antibiotics, and phototherapy versus sham light. However, as planned in our protocol, we produced additional 'Summary of findings' tables for our other major comparisons.
Our key results report on the efficacy of oral antibiotics for bacterial folliculitis and boils therapy. We selected the following as key clinical comparisons: cefadroxil versus flucloxacillin (see Table 4); cefdinir versus cefalexin (see Table 5); azithromycin versus cefaclor (see Table 6); and cefditoren pivoxil versus cefaclor (see Table 7).
When assessing achievement of clinical cure, defined as the clearance of all visible lesions of folliculitis or boils, cefdinir compared to cefalexin may make little to no difference (low‐certainty evidence). Similarly, but with moderate‐certainty evidence, cefditoren pivoxil probably makes little to no difference when compared to cefaclor. We are uncertain of the effect of both cefadroxil compared to flucloxacillin and azithromycin compared to cefaclor, due to very low‐certainty evidence.
Cefadroxil (compared to flucloxacillin) and cefditoren pivoxil (compared to cefaclor) may increase the risk of severe adverse events leading to withdrawal of treatment; however, for both of these results, the 95% confidence interval includes the possibility of both increased and reduced risk of serious adverse events (low‐certainty evidence). When compared to cefalexin, cefdinir may make little to no difference to the incidence of severe adverse events but, as above, the 95% CI is very wide and includes the possibility of both increased and reduced risk of serious adverse events (low‐certainty evidence). We are uncertain of the effect of azithromycin compared to cefaclor due to very low‐certainty evidence; two trials in this comparison did not report any severe adverse events.
Due to very low‐certainty evidence, we are uncertain of the effects of azithromycin compared to cefaclor in the risk of minor adverse events not leading to withdrawal of treatment. Cefadroxil (compared to flucloxacillin) and cefditoren pivoxil (compared to cefaclor) probably make little to no difference to this outcome (moderate‐certainty evidence). Although the study that assessed cefdinir compared to cefalexin did not report statistical data for this outcome, the authors reported that participants in both groups experienced the following minor adverse events: diarrhoea, nausea, and vaginal mycosis. Other adverse events reported by participants in the studies included in this review were gastrointestinal symptoms (e.g. stomach ache), vomiting, and rash; some of these adverse events led to participant withdrawal.
Our key comparisons did not provide data on recurrence of folliculitis or boils following completion of treatment or quality of life.
Overall completeness and applicability of evidence
The evidence we identified for inclusion in this review was insufficient to fully address our objective, that is to assess effects of interventions for people with bacterial folliculitis and boils.
The evidence for each of the majority of comparisons was based on a single trial, which precluded meta‐analysis. This meant that precision was low, and all findings should be interpreted with caution.
There was no evidence on topical antibiotics versus topical antiseptics; topical antibiotics versus systemic antibiotics; and phototherapy versus sham light, which were the key comparisons planned in our protocol. The included studies assessed six topical treatments, 16 oral treatments, and eight other treatments, either as the intervention or a comparator. There was almost no use of placebo groups in these trials, but it is generally accepted that antibiotics or other antibacterial treatments are necessary in folliculitis or boils, and there was no direct comparison between topical and oral antibiotics.
All of the studies assessing oral interventions (11 studies) compared different oral antibiotics, with five studies assessing cefaclor, the second‐generation cephalosporin. In fact, the most common category of antibiotics was the cephalosporins, a first‐line oral treatment. First‐ and third‐generation cephalosporins were assessed less frequently: one study assessed the first‐generation cephalosporins cefadroxil and cefalexin, and a second study assessed the newer third‐generation cephalosporins, cefdinir and cefditoren pivoxil. Consequently, we were unable to draw conclusions about treatments that target gram‐negative bacteria unresponsive to other cephalosporins. Seven studies included arms assessing either macrolide antibiotics, fluoroquinolone antibiotics, or penicillin or penicillin‐like antibiotics; three studies assessed each grouping, with the intervention evaluated as either a treatment or comparator. Many studies compared the efficacy of oral antibiotics, but in different trials the same study drug may have been given in different doses and intensity or assessed at different time points.
Only three studies assessed topical treatments: two studies compared different antibiotics against each other, and one study assessed a Traditional Chinese Medicine treatment. Common topical antibiotics, such as erythromycin or clindamycin, were not assessed by any study. Four studies assessed the following treatments: psoralen, fire cupping, incision and drainage, wound packing, and different types of skin graft. Phototherapy was another area of treatment evaluated by few studies, so we remain uncertain if phototherapy benefits people with chronic, non‐infective folliculitis.
Furthermore, treatment of bacterial folliculitis and boils is dependent on a number of factors, including age, severity, whether an infection is present, type of bacteria present, and a person's immune status. We had planned to assess a number of these factors in subgroup analyses, including paediatric versus adult participants, immunocompetent versus immunosuppressed participants, and MSSA versus MRSA. However, insufficient studies meant that data were not available to permit these analyses.
Regarding the representativeness of the study participants, bacterial folliculitis and boils have a worldwide prevalence, which is reflected in the setting of the trials. The trials were based in a total of 18 countries, including Asia, Europe, and America; over a third were set in East Asia. Bacterial folliculitis and boils affect both children and adults, and the studies included participants across the age spectrum: infants were enrolled in some studies, and the oldest participant was aged 88. Bacterial folliculitis is most common in adolescents and young men, and seven trials included participants as young as 13 years. Two other trials lowered the age for study inclusion and included participants as young as three and six years. A further two trials only included young participants (aged between 6 months and 12 years in 1 study, and between 1 and 25 years in another study). Our objective was limited because many trials enrolled participants with superficial skin and soft tissue infection, without specifying those who had the subgroup of folliculitis and boils. Furthermore, when studies did enrol people with folliculitis and boils as a subgroup, the age of these participants may not have been reported. Severity was not well reported either.
There was wide variation in treatment duration (range: 3 days to 6 weeks) and follow‐up (range: 3 days to 6 months).
When we found studies assessing our interventions of interest, they often did not evaluate our prespecified secondary outcomes. No studies assessed our key outcome quality of life, and only three of the 18 included studies assessed recurrence. However, just over 80% of the included studies assessed our primary efficacy outcome, clinical cure, and over two‐thirds of the studies assessed both major and minor adverse events.
Quality of the evidence
There were no available data for our planned comparisons of topical antibiotics versus topical antiseptics; topical antibiotics versus systemic antibiotics; and phototherapy versus sham light. Hence, we created 'Summary of findings' tables for four additional comparisons. We rated the certainty of the body of evidence as very low to low for most outcomes and moderate for a few outcomes.
Limitations in the design and implementation of available studies suggesting high likelihood of bias
The domains most frequently judged as at high risk were performance bias (10 (55.6%) out of 18 trials), followed by reporting bias (5 (27.8%) trials), then detection bias (3 (16.7%) trials). We assessed performance bias, reporting bias, and detection bias as high risk in these studies due to a lack of description of methods used for participant blinding; a lack of reporting of adverse events; and no blinding of outcome assessment, respectively. We assessed one trial with a high withdrawal rate (> 20% of participants) as having a high risk of attrition bias (Shenoy 1990).
Most of the included trials (16 (88.9%)) did not report the methods of randomisation and were classified as at unclear risk of selection bias. Twelve trials (66.7%) did not mention the methods of allocation concealment and were classified as having an unclear risk of bias. Eight trials (44.4%) did not describe the methods for blinding outcome assessors and were assessed as having an unclear risk of detection bias. In 10 trials, outcome efficacy analysis was based on PP data because ITT data were unavailable. We assessed one trial that did not mention dropouts or withdrawals as having an unclear risk of attrition bias.
For the comparisons cefadroxil versus flucloxacillin (Table 4) and cefdinir versus cefalexin (Table 5), we downgraded the certainty of evidence for high risk of performance bias because the participants were not blinded. For the comparison azithromycin versus cefaclor (Table 6), we downgraded the certainty of evidence twice because of a high risk of performance and detection bias.
Indirectness of evidence (indirect population, intervention, control, outcomes)
The trials included in our main comparisons focused on patients with bacterial folliculitis and boils, and the main outcome was clinical cure (the same as the primary outcome in this review). Consequently, we did not downgrade the certainty of the evidence for indirectness in the 'Summary of findings' tables.
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses)
Most comparisons included only one trial, and the only comparison for which there were two trials had low statistical heterogeneity (see Table 6). We therefore did not downgrade the certainty of evidence for inconsistency.
Due to a lack of relevant data, we were unable to conduct any of our planned subgroup analyses, such as paediatric versus adult participants; immunocompetent versus immunosuppressed participants; MSSA versus MRSA; or different dosages of an intervention.
Imprecision
For the cefadroxil versus flucloxacillin comparison (Table 4), we downgraded the certainty of evidence for the outcome clinical cure by two levels due to serious imprecision (not meeting optimal information size and the confidence interval contained 1). We also downgraded the certainty of evidence for the outcome severe adverse events leading to withdrawal of treatment by one level due to imprecision (the confidence interval contained 1).
For the cefdinir versus cefalexin comparison (Table 5), we downgraded the certainty of evidence for both severe adverse events leading to withdrawal of treatment and clinical cure by one level due to imprecision (the confidence interval contained 1).
For the azithromycin versus cefaclor comparison (Table 6), we downgraded the certainty of evidence by one level for imprecision due to not meeting the optimal information size for clinical cure; by one level for imprecision due to few events for severe adverse events leading to withdrawal of treatment; and by one level for imprecision due to the confidence intervals including 1 for minor adverse events not leading to withdrawal of treatment.
For the cefditoren pivoxil versus cefaclor comparison (Table 7), we downgraded the certainty of evidence by one level for clinical cure due to imprecision (just one modest‐size trial). We downgraded the certainty of evidence by two levels for severe adverse events leading to withdrawal of treatment due to serious imprecision (few events and the confidence of intervals contained 1.0), and by one level for minor adverse events not leading to withdrawal of treatment due to imprecision (the confidence of intervals contained 1).
Publication bias
We did not downgrade the certainty of the evidence for publication bias.
Potential biases in the review process
We attempted to conduct a comprehensive search for studies, but the fact that 16 studies are awaiting classification may be a source of potential bias.
We followed our protocol's search methods: we explored four databases and five trials registers, with no language restrictions, and also tried to contact authors for further relevant trials or unpublished data. We tried to minimise selection and publication bias. Many of the included trials were reported in Japanese and Chinese. Although our search had no language restrictions, but we did not search in the Japanese or Chinese language.
Severe adverse events are rare for bacterial folliculitis or boils, and it was difficult to conduct a complete search for adverse events. Other databases, such as Micromedex, may provide more information about adverse events with these interventions.
Agreements and disagreements with other studies or reviews
There are no systematic reviews or meta‐analysis of interventions for bacterial folliculitis or boils. Although most of the interventions were limited by small case numbers, and participants of interest were subgroups in skin or soft tissue infections, our review is the first systematic review to focus on the topic.
Authors' conclusions
Implications for practice.
We found insufficient evidence on the effects of interventions for people with bacterial folliculitis and boils. Approximately three‐quarters of the included studies assessed oral antibiotics, including beta‐lactams and quinolones, or topical antibacterial agents. However, these were not directly compared, so we could not establish whether there was any difference in efficacy between systemic and topical treatment based on the current evidence. The remaining studies evaluated Traditional Chinese Medicine, heat treatment, light therapy, wound packing, and skin grafting; conclusions regarding these treatments could not be drawn as they are based on evidence from single studies.
Due to very low‐certainty evidence, we could draw no conclusions about the effect of azithromycin compared to cefaclor on clinical cure, severe adverse events leading to withdrawal of treatment, or minor adverse events not leading to withdrawal of treatment.
Based on low‐certainty evidence, there may be little to no difference in clinical cure rate or severe adverse events when comparing cefdinir to cefalexin. The one study that assessed this comparison did not report statistical data for minor adverse events, but participants in both groups reported diarrhoea, nausea, and vaginal mycosis during therapy.
Based on moderate‐certainty evidence, there is probably little to no difference in minor adverse events when comparing the following:
cefadroxil against flucloxacillin; or
cefditoren pivoxil against cefaclor.
Based on low‐certainty evidence, there may be an increased risk of severe adverse events when cefadroxil is compared with flucloxacillin and cefditoren pivoxil is compared with cefaclor. However, the 95% confidence interval includes the possibility of both increased and reduced risk of serious adverse events. Vomiting, rashes, and gastrointestinal symptoms such as stomach ache were some of the adverse events reported in the included studies; some of these symptoms led to participant withdrawal.
Moderate‐certainty evidence indicates that there is probably little to no difference in clinical cure rate between cefditoren pivoxil and cefaclor, but we could draw no conclusions about the effect of cefadroxil compared to flucloxacillin for this outcome due to very‐low certainty evidence.
None of our key comparisons assessed quality of life or recurrence of folliculitis or boils following completion of treatment.
The 16 studies that are awaiting classification may alter the conclusions of the review once assessed.
Implications for research.
There were no trials comparing placebo with oral antibiotics or topical antibacterial agents, so we could not establish the efficacy of antibiotics (oral or topical) in the treatment of bacterial folliculitis or boils. The participants in most of the included trials had skin and soft tissue infection caused by a wide range of pathogens. It would be useful if further studies identified the relevant pathogen(s) and compared key uncertainties in practice such as topical antibiotics versus topical antiseptics and topical antibiotics versus oral antibiotics. The timing of outcome assessments varied amongst the included trials. If future trials had similar follow‐up duration, this would enable more comparability amongst included studies. Further trials will strengthen data if the outcomes include quality of life measures and recurrence rates.
To improve the quality of the evidence, trials should ensure participants, study personnel, and outcome assessors are blinded to the intervention where this is possible. In addition, trials should undertake sample size calculations to ensure that sufficient participants are included to detect any differences between treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 24 March 2021 | Amended | Republished to fix some typos in the Plain Language Summary and Description of studies |
History
Protocol first published: Issue 8, 2018 Review first published: Issue 2, 2021
Notes
Republished to fix some typos in the Plain Language Summary and Description of studies
Acknowledgements
The Cochrane Skin editorial base wishes to thank Sue Jessop, Cochrane Dermatology Editor for this review; Ben Carter, Statistical Editor; Jeremy M Hugh, clinical referee; Nji Mbaka Fon, consumer referee, as well as another consumer referee who wishes to remain anonymous; Lisa Winer who copy‐edited the review; and Nicole Pitcher who wrote the plain language summary.
Appendices
Appendix 1. Cochrane Skin Specialised Register (Cochrane Register of Studies Web, CRSW)
1. (boil*):ti,ab. AND INREGISTER 2. MESH DESCRIPTOR furunculosis AND INREGISTER 3. (furuncle* or furunculos*):ti,ab. AND INREGISTER 4. MESH DESCRIPTOR folliculitis AND INREGISTER 5. folliculiti*:ti,ab. AND INREGISTER 6. MESH DESCRIPTOR Carbuncle AND INREGISTER 7. carbuncle*:ti,ab. AND INREGISTER 8. (sycosis or sycoses):ti,ab. AND INREGISTER 9. (hair* follicle*):ti,ab. AND INREGISTER 10. (infect* or swell* or pus* or abscess or inflam*):ti,ab. AND INREGISTER 11. #9 AND #10 12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #11
Appendix 2. CENTRAL (the Cochrane Library) search strategy
#1 boil?:ti,ab #2 [mh furunculosis] #3 (furuncle* or furunculos*):ti,ab #4 [mh folliculitis] #5 folliculiti*:ti,ab #6 [mh Carbuncle] #7 carbuncle*:ti,ab #8 (sycosis or sycoses):ti,ab #9 ((hair? and follicle*) and (infect* or swell* or pus* or abscess or inflam*)):ti,ab #10 {or #1‐#9}
Appendix 3. MEDLINE (Ovid) search strategy
1. boil$1.ti,ab. 2. Furunculosis/ 3. (furuncle$ or furunculos$).ti,ab. 4. Folliculitis/ 5. folliculiti$.ti,ab. 6. CARBUNCLE/ 7. carbuncle$.ti,ab. 8. (sycosis or sycoses).ti,ab. 9. (hair$1 adj3 follicle$ adj5 (infect$ or swell$ or pus$ or abscess or inflam$)).ti,ab. 10. or/1‐9 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. clinical trials as topic.sh. 16. randomly.ab. 17. trial.ti. 18. 11 or 12 or 13 or 14 or 15 or 16 or 17 19. exp animals/ not humans.sh. 20. 18 not 19 21. 10 and 20
[Lines 11‐20: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 4. Embase (Ovid) search strategy
1. furunculosis/ 2. boil$1.ti,ab. 3. (furuncle$ or furunculos$).ti,ab. 4. folliculitis/ 5. folliculiti$.ti,ab. 6. carbuncle/ 7. carbuncle$.ti,ab. 8. (sycosis or sycoses).ti,ab. 9. (hair$1 adj3 follicle$ adj5 (infect$ or swell$ or pus$ or abscess or inflam$)).ti,ab. 10. or/1‐9 11. crossover procedure.sh. 12. double‐blind procedure.sh. 13. single‐blind procedure.sh. 14. (crossover$ or cross over$).tw. 15. placebo$.tw. 16. (doubl$ adj blind$).tw. 17. allocat$.tw. 18. trial.ti. 19. randomized controlled trial.sh. 20. random$.tw. 21. or/11‐20 22. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 23. human/ or normal human/ 24. 22 and 23 25. 22 not 24 26. 21 not 25 27. 10 and 26
[Lines 11‐26: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 5. Data extraction form
| Study characteristics | Data to be extracted | Instruction for data extraction |
| Study ID | (Surname of first author and publication year of first full report of study) | |
| Study information | Study title | Enter the title of the study. |
| Methods | Randomisation methods | How is the randomisation sequence generated? |
| Blinding | Are participants, outcome assessors, or providers blinded to which treatment is given? | |
| Numbers of recruitment locations | At how many study sites are participants recruited for the trial? | |
| Participants | Inclusion criteria | Enter the characteristics that the participants must have in this trial. |
| Exclusion criteria | Enter the characteristics that the participants cannot have if enrolled in this trial. | |
| Numbers of participants randomised | How many participants were randomised in this trial? | |
| Mean age (years) | Enter the mean age ± SD of participants assigned to each group. | |
| Sex (% male) | Enter the percentage of male participants assigned to each group. | |
| Numbers of participants analysed | Data from how many participants are analysed in this trial? | |
| Numbers of dropouts | How many randomised participants are lost to follow‐up during the study period? | |
| Dropout reasons | What are the reasons for participant dropouts? | |
| Interventions | Types of interventions | Enter the types and methods of interventions, for example, topical antibiotics, antiseptic agents, systemic antibiotics, phototherapy, or surgical interventions. |
| Names of medications or methods | Enter the names of the interventions, such as the generic name of drugs. | |
| Dosage | Enter the dose and frequency for drugs. Enter the duration and frequency for phototherapy. For surgical intervention, enter 'N/A'. | |
| Duration | How long do participants receive therapy? | |
| Time point | When are the outcomes measured? | |
| Outcomes | Primary outcomes | Enter data on primary outcomes. |
| Secondary outcomes | Enter data on secondary outcomes. | |
Appendix 6. Trialists contacted for missing or unpublished data
| Study | Enquiries | Reply |
| Manaktala 2009 | We sent the following request on 2 Feb 2019.
|
No reply. |
| Murakawa 2007 | We sent the following request on 2 Feb 2019.
|
No reply. |
| Narayanan 2014a, Narayanan 2014b, and Narayanan 2014c | We sent the following request on 2 Feb 2019.
|
No reply. |
| CTRI/2018/03/012411 | We sent the following request on 28 Jul 2019.
|
No reply. |
| Dey 2015 | We sent the following request on 28 Jul 2019.
|
No reply. |
| EUCTR 2016‐005105‐39 | We sent the following request on 28 Jul 2019.
|
No reply. |
| Chosidow 2003 | We sent the following request on 28 Jul 2019.
|
Reply on 3 Sep 2019, as follows.
|
| Chen 2011 | We sent the following request on 9 Sep 2019.
|
No reply. |
| NCT01032499 | We sent the following request on 15 Sep 2019.
|
No reply. |
Data and analyses
Comparison 1. Ofloxacin gel versus norfloxacin gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Sisomicin ointment versus gentamicin ointment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Dieda Xiaoyan Gao ointment versus ichthammol ointment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Erythromycin versus flucloxacillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Cefadroxil versus flucloxacillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Severe adverse events leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Cefdinir versus cefalexin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Clinical cure | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 6.1.1 Follliculitis | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.84, 1.63] |
| 6.1.2 Furunculosis | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.22] |
| 6.2 Severe adverse events leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Azithromycin versus cefaclor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Clinical cure subgroup | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.1 Azithromycin 250 mg once daily for 3 days vs cefaclor 250 mg 3 times daily for 7 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.2 Azithromycin 500 mg once daily for 3 days vs cefaclor 250 mg 3 times daily for 7 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.3 Azithromycin 10 mg/kg once daily for 3 days vs cefaclor 20 mg/kg/day in 3 divided doses for 10 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2 Clinical cure | 2 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.72, 1.40] |
| 7.3 Minor adverse events not leading to withdrawal of treatment | 2 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.38, 4.17] |
Comparison 8. Ciprofloxacin versus pentoxifylline plus ciprofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2 Recurrence of folliculitis or boil following completion of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Fleroxacin versus amoxicillin/clavulanate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.2 Severe adverse events leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.3 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Cefditoren pivoxil versus cefaclor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.2 Severe adverse events leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.3 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. S‐1108 versus cefaclor .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.2 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. SY 5555 versus cefaclor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.2 Severe adverse events leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.3 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 13. Grepafloxacin versus ofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.2 Minor adverse events not leading to withdrawal of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 14. Co‐trimoxazole plus 8‐MOP and sunlight versus co‐trimoxazole plus placebo and sunlight.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Lesion‐free rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.1.1 Day 45 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.1.2 Day 90 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 15. Fire cupping plus penicillin versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 16. Wound packing versus no wound packing following incision and drainage .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Pain score (48 h post‐incision and drainage) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.2 Recurrence rate (1 month) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 17. Primary STSG versus delay STSG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Survival of STSG | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arata 1988.
| Study characteristics | ||
| Methods | A randomised, double‐blind controlled trial | |
| Participants | County: Japan Setting: hospitals (multicentre) Study periods: from December 1985 to September 1986 Inclusion criteria:
Exclusion criteria:
A total of 157 participants (78 in the somycin (SISO) group and 79 in the GM group) were enrolled, and the clinical efficacy data of 136 participants (80 of whom were male (38 in the SISO group and 42 in the GM group) and 56 female (26 in the SISO group and 30 in the GM group); age from 0 to over 70 years old) were analysed, including 38 folliculitis patients (16 in the SISO group and 22 in the GM group; 25 were male and 13 were female). |
|
| Interventions | Somycin (SISO) (0.1% sisomicin sulfate) group: 0.1% SISO was applied over lesions 2 to 3 times daily for 7 days Gentamicin (0.1%) group: 0.1% gentamicin was applied over lesions 2 to 3 times daily for 7 days |
|
| Outcomes |
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We randomly assign the cases; in Groups A and C, SISO and GM are 3 cases, 2 cases, 2 cases, 3 cases, and in group B, SISO and GM are 3 cases, 4 cases, 4 cases and 3 cases." (author's translation) Comment: the method of randomisation of each group was not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation table was strictly stored by the controller." (author's translation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The same base as the marketing 0.1% gentamicin ointment test agent (white Vaseline and main liquid paraffin) was used, and its appearance was like the test drug." (author's translation) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The blinded physicians assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention to treat data were unavailable, and the outcome efficacy analysis was according to the pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Arata 1993.
| Study characteristics | ||
| Methods | A randomised, double‐blind trial | |
| Participants | Country: Japan Setting: hospitals Study periods: March 1991 to January 1992 Inclusion criteria:
Exclusion criteria:
There were 159 participants (cefditoren pivoxil (CDTR‐PI) group: 83 cases, cefaclor (CCL): group 76 cases), of which 145 cases were included in the efficacy analysis (73 in the CDTR‐PI group (46 with furuncle or boils) and 72 in the CCL group (47 with furuncle or boils)). |
|
| Interventions | Cefditoren pivoxil (CDTR‐PI) group: CDTR‐PI 200 mg 3 times per day for 7 days Cefaclor (CCL) group: CCL 250 mg 3 times per day for 7 days | |
| Outcomes |
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were divided into several groups and each group included 6 patients with group 2 disease and 4 patient with group 4 disease. Then they were divided into CDRP‐PI and CCL group randomly; each group had 3 group 2 patients and 2 group 4 patients [who] received CDRP‐PI and the same numbers received CCL group." (author’s translation) Comment: the method of randomisation in each group was not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "The controller kept the key codes hermetically." (author’s translation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In the CDTR‐PI group, two CDTR‐PI 100 mg tablets and one CCL‐like placebo capsule as one package was taken as one dose. In the CCL group, one dose included one CCL 250 mg capsule and two CCTR‐PI‐like placebo tablets." (author's translation) Quote: article title: "A double blind, double‐dummy comparative study of cefditoren pivoxil versus cefaclor in treatment of skin and skin structure infections" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In the CDTR‐PI group, two CDTR‐PI 100 mg tablets and one CCL‐like placebo capsule as one package was taken as one dose. In the CCL group, one dose included one CCL 250 mg capsule and two CCTR‐PI‐like placebo tablets." (author's translation) Quote: "double‐blindness" (author's translation) Comment: the blinded physicians assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The ITT data were unavailable, and the outcome efficacy analysis was according to the PP data. |
| Selective reporting (reporting bias) | Low risk | All of the primary outcomes, "General Improvement Level", "Bacteriological examination", and "Accompanying symptoms", were reported. Adverse events were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Arata 1994a.
| Study characteristics | ||
| Methods | A randomised, double‐blind, multicentre clinical trial | |
| Participants | Country: Japan Setting: hospitals (35 centres) Study period: July 1991 to July 1992 Inclusion criteria:
Exclusion criteria:
A total of 193 participants received the drugs (98 in the S‐1108 group and 95 in the cefaclor group); 183 (94.8%) (95 in the S‐1108 group and 88 in the cefaclor group) were included in the efficacy analysis. Focusing on folliculitis and boils, 132 participants were included in the efficacy analysis, including 68 receiving S‐1108 and 64 receiving cefaclor. 189 (97.9%) (96 in the S‐1108 group and 93 in the cefaclor group) were included in the safety analysis. |
|
| Interventions |
The active medicines and matching placebo used in this study were manufactured by Shionogi Pharmaceutical Co Ltd. |
|
| Outcomes |
|
|
| Funding source | Shionogi Research Laboratories, Shionogi & Co Ltd | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Method of administration: participants were allocated to assigned medications according to the sequence of numbers." (author's translation) Comment: method of random sequence generation not explicitly reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignment of agents: the control (Koi Nakajima) were allocated through the probabilistic operation." (author's translation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "By the same drug appearance, it could not be identified exteriorly; we kept the dummy double‐blind method." (author's translation) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded physicians assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention to treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Arata 1994b.
| Study characteristics | ||
| Methods | A randomised, double‐blind, multicentre trial | |
| Participants | Country: Japan Setting: hospitals (36 centres) Study periods: July 1992 to February 1993 Inclusion criteria:
Exclusion criteria:
A total of 363 participants received study medications (161 in the S‐1108 group and 162 in the cefaclor group), with 295 (81.3%) (145 in the S‐1108 group and 150 in the cefaclor group) included in the efficacy analysis. Focusing on folliculitis and boils, 81 participants, ranging in age from 16 to 88 years, were included in the efficacy analysis, 40 taking SY555 and 41 taking cefaclor. 302 (83.2%) (149 in the S‐1108 group and 153 in the cefaclor group) were included in efficacy analysis. Group 2: 45 in the S‐1108 group and 42 in the cefaclor group |
|
| Interventions | SY 5555 group: SY 5555 200 mg and cefaclor placebo 3 times per day for 7 days
Cefaclor group: cefaclor 250 mg and SY 5555 placebo 3 times per day for 7 days The active medicine and placebo used in this study were manufactured by Shionogi Pharmaceutical Co., Ltd. |
|
| Outcomes |
|
|
| Funding source | Santrie Co., Ltd. and Yamanouchi Pharmaceutical Co., Ltd. | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each group included 4 participants, and two of them were divided into SY 5555 group and the others into cefaclor group randomly." (author's translation) Comment: method of random sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "The key code was sealed and stored by the controller." (author's translation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We kept the double blind method by double dummy." (author's translation) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether the outcome assessors were blinded was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention to treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Arata 1995a.
| Study characteristics | ||
| Methods | A randomised, double‐blind clinical trial | |
| Participants | Country: Japan Setting: hospitals (15 centres) Study periods: June to December 1993 Inclusion criteria:
Exclusion criteria:
A total of 76 participants (24 in the azithromycin (AZT) 250 mg (L) group, 25 in the AZT 500 mg (H) group, 27 in the cefaclor (C) group) were enrolled in this study, with 68 (89.5%) (22 in L, 22 in H, 24 in C groups) in efficacy analysis and 74 (97.4%) (24 in L, 24 in H, 26 in C groups) in safety analysis. Focusing on boils, 20 participants (7 in L, 4 in H, 9 in C groups) were included in efficacy analysis. |
|
| Interventions | L group: Azithromycin (AZT) 250 mg oral once daily for 3 days H group: AZT 500 mg oral once daily for 3 days C group: cefaclor 250 mg oral 3 times a day for 7 days |
|
| Outcomes |
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Six participants were defined as one group, then they were assigned to each drug group averagely and randomly." (author's translation) Comment: method of random sequence in the group was not mentioned. |
| Allocation concealment (selection bias) | Low risk | Quote: "The key code was sealed and stored by the controller." (author's translation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We kept double‐blind method by combining unidentified appearance placebo tablets; they maintained group L unidentifiable from group H." (author's translation) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "We kept double blind method by combining unidentified appearance placebo tablets; they maintained group L unidentifiable from group H." (author's translation) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention to treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Arata 1997.
| Study characteristics | ||
| Methods | A randomised, double‐blind, multicentre trial | |
| Participants | Country: Japan Setting: hospitals (20 centres) Study period: April 1993 to August 1994 Inclusion criteria:
Exclusion criteria:
A total of 227 participants received study medications (114 in the grepafloxacin group and 113 in the ofloxacin group); 209 (92.1%) completed the study (105 in the grepafloxacin group and 104 in the ofloxacin group) and were included in efficacy analysis. Focusing on folliculitis and boils, 138 participants were included in efficacy analysis, 69 taking grepafloxacin and 69 taking ofloxacin. |
|
| Interventions |
The active medicine and placebo used in this study were manufactured by Otsuka Pharmaceutical Co, Ltd. |
|
| Outcomes |
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Four cases were designed as one group, and the drugs were given by the drugs list in the order of the acceptable patients." (author’s translation) Comment: the methods of randomisation to groups were not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "The controller stored the key code until the end of the test." (author’s translation) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both drugs were different in shape and usage, so that two kinds of placebo tablets with the same appearance as each drug were created, [which] kept the double‐blind method adopted." (author’s translation) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both drugs were different in shape and usage, so that two kinds of placebo tablets with the same appearance as each drug were created, [which] kept the double‐blind method adopted." (author’s translation) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention‐to‐treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Baig 1988.
| Study characteristics | ||
| Methods | A randomised, open clinical trial | |
| Participants | Country: United Kingdom Setting: clinics Study periods: not mentioned Inclusion criteria:
Exclusion criteria:
A total of 86 participants with boils (44 in the erythromycin group and 42 in the flucloxacillin group; 46 male, 40 female) received medication, all of whom completed treatment. |
|
| Interventions | Erythromycin: 500 mg oral twice daily for 10 days Flucloxacillin: 250 mg oral 4 times daily for 10 days | |
| Outcomes | 1. Clinical presentation on day 1 and day 10
2. Adverse events during study period (including withdrawal or not due to adverse effects) |
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Both studies were randomised, open parallel group." Comment: the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Both studies were randomised, open parallel group" and "those with boils or caruncles were treated with either 500 mg bid erythromycin pellets or 250 mg qds flucloxacillin." Comment: this is a open trial with different frequency of drug intake. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Both studies were randomised, open parallel group" and "those with boils or caruncles were treated with either 500 mg bid erythromycin pellets or 250 mg qds flucloxacillin." Comment: unblinded physicians performed outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 86 participants (44 in the erythromycin group and 42 in the flucloxacillin group) received medication, all of whom completed treatment. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety prespecified outcomes were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Beitner 1996.
| Study characteristics | ||
| Methods | A randomised, single‐blind, multicentre trial | |
| Participants | Country: Sweden Setting: hospital Study periods: 18 December 1992 to 16 November 1994 Inclusion criteria:
Exclusion criteria:
A total of 661 participants, aged 3 to 81 years old, enrolled in the study, and 642 in the intention‐to‐treat analysis of efficacy; only 327 of them (41 with furunculosis, 21 taking cefadroxil and 20 taking flucloxacillin) were included in the primary analysis of efficacy, and 651 for adverse events assessment. |
|
| Interventions | Cefadroxil group: oral cefadroxil tablets or suspension 40 mg/kg to a maximum dose of 1 g once daily for 10 days Flucloxacillin group: oral flucloxacillin 750 mg tablets twice daily or suspension 30 to 50 mg/kg administered in 2 or 3 daily doses to a maximum dose of 1.5 g for 10 days |
|
| Outcomes |
|
|
| Funding source | Bristol‐Myers Squibb | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this prospective single‐blind, comparative and randomized, multicentre trial" Comment: the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The database was closed and [a] clean file declared on 7 December 1994." Comment: the method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "For 10 days one group took cefadroxil (Cefamox, Bristol‐Myers Squibb) tablets or suspension 40 mg/Kg to a maximum dose of 1g once daily, while the other group took flucloxacillin (Heracillin, Astra) 750 mg tablets twice daily or suspension 30‐50 mg/kg administered in two or three daily doses to a maximum dose of 1.5g." Comment: the frequency and brand of medicine differed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: there was no description of the blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention‐to‐treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and adverse events were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Giordano 2006.
| Study characteristics | ||
| Methods | A randomised, investigator‐blinded, multicentre study | |
| Participants | Country: United States of America Setting: hospitals Study periods: 25 March 2005 to 22 July 2005 Inclusion criteria:
Exclusion criteria:
392 participants with USSSI were randomised to receive the study drug, and 391 participants took at least 1 dose of the study drug (191 in the cefdinir group and 200 in the cefalexin group; 44 with folliculitis and 30 with furunculosis); 365 of them (including 34 with folliculitis (14 taking cefdinir and 20 taking cefalexin) and 27 with furunculosis (13 taking cefdinir and 14 taking cefalexin)) competed the study. |
|
| Interventions | Cefdinir group: cefdinir capsules 300 mg twice a day for 10 days Cefalexin group: cefalexin capsules 250 mg 4 times per day for 10 days (Keflex, Eli Lilly and Company, Indianapolis, USA) |
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Funding source | This study was sponsored by Abbott Laboratories. | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization schedule was used to assign patients in a 1:1 ratio." Comment: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...receive either cefdinir capsules 300 mg twice a day (BID) for 10 days (Omnicef, Abbott Laboratories, North Chicago, IL, USA) or cephalexin capsules 250 mg four times per day (QID) for 10 days (Keflex, Eli Lilly and Company, Indianapolis, IN, USA)." "Furthermore, the patient was instructed not to disclose any details about the study drug (e.g. dosing frequency, taste, appearance, or packaging) to the investigator." Comment: participants took different medicines at different frequencies and were not blinded; however, personnel did not obtain information about the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To maintain investigator blinding, the study drug was dispensed by an unblinded third person who did not participate in the assessments of clinical response. Furthermore, the patient was instructed not to disclose any details about the study drug (e.g. dosing frequency, taste, appearance, or packaging) to the investigator." Comment: the investigator was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three hundred and ninety‐two patients with USSSI were randomized to receive the study drug and 391 patients took at least one dose of the study drug (191 in the cefdinir treatment group and 200 in the cephalexin treatment group)." Comment: a total of 365 (93.3%) participants (180 in the cefdinir group and 185 in the cefalexin group) competed the study. |
| Selective reporting (reporting bias) | Low risk | Efficacy, safety and compliance outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Iyer 2013.
| Study characteristics | ||
| Methods | A randomised controlled trial | |
| Participants | Country: India Setting: hospital Study periods: June 2007 to June 2010 Inclusion criteria:
Exclusion criteria:
A total of 60 participants (38 male, 22 female) were enrolled in the study. 30 participants in the study group had a mean age of 54.6, and 30 participants in the control group had a mean age of 51.9. 56 participants completed the study (30 in the study group and 26 in the control group). |
|
| Interventions | In the study group:
In the control group:
|
|
| Outcomes | Primary outcome:
Secondary outcome:
|
|
| Funding source | Not reported | |
| Declarations of interest | It is not based upon any communication with any society/meeting. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients, who fulfilled the inclusion criteria for the study, were randomly allotted to the control group and the study group." Comment: the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: delayed STSG could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the method of blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data were analysed for most participants enrolled in the study (56/60, 93.3%). |
| Selective reporting (reporting bias) | High risk | Comment: adverse effects were not mentioned. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Jin 1995.
| Study characteristics | ||
| Methods | A randomised trial | |
| Participants | Country: China Setting: hospitals (4 centres) Study periods: July to September 1993 Inclusion criteria:
Exclusion criteria:
A total of 134 participants aged 6 to 65 years old were enrolled in this study, including 60 with folliculitis (30 in the ofloxacin group and 30 in the norfloxacin group; 42 were male and 18 were female). All participants completed the study. |
|
| Interventions |
|
|
| Outcomes | Clinical efficacy:
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Simple randomized method" (author's translation) Comment: method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants used different drugs. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: method of blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Kessler 2012.
| Study characteristics | ||
| Methods | A prospective, randomised, single‐blind clinical trial | |
| Participants | Country: United States of America Setting: hospital Study periods: over 15 months Inclusion:
Exclusion:
A total of 56 participants received intervention (27 in the experimental group and 29 in the placebo group); data from 49 participants (33 male, 16 female; 22 in the experimental group and 27 in the placebo group) were analysed. |
|
| Interventions | Experimental group: participants underwent a routine incision and drainage procedure and received wound packing. Placebo group: participants underwent a routine incision and drainage procedure but did not receive wound packing. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Funding source | NYU Langone Health. NCT00746109 | |
| Declarations of interest | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Once a subject consented, he/she was randomized to be in either the packed or nonpacked group using numbered opaque sealed envelopes that were arranged via a blocked randomization scheme in blocks of 4, 6, or 8." |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the intervention was receiving wound packing or not. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The masked physician was also given a test of blinding and asked to guess which group the subject was part of." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data were analysed from most of the participants who received interventions (49/56, 87.5%). |
| Selective reporting (reporting bias) | High risk | Comment: adverse events of wound packing were not included as an outcome. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Montero 1996.
| Study characteristics | ||
| Methods | A randomised, open‐label, multicentre controlled trial | |
| Participants | Country: Colombia, Guatemala, Panama, and South Africa Setting: hospitals Study periods: not mentioned Inclusion criteria:
Exclusion criteria:
Of the 100 children enrolled in each treatment group, 98 were evaluable for clinical efficacy in the azithromycin group and 98 in the cefaclor group. There were 11 participants with furuncles, of which 4 received azithromycin and 7 received cefaclor. |
|
| Interventions |
|
|
| Outcomes | Clinical efficacy:
Bacteriological efficacy:
Adverse events: those occurring during the study were recorded and classified as mild, moderate, or severe. |
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned in a 1:1 ratio to receive either azithromycin (cherry‐ or banana‐flavoured suspension containing 200 mg azithromycin/5 mL) or cefaclor (250 mg/5 mL) oral suspension." Comment: method was not described clearly. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Azithromycin was administered once daily at a dose of 10 mg/kg for 3 days 1 h [hour] before or 2 h after a meal. Cefaclor was administered at a total daily dosage of 20 mg/kg in divided doses 8 hourly, for 10 days, irrespective of meal times." Comment: frequency of administration of medicine differed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention‐to‐treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Parsad 1997.
| Study characteristics | ||
| Methods | A randomised controlled trial | |
| Participants | Country: India Setting: hospital Study periods: not reported Inclusion criteria: patients with chronic superficial folliculitis who had not received topical or systemic treatment Of the 38 participants (age range: 18 to 39 years (mean age: 22.5 years)) enrolled in the study, 18 in group I and 17 in group II were evaluated. |
|
| Interventions |
|
|
| Outcomes | Clinical response: grading the lesions at the end of the second week
Relapse of the lesions in 6 months Adverse events |
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to treatment groups equally." Comment: method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Group I was given combination of ciprofloxacin and placebo for two weeks followed by placebo for another 4 weeks whereas patients in group II were given combination of ciprofloxacin and pentoxifylline for two weeks followed by pentoxifylline for 4 weeks." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: method of blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention‐to‐treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Shenoy 1990.
| Study characteristics | ||
| Methods | A randomised controlled trial | |
| Participants | Country: India Setting: hospital Study periods: not mentioned Inclusion criteria: patients with chronic folliculitis of the legs Exclusion criteria: not mentioned Total: 45 participants (25 in the study group and 20 in the placebo group) received drug therapy, of which 26 (16 in the study group and 10 in the placebo group) were evaluated at day 90. |
|
| Interventions |
|
|
| Outcomes | Clinical efficacy: free of lesions on days 15, 45, and 90 | |
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty five of these patients selected randomly, in addition received 20 mg of 8‐MOP at 8 AM followed by exposure to sunlight from 10 AM to 10.15 AM." Comment: method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In the control group, 8 MOP was substituted with a colour, size and weight matched placebo made of lactose and starch." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This double‐blind in vivo and in vitro study was undertaken to assess the effectiveness of this regime." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 45 participants (25 in the study group and 20 in the placebo group) received drug therapy, of which 26 (57.8%; 16 in study group and 10 in placebo group) were evaluated on day 90. Comment: only 57.8% of participants were evaluated on day 90 for the efficacy outcome. |
| Selective reporting (reporting bias) | High risk | Efficacy outcomes were prespecified and reported, but there was no reporting of safety outcomes. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Tassler 1993.
| Study characteristics | ||
| Methods | A randomised, open‐label, multicentre trial | |
| Participants | Countries: Germany, Argentina, Austria, Brazil, Belgium, Finland, France, United Kingdom, and Italy Setting: hospitals Study periods: not mentioned Inclusion criteria:
Exclusion criteria:
A total of 285 participants (190 in the fleroxacin group and 95 in the amoxicillin/clavulanate potassium (AMX/CP) group) were enrolled in the study, of which 172 (60.4%; 115 in the fleroxacin group and 57 in the AMX/CP group) were evaluated for efficacy, and 284 (99.6%; 189 in the fleroxacin group and 95 in the AMX/CP group) were evaluated for safety. There were 7 participants with folliculitis: 5 taking fleroxacin, and 2 taking AMX/CP. |
|
| Interventions | Group A: fleroxacin 400 mg orally once daily for 4 to 21 days Group B: amoxicillin/clavulanate potassium (500 mg/125 mg) 3 times daily for 4 to 21 days | |
| Outcomes |
Followed up after 3 to 5 days of therapy and 3 to 9 days after completion of therapy for assessment of bacteriologic, clinical, and safety parameters |
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was designed as a prospective, randomized, open‐label, multicenter trial..." and "a total of 285 patients were randomized to treatment in a 2:1 ratio." Comment: the methods of random sequence generation were not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the methods of allocation were not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was designed as a prospective, randomized, open‐label, multicenter trial..." and "patients were allocated in consecutive order of study entry to receive either two 200‐mg fleroxacin tablets once daily or one tablet of AMX/CP (500mg/125mg) three times daily." Comment: frequency of administration of medicine differed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded physicians performed outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention‐to‐treat data were unavailable, and the outcome efficacy analysis was according to pre‐protocol data. |
| Selective reporting (reporting bias) | Low risk | Both efficacy and safety outcomes were prespecified and reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Xu 1992.
| Study characteristics | ||
| Methods | A randomised trial | |
| Participants | Country: China Setting: hospital Study periods: not mentioned Inclusion criteria: patient with carbuncles and furuncles Exclusion criteria: not mentioned A total of 60 participants (30 in the Dieda Xiaoyan Gao group and 30 in the Yushi Zhigao group) were enrolled. |
|
| Interventions | Dieda Xiaoyan Gao group: Dieda Xiaoyan Gao ointment applied over the infective site once daily for 10 days Yushi Zhigao group: ichthammol ointment applied over the infective site once daily for 10 days | |
| Outcomes | Clinical efficacy:
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Similar cases were divided into two groups randomly." (author's translation) Comment: method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the ointments differed in appearance and odour. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: method of blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | High risk | The efficacy outcomes were reported but not prespecified. Safety outcomes were not reported or prespecified. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Xu 1999.
| Study characteristics | ||
| Methods | A randomised clinical trial | |
| Participants | Country: China Setting: hospital Study periods: not mentioned Inclusion criteria: patient with pus‐furuncle Exclusion criteria: not mentioned A total of 260 participants (aged 3 to 65 years; mean age: 34 years) were enrolled in the study, 148 in group A and 112 in group B. 142 were male and 118 female. |
|
| Interventions | Group A: participants received fire cupping after pus stopping naturally after incision, and wound care after fire cupping. Participants received penicillin 800,000 U intramuscular injection twice a day. Group B: participants received incision for pus flowing out and wound care once or twice daily. Participants received penicillin 800,000 U intramuscular injection twice a day. | |
| Outcomes | Clinical efficacy:
|
|
| Funding source | Not reported | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned incision and drainage randomly" (author's translation) Comment: method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The procedure could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: method of blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the numbers of participants who withdrew were not mentioned. |
| Selective reporting (reporting bias) | High risk | The efficacy outcome was reported but not prespecified. Safety outcomes were not mentioned. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arata 1995b | Wrong population: we could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Arata 2005 | Wrong population: participants had acupuncture acne, acute suppurative psoriasis, diffuse infections, erysipelas, cellulitis, and lymphangitis. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Ballantyne 1982 | This was an open and double‐blind study of treatment of infection of skin and soft tissue with cefadroxil, not a randomised controlled trial. The outcome was overall clinical and bacteriological cure rate. |
| Banerjee 1975 | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Blaszczyk‐Kostanecka 1998 | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Bryant 1965 | This was a prevention study. Participants with recurrent furunculosis were included and used vaccine to prevent the furunculosis onset. |
| ChiCTR1800017342 | Participants with mastitis were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Chosidow 2003 | Participants with superficial pyodermas (impetigo or secondary infection of a recent wound, carbuncle, suppurative paronychia) were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| CTRI/2014/01/004283 | This was a single‐arm trial, not a randomised controlled trial. |
| Dey 2015 | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Ellis‐Grosse 2005 | Participants with complicated skin and skin‐structure infections (cSSSI) were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Goldfarb 1987 | There was only one participant with folliculitis; others had impetigo, cellulitis, adenitis, and abscess. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Ji 1997 | Participants with perifolliculitis capitis abscedens et suffodiens were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Kamme 1974 | This was not a randomised controlled trial. |
| Manaktala 2009 | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Murakawa 2007 | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Nakagawa 1991 | This was not a randomised controlled trial. |
| Narayanan 2014a | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Narayanan 2014b | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| Narayanan 2014c | This study did not provide subgroup data for bacterial folliculitis and boils participants. |
| NCT00388310 | Participants with abscesses greater than 3 cm in diameter were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| NCT01537783 | Participants with cutaneous abscesses were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| NCT02600871 | Participants with cellulitis and abscesses were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Neldner 1991 | Participants with conditions such as cellulitis, superficial skin infection, and abscesses were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Parish 1984 | Participants with skin and skin structure infections (SSSI) were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Prasad 1996 | This was not a randomised controlled trial. |
| RBR‐333g2h | Participants with complicated skin and soft tissue infection were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Scott 1958 | This was not a randomised controlled trial. |
| Tanioku 1975 | Participants with other infective disease over skin were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Umashankar 2018 | Participants with pyoderma were included in the study. We could not retrieve subgroup data for bacterial folliculitis and boils participants. |
| Watanabe 1985 | This was not a randomised controlled trial. |
Characteristics of studies awaiting classification [ordered by study ID]
Balachandran 1995.
| Methods | A double‐blind, cross‐over study |
| Participants | Patients with chronic folliculitis of the legs |
| Interventions | Ciprofloxacin or placebo |
| Outcomes | Average remission time |
| Notes |
Bernard 1997.
| Methods | A multicentric, randomised, double‐blind, double‐placebo study |
| Participants | Both sexes, age 15 to 80 years, clinical diagnosis of superficial pyoderma (impetigo, wound infection within the last 15 days, furunculosis, carbuncle, perionyxis), informed consent |
| Interventions |
|
| Outcomes | The efficacy and tolerance of pristinamycin were statistically equivalent to that of oxacillin for all participants with superficial pyoderma. |
| Notes |
Beurey 1975.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Only the study title was available. |
Bilen 1998.
| Methods | Randomised controlled trial |
| Participants | Patients with various bacterial skin infections |
| Interventions |
|
| Outcomes |
|
| Notes |
Carr 1994.
| Methods | A randomised, double‐blind study |
| Participants | 617 patients with skin and soft tissue infections |
| Interventions |
|
| Outcomes |
|
| Notes |
Chen 2011.
| Methods | A randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Of 220 patients screened, 200 were enrolled in the study. 100 participants were randomly assigned to receive cefalexin and 100 to receive clindamycin. |
| Interventions | Intervention 1 (cefalexin group): participants took cefalexin 40 mg/kg per day in divided doses administered 3 times per day. Intervention 2 (clindamycin group): participants took clindamycin 20 or 40 mg/kg per day in divided doses administered 3 times per day. |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Notes | Location: Johns Hopkins in the United States Sponsor: National Institutes of Health (NIH) |
Fujita 1982.
| Methods | A double‐blind comparative study |
| Participants | A total of 174 evaluable patients with superficial suppurative skin and soft tissue infections |
| Interventions |
|
| Outcomes | The results indicate that cefadroxil is superior to L‐cephalexin in the effectiveness and utility evaluation for the treatment of furuncle, furunculosis, and carbuncle, whilst no statistically significant differences between groups were demonstrated in other disease categories. |
| Notes |
Gomez 1968.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Only the study title was available. |
Li 1990.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Only the study title was available. |
Lobo 1995.
| Methods | A randomised, comparative trial |
| Participants | 30 patients with staphylococci (folliculitis, furunculosis) and streptococci pyodermitis (cellulitis, erysipela) |
| Interventions |
|
| Outcomes | Both antibiotics showed similar therapeutic efficacy, but the participants had an expressive preference for the single‐dose regimen, which decisively interfered with their adherence to the trial. Adverse reactions were not observed. |
| Notes |
Macedo De Souza 1995.
| Methods | A randomised, prospective and comparative clinical trial |
| Participants | 28 patients with cutaneous infection completed the evaluation, 14 in each treatment group |
| Interventions |
|
| Outcomes |
|
| Notes |
Mattsson 1982.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Only the study title was available. |
Moessinger 1976.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Only the study title was available. |
NCT01032499.
| Methods | Multicentre clinical study, phase III, prospective, randomised |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | A: 1 tablespoon (15 mL) of taro elixir taken orally 3 times daily for breakfast, lunch, and dinner B: oral oxytetracycline |
| Outcomes | Primary outcome measure:
Secondary outcome measure:
|
| Notes | Locations: Brazil
Sponsors and collaborators: Laboratorios Goulart S.A. |
Pereira 1996.
| Methods | A clinical, randomised, prospective and comparative trial |
| Participants |
A total of 31 patients older than 14 years participated in this evaluation, divided into 2 therapeutic groups: roxithromycin group (17 participants) and cefalexin group (14 participants). |
| Interventions |
|
| Outcomes |
|
| Notes |
Welsh 1987.
| Methods | A randomised clinical trial was conducted in 60 patients presenting with primary and secondary bacterial skin infections to compare the clinical and bacteriologic efficacy of mupirocin in a polyethylene glycol vehicle (Bactroban 2% topical) with that of oral ampicillin |
| Participants | 32 participants with primary and secondary bacterial skin infections |
| Interventions |
|
| Outcomes |
|
| Notes |
Characteristics of ongoing studies [ordered by study ID]
CTRI/2015/01/005361.
| Study name | Comparative efficacy, safety and tolerability of fixed dose combination of cephalexin extended release (375 mg) and clavulanate potassium (125 mg) tablets with cephalexin extended release (375 mg) tablets in the treatment of uncomplicated skin and soft tissue infection |
| Methods | Randomised, parallel‐group trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention:
Control:
|
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 15 January 2015 |
| Contact information | Name: Dr Upasana Pal Telephone: 01244194217 Email: dr.upasana.pal@rsunpharma.com |
| Notes | Country: India Sponsor: Sun Pharmaceutical Industries Ltd Site: not mentioned |
CTRI/2018/03/012411.
| Study name | The comparative study of nadifloxacin and mupirocin in children with skin and soft tissue infection |
| Methods | An open‐label, randomised, parallel‐group trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 19 July 2017 |
| Contact information | Name: Dr Swapnil Janbandhu Phone: 9665041290 Email: janbandhu.swapnil117@gmail.com |
| Notes | Country: India Sponsor: Dr Swapnil Janbandhu Site: Lifepoint Multispecialty Hospital |
EUCTR 2008‐006151‐42.
| Study name | A comparison of oral flucloxacillin alone with combined oral phenoxymethylpenicillin and flucloxacillin for the treatment of uncomplicated skin and soft tissue infections |
| Methods | A phase IV, double‐blinded, placebo‐controlled, prospective randomised controlled trial |
| Participants | Eligible patients will include those aged > 12 years of age with uncomplicated skin and/or skin structure infections that can be treated with antibiotics for a period of 7 to 10 days. Infections may include, but are not limited to, the following clinical descriptors:
Inclusion criteria:
Exlusion criteria:
|
| Interventions | Study group 1: flucloxacillin 500 mg oral Study group 2: phenoxymethylpenicillin 500 mg oral Placebo group: placebo 500 mg oral |
| Outcomes |
|
| Starting date | 17 December 2009 |
| Contact information | Not mentioned |
| Notes | Country: Ireland Sponsor: Beaumont Hospital Site: emergency department of Beaumont Hospital Dublin |
EUCTR 2016‐005105‐39.
| Study name | Investigation of the effectiveness tolerability and safety of ilon Salbe classic in the treatment of acute inflammation of the hair follicle |
| Methods | Prospective, open, randomised, placebo‐comparator controlled, multicentre trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: ilon Salbe classic, maximum 2‐centimetre cord of ointment, twice daily, for a maximum of 7 days Intervention 2: Vaselin Salbe LAW, 100%, maximum 2‐centimetre cord of ointment, twice daily, for a maximum of 7 days Intervention 3: Polysept Lösung (PVI), maximum 5 mL, twice daily, for a maximum of 7 days |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | March 2017 |
| Contact information | Name: Arbeitskreis Klinische Prfungen PD Dr med Seiler GmbH Phone: 00490761479400 Email: info@akp‐freiburg.de |
| Notes | Country: Germany Sponsor: Cesra Arzneimittel GmbH Co KG Site: multicentre |
NCT01281930.
| Study name | Abscess packing versus wick placement after incision and drainage |
| Methods | A randomised, parallel, triple‐blind (participant, care provider, outcomes assessor) trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: wick placement into abscess cavity
Active comparator: full packing of abscess cavity
|
| Outcomes | Primary outcome measure:
Secondary outcome measure:
|
| Starting date | June 2009 |
| Contact information | Washington University School of Medicine Site: St. Louis Children's Hospital, St. Louis, Missouri, USA, 63110 |
| Notes | Country: USA Sponsors: Washington University School of Medicine Site: St. Louis Children's Hospital, St. Louis, Missouri, USA, 63110 |
Differences between protocol and review
We removed the planned determination of overall risk of bias for each outcome in the protocol and used the GRADE approach to assess the certainty of evidence of each outcome.
We found no randomised controlled trials (RCTs) comparing topical antibiotics versus topical antiseptics; topical antibiotics versus systemic antibiotics; or phototherapy versus sham light, which were of interest in the protocol for this review. Most RCTs evaluated the differences between different topical antibiotics or different systemic antibiotics. We considered oral antibiotics, especially cephalosporins, as clinically important, and they are universal treatments for bacterial folliculitis and boils. We therefore included the following comparisons in 'Summary of findings' tables: cefadroxil versus flucloxacillin; cefdinir versus cefalexin; azithromycin versus cefaclor; and cefditoren pivoxil versus cefaclor.
In the Methods: a number of planned methods could not be carried out due to the limited number of included studies. These included expressing standardised mean differences for continuous data; hazard ratios for time‐to‐event data; methods for dealing with cluster, cross‐over, and split‐body RCTs; assessing statistical heterogeneity in the meta‐analyses; and conducting sensitivity and subgroup analyses.
In the Methods > Criteria for considering studies for this review > Types of outcome measures, we clarified that "If a trial reported data at multiple time points within the short‐ or long‐term timeframe, we chose the longest time point."
In the Methods > Data collection and analysis > Assessment of heterogeneity, following editorial advice, we reclassified an I2 of > 50% as at least moderate heterogeneity.
In the Methods > Data collection and analysis > Dealing with missing data: we contacted the authors of studies less than 10 years old to ask for missing data. Where data were unavailable, we conducted an intention‐to‐treat analysis to recalculate the intervention effect estimates; we included all randomised participants in the analysis and assumed that those with missing dichotomous outcome data experienced treatment failure. If the intention‐to‐treat data were unavailable, we carefully evaluated other important numerical data as randomised participants as well as per‐protocol population and as‐treated and described this in the 'Risk of bias' assessment.
No data were available for the following subgroup analyses as described in the protocol.
Paediatric versus adult participants (further divided into bacterial culture‐proven or clinical diagnosis only).
Immunocompetent versus immunosuppressed participants (further divided into bacterial culture‐proven or clinical diagnosis).
Methicillin‐sensitive Staphylococcus aureus (MSSA) versus methicillin‐resistant S aureus (MRSA) (including Panton‐Valentine leukocidin (PVL) gene type).
Different dosages of an intervention.
Contributions of authors
CC was the contact person with the editorial base. HL, CC co‐ordinated contributions from the co‐authors and wrote the final draft of the review. HL, PL, CC screened papers against eligibility criteria. YT obtained data on ongoing and unpublished studies. HL, PL, CC appraised the risk of bias of papers. HL, PL, CC extracted data for the review and sought additional information about papers. HL, PL, CC entered data into Review Manager 5. HL, PL, CC analysed and interpreted data. HL, PL, CC worked on the Methods sections. HL drafted the clinical sections of the Background and responded to the clinical comments of the referees. HL, PL, CC responded to the methodology and statistics comments of the referees. SW was the consumer co‐author and checked the review for readability and clarity, as well as ensuring that outcomes are relevant to consumers.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Huang‐Shen Lin: none known. Pei‐Tzu Lin: none known. Yu‐Shiun Tsai: none known. Shu‐Hui Wang: none known. Ching‐Chi Chi: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Arata 1988 {published data only}
- SG-Ointment Research Group. Clinical evaluation of sisomicin ointment in dermatological infections. Double blind comparison with gentamicin ointment. Skin Research 1988;30(2):262-80. [CENTRAL: CN-00351134] [DOI: 10.11340/skinresearch1959.30.262] [DOI] [Google Scholar]
Arata 1993 {published data only}
- Arata J, Akiyama H, Abe Y, Ishibashi Y, Takehara K, Kurose N, et al. A double blind comparative study of cefditoren pivoxil versus cefaclor in treatment of skin and skin structure infections. Chemotherapy 1993;41(1):57-77. [CENTRAL: CN-00351839] [DOI: 10.11250/chemotherapy1953.41.57] [DOI] [Google Scholar]
Arata 1994a {published data only}
- Arata J, Akiyama H, Abe Y, Ishibashi Y, Takehara K, Tsuchida T, et al. A double-blind comparative study of S-1108 and cefaclor in skin and skin structure infections. Chemotherapy 1994;42(3):326-45. [CENTRAL: CN-00181738] [DOI: 10.11250/chemotherapy1953.42.326] [DOI] [Google Scholar]
Arata 1994b {published data only}
- Arata J, Kanzaki H, Abe Y, Torigoe R, Ohkawara A, Yamanaka K, et al. A multicenter, double-blind, double-placebo comparative study of SY 5555 versus cefaclor in the treatment of skin and skin structure infections. Chemotherapy 1994;42(6):740-60. [CENTRAL: CN-00755286] [DOI: 10.11250/chemotherapy1953.42.740] [DOI] [Google Scholar]
Arata 1995a {published data only}
- Arata J, Shimoe T, Torigoe R, Ohkawara A, Koizumi H, Ishibashi Y, et al. Clinical dose-finding study on azithromycin in the treatment of skin and skin structure infections. Chemotherapy 1995;43(9):837-50. [CENTRAL: CN-00168727] [DOI: 10.11250/chemotherapy1995.43.836] [DOI] [Google Scholar]
Arata 1997 {published data only}
- Arata J, Matsuura Y, Umemura S, Nagao H, Katayama H, Miyoshi K, et al. A multicenter, double-blind, double-placebo comparative study of grepafloxacin versus ofloxacin in the treatment of skin and skin structure infections. Japanese Journal of Chemotherapy 1997;45(7):506-24. [CENTRAL: CN-00193152] [DOI: 10.11250/chemotherapy1995.45.506] [DOI] [Google Scholar]
Baig 1988 {published data only}
- Baig A, Grillage MG, Welch RB. A comparison of erythromycin and flucloxacillin in the treatment of infected skin lesions in general practice. British Journal of Clinical Practice 1988;42(3):110-5. [CENTRAL: CN-00057224] [PMID: ] [PubMed] [Google Scholar]
Beitner 1996 {published data only}
- Beitner H. Cefadroxil compared with flucloxacillin for skin and soft tissue infection. Journal of Dermatological Treatment 1996;7(3):143-6. [CENTRAL: CN-00168899] [DOI: 10.3109/09546639609086875] [DOI] [Google Scholar]
Giordano 2006 {published data only}
- Giordano PA, Elston D, Akinlade BK, Weber K, Notario GF, Busman TA, et al. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Current Medical Research and Opinion 2006;22(12):2419-28. [CENTRAL: CN-00577996] [DOI: 10.1185/030079906X148355] [DOI] [PubMed] [Google Scholar]
Iyer 2013 {published data only}
- Iyer SP, Kadam P, Gore MA, Subramaniyan P. Excision of carbuncle with primary split-thickness skin grafting as a new treatment modality. International Wound Journal 2013;10(6):697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 1995 {published data only}
- Jin PY, Wang HQ, Liu XQ, Li HZ. Clinical efficacy of folliculitis ofloxacin gel for external use. Journal of Clinical Dermatology 1995;24(1):25-7. [CENTRAL: CN-00454366] [Google Scholar]
Kessler 2012 {published data only}
- Kessler DO, Krantz A, Mojica M. Randomized trial comparing wound packing to no wound packing following incision and drainage of superficial skin abscesses in the pediatric emergency department. Pediatric Emergency Care 2012;28(6):514-7. [DOI: 10.1097/PEC.0b013e3182587b20.] [NCT00746109] [DOI] [PubMed] [Google Scholar]
Montero 1996 {published data only}
- Montero L. A comparative study of the efficacy, safety and tolerability of azithromycin and cefaclor in the treatment of children with acute skin and/or soft tissue infections. Journal of Antimicrobial Chemotherapy 1996;37(Suppl C):125-31. [CENTRAL: CN-00130338] [DOI: 10.1093/jac/37.suppl_c.125] [DOI] [PubMed] [Google Scholar]
Parsad 1997 {published data only}
- Parsad D, Saini R, Negi SK. Pentoxifylline and ciprofloxacin in chronic folliculitis of legs. Indian Journal of Dermatology, Venereology and Leprology 1997;63(1):9-10. [CENTRAL: CN-01095084] [PMID: ] [PubMed] [Google Scholar]
Shenoy 1990 {published data only}
- Shenoy K, Srinivas CR, Sharma S, Shivananda PG, Shetty JN. Efficacy of cotrimoxazole and PUVA for the management of chronic folliculitus of legs. Indian Journal of Dermatology, Venereology and Leprology 1990;56(3):223-5. [CENTRAL: CN-01095482] [Google Scholar]
Tassler 1993 {published data only}
- Tassler H. Comparative efficacy and safety of oral fleroxacin and amoxicillin/clavulanate potassium in skin and soft tissue infections. American Journal of Medicine 1993;94(3A):159S-65S. [CENTRAL: CN-00091754] [PMID: ] [PubMed] [Google Scholar]
Xu 1992 {published data only}
- Xu GM, Li CM. Comparison of the curative effect of Dieda Xiaoyan Gao and Yushi Zhigao on carbuncles and furuncles. Chinese Traditional Patent Medicine 1992;14(11):48. [CENTRAL: CN-00792201] [Google Scholar]
Xu 1999 {published data only}
- Xu SX. The application of cupping on treating pus-furuncle swelling. Journal of Dermatology and Venereology 1999;21(2):21-2. [CENTRAL: CN-00796087] [Google Scholar]
References to studies excluded from this review
Arata 1995b {published data only}
- Arata J, Torigoe R, Ohkawara A, Koizumi H, Sato H, Furuya K, et al. A multicenter, double-blind, double placebo clinical trial of azithromycin versus cefaclor in the treatment of skin and skin structure infections. Japanese Journal of Chemotherapy 1995;43(11):1069-87. [Google Scholar]
Arata 2005 {published data only}
- Arata J, Shimizu H, Watanabe S, Miyachi Y, Iwatsuki K, Furue M, et al. Clinical evaluation of telithromycin in patients with skin and soft tissue infections. Phase III double-blind comparative study of telithromycin versus cefdinir. Japanese Journal of Chemotherapy 2005;53(3):183-206. [Google Scholar]
Ballantyne 1982 {published data only}
- Ballantyne F. Cefadroxil in the treatment of skin and soft tissue infections. Journal of Antimicrobial Chemotherapy 1982;10(Suppl B):143-7. [DOI] [PubMed] [Google Scholar]
Banerjee 1975 {published data only}
- Banerjee BN, Mandal SB, Dutta AK. Evaluation of furacin-s (nitrofurazone and hydrocortisone acetate) in the treatment of different dermatoses. Indian Journal of Dermatology, Venereology and Leprology 1975;41(6):209-14. [Google Scholar]
Blaszczyk‐Kostanecka 1998 {published data only}
- Blaszczyk-Kostanecka M, Dobozy A, Dominguez-Soto L, Guerrero R, Hunyadi J, Lopera J, et al. Comparison of two regimens of oral clindamycin versus dicloxacillin in the treatment of mild-to-moderate skin and soft-tissue infections. Current Therapeutic Research, Clinical and Experimental 1998;59(6):341-53. [Google Scholar]
Bryant 1965 {published data only}
- Bryant RE, Sanford JP, Alcoze T. Treatment of recurrent furunculosis with staphylococcal bacteriophage-lysed vaccine. Journal of the American Medical Association 1965;194(1):11-4. [PubMed] [Google Scholar]
ChiCTR1800017342 {published data only}
- ChiCTR1800017342. Study on the application of moxibustion combined with the comprehensive nursing of traditional Chinese medicine to the initial fever of breast carbuncle. www.chictr.org.cn/showprojen.aspx?proj=29143 (first received 25 July 2018).
Chosidow 2003 {published data only}
- Chosidow O, Bernard P, Berbis P, Humbert P, Crickx B, Jarlier V, ORPIC Study Investigator Group. Cloxacillin versus pristinamycin for superficial pyodermas: a randomized, open-label, non-inferiority study. Dermatology 2005;210(4):370-4. [DOI] [PubMed] [Google Scholar]
- Chosidow O, Bernard P, Berbis P, Humbert P, Crickx B, Jarlier V. Cloxacillin vs. pristinamycin to treat superficial pyodermas: randomized non-inferiority open trial. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):191. [Google Scholar]
- Chosidow O, Bernard PH, Berbis PH, Humbert PH, Crickx B, Jarlier V. Cloxacillin vs. pristinamycin to treat superficial pyodermas: randomised non-inferiority open trial. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):185. [Google Scholar]
CTRI/2014/01/004283 {published data only}
- CTRI/2014/01/004283. To evaluate the safety and efficacy of Unani formulations in the treatment of pustules/boils. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=6359&EncHid=&modid=&compid=%27,%276359det%27 (first received 6 January 2014).
Dey 2015 {published and unpublished data}
- Dey SK, Das AK, Sen S, Hazra A. Comparative evaluation of 2 g single dose versus conventional dose azithromycin in uncomplicated skin and skin structure infections. Indian Journal of Pharmacology 2015;47(4):365-9. [DOI: 10.4103/0253-7613.161254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis‐Grosse 2005 {published data only}
- Ellis-Grosse EJ, Babinchak T, Dartois N, Rose G, Loh E. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clinical Infectious Diseases 2005;41(5 Suppl):S341-53. [DOI] [PubMed] [Google Scholar]
Goldfarb 1987 {published data only}
- Goldfarb J, Aronoff SC, Jaffe A, Reed MD, Blumer JL. Sultamicillin in the treatment of superficial skin and soft tissue infections in children. Antimicrobial Agents and Chemotherapy 1987;31:663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 1997 {published data only}
- Ji JM, Li JX, Gong YW. Study of folliculitis treated by ultraviolet rays. Chinese Journal of Physical Therapy 1997;20(1):44-6. [Google Scholar]
Kamme 1974 {published data only}
- Kamme C, Ursing B. Serum levels and clinical effect of flucloxacillin in patients with staphylococcal infections. Scandinavian Journal of Infectious Diseases 1974;6(3):273-8. [DOI] [PubMed] [Google Scholar]
Manaktala 2009 {published data only}
- Manaktala C, Singh AK, Verma M, Sachdeva A, Sharma H, Roy A, et al. Efficacy and tolerability of cefditoren pivoxil in uncomplicated skin and skin structure infections in Indian patients. Indian Journal of Dermatology 2009;54(4):350-6. [DOI: 10.4103/0019-5154.57612.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murakawa 2007 {published data only}
- Murakawa G, Yeung-Yue K, Luszczyk N. Effectiveness of oral cefdinir once daily versus oral cephalexin 4 times daily for the treatment of uncomplicated skin and skin structure infections. Journal of the American Academy of Dermatology 2007;56(2):AB121. [DOI: 10.1016/j.jaad.2006.10.567] [DOI] [Google Scholar]
Nakagawa 1991 {published data only}
- Nakagawa K, Hamada T, Tanii T, Kono T, Kitajima J, Chanoki M, et al. Oral administration of cefpodoxime proxetil (CPDX-PR, BANAN) in the treatment of bacterial skin disorders: a randomized clinical trial. Skin Research 1991;33(4):469-84. [Google Scholar]
Narayanan 2014a {published data only}
- Narayanan V, Motlekar S, Kadhe G, Bhagat S. Efficacy and safety of nadifloxacin for bacterial skin infections: results from clinical and post-marketing studies. Dermatology and Therapy 2014;4(2):233-48. [DOI: 10.1007/s13555-014-0062-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayanan 2014b {published data only}
- Narayanan V, Motlekar S, Kadhe G, Bhagat S. Efficacy and safety of nadifloxacin for bacterial skin infections: results from clinical and post-marketing studies. Dermatology and Therapy 2014;4(2):233-48. [DOI: 10.1007/s13555-014-0062-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayanan 2014c {published data only}
- Narayanan V, Motlekar S, Kadhe G, Bhagat S. Efficacy and safety of nadifloxacin for bacterial skin infections: results from clinical and post-marketing studies. Dermatology and Therapy 2014;4(2):233-48. [DOI: 10.1007/s13555-014-0062-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00388310 {published data only}
- NCT00388310. Effective antibiotic treatment of MRSA. clinicaltrials.gov/ct2/show/NCT00388310 (first received 16 October 2006).
NCT01537783 {published data only}
- NCT01537783. Evaluation of a staphylococcus eradication protocol for patients who present to the ED with cutaneous abscess. clinicaltrials.gov/ct2/show/NCT01537783 (first received 13 March 2017).
NCT02600871 {published and unpublished data}
- NCT02600871. Skin and soft tissue infection (SSTI) study. clinicaltrials.gov/ct2/show/nct02600871 (first received 27 March 2018).
Neldner 1991 {published data only}
- Neldner KH. Double-blind randomized study of oral temafloxacin and cefadroxil in patients with mild to moderately severe bacterial skin infections. American Journal of Medicine 1991;91(6A):111S-4S. [DOI] [PubMed] [Google Scholar]
Parish 1984 {published data only}
- Parish LC, Aten EM. Treatment of skin and skin structure infections: a comparative study of Augmentin and cefaclor. Cutis 1984;34(6):567-70. [PubMed] [Google Scholar]
Prasad 1996 {published data only}
- Prasad PVS. Rifampicin and dapsone in superficial pustular folliculitis. Indian Journal of Dermatology, Venereology and Leprology 1996;62(1):16-8. [PubMed] [Google Scholar]
RBR‐333g2h {published data only}
- RBR-333g2h. Trial of the safety and efficacy of Dalbavancin versus active comparator in children with skin Infections [A phase 3, multicenter, open-label, randomized, comparator controlled trial of the safety and efficacy of Dalbavancin versus active comparator in pediatric subjects with acute bacterial skin and skin structure infections]. apps.who.int/trialsearch/Trial2.aspx?TrialID=RBR-333g2h (first received 9 October 2017).
Scott 1958 {published data only}
- Scott A, Waterworth PM. Treatment of boils with erythromycin and with antibiotic E129. British Medical Journal 1958;2(5088):83-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanioku 1975 {published data only}
- Tanioku K. A double blind evaluation of triacetyloleandomycin and erythromycin estolate in the field of dermatology. A collaborative study. Chemotherapy 1975;23(10):3166-78. [Google Scholar]
Umashankar 2018 {published data only}
- Umashankar N, Pemmanda B, Gopkumar P, Hemalatha AJ, Sundar PK, Prashanth HV. Effectiveness of topical green tea against multidrug-resistant Staphylococcus aureus in cases of primary pyoderma: an open controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2018;84(2):163-8. [DOI] [PubMed] [Google Scholar]
Watanabe 1985 {published data only}
- Watanabe S, Takizawa K, Shimada S, Yamada K, Nakagawa H, Kukita A, et al. Clinical and bacteriological evaluation of TMS-19-Q in superficial suppurative skin and soft tissue infection. Japanese Journal of Antibiotics 1985;38(3):375-94. [PubMed] [Google Scholar]
References to studies awaiting assessment
Balachandran 1995 {published data only}
- Balachandran C, Malpani S, Srinivas CR. Ciprofloxacin therapy in chronic folliculitis of legs. Indian Journal of Dermatology, Venereology and Leprology 1995;61(4):212-3. [PubMed] [Google Scholar]
Bernard 1997 {published data only}
- Bernard P, Vaillant L, Martin C, Beylot C, Quentin R, Touron D. Pristinamycin versus oxacillin in the treatment of superficial pyoderma. A multicenter randomized study in 293 outpatients. Annales de Dermatologie et de Venereologie 1997;124(5):384-9. [PubMed] [Google Scholar]
Beurey 1975 {published data only}
- Beurey J, Centeleghe S. Clinical trial of an ointment containing fluocinonide with neomycin (topsyne neomycin). MedGenMed: Medscape General Medicine 1975;1(2):89-91. [Google Scholar]
Bilen 1998 {published data only}
- Bilen N, Apaydin R, Mutlu B, Bayramgurler D. Two different dose regimen of roxythromycin treatment in bacterial infections of skin. Turkderm Deri Hastaliklari ve Frengi Arsivi 1998;32(4):222-5. [Google Scholar]
Carr 1994 {published data only}
- Carr WD, Wall AR, Georgala-Zervogiani S, Stratigos J, Gouriotou K. Fusidic acid tablets in patients with skin and soft-tissue infection: a dose-finding study. European Journal of Clinical Research 1994;5:87-95. [Google Scholar]
Chen 2011 {published data only}
- Chen AE, Carroll KC, Diener-West M, Ross T, Ordun J, Goldstein MS, et al. Randomized controlled trial of cephalexin versus clindamycin for uncomplicated pediatric skin infections. Pediatrics 2011;127(3):e573-80. [DOI: 10.1542/peds.2010-2053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujita 1982 {published data only}
- Fujita K, Eiichiro N, Hisashi T, Yusho M, Masanobu K, Tohru F, et al. Clinical evaluation of cefadroxil in the treatment of superficial suppurative skin and soft tissue infections - a double-blind study comparing to L-cephalexin. Rinsho Hyoka (Clinical Evaluation) 1982;10(1):175-200. [Google Scholar]
Gomez 1968 {published data only}
- Gomez JR, Gomez G. Comparative trial of erythromycin and tetracycline in common infections found in general practice. British Journal of Clinical Practice 1968;22(11):475-7. [PubMed] [Google Scholar]
Li 1990 {published data only}
- Li FF, Ma XT, Qian BR. A clinical summary of bodkin to Du channel for furunculosis in 1426 cases. Zhen Jiu Xue Bao [Journal of Acupuncture] 1990;6(4):1-2. [Google Scholar]
Lobo 1995 {published data only}
- Lobo JM, Guedes MM. Treatment of bacterial skin infections. An open, randomized and comparative study among roxithromycin and cephalexin. Revista Brasileira de Medicina 1995;52(9):1045-51. [Google Scholar]
Macedo De Souza 1995 {published data only}
- Macedo De Souza E, Da Silva VMCF. Clinical study of efficacy, safety and adherence to treatment of skin infections with roxithromycin compared to cephalexin. Revista Brasileira de Medicina 1995;52(7):799-803. [Google Scholar]
Mattsson 1982 {published data only}
- Mattsson L, Rombo L, Holmgren B. Vaccination against furunculosis with Staphylococcal vaccine. No difference in therapeutic effect can be noted between vaccine and placebo. Lakartidningen 1982;79(9):743-4. [PubMed] [Google Scholar]
Moessinger 1976 {published data only}
- Moessinger P. Trial of hepar sulfuris calcareum in treatment of furuncles and pyodermia. Allgemeine Homoeopathische Zeitung 1976;221(4):137-46. [Google Scholar]
NCT01032499 {published and unpublished data}
- NCT01032499. Open and comparative study to measure tolerability and efficacy of taro elixir (E01GOU-INH0109). clinicaltrials.gov/ct2/show/NCT01032499 (first received 15 December 2009).
Pereira 1996 {published data only}
- Pereira LC. Comparative clinical study between roxithromycin and cephalexin in the treatment of folliculitis, furunculosis and erysipelas/cellulitis. Revista Brasileira de Medicina 1996;53(1-2):81-6. [Google Scholar]
Welsh 1987 {published data only}
- Welsh O, Saenz C. Topical mupirocin compared with oral ampicillin in the treatment of primary and secondary skin infections. Current Therapeutic Research, Clinical and Experimental 1987;41(1):114-20. [Google Scholar]
References to ongoing studies
CTRI/2015/01/005361 {published data only}
- CTRI/2015/01/005361. Comparative efficacy, safety and tolerability of fixed dose combination of Cephalexin extended release (375 mg) and Clavulanate Potassium (125 mg) tablets with Cephalexin extended release (375 mg) tablets in the treatment of uncomplicated skin and soft tissue infection. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=9459 (first received 6 January 2015).
CTRI/2018/03/012411 {published data only}
- CTRI/2018/03/012411. The comparative study of Nadifloxacin and Mupirocin in children with skin and soft tissue infection. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/03/012411 (first received 7 March 2018).
EUCTR 2008‐006151‐42 {published data only}
- EUCTR 2008-006151-42. A comparison of oral flucloxacillin alone with combined oral phenoxymethylpenicillin and flucloxacillin for the treatment of uncomplicated skin and soft tissue infection. www.clinicaltrialsregister.eu/ctr-search/trial/2008-006151-42/IE (first received 10 December 2008).
EUCTR 2016‐005105‐39 {published data only}
- EUCTR 2016-005105-39. Investigation of the effectiveness tolerability and safety of ilon Salbe classic in the treatment of acute inflammation of the hair follicle folliculitis - prospective open randomized placebo-comparator controlled multicenter trial. www.clinicaltrialsregister.eu/ctr-search/trial/2016-005105-39/DE (first received 3 March 2017).
NCT01281930 {published data only}
- NCT01281930. Abscess packing versus wick placement after incision and drainage. clinicaltrials.gov/ct2/show/NCT01281930 (first received 24 January 2011).
Additional references
Ahmad 2017
- Ahmad H, Siddiqui SS. An unusually large carbuncle of the temporofacial region demonstrating remarkable post-debridement wound healing process: a case report. Wounds 2017;29(4):92-5. [PMID: ] [PubMed] [Google Scholar]
Al‐Saeed 2006
- Al-Saeed WY, Al-Dawood KM, Bukhari IA, Bahnassy AA. Prevalence and pattern of skin disorders among female school children in Eastern Saudi Arabia. Saudi Medical Journal 2006;27(2):227-34. [PMID: ] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Yitter Y, Flottorp G, et al GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI: 10.1136/bmj.328.7454.1490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Böni 2003
- Böni R, Nehrhoff B. Treatment of gram-negative folliculitis in patients with acne. American Journal of Clinical Dermatology 2003;4(4):273-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Bulat 2011
- Bulat V, Situm M, Dediol I, Ljubicić I, Bradić L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Collegium Antropologicum 2011;35(Suppl 2):147-51. [PMID: ] [PubMed] [Google Scholar]
Chi 2015
- Chi CC, Ko SH, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD011972. [DOI: 10.1002/14651858.CD011972] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 1 February 2018. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Craft 2012
- Craft N. Chapter 176. Superficial cutaneous infections and pyodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors(s). Fitzpatrick's Dermatology in General Medicine. 8th edition. New York: McGraw-Hill Companies, Inc, 2012:2134-6. [ISBN: 978-0-07-171755-7] [Google Scholar]
Davido 2013
- Davido B, Dinh A, Salomon J, Roux AL, Gosset-Woimant M, Pierre I, et al. Recurrent furunculosis: efficacy of the CMC regimen - skin disinfection (chlorhexidine), local nasal antibiotic (mupirocin), and systemic antibiotic (clindamycin). Scandinavian Journal of Infectious Diseases 2013;45(11):837-41. [DOI: 10.3109/00365548.2013.810815] [DOI] [PubMed] [Google Scholar]
Dufour 2002
- Dufour P, Gillet Y, Bes M, Lina G, Vandenesch F, Floret D, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clinical Infectious Diseases 2002;35(7):819-24. [DOI: 10.1086/342576] [DOI] [PubMed] [Google Scholar]
Fisher 2008
- Fisher RG, Chain RL, Hair PS, Cunnion KM. Hypochlorite killing of community-acquired methicillin-resistant Staphyloccus aureus. Pediatric Infectious Disease Journal 2008;27(10):934-5. [DOI: 10.1097/INF.0b013e318175d871] [DOI] [PubMed] [Google Scholar]
Frosini 2017
- Frosini SM, Bond R, Loeffler A, Larner J. Opportunities for topical antimicrobial therapy: permeation of canine skin by fusidic acid. BMC Veterinary Research 2017;13(1):345. [DOI: 10.1186/s12917-017-1270-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2018
- Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT, editor(s). Sanford Guide to Antimicrobial Therapy. 48th edition. Sperryville, VA: Antimicrobial Therapy, Inc., 2018. [Google Scholar]
Global Burden of Disease
- Institute for Health Metrics and Evaluation. Global Burden of Disease (GBD). vizhub.healthdata.org/gbd-compare/ (accessed 18 March 2018).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 14 July 2020. Available at gradepro.org.
Gunatheesan 2018
- Gunatheesan S. Folliculitis. www.dermcoll.edu.au/atoz/folliculitis/ (accessed 13 June 2018).
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Ibler 2014
- Ibler KS, Kromann CB. Recurrent furunculosis - challenges and management: a review. Clinical, Cosmetic and Investigational Dermatology 2014;2014(7):59-64. [DOI: 10.2147/CCID.S35302] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 1994
- Inoue E, Mitsuhashi S. In vitro antibacterial activity and beta-lactamase stability of SY5555, a new oral penem antibiotic. Antimicrobial Agents and Chemotherapy 1994;38(9):1974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawashima 2017
- Kawashima M, Nagare T, Doi M. Clinical efficacy and safety of benzoyl peroxide for acne vulgaris: comparison between Japanese and Western patients. Journal of Dermatology 2017;44(11):1212-8. [DOI: 10.1111/1346-8138.13996] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khilji 2002
- Khilji FA, Gonzalez M, Finlay AY. Clinical meaning of change in Dermatology Life Quality Index scores. British Journal of Dermatology 2001;147(Suppl 62):50. [DOI: 10.1046/j.1365-2133.147.s62.16.x] [DOI] [Google Scholar]
Koning 2002
- Koning S, Suijlekom-Smit LW, Nouwen JL, Verduin CM, Bernsen RM, Oranje AP, et al. Fusidic acid cream in the treatment of impetigo in general practice: double blind randomised placebo controlled trial. BMJ 2002;324(7331):203-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lally 2011
- Lally A, Casabonne D, Imko-Walczuk B, Newton R, Wojnarowska F. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. Journal of the European Academy of Dermatology and Venereology 2011;25(4):462-70. [DOI: 10.1111/j.1468-3083.2010.03814.x] [DOI] [PubMed] [Google Scholar]
Laureano 2014
- Laureano AC, Schwartz RA, Cohen PJ. Facial bacterial infections: folliculitis. Clinics in Dermatology 2014;2(6):711-4. [DOI: 10.1016/j.clindermatol.2014.02.009] [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez FA, Lartchenko S. Skin and soft tissue infections. Infectious Disease Clinics of North America 2006;20(4):759-72. [DOI: 10.1016/j.idc.2006.09.006] [DOI] [PubMed] [Google Scholar]
Micromedex 2018
- DRUGDEX®. IBM Micromedex® (electronic version). www.micromedexsolutions.com/ (accessed 13 June 2018).
Nagaraju 2004
- Nagaraju U, Bhat G, Kuruvila M, Ganesh SP, et al. Methicillin-resistant Staphylococcus aureus in community-acquired pyoderma. International Journal of Dermatology 2004;43(6):412-4. [DOI: 10.1111/j.1365-4632.2004.02138.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nisticò 2009
- Nisticò SP, Saraceno R, Carboni I, Chimenti S. Treatment of folliculitis with monochromatic excimer light (308 nm). Dermatology 2009;218(1):33-6. [DOI: 10.1159/000165627] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Dell 1998
- O'Dell ML. Skin and wound infections: an overview. American Family Physician 1998;57(10):2424-32. [PMID: 9614412] [PubMed] [Google Scholar]
Pasternack 2015
- Pasternack MS, Swartz MN. Cellulitis, necrotizing fasciitis, and subcutaneous tissue infections. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th edition. Vol. 95. St. Louis, MO: Elsevier, 2015:1194-215. [Google Scholar]
Petrofsky 2009
- Petrofsky JS, Bains G, Raju C, Lohman E, Berk L, Prowse M, et al. The effect of the moisture content of a local heat source on the blood flow response of the skin. Archives of Dermatology Research 2009;301(8):581-5. [DOI: 10.1007/s00403-009-0957-3] [DOI] [PubMed] [Google Scholar]
Rao 1992
- Rao JNK, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48(2):577-85. [DOI: 10.2307/2532311] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2011
- Schunemann HJ, Oxman AD, Higgins JP, Vist GE, Glazziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings’ tables. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from training.cochrane.org/handbook/archive/v5.1/.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shallcross 2015
- Shallcross LJ, Hayward AC, Johnson AM, Petersen I. Evidence for increasing severity of community-onset boils and abscesses in UK general practice. Epidemiology and Infection 2015;143(11):2426-9. [DOI: 10.1017/S0950268814003458] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shehab 2008
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clinical Infectious Diseases 2008;47(6):735-43. [DOI: 10.1086/591126] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Totsuka 1992
- Totsuka K, Shimizu K, Konishi M, Yamamoto S. Metabolism of S-1108, a new oral cephem antibiotic, and metabolic profiles of its metabolites in humans. Antimicrobial Agents and Chemotherapy 1992;36(4):757-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2017
- Tran K, Wright MD. Topical antibiotics for infected dermatitis: a review of the clinical effectiveness and guidelines (CADTH rapid response report: summary with critical appraisal). www.cadth.ca/topical-antibiotics-infected-dermatitis-review-clinical-effectiveness-and-guidelines (accessed 18 March 2018). [PMID: ] [PubMed]
Wahba‐Yahav 1992
- Wahba-Yahav AV. Intractable chronic furunculosis: prevention of recurrences with pentoxifylline. Acta Dermato-venereologica 1992;72(6):461-2. [PMID: ] [PubMed] [Google Scholar]
WebPlotDigitizer 2017 [Computer program]
- WebPlotDigitizer. Austin, TX: Ankit Rohatgi, 2017. Available at automeris.io/WebPlotDigitizer.
Zacherle 1982
- Zacherle BJ, Silver DS. Hot tub folliculitis: a clinical syndrome. Western Journal of Medicine 1982;137(3):191-4. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lin 2018
- Lin HS, Lin PT, Tsai YS, Wang SH, Chi CC. Interventions for bacterial folliculitis and boils (furuncles and carbuncles). Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD013099. [DOI: 10.1002/14651858.CD013099] [DOI] [PMC free article] [PubMed] [Google Scholar]


