Abstract
Background
Alcohol is consumed by over 2 billion people worldwide. It is a common substance of abuse and its use can lead to more than 200 disorders including hypertension. Alcohol has both acute and chronic effects on blood pressure. This review aimed to quantify the acute effects of different doses of alcohol over time on blood pressure and heart rate in an adult population.
Objectives
Primary objective
To determine short‐term dose‐related effects of alcohol versus placebo on systolic blood pressure and diastolic blood pressure in healthy and hypertensive adults over 18 years of age.
Secondary objective
To determine short‐term dose‐related effects of alcohol versus placebo on heart rate in healthy and hypertensive adults over 18 years of age.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to March 2019: the Cochrane Hypertension Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2), in the Cochrane Library; MEDLINE (from 1946); Embase (from 1974); the World Health Organization International Clinical Trials Registry Platform; and ClinicalTrials.gov. We also contacted authors of relevant articles regarding further published and unpublished work. These searches had no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) comparing effects of a single dose of alcohol versus placebo on blood pressure (BP) or heart rate (HR) in adults (≥ 18 years of age).
Data collection and analysis
Two review authors (ST and CT) independently extracted data and assessed the quality of included studies. We also contacted trial authors for missing or unclear information. Mean difference (MD) from placebo with 95% confidence interval (CI) was the outcome measure, and a fixed‐effect model was used to combine effect sizes across studies.
Main results
We included 32 RCTs involving 767 participants. Most of the study participants were male (N = 642) and were healthy. The mean age of participants was 33 years, and mean body weight was 78 kilograms.
Low‐dose alcohol (< 14 g) within six hours (2 RCTs, N = 28) did not affect BP but did increase HR by 5.1 bpm (95% CI 1.9 to 8.2) (moderate‐certainty evidence).
Medium‐dose alcohol (14 to 28 g) within six hours (10 RCTs, N = 149) decreased systolic blood pressure (SBP) by 5.6 mmHg (95% CI ‐8.3 to ‐3.0) and diastolic blood pressure (DBP) by 4.0 mmHg (95% CI ‐6.0 to ‐2.0) and increased HR by 4.6 bpm (95% CI 3.1 to 6.1) (moderate‐certainty evidence for all).
Medium‐dose alcohol within 7 to 12 hours (4 RCTs, N = 54) did not affect BP or HR.
Medium‐dose alcohol > 13 hours after consumption (4 RCTs, N = 66) did not affect BP or HR.
High‐dose alcohol (> 30 g) within six hours (16 RCTs, N = 418) decreased SBP by 3.5 mmHg (95% CI ‐6.0 to ‐1.0), decreased DBP by 1.9 mmHg (95% CI‐3.9 to 0.04), and increased HR by 5.8 bpm (95% CI 4.0 to 7.5). The certainty of evidence was moderate for SBP and HR, and was low for DBP.
High‐dose alcohol within 7 to 12 hours of consumption (3 RCTs, N = 54) decreased SBP by 3.7 mmHg (95% CI ‐7.0 to ‐0.5) and DBP by 1.7 mmHg (95% CI –4.6 to 1.8) and increased HR by 6.2 bpm (95% CI 3.0 to 9.3). The certainty of evidence was moderate for SBP and HR, and low for DBP.
High‐dose alcohol ≥ 13 hours after consumption (4 RCTs, N = 154) increased SBP by 3.7 mmHg (95% CI 2.3 to 5.1), DBP by 2.4 mmHg (95% CI 0.2 to 4.5), and HR by 2.7 bpm (95% CI 0.8 to 4.6) (moderate‐certainty evidence for all).
Authors' conclusions
High‐dose alcohol has a biphasic effect on BP; it decreases BP up to 12 hours after consumption and increases BP > 13 hours after consumption. High‐dose alcohol increases HR at all times up to 24 hours. Findings of this review are relevant mainly to healthy males, as only small numbers of women were included in the included trials.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Young Adult; Alcohol Drinking; Alcoholic Beverages; Bias; Blood Pressure; Blood Pressure/drug effects; Central Nervous System Depressants; Central Nervous System Depressants/administration & dosage; Central Nervous System Depressants/pharmacology; Cross-Over Studies; Ethanol; Ethanol/administration & dosage; Ethanol/pharmacology; Heart Rate; Heart Rate/drug effects; Randomized Controlled Trials as Topic; Sex Factors; Time Factors
Plain language summary
Alcohol has a biphasic effect on blood pressure and increases heart rate
Review question
We reviewed available evidence about the short‐term effects of different doses of alcoholic drinks compared to non‐alcoholic drinks on blood pressure and heart rate in adults (≥ 18 years) with both normal and raised blood pressure.
Background
Drinking excessive alcohol is considered one of the most common causes of raised blood pressure. We wanted to quantify the effects of a single dose of alcohol on blood pressure and heart rate within 24 hours of consumption.
Study characteristics
We included 32 randomised controlled trials involving 767 participants published up to March 2019. Although these trials included adults from 18 to 96 years of age with various health conditions, most study participants were young healthy males. The source of funding was not reported for a majority of the studies.
Key results
For low doses of alcohol, we found that one glass of alcohol had little to no effect on blood pressure and increased heart rate within six hours of drinking.
We are moderately certain that medium‐dose alcohol decreased blood pressure and increased heart rate within six hours of consumption. We did not see any significant change in blood pressure or heart rate after that, but the evidence was limited.
We are also moderately certain that high‐dose alcohol decreased blood pressure within six hours, and the effect lasted up to 12 hours. After that, blood pressure was found to be increased. Heart rate increased significantly after alcohol consumption and remained increased at all times measured.
Thus alcohol decreases blood pressure initially (up to 12 hours after ingestion) and increases blood pressure after that. Alcohol consistently increases heart rate at all times within 24 hours of consumption.
Summary of findings
Summary of findings 1. Effect of high‐dose alcohol compared to placebo .
| Effect of high‐dose alcohol compared to placebo | ||||
| Patient or population: adult participants Setting: ambulatory Intervention: high‐dose alcohol (> 30 g) Comparison: placebo | ||||
| Outcomes | Participants (RCTs) | Certainty of the evidence (GRADE) | Mean difference of high‐dose alcohol compared to placebo* (95% CI) | |
| Systolic blood pressure ‐ ≤ 6 hours | 418 (16) | ⊕⊕⊕⊝ Moderatea | ‐3.5 mmHg [‐6 to ‐0.5] | |
| Systolic blood pressure ‐ 7 to 12 hours | 54 (3) | ⊕⊕⊕⊝ Moderatea | ‐3.7 mmHg [‐6.9 to ‐0.5] | |
| Systolic blood pressure ‐ ≥ 13 hours | 154 (4) | ⊕⊕⊕⊝ Moderatea | 3.7 mmHg [2.3 to 5] | |
| Diastolic blood pressure ‐ ≤ 6 hours | 350 (14) | ⊕⊕⊝⊝ Lowa,b | ‐1.9 mmHg [‐3.9 to 0.04] | |
| Diastolic blood pressure ‐ 7 to 12 hours | 54 (5) | ⊕⊕⊝⊝ Lowa,b | ‐1.6 mmHg [‐4.1 to 0.9] | |
| Diastolic blood pressure ‐ ≥ 13 hours | 154 (4) | ⊕⊕⊕⊝ Moderatea | 2.4 mmHg [0.3 to 4.5] | |
| Heart rate ‐ ≤ 6 hours | 495 (17) | ⊕⊕⊕⊝ Moderatea | 5.5 bpm [4.3 to 6.7] | |
| Heart rate ‐ 7 to 12 hours | 144 (7) | ⊕⊕⊕⊝ Moderatea | 6.2 bpm [3 to 9.3] | |
| Heart rate ‐ ≥ 13 hours | 244 (8) | ⊕⊕⊕⊝ Moderatea | 2.7 bpm [0.8 to 4.6] | |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aUnclear risk of selection bias and attrition bias in more than one study.
b95% confidence interval around the best effect estimate includes both negligible effect and appreciable benefit.
Summary of findings 2. Effect of medium‐dose alcohol compared to placebo.
| Effect of medium‐dose alcohol compared to placebo | |||
| Patient or population: adult participants Setting: ambulatory Intervention: medium‐dose alcohol (15 to 30 g) Comparison: placebo | |||
| Outcomes | Participants (RCTs) | Certainty of the evidence (GRADE) | Mean difference of medium‐dose alcohol compared to placebo* (95% CI) |
| Systolic blood pressure ‐ ≤ 6 hours | 149 (10) | ⊕⊕⊕⊝ Moderatea | ‐5.63 mmHg [‐8.3 to ‐3] |
| Systolic blood pressure ‐ 7 to 12 hours | 54 (4 ) | ⊕⊕⊝⊝ Lowa,b,c | ‐3.2 mmHg [‐8.4 to 2] |
| Systolic blood pressure ‐ ≥ 13 hours | 66 (5) | ⊕⊕⊝⊝ Lowa,b | 0.6 mmHg [‐3.9 to 5.2] |
| Diastolic blood pressure ‐ ≤ 6 hours | 149 (10) | ⊕⊕⊕⊝ Moderatec | ‐4 mmHg [‐6 to ‐2] |
| Diastolic blood pressure ‐ 7 to 12 hours | 54 (4) | ⊕⊕⊝⊝ Lowa,b | ‐1.2 mmHg [‐4.3 to 1.9] |
| Diastolic blood pressure ‐ ≥ 13 hours | 66 (5) | ⊕⊕⊕⊝ Moderateb | 1.8 mmHg [‐0.9 to 4.5] |
| Heart rate ‐ ≤ 6 hours | 181 (12) | ⊕⊕⊕⊝ Moderatec | 4.6 bpm [3.1 to 6.1] |
| Heart rate ‐ 7 to 12 hours | 54 (4) | ⊕⊕⊝⊝ Lowa,b | 1.2 bpm [‐1.9 to 4.3] |
| Heart rate ‐ > 13 hours | 36 (3) | ⊕⊕⊝⊝ Lowa,b | 1.4 bpm [‐2.1 to 4.9] |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | |||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
aUnclear risk of selection bias in more than one study.
b95% confidence interval around the effect estimate includes both appreciable benefit and appreciable harm.
cUnclear risk of selection bias and attrition bias in more than one study.
Summary of findings 3. Effect of low‐dose alcohol compared to placebo.
| Effect of low‐dose alcohol compared to placebo | |||
| Patient or population: adult participants Setting: ambulatory Intervention: low‐dose alcohol (≥ 14 g) Comparison: placebo | |||
| Outcomes | Participants (RCTs) | Certainty of the evidence (GRADE) | Mean difference of low‐dose alcohol compared to placebo* (95% CI) |
| Systolic blood pressure ‐ ≤ 6 hours | 28 (2) | ⊕⊕⊝⊝ Lowa,b | ‐1.9 mmHg [‐8.4 to 5.4] |
| Diastolic blood pressure ‐ ≤ 6 hours | 28 (2 ) | ⊕⊕⊝⊝ Lowa,b | ‐1.5 mmHg [‐6.9 to 4] |
| Heart rate ‐ ≤ 6 hours | 28 (2) | ⊕⊕⊕⊝ Moderatea | 5.1 bpm [1.88 higher to 8.24] |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | |||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
aUnclear risk of selection bias.
b95% confidence interval around the best effect estimate includes both negligible effect and appreciable benefit.
Background
Description of the condition
Hypertension, or elevated blood pressure, is commonly defined as resting systolic blood pressure (SBP) above 140 mmHg or resting diastolic blood pressure (DBP) above 90 mmHg ‐ or both ‐ assuming that the person is not taking any antihypertensive medication (Poulter 2015). It is one of the most common health conditions; hypertension has an increased prevalence with increasing age and affects up to 31% of the world’s adult population (Mills 2016). Sustained hypertension is associated with increased risk of stroke, myocardial infarction, heart failure, renal failure, blindness, and cognitive impairment (Kannel 1972; WHO 2013). In 2015, approximately 10.7 million deaths around the world were estimated to be attributable to hypertension‐related health complications (GBD 2015).
Hypertension can be genetic or may be due to environmental factors such as poor diet, obesity, tobacco use, excessive alcohol consumption, and sedentary lifestyle (Weber 2014; WHO 2013). A population‐based study showed that the incidence of hypertension is higher in African descendants (36%) than in Caucasians (21%) (Willey 2014). Proper management of hypertension can lead to reduction in cardiovascular complications and mortality (Kostis 1997; SHEP 1991; Staessen 1999). Although pharmacological interventions can effectively reduce blood pressure, multiple studies have shown that a healthy lifestyle alone without any pharmacological interventions can greatly reduce the prevalence of hypertension (Appel 2003; Guitteau 2006). According to US guidelines for prevention, detection, evaluation, and management of high blood pressure, adults are advised to reduce weight and sodium intake, increase physical activity and potassium intake, cease smoking, and moderate alcohol intake to manage hypertension non‐pharmacologically (Reboussin 2018).
Description of the intervention
Alcohol has been a part of almost every human culture for a very long time (McGovern 2009). According to the World Health Organization (WHO), around 2.3 billion people globally drink alcohol, and most of them are from the European region. On average, drinkers consume 32.8 grams of pure alcohol per day, and beer (34.3%) is the most consumed alcoholic beverage (WHO 2018). In the United States, 14 grams of pure alcohol is considered as one standard drink or one unit, and the maximum daily limit for men and women is four and three drinks, respectively (NIAAA 2017). Exceeding this limit increases the risk of cardiovascular, hepatic, and nervous system disorders (Bellentani 1997; Fuchs 2001; Gao 2011; Lieber 1998; McCullough 2011; Nutt 1999; Welch 2011). Also, multiple studies have found associations between consumption of alcoholic beverages and specific cancers (Kushi 2012; Seitz 2007). Abuse of alcohol resulted in approximately 3 million deaths worldwide and 132.6 million disability‐adjusted life years (DALYs) in 2016 (WHO 2018).
Alcohol is water‐soluble and can cross biological membranes by passive diffusion. It reaches equilibrium quickly if the body water content is higher. The presence of food in the stomach slows down alcohol absorption by retarding gastric emptying. Hence, it is recommended to not drink alcoholic beverages on an empty stomach. Alcohol is predominantly metabolised by the alcohol dehydrogenase (ADH) enzyme system and to a lesser extent by cytochrome P‐450 2E1 in the liver. Alcohol is first metabolised to acetaldehyde and then is oxidised into acetyl coenzyme A (CoA) by aldehyde dehydrogenase (ALDH) (Cederbaum 2012). Although alcohol metabolism by the liver is well characterised, its metabolism in other parts of the body is not well defined. The enzyme catalase was found to be responsible for metabolising alcohol in the brain to produce acetaldehyde (Heit 2013). Acetaldehyde is highly reactive and has been associated with a wide range of physiological adverse effects (Zimatkin 2006).
Despite the potential negative effects of alcohol consumption, systematic reviews based on cohort studies show that light to moderate consumption of alcohol has a cardioprotective effect and may decrease mortality in adult men and women (Briasoulis 2012; Di Castelnuovo 2006; Plunk 2014; Taylor 2009).
How the intervention might work
The molecular mechanisms through which alcohol raises blood pressure are unclear. Alcohol can affect blood pressure through a variety of possible mechanisms. Previous research suggests that acute alcohol consumption affects the renin–angiotensin–aldosterone system (RAAS) by increasing plasma renin activity (Puddey 1985). The RAAS is responsible for maintaining the balance of fluid and electrolytes. An increase in plasma renin results in increased production of angiotensin I (AI), which is converted to angiotensin II (AII) by angiotensin‐converting enzyme (ACE). The hormone AII is a potent vasoconstrictor that stimulates aldosterone and vasopressin secretion from the adrenal gland, promoting sodium and water retention (Schrier 1999). As a result, peripheral resistance and blood volume are increased, leading to elevated arterial blood.
Several clinical trials in humans and studies conducted in animal models have reported stimulation of the sympathetic nervous system and increased noradrenaline after consumption of alcohol (Barden 2013; Grassi 1989; Randin 1995; Russ 1991; Zhang 1989). When noradrenaline stimulates the adrenergic receptors located in the heart muscles, heart rate and blood pressure are increased.
Alcohol has been reported to diminish baroreceptor sensitivity, which is a key factor in regulating blood pressure (Abdel‐Rahman 1985; Rupp 1996). Baroreceptors or stretch receptors are mechanoreceptors located on the arch of the aorta and the carotid sinus. They can detect changes in blood pressure and can maintain blood pressure by controlling heart rate, contractility, and peripheral resistance. Acute administration of alcohol stimulates the release of histamine and endorphin, which interferes with baroreflex sensitivity (Carretta 1988).
Another possible mechanism is the increase in plasma cortisol levels following heavy alcohol consumption (Jenkins 1968). Several studies have suggested a role for cortisol in alcohol‐induced hypertension (Husain 2014; Potter 1986). Cortisol is a type of steroid hormone, and the presence of excess cortisol has been associated with elevated blood pressure in normotensive individuals (Whitworth 1984; Whitworth 2005).
Alcohol can affect drinkers differently based on their age, sex, ethnicity, family history, and liver condition (Cederbaum 2012; Chen 1999; Gentry 2000; Thomasson 1995). Previous studies reported that women are affected more than men after drinking the same amount of alcohol because of their lower body weight and higher body fat. The blood alcohol concentration (BAC) rises faster in women because they have a smaller volume of distribution (Kwo 1998). In contrast, women eliminate alcohol from the body a little faster than men (Thomasson 2000). Different genetic variants of ADH and ALDH enzymes have been found to show strikingly different rates of alcohol metabolism among different races (Chen 1999; Peng 2014; Agarwal 1981).
Why it is important to do this review
Several systematic reviews based on cohort studies have concluded that alcohol intake has a considerable effect on blood pressure and on risk of hypertension (Chen 2008; Worm 2013). It has also been shown that heavy alcohol consumption causes hypertension and leads to left ventricular dysfunction and dilated cardiomyopathy. On the other hand, abundant epidemiological and clinical evidence shows that light to moderate drinking is associated with reduced risk of coronary heart disease (CHD), incidence of stroke, and total mortality among middle‐aged and elderly men and women (Abramson 2001;Briasoulis 2012; Di Castelnuovo 2006; Djoussé 2007;Jaubert 2014; Plunk 2014; Taylor 2009).
All these conclusions are based on findings of observational studies. Several RCTs have reported the magnitude of effect of alcohol on blood pressure, but because those trials are small, their findings are not sufficient to justify a strong conclusion. In 2005, McFadden and colleagues conducted a systematic review of RCTs, which investigated the haemodynamic effects of daily consumption of alcohol (McFadden 2005). Based on nine RCTs in which participants consumed alcohol repeatedly over days, these review authors reported that alcohol increases SBP by 2.7 mmHg and DBP by 1.4 mmHg. However, they excluded studies for which the duration of BP observation was less than 24 hours and articles published in non‐English languages. We believe that inclusion of those studies will provide useful information about the dose‐related magnitude and time‐course effect of alcohol on blood pressure in people with both normal and elevated blood pressure.
Objectives
Primary objectives
To determine short‐term dose‐related effects of alcohol versus placebo on systolic blood pressure and diastolic blood pressure in healthy and hypertensive adults over 18 years of age.
Secondary objective
To determine short‐term dose‐related effects of alcohol versus placebo on heart rate in healthy and hypertensive adults over 18 years of age.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) that compared alcohol to placebo or similar tasting non‐alcoholic beverages were included in this systematic review.
Types of participants
We included adult (≥ 18) participants of both sexes without any restriction on their health condition.
Types of interventions
We included any type of alcoholic beverage as the intervention arm. The dose of alcohol had to be reported by study authors for inclusion in the systematic review. Because there are no published standards for differentiating between low and medium doses of alcohol, we chose the alcohol content in one standard drink as the threshold between low dose and medium dose. Because the alcohol content in one standard drink varies among different countries (ranging from 8 g to 14 g), we chose the Canadian standard for an alcoholic beverage, which is 14 g of pure alcohol (CCSA). Accordingly, we considered up to 14 g of alcohol as a low dose of alcohol. To differentiate between medium and high doses, the Canadian Centre on Substance Use and Addiction (CCSA) identifies less than 30 g of alcohol for men and less than 20 g of alcohol for women as the threshold for low risk of alcohol intake (CCSA). Thus, in our review, we used up to 30 g alcohol intake for men and up to 20 g alcohol intake for women as a moderate dose, and above this limit as a high dose. In studies where sex‐specific results were not provided, we categorised dose based on the dominating sex in terms of study participation. In conclusion, we categorised doses of alcohol as follows.
Low dose (≤ 14 g of alcohol/≤ 1 standard drink).
Medium dose (> 14 g and ≤ 30 g of alcohol for men and > 14 g and ≤ 20 g of alcohol for women).
High dose (> 30 g of alcohol for men and > 20 g of alcohol for women).
Types of outcome measures
Primary outcomes
Change in resting seated systolic and diastolic blood pressures at three different time periods after alcohol intake: early (up to six hours); intermediate (7 to 12 hours); and late (≥ 13 hours)
Secondary outcomes
Change in resting heart rate at the same time periods as blood pressure outcomes above
Additional outcomes
Change in resting mean arterial pressure (MAP) at the same time periods as blood pressure outcomes above
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist searched the following databases without language, publication year, or publication status restrictions.
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 14 March 2019).
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2), in the Cochrane Library, via the Cochrane Register of Studies (CRS‐Web) (searched 14 March 2019).
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 14 March 2019).
Embase Ovid (from 1974 onwards) (searched 14 March 2019).
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 14 March 2019).
World Health Organization International Clinical Trials Registry Platform (www.who.it.trialsearch) (searched 14 March 2019).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. When appropriate, these were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.c. (Higgins 2011)). We present search strategies for major databases in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches of CAB Abstracts & Global Health, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), ProQuest Dissertations & Theses, and Web of Science for controlled trials
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials
When necessary, we contacted authors of key papers and abstracts to request additional information about their trials
Data collection and analysis
Selection of studies
We (ST and CT) independently screened the citations found through the database search using Covidence software (Covidence). We excluded articles if the citation seemed completely irrelevant or was identified as a review or observational study after the title and abstract were read. For remaining studies, we (ST and CT) retrieved full‐text articles for further assessment. Any disagreements regarding inclusion or exclusion of studies were resolved by discussion between review authors. The reason for exclusion was documented for each citation at the full‐text level. We also checked the list of references in the included studies and articles that cited the included studies in Google Scholar to identify relevant articles. We reported the flow of citation in Figure 1.
1.
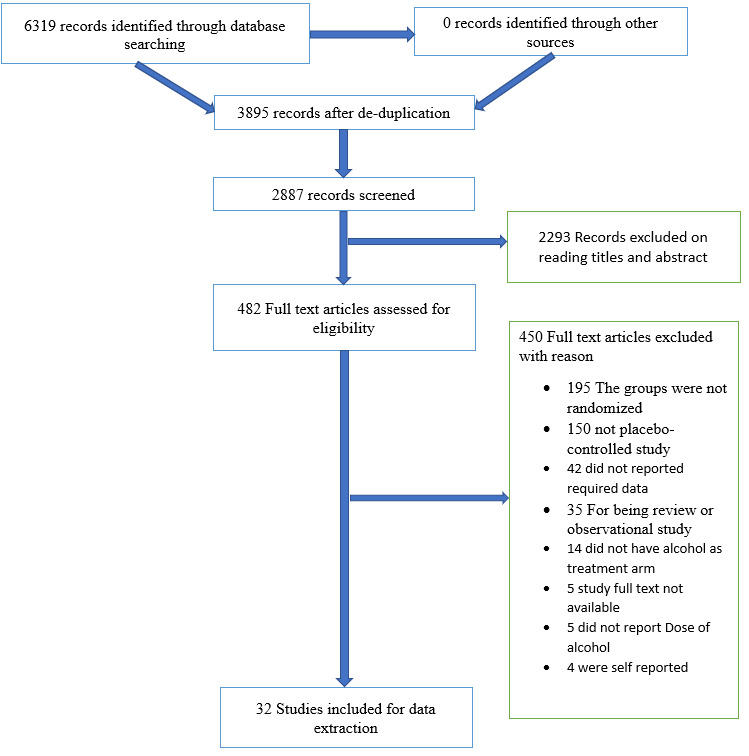
Study flow diagram.
Data extraction and management
Two review authors (ST and CT) performed data extraction independently using a standard data collection form, followed by a cross‐check. In cases of disagreement, the third review authors (JMW) became involved to resolve the disagreement. When necessary, we contacted the authors of studies for information about unclear study design. We recorded study design, type of masking, randomisation and allocation concealment methods, details of intervention and comparator, duration of intervention, baseline characteristics of participants, whether food was consumed before or during the intervention, numbers of participants included in the final analysis, outcomes and results, method and position of BP measurement, declaration of conflict of interest, funding source, and protocol registration number. All extracted data were entered and double‐checked in RevMan 5.3 software (Review Manager (RevMan)).
Assessment of risk of bias in included studies
We (ST and CT) assessed the risk of bias of included studies independently using the Cochrane risk of bias tool (version 1) according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation sequence concealment (selection bias).
Blinding of participants (performance bias).
Blinding of outcome assessors (detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other potential sources of bias (i.e. conflict of interest, funding source, registration of the study protocol).
We assessed selective reporting bias for each of the outcomes separately. For the other domains, we grouped outcomes together and provided only one judgement. We contacted study authors for missing or unclear information required for the risk of bias assessment and then reassessed the domains once the information was available.
Measures of treatment effect
All outcomes of interest in the review (BP and HR) produced continuous data. We calculated and reported mean difference (MD), with corresponding 95% confidence interval (95% CI).
Unit of analysis issues
Most of the studies included in the review had a cross‐over design. The carry‐over effect in a cross‐over trial can confound the effects of subsequent treatment. We recorded the washout period of each included study reported by study authors to decide if there was risk of a carry‐over effect. If it was appropriate to combine cross‐over trials with other trials, we used the recommended generic inverse variance approach of meta‐analysis. We tested the effect of cross‐over trials through sensitivity analysis by excluding them from the meta‐analysis to check if the effect estimate changed significantly.
For multi‐arm trials, if a study reported more than one intervention arm, we identified the relevant intervention arm and included that in the review. If studies reported more than one placebo group, we combined them into a single group when appropriate, using the formulae for combining groups reported in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We followed the same formulae for combining groups if a study reported two different types of alcoholic beverages containing the same amount of alcohol.
Dealing with missing data
General missing data
We contacted the study authors for missing or unclear information relevant to the review using contact information provided in their respective articles. If the dose of a study was not reported in the article and the study author did not respond to our request, we excluded that study.
Missing statistics
If a standard error (SE) was given instead of a standard deviation (SD), we used the formula SD = SE × square root of n (number of participants) to calculate the SD.
We also calculated SD if 95% CI, P value, or t value was reported in the included studies, according to Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If we were not able to get SD from the study authors or calculate SD from the values mentioned above, we imputed SD using the following hierarchy (listed from highest to lowest) (Musini 2014).
SD of change in blood pressure measured in a different position (e.g. lying down) than that of the blood pressure data used.
SD of blood pressure at the end of treatment.
SD of blood pressure at the end of treatment measured in a different position (e.g. lying down) than that of the blood pressure data used.
SD of blood pressure at baseline (unless this measure was used as an entry criterion).
Mean SD of change in blood pressure from other studies that studied the effects of alcohol.
Assessment of heterogeneity
We conducted a standard Chi² test through Review Manager Software 5.3 to test for heterogeneity (Review Manager (RevMan)). A P value of 0.1 or less was considered to show statistically significant heterogeneity. The I² statistic was used to interpret the level of heterogeneity (Higgins 2011). For the purposes of this review, if I² was greater than 50%, it was considered to show a substantial level of heterogeneity. Furthermore, we visually inspected the forest plot to check whether there were any non‐overlapping confidence intervals indicating heterogeneity. Last, we attempted to explore the reason for heterogeneity by looking for clinical and methodological differences between trials.
Assessment of reporting biases
We used funnel plots if there were more than 10 studies that contributed to a meta‐analysis to detect the extent of risk of reporting bias based on symmetry of the plot (Higgins 2011). We visually inspected the funnel plots. If the residual scatter plot resembles a symmetrical inverted funnel, we considered publication bias to be unlikely. Positive results are more likely to be published than negative results, which leads to potential publication bias. However, publication bias does not necessarily lead to asymmetry in funnel plots. Asymmetry in the funnel plot can also be due to inflated effects in smaller studies resulting from poor study design, heterogeneity, sampling variation, or chance. Therefore, we performed sensitivity analyses and searched for unpublished studies as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Data synthesis
We used Cochrane review manager software for all data analyses (Review Manager (RevMan)). We conducted meta‐analysis for the three dose groups (low dose, medium dose, and high dose of alcohol) separately. We considered statistical, clinical, and methodological heterogeneity between study populations and proceeded with the meta‐analysis if only we considered interventions, comparisons, and outcome measures similar enough to pool. When trials compared more than one dose of alcohol, we handled each comparison separately. Because all of our outcomes of interest provided continuous data, we used the inverse variance approach and a fixed‐effect model to combine effect sizes across studies.
Subgroup analysis and investigation of heterogeneity
We planned on performing subgroup analysis based on the following.
Normotensive participants (defined as SBP < 140 mmHg and DBP < 90 mmHg) versus hypertensive participants (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg).
Sex of participants.
It is recommended that there should be at least 10 studies reporting each of the subgroups in question (Deeks 2011). Among the 34 included studies, only four studies included hypertensive participants. So, it was not possible to conduct a subgroup analysis based on blood pressure. For the planned subgroup analysis based on sex, no study reported male and female participant data separately.
Sensitivity analysis
We performed the following sensitivity analyses.
We checked if blinding of participants and outcome assessors affected the effect estimate of BP and HR (blinded studies versus unblinded studies).
We checked the difference between effect estimates of outcomes given by the fixed‐effect model and the random‐effects model by conducting sensitivity analysis. We did this only when heterogeneity was substantial.
Summary of findings and assessment of the certainty of evidence
We used the GRADE approach (Grading of Recommendations, Assessment, Development and Evaluation) to assess the certainty of a body of evidence as high, moderate, low, or very low and provided review authors' comments to support our judgements as outlined in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions ((Guyatt 2011Higgins 2011). We included the outcomes SBP, DBP, and HR for each comparison. Both review authors (ST and CT) rated the certainty of evidence independently by examining risk of bias, indirectness, inconsistency, imprecision, and publication bias.
To assess risk of bias across studies, we rated the evidence as having no limitations, serious limitations, or very serious limitations while taking into account the extent that each trial contributes towards the magnitude of effect (weight) as based on its study sample size and mean difference.
We used GRADEpro software to construct a 'Summary of findings' table to compare outcomes including change in SBP and DBP and HR (GRADEpro 2014). In addition, we included illustrative risks to present findings for the most important outcome (change in systolic blood pressure).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach (Grading of Recommendations, Assessment, Development and Evaluation) (Guyatt 2011) to assess the certainty of the body evidence as high, moderate, low or very low and provide review authors' comments to support our judgements as outlined in the Cochrane Handbook for Systematic Reviews of Interventions chapter 12 (Higgins 2011). We included the outcomes SBP, DBP and HR for each comparison. Both reviewers (ST and CT) rated the certainty of evidence independently by examining risk of bias, indirectness, inconsistency, imprecision, and publication bias.
To assess the risk of bias across studies, we rated the evidence as having no limitations, serious limitations, or very serious limitations while taking into account the extent that each trial contributes towards the magnitude of effect (weight) as based on their study sample size and mean difference.
We used GRADEpro software (GRADEpro 2014) to construct a 'Summary of findings' table to compare outcomes including change in SBP and DBP and HR. In addition, we also included illustrative risks to present findings for the most important outcome (change in systolic blood pressure)
Results
Description of studies
Results of the search
The search was conducted up to March 2019 and resulted in 6869 citations. After de‐duplication and screening of titles and abstracts, we were left with 482 citations for further assessment. We retrieved full‐text articles for those citations and included 32 studies (Figure 1).
Included studies
Refer to Characteristics of included studies and Table 4 for further details regarding these studies.
1. Baseline characteristics.
| Study ID |
Randomised participants, N |
Mean age (range) | Mean body weight, kg | Health condition | Reported dose of alcohol |
Duration of intervention |
Baseline SBP (SD) | Baseline DBP (SD) | Baseline HR (SD) |
| Agewall 2000 | 12 | 31 (younger than 40 years old) | Not reported | Healthy, normotensive non‐smokers | 31.25 g | 10 minutes | 121 (6) | 79 (4) | 61 (7) |
| Barden 2013 | 24 | 56 (20 to 65) | Not reported | Healthy | 41 g | 30 minutes | 115 (11) | 72 (6) | 62 (7) |
| Bau 2005 | 100 | 20.7 (18 to 25) | Not reported | Healthy non‐smokers | 60 g | 30 minutes | 114.2 | 64.8 | 72.43 (10.9) |
| Bau 2011 | 70 | 20.7 (18 to 25) | Not reported | Healthy | 60 g | 30 minutes | Not reported | Not reported | 75 (10) |
| Buckman 2015 | 72 | 21.5 | Not reported | Healthy | 0.90 mL/kg for men 0.78 mL/kg for women |
15 minutes | 116.9 (13.5) | Not reported | 66.9 (9.9) |
| Chen 1986 | 20 | 19 to 32 | Not reported | Healthy, normotensive non‐smokers | Target to achieve blood level of 0.05% | Not reported (mentioned that fairly fast rate) |
118 (12.88) | 62.25 (5.1) | 63.13 (7.1) |
| Cheyne 2004 | 17 | 35 (21 to 46) | Not reported | Type 1 diabetes | 0.35 mg/kg BW | Not reported | 116.2 (18.7) | 66.8 (8.2) | 70 (12) |
| Dai 2002 | 40 | 19 to 25 years | 81.35 | Healthy | 0.5 g/kg | 5 minutes | 114.1 | 72.6 | 63.5 |
| Dumont 2010 | 14 | 22.1 (18 to 29) | Not reported | Regular user of MDMA, otherwise healthy | Target blood alcohol level 0.6%, equivalent of 2 to 3 alcoholic beverages. | 3 hours to maintain target BAC | Not reported | Not reported | 66 |
| Fantin 2016 | 18 | 34.2 (25 to 53) | 70.2 (53 to 85.6) | Healthy | 30 g | 10 hours | 110.3 (12) | 80 (8) | 75.5 (11.5) |
| Fazio 2004 | 10 | 22 (20 to 25) | Not reported | Healthy | 0.3 g/kg | 5 minutes | Not reported | Not reported | 68 |
| Foppa 2002 | 13 | 55 (43 to 65) | Not reported | Hypertensive and centrally obese | 23 g | 15 minutes | 130 | 83 | 72 (8.72) |
| Hering 2011 | 24 | 44 | Not reported | 13 hypertensive and 11 normotensive | 1 g/kg | 20 minutes | Hypertensive: 150
(21) Normotensive: 136 (13.2) |
Hypertensive: 91 (14.4) Normotensive: 76 (10) |
Hypertensive: 72 (7.2) Normotensive: 70 (10) |
| Karatzi 2005 | 15 | 52.4 | Not reported | Coronary artery disease | 30 g | Not reported | 109.8 (9.2) | 80.7 (10.8) | 67.1 (13.1) |
| Karatzi 2013 | 16 | 28.5 | 77.5 | Healthy non‐smokers | 20 g | 15 minutes | 115.4 (6.2) | 68.5 (5.4) | 60 (8.1) |
| Kawano 1992 | 13 | 55.2 (22 to 70) | 65.2 | Mild to moderate essential hypertension | 51 g | Not reported | 159 (18.8) | 91.3 (12) | 61.5 |
| Kawano 2000 | 10 | 54 (32 to 67) | 70 (60 to 78) | Mild essential hypertension | 55.3 g (1 mL/kg BW) | 60 minutes | 147 | 91 | 65 |
| Koenig 1997 | 15 | 20 to 35 | 76 | Healthy | 10 mL/kg BW | 30 minutes | 127 (11) | 80 (9.5) | Not reported |
| Kojima 1993 | 21 | 56.5 (33 to 73) | Not reported | Essential hypertension | 1 mL/kg BW | 30 minutes | 146 (18.33) | 89 (9.2) | 59 (9.2) |
| Mahmud 2002 | 8 | 21 to 40 | 70 | Healthy, normotensive non‐smokers | 56 g (0.8 g/kg of BW) | 10 minutes | 93.3 (10) | 67 (8) | Not reported |
| Maufrais 2017 | 24 | 23.3 | 62.9 | Healthy | 0.4 g/kg | 5 minutes | Not reported | Not reported | 69 (9.8) |
| Maule 1993 | 10 | 31 (22 to 51) | 68.1 (50 to 81) | Healthy | 34 g (0.5 g/kg BW) | 10 minutes | 122 | 70 | 62 (6.3) |
| Narkiewicz 2000 | 19 | 26 | Not reported | Healthy | 1 g/kg BW | 30 minutes | 111 (11.2) | 61 (7.5) | 57 (7.5) |
| Nishiwaki 2017 | 11 | 21.1 (20 to 22) | 62.6 | Healthy non‐smokers | 11 g and 19.25 g | 5 minutes | 123 (6.63) | 71 (6.63) | 59 (9.94) |
| Potter 1986 | 16 | 22 (20 to 30) | 77 | Healthy, normotensive | 0.75 g/kg | 15 minutes | 122.5 (11) | 72.5 (9) | 63.2 (8) |
| Rosito 1999 | 40 | 22.2 (19 to 30) | Not reported | Healthy | 60 g | 1 hour | 124.2 (10.7) | 76.2 (9.4) | 70.5 (12.6) |
| Rossinen 1997 | 20 | 39 to 68 | Not reported | Coronary artery disease and
myocardial ischaemia Patients were taking usual medicine |
1.25 g/kg | 1 hour 30 minutes | 132 (16) | Not reported | 69.5 |
| Stott 1987 | 10 | 18 to 31 | 56 to 101 | Healthy | 1.3 g/kg | 1 hour | 115.5 | 67 | 70.5 |
| Stott 1991 | 8 | 81 (70 to 96) | 68.4 | Normotensive | 0.5 g/kg | 15 minutes | 130 (18.3) | 77.5 (15.5) | 57 (7.5) |
| Van De Borne 1997 | 16 | 26 | Not reported | Healthy | 1 g/kg | 30 minutes | Not reported | Not reported | 59 (8) |
| Williams 2004 | 13 | 59 (48 to 70) | 86 | Coronary artery disease | 0.52 g/kg | 20 minutes | 135 (11) | 82 (7) | 55 (11) |
| Zeichner 1985 | 48 | 20.9 (19 to 23) | Not reported | Healthy | 1 g/kg | 20 minutes | 115.5 (10.2) | 70.4 (9.1) | 69.4 (12.10) |
BAC: blood alcohol concentration. BW: body weight. DBP: diastolic blood pressure. HR: heart rate. SBP: systolic blood pressure. SD: standard deviation.
Of the 32 included RCTs involving 767 participants, 26 trials used a cross‐over design and six used a parallel‐group design. Three studies were single‐blind, 12 were double‐blind, and 17 were open‐label studies. Most study participants were male (N = 642). The age of participants ranged from 18 to 96 years, and mean participant age was 33 years. Stott 1991 included relatively old participants (mean age 81, range 70 to 96 years) compared to the other studies. Only 14 out of 34 studies reported the mean body weight of participants. The mean body weight from those 14 studies was 78 kg. Most studies included healthy participants with normal blood pressure. Some studies included small numbers of participants with essential hypertension (10%) (Foppa 2002Hering 2011; Kawano 1992; Kawano 2000; Kojima 1993), type 1 diabetes (2.2%) (Cheyne 2004), coronary heart disease (6.3%) (Karatzi 2005Rossinen 1997Williams 2004) and regular user of methylenedioxymethamphetamine (MDMA) (2%) (Dumont 2010).
Different types of alcoholic beverages including red wine, white wine, beer, and vodka were used among 32 studies. Out of 32 studies, 23 used non‐alcoholic beverages including juice as placebo (Bau 2005; Bau 2011Buckman 2015; Cheyne 2004; Dai 2002; Dumont 2010; Fazio 2004; Foppa 2002; Hering 2011; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Maufrais 2017; Maule 1993; Narkiewicz 2000; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991; Van De Borne 1997; Williams 2004; Zeichner 1985); seven studies used de‐alcoholised wine as placebo (Agewall 2000; Barden 2013; Karatzi 2005; Karatzi 2013; Mahmud 2002; Nishiwaki 2017; Potter 1986); and two studies did not mention the type of control they used for alcohol (Chen 1986; Fantin 2016). The dose of alcohol ranged between 0.35 mg/kg and 1.3 g/kg, and alcohol was consumed over five minutes and over one hour and 30 minutes. It is important to note that the dose of alcohol was comparatively higher (≥ 60 g or ≥ 1 g/kg) in nine studies (Bau 2005; Buckman 2015; Hering 2011; Narkiewicz 2000; Rosito 1999; Rossinen 1997; Stott 1987; Van De Borne 1997; Zeichner 1985).
Excluded studies
We excluded 450 trials after reviewing the full‐text articles, and we recorded the reasons for exclusion (see table Characteristics of excluded studies table).
Risk of bias in included studies
Refer to Figure 2 and Figure 3 for the overall 'Risk of bias' assessment.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We (ST and CT) independently assessed risk of bias by following the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the risk of bias based on 11 domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) for systolic blood pressure (SBP), selective reporting (reporting bias) for diastolic blood pressure (DBP), selective reporting (reporting bias) for mean arterial blood pressure (MAP), selective reporting (reporting bias) for heart rate (HR), other bias (conflict of interest, industry sponsorship), and other bias (was the study registered in clinical trials.gov and was the protocol available). We classified each domain as being at low, high, or uncertain risk of bias.
In the case of disagreement, a third party (JMW) was involved to discuss and resolve the disagreement. In the case of uncertain information regarding the method of RCT, we contacted study authors via email to request clarification. Refer to Table 5 for further details regarding reasons and responses.
2. Contact with corresponding authors.
| Study ID | Reason for contact | Contacted? (Yes/No) | Response? (Yes/No) |
| Agewall 2000 | Method of allocation concealment used in RCT was not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Bau 2005 | Method of allocation concealment used in RCT was not mentioned | Yes | No |
| Bau 2011 | Method of allocation concealment used in RCT was not mentioned | Yes | No |
| Buckman 2015 | Method of allocation concealment used in RCT was not mentioned | Yes | Yes |
| Chen 1986 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Dai 2002 | Method of allocation concealment used in RCT was not mentioned | Yes | No |
| Dumont 2010 | Methods of randomisation and allocation concealment, blinding of participants and personnel, and blinding of outcome assessment used in RCT were not mentioned | Yes | No |
| Fantin 2016 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | No |
| Fazio 2004 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | No |
| Foppa 2002 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | Yes |
| Hering 2011 | Methods of allocation concealment used in RCT was not mentioned | Yes | Yes |
| Karatzi 2005 | Methods of randomisation and allocation concealment, blinding of participants and personnel, and blinding of outcome assessment used in RCT were not mentioned | Yes | No |
| Karatzi 2013 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | No |
| Kawano 1992 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes Comment ‐ contact information cannot be found in the study. However, we used contact information provided in Kawano 2000 |
No |
| Kawano 2000 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | No |
| Kojima 1993 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Mahmud 2002 | Methods of randomisation and allocation concealment, blinding of participants and personnel, and blinding of outcome assessment used in RCT were not mentioned | Yes | No |
| Maufrais 2017 | Method of allocation concealment used in RCT was not mentioned | Yes | No |
| Maule 1993 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Narkiewicz 2000 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | No |
| Nishiwaki 2017 | Methods of randomisation and allocation concealment used in RCT were not mentioned | Yes | No |
| Potter 1986 | Methods of randomisation and allocation concealment and blinding of outcome assessor used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Rossinen 1997 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Rosito 1999 | Methods of randomisation and allocation concealment and blinding of participants and personnel used in RCT were not mentioned | Yes | Yes |
| Stott 1987 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Stott 1991 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Van De Borne 1997 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Williams 2004 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
| Zeichner 1985 | Methods of randomisation and allocation concealment used in RCT were not mentioned | No Comment ‐ contact information cannot be found |
NA |
NA: not applicable. RCT: randomised controlled trial.
Allocation
We (ST and CT) assessed selection bias based on two categories: random sequence generation and allocation concealment.
Random sequence generation
For random sequence generation, we classified 22 included studies as having uncertain risk of bias (Agewall 2000; Chen 1986; Dumont 2010; Fantin 2016; Fazio 2004; Karatzi 2005; Karatzi 2013; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Mahmud 2002; Maule 1993; Narkiewicz 2000; Nishiwaki 2017; Potter 1986; Rossinen 1997; Stott 1987; Stott 1991; Van De Borne 1997; Williams 2004; Zeichner 1985). Even though these studies reported that participants were randomised to receive alcohol or placebo, the method of randomisation was not mentioned. Although three studies did not report the method of randomisation (Barden 2013; Buckman 2015; Dai 2002), their reported baseline characteristics were well matched. We classified them as having uncertain risk of bias. The remaining seven studies reported the method of randomisation used, hence we classified them as having low risk of bias. Random seed generation was used in Bau 2005 and Bau 2011 computer‐generated random selection of 4 × 4 Latin squares was used in Cheyne 2004 a third person unaware of research objectives or protocol prepared sealed randomised envelops in blocks of eight in Foppa 2002; a randomised computer‐generated number table was used in Hering 2011; a random sequence generator was used in Maufrais 2017; and a random number allocator was used in Rosito 1999. It is important to note that information regarding to the method of randomisation used in Foppa 2002 and Rosito 1999 was provided by the study author via email. Refer to Table 5 for further details.
For allocation concealment, we classified 28 included studies as having uncertain risk of bias because the method of allocation concealment was not reported (Agewall 2000; Bau 2005; Bau 2011; Buckman 2015; Chen 1986; Dai 2002; Dumont 2010; Fantin 2016; Fazio 2004; Hering 2011; Karatzi 2005; Karatzi 2013; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Mahmud 2002; Maufrais 2017; Maule 1993; Narkiewicz 2000; Nishiwaki 2017; Potter 1986; Rossinen 1997; Stott 1987; Stott 1991; Van De Borne 1997; Williams 2004; Zeichner 1985). We classified the remaining four studies as having low risk of bias. In Barden 2013, treatment allocation was performed by a statistician who was not involved in the trial. Opaque sealed randomised envelopes were used in Cheyne 2004 and Foppa 2002, and random number allocator was used in Rosito 1999. It is important to note that information regarding the method of allocation concealment used in Foppa 2002 and Rosito 1999 was provided by the study author via email. We also contacted Hering 2011, but the study author did not explicitly mention in the email the method of allocation concealment used. Thus, we classified this study as having uncertain risk of bias. Refer to Table 5 for further details.
Blinding
In the case of performance bias, we classified six studies as having low risk of bias, 19 studies as having high risk of bias, and seven studies as having unclear risk of bias.
We classified six studies as having low risk of performance bias (Dai 2002; Narkiewicz 2000; Nishiwaki 2017; Potter 1986; Rosito 1999; Van De Borne 1997). Nishiwaki 2017 was a single‐blinded study. In this study, all test drinks were poured into paper cups to achieve blinding of participants. We contacted the author of Rosito 1999 to request additional information regarding the method of blinding used. The study author explained the blinding method in detail in an email, so we classified this study as having low risk of bias.
We classified 19 studies as having high risk of performance bias. Of 19 studies, 17 were open‐label (Barden 2013; Buckman 2015; Chen 1986; Fantin 2016; Fazio 2004; Foppa 2002; Hering 2011; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Maufrais 2017; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985), hence these studies were not blinded for participants nor for personnel. It is important to note that 2 out of 19 studies were single‐blinded (Agewall 2000; Karatzi 2013). Personnel were blinded instead of participants in Karatzi 2013, and neither personnel nor participants were blinded in Agewall 2000, so we assessed these studies as having high risk of bias.
We classified seven studies as having unclear risk of performance bias (Bau 2005; Bau 2011; Cheyne 2004; Dumont 2010; Karatzi 2005; Mahmud 2002; Maule 1993). Bau 2005 and Bau 2011 mentioned only that investigators and volunteers were blinded to the content of the drink but did not mention the method of blinding used in these studies. Karatzi 2005 mentioned the method of blinding of participants, but it is not clear whether involved personnel were blinded as well. The method of blinding of participants and personnel was not mentioned in Dumont 2010, Mahmud 2002, and Maule 1993. In Cheyne 2004, participants were blinded to the content of the drink, but some reported that they were able to detect the alcohol by taste at the end of the study. Hence, we classified this study as having high risk of bias.
In the case of detection bias, we classified nine studies as having low risk of performance bias (Agewall 2000; Bau 2005; Bau 2011; Cheyne 2004; Dai 2002; Karatzi 2013; Narkiewicz 2000; Rosito 1999; Van De Borne 1997). All studies included an independent individual who was blinded to control and test groups to evaluate and analyse the data. We classified 17 studies as having high risk of bias because they were described as open‐label studies, and because the outcome assessor was not blinded (Barden 2013; Buckman 2015; Chen 1986; Fantin 2016; Fazio 2004; Foppa 2002; Hering 2011; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Maufrais 2017; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985). One study ‐ Nishiwaki 2017 (a single‐blinded study) ‐ ensured participant blinding but not blinding of outcome assessors. We classified five studies as having uncertain risk of detection bias. Karatzi 2005, Mahmud 2002, Maule 1993, and Potter 1986 did not mention the method of blinding of outcome assessors. Even though Dumont 2010 mentioned blinding of outcome assessors, it is not clear whether blinding of outcome assessment was maintained in the case of blood pressure and heart rate measurements.
Incomplete outcome data
We classified 18 studies as having low risk of attrition bias (Agewall 2000; Barden 2013; Bau 2005; Bau 2011; Dai 2002; Dumont 2010; Fantin 2016; Foppa 2002; Karatzi 2013; Kawano 1992; Kojima 1993; Mahmud 2002; Narkiewicz 2000; Potter 1986; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004). Dumont 2010, Karatzi 2013, Kawano 1992, and Williams 2004 reported reasons for participant withdrawal and excluded their data from the final analysis. Data were balanced across groups, hence missing data did not affect the final results.
We classified one study as having high risk of bias. Chen 1986 reported that two participants in the alcohol group dropped out of the study for unknown reasons, so data analyses were based on eight participants in the alcohol group and on 10 participants in the control group. Because the reasons behind withdrawal were not mentioned in this study, we considered this study to have high risk of bias.
We classified 13 studies as having uncertain risk of attrition bias because study authors did not explicitly mention whether all participants were included in the final analysis (Buckman 2015; Cheyne 2004; Fazio 2004; Hering 2011; Karatzi 2005; Kawano 2000; Koenig 1997; Maufrais 2017; Maule 1993; Nishiwaki 2017; Rosito 1999; Van De Borne 1997; Zeichner 1985).
Selective reporting
We (ST and CT) assessed reporting bias based on four categories: selective reporting of systolic blood pressure (SBP), selective reporting of diastolic blood pressure (DBP), selective reporting of mean arterial blood pressure (MAP), and selective reporting of heart rate (HR).
In the case of selective reporting of systolic blood pressure (SBP), we classified 25 studies as having low risk of bias because they recorded and reported SBP in the Results section or in the Figure (Barden 2013; Bau 2005; Buckman 2015; Chen 1986; Cheyne 2004; Dai 2002; Fantin 2016; Foppa 2002; Hering 2011; Karatzi 2005; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Mahmud 2002; Maule 1993; Narkiewicz 2000; Nishiwaki 2017; Potter 1986; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985). We classified seven studies as having high risk of bias (Agewall 2000; Bau 2011; Dumont 2010; Fazio 2004; Karatzi 2013; Maufrais 2017; Van De Borne 1997). Agewall 2000 measured blood pressure upon arrival of participants and did not measure blood pressure after the intervention. The aim of Bau 2011 was to determine the effects of alcohol on heart rate variability, so SBP was not measured in this study. Dumont 2010 measured blood pressure during the study period, but study authors did not provide the before and after measurement of SBP. They mentioned only that change in blood pressure was not significant. The aim of Fazio 2004 was to determine the effects of alcohol on blood flow volume and velocity. Blood pressure was also measured but was not reported. Study authors mentioned only that acute ethanol administration caused a transitory increase in BP at 20 minutes. Karatzi 2013Maufrais 2017 and Van De Borne 1997 measured blood pressure before and after treatment but did not report these measurements.
In the case of selective reporting of diastolic blood pressure (DBP), we classified 23 studies as having low risk of bias because they measured and reported DBP in the Results section or in the figure (Barden 2013; Bau 2005; Chen 1986; Cheyne 2004; Dai 2002; Fantin 2016; Foppa 2002; Hering 2011; Karatzi 2005; Kawano 1992; Kawano 2000; Koenig 1997; Kojima 1993; Mahmud 2002; Maule 1993; Narkiewicz 2000; Nishiwaki 2017; Potter 1986; Rosito 1999; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985). We classified nine studies as having high risk of bias (Agewall 2000; Bau 2011; Buckman 2015; Dumont 2010; Fazio 2004; Karatzi 2013; Maufrais 2017; Rossinen 1997; Van De Borne 1997). Agewall 2000 measured blood pressure upon participants' arrival and did not measure blood pressure after the intervention. The aim of Bau 2011 was to determine the effects of alcohol on heart rate variability, so study authors did not measure and report DBP. For Buckman 2015, blood pressure was recorded beat to beat continuously, but DBP was not reported. Dumont 2010 measured blood pressure during the RCT, but study authors did not provide the before and after measurement of DBP. They mentioned that changes in blood pressure were not significant. The aim of Fazio 2004 was to determine effects of alcohol on blood flow volume and velocity. Blood pressure was also measured but was not reported. Study authors mentioned that acute ethanol administration caused transitory increase in BP at 20 minutes. Rossinen 1997 measured blood pressure but selectively reported only SBP instead of reporting both SBP and DBP. Karatzi 2013Maufrais 2017 and Van De Borne 1997 measured blood pressure before and after treatment but did not report these measurements.
For selective reporting of mean arterial blood pressure (MAP), we classified 11 studies as having low risk of bias because MAP was measured and reported (Buckman 2015; Dumont 2010; Fazio 2004; Foppa 2002; Karatzi 2005; Karatzi 2013; Kojima 1993; Maufrais 2017; Maule 1993; Narkiewicz 2000; Van De Borne 1997). We classified 21 studies as having high risk of bias because they did not report MAP (Agewall 2000; Barden 2013; Bau 2005; Bau 2011; Chen 1986; Cheyne 2004; Dai 2002; Fantin 2016; Hering 2011; Kawano 1992; Kawano 2000; Koenig 1997; Mahmud 2002; Nishiwaki 2017; Potter 1986; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985).
For selective reporting for heart rate (HR), we classified only Koenig 1997 as having high risk of bias because heart rate was not reported. We classified the remaining 33 studies as having low risk of bias because heart rate was measured and reported.
Other potential sources of bias
We divided other potential sources of bias into two main categories: conflict of interest/industry sponsorship and registration with clinical trials.gov.
In the case of assessing conflicts of interest and industry sponsorship, we classified 21 studies as having low risk of bias because they reported industry sponsorship and had no conflicts of interest (Agewall 2000; Barden 2013; Bau 2005; Bau 2011; Buckman 2015; Cheyne 2004; Dai 2002; Dumont 2010; Fantin 2016; Hering 2011; Karatzi 2013; Kawano 1992; Kawano 2000; Kojima 1993; Maufrais 2017; Narkiewicz 2000; Nishiwaki 2017; Potter 1986; Van De Borne 1997; Williams 2004; Zeichner 1985). We classified 11 studies as having uncertain risk of bias because the funding source or conflicts of interest were not reported (Chen 1986; Fazio 2004; Foppa 2002; Karatzi 2005; Koenig 1997; Mahmud 2002; Maule 1993; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991).
In the case of registration at clinical trials.gov, we considered only one study to have low risk of bias (Barden 2013). The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR). We classified the remaining studies as having high risk of bias because the protocol was not registered and the study identifier was not reported. Therefore, it is difficult to determine a priori selection of primary and secondary outcome measures for the included studies.
Publication bias
We did not identify enough studies to construct a funnel plot for the outcomes under low doses of alcohol. We interpreted only funnel plots that were constructed based on studies reporting outcomes under medium dose and high dose of alcohol versus placebo comparisons.
We created a funnel plot using the mean difference (MD) from studies reporting effects of medium doses and high doses of alcohol on SBP, DBP, MAP, and HR against standard error (SE) of the MD to check for the existence of publication bias. Visual inspection of funnel plots shows that the effect estimate is equally distributed around the mean in Figure 4, Figure 5, Figure 6. Figure 7, and Figure 8. In Figure 9, Figure 10, and Figure 11, we observed slight asymmetry in the funnel plot that was probably due to heterogeneity rather than to publication bias. We noted some overlap of data points in some funnel plots, indicating that some of the included studies were of similar size. According to Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), a funnel plot asymmetry test should not be used if all studies are of similar size. In this review, most of the included studies are of similar size.
4.

Funnel plot of comparison: 2 Medium‐dose alcohol vs placebo, outcome: 2.4 Heart rate.
5.

Funnel plot of comparison: 3 High‐dose alcohol vs placebo, outcome: 3.1 Systolic blood pressure.
6.

Funnel plot of comparison: 3 High‐dose alcohol vs placebo, outcome: 3.2 Diastolic blood pressure.
7.

Funnel plot of comparison: 3 High‐dose alcohol vs placebo, outcome: 3.3 Mean arterial blood pressure.
8.

Funnel plot of comparison: 3 High‐dose alcohol vs placebo, outcome: 3.4 Heart rate.
9.

Funnel plot of comparison: 2 Medium‐dose alcohol vs placebo, outcome: 2.1 Systolic blood pressure.
10.

Funnel plot of comparison: 2 Medium‐dose alcohol vs placebo, outcome: 2.2 Diastolic blood pressure.
11.

Funnel plot of comparison: 2 Medium‐dose alcohol vs placebo, outcome: 2.3 Mean arterial blood pressure.
Effects of interventions
See: Table 1; Table 2; Table 3
Low dose
A dose of 14 grams of pure alcohol/ethanol or less was defined as a low dose of alcohol.
Effects of low doses of alcohol on SBP
≤ 6 hours after alcohol consumption: based on two studies in 28 participants (Cheyne 2004; Nishiwaki 2017), the mean decrease in SBP with a fixed‐effect model was 1.46 mmHg (95% confidence interval (CI) ‐8.38 to 5.42; P = 0.67). There was no heterogeneity (I² = 0%)
7 to 12 hours after alcohol consumption: unfortunately, none of the included studies reported results at this time interval
≥ 13 hours of alcohol consumption: unfortunately, none of the included studies reported results at this time interval.
Effects of low doses of alcohol on DBP
≤ 6 hours after alcohol consumption: two studies reported the early effect of alcohol consumption on DBP; the decrease in DBP was 1.46 mmHg (95% CI ‐6.91 to 3.99; P = 0.36) (Cheyne 2004; Nishiwaki 2017). There was no heterogeneity (I² = 0%)
7 to 12 hours after alcohol consumption: unfortunately, none of the included studies reported data at this time interval
≥ 13 hours of alcohol consumption: unfortunately, none of the included studies reported data at this time interval
Effects of low doses of alcohol on MAP
≤ 6 hours after alcohol consumption: based on MAP data from two studies (Cheyne 2004; Nishiwaki 2017), the mean decrease in DBP for MAP was 1.45 mmHg (95% CI ‐4.55 to 1.65; P = 0.36). There was no heterogeneity (I² = 0%)
7 to 12 hours after alcohol consumption: unfortunately, none of the included studies reported data at this time interval
≥ 13 hours of alcohol consumption: unfortunately, none of the included studies reported data at this time interval
Effects of low doses of alcohol on HR
≤ 6 hours after alcohol consumption: based on the early effect of a low dose of alcohol on HR data from two studies (Cheyne 2004; Nishiwaki 2017), HR was increased significantly by 5.06 bpm (95% CI 1.88 to 8.24; P = 0.002). There was no heterogeneity (I² = 0%)
7 to 12 hours after alcohol consumption: unfortunately, none of the included studies reported data at this time interval
≥ 13 hours after alcohol consumption: unfortunately, none of the included studies reported data at this time interval
Medium dose
Effects of medium doses of alcohol on SBP
-
≤ 6 hours after alcohol consumption: based on data from nine studies in 149 participants (Chen 1986; Fantin 2016; Foppa 2002; Karatzi 2005; Kawano 1992; Kawano 2000; Kojima 1993; Nishiwaki 2017; Rosito 1999), a medium dose of alcohol decreased SBP by 5.63 mmHg (95% CI ‐8.25 to ‐3.02; P < 0.001). The I² statistic value was 63% and the P value for the Chi² test was 0.004, indicating substantial heterogeneity across studies.
Due to the presence of substantial heterogeneity across studies, we also conducted this analysis using the random‐effects model. A medium dose of alcohol decreased SBP by 6.15 mmHg (95% CI ‐10.55 to ‐1.75; P = 0.006)
7 to 12 hours after consumption: four studies in 54 participants showed that a medium dose of alcohol decreased SBP by 3.22 mmHg (95% CI ‐8.37 to 1.93; P = 0.22) 7 to 12 hours after consumption of a medium dose of alcohol (Fantin 2016; Foppa 2002; Kawano 1992; Kawano 2000). There was no heterogeneity (I² = 0)
≥ 13 hours after consumption: based on data for 66 participants from four studies (Foppa 2002; Kawano 1992; Kawano 2000; Rosito 1999), the mean increase in SBP was 0.64 mmHg (95% CI ‐3.90 to 5.18; P = 0.78). There was no heterogeneity (I² = 0)
Effects of medium doses of alcohol on DBP
-
≤ 6 hours after alcohol consumption: based on data from nine studies in 149 participants (Chen 1986; Fantin 2016; Foppa 2002; Karatzi 2005; Kawano 1992; Kawano 2000; Kojima 1993; Nishiwaki 2017; Rosito 1999), a medium dose of alcohol decreased DBP by 4.01 mmHg (95% CI ‐6.02 to ‐2.00; P < 0.001). The I² statistic value was 59% and the P value for the Chi² test was 0.009, indicating substantial heterogeneity across studies.
Due to the presence of substantial heterogeneity across studies, we also conducted this analysis using the random‐effects model. A medium dose of alcohol decreased DBP by 3.76 mmHg (95% CI ‐7.02 to ‐0.50; P = 0.006)
7 to 12 hours after alcohol consumption: four studies in 54 participants reported a mean decrease in DBP of 1.19 mmHg (95% CI ‐4.29 to 1.90; P = 0.45) after 7 to 12 hours after consumption of a medium dose of alcohol (Fantin 2016; Foppa 2002; Kawano 1992; Kawano 2000). There was no heterogeneity (I² = 0)
≥ 13 hours after alcohol consumption: Based on data from 66 participants in four studies (Foppa 2002; Kawano 1992; Kawano 2000; Rosito 1999), the mean increase in DBP was 1.78 mmHg (95% CI ‐0.95 to 4.51; P = 0.64). There was no heterogeneity (I² = 0)
Effects of medium doses of alcohol on MAP
-
≤ 6 hours after alcohol consumption: based on data from 17 studies in 428 participants (Barden 2013; Bau 2005; Buckman 2015; Dai 2002; Dumont 2010; Hering 2011; Koenig 1997; Mahmud 2002; Maule 1993; Narkiewicz 2000; Potter 1986; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985), we found that a high dose of alcohol decreased MAP by 1.53 mmHg (95% CI ‐3.34 to 0.28; P = 0.10). There was evidence of low heterogeneity across studies (I² = 27%)
Due to the presence of substantial heterogeneity across studies, we also conducted this analysis using the random‐effects model. A medium dose of alcohol decreased MAP by 2.92 mmHg (95% CI –5.76 to ‐0.07; P = 0.04)
7 to 12 hours after alcohol consumption: based on data from four studies in 54 participants (Fantin 2016; Foppa 2002; Kawano 1992; Kawano 2000), the mean decrease in MAP was 2.11 mmHg (95% CI ‐4.69 to 0.48; P = 0.11) after 7 to 12 hours after consumption of a medium dose of alcohol. There was no heterogeneity (I² = 0)
≥ 13 hours after alcohol consumption: based on data from 66 participants from four studies (Foppa 2002; Kawano 1992; Kawano 2000; Rosito 1999), the mean increase in MAP was 1.43 mmHg (95% CI ‐1.18 to 4.04; P = 0.28). There was no heterogeneity (I² = 0)
Effects of medium doses of alcohol on HR
≤ 6 hours after alcohol consumption: 12 studies reported the effects of alcohol consumption in 181 participants (Agewall 2000; Chen 1986; Fantin 2016; Fazio 2004; Foppa 2002; Karatzi 2005; Karatzi 2013; Kawano 1992; Kawano 2000; Kojima 1993; Maufrais 2017; Nishiwaki 2017); the mean increase in HR was 4.62 bpm (95% CI 3.14 to 6.11; P < 0.01). There was no heterogeneity (I² = 0)
7 to 12 hours after alcohol consumption: based on data from four studies in 54 participants (Fantin 2016; Foppa 2002; Kawano 1992; Kawano 2000), the mean increase in HR was 1.22 bpm (95% CI –1.88 to 4.32; P = 0.44) after 7 to 12 hours after consumption of a medium dose of alcohol. There was no heterogeneity (I² = 0)
≥ 13 hours after alcohol consumption: based on three studies in 36 participants (Foppa 2002; Kawano 1992; Kawano 2000), the mean increase in HR was 1.37 bpm (95% CI ‐2.12 to 4.86; P = 0.44)
High dose
Effects of high doses of alcohol on SBP
≤ 6 hours after alcohol consumption: based on data from 418 participants in 16 studies (Barden 2013; Bau 2005; Buckman 2015; Dai 2002; Hering 2011; Koenig 1997; Mahmud 2002; Maule 1993; Narkiewicz 2000; Potter 1986; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985), high doses of alcohol decreased SBP by 3.49 mmHg (95% CI ‐6.03 to ‐0.95; P = 0.007). The I² statistic value was 45% and the P‐value for the Chi² test was 0.02, indicating moderate heterogeneity across studies
7 to 12 hours after alcohol consumption: based on data from 54 participants in three studies (Barden 2013; Rosito 1999; Stott 1987), high doses of alcohol decreased SBP by 3.72 mmHg (95% CI ‐6.97 to ‐0.48; P = 0.02). There was no heterogeneity (I² = 0)
≥ 13 hours after alcohol consumption: based on data from 154 participants in four studies (Barden 2013; Bau 2005; Rosito 1999; Stott 1987), high doses of alcohol increased SBP by 3.69 mmHg (95% CI 2.33 to 5.05; P < 0.001]. There was no heterogeneity (I² = 0)
Effects of high doses of alcohol on DBP
≤ 6 hours after alcohol consumption: based on data from 350 participants in 14 studies (Barden 2013; Bau 2005; Dai 2002; Hering 2011; Koenig 1997; Mahmud 2002; Maule 1993; Narkiewicz 2000; Potter 1986; Rosito 1999 ; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985), high doses of alcohol decreased DBP by 1.91 mmHg (95% CI ‐3.86 to 0.04; P = 0.05). The I² statistic value was 35% and the P‐value for the Chi² test was < 0.1, indicating low heterogeneity across studies
7 to 12 hours after alcohol consumption: based on data from 54 participants in three studies (Barden 2013; Rosito 1999; Stott 1987), high doses of alcohol decreased DBP by 1.71 mmHg (95% CI –4.59 to 1.77; P = 0.24). There was no heterogeneity (I² = 0)
≥ 13 hours after alcohol consumption: based on data from 154 participants in four studies (Barden 2013; Bau 2005; Rosito 1999; Stott 1987), high doses of alcohol increased DBP by 2.37 mmHg (95% CI 0.24 to 4.49; P = 0.03) after 13 hours of consumption. There was no heterogeneity (I² = 0)
Effects of high doses of alcohol on MAP
≤ 6 hours after alcohol consumption: based on data from 16 studies in 418 participants (Barden 2013; Bau 2005; Buckman 2015; Dai 2002; Dumont 2010; Hering 2011; Koenig 1997; Mahmud 2002; Maule 1993; Narkiewicz 2000; Potter 1986; Rosito 1999; Stott 1987; Stott 1991; Williams 2004; Zeichner 1985), high doses of alcohol decreased MAP by 1.79 mmHg (95% CI ‐3.72 to 0.13; P = 0.07). There was evidence of low heterogeneity across studies (I² = 30%)
7 to 12 hours after alcohol consumption: three studies reported the effects of high doses of alcohol consumption in 54 participants (Barden 2013; Rosito 1999; Stott 1987); the mean decrease in MAP was 2.47 mmHg (95% CI ‐5.69 to 0.75; P = 0.13). There was no heterogeneity (I² = 0)
≥ 13 hours after alcohol consumption: four studies reported the effects of high doses of alcohol in 154 participants (Barden 2013; Bau 2005; Rosito 1999; Stott 1987); the mean increase in MAP was 2.96 mmHg (95% CI 0.35 to 5.58, P = 0.02) ≥ 13 hours after consumption. There was no heterogeneity (I² = 0)
Effects of high doses of alcohol on HR
≤ 6 hours after alcohol consumption: 17 studies reported the effects of high doses of alcohol in 495 participants (Barden 2013; Bau 2005; Bau 2011; Buckman 2015; Dai 2002; Dumont 2010; Hering 2011; Maule 1993; Narkiewicz 2000; Potter 1986; Rosito 1999; Rossinen 1997; Stott 1987; Stott 1991; Van De Borne 1997; Williams 2004; Zeichner 1985); the mean increase in HR was 5.75 bpm (95% CI 3.99 to 7.51; P < 0.001). There was evidence of low heterogeneity across studies (I² = 34%)
7 to 12 hours after alcohol consumption: five studies reported the effects of high doses of alcohol in 144 participants (Barden 2013; Bau 2011; Rosito 1999; Rossinen 1997; Stott 1987); the mean increase in HR was 6.16 bpm (95% CI 3.04 to 9.28; P < 0.001). There was moderate heterogeneity across studies (I² = 44%)
≥ 13 hours after alcohol consumption: six studies reported the effects of high doses of alcohol in 244 participants (Barden 2013; Bau 2005; Bau 2011; Rosito 1999; Rossinen 1997; Stott 1987); the mean increase in HR was 2.70 bpm (95% CI 0.80 to 4.60; P = 0.005) ≥ 13 hours after consumption. There was no heterogeneity (I² = 0)
The above‐mentioned results are summarised in Table 1, Table 2, and Table 3.
Subgroup analysis
It is recommended that there should be at least 10 studies reporting each of the subgroups in question. Among the 32 included studies, only four studies included hypertensive participants (Kawano 1992; Kawano 2000; Kojima 1993; Foppa 2002). So, it was not appropriate to conduct a separate meta‐analysis based on that population.
For the planned subgroup analysis based on sex, no studies reported male and female participant data separately. Therefore, we were unable to perform a subgroup analysis based on the sex of participants.
Sensitivity analysis
As planned, we conducted sensitivity analyses to see if there was any significant difference between effect estimates of outcomes given by the fixed‐effect model and the random‐effects model, when substantial heterogeneity was present. The result is presented in Table 6; there was no significant difference between results given by the two models.
3. Sensitivity analysis: fixed‐effect model vs random‐effects model.
| Outcomes or subgroup | Mean difference, IV, fixed‐effect model, 95% CI | Mean difference, IV, random‐effects model, 95% CI |
| 2.1 SBP (≤ 6 hours) | ‐5.63 [‐8.25, ‐3.02] Heterogeneity: Chi² = 24.51, df = 9 (P = 0.004); I² = 63% Test for overall effect: Z = 4.22 (P < 0.0001) |
‐6.15 [‐10.55, ‐1.75] Heterogeneity: Chi² = 24.51, df = 9 (P = 0.004); I² = 63% Test for overall effect: Z = 2.74 (P = 0.006) |
| 2.2 DBP (≤ 6 hours) | ‐4.01 [‐6.02, ‐2.00] Heterogeneity: Chi² = 21.81, df = 9 (P = 0.009); I² = 59% Test for overall effect: Z = 3.91 (P < 0.0001) |
‐3.76 [‐7.02, ‐0.50] Heterogeneity: Chi² = 21.81, df = 9 (P = 0.009); I² = 59% Test for overall effect: Z = 2.26 (P = 0.02) |
| 2.3 MAP (≤ 6 hours) | ‐2.17 [‐3.68, ‐0.65] Heterogeneity: Chi² = 38.36, df = 12 (P = 0.0001); I² = 69% Test for overall effect: Z = 2.80 (P = 0.005) |
‐2.92 [‐5.76, ‐0.07] Heterogeneity: Chi² = 38.36, df = 12 (P = 0.0001); I² = 69% Test for overall effect: Z = 2.01 (P = 0.04) |
CI: confidence interval. DBP: diastolic blood pressure. IV: inverse variance. MAP: mean arterial pressure. SBP: systolic blood pressure.
We planned on conducting sensitivity analyses on studies based on their level of risk of bias (high‐risk studies versus low‐risk studies). Most of the included studies had similar risk of bias across all domains except for performance bias and detection bias, for which risk arises from blinding of participants, personnel, and outcome assessors. So, we decided to conduct a sensitivity analysis of the included studies based on the blinding condition (Table 7). We observed a greater reduction in blood pressure after a moderate dose of alcohol consumption for the unblinded studies, which was probably due to the presence of a heterogeneous population. For high‐dose alcohol studies, we did not find any significant difference between blinded and unblinded studies. Overall, the of studies did not influence outcomes.
4. Sensitivity analysis: blinded studies vs unblinded studies.
| Outcomes or subgroups | Blinded studies, mean difference, IV, fixed‐effect model, 95% CI | Unblinded, mean difference, IV, fixed‐effect model, 95% CI |
| 2.1 SBP (< 6 hours) | ‐4.56 [‐8.39, ‐0.73] Heterogeneity: Chi² = 2.14, df = 3 (P = 0.54); I² = 0% Test for overall effect: Z = 2.33 (P = 0.02) |
‐6.57 [‐10.15, ‐3.00] Heterogeneity: Chi² = 21.80, df = 5 (P = 0.0006); I² = 77% Test for overall effect: Z = 3.60 (P = 0.0003) |
| 2.2 DBP (< 6 hours) | ‐1.50 [‐4.89, 1.89] Heterogeneity: Chi² = 1.99, df = 3 (P = 0.57); I² = 0% Test for overall effect: Z = 0.86 (P = 0.39) |
‐5.37 [‐7.86, ‐2.87] Heterogeneity: Chi² = 16.57, df = 5 (P = 0.005); I² = 70% Test for overall effect: Z = 4.22 (P < 0.0001) |
| 2.3 MAP (< 6 hours) | ‐0.11 [‐3.39, 3.18] Heterogeneity: Chi² = 8.24, df = 4 (P = 0.08); I² = 51% Test for overall effect: Z = 0.06 (P = 0.95) |
‐4.93 [‐8.83, ‐1.02] Heterogeneity: Chi² = 21.61, df = 7 (P = 0.003); I² = 68% Test for overall effect: Z = 2.47 (P = 0.01) |
| 2.4 HR (< 6 hours) | 4.35 [2.31, 6.40] Heterogeneity: Chi² = 2.33, df = 3 (P = 0.51); I² = 0% Test for overall effect: Z = 4.17 (P < 0.0001) |
4.92 [2.77, 7.08] Heterogeneity: Chi² = 7.37, df = 7 (P = 0.39); I² = 5% Test for overall effect: Z = 4.48 (P < 0.00001) |
| 3.1 SBP (< 6 hours) | ‐3.80 [‐8.03, 0.43] Heterogeneity: Chi² = 21.01, df = 7 (P = 0.004); I² = 67% Test for overall effect: Z = 1.76 (P = 0.08) |
‐2.84 [‐5.53, ‐0.14] Heterogeneity: Chi² = 6.04, df = 7 (P = 0.54); I² = 0% Test for overall effect: Z = 2.06 (P = 0.04) |
| 3.2 DBP (< 6 hours) | ‐1.88 [‐4.73, 0.97] Heterogeneity: Tau² = 6.68; Chi² = 11.57, df = 6 (P = 0.07); I² = 48% Test for overall effect: Z = 1.29 (P = 0.20) |
‐1.99 [‐4.89, 0.90] Heterogeneity: Tau² = 3.87; Chi² = 8.16, df = 6 (P = 0.23); I² = 26% Test for overall effect: Z = 1.35 (P = 0.18) |
| 3.3 MAP (< 6 hours) | ‐1.62 [‐3.98, 0.74] Heterogeneity: Chi² = 10.45, df = 8 (P = 0.23); I² = 23% Test for overall effect: Z = 1.35 (P = 0.18) |
‐1.44 [‐4.46, 1.57] Heterogeneity: Chi² = 11.54, df = 7 (P = 0.12); I² = 39% Test for overall effect: Z = 0.94 (P = 0.35) |
| 3.4 HR (< 6 hours) | 4.82 [3.01, 6.63] Heterogeneity: Chi² = 5.79, df = 8 (P = 0.67); I² = 0% Test for overall effect: Z = 5.22 (P < 0.00001) |
6.62 [3.21, 10.03] Heterogeneity: Chi² = 16.68, df = 7 (P = 0.02); I² = 58% Test for overall effect: Z = 3.81 (P = 0.0001) |
CI: confidence interval. DBP: diastolic blood pressure. HR: heart rate. IV: inverse variance. MAP: mean arterial pressure. SBP: systolic blood pressure.
Discussion
This review summarises the acute effects of different doses of alcohol on blood pressure and heart rate in adults (≥ 18 years of age) during three different time intervals after ingestion of alcohol.
Summary of main results
Effects of low‐dose alcohol consumption
Low‐dose alcohol consumption had no effect on blood pressure (BP) within six hours, but we found only two trials that studied this dose and no trials that assessed BP after six hours. Low‐dose alcohol increased heart rate (HR) within six hours, suggesting that even one glass of wine increases HR. Unfortunately, we found no studies measuring HR more than six hours after the dose.
Effects of medium‐dose alcohol consumption
Medium‐dose alcohol decreased systolic blood pressure (SBP) by 5.6 mmHg and diastolic blood pressure (DBP) by 4 mmHg within the first six hours of consumption. Although the hypotensive effect of alcohol seemed to last up to 12 hours after drinking alcohol, and the effect was lost after 13 hours, the result was based on only four trials reporting intermediate (7 to 12 hours) and late (after 13 hours) effects of alcohol on BP.
Heart rate was increased by 4.6 bpm six hours after drinking alcohol compared to placebo. Intermediate (7 to 12 hours) and late (after 13 hours) effects of the medium dose of alcohol on HR were based on only four trials and were not statistically different compared to placebo.
Effects of high‐dose alcohol consumption
High‐dose alcohol decreased SBP by 3.49 mmHg within the first six hours, and by 3.77 mmHg between 7 and 12 hours after consumption. After 13 hours, high doses of alcohol increased SBP by 3.7 mmHg compared to placebo. DBP was not significantly affected up to 12 hours after drinking a high dose of alcohol, but there was a statistically significant increase in DBP during the ≥ 13 hour time interval after alcohol consumption.
High‐dose alcohol consumption increased HR by approximately 6 bpm in participants, and the effect lasted up to 12 hours. After that, HR was still raised in participants, but it averaged 2.7 bpm.
Dose‐dependent response
There is likely a dose‐response effect of alcohol on BP, as the effects of alcohol appeared to last longer with higher doses. However, we lacked data on the effects of low doses. We intended to find out the dose‐dependent changes in SBP, DBP, mean arterial pressure (MAP), and HR after consumption of a single dose of alcohol. Because the numbers of included studies that fell into our pre‐specified dose categories were not comparable, we were unable to conduct a comprehensive dose‐dependent analysis. Rosito 1999 tested the effects of 15 g, 30 g, and 60 g of alcohol on 40 young medical students. The decrease in SBP was greater with 30 g of alcohol seven hours after consumption compared to placebo and 15 g and 60 g alcohol‐consuming groups. In this study, alcohol had no significant effect on DBP in the four groups.
Possible mechanisms to explain the results
Many interrelated changes are possibly responsible for the biphasic effect of alcohol on blood pressure.
Acute alcohol consumption was found to reduce 20‐hydroxyeicosatetraenoic acid (20‐HETE) in Barden 2013. 20‐HETE is a signalling molecule with a wide range of effects on the cardiovascular system. It is a vasoconstrictor that inhibits sodium reabsorption in the proximal and distal tubules of the kidney. The reduction in 20‐HETE was greatest when the blood alcohol level was highest (Barden 2013; Collins 2005). Increased production of nitric oxide (NO) was also associated with acute alcohol consumption (Deng 2007; Rocha 2012). NO is released from the endothelium and is a potent vasodilator. Also, there is an inverse relationship between NO and 20‐HETE. Alcohol is often consumed mixed together with fruit juices, and the combination was found to promote insulin secretion (Steiner 2015). Insulin is known to signal a phosphorylation‐dependent mechanism resulting in the production of NO (Muniyappa 2007). Moreover, alcohol's metabolic byproduct acetaldehyde is another known vasodilator (Altura 1978; Gillespie 1967). Together, the reduction in vasoconstrictors and the increase in vasodilators could be the explanation for the drop in blood pressure, which lasted approximately 12 hours.
The blood alcohol level decreased over time, and 20‐HETE started to rise (Barden 2013). The hypertensive effect of alcohol after 13 hours of consumption could be the result of the rise in vasoconstrictors and the homeostatic response to restore blood pressure. Plasma renin activity was reported to be increased in Kawano 2000 as a late effect of alcohol consumption.
Heart rate was increased following alcohol consumption regardless of the dose of alcohol. Alcohol has been shown to slow down parasympathetic nervous activity and to stimulate sympathetic nervous activity. Hering 2011, Carter 2011, and Spaak 2008 reported an increase in muscle sympathetic nervous activity (MSNA), which persists for at least 10 hours after consumption. The vagus nerve is a component of the parasympathetic nervous system and is largely responsible for regulation of the heart rate at rest. Rossinen 1997 and Van De Borne 1997 reported withdrawal of vagal tone and reduced heart rate variability within an hour after alcohol consumption; this explains the increased heart rate. Buckman 2015, Van De Borne 1997, and Fazio 2001 also reported reduced baroreflex sensitivity following alcohol consumption. Impairment of baroreflex sensitivity results in failure to sense the increase in heart rate and maintenance of cardiovascular homeostasis. Kawano 2000 reported a reduction in plasma potassium levels after alcohol consumption, which might provide another reason for the increase in heart rate.
Clinical implications of the findings
This systematic review provides us with a better understanding of the time‐course of alcohol's acute effects on blood pressure and heart rate. This review included only short‐term randomised controlled trials (RCTs) investigating the effects of alcohol on blood pressure and heart rate. Acute alcohol consumption mimics the pattern of social drinking, and evidence indicates that even one glass of an alcoholic drink can increase heart rate. The magnitude of the effects of alcohol on blood pressure and heart rate varies, based possibly on genetic factors and on the amount of alcohol consumed.
Several long‐term observational studies have reported that light to moderate alcohol consumption is associated with a reduction in adverse cardiovascular events (Briasoulis 2012; Di Castelnuovo 2006; Plunk 2014; Taylor 2009). At the present time, the explanation for these possible beneficial effects of regular light to moderate alcohol consumption is unknown. In this review, consuming medium (moderate) doses of alcohol lowered blood pressure for possibly up to 12 hours after consumption. Perhaps regular light to moderate consumption of alcohol lowers blood pressure, and this is the explanation for the reduction in cardiovascular events seen in the observational studies.
On the other hand, this review shows that high doses of alcohol increased blood pressure after 13 hours and could lead to increased blood pressure the day after consumption. Thus regular consumption of high doses of alcohol could lead to a sustained increase in blood pressure and the adverse consequences associated with hypertension.
Hypertensive individuals who take antihypertensive drugs should be cautious about the timing of drinking alcohol because the combination of some antihypertensive drugs and alcohol was found to synergistically reduce blood pressure (Bailey 1989; Kawano 1999; Kawano 2000).
Overall completeness and applicability of evidence
The evidence synthesised in this review was collected from 32 RCTs in 767 participants. Of the 32 studies, two studied low‐dose alcohol, 12 studied medium‐dose alcohol, and 19 studied high‐dose alcohol. Rosito 1999 studied both medium and high doses of alcohol. The sample size in the meta‐analysis for low‐dose comparison was not adequate to assess the effects of low doses of alcohol on BP and HR; however, we believe that the direction of the change in BP and HR was correct. For medium doses and high doses of alcohol, participants represented a range in terms of age, sex, and health condition. Because the participant population comprised predominantly young and healthy normotensive men, the overall evidence generated in this review cannot be extrapolated to women and older populations with other comorbidities.
There was substantial heterogeneity in the meta‐analysis for effects of medium doses on SBP and DBP six hours after alcohol consumption (Analysis 2.1 and Analysis 2.2) due to the inclusion of Kawano 1992, Kawano 2000, and Kojima 1993. Participants in these studies were Japanese with mean age > 50 years and essential hypertension. The mean reduction in SBP and DBP was greater in these participants compared to participants from other studies ingesting medium doses of alcohol. The reason behind the greater reduction in blood pressure after consuming alcohol is probably the presence of genetic variants of the hormones alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) among Japanese participants. The presence of these gene variants can cause an increase in the blood acetaldehyde level to more than 10 times the normal level (Enomoto 1991; Peng 2014; Thomasson 1995). The ALDH2*2 allele is a gene variant of the enzyme ALDH and is almost exclusively present in the northeast Asian population (Wall 2016). Studies on liver extracts have reported that this particular allele of ALDH is dominant. The presence of this allele leads to reduced acetaldehyde metabolising activity in heterozygotes and no metabolising activity in homozygotes (Li 2009; Li 2012). In both cases, the blood acetaldehyde level after alcohol consumption was found to be greater than the normal range. An excessive amount of acetaldehyde in blood is responsible for facial flushing, which is a common adverse effect of alcohol consumption among east Asians. Acetaldehyde could be responsible for the hypotensive effects of alcohol (Altura 1978; Gillespie 1967), and excessive acetaldehyde could be the reason for the greater reduction in blood pressure reported in the aforementioned studies including Japanese populations.
2.1. Analysis.

Comparison 2: Medium‐dose alcohol vs placebo, Outcome 1: Systolic blood pressure
2.2. Analysis.

Comparison 2: Medium‐dose alcohol vs placebo, Outcome 2: Diastolic blood pressure
To check whether the age of participants had any role in the effects of alcohol on blood pressure, we selected studies with older participants (> 50 years) from the analysis (Analysis 2.1; Analysis 2.2; Analysis 3.1; Analysis 3.2). Studies with older participants showed a greater decrease in SBP and DBP compared to studies with younger participants (< 50 years) (results presented in Table 8). Although we do not have much confidence in the mean change in blood pressure in the older population due to fewer studies compared to studies with younger participants and the presence of heterogeneity, previous research suggests that age can affect the rate of elimination of alcohol from the body (Cederbaum 2012; Hahn 1983). With increasing age, the amount of body water and liver mass are decreased, leading to higher blood alcohol levels. Also, the metabolising enzymes such as cytochrome P‐4502E1 and ALDH diminish with age. Older populations are more likely to take more drugs to treat existing comorbidities, and the drug‐alcohol interaction can modify alcohol metabolism (Meier 2008). All these factors can lead to increased acetaldehyde in the body and greater reduction in blood pressure.
3.1. Analysis.

Comparison 3: High‐dose alcohol vs placebo, Outcome 1: Systolic blood pressure
3.2. Analysis.

Comparison 3: High‐dose alcohol vs placebo, Outcome 2: Diastolic blood pressure
5. Differences between older and younger participants.
| Analysis | Older participants (mean age ≥ 50), mean difference, IV, fixed‐effect model, 95% CI | Younger participants (mean age < 50), mean difference, IV, fixed‐effect model, 95% CI |
| 2.1 SBP (< 6 hours) | ‐11.25 [‐15.63, ‐6.87] Test for overall effect: Z = 5.04 (P < 0.00001); df = 3 (P = 0.03); I² = 66% |
‐2.52 [‐5.78, 0.74] Test for overall effect: Z = 1.52 (P = 0.13); df = 5 (P = 0.31); I² = 16% |
| 2.2 DBP (<6 hours) | ‐7.82 [‐11.08, ‐4.57] Test for overall effect: Z = 4.71 (P < 0.00001); df = 3 (P = 0.008); I² = 74% |
‐1.66 [‐4.22, 0.89] Test for overall effect: Z = 1.28 (P = 0.20); df = 5 (P = 0.91); I² = 0% |
| 3.1 SBP (<6 hours) | ‐6.71 [‐11.23, ‐2.18] Test for overall effect: Z = 2.91 (P = 0.004); df = 3 (P = 0.92); I² = 0% | ‐3.04 [‐4.87, ‐1.20]; Test for overall effect: Z = 3.24 (P = 0.001); df = 11 (P = 0.010); I² = 56% |
| 3.2 DBP (<6 hours) | ‐4.30 [‐7.32, ‐1.27] Test for overall effect: Z = 2.78 (P = 0.005); df = 2 (P = 0.25); I² = 27% | ‐1.67 [‐3.33, ‐0.01] Test for overall effect: Z = 1.97 (P = 0.05); df = 10 (P = 0.13); I² = 34% |
CI: confidence interval. DBP: diastolic blood pressure. IV: inverse variance. SBP: systolic blood pressure.
Rosito 1999 reported the effects of 15, 30, and 60 g of alcohol compared to placebo on healthy male volunteers. According to our pre‐specified dose categories, both 15 g and 30 g of alcohol fell under the medium dose category. Including both of these doses or de‐selecting either one of these doses from Rosito 1999 from Analysis 2.1 and Analysis 2.2 (medium doses of alcohol) resulted in the same statistically significant conclusion.
We identified Stott 1987 and Barden 2013 from Analysis 3.1 and Analysis 3.2 as having a considerably lower standard error (SE) of the mean difference (MD) compared to the other included studies. Assuming that the low SEs of MDs reported in Stott 1987 and Barden 2013 are errors and are not reliable, we replaced these measures with the average SE of MD from the rest of the included studies. The statistically significant conclusions remained the same.
Nine out of the 19 studies used comparatively high (≥ 60 g or ≥ 1 g/kg) doses of alcohol compared to the 10 other studies under the high dose of alcohol category, and consumption of very high doses of alcohol in these nine trials over the reported short duration mimics the pattern of binge drinking. Hence, we conducted additional analyses to see if the very high dose of alcohol (≥ 60 g or ≥ 1 g/kg) had any dose‐related effects compared to lower high doses of alcohol (31 to 59 g of alcohol) (see Table 9). Results suggest that the decrease in BP with very high doses of alcohol is greater compared to lower high doses of alcohol. The change in heart rate was similar in both comparisons. However, the result was heterogeneous; therefore, we are unable to make any implications from this.
6. Comparison between very high‐dose alcohol and lower high‐dose alcohol.
| Outcomes |
Very high dose (≥ 60 g) Mean difference, IV, fixed‐effect model, 95% CI |
Lower high dose (31 to 59 g) Mean difference, IV, fixed‐effect model, 95% CI |
| 3.1. SBP | ‐5.12 [‐7.32, ‐2.92] Test for overall effect: Z = 4.57 (P < 0.00001); df = 7 (P = 0.02); I² = 56% | ‐1.20 [‐3.90, 1.49] Test for overall effect: Z = 0.88 (P = 0.38); df = 7 (P = 0.47); I² = 0% |
| 3.2. DBP | ‐3.21 [‐5.49, ‐0.92] Test for overall effect: Z = 2.75 (P = 0.006); df = 5 (P = 0.22); I² = 29% | ‐1.65 [‐3.53, 0.23] Test for overall effect: Z = 1.72 (P = 0.09); df = 7 (P = 0.10); I² = 41% |
| 3.3. MAP | 2.17 [‐4.09, ‐0.25] Test for overall effect: Z = 2.21 (P = 0.03); df = 7 (P = 0.04); I² = 52% | ‐0.47 [‐2.83, 1.90] Test for overall effect: Z = 0.39 (P = 0.70); df = 8 (P = 0.63); I² = 0% |
| 3.4. HR | 5.43 [3.76, 7.11] Test for overall effect: Z = 6.35 (P < 0.00001); df = 9 (P = 0.76); I² = 0% | 6.09 [3.67, 8.51] Test for overall effect: Z = 4.93 (P < 0.00001); df = 6 (P = 0.005); I² = 67% |
CI: confidence interval. DBP: diastolic blood pressure. HR: heart rate. IV: inverse variance. MAP: mean arterial pressure. SBP: systolic blood pressure.
This is the first systematic review on this topic based on RCTs. Much of the current literature on alcohol does not mention the hypotensive effect of alcohol or the magnitude of change in BP or HR after alcohol consumption. This review will be useful for social and regular drinkers to appreciate the risks of low blood pressure within the first 12 hours after drinking.
Quality of the evidence
We graded the overall certainty of evidence using the GRADE approach via GRADEpro GDT software (GRADEpro 2014); we formulated summary of findings (SoF) tables.
We created three SoF tables to show the certainty of evidence and the summary of effects on outcomes of interest (SBP, DBP, and HR) for high (Table 1), medium (Table 2), and low doses (Table 3) of alcohol.
Ratings of the certainty of evidence ranged from moderate to low in this review, which suggests that the effect estimates of alcohol might be slightly different than the true effects. For high doses of alcohol, we found moderate‐certainty evidence showing a decrease in SBP and low‐certainty evidence suggesting a decrease in DBP within the first six hours and 7 to 12 hours after consumption. Moderate‐certainty evidence shows that SBP and DBP rise between 13 and 24 hours after alcohol ingestion.
For medium doses of alcohol, moderate‐certainty evidence shows a decrease in SBP and DBP six hours after alcohol consumption, and low‐certainty evidence suggests a decrease in SBP and DBP for 7 to 12 hours after alcohol consumption. After ≥ 13 hours of consumption, SBP and DBP were raised; the certainty of evidence was low and medium, respectively.
For low doses of alcohol, we found low‐certainty evidence suggesting that SBP, DBP, and MAP fall within the first six hours after alcohol consumption.
We also found moderate‐certainty evidence showing that alcohol raises HR within the first six hours of consumption, regardless of the dose of alcohol. Moderate‐certainty evidence indicates an increase in heart rate after 7 to 12 hours and ≥ 13 hours after high‐dose alcohol consumption, low certainty of evidence was found for moderate dose of alcohol consumption.
We did not consider the lack of blinding of participants as a downgrading factor for certainty of evidence because we do not think that it affected the outcomes of this systematic review. Changes in blood pressure and heart rate after alcohol consumption were not the primary outcomes of interest in most of the included studies. We do not think participants were anticipating any significant influence on blood pressure or heart rate after drinking.
We also did not rate the certainty of evidence based on the funding sources of studies or on lack of a registered protocol because we did not think this would affect the effect estimates for these outcomes. However, we noted the lack of description of randomisation and allocation concealment methods in most of the included studies as a reason for downgrading because of the possibility of selection bias.
Potential biases in the review process
We faced several limitations during the review process. First, there was the possibility of undesired bias and imprecision due to imputations of missing statistics. Most of the included studies did not report the standard error (SE)/standard deviation (SD) of the mean difference (MD) for the outcomes of interest. As described in our protocol, when we were unable to obtain the required SE/SD from study authors or by calculation from the reported P value or 95% CI, we imputed data according to the pre‐specified imputation hierarchy. We most often used the reported endpoint SE/SD value to impute the SE/SD of MD. This is known to provide a good approximation of the SD of change in BP so is unlikely to lead to bias. Also, only 10 out of 32 studies reported changes in MAP after alcohol consumption along with SE/SD (Buckman 2015; Dumont 2010; Foppa 2002; Karatzi 2005; Karatzi 2013; Kojima 1993; Maufrais 2017; Maule 1993; Narkiewicz 2000; Van De Borne 1997). So, we had to calculate missing MAP values from reported SBP and DBP values using the formula mentioned in the protocol and we imputed the SE/SD for those.
Second, lack of representation of the female population was notable in the included studies. Only 16 out of 32 studies included a total of 129 (16% of total participants) female participants (Agewall 2000; Buckman 2015; Chen 1986; Cheyne 2004; Dumont 2010; Fantin 2016; Foppa 2002; Hering 2011; Mahmud 2002; Maufrais 2017; Maule 1993; Narkiewicz 2000; Rossinen 1997; Stott 1987; Stott 1991; Zeichner 1985), and this number was very low compared to 638 male participants. Only four studies included almost equal numbers of male and female participants (Buckman 2015; Foppa 2002; Maufrais 2017; Zeichner 1985). Moreover, none of the studies reported male and female data separately. As a result, we were not able to quantify the magnitude of the effects of alcohol on men and women separately. This is unfortunate, as we have reason to believe that the effects of alcohol on BP might be greater in women.
Methodological differences between studies might have affected measurement of the reported outcomes. Recent research suggests that automated ambulatory blood pressure monitors are more reliable than manual sphygmomanometers, particularly because automated monitors reduce white coat anxiety (Mirdamadi 2017). Of the 32 included studies, seven studies used a manual mercury sphygmomanometer or a semi‐automated sphygmomanometer for BP measurement (Bau 2005; Dai 2002; Karatzi 2005; Kojima 1993; Potter 1986; Rossinen 1997; Van De Borne 1997). Mixing of various measurement techniques (manual, semi‐automated, and fully automated) in the meta‐analysis might have led to some of the heterogeneity.
Another reason behind the heterogeneity was probably the variation in alcohol intake duration and in the timing of measurement of outcomes across the included studies. Most studies gave participants 15 to 30 minutes to finish their drinks, started measuring outcomes sometime after that, and continued taking measurements for a certain period, but there were some exceptions. Chen 1986 did not report consumption duration nor timing of measurement of BP and HR. Dai 2002 gave participants five minutes to consume high doses of alcohol and measured outcomes immediately. On the other hand, Fantin 2016 allowed participants to continue drinking during the period of outcome measurement. These differences in alcohol consumption duration and in outcome measurement times probably contributed to the wide variation in blood pressure in these studies and affected overall results of the meta‐analysis.
We took several steps to minimise the risk of selection bias to identify eligible studies for inclusion in the review. We used highly sensitive search strategies. We also checked the lists of references in the included studies and articles that cited the included studies in Google Scholar to identify relevant articles. Furthermore, we contacted authors of included studies to obtain all relevant data when information was insufficient or missing.
Agreements and disagreements with other studies or reviews
We are aware of one systematic review on effects of alcohol on blood pressure that was published in 2005 (McFadden 2005). McFadden 2005 included both randomised and non‐randomised studies with a minimum of 24 hours of blood pressure observation after alcohol consumption. This systematic review searched only the MEDLINE database for relevant studies, hence it was not exhaustive. Review authors included nine studies involving a total of 119 participants, and the duration of these studies was between four and seven days. Participants in those studies consumed alcohol regularly during the study period, whereas in our systematic review, we included only studies in which participants consumed alcohol for a short period. Based on nine studies, McFadden 2005 reported that the mean increase in SBP was 2.7 mmHg and in DBP was 1.4 mmHg. Only three of these studies measured BP at various time points and found that alcohol has a hypotensive effect lasting up to five hours after alcohol consumption and a hypertensive effect 20 hours after alcohol consumption that lasts until the next day. These findings are consistent with our results. However, the reported early reduction in BP was 11. 6 mmHg for SBP and 7.9 mmHg for DBP in McFadden 2005. These estimates are almost twice as large as our results. The inclusion of non‐randomised studies in McFadden 2005, which are known to be at higher risk of bias, is likely the reason for the discrepancy in the magnitude of BP effects.
Authors' conclusions
Implications for practice.
The magnitude and direction of the effects of alcohol on blood pressure depend on the time after alcohol consumption. Moderate‐certainty evidence shows that acute consumption of medium to high doses of alcohol decreases blood pressure within the first six hours and for up to 12 hours after alcohol consumption. For times greater than 13 hours, high doses of alcohol consumption increased blood pressure. Low, moderate, and high alcohol consumption increased heart rate within the first six hours. High alcohol consumption also increased heart rate from 7 to 12 hours and after 13 hours. Most of the evidence from this review is relevant to healthy males, as these trials included small numbers of women (126 females compared to 638 males).
Implications for research.
This review did not find any eligible RCTs that reported the effects of alcohol on women separately. Because women could be affected differently by alcohol than men, future RCTs in women are needed. If future RCTs include both men and women, it is important that their blood pressure and heart rate readings are reported separately. Although eligible studies included East Asian, Latino, and Caucasian populations, they lacked African, South Asian, and Native Hawaiian/other Pacific Islander representation. Most of the hypertensive participants in the included studies were Japanese, so it is unclear if the difference in blood pressure between alcohol and placebo groups was due to the presence of genetic variants or the presence of hypertension. Large RCTs including both hypertensive and normotensive participants with various ethnic backgrounds are required to understand the effects of alcohol on blood pressure and heart rate based on ethnicity and the presence of hypertension. More RCTs are needed to study the effects of low‐dose alcohol to better delineate the dose‐response effects of alcohol on BP and heart rate. RCTs with measurements more than 24 hours after alcohol consumption are needed to see how long the effect of high‐dose acute alcohol consumption lasts.
History
Protocol first published: Issue 9, 2017 Review first published: Issue 7, 2020
Acknowledgements
We would like to acknowledge Douglas Salzwedel for designing the search strategy. We would also like to thank the Cochrane Hypertension Group members for their input.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily, and Versions(R) <1946 to July 13, 2018> Search Date: 14 July 2018 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp alcohol drinking/ 2 (alcohol$ or beer? or liquor? or spirit? or wine?).tw,kf. 3 or/1‐2 4 cardiovascular.mp. 5 hypertension/ 6 hypertens$.tw,kf. 7 exp blood pressure/ 8 (blood pressure or bp or dbp or sbp).mp. 9 exp heart rate/ 10 ((heart or pulse) adj2 rate?).tw,kf. 11 or/4‐10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 clinical trials as topic/ 17 randomly.ab. 18 trial.ti. 19 or/12‐18 20 animals/ not (humans/ and animals/) 21 19 not 20 22 3 and 11 and 21 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web) Search Date: 15 July 2018 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Alcohol Drinking EXPLODE ALL AND INSEGMENT #2 (alcohol* OR beer* OR liquor* OR spirit* OR wine) AND INSEGMENT #3 #1 OR #2 AND INSEGMENT #4 RCT:DE AND INSEGMENT #5 Review:ODE AND INSEGMENT #6 #4 OR #5 AND INSEGMENT #7 #3 AND #6 AND INSEGMENT ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search Date: 14 July 2018 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Alcohol Drinking EXPLODE ALL AND CENTRAL:TARGET #2 (alcohol* OR beer* OR liquor* OR spirit* OR wine) NEAR5 (consum* OR drink* OR usage OR use*):ti,ab AND CENTRAL:TARGET #3 #1 OR #2 AND CENTRAL:TARGET #4 cardiovascular AND CENTRAL:TARGET #5 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #6 hypertens* AND CENTRAL:TARGET #7 MESH DESCRIPTOR Blood Pressure EXPLODE ALL AND CENTRAL:TARGET #8 (blood pressure OR bp OR dbp OR sbp) AND CENTRAL:TARGET #9 MESH DESCRIPTOR Heart Rate EXPLODE ALL AND CENTRAL:TARGET #10 (heart OR pulse) NEAR2 rate* AND CENTRAL:TARGET #11 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 AND CENTRAL:TARGET #12 #3 AND #11 AND CENTRAL:TARGET ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2018 July 13> Search Date: 14 July 2018 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 drinking behavior/ (46148) 2 ((alcohol$ or beer? or liquor? or spirit? or wine?) adj10 (consum$ or drink$ or usage or use?)).tw. 3 or/1‐2 (172470) 4 cardiovascular.tw. (553583) 5 hypertens$.tw. (579052) 6 exp blood pressure/ (524780) 7 (blood pressure or bp or dbp or sbp).tw. 8 exp heart rate/ 9 ((heart or pulse) adj2 rate?).tw. 10 or/4‐9 11 randomized controlled trial/ 12 crossover procedure/ 13 double‐blind procedure/ 14 (randomi?ed or randomly).tw. 15 (crossover$ or cross‐over$).tw. 16 placebo.ab. 17 (doubl$ adj blind$).tw. 18 assign$.ab. 19 allocat$.ab. 20 or/11‐19 21 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 22 20 not 21 23 3 and 10 and 22 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 15 July 2018 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other terms: randomized Intervention/treatment: alcohol OR beer OR liquor OR spirits OR wine Study type: Interventional Studies (Clinical Trials) Outcome Measures: blood pressure OR heart rate ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 15 July 2018 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ randomized AND blood pressure AND alcohol randomized AND blood pressure AND beer randomized AND blood pressure AND liquor randomized AND blood pressure AND spirits randomized AND blood pressure AND wine randomized AND heart rate AND alcohol randomized AND heart rate AND beer randomized AND heart rate AND liquor randomized AND heart rate AND spirits randomized AND heart rate AND wine
Data and analyses
Comparison 1. Low‐dose alcohol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Systolic blood pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 ≤ 6 hours | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.48 [‐8.38, 5.42] |
| 1.2 Diastolic blood pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 ≤ 6 hours | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐6.91, 3.99] |
| 1.3 Mean arterial blood pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 ≤ 6 hours | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐4.55, 1.65] |
| 1.4 Heart rate | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 ≤ 6 hours | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 5.06 [1.88, 8.24] |
1.1. Analysis.

Comparison 1: Low‐dose alcohol vs placebo, Outcome 1: Systolic blood pressure
1.2. Analysis.

Comparison 1: Low‐dose alcohol vs placebo, Outcome 2: Diastolic blood pressure
1.3. Analysis.

Comparison 1: Low‐dose alcohol vs placebo, Outcome 3: Mean arterial blood pressure
1.4. Analysis.

Comparison 1: Low‐dose alcohol vs placebo, Outcome 4: Heart rate
Comparison 2. Medium‐dose alcohol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Systolic blood pressure | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 ≤ 6 hours | 9 | 260 | Mean Difference (IV, Fixed, 95% CI) | ‐5.63 [‐8.25, ‐3.02] |
| 2.1.2 7 to 12 hours | 4 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐3.22 [‐8.37, 1.93] |
| 2.1.3 ≥ 13 hours | 4 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐3.90, 5.18] |
| 2.2 Diastolic blood pressure | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 ≤ 6 hours | 9 | 260 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐6.02, ‐2.00] |
| 2.2.2 7 to 12 hours | 4 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐4.29, 1.90] |
| 2.2.3 ≥ 13 hours | 4 | 112 | Mean Difference (IV, Fixed, 95% CI) | 1.78 [‐0.95, 4.51] |
| 2.3 Mean arterial blood pressure | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 ≤ 6 hours | 12 | 360 | Mean Difference (IV, Random, 95% CI) | ‐2.92 [‐5.76, ‐0.07] |
| 2.3.2 7 to 12 hours | 4 | 108 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐4.69, 0.48] |
| 2.3.3 ≥ 13 hours | 4 | 112 | Mean Difference (IV, Random, 95% CI) | 1.43 [‐1.18, 4.04] |
| 2.4 Heart rate | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 ≤ 6 hours | 12 | 344 | Mean Difference (IV, Fixed, 95% CI) | 4.62 [3.14, 6.11] |
| 2.4.2 7 to 12 hours | 4 | 108 | Mean Difference (IV, Fixed, 95% CI) | 1.22 [‐1.88, 4.32] |
| 2.4.3 ≥ 13 hours | 3 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [‐2.12, 4.86] |
2.3. Analysis.

Comparison 2: Medium‐dose alcohol vs placebo, Outcome 3: Mean arterial blood pressure
2.4. Analysis.

Comparison 2: Medium‐dose alcohol vs placebo, Outcome 4: Heart rate
Comparison 3. High‐dose alcohol vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Systolic blood pressure | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 ≤ 6 hours | 16 | 620 | Mean Difference (IV, Random, 95% CI) | ‐3.49 [‐6.03, ‐0.95] |
| 3.1.2 7 to 12 hours | 3 | 88 | Mean Difference (IV, Random, 95% CI) | ‐3.72 [‐6.97, ‐0.48] |
| 3.1.3 ≥ 13 hours | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 3.69 [2.33, 5.05] |
| 3.2 Diastolic blood pressure | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 ≤ 6 hours | 14 | 532 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐3.86, 0.04] |
| 3.2.2 7 to 12 hours | 3 | 88 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐4.59, 1.17] |
| 3.2.3 ≥ 13 hours | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 2.37 [0.25, 4.49] |
| 3.3 Mean arterial blood pressure | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 ≤ 6 hours | 17 | 640 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐3.34, 0.28] |
| 3.3.2 7 to 12 hours | 3 | 88 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐5.69, 0.75] |
| 3.3.3 ≥ 13 hours | 4 | 188 | Mean Difference (IV, Random, 95% CI) | 2.96 [0.35, 5.58] |
| 3.4 Heart rate | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.4.1 ≤ 6 hours | 17 | 704 | Mean Difference (IV, Random, 95% CI) | 5.75 [3.99, 7.51] |
| 3.4.2 7 to 12 hours | 5 | 198 | Mean Difference (IV, Random, 95% CI) | 6.16 [3.04, 9.28] |
| 3.4.3 ≥ 13 hours | 6 | 298 | Mean Difference (IV, Random, 95% CI) | 2.70 [0.80, 4.60] |
3.3. Analysis.

Comparison 3: High‐dose alcohol vs placebo, Outcome 3: Mean arterial blood pressure
3.4. Analysis.

Comparison 3: High‐dose alcohol vs placebo, Outcome 4: Heart rate
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agewall 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial, single blinded, cross‐over design | |
| Participants | 12 adult volunteers (8 male and 4 female) with mean age of 31 years were included in the study Inclusion criteria:
|
|
| Interventions |
Both were consumed over 10 minutes |
|
| Outcomes |
Measured at baseline and 60 minutes after consumption of the drink |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The subjects drank 250 ml of red wine with or without alcohol over 10 min according to a randomized procedure” Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | “Subsequently the subjects were randomly allocated to drink over 10 min either 250 ml of a Cabernet Sauvignon/Merlot red wine containing 12·5% alcohol by volume or 250 ml of a de‐alcoholized red wine containing less than 0.5% alcohol by volume” Comment ‐ no information was provided on the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Images were digitally acquired from the videotape and measured in random order by a single observer blinded to the phase of the study and randomization code" Comment ‐ blinding of outcome assessors was probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All subjects returned for a second examination within one week” Comment ‐ all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ study authors did not report SBP |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP . |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SD |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "This study was supported by grants from the Swedish Medical Research Council, Swedish Medical Society" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Barden 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial, 3‐period open‐label cross‐over study | |
| Participants | 25 men with mean age of 56 years were recruited for the study Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “They were randomized using permuted block randomization by a statistician who was not involved with the study to drink a different beverage each day” Comment ‐ method of random sequence generation was not reported but reported baseline characteristics were well matched |
| Allocation concealment (selection bias) | Unclear risk | “Subjects enrolled after screening and medical examination by research nurse who requests treatment allocation from a statistician not involved in the trial” [from protocol] Comment ‐ method of allocation concealment was unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Twenty‐four men completed the study; one withdrew because of work commitments” Comment ‐ only 1 participant withdrew from the study and the reason was reported |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR in a figure |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "This work was funded by a project grant from the National Health and Medical Research Council of Australia" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | Low risk | "The trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN12608000467336" |
Bau 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, parallel‐group assignment | |
| Participants | 100 males with mean age of 20.74 ± 2.36 (SD) years (range 18 to 25 years) with no history of cardiovascular disease or medication use and non‐smokers Baseline characteristics:
|
|
| Interventions |
|
|
| Outcomes |
Measurements were made at 10 PM (4 hours after intervention) and at 7 AM (13 hours after intervention) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation of groups to either an alcohol containing drink or a similar nonalcoholic beverage was performed with a random seed generator" Comment: adequate randomisation was done and baseline characteristics of groups were well matched |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Investigators and volunteers were blinded to the content of the drink, with or without alcohol" Comment: even though study authors mentioned investigators and volunteers were blind to content of the drink, method of blinding was not mentioned in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The same sonographer, certified for heart and vascular ultrasound, blind to the control and test groups, evaluated all subjects" Comment: adequate blinding of outcome assessors was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "The Instituto Cardiovascular (ICARDIO) and Conselho Nacional de Desenvolvimento Cienti'fico e Tecnolo'gico (CNPq) funded this research" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Bau 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, parallel‐group assignment | |
| Participants | 70 healthy men with mean age of 20.7 ± 2.4 (SD) years (range 18 to 25 years) without cardiovascular disease, not using drugs with cardiovascular effects, and non‐smokers were recruited from general population for the study Baseline characteristics:
|
|
| Interventions |
|
|
| Outcomes |
Measured during pre‐ingestion; 1 to 4 hours after ingestion (high plasma levels of alcohol, with vasodilation and lower blood pressure); 4 to 10 hours after ingestion (vanishing of alcohol); and between 10 and 17 hours after ingestion (after elimination, when there is a late increase in blood pressure) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation to the alcohol and control groups was performed with a random seed generator" Comment ‐ baseline data between groups were matched. Adequate randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Investigators and volunteers were blinded to the content of the drink" Comment ‐ even though study authors mentioned investigators, and volunteers were blinded to the content of the drink, method of blinding was not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An investigator blinded in relation to the experimental groups performed the evaluations" Comment ‐ adequate blinding of outcome assessment was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ study authors did not report SBP |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SD |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "Grants from the Instituto Cardiovascular (ICARDIO) and Conselho Nacional de Desenvolvimento Cientı´fico e Tecnolo´gico (CNPq) funded this research. Disclosures: none" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Buckman 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial, parallel‐group assignment, open‐label study | |
| Participants | 72 healthy participants with average age of 21.7 years (SD = 0.9). Among participants, 44% were female Exclusion criteria:
|
|
| Interventions |
Each beverage was divided into 3 equal drinks, and participants were instructed to consume each beverage evenly over a 5‐minute period (total drinking time = 15 minutes) |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were randomly assigned to the alcohol, placebo, or no‐alcohol beverage group” "Upon arrival in the laboratory, weight, height, pregnancy status, and a zero blood alcohol concentration (BAC) were confirmed and participants were randomly assigned to the alcohol, placebo, or no‐alcohol beverage group, as described below" "Table 1 shows that groups were not statistically different in terms of demographics, family history of alcohol dependence, alcohol use, and mood" Comment ‐ adequate randomisation was probably done and baseline characteristics between groups were well matched |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Participants in the alcohol group (n = 24) were told that they would receive some amount of alcohol and were given mixer (orange, cranberry, and lime juice) with an active ethanol…” “Participants in the placebo group (n = 24) were told that they would be given some amount of alcohol and received mixer with a physiologically inactive dose of alcohol…” “The no‐alcohol control group (n = 24) were told that they would not be given alcohol and received 100% mixer” Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all participants were included in the final analysis (additional information from study author) |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SD |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP and SD |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SD |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "This study was funded with the support of K01AA017473, K02AA00325, K24AA021778, R21AA020367, and HHSN275201000003C" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Chen 1986.
| Study characteristics | ||
| Methods | Randomised controlled trial, parallel‐group assignment, open‐label study | |
| Participants | 20 college students, both male and female, between the ages of 19 and 32 years, participated in the study Inclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After the initial screening, subjects were randomly assigned to either an alcohol group or a control group" Comment ‐ method of randomisation was not reported; baseline characteristics of groups were not reported either |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Two subjects in the alcohol group dropped from the study for unknown reasons. Data analyses were based on 8 subjects in the alcohol group and 10 subjects in the control group" Comment ‐ 2 participants withdrew from alcohol‐receiving group; reasons for withdrawal are not mentioned |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP along with standard deviation (SD) |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP along with standard deviation (SD) |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR along with standard deviation (SD) |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any conflicts of interest or sponsorship |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Cheyne 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, cross‐over design | |
| Participants | 17 participants (14 male, 3 female) with mean age of 35 years (range 21 to 46 years), with type 1 diabetes, HbA1c 8.1% (1.4%) [mean (SD)], and duration of diabetes 19 years (12 years) were studied. All were drivers and drank alcohol (between 1 and 14 units per week). They did not have a history of alcoholism or drug abuse and were not taking any other medication apart from insulin | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects participated in four studies in a random order: (A) Euglycaemia (4.5 mmol/l) with placebo (B) Euglycaemia (4.5 mmol/l) with alcohol (C) Hypoglycaemia (2.8 mmol/l) with placebo (D) Hypoglycaemia (2.8 mmol/l) with alcohol" "(order determined using computer‐generated random selection of 4 × 4 Latin squares [15] and concealed using opaque envelopes" Comment ‐ adequate randomisation was done |
| Allocation concealment (selection bias) | Low risk | "(order determined using computer‐generated random selection of 4 × 4 Latin squares [15] and concealed using opaque envelopes" Comment ‐ adequate allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Subjects were blinded to the content of the drink and the prevailing blood glucose level, although some reported that they were able to detect alcohol by taste" Comment ‐ blinding of participants was not successful |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistant supervising cognitive function tests was blinded to both contents of the drink and blood glucose level Comment ‐ adequate blinding of outcome assessment was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ study authors did not report anything about patient withdrawal or whether all participants completed all 4 sessions |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP, along with the standard deviation (SD) |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP along with the standard deviation (SD) |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR along with the standard deviation (SD) |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "This study was supported by a grant from the South‐west NHS R&D executive" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Dai 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, cross‐over design | |
| Participants | 20 participants with high risk and 20 participants with low risk of alcoholism (total 40 participants) were included in the study. All participants were men and were between 19 and 25 years old Inclusion criteria: not reported Exclusion criteria:
|
|
| Interventions |
Beverage was consumed as a single drink within 5 minutes |
|
| Outcomes |
HR, SBP, and DBP measurements were taken at baseline and at 5, 20, 35, 50, 65, 95, 125, 155, 185, 215, and 245 minutes after ingestion of the drink |
|
| Notes | All participants were requested to abstain from alcohol for 48 hours before each testing session | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Each subject participated in four testing sessions administered in a random order; the sessions were double‐blinded with respect to the ingestion of the alcohol or placebo drink” “The HR and LR subjects were matched for age, body weight, education, smoking behavior, and drinking behavior, such as age at onset and quantity and frequency of drinking (Table 1)” Comment ‐ method of randomisation was not described but baseline characteristics were matched |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The lips of the glass were dipped in alcohol to give it the smell and taste of alcohol. Subjects initially could not distinguish between the placebo and alcohol drinks. However, in the absence of the pharmacological effects of ethanol, some subjects did suspect that they had consumed a placebo drink" Comment ‐ adequate attempt was made to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All measurements of the cardiovascular function were taken manually, while the subjects reclined in bed, by a research nurse blinded to the treatment and risk type of the subjects" Comment ‐ adequate blinding of outcome assessment was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total 20 HR and 20 LR subjects completed the study" Comment ‐ all 40 participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR in a figure |
| Other bias (conflict of interest, industry sponsorship) | Low risk | Comment ‐ study was supported by the Canadian Institutes on Health Research |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Dumont 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial, 4‐way, double‐blind, cross‐over design | |
| Participants | 16 healthy volunteers (9 male and 7 female) with mean age (SD) of 22.1 (2.9) years (range 18 to 29) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | Cardiovascular function assessed by:
Plasma concentration of:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Sixteen volunteers were randomly assigned to one of the four treatment sequences” Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment ‐ method of blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The operator of the breath alcoholmeter and the ethanol infusion pump was unblinded for alcohol treatment, but did not communicate with the study team or the subject about the results at any stage during the trial. A sham procedure including a mock‐spreadsheet was used on ethanol‐placebo‐occasions" Comment ‐ it is not mentioned whether heart rate and blood pressure were measured in a blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant had a mild adverse reaction (local vascular reaction) to the ethanol infusion, and 1 participant did not refrain from drug use; both (1 male and 1 female) were excluded from further participation and results obtained from these individuals were not included in the final data analysis Comment ‐ reasons for exclusion of 2 participants were reported and balanced across groups, so missing data should not affect the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ study authors did not provide the before and after measure of SBP. They mentioned that the change was not significant |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not provide the before and after measure of DBP. They mentioned that the change was not significant |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP in a figure |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR in a figure |
| Other bias (conflict of interest, industry sponsorship) | Low risk | This research was supported by a grant from ZonMW (31000062), the Netherlands, and complies with current laws |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Fantin 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label with cross‐over design | |
| Participants | 18 healthy adults (12 male and 6 female) with mean age 34.2 years (range 25 to 53 years) Exclusion criteria:
|
|
| Interventions | 250 mL of red wine (12% of ethanol) | |
| Outcomes | Heart rate, BP, and arterial stiffness, as assessed by QKD | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The subjects received the two days in random order” “The subjects were randomly allocated to either consuming alcohol on the first day (control day second) or the control day first” Comment ‐ method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Our study sample was composed of 21 healthy adult volunteer men and women. 3 of them decided not to go on with the study so the final sample was composed of 18 subjects" Comment ‐ study included only participants who finished the study, hence participant withdrawal did not affect results. 18 participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ SBP data with SD were reported |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ DBP data with SD were reported |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ MAP data were not reported |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ HR data with SD were reported |
| Other bias (conflict of interest, industry sponsorship) | Low risk | “The authors declare no conflict of interest” |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Fazio 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label with cross‐over design | |
| Participants | 10 young healthy and normotensive male volunteers with mean age of 22 ±2.1 (SD) years (range 20 to 25 years) | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “In random order the subjects drank 95% ethanol, 0.3 g/kg in 250 mL water, in the sitting position in 5 minutes or had 95% ethanol….” Comment ‐ method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ because this study was open‐label, outcome measurement was likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ SBP data with SD were not reported |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ DBP data with SD were not reported |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ MAP data with SD were reported |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ HR data with SD were reported |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any funding source or conflict of interest |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Foppa 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label with cross‐over design | |
| Participants | 13 hypertensive and centrally obese individuals (8 postmenopausal women and 5 men) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Measured at the following time intervals: postprandial (3 hours from intervention), daytime (1200 hours to 2200 hours), nighttime (2200 hours to 0700 hours), and overall (19‐hour interval, from intervention at 1200 hours to 0700 hours, Day 2) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “the randomization order in this crossover randomized clinical trial was done with sealed randomized envelopes in blocks of 8 (envelopes were prepared by a third person unaware of research objectives or protocol)" [information was provided by study author] Comment ‐ adequate random sequence generation was done |
| Allocation concealment (selection bias) | Low risk | “the randomization order in this crossover randomized clinical trial was done with sealed randomized envelopes in blocks of 8 (envelopes were prepared by a third person unaware of research objectives or protocol)" [information was provided by study author] Comment ‐ adequate allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all 13 participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SD in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP with SD in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP with SD |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR with SD in a figure |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any funding source or conflict of interest |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Hering 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label with cross‐over design | |
| Participants | Total 24 participants; 13 patients (8 men and 5 women) with essential hypertension and 11 normotensive controls (6 men and 5 women) matched for sex, age (44 ± 2 vs 43 ± 2 years, respectively; mean ± SEM), and BMI (29 ± 1 vs 28 ± 1 kg/m², respectively) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Administered over a period of 20 minutes |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The study design was randomized and placebo‐controlled with two experimental sessions (an alcohol session and a vehicle session). The sessions were performed at the same time of day on two separate days in random order” "The assignment of subjects to either begin the study protocol with an alcohol session or vehicle session was performed by a random allocation using the randomized computer generated number table" [information was given by study authors] Comment ‐ adequate random sequence generation was done and baseline characteristics were matched |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP with SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR with SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | “These studies were supported by NIH HL61560 and NIH FIRCA Award R03 TW0 1148. The authors are also supported by European Union LSHM‐CT‐2006‐037093 InGenious grant (K.N. and W.K.), and by Foundation for Polish Science TEAM/2008‐2/5 (K.N. and W.K.) and MISTRZ 8/2008 (K.N. and D.H.) grants” “There are no conflicts of interest” |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Karatzi 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, cross‐over design | |
| Participants | 15 male participants with coronary artery disease with mean age of 52.4 ± 9.7 (SD) years and BMI of 28.3 ± 1.8 kg/m² Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Assessed at fast and at 30, 60, and 90 minutes postprandially |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly allocated to drink either a glass of 250 mL of red wine or 250 mL of dealcoholized red wine" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “duo–trio tests* and a hedonic scale were used to test whether de‐alcoholised red wine could be well accepted by the subjects who would consume it, and whether it could be recognized compared with regular red wine” “results from the duo–trio tests and the hedonic scale analysis showed that the subjects could not distinguish the two wines, as they could not correctly recognize the presence or absence of alcohol” Comment ‐ it is not clear whether personnel who administered the intervention were blinded as well |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment ‐ it is not mentioned whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP with SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP with SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR with SEM |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any funding source or conflict of interest |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Karatzi 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial, single‐blinded, cross‐over design | |
| Participants | 17 healthy, non‐smoking male volunteers with a mean age of 28.5 ± 5.2 years and body mass index (BMI) of 24.4 ± 2.5 kg/m2 Exclusion criteria:
|
|
| Interventions |
Consumed within 15 minutes |
|
| Outcomes |
Assessed at fast and at 1 and 2 hours postprandially |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "all volunteers consumed in a randomized order either a) 400 ml of beer & 400 ml water, b) 800 ml of dealcoholized beer (same amount of polyphenols), or c) 67 ml of vodka & 733 ml water" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The same trained observer who was blinded for the type of intervention performed all measurements" Comment ‐ blinding of outcome assessors was probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Data from 16 subjects were available for the final analysis due to missing values related to poor quality of vascular recordings in at least one of the visits” Comment ‐ reasons for exclusion of a participant were reported and balanced across groups, so missing data should not affect the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ study authors did not report SBP |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP with 95% confidence interval |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR with 95% confidence interval |
| Other bias (conflict of interest, industry sponsorship) | Low risk | “The authors declare that they have no conflict of interest” |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Kawano 1992.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 16 Japanese men (22 to 70 years old) with essential hypertension. Mean age was 55.2 ± 3.3 years (mean ± SEM) | |
| Interventions |
Ingested at dinner |
|
| Outcomes | Ambulatory blood pressure and heart rate were measured every 30 minutes until noon the next day | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the order of alcohol intake day and control day was randomized to minimize the influences of various factors including the order effect" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data for three patients were excluded because the ambulatory recordings were not available for the entire period" Comment ‐ reasons for exclusion of 3 participants were reported and balanced across groups, so missing data should not affect the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SD |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP with SD |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR with SD |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "Supported by Research Grant for Cardiovascular Diseases 63C‐2 from the Ministry of Health and Welfare" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Kawano 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 10 Japanese hypertensive patients with mean age ± SEM of 54 ± 3 years and body weight 70 ± 2 kg Inclusion criteria:
|
|
| Interventions |
Consumed within 60 minutes
|
|
| Outcomes |
BP and HR were measured every 30 minutes for 24 hours |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The order of the alcohol and control days was randomized" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP with SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR with SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "This study was supported by a Research Grant for Cardiovascular Diseases 5A‐2 from the Ministry of Health and Welfare, and a grant from Takeda Medical Research Foundation" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Koenig 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 15 males between the ages of 20 and 35 years (average gross height 184 cm, weight 76 kg) were enrolled in the study Exclusion criteria:
|
|
| Interventions |
Consumed within 30 minutes |
|
| Outcomes |
Measured at baseline and at 2 and 3 hours after consumption |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SD in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP with SD in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | High risk | Comment ‐ study authors did not report HR |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not mention any funding source or declare any conflict of interest |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Kojima 1993.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 21 men with essential hypertension and mean (SD) age of 56.5 (11.8) were enrolled in the study Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Measured 30 minutes before and 2 hours after consumption |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “with a randomized, balanced crossover design, the effects of alcohol in the form of vodka made up to 500 ml with lime juice, were compared with a control drink..” Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Although serum insulin was measured in only 20 patients, the other parameters were measured in all 21 patients" Comment ‐ all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | “This study was supported in part by Research Grants for Cardiovascular Disease (63‐2C and 2C‐3) from the ministry of health and welfare“ |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Mahmud 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, cross‐over design | |
| Participants | 8 healthy normotensive individuals (3 men and 5 women) between the ages of 21 and 40 years with mean ± SEM body weight of 70 ± 3.9 kg | |
| Interventions |
Consumed within 10 minutes |
|
| Outcomes |
Measured at baseline and at 30, 60, and 90 minutes after ingestion of either drink |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant, studied while fasting, consumed 500 mL of red wine (0.8 g/kg ethanol) or 500 mL of red wine without alcohol within 10 minutes in a double‐blind, randomised, cross‐over fashion Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment ‐ method of blinding of participants and personnel was not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment ‐ method of blinding of outcome assessors was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all participants were included in the final analysis according to Figure 1 |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not mention any funding source nor declare any conflict of interest |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Maufrais 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 24 healthy young individuals (12 men, 12 women) of European descent with mean ± SD age of 23.3 ± 2.2 years and weight 62.9 ± 10.1 kg Exclusion criteria:
|
|
| Interventions | Participants ingested 1 of the following 4 drinks at a temperature of around 10°C (at a convenient pace over 5 minutes):
|
|
| Outcomes |
Measured before drinking and at 15, 30, 60, 90, and 120 minutes post drinking |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random sequence generator (http://www.random.org/sequences/) where the session order was determined for 24 test subjects before the study started" |
| Allocation concealment (selection bias) | Unclear risk | "The test subjects were not allowed to know the order of their sessions in advance" Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ study authors did not report SBP |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest" "This work was supported by the Swiss Foundation for Alcohol Research (project 276) and in part by the Swiss National Science Foundation" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Maule 1993.
| Study characteristics | ||
| Methods | Randomised controlled trial, single‐blind, cross‐over design | |
| Participants | 10 healthy individuals (6 male, 4 female) with mean age of 31 (range 22 to 51) and mean weight of 68.1 kg were included in the study | |
| Interventions |
Consumed within 10 minutes |
|
| Outcomes |
Blood pressure and heart rate were measured at baseline and every 5 minutes until the 45th minute |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “subjects were then randomized to receive a drink of either alcohol or placebo which was ingested with a straw over 10 mins in supine position” Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment ‐ it is not clear who was blinded or how blinding was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment ‐ it is not clear who was blinded or how blinding was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any conflict of interest nor sponsorship |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Narkiewicz 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blind, cross‐over design | |
| Participants | 19 healthy young volunteers (18 men and 1 women) with mean age ± SD of 26 ± 2 years participated in the study | |
| Interventions |
Consumed over a 30‐minute period |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Measurements were obtained by use of a randomized, double‐blind, placebo‐controlled design with 2 experimental sessions, a placebo session and an alcohol session" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A flavoring (Crystal Light) was added to these solutions to prevent the subjects from distinguishing alcohol from placebo" Comment ‐ blinding of participants was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Studies were analyzed by investigators blinded to session (alcohol or placebo)" Comment ‐ blinding of outcome assessors was probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Vasovagal responses or discomfort during LBNP in 5 subjects resulted in completion of studies examining the effects of both alcohol and placebo in only 14 subjects (13 men, 1 woman; mean age, 26±2 years)" Comment ‐ reasons for exclusion of participants were reported and balanced across groups, so missing data should not affect the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "Dr. Narkiewicz, a visiting research scientist from the Department of Hypertension and Diabetology, Medical University of Gdansk, Gdansk, Poland, was a recipient of an International Research John E. Fogarty Fellowship (NIH 3F05 TW05200) and a Perkins Memorial Award from the American Physiological Society. Dr. Somers is an Established Investigator of the AHA and a Sleep Academic Awardee of the NIH. Other support includes HL61560 and HL65176 (NIH). We thank Diane Davison, RN, MA, for technical assistance" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Nishiwaki 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial, single‐blind, cross‐over design | |
| Participants | 11 healthy young men with mean age ± SD of 21.1 ± 0.2 years, body weight 62.6 ± 2.6 kg | |
| Interventions |
Consumed within 5 minutes |
|
| Outcomes |
Measured at baseline and at 30, 60, and 90 minutes after ingestion |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All volunteers participated in four trials assigned in random sequence to four separate days" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All of the test drinks were poured into paper cups to maintain participant blinding (i.e. single‐blind study)" Comment ‐ blinding of participants was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "the same investigators performed all measurements" Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "The authors declare that there is no conflict of interests regarding the publication of this article" "This study was supported in part by a Grant‐in‐Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology to MN (JSPS KAKENHI Grant number JP 26750345)" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Potter 1986.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blind, cross‐over design | |
| Participants | 16 normotensive male medical students (8 with a family history of hypertension) with mean age of 22 years and mean body weight of 77 kg were included in the study | |
| Interventions |
Consumed within 15 minutes |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each subject then drank 600 ml of Barbican, an alcohol‐free lager (Canada Dry Rawlings, Northants, UK) kept at room temperature, with or without the addition of 50% alcohol in a double‐blind, random‐order crossover design" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "At the end of each part of the study, subjects were asked to indicate whether they thought they had received the placebo (alcohol‐free beer) or placebo with added alcohol" "Subjects were blinded to the order of treatment, and only eight of the 16 subjects recognized both stages correctly, indicating that the blinding procedure was reasonably successful" Comment ‐ blinding of participants was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment ‐ method of blinding of outcome assessors was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | .Comment ‐ study authors reported DBP and SEM in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM in a figure |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "Dr. Potter was supported by a research grant from the British Heart Foundation" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Rosito 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blind, parallel‐group assignment | |
| Participants | 40 male medical students with mean age of 22.2 years were included in the trial | |
| Interventions |
|
|
| Outcomes |
Measured every hour for 24 hours |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Between 11:00 am and noon, according to a random allocation and without knowledge of exact ethanol content, the study subjects ingested water, 15, 30, or 60 g of ethanol" "The randomization (with random number allocator) was done in blocks of four" (information was given by study authors upon contacting) Comment ‐ adequate randomisation was done |
| Allocation concealment (selection bias) | Low risk | "The randomization (with random number allocator) was done in blocks of four" (information was given by study authors upon contacting) Comment ‐ adequate allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment ‐ investigator who gave the drink was blinded to the content of alcohol, so adequate blinding of participants and personnel was done (information was given by study authors upon contacting) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The data were analyzed in a blinded fashion using analysis of variance (ANOVA) for repeated measurements and multiple factors to assess the effects of quantity of ethanol ingested, time after ingestion, and their interaction" Comment ‐ blinding of outcome assessment was probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM in a figure |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any conflict of interest nor sponsorship |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Rossinen 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 20 individuals (17 men and 3 women, aged 39 to 68 years) with angiographically verified coronary heart disease and electrocardiographic evidence of myocardial ischaemia were included in the study Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Participants were instructed to drink at an even pace over the 1 and 1/2 hours |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized to either alcohol or juice in a crossover manner" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SD |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SD |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any conflict of interest nor sponsorship |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Stott 1987.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 10 healthy individuals (7 male, 3 female) aged 18 to 31 years with body weight of 56 to 101 kg | |
| Interventions |
|
|
| Outcomes |
Measured at baseline (13.00), and at 14.00, 15.00, 16.00, 17.00, 19.00, and 22.00 hours that day; 09.00 and 14.00 hours the next day; and 14.00 hours for a further 5 days |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In a randomized, balanced, crossover study the effects of 1.3 g of alcohol/kg body weight, in the form of vodka made up to 1 litre with orange juice, were compared with 1 litre of orange juice made isocaloric with glucose (1 g alcohol = 7 kcal)" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Results were assessed independently by two observers” Comment ‐ study was not blinded for outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all 10 participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM in a figure |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any conflict of interest nor sponsorship |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Stott 1991.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 8 normotensive elderly participants (6 male, 2 female) with mean age of 81 years (range 70 to 96 years) and mean body weight of 68.4 kg were included in the study | |
| Interventions |
Each consumed over 15 minutes |
|
| Outcomes |
Baseline recording was taken at 1:00, and further recordings were taken at 1:30, 2:00, 3:00, and 4:00 PM |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In a randomized, balanced, crossover study design, the effects of 0.5 g of alcohol/kg body weight, in the form of vodka made up to 200 mls with unsweetened orange juice, were compared with the effects of 200 mls of orange juice alone, each consumed over 15 minutes" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment ‐ all 8 participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SEM in a figure |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SEM in a figure |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP and SEM |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM in a figure |
| Other bias (conflict of interest, industry sponsorship) | Unclear risk | Comment ‐ study authors did not report any conflict of interest nor sponsorship |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Van De Borne 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial, double‐blinded, cross‐over design | |
| Participants | 16 normal male individuals with mean age of 26 ± 4 (SD) years | |
| Interventions |
Consumed over 30 minutes |
|
| Outcomes |
Measured at baseline and at 45, 60, and 80 minutes after ingestion |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A flavoring (Crystal Light) was added to these solutions to prevent the subjects from distinguishing the alcohol from the vehicle session" Comment ‐ adequate blinding of participants and personnel was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Measurements were made by a single observer (P. van de B.) in a blinded fashion" "Statisical analysis was performed by an independent statistician" Comment ‐ adequate blinding of outcome assessment was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | High risk | Comment ‐ study authors did not report SBP |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | High risk | Comment ‐ study authors did not report DBP |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | Low risk | Comment ‐ study authors reported MAP and SEM in a figure |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SEM in a figure |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "These studies were also supported by a Grant‐in‐Aid from the American Heart Association, National Institutes of Health (NIH) grants HL‐14388 and HL‐24962, and an NIH Sleep Academic Award" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Williams 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, cross‐over design | |
| Participants | 14 men with mean age of 59 ± 7 (SD) years and body weight of 86 ± 13 (SD) kg were included in the study Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
BP and HR were measured at baseline and at 1 hour and 6 hours after consumption |
|
| Notes |
We calculated MAP from reported SBP and DBP. SD for the MAP had to be imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized to receive red wine (Stoneleigh Marlborough Pinot Noir, New Zealand, 1998) or white wine (Jackson Estate Marlborough Sauvignon Blanc, New Zealand, 1998) followed by the alternate wine with an interval of at least a week between each intervention" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One subject was excluded from the present study due to insufficient stored plasma for measurement of cytokines at all time points" Comment ‐ reasons for exclusion of participants were reported and balanced across groups, so missing data should not affect the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP with SD |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SD |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SD |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "Supported by the Cardiology Department Research Fund" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
Zeichner 1985.
| Study characteristics | ||
| Methods | Randomised controlled trial, open‐label, parallel‐group assignment | |
| Participants | 48 healthy psychology undergraduate students (24 male and 24 female) with mean age of 20.9 ± 2.18 (SD) years (range 19 to 23) | |
| Interventions |
All participants were told that they must consume the first drink within 10 minutes and the second drink within the subsequent 10 minutes |
|
| Outcomes |
Baseline and 40 minutes after alcohol consumption |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Healthy college age males and females classified as type A and Type B were randomly assigned to an alcohol group or a no alcohol group" Comment ‐ method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment ‐ study was not blinded for participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment ‐ study was not blinded for outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment ‐ it is not explicitly mentioned whether all participants were included in the final analysis |
| Selective reporting (reporting bias) For systolic blood pressure (SBP) | Low risk | Comment ‐ study authors reported SBP and SD |
| Selective reporting (reporting bias) For diastolic blood pressure (DBP) | Low risk | Comment ‐ study authors reported DBP and SD |
| Selective reporting (reporting bias) For mean arterial blood pressure (MAP) | High risk | Comment ‐ study authors did not report MAP |
| Selective reporting (reporting bias) For heart rate (HR) | Low risk | Comment ‐ study authors reported HR and SD |
| Other bias (conflict of interest, industry sponsorship) | Low risk | "This research was supported in part by a grant from the University of Georgia Research Foundation, Inc" |
| Other bias (was the study registered in clinical trials.gov/ was the protocol available?) | High risk | Comment ‐ protocol was not registered and study identifier was not reported |
ACE: angiotensin‐converting enzyme. ADH: alcohol dehydrogenase. AIx: augmentation index. BAC: blood alcohol concentration. BMI: body mass index. BP: blood pressure. bpm: beats per minute. CAD: coronary artery disease. DBA: diameter of brachial artery. DBP: diastolic blood pressure. DRW: de‐alcoholised red wine. DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. EtOH: ethanol. HbA1c: glycosylated haemoglobin. HETE: hydroxyeicosatetraenoic acid. HR: heart rate. HRV: heart rate variability. IL‐6: interleukin‐6. MAP: mean arterial pressure. MDMA: methylenedioxymethamphetamine. NE: norepinephrine. NSAID: non‐steroidal anti‐inflammatory drug. SBP: systolic blood pressure. SD: standard deviation. SEM: standard error of the mean. SNA: sympathetic nervous activity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 1979 | This study used the wrong study design. Heart rate measurements in this study were divided into 14 phases, and all 14 phases were affected by social anxiety (stress) |
| Abu‐AmshaCaccetta 2001 | This study used the wrong study design. Because the intervention was consumed continuously for 2 weeks and measurements were made only after 2 weeks, this study did not meet our inclusion criteria "Volunteers were randomly allocated to drink either: (i) 375 ml of red wine (Shiraz‐Grenache Blend, 13.3% alc/vol, 1200 mg/l polyphenols); or (ii) 375 ml of white wine (Deakin Estate, Chardonnay, 13.7% alc/vol, 345 mg/l polyphenols) 'polyphenol control'; or (iii) 500 ml of the same red wine but dealcoholized (< 2% alc/vol, 905 mg/l polyphenols) 'alcohol control,' each evening for 2 weeks, with a 1 week washout at the start of the study and between each beverage" |
| Adamsson 2011 | This study used the wrong intervention. Nordic diet containing 27%, 52%, 19%, and 2% of energy from fat, carbohydrate, protein, and alcohol, respectively, was used as an intervention in this study. Because only 2% alcohol was contributed in this diet, alcohol was not the major intervention in this study |
| Aguilar 2004 | This study used the wrong study design because alcohol consumption was self‐reported "Patients were classified as non‐drinkers, light to moderate drinkers or heavy drinkers based on alcohol consumption reported at baseline" |
| Aigner 2016 | This was a case control study, so this study used the wrong study design "We performed a case‐control study based on a sample of 3308 stroke patients aged 18 to 55 years" |
| Ajani 2000 | This study used the wrong study design because alcohol consumption was self‐reported "We used four categories of alcohol intake at baseline ‐ rarely/never, monthly, weekly, and daily" |
| Almeida 2014 | This study used the wrong study design because alcohol consumption was self‐reported "We then asked men whether they had drunk alcohol during the last year (yes/no). Those who answered yes were required to indicate how many standard drinks of alcohol they consumed each usual day (from Monday to Sunday)" |
| Andres‐Lacueva 2013 | This study used the wrong intervention because it examined the effect of red wine polyphenols rather than the effect of alcohol |
| Anil 2016 | Alcohol was not given as an intervention in this study, and this study focused on the dietary pattern; hence this study used the wrong intervention and the wrong study design |
| Apostolidou 2015 | This was not a randomised controlled trial and red wine tannat rather than alcohol was given as an intervention; hence this study used the wrong study design and the wrong intervention |
| Appel 2003 | This study used the wrong intervention because alcohol was not used as an intervention |
| Argani 2016 | Red grape seed extract (RGSE) was used as the intervention in this study; hence this study used the wrong intervention |
| Ariansen 2009 | Losartan‐ or atenolol‐based antihypertensive therapy was used as the intervention rather than alcohol in this study; hence this study used the wrong intervention |
| Ariansen 2012 | Losartan‐ or atenolol‐based antihypertensive therapy was used as the intervention rather than alcohol in this study; hence this study used the wrong intervention |
| Assaad 2006 | This study used the wrong study design because it was not a placebo‐controlled study "Participants were explicitly told what they drank, and no placebo control group was used" |
| AuYeung 2013 | This study used the wrong study design because it was a cohort study and alcohol consumption was self‐reported |
| Avellone 2006a | This study examined the effects of 2 different red wines. It was not placebo‐controlled; hence this study used the wrong study design |
| Avellone 2006b | This study compared the effects of 2 different red wines rather than using a placebo control group; hence this study used the wrong study design |
| Bae 2015 | Alcohol was not used as an intervention in this study. Bisphenol A (BPA) from canned beverages was examined in this study; hence this study used the wrong intervention |
| Bailey 1989 | This study used the wrong intervention and the wrong outcome. Alcohol was not the only intervention given in this study. Blood pressure and heart rate were recorded but only when felodipine was given |
| Bailey 2003 | Alcohol was not used as an intervention in this study; hence this study used the wrong intervention |
| Banini 2006 | The intervention provided in this study consisted of muscadine grape products rather than ethanol; hence this study used the wrong intervention |
| Barden 2007 | This study used the wrong study design because it examined the reduction effect of alcohol "[Participants] were randomized to either continue their usual alcohol intake or reduce their alcohol intake..." |
| Barden 2017 | Blood pressure was measured at baseline and after each intervention period (4 weeks), which was not considered an acute effect of alcohol; hence this study used the wrong study design |
| Baros 2008 | This study used the wrong study design. Alcohol was not given as an intervention in this study. Alcohol consumption was self‐reported by participants during a specific period of time |
| Barskova 2005 | Metformin rather than alcohol was used as an intervention in this study; hence this study used the wrong intervention |
| Beevers 1987 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| Beilin 1992 | This study was a review; hence this study used the wrong study design |
| Beilin 1994 | Alcohol was not used as an intervention in this study; hence this study used the wrong intervention |
| Beilin 1996 | This study was a review; hence this study used the wrong study design |
| Bengtsson 1973 | This was an epidemiological study; hence this study used the wrong study design |
| Berg 2005 | This was an epidemiological study; hence this study used the wrong study design |
| Berglund 1989 | Alcohol was not used as an intervention in this study. Decreasing the consumption of alcohol and changing diet (increasing ratio of polyunsaturated to saturated fat, and increasing potassium intake) were used as interventions in this study; hence this study used the wrong interventions |
| Bermudez 2015 | This study used the wrong study design. Alcohol consumption was measured by asking participants to estimate the number of alcoholic drinks they consumed within a month; hence this study was based on self‐report |
| Beulens 2005 | Intervention duration of this study was 3 weeks, and neither blood pressure nor heart rate was recorded; hence this study used the wrong study design and the wrong outcomes |
| Beyeler 1987 | This study used the wrong intervention. Alcohol was not given as the only intervention. It was given with disulfiram treatment |
| Bjorntorp 1999 | This study used the wrong intervention because alcohol was not provided as an intervention |
| Blankenhorn 1990 | Alcohol was not the main intervention in this study, and data collected were self‐reported; hence this study used the wrong intervention and the wrong study design |
| Bleich 2001 | The intervention duration was 6 weeks for each intervention, which was not considered an acute effect; hence this study used the wrong study design |
| Bold 2017 | Alcohol consumption and data collected were self‐reported; hence this study used the wrong study design |
| Bond 1984 | This study used the wrong study design. Even though alcohol was the major intervention, measurements of heart rate and blood pressure were made only after exercise, and when the heart rate of 150 beats/min was reached "The subject then ingested the experimental liquid in five equal parts over a 10 minute period. After a 30 minute absorption period, fingertip blood samples were collected for determining blood alcohol levels. After completion of the absorption period, work bouts on the ergometer were initiated. Measures of heart rate, blood pressure, oxygen uptake and ventilation were recorded each minute after a heart rate of 150 beats/min was reached" |
| Botden 2011 | Red wine polyphenols rather than alcohol were given as the intervention; hence this study used the wrong intervention |
| Botden 2012 | Red wine polyphenols rather than alcohol were given as the intervention; hence this study used the wrong intervention |
| Brader 2011 | Nordic diet rather than alcohol was given as an intervention; hence this study used the wrong intervention |
| Bradford 1990 | Lovastatin was given as an intervention in this study; hence this study used the wrong intervention |
| Braggio 1992 | This was not a randomised controlled trial and it was questionnaire‐based; hence this study used the wrong study design |
| Brainin 2016 | Lifestyle modification rather than alcohol was used as an intervention in this study; hence this study used the wrong intervention |
| Brasser 2004 | Alcohol was not given as the only intervention in this study. The main purpose of this study was to examine the effect of alcohol on acamprosate; hence this study used the wrong intervention |
| Brewer 2010 | Alcohol was not used as an intervention in this study; hence this study used the wrong intervention |
| Brien 1979 | Alcohol was given with calcium carbimide in this study; hence this study used the wrong intervention |
| Brown 1981 | Alcohol was not given as the only intervention in this study; hence this study used the wrong intervention |
| Brown 2010 | This randomised controlled trial was double‐blinded and placebo‐controlled. Even though heart rate was recorded, heart rate could not be analysed due to technical failure and was not reported in this study |
| Brugger‐Andersen 2009 | Alcohol consumption was self‐reported, and this study was not a randomised controlled trial; hence this study used the wrong study design |
| Brunelle 2007 | This study was neither a randomised controlled trial nor a placebo‐controlled trial; hence this study used the wrong study design |
| Bryson 2008 | This was an analysis of cohort study data; hence this study used the wrong study design |
| Bulpitt 1999 | This study examined the effects of alcohol reduction; hence this study used the wrong study design |
| Burke 2001 | Moderation of lifestyle was used as an intervention rather than alcohol; hence this study used the wrong intervention |
| Burke 2005 | Alcohol was not the main intervention in this study; hence this study used the wrong intervention |
| Burke 2006 | Moderation of lifestyle was used as an intervention rather than alcohol; hence this study used the wrong intervention |
| Campbell 1999 | Lifestyle modification was the main intervention in this study; hence this study used the wrong intervention |
| Carter 2011 | This study used the wrong study design. Even though it had met all inclusion criteria, measurements were influenced by lower body negative pressure (LBNP), which resulted in displacement of blood away from the upper body to the abdomen and the lower extremities; hence results of this study could not be considered "Subjects were then placed in the supine position, with the bottom portion of their body in a LBNP chamber" "Subjects underwent a 5‐min resting baseline, followed by a progressive LBNP protocol of 3 min each at ‐5, ‐10, ‐15, ‐20, ‐30, and ‐40 mmHg (pretreatment)" |
| Chagas 2016 | This was a cross‐sectional study; hence this study used the wrong study design |
| Chang 2011 | This study was interview‐based; hence this study used the wrong study design |
| Chaplin 2008 | Alcohol was not given as the intervention in this study; hence this study used the wrong intervention and the wrong study design |
| Chaudhuri 1994 | This study was not a randomised controlled trial; hence this study used the wrong study design |
| Chiadak 2017 | Data collected in this study were questionnaire‐based; hence this study used the wrong study design |
| Childs 2011 | Even though this study was a randomised controlled trial and was placebo‐controlled, neither blood pressure nor heart rate was reported after the intervention. Only baseline heart rate and blood pressure were measured |
| Chiva‐Blanch 2012a | The intervention duration of this study was 4 weeks, which was not considered an acute effect; hence this study used the wrong study design |
| Chiva‐Blanch 2012b | Blood pressure was measured at the end of the intervention duration of 4 weeks, which was not considered an acute effect; hence this study used the wrong study design |
| Chiva‐Blanch 2013a | Even though this study was a randomised controlled trial and a cross‐over study, it was not placebo‐controlled; hence this study used the wrong study design |
| Chiva‐Blanch 2013b | Blood pressure was measured at the end of the intervention duration of 4 weeks, which was not considered an acute effect; hence this study used the wrong study design. In addition, neither blood pressure nor heart rate was recorded; hence this study used the wrong outcomes |
| Chiva‐Blanch 2015 | The intervention duration of this study was 4 weeks, which was not considered an acute effect; hence this study used the wrong study design |
| Colby 2004 | The main purpose of this study was irrelevant to the interests of our review. This study compared tobacco to alcohol; hence this study used the wrong main intervention |
| Conen 2008 | This was a prospective study; hence this study used the wrong study design |
| Cordain 2000 | This study used the wrong study design because the intervention duration was 10 weeks, which was not considered an acute effect |
| Covault 2014 | This study used the wrong intervention because alcohol was not given as the only intervention "...pretreatment with 4mg dutasteride or placebo was paired with a moderate dose of alcohol (0.8 g/kg) or placebo beverage" |
| Cox 1990 | This study used the wrong study design because it examined the effects of alcohol reduction |
| Cox 1993 | This study used the wrong study design because it examined the effects of alcohol reduction |
| Croissant 2011 | This study was not placebo‐controlled; hence this study used the wrong study design |
| Cushing 2009 | This study used the wrong intervention as participants received placebo of PM 101 as the intervention "...receiving placebo (5% dextrose in water, n=112) or PM101 (bolus push, n=112)" |
| Cushman 1994 | This study used the wrong study design because it examined the effects of alcohol reduction |
| Cushman 1998 | The intervention of this study was reduction of alcohol; hence this study used the wrong intervention |
| Cutler 1991 | Reduction of alcohol was performed in this study and alcohol was not the main intervention; hence this study used the wrong study design and the wrong intervention |
| deLorenzo 1985 | This study used the wrong intervention because ketanserin was used as the main intervention |
| deLorenzo 1988 | This study used the wrong intervention because serotonin antagonist was used as the main intervention |
| Demmler 2013 | This was a cross‐sectional study; hence this study used the wrong study design |
| deRijke 1996 | This study used the wrong study design because it was not placebo‐controlled. Red wine was compared to white wine instead of to placebo |
| DiGarbo 2004 | This study used the wrong study design because it was not placebo‐controlled |
| Dimmitt 1998 | This study used the wrong study design because it examined the effects of alcohol reduction |
| Draijer 2015 | This study used the wrong intervention because grape extracts were used as the intervention instead of alcohol |
| Droste 2013a | This study used the wrong study design because the intervention duration was 20 weeks, which was not considered an acute effect |
| Droste 2013b | This study used the wrong study design because the intervention duration was 20 weeks, which was not considered an acute effect |
| Durocher 2011 | Study authors were contacted, but required data were not reported in the study |
| Eisenhofer 1987 | This study used the wrong outcomes because reported blood pressure was accompanied by information on the effects of cold pressor |
| Estevez 1995 | This study used the wrong comparator and was not placebo‐controlled. Alcohol was compared to ritanserin instead of to placebo; hence this study used the wrong study design |
| Estruch 2011 | This study used the wrong study design because the intervention duration was 28 days, which was not considered an acute effect |
| Farre 1993 | This study used the wrong intervention because alcohol was given with cocaine as the intervention |
| Farre 2014 | This study used the wrong comparator and was not placebo‐controlled. Alcohol was compared to soy extract in this study; hence this study used the wrong study design |
| Farre 2016 | This study used the wrong intervention because alcohol was given with mephedrone |
| Farre 2017 | This study used the wrong intervention because alcohol was given with mephedrone |
| Fazio 2001 | This study used the wrong study design because it was questionnaire‐based "The study was performed in 10 young healthy male volunteers whose weekly ethanol consumption was estimated by questionnaire to be less than 100g" |
| Flanagan 2002 | This study used the wrong study design because the intervention duration was 1 week, which was not considered an acute effect |
| Flechtner‐Mors 2004 | This study used the wrong study design because the intervention duration was 3 months, which was not considered an acute effect |
| Foltin 1988 | This study used the wrong intervention because alcohol was ingested with cocaine |
| Foppa 1999 | Full text of this study was not available, and contact information for study authors could not be found |
| Frisk‐Holmberg 1990 | This study used the wrong intervention because jian bu wan was given as the intervention instead of alcohol |
| Garcia‐Andrade 1997 | Even though this study met all the inclusion criteria, placebo data were not reported. Study author was contacted via email for additional information but this author refused to disclose unpublished data. Hence, we excluded this study |
| Gepner 2013 | This study used the wrong study design because the intervention duration was 6 months, which was not considered an acute effect |
| Gepner 2015 | This study used the wrong study design because the intervention duration was 2 years, which was not considered an acute effect |
| Gepner 2016 | This study used the wrong study design because the intervention duration was 6 months, which was not considered an acute effect |
| Giovannelli 2011 | This study used the wrong intervention because it provided de‐alcoholised red wine with different concentrations of polyphenols as the intervention instead of alcohol |
| Golan 2016 | This study used the wrong study design because the intervention duration was 2 years, which was not considered an acute effect |
| Golan 2017 | This study used the wrong study design because the intervention duration was 2 years, which was not considered an acute effect |
| Golan 2018 | This study used the wrong study design because the intervention duration was 2 years, which was not considered an acute effect |
| Gopane 2010 | This study used the wrong study design because it was a cross‐sectional study |
| Gourlay 2013 | Full text was not available for this study, and contact information for study authors was not found |
| Greyling 2016 | This study used grape juice and wine polyphenols as the intervention instead of alcohol; hence this study used the wrong intervention. In addition, this study used the wrong study design because the intervention duration was 8 weeks, which was not considered an acute effect |
| Hansen 2005 | This study used the wrong study design because the intervention duration was 4 weeks, which was not considered an acute effect |
| Hartmann 2017 | Full text was not available for this study, and contact information for study authors was not found |
| Hijmering 2007 | This study was not placebo‐controlled; hence this study used the wrong study design "In 45 minutes, three units of red wine or an alcoholic beverage with a low polyphenolic count [was] consumed" |
| Howes 1985 | The effect of alcohol on blood pressure was measured on the fifth day after 4 days of intervention; hence this study used the wrong study design |
| Howes 1986a | This study used the wrong study design as the intervention duration was 4 days |
| Howes 1986b | Even though this study met all the inclusion criteria, only data on the effect of alcohol with noradrenaline on blood pressure were reported, and no author contact information was provided. Hence, we excluded this study |
| Jacobs 2012 | This study used the wrong comparator because red wine was compared to grape juice extracts |
| Jain 2016 | This study used the wrong study design because the intervention duration was 2 years, which was not considered an acute effect |
| Jones 1979 | This study used the wrong intervention because it examined the effect of alcohol combined with hyperbaric air |
| Kaul 2010 | This study used the wrong study design because the intervention duration was 2 weeks, which was not considered an acute effect |
| Kawano 1998 | The effect of alcohol on blood pressure was measured after 4 days of intervention; hence this study used the wrong study design |
| Kawano 2002 | The alcohol reduction effect was examined in this study; hence this study used the wrong study design |
| Kawano 2004 | This study included 3 phases: control phase, alcohol phase, and recovery phase. The placebo‐controlled group was not present throughout the study; hence this study used the wrong study design |
| Kechagias 2015 | This study used the wrong study design because the intervention duration was 3 months, which was not considered an acute effect |
| Kelbaek 1987 | This study met all inclusion criteria. However, control group data were not reported, and no author contact information was provided. Thus, we excluded this study |
| Kelbaek 1988 | This was not a randomised controlled trial; hence this study used the wrong study design |
| Kino 1981 | This was not a randomised controlled trial; hence this study used the wrong study design |
| Koskinen 1991 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| Lachtermann 1999 | This study met all the inclusion criteria. However, heart rate and blood pressure were not reported, and no author contact information was provided. Thus, we excluded this study |
| Latella 2009 | This was a cross‐sectional study; hence this study used the wrong study design |
| Lavy 1994 | This study used the wrong study design because it was not placebo‐controlled. Red wine was compared to white wine instead of to placebo |
| Lee 2002 | This was a review; hence this study used the wrong study design |
| Lee 2016 | Data reported in this study were questionnaire‐based and were self‐reported; hence this study used the wrong study design |
| Li 2006 | This was a cross‐sectional study; hence this study used the wrong study design |
| Li 2016 | This was an observational analysis; hence this study used the wrong study design |
| Liang 2012 | This was an observational study; hence this study used the wrong study design |
| Malinski 2004 | Data reported in this study were self‐reported. This was a cross‐sectional study; hence this study used the wrong study design |
| Mammen 2018 | This study used the wrong study design. Alcohol was compared to placebo or to low‐dose polyphenol. The purpose of this study was irrelevant to the interest of this review |
| Marczinski 2018 | Alcohol was not given alone as the intervention. Energy drink was also given to participants. Thus, this study provided the wrong intervention |
| McCance‐Katz 1996 | This study used the wrong intervention because alcohol was given with cocaine |
| McCance‐Katz 2005 | This study used the wrong intervention because alcohol was given with cocaine |
| McCance‐Katz 2013 | This study used the wrong intervention because alcohol was given with ritonavir or efavirenz |
| McCaul 1991 | This study used the wrong intervention because alcohol was given with secobarbital |
| McDonagh 2018 | This study used the wrong intervention. Alcohol was not given as the only intervention in this study |
| McDougle 1995 | This study used the wrong intervention because yohimbine was provided as the main intervention instead of ethanol |
| Mezzano 2001 | This study used the wrong intervention and the wrong study design. Participants received either MD (Mediterranean‐type diet) or HFD (high‐fat diet) for 90 days, and both diets were supplemented with red wine on day 30 and on day 90. Thus, this study did not include a placebo control group, and the intervention duration was too long to be considered an acute effect |
| Mezzano 2003 | This study used the wrong intervention and the wrong study design. Participants received either MD (Mediterranean‐type diet) or HFD (high‐fat diet) for 90 days, and both diets were supplemented with red wine on day 30 and on day 90. Thus, this study did not include a placebo control group, and the intervention duration was too long to be considered an acute effect |
| Minami 2002 | This study used the wrong study design because the effect of alcohol reduction was examined |
| Mizushima 1990 | In this cross‐sectional study, alcohol consumption was self‐reported; hence this study used the wrong study design |
| Molnar 2009 | This study used the wrong study design because it was not a randomised controlled trial |
| Moreira 1998 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| Mori 2015 | This study used the wrong study design because the intervention duration was 4 weeks |
| Mori 2016 | This study used the wrong study design because the intervention duration was 4 weeks, which was not considered an acute effect |
| Movai 1989 | This was not a randomised placebo‐controlled study |
| Mukamal 2017 | This study used the wrong study design because the intervention duration was 6 months. Even though participants returned monthly for measurement, this was still not considered an acute effect |
| Naissides 2006a | This study used the wrong study design because the intervention duration was 6 weeks, which was not considered an acute effect |
| Naissides 2006b | This study used the wrong study design because the intervention duration was 6 weeks, which was not considered an acute effect |
| Newlin 1989 | This study was not a randomised controlled trial; hence this study used the wrong study design |
| Newlin 1990 | This study used the wrong outcomes because only heart rate change before and after the intervention was measured |
| Niaura 1988 | This was not a randomised controlled trial; hence this study used the wrong study design |
| Nicholas 2012 | Full text was not available for this study, and contact information for study authors was not found |
| Nicholas 2013a | Full text was not available for this study, and contact information for study authors was not found |
| Nicholas 2013b | Full text was not available for this study, and contact information for study authors was not found |
| Noad 2016 | Alcohol was not the intervention used in this study. Polyphenol was used as the intervention. Thus, this study used the wrong intervention |
| O'Malley 2014 | This study used the wrong study design because there was no placebo‐controlled group. Only low, medium, and high doses of alcohol were given to participants |
| Oda 2017 | This study used the wrong study design because alcohol consumption was self‐reported |
| Okamura 2001 | This was a cross‐sectional study; hence this study used the wrong study design |
| Papamichael 2006 | The effect of alcohol accompanied by smoking rather than the effect of alcohol alone was examined; hence this study used the wrong intervention |
| Papamichael 2008 | This study used the wrong intervention because alcohol was not given as the only intervention. It was accompanied by olive oil |
| Papaseit 2016 | This study used the wrong intervention because alcohol was given with mephedrone rather than alone |
| Park 2004 | This study used the wrong intervention. Concord grape juice was given as the intervention instead of alcohol |
| Parker 1990 | This study used the wrong study design because the effect of alcohol reduction was examined |
| Paz 1996 | This study used the wrong study design because it was not a randomised controlled trial |
| Perkins 1995 | This study used the wrong intervention because the combined effect of alcohol and nicotine was examined instead of the effect of alcohol alone |
| Perneger 1999 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| Petrone 2014 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| Pisa 2010 | This study was a cross‐sectional epidemiological survey; hence this study used the wrong study design |
| Pitsavos 2004 | This study was an epidemiological study; hence this study used the wrong study design |
| Potter 1984 | This study used the wrong study design. The intervention duration was 4 days, and measurements were made after the intervention duration; thus this was not considered an acute effect |
| Puddey 1985a | This study used the wrong study design. The intervention duration was 6 weeks, and measurements were made after the intervention duration; thus this was not considered an acute effect |
| Puddey 1985b | Alcohol reduction was examined in this study; hence this study used the wrong study design |
| Puddey 1985c | Alcohol reduction was examined in this study; hence this study used the wrong study design |
| Puddey 1986 | Alcohol reduction was examined in this study; hence this study used the wrong study design |
| Puddey 1987 | This study used the wrong study design because changes in alcohol consumption were self‐reported and the intervention duration was 6 weeks, which was not considered an acute effect |
| Puddey 1992 | This study used the wrong study design because alcohol reduction was examined and the intervention duration was 18 weeks, which was not considered an acute effect |
| Rada 2018 | This study used the wrong study design. The intervention duration was 4 weeks, and measurements were made after the intervention period, which was not considered an acute effect |
| Rajdl 2007 | This study used the wrong study design because it was not placebo‐controlled. This study examined only the effect of white wine but did not compare this to the placebo control |
| Rakic 1998 | Alcohol reduction effect was examined in this study; hence this study used the wrong study design |
| Retterstol 2005 | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Roache 2011 | This study used the wrong intervention because it examined the combined effect of cocaine and alcohol instead of the effect of alcohol only |
| Roth 2013 | This study used the wrong study design. The intervention duration was 1 month, and measurements were made at the end of the study, which was not considered an acute effect |
| Roth 2017 | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Roth 2018 | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Sagawa 2011 | This was not a randomised controlled trial; hence this study used the wrong study design |
| Saito 2003 | This was a cross‐sectional study; hence this study used the wrong study design |
| Sehested 1998 | This study was not placebo‐controlled; hence this study used the wrong study design. Participants were divided into 2 groups: Group A: alcohol mixed with 500 mL of juice; Group B: similar to Group A plus 750 mL of mineral water. Group B did receive alcohol even though it was mixed with mineral water; hence this was a placebo‐controlled group |
| Senault 2000 | This study used the wrong study design. The intervention duration was 14 days, and measurements were made at the end of the study, which was not considered an acute effect |
| Shai 2007 | This study used the wrong study design. The intervention duration was 3 months, and measurements were made at the end of the study, which was not considered an acute effect |
| Shai 2015 | This study used the wrong study design. The intervention duration was 2 years, and measurements were made at the end of the study, which was not considered an acute effect |
| Sierksma 2002 | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Sierksma 2004a | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Sierksma 2004b | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Spaak 2008 | This study used the wrong study design because alcohol was given repeatedly to reach a certain blood pressure level. Thus, alcohol was not given at a particular dose; dose varied with the blood alcohol level of participants |
| Stiffler 1999 | This study reported only the mean difference in heart rate between day and night; thus required data were not reported |
| Stream 2010 | Full text was not available for this study, and contact information for study authors was not found |
| Stream 2014 | Full text was not available for this study, and contact information for study authors was not found |
| Stuart 2013 | This study used the wrong intervention because alcohol was not the main intervention used "...received 40h of standard batterer program or the standard batterer program and 90 min alcohol intervention" |
| Stubbs 1995 | This study used the wrong intervention because the combined effect of alcohol and caffeine (coffee) was examined instead of the effect of alcohol alone |
| Taborsky 2012 | This study was not placebo‐controlled (compared red wine to white red); hence this study used the wrong study design |
| Taborsky 2014 | This study was not placebo‐controlled (compared red wine to white red); hence this study used the wrong study design |
| Takashima 1998 | This was a cross‐sectional study; hence this study used the wrong study design |
| Tinklenberg 1976 | This study did not include a placebo for the alcohol |
| Tome‐Carneiro 2012 | This study used the wrong study design. The intervention duration was 1 year, and measurements were made at the end of the study, which was not considered an acute effect. In addition, this study used the wrong intervention because grape supplement was used as the main intervention instead of alcohol |
| Tome‐Carneiro 2013 | This study used the wrong study design. The intervention duration was 1 year, and measurements were made at the end of the study, which was not considered an acute effect. In addition, this study used the wrong intervention because resveratrol‐containing grape extract was used as the main intervention instead of alcohol |
| TOMHS 1991 | Alcohol was not used as the main intervention in this study; hence this study used the wrong intervention |
| Toth 2012 | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Toth 2014 | This study used the wrong study design. The intervention duration was 3 weeks, and measurements were made at the end of the study, which was not considered an acute effect |
| Tresserra‐Rimbau 2013 | This study used the wrong study design. The intervention duration was 5 years, and measurements were made at the end of the study, which was not considered an acute effect |
| Tresserra‐Rimbau 2015 | This was a cross‐sectional study; hence this study used the wrong study design |
| Tuomilehto 1984 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| Turczynski 2001 | Full text was not available for this study, and contact information for study authors was not found |
| Ueshima 1987a | Full text was not available for this study, and contact information for study authors was not found |
| Ueshima 1987b | Effects of alcohol reduction were examined in this study; hence this study used the wrong study design |
| Ueshima 1988 | Effects of alcohol reduction were examined in this study; hence this study used the wrong study design |
| Ueshima 1993 | Effects of alcohol reduction were examined in this study; hence this study used the wrong study design |
| Uhart 2010 | This study was not placebo‐controlled; hence this study used the wrong study design |
| Urquiaga 2015 | This study used the wrong intervention because red wine grape pomace flour (WGPF) was given as the main intervention instead of alcohol |
| vanMierlo 2010 | This study used the wrong study design. The intervention duration was 2 weeks, and measurements were made at the end of the study, which was not considered an acute effect. In addition, this study used the wrong intervention because polyphenol‐rich solids were used as the main intervention instead of alcohol |
| Vatsalya 2011 | Full text was not available for this study, and contact information for study authors was not found |
| Vazquez‐Fresno 2012 | This study used the wrong study design. The intervention duration was 28 days, and measurements were made at the end of the study, which was not considered an acute effect |
| Vena 2018a | Alcohol was combined with oxytocin; hence this study used the wrong intervention |
| Vena 2018b | Alcohol was combined with oxytocin; hence this study used the wrong intervention |
| Wensing 2006 | This study used the wrong intervention because the combined effect of alcohol and vardenafil was examined instead of the effect of alcohol alone |
| Wilson 2014 | This study used the wrong study design as intervention duration was 6 months, which was not considered an acute effect of alcohol |
| Witkowska 2017 | This study used the wrong intervention because it examined the effect of red wine polyphenols rather than the effect of alcohol |
| Woerdeman 2018 | Red wine polyphenol was used as the intervention instead of alcohol; hence this study used the wrong intervention |
| Wray 2013 | Full text was not available for this study, and contact information of study authors was not found |
| Yang 2017 | Alcohol consumption was self‐reported in this study; hence this study used the wrong study design |
| YftachGepner 2015 | This study used the wrong study design. The intervention duration was 6 months, and measurements were made at the end of the study, which was not considered an acute effect |
| Yin 2007 | This study was questionnaire‐based; hence this study used the wrong study design |
| Yin 2015 | This study was questionnaire‐based; hence this study used the wrong study design |
| Zamora‐Ros 2006 | This study was not placebo‐controlled. Only red wine, white wine, ethanol as sparkling wine, and gin were compared in this study; hence this study used the wrong study design |
| Zheng 2012 | This study was not placebo‐controlled (compared traditional Chinese liquor and tea flavour liquor); hence this study used the wrong study design |
| Ziauddeen 2013 | This study used the wrong intervention. Alcohol was combined with mu‐opioid receptor antagonist GSK1521498 |
| Zilkens 2003 | Alcohol reduction was examined in this study; hence this study used the wrong study design |
| Zilkens 2005 | This study used the wrong study design. The intervention duration was 4 weeks, and measurements were made on the last or second‐last day of the study, which was not considered an acute effect |
BPA: bisphenol A. HFD: high‐fat diet. LBNP: lower body negative pressure. MD: Mediterranean‐type diet. RGSE: red grape seed extract. WGPF: wine grape pomace flour.
Characteristics of ongoing studies [ordered by study ID]
Polzein ongoing.
| Study name | Alcohol and Neural Cardiovascular Control in Binge Drinkers |
| Methods | Randomised, cross‐over, double‐blind, placebo‐based |
| Participants |
|
| Interventions | Intervention: alcohol Placebo: fluid control |
| Outcomes | Primary outcome measure
Secondary outcome measure: baroreflex function
Other outcome measures:
|
| Starting date | 21 May 2018 |
| Contact information | Responsible party: Michigan Technological University Joanne Polzein (jpolzien@mtu.edu) Cheryl Gherna (cagherna@mtu.edu) |
| Notes | Status: recruiting Estimated study completion date: September 2022 |
Differences between protocol and review
According to the published protocol, we intended to include only double‐blind RCTs in this review. Because higher doses of alcohol exert specific pharmacological effects on drinkers, we had a few double‐blind RCTs after the first screening. Considering the difficulty of masking in these types of studies, we decided to also include single‐blind and open‐label studies in the review.
Contributions of authors
James M Wright (JMW) formulated the idea, developed the basis of the protocol, and contributed to data analysis, interpretation of the final result, and editing of the final draft of the review.
Sara Tasnim (ST) and Chantel Tang (CT) drafted the protocol with help from JMW. Both ST and CT independently assessed studies for inclusion or exclusion and assessed the risk of bias of all included studies.
ST extracted data, checked data entry, conducted data analysis, interpreted study results, and drafted the final review.
CT checked data entry and contributed to drafting of the review.
Vijaya Musini (VM) contributed to data analysis, interpretation of the final result, and editing of the final draft of the review.
All review authors reviewed and approved the final version.
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology & Therapeutics, University of BC, Canada
infrastructure
External sources
-
British Columbia Ministry of Health, Canada
Therapeutics Initiative grant
Declarations of interest
Chantel Tang: none known.
Sara Tasnim: none known.
Vijaya Musini: none known.
James M Wright: none known.
New
References
References to studies included in this review
Agewall 2000 {published data only}
- Agewall S, Wright S, Doughty RN, Whalley GA, Duxbury M, Sharpe N. Does a glass of red wine improve endothelial function. European Heart Journal 2000;21:74-8. [DOI] [PubMed] [Google Scholar]
Barden 2013 {published data only}
- Barden AE, Croft KD, Beilin LJ, Phillips M, Ledowski T, Puddey IB. Acute effects of red wine on cytochrome P450 eicosanoids and blood pressure in men. Journal of Hypertension 2013;31:2195-202. [DOI: 10.1097/HJH.0b013e328364a27f] [DOI] [PubMed] [Google Scholar]
Bau 2005 {published data only}
- Bau PF, Bau CH, Naujorks AA, Rosito GA. Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol 2005;37:53-8. [DOI: 10.1016/j.alcohol.2005.10.034] [DOI] [PubMed] [Google Scholar]
Bau 2011 {published data only}
- Bau PF, Moraes RS, Bau CH, Ferlin EL, Rosito GA, Fuchs FD. Acute ingestion of alcohol and cardiac autonomic modulation in healthy volunteers. Alcohol 2011;45:123-9. [DOI: 10.1016/j.alcohol.2010.08.011] [DOI] [PubMed] [Google Scholar]
Buckman 2015 {published data only}
- Buckman JF, Eddie D, Vaschillo EG, Vaschillo B, Garcia A, Bates ME. Immediate and complex cardiovascular adaptation to an acute alcohol dose. Alcoholism: Clinical and Experimental Research 2015;39(12):2334-44. [DOI: 10.1111/acer.12912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1986 {published data only}
- Chen W, Lockhart JO. Does moderate level of alcohol consumption produce a relaxation effect? ERIC 1986:1-16.
Cheyne 2004 {published data only}
- Cheyne EH, Sherwin RS, Lunt MJ, Cavan DA, Thomast PW, Kerr D. Influence of alcohol on cognitive performance during mild hypoglycaemia; implications for Type 1 diabetes. Diabetic Medicine 2004;21:230-7. [DOI] [PubMed] [Google Scholar]
Dai 2002 {published data only}
- Dai X, Thavundayil J, Gianoulakis C. Differences in the responses of the pituitary beta-endorphin and cardiovascular system to ethanol and stress as a function of family history. Alcoholism: Clinical and Experimental Research 2002;26(8):1171-80. [DOI: 10.1097/01.ALC.0000024129.32956.38] [DOI] [PubMed] [Google Scholar]
Dumont 2010 {published data only}
- Dumont GJ, Kramers C, Sweep FC, Willemsen JJ, Touq DJ, Schoemaker RC, et al. Ethanol co-administration moderates 3,4-methylenedioxymethampthetamine effects on human physiology. Journal of Psychopharmacology 2010;24(2):165-74. [DOI: 10.1177/0269881108100020] [DOI] [PubMed] [Google Scholar]
Fantin 2016 {published data only}
- Fantin F, Bulpitt CJ, Zamboni M, Cheek E, Rajkumar C. Arterial compliance may be reduced by ingestion of red wine. Journal of Human Hypertension 2016;30:68-72. [DOI: 10.1038/jhh.2015.19] [DOI] [PubMed] [Google Scholar]
Fazio 2004 {published data only}
- Fazio M, Bardelli M, Fabris B, Macluso L, Fiammengo F, Vran F, et al. Large-artery hemodynamics after acute alcohol administration in young, healthy volunteers. Angiology 2004;55:139-45. [DOI] [PubMed] [Google Scholar]
Foppa 2002 {published data only}
- Foppa M, Fuchs F, Preissler L, Andrighetto A, Rosito G, Duncan BB. Red wine with the noon meal lowers post-meal blood pressure: a randomized trial in centrally obese, hypertensive patients. Journal of Studies on Alcohol and Drugs 2002;63:247-51. [DOI] [PubMed] [Google Scholar]
Hering 2011 {published data only}
- Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Potentiated sympathetic and hemodynamic responses to alcohol in hypertensive vs. normotensive individuals. Journal of Hypertension 2011;29:537-41. [DOI: 10.1097/HJH.0b013e328342b2a9] [DOI] [PubMed] [Google Scholar]
Karatzi 2005 {published data only}
- Karatzi KN, Papamichael CM, Karatzis EN, Papaioannou TG, Aznaouridis KA, Katsichti PP, et al. Red wine acutely induces favorable effects on wave reflections and central pressures in coronary artery disease patients. American Journal of Hypertension 2005;18:1161-7. [DOI: 10.1016/jamjhyper.2005.03.744] [DOI] [PubMed] [Google Scholar]
Karatzi 2013 {published data only}
- Karatzi K, Rontoyanni VG, Protogerou AD, Georgoulia A, Xenos K, Chrysou J, et al. Acute effects of beer on endothelial function and haemodynamics: a single blind, cross-over study in healthy volunteers. Nutrition 2013;29(9):1122-6. [DOI: 10.1016/j.nut.2013.02.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawano 1992 {published data only}
- Kawano Y, Abe H, Kojima S, Ashida T, Yoshida K, Imanishi M, et al. Acute depressor effect of alcohol in patients with essential hypertension. Hypertension 1992;20:219-26. [DOI] [PubMed] [Google Scholar]
Kawano 2000 {published data only}
- Kawano Y, Abe H, Kojima S, Takishita S, Omae T. Interaction of alcohol and an alpha1-blocker on ambulatory blood pressure in patients with essential hypertension. American Journal of Hypertension 2000;13:307-12. [DOI] [PubMed] [Google Scholar]
Koenig 1997 {published data only}
- König D, Berg A, Plarr K, Grathwohl D, Halle M, Keul J. Acute influence of beer in moderate doses on blood pressure regulation and electrolyte metabolism [Akuteinfluß von bier in moderater dosierung auf blutdruckregulation und elektrolystoffwechsel]. Aktuelle Ernährungsmedizin 1997;22:76-81. [Google Scholar]
Kojima 1993 {published data only}
- Kojima S, Kawano Y, Abe H, Sanai T, Yoshida K, Imanishi M, et al. Acute effects of alcohol ingestion on blood pressure and erythrocyte sodium concentration. Journal of Hypertension 1993;11:185-90. [DOI] [PubMed] [Google Scholar]
Mahmud 2002 {published data only}
- Mahmud A, Feely J. Divergent effect of acute and chronic alcohol on arterial stiffness. American Journal of Hypertension 2002;15:240-3. [DOI] [PubMed] [Google Scholar]
Maufrais 2017 {published data only}
- Maufrais C, Charriere N, Montani JP. Cardiovascular and cutaneous responses to the combination of alcohol and soft drinks: the way to orthostatic intolerance. Frontiers in Physiology 2017;8:860. [DOI: 10.3389/fphys.2017.00860] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maule 1993 {published data only}
- Maule S, Chaudhuri KR, Thomaides T, Pavitt D, McCleery J, Mathias CJ. Effects of oral alcohol on superior mesenteric artery blood flow in normal man, horizontal and tilted. Clincal Science 1993;84:419-25. [DOI] [PubMed] [Google Scholar]
Narkiewicz 2000 {published data only}
- Narkiewicz K, Cooley RL, Somers VK. Alcohol potentiates orthostatic hypotension implications for alcohol-related syncope. Circulation 2000;101:389-402. [DOI] [PubMed] [Google Scholar]
Nishiwaki 2017 {published data only}
- Nishiwaki M, Kora N, Matsumoto N. Ingesting a small amount of beer reduces arterial stiffness in healthy humans. Physiological Reports 2017;5(15):e13381. [DOI: 10.14814/phy2.13381] [DOI] [PMC free article] [PubMed] [Google Scholar]
Potter 1986 {published data only}
- Potter JF, Watson RD, Skan W, Beevers DG. The pressor and metabolic effects of alcohol in normotensive subjects. Hypertension 1986;8:625-31. [DOI: 10.1161/01.HYP.8.7.625] [DOI] [PubMed] [Google Scholar]
Rosito 1999 {published data only}
- Rosito GA, Fuchs FD, Duncan BB. Dose-dependent biphasic effect of ethanol on 24-h blood pressure in normotensive subjects. American Journal of Hypertension 1999;12:236-40. [DOI] [PubMed] [Google Scholar]
Rossinen 1997 {published data only}
- Rossinen J, Viitasalo M, Partanen J, Koskinen P, Kupari M, Nieminen MS. Effects of acute alcohol ingestion on heart rate variability in patients with documented coronary artery disease and stable angina pectoris. American Journal of Cardiology 1997;79:487-91. [DOI] [PubMed] [Google Scholar]
Stott 1987 {published data only}
- Stott DJ, Ball SG, Inglis GC, Davies DL, Fraser R, Murray GD, et al. Effects of a single moderate dose of alcohol on blood pressure, heart rate and associated metabolic and endocrine changes. Clincial Science 1987;73:411-6. [DOI] [PubMed] [Google Scholar]
Stott 1991 {published data only}
- Stott DJ, Dutton M, Murray GD, WIlliams BO, McInnes GT. Hemodynamic effects of a single moderate dose of alcohol in elderly subjects. Journal of Studies on Alcohol 1991;52:377-9. [DOI] [PubMed] [Google Scholar]
Van De Borne 1997 {published data only}
- Van de Borne P, Mark AL, Montano N, Mion D, Somers VK. Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension 1997;29:1278-83. [DOI: 10.1161/01.HYP.29.6.1278] [DOI] [PubMed] [Google Scholar]
Williams 2004 {published and unpublished data}
- Williams MJ, Sutherland WH, Whelan AP, McCormick MP, Jong SA. Acute effect of drinking red and white wines on circulating levels of inflammation-sensitive molecules in men with coronary artery disease. Metabolism 2004;53(3):318-23. [DOI] [PubMed] [Google Scholar]
Zeichner 1985 {published data only}
- Zeichner A, Edwards P, Cohen E. Acute effects of alcohol on cardiovascular reactivity to stress on college-age type a (coronary prone) individuals. Journal of Psychopathology and Behavioral Assessment 1985;7(1):75-89. [Google Scholar]
References to studies excluded from this review
Abrams 1979 {published data only}
- Abrams DB, Wilson GT. Effect of alcohol on social anxiety in women: cognitive versus physiological processes. Journal of Abnormal Psychology 1979;88(2):161-73. [DOI: 10.1037/0021-843X.88.2.161] [DOI] [PubMed] [Google Scholar]
Abu‐AmshaCaccetta 2001 {published data only}
- Caccetta RA, Burke V, Mori TA, Beilin LJ, Puddey IB, Croft KD. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free Radical Biology & Medicine 2001;30(6):636-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Adamsson 2011 {published data only}
- Adamsson V, Reumark A, Fredrikson IB, Hammarstrom E, Vessby J, Johansson G. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (Nordiet). Journal of Internal Medicine 2011;269:150-9. [DOI: 10.1111/j.1365-2796.2010.02290.x] [DOI] [PubMed] [Google Scholar]
Aguilar 2004 {published data only}
- Aguilar D, Skali H, Moyé LA, Lewis EF, Gaziano JM, Rutherford JD, et al. Alcohol consumption and prognosis in patients with left ventricular systolic dysfunction after a myocardial infarction. Journal of the American College of Cardiology 2004;43(11):2015-21. [DOI: 10.1016/j.jacc.2004.01.042] [DOI] [PubMed] [Google Scholar]
Aigner 2016 {published data only}
- Aigner A, Grittner U, Rolfs A, Norrving B, Siegerink B, Busch MA. To what extent do established risk factors explain stroke in the young, a case-control study. European Journal of Epidemiology 2016;31:S10. [DOI: 10.1007/s10654-016-0183-1] [DOI] [Google Scholar]
Ajani 2000 {published data only}
- Ajani UA, Gaziano M, Lotufo PA, Liu S, Hennekens CH, Buring JE, et al. Alcohol consumption and risk of coronary heart disease by diabetes status. Circulation 2000;102:500-5. [DOI: 10.1161/01.CIR.102.5.500] [DOI] [PubMed] [Google Scholar]
Almeida 2014 {published data only}
- Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Alcohol consumption and cognitive impairment in older men: a mendelian randomization study. Neurology 2014;82:1038-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andres‐Lacueva 2013 {published data only}
- Andres-Lacueva C, Urpi-Sarda M, Vazquez-Fresno R, Tulipani S, Rotches-Ribalta M, Boto-Ordonez M, et al. Red wine polyphenols and human health. Annals of Nutrition and Metabolism 2013;1:110. [Google Scholar]
Anil 2016 {published data only}
- Anil S, Charlton KE, Tapsell LC, Probst Y, Ndanuko R, Batterham MJ. Identification of dietary patterns associated with blood pressure in a sample of overweight Australian adults. Journal of Human Hypertension 2016;30:672-8. [DOI: 10.1038/jhh.2016.10] [DOI] [PubMed] [Google Scholar]
Apostolidou 2015 {published data only}
- Apostolidou C, Adamopoulos K, Lymperaki E, Iliadis S, Papapreponis P, Kourtidou-Papadeli C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clinical Nutrition ESPEN 2015;10:e224-33. [DOI: 10.1016/j.clnesp.2015.08.001] [DOI] [PubMed] [Google Scholar]
Appel 2003 {published data only}
- Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the Premier clinical trial. JAMA 2003;289(16):2083-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Argani 2016 {published data only}
- Argani H, Ghorbanihaghjo A, Vatankhahan H, Rashtchizadeh N, Raeisi S, Ilghami H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Medical Journal 2016;134(3):234-9. [DOI: 10.1590/1516-3180.2015.01702312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ariansen 2009 {published data only}
- Ariansen I, Wachtell K, Hecht Olsen M, Ibsen H, Devereux RB, Okin PM, et al. Impact of alcohol and smoking habits on the risk of new-onset atrial fibrillation in hypertensive patients with ECG left ventricular hypertrophy: the LIFE study. European Heart Journal 2009;30:160. [DOI] [PubMed] [Google Scholar]
Ariansen 2012 {published data only}
- Ariansen I, Reims HM, Gjesdal K, Hecht Olsen M, Ibsen H, Devereux RB, et al. Impact of alcohol habits and smoking on the risk of new-onset atrial fibrillation in hypertensive patients with ECG left ventricular hypertrophy: the LIFE study. Blood Pressure 2012;21:6-11. [DOI: 10.3109/08037051.2011.622978] [DOI] [PubMed] [Google Scholar]
Assaad 2006 {published data only}
- Assaad JM, Pihl RO, Séguin JR, Nagin D, Vitaro F, Tremblay RE. Heart rate response to alcohol and intoxicated aggressive behavior. Alcoholism: Clinical and Experimental Research 2006;30:774-82. [DOI: 10.1111/j.1530-0277.2006.00090.x] [DOI] [PubMed] [Google Scholar]
AuYeung 2013 {published data only}
- AuYeung SL, Jiang C, Cheng KK, Cowling BJ, Liu B, Zhang W, et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PloS ONE 2013;8(7):e68054. [DOI: 10.1371/jounral.pone.0068054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Avellone 2006a {published data only}
- Avellone G, Di Garbo V, Campisi D, De Simone R, Raneli G, Scaglione R, et al. Effects of moderate Sicilian red wine consumption on inflammatory biomarkers of atherosclerosis. European Journal of Clinical Nutrition 2006;60(1):41-7. [DOI: 10.1038/sj.ejcn.1602265] [DOI] [PubMed] [Google Scholar]
Avellone 2006b {published data only}
- Avellone G, Di Garbo V, Campisi D, Indovina A, Abruzzese G, Raneli G, et al. Moderate consumption of two Sicilian red wine inhibits platelet aggregation. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2006;165(2):79-85. [Google Scholar]
Bae 2015 {published data only}
- Bae S, Hong YC. Exposure to bisphenol A from drinking canned beverage increases blood pressure: randomized crossover trial. Hypertension 2015;65:313-9. [DOI: 10.1161/HYPERTENSIONAHA.114.04261] [DOI] [PubMed] [Google Scholar]
Bailey 1989 {published data only}
- Bailey DG, Spence JD, Edgar B, Bayliff CD, Arnold JM. Ethanol enhances the hemodynamic effects of felodipine. Clinical and Investigative Medicine 1989;12(6):357-62. [PMID: ] [PubMed] [Google Scholar]
Bailey 2003 {published data only}
- Bailey GD, Dresser GK, Bend JR. Bergamottin, lime juice, and red wine as inhibitors of cytochrome P450 3A4 activity: comparison with grapefruit juice. Clinical Pharmacology & Therapeutics 2003;73:529-37. [DOI: 10.1016/S009-9236(03)00051-1] [DOI] [PubMed] [Google Scholar]
Banini 2006 {published data only}
- Banini AE, Boyd LC, Allen JC, Allen HG, Sauls DL. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006;22(11-12):1137-45. [DOI] [PubMed] [Google Scholar]
Barden 2007 {published data only}
- Barden A, Zilkens RR, Croft K, Mori T, Burke V, Beilin LJ, et al. A reduction in alcohol consumption is associated with reduced plasma F2-isoprostanes and urinary 20-HETE excretion in men. Free Radical Biology & Medicine 2007;42(11):1730-6. [DOI: 10.1016/j.freeadbiomed.2007.03.004] [DOI] [PubMed] [Google Scholar]
Barden 2017 {published data only}
- Barden AE, Chavez V, Phillips M, Mas E, Beilin LJ, Croft KD, et al. A randomized trial of effects of alcohol on cytochrome P450 eicosanoids, mediators of inflammation resolution, and blood pressure in men. Alcoholism: Clinical and Experimental Research 2017;41(10):1666-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Baros 2008 {published data only}
- Baros AM, Wright TM, Latham PK, Miller PM, Anton RF. Alcohol consumption, %CDT, GGT and blood pressure change during alcohol treatment. Alcohol & Alcoholism 2008;43(2):192-7. [DOI: 10.1093/alcalc/agm156] [DOI] [PubMed] [Google Scholar]
Barskova 2005 {published data only}
- Barskova VG, Eliseev MS, Nasonov EL, Volkov AV, Tsapina TN, Zilov AV, et al. Use of metformin (siofor) in patients with gout and insulin resistance (pilot 6-month results). Ter Arkh 2005;77(12):44-9. [PMID: ] [PubMed] [Google Scholar]
Beevers 1987 {published data only}
- Beevers DG, Zezulka AV, Potter JF, Bannan LT, Maheswaran R, Gill JS. The clinical relevance of alcohol in blood pressure clinic. European Heart Jounral 1987;8(Suppl B):27-9. [DOI] [PubMed] [Google Scholar]
Beilin 1992 {published data only}
- Beilin LJ, Puddey IB. Alcohol and hypertension. Clinical and Experimental Hypertension: Theory and Practice 1992;14(1-2):119-38. [DOI] [PubMed] [Google Scholar]
Beilin 1994 {published data only}
- Beilin LJ, Burke V. Vegetarian diet components, proteins and blood pressure: which nutrients are important. Clinical and Experimental Pharmacology and Physiology 1995;22:195-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beilin 1996 {published data only}
- Beilin LJ, Puddey IB, Burke V. Alcohol and hypertension - kill or cure. Journal of Human Hypertension 1996;10(Suppl 2):S1-5. [PMID: ] [PubMed] [Google Scholar]
Bengtsson 1973 {published data only}
- Bengtsson C. Ischaemic heart disease in women: a study based on a randomized population sample of women and women with myocardial infarction in Goteborg, Sweden. American Surgeon Supplement 1973;549:128. [PMID: ] [PubMed] [Google Scholar]
Berg 2005 {published data only}
- Berg CM, Lissner L, Aires N, Lappas G, Toren K, Wilhelmsen L, et al. Trends in blood lipid levels, blood pressure, alcohol and smoking habits from 1985 to 2002: results from Intergene and GOT-MONICA. European Journal of Cardiovascular Prevention & Rehabilitation 2005;12(2):115-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Berglund 1989 {published data only}
- Berglund A, Andersson OK, Berglund G, Fagerberg B. Antihypertensive effect of diet compared with drug treatment in obese men with mild hypertension. BMJ 1989;299(6697):480-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bermudez 2015 {published data only}
- Bermúdez V, Martínez MS, Chávez-Castillo M, Olivar LC, Morillo J, Mejías JC, et al. Relationship between alcohol consumption and components of the metabolic syndrome in adult population from Maracaibo City, Venezuela. Advances in Preventive Medicine 2015;2015:352547. [DOI: 10.1155/2015/352547] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beulens 2005 {published data only}
- Beulens JW, Sierksma A, Schaafsma G, Kok FJ, Struys EA, Jakobs C, et al. Kinetics of homocysteine metabolism after moderate alcohol consumption. Alcoholism: Clinical and Experimental Research 2005;29(5):739-45. [DOI: 10.1097/01.ALC.0000163507.76773.1A] [DOI] [PubMed] [Google Scholar]
Beyeler 1987 {published data only}
- Beyeler C, Fisch HU, Preisig R. Cardiovascular and metabolic changes in the antabuse-alcohol reaction: basis for the diagnosis of degree of severity. Schweizerische Medizinische Wochenschrift 1987;117(2):52-60. [PMID: ] [PubMed] [Google Scholar]
Bjorntorp 1999 {published data only}
- Bjorntorp P, Holm G, Rosmond R. Hypothalmic arousal, insulin resistance and type 2 diabetes mellitus. Diabetic Medicine 1999;16(5):373-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Blankenhorn 1990 {published data only}
- Blankenhorn DH, Johnson RL, Mack WJ, El Zein HA, Vailas LI. The influence of diet on the appearance of new lesions in humans coronary arteries. JAMA 1990;263(12):1646-52. [PMID: ] [PubMed] [Google Scholar]
Bleich 2001 {published data only}
- Bleich S, Bleich K, Kroop S, Bittermann HJ, Degner D, Sperling W, et al. Moderate alcohol consumption in social drinkers raises plasma homocysteine levels: a contradiction to the 'French Paradox'. Alcohol and Alcoholism 2001;36(3):189-92. [DOI: 10.1093/alcalc/36.3.189] [DOI] [PubMed] [Google Scholar]
Bold 2017 {published data only}
- Bold KW, Epstein EE, McCrady BS. Baseline health status and quality of life after alcohol treatment for women with alcohol dependence. Addictive Behaviors 2017;64:35-41. [DOI: 10.1016/j.addbeh.2016.08.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 1984 {published data only}
- Bond V, Franks BD, Howley ET. Alcohol, cardiorespiratory function and work performance. British Journal of Sports Medicine 1984;18(3):203-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Botden 2011 {published data only}
- Botden IP, Draijer R, Westerhof BE, Rutten JH, Langendonk JG, Sijbrands EJ, et al. Red wine polyphenols do not lower peripheral or central blood pressure in mild hypertension: a randomized, double-blind crossover trial. Hypertension 2011;58(5):e165. [DOI: 10.1038/ajh.2012.25] [DOI] [Google Scholar]
Botden 2012 {published data only}
- Botden IP, Draijer R, Westerhof BE, Rutten JH, Langendonk JG, Sijbrands EJ, et al. Red wine polyphenols do not lower peripheral or central blood pressure in high normal blood pressure and hypertension. American Journal of Hypertension 2012;25(6):718-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brader 2011 {published data only}
- Brader L, Uusitupa M, Hermansen K. Beneficial effects of a healthy Nordic diet on ambulatory blood pressure in subjects with the metabolic syndrome: a Sysdiet sub study. Obesity Reviews 2011;12:230. [DOI] [PubMed] [Google Scholar]
Bradford 1990 {published data only}
- Bradford RH, Shear CL, Chremos AN, Dujovne C, Franklin FA, Hesney M, et al. Expanded clinical evaluation of lovastatin (EXCEL) study: design and patient characteristics of a double-blind, placebo-controlled study in patients with moderate hypercholesterolemia. American Journal of Cardiology 1990;66(8):44B-55B. [PMID: ] [DOI] [PubMed] [Google Scholar]
Braggio 1992 {published data only}
- Braggio JT, Pishkin V. Systolic blood pressure and neuropsychological test performance of alcoholics. Alcoholism: Clinical and Experimental Research 1992;16(4):726-33. [DOI: 10.1111/j.1530-0277.1992.tb00669.x] [DOI] [PubMed] [Google Scholar]
Brainin 2016 {published data only}
- Brainin M, Teuschl Y, Matz K, Dachenhausen A, Firlinger B, Reiter M, et al. Testing the intensity of multiple, complex interventions for life-style modification in randomized stroke trials. American Stroke Association 2016;42:no pagination. [Google Scholar]
Brasser 2004 {published data only}
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol effects during acamprosate treatment: a dose-response study in humans. Alcoholism: Clinical and Experimental Research 2016;42:1074-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brewer 2010 {published data only}
- Brewer J, Sinha R, Chen J, Michalsen R, Babuscio T, Nich C, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. In: The 72th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2010 June 12-17; Scottsdale, Arizona USA. 2010:18. [DOI] [PMC free article] [PubMed]
Brien 1979 {published data only}
- Brien JF, Peachey JE, Loomis CW, Rogers BJ. The calcium carbimide-ethanol interaction: effects of ethanol dose. Clinical Pharmacology & Therapeutics 1979;25(4):454-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 1981 {published data only}
- Brown ZW, Amit Z, Sutherland A, Rockman G, Selvaggi N, Ogren SO. An examination of possible interactions between zimelidine, a serotonin uptake inhibitor, and ethanol ingested conjointly in human subjects. Neuroscience Letters 1981;27(3):351-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 2010 {published data only}
- Brown J, Brignell CM, Dhiman SK, Curran HV, Kamboj SK. Acute effects of alcohol on memory: impact of emotional context and serial position. Neurobiology of Learning and Memory 2010;93:428-34. [DOI: 10.1016/j.nlm.2009.12.010] [DOI] [PubMed] [Google Scholar]
Brugger‐Andersen 2009 {published data only}
- Brugger-Andersen T, Ponitz V, Snapinn S, Dickstein K. Moderate alcohol consumption is associated with reduced long-term cardiovascular risk in patients following a complicated acute myocardial infraction. International Journal of Cardiology 2009;133(2):229-32. [DOI: 10.1016/j.ijcard.2007.12.046] [DOI] [PubMed] [Google Scholar]
Brunelle 2007 {published data only}
- Brunelle C, Pihl RO. Effects of conditioned reward and nonreward cues on the heart rate response to alcohol intoxication in male social drinkers. Alcoholism: Clinical and Experimental Research 2007;31(3):383-9. [DOI: 10.1111/j.1530-0277.2006.00318.x] [DOI] [PubMed] [Google Scholar]
Bryson 2008 {published data only}
- Bryson CL, Au DH, Sun H, Williams EC, Kivlanhan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Annals of Internal Medicine 2008;149(11):795-804. [DOI: 10.7326/0003-4819-149-11-200812020-00004] [DOI] [PubMed] [Google Scholar]
Bulpitt 1999 {published data only}
- Bulpitt CJ, Shipley MJ. Failure of alcohol reduction to lower blood pressure in the PATHS trial: prevention and treatment of hypertension study. Archives of Internal Medicine 1999;159(2):195-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burke 2001 {published data only}
- Burke V, Hodgson JM, Beilin LJ, Giangiulioi N, Rogers P, Puddey IB. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension 2001;38(4):821-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burke 2005 {published data only}
- Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. Journal of Hypertension 2005;23(6):1241-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burke 2006 {published data only}
- Burke V, Beilin L, Cutt H, Mansour J, Williams A, Mori T. A lifestyle program for treated hypertensives improves cardiovascular risk factors: a randomized controlled trial. Atherosclerosis 2006;Suppl 7(3):386. [PMID: 17208119]17208119 [Google Scholar]
Campbell 1999 {published data only}
- Campbell NR, Burgess E, Choi BC, Taylor G, Wilson E, Cleroux J, et al. Lifestyle modifications to prevent and control hypertension. 1. Methods and an overview of the Canadian recommendations. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Center for Disease Control at Health Canada, Heart and Stroke Foundation in Canada. Canadian Medical Association Journal 1999;160(9 Suppl):S1-6. [PMC free article] [PubMed] [Google Scholar]
Carter 2011 {published data only}
- Carter JR, Stream SF, Durocher JJ, Larson RA. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. American Journal of Physiology-Endocrinology and Metabolism 2011;300:E771-8. [DOI: 10.1152/ajpendo.00674.2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chagas 2016 {published data only}
- Chagas P, Mazocco L, Piccoli J, Ardenghi TM, Badimon L, Caramori PR, et al. Association of alcohol consumption with coronary artery disease severity. Clinical Nutrition ESPEN 2016;36(4):1036-9. [DOI: 10.1016/j.clnu.2016.06.017] [DOI] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang G, Fisher ND, Hornstein MD, Jones JA, Hauke SH, Niamkey N, et al. Brief intervention for women with risky drinking and medical diagnoses: a randomized controlled trial. Journal of Substance Abuse Treatment 2011;41(2):105-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaplin 2008 {published data only}
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcoholism: Clinical and Experimental Research 2008;32(7):1242-50. [PMID: 18482163 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhuri 1994 {published data only}
- Chaudhuri KR, Maule S, Thomaides, Pavitt D, Mathias CJ. Alcohol ingestion lowers supine blood pressure, causes splanchnic vasodilatation and worsens postural hypotension in primary autonomic failure. Journal of Neurology 1994;241(3):145-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiadak 2017 {published data only}
- Chiadak JD, Perret J, Womeni HM, Kuiate JR, Cullus P, Senterre C, et al. Pilot study of risk factors associated with cardiovascular disease in northern and southern Cameroonians. Cardiovascular Journal of Africa 2017;28(4):235-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Childs 2011 {published data only}
- Childs E, O'Connor S, De Wit H. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcoholism: Clinical and Experimental Research 2011;35(10):1794-803. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiva‐Blanch 2012a {published data only}
- Chiva-Blanch G, Urpi-Sarda M, Llorach R, Rotches-Ribalta M, Guillen M, Casas R, et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: a randomized clinical trial. American Journal of Clinical Nutrition 2012;95(2):326-34. [DOI: 10.3945/ajcn.111.022889] [DOI] [PubMed] [Google Scholar]
Chiva‐Blanch 2012b {published data only}
- Chiva-Blanch G, Urpi-Sarda M, Ros E, Arranz S, Valderas-Martinez P, Casas R, et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: short communication. Circulation Research 2012;111(8):1065-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiva‐Blanch 2013a {published data only}
- Chiva-Blanch G, Condines X, Roth I, Magraner E, Arranz S, Valderas-Martinez, et al. Moderate beer consumption increases the number of circulating endothelial progenitor cells in high cardiovascular risk males. Annals of Nutrition and Metabolism 2013;2:40. [Google Scholar]
Chiva‐Blanch 2013b {published data only}
- Chiva-Blanch G, Urpi-Sarda M, Ros E, Valderas-Martinez P, Casas R, Arranz S, et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clinical Nutrition 2013;32(2):200-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiva‐Blanch 2015 {published data only}
- Chiva-Blanch G, Magraner E, Condines X, Valderas-Martinez P, Roth I, Arranz S, et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: a randomized feeding trial. Nutrition, Metabolism and Cardiovascular Diseases 2015;25(1):36-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Colby 2004 {published data only}
- Colby SM, Rohsenow DJ, Monti PM, Gwaltney CJ, Gulliver SB, Abrams DB, et al. Effects of tobacco deprivation on alcohol cue reactivity and drinking among young adults. Addictive Behaviors 2004;29(5):879-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conen 2008 {published data only}
- Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA 2008;300(21):2489-96. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cordain 2000 {published data only}
- Cordain L, Melby CL, Hamamoto AE, O'Neill DS, Cornier MA, Barakat HA, et al. Influence of moderate chronic wine consumption on insulin sensitivity and other correlates of syndrome X in moderately obese women. Metabolism 2000;49(11):1473-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covault 2014 {published data only}
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzier HR. Dutasteride reduces alcohol's sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology 2014;231(17):3609-18. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 1990 {published data only}
- Cox KL, Puddey IB, Morton AR, Masarei JR, Vandongen R, Beilin LJ. Controlled comparison of effects of exercise and alcohol on blood pressure and serum high density lipoprotein cholesterol in sedentary males. Clinical and Experimental Pharmacology and Physiology 1990;17(4):251-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cox 1993 {published data only}
- Cox KL, Puddey IB, Morton AR, Beilin LJ, Vandongen R, Masarei JR. The combined effects of aerobic exercise and alcohol restriction on blood pressure and serum lipids: a two-way factorial study in sedentary men. Journal of Hypertension 1993;11(2):191-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
Croissant 2011 {published data only}
- Croissant B, Demmel R, Rist F, Olbrich R. Heart rate stress response dampening: the impact of alcohol, family history, and gender on at risk children and siblings. International Journal of Psychophysiology 2011;80(1):11-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cushing 2009 {published data only}
- Cushing DJ, Adams MP, Cooper WD, Zhang B, Lipicky RJ, Kowey PR. Evaluation of the effects of PM101, a cyclodextrin-based formulation of intravenous amiodarone, on blood pressure in healthy humans. American Journal of Cardiology 2009;104(8):1152-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cushman 1994 {published data only}
- Cushman WC, Cutler JA, Bingham SF, Harford T, Hanna E, Dubbert P, et al. Prevention and treatment of hypertension study (PATHS): rationale and design. American Journal of Hypertension 1994;7(9 Pt 1):814-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cushman 1998 {published data only}
- Cushman WC, Cutler JA, Hanna E, Bingham SF, Follmann D, Harford T, et al. Prevention and treatment of hypertension study (PATHS): effects of an alcohol treatment program in blood pressure. Archives of Internal Medicine 1998;158(11):1197-207. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cutler 1991 {published data only}
- Cutler JA. Randomized clinical trials of weight reduction in nonhypertensive persons. Annals of Epidemiology 1991;1(4):363-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
deLorenzo 1985 {published data only}
- Lorenzo A, Ceccanti M, Assogna G, Romeo M, Cavalieri G, Attilia ML. Ketanserin hypertension and chronic alcoholism DB study in 40 patients. Bollettino Chimico Farmaceutico 1985;124(10):125S-8S. [PMID: ] [PubMed] [Google Scholar]
deLorenzo 1988 {published data only}
- Lorenzo A, Ceccanti M, Assogna G, Romeo M, Cavaleri G, Attilia ML. Ketanserin, hypertension, and chronic alcoholism: double-blind study in forty patients. International Journal of Clinical and Pharmacological Research 1988;8(5):321-5. [PMID: ] [PubMed] [Google Scholar]
Demmler 2013 {published data only}
- Demmler KM, Ludwig C, Msuya J, Krawinkel MB. Lifestyle patterns are correlated with signs of the metabolic syndrome in Kilimanjaro region, Tanzania. Annals of Nutrition and Metabolism 2013;63:860. [Google Scholar]
deRijke 1996 {published data only}
- Rijke YB, Demacker PN, Assen NA, Sloots LM, Katan MB, Stalenhoef AF. Red wine consumption does not affect oxidizability of low-density lipoproteins in volunteers. American Journal of Clinical Nutrition 1996;63(3):329-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
DiGarbo 2004 {published data only}
- Di Garbo V, Campisi D, Alonzo G, Gambino L, Avellone G, De Simone R, et al. Effects of two Sicilian red wines on some cardiovascular risk factors [Effetto di due vini rossi siciliani su alcuni fattori di rischio cardiovascolare]. Italian Heart Journal 2004;5(5):382-8. [PubMed] [Google Scholar]
Dimmitt 1998 {published data only}
- Dimmitt SB, Rakic V, Puddey IB, Baker R, Oostryck R, Adams MJ, et al. The effects of alcohol on coagulation and fibrinolytic factors: a controlled trial. Blood Coagulation & Fibrinolysis 1998;9(1):39-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Draijer 2015 {published data only}
- Draijer R, Graaf Y, Slettenaar M, Groot E, Wright CI. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients 2015;7(5):3138-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Droste 2013a {published data only}
- Droste DW, lliescu C, Vaillant M, Gantenbein M, De Vremaeker N, Lieunard C, et al. A daily glass of red wine and lifestyle changes do not affect arterial blood pressure and heart rate in patients with carotid arteriosclerosis after 4 and 20 weeks. Cerebrovascular Research 2013;3(1):121-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Droste 2013b {published data only}
- Droste DW, Iliescu C, Vaillant M, Gantenbein M, De Bremaeker N, Lieunard C, et al. A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: results from a randomized controlled trial. Nutrition Journal 2013;3(1):147. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durocher 2011 {published data only}
- Durocher JJ, Larson RA, Carter JR. Acute alcohol ingestion lengthens sympathetic burst latency in humans. FASEB Journal. Conference: Experimental Biology 2011;25:No pagination. [Google Scholar]
Eisenhofer 1987 {published data only}
- Eisenhofer G, Lambie DG, Johnson RH. Effects of ethanol ingestion on blood pressure reactivity. Clinical Science (Colchester) 1987;72(2):251-4. [DOI] [PubMed] [Google Scholar]
Estevez 1995 {published data only}
- Estevez F, Parrillo S, Giusti M, Monti JM. Single-dose ritanserin and alcohol in healthy volunteers: a placebo-controlled trial. Alcohol 1995;12(6):541-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Estruch 2011 {published data only}
- Estruch R, Sacanella E, Mota F, Chiva-Blanch G, Antunez E, Casal E, et al. Moderate consumption of red wine, but not gin, decreases erthrocyte superoxide dismutase activity: a randomized cross-over trial. Nutrition, Metabolism, and Cardiovascular Disease 2011;21(1):46-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farre 1993 {published data only}
- Farre M, la Torre R, Llorente M, Lamas X, Ugena B, Segura J, et al. Alcohol and cocaine interactions in humans. Journal of Pharmacology and Experimental Therapeutics 1993;266(3):1364-73. [PMID: ] [PubMed] [Google Scholar]
Farre 2014 {published data only}
- Farre M, Papaseit E, Menoyo E, Perez M, Martin S, Martinez R, et al. Interactions between soy extract and alcohol. Basic and Clinical Pharmacology and Toxicology 2014;115:27. [Google Scholar]
Farre 2016 {published data only}
- Farre M, Perez-Mana C, De Souza E, Mateus J, Theunisen E, Kuypers K, et al. Interactions between mephedrone and alcohol in humans: cardiovascular and subjective effects. European Psychiatry 2016;33:S117. [DOI: 10.1016/j.eurpsy.2016.01.122] [DOI] [Google Scholar]
Farre 2017 {published data only}
- Farre M, Papaseit E, Perez-Mana C, De Souza Fernandes E, Mateus JF, Olesti E, et al. Interactions between alcohol and mephedrone in humans. Drug and Alcohol Dependence 2017;171:e61. [Google Scholar]
Fazio 2001 {published data only}
- Fazio M, Bardelli M, Macaluso L, Fiammengo F, Mattei PL, Bossi M, et al. Mechanics of the carotid artery wall and baroreflex sensitivity after acute ethanol administration in young healthy volunteers. Clinical Science 2001;101(3):253-60. [PMID: ] [PubMed] [Google Scholar]
Flanagan 2002 {published data only}
- Flanagan DE, Pratt E, Murphy J, Vaile JC, Petley GW, Godsland IF, et al. Alcohol consumption alters insulin secretion and cardiac autonomic activity. European Journal of Clinical Investigation 2002;32(3):187-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flechtner‐Mors 2004 {published data only}
- Flechtner-Mors M, Biesalski HK, Jenkinson CP, Adler G, Ditschuneit HH. Effects of moderate consumption of white wine on weight loss in overweight and obese subjects. International Journal of Obesity and Related Metabolic Disorders 2004;28(11):1420-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Foltin 1988 {published data only}
- Foltin RW, Fischman MW. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacology Biochemistry and Behavior 1988;31(4):877-83. [DOI: 10.1016/0091-3057(88)90399-1] [DOI] [PubMed] [Google Scholar]
Foppa 1999 {published data only}
- Foppa M, Fuchs FD Preissler L, Andrighetto A, Rosito GA, Duncan BB. Wine with the noon meal lowers post meal blood pressure. Randomized trial in centrally obese hypertensive patients. Hypertension 1999;33(4):119. [DOI] [PubMed] [Google Scholar]
Frisk‐Holmberg 1990 {published data only}
- Frisk-Holmberg M, Kerth P, Kleyn E, Synavae P. Effects of jian bu wan - a traditional Chinese medication - on behavioral and cardiovascular parameters after acute alcohol intake in normal subjects. Fundamentals of Clinical Pharmacology 1990;4(1):11-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Andrade 1997 {published data only}
- Garcia-Andrade C, Wall TL, Ehlers CL. The firewater myth and response to alcohol in Mission Indians. American Journal of Psychiatry 1997;154(7):983-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gepner 2013 {published data only}
- Gepner Y, Schwarzfuchs D, Golan R, Henkin Y, Harman-Boehm I, Witkow S, et al. Effects of moderate alcohol intake on 24-h blood pressure dynamics among patients with type 2 diabetes. Clinical Nutrition 2013;32:S166. [Google Scholar]
Gepner 2015 {published data only}
- Gepner Y, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Shelef I, et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized, controlled trial. Annals of Internal Medicine 2015;163(8):569-79. [PMID: 26458258 ] [DOI] [PubMed] [Google Scholar]
Gepner 2016 {published data only}
- Gepner Y, Henkin Y, Schwarzfuchs D, Golan R, Durst R, Shelef I, et al. Differential effect of initiating moderate red wine consumption on 24-h blood pressure by alcohol dehydrogenase genotypes: randomized trial in type 2 diabetes. American Journal of Hypertension 2016;29(4):476-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giovannelli 2011 {published data only}
- Giovannelli L, Pitozzi V, Luceri C, Giannini L, Toti S, Salvini S, et al. Effect of de-alcoholized wines with different polyphenol content on DNA oxidative damage, gene expression of peripheral lymphocytes, and haemorheology: an intervention study in post-menopausal women. European Journal of Nutrition 2011;50(1):19-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Golan 2016 {published data only}
- Golan R, Schwarzfuchs D, Gepner Y, Harman-Boehm I, Stampfer MJ, Rudich A, et al. The effect of moderate wine intake on carotid atherosclerosis in type 2 diabetes, a 2-year intervention study. European Heart Journal 2016;37(Suppl 1):733. [Google Scholar]
Golan 2017 {published data only}
- Golan R, Shelef I, Shemesh E, Henkin Y, Schwarzfuchs D, Gepner Y, et al. Effects of initiating moderate wine intake on abdominal adipose tissue in adults with type 2 diabetes: a 2-year randomized controlled trial. Public Health Nutrition 2017;20(3):549-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golan 2018 {published data only}
- Golan R, Shai I, Gepner Y, Harman-Boehm I, Schwarzfuchs D, Spence J, et al. Effect of wine on carotid atherosclerosis in type 2 diabetes: a 2-year randomized controlled trial. European Journal of Clinical Nutrition 2018;72(6):871-8. [DOI] [PubMed] [Google Scholar]
Gopane 2010 {published data only}
- Gopane RE, Pisa PT, Vorster HH, Kruger A, Margettes BM. Relationships of alcohol intake with biological health outcomes in an African population in transition: the transition and health during urbanisation in South Africa (THUSA) study. South African Journal of Clincial Nutrition 2010;23(3 Suppl 1):S16-21. [DOI: 10.1080/16070658.2010.11734297] [DOI] [Google Scholar]
Gourlay 2013 {published data only}
- Gourlay C, Couchman A, Chan J, Colrain I, Trinder J, Nicholas C. The acute effects of pre bedtime alcohol consumption on heart rate and blood pressure during sleep and at arousal. Sleep and Biological Rhythms 2013;2:29. [Google Scholar]
Greyling 2016 {published data only}
- Greyling A, Bruno RM, Draijer R, Mulder T, Thijssen DH, Taddei S, et al. Effects of wine and grape polyphenols on blood pressure, endothelial function and sympathetic nervous system activity in treated hypertensive subjects. Journal of Functional Foods 2016;27:448-60. [DOI: 10.1016/j.jff.2016.10.003] [DOI] [Google Scholar]
Hansen 2005 {published data only}
- Hansen AS, Marckmann P, Dragsted LO, Finne Nielsen IL, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. European Journal of Clinical Nutrition 2005;59(3):449-55. [PMID: 15674304 ] [DOI] [PubMed] [Google Scholar]
Hartmann 2017 {published data only}
- Hartmann JP, Lund MT. A randomized, single-blinded study of the effect of intake of repair beer during a hangover [En randomiseret og enkeltblindet undersøgelse af effekten af reparationsøl på tømmermænd]. Ugeskr Laeger 2017;179:2208-11. [PMID: ] [PubMed] [Google Scholar]
Hijmering 2007 {published data only}
- Hijmering ML, Lange DW, Lorsheyd A, Kraaijenhagen RJ, de Wiel A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Netherlands Journal of Medicine 2007;65(1):29-35. [PMID: ] [PubMed] [Google Scholar]
Howes 1985 {published data only}
- Howes LG. Pressure effect of alcohol. Lancet 1985;2(8459):835. [PMID: ] [DOI] [PubMed] [Google Scholar]
Howes 1986a {published data only}
- Howes LG, Reid JL. Changes in blood pressure and autonomic reflexes following regular, moderate alcohol consumption. Journal of Hypertension 1986;4(4):421-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Howes 1986b {published data only}
- Howes LG, MacGilchrist A, Hawksby C, Sumner D, Reid JL. Plasma [3H]-noradrenaline kinetics and blood pressure following regular, moderate ethanol consumption. British Journal of Clinical Pharmacology 1986;22(5):521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2012 {published data only}
- Jacobs DM, Fuhrmann JC, Dorsten FA, Rein D, Peters S, Velzen EJ, et al. Impact of short-term intake of red wine and grape polyphenol extract on the human metabolome. Journal of Agricultural and Food Chemistry 2012;60(12):3078-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain M, Wernick R. In abstinent adults with type 2 diabetes, a daily glass of wine (vs mineral water) improved cardiometabolic factors. Annals of Internal Medicine 2016;164(4):JC17. [DOI] [PubMed] [Google Scholar]
Jones 1979 {published data only}
- Jones AW, Jennings RD, Adolfson J, Hesser CM. Combined effects of ethanol and hyperbaric air on body sway and heart rate in man. Undersea Biomedical Research 1979;6(1):15-25. [PMID: ] [PubMed] [Google Scholar]
Kaul 2010 {published data only}
- Kaul S, Belcik T, Kalvaitis S, Jayaweera AR, Choi SW, Wei K. Effect of modest alcohol consumption over 1-2 weeks on the coronary microcirculation of normal subjects. European Journal of Echocardiography 2010;11(8):683-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawano 1998 {published data only}
- Kawano Y, Abe H, Takishita S, Omae T. Effects of alcohol restriction on 24-h ambulatory blood pressure in Japanese men with hypertension. American Journal of Medicine 1998;105(4):307-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kawano 2002 {published data only}
- Kawano Y, Pontes CS, Abe H, Takishita S, Omae T. Effects of alcohol consumption and restriction on home blood pressure in hypertensive patients: serial changes in the morning and evening records. Clinical and Experimental Hypertension 2002;24(1-2):33-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kawano 2004 {published data only}
- Kawano Y, Abe H, Kojima S, Takishita S, Matsuoka H. Effects of repeated alcohol intake on blood pressure and sodium balance in Japanese males with hypertension. Hypertension Research 2004;27(3):167-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kechagias 2015 {published data only}
- Kechagias S, Dernroth DN, Blomgren A, Hansson T, Isaksson A, Walther L, et al. Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: a prospective randomized study. Alcohol and Alcoholism 2015;50(4):399-406. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kelbaek 1987 {published data only}
- Kelbaek H, Gjorup T, Hartling OJ, Marving J, Christensen NJ, Godtfredsen J. Left ventricular function during alcohol intoxication and autonomic nervous blockade. American Journal of Cardiology 1987;59:685-8. [DOI] [PubMed] [Google Scholar]
Kelbaek 1988 {published data only}
- Kelbaek H, Heslet L, Skagen K, Munck O, Christensen NJ, Godtfredsen J. Cardiac function after alcohol ingestion in patients with ischemic heart disease and cardiomyopathy: a controlled study. Alcohol and Alcoholism 1988;23(1):17-21. [DOI: 10.1093/oxfordjournals.alcalc.a044749] [DOI] [PubMed] [Google Scholar]
Kino 1981 {published data only}
- Kino M, Imamitchi H, Morigutchi M, Kawamura K, Takatsu T. Cardiovascular status in asymptomatic alcoholics, with reference to the level of ethanol consumption. British Heart Journal 1981;46(5):545-51. [DOI: 10.1136/hrt.46.5.545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koskinen 1991 {published data only}
- Koskinen P, Kupari M. Alcohol consumption of patients with supraventricular tachyarrhythmias other than atrial fibrillation. Alcohol and Alcoholism 1991;26(2):199-206. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lachtermann 1999 {published data only}
- Lachtermann E, Turhanova L, Rodziewicz M, Stein-Hammer C, Schicketanz KH, Nagel D, et al. Moderate red and white wine consumption and the risk of cardiovascular disease [Veränderungen des KHK-Risikoprofils durch moderaten rotwein- im vergleich zu weißweinkonsum]. Herz Kreislauf 1999;31(1):25-31. [Google Scholar]
Latella 2009 {published data only}
- Latella MC, Di Casteinuovo A, Lorgeril M, Amout J, Cappuccio FP, Krogh V. Genetic variation of alcohol dehydrogenase type 1C (ADH1C) alcohol consumption, and metabolic cardiovascular risk factors: results from the IMMIDIET study. Atherosclerosis 2009;207(1):284-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lavy 1994 {published data only}
- Lavy A, Fuhrman B, Markel A, Dankner G, Ben-Amotz A, Presser D, et al. Effect of dietary supplementation of red or white wine on human blood chemistry, hematology and coagulation: favorable effect of red wine on plasma high-density lipoprotein. Annals of Nutrition and Metabolism 1994;38(5):287-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee WK, Regan TJ. Alcoholic cardiomyopathy: is it dose-dependent. Congestive Heart Failure 2002;8(6):303-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee SJ, Kim JG, Ko Y, Cho YJ, Hong KS, Park JM, et al. Binge drinking habit can increase risk for ischemic stroke in Korean men.. In: American Heart Assocition/ American Stroke Association. International Stroke Conference and State-of the Science Stroke Nursing Symposium; 2006 Feb 16-19; Los Angeles (CA). Los Angeles (CA), 2006.
Li 2006 {published data only}
- Li Y, Wang JG, Gao PJ, Wang GL, Qian YS, Zhu DL, et al. Interaction between body mass index and alcohol intake in relation to blood pressure in HAN and SHE Chinese. American Journal of Hypertension 2006;19(5):448-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li J, Salfati E, Patel C, Eaton C, Nassir R, Stefanick M, et al. A multi-ethnic mendelian randomization study of moderate alcohol use and the risk of atherosclerotic cardiovascular disease in the women's health initiative. Circulation 2016;133:A31. [Google Scholar]
Liang 2012 {published data only}
- Liang Y, Mente A, Yusuf S, Gao P, Sleight P, Zhu J, et al. Alcohol consumption and the risk of incident atrial fibrillation among people with cardiovascular disease. Canadian Medical Association Journal 2012;184(16):E857-66. [DOI: 10.1503/cmaj.120412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malinski 2004 {published data only}
- Malinski MK, Sesso HD, Lopez-Jimenez F, Burirng JE, Gaziano JM. Alcohol consumption and cardiovascular disease mortality in hypertensive men. Archives of Internal Medicine 2004;164(6):623-8. [DOI: 10.1001/archinte.164.6.623] [DOI] [PubMed] [Google Scholar]
Mammen 2018 {published data only}
- Mammen RR, Natinga Mulakal J, Mohanan R, Maliakel B, Illathu Madhavamenon K. Clove bud polyphenols alleviate alterations in inflammation and oxidative stress markers associated with binge drinking: a randomized double-blinded placebo-controlled crossover study. Journal of Medicinal Food 2018;21(11):1188-96. [PMID: 30234415 ] [DOI] [PubMed] [Google Scholar]
Marczinski 2018 {published data only}
- Marczinski CA, Fillmore MT, Stamates AL, Maloney SF. Alcohol-induced impairment of balance is antagonized by energy drinks. Alcoholism: Clinical and Experimental Research 2018;42(1):144-52. [PMID: 29112285 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCance‐Katz 1996 {published data only}
- McCance-Katz EF, Kosten TR, Jatlow PI. Clinical effects of repeated cocaine and alcohol use. In: Proceedings of the 149th Annual Meetig of the American Psychiatric Association; 1996 May 4-9, New York. New York, 1996.
McCance‐Katz 2005 {published data only}
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Substance Use & Misuse 2005;40(4):511-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
McCance‐Katz 2013 {published data only}
- McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM. Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. Journal of Addiction Medicine 2013;7(4):264-70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCaul 1991 {published data only}
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcoholism: Clinical and Experimental Research 1991;15(1):94-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
McDonagh 2018 {published data only}
- McDonagh ST, Wylie LJ, Morgan PT, Vanhatalo A, Jones AM. A randomised controlled trial exploring the effects of different beverages consumed alongside a nitrate-rich meal on systemic blood pressure. Nutrition and Health 2018;24(3):183-92. [PMID: 30099933 ] [DOI] [PubMed] [Google Scholar]
McDougle 1995 {published data only}
- McDougle CJ, Krystal JH, Price LH, Heninger GR, Charney DS. Noradrenergic response to acute ethanol administration in healthy subjects: comparison with intravenous yohimbine. Psychopharmacology (Berlin) 1995;118(2):127-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mezzano 2001 {published data only}
- Mezzano D, Leighton F, Martinez C, Marshall G, Cuevas A, Castillo O, et al. Complementary effects of Mediterranean diet and moderate red wine intake on haemostatic cardiovascular risk factors. European Journal of Clinical Nutrition 2001;55(6):444-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mezzano 2003 {published data only}
- Mezzano D, Leighton F, Strobel P, Martinez C, Marashall G, Cuevas A, et al. Mediterranean diet, but not red wine, is associated with beneficial changes in primary haemostasis. European Journal of Clinical Nutrition 2003;57(3):439-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Minami 2002 {published data only}
- Minami J, Yoshii M, Todoroki M, Nishikimi T, Ishimitsu T, Fukunaga T, et al. Effects of alcohol restriction on ambulatory blood pressure, heart rate, and heart rate variability in Japanese men. American Journal of Hypertension 2002;15(2 Pt 1):125-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mizushima 1990 {published data only}
- Mizushima S, Nara Y, Mano M, Sawamura M, Horie R, Yamori Y. Alcohol consumption as a risk factor for high blood pressure from the cardiovascular disease and alimentary comparison study, CARDIAC Cooperative Research Group. Journal of Cardiovascular Pharmacology 1990;16(Suppl 8):S35-7. [PMID: ] [PubMed] [Google Scholar]
Molnar 2009 {published data only}
- Molnar M, Boha R, Czigler B, Gaal ZA, Benyovszky M, Rona K, et al. The acute effect of low-dose alcohol on working memory during mental arithmetic: II: changes of nonlinear and linear EEG-complexity in the theta band, heart rate and electrodermal activity. International Journal of Psychophysiology 2009;73(2):138-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moreira 1998 {published data only}
- Moreira LB, Fuchs FD, Moraes RS, Bredemeier M, Duncan BB. Alcohol intake and blood pressure: the importance of time elapsed since last drink. Journal of Hypertension 1998;16(2):175-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mori 2015 {published data only}
- Mori TA, Burke V, Beilin LJ, Puddey IB. Randomized controlled intervention of the effects of alcohol on blood pressure in premenopausal women. Hypertension 2015;66(3):517-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mori 2016 {published data only}
- Mori TA, Burke V, Zilkens RR, Hodgson JM, Beilin LJ, Piddey IB. The effects of alcohol on ambulatory blood pressure and other cardiovascular risk factors in type 2 diabetes: a randomized intervention. Journal of Hypertension 2016;34(3):421-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Movai 1989 {published data only}
- Movai V, Nadhazi Z, Molnar G, Ungvary G, Folly G. Effect of acute alcohol intake on certain cardiovascular functions in healthy young males [Akut alkoholbevltel hatása egyes cardiovascularls funkclókra flatal egészséges férfiakon]. Orv Hetil 1989;130(52):2785-9. [PubMed] [Google Scholar]
Mukamal 2017 {published data only}
- Mukamal KJ, Na B, Mu L, Mantzoros CS, Manning WJ, Mittleman MA. Lessons and challenges from a 6-month randomized pilot study of daily ethanol consumption: research methodology and study design. Current Developments in Nutrition 2017;1(7):e000505. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naissides 2006a {published data only}
- Naissides M, Pal S, Mamo JC, James AP, Dhaliwal S. The effects of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. European Journal of Clinical Nutrition 2006;60(6):740-5. [DOI] [PubMed] [Google Scholar]
Naissides 2006b {published data only}
- Naissides M, Pal S, Mamo JC, James AP, Dhaliwal S. The effect of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. European Journal of Clinical Nutririon 2006;60(6):740-5. [DOI] [PubMed] [Google Scholar]
Newlin 1989 {published data only}
- Newlin DB. Placebo responding in the same direction as alcohol in women. Alcoholism: Clinical and Experimental Research 1989;13(1):36-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Newlin 1990 {published data only}
- Newlin DB, Byrne EA, Porges SW. Vagal mediation of the effect of alcohol on heart rate. Alcoholism: Clinical and Experimental Research 1990;14(3):421-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Niaura 1988 {published data only}
- Niaura R, Wilson GT, Westrick E. Self-awareness, alcohol consumption, and reduced cardiovascular reactivity. Psychosomatic Medicine 1988;50(4):360-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nicholas 2012 {published data only}
- Nicholas CL, Andrewes HE, Chan JK, Mayer BZ, Colrain IM, Trinder JA. The acute effects of pre bedtime alcohol consumption on heart rate and parasympathetic nervous system activity during wakefulness and sleep. Sleep 2012;35:A57-A8. [Google Scholar]
Nicholas 2013a {published data only}
- Nicholas CL, Coleman HE, Chan JK, Gourlay CG, Colrain IM, Trinder JA. The acute effects of alcohol intake and sleep deprivation on heart rate and parasympathetic activity in young adults. Sleep 2013;36:A71. [Google Scholar]
Nicholas 2013b {published data only}
- Nicholas CL, Abbie C, Gourlay CG, Chan JK, Colrain IM, Trinder JA. The acute effects of pre bedtime alcohol consumption on heart rate and blood pressure during sleep and at arousal. Sleep 2013;36:A335. [Google Scholar]
Noad 2016 {published data only}
- Noad RL, Rooney C, McCall D, Young IS, McCance D, McKinley MC, et al. Beneficial effect of a polyphenol-rich diet on cardiovascular risk: a randomized controlled trial. Heart 2016;102:1371-9. [DOI: 10.1136/heartjnl-2015-309218] [DOI] [PubMed] [Google Scholar]
O'Malley 2014 {published data only}
- O'Malley SS, Wu R, Gueorgieva R, Jatlow P. Bilirubin, an endogenous antioxidant, increases following alcohol consumption. Alcoholism: Clinical and Experimental Research 2014;1:198A. [Google Scholar]
Oda 2017 {published data only}
- Oda N, Kajikawa M, Maruhashi T, Iwamoto Y, Kishimoto S, Matsui S, et al. Endothelial function is impaired in relation to alcohol intake even in the case of light alcohol consumption in Asian men: flow-mediated dilation Japan (FMD-J) study. International Journal of Cardiology 2017;230:523-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Okamura 2001 {published data only}
- Okamura T, Hayakawa T, Kadowaki T, Amamoto K, Kita Y, Okayama A, et al. Estimated relative and absolute risk for cardiovascular disease according to alcohol consumption increase in Japanese general population. Nihon Arukoru Yakubutsu Igakkai Zasshi 2001;36(6):586-95. [PMID: ] [PubMed] [Google Scholar]
Papamichael 2006 {published data only}
- Papamichael C, Karatzi K, Karatzis E, Papaioannou TG, Katsichti P, Zampelas A, et al. Combined acute effects of red wine consumption and cigarette smoking on haemodynamics of young smokers. Journal of Hypertension 2006;24(7):1287-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Papamichael 2008 {published data only}
- Papamichael CM, Karatzi KN, Papioannou TG, Karatzis EN, Katsichti P, Sideris V, et al. Acute combined effects of olive oil and wine on pressure wave reflections: another beneficial influence of the Mediterranean diet antioxidants. Journal of Hypertension 2008;26(2):223-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Papaseit 2016 {published data only}
- Papaseit E, Perez-Mana C, De Souza E, Mateus JA, Theunissen E, Kuypers K, et al. Mephedrone effects on acute alcohol intoxication. Basic and Clinical Pharmacology and Toxicology 2016;119(Suppl 1):28. [Google Scholar]
Park 2004 {published data only}
- Park YK, Kim JS, Kang MH. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: double-blind, placebo controlled intervention trial. Biofactors 2004;22(1-4):145-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 1990 {published data only}
- Parker M, Puddey IB, Beilin LJ, Vandongen R. Two-way factorial study of alcohol and salt restriction in treated hypertensive men. Hypertension 1990;16(4):398-406. [DOI: 10.1161/01.HYP.16.4.398] [DOI] [PubMed] [Google Scholar]
Paz 1996 {published data only}
- Paz R, Jortner R, Tunick PA, Sclarovsky S, Eilat B, Perez JL, et al. The effects of the ingestion of ethanol on obstruction of the left ventricular outflow tract in hypertrophic cardiomyopathy. New England Journal of Medicine 1996;335(13):938-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perkins 1995 {published data only}
- Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, Stiller RL. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berlin) 1995;119(2):205-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perneger 1999 {published data only}
- Pernegar TV, Whelton PK, Puddey IB, Klag MJ. Risk of end-stage renal disease associated with alcohol consumption. American Journal of Epidemiology 1999;150(12):1275-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Petrone 2014 {published data only}
- Petrone AB, Gaziano JM, Djousse L. Alcohol consumption and risk of death in male physicians with heart failure. American Journal of Cardiology 2014;114(7):1065-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisa 2010 {published data only}
- Pisa PT, Kruger A, Vorster HH, Margetts BM, Loots DT. Alcohol consumption and cardiovascular disease risk in an African population in transition: the prospective urban and rural epidemiology (PURE) study. South African Journal of Clinical Nutrition 2010;23(3 Suppl 1):S29-37. [Google Scholar]
Pitsavos 2004 {published data only}
- Pitsavos C, Panagiotakos DB, Kontogianni MD, Chrysohoou C, Chloptsios Y, Zampelas A, et al. The J-shape association of ethanol intake with total homocysteine concentrations: the ATTICA study. Nutrition & Metabolism (London) 2004;1(1):9. [DOI: 10.1186/1743-7075-1-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Potter 1984 {published data only}
- Potter JF, Beevers DG. Pressor effect of alcohol in hypertension. Lancet 1984;1(8369):119-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puddey 1985a {published data only}
- Puddey IB, Beilin LJ, Vandongen R, Rouse IL. A randomized controlled trial of the effect of alcohol consumption on blood pressure. Clinical and Experimental Pharmacology and Physiology 1985;12(3):257-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puddey 1985b {published data only}
- Puddey IB, Beilin LJ, Vandongen R, Rouse IL, Rogers P. Evidence for a direct effect of alcohol consumption on blood pressure in normotensive men: a randomized controlled trial. Hypertension 1985;7(5):707-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puddey 1985c {published data only}
- Puddey IB, Vandongen R, Beilin LJ. Pressore effect of alcohol. Lancet 1985;2(8464):1119-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puddey 1986 {published data only}
- Puddey IB, Beilin LJ, Vandongen R. Effect of regular alcohol use on blood pressure control in treated hypertensive subjects: a controlled study. Clinical and Experimental Pharmacology and Physiology 1986;13(4):315-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puddey 1987 {published data only}
- Puddey IB, Beilin LJ, Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects: a randomized controlled trial. Lancet 1987;1(8534):647-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puddey 1992 {published data only}
- Puddey IB, Parker M, Beilin LJ, Vandongen R, Masarei JR. Effects of alcohol and caloric restrictions on blood pressure and serum lipids in overweight men. Hypertension 1992;20(4):533-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rada 2018 {published data only}
- Rada PQ, Blanch GC, Jauregui O, Estruch R, Raventos RL. Use of LC-HRMS based metabolomics to elucidate beer polyphenol health effects in a human cross over intervention trial. European Journal of Clinical Investigation 2018;48(Suppl 1):184. [Google Scholar]
Rajdl 2007 {published data only}
- Rajdl D, Racek J, Trefil L, Siala K. Effect of white wine consumption on oxidative stress markers and homocysteine levels. Physiology Research 2007;56(2):203-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rakic 1998 {published data only}
- Rakic V, Puddey IB, Burke V, Dimmitt SB, Beilin LJ. Influence of pattern of alcohol intake on blood pressure in regular drinkers: a controlled trial. Journal of Hypertension 1998;16(2):165-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Retterstol 2005 {published data only}
- Retterstol L, Berge KE, Braaten O, Eikvar L, Pedersen TR, Sandvik L. A daily glass of red wine: does it affect markers of inflammation. Alcohol and Alcoholism 2005;40(2):102-5. [PMID: 15642722 ] [DOI] [PubMed] [Google Scholar]
Roache 2011 {published data only}
- Roache JD, Kahn R, Newton TF, Wallace CL, Murff WL, De La Garza R, et al. A double-blind, placebo-controlled assessment of the safety of potential interactions between intravenous cocaine, ethanol, and oral disulfiram. Drug and Alcohol Dependence 2011;119(1-2):37-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2013 {published data only}
- Roth I, Chiva-Blanch G, Codines X, Magraner E, Casas R, Valderas-Martinez P, et al. Moderate beer consumption reduces the cardiovascular risk. Annals of Nutrition and Metabolism 2013;2:62. [Google Scholar]
Roth 2017 {published data only}
- Roth I, Casas R, Medina A, Ribo Coll M, Lamuela-Raventos RM, Estruch R. Acute effects of age wine, Spanish white wine, consumption increased circulating endothelial progenitor cells and reduced cardiovascular risk factors in men: a randomized intervention trial. Annals of Nutrition and Metabolism 2017;71(Suppl 2):940-1. [Google Scholar]
Roth 2018 {published data only}
- Roth I, Ribo-Coll M, Casas R, Estruch R. Consumption of aged wine has beneficial effects on blood pressure and plasma nitric oxide in men with high cardiovascular risk. Annals of Nutrition and Metabolism 2018;73(Suppl 2):44. [Google Scholar]
Sagawa 2011 {published data only}
- Sagawa Y, Kondo H, Matsubuchi N, Takemura T, Kanayama H, Kaneko Y, et al. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcoholism: Clinical and Experimental Research 2011;35(11):2093-100. [DOI: 10.1111/j.1530-0277.2011.01558.x] [DOI] [PubMed] [Google Scholar]
Saito 2003 {published data only}
- Saito K, Yokoyama T, Yoshiike N, Date C, Yamamoto A, Muramatsu M, et al. Do the ethanol metabolizing enzymes modify the relationship between alcohol consumption and blood pressure. Journal of Hypertension 2003;21(6):1097-105. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sehested 1998 {published data only}
- Sehested J, Heringlake M, Schmidt V. Neurohumoral cardiovascular responses to alcohol and their modulation by peroral fluid. American Journal of Cardiology 1998;81(6):761-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Senault 2000 {published data only}
- Senault C, Betoulle D, Luc G, Hauw P, Rigaud D, Fumeron F. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 2000;10(2):63-9. [PMID: ] [PubMed] [Google Scholar]
Shai 2007 {published data only}
- Shai I, Wainstein J, Harman-Boehm I, Raz I, Fraser D, Rudich A, et al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care 2007;30(12):3011-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shai 2015 {published data only}
- Shai I, Gepner Y, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, et al. What is the effect of wine intake in type 2 diabetes and does the wine color matter: a 2-year randomized controlled trial. Obesity Facts 2015;8:32. [Google Scholar]
Sierksma 2002 {published data only}
- Sierksma A, Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. European Journal of Clinical Nutrition 2002;56(11):1130-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sierksma 2004a {published data only}
- Sierksma A, Vermunt SH, Lankhuizen IM, Gaag MS, Scheek LM, et al. Effect of moderate alcohol consumption on parameters of reverse cholesterol transport in postmenopausal women. Alcoholism: Clinical and Experimental Research 2004;28(4):662-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sierksma 2004b {published data only}
- Sierksma A, Sarkola T, Eriksson CJ, Gaag MS, Grobbee DE, Hendriks HF. Effect of moderate alcohol consumption on plasma dehydroepiandrosterone sulfate testosterone, and estradiol levels in middle-aged men and postmenopausal women: a diet-controlled intervention study. Alcoholism: Clinical and Experimental Research 2004;28(5):780-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spaak 2008 {published data only}
- Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, et al. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. American Journal of Physiology: Heart and Circulatory Physiology 2008;294(2):H605-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stiffler 1999 {published data only}
- Stiffler B, Suter PM, Vetter W. Effect of alcohol on circadian blood pressure [Effekt des alkohols auf die blutdruck-tag-nacht-differenz]. Praxis 1999;88:1601-9. [PubMed] [Google Scholar]
Stream 2010 {published data only}
- Stream SF, Schwartz CE, Klein JC, Carter JR. Acute alcohol ingestion blunts arterial pressure but not sympathetic neural responses to orthostatic stress in humans. FASEB Journal. Conference: Experimental Biology 2010;24:no pagination. [Google Scholar]
Stream 2014 {published data only}
- Stream SF, Durocher JJ, Chen QH, Carter JR. Acute alcohol consumption elicits augmented sympathoexcitation in prehypertensive humans. Alcoholism: Clinical and Experimental Research 2014;38:163A. [Google Scholar]
Stuart 2013 {published data only}
- Stuart GL, Shorey RC, Moore TM, Ramsey SE, Kahler CW, O'Farrell TJ, et al. Randomized clinical trial examining the incremental efficacy of a 90-minute motivational alcohol intervention as an adjunct to standard batterer intervention for men. Addiction 2013;108(8):1376-84. [PMID: 23414253 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubbs 1995 {published data only}
- Stubbs TA, Macdonald IA. Systemic and regional haemodynamic effects of caffeine and alcohol in fasting subjects [corrected]. Clinical Autonomic Research 1995;5(3):123-7. [PMID: ] [PubMed] [Google Scholar]
Taborsky 2012 {published data only}
- Taborsky M, Ostadal P, Petrek M. A pilot randomized trial comparing long-term effects of red and white wines on biomarkers of atherosclerosis (in vino veritas: IVV trial). Bratisl Lek Listy 2012;113(3):156-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taborsky 2014 {published data only}
- Taborsky M, Ostadal P, Adam T, Rihova D. In vino veritas (IVV) study: randomized trial comparing long-term effects of red and white wine on markers of atherosclerosis and oxidative stress. European Heart Journal 2014;1:181. [Google Scholar]
Takashima 1998 {published data only}
- Takashima Y, Yoshida M, Kokaze A, Orido Y, Tsugane S, Ishikawa M, et al. Relationship of occupation to blood pressure among middle-aged Japanese men - the significance of the difference in body mass index and alcohol consumption. Journal of Epidemiology 1998;8(4):216-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tinklenberg 1976 {published data only}
- Tinklenberg JR, Roth WT, Kopell BS. Marijuana and ethanol: differential effects on time perception, heart rate, and subjective response. Psychopharmacology (Berlin) 1976;49(3):275-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tome‐Carneiro 2012 {published data only}
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, et al. One-year consumption of grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. American Journal of Cardiology 2012;110(3):356-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tome‐Carneiro 2013 {published data only}
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovascular Drug Therapy 2013;27(1):37-48. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TOMHS 1991 {published data only}
- The Treatment of Mild Hypertension Research Group. The treatment of mild hypertension study. A randomized, placebo-controlled trial of a nutritional-hygienic regimen along with various drug monotherapies. Archives of Internal Medicine 1991;151(7):1413-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Toth 2012 {published data only}
- Toth A, Sandor B, Papp J, Botor D, Horvath Z, Rabai M, et al. Red wine and hemorheology: complex results of in vitro and vivo studies in healthy volunteers. Biorheology 2012;49(2-3):109. [Google Scholar]
Toth 2014 {published data only}
- Toth A, Sandor B, Papp J, Rabai M, Botor D, Horvath Z, et al. Moderate red wine consumption improves hemorheological parameters in healthy volunteers. Clinical Hemorheology and Microcirculation 2014;56(1):13-23. [DOI] [PubMed] [Google Scholar]
Tresserra‐Rimbau 2013 {published data only}
- Tresserra-Rimbau A, Medina-Remon A, Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Lamuela-Raventos R. Cardiovascular risk factors and alcohol consumption within an elderly Spanish population at high cardiovascular risk. Annals of Nutrition and Metabolism 2013;62:68. [Google Scholar]
Tresserra‐Rimbau 2015 {published data only}
- Tresserra-Rimbau A, Medina-Remon A, Lamuela-Raventos RM, Bullo M, Salas-Salvado J, Corella D, et al. Moderate red wine consumption is associated with lower prevalence of the metabolic syndrome in the PREDIMED population. British Journal of Nutrition 2015;113 Suppl 2:S121-30. [DOI] [PubMed] [Google Scholar]
Tuomilehto 1984 {published data only}
- Tuomilehto J, Enlund H, Salonen JT, Nissinen A. Alcohol, patient compliance and blood pressure control in hypertensive patients. Scandinavian Journal of Social Medicine 1984;12(4):177-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Turczynski 2001 {published data only}
- Turczynski B, Chmiel B, Slowinska L, Olszowy Z. The influence of controlled ethanol consumption on whole blood and plasma viscosity. Wiadomosci Lekarskie 2001;54(7-8):409-17. [PubMed] [Google Scholar]
Ueshima 1987a {published data only}
- Ueshima H, Ogihara T, Baba S, Tabuchi Y, Mikawa K, Hashizume K, et al. The effect of reduced alcohol consumption on blood pressure: a randomized, controlled, single blind study EMT - adult EMT - blood pressure EMT - controlled study EMT - etiology EMT - human EMT - male EMT - normal human EMT - therapy EMT - adrenalin EMT - alcohol EMT - aldosterone EMT - hydrocortisone EMT - renin. Journal of Human Hypertension 1987;1(2):113-9. [PubMed] [Google Scholar]
Ueshima 1987b {published data only}
- Ueshima H, Ogihara T, Baba S, Tabuchi Y, Mikawa K, Hashizume K, et al. The effect of reduced alcohol consumption on blood pressure: a randomized, controlled, single blind study. Journal of Human Hypertension 1987;1(2):113-9. [PMID: ] [PubMed] [Google Scholar]
Ueshima 1988 {published data only}
- Ueshima H, Mikawa K, Baba S, Ozawa H, Tsushima M, Kawaguchi A, et al. Reduction in alcohol consumption lowers blood pressure: a randomized and crossover controlled study. Journal of Hypertension 1988;6:S743. [Google Scholar]
Ueshima 1993 {published data only}
- Ueshima H, Mikawa K, Baba S, Sasaki S, Ozawa H, Tsushima M, et al. Effect of reduced alcohol consumption on blood pressure in untreated hypertensive men. Hypertension 1993;21(2):248-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Uhart 2010 {published data only}
- Uhart M, McCaul ME, Weerts EM, Munro C, Breese C, Wand GS. GABRG1 and GABRA2 markers moderate the cardiovascular effects of alcohol. Alcoholism: Clinical and Experimental Research 2010;34(6):74A. [Google Scholar]
Urquiaga 2015 {published data only}
- Urquiaga I, D'Acuna S, Perez D, Dicenta S, Echeverria G, Rigotti A, et al. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: a randomized controlled trial. Biological Research 2015;48:49. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
vanMierlo 2010 {published data only}
- Mierlo LA, Zock PL, Knaap HC, Drajier R. Grape polyphenols do not affect vascular function in healthy men. Journal of Nutrition 2010;140(10):1769-73. [PMID: 20702747 ] [DOI] [PubMed] [Google Scholar]
Vatsalya 2011 {published data only}
- Vatsalya V, Momenan R, Hommer D, Ramchandani VA. Association between changes in heart rate and subjective responses during acute intravenous alcohol infusions in social drinkers. Alcoholism: Clinical and Experimental Research 2011;35:222A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vazquez‐Fresno 2012 {published data only}
- Vazquez-Fresno R, Llorach R, Alcaro F, Rodriguez MA, Vinaixa M, Chiva-Blanch G, et al. (1) H-NMR-based metabolomic analysis of the effect of moderate wine consumption on subjects with cardiovascular risk factors. Electrophoresis 2012;33(15):2345-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vena 2018a {published data only}
- Vena A, King A, Lee R, Wit H. Intranasal oxytocin does not modulate responses to alcohol in social drinkers. Alcoholism: Clinical and Experimental Research 2018;42(9):235A. [DOI: 10.1111/acer.13814] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vena 2018b {published data only}
- Vena A, King A, Lee R, Wit H. Intranasal oxytocin does not modulate responses to alcohol in social drinkers. Alcoholism: Clinical and Experimental Research 2018;42(9):1725-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wensing 2006 {published data only}
- Wensing G, Bauer R, Unger S, Rohde G, Heinig R. Somultaneous administration of vardenafil and alcohol does not result in a pharmacodynamic or pharmacokinetic interaction in healthy male subjects. International Journal of Clinical and Pharmacological Therapy 2006;44(5):216-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilson 2014 {published data only}
- Wilson GB, Wray C, McGovern R, Newbury-Birch D, McColl E, Crosland A, et al. Intervention to reduce excessive alcohol consumption and improve comorbidity outcomes in hypertensive or depressed primary care patients: two parallel cluster randomized feasibility trials. Trials 2014;15:235. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Witkowska 2017 {published data only}
- Witkowska AM, Zujko ME, Waskiewicz A, Szczesniewska D, Stepaniak U, Drygas W. Dietary antioxidant potential and polyphenol intake in Polish postmenopausal women diagnosed with cardiovascular diseases. European Journal of Preventive Cardiology 2017;24(1_suppl):S17-S24. [DOI: 10.1177/2047487317703525] [DOI] [Google Scholar]
Woerdeman 2018 {published data only}
- Woerdeman J, Dei Rio D, Calani L, Eringa EC, Smulders YM, Seme EH. Red wine polyphenols do not improve obesity-associated insulin resistance: a randomized controlled trial. Diabetes, Obesity, and Metabolism 2018;20:206-10. [DOI: 10.1111/dom.13044] [DOI] [PubMed] [Google Scholar]
Wray 2013 {published data only}
- Wray TB, Simons JS, Maisto SA. Effectsof alcohol consumption on heart rate, subjective drug effects, and sexual behavior in those experiencing high/low arousal. Alcoholism: Clinical and Experimental Research 2013;37:84A. [Google Scholar]
Yang 2017 {published data only}
- Yang Y, Zhang H, Huang W, Feng R, Feng P, Gu J, et al. The relationship of alcohol consumption with left ventricular mass in people 35 years old or older in rural areas of Western China. Journal of the American Society of Hypertension 2017;12:No pagination. [DOI] [PubMed] [Google Scholar]
YftachGepner 2015 {published data only}
- Yftach Gepner Y, Henkin Y, Schwarzfuchs D, Golan R, Durst R, Shelef I, et al. The effect of moderate red wine consumption on 24-h blood pressure trajectory in type 2 diabetes; a six month randomized controlled intervention trial. European Journal of Preventive Cardiology 2015;1:S179. [Google Scholar]
Yin 2007 {published data only}
- Yin R, Li H, Wu J, Lin W, Yang D Pan S, et al. Effects of alcohol consumption and other lifestyle behaviors on blood pressure for the middle-aged and elderly in the Guangxi Hei Yi Zhuang and Han population. Alcohol 2007;41(8):541-50. [DOI] [PubMed] [Google Scholar]
Yin 2015 {published data only}
- Yin RX, Aung LH, Long XJ, Yan TT, Cao XL, Huang F. Interactions of several single nucleotide polymorphisms and alcohol consumption on blood pressure levels. Biofactors 2015;41(5):339-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zamora‐Ros 2006 {published data only}
- Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventos RM, Estruch R, Vazquez-Agell M, Serrano-Martinez J, et al. Diagnostic performance of urinary resveratrol metabolites as a biomarker of moderate wine consumption. Clinical Chemistry 2006;52(7):1373-80. [DOI: 10.1373/clinchem.2005.065870] [DOI] [PubMed] [Google Scholar]
Zheng 2012 {published data only}
- Zheng JS, Yang J, Huang T, Hu XJ, Luo M, Li D. Effects of Chinese liquors on cardiovascular disease risk factors in healthy young humans. Scientific World Journal 2012;2012:372143. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziauddeen 2013 {published data only}
- Ziauddeen H, Nathan PJ, Dodds C, Maltby K, Miller SR, Waterworth D, et al. The effects of alcohol on the pharmacokinetics and pharmacodynamics of the selective mu-opioid receptor antagonist GSK1521498 in healthy subjects. Journal of Clinical Pharmacology 2013;53(10):1078-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zilkens 2003 {published data only}
- Zilkens RR, Rich L, Burke V, Beilin LJ, Watts GF, Puddey IB. Effects of alcohol intake on endothelial function in men: a randomized controlled trial. Journal of Hypertension 2003;21(1):97-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zilkens 2005 {published data only}
- Zilkens RR, Burke V, Hodgson JM, Barden A, Beilin LJ, Puddey IB. Red wine and beer elevate blood pressure in normotensive men. Hypertension 2005;45(5):874-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Polzein ongoing {published data only}
- Polzein J, Gherna C. Alcohol and neural cardiovascular control in binge drinkers. https://clinicaltrials.gov/ct2/show/NCT03567434 (accessed 25 June 2018).
Additional references
Abdel‐Rahman 1985
- Abdel-Rahman AR, Dar MS, Wooles WR. Effect of chronic ethanol administration on arterial baroreceptor function and pressor and depressor responsiveness in rats. Journal of Pharmacology and Experimental Therapeutics 1985;232(1):194-201. [PMID: ] [PubMed] [Google Scholar]
Abramson 2001
- Abramson JL, Williams SA, Krumholz HM, Vaccarino V. Moderate alcohol consumption and risk of heart failure among older persons. JAMA 2001;285(15):1971-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agarwal 1981
- Agarwal DP, Harada S, Goedde HW. Racial differences in biological sensitivity to ethanol: the role of alcohol dehydrogenase and aldehyde dehydrogenase isozymes. Alcoholism: Clinical and Experimental Research 1981;5(1):12-6. [DOI] [PubMed] [Google Scholar]
Altura 1978
- Altura BM, Carella A, Altura BT. Acetaldehyde on vascular smooth muscle: possible role in vasodilator action of ethanol. European Journal of Pharmacology 1978;52(1):73-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bellentani 1997
- Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. BMJ Journals (Gut) 1997;41(6):845-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Briasoulis 2012
- Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta‐analysis. Journal of Clinical Hypertension 2012;14(11):792-8. [DOI: https://doi.org/10.1111/jch.12008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carretta 1988
- Carretta R, Fabris B, Bardelli M, Muiesan S, Fishchetti F, Cesanelli R, et al. Acute effects of intravenous infusions of alcohol on baroreceptor sensitivity in essential hypertension. Cardiovascular Research 1988;22(3):226-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
CCSA
- National Alcohol Strategy Advisory Committee Working Group. What Is a Drink? Communicating Drink Information to the Consumer. Canadian Centre on Substance Abuse 2015.
Cederbaum 2012
- Cederbaum A. Alcohol metabolism. Clinical Liver Disease 2012;16(4):667-85. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1999
- Chen CC, Lu BB, Chen YC, Wang MF, Chang YC, Li TK, et al. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. American Journal of Human Genetics 1999;65(3):795-807. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008
- Chen L, Smith GD, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Medicine 2008;5(3):e52. [PMID: 18318597 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2005
- Collins XH, Harmon SD, Kaduce TL, Berst KB, Fang X, Moore SA, et al. ω-Oxidation of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral microvascular smooth muscle and endothelium by alcohol dehydrogenase 4. Journal of Biological Chemistry 2005;280(39):33157-64. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2018.Available at www.covidence.org.
Deeks 2011
- Deeks J, Higgins J, Altman D. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: Cochrane, 2011. [Google Scholar]
Deng 2007
- Deng XS, Deitrich RA. Ethanol metabolism and effects: nitric oxide and its interaction. Current Clinical Pharmacology 2007;2(2):145-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Castelnuovo 2006
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, De Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Archives of Internal Medicine 2006;166(22):2437-45. [DOI: 10.1001/archinte.166.22.2437] [DOI] [PubMed] [Google Scholar]
Djoussé 2007
- Djoussé L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians' Health Study I. Circulation 2007;115(1):34-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Enomoto 1991
- Enomoto N, Takase S, Yasuhara M, Takada A. Acetaldehyde metabolism in different aldehyde dehydrogenase-2 genotypes. Alcoholism: Clinical and Experimental Research 1991;15(1):141-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fuchs 2001
- Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension 2001;37(5):1242-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gao 2011
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141(5):1572-85. [DOI: 10.1053/j.gastro.2011.09.002] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2015
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1659-724. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentry 2000
- Gentry RT. Effect of food on the pharmacokinetics of alcohol absorption. Alcoholism: Clinical and Experimental Research 2000;24(4):403-4. [DOI: 10.1097/00000374-200004000-00003] [DOI] [PubMed] [Google Scholar]
Gillespie 1967
- Gillespie JA. Vasodilator properties of alcohol. British Medical Journal 1967;2(5547):274-7. [DOI: 10.1136/bmj.2.5547.274] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADEpro GDT. Version Accessed prior to 20 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.Available at gradepro.org.
Grassi 1989
- Grassi GM, Somers VK, Renk WS, Abboud FM, Mark AL. Effects of alcohol intake on blood pressure and sympathetic nerve activity in normotensive humans: a preliminary report. Journal of Hypertension Supplement 1989;7(6):S20-1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guitteau 2006
- Guitteau M, Rosenberg M. Lifestyle recommendations plus the DASH diet reduced hypertension in patients with above optimal blood pressure. ACP Journal Club 2006;145(2):42. [PMID: ] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AE, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Hahn 1983
- Hahn HK, Burch RE. Impaired ethanol metabolism with advancing age. Alcoholism: Clinical and Experimental Research 1983;7(3):299-301. [DOI: ] [DOI] [PubMed] [Google Scholar]
Heit 2013
- Heit C, Dong H, Chen Y, Thompson DC, Deitrich RA, Vasiliou VK. The role of CYP2E1 in alcohol metabolism and sensitivity in the central nervous system. Subcellular Biochemistry 2013;67:235-47. [DOI: 10.1007/978-94-007-5881-0_8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). London: Cochrane, 2011. Available from handbook.cochrane.org.
Husain 2014
- Husain K, Ansari RA, Ferder L. Alcohol-induced hypertension: mechanism and prevention. World Journal of Cardiology 2014;6(5):245-52. [DOI: 10.4330/wjc.v6.i5.245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaubert 2014
- Jaubert MP, Jin Z, Russo C, Schwartz JE, Homma S, Elkind MS, et al. Alcohol consumption and ambulatory blood pressure: a community-based study in an elderly cohort. American Journal of Hypertension 2014;27(5):688-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 1968
- Jenkins JS, Connolly J. Adrenocortical response to ethanol in man. BMJ Journals 1968;2(5608):804–5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kannel 1972
- Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. New England Journal of Medicine 1972;287(16):781-7. [DOI: 10.1056/NEJM197210192871601] [DOI] [PubMed] [Google Scholar]
Kawano 1999
- Kawano Y, HItoshi A, Kojima S, Takishita S, Omae T. Effects of propranolol on cardiovascular and neurohumoral actions of alcohol in hypertensive patients. Blood Pressure 1999;8(1):37-42. [DOI] [PubMed] [Google Scholar]
Kostis 1997
- Kostis JB, Davis BR, Cutler J, Grimm RH, Berge KG, Cohen JD, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA 1997;278(3):212-6. [PMID: ] [PubMed] [Google Scholar]
Kushi 2012
- Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA: A Cancer Journal for Clinicians 2012;62(1):30-67. [DOI: 10.3322/caac.20140] [DOI] [PubMed] [Google Scholar]
Kwo 1998
- Kwo PY, Ramchandani VA, O'Connor S, Amann D, Carr LG, Sandrasegaran K, et al. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology 1998;115(6):1552-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2009
- Li H, Borinskaya S, Yoshimura K, Kal’Ina N, Marusin A, Stepanov VA, et al. Refined geographic distribution of the oriental ALDH2* 504Lys (nee 487Lys) variant. Annals of Human Genetics 2009;73(3):335-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2012
- Li D, Zhao H, Gelernter J. Strong protective effect of the aldehyde dehydrogenase gene (ALDH2) 504lys (* 2) allele against alcoholism and alcohol-induced medical diseases in Asians. Human Genetics 2012;131(5):725-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lieber 1998
- Lieber CS. Hepatic and other medical disorders of alcoholism: from pathogenesis to treatment. Journal of Studies on Alcohol and Drugs 2015;59(1):9–25. [DOI] [PubMed] [Google Scholar]
McCullough 2011
- McCullough AJ, O'Shea RS, Dasarathy S. Diagnosis and management of alcoholic liver disease. Journal of Digestive Diseases 2011;12(4):257-62. [DOI: 10.1111/j.1751-2980.2010.00470.x] [DOI] [PubMed] [Google Scholar]
McFadden 2005
- McFadden CB, Brensinger CM, Berlin JA, Townsend RR. Systematic review of the effect of daily alcohol intake on blood pressure. American Journal of Hypertension 2005;18(2 Pt 1):276-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
McGovern 2009
- McGovern PE. Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages. Oakland (CA): University of California Press, 2009. [0520253795, 9780520253797] [Google Scholar]
Meier 2008
- Meier P, Seitz HK. Age, alcohol metabolism and liver disease. Current Opinion in Clinical Nutrition & Metabolic Care 2008;11(1):21-6. [DOI: 10.1097/MCO.0b013e3282f30564] [DOI] [PubMed] [Google Scholar]
Mills 2016
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134(6):441-50. [DOI: 10.1161/CIRCULATIONAHA.115.018912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirdamadi 2017
- Mirdamadi A, Etebari M. Comparison of manual versus automated blood pressure measurement in intensive care unit, coronary care unit, and emergency room. ARYA Atherosclerosis 2017;13(1):29-34. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Muniyappa 2007
- Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocrine Reviews 2007;28(5):463-91. [DOI] [PubMed] [Google Scholar]
Musini 2014
- Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003824.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIAAA 2017
- National Institute on Alcohol Abuse and Alcoholism. Understanding the impact of alcohol on human health and well-being: what is a standard drink? National Institute on Alcohol Abuse and Alcoholism 2017.
Nutt 1999
- Nutt D. Alcohol and the brain. Pharmacological insights for psychiatrists. British Journal of Psychiatry 1999;175:114-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peng 2014
- Peng Q, Gizer IR, Libiger O, Bizon C, Wilhelmsen KC, Schork NJ, Ehlers CL. Association and ancestry analysis of sequence variants in ADH and ALDH using alcohol-related phenotypes in a Native American community sample. American Journal of Medical Genetics 2014;165B(8):673-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plunk 2014
- Plunk AD, Syed-Mohammed H, Cavazos-Rehg P, Bierut LJ, Grucza RA. Alcohol consumption, heavy drinking, and mortality: rethinking the j-shaped curve. Alcoholism: Clinical and Experimental Research 2014;38(2):471-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poulter 2015
- Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 2015;386(9995):801-12. [DOI: 10.1016/S0140-6736(14)61468-9] [DOI] [PubMed] [Google Scholar]
Puddey 1985
- Puddey IB, Vandongen R, Beilin LJ, Rouse IL. Alcohol stimulation of renin release in man: its relation to the hemodynamic, electrolyte, and sympatho-adrenal responses to drinking. Journal of Clinical Endocrinology & Metabolism 1985;61(1):37-42. [DOI: 10.1210/jcem-61-1-37] [DOI] [PubMed] [Google Scholar]
Randin 1995
- Randin D, Vollenweider P, Tappy L, Jéquier E, Nicod P, Scherrer U. Suppression of alcohol-induced hypertension by dexamethasone. New England Journal of Medicine 1995;332:1733-8. [PMID: 10.1056/NEJM199506293322601] [DOI] [PubMed] [Google Scholar]
Reboussin 2018
- Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2018;71(19):2176-98. [DOI: 10.1016/j.jacc.2017.11.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, Cochrane, 2014.
Rocha 2012
- Rocha JT, Hipólito UV, Callera GE, Yogi A, dos Anjos Neto Filho M, Bendhack LM, et al. Ethanol induces vascular relaxation via redox-sensitive and nitric oxide-dependent pathways. Vascular Pharmacology 2012;56(1-2):74-83. [DOI] [PubMed] [Google Scholar]
Rupp 1996
- Rupp H, Brilla CG, Maisch B. Hypertension and alcohol: central and peripheral mechanisms. Herz 1996;21(4):258-64. [PMID: ] [PubMed] [Google Scholar]
Russ 1991
- Russ R, Abdel-Rahman AR, Wooles WR. Role of the sympathetic nervous system in ethanol-induced hypertension in rats. Alcohol 1991;8(4):301-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schrier 1999
- Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. New England Journal of Medicine 1999;341:577-85. [DOI: 10.1056/NEJM199908193410806] [DOI] [PubMed] [Google Scholar]
Seitz 2007
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Reviews. Cancer 2007;7(8):599-612. [DOI: 10.1038/nrc2191] [DOI] [PubMed] [Google Scholar]
SHEP 1991
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991;265(24):3255-64. [PMID: ] [PubMed] [Google Scholar]
Staessen 1999
- Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, Leeuw PW, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic hypertension in Europe trial investigators. JAMA 1999;282(6):539-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Steiner 2015
- Steiner J, Crowell K, Lang C. Impact of alcohol on glycemic control and insulin action. Biomolecules 2015;5(4):2223-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2009
- Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, et al. Alcohol and hypertension: gender differences in dose–response relationships determined through systematic review and meta‐analysis. Addiction 2009;104(12):1981-90. [DOI: https://doi.org/10.1111/j.1360-0443.2009.02694.x] [DOI] [PubMed] [Google Scholar]
Thomasson 1995
- Thomasson HR, Beard JD, Li TK. ADH2 gene polymorphisms are determinants of alcohol pharmacokinetics. Alcoholism: Clinical and Experimental Research 1995;19(6):1494-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomasson 2000
- Thomasson H. Alcohol elimination: faster in women? Alcoholism: Clinical and Experimental Research 2000;24(4):419-20. [PMID: ] [PubMed] [Google Scholar]
Wall 2016
- Wall TL, Luczak SE, Hiller-Sturmhöfel S. Biology, genetics, and environment: underlying factors influencing alcohol metabolism. Alcohol Research: Current Reviews 2016;38(1):59-68. [PMC free article] [PubMed] [Google Scholar]
Weber 2014
- Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. Journal of Clinical Hypertension (Greenwich) 2014;16(1):14-26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2011
- Welch KA. Neurological complications of alcohol and misuse of drugs. Practical Neurology 2011;11(4):206-19. [DOI: 10.1136/practneurol-2011-000062] [DOI] [PubMed] [Google Scholar]
Whitworth 1984
- Whitworth JA, Saines D, Scoggins BA. Blood pressure and metabolic effects of cortisol and deoxycorticosterone in man. Clinical and Experimental Hypertension. Part A: Theory and Practice 1984;6(4):795-809. [DOI: 10.3109/10641968409044039] [DOI] [PubMed] [Google Scholar]
Whitworth 2005
- Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vascular Health and Risk Management 2005;1(4):291-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
WHO 2018
- World Health Organization. Global Status Report on Alcohol and Health 2018. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
Willey 2014
- Willey JZ, Moon YP, Kahn E, Rodriguez CJ, Rundek T, Cheung K, et al. Population attribution risks of hypertension and diabetes for cardiovascular disease and stroke in the Northern Manhattan study. Journal of the American Heart Association 2014;3:e001106. [DOI: 10.1161/JAHA.114.001106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worm 2013
- Worm N, Belz GG, Stein-Hammer C. Moderate wine consumption and prevention of coronary heart disease. Deutsche Medizinische Wochenschrift 2013;138(51/52):2653-7. [DOI: 10.1055/s-0033-1359900] [DOI] [PubMed] [Google Scholar]
Zhang 1989
- Zhang X, Abdel-Rahman AA, Wooles WR. Impairment of baroreceptor reflex control of heart rate but not sympathetic efferent discharge by central neuroadministration of ethanol. Hypertension 1989;14(3):282-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zimatkin 2006
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcoholism: Clinical and Experimental Research 2006;30(9):1500-5. [DOI: 10.1111/j.1530-0277.2006.00181.x] [DOI] [PubMed] [Google Scholar]


