Abstract
Background
Populations experiencing homelessness have high rates of tobacco use and experience substantial barriers to cessation. Tobacco‐caused conditions are among the leading causes of morbidity and mortality among people experiencing homelessness, highlighting an urgent need for interventions to reduce the burden of tobacco use in this population.
Objectives
To assess whether interventions designed to improve access to tobacco cessation interventions for adults experiencing homelessness lead to increased numbers engaging in or receiving treatment, and whether interventions designed to help adults experiencing homelessness to quit tobacco lead to increased tobacco abstinence. To also assess whether tobacco cessation interventions for adults experiencing homelessness affect substance use and mental health.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register, MEDLINE, Embase and PsycINFO for studies using the terms: un‐housed*, homeless*, housing instability, smoking cessation, tobacco use disorder, smokeless tobacco. We also searched trial registries to identify unpublished studies. Date of the most recent search: 06 January 2020.
Selection criteria
We included randomized controlled trials that recruited people experiencing homelessness who used tobacco, and investigated interventions focused on the following: 1) improving access to relevant support services; 2) increasing motivation to quit tobacco use; 3) helping people to achieve abstinence, including but not limited to behavioral support, tobacco cessation pharmacotherapies, contingency management, and text‐ or app‐based interventions; or 4) encouraging transitions to long‐term nicotine use that did not involve tobacco. Eligible comparators included no intervention, usual care (as defined by the studies), or another form of active intervention.
Data collection and analysis
We followed standard Cochrane methods. Tobacco cessation was measured at the longest time point for each study, on an intention‐to‐treat basis, using the most rigorous definition available. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for smoking cessation for each study where possible. We grouped eligible studies according to the type of comparison (contingent reinforcement in addition to usual smoking cessation care; more versus less intensive smoking cessation interventions; and multi‐issue support versus smoking cessation support only), and carried out meta‐analyses where appropriate, using a Mantel‐Haenszel random‐effects model. We also extracted data on quit attempts, effects on mental and substance‐use severity, and meta‐analyzed these outcomes where sufficient data were available.
Main results
We identified 10 studies involving 1634 participants who smoked combustible tobacco at enrolment. One of the studies was ongoing. Most of the trials included participants who were recruited from community‐based sites such as shelters, and three included participants who were recruited from clinics. We judged three studies to be at high risk of bias in one or more domains. We identified low‐certainty evidence, limited by imprecision, that contingent reinforcement (rewards for successful smoking cessation) plus usual smoking cessation care was not more effective than usual care alone in promoting abstinence (RR 0.67, 95% CI 0.16 to 2.77; 1 trial, 70 participants). We identified very low‐certainty evidence, limited by risk of bias and imprecision, that more intensive behavioral smoking cessation support was more effective than brief intervention in promoting abstinence at six‐month follow‐up (RR 1.64, 95% CI 1.01 to 2.69; 3 trials, 657 participants; I2 = 0%). There was low‐certainty evidence, limited by bias and imprecision, that multi‐issue support (cessation support that also encompassed help to deal with other challenges or addictions) was not superior to targeted smoking cessation support in promoting abstinence (RR 0.95, 95% CI 0.35 to 2.61; 2 trials, 146 participants; I2 = 25%). More data on these types of interventions are likely to change our interpretation of these data. Single studies that examined the effects of text‐messaging support, e‐cigarettes, or cognitive behavioral therapy for smoking cessation provided inconclusive results. Data on secondary outcomes, including mental health and substance use severity, were too sparse to draw any meaningful conclusions on whether there were clinically‐relevant differences. We did not identify any studies that explicitly assessed interventions to increase access to tobacco cessation care; we were therefore unable to assess our secondary outcome ‘number of participants receiving treatment'.
Authors' conclusions
There is insufficient evidence to assess the effects of any tobacco cessation interventions specifically in people experiencing homelessness. Although there was some evidence to suggest a modest benefit of more intensive behavioral smoking cessation interventions when compared to less intensive interventions, our certainty in this evidence was very low, meaning that further research could either strengthen or weaken this effect. There is insufficient evidence to assess whether the provision of tobacco cessation support and its effects on quit attempts has any effect on the mental health or other substance‐use outcomes of people experiencing homelessness. Although there is no reason to believe that standard tobacco cessation treatments work any differently in people experiencing homelessness than in the general population, these findings highlight a need for high‐quality studies that address additional ways to engage and support people experiencing homelessness, in the context of the daily challenges they face. These studies should have adequate power and put effort into retaining participants for long‐term follow‐up of at least six months. Studies should also explore interventions that increase access to cessation services, and address the social and environmental influences of tobacco use among people experiencing homelessness. Finally, studies should explore the impact of tobacco cessation on mental health and substance‐use outcomes.
Plain language summary
What types of interventions benefit people experiencing homelessness to quit smoking?
Background
People experiencing homelessness are more likely to use tobacco, and face many problems that make it difficult for them to quit. Health problems caused by using tobacco are among the leading causes of death among this population, so there is a need to find new ways to reduce tobacco use in people experiencing homelessness. Healthcare guidance says that treatment to quit tobacco smoking should include some form of counseling or support, plus medicines designed to help people stop smoking. However, this treatment is often not provided or used among people experiencing homelessness. Our review looked at whether systems designed to help adults experiencing homelessness to get treatments to quit tobacco, and treatments designed to help adults experiencing homelessness to quit tobacco lead to more use of treatments and more people quitting tobacco use. We also looked at whether treatments to help adults experiencing homelessness to quit tobacco changed their use of other drugs and their mental health.
Study characteristics
We included 10 studies involving 1634 participants. One of these studies is still being carried out, but the other nine have been completed. All participants were tobacco smokers, aged 18 years or older, and had experienced homelessness. Most participants were recruited from places within the community, such as homeless shelters, but some were also recruited from healthcare clinics. All studies offered participants some form of counseling support to quit smoking, and eight of these studies also offered stop‐smoking medicines. The treatments tested in the included studies were: e‐cigarettes, text‐message support, rewards for stopping smoking, more intensive counseling support, treatments focused on other lifestyle challenges plus smoking, and cognitive behavioral therapy. The evidence is up to date to January 2020.
Key results
There was not enough information to decide whether stop‐smoking treatments targeted specifically at people experiencing homelessness made them more likely to quit smoking than standard treatment to stop smoking.There was also not enough information to determine whether these treatments affected the mental health or drug use of people experiencing homelessness.
Quality of evidence
We judged all of the information included in this review to be either of low or of very low quality. This is because the studies included in this review were small, and there were problems with how some of the included studies were carried out. This means it is difficult to know whether these interventions help people who experience homelessness to quit smoking. The findings of this review are very likely to change as new studies are completed.
Summary of findings
Background
Description of the condition
Tobacco use is disproportionately concentrated among low‐income populations, with rates exceeding that of the general population at least two‐fold (Jamal 2015). Among low‐income populations, such as people experiencing homelessness, estimated smoking prevalence ranges between 57% and 82% (Baggett 2013a; Soar 2020). Individuals with severe mental‐health disorders, substance‐use disorders, or both, who belong to racial or ethnic minority groups, who are older, or who self‐identify as a gender and sexual minority are disproportionately represented in populations experiencing homelessness (Culhane 2013; Fazel 2014). A systematic review has concluded that the most common mental health disorders among populations experiencing homelessness were drug (range 5% to 54%) and alcohol dependence (range 8% to 58%), and that the prevalence of psychosis (range 3% to 42%) was as high as that of depression (range 0% to 59%) (Fazel 2008). These populations carry a high burden of tobacco use and tobacco‐caused morbidity and mortality (Schroeder 2009). Persons experiencing homelessness are three to five times more likely to die prematurely than those who are not homeless (Baggett 2015; Hwang 2009), and tobacco‐caused chronic diseases are the leading causes of morbidity and mortality among those aged 45 and older (Baggett 2013b). Among younger homeless‐experienced adults (aged less than 45 years), the incidence of tobacco‐caused chronic diseases is three times higher than the incidence in age‐matched non‐homeless adults (Baggett 2013b).
Persons experiencing homelessness have distinctive tobacco use behaviors associated with low income, substance‐use comorbidities, and housing instability that affect their likelihood of successfully quitting. Epidemiological studies of tobacco use among this population have shown that most adults experiencing homelessness initiate smoking before the age of 16 (Arnsten 2004). Among studies in people experiencing homelessness in the US, average daily cigarette consumption is between 10 and 13 cigarettes a day, and more than one‐third smoke their first cigarette within 30 minutes of waking (Okuyemi 2006a; Vijayaraghavan 2015; Vijayaraghavan 2017). In a study in the UK among people experiencing homelessness, cigarette consumption was much higher (19 cigarettes a day) than that reported in the US (Dawkins 2019). People experiencing homelessness also have high rates of concurrent use of alternative tobacco products such as little cigars, smokeless tobacco, and e‐cigarettes (Baggett 2016a; Neisler 2018). They also engage in high‐risk smoking practices, including exposure compensation when reducing cigarettes smoked per day and smoking cigarette butts (Garner 2013; Vijayaraghavan 2018). Smoking norms include sharing cigarettes, which may increase the risk of viral infections and stigma, and these practices may also reduce the effects of policy interventions such as increased taxes (Garner 2013; Vijayaraghavan 2018). Individuals experiencing homelessness also face significant barriers to cessation, including disproportionately high rates of post‐traumatic stress disorder (PTSD), which can lead to positive associations with smoking (Baggett 2016b). Tobacco cessation is challenging for people who have to navigate the stressors of homelessness (Baggett 2018; Chen 2016), high levels of nicotine dependence, limited access to cessation treatment and smoke‐free living environments (Vijayaraghavan 2016c; Vijayaraghavan 2016b). Integrating tobacco dependence treatment into existing services for homeless‐experienced adults remains challenging (Vijayaraghavan 2016b). Staff members may not support quit attempts (Apollonio 2005; Garner 2013), and homeless‐experienced adults do not have consistent access to services or information technologies used to improve access to cessation interventions (McInnes 2013).
Despite these challenges, over 40% of adults experiencing homelessness report making a quit attempt in the past year (Baggett 2013c; Connor 2002), and in the UK studies suggest a high desire to quit smoking, and a preference for use of both traditional cessation aids and e‐cigarettes (Dawkins 2019). However, most of these people will relapse to smoking, with estimates of the quit ratio (i.e. the ratio of former‐to‐ever smokers) between 9% and 13%, compared to 50% in the general population (Baggett 2013c; Vijayaraghavan 2016c).
Homeless populations have historically been neglected in population‐wide tobacco control efforts; however, there has been increasing interest in studying the correlates of tobacco use and cessation behaviors for these populations, and in discovering how these individuals may differ from the general population (Goldade 2011; Okuyemi 2013). Typically high levels of nicotine dependence among adults experiencing homelessness are associated with low likelihood of quitting (Vijayaraghavan 2014). Proximity to a shelter during the week after a quit attempt has been associated with a higher risk of relapse, thought to occur because of increased exposure to environmental cues to smoking (Businelle 2014a; Reitzel 2011). In contrast, staying in a shelter, as opposed to on the street, has been associated with quitting smoking (Vijayaraghavan 2016c), possibly due to exposure to shelter‐based smoke‐free policies. Studies have shown that engaging in smoking cessation does not adversely affect substance‐use behaviors (Apollonio 2016), and has increased the number of days abstinent from alcohol (Reitzel 2014). More recent research efforts in the US, UK and Ireland have focused on designing interventions to reduce smoking initiation among youth experiencing homelessness (Shadel 2014), interventions to improve quit rates among adults experiencing homelessness (Baggett 2017; Carpenter 2015; Ojo‐Fati 2015; Okuyemi 2006; Okuyemi 2013; Rash 2018), and the implementation of harm reduction approaches, including harm reduction counseling and the use of e‐cigarettes as smoking cessation aids (Collins 2018; Collins 2019; Dawkins 2019; Dawkins 2020; Scheibein 2020).
Description of the intervention
Interventions designed to support people to stop using tobacco can work to motivate people to attempt to stop using tobacco ('cessation induction'), or to support people who have already decided to stop to achieve abstinence ('aid to cessation'). However, many people who are homeless face barriers to using regular services, such as healthcare services, through which these types of cessation interventions are offered. The availability of support to assist a quit attempt can itself create motivation to quit (Aveyard 2012). Thus one approach to supporting people experiencing homelessness to quit tobacco might be to provide an easily accessible, engaging cessation service that can operate both to make quitting seem more desirable and to provide treatment for those who have already decided to stop.
The combination of behavioral counseling and pharmacotherapy (nicotine replacement therapy, bupropion, or varenicline) is the gold standard for individually‐tailored smoking‐cessation treatment in the general population (Stead 2016). However, the vast majority of quit attempts made by people experiencing homelessness are unassisted (Vijayaraghavan 2016c). There is evidence that preference for cessation aids may vary by cigarette consumption in people who smoke and are experiencing homelessness, with light smokers (0 to 10 cigarettes a day) preferring counseling over medication, in contrast to moderate/heavy smokers (more than 10 cigarettes a day) (Nguyen 2015). Recent studies from outside the US also suggest a preference for e‐cigarettes for smoking cessation among people experiencing homelessness (Collins 2018; Collins 2019; Dawkins 2019; Dawkins 2020; Scheibein 2020).
How the intervention might work
Cessation‐induction interventions directed at tobacco users who are not ready to quit rely on pharmacological, behavioral, or combination interventions to increase motivation and intention to quit, with an eventual goal of abstinence. Interventions may include nicotine therapy sampling to induce practice quit attempts, as described in Carpenter 2011, e‐cigarettes as a smoking cessation aid (Dawkins 2020; Hartmann‐Boyce 2020), or motivational interviewing to induce cessation‐related behaviors among tobacco users who are not motivated to quit, as examined in Catley 2016.
Tobacco‐dependence treatment can provide motivation and support for change through pharmacotherapy (Cahill 2013), counseling (Lancaster 2017), financial incentives (Notley 2019), or a combination of these (Stead 2016). Pharmacotherapy can reduce the urge to smoke and can decrease nicotine withdrawal symptoms with nicotine replacement therapy (NRT), varenicline, or bupropion (Cahill 2013), and counseling can provide support and motivation to make a quit attempt and quit completely (Lancaster 2017). For individuals with severe tobacco dependence, such as people experiencing homelessness, multicomponent interventions that include behavioral counseling, combination pharmacotherapy, and other adjunctive methods such as financial incentives (as discussed in Businelle 2014; Baggett 2017; Rash 2018) or mobile support (as offered in Carpenter 2015) may be beneficial. There is no reason to believe that these treatments would work differently in people experiencing homelessness than in the general population. However, as many quit attempts in this population are currently unassisted, and people experiencing homelessness face so many life challenges and stressors, more may need to be done to remove barriers to treatment, facilitate access, and promote engagement with cessation support.
Why it is important to do this review
People experiencing homelessness have unique tobacco‐use characteristics, including a higher likelihood of irregular smoking patterns, reduced exposure to clean indoor air policies, and reliance on 'used' cigarettes (Baggett 2016a; Garner 2013; Vijayaraghavan 2018). They receive limited support for cessation from service providers (Apollonio 2005; Garner 2013). Many countries have identified homeless‐experienced adults as a high‐risk group in need of targeted interventions (Fazel 2014). Tobacco use is the single most preventable cause of mortality among adults experiencing homelessness (Baggett 2015). Past efforts to promote tobacco cessation among populations experiencing homelessness have yielded mixed results that make it difficult to assess which types of tobacco‐dependence treatments promote abstinence. Our findings will synthesize evidence to date and will try to identify interventions that increase quit attempts and abstinence, as well as improve access to treatment, for this vulnerable population. We will also explore whether cessation interventions affect mental health or substance‐use outcomes among this population.
Objectives
To assess whether interventions designed to improve access to tobacco cessation interventions for adults experiencing homelessness lead to increased numbers engaging in or receiving treatment, and whether interventions designed to help adults experiencing homelessness to quit tobacco lead to increased tobacco abstinence. To also assess whether tobacco cessation interventions for adults experiencing homelessness affect substance use and mental health.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and cluster‐RCTs, with no exclusions based on language of publication or publication status.
Types of participants
Eligible participants include homeless and unstably‐housed adults, aged 18 years or older. This was defined by criteria specified by individual studies, but was in line with one or more of the following criteria for homelessness (ANHD 2018; Council to Homeless Persons 2018; Fazel 2014):
Individuals and families who do not have a fixed, regular, and adequate night‐time residence, including individuals who live in emergency shelters for homeless individuals and families, and those who live in places not meant for human habitation;
Individuals and families who will imminently lose their main night‐time residence;
Unaccompanied young adults and families with children and young people who meet other definitions of homelessness;
Individuals and families who are fleeing or attempting to flee domestic violence, dating violence, sexual assault, stalking, or other dangerous or life‐threatening conditions that relate to violence against an individual or family member;
Individuals and families who live in transitional shelters or housing programs;
Individuals and families who are temporarily living with family or friends;
Individuals and families who are living in overcrowded conditions.
Participants also had to be tobacco users who may or may not have been motivated to quit at the time of enrolment into the study. We did not classify e‐cigarette users as tobacco users for the purposes of this review, but sought to include studies recruiting users of smokeless tobacco, where they existed.
Types of interventions
We deemed the following types of intervention eligible for inclusion:
Interventions focused on building capacity for tobacco cessation services (e.g. providing education or training to provide cessation support to staff working with people who are homeless), or improving access to tobacco cessation services in clinical and non‐clinical settings for homeless adults;
Interventions focused on increasing motivation to quit (e.g. through motivational interviewing or NRT sampling);
Interventions aimed to help people to make a quit attempt to achieve abstinence, including but not limited to behavioral support, tobacco cessation pharmacotherapies, contingency management, and app‐based interventions;
Interventions focused on transitions to long‐term nicotine use that did not involve tobacco.
Eligible control groups could receive no intervention, 'usual care', as defined by individual studies, or another form of the interventions specified above.
Types of outcome measures
Primary outcomes
-
Tobacco abstinence assessed at three time points:
Short‐term abstinence: < 3 months after quit day;
Medium‐term abstinence: ≥ 3 months and < 6 months after quit day;
Long‐term abstinence: ≥ 6 months after quit day.
We conducted separate subgroup analyses for each time point. We used the strictest definition of abstinence available in each study, with preference for continuous or prolonged (allowing a grace period for slips) abstinence over point prevalence abstinence. Where possible, we extracted biochemically‐verified rates (e.g. breath carbon monoxide (CO), urinary/saliva cotinine) over self‐report. We assessed abstinence on an intention‐to‐treat basis, using the number of people randomized as the denominator.
Secondary outcomes
Number of participants receiving treatment, i.e. the number of participants engaged in cessation treatment. We only planned to assess this outcome for studies where the intervention tested aimed to improve access to tobacco cessation treatment. As we did not identify any studies of this type, we could not assess this outcome for this version of the review.
Number of people making at least one quit attempt (as defined by included studies).
Abstinence from alcohol and other drugs, as defined by self‐reported drug use or through biochemical validation (or both), at the longest follow‐up reported in the study.
Point prevalence or continuous estimates (e.g. questionnaire scores) of mental illnesses (including major depressive disorder, generalized anxiety disorder, PTSD, schizophrenia, and bipolar disorder) as defined by previously‐validated survey instruments or physician diagnosis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Tobacco Addiction Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), and MEDLINE up to 06 January 2020. The MEDLINE search strategy is provided in Appendix 1. The Specialized Register includes reports of tobacco‐related trials identified through research databases, including MEDLINE, Embase, and PsycINFO, as well as through trial registries and handsearching of journals and conference abstracts. For a detailed account of searches carried out to populate the Register, see the Cochrane Tobacco Addiction Group's website.
Searching other resources
We also searched conference abstracts from meetings of the Society for Research on Nicotine and Tobacco, and contacted investigators in the field about unpublished studies. We searched for registered unpublished trials through the National Institutes of Health clinical trials registry (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/).
Data collection and analysis
Selection of studies
We merged search results using reference management software and removed all duplicate records. Two review authors (from MV, HE, KF) independently examined all titles and abstracts to identify potentially relevant articles, and subsequently retrieved and independently examined the full‐text articles to assess adherence to the eligibility criteria. Where disagreements arose we resolved them through discussion with a third review author (DA).
Data extraction and management
Two review authors (from MV, HE, KF) independently extracted data in duplicate. We contacted study authors to obtain any missing outcome data. Once outcome data had been extracted, one review author (MV) entered them into Review Manager 5, and another (HE) checked them (Higgins 2020). We extracted the following information from study reports using a template developed by DA and modified by MV.
Source, including study ID, report ID, review author ID, citation, contact details, and country.
Methods, including study design, study objectives, study site, study dates, blinding, and sequence generation.
Participant characteristics, including total number enrolled and number in each group, setting, eligibility criteria, age, sex, race/ethnicity, sociodemographics, tobacco use (type, dependence level, amount used), mental illness, substance use, other comorbidities, and current residence (unsheltered, sheltered, single‐room occupancy, hotel or temporary residence, or supportive housing).
Interventions, including total number of intervention groups and comparisons of interest, specific intervention, intervention details, and integrity of the intervention.
Outcomes, including definition, unit of measurement, and time points collected and reported.
Results, including participants lost to follow‐up, summary data for each group, and subgroup analyses.
Miscellaneous items, including study author conflicts of interest, funding sources, and correspondence with study authors.
Assessment of risk of bias in included studies
Two review authors (from MV, HE, KF) independently assessed risks of bias for each included study, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Risk of bias was categorized as low risk, high risk or unclear risk for each domain, with the latter category indicating insufficient information to judge risk of bias. We planned to assess the following domains: selection bias (including sequence generation and allocation concealment), blinding (performance bias and detection bias), attrition bias (incomplete outcome data), and any other bias. However, as all but one of the studies investigated behavioral interventions,which are impossible to blind, we only assessed performance bias for one study, in line with the guidance from the Cochrane Tobacco Addiction Group. In future updates we will continue to assess only performance bias for studies that solely test the effect of pharmacotherapies or e‐cigarettes.
Measures of treatment effect
We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for the primary outcome (i.e. abstinence) for each included study. The risk ratio was defined as (number of participants in the intervention group who achieve abstinence/total number of people randomized to the intervention group)/(number of participants in the control group who achieve abstinence/total number of people randomized to the control group). We used intention‐to‐treat analyses, assuming that all participants lost to follow‐up were still smoking. For dichotomous secondary outcomes, such as the number of people making a quit attempt and abstinence from substance use, we calculated the RR with its 95% CI for each study, assuming that those lost to follow‐up had either failed to make a quit attempt or were not abstinent. For any continuous measurements of our secondary substance‐use or mental‐illness outcomes, we calculated the mean difference (MD) and CI for each study, using complete‐case analyses.
Unit of analysis issues
In all cases the unit of analysis was the individual. For cluster‐randomized trials we planned to assess whether study authors adjusted for the clustering, and whether this had an impact on the overall result. Where clustering appeared to have little impact on the results we planned to use unadjusted quit‐rate data, but where clustering did appear to have an impact on results we planned to adjust for this using the intra‐class correlation (ICC). However, none of the trials used cluster randomization; one of the trials set out to do so, but ultimately randomization did not occur, which was accounted for in 'Risk of bias' assessments (Dawkins 2020).
Dealing with missing data
Where outcome data were missing, we tried to contact study authors to request the data. For all outcomes apart from mental health we assumed that participants lost to follow‐up were continuing smokers, were still using other substances, or did not make a quit attempt. For the mental‐health outcome and for continuous measures of substance use, we conducted complete‐case analyses.
Assessment of heterogeneity
We assessed any clinical or methodological heterogeneity between studies within comparisons, to judge whether it was appropriate to conduct meta‐analyses (Higgins 2020). We then assessed statistical heterogeneity using the I2 statistic for each meta‐analysis. This represents the percentage of the effect that is attributable to the true variance between studies versus chance alone (Higgins 2020). We considered an I2 value greater than 50% as evidence of substantial heterogeneity.
Assessment of reporting biases
We assessed the potential for reporting bias (selective reporting of outcomes) for each study through our 'Risk of bias' assessment (described above), and planned to assess publication bias (publication or non‐publication of studies depending on the direction of outcome effects) using funnel plots where possible. However, as none of our analyses included 10 or more studies this was not possible. We attempted to minimize publication bias by searching clinical trial registers, and by including studies that remained ongoing and where results were not yet published.
Data synthesis
We grouped studies according to common comparisons. Where appropriate, we used Mantel‐Haenszel random‐effects methods to calculate the pooled, summary, weighted RR (95% CIs), or inverse‐variance random‐effects methods to calculate pooled, summary, weighted MDs (95% CIs) or standardized mean differences (SMDs) and their 95% CIs.
Subgroup analysis and investigation of heterogeneity
Where possible, we planned to carry out subgroup analyses to examine whether intervention effects differed based on the following characteristics:
Intensity of treatment (e.g. number of counseling sessions);
Participants' residential history (sheltered versus unsheltered);
Participants' substance‐use history;
Participants' diagnosis of mental‐health disorders; and
Participants' use of non‐cigarette tobacco and nicotine products.
However, this was not possible, due to the paucity of studies and data identified for each comparison.
Sensitivity analysis
We conducted prespecified sensitivity analyses by excluding studies with high risk of bias (judged to be at high risk for one or more of the domains assessed) from meta‐analyses where relevant.
We also carried out post hoc sensitivity analyses in response to reviewers' comments. A reviewer pointed out that there may be higher rates of loss to follow‐up in studies recruiting people who smoke and experience homelessness than in people who smoke in the general population. Loss to follow‐up could therefore be for reasons other than a failed quit attempt, meaning the established practice of assuming that participants lost to follow‐up are smoking may be flawed in this population and could impact on results. In response, we carried out analyses of the primary outcome (tobacco abstinence) for each comparison using complete‐case analysis, where possible, to see whether this had any impact on the interpretation of results.
Summary of findings and assessment of the certainty of the evidence
We produced a 'Summary of findings' table (Higgins 2020), presenting the primary outcome (tobacco use abstinence at all time points), absolute and relative magnitude of effects, numbers of participants, and numbers of studies contributing to these outcomes, for each meta‐analyzed comparison. Two independent review authors (MV, NL) also carried out GRADE assessments of the certainty of evidence. Using GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias), we graded the certainty of the evidence as very low, low, moderate, or high, and provided footnotes to explain reasons for any downgrading.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
Our search resulted in 87 citations. After removing 35 duplicates, we had 52 citations to screen by title and abstract. We found 13 citations to be ineligible, leaving 39 citations for full‐text screening. At this stage we excluded 29 citations, leaving 10 included studies; nine were completed studies and one was ongoing. This ongoing study is likely to be relevant for inclusion once completed (Tucker 2020). See Figure 1 for study flow information relating to the most recent search.
1.
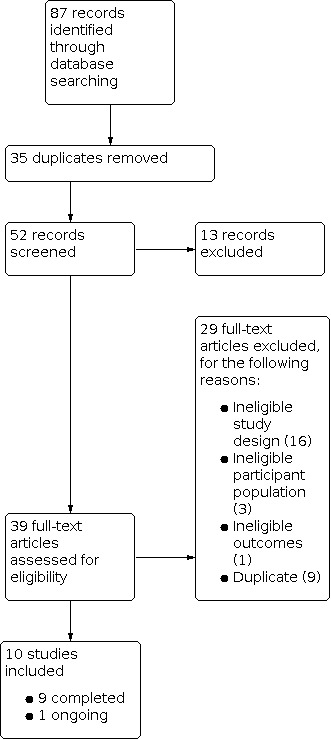
Study flow diagram.
Included studies
This review includes nine completed RCTs, representing 1634 participants. All except one trial was conducted in the USA; the remaining trial was conducted in the UK. All but one of the completed trials had reported outcome data at time of searching; Ojo‐Fati 2015 had published their protocol, and their trial registry entry reported that the trial had been completed, but outcome data were not published.
Participants
All participants were tobacco smokers, over 18 years of age, and had experienced homelessness, as defined under Types of participants. Three studies included participants recruited from clinical settings (Baggett 2018; Burling 2001; NCT02245308), and six studies included participants recruited from service settings, such as homeless shelters or transitional housing (Dawkins 2020; Ojo‐Fati 2015; Okuyemi 2006; Okuyemi 2013; Rash 2018; Spector 2007). Two studies included participants who had co‐occurring substance‐use disorders (Burling 2001; Ojo‐Fati 2015). Two studies included participants who had expressed readiness or motivation to quit within one month (Baggett 2018; NCT02245308), while the other studies did not have motivation to quit as an inclusion criterion.
Intervention
All but one of the studies included in this review aimed to test a behavioural smoking cessation intervention. Two studies (Baggett 2018; Rash 2018) specifically tested the effect of offering rewards for successful smoking cessation (contingent reinforcement); three studies investigated more versus less intensive support (Burling 2001; NCT02245308; Okuyemi 2013); two studies (Burling 2001; Okuyemi 2006) looked at the effect of offering multi‐issue support (i.e. to tackle other life challenges as well as smoking), as opposed to smoking cessation support alone; one study looked at text message‐based support as an adjunct to usual care (Baggett 2018); and one compared cognitive behavioral therapy to a form of empathic support for smoking cessation (Spector 2007).
Dawkins 2020 aimed to test the effects of providing e‐cigarettes to participants experiencing homelessness, alongside very minimal behavioural support effects, in comparison to a flyer providing the details of available smoking cessation resources.
Behavioral support
All studies included in this review offered a form of behavioral intervention. All but one study offered either one‐on‐one counseling (Baggett 2018; Okuyemi 2013; Rash 2018; Spector 2007; NCT02245308; Okuyemi 2006) or group and one‐on‐one counseling (Ojo‐Fati 2015; Burling 2001). Participants were offered a variety of styles of counseling, with two studies offering motivational interviewing (Okuyemi 2006; Okuyemi 2013), three offering cognitive behavioral therapy (Burling 2001; NCT02245308; Spector 2007), and one offering a mix of motivational interviewing and cognitive behavioral therapy (Baggett 2018). While most counseling sessions focused on smoking cessation and relapse prevention, a few studies integrated smoking cessation counseling with substance use treatment (Burling 2001; Ojo‐Fati 2015; Okuyemi 2006). Burling 2001 and Okuyemi 2006 offered group or one‐on‐one counseling that focused on smoking cessation within the context of substance use, whereas Ojo‐Fati 2015 included one‐on‐one counseling for both alcohol and tobacco use. Dawkins 2020 offered participants only very minimal support in the intervention arm, with center staff meeting with participants once a week to provide e‐cigarette liquid and to troubleshoot e‐cigarette use; however, the study was not designed to test the effects of this support.
Contingent reinforcement interventions
As mentioned above, two studies specifically investigated the isolated effect of contingency management for smoking cessation in people experiencing homelessness (Baggett 2018; Rash 2018). Contingent reinforcement refers to offering money or goods for abstinence, to promote smoking cessation. Baggett 2018 offered escalating financial rewards to participants contingent upon abstinence, whereas Rash 2018 offered draws from a prize bowl for each negative CO measure submitted (prizes included money or material goods of varying values). NCT02245308 also offered contingent reinforcement, with financial incentives provided for uploading a video that showed the participant providing a negative CO reading. However, as the intervention being tested was multicomponent it was impossible to separate out the independent effect of the contingent reinforcements.
Modality
All studies that offered behavioral support did so in person, with the exception of NCT02245308, that offered cognitive behavioral therapy by telephone. In‐person sessions were offered one‐on‐one (Baggett 2018; Okuyemi 2006; Okuyemi 2013; Rash 2018; Spector 2007) or in groups (Burling 2001; Ojo‐Fati 2015). Two studies offered both one‐on‐one and group sessions (Burling 2001; Ojo‐Fati 2015); they offered one‐on‐one sessions during the early phase of the intervention and group sessions toward the latter phase of the intervention. It is unclear whether the support in Dawkins 2020 was offered one‐on‐one or in a group. In one study, one of the intervention arms included a motivational text‐message program; participants received between one and five automated texts a day, beginning on the quit day and lasting for the duration of the intervention (Baggett 2018).
Intensity
Most of the studies providing counseling offered this for at least four weeks, with one study offering bi‐weekly counseling for four weeks (Rash 2018). One study offered daily one‐on‐one counseling sessions for 30 to 45 minutes a day during the five‐week pre‐quit and two‐week post‐quit phases of the intervention, followed by bi‐weekly one‐on‐one counseling during the final two weeks of the intervention (Burling 2001). In addition to one‐on‐one counseling, Burling 2001 offered weekly group‐counseling sessions that were either focused on smoking alone or smoking within the context of substance use. Three studies offered participants weekly one‐on‐one counseling lasting for 15 to 20 minutes (Baggett 2018; Okuyemi 2006; Spector 2007). Okuyemi 2013 offered six individual motivational‐interviewing sessions lasting for 15 to 20 minutes. In Ojo‐Fati 2015, participants received weekly one‐on‐one counseling sessions focused on smoking and alcohol for 30 minutes each, followed by weekly group counseling for three months. NCT02245308 offered participants 10 telephone‐counseling sessions. Three studies compared more intensive to less intensive behavioral interventions (Burling 2001; NCT02245308; Okuyemi 2013). Burling 2001 compared their intensive nine‐week support program with one‐off brief advice detailing the cessation treatments available through the hospital; NCT02245308 compared their 10 x 30‐to‐45‐minute counseling sessions to a referral to standard Department of Veterans Affairs Medical Center support, which varied in what it offered; and Okuyemi 2013 compared their six x 15‐to‐20‐minute support sessions to a one‐off 10‐to‐15‐minute session of brief advice.
Providers
Counseling was delivered by master's or doctoral‐level counselors (Burling 2001), a trained tobacco‐treatment specialist (Baggett 2018), trained counselors (Okuyemi 2006; Okuyemi 2013, Ojo‐Fati 2015; NCT02245308), medical students (Spector 2007), or research staff (Rash 2018). In Dawkins 2020 the support for e‐cigarette use was provided by staff at the homeless shelters where the study took place. Staff in each of the four centers received one education and training session, following the recommendations of the UK National Centre for Smoking Cessation and Training. This was designed to ensure that center staff had a basic knowledge of the issues that surround smoking and cessation, and to optimize the delivery of the trial interventions.
Pharmacotherapy and e‐cigarettes
All but one study (Spector 2007) included in this review offered pharmacotherapy or e‐cigarettes. However, Dawkins 2020 was the only study that set out to specifically test its effects, with only the intervention arm receiving e‐cigarettes. Participants received an e‐cigarette starter kit, with a choice of nicotine dose and flavor, and were offered weekly support to use the e‐cigarette. The other studies offered pharmacotherapy, but this was provided in all study arms. Most studies offered nicotine replacement therapy (NRT) in the form of patches or gum or both (Baggett 2018; Burling 2001; Ojo‐Fati 2015; Okuyemi 2006; Okuyemi 2013; Rash 2018), and one study offered both NRT and bupropion (NCT02245308).
Comparator
We grouped studies according to the type of intervention offered. In the studies that offered contingent reinforcement alongside 'usual care', the comparator group only received 'usual care' (Baggett 2018; NCT02245308; Rash 2018). In some cases, comparators included interventions of lower intensity (Burling 2001; NCT02245308; Okuyemi 2013), or interventions that included only smoking‐cessation counseling, without any additional components (Burling 2001; Ojo‐Fati 2015; Okuyemi 2006). The comparator group in Dawkins 2020 was offered a printed flyer of smoking‐cessation resources, whereas in Spector 2007 the comparator group was offered empathetic support for smoking cessation.
Outcomes
Primary
One study described measuring abstinence at four weeks follow‐up (Spector 2007). This outcome measure was not fully reported due to high participant dropout, but we were able to obtain this information from the study investigators. One study measured abstinence at eight weeks (Baggett 2018), five measured abstinence at six months (Dawkins 2020; NCT02245308; Rash 2018; Okuyemi 2006; Okuyemi 2013), and two measured abstinence at 12 months (Burling 2001; Ojo‐Fati 2015). However, Ojo‐Fati 2015 did not report any outcome data and we were unable to obtain this information from the investigators. All studies that reported abstinence verified this biochemically, either through exhaled CO or urinary/salivary cotinine.
Secondary
Only one study reported past‐month 24‐hour quit attempts (Baggett 2018). Four studies reported secondary outcomes related to mental health or substance‐use disorders (Baggett 2018; Burling 2001; Dawkins 2020; Okuyemi 2006). One study reported measuring alcohol use severity but these data are not currently published (Ojo‐Fati 2015). Several measures were used to evaluate substance use and mental‐health outcomes, including the Addiction Severity Index (Baggett 2018), biochemically‐verified drug and alcohol abstinence at 12 months (Burling 2001), generalized anxiety disorder scale, patient health questionnaire scale, AUDIT for alcohol use, and severity of dependence scale (Dawkins 2020).
We also set out to assess engagement in treatment, but we did not find any trials that explored this or improving access to cessation treatment, and are therefore unable to report on this outcome in this version of the review.
Excluded studies
We list 29 studies that we thought were relevant, but were excluded, with reasons outlined in the Characteristics of excluded studies table. Reasons for exclusion include ineligible study design, ineligible study population and ineligible outcomes. Nine of the citations originally identified were duplicates of those in the 'Excluded studies' table.
Risk of bias in included studies
Full details of the 'Risk of bias' assessments are provided for each trial within the Characteristics of included studies tables, and a summary of decisions are available in Figure 2. We rated none of the studies at low risk of bias for all domains; we judged six studies to be at unclear risk of overall bias (Baggett 2018; NCT02245308; Ojo‐Fati 2015, Okuyemi 2006; Okuyemi 2013; Rash 2018), and three studies at high risk for at least one domain (Burling 2001; Dawkins 2020; Spector 2007).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed selection bias through evaluating methods of random‐sequence generation and allocation concealment for each study. We rated two studies at low risk for random‐sequence generation (Baggett 2018; Rash 2018), one at high risk (Dawkins 2020), and the remainder at unclear risk. We judged all studies to be at unclear risk for allocation concealment. We judged studies to have an unclear risk of bias when authors provided insufficient information about the methods used. Dawkins 2020 was considered high risk for random‐sequence generation because the investigators originally planned to randomize study sites, but had to switch to a pragmatic approach to allocating sites to treatment and control groups, based on center readiness and researcher availability.
Blinding
We only assessed performance bias for one study, in line with the Cochrane Tobacco Addiction Group's guidance on assessing studies with behavioral components. This is because it is impossible to blind staff or participants to a behavioral intervention. The one study for which we assessed performance bias investigated the effect of providing participants with an e‐cigarette for smoking cessation (Dawkins 2020). We deemed the study to be at high risk of performance bias, as participants were not provided with placebo treatment (i.e. a non‐nicotine e‐cigarette) in the control group, and instead received minimal behavioral support and a referral to other stop‐smoking services. We assessed detection bias by assessing the blinding of outcome assessment through biochemical verification of the abstinence outcome; in the case of no verification we would have also considered whether the amount of contact with participants was matched between study arms. We judged all studies to be at low risk of detection bias. In most cases this was because cessation outcomes were biochemically verified; however, Spector 2007 did not require verification as none of the participants reported having quit.
Incomplete outcome data
We judged studies to be at low risk of attrition bias where the numbers of participants lost to follow‐up were reported, the overall number lost to follow‐up was not more than 50%, and the difference in loss to follow‐up between groups was no greater than 20%. This is in accordance with the guidance on 'Risk of bias' assessment produced by the Cochrane Tobaccco Addiction Group for smoking cessation studies. Based on these criteria, we rated Baggett 2018; Okuyemi 2006; Okuyemi 2013; and Rash 2018 at low risk. We judged NCT02245308, Ojo‐Fati 2015 and Spector 2007 as at unclear risk, as full data on losses to follow‐up were not reported. We judged Burling 2001 and Dawkins 2020 to be at high risk. In these studies, the total number of losses to follow‐up was over 50% (Burling 2001), or there were unequal losses between study arms (Dawkins 2020).
Selective reporting
We judged studies to be at risk of selective reporting if reported outcomes were different from those listed in a protocol or on a pre‐trial registry. We judged three trials as low risk as outcomes matched those listed in the pre‐trial registry (Baggett 2018; Dawkins 2020; NCT02245308). We judged five trials as being at unclear risk, as we either could not find any evidence that the trial was preregistered (Burling 2001; Okuyemi 2006; Okuyemi 2013; Rash 2018), or the outcome data were not published, but may yet be in the future (Ojo‐Fati 2015). We judged Spector 2007 as high risk, as smoking cessation outcome data were not reported clearly by study groups.
Other potential sources of bias
There was one potential additional source of bias in Baggett 2018. Assessment of abstinence for the receipt of incentives was based on verification by CO, but participants who had CO‐verified abstinence self‐reported short‐term relapses, suggesting that the assessment of abstinence using point‐prevalence CO verification may have overestimated abstinence. However, as we cannot be sure of this we judged the potential risk to be unclear.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Contingent reinforcement in addition to usual smoking cessation care in people experiencing homelessness.
| Contingent reinforcement as an addition to usual smoking cessation care in people experiencing homelessness | ||||||
| Patient or population: people experiencing homelessness Setting: homeless shelter and healthcare clinic for people experiencing homelessness (USA) Intervention: contingent reinforcement in addition to usual care Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with contingent reinforcement in addition to usual care | |||||
| Smoking abstinence assessed with: biochemical verification Follow‐up: range 2 months to 6 months | Study population | RR 0.67 (0.16 to 2.77) | 120 (2 RCTs) | ⊕⊕⊝⊝ LOWa | Of the 2 studies included in this analysis 1 of the studies (Baggett 2018 ‐ 2‐month follow‐up) had no events and therefore the risk ratio for this study was not estimable. The effect estimate is calculated from Rash 2018 only (6‐month follow‐up) | |
| 7 per 100 | 5 per 100 (1 to 19) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels due to imprecision: the range of potential effect estimates spans from harm to substantial benefit and the number of events was extremely low (n = 7).
Summary of findings 2. More compared to less intensive behavioral smoking cessation support in people experiencing homelessness.
| More compared to less intensive behavioral smoking cessation support in people experiencing homelessness | ||||||
| Patient or population: people experiencing homelessness Setting: homeless shelter, veteran medical centre, residential drug and alcohol rehabilitation center for people experiencing homelessness (USA) Intervention: more intensive behavioral support Comparison: less intensive behavioral support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with less intensive support | Risk with more intensive support | |||||
| Smoking abstinence assessed with: biochemical validation Follow‐up: 6 months | Study population | RR 1.64 (1.01 to 2.69) | 657 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | ‐ | |
| 7 per 100 | 12 per 100 (7 to 19) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias: one of the three studies (Burling 2001) is deemed to be at high risk of bias (the other two studies are at unclear risk). Removing this study results in the lower limit of the confidence interval falling below 1, although the point estimate still suggests a benefit of more intensive intervention.
bDowngraded two levels due to imprecision: confidence intervals encompass estimates that indicate both no benefit and a potential benefit of more intensive intervention. The number of events is very low (n = 61).
Summary of findings 3. Multi‐issue support compared to smoking cessation support only in people experiencing homelessness.
| Multi‐issue support compared to smoking cessation support only in people experiencing homelessness | ||||||
| Patient or population: people experiencing homelessness Setting: homeless shelter, residential drug and alcohol rehabilitation centre for people experiencing homelessness (USA) Intervention: multi‐issue support (i.e. other addictions, difficult life events) Comparison: smoking cessation support only | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with smoking cessation support only | Risk with multi‐issue support | |||||
| Smoking abstinence assessed with: biochemical validation Follow‐up: range 6 months to 12 months | Study population | RR 0.95 (0.35 to 2.61) | 146 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | ‐ | |
| 15 per 100 | 14 per 100 (5 to 39) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias: one of the two studies (Burling 2001) was deemed to be at high risk of bias (the second study was judged to be at unclear risk). The removal of this study changed the direction of the pooled effect estimate from favoring smoking‐only support to favoring multi‐issue support, although in both cases the CIs encompassed one, indicating the potential for both harm and benefit of either approach over the other.
bDowngraded two levels due to imprecision: the confidence intervals illustrate the potential for both substantial benefit and harm of multi‐issue support as opposed to smoking‐only support.
1. 'Contingent reinforcement in addition to usual smoking cessation care in people experiencing homelessness' comparison
See: Table 1 for the: 'Contingent reinforcement in addition to usual smoking cessation care in people experiencing homelessness' comparison.
Smoking cessation outcome
We compared receipt of contingent reinforcement in addition to usual care versus usual care alone (Analysis 1.1). Of the two studies included in this analysis, one (Baggett 2018: two‐month follow‐up) had no events, and therefore the RR for this study was not estimable (Analysis 1.1.2). The effect estimate is therefore calculated from Rash 2018 only (six‐month follow‐up, Analysis 1.1.1), giving an RR of 0.67 (95% CI 0.16 to 2.77; 1 trial, 70 participants; I2 = N/A), suggesting no clear evidence of a benefit. However, this result should be treated with caution as there is substantial imprecision due to the low number of contributing participants.
1.1. Analysis.

Comparison 1: Contingent reinforcement (CR) as adjunct, Outcome 1: Smoking abstinence
Change in other drug use
Only one study provided information to assess change in alcohol and other substance use at eight weeks (Baggett 2018). This study used the Addiction Severity Index to assess alcohol and substance use, and had a total of 50 participants. The point estimate for change in alcohol use was MD 0.02 (95% CI −0.05 to 0.09; 1 trial, 50 participants; I2 = N/A) and change in substance use was MD 0.01 (95% CI −0.03 to 0.05; 1 trial, 50 participants; I2 = N/A; Analysis 1.2). In both cases the CI spanned zero, but the size of the estimates provides little evidence of any clinically meaningful benefit or harm.
1.2. Analysis.

Comparison 1: Contingent reinforcement (CR) as adjunct, Outcome 2: Change in other drug use (past month severity at 8 weeks)
Change in mental health
Only one study provided information to assess change in mental‐health severity (Baggett 2018), with a total of 50 participants (Analysis 1.3). The MD for change in mental‐health severity at eight‐week follow‐up was 0.12 (95% CI 0.01 to 0.23; 1 trial, 50 participants; I2 = N/A), suggesting no benefit from contingent reinforcement for mental‐health severity.
1.3. Analysis.

Comparison 1: Contingent reinforcement (CR) as adjunct, Outcome 3: Change in mental health (past month severity at 8 weeks)
Number making a quit attempt for 24 hours
Only one study provided information to assess the number of people making a quit attempt for 24 hours (Baggett 2018), with a total of 50 participants (Analysis 1.4). The point estimate for the RR was 1.25 (95% CI 0.74 to 2.10; 1 trial, 50 participants; I2 = N/A). The substantial imprecision suggests the potential for contingent reinforcement to both reduce or increase the number of people making a quit attempt, and so should be treated with caution.
1.4. Analysis.

Comparison 1: Contingent reinforcement (CR) as adjunct, Outcome 4: Number making a quit attempt for 24 hours or more (at 8 weeks)
2. 'More compared to less intensive behavioral smoking cessation support in people experiencing homelessness' comparison
See: Table 2 for the 'More compared to less intensive behavioral smoking cessation support in people experiencing homelessness' comparison.
Smoking cessation outcome
We compared more versus less intensive behavioral smoking‐cessation support, and include three studies with 657 participants (Analysis 2.1). The pooled RR for smoking abstinence at six months was 1.64 (95% CI 1.01 to 2.69; 3 trials, 657 participants; I2 = 0%). This pooled estimate suggests a potential benefit of more intensive behavioral smoking‐cessation support to increase abstinence at six months. However, we rated one of the studies at high risk of bias (Burling 2001); removing this study from the analysis resulted in a pooled RR of 1.70 (95% CI 0.96 to 3.02). While the point estimate still suggests a benefit of more intensive support, the lower limit of the CI is less than 1, which may signify no benefit. This result should therefore be treated with caution.
2.1. Analysis.

Comparison 2: More versus less intensive behavioural support, Outcome 1: Smoking abstinence
Drug and Alcohol abstinence
Only one study reported biochemically‐verified drug and alcohol abstinence at 12 months (Burling 2001) (Analysis 2.2). The point estimate for the RR was 1.19 (95% CI 0.78 to 1.83; 1 trial, 100 participants; I2 = N/A). As there is substantial imprecision demonstrated by the confidence interval, this result shows that more intensive smoking cessation treatment has the potential to both increase or decrease the likelihood of drug or alcohol abstinence.
2.2. Analysis.

Comparison 2: More versus less intensive behavioural support, Outcome 2: Drug and alcohol abstinence
3. 'Multi‐issue support versus smoking support only in people experiencing homelessness' comparison
See: Table 3 for the 'Multi‐issue support compared to smoking cessation support only in people experiencing homelessness' comparison.
Smoking cessation outcome
We compared interventions that integrated smoking‐cessation counseling with other issues or ongoing substance use versus interventions that focused only on smoking cessation (Analysis 3.1). We included two studies, with a total of 146 participants. The resulting pooled RR of 0.95 (95% CI 0.35 to 2.61; 2 trials, 146 participants; I2 = 25%) favored a focus of smoking cessation alone, as opposed to multi‐issue support. However, the confidence interval is very wide, encompassing both strongly beneficial effects of multi‐issue support and strongly detrimental effects on cessation. In addition, we deemed one of the studies to be at high risk of bias (Burling 2001). Removing this study from the analysis yielded an RR of 2.00 (95% CI 0.41 to 9.87; 1 trial, 46 participants; I2 = N/A), thus changing the direction of the point estimate to favor multi‐issue support and increasing imprecision. This estimate should therefore be treated with caution.
3.1. Analysis.

Comparison 3: Multi‐issue support versus smoking support only, Outcome 1: Smoking abstinence
Drug and alcohol abstinence
Both studies reported on drug and alcohol abstinence outcomes (Burling 2001; Okuyemi 2006). Burling 2001 reported alcohol and substance‐use abstinence (Analysis 3.2), with a RR of 0.68 (95% CI 0.42 to 1.09; 1 trial, 100 participants; I2 = N/A) at 12 months. Okuyemi 2006 (28 participants) evaluated the number of days alcohol was drunk within the last 30 days: MD 3.20 (95% CI −4.44 to 10.84); days of binge‐drinking within the last 30 days (MD −4.95, 95% −12.02 to 2.12); number of alcoholic drinks/day (MD −0.2, 95% −2.26 to 1.86); number of days of marijuana used in the past 30 days (MD 12.6, 95% CI 5.28 to 19.92); days of cocaine use within the past 30 days (MD 10.25, 95% CI 1.49 to 19.01) (Analysis 3.3). In all cases the confidence intervals were very wide, making it difficult to draw clear conclusions; however, the two analyses of marijuana and cocaine use respectively did suggest a benefit of focusing on smoking cessation support only.
3.2. Analysis.

Comparison 3: Multi‐issue support versus smoking support only, Outcome 2: Drug and alcohol abstinence
3.3. Analysis.

Comparison 3: Multi‐issue support versus smoking support only, Outcome 3: Change in other drug use (at 6 months)
4. Other interventions
For studies that investigated interventions that could not be grouped into the comparisons above, we report on outcomes separately below.
Text support as an adjunct to combination behavioral and pharmacotherapy smoking‐cessation support
One study offered smoking cessation‐focused text support in one of the intervention arms as an adjunct to behavioral counseling and pharmacotherapy (Baggett 2018). However, this study reported no quitters in either study arm at eight weeks follow‐up, meaning it was impossible to calculate an RR for smoking cessation. Baggett 2018 found substantial imprecision around the estimate of the effect of the text‐based intervention on the number of people making a quit attempt for 24 hours: RR 0.83 (95% CI 0.44 to 1.56; 1 trial, 50 participants; I2 = N/A; Analysis 4.1).
4.1. Analysis.

Comparison 4: Text support as an adjunct, Outcome 1: Number making a quit attempt for 24 hour or more (at 8 weeks)
Past‐month severity of alcohol and substance use at eight weeks follow‐up was also reported for this study (Baggett 2018). The point estimate suggests a potential benefit of the addition of a smoking cessation‐focused text‐messaging intervention to reduce alcohol use: MD −0.22 (95% −0.79 to 0.35; 1 trial, 48 participants; I2 = N/A; Analysis 4.2); however, the wide CI also indicates the possibility of no benefit. There was no evidence for a benefit observed for the substance‐use outcome: MD 0.23 (95% CI −0.34 to 0.80; 1 trial, 48 participants; I2 = N/A; Analysis 4.2).
4.2. Analysis.

Comparison 4: Text support as an adjunct, Outcome 2: Other drug use (past month severity at 8 weeks)
The text‐based intervention also showed no evidence for an effect on the severity of mental‐health issues at eight weeks follow‐up: MD 0.00 (95% CI −0.11 to 0.11; 1 trial, 48 participants; I2 = N/A) (Analysis 4.3; Baggett 2018).
4.3. Analysis.

Comparison 4: Text support as an adjunct, Outcome 3: Mental health (past month severity at 8 weeks)
E‐cigarette with support versus minimal cessation support
One study offered e‐cigarettes for smoking cessation (Dawkins 2020), with 70 participants. Smoking cessation abstinence was assessed at six months, resulting in an RR of 4.71 (95% 0.25 to 88.30; 1 trial, 70 participants; I2 = N/A). However, the wide CI suggests the potential for no benefit, as well as a substantial benefit (Analysis 5.1). We judged the contributing study to be at high risk of bias, as although the aim was to randomize participants, this proved not to be possible.
5.1. Analysis.

Comparison 5: E‐cigarette versus usual care, Outcome 1: Smoking abstinence
Dawkins 2020 also allowed us to compare the change in alcohol‐use severity and substance‐use severity between treatment arms (Analysis 5.2). The MD for alcohol‐use severity was −1.00 (95% CI −9.9 to 7.9) and for substance‐use severity was 0.38 (95% CI −4.86 to 5.62). These estimates should be treated with caution, due to the substantial imprecision, and high risk of bias.
5.2. Analysis.

Comparison 5: E‐cigarette versus usual care, Outcome 2: Change in other drug use
The measured change in anxiety and depression symptoms also found no evidence for a clear benefit of the e‐cigarette intervention on either of these outcomes (Analysis 5.3).
5.3. Analysis.

Comparison 5: E‐cigarette versus usual care, Outcome 3: Change in mental health symptoms
Cognitive behavioral therapy versus empathic support
Spector 2007 offered nine sessions of CBT versus empathic support. However, the study only randomized three people to the intervention arm and eight to the control arm. As no one successfully quit in the intervention or control arm, the RR for smoking cessation was not estimable.
For each comparison we carried out a sensitivity analysis for the primary outcome (tobacco abstinence), calculating the RR and 95% CI for each study using a complete‐case analysis (Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5). This was possible for six of the eight studies originally analyzed (NCT02245308 and Spector 2007 did not provide information on the numbers of participants lost to follow‐up in individual study arms). The calculated RRs and 95% CIs were similar in all cases, and resulted in the same interpretation of results as the effect estimates and CIs calculated using intention‐to‐treat analyses (where missing was deemed equal to smoking). Where it was possible to calculate a pooled effect estimate and 95% CI for a comparison, including the same studies as in the original analysis (Analysis 7.3; RR 0.90, 95% CI 0.40 to 2.04), these were very similar to the effects estimated in the original analysis (Analysis 3.1; RR 0.95, 95% CI 0.35 to 2.61).
7.1. Analysis.

Comparison 7: Sensitivity analysis: abstinence outcome, complete case analysis, Outcome 1: Contingent reinforcement (CR) as adjunct
7.2. Analysis.

Comparison 7: Sensitivity analysis: abstinence outcome, complete case analysis, Outcome 2: More versus less intensive behavioral support
7.3. Analysis.

Comparison 7: Sensitivity analysis: abstinence outcome, complete case analysis, Outcome 3: Multi‐issue support versus smoking support only
7.4. Analysis.

Comparison 7: Sensitivity analysis: abstinence outcome, complete case analysis, Outcome 4: Text support as an adjunct
7.5. Analysis.

Comparison 7: Sensitivity analysis: abstinence outcome, complete case analysis, Outcome 5: E‐cigarette versus usual care
Discussion
Summary of main results
This review includes nine completed trials that address tobacco use among people experiencing homelessness. Almost all studies offered treatment as a form of behavioral support and pharmacotherapy or electronic cigarettes, although the components differed substantially across trials. Based on common components of some of the studies, we attempted to investigate three main treatment variations: contingent reinforcement as an adjunct to usual care (consisting of counseling and nicotine replacement therapy); more versus less intensive behavioral support; and multi‐issue support (including smoking cessation) versus smoking cessation support alone. For our first investigation of contingent reinforcement, we were unable to pool smoking cessation data from the two relevant trials, as one trial did not identify any abstinent participants at their eight‐week follow‐up. The remaining trial found no clear evidence that contingent reinforcement increased quitting in people experiencing homelessness. However, there was substantial imprecision in the findings, given the small number of events and short intervention duration, and we judged this evidence to be of low certainty.
Our pooled analysis investigating the effects of more versus less intensive behavioral support suggested a potential benefit of more intensive interventions on increasing abstinence at six months compared to less intensive support. However, we judged this finding to be of very low certainty, due to risk of bias and substantial imprecision, meaning that we have very little confidence in the effect estimate, and that the true effect is likely to differ substantially from the estimate of effect.
For our comparison of treatment focused on multiple issues (for example, smoking cessation in addition to drug and alcohol dependence or significant life events) versus treatment targeted at smoking cessation alone, the evidence was again deemed to be of very low certainty and gave no clear indication that either approach was more successful in helping people experiencing homelessness to quit smoking.
Remaining single studies examined the use of text‐messaging support, e‐cigarettes, and cognitive behavioral therapy for smoking cessation in people experiencing homelessness. However, in all cases studies were very small or there were methodological issues, or both, making it impossible to draw meaningful conclusions. Similarly, data on our secondary outcomes, i.e. quit attempts, drug and alcohol abstinence, and mental illness, were too sparse to conclude whether any of the interventions tested were having clinically significant effects. It is also possible that rates of drug and alcohol abstinence may be low because participants staying in shelters may be required to abstain from these substances to access shelter services. We did not identify any studies that explicitly aimed to improve access to smoking‐cessation treatment for people experiencing homelessness, and we are therefore unable to assess our secondary outcome, 'number of participants receiving treatment'.
Overall completeness and applicability of evidence
The searches conducted for this review were broad, and to our knowledge include all studies that mentioned smoking or tobacco cessation among people experiencing homelessness. We searched trial registers as well as medical databases to identify any ongoing or completed but unpublished registered studies.
All of the included studies were conducted in the USA, except for one based in the UK. This means that results may not be generalizable outside of these countries and their respective systems for supporting people experiencing homelessness. All studies explicitly focused on people experiencing homelessness, and drew from populations meeting our prespecified definition (ANHD 2018; Council to Homeless Persons 2018; Fazel 2014; Types of participants). We used the most stringent definition of tobacco abstinence (biochemically‐verified at the longest measured time point, with a preference for continuous abstinence over point prevalence abstinence), in line with the guidance from the Cochrane Tobacco Addiction Group. In doing so, it is possible that we may have underestimated the effects of the intervention on shorter‐term quit attempts. However, as long‐term smoking abstinence is necessary to lead to all of the associated health benefits of quitting smoking, we deem this to be appropriate. A number of the included studies did not report on our secondary outcomes, such as the number making a quit attempt. This outcome is useful, as it could give us an idea of whether quit rates are low because people fail to engage with the treatment in the first place, or because they relapse after initially managing to stop. This would allow future interventions to be targeted more specifically to either engagement or relapse prevention.
We set out to include studies that recruited any type of tobacco user, but we only identified studies aimed at helping users of combustible tobacco to quit. In addition, although we would have deemed studies that investigated interventions aiming to increase engagement with tobacco cessation treatment as eligible, we did not find studies that specifically set out to do so. As there is no reason to believe that established effective tobacco cessation treatments, such as behavioral support, nicotine replacement therapy and varenicline, would vary in efficacy in people experiencing homelessness when compared to the general population, the development of interventions to improve access and adherence to treatments we know to be effective might be especially useful. Commonly‐reported barriers to cessation and reasons for relapse are reported to include the social and environmental context of tobacco use (Pratt 2019), heavy nicotine dependence (Vijayaraghavan 2014), and low social support for quitting. Few of these trials incorporated intervention components that might have specifically addressed these barriers. Other components that might be worth exploring in future interventions include long‐term combination NRT to support multiple quit attempts, e‐cigarettes (given increasing evidence of their benefits as a cessation aid (Hartmann‐Boyce 2020), and the preference for e‐cigarettes for smoking cessation among some smokers experiencing homelessness (Dawkins 2020; Scheibein 2020)), varenicline (which evidence suggests is the most effective smoking cessation pharmacotherapy (Cahill 2013)), interventions designed to increase medication adherence, and behavioral‐counseling approaches that include community‐based outreach or peer support to increase engagement in cessation, as well as continued adherence. These types of interventions merit further exploration among people experiencing homelessness who face competing priorities and may benefit from multi‐modal approaches to cessation.
The Cochrane Tobacco Addiction Group’s reviews usually only include studies with at least six month follow‐up, in order to assess the long‐term efficacy of interventions. As many people who make successful early quit attempts lapse within six months, short‐term quit outcomes may overestimate the success of interventions. However, in this review we made the decision to include studies with a shorter follow‐up, due to a paucity of long‐term data. This has specific limitations for this population; people experiencing homelessness who are smokers may also have co‐occurring mental‐health or substance‐use disorders, and may benefit from more sustained interventions, or may lapse early on in an attempt and need to make multiple quit attempts before being successful. This could mean that looking at short‐term quit rates in this instance underestimates intervention effects, and this should be taken into account. As further long‐term evidence accumulates we will consider removing the shorter‐term evidence (less than six months) from this review. While there are challenges to retaining people experiencing homelessness in long‐term studies, previous studies have highlighted key strategies to increase enrolment and retention of people experiencing homelessness into clinical trials, and could be used as guidance for using community‐based participatory research methods (Goldade 2011). These strategies include incentivizing attendance for check‐in visits between study follow‐up assessments, hiring people with lived experiences of homelessness to assist with recruitment and retention efforts, obtaining multiple forms of contact, and at times, physically going to sites where people stay or spend time (Goldade 2011). These and other efforts have increased retention rates in longitudinal studies with people experiencing homelessness to more than 75% at six‐month follow‐up (Vijayaraghavan 2014; Vijayaraghavan 2016c).
Quality of the evidence
Of the nine studies included in this review, we judged three to be at high risk of bias, with the remaining studies at unclear risk in one or more domains. If studies did not present information to determine bias in one or more domains, we judged these studies to be at unclear risk. For these studies, it is not clear whether there is a true risk of bias or just a lack of reporting. We conducted sensitivity analyses to remove studies that were deemed to be at high risk of bias. For the comparison of more intensive versus less intensive support, removal of the study did not change the direction of the pooled RR estimate for cessation at longest follow‐up, but did reduce the precision of the estimate, meaning that the CIs no longer only indicated a benefit. For the comparison of multi‐issue support compared to smoking cessation‐only support, removal of the study at high risk changed the direction of the RR estimate for quitting, so that it favored multi‐issue support over smoking cessation support alone. These findings highlight their substantial imprecision, and warrant the need for more research among people experiencing homelessness.
We assessed the certainty of the evidence by creating 'Summary of findings' tables for the evidence investigating contingent reinforcement as an adjunct to usual care, more compared to less intensive behavioral smoking cessation, and multi‐issue support compared to smoking cessation support alone. We carried out GRADE ratings for the smoking cessation outcome for each comparison (Table 1; Table 2; Table 3). We judged evidence contributing to the abstinence outcome for the contingent reinforcement comparison to be of low certainty due to imprecision in the estimate (Table 1). One of the studies did not have any events to contribute to the comparison, and the RR for cessation was derived from only one small study. The CI spanned the line of no effect, suggesting a potential for benefit but also no benefit or substantial harm. We judged evidence contributing to the abstinence outcome for the behavioral‐support intensity comparison to be of very low certainty (Table 2). One of the studies included in this comparison was at high risk of bias, and removing this study from the analysis yielded a wide CI, highlighting the substantial imprecision of the estimate. Again, the CI spanned the line of no effect, suggesting a potential for benefit, but also no benefit or substantial harm. We judged evidence contributing to the abstinence outcome for the multi‐issue support comparison to be of very low certainty (Table 3). One of the studies was at high risk of bias, and removal of this study reversed the direction of the RR, suggesting a benefit from multi‐issue support compared to smoking support alone. However, the CI spanned the line of no effect, suggesting that the multi‐issue support could provide a potential benefit over smoking cessation support alone, as well as a potential for no benefit.
Most studies contributing data to these clinical trials had small sample sizes, which impacts our ability to measure a precise effect. There is a need for well‐powered clinical trials of interventions to reduce tobacco use among people experiencing homelessness.
Potential biases in the review process
To conduct this review, we followed standard Cochrane methods, which are considered to be robust. For the 'Risk of bias' outcome assessment, we followed the standard methods used for Cochrane Tobacco Addiction Review Group cessation reviews; we assessed selection bias, detection bias, attrition bias and other types of biases for each study, and only assessed performance bias for the one study that tested a pharmacological or e‐cigarette intervention. In an attempt to avoid publication bias, we searched trial registries to identify any ongoing or unpublished studies, and thereby identified one trial whose results were not published and another that was ongoing. We included one study where measures of long‐term cessation were intended, but not included because the study randomized only three participants to the intervention group and there were no quit attempts reported for either the intervention or control groups (Spector 2007). Results have not yet been reported for one of the included studies (Ojo‐Fati 2015). The trial registry states that this study was only completed in 2018; it could therefore be that publication is still pending. We will continue to monitor this study in future updates. We were unable to create funnel plots for any of our comparisons, as none included 10 or more studies, as is recommended by the Cochrane Handbook (Higgins 2020).
We carried out sensitivity analyses to test the assumption that participants lost to follow‐up were smoking by carrying out complete‐case analyses for the primary outcome, as well as the original prespecified intention‐to‐treat analyses. In all cases these analyses found no evidence for a difference in the interpretation of effects across the two types of analysis. This suggests that in the studies that we were able to analyze there was no evidence that the reasons for being lost to study follow‐up varied across study arms, and were therefore a function of the intervention participants received.
Agreements and disagreements with other studies or reviews
A recent review among people experiencing homelessness (Soar 2020) sought to: 1) estimate smoking prevalence; 2) determine the efficacy of smoking cessation and reduction interventions for people experiencing homelessness; and 3) describe barriers to and facilitators of smoking cessation and reduction. This systematic review included 53 studies, of which 46 were conducted in the USA. They found no clear evidence on cessation methods that worked best for people experiencing homelessness, but concluded that multimodal approaches that included adjuncts to usual care appeared to enhance smoking quit rates.
In addition, a narrative review investigating disadvantaged groups more generally included information on the effects of tobacco‐cessation interventions on people experiencing homelessness (Wilson 2017), and concluded that multicomponent interventions with a combination of behavioral counseling and pharmacotherapy or contingent reinforcement were effective in reducing tobacco use or promoting cessation among low‐income smokers and smokers with mental‐health disorders. However, only one trial contributed to this review that focused solely on people experiencing homelessness (Okuyemi 2013). We include this trial in our review and judge it to be at unclear risk of bias, due to uncertainty about randomization procedures and prespecification of outcomes. Consistent with Wilson 2017, we found an indication of some benefit from more intensive interventions compared to less intensive interventions. However, thorough GRADE assessments of our primary outcome across all key comparisons indicate that the certainty of this evidence is low or very low, making it inappropriate to draw any clear conclusions.
Other reviews that have assessed similar interventions in a general population have found clearer and more beneficial effects. A recent Cochrane Review of incentives for smoking cessation (Notley 2019) found high‐certainty evidence that providing incentives was more effective than not providing incentives (RR 1.49, 95% CI 1.28 to 1.73; 30 trials, 21,627 participants; I2 = 33%), and a Cochrane Review looking at behavioral support as an addition to pharmacological support found high‐certainty evidence that increasing the amount of behavioral support is likely to increase the chance of success by about 10% to 20%, based on a pooled estimate from 65 trials (Hartmann‐Boyce 2019). A recent Cochrane Review of e‐cigarettes for smoking cessation in the general population found very low‐certainty evidence to suggest that e‐cigarettes with nicotine might help to increase quit rates compared to behavioral support only or no support (Hartmann‐Boyce 2020). However, the small number of trials, the wide confidence intervals and low event rates highlight the need for higher‐quality studies to examine the potential benefits of e‐cigarettes as cessation aids, both among the general population and for persons experiencing homelessness. The latter two results are in line with our findings, but the former result for incentives differs considerably. One of the main reasons the effects in this review may diverge is the vast difference in the number of participants included in our analyses; our contingent‐reinforcement analysis included only 120 participants and was very imprecise. Although our effect estimate suggests a potential harm of offering incentives, the upper limit of the confidence interval still incorporates significant benefit; future updates could bring the result more into line with that found in the general population. However, it is also possible that the people experiencing homelessness who have higher levels of nicotine dependence may derive less benefit from incentives under these circumstances, or that engagement with services is so low that interventions are simply not delivered in the same way as they would be in the general population, thereby reducing their benefit.
Authors' conclusions
Implications for practice.
There is insufficient RCT evidence to assess the effects of any smoking‐cessation interventions in people specifically experiencing homelessness. However, interventions were assessed in addition to usual care (generally comprising behavioral and pharmacological support), and there is no reason to think that standard cessation support is any less effective for people experiencing homelessness than for the general population.
There is no RCT evidence assessing the effects of increasing the accessibility of smoking‐cessation interventions for people experiencing homelessness.
Although there was some evidence to suggest a modest benefit of more intensive behavioral interventions when compared to less intensive interventions, our certainty in this evidence was very low, meaning that further research could either strengthen or weaken this effect.
There is insufficient evidence to assess whether the provision of smoking‐cessation support has any effects on the mental health or other substance‐use outcomes of people experiencing homelessness.
Implications for research.
More high‐quality studies are needed, investigating ways to help people experiencing homelessness to give up smoking in the long term. These studies should have sufficient statistical power and retain participants for long‐term follow‐up of at least six months.
Future studies should explore mental health and substance‐use outcomes, as these are especially relevant to people experiencing homelessness.
Studies carried out in homeless populations suffer substantially through lack of engagement and attrition. Further studies should aim to enhance engagement with smoking‐cessation services and maximize adherence. This could include interventions with peer support or community‐based support to address the social and environmental context of smoking.
History
Protocol first published: Issue 9, 2019 Review first published: Issue 12, 2020
Acknowledgements
The review authors would like to thank Professor Paul Aveyard, and Dr Jonathan Livingstone‐Banks for their thoughtful review of draft versions of this review. We gratefully acknowledge Professor Lynne Dawkins and Dr Sharon Cox, who provided additional unpublished information about their study (Dawkins 2020). We also thank Dr Jamie Hartmann‐Boyce and Annika Theodoulou for sharing their data extraction of this study for their Cochrane Review Electronic cigarettes for smoking cessation. We gratefully acknowledge peer review by Debbie Robson, RMN, PhD, Senior Research Fellow, Nicotine Research Group, King’s College London and Dr Sharon Cox, Senior Research Fellow, University College London, and consumer review by Lee Bromhead.
Nicola Lindson is Managing Editor of the Cochrane Tobacco Addiction Group, which is funded by the National Institute for Health Research (NIHR), via Cochrane Infrastructure Grant funding. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Appendices
Appendix 1. MEDLINE search strategy
(un‐housed* OR homeless* OR "unstably housed" OR runaway OR "homeless persons"[mesh] OR housing instability)
((smoking cessation.mp. OR exp Smoking Cessation/) OR "Tobacco‐Use‐Cessation"/ OR "Tobacco‐Use‐Disorder"/ OR Tobacco‐Smokeless/ OR exp Tobacco‐/ OR ((quit$ or stop$ or cease$ or giv$) adj5 smoking).ti,ab.)) OR exp Smoking/)
((randomised controlled trial[pt]) OR (controlled clinical trial[pt]) OR (clinical trial[pt])) OR ((pragmatic clinical trial)) NOT (animals[mh]))
1 AND 2 AND 3
Data and analyses
Comparison 1. Contingent reinforcement (CR) as adjunct.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Smoking abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 Long‐term abstinence (≥ 6 m) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.2 Short‐term abstinence (< 3 m) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Change in other drug use (past month severity at 8 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Alcohol | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 Substance use | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Change in mental health (past month severity at 8 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Number making a quit attempt for 24 hours or more (at 8 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. More versus less intensive behavioural support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Smoking abstinence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Long‐term abstinence (≥ 6 m) | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.01, 2.69] |
| 2.2 Drug and alcohol abstinence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Multi‐issue support versus smoking support only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Smoking abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Long‐term abstinence (≥ 6 m) | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.35, 2.61] |
| 3.2 Drug and alcohol abstinence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Change in other drug use (at 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 Days drank alcohol (within last 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.2 Days of binge‐drinking (within last 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.3 Number of alcoholic drinks/day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.4 Days of marijuana use (within the last 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.5 Days of cocaine use (within last 30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4 Change in perceived stress | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.92, 0.56] |
3.4. Analysis.

Comparison 3: Multi‐issue support versus smoking support only, Outcome 4: Change in perceived stress
Comparison 4. Text support as an adjunct.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Number making a quit attempt for 24 hour or more (at 8 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 Other drug use (past month severity at 8 weeks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.1 Alcohol | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.2 Substance use | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Mental health (past month severity at 8 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. E‐cigarette versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Smoking abstinence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1.1 Long‐term abstinence (≥ 6 m) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Change in other drug use | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.1 Alcohol use severity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.2 Substance use severity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Change in mental health symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.1 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.2 Anxiety | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Cognitive behavioral therapy versus empathic support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Smoking abstinence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1.1 Short‐term abstinence (< 3m) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
6.1. Analysis.

Comparison 6: Cognitive behavioral therapy versus empathic support, Outcome 1: Smoking abstinence
Comparison 7. Sensitivity analysis: abstinence outcome, complete case analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Contingent reinforcement (CR) as adjunct | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1.1 Long‐term abstinence (≥ 6 months) | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.17, 2.68] |
| 7.1.2 Short‐term abstinence (< 3 months) | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.2 More versus less intensive behavioral support | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2.1 Long‐term abstinence (≥ 6 months) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.3 Multi‐issue support versus smoking support only | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.3.1 Long‐term abstinence (≥ 6 months) | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.40, 2.04] |
| 7.4 Text support as an adjunct | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.4.1 Short‐term abstinence (< 3 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.5 E‐cigarette versus usual care | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.5.1 Long‐term abstinence (≥ 6 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baggett 2018.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: October 2015 to June 2016 Location: USA Setting: Boston Healthcare for the Homeless Program headquarters Recruitment: Participants were recruited by in‐person advertisement in the Boston Healthcare for the Homeless clinic lobby, flyers posted in clinics, and referrals from clinicians |
|
| Participants |
N = 75 randomized (83 originally enrolled) Participant characteristics:Age (mean, SD): 46.6 (9.1); Sex (female, n, %): 26 (52.0); Race/ethnicity (n, %): White 21 (42.0), Black 16 (32.0), Hispanic 11 (22.0), Other 2 (4.0); smokers ready to quit smoking within the next month; currently homeless (defined as usually staying in an emergency shelter, transitional shelter, abandoned building, place of business, car or other vehicle, church or mission, hotel or motel, or anywhere outside during the past 7 days, or if currently in a residential treatment program, in the 7 days prior to program entry. Additionally, individuals were considered currently homeless if they usually stayed in somebody else’s place in the past 7 days because of not having their own place to stay). Inclusion criteria included readiness to quit smoking within the next month |
|
| Interventions | The QUIT (Quitting with Usual Care, Incentives, and Technology) Smoking Study ‐ a 3‐arm pilot randomized controlled trial Control group (N = 25): Transdermal nicotine patch and weekly in‐person counseling for 8 weeks. Participants received a mobile phone with a QWERTY keyboard and a 2.4‐inch display. The phones were activated by study staff at the time of randomization and loaded with a prepaid 2‐month voice and text plan Intervention 1 ‐ financial incentives (N = 25): As control group, plus contingent financial rewards for smoking abstinence ‐ Escalating‐value financial rewards for smoking abstinence, verified by exhaled carbon monoxide < 8 ppm. Reward values started at USD 15 and increased in USD 5 increments with each successive abstinent measurement, up to a maximum of USD 35. Non‐abstinence or non‐attendance resulted in no payment and reset the subsequent payment to the starting value of USD 15. The maximum amount that participants could earn for smoking abstinence was USD 440 Intervention 2 ‐ text messaging (N = 25): As control group, plus text messages, delivered by SmokefreeTXT to support smoking abstinence. At enrolment, SmokefreeTXT prompted participants to set a quit date within the next 2 weeks. The program then sent 1 ‐ 5 text messages per day starting up to 2 weeks before the quit date and continuing until 6 weeks after the quit date. Message content provided encouragement, advice, and tips for quitting smoking, and was not tailored to homeless or low‐income individuals. SmokefreeTXT messages were unidirectional, but beginning on the quit date, a subset (approximately 23%) of messages were interactive in nature and solicited brief participant responses |
|
| Outcomes |
Primary outcome: Biochemically‐verified 7‐day point prevalence smoking abstinence at 8 weeks follow‐up Secondary outcome measures of interest:
|
|
| Declarations of Interest |
Sponsorship source: This study was supported by award K23DA034008 (Baggett) from the National Institute on Drug Abuse at the National Institutes of Health, by the Massachusetts General Hospital Department of Medicine Transformative Scholars Program (Baggett), and by award P20GM103644 (Higgins) from the National Institute of General Medical Sciences at the National Institutes of Health Study author conflicts of interest: Dr. Baggett receives royalty payments from UpToDate for authorship of a topic review on the health care of homeless people in the United States. Dr. Rigotti has a research grant from and has consulted without pay for Pfizer regarding smoking cessation. She receives royalties from UpToDate for authorship of topic reviews on smoking cessation |
|
| Notes | The authors kindly supplied quit attempt data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was computer‐generated in random permuted blocks of 3,6,9,12, or 15 and concealed from study staff |
| Allocation concealment (selection bias) | Unclear risk | The study report states that the allocation sequence was concealed from study staff, but provides no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking abstinence was biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/25 (4%) participants in the control arm, 0/25 (0%) participants in the financial incentives arm and 1/25 (4%) of the texts arm were lost to follow‐up. Therefore, losses were small and similar across study arms |
| Selective reporting (reporting bias) | Low risk | Authors report on all the outcomes described in the trial registry entry |
| Other bias | Unclear risk | Assessment for receipt of incentives was based on CO‐verified abstinence, but participants who had CO‐verified abstinence reported short‐term relapses by self‐report, suggesting the PPA may be overestimating abstinence |
Burling 2001.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: Not reported Location: USA Setting: Palo Alto Veterans Affairs Health Care System Recruitment: Volunteer participants were recruited from a residential rehabilitation program for homeless veterans at the Palo Alto Department of Veterans Affairs Health Care System |
|
| Participants |
N = 150 Participant characteristics (Treatment acceptors):Age (Mean, SD) :39.6 (6.2); Sex (% female): 5; Race/ethnicity: 44% White, 44% African American, 5% Hispanic, 5% Other; drug and alcohol dependent cigarette smokers in a residential rehabilitation program for homeless veterans at the Palo Alto Department of Veterans Affairs Health Care System |
|
| Interventions |
Usual care (N = 50): participants were informed about the treatments offered by the general hospital‐wide smoking cessation support program Multicomponent smoking treatment (MST) group (N = 50): Smoking cessation treatment consisted of 5 weeks of prequit treatment and 4 weeks of postquit treatment. The prequit counseling was delivered daily, face‐to‐face, and lasted about 30 ‐ 45 minutes per day. The first 2 weeks of postquit support involved daily counseling while participants used 14‐mg nicotine patches and the second week involved bi‐weekly counseling while participants took the 7 mg patch MST + generalization training (G) group (N = 50): The structure of the program was identical to MST; however, content for the counseling focused on smoking cessation in the context of drug and alcohol dependence |
|
| Outcomes |
Primary outcome: Postquit continuous smoking abstinence rates at 12 months follow‐up, biochemically verified using expired CO and urine cotinine. Secondary outcome:
|
|
| Declarations of Interest |
Sponsorship source: This research was partially supported by National Institute on DrugAbuse Grant DA07426. Although it provided no direct funding, this research was partially supported by the Veterans Affairs Conflicts of interest: None reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assignment is described as random, but no further details are given |
| Allocation concealment (selection bias) | Unclear risk | None noted; unclear if study staff were blinded to concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking abstinence outcomes were biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46% of the MST group and 44% of the MST+G did not complete the 9‐week smoking treatment protocol. Losses to treatment were mainly due to discharge from inpatient treatment. Although dropout was similar between arms, overall dropout was therefore high |
| Selective reporting (reporting bias) | Unclear risk | This trial was not registered so we are unable to compare whether reported outcomes matched outcomes in the trial registry |
Dawkins 2020.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomized controlled trial (although randomization was ultimately not possible) Study dates: October 2018 to March 2020 Location: UK Setting: 4 UK homeless shelters Recruitment: Participants accessing homeless center services and actively engaging with the service were recruited |
|
| Participants |
N = 80 Participant characteristics:Age (Mean, SD) :42.7 (10.8); Sex (% female): 35; adult smokers accessing homeless support services on a regular basis and known to staff, including those who reported mental illness or substance dependence |
|
| Interventions |
Control arm (N = 32): written information on quitting smoking (adapted from NHS Choices); signposting to the local stop‐smoking service (SSS) by centre staff Intervention arm (N = 48): electronic cigarette starter kit, comprising tank‐style refillable electronic cigarette with a choice of nicotine strength e‐liquid (12 mg/mL or 18 mg/mL) and flavors (fruit, menthol, tobacco), a 'tips and tricks' leaflet and support from center staff, who met once a week to provide e‐liquid and troubleshoot EC use |
|
| Outcomes |
Primary outcome: exhaled CO‐verified sustained (from 2 weeks after quit day) smoking abstinence at 24‐weeks follow‐up Secondary outcomes:
|
|
| Declarations of Interest |
Sponsorship source: funded by the National Institute for Health Research (Public Health Stream 17/44/29) Conflicts of Interest: SC, AF, JL, CB, AT, DR, IU, LB, SP have no competing interests. H has received research grant from and provided consultancy to Pfizer. LD has provided consultancy for the pharmaceutical industry relating to the development of smoking cessation products |
|
| Notes | Authors provided information prior to peer review by personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Thus the actual allocation of centres to each arm was a pragmatic decision based on centre readiness and staff/researcher availability though we balance potential confounders and differences in environment by ensuring each cluster (EC and UC) contained one day centre and one residential unit.” Comment: Intention was to randomize but were unable to due to practical constraints |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants joined after cluster randomisation… Allocation was concealed to participants until after the baseline assessment.” Comment: But unclear if allocation was concealed for those recruiting, and allocation would have been known to new participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The electronic cigarette intervention was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence was biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/48 (27.1%) participants in the intervention arm and 20/32 (62.5%) in the control arm were lost to follow‐up. There was therefore differential loss to follow‐up between study arms |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes reported |
NCT02245308.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: October 2014 to March 2018 Location: USA Setting: Durham Veterans Affairs Medical Center (DVAMC), Durham, NC Recruitment: Eligible veterans identified through data pulls from DVAMC's electronic health record. Study staff also worked closely with DVAMC staff to identify potential participants. Participants were also recruited using flyers, business cards, and brochures. Staff members visited local housing facilities (e.g. shelters, transitional housing) to identify potential participants |
|
| Participants |
N = 127 Participant characteristics: Age (Mean, SD): 54.75 (8.99); Sex (n, % female): 9, 7.1%; Race/ethnicity (n, % ): White 31 (24.4%), Black 85 (66.9%, ), American Indian or Alaskan Native 4 (3.1%), more than one race 7 (5.5%). Participants were homeless, enrolled in the DVAMC for ongoing care, current smokers (at least 10 cigarettes or equivalent), willing to quit smoking in the next 30 days |
|
| Interventions |
Control group (N = 63): Participants assigned to the active control arm were referred to the DVAMC specialty smoking cessation clinic for standard treatment, which may include group counseling, individual counseling, self‐help materials, and smoking cessation aids (nicotine replacement therapy or bupropion) Abstinence reinforcement therapy (ART; N = 64): Participants received guideline‐based cognitive behavioral counseling for smoking cessation, attended a telemedicine clinic for access to smoking cessation aids, and received intensive behavioral therapy for smoking cessation by mobile contingency management (mCM). mCM is a behavioral intervention designed to provide positive reinforcement for remaining abstinent from smoking. Participants are loaned a smart phone and trained to take video of themselves taking a carbon monoxide reading. Anytime participants uploaded a video they get a monetary reward. Participants also received nicotine replacement in the form of Nicorette gum or lozenge or bupropion |
|
| Outcomes |
Primary outcomes: Biochemically‐verified abstinence (using salivary cotinine) at 6 months Secondary outcomes: none reported |
|
| Declarations of Interest |
Sponsorship source: Veteran Affairs office of Research and Development Conflicts of interest: Unknown |
|
| Notes | Some study outcomes are published in the clinical trials registry, but no results are published in the peer‐reviewed literature at the time of writing this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedures are not reported in the trial registry entry |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedures are not reported in the trial registry entry |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence outcomes are biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in the trial registry entry |
| Selective reporting (reporting bias) | Low risk | Authors report on all stated relevant outcomes |
Ojo‐Fati 2015.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: January 2015 to October 2018 Location: USA Setting: Homeless emergency shelters and transitional housing units in Minneapolis‐St. Paul, MN Recruitment: Participants recruited from sites using promotional flyers, announcements about the study during peak times of use at the shelters, and word of mouth from current participants and study staff to shelter users |
|
| Participants |
N = 645 Participant characteristics: Not reported yet Eligibility criteria include currently homeless, and AUDIT score ≥ 7, adult current daily cigarette smoker (defined as smoking at least 1 cigarette a day for the last 7 days). Eligibility criteria including willingness to participate in counseling and use NRT |
|
| Interventions | All 3 groups received bi‐weekly supplies of NRT (21‐mg patch + gum and/or lozenge) for a total of 12 weeks beginning at week 4 Integrated intensive smoking intervention using cognitive behavioral therapy plus alcohol intervention (IntS+A; N = 215): Participants received alcohol and smoking counseling during each session. Each session included 2 x 30‐minute segments of distinct alcohol counseling and smoking intervention. Participants received weekly sessions for 3 months, and then monthly group sessions for the next 3 months Intensive smoking intervention using CBT (N = 215): Participants received weekly individual smoking counseling for the first 3 months, followed by monthly group sessions for the next 3 months. They also received brief alcohol counseling Usual care (N = 215): Participants received brief 20‐minute one‐time counseling for smoking and alcohol cessation. The smoking cessation counseling was based on the 5As model (ask, advise, assess, assist and arrange) |
|
| Outcomes |
Primary outcome: Cotinine‐verified 7‐day point prevalence smoking abstinence at 52 weeks follow‐up Secondary outcome:
|
|
| Declarations of Interest |
Sponsorship source: This project is being funded by a grant from the National Heart, Lung, and Blood Institute (grant R01 HL081522). This research was also supported by the National Cancer Institute of the National Institutes of Health (NIH) under award number R25CA163184 Conflicts of interest: None reported |
|
| Notes | The study protocol is published, and the clinical trials registry states that the study was completed 31 October 2018, but the study results are not yet published or available on the clinical trials registry entry. We contacted authors, and have not received any information from them | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A block randomisation with three or six random blocks will be used to improve balance. The statistician developed the randomisation scheme for the study. In this scheme, each participant is randomised on‐site by study staff using the established protocol during the enrolment visit". Comment: Insufficient information to make a judgment. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment methods |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study results are not available so we cannot make a judgment |
| Selective reporting (reporting bias) | Unclear risk | Study results are not available so we cannot make a judgment |
Okuyemi 2006.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: February 2004 to December 2004 Location: USA Setting: 13 homeless facilities in Kansas City area, Missouri Recruitment: Smokers were recruited by flyers posted and distributed at 13 homeless service facilities in the Kansas City area. Recruitment took place in 3 waves |
|
| Participants |
N = 46 Participant characteristics (smoking plus):Age (mean, SD): 43.7 (9.8); Sex (female, %): 34.8; Race/ethnicity (%): White 21.7, Black 69.6, Other 8.7. Adult smokers, interested in quitting in the next 2 weeks, and homeless. A homeless person was defined as any individual (a) who lacked a fixed, regular, and adequate night‐time residence, and (b) whose primary night‐time residence was a supervised publicly‐ or privately‐operated shelter designed to provide temporary living accommodations, transitional housing, or other supportive housing programs or a public or private place not meant for human habitation (e.g. on the streets or in abandoned buildings, tents, or automobiles). Eligibility criteria included willingness to quit in the next 2 weeks |
|
| Interventions | All study participants received a 2‐week supply of a choice of NRT (either nicotine patch or lozenge). Participants were instructed to begin using NRT from the day after their first visit for up to 8 weeks Smoking only (N = 23): participants received 5 x 30‐ to 45‐minute in‐person individual motivational interviewing sessions addressing smoking behaviors Smoking plus (N = 23): Participants received motivational interviewing cessation counseling but in the context of other barriers to quitting, including other addictions or life events affecting their motivation to quit |
|
| Outcomes |
Primary outcome: 7‐day PPA, verified by CO and salivary cotinine, at 26‐week follow‐up Secondary outcome:
|
|
| Declarations of Interest |
Sponsorship source: None reported Conflicts of interest: None reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information on sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Information on allocation concealment methods were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking outcomes were biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28/41 (68%) were included in the final analysis at 26 weeks. 10/26 (38.4%) were lost to follow‐up at 26 weeks in the smoking‐only group versus 8/26 (30.7%) in the smoking‐plus group. Overall loss was therefore less than 50% and was similar between groups |
| Selective reporting (reporting bias) | Unclear risk | Unclear as trial was not preregistered |
Okuyemi 2013.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial (the Power to Quit study) Study dates: September 2007 ‐ March 2011 Location: USA Setting: Homeless shelters and transitional housing units in Minneapolis, USA Recruitment: Participants were recruited at health fairs and through flyers and informational sessions at the homeless shelters |
|
| Participants |
N = 430 Participant characteristicsAge (Mean, SD): 44.9 (9.9); Sex (% female): 25.3; Race/ethnicity: 35.6% White, 56.3% African American, 2.3% Hispanic, 2.3% Native American/Alaskan Native, 2.3% Other; currently homeless (based upon the Stewart B. McKinney Act passed by the US congress in 1987 in which homelessness was defined as anyone lacking “a fixed, regular and adequate nighttime residence;” or anyone staying at “a supervised publicly or privately operated shelter designed to provide temporary living accommodations, transitional housing, or other supportive housing program or a public or private place not meant for human habitation"), cigarette smokers (confirmed with exhaled CO measure of 5 ppm or more, willing to take part in smoking cessation treatment |
|
| Interventions | All participants received nicotine replacement therapy in the form of nicotine patches (21 mg) during the duration of the intervention and a health educational resource called The Power to Quit: A Quit Smoking Guide Standard care (N = 214): Participants received one‐time brief smoking cessation counseling (10 ‐ 15 minutes) at the onset of the intervention Intervention arm (N = 216): Participants received 6 x face‐to‐face individual MI sessions, each lasting 15 to 20 minutes that took place at baseline, and on the 1st, 2nd, 4th, 6th, and 8th week of the study |
|
| Outcomes |
Primary outcome: 7‐day PPA, 26 weeks. Biochemically verified using exhaled CO (cut‐off ≤ 10 ppm). Salivary cotinine testing was carried out if expired CO was > 10 and if participants self‐reported abstinence. A cut‐off of ≤ 20 ng/ml was used to verify abstinence using salivary cotinine Secondary outcome: None of our secondary outcomes are reported (although mental health and substance‐abuse outcomes were measured, these were only analyzed by cessation status, not by intervention group) |
|
| Declarations of Interest |
Sponsorship source: This study was supported by the National Institutes of Health [grant numbers R25CA163184 and R01HL081522] and the Veterans Affairs Advanced Fellowship in Clinical and Health Services Research through the Center for Chronic Disease Outcomes Research Conflicts of interest: None reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not provided |
| Allocation concealment (selection bias) | Unclear risk | The authors do not discuss allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was biochemically verified using exhaled CO and cotinine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47/216 (22%) in MI intervention group and 59/214 (28%) in control group lost to follow‐up. Rates were therefore similar between groups |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not reported in the trial registry |
Rash 2018.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: October 2012 to July 2016 Location: USA Setting: Homeless shelter Recruitment: Participants were recruited from local facilities that serve the homeless population using flyers, referrals and word‐of‐mouth efforts |
|
| Participants |
N = 70 Participant characteristics:Age (mean, SD): 45.1; Sex ( n (% female): 25 (35.7%); Race/ethnicity (n,%): White 34 (48.6%), Black/African American 25 (35.7%), Other: 11 (15.7%); receiving services at a homeless facility or otherwise meeting the federal definition of homeless, cigarette smokers (exhaled CO reading of ≥ 6 ppm and urine cotinine reading consistent with > 100 ng/ml); self‐reported interest in quitting cigarette smoking |
|
| Interventions |
Standard care (N = 33): Participants started treatment on their quit date. The first intensive phase (weeks 1 ‐ 4) included twice‐daily CO monitoring, twice‐weekly counseling, and NRT (transdermal nicotine, dosages started at 21mg and tapered over the course of 8 weeks) for the first 4 weeks post‐quit day. In the second phase (weeks 5 ‐ 8), only NRT continued. Participants received USD 2 for each CO breath sample submitted, non‐contingent on smoking status Standard care with contingency management (N = 37): Participants received all aspects of standard care except non‐contingent USD 2 payments. Instead, participants earned draws from a prize bowl for each negative (CO ≤ 4 ppm) sample submitted. Draws started at 1 on the first session of the quit day and escalated by 1 up to a cap of 4 draws in each session for consecutive negative samples |
|
| Outcomes |
Primary outcome: 7 day PPA assessed at 24‐week follow‐up. Biochemically‐verified by negative urine cotinine (≤ 100 ng/mL) readings Secondary outcomes: none measured |
|
| Declarations of Interest |
Sponsorship source: This report was supported in part by the following National Institutes of Health grants: R21‐DA031897, P50‐DA009241, P60‐AA03510, R01‐HD075630, R01‐AA021446, R01‐AA023502, and R01‐DA013444. Additional support was provided by the Connecticut Institute for Clinical and Translational Science (CICATS) at the University of Connecticut Conflicts of interest: None reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using computerized urn procedure |
| Allocation concealment (selection bias) | Unclear risk | No information provided for allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation was biochemically verified using urine cotinine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At week 24, 24/33 (73%) in the standard‐care arm and 27/37 (73%) in the contingency‐management arm completed the intervention. Loss to follow‐up was therefore low and similar between arms |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not reported in trial registry entry |
Spector 2007.
| Study characteristics | ||
| Methods |
Study design: Randomized controlled trial Study dates: January 2004 to October 2005 Location: USA Setting: Homeless shelter Recruitment: Participants were recruited by case managers and nurse practitioner from a local county homeless shelter |
|
| Participants |
N = 11 Participant characteristics:Mean age (Mean, SD): 40.6 (10.8); Sex (n, % female): 2 (18.1%); Race/ethnicity (n, %): none reported. Eligibility criteria include being homeless, and an adult current smoker |
|
| Interventions | All participants received 9 x 20‐minute sessions of counseling over 3 weeks. Counseling was delivered by medical students Cognitive behavioral therapy (N = 3): The CBT was administered as individual therapy and included topics such as 1) Introduction, 2) Preparing to quit, 3) Quitting, 4) Staying off cigarettes, 5) Relapse prevention, 6) Healthy management of reality, 7) Thoughts and mood, 8) People and mood, and 9) Preventative lifestyle Unstructured support (N = 8): This support included being emphatic and supportive while talking about smoking, without providing specific guidance or encouragement to quit |
|
| Outcomes |
Primary outcome: Biochemically‐verified smoking cessation approximately 4 weeks post‐baseline (this is not reported in the paper, reportedly due to high levels of dropout, but we acquired the information from the authors) Secondary outcomes: none measured |
|
| Declarations of Interest |
Sponsorship source: Blue Cross Blue Shield of Michigan Foundation Student Award Program and the David E. Rogers Fellowship of the New York Academy of Medicine provided financial support to Dr. Spector Conflicts of interest: None reported |
|
| Notes | Denominators were provided through communication with the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No participants reported quitting and so biochemical verification was not required |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/11 (55%) overall completed the 9‐session protocol. Numbers followed‐up were not reported by study arm. |
| Selective reporting (reporting bias) | High risk | Quote: "The study outcomes (smoking reduction or cessation) were designed to be within‐subject repeated measure analyses to determine the efficacy over time and across interventions (CBT versus unstructured support). Because of small numbers per treatment group, we were unable to compare CBT versus unstructured support formats; our final analysis examined pre‐ and post‐differences (including the number of cigarettes and amount of carbon monoxide [CO]) using a pooled analysis with all six completers." Comment: Due to the small number of people who completed the analysis between‐group differences were not reported |
AUDIT: alcohol use disorders identification test: CO: carbon monoxide; EC: electronic cigarette; GAD: generalized anxiety disorder; PHQ: patient health questionnaire; PPA: point prevalence abstinence; SDS: severity of dependence scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beckham 2018 | Ineleigible study design |
| Bonevski 2011 | Ineligible participant population |
| Bonevski 2012 | Ineligible study design |
| Businelle 2014 | Ineligible study design ‐ non‐random assignment |
| Businelle 2015 | Ineligible study design |
| Carpenter 2015 | Ineligible study design |
| Chen 2015 | Commentary paper. Not a study |
| Collins 2019 | Ineligible study design |
| Hamner 2015 | Commentary paper. Not a study |
| Hickman 2015 | Ineligible participant population |
| Nyamathi 2012 | Study did not assess tobacco smoking cessation. |
| Power 2015 | Ineligible study design |
| Santa Ana 2016 | Ineligible study design ‐ non‐random assignment |
| Segan 2015 | Ineligible study design |
| Shadel 2015 | Commentary paper. Not a study |
| Shelley 2010 | Ineligible study design |
| Sherman 2016 | Ineligible study population |
| Tucker 2015 | Ineligible study design |
| Vijayaraghavan 2016a | Ineligible study design |
Characteristics of ongoing studies [ordered by study ID]
Tucker 2020.
| Study name | Text messaging‐based smoking cessation program for homeless youth |
| Methods | Randomized controlled trial |
| Participants | Homeless youth |
| Interventions | Control: 30‐minute group‐based smoking cessation counseling session based on the 5A's approach (Ask; Advise; Assess; Assist; Arrange) and free nicotine replacement Intervention: as control condition, plus a 6‐week text messaging intervention |
| Outcomes | 90‐day continuous abstinence at 3 month follow‐up |
| Starting date | May 2019 |
| Contact information | jtucker@rand.org |
| Notes | Due to complete May 2020 |
Differences between protocol and review
We planned to include interventions that increased access to cessation treatment, but we did not find any intervention of this type.
Contributions of authors
The protocol was conceived and prepared by MV, HE, and DA.
MV, HE, KF, and DA were involved in study screening and eligibility and assessment, data extraction, and 'Risk of bias' assessment.
NL provided statistical expertise.
MV and NL wrote the first draft and all authors commented on the draft.
Sources of support
Internal sources
-
Univeristy of California, San Francisco, San Francisco Cancer Initiative, USA
This work was supported by the San Francisco Cancer (SF CAN) Initiative, a collaborative community effort initiated by the UCSF HDFCCC to reduce the cancer burden across the city and beyond [www.sfcancer.org].
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK
External sources
-
Tobacco Related Disease Reseach Program, USA
Grant no.: 25IP‐0015S
-
NIHR, UK
The Cochrane Tobacco Addiction Group is funded by an NIHR Infrastructure Grant
Declarations of interest
Maya Vijayaraghavan has no conflicts of interest to report. MV has one pending grant application on the topic of smoke‐free policies in permanent supportive housing for formerly homeless populations, and was recently awarded a grant by the Tobacco Related Disease Research Program to study extended contingent reinforcement interventions for long‐term abstinence for people experiencing homelessness.
Holly Elser has no conflicts of interest.
Kate Frazer has no conflicts of interest. KF is the co‐investigator on a grant from the Irish Cancer Society awarded January 2020 for 18 months: Smoking cessation for cancer patients in Ireland A scoping and feasibility initiative.
Nicola Lindson has no conflicts of interest.
Dorie Apollinio has no conflicts of interest.
New
References
References to studies included in this review
Baggett 2018 {published data only}
- Baggett TP, Chang Y, Yaqubi A, McGlave C, Higgins ST, Rigotti NA. Financial incentives for smoking abstinence in homeless smokers: a pilot randomized controlled trial. Nicotine & Tobacco Research 2018;20(12):1442-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett TP, McGlave C, Kruse GR, Yaqubi A, Chang Y, Rigotti NA. SmokefreeTXT for homeless smokers: pilot randomized controlled trial. JMIR mHealth 2019;7(6):e13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett TP, Yaqubi A, Berkowitz SA, Kalkhoran SM, McGlave C, Chang Y, et al. Subsistence difficulties are associated with more barriers to quitting and worse abstinence outcomes among homeless smokers: evidence from two studies in Boston, Massachusetts. BMC Public Health 2018;18(1):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlave CC, Kruse G, Yaqubi A, Chang Y, Rigotti N, Baggett T. Smokefree TXT for homeless smokers: a pilot RCT with a mixed methods analysis. In: 7th Annual Meeting of the MGH Scientific Advisory Committee; 2018 03 28; Massachusetts General Hospital, USA. 2018.
- NCT02565381. A pilot randomized controlled trial of financial incentives, text messaging, and usual care for homeless smokers (QUIT). clinicaltrials.gov/ct2/show/NCT02565381 (first received 01 October 2015).
Burling 2001 {published data only}
- Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance-dependent inpatients. Journal of Consulting and Clinical Psychology 2001;69(2):295-304. [DOI: 10.1037//0022-006x.69.2.295] [DOI] [PubMed] [Google Scholar]
Dawkins 2020 {published data only}
- Dawkins L, Bauld L, Ford A, Robson D, Hajek P, Parrott S, et al. A cluster feasibility trial to explore the uptake and use of e-cigarettes versus usual care offered to smokers attending homeless centres in Great Britain. PLOS One 2020;15:e0240968. [DOI: 10.1371/journal.pone.0240968] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L. Exploring the uptake and use of e-cigarettes offered to adults accessing homeless centres in the UK: quantitative results. In: UK Electronic Cigarette Research Forum; 2020 01 17; London: UK. London: Cancer Research UK, 2020.
- ISRCTN14140672. Exploring the use and uptake of e-cigarettes for homeless smokers. www.isrctn.com/ISRCTN14140672 (first received 25 September 2019).
NCT02245308 {published data only}
- NCT02245308. Abstinence reinforcement therapy for homeless veteran smokers. clinicaltrials.gov/ct2/show/NCT02245308 (first received 19 September 2014).
Ojo‐Fati 2015 {published data only}
- NCT01932996. Enhancing smoking cessation in the homeless population. clinicaltrials.gov/ct2/show/NCT01932996 (first received 30 August 2013).
- Ojo-Fati O, John F, Thomas J, Joseph AM, Raymond NC, Cooney NL et al. Integrating smoking cessation and alcohol use treatment in homeless populations: study protocol for a randomized controlled trial. Trials 2015;16:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuyemi 2006 {published data only}
- Okuyemi KS, Thomas L, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: a pilot clinical trial. Nicotine & Tobacco Research October 2006;8(5):689-99. [DOI] [PubMed] [Google Scholar]
Okuyemi 2013 {published data only}
- Goldade K, Des Jarlais D, Everson-Rose SA, Guo H, Thomas J, Gelberg L, et al. Knowing quitters predicts smoking cessation in a homeless population. American Journal of Health Behavior 2013;37(4):517-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldade K, Whembolua G, Thomas J, Eischen S, Guo H, Connett J, et al. Designing a smoking cessation intervention for the unique needs of homeless persons: a community-based randomized clinical trial. Clinical Trials 2011;8(6):744-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00786149. Improving varenicline adherence and outcomes in homeless smokers. clinicalTrials.gov/show/NCT00786149 (first received 6 November 2008).
- Ojo-Fati O, Joseph AM, Ig-Izevbekhai J, Thomas JL, Everson-Rose SA, Pratt R, et al. Practical issues regarding implementing a randomized clinical trial in a homeless population: strategies and lessons learned. Trials 2017;18(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo-Fati O, Thomas JL, Vogel RI, Ogedegbe O, Jean-Louis G, Okuyemi KS. Predictors of adherence to nicotine replacement therapy (nicotine patch) among homeless persons enrolled in a randomized controlled trial targeting smoking cessation. Journal of Family Medicine 2016;3(7):1-13. [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua G, Thomas JL, Eischen S, Guo H, et al. Smoking characteristics and comorbidities in the power to quit randomized clinical trial for homeless smokers. Nicotine & Tobacco Research 2013;15(1):22-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Guo H, et al. Effects of motivational interviewing and the nicotine patch for smoking cessation among homeless smokers (PA7-2). In: Society for Research on Nicotine and Tobacco 18th Annual Meeting; 2012 Mar 13-16; Houston Texas. 2012.
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108(6):1136-44. [DOI: 10.1111/add.12140 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker EA, Hennrikus DJ, Erickson DJ, Call KT, Forster JL, Okuyemi KS. Trends in self-efficacy to quit and smoking urges among homeless smokers participating in a smoking cessation RCT. Addictive Behaviors 2018;78:43-50. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel LR, Nguyen N, Eischen S, Thomas J, Okuyemi KS. Is smoking cessation associated with worse comorbid substance use outcomes among homeless adults? Addiction 2014;109(12):2098-104. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CM, Sharif F, Eischen S, Thomas J, Wang Q, Guo H, et al. Retention of homeless smokers in the power to quit study. Nicotine & Tobacco Research 2015;17(9):1104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CD, Rogers CR, Okuyemi KS. Depression symptoms among homeless smokers: effect of motivational interviewing. Substance Use & Misuse 2016;51(10):1393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C, Sewali B, Olayinka A, Eischen S, Wang Q, Guo H, et al. Smoking cessation in homeless populations: who participates and who does not. Nicotine & Tobacco Research 2014;16(3):369-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rash 2018 {published data only}
- NCT01736982. Contingency management for smoking cessation in the homeless. clinicaltrials.gov/ct2/show/NCT01736982 (first received 29 November 2012).
- Rash CJ, Petry NM, Alessi SM. A randomized trial of contingency management for smoking cessation in the homeless. Psychology of Addictive Behaviors 2018;32(2):141-8. [DOI: 10.1037/adb0000350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spector 2007 {published data only}
- Spector A, Alpert H, Karam-Hage M. Smoking cessation delivered by medical students is helpful to homeless population. Academic Psychiatry 2007;31(5):402-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beckham 2018 {published data only}
- Beckham JC, Kirby AC, Tise N, Carpenter VL, Dedert EA, McNiel JM, et al. A learning collaborative model for implementation of smoking cessation among homeless veterans. Journal of Smoking Cessation 2018;13(3):129-36. [Google Scholar]
Bonevski 2011 {published data only}
- Bonevski B, Paul C, D'Este C, Sanson-Fisher R, West R, Girgis A, et al. RCT of a client-centred, caseworker-delivered smoking cessation intervention for a socially disadvantaged population. BMC Public Health 2011;11(1):70-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonevski 2012 {published data only}
- Bonevski B, Baker A, Twyman L, Paul C, Bryant J. Addressing smoking and other health risk behaviours using a novel telephone-delivered intervention for homeless people: a proof-of-concept study. Drug and Alcohol Review 2012;31(5):709-13. [DOI] [PubMed] [Google Scholar]
Businelle 2014 {published data only}
- Businelle MS, Kendzor DE, Kesh A, Cuate EL, Poonawalla IB, Reitzel LR, et al. Small financial incentives increase smoking cessation in homeless smokers: a pilot study. Addictive Behaviors 2014;39(3):717-20. [DOI] [PubMed] [Google Scholar]
Businelle 2015 {published data only}
- Businelle MS, Poonawalla IB, Kendzor DE, Rios DM, Cuate EL, Savoy EJ, et al. Smoking policy change at a homeless shelter: attitudes and effects. Addictive Behaviors 2015;40:51-6. [DOI] [PubMed] [Google Scholar]
Carpenter 2015 {published data only}
- Carpenter VL, Hertzberg JS, Kirby AC, Calhoun PS, Moore SD, Dennis MF, et al. Multicomponent smoking cessation treatment including mobile contingency management in homeless veterans. Journal of Clinical Psychiatry 2015;76(7):959-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen TC, Myers MG. A comprehensive approach to tobacco cessation for the homeless. Journal of Clinical Psychiatry 2015;76(7):e908-10. [DOI] [PubMed] [Google Scholar]
Collins 2019 {published data only}
- Collins SE, Nelson LA, Stanton J, Mayberry N, Ubay T, Taylor EM, et al. Harm reduction treatment for smoking (HaRT-S): findings from a single-arm pilot study with smokers experiencing chronic homelessness. Substance Abuse 2019;40(2):229-39. [DOI] [PubMed] [Google Scholar]
Hamner 2015 {published data only}
- Hamner MB. Smoking cessation in homeless veterans. Journal of Clinical Psychiatry 2015;76(7):e911-2. [DOI] [PubMed] [Google Scholar]
Hickman 2015 {published data only}
- Hickman NJ 3rd, Delucchi KL, Prochaska JJ. Treating tobacco dependence at the intersection of diversity, poverty, and mental Illness: a randomized feasibility and replication trial. Nicotine & Tobacco Research 2015;17(8):1012-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Hickman N, Gali K, Orozco N, Prochaska JJ. Maximizing retention with high risk participants in a clinical trial. American Journal of Health Promotion 2014;28(4):268-74. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyamathi 2012 {published data only}
- Nyamathi A, Branson C, Kennedy B, Salem B, Khalilifard F, Marfisee M, et al. Impact of nursing intervention on decreasing substances among homeless youth. American Journal on Addictions 2012;21(6):558-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Power 2015 {published data only}
- Power J, Mallat C, Bonevski B, Nielssen O. An audit of assessment and outcome of intervention at a quit smoking clinic in a homeless hostel. Australasian Psychiatry 2015;23(5):528-30. [DOI] [PubMed] [Google Scholar]
Santa Ana 2016 {published data only}
- Santa Ana EJ, LaRowe S, Hartwell KJ, Lamb K. Impact of group motivational interviewing on enhancing treatment engagement for homeless veterans with nicotine dependence and other substance use disorders. American Journal on Addictions 2016;25(7):533-41. [DOI: https://doi.org/10.1111/ajad.12426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Segan 2015 {published data only}
- Segan CJ, Maddox S, Borland R. Homeless clients benefit from smoking cessation treatment delivered by a homeless persons' program. Nicotine & Tobacco Research 2015;17(8):996-1001. [DOI: 10.1093/ntr/ntv062] [DOI] [PubMed] [Google Scholar]
Shadel 2015 {published data only}
- Shadel WG, Tucker JS, Golinelli D. Readjusting our priorities: helping homeless youth quit smoking. American Journal of Preventive Medicine 2015;49(6):970-3. [DOI] [PubMed] [Google Scholar]
Shelley 2010 {published data only}
- Shelley D, Cantrell J, Wong S, Warn D. Smoking cessation among sheltered homeless: a pilot. American Journal of Health Behavior 2010;34(5):544-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2016 {published data only}
- NCT01363245. Effectiveness of smoking-cessation interventions for urban hospital patients. clinicaltrials.gov/ct2/show/NCT01363245 (first received 1 June 2011).
- Rogers ES, Friedes R, Jakes A, Grossman E, Link A, Sherman SE. Long-term abstinence and predictors of tobacco treatment uptake among hospitalized smokers with serious mental illness enrolled in a smoking cessation trial. Journal of Behavioral Medicine 2017;40(5):750-9. [DOI: 10.1007/s10865-017-9844-0] [DOI] [PubMed] [Google Scholar]
- Sherman SE, Link AR, Rogers ES, Krebs P, Ladapo JA, Shelley DR, et al. Smoking-cessation interventions for urban hospital patients: a randomized comparative effectiveness trial. American Journal of Preventive Medicine 2016;51(4):566-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2015 {published data only}
- Tucker JS, Shadel WG, Golinelli D, Ewing B, Mullins L. Motivation to quit and interest in cessation treatment among homeless youth smokers. Nicotine & Tobacco Research 2015;17(8):990-5. [DOI] [PubMed] [Google Scholar]
Vijayaraghavan 2016a {published data only}
- Vijayaraghavan M, Guydish J, Pierce JP. Building tobacco cessation capacity in homeless shelters: a pilot study. Journal of Community Health 2016;41(5):998-1005. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Tucker 2020 {published and unpublished data}
- NCT03874585. Text messaging-based smoking cessation program for homeless youth. clinicaltrials.gov/ct2/show/NCT03874585 (first received 14 March 2019).
- Tucker JS, Pedersen ER, Linnemayr S, Shadel WG, DeYoreo M, Zutshi R. A text message intervention for quitting cigarette smoking among young adults experiencing homelessness: study protocol for a pilot randomized controlled trial. Addiction Science & Clinical Practice 2020;15(1):1-13. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ANHD 2018
- Association for Neighborhood and Housing Development. Growing Overcrowding, Growing Risk of Homelessness Shown in New Affordable Housing Neighborhood Risk Analysis. anhd.org/press-release/growing-overcrowding-growing-risk-homelessness-shown-new-affordable-housing (accessed 31 July 2019).
Apollonio 2005
- Apollonio DE, Malone RE. Marketing to the marginalised: tobacco industry targeting of the homeless and mentally ill. Tobacco Control 2005;14(6):409-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Apollonio 2016
- Apollonio DE, Philipps R, Bero LA. Interventions for tobacco use cessation in people in treatment for or recovery from substance use disorders. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD010274. [DOI: 10.1002/14651858.CD010274.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnsten 2004
- Arnsten JH, Reid K, Bierer M, Rigotti N. Smoking behavior and interest in quitting among homeless adults. Addictive Behaviors 2004;29(6):1155-61. [DOI] [PubMed] [Google Scholar]
Aveyard 2012
- Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction 2012;107(6):1066-73. [DOI: 10.1111/j.1360-0443.2011.03770.x] [DOI] [PubMed] [Google Scholar]
Baggett 2013a
- Baggett TP, Tobey ML, Rigotti NA. Tobacco use among homeless people - addressing the neglected addiction. New England Journal of Medicine 2013;369(3):201-4. [PMID: PMID: 23863048] [DOI] [PubMed] [Google Scholar]
Baggett 2013b
- Baggett TP, Hwang SW, O'Connell JJ, Porneala BC, Stringfellow EJ, Orav EJ, et al. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Internal Medicine 2013;173(3):189-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baggett 2013c
- Baggett TP, Lebrun-Harris LA, Rigotti NA. Homelessness, cigarette smoking and desire to quit: results from a US national study. Addiction 2013;108(11):2009-18. [PMID: PMID: 23834157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baggett 2015
- Baggett TP, Chang Y, Singer DE, Porneala BC, Gaeta JM, O'Connell JJ, et al. Tobacco-, alcohol-, and drug-attributable deaths and their contribution to mortality disparities in a cohort of homeless adults in Boston. American Journal of Public Health 2015;105(6):1189-97. [PMID: PMID: 25521869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baggett 2016a
- Bagget TP, Campbell EG, Chang Y, Rigotti NA. Other tobacco product and electronic cigarette use among homeless cigarette smokers. Addictive Behaviors 2016;60:124-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baggett 2016b
- Baggett TP, Campbell EG, Chang Y, Magid LM, Rigotti NA. Posttraumatic stress symptoms and their association with smoking outcome expectancies among homeless smokers in Boston. Nicotine & Tobacco Research 2016;18(6):1526-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baggett 2017
- Baggett TP, Chang Y, Yaqubi A, McGlave C, Higgins ST, Rigotti NA. Financial incentives for smoking abstinence in homeless smokers: a pilot randomised controlled trial. Nicotine & Tobacco Research 2018;20(12):1442-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Businelle 2014a
- Businelle MS, Ma P, Kendzor DE, Reitzel LR, Chen M, Lam CY, et al. Predicting quit attempts among homeless smokers seeking cessation treatment: an ecological momentary assessment study. Nicotine & Tobacco Research 2014;16(10):1371-8. [PMID: PMID: 24893602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009329. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2011
- Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Archives of Internal Medicine 2011;171(21):1901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catley 2016
- Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, et al. A randomized trial of motivational interviewing cessation induction among smokers with low desire to quit. American Journal of Preventive Medicine 2016;50(5):573-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016
- Chen JS, Nguyen AH, Malesker MA, Morrow LE. High-risk smoking behaviors and barriers to smoking cessation among homeless individuals. Respiratory Care 2016;61(5):640-5. [PMID: PMID: 26860400] [DOI] [PubMed] [Google Scholar]
Collins 2018
- Collins SE, Orfaly VE, Wu T, Chang S, Hardy R, Nash A, et al. Content analysis of homeless smokers ' perspectives on established and alternative smoking interventions. International Journal of Drug Policy 2018;51:10-17. [DOI] [PubMed] [Google Scholar]
Connor 2002
- Connor SE, Cook RL, Herbert MI, Neal SM, Williams JT. Smoking cessation in a homeless population: there is a will, but is there a way? Journal of General Internal Medicine 2002;17(5):369-72. [PMID: PMID: 12047734] [DOI] [PMC free article] [PubMed] [Google Scholar]
Council to Homeless Persons 2018
- Council to Homeless Persons. No room to breathe; why severe overcrowding is a form of homelessness. 2018. chp.org.au/no-room-to-breathe-why-severe-overcrowding-is-a-form-of-homelessness/ (accessed 31 July 2019).
Culhane 2013
- Culhane DP, Metraux S, Byrne T, Stino M, Bainbridge J. The age structure of contemporary homelessness: evidence and implications for public policy. Analysis of Social Issues and Public Policy 2013;13(1):228-44. [Google Scholar]
Dawkins 2019
- Dawkins L, Ford A, Bauld L, Balaban S, Tyler A, Cox S. A cross sectional survey of smoking characteristics and quitting behaviour from a sample of homeless adults in Great Britain. Addictive Behaviors 2019;95:35-40. [DOI] [PubMed] [Google Scholar]
Fazel 2008
- Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Medicine 2008;5:e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fazel 2014
- Fazel S, Geddes JR, Kushel M. The health consequences of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014;384(9953):1529-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garner 2013
- Garner L, Ratschen E. Tobacco smoking, associated risk behaviours, and experience with quitting: a qualitative study with homeless smokers addicted to drugs and alcohol. BMC Public Health 2013;13:951. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldade 2011
- Goldade K, Whemboula G, Thomas J, Eischen S, Guo H, Connett J, et al. Designing a smoking cessation intervention for the unique needs of homeless persons: a community-based randomized clinical trial. Clinical Trials 2011;8(6):744-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2019
- Hartmann‐Boyce J, Hong B, Livingstone‐Banks J, Wheat H, Fanshawe TR. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009670. [DOI: 10.1002/14651858.CD009670.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2020
- Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A et al. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. www.training.cochrane.org/handbook.
Hwang 2009
- Hwang SW, Wilkins R, Tjepkema M, O'Campo PJ, Dunn JR. Mortality among resident of shelters, rooming houses, and hotels in Canada: 11-year follow-up study. BMJ 2009;339:b4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamal 2015
- Jamal A, Homa DM, O'Conner E, Babb SD, Caraballo RS, Singh T, et al. Current cigarette smoking among adults - United States, 2005-2014. Morbidity and Mortality Weekly Report 2015;64:1233-40. [DOI] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD001292. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
McInnes 2013
- McInnes DK, Li AE, Hogan TP. Opportunities for engaging low-income, vulnerable populations in health care: a systematic review of homeless persons’ access to and use of information technologies. American Journal of Public Health 2013;103(Suppl 2):e11-24. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neisler 2018
- Neisler J, Retizel LR, Garey L, Kendzor DE, Hebert ET, Vijayaraghavan M, et al. Concurrent nicotine and tobacco product use among homeless smokers and associations with cigarette dependence and other factors related to quitting. Drug & Alcohol Dependence 2018;185:133-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2015
- Nguyen MA, Reitzel LR, Kendzor DE, Businelle MS. Perceived cessation treatment effectiveness, medication preferences, and barriers to quitting among light and moderate/heavy homeless smokers. Drug & Alcohol Dependence 2015;153:341-5. [PMID: PMID: 26072221] [DOI] [PubMed] [Google Scholar]
Notley 2019
- Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J. Incentives for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD004307. [DOI: 10.1002/14651858.CD004307.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okuyemi 2006a
- Okuyemi KS, Caldwell AR, Thomas JL, Born W, Richter KP, Nollen N, et al. Homelessness and smoking cessation: insights from focus groups. Nicotine & Tobacco Research 2006;8(2):287-96. [DOI] [PubMed] [Google Scholar]
Pratt 2019
- Pratt R, Pernat C, Kerandi L, Kmiecik A, Strobel-Ayres C, Joseph A, et al. Its a hard thing to manage when you're homeless: the impact of the social environment on smoking cessation for smokers experiencing homelessness. BMC Public Health 2019;19(1):635. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitzel 2011
- Reitzel LR, Cromley EK, Li Y, Cao Y, Dela Mater R, Mazas CA, et al. The effect of tobacco outlet density and proximity on smoking cessation. American Journal of Public Health 2011;101(2):315-20. [PMID: PMID: 21164089 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitzel 2014
- Reitzel LR, Nguyen N, Eischen S, Thomas J, Okuyemi KS. Is smoking cessation associated with worse comorbid substance use outcome among homeless adults? Addiction 2014;109(12):2098-104. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheibein 2020
- Scheibein F, McGirr K, Morrison A, Roche W, Wells J. An exploratory non-randomized study of 3-month electronic nicotine delivery system (ENDS) intervention with people accessing a homeless supported temporary accomodation services (STA) in Ireland. Harm Reduction Journal 2020;17(73):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroeder 2009
- Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annual Review of Public Health 2009;31:297-314. [DOI] [PubMed] [Google Scholar]
Shadel 2014
- Shadel WG, Tucker JS, Mullins L, Staplefoot L. Providing smoking cessation programs to homeless youth: the perspective of service providers. Journal of Substance Abuse Treatment 2014;47(4):251-7. [DOI] [PubMed] [Google Scholar]
Soar 2020
- Soar K, Dawking L, Robson D, Cox S. Smoking amongst adults experiencing homelessness: a systematic review of prevalence rates, interventions, barriers and facilitators to quitting and staying quit. Journal of Smoking Cessation 2020;15:94-108. [Google Scholar]
Stead 2016
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD008286. [DOI: 10.1002/14651858.CD008286.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijayaraghavan 2014
- Vijayaraghavan M, Penko J, Vittinghoff E, Bangsberg DR, Miaskowski C, Kushel MB. Smoking behaviours in a community-based cohort of HIV-infected indigent adults. AIDS and Behavior 2014;18(3):535-43. [PMID: PMID: 23918243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijayaraghavan 2015
- Vijayaraghavan M, Pierce JP. Interest in smoking cessation related to a smoke-free policy among homeless adults. Journal of Community Health 2015;40(4):686-91. [PMID: PMID: 25559109 ] [DOI] [PubMed] [Google Scholar]
Vijayaraghavan 2016b
- Vijayaraghavan M, Hurst S, Pierce P. Implementing tobacco control programs in homeless shelters: a mixed-methods study. Health Promotion Practice 2016;17(4):501-11. [PMID: PMID: 26678988] [DOI] [PubMed] [Google Scholar]
Vijayaraghavan 2016c
- Vijayaraghavan M, Tieu L, Ponath C, Guzman D, Kushel M. Tobacco cessation behaviors among older homeless adults: results from the HOPE HOME study. Nicotine & Tobacco Research 2016;18(8):1733-9. [PMID: PMID: 26920648] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijayaraghavan 2017
- Vijayaraghavan M, Hurst S, Pierce JP. A qualitative examination of smoke-free policies and electronic cigarettes among sheltered homeless adults. American Journal of Health Promotion 2017;31(3):243-50. [PMID: PMID: 26559719] [DOI] [PubMed] [Google Scholar]
Vijayaraghavan 2018
- Vijayaraghavan M, Olsen P, Weeks J, McKelvey L, Ponath C, Kushel M. Older African American homeless-experienced smokers’ attitudes toward tobacco control policies - results from the HOPE HOME study. American Jornal of Health Promotion 2018;32(2):381-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2017
- Wilson A, Guillaumier A, George J, Denham A, Bonevski B. A systematic narrative review of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups (2010-2017). Expert Review of Respiratory Medicine 2017;11(8):617-30. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Vijayaraghavan 2019
- Vijayaraghavan M, Elser H, Apollonio D. Interventions to reduce tobacco use in people experiencing homelessness. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013413. [DOI: 10.1002/14651858.CD013413] [DOI] [PMC free article] [PubMed] [Google Scholar]


