Abstract
Background
Antibiotics provide only modest benefit in treating sore throat, although their effectiveness increases in people with positive throat swabs for group A beta‐haemolytic streptococci (GABHS). It is unclear which antibiotic is the best choice if antibiotics are indicated. This is an update of a review first published in 2010, and updated in 2013, 2016, and 2020.
Objectives
To assess the comparative efficacy of different antibiotics in: (a) alleviating symptoms (pain, fever); (b) shortening the duration of the illness; (c) preventing clinical relapse (i.e. recurrence of symptoms after initial resolution); and (d) preventing complications (suppurative complications, acute rheumatic fever, post‐streptococcal glomerulonephritis). To assess the evidence on the comparative incidence of adverse effects and the risk‐benefit of antibiotic treatment for streptococcal pharyngitis.
Search methods
We searched the following databases up to 3 September 2020: CENTRAL (2020, Issue 8), MEDLINE Ovid (from 1946), Embase Elsevier (from 1974), and Web of Science Thomson Reuters (from 2010). We also searched clinical trial registers on 3 September 2020.
Selection criteria
Randomised, double‐blind trials comparing different antibiotics, and reporting at least one of the following: clinical cure, clinical relapse, or complications and/or adverse events.
Data collection and analysis
Two review authors independently screened trials for inclusion and extracted data using standard methodological procedures as recommended by Cochrane. We assessed the risk of bias of included studies according to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions, and used the GRADE approach to assess the overall certainty of the evidence for the outcomes. We have reported the intention‐to‐treat analysis, and also performed an analysis of evaluable participants to explore the robustness of the intention‐to‐treat results.
Main results
We included 19 trials reported in 18 publications (5839 randomised participants): six trials compared penicillin with cephalosporins; six compared penicillin with macrolides; three compared penicillin with carbacephem; one compared penicillin with sulphonamides; one compared clindamycin with ampicillin; and one compared azithromycin with amoxicillin in children. All participants had confirmed acute GABHS tonsillopharyngitis, and ages ranged from one month to 80 years. Nine trials included only, or predominantly, children. Most trials were conducted in an outpatient setting. Reporting of randomisation, allocation concealment, and blinding was poor in all trials. We downgraded the certainty of the evidence mainly due to lack of (or poor reporting of) randomisation or blinding, or both; heterogeneity; and wide confidence intervals.
Cephalosporins versus penicillin
We are uncertain if there is a difference in symptom resolution (at 2 to 15 days) for cephalosporins versus penicillin (odds ratio (OR) for absence of symptom resolution 0.79, 95% confidence interval (CI) 0.55 to 1.12; 5 trials; 2018 participants; low‐certainty evidence). Results of the sensitivity analysis of evaluable participants differed (OR 0.51, 95% CI 0.27 to 0.97; 5 trials; 1660 participants; very low‐certainty evidence). We are uncertain if clinical relapse may be lower for cephalosporins compared with penicillin (OR 0.55, 95% CI 0.30 to 0.99; number needed to treat for an additional beneficial outcome (NNTB) 50; 4 trials; 1386 participants; low‐certainty evidence). Very low‐certainty evidence showed no difference in reported adverse events.
Macrolides versus penicillin
We are uncertain if there is a difference between macrolides and penicillin for resolution of symptoms (OR 1.11, 95% CI 0.92 to 1.35; 6 trials; 1728 participants; low‐certainty evidence). Sensitivity analysis of evaluable participants resulted in an OR of 0.79, 95% CI 0.57 to 1.09; 6 trials; 1159 participants). We are uncertain if clinical relapse may be different (OR 1.21, 95% CI 0.48 to 3.03; 6 trials; 802 participants; low‐certainty evidence).
Azithromycin versus amoxicillin
Based on one unpublished trial in children, we are uncertain if resolution of symptoms is better with azithromycin in a single dose versus amoxicillin for 10 days (OR 0.76, 95% CI 0.55 to 1.05; 1 trial; 673 participants; very low‐certainty evidence). Sensitivity analysis for per‐protocol analysis resulted in an OR of 0.29, 95% CI 0.11 to 0.73; 1 trial; 482 participants; very low‐certainty evidence). We are also uncertain if there was a difference in relapse between groups (OR 0.88, 95% CI 0.43 to 1.82; 1 trial; 422 participants; very low‐certainty evidence). Adverse events were more common with azithromycin compared to amoxicillin (OR 2.67, 95% CI 1.78 to 3.99; 1 trial; 673 participants; very low‐certainty evidence).
Carbacephem versus penicillin
There is low‐certainty evidence that compared with penicillin, carbacephem may provide better symptom resolution post‐treatment in adults and children (OR 0.70, 95% CI 0.49 to 0.99; NNTB 14.3; 3 trials; 795 participants).
Studies did not report on long‐term complications, so it was unclear if any class of antibiotics was better in preventing serious but rare complications.
Authors' conclusions
We are uncertain if there are clinically relevant differences in symptom resolution when comparing cephalosporins and macrolides with penicillin in the treatment of GABHS tonsillopharyngitis. Low‐certainty evidence in children suggests that carbacephem may be more effective than penicillin for symptom resolution. There is insufficient evidence to draw conclusions regarding the other comparisons in this review. Data on complications were too scarce to draw conclusions. These results do not demonstrate that other antibiotics are more effective than penicillin in the treatment of GABHS pharyngitis. All studies were conducted in high‐income countries with a low risk of streptococcal complications, so there is a need for trials in low‐income countries and Aboriginal communities, where the risk of complications remains high.
Plain language summary
Different antibiotics for group A streptococcal pharyngitis
Review question
We wanted to know which antibiotic was more effective in treating sore throats caused by bacteria (group A beta‐haemolytic streptococci (GABHS)).
Background
Most sore throats are caused by viruses, but many people carry throat bacteria, which sometimes causes bacterial throat infection.
GABHS infection can have serious complications including rheumatic fever and kidney disease. Antibiotics are often prescribed to prevent complications, but provide modest benefit for sore throat, even if GABHS are present. Most throat infections resolve without treatment, and complication risks are extremely low for most people in high‐income countries. However, sometimes antibiotics are needed. Penicillin, an inexpensive antibiotic, has been used to treat GABHS for many years. GABHS resistance to penicillin is rare.
Search date
We searched the literature to 3 September 2020.
Study characteristics
We included 19 trials (18 publications) that involved 5839 people. The included trials studied different antibiotics for people with sore throat who tested positive for GABHS, and were aged from one month to 80 years. Nine trials included only children, and 10 trials included people aged 12 years or older. Most studies were published over 15 years ago, and all but one reported on outcome measures relevant for patients.
Study funding sources
Twelve trials reported funding from drug companies. Authors of six trials (in five publications) were employed by drug companies. Seven trials (in six publications) did not report funding sources.
Key results
Antibiotic effects were similar, and all antibiotics caused side effects (such as nausea and vomiting, rash), but there was no strong evidence to show meaningful differences between antibiotics. Studies did not report on long‐term complications, therefore it was unclear if any class of antibiotics was better in preventing serious but rare complications.
All studies were performed in high‐income countries with a low risk of streptococcal complications, so there is a need for trials in low‐income countries and Aboriginal communities, where the risk of complications remains high. Our review supports the use of penicillin as a first‐choice antibiotic in people with throat infections caused by GABHS.
Certainty of the evidence
We judged the certainty of the evidence as low or very low for all outcomes when macrolides or cephalosporins were compared with penicillin. We have concerns about the rigour of the study methods, the fact that estimates were not very precise and about the differences between studies.
Summary of findings
Background
Description of the condition
Pharyngitis is a common upper respiratory tract infection for which antibiotics are often prescribed. Patients usually consult a physician with the complaint of sore throat. A previous Cochrane Review comparing the effect of antibiotics to placebo in participants with or without GABHS sore throat pointed to the self‐limiting nature of an acute sore throat (even in cases of positive GABHS culture) (Spinks 2013).
Description of the intervention
Antibiotics provide only modest benefit when prescribed for sore throat (Spinks 2013). The effect of antibiotic treatment was increased in participants with positive throat swabs for GABHS. Only a small proportion of individuals with a sore throat are streptococci‐positive. Nevertheless, in many countries antibiotics are prescribed for most people who have a sore throat (Cars 2001; Linder 2001). Given the high consumption of antibiotics for this condition, a rational approach would be to reserve treatment with antibiotics for those with proven presence, or a high likelihood of GABHS (Cooper 2001; Snow 2001). However, clinical scoring systems are somewhat limited in their ability to correctly target GABHS‐positive patients (McIsaac 1998), and the usefulness of rapid assay tests depends on the prevalence of GABHS in the population (Sonnad 1999). Justification of its cost‐effectiveness is unclear (Gerber 2004; Neuner 2003). The slight benefit of treatment with antibiotics in patients with GABHS sore throat may be considered relevant. When antibiotics are indicated, a choice needs to be made.
How the intervention might work
When prescribing an antibiotic, several factors need to be considered, such as the comparative benefit‐harm balance, costs, and local antimicrobial resistance patterns. Many guidelines recommend penicillin as a first choice, with erythromycin preferred for people who are allergic to penicillin (Cooper 2001; Snow 2001). To date, resistance of GABHS to penicillin has only been documented incidentally (Devi 2011; Gerber 2009; Ibrahim 2014), and resistance to erythromycin is still low (Cooper 2001). Considering the growing problem of antibiotic resistance for other pathogens, this responsiveness of GABHS should not be endangered (Wise 1998). Penicillin and erythromycin are inexpensive and the most cost‐effective option. Despite this, physicians continue to prescribe broad‐spectrum antibiotics, including recently marketed ones. It is not clear if these antibiotics have any substantial clinical benefit over penicillin (and erythromycin).
Why it is important to do this review
Antimicrobial resistance is a global emergency warranting the judicious and appropriate use of antibiotics, especially for self‐limiting conditions (O'Neill 2016). International guidelines recommend using penicillin as the first choice when choosing to treat people with an acute sore throat (suspected to be caused by GABHS) with antibiotics (eTG 2019; Matthys 2007). However, some argue that cephalosporins are more effective and should therefore be preferred (Casey 2004). Many physicians argue that the occurrence of penicillin allergy should be taken into account when choosing an antibiotic. In this review we sought evidence of penicillin allergy in the available trials. In addition, in the presence of documented penicillin allergy, the side effect profile of eligible antibiotics can guide choice. The burden of GABHS is higher in some communities, such as low‐income countries, or first nations populations in Australia. Appropriate treatment in the context of strong antimicrobial stewardship is needed to provide healthcare providers with sufficient information to make an evidence‐based choice (May 2016). Both treatment benefits and adverse events need to be compared and taken into account.
Objectives
To assess the comparative efficacy of different antibiotics in: (a) alleviating symptoms (pain, fever); (b) shortening the duration of the illness; (c) preventing clinical relapse (i.e. recurrence of symptoms after initial resolution); and (d) preventing complications (suppurative complications, acute rheumatic fever, post‐streptococcal glomerulonephritis). To assess the evidence on the comparative incidence of adverse effects and the risk‐benefit of antibiotic treatment for streptococcal pharyngitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind, controlled trials comparing at least two different classes of antibiotics, and reporting at least one of the following: clinical cure, clinical relapse, or complications and/or adverse events.
Types of participants
Adults and children of all ages presenting with symptoms of sore throat and with an infection caused by GABHS confirmed by a throat culture, rapid test, or both.
Types of interventions
Antibiotics of one class compared with another class.
Types of outcome measures
The focus was on outcome measures relevant for patients.
Primary outcomes
Resolution of symptoms (cure or improvement of signs and symptoms, which could include sore throat, fever, feeling ill, etc.) post‐treatment.
Secondary outcomes
Sore throat.
Fever.
Duration of illness.
Incidence of relapse.
Incidence of complications (suppurative complications, acute rheumatic fever, post‐streptococcal glomerulonephritis).
Adverse events.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 8), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register (searched 3 September 2020), MEDLINE Ovid (1946 to 3 September 2020), Embase Elsevier (1974 to 3 September 2020), and Web of Science Thomson Reuters (2010 to 3 September 2020). We also searched clinical trials registers: the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/default.aspx) and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) for completed and ongoing trials. We used the terms streptococcal AND pharyngitis (latest search 3 September 2020). Search strategies for previous versions of the review are presented in Appendix 1. Details of the current search strategy for MEDLINE and CENTRAL are in Appendix 2; for Embase in Appendix 3; and for Web of Science in Appendix 4.
We did not impose any language or publication restrictions.
Searching other resources
We also searched the reference sections of the identified reviews and trials for additional trials; independent sources of drug information (journals of the International Society of Drug Bulletins (electronically and by hand)); and proceedings of meetings and conferences for additional references to trials. We contacted pharmaceutical companies producing antibiotics applied in the treatment of pharyngitis for published or unpublished trials on their products, and experts in the field for additional references.
Data collection and analysis
Selection of studies
In this update two review authors (MVD, ADS) independently assessed all trials identified by the search with relevant titles or abstracts, or both, to determine which potentially met the inclusion criteria. We reviewed the full texts of these potentially eligible papers to assess them for inclusion in our review. We excluded all trials that did not meet our inclusion criteria. We list trials that were assessed for inclusion by reading the full texts but subsequently excluded in the Characteristics of excluded studies table. We reported the search results in a PRISMA flow diagram (Figure 1).
1.
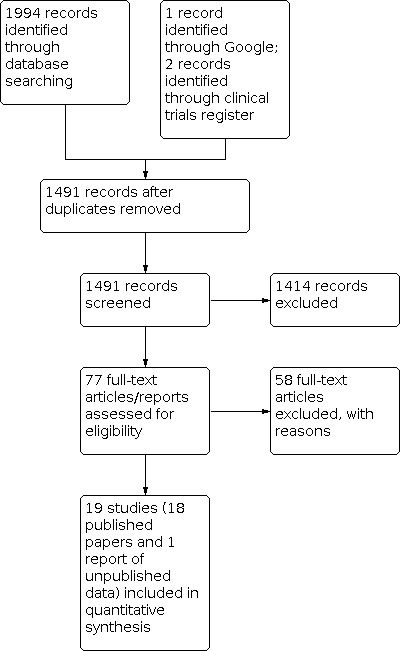
Study flow diagram.
Data extraction and management
In this update two review authors (MVD, ADS) independently extracted data in pairs, using a standard checklist developed specifically for the review. The data extraction form included the following general information: published/unpublished, title, authors, source, contact address, country, language of publication, year of publication, duplicate publications, sponsoring, and setting. It also included data on the following categories.
Methods: randomisation procedure, allocation, blinding (participants, people administering treatment, outcome assessors), duration of study, design, analysis (intention‐to‐treat (ITT)).
Participants: number, age, diagnostic criteria, history, baseline characteristics.
Interventions: dose, route, timing, duration; comparison group.
Outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow‐up.
Results: for outcomes and times of assessment (including a measure of variation).
Assessment of risk of bias in included studies
In this update two review authors (MVD, ADS) independently assessed the methodological quality of the included trials using Cochrane's 'Risk of bias' tool (Higgins 2011). We assessed risk of bias for the following domains: selection bias (random number generation and allocation concealment), performance and detection bias (blinding), attrition bias (incomplete outcome data), and reporting bias (selective reporting). We assessed studies as low risk of bias (methods clearly described and deemed adequate); high risk of bias (methods described and inadequate or not described and deemed likely to be inadequate); or unclear risk of bias (insufficient information to assess the methods, but no obvious indication for use of inadequate methods).
Measures of treatment effect
For dichotomous outcomes, we expressed results as odds ratios (ORs) with 95% confidence intervals (CIs). For statistically significant results, we calculated number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) where possible.
Unit of analysis issues
We did not include any cluster‐randomised studies. All included studies reported outcomes at the level of the randomised unit, the individual participant.
Dealing with missing data
We assessed the impact of missing data on the overall outcome of the meta‐analysis by comparing analysis of on‐treatment (or evaluable participants) and ITT data.
Assessment of heterogeneity
We assessed heterogeneity amongst trials by calculating a Chi² test (significance defined as P < 0.10) and I² statistic (Higgins 2003).
Assessment of reporting biases
We did not identify a sufficient number of studies to assess the presence of publication bias by means of a funnel plot.
Data synthesis
We pooled dichotomous data using a random‐effects model (DerSimonian 1986). In the absence of statistical heterogeneity (using a cut‐off point of I² < 20%), we also pooled data using the fixed‐effect model and compared results (Mantel 1959). We used Review Manager 5 software for pooling (Review Manager 2020).
We performed analyses according to ITT analysis, meaning that the number of participants randomised was used as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. The outcome of relapse incidence was analysed by including only evaluable participants; an ITT analysis would have seriously overestimated the importance of relapse, and results would not be relevant to clinical practice.
Subgroup analysis and investigation of heterogeneity
We stratified the trials into subcategories according to the comparisons between different classes of antibiotics. We performed subgroup analyses for trials with children versus adults.
Sensitivity analysis
We assessed the impact of missing data by performing analyses of on‐treatment (or evaluable) participants and comparing results with the ITT analyses. We assessed the impact of heterogeneity on the overall effect estimate by first pooling all studies and subsequently removing studies one by one, starting with the studies that appeared (by inspection of the forest plot) to be contributing to the heterogeneity. A meaningful sensitivity analysis of the impact of heterogeneity was only possible for resolution of symptoms in the comparison of cephalosporins versus penicillin.
Summary of findings and assessment of the certainty of the evidence
We created four 'Summary of findings' tables for the following comparisons: cephalosporins versus penicillin (Table 1; Analysis 1.1; Analysis 1.2; Analysis 1.7; Analysis 1.9); macrolides versus penicillin (Table 2; Analysis 2.1; Analysis 2.2; Analysis 2.6; Analysis 2.7); azithromycin versus amoxicillin (Table 3; Analysis 3.1; Analysis 3.3; Analysis 3.5); and carbacephem versus penicillin (Table 4; Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4). We used the GRADE approach to assess the overall certainty of evidence for the pooled studies (Atkins 2004), employing GRADEpro GDT software (GRADEpro GDT). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We assessed the certainty of evidence for the primary outcome (resolution of symptoms, both ITT and evaluable participant analysis) and secondary outcomes (incidence of relapse and incidence of adverse events). We justified all decisions to down‐ or upgrade the certainty of the evidence using footnotes to aid the reader's understanding of the review where necessary.
Summary of findings 1. Cephalosporins versus penicillin for group A streptococcal pharyngitis.
| Cephalosporinsversus penicillin for group A streptococcal pharyngitis | ||||||
| Patient or population: group A streptococcal pharyngitis Setting: outpatients Intervention: cephalosporin Comparison: penicillin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with penicillin | Risk with cephalosporin | |||||
| Resolution of symptoms post‐treatment (ITT analysis) | Study population | OR 0.79 (0.55 to 1.12) | 2018 (5 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcome measured at 2 to 15 days or more post‐treatment. Subgroup analyses: Adults: OR 0.78 (0.60 to 1.01; 2 trials; 1163 participants; low‐certainty evidence) Children: OR 0.83 (0.40 to 1.73; 3 trials; 855 participants; very low‐certainty evidence) Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 245 per 1000 | 204 per 1000 (151 to 267) | |||||
| Resolution of symptoms post‐treatment (evaluable participants) | Study population | OR 0.51 (0.27 to 0.97) | 1660 (5 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | Outcome measured at 2 to 15 days or more post‐treatment. Note: The 'evaluable participants' analysis includes only those randomised participants for whom an outcome was reported. |
|
| 112 per 1000 | 60 per 1000 (33 to 109) | |||||
| Incidence of relapse (evaluable participants) | Study population | OR 0.55 (0.30 to 0.99) | 1386 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcome measured at 17 to 90 days post‐treatment. Note: The 'evaluable participants' analysis includes only those randomised participants for whom an outcome was reported. |
|
| 46 per 1000 | 26 per 1000 (14 to 45) | |||||
| Adverse events (ITT analysis) | Study population | OR 0.94 (0.27 to 3.25) | 1279 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. | |
| 193 per 1000 | 184 per 1000 (61 to 438) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level due to unclear randomisation and blinding. bDowngraded 1 level due to wide confidence intervals. cDowngraded 1 level due to heterogeneity.
1.1. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 1: Resolution of symptoms post‐treatment (ITT analysis)
1.2. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 2: Resolution of symptoms post‐treatment (evaluable participants)
1.7. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 7: Incidence of relapse (evaluable participants)
1.9. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 9: Adverse events (ITT analysis)
Summary of findings 2. Macrolides versus penicillin for group A streptococcal pharyngitis.
| Macrolides versus penicillin for group A streptococcal pharyngitis | ||||||
| Patient or population: group A streptococcal pharyngitis Settings: outpatients Intervention: macrolide Comparison: penicillin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with penicillin | Risk with macrolide | |||||
| Resolution of symptoms post‐treatment (ITT analysis) | Study population | OR 1.11 (0.92 to 1.35) | 1728 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcome measured at 2 to 20 days post‐treatment. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 423 per 1000 | 448 per 1000 (402 to 497) | |||||
| Resolution of symptoms post‐treatment (evaluable participants) | Study population | OR 0.79 (0.57 to 1.09) | 1159 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcome measured at 2 to 20 days post‐treatment. Note: The 'evaluable participants' analysis includes only those randomised participants for whom an outcome was reported. |
|
| 172 per 1000 | 141 per 1000 (106 to 185) | |||||
| Incidence of relapse (evaluable participants) | Study population | OR 1.21 (0.48 to 3.03) | 802 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcome measured between 15 and 56 days post‐treatment. Note: The 'evaluable participants' analysis includes only those randomised participants for whom an outcome was reported. |
|
| 44 per 1000 | 53 per 1000 (22 to 123) | |||||
| Adverse events (ITT analysis) | Study population | OR 1.19 (0.82 to 1.73) | 1727 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | A subgroup analysis based on 1 trial with 489 participants shows that children experienced more adverse events with macrolides compared with penicillin (OR 2.33, 95% CI 1.06 to 5.15). However, the test for subgroup differences was not significant. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 324 per 1000 | 363 per 1000 (282 to 453) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level due to unclear randomisation. bDowngraded 1 level due to wide confidence intervals.
2.1. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 1: Resolution of symptoms post‐treatment (ITT analysis)
2.2. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 2: Resolution of symptoms post‐treatment (evaluable participants only)
2.6. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 6: Incidence of relapse (evaluable participants)
2.7. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 7: Adverse events (ITT analysis)
Summary of findings 3. Azithromycin versus amoxicillin for group A streptococcal pharyngitis.
| Azithromycin versus amoxicillin for group A streptococcal pharyngitis | ||||||
| Patient or population: group A streptococcal pharyngitis Setting: paediatric outpatient departments Intervention: azithromycin Comparison: amoxicillin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with amoxicillin | Risk with azithromycin | |||||
| Clinical cure (ITT analysis) | Study population | OR 0.76 (0.55 to 1.05) | 673 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Outcomes measured at 24 to 28 days after commencing treatment. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 351 per 1000 | 291 per 1000 (229 to 362) | |||||
| Clinical cure (bacteriological per protocol analysis) | Study population | OR 0.29 (0.11 to 0.73) | 482 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Outcomes measured at 24 to 28 days after commencing treatment. Note: The 'bacteriological per protocol population' was defined as those with GABHS‐positive culture within 48 hours of treatment start, at least eight days of treatment (compliance), and available data at baseline. |
|
| Relapse (ITT analysis) | Study population | OR 0.75 (0.55 to 1.02) | 673 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Outcomes measured at 38 to 45 days after commencing treatment. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 455 per 1000 | 385 per 1000 (315 to 460) | |||||
| Relapse (bacteriological per protocol analysis) | Study population | OR 0.88 (0.43 to 1.82) | 422 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Outcomes measured at 38 to 45 days after commencing treatment. Note: The 'bacteriological per protocol population' was defined as those with GABHS‐positive culture within 48 hours of treatment start, at least eight days of treatment (compliance), and available data at baseline. |
|
| Adverse events (ITT analysis) | Study population | OR 2.67 (1.78 to 3.99) | 673 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Outcomes measured at 38 to 45 days after commencing treatment. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 125 per 1000 | 276 per 1000 (203 to 363) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level due to unclear randomisation. bDowngraded 1 level due to wide confidence interval. cDowngraded 1 level due to unpublished data only.
3.1. Analysis.

Comparison 3: Azithromycin versus amoxicillin, Outcome 1: Clinical cure at 24 to 28 days (ITT)
3.3. Analysis.

Comparison 3: Azithromycin versus amoxicillin, Outcome 3: Relapse on day 38 to 45 (ITT)
3.5. Analysis.

Comparison 3: Azithromycin versus amoxicillin, Outcome 5: Adverse events (all participants)
Summary of findings 4. Carbacephem versus penicillin for group A streptococcal pharyngitis.
| Carbacephem versus penicillin for group A streptococcal pharyngitis | ||||||
| Patient or population: group A streptococcal pharyngitis Setting: outpatients Intervention: carbacephem Comparison: penicillin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with penicillin | Risk with carbacephem | |||||
| Resolution of symptoms post‐treatment (ITT analysis) | Study population | OR 0.70 (0.49 to 0.99) | 795 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcomes measured at 3 to 6 days post‐treatment. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 381 per 1000 | 301 per 1000 (232 to 379) | |||||
| Resolution of symptoms post‐treatment (evaluable participants) | Study population | OR 0.62 (0.38 to 1.01) | 602 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcomes measured at 3 to 6 days post‐treatment. Note: The 'evaluable participants' analysis includes only those randomised participants for whom an outcome was reported. |
|
| 160 per 1000 | 106 per 1000 (67 to 161) | |||||
| Incidence of relapse (evaluable participants) | Study population | OR 1.27 (0.64 to 2.50) | 523 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcomes measured at 28 to 45 days post‐treatment. Note: The 'evaluable participants' analysis includes only those randomised participants for whom an outcome was reported. |
|
| 63 per 1000 | 78 per 1000 (41 to 143) | |||||
| Adverse events (ITT analysis) | Study population | OR 1.08 (0.75 to 1.55) | 795 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | Outcomes measured at 28 to 45 days post‐treatment. Note: The ITT analysis uses the number of participants randomised as the denominator for each outcome. We considered the participants for whom an outcome was not reported as treatment failures. |
|
| 178 per 1000 | 189 per 1000 (140 to 251) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level due to unclear randomisation. bDowngraded 1 level due to wide confidence interval.
4.1. Analysis.

Comparison 4: Carbacephem versus penicillin, Outcome 1: Resolution of symptoms post‐treatment (ITT analysis)
4.2. Analysis.

Comparison 4: Carbacephem versus penicillin, Outcome 2: Resolution of symptoms post‐treatment (evaluable participants)
4.3. Analysis.

Comparison 4: Carbacephem versus penicillin, Outcome 3: Incidence of relapse (evaluable participants)
4.4. Analysis.

Comparison 4: Carbacephem versus penicillin, Outcome 4: Adverse events (ITT analysis)
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We retrieved 136 search results from our electronic searches in July 2010, van Driel 2010, and 249 in October 2012, van Driel 2013, but no new trials were included. In the 2016 update we retrieved 474 records from our electronic searches until March 2016, and included one new trial (van Driel 2016). In this 2020 update we retrieved 629 records from our electronic searches to 3 September 2020. We did not identify any new trials for inclusion.
We identified one additional trial through a Google search (Muller 1992). We identified two references to completed (unpublished) studies on ClinicalTrials.gov in the 2016 search (NCT00393744; NCT00643149).
We reviewed a total of 77 trials for this review. Of these, 21 met the predefined inclusion criteria. Two of the 21 papers reported different outcomes of the same study and were considered as one single study (Norrby 2002). The unpublished report of one study registered and marked as completed on ClinicalTrials.gov was made available by Pfizer upon request in 2013 and was included in the 2016 update (NCT00643149). Of the two additional studies that we identified in the March 2016 search, we excluded one (Stillerman 1970), and one which was initially available in abstract form only was excluded after review of the full paper in the 2020 update (Eslami 2014). See the PRISMA flow diagram (Figure 1) (Moher 2009).
Included studies
We included 18 trials in the first version of this review (van Driel 2010). Henness 1982 reported two separate trials, and we split this into two parts to clarify which trial was assessed (Henness 1982‐study 1; Henness 1982‐study 2). We identified one new study in the 2012 update (NCT00643149). We did not add any new studies in the 2016 or this 2020 update. As data became available we included the unpublished study (NCT00643149) in this 2020 update, resulting in a total of 19 trials in the current review. Most of the included trials were conducted in the 1990s; three were conducted in the 1980s (Henness 1982‐study 1; Henness 1982‐study 2; Randolph 1985); and two in the 1970s (Jackson 1973; Trickett 1973). Only two trials were more recent (NCT00643149; Norrby 2002). All but one trial reported clinical outcome (Henness 1982‐study 2).
Contacting pharmaceutical companies did not result in any additional published or unpublished data (only one company replied), nor did contacting authors or experts in the field. We identified the NCT00643149 study through searching a clinical trials register, and we subsequently obtained a report from the manufacturer.
All but two of the included studies compared penicillin with another antibiotic class. Henness 1982 compared penicillin V with cefadroxil in both study 1 and study 2, but added two additional study arms in study 2 (erythromycin, benzathine penicillin G/procaine penicillin). Jackson 1973 compared clindamycin with ampicillin, and NCT00643149 compared azithromycin with amoxicillin.
The included trials investigated a total of 5839 randomised participants with acute GABHS tonsillopharyngitis. Participants' ages ranged from one month to 80 years. Nine trials included only, or predominantly, children (Disney 1992a; Disney 1992b; Henness 1982‐study 1; Henness 1982‐study 2; Jackson 1973; NCT00643149; O'Doherty 1996; Randolph 1985; Reed 1991). Ten trials included participants who were at least 12 years of age or older (Bachand 1991; Carbon 1995; Levenstein 1991; McCarty 1992a; Muller 1992; Nemeth 1999; Norrby 2002; Stein 1991; Trickett 1973; Watkins 1997). In Reed 1991, approximately 80% of participants were under 15 years of age, and were therefore included in the subgroup analysis for children. In Muller 1992, 90% of participants were aged over 12 years; however, because results were not stratified by age group, this study was included in the adult subgroup analysis.
All of the included trials involved only participants with confirmed acute GABHS tonsillopharyngitis. Confirmation of the presence of GABHS in participants with clinical signs of tonsillopharyngitis was mostly performed first by a rapid immunoassay test and reconfirmed with a throat culture. In five trials, the confirmation of GABHS tonsillopharyngitis was carried out only by a throat culture (Henness 1982‐study 1; Henness 1982‐study 2; Jackson 1973; Randolph 1985; Trickett 1973), and in two trials only with a rapid immunoassay test (O'Doherty 1996; Stein 1991). All but one trial (Henness 1982‐study 2) reported on clinical outcomes. Trickett 1973 reported only bacteriological outcomes to assess efficacy, but was included in the meta‐analysis on adverse effects.
Clinical outcomes, in most studies defined as complete resolution of signs and symptoms (Characteristics of included studies), were assessed at various time points, but mostly measured between five to 10 days following the end of antibiotic treatment. Consequently, post‐treatment the outcome 'post‐treatment clinical efficacy' (i.e. assessment of signs and symptoms after completion of the treatment course) was pooled. Randolph 1985 reported clinical effect within the first 24 hours of treatment. NCT00643149 assessed clinical effects on days 24 to 28 after starting the study drug. Three trials reported on specific symptoms, such as sore throat and fever (Bachand 1991; Levenstein 1991; Randolph 1985). No studies reported data on the duration of illness. Henness 1982‐study 2 did not report any clinical outcomes.
Twelve trials reported the incidence of clinical relapse (Bachand 1991; Carbon 1995; Disney 1992a; Disney 1992b; Levenstein 1991; McCarty 1992a; Muller 1992; Nemeth 1999; Norrby 2002; O'Doherty 1996; Reed 1991; Stein 1991). The definition of clinical relapse varied slightly, from "pretreatment signs and symptoms resolved but reappeared", Bachand 1991; Carbon 1995; Disney 1992b; Levenstein 1991; McCarty 1992a; Muller 1992; Nemeth 1999; Norrby 2002; Stein 1991, or "initial improvement or alleviation of symptoms, but subsequent worsening or recurrence", McCarty 1992a; Watkins 1997, to "new infection with different serotype" (Disney 1992a). One study defined clinical cure as "clinical improvement within first 24 hours of therapy and all follow‐up cultures no S pyogenes" (Henness 1982‐study 1). Two studies used the physician's assessment of symptoms as outcome (Randolph 1985; Reed 1991).
Four trials reported complications occurring during longer follow‐up (Carbon 1995; Jackson 1973; McCarty 1992a; Muller 1992). Fifteen trials mentioned adverse effects reported during treatment. Jackson 1973 only reported bacteriological outcomes and clinical adverse events.
The use of antipyretic analgesics was allowed in four trials (Bachand 1991; Disney 1992b; Muller 1992; Watkins 1997), prohibited in two (Carbon 1995; Randolph 1985), and not stated in the other 13 trials.
The percentage of participants who dropped out before outcome measurement varied. Some trials seemed not to have any dropouts (Henness 1982‐study 1; Henness 1982‐study 2; Randolph 1985), or lost 20% or fewer of the randomised participants at the time of outcome evaluation (Carbon 1995; Disney 1992b; Jackson 1973; Levenstein 1991; NCT00643149; Norrby 2002; Reed 1991). Six studies reported dropout rates of between 20% and 30% (Bachand 1991; McCarty 1992a; Muller 1992; Nemeth 1999; O'Doherty 1996; Stein 1991), and in Watkins 1997, reportedly 38% of participants dropped out before the end of the study. The most commonly reported reason for dropout was negative culture for GABHS.
Excluded studies
We excluded 58 studies. The most common reason for exclusion (38 trials) was no or inadequate blinding (Adam 1994; Adam 1995; Adam 1996; Adam 2000a; Adam 2000b; Adam 2001; Aujard 1995; Bottaro 2012; Cohen 2002; Denny 1953; Dykhuizen 1996; Eslami 2014; Esposito 2002; Feder 1999; Gerber 1986; Gooch 1993; Hamill 1993; Holm 1991; Howe 1997; Kuroki 2013; Lennon 2008; McCarty 1992b; McCarty 1994; Milatovic 1991; Milatovic 1993; NCT00393744; Pacifico 1996; Perkins 1969; Pichichero 2000; Pichichero 2008; Portier 1990; Portier 1994; Sakata 2008; Shapera 1973; Shvartzman 1993; Stillerman 1986; Tack 1997; Tack 1998; Uysal 2000). Seven trials did not compare at least two different classes of antibiotics (Breese 1974; Disney 1979; Matsen 1974; McIsaac 2004; Rimoin 2011; Siegel 1961; Zwart 2000). In two trials, the included participants did not exclusively have acute GABHS tonsillopharyngitis (Davies 1995; Standaert 1997), and one trial included participants with recurrent tonsillitis (Roos 1997). Two trials did not report any clinical outcomes (Gerber 1999a; Stillerman 1970); one study was a meta‐analysis (Llerena 2011); two studies were reviews (Stelter 2014; Van Brusselen 2014); and four studies were not randomised controlled trials (Del Mar 2008; De Meyere 1992; Granizio 2008; Haverkorn 1971).
Risk of bias in included studies
'Risk of bias' assessment is reported in Characteristics of included studies and illustrated in Figure 2 and Figure 3. Only three trials reported ITT analysis for efficacy outcomes (Disney 1992a; Norrby 2002; Randolph 1985). One trial reported carrying out an ITT analysis, but postrandomisation exclusions were not included in the efficacy analysis (Carbon 1995). All trial authors used an ITT analysis for adverse effects.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All trials were randomised, but only four described methods of randomisation or allocation concealment, or both (Jackson 1973; Randolph 1985; Reed 1991; Watkins 1997).
Random sequence generation was described and deemed adequate in two studies (Randolph 1985; Watkins 1997), and not described (assessed as unclear risk) in the remaining studies.
Allocation concealment was described and assessed as adequate in four studies (Jackson 1973; Randolph 1985; Reed 1991; Watkins 1997), and not described (assessed as unclear risk) in the other studies.
Blinding
All trials were double‐blinded, and methods of blinding were described in 14 trials (Disney 1992a; Disney 1992b; Jackson 1973; Levenstein 1991; McCarty 1992a; Muller 1992; NCT00643149; Norrby 2002; O'Doherty 1996; Randolph 1985; Reed 1991; Stein 1991; Trickett 1973; Watkins 1997).
Blinding of participants and personnel was reported and assessed as low risk of bias in 15 trials (Bachand 1991; Disney 1992a; Disney 1992b; Jackson 1973; Levenstein 1991; McCarty 1992a; Muller 1992; NCT00643149; Norrby 2002; O'Doherty 1996; Randolph 1985; Reed 1991; Stein 1991; Trickett 1973; Watkins 1997). In four studies (Carbon 1995; Henness 1982‐study 1; Henness 1982‐study 2; Nemeth 1999), this was not reported, and the studies were assessed as at unclear risk of bias.
Blinding of outcome assessors was reported and assessed as low risk of bias in only one trial (Randolph 1985). This was not reported and hence assessed as unclear risk of bias in all the other included studies.
Incomplete outcome data
The postrandomisation dropout rate was high in most trials. In 12 trials, the proportion of dropouts was more than 20% (Bachand 1991; Henness 1982‐study 1; Jackson 1973; Levenstein 1991; McCarty 1992a; Muller 1992; NCT00643149; Nemeth 1999; O'Doherty 1996; Reed 1991; Stein 1991; Watkins 1997), ranging from 21.5% in McCarty 1992a to 48.5% in Levenstein 1991. Most trials included only participants with complete outcome data in the outcome analysis. This may have had an important impact on the effect measured, therefore these studies were assessed as at high risk of attrition bias.
Only four trials reported an ITT analysis with all randomised participants included in the analysis of the clinical outcome (Disney 1992a; Disney 1992b; Norrby 2002; Randolph 1985). These trials had minimal to no dropouts (0 or 1 participant) and were assessed as at low risk of attrition bias. We also assessed Carbon 1995, Henness 1982‐study 2, and Trickett 1973 as at low risk of attrition bias due to a low postrandomisation dropout rate.
None of the studies were assessed as at unclear risk of attrition bias.
Selective reporting
We assessed all included studies as at unclear risk of reporting bias, as pre‐publication protocols were not available.
Other potential sources of bias
Eleven published trials reported sponsorship by a pharmaceutical company (Disney 1992a; Disney 1992b; Jackson 1973; McCarty 1992a; Muller 1992; Nemeth 1999; Norrby 2002; Randolph 1985; Reed 1991; Trickett 1973; Watkins 1997). NCT00643149 was unpublished and was obtained from the company that conducted the trial (Pfizer). The authors of six trials (in five publications) were reported to be employees of a pharmaceutical company (Bachand 1991; Henness 1982‐study 1; Henness 1982‐study 2; NCT00643149; Nemeth 1999; Watkins 1997); in three of these trials, the employing pharmaceutical company was not reported as a funding source (Bachand 1991; Henness 1982‐study 1; Henness 1982‐study 2). We assessed the 14 trials (in 13 publications) reporting pharmaceutical company funding and/or including a pharmaceutical company employee in the authorship as at high risk of other bias (Bachand 1991; Disney 1992a; Disney 1992b; Henness 1982; Jackson 1973; McCarty 1992a; Muller 1992; NCT00643149; Nemeth 1999; Norrby 2002; Randolph 1985; Reed 1991; Watkins 1997). Trickett 1973 reported only receiving medication from a pharmaceutical company. Five trials (Carbon 1995; Henness 1982; Levenstein 1991; O'Doherty 1996; Stein 1991; Trickett 1973) did not report funding sources or pharmaceutical company authorship, and were assessed as unclear risk of bias for this domain.
Six trials mentioned that ethics approval was obtained for the study (Bachand 1991; Levenstein 1991; Muller 1992; Nemeth 1999; Norrby 2002; O'Doherty 1996), and seven trials reported that informed consent was obtained from participants or guardians (Levenstein 1991; McCarty 1992a; Muller 1992; Nemeth 1999; Norrby 2002; O'Doherty 1996; Reed 1991).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1: cephalosporins versus penicillin
Six trials contributed to the pooled analysis within this comparison (Carbon 1995; Disney 1992a; Henness 1982‐study 1; Nemeth 1999; Randolph 1985; Reed 1991). We assessed the overall certainty of evidence for the primary outcome, resolution of symptoms post‐treatment, as low for the ITT analysis in the total study population and in the subgroup analysis for adults, but very low for the analysis of evaluable participants and ITT analysis in children. We assessed the certainty of the pooled effect estimate as low for the outcome incidence of relapse (evaluable participants) and very low for the outcome adverse events (ITT analysis). We downgraded the certainty due to unclear randomisation and blinding, wide confidence intervals, and heterogeneity amongst studies when pooled. See Table 1.
Primary outcome
1. Resolution of symptoms post‐treatment
Six trials reported on the resolution of symptoms at various time points (Carbon 1995; Disney 1992a; Henness 1982‐study 1; Nemeth 1999; Randolph 1985; Reed 1991). See Table 1.
Five trials measured resolution of symptoms at the end of treatment (2 to 15 days or more post‐treatment): two trials in adults (Carbon 1995; Nemeth 1999), and three in children (Disney 1992b; Henness 1982‐study 1; Reed 1991). The ITT analysis included 2018 participants and showed no difference between treatments (odds ratio (OR) 0.79, 95% confidence interval (CI) 0.55 to 1.12; 5 trials; 2018 participants; low‐certainty evidence; Analysis 1.1; Table 1). The effect in adults (OR 0.78, 95% CI 0.60 to 1.01; 2 trials; 1163 participants; low‐certainty evidence; Analysis 1.1) was similar to that in children (OR 0.83, 95% CI 0.40 to 1.73; 3 trials; 855 participants; very low‐certainty evidence; Analysis 1.1); however, the test for subgroup differences was not significant (P = 0.87).
The result of the analysis of evaluable participants only showed an effect in favour of treatment with cephalosporins (OR 0.51, 95% CI 0.27 to 0.97; absolute risk difference (ARD) 0.05; NNTB 20; 5 trials; 1660 participants; very low‐certainty evidence; Analysis 1.2; Table 1). However, the estimates of effect in adults (OR 0.56, 95% CI 0.24 to 1.32; 2 trials; 880 participants; very low‐certainty evidence; Analysis 1.2) and in children (OR 0.46, 95% CI 0.14 to 1.52; 3 trials; 780 participants; very low‐certainty evidence; Analysis 1.2), when analysed separately, revealed no statistically significant differences between treatment groups.
One trial in children also reported resolution of symptoms within 24 hours of treatment (Randolph 1985), and found no difference between treatment groups (OR 0.97, 95% CI 0.34 to 2.74; 1 trial; 138 participants; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 3: Resolution of symptoms within 24 hours of treatment (ITT analysis)
We analysed the studies with reported pharmaceutical company sponsorship separately for the outcome resolution of symptoms post‐treatment. Two studies that did not report funding sources showed a statistically significant effect in favour of cephalosporins (OR 0.47, 95% CI 0.27 to 0.81; ARD 0.02; NNTB 50; 2 trials; 769 participants; very low‐certainty evidence; Analysis 1.4) (Carbon 1995; Disney 1992a). Pooling sponsored studies did not result in a significant difference between antibiotic groups (OR 0.90, 95% CI 0.70 to 1.16; 3 trials; 1249 participants; very low‐certainty evidence; Analysis 1.4) (Henness 1982‐study 1; Nemeth 1999; Reed 1991).
1.4. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 4: Resolution of symptoms ITT (subgroup sponsored versus no sponsor reported)
A sensitivity analysis revealed that in the ITT analysis, the trial by Disney 1992a contributed to the heterogenity of the analysis in children. However, removing this trial from the analysis did not result in a significant change in the overall outcome. In a similar analysis for the evaluable participants only, the trial by Reed 1991 appeared to contribute the most to the heterogeneity. After removal of this trial, the I² statistic was no longer important. Pooling the two remaining trials in children showed a statistically significant benefit in favour of cephalosporins in children. However, the overall effect on all participants remained non‐significant.
Secondary outcomes
1. Sore throat
One trial in children found no difference between treatment groups for resolution of sore throat (OR 0.97, 95% CI 0.23 to 4.04; 1 trial; 138 participants; very low‐certainty evidence; Analysis 1.5) (Randolph 1985).
1.5. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 5: Sore throat (ITT analysis)
2. Fever
One trial in children found no difference between treatment groups for resolution of fever (OR 0.97, 95% CI 0.19 to 4.98; 1 trial; 138 participants; very low‐certainty evidence; Analysis 1.6) (Randolph 1985).
1.6. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 6: Fever (ITT analysis)
3. Duration of illness
Not reported.
4. Incidence of relapse
In four trials that reported the incidence of clinical relapse in evaluated participants (Carbon 1995; Disney 1992a; Nemeth 1999; Reed 1991), treatment with cephalosporins resulted in less relapse than treatment with penicillin in the total population (OR 0.55, 95% CI 0.30 to 0.99; ARD 0.02; NNTB 50; 4 trials; 1386 participants; low‐certainty evidence). This was due to a difference in two trials in adults (OR 0.42, 95% CI 0.20 to 0.88; ARD 0.03; NNTB 33.3; 2 trials; 770 participants; low‐certainty evidence; Analysis 1.7; Table 1) (Carbon 1995; Nemeth 1999). There was no difference in two trials in children (OR 0.89, 95% CI 0.33 to 2.45; 2 trials; 616 participants; low‐certainty evidence; Analysis 1.7) (Disney 1992a; Reed 1991).
5. Incidence of complications
In one trial in adults (244 participants), no complications were reported in the cephalosporin group (119 participants) or the penicillin group (125 participants) (Carbon 1995; Analysis 1.8).
1.8. Analysis.

Comparison 1: Cephalosporins versus penicillin, Outcome 8: Complications (ITT analysis)
6. Adverse events
Three trials in adults reported the incidence of adverse effects (Carbon 1995; Nemeth 1999; Reed 1991). There was significant heterogeneity amongst the trials. In the cephalosporin group, 212 of 788 participants reported adverse events, compared with 87 of 491 in the penicillin group. There was no difference between treatments (OR 0.94, 95% CI 0.27 to 3.25; 3 trials; 1279 participants; very low‐certainty evidence; Analysis 1.9; Table 1).
The reported adverse events were predominantly gastrointestinal (diarrhoea, nausea and vomiting, constipation), but also vaginal moniliasis and headaches have been reported with both antibiotic classes (Carbon 1995; Nemeth 1999). Reed 1991 did not report the nature of the adverse events. None of the adverse events were serious. Carbon 1995 reported one participant with penicillin allergy.
Comparison 2: macrolides versus penicillin
Six trials contributed to the pooled analysis within this comparison (Bachand 1991; Levenstein 1991; Norrby 2002; O'Doherty 1996; Stein 1991; Watkins 1997). We assessed the overall certainty of the evidence for the primary outcome, resolution of symptoms, and for the secondary outcomes as low. We downgraded the certainty of the evidence two levels due to unclear randomisation and wide confidence intervals. See Table 2.
Primary outcome
1. Resolution of symptoms post‐treatment
Five trials in adults (Bachand 1991; Levenstein 1991; Norrby 2002; Stein 1991; Watkins 1997), and one in children (O'Doherty 1996), investigated the resolution of symptoms at various time points post‐treatment. In the ITT analysis, there were no differences between the treatment groups (OR 1.11, 95% CI 0.92 to 1.35; 6 trials; 1728 participants; low‐certainty evidence; Analysis 2.1; Table 2). The estimate of effect in adults (OR 1.07, 95% CI 0.86 to 1.34; 5 trials; 1239 participants; low‐certainty evidence; Analysis 2.1) was similar to that in children (OR 1.25, 95% CI 0.85 to 1.84; 1 trial; 489 participants; low‐certainty evidence; Analysis 2.1). The test for subgroup differences was not significant (P = 0.51). The analysis of evaluable participants only did not result in any significant differences between treatment groups (OR 0.79, 95% CI 0.57 to 1.09; 6 trials; 1159 participants; low‐certainty evidence; Analysis 2.2; Table 2). The estimate for the five trials in adults was OR 0.88 (95% CI 0.59 to 1.31; 5 trials; 801 participants; low‐certainty evidence; Analysis 2.2) and for one trial in children was OR 0.64 (95% CI 0.36 to 1.11; 1 trial; 358 participants; low‐certainty evidence; Analysis 2.2).
ITT analysis of pharmaceutical industry‐sponsored trials versus trials that did not report funding sources did not show significant differences in results: trials with no sponsor reported (OR 1.11, 95% CI 0.84 to 1.48; 3 trials; low‐certainty evidence; Analysis 2.3) and sponsored studies (OR 1.12, 95% CI 0.85 to 1.46; 3 trials; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 3: Resolution of symptoms ITT (subgroup sponsored versus no sponsor reported)
Secondary outcomes
1. Sore throat
Two trials reported resolution of sore throat in adults and found no difference between the treatments (OR 0.97, 95% CI 0.64 to 1.46; 2 trials; 371 participants; low‐certainty evidence; Analysis 2.4) (Bachand 1991; Levenstein 1991).
2.4. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 4: Sore throat post‐treatment (ITT analysis)
2. Fever
Two trials with 371 adult participants reported resolution of fever at 2 to 10 days post‐treatment (Bachand 1991; Levenstein 1991). All participants in both groups were free of fever at the time of evaluation (45 participants in the macrolide group and 39 participants in the penicillin group; OR 1.05, 95% CI 0.69 to 1.59; 2 trials; 371 participants; low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Macrolides versus penicillin, Outcome 5: Fever post‐treatment (ITT analysis)
3. Duration of illness
Not reported.
4. Incidence of relapse
Six trials (802 participants) evaluated incidence of clinical relapse: five trials in adults (Bachand 1991; Levenstein 1991; Norrby 2002; Stein 1991; Watkins 1997), and one in children (O'Doherty 1996). Twenty‐two of 441 participants in the macrolide group and 16 of 361 in the penicillin group reported relapse at day 15 to 56 post‐treatment. The difference was not statistically significant (OR 1.21, 95% CI 0.48 to 3.03; 6 trials; 802 participants; low‐certainty evidence; Analysis 2.6; Table 2). In the trials in adults, the OR was 0.90 (95% CI 0.34 to 2.39; 5 trials; 495 participants; low‐certainty evidence; Analysis 2.6). In the only trial in children, the OR was 3.10 (95% CI 0.67 to 14.25; very low‐certainty evidence; Analysis 2.6).
5. Incidence of complications
Not reported.
6. Adverse events
In the six trials (1727 participants), five in adults and one in children (O'Doherty 1996), that reported on the incidence of adverse events, there were no statistically significant differences between treatment groups: 282 events were reported in the macrolide group and 251 in the penicillin group (OR 1.19, 95% CI 0.82 to 1.73; 6 trials; 1727 participants; low‐certainty evidence; Table 2). In the trial in children, macrolides seemed to cause more adverse events than penicillin (OR 2.33, 95% CI 1.06 to 5.15; 489 participants; NNTH 17.2; low‐certainty evidence; Analysis 2.7). However, the test for subgroup differences was not significant.
The reported adverse events were predominantly gastrointestinal (diarrhoea, nausea and vomiting, constipation, abdominal pain), but vaginal moniliasis and headaches and dizziness were also reported with both antibiotic classes. Rash was reported in participants taking penicillin (O'Doherty 1996). Most studies did not report any serious adverse events, but Levenstein 1991 reported two serious events: depression and balanitis.
Comparison 3: azithromycin versus amoxicillin
One trial (unpublished data provided by Pfizer) studied the effect of a single dose of azithromycin versus 10 days of amoxicillin in 673 children (NCT00643149). We downgraded the certainty of the evidence for all outcomes by three levels due to poor reporting of randomisation, wide confidence intervals (low precision), and potential publication bias. See Table 3.
Primary outcome
1. Resolution of symptoms post‐treatment
The clinical cure rate was reported for the 'bacteriological per protocol population' only, which was defined as those with GABHS‐positive culture within 48 hours of treatment start, at least eight days of treatment (compliance), and available data at baseline. Effects were measured at 24 to 28 days after commencing treatment and on days 38 to 42.
Resolution of symptoms was not different between azithromycin and amoxicillin in the ITT analysis (OR 0.76, 95% CI 0.55 to 1.05; 1 trial; 673 participants; very low‐certainty evidence; Analysis 3.1; Table 3). In the bacteriological per‐protocol analysis, in the azithromycin group, 239/245 participants achieved clinical cure at the first evaluation point versus 218/237 in the amoxicillin group (OR 0.29, 95% CI 0.11 to 0.73; NNTB 18; 1 trial; 482 participants; very low‐certainty evidence; Analysis 3.2). The 'bacteriological per protocol population' was defined as those with GABHS‐positive culture within 48 hours of treatment start, at least eight days of treatment (compliance), and available data at baseline.
3.2. Analysis.

Comparison 3: Azithromycin versus amoxicillin, Outcome 2: Clinical cure at 24 to 28 days (bacteriological per protocol population)
Secondary outcomes
1. Sore throat
Not reported.
2. Fever
Not reported.
3. Duration of illness
Not reported.
4. Incidence of relapse
Between days 38 to 45 after treatment commencement (long‐term follow‐up), the per‐protocol population was reduced to 223 in the azithromycin group and 199 in the amoxicillin group. The incidence of relapse did not differ between groups in the ITT analysis (OR 0.75, 95% CI 0.55 to 1.02; 1 trial; 673 participants; very low‐certainty evidence; Analysis 3.3; Table 3) or the bacteriological per protocol population (16/223 in the azithromycin group versus 16/199 in the amoxicillin group; OR 0.88, 95% CI 0.43 to 1.82; 1 trial; 422 participants; very low‐certainty evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3: Azithromycin versus amoxicillin, Outcome 4: Relapse on day 38 to 45 (bacteriological per protocol)
5. Incidence of complications
Not reported.
6. Adverse events
In total, 57.5% of participants in the azithromycin group and 56.3% in the amoxicillin group reported experiencing an adverse event. However, reported treatment‐related adverse events were more prevalent in the azithromycin group (27.6%) than in the amoxicillin group (12.5%): OR 2.67 (95% CI 1.78 to 3.99; 1 trial; 673 participants; very low‐certainty evidence; Analysis 3.5; Table 3). The most commonly reported adverse events were related to the digestive system (diarrhoea, nausea, vomiting, abdominal pain) and occurred more frequently in participants treated with azithromycin (34.1%) than in those treated with amoxicillin (16.1%). Rash was more common in the amoxicillin group (3.0% versus 0.6% in the azithromycin group). No deaths or serious adverse events were reported.
Comparison 4: carbacephem versus penicillin
We included three trials (795 participants) in this comparison: one in children (Disney 1992b), one in adults (McCarty 1992a), and one in a mixed population of adults and children (but predominantly adults; 90% were aged over 12 years) (Muller 1992). We downgraded the certainty of the evidence for all outcomes by two levels due to poor reporting of randomisation and wide confidence intervals (imprecision). See Table 4.
Primary outcome
1. Resolution of symptoms post‐treatment
In the ITT analysis, more participants reported resolution of symptoms in the carbacephem group than in the penicillin group (OR for the absence of symptom resolution post‐treatment 0.70, 95% CI 0.49 to 0.99; ARD 0.07; NNTB 14.3; 3 trials; 795 participants; low‐certainty evidence; Analysis 4.1; Table 4). There was no difference in adults (OR 0.75, 95% CI 0.46 to 1.22; 2 trials; 562 participants; low‐certainty evidence; Analysis 4.1). There was a beneficial effect from carbacephem in children (OR 0.57, 95% CI 0.33 to 0.99; ARD 0.12; NNTB 8.3; 1 trial; 233 participants; low‐certainty evidence; Analysis 4.1). However, the test for subgroup differences was not significant.
The analysis of evaluable participants showed no differences between treatment groups (OR 0.62, 95% CI 0.38 to 1.01; 3 trials; 602 participants; low‐certainty evidence; Analysis 4.2; Table 4).
Secondary outcomes
1. Sore throat
Not reported.
2. Fever
Not reported.
3. Duration of illness
Not reported.
4. Incidence of relapse
There were no differences in the incidence of clinical relapse between carbacephem and penicillin groups (21 events in 267 participants treated with carbacephem, and 16 events in 256 participants treated with penicillin: OR 1.27, 95% CI 0.64 to 2.50; 3 trials; 523 participants; low‐certainty evidence; Analysis 4.3; Table 4).
5. Incidence of complications
Not reported.
6. Adverse events
There were no differences in reported adverse events between treatments (75 events in 396 participants treated with carbacephem, and 71 events in 399 participants treated with penicillin: OR 1.08, 95% CI 0.75 to 1.55; 3 trials; 795 participants; low‐certainty evidence; Analysis 4.4; Table 4). Muller 1992 reported that one participant was hospitalised for surgical drainage of a tonsillar abscess in the group treated with loracarbef one day after initiating therapy.
Reported adverse events were predominantly gastrointestinal (diarrhoea, nausea, vomiting) in all treatment groups. Headaches were reported in McCarty 1992a and Muller 1992, and vaginal moniliasis in McCarty 1992a. Rashes were reported in both treatment groups (Disney 1992b; Muller 1992).
Comparison 5: clindamycin versus ampicillin
Jackson 1973 compared treatment with clindamycin to ampicillin (314 participants). The only clinical outcome reported was adverse events. We downgraded the certainty of the evidence by two levels due to poor reporting of randomisation and wide confidence intervals (imprecision).
Primary outcome
1. Resolution of symptoms post‐treatment
Not reported.
Secondary outcomes
1. Sore throat
Not reported.
2. Fever
Not reported.
3. Duration of illness
Not reported.
4. Incidence of relapse
Not reported.
5. Incidence of complications
Not reported.
6. Adverse events
Adverse events were reported in 6 of 156 participants in the clindamycin group and 14 of 158 participants in the ampicillin group. The difference was not statistically significant (OR 0.41, 95% CI 0.15 to 1.10; 1 trial; 314 participants; low‐certainty evidence; Analysis 5.1). Gastrointestinal adverse events (nausea or vomiting and loose stools) and rash or urticaria occurred in both treatment groups. No other events were reported.
5.1. Analysis.

Comparison 5: Clindamycin versus ampicillin, Outcome 1: Adverse events (ITT analysis)
Comparison 6: sulphonamides versus penicillin
We included one trial in adults (87 participants) in this comparison (Trickett 1973), which reported only on adverse events. We downgraded the certainty of the evidence for this outcome by three levels due to poor reporting of randomisation and allocation concealment and very wide confidence intervals (imprecision).
Primary outcome
1. Resolution of symptoms post‐treatment
Not reported.
Secondary outcomes
1. Sore throat
Not reported.
2. Fever
Not reported.
3. Duration of illness
Not reported.
4. Incidence of relapse
Not reported.
5. Incidence of complications
Not reported.
6. Adverse events
Trickett 1973 reported eight events in the sulphonamides group and six events in the penicillin group (Analysis 6.1). They found no difference between sulphonamide and penicillin (OR 1.37, 95% CI 0.43 to 4.34; 1 trial; 87 participants; very low‐certainty evidence; Analysis 6.1). Gastrointestinal disturbances, rash, (reversible) leukopenia, and (reversible) liver and kidney function disturbances were reported in both treatment groups.
6.1. Analysis.

Comparison 6: Sulfonamide versus penicillin, Outcome 1: Adverse events (ITT analysis)
Penicillin allergy
We assessed the reporting of penicillin allergy in all included trials. Carbon 1995 reported one participant with a "severe allergic reaction" in the penicillin group, but provided no further details. Muller 1992 reported that one participant developed a rash and another experienced vomiting, both attributed to use of penicillin (although participants were then successfully switched to amoxicillin/clavulanate). However, in the loracarbef group, one participant discontinued treatment because of a rash. Trickett 1973 reported one participant with a rash in the penicillin group, but two participants reported a rash in the trimethoprim/sulfamethoxazole group. None of the other included trials specifically reported penicillin allergy.
Discussion
Summary of main results
Our meta‐analysis found generally low‐certainty evidence (as per the GRADE assessment) that did not show clinically important differences in clinical outcomes when different classes of antibiotics were compared with penicillin in adults and children with pharyngitis caused by GABHS.
Resolution of symptoms
ITT analysis did not show any difference in resolution of symptoms between cephalosporins and penicillin. When only evaluable participants were included in the analysis (i.e. participants for whom an outcome was known), there seemed to be a benefit of cephalosporins over penicillin with regard to resolution of symptoms after treatment (NNTB 20). Subgroup analysis of adults and children (aged between one month and 17 years) did not reveal any significant differences, but this could be attributed to lack of sufficient power.
ITT analysis of carbacephem versus penicillin showed a benefit of carbacephem with regard to resolution of symptoms after treatment (NNTB 14.3). There was no significant benefit in the (large) adult subgroup, and the effect may be largely based on an observed effect in children (aged between six months and 12 years) (NNTB 8.3). The analysis of evaluable participants only did not reach statistical significance (but the estimated NNTB was likely to be high).
Pooling of trials comparing macrolides with penicillin did not result in any differences between groups in terms of resolution of symptoms. Only one unpublished trial in children aged between two and 12 years that compared a single dose of azithromycin with 10 days of amoxicillin found that more children on azithromycin were cured after 24 to 28 days than with amoxicillin. However, this effect was no longer significant in the ITT analysis.
Other comparisons with penicillin (clindamycin or sulphonamides) did not report clinical outcomes for this meta‐analysis.
Relapse
The incidence of relapse in evaluable participants seemed to be lower in participants treated with cephalosporins compared with those treated with penicillin, but the event rate was low (approximately 3.5%), and the NNTB was quite high (NNTB 50). There were no differences in relapse rate between other antibiotics and penicillin.
Adverse events
Adverse events occurred at a similar rate in all treatment groups, except in children treated with macrolides, who seemed to experience more adverse events than those treated with penicillin (although this difference was not statistically significant, most likely due to insufficient power) or amoxicillin or ampicillin.
The results of our meta‐analysis need to be considered in the context of morbidity (including serious complications) prevalence, concerns about rising antibiotic resistance, and economic constraints in all healthcare systems.
Penicillin allergy
Incidence of penicillin allergy was reported poorly if at all in the included trials. When a rash is reported in the penicillin group, this is often also reported in the comparator group. The limited information about penicillin allergy may reflect the low incidence in the general population. Albin 2014 found that penicillin allergy was reported in 11.5% of patients in a retrospective chart review, but only 11.8% of those with a documented allergy had experienced an anaphylactic reaction. The incidence of true anaphylaxis has been reported as less than 0.01% (Bhattacharya 2010). It is also possible that patients with known penicillin allergies were excluded from the trials, resulting in a low incidence of allergies during the trial. This exclusion was only explicitly mentioned in a few of the included studies.
Overall completeness and applicability of evidence
Although we searched several databases and scrutinised all references listed in identified reviews and publications of trials, we may have missed some trials. We contacted experts and pharmaceutical companies. One pharmaceutical company responded, but this did not result in additional data. An updated search in 2012 identified an unpublished study, and a report was provided by the manufacturer in 2013 (NCT00643149). This study was included in the 2016 update, but we did not identify any new published or unpublished trials in a new search. As an analysis of unpublished data used in Cochrane Reviews suggested that searching for unpublished data generally does not uncover new data that are important to the conclusion of the review (van Driel 2009), the lack of further unpublished data may not have had an important impact on the results of our review.
Our meta‐analysis focused on clinical outcomes. Reviews that report bacteriological outcomes point to the superiority of cephalosporins over penicillin with regard to eradication of GABHS (Brunton 2006; Casey 2004). However, this does not take clinical presentation into account. Gerber 1999a found no difference in bacteriologic treatment success rates between cefadroxil and penicillin groups amongst participants classified clinically as likely to have true GABHS pharyngitis, but cephalosporins seemed to be more successful in eradicating GABHS in patients classified as clinically likely to be streptococcal carriers. Contamination of treatment groups by such chronic GABHS carriers contributes to the apparent superiority of cephalosporins in studies focusing on bacteriological outcomes (Shulman 2004); this is of very limited clinical relevance. To our knowledge, chronic streptococcal carriage is not linked to higher risk of developing GABHS pharyngitis, hence eradication of streptococci in carriers is not a treatment goal. Information on complications was scarcely reported, therefore we could not draw any conclusions regarding this outcome.
Our review included studies involving children and adults, but the age ranges of participants in each study varied widely, and there was significant overlap. It was therefore not always possible to perform subgroup analyses based on age groups. We were unable to draw conclusions about specific age groups. This would have been clinically relevant because GABHS is more common in children aged between five and 15 years (Worrall 2007).
Quality of the evidence
A strength of our review is that we included only randomised and double‐blinded trials. This was intended to minimise risk of bias related to participant selection and reporting of outcomes. However, despite the low risk of bias due to methodology, reporting of findings and transparency of analyses in the trials were often unsatisfactory. Participant characteristics were poorly reported and outcomes reported poorly or not defined at all. Dropout rates in some studies were very high (> 20%).
The overall risk of bias in the included studies was difficult to assess because the process of randomisation and blinding was not described in most studies. For instance, only four studies described the method used to conceal allocation (Jackson 1973; Randolph 1985; Reed 1991; Watkins 1997).
It is surprising that resolution of sore throat, a key symptom in GABHS pharyngitis and an important reason for patients to consult their doctor (van Driel 2006), was only reported as a separate outcome in one study (McCarty 1992a). However, most studies assessed our primary outcome, which is a composite endpoint consisting of a combination of symptoms including sore throat, fever, and feeling unwell. This is of course also of clinical relevance to patients.
The overall certainty of the pooled evidence assessed using the GRADE approach was low for all outcomes in the comparison of macrolides versus penicillin, and low or very low for the comparison of cephalosporins versus penicillin. We downgraded the certainty of evidence mainly due to lack of or poor reporting of randomisation or blinding, or both; heterogeneity; and wide confidence intervals.
Potential biases in the review process
Pooling of outcomes was hampered by differences in outcome definitions amongst studies. Most trials measured clinical outcomes within two weeks of the end of antibiotic treatment and were therefore pooled for the outcome resolution of symptoms post‐treatment. We considered the trial that reported symptom resolution within the first 24 hours of treatment separately. Very few trials reported on specific symptoms related to acute GABHS tonsillopharyngitis. Because symptom resolution is a subjective outcome, the interpretation may differ amongst trials, and pooling may therefore be inappropriate. However, differences between comparison groups in the same trial were not affected as they were measured in the same population.
We used ITT analysis of the selected outcomes for our meta‐analyses. However, this may have underestimated the efficacy of treatment. Most trials reported numbers of participants randomised, but included only the evaluated participants in the outcome analysis. When reported, a common reason for postrandomisation exclusion was negative throat culture, suggesting that another pathogen caused the signs and symptoms of acute tonsillopharyngitis. Including these GABHS‐negative participants in the analysis could bias the results if exclusion was not similar in both treatment groups. Some trials reported exclusions per group and show that this is not the case. When comparing two efficacious treatments, this potential underestimation did not seem relevant because it did not influence conclusions. However, for trials that did not report this, it was not possible to know if selective exclusions occurred. We checked if the analysis method influenced outcomes by performing both ITT and analysis of evaluable participants for the outcome resolution of symptoms post‐treatment. This showed different results in two comparisons. When cephalosporins and penicillin were compared, ITT analysis yielded a non‐significant result, whereas analysis of evaluable participants showed a benefit of cephalosporins over penicillin. The opposite occurred in the analysis of effect on the same outcome in participants treated with carbacephem versus penicillin: ITT analysis showed a statistically significant difference, and the evaluable participants analysis did not, most likely due to a reduction in the number of participants included in the analysis (resulting in reduced statistical power). Analysing only evaluable participants implies a high risk of bias, as there may have been a selective dropout. On the other hand, the ITT analysis can be considered as a conservative estimate of the true effect.
The estimated ORs suggested that large benefits could be expected when treating patients with cephalosporins or carbacephems. However, these supposedly impressive effects expressed as a relative measure of risk (ORs) do not always translate into a clinically meaningful difference. For example, the estimated OR of 0.55 for the incidence of relapse in cephalosporins compared with penicillin suggests that the risk of relapse could be halved by treating patients with cephalosporins. However, the associated absolute risk difference is 0.02, resulting in an NNTB of 50, which means that 50 patients need to be treated with broad‐spectrum, more expensive antibiotics to prevent one additional relapse.
Calculating the absolute risk difference and the NNTB is therefore a useful method to assess the clinical importance of a relative risk. However, the interpretation of the NNTBs (how many patients needed to treat is acceptable) is not clear‐cut and depends on assessment of benefit and harm, as well as cost‐effectiveness.
All included trials were performed in high‐income countries. The incidence of suppurative and other complications (which are rare in high‐income countries), as well as antimicrobial resistance rates, may be different in low‐income countries or specific communities with high prevalence of GABHS tonsillitis (Hanna 2010). Studies performed in low‐income and high‐prevalence communities are therefore needed.
Agreements and disagreements with other studies or reviews
We found that although there seems to be some benefit of antibiotics with a wider spectrum, such as cephalosporins and carbacephem, this observed effect is not consistent across analysis methods and subgroups. Cephalosporins showed benefit regarding resolution of symptoms only in the analysis of evaluable participants, and carbacephem is superior to penicillin for this outcome only in the ITT analysis (attributable to an effect in children treated with a carbacephem). The NNTBs associated with the observed effects were relatively high (20 for treatment with cephalosporins compared with penicillin), except perhaps for the effect of carbacephem in children (NNTB 8.3). There was no clinically meaningful difference between penicillin and the other classes of antibiotics studied with regard to rate of clinical relapse. However, cephalosporins seemed to reduce the relapse rate (NNTB 50), especially in adults (NNTB 33.3).
The effects observed in cephalosporins and carbacephems and not in the other antibiotic classes can be explained by the fact that although they are considered different classes of antibiotics, carbacephems chemically closely resemble cephalosporins (Cooper 1992).
An unpublished study, NCT00643149, concluded that a single dose of azithromycin was superior to 10 days of amoxicillin in children. However, the analysis was based on a per‐protocol population that had completed at least eight days of treatment. Results were based on those patients who responded bacteriologically, thus censoring patients with strains resistant to the allocated antibiotic. Because eradication rates were higher in the azithromycin arm, this may have biased the analysis. The ITT analysis, which underestimates the effect, did not show any difference between groups. In addition, amoxicillin may not be an appropriate choice for the treatment of GABHS pharyngitis/tonsillitis, considering the implications of using wide‐spectrum antibiotics on resistance in the community.
Interpretation of these findings for clinical practice is not straightforward. One could argue that our meta‐analysis points to a superior efficacy of cephalosporins over penicillin, especially in adults, where the upper limit of the 95% CI is 1.01 (P = 0.06) in the ITT analysis. The population size may not have been large enough to reach statistical significance. This finding is in line with an earlier review that concluded that cephalosporins are superior to penicillin in treating GABHS pharyngitis, and therefore cephalosporins should be considered first choice (Casey 2004). However, in our review the absolute difference between the cephalosporin or penicillin, although not statistically significant, was 2.5%, which implies an NNTB of 40. Treating 40 patients with cephalosporins instead of penicillin would incur additional costs to healthcare systems and add to the risk of developing antibiotic resistance, especially in broad‐spectrum antibiotics such as cephalosporins.
The observed superior effect of cephalosporins in reducing the rate of relapse has been reported elsewhere (Casey 2004). However, in our review it was only observed in adults and may be biased by the rather liberal definition of relapse in the study accounting for 49% of weighting in the meta‐analysis (Nemeth 1999): "worsening of, or absence of significant remission of, signs and symptoms 17 to 24 days post‐therapy or need for further AB therapy", whereas in other studies "recurrence of symptoms" after initial remission was required. The NNTB of 33 patients that need to be treated with cephalosporins rather than penicillin to prevent one patient experiencing relapse illustrates the limited clinical relevance of this statistically significant result.
How can the differences between Casey's meta‐analysis and ours be explained? Casey 2004 included 35 trials, two‐thirds of which were not blinded, and reporting of randomisation and losses to follow‐up was very poor, implying a high risk of bias (Gerber 2004). By restricting inclusion to double‐blinded trials, we ruled out one source of potential bias and improved methodological rigour. The Casey 2004 subgroup analysis of double‐blinded studies generated an OR similar to ours (although with a much narrower CI: OR 0.43, 95% CI 0.25 to 0.71), but included studies with carbacephems, which have been advertised as a separate class of antibiotics (Cooper 1992). Casey 2004 reported an analysis of evaluable patients, whereas ITT analysis may be more appropriate especially with important numbers of dropouts (which is the case in many of the trials included in our review). The trial populations included in Casey 2004, as in ours, may have been contaminated with chronic carriers of GABHS who had intercurrent viral pharyngitis (Gerber 2004), but it was not clear if this has implications for clinical practice. Gerber 1999b argued that the superior effectiveness of cephalosporins over penicillin observed in some studies may reflect a greater ability to eradicate the streptococcal carrier state rather than actual superior effectiveness of "bona fide acute GABHS pharyngitis".
We found no differences in the incidence of adverse events, and data on long‐term follow‐up and occurrence of complications were insufficient. Costs and antimicrobial resistance patterns are therefore important in making treatment choices.
Authors' conclusions
Implications for practice.
Our review did not find evidence for clinically important differences in clinical outcomes when different classes of antibiotics were compared with penicillin in adults and children with pharyngitis caused by group A beta‐haemolytic streptococci (GABHS). The finding that carbacephems and cephalosporins may have some benefit over penicillin in terms of resolution of symptoms and prevention of relapse was inconsistent across analysis methods (only statistically significant for the evaluable participants analysis), and the number needed to treat for an additional beneficial outcome was substantial. This means our findings support current guideline recommendations for the treatment of patients with GABHS tonsillopharyngitis, which list penicillin as first choice. Moreover, the occurrence of adverse events may not be different between antibiotic groups, and data on the incidence of complications were too few to draw conclusions.
As other reviews have shown, antibiotics have a limited effect in the treatment of patients with acute sore throat, even in the presence of GABHS. However, if antibiotics are to be prescribed, low‐certainty evidence supports guidelines recommending penicillin as first choice for both adults and children. This takes into consideration the costs of antibiotics and the favourable antimicrobial resistance pattern of penicillin.
Implications for research.
The observed differences in clinical efficacy between adults and children needs further exploration. The currently available studies included different age ranges, which makes it difficult to identify differential effects in various age groups. Individual participant data were unavailable, therefore future studies reporting effects in distinct age groups may provide clinically relevant information. Prevention of serious complications such as acute rheumatic fever and acute glomerulonephritis are often mentioned as arguments in favour of antibiotic use. However, the current data do not provide information about the impact of different antibiotics for the prevention of complications. Further studies with longer follow‐up may be able to address this issue. Because these complications seem to be more prevalent in low‐income and high‐risk communities (e.g. Australian Indigenous communities), studies in these specific high‐risk communities are needed. Economic analysis of the cost‐effectiveness of different treatment options may provide additional guidance for making treatment choices.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2020 | New citation required but conclusions have not changed | We did not identify any new trials for inclusion. We excluded one trial previously awaiting classification (Eslami 2014). |
| 3 September 2020 | New search has been performed | We updated the searches on 3 September 2020. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 25 March 2016 | New search has been performed | We updated the searches and identified two new studies. We excluded one of the studies (Stillerman 1970). Further details have been requested from the authors of the other identified study (Eslami 2014), which is currently inserted in the 'Studies awaiting classification' section. This review update includes the Pfizer 2011 study that was identified in the 2012 review publication and had been awaiting classification until data became available. We identified three new trials for exclusion (Kuroki 2013; Stelter 2014; Van Brusselen 2014). |
| 25 March 2016 | New citation required but conclusions have not changed | The review conclusions remain unchanged. |
| 19 October 2012 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 19 October 2012 | New search has been performed | The updated searches identified five new references. We excluded four studies (Bottaro 2012; Llerena 2011; NCT00393744; Rimoin 2011), and requested results from one completed unpublished study (NCT00643149). |
Acknowledgements
We thank the Cochrane Acute Respiratory Infections Group, particularly Liz Dooley and Chris Del Mar, for their support. We thank Warren McIsaac, Amy Zelmer, Mark Jones, and Paul Little for their valuable comments. For the 2016 update, we thank the following people for commenting on the updated review: Noorin Bhimani, Rashmi Das, Mark Jones, and Paul Little.
We also thank Natalja Keber (NK) who assisted with study selection and data extraction for the original version of this review (published in 2010), and Hilde Habraken (HH) who contributed to all versions of this review and contributed to the selection and data extraction until 2016.
Appendices
Appendix 1. Previous searches
Our 2012 review update used the search strategy described below. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 10, part of The Cochrane Library, www.thecochranelibrary.com (accessed 19 October 2012), which includes the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to October week 4, 2012), EMBASE (1974 to October 2012) and Web of Science (2010 to October 2012).
In 2010 we searched The Cochrane Library, Cochrane Central Register of Controlled Trials (CENTRAL 2010, Issue 3) which includes the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to July Week 4, 2010) and EMBASE (1974 to August 2010).
The following search strategy was used to search MEDLINE and CENTRAL. The MEDLINE search terms were combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2009). The search terms were adapted for EMBASE (Appendix 3).
MEDLINE (Ovid) 1 exp Pharyngitis/ 2 pharyngit*.tw. 3 Nasopharyngitis/ 4 nasopharyngit*.tw. 5 rhinopharyngit*.tw. 6 tonsillit*.tw. 7 tonsillopharyngit*.tw. 8 sore throat*.tw. 9 (strep* adj3 throat*).tw. 10 Streptococcal Infections/ 11 "group a beta hemolytic streptococc*".tw. 12 "group a beta haemolytic streptococc*".tw. 13 gabhs.tw. 14 or/10‐13 15 throat*.tw. 16 14 and 15 17 1 or 2 or 3 or 4 or 5 or 7 or 8 or 9 or 16 18 exp Anti‐Bacterial Agents/ 19 (antibacterial* or anti bacterial*).tw. 20 antibiotic*.tw. 21 or/18‐20 22 17 and 21
There were no language or publication restrictions.
Appendix 2. MEDLINE and CENTRAL search strategy
MEDLINE (Ovid)
1 exp Pharyngitis/ 2 pharyngit*.tw. 3 Nasopharyngitis/ 4 nasopharyngit*.tw. 5 rhinopharyngit*.tw. 6 tonsillit*.tw. 7 tonsillopharyngit*.tw. 8 sore throat*.tw. 9 (throat* adj3 (infect* or inflam*)).tw. 10 (strep* adj3 (throat* or pharyng*)).tw. 11 Streptococcal Infections/ 12 Streptococcus pyogenes/ 13 ("group a" adj5 streptococc*).tw. 14 gabhs.tw. 15 or/11‐14 16 (throat* or pharyng*).tw. 17 15 and 16 18 1 or 2 or 3 or 4 or 5 or 7 or 8 or 9 or 10 or 17 19 exp Anti‐Bacterial Agents/ 20 (antibacterial* or anti bacterial*).tw. 21 antibiotic*.tw. 22 exp beta‐lactams/ 23 exp aminoglycosides/ 24 exp Macrolides/ 25 exp Quinolones/ 26 exp Sulfonamides/ 27 exp Tetracyclines/ 28 (aminoglycoside* or amoxicillin* or amoxycillin* or ampicillin* or azithromycin* or benzylpenicillin* or beta‐lactam* or betalactam* or cefaclor* or cefadroxil or cefalexin or cefdinir or cefditoren or cefixime or cefpodoxime or cefprozil or ceftibuten or ceftriaxone or cefuroxime or cephalosporin* or clarithromycin or clavulanic acid* or clindamycin or co‐amoxyclav* or doripenem or doxycycline or eratapenem or erythromycin or imipenem or lincomycin or macrolide* or meropenem or moxifloxacin or penicillin* or phenoxymethylpenicillin* or piperacillin* or quinolone* or roxithromycin* or sulfamethoxazole* or sulfonimide* or tetracycline* or ticarcillin or trimethoprim*).tw,nm. 29 or/19‐28 30 18 and 29
The MEDLINE search terms were combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011)
Appendix 3. Embase.com (Elsevier) search strategy
#31 #22 AND #30 #30 #25 NOT #29 #29 #26 NOT #28 #28 #26 AND #27 #27 'human'/de #26 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #25 #23 OR #24 #24 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #23 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #22 #16 AND #21 #21 #17 OR #18 OR #19 OR #20 #20 aminoglycoside*:ab,ti OR amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR ampicillin*:ab,ti OR azithromycin*:ab,ti OR benzylpenicillin*:ab,ti OR 'beta‐lactam':ab,ti OR 'beta‐lactams':ab,ti OR betalactam*:ab,ti OR cefaclor*:ab,ti OR cefadroxil:ab,ti OR cefalexin:ab,ti OR cefdinir:ab,ti OR cefditoren:ab,ti OR cefixime:ab,ti OR cefpodoxime:ab,ti OR cefprozil:ab,ti OR ceftibuten:ab,ti OR ceftriaxone:ab,ti OR cefuroxime:ab,ti OR cephalosporin*:ab,ti OR clarithromycin:ab,ti OR 'clavulanic acid':ab,ti OR clindamycin:ab,ti OR 'co‐amoxyclav':ab,ti OR doripenem:ab,ti OR doxycycline:ab,ti OR eratapenem:ab,ti OR erythromycin:ab,ti OR imipenem:ab,ti OR lincomycin:ab,ti OR macrolide*:ab,ti OR meropenem:ab,ti OR moxifloxacin:ab,ti OR penicillin*:ab,ti OR phenoxymethylpenicillin*:ab,ti OR piperacillin*:ab,ti OR quinolone*:ab,ti OR roxithromycin*:ab,ti OR sulfamethoxazole*:ab,ti OR sulfonimide*:ab,ti OR tetracycline*:ab,ti OR ticarcillin:ab,ti OR trimethoprim*:ab,ti #19 'beta lactam antibiotic'/exp OR 'aminoglycoside antibiotic agent'/exp OR 'macrolide'/exp OR 'quinolone derivative'/exp OR 'sulfonamide'/exp OR 'tetracycline derivative'/exp #18 antibiotic*:ab,ti OR antibacterial*:ab,ti OR 'anti‐bacterial':ab,ti OR 'anti‐bacterials':ab,ti #17 'antibiotic agent'/exp #16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #15 #15 #13 AND #14 #14 throat*:ab,ti OR pharyngit*:ab,ti #13 #9 OR #10 OR #11 OR #12 #12 gabhs:ab,ti #11 ('group a' NEXT/5 streptococc*):ab,ti #10 'streptococcus pyogenes'/de #9 'streptococcus infection'/de OR 'group a streptococcal infection'/de #8 (strep* NEAR/3 (throat* OR pharyngit*)):ab,ti #7 'streptococcal pharyngitis'/de #6 'sore throat':ab,ti OR 'sore throats':ab,ti OR (throat* NEAR/3 (infect* OR inflam*)):ab,ti #5 'sore throat'/de #4 tonsillit*:ab,ti OR tonsillopharyngit*:ab,ti #3 'tonsillitis'/de #2 pharyngit*:ab,ti OR nasopharyngit*:ab,ti OR rhinopharyngit*:ab,ti #1 'pharyngitis'/de OR 'rhinopharyngitis'/de OR 'viral pharyngitis'/de
Appendix 4. Web of Science (Thomson Reuters) search strategy
| # 6 | 18 | #4 AND #3 Refined by: Publication Years=( 2011 OR 2010 OR 2012 ) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 5 | 297 | #4 AND #3 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 4 | 1,296,034 | Topic=(random* or placebo* or crossover* or "cross over" or ((singl* or doubl*) NEAR/1 blind*) or allocat*) OR Title=(trial) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 3 | 1,398 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 2 | 350,460 | Topic=(antibiotic* or anti‐bacterial* or antibacterial* or aminoglycoside* or amoxicillin* or amoxycillin* or ampicillin* or azithromycin* or benzylpenicillin* or beta‐lactam* or betalactam* or cefaclor* or cefadroxil or cefalexin or cefdinir or cefditoren or cefixime or cefpodoxime or cefprozil or ceftibuten or ceftriaxone or cefuroxime or cephalosporin* or clarithromycin or "clavulanic acid*" or clindamycin or co‐amoxyclav* or doripenem or doxycycline or eratapenem or erythromycin or imipenem or lincomycin or macrolide* or meropenem or moxifloxacin or penicillin* or phenoxymethylpenicillin* or piperacillin* or quinolone* or roxithromycin* or sulfamethoxazole* or sulfonimide* or tetracycline* or ticarcillin or trimethoprim*) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 1 | 2,840 | Topic=(pharyngit* or nasopharyngit* or rhinopharyngit* or tonsillit* or tonsillopharyngit* or "sore throat" or "sore throats" or (throat NEAR/2 (infect* or inflam*))) AND Topic=(streptococc* or gabhs) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
Data and analyses
Comparison 1. Cephalosporins versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Resolution of symptoms post‐treatment (ITT analysis) | 5 | 2018 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.12] |
| 1.1.1 Adults | 2 | 1163 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.01] |
| 1.1.2 Children | 3 | 855 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.40, 1.73] |
| 1.2 Resolution of symptoms post‐treatment (evaluable participants) | 5 | 1660 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 1.2.1 Adults | 2 | 880 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.24, 1.32] |
| 1.2.2 Children | 3 | 780 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.14, 1.52] |
| 1.3 Resolution of symptoms within 24 hours of treatment (ITT analysis) | 1 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.34, 2.74] |
| 1.3.1 Children | 1 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.34, 2.74] |
| 1.4 Resolution of symptoms ITT (subgroup sponsored versus no sponsor reported) | 5 | 2018 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.12] |
| 1.4.1 Sponsor not reported | 2 | 769 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.81] |
| 1.4.2 Sponsored studies | 3 | 1249 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.70, 1.16] |
| 1.5 Sore throat (ITT analysis) | 1 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.04] |
| 1.6 Fever (ITT analysis) | 1 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.19, 4.98] |
| 1.7 Incidence of relapse (evaluable participants) | 4 | 1386 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 0.99] |
| 1.7.1 Adults | 2 | 770 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.20, 0.88] |
| 1.7.2 Children | 2 | 616 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.33, 2.45] |
| 1.8 Complications (ITT analysis) | 1 | 244 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Adverse events (ITT analysis) | 3 | 1279 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.27, 3.25] |
Comparison 2. Macrolides versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Resolution of symptoms post‐treatment (ITT analysis) | 6 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.35] |
| 2.1.1 Adults | 5 | 1239 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.34] |
| 2.1.2 Children | 1 | 489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.84] |
| 2.2 Resolution of symptoms post‐treatment (evaluable participants only) | 6 | 1159 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.09] |
| 2.2.1 Adults | 5 | 801 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 2.2.2 Children | 1 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.11] |
| 2.3 Resolution of symptoms ITT (subgroup sponsored versus no sponsor reported) | 6 | 1728 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.35] |
| 2.3.1 Sponsor not reported | 3 | 860 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.48] |
| 2.3.2 Sponsored studies | 3 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.85, 1.46] |
| 2.4 Sore throat post‐treatment (ITT analysis) | 2 | 371 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.46] |
| 2.5 Fever post‐treatment (ITT analysis) | 2 | 371 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.59] |
| 2.6 Incidence of relapse (evaluable participants) | 6 | 802 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.48, 3.03] |
| 2.6.1 Adults | 5 | 495 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.34, 2.39] |
| 2.6.2 Children | 1 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 3.10 [0.67, 14.25] |
| 2.7 Adverse events (ITT analysis) | 6 | 1727 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.82, 1.73] |
| 2.7.1 Adults | 5 | 1238 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.50] |
| 2.7.2 Children | 1 | 489 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [1.06, 5.15] |
Comparison 3. Azithromycin versus amoxicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Clinical cure at 24 to 28 days (ITT) | 1 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.05] |
| 3.1.1 Children | 1 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.05] |
| 3.2 Clinical cure at 24 to 28 days (bacteriological per protocol population) | 1 | 482 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.73] |
| 3.2.1 Children | 1 | 482 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.73] |
| 3.3 Relapse on day 38 to 45 (ITT) | 1 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| 3.3.1 Children | 1 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| 3.4 Relapse on day 38 to 45 (bacteriological per protocol) | 1 | 422 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.82] |
| 3.4.1 Children | 1 | 422 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.82] |
| 3.5 Adverse events (all participants) | 1 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.78, 3.99] |
| 3.5.1 Children | 1 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.78, 3.99] |
Comparison 4. Carbacephem versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Resolution of symptoms post‐treatment (ITT analysis) | 3 | 795 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.49, 0.99] |
| 4.1.1 Adults | 2 | 562 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.46, 1.22] |
| 4.1.2 Children | 1 | 233 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.99] |
| 4.2 Resolution of symptoms post‐treatment (evaluable participants) | 3 | 602 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 4.2.1 Adults | 2 | 410 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.31, 1.13] |
| 4.2.2 Children | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.38] |
| 4.3 Incidence of relapse (evaluable participants) | 3 | 523 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.64, 2.50] |
| 4.4 Adverse events (ITT analysis) | 3 | 795 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.75, 1.55] |
Comparison 5. Clindamycin versus ampicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Adverse events (ITT analysis) | 1 | 314 | Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.15, 1.10] |
Comparison 6. Sulfonamide versus penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Adverse events (ITT analysis) | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.43, 4.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bachand 1991.
| Study characteristics | ||
| Methods | RCT, randomised 1:1 Double‐blinded Double‐dummy |
|
| Participants | Number of randomised participants: 128 (108 Streptococcus pyogenes positive) Number of participants evaluated: 90 Number of dropouts: 38 (29.7%) Setting: 17 clinical centres in the USA Age: 12 to 62 years Diagnosis: rapid immunoassay test, throat culture Inclusion criteria: confirmed GABHS pharyngitis Exclusion criteria: risk for pregnancy or lactation, weight < 34 kg, no sore throat with at least 1 sign of streptococcal pharyngitis, negative rapid immunoassay test, overall poor health, hypersensitivity to erythromycin or penicillin, renal impairment or hepatic disease, history of rheumatic fever or cardiac valvular disease, rash suggestive of scarlet fever, active eye inflammation, treated with systemic antibiotic within 2 weeks/an investigational drug within 4 weeks/long‐acting injectable penicillin within 6 weeks prior to trial, concurrent antimicrobial agents | |
| Interventions | Groups: clarithromycin, 250 mg (2 x 125 mg) caps 12‐hourly (n = 65); penicillin VK 250 mg (2 x 125 mg) caps 6‐hourly (n = 63) Duration of therapy: 80% > 10 days Duration of follow‐up: 15 to 56 days | |
| Outcomes | Clinical outcomes at 2 to 10 days post‐treatment: cure (pre‐treatment signs and symptoms resolved and pathogen eradicated); improvement (pre‐treatment signs and symptoms improved but not resolved); failure (pre‐treatment signs and symptoms not improved or worsened and pathogen persisted); indeterminate (response could not be assigned); relapse/recurrence (pre‐treatment signs and symptoms resolved but reappeared and pathogen recurred) Relapse at 15 to 56 days post‐treatment Adverse effects Bacteriological outcomes Serology | |
| Notes | Funding: not reported, but author is employee of Abbott International Ltd. Ethics approval: "the protocol was approved by local ethics committees" No ITT for efficacy reported No ITT reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised (1:1)". Not described how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "To maintain the double‐blind nature of the study, placebos were administered and all drugs were placed in identical grey opaque capsules." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26 participants prematurely discontinued, and 38 were excluded from efficacy analysis (reasons reported). 29.7% postrandomisation dropout No ITT analysis (128 randomised and 90 included in efficacy analysis) |
| Selective reporting (reporting bias) | Unclear risk | "There was no evidence of investigator bias in any of the analyses." |
| Other bias | High risk | Funding: not reported, but author is employee of Abbott International Ltd. |
Carbon 1995.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled: 250 Number of participants randomised: 240 Number of participants evaluated: 236 Number of dropouts: 4 (2%) Setting: 60 French general practice clinics Age: > 15 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: fever =/> 38 °C, odynophagia, erythema or purulent exudate of pharynx, at least 1 tender submaxillary lymph node, rapid antigen test positive for GABHS, followed by positive throat culture Exclusion criteria: allergy to beta‐lactams, pregnancy, lactation, chronic tonsillitis, antibiotics in 5 days preceding randomisation, no written consent | |
| Interventions | Groups: cefotiam hexetil (CTM), 200 mg twice a day for 5 days and a penicillin V (PEV)‐like placebo 3 times a day for 10 days (n = 119); penicillin V (PEV) megaunit (600 mg) 3 times a day for 10 days and CTM‐like placebo twice a day for 5 days (n = 125) Duration of treatment: 15 days Duration of follow‐up: 90 days | |
| Outcomes | Clinical outcomes: success = cure (complete resolution of fever and symptoms) on days 10 and 30 or improvement on day 10 and cure on day 30 without further antibiotics Failure = no response to therapy on day 10, or improvement on day 10 but required further antibiotic or relapsed (recurrence of fever or symptoms, or both), or cured on day 10 but subsequent relapse Relapse assessed on day 90 Adverse effects Bacteriological outcomes | |
| Notes | Funding: not reported Ethics approval: not mentioned Described as ITT analysis for efficacy, but postrandomisation exclusions not included in analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised", but no description of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Reported as "double blind, double dummy", but no description of how blinding of different administration frequency and duration was maintained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 4 lost to follow‐up (all in penicillin group) No ITT analysis (although reported in table that ITT, the numbers do not correspond to ITT) |
| Selective reporting (reporting bias) | Unclear risk | Only clinical success reported, no specific symptoms. Adverse events reported, but no ITT analysis. 3 participants in each group discontinued because of adverse events. |
| Other bias | Unclear risk | Funding: not reported |
Disney 1992a.
| Study characteristics | ||
| Methods | RCT Double‐blinded | |
| Participants | Number of participants eligible: 654 Number of participants randomised: 525 Number of participants evaluated: 525 Number of dropouts: not specified Setting: 7 paediatric practices in the USA Age: 4 to 17 years Diagnosis: clinical tonsillitis or pharyngitis, throat cultures Inclusion criteria: clinical tonsillopharyngitis and throat cultures strongly positive for GABHS Exclusion criteria: concurrent enrolment of siblings, 2 or more sore throats in previous 6 months, treated with antibiotic in previous 2 weeks, throat culture negative for GABHS | |
| Interventions | Groups: cephalexin 27 mg/kg 4 times per day (n = 263); penicillin 27 mg/kg 4 times per day (n = 262) Duration of treatment: 10 days Duration of follow‐up: 32 to 35 days | |
| Outcomes | Clinical outcomes: clinical failure (not defined) at 32 to 35 days Clinical relapse (new infection with different serotype) Bacteriological outcomes Antistreptolysin‐O titres Anti‐DNase B titres | |
| Notes | Funding: grant from Lilly Research Laboratories, Indianapolis, IN, USA Ethics approval: not mentioned ITT analysis on 525 participants completing the protocol, no information on dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised", but no description of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | "The participants were assigned...on a random schedule supplied by Eli Lilly and Co." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "...the physician and parents were not appraised as to who was in which group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No description of dropouts, 525 of 525 randomised participants reported ITT analysis for clinical outcome |
| Selective reporting (reporting bias) | Unclear risk | Only clinical (and bacteriological) failure reported, no symptoms specified. No reporting of adverse events |
| Other bias | High risk | Funding: grant from Lilly Research Laboratories, Indianapolis, IN, USA |
Disney 1992b.
| Study characteristics | ||
| Methods | RCT, randomised 1:1 Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled: 233 (19 negative culture) Number of evaluated participants: 192 Number of dropouts: 31 (13%) Setting: 11 paediatric offices in the USA Age: 6 months to 12 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: clinical diagnosis of acute streptococcal pharyngitis/tonsillitis, inflammation and swelling, with or without fever =/> 38 °C or exudate, rapid antigen test or throat culture positive for GABHS, history of compliance Exclusion criteria: history of renal impairment (serum creatinine ≥ 177 µmol/L, 2.0 mg/dL), any condition that could preclude evaluation of response, requirement for systemic antibiotic, any antibiotic therapy within 3 days of start, hypersensitivity to penicillins and/or cephalosporins | |
| Interventions | Groups: loracarbef oral suspension, 15 mg/kg/day 2 divided doses, or 200 mg caps 2 per day (participant > 25 kg) (n = 120); penicillin VK oral suspension 20 mg/kg/day 4 doses, daily maximum 500 mg or 250 mg caps 4 per day (participant > 25 kg) (n = 113) Duration of treatment: 10 days Duration of follow‐up: 4 to 5 weeks | |
| Outcomes | Clinical outcomes at 3 to 5 days post‐treatment: cure (absence of presenting signs/symptoms); significant improvement (persistence of signs/symptoms); failure (insignificant change in signs/symptoms); relapse (recurrence of 1 or more signs/symptoms) Relapse at 5 to 6 weeks post‐treatment Adverse effects Bacteriological outcomes | |
| Notes | Funding: Eli Lilly Company Ethics approval: not mentioned No ITT reported for efficacy, but ITT for adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised (1:1)", but no reporting of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo was administered twice daily to the loracarbef group to maintain double blind conditions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "unevaluable": 16 in loracarbef group and 25 in penicillin group (negative pre‐therapy culture, insufficient therapy, incomplete data, lost to follow‐up, late for visit, concomitant use of other antibiotic) No ITT for clinical outcome |
| Selective reporting (reporting bias) | Unclear risk | ITT for adverse events |
| Other bias | High risk | Funding: Eli Lilly Company |
Henness 1982.
| Study characteristics | ||
| Methods | Study 1: RCT Double‐blinded Study 2: RCT Double‐blinded |
|
| Participants | Study 1: Number of participants randomised: 214 (47 no Streptococcus pyogenes) Number of evaluated participants: 162 (75.7%) Number of dropouts: 3 lost to follow‐up from evaluable participants Setting: private paediatric practices in the USA Age: 1 to 16 years Diagnosis: throat culture Inclusion criteria: acute untreated tonsillopharyngitis Exclusion criteria: not reported Study 2: Number of participants randomised: 198 Number of evaluated participants: 198 Number of dropouts: 0? Setting: private paediatric practices in the USA Age: 1 to 16 years Diagnosis: throat culture Inclusion criteria: acute untreated tonsillopharyngitis Exclusion criteria: not reported |
|
| Interventions | Study 1: Groups: penicillin V suspension 8 mg/kg every 6 hours (n = 114); cefadroxil suspension 15 mg/kg twice daily (n = 100) Duration of treatment: 10 days Duration of follow‐up: 27 to 43 days Study 2: Groups: penicillin V suspension 10 mg/kg every 8 hours (n = 50); cefadroxil suspension 15 mg/kg twice daily (n = 50); erythromycin 15 mg/kg orally twice daily (n = 49); benzathine penicillin G (900,000 units) and procaine penicillin (300,000 units) once intramuscular Duration of treatment: 10 days for all oral treatments Duration of follow‐up: 27 to 43 days |
|
| Outcomes | Study 1: Clinical outcomes: cure (clinical improvement within first 24 hours of therapy and all follow‐up cultures no S pyogenes); failure (illness consistent with streptococcal infection and positive throat culture at 4 days post‐therapy); carrier (asymptomatic with same type S pyogenes in throat culture obtained between 5 to 33 days post‐therapy) Bacteriological outcomes Complete blood counts Urinalysis Streptozyme titres Susceptibility studies Study 2: Clinical outcomes: not reported Bacteriological outcomes Streptozyme titres Susceptibility |
|
| Notes | Study 1: Funding: not mentioned, author employee of Mead Johnson Pharmaceutical Division, Evansville, IN, USA Ethics approval: not mentioned First study in the publication No ITT reported Study 2: Funding: not mentioned, author employee of Mead Johnson Pharmaceutical Division, Evansville, USA Ethics approval: not mentioned Second study in the publication No ITT reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study 1: Reported as "randomised", but no description of randomisation sequence Study 2: Reported as "randomised", but no description of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Study 1: "...participants were assigned randomly..." Study 2: Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study 1: Reported as "double blind", but no description of blinding Study 2: Reported as "double blind", but no description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study 1: 52 participants discontinued (cefadroxil 35 and penicillin 17); reasons: negative culture (total 47; cefadroxil 31 and penicillin 16), lost to follow‐up (total 3; cefadroxil 2 and penicillin 1), other (total 2; cefadroxil 2 and penicillin 0). 24.3% postrandomisation dropout No ITT analysis for clinical outcomes Study 2: No dropouts described; according to reported numbers no participants dropped out. |
| Selective reporting (reporting bias) | Unclear risk | Study 1: Only clinical (and bacteriological) cure reported, no specific symptoms; no ITT. Adverse events not reported. Study 2: No clinical outcomes reported. |
| Other bias | High risk | Author is an employee of Mead Johnson Pharmaceutical Division, Evansville, IN, USA. |
Jackson 1973.
| Study characteristics | ||
| Methods | RCT Double‐blinded | |
| Participants | Number of participants randomised: 314 (95 negative culture excluded from analysis) Number of participants evaluated: 207 (70%) Number of dropouts: 12 reported Setting: not described Age: not described Diagnosis: throat culture Inclusion criteria: child in weight range 11.4 to 45.4 kg, pharyngitis, positive culture or white blood count > 10,000 Exclusion criteria: allergy to penicillin or lincomycin, received any antibiotics within previous 6 weeks | |
| Interventions | Groups: clindamycin daily dose 150 to 450 mg (n = 156); ampicillin daily dose 750 to 2000 mg (n = 158) Duration of treatment: 10 days Duration of follow‐up: 26 to 28 days post‐therapy | |
| Outcomes | Adverse effects Bacteriological outcomes | |
| Notes | Funding: Upjohn Company Ethics approval: not mentioned ITT for adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised", but no description of randomisation sequence |
| Allocation concealment (selection bias) | Low risk | "Labels for each group were randomised, sealed in sequentially numbered envelopes,..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 95 negative cultures excluded after randomisation; 12 positive cultures excluded due to failure to return first follow‐up culture (clindamycin 7 and ampicillin 5). 30% postrandomisation dropout |
| Selective reporting (reporting bias) | Unclear risk | Only clinical outcome for poststreptococcal sequelae ITT for adverse events |
| Other bias | High risk | Funding: Upjohn Company |
Levenstein 1991.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled: 243 (82 Streptococcus pyogenes negative) Number of participants evaluated in clinical outcome analysis: 125 (51.4%) Number of dropouts: 28 (12%) Setting: multicentre (Australia, New Zealand, Chile, South Africa) outpatient clinics Age: 13 to 59 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: body weight =/> 50 kg, ability to swallow capsules, sore throat with at least 1 other sign of streptococcal pharyngitis (pharyngeal erythema/exudate, cervical lymph node tenderness, fever), positive rapid immunoassay for GABHS antigen Exclusion criteria: hypersensitivity to erythromycin or penicillin, previous course of clarithromycin or penicillin VK in this trial, renal impairment or history of glomerulonephritis, history of hepatic disease or liver enzyme elevation, history of cardiac valvular disease, rash symptomatic of scarlet fever, history of allergies or asthma, or both | |
| Interventions | Groups: clarithromycin, 250 mg capsules every 12 hours (n = 128); penicillin VK, 250 mg capsules every 6 hours (n = 115) Duration of treatment: clarithromycin 8 to 10 days; penicillin VK 10 to 14 days Duration of follow‐up: 15 to 56 days | |
| Outcomes | Clinical outcomes at 2 to 10 days post‐treatment: cure (pre‐treatment signs and symptoms resolved); improvement (symptoms improved but not totally resolved); failure (symptoms not improved or worsened); indeterminate (clinical response could not be assigned because of non‐compliance or other reasons) Relapse 15 to 56 days post‐treatment Adverse effects Bacteriological outcomes Blood haematology and chemistry Urinalysis | |
| Notes | Funding: not reported Informed consent obtained Ethics approval: "the study was approved by local ethics committees" No ITT for efficacy, but ITT for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised" but no description of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Description of medication and placebo to ensure blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts accounted for the bacteriological outcome analysis, but not for the clinical outcome analysis (only 125 of 243 randomised participants included in clinical outcome analysis). 48.6% postrandomisation dropout No ITT for clinical outcomes |
| Selective reporting (reporting bias) | Unclear risk | Safety analysis on all 243 randomised participants; clinical and bacteriological outcomes on only 125 participants |
| Other bias | Unclear risk | Funding: not reported |
McCarty 1992a.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of enrolled participants: 218 Number of participants randomised: 218 (31 negative culture) Number of participants evaluated: 171 (78.4%) Number of dropouts: 47 (22%) Setting: 12 study centres in North America Age: > 12 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: clinical diagnosis of streptococcal pharyngitis or tonsillitis: inflammation of pharynx and tonsils with pain in the throat, with or without fever or exudate, rapid antigen test or throat culture positive for GABHS Exclusion criteria: pregnancy, lactation, history of renal impairment (serum creatinine levels ≥ 177 µmol/L, 2.0 mg/dL), physical or mental condition that might preclude evaluation of response, possible future need for other systemic antibiotic during study, use of antibiotic therapy within 3 days of pre‐therapy evaluation, use of other investigational agents within previous 28 days, hypersensitivity to beta‐lactam antibiotic | |
| Interventions | Groups: loracarbef oral suspension 15 mg/kg/day 2 doses, daily maximum 375 mg, or 200 mg capsules 2 per day (n = 107); penicillin VK oral suspension 20 mg/kg/day 4 doses daily maximum 500 mg, or 250 mg capsules 4 per day (n = 111) Duration of treatment: 10 days Duration of follow‐up: 28 to 35 days | |
| Outcomes | Clinical outcomes at 3 to 5 days post‐treatment: cure (total alleviation of difficulty in swallowing, pharyngeal pain); improvement (substantial improvement in signs and symptoms); failure (signs and symptoms not substantially alleviated); relapse (initial improvement or alleviation of symptoms, but subsequent worsening or recurrence); unable to evaluate Relapse at 28 to 35 days post‐treatment Adverse effects Bacteriological outcomes | |
| Notes | Funding: Eli Lilly and Company Informed consent obtained. Ethics approval: not mentioned No ITT reported for efficacy, but ITT reported for adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised"; no description of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "In order to maintain blinding, placebo was administered twice daily to participants in the loracarbef group so that all participants received 4 doses daily." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: 18 in loracarbef group and 29 in penicillin group. Reasons for dropout: negative culture (loracarbef 12 and penicillin 19), insufficient therapy, incomplete data, use of other antibiotic, non‐compliance, lack of post‐therapy culture 21.6% postrandomisation dropout No ITT for clinical outcome |
| Selective reporting (reporting bias) | Unclear risk | ITT for adverse events analysis |
| Other bias | High risk | Funding: Eli Lilly and Company |
Muller 1992.
| Study characteristics | ||
| Methods | RCT Double‐blind | |
| Participants | Number of enrolled participants: 344 Number of participants randomised: 344 Number of participants evaluated: 239 (69.5%) Number of dropouts: 105 (31%) Setting: study centres in Europe and Israel Age: 3 to 80 years (mean 28.2) 10.8% < 12 years, 2.0% > 65 years Diagnosis: rapid antigen test and confirmed by throat culture Inclusion criteria: clinical diagnosis of streptococcal pharyngitis or tonsillitis and a positive rapid streptococcal antigen test. Selections were made on the basis of a demonstrated history of therapeutic compliance on the part of the patient or the patient's parent/guardian, or both. Exclusion criteria: pregnant or nursing or history of renal impairment; any condition, including significant underlying disease or concomitant infection, which in the opinion of the investigator could have precluded evaluation of response; anticipated need for systemic antibiotics; use of antibiotic < 3 days; or hypersensitivity to penicillins or cephalosporins, or both | |
| Interventions | Groups: 1) loracarbef (n = 169) suspension of 15 mg/kg/day in 2 divided doses up to a maximum daily dose of 375 mg or as a 200 mg capsule twice daily, with placebo twice daily to maintain double‐blind conditions 2) penicillin V (n = 175 suspension of 20 mg/kg/day in 4 divided doses up to a maximum daily dose of 500 mg or as 250 mg capsules) 4 times daily Duration of treatment: 10 days Duration of follow‐up: 38 to 45 days Concomitant medication for treatment of underlying diseases or conditions was allowed with the exception of systemic antibiotics. Paracetamol was used during therapy by 5.5% of the participants. |
|
| Outcomes | Clinical outcomes at days 4 to 6: participants' symptomatic responses and adherence to the treatment regimen; at days 13 to 15: physical examination to determine symptomatic response to therapy; at days 38 to 45: physical examination to evaluate possible recurrence of pharyngitis or tonsillitis. Throat cultures were required at every observation period. Global symptomatic response based on symptom score (difficulty in swallowing, pharyngeal pain, pharyngeal redness, tonsillar inflammation, tonsillar swelling, and temperature): cure, improvement (substantial), failure, relapse, or unable to evaluate Relapse: no definition given A participant was discontinued from the study if the pathogen isolated from initial culture was resistant to study antibiotic; if there was obvious symptomatic failure of the study antibiotic at any time during treatment; if there was a significant adverse event or a clinically significant alteration in a laboratory parameter; if a participant or parent/guardian wished to withdraw from the study; if the blinding was broken for safety reasons; or if the participant had an elevated pre‐therapy serum creatinine. Adverse events: at least 1 adverse event was reported by loracarbef = 22 (13.0%) and penicillin V = 19 (10.9%) participants. Headache and nausea/vomiting were the only 2 events reported during therapy by more than 2% of the total population. Headache was reported by loracarbef = 5/169 (3.0%) and by penicillin V = 4/175 (2.3%) (P = 0.696). Nausea or vomiting was reported by loracarbef = 2/169 (1.2%) and by penicillin V = 5/175 (2.9%) (P = 0.272). Few participants (approximately 5% of the total population) reported adverse events during the 28‐ to 35‐day post‐therapy follow‐up period. | |
| Notes | Funding: grants from Lilly Research Centre Ltd. Informed consent obtained. Ethics: "conducted according to ethical committee guidelines, including the Declaration of Helsinki (1983 Venice Amendment)" No ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "with placebo twice daily to maintain double‐blind conditions." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 54/169 (31.9%) loracarbef‐treated and 51/115 (29.1%) penicillin‐treated participants did not qualify for efficacy evaluation. The most common reasons for disqualification in each therapy group were bacteriological (loracarbef = 37, penicillin V = 3); 12 participants in each group received either insufficient therapy, had no follow‐up data (lost to follow‐up), or had incomplete data; loracarbef = 3 and penicillin V = 1 participants were disqualified from the efficacy analysis due to protocol violations; loracarbef = 1 participant was disqualified for efficacy evaluation due to the use of another antibiotic during the study period, and loracarbef = 1 participant could not be evaluated because the post‐therapy evaluation was performed 22 days after discontinuing therapy. |
| Selective reporting (reporting bias) | Unclear risk | All indicated outcomes are reported. |
| Other bias | High risk | Funding: grants from Lilly Research Centre Ltd. |
NCT00643149.
| Study characteristics | ||
| Methods | RCT non‐inferiority trial 15 May 2003 to 22 May 2004 |
|
| Participants | Number of participants enrolled: target 626 (313 per arm)
Number of participants randomised: 693 Number of evaluated (treated) participants: 673 (337 azithromycin and 336 amoxicillin) Number of participants discontinued: 125 (56 azithromycin and 69 amoxicillin) Age: children 2 to 12 years Setting: multicentre: 33 centres in North America (6 sites in Canada, 19 in the USA), Latin America (3 sites in Costa Rica, 1 in Guatamala), and India (4 sites); paediatric outpatients Acute pharyngitis/tonsillitis based on "erythematous pharyngeal mucosa or thick exudate covering the pharynx and tonsillar area, and at least one of the following signs or symptoms: sore/scratchy throat; pain on swallowing; chills and/or fever; cervical adenopathy; scarlet fever rash on the face and skin folds, or red tongue with prominent papillae ("strawberry tongue")." Positive rapid antigen detection test or positive culture for GABHS GABHS pharyngitis/tonsillitis (tested for susceptibility to azithromycin and amoxicillin) |
|
| Interventions | Azithromycin Sustained Release 60 mg/kg single dose (n = 337); bacteriological per protocol population (n = 245) Amoxicillin 45 mg/kg twice daily for 10 days (n = 336); bacteriological per protocol population (n = 237) |
|
| Outcomes | Bacteriological cure (primary outcome) Clinical success Compliance Adverse events Time points of assessment: "Test of Cure" at 24 to 28 days after starting study drug, and long‐term follow‐up on days 38 to 45 |
|
| Notes | Report provided by Pfizer. Study supported and conducted by Pfizer. Protocol No: A0661071 Outcomes only reported for "Bacteriological Per Protocol Population", i.e. positive GABHS culture at recruitment or within 48 hours of starting treatment, at least 8 days of study medication and assessment at baseline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo matched to the active treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total 693 randomised; 20 were not treated due to insufficient drug supply at study site (no more information given). Of 673 participants treated, 125 discontinued (56 in azithromycin group and 69 in amoxicillin group); reasons for discontinuation provided (more dropout due to adverse events in azithromycin arm (4.7% versus 0.9%) and more lack of efficacy in amoxicillin arm (8.3% versus 3.3%)). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. |
| Other bias | High risk | Study supported and conducted by Pfizer. |
Nemeth 1999.
| Study characteristics | ||
| Methods | RCT, randomised 1:1:1 Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled: 919 Number of positive throat cultures susceptible to study drugs: 725 Number of participants evaluated: 644 Number of dropouts: 275 (30%) Setting: 25 study centres in the USA and Canada Age: =/> 13 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: throat culture positive for GABHS, at least 1 clinical sign or symptom of pharyngitis Exclusion criteria: pregnancy, history of rheumatic fever or rheumatic heart disease, peritonsillar abscess or invasive disease, hypersensitivity to beta‐lactam drugs, hepatic disease, hepatic enzyme levels or serum creatinine > 2 times upper limit of normal, another systemic antibiotic within 3 days before first dose of study medication or for which < 5 half‐lives had elapsed, enrolled in this study previously, received another investigational drug within 4 weeks before study admission | |
| Interventions | Groups: cefdinir 600 mg 4 times a day (n = 305); cefdinir 300 mg twice a day (n = 304); penicillin V 250 mg 4 times a day (n = 310) Duration of treatment: 10 days Duration of follow‐up: 17 to 24 days post‐therapy | |
| Outcomes | Clinical outcomes at day 4 to 9 after treatment: cure (all signs and symptoms absent or in satisfactory remission and no further antibiotic therapy required); failure (absence of significant remission of signs and symptoms or need for further antibiotic therapy); relapse (worsening of, or absence of significant remission of, signs and symptoms 17 to 24 days post‐therapy or need for further antibiotic therapy) Relapse at day 17 to 24 after treatment Adverse effects Bacteriological outcomes | |
| Notes | Funding: Parke‐Davis Pharmaceutical Research, Ann Arbor, MI, USA (first author is employee) Informed consent obtained. Ethics approval: institutional review board approval obtained at each site No ITT for efficacy reported, but ITT for adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised", but no description of the randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly assigned in a 1:1:1 ratio." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "All participants took the same number of capsules daily. All regimens were administered for 10 days." No description of the appearance of the capsules |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts 275: no GABHS at admission culture (194); failure to return or non‐compliance (not specified in which group) 30% dropout No ITT analysis for clinical outcomes |
| Selective reporting (reporting bias) | Unclear risk | Only clinical cure reported, no symptoms specified. Adverse events analysed by ITT: 21 participants discontinued due to adverse events (cefdinir = 17, penicillin V = 4); difference between groups is not significant. |
| Other bias | High risk | Funding: Parke‐Davis Pharmaceutical Research, Ann Arbor, MI, USA (first author is employee) |
Norrby 2002.
| Study characteristics | ||
| Methods | RCT, randomised 1:1 Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled: 398 Number of participants randomised: 396 (1 negative culture) Number of participants evaluated: 395 Number of dropouts: 34 (9%) Setting: 62 centres in 10 countries (Europe, New Zealand, South Africa) Age: 15 to 74 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: clinical signs and symptoms of acute pharyngitis/tonsillitis, including sore throat and 1 or more others; presumed diagnosis of acute GABHS pharyngitis/tonsillitis based on positive rapid antigen detection test or throat culture within 24 hours prior to starting study medication Exclusion criteria: infection of deep tissues of upper respiratory tract or subpharyngeal respiratory tract; head or neck cancer; history of rheumatic heart disease or valve disease, infectious mononucleosis, rash; immunocompromised, impaired renal or hepatic function, history of heart rhythm diseases, severe hypokalaemia, any concomitant condition likely to preclude assessment of treatment response; non‐streptococcal or viral pharyngitis/tonsillitis, chronic streptococcal carrier, environmental risk of reinfection, treatment with penicillin V, systemic or local antibiotic within 7 days prior to study entry; pregnancy, lactation, hypersensitivity to study antibiotic, infection with a pathogen known to be resistant to study drugs, concurrent treatment with other antibiotic or probenecid, or any medication that may interact with study medication | |
| Interventions | Groups: telithromycin 800 mg oral once daily (n = 198); penicillin V 500 mg oral 3 times daily (n = 197) Duration of treatment: telithromycin 5 days; penicillin V 10 days Duration of follow‐up: 38 to 45 days | |
| Outcomes | Clinical outcomes at day 16 to 20: cure (improvement, disappearance, or return to preinfection state of all infection‐related signs and symptoms, without additional antibiotic); failure (infection‐related signs and symptoms unchanged or worsened, or clinical improvement but required additional antibiotic, developed new clinical findings consistent with active infection); indeterminate (missing post‐treatment information, discontinued early for reasons unrelated to study drug) Relapse at day 38 to 45 Adverse effects Bacteriological outcomes Blood haematology Urinalysis Mean symptom score reported in second publication; no SD reported. | |
| Notes | Funding: Aventis Pharma Informed consent obtained. Ethics approval: "approved by and independent ethics committee in each country" Modified ITT (1 participant with negative GABHS excluded) 2 publications of same study with different outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Reported as "randomised (1:1)"; randomisation not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Blinding was maintained by masking the tablets in capsules and matching placebo capsules where appropriate." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT for clinical outcomes excluded 1 randomised participant with negative culture; 34 participants discontinued, mainly due to withdrawal of consent or adverse events; not clear how these reasons were distributed in the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | Cure was predefined clinical outcome; adverse events reported. |
| Other bias | High risk | Funding: Aventis Pharma |
O'Doherty 1996.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled: 489 (92 negative culture) (azithromycin 20 mg = 160; azithromycin 10 mg = 166; penicillin V = 163) Number of participants evaluated: 358 Number of dropouts: 131 excluded (azithromycin 20 mg = 57; azithromycin 10 mg = 43; penicillin V = 31) (27%) Setting: 19 outpatient clinical centres in Europe Age: 2 to 13 years Diagnosis: clinical examination, rapid antigen test Inclusion criteria: clinical signs and symptoms suggestive of GABHS pharyngitis/tonsillitis, rapid antigen test positive for GABHS Exclusion criteria: within 72 hours prior to the study other antibiotic which could interfere with evaluation of therapy, hypersensitivity to macrolide or beta‐lactam antibiotic, terminal illness or other serious disease, any gastrointestinal condition that might affect drug absorption, other investigational drug in the previous month or long‐acting penicillin injections within the previous 6 weeks | |
| Interventions | Groups: azithromycin suspension single oral dose 10 mg/kg (n = 166); azithromycin suspension 1 single dose 20 mg/kg (n = 160); penicillin V solution 50 mg/mL orally 4 times daily (total daily dose 500 to 1000 mg) (n = 163) Duration of treatment: azithromycin 3 days; penicillin V 10 days Duration of follow‐up: 28 to 30 days | |
| Outcomes | Clinical outcomes at day 12 to 14: cure, improvement, failure, relapse Relapse at day 28 to 30 Adverse effects Bacteriological outcomes Blood haematology and chemistry Urinalysis | |
| Notes | Funding: not reported Informed consent obtained. Ethics approval: institutional review board approval obtained Definition of outcomes not reported. No ITT for efficacy, but ITT for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised", but no description of randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Matched placebo suspensions or solutions were administered to maintain blinding of the study." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout 131 participants: absence of pathogen (azithromycin 20 mg = 36; azithromycin 10 mg = 30; penicillin = 26), deviation from protocol (azithromycin 20 mg = 10; azithromycin 10 mg = 8; penicillin = 3), adverse event (azithromycin 20 mg = 11; azithromycin 10 mg = 5; penicillin = 2) 27% postrandomisation dropout No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Only clinical (and bacteriological) cure reported, no specific symptoms in outcome analysis. Adverse events reported with ITT analysis. |
| Other bias | Unclear risk | Funding: not reported |
Randolph 1985.
| Study characteristics | ||
| Methods | RCT Double‐blinded | |
| Participants | Number of eligible participants: 260 Number of randomised participants: 194 Number of participants evaluated: 194 Number of dropouts: 0 Setting: a private paediatric office Age: 2 to 20 years Diagnosis: throat culture Inclusion criteria: clinically suggestive GABHS pharyngitis Exclusion criteria: history of hypersensitivity to penicillin or cephalosporins, antibiotic within previous 72 hours | |
| Interventions | Groups: cefadroxil 250 mg in 3 doses over next 18 to 24 hours (n = 70); penicillin V 250 mg in 3 doses over next 18 to 24 hours (n = 68); placebo (n = 56) Duration of treatment: 10 days Duration of follow‐up: 4 weeks (only results from examination 18 to 24 hours after initiation of treatment reported) | |
| Outcomes | Clinical outcomes 24 hours after treatment start assessed by physician: improvement Sore throat (numbers only reported in graph) Fever (numbers only reported in graph) Bacteriological outcomes | |
| Notes | Funding: Mead Johnson and Company Ethics approval: not mentioned ITT analysis reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All participants were then assigned by a table of random numbers..." |
| Allocation concealment (selection bias) | Low risk | "Randomization of treatment regimens was performed by a study nurse so that the evaluating physician, parents and participants were unaware of which agent was dispensed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts (all randomised participants evaluated) |
| Selective reporting (reporting bias) | Unclear risk | Specific signs and symptoms reported. No reporting of adverse events |
| Other bias | High risk | Funding: Mead Johnson and Company |
Reed 1991.
| Study characteristics | ||
| Methods | RCT Double‐blinded | |
| Participants | Number of participants enrolled and randomised: 116 Number of evaluated participants: 93 Number of dropouts: 23 (20%) Setting: 4 primary care offices in the USA Age: > 1 month Diagnosis: rapid test, throat culture Inclusion criteria: sore throat or poor eating, rapid test positive for GABHS Exclusion criteria: allergy to penicillin or cephalosporins, pregnancy, history of renal or hepatic impairment, significant underlying disease or concomitant infection that could preclude evaluation of response to treatment, antibiotic in the previous 3 days | |
| Interventions | Groups: cefaclor 20 mg/kg/day in 3 doses (n = 60); penicillin VK 20 mg/kg/day in 3 doses (n = 56) Duration of treatment: 10 days Duration of follow‐up: 28 to 30 days post‐therapy | |
| Outcomes | Clinical outcomes (not defined; according to clinician's impression at 2 days after treatment completion): cure, improvement, relapse, failure Relapse at day 28 to 30 Adverse effects Bacteriological outcomes Beta‐lactamase enzyme production | |
| Notes | Funding: Eli Lilly and Company, Indianapolis, IN, USA Informed consent obtained. Ethics approval not mentioned. No ITT reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "The patient was given a prescription that used a code number to identify the medication to be used." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The identity of the antibiotic was unknown to the physician and to the patient, and was randomised by a coding sheet that was available only to the pharmacists dispensing the study medication." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts 23: no GABHS on culture (cefaclor 6 and penicillin 2), insufficient therapy (cefaclor 0 and penicillin 1), no follow‐up culture (cefaclor 3 and penicillin 0), other antibiotic (cefaclor 1 and penicillin 2), could not be evaluated according to investigator (cefaclor 3 and penicillin 5) 20% postrandomisation dropout No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Only clinical (and bacteriological) outcomes reported, no specific symptom outcomes reported. Adverse events reported; no ITT analysis. |
| Other bias | High risk | Funding: Eli Lilly and Company, Indianapolis, IN, USA |
Stein 1991.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of participants enrolled and randomised: 128 (clarithromycin 65 and penicillin 63) Number of participants with Streptococcus pyogenes: 109 Number of participants evaluated: 95 (clarithromycin 47 and penicillin 48) Number of dropouts: 33 (26%) Setting: multicentre (not specified) Age: 12 to 58 years Diagnosis: clinical examination, rapid immunoassay test Inclusion criteria: signs and symptoms of streptococcal throat infection, rapid immunoassay test positive for GABHS antigen Exclusion criteria: age < 12 years, pregnancy, lactation, hypersensitivity to erythromycin or penicillin, receiving antibiotics, impaired renal or liver function | |
| Interventions | Groups: clarithromycin 250 mg capsule every 12 hours (n = 65); penicillin V 250 mg capsule every 6 hours (n = 63) Duration of treatment: 10 days Duration of follow‐up: 29 to 35 days | |
| Outcomes | Clinical outcomes at day 5 to 7 and at day 14 to 16: cure (complete resolution of signs and symptoms); improved (considerable resolution of presenting signs and symptoms); failure (no improvement) Relapse at day 29 to 35 Adverse effects Bacteriological outcomes Blood haematology and chemistry Urinalysis Serology (antistreptolysin‐O titres, anti‐DNase B titres) | |
| Notes | Funding: not reported Ethics approval: not mentioned No ITT for efficacy, but ITT for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random number code" was used, but unclear how it was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "In order to maintain blinding of the study placebo capsules were alternated with clarithromycin capsules every six hours." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts 33 (26%); no description of reasons; no ITT for clinical outcomes |
| Selective reporting (reporting bias) | Unclear risk | Clinical (and bacteriological) cure rate reported, no specific symptoms. Adverse events reported with ITT analysis. |
| Other bias | Unclear risk | Funding: not reported |
Trickett 1973.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of enrolled participants: 96 Number of participants evaluated: 87 Number of dropouts: 9 (9%) Setting: 3 institutions (regular clinics + emergency rooms) Age: > 16 years Diagnosis: throat culture Inclusion criteria: acute sore throat suggestive of acute streptococcal pharyngitis and/or tonsillitis, throat culture positive for GABHS Exclusion criteria: pregnancy, breastfeeding, antibiotic other than study drugs during the trial period, inadequate folate reserves, malabsorption syndrome, haemolytic anaemia, anticonvulsant therapy (dilantin, primidone), antibiotic 1 week preceding acute streptococcal infection, renal insufficiency, abnormal liver function, low platelets, total white cells, neutrophils, haemoglobin, haematocrit; glucose‐6‐phosphate dehydrogenase deficiency, systemic lupus erythematosus, history of idiosyncratic or allergic reactions to any of the drugs | |
| Interventions | Groups: sulfamethoxazole (SMZ) 400 mg and trimethoprim (TMP) 80 mg 2 tablets 4 times per day (n = 48); penicillin G 250 mg 1 tablet 4 times per day (n = 48) Duration of therapy: 10 days Duration of follow‐up: 28 days | |
| Outcomes | No clinical outcomes reported Adverse effects Bacteriological outcomes Urinalysis Creatinine Liver function: serum glutamic oxaloacetic transaminase (SGOT) or aspartate transaminase (AST) | |
| Notes | Funding: medication supplied by Hoffmann‐La Roche Inc. Ethics approval: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomised", but no description of randomisation sequence; "both groups were evenly matched as to age, sex, physical condition, and concurrent diagnoses." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all test medications were supplied in individually coded bottles of identical appearance and were administered according to the randomised double blind code." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 dropouts: lost to follow‐up, failed to take medication, or negative on strep A tests (not specified per group) No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Cure rates reported, not individual symptoms. Adverse events mentioned but not tested. |
| Other bias | Unclear risk | Funding: medication supplied by Hoffmann‐La Roche Inc. |
Watkins 1997.
| Study characteristics | ||
| Methods | RCT Double‐blinded Double‐dummy | |
| Participants | Number of participants randomised: 345 (dirithromycin 170 and penicillin 175) Number of participants evaluated: 257 (dirithromycin 121 and penicillin 136) Number of dropouts: 66 in each group (38%) Setting: 15 clinical centres in North America Age: > 12 years Diagnosis: rapid antigen test, throat culture Inclusion criteria: weight > 81 lb, positive throat culture, informed consent, ability to return for follow‐up, negative pregnancy test and use of a reliable method of contraception during therapy and for 30 days thereafter Exclusion criteria: any condition precluding evaluation of response to treatment, systemic antibiotic other than the study antibiotic; hypersensitivity to macrolides, penicillins, cephalosporins; pregnancy, breastfeeding; systemic antibiotic in 7 days before study; participation in a previous dirithromycin study or any study involving and investigational drug in the 30 days prior to this study | |
| Interventions | Groups: dirithromycin, 500 mg once daily (n = 170); penicillin VK 250 mg 4 times daily (n = 175) Duration of treatment: 10 days Duration of follow‐up: 3 to 5 weeks post‐treatment | |
| Outcomes | Clinical outcomes 3 to 5 days post‐treatment: cure (elimination of signs and symptoms); improvement (significant but incomplete resolution of signs and symptoms); relapse (worsening of signs and symptoms after initial improvement); failure (no improvement in signs and symptoms during treatment) Clinical relapse at 3 to 5 weeks post‐treatment not reported Adverse effects Bacteriological outcomes | |
| Notes | Funding: Eli Lilly and Company (2 authors are employees) Ethics approval: not mentioned No ITT for efficacy, but ITT for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by computer program. |
| Allocation concealment (selection bias) | Low risk | "The randomisation list was not provided to the investigators until the study was complete." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double dummy design"; "This was accomplished by giving two bottles to each patient, one containing 20 tablets (dirithromycin or placebo) and one containing 40 capsules (penicillin or placebo)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Description of dropouts in each group: lack of efficacy (dirithromycin 20; penicillin 26), lost to follow‐up (dirithromycin 4; penicillin 1), participant's decision (dirithromycin 3; penicillin 0), entry criteria exclusion (dirithromycin 25; penicillin 22), protocol violation (dirithromycin 8; penicillin 8), adverse event (dirithromycin 6; penicillin 9) 38% postrandomisation dropout No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Only clinical cure reported, no specific symptoms. Adverse events reported with ITT. |
| Other bias | High risk | Funding: Eli Lilly and Company (2 authors are employees) |
CTM: cefotiam hexetil GABHS: group A beta‐haemolytic streptococcus ITT: intention‐to‐treat lb: pound weight Penicillin VK: penicillin V potassium PEV: penicillin V RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1994 | Not double‐blinded |
| Adam 1995 | Not double‐blinded |
| Adam 1996 | Not double‐blinded |
| Adam 2000a | Not double‐blinded |
| Adam 2000b | Not double‐blinded |
| Adam 2001 | Not double‐blinded |
| Aujard 1995 | Not double‐blinded |
| Bottaro 2012 | Open‐label study |
| Breese 1974 | Did not compare 2 different classes of antibiotics |
| Cohen 2002 | Not double‐blinded |
| Davies 1995 | Not only acute GABHS tonsillopharyngitis |
| Del Mar 2008 | Commentary of an RCT |
| De Meyere 1992 | Not an RCT |
| Denny 1953 | Not double‐blinded |
| Disney 1979 | Did not compare 2 different classes of antibiotics |
| Dykhuizen 1996 | Not double‐blinded |
| Eslami 2014 | Not double‐blinded |
| Esposito 2002 | Not double‐blinded |
| Feder 1999 | Not double‐blinded |
| Gerber 1986 | Not double‐blinded |
| Gerber 1999a | Did not report any clinical outcomes |
| Gooch 1993 | Not double‐blinded |
| Granizio 2008 | Pooled analysis; not original studies |
| Hamill 1993 | Not double‐blinded |
| Haverkorn 1971 | Not an RCT Did not compare 2 different classes of antibiotics |
| Holm 1991 | Not double‐blinded |
| Howe 1997 | Not double‐blinded |
| Kuroki 2013 | Open‐label study |
| Lennon 2008 | Not double‐blinded (investigator‐blinded only) |
| Llerena 2011 | Meta‐analysis |
| Matsen 1974 | Did not compare 2 different classes of antibiotics |
| McCarty 1992b | Not double‐blinded |
| McCarty 1994 | Not double‐blinded |
| McIsaac 2004 | Did not compare 2 different classes of antibiotics |
| Milatovic 1991 | Not double‐blinded |
| Milatovic 1993 | Not double‐blinded |
| NCT00393744 | Not double‐blinded |
| Pacifico 1996 | Not double‐blinded |
| Perkins 1969 | Not double‐blinded |
| Pichichero 2000 | Not double‐blinded |
| Pichichero 2008 | Not double‐blinded (investigator‐blinded only) |
| Portier 1990 | Not double‐blinded |
| Portier 1994 | Not double‐blinded |
| Rimoin 2011 | Did not compare 2 different classes of antibiotics |
| Roos 1997 | Recurrent sore throat |
| Sakata 2008 | Not double‐blinded |
| Shapera 1973 | Not double‐blinded |
| Shvartzman 1993 | Not double‐blinded |
| Siegel 1961 | Did not compare 2 different classes of antibiotics |
| Standaert 1997 | Not only acute GABHS tonsillopharyngitis |
| Stelter 2014 | Review of results of tonsillectomy |
| Stillerman 1970 | No information on blinding and no data on clinical outcomes |
| Stillerman 1986 | Not double‐blinded |
| Tack 1997 | Not double‐blinded |
| Tack 1998 | Not double‐blinded |
| Uysal 2000 | Not double‐blinded |
| Van Brusselen 2014 | Review of tonsillitis guidelines |
| Zwart 2000 | Did not compare 2 different classes of antibiotics |
GABHS: group A beta‐haemolytic streptococci RCT: randomised controlled trial
Differences between protocol and review
In the 2010 review, outcomes were split into primary and secondary. The composite outcome 'resolution of symptoms' was included as a primary outcome.
In the 2013 update, the risk of bias assessment tool was changed from the Jadad score to the Cochrane 'Risk of bias' assessment tool. We also included a GRADE assessment using GRADEpro GDT software, and added a description of the GRADE assessment of the overall certainty of the evidence to the Methods section and text of the review.
Following advice from the Statistical Editor, we changed the analysis method for pooling to a random‐effects model as the default. To be consistent with our protocol (van Driel 2003), we also used a fixed‐effect model if there was no substantial heterogeneity, and compared results in a sensitivity analysis. This was mentioned as a sensitivity analysis in the protocol (van Driel 2003), and is now described in the Methods section as a subgroup analysis.
We performed subgroup analyses for adults and children where appropriate because this is relevant to clinicians; this was added to the Methods section.
Sensitivity analysis: our protocol planned sensitivity analyses for participants in different settings, per carrier status, or diagnostic criteria (throat culture or rapid test), publication status (published versus unpublished studies, studies published as abstract versus full‐text articles, year of publication). These were replaced with sensitivity analysis of the impact of heterogeneity and of applying a random‐effects and fixed‐effect model.
Sensitivity analysis according to methodological quality rated on the Jadad score, van Driel 2003, was abandoned with the introduction of the Cochrane 'Risk of bias' assessment.
The 2016 author team was changed to include Sarah Thorning as an author. Natalja Keber no longer contributed to the review and was removed as an author.
The outcome 'incidence of relapse' was added to the 'Summary of findings' table for cephalosporins compared to penicillin.
Hilde Habraken was removed as an author for the 2020 update, as she was no longer able to contribute to this review.
Contributions of authors
MVD wrote the protocol. All authors contributed to final editing of the protocol. ST conducted all searches for this review. MVD and ADS reviewed the searches for the review updates. MVD, ADS, and NK independently performed 'Risk of bias' assessment. MVD, NK and ADS performed data extraction. MVD analysed the data. MVD wrote the draft review and addressed the peer‐reviewers' comments. MVD updated the review. All review authors contributed to the discussion and the editing.
Sources of support
Internal sources
-
None received, Other
N/A
External sources
-
None received, Other
N/A
Declarations of interest
Mieke L van Driel: none known An IM De Sutter: none known Sarah Thorning: none known Thierry Christiaens: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bachand 1991 {published data only}
- Bachand RT Jr. A comparative study of clarythromycin and penicillin VK in the treatment of outpatients with streptococcal pharyngitis. Journal of Antimicrobial Chemotherapy 1991;27(Suppl A):75-82. [DOI] [PubMed] [Google Scholar]
Carbon 1995 {published data only}
- Carbon C, Chatelin A, Bingen E, Zuck P, Rio Y, Guetat F, et al. A double-blind randomized trial comparing the efficacy and safety of a 5-day course of cefotiam hexetil with that of a 10-day course of penicillin V in adult patients with pharyngitis caused by group A beta-haemolytic streptococci. Journal of Antimicrobial Chemotherapy 1995;35(6):843-54. [DOI] [PubMed] [Google Scholar]
Disney 1992a {published data only}
- Disney FA, Dillon H, Blumer JL, Dudding BA, McLinn SE, Nelson DB, et al. Cephalexin and penicillin in the treatment of group A beta-hemolytic streptococcal throat infections. American Journal of Diseases of Children 1992;146(11):1324-7. [DOI] [PubMed] [Google Scholar]
Disney 1992b {published data only}
- Disney FA, Hanfling MJ, Hausinger SA. Loracarbef (LY163892) vs. penicillin VK in the treatment of streptococcal pharyngitis and tonsillitis. Pediatric Infectious Disease Journal 1992;11(Suppl 8):20-6. [DOI] [PubMed] [Google Scholar]
Henness 1982 {published data only}
- Henness DM. A clinical experience with cefadroxil in upper respiratory tract infection. Journal of Antimicrobial Chemotherapy 1982;10(Suppl B):125-35. [DOI] [PubMed] [Google Scholar]
Jackson 1973 {published data only}
- Jackson H. A comparative study of clindamycin palmitate and ampicillin in the treatment of group A beta hemolytic streptococcal pharyngitis. Clinical Pediatrics 1973;12(8):501-3. [DOI] [PubMed] [Google Scholar]
Levenstein 1991 {published data only}
- Levenstein JH. Clarythromycin versus penicillin in the treatment of streptococcal pharyngitis. Journal of Antimicrobial Chemotherapy 1991;27(Suppl A):67-74. [DOI] [PubMed] [Google Scholar]
McCarty 1992a {published data only}
- McCarty J. Loracarbef versus penicillin VK in the treatment of streptococcal pharyngitis and tonsillitis in an adult population. American Journal of Medicine 1992;92(Suppl 6A):74-9. [DOI] [PubMed] [Google Scholar]
Muller 1992 {published data only}
- Muller O, Spirer Z, Wettich K. Loracarbef versus penicillin V in the treatment of streptococcal pharyngitis and tonsillitis. Infection 1992;20(5):301-8. [DOI] [PubMed] [Google Scholar]
NCT00643149 {unpublished data only}
- NCT00643149. A multicenter, randomized, double-blind, double-dummy study of azithromycin SR versus amoxicillin for the treatment of strep throat In children. clinicaltrials.gov/ct2/show/NCT00643149 (first received 19 March 2008).
Nemeth 1999 {published data only}
- Nemeth MA, McCarty J, Gooch WM 3rd, Henry D, Keyserling CH, Tack KJ. Comparison of cefdinir and penicillin for the treatment of streptococcal pharyngitis. Clinical Therapeutics 1999;21(11):1873-81. [DOI] [PubMed] [Google Scholar]
Norrby 2002 {published data only}
- Norrby SR, Chang J, Stewart JA, Brumpt I, Conway DP. Relief of symptoms in patients with group A b-hemolytic streptococcus tonsillopharyngitis: comparison between telithromycin and penicillin V. Scandinavian Journal of Infectious Diseases 2003;35(4):223-5. [DOI] [PubMed] [Google Scholar]
- Norrby SR, Rabie WJ, Bacart P, Mueller O, Leroy B, Rangaraju M, et al. Efficacy of short-course therapy with the ketolide telithromycin compared with 10 days of penicillin V for the treatment of pharyngitis/tonsillitis. Scandinavian Journal of Infectious Diseases 2002;33(12):883-90. [DOI] [PubMed] [Google Scholar]
O'Doherty 1996 {published data only}
- O'Doherty B, Paediatric Azithromycin Study Group. Azithromycin versus penicillin V in the treatment of paediatric patients with acute streptococcal pharyngitis/tonsillitis. European Journal of Clinical Microbiology and Infectious Diseases 1996;15(9):718-24. [DOI] [PubMed] [Google Scholar]
Randolph 1985 {published data only}
- Randolph MF, Gerber MA, DeMeo KK, Wright BS. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. Journal of Pediatrics 1985;106(6):870-5. [DOI] [PubMed] [Google Scholar]
Reed 1991 {published data only}
- Reed BD, Huck W, Zazove P. Treatment of beta-hemolytic streptococcal pharyngitis with cefaclor or penicillin; efficacy and interaction with beta-lactamase-producing organisms in the pharynx. Journal of Family Practice 1991;32(2):138-44. [PubMed] [Google Scholar]
Stein 1991 {published data only}
- Stein GE, Christensen S, Mummaw N. Comparative study of clarythromycin and penicillin V in the treatment of streptococcal pharyngitis. European Journal of Clinical Microbiology and Infectious Diseases 1991;10(11):949-53. [DOI] [PubMed] [Google Scholar]
Trickett 1973 {published data only}
- Trickett PC, Dineen P, Mogabgab W. Clinical experience: respiratory tract. Trimethoprim-sulfamethoxazole versus penicillin G in the treatment of group A beta-hemolytic streptococcal pharyngitis and tonsillitis. Journal of Infectious Diseases 1973;128(Suppl):693-5. [DOI] [PubMed] [Google Scholar]
Watkins 1997 {published data only}
- Watkins VS, Smietana M, Conforti PM, Sides GD, Huck W. Comparison of dirithromycin and penicillin for treatment of streptococcal pharyngitis. Antimicrobial Agents and Chemotherapy 1997;41(1):72-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1994 {published data only}
- Adam D, Hostalek U. Effectiveness and tolerance of cefixime in comparison with penicillin V in bacterial pharyngitis and tonsillitis in children. Cefixime Study Group. Klinische Padiatrie 1994;206(1):26-9. [DOI] [PubMed] [Google Scholar]
Adam 1995 {published data only}
- Adam D, Hostalek U, Troster K. 5-day cefixime therapy for bacterial pharyngitis and/or tonsillitis: comparison with 10-day penicillin V therapy. Infection 1995;22(Suppl 2):83-6. [DOI] [PubMed] [Google Scholar]
Adam 1996 {published data only}
- Adam D, Scholz H, the Pharyngitis Study Group. Five days of erythromycin estolate versus ten days of penicillin V in the treatment of group A Streptococcal tonsillopharyngitis in children. European Journal of Clinical Microbiology and Infectious Diseases 1996;15(9):712-7. [DOI] [PubMed] [Google Scholar]
Adam 2000a {published data only}
- Adam D, Scholz H, Helmerking M. Comparison of short-course (5 day) cefuroxime axetil with a standard 10 day oral penicillin V regimen in the treatment of tonsillopharyngitis. Journal of Antimicrobial Chemotherapy 2000;45(Suppl):23-30. [DOI] [PubMed] [Google Scholar]
Adam 2000b {published data only}
- Adam D, Scholz H, Helmerking M. Short-course antibiotic treatment of 4782 culture-proven cases of group A streptococcal tonsillopharyngitis and incidence of poststreptococcal sequelae. Journal of Infectious Diseases 2000;182(2):509-16. [DOI] [PubMed] [Google Scholar]
Adam 2001 {published data only}
- Adam D, Scholz H, Helmerking M. Five days ceftibuten versus 10 days penicillin in the treatment of 2099 patients with A-streptococcal tonsillopharyngitis [Fünf tage ceftibuten im vergleich zu zehn tagen penicillin V in der therapie der A-streptokokken-tonsillopharyngitis]. Fortschritte der Medizin 2001;119(Suppl 2):63-70. [PubMed] [Google Scholar]
Aujard 1995 {published data only}
- Aujard Y, Boucot I, Brahimi N, Chiche D, Bingen E. Comparative efficacy and safety of four-day cefuroxime axetil and ten-day penicillin treatment of group A beta-hemolytic streptococcal pharyngitis in children. Pediatric Infectious Disease Journal 1995;14(4):295-300. [DOI] [PubMed] [Google Scholar]
Bottaro 2012 {published data only}
- Bottaro G, Biasci P, Giudice ML, Mele G, Montanari G, Napoleone E, et al. 5 days cefaclor vs. 10 days amoxicillin/clavulanate in the treatment of childhood streptococcal pharyngitis. Data from a randomized clinical trial. Minerva Pediatrica 2012;64(3):341-6. [PubMed] [Google Scholar]
Breese 1974 {published data only}
- Breese BB, Disney FA, Talpey WB, Green JL. Treatment of streptococcal pharyngitis with amoxicillin. Journal of Infectious Diseases 1974;129(Suppl):178-80. [DOI] [PubMed] [Google Scholar]
Cohen 2002 {published data only}
- Cohen R, Reinert P, De La Rocque F, Levy C, Boucherat M, Robert M, et al. Comparison of two dosages of azithromycin for three days versus penicillin V for ten days in acute group A streptococcal tonsillopharyngitis. Pediatric Infectious Disease Journal 2002;21:297-303. [DOI] [PubMed] [Google Scholar]
Davies 1995 {published data only}
- Davies HD, Low DE, Schwartz B, Scriver S, Fletcher A, O'Rourke K, et al. Evaluation of short-course therapy with cefixime or rifampin for eradication of pharyngeally carried group A Streptococci. Clinical Infectious Diseases 1995;21:1294-6. [DOI] [PubMed] [Google Scholar]
Del Mar 2008 {published data only}
- Del Mar C. Once-daily amoxycillin eradicates group A beta-hemolytic strep as well as penicillin twice a day. Journal of Pediatrics 2008;153(5):725. [DOI] [PubMed] [Google Scholar]
De Meyere 1992 {published data only}
- Meyere M, Mervielde I, Bogaert M. Use of antibiotics in acute sore throat [Het nut van antibiotica bij acute keelpijn]. Nederlands Tijdschrift Voor Geneeskunde 1992;136(47):2314-7. [PubMed] [Google Scholar]
Denny 1953 {published data only}
- Denny FW, Wannamaker LW, Hahn EO. Comparative effects of penicillin, aureomycin and terramycin on streptococcal tonsillitis and pharyngitis. Pediatric Infectious Disease 1953;11:7-14. [PubMed] [Google Scholar]
Disney 1979 {published data only}
- Disney FA, Breese BB, Francis AB, Green JL. The use of cefaclor in the treatment of beta-haemolytic streptococcal throat infections in children. Postgraduate Medical Journal 1979;55(Suppl 4):50-2. [PubMed] [Google Scholar]
Dykhuizen 1996 {published data only}
- Dykhuizen RS, Golder D, Reid TM, Gould IM. Phenoxymethyl penicillin versus co-amoxiclav in the treatment of acute streptococcal pharyngitis, and the role of beta-lactamase activity in saliva. Journal of Antimicrobial Chemotherapy 1996;37:133-8. [DOI] [PubMed] [Google Scholar]
Eslami 2014 {published data only}
- Eslami ST, Nassirian A, Nassirian H, Hatami E, Sobhani E, Najibpour R. Comparing performance of amoxicillin and intramuscular benzathine penicillin in relieving manifestations of streptococcal pharyngitis in children. Ghana Medical Journal 2014;48(4):185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2002 {published data only}
- Esposito S, Marchisio P, Bosis S, Droghetti R, Mattina R, Principi N, et al. Comparative efficacy and safety of 5-day cefaclor and 10-day amoxycillin treatment of group A streptococcal pharyngitis in children. International Journal of Antimicrobial Agents 2002;20:28-33. [DOI] [PubMed] [Google Scholar]
Feder 1999 {published data only}
- Feder HM Jr, Gerber MA, Randolph MF, Stelmach PS, Kaplan EL. Once-daily therapy for streptococcal pharyngitis with amoxicillin. Pediatrics 1999;103:47-51. [DOI] [PubMed] [Google Scholar]
Gerber 1986 {published data only}
- Gerber MA, Randolph MF, Chanatry J, Wright LL, Anderson LR, Kaplan EL. Once daily therapy for streptococcal pharyngitis with cefadroxil. Journal of Pediatrics 1986;109(3):531-7. [DOI] [PubMed] [Google Scholar]
Gerber 1999a {published data only}
- Gerber MA, Tanz RR, Kabat W, Bell GL, Siddiqui PN, Lerer TJ, et al. Potential mechanisms for failure to eradicate group A streptococci from the pharynx. Pediatrics 1999;104(4):911-7. [DOI] [PubMed] [Google Scholar]
Gooch 1993 {published data only}
- Gooch WM 3rd, McLinn SE, Arnovitz GH, Pichichero ME, Kumar A, Kaplan A, et al. Efficacy of cefuroxime axetil suspension compared with that of penicillin V suspension in children with group A streptococcal pharyngitis. Antimicrobial Agents and Chemotherapy 1993;37(2):159-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Granizio 2008 {published data only}
- Granizio JJ, Gimenez MJ, Barberan J, Coronel J, Gimeno M, Aguilar L. Efficacy of cefditoren in the treatment of upper respiratory tract infections: a pooled analysis of six clinical trials. Revista Espanola de Quimioterapia 2008;21(1):14-21. [PubMed] [Google Scholar]
Hamill 1993 {published data only}
- Hamill J. Multicentre evaluation of azithromycin and penicillin V in the treatment of acute streptococcal pharyngitis and tonsillitis in children. Journal of Antimicrobial Chemotherapy 1993;31(Suppl E):89-94. [DOI] [PubMed] [Google Scholar]
Haverkorn 1971 {published data only}
- Haverkorn MJ, Valkenburg HA, Goslings WR. Streptococcal pharyngitis in the general population. I. A controlled study of streptococcal pharyngitis and its complications in the Netherlands. Journal of Infectious Diseases 1971;124(4):339-47. [DOI] [PubMed] [Google Scholar]
Holm 1991 {published data only}
- Holm SE, Roos K, Stromberg A. A randomized study of treatment of streptococcal pharyngotonsillitis with cefadroxil or phenoxymethylpenicillin (penicillin V). Pediatric Infectious Disease Journal 1991;10(Suppl 10):68-71. [DOI] [PubMed] [Google Scholar]
Howe 1997 {published data only}
- Howe RW, Millar MR, Coast J, Whitfield M, Peters TJ, Brookes S. A randomized controlled trial of antibiotics on symptom resolution in patients presenting to their general practitioner with a sore throat. British Journal of General Practice 1997;47:280-4. [PMC free article] [PubMed] [Google Scholar]
Kuroki 2013 {published data only}
- Kuroki H, Ishiwada N, Inoue N, Ishikawa N, Suzuki H, Himi K, et al. Comparison of clinical efficacy between 3-day combined clavulanate/amoxicillin preparation treatment and 10-day amoxicillin treatment in children with pharyngolaryngitis or tonsillitis. Journal of Infection and Chemotherapy 2013;19:12-9. [DOI] [PubMed] [Google Scholar]
Lennon 2008 {published data only}
- Lennon DR, Farrell E, Martin DR, Stewart JM. Once-daily amoxicillin versus twice-daily penicillin V in group A beta-hemolytic streptococcal pharyngitis. Archives of Disease in Childhood 2008;93(6):474-8. [DOI] [PubMed] [Google Scholar]
Llerena 2011 {published data only}
- Llerena Santa Cruz ED, Buñuel Álvarez JC, Porcar Farrán D, Solà Pou J, Fortea Gimeno E, Cortés Marina RB, et al. Treatment of streptococcal tonsillitis with once-a-day amoxicillin: a meta-analysis. Anales De Pediatria 2011;75(5):298-306. [DOI] [PubMed] [Google Scholar]
Matsen 1974 {published data only}
- Matsen JM, Torstenson O, Siegel SE, Bacaner H. Use of available dosage forms of cephalexin in clinical comparison with phenoxymethyl penicillin and benzathine penicillin in the treatment of streptococcal pharyngitis in children. Antimicrobial Agents and Chemotherapy 1974;6(4):501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarty 1992b {published data only}
- McCarty JM, Renteria A. Treatment of pharyngitis and tonsillitis with cefprozil: review of three multicenter trials. Clinical Infectious Diseases 1992;14(Suppl 2):224-30. [DOI] [PubMed] [Google Scholar]
McCarty 1994 {published data only}
- McCarty JM. Comparative efficacy and safety of cefprozil versus penicillin, cefaclor and erythromycin in the treatment of streptococcal pharyngitis and tonsillitis. European Journal of Clinical Microbiology and Infectious Diseases 1994;13(10):846-50. [DOI] [PubMed] [Google Scholar]
McIsaac 2004 {published data only}
- McIsaac WJ, Kellner JD, Aufricht P, Vanjaka A, Low DE. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA 2004;291(13):1587-95. [DOI] [PubMed] [Google Scholar]
Milatovic 1991 {published data only}
- Milatovic D. Evaluation of cefadroxil, penicillin and erythromycin in the treatment of streptococcal tonsillopharyngitis. Pediatric Infectious Disease Journal 1991;10(Suppl):61-3. [DOI] [PubMed] [Google Scholar]
Milatovic 1993 {published data only}
- Milatovic D, Adam D, Hamilton H, Materman E. Cefprozil versus penicillin V in the treatment of streptococcal tonsillopharyngitis. Antimicrobial Agents and Chemotherapy 1993;37(8):1620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00393744 {published and unpublished data}
- NCT00393744. Efficacy study of pristinamycin versus amoxicillin to treat tonsillitis induced by streptococcus in children. clinicaltrials.gov/ct2/show/NCT00393744 (first received 27 October 2006).
Pacifico 1996 {published data only}
- Pacifico L, Scopetti F, Ranucci A, Pataracchia M, Savignoni F, Chiesa C. Comparative efficacy and safety of 3-day azithromycin and 10-day penicillin V treatment of group A beta-hemolytic streptococcal pharyngitis in children. Antimicrobial Agents and Chemotherapy 1996;40(4):1005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkins 1969 {published data only}
- Perkins RL, Glontz GE, Saslaw S. Cephaloglycin: crossover absorption studies and clinical evaluation. Clinical Pharmacology and Therapeutics 1969;10(2):244-9. [DOI] [PubMed] [Google Scholar]
Pichichero 2000 {published data only}
- Pichichero ME, Gooch WM 3rd. Comparison of cefdinir and penicillin V in the treatment of pediatric streptococcal tonsillopharyngitis. Pediatric Infectious Disease Journal 2000;19(Suppl 12):171-3. [DOI] [PubMed] [Google Scholar]
Pichichero 2008 {published data only}
- Pichichero ME, Casey JR, Block SL, Guttendorf R, Flanner H, Markowitz D, et al. Pharmacodynamic analysis and clinical trial of amoxicillin sprinkle administered once daily for 7 days compared to penicillin v potassium administered four times daily for 10 days in the treatment of tonsillopharyngitis due to streptococcus pyogenes in children. Antimicrobial Agents and Chemotherapy 2008;52(7):2512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Portier 1990 {published data only}
- Portier H, Chavanet P, Gouyon JB, Guetat F. Five day treatment of pharyngotonsillitis with cefpodoxime proxetil. Journal of Antimicrobial Chemotherapy 1990;26(Suppl E):79-85. [DOI] [PubMed] [Google Scholar]
Portier 1994 {published data only}
- Portier H, Chavanet P, Waldner-Combernoux A, Kisterman JP, Grey PC, Ichou F, et al. Five versus ten days treatment of Streptococcal pharyngotonsillitis: a randomized controlled trial comparing cefpodoxime proxetil and phenoxymethyl penicillin. Scandinavian Journal of Infectious Diseases 1994;26(1):59-66. [DOI] [PubMed] [Google Scholar]
Rimoin 2011 {published data only}
- Rimoin AW, Hoff NA, Fischer Walker CL, Hamza HS, Vince A, Abdel Rahman N, et al. Treatment of streptococcal pharyngitis with once-daily amoxicillin versus intramuscular benzathine penicillin G in low-resource settings: a randomized controlled trial. Clinical Pediatrics 2011;50(6):535-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roos 1997 {published data only}
- Roos K, Larsson P. Loracarbef versus phenoxymethylpenicillin in the treatment of recurrent streptococcal pharyngotonsillitis. Scandinavian Journal of Infectious Diseases 1997;29(2):141-5. [DOI] [PubMed] [Google Scholar]
Sakata 2008 {published data only}
- Sakata H. Comparative study of 5-day cefcapene-pivoxil and 10-day amoxicillin or cefcapene-pivoxil for treatment of group A streptococcal pharyngitis in children. Journal of Infection and Chemotherapy 2008;14(3):208-12. [DOI] [PubMed] [Google Scholar]
Shapera 1973 {published data only}
- Shapera RM, Hable KA, Matsen JM. Erythromycin therapy twice daily for streptococcal pharyngitis. Controlled comparison with erythromycin or penicillin phenoxymethyl four times daily or penicillin G benzathine. JAMA 1973;226(5):531-5. [PubMed] [Google Scholar]
Shvartzman 1993 {published data only}
- Shvartzman P, Tabenkin H, Rosentzwaig A, Dolginov F. Treatment of streptococcal pharyngitis with amoxycillin once a day. BMJ 1993;306(6886):1170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 1961 {published data only}
- Siegel AC, Johnson EE, Stollerman GH. Controlled studies of streptococcal pharyngitis in a pediatric population. New England Journal of Medicine 1961;265(12):559-65. [Google Scholar]
Standaert 1997 {published data only}
- Standaert BB, Finney KM, Taylor MT, Coleman RT, Horowitz CL, Walter SM, et al. Comparison between cefprozil and penicillin to eradicate pharyngeal colonization of group A beta-hemolytic streptococci. Pediatric Infectious Disease Journal 1998;17(1):39-43. [DOI] [PubMed] [Google Scholar]
Stelter 2014 {published data only}
- Stelter K. Tonsillitis and sore throat in childhood. Laryngologie, Rhinologie, Otologie 2014;93(Suppl):84-102. [DOI] [PubMed] [Google Scholar]
Stillerman 1970 {published data only}
- Stillerman M. Comparison of cephaloglycin and penicillin in streptococcal pharyngitis. Clinical Pharmacology and Therapeutics 1970;11(2):205-12. [DOI] [PubMed] [Google Scholar]
Stillerman 1986 {published data only}
- Stillerman M. Comparison of oral cephalosporins with penicillin therapy for group A streptococcal pharyngitis. Pediatric Infectious Disease Journal 1986;5(6):649-54. [DOI] [PubMed] [Google Scholar]
Tack 1997 {published data only}
- Tack KJ, Hendrick JA, Rothstein E, Nemeth MA, Keyserling C, Pichichero ME. A study of 5-day treatment for streptococcal pharyngitis in children. Cefdinir Pediatric Study Group. Archives of Pediatric and Adolescent Medicine 1997;151(1):45-9. [DOI] [PubMed] [Google Scholar]
Tack 1998 {published data only}
- Tack KJ, Henry DC, Gooch WM, Brink DN, Keyserling CH, the Cefdinir Pharyngitis Study Group. Five-day cefdinir treatment for streptococcal pharyngitis. Antimicrobial Agents and Chemotherapy 1998;42(5):1073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uysal 2000 {published data only}
- Uysal S, Sanack R, Sunbul M. A comparison of the efficacy of cefuroxime axetil and intramuscular benzathine penicillin for treating streptococcal tonsillopharyngitis. Annals of Tropical Paediatrics 2000;20:199-202. [DOI] [PubMed] [Google Scholar]
Van Brusselen 2014 {published data only}
- Van Brusselen D, Vlieghe E, Schelstraete P, De Meulder F, Vandeputte C, Garmyn K, et al. Streptococcal pharyngitis in children: to treat or not to treat? European Journal of Paediatrics 2014;173(10):1275-83. [DOI] [PubMed] [Google Scholar]
Zwart 2000 {published data only}
- Zwart S, Sachs AP, Ruijs GJ, Gubbels JW, Hoes AW, Melker RA. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults. BMJ 2000;320(7228):150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Albin 2014
- Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy and Asthma Proceedings 2014;35:489–94. [DOI: 10.2500/aap.2014.35.3791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharya 2010
- Bhattacharya S. The facts about penicillin allergy: a review. Journal of Advanced Pharmaceutical Technology & Research 2010;1(1):11-7. [PMC free article] [PubMed] [Google Scholar]
Brunton 2006
- Brunton S, Pichichero M. Considerations in the use of antibiotics for streptococcal pharyngitis. Journal of Family Practice 2006;55(Suppl 7):9-16. [PubMed] [Google Scholar]
Cars 2001
- Cars O, Mölstad S, Melander A. Variation in antibiotic use in the European Union. Lancet 2001;357(9271):1851-3. [DOI] [PubMed] [Google Scholar]
Casey 2004
- Casey JR, Pichichero ME. Meta-analysis of cephalosporin versus penicillin treatment of group A streptococcal tonsillopharyngitis in children. Pediatrics 2004;113:866–82. [DOI] [PubMed] [Google Scholar]
Cooper 1992
- Cooper RD. The carbacephems: a new beta-lactam antibiotic class. American Journal of Medicine 1992;92(Suppl):2-6. [DOI] [PubMed] [Google Scholar]
Cooper 2001
- Cooper RJ, Hoffman JR, Bartlett JG, Besser JG, Gonzales R, Hickner JM, et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Annals of Internal Medicine 2001;134(6):509-17. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Devi 2011
- Devi U, Borah PK, Mahanta J. The prevalence and antimicrobial susceptibility patterns of beta-hemolytic streptococci colonizing the throats of schoolchildren in Assam, India. Journal of Infection in Developing Countries 2011;5(11):804-8. [DOI] [PubMed] [Google Scholar]
eTG 2019
- eTG Complete. Therapeutic Guidelines: Antibiotic. https://www.tg.org.au/ (accessed 12 March 2021).
Gerber 1999b
- Gerber MA, Tanz RR, Kabat W, Bell GL, Lerer TJ, Lepow ML, et al. Potential mechanisms for failure to eradicate group A streptococci from the pharynx. Pediatrics 1999;104:911-7. [DOI] [PubMed] [Google Scholar]
Gerber 2004
- Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clinical Microbiology Reviews 2004;17(3):571-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerber 2009
- Gerber MA, Baltimore RS, Eaton CB, Gewitz MS, Rowley AH, Shulman ST, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis. A scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation 2009;119(11):1541. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version (accessed 12 March 2021). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Hanna 2010
- Hanna J, Clark MF. Acute rheumatic fever in Indigenous people in North Queensland: some good news at last? Medical Journal of Australia 2010;192(10):581-4. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DJ. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Ibrahim 2014
- Ibrahim SB, El-Sokkary RH, Elhewala AA, El-Anwar MW, Awad WM, Hamed AM, et al. Emerging resistance to erythromycin and penicillin among streptococcus pyogenes isolates in Zagazig, Egypt. International Journal of Current Microbiology and Applied Sciences 2014;3(10):750-6. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Linder 2001
- Linder JA, Stafford RS. Antibiotic treatment of adults with sore throat by community primary care physicians. A National Survey, 1989-1999. JAMA 2001;286(10):1181-6. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719-48. [PubMed] [Google Scholar]
Matthys 2007
- Matthys J, De Meyere M, Driel ML, De Sutter A. Differences among international pharyngitis guidelines: not just academic. Annals of Family Medicine 2007;5:436-43. [DOI: 10.1370/afm.741] [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2016
- May PJ, Bowen AC, Carapetis JR. The inequitable burden of group A streptococcal diseases in Indigenous Australians. Medical Journal of Australia 2016;205(5):201-3. [DOI: 10.5694/mja16.00400] [DOI] [PubMed] [Google Scholar]
McIsaac 1998
- McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. Canadian Medical Association Journal 1998;158:75-83. [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Neuner 2003
- Neuner JM, Hamel MB, Phillips RS, Bona K, Aronson MD. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Annals of Internal Medicine 2003;139(2):113-22. [DOI] [PubMed] [Google Scholar]
O'Neill 2016
- O'Neill J. Tackling drug-resistant infections globally: final report and recommendations. amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Shulman 2004
- Shulman ST, Gerber MA. So what's wrong with penicillin for strep throat? Pediatrics 2004;113:1816-9. [DOI: DOI: 10.1542/peds.113.6.1816] [DOI] [PubMed] [Google Scholar]
Snow 2001
- Snow V, Mottur-Pilson C, Cooper RJ, Hoffman JR. Principles of appropriate antibiotic use for acute pharyngitis in adults. Annals of Internal Medicine 2001;134(6):506-8. [DOI] [PubMed] [Google Scholar]
Sonnad 1999
- Sonnad SS, Zarkower N, Varney G. Rapid antigen testing for group A beta-hemolytic Streptococcus: a meta-analysis evaluation of test performance (Meeting Abstract). Annual Meeting of the International Society of Technology Assessment in Health Care 1999;15:122. [PMID: ] [Google Scholar]
Spinks 2013
- Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD000023. [DOI: 10.1002/14651858.CD000023.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Driel 2006
- Driel ML, De Sutter A, Deveugele M, Peersman W, Butler CC, De Meyere M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Annals of Family Medicine 2006;4:494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Driel 2009
- Driel ML, De Sutter A, De Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. Journal of Clinical Epidemiology 2009;62(8):838-44. [DOI] [PubMed] [Google Scholar]
Wise 1998
- Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P, et al. Antimicrobial resistance. BMJ 1998;317(7159):609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Worrall 2007
- Worrall GJ. Acute sore throat. Canadian Family Physician 2007;53:1961-2. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
van Driel 2003
- Driel ML, De Sutter AIM, Keber N, Habraken H, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD004406. [DOI: 10.1002/14651858.CD004406] [DOI] [PubMed] [Google Scholar]
van Driel 2010
- Driel ML, De Sutter AIM, Keber N, Habraken H, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD004406. [DOI: 10.1002/14651858.CD004406.pub2] [DOI] [PubMed] [Google Scholar]
van Driel 2013
- Driel ML, De Sutter AIM, Keber N, Habraken H, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD004406. [DOI: 10.1002/14651858.CD004406.pub3] [DOI] [PubMed] [Google Scholar]
van Driel 2016
- Driel ML, De Sutter AIM, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD004406. [DOI: 10.1002/14651858.CD004406.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


