Rice straw is an important renewable biomass resource. However, due to its slow degradation returning to the field, and the environmental pollution of conventional straw burning, it is highly desired for more efficient utilization of rice straw resource. The complex structure of rice cell wall composition impedes the efficient utilization of straw biomass. Developing new strategies to improve rice straw utilization efficiency is attractive. Glucuronoarabinoxylan (GAX) and arabinoxylan (AX) are the common types of xylan in rice, which have β1,4‐xylose backbone modified with arabinose (Ara) and/or glucuronic acid side chains. The UDP‐xylose epimerase (UXE) and xylan arabinosyl‐transferase (XAT) are the major contributors to Ara side chain biosynthesis in Golgi apparatus. UXE catalyses isomerization of UDP‐Xyl to UDP‐Arap, the first step of UDP‐Arap biosynthesis, and Arap is then transformed to Araf by UDP‐arabinopyranose mutase (UAM) in cytosol. XAT catalyses arabinosyl addition to the xylan backbone to form GAX/AX. Previous reports indicate that either higher Ara content or lower ferulic acid of rice xylan side chain would increase the enzymatic hydrolysis efficiencies (Casler and Jung, 2006; Li et al., 2015). Meanwhile, it has also proposed an idea of reducing arabinose side chains to improve saccharification (Konishi et al., 2011). Thus, we hypothesize that specifically and intensively altering degree of xylan side chain substitutions might improve the utilization efficiency of rice straw. In present study, both rice UXE and XAT knockout mutants were generated using CRISPR technology; subsequently, the saccharification efficiencies of both mutant lines, compared to the wild types (WT), were explored.
We designed three CRISPR/CAS9 constructs, each targets a pair of OsUXE or OsXAT genes in rice. After obtaining transgenic rice plants, we amplified and sequenced OsUXE or OsXAT genes from 30 independently regenerated lines. Finally, double mutants, at least two alleles at different mutation, were obtained and named as uxe1uxe2, uxe1uxe3 and xat2xat3, respectively (Figure 1a, b). Phenotypic analysis revealed that the growth and development of both uxe and xat double mutants were not obviously affected in rice (Figure 1c), which is similar to the wheat TaXAT2 mutant (Anders et al., 2012) but different with UAM RNAi lines that show dwarf and infertile phenotypes (Konishi et al., 2011). Sections of stems confirmed growth of secondary cell walls in the epidermis, phloem and xylem vessels, with no significant change compared to the WT (Figure 1d). These findings indicated that cell wall morphology was not affected in the mutant lines. Previous studies revealed that disruption of uxe resulted in significant reduction in the amount of Ara in the cell walls of Arabidopsis (Burget et al., 2003; Sumiyoshi et al., 2013).Therefore, the amount of Ara in the transgenic rice cell walls was determined. Compared with the WT (4.15%), by ion chromatography measurements the arabinose content of uxe1uxe2 and xat2xat3 decreased to 2.19% and 2.78%, respectively (Figure 1e). By contrast, the amount of xylose and glucose did not change in any of the mutant lines, indicating that UXE and XAT mutations have no effect on the glycan backbone of GAX or AX. Why the UXEs mutants were still with Ara in their cell walls? One of the reasons might be due to the remaining UXE. Rice has three OsUXEs. Determining the total activity of UXE in the two uxe double mutants indeed illustrated the residual activity of UXE in those double mutants (Figure 1f). In rice, OsXAT2 and OsXAT3 are homologous to TaXAT2 (Anders et al., 2012). The rice xat2xat3 double mutant showed the reduction of the Ara content, which may be due to the potential redundant functions of other members of the GT61 family. Previous UAM RNAi rice line with a significant reduction in Ara content of the cell wall showed severe developmental defects (Konishi et al., 2011), whereas the traits of growth in our double mutants only exhibited a slight change, except seeds per ears in xat2xat3 mutant (Figure 1g). Such discrepancy might be caused with different target genes, the rice varieties and/or growth conditions. To further confirm the decrease in Ara content in our mutants, 2D‐HSQC was used to detect the CH‐related signal peaks of hemicellulose (Figure 1h). The chemical shifts of X‐1, X‐4, X‐3, X‐2 and X‐5 of the 1, 4‐β‐D xylose unit were 101.77/4.30 ppm, 75.60/3.63 ppm, 74.09/3.36 ppm, 72.81/3.15 ppm and 63.43/3.95 ppm, respectively (Sun et al., 1996), and all xylose (X) signals showed almost no obvious alteration. The weak signal peak (green) from 4‐O methyl glucuronic acid and the unchanged structure of the xylose backbone were consistent in all samples. However, the samples of mutants showed weaker signals assigned to α‐L‐Ara in A1‐A5 (Figure 1h), indicative of reduction of Ara content. Our 2D‐HSQC results were consistent with the results of hemi‐cellulosic monosaccharide composition analysis, further confirming that Ara content was decreased in mutants.
Figure 1.
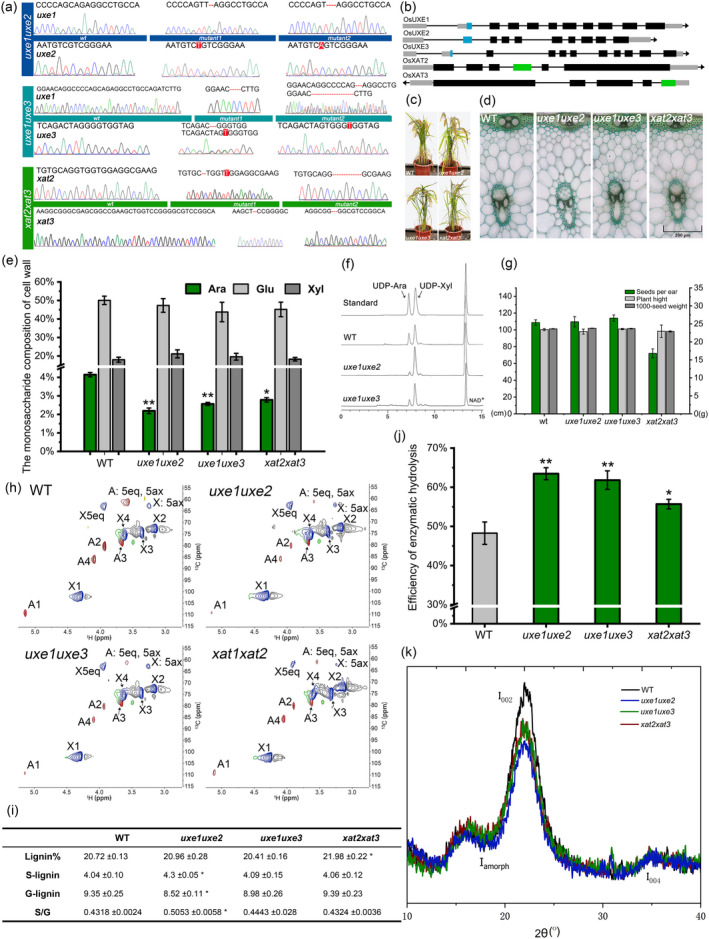
The cell wall changes of uxe and xat mutants improve the ability of straw to be hydrolysed. (a) The mutation site of the mutant (by CRISPR/Cas 9). Red markers represent mutations and dashed lines represent missing bases. (b) Schematic representation of targets in genes. Block shows exons and coloured section indicates target position. Line represents introns. (c) Photographs of plant growth. (d) Cross sectioning of rice stems. Toluidine blue dye was used. (e) Determination of amounts of arabinose, xylose and glucose in plant stem cell wall. Results are shown as ratio of monosaccharides to cell wall weight. (f) Enzyme activity of UXEs among uxes mutants and WT by extracted microsome with method referenced by Gu et al. (https://doi.org/10.1099/mic.0.040758‐0). (g) Growth condition statistics of mutants in the field. (h) 2D‐HSQC comparative of hemicellulose. Abbreviations: A, Araf; X, Xylp; (i) determination of amount of lignin of plant stem cell wall by pyGC‐MS. S (%) and G (%) data indicate proportion of syringyl units and guaiacyl units in total composition, respectively. (j) Enzymatic hydrolysis efficiency of cellulose in rice mutants. (k) XRD of uxes and xats. Scanning speed: 5°/min. Three biological replicates were used as test samples, and error bars are based on three data results. Asterisk indicates that sample is significant compared to WT (‘*’, P < 0.05; ‘**’, P < 0.01; Student's t‐test).
Ara is a vital component mediating linkage between xylan and lignin, because ferulic acid at the xylan Araosyl side chain links with monolignols. We used pyGC‐MS to determine the lignin content and composition. The amount of lignin in mutants was largely consistent with that of the WT plants, except for xat2xat3 showing an increase (Figure 1i). The G‐lignin unit was decreased but S/G ratio was increased in uxe double mutants. Normally, not only S/G ratio, but also Ara content and G‐type lignin are significantly correlated with enzymatic saccharification (Li et al., 2015; Wang et al., 2016; Wu et al., 2013). So saccharification was analysed with three double‐mutant rice straw. As expected, all mutants exhibited significant increase in simple sugar release (Figure 1j). In particular, the saccharification efficiency of the uxe1uxe2 mutants improved by 15.19%, while xat2xat3 mutants showed an increase of 7.42%. Due to part of Ara in GAX and ferulic acid is considered to affect digestibility of cellulose (Casler and Jung, 2006), a large amount of Ara loss will loosen the cell wall structure, thereby increasing the accessibility of cellulase (Marriott et al., 2016; Wu et al., 2013).
Previous reports indicate that moderate increase of arabinose may have a negative effect on the cellulose crystallinity, which in turn affects enzymatic hydrolysis (Li et al., 2015). To explain our double mutants with improved saccharification efficiency, cellulose crystallinity was examined. As expected, the uxe1uxe2, uxe1uxe3 and xat2xat3 double mutants with less Ara showed reduced cellulose crystallinity (Figure 1k), which implies that excessive reduction of the Ara content in xylan also impairs the cellulose crystalline structures. In turn, it makes the cell walls of both uxe and xat rice mutants more hydrolysis‐prone. These results confirm our assumption that xylan with extremely low Ara side chain also has a positive effect on enzymatic hydrolysis.
In summary, knockout of uxe and xat by CRISPR / Cas9 resulted in a decrease in cell wall arabinose content. The alterations in cellulose crystallinity and enzymolysis efficiency of the mutant indicate that the cell wall structure in the mutants is loose and easier to use. We provide an environmentally friendly way of using rice straw efficiently.
Accession number
LOC4342364 (OsUXE1), LOC4337011 (OsUXE2), LOC4344584 (OsUXE3), LOC4329205 (OsXAT2), LOC4333275 (OsXAT3).
Conflict of interest
No conflict of interests to declare.
Author contributions
C. C. and A‐M. W. contributed to project design. C. C., X‐H. Z. and X‐C. W. performed the experiments and data analysis. J‐X. F. and H‐L. L presented technique supports. C. C., A‐M. W. and B. W. wrote the manuscript. A‐M. W. and J‐X. F. revised the article.
Acknowledgements
We are thankful to Joshua Heazlewood and Berit Ebert from the University of Melbourne, Australia, for critical reading. This work was supported by the National Natural Science Foundation of China (Grant Numbers 31670670, 31870653, 31811530009), the Autonomous Research Project of State Key Laboratory for Conservation and Utilization of Subtropical Agro‐bioresources (SKLCUSA‐a201923), and the open Foundation (No. 491170K201703) of Jiangsu Provincial Key Laboratory of Agrobiology.
Chen, C. , Zhao, X. , Wang, X. , Wang, B. , Li, H. , Feng, J. and Wu, A. (2021) Mutagenesis of UDP‐xylose epimerase and xylan arabinosyl‐transferase decreases arabinose content and improves saccharification of rice straw. Plant Biotechnol J., 10.1111/pbi.13552
References
- Anders, N. , Wilkinson, M.D. , Lovegrove, A. , Freeman, J. , Tryfona, T. , Pellny, T.K. , Weimar, T. et al. (2012) Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc. Natl Acad. Sci. USA, 109, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burget, E.G. , Verma, R. , Molhoj, M. and Reiter, W.D. (2003) The biosynthesis of L‐arabinose in plants: Molecular cloning and characterization of a Golgi‐localized UDP‐D‐xylose 4‐epimerase encoded by the MUR4 gene of Arabidopsis. Plant Cell, 15, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casler, M.D. and Jung, H.J.G. (2006) Relationships of fibre, lignin, and phenolics to in vitro fibre digestibility in three perennial grasses. Anim. Feed Sci. Technol. 125, 151–161. [Google Scholar]
- Konishi, T. , Aohara, T. , Igasaki, T. , Hayashi, N. , Miyazaki, Y. , Takahashi, A. , Hirochika, H. et al. (2011) Down‐regulation of UDP‐arabinopyranose mutase reduces the proportion of arabinofuranose present in rice cell walls. Phytochemistry 72, 1962–1968. [DOI] [PubMed] [Google Scholar]
- Li, F. , Zhang, M. , Guo, K. , Hu, Z. , Zhang, R. , Feng, Y. , Yi, X. et al. (2015) High‐level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol. J. 13, 514–525. [DOI] [PubMed] [Google Scholar]
- Marriott, P.E. , Gomez, L.D. and McQueen‐Mason, S.J. (2016) Unlocking the potential of lignocellulosic biomass through plant science. New Phytol. 209, 1366–1381. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi, M. , Nakamura, A. , Nakamura, H. , Hakata, M. , Ichikawa, H. , Hirochika, H. , Ishii, T. et al. (2013) Increase in cellulose accumulation and improvement of saccharification by overexpression of arabinofuranosidase in rice. PLoS One 8, e78269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, R. , Lawther, J.M. and Banks, W.B. (1996) Fractional and structural characterization of wheat straw hemicelluloses. Carbohyd. Polym. 29, 325–331. [Google Scholar]
- Wang, Y. , Fan, C. , Hu, H. , Li, Y. , Sun, D. , Wang, Y. and Peng, L. (2016) Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol. Adv. 34, 997–1017. [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Zhang, M. , Wang, L. , Tu, Y. , Zhang, J. , Xie, G. , Zou, W. et al. (2013) Biomass digestibility is predominantly affected by three factors of wall polymer features distinctive in wheat accessions and rice mutants. Biotechnol. Biofuels, 6, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]


