Abstract
Objectives
Protecting healthcare workers (HCWs) from coronavirus disease-19 (COVID-19) is critical to preserve the functioning of healthcare systems. We therefore assessed seroprevalence and identified risk factors for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) seropositivity in this population.
Methods
Between 22 June 22 and 15 August 2020, HCWs from institutions in northern/eastern Switzerland were screened for SARS-CoV-2 antibodies. We recorded baseline characteristics, non-occupational and occupational risk factors. We used pairwise tests of associations and multivariable logistic regression to identify factors associated with seropositivity.
Results
Among 4664 HCWs from 23 healthcare facilities, 139 (3%) were seropositive. Non-occupational exposures independently associated with seropositivity were contact with a COVID-19-positive household (adjusted OR 59, 95% CI 33–106), stay in a COVID-19 hotspot (aOR 2.3, 95% CI 1.2–4.2) and male sex (aOR 1.9, 95% CI 1.1–3.1). Blood group 0 vs. non-0 (aOR 0.5, 95% CI 0.3–0.8), active smoking (aOR 0.4, 95% CI 0.2–0.7), living with children <12 years (aOR 0.3, 95% CI 0.2–0.6) and being a physician (aOR 0.2, 95% CI 0.1–0.5) were associated with decreased risk. Other occupational risk factors were close contact to COVID-19 patients (aOR 2.7, 95% CI 1.4–5.4), exposure to COVID-19-positive co-workers (aOR 1.9, 95% CI 1.1–2.9), poor knowledge of standard hygiene precautions (aOR 1.9, 95% CI 1.2–2.9) and frequent visits to the hospital canteen (aOR 2.3, 95% CI 1.4–3.8).
Discussion
Living with COVID-19-positive households showed the strongest association with SARS-CoV-2 seropositivity. We identified several potentially modifiable work-related risk factors, which might allow mitigation of the COVID-19 risk among HCWs. The lower risk among those living with children, even after correction for multiple confounders, is remarkable and merits further study.
Keywords: COVID-19, Healthcare workers, Risk factors, Seroprevalence, Switzerland
Introduction
Coronavirus disease 2019 (COVID-19) is currently afflicting healthcare systems around the globe. As of 9 March 2021, over 2.6 million COVID-19 deaths have been reported worldwide [1]. In Switzerland, over 550 000 COVID-19 cases have been reported, more than 23 000 patients have been hospitalized and over 9000 have died [2]. Seroprevalence studies among Swiss healthcare workers (HCWs) performed in March and April 2020 have shown a low prevalence of 1% in the eastern part of the country, and a higher prevalence of around 10% in the western part [3,4]. Studies from different countries suggest that HCWs are at increased risk to acquire severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) compared with the general population [[5], [6], [7]]. In the UK, HCWs and their household contacts accounted for a sixth of all COVID-19 cases admitted to the hospital for those aged 18–65 years. This risk was increased for HCWs involved in patient care [8]. Considering these data, it is imperative to better understand risk factors for SARS-CoV-2 acquisition among HCWs to better protect them from infection.
In this multicentre study from Switzerland, we aimed to assess the prevalence of antibodies against SARS-CoV-2 among HCWs with and without patient contact. Additionally, we identified non-occupational and occupational factors associated with seropositivity to inform prevention recommendations for this population.
Materials and methods
Study design and participants
We initiated a multicentre cross-sectional study between 22 June and 15 August 2020 in healthcare institutions located in northern and eastern Switzerland. COVID-19 incidence was very low (7-day average between 0.6 and 3.2 cases/100 000 population) in Switzerland during the recruitment phase [2]. Acute care hospitals, rehabilitation clinics, and geriatric and psychiatric clinics were asked to participate. Employees aged 16 years or older were invited to enrol into the study via institutional webpages. Employees registered online and provided electronic consent. The study was approved by the local ethics committee (#2020-00502).
Questionnaire and definitions
We implemented a multimodular digital web-based questionnaire for institutions (including questions about facility structure) and participants. Participants received an email invitation to the questionnaire after blood draw for serology (but before being informed of the test result) and were asked about anthropometric data, occupational and non-occupational risk exposures and previous SARS-CoV-2 nasopharyngeal swabs. Household contacts were defined as people living in the same household or intimate partners; close contact to COVID-19 patients was assumed for those with contact >15 minutes within 2 meters with or without personal protective measures (PPE); aerosol-generating procedures (AGPs) were defined according to guidelines of the Swiss Centre for Infection Prevention (Swissnoso) and included mainly intubation, tracheotomy, non-invasive ventilation and bronchoscopy. Poor knowledge of standard precautions was assumed for those who correctly identified fewer than three measures out of (hand hygiene, surgical mask in case of respiratory symptoms, gowns in case of potential contamination with body fluids, cough etiquette and vaccination). Low protection while caring for COVID-19 patients was assumed for those reporting fewer than three measures out of face masks, gloves, gowns and goggles.
Sample processing
Upon registration, a venous blood sample was collected on site. Total antibodies directed against the nucleocapsid-(N)-protein of SARS-CoV-2 were detected by an electro-chemiluminescence immunoassay (ECLIA, Roche Diagnostics, Rotkreuz, Switzerland) on a COBAS 6000 instrument [9]. For this test, a sensitivity and specificity of 88% (at 3 weeks after infection) and >99%, respectively, have been reported [10]. A subgroup of samples with a positive signal in the ECLIA (at a cut-off index, COI, ≥1) were also tested with an enzyme-linked immunosorbent assay (ELISA, Euroimmune, Germany, detection each of IgG and IgA antibodies against S1 domain of the spike-(S)-protein including the immunologically relevant receptor binding domain). Seropositivity cut-offs were applied following manufacturer recommendations. Seropositivity was defined as a positive result in the ECLIA.
Statistical analysis
The relative frequency of participants with positive and negative serology was compared between levels of baseline characteristics, non-occupational risk factors and occupational risk factors. Fisher's exact test was used for dichotomous factors or factors with a reference level, comparing each level to the reference. Individuals with missing data were removed from the analysis of the respective variable. Logistic regression was used for numeric and ordinal variables. To evaluate which characteristics appeared to influence seropositivity after adjusting for possible confounders, age, sex, body mass index (BMI), blood group, smoking status, comorbidities as well as non-occupational and occupational risk factors were entered additively into a multivariable logistic regression model with serostatus at baseline (positive or negative) as response (see Table S2 for the definition and coding of covariables and Table S3 for methodical details of variable selection, model fitting and assessment). To assess whether spatial proximity or clustering of observations confounded the effects of the risk factors, we fitted two additional models including place of residence (seven predefined regions) and institution either as fixed effects or as random effects. An additional sensitivity analysis (complete case analysis) was performed excluding observations with missing data (Table S4). Analyses were performed with R statistical software, version 4.0.2.
Results
Baseline characteristics
We included 17 institutions on 23 sites across northern and eastern Switzerland, thereof 19 inpatient sites (14 acute care; one geriatric clinic; one rehabilitation clinic; three psychiatric clinics) and four outpatient clinics (three psychiatric facilities; one blood donation centre). The total of represented patient beds was 3523 (thereof 106 ICU beds) (Table 1 ).
Table 1.
Characteristics of the institutions from which participants were recruited (n = 17) including number of sites (different cities), number of beds, total number of healthcare workers working in institution, number of study participants and seropositivity
| Type of institution | Sites (n) | Inpatients (yes vs. no) | Beds (n) | ICU beds (n) | HCWs (n) | HCWs in study (n) | HCWs in study (%) | Seropositive HCWs (n) | Seropositive HCWs (%) |
|---|---|---|---|---|---|---|---|---|---|
| Total | 23 | NA | 3523 | 106 | 17 060 | 4664 | 27 | 139 | 3.0 |
| Acute care A | 3 | yes | 765 | 36 | 5930 | 1074 | 18 | 37 | 3.4 |
| Acute care B | 1 | yes | 370 | 10 | 2245 | 1023 | 46 | 39 | 3.8 |
| Acute care C | 3 | yes | 304 | 7 | 1367 | 534 | 39 | 9 | 1.7 |
| Acute care D | 1 | yes | 74 | 0 | 362 | 109 | 30 | 3 | 2.8 |
| Acute care E | 1 | yes | 46 | 0 | 178 | 66 | 37 | 1 | 1.5 |
| Acute care F | 1 | yes | 246 | 9 | 749 | 169 | 23 | 7 | 4.1 |
| Acute care G | 1 | yes | 310 | 12 | 740 | 171 | 23 | 3 | 1.8 |
| Acute care H | 1 | yes | 330 | 18 | 1788 | 448 | 25 | 18 | 4.0 |
| Acute care I | 1 | yes | 129 | 6 | 525 | 159 | 30 | 4 | 2.5 |
| Acute care J | 1 | yes | 100 | 8 | 632 | 109 | 17 | 3 | 2.8 |
| Geriatric acute care K | 1 | yes | 98 | 0 | 265 | 123 | 46 | 3 | 2.4 |
| Rehabilitation clinic L | 1 | yes | 135 | 0 | 510 | 168 | 33 | 7 | 4.2 |
| Psychiatric clinic M | 1 | yes | 242 | 0 | 360 | 190 | 53 | 1 | 0.5 |
| Psychiatric clinic N | 1 | yes | 150 | 0 | 391 | 108 | 28 | 1 | 0.9 |
| Psychiatric clinic O | 1 | yes | 224 | 0 | 780 | 98 | 13 | 1 | 1.0 |
| Psychiatry P | 3 | no | NA | NA | 178 | 88 | 49 | 2 | 2.3 |
| Blood donation Q | 1 | no | NA | NA | 60 | 27 | 45 | 0 | 0.0 |
ICU, Intensive Care Unit; HCWs, healthcare workers.
Among 17 060 potentially eligible HCWs, median age was 40 years, 76% were female, 40% were nurses and 15% physicians. Of these, 4664 (27%) participated in the study. Median age of participating HCWs was 38 years (range 16–73); 3654 (78%) were female. The majority were nurses (n = 2126; 46%) followed by physicians (n = 776; 17%); 3676 (79%) reported having patient contact (Table 2 ).
Table 2.
Distribution of baseline characteristics, non-occupational and occupational factors, and self-reported PCR results among the study participants, and distribution of serostatus for each level of the factors (n and % if not stated otherwise)
| Total n = 4664 | Missing valuesa | Seropositive n = 139 | Seronegative n = 4525 | OR with 95% CIb | p | |
|---|---|---|---|---|---|---|
| Gender | 27 | |||||
| Female | 3654 | 105 (2.9%) | 3549 (97.1%) | ref | — | |
| Male | 983 | 34 (3.5%) | 949 (96.5%) | 1.21 (0.79–1.81) | 0.343 | |
| Age, median (IQR), OR per 10 years | 38.3 (29.7–49.5) | 10 | 35.5 (26.8–46.8) | 38.4 (29.7–49.6) | 0.83 (0.71–0.96) | 0.012 |
| BMI, median (IQR), OR per unit | 23.4 (21.3–26.2) | 11 | 24.2 (22.2–27.1) | 23.4 (21.3–26.1) | 1.03 (1.00–1.07) | 0.078 |
| Smoking status | 0 | |||||
| Never | 2891 | 96 (3.3%) | 2795 (96.7%) | ref | — | |
| Active | 822 | 16 (1.9%) | 806 (98.1%) | 0.58 (0.32–0.99) | 0.049 | |
| Former | 951 | 27 (2.8%) | 924 (97.2%) | 0.85 (0.53–1.33) | 0.525 | |
| Comorbidity | 0 | |||||
| No | 3021 | 80 (2.6%) | 2941 (97.4%) | ref | — | |
| Yes | 1643 | 59 (3.6%) | 1584 (96.4%) | 1.37 (0.96–1.95) | 0.072 | |
| Blood group (OR: one group vs. all others) | 65 | |||||
| A | 1396 | 51 (3.7%) | 1345 (96.3%) | 1.37 (0.95–1.97) | 0.090 | |
| AB | 161 | 6 (3.7%) | 155 (96.3%) | 1.27 (0.45–2.91) | 0.482 | |
| B | 354 | 14 (4.0%) | 340 (96.0%) | 1.38 (0.72–2.43) | 0.254 | |
| 0 | 1383 | 25 (1.8%) | 1358 (98.2%) | 0.51 (0.32–0.80) | 0.002 | |
| I don't know | 1305 | 41 (3.1%) | 1264 (96.9%) | 1.08 (0.73–1.58) | 0.701 | |
| Influenza vaccine 2019/2020 | 89 | |||||
| No | 3159 | 102 (3.2%) | 3057 (96.8%) | ref | — | |
| Yes | 1416 | 35 (2.5%) | 1381 (97.5%) | 0.76 (0.50–1.13) | 0.189 | |
| BCG vaccine | 66 | |||||
| No | 1586 | 55 (3.5%) | 1531 (96.5%) | ref | — | |
| Yes | 1908 | 49 (2.6%) | 1859 (97.4%) | 0.73 (0.49–1.11) | 0.134 | |
| I don't know | 1104 | 34 (3.1%) | 1070 (96.9%) | 0.88 (0.56–1.39) | 0.661 | |
| No of respiratory tract infections/year | 0 | |||||
| 0 or 1 | 3862 | 105 (2.7%) | 3757 (97.3%) | ref | — | |
| 2 to 4 | 776 | 31 (4.0%) | 745 (96.0%) | 1.49 (0.96–2.26) | 0.062 | |
| 5+ | 26 | 3 (11.5%) | 23 (88.5%) | 4.66 (0.88–15.8) | 0.034 | |
| No of persons in household | 0 | |||||
| 1 (OR per person) | 814 | 17 (2.1%) | 797 (97.9%) | 0.94 (0.82–1.08) | 0.383 | |
| 2 | 1660 | 64 (3.9%) | 1596 (96.1%) | |||
| 3 | 778 | 22 (2.8%) | 756 (97.2%) | |||
| 4 | 957 | 29 (3.0%) | 928 (97.0%) | |||
| 5+ | 455 | 7 (1.5%) | 448 (98.5%) | |||
| No of children ≤12 years | 0 | |||||
| 0 (OR per person) | 3526 | 120 (3.4%) | 3406 (96.6%) | 0.70 (0.52–0.90) | 0.010 | |
| 1 | 492 | 6 (1.2%) | 486 (98.8%) | |||
| 2 | 509 | 12 (2.4%) | 497 (97.6%) | |||
| 3+ | 137 | 1 (0.7%) | 136 (99.3%) | |||
| Confirmed COVID-19 case in household | 0 | |||||
| No | 4585 | 95 (2.1%) | 4490 (97.9%) | ref | — | |
| Yes | 79 | 44 (55.7%) | 35 (44.3%) | 59.1 (35.4–99.9) | <0.001 | |
| Symptomatic household contact | 0 | |||||
| No | 3269 | 62 (1.9%) | 3207 (98.1%) | ref | — | |
| Yes | 1395 | 77 (5.5%) | 1318 (94.5%) | 3.02 (2.12–4.32) | <0.001 | |
| Visit to a COVID-19 hotspotc | 0 | |||||
| No | 4413 | 122 (2.8%) | 4291 (97.2%) | ref | — | |
| Yes | 251 | 17 (6.8%) | 234 (93.2%) | 2.55 (1.42–4.35) | 0.002 | |
| Leisure activities (currently; OR for with vs. without activity)d | 0 | |||||
| Visit to restaurant/bar | 2783 | 84 (3.0%) | 2699 (97.0%) | 1.03 (0.72–1.49) | 0.930 | |
| Sport club | 833 | 28 (3.4%) | 805 (96.6%) | 1.17 (0.74–1.79) | 0.499 | |
| Fitness/yoga classes | 1462 | 49 (3.4%) | 1413 (96.6%) | 1.20 (0.82–1.73) | 0.309 | |
| Theatre/concerts | 112 | 4 (3.6%) | 108 (96.4%) | 1.21 (0.32–3.27) | 0.577 | |
| Cinema | 290 | 14 (4.8%) | 276 (95.2%) | 1.72 (0.90–3.05) | 0.071 | |
| Religious gatherings | 228 | 6 (2.6%) | 222 (97.4%) | 0.87 (0.31–1.99) | 1.000 | |
| Singing in choir | 59 | 2 (3.4%) | 57 (96.6%) | 1.14 (0.13–4.41) | 0.695 | |
| Active group musician | 110 | 4 (3.6%) | 106 (96.4%) | 1.24 (0.33–3.33) | 0.570 | |
| No of leisure activities above | 0 | |||||
| 0 (OR per activity) | 1045 | 25 (2.4%) | 1020 (97.6%) | 1.13 (0.95–1.34) | 0.169 | |
| 1 | 1875 | 55 (2.9%) | 1820 (97.1%) | |||
| 2 | 1320 | 46 (3.5%) | 1274 (96.5%) | |||
| 3 | 342 | 9 (2.6%) | 333 (97.4%) | |||
| 4+ | 82 | 4 (4.9%) | 78 (95.1%) | |||
| No of shopping trips per week (currently) | 174 | |||||
| 0 (OR per trip) | 34 | 2 (5.9%) | 32 (94.1%) | 1.03 (0.87–1.21) | 0.753 | |
| 1 | 1212 | 34 (2.8%) | 1178 (97.2%) | |||
| 2 | 1631 | 46 (2.8%) | 1585 (97.2%) | |||
| 3 | 963 | 33 (3.4%) | 930 (96.6%) | |||
| 4+ | 650 | 19 (2.9%) | 631 (97.1%) | |||
| Profession (OR: one profession vs. all others) | 209 | |||||
| Nurse | 2257 | 88 (3.9%) | 2169 (96.1%) | 1.87 (1.31–2.71) | <0.001 | |
| Physician | 776 | 8 (1.0%) | 768 (99.0%) | 0.30 (0.13–0.61) | <0.001 | |
| Administration/Secretary | 472 | 8 (1.7%) | 464 (98.3%) | 0.53 (0.22–1.09) | 0.087 | |
| Physiotherapist | 181 | 7 (3.9%) | 174 (96.1%) | 1.33 (0.52–2.87) | 0.498 | |
| Other | 769 | 16 (2.1%) | 753 (97.9%) | 0.65 (0.36–1.11) | 0.130 | |
| Speciality, if any (OR: one speciality vs. all others)a | 0 | |||||
| Internal Medicine | 995 | 31 (3.1%) | 964 (96.9%) | 1.06 (0.68–1.61) | 0.753 | |
| Surgery/Orthopaedics | 475 | 14 (2.9%) | 461 (97.1%) | 0.99 (0.52–1.74) | 1.000 | |
| Intensive care | 289 | 5 (1.7%) | 284 (98.3%) | 0.56 (0.18–1.35) | 0.280 | |
| Emergency department | 272 | 9 (3.3%) | 263 (96.7%) | 1.12 (0.50–2.23) | 0.712 | |
| Other | 585 | 18 (3.1%) | 567 (96.9%) | 1.04 (0.59–1.73) | 0.896 | |
| Work percentage (i.e. employment level) | 0 | |||||
| >80% | 2690 | 90 (3.3%) | 2600 (96.7%) | ref | — | |
| ≤80% | 1974 | 49 (2.5%) | 1925 (97.5%) | 0.74 (0.51–1.06) | 0.098 | |
| Patient contact | 269 | |||||
| No | 719 | 12 (1.7%) | 707 (98.3%) | ref | — | |
| Yes | 3676 | 115 (3.1%) | 3561 (96.9%) | 1.23 (0.85–1.77) | 0.263 | |
| Involved in AGP | 0 | |||||
| No | 3228 | 90 (2.8%) | 3138 (97.2%) | ref | — | |
| Yes | 1436 | 49 (3.4%) | 1387 (96.6%) | 1.90 (1.04–3.81) | 0.037 | |
| No of correct standard precaution measures | 0 | |||||
| 0 to 2 | 1073 | 44 (4.1%) | 1029 (95.9%) | Ref | — | |
| 3 or 4 | 2229 | 55 (2.5%) | 2174 (97.5%) | 0.59 (0.39 – 0.91) | 0.012 | |
| 5 | 1362 | 40 (2.9%) | 1322 (97.1%) | 0.71 (0.45 – 1.12) | 0.146 | |
| Adherence to standard precautions | 0 | |||||
| Almost always | 2829 | 76 (2.7%) | 2753 (97.3%) | ref | — | |
| If I remember | 1227 | 37 (3.0%) | 1190 (97.0%) | 1.13 (0.73–1.70) | 0.604 | |
| Often not possible | 320 | 10 (3.1%) | 310 (96.9%) | 1.17 (0.53–2.30) | 0.589 | |
| Poorly | 43 | 2 (4.7%) | 41 (95.3%) | 1.77 (0.20–7.02) | 0.327 | |
| No answer | 245 | 14 (5.7%) | 231 (94.3%) | 2.19 (1.13–3.99) | 0.015 | |
| Caring for COVID-19 patients | 254 | |||||
| No | 2348 | 40 (1.7%) | 2308 (98.3%) | ref | — | |
| Yes | 2062 | 85 (4.1%) | 1977 (95.9%) | 2.48 (1.68–3.73) | <0.001 | |
| Physical contact with COVID-19 patienta | 1 | |||||
| No (only distant contact) | 732 | 16 (2.2%) | 716 (97.8%) | ref | — | |
| Yes | 1329 | 69 (5.2%) | 1260 (94.8%) | 2.45 (1.39–4.56) | 0.001 | |
| Exposure to coughing or sneezing by COVID-19 patienta | 1 | |||||
| No | 1544 | 52 (3.4%) | 1492 (96.6%) | ref | — | |
| Yes | 517 | 33 (6.4%) | 484 (93.6%) | 1.96 (1.21–3.12) | 0.005 | |
| Protection during close contact; OR for with vs. without each protectiona,d | 0 | |||||
| Any face mask | 1275 | 59 (4.6%) | 1216 (95.4%) | 0.21 (0.10–0.50) | <0.001 | |
| Gloves | 1125 | 49 (4.4%) | 1076 (95.6%) | 0.42 (0.24–0.76) | 0.003 | |
| Gown | 979 | 41 (4.2%) | 938 (95.8%) | 0.50 (0.30–0.86) | 0.008 | |
| Goggles | 931 | 39 (4.2%) | 892 (95.8%) | 0.54 (0.32–0.91) | 0.015 | |
| None | 47 | 8 (17.0%) | 39 (83.0%) | 4.10 (1.58–9.40) | 0.002 | |
| No of protection measures abovea | 0 | |||||
| 0 (OR per measure) | 44 | 8 (18.2%) | 36 (81.8%) | 0.73 (0.61–0.87) | <0.001 | |
| 1 | 147 | 12 (8.2%) | 135 (91.8%) | |||
| 2 | 116 | 6 (5.2%) | 110 (94.8%) | |||
| 3 | 157 | 8 (5.1%) | 149 (94.9%) | |||
| 4 | 865 | 35 (4.0%) | 830 (96.0%) | |||
| Contacts with COVID-19 positive co-worker | 254 | |||||
| No answer/don't know | 1212 | 31 (2.6%) | 1181 (97.4%) | 1.15 (0.71–1.82) | 0.564 | |
| None | 2548 | 57 (2.2%) | 2491 (97.8%) | ref | — | |
| 1–2 times | 474 | 25 (5.3%) | 449 (94.7%) | 2.43 (1.44–4.01) | 0.001 | |
| 3 or more times | 176 | 12 (6.8%) | 164 (93.2%) | 3.20 (1.53–6.17) | 0.001 | |
| Frequency of meals in staff canteen | 29 | |||||
| Never | 765 | 10 (1.3%) | 755 (98.7%) | ref | — | |
| Occasionally | 659 | 17 (2.6%) | 642 (97.4%) | 2.00 (0.86–4.92) | 0.083 | |
| Weekly | 1184 | 45 (3.8%) | 1139 (96.2%) | 2.98 (1.47–6.68) | 0.001 | |
| Daily | 2027 | 66 (3.3%) | 1961 (96.7%) | 2.54 (1.29–5.57) | 0.004 | |
| Self-reported PCR resultsa | ||||||
| Negative | 792 | 17 (2.1%) | 775 (97.9%) | ref | — | |
| Positive | 72 | 66 (91.7%) | 6 (8.3%) | 501 (191–1315) | <0.001 |
Odds ratio (and 95% confidence interval) of being seropositive for participants with a certain characteristic compared to those without it or with a reference level (denoted as “ref”), or for the increase in seropositivity per unit increase in numeric/ordinal characteristics. OR, odds ratio; CI, confidence interval; IQR, interquartile range; BMI, body mass index; BCG, bacillus Calmette-Guerin; AGP, aerosol-generating procedure; COVID-19, coronavirus disease-2019; PCR, polymerase chain reaction.
Some questions have only been asked to a subgroup of participants, therefore the total number of answers for these questions does not add up to n = 4664. See Table S2 how missing values were handled for each variable; see Table S4 for complete case analysis.
OR (and 95% CI) and p-value derived from Fisher's Exact test for categorical characteristics or from logistic regression for numeric characteristics.
COVID-19 hotspots before April 2020 (i.e. Northern Italy, Austrian ski resorts or Alsace).
More than one answer possible.
Seropositivity and self-reported PCR results
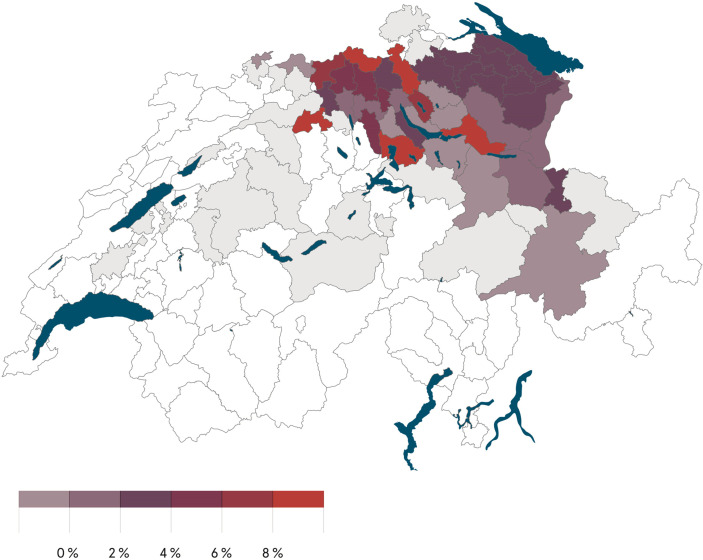
Overall, seropositivity was 3% (139/4664). Among these 139, 88 (63%) were tested with the confirmatory ELISA with 88 samples showing either positive IgA or IgG. At the institutional level, seropositivity ranged from 0.5% to 4.2% for inpatient, and 0% to 2.3% for outpatient facilities (Table 1). Seropositivity by district ranged from 0% to 13% and was lower in eastern than in northern Switzerland (Fig. 1 ).
Fig. 1.
SARS-CoV-2 seropositivity by district (place of residence of healthcare workers) in northern and eastern Switzerland (in grey: no seroprevalence indicated for districts with less than 10 participants).
A previous PCR result was reported by 864 of 4664 (18.5%) participants. Of 72 participants with positive PCR, 66 (92%) were seropositive, whereas 17/792 (2.2%) participants with negative PCR had a positive serology (Table 2).
Non-occupational factors associated with seropositivity
Exposure to COVID-19 confirmed (55.7% vs. 2.1%, p < 0.001) or symptomatic, not confirmed household contacts (5.5% vs. 1.9%, p < 0.001) was strongly associated with seropositivity. Visiting a known COVID-19 hotspot in Austria (but not Italy or France) was clearly associated with seropositivity (6.8% vs. 2.8%, p 0.002). Seroprevalence was lower among those with blood group 0 vs. non-0 (1.8% vs. 3.5%, p 0.002) and for those living with children aged 12 or younger (1.7% vs. 3.4%, p 0.002) (Tables 2 and S2).
Occupational factors associated with seropositivity
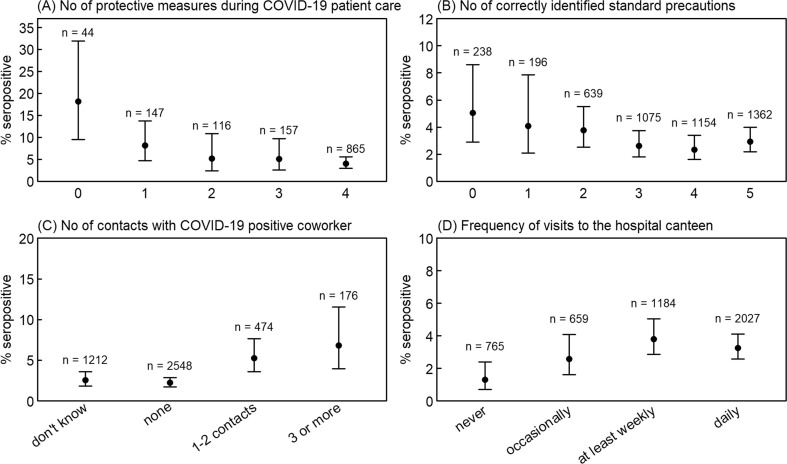
Nurses had higher (3.9%), physicians lower (1.0%) seropositivity rates; no differences were noted between medical specialities. Seroprevalence was higher among those with patient contact (3.1% vs. 1.7%, p 0.037), particularly contact to confirmed COVID-19 patients (4.1% vs. 1.7%, p < 0.001). Workers indicating low protection while caring for COVID-19 patients (5.8% vs. 3.5%, p 0.019) and those with poor knowledge of hygiene standards had higher seropositivity (4.1% vs. 2.6%, p 0.018) (Fig. 2 A,B). Numbers of unprotected contacts to COVID-19 confirmed or symptomatic co-workers were associated with seropositivity (Fig. 2C). Workers who never/occasionally visited the hospital canteen had a lower seroprevalence compared to those with weekly/daily visits (1.9% vs. 3.5%, p 0.004) (Fig. 2D). This effect was consistent across institutions and professions (Table S1).
Fig. 2.
SARS-CoV-2 seropositivity according to four occupational factors, with 95% Wilson confidence intervals: (A) number of protective measures used (among face mask, gown, gloves, goggles) while caring for COVID-19 patients; (B) number of correctly identified elements of standard precautions (among hand hygiene, cough etiquette, surgical mask in case of respiratory symptoms, vaccinations, donning of gowns if potential contact with body fluids); (C) number of contacts with COVID-19-positive co-workers; (D) frequency of meals in the hospital canteen.
Multivariable analyses
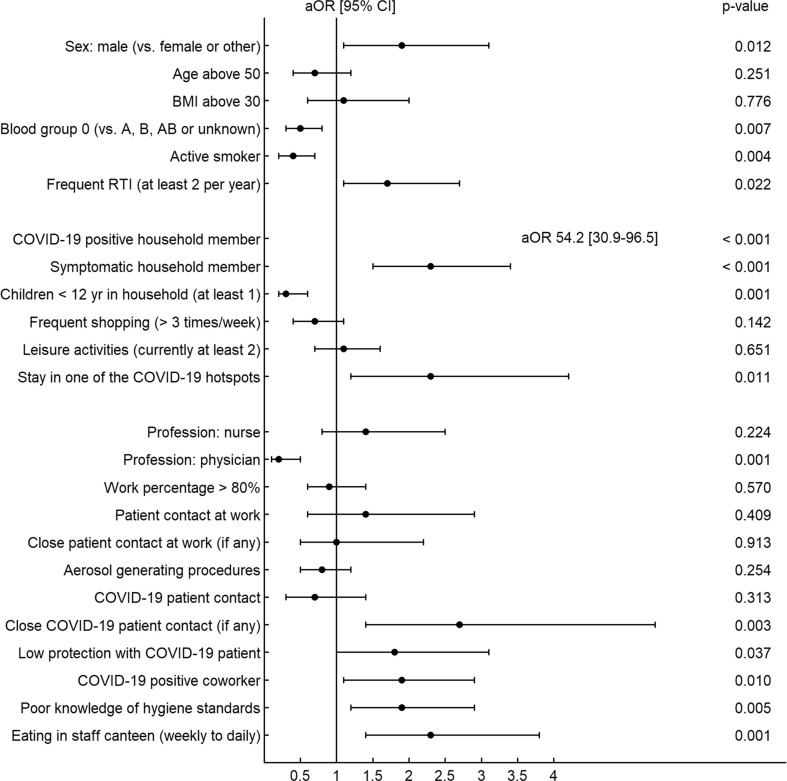
In multivariable analysis, exposure to a COVID-19-positive household member remained the strongest risk factor for seropositivity with an adjusted odds ratio (aOR) of 59 (95% CI 33–106) (Fig. 3 and Table S3). There was an increased risk associated with male sex (aOR 1.9, 95% CI 1.1–3.1) and stay in a COVID-19 hotspot (aOR 2.3, 95% CI 1.2–4.2), whereas blood group 0 (aOR 0.5, 95% CI 0.3–0.8), active smoking (aOR 0.4, 95% CI 0.2–0.7) and living with children <12 years (aOR 0.3, 95% CI 0.2–0.6) were all associated with decreased risk after correcting for multiple confounder variables.
Fig. 3.
Forest plot showing the association of baseline, occupational and non-occupational risk factors with seropositivity based on multivariable logistic regression analysis. For this analysis, variables from Table 2 were dichotomized and combined into an additive model. Adjusted odds ratios (aOR) were derived from model coefficients, and 95% CI were obtained though the profile likelihood. See Table S3 for further details on model definition.
Physicians had lower risk than other professions (aOR 0.2, 95% CI 0.1–0.5). Other significant occupational factors included close contact with a COVID-19 patient (aOR 2.7, 95% CI 1.4–5.4), exposure to a COVID-19-positive co-worker (aOR 1.9, 95% CI 1.1–2.9), poor knowledge of standard precautions (aOR 1.9, 95% CI 1.2–2.9), likewise having weekly/daily (vs. rarely/never) meals in hospital canteens (aOR 2.3, 95% CI 1.4–3.8). Sensitivity analyses showed no relevant confounding by geographic region or healthcare institution (Table S3), nor did the exclusion of missing values (complete-case analysis) cause relevant changes to point-estimates and significance levels for the effects of the risk factors (Table S4).
Discussion
In this cross-sectional study of 4664 Swiss HCWs, 3% of participants had SARS-CoV-2 antibodies. The main findings are that exposure to a COVID-19-positive household member is the strongest risk factor for seropositivity. Meanwhile, living with children under the age of 12, is clearly associated with decreased risk, even after correction for multiple confounders. We also identified several work-related exposures associated with seropositivity which might serve as leverage further decreasing the risk of SARS-CoV-2 acquisition among HCWs.
We confirm findings from other studies showing that COVID-19-positive household contacts are the main source of SARS-CoV-2 infection for HCWs [11,12]. Our findings are consistent with a Dutch study that concluded that nosocomial transmissions seemed rather uncommon and that multiple hospital introductions from the community are probably responsible for most COVID-19 cases among patients and HCWs, at least in a low-prevalence setting [13]. Of course, this association is most certainly overestimated given that the directionality of virus transmission cannot be definitely assessed with our study design.
An important finding of our study is that participants living with children aged <12 years were less likely to be seropositive. Findings from a Scottish study among over 300 000 HCW households [14] and a population-based UK cohort [15] are consistent with our results. An intriguing hypothesis is that frequent infections in childhood with endemic coronaviruses (e.g. HCoV-OC43) might confer partial cross-immunity to SARS-CoV-2. In agreement, youth and adults aged 15 to 44 years, who are more likely to live with young children, had higher antibody titres against the HCoV-OC43 nucleocapsid protein than older adults [16]. Also, supporting the notion of a rather immunological than a purely epidemiological phenomenon, a German study among over 4000 COVID-19 patients suggested a less complicated disease course for those with frequent contact to children [17]. However, the protective role of humoral and cellular immunity against endemic coronaviruses regarding SARS-CoV-2 acquisition has to be confirmed in prospective studies.
Interestingly, a stay in an Austrian ski resort where at least one COVID-19 superspreading event had occurred in February/March 2020 was an independent risk factor for seropositivity [18]. Several studies have by now identified an association between the ABO blood group system and acquisition of COVID-19. Consistently, blood group O is considered to have a protective effect as shown in our study, whereas people with a non-O blood group (mostly A) seem to carry an increased risk [19]. We also observed a lower seroprevalence among active smokers, confirming findings of a meta-analysis [20]. However, it seems not justified at all to deduce a protective role of smoking from these results given the greater risk of worse outcomes among smokers with COVID-19 [20]. As recently speculated, this “smoking paradox” (lower risk of SARS-CoV-2 infection for current smokers, but greater risk for worse outcomes in case of COVID-19) might be the result of a confounder effect [21]. Another potential bias in our study is that smoking status was self-reported.
An important question is whether HCWs caring for COVID-19 patients are in fact at increased risk for acquiring the disease themselves. A recent meta-analysis concluded that HCWs do indeed have an increased risk compared with the general population [22]. Also, frontline HCWs in Denmark showed higher seroprevalences than other HCWs [23]. Our study confirms these findings, at least for those with close contact to COVID-19 patients. Interestingly, physicians were less likely to be seropositive than other professions, as shown previously [24]. This could be possibly explained by less patient exposure for physicians than nurses [25]. As shown by Galanis et al., male HCWs had an increased risk in our study [22]. As opposed to other studies [22], a lower level of protection was not significantly associated with seropositivity in our analysis, probably because of the restrictive definition of low protection. Due to the cross-sectional study design we cannot draw valid conclusions regarding the individual benefit of single protective measures such as gloves, gowns or goggles. However, participants performing AGPs and those working in intensive care or emergency rooms did not have an increased risk for COVID-19, suggesting that current safety measures are sufficient for these high-risk HCWs. Of note, poor knowledge of standard hygiene precautions was associated with detection of SARS-CoV-2 antibodies, supporting efforts to continuously educate HCWs regarding basic infection prevention concepts.
We identified other work-related COVID-19 risks for HCWs. Exposure to ill co-workers is a known risk factor for respiratory illness in HCWs, not only for COVID-19 but also for other respiratory viral diseases [26]. Across all participating institutions, we identified visits to the hospital canteen as potential risk factor for seropositivity. We found one other study which reported staying in the same HCWs break room and eating in proximity to other HCWs as risk factor for SARS-CoV-2 transmission [27]. Visiting restaurants other than hospital canteens has previously been shown to be potentially associated with higher risk of SARS-CoV-2 acquisition [[28], [29], [30]]; however, this was not the case in our data. This discrepancy could be explained by the fact that (a) the visitor turnover of hospital canteens is much higher than in other eating places and (b) that the probability of a HCWs being infectious is higher than for an average visitor to other restaurants. We therefore suggest that hospitals should revisit and potentially reinforce the safety concepts of their canteens and food courts.
Our study has several limitations. First, causality cannot be inferred between exposures and seropositivity. Second, only one-quarter of eligible HCWs were included in our study, which might have biased our results. For example, seroprevalence among all eligible HCWs might be slightly lower, because nurses, who were more likely to be seropositive, were overrepresented in our study. Third, we relied on mostly self-reported data in our questionnaire, which are subject to recall and other bias. Fourth, we observed several missing or unknown values in our dataset. Yet, results of the complete case analysis were very similar to the figures obtained from the full model. Strengths of the study are its large sample size, the inclusion of different types of healthcare institutions across a large geographic area, and consideration of not only occupational but a broad range of non-occupational risk factors. In particular the latter differentiates our study from most other seroprevalence studies performed among HCWs.
To conclude, having a COVID-19-positive household member had the strongest impact on SARS-CoV-2 seropositivity among our HCWs. However, we identified several modifiable work-related risks, including contact to COVID-19 co-workers, poor knowledge of standard hygiene precautions, and possibly frequent visits to hospital canteen. Living with children below 12 years of age was independently associated with decreased risk, an extraordinary finding suggesting an increased role of cross-immunity.
Transparency declaration
None of the co-authors reports any conflict of interest. This work was supported by the Swiss National Sciences Foundation (grant number 31CA30_196544; grant number PZ00P3_179919 to PK), the Federal Office of Public Health (grant number 20.008218/421–28/1), the Health Department of the Canton of St. Gallen, and the research fund of the Cantonal Hospital of St. Gallen.
Acknowledgements
We would like to warmly thank the large number of employees of the participating health care institutions who either took part in this study themselves or supported it. Furthermore, we thank the laboratory staff for shipment, handling and analysis of the blood samples. In particular, we acknowledge the organizational core team Simone Kessler and Susanne Nigg, who kept all strings between the participating centres and the laboratory and without whom this study would not have been possible.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.014.
Authors contributions
All authors contributed to the conceptualization of the study. C.R.K. and P.K. supervised the study. C.R.K., O.L., T.E., P.V. and P.K. were responsible for data curation. T.E. was responsible for project administration. R.P., J.S., D.F., A.B., E.L., C.M., P.R., R.S., D.V., B.W., U.B., L.R. and A.F. contributed to the investigations. L.R. provided laboratory resources. SG was responsible for the formal analysis and data visualizations. C.R.K., P.V., and P.K. were responsible for funding acquisition. C.R.K., R.P. and P.K. wrote the original draft, which was critically reviewed and edited by all authors.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.COVID-19 map Johns hopkins coronavirus resource center. https://coronavirus.jhu.edu/map.html Available at:
- 2.Swiss Federal Office of Public Health Current situation in Switzerland -Daily report. https://www.covid19.admin.ch/en/overview?ovTime=total Available at:
- 3.Kohler P.P., Kahlert C.R., Sumer J., Flury D., Güsewell S., Leal-Neto O.B. Prevalence of SARS-CoV-2 antibodies among Swiss hospital workers: results of a prospective cohort study. Infect Control Hosp Epidemiol. 2022;42:604–608. doi: 10.1017/ice.2020.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudberg A.-S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.-G., Ma W. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant J.J., Wilmore S.M.S., McCann N.S., Donnelly O., Lai R.W.L., Kinsella M.J. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah A.S.V., Wood R., Gribben C., Caldwell D., Bishop J., Weir A. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ. 2020:m3582. doi: 10.1136/bmj.m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron R.C., Risch L., Weber M., Thiel S., Grossmann K., Wohlwend N. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: a population-based study. Clin Chem Lab Med. 2020;58:2131–2140. doi: 10.1515/cclm-2020–0978. [DOI] [PubMed] [Google Scholar]
- 10.Mahase E. Covid-19: two antibody tests are “highly specific” but vary in sensitivity, evaluations find. BMJ. 2020;369 doi: 10.1136/bmj.m2066. [DOI] [PubMed] [Google Scholar]
- 11.Steensels D., Oris E., Coninx L., Nuyens D., Delforge M.-L., Vermeersch P. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh W.C., Naing L., Chaw L., Rosledzana M.A., Alikhan M.F., Jamaludin S.A. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikkema R.S., Pas S.D., Nieuwenhuijse D.F., O’Toole Á., Verweij J., van derLinden A. COVID-19 in health-care workers in three hospitals in the south of The Netherlands: a cross-sectional study. Lancet Infect Dis. 2020;20:1273–1280. doi: 10.1016/S1473-3099(20)30527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood R., Thomson E.C., Galbraith R., Gribben C., Caldwell D., Bishop J. Sharing a household with children and risk of COVID-19: a study of over 300,000 adults living in healthcare worker households in Scotland. Arch Dis Child. 2021 Mar 18 doi: 10.1136/archdischild-2021-321604. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes H., Morton C.E., Bacon S., McDonald H.I., Minassian C., Brown J.P. Association between living with children and outcomes from COVID-19: an OpenSAFELY cohort study of 12 million adults in England. BMJ. 2021;372:n628. doi: 10.1136/bmj.n628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X., Zhou H., Wu C., Xiao Y., Ren L., Paranhos-Baccalà G. Antibody against nucleocapsid protein predicts susceptibility to human coronavirus infection. J Infect. 2015;71:599–602. doi: 10.1016/j.jinf.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dugas M., Schrempf I.-M., Ochs K., Frömmel C., Greulich L., Neuhaus P. Association of contact to small children with a mild course of COVID-19. Int J Infect Dis. 2020;100:314–315. doi: 10.1016/j.ijid.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreidl P., Schmid D., Maritschnik S., Richter L., Borena W., Genger J.-W. Emergence of coronavirus disease 2019 (COVID-19) in Austria. Wien Klin Wochenschr. 2020;132:645–652. doi: 10.1007/s00508-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Severe Covid-19 GWAS Group Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons D., Shahab L., Brown J., Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7) Addict Abingdon Engl. 2020 doi: 10.1111/add.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eakin M.N., Neptune E. Smoking and COVID-19: the real deal. Ann Am Thorac Soc. 2021 Mar 1 doi: 10.1513/AnnalsATS.202012-1537PS. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai X., Wang M., Qin C., Tan L., Ran L., Chen D. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen B., Hyman S., Rosenberg L., Larson E. Frequency of patient contact with health care personnel and visitors: implications for infection prevention. Jt Comm J Qual Patient Saf Jt Comm Resour. 2012;38:560–565. doi: 10.1016/s1553-7250(12)38073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckrell S., Coleman B.L., McNeil S.A., Katz K., Muller M.P., Simor A. Sources of viral respiratory infections in Canadian acute care hospital healthcare personnel. J Hosp Infect. 2020;104:513–521. doi: 10.1016/j.jhin.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Çelebi G., Pişkin N., Bekleviç A.Ç., Altunay Y., Keleş A.S., Tüz M.A. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control. 2020;48:1225–1230. doi: 10.1016/j.ajic.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J., Gu J., Li K., Xu C., Su W., Lai Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher K.A., Tenforde M.W., Feldstein L.R., Lindsell C.J., Shapiro N.I., Files D.C. Community and close contact exposures associated with covid-19 among symptomatic adults ≥18 years in 11 outpatient health care facilities – United States, July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1258–1264. doi: 10.15585/mmwr.mm6936a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lentz R.J., Colt H., Chen H., Cordovilla R., Popevic S., Tahura S. Assessing coronavirus disease 2019 (COVID-19) transmission to healthcare personnel: the global ACT-HCP case-control study. Infect Control Hosp Epidemiol. 2021;42:381–387. doi: 10.1017/ice.2020.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.