Abstract
Background:
For individuals with Alcohol Use Disorder (AUD), long term recovery is difficult in part due to symptoms of anxiety that occur during early abstinence and can trigger relapse. Research in rodent models of AUD has identified the bed nucleus of the stria terminalis (BNST), a small, sexually dimorphic, subcortical region, as critical for regulating anxiety-like behaviors during abstinence, particularly in female mice. Furthermore, prolonged alcohol use and subsequent abstinence alter BNST afferent and efferent connections to other brain regions. To our knowledge, however, no studies of early abstinence have investigated BNST structural connectivity in humans during abstinence; this study addresses that gap.
Methods:
Nineteen participants with AUD currently in early abstinence and 20 healthy controls completed a diffusion tensor imaging (DTI) scan. BNST structural connectivity was evaluated using probabilistic tractography. A linear mixed model was used to test BNST network connectivity for between groups differences. Exploratory analyses were conducted to test for correlations between BNST connectivity and alcohol use severity and anxiety within the abstinence group. Sex was included as a factor for all analysis.
Results:
The BNST showed stronger structural connectivity with the BNST network in early abstinence women compared to control women, which was not seen in men. Women also showed region-specific differences, with stronger BNST-hypothalamus structural connectivity but weaker vmPFC-BNST structural connectivity compared to men. Exploratory analyses also demonstrated a relationship between alcohol use severity and vmPFC-BNST structural connectivity that was moderated by sex.
Conclusions:
This study is the first to demonstrate BNST structural connectivity differences in early abstinence and revealed key sex differences. The sex-specific differences in BNST structural connectivity during early abstinence could underlie known sex differences in abstinence symptoms and relapse risk and help develop potential sex-specific treatments.
Keywords: BNST, DTI, AUD, abstinence, structural connectivity
Introduction
For individuals suffering from Alcohol Use Disorder (AUD), maintaining abstinence often remains elusive, particularly when faced with stressful or uncertain circumstances. Following extended periods of sustained alcohol use, individuals with AUD undergo alterations in brain structure and neural function to maintain allostasis in the presence of alcohol (for review see Koob and Le Moal, 2001; Koob and Volkow, 2016). These alterations persist even when alcohol use is decreased or discontinued, as evidenced by the neural hyperexcitability seen in the first few days of abstinence when patients are at risk of autonomic dysregulation, seizures, and even death. Many symptoms of acute withdrawal are associated with alcohol-related changes in GABA signaling; GABA receptors are downregulated to counteract the depressant effect of alcohol; the subsequent cessation of alcohol leads to hyperexcitability (Koob and Le Moal, 2001). However, as the GABA-mediated withdrawal phase of the first few days subsides, longer-lasting effects emerge as a result of alterations in anxiety-associated brain regions (Centanni et al., 2019b; Koob and Volkow, 2016). The dysregulation of these brain regions has widespread consequences, including production of downstream stress hormones and abnormal stress responses (Koob and Le Moal, 2001). As a result, many people who become sober experience months or even years of heightened symptoms of anxiety and stress-reactivity (Heilig et al., 2010), lasting long past when physical withdrawal symptoms subside. Early abstinence refers to the phase following withdrawal when symptoms of anxiety and stress remain heightened. During this phase, the anxiety symptoms of abstinence can be transiently alleviated by alcohol, creating a powerful motivation to resume drinking through negative reinforcement mechanisms (e.g. Ramchandani et al., 2018). Thus, understanding the neural changes of abstinence-related anxiety is critical for identifying contributions to relapse and, ultimately, finding strategies to prevent relapse.
Substantial work in rodent models of abstinence has identified the bed nucleus of the stria terminalis (BNST) as a critical brain region contributing to anxiety-like behavior during abstinence (Centanni et al., 2019a; Koob and Volkow, 2016). The BNST is a small, sexually dimorphic, subcortical brain region that is part of the extended amygdala (Alheid and Heimer, 1988). The BNST has a variety of functions including arousal, hormone production, and the generation and maintenance of the stress response (for reviews see Avery et al., 2016; Ch’ng et al., 2018; Pleil and Skelly, 2018). In rodent models of chronic alcohol use, abstinent rodents have greater activity in stress-related BNST neurons (e.g. Centanni et al., 2019b). Activation of BNST neurons is associated with greater anxiety-like behavior during abstinence (Centanni et al., 2019b), and inhibiting BNST neurons is associated with less stress-induced reinstatement (e.g. Erb and Stewart, 1999). These rodent studies provide examples of how abstinence alters BNST function. However, the development of increasingly complex methodological tools has provided opportunities to study how the BNST interacts with other brain regions and how alterations in these networks influence BNST-driven abstinence behaviors. For example, a recent study showed that projections from the anterior insula to the BNST regulate anxiety-like behavior during abstinence (Centanni et al., 2019b). In addition, inhibition of projections from the central amygdala to the BNST is associated with less alcohol self-administration and withdrawal symptoms in alcohol-dependent rats (de Guglielmo et al., 2019). Such findings reinforce the interconnected nature of the brain and the importance of studying larger brain networks to understand complex behavior such as those seen in alcohol abstinence.
Consistent with animal models, studies of humans in the early phase of abstinence provide preliminary evidence for alterations in the extended amygdala (Braus et al., 2001; De Rosa et al., 2004; Sullivan et al., 2005; Wrase et al., 2008). Structural connectivity studies during abstinence have examined major white matter tracts and shown less structural integrity in several tracts including the corpus callosum, cingulate gyrus, and fornix (e.g. Monnig et al., 2015; Pfefferbaum et al., 2006; Pfefferbaum & Sullivan, 2002; Yeh et al., 2009; Zou et al., 2017). However, little is known about white matter connections between specific brain regions implicated in abstinence, for example, the BNST. One reason for this dearth of studies is that in vivo imaging of the BNST in humans has only recently been possible because of the BNST’s small size and limited resolution of neuroimaging methods. Our laboratory conducted the first systematic study of human BNST connectivity, in which the BNST’s major structural and functional connections were mapped (Avery et al 2014). The structural and functional connectivity patterns have been largely replicated by other groups (Gorka et al., 2017; Kamali et al., 2016, 2015; Krüger et al., 2015; McMenamin et al., 2014; Tillman et al., 2018; Torrisi et al., 2015). More recent studies have expanded upon our knowledge of BNST circuits, including a detailed mapping of BNST structural connectivity with insula subregions (Flook et al., 2020). These initial studies have also begun to describe sex differences in BNST structural connectivity with the majority of regions showing stronger structural connectivity with the BNST in women (e.g. Avery et al., 2014; Flook et al., 2020), expanding on known anatomical (Allen and Gorski, 1990) and functional (e.g. Fetterly et al., 2019) sex differences. While there have been studies of BNST function in different psychiatric disorders (for examples see Brinkmann et al., 2017; Clauss et al., 2019; Torrisi et al., 2019), to date there have yet to be any studies of BNST structural connectivity in AUD.
The aim of this study is to evaluate structural connectivity differences in the BNST network during abstinence from Alcohol Use Disorder (AUD). First, the hypothesis tested was that individuals with AUD in early abstinence have stronger structural connectivity between the BNST and the BNST network: a collection of brain regions with structural and functional connectivity with the BNST and known associations with anxiety and AUD. Although many studies show weaker white matter integrity during abstinence (e.g. Monnig et al., 2015; Pfefferbaum et al., 2006), the BNST network structural connectivity is hypothesized to be strengthened, reflecting the heightened BNST responses and greater anxiety symptoms during abstinence. Second, an exploratory analysis was conducted to determine whether BNST structural connectivity in individuals with AUD is associated with self-reported alcohol use severity or anxiety. The results of this preliminary investigation into BNST network alterations in abstinence are a critical step in elucidating the role of anxiety in alcohol use disorder.
Methods
Participants:
Study participants were 20 individuals during abstinence (30–180 days) from an AUD and 20 light social drinkers (control). Participants in the abstinence group were recruited using advertisements, an email distribution list, and referrals from a local rehabilitation center in Nashville, TN. Participants for the control group were recruited using an email distribution list. Participants were included if they were between 21–40 years of age, had no major medical illness or history of traumatic brain injury, could pass an MRI safety screen, and had no current or lifetime psychotic disorder. Additionally, abstinence participants were required to meet criteria for an AUD within the past year, as determined by the SCID IV, and be 30–180 days sober at the time of the initial study visit. A minimum of thirty days of sobriety was chosen to provide a clear separation between the stages of physical withdrawal and early abstinence. As some studies show brain differences are associated with abstinence length (for recent example see Blaine et al., 2020), an analysis confirmed there was no effect of days of abstinence or interactions (results in Supplementary Materials, all p > 0.3). Exclusion criteria for the healthy controls included: use of psychoactive medication (last 6 months), lifetime history of alcohol or substance abuse, and current psychiatric disorder. In addition, controls were light social drinkers and were excluded if they reported either binge drinking or no alcohol use for the year prior to enrolling in the study. Abstinence participants were excluded for current drug or alcohol use (except nicotine), current psychiatric disorder other than AUD, depression, or anxiety, or use of psychoactive medication use other than a stable dose of SSRI/SNRIs. Abstinence from drugs of abuse was self-reported and confirmed at the initial and MRI study visits using a breathalyzer, urine drug screen, and urine ETG test. Over the course of the recruitment, three participants withdrew between study visits due to self-reported relapse. The Vanderbilt University Institutional Review Board approved the study and written informed consent was obtained after providing subjects with a complete description of the study. One abstinence participant was unable to complete the scan; thus, the final sample sizes were 19 (abstinence) and 20 (controls).
Alcohol and Anxiety Measures:
Alcohol use was evaluated using the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1989; see Table 1), a 10-item self-report of alcohol consumption and alcohol-related behaviors and consequences. In addition, the lifetime drinking history (Skinner and Sheu, 1982) was used to calculate the quantity-frequency index (QFI) of each participant’s current (control group) or most recent (abstinence group) drinking phase. QFI was calculated by taking the frequency (number of drinking days per month) and multiplying by the typical number of drinks consumed on a drinking day.
Table 1:
Study Participant Characteristics
| Healthy Controls | Abstinence | |
|---|---|---|
| N | 20 | 19 |
| N (%) | ||
| Women | 11 (55%) | 9 (47%) |
| White | 13 (65%) | 15 (79%) |
| Mean (SD) | ||
| Age, years | 29.0 (4.4) | 31 (5.8) |
| AUDIT score | 2.8 (1.8) | 25.9 (9.2) |
| Anxiety composite | −0.61 (−0.37) | 0.63 (0.78) |
| Days abstinent at scan | N/A | 127.4 (47.8) |
| Quantity frequency index, per month | 3.76 (3.01) | 206.65 (297.23)# |
Data missing from 1 subject
Anxiety was measured using well-established questionnaires. Each questionnaire also has good internal reliability in this sample (Cronbach’s α provided in parentheses). The questionnaires included: the State-Trait Anxiety Inventory (Cronbach’s α = 0.94; Spielberger et al., 1983), Brief Fear of Negative Evaluation (Cronbach’s α = 0.95; Leary, 1983), Beck Anxiety Inventory (Cronbach’s α = 0.94, Beck et al., 1988), Intolerance of Uncertainty Scale (Cronbach’s α = 0.97; Carleton et al., 2007), Liebowitz Social Anxiety Scale (Cronbach’s α = 0.97; Liebowitz, 1987), and Penn State Worry Questionnaire (Cronbach’s α = 0.95; Meyer et al., 1990). The anxiety measures were moderately correlated for both groups (average r = 0.5); therefore, a composite anxiety score was created by averaging standardized scores (M = 0, SD = 1) for each questionnaire (see Table 1).
Data Acquisition:
Neuroimaging data were acquired on a 3T Philips Intera Achieva scanner (32-channel receive head coil, single-band imaging; Philips Healthcare, Andover, MA) located at the Vanderbilt University Institute for Imaging Sciences. Standard T1-weighted structural scans were collected for anatomical information (voxel size = 0.9mm3, echo time = 4.6ms, TR = 9.1ms). For structural connectivity, high-angular radial diffusion-weighted imaging (HARDI) scans (2.5-mm isotropic resolution, 60 directions, b value = 2000 s/mm, 5 b0 images) were collected with a SENSE factor of 2.2 to reduce echo time and echo-planar image distortions.
Data Processing:
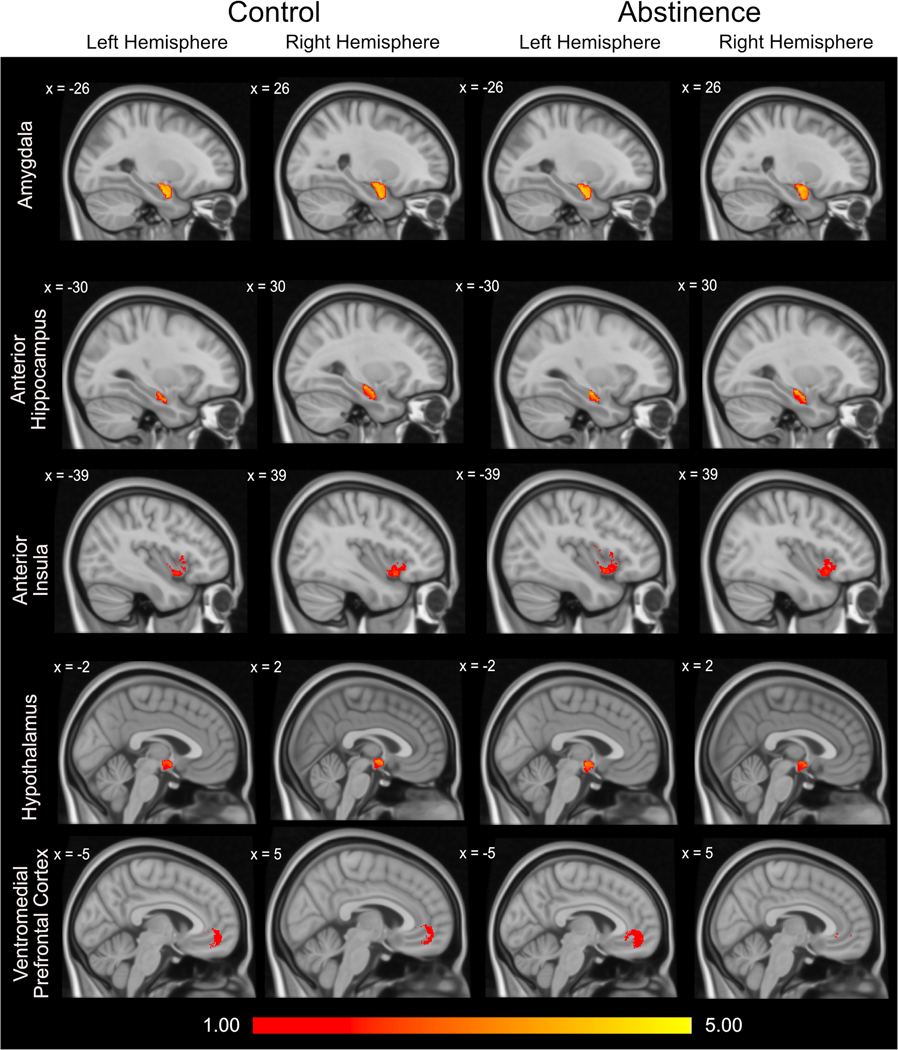
Structural connectivity analysis was performed using FMRIB Software Library (FSL, version 5.0; Oxford Centre for Functional MRI of the Brain (FMRIB), UK; http://www.fmrib.ox.ac.uk/fsl/). Diffusion data were eddy current corrected, skull-stripped, and visually inspected for significant artifacts (Andersson et al., 2003; Smith, 2002). Diffusion tensors were then fitted at each voxel and probabilistic fiber tractography was performed to evaluate structural connectivity for each hemisphere between the ipsilateral BNST and selected regions of interest (ROIs, see Supplementary Figure 1). In accordance with standard FSL analysis procedures, the subject weighted diffusion images were preprocessed in subject space. ROI masks were transformed from MNI space to native subject space using FLIRT, an FSL linear registration tool. ROIs were selected based on three criteria: 1) known structural connectivity to the BNST; 2) evidence for altered function in people with anxiety disorders; and 3) evidence for altered function in AUD. Five brain regions were identified as fitting these criteria: amygdala, anterior hippocampus, ventromedial prefrontal cortex (vmPFC), hypothalamus, and anterior insula. Our published mask was used for the BNST (Avery et al., 2014). The hypothalamus was created using the same methods as the BNST. The Harvard-Oxford atlas templates were used for the hippocampus, which was then manually segmented into the anterior hippocampus using the uncus as the anterior/posterior boundary, and the amygdala. For the anterior insula, a mask developed by Farb (2013) was used that has previously been used to demonstrate anterior insula structural connectivity with the BNST (Flook et al., 2020). Finally, the vmPFC mask was defined based on a 10mm sphere surrounding the peak point of vmPFC-BNST connectivity from our previous study [x = 0, y = 43, z = −9] (Avery et al., 2014). After transformation to native space, ROIs were visually inspected for each participant to ensure that the ROIs were in the correct anatomical location. Fiber tracking was initiated from every voxel within the BNST (samples = 5000) and the number of streamlines connecting to each ROI was recorded. The average number of streamlines per voxel in each ROI was log transformed for analysis; this method inherently adjusts for positive skew and variance in BNST and ROI volumes between subjects. The transformed data were normally distributed as evidenced by a non-significant Kolmogorov-Smirnoff test. Figure 1 shows the voxel-wise connectivity values (log-transformed) within each ROI.
Figure 1.
Averaged group voxel-wise maps for each ROI. Values represent the log transformed number of streamlines that extend from the BNST to each voxel in the ROI. Values were thresholded such that only values of one or greater are included.
Statistical Analysis:
The primary analysis was a linear mixed model (p < 0.05) with region (BNST network ROIs), group (abstinence/control), and sex (men/women) as fixed factors. Subject was included as a random effect variable. Hemisphere was included as a covariate of no interest to control for hemispheric differences. The omnibus test, with region as a factor, provided control for Type I error. Significant effects and interactions were investigated in post-hoc analyses. Effect sizes were calculated for this analysis conducted using eta-squared for the model and Cohen’s d for the post-hoc analysis.
As little is known about how these regions function in the network, next a planned, a priori investigation of individual regions was conducted to better understand the role of each region within the BNST network. This was accomplished using a linear mixed model for each region with group and sex as fixed factors and subject as a random effect variable. Hemisphere was included as a covariate of no interest to control for hemispheric differences. Effect sizes were calculated for this analysis using eta-squared.
Given the heterogeneity of alcohol severity and anxiety symptoms in AUD, exploratory analysis investigating the effect of these variables on BNST network structural connectivity was conducted in the abstinence group using a linear mixed model. The effects of alcohol use severity and anxiety were separately investigated as a fixed factor in a linear mixed model (p < 0.05) along with region (amygdala/anterior hippocampus/anterior insula/hypothalamus/vmPFC) and sex (men/women). Subject was included as a random effects variable. To account for hemispheric differences, hemisphere was included as a covariate of no interest. Effect sizes were calculated for this analysis conducted using eta-squared and Cohen’s d.
All analyses were conducted in R (R Core Team, 2017) using the lme4 (Bates et al., 2015) package for linear mixed models and the emmeans (Lenth, 2019) package for post-hoc analysis. Post-hoc p values were adjusted in emmeans using the Tukey method for family-wise comparisons. For all models, sex was included as previous literature has demonstrated sex differences during abstinence, in which women are more likely to experience anxiety (e.g. Becker & Koob, 2016; Erol & Karpyak, 2015), and BNST structural connectivity, in which women have stronger BNST structural connectivity with many but not all other regions (Avery et al., 2014; Flook et al., 2020). Hemisphere was included as a control variable as recent findings indicate BNST structural connectivity differences by hemisphere (Flook et al., 2020).
Results
Participant Demographics:
Participant characteristics are shown in Table 1 (and Supplementary Table 1). The groups did not significantly differ on age (t = 1.22, p = 0.23), sex (χ2(1,38) = 0.23, p = 0.63), or race/ethnicity (white vs nonwhite; χ2(1,38) = 0.936, p = 0.33). For all participants, gender identity reported was consistent with sex assigned at birth. The abstinence group had higher AUDIT (t = 11.01, p < 0.001, d = 3.48) and anxiety (t = 6.40, p < 0.001, d = 2.03) scores compared to the control group.
Group Differences in BNST Network Structural Connectivity:
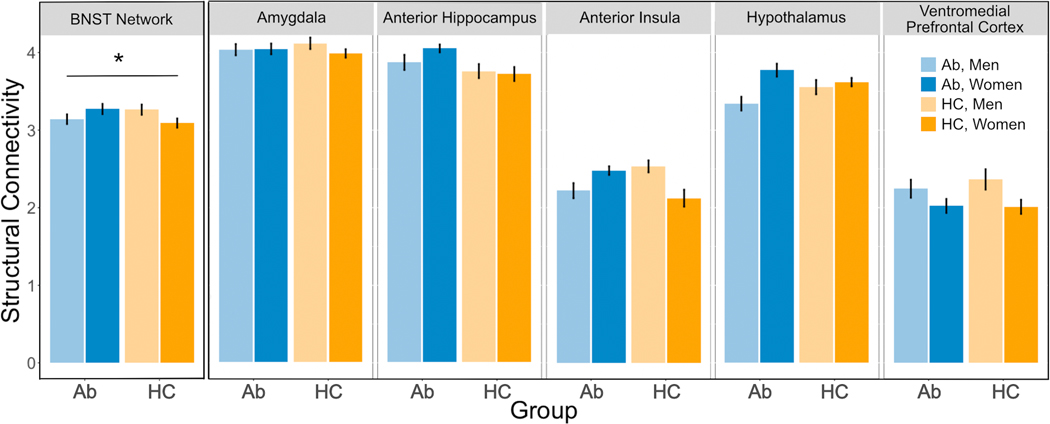
To determine whether BNST network structural connectivity was altered during abstinence, a linear mixed model was performed with region, group, and sex as factors. BNST structural connectivity differed by group and sex (Figure 2, group x sex interaction: F(1,35) = 5.68, p = 0.02, η2 = 0.14). Post-hoc analysis revealed a significant effect of group for women (t = 2.03, p = 0.050, d = 0.91), with stronger structural connectivity in the abstinence group compared to controls, but no group difference in men (t = −1.35, p = 0.19, d = 0.60).
Figure 2.
BNST network structural connectivity by sex and group both as a combined BNST network and by region. Data presented as mean connectivity with standard error bars. Ab: Abstinence; HC: Control * p< .05
There was also a significant main effect of region (F(4, 334) = 274.6, p < 0.001, η2 = 0.19) that was qualified by a region x sex interaction (F(4,334) = 3.45, p = 0.009, η2 = 0.010). Post-hoc analyses demonstrate stronger BNST-hypothalamus structural connectivity in women compared to men (t = −2.16, p = 0.03, d = 0.67) and stronger vmPFC-BNST structural connectivity in men compared to women (t = 2.51, p = 0.01, d = 0.78). No other regions had a significant sex difference (amygdala: t = 0.53, p = 0.60, d = 0.17; anterior hippocampus: t = −0.62, p = 0.53, d = 0.20; anterior insula: t = 0.66, p = 0.51, d = 0.22).
There was no main effect of group (F(1, 35) = 0.21, p = 0.65, η2 = 0.006) or sex (F(1, 35) = 0.11, p = 0.75, η2 = 0.003). There was also no region x group interaction (F(4, 334) = 1.08, p = 0.36, η2 = 0.003) or region x group x sex interaction (F(4, 334) = 1.09, p = 0.36, η2 = 0.003).
Region-Specific Group Differences in BNST Network Structural Connectivity:
As BNST structural connectivity with the regions of the BNST network is uncharacterized in abstinence, in addition to analysis of the network as a whole, a preliminary, a priori investigation of each region was performed. Linear mixed models were performed with group and sex for each ROI. Structural connectivity values by region, group, and sex are provided in Figure 2. These analyses are for illustrative purposes; to this end, effect sizes are provided (Table 2). Significance values are not provided to avoid “double dipping”. The group effect size is largest for the anterior hippocampus and smallest for the amygdala. The effect size for sex is largest for the hypothalamus and vmPFC, and the effect size for the group x sex interaction is largest for the anterior insula.
Table 2:
BNST Network Connectivity by Region
| Group Effect | Sex Effect | Group x Sex | ||||
|---|---|---|---|---|---|---|
| Region | F | η2 | F | η2 | F | η2 |
| Amygdala | 0.01 | 0.000 | 0.39 | 0.011 | 0.53 | 0.015 |
| Anterior Hippocampus | 3.30 | 0.086 | 0.34 | 0.010 | 0.80 | 0.022 |
| Anterior Insula | 0.03 | 0.001 | 0.35 | 0.010 | 6.88 | 0.164 |
| Hypothalamus | 0.07 | 0.002 | 4.74 | 0.119 | 2.59 | 0.069 |
| vmPFC | 0.13 | 0.004 | 3.50 | 0.091 | 0.18 | 0.005 |
Individual Differences in BNST Network Structural Connectivity:
To evaluate if alcohol use severity or anxiety symptoms were associated with BNST network structural connectivity, exploratory analyses were conducted in the abstinence group.
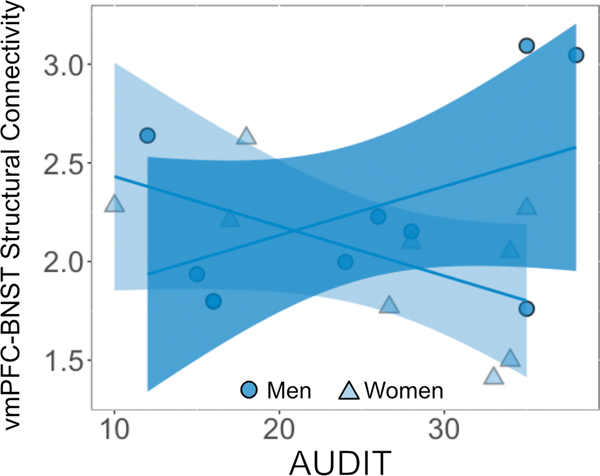
For the alcohol use severity analysis, there was a main effect of region (F(4, 154) = 17.67, p < 0.001, η2 = 0.08) that was qualified by a three-way interaction between region, sex, and AUDIT (F(4,154) = 3.33, p = 0.01, η2 = 0.02). Post hoc analysis demonstrated a sex difference in the relationship between alcohol use severity and BNST structural connectivity with the vmPFC (Figure 3, t = 2.8, p = 0.005, d = 0.52), where women had a negative trend (r = −0.35, p = 0.15) and men had a positive trend (r = 0.35, p = 0.13) though neither were significant. The other regions did not show a significant sex difference in alcohol use severity and BNST structural connectivity (amygdala: t = 0.69, p = 0.49; anterior hippocampus: t = −1.00, p = 0.32; anterior insula: t = −1.63, p = 0.11; hypothalamus: t = 0.19, p = 0.85). There was no main effect of sex (F(1,15) = 0.91, p = 0.35, η2 = 0.06) or AUDIT (F(1,15) = 2.11, p = 0.17, η2 = 0.12). There were no region x sex (F(4,154) = 1.78, p = 0.13, η2 = 0.011), region x AUDIT (F(4,154) = 1.35, p = 0.25, η2 = 0.008), or sex x AUDIT (F(1,15) = 0.17, p = 0.68, η2 = 0.011) interactions.
Figure 3.
The relationship between alcohol use severity and structural vmPFC-BNST connectivity in abstinence. The relationship between vmPFC-BNST connectivity and AUDIT score was mediated by sex (men: r = 0.35; women: r = −0.35). As there was no effect of hemisphere, data represent the averages between hemisphere from each subject for clarity. The line and shaded region represent the linear best fit with 95% confidence intervals for men and women.
The analysis of anxiety symptoms demonstrated a main effect of region (F(4,154) = 50.28, p < 0.001, η2 = 0.14). Post hoc pair-wise analyses comparing regions showed that the amygdala and anterior hippocampus had similar structural connectivity (t = 0.56, p = 0.98, d = 0.09) that was stronger than all other regions (all ps < 0.003, ds > 0.59). The hypothalamus had stronger structural connectivity than the anterior insula and vmPFC (both p < 0.001, d > 1.50), and the anterior insula and vmPFC had similar structural connectivity (t = 1.78, p = 0.32, d = 0.29). There was no main effect of sex (F(1,15) = 3.49, p = 0.08, η2 = 0.19) nor significant effect of anxiety (F(1,15) = 0.02, p = 0.89, η2 = 0.001). There were no region x sex (F(4,154) = 1.33, p = 0.26, η2 = 0.008), region x anxiety (F(4,154) = 0.61, p = 0.66, η2 = 0.003), sex x anxiety (F(1,15) = 1.93, p = 0.11, η2 = 0.11), or region x sex x anxiety (F(4,154) = 0.21, p = 0.93, η2 = 0.001) interactions.
Discussion
The present study investigated BNST network structural connectivity in humans in the early stages of abstinence from AUD. The study findings provide exciting preliminary evidence for different BNST network structural connectivity in abstinence. Importantly, prominent sex differences were evident. These findings are consistent with a growing literature highlighting sex differences in AUD, although they were not explicitly predicted in this study. Finally, BNST network structural connectivity was associated with individual differences in alcohol use severity but not anxiety.
This study provides the first evidence of BNST network structural connectivity differences in abstinence; specifically, women in abstinence had stronger BNST network structural connectivity compared to controls. Although sex differences were not predicted, these findings are not surprising. First, the BNST is one of the most sexually dimorphic brain regions in both size and function (e.g. Allen and Gorski, 1990; Fetterly et al., 2019). Second, our laboratory has previously demonstrated that the majority of regions have stronger BNST structural connectivity in women compared to men (Avery et al., 2014). Third, sex differences have been reported in white matter studies of abstinence, with most studies reporting less white matter integrity in men but not women (e.g. Oscar Berman & Song, 2011; Rivas-Grajales et al., 2018; Seitz et al., 2017). The results of the present study expand on previous findings of sex differences and provide novel evidence for BNST structural connectivity differences between women and men during abstinence. These differences are intriguing given known clinical and behavioral sex differences in abstinence. For example, during abstinence, women experience greater levels of anxiety (Conway et al., 2006) and are more likely to relapse as a result of these symptoms (Becker and Koob, 2016). Thus, it is critical to understand how sex-related differences in the BNST network abstinence could contribute to behavioral changes in response to anxiety.
The vmPFC showed overall stronger BNST structural connectivity in men compared to women, and vmPFC-BNST structural connectivity was positively correlated with alcohol use severity in abstinent men but negatively correlated in abstinent women. The vmPFC has a known regulatory role in anxiety and alcohol use disorder (Blaine et al., 2017; Hiser and Koenigs, 2018; Myers-Schulz and Koenigs, 2012; Seo et al., 2013). Specific to the vmPFC-BNST connection, non-human primate studies show that vmPFC lesions decrease BNST activity and anxiety-related behavior (Fox et al., 2010), and altered BNST and vmPFC metabolism is associated with anxiety-related behaviors (Fox et al., 2015). Lesions of the vmPFC are associated with lower BNST activity in humans (Motzkin et al., 2015). Given the regulatory role of the vmPFC, weaker structural connectivity in women could underlie clinical differences in risk of anxiety disorders (McLean et al., 2011) or anxiety symptoms during abstinence (Becker and Koob, 2016; Hogle and Curtin, 2006). Furthermore, in the abstinence group, women with greater alcohol use severity had weaker vmPFC-BNST structural connectivity. This relationship could suggest heavier drinking is associated with weaker vmPFC-BNST structural connectivity in women or that more vmPFC-BNST structural connectivity protects against heavier drinking in women.
Sex differences were also seen in BNST-hypothalamus structural connectivity. Women had stronger BNST structural connectivity with the hypothalamus compared to men. While BNST-hypothalamus structural connectivity has not been shown previously in humans, rodent studies demonstrate the BNST has robust projects to the hypothalamus (for examples see Dong et al., 2001; Yamamoto et al., 2018). Interestingly, Polston and colleagues showed sex differences in this projection, with male rodents displaying a greater amount of inhibitory BNST neurotransmission into the hypothalamus (Polston et al., 2004). BNST projections trigger hypothalamic CRF release and activate the downstream stress response (Crestani et al., 2013; Dabrowska et al., 2016, 2011). In fact, a recent study demonstrated that abstinence is associated with greater excitability in neurons projecting from the BNST to the hypothalamus (Pati et al., 2020). Stronger BNST-hypothalamus structural connectivity in women could help explain the biological basis for why women are more likely to have an anxiety diagnosis (McLean et al., 2011) and why women with AUD are the most likely to struggle with anxiety during abstinence (e.g. Becker & Koob, 2016; Hogle & Curtin, 2006).
Anxiety symptoms in the abstinence group did not correlate with BNST network structural connectivity. Rates of anxiety are elevated during abstinence from AUD, particularly in women (e.g. Becker & Koob, 2016; Hogle & Curtin, 2006). The preliminary results of this study suggest that individual differences in BNST structural connectivity are not associated with individual differences in anxiety symptoms. It is possible that the sex differences in both anxiety symptoms and structural connectivity in this sample in the abstinence group made it more difficult to observe individual differences.
The primary limitation of this study is the relatively small within-sex sample size. False negative results can often occur in small samples when the effects are small to medium in size. The potential consequence of false negative findings is that important findings may be missed. To address this, the effect sizes for each statistical test are included, showing that many of the non-significant findings of this study would have required much larger sample sizes and therefore are unlikely to be false negatives. Small sample sizes can also lead to increased false positive results. To reduce the likelihood of false positives due to Type I error, the analysis was restricted to regions of interest that are known to have structural connections to the BNST in other species (Poldrack et al., 2017) and used an omnibus statistical test to reduce multiple comparisons. Furthermore, point estimates, such as correlations, from small samples sizes often have large confidence intervals (Schönbrodt and Perugini, 2013). Therefore, it will be important to replicate the current study findings using a larger sample. The findings of regional differences in connectivity and sex differences are consistent with prior BNST and clinical studies (Allen and Gorski, 1990; Avery et al., 2014; Becker and Koob, 2016; Flook et al., 2020; Hogle and Curtin, 2006), providing convergent validation. However, it will be important for these study findings to be replicated in a larger study with the statistical power to support a priori test of sex differences.
Conclusions and future directions
These findings provide preliminary evidence for alterations in BNST network structural connectivity during abstinence. In addition, intriguing sex differences were observed; women in the abstinence group had stronger overall BNST network structural connectivity than the control group, a difference that was not seen in men. These findings represent a critical first step towards understanding sex-specific white matter differences in abstinence and relationships with alcohol severity and anxiety.
The exciting results of this study promote several important future directions. First, abstinence is a dynamic process. Future studies are needed to characterize this process and identify critical transition points between phases, for example between early and protracted abstinence. Second, future studies should investigate BNST functional connectivity, both intrinsic (“at rest”) connectivity and task-based connectivity, for example in response to stress or threat to determine the functional consequences of the structural alterations. Functional studies are a critical complement to structural connectivity studies, as structural connectivity studies are unable to detect if changes are associated with excitatory or inhibitory pathways and might have unexpected functional outcomes. Furthermore, task-based functional connectivity studies can illuminate the importance of subtle changes in connectivity that can only be detected when activated by a certain stimulus or task. Third, future studies should expand on the association between individual characteristics and connectivity. Structural connectivity studies often demonstrate significant individual variability that could reflect meaningful individual differences (for example see Kumar et al 2017). Thus, a future, larger study of BNST structural connectivity in abstinence could be used to better understand the role of various factors in anxiety during abstinence, such as family history, genetics, age of first alcohol use, or history of trauma. One such important factor is the contribution of nicotine use or dependence, which is thought to decrease alcohol withdrawal symptoms and could influence abstinence (for review see Cosgrove et al., 2011). In addition, many individuals are in recovery from polysubstance use, which could impact abstinence and should be considered in future studies. Furthermore, understanding which of these individual factors impact structural connectivity could identify subgroups of patients for which individualized treatments could be developed. A better understanding of this heterogeneity could lead to individualized treatments reflecting the unique circumstances of patients with AUD and help prevent relapse.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Hannah Gardner for her contributions to study recruitment and data collection, and Cumberland Heights for their support in recruitment. The authors would also like to acknowledge the funding sources that supported this work: the National Institute of Alcohol Abuse and Alcoholism (R21AA025385 to JUB and DGW; F30AA027418 to EF), the National Institute of Mental Health (T32MH018921 to EF and BF), the National Institute of General Medical Sciences (T32GM007347 to EF), and the Vanderbilt Institute for Clinical and Translational Research (VR54926 to EF).
References
- Alheid GF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27:1–39. [DOI] [PubMed] [Google Scholar]
- Allen LS, Gorski RA (1990) Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 302:697–706. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20:870–888. [DOI] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU (2016) The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41:126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU (2014) BNST neurocircuitry in humans. Neuroimage 91:311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, de la Fuente JR, Saunders JB, Grant M (1989) AUDIT: The Alcohol Use Disorders Identification Test: guidelines for use in primary care., First. ed. Geneva, World Health Organization. [Google Scholar]
- Bates D, Machler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using {lme4}. J Stat Softw 67:1–48. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex differences in animal models: focus on addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Seo D, Sinha R (2017) Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol 22:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, Sinha R (2020) Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. Am J Psychiatry 177:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A (2001) Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm 108:887–894. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Tupak S V, Becker MPI, Herrmann MJ, Straube T (2017) Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychol Med 1–14. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Norton MAPJ, Asmundson GJG (2007) Fearing the unknown: a short version of the Intolerance of uncertainty scale. J Anxiety Disord 21:105–117. [DOI] [PubMed] [Google Scholar]
- Centanni SW, Bedse G, Patel S, Winder DG (2019a) Driving the downward spiral: alcohol-induced dysregulation of extended amygdala circuits and negative affect. Alcohol Clin Exp Res 43:2000–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni SW, Morris BD, Luchsinger JR, Bedse G, Fetterly TL, Patel S, Winder DG (2019b) Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology 44:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng S, Fu J, Brown RM, McDougall SJ, Lawrence AJ (2018) The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog Neuro-Psychopharmacology Biol Psychiatry 87:108–125. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM, Blackford JU (2019) Social anxiety is associated with BNST response to unpredictability. Depress Anxiety 36:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF (2006) Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders. J Clin Psychiatry 67:247–258. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, Mason GF, Bois F, O’Malley SS, Krystal JH (2011) Neuroimaging insights into the role of cortical GABA systems and the influence of nicotine on the recovery from alcohol dependence. Neuropharmacology 60:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves F, Gomes F, Resstel L, Corrêa FMA, Herman J (2013) Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol 11:141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo J-D, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG (2011) Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 36:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG (2016) Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol 28: 10.1111/jne.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford EF, Simpson S, Schweitzer P, Koob GF, Messing RO, George O (2019) Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun 10:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan E V (2004) The human basal forebrain integrates the old and the new. Neuron 41:825–837. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW (2001) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev 38:192–246. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J (1999) A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19:RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Karpyak VM (2015) Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend 156:1–13. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK (2013) Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex 23:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterly TL, Basu A, Nabit BP, Awad E, Williford KM, Centanni SW, Matthews RT, Silberman Y, Winder DG (2019) α2A-adrenergic receptor activation decreases parabrachial nucleus excitatory drive onto BNST CRF neurons and reduces their activity in vivo. J Neurosci 39:472 LP – 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook EA, Feola B, Avery SN, Winder DG, Woodward ND, Heckers S, Blackford JU (2020) BNST-insula structural connectivity in humans. Neuroimage 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, Converse AK, Alexander A, Davidson RJ, Blangero J, Rogers J, Kalin NH (2015) Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci 112:9118 LP – 9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH (2010) Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci 30:7023–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Torrisi S, Shackman AJ, Grillon C, Ernst M (2017) Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroimage 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC (2010) Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, Koenigs M (2018) The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry 83:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ (2006) Sex differences in negative affective response during nicotine withdrawal. Psychophysiology 43:344–356. [DOI] [PubMed] [Google Scholar]
- Kamali A, Sair HI, Blitz AM, Riascos RF, Mirbagheri S, Keser Z, Hasan KM (2016) Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct Funct 221:3561–3569. [DOI] [PubMed] [Google Scholar]
- Kamali A, Yousem DM, Lin DD, Sair HI, Jasti SP, Keser Z, Riascos RF, Hasan KM (2015) Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neurosci Lett 608:45–50. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger O, Shiozawa T, Kreifelts B, Scheffler K, Ethofer T (2015) Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex 66:60–68. [DOI] [PubMed] [Google Scholar]
- Kumar K, Desrosiers C, Siddiqi K, Colliot O, Toews M. (2017) Fiberprint: A subject fingerprint based on sparse code pooling for white matter fiber analysis. Neuroimage, 157, 242–259. [DOI] [PubMed] [Google Scholar]
- Leary MR (1983) A brief version of the Fear of Negative Evaluation Scale. Personal Soc Psychol Bull 9:371–376. [Google Scholar]
- Lenth R (2019) emmeans: Estimated Marginal Means, aka Least-Squares Means. [Google Scholar]
- Liebowitz MR (1987) Social Phobia. Mod Probl Pharmacopsychiatry 22:141–173. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG (2011) Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, Pessoa L (2014) Network organization unfolds over time during periods of anxious anticipation. J Neurosci 34:11261–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD (1990) Development and validation of the penn state worry questionnaire. Behav Res Ther 28:487–495. [DOI] [PubMed] [Google Scholar]
- Monnig MA, Yeo RA, Tonigan JS, McCrady BS, Thoma RJ, Sabbineni A, Hutchison KE (2015) Associations of white matter microstructure with clinical and demographic characteristics in heavy drinkers. PLoS One 10:e0142042–e0142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Oler JA, Kalin NH, Baskaya MK, Koenigs M (2015) Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex 64:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M (2012) Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry 17:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar Berman M, Song J (2011) Brain volumetric measures in alcoholics: a comparison of two segmentation methods. Neuropsychiatr Dis Treat 7:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati D, Marcinkiewcz CA, DiBerto JF, Cogan ES, McElligott ZA, Kash TL (2020) Chronic intermittent ethanol exposure dysregulates a GABAergic microcircuit in the bed nucleus of the stria terminalis. Neuropharmacology 168:107759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2006) Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging 27:994–1009. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV (2002) Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 15:708–718. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Skelly MJ (2018) CRF modulation of central monoaminergic function: Implications for sex differences in alcohol drinking and anxiety. Alcohol 72:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline J-B, Vul E, Yarkoni T (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston EK, Gu G, Simerly RB (2004) Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic gabaergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience 123:793–803. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017) R: A language and environment for statistical computing. [Google Scholar]
- Ramchandani VA, Stangl BL, Blaine SK, Plawecki MH, Schwandt ML, Kwako LE, Sinha R, Cyders MA, O’Connor S, Zakhari S (2018) Stress vulnerability and alcohol use and consequences: from human laboratory studies to clinical outcomes. Alcohol 72:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Grajales AM, Sawyer KS, Karmacharya S, Papadimitriou G, Camprodon JA, Harris GJ, Kubicki M, Oscar-Berman M, Makris N (2018) Sexually dimorphic structural abnormalities in major connections of the medial forebrain bundle in alcoholism. NeuroImage Clin 19:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbrodt FD, Perugini M (2013) At what sample size do correlations stabilize? J Res Pers 47:609–612. [Google Scholar]
- Seitz J, Sawyer KS, Papadimitriou G, Oscar-Berman M, Ng I, Kubicki A, Mouradian P, Ruiz SM, Kubicki M, Harris GJ, Makris N (2017) Alcoholism and sexual dimorphism in the middle longitudinal fascicle: a pilot study. Brain Imaging Behav 11:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong K-I, Constable RT, Sinha R (2013) Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu W-J (1982) Reliability of alcohol use indices. J Stud Alcohol 43:1157–70. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA, Mind Garden. [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A (2005) Striatal and forebrain nuclei volumes: Contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry 57:768–776. [DOI] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, Shackman AJ (2018) Intrinsic functional connectivity of the central extended amygdala. Hum Brain Mapp 39:1291–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Alvarez GM, Gorka AX, Fuchs B, Geraci M, Grillon C, Ernst M (2019) Resting-state connectivity of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in clinical anxiety. J Psychiatry Neurosci 44:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, Grillon C, Ernst M (2015) Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Hum Brain Mapp 36:4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A (2008) Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry 165:1179–1184. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Ahmed N, Ito T, Gungor NZ, Pare D (2018) Optogenetic study of anterior BNST and basomedial amygdala projections to the ventromedial hypothalamus. eNeuro 5:ENEURO.0204–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P-H, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ (2009) Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res 173:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Murray DE, Durazzo TC, Schmidt TP, Murray TA, Meyerhoff DJ (2017) Effects of abstinence and chronic cigarette smoking on white matter microstructure in alcohol dependence: Diffusion tensor imaging at 4T. Drug Alcohol Depend 175:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.