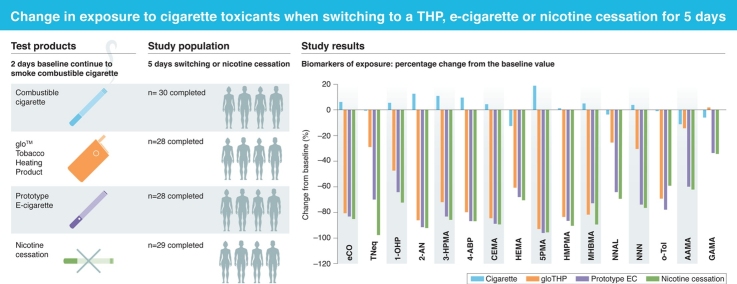
Graphical abstract
Keywords: Smoking, Biomarker of exposure, Tobacco heating product, e-Cigarette, Harm reduction
Highlights
-
•
Cigarettes compared to tobacco heating product and e-cigarette in a clinical study.
-
•
Biomarkers of Exposure were analysed over five days of switching.
-
•
Electronic cigarettes and tobacco heating products substantially reduce exposure.
-
•
The evidence enables placement of these products on an exposure continuum.
Abstract
Background
Cigarette smoking is associated with a number of diseases, such as cancer and cardiovascular diseases. Recently, there has been an increase in the use of electronic cigarettes (ECs) and tobacco-heating products (THPs) as an alternative to cigarettes, which may reduce the health burden associated with smoking. However, an exposure continuum when smokers switch to ECs or THPs compared to complete smoking cessation is not well established.
Methods
148 healthy smokers were randomized to either continue smoking cigarettes, switch to using the glo THP or a prototype EC, or completely quit any nicotine or tobacco product use for 5 days, after a 2-day baseline period. During this study breath and 24-h urine samples were collected for Biomarker of Exposure (BoE) analysis.
Results
After a 5-day switching period BoE levels showed a substantial significant decrease in levels from baseline in the groups using the glo THP, the prototype EC, and having quit all nicotine and tobacco use. On an exposure continuum, smokers who completely quit nicotine had the lowest levels of assessed BoEs, followed by those who switched to the EC and then those who switched to glo THP use. Participants who continued to smoke had the highest levels of BoEs.
Conclusions
THP or EC use over a 5-day period resulted in significant reductions in exposure to smoke toxicants, in some cases to levels similar to those for nicotine cessation. These results show that on an exposure continuum, nicotine cessation gives the greatest reduction in exposure to tobacco smoke toxicants, closely followed by the EC and the glo THP. These significant reductions in exposure to toxicants suggest that the glo THP and EC have the potential to be Reduced Risk Products.
Study Registration
ISRCTN80651909.
1. Implications
This clinical study is one of the first to investigate the exposure continuum with the glo tobacco heating product (THP), an e-cigarette (EC), continued smoking and nicotine cessation. Conventional cigarette smokers switching from combustible cigarettes to either a THP or an EC in this study demonstrated significant reductions in exposure to smoke toxicants. For a number of these toxicants, the reductions were similar to those seen in participants who quit smoking and ceased nicotine use. This suggests that both the EC and the THP used in this study are Potentially Reduced Risk Products (PRRPs) when used to replace conventional cigarette smoking completely.
2. Introduction
It is well established that cigarette smoking is a leading cause of diseases, such as cardiovascular disease, lung disease and cancer. Cigarette smoking is harmful largely because of the complex mixture of more than 6500 chemical constituents that are released during the combustion process [1]. These chemicals include a number of International Agency for Research on Cancer (IARC) Group 1 carcinogens, as well as other potentially harmful toxicants. Although there has been a global decline in smoking prevalence in the past five decades [2], the health burden associated with cigarette smoking is still high. Potentially Reduced Risk Products (PRRPs), such as tobacco-heating products (THPs) and electronic cigarettes (ECs), and reduced risk products, such as snus, hold great potential for reducing the harms associated with cigarette smoking by delivering nicotine in an aerosol in which harmful cigarette smoke toxicants are either absent or greatly reduced [3,4]. Although smoking cessation is still the gold standard to reduce the health burden associated with smoking as the epidemiological outcomes of cessation are well established [5], it is important to build a comprehensive set of scientific evidence on non-combustible novel tobacco and nicotine products in order to understand where they sit on an exposure continuum in relation to cessation.
Health-related claims on these PRRPs, such as ‘reduced exposure’ and ‘reduced risk’, should be substantiated using a weight-of-evidence approach based on a comprehensive scientific assessment. The US Food and Drug Administration (FDA) has provided draft guidance outlining a framework to assess Modified Risk Tobacco Products (MRTP). Based on this guidance, a scientific assessment framework [6] has been developed and used to assess the potential of ECs and THPs to reduce risk relative to smoking. This framework consists of pre-clinical, clinical and population studies.
For ECs, a number of studies have shown that they are considerably less toxic than conventional cigarettes due to reductions in exposure to chemical toxicants [[7], [8], [9]]. Both Public Health England and the UK Royal College of Physicians [10,11] have extensively reviewed data on ECs. On a pre-clinical assessment basis, the potential chemical, toxicological, mutagenic, cytotoxic and tumour-promoting properties of ECs have been assessed. Compared to University of Kentucky reference cigarettes (3R4F), ECs have been shown to have reduced chemical toxicants [8], and in vitro studies showed reduced levels of DNA damage [12,13], mutagenicity [[13], [14], [15]], cytotoxicity [7], carcinogenicity [16] and oxidative stress [17].
In contrast to ECs, there has been less public health and academic research on the properties of THPs; however, in-house assessments at British American Tobacco (BAT) of the chemicals found in the aerosol from a novel THP (glo, THP1.0 T) that electronically heats tobacco to a temperature of 240 °C revealed significant reductions in levels of many chemical toxicants when compared to those found in conventional cigarette smoke [18,19], and more recent studies on a novel heated tobacco product support this greatly reduced toxicant profile [20]. In addition, glo (THP1.0 T) showed an absence or substantially reduced responses in mutagenic, cytotoxic, oxidative stress and tumour-promoting endpoints compared to University of Kentucky 3R4F reference cigarettes when assessed with both in vitro toxicological and contemporary screening assays [17,[21], [22], [23]]. Furthermore, risk assessments carried out on a novel heated tobacco product demonstrated a >90 % non-cancer and cancer risk reduction compared to cigarette smoking [20].
Clinical studies measuring Biomarkers of Exposure (BoE) are an important way to determine if the observations in vitro translate to a reduction in exposure to cigarette smoke toxicants when smokers switch to using PRRPs. McNeil and Munafo (2013) [24] suggested the concept of a risk continuum for tobacco and nicotine products, with cigarettes placed at the high-risk end of the continuum and nicotine replacement therapy at the low-risk end.
This paper reports on the results of a clinical study which incorporates participants switching to a THP, a prototype EC, continuing to smoke, or abstaining from smoking or any other nicotine product use. This is the first study to investigate an EC and THP within the same study and, as such, it is the first study to assess the exposure continuum between these PRRPs relative to nicotine cessation. To investigate the exposure continuum, 16 BoEs in urine and exhaled breath were selected based on the FDA list of Harmful or Potentially Harmful Constituents in tobacco products and tobacco smoke [25]. This assessment provides insights into the potential reduced exposure profiles of the glo THP and an EC relative to nicotine cessation. A fifth arm in this study involved participants switching to a non-BAT commercial product as a benchmark, this data is not reported in this paper as we wished to focus on the exposure continuum.
3. Methods
This study followed the same study design used in a previous clinical study conducted in Japan, which investigated the glo THP (study registrations ISCTRN14301360 and UMIN000024988). A full description of the Japan study protocol has been published [26]. There were a few differences with respect to the previously mentioned protocol, the main differences being that this study was conducted in the UK, at a single site and included an arm that involved switching to a prototype EC.
3.1. Primary objectives
The primary objective of this study was to quantitatively assess within-arm changes in 16 BoE, two biomarkers of biological effect (BoBE) and a urinary biomarker to investigate potential changes in nicotine metabolism (assessed by the nicotine molar metabolic ratio) following a forced switch from a conventional cigarette to a PRRP or nicotine cessation.
3.2. Secondary objectives
Secondary objectives were to assess differences between arms in BoE and BoBE following a forced switch from a conventional cigarette to a PRRP or nicotine cessation as well as monitor the safety profile of participants using the glo THP, the EC or conventional cigarettes as well as in participants undergoing nicotine cessation.
3.3. Study design
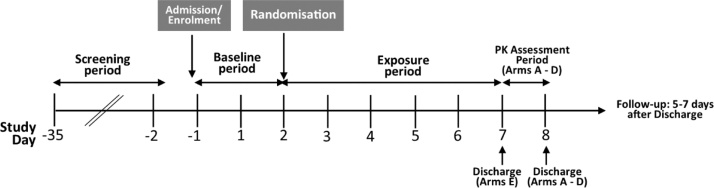
This study was a randomized, controlled, five-arm, parallel group, open-label study conducted at a single site in Belfast, UK. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice (International Conference on Harmonisation E6 Consolidated Guidance) and local laws. The study was registered on the ISRCTN registry (ISRCTN80651909). Favourable ethical opinion for the study was given by the Office for Research Ethics Committees Northern Ireland (ORECNI, reference number 17/NI/0065). This was a confined, forced switching study design with a two-day baseline period and a five-day period where participants either continued to smoke, switch to one of the study PRRPs or abstain for all nicotine and tobacco use [26]. A schematic illustration of the design of this study can be found in Fig. 1.
Fig. 1.
Study design schematic. Participants completed a two-day baseline period during which they continued to smoke combustible cigarettes before being randomised to one of the five study arms in the exposure period.
3.4. Participants
All participants were healthy adult male and female non-mentholated cigarette smokers and eligibility for enrolment in the study was assessed during a screening visit and based on a set of inclusion/exclusion criteria [26]. The main inclusion criteria were that potential participants were current smokers of commercially manufactured cigarettes of International Organisation for Standardization (ISO) tar levels of 6–10 mg/cig tar, inclusive, consuming at least 10 and up to 30 cigarettes per day, inclusive, had smoked for at least 3 years and were aged 21–55 years. Smoking status was verified using exhaled breath carbon monoxide [eCO; >10 ppm] and a urinary cotinine test [>200 ng/mL].
3.5. Investigational products
All study cigarettes, ECs/e-liquid cartridges and THP devices/tobacco consumables were provided by the Sponsor free of charge. During the baseline period, all participants were provided with a 7 mg/cig ISO tar combustible tobacco non-menthol cigarette (K594, Lucky Strike Regular, control product). For the exposure period of the study, a single study product was allocated to each participant. These products were either the 7 mg/cig ISO tar combustible non-menthol cigarette (K594), the glo THP with non-menthol Neostiks (THP1.0 T), or a prototype EC with ‘Twilight Tobacco’ flavoured e-liquid (IS1.0[TT]) (Supplementary Table 1). The prototype EC is a closed system device, with a power output of 10 W and the e-liquid contained in replaceable disposable cartridges. The glo THP used in this study is an electronic device with a heating chamber into which a specially designed tobacco rod (the Neostik) can be inserted and on activation this heats the tobacco rod to a temperature of 240 °C [18]. This allows the user to puff on the filter end of the tobacco rod as they would with a conventional cigarette.
A further group abstained from using any tobacco or nicotine products during the exposure period. Aerosol emissions for the THP used in this study have been published previously [19,26]. For the batch of Neostiks used for the glo THP in this study, emissions analysis was carried out for the nine toxicants proposed by the World Health Organization Study Group on Tobacco Product Regulation [27] for mandated reduction in cigarettes (Supplementary Table 2) to ensure levels of these constituents were reduced by at least 90 % in comparison to University of Kentucky 3R4F reference cigarettes. For the comparator combustible cigarettes manufactured for this study, smoke constituents are reported in Supplementary Table 3. The prototype EC with Twilight Tobacco flavour e-liquid emissions for the TobReg nine toxicants [27] are reported in Supplementary Table 4.
3.6. Study procedures
At screening, participants completed a tobacco use history questionnaire and the Fagerström Test for Cigarette Dependence [28] as well as being assessed to ensure that they met all inclusion/exclusion criteria. Participants who met all inclusion/exclusion criteria were enrolled into the study (Day –1). For the first 2 days (Days 1 and 2), the baseline period, participants smoked conventional cigarette control products (K594). For eCO analysis, a portable meter (Micro + Smokerlyzer, Bedfont Scientific Ltd., Maidstone, UK) was used in the afternoon on both days. A 24-h urine collection was also taken from Day 1 to Day 2 and a blood sample was taken on Day 2 for BoE and BoBE analyses. At the end of the baseline period on Day 2 and according to the randomisation code, participants either continued to smoke the conventional cigarettes, switched to using the glo THP (THP1.0 T), or the prototype EC (IS1.0[TT]), or a non-BAT commercial product (data not reported), or abstained from all nicotine and tobacco (nicotine cessation) use for 5 days (Day 2 to Day 7). During this exposure period, eCO was measured in the afternoon on all days and 24-h urine samples were collected from Day 2 to Day 3, Day 4 to Day 5 and Day 6 to Day 7. Blood samples were also collected on Day 5 and Day 7. Each participant’s consumption of either cigarettes or glo THP Neostiks was limited to 120 % of their usual cigarette consumption as self-reported at screening.
The study products were dispensed by clinic staff each time a participant requested to smoke a cigarette or use the glo THP or EC. Participants randomised to the cessation arm were not permitted cigarettes/THPs/ECs, were housed separately to the cigarette, THP and EC arms, and were not allowed to enter the smoking rooms. For the prototype EC, the number of products used per day is defined as the number of times the participant requested to use the EC each day.
During the entire confinement period participants received a standardised controlled diet which excluded cruciferous vegetables, and grilled, smoked, fried or barbequed food items to avoid interference with the study endpoints.
At the end of the 5-Day exposure period, participants in the nicotine cessation group were discharged from the clinic. The continue to smoke and switching groups remained in the clinic for a further day to undergo a nicotine pharmacokinetic assessment with their assigned product. The nicotine pharmacokinetic data are not reported in this manuscript.
After the completion of the nicotine pharmacokinetic assessment, participants were discharged from the clinic. Follow-up telephone calls to all participants were conducted 5 days after discharge to ensure any potential subsequent adverse events (AEs) were captured.
3.7. Urinary BoE analysis
The 24-h urine samples collected from Days 1–2, Days 3–4 and Days 5–6 during the study were analysed for BoE to selected cigarette smoke constituents. The BoEs analysed in this study are shown in Table 1. All BoE analysis used LC-MS/MS methods apart from 4-aminobiphenyl, 2- aminonaphthalene and o- toluidine which used a GC-MS method as described in Gale et al. 2017 [26].
Table 1.
Tobacco smoke constituents and their urinary Biomarkers of Exposure used in this study.
| Tobacco Smoke constituent | Biomarker of Exposure (Urinary Metabolites) | BoE Acronym |
|---|---|---|
| Urinary Nicotine (Total Nicotine Equivalents) | Nicotine, Cotinine, 3-hydroxycotinine, and their glucuronide conjugates | TNeq |
| NNK | Total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol | Total NNAL |
| NNN | Total N-nitrosonornicotine | Total NNN |
| Acrolein | 3-hydroxypropylmercapturic acid | 3-HPMA |
| Crotonaldehyde | 3-hydroxy-1-methylpropylmeracpturic acid | 3-HMPMA |
| Benzene | S-phenylmercapturic acid | S-PMA |
| 1,3-Butadiene | monohydroxybutenyl-mercapturic acid | MHBMA |
| Acrylonitrile | 2-cyanoethylmercapturic acid | CEMA |
| 4-aminobiphenyl | 4-aminobiphenyl | 4-ABP |
| o-toluidine | o-toluidine | o-tol |
| 2-aminonaphthalene | 2-aminonaphthalene | 2-AN |
| Pyrene | 1-hydroxypyrene | 1-OHP |
| Ethylene Oxide | 2-hydroxyethylmercapturic acid | HEMA |
| Acrylamide | N-acetyl-S-(2-carbamoylethyl)cysteine & N-acetyl-S-(2-hydroxy-2-carbamoylethyl)cysteine | AAMA & GAMA |
3.8. Other biomarkers
In addition, three further biomarkers were analysed: two BoBE (white blood cell count, urinary 8-epi-PGF2α Type III) as well as the nicotine molar metabolic ratio (ratio of nicotine metabolites 3-hydroxycotinine to cotinine) in urine. Two of these BoBEs, white blood cell count and urinary 8-epi-PGF2α Type III, were analysed as they are short-term biomarkers that may change over the study period with smoking abstinence. The nicotine molar ratio was assessed to monitor participant’s nicotine metabolism rates as this may have affected study outcomes.
All laboratory analyses were carried out at Celerion Laboratories (Lincoln, NE, USA) or ABF GmbH (Munich, Germany). Details of the bioanalytical methods have been published previously [26].
3.9. AEs, medical history, and concomitant medication
Safety assessments included AEs, serious AEs (SAEs), vital signs, ECG, spirometry, clinical chemistry, haematology, urinalysis, physical examinations and use of concomitant medications. AEs were recorded from time of signing the informed consent form until the end of the follow-up period after discharge from the clinic. AEs, concomitant diseases, and medical/surgical history were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 20.0).
3.10. Sample size determination
An intended sample size of 30 participants per arm was set for this study, with an anticipated number of 26 participants per arm to complete the study. This sample size was based on powering the primary objective of within-arm comparisons of biomarker levels between baseline and end of study (Days 6–7). The calculation was based on the number of pairs required to perform a paired t test with 80 % power to show a decrease in biomarker levels of 40 % or more compared with historical biomarker data available for a 7 mg/cig ISO tar conventional cigarette [29,30]. Based on the biomarker requiring the most pairs to power (eCO, requiring 26 pairs) and allowing for 12 % attrition, a sample size of 30 was determined to be adequate. For the secondary objectives a sample size of 30 was also determined to be able to provide sufficient power for between-group comparisons, based on a minimum of 40 % reduction in BoE.
3.11. Randomisation
A randomisation scheme was provided for the clinical site to recruit 30 participants for each arm, giving a total of 150 participants. A dual randomisation was used whereby participants were randomised either to Arms C or E (the non-BAT commercial product or nicotine cessation), or Arms A, B, or D (control cigarette, THP1.0 T or IS1.0[TT], respectively). This randomisation was generated using SAS® version 9.3 and consisted of 30 blocks with each block being 5 in size and comprising one allocation to each of the study arms.
3.12. Statistical methods
Summary statistics and statistical analyses were performed for the relevant safety, intent to treat and/or per protocol analysis populations, as specified in the Statistical Analysis Plan. Missing values were not imputed, and values below the analytical limit of quantification (LOQ) were replaced with half the value of the lower LOQ. All data analyses were performed using SAS® Version 9.3.
Statistical summaries were presented as both absolute change from baseline and percentage change from baseline. Absolute change from baseline was defined as the mass recovered for each biomarker at post-baseline “Day 7” subtracted from the baseline mass. Of note, for urine biomarkers, baseline was defined as urine collected from 19:00 h of Day 1 to 19:00 on Day 2 and for “Day 7” as urine collected from 19:00 h of Day 5 to 19:00 on Day 6. However, for eCO, baseline was determined as the mean of the two values taken prior to randomisation (i.e. Day –1 and Day 2). Percentage biomarker change was calculated as 100 x (biomarker change/baseline value).
Within-arm changes in biomarker concentrations were assessed using a mixed model with arm, day and the interaction between arm*day as fixed effects and participant (arm) as random effects. Contrast between baseline and “Day 7” were performed using the interaction term. For the secondary objective, the endpoint used was change from baseline in a mixed model ANOVA comparing the different arms and adjusted for baseline values. For all statistical comparisons, the difference in the changes from baseline between two products was presented along with 95 % confidence intervals.
Multiple comparisons adjustments were performed according to Holm’s procedure, based on pre-allocated alpha levels assigned to different sets of comparisons. Firstly, alpha at 0.05 was allocated to test within-cohort changes for the test groups (comparisons for arms B and D) for all 19 biomarkers (16 BoEs, 2 BoBEs and Nicotine Metabolic Molar Ratio) separately from the rest of the control groups comparisons (Arms A, C and E). Secondly, the alpha was divided equally between test and control comparisons (α = 0.025). For the test product comparisons, the values α = 0.025/38 = 0.000657 and α = 0.025/57 = 0.000438 were used for controls (for their respective smallest p-values).
4. Results
4.1. Participant demographics
A total of 148 participants entered the study on Day –1 and were randomised into one of five study arms. Of these participants, 143 completed the study in accordance with the protocol and five participants withdrew consent for personal or ‘other’ reasons.
Table 2 shows the demographic details of the study participants. A consort diagram detailing screening, randomisation and completions for the study is shown in Supplementary Fig. 1. The mean age between the study groups was similar, ranging from 32.8–37.4 years of age. Smoking history and Fagerström score summary data are shown in Supplementary Table 5.
Table 2.
Demographic Summary (Safety Population).
| Study Arm |
|||||||
|---|---|---|---|---|---|---|---|
| Trait | Category/Statistics | A | B | C | D | E | Overall |
| n | 30 | 30 | 29 | 30 | 29 | 148 | |
| Age (years) | Mean (SD) | 35.6 (8.93) | 37.4 (11.48) | 32.8 (8.78) | 36.7 (9.10) | 37.2 (9.09) | 35.9 (9.55) |
| Sex | Female | 8 (27 %) | 14 (47 %) | 12 (41 %) | 15 (50 %) | 12 (41 %) | 61 (41 %) |
| Male | 22 (73 %) | 16 (53 %) | 17 (59 %) | 15 (50 %) | 17 (59 %) | 87 (59 %) | |
| Race | White | 30 (100 %) | 30 (100 %) | 29 (100 %) | 30 (100 %) | 29 (100 %) | 148 (100 %) |
| Ethnicity | Not Hispanic or Latino | 30 (100 %) | 30 (100 %) | 29 (100 %) | 30 (100 %) | 29 (100 %) | 148 (100 %) |
| Weight Male (kg) | Mean (SD) | 77.4 (11.09) | 75.1 (12.91) | 84.5 (6.47) | 79.1 (9.62) | 77.5 (13.19) | 78.7 (11.15) |
| Weight Female (kg) | Mean (SD) | 68.9 (6.89) | 66.1 (7.82) | 62.7 (8.08) | 65.3 (12.58) | 65.1 (9.07) | 65.4 (9.27) |
| BMI Male (kg/m²) | Mean (SD) | 25.078 (3.0683) | 24.626 (3.1230) | 26.472 (2.3689) | 24.995 (2.4491) | 25.336 (3.0708) | 25.303 (2.8566) |
| BMI Female (kg/m²) | Mean (SD) | 25.799 (3.0382) | 24.609 (2.3915) | 24.216 (2.3407) | 24.525 (2.7678) | 24.774 (2.2822) | 24.700 (2.5063) |
Arm A: Conventional cigarettes from Day -1 to Day 8 Arm B: Conventional cigarettes from Day -1 to evening of Day 2 followed by Glo device with Neostiks through Day 8 Arm C: Conventional cigarettes from Day -1 to evening of Day 2 followed by a non-BAT commercial product through Day 8 Arm D: Conventional cigarettes from Day -1 to evening of Day 2 followed by the Prototype e-cigarette with Twilight Tobacco flavour e-liquid through Day 8 Arm E: Conventional cigarettes from Day -1 to evening of Day 2 followed by nicotine cessation through Day 8 BMI = Body mass index Age was calculated at the time of informed consent.
Participants’ chosen cigarette brands were within the ISO NFDPM (‘tar’) rating of 6–10 mg/cig, and they had smoked these brands for at least 6 months prior to screening. All participants smoked between 10 and 30 cigarettes daily. The number of cigarettes smoked daily, as reported at screening by the participants, was similar in all study groups, ranging from a mean of 19.7–21.5 cigarettes per day.
4.2. Biomarkers of exposure
A total of 143 participants completed the study in accordance with the protocol. However, due to a sample from one participant in the nicotine cessation arm being discarded in error, analysis for some of the urinary biomarkers were not carried out and only TNeq and 1-OHP were reported for this participant. In addition, one other participant in the nicotine cessation arm had an inexplicably high value for o-toluidine on Day 6–7 of the study and, therefore, a sensitivity test was carried out to show analysis with and without this value present.
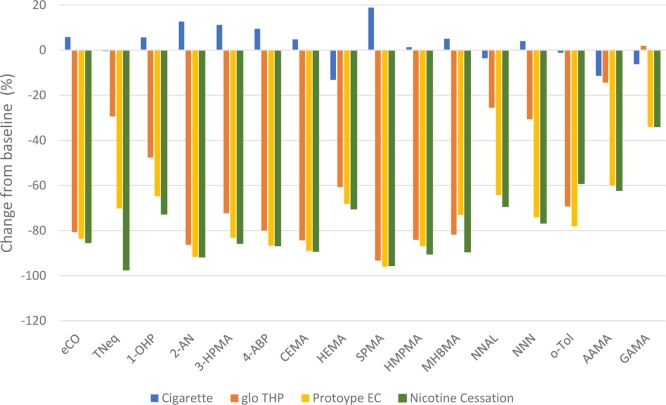
Changes in BoE levels between baseline and Days 6–7 of the exposure period are presented in Fig. 2. Absolute values and statistical analysis for within-arm changes for all study arms are presented in Supplementary Table 6, and the statistical comparison between arms in Supplementary Table 7.
Fig. 2.
Biomarker of Exposure (BoEs) changes between baseline and Days 6 to 7. Data are mean values expressed as the percentage change from the baseline value. All data, except for eCO, were calculated using biomarker levels from 24-h urine collections at baseline and on Days 6 to 7. eCO levels were calculated from data captured at baseline and on Day 6 to 7. eCO - exhaled carbon monoxide; TNeq - total nicotine equivalents (nicotine, cotinine, 3-hydroxycotinine and their glucuronide conjugates); 1-OHP - 1-hydroxypyrene; 2-AN - 2-aminonaphthalene; 3-HPMA - 3-hydroxypropylmercapturic acid; 4-ABP - 4-aminobiphenyl; CEMA - 2-cyanoethylmercapturic acid; HEMA - 2-hydroxyethylmercapturic acid; S-PMA - S-phenylmercapturic acid; HMPMA - 3-hydroxy-1-methylpropylmercapturic acid; MHBMA - monohydroxybutenyl-mercapturic acid; NNAL - 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNN - N-nitrosonornicotine; o-tol - o-toluidine; AAMA - N-acetyl-S-(2-carbamoylethyl)cysteine; GAMA - N-acetyl-S-(2-hydroxy-2-carbamoylethyl)cysteine.
Following a switch from smoking combustible cigarettes to using the prototype EC (IS1.0[TT]), there were significant mean reductions in all classes of BoEs, which include gas phase compounds (eCO; -83.6 %), total nicotine (TNeq; -70.2 %), a polycyclic aromatic hydrocarbon (PAH) (1-OHP; -64.7 %), aromatic amines (4-ABP, 2-AN & o-tol; -78.1 to -91.7 %), tobacco specific nitrosamines (TSNAs NNAL & NNN; -64.2 & -74.1 %), acrylamide (GAMA & AAMA; -34.0 & -59.9 %), volatile carbonyls (3-HPMA & HMPMA; -83.2 & -86.9 %), acrylonitrile (CEMA; -89.0 %), ethylene oxide (HEMA; -68.1 %) and other volatile compounds (SPMA & MHBMA; -96.0 & -73.0 %).
In the glo THP group, significant reductions were also observed for gas phase compounds (eCO; -80.6 %), total nicotine (TNeq;-29.3 %), PAH (1-OHP; -47.6 %), aromatic amines (4-ABP, 2-AN & o-tol; -69.3 % to -86.3 %), TSNAs (NNAL & NNN; -25.5 & -30.6 %), acrylamide (GAMA & AAMA; 1.9 & -14.3 %) and volatile carbonyls (3-HPMA & HMPMA; -72.3 & -84.0 %), acrylonitrile (CEMA -84.3 %), ethylene oxide (HEMA -60.7 %) and other volatile compounds (SPMA & MHBMA; -93.3 & -81.8 %). For the biomarker for acrylamide (GAMA), the mean percentage change from baseline to Day 7 was negligible (1.9 %), however, when assessed as a concentration change from baseline, GAMA levels showed a reduction of 5,728.4 ng/24 h for the glo THP arm. This absolute change from baseline was statistically significant even after adjustment for multiplicity (p = 0.001011) (Supplementary Table 6).
For the nicotine cessation arm, significant reductions were seen for all BoEs between baseline and Day 7. For eCO there was a reduction of 85.3 % and a reduction of 97.4 % for urinary nicotine (TNeq). For the other urinary BoEs: for the PAH biomarker 1-OHP there was a reduction of 72.6 %, while the aromatic amines 4-ABP, 2-AN & o-tol (sensitivity test) were reduced by between 59.1 and 91.7 %. The TSNAs (NNAL and NNN) were reduced by 69.3 and 76.6 %, respectively. The acrylamide BoEs AAMA and GAMA were reduced by 62.2 and 33.9 %, respectively. Levels of the volatile carbonyls (3-HPMA & HMPMA) were reduced by 85.6 and 90.4 %, respectively, while the acrylonitrile BoE (CEMA) and the ethylene oxide BoE (HEMA) were reduced by 89.1 and 70.3 %, respectively. Finally, for the other volatile compounds, i.e. benzene and 1,3-butadiene (SPMA & MHBMA), the BoE were reduced by 95.5 and 89.4 %, respectively.
All urinary and exhaled BoE, except o-toluidine, assessed following the switch from conventional cigarette to either the glo THP (THP1.0 T), the prototype EC (IS1.0[TT]) or to the nicotine cessation arm were significantly decreased from baseline levels at Days 6–7 after multiple comparison adjustments. For o-toluidine this was significant only after the removal of an inexplicably high value in the nicotine cessation arm (without the value, p < 0.000001; including the inexplicably high value gave p = 0.007153). For the participants that remained using the conventional cigarettes for the duration of the study, there was no significant change in any of the BoEs. In addition, the Nicotine Molar Metabolic Ratio, which was incorporated to identify any differences in the metabolism of nicotine, was not significantly changed between baseline and Days 6–7 for all arms of the study. For the BoBEs, white blood cell count was significantly reduced between baseline and Day 7 for the glo THP and the prototype EC arms. However, there was no significant change in the nicotine cessation or in the control cigarette arm. For 8-epi-PGF2α Type III, there was no significant change in all arms except the glo THP arm, which showed a significant decrease in the urinary levels of this BoBE.
To assess the exposure continuum the mean percentage change from baseline for all BoEs except TNeq were calculated. This showed that the biggest reduction in exposure to cigarette toxicants was experienced by the nicotine cessation arm (-77.2 %) followed closely by the EC arm (-75.5 %) and the glo THP arm (-60.6 %). While for the continue to smoke arm there was a slight increase in exposure to cigarette smoke toxicants (2.9 %).
4.3. Product consumption
Overall, the mean number of products used during each urine collection interval tended to be highest in the glo THP arm, followed by the continue to smoke arm, with the prototype EC arm as the lowest (Supplementary Fig. 2). However, as the study went on, it was noted that the average amount of e-liquid used increased each day, with highest use being on day 7, and could reflect that participants used the product for a longer period per session.
5. Safety
A summary of the AEs that occurred during the study can be found in Supplementary Table 8. All participants that were enrolled in and completed the study were included in the biomarker analysis.
Overall, 61 (41 %) participants experienced a total of 82 AEs in this study. Headache was the most frequently reported AE, experienced by a total of 20 (14 %) participants. The majority of AEs were mild or moderate in severity, with six severe events, including five events of presyncope. Of note, all five of the severe presyncope events occurred during blood draws or intravenous cannula insertion and were considered not product related.
One subject experienced the SAE of tachycardia (verbatim term: decompensated tachycardia) in Arm C, which resolved in 52 min and was considered product related. The subject recovered and completed the study per protocol. No participants were discontinued due to AEs. There were no deaths reported in this study.
6. Discussion
This study investigated exposure to cigarette smoke toxicants when smokers switched to an EC or THP or completely quit nicotine or tobacco product use for 5 days. As such, this is the first study to report the exposure continuum between ECs and THPs relative to nicotine cessation. To investigate the exposure continuum, 16 (urinary and exhaled breath) harmful and potentially harmful toxicants were selected from the FDA list of toxicants, with the exception of pyrene, which was used as a surrogate BoE for Benzo[a]pyrene [25,31].
These biomarkers have been shown to be well described in smokers and non-smokers and are widely used for assessing exposure in humans as a result of using tobacco products [32,33]. All urinary and exhaled BoE assessed following the switch from a conventional cigarette to EC or THP use or nicotine cessation showed substantial decreases in levels from baseline to Day 7. Critical evaluation of these findings and their implications for placing ECs and THPs on an exposure continuum relative to nicotine cessation is discussed below.
Levels of urinary BoE and eCO showed reductions of –34.0 % to –96.0 % in participants who switched to the EC. For most of these BoEs there were similar reductions in levels in participants who abstained from any tobacco or nicotine use for 5 days, ranging from 33.9–97.4%. Similar reductions in potentially harmful smoke constituents in participants switching exclusively to ECs were reported by Walele et al., (2018) [34], Goniewicz et al., (2016) [35], D’Ruiz et al., (2016) [36] and Jay et al. (2020) [37]. Indeed, in the case of two of those studies [36,37], nicotine cessation arms were implemented in the study design and showed similar reductions in levels of most BoEs to the exclusive e-cigarette use arms.
For the majority of the BoEs, reductions were not as high with the THP as with the EC, placing the THP category slightly higher on the exposure continuum. In the glo THP arm, all BoEs were statistically significantly lower after 5 days’ use relative to baseline levels, with the exception of o-toluidine. However, this latter test became significant when a sensitivity analysis was conducted with one participant’s inexplicably high o-toluidine value removed from the nicotine cessation group. With this value included, the nicotine cessation arm showed an increase of 274.7 %, thus, demonstrating the effect of this inexplicably high value on the data. A similar clinical study, conducted in Japan on the glo THP, reported that all BoEs, including o-toluidine, were statistically significantly reduced in comparison to baseline levels [38]. Overall, our results are consistent with previous studies assessing BoEs following a switch from conventional combustible cigarettes to THPs [[38], [39], [40]]. These results also support the Public Health England report on current research on THPs. Although the report highlights the need for more research on THPs, existing evidence found that “compared with cigarettes, THPs are likely to expose users and bystanders to lower levels of particulate matter and harmful and potentially harmful compounds” [41].
In terms of the exposure continuum, reductions in BoEs, when calculated as mean percent change from baseline for all BoEs except TNeq, were highest for the nicotine cessation arm (-77.2 %) as would be expected. The EC (-75.5 %) was the closest to the nicotine cessation arm followed by the glo THP arm (-60.6 %), this is also expected with the levels of emissions for most smoke toxicants being higher in THPs than ECs.
For the two BoBEs, significant changes were observed in the glo THP arm, but these changes were not significant in the nicotine cessation arm. However, a quantitative risk assessment approach by Hirn et al. comparing non-cancer and cancer risk estimates for emissions generated by a THP with smoke from a reference cigarette (3R4F) have shown a 90 % reduction in cancer and non-cancer risk estimates [20]. Therefore, further studies are required to investigate whether long-term switching to these PRRPs can result in significant changes in these biomarkers of potential harm.
The results from this clinical study are consistent with findings from emissions studies, which have shown that the glo THP, a novel heated tobacco product and ECs generate less harmful chemical toxicants than reference cigarettes [7,9,19,20]. While TNeq and TSNAs are tobacco specific, it is important to note that exposure to the assessed BoEs can be confounded by environmental and dietary factors. However, as this study was a confined design, we believe we have minimised the impact of confounding variables on the BoEs analysed. Another benefit of the confined study design is that compliance following the randomisation period was controlled. This type of clinical study generally involves investigation of numerous endpoints that could lead to detection of significant outcomes just by chance. In our study, we adjusted for multiple comparisons to make sure it was controlled for type I error, and hence preserved confidence in the significance of results.
Government health authorities and research agencies have encouraged complete abstinence of smoking and nicotine use, regardless of relative risk [42]. These efforts include long-term treatment, support, or nicotine replacement therapy to enable users to sustain abstinence from smoking. However, complete cessation from cigarette smoking is difficult and it remains a leading cause of disease and health burden [42,43]. The principle of tobacco harm reduction is that, compared with continuing to smoke conventional cigarettes, adult smokers who do not wish to quit smoking completely should be given the opportunity to switch to PRRPs. The substitution of cigarette smoking with the use of PRRPs could be a realistic compromise. ECs are already proving to be successful as an effective means to sustained smoking cessation [[44], [45], [46]].
Biomarker studies are essential to characterising the risk continuum among nicotine products. Two components – expected risk profiles between products and behavioural patterns – are the main components of population-level assessments. These are used to create projections about the potential health effects of launching these PRRPs, especially in view of MRTP applications, and help support guidance policy [47].
7. Limitations
Extrapolating the findings from this study to PRRP use in the general population of smokers may be constrained by a few limitations. Firstly, this study was conducted in a confined controlled environment and this may not reflect real-world use of PRRPs. In addition, a never smoker group was not included in this study; this would give an indication of levels of these toxicants generally found in the environment. Therefore, further studies are required to investigate use of these products in an ambulatory setting and whether reductions in exposure to tobacco smoke toxicants are sustained over a longer period of time and how they compare to never smokers. Secondly, this study does not cover the potential risk of tobacco-related disease and further studies are required to investigate whether long-term switching to these PRRPs can result in reduced disease risk. Finally, this does not address the effects of potential dual use of PRRPs along with combustible cigarettes; further studies would be required to understand the potential effects of consumers reducing combustible cigarette usage and substituting this with PRRP use.
8. Conclusions
This study demonstrates that substantial reductions in exposure to cigarette smoke toxicants were achieved when smokers switched to the glo THP or the prototype EC over a 5 day period, in some cases to a similar level of reduction as cessation. It also demonstates that in terms of the levels of exposure to tobacco smoke constituents and the exposure continuum the greatest reductions across all the BoEs were with the EC followed by the glo THP, all of which demonstrated significant reductions in comparison to smoking combustible cigarettes. This is the first study to investigate both the THP and EC categories in an exposure continuum and demonstrates that cigarette smoking has the highest levels of exposure to smoke toxicants with a substantial reduction in levels with the glo THP, while ECs have the lowest potential exposure to toxicants of these PRRPs.
Author statement
Michael McEwan: Conceptualization, Methodology, Writing - Original Draft, Visualization, Project administration Nathan Gale: Methodology, Writing - Review & Editing, Project administration James K. Ebajemito: Validation, Visualization Oscar M. Camacho: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing - Original Draft George Hardie: Writing - Review & Editing, Visualization, Supervision Christopher J. Proctor: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Funding acquisition James Murphy: Conceptualization, Methodology, Writing - Review & Editing, Funding acquisition
Funding
This study was sponsored by British American Tobacco (Investments) Limited (BAT).
Declaration of Competing Interest
All authors except CJP are current employees of British American Tobacco (Investments) Limited. CJP is a consultant contracted by British American Tobacco (Investments) Limited.
Acknowledgements
We would like to acknowledge the support from Lois Mollison-Ball and Rory Fraser for coordinating the supply of products for this study.
Edited by Dr. A.M Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.05.003.
Contributor Information
Michael McEwan, Email: mike_mcewan@bat.com.
Nathan Gale, Email: nathan_gale@bat.com.
James K. Ebajemito, Email: james_ebajemito@bat.com.
Oscar M. Camacho, Email: oscar_m_camacho@bat.com.
George Hardie, Email: george_hardie@bat.com.
Christopher J. Proctor, Email: dr-chris-proctor@hotmail.com.
James Murphy, Email: murphyj12@rjrt.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Perfetti T., Rodgman A. The complexity of tobacco and tobacco smoke. Beitr. Tabaforsch. 2011;24(5):215–232. doi: 10.2478/cttr-2013-0902. [DOI] [Google Scholar]
- 2.Ng M., Freeman M.K., Fleming T.D., Robinson M., Dwyer-Lindgren L., Thomson B., Wollum A., Sanman E., Wulf S., Lopez A.D., Murray C.J., Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 3.Fearon I.M., Eldridge A.C., Gale N., McEwan M., Stiles M.F., Round E.K. Nicotine pharmacokinetics of electronic cigarettes: A review of the literature. Regul. Toxicol. Pharmacol. 2018;100:25–34. doi: 10.1016/j.yrtph.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson K., Martinez J., Larroque S., Jones I.W., Paschke T. Nicotine pharmacokinetics of electronic cigarettes: a pooled data analysis from the literature. Toxicol. Rep. 2020;8:84–95. doi: 10.1016/j.toxrep.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services . Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2014. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General. [Google Scholar]
- 6.Murphy J., Gaҫa M., Lowe F., Minet E., Breheny D., Prasad K., Camacho O.M., Fearon I.M., Liu C., Wright C., McAdam K., Proctor C. Assessing modified risk tobacco and nicotine products: description of the scientific framework and assessment of a closed modular electronic cigarette. Regul. Toxicol. Pharmacol. 2017;90:342–357. doi: 10.1016/j.yrtph.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Azzopardi D., Patel K., Jaunky T., Santopietro S., Camacho O.M., McAughey J., Gaҫa M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Methods. 2016;26(6):477–491. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margham J., McAdam K., Forster M., Liu C., Wright C., Mariner D., Proctor C. Chemical composition of aerosol from an E-Cigarette: a quantitative comparison with cigarette smoke. Chem. Res. Toxicol. 2016;29(10):1662–1678. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 9.Wang G., Liu W., Song W. Toxicity assessment of electronic cigarettes. Inhal. Toxicol. 2019;31(7):259–273. doi: 10.1080/08958378.2019.1671558. [DOI] [PubMed] [Google Scholar]
- 10.McNeil A., Brose L.S., Calder R., Hitchman S.C., Hajek P., McRobbie H. 2015. E-cigarettes: An Evidence Update. A Report Commissioned by Public Health England. August. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457102/Ecigarettes_an_evidence_update_A_report_commissioned_by_Public_Health_England_FINAL.pdf [Accessed 24th September 2019] [Google Scholar]
- 11.Royal College of Physicians (RCP) 2016. Nicotine without Smoke: Tobacco Harm Reduction. April. Available at https://www.rcplondon.ac.uk/sites/default/files/media/Documents/Nicotine%20without%20smoke.pdf [Accessed 24th September 2019] [Google Scholar]
- 12.Thorne D., Larard S., Baxter A., Meredith C., Gaҫa M. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the yH2AX assay and applied dose measurements. Toxicol. Lett. 2017;265:170–178. doi: 10.1016/j.toxlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Breheny D., Thorne D., Baxter A., Bozhilova S., Jaunky T., Santopietro S., Taylor M., Terry A., Gaça M. The in vitro assessment of a novel vaping technology. Toxicol. Rep. 2020;7:1145–1156. doi: 10.1016/j.toxrep.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorne D., Crooks I., Hollings M., Seymour A., Meredith C., Gaҫa M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strain TA98 and TA100. Mutat. Res. 2016;812:29–38. doi: 10.1016/j.mrgentox.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Thorne D., Hollings M., Seymour A., Adamson J., Dalrymple A., Ballantyne M., Gaҫa M. Extreme testing of undiluted e-cigarette aerosol in vitro using an Ames air-agar-interface technique. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018;828:46–54. doi: 10.1016/j.mrgentox.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Breheny D., Oke O., Pant K., Gaҫa M. Comparative tumour promotion assessment of e-cigarette and cigarettes using the in vitro Bhas 42 cell transformation assay. Environ. Mol. Mutagen. 2017;58(4):190–198. doi: 10.1002/em.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito S., Taylor M., Mori S., Thorne D., Nishino T., Breheny D., Gaça M., Yoshino K., Proctor C. An inter-laboratory in vitro assessment of cigarettes and next generation nicotine delivery products. Toxicol. Lett. 2019;315:14–22. doi: 10.1016/j.toxlet.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Eaton D., Jakaj B., Forster M., Nicol J., Mavropoulou E., Scott K., Liu C., McAdam K., Murphy J., Proctor C.J. Assessment of tobacco heating product THP1.0. Part 2: Product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol. 2018;93:4–13. doi: 10.1016/j.yrtph.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Forster M., Fiebelkorn S., Yurteri C., Mariner D., Liu C., Wright C., McAdam K.G., Murphy J., Proctor C.J. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018;93:14–33. doi: 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Hirn C., Kanemaru Y., Stedeford T., Paschke T., Baskerville-Abraham I. Comparative and cumulative quantitative risk assessments on a novel heated tobacco product versus the 3R4F reference cigarette. Toxicol. Rep. 2020;7:1502–1513. doi: 10.1016/j.toxrep.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaunky T., Adamson J., Santopietro S., Thorne D., Breheny D., Proctor C. Assessment of tobacco heating product THP1.0 Part 5: in vitro dosimetric and cytotoxic assessment. Regul. Toxicol. Pharmacol. 2018;93:52–61. doi: 10.1016/j.yrtph.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Taylor M., Thorne D., Carr T., Breheny D., Walker P., Proctor C., Gaça M. Assessment of tobacco heating product THP1.0 Part 6: a comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharmacol. 2018;93:62–70. doi: 10.1016/j.yrtph.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Thorne D., Breheny D., Proctor C., Gaҫa M. Assessment of tobacco heating product THP1.0 Part 7: comparative in vitro toxicological evaluation. Regul. Toxicol. Pharmacol. 2018;93:71–83. doi: 10.1016/j.yrtph.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 24.McNeill A., Munafò M.R. Reducing harm from tobacco use. J. Psychopharmacol. 2013;27(1):13–18. doi: 10.1177/0269881112458731. [DOI] [PubMed] [Google Scholar]
- 25.Department of Health and Human Services, Food and Drug Administration Harmful and potentially harmful constituents in tobacco products and tobacco smoke. Federal Reg. 2012;77(64):20034–20037. [Google Scholar]
- 26.Gale N., McEwan M., Eldridge A., Sherwood N., Bowen E., McDermott S., Holmes E., Hedge A., Hossak S., Camacho O.M., Errington G., McAughey J., Murphy J., Lui C., Proctor C.J., Fearon I.M. A randomised, controlled, two-centre open-label study in healthy Japanese subjects to evaluate the effect on biomarkers of exposure of switching from a conventional cigarette to a tobacco heating product. BMC Public Health. 2017;17(1):673. doi: 10.1186/s12889-017-4678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns D.M., Dybing E., Gray N., Hecht S., Anderson C., Sanner T., O’Connor R., Djordjevic M., Dresler C., Hainaut P., Jarvis M., Opperhuizen A., Straif K. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob. Control. 2008;17:132–141. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagerström K. Determinants of tobacco use and renaming the FTND to the fagerström test for cigarette dependence. Nicotine Tob. Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 29.Dittrich D.J., Fieblekorn R.T., Bevan M.J., Rushforth D., Murphy J.J., Ashley M., McAdam K.G., Liu C., Proctor C.J.A. Pproaches for the design of reduced toxicant emission cigarettes. Springerplus. 2014;3(1):374. doi: 10.1186/2193-1801-3-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepperd C.J., Newland N., Eldridge A., Haswell L., Lowe F., Papadopoulou E., Camacho O., Proctor C.J., Graff D., Meyer I. Changes in levels of biomarkers of exposure and biological effect in a controlled study of smokers switched from conventional cigarettes to reduced-toxicant-prototype cigarettes. Regul. Toxicol. Pharmacol. 2015;72(2):273–291. doi: 10.1016/j.yrtph.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Yuki D., Takeshige Y., Nakaya K., Futamura Y. Assessment of the exposure to harmful and potentially harmful constituents in healthy Japanese smokers using a novel tobacco vapour product compared with conventional cigarettes and smoking abstinence. Regul. Toxicol. Pharmacol. 2018;96:127–134. doi: 10.1016/j.yrtph.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Scherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Exp. Toxicol. Pathol. 2006;58(2-3):101–124. doi: 10.1016/j.etp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Gregg E.O., Minet E., McEwan M. Urinary biomarkers of smokers’ exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach. Biomarkers. 2013;18(6):467–486. doi: 10.3109/1354750X.2013.821523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walele T., Bush J., Koch A., Savioz R., Martin C., O’Connell G. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regul. Toxicol. Pharmacol. 2018;92:226–238. doi: 10.1016/j.yrtph.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Goniewicz M.L., Gawron M., Smith D.M., Peng M., Jacob P., III, Benowitz N.L. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob. Res. 2017;19(2):160–167. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Ruiz C.D., Graff D.W., Robinson E. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health. 2016;16:543. doi: 10.1186/s12889-016-3236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jay J., Pfaunmiller E.L., Huang N.J., Cohen G., Graff D.W. Five-day changes in biomarkers of exposure among adult smokers after completely switching from combustible cigarettes to a nicotine-salt pod system. Nicotine Tob. Res. 2020;22(8):1285–1293. doi: 10.1093/ntr/ntz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gale N., McEwan M., Eldridge A.C., Fearon I.M., Sherwood N., Bowen E., McDermott S., Holmes E., Hedge A., Hossak S., Wakenshaw L., Glew J., Camacho O.M., Errington G., McAughey J., Murphy J., Lui C., Proctor C.J. Changes in biomarkers of exposure on switching from a conventional cigarette to Tobacco Heating Products: A randomized, controlled study in healthy Japanese subjects. Nicotine Tob. Res. 2018;21(9):1220–1227. doi: 10.1093/ntr/nty104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lüdicke F., Baker G., Magnette J., Picavet P., Weitkunat R. Reduced exposure to harmful and potentially harmful smoke constituents with the tobacco heating system 2.1. Nicotine Tob. Res. 2017;19(2):168–175. doi: 10.1093/ntr/ntw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haziza C., de La Bourdonnaye G., Skiada D., Ancerewicz J., Baker G., Picavet P., Lüdicke F. Biomarker of exposure level data set in smokers switching from conventional cigarettes to Tobacco Heating System 2.2, continuing smoking or abstaining from smoking for 5 days. Data Brief. 2017;10:283–293. doi: 10.1016/j.dib.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNeill A., Brose L., Calder R., Bauld L., Robson D. 2018. Evidence Review of e-cigarettes and Heated Tobacco Products, a Report Commissioned by Public Health England. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/684963/Evidence_review_of_e-cigarettes_and_heated_tobacco_products_2018.pdf [Accessed 24th September 2019] [Google Scholar]
- 42.World Health Organization . 2011. WHO Report on the Global Tobacco Epidemic 2011: Warning About the Dangers of Tobacco. July 2011. Available at https://www.who.int/tobacco/global_report/2011/en/ [Accessed 24th September 2019] [Google Scholar]
- 43.GDB 2016 Risk Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2016;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown J., West R., Beard E., Michie S., Shahab L., McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: findings from a general population survey of smokers. Addict. Behav. 2014;39(6):1120–1125. doi: 10.1016/j.addbeh.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brose L.S., Hitchman S.C., Brown J., West R., McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160–1168. doi: 10.1111/add.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajek P., Phillips-Waller A., Przulj D., Pesola F., Smith K.M., Bisal N., Li J., Parrott S., Sasieni P., Dawkins L., Ross L., Goniewicz M., Wu Q., McRobbie H.J. A randomized trial of E-Cigarettes versus nicotine-replacement therapy. N. Engl. J. Med. 2019;380:629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 47.Hill A., Camacho O.M. A system dynamics modelling approach to assess the impact of launching a new nicotine product on population health outcomes. Regul. Toxicol. Pharmacol. 2017;86:265–278. doi: 10.1016/j.yrtph.2017.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.