Abstract
Culex species are the most widespread mosquito species across the world and are known to be highly opportunistic, feeding on humans and livestock. They are known to acquire the potential to transmit zoonotic diseases, including Rift Valley Fever (RVF). However, despite their public health significance, they remain understudied in North-western Nigeria, compared to Anophelines. This study was therefore aimed at determining the relative abundance and Multiplex polymerase chain reaction (Multiplex PCR) identification of members of the Culex pipiens complex, in Kura Local Government Area (LGA), North-western, Nigeria. Adult mosquitoes were collected using Center for Disease Control (CDC) miniature light traps from August to October 2019. Mosquitoes were identified using morphological identification keys. Members of the Culex pipiens complex were further identified using Multiplex PCR to assess the presence of sibling species. A total of 413 mosquitoes, belonging to 3 genera, Culex, Anopheles and Aedes were collected. Of this figure, 120 Culex spp. females were collected. Homes with livestock had the highest occurrence of mosquitoes, 123 (61.19%) compared to those without livestock, 78 (38.81%). There was no statistical difference among the two (2) categories of homes (P ≥ 0.005). Culicoides spp. were the most common with 130 collected (65.38%). Again, homes with livestock had the highest occurrence, 85 whilst homes without livestock had 45 of the other flies caught. Multiplex- PCR revealed no expected bands for Cx. quinquefasciatus and Cx. pipiens from the DNA obtained from field collected mosquitoes as confirmed by using genomic DNA of an insectary Culex quinquefasciatus as control. Cx. spp. is presently regarded as a biting nuisance having no significant epidemiological importance. Efforts at its control should be intensified before it is too late. This study provides useful information on the occurrence and multiplex PCR of Culex spp in Kura Local Government Area, North-western Nigeria. These results have implications for the control of Culex spp. mosquito populations and the spread of human, livestock and avian diseases.
Keywords: Aedes, Anophilines, Culex, Culicoides, Multiplex PCR, Nigeria
1. Introduction
Culex mosquitoes constitute a wide range of mosquitoes generally involved in the transmission of mosquito- borne arboviruses including; Sindbis virus, West Nile Virus, Equine encephalitis, St Louis, Oro-pouche fever, Avian malaria, Lymphatic filariasis and Rift valley fever (RVF) (Linthicum et al., 2016; Bellone and Anna-Bella, 2020). One of the most important groups in the Culex genus is Culex pipiens complex which comprises six members: Cx. quinquifasciatus Say, Cx. pallens Coquillet, Cx. australicus Dobrotworsky and Drummond, Cx. pipiens Linneaus, Cx. globocoxitus Dobrotworsky and Cx. molestus Forskll (Zittra et al., 2019). Members of the Culex pipiens complex are globally distributed important disease vectors (Nchoutpouen et al., 2019). Species of the Culex pipiens complex particularly Cx. quinquefasciatus are predominant in the urban environment notably in Africa including Nigeria where suitable environmental conditions created by rapid unplanned urbanization is contributing to their proliferation (Brown et al., 2008). Of the arboviral diseases, Rift Valley Fever Virus (RVFV) is transmitted by mosquitoes between animals and humans, particularly belonging to the Aedes, Culex and Anopheles genera (Seufi and Galal, 2010). Although there is no recent report on RVF outbreak in Nigeria, numerous studies revealed the occurrence of RVFV in Jigawa and Katsina (Adamu et al., 2020), Oyo (Opayele et al., 2018), Niger (Alhaji et al., 2018), Lagos and Borno (Olaleye et al., 1996), Kaduna and Sokoto States (Ezeifeka et al., 1982). Vector identification and control is quintessential to the success of the eradication of the RVF and other arboviral diseases. Therefore, the strategy of mosquito control is highly dependent on the vector competence of the Culex pipiens complex vector (Fall et al., 2014). However, this vector competence has not been evaluated, since it is quite difficult to distinguish Cx. pipiens, Cx. pallens, Cx. antennatus and Cx. quinquefasciatus morphologically (Shaikevich et al., 2016). Previous studies revealed the significance of molecular techniques associated with more precision and reliability than older morphological differentiation methods of species complex including multiplex polymerase reaction (Shahhosseini et al., 2018; Kayedi et al., 2020; Adugna et al., 2020; Kirby et al., 2008).
Knowledge on the particular species and siblings of these potential arboviral vectors is quintessential in the design of effective control strategies. There has been no published data on the relative abundance and molecular identification of Culex pipiens complex, in North-western Nigeria. The aim of this study therefore was to investigate the relative abundance and molecularly identufy Culex pipiens complex, the potential vectors of Rift valley fever (RVF), Lymphatic filariasis and West Nile Virus (WNV) in Kura LGA, North-western Nigeria.
2. Materials and methods
2.1. Study area
Kano State is located at latitude 11°30 U N and longitude 8°30 U E with a land area of 20,760 km2 (Adamu and Bassey, 2010). Its vegetation falls mostly within the Sudan Savanna Zone (Plate 1). Annual average rainfall in the area ranges from 884 to 1200 mm (from north to south of the state) which is characterized by one peak period (mono-modal), usually attained in August (Tanko and Momale, 2013). The rainy season is from May to October. The temperature is, on average, warm to hot throughout the year at about 25 ± 7 °C (Olofin and Tanko, 2002). The economy of the state is predominantly agricultural production with over 75% of the population engaged in farming. Livestock are also being raised especially cattle, sheep and goats (Haruna and Murtala, 2005).
Plate 1.
Map of Nigeria showing the geographical location of Kano State (A). Map of Kano State showing the study site: Kura Local Government Area (B).
2.2. Mosquito collection
Adult Mosquitoes were caught for three consecutive nights monthly (August–October 2019) during a visit Imawa village, North-western Nigeria using the CDC light trap (Model 512, J.W. Hock Ltd., Gainesville, Florida, USA).
2.2.1. Description of catch method in this study
Each trap was suspended from the roof about 1.8 m above the floor and/or the tree near the livestock pens. Collections were made between 18.00 h- 6.00 am in each of the homes with and homes without livestock selected as mosquito collection sites according to the method used by Obenauer et al. (Obenauer et al., 2013). The traps were modified with carbon-dioxide (CO2) and chemical lure according to the methods described in Obenauer et al. (Obenauer et al., 2013).
2.2.2. Preservation and morphological identification
All mosquitoes harvested in the traps were preserved in EDTA bottles in 70% alcohol and transported to the Entomology Laboratory, Department of Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria. Adult Mosquitoes were sorted according to their genera; Anopheles, Aedes and Culex spp. using keys described by Becker et al. (Becker et al., 2012) and, analysed with the aid of stereomicroscope. Female mosquitoes belonging to Culex pipiens complex were stored on silica in Eppendorf tubes and transported to the National Arbovirus and Vector Research Center (NAVRC), Enugu State, Nigeria for further molecular analysis.
2.3. Molecular identification of Culex pipiens complex
2.3.1. Isolation of genomic DNA
The extraction of Genomic DNA from the female Culex spp. of mosquitoes from was carried out using ZymoResearch DNA kit extraction (Cat. D 6015, ZymoResearch, California, CA, USA). The concentration and purity of the isolated DNA was analysed using a Nanodrop™ 1000 Spectrophotometer.
2.3.2. Multiplex polymerase chain reaction (PCR)
Molecular identification of Culex pipiens complex was conducted using the locus ACE2 gene of the mosquito through multiplex polymerase chain reaction as described by Smith and Fonseca (Smith and Fonseca, 2004).
Previously designed primers ACEpip for Culex pipiens (5′-GGA AAC AAC GAC GTA TGT ACT-3)′, ACEquin for Culex quinquefasciatus - (5′-CCTTCTTGAATGG CTG TGGCA-3); and the reverse primers B1246s (5′-TGGAGCCTCCTCTTCACGGC-3) (Nchoutpouen et al., 2019; Motayo et al., 2016). Multiplex PCR was performed in a 50 μl volume using 1 unit Taq 2× Master mix 25 μl (Cat. # K0701, Thermoscientific, Pittsburgh, PA, USA), 6 μl of genomic DNA template, 2 μl of each of following primers: ACE pip for Cx. pipiens, ACE quin for Cx. quinquefasciatus, and B1246s and 13 μl nuclease free water. The PCR reaction was performed with an Initial denaturation at 950C for 5 min, followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min with a final 5 min extension at 72 °C (Nchoutpouen et al., 2019).
2.3.3. Gel electrophoresis
The amplified DNA were loaded onto an agarose gel (2%) with the 1000-bp ladder loading marker (Bio-Rad, Richmond, Calif., USA), stained with ethidium bromide (Amresco Inc., Solon, Ohio, USA), run for 45 min at 120 V, and visualized on a UV trans-illuminator (Sigma-Aldrich Chemie GmbH, Germany). The gels showing only primer dimers indicating that DNAs were extracted but are not specific for the primers added were conducted in triplicates individually (Supplementary materials).
2.4. Data analysis
The data obtained from the studies were expressed as percentages and presented as tables. Statistical Package for Social Sciences (SPSS Version 20.0) was used for the analysis and Chi- square test association was used and values of P ≤ 0.05 were considered statistically significant.
3. Result
3.1. Entomological data
A total of (n = 413) adult mosquitoes, belonging to 3 genera, Culex, Anopheles and Aedes were caught and collected, of which Culex spp. had the highest occurrences; 201 species. Female were 120 (59.70%), while male were 81(40.29%). This was followed by Anopheles spp.; 117 species collected with 73 (36.32%) being female and 44 (21.89%) male. Aedes spp. were the lowest occurrence 95; female 62 (30.85%) and male were 33(16.41%) (Table 1).
Table 1.
Relative occurrence and sex distribution of mosquitoes caught in the study area.
| Sex |
|||||
|---|---|---|---|---|---|
| Genus | Total number of collected species | Female (%) | Male | (%) | |
| Culex | 201 | 120 | 59.70 | 81 | 40.29 |
| Anopheles | 117 | 73 | 36.32 | 44 | 21.89 |
| Aedes | 95 | 62 | 30.85 | 33 | 16.41 |
| Total | 413 | 255 | 158 | ||
3.2. Distribution of mosquitoes in the study area
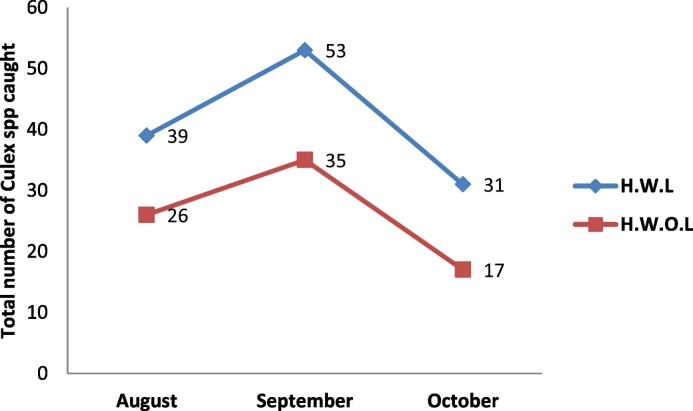
Monthly distribution of Culex spp. caught in the study area. Chi-square analysis of variance was used to analyze the data. In August, 65 Culex spp. were caught comprising of home with livestock and home without livestock. This indicates that the calculated chi-square of 6.00 and the P.value of 0.199 which is less than the calculated chi-square. The implication here is that August had highest calculated chi-square and P. value compared to the months of September and October (Fig. 1).
Fig. 1.
Monthly distribution of Culex spp. in homes with livestock (H.W.L) and homes without livestock (H.W.O.L) in the study area.
To the best of our knowledge in Kano, northwestern Nigeria, Culicoides spp. were caught (Supplementary materials). This is significant because of its zoonotic and public health importance.
4. Discussions
The mosquitoes belonging to the genera Culex (Table 1) and Culicoides (Table 3) collected and identified in Kura Local Government Area, North-western Nigeria are capable of transmitting pathogens causing many different arbo-viral diseases (Linthicum et al., 2016; Opayele et al., 2018; Nyakarahuka et al., 2018; Lagare et al., 2019). Little is known about the occurrence of Cx. pipiens complex mosquitoes in Kura Local Government Area. This information is quintessential for assessing the epidemic potential of RVF in Kano State. Vector control in the study area is majorly through the use of insecticides. Among the species collected, Culex spp. had the highest occurrences; 201 species. Females had the highest occurrences 120 (59.70%), while male had 81(40.29%). Similar high occurrences were recorded for Culex spp. (52.5%) in Makurdi, Nigeria (Msugh-Ter et al., 2017) but lower than the results recorded in Minna, Nigeria (24%) (Rabi'u and Ahmed, 2019).
These studies displayed for the first time ever the relative abundance and molecular characterisation of Cx. pipiens complex in Kura Local Government Area, North-western Nigeria, this information is necessary for establishing effective surveillance and targeted control programs to prevent or control RVF outbreaks. The abundance of hybrid mosquitoes increased in the area is between August and October associated with a decrease in temperature (Fig. 1).
Even though the multiplex PCR aimed at the molecular identification of the Culex pipiens complex and therefore the specific siblings and perhaps hybrid spp. are still not very clear (Supplementary materials), although this may not represent the true picture as the major limitations to this study were a smaller sample size of the field mosquitoes collected and our inability to consistently test each sampled field mosquito using PCR due to high cost. Hence, only a small proportion of identified mosquito species were tested using PCR. The presence of Culex mosquitoes encountered in the present study should be a source of concern to residents and the Kano State Government. It was noticeable that greater proportion of Culex mosquitoes than Aedes were collected (Table 1). A probable reason for this is that the former are usually more active from dusk to dawn (night-biting species) unlike Aedes that are mostly day-biting species (World Health Organization (WHO), 2013) (Table 3). Baba et al., 2006 in Nigeria, (Özer et al., 2007) in Turkey, LaBeaud et al. (LaBeaud et al., 2011) in Kenya, and Vaux et al. (Vaux et al., 2015) in the United Kingdom also collected greater proportion of Culex mosquitoes in their studies. This study revealed the abundance of Culicines for the first time in the study area and was found to be higher than other mosquito genera in houses with livestock. This is primarily because presence of livestock attracts Culicines into a compound as previously shown (Kirby et al., 2008; Forattini et al., 1993). More significantly however, RVF was previously isolated from Culicoides spp. and Culex antennatus in Ibadan, Nigeria (Fagbami and Ojeh, 1983; Oluwayelu et al., 2018).
5. Conclusions
This study provides an insight for the first time into the relative abundance of Culex mosquito species in Kura Local Government Area, Northwestern Nigeria. And is suggestive of the prevalence of vector-borne diseases such as yellow fever, dengue fever and filariasis.
in the study area. Even though the multiplex PCR aimed at the molecular identification of the Culex spp. and therefore the specific species perhaps hybrid spp. are still not very clear, this work shows the importance of the occurrence of these potential arboviral vectors in the study area. Of significance also was the identification of Culicoides spp. for the first time in the study area. Therefore, intensive vector control programmes and public enlightenment especially on human activities that encourage mosquito breeding are recommended. Further studies through designing and validating primers for Culex antennatus; an important species previously associated with transmission of RVF in Nigeria to reveal if these species are still in existence is recommended. It will also be interesting if the harvested Culex spp. mosquitoes are investigated using Real Time – Polymerase Chain Reaction (RT-PCR), to determine or otherwise the presence of the Rift Valley Virus in the study area.
Declaration of competing interest
The authors declare that there are no competing interests regarding the publication of this paper.
Acknowledgements
The authors thank Dr. C.S. Wilding, of Liverpool John Moores University, Liverpool L2 2QP, United Kingdom, for his helpful comments on the manuscript. We also thank Staff of the Center for Infectious Diseases, Bayero University, Kano and Molecular Biology unit of the National Arbovirus and Vector Research Center (NAVRC), Enugu State, Nigeria for their contribution in the conduct of this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parepi.2021.e00213.
Appendix A. Supplementary data
Supplementary material
References
- Adamu A.U., Bassey S.E. Incidence of Strongyloides stereoralis infection in Ungogo, Nassarwa, Dala and Fagge Local Government Areas of Kano State, Nigeria. Bayero J. Pure Appl. Sci. 2010;3:76–80. [Google Scholar]
- Adamu A.M., Yila S.A., Allam L., Sackey A., Alhaji N.B., Garba B.S., Owolodun O.A., Idoko S.I. Serological evidence of Rift Valley Fever infection and risk factors among one humped camels (Camelus dromedarius) in Northern Nigeria. BioRxiv. 2020 doi: 10.1101/2020.01.10.901470. preprint paper. [DOI] [Google Scholar]
- Adugna T., Getu E., Yewhalaw D. Species diversity and distribution of Anopheles mosquitoes in Bure district, Northwestern Ethiopia. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaji N.B., Babalobi O.O., Isola T.O. A quantitative exploration of nomadic pastoralists’ knowledge and practices towards Rift Valley fever in Niger state, North-central Nigeria: the associated socio-cultural drivers. One Health. 2018;6:16–22. doi: 10.1016/j.onehlt.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M.M., Saron M.-F., Diop O., Mathiot C., Adeniji J.A., Olaleye O.D. West Nile virus in mosquitoes and febrile patients in a semi-arid zone, Nigeria. J. Am. Sci. 2006;2(2):28–34. [Google Scholar]
- Becker N., Jöst H., Ziegler U., Eiden M., Höper D., Emmerich P., Hoffmann B. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone R., Anna-Bella F. Temperature in shaping mosquito-borne viruses transmission. Front. Microbiol. 2020;11:2388. doi: 10.3389/fmicb.2020.584846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H.E., Childs J.E., Diuk-Wasser M.A., Fish D. Ecologic factors associated with West Nile virus transmission, North eastern United States. Emerg. Infect. Dis. 2008;14(10):1539–1545. doi: 10.3201/eid1410.071396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeifeka G.O., Umoh J.U., Belino E.D., Ezeokoli C.D. A serological survey for Rift Valley fever antibody in food animals in Kaduna and Sokoto states of Nigeria. Int. J. Zoonoses. 1982;9(2):147–151. [PubMed] [Google Scholar]
- Fagbami A.H., Ojeh C. Arthropod-borne viral infections of livestock in Nigeria. Trop. Vet. 1983;1(2):61–69. [Google Scholar]
- Fall G., Diallo M., Loucoubar C., Faye O. Vector competence of Culex neavei and Culex quinquefasciatus (Diptera: Culicidae) from Senegal for lineages 1, 2, Koutango and a putative new lineage of West Nile virus. Am. J. Trop. Med. Hyg. 2014;90(4):747–754. doi: 10.4269/ajtmh.13-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forattini O.P., Kakitani I., Massad E., Marucci D. Studies on mosquitoes (Diptera: Culicidae) and anthropic environment: 4-survey of resting adults and synanthropic behaviour in south-eastern, Brazil. Rev. Saude Publica. 1993;27:398–411. doi: 10.1590/s0034-89101993000600002. [DOI] [PubMed] [Google Scholar]
- Haruna U., Murtala N.A. 2005. Commodity Chain Analysis of Cattle Marketing in Nigeria; A Case Study of KRIP Area Kano State. A Report Submitted to ADENI Project/NAERLS; pp. 13–14. [Google Scholar]
- Kayedi M.H., Sepahvand F., Mostafavi E., Chinikar S., Mokhayeri H., Chegheni Sharafi A., Wong G. Morphological and molecular identification of Culicidae mosquitoes (Diptera: Culicidae) in Lorestan province, WesternIran. Heliyon. 2020;6(8) doi: 10.1016/j.heliyon.2020.e04480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby M.J., West P., Green C., Jasseh M., Lindsay S.W. Risk factors for house-entry by Culicine mosquitoes in a rural town and satellite villages in the Gambia. Parasit. Vectors. 2008;1(1):41. doi: 10.1186/1756-3305-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeaud A.D., Sutherland L.J., Muiruri S., Muchiri E.M., Gray L.R., Zimmerman P.A., King C.H. Arbovirus prevalence in mosquitoes, Kenya. Emerg. Infect. Dis. 2011;17(2):233. doi: 10.3201/eid1702.091666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagare A., Fall G., Ibrahim A., Ousmane S., Sadio B., Abdoulaye M., Zaneidou M. First occurrence of Rift Valley fever outbreak in Niger, 2016. Vet. Med. Sci. 2019;5(1):70–78. doi: 10.1002/vms3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum K.J., Britch S.C., Anyamba A. Rift Valley fever: an emerging mosquito-borne disease. Annu. Rev. Entomol. 2016;61:395–415. doi: 10.1146/annurev-ento-010715-023819. [DOI] [PubMed] [Google Scholar]
- Motayo B.O., Onoja B.A., Faneye A.O., Adeniji J.A. Seasonal abundance and molecular identification of West Nile virus vectors, Culex pipens and Culex quinquefasciatus (Diptera: Culicidae) in Abeokuta, South-West, Nigeria. Afr. Health Sci. 2016;16(1):135–140. doi: 10.4314/ahs.v16i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msugh-Ter M.M., Palegh A., Aondowase D.A. Anopheles and Culex Mosquito species diversity and its epidemiological implications in the Makurdi area of Benue state, North Central Nigeria. Ann. Exp. Biol. 2017;5(1):9–13. [Google Scholar]
- Nchoutpouen E., Talipouo A., Djiappi-Tchamen B., Djamouko-Djonkam L., Kopya E., Ngadjeu C.S. Culex species diversity, susceptibility to insecticides and role as potential vector of Lymphatic filariasis in the city of Yaoundé, Cameroon. PLoS Negl. Trop. Dis. 2019;13(4) doi: 10.1371/journal.pntd.0007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakarahuka L., Maurice A.D.S., Purpura L., Ervin E., Balinandi S., Tumusiime A., Klena J.D. Prevalence and risk factors of Rift Valley fever in humans and animals from Kabale district in southwestern Uganda, 2016. PLoS Negl. Trop. Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauer P., Abdel-Dayem M., Stoops C., Villinski J., Tageldin R., Fahmy N. Field responses of Anopheles gambiae complex (Diptera: Culicidae) in Liberia using yeast-generated carbon dioxide and synthetic lure-baited light traps. J. Med. Entomol. 2013;50(4):863–870. doi: 10.1603/me12174. [DOI] [PubMed] [Google Scholar]
- Olaleye O.D., Tomori O., Schmitz H. Rift Valley fever in Nigeria: infections in domestic animals. Rev. Sci. Tech. OIE. 1996;15(3):937–946. doi: 10.20506/rst.15.3.966. [DOI] [PubMed] [Google Scholar]
- Olofin E.A., Tanko A.I. Department of Geography, Bayero University; Kano: 2002. Laboratory of Areal Differentiation: Metropolitan Kano Geographical Perspective. [Google Scholar]
- Oluwayelu D., Adebiyi A., Tomori O. Endemic and emerging arboviral diseases of livestock in Nigeria: a review. Parasit. Vectors. 2018;11(1):1–12. doi: 10.1186/s13071-018-2911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opayele A.V., Odaibo G.N., Olaleye O.D. Rift valley fever virus infection among livestock handlers in Ibadan, Nigeria. J. Immunoass. Immunochem. 2018;39(6):609–621. doi: 10.1080/15321819.2018.1525739. [DOI] [PubMed] [Google Scholar]
- Özer N., Ergünay K., Simsek F., Kaynas S., Alten B., Caglar S.S., Ustacelebi S. West Nile virus studies in the Sanliurfa Province of Turkey. J. Vector Ecol. 2007;32(2):202–206. doi: 10.3376/1081-1710(2007)32[202:wnvsit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rabi’u H.M., Ahmed A. A preliminary study on the abundance and species composition of mosquitoes breeding in discarded automobile tyres in Minna, Niger State, Nigeria. Int. J. Mosq. Res. 2019;6(1):119–123. [Google Scholar]
- Seufi A.M., Galal F.H. Role of Culex and Anopheles mosquito species as potential vectors of rift valley fever virus in Sudan outbreak, 2007. BMC Infect. Dis. 2010;10(1):65. doi: 10.1186/1471-2334-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahhosseini N., Kayedi M.H., Sedaghat M.M., Racine P.T., Kobinger G., Moosa-Kazemi S.H. DNA barcodes corroborating identification of mosquito species and multiplex real-time PCR differentiating Culex pipiens complex and Culex torrentium in Iran. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikevich E.V., Vinogradova E.B., Bouattour A., De Almeida A.P.G. Genetic diversity of Culex pipiens mosquitoes in distinct populations from Europe: contribution of Cx. quinquefasciatus in Mediterranean populations. Parasit. Vectors. 2016;9(1):47. doi: 10.1186/s13071-016-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Fonseca D.M. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae) Am. J. Trop. Med. Hyg. 2004;70(4):339–345. [PubMed] [Google Scholar]
- Tanko A.I., Momale S.B. Adonis and Abbey Publishers Ltd; New York, USA: 2013. Geography of Kano Region; p. 33. [Google Scholar]
- Vaux A.G., Gibson G., Hernandez-Triana L.M., Cheke R.A., McCracken F., Jeffries C.L., Leach S. Enhanced West Nile virus surveillance in the North Kent marshes, UK. Parasit. Vectors. 2015;8(1):1–8. doi: 10.1186/s13071-015-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO; Geneva: 2013. World Malaria Report 2013.http://www.who.int/malaria/publications/world_malaria_report_2013/en/ [Google Scholar]
- Zittra C., Moog O., Christian E., Fuehrer H.P. DNA-aided identification of Culex mosquitoes (Diptera: Culicidae) reveals unexpected diversity in underground cavities in Austria. Parasitol. Res. 2019;118(5) doi: 10.1007/s00436-019-06277-y. 1385–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material