Summary
Recent engineering technologies have transformed traditional perspectives of cancer to include the important role of the extracellular matrix (ECM) in recapitulating the malignant behaviors of cancer cells. Novel biomaterials and imaging technologies have advanced our understanding of the role of ECM density, structure, mechanics, and remodeling in tumor cell-ECM interactions in cancer biology and have provided new approaches in the development of cancer therapeutics. Here, we review emerging technologies in cancer ECM biology and recent advances in engineered systems for evaluating cancer therapeutics and provide new perspectives on how engineering tools present an opportunity for advancing the modeling and treatment of cancer. This review offers the cell biology and cancer cell biology communities insight into how engineering tools can improve our understanding of cancer ECM biology and therapeutic development.
Subject areas: Biological sciences, Cell biology, Cancer
Graphical abstract
Biological sciences; Cell biology; Cancer
Introduction
The extracellular matrix (ECM) is most commonly defined as a complex 3D network of macromolecules that play an essential role in all tissues by providing both structural support and essential biochemical interactions to its cellular constituents. Once believed to be merely an intercellular filling, the ECM is a physiologically active component of living tissue, regulating diverse cellular processes during embryogenesis, tissue repair, homeostasis, and pathogenesis. The native ECM acts as an environmental niche that is actively interacting with the cells in a dynamic and tissue-specific manner. Fundamentally, the ECM primarily comprises of collagen, enzymes, glycosaminoglycans, and glycoproteins, such as laminin and fibronectin. These collections of highly conserved bioactive, structural and functional molecules regulate cellular functions by providing structural support, biological signaling, modulation of immune responses, and recruitment of cells. Importantly, ECM composition, biomechanics, and in most cases anisotropy are precisely tuned to serve a particular tissue-specific purpose (Bonnans et al., 2014; Badylak et al., 2009).
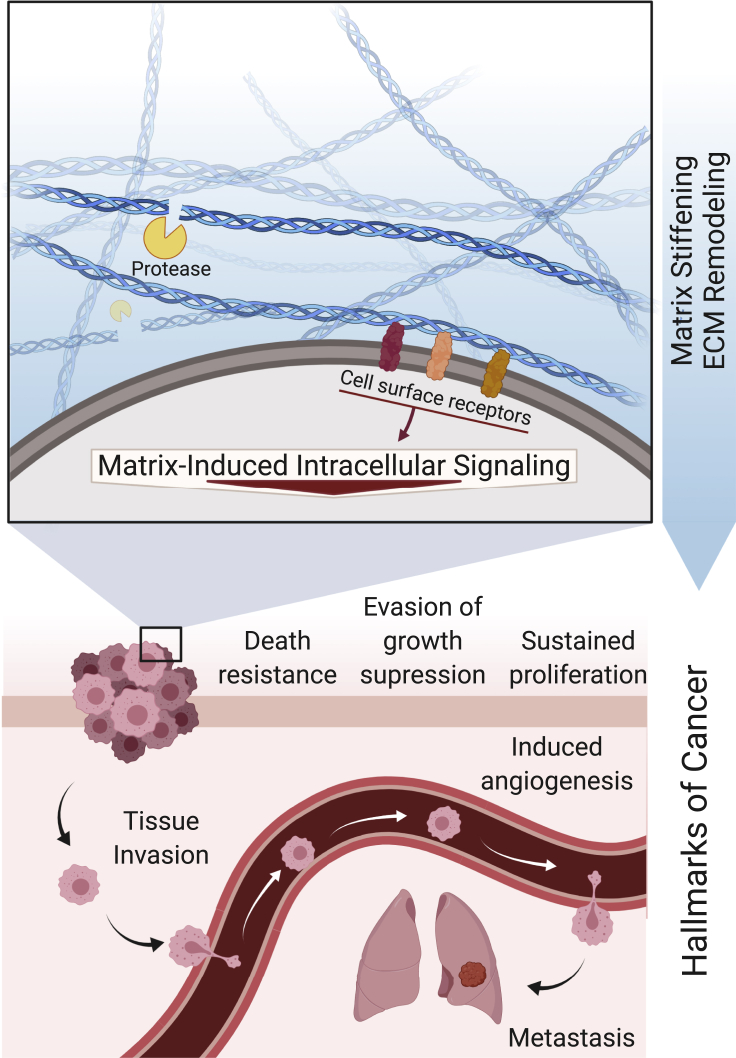
Interactions between cells and ECM components involve mechanical and biochemical signals that are responsible for fundamental cellular activities such as cell-cell communication, cell adhesion, proliferation, and differentiation. Dysregulation of cell-ECM interactions produces changes to ECM composition and remodeling, disturbing tissue homeostasis and contributing to pathological conditions, such as cancer (Bonnans et al., 2014; Chaudhuri et al., 2020; Nia et al., 2020). Indeed, it is now well established that an altered ECM is capable of modulating hallmarks of cancer by supporting sustained proliferation of cancer cells, evasion of growth suppression, resistance to cell death, angiogenesis, tissue invasion, and ultimately metastasis (Figure 1) (Pickup et al., 2014; Hanahan and Weinberg, 2000). Discrete biophysical and biochemical cues from the tumor-associated ECM (e.g. increased stiffness, changes in presentation of cell adhesion sites) influence the intrinsic mechanisms of cancer development and resistance to therapy (Pickup et al., 2014). The number of clinical and basic science studies on the relevance of ECM properties in cancer has greatly increased over the last decade in an attempt to understand the dynamic reciprocal feedback between the ECM and tumor cells that both change in space and time (Laklai & et al., 2016; Fernández-Sánchez & et al., 2015; Gan & et al., 2018; Liu et al., 2018; Chen & et al., 2019; Stephan & et al., 2015; Chakravarthy et al., 2018).
Figure 1.
Tumor ECM
From tumor initiation to metastasis, the ECM influences each of the classically defined and emerging hallmarks of cancer as first described by Hanahan and Weinberg, 2000 and amended in 2011. Dysregulated changes in ECM properties, such as increased stiffness and protease-mediated remodeling, mediate cell receptor-ECM interactions that activate intracellular signaling pathways that promote sustained cancer cell proliferation, evasion of growth suppression, death resistance, induced angiogenesis, tissue invasion, and metastasis. Created with BioRender.com
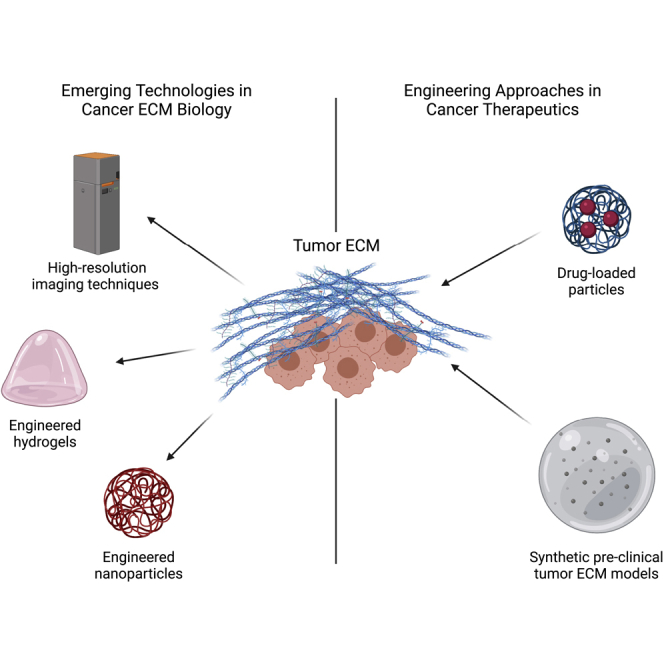
Here, we discuss the current and emerging technologies that are serving as tools for the understanding of the role of dysregulated ECM properties in cancer and as platforms for developing novel cancer therapeutics. We first review the basic science findings on how ECM composition, structure, biomechanics, remodeling, and interactions with tumor cells contribute to modeling of cancer, with an emphasis on biomaterials and high-resolution imaging techniques. Moreover, as many of these technologies offer high tunability, we review recent advances in their adaptation to novel approaches in cancer therapeutics, with an emphasis on engineered and microstructures/nanostructures, such as hydrogels and nanoparticles. Finally, we provide new perspectives on how engineering tools present an opportunity to move forward the understanding of the role of ECM properties in cancer, as well as for the treatment of cancer.
Material properties of the cancer extracellular matrix
The ECM is a biological material that is composed of a meshwork of proteins, where each protein component has a particular structure and biological function. When presented in combination, these proteins orchestrate a cascade of cellular responses that direct tissue organization and function, which can translate to embryogenesis, tissue repair, homeostasis, and pathogenesis. The ECM can be described in terms of material properties such as stiffness, protein composition, and organization. The interrelated ECM characteristics are constantly modified and restructured by harbored cells that conversely respond to ECM signals via cell adhesion receptors and enzymes, forming a feedback loop that results in a dynamically evolving ECM in its native environment. This feedback loop is altered in cancer, underlining the important role of cell-ECM interactions in the initiation and progression of this harmful disease (Figure 1).
As the complex, interrelated ECM physicochemical properties are difficult to uncouple using conventional cell and molecular biology methods, unconventional approaches are needed to better understand the independent role of material properties of the ECM in cancer. In this section, we describe emerging technologies that can help elucidate the contributions of aberrant changes in properties of tumor ECM to tumorigenesis and cancer progression.
High-resolution imaging techniques reveal the role of ECM protein organization in cancer progression and diagnosis
It is well known that aberrant changes in ECM stiffness provide biochemical and mechanical support for tumor progression (Lu et al., 2012). As ECM stiffness can be affected by changes in protein organization, structure, and density, recent focus has shifted to the underlying physical mechanisms that affect ECM stiffness in cancer, as this knowledge can lead to new diagnostic methods. Current clinical tomographic modalities such as computed tomography, magnetic resonance imaging, and positron emission tomography are limited by macro level resolution (∼1 mm) that cannot capture the submicron characteristics of ECM components (Campagnola, 2011). In this context, high-resolution nonlinear optical microscopy techniques, such as second-harmonic generation (SHG), coherent anti-Stokes Raman, and multiphoton excitation, which eliminate the pitfalls of both noninvasive imaging (lack of resolution/specificity) and traditional histology (subjectivity), have shown potential as quantitative optical microscopy approaches to quantify tissue structural changes during disease progression (Campagnola, 2011). For instance, although SHG imaging was first reported in 1986, in the past decade, it has emerged as a potential powerful tool for non-invasive early cancer diagnostics by enabling visualization and quantitation of collagen-based changes at cellular resolution in pancreatic (Drifka & et al., 2015), breast (Ambekar et al., 2012), ovarian (Tilbury and Campagnola, 2015; Nadiarnykh et al., 2010), and many other types of cancer (Campagnola and Dong, 2011; Drifka & et al., 2016; Chen & et al., 2010).
Drifka et al.(Drifka & et al., 2015) utilized SHG imaging to demonstrate differences in stromal collagen organization and structure (both of which have been correlated to ECM stiffness (Bonnans et al., 2014; Leonard & et al., 2019)) in human pancreatic ductal adenocarcinoma (PDAC) tissues compared with non-neoplastic tissues. Their work demonstrated that there is a characteristic and quantifiable collagen alignment surrounding the malignant epithelium that allows distinction of PDAC tissue from benign tissue, providing unprecedented insight into tumor growth and metastasis. In breast cancer, stromal collagen topology also correlates with great propensity to tumor metastasis and negative prognosis (Provenzano & et al., 2006; Conklin & et al., 2011). In fact, an in vitro study using 3D culture of breast cancer cells within collagen matrices suggested that the pathological consequence of collagen alignment is increased ECM stiffness (Riching & et al., 2014). Another study utilizing SHG imaging of biopsied tissue sections of breast cancer found that collagen alignment contributed to tumor cell migration toward the vasculature (Provenzano & et al., 2006; Fraley & et al., 2015), emphasizing the significance of visualizing biological structures of the tumor ECM in all their complexity for better assessment of clinical outcomes and to inform the design of in vitro pre-clinical cancer models that better recapitulate features of the tumor ECM.
Multiplexed imaging methods are becoming increasingly important as imaging tools for both basic science and clinical research (Baharlou et al., 2019). Recently, mass cytometry imaging (MCI) approaches, which enable simultaneous visualization of up to 40 proteins, have been developed to provide information at subcellular resolution regarding complex single-cell phenotypes and their spatial context (Jackson & et al., 2020; Ali & et al., 2020). This information is currently not reflected in the histological stratification that is the foundation of many clinical decisions. Several studies have used this powerful imaging technique to reveal that distinct combinations of multicellular phenotypes in breast cancer tissue can be medically relevant, suggesting that tissue architecture and organization could influence disease outcomes (Jackson & et al., 2020; Ali & et al., 2020). In this context, mass spectrometry-based strategies have great potential for investigating how local changes in ECM protein structure influence cancer progression (Angel & et al., 2018). A detailed understanding of the native ECM protein architecture, such as chemical features and localization of specific collagen isoforms in the complex tumor setting, can facilitate the design of in vitro tumor ECM models that preserve innate tumor ECM characteristics and thus better recreate the tumor microenvironment. Insights into biomaterial design have become increasingly important as new studies highlight the impact of early protein deposition (nascent protein) on cell behavior within 3D in vitro hydrogel models (Loebel et al., 2019; Chen et al., 2017), which may have significant implications for in vitro cancer cell signaling studies.
Consequently, the continued application of high-resolution imaging in cancer research and diagnostics can inform clinical outcomes and the design and implementation of 3D in vitro pre-clinical models that recapitulate the contributions of ECM topology and organization to the progression and metastasis of cancer. Conversely, high-resolution imaging techniques can further reveal the nascent functional organization of ECM proteins, their contribution to cancer progression, and utilization of cell regulation by initial matrix composition in engineered tumor models.
Engineered 3D hydrogels elucidate the role of ECM protein density in cancer cell migration
In addition to protein topology, ECM stiffness is directly related to changes in protein density. In cancer, increased protein density is present due to excessive protein deposition by cancer cells and cancer-associated fibroblasts. Cancer cells are able to sense changes in ECM protein density and respond in ways that lead to classically defined tumorigenic events, such as tumor cell invasion and metastasis (Chaudhuri & et al., 2014; Rice & et al., 2017; Sapudom and Pompe, 2018). For instance, during gastric cancer (GC) progression, increased ECM deposition, notably type I collagen, correlates with an overall increase in expression of the mesenchymal phenotype, as the intestinal epithelium exhibits impaired cell-cell adhesion and enhanced cell-ECM adhesion (Jang & et al., 2018). For decades, biological matrices, such as type I collagen gels, have been used as in vitro 3D culture platforms for cancer cells to better understand the underlying mechanisms of cancer progression in response to matrix density (Thakuri et al., 2017). For instance, Jang et al. utilized a density-varying type I collagen matrix to demonstrate that, in response to greater collagen density, human gastric adenocarcinoma cells showed an overall increase in a mesenchymal phenotype via increased cell proliferation, increased integrin-mediated cell-ECM interactions, and reduced intercellular adhesion to the E-cadherin/β-catenin complex via phosphorylated FAK and ERK signaling (Jang & et al., 2018; Sulzmaier et al., 2014).
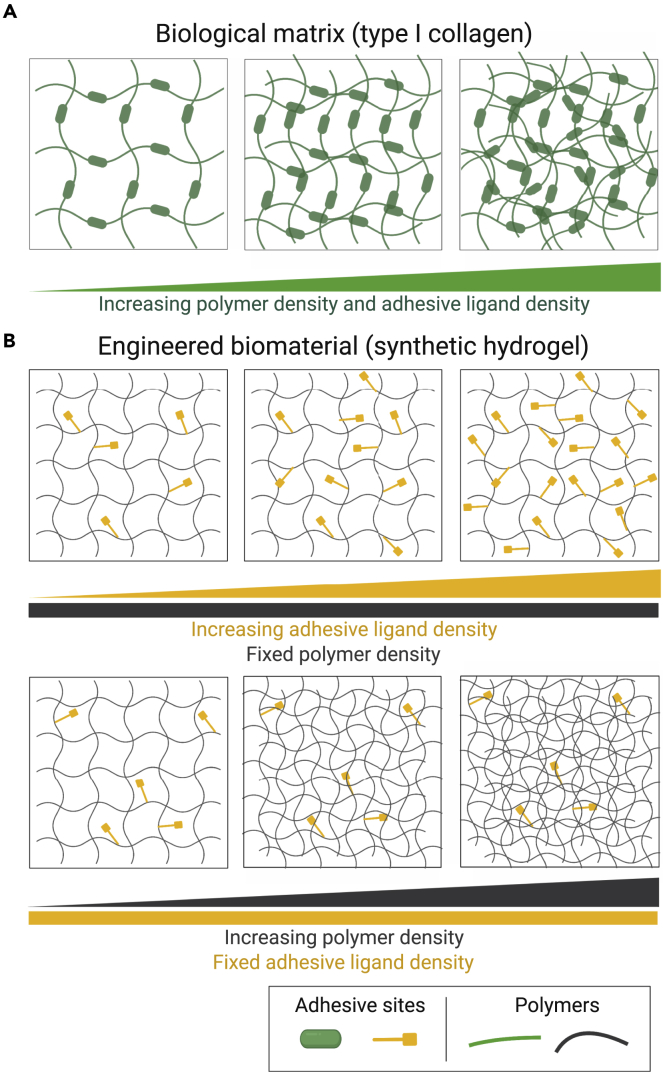
Despite these advances, biological matrices are inherently limited by the inability to decouple their biochemical and biomechanical properties (Yu & et al., 2005; Hughes et al., 2010). For instance, an increase in matrix density by changes to the bulk concentration of collagen gels unavoidably alters other matrix properties, such as protein fiber structure and adhesive ligand density (Figure 2A) (Cruz-Acuña and García, 2016). Therefore, although an increased bulk concentration of collagen matrices promoted epithelial GC cell malignancy via changes in cell-ECM adhesion and intercellular integrity, it is unknown whether this effect was mediated by differences in biochemical or mechanical matrix properties between different collagen gel formulations. These limitations motivate the need for engineered biomaterial designs that allow independent control over physicochemical properties while bearing appropriate features to support in vitro tumor culture models.
Figure 2.
Tunability of physicochemical matrix properties of biological and synthetic matrices
(A) Biological matrices such as type I collagen gels allow changes in matrix stiffness by increasing polymer density. However, increasing polymer density also changes adhesive ligand density, as well as fiber structure and organization.
(B) Engineered hydrogel matrices, such as PEG-based hydrogels, allow independent control over polymer and adhesive ligand densities. Created with BioRender.com. Adapted from Experimental Cell Research, Volume 377, Cruz-Acuña, R & García, AJ, Engineered materials to model human intestinal development and cancer using organoids, Pages 109–114 (2019), with permission from Elsevier.
Indeed, several groups have reported seminal work in studying the individual contributions of matrix properties to cancer progression and metastasis with the use of engineered biomaterials (e.g. synthetic 3D hydrogels; Figure 2B) with tunable physicochemical properties that overcome many of the limitations of natural matrices (Beck et al., 2013; Singh & et al., 2015; Singh et al., 2014; Casey & et al., 2017; Cassereau et al., 2015; Micek et al., 2020). Beck et al. (Beck et al., 2013) developed a composite hydrogel to characterize the contributions of matrix stiffness in mammary tumor cell dissemination independent of biochemical signals. The hydrogel was composed of poly(ethylene glycol) (PEG) networks that facilitated precise control over the mechanical properties of the matrix (Figure 2B) and could be used in combination with Matrigel to resemble the laminin-rich basement membrane. PEG-Matrigel composite hydrogels with a narrow range of matrix elasticity and adhesive peptide density supported dissemination of malignant mammary epithelial cells. However, as Matrigel is limited by lot-to-lot variabilities and contains unspecified tumor molecules, other groups have focused on engineering fully defined synthetic hydrogels with ECM-mimicking components such as adhesive peptide sequences or matrix metalloproteinase (MMP)-degradable cross-links that provide bioactivity to the otherwise biologically inert 3D matrix. For instance, peptide-functionalized PEG hydrogels have served as a 3D platform for investigating aspects of tumor cell migration in human melanoma patient-derived xenograft tumors in response to changes in polymer density (Singh & et al., 2015). Moreover, as the typical structure of PEG-based hydrogel systems is characterized as nanoporous, other studies have focused on designing ECM-inspired 3D hydrogel platforms that recapitulate the microscale porosity (Chiellini & et al., 2016), fibrillar structure (Ashworth & et al., 2020), and anisotropy (Prince and Kumacheva, 2019) of tumor ECM to better understand the independent role of polymer density in cancer cell migration.
Advances in decellularization methods have also encouraged attention to tissue-derived, decellularized ECM in vitro scaffolds to model the role of matrix properties in development and disease, including cancer (Guruswamy Damodaran and Vermette, 2018). These matrices can be obtained from animal or patient-derived tissues, thus containing native ECM proteins and proteoglycans and maintaining the original ECM structure, which play a major role in matrix integrity (Fitzpatrick and McDevitt, 2015; Landberg & et al., 2020). Indeed, biological scaffolds have been useful tools to uncover unique information about the malignancy-inducing properties of the tumor microenvironment and can potentially be used for the identification of effective cancer treatments (Landberg & et al., 2020; Yi & et al., 2019; Sensi & et al., 2020). Yet, the biological complexity of cell-derived ECM has presented challenges to controlling the modulation of matrix properties without affecting the integrity of the biomolecules. Consequently, novel approaches to chemically functionalize these natural matrices, such as incorporation of azide moieties via metabolic glycoengineering (Gutmann et al., 2018; Ruff & et al., 2017), may provide a solution by facilitating covalent integration of biologically active groups and construction of hybrid hydrogels that allow improved control over matrix properties (Fitzpatrick and McDevitt, 2015; Petrou & et al., 2020). Chemical modifications of decellularized ECM scaffolds have increased their attractiveness as an engineered biomaterial platform for the study of dynamic ECM-directed functions of cancer cells, while preserving their inherent tissue-specific bioactivity and structural properties.
Altogether, these studies reveal that a specific combination of biophysical and biochemical signals can induce cancer cell dissemination and migration, thus underscoring the advantages of utilizing engineered hydrogels as in vitro 3D scaffolds to dissect the independent contribution of matrix properties in cancer cell migration while recapitulating the native tumor ECM. Furthermore, the continued evolution of 3D hydrogel designs toward biomaterials that can structurally and mechanically recapitulate the tumor ECM and overcome the limitations of biological matrices is paramount to future insights into cancer progression in the context of tumor ECM properties. A recent review by Micek et al. (Micek et al., 2020) provides detailed discussion of this approach.
Engineered nanoparticles dissect the effect of loss of cell-ECM mechanoreciprocity in tumorigenesis
Accumulating evidence demonstrates that ECM properties can directly regulate tissue homeostasis (Sawada & et al., 2006; Wang et al., 2019). For instance, cells can sense ECM mechanical forces through mechanoreceptors and respond by exerting reciprocal cytoskeletal-dependent force by a process termed “mechanoreciprocity” (Butcher et al., 2009; van Helvert et al., 2018). Simply put, mechanoreciprocity occurs when cells modify their behavior and remodel their microenvironment as a response to forces exerted by the ECM. This adaptation has been proposed to involve a combination of epigenetic chromatin remodeling events and direct physical links between the ECM and cell nucleus that regulate gene expression (Ingber, 2006; Maniotis et al., 1997). However, when mechanoreciprocity is lost, these gene regulatory processes are altered and can promote cancer progression (Paszek & et al., 2005). Thus, the changing force that cells experience needs to be considered when trying to understand the complex nature of tumorigenesis.
Recent work has shown that mechanical strain, such as that produced by the tumor microenvironment, leads to oncogene expression in non-tumorous colon explants through the activation of the mechanoresponsive β-catenin oncogenic pathway in normal epithelial cells (Whitehead & et al., 2008). Thus, Fernández-Sánchez et al. (Fernández-Sánchez & et al., 2015) developed a method that allows the delivery of defined mechanical pressure in vivo, by implanting a magnet near the mouse colon that generates a magnetic force on nanoparticle-containing liposomes in the mesenchymal tissue. The engineered magnetic liposomes delivered pressure to the colon that quantitatively mimicked the endogenous early tumor growth stress activating tumorigenic mechanosensitive pathways, including phosphorylation and nuclear translocation of β-catenin. Mechanical activation of the tumorigenic β-catenin pathway led to the formation of early tumorous aberrant crypt foci. This study provides new insights regarding how the loss of mechanoreciprocity contributes to early events in tumorigenesis in normal tissues neighboring a tumor, establishing an unstable positive feedback loop between oncogene expression and tumor induction. This study established an innovative approach to dissect the role of mechanical stimulation to activate oncogenic pathways without changing other interrelated material properties (e.g. polymer density, topography). This is particularly important as an increasing number of studies report new correlations between increased expression of mechanoreceptors/activation of mechanoreceptor-linked oncogenic pathways relevant to the diagnosis and prognosis of distinct types of cancers (Gan & et al., 2018; Ren et al., 2014; Gong & et al., 2019; Wei & et al., 2015). Therefore, increasing efforts in the utilization of advanced engineering approaches that dissect the independent contributions of matrix properties in cancer can provide insights into better understanding the underlying mechanisms of cancer and potentially find novel therapeutic targets.
Dynamic hydrogels elucidate the role of ECM remodeling in cancer progression
ECM remodeling is a dynamic process in which cells change the overall abundance, concentration, structure, and organization of individual ECM protein components. The dynamic production and degradation of proteins of the ECM directly impact the three-dimensional spatial topology of the matrix around cells, its biochemical and biophysical properties, and consequently its effect on cell fate. ECM remodeling is an essential and tightly regulated physiological process in development and plays an important role in the maintenance of tissue homeostasis through a myriad of cell-ECM interactions (Bonnans et al., 2014). ECM remodeling is dysregulated in pathologic conditions such as inflammatory diseases, tissue fibrosis, and cancer (Winkler et al., 2020).
Proteolytic enzymes play a central role in remodeling the ECM in development, maintenance of tissue homeostasis, and disease by catalyzing the reactions that culminate in alterations in the chemical structure of ECM protein components (Lei & et al., 2020). MMPs are zinc-dependent endopeptidases and represent one of the major classes of enzymes involved in ECM remodeling during tumor progression. Elevated levels of distinct subtypes of MMPs have been established as prognostic indicators in cancer as MMPs facilitate cancer cell survival and invasion, metastasis development and angiogenesis, and malignant transformation (Palumbo & et al., 2020; Vihinen and Kähäri, 2002). In esophageal carcinoma (EC), MMPs function as potential biomarkers as distinct MMP subtypes, such as MMP-1, -7, -9, and -14, have been associated with EC diagnosis and/or prognosis (Yamashita et al., 2001; Yamashita et al., 2000; Garalla & et al., 2018; Ohashi et al., 2000; Lu & et al., 2019).
In PDAC, upregulation of a wide number of MMP subtypes has been associated with poor prognosis; however, the generalized notion that MMPs drive PDAC progression is still arguable, due to inconsistent insights on underlying mechanisms and the lack of appropriate pre-clinical models (Slapak et al., 2020). Increased expressions of MMP-2, -7, and -14 have been well established to potentiate tumor growth and/or metastasis in multiple independent studies (Koikawa & et al., 2018; Lu & et al., 2012; Resovi & et al., 2018; Krantz & et al., 2011). To investigate cancer cell responses to ECM in vitro, most studies utilized non-physiologically stiff 2D tissue culture plates that lack 3D cellular interactions or 3D hydrogels with static or degrading mechanical properties (Kirschner and Anseth, 2013). Unfortunately, these convenient culture platforms do not capture the dynamic landscape of a stiffening tumor microenvironment (Cruz-Acuña and García, 2019). In this context, Liu and colleagues engineered a dynamic gelatin-hyaluronic acid hybrid hydrogel system that allowed enzyme-triggered stiffening and degradation to understand the effects of MMP activation during matrix stiffening in PDAC cell fate in a 3D environment (Liu et al., 2018). This 3D cell-laden, engineered matrix overcomes limitations of current in vitro models by establishing a physiologically relevant protein composition and cell-directed, dynamic physicochemical properties of the matrix. The bioactive peptide sequences on gelatin permitted cell adhesion and MMP-mediated local matrix cleavage, and gelatin-conjugated moieties allowed sensitivity to on-demand cross-linking, which led to physiologically relevant stiffening in the presence of PDAC cells. Importantly, hyaluronic acid (HA), an essential tumor matrix glycoprotein, was modularly and covalently incorporated into the hydrogel. HA has a leading role in PDAC development (Sato et al., 2016; Heeg & et al., 2016); however, a recent clinical trial failed to show its promise as a potential therapeutic target (Doherty et al., 2017). Notably, the authors reported that upon matrix stiffening, cell-directed matrix remodeling was mediated by increased levels of MMP-2 and -7, consistent with some previous reports (Slapak et al., 2020; Koikawa & et al., 2018; Lu & et al., 2012; Resovi & et al., 2018). Stiffening of HA-containing hydrogels promoted upregulation of a number of canonical oncogenes associated with PDAC tumorigenesis and metastasis, such as KRAS, SRC, and NOTCH1, in encapsulated cancer cells. These findings are significant as they suggest that engineered hydrogels can potentially be used as pre-clinical in vitro models to study the influence of matrix components on the expression and activity of MMPs or as tunable platforms to test the efficacy of anti-MMP therapeutics for cancer treatment.
Moreover, biomaterial designs that allow cell-mediated enzymatic degradation or light-mediated reactions that enabled matrix mechanics to change in the presence of cells are often irreversible mechanisms, leading to hydrogel erosion over time or the inability to recapitulate the cyclical changes in ECM mechanics and remodeling. Therefore, to recapitulate the dynamic nature of the ECM, many reversible chemistries have been incorporated into hydrogels to enable the spatiotemporally controlled addition and removal of biochemical signals and, ultimately, regulate cell spreading and dynamic biochemical ligand presentation and matrix mechanics (Rosales and Anseth, 2016). For example, hydrogels can be designed to have reversible dynamic linkages that lead to viscoelastic properties, recapitulating the dynamic properties of the ECM (Wang and Heilshorn, 2015). As progress has been made in the design of matrices for 3D cell cultures, reversible hydrogels will surely play a key role as in vitro 3D scaffolds for studies in cancer biology.
Engineering approaches in cancer therapeutics
As reviewed so far, emerging technologies have supplemented basic science observations to provide insights on the role of the ECM in cancer biology. The adaptability of engineered biomaterials has facilitated translational approaches in cancer therapeutics, such as the use of nanoparticles for drug delivery or tunable hydrogel designs for drug testing. These new approaches seek to overcome some of the limitations of conventional technologies, such as the off-target toxicity of antineoplastic agents and the inability of current in vitro pre-clinical models used for drug development to faithfully recapitulate the heterogeneity of the tumor ECM. We review these and other emerging engineering approaches in cancer therapeutics and provide a perspective on how engineering tools present an opportunity for improved treatment of cancer.
Engineered nanostructures/microstructures for cancer therapy and normalization of tumor ECM
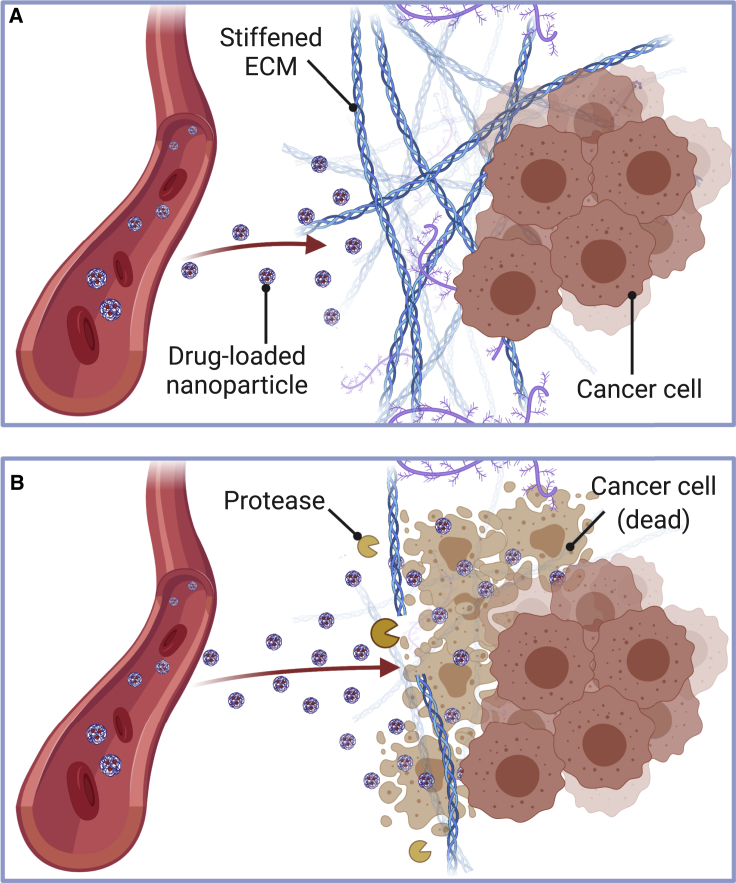
Systemic delivery of antineoplastic agents has been one of the key methods of cancer treatment, and numerous technologies have been used to deliver chemotherapy systemically, such as oral capsules, injections of nanoparticles/microparticles, and engineered cell infusion (Chew and Danti, 2017). However, even with these new approaches, systemic delivery has many limitations, including off-target cell and organ toxicity, and low efficacy of the delivered drug due to its short half-life. A major contributor to these limitations is the dense and aberrant tumor ECM, particularly in solid tumors, which makes certain areas of the tumor difficult to access and treat (Figure 3A) (Abyaneh et al., 2020). Therefore, there is an ongoing search for novel targeted approaches to fight cancer with insignificant off-target toxicity and prolonged drug circulation, many of which involve targeting the tumor ECM. Indeed, biomaterials have emerged in cancer therapeutics to address some of these limitations, such as nanostructures/microstructures for drug targeting and delivery (Gill & et al., 2018; Pesoa & et al., 2018; Wu & et al., 2018), diagnostic applications (Mani et al., 2009), and delivery of contrast agents (Kim et al., 2014).
Figure 3.
Tumor ECM represents a physical barrier for nanoparticle-based therapy delivery
(A) Solid tumors possess a dense ECM and increased stiffness that blocks adequate penetration of nanoparticles into the tumor.
(B) Proteolytic molecules such as MMPs, hyaluronidases, and collagenases degrade the ECM. These can be exploited pharmacologically as antifibrotic agents to enhance nanoparticle delivery to tumors. Created with BioRender.com.
Engineered nanostructures/microstructures have emerged as tunable platforms for therapeutic delivery of combination therapies for different types of cancer (Gill & et al., 2018; Zou & et al., 2017; Tseng & et al., 2016; Rhines & et al., 2003). For instance, Gil et al. (Gill & et al., 2018) developed a nanoparticle carrier for the co-delivery of a ruthenium-based radiosensitizer and a therapeutic radionuclide to attain combinational targeted therapeutic effects in esophageal cancer cells that overexpress the epidermal growth factor (EGF) receptor. In their study, poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles were loaded with Ru1 (Ru(phen)2(tpphz)2+), a member of the ruthenium(II) polypyridyl complexes, and coated with the radionuclide Indium-111 (111-In) to facilitate cellular internalization and direct visualization of nanoparticle accumulation in vivo and supplemented by EGF, a targeting ligand for EGF receptor-expressing cells. The study demonstrated in an in vivo tumor xenograft model that nanoparticle co-delivery of Ru1 and 111-In resulted in a synergistic therapeutic effect of increased esophageal cancer cell death through DNA damage enhancement and significantly decreased off-target effects on cells with normal EGF receptor levels. A major advantage of this material is its “tunable” synthesis, such that the loaded drug, targeting peptide, and radionuclide can be varied to account for the molecular characteristics of a specific cancer. A major limitation of this technology is the low accumulation of nanoparticles in solid tumors after intravenous injection and high liver and spleen accumulations as a consequence of nanoparticle design characteristics and biophysical barriers to nanoparticle transport such as ECM stiffening (Figure 3A) (Wilhelm & et al., 2016). Other studies have focused on direct targeting and depletion of ECM molecules to improve the intratumoral delivery and efficacy of anticancer drugs in pre-clinical settings, suggesting that combining nanoparticle-based therapeutics with antifibrotic strategies may help improve targeted cancer therapies (Figure 3B) (Gourd, 2018; Zhang & et al., 2016; Dong & et al., 2019; Hauge and Rofstad, 2020).
Collagen is the major structural component of the ECM and its increased deposition is a significant contributor to cancer; there has been great interest in developing therapeutic strategies that target collagen in the tumor ECM (Figure 3B) (Abyaneh et al., 2020; Whatcott et al., 2015; Lampi and Reinhart-King, 2018). These approaches, which include degradation of stromal collagen (Goodman et al., 2007; Zinger & et al., 2019; Dolor and Szoka Jr, 2018), inhibition of collagen synthesis (Juárez & et al., 2012; Chauhan & et al., 2013; Zion & et al., 2009) or cross-linking (Kanapathipillai & et al., 2012; Miller & et al., 2015), and blocking of collagen-integrin receptor interactions (Schnittert et al., 2018), seek to remodel the microenvironment to resemble that of healthy tissue (often referred to as “normalizing the tumor microenvironment”) and thus further improve treatment of solid tumors (Abyaneh et al., 2020). For instance, Zinger et al. (Zinger & et al., 2019) demonstrated that a pretreatment based on a collagenase-loaded nanoparticle system digests the dense PDAC collagen stroma and increases drug penetration into the pancreatic tumor, resulting in improved PDAC treatment. The authors showed that, upon intravenous injection of collagenase nanoparticles in an in vivo PDAC xenograft model, the nanoparticle carrier (100-nm liposomes) protected the collagenase from early deactivation in the plasma, modulated the enzyme release profile inside the tumor, and was more effective than the free enzyme in degrading the tumor ECM. To test the therapeutic efficacy of collagenase nanoparticle treatment, tumor-bearing mice were administered with the collagenase nanoparticles 24 hr before intravenous administration of a dose of therapeutic paclitaxel nanoparticles. PDAC tumors, pretreated with the collagenase nanoparticle followed by paclitaxel treatment, were 87% smaller than tumors pretreated with empty nanoparticles. Interestingly, degradation of the PDAC collagen stroma did not increase the number of circulating tumor cells or metastases, with minimal harm to healthy organs. This strategy suggests that direct targeting and depletion of ECM molecules can improve the intratumoral delivery and efficacy of anticancer drugs in pre-clinical settings and that combining nanoparticle-based therapeutics with antifibrotic strategies may improve targeted cancer therapies (Figure 3B). A recent review by Dolor et al. (Dolor and Szoka Jr, 2018) provides detailed discussion of this approach.
It is worth noting that normalizing the tumor microenvironment by collagen degradation is not without its caveats. It can result in the release of cytokines and growth factors embedded within the stromal ECM and the recruitment of inflammatory cells that can lead to disease progression (Abyaneh et al., 2020; Parks et al., 2004). Therefore, other groups have instead focused on inhibiting ECM protein synthesis by utilizing cancer-associated fibroblast-reprogramming agents, referred to as “mechanotherapeutics”, to promote the development of a quiescent stromal phenotype and reduce ECM deposition to near-normal values (Sherman & et al., 2014). Mechanotherapeutics can also alleviate solid stresses, thus decompressing tumor blood vessels, which results in improved levels of tumor perfusion and increased transport of nanomedicines (Martin et al., 2019). This novel approach has gain increased attention for improving cancer nanotherapy and immunotherapy. A recent review by Martin et al. (Martin et al., 2020) provides detailed discussion of this approach.
Nanoparticle-based approaches are only one of the many engineering methods continuously researched to facilitate localized therapy delivery to the cancer site. Another approach is engineered injectable hydrogel systems suitable for chemotherapy and radiotherapy (Cirillo et al., 2019) or immunotherapy delivery (Weiden et al., 2018; Gosselin et al., 2018). A drug-loaded, thermosensitive PLGA-PEG-PLGA gel has been proposed for the treatment of osteosarcoma (Yang & et al., 2018), whereas injectable macroporous alginate gels loaded with chemokines, adjuvants, and chemotherapeutic drugs have been proposed as chemoimmunotherapy cancer vaccines for poorly immunogenic tumors, including triple-negative breast cancers (Wang & et al., 2020). Implantable biomaterial devices for the local delivery of chemotherapy have been studied, such as chemotherapy drug-loaded ethylene-vinyl acetate copolymer disks in in vivo intracranial glioma models (Bota et al., 2007) and paclitaxel-loaded PLGA fibrous meshes that allow sustained, long-term drug release in a glioma in vivo model (Ranganath and Wang, 2008). Overall, these approaches have limitations, but as the field gains new insights regarding the biological barriers of cancer therapy administration, the tunability of engineered biomaterials can be utilized to overcome biological barriers and the limitations of traditional therapeutics.
Engineered hydrogels as in vitro pre-clinical models for anticancer drug screening
High-throughput sequencing techniques have been used to generate large-scale genomics data to predict cancer behavior and response to therapeutics (Thakuri et al., 2017). These studies are limited by the use of cancer cell lines, which cannot replicate the intratumoral heterogeneity of patient tumors and acquire culture-induced genetic drift that reduces their ability to recapitulate the genomic aberrations observed in tumors (Fatehullah et al., 2016). Additionally, non-physiologically stiff substrates and the lack of 3D cellular interactions are some of the discrepancies of 2D culture systems to native tumor microenvironments (Thakuri et al., 2017). Thus, additional work is imperative to develop physiologically relevant 3D culture models that recapitulate with fidelity the human cancer microenvironment in order to elucidate underlying mechanisms and translate them into therapies. In this context, engineered hydrogels are an important component of 3D organoid culture systems, particularly to introduce microenvironment signals necessary to model human development and disease (Cruz-Acuña and García, 2019; Gjorevski et al., 2014; Cruz-Acuña & et al., 2017; Cruz-Acuña & et al., 2018; Gjorevski & et al., 2016; Dye & et al., 2016). Indeed, hydrogels introduce a tunable physicochemical matrix signal that has been investigated in tumor progression and metastasis (Beck et al., 2013; Casey, 2017; Fong et al., 2013). Furthermore, patient-derived xenograft (PDX) models have become an attractive pre-clinical model to study cancer biology and predict responses to anticancer drugs, as they retain the biological characteristics of the primary tumor and recapitulate patient's drug responses in the clinic (Hidalgo & et al., 2014; Gao & et al., 2015). Through the emerging integration of biomaterials design and organoid biology, scientists are seeking to find novel physiologically relevant 3D culture models that overcome the limitations of the current model systems.
In a complementary approach, Fong et al. (Fong & et al., 2018) generated an in vitro system for the culture of human hepatocellular carcinoma (HCC)-PDX organoids derived from 14 different PDX lines from patients using a macroporous cellulosic sponge system that facilitates drug testing. Whole exome and RNA sequencing validated the concordance in genomic and transcriptomic profiles and the intratumoral heterogeneity between the matched in vitro and in vivo HCC-PDX pairs. This group had previously shown that this hydrogel system prevents cell death in the organoid inner core due to diffusion limitations, displays in vivo-like mechanical stiffness, has minimal drug absorption, and is readily scalable, overcoming the limitations of natural protein matrices (e.g. type I collagen) (Nugraha & et al., 2011). Importantly, the engineered biomaterial supported HCC-PDX organoid response to treatment with a multi-kinase inhibitor (Llovet & et al., 2008), suggesting that organoids generated in the biomaterial can be used for pre-clinical drug screening. Finally, these observations suggest that the physicochemical properties of the matrix establish the engineered hydrogel as a potential platform for high-throughput, large-scale drug safety testing applications. However, the physical structure of the hydrogel was supported by non-degradable cross-links, which may have limited the proliferation of the organoids and, most importantly, fails to recapitulate the dynamics of cell-directed matrix remodeling and the contributions of other cellular components of the native tumor microenvironment to tumor cell response. Other technologies that incorporate a vascularized microenvironment, other cell types (mesenchymal stem cells, endothelial cells), and microfluidic connections (Bersini & et al., 2014; Jeon & et al., 2015; Zervantonakis & et al., 2012) have recently emerged as potential platforms for pre-clinical drug screening models, overcoming some of the limitations of current static hydrogel systems.
Overall, engineered 3D biomaterial scaffolds have emerged as a highly advantageous alternative to natural matrices for the in vitro modeling and study of human development and disease in combination with 3D organoid systems. As we continue to gain insights regarding the complex interrelation of ECM physicochemical properties and the contribution of stromal and immune cells to cancer initiation and progression, we require new 3D biomaterial designs that incorporate these important contributions in order to develop more suitable in vitro pre-clinical models that can be used for high-throughput drug screening.
Outlook for technological approaches to understand the role of extracellular matrix properties in cancers and therapeutics
The studies we have reviewed are illustrations of recent work that provided novel insights into cancer initiation, progression, and therapeutic effects with the use of advanced biotechnological and engineering approaches, contributing to the advancement of cancer research. These approaches have facilitated a better understanding of the individual contributions of ECM properties to cancer biology that is virtually not attainable through the use of traditional approaches, validating how these approaches can be built upon basic and translational science observations in cancer. As we utilize these techniques to dissect ECM properties, we venture into the paradoxically complex interrelation of ECM physicochemical properties and their contributions to tumorigenesis and cancer progression. New answers are accompanied by new questions, which inspire the application of current or new technologies for the first time in cancer research.
This review highlights the increasing interest for in vitro 3D models of tumor microenvironments that recapitulate the physicochemical matrix properties of the tumor ECM in combination with 3D organoid systems, while overcoming the limitations of costly and labor-intensive in vivo models.
The field has come a long way in the past decade with the application of tunable 3D hydrogel matrices that allow independent control over matrix properties; however, existing models still fail to faithfully recapitulate the contributions of supporting cellular components of the native tumor microenvironment (e.g. mesenchymal cells, immune cells, fibroblasts) to cancer, limiting the potential of these innovative in vitro models. Biomaterial designs that can support additional cellular constituents, in addition to cancer cells, can then serve as platforms to model a more comprehensive interplay of the native tumor microenvironment. In fact, an in vitro study using a 3D biological matrix has demonstrated that co-culture of patient-derived organoids with native immune cells can model cancer cell-immune cell interactions (Neal & et al., 2018). However, as natural 3D matrices are unable to uncouple physicochemical matrix properties, new biomaterial designs that can support multiple cellular systems while maintaining tunability of material properties are needed to model a comprehensive interplay of the native tumor microenvironment. Perhaps materials that present multiple adhesive ligand types at specific densities and mimic the structural properties of ECM proteins (e.g. fibrillar structure) can better support such co-culture of different cellular constituents that participate in cancer. In this context, glycoengineered decellularized matrices that present azide moieties (Gutmann et al., 2018; Ruff & et al., 2017) may provide a solution by facilitating covalent integration of biologically active groups and construction of hybrid hydrogels that allow independent control over matrix properties (Fitzpatrick and McDevitt, 2015; Petrou & et al., 2020). Another interesting possibility is the use of hybrid materials where a synthetic core structure (providing precise control over structural and biomechanical properties) is blended by tissue-specific ECM (providing biological signaling). Therefore, as biomaterial scientists acquire more detailed understanding of the intratumor complexity (e.g. phenotypic and spatial heterogeneity; nascent protein organization) via high-resolution imaging (e.g. mass cytometry or SHG), they can potentially design in vitro tumor ECM models that better recapitulate the native tumor microenvironment.
The application of engineering approaches into cancer therapeutics has provided new possibilities for their adaptation as therapy delivery and pre-clinical drug screening platforms. As emphasized here, current nanoparticle-based approaches for therapy delivery have focused on developing drug carriers that allow improved targeted delivery. Although improvements in patient safety and morbidity have led to clinical approval of nanoparticle platforms (O'Brien & et al., 2004; Gradishar & et al., 2005), biological barriers to drug transport, such as tumor ECM stiffening (Figure 3A), prevent targeted, local accumulation of nanotherapeutics at cancer sites, limiting these platforms to offer only marginal improvements over current chemotherapy treatments. In this context, the implementation of innovative designs such as the use of nontraditional geometries or functionalization with biomimetic membranes and depletion of tumor ECM molecules (Figure 3B) may help overcome biological barriers by improving vascular dynamics, phagocytic uptake evasion, and tumor fibrosis, thus offering advantages over conventional nanoparticle formulations.
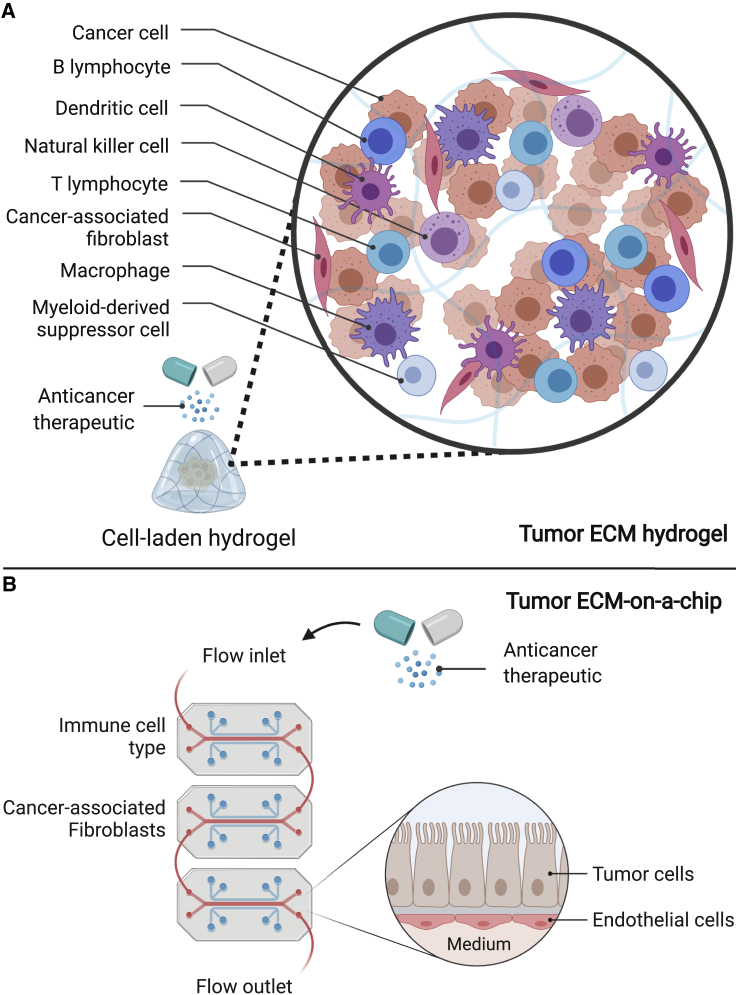
In parallel, the utilization of engineered biomaterials for in vitro pre-clinical drug screening also faces challenges. In order to represent suitable models for drug screening, these matrices are required to have a particular narrow range of matrix elasticity and adhesive peptide density, while supporting cell-directed remodeling of the matrix. In addition, these materials need to have minimal drug absorption and be readily scalable for high-throughput, large-scale drug safety testing applications. Importantly, biomaterial designs that support additional cellular components of the tumor microenvironment, in addition to cancer cells, can then serve as platforms to model a more comprehensive interplay of the native tumor microenvironment during therapy (Figure 4A). For instance, integrated engineering platforms, such as organ-on-a-chip models that allow predictive drug response from bioengineered tissues (Chramiec & et al., 2020), could be readily extended to model anticancer drug responses of tumor cells in communication with tumor microenvironments to better predict clinical outcomes (Figure 4B).
Figure 4.
Future considerations of engineered approaches for pre-clinical drug testing applications
Biomaterials used for pre-clinical drug testing are required to have a particular narrow range of physicochemical matrix properties, while supporting intrinsic cellular processes, and have minimal drug absorption.
The innate tunability and high-throughput potential of (A) engineered hydrogels and (B) microfluidic devices should be exploited to support additional cellular components of the tumor microenvironment, among cancer cells, to model a more comprehensive interplay of the native tumor microenvironment during therapy. Created with BioRender.com.
Alternatively, 3D bioprinted vascularized tumor models that replicate cancer dissemination via growth factor-induced migration of tumor and endothelial cells (Meng & et al., 2019) could be exploited as platforms for pre-clinical drug screening and to test patient-specific strategies for diagnosis and therapeutics. Importantly, the design of these engineered platforms and tumor tissues can potentially be better informed by MCI in the context of the spatial distribution of anticancer drugs at subcellular resolutions (Baharlou et al., 2019; Chang & et al., 2016).
As we gain new insights into the contributions of ECM properties in tumorigenesis, cancer progression, and cancer therapeutics, new engineering approaches will continue to be developed and improved to support advancements in translational medicine and tumor models and the innovative therapeutic options that are not attainable through the use of traditional techniques.
Acknowledgments
This work was supported by the NIH (P01CA098101 and U54CA163004). All Figures were created with BioRender.com
Author contributions
Conceptual development was done by R.C.-A., J.A.B., and A.K.R. Literature review was conducted by R.C.-A. All authors contributed to writing and final editing of the manuscript. Funding was obtained by A.K.R.
Declaration of interests
The authors declare no competing interests.
References
- Abyaneh H.S., Regenold M., McKee T.D., Allen C., Gauthier M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics. 2020;10:1960–1980. doi: 10.7150/thno.39995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H.R., Jackson H.W., Zanotelli V.R.T., Danenberg E., Fischer J.R., Bardwell H., Provenzano E., Ali H.R., Al Sa’d M., Alon S. Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nat. Cancer. 2020;1:163–175. doi: 10.1038/s43018-020-0026-6. [DOI] [PubMed] [Google Scholar]
- Ambekar R., Lau T.-Y., Walsh M., Bhargava R., Toussaint K.C., Jr. Quantifying collagen structure in breast biopsies using second-harmonic generation imaging. Biomed. Opt. Express. 2012;3:2021–2035. doi: 10.1364/BOE.3.002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P.M., Comte-Walters S., Ball L.E., Talbot K., Mehta A., Brockbank K.G.M., Drake R.R. Mapping extracellular matrix proteins in Formalin-Fixed, Paraffin-Embedded tissues by MALDI imaging mass Spectrometry. J. Proteome Res. 2018;17:635–646. doi: 10.1021/acs.jproteome.7b00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth J.C., Thompson J.L., James J.R., Slater C.E., Pijuan-Galitó S., Lis-Slimak K., Holley R.J., Meade K.A., Thompson A., Arkill K.P. Peptide gels of fully-defined composition and mechanics for probing cell-cell and cell-matrix interactions in vitro. Matrix Biol. 2020;85–86:15–33. doi: 10.1016/j.matbio.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak S.F., Freytes D.O., Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Baharlou H., Canete N.P., Cunningham A.L., Harman A.N., Patrick E. Mass cytometry imaging for the study of human diseases—applications and data analysis strategies. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.02657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J.N., Singh A., Rothenberg A.R., Elisseeff J.H., Ewald A.J. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials. 2013;34:9486–9495. doi: 10.1016/j.biomaterials.2013.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S., Jeon J.S., Dubini G., Arrigoni C., Chung S., Charest J.L., Moretti M., Kamm R.D. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota D.A., Desjardins A., Quinn J.A., Affronti M.L., Friedman H.S. Interstitial chemotherapy with biodegradable BCNU (Gliadel) wafers in the treatment of malignant gliomas. Ther. Clin. Risk Manag. 2007;3:707–715. [PMC free article] [PubMed] [Google Scholar]
- Butcher D.T., Alliston T., Weaver V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola P. Second harmonic generation imaging microscopy: applications to diseases diagnostics. Anal. Chem. 2011;83:3224–3231. doi: 10.1021/ac1032325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola P.J., Dong C.-Y. Second harmonic generation microscopy: principles and applications to disease diagnosis. Laser Photon. Rev. 2011;5:13–26. doi: 10.1002/lpor.200910024. [DOI] [Google Scholar]
- Casey J., Yue X., Nguyen T.D., Acun A., Zellmer V.R., Zhang S., Zorlutuna P. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 2017 doi: 10.1088/1748-605X/aa5d5c. [DOI] [PubMed] [Google Scholar]
- Cassereau L., Miroshnikova Y.A., Ou G., Lakins J., Weaver V.M. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. J. Biotechnol. 2015;193:66–69. doi: 10.1016/j.jbiotec.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy A., Khan L., Bensler N.P., Bose P., De Carvalho D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018;9:4692. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Ornatsky O.I., Siddiqui I., Straus R., Baranov V.I., Hedley D.W. Biodistribution of cisplatin revealed by imaging mass cytometry identifies extensive collagen binding in tumor and normal tissues. Sci. Rep. 2016;6:36641. doi: 10.1038/srep36641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O., Cooper-White J., Janmey P.A., Mooney D.J., Shenoy V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584:535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O., Koshy S.T., Branco da Cunha C., Shin J.-W., Verbeke C.S., Allison K.H., Mooney D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V., Stylianopoulos T., Mousa A.S., Han X., Adstamongkonkul P. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-W.E., Pedron S., Harley B.A.C. The combined influence of hydrogel stiffness and matrix-Bound hyaluronic acid Content on glioblastoma invasion. Macromol. Biosci. 2017;17:1700018. doi: 10.1002/mabi.201700018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Chen S., Wu H., Lee W., Liao Y., Sun C. In vivo virtual biopsy of human skin by using noninvasive higher harmonic generation microscopy. IEEE J. Sel. Top. Quan. Electron. 2010;16:478–492. doi: 10.1109/JSTQE.2009.2031987. [DOI] [Google Scholar]
- Chen W., Zhou Y., Zhi X., Ma T., Liu H., Chen B.W., Zheng X., Xie S., Zhao B., Feng X. Delivery of miR-212 by chimeric peptide-condensed supramolecular nanoparticles enhances the sensitivity of pancreatic ductal adenocarcinoma to doxorubicin. Biomaterials. 2019;192:590–600. doi: 10.1016/j.biomaterials.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Chew S.A., Danti S. Biomaterial-based implantable devices for cancer therapy. Adv. Healthc. Mater. 2017;6:1600766. doi: 10.1002/adhm.201600766. [DOI] [PubMed] [Google Scholar]
- Chiellini F., Puppi D., Piras A.M., Morelli A., Bartoli C., Migone C. Modelling of pancreatic ductal adenocarcinoma in vitro with three-dimensional microstructured hydrogels. RSC Adv. 2016;6:54226–54235. doi: 10.1039/C6RA08420F. [DOI] [Google Scholar]
- Chramiec A., Teles D., Yeager K., Marturano-Kruik A., Pak J., Chen T., Hao L., Wang M., Lock R., Tavakol D.N. Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab Chip. 2020;20:4357–4372. doi: 10.1039/D0LC00424C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo G., Spizzirri U.G., Curcio M., Nicoletta F.P., Iemma F. Injectable hydrogels for cancer therapy over the last decade. Pharmaceutics. 2019;11:486. doi: 10.3390/pharmaceutics11090486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin M.W., Eickhoff J.C., Riching K.M., Pehlke C.A., Eliceiri K.W., Provenzano P.P., Friedl A., Keely P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña R., García A.J. Engineered materials to model human intestinal development and cancer using organoids. Exp. Cell Res. 2019;377:109–114. doi: 10.1016/j.yexcr.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña R., García A.J. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña R., Quirós M., Farkas A.E., Dedhia P.H., Huang S., Siuda D., García-Hernández V., Miller A.J., Spence J.R., Nusrat A., García A.J. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017 doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña R., Quirós M., Huang S., Siuda D., Spence J.R., Nusrat A., García A.J. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 2018 doi: 10.1038/s41596-018-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G.J., Tempero M., Corrie P.G. HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Futur. Oncol. 2017;14:13–22. doi: 10.2217/fon-2017-0338. [DOI] [PubMed] [Google Scholar]
- Dolor A., Szoka F.C., Jr. Digesting a path forward: the utility of collagenase tumor treatment for improved drug delivery. Mol. Pharm. 2018;15:2069–2083. doi: 10.1021/acs.molpharmaceut.8b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Liu H.-J., Feng H.-Y., Yang S.-C., Liu X.-L., Lai X., Lu Q., Lovell J.F., Chen H.-Z., Fang C. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 2019;19:997–1008. doi: 10.1021/acs.nanolett.8b04236. [DOI] [PubMed] [Google Scholar]
- Drifka C.R., Loeffler A.G., Mathewson K., Mehta G., Keikhosravi A., Liu Y., Lemancik S., Ricke W.A., Weber S.M., Kao W.J., Eliceiri K.W. Comparison of picrosirius red staining with second harmonic generation imaging for the quantification of clinically relevant collagen fiber features in histopathology Samples. J. Histochem. Cytochem. 2016;64:519–529. doi: 10.1369/0022155416659249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drifka C.R., Tod J., Loeffler A.G., Liu Y., Thomas G.J., Eliceiri K.W., Kao W.J. Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod. Pathol. 2015;28:1470–1480. doi: 10.1038/modpathol.2015.97. [DOI] [PubMed] [Google Scholar]
- Dye B.R., Dedhia P.H., Miller A.J., Nagy M.S., White E.S., Shea L.D., Spence J.R. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife. 2016;5 doi: 10.7554/eLife.19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- Fernández-Sánchez M.E., Barbier S., Whitehead J., Béalle G., Michel A., Latorre-Ossa H., Rey C., Fouassier L., Claperon A., Brullé L. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick L.E., McDevitt T.C. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater. Sci. 2015;3:12–24. doi: 10.1039/C4BM00246F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong E.L.S., Lamhamedi-Cherradi S.-E., Burdett E., Ramamoorthy V., Lazar A.J., Kasper F.K., Farach-Carson M.C., Vishwamitra D., Demicco E.G., Menegaz B.A. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong E.L.S., Toh T.B., Lin Q.X.X., Liu Z., Hooi L., Mohd Abdul Rashid M.B., Benoukraf T., Chow E.K.-H., Huynh T.H., Yu H. Generation of matched patient-derived xenograft in vitro-in vivo models using 3D macroporous hydrogels for the study of liver cancer. Biomaterials. 2018;159:229–240. doi: 10.1016/j.biomaterials.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Fraley S.I., Wu P., He L., Feng Y., Krisnamurthy R., Longmore G.D., Wirtz D. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci. Rep. 2015;5:14580. doi: 10.1038/srep14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Meng J., Xu M., Liu M., Qi Y., Tan C., Wang Y., Zhang P., Weng W., Sheng W. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric cancer. Oncogene. 2018;37:744–755. doi: 10.1038/onc.2017.363. [DOI] [PubMed] [Google Scholar]
- Gao H., Korn J.M., Ferretti S., Monahan J.E., Wang Y., Singh M., Zhang C., Schnell C., Yang G., Zhang Y. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015;21:1318. doi: 10.1038/nm.3954. https://www.nature.com/articles/nm.3954#supplementary-information [DOI] [PubMed] [Google Scholar]
- Garalla H.M., Lertkowit N., Tiszlavicz L., Reisz Z., Holmberg C., Beynon R., Simpson D., Varga A., Kumar J.D., Dodd S. Matrix metalloproteinase (MMP)-7 in Barrett’s esophagus and esophageal adenocarcinoma: expression, metabolism, and functional significance. Physiol. Rep. 2018;6 doi: 10.14814/phy2.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M.R., Menon J.U., Jarman P.J., Owen J., Skaripa-Koukelli I., Able S., Thomas J.A., Carlisle R., Vallis K.A. 111In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells. Nanoscale. 2018;10:10596–10608. doi: 10.1039/C7NR09606B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N., Ranga A., Lutolf M.P. Bioengineering approaches to guide stem cell-based organogenesis. Development. 2014;141:1794–1804. doi: 10.1242/dev.101048. [DOI] [PubMed] [Google Scholar]
- Gjorevski N., Sachs N., Manfrin A., Giger S., Bragina M.E., Ordóñez-Morán P., Clevers H., Lutolf M.P. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- Gong Y.-Z., Ruan G.-T., Liao X.-W., Wang X.-K., Liao C., Wang S., Gao F. Diagnostic and prognostic values of integrin α subfamily mRNA expression in colon adenocarcinoma. Oncol. Rep. 2019;42:923–936. doi: 10.3892/or.2019.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T.T., Olive P.L., Pun S.H. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int. J. Nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- Gosselin E.A., Eppler H.B., Bromberg J.S., Jewell C.M. Designing natural and synthetic immune tissues. Nat. Mater. 2018;17:484–498. doi: 10.1038/s41563-018-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourd E. PEGPH20 for metastatic pancreatic ductal adenocarcinoma. Lancet Oncol. 2018;19:e81. doi: 10.1016/S1470-2045(17)30953-1. [DOI] [PubMed] [Google Scholar]
- Gradishar W.J., Tjulandin S., Davidson N., Shaw H., Desai N., Bhar P., Hawkins M., O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated Castor oil–based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- Guruswamy Damodaran R., Vermette P. Tissue and organ decellularization in regenerative medicine. Biotechnol. Prog. 2018;34:1494–1505. doi: 10.1002/btpr.2699. [DOI] [PubMed] [Google Scholar]
- Gutmann M., Braun A., Seibel J., Lühmann T. Bioorthogonal modification of cell derived matrices by metabolic glycoengineering. ACS Biomater. Sci. Eng. 2018;4:1300–1306. doi: 10.1021/acsbiomaterials.8b00264. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hauge A., Rofstad E.K. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020;18:207. doi: 10.1186/s12967-020-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg S., Das K.K., Reichert M., Bakir B., Takano S., Caspers J., Aiello N.M., Wu K., Neesse A., Maitra A. ETS-transcription factor ETV1 regulates stromal expansion and metastasis in pancreatic cancer. Gastroenterology. 2016;151:540–553.e14. doi: 10.1053/j.gastro.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M., Amant F., Biankin A.V., Budinská E., Byrne A.T., Caldas C., Clarke R.B., de Jong S., Jonkers J., Mælandsmo G.M. Patient-Derived xenograft models: an emerging platform for translational cancer Research. Cancer Discov. 2014;4:998. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.S., Postovit L.M., Lajoie G.A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- Ingber D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Jackson H.W., Fischer J.R., Zanotelli V.R.T., Ali H.R., Mechera R., Soysal S.D., Moch H., Muenst S., Varga Z., Weber W.P., Bodenmiller B. The single-cell pathology landscape of breast cancer. Nature. 2020;578:615–620. doi: 10.1038/s41586-019-1876-x. [DOI] [PubMed] [Google Scholar]
- Jang M., Koh I., Lee J.E., Lim J.Y., Cheong J.-H., Kim P. Increased extracellular matrix density disrupts E-cadherin/β-catenin complex in gastric cancer cells. Biomater. Sci. 2018;6:2704–2713. doi: 10.1039/C8BM00843D. [DOI] [PubMed] [Google Scholar]
- Jeon J.S., Bersini S., Gilardi M., Dubini G., Charest J.L., Moretti M., Kamm R.D. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez P., Mohammad K.S., Yin J.J., Fournier P.G.J., McKenna R.C., Davis H.W., Peng X.H., Niewolna M., Javelaud D., Chirgwin J.M. Halofuginone inhibits the establishment and progression of melanoma bone metastases. Cancer Res. 2012;72:6247–6256. doi: 10.1158/0008-5472.CAN-12-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapathipillai M., Mammoto A., Mammoto T., Kang J.H., Jiang E., Ghosh K., Korin N., Gibbs A., Mannix R., Ingber D.E. Inhibition of mammary tumor growth using Lysyl Oxidase-Targeting nanoparticles to modify extracellular matrix. Nano Lett. 2012;12:3213–3217. doi: 10.1021/nl301206p. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Park W., Hu J., Bae Y.H., Na K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials. 2014;35:337–343. doi: 10.1016/j.biomaterials.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Kirschner C.M., Anseth K.S. Hydrogels in healthcare: from static to dynamic material microenvironments. Acta Mater. 2013;61:931–944. doi: 10.1016/j.actamat.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koikawa K., Ohuchida K., Ando Y., Kibe S., Nakayama H., Takesue S., Endo S., Abe T., Okumura T., Iwamoto C. Basement membrane destruction by pancreatic stellate cells leads to local invasion in pancreatic ductal adenocarcinoma. Cancer Lett. 2018;425:65–77. doi: 10.1016/j.canlet.2018.03.031. [DOI] [PubMed] [Google Scholar]
- Krantz S.B., Shields M.A., Dangi-Garimella S., Cheon E.C., Barron M.R., Hwang R.F., Rao M.S., Grippo P.J., Bentrem D.J., Munshi H.G. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-β signaling. Mol. Cancer Res. 2011;9:1294–1304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laklai H., Miroshnikova Y.A., Pickup M.W., Collisson E.A., Kim G.E., Barrett A.S., Hill R.C., Lakins J.N., Schlaepfer D.D., Mouw J.K. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi M.C., Reinhart-King C.A. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- Landberg G., Fitzpatrick P., Isakson P., Jonasson E., Karlsson J., Larsson E., Svanström A., Rafnsdottir S., Persson E., Gustafsson A. Patient-derived scaffolds uncover breast cancer promoting properties of the microenvironment. Biomaterials. 2020;235:119705. doi: 10.1016/j.biomaterials.2019.119705. [DOI] [PubMed] [Google Scholar]
- Lei Z., Jian M., Li X., Wei J., Meng X., Wang Z. Biosensors and bioassays for determination of matrix metalloproteinases: state of the art and recent advances. J. Mater. Chem. B. 2020;8:3261–3291. doi: 10.1039/C9TB02189B. [DOI] [PubMed] [Google Scholar]
- Leonard B.C., Cosert K., Winkler M., Marangakis A., Thomasy S.M., Murphy C.J., Jester J.V., Raghunathan V.K. Stromal collagen arrangement correlates with stiffness of the canine cornea. Bioeng. (Basel, Switzerland) 2019;7:4. doi: 10.3390/bioengineering7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-Y., Korc M., Lin C.-C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials. 2018;160:24–36. doi: 10.1016/j.biomaterials.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.-F., de Oliveira A.C., Santoro A., Raoul J.-L., Forner A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Loebel C., Mauck R.L., Burdick J.A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 2019;18:883–891. doi: 10.1038/s41563-019-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Zheng M., Zhu Y., Sha M., Wu Y., Han X. Selection of peptide inhibitor to matrix metalloproteinase-2 using phage display and its effects on pancreatic cancer cell lines PANC-1 and CFPAC-1. Int. J. Biol. Sci. 2012;8:650–662. doi: 10.7150/ijbs.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Bhat A.A., Peng D., Chen Z., Zhu S., Hong J., Maacha S., Yan J., Robbins D.J., Washington M.K. APE1 upregulates MMP-14 via redox-sensitive ARF6-mediated recycling to promote cell invasion of esophageal adenocarcinoma. Cancer Res. 2019;79:4426–4438. doi: 10.1158/0008-5472.CAN-19-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V., Chikkaveeraiah B.V., Patel V., Gutkind J.S., Rusling J.F. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano. 2009;3:585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis A.J., Bojanowski K., Ingber D.E. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J. Cell. Biochem. 1997;65:114–130. doi: 10.1002/(SICI)1097-4644. [DOI] [PubMed] [Google Scholar]
- Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.D., Seano G., Jain R.K. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol. 2019;81:505–534. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Meyer C.M., Joung D., Vallera D.A., McAlpine M.C., Panoskaltsis-Mortari A. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 2019;31:1806899. doi: 10.1002/adma.201806899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micek H.M., Visetsouk M.R., Masters K.S., Kreeger P.K. Engineering the extracellular matrix to model the evolving tumor microenvironment. iScience. 2020;23:101742. doi: 10.1016/j.isci.2020.101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.W., Morton J.P., Pinese M., Saturno G., Jamieson N.B., McGhee E., Timpson P., Leach J., McGarry L., Shanks E. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 2015;7:1063–1076. doi: 10.15252/emmm.201404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiarnykh O., LaComb R.B., Brewer M.A., Campagnola P.J. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer. 2010;10:94. doi: 10.1186/1471-2407-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J.T., Li X., Zhu J., Giangarra V., Grzeskowiak C.L., Ju J., Liu I.H., Chiou S.-H., Salahudeen A.A., Smith A.R. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–1988.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nia H.T., Munn L.L., Jain R.K. Physical traits of cancer. Science. 2020;80-:370. doi: 10.1126/science.aaz0868. eaaz0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugraha B., Hong X., Mo X., Tan L., Zhang W., Chan P.-M., Kang C.H., Wang Y., Beng L.T., Sun W. Galactosylated cellulosic sponge for multi-well drug safety testing. Biomaterials. 2011;32:6982–6994. doi: 10.1016/j.biomaterials.2011.05.087. [DOI] [PubMed] [Google Scholar]
- Ohashi K., Nemoto T., Nakamura K., Nemori R. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer. 2000;88:2201–2209. [PubMed] [Google Scholar]
- O’Brien M.E.R., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A., Catane R., Kieback D.G., Tomczak P., Ackland S.P. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- Palumbo A., Meireles Da Costa N., Pontes B., Leite de Oliveira F., Lohan Codeço M., Ribeiro Pinto F.L., Nasciutti E.L. Esophageal cancer development: crucial clues arising from the extracellular matrix. Cells. 2020;9 doi: 10.3390/cells9020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W.C., Wilson C.L., López-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pesoa J.I., Rico M.J., Rozados V.R., Scharovsky O.G., Luna J.A., Mengatto L.N. Paclitaxel delivery system based on poly(lactide-co-glycolide) microparticles and chitosan thermo-sensitive gel for mammary adenocarcinoma treatment. J. Pharm. Pharmacol. 2018;70:1494–1502. doi: 10.1111/jphp.13006. [DOI] [PubMed] [Google Scholar]
- Petrou C.L., D’Ovidio T.J., Bölükbas D.A., Tas S., Brown R.D., Allawzi A., Lindstedt S., Nozik-Grayck E., Stenmark K.R., Wagner D.E., Magin C.M. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J. Mater. Chem. B. 2020;8:6814–6826. doi: 10.1039/D0TB00613K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince E., Kumacheva E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019;4:99–115. doi: 10.1038/s41578-018-0077-9. [DOI] [Google Scholar]
- Provenzano P.P., Eliceiri K.W., Campbell J.M., Inman D.R., White J.G., Keely P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath S.H., Wang C.-H. Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials. 2008;29:2996–3003. doi: 10.1016/j.biomaterials.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Ren J., Xu S., Guo D., Zhang J., Liu S. Increased expression of α5β1-integrin is a prognostic marker for patients with gastric cancer. Clin. Transl. Oncol. 2014;16:668–674. doi: 10.1007/s12094-013-1133-y. [DOI] [PubMed] [Google Scholar]
- Resovi A., Bani M.R., Porcu L., Anastasia A., Minoli L., Allavena P., Cappello P., Novelli F., Scarpa A., Morandi E. Soluble stroma-related biomarkers of pancreatic cancer. EMBO Mol. Med. 2018;10 doi: 10.15252/emmm.201708741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhines L.D., Sampath P., DiMeco F., Lawson H.C., Tyler B.M., Hanes J., Olivi A., Brem H. Local immunotherapy with Interleukin-2 delivered from biodegradable polymer microspheres combined with interstitial chemotherapy: a novel treatment for experimental malignant glioma. Neurosurgery. 2003;52:872–880. doi: 10.1227/01.NEU.0000053211.39087.D1. [DOI] [PubMed] [Google Scholar]
- Rice A.J., Cortes E., Lachowski D., Cheung B.C.H., Karim S.A., Morton J.P., Del Río Hernández A. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6:e352. doi: 10.1038/oncsis.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riching K.M., Cox B.L., Salick M.R., Pehlke C., Riching A.S., Ponik S.M., Bass B.R., Crone W.C., Jiang Y., Weaver A.M. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales A.M., Anseth K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016;1:15012. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff S.M., Keller S., Wieland D.E., Wittmann V., Tovar G.E.M., Bach M., Kluger P.J. clickECM: development of a cell-derived extracellular matrix with azide functionalities. Acta Biomater. 2017;52:159–170. doi: 10.1016/j.actbio.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Sapudom J., Pompe T. Biomimetic tumor microenvironments based on collagen matrices. Biomater. Sci. 2018;6:2009–2024. doi: 10.1039/C8BM00303C. [DOI] [PubMed] [Google Scholar]
- Sato N., Kohi S., Hirata K., Goggins M. Role of hyaluronan in pancreatic cancer biology and therapy: once again in the spotlight. Cancer Sci. 2016;107:569–575. doi: 10.1111/cas.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Tamada M., Dubin-Thaler B.J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M.P. Force sensing by mechanical extension of the src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittert J., Bansal R., Storm G., Prakash J. Integrins in wound healing, fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 2018;129:37–53. doi: 10.1016/j.addr.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Sensi F., D’Angelo E., Piccoli M., Pavan P., Mastrotto F., Caliceti P., Biccari A., Corallo D., Urbani L., Fassan M. Recellularized colorectal cancer patient-derived scaffolds as in vitro pre-clinical 3D model for drug screening. Cancers (Basel) 2020;12:681. doi: 10.3390/cancers12030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M.H., Yu R.T., Engle D.D., Ding N., Atkins A.R., Tiriac H., Collisson E.A., Connor F., Van Dyke T., Kozlov S. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.P., Schwartz M.P., Lee J.Y., Fairbanks B.D., Anseth K.S. A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration. Biomater. Sci. 2014;2:1024–1034. doi: 10.1039/C4BM00022F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.P., Schwartz M.P., Tokuda E.Y., Luo Y., Rogers R.E., Fujita M., Ahn N.G., Anseth K.S. A synthetic modular approach for modeling the role of the 3D microenvironment in tumor progression. Sci. Rep. 2015;5:17814. doi: 10.1038/srep17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slapak E.J., Duitman J., Tekin C., Bijlsma M.F., Spek C.A. Matrix Metalloproteases in pancreatic ductal adenocarcinoma: key drivers of disease progression? Biology (Basel) 2020;9:80. doi: 10.3390/biology9040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan S.B., Taber A.M., Jileaeva I., Pegues E.P., Sentman C.L., Stephan M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzmaier F.J., Jean C., Schlaepfer D.D. FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakuri P.S., Liu C., Luker G.D., Tavana H. Biomaterials-Based approaches to tumor Spheroid and organoid modeling. Adv. Healthc. Mater. 2017;7:1700980. doi: 10.1002/adhm.201700980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbury K., Campagnola P.J. Applications of second-harmonic generation imaging microscopy in ovarian and breast cancer. Perspect. Medicin. Chem. 2015;7:21–32. doi: 10.4137/PMC.S13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y.-Y., Su C.-H., Yang S.-T., Huang Y.-C., Lee W.-H., Wang Y.-C., Liu S.-C., Liu S.-J. Advanced interstitial chemotherapy combined with targeted treatment of malignant glioma in rats by using drug-loaded nanofibrous membranes. Oncotarget. 2016;7:37. doi: 10.18632/oncotarget.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helvert S., Storm C., Friedl P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018;20:8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihinen P., Kähäri V.-M. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int. J. Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- Wang H., Heilshorn S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015;27:3717–3736. doi: 10.1002/adma.201501558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Najibi A.J., Sobral M.C., Seo B.R., Lee J.Y., Wu D., Li A.W., Verbeke C.S., Mooney D.J. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat. Commun. 2020;11:5696. doi: 10.1038/s41467-020-19540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang L., Liang F., Chen Y., Yang G. Integrin alpha x stimulates cancer angiogenesis through PI3K/Akt signaling–mediated VEGFR2/VEGF-A overexpression in blood vessel endothelial cells. J. Cell. Biochem. 2019;120:1807–1818. doi: 10.1002/jcb.27480. [DOI] [PubMed] [Google Scholar]
- Wei S.C., Fattet L., Tsai J.H., Guo Y., Pai V.H., Majeski H.E., Chen A.C., Sah R.L., Taylor S.S., Engler A.J., Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden J., Tel J., Figdor C.G. Synthetic immune niches for cancer immunotherapy. Nat. Rev. Immunol. 2018;18:212–219. doi: 10.1038/nri.2017.89. [DOI] [PubMed] [Google Scholar]
- Whatcott C.J., Han H., Von Hoff D.D. Orchestrating the tumor microenvironment to Improve survival for patients with pancreatic cancer: normalization, Not destruction. Cancer J. 2015;21:299–306. doi: 10.1097/PPO.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead J., Vignjevic D., Fütterer C., Beaurepaire E., Robine S., Farge E. Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2008;2:286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- Winkler J., Abisoye-Ogunniyan A., Metcalf K.J., Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Fowler A.J., Garmon C.B., Fessler A.B., Ogle J.D., Grover K.R., Allen B.C., Williams C.D., Zhou R., Yazdanifar M. Treatment of pancreatic ductal adenocarcinoma with tumor antigen specific-targeted delivery of paclitaxel loaded PLGA nanoparticles. BMC Cancer. 2018;18:457. doi: 10.1186/s12885-018-4393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Mori M., Kataoka A., Inoue H., Sugimachi K. The clinical significance of MMP-1 expression in oesophageal carcinoma. Br. J. Cancer. 2001;84:276–282. doi: 10.1054/bjoc.2000.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Mori M., Shiraishi T., Shibuta K., Sugimachi K. Clinical significance of matrix metalloproteinase-7 expression in esophageal carcinoma. Clin. Cancer Res. 2000;6:1169–1174. [PubMed] [Google Scholar]
- Yang Z., Yu S., Li D., Gong Y., Zang J., Liu J., Chen X. The effect of PLGA-based hydrogel scaffold for improving the drug maximum-tolerated dose for in situ osteosarcoma treatment. Colloids Surf. B Biointerfaces. 2018;172:387–394. doi: 10.1016/j.colsurfb.2018.08.048. [DOI] [PubMed] [Google Scholar]
- Yi H.-G., Jeong Y.H., Kim Y., Choi Y.-J., Moon H.E., Park S.H., Kang K.S., Bae M., Jang J., Youn H. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019;3:509–519. doi: 10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- Yu W., Datta A., Leroy P., O’Brien L.E., Mak G., Jou T.-S., Matlin K.S., Mostov K.E., Zegers M.M.P. β1-Integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervantonakis I.K., Hughes-Alford S.K., Charest J.L., Condeelis J.S., Gertler F.B., Kamm R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Jiang T., She X., Shen S., Wang S., Deng J., Shi W., Mei H., Hu Y., Pang Z., Jiang X. Fibrin degradation by rtPA enhances the delivery of nanotherapeutics to A549 tumors in nude mice. Biomaterials. 2016;96:63–71. doi: 10.1016/j.biomaterials.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Zinger A., Koren L., Adir O., Poley M., Alyan M., Yaari Z., Noor N., Krinsky N., Simon A., Gibori H. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13:11008–11021. doi: 10.1021/acsnano.9b02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion O., Genin O., Kawada N., Yoshizato K., Roffe S., Nagler A., Iovanna J.L., Halevy O., Pines M. Inhibition of transforming growth factor β signaling by halofuginone as a modality for pancreas fibrosis Prevention. Pancreas. 2009;38 doi: 10.1097/MPA.0b013e3181967670. [DOI] [PubMed] [Google Scholar]
- Zou L., Wang D., Hu Y., Fu C., Li W., Dai L., Yang L., Zhang J. Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget. 2017;8:36. doi: 10.18632/oncotarget.19728. [DOI] [PMC free article] [PubMed] [Google Scholar]