Abstract
Introduction
This study evaluates the impact of the COVID-19 pandemic on testing for common sexually transmitted infections. Specifically, changes are measured in chlamydia and gonorrhea testing and case detection among patients aged 14–49 years during the COVID-19 pandemic.
Methods
U.S. chlamydia and gonorrhea testing and positivity were analyzed on the basis of >18.6 million tests (13.6 million tests for female patients and 4.7 million tests for male patients) performed by a national reference clinical laboratory from January 2019 through June 2020.
Results
Chlamydia and gonorrhea testing reached a nadir in early April 2020, with decreases (relative to the baseline level) of 59% for female patients and 63% for male patients. Declines in testing were strongly associated with increases in weekly positivity rates for chlamydia (R2=0.96) and gonorrhea (R2=0.85). From March 2020 through June 2020, an expected 27,659 (26.4%) chlamydia and 5,577 (16.5%) gonorrhea cases were potentially missed.
Conclusions
The COVID-19 pandemic impacted routine sexually transmitted infection services, suggesting an increase in syndromic sexually transmitted infection testing and missed asymptomatic cases. Follow-up analyses will be needed to assess the long-term implications of missed screening opportunities. These findings should serve as a warning for the potential sexual and reproductive health implications that can be expected from the overall decline in testing and potential missed cases.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic required the implementation of prevention measures that changed how people interact with one another and with healthcare services, including seeking screening and care for sexually transmitted infections (STIs). Chlamydia and gonorrhea are the 2 most common notifiable diseases in the U.S., and their rates have continually increased since 2013.1, 2, 3 Increasing rates of these STIs are due to myriad factors, such as expanding social and sexual networks and decreased funding for already strained sexual health services.1 , 4, 5, 6
The Centers for Disease Control and Prevention (CDC) provided guidance on May 13, 2020 for sexual health services during the pandemic.7 For STI clinics that remained open, CDC recommended prioritizing patients on the basis of symptoms and risk, with routine screening visits deferred until after the emergency response.7 The guidance also recommended providing phone- or telemedicine-based triage, including syndromic management, identifying additional at-risk individuals, and referral to other clinics and pharmacies that can provide healthcare services.7 In addition to these direct patient-care restrictions, some STI disease intervention specialists, who routinely provide partner management, were redeployed to perform contact tracing for the COVID-19 pandemic.8 , 9
The impact of both delayed healthcare screening and redeployment of disease intervention specialist personnel to combat the pandemic may have negative outcomes on STI prevention and control, comparable with what has been noted in cancer diagnosis and treatment.10 Similar to early stages of cancer, chlamydia and gonorrhea are often asymptomatic (~80% of female cases), and therefore age- and sexual behavior‒based screening is recommended.11 Unlike cancer, the long-term sequelae of STIs are often unrecognized and include a higher risk of HIV infection, pelvic inflammatory disease, infertility, ectopic pregnancy, and other adverse outcomes of pregnancy.1 During the COVID-19 pandemic, the likely shift of testing toward symptomatic patients may be reducing the ability to identify asymptomatic cases, particularly cases of chlamydia in male and female individuals. The objective of this study is to evaluate the impact of the COVID-19 pandemic on testing and case detection for chlamydia and gonorrhea among patients aged 14–49 years during the onset of the COVID-19 pandemic using data obtained from a national reference clinical laboratory.
METHODS
Study Population
Deidentified results from all patients aged 14–49 years with test results (positive or negative) from the first full calendar week of January 2019 (beginning on January 6, 2019) through the week ending on June 27, 2020 were selected for potential inclusion. This age group was selected because it accounts for the largest proportion (>90%) of STI cases in the U.S.1 Follow-up specimens with consecutive positive results within a 90-day period were excluded because they may have represented unresolved infections previously identified. Specimens were required to have a unique company-wide patient identifier to enable the analysis of consecutive positive tests. Community spread of COVID-19 was presumed to have started by February 28, 2020, and a national emergency was declared on March 13, 2020.12 , 13 For simplification, March 1–June 27, 2020 (the period that has the latest data available) was used as the COVID-19 pandemic period, with the preceding 60-week period defined as the baseline period.
Measures
Specimens from urogenital, anorectal, or oropharyngeal sites were tested for chlamydia and gonorrhea using the Aptima Combo 2 Assay (Hologic, San Diego, CA). Equivocal test results were excluded. For some patients, specimens from multiple anatomic sites (urogenital, pharyngeal, or rectal) collected during the same examination were tested. A positive result could have come from any site, but each visit was counted as a single event regardless of the number of anatomic sites tested per encounter.
Patients were classified by sex (female and male), age group at the time of testing (14–19, 20–24, 25–30, 31–34, 35–39, 40–44, and 45–49 years), and HHS region. When the patient's state of residence information was missing, the account state was used, where available. Patients without data for sex or state were included in the overall analyses but were excluded in the stratified analyses.
Statistical Analysis
Comparisons of proportions were conducted using chi-square tests. To enable an analysis of anticipated positive specimens, a simple linear trend formula14 was calculated using weekly positive cases during the baseline period and then projected forward in time through the COVID-19 pandemic period. Separate linear trend formulas for baseline positive cases were created for each sex/age-group combination and each HHS region for the stratified analyses. Missing positive specimens were calculated as the difference between the number of anticipated and observed positive specimens. The 95% CIs for missing positive specimens shown in Table 1 were calculated as the difference between the 95% CIs of anticipated positive specimens and the number of observed positive specimens. A separate, additional analysis of the association between aggregate weekly positivity rates and testing volumes during the pandemic (both calculated as a percentage of baseline statistics) included unadjusted linear regression models and correlation coefficients (R 2). Data analyses were performed using SAS Studio, version 3.6, on SAS, version 9.4. This Quest Diagnostics Health Trends study was deemed exempt by the Western IRB (Puyallup, Washington).
Table 1.
Testing Volume, Positivity Rate, and Projected Missing Positives by Age, Sex, and HHS Regions
| Chlamydiaa |
Gonorrheab |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years), sex, or region | Testing, n | Baseline positivity | Trough volume (% baseline)c | Peak positivity (% baseline)c | Missing positive (95% CI) | Testing, n | Baseline positivityc | Trough volume (% baseline)c | Peak positivity (% baseline) | Missing positive (95% CI) |
| Female, total | 6,999,676 | 4.0 | 41.1 | 121.1 | 16,946 (11,865, 22,027) | 6,949,408 | 0.7 | 41.6 | 173.1 | 1,613 (700, 2,525) |
| 14−19 | 1,028,945 | 8.2 | 34.3 | 134.7 | 5,622 (3,913, 7,330) | 994,634 | 1.2 | 35.2 | 210.0 | 516 (249, 783) |
| 20−24 | 1,697,071 | 6.3 | 41.9 | 123.9 | 5,978 (4,091, 7,864) | 1,666,716 | 0.9 | 42.6 | 186.7 | 273 (19, 565) |
| 25−29 | 1,463,335 | 3.4 | 46.5 | 125.1 | 2,674 (1,753, 3,595) | 1,457,186 | 0.7 | 46.9 | 161.8 | 226 (0, 452) |
| 30−34 | 1,136,603 | 1.9 | 48.1 | 120.0 | 1,248 (802, 1,695) | 1,138,372 | 0.5 | 48.4 | 162.5 | 272 (122, 422) |
| 35−39 | 799,645 | 1.4 | 41.3 | 117.6 | 748 (521, 975) | 805,042 | 0.4 | 41.5 | 189.2 | 133 (37, 228) |
| 40−44 | 509,665 | 1.1 | 32.0 | 169.2 | 401 (279, 523) | 516,623 | 0.4 | 32.1 | 273.5 | 137 (79, 195) |
| 45−49 | 364,412 | 0.8 | 26.3 | 159.7 | 275 (188, 363) | 370,835 | 0.3 | 26.6 | 257.3 | 56 (10, 101) |
| Male, total | 2,322,486 | 6.7 | 36.9 | 143.4 | 10,697 (8,483, 12,910) | 2,364,135 | 3.4 | 36.7 | 185.4 | 3,965 (2,877, 5,052) |
| 14−19 | 371,944 | 7.3 | 26.5 | 188.7 | 2,054 (1,607, 2,502) | 363,390 | 2.2 | 27.1 | 293.7 | 202 (59, 345) |
| 20−24 | 454,618 | 11.2 | 38.5 | 144.7 | 2,982 (2,246, 3,719) | 454,160 | 4.1 | 38.7 | 216.0 | 481 (232, 731) |
| 25−29 | 463,630 | 7.5 | 39.0 | 146.4 | 2,252 (1,741, 2,763) | 472,947 | 4.1 | 38.6 | 186.1 | 921 (608, 1,234) |
| 30−34 | 372,541 | 5.5 | 40.0 | 141.5 | 1,418 (1,056, 1,780) | 385,516 | 3.7 | 39.5 | 193.6 | 850 (627, 1,072) |
| 35−39 | 281,827 | 4.2 | 38.9 | 141.9 | 951 (722, 1,179) | 293,504 | 3.1 | 38.3 | 164.0 | 763 (563, 964) |
| 40−44 | 206,278 | 3.4 | 39.4 | 150.9 | 614 (479, 749) | 215,568 | 2.7 | 38.4 | 160.4 | 526 (381, 671) |
| 45−49 | 171,648 | 2.6 | 36.4 | 140.1 | 425 (325, 525) | 179,050 | 2.3 | 35.5 | 160.2 | 222 (115, 329) |
| HHS regions | ||||||||||
| (1) CT, MA, ME, NH, RI, VT | 642,044 | 3.4 | 26.0 | 142.6 | 2,166 (1,791, 2,541) | 640,186 | 0.8 | 25.9 | 229.3 | 473 (326, 619) |
| (2) NJ, NY | 1,461,888 | 3.3 | 23.1 | 152.2 | 5,247 (4,492, 6,001) | 1,418,886 | 1.0 | 23.8 | 265.8 | 1,111 (862, 1,361) |
| (3) DE, DC, MD, PA, VA, WV | 692,069 | 4.2 | 35.3 | 137.7 | 2,597 (2,006, 3,187) | 657,393 | 0.9 | 38.1 | 206.8 | 511 (352, 671) |
| (4) AL, FL, GA, KY, MS, NC, SC, TN | 2,045,747 | 5.4 | 49.0 | 126.5 | 3,703 (1,816, 5,591) | 2,082,178 | 1.6 | 48.2 | 148.8 | 678 (170, 1,186) |
| (5) IL, IN, MI, MN, OH, WI | 893,619 | 6.0 | 38.1 | 130.7 | 4,333 (3,384, 5,282) | 885,743 | 2.1 | 39.0 | 177.7 | 1,350 (981, 1,720) |
| (6) AR, LA, NM, OK, TX | 855,486 | 6.5 | 50.4 | 126.6 | 1,715 (768, 2,663) | 877,145 | 1.9 | 49.6 | 190.3 | −118 (−376, 139) |
| (7) IA, KS, MO, NE | 280,574 | 6.4 | 48.6 | 127.0 | 1,011 (714, 1,309) | 279,574 | 2.0 | 48.3 | 183.1 | 171 (41, 300) |
| (8) CO, MT, ND, SD, UT, WY | 110,089 | 5.8 | 46.9 | 130.3 | 441 (291, 591) | 113,898 | 1.7 | 45.6 | 186.0 | 67 (14, 121) |
| (9) AZ, CA, HI, NV | 2,171,039 | 4.2 | 44.1 | 117.8 | 5,707 (3,967, 7,446) | 2,178,722 | 1.2 | 44.0 | 164.7 | 1,164 (690, 1,637) |
| (10) AK, OR, ID, WA | 165,376 | 5.2 | 46.2 | 141.9 | 633 (448, 818) | 175,661 | 1.6 | 44.9 | 219.1 |
|
Note: Owing to differences in stratified linear positive specimen trends, the stratified subgroups arrive at different estimates for missing positive specimens from the totals for male and female.
Chlamydia testing: 7,529 missing sex data; 11,760 missing HHS region data.
Gonorrhea testing: 8,009 missing sex data; 12,118 missing HHS region data.
Trough volume and peak positivity were measured as the percentage of the baseline mean weekly values.
AK, Alaska; AL, Alabama; AR, Arkansas; AZ, Arizona; CA, California; CO, Colorado; CT, Connecticut; DC, District of Columbia; DE, Delaware; FL, Florida; GA, Georgia; HI, Hawaii; IA, Iowa; ID, Idaho; IL, Illinois; IN, Indiana; KS, Kansas; KY, Kentucky; LA, Louisiana; MA, Massachusetts; MD, Maryland; ME, Maine; MI, Michigan; MN, Minnesota; MO, Missouri; MS, Mississippi; MT, Montana; NC, North Carolina; ND, North Dakota; NE, Nebraska; NH, New Hampshire; NJ, New Jersey; NM, New Mexico; NV, Nevada; NY, New York; OH, Ohio; OK, Oklahoma; OR, Oregon; PA, Pennsylvania; RI, Rhode Island; SC, South Carolina; SD, South Dakota; TN, Tennessee; TX, Texas; UT, Utah; VA, Virginia; VT, Vermont; WA, Washington; WI, Wisconsin; WV, West Virginia; WY, Wyoming.
RESULTS
Patients resided in all the U.S. states and the District of Columbia. A total of 9,329,691 chlamydia and 9,321,572 gonorrhea test results were assessed. Most patient specimens (92.9%) were tested for chlamydia and gonorrhea simultaneously; 18,110,634 (97.1%) results were from specimens that were urogenital, 322,534 (1.7%) were from those that were pharyngeal, and 218,095 (1.2%) were from those that were rectal. Approximately three quarters of tested specimens were from female patients. Patients aged <30 years represented 58.8% and 58.1% of the chlamydia and gonorrhea testing, respectively (Appendix Table 1, available online).
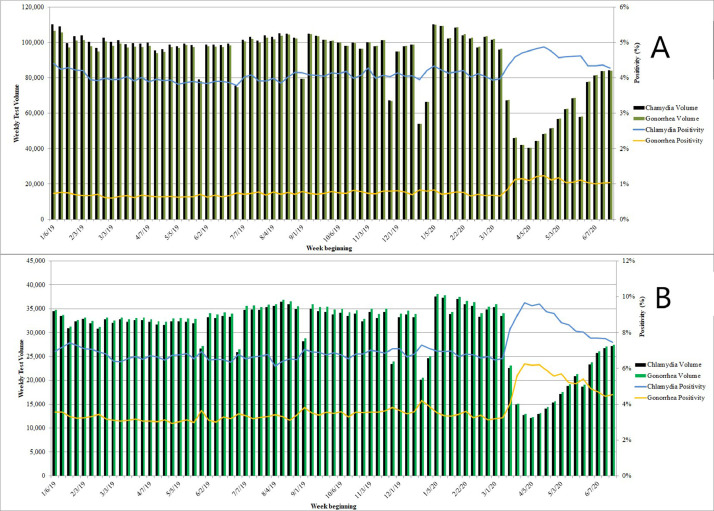
Because cotesting for chlamydia and gonorrhea is the norm in the U.S., volumes for chlamydia and gonorrhea were virtually identical to each other throughout the study period. During the baseline period, the mean weekly test volume was 131,114 (SD=13,431) for chlamydia and 130,831 (SD=13,325) for gonorrhea; the mean positivity rates were 4.7% and 1.4%, respectively. This translated into weekly means of 6,195 (SD=669) cases of chlamydia and 1,819 (SD=196) cases of gonorrhea. During the pandemic period, test volume decreased, whereas positivity rates increased (Figure 1 ). At the nadir, the week starting on April 5, 2020, test volumes had declined to 40% of baseline: decreases of approximately 59% for female patients and 63% for male patients. Chlamydia and gonorrhea positivity rates peaked in the following week, at 5.9% for chlamydia and 2.4% for gonorrhea. For both chlamydia and gonorrhea, there was a strong relationship between the weekly test volume and positivity rates as a percentage of mean baseline statistics during the COVID-19 pandemic (R 2=0.96 for chlamydia and 0.85 for gonorrhea). However, because of reduced testing volumes, the increased positivity rates translated to total case detection of only 3,381 chlamydia and 1,356 gonorrhea cases. During the final week of the study period, test volumes had more than doubled from the trough but were still only 85% of the baseline values, whereas positivity rates for chlamydia and gonorrhea remained significantly higher than the baseline levels (5.1% and 1.9%, respectively; p<0.001 for both).
Figure 1.
Weekly test volume and positivity for Chlamydia trachomatis and Neisseria gonorrhoeae from the first full calendar week in January 2019 through the week starting on June 21, 2020 for (A) females and (B) males.
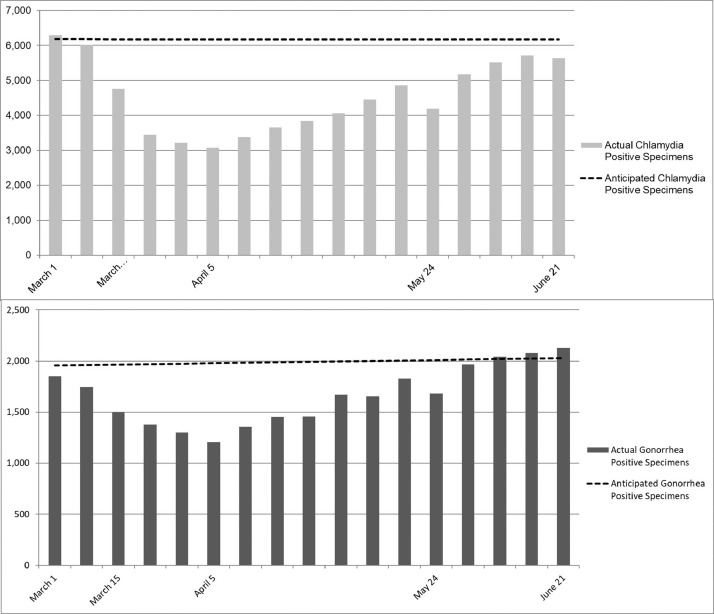
The anticipated number of positive cases was calculated by extrapolating the linear trend in the number of positives from the 60 weeks preceding March 1, 2020 (Figure 2 ). The anticipated numbers of positive cases during the COVID-19 pandemic period in this study were 104,930 for chlamydia and 33,862 for gonorrhea. However, the observed number of cases during this time period was 77,271 for chlamydia and 28,285 for gonorrhea. Thus, the estimated number of potentially missed positive specimens during the COVID-19 pandemic period was 27,659 (95% CI=20,429, 34,888) for chlamydia (26.4%, 95% CI=19.5, 33.3) and 5,577 (95% CI= 3,637, 7,517) for gonorrhea (16.5%, 95% CI=10.7, 22.2).
Figure 2.
Actual versus anticipated positive specimens for (A) Chlamydia trachomatis and (B) Neisseria gonorrhoeae with a baseline trend.
Note: Anticipated C. trachomatis‒positive specimens=6,213.3−0.594*week; difference=27,659. Anticipated N. gonorrhoeae‒positive specimens=1,681.9+4.493*week; difference=5,577.
Male patients had higher baseline positivity rates than female patients for both chlamydia (6.8% vs 4.0%, p<0.001) and gonorrhea (3.4% vs 0.7%, p<0.001). During the COVID-19 pandemic period, positivity rates increased significantly among male patients (chlamydia: to 8.0%, 18% increase, p<0.001; gonorrhea: to 4.8%, 41% increase, p<0.001) and female patients (chlamydia: to 4.4%, 10% increase, p<0.001; gonorrhea: to 1.0%, 43% increase, p<0.001). The proportion of missed positive specimens compared with the anticipated number was higher for male patients than for female patients for both chlamydia (27.6% vs 25.6%, p<0.001) and gonorrhea (18.8% vs 12.6%, p<0.001) (Table 1).
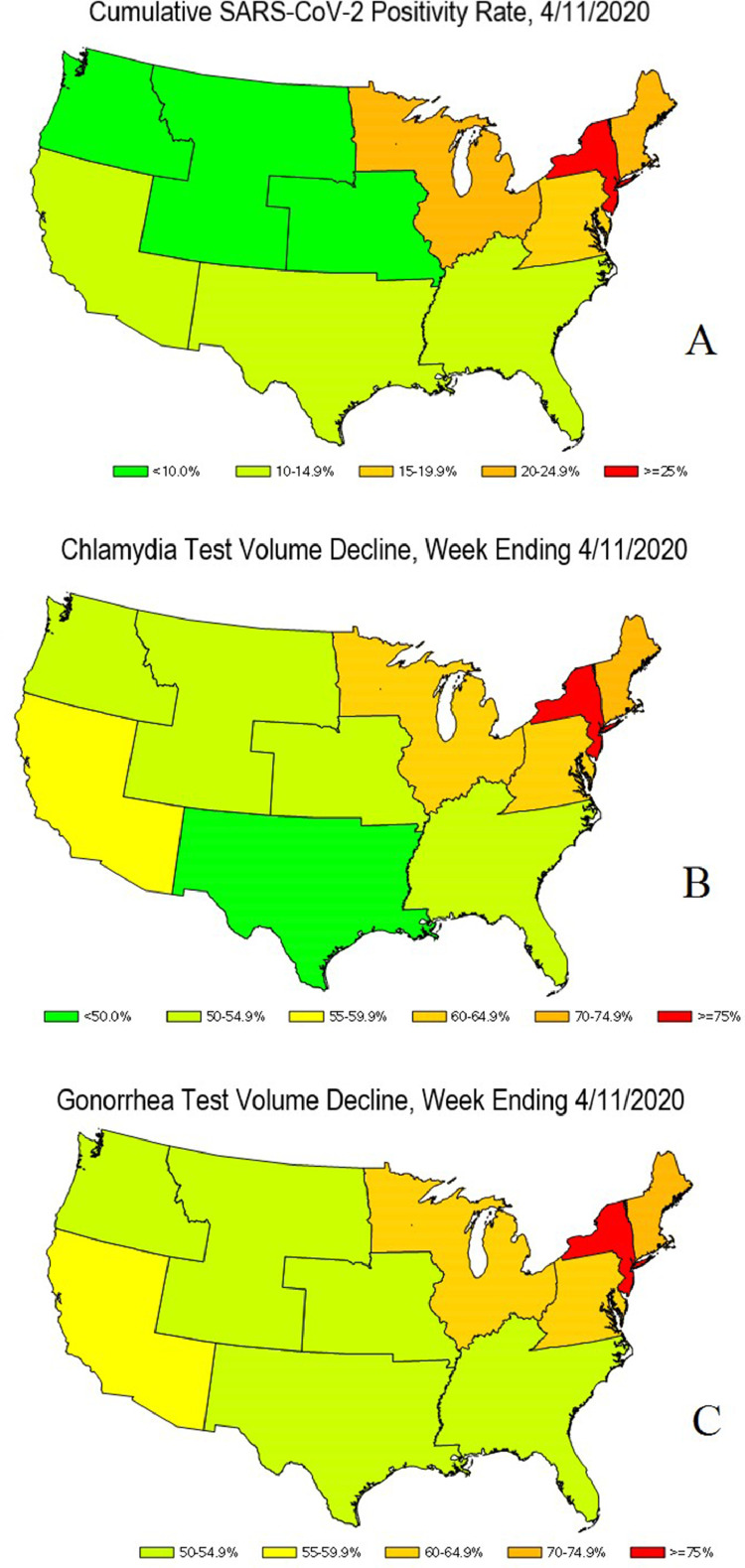
The largest trough decline in chlamydia and gonorrhea testing volume (>76% for both) occurred in HHS Region 2 (New York and New Jersey), which coincided with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) case peaks in this region.15 In addition, this region also had the largest increase in peak weekly positivity (52% for chlamydia, 166% for gonorrhea) and the largest proportion of missing chlamydia-positive cases (43.4%). Notable regional differences also occurred in HHS Region 1 (Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, and Vermont), where the largest proportion of missing gonorrhea-positive cases (32.3%) were found. Finally, there were more gonorrhea-positive specimens than anticipated for HHS Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) during the COVID-19 pandemic period (Table 1). Heat maps of U.S. regions illustrating SARS-CoV-2 positivity and chlamydia/gonorrhea-testing rates at trough testing period are shown in Figure 3 .
Figure 3.
Heatmaps of the U.S., April 11, 2020. (A) Cumulative SARS-CoV-2‒positivity rate. (B) Chlamydia test volume decline. (C) Gonorrhea test volume decline.
DISCUSSION
The COVID-19 pandemic has necessitated far-reaching measures to help curb morbidity and mortality from SARS-CoV-2 infection, including negatively impacted STI case detection. These measures included stay-at-home orders, shifts to telemedicine, closures of clinical services, and reallocation of resources as health care adapted to rapidly changing recommendations from CDC and other professional societies. These changes in patient care services were made to facilitate social distancing, increase SARS-CoV-2 testing, and increase resources for COVID-19 contact tracing. This shift of services, although necessary, has also affected non‒COVID-19 health issues, such as STI management.
Testing for chlamydia and gonorrhea decreased by approximately 59% for female patients and 63% for male patients in early April 2020 before gradually rebounding to approximately 15% below the baseline value by the final week of the study period. Declines were consistent across sex and age groups except among female patients aged 40–49 years. This group had a larger decline in testing with a higher positivity rate for both gonorrhea and chlamydia, which is currently unexplained. Anecdotally, this decline in testing may be related to provider comfort in prescribing medications by telemedicine in this population. These female patients are more likely to be established patients and more likely to have vaginitis owing to lower STI risk. The resultant higher positivity rates are likely due to failure of vaginitis therapy, requiring further testing. This association was not isolated because there was a strong association between the higher weekly positivity rates and the declines in testing volumes among all age groups. However, despite this increase in positivity rates, case detection was adversely affected, with drops of 50% for chlamydia and 39% for gonorrhea during the trough period.
The immediate drop in observed positive cases in March, in theory, should not be reflective of a drop in true prevalence, even if sexual practices changed during the pandemic. Sexual practices likely were unaffected until after the declaration of the national emergency on March 13, 2020, if at all. The slow replication cycle of these 2 STIs and the likelihood of a delayed onset of symptoms of about 10 days for gonorrhea and 3–6 weeks for chlamydia16 , 17 would mean that a drop in cases would not be seen for several weeks after the initiation of stay-at-home orders. Moreover, this drop in cases likely represents the shift to a higher percentage of symptomatic testing with a lower percentage of risk-based screening, which would explain the increased positivity.
The likely decrease in risk-based screening, although necessary, ultimately means that there will be future negative health outcomes from the large number of missed cases, owing to the often asymptomatic nature of both chlamydia and gonorrhea.11 For example, a meta-analysis examining large study cohorts showed that the pelvic inflammatory disease rate attributable to untreated chlamydia infections was >37-fold higher among female patients with deferred chlamydia screening due to lack of symptoms than among those who had received active screening regardless of symptoms.18 In addition, syndromic management of STIs results in poor STI control, which has historically coincided with HIV epidemics.19 With nearly 80% of chlamydia and gonorrhea infections reported as asymptomatic among female patients, the estimated 33,236 missed cases of both STIs (26.4% of anticipated chlamydia cases and 16.5% of anticipated gonorrhea cases) is a reasonable approximation for the laboratory during the COVID-19 time period. The total number of missed cases nationally is likely much larger given that this diagnostic laboratory represents approximately 18% of all positive cases reported by CDC during the baseline period (not the COVID-19 period). Thus, the true number of missed cases in the U.S. anticipated during the COVID-19 period included in this study is likely >150,000. Because the impact of social distancing guidelines remains unknown, the ramifications of this large number of missed cases are not yet fully understood. However, the situation is not likely to improve soon given the ongoing challenges with reagent and supply allocation for testing, combined with the current shortage of STI testing collection devices available in the market since August 2020.20
The spatiotemporal progression of COVID-19 in the U.S. started with New York, New Jersey, and New England, which received the earliest and largest burden of COVID-19 cases, whereas other regions such as the central U.S. were relatively spared. To better understand these geographic disparities and the impact on STI testing and rates, a heat map was created (Figure 3), and it examined STI testing by region. As noted earlier, the largest trough decline (>76% for both chlamydia and gonorrhea) as well as the largest peak weakly positivity rate occurred in HHS Region 2 (New York and New Jersey), which coincided with early SARS-CoV-2 case peaks. As service likely transitioned toward symptomatic STI care, the largest proportions of missing chlamydia-positive cases (43.4%) were also noted in this region. HHS Region 1 (Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, and Vermont) had the largest proportion of missing gonorrhea-positive cases (32.3%), the reasons for which are unclear and could potentially be related to proximity to the epicenter of the pandemic and transition of services or fear of seeking care. These preliminary data, provided in the form of a heat map, show a strong visual representation of the association between the trough in chlamydia testing and the rate of SARS-CoV-2‒positive cases (Figure 3).
Limitations
A limitation of this study is the lack of clinical information for the positive cases. Thus, the authors were unable to associate cases with symptoms or specific populations (e.g., men who have sex with men). A major strength of this study is the analysis of testing and positivity rates at a large scale from patients residing in all the U.S. states and the District of Columbia. To the authors’ knowledge, these data represent the largest national study to date that includes both positive and negative STI test results. These data allow a nuanced understanding of the impact of the COVID-19 pandemic on U.S. STI testing and prevalence. Although the data presented in this paper represent a relatively large share of all testing in the U.S., shifts in testing to alternative laboratories during the pandemic are possible, although unlikely. Infection rates may have changed owing to social distancing and other factors during the pandemic. However, the authors are unaware of evidence to support such a change, and there is evidence to support the notion that sex and casual encounters were still occurring.21, 22, 23, 24
CONCLUSIONS
The unprecedented decline in STI testing has already had an impact on the perception of the STI epidemic in the U.S. The National Coalition for STD Directors highlighted the drop in STI cases, with predictions of major declines in STI rates due to social distancing.9 , 20 In addition, there have been international reports of a decline in STIs19 , 21 during the pandemic. These reports and predictions may not have fully accounted for the relationship among case rates, missed cases, and the declines in STI testing, especially in the context of potential downstream consequences such as pelvic inflammatory disease and HIV. Further studies are warranted to understand the long-term implications of COVID-19 on STI rates. Thus, the findings should serve as a warning of the potential sexual and reproductive health complications that can be expected because of limited STI screening services in the U.S.
ACKNOWLEDGMENTS
The authors thank Ron Kagan and Cara Exten for their contributions to this article.
Quest Diagnostics (Secaucus, NJ) did not have any additional role in the study design and in the collection, analysis, or interpretation of the data.
Quest Diagnostics provided support in the form of salaries for JKN, HWK, EMM, and DA.
All authors contributed to the writing, reviewing, and revising of the manuscript for intellectual and technical content. CNP, EMM, and BVDP conceptualized the research idea. CNP, EMM, BVDP, HWK, and JKN contributed to the study design. JKN performed all data analysis.
The content of this manuscript has not been presented elsewhere.
JKN, HWK, EMM, and DA are employees of Quest Diagnostics. HWK, EMM, and DA own stocks in Quest Diagnostics. BVDP has received grant support to her institution and consulting fees or speaking honorarium from Abbott, BD Diagnostics, binx health, BioFire, Cepheid, Hologic, Rheonix, Roche, and SpeeDx. No other financial disclosures were reported.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2021.03.009.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Sexually transmitted disease surveillance 2018. Centers for Disease Control and Prevention. https://www.cdc.gov/std/stats18/default.htm. Updated January 25, 2021. Accessed April 5, 2021.
- 2.2015 STD surveillance report. Centers for Disease Control and Prevention. https://www.cdc.gov/nchhstp/newsroom/2016/2015-std-surveillance-report.html. Updated October 19, 2016. Accessed August 27, 2020.
- 3.NCHHSTP Newsroom, Centers for Disease Control and Prevention; Atlanta, GA: October 8, 2019. New CDC report: STDs continue to rise in the U.S. [press release]https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report-press-release.html [Google Scholar]
- 4.Enomoto C, Noor S, Widner B. Is social media to blame for the sharp rise in STDs? Soc Sci. 2017;6(3):78. doi: 10.3390/socsci6030078. [DOI] [Google Scholar]
- 5.Traeger MW, Schroeder SE, Wright EJ. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67(5):676–686. doi: 10.1093/cid/ciy182. [DOI] [PubMed] [Google Scholar]
- 6.Chen A. STD infections rise to new highs after states close health clinics. NPR. October 20, 2016 https://www.npr.org/sections/health-shots/2016/10/20/498719092/std-infections-rise-to-new-highs-after-states-close-health-clinics [Google Scholar]
- 7.Bachman LH. HHS, Public Health Service, Centers for Disease Control and Prevention; Washington, DC: Published May 13, 2020. Dear colleague letter - clarification.https://www.cdc.gov/std/dstdp/dcl-clarification-may2020.pdf [Google Scholar]
- 8.National Coalition of STD Directors; Washington, DC: March 23, 2020. Updated: starved public health system in distress, STD first responders spring into action against COVID-19 [press release]https://www.ncsddc.org/update-starved-public-health-system-in-distress-std-first-responders-spring-into-action-against-covid-19/ [Google Scholar]
- 9.Bolan G. DSTDP director's update – July 2020. National Coalition of STD Directors. July 23, 2020. https://www.ncsddc.org/dstdp-directors-update-july-2020/. Accessed August 12, 2020.
- 10.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of us patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic [published correction appears in JAMA Netw Open. 2020;3(9):e2020927] JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detels R, Green AM, Klausner JD. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis. 2011;38(6):503–509. doi: 10.1097/olq.0b013e318206c288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.President Trump declares state of emergency for COVID-19 . March 25, 2020. National Conference of State Legislatures.https://www.ncsl.org/ncsl-in-dc/publications-and-resources/president-trump-declares-state-of-emergency-for-covid-19.aspx [Google Scholar]
- 13.Update and interim guidance on outbreak of coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://emergency.cdc.gov/han/2020/HAN00428.asp. Updated February 28, 2020. Accessed February 22, 2021.
- 14.Altman DG. Practical Statistics for Medical Research. Chapman & Hall; London, United Kingdom: 1991. Comparing groups – continuous data. [Google Scholar]
- 15.Workbook : NYS-COVID19-tracker. New York State Department of Health.https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n. Updated May 9, 2021. Accessed August 27, 2020.
- 16.STD modules: gonorrhea. National STD Curriculum.https://www.std.uw.edu/custom/self-study/gonorrhea/1. Updated December 29, 2020. Accessed August 12, 2020.
- 17.STD modules: chlamydia. National STD Curriculum. https://www.std.uw.edu/custom/self-study/chlamydia. Updated December 29, 2020. Accessed August 12, 2020.
- 18.Davies B, Turner KM, Leung S. Comparison of the population excess fraction of Chlamydia trachomatis infection on pelvic inflammatory disease at 12-months in the presence and absence of chlamydia testing and treatment: systematic review and retrospective cohort analysis. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steen R, Wi TE, Kamali A, Ndowa F. Control of sexually transmitted infections and prevention of HIV transmission: mending a fractured paradigm. Bull World Health Organ. 2009;87(11):858–865. doi: 10.2471/blt.08.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann L, Bolan G. HHS, Public Health Service, Centers for Disease Control and Prevention; Washington, DC: Published September 8, 2020. Dear colleague letter: DSTDP lab and drug shortages.https://www.cdc.gov/std/general/DCL-Diagnostic-Test-Shortage.pdf [Google Scholar]
- 21.de Miguel, Buckley R, Trigo E, de la Calle-Prieto F, Arsuaga M, Díaz-Menéndez M. Social distancing to combat COVID-19 led to a marked decrease in food-borne infections and sexually transmitted diseases in Spain. J Travel Med. 2020;27(8):taaa134. doi: 10.1093/jtm/taaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob L, Smith L, Butler L. Challenges in the practice of sexual medicine in the time of COVID-19 in the United Kingdom. J Sex Med. 2020;17(7):1229–1236. doi: 10.1016/j.jsxm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tregenza H. Coronavirus shutdown could be responsible for decrease in sexually transmitted infection rates in the ACT. ABC News. May 19, 2020 https://www.abc.net.au/news/2020-05-20/coronavirus-shutdown-leads-to-apparent-drop-in-stis-in-canberra/12264908 [Google Scholar]
- 24.Barbee LA, Dombrowski JC, Hermann S. Sex in the time of COVID”: clinical guidelines for sexually transmitted disease management in an era of social distancing. Sex Transm Dis. 2020;47(7):427–430. doi: 10.1097/OLQ.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.