Abstract
Cancer has become one of the greatest threats to human health, and new technologies are urgently needed to further clarify the mechanisms of cancer so that better detection and treatment strategies can be developed. At present, extensive genomic analysis and testing of clinical specimens shape the insights into carcinoma. Nevertheless, carcinoma of humans is a complex ecosystem of cells, including carcinoma cells and immunity-related and stroma-related subsets, with accurate characteristics obscured by extensive genome-related approaches. A growing body of research shows that sequencing of single-cell RNA (scRNA-seq) is emerging to be an effective way for dissecting human tumor tissue at single-cell resolution, presenting one prominent way for explaining carcinoma biology. This review summarizes the research progress of scRNA-seq in the field of tumors, focusing on the application of scRNA-seq in tumor circulating cells, tumor stem cells, tumor drug resistance, the tumor microenvironment, and so on, which provides a new perspective for tumor research.
Keywords: single-cell RNA sequencing, carcinoma, carcinoma stem cells, tumor resistance, tumor microenvironment
Graphical abstract
Sequencing of single-cell RNA (scRNA-seq) is emerging to be an effective way for dissecting human tumor tissue at single-cell resolution, presenting one prominent way for explaining carcinoma biology. This review summarizes the research progress of scRNA-seq in the field of tumors, which provides a new perspective for tumor research.
Introduction
During the last three decades, carcinoma studies have largely discussed somatic gene-centric mutations (so-called oncogenes) that target their functional characteristics and biochemical activity.1,2 Thus far, multiple targeted therapies have been approved to treat multiple tumors, and more are in development or in early clinical trials. However, with the extensive use of targeted therapies, common themes of treating relapse and drug resistance have been proposed.3,4 Carcinoma refers to a selectively proliferating, invasive somatic mutant phenotype. There is an implicit concept that following the evolution path of carcinoma, genetically complex groups of different individual carcinoma cells may develop and interact in a dynamic manner with each other. Therefore, studying this potential intratumoral genetic heterogeneity is of great significance for the selection of anti-target treatment methods.
Indeed, as next-generation sequencing (NGS) technology is emerging, sequencing of large amounts of DNA and RNA retrieved from carcinoma tissue can be conducted in depth, fine-grained studies of intra-tumor genetic heterogeneity can be carried out, and computational inferences of subclonality can be achieved.5,6 However, as impacted by practical and technical limitations, deep sequencing alone is insufficient to fully explore the genomic and transcriptomic heterogeneity of carcinoma. Individual cells are the basic substrates for the mechanisms of mutation and selection at work to evolve complex structures known as tumor blocks. For this reason, gaining insights into individual carcinoma cells under the individual condition and, overall, into dynamically related systems of interaction (carcinoma microenvironment) will indeed further clarify therapy-related resistance and biology of tumors generally.
The quickly advancing method of single-cell RNA sequencing (scRNA-seq), by exhibiting its ability for characterizing the epigenome, transcriptome, and genome of one individual cell, profoundly presents insights into genetics and tumor biology, and it will enable us to understand the changes in the various stages of tumor progression to advanced metastatic disease.7,8 In addition, the clinical application of scRNA-seq may profoundly alter the way we treat carcinoma. Much information has been gathered using the mentioned techniques, and, although numerous difficulties still exist, it is considered that the capacity exhibited by scRNA-seq will continuously drive innovation and yield methods to solve current issues that profoundly increase our understanding of the disease. In this review, we discuss the topic of scRNA-seq in the carcinoma field and highlight the use of emerging scRNA-seq approaches to circulating tumor cells (CTCs), tumor drug resistance, and the tumor microenvironment (TME).
What is scRNA-seq?
RNA sequencing (either cell collections or individual cells) is one effective approach to investigate gene expressing modes, involving the reverse transcribing of RNA to cDNA, followed by DNA sequencing under significant throughput. Genes exhibiting a significant expression state in the sample generate greater amounts of DNA sequence readings, cDNA and RNA, as opposed to those with a lower expressing state. For this reason, RNA sequencing presents a digital gene expressing state reading, of which DNA sequence readings have a number abiding by the expressing level of one gene inside the sample. The scRNA-seq idea is consistent with that mentioned, although an individual cell should first receive the isolation because of its tiny RNA content, which requires an effective amplifying procedure for producing enough cDNA as an attempt to sequence protein. Under conventional RNA sequencing, the more considerable amount of reads on the sequence of DNA of one gene represents the greater expressing state of such a gene in the cell.9, 10, 11 It is noteworthy that the current scRNA-seq approach can determine the expression levels of all genes.
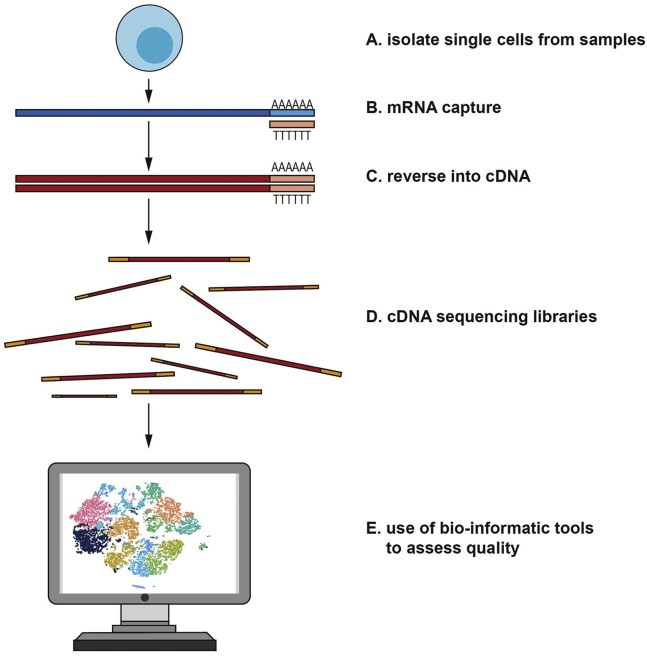
Experiment-related workflow of scRNA-seq techniques
In summary, existing scRNA-seq technologies overall have a general workflow as follows: a sample preparing process, an individual cell capturing process, a reverse transcribing process, an amplifying process, a library preparing process, and a sequencing and analyzing process (Figure 1).12 The sample preparing process is critical for producing reliable individual-cell transcriptome information. A key step in the sample preparation process, especially in terms of dense tissue and three-dimensional-like organ models, refers to individual-cell dissociation, usually done based on enzymatic conditions with mild mechanical agitation for limiting noise in background and extreme cell lysis. Moreover, proteolytic enzymes (e.g., trypsin, collagenase, and free enzymes) and digestion times are required to be rigorously selected for maximizing individual cell production and minimizing apoptosis. If a cell received full dissociation, it can receive the isolation to an individual cell by drawing upon various cell capturing technologies (e.g., the plate-related cell isolating process to droplet-based methods). Maintaining large numbers of isolated living cells shows critical significance for improving data quality. In addition, the approach of cell capturing commonly depends on the target sample nature. Common single-cell separation technologies include limiting dilution, micromanipulation, flow-activated cell sorting (FACS), and laser capture microdissection. Limiting dilution is done with pipette dilution to isolate individual cells. When using this method, usually when the concentration is diluted to 0.5 cells per aliquot, one can only complete about one third of the preparation wells, so this method is not very effective. Micromanipulation is a classic method of extracting cells from early embryos or uncultured microorganisms.13,14 Microscopically guided capillary straws have been used to extract individual cells from suspensions. However, these methods are time-consuming and have a low throughput. Therefore, this method is not often used. Recently, FACS has been a commonly used technology to isolate a high purity of single cells. When the target cells express very low levels of markers, FACS is also the preferred method. In this way, the cells are first labeled with fluorescent monoclonal antibodies. These antibodies have an ability to identify specific surface markings and are able to classify different groups. In addition, negative selection can be used for unstained populations. In this case, based on the predetermined fluorescence parameters, an electric charge is applied to the cell of interest using an electrostatic deflection system, and the cell is magnetically isolated.15 The potential limitations of these techniques include the need for a large initial amount of cells (it is difficult to isolate cells from a low input of less than 10,000) and the need for monoclonal antibodies against the target protein of interest. For laser capture microdissection, cells are isolated from solid samples using a laser system assisted by a computer system.16 When successfully captured, a single cell is cleaved and treated to produce a first strand of cDNA by the reverse transcribing process, with a second strand synthesizing process and a PCR amplifying process conducted subsequently. When PCR amplifying and library preparing processes are conducted, the samples are sequenced. The data from sequencing are then analyzed, rather than simply quantitative analysis of the gene expressing state, for including deep studies on cellular heterogeneity, pedigree transformation, and relationships between cells.12,17,18
Figure 1.
Experiment-related workflow of single-cell RNA sequencing (scRNA-seq) techniques
Advances of individual-cell RNA sequencing in carcinoma
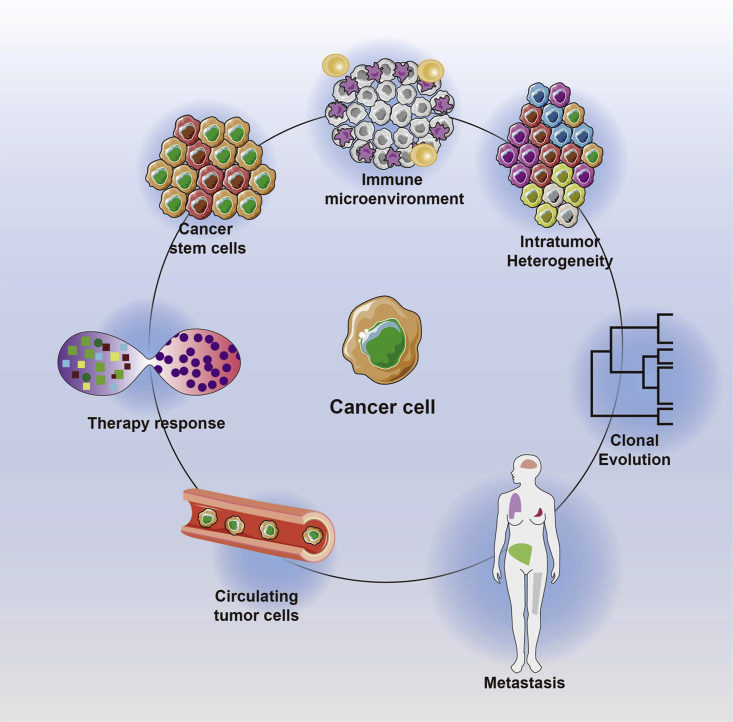
Individual-cell gene expressing profiling has quickly turned out to be one of the normal analysis-related techniques. It has received extensive studies in numerous subjects. Herein, this technique’s use in carcinoma is largely discussed (Figure 2). We comprehensively reviewed the application of single-cell sequencing in cancer and found that this technique mainly plays an important role in the aspects of CTCs, carcinoma stem cells (CSCs), the TME, and tumor drug resistance, so we review previous research mainly around these four aspects.
Figure 2.
Advances of individual-cell RNA sequencing in carcinoma
scRNA-seq and CTCs
CTCs receive detection in most epithelial carcinomas and stand for carcinoma cells that have been obtained when passing via the bloodstream.19, 20, 21 Such detection is currently thought to be key in mediating blood-borne transmission of carcinoma, whereas CTCs may not be easy to detect in the analysis of primary or metastatic tumor populations. The elements that cause primary tumors to produce CTCs are unknown, including parts of the carcinoma cells that are injected in an active manner in the bloodstream intravenously and those that are passively shed due to damage to the tumor’s vascular system. Compared with ordinary cells in the blood, CTC is extremely rare. There are fewer CTCs that can cause distant metastasis, which indicates that a large number of CTCs die in the blood, and only a small part is related to metastasis. However, emerging scRNA-seq brings new hope for CTC detection. We summarize the applications of scRNA-seq techniques in CTC-related aspects to date and present them in Table 1.
Table 1.
Application of scRNA-seq in CTCs
| Tumor | Application of scRNA-seq | Research achievement | Reference |
|---|---|---|---|
| Breast cancer | scRNA-seq was performed on the CTC clusters and on the individual CTCs in breast cancer patients and the date was matched in a single blood sample | in mouse models, albumin knockout eliminated the formation of CTC clusters and inhibited lung metastasis; in breast cancer patients, both the abundance of CTC clusters and the elevation of tumor protein levels predict adverse outcomes | 22 |
| Breast cancer | scRNA-seq of CTCs isolated from blood samples of patients with metastatic estrogen receptor (ER)+ breast cancer was performed to compare the progression of bone and internal organs | the cellular pathway activated by CTCs includes the androgen receptor (AR) signal pathway; expression of the AR gene and its structural active splicing variants AR-V7 increased significantly | 23 |
| Breast cancer | individual CTC-associated WBCs and corresponding cancer cells in each CTC-WBC cluster were isolated and identified from breast cancer patients and mouse models and scRNA-seq was carried out | the association between neutrophils and CTCs promotes development of the cell cycle in the bloodstream and expands the metastatic potential of CTCs | 24 |
| Breast cancer | Hydro-Seq, a scalable fluid dynamic scRNA-seq bar code technology, was used on 666 CTC samples taken from 21 breast cancer patients | drug therapy targets for breast cancer were identified and individual cells expressing tumor stem cell (CSCs) and epithelial/mesenchymal cell state transition markers were tracked; transcriptome analysis of these cells provides insight into the targeted therapy and process of tumor metastasis | 25 |
| Breast cancer | scRNA-seq was used to extract CTCs from concentrated CTCs and carryover PBMCs | transcriptome analysis identified two types of CTC, one rich in estrogen reactivity and increased proliferation, and the other rich in decreased proliferation and EMT; immune avoidance was enhanced in the CTC population with EMT characteristics | 26 |
| Pancreatic cancer | single CTCs were isolated by epitope-independent microfluidic capture in a mouse model of pancreatic cancer and then scRNA-seq was performed | both mouse and human pancreas CTCs highly express interstitial-derived extracellular matrix (ECM) protein, including SPARC, and knockout in cancer cells can inhibit cell migration and invasion | 27 |
| Pancreatic cancer | a mouse model of xenotransplantation from highly metastatic pancreatic ductal adenocarcinoma (PDAC) patients was established and scRNA-seq was performed on circulating tumor cells isolated from human histocompatibility leukocyte antigen (HLA) | isolated CTCs are highly tumorigenic and have the potential of metastasis; the expression profile of a CTC is different from that of the matched primary and metastatic tumors, and it is characterized by low expression of genes related to cell cycle and extracellular matrix | 28 |
| Hepatocellular carcinoma | a sequential combination of image flow cytometry and high-density scRNA-seq was described for the identification of CTCs in hepatocellular carcinoma (HCC) patients | the genome-wide expression profile of CTCs using this method shows heterogeneity of CTCs and helps to detect known oncogenic drivers of HCC, such as IGF2 | 29 |
| Vesicular rhabdomyosarcoma | scRNA-seq was performed on the CTCs from a child with vesicular rhabdomyosarcoma | CTCs are easily detected at diagnosis, and their levels decrease with the success of treatment and can be detected in the blood of patients without radiological evidence of dominant metastasis | 30 |
| Multiple myeloma | single CTC RNA sequencing was used for classification of multiple myeloma (MM) and quantitative evaluation of genes related to prognosis | CTCs provide the same genetic information as bone marrow multiple myeloma cells, and in some cases are even more sensitive than bone marrow biopsy to reveal mutations | 31 |
| Prostate cancer | single CTCs isolated from the blood of patients with metastatic prostate cancer and single prostate cancer cell line LNCaP cells added to the blood of healthy donors were analyzed by mRNA sequencing | the CTC RNA of patients with prostate cancer showed obvious signs of degradation, and the transcriptional characteristics of prostate cancer tissues could be easily detected by a single-CTC RNA sequencing | 32 |
| – | the single-cell expression profiles of publicly available circulating tumor cells were collated | CTCs that span cancer exist in an almost perfect continuum of EMT; comprehensive analysis of CTC transcripts also highlighted the reverse gene expression patterns between PD-L1 and major histocompatibility complex (MHC), which are associated with cancer immunotherapy | 33 |
| – | longitudinal isolated CTCs from animals treated with the BET inhibitor JQ1 for more than 4 days were analyzed by scRNA-seq | the details of the evolution of CTCs over time are revealed, which prove the change of CTCs as a biomarker of drug response and are helpful for future research to understand the role of CTCs in metastasis | 34 |
Aceto et al.22 used in vivo flow cytometry and scRNA-seq for studying CTCs in cases subjected to metastatic breast carcinoma (BC) and inside mouse tumor systems. They found that in mouse models, oligonucleotides from primary tumor cells take up one rare but significantly metastatic subset of CTCs in comparison with an individual circulating BC cell. scRNA-seq of the human breast CTC cluster determined that plakoglobin is the critical medium of aggregation of tumor cells, showing the expression inside one heterogeneous mode in the primary tumor. The expression inhibition of plakoglobin in a mouse model inhibited the forming process of CTC clusters and reduced transfer diffusion. In cases subjected to BC, according to the process to match CTC clusters inside one individual blood sample and individual-CTC resolution RNA sequencing, the cell-linking component plakoglobin exhibited high differential expression. For this reason, CTC clusters cover primary tumor cells’ multicellular clusters, which bind jointly by exploiting intercellular globin adhesion, significantly facilitating metastasis and carcinoma spread.22 Subsequently, some research has suggested scRNA-seq application in detecting CTCs. Miyamoto et al.35 established scRNA-seq profiles of 77 intact CTCs isolated from 13 patients by using microfluidic enrichment. Single CTCs from each individual display considerable heterogeneity, including expression of androgen receptor (AR) gene mutations and splicing variants. According to the retrospective study on CTCs from cases advancing through the treating process exploiting one AR inhibiting element, as opposed to untreated cases, activation of noncanonical Wnt signaling is indicated. The ectopic expressing state exhibited by Wnt5a in prostate carcinoma cells downregulates the antiproliferation-related influence exerted by the AR inhibiting process, while its suppressing process in drug-resistant cells recovers partial sensitivity, a correlation also evident in an established mouse model. For this reason, the single-cell analyzing process on prostate CTCs suggests heterogeneity in signaling channels capable of causing treatment failure.35 In addition, Szczerba et al.24 isolated and characterized individual CTC-associated white blood cells (WBCs), as well as corresponding carcinoma cells in the respective CTC-WBC cluster, from cases with BC and from mouse models. scRNA-seq was used for indicating a relationship of CTCs to neutrophils in most of the mentioned cases. Under the comparative process of the transcriptome profiles of CTCs displaying an association with neutrophils against those of CTCs independently, they found several genes with differential expression, outlining cell cycle progression and accelerating metastasis.24 Interestingly, Cheng et al.25 developed Hydro-Seq, a technique for scalable hydrodynamic scRNA-seq barcoding as an attempt to conduct CTC analysis with large throughput. The high cell-capture efficiency and contamination removal capability of Hydro-Seq successfully carried out scRNA-seq of 666 CTCs from 21 BC patient samples under significant throughput. A transcriptome study related to these cells clarified the process to monitor target therapeutic methods and procedures for the tumor metastatic process.25 For the above-mentioned reason, the popularity of scRNA-seq makes CTC detection more accurate than before.
scRNA-seq and CSCs
CSCs (i.e., tumor-initiating cells) refer to one function-related cellular heterogeneity source in tumors. Given the CSC system, CSCs reach the top function-related cellular hierarchy, acting as cells’ subdivided-population, contributing to the tumor-initiating process and to continuing tumorigenesis, whereas non-CSC populations exhibit no tumorigenicity. It is noteworthy that CSCs are responsible for a therapeutically related resisting property and a tumor-relapsing process.36, 37, 38 As a new and powerful tool for cancer research, single-cell sequencing technology also has many applications in CSC-related research fields. We review and summarize the related studies and present them in Table 2. It is extensively documented that BC metastasis is caused by cells receiving epithelial-to-mesenchymal-transition (EMT) and CSCs. With the use of one microfluidic tool enriching migration-related BC cells and exhibiting an enhanced capacity for a tumor-forming process and metastatic process, based on high-throughput scRNA-seq, Chen et al.67 found genes with differential expression inside migration-related cells. Migratory cells exhibited overall signatures of the EMT and CSCs with variable expression of marker genes, and they retained expression profiles of the EMT over time. Based on single-cell resolution, they reported intermediate EMT states and significant epithelial and mesenchymal sub-populations of migration cells, demonstrating that BC cells can migrate rapidly while retaining an epithelial state.67 Since there are few breast CSCs (BCSCs), performing transcriptome sequencing on them has thus far been difficult to achieve. With the emergence of scRNA-seq, Lei et al.68 carried out a relevant study. They prepared single-cell suspensions, which were sorted using flow cytometry from breast tumor tissue and adjacent normal breast tissue from two HER2-positive patients. They acquired CD44 mammary cells, mammary cells, BC cells, as well as BCSCs. Based on bioinformatics, 404 BCSC genes with differential expression were found according to the HER2-positive tumors and preliminary explored transcriptome characteristics of BCSCs. Lastly, based on public database querying, CA12 was reported as one novel prognosis biomarking element in HER2-positive BC, showing prognosis significance for all BC types.68 Moreover, Zhao et al.49 conducted scRNA-seq on a chromosomally unstable glioblastoma CSC line as well as one control normal, diploid neural stem cell (NSC) line for delving into the effect of copy number variations (CNVs) as impacted by chromosomal instability (CIN) on gene expression. From the gene expression data, they computationally inferred great-scale CNVs in individual cells. As proven by the results, CIN contributes to gene expression variations in a direct manner, as well as both genetic and transcription-related heterogeneity of glioblastoma CSCs. It is noteworthy that one gene signature originating according to the subset of genes with buffered expression against copy number variations displays an association to tumor grade and exhibits prognosis significance to case survival, capable of facilitating case diagnosis and treatment processes.49
Table 2.
The application of scRNA-seq in cancer stem cells
| Tumor | Application of scRNA-seq | Research achievement | Reference |
|---|---|---|---|
| Breast cancer | an efficient cell state enriched for an independent breast stem cell expression module was revealed by using unlabeled algorithm from scRNA-seq data of human breast epithelial cells | in basal breast cancer, bipotent stem cell-like status is associated with clinical outcomes and is characterized by overexpression of YBX1 and ENO1, which regulate the risk of basal breast cancer | 39 |
| Breast cancer | the cells with different CSC attributes in breast cancer cell line MDA-MB-231 were enriched and scRNA-seq was carried out | fourteen significantly upregulated genes were found in a CSC population; some of these potential breast CSC markers are associated with reported stem cell characteristics and clinical survival data | 40 |
| Breast cancer | cell distribution during the whole development stage of the normal mammary gland was quantitatively determined by single cells | the results highlight the phenotypic plasticity of normal breast stem cells and provide insights into the relationship between hybrid cell populations, dryness, and cancer | 41 |
| Breast cancer | significant advances in scRNA-seq have been used to solve the heterogeneity of breast epithelial cells | the existence of a population of breast epithelial stem cells can mediate breast development and homeostasis; the rigid boundaries of separated stem cells, progenitor cells, and differentiated epithelial cell populations are deconstructed | 42 |
| Triple-negative breast cancer | full-length scRNA-seq of the triple-negative breast cancer (TNBC) cell line SUM149 was carried out on the Polaris platform of Fluidigm | three cell populations expressing stem cell types were identified, including epithelial-mesenchymal transformed cancer stem cells, mesenchymal-epithelial transformed stem cells, and double epithelial-mesenchymal transformed stem cells | 43 |
| Breast cancer | scRNA-seq was performed on breast cancer | CK5+ cells are rich in characteristics of cancer stem cells; CK5 actively reshapes cell morphology, and blocking CK5-catenin interaction may reverse the harmful characteristics of CK5+ breast cancer cells | 44 |
| Breast cancer | DNA sequencing of parental tumor cells and successive generation spheres derived from breast cancer stem cells (BCSCs) as well as the frequency of increasing sequences were performed at the single-cell level | there was a significant difference in the transcriptional group of cancer cells at the single-cell level of BCSCs; breast cancer patients with a high risk of recurrence showed higher expression levels of BCSC markers than did breast cancer patients with a low risk of recurrence | 45 |
| Glioblastoma | scRNA-seq was performed on 53,586 adult glioblastoma cells and 22,637 normal human fetal brain cells | a conservative glial ancestor-like cell was found in the center of the neural tertiary cancer level; this progenitor cell group contains most of the circulating cells of cancer cells and often is the initiator of other cell types | 46 |
| Glioblastoma | pseudo-transient sequencing of a single tumor cell reconstructed the branching trajectory and validated the results in the glioblastoma multiforme (GBM) independent single-cell dataset | the pathway of gradual transformation of GSCs into invasive cells is determined, which is called the “stem cell-invasive pathway” | 47 |
| Glioblastoma | the surgical specimens of seven patients with malignant gliomas and the single-cell sequencing data of glioma stem cells (GSCs) co-cultured with peripheral blood leukocytes were used for analysis | astrocytes in glioblastoma are mainly derived from differentiated GSCs, while oligodendrocytes are mainly derived from different precursor cells; the immune checkpoint interaction between GSCs and immune cells changes from stimulation to inhibition | 48 |
| Glioblastoma | scRNA-seq was performed on a glioblastoma stem cell line with chromosome instability and a normal diploid neural stem cell line | in chromosomal unstable CSCs, the proportion of gene expression in large genomic regions is proportional to the copy number of the whole chromosome; the difference in the expression of most genes between normal neural stem cells and glioblastoma CSCs is largely caused by the change of copy number | 49 |
| Hepatocellular carcinoma | scRNA-seq analysis of mouse PROM1+ cells revealed the transcriptional structure of ductular reaction progenitors (DRPs) and tumor-initiating stem cell-like cells (TICs) as duct reaction progenitor cells | PROM1 is one of the few clinically related progenitor cell markers in human alcoholic hepatitis (AH), HCC, and mouse liver TICs | 50 |
| Hepatocellular carcinoma | scRNA-seq was used to dissect the heterogeneity within the tumor, and the landscape of the single-cell transcriptome was analyzed to detect meaningful rare cell subsets | EPCAM+ cells upregulated the expression of a variety of oncogenes and CD24+/CD44+-enriched cell subsets with specific signature genes, indicating that there is a new stem cell-related cell subclone in HCC; knocking down the marker gene CTSE of CD24+/CD44+ cells significantly reduced the self-renewal ability of HCC cells in vitro, and further confirmed the stem cell-related role of CTSE in tumorigenicity in nude mice | 51 |
| Gastric cancer | scRNA-seq was used to construct a map of 32,332 high-quality cells from antral mucosal biopsies of patients with pregastric malignancy and an early gastric cancer cascade | during metaplasia, glandular mucinous cells tend to acquire an enter-like stem cell phenotype | 52 |
| Chronic myeloid leukemia | high-sensitive mutation detection combined with single-cell full transcriptome analysis was used to analyze more than 2,000 stem cells in patients with chronic myeloid leukemia (CML) during the whole course of the disease | this single-cell method is used to identify the explosion crisis-specific stem cell population, which also exists in the subcloning of CML stem cells in the chronic phase of patients with subsequent explosion crisis | 53 |
| Squamous cell carcinoma | scRNA-seq and cell line tracing were applied to a skin cancer model of squamous cell carcinoma (SCC) | after binding to cytotoxic T lymphocyte antigen-4 (CTLA4), tumor-initiating stem cells (TSCs) expressing CD80 directly inhibited the activity of cytotoxic T cells; after CTLA4 or transforming growth factor (TGF)-blocking immunotherapy or CD80 ablation, TSCs becomes vulnerable and reduces tumor recurrence after adoptive cytotoxic T cell transfer (ACT) treatment | 54 |
| Pancreatic cancer | the whole-genome expression profiles of CTCs and matched primary tumors were compared in a mouse pancreatic cancer model; single CTCs were isolated using epitope-independent microfluidic capture and then using scRNA-seq | CTCs aggregate separately from primary tumor and tumor-derived cell lines, showing low proliferation markers, Aldh1a2 enrichment of stem cell-related genes, biphenotypic expression of epithelial and mesenchymal markers, Igfbp5 expression, and gene transcription enriched at the epithelial-matrix interface | 27 |
| Intestinal neoplasms | scRNA-seq analysis was used to characterize the heterogeneity of intestinal mesenchymal cells | the mechanism of paracrine regulation of tumor initiation stem cells by fibroblasts expressing Ptgs2 in pericytes through the PGE2-Ptger4-Yap signal axis was shown | 55 |
| Lung cancer | scRNA-seq data of 11,485 lung cancer cells were retrieved from the Gene Expression Omnibus for classification and analysis | fourteen cell types were gathered; among them, CD4+ T cells, B cells, plasma cells, natural killer (NK) cells, and tumor stem cells were in the top five | 56 |
| Chronic myelomonocytic leukemia | scRNA-seq of 6,826 Lin−CD34+ CD38 stem cells from chronic myelomonocytic leukemia (CMML) patients and healthy controls using droplet-based, ultra-high-throughput 10X platform | the transcriptome of CMML stem cells is characterized by increased expression of myeloid and cell cycle genes; CMML-1 and CMML-2 stem cells are different in transcriptome and pseudo-time; CMML patients have different sizes of normal transcriptional stem cell subsets | 57 |
| Renal cell carcinoma | Two sets of primary and metastatic (15,208) cells collected from renal tubular renal carcinoma were analyzed by scRNA-seq | 1,068 CSC populations were identified; these CSCs are located at the center of the differentiation process, transform into collecting duct renal cell carcinoma (CDRCC) primary cells and metastatic cells in space and time sequences, and play a key role in promoting bone destruction in the bone metastasis microenvironment, showing a positive feedback cycle | 58 |
| Central nervous system atypical teratoid/rhabdoid tumors | scRNA-seq data of atypical teratoid/rhabdoid tumor (ATRT)-06 globules processed by 4SC-202 were analyzed | there is a decrease in the number of cells overexpressing stem cell-related genes, including SOX2; 4SC-202 has cytotoxicity and cell inhibition to ATRTs | 59 |
| Medulloblastoma | the medulloblastoma was lineage tracked and single-cell analysis was performed | Sox2+ cells belong to the Atoh1+ lineage, are widely involved in adult granular neurons, and are similar to Sox2+ tumor cells; the constitutive activation of the sonic hedgehog (SHH) pathway leads to their abnormal persistence in the external germinal layer (EGL) and rapid tumor onset | 60 |
| – | scRNA-seq was used to define the classification of mouse bone marrow stromal cells and malignant tumor interference | individual populations in stem cell niches that regulate hematopoietic regeneration can cause leukemia; tissue stroma responds to malignant cells by damaging normal parenchymal cells | 61 |
| – | a single-cell transcriptome was used to study the divergent mode of chemotherapy resistance of tumor cells | tumor evolution may be driven by epigenetic plasticity mediated by stem cell transformation | 62 |
| – | scRNA-seq of airway epithelial cells was performed | the deletion of p53 changes the differences of molecular phenotype and cell cycle regulatory genes of progenitor cells | 63 |
| – | dynamic scRNA-seq of mouse tissue tumor granules blocked by PD-1 was carried out | these cells express Snai1 and stem cell antigen-1 (Sca-1) and show mixed epithelial-mesenchymal characteristics in a stem cell-like state | 64 |
| – | the transcriptional changes at single-cell resolution after KRAS activation in different groups of samples were compared | alveolar epithelial progenitor cells expressing carcinogenic KRAS decreased the expression of genes recognized by mature lineages | 65 |
| – | scRNA-seq analysis was performed on human preimplantation embryos | the awakening of scarce regulatory stemness networks in differentiated cells is related to the development of different spectra of genomic aberrations in many types of clinically fatal malignant tumors, which leads to the emergence of drug-resistant cancer phenotypes | 66 |
scRNA-seq and tumor resistance
Tumor drug resistance refers to one enduring topic, and scRNA-seq progress has led to the creation of effective tools for solving the problem of drug resistance. So far, many studies have used single-cell sequencing techniques to explore the problems in the field of tumor drug resistance, which we discuss in Table 3. Kim et al.69 used single-cell DNA and RNA sequencing in addition to bulk exome sequencing to profile longitudinal samples according to 20 triple-negative BC (TNBC) patients during neoadjuvant chemotherapy (NAC). Deep-exome sequencing identified 10 patients in which NAC led to clonal extinction and 10 patients in which clones persisted after treatment. In eight patients, they performed a more detailed study based on single-cell DNA sequencing for analyzing 900 cells and individual-cell RNA sequencing for the investigation of 6,862 cells. Their data showed that resistant genotypes were pre-existing and adaptively selected by NAC, while transcription-related profiles were acquired by reprogramming in response to chemotherapy in TNBC patients.93 Kim et al.100 isolated 34 patient-derived xenograft (PDX) tumor cells from a lung adenocarcinoma patient tumor xenograft. Individual tumor cells were subjected to scRNA-seq for gene expression profiling and expressed mutation profiling. Fifty tumor-specific single-nucleotide variations, including KRAS, were observed to be heterogeneous in individual PDX cells. Semi-supervised clustering, based on KRAS mutant expression and a risk score representing expression of 69 lung adenocarcinoma-prognostic genes, classified PDX cells into four groups. These results suggest that scRNA-seq on viable PDX cells identified a candidate tumor cell subgroup associated with anti-carcinoma drug resistance. For the mentioned reason, scRNA-seq refers to one effective method of finding special tumor cell-particular gene expression profiles that could facilitate the development of optimized clinical anti-carcinoma strategies.100
Table 3.
The application of scRNA-seq in tumor resistance
| Tumor | Application of scRNA-seq | Research achievement | Reference | |
|---|---|---|---|---|
| Triple-negative breast cancer | scRNA-seq and exome sequencing were used to analyze longitudinal samples of 20 TNBC patients undergoing neoadjuvant chemotherapy | the chemotherapy-resistant genotype of TNBC is preexisting and adaptively selected by NAC, while the transcription profile of TNBC patients after chemotherapy is obtained by reprogramming | 69 | |
| Triple-negative breast cancer | scRNA-seq was performed on >1,500 cells of six major TNBCs | different malignant cell subsets shared by multiple tumors were identified, including a subgroup associated with multiple therapeutic drug resistance, characterized by the activation of glucose and lipid metabolism and related innate immune pathways | 70 | |
| Breast cancers | the contribution of genetic diversity and transcriptional plasticity in early and late endocrine therapy (ET) at single-cell resolution was analyzed | a rare pre-adapted (PA) cell subset undergoes further transcriptional recombination and copy number changes to achieve complete resistance; a multi-stage development model of ET resistance was proposed | 71 | |
| Breast cancer | scRNA-seq, cell barcoding, and mathematical modeling have been used to study endocrine resistance in breast cancer | endocrine resistance is due to the selection of cells with existing genetic differences, while the resistance of KDM5 inhibitors is acquired; phenotypic heterogeneity is associated with drug resistance, and KDM5A/B is a key regulator of this process | 72 | |
| Breast cancer | cells were enriched with different degrees of CSC properties in breast cancer cell line MDA-MB-231, and scRNA-seq was performed | fourteen significantly upregulated genes were found in the CSC population, which is very important for the study of metastasis and treatment of drug resistance | 40 | |
| Breast cancer | scRNA-seq was used to map the entire ER+ breast cancer microenvironment | a new subgroup of CD63+ carcinoma-associated fibroblasts (CAFs) is revealed to induce breast cancer resistance to tamoxifen through exosome Mir-22, suggesting that CD63+ CAFs may be a new therapeutic target for improving tamoxifen sensitivity | 73 | |
| Breast cancer | scRNA-seq analysis was used on samples from patients with progressive ER+ metastatic breast cancer | histone deacetylase (HDAC) inhibition can block chemotherapy-induced drug resistance phenotypes in refractory breast cancer cells through a “one-two punch” strategy | 74 | |
| Breast cancer | scRNA-seq of CTCs isolated from blood samples of patients with metastatic ER+ breast cancer was performed to compare the progression of bone and internal organs | the expression of AR in tumor cells was related to the treatment time of aromatase inhibitors, suggesting that it was related to resistance to acquired endocrine therapy | 23 | |
| Breast cancer | the large-volume scRNA-seq technique was used to track the evolution of genetic and phenotypic subclones of four kinds of breast cancer in order to better understand how breast cancer develops drug resistance | after treatment, the tumor acquired a malignant phenotype, including enhanced matrix and growth factor signals, which may promote drug resistance | 75 | |
| Breast cancer | scRNA-seq was used in breast cancer | the results underscore the need to consider lineage-specific tumor-propagating cells (TPCs) and grade composition of breast tumors, as these heterogeneous subsets may have different treatment sensitivities | 76 | |
| Breast cancer | scRNA-seq was performed on invasive ductal breast carcinoma (IDC) and invasive lobular breast carcinoma (ILC) cell lines and a group of CRISPR-Cas9-mediated E-cadherin gene knockout IDC cell lines (T47D) | remodeling of the transcriptional membrane system is similar to that of ILCs in the activation of regulatory factors, and it increases the sensitivity to interferon (IFN)-H-mediated growth inhibition by activating IRF1 | 77 | |
| Melanoma | scRNA-seq and computational analysis were used to study the status of malignant cells promoting immune evasion in 33 melanoma tumors | CDK4/6 inhibition combined with immunotherapy reduces melanoma tumor growth, and the study provides a high-resolution picture of immune checkpoint inhibitor (ICI)-resistant cell status | 78 | |
| Lung adenocarcinoma | scRNA-seq data of patients with lung adenocarcinoma were analyzed to characterize the heterogeneity of genes related to immune response in the tumor | downregulation of the IFN-H signal pathway gene corresponds to the phenotype of acquired drug resistance | 79 | |
| High-grade serous ovarian cancer | about 11,000 cells from 11 ascites patients with high-grade serous ovarian cancer (HGSOC) were analyzed by scRNA-seq | inhibition of the expression of the JAK/STAT pathway in malignant cells and tumor-associated fibroblasts showed strong anti-tumor activity, which further clarified the mechanism of drug resistance in patients with ascites | 80 | |
| Muscle-invasive urothelial bladder cancer | scRNA-seq was used to describe the tumor landscape of a case of chemotherapy-resistant metastatic muscle-invasive urothelial bladder cancer (MIUBC) | clinical application of the PD-L1 inhibitor atezolizumab resulted in a good acquired response to patients resistant to tipifarnib | 81 | |
| Prostate cancer | scRNA-seq was performed on docetaxel-sensitive and drug-resistant variants of DU145 and PC3 prostate cancer cell lines | NUPR1 was identified as a mediator of drug resistance in prostate cancer, providing a theoretical basis for exploring the reversal of docetaxel resistance of NUPR1 and its target genes | 82 | |
| Glioblastoma multiforme | scRNA-seq was used on GBM | GBM shows four subtypes, among which proneural and mesenchymal are the main types, with the latter showing stronger invasive and treatment resistance | 83 | |
| Glioma | combining scRNA-seq data with clinical bulk gene expression data, a computational pipeline was developed for identifying prognostic and predictive characteristics linking cancer cells to microenvironmental cells; the pipeline was applied to glioma scRNA-seq data | multilayer network biomarker (MNB) can predict the sensitivity or drug resistance of glioma patients to molecular targeted therapy | 84 | |
| Chronic myeloid leukemia | high-sensitivity mutation detection combined with the same single-cell full transcriptometric analysis was used to analyze more than 2,000 stem cells from patients with CML throughout the course of the disease | a CML-SCs subgroup with distinct molecular characteristics was identified, which persisted selectively during long-term treatment and was related to drug resistance | 53 | |
| Acute lymphoblastic leukemia | scRNA-seq and several complementary genomic maps were used to compare the differentiation of normal B lines and the status of leukemic cells in vivo | abundance of the G1 cell cycle state at diagnosis and lack of differentiation-associated regulatory network changes during the induction of chemotherapy represent features of chemoresistance | 85 | |
| Acute lymphoblastic leukemia | full-length scRNA-seq was performed on malignant and microenvironmental cells | lineage plasticity and stemness have been used as the cause of cancer treatment resistance, the mechanism of which is the activation of NOTCH1 mutations in recurrent/refractory early T cell progenitor cells | 86 | |
| Acute lymphoblastic leukemia | scRNA-seq data and several complementary genomic maps were used to compare normal B-line differentiation and leukemic cell status in vivo | the G1 cell cycle state is abundant, and there is a lack of differentiation-related regulatory network changes during induced chemotherapy, which is a characteristic of chemotherapeutic resistance | 87 | |
| Melanoma | the published scRNA-seq dataset was analyzed from melanoma patients treated with immune checkpoint therapy (ICT) | identified and validated a feature of immune cells; ImmuneCells.Sig can represent a valuable tool for clinical decision-making in patients receiving immunotherapy | 88 | |
| Tubular renal cell carcinoma | scRNA-seq analysis was performed on 15,208 cells from a pair of primary and metastatic sites that collected tubular renal cell carcinoma | effective inhibitors of PARP, PIGF, HDAC2, and FGFR targeting CSCs may be potential therapeutic strategies for CDRCC | 58 | |
| Basal cell carcinoma | three prognostic surface markers (LYPD3, TACSTD2, and LY6D) associated with drug resistance to nuclear myocardin-related transcription factor (nMRTF) and Smoothened (SMO) inhibitors were identified using scRNA-seq from the patient’s tumor | basal cell carcinoma (BCC) is dependent on hedgehog (Hh)/Gli signaling pathways, but can form SMO inhibitor resistance mechanisms, with AP-1 and TGF reagent groups synergistically driving the activation of nMRTF; the JNK/AP-1 signal promotes nMRTF activity through chromatin accessibility and Smad3 DNA binding, leading to Rho guanine exchange factor (RhoGEF) transcription; small molecule AP-1 inhibitors target human BCC LYPD3+/TACSTD2+/LY6D+ nMRTF in vitro, opening a way for improved combination therapy | 89 | |
| Merkel cell carcinoma | scRNA-seq was used in two patients with metastatic Merkel cell carcinoma (MCC) | dynamic transcriptional suppression of specific HLA genes targeting viral epitopes is present in drug-resistant tumors under intense immune pressure mediated by CD8 | 90 | |
| Merkel cell carcinoma | scRNA-seq was used in the MCC study | domatinostat not only has a direct anti-tumor effect, but it also can restore the surface expression of HLA class I on MCC cells, thus restoring the sensitivity of surviving MCC cells to the recognition and elimination of homologous cytotoxic T cells | 91 | |
| Kidney cancer | a comprehensive immunoassay was performed for established RENCA tumors of renal cell carcinoma by multiparametric flow cytometry, tumor cytokine profile, and scRNA-seq | IL-1B blocking and ICIs or tyrosine kinase inhibitors (TKIs) reshape the intermedullary chamber through a non-redundant and relatively independent T cell mechanism; IL-1B is the upstream mediator of adaptive bone marrow resistance | 92 | |
| Pancreatic ductal adenocarcinoma | scRNA-seq was used to comprehensively study the tumor and tumor microenvironmental content of PDAC tumors in both humans and mice | CAFs are involved in the drug resistance of PDAC; MHC class II can provide antigens to CD4+ T cells, which express CAFs and potentially regulate the immune response of PDAC | 93 | |
| Pancreatic ductal adenocarcinoma | single-cell transcriptology was used to draw an image of fibroblasts during the progression of PDAC in an animal model | the LRRC15CAF pedigree driven by TGF-β is associated with adverse results in immunotherapy trial data containing multiple solid tumors, and it is the target of combined therapy | 94 | |
| – | scRNA-seq analysis was performed on human preimplantation embryos | the awakening of scarce regulatory stemness networks in differentiated cells is related to the development of different spectra of genomic aberrations in many types of clinically fatal malignant tumors, which leads to the emergence of drug-resistant cancer phenotypes | 66 | |
| – | pretreatment tumor biopsy combined with scRNA-seq was used to reveal the underlying mechanisms of avelumab drug resistance | in macrophage cells with extensive immunosuppression, there were pro-inflammatory populations promoting a PD-L1 blocking response | 95 | |
| – | according to scRNA-seq, the tumor microenvironment (TME) was divided into an inflammatory immunotherapy response type and an inflammatory non-response type | the expression of IL-2 signal pathway-related genes in the inflammatory microenvironment is upregulated, while the PPAR signal pathway-related genes and multiple epigenetic pathway-related genes are suppressed and upregulated, respectively, suggesting the possible mechanism of immunotherapy resistance | 96 | |
| – | single-cell transcriptomics were used to study the divergent patterns of chemotherapy resistance in tumor cells | a high degree of intratyphogenic heterogeneity (ITH) facilitates the selection of existing drug-resistant cells, while phenotypic homogeneous cells participate in recessive epigenetic mechanisms for transdifferentiation under drug selection; drug-induced adaptation was achieved after the loss of the stem-factor SOX2 and the acquisition of SOX9, and JQ1-mediated BRD4 inhibition could reverse drug-induced adaptation | 62 | |
| – | bulky and scRNA-seq analysis revealed the inherent characteristics and diversity of unconventional T cell (UTC) antibodies associated with neutrophil-dependent anti-sarcoma immunity | neutrophil-driven UTCs toward zero polarization and type 1 immunity are essential for resistance to murine sarcoma and selective human tumors | 97 | |
| – | the transcriptomic response of cultured colon cancer cell lines to DNA damage induced by 5-fluorouracil (5-FU) was described by scRNA-seq | after 5-FU treatment, a single colon cancer cell population was treated with three different transcriptional phenotypes, which correspond to different cell fate responses: apoptosis, cell cycle checkpoints, and stress resistance | 98 | |
| – | dynamic scRNA-seq was performed on tissue-type tumor globules in mice undergoing PD-1 blockade | a discrete subset of immunotherapy persister cells (IPCs) was identified to be resistant to CD8 T cell-mediated killing; PD-1 blockade and BIRC2/3 antagonism combined to reduce IPCs | 64 | |
| – | single-cell transcriptome data of human melanoma and non-small cell lung cancer (NSCLC) specimens were analyzed | TOX promotes the depletion of CD8 T cells in tumor by upregulating IC molecules, suggesting that TOX inhibition may hinder T cell depletion and improve the efficacy of ICIs | 99 | |
The property of resisting oncogene-targeted therapies consists of discrete drug-tolerant persister cells, initially reported by in vitro assays. Sehgal et al.64 performed dynamic scRNA-seq of murine organotypic tumor spheroids undergoing PD-1 blockade and identified a discrete sub-population of immunotherapy persister cells (IPCs) that resisted CD8 T cell-mediated killing. The mentioned cells showed the expression of Snai1 and stem cell antigen-1 (Sca-1), while exhibiting hybrid epithelial-mesenchymal characteristics pertaining to one state similar to stem cells. IPCs received the expansion via interleukin (IL)-6, whereas they exhibited vulnerability to tumor necrosis factor (TNF)-α-triggered cytotoxicity, by drawing upon Birc3 and Birc2 to be the surviving element. The process of integrating PD-1 blockade to Birc2/3 antagonism in mice led to the reduction of IPCs and the enhancement of tumor cell killing in vivo, thereby leading to durable responsiveness matching TNF cytotoxicity thresholds in vitro.101 Indeed, the present report is one of those studying PD-1 resistance based on scRNA-seq. Wu et al.102 surveyed the profiles of various populations of T cells and T cell receptors (TCRs) in tumors, normal adjacent tissue, and peripheral blood by performing deep scRNA-seq. They found clear evidence of clonotypic expansion of effector-like T cells not only within the tumor but also in normal adjacent tissue. Patients with gene signatures of such clonotypic expansion respond best to anti-PDL1 therapy. It is noteworthy that clonotypes with expansion identified in the tumor and common nearby tissue are also capable of receiving typical detection in peripheral blood, indicating one convenient method regarding the patient-identifying process. According to the processes of analyzing their information as well as a number of external datasets, intratumoral T cells, particularly in responsive cases, are supplemented again with fresh, non-exhausted replacement cells from sites out of the tumor, demonstrating sustaining activity exhibited by the carcinoma immunity cycle in the mentioned cases. The relevant acceleration is likely to display the relationship to clinically related response.102
For gaining insights into the heterogeneous characteristics exhibited by individual carcinoma cells, gene expression levels and the divergence among various individual cells require in-depth studies. Suzuki et al.103 analyzed a total of 336 scRNA-seq libraries from seven cell lines. The results are highly robust regarding both average expression levels and the relative gene expression differences between individual cells. Gene expression diversity is characteristic depending on genes and pathways. As indicated by the investigation of single cells under the treatment of the multiple-tyrosine kinase inhibiting element vandetanib, when the ribosome genes and numerous housekeeping genes lead to the reduction of their expression diversity relatively in the drug-treating process, the genes under the direct targeting by vandetanib (the RET and EGFR genes) continue to be stable. Rigid transcription-related control of the mentioned genes is not likely to enable plastic variations of their expression state following the drug-treating process or during the course of the cell-acquiring process of drug resistance. Feature modes inside gene expression divergence, probably not suggested from transcriptome study regarding bulk cells, are likely to critically impact the relevant process during the drug resistance acquiring process of cells, which may be achieved by presenting a cell reservoir for gene expression programs.103
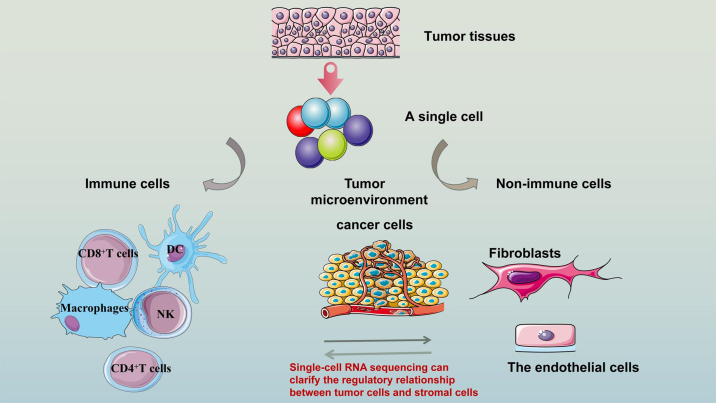
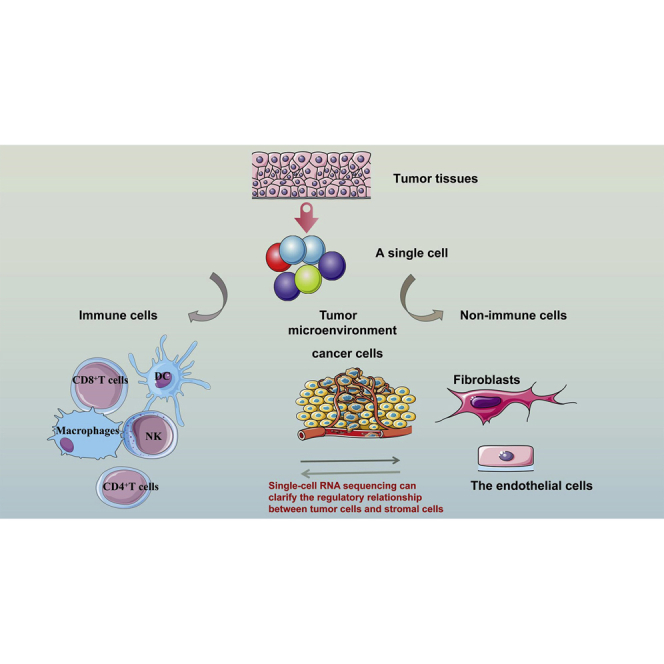
scRNA-seq and the TME
In fact, the essence of scRNA-seq is to detect the microenvironment of tumors in different states. We discuss immune cells and non-immune cells separately to better clarify the new analysis brought about by scRNA-seq (Figure 3).
Figure 3.
scRNA-seq and the tumor microenvironment
Immune cells
The tumor immune microenvironment refers to one complex integrated system, and one of its core features is immune function. The tumor immune microenvironment covers various tumor-infiltrating immune cells (e.g., T lymphocytes, B lymphocytes, natural killer [NK] cells, mast cells, and myeloid suppressor cells).104,105 On the one hand, these immune cells inhibit and kill the anti-tumor immunity of tumor cells, and, on the other hand, they promote tumor development and immune escape. In existing tumor research, the tumor immune microenvironment turns out to be one vital research focus. Conventional gene sequencing approaches are capable of merely obtaining the results of average gene expression information of different types of cells in tissue samples, which involve tumor cells and the microenvironment around tumor cells. The mentioned analyses are general analyses of significant tumor samples, failing to effectively identify and describe the type and status of individual immune cells in the TME. This approach is likely to conceal much key information. Single-cell sequencing technology with second-generation sequencing technology to analyze the DNA, RNA, or DNA methylation level of a single cell reveal its genome, transcriptome, and epigenetic characteristics, respectively, and delineate the function and state of a single cell. scRNA-seq is capable of identifying the heterogeneity of cells in solid tumors, but it also helps to clarify the molecular mechanism of tumor microenvironmental immune cells’ action on tumor cell generation, development, metastasis, drug resistance, and immune escape as an attempt to conduct the clinical diagnosing process, treating process, and prognostic process regarding solid tumors more accurately.
The quantity of tumor-infiltrating lymphocytes (TILs) in BC is a robust prognostic factor for improved patient survival, particularly in triple-negative and HER2-overexpressing BC subtypes. Alhough T cells are the predominant TIL population, the relationship between quantitative and qualitative differences in T cell subpopulations and patient prognosis remains unknown. Savas et al.106 carried out scRNA-seq of 6,311 T cells under isolation according to BCs of humans. They suggested noticeable heterogeneity inside the infiltrating T cell population. Thus, BCs with a high number of TILs covered CD8+ T cells displaying a tissue-resident memory T cell-differentiating process, and these CD8+ T cells displayed significantly considerable effector proteins and immune checkpoint molecules. A CD8+ T gene signature developed from the scRNA-seq data displayed noticeable relationships to optimized patient surviving state in early phase TNBC and provided more effective prognostication than did CD8 expression alone.106 Zheng et al.107 carried out deep scRNA-seq on 5,063 single T cells under isolation according to peripheral blood, tumor, and adjacent normal tissues from six hepatocellular carcinoma (HCC) cases. The transcription-related profiles of these individual cells were coupled with assembled TCR sequences. Specific subsets (e.g., exhausted CD8+ T cells and regulatory T cells [Tregs]) are favorably enriched and potentially clonally expanded in HCC, and they identified signature genes for each subset. One of the genes, layilin, exhibits an upregulation of activated CD8+ T cells and Tregs and represses the CD8+ T cell functions in vitro. Such a compendium of transcriptome data helps clarify the immune landscape in carcinomas.107
Hypoxia refers to one common feature of solid tumors, and cells adapt by increasing the transcription factor hypoxia-inducible factor-1α (HIF-1α). Ni et al.108 defined the transcription-related landscape of mouse tumor-infiltrating NK cells by using scRNA-seq. Conditional deletion of Hif1a in NK cells resulted in reduced tumor growth, elevated expression of activation markers and effector molecules, and an enriched nuclear factor κB (NF-κB) pathway in tumor-infiltrating NK cells. IL-18 from myeloid cells was required to activate NF-κB and enhance HIF-1α NK cells’ anti-tumor activity. Extended culture with an HIF-1α inhibitor increased human NK cell responses. Thus, inhibition of HIF-1α unleashes NK cell anti-tumor activity and could be exploited for carcinoma therapy.108
According to the hypothesis of Sathe et al.,109 the scRNA-seq analyzing process regarding gastric carcinoma together with paired normal tissue and peripheral blood mononuclear cells (PBMCs) could identify critical elements of cellular deregulation not apparent with other approaches. This study carried out scRNA-seq onto seven patients subjected to gastric carcinoma and one case subjected to intestinal metaplasia. As revealed from the results, tumor epithelium suggested copy number variations, a distinct gene expression program from normal, with intratumoral heterogeneity. The gastric carcinoma TME was significantly enriched for stromal cells, macrophages, dendritic cells (DCs), and Tregs. TME-exclusive stromal cells expressed distinct extracellular matrix components more than normal. Macrophages exhibited transcription-related heterogeneity and displayed no consistency with one binary M1/M2 paradigm. Tumor DCs followed one special gene expression program as opposed to PBMC DCs. TME-specific cytotoxic T cells received exhaustion based on two heterogeneous subsets. Helper, cytotoxic T, regulatory T, and NK cells expressed multiple immune checkpoint or co-stimulatory molecules. A TME-exclusive intercellular communicating process was suggested from the receptor-ligand analyzing process.109
Chung et al.110 performed scRNA-seq to BC and studied 515 cells from 11 patients. Inferred copy number variations from the scRNA-seq data were used to separate carcinoma cells from non-carcinoma cells. At a single-cell resolution, carcinoma cells displayed common signatures within the tumor as well as intratumoral heterogeneity regarding BC subtype and crucial carcinoma-related pathways. Most of the non-carcinoma cells are immune cells, with three distinct clusters of T lymphocytes, B lymphocytes, and macrophages. T lymphocytes and macrophages both displayed immunosuppressive characteristics, i.e., T cells with a regulatory or an exhausted phenotype and macrophages with an M2 phenotype. These results illustrate that the BC transcriptome has a wide range of intratumoral heterogeneity, which is shaped by the tumor cells and immune cells in the surrounding microenvironment.110
Non-immune cells
As a key component of the TME, stromal tissue is of great significance to carcinoma. In recent years, studies have shown that the microenvironment is an “accomplice” of tumor drug resistance and has become one of the key factors in disease prevention and treatment. scRNA-seq can present insights into the role of non-immune cells, including stromal cells, fibroblasts, and epithelial cells, in carcinoma progression.
Carcinoma-associated fibroblasts (CAFs) refer to a major component of the TME whose origin and role in shaping disease initiation, progression, and treatment response are not clear for large heterogeneity.111 Bartoschek et al.146 gave a definition of three clear subpopulations of CAFs by using one negative selection strategic process combined with scRNA-seq of 768 transcriptomes of mesenchymal cells according to one genetically engineered mouse model of BC. In experimental models of several cancers and human tumors, transcription-related and protein-level validation have shown that the CAF subclass exists spatially isolated, which can be attributed to different origins, covering transformed epithelium, mammary fat pads, and perivascular niches. The genetic profile of the respective CAF subtype is associated with a unique functional program and has an independent prognostic capability in the clinical cohort through its association with metastatic disease.112 Lin et al.113 used scRNA-seq for profiling single-cell transcriptomes according to dissociated primary tumors or metastasis-related biopsies of patients with pancreatic ductal adenocarcinoma (PDAC) and revealed distinct cell types in primary and metastatic PDAC tissues, including tumor cells, endothelial cells, CAFs, and immune cells. The carcinoma cells showed high inter-patient heterogeneity, whereas the stromal cells were more homogeneous across patients. Immune infiltration varies significantly from patient to patient, with most immune cells being macrophages and exhausted lymphocytes.113
Breast cancer takes places in epithelial cells of the mammary gland, where genetic changes lead to a loss of homeostasis. Several different epithelial subsets have been proposed, but a full understanding of the heterogeneity and differentiation levels of the human breast has not been realized. Nguyen et al.114 used scRNA-seq to profile the transcriptomes of 25,790 primary human breast epithelial cells isolated from reduction mammoplasties of seven individuals. Unbiased cluster analysis revealed the presence of three distinct epithelial cell populations, one basal cell type and two luminal cell types, identified to be l1-secreting and hormone-responsive L2 cells. Pseudotemporal reconstruction of differentiation trajectories produces one continuous lineage hierarchy that closely connects the basal lineage to the two differentiated luminal branches. The relevant overall cell atlas clarifies the human breast epithelium’s cellular blueprint and underpins the understanding of how the system goes awry during BC.114
Carcinoma cells are embedded in the TME, a complex ecosystem of stromal cells. Lambrechts et al.115 presented a 52,698-cell catalog of the TME transcriptome in human lung tumors at single-cell resolution, which was validated in independent samples where 40,250 additional cells were sequenced. Results underwent a comparison with matched nonmalignant lung samples. As a result, they found a highly complex TME that profoundly molds stromal cells. They identified 52 subtypes of stromal cells, including new subsets of cells hitherto thought to be homogeneous, as well as transcription factors for their heterogeneity. As an example, these researchers found that fibroblasts expressed different collagen proteins, endothelial cells downregulated immune cell homing, and genes co-regulated with established immune checkpoint transcripts and were associated with T cell activity. For this reason, by providing a comprehensive catalog of stromal cell types, and by characterizing their phenotypes and cooperative behavior, this resource provides a deeper insight into cancer biology that will help advance lung cancer diagnosis and treatment.115
CTCs are the main way of blood transmission of tumors. The application of single-cell sequencing in CTCs undoubtedly provides a new and powerful method for the diagnosis and prognosis of cancer. Similarly, as the initiating factor of tumorigenesis and the driving factor of tumor development, the application of single-cell sequencing technology provides a powerful guarantee to unravel the mystery of the mechanism of CSCs. In addition, tumor drug resistance, a factor leading to poor prognosis, has also been further studied because of the application of single-cell sequencing technology. If we want to make a breakthrough in the above three aspects, we cannot avoid explorations in the field of the TME. Therefore, the applications of single-cell sequencing technology in the fields of CTCs, CSCs, tumor drug resistance, and the TME make it a powerful tool, which can effectively explore the internal mechanisms of tumors from many aspects, such as tumor occurrence and development and tumor diagnosis, prognosis, and treatment, and provide a new research perspective for conquering tumors (Table 4).
Table 4.
The application of scRNA-seq in the TME
| Tumor | Application of scRNA-seq | Research achievement | Reference |
|---|---|---|---|
| Immune-related | |||
| Lung cancer | scRNA-seq was performed on 49 clinical biopsy specimens from 30 patients with metastatic lung cancer before and during targeted therapy | cancer cells that survived after treatment of residual disease (RD) expressed alveolar regenerating cell signals, suggesting that treatment induced primitive cell state transformation, while cancer cells with treatment of progressive disease (PD) upregulated canine urine, plasminogen, and gap junction pathways; T lymphocytes were active in the RD group, macrophages were decreased, and PD showed immunosuppressive cell status | 127 |
| Non-small cell lung cancer | 3,110 peripheral T cells from patients with non-small cell lung cancer (NSCLC) before and after programmed PD-1 blockage were analyzed by scRNA-seq | tumor-related CD4 T cell clones have higher cytotoxic activity than do CD8 T cell clones; 25 genes that were significantly upregulated or downregulated during the progression of the disease were also detected | 128 |
| Lung adenocarcinoma | a single-cell transcriptome map of metastatic lung adenocarcinoma is established | a cancer cell subtype that deviates from normal differentiation and dominates the metastatic stage was identified; as T cells are depleted, the normal population of medullary resident cells is gradually replaced by mononuclear-derived macrophages and dendritic cells (DCs) | 129 |
| Lung adenocarcinoma | scRNA-seq data from lung adenocarcinoma patients and cell lines were analyzed to characterize the heterogeneity of immune response-related genes within the tumor | IFN-γ signaling pathway genes are heterologously expressed in individual cancer cells and include other genes of MHC class II genes. Downregulation of IFN signaling pathway genes in cell lines corresponded to acquired resistance phenotypes | 79 |
| Hepatocellular carcinoma | 5,063 single T cells isolated from peripheral blood, tumor, and adjacent normal tissues of six patients with HCC were deeply sequenced by scRNA-seq | the gene laylin upregulates activated CD8+ T cells and Tregs and inhibits the function of CD8+ T cells in vitro | 107 |
| Pancreatic ductal adenocarcinoma | analysis of the allogeneic PDAC model with immune activity by scRNA-seq and polychromatic fluorescence-activated cell sorting (FACS) | oncolytic herpes simplex virus-1 (OHSV) can downregulate tumor-associated macrophages and increase the percentage of tumor-infiltrating lymphocytes; the combination of OHSV and the immune checkpoint regulator can prolong the lifespan of tumor-bearing mice | 130 |
| Pre-B cell acute lymphoblastic leukemia | scRNA-seq was used to describe the kinetics of the mouse bone marrow microenvironment (BMM) during the progression of B cell acute lymphoblastic leukemia (B-ALL) | the expression of tumor suppressor long non-coding RNA (lncRNA) Neat1 was decreased and the transcription rate was interrupted; monocyte-DC precursors continue to be active in the progression of B-ALL | 131 |
| Intrahepatic cholangiocarcinoma | 8 human intrahepatic cholangiocarcinomas (ICCs) and adjacent samples were sequenced by scRNA-seq to clarify the complete transcriptional map and intercellular communication network | tumor-infiltrating CD4 Tregs showed high immunosuppressive characteristics; CD146+ vascular CAFs (VCAFs) have high expression of microvascular characteristics and a high level of IL-6; IL-6 secreted by VCAFs can induce clear epigenetic changes in ICC cells; miR-9-5p can induce VCAFs to overexpress IL-6 and promote tumor progression | 132 |
| Melanoma | the interstitial septum and draining lymph nodes (LNs) across tumor development points in mouse melanoma were examined using scRNA-seq | before the expression of PD-1 and LAG3, immature lymphocytes from LNs experienced activation and clonal expansion in the tumor, while tumor-related myeloid cells promoted the formation of an inhibitory niche | 133 |
| Head and neck cancer | activated B cells, germinal center B cells, and antibody-secreting cells (ASCs) in the TME were detected by scRNA-seq | compared with the tumor parenchyma, B cells and ASCs are preferentially located in the tumor stroma, forming a good activated B cell cluster, indicating that the germinal center reaction is underway | 85 |
| Triple-negative breast cancer | five TNBCs were analyzed by scRNA-seq, and two CAF and two perivascular-like (PVL) subpopulations were found | inflammatory CAFs are related to the dysfunction of cytotoxic T cells, and there is a strong correlation between differentiated PVL cells and rejection | 134 |
| Esophageal cancer | using scRNA-seq, more heterogeneity of depleted T cells in esophageal cancer was reported than previously elucidated | GF2 is an important regulator of SPRY1 expression and participates in establishment of the dysfunctional state of CD8+ T cells in esophageal cancer | 135 |
| Bladder cancer | scRNA-seq was performed on eight bladder cancer (BC) tumor samples and three paracancer samples | nineteen different cell types were found in the BC TME; tumor cells downregulate MHC class II, monocytes show M2 polarization and differentiation in the tumor area, and LAMP3+ DC subsets may be able to recruit Tregs; the above changes may be involved in the formation of the immunosuppressive TME | 136 |
| Colorectal cancer | intestinal tissue was analyzed by scRNA-seq to determine the cellular source of lymphotoxin | the lymphotoxin signal regulates the expression of IL22BP in the colon, and the decrease of the IL22BP level in human colon tumors is related to the shortening of survival time; the LTbR signal regulates the expression of IL22BP in mouse colonic tumors and cultured human DCs | 137 |
| – | the transcription profile of mouse tumor invasive NK cells was defined by scRNA-seq | antitumor activity of NK cells can be inhibited by hypoxia-inducible factor-1, which can be used in tumor therapy | 108 |
| – | full-length scRNA-seq was applied to three tumor types to provide a comprehensive single T cell data resource for understanding various characteristics of tumor-infiltrating T cells | the conserved and cancer-type-specific T cell subpopulations and developmental patterns were revealed, and detailed molecular portraits of T cell clusters associated with tumor immunity were provided, revealing the composition, heterogeneity, and cellular and molecular mechanisms underlying the formation of tumor immune microenvironments | 138 |
| – | a subgroup of CD4T cells transduced by CASTAT5 was identified by scRNA-seq analysis | STAT5 is an effective candidate for T cell engineering, which can produce multifunctional, anti-fatigue, and anti-tumor CD4T cells to enhance adoptive T cell therapy for cancer | 139 |
| – | scRNA-seq was performed on CD45 immune cells in a homogeneous tumor model using a 10X genomic approach | targeting CD47 induces atrioventricular remodeling of tumor-infiltrating immune cells in PDAC; different PDAC mouse models had different responses to CD47 and anti-PD-L1 blocking due to the different effects of combination therapy on infiltrating immune cells and key immune-activating genes in the TME established by different PDAC cell lines | 140 |
| Others | |||
| Prostate cancer | the scRNA-seq method was used to identify prostate cancer cell types and corresponding marker genes | fifteen cell groups including three luminal clusters with different expression profiles were identified in prostate cancer tissues | 141 |
| Pancreatic cancer | scRNA-seq was used to identify differentially expressed genes (DEGs) associated with intracellular APE1 protein levels | several new genes and pathways were identified that were affected by APE1, as well as tumor subtype specificity; these findings will enable hypothesis-driven approaches to produce combination therapies in an innovative way | 142 |
| Gastric adenocarcinoma | unbiased transcriptome scRNA-seq analysis was performed on 27,677 cells from nine gastric adenocarcinoma tumors and three non-tumor samples | five subsets of cells with different expression profiles were identified | 143 |
| Triple-negative breast cancer | deep and full-length scRNA-seq was performed on Fluidigm’s Polaris platform | the cell, transcriptional, and isomer heterogeneity of TNBC cell line SUM149 was revealed | 43 |
| Breast cancer | scRNA-seq was used to study the transcriptome of breast cancer cells, and various bioinformatics analyses were used to determine the pathway of change | the results revealed the reprogramming of tumor-draining LNs (TDLNs) at the single-cell level in the spontaneous breast cancer model, and they suggested that the changes of immunomodulatory and metabolic switches are the key changes in the niche of breast cancer cells before they are ready for metastasis | 144 |
| Breast cancer | scRNA-seq and xenotransplantation models of breast cancer patient origin were used to identify the overall transcriptional changes of rare metastatic cells during seeding | both primary tumors and micrometastases exhibit transcriptional heterogeneity, but micrometastases have a unique transcriptome program that is conservative in patient-derived xenotransplantation models and has a high predictive power for low patient survival | 145 |
| Breast cancer | scRNA-seq of 768 mesenchymal cell transcripts from a genetically engineered mouse model of breast cancer was performed using a negative selection strategy | three distinct CAF subgroups were defined that could be attributed to different sources, including perivascular niches, breast fat pads, and transformed epithelial cells | 146 |
| Ovarian cancer | scRNA-seq was used to characterize subsets of ovarian surface epithelial (OSE) cells after exposure to estradiol and to determine the transcriptional dynamics involved in its emergence | estradiol-treated cells are characterized by upregulation of genes associated with proliferation, metabolism, and survival pathways | 112 |
| Esophageal cancer | scRNA-seq was used to analyze 368 single cells from three esophageal squamous cell carcinoma (ESCC) and two esophageal adenocarcinoma (EAC) tumors | cancer cells from the heterogeneous cell population are distinguished and genetic characteristics and key cancer-related signaling pathways associated with ESCC and EAC are identified | 147 |
| Renal cell carcinoma | scRNA-seq was used to examine the heterogeneity within a pair of primary renal cell carcinomas and their lung metastases | activation of drug-targeted pathways showed considerable variability between primary and metastatic sites and between individual cancer cells within each site | 148 |
| Neuroblastoma | transcripts of adrenal neuroblastoma (NB) were obtained from 160,910 cells of 16 patients by scRNA-seq, and the transcripts of developmental cells probably originating from NB were obtained from 12,103 cells of human embryo and fetal adrenal gland | most adrenal NB tumor cells transcriptionally mirror noradrenergic chromaffin cells. Malignant states also recapitulate the proliferation/differentiation status of chromaffin cells in the process of normal development. | 149 |
| Lung adenocarcinoma | scRNA-seq was used to compare ground glass nodule adenocarcinoma (GGN-ADC) and solid adenocarcinoma (SADC) in order to fully understand GGNs | the signal pathways related to cell proliferation are downregulated in GGN-ADC, and the roles of stromal cells in GGN-ADC and SADC are different; in GGN-ADC, the signal pathway of angiogenesis is downregulated | 150 |
| Paget’s disease | scRNA-seq technique was used in Paget’s disease (PD) of breast and extramammary PD | scRNA-seq identified different cell states, new biomarkers, and signaling pathways, including mTOR associated with extramammary PD | 151 |
| Glioma | create a single-cell tumor-host interaction tool (ScTHI) to identify significantly activated ligand-receptor interactions across cell clusters from scRNA-seq data | the results provide a complete map of active tumor-host interaction pairs in gliomas, which can be used for treatment to reduce immunosuppression of the brain TME | 152 |
| Acute lymphoblastic leukemia | scRNA-seq and several complementary genomic maps were used to compare the differentiation of normal B lines and the status of leukemic cells in vivo | the activity of multiple ETS transcription factors increased in leukemic cells states; the accompanying gene expression changes associated with NK cell inactivation and depletion in the leukemic immune microenvironment | 87 |
| Epithelial cancers | the transcriptional changes at single-cell resolution after KRAS activation in different groups of samples were compared | in all four groups of samples, the mature pedigree identity gene expression of alveolar epithelial progenitor cells expressing carcinogenic KRAS was decreased | 65 |
| Melanoma | scRNA-seq data from patients with melanoma showed that SDC-3 was expressed on tumor-related macrophages, cancer cells, and vascular endothelial cells | hypoxia (1% oxygen) or IFN-γ can stimulate the expression of SDC-3 in RAW-264.7-derived macrophages and associate the expression of SDC-3 with the pro-inflammatory response; the expression of Syndecan-3 is related to the increase of overall survival rate of patients with hypoxic melanoma | 153 |
| Colorectal cancer | the early development model of colorectal cancer (CRC) was established, and the transcriptome analysis of scRNA-seq data was carried out | CCDC85B is a major regulatory factor, which can transform fast-growing subtype cells into slow-growing subtype cells | 154 |
| – | the cellular composition and molecular characteristics of laryngeal squamous cell carcinoma were analyzed by scRNA-seq | keratinocyte-like cells are located in the center of the tumor, while malignant proliferating cells are located at the edge of the tumor; malignant proliferative cells are associated with poor prognosis | 155 |
Combination of scRNA-seq and other methods
Although scRNA-seq is very useful, RNA expression measurements do not provide information about protein abundance or post-translational modification. Recently conducted studies in samples of human brain exploited multiplexed individual-cell techniques, including mass cytometry (cytometry by time-of-flight [CyTOF]) and scRNA-seq, for overall molecular viewing of human microglia in healthy and diseased brains. CyTOF refers to one effective process for studying a high-dimension-based protein expression state of human microglia (huMG) under the individual-cell condition. Such a technique makes quantification with high throughput more possible under an individual-cell resolution. CyTOF is able to use the combination with scRNA-seq to conduct an overall process of analysis, since it enables individual-cell analysis of post-translation-related protein modifications, clarifying cell signaling dynamics in targeted cells. Moreover, imaging mass cytometry (IMC) now turns out to be acquired in the market, and it is conducive to exploring several cell types in brain sections of humans. IMC exploits mass spectrometry for acquiring spatial information of cell-cell interactions on tissue sections, based on the use of more than 40 markers simultaneously.116
In situ visualization of transcriptome is verified as one effective process to investigate scRNA-seq information, presenting one extra space dimension for investigating multiplexed gene expression, cell type, disease architectures, or even information-promoted discovery processes. The in situ sequencing (ISS) approach with the use of padlock probes and a rolling circle amplification process is adopted for resolving gene transcripts in a spatial manner inside tissue sections of a range of origins. The next iteration of ISS, hybridization-related ISS (HybISS), was presented by Gyllborg et al.117 Probe design modifications enable one novel barcoding mechanism through sequence-by-hybridization chemistry to achieve the optimized space detecting process of RNA transcript. One molecular method was developed by Asp et al.,118 revealing the overall transcription-related landscape pertaining of cell types in the embryonic heart under three developing phases, as well as mapping a cell-type-particular gene expression state for particular anatomically related domains. Space transcriptomics found special gene profiles representing prominent anatomically related places in the respective developing phase. Embryonic cardiac cell types of humans reported through the use of scRNA-seq verified and supplemented embryonic cardiac gene expression state space annotation. Next, the authors used ISS for refining the mentioned results and creating one space subcellular map in terms of the three developing phases. They developed one public human developing heart web resource for facilitating subsequent analyses over human cardiogenesis.118 In addition, Moncada et al.119 integrated a one microarray-related space transcriptomics approach revealing space patterns of gene expression with one spot arrays, respectively obtaining the transcriptomes of several nearby cells, while scRNA-seq was produced according to the identical sample. For annotating the accurate cell compositions of prominent tissue places, one approach to conduct the multimodal intersection analysis process was presented. Based on a multimodal cross-analysis of cells in major pancreatic tumors, including ductal cells, macrophages, dendritic cells, and cancer cells, we found that the subsets restricted enrichment in a spatial manner and restricted significant co-enrichment with other cell types.119
Use of individual-cell RNA sequencing inside different fields
Individual-cell gene expression profiling is quickly turning out to be a normal way of analysis in numerous subjects, including organ development, immunity, metabolism, and microorganisms, to name just a few (Figure 4).101,120 scRNA-seq refers to one effective way to delve into the developmental process. Early in the organ developmental process, cell collections commonly take place, appearing to be the same in a histological manner, whereas the process shows differentiation inside many different orientations for forming clear cell varieties. A closer look at the precise gene expression processes that drive these different pathways of differentiation will improve understanding of organ development. In this case, it is not appropriate to examine the gene expression analysis of the cell pool, as the analysis will eventually lead to cell pools that differentiate into different pathways. However, single-cell studies can identify different gene expression patterns associated with different lineage decisions in neighboring cells.121 The critical uses of scRNA-seq in immunology are to untangle cellular heterogeneity, cell development and differentiation, and hematopoietic and gene-regulatory networks to predict immunity. scRNA-seq is now exploited for delving into the development of hematopoietic stem cells and downstream progenitors to immune cells under differentiation.122,123 Individual-cell sequencing presenting V(D)J transcript information is being used to study lymphocyte antigen receiving elements, including B cell receptor (BCR) and TCR sequences. One computing approach, TraCeR, has been developed for reconstructing the TCR sequence and revealing the clonal associations of cells. The combination of the TCR reconstruction process and T cell transcription analysis is used for mapping the activation kinetics of T cells inside a mouse Salmonella infection system.124 Carmona et al.125 analyzed the evolutionary conservation of genes in human and mouse immune cell types, thereby identifying three T cell populations in zebrafish. Based on use of the locus reconstruction process, novel immunity-particular genes are found, consisting of emerging receptors similar to immunoglobulin. Of course, scRNA-seq is mainly used in the field of oncology, and we will introduce these applications in the next chapter. Thus far, the scRNA-seq analysis process is conducted largely in terms of higher organisms. Specific to microorganisms, scRNA-seq receives limited applications, primarily since the stiff cell wall can prevent valid lysis. Noticeably, a smaller amount of initiating RNA material is acquired, and the RNA has insufficient polyadenylate-related tails in terms of general mRNA molecule priming. On the whole, the detection process of existing scRNA-seq techniques exhibits less efficiency, and subsequent development or process improvement of the mentioned techniques is needed to delve into the microbial field under individual-cell resolution.126
Figure 4.
Use of individual-cell RNA sequencing inside different fields
Conclusions and perspective
There has been a great deal of interest in using scRNA-seq to provide answers to biological and medically related questions for humans in recent years. Such a technology provides an interesting insight into the basic processes of various developmental, physiological, and disease systems. During this period, many studies initiated new scRNA-seq programs and methodologies and reported on specific measures to explore medical diseases. Although scRNA-seq has progressed remarkably, further breakthroughs in the technology and its use are expected to be made. First, scRNA-seq studies are largely inclined to examine freshly isolated cells. We hope that more studies will use scRNA-seq to explore cryopreserved and fixed tissue samples, which will further open the technique for clinical research. Second, although numerous scRNA-seq studies have used expensive hardware, covering drop-based platforms and microfluidics, subsequent studies are expected to downregulate costs through reaction volume reductions and to probably avoid custom devices.
We expect that by using proteomics, epigenomics will lead to many new combinatorial approaches and the analysis of non-coding RNA species with scRNA-seq. This study speculates that we will get closer to a true global examination of single cells in the next decade for mRNAs, as well as genomes, epigenomes, proteomics, and metabolomics.
Finally, it is considered that in the next 5 years or so, there will be several clinical applications of scRNA-seq. First, the presence of rare malignant and chemical-resisting carcinoma cells may be assessed in removed tumors routinely. This information will provide important diagnostic information and guide decisions about treatment. Second, scRNA-seq assessment will provide in-depth information on immune cell responses, which will again inform diagnosis and treatment options. Third, the relatively small number of cells present in a range of other tissue biopsies (e.g., those from the surface of the skin and intestinal mucosa) would be ideal for presenting molecular data, probably aiding the diagnostic process, disease progression, as well as the appropriate treatment process. Thus, scRNA-seq will stand out from professional research laboratories and will be an established tool that both basic scientists and clinicians can use. Subsequent relevant research will seek to shed light on the above-mentioned processes, leading to the development of maps describing gene expressing throughout human cells and contributing to the field of personalized medicine.
Although the application prospect of single-cell sequencing technology in the field of cancer is very considerable, through the review of more than 100 studies in this paper, we also found its inevitable problems and challenges. One of the main reasons is that single-cell collection is tricky because the number of sample materials used is small, but analysis still requires a sufficient number of cells to ensure that all cell types are labeled. Second, the analysis of single-cell sequencing data is still challenging. At present, there are limited guidelines on how to define quality control standards, remove technical artifacts, and interpret results. Moreover, with the expansion of the scale of the experiment, the burden of data analysis increases. At the same time, the use of single-cell sequencing is limited in part because of its high cost. Most of the instruments and reagents required are expensive. The wide application of single-cell sequencing still has a long way to go. Significant investment will be required to unleash its full potential in research, drug discovery, and diagnosis. Nevertheless, there is still momentum in this field, and once it has solved the challenges, there is no doubt that single-cell sequencing will pave the way for breakthrough innovation in personalized medicine.
scRNA-seq is now overall used for various tumors. With the accumulation of huge datasets, the problem of how to make full use of them arises. Many studies will indeed benefit from a concentrated repository in which information can receive easy access under the cell condition rather than only at the sequence condition. We expect significant advances in bioinformatics and computation methods during the next few years. We are most looking forward to the sharing of scRNA-seq data around the world, which will help speed up the solution of the carcinoma problem.
Acknowledgments
Author contributions
Guangshun Sun, Z.L., D.R., and H.Z. were responsible for gathering information on the related research and designing the review. X.S., W.Y., W.Z., Guoqiang Sun, and F.W. were responsible for gathering information of the related research and making the drawings. Furthermore, there are three corresponding authors in this manuscript. W.T. contributed to information interpretation, editing, and critical revision of the manuscript. H.C. and Y.S. contributed to study design and critical revision of the manuscript. W.T., H.C., and Y.S. were also responsible for handling the revisions and re-submission of revised manuscripts. All authors read and approved the final manuscript
Declaration of interests
The authors declare no competing interests.
Contributor Information
Hongyong Cao, Email: caohongy6167@163.com.
Weiwei Tang, Email: 1243773473twww@sina.com.
Yangbai Sun, Email: drsunyb@fudan.edu.cn.
References
- 1.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyer J., Kwong L.N., Lowe S.W., Chin L. Non-germline genetically engineered mouse models for translational cancer research. Nat. Rev. Cancer. 2010;10:470–480. doi: 10.1038/nrc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma C.X., Reinert T., Chmielewska I., Ellis M.J. Mechanisms of aromatase inhibitor resistance. Nat. Rev. Cancer. 2015;15:261–275. doi: 10.1038/nrc3920. [DOI] [PubMed] [Google Scholar]
- 4.Lito P., Rosen N., Solit D.B. Tumor adaptation and resistance to RAF inhibitors. Nat. Med. 2013;19:1401–1409. doi: 10.1038/nm.3392. [DOI] [PubMed] [Google Scholar]
- 5.Morganti S., Tarantino P., Ferraro E., D’Amico P., Duso B.A., Curigliano G. Next generation sequencing (NGS): A revolutionary technology in pharmacogenomics and personalized medicine in cancer. Adv. Exp. Med. Biol. 2019;1168:9–30. doi: 10.1007/978-3-030-24100-1_2. [DOI] [PubMed] [Google Scholar]
- 6.Garziera M., Roncato R., Montico M., De Mattia E., Gagno S., Poletto E., Scalone S., Canzonieri V., Giorda G., Sorio R. New challenges in tumor mutation heterogeneity in advanced ovarian cancer by a targeted next-generation sequencing (NGS) approach. Cells. 2019;8:584. doi: 10.3390/cells8060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang F., Barbacioru C., Wang Y., Nordman E., Lee C., Xu N., Wang X., Bodeau J., Tuch B.B., Siddiqui A. mRNA-seq whole-transcriptome analysis of a single cell. Nat. Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 8.Navin N., Kendall J., Troge J., Andrews P., Rodgers L., McIndoo J., Cook K., Stepansky A., Levy D., Esposito D. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potter S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat. Rev. Nephrol. 2018;14:479–492. doi: 10.1038/s41581-018-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow M., Gillis J. Single cell RNA-sequencing: Replicability of cell types. Curr. Opin. Neurobiol. 2019;56:69–77. doi: 10.1016/j.conb.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegenhain C., Vieth B., Parekh S., Reinius B., Guillaumet-Adkins A., Smets M., Leonhardt H., Heyn H., Hellmann I., Enard W. Comparative analysis of single-cell RNA sequencing methods. Mol. Cell. 2017;65:631–643.e4. doi: 10.1016/j.molcel.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Haque A., Engel J., Teichmann S.A., Lönnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017;9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brehm-Stecher B.F., Johnson E.A. Single-cell microbiology: Tools, technologies, and applications. Microbiol. Mol. Biol. Rev. 2004;68:538–559. doi: 10.1128/MMBR.68.3.538-559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo F., Li L., Li J., Wu X., Hu B., Zhu P., Wen L., Tang F. Single-cell multi-omics sequencing of mouse early embryos and embryonic stem cells. Cell Res. 2017;27:967–988. doi: 10.1038/cr.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julius M.H., Masuda T., Herzenberg L.A. Demonstration that antigen-binding cells are precursors of antibody-producing cells after purification with a fluorescence-activated cell sorter. Proc. Natl. Acad. Sci. USA. 1972;69:1934–1938. doi: 10.1073/pnas.69.7.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichterwitz S., Chen G., Aguila Benitez J., Yilmaz M., Storvall H., Cao M., Sandberg R., Deng Q., Hedlund E. Laser capture microscopy coupled with Smart-seq2 for precise spatial transcriptomic profiling. Nat. Commun. 2016;7:12139. doi: 10.1038/ncomms12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y., Zhang K. Tools for the analysis of high-dimensional single-cell RNA sequencing data. Nat. Rev. Nephrol. 2020;16:408–421. doi: 10.1038/s41581-020-0262-0. [DOI] [PubMed] [Google Scholar]
- 18.Luecken M.D., Theis F.J. Current best practices in single-cell RNA-seq analysis: A tutorial. Mol. Syst. Biol. 2019;15:e8746. doi: 10.15252/msb.20188746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan J., Nedosekin D.A., Galanzha E.I., Zharov V.P. Detection of apoptotic circulating tumor cells using in vivo fluorescence flow cytometry. Cytometry A. 2019;95:664–671. doi: 10.1002/cyto.a.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L., Mao X., Grey A., Scandura G., Guo T., Burke E., Marzec J., Abdu S., Stankiewicz E., Davies C.R. Noninvasive detection of clinically significant prostate cancer using circulating tumor cells. J. Urol. 2020;203:73–82. doi: 10.1097/JU.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 21.Troncarelli Flores B.C., Souza E Silva V., Ali Abdallah E., Mello C.A.L., Gobo Silva M.L., Gomes Mendes G., Camila Braun A., Aguiar Junior S., Thomé Domingos Chinen L. Molecular and kinetic analyses of circulating tumor cells as predictive markers of treatment response in locally advanced rectal cancer patients. Cells. 2019;8:641. doi: 10.3390/cells8070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aceto N., Bardia A., Miyamoto D.T., Donaldson M.C., Wittner B.S., Spencer J.A., Yu M., Pely A., Engstrom A., Zhu H. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aceto N., Bardia A., Wittner B.S., Donaldson M.C., O’Keefe R., Engstrom A., Bersani F., Zheng Y., Comaills V., Niederhoffer K. AR expression in breast cancer CTCs associates with bone metastases. Mol. Cancer Res. 2018;16:720–727. doi: 10.1158/1541-7786.MCR-17-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczerba B.M., Castro-Giner F., Vetter M., Krol I., Gkountela S., Landin J., Scheidmann M.C., Donato C., Scherrer R., Singer J. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y.H., Chen Y.C., Lin E., Brien R., Jung S., Chen Y.T., Lee W., Hao Z., Sahoo S., Min Kang H. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat. Commun. 2019;10:2163. doi: 10.1038/s41467-019-10122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brechbuhl H.M., Vinod-Paul K., Gillen A.E., Kopin E.G., Gibney K., Elias A.D., Hayashi M., Sartorius C.A., Kabos P. Analysis of circulating breast cancer cell heterogeneity and interactions with peripheral blood mononuclear cells. Mol. Carcinog. 2020;59:1129–1139. doi: 10.1002/mc.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting D.T., Wittner B.S., Ligorio M., Vincent Jordan N., Shah A.M., Miyamoto D.T., Aceto N., Bersani F., Brannigan B.W., Xega K. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitrov-Markov S., Perales-Patón J., Bockorny B., Dopazo A., Muñoz M., Baños N., Bonilla V., Menendez C., Duran Y., Huang L. Discovery of new targets to control metastasis in pancreatic cancer by single-cell transcriptomics analysis of circulating tumor cells. Mol. Cancer Ther. 2020;19:1751–1760. doi: 10.1158/1535-7163.MCT-19-1166. [DOI] [PubMed] [Google Scholar]
- 29.D’Avola D., Villacorta-Martin C., Martins-Filho S.N., Craig A., Labgaa I., von Felden J., Kimaada A., Bonaccorso A., Tabrizian P., Hartmann B.M. High-density single cell mRNA sequencing to characterize circulating tumor cells in hepatocellular carcinoma. Sci. Rep. 2018;8:11570. doi: 10.1038/s41598-018-30047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M., Zhu P., McCarty G., Meyer C.F., Pratilas C.A., Levin A., Morris C.D., Albert C.M., Jackson K.W., Tang C.M., Loeb D.M. Size-based detection of sarcoma circulating tumor cells and cell clusters. Oncotarget. 2017;8:78965–78977. doi: 10.18632/oncotarget.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohr J.G., Kim S., Gould J., Knoechel B., Drier Y., Cotton M.J., Gray D., Birrer N., Wong B., Ha G. Genetic interrogation of circulating multiple myeloma cells at single-cell resolution. Sci. Transl. Med. 2016;8:363ra147. doi: 10.1126/scitranslmed.aac7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cann G.M., Gulzar Z.G., Cooper S., Li R., Luo S., Tat M., Stuart S., Schroth G., Srinivas S., Ronaghi M. mRNA-seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS ONE. 2012;7:e49144. doi: 10.1371/journal.pone.0049144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyer A., Gupta K., Sharma S., Hari K., Lee Y.F., Ramalingam N., Yap Y.S., West J., Bhagat A.A., Subramani B.V. Integrative analysis and machine learning based characterization of single circulating tumor cells. J. Clin. Med. 2020;9:1206. doi: 10.3390/jcm9041206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamza B., Ng S.R., Prakadan S.M., Delgado F.F., Chin C.R., King E.M., Yang L.F., Davidson S.M., DeGouveia K.L., Cermak N. Optofluidic real-time cell sorter for longitudinal CTC studies in mouse models of cancer. Proc. Natl. Acad. Sci. USA. 2019;116:2232–2236. doi: 10.1073/pnas.1814102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto D.T., Zheng Y., Wittner B.S., Lee R.J., Zhu H., Broderick K.T., Desai R., Fox D.B., Brannigan B.W., Trautwein J. RNA-seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–1356. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najafi M., Farhood B., Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019;234:8381–8395. doi: 10.1002/jcp.27740. [DOI] [PubMed] [Google Scholar]
- 37.De Francesco E.M., Sotgia F., Lisanti M.P. Cancer stem cells (CSCs): Metabolic strategies for their identification and eradication. Biochem. J. 2018;475:1611–1634. doi: 10.1042/BCJ20170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phi L.T.H., Sari I.N., Yang Y.G., Lee S.H., Jun N., Kim K.S., Lee Y.K., Kwon H.Y. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018:5416923. doi: 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W., Morabito S.J., Kessenbrock K., Enver T., Meyer K.B., Teschendorff A.E. Single-cell landscape in mammary epithelium reveals bipotent-like cells associated with breast cancer risk and outcome. Commun. Biol. 2019;2:306. doi: 10.1038/s42003-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonasson E., Ghannoum S., Persson E., Karlsson J., Kroneis T., Larsson E., Landberg G., Ståhlberg A. Identification of breast cancer stem cell related genes using functional cellular assays combined with single-cell RNA sequencing in MDA-MB-231 cells. Front. Genet. 2019;10:500. doi: 10.3389/fgene.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thong T., Wang Y., Brooks M.D., Lee C.T., Scott C., Balzano L., Wicha M.S., Colacino J.A. Hybrid stem cell states: Insights into the relationship between mammary development and breast cancer using single-cell transcriptomics. Front. Cell Dev. Biol. 2020;8:288. doi: 10.3389/fcell.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anstine L.J., Keri R. A new view of the mammary epithelial hierarchy and its implications for breast cancer initiation and metastasis. J. Cancer Metastasis Treat. 2019;5:50. doi: 10.20517/2394-4722.2019.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S., Zhang H., Fouladdel S., Li H., Keller E., Wicha M.S., Omenn G.S., Azizi E., Guan Y. Cellular, transcriptomic and isoform heterogeneity of breast cancer cell line revealed by full-length single-cell RNA sequencing. Comput. Struct. Biotechnol. J. 2020;18:676–685. doi: 10.1016/j.csbj.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGinn O., Ward A.V., Fettig L.M., Riley D., Ivie J., Paul K.V., Kabos P., Finlay-Schultz J., Sartorius C.A. Cytokeratin 5 alters β-catenin dynamics in breast cancer cells. Oncogene. 2020;39:2478–2492. doi: 10.1038/s41388-020-1164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong M., Deng Z., Yang M., Xu C., Zhang X., Zhang Q., Liao Y., Deng X., Lv D., Zhang X. Transcriptomic but not genomic variability confers phenotype of breast cancer stem cells. Cancer Commun. (Lond.) 2018;38:56. doi: 10.1186/s40880-018-0326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couturier C.P., Ayyadhury S., Le P.U., Nadaf J., Monlong J., Riva G., Allache R., Baig S., Yan X., Bourgey M. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 2020;11:3406. doi: 10.1038/s41467-020-17186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang B., Xu J., Hu J., Guo F., Wan L., Cheng M., Pang L. Single-cell RNA-seq reveals the invasive trajectory and molecular cascades underlying glioblastoma progression. Mol. Oncol. 2019;13:2588–2603. doi: 10.1002/1878-0261.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhai Y., Li G., Li R., Chang Y., Feng Y., Wang D., Wu F., Zhang W. Single-cell RNA-sequencing shift in the interaction pattern between glioma stem cells and immune cells during tumorigenesis. Front. Immunol. 2020;11:581209. doi: 10.3389/fimmu.2020.581209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y., Carter R., Natarajan S., Varn F.S., Compton D.A., Gawad C., Cheng C., Godek K.M. Single-cell RNA sequencing reveals the impact of chromosomal instability on glioblastoma cancer stem cells. BMC Med. Genomics. 2019;12:79. doi: 10.1186/s12920-019-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu R., Pan S., Chen Y., Nakano Y., Li M., Balog S., Tsukamoto H. Fate and functional roles of Prominin 1+ cells in liver injury and cancer. Sci. Rep. 2020;10:19412. doi: 10.1038/s41598-020-76458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho D.W., Tsui Y.M., Sze K.M., Chan L.K., Cheung T.T., Lee E., Sham P.C., Tsui S.K., Lee T.K., Ng I.O. Single-cell transcriptomics reveals the landscape of intra-tumoral heterogeneity and stemness-related subpopulations in liver cancer. Cancer Lett. 2019;459:176–185. doi: 10.1016/j.canlet.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P., Yang M., Zhang Y., Xiao S., Lai X., Tan A., Du S., Li S. Dissecting the single-cell transcriptome network underlying gastric premalignant lesions and early gastric cancer. Cell Rep. 2020;30:4317. doi: 10.1016/j.celrep.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Giustacchini A., Thongjuea S., Barkas N., Woll P.S., Povinelli B.J., Booth C.A.G., Sopp P., Norfo R., Rodriguez-Meira A., Ashley N. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med. 2017;23:692–702. doi: 10.1038/nm.4336. [DOI] [PubMed] [Google Scholar]
- 54.Miao Y., Yang H., Levorse J., Yuan S., Polak L., Sribour M., Singh B., Rosenblum M.D., Fuchs E. Adaptive immune resistance emerges from tumor-initiating stem cells. Cell. 2019;177:1172–1186.e14. doi: 10.1016/j.cell.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roulis M., Kaklamanos A., Schernthanner M., Bielecki P., Zhao J., Kaffe E., Frommelt L.S., Qu R., Knapp M.S., Henriques A. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580:524–529. doi: 10.1038/s41586-020-2166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong R., Chen D., Cao S., Li J., Han B., Zhong H. Immune cell infiltration features and related marker genes in lung cancer based on single-cell RNA-seq. Clin. Transl. Oncol. 2021;23:405–417. doi: 10.1007/s12094-020-02435-2. [DOI] [PubMed] [Google Scholar]
- 57.Wiseman D.H., Baker S.M., Dongre A.V., Gurashi K., Storer J.A., Somervaille T.C., Batta K. Chronic myelomonocytic leukaemia stem cell transcriptomes anticipate disease morphology and outcome. EBioMedicine. 2020;58:102904. doi: 10.1016/j.ebiom.2020.102904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan X.W., Zhang H., Xu D., Chen J.X., Chen W.J., Gan S.S., Qu F.J., Chu C.M., Cao J.W., Fan Y.H. Identification of a novel cancer stem cell subpopulation that promotes progression of human fatal renal cell carcinoma by single-cell RNA-seq analysis. Int. J. Biol. Sci. 2020;16:3149–3162. doi: 10.7150/ijbs.46645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman M.M., Zylla J.S., Bhattacharya S., Calar K., Hartman T.W., Bhardwaj R.D., Miskimins W.K., de la Puente P., Gnimpieba E.Z., Messerli S.M. Analysis of dual class I histone deacetylase and lysine demethylase inhibitor domatinostat (4SC-202) on growth and cellular and genomic landscape of atypical teratoid/rhabdoid. Cancers (Basel) 2020;12:756. doi: 10.3390/cancers12030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selvadurai H.J., Luis E., Desai K., Lan X., Vladoiu M.C., Whitley O., Galvin C., Vanner R.J., Lee L., Whetstone H. Medulloblastoma arises from the persistence of a rare and transient Sox2+ granule neuron precursor. Cell Rep. 2020;31:107511. doi: 10.1016/j.celrep.2020.03.075. [DOI] [PubMed] [Google Scholar]
- 61.Baryawno N., Przybylski D., Kowalczyk M.S., Kfoury Y., Severe N., Gustafsson K., Kokkaliaris K.D., Mercier F., Tabaka M., Hofree M. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177:1915–1932.e16. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma A., Cao E.Y., Kumar V., Zhang X., Leong H.S., Wong A.M.L., Ramakrishnan N., Hakimullah M., Teo H.M.V., Chong F.T. Longitudinal single-cell RNA sequencing of patient-derived primary cells reveals drug-induced infidelity in stem cell hierarchy. Nat. Commun. 2018;9:4931. doi: 10.1038/s41467-018-07261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McConnell A.M., Yao C., Yeckes A.R., Wang Y., Selvaggio A.S., Tang J., Kirsch D.G., Stripp B.R. p53 regulates progenitor cell quiescence and differentiation in the airway. Cell Rep. 2016;17:2173–2182. doi: 10.1016/j.celrep.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Sehgal K., Portell A., Ivanova E.V., Lizotte P.H., Mahadevan N.R., Greene J.R., Vajdi A., Gurjao C., Teceno T., Taus L.J. Dynamic single-cell RNA sequencing identifies immunotherapy persister cells following PD-1 blockade. J. Clin. Invest. 2021;131:135038. doi: 10.1172/JCI135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dost A.F.M., Moye A.L., Vedaie M., Tran L.M., Fung E., Heinze D., Villacorta-Martin C., Huang J., Hekman R., Kwan J.H. Organoids model transcriptional hallmarks of oncogenic KRAS activation in lung epithelial progenitor cells. Cell Stem Cell. 2020;27:663–678.e8. doi: 10.1016/j.stem.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glinsky G.V. Single cell genomics reveals activation signatures of endogenous SCAR’s networks in aneuploid human embryos and clinically intractable malignant tumors. Cancer Lett. 2016;381:176–193. doi: 10.1016/j.canlet.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y.C., Sahoo S., Brien R., Jung S., Humphries B., Lee W., Cheng Y.H., Zhang Z., Luker K.E., Wicha M.S. Single-cell RNA-sequencing of migratory breast cancer cells: Discovering genes associated with cancer metastasis. Analyst (Lond.) 2019;144:7296–7309. doi: 10.1039/c9an01358j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lei B., Zhang X.Y., Zhou J.P., Mu G.N., Li Y.W., Zhang Y.X., Pang D. Transcriptome sequencing of HER2-positive breast cancer stem cells identifies potential prognostic marker. Tumour Biol. 2016;37:14757–14764. doi: 10.1007/s13277-016-5351-0. [DOI] [PubMed] [Google Scholar]
- 69.Kim C., Gao R., Sei E., Brandt R., Hartman J., Hatschek T., Crosetto N., Foukakis T., Navin N.E. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell. 2018;173:879–893.e13. doi: 10.1016/j.cell.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karaayvaz M., Cristea S., Gillespie S.M., Patel A.P., Mylvaganam R., Luo C.C., Specht M.C., Bernstein B.E., Michor F., Ellisen L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018;9:3588. doi: 10.1038/s41467-018-06052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong S.P., Chan T.E., Lombardo Y., Corleone G., Rotmensz N., Bravaccini S., Rocca A., Pruneri G., McEwen K.R., Coombes R.C. Single-cell transcriptomics reveals multi-step adaptations to endocrine therapy. Nat. Commun. 2019;10:3840. doi: 10.1038/s41467-019-11721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinohara K., Wu H.J., Sébastien Vigneau, McDonald T.O., Igarashi K.J., Yamamoto K.N., Madsen T., Fassl A., Egri S.B., Papanastasiou M. KDM5 histone demethylase activity links cellular transcriptomic heterogeneity to therapeutic resistance. Cancer Cell. 2019;35:330–332. doi: 10.1016/j.ccell.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao Y., Li X., Zeng C., Liu C., Hao Q., Li W., Zhang K., Zhang W., Wang S., Zhao H. CD63+ cancer-associated fibroblasts confer tamoxifen resistance to breast cancer cells through exosomal miR-22. Adv. Sci. (Weinh.) 2020;7:2002518. doi: 10.1002/advs.202002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chi F., Liu J., Brady S.W., Cosgrove P.A., Nath A., McQuerry J.A., Majumdar S., Moos P.J., Chang J.T., Kahn M., Bild A.H. A “one-two punch” therapy strategy to target chemoresistance in estrogen receptor positive breast cancer. Transl. Oncol. 2021;14:100946. doi: 10.1016/j.tranon.2020.100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brady S.W., McQuerry J.A., Qiao Y., Piccolo S.R., Shrestha G., Jenkins D.F., Layer R.M., Pedersen B.S., Miller R.H., Esch A. Combating subclonal evolution of resistant cancer phenotypes. Nat. Commun. 2017;8:1231. doi: 10.1038/s41467-017-01174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeo S.K., Zhu X., Okamoto T., Hao M., Wang C., Lu P., Lu L.J., Guan J.L. Single-cell RNA-sequencing reveals distinct patterns of cell state heterogeneity in mouse models of breast cancer. eLife. 2020;9:e58810. doi: 10.7554/eLife.58810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen F., Ding K., Priedigkeit N., Elangovan A., Levine K.M., Carleton N., Savariau L., Atkinson J.M., Oesterreich S., Lee A.V. Single-cell transcriptomic heterogeneity in invasive ductal and lobular breast cancer cells. Cancer Res. 2021;81:268–281. doi: 10.1158/0008-5472.CAN-20-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jerby-Arnon L., Shah P., Cuoco M.S., Rodman C., Su M.J., Melms J.C., Leeson R., Kanodia A., Mei S., Lin J.R. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell. 2018;175:984–997.e24. doi: 10.1016/j.cell.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma K.Y., Schonnesen A.A., Brock A., Van Den Berg C., Eckhardt S.G., Liu Z., Jiang N. Single-cell RNA sequencing of lung adenocarcinoma reveals heterogeneity of immune response-related genes. JCI Insight. 2019;4:e121387. doi: 10.1172/jci.insight.121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izar B., Tirosh I., Stover E.H., Wakiro I., Cuoco M.S., Alter I., Rodman C., Leeson R., Su M.J., Shah P. A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 2020;26:1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee H.W., Chung W., Lee H.O., Jeong D.E., Jo A., Lim J.E., Hong J.H., Nam D.H., Jeong B.C., Park S.H. Single-cell RNA sequencing reveals the tumor microenvironment and facilitates strategic choices to circumvent treatment failure in a chemorefractory bladder cancer patient. Genome Med. 2020;12:47. doi: 10.1186/s13073-020-00741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schnepp P.M., Shelley G., Dai J., Wakim N., Jiang H., Mizokami A., Keller E.T. Single-cell transcriptomics analysis identifies nuclear protein 1 as a regulator of docetaxel resistance in prostate cancer cells. Mol. Cancer Res. 2020;18:1290–1301. doi: 10.1158/1541-7786.MCR-20-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teng J., da Hora C.C., Kantar R.S., Nakano I., Wakimoto H., Batchelor T.T., Chiocca E.A., Badr C.E., Tannous B.A. Dissecting inherent intratumor heterogeneity in patient-derived glioblastoma culture models. Neuro-oncol. 2017;19:820–832. doi: 10.1093/neuonc/now253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J., Guan M., Wang Q., Zhang J., Zhou T., Sun X. Single-cell transcriptome-based multilayer network biomarker for predicting prognosis and therapeutic response of gliomas. Brief. Bioinform. 2020;21:1080–1097. doi: 10.1093/bib/bbz040. [DOI] [PubMed] [Google Scholar]
- 85.Wieland A., Patel M.R., Cardenas M.A., Eberhardt C.S., Hudson W.H., Obeng R.C., Griffith C.C., Wang X., Chen Z.G., Kissick H.T. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature. 2020 doi: 10.1038/s41586-020-2931-3. Published online November 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anand P., Guillaumet-Adkins A., Dimitrova V., Yun H., Drier Y., Sotudeh N., Rogers A.J., Ouseph M.M., Nair M., Potdar S. Single cell RNA-seq reveals developmental plasticity with coexisting oncogenic and immune evasion programs in ETP-ALL. Blood. 2020 doi: 10.1182/blood.2019004547. Published online November 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mehtonen J., Teppo S., Lahnalampi M., Kokko A., Kaukonen R., Oksa L., Bouvy-Liivrand M., Malyukova A., Mäkinen A., Laukkanen S. Single cell characterization of B-lymphoid differentiation and leukemic cell states during chemotherapy in ETV6-RUNX1-positive pediatric leukemia identifies drug-targetable transcription factor activities. Genome Med. 2020;12:99. doi: 10.1186/s13073-020-00799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong D., Wang Y., You M. A gene expression signature of TREM2hi macrophages and γδ T cells predicts immunotherapy response. Nat. Commun. 2020;11:5084. doi: 10.1038/s41467-020-18546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao C.D., Haensel D., Gaddam S., Patel T., Atwood S.X., Sarin K.Y., Whitson R.J., McKellar S., Shankar G., Aasi S. AP-1 and TGFß cooperativity drives non-canonical Hedgehog signaling in resistant basal cell carcinoma. Nat. Commun. 2020;11:5079. doi: 10.1038/s41467-020-18762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paulson K.G., Voillet V., McAfee M.S., Hunter D.S., Wagener F.D., Perdicchio M., Valente W.J., Koelle S.J., Church C.D., Vandeven N. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat. Commun. 2018;9:3868. doi: 10.1038/s41467-018-06300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song L., Bretz A.C., Gravemeyer J., Spassova I., Muminova S., Gambichler T., Sriram A., Ferrone S., Becker J.C. The HDAC inhibitor domatinostat promotes cell-cycle arrest, induces apoptosis, and increases immunogenicity of Merkel cell carcinoma cells. J. Invest. Dermatol. 2021;141:903–912.e4. doi: 10.1016/j.jid.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aggen D.H., Ager C.R., Obradovic A.Z., Chowdhury N., Ghasemzadeh A., Mao W., Chaimowitz M.G., Lopez-Bujanda Z.A., Spina C.S., Hawley J.E. Blocking IL1 beta promotes tumor regression and remodeling of the myeloid compartment in a renal cell carcinoma model: Multi-dimensional analyses. Clin. Cancer Res. 2021;27:608–621. doi: 10.1158/1078-0432.CCR-20-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A., Teinor J.A., Belleau P., Biffi G., Lucito M.S. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dominguez C.X., Müller S., Keerthivasan S., Koeppen H., Hung J., Gierke S., Breart B., Foreman O., Bainbridge T.W., Castiglioni A. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020;10:232–253. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 95.Qu Y., Wen J., Thomas G., Yang W., Prior W., He W., Sundar P., Wang X., Potluri S., Salek-Ardakani S. Baseline frequency of inflammatory Cxcl9-expressing tumor-associated macrophages predicts response to avelumab treatment. Cell Rep. 2020;32:108115. doi: 10.1016/j.celrep.2020.108115. [DOI] [PubMed] [Google Scholar]
- 96.Wang B., Liu M., Ran Z., Li X., Li J., Ou Y. Analysis of gene signatures of tumor microenvironment yields insight into mechanisms of resistance to immunotherapy. Front. Bioeng. Biotechnol. 2020;8:348. doi: 10.3389/fbioe.2020.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ponzetta A., Carriero R., Carnevale S., Barbagallo M., Molgora M., Perucchini C., Magrini E., Gianni F., Kunderfranco P., Polentarutti N. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178:346–360.e24. doi: 10.1016/j.cell.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park S.R., Namkoong S., Friesen L., Cho C.S., Zhang Z.Z., Chen Y.C., Yoon E., Kim C.H., Kwak H., Kang H.M., Lee J.H. Single-cell transcriptome analysis of colon cancer cell response to 5-fluorouracil-induced DNA damage. Cell Rep. 2020;32:108077. doi: 10.1016/j.celrep.2020.108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim K., Park S., Park S.Y., Kim G., Park S.M., Cho J.W., Kim D.H., Park Y.M., Koh Y.W., Kim H.R. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med. 2020;12:22. doi: 10.1186/s13073-020-00722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim K.T., Lee H.W., Lee H.O., Kim S.C., Seo Y.J., Chung W., Eum H.H., Nam D.H., Kim J., Joo K.M., Park W.Y. Single-cell mRNA sequencing identifies subclonal heterogeneity in anti-cancer drug responses of lung adenocarcinoma cells. Genome Biol. 2015;16:127. doi: 10.1186/s13059-015-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu X., Zhang L., Chaudhry A., Rapaport A.S., Ouyang W. Unravelling the heterogeneity and dynamic relationships of tumor-infiltrating T cells by single-cell RNA sequencing analysis. J. Leukoc. Biol. 2020;107:917–932. doi: 10.1002/JLB.6MR0320-234R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu T.D., Madireddi S., de Almeida P.E., Banchereau R., Chen Y.J., Chitre A.S., Chiang E.Y., Iftikhar H., O’Gorman W.E., Au-Yeung A. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579:274–278. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki A., Matsushima K., Makinoshima H., Sugano S., Kohno T., Tsuchihara K., Suzuki Y. Single-cell analysis of lung adenocarcinoma cell lines reveals diverse expression patterns of individual cells invoked by a molecular target drug treatment. Genome Biol. 2015;16:66. doi: 10.1186/s13059-015-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stankovic B., Bjørhovde H.A.K., Skarshaug R., Aamodt H., Frafjord A., Müller E., Hammarström C., Beraki K., Bækkevold E.S., Woldbæk P.R. Immune cell composition in human non-small cell lung cancer. Front. Immunol. 2019;9:3101. doi: 10.3389/fimmu.2018.03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hadler-Olsen E., Wirsing A.M. Tissue-infiltrating immune cells as prognostic markers in oral squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer. 2019;120:714–727. doi: 10.1038/s41416-019-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Savas P., Virassamy B., Ye C., Salim A., Mintoff C.P., Caramia F., Salgado R., Byrne D.J., Teo Z.L., Dushyanthen S., Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 107.Zheng C., Zheng L., Yoo J.K., Guo H., Zhang Y., Guo X., Kang B., Hu R., Huang J.Y., Zhang Q. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 108.Ni J., Wang X., Stojanovic A., Zhang Q., Wincher M., Bühler L., Arnold A., Correia M.P., Winkler M., Koch P.S. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK cell activity. Immunity. 2020;52:1075–1087.e8. doi: 10.1016/j.immuni.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Sathe A., Grimes S.M., Lau B.T., Chen J., Suarez C., Huang R.J., Poultsides G., Ji H.P. Single-cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin. Cancer Res. 2020;26:2640–2653. doi: 10.1158/1078-0432.CCR-19-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chung W., Eum H.H., Lee H.O., Lee K.M., Lee H.B., Kim K.T., Ryu H.S., Kim S., Lee J.E., Park Y.H. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017;8:15081. doi: 10.1038/ncomms15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao Z., Tan Z.W., Zhu P., Tan N.S. Cancer-associated fibroblasts in tumor microenvironment—Accomplices in tumor malignancy. Cell. Immunol. 2019;343:103729. doi: 10.1016/j.cellimm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Vuong N.H., Cook D.P., Forrest L.A., Carter L.E., Robineau-Charette P., Kofsky J.M., Hodgkinson K.M., Vanderhyden B.C. Single-cell RNA-sequencing reveals transcriptional dynamics of estrogen-induced dysplasia in the ovarian surface epithelium. PLoS Genet. 2018;14:e1007788. doi: 10.1371/journal.pgen.1007788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin W., Noel P., Borazanci E.H., Lee J., Amini A., Han I.W., Heo J.S., Jameson G.S., Fraser C., Steinbach M. Single-cell transcriptome analysis of tumor and stromal compartments of pancreatic ductal adenocarcinoma primary tumors and metastatic lesions. Genome Med. 2020;12:80. doi: 10.1186/s13073-020-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen Q.H., Pervolarakis N., Blake K., Ma D., Davis R.T., James N., Phung A.T., Willey E., Kumar R., Jabart E. Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat. Commun. 2018;9:2028. doi: 10.1038/s41467-018-04334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., Bassez A., Decaluwé H., Pircher A., Van den Eynde K. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 116.Fernández-Zapata C., Leman J.K.H., Priller J., Böttcher C. The use and limitations of single-cell mass cytometry for studying human microglia function. Brain Pathol. 2020;30:1178–1191. doi: 10.1111/bpa.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gyllborg D., Langseth C.M., Qian X., Choi E., Salas S.M., Hilscher M.M., Lein E.S., Nilsson M. Hybridization-based in situ sequencing (HybISS) for spatially resolved transcriptomics in human and mouse brain tissue. Nucleic Acids Res. 2020;48:e112. doi: 10.1093/nar/gkaa792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Asp M., Giacomello S., Larsson L., Wu C., Furth D., Qian X., Wärdell E., Custodio J., Reimegård J., Salmén F. A spatiotemporal organ-wide gene expression and cell atlas of the developing human heart. Cell. 2019;179:1647–1660.e19. doi: 10.1016/j.cell.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 119.Moncada R., Barkley D., Wagner F., Chiodin M., Devlin J.C., Baron M., Hajdu C.H., Simeone D.M., Yanai I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020;38:333–342. doi: 10.1038/s41587-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 120.Ranzoni A.M., Strzelecka P.M., Cvejic A. Application of single-cell RNA sequencing methodologies in understanding haematopoiesis and immunology. Essays Biochem. 2019;63:217–225. doi: 10.1042/EBC20180072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong H., He A. Single-cell transcriptomic analysis of cardiac progenitor differentiation. Curr. Cardiol. Rep. 2020;22:38. doi: 10.1007/s11886-020-01285-2. [DOI] [PubMed] [Google Scholar]
- 122.Nestorowa S., Hamey F.K., Pijuan Sala B., Diamanti E., Shepherd M., Laurenti E., Wilson N.K., Kent D.G., Göttgens B. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood. 2016;128:e20–e31. doi: 10.1182/blood-2016-05-716480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martinez-Jimenez C.P., Eling N., Chen H.C., Vallejos C.A., Kolodziejczyk A.A., Connor F., Stojic L., Rayner T.F., Stubbington M.J.T., Teichmann S.A. Aging increases cell-to-cell transcriptional variability upon immune stimulation. Science. 2017;355:1433–1436. doi: 10.1126/science.aah4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stubbington M.J.T., Lönnberg T., Proserpio V., Clare S., Speak A.O., Dougan G., Teichmann S.A. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods. 2016;13:329–332. doi: 10.1038/nmeth.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carmona S.J., Teichmann S.A., Ferreira L., Macaulay I.C., Stubbington M.J., Cvejic A., Gfeller D. Single-cell transcriptome analysis of fish immune cells provides insight into the evolution of vertebrate immune cell types. Genome Res. 2017;27:451–461. doi: 10.1101/gr.207704.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y., Gao J., Huang Y., Wang J. Recent developments in single-cell RNA-seq of microorganisms. Biophys. J. 2018;115:173–180. doi: 10.1016/j.bpj.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maynard A., McCoach C.E., Rotow J.K., Harris L., Haderk F., Kerr D.L., Yu E.A., Schenk E.L., Tan W., Zee A. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–1251.e22. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang F., Bai H., Gao R., Fei K., Duan J., Zhang Z., Wang J., Hu X. Dynamics of peripheral T cell clones during PD-1 blockade in non-small cell lung cancer. Cancer Immunol. Immunother. 2020;69:2599–2611. doi: 10.1007/s00262-020-02642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim N., Kim H.K., Lee K., Hong Y., Cho J.H., Choi J.W., Lee J.I., Suh Y.L., Ku B.M., Eum H.H. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 2020;11:2285. doi: 10.1038/s41467-020-16164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L., Wang W., Wang R., Zhang N., Shang H., Bi Y., Chen D., Zhang C., Li L., Yin J. Reshaping the immune microenvironment by oncolytic herpes simplex virus in murine pancreatic ductal adenocarcinoma. Mol. Ther. 2021;29:744–761. doi: 10.1016/j.ymthe.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anderson D., Skut P., Hughes A.M., Ferrari E., Tickner J., Xu J., Mullin B.H., Tang D., Malinge S., Kees U.R. The bone marrow microenvironment of pre-B acute lymphoblastic leukemia at single-cell resolution. Sci. Rep. 2020;10:19173. doi: 10.1038/s41598-020-76157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang M., Yang H., Wan L., Wang Z., Wang H., Ge C., Liu Y., Hao Y., Zhang D., Shi G. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020;73:1118–1130. doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 133.Davidson S., Efremova M., Riedel A., Mahata B., Pramanik J., Huuhtanen J., Kar G., Vento-Tormo R., Hagai T., Chen X. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 2020;31:107628. doi: 10.1016/j.celrep.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu S.Z., Roden D.L., Wang C., Holliday H., Harvey K., Cazet A.S., Murphy K.J., Pereira B., Al-Eryani G., Bartonicek N. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020;39:e104063. doi: 10.15252/embj.2019104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen Q.Y., Li Y.N., Wang X.Y., Zhang X., Hu Y., Li L., Suo D.Q., Ni K., Li Z., Zhan J.R. Tumor fibroblast-derived FGF2 regulates expression of SPRY1 in esophageal tumor-infiltrating T cells and plays a role in T-cell exhaustion. Cancer Res. 2020;80:5583–5596. doi: 10.1158/0008-5472.CAN-20-1542. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z., Zhou L., Liu L., Hou Y., Xiong M., Yang Y., Hu J., Chen K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020;11:5077. doi: 10.1038/s41467-020-18916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kempski J., Giannou A.D., Riecken K., Zhao L., Steglich B., Lücke J., Garcia-Perez L., Karstens K.F., Wöstemeier A., Nawrocki M. IL22BP mediates the antitumor effects of lymphotoxin against colorectal tumors in mice and humans. Gastroenterology. 2020;159:1417–1430.e3. doi: 10.1053/j.gastro.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ren X., Zhang Z. Understanding tumor-infiltrating lymphocytes by single cell RNA sequencing. Adv. Immunol. 2019;144:217–245. doi: 10.1016/bs.ai.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 139.Ding Z.C., Shi H., Aboelella N.S., Fesenkova K., Park E.J., Liu Z., Pei L., Li J., McIndoe R.A., Xu H. Persistent STAT5 activation reprograms the epigenetic landscape in CD4+ T cells to drive polyfunctionality and antitumor immunity. Sci. Immunol. 2020;5:eaba5962. doi: 10.1126/sciimmunol.aba5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan Y., Lu F., Fei Q., Yu X., Xiong P., Yu X., Dang Y., Hou Z., Lin W., Lin X. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J. Hematol. Oncol. 2019;12:124. doi: 10.1186/s13045-019-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma X., Guo J., Liu K., Chen L., Liu D., Dong S., Xia J., Long Q., Yue Y., Zhao P. Identification of a distinct luminal subgroup diagnosing and stratifying early stage prostate cancer by tissue-based single-cell RNA sequencing. Mol. Cancer. 2020;19:147. doi: 10.1186/s12943-020-01264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shah F., Goossens E., Atallah N.M., Grimard M., Kelley M.R., Fishel M.L. APE1/Ref-1 knockdown in pancreatic ductal adenocarcinoma—Characterizing gene expression changes and identifying novel pathways using single-cell RNA sequencing. Mol. Oncol. 2017;11:1711–1732. doi: 10.1002/1878-0261.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang M., Hu S., Min M., Ni Y., Lu Z., Sun X., Wu J., Liu B., Ying X., Liu Y. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2021;70:464–475. doi: 10.1136/gutjnl-2019-320368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Y.L., Chen C.H., Chen J.Y., Lai Y.S., Wang S.C., Jiang S.S., Hung W.C. Single-cell analysis reveals immune modulation and metabolic switch in tumor-draining lymph nodes. OncoImmunology. 2020;9:1830513. doi: 10.1080/2162402X.2020.1830513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davis R.T., Blake K., Ma D., Gabra M.B.I., Hernandez G.A., Phung A.T., Yang Y., Maurer D., Lefebvre A.E.Y.T., Alshetaiwi H. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat. Cell Biol. 2020;22:310–320. doi: 10.1038/s41556-020-0477-0. [DOI] [PubMed] [Google Scholar]
- 146.Bartoschek M., Oskolkov N., Bocci M., Lövrot J., Larsson C., Sommarin M., Madsen C.D., Lindgren D., Pekar G., Karlsson G. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu H., Yu J., Li Y., Hou Q., Zhou R., Zhang N., Jing Z., Jiang M., Li Z., Hua Y. Single-cell RNA sequencing reveals diverse intratumoral heterogeneities and gene signatures of two types of esophageal cancers. Cancer Lett. 2018;438:133–143. doi: 10.1016/j.canlet.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 148.Kim K.T., Lee H.W., Lee H.O., Song H.J., Jeong E., Shin S., Kim H., Shin Y., Nam D.H., Jeong B.C. Application of single-cell RNA sequencing in optimizing a combinatorial therapeutic strategy in metastatic renal cell carcinoma. Genome Biol. 2016;17:80. doi: 10.1186/s13059-016-0945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dong R., Yang R., Zhan Y., Lai H.D., Ye C.J., Yao X.Y., Luo W.Q., Cheng X.M., Miao J.J., Wang J.F. Single-cell characterization of malignant phenotypes and developmental trajectories of adrenal neuroblastoma. Cancer Cell. 2020;38:716–733.e6. doi: 10.1016/j.ccell.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 150.Lu T., Yang X., Shi Y., Zhao M., Bi G., Liang J., Chen Z., Huang Y., Jiang W., Lin Z. Single-cell transcriptome atlas of lung adenocarcinoma featured with ground glass nodules. Cell Discov. 2020;6:69. doi: 10.1038/s41421-020-00200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Song Y., Guerrero-Juarez C.F., Chen Z., Tang Y., Ma X., Lv C., Bi X., Deng M., Bu L., Tian Y. The Msi1-mTOR pathway drives the pathogenesis of mammary and extramammary Paget’s disease. Cell Res. 2020;30:854–872. doi: 10.1038/s41422-020-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Caruso F.P., Garofano L., D’Angelo F., Yu K., Tang F., Yuan J., Zhang J., Cerulo L., Pagnotta S.M., Bedognetti D. A map of tumor-host interactions in glioma at single-cell resolution. Gigascience. 2020;9:giaa109. doi: 10.1093/gigascience/giaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prieto-Fernández E., Egia-Mendikute L., Bosch A., García Del Río A., Jimenez-Lasheras B., Antoñana-Vildosola A., Lee S.Y., Palazon A. Hypoxia promotes syndecan-3 expression in the tumor microenvironment. Front. Immunol. 2020;11:586977. doi: 10.3389/fimmu.2020.586977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Choi J., Gong J.R., Hwang C.Y., Joung C.Y., Lee S., Cho K.H. A systems biology approach to identifying a master regulator that can transform the fast growing cellular state to a slowly growing one in early colorectal cancer development model. Front. Genet. 2020;11:570546. doi: 10.3389/fgene.2020.570546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Song L., Zhang S., Yu S., Ma F., Wang B., Zhang C., Sun J., Mao X., Wei L. Cellular heterogeneity landscape in laryngeal squamous cell carcinoma. Int. J. Cancer. 2020;147:2879–2890. doi: 10.1002/ijc.33192. [DOI] [PubMed] [Google Scholar]