Abstract
Physical activity may improve cognitive function in women with breast cancer. In a cross-sectional study, we explored the relationship between cognitive function and physical activity (actigraph) and cardiorespiratory fitness (sub-maximal graded exercise test) in 73 postmenopausal women with early stage breast cancer prior to the initiation of systemic adjuvant therapy. Cognitive function was assessed with a standardized battery of neurocognitive measures assessing eight domains. Data were analyzed using partial correlations, controlling for age and total hours of actigraph wear-time. Women were, on average, 63.71 (± 5.3) years of age with 15.47 (± 2.48) years of education. For physical activity, greater average number of steps per day were associated with better attention (r=.262, p=.032) and psychomotor speed (r=.301, p=.011); greater average hours of moderate and moderate/vigorous intensity physical activity were associated with better visual memory (r=.241, p=.049; r=.241, p=.049, respectively); and greater average daily energy expenditure was associated with better visual memory (r=.270, p=.027) and psychomotor speed (r=.292, p=.017). For fitness, higher peak maximum VO2 was associated with better concentration (r=.330, p=.006), verbal memory (r=.241, p=.048) and working memory (r=.281, p=.019). These results suggest that higher levels of physical activity and cardiorespiratory fitness are associated with better cognitive function in postmenopausal women with breast cancer. Randomized controlled trials (RCT) to examine whether physical activity improves cognitive function in women with breast cancer are warranted. These RCTs should also determine the mechanisms of the influence of physical activity on cognitive function.
Clinical trials registration number:
NCT02793921; Date: May 20, 2016
Keywords: Cognitive function, breast cancer, physical activity, cardiorespiratory fitness
Introduction
In the United States, breast cancer is the most common cancer diagnosed among women, there are currently over 3.5 million breast cancer survivors and it is estimated that 276,480 new cases of invasive breast cancer will be diagnosed in women in 2020 [1]. Over 75% of women with breast cancer are postmenopausal at diagnosis.[2] Women with breast cancer often experience changes in cognitive function related to both disease and treatment that significantly impacts their quality of life and functional ability, [3] compromises their psychological well-being and interferes with work, decision making, and self-management including adherence to cancer therapy [4, 5]. Postmenopausal women may be particularly vulnerable to cognitive decline with breast cancer and its treatment. Mounting evidence suggests that cancer and cancer therapy may accelerate normal aging, an outcome of which is a deterioration in cognitive function.[6–9] Thus, postmenopausal women, experiencing normal age-related cognitive decline, may experience an acceleration of that decline with breast cancer and its treatment. Indeed, our group demonstrated that, compared to age and education-matched healthy women, postmenopausal women with breast cancer had worse executive function prior to initiating systemic adjuvant therapy that persisted through the first 18 months of therapy [10]. The basis for worse pre-therapy cognitive function is not clear [11, 12].
Increasing evidence suggests that being physically inactive with low levels of cardiorespiratory fitness are related to worse cognitive function in multiple populations of adults without cancer. Physical inactivity is a major risk factor for dementia in the US,[13] and predicts normal age-related cognitive decline [14]. These same factors put postmenopausal women at a higher risk for breast cancer, are associated with poorer disease outcomes, and may be related to worse cognitive function in women with the disease [15]. Being physically inactive with low cardiorespiratory fitness may also contribute to worse pre-therapy cognitive function. However, little research has examined these relationships in postmenopausal women with breast cancer prior to the initiation of systemic adjuvant therapy. The purpose of this study was to explore the relationship between cognitive function and physical activity and cardiorespiratory fitness in postmenopausal women with early stage breast cancer before they begin systemic adjuvant therapy, aromatase inhibitors with or without chemotherapy.
Methods
Using a cross-sectional design, we assessed cognitive function, cardiorespiratory fitness, and physical activity in 73 postmenopausal women with early stage breast cancer. All data used for this investigation were collected prior to randomization (post-breast cancer diagnosis; pre-adjuvant therapy) from the Exercise Program in Cancer and Cognition (EPICC) study, an ongoing randomized, controlled trial of the use of aerobic exercise to improve cognitive function and brain health in women with breast cancer (R01 CA196762). Human subjects’ approval was obtained from the University of Pittsburgh Human Research Protection Office, all participants provided written informed consent. A detailed description of EPICC study methods has been reported elsewhere [16].
Women with breast cancer were recruited from the Comprehensive Breast Cancer Program of the UPMC Hillman Cancer Center and UPMC Cancer Centers between 2015 and 2019. Eligible women were postmenopausal, younger than 80 years of age, diagnosed with stage 0, 1, 2 or 3a breast cancer, eligible to receive, but have not yet begun aromatase inhibitor therapy, and English-speaking with a minimum of 8 years of education. Women were excluded who had evidence of distant metastases, history of neurological illness or cancer, self-reported hospitalization for psychiatric illness within 2 years, eating disorders, history of substance abuse, use of an assisted walking device, reconstructive surgery, breast cancer surgery complication, history of falls or balance problems, or any significant medical condition that would make exercise unsafe.
Measures
Cognitive function was measured with a battery of validated neurocognitive measures, with demonstrated sensitivity to cognitive function in women with breast cancer [17, 18]. The battery assesses multiple cognitive domains and includes measures from the Cambridge Neuropsychological Test Automated Battery (CANTAB) [19–21]. Due to the high number of scores produced from this battery, we performed empirically-based factor analysis with orthogonal rotation, a data reduction approach, to reduce the number of outcome variables. This procedure yielded eight factors. (Table 1) Mean z-scores for the eight factors were computed as the average of the participant’s z-scores of individual scores from the tests in the battery of neurocognitive measures and relative to our normative data from women without breast cancer matched on age and years of education to breast cancer cohorts from a previous study [10]. Higher positive z-scores indicate better performance relative to the control group at baseline. Based upon evidence of variables that may influence the relationships being examined[22–24], we also assessed potential covariates including age, years of education, pain (Brief Pain Inventory)[25], fatigue (PROMIS Fatigue Short Form)[26], depressive symptoms (Beck Depression Inventory II)[27], anxiety (PROMIS Emotional Distress Anxiety Short Form)[28], armband wear time[29], and body mass index (BMI)[30].
Table 1.
Eight factors with corresponding measures in neurocognitive battery.
| Attention | Visual Working Memory |
| CANTAB* Rapid Visual Information Processing (Total hits, A‘, and mean latency)[19] | CANTAB Stockings of Cambridge (Mean subsequent thinking time)[19] |
| Rey Complex Figure (Immediate and delayed recall)[68] | |
| Concentration | |
| Digit Vigilance Test (Time and errors)[65] | Executive Function |
| CANTAB Stockings of Cambridge (Mean Initial thinking time and problems solved in minimum moves)[19] | |
| Verbal Memory | |
| Auditory Verbal Learning Test (Trial 6 #, delay and total correct)[66] | CANTAB Spatial Working Memory (Errors and strategy)[19] |
| Rivermead Story (Immediate and delayed recall)[67] | |
| Psychomotor Speed | |
| Visual Memory | Grooved Pegboard (Dominant and non-dominant hand time)[69] |
| CANTAB Paired Associate Learning (Stages completed and total errors adjusted)[19] | |
| Digit Symbol Substitution[70] | |
| Rey Complex Figure (Copy)[68] | |
| Mental Flexibility | |
| Delis Kaplan Color Word Interference Test (Scaled scores 1 and 2, composition scaled score, inhibition switching norming method scaled score)[71] |
CANTAB = Cambridge Neuropsychological Test Automated Battery
Cardiorespiratory fitness was measured using a sub-maximal graded exercise test which requires walking on a treadmill between 2.0 and 4.0 mph as the grade increases 1% each minute. Test speed was self-selected by the participant at increments of 0.5 mph within the 2.0–4.0 mph range. Prior to the test, height and body mass were recorded and the participant was fitted with a mouthpiece and nose clip to collect expired air. The test was terminated when the participant reached 85% of her age-predicted maximal heart rate (220–age), a Borg rating of perceived exertion [31] of 15 or greater in participants taking a beta blocker, or volitional exhaustion. Oxygen consumption was analyzed using a ParvoMedics metabolic cart and data were extrapolated to estimate maximal oxygen consumption (Peak VO2).
Physical activity was assessed with the SenseWear Armband (BodyMedia, Inc.) [32]. Following the completion of cardiorespiratory fitness testing, the device was placed on the upper arm and participants were instructed to wear it at all times (other than showering, swimming, or bathing) for seven consecutive days. Minute-by minute data were used to quantify participants’ average hours of physical activity per day and daily active energy expenditure. Bhammar (2013) reported excellent test-retest reliability for the SenseWear Armband for energy expenditure (r=0.94) and for total energy expenditure (0.95); the coefficient of variation for the test retest reliability for total energy expenditure was estimated at 6.3% (95% CI: 4.7 – 9.5%) [33].
Statistical Analysis.
Exploratory analyses were first performed to screen data for any anomalies (e.g., outliers, violations of statistical assumptions, etc.) that may invalidate results and potential covariates. Descriptive analyses were then performed to summarize the demographic and clinical characteristics and the main variables of interest of the sample. Measures of central tendency and dispersion (means and standard deviations [SDs]) were computed for continuous type variables (e.g., age, BMI) and frequency counts and percentages summarized categorical characteristics (e.g., race). Potential covariates/confounders identified from the literature, such as age, armband wear time, BMI, education, IQ, depressive symptoms, anxiety, fatigue, pain, were screened for possible inclusion in analyses. Controlling for the identified covariates of age and total wear time for BodyMedia Sensewear armband, partial correlational analyses were conducted to explore the associations between cognitive function domains and the selected measures of physical activity and fitness. This analytic approach allowed us to “partial out” the effects of identified extraneous covariates (age, armband wear time) when examining the associations between our targeted predictors (physical activity and cardiorespiratory fitness) and our outcomes (objectively measured cognitive function) of interest. As this was a secondary analysis using currently available baseline (pre-randomization) data from an ongoing randomized controlled trial that is still accruing participants, the maximum sample size for this investigation was fixed at 73 which would allow for the detection of population partial correlations (based on the correlation of residuals) as small as 0.32 with 0.80 power when testing hypotheses at a significance level of .05 (two-tailed).
Results
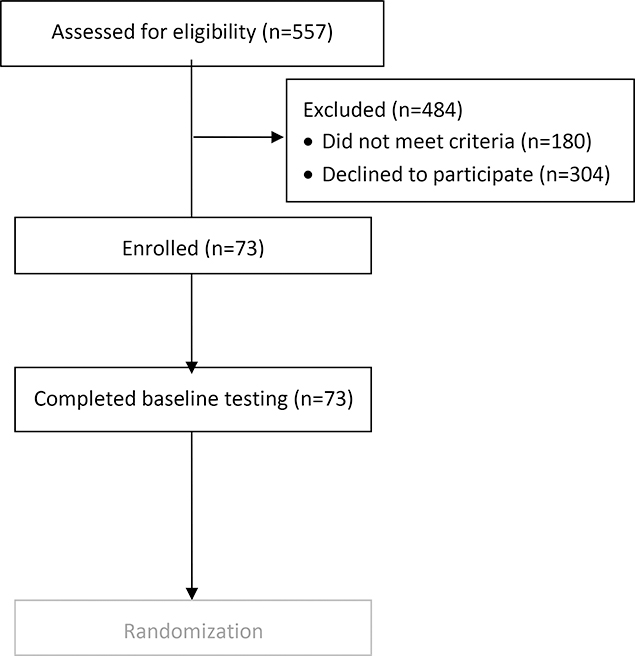
Figure 1 summarizes the number of women assessed for study eligibility, the number enrolled and reasons for exclusion. Women cited time constraints as the major reason for participation refusal[. Seventy-three women were enrolled in the study. On average, participants were 63.71 years of age with 15.47 years of education and white (89%) (Table 1). They had stage 1 disease (64.4%), obesity [BMI = 31.06 (6.61)] and their average maximum peak VO2 was low [1.38 (0.29)]. Participants had low pain severity and minimal depressive symptoms, anxiety and fatigue. Their cognitive function performance did not differ markedly from our normative data across domains.
Figure 1.
Flow chart summarizing assessment for eligibility, exclusion and enrollment.
Controlling for age and total armband wear time, fitness level and physical activity were significantly related to performance in multiple cognitive domains, including attention, concentration, psychomotor speed, verbal and visual memory and working memory (Table 2). Lower maximum values of peak VO2 were associated with worse concentration (p = .006), verbal memory (p = .048) and visual working memory (p=.019) and there was a trend for an association with worse psychomotor speed (p=.074). Fewer average number of steps per day were associated with worse attention (p = .032) and psychomotor speed (p = .011). Neither average hours of light (1.5 – 3.0 METS) nor vigorous (6.0 – 9.0 METS) intensity physical activity were associated with any domain of cognitive function (p≥.050). Fewer average hours of moderate-intensity physical activity (3.0 – 6.0 METS) per day were associated with worse visual memory (p = .049) and there was a trend toward an association with worse psychomotor speed (p = .076). Similarly, fewer average hours of moderate/vigorous intensity physical activity (3.0 – 9.0 METS) were associated with worse visual memory (p = .049) and there was a trend toward an association with worse psychomotor speed (p = .069). And finally, lower average daily energy expenditure was associated with worse visual memory (p = .027) and psychomotor speed (p = .017).
Table 2.
Sample characteristics (N=73)
| Mean ± SD or n (%) | |
|---|---|
| Age (years) | 63.71 ± 5.34 |
| Race | |
| White | 65 (89.0) |
| Black | 5 (6.9) |
| Multi-racial | 3 (4.2) |
| Education (years) | 15.47 ± 2.48 |
| Stage of breast cancer (n=72) | |
| DCIS | 15 (20.8) |
| Stage I | 47 (64.4) |
| Stage IIa | 9 (12.3) |
| Stage IIb | 2 (2.7) |
| Body mass index (kg/m2) | 31.05 ± 6.61 |
| Beck Depression Index II (Total Score) | 5.47 ± 5.14 |
| PROMIS Emotional Distress Anxiety Short Form (Total Raw Score) | 12.24 ± 5.31 |
| PROMIS Fatigue Short Form (Total Raw Score) | 18.24 ± 7.17 |
| Brief Pain Inventory (Mean Severity) | 1.62 ± 1.78 |
| Fitness/Physical Activity | |
| Total hours of armband wear time | 158.66 ± 34.79 |
| Peak VO2 maximum value | 1.38 ± 0.29 |
| Average steps/day | 5,345.75 ± 2,614.38 |
| Average hours light physical activity/day (1.5 – 3.0 METS) | 3.74 ± 1.46 |
| Average hours moderate physical activity/day (3.0 – 6.0 METS) | 0.77 ± 0.57 |
| Average hours vigorous physical activity/day (6.0 – 9.0 METS) | 0.01 ± 0.04 |
| Average hours moderate/vigorous physical activity (3.0 – 9.0 METS) | 0.79 ± 0.57 |
| Average daily energy expenditure (kcals > 3.0 METS) | 228.19 ± 163.23 |
| Cognitive Function Composite/Domain Z-scores* | |
| Verbal Memory | −0.20 ± 0.68 |
| Mental Flexibility | −0.17 ± 0.82 |
| Psychomotor Speed | 0.01 ± 0.71 |
| Attention | −0.09 ± 0.81 |
| Visual Memory | 0.11 ± 0.86 |
| Executive Function | −0.04 ± 0.59 |
| Working Memory | 0.06 ± 0.75 |
| Concentration | −0.10 ± 0.65 |
Z-scores relative to normative data from women without breast cancer matched on age and education to breast cancer cohorts
SD=standard deviation
Discussion
Our results suggest that women with breast cancer who are physically inactive or with lower fitness levels are more likely to have worse cognitive function, specifically in the attention, concentration, psychomotor speed, verbal and visual memory and working memory domains. Mounting evidence suggests that women with breast cancer engage in less physical activity and more sedentary behavior compared to women without the disease [34]. In fact, women with breast cancer reduce their physical activity after diagnosis [35]. In addition, compared to women without the disease, women with breast cancer have low cardiorespiratory fitness that declines after adjuvant therapy[36] and persists up to seven years after the completion of therapy [37]. We did not observe that vigorous intensity physical activity was associated with cognitive function. However, few women in our study engaged in vigorous levels of physical activity and those who did, did so for very brief periods of time (range 1.2 to 30 minutes). When we combined moderate and vigorous intensity physical activity, the results were quite similar.
Higher levels of physical activity and fitness have been associated with better cognitive function, particularly executive function, memory and psychomotor speed, in community-dwelling older adults and in adults with chronic disease [38, 39]. However, few studies have examined the relationship between these factors and cognitive function in women with breast cancer, particularly in postmenopausal women [40]. In a cross-sectional study, Marinac (2015) explored the relationship between physical activity, measured with the ActiGraph GT3X+accelerometer (ActiGraph, Pensecola, FL) and objectively measured cognitive function in 136 women with early stage breast cancer within five years of diagnosis and found improved processing speed (r = 0.20; p = .02) with moderate intensity physical activity [29]. Among the cognitive domains that we explored, psychomotor speed was also associated with moderate and moderate/vigorous-intensity physical activity as well as the average number of steps per day and average daily energy expenditure greater than 3.0 kcals. In addition, we found significant associations between greater physical activity and better attention and visual memory. The bases for our findings of relationships between physical activity and more cognitive domains may in part be due to measurement differences between the studies. Marinac and colleagues used a different cognitive battery that assessed processing speed, executive function and verbal and nonverbal memory, whereas, our battery measured a broader number of cognitive domains. Approaches to the assessment of physical activity also differed, with Marinac using a hip-worn device versus our use of an armband. Finally, the assessments in our study were limited to a narrow timeframe, after a breast cancer diagnosis and prior to the initiation of systemic adjuvant therapy, whereas women in the Marinac study were assessed up to five years post-breast cancer diagnosis ( years).
We also compared the magnitude of the associations observed in our study to the results reported by Marinac. We observed small to moderate effects (r = .070 to .330) for the relationship between physical activity and cognitive function, with the strongest effects for the relationships between physical activity and psychomotor speed, visual memory and visual working memory. Marinac and colleagues (2015) found small associations for processing speed (r = 0.20, p=.02). Differences in in cognitive measures and in samples may help to explain these dissimilarities in magnitude of associations. The sample in Marinac’s study had a lower mean BMI (28.7 vs. 31.05) and while the average age of their sample ( years) was similar to ours ( years), it is not clear that their sample was limited to postmenopausal women. In addition, their assessments occurred an average of 2.1 years post-breast cancer diagnosis, after women completed chemotherapy and while many were taking endocrine therapy. Our assessments occurred within months of diagnosis and before women began their adjuvant therapy. Moreover, Marinac controlled for accelerometer wear-time, sedentary time and primary language spoken in their correlational analyses, we controlled for age and total armband wear time [29].
Finally, few studies have examined the relationship between cardiorespiratory fitness and cognitive function in women with breast cancer. In a cross-sectional study, Crowgey (2014) assessed cardiorespiratory fitness with a cardiopulmonary exercise test (12-lead electrocardiography, peak oxygen consumption, VO2peak) and cognitive function with a computerized battery of objective measures in 37 women with women with early stage breast cancer. They found weak, non-significant correlations between cardiorespiratory fitness and cognitive function with the strongest effects for visual memory (r = 0.20) [41], whereas we saw small effects for the relationship between cardiorespiratory fitness and cognitive function (r = .074 to .330). In addition to differences in the approach to the assessment of cognitive function between our studies, the sample in Crowgey et al was younger (52 ± 12 years), with a lower BMI (27 ± 5) and an average of 21.7 months post-diagnosis. Moreover the average VO2peak in their study was 23.5 ± 6.3, higher than what we observed in our study, 17.08 ± 3.62 [41].
Evidence suggests that postmenopausal women may be more vulnerable to decline in cognitive function with accelerated aging with breast cancer and cancer therapy [8, 9]. This acceleration may be compounded by being physically inactive and obese, a risk factor for breast cancer in postmenopausal women [42]. It must be noted that our assessments occurred soon after diagnosis for breast cancer and prior to the initiation of systemic adjuvant therapy. Additional research is needed to confirm the relationships we observed and to determine whether these relationships persist during and after the conclusion of adjuvant therapy. Studies with age-matched controls are also needed to test the accelerated aging hypothesis and to examine the mechanisms underlying the potential acceleration of aging and cognitive decline in women with breast cancer. These studies will provide essential information as the basis of interventions to mitigate accelerated aging and improve cognitive function in women with the disease.
Our study is limited by its cross-sectional design. However, our results and the work of others suggest that longitudinal studies and randomized controlled trials (RCT) examining the influence of physical activity on cognitive function are needed to confirm these results in women with breast cancer. Results of two randomized controlled trials indicated improved Trail Making Test performance with resistance exercise versus control [43–45]. In another 24-week RCT in women with early stage breast cancer, 3 months to 3 years post-adjuvant therapy, women who engaged in aerobic exercise (n=10) showed improved Trail Making Test A performance compared to controls (n=9) [46]. Hartman (2018) also found improved processing speed with 12 weeks of aerobic exercise in women with BC (n=43), compared to controls (n=44). No other group differences in subjective or objective cognitive function were found, perhaps due to the short intervention duration [47]. Research is also needed to focus on these relationships in older women with breast cancer. In one of the few studies of exercise in older cancer survivors, Miki (2014) found improved “cognitive function” (Frontal Assessment Battery) with 4 weeks of aerobic exercise (n=38) vs. control (n=40) in elderly patients (≥ 65 y/o) with breast and prostate cancer [48].
Research is also needed to determine the mechanisms of the influence of physical activity and fitness on cognitive function in women with breast cancer. These factors may be associated with improved cognitive function by mitigating accelerated aging in individuals with cancer. Compelling evidence suggests that cancer and cancer therapy accelerate aging with increased inflammation, greater senescent cell load, persistent DNA damage with reduced repair capacity and changes in brain structure and function [8, 49]. An outcome of the acceleration of these aging processes may be a cognitive decline. Moreover, symptoms experienced by postmenopausal women as they age, including fatigue, depressive symptoms, anxiety, low sleep quality, and pain are commonly exacerbated with breast cancer and its therapy and may contribute to cognitive decline [50].
Physical activity may improve cognitive function by targeting similar pathways affected in accelerated aging. Physical activity may increase cerebral blood flow [51], may be associated with increased levels of neurotrophins such as brain-derived neurotrophic factor (BDNF) [52]. Moreover, in our neuroimaging studies in healthy older adults, we demonstrated that only modest amounts of exercise are capable of increasing hippocampal volume and modifying intrinsic brain connectivity in the prefrontal cortex and hippocampus that are affected in the course of normal aging [53, 54]. Physical activity may also reduce pro-inflammatory production and promote anti-inflammatory responses [55]. For example, in a RCT of 87 healthy older adults, moderate intensity aerobic activity was associated with significant reductions in IL-6, CRP, and IL-18 compared to a flexibility control group and these changes were correlated with reduced depressive symptoms [56].
Physical activity may also improve cognitive function by mitigating age-related symptoms. Two meta-analyses of RCTs in women with breast cancer found that exercise reduces fatigue, depressive symptoms, and anxiety [23, 57]. Recent reviews have also shown that physical activity is effective in reducing fatigue during and after cancer treatment [23, 40, 58, 59] and that it is more effective in reducing fatigue and depressive symptoms than pharmacological interventions in women with breast cancer [24, 60]. Physical activity has also been demonstrated to reduce pain and improve sleep quality in women with the disease [61, 62].
Limitations of this study include the cross-sectional design that precludes our ability to test causal relationships among the variables. Moreover, while we included objective measures of cognitive function, physical activity, and cardiorespiratory fitness, we acknowledge that our study may be limited by selection bias and imprecision. The sample size for this ongoing study is small and we are not able to test for selections bias. However, we did compare the characteristics of our sample to the broader population of postmenopausal women with breast cancer in the United States. The average age at diagnosis of breast cancer in the U.S. is 55 years.[42] Our participants were older ( years), however, we only enrolled women who were postmenopausal at diagnosis. In addition, our participants were obese and relatively sedentary. These characteristics match those of the average postmenopausal women with breast cancer in the U.S.[63, 64]. Finally, our sample was largely white and well-educated thus limiting the generalizability of the results to a less diverse population of postmenopausal women with breast cancer. Despite these limitations, our results suggest positive correlations between physical activity and cognitive function, the magnitude of these effects were small to moderate. As this is a cross-sectional study we cannot determine causal directions between physical activity and cognitive performance. An alternative interpretation of our results is that individuals with better cognitive performance elect to engage in greater amounts of regular physical activity; to date there are no data supporting this hypothesis. Future RCTs will be better positioned to test these alternative hypotheses. Moreover, clinical trials are needed to examine the influence of physical activity on cognitive function and the mechanisms underlying the benefits of physical activity for cognitive function in postmenopausal women with breast cancer. These trials should determine the influence of different physical activity modes and intensities and examine the mechanisms of the influence of physical activity on cognitive function in women with breast cancer.
Table 3.
Partial correlations between cardiorespiratory fitness, physical activity and cognitive function, controlling for age and total hours of actigraph wear time.
| Fitness or Physical Activity Measure | Statistic | Attention Composite | Concentration Composite | Verbal Memory Composite | Visual Memory Composite | Working Memory Composite | Executive Function Composite | Psychomotor Speed Composite | Mental Flexibility Composite |
|---|---|---|---|---|---|---|---|---|---|
| Peak VO2 Maximum Value | Correlation | .173 | .330 | .241 | .152 | .281 | .143 | .219 | .074 |
| p-value | .161 | .006 | .048 | .218 | .019 | .244 | .074 | .548 | |
| Average Steps/Day | Correlation | .262 | .105 | .008 | .159 | −.045 | −.137 | .309 | .144 |
| p-value | .032 | .395 | .949 | .200 | .715 | .266 | .011 | .240 | |
| Average Hours Light Physical Activity/Day (1.5 – 3.0 METS) | Correlation | −.035 | −.200 | .041 | .156 | .019 | −.160 | −.047 | .131 |
| p-value | .779 | .101 | .740 | .207 | .875 | .191 | .708 | .288 | |
| Average Hours Moderate Physical Activity/Day (3.0 – 6.0 METS) | Correlation | .103 | −.049 | .104 | .241 | .155 | −.006 | .218 | .166 |
| p-value | .409 | .689 | .400 | .049 | .205 | .961 | .076 | .175 | |
| Average Hours Vigorous Physical Activity/Day (6.0 – 9.0 METS) | Correlation | .119 | .134 | .088 | .102 | .029 | −.011 | .183 | −.050 |
| p-value | .336 | .275 | .473 | .413 | .810 | .931 | .138 | .686 | |
| Average Hours Moderate/Vigorous Activity/Day | Correlation | .108 | −.038 | .108 | .241 | .152 | −.006 | .223 | .156 |
| p-value | .385 | .761 | .383 | .049 | .214 | .959 | .069 | .203 | |
| Average Daily Energy Expenditure (Kcals > 3.0 METS) | Correlation | .174 | .013 | .154 | .270 | .193 | .010 | .292 | .150 |
| p-value | .159 | .915 | .211 | .027 | .112 | .938 | .017 | .223 | |
Acknowledgments
DECLARATIONS:
Funding (National Cancer Institute, R01 CA196762)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Catherine M. Bender, University of Pittsburgh School of Nursing, Pittsburgh, PA, USA.
Susan M. Sereika, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
Amanda L. Gentry, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
Jennie E. Duquette, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
Frances E. Casillo, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
Anna Marsland, University of Pittsburgh, School of Arts and Sciences, Pittsburgh, PA, USA.
Adam M. Brufsky, University of Pittsburgh, School of Medicine, Pittsburgh, PA, USA.
Steven Evans, University of Pittsburgh, School of Medicine, Pittsburgh, PA, USA.
Vikram C. Gorantla, University of Pittsburgh, School of Medicine, Pittsburgh, PA, USA.
Tara L. Grahovac, St. Clair Hospital, Department of Surgery, Pittsburgh, PA, USA.
Priscilla F. McAuliffe, University of Pittsburgh, School of Medicine, Pittsburgh, PA, USA.
Jennifer G. Steiman, University of Pittsburgh, School of Medicine, Pittsburgh, PA, USA.
Yehui Zhu, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
Kirk I. Erickson, University of Pittsburgh, School of Arts and Sciences, Pittsburgh, PA, USA.
References
- [1].Siegel RL, Miller KD, and Jemal A, “Cancer statistics, 2020,” CA Cancer J Clin, vol. 70, no. 1, pp. 7–30, January 2020, doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- [2].DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, and Jemal A, “Breast cancer statistics, 2015: Convergence of incidence rates between black and white women,” CA Cancer J Clin, vol. 66, no. 1, pp. 31–42, January-February 2016, doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- [3].Von Ah D et al. , “Cognitive function in breast cancer survivors compared to healthy age- and education-matched women,” Clin Neuropsychol, vol. 23, no. 4, pp. 661–74, May 2009, doi: 10.1080/13854040802541439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bender CM and Thelen BD, “Cancer and cognitive changes: the complexity of the problem,” (in eng), Semin Oncol Nurs, vol. 29, no. 4, pp. 232–7, November 2013, doi: 10.1016/j.soncn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- [5].Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, and Ryan CM, “The impact of cognitive function on medication management: three studies,” (in eng), Health Psychology, Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t vol. 29, no. 1, pp. 50–5, January 2010, doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Henderson TO, Ness KK, Cohen HJ, “Accelerated aging among cancer survivors: From pediatrics to geriatrics,” American Society of Clinical Oncology, pp. 423–430, 2014. [DOI] [PubMed] [Google Scholar]
- [7].Hurria A, Patel SK, Mortimer J, Luu T, Somlo G, Katheria V, Ramani R, Hansen K, Feng T, Chuang C, Geist CL, Silverman DHS, “The effect of aromatase inhibition on the cognitive function of older patients with breast cancer.,” Clinical Breast Cancer, vol. 14, no. 2, pp. 132–140, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hurria A, Jones L, and Muss HB, “Cancer Treatment as an Accelerated Aging Process: Assessment, Biomarkers, and Interventions,” Am Soc Clin Oncol Educ Book, vol. 35, pp. e516–22, 2016, doi: 10.14694/EDBK_156160. [DOI] [PubMed] [Google Scholar]
- [9].Mandelblatt JS et al. , “Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance),” Cancer, July 22 2016, doi: 10.1002/cncr.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bender C et al. , “Patterns of change in cognitive function with anastrozole therapy,” Cancer, vol. 121, no. 15, pp. 2627–2636, 2015, doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahles TA et al. , “Cognitive function in breast cancer patients prior to adjuvent treatment,” Breast Cancer Research and Treatment, vol. 110, no. 1, pp. 143–152, 2008, doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, and Meyers M, “‘Chemobrain’ in breast carcinoma? A prologue,” Cancer, vol. 101, no. 3, pp. 466–475, 2004, doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- [13].Barnes DE and Yaffe K, “The projected effect of risk factor reduction on Alzheimer’s disease prevalence,” Lancet neurology, Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review vol. 10, no. 9, pp. 819–28, September 2011, doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sofi F et al. , “Physical activity and risk of cognitive decline: a meta-analysis of prospective studies,” Journal of internal medicine, Meta-Analysis Review vol. 269, no. 1, pp. 107–17, January 2011, doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- [15].Brown JC, Winters-Stone K, Lee A, and Schmitz KH, “Cancer, physical activity, and exercise,” Compr Physiol, vol. 2, no. 4, pp. 2775–809, October 2012, doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gentry AL, Erickson KI, Sereika SM, Casillo FE, Crisafio ME, Donohue PT, Grove GA, Marsland AL, Watt JC, Bender CM, “Protocol for Exercise Program in Cancer and Cognition (EPICC): A randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy,” Contemporary Clinical Trials, vol. 67, pp. 109–115, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bender CM, Merriman JD, Sereika SM, Gentry AL, Casillo F, Koleck TA, Rosenzweig MQ, Brufsky AM, McAuliffe P, Zhu Y, Conley YP, “Trajectories of Cognitive Function and Associated Phenotypic and Genotypic Factors in Breast Cancer,” Oncology Nursing Forum, vol. 45, no. 3, pp. 308–326, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bender CM et al. , “Patterns of change in cognitive function with anastrozole therapy,” Cancer, vol. 121, no. 15, pp. 2627–36, August 1 2015, doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robbins TW, James M, Owen A, Sahakian BJ, McInnes L, and Rabbitt PM, “Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers,” Dementia, vol. 5, pp. 266–281, 1994. [DOI] [PubMed] [Google Scholar]
- [20].Bender C, Sereika S, Berga SM., Vogel V, Brufsky AM, Ryan CM, “Cognitive impairment associated with adjuvant therapy in women with breast cancer.,” Psycho-Oncology, vol. 15, pp. 422–430., 2006. [DOI] [PubMed] [Google Scholar]
- [21].Bender CM, Sereika SM., Brufsky AM, Vogel VG, Berga SL, Rastogi P, Cohen SM, Casillo FE, Ryan CM, “Memory impairments with adjuvant anastrozole versus tamoxifen in women with early stage breast cancer.,” Menopause, vol. 14, no. 6, pp. 995–998, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lavallee JF, Abdin S, Faulkner J, and Husted M, “Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: A qualitative metasynthesis,” Psychooncology, vol. 28, no. 3, pp. 468–476, March 2019, doi: 10.1002/pon.4980. [DOI] [PubMed] [Google Scholar]
- [23].Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, and Hayes SC, “A Systematic Review and Meta-Analysis of the Safety, Feasibility, and Effect of Exercise in Women With Stage II+ Breast Cancer,” Arch Phys Med Rehabil, vol. 99, no. 12, pp. 2621–2636, December 2018, doi: 10.1016/j.apmr.2018.03.026. [DOI] [PubMed] [Google Scholar]
- [24].Wirtz P and Baumann FT, “Physical Activity, Exercise and Breast Cancer - What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review,” Breast Care (Basel), vol. 13, no. 2, pp. 93–101, April 2018, doi: 10.1159/000488717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cleeland CS, “Pain assessment in cancer,” in Effect of Cancer on Quality of Life Osoba D Ed. Boca Raton: CRC Press, Inc., 1991, pp. 293–305. [Google Scholar]
- [26].Cook KF et al. , “PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions,” J Clin Epidemiol, vol. 73, pp. 89–102, May 2016, doi: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beck AT, Steer RA, and Brown GK, Beck Depression Inventory-II. San Antonio: The Psychological Corporation, 1996. [Google Scholar]
- [28].Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, and Cella D, “Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger.,” Assessment, vol. 18, no. 3, pp. 263–83, 2011, doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marinac CR, Godbole S, Kerr J, Natarajan L, Patterson RE, and Hartman SJ, “Objectively measured physical activity and cognitive functioning in breast cancer survivors,” J Cancer Surviv, vol. 9, no. 2, pp. 230–8, June 2015, doi: 10.1007/s11764-014-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu Z and Lippa CF, “Association of Metabolic Syndrome and Inflammation with Cognitive Decline in Adults Aged 60 Years and Older: Findings from a National Health Survey in the United States,” Neurosci J, vol. 2013, p. 846027, 2013, doi: 10.1155/2013/846027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Borg GA, “Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise.,” Medicine and Science in Sports and Exercise, vol. 14, pp. 377–318, 1982. [PubMed] [Google Scholar]
- [32].Liden CB et al. , “Accuracy and reliability of the Sensewear™ armband as an energy expenditure assessment device,” BodyMedia Inc White Papers, vol. 12, pp. 1–15, 2002. [Google Scholar]
- [33].Bhammar, BJ. DS; Tucker WJ; Baez JC; Gaesser GA, “BActiheart and Actigraph in Adults Physical Activity and Energy Expenditure Measurements Using Sensewear Armband.,” in Meeting of the American College of Sports Medicine., Indianapolis, IN, May 2013 2013. [Google Scholar]
- [34].Thraen-Borowski KM, Trentham-Dietz A, Edwards DF, Koltyn KF, and Colbert LH, “Dose-response relationships between physical activity, social participation, and health-related quality of life in colorectal cancer survivors,” J Cancer Surviv, vol. 7, no. 3, pp. 369–78, September 2013, doi: 10.1007/s11764-013-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Irwin ML et al. , “Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study,” Cancer, vol. 97, no. 7, pp. 1756–57, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lakoski SG et al. , “The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study,” Breast cancer research and treatment, vol. 138, no. 3, pp. 909–16, April 2013, doi: 10.1007/s10549-013-2478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peel AB, Thomas SM, Dittus K, Jones LW, and Lakoski SG, “Cardiorespiratory fitness in breast cancer patients: a call for normative values,” J Am Heart Assoc, vol. 3, no. 1, p. e000432, January 13 2014, doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cai H, Li G, Hua S, Liu Y, and Chen L, “Effect of exercise on cognitive function in chronic disease patients: a meta-analysis and systematic review of randomized controlled trials,” Clin Interv Aging, vol. 12, pp. 773–783, 2017, doi: 10.2147/CIA.S135700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Colcombe S and Kramer AF, “Fitness effects on the cognitive function of older adults: A meta-analytic study,” (in English), Psychological Science, vol. 14, no. 2, pp. 125–130, March 2003, doi: Doi 10.1111/1467-9280.T01-1-01430. [DOI] [PubMed] [Google Scholar]
- [40].Myers JS, Erickson KI, Sereika SM, and Bender CM, “Exercise as an Intervention to Mitigate Decreased Cognitive Function From Cancer and Cancer Treatment: An Integrative Review,” Cancer Nurs, vol. 41, no. 4, pp. 327–343, Jul/Aug 2018, doi: 10.1097/NCC.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Crowgey T et al. , “Relationship between exercise behavior, cardiorespiratory fitness, and cognitive function in early breast cancer patients treated with doxorubicin-containing chemotherapy: a pilot study,” Applied Physiology, Nutrition, and Metabolism, vol. 39, no. 6, pp. 724–729, 2014, doi: 10.1139/apnm-2013-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].A. C. Society, Breast Cancer Facts & Figures 2017–2018. Atlanta: American Cancer Society, Inc., 2017. [Google Scholar]
- [43].Furmaniak AC, Menig M, and Markes MH, “Exercise for women receiving adjuvant therapy for breast cancer (Review),” Cochrane Database of Systematic Reviews, no. 9, 2016, doi: 10.1002/14651858.CD005001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, and Steindorf K, “Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial,” Int J Cancer, vol. 137, no. 2, pp. 471–80, July 15 2015, doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- [45].Steindorf K et al. , “Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life,” Ann Oncol, vol. 25, no. 11, pp. 2237–43, November 2014, doi: 10.1093/annonc/mdu374. [DOI] [PubMed] [Google Scholar]
- [46].Campbell K, Kam JWY, Neil-Sztramko SE, Liu-Ambrose T, Handy TC, Lim HJ, Hayden S, Hsu L, Kirkham AA, Gotay CC, McKenzie DC, Boyd LA., “Effect of aerobic exercise on cancer-associated cognitive impairment: a proof-of-concept RCT.,” Psychooncology, vol. 27, pp. 53–60, 2018. [DOI] [PubMed] [Google Scholar]
- [47].Hartman SJ et al. , “Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study.,” Cancer Cytopathol, vol. 124, no. 1, pp. 192–202, 2018, doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miki E, Kataoka T, and Okamura H, “Feasibility and efficacy of speed-feedback therapy with a bicycle ergometer on cognitive function in elderly cancer patients in Japan,” Psychooncology, vol. 23, no. 8, pp. 906–13, August 2014, doi: 10.1002/pon.3501. [DOI] [PubMed] [Google Scholar]
- [49].Hurria A et al. , “The effect of aromatase inhibition on the cognitive function of older patients with breast cancer,” Clin Breast Cancer, vol. 14, no. 2, pp. 132–40, April 2014, doi: 10.1016/j.clbc.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tometich DB et al. , “Pretreatment Psychoneurological Symptoms and Their Association With Longitudinal Cognitive Function and Quality of Life in Older Breast Cancer Survivors,” J Pain Symptom Manage, vol. 57, no. 3, pp. 596–606, March 2019, doi: 10.1016/j.jpainsymman.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Steventon JJ, Foster C, Furby H, Helme D, Wise RG, Murphy K, “Hippocampal Blood Flow Is Increased After 20 min of Moderate-Intensity Exercise,” Cereb Cortex, vol. 30, no. 2, pp. 525–533, 2020, doi: 10.1093/cercor/bhz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Erickson KI, Miller DL, and Roecklein KA, “The aging hippocampus: Interactions between exercise, depression, and BDNF,” The Neuroscientist, vol. 18, no. 1, pp. 82–97, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Erickson KI, “Therapeutic effects of exercise on cognitive function,” (in eng), J Am Geriatr Soc, Editorial vol. 61, no. 11, pp. 2038–9, November 2013, doi: 10.1111/jgs.12529. [DOI] [PubMed] [Google Scholar]
- [54].Erickson KI, Gildengers AG, and Butters MA, “Physical activity and brain plasticity in late adulthood,” (in eng), Dialogues Clin Neurosci, Research Support, N.I.H., Extramural Review vol. 15, no. 1, pp. 99–108, March 2013. [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/23576893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cerqueira E, Marinho DA, Neiva HP, and Lourenco O, “Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review,” Frontiers in physiology, vol. 10, p. 1550, 2019, doi: 10.3389/fphys.2019.01550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kohut ML MD, Russell DW, et al. , “erobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults.,” Brain, Behavior and Immunity, vol. 20, no. 3, pp. 201–209, 2006. [DOI] [PubMed] [Google Scholar]
- [57].Fuller JT, Hartland MC, Maloney LT, and Davison K, “Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials,” Br J Sports Med, vol. 52, no. 20, p. 1311, October 2018, doi: 10.1136/bjsports-2017-098285. [DOI] [PubMed] [Google Scholar]
- [58].Hilfiker R et al. , “Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis,” Br J Sports Med, vol. 52, no. 10, pp. 651–658, May 2018, doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Juvet LK et al. , “The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis,” Breast, vol. 33, pp. 166–177, June 2017, doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- [60].Mustian KM et al. , “Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis,” JAMA Oncol, vol. 3, no. 7, pp. 961–968, July 1 2017, doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fang YY, Hung CT, Chan JC, Huang SM, and Lee YH, “Meta-analysis: Exercise intervention for sleep problems in cancer patients,” Eur J Cancer Care (Engl), vol. 28, no. 5, p. e13131, September 2019, doi: 10.1111/ecc.13131. [DOI] [PubMed] [Google Scholar]
- [62].Irwin ML et al. , “Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors,” J Clin Oncol, vol. 33, no. 10, pp. 1104–11, April 1 2015, doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hildebrand JS GS, Campbell PT, Gaudet MM, Patel AV, “Recreational physical activity and leisure-time sitting in relation to postmenopausal breast cancer risk.,” Cancer Epidemiol Biomarkers Prev., vol. 22, pp. 1906–1912., 2013. [DOI] [PubMed] [Google Scholar]
- [64].La Vecchia C GS, Hortobagyi GN, Chabner B, “Overweight, obesity, diabetes, and risk of breast cancer: interlocking pieces of the puzzle.,” Oncologist, vol. 16, no. 726–729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lafayette clinical repeatable neuropsychological test battery. Sagamore: Lafayette Clinical Instrument Company, 1989. [Google Scholar]
- [66].Rey A, “L’examen psychologique dans les cas d’encephalopathie traumatique,” Archives de Psychologie, vol. 122, pp. 382–340, 1964. [Google Scholar]
- [67].Cockburn J and Smith PT, “Correlates of everyday memory among residents of Part III homes,” British Journal of Clinical Psychology, vol. 32, no. Pt 1, pp. 75–7, 1993, doi: 10.1111/j.2044-8260.1993.tb01029.x. [DOI] [PubMed] [Google Scholar]
- [68].Osterrieth PA, “Test of copying a complex figure; contribution to the study of perception and memory,” Archives de Psychologie, vol. 30, pp. 206–356, 1944. [Google Scholar]
- [69].Klove H, “Clinical neuropsychology.,” in The Medical Clinics of North America, Forster FM Ed. New York: Saunders, 1963. [PubMed] [Google Scholar]
- [70].Wechsler D, Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: PsychCorp, 2008. [Google Scholar]
- [71].Delis DC, Kaplan E, and Kramer JH, Delis-Kaplan (D-KEFS) Executive Function System, Examiners Manual. San Antonio: The Psychological Corporation, 2001. [Google Scholar]