Abstract
PURPOSE:
To detect keratoconus using optical coherence tomography (OCT) corneal map parameters and patterns.
SETTING:
Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, USA
DESIGN:
Cross-sectional observational study.
METHODS:
A spectral-domain OCT was used to acquire corneal and epithelial thickness maps in normal, manifest keratoconic, subclinical keratoconic, and forme fruste keratoconic (FFK) eyes. A two-step decision tree was designed. An eye will be classified as keratoconus if both decision tree conditions are met: First, at least one of the four quantitative corneal thickness (minimum, minimum-maximum, superonasal-inferotemporal) and epithelial thickness (standard deviation) map parameters exceed cutoff values. Second, presence of both concentric thinning pattern on the epithelial thickness map and coincident thinning patterns on corneal and epithelial thickness maps by visual inspection.
RESULTS:
The study was compromised of 54 eyes from 29 normal participants, 91 manifest keratoconic eyes from 65 patients, 12 subclinical keratoconic eyes from 11 patients, and 19 FFK eyes from 19 patients. The decision tree correctly classified all normal eyes (100% specificity), and had good sensitivities for detecting manifest keratoconus (97.8%), subclinical keratoconus (100.0%), and FFK (73.7%).
CONCLUSIONS:
The two-step decision tree provided a useful tool to detect keratoconus including cases at early disease stages (subclinical keratoconus and FFK). OCT corneal and epithelial thickness map parameters and patterns can be used in conjunction with topography to improve keratoconus screening.
INTRODUCTION
Keratoconus is a corneal dystrophy characterized by progressive stromal thinning and localized protrusion, resulting in refractive aberrations and decreased quality of vision.1, 2 Unrecognized early-stage keratoconus, including subclinical keratoconus and forme fruste keratoconus (FFK), is considered the primary risk factor for the development of iatrogenic corneal ectasia which is a rare but serious complication after laser refractive surgeries such as laser-assisted in situ keratomileusis, (LASIK), photorefractive keratectomy (PRK) and small-incision lenticule extraction (SMILE).3, 4
Corneal topography is commonly utilized in the documentation of, and assistance in the clinical diagnosis and classification of keratoconus.5 However, post-LASIK corneal ectasia could still occur even when the preoperative topography map appeared normal.6 It tends to happen more in younger patients, who might have early-stage keratoconus that has not yet manifested on corneal topography.3 One limitation of anterior topography is that the corneal epithelium can thin over the location of corneal steepening, thus masking early ectasia.7–9 This compensatory epithelial thickness modulation hampers the topographic diagnosis of keratoconus, but is itself an important diagnostic clue that could be detected by ultrahigh-frequency ultrasound and optical coherence tomography (OCT).7, 10–14
Fourier-domain OCT is a non-contact high-resolution imaging technique that is now widely available for corneal imaging. Automated software has been developed to precisely delineate the corneal interfaces and map both pachymetric and epithelial thicknesses.11, 15 Several corneal thickness-based diagnostic parameters are now available on commercial OCT systems. These parameters demonstrated good accuracy for identifying manifest keratoconus, but their success in detecting FFK was limited.16, 17
We have observed that even in the early stages, keratoconus could be recognized by their characteristic patterns on OCT pachymetric and epithelial thickness maps as focal thinning at the same or nearby locations.14 We hypothesize that the recognition of this pattern could enhance diagnostic accuracy beyond that afforded by simple quantitative parameters. In this study, we developed a two-step decision tree method that combines formal criteria using OCT pachymetric and corneal epithelial thickness parameters and map patterns. This new diagnostic approach was tested in a clinical study that included keratoconus at all stages of severity.
MATERIALS AND METHODS
Subjects
Participants in this cross-sectional observational study were recruited at the Casey Eye Institute at Oregon Health and Science University (OHSU), Portland, Oregon. The study followed the tenets of the Declaration of Helsinki and was in accordance with the U.S. Health Insurance Portability and Accountability Act of 1996. The institutional review board of OHSU approved the study protocol. All participants were at least 18 years old and provided written informed consent.
A clinical diagnosis of keratoconus was established using a combination of corrected distance visual acuity (CDVA), slit-lamp physical findings, topographic patterns, and the quantitative topography KISA% index.18 Each keratoconic eye was assigned to one of the three keratoconic subgroups according to following classification scheme:1, 19, 20
Manifest keratoconus: Slit-lamp findings associated with keratoconus (Vogt’s striae, Fleischer’s ring, Munson’s sign, iron ring, Rizzuti’s sign and apparent focal corneal bulging and thinning) or CDVA worse than 20/20; topography characteristic of keratoconus (asymmetric bowtie with a skewed radial axis, central or inferior steep zone, claw-shape); and KISA% > 100%.
Subclinical keratoconus: CDVA equal to or better than 20/20; no slit-lamp findings of keratoconus; topography characteristic of keratoconus or pellucid marginal degeneration; and KISA% > 100%.
FFK: The better eye of asymmetric keratoconus patients, CDVA equal to or better than 20/20; no slit-lamp findings of keratoconus; and KISA% < 100%.
Age-matched normal participants were recruited from volunteers and patients seeking refractive surgery consultation. All normal eyes had CDVA equal to or better than 20/20; no signs of keratoconus on slit-lamp examination; regular axial power map topography pattern (round, oval, symmetric bow tie, etc); KISA% < 100%; no ocular pathology other than myopia or hyperopia.
Exclusion criteria were previous corneal surgeries, recent contact lens usage (soft contact lens within 1 week or rigid gas permeable lens within 3 weeks), inability to give informed consent, or inability to maintain stable fixation for imaging.
Severe keratoconus with corneal scarring has unpredictable corneal and epithelial thickness patterns and does not pose challenge for clinical diagnosis. Therefore, these cases were excluded from the data analysis.
Optical Coherence Tomography
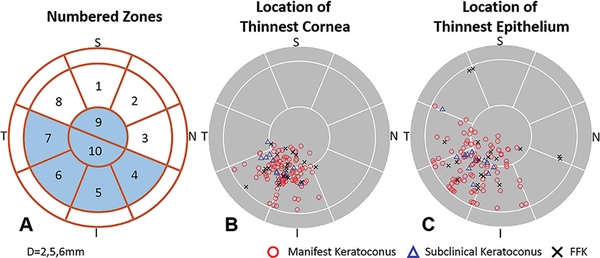
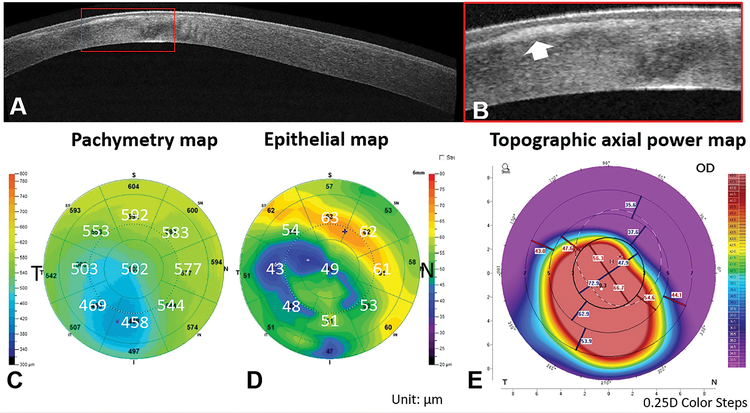
A spectral-domain OCT system (Avanti, Optovue, Inc. Fermont, CA, USA) with a corneal adaptor lens was used to acquire pachymetry (corneal thickness) and epithelial thickness maps. It has a working wavelength of 840 nm and operates at a scan speed of 70,000 axial scans per second. A “Pachymetry+Cpwr” scan pattern (6.0 mm scan diameter, 8 radials, 1024 axial scans for each radial) centered on the pupil was used to map the cornea. The subject’s head was stabilized with a chin-forehead rest. An internal fixation target facilitated gaze stabilization during scan acquisition. Each eye was scanned at least 2 times during a single visit to ensure the quality of OCT images. OCT thickness maps and reports were generated using Avanti OCT software (ReVue version 2018.0.04, Optovue, Inc. Fremont, CA, USA). All corneal and epithelial boundaries were visually inspected and corrected for segmentation errors. OCT maps were divided into zones by octants and annular rings (2.0, 5.0, and 6.0mm diameters, Figure 1A). The Minimum thickness locations were marked on corneal and epithelial thickness maps using an asterisk (*). The color scale of the corneal thickness map was in 21-μm steps and the epithelial map was in 2.5-μm steps.
Figure 1.
Optical coherence tomography (OCT) map zones (A). Plots of minimum pachymetry map locations (B) and minimum epithelial thickness map locations (C). The circles overlaid on the maps had diameters of 2.0, 5.0 and 6.0mm (I=inferior; N=nasal; S=superior; T=temporal)
Four OCT pachymetric and epithelial parameters that best characterize keratoconic corneal thickness changes according to our previous studies11, 21, 22 were chosen to serve as quantitative indices in this study:
Pachymetric Minimum (Min): the minimum corneal thickness.
Pachymetric Minimum-Maximum (Min-Max): the minimum corneal thickness minus the maximum corneal thickness.
Pachymetric superonasal- inferotemporal (SN-IT): the average thickness of the superonasal octant minus that of the inferotemporal octant between the 2 and 5 mm diameter rings.
Epithelial standard deviation (Std Dev): the standard deviation of the epithelial thickness.
All parameters were calculated inside the 5 mm diameter zone. The decision tree OCT parameter cutoff values were determined using the 25th percentile (pachymetric Min and Min-Max) or the 75th percentile (pachymetric SN-IT and epithelial Std Dev) from measurements acquired in the normal group.
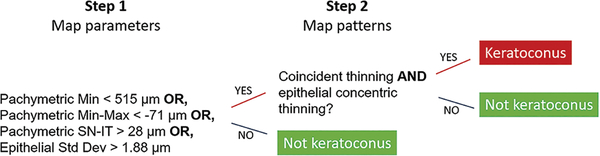
Two-step Decision Tree
A two-step decision tree was designed to identify the keratoconic cornea (Figure 2).
Figure 2.
A two-step decision tree to distinguish keratoconus from normal eyes.
Step 1 utilizes quantitative OCT pachymetric and epithelial thickness map parameters. If any of the four parameters listed in the previous section exceeds the cutoff, the eye is suspicious for keratoconus and proceeds to Step 2. If none of the four parameters exceeds the cutoff, then the eye is considered normal and does not require Step 2 examination.
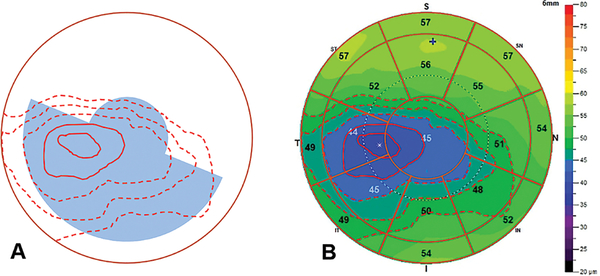
Step 2 requires a human grader to visually inspect the corneal and epithelial thickness maps and search for characteristic keratoconic map patterns of coincident thinning and concentric epithelial thinning. The coincident thinning pattern requires that the point of minimum pachymetry is located inside the analytic zones (inferior and temporal zones of 4–7 or central zones 9–10, Figure 1A), and the point of thinnest epithelium is in the same or adjacent zones relative to the minimum pachymetry. The epithelial concentric thinning pattern requires more than two color-scale step changes (>5 μm) with at least one complete ring around the thinnest point inside the analytic zones on the epithelial thickness map (Figure 3).
Figure 3.
Concentric thinning pattern (A) on the epithelial thickness map (B) of a keratoconic eye. This example has 6 color-step change and two complete rings (marked with solid red lines) around the thinnest epithelium inside the analytic zones (shaded in light blue on A).
Topography
Corneal topography was obtained using either Orbscan II (Bausch & Lomb Corp. Rochester, NY, USA) or Pentacam (Oculus Inc. Wetzlar, Germany). Central keratometry (central K, average of the steep and flat simulated keratometry [Sim-K] readings), inferior-superior dioptric asymmetry (I-S), Sim-K astigmatism (AST), and the skewed radial axis (SRAX) index were recorded for all eyes. The topography-based keratoconus percentage index (KISA%) was calculated using following formula as described in the literature.18, 20
Statistical Analysis
Repeated measurements of OCT parameters were averaged before calculating the population mean ± standard deviation (SD) of each corneal and epithelial thickness map parameter in keratoconic and normal groups. The D’Agostino-Pearson normality test was used to verify data normality. Two-sided t-tests were used to compare the arithmetic mean of each keratoconic group against the control group if data normality was confirmed. Otherwise, a non-parametric Mann-Whitney test was used to compare medians. If both eyes of a subject were involved in the study, a randomly selected eye was chosen for the t-test or Mann-Whitney test.
Receiver operating characteristic (ROC) curve analyses were performed to evaluate the diagnostic accuracy of the four quantitative OCT parameters. The sensitivity values of individual quantitative OCT parameters were determined from the spline-interpolated ROC curves at 99% specificity.
One best quality (strongest signal intensity and best pupil centration) OCT scan of each eye was chosen to evaluate for keratoconus using the decision tree method. The OCT parameters were exported and compared with the cutoff values. The OCT pachymetric and epithelial thickness map patterns were reviewed by a human grader who was masked from the clinical diagnosis information. The order of the eyes was randomized for grading. The decision tree method provided a binary output (keratoconus or not keratoconus). Sensitivity, specificity, and accuracy were calculated to evaluate its performance in keratoconus detection. The McNemar test was utilized to compare the sensitivity of individual OCT parameters with that of the decision tree in each of the three keratoconus groups.
In this study, descriptive and other statistical analyses were performed using Excel 2016 software (Microsoft Corp. Redmond, WA, USA) and MedCalc 19.0 software (MedCalc Software Corp. San Francisco, CA, USA). The significance level was set at a P value less than 0.05 for all tests.
RESULTS
Five severe keratoconic eyes with corneal scars were excluded from further analysis. Data from 54 eyes of 29 normal participants (19 men, 10 women), 91 manifest keratoconic eyes of 65 patients (51 men, 14 women), 12 subclinical keratoconic eyes of 11 patients (7 men, 4 women) and 19 FFK eyes of 19 patients (13 men, 6 women) were analyzed. No statistically significant difference in age was detected between normal and two keratoconic (manifest and subclinical) groups. The FFK group was younger than the normal control. The average age was 40.2 ± 13.5 (range 23 to 76) years for normal controls, 37.2 ± 14.0 (range 20 to 81) years for the manifest keratoconus group (p =0.32), 35.4 ± 11.9 (range 21 to 57) years for subclinical keratoconus subjects (p =0.29), and 32.5 ± 11.8 (range 21 to 57) years for FFK subjects (p =0.05).
Descriptive statistics of the OCT pachymetric (Min, Min-Max, SN-IT) and epithelial (Std Dev) map parameters are listed in Table 1. All four parameters measured in the keratoconic groups were significantly different from those of the normal controls (p < 0.001). The cutoffs for Step 1 of the decision tree were <515 μm (pachymetric Min), <−71 μm (pachymetric Min-Max), >28 μm (pachymetric SN-IT), and >1.7 μm (epithelial Std Dev) for the four individual OCT parameters.
Table 1.
Statistics of Optical Coherence Tomography Parameters
| Pachymetric Min (μm) | Pachymetric Min-Max (μm) | Pachymetric SN-IT (μm) | Epithelial Std Dev (μm) | |
|---|---|---|---|---|
| Normal | 531.6±24.9 | −57.1±11.2 | 20.4±9.1 | 1.5±0.6 |
| Manifest keratoconus | 438.5±45.0* | −126.4±49.5* | 71.8±27.8* | 6.0±2.3† |
| Subclinical keratoconus | 458.5±48.4* | −110.3±15.3* | 68.8±15.3* | 4.8±1.5† |
| FFK | 490.3±34.1* | −77.9±22.7* | 43.2±22.1* | 2.5±1.2† |
| Step-1 cutoff values | < 515 | < −71 | > 28 | > 1.7 |
2-tailed t-test p<0.0001
2-tailed Mann-Whitney test p<0.001, compared to normal group.
Values reported as average ± standard deviation
Abbreviations: FFK = forme fruste keratoconus; Min = minimum; Min-Max = minimum – maximum; SN-IT= superonasal – inferotemporal; Std Dev = standard deviation.
Case Reports
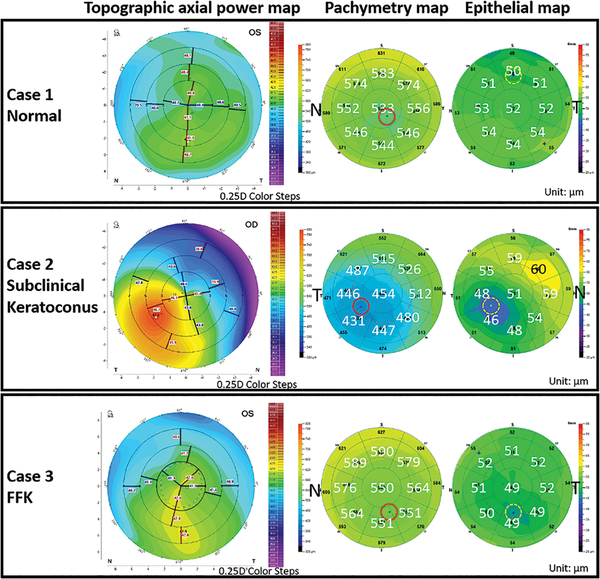
Case 1: Normal
The top row of Figure 4 shows the left eye of a normal 29-year-old male who had a CDVA of 20/15. The topographic map had a normal asymmetric bow-tie pattern with an I-S difference of 0.7 D. The SimKs were 40.9D (steep) and 40.3D (flat). The topographic KISA% value was 4.7%. One of the OCT quantitative parameters (pachymetric Min-Max= −78.3μm) exceeded the cutoff value. This eye appeared suspicious for keratoconus at decision tree Step 1, but was ruled out for keratoconus at Step 2 for three reasons. First, the thinnest epithelium was located superiorly. Second, the OCT epithelial thickness map did not show a concentric thinning pattern. Third, the thinnest corneal and epithelial locations were not in adjacent map zones.
Figure 4.
Topographic axial power maps and OCT pachymetry and epithelial thickness maps for normal, subclinical keratoconus, and forme fruste keratoconus (FFK) case examples.
In the normal eye (Case 1, top row), the steepest cornea was located inferiorly by topography, not located in the same region as the thinnest cornea (centrally) or thinnest epithelium (superiorly). In the subclinical keratoconic eye (Case 2, second row), the topography map showed inferotemporal corneal steepening. The thinnest cornea and epithelium were situated at nearby locations. There was a 6 color-step concentric thinning pattern on the epithelial thickness map. In the FFK eye (Case 3, bottom row), the topography map illustrated an asymmetric bowtie pattern with a skewed axis. The location of the thinnest point on the pachymetry and epithelial maps agreed with the location of inferior corneal steepening. There was a 3 color-step concentric thinning pattern on the epithelial thickness map. The color scale of the topographic maps was in 0.25-diopter steps. The color scale of the pachymetry map was in 21-μm steps and the epithelial map was in 2.5-μm steps. Red circles marked the minimum corneal thickness locations and yellow dashed circles marked the thinnest epithelium locations.
Case 2: Subclinical keratoconus
A 42-year-old female presented with subclinical keratoconus in her right eye (Figure 4 middle row). Her CDVA was 20/15. The topographic map had an asymmetric bowtie pattern with a skewed axis, SimKs were 43.4 D and 41.9 D, and the KISA% value was 276%. All four OCT quantitative variables exceeded the cutoff values (pachymetric Min = 423 μm, Min-Max = −124.5 μm, SN-IT = 95.1 μm, and epithelial Std Dev = 5.6 μm). Moreover, the epithelial thickness map had a concentric thinning pattern with 6 color-step change. The minimum corneal and epithelial thicknesses were both located in the inferotemporal octant. This eye was identified as keratoconus using the decision tree method.
Case 3: FFK
A 27-year-old female patient presented with FFK in her left eye (Figure 4, bottom row). Her CDVA was 20/20. The topographic map presented an asymmetric bow-tie pattern with inferior steepening and skewed axes. However, the topographic distortions did not meet the formal criteria for keratoconus, with a KISA% value of 16.1%. The SimKs were 46.0 D and 41.5 D. Two OCT parameters exceeded the cutoff values (pachymetric Min-Max = −74.9 μm, SN-IT = 40.1μm). The epithelial thickness map presented a concentric thinning pattern with 3 color-step change. Both the thinnest cornea and thinnest epithelium were found in the inferior octant. This eye was identified as keratoconus using the decision tree method. The right eye of the patient had manifest keratoconus (not shown in the figure).
Case 4: A severe keratoconic case excluded from the data analysis
A 31-year-old male presented with keratoconus in his right eye (Figure 5). His CDVA was 20/100. The SimKs were 60.8 D and 58.7 D. The OCT images showed dense corneal scars (Figure 5 A and B) due to subepithelial fibrosis. The epithelial thickness map was altered and did not follow the concentric thinning pattern characteristic to keratoconus. This case was excluded from statistical analysis.
Figure 5.
The OCT cross-sectional image (A-B), pachymetric (C), epithelial thickness (D), and topography (E) maps of a severe keratoconic eye (Case 4). The OCT image illustrated uneven corneal epithelial thickness due to subepithelial fibrosis (marked by red rectangle in A and magnified in B). The epithelial thickness map showed an irregular thinning pattern with multiple thinning centers (D).
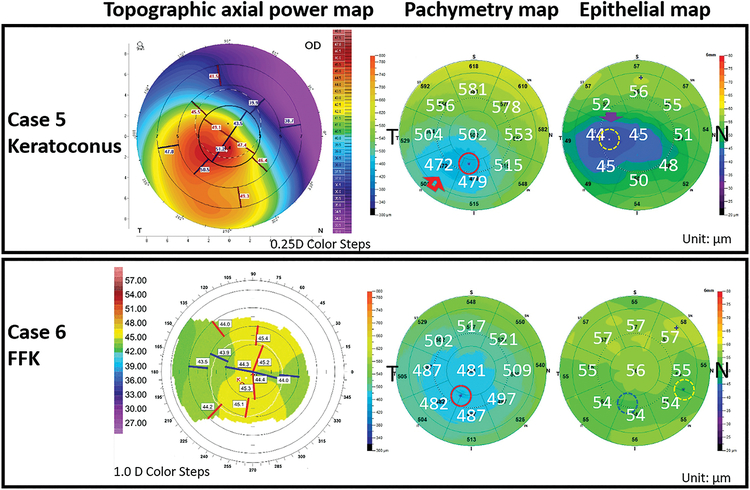
Case 5: A keratoconic eye misclassified by the decision tree method
A 52-year-old male presented with keratoconus in his right eye (Figure 6, top row). His CDVA was 20/25. The SimKs were 48.3 D and 47.2D and the KISA% value was 267.9%. All four OCT-based indices exceeded the cutoff values (pachymetric Min=459.9 μm, Min-Max=−145.4 μm, SN-IT=106.5 μm and epithelial Std Dev=5.2 μm), the eye was suspicious for keratoconus at Step 1. The epithelial thickness map did show a concentric thinning pattern around the thinnest epithelium, but the thinnest cornea and the thinnest epithelium locations were separated by the inferotemporal octant, located neither in the same nor adjacent zones. The decision tree misclassified this case as not keratoconus. The distance between the two thinnest points was relatively small (1.34 mm), but unfortunately straddled two sector boundary lines.
Figure 6.
Examples of misclassified keratoconic cases. Both eyes were suspicious for keratoconus according to decision tree step 1 output. But their thickness map patterns did not meet the criteria for keratoconus in step 2 of the decision tree. A keratoconic eye (Case 5, top row) was misclassified by the decision tree because the thinnest cornea and epithelium were separated by more than one map zone. Both pachymetric and epithelial thickness maps demonstrated a clear concentric thinning pattern (arrows). An FFK eye (Case 6, bottom row) was misclassified by the decision tree due to 2 sectors separating the thinnest cornea (red circle) and epithelium (yellow dotted circle). There was a second area of thin epithelium (blue dotted circle) that was co-located with the thinnest cornea.
Case 6: An FFK eye misclassified by the decision tree
A 30-year-old male presented with FFK in his right eye (Figure 6). His CDVA was 20/15, the Sim-Ks were 44.5 D and 43.5D. The KISA% value was 8.7%. Two pachymetric variables (Min=470.4 μm, SN-IT=31.4 μm) exceeded their cutoff values. However, the thinnest cornea and the thinnest epithelium locations were separated by 2 octants. There was an area of relative epithelial thinning (but not the thinnest) that coincided with the thinnest corneal thickness. Unfortunately, this did not meet the formal diagnostic criteria. The decision tree misclassified this case as normal.
The sensitivity of the diagnostic OCT parameters is listed in Table 2. Although the individual parameters had limited sensitivity, their OR-logic combination in the first step of the decision tree was 100% sensitive in detecting all 3 categories of keratoconus.
Table 2.
Sensitivity of Individual Optical Coherence Tomography Parameters in Keratoconus Detection.
| Pachymetric SN-IT | Pachymetric Min | Pachymetric Min-Max | Epithelial Std Dev | |
|---|---|---|---|---|
| Manifest keratoconus | 87.8%* | 92.2% | 91.1%* | 93.3% |
| Subclinical keratoconus | 100% | 75.0% | 100% | 100% |
| FFK | 57.9% | 47.4% | 26.3%* | 26.3%* |
McNemar test p<0.05, compared to the decision tree.
Abbreviations: FFK = forme fruste keratoconus; Min = minimum; Min-Max = minimum – maximum; SN-IT= superonasal-inferotemporal; Std Dev = standard deviation.
The results of map pattern recognition by a human grader are summarized in Table 3. Compared to normal, the keratoconic eyes had significantly more color step changes inside the analytic zones (inferior and temporal zones of 4–7 or central zones 9–10) on both corneal and epithelial thickness maps (p < 0.0001). Almost all corneas (96.3% of normal and 100% of keratoconic eyes) demonstrated a concentric thinning pattern on corneal thickness maps. In contrast, only a small portion of normal eyes (7.4%) showed concentric thinning patterns on the epithelial thickness maps. About a quarter of normal eyes (27.8%), most manifest keratoconic (97.8%), and all subclinical keratoconic eyes exhibited minimum pachymetric and epithelial thickness locations in the same or adjacent map zones. The combination of these two map patterns - coincident thinning and concentric epithelial thinning – captured a small percentage of normal eyes (5.6%) while identifying the vast majority of manifest and subclinical keratoconic eyes (98.1%). The combined map pattern criteria also showed good sensitivity in FFK detection (73.7%).
Table 3.
Characteristics of Pachymetric and Epithelial Thickness Map Patterns
| Pachymetric color steps | Epithelial color steps | Pachymetric Concentric Thinning #eyes (%) | Epithelial Concentric Thinning #eyes (%) | Coincident Thinning #eyes (%) | Coincident Thinning & Epithelial Concentric Thinning #eyes (%) | |
|---|---|---|---|---|---|---|
| Normal | 3.4±0.8 | 3.2±1.3 | 52 (96.3%) | 4 (7.4%) | 15 (27.8%) | 3 (5.6%) |
| Manifest keratoconus | 6.4±2.0* | 8.6±3.6* | 91 (100%) | 91 (100%) | 89 (97.8%) | 89 (97.8%) |
| Subclinical keratoconus | 6.3 ± 1.3* | 8.5±3.9* | 12 (100%) | 12 (100%) | 12 (100%) | 12 (100%) |
| FFK | 5.8±2.0* | 7.8±3.3* | 19 (100%) | 14 (73.7%) | 17 (89.5%) | 14 (73.7%) |
2-tailed t-test p<0.0001, compared to normal group.
Abbreviations: FFK = forme fruste keratoconus.
The stepwise results of the decision tree method are summarized in Table 4. Step 1 of the decision tree identified 100% cases in all three keratoconic groups. Step 2 of the decision tree method ruled out all suspicious but normal cases. Working together, the two steps of the decision tree provided accurate disease screening in 97.8% (89) of manifest keratoconus eyes, 100% (12) of subclinical keratoconus eyes, 73.7% (14) of FFK eyes, and 100% (54) of normal eyes. 2.2% (2) manifest keratoconus and 26.3% (5) FFK eyes were misclassified.
Table 4.
Stepwise Results of the Two-Step Decision Tree
| Step 1 # (%) of Eyes Suspicious for Keratoconus | Step 2 # (%) of Eyes Identified as Keratoconus | |
|---|---|---|
| Normal | 28(51.9%) | 0(0%) |
| Manifest keratoconus | 91(100%) | 89 (97.8%) |
| Subclinical keratoconus | 12(100%) | 12 (100%) |
| FFK | 19(100%) | 14 (73.7%) |
Abbreviations: FFK = forme fruste keratoconus.
Overall, the two-step decision tree demonstrated excellent diagnostic accuracy in keratoconus screening. It provided perfect specificity (100%), excellent sensitivity in manifest keratoconus (97.8%) and subclinical keratoconus (100.0%) detection, and good sensitivity in FFK detection (73.7%).
DISCUSSION
In this study, we developed a two-step decision tree method to detect keratoconus which combined criteria from quantitative OCT parameters and map pattern recognition. We also evaluated the diagnostic performance of the decision tree in a clinical study involving manifest keratoconus, subclinical keratoconus, and FFK.
Corneal thinning and compensatory epithelial thickness modulation around the location of corneal steepening are characteristics of keratoconus.1, 10, 11, 21, 22 Four individual OCT thickness map-based parameters were chosen to be included in the first step of the decision tree for several reasons. These 4 parameters work synergistically to detect the characteristic changes in keratoconic corneas: pachymetric Min and Min-Max capture corneal focal thinning; pachymetric SN-IT detect asymmetric thinning; and epithelial Std Dev measures the unevenness of the corneal epithelium. The numerical value of these parameters can be retrieved directly from the Avanti OCT corneal thickness map report. These parameters demonstrated the best diagnostic accuracy among all individual parameters currently available on OCT map reports.21, 22 The combination of the 4 individual parameters showed excellent sensitivity (100%) in Step 1 of the decision tree (Table 4). The sensitivity of the 4 parameters combined was higher than any of the individual parameters in keratoconus screening (Table 2).
Another unique feature of keratoconus is the coincident pachymetric and epithelial focal thinning.23, 24 This feature was incorporated in Step 2 of the decision tree method. Applying the coincident thinning criteria alone gave a high sensitivity (98.1%, Table 3) in manifest and subclinical keratoconus detection, however the specificity was low (72.2%). We found that epithelial concentric thinning is a highly specific (92.6% specificity) map pattern for keratoconus detection. Combining the coincident thinning and epithelial concentric thinning criteria together improved the accuracy of keratoconus detection and increased the robustness of the map pattern recognition.
Step 1 of the decision tree method was very sensitive (100% sensitivity) in detecting possible keratoconus related corneal and epithelial thickness changes. Step 2 of the decision tree ensured the specificity of the method. Working together, the two steps of the decision tree provided excellent disease screening that achieved 100% specificity and excellent sensitivity in manifest (97.8%) and subclinical (100%) keratoconus. We also showed that even FFK could be recognized by the decision tree method with good sensitivity (73.7%).
The Global Consensus on Keratoconus and Ectatic Diseases suggested that tomography is currently the best test to diagnose early keratoconus which analyzes the anterior and posterior cornea and produces a corneal thickness map.25 The Belin-Ambrosio Enhanced Ectasia Display (BAD) on Pentacam (Oculus GmbH, Wetzlar, Germany) utilizes both anterior and posterior elevation data and pachymetric data to screen for ectatic changes. Nine BAD-based tomographic parameters were combined into an overall “D” reading using regression analysis. A high sensitivity of 99% and a high specificity of 97.5% were reported for the “D” reading to identify keratoconic corneas that differ significantly from normal.26 Dr. Golan and colleagues used logistic regression to determine a best model combination of 9 out of 87 corneal metrics measured by a Dual Scheimpflug/Placido device (Galilei G4, Ziemer Ophthalmic Systems AG, Port, Switzerland). Among those 9 metrics included, 5 related to corneal pachymetry. The model yielded 90.3% sensitivity and 92.6% specificity in a study to distinguish unaffected eyes (similar to the FFK eyes in our study) in patients with asymmetric keratoconus (AKC) from normal eyes.27 Dr. Ambrosio’s group investigated artificial intelligence models to detect corneal ectasia using Pentacam keratometric, topometric, and tomographic indices. A random forest model provided the highest accuracy with sensitivity of 85.2% for the unaffected eye of AKC patients and 96.6% specificity.28 Luz and collaborators used a logistic regression model to combine Pentacam tomography measurements and Ocular Response Analyzer (Reichert Ophthalmic Instruments, Depew, USA) biomechanical variables and achieved a high sensitivity of 85.7% and specificity of 98.7% for distinguishing FFK.29 Dr. Randleman’s group used logistic regression analysis to evaluate Scheimpflug (Pentacam) and spectral-domain OCT (RTVue, Optovue, Inc., Fremont, USA) measurements for unaffected eyes of AKC patients detection.30 They reported that combined OCT corneal and epithelial thickness metrics (sensitivity 89%, specificity 89%) performed better than combined Scheimpflug metrics (sensitivity 83%, specificity 83%). A combination of variables from OCT and Scheimpflug technologies performed optimally (sensitivity 100%, specificity 100%) in their study population. The most significant machine-derived variables across the models included metrics agree with those used in our study such as pachymetry minimum and epithelial standard deviation. Other impactful variables in their combined models included anterior asymmetry and anterior curvature metrics from Scheimpflug imaging. Their result suggests that adding corneal shape-based topometric measurements to our decision tree method may further improve its diagnostic accuracy.
The primary limitation of our method is the reliability of the epithelial thickness map. In particular, one of the two false negative examples indicated that the concentric and coincident epithelial thinning criteria could be disrupted by other patterns of epithelial irregularity. Keratonconic eyes with dry eye or contact lens-induced epitheliopathy could fail to meet our decision tree criteria due to a multifocal pattern of epithelial thinning superimposed on the keratoconic concentric pattern. Thus, we recommend that in patients with epitheliopathy, more flexibility be used in the interpretation of the epithelial map and more reliance be placed on the corneal thickness map. The false positive diagnosis of keratoconus in patients with corneal epitheliopathy is also a theoretical concern, although it would be unlikely for epitheliopathy to mimic the coincident concentric thinning pattern characteristic of keratoconus. We did not see any such cases in the current study. Overall, we advise caution in applying our decision tree method to patients with significant corneal epitheliopathy. In these patients, it would be better to focus diagnostic consideration on the corneal thickness map and the anterior and posterior topography maps. Another limitation of our study is that the Step 1 cutoff values were determined using quartile measurements from the same group of normal eyes used for validation. Independent study is needed to further validate the specificity of the decision tree method.
There are several potential improvements for our method. First, the current version of Optovue OCT software did not provide a quantitative measurement of the distance between the minimum thickness locations for pachymetric and epithelial thickness maps. The current coincident thinning map pattern was defined using the same or adjacent map zones criteria, which is less precise than the direct distance measure. If a quantitative distance measure of coincident thinning was available, Case 5 could have been correctly classified. Second, the current Optovue OCT software does not provide the epithelial pattern standard deviation parameter, which we have previously found to outperform simple standard deviation in keratoconus diagnostic accuracy.11, 21 Third, recognition of the concentric epithelial thinning pattern currently relies on human visual inspection. Pattern recognition using artificial intelligence techniques could potentially be more reliable than humans.
In summary, the two-step decision tree method provided a useful tool to detect keratoconus, including FFK cases that would go undetected using standard topography-based indices. This study provides evidence that OCT corneal and epithelial thickness map parameters and patterns provide diagnostic information, in addition to topography, for characterizing corneal ectatic conditions. Therefore, these maps can be used in conjunction with topography to potentially improve diagnostic accuracy for keratoconus.
WHAT WAS KNOWN
Abnormal corneal thinning is a characteristic of keratoconus.
Corneal epithelial thickness changes around the corneal steepening location can mask early signs of keratoconus on anterior topography.
WHAT THIS PAPER ADDS
Coincident thinning patterns on corneal and epithelial thickness maps and concentric thinning pattern on the epithelial thickness map are unique patterns of keratoconus.
OCT corneal and epithelial thickness map parameters and patterns can be used in conjunction with topography to improve keratoconus screening.
Acknowledgments
Financial Supports: Supported by the National Institutes of Health, Bethesda, MD (R01EY028755, R01EY029023, T32EY023211, P30EY010572, Pavlatos, Huang, Li); a research grant and equipment support from Optovue, Inc., Fremont, CA (Huang, Li); unrestricted grants to Casey Eye Institute from Research to Prevent Blindness, Inc., New York, NY (Pavlatos, Chamberlain, Huang, Li); National Natural Science Foundation of China, Beijing, China (81900830, Yang). The sponsors did not participate in the data collection, data management, or data analysis in the present study.
Footnotes
Financial Disclosures: Oregon Health and Science University (OHSU) and Drs. Li and Huang have a significant financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998; 42: 297–319 [DOI] [PubMed] [Google Scholar]
- 2.Galvis V, Tello A, Barrera R, Nino CA. Inflammation in Keratoconus. Cornea 2015; 34: e22–23 [DOI] [PubMed] [Google Scholar]
- 3.Bohac M, Koncarevic M, Pasalic A, Biscevic A, Merlak M, Gabric N, Patel S. Incidence and Clinical Characteristics of Post LASIK Ectasia: A Review of over 30,000 LASIK Cases. Semin Ophthalmol 2018; 33: 869–877 [DOI] [PubMed] [Google Scholar]
- 4.Moshirfar M, Albarracin JC, Desautels JD, Birdsong OC, Linn SH, Hoopes PC Sr. Ectasia following small-incision lenticule extraction (SMILE): a review of the literature. Clin Ophthalmol 2017; 11: 1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belin MW, Ambrosio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol 2013; 61: 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosio R Jr., Dawson DG, Salomao M, Guerra FP, Caiado AL, Belin MW. Corneal ectasia after LASIK despite low preoperative risk: tomographic and biomechanical findings in the unoperated, stable, fellow eye. J Refract Surg 2010; 26: 906–911 [DOI] [PubMed] [Google Scholar]
- 7.Kanellopoulos AJ, Asimellis G. Anterior segment optical coherence tomography: assisted topographic corneal epithelial thickness distribution imaging of a keratoconus patient. Case Rep Ophthalmol 2013; 4: 74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman RH, Urs R, Roychoudhury A, Archer TJ, Gobbe M, Reinstein DZ. Epithelial remodeling as basis for machine-based identification of keratoconus. Invest Ophthalmol Vis Sci 2014; 55: 1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Stojanovic A, Wang X, Liang J, Hu D, Utheim TP. Epithelial Thickness Profile Change After Combined Topography-Guided Transepithelial Photorefractive Keratectomy and Corneal Cross-linking in Treatment of Keratoconus. J Refract Surg 2016; 32: 626–634 [DOI] [PubMed] [Google Scholar]
- 10.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg 2009; 25: 604–610 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology 2012; 119: 2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanellopoulos AJ, Asimellis G. OCT corneal epithelial topographic asymmetry as a sensitive diagnostic tool for early and advancing keratoconus. Clin Ophthalmol 2014; 8: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temstet C, Sandali O, Bouheraoua N, Hamiche T, Galan A, El Sanharawi M, Basli E, Laroche L, Borderie V. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract Refract Surg 2015; 41: 812–820 [DOI] [PubMed] [Google Scholar]
- 14.Schallhorn JM, Tang M, Li Y, Louie DJ, Chamberlain W, Huang D. Distinguishing between contact lens warpage and ectasia: Usefulness of optical coherence tomography epithelial thickness mapping. J Cataract Refract Surg 2017; 43: 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg 2013; 29: 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda S, Beheregaray S, Hoshi S, Yamanari M, Lim Y, Hiraoka T, Yasuno Y, Oshika T. Comparison of three-dimensional optical coherence tomography and combining a rotating Scheimpflug camera with a Placido topography system for forme fruste keratoconus diagnosis. Br J Ophthalmol 2013; 97: 1554–1559 [DOI] [PubMed] [Google Scholar]
- 17.Catalan S, Cadarso L, Esteves F, Salgado-Borges J, Lopez M, Cadarso C. Assessment of Corneal Epithelial Thickness in Asymmetric Keratoconic Eyes and Normal Eyes Using Fourier Domain Optical Coherence Tomography. J Ophthalmol 2016; 2016: 5697343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg 1999; 25: 1327–1335 [DOI] [PubMed] [Google Scholar]
- 19.Shetty R, Rao H, Khamar P, Sainani K, Vunnava K, Jayadev C, Kaweri L. Keratoconus Screening Indices and Their Diagnostic Ability to Distinguish Normal From Ectatic Corneas. Am J Ophthalmol 2017; 181: 140–148 [DOI] [PubMed] [Google Scholar]
- 20.Steinberg J, Aubke-Schultz S, Frings A, Hulle J, Druchkiv V, Richard G, Katz T, Linke SJ. Correlation of the KISA% index and Scheimpflug tomography in ‘normal’, ‘subclinical’, ‘keratoconus-suspect’ and ‘clinically manifest’ keratoconus eyes. Acta Ophthalmol 2015; 93: e199–207 [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Chamberlain W, Tan O, Brass R, Weiss JL, Huang D. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J Cataract Refract Surg 2016; 42: 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Meisler DM, Tang M, Lu AT, Thakrar V, Reiser BJ, Huang D. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology 2008; 115: 2159–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang M, Li Y, Chamberlain W, Louie DJ, Schallhorn JM, Huang D. Differentiating keratoconus and corneal warpage by analyzing focal change patterns in corneal topography, pachymetry, and epithelial thickness maps. Invest Ophthalmol Vis Sci 2016; 57: OCT544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schallhorn JM, Tang M, Li Y, Louie DJ, Chamberlain W, Huang D. Distinguishing between contact lens warpage and ectasia: the utility of optical coherence tTomography epithelial thickness mapping. J Cataract Refract Surg 2017; 43: 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrosio R Jr., Guell JL, Malecaze F, Nishida K, Sangwan VS, Group of Panelists for the Global Delphi Panel of K, Ectatic D. Global consensus on keratoconus and ectatic diseases. Cornea 2015; 34: 359–369 [DOI] [PubMed] [Google Scholar]
- 26.Belin MW, Villavicencio OF, Ambrosio RR Jr. Tomographic parameters for the detection of keratoconus: suggestions for screening and treatment parameters. Eye Contact Lens 2014; 40: 326–330 [DOI] [PubMed] [Google Scholar]
- 27.Golan O, Piccinini AL, Hwang ES, De Oca Gonzalez IM, Krauthammer M, Khandelwal SS, Smadja D, Randleman JB. Distinguishing Highly Asymmetric Keratoconus Eyes Using Dual Scheimpflug/Placido Analysis. Am J Ophthalmol 2019; 201: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes BT, Ramos IC, Salomao MQ, Guerra FP, Schallhorn SC, Schallhorn JM, Vinciguerra R, Vinciguerra P, Price FW, Jr., Price MO, Reinstein DZ, Archer TJ, Belin MW, Machado AP, Ambrosio R Jr., Enhanced Tomographic Assessment to Detect Corneal Ectasia Based on Artificial Intelligence. Am J Ophthalmol 2018; 195: 223–232 [DOI] [PubMed] [Google Scholar]
- 29.Luz A, Lopes B, Hallahan KM, Valbon B, Ramos I, Faria-Correia F, Schor P, Dupps WJ Jr., Ambrosio R Jr. Enhanced Combined Tomography and Biomechanics Data for Distinguishing Forme Fruste Keratoconus. J Refract Surg 2016; 32: 479–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang ES, Perez-Straziota CE, Kim SW, Santhiago MR, Randleman JB. Distinguishing Highly Asymmetric Keratoconus Eyes Using Combined Scheimpflug and Spectral-Domain OCT Analysis. Ophthalmology 2018; 125: 1862–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]