Abstract
Plant pathogenic oomycetes cause significant impact on agriculture and, therefore, their management is utmost important. Though conventional methods to combat these pathogens (resistance breeding and use of fungicides) are available but these are limited by the availability of resistant cultivars due to evolution of new pathogenic races, development of resistance in the pathogens against agrochemicals and their potential hazardous effects on the environment and human health. This has fuelled a continual search for novel and alternate strategies for management of phytopathogens. The recent advances in oomycetes genome (Phytophthora infestans, P. ramorum, P. sojae, Pythium ultimum, Albugo candida etc.) would further help in understanding host–pathogen interactions essentially needed for designing effective management strategies. In the present communication the novel and alternate strategies for the management of oomycetes diseases are discussed.
Keywords: Oomycetes, Phytophthora, Pythium, RNAi, Transgenics, Molecular markers, Gene editing
Introduction
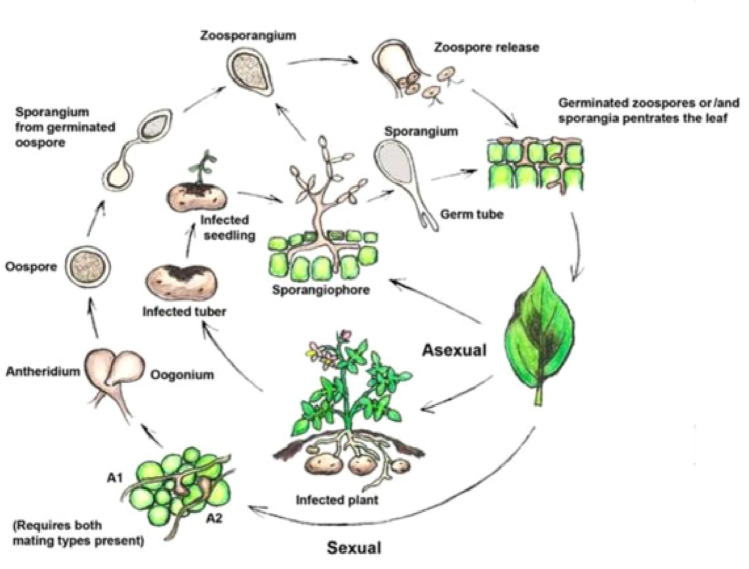
Oomycetes are fungal-like eukaryotes classified as Stramenopiles within the Kingdom Chromista and Super Kingdom Chromalveolate, and are phylogenetically grouped with diatoms and brown algae (Beakes et al. 2012; Cavalier-Smith and Chao 2006). They are among the most problematic group of disease causing organisms and belong to two orders, namely Saprolegniales (Aphanomyces) and Peronosporales (Pythium, Phytophthora, Bremia, Peronospora, Plasmopara, Pseudoperonospora, Peronosclerospora, Sclerophthora, Sclerospora and Albugo species). The diseases they cause include foliar blights, damping off, stem and root rots, fruit rots, downy mildews, white rusts etc. which result in major economic losses and serious damage to natural ecosystems. The best studied oomycetes is Phytophthora to which most notorious species such as P. infestans, known for triggering the Irish potato famine; P. palmivora, causing cocoa black pod; P. ramorum, threatening tree species; P. sojae, causing stem and root rot of soybean etc. belong (Kamoun et al. 2015; Judelson and Blanco 2005). The list of economically important oomycetes species is given in the Table 1. The oomycetes are remarkably diverse in lifestyles such as biotrophic, necrotrophic or hemibiotrophic. Many biotrophic oomycetes like downy mildews (Hyaloperonospora arabidopsidis, Hyaloperonospora parasitica and Plasmopara viticola), as well as, Albugo candida, causal agent of white rust, are completely reliant on host tissues and keep their hosts alive for their own survival. The hemibiotrophic oomycetes, such as Phytophthora spp., initially grow as biotrophs but later switching to a necrotrophic phase. The necrotrophs such as Pythium species have the ability to survive as facultative (Fawke et al. 2015). The dispersal of asexual sporangia of oomycetes takes place through wind or water. Germination of sporangia can occur either directly, forming invasive hyphae, or indirectly, releasing motile zoospores, which are chemotactically or electrotactically attached to the surface of host plants (Tyler 2002). Zoospores swim until reaching the plant surface, shed their flagella and encyst, firmly attaching themselves to the plant surface. Upon germination of a zoospore, a germ tube enlarges and grows across the plant surface until the development of an appresorium. In general, oomycete appresoria function in the penetration of the host cells. Exceptions to this include Albugo candida, which enters through stomata and then forms appresoria to penetrate the host cells, and Aphanomyces euteiches, which does not form distinct appresoria (Kemen and Jones 2012). The disease cycle of late blight pathogen of potato is illustrated in Fig. 1. The wide host range of these oomycetes and nature of damage they cause, pose a serious challenge for their sustainable management. To reduce the plant losses, biologists have developed resistant plants using disease resistance breeding, transgenes, cis-genes, RNA silencing, marker-assisted selection (MAS) and protoplast fusion.
Table 1.
Economically important oomycetes species
| S. no. | Species | S. no. | Species |
|---|---|---|---|
| 1 | Phytophthora infestans | 16 | Aphanomyces euteiches |
| 2 | Hyaloperonospora arabidopsidis | 17 | Albugo laibachii |
| 3 | Phytophthora ramorum | 18 | Bremia lactucae |
| 4 | Phytophthora sojae | 19 | Phytophthora palmivora |
| 5 | Phytophthora capsici | 20 | Pseudoperonospora cubensis |
| 6 | Plasmopara viticola | 21 | Plasmopara halstedii |
| 7 | Pytophthora cinnamomi | 22 | Peronophythora litchi |
| 8 | Phytophthora parasitica | 23 | Peronosclerospora sorghi |
| 9 | Pythium ultimum | 24 | Peronospora belbahrii |
| 10 | Albugo candida | 25 | Phytophthora alni |
| 11 | Phytophthora brassicae | 26 | Phytophthora cactorum |
| 12 | Phytophthora meadii | 27 | Phytophthora phaseoli |
| 13 | Phytophthora plurivora (formerly P. citricola) | 28 | Plasmopara obducen |
| 14 | Pythium aphanidermatum | 29 | Pythium oligandrum |
| 15 | Sclerophthora rayssiae | 30 | Hyaloperonospora brassicae |
Source: Kamoun et al. 2015
Fig. 1.
Life cycle of Phytophthora infestans (From public domain internet source)
Host–pathogen mediated resistance
Introgression of RB gene/cis-genics for management of late blight
Deployment of resistant varieties is the best option for management of late blight. Although race-specific resistance derived from Solanum demissum (11R genes) through conventional breeding has yielded late blight resistant cultivars but the resistance is not durable (Fry 2008). Due to development of new matching races that overcome the introgressed resistance, race non-specific and durable resistance is now being preferred. Intensive long-term efforts have resulted in the identification of numerous resistance genes from wild potato species and their integration into potato. Though, S. bulbocastanum (2n = 2× = 24) has provided another source of resistance (RB/Rpi-blb1) (Helgeson et al. 1998; Naess et al. 2001; Song et al. 2003; van der Vossen et al. 2003) but its transfer through classical breeding to cultivated potato is hampered due of differences in ploidy and endosperm balance number (Carputo and Frusciante 2011). Somatic hybrids developed from this wild species exhibited same level of blight resistance as that of parental S. bulbocastanum clone PT29 (Naess et al. 2001).
Introgression of RB gene into Indian potato cultivars (Kufri Bahar and Kufri Jyoti) was attempted by crossing with the RB-transgenic Katahdin lines SP904 and SP951. The F1 progenies were screened for presence of RB gene and positive lines were taken forward. Results have shown that the efficacy of R gene largely depends on the genetic background of the recipient genotype and not solely on its presence in the variety (Shandil et al. 2017). Further, the results of downstream analysis of RB gene reveal the involvement of immune priming plant receptors in stability and functionality of RB to induce resistance against P. infestans as highest expression of NBS-LRR along with ptotease, protein esterase inhibitors, chaperones and reactive oxygen species genes was observed (Sundaresha et al. 2018).
Potato late blight resistant genes R3a, R1 and RB have been cloned and transferred into tomato plants. The transformants showed hypersensitive response (HR) to race 0, R3a (potato late blight); R1 showed resistance to some tomato late blight isolates, while RB showed resistance to all 5 isolates. Hence, Jia et al. (2009) suggested that RB can possibly be deployed for tomato late blight management.
Transgenic approach: RNA silencing to plant disease resistance
Earlier conventional methods including cross protection and host resistance were used to protect plant infections. The concept of pathogen-derived resistance introduced by the Beachy Lab (Prins et al. 1995) has given a new dimension for the management of plant diseases. This concept has triggered multiple strategies to engineer plants (Prins et al. 2008) by protein-mediated and RNA-mediated resistance. The mechanisms of protein-mediated resistance are not yet clear; whereas, the RNA-mediated mechanism (RNA silencing) has become a powerful tool for functional genomics, disease resistance and qualitative crop improvement. The summary of RNAi silencing in oomycetes is presented in Table 2.
Table 2.
RNA silencing: A tool for the management of oomycetes in various crops
| Host | Target pathogen | Targeted gene(s) | Comments | References |
|---|---|---|---|---|
| Solanum tuberosum (Potato) | Phytophthora infestans | G-protein β-subunit encoding gene (Pigpb 1) |
Silenced transformants failed to sporulate Reduced disease symptoms and sporulation |
Latijnhouwers and Govers (2003), Jahan et al. (2015) |
| Cdc 14 coding gene (PiCdc14) | Transformants showed reduced sporulation | Ah-Fong and Judelson (2003) | ||
| G-protein α-subunit gene (Pigpa 1) | Transformants exhibited reduced zoospore production and infection on potato leaves | Latijnhouwers et al. (2004) | ||
| GFP inf1and cdc14 | GFP, inf1 and cdc14 expression levels were reduced after exposure to dsRNA | Whisson et al. (2005) | ||
| bZIP transcription factor (Pibzp1) | Silenced transformants showed abnormal zoospore movement, failed to develop appressoria and were incapable of infecting tomato leaflets | Blanco and Judelson (2005) | ||
| Nuclear LIM interactor-interacting factors (NIFC1 and NIFC2) | Zoospore cyst germination was impaired by 60% in silenced NIFC transformants. Silencing occurred at the transcription level | Judelson and Tani (2007) | ||
| Inf1 | Hairpin most efficient method of silencing | Ah-Fong et al. (2008) | ||
| Putative glycosylated protein (Pihmp1) | Silenced lines showed loss of pathogenicity | Avrova et al. (2008) | ||
| Putative ATP-dependent DEAD-box RNA-helicase gene (Pi-RNH1) | Pi-RNH1-silenced lines formed large aberrant zoospores that had multiple flagella and underwent partial cleavage | Walker et al. (2008) | ||
| Four members of the CesA encoding for cellulose synthase genes |
Silenced strains contained disrupted cell wall surrounding appressoria Cellulose content of the silenced strains was > 50% lower than that of non-silenced strains |
Grenville-Briggs et al. (2008) | ||
| Effector protein (PiAVR3a) | A PiAVR3a-silenced line (CS12) was significantly reduced in pathogenicity on Solanum tubersonum cv Bintje (susceptible) and on Nicotinia benthamiana | Bos et al. (2010) | ||
| Dicer-like (Pidcl1), Argonaute (Piago1/2) and Histone deacetylase (Pihda1) |
Stable Ns (Non-sporulating, Picdc14-silenced) transformant protoplasts were treated with dsRNA homologous to Pidcl1, Piago1/2 and Pihda1 Regenerated lines of Pidcl1, Piago1/2 and Pihda1 showed an obvious sporulation |
Vetukuri et al. (2011a, b) | ||
| Cutinase | Significant resistance against the pathogen in the RNAi plants, as evident from reduced foliar disease symptoms | Niblett and Bailey (2012) | ||
| Cellulose synthase A2 (PiCESA2) | P. infestans encountered difficulty in establishing the infection process but the few spores that managed to penetrate the mesophyll cells continued to colonize the leaves, thus leading to. ‘incomplete’ inhibition | Jahan et al. (2015) | ||
| Pectinesterase (PiPEC) | P. infestans encountered difficulty in establishing the infection process but the few spores that managed to penetrate the mesophyll cells continued to colonize the leaves, thus leading to. ‘incomplete’ inhibition | Jahan et al. (2015) | ||
| Glyceraldehyde 3-phosphate dehydrogenase (PiGAPDH) | P. infestans encountered difficulty in establishing the infection process but the few spores that managed to penetrate the mesophyll cells continued to colonize the leaves, thus leading to. ‘incomplete’ inhibition | Jahan et al. (2015) | ||
| P. parasitica var. nicotianae | A coding gene considered to be involved in cellulose-binding (CB), elicitor (E) of defence in plants and lectin (L)-like activities (CBEL) | Silenced transformants showed reduced attachment to cellulosic surfaces and cell wall thickening | Gaulin et al. (2002) | |
| Glycine max (Soybean) | P. sojae | Heterotrimeric G-protein α subunit (PsGPA1) |
Silenced PsGPA1 lines had abnormal zoospore chemotaxis, encystment and germination Silenced transformants were unable to infect soybean |
Hua et al. (2008) |
| C2H2 zinc finger transcription factor (PsCZF1) |
Hyphal growth rate of silenced transformants was reduced about 50% and oospore production, zoospore and cyst germination were impaired Silenced strains lost virulence in soybean |
Wang et al. (2009) | ||
| MAP kinase encoding gene (PsSAK1) |
Silenced transformants showed faster encystment, reduced germination rate and longer germtubes compared with wild type Transformants were unable to colonize wounded and unwounded soybean leaves |
Li et al. (2010) | ||
| Putative seven-transmembrane G-protein-coupled receptor (GPR11) |
Zoospore release from sporangia was drastically impaired as well as zoospore encystment and germination Silenced transformants lost pathogenicity to soybean |
Wang et al. (2010) | ||
| PsYKT6, a conserved member gene of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) |
Silencing of PsYKT6 revealed involment of this gene in asexual development, sexual reproduction and pathogenesis on soybean cultivars Antisense constructs were stable in all three transformants |
Zhao et al. (2011) | ||
| Crinkling-and necrosis-inducing proteins (CRN) (PsCRN63 and PsCRN115) | Silenced transformants were unable to suppress host cell death | Liu et al. (2011) | ||
| Cutinase | Significant resistance against the pathogen in the RNAi plants, as evident from reduced foliar disease symptoms | Niblett and Bailey (2012) | ||
| Nicotiana tabacum (Tobacco) | Phytophthora nicotianae, | Cutinase | Significant resistance against the pathogen in the RNAi plants, as evident from reduced foliar disease symptoms | Niblett and Bailey (2012) |
| Peronospora tabacina | Cutinase | Significant resistance against the pathogen in the RNAi plants, as evident from reduced foliar disease symptoms | Niblett and Bailey (2012) | |
| Arabidopsis thaliana | Hyaloperonospora arabidopsidis | HpasRNAs | Reduced disease level | Dunker et al. (2020) |
The applicability of RNAi in P. infestans was investigated by targeting the gene inf1, which encodes the secreted protein INF1 and demonstrated internuclear transfer of signals from transgenic silenced nuclei to wild-type nuclei (van West et al. 1999). The expression of cell cycle regulator Cdc14 has been shown during sporulation but not at hyphal growth in P. infestans using RNAi (Ah-Fong and Judelson 2003). Signal-transduction pathways that govern development and pathogenicity in P. infestans was investigated by silencing Pipa1, coding for PiGPA1 (Gα subunit) that resulted in severely impaired virulence in the PiGPA1-deficient mutants due to defective zoospore motility, reduced zoospore release, and defective appresorium development (Latijnhouwers et al. 2004).
Sporangial cleavage of P. infestans is initiated by a cold shock that compartmentalizes single nuclei within each zoospore. Comparative analysis of EST revealed a 140-fold increased expression of Pi-RNH1 in young zoospores compared to uncleaved sporangia. The role of Pi-RNH1 in zoospore development was determined by RNAi that resulted in production of partially cleaved large aberrant zoospores having multiple flagella with silencing efficiencies up to 99%. Transmission electron microscopy revealed large fused cytoplasmic vesicles sensitive to osomotic pressure and that often ruptured upon release from sporangia in the silenced lines. These findings indicate that Pi-RNH1is involved in zoospore development (Walker et al. 2008).
Cellulose is an important structural compound that provides strength and rigidity to cells of oomycetes. Four cellulose synthase genes (CesA), whose expression is shown to up-regulate during pre- and early infection stages, have been functionally characterized in potato—P. infestans system. Inhibition of cellulose synthesis by 2, 6-dichlorobenzonitrile leads to reduction in the number of normal germ tubes, severe disruption of the cell wall, and a complete loss of pathogenicity. Silencing of the entire gene family by RNAi in P. infestans led to 50% reduction in cellulose content in silenced lines compared to non-silenced lines. This clearly indicates the role of cellulose synthesis in P. infestans infection and involvement of isolated genes in cellulose biosynthesis (Grenville-Briggs et al. 2008).
Silencing of transposons
Transposable elements are generally regarded as selfish or junk DNA, but they have had a profound influence on the evolution of the genomes of many plant pathogens, which is exemplified by P. infestans and closely related species having expanded genomes due to extensive transposon amplification (Raffaele et al., 2010). The genome of P. infestans is organized into gene-rich and gene-poor islands. The gene-poor islands contain highly repetitive DNA that is rich in transposable elements (Haas et al. 2009). Effector genes are preferentially located in this region (Haas et al. 2009; Raffaele et al. 2010). P. infestans has the largest known oomycete genome at 240 Mb (Haas et al. 2009) having 74% of the genome with highly repeated sequences. Vetukuri et al. (2011a, b) identified small non-coding RNAs of 40nt that were homologous to a non-autonomous P. infestansSINE called infSINEm and have been hypothesized to be involved in silencing the expression of infSINEm. Any P. infestans sequence transcriptionally fused to infSINEm, together with its endogenous copy, would also be subject to silencing. In their study, Whisson et al. (2012) have reported silencing of infSINEm-PiAvr3a fusion in transgenic lines of P. infestans.
Silencing of effectors in P. infestans through proximity to transposons
Studies have shown that a transposable element-derived sequence silenced via sRNAs, can potentially bi-directionally silence nearby sequences in P. infestans (Whisson et al. 2012). Silencing of P. infestans NIF transcription factors leads to formation of heterochromatin at the affected locus (Judelson and Tani 2007); and can spread outwards up to 300 bp from the silenced locus, but was also detectable up to 600 bp. This indicates that genes located within 300 bp results in reduction in expression due to heterochromatin formation. In P. infestans, PiAvr3a is one of the effectors that are considered essential for pathogenicity (Vetukuri et al. 2011a, b). However, report on PiAvr2 indicates that transposon-initiated silencing of effector may also occur naturally (Gilroy et al. 2011). A sequence variant (PiAvr2-like), that is not recognised by R2, was expressed by virulent genotype of P. infestans on potato with R2 gene. Virulent genotypes are typically homozygous for PiAvr2-like, while avirulent genotypes may be homozygous or heterozygous. The sequence and organisation of the genomic region encompassing the PiAvr2 locus is highly variable between virulent and avirulent alleles, and is bounded by transposable element-derived sequences. The nearest transposon, which is within the range of heterochromatin formation (231 bp from the 3’ end of PiAvr2), was determined experimentally (Judelson and Tani 2007). Of the 563 RXLR effectors predicted from the P. infestans genome, 35 are located within 300 bp, and 106 within 600 bp, while 283 are located within 2 kb of a transposon-derived sequence. PiAvr2, PiAvr4, PiAvrBlb1, and PiAvrBlb2 are found within 2 kb of transposon-derived sequences (Whisson et al. 2012).
Transcriptional inactivation of avirulence effectors has also been observed in soybean root rot pathogen (P. sojae) and genotypes that exhibit transcriptional inactivation of the PsAvr1a, 1b and 3al5 avirulence genes have been identified (Dong et al. 2011; Qutob et al. 2009; Shan et al. 2004).
Host-induced gene silencing (HIGS)
Transformation of an RNAi construct into a host plant leads to Host- Induced Gene Silencing in plant- fungal pathosystems which targets a fungal virulence or essential gene. The resulting dsRNA down-regulates the gene of interest in the fungus upon infection resulting in more resistant plants. HIGS has been proved to be successful in various fungal patho systems and for insect pests also. Host-delivered RNAi (HD-RNAi) strategy is successfully used to target pathogenicity genes in barley powdery mildew fungus (Blumeria graminis) (Nowara et al. 2010) and in Puccinia (Yin et al. 2010). Niblett and Bailey (2012) also used HIGS to transform potato against P. infestans for elicitin and ribosomal RNA genes.
The cytoplasmic effector protein, RXLR is reported to suppress hypersensitive cell death during infection by regulating host processes. P. infestans gene, Avr3a, carrying RXLR motif, causes pathogenicity by suppressing host cell death. Therefore, silencing of this gene could lead to pathogen death and/or loss of virulence and can impart late blight resistance. Avr3a, the P. infestans gene responsible for pathogenicity in potato, by suppression of host cell death has been proved by Bos et al. (2010). The silencing of RXLR effector Avr3a through RNAi (amiRNA & siRNA) technology imparted moderate resistance to late blight suggesting that silencing of single effector is not sufficient to impart complete resistance but cumulative effect of effector genes needs to be considered (Sanju et al. 2015; Thakur et al. 2015). Phenotyping of siRNA derived lines (Kufri Khyati 1037 and Kufri Khyati 1129) and amiRNA derived line (Kufri Khyati 2.1358) exhibited less disease development and inoculum production. The reduced expression of Avr3a transcripts in these lines coincided with less disease severity. Though, both techniques had remarkable effect on Avr3a pathogenicity, but siRNA mediated silencing was superior over amiRNA when tested through detached leaf assay (Sanju et al. 2016).
Homology-dependent gene-silencing (HDGS)
Now it is clear that different organisms react to foreign nucleic acids when introduced by gene-silencing mechanisms (Cogoni and Macino 2000). Gene silencing is achieved via diverse strategies. Homologous sequences are inactivated either at a transcriptional level or at a post-transcriptional level by degrading sequence-specific mRNA. This is because of the fact that various homology-dependent gene-silencing phenomenon correspond to host defence responses against parasitic nucleic acids such as transposons, RNA, or DNA viruses and viroids (Matzke et al. 2000). Genetic and biochemical studies have revealed commonality between fungi, plants and animals for homology-dependent gene silencing (Cogoni and Macino 2000).
Phytophthora sojae
Role of isoflavonoids in imparting resistance against root rot pathogen (Phytophthora sojae) in soybean (Glycine max) was investigated by silencing genes involved in their biosynthesis and controlling their elicitation. Silencing of isoflavone synthase (IFS) or chalcone reductase (CHR) genes resulted in breakdown of Rps-mediated resistance to race 1 in 'W79' (Rps 1c) or 'W82' (Rps 1 k) due to suppression of hypersensitive (HR) cell death and cell death-associated activation of hydrogen peroxide and peroxidase. P. sojae cell wall glucan elicitor that triggers cell death response in roots was also suppressed by silencing either CHR or IFS. Silencing of the elicitor-releasing endoglucanase (PR-2) led to the loss of HR cell death, isoflavone and cell death response to cell wall glucan elicitor and race-specific resistance to P. sojae. These results suggest that release of active fragments from a general resistance elicitor is necessary for HR cell death in soybean roots carrying resistance genes at the Rps 1 locus, and that this response is regulated through accumulation of cell wall glucan elicitor (Graham et al. 2007; Subramanian et al. 2005).
PsSAK1 is a novel group of mitogen activated protein kinase (MAPK) that is highly conserved in oomycetes and have shown to up-regulate in zoospores, cysts and during early infection in P. sojae. To demonstrate the function, the silencing of expression of PsSAK1 did not impair hyphal growth, sporulation, or oospore production but severely affected zoospore development. The silenced mutants produced much longer germ tubes but could not colonize soybean leaves indicating the role of PsSAK1 in zoospore development and pathogenicity in P. sojae (Li et al. 2010).
Phytophthora nicotianae
The role of cell wall invertase (cwINV) in plant defence was investigated by comparing wild-type tobacco (Nicotiana tabacum) Samsum NN (SNN) with RNAi-mediated repression of cwINV (SNN::cwINV) plants. The P. nicotianae infection resulted in increase in cwINV in leaves of the resistant wild type while it was absent in SNN::cwINV. Besides, reduction and delay in defence-related callose deposition at cell-to-cell interfaces, decline in sugar export, and accumulation of apoplastic carbohydrates were observed. Expression of PR proteins and increase in phenylalanine ammonia lyase and glucose-6-phosphate-dehydrogenase activities were alleviated but formation of H2O2 and development of hypersensitive lesions were weak, and the pathogen was able to sporulate. This indicates the role of tobacco cwINV in acquisition of carbohydrates in photosynthetically active leaves during plant-pathogen interactions and that the hypersensitive reaction ensures successful defence (Essmann et al. 2008).
The oxidative burst and subsequent formation of hypersensitive lesions in source leaves of resistant tobacco upon P.nicotianae infection can be prevented by inhibition of glucose-6-phosphate dehydrogenase (G6PDH) or NADPH oxidases. When a plastidic isoform of the G6PDH-encoding gene (G6PD) displaying high NADPH tolerance was engineered for cytosolic expression (cP2) in a susceptible cultivar, showed early oxidative bursts, callose deposition, and changes in metabolic parameters. Further, RNAi suppression of endogenous cytosolic G6PD isoforms enhanced uniform defence responses, drought tolerance and flowering. As isoenzyme replacement of G6PDH in the cytosol is beneficial, this can be used as a tool to enhance stress tolerance in general (Scharte et al. 2009).
Marker assisted selection
Pyramiding late blight resistance genes in potato through MAS
A few wild potato species had been exploited to introgress race-specific resistance into the cultivated gene pool by conventional breeding and S. demissum is one that was extensively utilized during the 1950s and 1960s to breed resistant cultivars (Bradshaw and Ramsay 2005) that’s why most of the current potato cultivars have resistance genes from S. demissum. Besides S. demissum, other species viz., S. stoloniferum, S. phureja and S. tuberosum subsp. andigena had also been used to introgress R genes into common potato across the world (Bradshaw et al. 2006). Later, search was initiated for minor genes that could provide durable resistance to late blight but with mixed results globally. However, with the availability of new tools at molecular level, it was found that both major and minor (durable) resistances are not diverse and it is the same part of the genome that control both these type of resistances (Gebhardt and Valkonen 2001). This fact renewed the interest of breeders in these hitherto abandoned R genes necessating the search for new sources of resistance in wild gene pools and their faster deployment through marker-assisted selection (MAS).
Conventional breeding methods are of primary importance but are too slow. To breed potato cultivars with durable resistance, it is necessary to combine multiple resistance (R) genes and/or quantitative trait loci (QTLs) against P. infestans. Two QTLs in a diploid mapping population of Solanum spegazzinii (susceptible) × S. chacoense (resistant) contributing to quantitative resistance against late blight have been identified (Chakrabarti et al. 2014). Several R genes could probably prolong the life of late blight resistance as single R genes are easily overcome by this ever evolving pathogen. The large gene pool of Solanum species provides opportunities to explore new R genes against P. infestans that can be introgressed through new approaches like MAS.
The gene R1 is one of the P. infestans race-specific R genes introgressed through traditional breeding but was quickly defeated by matching virulence in P. infestans even in gene pyramiding systems (Hein et al. 2009). This stimulated the breeders to re-orient their breeding strategies towards durable field resistance (van der Vossen et al. 2003) by stacking multiple resistance genes. A series of R genes have been mapped from wild species and the list of the same is given in Table 3. Besides, there are a few more wild species including S. urubambae, S. violaceimamoratum, S. cantense, S. cajamarquense, S. orophilum, S. velaedei, etc. that had shown a high degree of resistance, are yet to be characterized (Hermanova et al. 2007).
Table 3.
List of R genes mapped/cloned against oomycetes pathogens
| Target pathogen | Gene | Source/Mapped from | References |
|---|---|---|---|
| Phytophthora infestans | R1 | S. demissum | Leonards-Schippers et al. (1992) |
| R2 | S. demissum | Li et al. (1998) | |
| R6, R7, R3a, R3b, R10, R11 | S. demissum | Bradshaw et al. (2006); El-Kharbotly et al. (1996); Huang et al. (2004) | |
| R8 | S. demissum | Jo et al. (2011) | |
| RB/Rpi-blb1, Rpi-blb2, Rpi-blb3, Rpi-bt1, Rpi-abpt | S. bulbocastanum | Hermanova et al. (2007) | |
| Rpi-bst1 | S. brachistotrichum | ||
| Rpi-edn1.1 | S. edinense | ||
| Rpi-hjt1.1, Rpi-hjt1.2 and Rpi-hjt1.3 | S. hjertingii | ||
| Rpi-mcd1 | S. microdontum | ||
| Rpi-snk1.1 and Rpi-snk1.2 | S. schenckii | ||
| Rpi-ver1 | S. verrucosum | ||
| Rpi-pnt1 | S. pinnatisectum | ||
| Rpi-sto1 and Rpi-sto2 | S. stoloniferum | ||
| Rpi-pta1 | S. papita | ||
| Rpi-plt1 | S. polytrichon | ||
| Rpi-mcq1 | S. mochiquense | ||
| Rpi-phu1 | S. phureja | ||
| Rpi-vnt1.1, Rpi-vnt1.2, Rpi-vnt1.3 | S. venturii | ||
| Rpi-dlc1 | S. dulcamara | ||
| Rpi-ber1 and Rpi-ber2 | S. berthaultii | ||
| Rpi-avl1 | S. avilesi | ||
| Rpi-cap1 | S. capsicibaccatum | ||
| Rpi-qum1 | S. circaeifolium spp. quimense | ||
| Albugo candida | WRR4 | Arabidopsis thaliana | Borhan et al. (2008) |
| Hyaloperonospora arabidopsidis | RPP1, RPP2, RPP4, RPP5, RPP7, RPP8, RPP13 | Arabidopsis thaliana | Botella et al. (1998); Krasileva et al. (2010); Sohn et al. (2007); Tör et al. (1994); Parker et al. (1997); McDowell et al. (1998); Bittner-Eddy et al. (2000) |
| Peronospora manshurica | Rpm | Glycine max | Lim et al. (1984) |
| Phytophthora cinnamomi | T1R1 | Arabidopsis thaliana | Ruegger et al. (1998) |
| Phytophthora sojae | Rps1d, Rps1b | Glycine max | Na et al. (2013); Shan et al. (2004) |
| Plasmopara viticola | Rpv1, Rpv2, Rpv3, Rpv10 | Vitis vinifera | Na et al. (2013); Shan et al. (2004); Merdinoglu et al. (2003); Bellin et al. (2009); Casagrande et al. (2011); Schwander et al. (2012) |
Four genes within S. demissum viz., R1 (Ballvora et al. 2002), R2 (Lokossou et al. 2009), R3a (Huang et al. 2005), and R3b (Li et al. 2011) have been cloned. Besides, about a dozen functional late blight R genes having source other than S. demissum like, Rpi-blb1 (van der Vossen et al. 2003), Rpi-blb2 (van der Vossen et al. 2005), RBver (Liu and Halterman 2006), Rpi-stol1 and Rpi-pta1 (Vleeshouwers et al. 2008), Rpi-vnt1.1 and Rpi-vnt1.3 (Foster et al. 2009; Pel et al. 2009), Rpi-blb3 and Rpi-bt1 (Oosumi et al. 2009) have also been cloned. New RB homologous fragments of resistance genes from the wild potato species have been identified (Tiwari et al. 2015; Srivastava et al. 2016) and classified. These studies indicate that RGA1-blb and Rpi-blb1 are well conserved in the wild potato species during the course of evolution and the newly identified R-gene homologues can be utilized as a novel sourse of genes for late blight resistance breeding. Many more R genes are expected to be cloned that could be deployed in potato breeding which would pave the way for effective Marker Assisted Selection (MAS).
MAS as a tool for multiple genes pyramiding
DNA-based molecular markers offer a remarkable promise for potato breeding. Introgression of genes through MAS allows breeders to track the gene of interest and thus has become a complementary tool in selecting the desired traits (Li et al. 2013) and economically feasible. Many molecular markers linked to late blight resistance are now available for rapid and efficient selection (Table 4). Now selection of desirable lines based on genotype rather than phenotype, analysing plants at the seedling stage for multiple characters, minimizing linkage drag and rapidly recovering a recurrent parent’s genotype has become reality through MAS (Collard and Mackill 2008).
Table 4.
Molecular markers of R genes/QTLs for MAS against oomycetes pathogens
| Pathogen | Gene/QTL | Marker/primer | Marker type | Primer sequence (5′ → 3′) | References |
|---|---|---|---|---|---|
| Phytophthora infestans | R1 | R1-1205 | SCAR | CACTCGTGACATATCCTCACTA | Sokolova et al. (2011) |
| GTAGTACCTATCTTATTTCTGCAAGAAT | |||||
| BA47f2 | SCAR | TAACCAACATTATCTTCTTTGCC | Gebhardt et al. (2004) | ||
| GAATTTGGAGAGGGGTTTGCTG | |||||
| CosA | SCAR | CTCATTCAAAATCAGTTTTGATC | Gebhardt et al. (2004) | ||
| GAATGTTGAATCTTTTTGTGAAGG | |||||
| R1F/R (76-2sf2/76-2SR) | AS | CACTCGTGACATATCCTCACTA | Ballvora et al. (2002) | ||
| CAACCCTGGCATGCCACG | |||||
| GP76 | SCAR | ATGAAGCAACACTGATGCAA | Oberhagemann et al. (1999) | ||
| TTCTCCAATGAACGCAAACT | |||||
| SPUD237 (AluI) | CAPS | TTCCTGCTGATACTGACT | De Jong et al. (1997) | ||
| AGAAAACC AGCCAAGGAAAAGCTAGCATCCAAG | |||||
| GP21 (AluI) | CAPS | AGTGAGCCAGCATAGCATTACTTG | De Jong et al. (1997) | ||
| GGTTGGTGGCCTATTAGCCATGC | |||||
| GP179 | SCAR | GGTTTTAGTGATTGTGCTGC | Meksem et al. (1995) | ||
| AATTTCAGACGAGTAGGCACT | |||||
| R3 (R3a & R3b) | R3-1380 | SCAR | TCCGACATGTATTGATCTCCCTG | Sokolova et al. (2011) | |
| AGCCACTTCAGCTTCTTACAGTAGG | |||||
| SHa-F/ SHa-R | AS | ATCGTTGTCATGCTATGAGATTGTT | Huang et al. (2005) | ||
| CTTCAAGGTAGTGGGCAGTATGCTT | |||||
| R3bF4/R3bR5 | AS | GTCGATGAATGCTATGTTTCTCGAGA | Rietman (2011) | ||
| ACCAGTTTCTTGCAATTCCAGATTG | |||||
| RB/Rpi-blb1 | RB-629/638 | SCAR | AATCAAATTATCCACCCCAA | Sokolova et al. (2011) | |
| CTTTTAAATCAAGTATTGGGAGGACTGAAAGGT | |||||
| RB-1223 | SCAR | ATGGCTGAAGCTTTCATTCAAGTTCTG | Pankin et al. (2011) | ||
| CAAGTATTGGGAGGACTGAAAGGT | |||||
| CT88 (Primer1/primer 1′) | SCAR | CACGAGTGCCCTTTTCTGAC | Colton et al. (2006) | ||
| ACAATTGAATTTTTAGACTT | |||||
| Rpi-abpt | R2-F1/R2-R3 | AS | GCTCCTGATACGATCCATG | Kim et al. (2012) | |
| ACGGCTTCTTGAATGAA | |||||
| Th2 | CAPS | AGGATTTCAGTATGTCTCG | Park et al. (2005) | ||
| TCCATTGTTGATTGCCCCT | |||||
| Rpi-ber1 | CT214 (DdeI) | CAPS | GAACGCGAAAGAGTGCTGATAG | Tan et al. (2010) | |
| CCCGCTGCCTATGGAGAGT | |||||
| TG63(Bme1390I) | CAPS | TCCAATTGCCAGACGAA | Tan et al. (2010) | ||
| TAGAGAAGGCCCTTGTAAGTTT | |||||
| Q133 | SCAR | ATCATCTCCTCAAAGAATCAAG | Tan et al. (2010) | ||
| ATCTCCCCATTGACAACCAA | |||||
| Rpi-mcd1 | TG339 (MnlI) | CAPS | GCTGAACGCTATGAGGAGATG | Tan et al. (2010) | |
| TGAGGTTATCACGCAGAAGTTG | |||||
| Rpi-phu1 | GP94 (OPB07 + TG/GT) | RAPD | GAAACGGGTG + TG/GT | Sliwka et al. (2006) | |
| QTL_phu-stn | OPA17 | RAPD | GACCGCTTGT | Wickramasinghe et al. (2009) | |
| OPA03 | RAPD | AGTCAGCCAC | Wickramasinghe et al. (2009) | ||
| GP198F/R | SCAR | GTAATTTGCGAGGAAGGAGAAG | Wickramasinghe et al. (2009) | ||
| TCACTTTGGTGCTTCTGTCG | |||||
| GP198F-1/R | AS | TTTGCTTACTCTTGTTGTATG | Wickramasinghe et al. (2009) | ||
| TCACTTTGGTGCTTCTGTCG | |||||
| Rpi-sto1 | Ssto-448 | SCAR | GTGGAACGCCGTCCATCCTTAG | Sokolova et al. (2011) | |
| TGCATAGGTGGTTAGATGTATGTTTGATTA | |||||
| Rpi-avl1 | N2527 | AS | GAAACACAGGGGAATATTCACC | Verzaux (2010) | |
| CCATRTCTTGWATTAAGTCATGC | |||||
| Rpi-cap1 | CP58 (MspI) | CAPS | ATGTATGGTTCGGGATCTGG | Jacobs et al. (2010) | |
| TTAGCACCAACAGCTCCTCT | |||||
| Rpi-dlc1 | GP101 (AluI) | CAPS | GGCATTTCTATGGTATCAGAG | Golas et al. (2010) | |
| GCTTAACATGCAAAGGTTAAA | |||||
| S1d5-a | AS | CGCCTCTTTCTCTGAATTTC | Golas et al. (2010) | ||
| GATCTGGGATGGTCCATTC | |||||
| Rpi-mcq1 | TG328 (AluI) | CAPS | AATTAAATGGAGGGGGTATC | Smilde et al. (2005) | |
| GTAGTATTCTAGTTAAACTACC | |||||
| Rpi-snk1.1 and Rpi-snk1.2 | Th21 (MboI) | CAPS | ATTCAAAATTCTAGTTCCGCC | Jacobs et al. (2010) | |
| AACGGCAAAAAAGCACCAC | |||||
| Rpi-ver1 | CD67 (HpyCH4I, SsiI) | CAPS | CCCCTGCAAATCCGTACATA | Jacobs et al. (2010) | |
| CCATACGAGTTGAGGGATCG | |||||
| Rpi-vnt1.1 | TG35 (HhaI/XapI) | CAPS | CACGGAGACTAAGATTCAGG | Pel et al. (2009) | |
| TAAAGGTGATGCTGATGGGG | |||||
| Rpi-vnt1.3 | NBS3B | AS | CCTTCCTCATCCTCACATTTAG | Pel et al. (2009) | |
| GCATGCCAACTATTGAAACAAC | |||||
| R2 | R2-800 | PCR | TACTAACCTTTTCCTAGATG | Mori et al. (2011) | |
| AAAACTTTCACGCACCCATAGGA | |||||
| R8 | R8 | PCR | AACAAGAGATGAATTAAGTCGGTAGC | Vossen et al. (2016) | |
| AGAACTTTCTCACAGCTTTT | |||||
| Rpi2 | SpCP56 | SCAR | GGCATATTACTTGAATCCAATA | Yang et al. (2017) | |
| AGCAGAGGAGCACCATACT | |||||
| SpGot2-1 | CAPS Msp1 | AAGCGCTTTTGTCCTGATTC | Yang et al.(2017) | ||
| TTCCCCGAGCCATAGAGTT | |||||
| SpGro1 | CAPS Taq1 | GAGTTGAGGTGGCTTGATTGG | Yang et al. (2017) | ||
| CAACATTGGATATTTGGTTAGTTGA | |||||
| SpCT226 | CAPS Tru1 | GGCATATTACTTGAATCCAATA | Yang et al. (2017) | ||
| AGCAGAGGAGCACCATACT | |||||
| SpT1756 | CAPS Msp1 | GTTGATGCCTGTGTCGTCGTT | Yang et al. (2017) | ||
| TAATGCGTCCTTCTTCGTCAATCT | |||||
| SpAFLP2 | CAPS Tail1 | CAACAAATAGGCATGTGAAGAAC | Yang et al. (2017) | ||
| GACCCCACCAACCCAAAAAGAC | |||||
| SpTG572 | SCAR | TCCTTGATCCCCTCCCTTTGGTGTGA | Yang et al. (2017) | ||
| TCATTTCCAGCCAAGCTATTTAC | |||||
| SpAFLP1 | CAPS Taq1 | TCAGAGGACACGAGATTACG | Yang et al.(2017) | ||
| ATACCATCCTCATTTTCT TTGTTA | |||||
| SpAL21 | CAPS Taq1 | TTTTTGGGCTTGTCCTCTGC | Yang et al. (2017) | ||
| GATCCCCTTCTGCGTGGT | |||||
| SpGP127 | CAPS Trul1 | GGTGATCGACTGGAACGTCT | Yang et al. (2017) | ||
| CTGCAGATTCCTCGCATACT | |||||
| SpTG20 | CAPS Rsa1 | GAGTGGCATGGTGTGGCAGTTCT | Yang et al. (2017) | ||
| TCTTTGTGGGGTTTTGGAGTTTTT | |||||
| R9a | CDPHero33 | CDP/HaeIII | RRAGATTCAGCCATKGARATTAAGAAA | Jo et al. (2011) | |
| CDPTm22 | CDP/MseI | GCCAAATAGTATTGTCAAGCTC | Jo et al. (2011) | ||
| CDPTm26 | CDP/MseI | CATTTCTCTCTGGAGCCAATC | Verzaux (2010) | ||
| CDPTm27 | CDP/MseI | CAAGTTTGTCGCAGAGATTGA | Verzaux (2010) | ||
| CDPSw58 | CDP/MseI | AAGGATGCGACCGTATTGACCTCAT | Jo et al. (2015) | ||
| CDPSw59 | CDP/MseI | AAGGATGCGACCGTATTGACCTCAT | Jo et al.(2015) | ||
| CDPSw510 | CDP/MseI | AAGGATGCGACCGTATTGACCTCAT | Jo et al. (2015) | ||
| 184–81 | CAPS/RsaI | CCACCGTATGCTCCGCCGTC | Jo et al. (2011) | ||
| GTTCCACTTAGCCTTGTCTTGCTCA | |||||
| Stm1021 | SSR | GGAGTCAAAGTTTGCTCACATC | Collins et al. (1999) | ||
| CACCCTCAACCCCCATATC | |||||
| Phytophthora sojae | Rps | SSR | TTGTGATCGTTTGGGATGAG | Li et al. (2017) | |
| GGAAAAGGATGCATAGCAAAA | |||||
| GGTAGATCCAGGAGCTTGAGTCAG | |||||
| GCGCATCTCACTGCACTTGATTTT | |||||
| GCGACGCGCTAGTCTTATTT | |||||
| GCGGATGGCTTTTACTTT | |||||
| AAAACCTCGTTCCCACTGTT | |||||
| TCTTCCTTGGACTCCTCGAA | |||||
| Rps 9 | SSR | GGTAGATCCAGGAGCTTGAGTCAG | Wu et al. (2011) | ||
| GCGCATCTCACTGCACTTGATTTT | |||||
| Rps1 | SSR | GAAATGCCCAGAAAAACCTAATAAC | Demirbas et al. (2001) | ||
| TGAAGCAACAAAATAGAGGAATAGAG | |||||
| GCGCTATTCCTATCACAACACA | |||||
| TAGGGTTGTCACTGTTTTGTTCTTA | |||||
| CATGCATATTGACTTCATTATT | |||||
| CCAAGCGGGTGAAGAGGTTTTT | |||||
| GCGCCCAAACCTATTAAGGTATGAACA | |||||
| GCGGGTCAGAAGATGCTACCAAACTCT | |||||
| Rps2 | SSR | GCGCTATCCGATCCATATGTG | |||
| TGATTTCGCTAGGTAAAATCA | |||||
| Rps3 | GGGTTATCCTCCCCAATA | ||||
| ATATGGGATGATAAGGTGAAA | |||||
| Rps4 | GCGAATACATAAAACTCAAATTCAAATCATA | ||||
| GCGTTCTATAAATTTCATTCATAGTTTCAAT | |||||
| Rps6 | CGCGATCATGTCTCTG | ||||
| GGGAGTTGGTGTTTTCTTGTG | |||||
| Phytophthora capsici | Phyto 5.2 | SCAR | CCA TAA GGG TTGGTA AAT TTA CAA AG | Quirin et al. (2005) | |
| TCG AGAGATAAT TCA GAT AGTATA ATC | |||||
| Phyto 5.2 | SSR | CAGCGTTCTATCGTCTCAAATG | Minamiyama et al. (2007) | ||
| TTGACAAACCAGAAATTGATCG | |||||
| CaPhyto | SSR | TCCAGCCATCCATTATTTCAT | Wang et al. (2016) | ||
| ATCCCGAACTGCCAATAATTA | |||||
| Phyto5SAR | SNP | TTGATAGCCCCTGGTAAAGA | Liu et al. (2014) | ||
| GTGGTGATATTCAAACGGG | |||||
| PhR 10 | SSR | CAATCCAAACAAGTCCTAAG | Xu et al. (2016) | ||
| GGTGCAATTGAAAATCTAAG | |||||
| Plasmopara viticola | Rpv 12 | SSR | TGAAAGTCGATGGAATGTGC | Venuti et al. (2013) | |
| TTGCATCTCCCTTCTCAATG |
Pyramiding of R genes into single host cultivar by combining different R genes, defeated Rpi genes or alleles of single gene through molecular markers have facilitated selection procedure. Progress in molecular biology during the last two decades has made it easy to zoom into chromosomes, subsequently, exact position of most of the genes within the set of R1-R11 have been assigned. At least fifteen linkage maps have been constructed in experimental populations of diploid potato with DNA-based markers. The potato molecular map is saturated with more than 500 markers uniformly distributed on all 12 chromosomes and under such a scenario, marker-assisted selection (MAS) would prove helpful to stack multiple late blight resistance genes in commercial cultivars.
Breeding for resistance in potato through pyramiding started long back, but exact quantification of the resistance effect was not reported. Pentland dell, Escorts were some early potato cultivars developed through conventional breeding strategies. Though, allele dosage effect in duplex R3 or triplex R3 genotypes compared to simplex R3 genotypes was studied in P. infestans resistance but no additive effect was observed (Toxopeus 1956). Further, studies revealed an additive effect of stacking of P. infestans genes RPi-mcd1 and RPi-ber from S. microdontum and S. berthaultii in a diploid S. tuberosum population (Tan et al. 2010). ‘Sarpo Mira’ a potato cultivar containing a natural pyramid of at least five different R genes such as R3a, R3b, R4, and Rpi-Smira1, and Rpi-Smira2, have also been introduced with enhanced level of resistance (Kim et al. 2012; White and Shaw 2010). Broad-spectrum late blight resistance in potato differential set plant MaR9 has been shown by Kim et al. (2012), which is conferred by multiple stacked seven R genes including Rpi-abpt, R1, R3a, R3b, R4, R8, and R9 (Sokolova et al. 2011).
The R genes viz., Rpi-blb (Rpi-blb1/RB, Rpi-blb2 and Rpi-blb3) developed from S. bulbocastanum provide broad spectrum resistance and MAS for RB gene has been successfully applied in potato breeding. Besides, MAS for other R genes (Rpi-ber, Rpi-mcd1, Rpi-phu1 and Rpi-sto1; and QTL_phu-stn) have also been successfully demonstrated. CAPS and SCAR markers linked to Rpi-ber gene for MAS have been developed (Tan et al. 2010) and there are many more molecular markers that are closely linked/co-segregating to genes imparting late blight resistance.
Using MAS approach, R genes (R1, R2 and R3a) have been identified in indigenous and exotic collection of Indian potato genotypes. Seventeen genotypes possessed R1 gene, 18 genotypes were having R2 gene and 41 genotypes have R3a. Besides, 17 genotypes had combinations of either of the genes (R1 & R2), (R1 & R3a), (R2 & R3a) tested through detached leaf method to corroborate the results of molecular marker analysis. Most of the genotypes having multiple R genes were either resistant or moderately resistant. The identified genotypes have been put in use to combine R1, R2 and R3a genes in single host background. Besides, genotypes had also been characterized for presence of late blight resistance (R1, R2, R3a), PVY (RYadg, RYsto) and potato cyst nematodes (H1, HC, QRL and Gro1-4) and 14 genotypes possessing multiple disease resistance have been identified (Sharma et al. 2014) and are put in use as parents to expedite potato resistance breeding programme.
RAPD and bulk segregant analysis (BSA) approaches used to characterize the molecular markers linked to the P. infestans resistance gene Ph-3 in tomato resulted in identification of one RAPD marker (UBC#602) tightly linked to the said gene and could be successfully converted into a co-dominant SCAR marker. The SCAR marker SCU602 used to analyse 96 F2 progenies yielded the expected 1:2:1 Mendelian segregation ratio. When tomato inbred lines (41) were screened using the SCAR and a reference marker linked to the Ph-3 gene, both markers gave the same results. When SCU602 was further validated for association to resistance and its potential in MAS, it could identify three genotypes harbouring the resistance allele; thus, this SCAR marker can be used for the selection of the Ph-3 gene against P. infestans (Hai et al. 2013).
MAS-Phytophthora sojae
Many resistance genes (Rps) are known to provide protection against root and stem rot pathogen of soybean (P. sojae) but Rps8 confers resistance to most P. sojae isolates and has recently been mapped. Bulk segregant analysis of the whole soybean genome and mapping experiments revealed that Rps8 gene maps closely to the disease resistance gene-rich Rps 3 region (Sandhu et al. 2005). Partial resistance to P. sojae in soybean is exhibited as a reduced level of root rot and this resistance is effective against all races of the pathogen. When recombinant inbred soybean populations (Conrad × ‘Sloan’, Conrad × ‘Harosoy’, and Conrad × ‘Williams’) were evaluated for root rot following inoculation with P. sojae and SSR markers were used to identify putative QTLs; two putative QTLs positioned on soybean molecular linkage groups (MLGs) F and Dlb + W were identified in Conrad. The results indicate that MAS may be utilized for combining both Rps genes with partial resistance into high yielding cultivars (Burnham et al. 2003).
MAS-Phytophthora palmivora
When two related segregating populations of Theobromo cacao developed by crossing two susceptible cacao clones of Catongo (a highly homozygous genotype) and Pound 12 (a highly heterozygous genotype) and by backcrossing were analysed for their resistance to P. palmivora, six QTLs were detected in the F1 and BC1 populations. One QTL was found in both populations explaining 48% of the phenotypic variance in the F1 population and thus appeared to be a major component of disease resistance. QTL conferring increased resistance to Phytophthora were identified in both susceptible parents, suggesting the presence of transgressive traits and the possibility of selection in cacao (Crouzillat et al. 2000).
When a heterozygous F1 mapping population of cacao (T. cacao L.) was created by crossing ‘Pound 7’ × ‘UF 273’ and evaluated for black pod (P. palmivora) resistance, three QTLs were found on linkage group LG4, 8, and 10, with the most favourable alleles coming from Pound 7 (Brown et al. 2007).
MAS-Phytophthora nicotianae
Bulked segregant (BSA) and RAPD analyses were used to identify markers linked to the black shank (P. nicotianae var parasitica) resistance gene, Ph, from flue-cured tobacco (N. tabacum) cv Coker 371-Gold. RAPD markers (OPZ-5770 in coupling and OPZ-7370 in repulsion phase linkage) were used to select plants homozygous for the Ph gene that were used for further backcrossing to the widely grown flue-cured cv K326. Evaluation of K326-BC4S1 lines and their test cross hybrids to a susceptible tester confirmed linkage between Ph and OPZ-5770 and complete linkage between 26 RAPD markers and the Ph gene was confirmed in the K326-BC5 generation. RAPD phenotypes were stable across generation and ploidy levels and hence, these RAPD markers are useful in MAS for Ph (Johnson et al. 2002).
MAS-Phytophthora capsici
QTL analysis of a double-haploid population of Capsicum annuum, obtained by anther culture of an F1 hybrid between ‘K9-11’ (susceptible to P. capsici) and ‘AC2258’ (resistant to P. capsici) following inoculation with P. capsici, resulted in identification of three QTLs on LG1, LG6 and LG7. The QTL with the highest LOD score (67.02) detected on LG7, explained 82.7% of the phenotypic variance, was designated as Phyt-1; while the second QTL (Phyt-2) was found on LG1 which explained 6.4% phenotypic variance with a LOD score of 2.54. The other QTL (Phyt-3) was found on LG6 which explained 5.6% of the phenotypic variance with a LOD score of 2.20. The nearest marker to Phyt-1 was an AFLP marker (M10E3-6) and to Phyt-2 was RAPD (RP13-1) marker. The lines with a high resistance could be efficiently selected using two markers, i.e. M10E3-6 and marker RP13-1, simultaneously and can be useful for MAS to breed sweet pepper cultivars with high resistance to P. capsici (Sugita et al. 2006).
Similar studies were carried out using a segregating double-haploid (DH) population developed by anther culture of an F1 plant crossed between susceptible (‘Manganji’) and resistant (‘Criollo de Morelos 334’) lines of pepper (C. annuum) and a high density SSR-based map was constructed. Interval mapping for the resistance to P. capsici detected a common major QTL on LG 15 and flanked with an SSR marker, CAMS420. Besides, seven SSR markers were also located within 21 cM intervals from the peak of this QTL. The present linkage markers may widen the choice in MAS in breeding for Phytophthora rot resistant pepper cultivars (Minamiyama et al. 2007).
Resistance to Phytophthora root rot (P. capsici) is conditioned by a number of QTLs and resistance can be enhanced by pyramiding resistance alleles through molecular markers. An F8 recombinant inbred line population in combination with bulk segregant analysis was used to utilize Phytophthora root rot resistance into SCAR markers and one marker was successfully converted into a co-dominant SCAR marker SA133-4 linked to the trait. Genetic linkage analysis revealed that both SCAR and RAPD markers are located on chromosome 5 of pepper and QTL analysis showed that SA 133–4 and UBC 553 were linked to Phytophthora root rot resistance (Hai et al. 2013).
MAS-Phytophthora fragariae
Root rot of raspberry (P. fragariae var rubi) is a major production constraint throughout the world. Evaluation of BC1 population of (‘Latham’ × ‘Titan’) × ‘Titan’ for root and shoot symptoms along with genetic mapping and QTL analysis could result in identification of two major QTL for root rot resistance. Sequence specific PCR markers were developed for these regions with success rate of 76% for predicting resistance in these cultivars (Weber et al. 2008).
MAS-Phytophthora cactorum
Mapping of QTL controlling resistance to P. cactorum in strawberry (Fragaria × ananassa, 2n = 8× = 56) was done using segregating population of a cross between ‘Capitola’ and ‘CF1116’ and five QTLs were identified for resistance to P. cactorum (Lerceteau-Kohler et al. 2004).
MAS-Pseudoperonospora
RAPD markers linked to the downy mildew (Pseudoperonospora cubensis) resistance gene in cucumber (Cucumis sativus) have been identified. Of the 135 polymorphic RAPD markers identified from 960 primers, five (G14800, X151100, AS5800, BC5191100, and BC5261000) are linked to downy mildew resistance (Horejsi et al. 2000). Besides, five QTLs for resistance to downy mildew i.e. dm1.1, dm5.1, dm5.2, dm5.3, and dm6.1 have also been identified which are located on chromosome 1 (dm1.1), 6 (dm6.1) and 5(dm5.1, dm5.2, dm5.3) (Zhang et al., 2013). Screening of introgression lines derived from interspecific hybridization between C. sativus x C. hystrix (wild) resulted in identification of one introgression line (IL52) with high downy mildew resistance. When the same line was used as a resistant parent to make an F2 population with susceptible parent (Changchunmici), and three QTLs for downy mildew resistance were identified on chromosome 5 and 6 (Pang et al. 2013).
MAS-Aphanomyces
Identification of QTLs associated with resistance to Aphanomyces root rot in pea is essential to facilitate breeding and to understand inheritance of partial resistance. The recombinant inbred lines (RIL) were genotyped using different markers and three QTLs (Aph1, Aph2, Aph3) have been identified (Pilet-Nayel et al. 2003, 2005). While deciphering the diversity and stability of resistance QTL towards pathogen variability and environments, Hamon et al. (2011) identified 135 additive-effects QTLs corresponding to 23 genomic regions and 13 significant epistatic interactions associated with partial resistance. Out of 23 additive- effect genomic regions, five were consistently detected and showed highly stable effects towards pathogen strains, environments, resistance criteria, condition tests and RIL population.
MAS-Peronospora
Markers linked to downy mildew (P. manshurica) resistance in soybean were identified by crossing a resistant cultivar ‘AGS129’ with a susceptible cultivar ‘Nakhon Sawan 1’ and by performing bulked segregant analysis. Primer OPH-02 and OPP-10 generated OPH021250 and OPP-10831 fragments in donor parent and resistant bulks, but not in the recurrent parent and susceptible ones. Co-segeregation analysis confirmed the association of both markers to the Rpmx gene that control downy mildew resistance (Chowdhury et al. 2002).
The identification of downy mildew resistance gene Pp523 in broccoli led to the construction of a genetic map. Bulked segregating analysis resulted in identification of a group of molecular markers flanking that are closely linked in coupling to the resistance gene. Two markers linked in coupling, OPK17_980 and AT.CTA_133/134, are located at each side from the resistance gene can be used for marker-assisted selection (Farinho et al. 2004). Four AFLP markers closely linked to the downy mildew (P. destructor) resistance gene in onion have been identified that can be used in MAS (Scholten et al. 2007).
Protoplast fusion
This technique offers an opportunity to circumvent sexual reproduction barriers and allows transfer of nuclear and cytoplasmic genes to enrich the gene pool of cultivated species that promotes crop improvement in existing cultivars. Although, related or distantly related genera of cultivated crops are reservoirs of genes having desirable traits (Liu et al. 2005) but due to sexual incompatibility these elite traits cannot be transferred by conventional breeding methods (Aleza et al. 2010).
Phytophthora
Hexaploid hybrids resulting from mesophyll protoplasts fusion of Solanum brevidens and S. tuberosum showed resistance to race 0 of P. infestans (Helgeson et al. 1986). Similarly, hybrids from several combinations of S. brevidens (resistant to PLRV and PVY and frost, but non tuberous) and S. tuberosum (with various flesh colours and degrees of late blight resistance) were produced by protoplast fusion. Plants tested in the field showed stable expression of disease resistance genes (Austin et al. 1987). Hybrids developed from somatic fusion of diploid S. tuberosum and wild diploid S. circaeifolium were completely resistant to P. infestans (Mattheji et al. 1991). Louwes et al. (1992) reported that 75% of hybrids developed from S. tuberosum ssp. tuberosum and S. ciraeifolium ssp. ciraeifolium were highly resistant to P. infestans. Potato developed by electrofusion of protoplasts of 2 dihaploid S. tuberosum liners and S. brevidens was diploid but non-tuber bearing. Most of the hybrids were aneuploids at the tetraploid (4x) or hexaploid (6x) levels. Resistance to P. infestans differed between hybrids but was on an average better than that of parental genotypes (Rokka et al. 1994). Nine S. nigrum ( +) ZEL-1136 hybrids had significantly higher late blight resistance than that of S. nigrum while six clones had resistance similar to that of S. nigrum (Zimnoch-Guzowska et al. 2003).
Thieme et al. (2004) produced symmetric interspecific somatic hybrids between wild Solanum species, which belong to the series Pinnatisecta, Etuberosa and Solanum tuberosum cvs or potato breeding clones, that were resistant to foliage and tuber blight. Similarly, when interspecific hybrids produced by protoplast electrofusion of potato cv Delikat (S. tuberosum) and S. tarnii, a wild diploid tuber-bearing Mexican species were backcrossed with cv Delikat, most of the somatic hybrids exhibited high levels of foliage blight resistance (Thieme et al. 2008). Szczerbakowa et al. (2010) generated interspecific somatic hybrids between a diploid clone DG81-68 (susceptible to P. infestans) and Solanum x michoacanum (a resistant diploid tuber-bearing species) that showed variations in late blight resistance with enhanced resistance characteristics for three tetraploid hybrids. Interspecific somatic hybrids produced between the dihaploid S. tuberosum and the wild species S. pinnatisectum via protoplast fusion showed high foliage blight resistance (Sarkar et al. 2011). These somatic hybrids possess potential source of late blight resistance and are used for in situ hybridization in potato breeding (Tiwari et al. 2013; Sarkar et al. 2013).
The clones arised from crosses or somatic hybridization with the wild species S. bulbocastanum, S. circaeifolium and S. okadae displayed the lowest values of the AUDPC in comparison to standard varieties (Barquero et al. 2005). Similarly, somatic hybrids obtained from the cell fusion of S. tuberosum and the diploid species S. pinnatisectum, S. cardiophyllum, and S. chacoense showed higher level of blight resistance compared to S. tuberosum, indicating the significance of protoplast system in potato breeding programme (Chen et al. 2008). Nine somatic hybrids (S. pinnatisectum with S. tuberosum) were found to be highly resistant to P. infestans and level of resistance was not related to either ploidy level or type of cytoplasm (Polzerova et al. 2011).
Solanum x michoacanum (mch), a wild diploid (2n = 2× = 24) potato species derived from spontaneous cross of S. bulbocastanum and S. pinnatisectum, has one endosperm balance number (EBN) and resistant to P. infestans. This resistance was introgressed to S. tuberosum by genepool somatic hybridization and somatic hybrids were evaluated for morphological features, flowering, pollen stainability, tuberization and ploidy level, and for late blght resistance. After two seasons of testing, three somatic hybrids and 109 4× mch were found resistant, that forms a promising material for resistance breeding (Smyda et al. 2013).
Somatic hybrid developed from Citrus sinensis cv Hamlin + Poncirus trifoliate cv Flying Dragon had the greatest resistance to Phytophthora (Gan et al. 1995). Similarly, somatic hybrids of sweet orange-lemon parentage produced by protoplast fusion were consistently resistant to Phytophthora (Grosser et al. 1998). Citrus somatic hybrids namely ‘Cleopatra’ mandarin + Sour orange, ‘Rangpur’ lime + Sunki mandarin, ‘Cleopatra’ mandarin + ‘Volkamer’ lemon, ‘Ruby Blood’ sweet orange + ‘Volkamer’ lemon, ‘Rohde Red’ sweet orange + ‘Volkamer’ lemon, and ‘Caipira’ sweet orange + ‘Volkamer’ lemon had less trunk rot occurrence as compared to diploid citrus rootstock; whereas the somatic hybrids ‘Cleopatra’ mandarin + ‘Volkamer’ lemon, Cleopatra’ mandarin + Sour orange, ‘Caipira’ sweet orange + ‘Volkamer’ lemon and ‘Caipira’ sweet orange + ‘Rangpur’ lime were tolerant to root rot (Mourao-Filho et al. 2008). The somatic hybrid ‘Hamlin’ sweet orange + ‘Indian Red’ pummel was tolerant to P. nicotianae (Bassan et al. 2010).
Bu et al. (1993) developed an interspecific somatic hybrid between Nicotiana tabacum and N. rustica by protoplast fusion. After 3 generations of backcrosses to N. tabacum, 5 generations of selfing and some selection, a new breeding line, ((TR13 × Dabarijin 599)BC2S1 × NC82)S4, was obtained which has resistance to black shank (P. nicotanae var. parasitica) disease.
Peronospora
When somatic hybrids derived by fusing protoplasts from N. rustica var. Chlorotica (chlorophyll-deficient) and N. tabacum (albino mutant) were backcrossed to N. tabacum cvs for three generations, it resulted in hybrids resistant to P. tabacina (Pandeya et al. 1986). Sexual barrier between Nicotiana x sanderae and N. debneyi was overcome by protoplast fusion which resulted in asymmetrical nuclear hybridity with loss of chromosomes, although importantly, somatic hybrids were fertile, stable and resistant to P. tabacina (Patel et al. 2011).
Pythium
Somatic hybridization is one of the best alternatives to select heterozygous potato plants through combination of dihaploid genomes that introgressed genes of interest which can be used in agriculture if they are in agreement with agronomic criteria. Protoplast electrofusion of Aminca-Cardinal and Cardinal Nicola resulted in tetraploid (2n = 4× = 48) having improved tolerance to Pythium aphanidermatum during tuber storage (Nouri-Ellouz et al. 2006).
Genome editing
Biotechnology is advancing to the point where it is viable to alter the DNA encoded within a cell and this process is known as genome editing or gene editing. This has provided investigators with the ability to rapidly and economically introduce sequence-specific modifications into the genomes of a broad spectrum of cell types and organisms. Unlike early genetic engineering techniques that randomly insert genetic material into a host genome, genome editing targets insertion to site specific locations. The core of genome editing technology is the use of sequence-specific nucleases for recognizing specific DNA sequences and producing double stranded DNA breaks (DSBs) at targeted sites. DSBs are repaired mainly via two pathways: the non-homologous ending joining (NHE pathway) and the homologous recombination (HR) pathways (Voytas and Gao 2014). Currently, there are four major types of sequence-specific nucleases for genome editing: zinc finger nucleases (ZFNs), transcription activators such as effector nucleases (TALENs), clustered regularly interspaced short palindronic repeats/CRISPR-associated protein (CRISPR/cas) system, and homing endonucleases or meganucleases.
CRISPR is one particular method of gene editing that is showing enormous potential. CRISPR, was first developed in 2012 at the University of California, Berkeley, was adapted from a naturally occurring gene editing system in bacteria. By editing plant genomes, their resistance to biotic/abiotic threats can be increased, leading to higher yields and less dependence on harmful chemical interventions. Host genetic improvement can be accomplished by precise genome editing techniques, such as CRISPR/Cas-9 technology. A recent study revealed that mutation of a single gene in Arabidopsis, DMR6 (downy mildew resistance 6), led to increased salicyclic acid levels and resistance to several plant pathogens, including bacteria and oomycetes (Zeilmaker et al. 2015). Interestingly, the tomato orthologus SIDMR6-1 is also upregulated in response to infection by Pseudomonas syringae pv tomato and Phytophthora capsici. Null mutants of SIDMR6-1 generated via CRISPR/Cas9 system showed resistance to P. syringae, P. capsici and Xanthomonas spp. without detrimental effects on tomato growth and development (de Toledo Thomazella et al. 2016). Together, these results suggest that knocking out DMR6 may be a promising strategy to confer broad spectrum disease resistance to plants. Fang and Tyler (2016) described a CRISPR/Cas9 system enabling rapid and efficient genome editing in P. sojae. They tested the effects of the CRISPR/Cas-9 induced Avr4/6 mutations on P. sojae recognition by plants containing the Rps4 and Rps6 loci. Five homozygous NHEJ and two homozygous HDR mutatnts were inoculated onto hypocotyls of soybean isolines containing Rps4 or Rps6 in Williams and Harosoy backgrounds and observed increased killing of Rps4–or Rps6 containing soybean seedlings in both the backgrounds by the frame shifted mutants (T32-3 & T11-25); though the killing was less as compared to rps plants lacking Rps4/6 plants. These mutants were scored as intermediate on Rps4/6. Similar observations were recorded for other NHEJ mutants containing in-frame deletions. The Avr4/6 mutant having two amino acid deletions (T11-1) showed an intermediate phenotype that was close to fully virulent on Rps4 plants; whereas mutants with single amino acid deletion (18–1 & 18–2) showed intermediate to avirulent phenotypes. Same trend was observed for HDR mutants (T12-1 & T39-1). Thus, they validated the contribution of RXLR effector gene Avr4/6 to recognition by soybean R-gene loci Avr4/6 by efficient disruption and replacement of an effector gene using CRISPR/Cas9.
Oxathiapiprolin is a novel fungicide having excellent efficacy against plant oomycete diseases. But due to its single-site mode of action, it readily selected resistant mutants in P. capsici and P. nicotianae in laboratory (Andreassi et al. 2013; Bittner et al. 2017; Miao et al. 2016). Point mutation (G770V and G839W) in oxysterol binding protein-related protein1 (ORP1) have been detected in oxathiapiprolin-resistant P. capsici isolates (PcORP1). Miao et al. (2018) confirmed mutation in ORP1 by genome editing using CRISPR/Cas-9 in P. capsici and P. sojae.
Fister et al. (2018) targeted the cacao (Theobroma cacao) Non-Expressor of Pathogenesis-Related (TcNPR3) gene, a suppressor of the defence response, using Agrobacterium to introduce CRISPR/Cas 9 system into leaf tissue, and identified the presence of deletion in 27% of TcNPR3 copies in the treated tissues. The edited tissue exhibited an increased resistance to infection with Phytophthora tropicalis and elevated expression of downstream defence genes.
Pettongkhado et al. (2020) identified a glycoprotein (ppal15kDa) from P. palmivora which plays an important role in development and pathogenicity. Mutants generated via CRISPR/Cas-9 mediated gene editing compromised in infectivity on Nicotiana benthamiana and papaya. The mutants were affected in development as they produced smaller sporangia, shorter germ tubes, and fewer appressoria.
Conclusion
It is apparent that biotechnology not only has potential for more rational management of plant diseases but has also started giving dividends in at least some model plant patho systems. In the years to come, biotechnology will play more vital role in multifarious aspects of plant improvement including better management of oomycetes diseases. The resistance can be imparted against oomycetes by host–pathogen mediated resistance, transgenic approach, marker-assisted selection, protoplast fusion and genome editing. Some techniques have been sufficiently refined for their present day use, others need to be undertaken / exploited for plant diseases management in near future.
References
- Ah- Fong A, Judelson HS. Cell cycle regulator Cdc14 is expressed during sporulation but not hyphal growth in the fungus-like oomycete Phytophthora infestans. Mol Microbiol. 2003;50:487–494. doi: 10.1046/j.1365-2958.2003.03735.x. [DOI] [PubMed] [Google Scholar]
- Ah-Fong AMV, Bormann-Chung CA, Judelson HS. Optimization of transgene-mediated silencing in Phytophthora infestans and its association with small interfering RNAs. Fungal Genet Biol. 2008;45(8):1197–1205. doi: 10.1016/j.fgb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Aleza P, Juarez J, Ollitrault P, Navarro L. Polyembryony in non-apomictic citrus genotypes. Ann Bot. 2010;106:533–545. doi: 10.1093/aob/mcq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassi JL, Gutteridge S, Pember SO, Sweigard JA (2013) Detection and screening method and materials useful in performance thereof. International Patent No. Wo2013009971. World Intellactual Property Organization, Geneva
- Austin S, Helgeson JP, Wettstein (1987) Interspecific somatic fusions between Solanum brevidens and S. tuberosum. In: Von D and Chua NH (eds) Plant molecular biology. Proceedings of NATO advanced study institute series A: life sciences (140). Carlsberg Lab., Copenhagen, Denmark, pp 209–222
- Avrova AO, Boevink PC, Young V, Grenvilla-Briggs LJ, van West P, Birch PRJ, Whisson SC. A novel Phytophthora infestans haustorium-specific membrane protein is required for infection in potato. Cell Microbiol. 2008;10(11):2271–2284. doi: 10.1111/j.1462-5822.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- Ballvora A, Ercolano MR, Weiss J, Meksem K, Bormann CA, Oberhagemann P, Salamini F, Gebhardt C. The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/ NBS/LRR class of plant resistant genes. Plant J. 2002;30:361–371. doi: 10.1046/j.1365-313x.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- Barquero M, Gomez L, Brenes A. Resistance to late blight (Phytophthora infestans) in promising potato clones in Costa Rica. Agron Costarric. 2005;29(3):31–45. [Google Scholar]
- Bassan MM, Mourao-Filho F, de AA, Mendes BM J, Freire BFS, Cantuarias- Aviles TE, Beltrame AB (2010) Reaction of citrus somatic hybrids to the infection by Phytophthora nicotianae. Rev Bras Frutic 32(2): 429–435
- Beakes GW, Glocking SL, Sekimoto S. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma. 2012;249:3–19. doi: 10.1007/s00709-011-0269-2. [DOI] [PubMed] [Google Scholar]
- Bellin D, Peressotti E, Merdinoglu D, Wiedemann-Merdinoglu S, Adam-Blondon AF, Cipriani G, Morgante M, Testolin R, Di Gaspero G. Resistance to Plasmopara viticola in grapevine ‘Bianca’ is controlled by a major dominant gene causing localised necrosis at the infection site. Theor Appl Genet. 2009;120:163–176. doi: 10.1007/s00122-009-1167-2. [DOI] [PubMed] [Google Scholar]
- Bittner RJ, Sweigard JA, Mila AL. Assessing the resistance potential of Phytophthora nicotianae, the causal agent of black shank of tobacco, to oxathiapiprolin with laboratory mutants. Crop Prot. 2017;102:63–71. [Google Scholar]
- Bittner-Eddy PD, Crute IR, Holub EB, Beynon JL. RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J. 2000;21:177–188. doi: 10.1046/j.1365-313x.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- Blanco FA, Judelson HS. A bZIP transcription factor from Phytophthora interacts with a protein kinase and is required for zoospore motility and plant infection. Mol Microbiol. 2005;56(3):638–648. doi: 10.1111/j.1365-2958.2005.04575.x. [DOI] [PubMed] [Google Scholar]
- Borhan MH, Gunn N, Cooper A, Gulden S, Tör M, Rimmer SR, Holub EB. WRR4 encodes a TIR-NB-LRR protein that confers broadspectrum white rust resistance in Arabidopsis thaliana to four physiological races of Albugo candida. Mol Plant Microbe Interact. 2008;21:757–768. doi: 10.1094/MPMI-21-6-0757. [DOI] [PubMed] [Google Scholar]
- Bos JI, Armstrong MR, Gilroy EM, Boevink PC, Hein I, Taylor RM, Zhendong T, Engelhardt S, Vetukuri RR, Harrower B, Dixelius C, Bryan G, Sadanandom A, Whisson SC, Kamoun S, Birch P. Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc National Acad Sci USA. 2010;107:9909–9914. doi: 10.1073/pnas.0914408107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella MA, Parker JE, Frost LN, Bittner-Eddy PD, Beynon JL, Daniels MJ, Holub EB, Jones JD. Three genes of the Arabidopsis RPP1 complex resistance locus recognize distinct Peronospora parasitica avirulence determinants. Plant Cell. 1998;10:1847–1860. doi: 10.1105/tpc.10.11.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw JE, Ramsay G. Utilisation of the commonwealth potato collection in potato breeding. Euphytica. 2005;146:9–19. [Google Scholar]
- Bradshaw JE, Bryan GJ, Lees AK, McLean K, Solo-mon-Blackburn RM. Mapping the R10 and R11 genes for resistance to late blight (Phytophthora infestans) present in the potato (Solanum tuberosum) R-gene differentials of Black. Theor Appl Genet. 2006;112:744–751. doi: 10.1007/s00122-005-0179-9. [DOI] [PubMed] [Google Scholar]
- Brown JS, Phillips-Mora W, Power EJ, Krol C, Cervantes-Martinez C, Motamayor JC, Schnell RJ. Mapping QTLs for resistance to frosty pod and black pod diseases and horticultural traits in theobroma cacao L. Crop Sci. 2007;47(5):1851–1858. [Google Scholar]
- Bu GZ, Gong ML, Ding CM, Yao SM, Zhang LL (1993) A new breeding line of tobacco selected by somatic hybridization between N. tabacum and N. rustica. Proc First Asia Pacific Conf Agricul Biotech, Beijing, China, pp 273–278
- Burnham KD, Dorrance AE, VanToai TT, St Martin SK. Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci. 2003;43(5):1610–1671. [Google Scholar]
- Carputo D, Frusciante L (2011) Classical genetics and traditional breeding. In: Bradeen JM, Kole C (eds) Genetics, Genomics and Breeding of Potato, 20—40. CRC Press, New York
- Casagrande K, Falginella L, Castellarin SD, Testolin R, Di Gaspero G. Defence responses in Rpv3-dependent resistance to grapevine downy mildew. Planta. 2011;234:1097–1109. doi: 10.1007/s00425-011-1461-5. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T, Chao E. Phylogeny and megasystematics of phagtrophic heterokonts (Kingdom Chromista) J Mol Evol. 2006;62:388–420. doi: 10.1007/s00239-004-0353-8. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, Singh BP, Thakur G, Tiwari JK, Kaushik SK, Sharma S, Bhardwaj V. QTL analysis of late blight resistance in a diploid potato family of Solanum spegazzinii × S. chacoense. Potato Res. 2014;57:1–11. [Google Scholar]
- Chen Q, Li HY, Shi YZ, Beasley D, Bizimungu B, Goettel MS. Development of an effective protoplast fusion system for production of new potatoes with disease and insect resistance using Mexican wild potato species as gene pools. Can J Plant Sci. 2008;88(4):611–619. [Google Scholar]
- Chowdhury AK, Srinives P, Saksoong P, Tongpamnak P. RAPD markers linked to resistance to downy mildew disease in soybean. Euphytica. 2002;128(1):55–60. [Google Scholar]
- Cogoni C, Macino G. Gene silencing across kingdoms. Curr Opin Genet Dev. 2000;10:638–643. doi: 10.1016/s0959-437x(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Collard BCY, Mackill DJ. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Phil Trans Royal Soc B Biol Sci. 2008;363:557–572. doi: 10.1098/rstb.2007.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Milbourne D, Ramsay L, Meyer R, Chatot-Balandras C, Oberhagemann P, De Jong W, Gebhardt C, Bonnel E, Waugh R. QTL for field resistance to late blight in potato are strongly correlated with maturity and vigour. Mol Breed. 1999;5:387–398. [Google Scholar]
- Colton LM, Groza HI, Wielgus SM, Jiang J. Marker-assisted selection for the broad-spectrum potato late blight resistance conferred by gene RB derived from a wild potato species. Crop Sci. 2006;46:589–594. [Google Scholar]
- Crouzillat D, Phillips W, Fritz PJ, Petiard V. Quantitative trait loci analysis in Theobroma cacao using molecular markers. Inheritance of polygenic resistance to Phytophthora palmivora in two related cacao populations. Polygenic resistance to Phytophthora palmivora in cacao. Euphytica. 2000;14(1):25–36. [Google Scholar]
- De Jong W, Forsyth A, Leister D, Gebhardt C, Baulcombe DC. A potato hypersensitive resistance gene against potato virus X maps to a resistance gene cluster on chromosome 5. Theor Appl Genet. 1997;95:246–252. [Google Scholar]
- De Toledo Thomazella DP, Brail Q, Dahlbeck D, Staskawicz BJ. CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad spectrum disease resistance. BioRxiv. 2016 doi: 10.1101/064824. [DOI] [Google Scholar]
- Demirbas A, Rector BG, Lohnes DG, Fioritto RJ, Graef GL, Cregan PB, Shoemaker RC, Specht JE. Simple sequence repeat markers linked to the soybean Rps genes for Phytophthora resistance. Crop Sci. 2001;41(4):1220–1227. [Google Scholar]
- Dong S, Yu D, Cui L, Qutob D, Tedman-Jones J, Kale SD, Tyler BM, Wang Y, Gijzen M. Sequence variants of the Phytophthora sojae RXLR effector Avr3al5 are differentially recognized by Rps3a and Rps5 in soybean. PLoS ONE. 2011;6:e20172. doi: 10.1371/journal.pone.0020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker F, Trutzenberg A, Rothenpieler JS, Kuhn S, Prols R, Schreiber T, Tissier A, Kemen A, Kemen E, Huckelhoven R, Weiberg A. Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. Life. 2020;9:e56096. doi: 10.7554/eLife-56096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kharbotly A, Palomino Sanchez C, Salamini F, Jacobsen E, Gebhardt C. R6 and R7 alleles of potato conferring race-specific resistance to Phytophthora infestans (Mont.) de Bary identified genetic loci clustering with the R3 locus on chromosome XI. Theor Appl Genet. 1996;92:880–884. doi: 10.1007/BF00221901. [DOI] [PubMed] [Google Scholar]
- Essmann J, Schmitz-Thom I, Schon H, Sonnewald S, Weis E, Scharte J. RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 2008;147(3):1288–1299. doi: 10.1104/pp.108.121418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Tyler BM. Efficient disruption and replacement of an effector gene in the oomycete Phytophthora sojae using CRISPR/Cas9. Mol Pl Pathol. 2016;17(1):127–139. doi: 10.1111/mpp.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinho M, Coelho P, Carlier J, et al. Mapping of a locus for adult plant resistance to downy mildew in broccoli (Brassica oleracea convar. italica) Theor Appl Genet. 2004;109:1392–1398. doi: 10.1007/s00122-004-1747-0. [DOI] [PubMed] [Google Scholar]
- Fawke S, Doumane M, Schornack S. Oomycete interactions with plants: infection strategies and resistance principles. Microbiol Mol Biol Rev. 2015;79:263–280. doi: 10.1128/MMBR.00010-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fister AS, Landherr L, Maximova SN, Guiltinan MJ. Transient-expression of CRISPR/Cas 9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SJ, Park TH, Pel M, Brigneti G, Sliwka J, Jagger L, van der Vossen E, Jones JDG. Rpi-vnt1.1, a Tm-22 homo-log from Solanum venturii, confers resistance to potato late blight. Mol Plant-Microbe Intert. 2009;22:589–600. doi: 10.1094/MPMI-22-5-0589. [DOI] [PubMed] [Google Scholar]
- Fry W. Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol. 2008;9:385–402. doi: 10.1111/j.1364-3703.2007.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Sun Zhong H, Deng Xiu X, Zhang WC. Studies on the resistance of citrus somatic hybrids. Acta Hortic Sin. 1995;22(3):209–214. [Google Scholar]
- Gaulin E, Jauneau A, Villalba F, Rickauer M, Esquerre-Tugaye MT, Bottin A. The CBEL glycoprotein of Phytophthora parasitica var nicotianae is involved in cell wall deposition and adhesion to cellulosic substrates. J Cell Sci. 2002;115:4565–4575. doi: 10.1242/jcs.00138. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Valkonen JPT. Organization of genes controlling disease resistance in the potato genome. Annu Rev Phytopathol. 2001;39:79–102. doi: 10.1146/annurev.phyto.39.1.79. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Ballvora A, Walkemeier B, Oberhagemann P, Scluler K. Assessing genetic potential in germplasm collections of crop plants by marker-trait association: a case study for potatoes with quantitative variation of resistance to late blight and maturity type. Mol Breed. 2004;13:93–102. [Google Scholar]
- Gilroy EM, Breen S, Whisson SC, Squires J, Hein I, Kaczmarek M, Turnbull D, Boevink PC, Lokossou A, Cano LM, Morales J. Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2-like in Phytophthora infestans determine virulence on R2 plants. New PhIytol. 2011;191(3):763–776. doi: 10.1111/j.1469-8137.2011.03736.x. [DOI] [PubMed] [Google Scholar]
- Golas TMA, Sikkema A, Gros J, Feron RMC, van der Berg RG, van der Weerden GM, Mariani C, Allefs JJHM. Identification of a resistance gene Rpi-dlc1 to Phytophthora infestans in European accessions of Solanum dulcamara. Theor Appl Genet. 2010;120:797–808. doi: 10.1007/s00122-009-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TL, Graham MY, Subramanian S, Yu O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisoflavonois suppresses race-specific resistance and hypersensitive cell death in Phytophthora sojae infected tissues. Plant Physiol. 2007;144(2):728–740. doi: 10.1104/pp.107.097865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenville-Briggs LJ, Anderson VL, Fugelstad J, Avrova AO, Bouzenzana J, Williams A, Wawra S, Whisson SC, Birch PRJ, Bulone V, van West P. Cellulose synthesis in Phytophthora infestans is required for normal appressorium formation and successful infection of potato. Plant Cell. 2008;20(3):720–738. doi: 10.1105/tpc.107.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser JW, Gmitter FG, Jr, Castle WS, Chandler JL. Somatic hybridization: a new approach to citrus rootstock improvement. Fruits. 1998;53(5):331–334. [Google Scholar]
- Haas BJ, Kamoun S, Zody MC, Jiang RH, Handsaker RE, Cano LM, Grabherr M, Kodira CD, Raffaele S, Torto-Alalibo T, Bozkurt TO, Ah-Fong AM, Alvarado L, Anderson VL, Armstrong MR, Avrova A, Baxter L, Beynon J, Boevink PC, Bollmann SR, Bo JI, Bulone V, Cai G, Cakir C, Carrington JC, Chawner M, Conti L, Costanzo S, Ewan R, Fahlgren N, et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature. 2009;461:393–398. doi: 10.1038/nature08358. [DOI] [PubMed] [Google Scholar]
- Hai THT, Kim JH, Cho MC, Chae SY, Lee HE. Identification and development of molecular markers linked to Phytophthora root rot resistance in pepper (Capsicum annuum L.) Eur J Plant Pathol. 2013;135(2):289–297. [Google Scholar]
- Hamon C, Baranger A, Coyne CJ, McGee RJ, Goff I, le, L’Anthoene V, Esnault R, Riviere JP, Klein A, Mangin P, PcPhee KE, Roux-Duparque M, Porter L, Miteul H, Lesne A, Morin G, Onfroy C, Moussart, A, Tivoli B, Delmourme R, Plet-Nayel M L (2011) New consistent QTL in pea associated with partial resistance to Aphanomyces euteiches in multiple French and American environments. Theor Appl Genet 123(2): 261-–281 [DOI] [PubMed]
- Hein I, Birch PRJ, Danan S, Lefebvre V, Odeny DA, Geb-hardt C, Trognit F, Bryan GL. Progress in mapping and cloning qualitative and quantitative resistance against Phytophthora infestans in potato and its wild relatives. Potato Res. 2009;52:215–227. [Google Scholar]
- Helgeson JP, Hunt GJ, Haberlach GT, Austin S. Somatic hybrids between Solanum brevidens and Solanum tuberosum: expression of a late blight resistance gene and potato leaf roll resistance. Plant Cell Rep. 1986;5(3):212–214. doi: 10.1007/BF00269122. [DOI] [PubMed] [Google Scholar]
- Hermanova V, Barta J, Curn V. Wild potato species: characterization and biological potential for potato breeding. Czech J Genet Plant Breed. 2007;43:73–81. [Google Scholar]
- Horejsi T, Staub JE, Thomas C. Linkage of random amplified polymorphic DNA markers to downy mildew resistance in cucumber (Cucumis sativus L.) Euphytica. 2000;115:105–113. [Google Scholar]
- Hua C, Wang Y, Zheng X, Dou D, Zhang Z, Govers F. A Phytophthora sojae G-protein alpha subunit is involved in chemotaxis to soybean isoflavones. Eukaryot Cell. 2008;7:2133–2140. doi: 10.1128/EC.00286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Vivianne GAA, Vleeshouwers J, Werij S, Ronald C, Hutten B, Van Herman E, Richard GFV, Jacobsen E. The R3 resistance to Phytophthora infestans in potato is conferred by two closely linked R genes with distinct specificities. Mol Plant-Microb Inter. 2004;17:428–435. doi: 10.1094/MPMI.2004.17.4.428. [DOI] [PubMed] [Google Scholar]
- Huang S, van der Vossen EAG, Kuang H, Vleeshouwers VGAA, Zhang N, Borm TJA, van Eck HJ, Baker B, Jacobsen E, Visser RGF. Comparative genomics enabled the cloning of the R3a late blight resistance gene in potato. Plant J. 2005;42:251–261. doi: 10.1111/j.1365-313X.2005.02365.x. [DOI] [PubMed] [Google Scholar]
- Jacobs MMJ, Vosman JB, Vleeshouwers VGAA, Viser RGF, Henken B, van der Berg RG. A novel approach to locate Phytophthora infestans resistance genes on the potato genetic map. Theor Appl Genet. 2010;120:785–796. doi: 10.1007/s00122-009-1199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan SN, Åsman AK, Corcoran P, Fogelqvist J, Vetukuri RR, Dixelius C. Plant-mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans. J Exp Bot. 2015;66:2785–2794. doi: 10.1093/jxb/erv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia ZQ, Cui YH, Li Y, Yang YH, Huang SW, Du YC. Expression the potato late blight resistant gene R3a, R1 and RB in tomato. Acta Hortic Sin. 2009;36:1153–1160. [Google Scholar]
- Jo KR, Arens M, Kim TY, Jongsma MA, Visser RGF, Jacobsen E, Vossen JH. Mapping of the S. demissum late blight resistance gene R8 to a new locus on chromosome IX. Theor Appl Genet. 2011;123:1331–1340. doi: 10.1007/s00122-011-1670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo KR, Visser RGF, Jacobsen E, Vossen JH. Characterisation of the late blight resistance in potato differential MaR9 reveals a qualitative resistance gene, R9a, residing in a cluster of Tm-2 2 homologs on chromosome IX. Theor Appl Genet. 2015;128:931–941. doi: 10.1007/s00122-015-2480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES, Wolff MF, Wernsman EA, Rufty RC. Marker-assisted selection for resistance to black shank disease in tobacco. Plant Dis. 2002;86(12):1303–1309. doi: 10.1094/PDIS.2002.86.12.1303. [DOI] [PubMed] [Google Scholar]
- Judelson HS, Blanco FA. The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol. 2005;3:47–58. doi: 10.1038/nrmicro1064. [DOI] [PubMed] [Google Scholar]
- Judelson HS, Tani S. Transgene-induced silencing of the zoosporogenesis-specific NIFC gene cluster of Phytophthora infestans involves chromatin alterations. Eukaryot Cell. 2007;6:1200–1209. doi: 10.1128/EC.00311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun S, Furzer O, Jonathan DGJ, Judelson HS, Gul SA, Ronaldo JDD, Roy SG, Schena L, Antonios Z, Franck P. The top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol. 2015;16:413–434. doi: 10.1111/mpp.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemen E, Jones JD. Obligate biotroph parasitism: can we link genomes to life styles? Trends Plant Sci. 2012;17:448–457. doi: 10.1016/j.tplants.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee HR, Jo KR, Mortazavian SMM, Huigen DJ, Evenhuis B, Kessel G, Visser RGF, Jacobsen E, Vossen JH. Broad spectrum late blight resistance in potato differential set plants MaR8 and MaR9 is conferred by multiple stacked R genes. Theor Appl Genet. 2012;124:923–935. doi: 10.1007/s00122-011-1757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasileva KV, Dahlbeck D, Staskawicz BJ. Activation of an Arabidopsis resistance protein is specified by the in planta association of its leucine-rich repeat domain with the cognate oomycete effector. Plant Cell. 2010;22:2444–2458. doi: 10.1105/tpc.110.075358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latijnhouwers M, Govers F. A Phytophthora infestans G-protein beta subunit is involved in sporangium formation. Eukaryot Cell. 2003;2(5):971–977. doi: 10.1128/EC.2.5.971-977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latijnhouwers M, Ligterink W, Vleeshouwers VG, van West P, Grovers F. A alpha subunit controls zoospore motility and virulence in the potato late blight pathogen Phytophthora infestans. Mol Microbiol. 2004;51:925–936. doi: 10.1046/j.1365-2958.2003.03893.x. [DOI] [PubMed] [Google Scholar]
- Leonards-Schippers C, Gieffers W, Salamini F, Gebhardt C. The R1 gene conferring race specific resistance to Phytophthora infestans in potato is located on potato chromosome V. Mol Gen Genet. 1992;233:278–283. doi: 10.1007/BF00587589. [DOI] [PubMed] [Google Scholar]
- Lerceteau-Kohler E, Moing A, Guerin G, Maucourt M, Renaud C, Rolin D, Roudeillac P, Denoyes-Rothan B. QTL analysis for fruit quality traits and resistance to Colletotrichum acutatum and Phytophthora cactorum in octoploid strawberry (Fragaria × ananassa) Acta Hortic. 2004;649:93–97. [Google Scholar]
- Li X, van Eck HJ, Rouppe van der Voort JNAM, Huigen DJ, Stam P, Jacobsen E. Autotetraploids and genetic mapping using common AFLP markers: the R2 allele conferring resistance to Phytophthora infestans mapped on potato chromosome 4. Theor Appl Genet. 1998;96:1121–1128. [Google Scholar]
- Li XP, Han YP, Teng W, Zhang SZ, Yu KF, Poysa V, Anderson T, Ding JJ, Li WB. Pyramided QTL underlying tolerance to Phytophthora root rot in mega environments from soybean cultivars ‘Conrad’ and ‘Hefeng 25’. Theor Appl Genet. 2010;121(4):651–658. doi: 10.1007/s00122-010-1337-2. [DOI] [PubMed] [Google Scholar]
- Li G, Huang S, Guo X, Li Y, Yang Y, Guo Z, Kuang H, Rietman H, Bergervoet M, Vleeshouwers VGAA, van der Vossen E, Qu D, Visser RGF, Jacobsen E, Vossen JH. Cloning and characterization of R3b; members of the R3 superfamily of late blight resistance genes show sequence and functional divergence. Mol Plant Microbe Inter. 2011;24:1132–1142. doi: 10.1094/MPMI-11-10-0276. [DOI] [PubMed] [Google Scholar]
- Li L, Tacke E, Lu J, Walkemeier B, Gebhardt C. Validation of candidate gene markers for marker-assisted selection of potato cultivars with improved tuber quality. Theor Appl Genet. 2013;126:1039–1052. doi: 10.1007/s00122-012-2035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun S, Zhong C, Wang X, Wu X, Zhu Z. Genetic mapping and development of co-segregating markers of RpsQ, which provides resistance to Phytophthora sojae in soybean. Theor Appl Genet. 2017;130(6):1223–1233. doi: 10.1007/s00122-017-2883-7. [DOI] [PubMed] [Google Scholar]
- Lim S, Bernard R, Nickell C, Grey L. New physiological race of Peronospora manschurica virulent to the gene Rpm in soybeans. Plant Dis. 1984;68:71–72. [Google Scholar]
- Liu Z, Halterman D. Identification and characterization of RB-orthologous genes from the late blight resistant wild potato species Solanum verrucosum. Physiol Mol Plant Pathol. 2006;69:230–239. [Google Scholar]
- Liu JH, Xu XY, Deng XX. Intergeneric somatic hybridization and its application to crop genetic improvement. Plant Cell Tiss Org. 2005;82:19–44. [Google Scholar]
- Liu T, Ye W, Ru Y, Yang X, Gu B, Tao K, Lu S, Dong S, Zheng X, Shan W, Wang Y, Dou D. Two host cytoplasmic effectors are required for pathogenesis of Phytophthora sojae by suppression of host defenses. Plant Physiol. 2011;155:490–501. doi: 10.1104/pp.110.166470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WY, Kang JH, Jeong HS, Choi HJ, Yang HB, Kim KT, Choi D, Choi GJ, Jahn M, Kang BC. Combined use of bulked segregant analysis and microarrays reveals SNP markers pinpointing a major QTL for resistance to Phytophthora capsici in pepper. Theor Appl Genet. 2014;127(11):2503–2513. doi: 10.1007/s00122-014-2394-8. [DOI] [PubMed] [Google Scholar]
- Lokossou A, Park TH, Van Arkel G, Arens M, Ruyter-Spira C, Morales J, Steve C, Whisson SC, Birch PRJ, Visser RGF, Jacobsen E, VanderVossen EAG. Exploiting knowledge of R/Avr genes to rapidly Clone a new LZ-NBS-LRR family of late blight resistance genes from potato linkage group IV. Mol Plant-Microbe Inter. 2009;22:630–641. doi: 10.1094/MPMI-22-6-0630. [DOI] [PubMed] [Google Scholar]
- Louwes KM, Hoekstra R, Mattheij WM. Interspecific hybridization between the cultivated potato Solanum tuberosum subspecies tuberosum L. and the wild species S. circaeifolium subsp. circaeifolium Bitter exhibiting resistance to Phytophthora infestans (Mont.) de Bary and Globodera pallida (Stone) Berhrens. 2. Sexual Hybrids Theor Appl Genet. 1992;84:362–370. doi: 10.1007/BF00229495. [DOI] [PubMed] [Google Scholar]
- Mattheji WM, Louwes KM, Eijlander RE, de Koning JRA, Puite KJ. Transfer of resistance to Phytophthora infestans and Globodera pallida to the potato gene pool by interspecific somatic hybridization. Physiol Plant. 1991;82(1):A23. [Google Scholar]
- Matzke MA, Mette MF, Matzke AJ. Transgene silencing by the host genome defense: implications for the evolution of epigenetic control mechanisms in plants and vertebrates. Plant Mol Biol. 2000;43:401–415. doi: 10.1023/a:1006484806925. [DOI] [PubMed] [Google Scholar]
- McDowell JM, Dhandaydham M, Long TA, Aarts MG, Goff S, Holub EB, Dangl JL. Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell. 1998;10:1861–1874. doi: 10.1105/tpc.10.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meksem K, Leister D, Peleman J, Zabeau M, Salamini F, Gebhardt C. A high-resolution map of the vicinity of the R1 locus on chromosome V of potato based on RFLP and AFLP markers. Mol Gen Genet. 1995;249:74–81. doi: 10.1007/BF00290238. [DOI] [PubMed] [Google Scholar]
- Merdinoglu D, Wiedemann-Merdinoglu S, Coste P, Dumas V, Haetty S, Butterlin G, Greif C (2003) Genetic analysis of downy mildew resistance derived from Muscadinia rotundifolia. pp 451–456. In: Proceedings of the 8th International Conference on Grape Genetics and Breeding, Kecskemet, Hungary, 26 August 2002 to 31 August 2002.
- Miao JQ, Cai M, Dong X, Liu L, Lin D, Zhang C, Pang ZL, Liu XL. Resistance assessment for oxathiapiprolin in Phytophthora capsici and the deletion of a point mutation (G769W) in PcORP1 and confers resistance. Front Microbiol. 2016;7:615. doi: 10.3389/fmicb.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Chi Y, Lin D, Tyler BM, Liu X. Mutations in ORP1 conferring oxathiapiprolin resistance confirmed by genome editing using CRISPR/Cas 9 in P. capsici and P. sojae. Phytopathology. 2018;108:1412–1419. doi: 10.1094/PHYTO-01-18-0010-R. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Tsuro M, Kubo T, Hirai M. QTL analysis for resistance to Phytophthora capsici in pepper using a high density SSR-based map. Breed Sci. 2007;57(2):129–134. [Google Scholar]
- Mori K, Sakamoto Y, Mukojima N, Tamiya S, Nakao T, Ishii T, Hosaka K. Development of a multiplex PCR method for simultaneous detection of diagnostic DNA markers of five diseases and pests resistance genes in potato. Euphytica. 2011;180:347–355. [Google Scholar]
- Mourao-Filho F, de AA, Pio R, Mendes BMJ, Azevedo FAde, Schinor EH, Entelmann FA, Alves ASR, Cantuarias TE (2008) Evaluation of citrus somatic hybrids for tolerance to Phytophthora nicotianae and citrus tristeza virus. Sci Hortic 115(3): 301-308
- Na R, Yu D, Qutob D, Zhao J, Gijzen M. Deletion of the Phytophthora sojae avirulence gene Avr1d causes gain of virulence on Rps1d. Mol Plant Microbe Interact. 2013;26:969–976. doi: 10.1094/MPMI-02-13-0036-R. [DOI] [PubMed] [Google Scholar]
- Naess SK, Bradeen JM, Wielgus SM, Haberlach GT, McGrath JM, Helgeson JP. Analysis of the introgression of Solanum bulbocastanum DNA into potato breeding lines. Mol Genet Genom. 2001;265:694–704. doi: 10.1007/s004380100465. [DOI] [PubMed] [Google Scholar]
- Niblett CL, Bailey AM. Potential applications of gene silencing or RNA interference (RNAi) to control disease and insect pests of date palm. Emir J Food Agr. 2012;24:462–469. [Google Scholar]
- Nouri-Ellouz O, Gargouri-Bouzid R, Sihachakr D, Triki MA, Ducreux G, Drira N, Lakhoua L. Production of potato intraspecific somatic hybrids with improved tolerance to PVY and Pythium aphanidermatum. J Plant Physiol. 2006;163(12):1321–1332. doi: 10.1016/j.jplph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Nowara D, Gay A, Laccome C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P. HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell. 2010;22:3130–3141. doi: 10.1105/tpc.110.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhagemann P, Chatot-Balandras C, Schafer-Pregl R, Wegener D, Palomino C, Salamini F, Bonnel E, Gebhardt C (1999) A genetic analysis of quantitative resistance to late blight in potato: towards marker-assisted selection. Mol Breed 5: 399-415
- Oosumi T, Rockhold DR, Maccree MM, Deahl KL, McCue KF, Belknap WR. Gene Rpi-bt1 from Solanum bulbocastanum confers resistance to late blight in transgenic potatoes. Am J Potato Res. 2009;86:456–465. [Google Scholar]
- Pandeya RS, Douglas GC, Keller WA, Setterfield G, Patrick ZA. Somatic hybridization between Nicotiana rustica and N. tabacum: development of tobacco breeding strains with disease resistance and elevated nicotine content. Zeit Pflanzen. 1986;96(4):346–352. [Google Scholar]
- Pang X, Zhou XH, Wan HJ, Chen JF. QTL mapping of downy mildew resistance in an introgression line derived from interspecific hybridization between cucumber and Cucums hystrix. J Phytopathol. 2013;161(7/8):536–543. [Google Scholar]
- Park TH, Vleeshouwers GAA, Hutten RCB, van Eck HJ, van der Vossen E, Jacobsen E, Visser RGF. High-resolution mapping and analysis of the resistance locus Rpi-abpt against Phytophthora infestans in potato. Mol Breed. 2005;16:33–43. [Google Scholar]
- Parker JE, Coleman MJ, Szabò V, Frost LN, Schmidt R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JD. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the Toll and interleukin-1 receptors with N and L6. Plant Cell. 1997;9:879–894. doi: 10.1105/tpc.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Power JB, Anthony P, Badakshi F, Heslop-Harrison JS, Davey MR. Somatic hybrid plants of Nicotiana × sanderae (+) N. debneyi with fungal resistance to Peronospora tabacina. Ann Bot. 2011;108(5):809–819. doi: 10.1093/aob/mcr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pel MA, Foster SJ, Park TH, Rietman H, van Arkel G, Jones JDG, Eck HJV, Jacobsen E, Visser RGF, van der Vossen EAG. Mapping and cloning of late blight resistance genes from Solanum venturii using an interspecific candidate gene approach. Mol Plant-Microbe Inter. 2009;22:601–615. doi: 10.1094/MPMI-22-5-0601. [DOI] [PubMed] [Google Scholar]
- Pettongkhao S, Navet N, Schornack S, Tian M, Churngchow N. A secreted protein of 15kDa plays an important role in Phytophthora palmivora development and pathogenicity. Sci Rep. 2020;10:2319. doi: 10.1038/s41598-020-59007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet-Nayel ML, Muehlbauer FJ, McGee RJ, Kraft JM, Baranger A, Coyne CJ. Quantitative trait loci for partial resistance to Aphanomyces root rot in pea. Theor Appl Genet. 2003;106(1):28–39. doi: 10.1007/s00122-002-0985-2. [DOI] [PubMed] [Google Scholar]
- Pilet-Nayel ML, Muehlbauer FJ, McGee RJ, Kraft JM, Baranger A, Coyne CJ. Consistent quantitative trait loci in pea for partial resistance to Aphanomyces euteiches isolates from the United States and France. Phytopathology. 2005;95(11):1287–1293. doi: 10.1094/PHYTO-95-1287. [DOI] [PubMed] [Google Scholar]
- Polzerova H, Patzak J, Greplova M. Early characterization of somatic hybrids from symmetric protoplast electrofusion of Solanum pinnatisectum Dun. and Solanum tuberosum L. Plant Cell Tissu Org. 2011;104(2):163–170. [Google Scholar]
- Prins M, de Haan P, Luyten R, van Veller M, van Grinsven MQ, Goldbach R. Broad resistance to tospoviruses in transgenic tobacco plants expressing three tospoviral nucleoprotein gene sequences. Mol Plant-Microbe Inter. 1995;8:85–91. doi: 10.1094/mpmi-8-0085. [DOI] [PubMed] [Google Scholar]
- Prins M, Laimer M, Noris E, Schubert J, Wassenegger M, Tepfer M. Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol. 2008;9:73–83. doi: 10.1111/j.1364-3703.2007.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin EA, Ogundiwin EA, Prince JP, Mazourek M, Briggs MO, Chlanda TS, Kim KT, Falise M, Kang BC, Jahn MM (2005) Development of sequence characterized amplified region (SCAR) primers for the detection of Phyto 5.2, a major QTL for resistance to Phytophthora capsici Leon. in pepper. Theor Appl Genet 110(4): 605–612. [DOI] [PubMed]
- Qutob D, Tedman-Jones J, Dong S, Kuflu K, Pham H, Wang Y, Dou D, Kale SD, Arredondo FD, Tyler BM, Gijzen M. Copy number variation and transcriptional polymorphisms of Phytophthora sojae RXLR effector genes Avr1a and Avr3a. PLoS ONE. 2009;4:e5066. doi: 10.1371/journal.pone.0005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Farrer RA, Cano LM, Studholme DJ, MacLean D, Thines M, Jiang RH, Zody MC, Kunjeti SG, Donofrio NM, Meyers BC. Genome evolution following host jumps in the Irish potato famine pathogen lineage. Science. 2010;330:1540–1543. doi: 10.1126/science.1193070. [DOI] [PubMed] [Google Scholar]
- Rietman H (2011) Putting the Phytophthora infestans genome sequence at work: identification of many new R and Avr genes in Solanum. Ph.D. theiss. Wageningen University, Wageningen, the Netherlands
- Rokka VM, Xu YS, Kankila J, Kuusela A, Pulli S, Pehu E. Identification of somatic hybrids of dihaploid Solanum tuberosum lines and S. brevidens by species specific RAPD patterns and assessment of disease resistance of the hybrids. Euphytica. 1994;80(3):207–217. [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu D, Schallock KG, Rivera-Velez N, Lundeen P, Cianzio S, Bhattacharyya MK. Soybean Phytophthora resistance gene Rps8 maps closely to the Rps3 region. J Hered. 2005;96(5):536–541. doi: 10.1093/jhered/esi081. [DOI] [PubMed] [Google Scholar]
- Sanju S, Siddappa S, Thakur A, Shukla PK, Srivastava N, Pattanayak D, Sharma S, Singh BP. Host-mediated gene silencing of a single effector gene from the potato pathogen Phytophthora infestans imparts potential resistance to late blight disease. Funct Integr Genom. 2015;15(6):697–706. doi: 10.1007/s10142-015-0446-z. [DOI] [PubMed] [Google Scholar]
- Sanju S, Thakur A, Siddappa S, Sharma S, Shukla PK, Srivastava N, Pattanayak D, Singh BP. In-vitro detached leaf assay of host-mediated RNAi lines carrying Phytophthora infestans Avr3a effector gene for late blight resistance. Potato J. 2016;43(1):30–37. [Google Scholar]
- Sarkar D, Tiwari JK., Sharma S, Poonam, Sharma S, Gopal J, Singh BP, Luthra SK, Pandey SK, Pattanayak D (2011) Production and characterization of somatic hybrids between Solanum tuberosum L. and S. pinnatisectum Dun. Plant Cell Tiss Org 107(3): 427–440.
- Sarkar D, Tiwari JK, Sharma S, Poonam, Sharma S, Gopal J, Singh BP, Luthra SK, Pandey SK, Pattanayak D (2013) P-7 (IC0590090; INGR11051), a potato (Solanum tuberosum (+) S. pinnatisectum tetraploid, somatic male fertile hybrid conferring resistance to potato late blight introgressed from S. pinnatisectum. Indian J Plant Genetic Resour 26(1): 93–94.
- Scharte J, Schon H, Tjaden Z, Weis E, Schaewen A. Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proc Natl Acad Sci, USA. 2009;106(19):8061–8066. doi: 10.1073/pnas.0812902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten OE, Heusden AW, Khrustaleva LI, Burger-Meijer K, Mank RA, Antonise RGC, Harrewijn JL, Haecke W, Oost EH, Peters RJ, Kik C. The long and winding road leading to the successful introgression of downy mildew resistance into onion. Euphytica. 2007;156(3):345–353. [Google Scholar]
- Schwander F, Eibach R, Fechter I, Hausmann L, Zyprian E, Töpfer R. Rpv10: a new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor Appl Genet. 2012;124:163–176. doi: 10.1007/s00122-011-1695-4. [DOI] [PubMed] [Google Scholar]
- Shan W, Cao M, Leung D, Tyler BM. The Avr1b locus of Phutophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Mol Plant-Microbe Inter. 2004;17:394–403. doi: 10.1094/MPMI.2004.17.4.394. [DOI] [PubMed] [Google Scholar]
- Shandil RK, Chakrabarti SK, Singh BP, Sharma S, Sundaresha S, Kaushik SK, Bhatt AK, Sharma NN. Genotypic background of the recipient plant is crucial for conferring RB gene mediated late blight resistance in potato. BMC Genet. 2017;18:1–8. doi: 10.1186/s12863-017-0490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Bhardwaj V, Dalamu D, Kaushik SK, Singh BP, Sharma S, Umamaheshwari R, Baswaraj R, Kumar V, Gebhardt C. Identification of elite potato genotypes possessing multiple disease resistance genes through molecular approaches. Sci Hortic. 2014;179:204–211. [Google Scholar]
- Sliwka J, Jaknczum H, Lebecka R, Marczewski W, Gebrhardt C, Zimnoch-Guzowska E. The novel, major locus Rpi-phu 1 for late blight resistance maps to potato chromosome IX and is not correlated with long vegetation period. Theor Appl Genet. 2006;113:685–695. doi: 10.1007/s00122-006-0336-9. [DOI] [PubMed] [Google Scholar]
- Smilde WD, Brigneti G, Jagger L, Perkins S, Jones JD. S. mochiquense chromosome IX carries a novel late blight resistance gene Rpi-moc1. Theor Appl Genet. 2005;110:252–258. doi: 10.1007/s00122-004-1820-8. [DOI] [PubMed] [Google Scholar]
- Smyda P, Jakuczun H, Debski K, Sliwka J, Thieme R, Nachtigall M, Wasilewicz-Flis I, Zimnoch-Guzowska E (2013) Development of somatic hybrids Solanum x michoacanum Bitter. (Rydb.) (+) S. tuberosum L. and autofused 4x S. michoacanum plants as potential sources of late blight resistance for potato breeding. Plant Cell Rep 32(8): 1231–1241. [DOI] [PMC free article] [PubMed]
- Sohn KH, Lei R, Nemri A, Jones JD. The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell. 2007;19:4077–4090. doi: 10.1105/tpc.107.054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova E, Pankin A, Beketova M, Kuznetsova M, Spiglazova S, Rogozina E, Yashina I, Khavkin E. SCAR markers of the R-genes and germplasm of wild Solanum species for breeding late blight-resistant potato cultivars. Plant Genet Resour. 2011;9:309–312. [Google Scholar]
- Song J, Bradeen JM, Naess SK, Raasch JA, Wielgus SM, Haberlach GT, Liu J, Kuang H, Austin-Phillips S, Buel CR, Helgeson JP, Jiang J. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc Natl Acad Sci, USA. 2003;100:9128–9133. doi: 10.1073/pnas.1533501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Singh BP, Kaushik SK, Bhardwaj V, Tiwari JK, Sharma S. Identification of late blight resistance gene homologous in wild Solanum species. Proc Natl Acad Sci India Sect B Biol Sci. 2016;88(2):789–796. [Google Scholar]
- Subramaniam S, Graham MY, Yu O, Graham TL. RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiol. 2005;137(4):1345–1353. doi: 10.1104/pp.104.057257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita T, Yamaguchi K, Kinoshita T, Yuji K, Sugimura Y, Nagata R, Kawasaki S, Todoroki A. QTL analysis for resistance to Phytophthora blight (Phytophthora capsici Leon.) using an intraspecific double haploid population of Capsicum annuum. Breed Sci. 2006;56(2):137–145. [Google Scholar]
- Sundaresha S, Sharma S, Shandil RK, Sharma S, Thakur V, Bhardwaj V, Kaushik SK, Singh BP, Chakrabarti SK. An insight into downstream analysis of RB gene in F1 RB potato lines imparting field resistance to late blight. Funct Plant Biol. 2018;45(10):1026–1037. doi: 10.1071/FP17299. [DOI] [PubMed] [Google Scholar]
- Szczerbakova A, Tarwacka J, Oskiera M, Jakuczun H, Wielgat B. Somatic hybridization between the diploids of S. × michoacanum and S.tuberosum. Acta Physiol Plant. 2010;32(5):867–873. [Google Scholar]
- Tan MYA, Hutten RCB, Visser RGF, van Eck HJ. The effect of pyramiding Phytophthora infestans resistance genes Rpi-mcd1 and Rpi-ber in potato. Theor Appl Genet. 2010;121:117–125. doi: 10.1007/s00122-010-1295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Sanju S, Sundaresha S, Srivastava N, Shukla PK, Pattanayak D, Sharma S, Singh BP. Artificial microRNA mediated gene silencing of Phytophthora infestans single effector Avr3a gene imparts moderate type of late blight resistance in potato. Plant Pathol J. 2015;14(1):1–12. [Google Scholar]
- Thieme R, Darsow U, Rakosy TL, Kang-Zhen S, Gavrilenko T, Antonova O, Heimbach U, Thieme T. Use of somatic hybridization to transfer resistance to late blight and potato virus Y (PVY) into cultivated potato. Plant Breed Seed Sci. 2004;50:113–118. [Google Scholar]
- Thieme R, Rakosy-Tican E, Gavrilenko T, Antonova O, Schubert J, Nachtigall M, Heimbach U, Thieme T. Novel somatic hybrids (Solanum tuberosum L. + Solanum tarnii) and their fertile BC1 progenies express extreme resistance to potato virus Y and late blight. Theor Appl Genet. 2008;116(5):691–700. doi: 10.1007/s00122-007-0702-2. [DOI] [PubMed] [Google Scholar]
- Tiwari JK, Poonam KV, Singh BP, Sharma S, Luthra SK, Bhardwaj V. Evaluation of potato somatic hybrid of dihaploid Solanum tuberosum (+) S. pinnatisectum for late blight resistance. Potato J. 2013;40(2):176–179. [Google Scholar]
- Tiwari JK, Devi S, Sharma S, Chandel P, Rawat S, Singh BP. Allele mining in Solanum germplasm: cloning and characterization of RB-homologous gene fragments from late blight resistant wild potato species. Plant Mol Biol Rep. 2015;33(5):1584–1598. [Google Scholar]
- Tör M, Holub E, Brose E, Musker R, Gunn N, Can C, Crute I, Beynon J. Map positions of three loci in Arabidopsis thaliana associated with isolate-specific recognition of Peronospora parasitica (downy mildew) Mol Plant Microbe Interact. 1994;7:214–222. [Google Scholar]
- Toxopeus HJ. Reflections on the origin of new physiologic races in Phytophthora infestans and the breeding for resistance in potatoes. Euphytica. 1956;5:221–237. [Google Scholar]
- Tyler BM. Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu Rev Phytopathol. 2002;40:137–167. doi: 10.1146/annurev.phyto.40.120601.125310. [DOI] [PubMed] [Google Scholar]
- van der Vossen EAG, Sikkema ABL, Hekkert J, Gros P, Stevens M, Muskens D, Wouters A, Pereira W, Stiekema AS. An ancient R gene from the wild potato species Solanum bulbocastanum confers broad-spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J. 2003;36:867–882. doi: 10.1046/j.1365-313x.2003.01934.x. [DOI] [PubMed] [Google Scholar]
- van der Vossen EAG, Gros J, Sikkema A, Muskens M, Wouters D, Wolters P, Pereira A, Allefs S. The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog confer-ring broad spectrum late blight resistance in potato. Plant J. 2005;44:208–222. doi: 10.1111/j.1365-313X.2005.02527.x. [DOI] [PubMed] [Google Scholar]
- van West P, Kamoun S, van’t Klooster JW, Govers F (1999) Internuclear gene silencing in Phytophthora infestans. Mol Cell 3: 339–348 [DOI] [PubMed]
- Venuti S, Copetti D, Foria S, Falginella L, Hoffmann S, Bellin D, Cindric P, Kozma P, Scalabrin S, Morgante M, Testolin R, Gaspero GD. Historical introgression of the downy mildew resistance gene Rpv12 from the Asian species Vitis amurensis into grapevine varieties. PLoS ONE. 2013;8(4):e61228. doi: 10.1371/journal.pone.0061228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzaux E (2010) Resistance and susceptibility to late blight in Solanum: gene mapping cloning and stacking. Ph.D Thesis, Wageningen University, The Netherlands
- Vetukuri RR, Avrova AO, Grenville-Briggs LJ, van West P, Soderbom F, Savenkov EI, Whisson SC, Dixelius C. Evidence for involvement of dicer-like, argonaute and histone deacetylase proteins in gene silencing in Phytophthora infestans. Mol Plant Pathol. 2011;12:772–785. doi: 10.1111/j.1364-3703.2011.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetukuri RR, Tian Z, Avrova AO, Savenkov EI, Dixelius C, Whisson SC. Silencing of the PiAvr3a effector encoding gene from Phytophthora infestans by transcriptional fusion to a short interspersed element. Fungal Biol. 2011;115:1225–1233. doi: 10.1016/j.funbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Vleeshouwers VGAA, Rietman H, Krenek P, Champouret N, Young C, Oh SK, Wang M, Bouwmeester K, Vosman B, Visser RGF, Jacobsen E, Govers F, Kamoun S, van der Vossen EAG. Effector genomics accelerates discovery and functional pro-filing of potato disease resistance and Phytophthora infestans avirulence genes. PLoS ONE. 2008;3:e2875. doi: 10.1371/journal.pone.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen JH, van Arkel G, Bergervoet M, Jo KR, Jacobsen E, Visser RG. The Solanum demissum R8 late blight resistance gene is an Sw-5 homologue that has been deployed worldwide in late blight resistant varieties. Theor Appl Genet. 2016;129:1785–1796. doi: 10.1007/s00122-016-2740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas DF, Gao C. Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol. 2014;12:e1001877. doi: 10.1371/journal.pbio.1001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CA, Koppe M, Grenville-Briggs LJ, Avrova AO, Horner NR, McKinnon AD, Whisson SC, Birch PRJ, van West P. A putative DEAD-box RNA-helicase is required for normal zoospore development in the late blight pathogen Phytophthora infestans. Fungal Genet Biol. 2008;45(6):954–962. doi: 10.1016/j.fgb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dou D, Wang X, Li A, Sheng A, Hua C, Cheng B, Chen X, Zheng X, Wang Y. The PsCZF1 gene encoding a C2H2 zinc finger protein is required for growth, development and pathogenesis in Phytophthora sojae. Microbiol Pathogen. 2009;47:78–86. doi: 10.1016/j.micpath.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li A, Wang X, Zhang X, Zhao W, Dou D, Zheng Y, Wang Y. GPR11, a putative seven-transmembrane G protein-coupled receptor, controls zoospore development and virulence of Phytophthora sojae. Eukaryot Cell. 2010;9(2):242–250. doi: 10.1128/EC.00265-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang L, Guo J, Yang W, Shen H. Molecular mapping of a gene conferring resistance to Phytophthora capsici Leonian race 2 in pepper line PI201234 (Capsicum annuum L.) Mol Breed. 2016;36(6):66. [Google Scholar]
- Weber CA, Pattison J, Samuelian S. Marker assisted selection for resistance to root rot in red raspberry caused by Phytophthora fragariae var. rubi. Acta Hortic. 2008;777:311–316. [Google Scholar]
- Whisson SC, Avrova AO, Lavrova O, Pritchard L. Families of short interspersed elements in the genome of the oomycete plant pathogen, Phytophthora infestans. Fungal Genet Biol. 2005;42:351–365. doi: 10.1016/j.fgb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Whisson SC, Vetukuri RR, Avrova AO, Dixelius C. Can silencing of transposons contribute to variation in effector gene expression in Phytophthora infestans? Mob Genet Elements. 2012;2:110–114. doi: 10.4161/mge.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, Shaw D. Breeding for host resistance: the key to sustainable potato production. PPO-Special Rep. 2010;14:125–130. [Google Scholar]
- Wickramasinghe WMDK, Qu XS, Costanzo S, Haynes KG, Christ BJ. Development of PCR-based markers linked to quantitative resistance to late blight in a diploid hybrid potato population of S. phureja × S. stanotomum. Am J Potato Res. 2009;86:188–195. [Google Scholar]
- Wu XL, Zhang BQ, Shi S, Zhao JM, Feng Y, Na G, Gai JY, Han X. Identification, genetic analysis and mapping of resistance to Phytophthora sojae of Pm28 in soybean. Agric Sci China. 2011;10(10):1506–1511. [Google Scholar]
- Xu X, Chao J, Cheng X, Wang R, Sun B, Wang H, Luo S, Xu X, Wu T, Li Y. Mapping of a novel race specific resistance gene to phytophthora root rot of pepper (Capsicum annuum) using bulked segregant analysis combined with specific length amplified fragment sequencing strategy. PLoS ONE. 2016;11(3):e0151401. doi: 10.1371/journal.pone.0151401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang D, Xu Y, Zhao H, Wang L, Cao X, Chen Y, Chen Q. A new resistance gene against potato late blight originating from Solanum pinnatisectum located on potato chromosome 7. Front Plant Sci. 2017;8:1729. doi: 10.3389/fpls.2017.01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Jugenson J, Hulbert S. Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiforms f.sp. tritici. Mol Plant-Microbe Interact. 2010;24:554–561. doi: 10.1094/MPMI-10-10-0229. [DOI] [PubMed] [Google Scholar]
- Zeilmaker T, Ludwig NR, Elberse J, Seidl MF, Berke L, Van Doorn A, Schuurink RC, Snel B, Van den Ackerveken G. Downy mildew resistant 6 and DMR 6-Like oxygenase 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J. 2015;81:210–222. doi: 10.1111/tpj.12719. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu MM, Miao H, Zhang SQ, Yang Y, Xie B, Wehner T, Gu XF. Chromosomal mapping and QTL analyses of resistance to downy mildew in Cucumis sativus. Plant Dis. 2013;97:245–251. doi: 10.1094/PDIS-11-11-0941-RE. [DOI] [PubMed] [Google Scholar]
- Zhao W, Dong S, Ye W, Hua C, Meijer HJG, Dou X, Govers F, Wang Y. Genome-wide identification of Phytophthora sojae SNARE genes and functional characterization of the conserved SNARE PsYKT6. Fungal Genet Biol. 2011;48:241–251. doi: 10.1016/j.fgb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Zimnoch-Guzowska E, Lebecka R, Krysczuk A, Maciejewska U, Szczerbakowa A, Wielgat B. Resistance to Phytophthora infestans in somatic hybrids of Solanum nigrum L. and diploid potato. Theor Appl Genet. 2003;107:l43–148. doi: 10.1007/s00122-003-1221-4. [DOI] [PubMed] [Google Scholar]