Abstract
Resting and activated subpopulations of CD4+CD25+CD127loT regulatory cells (Treg) and CD4+CD25+CD127+ effector T cells in MS patients and in healthy individuals were compared. Peripheral blood mononuclear cells isolated using Ficoll Hypaque were stained with monoclonal antibodies and analysed by flow cytometer. CD45RA and Foxp3 expression within CD4+ cells and in CD4+CD25+CD127loT cells identified Population I; CD45RA+Foxp3+, Population II; CD45RA−Foxp3hi and Population III; CD45RA−Foxp3+ cells. Effector CD4+CD127+ T cells were subdivided into Population IV; memory /effector CD45RA− CD25−Foxp3− and Population V; effector naïve CD45RA+CD25−Foxp3−CCR7+ and terminally differentiated RA+ (TEMRA) effector memory cells. Chemokine receptor staining identified CXCR3+Th1-like Treg, CCR6+Th17-like Treg and CCR7+ resting Treg. Resting Treg (Population I) were reduced in MS patients, both in untreated and treated MS compared to healthy donors. Activated/memory Treg (Population II) were significantly increased in MS patients compared to healthy donors. Activated effector CD4+ (Population IV) were increased and the naïve/ TEMRA CD4+ (Population V) were decreased in MS compared to HD. Expression of CCR7 was mainly in Population I, whereas expression of CCR6 and CXCR3 was greatest in Populations II and intermediate in Population III. In MS, CCR6+Treg were lower in Population III. This study found MS is associated with significant shifts in CD4+T cells subpopulations. MS patients had lower resting CD4+CD25+CD45RA+CCR7+ Treg than healthy donors while activated CD4+CD25hiCD45RA−Foxp3hiTreg were increased in MS patients even before treatment. Some MS patients had reduced CCR6+Th17-like Treg, which may contribute to the activity of MS.
Subject terms: Autoimmunity, Multiple sclerosis, Lymphocyte activation
Introduction
The cause of Multiple Sclerosis (MS) is not fully understood. Auto-reactive inflammatory cells including effector T cells (Th1, Th17, CD8+ Cytotoxic T cells), activated B cells, and plasma cells producing autoantibodies infiltrate the central nervous system (CNS). This leads to patchy myelin and axonal damage in the CNS. Susceptibility to MS is strongly linked to Class II MHC haplotypes1,2, which present antigens to CD4+T cells. Autoimmunity can occur due to failure of Treg that express Foxp3 and have low expression of CD1273. Many genes associated with MS relate to CD4+CD25+Foxp3+ T regulatory cells (Treg), including CD25, Ctla4, CD127, Il101,2. Treg have the potential to limit inflammation in MS.
There are conflicting reports on Treg in blood of MS patients. A meta-analysis of eight papers reported CD4+CD25+Foxp3+Treg in blood were not reduced in MS4. Other studies found Treg function is not affected in MS5–8.
There are reports of defects in/or deficiency of Treg in blood7–20, brain and CSF13 of MS patients. Treg in MS patients have reduced message and protein expression of Foxp310,12,21–23, are functionally impaired9–12,14,23,24 with poor suppression of T cell responses to myelin basic protein25,26.
In contrast, higher Treg compared to healthy donors (HD)20 are described in secondary progressive MS patients. Higher frequency of Treg have been observed in cerebrospinal fluid (CSF) compared to blood in MS10,13,24, whereas others reported no difference in CSF and blood25.
Human Treg are best defined as CD4+CD25+CD127loFoxp3+ but use of Treg makers is not consistent. In 29 studies we identified of CD4+CD25+Treg in blood of MS patients5–12,14–17,19,20,22–25,27–37, only 20 stained for Foxp36–8,10–12,14,15,17,19,22–24,28–32,34,36 and 14 for CD1276,11,14,16,19,20,23,30,31,33–37. CD4+CD25+CD127lo Foxp3+T cells are a heterogeneous population that can include Treg but also includes activated effector cells that are transiently induced to express CD25 and Foxp3 but this Foxp3 expression is unstable38–41. Thus, many studies on CD4+CD25+Foxp3+T cells include a proportion of activated T cells that do not suppress.
A variety of markers have been used to subdivide Treg, including CD31 expression as a marker of Treg of recent thymic origin14,29. Attempts to identify activated Treg include 11 studies of CD45RA/RO expression11,14,22,24,27,29,31,33–35,37, eight of CD39 expression7,16,22,28,30,32,36,37, four of HLA-DR expression27,33,35,37, one of CD161 expression34, and one of PD-1 expression22.
Relevant to this study, CD4+CD25+CD127lo Foxp3+T cells can be divided into subpopulations by their expression of CD45RA, an isoform of CD45. Expression of CD45RA is reduced as Treg are activated42,43 and other isoforms of CD45 including CD45RB, CD45RC and CD45RO44 are expressed. Treg activation also increases expression of CD25 and Foxp3.
CD4+T cells can be divided into five populations using CD45RA and either Foxp3 or CD25 expression37,43 (Fig. 1A). Population I mainly contains thymus derived resting Treg that express CD45RA, CD25 and Foxp3 and is large at birth but decreases with age42. Population II identifies activated Treg that are CD45RA− with high expression of CD25 and Foxp3. These cells are activated/memory Treg. Population III includes cells that are CD45RA− and have low to intermediate expression of Foxp3 and CD25. Population III includes effector CD4+T cells with transient expression of CD25 and Foxp3, that are activated to secrete inflammatory cytokines and do not suppress43. Population IV includes activated effector CD4+T cells that do not express CD45RA, CD25 or Foxp3. Population V identifies CD4+ cells with high CD45RA expression and no CD25 and Foxp3.
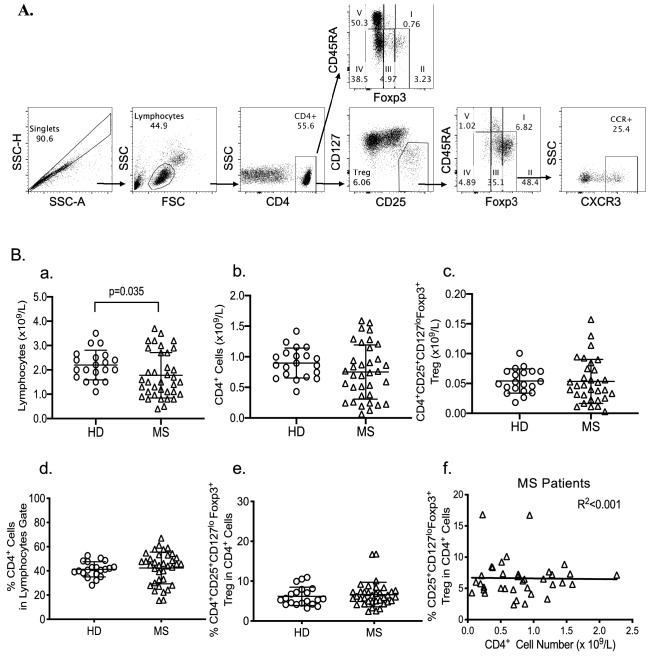
Figure 1.
(A) Gating Strategy for analysis of CD4+ and Treg subpopulations: PBMC isolated from a MS patient were stained for multicolour flow cytometry. Cells were first gated on SSC-A vs. SSC-H to exclude doublets, then lymphocytes were gated based on forward scatter (FSC-A) and side-scatter (SSC-A). CD4+ cells within the lymphocyte population were gated. Staining of CD25 vs. CD127 identified CD4+CD25+CD127loTreg within CD4 gate. Subpopulations were then identified based on Foxp3 vs. CD45RA expression either from CD4+ gate or from CD4+CD25+CD127loTreg gate. Within CD4+ cells five different populations were studied as defined by Miyara et al.43. Treg are thought to be located within populations I, II and III, with activated Th-like Treg thought to lie within population II. Populations IV and V are Foxp3− and CD25− and are respectively defined as activated/memory (CD45RA−) and effector CD4+ cells (CD45RA+). Populations I, II and III were then analysed for chemokine receptor expression (CXCR3, CCR6 or CCR7) to examine frequencies of specific Th-like Treg subsets, showing CXCR3 Th1-like Treg as an example. (B): Comparison of percentage of lymphocyte subsets in HD and MS patients. PBMC from MS patients (n = 36) were compared to PBMC from HD (n = 20) for lymphocyte count (a), CD4+cell count (b) and their proportion within lymphocytes (d), and CD4+CD25+CD127loFoxp3+ Treg count (c) and their proportion within CD4+cells (e). Regression analysis for Treg proportion within CD4+ cells vs. CD4+ cell numbers did not change with change in CD4+ cell numbers (f).
Activation of CD4+ T cells would expand Population IV while activation of Treg would increase Population II and III and may deplete Population I.
The five subpopulations identified by CD45RA, Foxp3 or CD25 expression can be subdivided by staining for other cell surface molecules. In this study, we examined key chemokine receptor (CCR) expression, which changes with activation of T cells. In Population I, expression of CCR7 identifies resting Treg that are recent thymic immigrants, whereas cells in Population I lacking CCR7 expression are effector memory (EM) Treg45,46.Terminally differentiated effector memory cells that re express CD45RA, but not CCR7, are known as TemRA cells47 and are found in Population V together with naïve CD4+ cells that are CD45RA+CCR7+.
Depending on the stimulating antigen, naïve CD4+ T helper lineage cells can be activated by cytokines to Th1, Th2, Th17 and Tfh effector cells. Th1 and Th17 cells mediate autoimmunity including MS48. Chemokine receptors expressed by activated T effector cells promote their migration to inflamed tissue. Th1 cells express CXCR349 and Th17 cells express CCR650.
Similar to T effector cell activation, CD4+CD25+Foxp3+Treg can be activated by antigen and cytokines of the ongoing effector response51,52 to develop into Th1-like, Th2-like, Th17-like or Tfh-like Treg53–56. Activated Treg express the same chemokine receptors as their associated activated T cells53. Treg expressing CXCR3 or CCR6 respectively migrate to sites of Th1 or Th17 inflammation in tissue57,58.
We examined peripheral blood lymphocytes, either CD4+ cells to identify Populations I-V or CD4+CD25+Foxp3+CD127lo Treg to identify Populations I–III. We also studied CCR expression within each of subpopulations of CD4+ cells and Treg. We compared these blood lymphocytes of healthy donors (HD) to MS patients to examine if this method of monitoring may reveal changes that could provide insights into the immune pathogenesis of MS and the effects of therapy. Three groups of MS patients were recruited; (i) patients newly diagnosed with MS that had received no treatment, (ii) patients whose MS had been inactive and who had received no immunomodulating therapy for three months and (iii) patients with clinical activity of MS that were on immunomodulating therapy.
MS patients’ blood had lower Population I (CD45RA+Foxp3+) and increased Population II (CD45RA−Foxp3hi). MS patients had less CCR6+ cells in Population III and some MS patients had lower numbers of CCR6+ cells in Population II. MS patients’ blood also had less CD4+ Population V including CD45RA+CCR7+ effector cells and TemRA cells and more activated/memory effector CD4+T cells (Population IV). These changes were evident in all three clinical categories and appear to be due to the disease of MS, not other factors.
Human ethics and subjects
This study was approved by the Human Ethics Committee of the South Western Sydney Local Health District at Liverpool Hospital, Liverpool, NSW Australia, and complied with the Declaration of Helsinki-Ethical Principles for Medical Research involving Human Subjects. All subjects voluntarily gave informed written consent.
HD were recruited by an internal email invitation within the hospital. All volunteers were screened to confirm the absence of diseases such as autoimmune conditions, HIV, other infections and anaemia. 20 healthy donors (HD) and 36 MS patients were recruited, and their characteristics are detailed in Table 1.
Table 1.
Characteristics of healthy donors (HD) and multiple sclerosis (MS) patients.
| Total HD | MS | |||||
|---|---|---|---|---|---|---|
| Total MS | Active3 | Non-active4 | Recent treatment | No recent treatment5 | ||
| N | 20 | 36 | 22* | 12* | 15* | 20* |
| Age-mean (range) years | 41.7 (21–69) | 42.5 (21–64) | 40 (21–61) | 45.6 (26–64) | 42.2 (21–61) | 42.1 (22–64) |
| Male | 6 | 9 | 6 | 2 | 3 | 6 |
| Female | 14 | 27 | 16 | 10 | 12 | 14 |
| Disease Duration1 years | N/A | 8.9 (0.1–25.2) | 7.9 (0.1–25.2) | 11.5 (4.8–24.9) | 9.2 (0.1–25.3) | 8.3 (0.1–19.4) |
| EDSS2 | N/A | 3.71 (0–8.5) | 3.24 (0–6) | 4.2 (1–6.5) | 3.04 (0–6) | 4.21 (1–6.5) |
1Disease duration calculated from very first onset of symptoms to time of study.
2EDSS is expanded disability status scale.
3Active MS defined as relapse or clinical progression of MS in 3 months before study.
4Non active MS, no progression or relapse in last 6 months.
5No recent treatment—no immunomodulatory therapy received in 3 months before study.
*Some clinical data not available for this post-hoc analysis.
All MS patients had a clinical diagnosis of MS made according to the McDonald Criteria 201759. Total 36 MS patients were recruited including recently diagnosed MS patients and patients who had received prior treatment with immunomodulatory drugs. Patients were assigned to three groups: (i) a treatment naïve group of 12 patients who had never received treatment with immune modulating therapies, including interferon β (Treatment Naïve), (ii) 8 MS patients who were clinically stable and had not received immune modulating therapy in the last three months, (iii) 15 MS patients who had received immune modulating therapy in the last three months (on therapy). For some analysis, Group I and II were combined as one group of patients not currently on immune modulating therapy and clinically stable (off therapy, n = 20). MS patients were defined to have active disease if they had a relapse or deterioration of symptoms within the last 3 months of sampling.
Immune modulating therapy included 15 patients given interferon-β, all had stopped this therapy 18 months to 10 years prior. Four patients had received glatiramer acetate (Copaxone, Teva Pharma Australia), which had been stopped 3-6 years ago. Eight patients had received natalizumab (Tysabri, Biogen Australia): two stopped 4 weeks prior sampling, the others 8 months to 6 years prior. 14 patients had been treated with fingolimod (Gilenya, Novartis Australia), nine had stopped this therapy in the previous 3 months, the other five stopped 2-4 years prior sampling. Five patients had received dimethyl fumarate (Tecfidera, Biogen Idec Australia), two were currently on the drug and three stopped over a year ago. Five patients had been treated with Rituximab (Mabthera, Roche, Australia) 7–12 months prior sampling. Four patients were treated with alemtuzumab (Lemtrada, Sanofi Aventis Australia), two a year prior and the other two 7–8 years ago. Two patients had been treated with cladribine (Mavenclad, Merck Australia), one remained on the drug, and the other had it a year prior sampling. One patient received IvIg (CSL, Melbourne Australia) 4 months prior. One patient received teriflunomide (Aubagio, Sanofi Aventis, Australia) 3 years prior. One patient had received methotrexate and another an unsuccessful stem cell transplant in Mexico, both several years prior. No patient had received anti-CD25 monoclonal antibody therapy.
Materials and methods
Cell isolation
Peripheral blood lymphocyte counts (PBL) were assessed by a clinical haematological laboratory. Peripheral blood mononuclear cells (PBMC) were isolated from 30ml of fresh whole blood using Ficoll-Hypaque density gradient centrifugation (Ficoll-PaqueTM PLUS, GE Healthcare Bio-Sciences AB).
Immunostaining
Monoclonal antibodies used were to CD4 (RPA-T4, APC-Cy7), CD127 (HIL-7R-M21, PE- Cy7), CD25 (M-A251, PE-Cy7), CXCR3 (IC6, PE), CCR6 (11A, PE), CCR7 (150503, PE); all purchased from BD Biosciences (North Ryde, NSW, Australia), CD45RA (HI100, APC) (Biolegend, San Diego, CA) and Foxp3 (PCH101, AF488) (eBioscience, San Diego, CA).
Fresh PBMC (1 × 106) were stained for chemokine receptors for 15 minutes at room temperature in dark before staining for CD4, CD25, CD127 and CD45RA for 30 minutes on ice. Cells were washed, fixed and permeabilized according to the manufacturer’s protocol (eBioscience) then stained for intracellular Foxp3.
Phenotypic analysis was performed on stained PBMC using a FACSCanto II flow cytometer (BD Biosciences) and FACS DIVA 8.0 software. Data was analysed following the gating strategy shown in Fig. 1A using FloJo v10 software (Tree Star, Ashland, OR). Lymphocyte populations were gated after exclusion of doublets based on SSC-A vs. SSC-H.
CD4+ cells were divided into five populations based on CD45RA and Foxp3 expression as described by Miyara et al.43. The percentage of each population was also calculated within CD4+CD25+CD127loTreg, which mainly has cells in Population I, Population II and Population III (Fig. 1A).
Each population was further examined for the Th-like Treg phenotype using expression of chemokine receptors; CXCR3 for Th1-like Treg, CCR6 for Th17-like Treg and CCR7 for circulating naïve Treg. The gating for chemokine receptor expressing cells was based on a Flourescence Minus One (FMO) control.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 8.0.2 and IBM SPSS Statistics 25. For comparison of unpaired data, the Mann–Whitney U test was used. The Kruskal–Wallis Test was used in cases where multiple independent groups were assessed. Linear regression analysis was performed to compare the effect of age and disease duration with Treg parameters. Results were expressed as mean ± SEM unless otherwise specified and significance was p < 0.05.
Results
Comparison of peripheral lymphocyte counts (PBL) in HD and MS patients
The demographic characteristics of HD and MS patients are summarised in Table 1. HD had higher PBL counts than MS patients, 2.19 ± 0.60 (mean ± SEM) vs. 1.77 ± 0.15 × 109/L (p = 0.035) (Fig. 1Ba). MS patients with no previous therapy had PBL counts of 2.45 ± 0.25 × 109/L that was not significantly different to HD. Compared to HD, PBL counts were lower in MS patients whose disease was inactive (p = 0.004) or had treatment in last three months (p = 0.012), but not in those not treated in last three months (p = 0.054). Regression analysis of PBL counts showed no significant effect of age, disease duration or time since last flare of MS (R2 = 0.053, p = 0.316).
Comparison of peripheral CD4+T cell counts in HD and MS patients
CD4+ cell counts for HD and MS were not significantly different (Fig. 1Bb). Patients with active MS had lower CD4+ counts (0.602 ± 0.074 × 109/L) whereas MS patients with no previous therapy had CD4+ counts (1.065 ± 0.154 × 109/L) similar to HD (0.990 ± 0.088 × 109/L). There was no difference in the proportion of CD4+ cells in PBL between MS patients and HD (42.14% ± 13.14% vs. 41.26% ± 6.19%, p = 0.339) though the variation within MS patients was greater than in HD (Fig. 1Bd).
Patients with active MS had higher numbers and proportions of CD4+ cells compared to those with inactive disease (p = 0.02). CD4+ cell counts or proportion of CD4+ cells (p = 0.184) were not different in MS patients that received therapy in last three months compared to MS patients who received no therapy in the last three months. Regression analysis found neither the number nor the proportion of CD4+ cells in MS patients correlated with age, disease duration or time since last flare of MS (R2 = 0.109, p = 0.087).
Comparison of peripheral CD4+CD25+CD127loT cells in HD and MS patients
The number of CD4+CD25+CD127loFoxp3+cells in MS was not significantly different to HD (p = 0.472, Fig. 1Bc). However, the spread of counts was wider in MS patients as a proportion of MS patients had either low or higher absolute numbers. There was no difference between HD and MS in the proportion within CD4+ cells of CD4+CD25+CD127lo (7.20 ± 0.546% vs. 7.48 ± 0.502%, p = 0.815) or of CD4+CD25+CD127loFoxp3+ (6.19 ± 0.516 vs. 6.62 ± 0.516%, p = 0.573, Fig. 1Be).
Regression analysis of CD4+ cell numbers with proportion of Treg in CD4+ cells in MS patients showed no correlation (R2 = 0.001) (Fig. 1Bf). This demonstrated that the ratio of Treg as a proportion of CD4+ cells did not relate to total CD4+ cell number. Thus, all results were expressed either as proportions of either CD4+ or of CD4+CD25+CD127loTreg.
Comparison of the three Treg populations based on CD45RA and Foxp3 expression in HD and MS patients
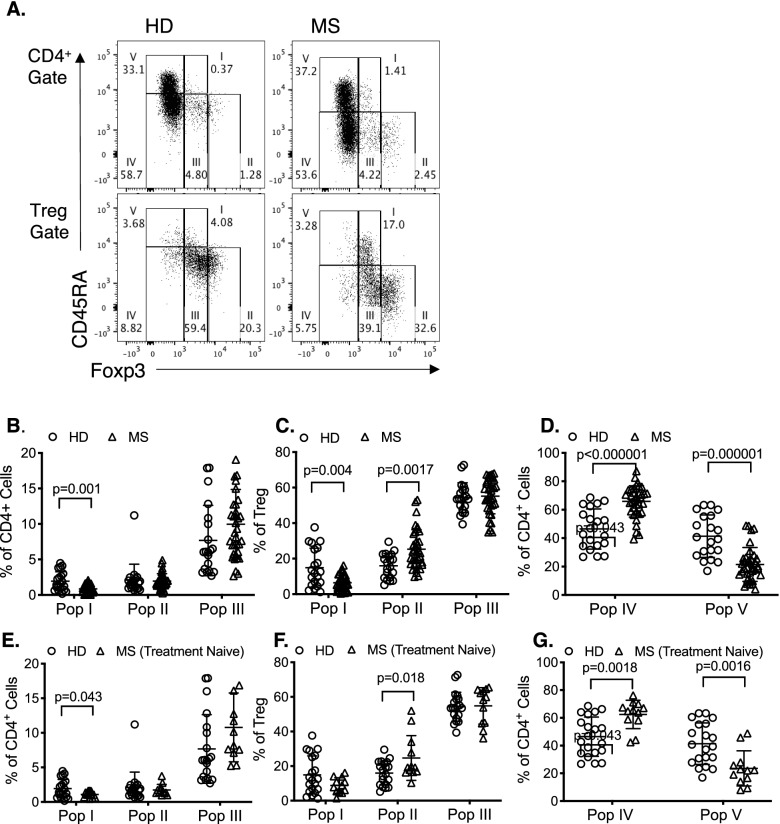
We next examined if there were changes in the subpopulations of CD4+ cells and CD4+CD25+CD127lo Treg as described by Miyara et al43 for CD4+ cells identifying resting and activated Treg by staining for CD45RA and Foxp3/CD25. A representative plot of five subpopulations within CD4+ cells and CD4+CD25+CD127loTreg population is shown as Fig. 2A. To identify if changes demonstrated in studies of all MS patients (Fig. 2B–D), were a marker of immune changes intrinsic to MS or a consequence of immune modifying therapy, we compared CD4+ and CD4+CD25+CD127lo Treg from treatment naïve MS patients (n = 12) and HD (Fig. 2E–G).
Figure 2.
Comparison of Treg subpopulations in HD and MS patients. Subpopulations were gated based on CD45RA and Foxp3 either within CD4+ cells (Population I-V) or CD4+CD25+CD127loTreg (population I–III). Values were expressed as the percentage of cells within each population. (A) Representative FACS profile of PBMC from HD (Left Column) and an MS patients (Right Column) gated on CD4+ (Top Row) and CD4+CD25+CD127loTreg (Bottom Row) showing increased frequency of Foxp3hiCD45RA− cells in Population II in MS patients compared to HD; 2.45% vs. 1.28% when gated on CD4+, and 32.6% vs. 20.3% when gated on CD4+CD25+CD127lo Treg. (B–D) shows comparison of all MS patients (n = 36) with HD (n = 20). (B) CD4+ cells had lower proportion of Population I (p = 0.001) in MS (n = 36) than HD (n = 20). (C) As proportion of CD4+CD25+CD127loTreg, significant difference was noted both in population I and II, Population I was significantly lower (p = 0.004) and Population II was significantly greater (p = 0.0017) in MS (n = 36) than HD (n = 20). (D) Proportion of Population IV (activated/memory effectors) in CD4+ was greater in MS (n = 36) compared to HD (n = 20) (p < 0.000001). Proportion of Population V (Foxp3−CD45RA+) within CD4+ cells was significantly lesser in MS than in HD (p < 0.000001). (E–G) shows comparison of subpopulations in treatment naïve MS patients (n = 12) to HD (n = 20) using similar analysis as in B–D. (E) Treatment naïve MS patients had significantly lower proportion of cells in Population I in CD4+ gated cells than HD (p = 0.043). There was no significant difference between Population II or Population III. (F) In CD4+CD25+CD127loTreg gate Population II was significantly greater in untreated MS than HD (p = 0.018) but there was no difference in population I or III although Population I was low. (G) In CD4+ gated cells, MS patients had more Population IV (p = 0.0018) and less Population V (p = 0.0016) than HD.
CD45RA+Foxp3+T cell (Population I) proportion within CD4+ cells was significantly smaller in MS than HD when comparing either all MS patients (p = 0.001) (Fig. 2B) or treatment naïve MS patients (p = 0.043) (Fig. 2E).
Within CD4+CD25+CD127loTreg, Population I was also significantly smaller in MS than HD (p = 0.004) (Fig. 2C). Population I was significantly lower in MS patients who were off treatment in last three months compared to HD (p = 0.008), but not for those who were on treatment in the last three months (data not shown).
CD4+CD25+CD45RA−Foxp3hi cells (Population II), the most suppressive of the three Populations43, was significantly increased in CD4+CD25+CD127loTreg in all MS patients (n = 36, p = 0.0017) (Fig. 2C) and treatment naïve MS patients (n=12, p < 0.018) (Fig. 2F) compared to HD. However, there was no difference between MS and HD (p = 0.532) in Population II of CD4+T cells either comparing all MS (Fig. 2B) or treatment naïve patients to HD (Fig. 2E).
There was no significant difference of Population III in either CD4+ cells (Fig. 2B,E) or CD4+CD25+CD127loTreg (Fig. 2C,F), when comparing all MS or treatment naïve MS to HD. Population III includes activated effector CD4+ that are activated and transiently express Foxp3 and CD2541,60.
Changes in effector CD4+T cells in MS compared to HD
Comparing all MS patients with HD, Population V containing naïve CD4+CD45RA+Foxp3− and TemRA cells was significantly lower in MS patients (21.48 ± 11.90%) than in HD (41.34 ± 14.07%) (p = 0.000001) (Fig. 2D). The TemRA CD4+CD245RA+CCR7− cells in Population V, were 12.25 ± 23.30% in MS and 5.16 ± 8.46 in HD. Population IV, CD4+CD45RA−Foxp3− activated effector T cells, was increased in all MS (65.85 ± 11.02%) compared to HD (46.42 ± 14.45%) (p < 0.000001) (Fig. 2D).
These differences were also observed when treatment naïve MS patients (n=12) were compared to HD (Fig. 2G). Population IV was significantly increased in MS (p = 0.0018) and Population V was significantly decreased in MS patients (p = 0.0016), (Fig. 2G).
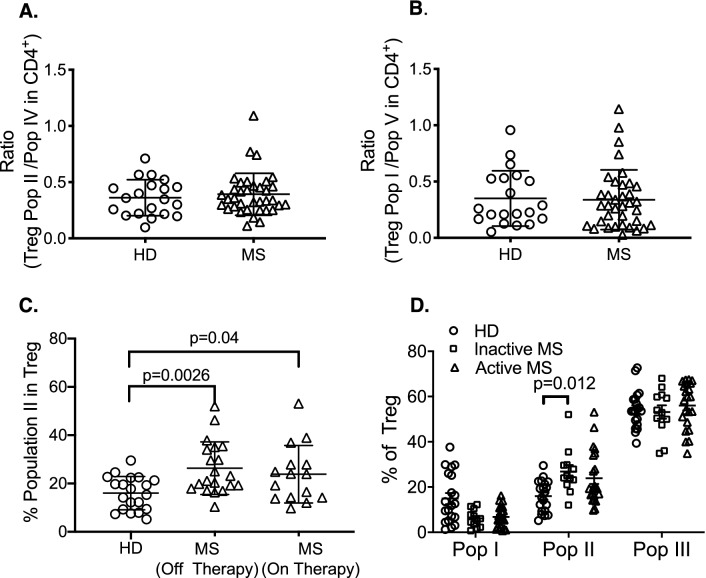
The ratio of Population I to Population V was not different between HD and MS (p = 0.867) (Fig. 3B). The ratio of activated Treg Population II to activated effector Population IV also showed no difference between HD and MS (p = 0.188) (Fig. 3A).
Figure 3.
Relationship of Treg to effector cell subpopulations and treatment in HD and MS patients. (A) Ratio of Treg Population II to activated/memory effector cells in population IV was examined. No significant difference was observed in MS (n = 36) compared to HD (n = 20). (B) Ratio of Treg Population I to effector cells in Population V found no significant difference between HD (n = 20) and MS patients (n = 36). (C) Comparison of Population II in Treg in HD (n = 20) and MS patients that either had no recent immunomodulatory therapy (n = 20) or received immunomodulatory therapy in last three months (n = 15). Significant differences were found between HD and MS patients who were off therapy (p = 0.0026) or were on immune modulating therapy for MS in the last three months of sampling (p = 0.04). (D) Comparison of Treg subpopulations in HD (n = 20), to patients with active MS (n = 22) and those who did not have active MS (n = 12). MS was classified as active if patients reported a clinical relapse or deterioration of symptoms within the last 3 months. Significant difference was observed in Treg Population II in MS patients with inactive disease compared to HD(p = 0.012). Patients with active MS, did not have an increase in Population II, albeit five of 20 patients had increased number of Population II above mean value for HD.
MS patients who were off treatment for three months had significantly higher Population II than HD (p = 0.026) (Fig. 3C), as did MS patients who were on treatment (p = 0.04).
Treg Population II increased significantly in patients with inactive MS compared to HD (p = 0.012) but not with active MS (Fig. 3D).
Changes in populations with age: comparison of HD and MS patients
Previous studies showed CD45RA+CD25+Foxp3+ naïve Treg decrease with age. Within the CD4+ gate there was a significant decrease in the Population I (R2 = 0.571, p < 0.001) with age in HD but not MS (data not shown).
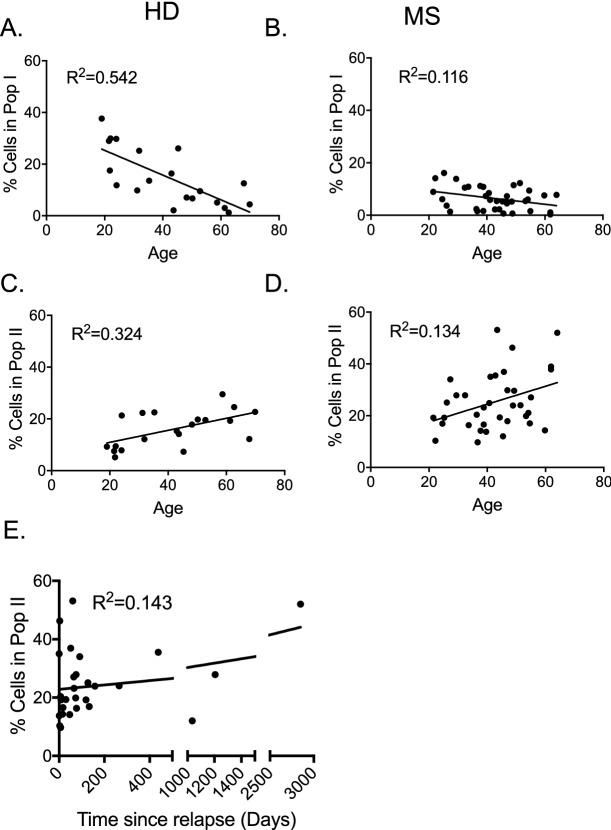
Within the CD4+CD25+CD127loTreg gate, Population I decreased with age in HD (R2 = 0.542, p < 0.001) and to a lesser degree in MS (R2 = 0.116, p = 0.042) (Fig. 4A,B). The decline with age was less apparent in MS patients (p = 0.042) compared to HD (p < 0.001).
Figure 4.
Regression analysis of Populations I and II in CD4+CD25+CD127loTreg. The proportion of Treg in Populations I and II were analysed for correlation with age of HD and MS patients. (A,B) Linear regression analysis of Populations I with age in HD (n = 20) (A) and MS (n = 36) (B). In HD, Population I decreased with age (R2 = 0.542) but not in MS patients (R2 = 0.116). (C,D) Linear regression analysis of Populations II with age in HD (n = 20) (C) and MS (n = 36) (D). Population II increased with age for HD (R2 = 0.324) and MS (R2 = 0.134). (E) Linear regression analysis of Population II with time since last MS relapse showed correlation (R2 = 0.143) (p < 0.05).
Population II (CD45RA−Foxp3hi) within the CD4+ gate showed a significant increase with age in MS patients (R2 = 0.237, p = 0.003), but not in HD (R2 = 0.034, p = 0.436)(data not shown). Within the CD4+CD25+CD127loTreg gate, Population II increased with age in both MS (R2 = 0.134, p = 0.028) and HD (R2 = 0.324, p = 0.009) (Fig. 4C,D).
Population III, in both CD4+ and CD4+CD25+CD127loTreg gates had no significant relationship with age in HD or MS patients (data not shown).
Changes in populations with disease duration in MS patients
Population I significantly decreased with MS duration within both, the CD4+ (R2 = 0.151, p = 0.026) and CD4+CD25+CD127loTreg gates (R2 = 0.16, p = 0.021) (data not shown). No significant changes in Population II with disease duration were identified, however.
Linear regression analysis of Treg with time since last flare of MS showed no correlation for Population I (data not shown), but Population II increased with length of clinical remission (R2 = 0.143, p = 0.047) (Fig. 4E). Population III in the CD4+CD25+CD127loTreg population increased with time since last clinical relapse (R2 = 0.151, p = 0.041).
Chemokine receptor expression by the three Populations of Treg
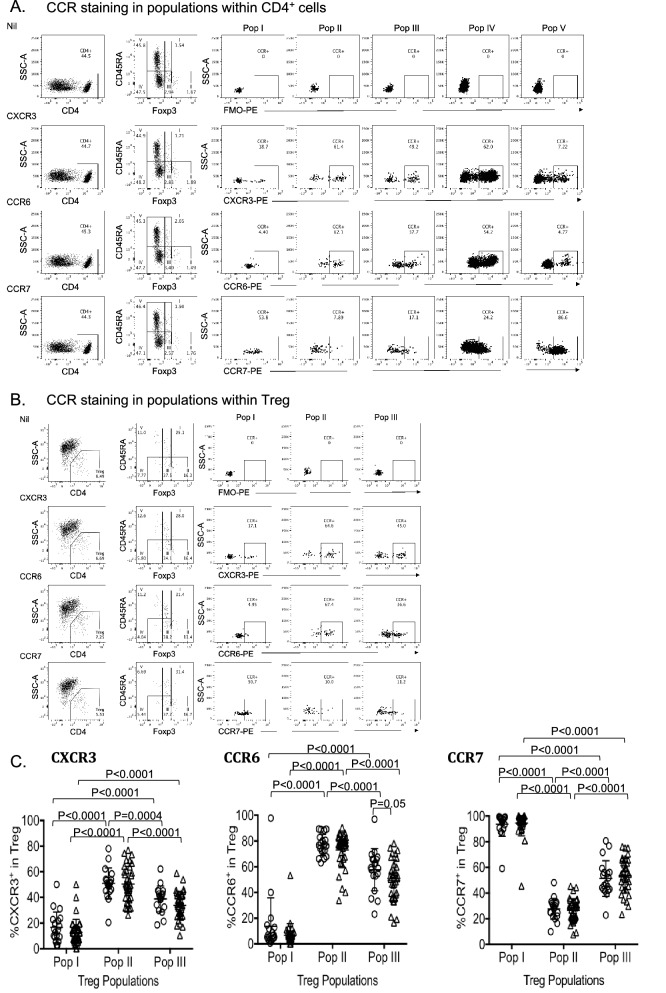
Representative plots as Fig. 5A,B show chemokine receptor positive cells in Populations I-V of CD4+ cells and in Treg Population 1–III respectively. The gate for CCR+ cells is set based on chemokine receptor FMO control in respective subpopulations, top row in Fig. 5A,B.
Figure 5.
Chemokine receptor expression in Treg Populations I–III in HD and MS patients. (A) Representative FACS analysis for CXCR3, CCR6 and CCR7 in CD4+ Populations I-V. Expression of chemokine receptors (CCR) was examined in the five Populations of CD4+ cells by using FMO control for CCR monoclonal antibody fluorochrome (top row). (B) Representative FACS analysis for CXCR3, CCR6 and CCR7 in Populations I–III within CD4+CD25+CD127loTreg. Chemokine receptors (CCR) expression was examined in the three Treg Populations using FMO control for CCR monoclonal antibody fluorochrome (top row). (C) Comparison of chemokine receptor expression in Treg Populations I–III in HD (Ο, n = 20) and MS patients (Δ, n = 31). The proportion of cells expressing CXCR3, CCR6 and CCR7 was examined within CD4+CD25+CD127loTreg populations I-III that were identified based on Foxp3 vs. CD45RA expression as outlined in Fig. 2A. Values shown as the percentage of cells expressing respective CCR within each population. Both in HD (n = 19) and in MS patients (n = 35), expression of CXCR3 and CCR6 was higher in Treg Population II compared to Population I (p < 0.0001) or in Population III (p < 0.0001). Population III also had higher expression of CXCR3 and CCR6 compared to Population I (p < 0.0001). CCR7 expression in Treg Population II in both HD (n = 19) and MS (n = 33) was significantly lower compared to Treg Population I (p < 0.0001) or Population III (p < 0.0001). Population III also had significantly lower CCR7 expression compared to Treg Population I in both MS and HD. Approximately half of the MS patients have low CXCR3 and CCR6 expression in Population II. CCR6 expression was lower in Population III in MS compared to HD (p = 0.05).
CCR7 was expressed by 90% of Population I but only a small portion of Population II and III (Fig. 5C). This is consistent with CCR7+ naïve Treg recirculating from blood to lymphoid tissues. In Population I, lack of expression of CCR7 identifies TemRA Treg, which in MS were 5.55 + 9.74% (n=33), similar to 6.43 + 9.23% (n=19) in HD.
CCR7 was significantly lower in Population II (26.52±1.60%) compared to Population I (94.45±1.70%, p < 0.0001) and Population III (53.63±2.37%, p < 0.0001). Population III also had lower expression of CCR7 than Population I (p < 0.0001) (Fig. 5C).
Majority of CD4+ T cells in Population V express CCR7, which promotes their migration into secondary lymphoid tissues.Within Population V, there are CD4 TemRA cells that express CD45RA but not CCR747,61. These are not naïve T cells. In this study MS patients had 12.25 + 23.3% of Population V that were CCR7− compared to 5.16 + 8.46% in HD.
Activated Treg express chemokine receptors that promote their migration to sites of inflammation. CXCR3 promotes migration to sites of Th1 inflammation and CCR6 to sites of Th17 inflammation53. In both HD and MS patients, within CD4+CD25+CD127loTreg gate, the highest expression of CXCR3 was in Population II (50.49±2.28%) compared to Population I (12.63±1.72%, p < 0.0001) and Population III (33.8±1.73%, p < 0.0001) (Fig. 5C). Population III had higher expression than Population I (p < 0.0001).
CCR6 was significantly higher in Population II (70.99±3.01%) compared to Population I (6.54 ± 1.48%, p < 0.0001), and Population III (47.93±2.94%, p < 0.0001). Population III had higher proportion of CCR6+ cells than Population I (p < 0.0001) (Fig. 5C).
The staining methods used in this study did not allow identification of CXCR3/CCR6 double positive cells. In our ongoing studies, we examined a group of MS patients with clinically active disease (n=10) to HD (n=10). In Population II, CXCR3+CCR6+ cells were 20.2 + 20.1% in MS compared to 13.1 + 7.9% in HD (NSD, p = 0.31). In Population III, CXCR3/CCR6 double positive cells were 6.2 + 4.8% in MS and 8.0 + 9.1% in HD. Thus, a small population of CXCR3+CCR6+ cells can be identified in HD and MS.
CCR6 expression is decreased in Treg of MS patients
A significant decrease in the number of CCR6+ cells in MS patients was observed within Population III of CD4+CD25+CD127loTreg compared to HD (p = 0.05) (Fig. 5C). Some MS patients had low expression of CCR6 in Population II. Reduced frequency of CCR6+ Treg did not associate with activity of MS, treatment or length of MS (data not shown). There were a few cases of low CCR6 expression in untreated, in any clinical groups. A larger cohort is required to resolve the meaning of this observation.
CXCR3 or CCR7 expression in subpopulations of CD4+ cells or CD4+CD25+CD127loTreg did not show any significant difference between MS and HD (Fig. 5C).
Discussion
This study identified trends in subpopulations of CD4+ cells that may have pathogenic significance in MS. As Treg function on ratio basis to effector CD4+ cells, we focused on the proportion of Treg subpopulations within whole CD4+ cells gate or within CD4+CD25+CD127loTreg gate and not on the absolute number of Treg. CD45RA expression was used to distinguish resting from activated/memory cells. Subtypes of activated Treg were analysed by expression of Th17 and Th1 associated chemokine receptors. Both activated/memory Treg (Population II in CD4+CD25+CD127loTreg) and activated effector T cells (Population IV in CD4+) were increased in MS. Both CD4+CD45RA+CD25−Foxp3− cells (Population V in CD4+) and CD4+CD25+Foxp3+CD127loTreg (Population I in Treg) were reduced in MS.
Many early studies in MS assumed CD4+CD25+Treg were a homogenous population of naïve Foxp3+Treg. In 29 studies we identified that examined CD4+CD25+Treg either without or with Foxp3/low CD127 expression in MS compared to healthy donors; 12 studies found no difference in MS compared to HD in frequency5,6,9,24,25,27–30,32,34,37 while nine found reduced Treg7,10,11,14–17,19,20, and three found reduced Treg during relapses8,12,19. Other studies reported reduced Treg in stable MS but an increase in Treg associated with relapses7 and progressive MS20. Three of 29 studies did not compare Treg frequency to HD, but studied function of Treg23 or monitored Treg in MS patients in response to therapy33,35. We also found no difference between CD4+CD25+Foxp3+CD127lo T cells in MS and HD.
Our main findings were differences in subpopulations of Treg identified by expression of CD45RA or chemokine receptors. We found a shift from resting to activated/ memory Treg in MS patients. The most consistent finding is reduced numbers of resting CD4+CD25+CD45RA+Foxp3+Treg in MS relative to age-matched controls. Resting Treg decrease with age in HD, accompanied by an increase in activated/memory Treg62, likely due to natural exposure to various antigens throughout life activating resting Treg. The reduction in CD45RA+ cells in both Population I (resting Treg) and V (CD4+ effectors) may be due to activation of resting cells by the autoimmune response. It was not due to loss of TemRA effector or regulatory cells. We observed a decline in resting Treg with age in MS patients as well, as occurs in HD.
In 11 studies of CD45RA/RO expression by Treg in blood of MS patients11,14,22,24,27,29,31,33–35,37, two studies used CD45RA for longitudinal monitoring of Treg in MS but did not compare to HD33,35. Two others did not report resting Treg numbers24,27. Of the other seven studies, five showed lower numbers of CD45RA+ cells in MS compared to HD11,14,29,34,37. One found reduction in both CD45RA+Treg and CD45RA−Treg22 and the other no reduction in resting Treg31.
Resting Treg have been reported as lower than in HD in newly diagnosed MS in both children14 and in adults29. In adults, there is no change in total Treg, but reduced CD31+Treg and increased memory Treg in adults29. In paediatric MS, resting Treg and CD31+RTE Treg were reduced while memory Treg increased14. Our study confirmed reduced resting Treg in MS patients with no prior immune modifying therapy, as well as in established MS. Whether depletion of resting Treg is a contributor to induction of MS or a consequence of the auto immune responses, needs to be resolved. Depletion of resting Treg did not appear to be a consequence of therapy, however. A minority of Population I Treg did not express CCR7, and are effector memory Treg. Their proportion in Population I was not different in MS compared to HD, and these cells were thus also relatively depleted.
We found nine studies identifying memory/activated Treg in MS patients by expression of CD45RO or lack of CD45RA. Five reported an increase in activated/memory Treg14,29,31,34,37, two found no difference24,27, and two reported reduced memory Treg10,22 in MS patients. Only four of the nine studies in MS of CD45RA/RO expression by Treg, described three Treg population based on CD45RA and Foxp3/CD25 expression in MS or CIS22,31,34,37. Our findings are in agreement with Ciccocioppo et al 2019 who, like our study, found no difference in MS or HD in total CD4+CD25+CD127loTreg, but higher activated Treg (Population II, CD45RA−CD25hi) and lower resting Treg (Population I, CD45RA+CD25+) in MS37. Jones et al34 showed an increase in Population III but not Population II in CIS patients. Sambucci et al22, showed a reduction in memory Treg but did not identify changes in Population II. Bjerg et al31 reported higher memory Treg associated with lower EDSS score.
In this study, activated/memory CD45RA− Treg were increased, particularly in the CD25hi and Foxp3hi cells. Population II was greater in MS patients without clinical activity and increased the longer they were not clinically active. This is consistent with the increased activated Treg pool controlling MS, as reported29,37.
The CD45RA+ and CD45RO+ subsets of Treg, the naïve and memory Treg respectively, are affected differently in MS. In the acute phase of MS, suppressive function is impaired in both subsets, but activated/memory Treg recover in chronic MS11. This is accompanied by an increased activated/ memory Treg frequency in chronic patients, potentially explaining the observed recovery of Treg in secondary progressive MS5,63 and the positive correlation between MS duration and Treg frequency11,15. However, in chronic disease the number of resting Treg remains low. Higher proportions of activated/memory Treg are also found in CSF of MS patients compared to their peripheral blood10,12,24.
Previous studies on activated Treg in MS assayed other markers, but none found a consistent increase in Treg. CD39, a rate-limiting enzyme in ATP/ADP–AMP–adenosine pathway, produces adenosine, inhibits activated T effector cells and thereby limits immune inflammation. CD39 promotes a major pathway for Treg inhibition of autoimmunity. In eight studies of CD39 expression by Treg in MS, five reported reduced CD39+Treg7,16,22,28,30, one no difference37 and two increased numbers32,36. Most studies examined CD39 expression by the whole Treg population. Dalla Libera et al.7 showed CD4+CD25+Foxp3+ or CD4+CD25+CD39+ or CD4+CD39+Foxp3+ are reduced in stable RRMS but restored to normal in acute relapse in RRMS. Alvarez-Sanchez et al.36 showed increased CD39+ cells in CD4+CD25+CD127loFoxp3+ in RRMS compared to HD. CD39+Treg have been found to be associated with relapsing-remitting MS and are increased in relapsing patients and is significantly correlated with EDSS score36. Patients have reduced CD39+Treg in a stable phase but in acute relapse had comparable CD39+Treg to HD28. Of the two studies examining CD39+ cell numbers in conjunction with CD45RA/RO, one reported reduced CD39+ cells in memory/activated Treg22. The other study found no difference in CD39 expression in MS and HD within total Treg, secreting Treg (Population III) or activated Treg (Population II)37. CD39+Treg were reduced in stable RRMS patients but their suppressive ability was not compromised7. Others reported that CD39+Treg have reduced capacity to suppress IL-17 production30. HD have marked variation ranging from 2% to 60% CD39+ cells within the CD4+CD25hi Treg population28.
Another marker of activated Treg is expression of class II MHC64,65. In four studies in MS27,33,35,37, two monitored HLA-DR+ Treg in response to therapy and made no comparison to HD33,35 and one found no difference in HLA-DR+Treg27. Only one study reported an increase in HLA-DR+ Treg within total and activated/memory Treg in MS compared to HD37. There is one study showing increased PD-1 expression on Treg in MS compared to in HD22. Overall the published studies do not show an increase in these activated Treg in MS patients.
In MS patients, CCR6 expression was lower in Population III, and for a fraction of patients in Population II when gating was on CD4+CD25+CD127lo cells, but not when gating was on all CD4+ cells. CCR6 is the chemokine receptor of Th17 cell responses. Th17 cells contribute to auto-inflammation in MS48, and infiltration of CCR6+CXCR3+ Th1 and Th17 effector memory cells into the CNS occurs in early disease activity in MS66. Given the role of CCR6 in chemotaxis67 with homeostatic accumulation of CCR6+Treg into the CNS following EAE induction in mice68, the decreased expression of CCR6 by CD4+CD25+CD45RA− Treg may lead to the reduced migration of these Treg to inflammatory lesions.
Jones et al. found an increase in Th17-like Treg in CIS patients34. They defined Treg as CD4+Foxp3+CXCR5− and hence their analysis would have excluded cells that may co-express both CXCR5 and CCR6. They also studied another marker of Th17 cells, CD161, in CIS34, These Th17-like Treg may control the Th17 effector response in CIS and prevent progression to MS. We found no differences in expression of CXCR3 the Th1 CCR. In a pilot study, we found a minority of cells in Population II and III express both CXCR3 and CCR6, but this was similar in HD and MS.
Our study identified several differences between MS patients and HD that may prove useful in monitoring MS. The proportion of activated/memory CD45RA−Foxp3hiTreg was increased in MS and appeared to be greatest in patients with longer clinical remissions. Increased activated/memory Treg may prove to be a marker of stable MS, with a reduced risk of relapse. CCR6 expression was also low in activated Treg and a relative deficiency of these cells may allow progression of MS.
This report is based on a limited number of patients and requires confirmation in a larger longitudinal study examining the subsets of activated/ memory Treg, identified in this study. The effects of therapy on the Treg populations and disease activity were not assessed.
However, this study including a large proportion of patients who never had immune modulating therapy, or who had no immune modulating therapy within three months prior sampling, confirmed that resting Treg are depleted in MS without immune modulating therapy, at a rate greater than natural attrition of these cells with age. Whether this makes patients more prone to develop MS or is a consequence of accelerated activation of Treg by the autoimmune response remains to be resolved.
Our findings identify an increased proportion of CD25hiFoxp3hi Treg in many MS patients a finding supported by other smaller studies31,37. Similar changes were reported in sarcoidosis, a Th1 mediated disease, but not in SLE43. This suggests activated/memory Treg, which should include autoantigen specific Treg are generated to control immune inflammation in MS.
The decreased frequency of CCR6+ cells in Population III and low numbers of CCR6+ activated/memory Treg in Population II in some MS patients, suggested that failure to produce Th17-like Treg may contribute to lack of control of inflammation in MS.
The methodology will allow more detailed studies of Treg in MS especially with the highly activated Treg in Population II, which can be further divided into Th1 and Th17-like Treg, and other markers of activated Treg such as CD39, Class II MHC and PD1. Whether the increased proportion of activated/memory Treg leads to inhibition of immune inflammation and disease activity requires resolution. It may potentially guide therapy.
Acknowledgements
This work was supported by a generous donation from Bob and Jack Ingham of Ingham Enterprises and an anonymous donor. Support from NHMRC Australia, Liverpool Hospital, University of New South Wales Australia, and South Western Sydney Area Research Foundation is acknowledged. The assistance of Catherine Robinson, Meena Sharma, Sue Baker, Bhumika Limbu is greatly appreciated.
Abbreviations
- CIS
Clinically isolated syndrome
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- FACS
Fluorescence-activated cell sorting
- HD
Healthy donor
- IFN-γ
Interferon-gamma
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- PBL
Peripheral blood lymphocytes
- Treg
T-regulatory cell
Author contributions
S.J.H. initiated and designed the study, supervised the conduct of experiments and analysis, and contributed to writing the paper. N.D.V. assisted in planning, designed experiments and FACS analysis panels as well as conduct of assays, She contributed to analysis and interpretation of experiments and preparation of the manuscript. C.C. initiated and conducted the experiments and FACS analysis. He analysed data, prepared illustrations and contributed to preparation of the manuscript. A.L. initiated and conducted the experiments and FACS analysis. He analysed data, prepared illustrations and contributed to preparation of the manuscript. G.T. assisted with the experiments and their analysis. He contributed to the preparation of the manuscript. B.M.H. initiated to design and organization of experiments. He reviewed all data, the preparation of illustrations and the manuscript. We examined changes in subsets of CD4+ T cells in multiple sclerosis (MS) patients, especially identifying subpopulations of CD4+CD25+Foxp3+CD127loT cells by their expression of CD45RA and the intensity of Foxp3 and CD25 expression to identify resting from activated Treg. These subpopulations were further subdivided by expression of chemokine receptors that identify naive Treg, and Th1 and Th17 associated Treg. We found depletion of resting Treg and increased activated Treg especially in patients with no recent relapses. This provides a basis for further assessment of activated Treg in MS and the potential to monitor remission and effects of therapy in MS.
Funding
This work was funded by donations from an anonymous donor and local Health resources. UNSW supported the studies of medical students, Mr Chiu and Mr Lam.
Competing interests
No authors have conflicts or financial interests directly related to this work. SJH receives support for clinical trials, consultancies and travel from Merck, Biogen, Atara, Genzyme and Novartis.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nirupama D. Verma, Email: nirupama.verma@unsw.edu.au
Suzanne J. Hodgkinson, Email: s.hodgkinson@unsw.edu.au
References
- 1.International MHC and Autoimmunity Genetics Network. Rioux JD, Goyette P, et al. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc. Natl. Acad. Sci. USA. 2009;106:18680–5. doi: 10.1073/pnas.0909307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australia and New Zealand Multiple Sclerosis Genetics Consortium A Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat. Genet. 2009;41:824–8. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 3.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noori-Zadeh A, Mesbah-Namin SA, Bistoon-Beigloo S, et al. Regulatory T cell number in multiple sclerosis patients: A meta-analysis. Mult. Sclerosis Relat. Disorders. 2016;5:73–76. doi: 10.1016/j.msard.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Venken K, Hellings N, Hensen K, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J. Neurosci. Res. 2006;83:1432–1446. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- 6.Michel L, Berthelot L, Pettré S, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J. Clin. Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalla Libera D, Di Mitri D, Bergami A, et al. T regulatory cells are markers of disease activity in multiple sclerosis patients. PLoS ONE. 2011;6:e21386. doi: 10.1371/journal.pone.0021386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodi M, Dimisianos N, de Lastic AL, et al. Regulatory cell populations in relapsing- remitting multiple sclerosis (RRMS) patients: effect of disease activity and treatment regimens. Int. J. Mol. Sci. 2016;17:E1398. doi: 10.3390/ijms17091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venken K, Hellings N, Thewissen M, et al. Compromised CD4+ CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2007;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J. Immunol. 2008;180:6411–20.. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 12.Frisullo G, Nociti V, Iorio R, et al. Regulatory T cells fail to suppress CD4T+-bet+ T cells in relapsing multiple sclerosis patients. Immunology. 2009;127:418–428. doi: 10.1111/j.1365-2567.2008.02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritzsching B, Haas J, König F, et al. Intracerebral human regulatory T cells: analysis of CD4+ CD25+ FOXP3+ T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PLoS ONE. 2011;6:e17988. doi: 10.1371/journal.pone.0017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balint B, Haas J, Schwarz A, et al. T-cell homeostasis in pediatric multiple sclerosis: old cells in young patients. Neurology. 2013;81:784–792. doi: 10.1212/WNL.0b013e3182a2ce0e. [DOI] [PubMed] [Google Scholar]
- 15.Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh-Esfahani S-H, Gharagozloo M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. J. Neuroimmunol. 2013;262:106–112. doi: 10.1016/j.jneuroim.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Serpero LD, Filaci G, Parodi A, et al. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J Neuroimmune Pharmacol. 2013;8:1106–1113. doi: 10.1007/s11481-013-9465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouchaki E, Salehi M, Reza Sharif M, Nikoueinejad H, Akbari H. Numerical status of CD4+CD25+FoxP3+ and CD8+CD28- regulatory T cells in multiple sclerosis, Iran. J. Basic Med. Sci. 2014;17:250–255. [PMC free article] [PubMed] [Google Scholar]
- 18.Trinschek B, Luessi F, Gross CC, Wiendl H, Jonuleit H. Interferon-beta therapy of multiple sclerosis patients improves the responsiveness of T Cells for immune suppression by regulatory T cells. Int. J. Mol. Sci. 2015;16:16330–16346. doi: 10.3390/ijms160716330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lifshitz GV, Zhdanov DD, Lokhonina AV, et al. Ex vivo expanded regulatory T cells CD4+CD25+FoxP3+CD127Low develop strong immunosuppressive activity in patients with remitting-relapsing multiple sclerosis. Autoimmunity. 2016;49:388–396. doi: 10.1080/08916934.2016.1199020. [DOI] [PubMed] [Google Scholar]
- 20.Khosravi M, Majdinasab N, Amari A, Ghadiri AA. Increased frequency of CD4 + CD25 high CD127 low / - regulatory T cells in patients with multiple sclerosis. Gene Rep. 2019;17:100456. doi: 10.1016/j.genrep.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J. Neurosci. Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 22.Sambucci M, Gargano F, De Rosa V, et al. FoxP3 isoforms and PD-1 expression by T regulatory cells in multiple sclerosis. Sci. Rep. 2018;8:3674. doi: 10.1038/s41598-018-21861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone F, De Rosa V, Carrieri PB, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 24.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp. immunol. 2007;147:412–418. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas J, Hug A, Viehöver A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur. J. Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 26.Venken K, Thewissen M, Hellings N, et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J. Immunol. Methods. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Putheti P, Pettersson A, Soderstrom M, Link H, Huang YM. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease- modulating drugs. J. Clin. Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 28.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 29.Haas J, Fritzsching B, Trübswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J. Immunol. 2007;179:1322–1330. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 31.Bjerg L, Brosbøl-Ravnborg A, Tørring C, et al. Altered frequency of T regulatory cells is associated with disability status in relapsing-remitting multiple sclerosis patients. J. Neuroimmunol. 2012;249:76–82. doi: 10.1016/j.jneuroim.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Muls NGV, Dang HA, Sindic CJM, van Pesch V. Regulation of Treg-associated CD39 in multiple sclerosis and effects of corticotherapy during relapse. Multiple Sclerosis J. 2015;21:1533–1545. doi: 10.1177/1352458514567215. [DOI] [PubMed] [Google Scholar]
- 33.Teniente-Serra A, Hervás JV, Quirant-Sánchez B, et al. Baseline differences in minor lymphocyte subpopulations may predict response to fingolimod in relapsing-remitting multiple sclerosis patients. CNS Neurosci Ther. 2016;22:584–592. doi: 10.1111/cns.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones AP, Trend S, Byrne SN, et al. Altered regulatory T-cell fractions and Helios expression in clinically isolated syndrome: clues to the development of multiple sclerosis. Clin. Transl. Immunol. 2017;6:e143. doi: 10.1038/cti.2017.18.eCollectionMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quirant-Sánchez B, Hervás-García JV, Teniente-Serra A, et al. Predicting therapeutic response to fingolimod treatment in multiple sclerosis patients. CNS Neurosci. Ther. 2018;24:1175–84. doi: 10.1111/cns.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Álvarez-Sánchez N, Cruz-Chamorro I, Díaz-Sánchez M, Lardone PJ, Guerrero JM, Carrillo-Vico A. Peripheral CD39-expressing T regulatory cells are increased and associated with relapsing-remitting multiple sclerosis in relapsing patients. Sci. Rep. 2019;9:2302. doi: 10.1038/s41598-019-38897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciccocioppo F, Lanuti L, Pierdomenico L, et al. The characterization of regulatory T-Cell profiles in Alzheimer's disease and multiple sclerosis. Sci. Rep. 2019;9:8788. doi: 10.1038/s41598-019-45433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron U, Floess S, Wieczorek G, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur. J. Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 39.Allan SE, Crome SQ, et al. Activation induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 40.Roncarolo M-G, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur. J. Immunol. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 42.Seddiki N, Santner-Nanan B, Tangye SG, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 43.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Aversa G, Waugh JA, Hall BM. A monoclonal antibody (A6) recognizing a unique epitope restricted to CD45RO and RB isoforms of the Leukocyte Common Antigen Family identifies functional T cell subsets. Cell Immunol. 1994;158:314–328. doi: 10.1006/cimm.1994.1279. [DOI] [PubMed] [Google Scholar]
- 45.Tosello V, Odunsi K, Souleimanian NE, et al. Differential expression of CCR7 defines two distinct subsets of human memory CD4+CD25+ Tregs. Clin. Immunol. (Orlando, Fla) 2008;26:291–302. doi: 10.1016/j.clim.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Matteucci E, Bartola LD, Giampietro O. Regulatory T cells with effector memory phenotype and glycaemic control in adult type I diabetes mellitus. J Diabetes Metab. 2013; S12.
- 47.Hoffmann J, Fiser K, Weaver J, et al. High-Throughput 13-parameter immunophenotyping identifies shifts in the circulating T-cell compartment following reperfusion in patients with acute myocardial infarction. PLoS ONE. 2012;7:e47115. doi: 10.1371/journa.pone.0047155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal BM. Stage-specific immune dysregulation in multiple sclerosis. J. Interferon Cytokine Res. 2014;34:633–640. doi: 10.1089/jir.2014.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T Helper Cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall BM. T cells: soldiers and spies-the surveillance and control of effector T Cells by regulatory T Cells. Clin. J. Am. Soc. Nephrol. 2015;10:2050–2064. doi: 10.2215/CJN.06620714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall BM, Tran GT, Verma ND, et al. Do natural T regulatory cells become activated to antigen specific t regulatory cells in transplantation and in autoimmunity? Front. Immunol. 2013;4:208. doi: 10.3389/fimmu.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mimic Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma ND, Plain KM, et al. CD4+CD25+T cells alloactivated ex vivo by IL-2 or IL-4, become potent alloantigen specific inhibitors of rejection with different phenotypes, suggesting Th1 and Th2 responses activate by separate pathways. Blood. 2009;113:479–87. doi: 10.1182/blood-2008-05-156612. [DOI] [PubMed] [Google Scholar]
- 55.Verma ND, Hall BM, Plain KM, et al. Interleukin-12 (IL-12p70) Promotes Induction of Highly Potent Th1-Like CD4+CD25+ T Regulatory Cells That Inhibit Allograft Rejection in Unmodified Recipients. Front. Immunol. 2014;9:190. doi: 10.3389/fimmu.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma ND, Davidson C, Robinson CM, et al. IL-13 prolongs allograft survival; associated with inhibition of macrophage cytokine activation. Transpl. Immunol. 2007;17:178–186. doi: 10.1016/j.trim.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 60.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin immunol (Orlando, Fla). 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orlando V, La Manna MP, Goletti D, et al. Human CD4 T-cells with a naive phenotype produce multiple cytokines during mycobacterium tuberculosis infection and correlate with active disease. Front. Immunol. 2018;9:1119. doi: 10.3389/fimmu.2018.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santner-Nanan B, Seddiki N, Zhu E, et al. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int. Immunol. 2008;20:375–383. doi: 10.1093/intimm/dxm151. [DOI] [PubMed] [Google Scholar]
- 63.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall BM, Pearce NW, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J. Exp. Med. 1990;171:141–57. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J. Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 66.van Langelaar J, van der Vuurst de Vries RM, Janssen M, et al. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain. 2018;141:1334–49. doi: 10.1093/brain/awy069. [DOI] [PubMed] [Google Scholar]
- 67.Yamazaki T, Yang XO, Chung Y, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]