Abstract
This study aimed to investigate the hydration influence on the autonomic responses of coronary artery disease subjects in the immediate recovery period after a cardiovascular rehabilitation session, in view of the risks of a delayed autonomic recovery for this population. 28 males with coronary artery disease were submitted to: (I) Maximum effort test; (II) Control protocol (CP), composed by initial rest, warm-up, exercise and passive recovery; (III) Hydration protocol (HP) similar to CP, but with rehydration during exercise. The recovery was evaluated through the heart rate (HR) variability, HR recovery and by the rate of perceived exertion and recovery. The main results revealed that the vagal reactivation occurred at the first 30 s of recovery in HP and after the first minute in CP. A better behavior of the HR at the first minute of recovery was observed in HP. The rate of perceived exertion had a significant decrease in the first minute of recovery in HP, while in CP this occurred after the third minute. In conclusion, despite an anticipated vagal reactivation found at HP, these results should be analyzed with caution as there were no significant differences between protocols for all variables and the effect sizes were small.
Subject terms: Cardiology, Rehabilitation
Introduction
Subjects with coronary artery disease (CAD) present an autonomic modulation impairment characterized by a decreased vagal activity and a sympathetic hyperactivity1, which is related to a risen risk of ischemic events, and a higher mortality rate2. Thus, the insertion of this population in cardiac rehabilitation programs (CRP) is essential, as physical exercise promotes positive modifications in the autonomic modulation3.
Despite the well-known physical exercise benefits, and although the rate of occurrence of major cardiovascular events during cardiac rehabilitation in stable patients is very low4,5, the post-exercise period represents a vulnerable state of the cardiovascular system, in which arrhythmias, syncope, and sudden death may occur6,7. During the early recovery phase, the autonomic nervous system (ANS) plays a central role in the cardiovascular deceleration, by rapidly decreasing the heart rate (HR) through vagal reactivation within the first minutes of recovery8. However, due to the sympathetic hyperactivity related to CAD, the vagal reentry is delayed in this population9. Thus, the HR, the cardiac workload and the myocardium oxygen consumption remain higher for a longer period, which favors the appearance of sudden events during the recovery10.
Given the risks related to a delayed vagal reactivation and HR reduction, the study of strategies capable of promoting a more efficient recovery on this population immediately after exercise is essential, as a faster recovery is related to a lower risk of sudden events2 and may contribute to the safety of CRP.
Considering that the fluid loss due to transpiration11 can negatively influence the ANS12 and directly affects the baroreflex mechanisms, contributing to the slower HR recovery after the exercise13, the fluid intake has been suggested as a strategy to accelerate the immediate14–17 and long-term18–20 autonomic and HR recovery after exercise. However, this topic has only been studied in healthy populations.
Thus, we highlight the importance of studying the influence of hydration in CAD subjects. Since this technic can be easily implemented in CRP as a strategy to decrease post-exercise risks, opening an important field of research in clinical practice, that may influence the current techniques used in these programs.
Considering these aspects, the study aimed to investigate the influence of hydration in the autonomic modulation of CAD patients, through HR recovery and HR variability, at the immediate recovery period of a cardiac rehabilitation session. Also, as secondary outcomes, its influence in the rate of perceived exertion, discomfort and recovery.
Method
Trial design and setting
This crossover clinical trial followed the Consolidated Standards of Reporting Clinical Trials (CONSORT) extension to randomized crossover trials21, and was registered at clinicaltrial.gov (NCT 03198806 – 26/06/2017).
The experimental procedure was divided into three phases with a minimal interval of 48 h between them and was performed at the Physical Therapy Study and Care Center—Presidente Prudente, São Paulo—Brazil. The phases were: Phase I. Cardiopulmonary Exercise Test; Phase II. Control Protocol (CP); and Phase III. Hydration Protocol (HP).
The volunteers were initially oriented to maintain their habitual physical activity habits while participating in the study, but to avoid vigorous physical activity and consumption of stimulant substances for 24 h before each phase, and to consume a light meal two hours before each phase22.
All procedures were approved by the Committee for Ethics and Research of the São Paulo State University, School of Technology and Science—UNESP (CAAE: 54864716.8.0000.5402) and followed the Helsinki Declaration. The volunteers were informed about the procedures and objectives of this study and, after agreeing to participate, signed the consent form.
Participants
Male patients of CRP who were previously diagnosed by their cardiologists with ischemic coronary artery disease and left ventricular ejection fraction higher than 50% were invited to participate in the study, after the previous analysis of the medical record regarding the inclusion/exclusion criteria.
Participants with less than 3 months of participation in CRP, those who were alcoholics and/or smokers, subjects with respiratory diseases, unstable angina, non-controlled hypertension, significant valvular disease, non-controlled metabolic disease, and/or neurological problems that could preclude the protocol execution were not included. The exclusion criteria were: abnormal hemodynamic responses during the cardiopulmonary exercise test23, presence of a series of RR intervals with less than 95% of sinus heartbeats22 and non-attendance to one of the protocol phases.
Interventions
Cardiopulmonary exercise test
To define the exercise load and to evaluate the hemodynamic responses, the volunteers performed a maximum stress test on a treadmill (INBRASPORT), according to Bruce’s protocol, that was preceded by 5 min of warming-up at 2.5 km/h24. The test was conducted by a physician and was interrupted by voluntary exertion and/or ischemia signs and/or severe arrhythmia. To determine the oxygen uptake (VO2), the exhaled gases were analyzed by the Quark PFT (COSMED) system25 calibrated with volumes and gases of known concentration. The exercise load used in Phases II and III was set as 60–80% of the HR reached at the anaerobic threshold. For those who did not reach the anaerobic threshold during the test, the oxygen uptake peak was considered23,26. This intensity is considered as low-moderate, which is safe and commonly used in CRP26. Exercises performed above the anaerobic threshold may produce negative acute responses in subjects with CAD, such as metabolic acidosis, hyperventilation, and reduction in the capacity of performing the exercise26.
Control and hydration protocols
The protocols were performed between 1:00 and 6:00 pm to avoid the circadian variation and the temperature and humidity of the room were controlled (22–25 °C and 40–60%, respectively)22.
After volunteer arrival, a urine sample was collected to identify the hydration status. Also, body mass and height were measured with a digital scale (BALMAK) and a stadiometer (SANNY), respectively.
After these procedures, the volunteers remained in the orthostatic position for 10 min. Then, they performed 15 min of warming-up, composed by stretching and global exercises, followed by 40 min of treadmill exercise, that was stopped without a cool-down period. After this, volunteers remained on the treadmill in orthostatic position for 10 min performing the passive recovery. After recovery, another urine sample was collected, and the body mass was measured. The axillar temperature was measured with a thermometer (G-TECH) before and immediately after exercise.
During the HP, volunteers ingested four equal portions of mineral water (BONAFONT) every 10 min during the treadmill exercise, as done in other studies18–20.
Hydration condition
The volunteers were instructed to ingest 500 ml of water two hours before the protocols to ensure an initial hydrated state27. The amount of water administered during the HP was obtained through the body mass difference verified before and after the CP, considering that one gram is equivalent to one milliliter of fluid lost, as described by Armstrong et al.28 and applied by other studies18–20.
The dynamic hydration status before and after the protocols was assessed by the urine analysis (Combur 10 M ROCHE). The urine specific density was used as a marker of the hydration level, and values above 1.020 classified the volunteer as dehydrated28.
Outcomes
Heart rate variability (HRV)
The vagal reactivation and the autonomic modulation were evaluated through HRV29, a non-invasive method based on the oscillations between consecutive RR intervals30 recorded through a HR monitor (POLAR ELECTRO)31.
To assess the vagal reactivation, the RMSSD index (root mean square of successive RR interval differences) was analyzed in 30-s intervals (RMSSD30) as proposed by Goldberger et al.32 and applied by other studies14,15,33. The last 30 s of the initial rest (M1), the last 30 s of the exercise (M2), and the first 2 min of the recovery, divided into four windows of 30 s each (M3, M4, M5, and M6), were considered.
This analysis was complemented by the evaluation of the autonomic modulation during the first 10 min of recovery. The last 5 min of the initial rest (REST) and the 10th-minute recovery divided into REC1 (0–5th minute) and REC2 (5th–10th minute) were considered. The HRV was analyzed in the time and frequency domains, as follows: LF (low-frequency component—0,04 to 0,15 Hz) and HF (high-frequency component—0,15 to 0,40 Hz) in milliseconds squared (ms2); LF/HF ratio; SDNN (standard deviation of all normal RR intervals); and RMSSD30.
The series of RR intervals were submitted to a digital filtration through the Polar Pro Trainer Software® (version 5.0, Polar Inc., Kempele, Finland)34 and only the series with more than 95% of sinus heartbeat were included. Also, visual analysis was performed to ensure the absence of artifacts or cardiac arrhythmias that could interfere in the analysis22. The indexes were calculated with the software Kubios HRV—version 2.035. This analysis was performed by an experienced and blinded researcher.
Heart rate recovery (HRR)
The parasympathetic reactivation after exercise was also indirectly evaluated by the HRR29. In order to determine the HRR, the HR was measured at three moments: at the peak of effort (HRpeak), at the first (HR1) and the second (HR2) minutes of recovery.
The HRpeak was defined as the mean of 5 RR intervals, considering two values before and two values after the HRpeak. The same procedure was used to obtain the HR1 and HR2. The HRR was defined by the difference between the HRpeak and HR1 (HRpeak—HR1 = HRR1) and between HRpeak and HR2 (HRRpeak—HR2 = HRR2)36. This procedure was also performed by an experienced and blinded researcher.
Rate of perceived exertion, recovery, and discomfort
The rate of perceived exertion (RPE) was evaluated by the Borg scale, an easy-to-use clinical tool commonly applied at the CRP to estimate the whole-body perceived effort, and it is known for indirectly correlating with the HR37. The Borg CR10 scale was used to define the perceived discomfort during the protocols. These scales were applied at the 10th minute of the rest, 15th and 35th minute of the exercise and 1st, 2nd, 3rd, 7th, and 10th minute of the recovery.
The rate of perceived recovery (RPR) was evaluated by a 10-point Likert scale, in which 1 corresponds to no recovery and 10 to fully recovered38 and it was applied during the recovery period.
Statistical analysis
The normality of data was determined by the Shapiro–Wilk test. To body mass, urine specific density, and axillar temperature, the comparison between the moments of the same protocol were performed by two-tailed paired Student’s t-test or Wilcoxon test. For the analysis between the protocols, two-tailed unpaired Student’s t-test or Mann–Whitney test were performed.
The comparison of HRR, HRV, RPE, RPR, and Borg CR10 between the protocols and moments was performed by two-way repeated-measures ANOVA. The data were checked for sphericity violation using Mauchly’s test and the Greenhouse–Geisser correction was considered when the sphericity was violated.
To analyze the moments during the same protocol, the Bonferroni post hoc test for parametric distribution or Dunnet post hoc test for non-parametric distributions were applied. The partial eta-squared effect size (η2P) was calculated for the ANOVA results (small ≤ 0.05; medium between 0.06 and 0.13; large ≥ 0.14)39. The HRR1 and HRR2 were analyzed between groups by the two-tailed unpaired Student’s t-test or Mann–Whitney test and the intragroup analysis was performed by the two-tailed paired Student’s t-test or Wilcoxon test. The Cohen’s d effect size was calculated for these analyses (small < 0.50; medium between 0.50 and 0.70; large between 0.80 and 1.20; very large ≥ 1.30)39.
The statistical significance was set at 5%. The analyses were performed using IBM SPSS Statistics—version 22.0 based on a coded data sheet by a blinded researcher.
Sample size
The sample size was based on the RMSSD index. The magnitude of significant difference assumed was 12 ms, considering a standard deviation of 16 ms36, with alfa risk of 5% and a beta risk of 80%, which resulted in a sample size of 28 volunteers. Considering the possible sample losses, we added 10% to the sample size calculated, totalizing 31 volunteers.
Results
Sample characterization and hydration condition
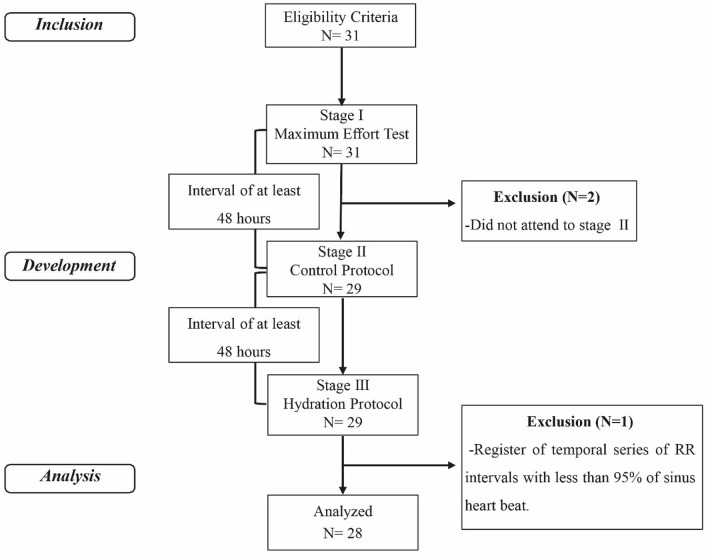
In total, 31 subjects were recruited (Fig. 1). After sample losses, 28 subjects were analyzed (Table 1).
Figure 1.
Protocol design.
Table 1.
Sample characterization (N = 28).
| Variables | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Age (years) | 63.61 ± 8.48 | 45.00 | 83.00 |
| Period of treatment in CR (years) | 3.27 ± 3.33 | 0.27 | 11.98 |
| Weight (kg) | 80.42 ± 12.94 | 58.00 | 108.50 |
| Height (m) | 1.71 ± 0.05 | 1.58 | 1.81 |
| BMI (kg/m2) | 27.44 ± 4.25 | 20.62 | 39.37 |
| SBP (mmHg) | 120.36 ± 8.38 | 110.00 | 150.00 |
| DBP (mmHg) | 77.50 ± 8.44 | 60.00 | 100.00 |
| Resting HR (bpm) | 72.50 ± 11.64 | 55.00 | 96.00 |
| Work load (km/h) | 4.92 ± 0.69 | 3.30 | 6.00 |
| Maximal stress test | |||
| VO2peak (ml/min/kg) | 26.10 ± 5.27 | 14.28 | 37.27 |
| Maximum HR (bpm) | 141.57 ± 20.70 | 103.00 | 173.00 |
| Risk factors N(%) | |||
| Diabetes | 11 (39.3%) | ||
| Dyslipidemia | 18 (64.3%) | ||
| Ex-smoker | 15 (53.6%) | ||
| High blood pressure | 25 (89.3%) | ||
| Family history | 20 (71.4%) | ||
| Obesity | 5 (17.9%) | ||
| Medicines N(%) | |||
| Anti-platelet drugs | 22 (78.6%) | ||
| ARA II | 10 (35.7%) | ||
| Ca+ channel blockers | 8 (28.6%) | ||
| K+ channel blockers | 1 (3.6%) | ||
| Beta bockers | 18 (64.3%) | ||
| Diuretics | 7 (25.0%) | ||
| Statins | 23 (82.1%) | ||
| Hypoglycemic agents | 7 (25.0%) | ||
| ACE inhibitor | 5 (17.9%) | ||
| Others | 8 (28.6%) | ||
| Vasodilator | 2 (7.1%) | ||
CR cardiac rehabilitation, kg kilogram, m meters, kg/m2 kilogram/meter2, mmHg millimeters of mercury, bpm beats per minute, ml/min/kg milliliter/minute/kilogram, km/h kilometer/hour, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, VO2peak peak oxygen consumption, ARA II angiotensin II receptor antagonists, ACE angiotensin-converting enzyme, Ca+ calcium, K+ potassium.
The body mass, axillar temperature, and urine specific density values are presented in Table 2. A significant reduction of the body mass was observed at the end of CP (0.345 ± 0.121 kg). Significant differences between the protocols for axillary temperature and specific density were not found.
Table 2.
Sample characterization in relation to body mass, axillar temperature, and urine specific density before and after protocols (N = 28).
| Variables | Moment | Control protocol | Hydration protocol |
|---|---|---|---|
| Body mass (kg) | Initial | 80.98 ± 12.71 | 80.98 ± 12.82 |
| Final | 80.64 ± 12.70a | 80.97 ± 12.80 | |
| Axillar temperature (°C) | Initial | 35.20 ± 0.54 | 35.02 ± 0.74 |
| Final | 34.90 ± 0.97 | 34.82 ± 0.98 | |
| Urine specific density | Initial | 1.016 ± 0.004 | 1.020 ± 0.035 |
| Final | 1.016 ± 0.004 | 1.014 ± 0.003 |
Mean ± standard deviation; kg kilogram, °C degree Celsius.
aDifference between the initial body mass in the control protocol; pvalue = 0.000 (two-talied paired student t test).
Heart rate variability and heart rate recovery analysis
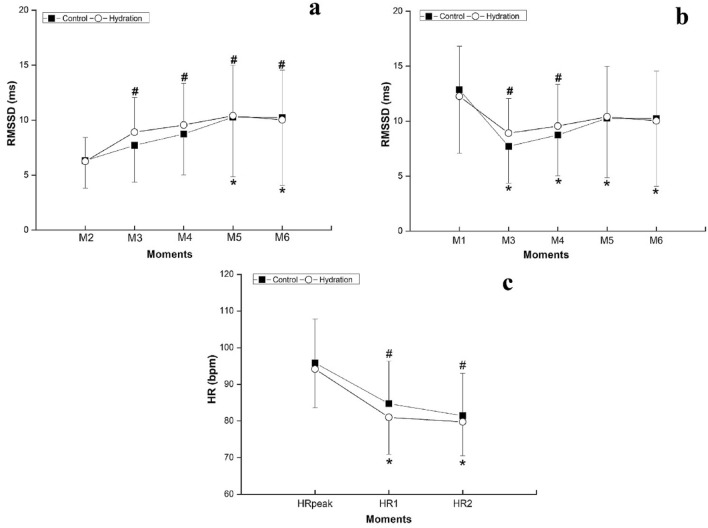
The RMSSD30 during the early recovery period was compared with the values at rest (Fig. 2b) and at the end of the exercise (Fig. 2a), to address the parasympathetic return to the rest values and the vagal reactivation, respectively. For both analysis, there were no significant differences between the protocols (2a: pvalue = 0.671; η2P = 0.003—small effect size/2b: pvalue = 0.791; η2P = 0.001—small effect size) and at the moments vs protocols interaction (2a: pvalue = 0.610; η2P = 0.012—small effect size/2b: pvalue = 0.469; η2P = 0.016—small effect size). However, there were differences between the moments (2a: pvalue < 0.001; η2P = 0.267—large effect size/2b: pvalue = 0.001; η2P = 0.237—large effect size).
Figure 2.
Comparison of RMSSD30 at exercise (a) and initial rest (b) and comparison between HRpeak and HR1 and HR2 (c). Legend: M1 = last 30 s of initial rest; M2 = last 30 s of exercise; M3 = 0–30 s, M4 = 30–60 s, M5 = 60–90 s, M6 = 90–120 s of recovery; HRpeak heart rate at the peak of exercise, HR1 heart rate after 1 min of recovery, HR2 heart rate after 2 min of recovery. *Difference between exercise (a)/initial rest (b) and recovery on control protocol (pvalue < 0.001); #Difference between exercise (a)/initial rest (b) and recovery on hydration protocol (pvalue < 0.05).
For the HR analysis (Fig. 2c), no significant differences were found between the protocols (pvalue = 0.583; η2P = 0.013—small effect size) and at the moments vs protocols interaction (pvalue = 0.305; η2P = 0.021—small effect size). However, between the moments significant differences were found (pvalue = 0.001; η2P = 0.781—large effect size).
In the CP significant differences between HRR1 (11.11 ± 6.57) and HRR2 (14.40 ± 5.41; pvalue < 0.0001; Cohen’s d = 0.55—medium effect size) were observed. However, for HP there were no differences between HRR1 (13.18 ± 8.02) and HRR2 (14.41 ± 6.60; pvalue = 0.132; Cohen’s d = 0.17—small effect size). Comparisons of HRR1 and HRR2 between the protocols showed no significant statistical differences (HRR1: pvalue = 0.237; Cohen’s d = 0.28—small effect size/HRR2: pvalue = 0.554; Cohen’s d = 0.00—small effect size).
The results of the 5 min analysis of SDNN, RMSSD, LF, HF, LF/HF are presented in Table 3. There were no statistical differences between the protocols.
Table 3.
Heart rate variability behavior for the 5-min windows in both protocols (N = 28).
| Variable | Protocol | REST | REC1 | REC2 | ANOVA results pvalue (η2P) |
|---|---|---|---|---|---|
| SDNN | Control | 29.31 ± 10.88 | 38.88 ± 13.34*** | 29.10 ± 10.46 | M: 0.000 (0.335) |
| Hydration | 31.43 ± 11.94 | 40.18 ± 12.26*** | 31.90 ± 11.94 | I: 0.864 (0.003) | |
| RMSSD | Control | 14.07 ± 6.68 | 9.96 ± 4.44*** | 10.73 ± 4.50*** | M: 0.000a (0.370) |
| Hydration | 14.91 ± 6.35 | 10.94 ± 4.09*** | 11.90 ± 5.20*** | I: 0.953 (0.001) | |
| LF (ms2) | Control | 232.82 ± 194.8 | 219.32 ± 310.41 | 321.14 ± 266.42 | M: 0.013 (0.079) |
| Hydration | 244.78 ± 194.33 | 299.53 ± 274.67 | 361.32 ± 341.09 | I: 0.614 (0.009) | |
| HF (ms2) | Control | 66.25 ± 72.20 | 37.64 ± 50.78* | 32.93 ± 28.20 | M: 0.000 (0.235) |
| Hydration | 73.14 ± 64.95 | 36.18 ± 29.17*** | 43.68 ± 48.17 | I: 0.627 (0.009) | |
| LF/HF | Control | 6.22 ± 4.68 | 7.46 ± 5.05 | 14.22 ± 17.72*** | M: 0.000 (0.204) |
| Hydration | 5.45 ± 4.57 | 9.90 ± 7.99** | 11.32 ± 8.54*** | I: 0.133 (0.037) |
Mean ± standard deviation. Bold values: significantly different from REST.
ms2 millisecond squared, REST last 5 min of initial rest, REC1 first 5 min of recovery, REC2 5th–10th minute of recovery, M moments, I interaction moments versus protocol.
***pvalue < 0.001; **pvalue < 0.01; *pvalue < 0.05.
Rate of perceived exertion and recovery analysis
For the RPE and RPR (Fig. 3), there were no significant differences between the protocols (RPE: pvalue = 0.302, η2P = 0,020—small effect size; RPR: pvalue = 0.390, η2P = 0.014—small effect size) and for the moments vs protocols interaction (RPE: pvalue = 0.360, η2P = 0.018—small effect size; RPR: pvalue = 0.743, η2P = 0.005—small effect size). However, for the moments, differences were observed for both scales (RPE: pvalue = 0.001, η2P = 0.519—large effect size; RPR: pvalue = 0.001, η2P = 0.607—large effect size).
Figure 3.

Mean and standard deviation for the rate of perceived recovery (a) and exertion (b) scale in both protocols. Legend: Rec 1 = 1st minute of recovery; Rec 3 = 3rd minute of recovery; Rec 5 = 5th minute of recovery; Rec 7 = 7th minute of recovery; Rec 10 = 10th minute of recovery; Res = initial rest; Ex 15 = 15th of treadmill exercise; Ex 35 = 35th minute of treadmill exercise; RPR rate of perceived recovery; RPE rate of perceived exertion. *Difference between Rec 1 and other moments of recovery (a)/initial rest and recovery (b) of control protocol (pvalue < 0.001); #Difference between Rec 1 and other moments of recovery (a)/initial rest and recovery (b) of hydration protocol (pvalue < 0.001).
Discussion
The results revealed that the hydration did not promote significant differences between the protocols for the vagal reactivation, autonomic modulation recovery, RPE, and RPR after a moderate-intensity session of exercise. However, small anticipation of vagal reactivation, autonomic modulation recovery, and RPE was observed when the hydration protocol was performed.
It is reported that hypohydration/dehydrated states promote a linear increase in body temperature40. However, it was not observed in the present study. This suggests that the exercise model used in CRP, even without fluid replacement, does not promote significant hypohydration/dehydration, which contributes to the safety of these programs, considering the deleterious effects of dehydration in the cardiovascular system. However, the hydration strategy used in our study was efficient to avoid fluid losses, as the body mass did not change in the HP.
The analysis of the RMSSD30, a marker of the parasympathetic modulation30, demonstrated the physiological gradual increase of vagal activity during recovery8. In the HP, the vagal reactivation may have occurred sooner, since, after the first minute of recovery, the RMSSD30 was no longer significantly different from the rest and, at the first 30 s of recovery the parasympathetic modulation was significantly higher than at the exercise. In the CP, the RMSSD30 did not return to the rest values and only became significantly higher than the exercise after the first minute of recovery. However, these results should be analyzed with caution due to the non-significant difference between the protocols and small effect sizes.
After exercise, the decrease of HR depends on the vagal reactivation8. The higher HRR1 observed at HP, even without significant difference between protocols, suggests a small acceleration of the parasympathetic reactivation at HP, in accordance with the RMSSD30 results. The low HRR1 and high HRR2 in the CP evidence a slower HR recovery when the volunteers did not ingest water. This response was not observed in HP, where HRR1 was as high as HRR2. Also, this faster HR reduction could explain the faster reduction of the RPE after exercise with water intake, since it is reported that the RPE scale correlates to the HR37.
The HRV indexes analyzed in 5-min windows had a physiological and similar response in both protocols. The significant increase of SDNN, which represents the global modulation at REC1 and its return to the rest value at REC2, in both protocols may be related to the vagal reactivation, evidenced in this study through the RMSSD30. In addition, the passive recovery in the orthostatic position for 10 min was insufficient to promote the total recovery of the parasympathetic modulation, even with the hydration.
This is the first study to investigate the influence of hydration in the immediate autonomic recovery of CAD subjects. This topic was previously studied in health and young males, who ingested 500 ml of water in a single dose immediately after moderate and high-intensity exercises14,15,17 and had a positive effect on the vagal reactivation and autonomic modulation recovery.
However, in our study, the volunteers ingested an individualized amount of water in fractionated doses throughout the exercise, as suggested by the American College of Sports Medicine23. The total amount of water ingested was 0.345 ± 0.11L, which was sufficient to avoid the fluid losses and, even if not significant, it promoted small anticipation of the recovery. However, if it is enough to minimize the appearance of sudden events during the recovery period is still an aspect that needs to be further studied.
Furthermore, another factor that may be responsible for the low effect of the hydration strategy used in our study is the exercise intensity proposed. The exercise in our experiment was performed bellow the anaerobic threshold, that classifies it as low-moderate intensity26, and it is known in the literature that, in healthy and young subjects, the higher the exercise intensity, the higher the sweating rate, fluid losses40, and the cardiovascular41 and autonomic12 perturbations. Thus, this aspect may justify the low effect of the hydration in the vagal reactivation, HRR and RPE.
As a study limitation, the randomization of the protocols was not possible because the amount of water ingested was obtained by assessing body mass loss after the CP. However, all participants were familiar with the proposed exercise protocol, so we believe that the exercise model performed in the study did not influence the outcomes analyzed.
Despite the small and non-significant effects between protocols found in the present study in the immediate recovery period of a cardiac rehabilitation session, the investigation of new hydration strategies capable of accelerating the recovery in CAD subjects is important and should be encouraged, since those individuals present an autonomic impairment1 and have a less efficient recovery after exercise. Therefore, from our preliminary results, new studies that include larger samples and that insert randomization of different hydration strategies during exercise, can be designed. In addition, it may be interesting to analyze a longer recovery period to understand the impact of hydration strategies on the autonomic behavior in the recovery period of CAD patients.
Conclusions
The hydration protocol performed did not promote significative changes in the vagal reactivation, autonomic modulation recovery, and rate of perceived exertion and recovery, when compared to the control protocol. However, the hydration protocol avoided the fluid losses induced by exercise and promoted small anticipation of vagal reactivation and autonomic modulation recovery and a small reduction at the rate of perceived exertion.
Acknowledgements
We would like to thank the Foundation for Research Support of the State of São Paulo (FAPESP) and the Laboratory of Cell Physiology of Exercise (LAFICE) for having provided the space and equipment.
Author contributions
M.J.L.L., A.K.F.d.S., L.M.V., L.C.M.V. wrote the main manuscript text. M.J.L.L., A.K.F.d.S., L.A.S., F.R., L.M.V. were responsible for designing the study. M.J.L.L., A.K.F.d.S., L.A.S., F.R., D.A.G.C. monitored the study development. M.J.L.L., L.M.V., L.C.M.V. decided and performed the analytic strategy. M.J.L.L., L.A.S., F.R., D.A.G.C. collected and handled the data. All authors reviewed the manuscript.
Funding
This work was supported by the Foundation for Research Support of the State of São Paulo (FAPESP) under Grant Number 2017/03142–1 and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under Grant Number 401258/2016–5.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur. Heart J. 2015;36:1974–1982. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu S, et al. Heart rate recovery and risk of cardiovascular events and all-cause. J. Am. Heart Assoc. 2017;6:1–12. doi: 10.1161/JAHA.117.005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu C, Hsieh P, Hsiao S, Chien M. Effects of exercise training on autonomic function in chronic heart failure: systematic review. Biomed. Res. Int. 2015;2015:1–8. doi: 10.1155/2015/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feiereisen P, Delagardelle C. Retrospective analysis of cardiac events during cardiac rehabilitation at Centre Hospitalier de Luxembourg during 2014 and 2015. Bull. Soc. Sci. Med. Grand. Duche Luxemb. 2016;2:13–25. [PubMed] [Google Scholar]
- 5.Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch. Intern. Med. 2006;166:2329–2334. doi: 10.1001/archinte.166.21.2329. [DOI] [PubMed] [Google Scholar]
- 6.Romero SA, Minson CT, Halliwill XJR. Recovery from exercise the cardiovascular system after exercise. J. Appl. Physiol. 2017;122:925–932. doi: 10.1152/japplphysiol.00802.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luttrell MJ, Halliwill JR. Recovery from exercise: vulnerable state, window of opportunity, or crystal ball? Front. Physiol. 2015;6:1–6. doi: 10.3389/fphys.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peçanha T, Silva-Júnior ND, Forjaz CLDM. Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging. 2014;34:327–339. doi: 10.1111/cpf.12102. [DOI] [PubMed] [Google Scholar]
- 9.Imai K, et al. Vagally mediated heart rate recovery after exercise is accelerated in athlestes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 10.Thompson PD, et al. Exercise and acute cardiovascular events: placing the risks into perspective a scientific statement from the American heart association council on nutrition, physical activity, and metabolism and the council on clinical cardiology. Circulation. 2007;115:2358–2369. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 11.Shibasaki M, Wilson TE, Crandall CG. A physiological systems approach to human neural control and mechanisms of eccrine sweating during heat stress and exercise. J. Appl. Physiol. 2006;100:1692–1701. doi: 10.1152/japplphysiol.01124.2005. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Sepúllveda M, et al. Hydration status after exercise affect resting metabolic rate and heart rate variability. Nutr. Hosp. 2015;31:1273–1277. doi: 10.3305/nh.2015.31.3.8523. [DOI] [PubMed] [Google Scholar]
- 13.Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J. Physiol. 2003;552:635–644. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira TP, Ferreira RB, de Mattos RA, da Silva JP, de Lima JRP. Influence of water intake on post-exercise heart rate variability recovery. J. Exerc. Physiol. Online. 2011;14:97–105. [Google Scholar]
- 15.Peçanha T, et al. Water intake accelerates parasympathetic reactivation after high-intensity exercise. Int. J. Sport Nutr. Exerc. 2014;24:489–496. doi: 10.1123/ijsnem.2013-0122. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira AL, et al. The role of water intake on cardiac vagal reactivation after upper-body resistance exercise. Int. J. Sport Med. 2015;36:204–208. doi: 10.1055/s-0034-1389971. [DOI] [PubMed] [Google Scholar]
- 17.Vianna LC, Oliveira RB, Silva BM, Ricardo DR, Araújo CGS. Water intake accelerates post-exercise cardiac vagal reactivation in humans. Eur. Appl. Physiol. 2008;102:283–288. doi: 10.1007/s00421-007-0584-7. [DOI] [PubMed] [Google Scholar]
- 18.Moreno IL, et al. Effects of an isotonic beverage on autonomic regulation during and after exercise. J. Int. Soc. Sport Nutr. 2013;10:1–10. doi: 10.1186/1550-2783-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderlei FM, Moreno IL, Carlos L, Vanderlei M, Pastre CM. Effects of different protocols of hydration on cardiorespiratory parameters during exercise and recovery. Int. Arch. Med. 2013;6:1–10. doi: 10.1186/1755-7682-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderlei FM, et al. Comparison of the effects of hydration with water or isotonic solution on the recovery of cardiac autonomic modulation. Int. J. Sport Nutr. Exerc. 2015;25:145–153. doi: 10.1123/ijsnem.2014-0004. [DOI] [PubMed] [Google Scholar]
- 21.Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. doi: 10.1136/bmj.l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catai AM, et al. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz. J. Phys. Ther. 2019;20:91–102. doi: 10.1016/j.bjpt.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. Wolters Kluwer; 2014. [DOI] [PubMed] [Google Scholar]
- 24.Meneghelo RS, et al. III Diretrizes da Sociedade Brasileira de Cardiologia Sobre Teste Ergométrico. Rev. Bras. Fisioter. 2011;95:1–26. doi: 10.1590/S0066-782X2010000800001. [DOI] [PubMed] [Google Scholar]
- 25.Cabral-Santos C, et al. Similar anti-inflammatory acute responses from moderate-intensity continuous and high-intensity intermittent exercise. J. Sport Sci. Med. 2015;14:849–856. [PMC free article] [PubMed] [Google Scholar]
- 26.Mezzani A, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitat. Eur. J. Prev. Cardiol. 2013;20:442–467. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]
- 27.Sawka MN, et al. Exercise and fluid replacement. Med. Sci. Sport Exerc. 2007;39:377–390. doi: 10.1249/01.mss.0000272779.34140.3b. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong LE. Hydration assessment techniques. Nutr. Rev. 2005;63:S40–S54. doi: 10.1111/j.1753-4887.2005.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 29.Peçanha T, et al. Methods of assessment of the post-exercise cardiac autonomic recovery: a methodological review. Int. J. Cardiol. 2017;227:795–802. doi: 10.1016/j.ijcard.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 30.Vanderlei LCM, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir Cardiovasc. 2009;24:205–217. doi: 10.1590/S0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 31.Vanderlei LCM, Silva RA, Pastre CM, Azevedo FM, Godoy MF. Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz. J. Med. Biol. Res. 2008;41:854–859. doi: 10.1590/S0100-879X2008005000039. [DOI] [PubMed] [Google Scholar]
- 32.Goldberger JJ, et al. Assessment of parasympathetic reactivation after exercise. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2446–H2452. doi: 10.1152/ajpheart.01118.2005. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez MG, Ribeiro F, Gomes RL, Valenti VE, Vanderlei LCM. Impact of musical auditory stimulation applied during and after aerobic exercise on vagal reentry in recovery period. J. Cardiol. Ther. 2017;4:588–593. doi: 10.17554/j.issn.2309-6861.2017.04.123-2. [DOI] [Google Scholar]
- 34.Nunan D, et al. Validity and reliability of short-term heart-rate variability from the Polar S810. Med. Sci. Sports Exerc. 2009;41:243–250. doi: 10.1249/MSS.0b013e318184a4b1. [DOI] [PubMed] [Google Scholar]
- 35.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput. Methods Prog. Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Chen J-L, et al. Parasympathetic nervous activity mirrors recovery status in weightlifting performance after training. J. Strength Cond. Res. 2011;25:1546–1552. doi: 10.1519/JSC.0b013e3181da7858. [DOI] [PubMed] [Google Scholar]
- 37.Borg GAV. Psychophysical bases of perceived exertion. Med. Sci. Sport Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 38.Laurent C, et al. A practical approach to monitoring recovery: development of a perceived recovery status scale. J. Strength Cond. Res. 2011;25:620–628. doi: 10.1519/JSC.0b013e3181c69ec6. [DOI] [PubMed] [Google Scholar]
- 39.Maher JM, Markey JC, Ebert-may D. The other half of the story: effect size analysis in quantitative research. CBE Life Sci. Educ. 2013;12:345–351. doi: 10.1187/cbe.13-04-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mcdermott BP, et al. National athletic trainers’ association position statement: fluid replacement for the physically active. J. Athl. Train. 2017;52:877–895. doi: 10.4085/1062-6050-52.9.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González-Alonso J, Mora-Rodríguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am. J. Physiol. Heart Circ. Physiol. 2000;278:321–330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]