Abstract
Two trials were designed to investigate the impacts of egg storage time and maternal dietary vitamin E (VE) supplementation on the growth performance and antioxidant capacity of progeny chicks. In total 512 Ross 308 broiler breeder hens (71-wk-old) were assigned to 2 dietary VE treatments (6 and 100 mg/kg) for 14 wk. Progeny chicks used in trials 1 and 2 were originated from eggs laid at week 10 (stored 0 d) and week 8 (stored 14 d), and week 14 (stored 0 d) and week 12 (stored 14 d), respectively. The 4 groups in trial 1 consisted of 2 levels of maternal VE (6 and 100 mg/kg) and 2 egg storage time (0 and 14 d). The 8 groups in trial 2 consisted of 2 levels of maternal VE (6 and 100 mg/kg), 2 egg storage time (0 and 14 d) and progeny sex (male and female). In trial 1, egg storage decreased the body weight, the liver total superoxide dismutase and total antioxidant capacity of 21-day-old offspring (P < 0.05), and the body weight gain and feed intake from 8 to 21 d and 1 to 21 d (P < 0.05); and increased the serum and liver malonaldehyde (MDA) of 7-day-old offspring and the ratio of feed: gain (F/G) from 1 to 7 d (P < 0.05). Maternal VE (100 vs. 6 mg/kg) decreased the F/G from 1 to 7 d and increased the serum total superoxide dismutase of 21-day-old offspring (P < 0.05). In trial 2, egg storage decreased the body weight of 42-day-old offspring, and the body weight gain and feed intake from 22 to 42 d and 1 to 42 d (P < 0.05); and increased the serum and liver MDA of 21- and 42-day-old offspring (P < 0.05). Maternal VE (100 vs. 6 mg/kg) reduced the serum MDA of 7-day-old offspring (P < 0.05). Interactively, maternal VE (100 vs. 6 mg/kg) reduced the serum MDA of offspring originated from stored eggs (P < 0.05), but not for that of offspring originated from unstored eggs in the two trials. It can be concluded that egg storage (14 vs. 0 d) decreased the growth performance and antioxidant capacity of offspring, while maternal dietary VE (100 vs. 6 mg/kg) supplementation could partly alleviate the reduction of antioxidant capacity (except for growth performance) of offspring induced by egg storage for the early phase post-hatch.
Key words: egg storage, maternal vitamin E, performance, antioxidant capacity, offspring
INTRODUCTION
As a major lipid-soluble antioxidant, vitamin E (VE) has revealed protection against oxidative stress (Shirpoor et al., 2009). However, VE cannot be synthesized by poultry, and exclusively depend on the dietary supplementation. According to NRC (1994), the recommendation of dietary VE for broiler breeder is 6 mg/kg. Previous studies indicated that the live performance of broiler breeder hens, egg characteristics, hatchability, and hatched chick quality were not affected by dietary VE supplemented range from 0 to 150 mg/kg (Hossain et al., 1998; Shahriar et al., 2007; Urso et al., 2015; Lin et al., 2017; Yaripour et al., 2018). Devine et al. (2012) and Liu et al. (2018) indicated that ovarian aging induced by oxidative stress is responsible for the decreased live performance of aged hens. Broiler breeder dietary supplementation of VE range from 0 to 365 mg/kg showed a positive effect on an increase in the antioxidant capacity of egg yolks, as well as an increase in development of embryo and hatchling (Surai et al., 1999; Surai, 2000; Tsai et al., 2008). Meanwhile, as hatched chicks have limited ability to assimilate the dietary VE (Surai and Fisinin, 2012), it seems that the antioxidant capacity of hatched chicks appear to be particularly important for the early growth (Surai et al., 2016). However, few trials have demonstrated that dietary VE supplemented range from 10 to 300 mg/kg showed no influence on the performance of offspring (Hossain et al., 1998; Siegel et al., 2006).
The reproductive performance of broiler breeder is influenced by numerous factors, primarily including bird strain, age, diet, flock and hatchery management (Chang et al., 2016). Fertilized eggs are usually stored for a few days before hatching in commercial poultry production. The total length of egg storage duration dependent upon the egg transporting distance, capacity of hatchery, and market demand of hatched chicks (Reijrink et al., 2008). Generally, short egg storage duration (≤ 7 d) seems to present little adverse influence on the hatchability (Elibol et al., 2002; Reijrink et al., 2009), and it even could increase the hatchability of eggs originated from young flocks (Pokhrel et al., 2018). However, egg storage duration (> 7 d) resulted in an increase in embryonic mortality and a decline in hatchability (Dymond et al., 2013; Gharib, 2013; Ebeid et al., 2017; Nasri et al., 2020). Besides, the quality of hatched chicks and their performance post-hatch were also impaired by prolonged egg storage (Tona et al., 2003; Tona et al., 2004; Petek and Dikmen, 2006; Damaziak et al., 2018).
Ebeid et al. (2017) demonstrated that egg storage (14 vs. 4 d) damaged the antioxidant capacity of hatched chicks, which suggested that oxidative stress happened in the process of egg storage. In our previous study, we have observed that maternal VE (100 vs. 6 mg/kg) supplementation could partly alleviate the decrease of hatchability traits and antioxidant capacity of hatched chicks induced by egg storage (Yang et al., 2020a). Yet, it is not clear whether or not oxidative stress is involved in the impact of egg storage on the performance of offspring, and maternal dietary VE supplementation can further exert positive effects in this process. Therefore, the goal of the present study was to investigate the impacts of egg storage duration and maternal dietary VE supplementation on the performance and antioxidant capacity of progeny chicks.
MATERIALS AND METHODS
The animal procedures involved in the present study were approved by the Animal Care and Use Committee, Sichuan Agricultural University.
Experimental Design and Management
Five hundred and twelve Ross 308 broiler breeder hens (71-wk-old, induced molting at the age of 61 wk) were allocated into two dietary VE groups (6 and 100 mg/kg of feed, Table 1), with 8 replicates of 32 hens in each replicate for 14 wk. The management of breeders was the same as the description of Yang et al. (2020a). Qualified eggs (all eggs except abnormal, cracked, shell-less, dirty eggs and small eggs below 52 g) per group were checked and collected over 5-d duration at weeks 8, 10, 12 and 14. At weeks 8 and 12, half of collected eggs were divided and stored for an addition of 14 d and turned every 4 h during storage until incubated.
Table 1.
Composition and nutritional levels of the basal diet (%, as fed-basis).1
| Item | Broiler breeder | Offspring |
||
|---|---|---|---|---|
| 1 to 21 d | 22 to 42 d | |||
| Ingredient | ||||
| Corn | 69.50 | 69.50 | 50.86 | 51.07 |
| Soybean meal, 43% | 19.00 | 19.00 | 30.42 | 22.28 |
| Soybean oil | 1.00 | 1.00 | 2.36 | 3.90 |
| Wheat flour | — | — | 4.00 | 6.00 |
| Gluten meal | — | — | 3.00 | 4.00 |
| Rapeseed meal | — | — | 2.00 | 3.60 |
| Corn distiller dried grains with solubles | — | — | 3.00 | 5.00 |
| Calcium hydrophosphate | 1.14 | 1.14 | 1.76 | 1.56 |
| Limestone | 8.25 | 8.25 | 1.10 | 1.12 |
| Sodium chloride | 0.30 | 0.30 | 0.32 | 0.31 |
| Vitamin and mineral premix2 | 0.50 | 0.50 | 0.20 | 0.20 |
| DL-Methionine, 99% | 0.11 | 0.11 | 0.27 | 0.21 |
| L-Lysine hydrochloride, 98.5% | 0.08 | 0.08 | 0.45 | 0.51 |
| Threonine, 98.5% | 0.02 | 0.02 | 0.14 | 0.14 |
| Choline chloride, 50% | 0.10 | 0.10 | 0.12 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutritional composition | ||||
| Metabolizable energy (kcal/kg) | 2780.00 | 2780.00 | 2925.00 | 3050.00 |
| Crude protein | 13.80 | 13.80 | 21.80 | 20.00 |
| Available phosphorus | 0.30 | 0.30 | 0.45 | 0.42 |
| Calcium | 3.40 | 3.40 | 0.95 | 0.90 |
| Digestible methionine | 0.32 | 0.32 | 0.57 | 0.52 |
| Digestible lysine | 0.66 | 0.66 | 1.25 | 1.15 |
| Digestible methionine + cystine | 0.53 | 0.53 | 0.89 | 0.82 |
| Digestible threonine | 0.46 | 0.46 | 0.81 | 0.75 |
| Vitamin E (mg/kg) | 6.00 | 100.00 | 100.00 | 100.00 |
| Determined values | ||||
| Crude protein | 13.98 | 13.92 | 22.05 | 20.14 |
| Total phosphorus | 0.46 | 0.47 | 0.65 | 0.61 |
| Calcium | 3.55 | 3.63 | 0.96 | 0.88 |
| Vitamin E (mg/kg) | 10.50 | 106.20 | 92.70 | 101.30 |
Breeder diet: a basal diet was firstly prepared without any vitamin E addition, which was divided into two equal portions, and then vitamin E (DL-α-Tocopherol Acetate) was added to each part at rate of 6 and 100 mg/kg and mixed to provide the two breeder dietary treatments. Broiler diet: supplied with 100 mg/kg vitamin E (DL-α-Tocopherol Acetate).
Supplied per kg feed. Broiler breeder: VA, 12000 IU; VD3, 4000 IU; VE, 0.00 mg; VK3, 4.0 mg; VB12, 0.02 mg; thiamin, 3.0 mg; pyridoxine, 7.2 mg; riboflavin, 11.5 mg; niacin, 47.1 mg; folic acid, 10.8 mg; biotin, 0.6 mg; pantothenic acid, 21.6 mg; copper, 20 mg; iron, 80 mg; manganese, 82.5 mg; selenium, 0.3 mg; zinc, 100 mg; iodine, 1.2 mg. Offspring: VA, 10000 IU; VD3, 4000 IU; VE, 100 mg; VK3, 3 mg; VB12, 0.02 mg; thiamin, 3.0 mg; pyridoxine, 4 mg; riboflavin, 10 mg; niacin, 65 mg; folic acid, 2 mg; biotin, 0.2 mg; pantothenic acid, 15 mg; copper, 10 mg; iron, 80 mg; manganese, 120 mg; selenium, 0.3 mg; zinc, 100 mg; iodine, 1.0 mg.
The randomized complete block design was used in this study. In trial 1, the 2 × 2 factorial arrangement was applied, and the 4 groups consisted of 2 egg storage time (0 and 14 d) and 2 levels of maternal VE (6 and 100 mg/kg). Eggs stored for 0 d were collected at week 10 and eggs stored for 14 d with 22-24°C (common storage temperature in China as storage time ≤ 7 days) were collected at week 8. At hatch, 104 chicks originated from each hatched treatment were selected, weighed and allocated to 8 replicates with 13 chicks (5 males and 8 females) per pen (1.0 m × 2.0 m). Trial 1 lasted for 21 days. In trial 2, the 2 × 2 × 2 factorial arrangement was applied, and the 8 groups consisted of 2 egg storage time (0 and 14 d), 2 levels of maternal VE (6 and 100 mg/kg) and progeny sex (male and female). Eggs stored for 0 d were collected at week 12 and eggs stored for 14 d with 16 to 18°C were collected at week 10. At hatch, 160 chicks originated from each hatched treatment were selected, sexed, weighed and allocated to 4 male replicates and 4 female replicates with 20 chicks per pen (1.0 m × 2.0 m). Trial 2 lasted for 42 d.
All chicks in the two trials received the same commercial pellet diets with an addition of 100 mg/kg VE (Table 1). The house temperature was initially set at 33 ± 1°C and then gradually reduced to 21 to 22°C after 35 days with a reduction of 0.3°C per day. Water and feed were supplied ad libitum. The two trials were conducted in a commercial broiler farm located at Shehong city (Sichuan Province, China).
The breeder and broiler diets were both formulated based on Ross 308 recommendation. For feed samples, crude protein (method 990.03), total phosphorus (method 964.06), and Calcium (method 935.13) were determined as described by AOAC International (2006). VE content was determined using the high-performance liquid chromatography method (Ministry of Health of the People's Republic of China and Standardization Administration of China, 2008).
Feed Intake and Body Weight
Feed intake (FI) and body weight (BW) per pen were determined at 1, 7, and 21 d of age in trial 1, and at 1, 7, 21, and 42 day of age in trial 2. Body weight gain (BWG) and adjusted feed conversion ratio (F/G) based on mortality per pen were calculated.
Sample Collection and Analysis
Two birds (one male and one female) from each pen in trial 1 (at 7 and 21 d of age) and one bird from each pen in trial 2 (at 7, 21, and 42 d of age) were randomly selected for the collection of liver and jugular vein blood samples. The preparation of serum and liver supernatant, and then the determination of total antioxidant capacity (T-AOC), malonaldehyde (MDA) content and total superoxide dismutase (T-SOD) activity in the liver and serum samples were in accordance with the description of Yang et al. (2020b).
Statistical Analysis
Data of trial 1 and 2 were analyzed by ANOVA as 2 × 2 and 2 × 2 × 2 factorial, respectively, using generalized linear model procedures of IBM SPSS Statistics 21.0. The main effects (egg storage time and maternal VE levels in trial 1; egg storage time, maternal VE levels and progeny sex in trial 2) and their interactions were investigated. When the interactions were significantly difference, Tukey's test was applied. Statistical significance is based on P-value < 0.05.
RESULTS
Growth Performance and Antioxidant Capacity in Trial 1
Results presented in Table 2 indicated that there was no significant interaction between maternal VE levels (100 vs. 6 mg/kg) and egg storage time (14 vs. 0 d) on the growth performance of offspring (P > 0.05). Egg storage time (14 vs. 0 d) decreased the BWG and FI of offspring from 8 to 21 d and 1 to 21 d, and the BW of 21-day-old offspring (P < 0.05); and increased the F/G of offspring from 1 to 7 d (P < 0.05). Maternal VE (100 vs. 6 mg/kg) reduced the F/G of offspring from 1 to 7 d (P < 0.05), and showed a trend in increasing the BW of 7-day-old offspring (P = 0.080) and the F/G of offspring from 1 to 7 d (P = 0.079).
Table 2.
The impacts of egg storage time (0 and 14 d) and maternal dietary vitamin E (6 and 100 mg/kg) on the growth performance of progeny chicks (trial 1).
| Items | BW (g) |
BWG (g) |
FI (g) |
F/G |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 7 d | 21 d | 1–7 d | 8–21 d | 1–21 d | 1–7 d | 8–21 d | 1–21 d | 1–7 d | 8–21 d | 1–21 d | |
| Egg storage (d) | ||||||||||||
| 0 | 45.7 | 167.2 | 892.3a | 121.5 | 725.1a | 846.6a | 142.4 | 995.3a | 1134.2a | 1.173b | 1.373 | 1.340 |
| 14 | 45.8 | 160.3 | 867.4b | 114.6 | 707.1b | 821.7b | 138.7 | 963.5b | 1099.6b | 1.214a | 1.363 | 1.339 |
| SEM | 0.1 | 2.5 | 8.1 | 2.5 | 6.0 | 8.1 | 2.7 | 7.7 | 9.7 | 0.013 | 0.005 | 0.004 |
| Vitamin E (mg/kg) | ||||||||||||
| 6 | 45.7 | 160.6 | 873.0 | 114.8 | 712.4 | 827.2 | 139.2 | 971.7 | 1108.3 | 1.215a | 1.364 | 1.340 |
| 100 | 45.7 | 167.0 | 886.8 | 121.3 | 719.8 | 841.0 | 141.9 | 987.1 | 1125.5 | 1.172b | 1.371 | 1.338 |
| SEM | 0.1 | 2.5 | 8.1 | 2.5 | 6.0 | 8.1 | 2.7 | 7.7 | 9.7 | 0.013 | 0.005 | 0.004 |
| P-value | ||||||||||||
| Egg storage | 0.544 | 0.064 | 0.039 | 0.059 | 0.042 | 0.039 | 0.334 | 0.007 | 0.014 | 0.041 | 0.136 | 0.802 |
| Vitamin E | 0.986 | 0.080 | 0.241 | 0.079 | 0.388 | 0.240 | 0.470 | 0.167 | 0.204 | 0.033 | 0.274 | 0.786 |
| Egg storage × Vitamin E | 0.722 | 0.114 | 0.188 | 0.116 | 0.258 | 0.190 | 0.104 | 0.314 | 0.201 | 0.698 | 0.699 | 0.546 |
Different superscript alphabets in the same column means significantly different (P < 0.05).
Abbreviations: BW, body weight; BWG, body weight gain; FI, feed intake; F/G, feed conversion ratio; SEM, standard error of the mean.
As shown in Table 3, maternal VE (100 vs. 6 mg/kg) reduced the serum T-SOD activity of 21-day-old male offspring (P < 0.05). An interaction was observed between maternal VE levels (100 vs. 6 mg/kg) and egg storage time (14 vs. 0 d) on the serum MDA content of 7-day-old female offspring (P < 0.05). No difference was found between maternal VE levels (100 vs. 6 mg/kg) on the serum MDA content of 7-day-old female offspring originated from unstored eggs (P > 0.05). However, maternal VE (100 vs. 6 mg/kg) reduced the serum MDA content of 7-day-old female offspring originated from stored eggs (P < 0.05). As shown in Table 4, egg storage time (14 vs. 0 d) increased the liver MDA content of 7-day-old female offspring (P < 0.05), and decreased the liver T-AOC of 21-day-old female offspring and the liver T-SOD activity of 21-day-old male offspring (P < 0.05).
Table 3.
The impacts of egg storage time (0 and 14 d) and maternal dietary vitamin E (6 and 100 mg/kg) on the serum antioxidant capacity of progeny chicks (trial 1).
| Male |
Female |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-AOC (μmol/mL) |
MDA (nmol/mL) |
T-SOD (U/mL) |
T-AOC (μmol/mL) |
MDA (nmol/mL) |
T-SOD (U/mL) |
||||||||
| Items | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | |
| Egg storage (d) | Vitamin E (mg/kg) | ||||||||||||
| 0 | 6 | 1.10 | 0.99 | 3.48 | 3.60 | 233.7 | 254.7 | 1.05 | 0.87 | 2.90b | 3.58 | 225.4 | 307.8 |
| 0 | 100 | 1.16 | 1.09 | 3.27 | 3.61 | 242.2 | 315.6 | 0.98 | 0.89 | 3.04b | 3.60 | 267.8 | 282.2 |
| 14 | 6 | 1.26 | 1.26 | 3.20 | 3.85 | 251.4 | 243.2 | 0.99 | 0.84 | 4.00a | 3.68 | 233.2 | 220.9 |
| 14 | 100 | 1.10 | 1.18 | 3.12 | 3.73 | 265.4 | 302.0 | 1.14 | 0.90 | 3.09b | 3.46 | 216.8 | 265.0 |
| SEM | 0.059 | 0.062 | 0.068 | 0.069 | 11.0 | 11.3 | 0.054 | 0.033 | 0.109 | 0.087 | 14.1 | 15.9 | |
| Egg storage (d) | |||||||||||||
| 0 | 1.13 | 1.04 | 3.37 | 3.61 | 237.9 | 285.0 | 1.01 | 0.88 | 2.97b | 3.59 | 246.6 | 295.0 | |
| 14 | 1.18 | 1.22 | 3.16 | 3.82 | 258.4 | 272.6 | 1.07 | 0.87 | 3.55a | 3.57 | 225.0 | 243.0 | |
| SEM | 0.083 | 0.087 | 0.096 | 0.097 | 15.6 | 15.9 | 0.076 | 0.046 | 0.155 | 0.124 | 20.0 | 22.5 | |
| Vitamin E (mg/kg) | |||||||||||||
| 6 | 1.18 | 1.12 | 3.34 | 3.73 | 242.5 | 248.9b | 1.02 | 0.86 | 3.45 | 3.63 | 229.3 | 264.3 | |
| 100 | 1.13 | 1.14 | 3.19 | 3.70 | 253.8 | 308.8a | 1.06 | 0.89 | 3.06 | 3.53 | 242.3 | 273.6 | |
| SEM | 0.083 | 0.087 | 0.096 | 0.097 | 15.6 | 15.9 | 0.076 | 0.046 | 0.155 | 0.124 | 20.0 | 22.5 | |
| P-value | |||||||||||||
| Egg storage | 0.692 | 0.159 | 0.122 | 0.139 | 0.363 | 0.582 | 0.641 | 0.888 | 0.013 | 0.932 | 0.452 | 0.113 | |
| Vitamin E | 0.661 | 0.912 | 0.300 | 0.825 | 0.615 | 0.013 | 0.699 | 0.579 | 0.089 | 0.572 | 0.649 | 0.772 | |
| Egg storage × Vitamin E | 0.343 | 0.466 | 0.625 | 0.770 | 0.901 | 0.963 | 0.300 | 0.756 | 0.024 | 0.498 | 0.308 | 0.282 | |
Different superscript alphabets in the same column means significantly different (P < 0.05).
Abbreviations: T-AOC, total antioxidant capacity; MDA, malonaldehyde; T-SOD, total superoxide dismutase; SEM, standard error of the mean.
Table 4.
The impacts of egg storage time (0 and 14 d) and maternal dietary vitamin E (6 and 100 mg/kg) on the liver antioxidant capacity of progeny chicks (trial 1).
| Male |
Female |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-AOC (μmol/10 mgprot) |
MDA (nmol/mgprot) |
T-SOD (U/mgprot) |
T-AOC (μmol/10 mgprot) |
MDA (nmol/mgprot) |
T-SOD (U/mgprot) |
|||||||
| Items | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d | 7 d | 21 d |
| Egg storage (d) | ||||||||||||
| 0 | 1.05 | 1.08 | 0.33 | 0.56 | 458.0 | 639.9a | 1.09 | 1.07a | 0.38b | 0.54 | 553.9 | 588.7 |
| 14 | 1.01 | 1.07 | 0.34 | 0.54 | 477.1 | 589.9b | 1.08 | 0.97b | 0.46a | 0.52 | 574.6 | 574.5 |
| SEM | 0.020 | 0.032 | 0.020 | 0.030 | 13.3 | 15.2 | 0.028 | 0.024 | 0.025 | 0.031 | 13.2 | 15.9 |
| Vitamin E (mg/kg) | ||||||||||||
| 6 | 1.02 | 1.04 | 0.32 | 0.54 | 470.6 | 626.6 | 1.11 | 1.04 | 0.41 | 0.51 | 570.3 | 593.0 |
| 100 | 1.04 | 1.10 | 0.34 | 0.56 | 464.5 | 603.3 | 1.06 | 1.00 | 0.43 | 0.56 | 558.2 | 570.1 |
| SEM | 0.020 | 0.032 | 0.020 | 0.030 | 13.3 | 15.2 | 0.028 | 0.024 | 0.025 | 0.031 | 13.2 | 15.9 |
| P-value | ||||||||||||
| Egg storage | 0.153 | 0.742 | 0.755 | 0.608 | 0.319 | 0.028 | 0.781 | 0.009 | 0.044 | 0.721 | 0.274 | 0.531 |
| Vitamin E | 0.568 | 0.169 | 0.478 | 0.736 | 0.748 | 0.288 | 0.201 | 0.207 | 0.533 | 0.229 | 0.523 | 0.316 |
| Egg storage × Vitamin E | 0.060 | 0.153 | 0.090 | 0.941 | 0.110 | 0.573 | 0.212 | 0.428 | 0.557 | 0.163 | 0.420 | 0.692 |
Different superscript alphabets in the same column means significantly different (P < 0.05).
Abbreviations: T-AOC, total antioxidant capacity; MDA, malonaldehyde; T-SOD, total superoxide dismutase; SEM, standard error of the mean.
Growth Performance and Antioxidant Capacity in Trial 2
As shown in Table 5, the performance of female and male offspring was significantly different (P < 0.05). Egg storage time (14 vs. 0 d) decreased the BW of 42-day-old offspring, the BWG of offspring from 22 to 42 d and 1 to 42 d, and the FI of offspring from 8 to 21 d, 22 to 42 d and 1 to 42 d (P < 0.05). Maternal VE levels (100 vs. 6 mg/kg) showed a trend in increasing the BW of 7-d-old offspring (P = 0.071) and the BWG of offspring from 1 to 7 d (P = 0.069). Interaction on the growth performance of offspring was not observed between egg storage time (14 vs. 0 d), maternal VE levels (100 vs. 6 mg/kg) and progeny sex (P > 0.05).
Table 5.
The impacts of egg storage time (0 and 14 d), maternal dietary vitamin E (6 and 100 mg/kg) and progeny sex (male and female) on the growth performance of progeny chicks (trial 2).
| Items | BW (g) |
BWG (g) |
FI (g) |
F/G |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 7 d | 21 d | 42 d | 1–7 d | 8-21d | 22–42d | 1–42d | 1–7 d | 8–21 d | 22–42 d | 1–42 d | 1–7 d | 8–21 d | 22–42 d | 1–42 d | |
| Sex | ||||||||||||||||
| Male | 44.6a | 163.6a | 911.8a | 3188.1a | 118.9a | 748.2a | 2276.4a | 3143.5a | 140.6a | 1005.1a | 3815.1a | 4933.1a | 1.183b | 1.344b | 1.677b | 1.570b |
| Female | 44.1b | 153.7b | 821.4b | 2655.1b | 109.6b | 667.7b | 1833.8b | 2611.0b | 133.9b | 927.3b | 3245.4b | 4286.2b | 1.222a | 1.389a | 1.770a | 1.642a |
| SEM | 0.02 | 1.4 | 5.2 | 17.5 | 1.4 | 4.3 | 16.6 | 17.5 | 1.6 | 5.1 | 23.0 | 25.7 | 0.007 | 0.007 | 0.007 | 0.005 |
| Egg storage (d) | ||||||||||||||||
| 0 | 44.4 | 160.1 | 869.7 | 2953.9a | 115.7 | 709.7 | 2084.1a | 2909.5a | 139.0 | 973.9a | 3570.5a | 4660.4a | 1.202 | 1.374 | 1.719 | 1.606 |
| 14 | 44.4 | 157.2 | 863.4 | 2889.4b | 112.8 | 706.2 | 2026.0b | 2845.0b | 135.6 | 958.5b | 3490.0b | 4558.9b | 1.202 | 1.359 | 1.727 | 1.605 |
| SEM | 0.02 | 1.4 | 5.2 | 17.5 | 1.4 | 4.3 | 16.6 | 17.5 | 1.6 | 5.1 | 23.0 | 25.7 | 0.007 | 0.007 | 0.007 | 0.005 |
| Vitamin E (mg/kg) | ||||||||||||||||
| 6 | 44.4 | 156.8 | 864.9 | 2912.4 | 112.4 | 708.1 | 2047.6 | 2868.1 | 135.6 | 967.6 | 3512.1 | 4593.6 | 1.207 | 1.368 | 1.721 | 1.605 |
| 100 | 44.4 | 160.5 | 868.3 | 2930.8 | 116.1 | 707.8 | 2062.6 | 2886.5 | 139.0 | 964.7 | 3548.4 | 4625.7 | 1.198 | 1.365 | 1.726 | 1.606 |
| SEM | 0.02 | 1.4 | 5.2 | 17.5 | 1.4 | 4.3 | 16.6 | 17.5 | 1.6 | 5.1 | 23.0 | 25.7 | 0.007 | 0.007 | 0.007 | 0.005 |
| P-value | ||||||||||||||||
| Sex | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
| Egg storage | 0.590 | 0.158 | 0.397 | 0.015 | 0.153 | 0.569 | 0.021 | 0.015 | 0.136 | 0.043 | 0.021 | 0.010 | 0.883 | 0.132 | 0.449 | 0.959 |
| Vitamin E | 0.983 | 0.071 | 0.646 | 0.463 | 0.069 | 0.964 | 0.528 | 0.463 | 0.132 | 0.693 | 0.275 | 0.386 | 0.404 | 0.762 | 0.577 | 0.877 |
| Sex × Egg storage | 0.812 | 0.423 | 0.501 | 0.976 | 0.415 | 0.576 | 0.808 | 0.976 | 0.611 | 0.601 | 0.476 | 0.432 | 0.494 | 0.995 | 0.187 | 0.195 |
| Sex × Vitamin E | 0.880 | 0.563 | 0.784 | 0.699 | 0.559 | 0.884 | 0.622 | 0.699 | 0.454 | 0.570 | 0.385 | 0.560 | 0.902 | 0.704 | 0.635 | 0.719 |
| Egg storage × Vitamin E | 0.779 | 0.663 | 0.365 | 0.875 | 0.660 | 0.335 | 0.651 | 0.874 | 0.584 | 0.493 | 0.699 | 0.818 | 0.911 | 0.742 | 0.962 | 0.810 |
| Sex × Egg storage × Vitamin E | 0.914 | 0.962 | 0.704 | 0.150 | 0.962 | 0.651 | 0.105 | 0.150 | 0.811 | 0.600 | 0.255 | 0.277 | 0.703 | 0.287 | 0.172 | 0.376 |
Different superscript alphabets in the same column means significantly different (P < 0.05).
Abbreviations: BW, body weight; BWG, body weight gain; FI, feed intake; F/G, feed conversion ratio; SEM, standard error of the mean.
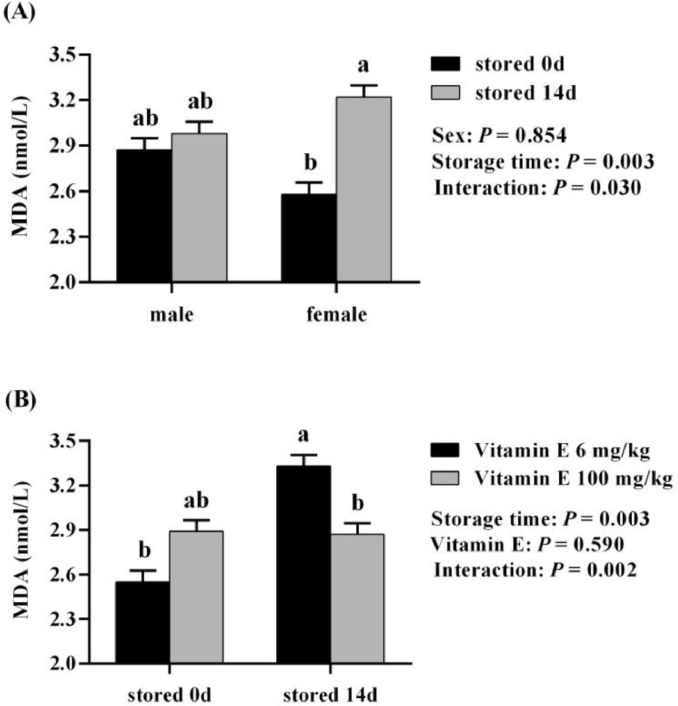
Results presented in Tables 6 and 7 indicated that egg storage time (14 vs. 0 d) increased the serum MDA of 21-day-old offspring (P < 0.05) and the serum and liver MDA of 42-d-old offspring (P < 0.05), and decreased the serum T-AOC of 42-day-old offspring (P = 0.060). Maternal VE (100 vs. 6 mg/kg) reduced the serum MDA of 7-day-old offspring (P < 0.05). Interactions were observed (P < 0.05) between progeny sex and egg storage duration (14 vs. 0 d); and maternal VE levels (100 vs. 6 mg/kg) and egg storage duration (14 vs. 0 d) on the serum MDA of 21-day-old offspring (Figure 1A and 1B). Egg storage time (14 vs. 0 d) increased the serum MDA of 21-day-old female offspring (P < 0.05), but not for that of 21-day-old male offspring. Maternal VE (100 vs. 6 mg/kg) reduced the serum MDA of 21-day-old offspring originated from stored eggs (P < 0.05), but not for that of offspring originated from un-stored eggs.
Table 6.
The impacts of egg storage time (0 and 14 d), maternal dietary vitamin E (6 and 100 mg/kg) and progeny sex (male and female) on the serum antioxidant capacity of progeny chicks (trial 2).
| Items | T-AOC (μmol/mL) |
MDA (nmol/mL) |
T-SOD (U/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 d | 21 d | 42 d | 7 d | 21 d | 42 d | 7 d | 21 d | 42 d | |||
| Sex | Egg storage (d) | Vitamin E (mg/kg) | |||||||||
| Male | 0 | 6 | 1.02 | 1.23 | 1.37 | 3.54 | 2.79 | 2.57 | 217.8 | 291.6 | 309.7 |
| Male | 0 | 100 | 0.92 | 0.95 | 1.37 | 3.30 | 2.95 | 2.73 | 224.0 | 289.8 | 277.8 |
| Male | 14 | 6 | 0.88 | 1.03 | 1.23 | 3.52 | 3.18 | 2.93 | 218.8 | 310.3 | 295.2 |
| Male | 14 | 100 | 1.07 | 1.07 | 1.11 | 3.19 | 2.78 | 2.62 | 279.0 | 317.5 | 286.5 |
| Female | 0 | 6 | 1.01 | 1.04 | 1.20 | 3.47 | 2.32 | 2.52 | 276.5 | 293.6 | 362.8 |
| Female | 0 | 100 | 0.92 | 0.9 | 1.37 | 3.23 | 2.84 | 2.65 | 317.7 | 278.2 | 372.1 |
| Female | 14 | 6 | 1.11 | 1.16 | 1.04 | 3.61 | 3.48 | 2.98 | 242.7 | 338.5 | 273.5 |
| Female | 14 | 100 | 0.98 | 1.05 | 1.18 | 2.96 | 2.96 | 3.03 | 252.4 | 235.1 | 285.3 |
| SEM | 0.024 | 0.037 | 0.048 | 0.071 | 0.057 | 0.060 | 13.1 | 9.0 | 15.3 | ||
| Sex | |||||||||||
| Male | 0.97 | 1.07 | 1.27 | 3.39 | 2.92 | 2.71 | 234.9 | 302.3 | 292.3 | ||
| Female | 1.00 | 1.04 | 1.20 | 3.32 | 2.90 | 2.80 | 272.3 | 286.4 | 323.4 | ||
| SEM | 0.034 | 0.053 | 0.068 | 0.101 | 0.081 | 0.085 | 18.6 | 12.7 | 21.7 | ||
| Egg storage (d) | |||||||||||
| 0 | 0.97 | 1.03 | 1.33 | 3.39 | 2.72b | 2.62b | 259.0 | 288.3 | 330.6 | ||
| 14 | 1.01 | 1.08 | 1.14 | 3.32 | 3.10a | 2.89a | 248.2 | 300.4 | 285.2 | ||
| SEM | 0.034 | 0.053 | 0.068 | 0.101 | 0.081 | 0.085 | 18.6 | 12.7 | 21.7 | ||
| Vitamin E (mg/kg) | |||||||||||
| 6 | 1.00 | 1.12 | 1.21 | 3.53 | 2.94 | 2.75 | 238.9 | 308.5 | 310.3 | ||
| 100 | 0.97 | 0.99 | 1.25 | 3.17 | 2.88 | 2.76 | 268.3 | 280.2 | 305.4 | ||
| SEM | 0.034 | 0.053 | 0.068 | 0.101 | 0.081 | 0.085 | 18.6 | 12.7 | 21.7 | ||
| P-value | |||||||||||
| Sex | 0.519 | 0.656 | 0.466 | 0.619 | 0.854 | 0.483 | 0.166 | 0.382 | 0.320 | ||
| Egg storage | 0.413 | 0.521 | 0.060 | 0.637 | 0.003 | 0.034 | 0.684 | 0.507 | 0.151 | ||
| Vitamin E | 0.503 | 0.115 | 0.635 | 0.018 | 0.590 | 0.948 | 0.275 | 0.127 | 0.874 | ||
| Sex × Egg storage | 0.472 | 0.241 | 0.898 | 0.997 | 0.030 | 0.239 | 0.152 | 0.538 | 0.177 | ||
| Sex × Vitamin E | 0.134 | 0.987 | 0.269 | 0.589 | 0.605 | 0.504 | 0.883 | 0.096 | 0.619 | ||
| Egg storage × Vitamin E | 0.225 | 0.275 | 0.700 | 0.383 | 0.002 | 0.276 | 0.833 | 0.282 | 0.835 | ||
| Sex × Egg storage × Vitamin E | 0.106 | 0.343 | 0.837 | 0.583 | 0.310 | 0.440 | 0.423 | 0.189 | 0.869 | ||
Different superscript alphabets in the same column means significantly different (P < 0.05).
Abbreviations: T-AOC, total antioxidant capacity; MDA, malonaldehyde; T-SOD, total superoxide dismutase; SEM, standard error of the mean.
Table 7.
The impacts of egg storage time (0 and 14 d), maternal dietary vitamin E (6 and 100 mg/kg) and progeny sex (male and female) on the liver antioxidant capacity of progeny chicks (trial 2).
| Items | T-AOC (μmol/10 mgprot) |
MDA (nmol/mgprot) |
T-SOD (U/mgprot) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 d | 21 d | 42 d | 7 d | 21 d | 42 d | 7 d | 21 d | 42 d | |
| Sex | |||||||||
| Male | 1.23 | 1.14 | 1.16 | 0.33 | 0.50 | 0.68 | 495.6 | 503.4 | 543.2 |
| Female | 1.24 | 1.13 | 1.15 | 0.37 | 0.51 | 0.62 | 527.1 | 490.9 | 538.9 |
| SEM | 0.030 | 0.032 | 0.030 | 0.026 | 0.028 | 0.030 | 14.8 | 17.9 | 27.2 |
| Egg storage (d) | |||||||||
| 0 | 1.25 | 1.11 | 1.12 | 0.36 | 0.50 | 0.58b | 517.4 | 503.8 | 562.1 |
| 14 | 1.22 | 1.16 | 1.18 | 0.34 | 0.51 | 0.72a | 505.3 | 490.4 | 520.0 |
| SEM | 0.030 | 0.032 | 0.030 | 0.026 | 0.028 | 0.030 | 14.8 | 17.9 | 27.2 |
| Vitamin E (mg/kg) | |||||||||
| 6 | 1.23 | 1.16 | 1.13 | 0.38 | 0.50 | 0.65 | 495.2 | 515.4 | 527.4 |
| 100 | 1.24 | 1.10 | 1.17 | 0.32 | 0.51 | 0.65 | 527.5 | 478.9 | 554.7 |
| SEM | 0.030 | 0.032 | 0.030 | 0.026 | 0.028 | 0.030 | 14.8 | 17.9 | 27.2 |
| P-value | |||||||||
| Sex | 0.907 | 0.835 | 0.796 | 0.283 | 0.812 | 0.202 | 0.147 | 0.624 | 0.910 |
| Egg storage | 0.433 | 0.292 | 0.152 | 0.565 | 0.812 | 0.002 | 0.568 | 0.602 | 0.283 |
| Vitamin E | 0.770 | 0.170 | 0.425 | 0.127 | 0.739 | 0.953 | 0.137 | 0.163 | 0.484 |
| Sex × Egg storage | 0.326 | 0.609 | 0.819 | 0.457 | 0.558 | 0.430 | 0.754 | 0.301 | 0.762 |
| Sex × Vitamin E | 0.369 | 0.771 | 0.246 | 0.478 | 0.083 | 0.814 | 0.514 | 0.911 | 0.199 |
| Egg storage × Vitamin E | 0.169 | 0.499 | 0.441 | 0.588 | 0.112 | 0.235 | 0.286 | 0.239 | 0.814 |
| Sex × Egg storage × Vitamin E | 0.217 | 0.729 | 0.647 | 0.709 | 0.196 | 0.397 | 0.236 | 0.971 | 0.420 |
Different superscript alphabets in the same column means significantly different (P < 0.05).
Abbreviations: T-AOC, total antioxidant capacity; MDA, malonaldehyde; T-SOD, total superoxide dismutase; SEM, standard error of the mean.
Figure 1.
Interactions of progeny sex and egg storage time (A), and egg storage time and maternal dietary vitamin E (B) on the serum MDA content of 21-d-old offspring in trial 2. ab Mean values with different letters are significantly different (P < 0.05).
DISCUSSION
In this study, egg storage decreased the growth performance of offspring, which was consistent with previous researches (Tona et al., 2003; Tona et al., 2004; Petek and Dikmen, 2006). Ebeid et al. (2017) indicated that egg storage (14 vs. 4 d) reduced the weight of hatching chicks and the ratio of first-grade hatched chicks. Damaziak et al. (2018) indicated that egg storage (12 vs. 5 d) decreased both the weight of hatching chicks and the subsequent BW of offspring. We also observed that egg storage decreased the weight of hatching chicks (Yang et al., 2020a) and the subsequent BW of offspring in the present study. It seems that the decreased BW of offspring induced by egg storage was probably related to the decreased quality of hatched chicks. Furthermore, as egg storage time was prolonged from 0 d to 14 d, the decreased BW of offspring was associated with the decreased FI of offspring in the current study. Thus, we speculated that when egg storage duration was prolonged, the quality of hatched chicks and then the FI of offspring decreased, therefore the BW of offspring decreased. In addition, the decreased BW of offspring induced by egg storage was observed at 21 and 42 d of age in trial 1 and 2, respectively, and a tendency of BW reduction even could be observed at 7 d of age in trial 1. It was considered that the impact of different egg storage temperature on the quality of hatched chicks was responsible for the different results of BW observed in the two trials, as the quality of hatched chicks originated from stored eggs in trial 2 was better than that of chicks in trial 1 (Yang et al., 2020a). Moreover, due to the highest egg storage temperature, the early F/G of offspring was increased by the prolonged egg storage in trial 1.
Commercial poultry production usually faces various stresses, especially the oxidative stress. As a stable product of lipid peroxidation, the content of MDA reflects the attacking degree of free radical. The T-AOC reflects the total levels of non-enzymatic antioxidants, such as selenium, ascorbic acid, VE and carotenoids (Surai et al., 2016). The T-SOD plays a vital role in the conversion of O2- into H2O2. According to Ebeid et al. (2017), egg storage (14 vs. 4 d) reduced the serum T-AOC and increased the serum MDA content of hatched chicks. However, no report involving in an effect of long-term egg storage on the antioxidant capacity of offspring post-hatch was found. In this study, egg storage reduced the antioxidant capacity of both the serum and liver of offspring. This could be another important reason for the detrimental growth performance of offspring induced by egg storage.
There were few studies reported that maternal VE levels did not impact the growth performance of offspring (Hossain et al., 1998; Siegel et al., 2006). However, these studies were conducted 15 y ago. In the present study, maternal VE levels trended to improve the growth performance of first week post-hatch. The recent research also indicated that in ovo feeding of VE to 17.5-day-old broiler embryo provided the better development of small intestine and proantioxidant enzymes, which had contributed to the performance improvement of offspring (Araújo et al., 2018). Nevertheless, in the case of present study, we did not find that maternal VE levels had improved the growth performance of offspring originated from stored eggs. Similarly, Damaziak et al. (2018) did not observed that the egg turning and the incubation with short periods during egg storage had presented a positive influence on the BW of 42-day-old offspring. It seems that the decreased growth performance of offspring induced by egg storage is difficult to be rescued by nutritional or physical strategies, and much more studies need to be conducted to evaluate it.
Maternal VE supplementation is effectively deposited in egg yolks and then further in tissues of developing embryo and hatched chicks, which determine the VE status of offspring at least for the first 7 d post-hatch, and improve the resistance of chick tissues to oxidative stress (Surai and Fisinin, 2012). As the maternal VE levels increased, the VE levels in the tissues of 5- and 10-day-old offspring increased and the MDA content decreased (Surai, 2000). Consistently, our results also demonstrated that increasing maternal dietary VE levels enhanced the antioxidant capacity of offspring, and especially showed the positive effects on reducing the serum MDA content of offspring originated from stored eggs, but not for that of offspring originated from unstored eggs. It suggested that maternal VE supplementation was beneficial to the improvement of antioxidant capacity of offspring in the case of egg storage.
Due to the difference of species, the serum antioxidant capacity of White Leghorn type chickens was higher in male than female (Maurice and Lightsey, 2007). However, no sex effect was observed in antioxidant status of broiler in previous (Jia et al., 2014) and present studies. We interestingly found that the damage of antioxidant capacity induced by egg storage was higher in female offspring than that of in male offspring in trial 1, and egg storage only increased the serum MDA content of 21-day-old female (except for male) offspring in trial 2. These results indicated that the adverse impact of egg storage on the antioxidant capacity of offspring revealed a gender difference. Further studies are needed to determine the progeny sex effect of egg storage time.
In conclusion, egg storage (14 vs. 0 d) decreased the growth performance and antioxidant capacity of offspring, while maternal dietary VE (100 vs. 6 mg/kg) supplementation could partly alleviate the reduction of antioxidant capacity (except for growth performance) of offspring induced by egg storage for the early phase post-hatch.
ACKNOWLEDGMENTS
This work was supported by Sichuan Provincial Science and technology Project for modern broiler Industry Production Chains (2016NZ00032012NZ0037) and National Key R & D Program (2016YFD0501202)
Disclosures
The authors declare no conflicts of interest.
REFERENCES
- AOAC International . 17th ed. Assoc. Off. Anal. Chem. Int.; Gaithersburg, MD: 2006. Official Methods of Analysis. [Google Scholar]
- Araújo I.C.S., Café M.B., Noleto R.A., Martins J.M.S., Ulhoa C.J., Guareshi G.C., Reis M.M., Leandro N.S.M. Effect of vitamin E in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult. Sci. 2018;98:3652–3661. doi: 10.3382/ps/pey439. [DOI] [PubMed] [Google Scholar]
- Chang A., Halley J., Silva M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016;56:1254–1262. [Google Scholar]
- Damaziak K., Paweska M., Gozdowski D., Niemiec J. Short periods of incubation, egg turning during storage and broiler breeder hens age for early development of embryos, hatching results, chicks quality and juvenile growth. Poult. Sci. 2018;97:3264–3276. doi: 10.3382/ps/pey163. [DOI] [PubMed] [Google Scholar]
- Devine P.J., Perreault S.D., Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond J., Vinyard B., Nicholson A.D., French N.A., Bakst M.R. Short periods of incubation during egg storage increase hatchability and chick quality in long-stored broiler eggs. Poult. Sci. 2013;92:2977–2987. doi: 10.3382/ps.2012-02816. [DOI] [PubMed] [Google Scholar]
- Ebeid T.A., Twfeek F.A., Assar M.H., Bealish A.M., Abd El-Karim R.E., Ragab M. Influence of pre-storage incubation on hatchability traits, thyroid hormones, antioxidative status and immunity of newly hatched chicks at two chicken breeder flock ages. Animal. 2017;11:1966–1974. doi: 10.1017/S1751731117000738. [DOI] [PubMed] [Google Scholar]
- Elibol O., Peak S.D., Brake J. Effect of flock age, length of egg storage, and frequency of turning during storage on hatchability of broiler hatching eggs. Poult. Sci. 2002;81:945–950. doi: 10.1093/ps/81.7.945. [DOI] [PubMed] [Google Scholar]
- Gharib H.B. Effect of pre-storage heating of broiler breeder eggs, stored for long periods, on hatchability and chick quality. Egyptian J. Anim. Prod. 2013;50:174–184. [Google Scholar]
- Hossain S.M., Barreto S.L., Bertechini A.G., Rios A.M., Silva C.G. Influence of dietary Vitamin E level on egg production of broiler breeders, and on the growth and immune response of progeny in comparison with the progeny from eggs injected with Vitamin E. Anim. Feed Sci. Tech. 1998;73:307–317. [Google Scholar]
- Jia R., Bao Y.H., Zhang Y., Ji C., Zhao L.H., Zhang J.Y., Gao C.Q., Ma Q.G. Effects of dietary α-lipoic acid, acetyl-L-carnitine, and sex on antioxidative ability, energy, and lipid metabolism in broilers. Poult. Sci. 2014;93:1–9. doi: 10.3382/ps.2014-03921. [DOI] [PubMed] [Google Scholar]
- Lin X.J., Jiang S.Q., Li L., Chen F., Gou Z.Y., Wu Q.W., Fan Q.L., Jiang Z.Y. Effects of dietary vitamin E and selenoyeast on laying performance, hatching performance and vitamin E and selenium deposition in egg of yellow-feathered broiler breeders. Chin. J. Anim. Nutr. 2017;29:1515–1526. [Google Scholar]
- Liu X., Xin L., Mi Y., Jian L., Zhang C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018;2018:1–16. doi: 10.1155/2018/9390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice D.V, Lightsey. S.F. Sexual difference in ascorbic acid synthesis, tissue ascorbic acid and plasma total antioxidant capacity in mature chickens. Br. Poult. Sci. 2007;48:519–523. doi: 10.1080/00071660701455821. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the People's Republic of China and Standardization Administration of China . China Standard Press; Beijing, China: 2008. Determination of Vitamin E in Feeds-High-Performance Liquid Chromatography (GB/T 17812-2008) [Google Scholar]
- Nasri H., Brand H., Najjar T., Bouzouaia M. Interactions between egg storage duration and broiler breeder age on egg fat content, chicken organ weights, and growth performance. Poult. Sci. 2020;99:4607–4615. doi: 10.1016/j.psj.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Petek M., Dikmen. S. The effects of prestorage incubation and length of storage of broiler breeder eggs on hatchability and subsequent growth performance of progeny. Czech J. Anim. Sci. 2006;51:73–77. [Google Scholar]
- Pokhrel N., Cohen B.T., Genin O., Ruzal M., Sela-Donenfeld D., Cinnamon Y. Effects of storage conditions on hatchability, embryonic survival and cytoarchitectural properties in broiler from young and old flocks. Poult. Sci. 2018;97:1429–1440. doi: 10.3382/ps/pex393. [DOI] [PubMed] [Google Scholar]
- Reijrink I.A.M., Meijerhof R., Kemp B., Van D.B.H. The chicken embryo and its micro environment during egg storage and early incubation. World Poult. Sci. J. 2008;64:581–598. [Google Scholar]
- Reijrink I.A., Meijerhof R., Kemp B., Graat E.A., Van D.B.H. Influence of prestorage incubation on embryonic development, hatchability, and chick quality. Poult. Sci. 2009;88:2649–2660. doi: 10.3382/ps.2008-00523. [DOI] [PubMed] [Google Scholar]
- Shahriar H.A., Shivazad M., Chamani M., Nazer-Adl K., Nezhad Y.E. Effects of dietary fat type and different levels of vitamin E on performance and some of eggs characters of broiler breeder. J. Anim. Vet. Adv. 2007;7:887–892. [Google Scholar]
- Shirpoor A., Minassian S., Salami S., Khademansari M.H., Ghaderipakdel F., Yeghiazaryan M. Vitamin E protects developing rat hippocampus and cerebellum against ethanol-induced oxidative stress and apoptosis. Food Chem. 2009;113:115–120. [Google Scholar]
- Siegel P.B., Blair M., Gross W.B., Meldrum B., Larsen C., Boaamponsem K., Emmerson D.A. Poult performance as influenced by age of dam, genetic line, and dietary vitamin E. Poult. Sci. 2006;85:939–942. doi: 10.1093/ps/85.5.939. [DOI] [PubMed] [Google Scholar]
- Surai P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000;41:235–243. doi: 10.1080/713654909. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Noble R.C., Speake B.K. Relationship between vitamin E content and susceptibility to lipid peroxidation in tissues of the newly hatched chick. Br. Poult. Sci. 1999;40:406–410. doi: 10.1080/00071669987520. [DOI] [PubMed] [Google Scholar]
- Surai P., Fisinin. V.I. Feeding breeders to avoid oxidative stress in embryos. Word's Poultry Congress, 5-9 August-2012; Salvador-Bahia-Brazil; 2012. [Google Scholar]
- Surai P.F., Fisinin V.I., Karadas F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016;2:1–11. doi: 10.1016/j.aninu.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona K., Bamelis F., De K.B., Bruggeman V., Moraes V.M., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., De Ketelaere B., Decuypere E., Bruggeman V. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick posthatch growth to forty-two days. J. Appl. Poult. Res. 2004;13:10–18. [Google Scholar]
- Tsai H.L., Chang S.K.C., Lin Y.F., Chang S.J. Beneficial effects of maternal vitamin E supplementation on the antioxidant system of the neonate chick brain. Asian-Austral. J. Anim. 2008;21:225–231. [Google Scholar]
- Urso U.R., Dahlke F., Maiorka A., Bueno I.J., Schneider A.F., Surek D., Rocha C. Vitamin E and selenium in broiler breeder diets: Effect on live performance, hatching process, and chick quality. Poult. Sci. 2015;94:976–983. doi: 10.3382/ps/pev042. [DOI] [PubMed] [Google Scholar]
- Yang J., Ding X.M., Bai S.P., Wang J.P., Zeng Q.F., Peng H.W., Su Z.W., Xuan Y., Zhang K.Y. The effects of broiler breeder dietary vitamin E and egg storage time on the quality of eggs and newly hatched chicks. Animals. 2020;10:1409. doi: 10.3390/ani10081409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ding X.M., Bai S.P., Wang J.P., Zeng Q.F., Peng H.W., Su Z.W., Xuan Y., Zhang K.Y. Effects of maternal dietary vitamin E on the egg characteristics, hatchability and offspring quality of prolonged storage eggs of broiler breeder hens. J. Anim. Physiol. Anim. Nutr. 2020;104:1384–1391. doi: 10.1111/jpn.13371. [DOI] [PubMed] [Google Scholar]
- Yaripour Mehrdad, Seidavi Alireza, Dadashbeiki Mohammad, Laudadio Vito, Tufarelli, Vincenzo Impact of dietary supra-nutritional levels of vitamins A and E on fertility traits of broiler breeder hens in late production phase. Agriculture. 2018;8:149–159. [Google Scholar]