Abstract
Our lungs are exposed daily to airborne pollutants, particulate matter, pathogens as well as lung allergens and irritants. Exposure to these substances can lead to inflammatory responses and may induce endogenous oxidant production, which can cause chronic inflammation, tissue damage and remodeling. Notably, the development of asthma and Chronic Obstructive Pulmonary Disease (COPD) is linked to the aforementioned irritants. Some inhaled foreign chemical compounds are rapidly absorbed and processed by phase I and II enzyme systems critical in the detoxification of xenobiotics including the glutathione-conjugating enzymes Glutathione S-transferases (GSTs). GSTs, and in particular genetic variants of GSTs that alter their activities, have been found to be implicated in the susceptibility to and progression of these lung diseases. Beyond their roles in phase II metabolism, evidence suggests that GSTs are also important mediators of normal lung growth. Therefore, the contribution of GSTs to the development of lung diseases in adults may already start in utero, and continues through infancy, childhood, and adult life. GSTs are also known to scavenge oxidants and affect signaling pathways by protein-protein interaction. Moreover, GSTs regulate reversible oxidative post-translational modifications of proteins, known as protein S-glutathionylation. Therefore, GSTs display an array of functions that impact the pathogenesis of asthma and COPD.
In this review we will provide an overview of the specific functions of each class of mammalian cytosolic GSTs. This is followed by a comprehensive analysis of their expression profiles in the lung in healthy subjects, as well as alterations that have been described in (epithelial cells of) asthmatics and COPD patients. Particular emphasis is placed on the emerging evidence of the regulatory properties of GSTs beyond detoxification and their contribution to (un)healthy lungs throughout life. By providing a more thorough understanding, tailored therapeutic strategies can be designed to affect specific functions of particular GSTs.
Keywords: Glutathione S-Transferases, Protein S-Glutathionylation, COPD, Asthma, RNA sequencing
Abbreviations
- GSH
Glutathione
- GST
Glutathione S-transferase
- PSSG
Protein S-glutathionylation
- COPD
Chronic Obstructive Pulmonary Disease
- MAPK
Mitogen Activated Protein Kinase pathway
- Cys
Cysteine
- Prdx
Peroxiredoxin
- Glrx
Glutaredoxin
- MAPK
Mitogen activated protein kinase
- JNK
c-Jun N-terminal kinase
- TRAF2
Tumor necrosis factor receptor associated factor 2
- H2O2
Hydrogen peroxide
- SOH
Sulfenic acid
- PM
Particulate matter
- AHR
Airway hyperresponsiveness
- BALF
Bronchoalveolar lavage fluid
- NF-κB
Nuclear factor kappa B
- Keap1
Kelch-like ECH-associated protein 1
- Nrf2
Nuclear factor erythroid 2–related factor
- STAT3
Signal transducer and activator of transcription 3
1. Introduction
Our lungs are constantly exposed to air and airborne pollutants (including NO2, ozone), particulate matter, pathogens as well as respiratory allergens and irritants. Many of these agents are free radicals or initiate free radical reactions. Exposure to these exogenous substances gives rise to oxidative stress which can cause pro-inflammatory responses in the lung, and may subsequently induce endogenous oxidant production [[1], [2], [3], [4], [5]]. The tripeptide glutathione (γ-L-glutamyl-cysteinyl-glycine) is a non-protein thiol in cells, as well as in plasma and extracellular fluid of lungs, and is considered an in important redox buffer that can exist in a thiol-reduced form (GSH) or an oxidized form (GSSG) [6]. GSH can be consumed by enzymes that have antioxidant functions [7]. Glutathione peroxidases (GPXs) detoxify hydrogen peroxide and reduce oxidized lipids [[8], [9], [10], [11], [12]]. Glutathione reductase (GR) recycles GSSG to maintain an appropriate intracellular GSH level within cells [13,14]. Glutathione S-transferases (GSTs) protect cells from environmental exposures by their detoxification function to catalyze the conjugation of GSH [[15], [16], [17], [18]]. Foreign chemical compounds inhaled into the lung are rapidly absorbed and processed for detoxification by phase I and II metabolizing enzymes. Notably, enzymes engaged in Phase I metabolism, involving the cytochrome P450 system, target these compounds by catalyzing different reactions including hydroxylation, oxidation and reduction to protect the cell [[19], [20], [21]]. Subsequently, during Phase II metabolism, Phase II enzymes, the GSTs and microsomal epoxide hydrolases, can catalyze the conjugation of Phase I modified xenobiotics to an endogenous water-soluble substrate, such as reduced GSH, or glycine. This will result in less toxic metabolites, which can then be more easily transported out of the cell by different transmembrane efflux pumps. Some compounds will not go through Phase I metabolism, but enter Phase II metabolism directly.
Oxidants, also known as reactive oxygen species (ROS) (i.e. hydrogen peroxide, superoxide anion, hydroxyl radicals, and nitric oxide radicals) are produced by living organisms as a result of normal cellular metabolism [[22], [23], [24], [25]]. At physiological concentrations, they function as signaling molecules to regulate (patho)physiological processes [26,27]. However, in excessive concentrations, oxidants that overwhelm the antioxidant defense system induce adverse modifications to cell components such as lipids, proteins, and DNA [28]. This shift in balance between oxidants and antioxidants in favor of oxidants is termed oxidative stress [29]. Aside from protecting cells from environmental toxins, GSTs also protect cells from oxidative stress [15,16,30]. Oxidative stress contributes to the development of chronic lung diseases including asthma and Chronic Obstructive Pulmonary Disease (COPD) [31]. Ambient outdoor air pollution from the use of biomass fuels, ozone, and tobacco smoking have been associated with decreased lung volume and growth [[3], [4], [5]]. Epidemiological studies demonstrate that exposure to ambient air pollutants induces episodes of acute respiratory exacerbations and bronchitis, which increases the progress of asthma and COPD [[32], [33], [34], [35]].
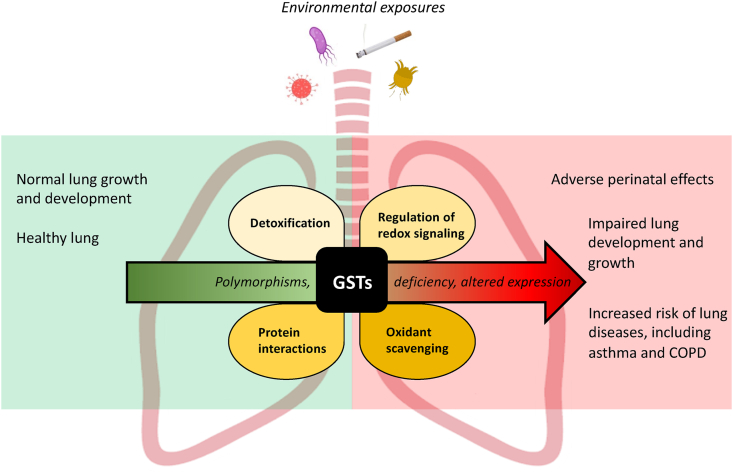
Genetic variations, including polymorphisms in cytosolic human GSTs, can change a person's ability to cope with inhaled environmental stressors and toxins, and can thereby contribute to the risk to develop inflammatory lung diseases associated with these exposures, as well as aggravate the course of these conditions [[36], [37], [38]]. Examples include the increased risk for asthma and COPD in subjects carrying null variants of the Mu and Theta class of GST, which lead to a loss of protein expression and therefore detoxification activity [[39], [40], [41], [42], [43], [44]]. The Glutathione S-transferase P (GSTP) class Ile105Val polymorphism, which also results in decreased GST activity towards chloro-2,4-dinitrobenzene (CDNB) [45] has also been linked to asthma and COPD in some studies [46,47]. In this review we will briefly describe the characteristics and main functions of the different classes of human cytosolic GSTs, and focus on their impact on the redox state. We will provide an overview of their implications in lung development and the diseases asthma and COPD (Fig. 1).
Fig. 1.
Schematic overview of the main known functions of human cytosolic Glutathione S-transferases and their contribution to lung diseases. Illustrated are the main known functions of human cytosolic GSTs in the lung upon exposure to environmental factors. However, GST polymorphisms, deficiency and/or altered expression (and activity) of GSTs in the lung may contribute to adverse perinatal effects, impaired lung development and growth as well as increase the risk of the development of the lung diseases asthma and COPD. GSTs: Glutathione S-transferases.
2. Historical overview of glutathione S-transferases
Despite their most well-known function as a family of Phase II detoxification enzymes, GSTs were first introduced as ligandins in the 1970's, as GSTs were known to bind toxins and function as transport proteins [48,49]. GSTs are present in different subcellular compartments including the cytosol, endoplasmic reticulum (ER), mitochondria, nucleus and plasma membranes. Human GSTs can be divided into distinct families, namely cytosolic, mitochondrial, also known as the kappa class, and microsomal GSTs, integral membrane proteins, also known as MAPEG (membrane-associated proteins involved in eicosanoid and glutathione metabolism) [49,50]. Cytosolic GSTs are further classified based on sequence similarities, and physical and structural properties. In humans, the cytosolic GSTs can be further divided into 7 functional classes: Alpha, Mu, Omega, Pi, Theta, Sigma and Zeta [50]. Mitochondrial GSTs are soluble enzymes and have structural similarities with cytosolic GSTs, whereas microsomal GSTs are evolutionarily unrelated to the other classes of GSTs. For the scope of this review we will only discuss the human cytosolic GSTs. GST Sigma remains uncharacterized and will not be discussed.
3. Human cytosolic glutathione S-transferases families
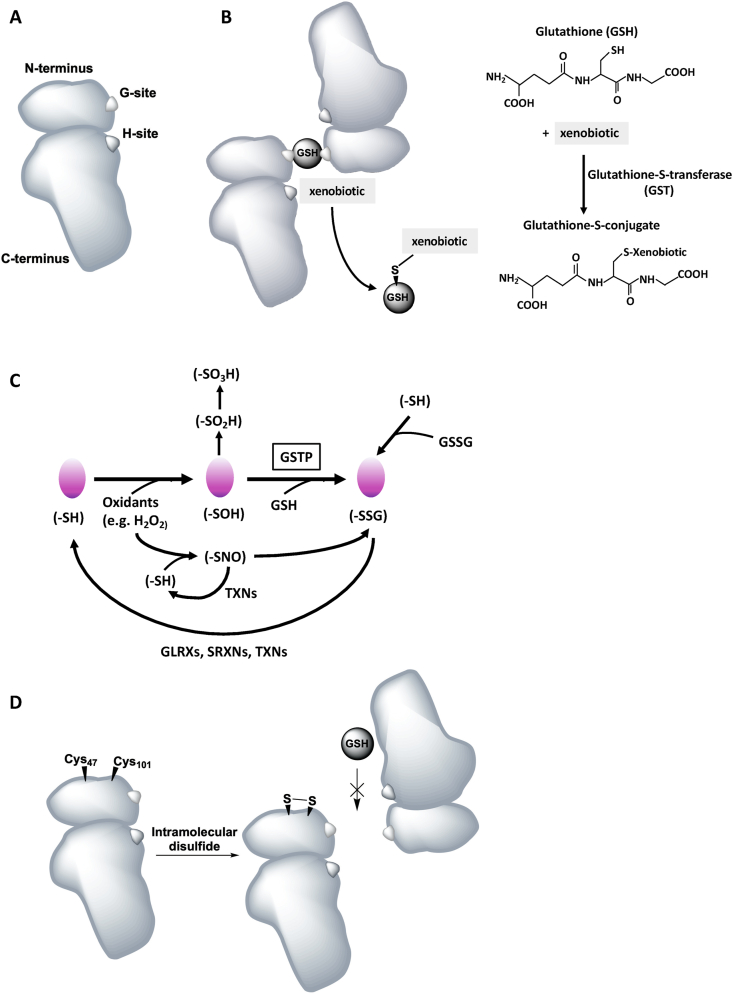
Mammalian cytosolic GSTs are the best-characterized group of GST proteins. The multiple members in each class share a common fold, and the GST isoenzymes within a class typically share more than 40% sequence homology, while less than 25% sequence homology occurs between classes [51,52]. GSTs are dimeric enzymes with, in most cases, both subunits being from the same class of GST (and forming homodimers) (Fig. 2A and B) [53]. The monomers are approximately 25–26 kDa (kDA) in size [49]. The GST monomer consists of two distinct domains, the N-terminal thioredoxin-like domain, which comprises about 1/3 of the protein, and a C-terminal alpha-helical domain [54]. The N-terminal thioredoxin-like domain is highly conserved in all GST isozymes and contains a redox active CXXC motif [51]. This structure is common to several proteins from a thioredoxin fold superfamily, including glutaredoxins (GLRXs), GPXs, and peroxiredoxins, which bind cysteine or GSH with high affinity [11,12,[55], [56], [57], [58]]. The highly specific GSH binding site, the G-site, is located within the N-terminal domain [51]. The C-terminal domain and a loop from the N-terminal domain together function to shape the co-substrate binding site, the H-site (xenobiotic binding site), which binds various hydrophobic and electrophilic substrates [51,59], and is proposed to be adjacent to the G-site [60]. Dimeric GSTs enhance protein stability, and GST catalysis occurs by binding the substrate at the H-site, thereby attacking xenobiotics, and binding GSH at the G-site which together form the well-conserved active site of the enzyme (Fig. 2B). Near the N-terminus, a specific amino acid residue activates the thiol cysteinyl side chain by attack of the sulfur atom of GSH and lowers its pKa through hydrogen-bonding. In the GST Alpha, Mu, and P isoenzymes, the activating residue constitutes a conserved tyrosine (Y-GSTs), while in the Theta and Zeta classes this is a serine, and in the Omega class a cysteine (S/C-GSTs) [[61], [62], [63], [64], [65]]. As such, unlike the other GSTs, the active site cysteine of GST Omega (GSTO) is able to form a disulfide bond with GSH. These residues have been shown to be essential for catalytic activity, as replacement of specific conserved residues by site-directed mutagenesis of for example tyrosine 7, lower GSTP activity toward CDNB, and ethacrynic acid [66]. CDNB is the most common GST substrate used in classical GST activity assays, however, not all GSTs can use CDNB as a substrate, and thus the activity of certain GSTs may be underestimated or even undetected when using this substrate. The activity of GSTs is moreover dependent upon GSH supply from γ-glutamylcysteine synthetase and glutathione synthetase, and on transporters to remove GSH conjugates from cells. Compounds that induce GSTs (often GST substrates) or that are recognized as substrates share a common chemical signal, namely a carbon-carbon double bond, adjacent to an electron-withdrawing group. The variability in co-substrates between the different GSTs is mainly reflected by different amino acids residues in the H-site [54]. In addition to the variations in co-substrates, the different classes of human cytosolic GSTs are expressed in different organs.
Fig. 2.
Schematic representation of the structure of a Glutathione S-transferase P molecule and the catalytic mechanisms of conjugation of GSTs to xenobiotics and protein S-glutathionylation. A, Schematic of a GSTP monomer with the N- and C-terminal domains including the glutathione binding site, the G-Site, and the xenobiotic binding site, the H-Site. B, Schematic of a GSTP dimer (forming homodimers) binding a GSH molecule on the G-sites in each monomer. Schematic vs molecular representation of the conjugation of GSTS to activated xenobiotics using reduced GSH. C, Catalytic cycle of PSSG and deglutathionylation thought to contribute to the pathogenesis of chronic lung diseases. PSSG can be induced by different biochemical events depending on multiple factors, and can occur spontaneously or is catalyzed by GSTs, notably GSTP. D, GSTP contains cysteines residues regulating its catalytic activity. Intra-subunit disulfide bond formation between Cys47 and Cys101 residues results in steric hindrance for GSH binding. All figures are schematic representations and the actual position of the binding sites and cysteine residues may deviate from the original 3D crystal structure. H2O2: hydrogen peroxide; –SH: protein thiol; –SOH: protein sulfenic acid; –SO2H: protein sulfinic acid; –SO3H: protein sulfonic acid; GSH: glutathione; -SSG: S-glutathionylation; GSSG: glutathione disulfide; –SNO: S-nitrosylation; GLRXs: glutaredoxins; SRNX: sulfiredoxins; TXNs: thioredoxins.
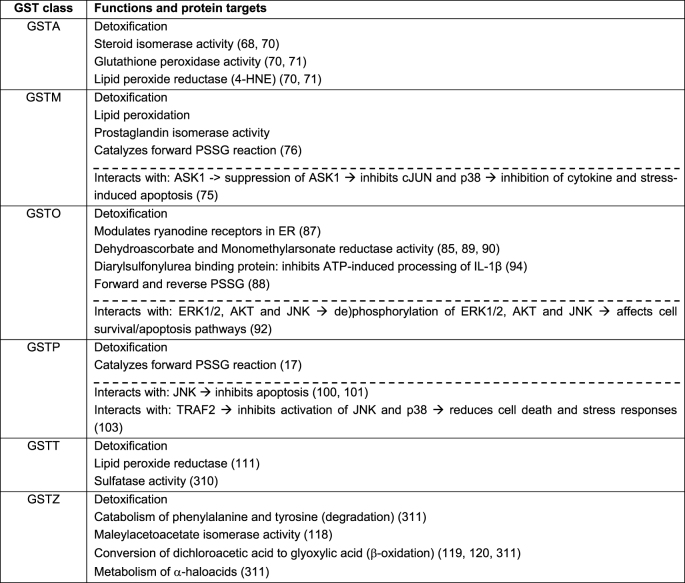
In addition to their roles in xenobiotic metabolism through GSH conjugation, GSTs have other catalytic activities (Table 1). For example, GSTM, GSTP, and GSTO have been shown to control the activity of members of the mitogen activated protein kinase (MAPK) pathway [67]. Some GSTs play an important role in biosynthesis of hormones (steroid hormone isomerase activity), are able to degrade tyrosine, display glutathione peroxidase activity, and may catalyze the reduction of organic hydroperoxides to their corresponding alcohols [50,68]. GSTs also have biological functions independently of their catalytic activity (Table 1). GSTs are capable of binding non-substrate ligands by forming protein-protein interactions with critical proteins involved in controlling stress responses, apoptosis, and proliferation [67]. The ligand binding moreover facilitates the intracellular transport of numerous hydrophobic and amphipathic compounds such as bilirubin, heme, steroids and bile salt. This binding often results in the inhibition of GST's activity. Furthermore, GSTs have been shown to regulate protein structure and function by their capability of conjugating GSH to proteins in a process called protein S-glutathionylation (PSSG) (Fig. 2C) [17,69]. Based upon the aforementioned versatility of GSTs, the potential roles of GSTs in the susceptibility to and progression of various chronic lung diseases, thus likely extends beyond the classic xenobiotic metabolism, which we will highlight in a later section. First, we will briefly describe the main characteristics, isotypes, functions as well as expression profile per class of human cytosolic GST.
Table 1.
Table describes the main functions of the different human cytosolic GST isoenzymes. These functions include detoxification, enzymatic functions, and protein S-glutathionylation. Moreover, the table describes the known interacting/target proteins per GST and the main resultant function/effect on the signaling pathway (below the dashed line). The arrows indicate the subsequent effect and/or outcome. 4-HNE: 4-hydroxy-2-nonenal; PSSG: Protein S-glutathionylation; ASK1: apoptosis signal-regulating kinase 1; JNK: c-Jun N-terminal kinase; ERK: Extracellular signal-regulated kinases; Akt: Protein Kinase B; ATP: Adenosine Triphosphate; IL-1β: Interleukin-1beta; TRAF2: tumor necrosis factor receptor associated factor 2.
3.1. Glutathione S-transferase alpha
GST Alpha (GSTA) is expressed in a wide variety of tissues with high expression in the liver, kidneys, adrenal glands, intestine, and in the testis, and at medium-low levels in a wide range of other tissues including the lung [70]. The GST Alpha class genes are located in a cluster mapped to chromosome 6 and contains five different isoforms: GSTA1, GSTA2, GSTA3 (expression is rare), GSTA4, and GSTA5, although GSTA5 has never been confirmed as a functional gene [70]. Of the GSTA isoforms, the GSTA4 protein is identical in length and shares 54% sequence identity to human GSTA1 and GSTA2.
Aside from being a Phase II detoxification enzyme, the human Alpha class genes are known to metabolize bilirubin and heme in the liver. GSTA enzymes furthermore display GPX activity which helps to protect cells and tissues through detoxification of oxidants and lipid peroxidation products. The GSTA enzymes are moreover known to possess glutathione-dependent steroid isomerase activity (especially GSTA3) [68,70] and have activity towards polycyclic aromatic hydrocarbons, epoxides and alkenyl products of lipid peroxidation (4-hydroxynonenal), especially GSTA4 [70,71].
Functional, allelic, single nucleotide polymorphisms (SNP) occur in an SP1-binding element of GSTA1 and in the coding regions of GSTA2 and GSTA3, leading to either low expression or reduced activity towards among others fatty acid hydroperoxides [70]. These properties suggest Alpha class GSTs to be involved in susceptibility to diseases with an environmental component (such as cancer, asthma, and cardiovascular disease).
3.2. Glutathione S-transferase Mu
Five isoforms of the GST Mu (GSTM) class, GSTM1 to GSTM5, exist, which are located on chromosome 1 [72,73] and are expressed in different organs. GSTM is highly expressed in most tissues including the liver, lungs, muscle, kidney, and brain. GSTM uses CDNB, and participates in the detoxification of carcinogenic compounds such as polycyclic aromatic hydrocarbons, aromatic amines and other organic compounds, including benzone(a)pyrene, styrene-7,8-oxide, and trans-stilbene oxide [74]. GSTM1 has been shown to bind (non-substrate dependent) and suppress apoptosis signal-regulating kinase 1 (ASK1) activity [75], a MAPK kinase kinase (MAPKKK) that activates c-Jun N-terminal kinase (JNK), a protein important in stress responses and pro-apoptotic signaling, and p38 pathways, that regulate cytokine and stress-induced apoptosis. Moreover, GSTM1 has been shown to catalyze the forward PSSG reaction [76], although not much is known about its specific PSSG targets.
The genes encoding the Mu class of enzymes are known to be polymorphic, especially GSTM1 exists as genetic variants. GSTM1 Lys173Asn (rs1065411) encodes proteins that form active mono- and heterodimeric enzymes. dGSTM1*1 × 2 is a unique variant in which the GSTM1 gene is duplicated [77]. The GSTM1 null variant on the other hand contains a homologous deletion of a 16 kb segment, which leads to a loss of protein expression and detoxification activity in homozygotes [78,79]. The prevalence of the null genotype is very high in the general population (up to 66%; in Caucasians ~ 50%), but varies across ethnicities [80,81]. In context of the lung, the GSTM null phenotype is associated with an increased risk of lung cancer, especially in Asians [39,82] and has also been linked with an increased risk of inflammatory lung diseases [43,80,83,84].
3.3. Glutathione S-transferase omega
GSTO is mainly expressed in the liver, notably in macrophages, glial, and endocrine cells, as well as in other tissues including for example the kidney, lung, and gallbladder [85]. The GSTO class has two isoforms, GSTO1, and GSTO2, located on chromosome 10 [64], and a third sequence appears to be a reverse-transcribed pseudogene, GSTO3p, identified on chromosome 3 [85]. GSTO1 and GSTO2 are two homo-dimeric proteins that display 64% sequence homology [86]. GSTO2 is strongly expressed in the testis, liver, kidney, and skeletal muscle, whereas GSTO1 is more abundantly expressed in the heart, in gastrointestinal tissues, and moderately in the lung [85]. The Omega class is structurally and functionally distinct from other eukaryotic GSTs, as it comprises a unique 19 residue N-terminus, which is similar to the tertiary structure of GLRXs, the main deglutathionylation enzymes (described in a later section).
Interestingly, GSTO enzymes have poor activity towards common GST substrates, such as CDNB. GSTO enzymes exhibit functions in cellular redox homeostasis as well as enzymatically modulating ryanodine receptors, calcium channels in the ER [87]. GSTO1 plays an important role in the S-glutathionylation cycle as it functions both as a catalyst of the forward reaction or as a deglutathionylation enzyme depending on the specific conditions [88]. Moreover, GSTO2 displays high glutathione-dependent thioltransferase and dehydroascorbate reductase activities, which are activities more characteristic of the GLRXs. Another novel activity of GSTO1 is the reduction of monomethylarsonic acid to monomethylarsonous acid (monomethylarsonate reductase activity), the rate-limiting step in the biotransformation of inorganic arsenic [85,89,90]. GSTO1 was originally identified as the ubiquitin ligase, p28, and is associated with anti-cancer drug resistance [91]. Additionally, it has been suggested that GSTO1 affects cell survival by activating survival and inhibiting apoptotic signaling pathways, presumably due to binding to and (de)phosphorylation of ERK1/2, AKT and JNK [92]. Moreover, GSTO1 is shown to translocate to the nucleus after heat shock and other stress conditions, but its function in the nucleus remains unclear [93]. Lastly, the GSTO1 isoform has been identified as a diarylsulfonylurea binding protein and it is suggested that this interaction is responsible for inhibition of ATP-induced interleukin 1 beta (IL-1β) posttranslational processing [94].
Based on the use of overexpression constructs it is believed that the GSTO1 Ala140Asp polymorphic variant (rs4925) protein is expressed at a higher level compared to the wild-type protein [86]. However, heat stability or activity towards CDNB or hydroxyethyl disulfide (measuring glutathione-dependent thioltransferase activity) is similar between wild-type and GSTO1 Ala140Asp variants [85]. Another group in contrast did find repressed thioltransferase activity of the Asp140 variant of GSTO1 (25% lower), but a difference in kinetics of its monomethyl arsenate reductase activity was not observed [95]. When using more specific substrates, the kinetics of S-glutathionylation were found to be increased and deglutathionylation repressed for the Asp140 variant of GSTO1 [88]. Protein expression of the GSTO2 Asn142Asp polymorphism (GSTO2*N142D; rs156697) was reduced to 76% compared to wild-type [86]. Due to the insolubility of GSTO2, the activity of variants has not been assessed. In a genome wide-analysis, top ranked relations were found between the non-synonymous coding homozygous GSTO2 Asn142 and lung function in adults [96].
3.4. Glutathione S-transferase P
Only one isoform of the GSTP class is known to be expressed in humans, namely GSTP1, which is located on chromosome 11 [18,97,98]. GSTP1 is the GST most commonly expressed outside the liver, with main expression in the heart, lung, and brain. GSTP accounts for over 90% of the GST activity toward CDNB in the lung, with the remaining activity attributed to GSTM1 and GSTT1 [99]. In addition, GSTP can bind to JNK by direct protein-protein interaction in a non-substrate dependent manner, thereby inhibiting the kinase activity and protecting cells against (H2O2-induced) cell death [[100], [101], [102]]. Another ligand binding partner of GSTP is tumor necrosis factor receptor associated factor 2 (TRAF2), a member of the TNF-α induced signaling, which in turn activates p38 and JNK [103], and is inhibited by GSTP binding. Moreover, GSTP is known to catalyze the forward PSSG reaction [17], which we will describe in more detail in the next section.
The GSTP gene is also known to be polymorphic [104]. In the context of the lung, the GSTP1 Ile105Val variant (rs1695) is one of the most commonly studied polymorphisms linked to chronic lung diseases, and has a decreased GST activity towards CDNB [45,105].
3.5. Glutathione S-transferase theta
GST Theta (GSTT) is predominantly found in the liver (hepatocytes), and is also expressed in the kidney (renal proximal tubule cells), gastrointestinal tract, and lung [[106], [107], [108]]. The Theta class includes the isoforms GSTT1 and GSTT2, which are both located on chromosome 22 [109,110]. GSTT1 and GSTT2 share 55% amino acid sequence identity. GSTT1 is important in phase II biotransformation of drugs and chemicals, and is involved in the detoxification of substrate intermediates which are produced during oxidative stress such as peroxidized lipids [111]. GSTT also participate in the detoxification of smoke-derived small hydrocarbons such as ethylene oxide, as well as epoxy butanes and methyl bromide [41,112,113].
Similar to the Mu class of GSTs, a null allele of GSTT1 exists, which contains a deletion of approximately 54 kb, and results in loss of enzymatic activity [[40], [41], [42]]. Individuals homozygous for this allele are at an increased risk for malignancies (head, neck, oral cavity) since the deletion results in decreased detoxification capacity of possible carcinogens [114]. The prevalence of the null genotype varies across ethnic groups with the highest prevalence in Asians (Caucasians 13–26%; Asians 35–52%) [81,115], and it has been linked to chronic lung diseases [43,83,84,116].
3.6. Glutathione S-transferase zeta
GST Zeta (GSTZ) is found in a wide variety of endocrine tissues including the liver, stomach, and testis as well as in the pancreas, and is located in the cytosol as well as in the mitochondria. The gene spans approximately 10.9 kb, is composed of 9 exons, and is located on chromosome 14 [117].
Besides its role in detoxification, GSTZ also plays a role in the catabolism of tyrosine. In particular, GSTZ catalyzes the cis-trans isomerization of maleylacetoacetate to fumarylacetoacetate, therefore has GSTZ also been described as maleylacetoacetate isomerase (MAAI) [118]. Interestingly, GSTZ1 shows the closest sequence similarity to GSTO1. However, GSTZ is the only enzyme in the GST family that plays a role in β-oxidation by catalyzing processes in the intermediary metabolism including the conversion of dichloroacetic acid to glyoxylate in a reaction that requires but does not consume GSH [119,120]. GSTZ is moderately expressed in the lung, but research efforts on GSTZ are very limited and studies are needed to examine the involvement of GSTZ in the lung.
4. Protein S-glutathionylation and deglutathionylation
Changes in the redox environment have long been implicated in the pathophysiology of many pathological conditions, especially the imbalance between antioxidant/oxidant production and scavenging. Consequently, damage by oxidants includes irreversible oxidations of cysteines within proteins. The original thought of oxidants being ‘bad actors’ and damaging has shifted since we now know that low levels of oxidants such as hydrogen peroxide (H2O2) and nitric oxide (NO) regulate processes important for maintaining cellular homeostasis [23]. Aside from being Phase II detoxification enzymes and the additional aforementioned functions, certain GSTs also play an important role in the process of PSSG by conjugating GSH to selective proteins (Fig. 2C). PSSG is a reversible post-translational modification [121], which regulates protein structure and function as it alters the shape, charge and size of the target protein. The process of PSSG can occur spontaneously/non-enzymatically, but can also be catalyzed enzymatically, by specifically the GSTs, GSTP [17,69], GSTO [88], and to a lesser extent GSTM [76]. Non-enzymatic PSSG depends on the availability of GSH/GSSG and occurs via thiol-disulfide exchange reactions between GSSG and a protein cysteinyl residue or via reaction of GSH with an oxidized thiol derivative such as sulfenic acid (-SOH), thiyl radical (-S•) or S-nitrosyl (-SNO) [122]. Depending on the protein, and targeted cysteine residue, PSSG can either activate or inhibit its function. GSTs have several substrates for glutathionylation which include transcription factors, kinases, structural proteins and enzymes involved in metabolism [123].
It is thought that both non-enzymatic, as well as enzymatically catalyzed formation of PSSG involves intermediate sulfenylation, the reaction of protein cysteines, containing a sulfhydryl side chain (SH), with an oxidant such as hydrogen peroxide (H2O2) to form a sulfenic acid (-SOH) intermediate (Fig. 2C). This SOH intermediate can be stabilized, or can give rise to sulfenamides (SN), or disulfide bonds (S–S) which can occur within a protein or between proteins [124]. The SOH intermediate is believed to be recognized by GSH-bound GSTP (or other GSTs), which in turn catalyzes the S-glutathionylation of the protein cysteine. The S-glutathionylated protein can interact with another GSH moiety to release oxidized GSH (GSSG), which regenerates the reduced protein thiol. This latter reaction is catalyzed by GLRXs under physiological conditions. The redox-dependent PSSG reaction is thought to protect proteins from further irreversible overoxidation to sulfinic (SO2H) and sulfonic acids (SO3H), but it has also been shown to play critical roles in the regulation of protein function. Worthy to mention is that only certain cysteine residues have been signified as ‘reactive’, meaning they can be readily oxidized, reduced and otherwise modified. Reactive cysteines are characterized as having a lower pKa (≤7) than that typical of cysteine, which ranges from 8 to 8.5. Detailed information on cysteine chemistry and modifications can be found elsewhere [122,[124], [125], [126]].
The regulatory role of GSTP in the forward reactions of PSSG is based on the catalytic activity of the enzyme. GSTP contains 4 cysteines residues: Cys14, 47, 101, and 169. Intramolecular disulfide bonds have been shown to occur between Cys47 and Cys101, and disulfide formation also seemed to occur preferably between Cys14 and Cys169 when a disulfide bond between Cys47 and Cys101 had already been formed [127] (Fig. 2D). Disulfide bond formation (intra-subunit) between Cys47 and Cys101 results in steric hindrance for GSH binding, which implies that these residues are located in an important region for GSH binding. Disulfide bonds can also be formed between Cys47 residues in different subunits, which can result in conformational change and inactivation of GSTP [127]. GSTP itself can be auto-S-glutathionylated on Cys47 and Cys101, changing its secondary structure with an impact on the structures within the monomer of GSTP [60]. This can reduce the catalytic activity of GSTP [17] and affect the interaction of GTSP with ligand-binding proteins. For example, Cys47 and Cys101 in GSTP have been shown to be critical for the interaction and regulation of JNK. S-glutathionylation on both cysteines thus acts as an oligomer switch and can cause GSTP multimerization and inactivation of the enzyme [17].
Whereas the forward PSSG reaction can be catalyzed by GSTs, in mammals, GLRXs, or thioltransferases are the main deglutathionylating enzymes (Fig. 2C) [128,129]. GLRXs are part of the family of thioltransferases [129], and there are four known GLRXs in mammalian cells; GLRX1 (cytosolic), GLRX2 (mitochondrial/nuclear), GLRX3/PICOT (cytosolic) and GLRX5 (mitochondrial), of which GLRX1 is the most efficient at deglutathionylating proteins. For the current knowledge on GLRXs, their impact on lung diseases and PSSG targets relevant to these lung diseases, we direct the reader to another review that we recently published [56]. Other enzymes that have been implicated in catalyzing the reverse reaction under certain conditions, include sulfiredoxins [130], as well as GSTO1 [88]. The GSH binding of GSTO1 distinguishes this GST from typical GSTs as the active site cysteine residue, Cys32, in GSTO1 creates a mixed disulfide with GSH. Mutating Cys32 ablated the deglutathionylation activity of GSTO1 [88]. β-actin is a protein that is specifically deglutathionylated by GSTO1 [88].
5. Glutathione S-transferases and their implication during early life and in lung diseases
5.1. GST presence in the lungs
Prenatal as well as postnatal environmental and lifestyle exposures may affect lung development, in part due to increased oxidant production. Especially at birth, newborns are highly susceptible to increases in oxidative stress levels, as for example the partial oxygen pressure increases from 20 to 25 mm Hg in utero to 100 mm Hg in the extra uterine environment, because of the increased metabolic activity to maintain breathing, and body temperature. This abrupt change exposes newborns to high oxidative stress levels [131]. Pre-term birth is associated with increased oxidative stress as higher levels of oxidative stress markers have been reported in pre-term compared to full-term newborns [132]. Moreover, pre-term infants often require assistance to breathe which includes the need for supplemental oxygen and ventilatory support. The amount of supplemental oxygen is crucial as too much oxygen has been shown to increase oxidative stress, and ‘pure’ (100%) oxygen may cause inflammation, and emphysema in the lungs of newborn mice [133]. Additionally, increased oxidative stress is even thought to cause pre-term birth [132,134], although the contribution of oxidative stress itself is hard to interpret since other factors may also contribute. Antioxidant systems in the lungs are therefore crucial in maintaining perinatal redox balance, and it is surprising that very few studies have investigated the expression profile of antioxidants including the cytosolic GSTs pre-, during and post-birth. In mouse lungs, GSTs are expressed at low levels before birth (with the exception of GSTP which showed high levels during gestation), are highly increased within one week after birth, and decrease again two to three weeks after birth [135]. Overall GST activity in the distal airways increases over this time window, which may be an adaptation to the external environment. During human lung development, GSTP is the predominantly expressed GST isoenzyme up to 13 weeks of gestation [136]. Another study showed that in a 14-week old fetus, GSTP is present in all differentiated epithelial cells [137]. As development proceeds with the differentiation of progenitor cells into type I and type II pneumocytes, the appearance of GSTP negative cells coincides. Two independent studies found that GSTA and GSTM expression was continuous throughout development in the lung, albeit at respectively moderate to low levels [136,138].
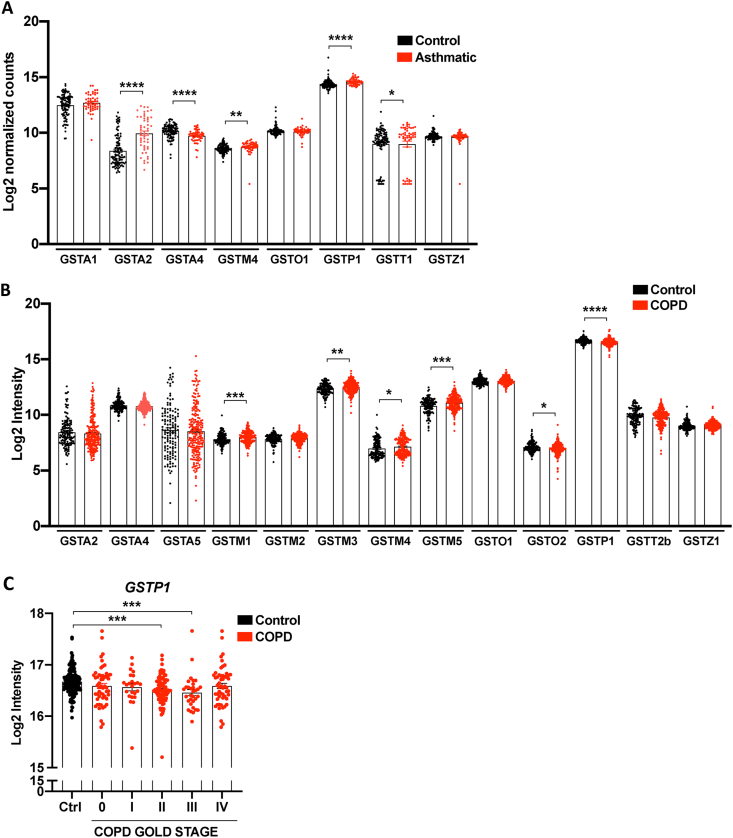
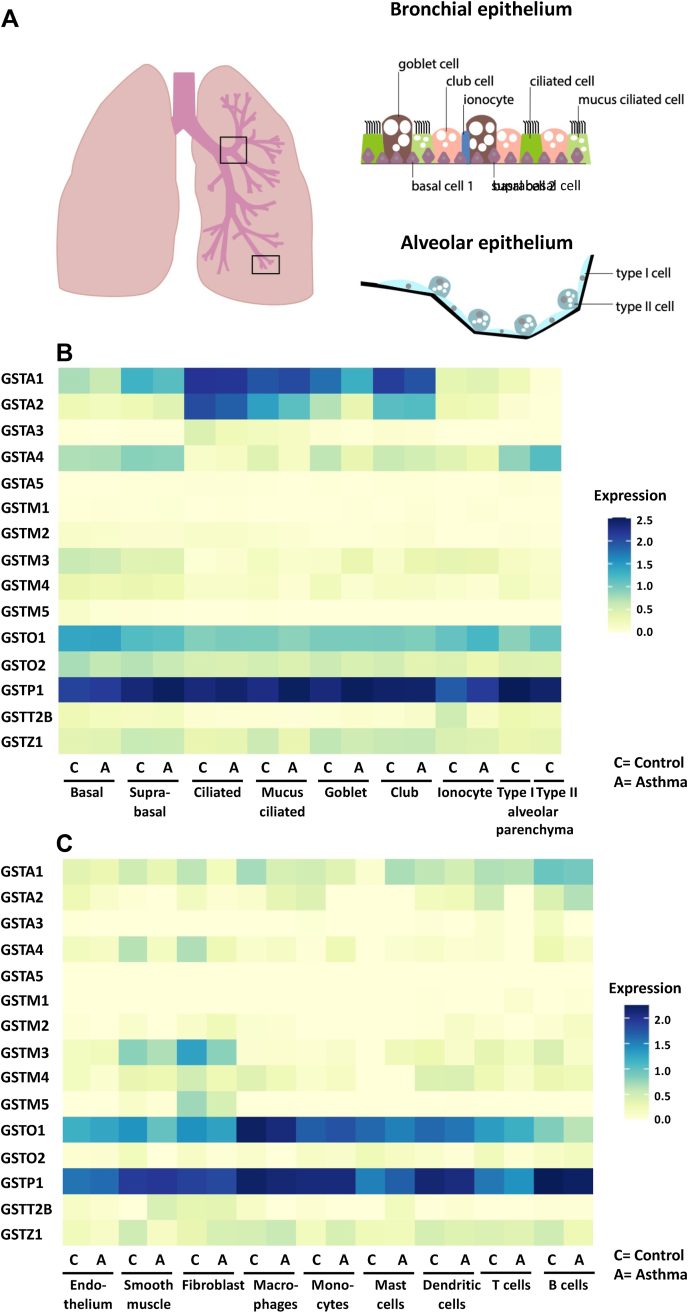
In humans, the expression of the different GSTs during lung development in early life remains poorly examined. However, a number of studies have examined the distribution of the different GSTs in easily obtained nasal epithelial cells and lung tissue in adults. Re-analysis (retrospective) of GST transcripts from a publicly available RNA sequence (RNA seq) dataset from nasal brushings of asthmatic patients (red; n = 53) compared with healthy subjects (black; n = 97) [139] showed expression of GSTA1, GSTA2, GSTA4, GSTM4, GSTO1, GSTP1, GSTT1, and GSTZ1 in healthy control subjects (Fig. 3A). Re-analysis of microarray gene expression data from the publicly available Lung Genomics Research Consortium (LGRC) showed expression of most GST isoforms with the exception of GSTA1, GSTA3, and the GSTT class in non-COPD control subjects (Fig. 3B). In lung tissue as well as in nasal epithelium, GSTP1 mRNA levels were most abundant. GSTs are highly expressed in the airway epithelium, but it is unknown whether GSTs exert unique functions among the various epithelial cell subtypes. Recently described single cell RNA seq databases begin to shed light onto this question. We therefore examined the expression of GST isoforms in a recently published single cell RNA seq database that profiled single cells of bronchial biopsies as well as lung parenchyma (small respiratory airways and alveoli) upon lung resection surgery from healthy subjects (Fig. 4) [140]. The airway epithelium is in direct contact with inhaled air and consists of basal cells (progenitor cells that exhibit the capacity to self-renew and give rise to multiple types of differentiated airway epithelial cells), ciliated cells (move liquid over surface, to keep the airways clean of mucus and dirt), goblet cells (a mucus secreting cell), and club cells (previously known as Clara cells that protect the bronchiolar epithelium by secreting proteins including CCSP) (Fig. 4A) [141]. Two discrete cell states were identified in basal, and ciliated epithelial cells. Basal cells were less-mature than suprabasal cells, which were more apically localized. Mucus ciliated cells are highly similar to ciliated cells, but co-express a number of mucus genes [140]. Fig. 4B shows that GSTP1 is the most abundantly expressed GST in all respiratory epithelial cell types in controls, including type I and type II alveolar epithelial cells, and is moreover expressed in pulmonary ionocytes, a newly identified Foxi1+ cell type which plays a role in clearing mucus from the airways [142,143]. GSTO1 is also widely expressed in lung epithelial cell types, specifically in basal and suprabasal cells, as well as in ionocytes and type II alveolar cells in the parenchyma, while GSTO2 is only moderately expressed in basal and suprabasal cells. GSTA1 is highly expressed in ciliated, mucus ciliated, goblet and club cells and moderately expressed in basal and suprabasal cells. GSTA2 is expressed in ciliated, and mucus ciliated cells, as well as in club cells, and to a lesser extent in goblet cells, while GSTA4 was mainly expressed in basal and suprabasal cells, and also in type I and II epithelial cells in the parenchyma. Besides expression in epithelial cells, GSTM3, GSTO1, and GSTP1 were highly expressed in fibroblasts and in smooth muscle cells in control subjects (Fig. 4C). Similarly, GSTA1, and GSTA4 were also expressed in smooth muscle cells and fibroblasts, as well as GSTM5 in fibroblasts but to a lesser extent. GSTO1, and GSTP1 were also highly expressed in the endothelium. GSTs are also detectable in immune cells (Fig. 4C). Especially GSTP1, and GSTO1 were expressed in a variety of immune cells including mast cells, macrophages, monocytes, as well as B and T lymphocytes, and dendritic cells. GSTA1 was moderate expressed in macrophages, as well as T and B lymphocytes.
Fig. 3.
Transcript levels of cytosolic Glutathione S-transferases in patients with asthma and COPD. A, Control vs Asthma: Retrospective analysis of GST transcripts from a publicly available RNA sequence dataset from RNA isolated from nasal brushing of asthmatic patients (red; n = 53) compared with healthy subjects (black; n = 97). A raw number of counts for each gene was generated from the RNA-seq database, and these data were subsequently normalized by a log2 transformation of the data (log2 normalized counts). The full description of RNA isolation and processing from nasal brushings, and the process of the raw RNA sequence data are published here [139]. B, Control vs COPD. Retrospective analysis of GST transcripts was obtained from publicly available microarray gene expression data from the Lung Genomics Research Consortium (LGRC) for COPD patients (red; n = 219), and non-diseased control tissues (black; n = 137). The intensity measurement of the microarray data was log2 normalized (log2 Intensity) and is available in the Gene Expression Omnibus (GEO) database accession no. GSE47460. Results are shown as average ± SEM. Statistical significance was calculated using a Mann–Whitney U Test. *p-value <0.05; **p-value <0.01; ***p-value <0.001; ****p-value <0.0001. C, Differences in GSTP transcripts between GOLD stages of COPD patients from the GLRC described in B. GSTP is shown only as only GSTP showed differences between GOLD stages of COPD patients. COPD: chronic obstructive pulmonary disease. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Airway and lung epithelium transcripts expression of cytosolic Glutathione S-transferases in patients with asthma and controls. A, Representative overview of the different epithelial cells in the bronchial epithelium (top), including basal, suprabasal, goblet, club, ciliated, mucus ciliated cells, and ionocytes, as well as the Type I and Type II cells in the alveolar epithelium (bottom). B–C, Heatmap of normalized gene expression of GST genes, which has been re-analyzed from a recently published single cell RNA seq dataset [140] that can be explored in the web portal www.lungcellatlas.org from expressing clusters of bronchial and alveolar epithelial cells (B), and other structural cells including immune cells, smooth muscle, fibroblasts, and endothelium in airway and lung epithelium (C) split by asthma and control. The type I and type II alveolar cells in the parenchyma were only analyzed in controls [140]. The single cell data consists of 22933 cells combined from bronchial biopsies from six healthy controls, six persistent asthma donors with chronic, childhood-onset asthma, and rejected lung donor material from six healthy deceased controls (alveolar cells). Six macroscopically adequate endobronchial biopsies were collected for the described published study [140], located between the third and sixth generation of the right lower and middle lobe. Extracted biopsies were processed directly thereafter with a delay of maximum 1 h. For the precise methods on patients recruitment, lung tissue and sample processing, and data quality control we refer the reader here [140], and the clinical characteristics of asthmatics and healthy controls can be found in supplementary table 3 [140]. The 10x Genomics raw seq data was processed with CellRanger version 2, ambient RNA corrected and normalized and analyzed using SoupX [309] and the R software package Seurat, as described here [140]. C= Control; A = Asthma.
Some of these new observations from the single-cell RNA seq study are a corroboration of much earlier studies examining the location of GSTs in lung tissue based upon immunohistochemistry. A study from the early 1990's indeed found that GSTP and GSTA were the most abundant GSTs in human lung tissue, and that these GSTs were primarily present in the bronchial epithelium [144,145]. It was furthermore shown that GSTP and GSTM were present in the distal airspaces, namely in alveolar type I and II cells and macrophages [145]. GSTM1 was present at low levels in the lung, whereas GSTM2 was minimally present in the epithelium, and GSTM3 was located in the airway epithelium and smooth muscle of the lung [144]. The GSTO1 protein was found primarily in apical parts of epithelial cells, in murine mouse lungs [146], while another study claimed, in humans, that GSTO1 was mainly expressed in alveolar macrophages, and to a lesser extent in airway and alveolar epithelium [147]. GSTT was found to be poorly expressed in the lung, and the expression of GSTZ was moderate. Moreover, GSTs were also present in extracellular fluids as GSTA1, GSTM, GSTP, and GSTO1 were detectable in sputum supernatants of human subjects and GSTA, GSTO1, and GSTP1 were furthermore found in bronchoalveolar lavage fluid (BALF) and plasma [145,147,148]. The extracellular presence and function of GSTs is of particular interest given the importance of GSH and regulatory enzymes in the protection of lung cells and tissues from the extracellular space. The unique expression profiles of GSTs in lung cells and within populations of airway epithelial cells point to specific functions of the diverse GSTs, which remain to be unraveled.
5.2. Importance of GSTs in lung development and lung function during early life events
Normal lung growth and development during early life and childhood are essential to reach maximal attainable adult lung function. Prenatal, postnatal, early life, and ongoing childhood environmental exposures as well as genetic GST polymorphisms can affect lung development, lung maturation and adult lung injury and repair, events which are highly associated with each other [149,150]. Reduced lung growth results in a lower attained lung volume which increases adverse effects from exposure to respiratory toxins, the risks for acute symptoms from exacerbations of asthma or respiratory infections, and risks for chronic diseases such as COPD. Indeed, studies have shown that exposure to for example air pollution or tobacco smoke especially in association with GST polymorphisms affects pre- and postnatal lung development, increases the risk of asthma (symptoms) at younger age, and increases the risk for decreased lung function in adolescence which increased the susceptibility of developing COPD [4,[151], [152], [153]].
Interestingly, there are some indications that GST polymorphisms are associated with the development of bronchopulmonary dysplasia (BPD), a severe pulmonary complication of premature birth. BPD is clinically defined as a dependence on oxygen past the 36th week of gestation and is characterized by an arrest in alveolarization and a reduced vascular network [154]. The GSTM1 null and combined GSTM1/GSTT1 null genotypes [155], as well as the GSTP1 Ile105 allele [156] have been associated with BPD, although some studies failed to confirm these associations [[157], [158], [159]]. Importantly, these infants have a life-long risk to develop lung diseases, from asthma in childhood to adult lung diseases, such as asthma and COPD at more advanced age [151,152].
Polymorphisms in GSTs of both the child and the mother during pregnancy are associated with lung function and the development of asthma in later life. It was shown that the presence of the GSTT1 null allele in both the mother and the child was associated with a lower lung function and increased airways hyperresponsiveness (AHR) in the first year of life. Maternal smoking during pregnancy further exacerbated these effects [160]. The detoxification capacity of both the mother and the child thus play a role in preventing adverse effects on early life lung development. Maternal exposure to particulate matter with a diameter of less than 2.5 μm (PM2.5) at 35–40 weeks of gestation was found to be associated with decreased FEV1 and FVC in early childhood (7 years of age), in particular in boys. In conjunction, this study demonstrated an increased methylation of the GSTP1 promotor in nasal epithelial cells at age 7 when prenatal exposure to PM2.5 was high in this same critical time-window (35–40 weeks) of pregnancy. A borderline significant association between GSTP1 methylation and decreased FEV1 was furthermore established [161]. In addition to the contribution of inherited genetic variants, this study demonstrates the role of epigenetic regulation of GSTP1 expression by pollutants in lung function development.
In addition to direct effects of GST polymorphisms on the toxic effects of smoking of adults and the development of disease, a number of studies identified an interaction between GST variants, maternal smoking and lung function in children. Early-life wheezing was more prevalent in children with the wild-type GSTP1 genotype whose mothers smoked [162]. Similar results were obtained for the presence of the null allele of GSTM1, which in combination with maternal smoking led to a reduced lung function [163], and strongly increased the risk to develop asthma and asthma-associated symptoms [153,[164], [165], [166]]. GSTM and GSTP1 Ile105Val variants have been associated with reduced lung function and growth until adolescence, independent of smoking [105,167,168]. Moreover, GSTT1 and GSTM1 gene deletions are associated with chronic bronchitis in children [169].
Exposure to air pollution (NO2 and PM2.5) at birth and early life was also associated with increased asthma incidence through adolescence, showing the vulnerability of children in the perinatal period and the development of asthma at a later age [170]. Moreover, 3–12 year old children with either the GSTM1 null genotype or who were homozygous for the GSTP1 Val105 allele, were more susceptible to asthma associated with environmental tobacco smoke exposure than those without these GST polymorphisms [171]. The effect of environmental tobacco smoke was shown to be cumulative over time, as in 13 to 21-year olds with the GSTM1 null allele, lung function declined with age as the peak expiratory flow rate was substantially reduced compared to 3–12 years old.
In 2007, the Framingham Heart study published results of their genome-wide analyses in relation to lung function in adults. Top ranked relations were found between the non-synonymous coding homozygous GSTO2 Asn142 and FEV1, as well as FVC [96]. This association was later confirmed in a larger study, which additionally found an interaction between lung function, GSTO2 Asn142Asp and environmental tobacco smoke exposure [172]. Surprisingly, the interaction of GSTO2 Asp142 with in utero exposure to tobacco smoke associated with higher FEV1 [172]. Moreover, unique studies involving human experimental exposures have demonstrated that adult subjects with the null genotype of GSTM1 show an increased susceptibility to the development of neutrophilic bronchitis in response to ozone, endotoxin, particulate matter and wood smoke [5,[173], [174], [175]]. Mechanistically, knock-down of GSTM1 increased IL-8 release by bronchial epithelial cells in response to ozone as well as diesel particles, involving enhanced nuclear factor kappa B (NF-κB) activation and ROS production, and enhanced Erk/PI3K/Akt activation and ROS production respectively [15,176]. Importantly, a similar increase in IL-8 release was noted by epithelial cells derived from donors with the GSTM1 null genotype [15]. Thus, the latter studies suggest that GSTM1 appears to provide protection from a variety of insults and to protect from inflammation.
Numerous studies have shown that air pollution can cause acute and chronic mortality, often related to lung damage [177,178]. Indeed, it has been estimated that ambient and household air pollution accounts indirectly for more than 6 million deaths per year, for which 50% is accountable due to COPD [179]. Although it is unambiguous that PM and ambient air pollution are harmful for our lungs, a recent manuscript questioned the studies performed on the detrimental effects of air pollution on the development of chronic lung diseases [180], and suggested caution regarding the interpretation of claims that exacerbations and deaths were caused by particulate air pollution. More studies will be required to test the individual impact of each component (pollutant) to the susceptibility in developing disease and affecting mortality.
Alterations in lung development and maturation can increase the susceptibility to respiratory infections, acute exacerbations and even chronic lung disease. Moreover, children with GST polymorphisms are significantly more likely to have increased risk of acute respiratory illness if they are born to mothers who smoke during pregnancy or if they have been exposed to environmental tobacco smoke [153,160,163,164]. In the next section we will review studies that have implicated GSTs in the pathogenesis of asthma and COPD.
6. Asthma
Asthma is a complex pulmonary disorder characterized by mucus metaplasia, airways hyperresponsiveness (AHR), airway remodeling, and inflammation, which affects approximately 300 million people worldwide [181]. Asthma can be further divided into subgroups including exercise-induced asthma, occupational asthma, and allergy-induced asthma [182]. Asthma also occurs in settings of obesity wherein the disease is notably severe [183,184]. Common symptoms include shortness of breath, wheezing and/or coughing, as well as chest tightness. Exposure to various irritants and allergens, e.g. airborne allergens (pollen, dust mites, mold spores), respiratory infections, physical activity, cold air, air pollutants such as smoke, and stress can trigger aforementioned symptoms of asthma. Oxidative stress has been speculated to be one of the main risk factors for asthma development [185].
Asthma is linked to chronic inflammation, and involves numerous cell types of the innate and adaptive immune system, including eosinophils, neutrophils, activated mast cells and type II immune cells, along others [186,187]. The pathophysiology of asthma is characterized by structural changes in the airways, such as subepithelial fibrosis, mucus metaplasia, smooth muscle hypertrophy and hyperplasia, increased blood vessel formation as well as dysregulation of epithelial barrier function [186]. Asthma can develop at any age and due to the heterogeneity of the disease, it is hard to define and characterize patients with asthma [188]. Current treatment for asthmatics include corticosteroids, and bronchodilators, which reduce symptoms, but do not cure disease. Moreover, these treatments are mostly effective in patients with typical asthma characteristics (atopic asthma) including eosinophilic inflammation, TH2 immunity and acute exacerbations [186]. Importantly, a subgroup of asthma patients with severe disease do not respond to these current treatments. The onset of asthma in these patients with severe disease is often independent of allergy and not always associated with eosinophilic inflammation. Conversely, these patients may have neutrophilic inflammation, are often steroid resistant and their disease is associated with other factors including environmental and lifestyle factors [189].
7. COPD
COPD, one of the most common lung diseases in the world, is a chronic inflammatory lung disease characterized by airflow obstruction. Approximately 251 million people are affected by COPD globally which is about 8–10% of the adult population in developed countries [190]. The World Health Organization has projected that COPD will become the 3rd leading cause of death globally by 2030 unless vital action is taken to reduce underlying risk factors [191]. Main symptoms include difficulty breathing, wheezing and coughing, and excessive mucus production. Initially these symptoms are mild and often leave the disease undiagnosed. But as the disease progresses, they lead to major limitations in the execution of daily activities and negatively impact patients’ quality of life. Current treatments alleviate some of the symptoms and slow disease progression, but the progression is not halted or reversed. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common, preventable and treatable disease that is characterized by persistent airflow limitation that is due to airway and/or alveolar abnormalities caused by significant exposure to noxious particles or gasses [192]. The severity of the disease is defined in GOLD stages (I- > IV; A- > D) from mild to more severe disease [192,193].
The primary exposure linked to the development of COPD is cigarette smoke, followed by air pollution, chemical dusts and fumes. Despite the strong association with smoking, it is important to note that approximately 25% of COPD patients never smoked, and that the majority of smokers is spared from the disease [194]. Genetic predisposition and gene-environment interactions thus play an important role in determining disease susceptibility. As COPD usually develops after the age of 40 and resembles features of normal lung aging, it is coined a disease of accelerated lung aging [195,196]. Because of the increase in the aging population, the still rising smoking prevalence in many countries, as well as the diminished air quality, the number of patients with COPD is likely to increase in the coming years.
The two most common conditions contributing to the loss of lung function in COPD are emphysema and chronic bronchitis [197]. Chronic bronchitis represents an inflammatory state of the airways, which is associated with thickening of the bronchial wall and excess mucus production. In emphysema, damage to pneumocytes and proteolytic degradation of alveolar membranes lead to loss of alveolar septae. This reduces the surface area for gas exchange and leaves the small airways more prone to collapse. COPD is thus a complex clinical condition in which different components and mechanisms represent and contribute to the pathophysiology, as well as to its clinical presentation [192,193,198]. Chronic inflammation and oxidative stress resulting from the exposure to noxious gasses and particles, and persisting after exposure ceases, are considered major drivers of the irreversible damage and aberrant repair that characterize the different pathological features.
The initial symptoms of COPD and asthma are very similar, and therefore sometimes difficult to distinguish during early stages of disease. One of the main differences between COPD and asthma is that COPD is mainly caused by irreversible damage elicited by smoke or other noxious inhaled particles and consequent chronic inflammation, and usually develops after the age of 40, whereas asthma is mainly caused by allergen-driven inflammatory reactions and can develop at any age [188,196]. The main inflammatory cells involved in COPD include neutrophils and macrophages, while smokers additionally have Th1 lymphocyte involvement, although some people with COPD also have eosinophil involvement, the predominant inflammatory cells in asthma. Similar to asthmatic patients with severe disease, current treatments for COPD patients reduce symptoms but do not recover the irreversible loss in functional lung tissue [192,193]. There is an urgent need to gain more insights into the underlying mechanisms of both chronic lung diseases to develop new treatment strategies to increase the quality of life and survival of patients with chronic lung diseases.
As mentioned in the previous section, GSTs display many important functions through with they may influence lung disease susceptibility. In the next section we will review studies performed per class of GST, including the different GST isoenzymes and the known polymorphisms relevant to asthma and COPD.
8. Glutathione S-transferases in asthma
8.1. GSTA in asthma
The studies that examined GSTA in asthma are very limited, which is surprising since the GSTA1/2 enzyme was found to be abundantly expressed in human lung tissue [144]. One study reported the upregulation of GSTA1 mRNA expression in induced sputum cells of asthmatics compared to healthy controls (Table 2). This study also showed that GSTA was highly expressed in peribronchial inflammatory cells and in epithelial cells in ovalbumin-treated mice, although the mRNA expression in the whole lung was not different between ovalbumin-treated mice and their controls [199]. RNA seq data showed that GSTA2 was upregulated, and GSTA4 was downregulated in nasal brushings from asthmatics compared to controls (Fig. 3A). Moreover, an earlier study highlighted GSTA1 as a susceptibility locus for asthma, including the GSTA1*‐69C/T (rs3957357) polymorphism. Individuals with the −69T allele exhibited lower GSTA1 expression [200]. In two studies by the same group, asthmatics displayed a higher prevalence of the GSTA1*‐69C/T genotype compared to controls, and the GSTA1*-69T polymorphism was associated with an increased risk of asthma and allergies in Italian adults (OR = 3.45; 95% CI: 1.80–6.62) [201,202] (Table 3).
Table 2.
mRNA and protein expression differences between COPD or asthma vs control.
| GST | Asthma vs control mRNA | Asthma vs control protein | COPD vs control mRNA | COPD vs control protein |
|---|---|---|---|---|
| GSTA | Increased in lung lysates of mild -moderate COPD patients compared to very severe disease and controls; GSTA higher in induced sputum [147]; GSTA + central airway epithelial cells decreased in GOLD stage IV patients. | |||
| GSTA1 | Increased in sputum [199] | Decreased in BALF [223] | ||
| GSTA2 | Decreased in BALF [223] | |||
| GSTM1 | Not different in sputum [199] | Lower in lung tissue [225]; Increased in microarray from bronchial brushing [226]; | ||
| GSTM3 | Increased in microarray from bronchial brushing [226] | |||
| GSTO1 | Decreased in lung tissue and sputum compared to smokers and never smoker controls [147] | |||
| GSTP1 | Downregulation in nasal epithelial cells in children [208], no difference in induced sputum samples [199] | Decreased in peripheral lung [225] | Increased in whole lung homogenates in patients with mild disease, number of GSTP + cells did not differ [147]; lower in BALF [223] | |
| GSTT1 | Not different [199] |
Table describes the differences in mRNA and protein expression of the different GST isoenzymes between COPD patients and controls or asthmatic patients and control subjects. COPD: Chronic Obstructive Pulmonary Disease; GST: Glutathione S-transferase; BALF: Broncho alveolar lavage fluid.
Table 3.
GST polymorphisms and their contribution to GST function, and the risk of the variant allele (compared to wild-type allele) on the chronic lung diseases asthma and COPD.
| Genetic variant | Function | Asthma | COPD |
|---|---|---|---|
| GSTA1* −69C/T, rs3957357 | Promotor variant, reduced protein expression [70] | Increased risk in adults [201,202]; Associated with allergies [202] | No data |
| GSTM1 G > C rs412543 GSTM1 null |
Promotor variant 16 kb deletion – loss of protein expression [78,79] |
Increased risk in children [104] Increased risk to (atopic) children & adults [204,205], especially in combination with exposure to tobacco smoke [164], meta-analysis [43,84,203]; no association [206,219,220]; increased risk but not when only restricted to larger studies [203] |
Increased risk, meta-analysis [83,234,238,240]; no association [44] |
| GSTO1 Ala140Asp (A140D), 419C > A, rs4925 GSTO1-E155del GSTO2 Asn142Asp (N142D), 424A > G, rs156697 |
Coding sequence variant with variable effects on activity [85,86,88,95] Coding sequence variant with reduced expression [86] |
No association [201] No association [201] Increased risk in adults [201,202] |
Increased risk as haplotype with rs156697 [227] Increased risk [227] |
| GSTP Ile105Val (L105V), 313A > G, rs1695 GSTP1 Ala114Val (A114V), 341C > T, rs1138272 |
Coding sequence variant with decreased activity [45] Coding sequence variant with not known activity |
Decreased risk [204,205,213]; no association [40,216,220]; increased risk to (atopic) asthma in children & adults [46,116]; increased in combination with environmental exposure [214,215]; No significant association in meta-analysis [219] Increased risk at young age [214] No association in children [216] |
Decreased risk [232,233]; increased risk [229] – in particular in α1-antitrypsin deficiency [230] – meta-analysis [236]. No association in meta-analysis [47,235] |
| GSTT1 null | Whole or partial ~ 54 kb deletion - loss of protein expression [41,42] | Increased risk to asthma attack in children [220], increased risk to atopic asthma [204], meta-analysis [43,84,219]; no association [206]; increased risk but not when only restricted to larger studies [203] | Increased risk [44], especially for emphysema [237], meta-analysis [83] |
Table describes different GST polymorphisms that have been associated with asthma or COPD in the literature. COPD: Chronic Obstructive Pulmonary Disease; GST: Glutathione S-transferase.
8.2. GSTM in asthma
GSTM1 genes have been associated with an increased risk of asthma in children and adults [40]. In the Children's Health Study in the 1990's, the GSTM1 null genotype was associated with deficits in annual growth rates (FVC: 0.21%; 95% CI: 0.40, −0.03, and FEV1:−0.27%; 95% CI, −0.50, −0.04) [105]. Moreover, as mentioned before, the GSTM1 null genotype has been linked to an increased risk of inflammatory lung diseases [80] including asthma, and atopy, a predisposition towards developing certain allergic hypersensitivity reactions [84,[203], [204], [205]], especially in combination with environmental exposure [164]. Asthma patients display a higher prevalence of the GSTM1 null genotype compared to healthy individuals (63.4% vs. 40.8%; OR = 2.34; 95% CI, 1.31–4.20) [116], although this prevalence varies across ethnic groups [206]. However, there has been extreme between-study variability and publication bias, and the association between GSTM1 and asthma sometimes disappeared when meta-analysis were restricted to the larger studies [203]. In a recent ‘updated’ meta-analysis, including 26 case-control studies, a significant association was found for the GSTM1 null polymorphism (OR = 1.452; 95% confidence interval (CI): 1.192–1.770) and the risk of asthma in both children (OR = 1.368; 95% CI: 1.051–1.781) and adults (OR = 1.859; 95% CI: 1.183–2.921) [43]. Based on subgroup analysis by ethnicity, a significant association for GSTM1 and the risk of asthma was found in Europeans (OR = 1.303; 95% CI: 1.018–1.667), Africans (OR = 2.175; 95% CI: 1.560–3.031) and Latin-Americans (OR = 2.265; 95% CI: 1.375–3.729). One study so far also reported an association between the GSTM1 polymorphism rs412543 (G > C) and asthma [104] (Table 3). GSTM4 transcript levels were increased in nasal brushings from asthmatic patients compared to control subjects (Fig. 3A), while GSTM1 mRNA levels were shown not to be different in induced sputum samples from asthmatics compared to healthy controls (Table 2) [199]. These data on transcript levels are in contrast with the results of the meta-analysis that suggest that subjects with the GSTM1 null polymorphism may display poorer lung function and may be of an increased risk of asthma.
8.3. GSTO in asthma
Although GSTO is not highly expressed in the lung, its expression can be induced in mouse lungs by allergens and arsenic, a component of cigarette smoke. The latter is of interest as GSTO1 also metabolizes arsenic by its monomethylarsonate reductase activity [90]. Gsto1 mRNA expression, but not protein, was indeed increased in an OVA-model of allergic airways disease [146]. Recently, it has been shown in a mouse model of allergic airways disease (involving the house dust mite allergen), that Gsto1 deficiency promoted a M2-macrophage phenotype (macrophages associated with wound healing and tissue repair), and increased nuclear HIF-1α levels in the lung, in association with increased eotaxin-induced eosinophilic airway inflammation [207]. In humans, the distribution of the GSTO1 genotypes, GSTO1 Ala140Asp and GSTO1*E155del, was nearly equal between control subjects and asthmatics [201] (Table 3). Italian subjects with the GSTO2 Asn142Asp genotype were shown to have an increased risk of asthma (Table 3) [201,202]. The in vivo mouse data suggests that Gsto1 may be protective during allergic airway disease. However, further studies with a larger sample size of human subjects are required to determine the contributory role of GSTO1 and GSTO2 and their polymorphisms to asthma pathology.
8.4. GSTP in asthma
The mRNA expression of GSTP has been examined in different animal models of asthma with contrasting results. One study reported increases in total GST activity in BAL fluid and Gstp1 mRNA expression in lung tissue using an ovalbumin mouse model of allergic airways disease [199]. In contrast, another study showed that GSTP1 transcript levels were down regulated and GST activity was decreased in lung tissues from wild-type mice challenged with house dust mite [208]. These latter results in mice are in line with the downregulation of GSTP1 in nasal epithelial cells from asthmatic children (Table 2) [208]. In contrast, RNA seq data show increased GSTP mRNA levels in nasal brushings of asthmatics (Fig. 3A) while another other study reported no difference in GSTP1 mRNA expression between induced sputum samples of asthmatics and healthy controls [199]. The discrepancy in data from the mouse studies might be attributable due to differences in the allergen challenge regimen and differences in strains of mice used [199]. Gstp knock-out mice also showed enhanced AHR, eosinophilia, airway remodeling, and goblet cell hyperplasia, although these results were also strain dependent, and only minor effects were observed in mice chronically stimulated with OVA [209]. Interestingly, GSTP1 has been recently reported to increase the proteolytic activity of the protease Der p1 in the house dust mite allergen, suggesting that GSTP may promote house dust mite-induced immune responses [210].
Multiple studies have examined the impact of GSTP1 polymorphisms on the risk of asthma development and lung function. For example the noncoding polymorphisms rs1871042 (C > T) and rs947895 (C > A) were associated with lower odds of asthma [104]. The haplotype corresponding to GSTP1 rs6591256, rs17593068, rs1695, rs1871042, rs947895 was associated with a nearly five-fold increase in the odds of asthma (OR = 4.8, p = 0.007) showing the complexity between GST variants and the relation with risk of disease. In particular, the GSTP1 Ile105Val polymorphism (rs1695, A > G) has been analyzed in a number of genetic association studies with conflicting outcomes [40,46,162,203,205,[211], [212], [213]] (Table 3). The GSTP1 Ile105Val polymorphism modifies the substrate affinity of the GSTP1 enzyme, and decreases GST activity towards CDNB [45]. Notable, individuals homozygous for the GSTP1 105Val genotype have an altered catalytic activity depending on substrates compared with individuals homozygous for the GSTP1 105Ile allele. Children (with asthma) homozygous for the GSTP1 Val105 allele show slower lung function growth [105]. Furthermore, the Val105 allele appears to play an important role in lung physiology in combination with environmental exposure. It was for instance associated with an increased susceptibility to breathing difficulties caused by ozone and an increased risk of sensitization to any allergen when exposed to elevated levels of traffic NO(x) [104,214] and asthma when exposed to PM2.5 or ozone [215]. A study in German children could however not establish an association between the Ile105Val, as well as the Ala114Val (A114V; rs1138272) polymorphisms and bronchial asthma or AHR (Table 3) [216]. Surprisingly, both the wild-type and mutated allele have been associated with early-life symptoms an increased risk of asthma or atopy [46,116,162,217,218]. These different outcomes might be accounted for by differences in ethnicities, age, and urbanization [213]. However, when meta-analyses was performed on 28 different studies, including these independent genetic associations no significant association was found with asthma susceptibility for the GSTP1 Ile105Val polymorphisms, although it is worth mentioning that high between-study heterogeneity was identified [219].
8.5. GSTT in asthma
Assessment of GSTT1 mRNA level revealed that its expression was not different in induced sputum samples between asthmatics and healthy controls in one study (Table 2) [199]. The RNA seq data revealed increases in GSTT1 mRNA in nasal brushings of asthmatics compared to controls (Fig. 3A). Just like GSTM1, atopic asthmatics displayed a higher prevalence of the GSTT1 null genotype [116], and the GSTT1 null genotypes have also been associated with (atopic) asthma and asthmatic symptoms (wheezing, cough, asthma attack) [40,164,204,220]. Meta-analyses however have shown contradicting results [84,203]. An ‘updated’ meta-analysis study including 26 case-control studies reported a significant association between polymorphisms in GSTT1 and the risk of asthma development in adults (OR = 2.312; 95%CI: 1.204–4.439) [43]. Based on subgroup analysis by ethnicity, a significant increased risk was found only in Asians (OR = 2.105; 95% CI: 1.101–4.025) and Russians (OR = 2.747; 95% CI: 1.071–7.046). In the same year, another meta-analysis study was published that included independent genetic studies using fixed, and random effects models, and also reported a significant association between asthma susceptibility and GSTT1 null phenotype (pooled OR = 1.33, 95% Cl = 1.10–1.60) [219] (Table 3).
9. Glutathione S-transferases in COPD
9.1. GSTA in COPD
Genetic variants in GSTA occur at a very low frequency and have not been examined in relation to COPD susceptibility. A microarray study on airway epithelium found an upregulation of GSTA2 mRNA in smokers compared to non-smokers [221]. These findings are in line with reports of enhanced GSTA2 mRNA expression in bronchial biopsy material of smokers compared to non-smokers [222]. Despite these consistent observations on GSTA2 mRNA, lung GSTA protein levels are not different between control smokers and non-smokers. On the other hand, increased levels were observed in whole lung lysates of mild/moderate COPD patients compared to patients with very severe disease and controls (Table 2). Importantly, the antibody used in this study showed two distinct bands on Western blot of whole lung lysates, which could represent GSTA1 and GSTA2. This was however not elucidated, nor were both bands quantified separately. In contrast, the number of GSTA positive central airway epithelial cells was decreased only in GOLD stage IV patients. Furthermore one GSTA immunoreactive band could be detected in induced sputum, and was shown to be increased in patients with chronic bronchitis and in patients with moderate to severe COPD compared to controls [147]. A proteomics study on BALF in contrast found both GSTA1 and GSTA2 decreased in COPD patients compared to controls. Because of the smaller scale of the study and focus on identifying biomarkers that could discriminate between COPD and lung cancer, in which GSTA1 and GSTA2 were increased as well, no relationship with disease severity were reported [223]. In aggregate, these data on GSTA(2) seem to fit the paradigm that this enzyme is elevated as part of the protective defense system against smoke components.
9.2. GSTM in COPD
Functional genetic variants of GSTM1 have been found to be protective in COPD [224]. Conversely, the GSTM1 null genotype has been found to be associated with COPD susceptibility in various studies, with some exceptions (Table 3). Reasons for discrepancies between studies may include the sample size, methodologies used and variants between ethnic backgrounds. For this reason, meta-analyses have been performed which will be discussed below.
One study examined GSTM1 gene expression in lung tissue of COPD patients compared to never-smoking and smoking controls as part of a candidate inflammatory and antioxidant gene study approach. They found significantly higher GSTM1 mRNA expression in non-COPD smokers compared to both never smoking controls, as well as COPD patients (Table 2). No significant correlations between GSTM1 mRNA expression and lung function were observed [225]. A microarray study on bronchial brushing also found increased GSTM1 mRNA levels in healthy smokers compared to non-smokers, but also found levels to further increase in COPD patients [226]. Another microarray study, focused specifically on antioxidant genes in airway epithelium, did not find such an upregulation of GSTM1 (or M3 or M4) mRNA in smokers compared to non-smokers [221]. A similar lack of induction of GSTM1 mRNA by smoking was reported in BAL cells and bronchial biopsies [222]. We are unaware of studies that have examined GSTM1 protein or activities in lung tissue of COPD patients.
Protein level of GSTM3 in the lung was more abundant in current smokers than ex-smokers [144]. Like GSTM1, GSTM3 mRNA was also increased in healthy smokers compared to non-smokers, and further increased in COPD patients in a microarray study on bronchial brushing [226]. Similarly, the microarray gene expression data available from the Lung Genomics Research Consortium (LGRC) also showed increases in GSTM1, GSTM3, GSTM4, and GSTM5 in lung tissues from COPD patients (Fig. 3B).
9.3. GSTO in COPD
Microarray data from lung tissue found a downregulation of GSTO2 mRNA in COPD patients compared to controls (Fig. 3B). The top-ranked association between lung function and GSTO2 (rs156697) that was observed in the Framingham Heart study prompted us to examine GSTO polymorphisms in COPD [96]. In a case-control study we did not demonstrate an association between lung function and GSTO1 Asp140Ala or GSTO2 Asn142Asp. However, an increased risk of COPD was found for the GSTO2 142Asp allele, as well as the GSTO1 140Asp/GSTO2 142Asp haplotype (OR = 1.39 95%CI 1.00–1.93, and OR = 2.40, 95%CI 1.43–4.02, respectively) [227].
Only protein expression of GSTO1 has been examined in COPD. In lung tissue homogenates of COPD patients, the GSTO1 protein expression was significantly lower compared to smoking and never smoking controls (Table 2) [147]. No significant relations to lung function parameters were found. Interestingly, GSTO1 could also be detected constitutively in cell culture supernatant of macrophage or bronchial epithelial cell lines and in induced sputum supernatant, suggesting that GSTO1 exerts functions extracellularly. The sputum of COPD patients also contained less GSTO1 compared to sputum of controls [147].
9.4. GSTP in COPD
Microarray data from the LGRC also showed a downregulation of GSTP mRNA in COPD patients, particularly in GOLD Stage II, and III (Fig. 3B and C). In line, mRNA levels of GSTP were reported to be downregulated in peripheral lung tissue of COPD patients compared to non-smoking controls and non-COPD smokers (Table 2) [225]. Furthermore, GSTP mRNA levels significantly correlated with parameters of pulmonary function and negatively correlated with cigarette smoking history. Contrasting the mRNA expression data in whole lung homogenates, the protein level of GSTP1 was increased in COPD patients with mild disease compared to both smoking and non-smoking controls, whereas the number of GSTP positive cells analyzed by IHC did not differ between these groups (Table 2) [147]. According to the mRNA data, a proteomics study on BALF reported lower levels of GSTP1 in patients with COPD compared to controls [223]. Overall, these results suggest that an impaired GSTP1-mediated xenobiotic-metabolizing activity may be correlated with pathophysiological changes in COPD. In line with this possibility, GSTP has been reported to exert protective properties against cigarette smoke-induced cell death in fibroblasts [228].
As previously described, several genetic variations have been detected in GSTP1. In particular the frequencies of the GSTP1 Ile105Val genotypes in COPD and risk of disease have been performed in a number of genetic association studies in different populations. However, these studies reported conflicting results, probably due to small sample sizes and different ethnic backgrounds [47,[229], [230], [231], [232], [233]]. Interestingly, the wild-type as well as the mutant allele have been associated with an increased risk for COPD, although some studies also failed to find any association at all [229,232,233]. Also, meta-analyses result in divergent outcomes with respect to the associations between this GSTP polymorphism and the risk for COPD. One meta-analysis reported that the lle105Val polymorphism in GSTP1 was not protective against COPD development in Asian populations [234,235]. Conversely, another meta-analysis study reported a significant correlation between the GSTP1 Val/Val and COPD [236]. The most recent and extensive analysis to date which included a total of 1892 cases and 2012 controls found no significant correlation between the GSTP1 lle/Val polymorphism and COPD risk in general, nor in any ethnic subgroups [47]. The relationship between GSTP1 polymorphisms and COPD development still remains unclear. Further studies involving larger populations and careful control with respect to age, sex, ethnicity and smoking behavior will be required to address the link between GSTP1 polymorphisms and COPD risk.
9.5. GSTT in COPD
The GSTT1 null genotype has been associated with COPD susceptibility in general [44], and emphysema in particular [237]. Similar to aforementioned conflicting data with other GSTs, not all studies could confirm the association between loss of GSTT1 and enhanced COPD risk [83,238]. No studies to date have examined GSTT1 beyond the null polymorphism. The allele has not been identified as a risk factor for COPD or lung function decline in GWAS analyses, nor has the mRNA been reported in COPD. The protein and activity levels in lung tissue remain furthermore unexplored.
9.6. Meta-analyses on genetic GSTT and GSTM polymorphisms and COPD
Because of the inconsistencies between genetic studies, and the inconclusive outcomes on the association between GST polymorphism and COPD, a number of meta- and pooled analyses have been performed. A recent meta-analysis of the association of the null genotypes of GSTM1 and GSTT1 with COPD based on 37 case-control studies, including 4674 COPD patients and 5006 controls, showed that both null genotypes were more frequent in COPD patients compared to controls. A significant relation was furthermore found between these individual polymorphisms, as well as the combined presence of both null polymorphisms and COPD risk, with divergent effects in different ethnic groups [83,115,234,[238], [239], [240]].
Combined GSTM1 and GSTT1 deficiency has been linked with accelerated age-related decline of lung function in males, irrespective of smoking status [241]. Additional gene-gene interactions have been reported between GSTM1 and MMP1, 9 and 12, modifying not only the risk of COPD, but also the age of onset, as well as the severity of the disease [242]. In combination with microsomal epoxide hydrolase genetic variants, the risk of COPD is further increased for GSTM1 null and GSTP1 Ile105 [243,244]. Furthermore, overall plasma GST activity decreased in COPD patients compared to control [245]. In the same study, the GST polymorphisms that associated with COPD were GSTM1 null and Val105Val GSTP1, although this association was only apparent when the GST genotypes were combined.
10. Differential expression of GSTs in epithelial cells in asthma and COPD
Cytosolic GSTs are differentially and heterogeneously expressed in the lung; in specific bronchial- and alveolar epithelial cells, as well as in various immune and mesenchymal cells (Table 2/Fig. 3, Fig. 4). We will focus here on their differential expression in epithelial cells as they display the most robust expression of GSTs and play important roles in lung pathologies. Upon activation, lung epithelial cells secrete pro-inflammatory mediators that recruit, activate, and/or promote differentiation and survival of immune cells, they release growth factors that control airway remodeling, and play an important role in lung repair through their (trans)differentiation capacities [246]. The differential gene expression of GSTs could be associated with their respective physiological roles of basal, ciliated, club, and goblet cells in maintaining lung homeostasis, as well as with the versatile roles in which epithelial cells contribute to disease pathogenesis. For example, GSTs have been linked to epithelial plasticity, which is a key pathogenic feature in asthma as well as COPD. Indeed, epithelial plasticity often contributes to airway remodeling including mucus metaplasia in asthma, and squamous metaplasia in COPD [246]. GSTA has been shown to promote epithelial-mesenchymal transition (EMT), the process whereby epithelial cells lose their cell polarity and cell-cell adhesion, and transform into mesenchymal cells, in lung cancer cells [247], and GSTO has been shown to inhibit membrane localization of E-cadherin [248]. The regulatory properties of GSTs and their possible contribution to airway epithelial (dys)function in asthma and COPD will be described in the next sections.
Although the differential expression of GSTs in epithelial subtypes has not been investigated in COPD, multiple studies have examined the expression of cytosolic GSTs in (small) airway epithelial cells derived from healthy smokers compared to non-smokers. In small airway epithelium from healthy and smoking subjects combined, GSTA1 was reported to be the most abundantly expressed isoenzyme of the GSTA class, followed by GSTA2, GSTA3, and GSTA5, data which are comparable to our GST transcript analysis of the publicly available RNA seq dataset in Fig. 3. Increased expression of GSTA2, GSTA1, as well as hypomethylation and upregulation of GSTM1 and GSTM5 were reported in small airway epithelium from smokers vs non-smokers [[249], [250], [251]]. In smokers moreover, decreases in mRNA of GSTO2 in airway basal cells were reported, in association with decreased GSH levels and a decreased GSH/GSSG ratio [252]. These data show that the small airway epithelium exhibit changes in GST gene expression in response to cigarette smoking, which could play a role in the development of smoking-associated lung disease [108]. Interestingly, our analysis of the previously published RNA seq database revealed, that the epithelial subtype expression of specific GSTs differs between healthy subjects and asthmatics (Fig. 4). However, because these data are obtained by using single cell RNA-seq data from individual epithelial cells (from 6 asthmatics and 6 controls), we were unable to perform justifiable statistical analyses on these data, due to the low expression of the GST genes in individual epithelial cells and the number of patients in the analyses. We therefore limit ourselves here to describing the most striking observations from the analysis. Further experiments should be performed to confirm these findings. As mentioned before, GSTA1, GSTO1, and GSTP1 are the main GSTs expressed in the different epithelial cell types. Interestingly, although the mRNA expression of GSTP1 is already high in all subtypes of airway epithelial cells, the expression seems to elevate further in most epithelial subtypes in asthmatics (Fig. 4B). In asthmatics, GSTA1 expression seems to be decreased in basal, suprabasal, goblet, and in club cells, while its expression may be slightly increased in mucus ciliated cells compared to controls, which is in line with the RNA seq data in nasal brushings (Fig. 3A), whereas GSTA2 expression appears to be decreased in ciliated, mucus ciliated and goblet cells in asthmatics compared to controls. The expression of GSTO1 is found to be higher in ionocytes of asthmatics compared to control. In smooth muscle, GSTA4, GSTM3, and GSTO1 are observed to be more highly expressed in controls than in asthmatics (Fig. 4C). Interestingly, in asthmatics, the expression of GSTA1, GSTA4, GSTM3, GSTM4, GSTM5, GSTO1 (and to a lesser extent GSTP1) may be decreased in fibroblasts compared to controls. As described before, GSTs are less ubiquitously expressed in immune cells (Fig. 4C). The expression of both GSTA1 and GSTO1 seems to be decreased in macrophages from asthmatics versus healthy controls. Furthermore, mast cells display elevated expression levels of GSTA1 and GSTP1, and lower expression of GSTO1 in asthmatics versus controls. In asthmatics, dendritic cell expression of GSTA1, and GSTO1 appears to be decreased in comparison to healthy controls. Lastly, T- and B-lymphocytes isolated from asthmatics seem to display reduced expression of GSTO1, versus healthy controls, while GSTP1 expression is decreased in T-lymphocytes. These preliminary, observational findings of the differences in expression profile of each GST isoform in epithelial subtypes between asthmatics and controls should be further explored to examine and expand the knowledge on the precise role of different GSTs in a particular cell type during homeostasis, as well as in the responses to for example allergens, and inhaled pollutants.
11. Biochemical reactions regulated by GSTs and their contributions to asthma and COPD
Antioxidant systems are crucial to detoxify the constant exposure to toxic compounds. It is tempting to speculate that GSTs could be upregulated as part of an adaptive and protective response to detoxify these compounds and to scavenge the excessive levels of oxidant production/oxidative stress. Conversely, a failure of the lungs to respond proportionally to these challenges would result in damage, and disease development and progression. This failure can have a genetic or epigenetic origin (as previously reviewed), or can occur at the transcriptional or translational level. With respect to failure at the transcriptional level, a disturbance in (detoxification) protection against smoke components is often seen in more severe disease in COPD, and is related to Nuclear factor erythroid 2–related factor (Nrf2) repression. Nrf2 is a transcription factor that regulates numerous antioxidant and cytoprotective genes, including GSTs, and protect against oxidative damage triggered by injury and inflammation. The role of Nrf2 in controlling the expression of GSTs has been shown in studies using the homozygous Nrf2−/− mouse model, where it was found that the constitutive as well as inducible (by the antioxidants butylated hydroxyanisole, and ethoxyquin) expression of Gsta1, Gsta2, Gstm1, Gstm2, Gstm3, and Gstm4 was impaired in the liver [253,254]. A recent comprehensive proteomic analysis moreover reported that GSTM1, GSTM2, GSTM3, GSTA3, GSTA4, and GSTP1 were all expressed at a constitutively lower level in the liver of Nrf2−/− mice [255]. However, it remains to be determined whether Nrf2 deficiency also alters GST expression in the lung. Previous studies have shown that a GSH deficiency and the exposure of cells to ROS or pro-oxidants enhances the induction of GSTs by activating Nrf2 [256]. For example, it has been shown that increased concentrations of the lipid peroxidation product 4-HNE, leads to modification of cysteine residues in the Nrf2 inhibitor, Kelch-like ECH-associated protein 1 (Keap1), thereby stabilizing Nrf2 and allowing its nuclear accumulation. Increased GSTA4 transcription in association with enhanced GSH levels thereby result in an increased capacity to metabolize 4-HNE. Conversely, GSTs can negatively regulate Nrf2 activity by protecting Keap1 from cysteine modifications that are required to stabilize and release the transcription factor. GSTs thus comprise a negative feedback system that indirectly controls the levels of other antioxidant and drug-metabolizing enzymes that are regulated through the Keap1/Nrf2 pathway. In the context of chronic lung diseases, NRF2 levels were decreased in lung tissue of emphysema patients [257]. In mice, Nrf2 absence enhanced the susceptibility to smoke-induced emphysema [258], while activation of Nrf2 attenuated emphysema development [259]. However, it is unknown whether GSTs play a role herein. Nrf2 deficient mice also show increased susceptibility to asthma, and cell-specific activation of Nrf2 in club cells significantly reduced allergen-induced oxidative stress, inflammation, mucus, and airway hyperresponsiveness [260], which is of particular interest given that Gsta1, Gsta2, and Gstp1 are inducible by Nrf2 and all highly expressed in club cells (Fig. 4). A disturbed detoxification may partially be caused by a lack of Nrf2-dependent upregulation of GSTs. This could be restored by dietary and natural compounds such as flavonoids, which have been shown to modulate the activity and/or expression of GSTs, and curcumin, which has been attributed antioxidant-like properties in part through activation of Nrf2 [261]. Nonetheless, these compounds do not specifically induce GSTs via Nrf2.
11.1. Contribution of GSTs through enzymatic activities and ligand binding
In addition to the classical detoxification function of GSTs, alterations in GST expression and/or enzymatic activity, regardless of the isoform, may affect their ability to scavenge oxidants, regulate biological processes (e.g. protein-protein interaction), and affect redox signaling through modulation of PSSG (Table 1, Table 2). GSTs display an array of regulatory mechanisms of pathways involved in asthma and COPD. Since GST isoforms have diverging functions, the relative contribution of a given isoform to chronic lung disease may differ. For instance, lipid peroxidation, a consequence of oxidative stress, induces pulmonary inflammation, and is therefore believed to contribute to asthma and COPD pathophysiology [262,263]. Isoenzymes of mainly the GSTA, but also GSTM, and GSTT classes use lipid peroxidation products generated by ROS. The upregulation of GSTA2 mRNA (and GSTA1 mRNA; Fig. 3A) in asthmatics as well as the upregulation of GSTT and GSTM isoenzymes in asthma and COPD (Fig. 3A and B) could be a response to cope with the elevated level of lipid peroxidation in these patients. GSTs also act as ligandins (non-enzymatic protein-protein interaction) regulating inflammation and (programmed) cell death. GSTM, GSTO, and GSTP regulate the activity of members of the MAPK pathway, especially JNK and TRAF2. The regulatory effect on JNK is of considerable interest as JNK has been shown to play a role in allergen-induced inflammation and remodeling associated with bronchial hyperresponsiveness [264]. Moreover, activation of JNK signaling was present in lungs of patients with COPD and was shown to be involved in TNFα-driven extracellular matrix remodeling [265]. The precise link between JNK and GSTP in the pathology of asthma and COPD remains to be examined, especially given that GSTP1 is differentially regulated in both diseases (decreased in COPD; increased in asthma) (Fig. 3, Fig. 4). The Omega class of GSTs has furthermore been shown to enzymatically modulate ryanodine receptors, which may be a novel target to modulate airway reactivity in cigarette smoke linked diseases such as COPD, as exposure to acute cigarette smoke selectively altered small airway contraction and down-regulated ryanodine receptors in airway smooth muscle [266]. Furthermore, the GSTO2 Asn142Asp genotype has been associated with age-related defects in smokers such as the development of cataracts due to inefficient ascorbate regeneration [267]. Based on its function in β-oxidation, changes in GSTZ levels in the lung may regulate fatty acid metabolism and related mitochondrial (dys)function, which have previously been linked to chronic lung diseases such as asthma and COPD [[268], [269], [270]].
11.2. Contribution of GSTs in PSSG
Changes in redox balance may contribute to the pathogenesis of chronic lung diseases including asthma and COPD. Notably, dysregulation of reversible oxidation of protein cysteines including PSSG is thought to promote chronic lung diseases as it may affect cellular pathways which play an important role in the lung including cell proliferation, inflammation, metabolism and apoptosis. The importance of PSSG in particular the lung epithelium is beginning to be elucidated. Studies from our laboratory have shown that overall PSSG levels are increased in lungs of mice with allergic airways disease exposed to the house dust mite allergen compared to control lungs [271]. On the other hand, PSSG levels were decreased in sputum samples from eosinophilic and neutrophilic asthmatics compared to healthy controls, although this should be confirmed in a larger study [272]. Moreover, others have reported that the exposure to diesel exhaust co-administered with house dust mite allergen promotes PSSG in mice with allergic airways disease [273]. Although PSSG levels have not yet been examined in COPD patients, exposure of lung epithelial cells to cigarette smoke extract increased PSSG [274], while PSSG was shown to be decreased in lungs of mice exposed to cigarette smoke extract [275]. In BALF, as well as in macrophages isolated from smoke-exposed mice, an increase in PSSG was observed [274]. PSSG seems thus to be distinctly regulated in different regions and cellular compartments of the lungs in response to cigarette smoke, which could be related to differences in GST expression and/or activity in these various compartments and cell types.
As previously mentioned, especially GSTP, and to a lesser extent GSTO and GSTM, have been shown to catalyze the forward PSSG reaction [17,76,88]. GSTO has also been shown to deglutathionylate proteins, and the GSTO1 Ala140Asp polymorphism was found to have increased activity for the forward PSSG reactions, while deglutathionylation was repressed [88]. The biological contribution of this polymorphism still has to be further confirmed, and so far, no association is found between this GSTO1 variant and asthma or COPD (Table 3). With respect to specific targets, GSTP and GSTM can directly bind (enzymatically) and glutathionylate adenosine monophosphate activated protein kinase (AMPK), leading to its activation in vitro [76]. AMPK is a key enzyme in the regulation of cellular energy homeostasis, and interestingly, also modulates inflammatory responses. AMPK has been shown to suppress airway smooth muscle cells thereby decreasing airway remodeling [276]. Furthermore, AMPK has been shown to decrease lung inflammation and emphysema by reducing IL-8 production in airway cells [277]. GSTP moreover promotes SRC-glutathionylation, which was essential for GSTP to inhibit SRC phosphorylation and activation [278]. Glutathionylation of SRC regulates VE-cadherin stabilization, a key transmembrane adhesive protein in endothelium adherens junctions, thereby maintaining endothelial barrier function. SRC is a non-receptor tyrosine kinase protein which has been shown to play an essential role in mucin secretion induced by pathogens, and it can promote airway smooth muscle cell growth and migration which occur in airway remodeling found in asthma and COPD [279,280]. Furthermore, SRC has been shown to regulate the allergic inflammatory response via epithelial growth factor receptor (EGFR) and subsequent downstream activation of multiple pathways including ERK1/2, PI3Kδ/AKT and NF-κB [281]. These results suggest that GSTP (and GSTM)-induced PSSG of AMPK and SRC may act on airway remodeling and inflammation. Additionally, glutathionylation of actin and tubulin inhibits their polymerization, thereby altering cell structure and affecting morphological polarity and migration of neutrophils in response to chemotactic gradients or cell growth [[282], [283], [284]]. Moreover, neutrophils from mice lacking Glrx1 displayed impaired recruitment to sites of inflammation and reduced bacterial capability [284]. It remains to be determined whether the GST-controlled forward reaction similarly regulates neutrophil migration.
Members of NF-κB, a family of transcription factors that promote the activation of pro-inflammatory responses [285], have been shown to be inhibited by glutathionylation including RelA, and p50 [[286], [287], [288]]. Notably, we have shown that, upon LPS-induced lung inflammation, the activity of inhibitory kappa B kinase beta (IKKβ), a key activator of the NF-κB signaling pathway, was inhibited by GSTP-mediated PSSG, resulting in decreased levels of pro-inflammatory mediators in epithelial cells, which was rescued by GLRX1 [286]. IL-1β is also a pro-inflammatory cytokine related to asthma pathology, and PSSG has been shown to protect inhibition of IL-1β by overoxidation thereby maintaining IL-1β activity [289]. This is of interest as it has recently been reported that human nasal mucociliary epithelial cells exposed to PM2.5 elicited a dose-dependent transcriptomic response with an upregulation of IL-1 (α and β) expression [290]. Moreover, our laboratory has recently demonstrated that increases in IL-1β-dependent glycolysis (e.g. metabolic reprogramming) resulted in an induced inflammatory response in epithelial cells during allergic airways disease [291]. We also showed that the induced pro-inflammatory signaling was in part driven by the glycolysis inactive form of Pyruvate Kinase M2 (PKM2) through, in part, phosphorylation of signal transducer and activator of transcription 3 (STAT3), an important protein in the regulation of cell proliferation, differentiation, apoptosis and inflammation [292]. This is of considerable interest as metabolic enzymes have been shown to be reversibly inactivated by PSSG, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolysis enzyme, and α-ketoglutarate dehydrogenase (KGDH), a TCA cycle protein, as well Pyruvate Kinase in the liver (PKL) [[293], [294], [295]]. Furthermore, STAT3 has been shown to be glutathionylated resulting in inhibition of its phosphorylation [296,297]. GSTP has already been shown to interact with STAT3, thereby inhibiting the STAT3-signaling pathway [298]. The observed metabolic reprogramming during allergic airways disease may therefore be attributable to changes in (GSTP)-controlled PSSG.
Lastly, it has been shown that GSTP catalyzes the first step in the reduction of the sulfenylated peroxidatic cysteine of PRDX6 (1-cysPRX), a member of the peroxiredoxin family, by binding the sulfenic acid form of Cys47 and glutathionylating the active site cysteine [299,300]. PRDX6 is involved in redox regulation of the cell as it can for example reduce H2O2, short chain fatty acids and hydroperoxides. Oxidation of the catalytic cysteine of 1-cysPRX has been associated with loss of peroxidase activity [299,301]. Heterodimerization of 1-cysPRX with GSTP mediates the glutathionylation of the previously oxidized cysteine thus restoring its peroxidase activity. PRDX6 is unique since PRDX6 uses GSH as an electron donor, whereas PRDX1-5 used thioredoxin as an electron donor. Interestingly, PRDX6 is the highest expressed PRDX of the PRDX family in the epithelium of human lungs [302], and similarly GSTP is the most abundantly expressed GST in lung tissue, especially in the epithelium [144].
Given that GSTP is the most abundantly expressed GST in the lung epithelium, displaying differential expression in asthmatics and COPD patients compared to controls, coupled to its important role in PSSG chemistry, a prominent role for GSTP in lung epithelial pathology is suggested. However, the implications of PSSG, the proteins that are targeted via glutathionylation and the GSTs that control them in epithelia of asthmatics and COPD patients remains a much unexplored area of research.
12. Future directions
In this review we highlighted the current knowledge on GSTs in the susceptibility to and progression of asthma and COPD, as well as their contribution to lung growth and development. Despite poor reproducibility, genetic studies support the prevailing concept that GST deficiencies (notably the GSTT and GSTM null polymorphisms) are associated with an increased risk for the development of asthma and COPD, which could be related to adverse perinatal effects, as well as diminished lung development and growth. Gene-environment interactions, as well as epigenetic modulation likely contribute to these risks. In contrast, mRNA and/or protein expression of most GSTs are upregulated in asthma and COPD patients, as well as in smokers, compared to controls (Fig. 3, Table 2). Although there are some exceptions, these expression data are in line with the notion that GSTs are upregulated during chronic lung diseases as part of an adaptive and protective response to the disease-triggering exposures. However, since GSTs have effects beyond detoxification, including scavenging of oxidants, ligand binding, and regulating redox signaling (Table 1), this upregulation might not inherently be of a protective nature. Similarly, downregulation of GSTs may have detrimental effects beyond attenuated detoxification. Generic knock-out studies at this point fail to unravel the contribution of each of these divergent functions of each (sub)class of GST to the outcome in disease models. Future studies should therefore extend their analyses to include these alternative functions. For example, the most prominently expressed GST in human lung tissue, GSTP1, accounts for over 90% of the lung's activity towards CDNB, but also regulates various pathways that play a role in lung pathologies through S-glutathionylation, as well as by non-enzymatic protein-protein interactions. GSTP is overexpressed in a wide variety of tumors. Since it was thought to be involved in the resistance to several anticancer drugs, and since its interaction with JNK results in inhibition of apoptosis, a number of GST(P) inhibitors have been synthesized. Ethacrynic acid, an inhibitor of all classes of GST isoenzymes, Terrapin 199, a GSH analog-based GSTP inhibitor, and TLK199, a small peptide GSH-analog, are examples of agents that have been examined to inhibit GSTP [54,303,304]. TLK199 prevents the binding of GSTP and JNK, and its activated form that has anti-GSTP activity, TLK117 [305], was shown to halt the progression of fibrosis in mice in association with decreased levels of PSSG [306]. On the other hand, in the context of e.g. lung diseases, inhibition of JNK by GSTP can be beneficial due to its contribution to matrix remodeling. TLK286 is an example of a prodrug that activates GSTP [307,308]. However, clinical trials have not yielded new drugs that are widely used clinically to date. We posit that given the wealth of new information about the contributions of GSTs to a variety of diseases, it is an exciting time to re-consider clinical development of compounds that specifically interfere with the various functions of GSTs including the disruption of binding to unique target proteins. Future studies could also include examining the effect of genetic polymorphisms of GSTs on their alternative functions in allergic asthma and COPD. Furthermore, studies could focus on gene-editing of GST isoforms in specific target cells to examine and expand the knowledge into the specific functions and contribution to disease.
13. Conclusion
In conclusion, the purpose and importance of the differential and heterogeneous expression of GSTs in the lung remains to be further explored. In order to better understand the contribution of GSTs to (normal) lung growth and development, as well as to the onset of lung diseases at later age, more detailed insights are needed with respect to their complex regulation, their cellular distribution and versatility of effector functions. Only through such thorough and precise understanding can tailored therapeutic strategies be designed to affect specific functions of particular GSTs that may help alleviate the burden, or even prevent the development, of chronic lung diseases.
Declaration of competing interest
Yvonne Janssen-Heininger and Niki Reynaert hold patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents” (YJ-H, NR), United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins” (YJ-H, NR), United States Patents 9,907,828 and 10, 688, 150 “Treatments of oxidative stress conditions” (YJ-H). Yvonne Janssen-Heininger has received consulting fees from Celdara Medical LLC for the contributions to the proposed commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Acknowledgments
This work was supported by grants NIH, R35HL135828 (Y-JH), NIH R01HL137268, Dutch Lung Foundation 5.1.17.166 (NLR and Y-JH) and 6.1.16.088 (NLR) and an unrestricted grant from Chiesi. We acknowledge Wren Wagers for her contribution regarding Fig. 4A.
Contributor Information
Yvonne M.W. Janssen-Heininger, Email: yvonne.janssen@uvm.edu.
Niki L. Reynaert, Email: n.reynaert@maastrichtuniversity.nl.
References
- 1.Church D.F., Pryor W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardi C., Valacchi G. Cigarette smoke and ozone effect on murine inflammatory responses. Ann. N. Y. Acad. Sci. 2012;1259:104–111. doi: 10.1111/j.1749-6632.2012.06605.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein J.A., Alexis N., Barnes C., Bernstein I.L., Bernstein J.A., Nel A. Health effects of air pollution. J. Allergy Clin. Immunol. 2004;114(5):1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Burbank A.J., Sood A.K., Kesic M.J., Peden D.B., Hernandez M.L. Environmental determinants of allergy and asthma in early life. J. Allergy Clin. Immunol. 2017;140(1):1–12. doi: 10.1016/j.jaci.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbank A.J., Vadlamudi A., Mills K.H., Alt E.M., Wells H., Zhou H. The glutathione-S-transferase mu-1 null genotype increases wood smoke-induced airway inflammation. J. Allergy Clin. Immunol. 2019;143(6):2299–2302. doi: 10.1016/j.jaci.2019.02.006. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol. Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 7.Ye Z.W., Zhang J., Townsend D.M., Tew K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta. 2015;1850(8):1607–1621. doi: 10.1016/j.bbagen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brigelius-Flohe R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830(5):3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2011;15(7):1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H.J., Liu J.Q., Bock A., Li J., Luo G.M., Shen J.C. Engineering glutathione transferase to a novel glutathione peroxidase mimic with high catalytic efficiency. Incorporation of selenocysteine into a glutathione-binding scaffold using an auxotrophic expression system. J. Biol. Chem. 2005;280(12):11930–11935. doi: 10.1074/jbc.M408574200. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn C.C. Biological production, detection, and fate of hydrogen peroxide. Antioxidants Redox Signal. 2018;29(6):541–551. doi: 10.1089/ars.2017.7425. [DOI] [PubMed] [Google Scholar]
- 12.Brigelius-Flohe R., Flohe L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxidants Redox Signal. 2020;33(7):498–516. doi: 10.1089/ars.2019.7905. [DOI] [PubMed] [Google Scholar]
- 13.Pannala V.R., Bazil J.N., Camara A.K.S., Dash R.K. A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radical Biol. Med. 2013;65:1385–1397. doi: 10.1016/j.freeradbiomed.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., Ahn Y.J., Asmis R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox biology. 2020;31:101410. doi: 10.1016/j.redox.2019.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu W., Doreswamy V., Diaz-Sanchez D., Samet J.M., Kesic M., Dailey L. GSTM1 modulation of IL-8 expression in human bronchial epithelial cells exposed to ozone. Free Radic. Biol. Med. 2011;51(2):522–529. doi: 10.1016/j.freeradbiomed.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes J.D., Strange R.C. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic. Res. 1995;22(3):193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 17.Townsend D.M., Manevich Y., He L., Hutchens S., Pazoles C.J., Tew K.D. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009;284(1):436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson C.J., Wolf C.R. Disruption of the glutathione transferase pi class genes. Methods Enzymol. 2005;401:116–135. doi: 10.1016/S0076-6879(05)01007-4. [DOI] [PubMed] [Google Scholar]
- 19.Wolf C.R., Mahmood A., Henderson C.J., McLeod R., Manson M.M., Neal G.E. Modulation of the cytochrome P450 system as a mechanism of chemoprotection. IARC Sci. Publ. 1996;(139):165–173. [PubMed] [Google Scholar]
- 20.Guengerich F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001;14(6):611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 21.Castell J.V., Donato M.T., Gomez-Lechon M.J. Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp. Toxicol. Pathol. 2005;57(Suppl 1):189–204. doi: 10.1016/j.etp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad A., Shameem M., Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann. Thorac. Med. 2012;7(4):226–232. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 24.Mates J.M., Perez-Gomez C., Nunez de Castro I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999;32(8):595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 25.Murphy M.P., Holmgren A., Larsson N.G., Halliwell B., Chang C.J., Kalyanaraman B. Unraveling the biological roles of reactive oxygen species. Cell Metabol. 2011;13(4):361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279(6):L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 27.Forman H.J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton G.J., Jauniaux E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011;25(3):287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma R., Yang Y., Sharma A., Awasthi S., Awasthi Y.C. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxidants Redox Signal. 2004;6(2):289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L., Diaz P.T., Best T.M., Stimpfl J.N., He F., Zuo L. Molecular characterization of redox mechanisms in allergic asthma. Ann. Allergy Asthma Immunol. 2014;113(2):137–142. doi: 10.1016/j.anai.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Bauer R.N., Diaz-Sanchez D., Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J. Allergy Clin. Immunol. 2012;129(1):14–24. doi: 10.1016/j.jaci.2011.11.004. quiz 5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazenq J., Dubus J.C., Gaudart J., Charpin D., Nougairede A., Viudes G. Air pollution and children's asthma-related emergency hospital visits in southeastern France. Eur. J. Pediatr. 2017;176(6):705–711. doi: 10.1007/s00431-017-2900-5. [DOI] [PubMed] [Google Scholar]
- 34.Byers N., Ritchey M., Vaidyanathan A., Brandt A.J., Yip F. Short-term effects of ambient air pollutants on asthma-related emergency department visits in Indianapolis, Indiana, 2007-2011. J. Asthma. 2016;53(3):245–252. doi: 10.3109/02770903.2015.1091006. [DOI] [PubMed] [Google Scholar]
- 35.Noh J., Sohn J., Cho J., Cho S.K., Choi Y.J., Kim C. Short-term effects of ambient air pollution on emergency department visits for asthma: an assessment of effect modification by prior allergic disease history. J Prev Med Public Health. 2016;49(5):329–341. doi: 10.3961/jpmph.16.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirivarasai J., Wananukul W., Kaojarern S., Chanprasertyothin S., Thongmung N., Ratanachaiwong W. Association between inflammatory marker, environmental lead exposure, and glutathione S-transferase gene. BioMed Res. Int. 2013;2013:474963. doi: 10.1155/2013/474963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Araujo R.M., de Melo C.F., Neto F.M., da Silva J.N., Soares L.F., de Arruda Cardoso Smith M. Association study of SNPs of genes IFNGR1 (rs137854905), GSTT1 (rs71748309), and GSTP1 (rs1695) in gastric cancer development in samples of patient in the northern and northeastern Brazil. Tumour Biol. 2014;35(5):4983–4986. doi: 10.1007/s13277-014-1656-z. [DOI] [PubMed] [Google Scholar]
- 38.Hollman A.L., Tchounwou P.B., Huang H.C. The association between gene-environment interactions and diseases involving the human GST superfamily with SNP variants. Int. J. Environ. Res. Publ. Health. 2016;13(4):379. doi: 10.3390/ijerph13040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X., Li Z., Zhang Z., Zhang W., Li W., Xiao Z. Meta-analysis of GSTM1 null genotype and lung cancer risk in Asians. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. : Int. Med. J. Exp. Clin. Res. 2014;20:1239–1245. doi: 10.12659/MSM.890490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brasch-Andersen C., Christiansen L., Tan Q., Haagerup A., Vestbo J., Kruse T.A. Possible gene dosage effect of glutathione-S-transferases on atopic asthma: using real-time PCR for quantification of GSTM1 and GSTT1 gene copy numbers. Hum. Mutat. 2004;24(3):208–214. doi: 10.1002/humu.20074. [DOI] [PubMed] [Google Scholar]
- 41.Pemble S., Schroeder K.R., Spencer S.R., Meyer D.J., Hallier E., Bolt H.M. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J. 1994;300(Pt 1):271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidegard J., Vorachek W.R., Pero R.W., Pearson W.R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc. Natl. Acad. Sci. U. S. A. 1988;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang S., Wei X., Gong C., Wei J., Chen Z., Chen X. Significant association between asthma risk and the GSTM1 and GSTT1 deletion polymorphisms: an updated meta-analysis of case-control studies. Respirology. 2013;18(5):774–783. doi: 10.1111/resp.12097. [DOI] [PubMed] [Google Scholar]
- 44.Mehrotra S., Sharma A., Kumar S., Kar P., Sardana S., Sharma J.K. Polymorphism of glutathione S-transferase M1 and T1 gene loci in COPD. Int. J. Immunogenet. 2010;37(4):263–267. doi: 10.1111/j.1744-313X.2010.00918.x. [DOI] [PubMed] [Google Scholar]
- 45.Watson M.A., Stewart R.K., Smith G.B., Massey T.E., Bell D.A. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19(2):275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 46.Kamada F., Mashimo Y., Inoue H., Shao C., Hirota T., Doi S. The GSTP1 gene is a susceptibility gene for childhood asthma and the GSTM1 gene is a modifier of the GSTP1 gene. Int. Arch. Allergy Immunol. 2007;144(4):275–286. doi: 10.1159/000106316. [DOI] [PubMed] [Google Scholar]
- 47.Yang L., Li X., Tong X., Fan H. Association between glutathione S-transferase P1 Ile (105) Val gene polymorphism and chronic obstructive pulmonary disease: a meta-analysis based on seventeen case-control studies. Meta Gene. 2015;6:59–64. doi: 10.1016/j.mgene.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habig W.H., Pabst M.J., Fleischner G., Gatmaitan Z., Arias I.M., Jakoby W.B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc. Natl. Acad. Sci. U. S. A. 1974;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes J.D., Ju Flanagan, Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 50.Wu B., Dong D. Human cytosolic glutathione transferases: structure, function, and drug discovery. Trends Pharmacol. Sci. 2012;33(12):656–668. doi: 10.1016/j.tips.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Oakley A. Glutathione transferases: a structural perspective. Drug Metab. Rev. 2011;43(2):138–151. doi: 10.3109/03602532.2011.558093. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong R.N. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 1997;10(1):2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 53.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. 2013;1830(5):3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Allocati N., Masulli M., Di Ilio C., Federici L. Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7(1):8. doi: 10.1038/s41389-017-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroder E., Ponting C.P. Evidence that peroxiredoxins are novel members of the thioredoxin fold superfamily. Protein Sci. 1998;7(11):2465–2468. doi: 10.1002/pro.5560071125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chia S.B., Elko E.A., Aboushousha R., Manuel A.M., van de Wetering C., Druso J.E. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am. J. Physiol. Cell Physiol. 2019;318(2):C304–C327. doi: 10.1152/ajpcell.00410.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bushweller J.H., Billeter M., Holmgren A., Wuthrich K. The nuclear magnetic resonance solution structure of the mixed disulfide between Escherichia coli glutaredoxin(C14S) and glutathione. J. Mol. Biol. 1994;235(5):1585–1597. doi: 10.1006/jmbi.1994.1108. [DOI] [PubMed] [Google Scholar]
- 58.Elko E.A., Cunniff B., Seward D.J., Chia S.B., Aboushousha R., van de Wetering C. Peroxiredoxins and beyond; redox systems regulating lung physiology and disease. Antioxidants Redox Signal. 2019;31(14):1070–1091. doi: 10.1089/ars.2019.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pljesa-Ercegovac M., Savic-Radojevic A., Matic M., Coric V., Djukic T., Radic T. Glutathione transferases: potential targets to overcome chemoresistance in solid tumors. Int. J. Mol. Sci. 2018;19(12) doi: 10.3390/ijms19123785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tew K.D., Manevich Y., Grek C., Xiong Y., Uys J., Townsend D.M. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic. Biol. Med. 2011;51(2):299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinning I., Kleywegt G.J., Cowan S.W., Reinemer P., Dirr H.W., Huber R. Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the Mu and Pi class enzymes. J. Mol. Biol. 1993;232(1):192–212. doi: 10.1006/jmbi.1993.1376. [DOI] [PubMed] [Google Scholar]
- 62.Polekhina G., Board P.G., Blackburn A.C., Parker M.W. Crystal structure of maleylacetoacetate isomerase/glutathione transferase zeta reveals the molecular basis for its remarkable catalytic promiscuity. Biochemistry. 2001;40(6):1567–1576. doi: 10.1021/bi002249z. [DOI] [PubMed] [Google Scholar]
- 63.Rossjohn J., McKinstry W.J., Oakley A.J., Verger D., Flanagan J., Chelvanayagam G. Human theta class glutathione transferase: the crystal structure reveals a sulfate-binding pocket within a buried active site. Structure. 1998;6(3):309–322. doi: 10.1016/s0969-2126(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 64.Board P.G., Coggan M., Chelvanayagam G., Easteal S., Jermiin L.S., Schulte G.K. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000;275(32):24798–24806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 65.Atkinson H.J., Babbitt P.C. Glutathione transferases are structural and functional outliers in the thioredoxin fold. Biochemistry. 2009;48(46):11108–11116. doi: 10.1021/bi901180v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong K.H., Nishida M., Inoue H., Takahashi K. Tyrosine-7 is an essential residue for the catalytic activity of human class PI glutathione S-transferase: chemical modification and site-directed mutagenesis studies. Biochem. Biophys. Res. Commun. 1992;182(3):1122–1129. doi: 10.1016/0006-291x(92)91848-k. [DOI] [PubMed] [Google Scholar]
- 67.Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010;17(9):1373–1380. doi: 10.1038/cdd.2010.80. [DOI] [PubMed] [Google Scholar]
- 68.Tars K., Olin B., Mannervik B. Structural basis for featuring of steroid isomerase activity in alpha class glutathione transferases. J. Mol. Biol. 2010;397(1):332–340. doi: 10.1016/j.jmb.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 69.Grek C.L., Zhang J., Manevich Y., Townsend D.M., Tew K.D. Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 2013;288(37):26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coles B.F., Kadlubar F.F. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. Methods Enzymol. 2005;401:9–42. doi: 10.1016/S0076-6879(05)01002-5. [DOI] [PubMed] [Google Scholar]
- 71.Johansson A.S., Mannervik B. Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J. Biol. Chem. 2001;276(35):33061–33065. doi: 10.1074/jbc.M104539200. [DOI] [PubMed] [Google Scholar]
- 72.Pearson W.R., Vorachek W.R., Xu S.J., Berger R., Hart I., Vannais D. Identification of class-mu glutathione transferase genes GSTM1-GSTM5 on human chromosome 1p13. Am. J. Hum. Genet. 1993;53(1):220–233. [PMC free article] [PubMed] [Google Scholar]
- 73.Ross V.L., Board P.G., Webb G.C. Chromosomal mapping of the human Mu class glutathione S-transferases to 1p13. Genomics. 1993;18(1):87–91. doi: 10.1006/geno.1993.1429. [DOI] [PubMed] [Google Scholar]
- 74.Ketterer B., Harris J.M., Talaska G., Meyer D.J., Pemble S.E., Taylor J.B. The human glutathione S-transferase supergene family, its polymorphism, and its effects on susceptibility to lung cancer. Environ. Health Perspect. 1992;98:87–94. doi: 10.1289/ehp.929887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho S.G., Lee Y.H., Park H.S., Ryoo K., Kang K.W., Park J. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem. 2001;276(16):12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- 76.Klaus A., Zorman S., Berthier A., Polge C., Ramirez S., Michelland S. Glutathione S-transferases interact with AMP-activated protein kinase: evidence for S-glutathionylation and activation in vitro. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Evans D.A., Seidegard J., Narayanan N. The GSTM1 genetic polymorphism in healthy Saudi Arabians and Filipinos, and Saudi Arabians with coronary atherosclerosis. Pharmacogenetics. 1996;6(4):365–367. doi: 10.1097/00008571-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Xu S., Wang Y., Roe B., Pearson W.R. Characterization of the human class Mu glutathione S-transferase gene cluster and the GSTM1 deletion. J. Biol. Chem. 1998;273(6):3517–3527. doi: 10.1074/jbc.273.6.3517. [DOI] [PubMed] [Google Scholar]
- 79.Parl F.F. Glutathione S-transferase genotypes and cancer risk. Canc. Lett. 2005;221(2):123–129. doi: 10.1016/j.canlet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Wu W., Peden D., Diaz-Sanchez D. Role of GSTM1 in resistance to lung inflammation. Free Radic. Biol. Med. 2012;53(4):721–729. doi: 10.1016/j.freeradbiomed.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garte S., Gaspari L., Alexandrie A.K., Ambrosone C., Autrup H., Autrup J.L. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol. Biomark. Prev. 2001;10(12):1239–1248. [PubMed] [Google Scholar]
- 82.Ye Z., Song H., Higgins J.P., Pharoah P., Danesh J. Five glutathione s-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of 130 studies. PLoS Med. 2006;3(4):e91. doi: 10.1371/journal.pmed.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding Z., Wang K., Li J., Tan Q., Tan W., Guo G. Association between glutathione S-transferase gene M1 and T1 polymorphisms and chronic obstructive pulmonary disease risk: a meta-analysis. Clin. Genet. 2019;95(1):53–62. doi: 10.1111/cge.13373. [DOI] [PubMed] [Google Scholar]
- 84.Saadat M., Ansari-Lari M. Genetic polymorphism of glutathione S-transferase T1, M1 and asthma, a meta-analysis of the literature. Pakistan J. Biol. Sci. 2007;10(23):4183–4189. doi: 10.3923/pjbs.2007.4183.4189. [DOI] [PubMed] [Google Scholar]
- 85.Whitbread A.K., Tetlow N., Eyre H.J., Sutherland G.R., Board P.G. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics. 2003;13(3):131–144. doi: 10.1097/00008571-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Mukherjee B., Salavaggione O.E., Pelleymounter L.L., Moon I., Eckloff B.W., Schaid D.J. Glutathione S-transferase omega 1 and omega 2 pharmacogenomics. Drug Metab. Dispos. 2006;34(7):1237–1246. doi: 10.1124/dmd.106.009613. [DOI] [PubMed] [Google Scholar]
- 87.Dulhunty A., Gage P., Curtis S., Chelvanayagam G., Board P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J. Biol. Chem. 2001;276(5):3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- 88.Menon D., Board P.G. A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J. Biol. Chem. 2013;288(36):25769–25779. doi: 10.1074/jbc.M113.487785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitbread A.K., Masoumi A., Tetlow N., Schmuck E., Coggan M., Board P.G. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005;401:78–99. doi: 10.1016/S0076-6879(05)01005-0. [DOI] [PubMed] [Google Scholar]
- 90.Schmuck E.M., Board P.G., Whitbread A.K., Tetlow N., Cavanaugh J.A., Blackburn A.C. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: implications for arsenic metabolism and the age-at-onset of Alzheimer's and Parkinson's diseases. Pharmacogenetics Genom. 2005;15(7):493–501. doi: 10.1097/01.fpc.0000165725.81559.e3. [DOI] [PubMed] [Google Scholar]
- 91.Kodym R., Calkins P., Story M. The cloning and characterization of a new stress response protein. A mammalian member of a family of theta class glutathione s-transferase-like proteins. J. Biol. Chem. 1999;274(8):5131–5137. doi: 10.1074/jbc.274.8.5131. [DOI] [PubMed] [Google Scholar]
- 92.Piaggi S., Raggi C., Corti A., Pitzalis E., Mascherpa M.C., Saviozzi M. Glutathione transferase omega 1-1 (GSTO1-1) plays an anti-apoptotic role in cell resistance to cisplatin toxicity. Carcinogenesis. 2010;31(5):804–811. doi: 10.1093/carcin/bgq031. [DOI] [PubMed] [Google Scholar]
- 93.Piaggi S., Marchi S., Ciancia E., Debortoli N., Lazzarotti A., Saviozzi M. Nuclear translocation of glutathione transferase omega is a progression marker in Barrett's esophagus. Oncol. Rep. 2009;21(2):283–287. [PubMed] [Google Scholar]
- 94.Laliberte R.E., Perregaux D.G., Hoth L.R., Rosner P.J., Jordan C.K., Peese K.M. Glutathione s-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1beta posttranslational processing. J. Biol. Chem. 2003;278(19):16567–16578. doi: 10.1074/jbc.M211596200. [DOI] [PubMed] [Google Scholar]
- 95.Tanaka-Kagawa T., Jinno H., Hasegawa T., Makino Y., Seko Y., Hanioka N. Functional characterization of two variant human GSTO 1-1s (Ala140Asp and Thr217Asn) Biochem. Biophys. Res. Commun. 2003;301(2):516–520. doi: 10.1016/s0006-291x(02)03066-8. [DOI] [PubMed] [Google Scholar]
- 96.Wilk J.B., Walter R.E., Laramie J.M., Gottlieb D.J., O'Connor G.T. Framingham Heart Study genome-wide association: results for pulmonary function measures. BMC Med. Genet. 2007;8(Suppl 1):S8. doi: 10.1186/1471-2350-8-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Board P.G., Webb G.C., Coggan M. Isolation of a cDNA clone and localization of the human glutathione S-transferase 3 genes to chromosome bands 11q13 and 12q13-14. Ann. Hum. Genet. 1989;53(3):205–213. doi: 10.1111/j.1469-1809.1989.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 98.Cowell I.G., Dixon K.H., Pemble S.E., Ketterer B., Taylor J.B. The structure of the human glutathione S-transferase pi gene. Biochem. J. 1988;255(1):79–83. doi: 10.1042/bj2550079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fryer A.A., Hume R., Strange R.C. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim. Biophys. Acta. 1986;883(3):448–453. doi: 10.1016/0304-4165(86)90283-7. [DOI] [PubMed] [Google Scholar]
- 100.Adler V., Yin Z., Fuchs S.Y., Benezra M., Rosario L., Tew K.D. Regulation of JNK signaling by GSTp. EMBO J. 1999;18(5):1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang T., Arifoglu P., Ronai Z., Tew K.D. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 2001;276(24):20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 102.Elsby R., Kitteringham N.R., Goldring C.E., Lovatt C.A., Chamberlain M., Henderson C.J. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J. Biol. Chem. 2003;278(25):22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- 103.Wu Y., Fan Y., Xue B., Luo L., Shen J., Zhang S. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene. 2006;25(42):5787–5800. doi: 10.1038/sj.onc.1209576. [DOI] [PubMed] [Google Scholar]
- 104.Joubert B.R., Reif D.M., Edwards S.W., Leiner K.A., Hudgens E.E., Egeghy P. Evaluation of genetic susceptibility to childhood allergy and asthma in an African American urban population. BMC Med. Genet. 2011;12:25. doi: 10.1186/1471-2350-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gilliland F.D., Gauderman W.J., Vora H., Rappaport E., Dubeau L. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am. J. Respir. Crit. Care Med. 2002;166(5):710–716. doi: 10.1164/rccm.2112065. [DOI] [PubMed] [Google Scholar]
- 106.Simic T., Savic-Radojevic A., Pljesa-Ercegovac M., Matic M., Mimic-Oka J. Glutathione S-transferases in kidney and urinary bladder tumors. Nat. Rev. Urol. 2009;6(5):281–289. doi: 10.1038/nrurol.2009.49. [DOI] [PubMed] [Google Scholar]
- 107.de Bruin W.C., Wagenmans M.J., Peters W.H. Expression of glutathione S-transferase alpha, P1-1 and T1-1 in the human gastrointestinal tract. Jpn. J. Canc. Res. 2000;91(3):310–316. doi: 10.1111/j.1349-7006.2000.tb00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Butler M.W., Hackett N.R., Salit J., Strulovici-Barel Y., Omberg L., Mezey J. Glutathione S-transferase copy number variation alters lung gene expression. Eur. Respir. J. 2011;38(1):15–28. doi: 10.1183/09031936.00029210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Webb G., Vaska V., Coggan M., Board P. Chromosomal localization of the gene for the human theta class glutathione transferase (GSTT1) Genomics. 1996;33(1):121–123. doi: 10.1006/geno.1996.0167. [DOI] [PubMed] [Google Scholar]
- 110.Tan K.L., Webb G.C., Baker R.T., Board P.G. Molecular cloning of a cDNA and chromosomal localization of a human theta-class glutathione S-transferase gene (GSTT2) to chromosome 22. Genomics. 1995;25(2):381–387. doi: 10.1016/0888-7543(95)80037-m. [DOI] [PubMed] [Google Scholar]
- 111.Josephy P.D., Kent M., Mannervik B. Single-nucleotide polymorphic variants of human glutathione transferase T1-1 differ in stability and functional properties. Arch. Biochem. Biophys. 2009;490(1):24–29. doi: 10.1016/j.abb.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 112.Meyer D.J., Coles B., Pemble S.E., Gilmore K.S., Fraser G.M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem. J. 1991;274(Pt 2):409–414. doi: 10.1042/bj2740409. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wiencke J.K., Pemble S., Ketterer B., Kelsey K.T. Gene deletion of glutathione S-transferase theta: correlation with induced genetic damage and potential role in endogenous mutagenesis. Cancer Epidemiol. Biomark. Prev. 1995;4(3):253–259. [PubMed] [Google Scholar]
- 114.Joseph T., Kusumakumary P., Chacko P., Abraham A., Radhakrishna Pillai M. Genetic polymorphism of CYP1A1, CYP2D6, GSTM1 and GSTT1 and susceptibility to acute lymphoblastic leukaemia in Indian children. Pediatr. Blood Canc. 2004;43(5):560–567. doi: 10.1002/pbc.20074. [DOI] [PubMed] [Google Scholar]
- 115.Saitou M., Ishida T. Distributions of the GSTM1 and GSTT1 null genotypes worldwide are characterized by latitudinal clines. Asian Pac. J. Cancer Prev. APJCP. 2015;16(1):355–361. doi: 10.7314/apjcp.2015.16.1.355. [DOI] [PubMed] [Google Scholar]
- 116.Tamer L., Calikoglu M., Ates N.A., Yildirim H., Ercan B., Saritas E. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology. 2004;9(4):493–498. doi: 10.1111/j.1440-1843.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 117.Blackburn A.C., Woollatt E., Sutherland G.R., Board P.G. Characterization and chromosome location of the gene GSTZ1 encoding the human Zeta class glutathione transferase and maleylacetoacetate isomerase. Cytogenet. Cell Genet. 1998;83(1–2):109–114. doi: 10.1159/000015145. [DOI] [PubMed] [Google Scholar]
- 118.Fernandez-Canon J.M., Baetscher M.W., Finegold M., Burlingame T., Gibson K.M., Grompe M. Maleylacetoacetate isomerase (MAAI/GSTZ)-deficient mice reveal a glutathione-dependent nonenzymatic bypass in tyrosine catabolism. Mol. Cell Biol. 2002;22(13):4943–4951. doi: 10.1128/MCB.22.13.4943-4951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tong Z., Board P.G., Anders M.W. Glutathione transferase zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem. J. 1998;331(Pt 2):371–374. doi: 10.1042/bj3310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anderson W.B., Board P.G., Anders M.W. Glutathione transferase zeta-catalyzed bioactivation of dichloroacetic acid: reaction of glyoxylate with amino acid nucleophiles. Chem. Res. Toxicol. 2004;17(5):650–662. doi: 10.1021/tx034099+. [DOI] [PubMed] [Google Scholar]
- 121.Janssen-Heininger Y.M., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janssen-Heininger Y.M., Nolin J.D., Hoffman S.M., van der Velden J.L., Tully J.E., Lahue K.G. Emerging mechanisms of glutathione-dependent chemistry in biology and disease. J. Cell. Biochem. 2013;114(9):1962–1968. doi: 10.1002/jcb.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghezzi P. Regulation of protein function by glutathionylation. Free Radic. Res. 2005;39(6):573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 124.Klomsiri C., Karplus P.A., Poole L.B. Cysteine-based redox switches in enzymes. Antioxidants Redox Signal. 2011;14(6):1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoffman S., Nolin J., McMillan D., Wouters E., Janssen-Heininger Y., Reynaert N. Thiol redox chemistry: role of protein cysteine oxidation and altered redox homeostasis in allergic inflammation and asthma. J. Cell. Biochem. 2015;116(6):884–892. doi: 10.1002/jcb.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen H., Tsuchida S., Tamai K., Sato K. Identification of cysteine residues involved in disulfide formation in the inactivation of glutathione transferase P-form by hydrogen peroxide. Arch. Biochem. Biophys. 1993;300(1):137–141. doi: 10.1006/abbi.1993.1019. [DOI] [PubMed] [Google Scholar]
- 128.Chrestensen C.A., Starke D.W., Mieyal J.J. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J. Biol. Chem. 2000;275(34):26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 129.Shelton M.D., Chock P.B., Mieyal J.J. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxidants Redox Signal. 2005;7(3–4):348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 130.Findlay V.J., Townsend D.M., Morris T.E., Fraser J.P., He L., Tew K.D. A novel role for human sulfiredoxin in the reversal of glutathionylation. Canc. Res. 2006;66(13):6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin A., Faes C., Debevec T., Rytz C., Millet G., Pialoux V. Preterm birth and oxidative stress: effects of acute physical exercise and hypoxia physiological responses. Redox Biol. 2018;17:315–322. doi: 10.1016/j.redox.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moore T.A., Ahmad I.M., Zimmerman M.C. Oxidative stress and preterm birth: an integrative review. Biol. Res. Nurs. 2018;20(5):497–512. doi: 10.1177/1099800418791028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bonikos D.S., Bensch K.G., Northway W.H., Jr. Oxygen toxicity in the newborn. The effect of chronic continuous 100 percent oxygen exposure on the lungs of newborn mice. Am. J. Pathol. 1976;85(3):623–650. [PMC free article] [PubMed] [Google Scholar]
- 134.Richardson L.S., Vargas G., Brown T., Ochoa L., Sheller-Miller S., Saade G.R. Discovery and characterization of human amniochorionic membrane microfractures. Am. J. Pathol. 2017;187(12):2821–2830. doi: 10.1016/j.ajpath.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fanucchi M.V., Buckpitt A.R., Murphy M.E., Storms D.H., Hammock B.D., Plopper C.G. Development of phase II xenobiotic metabolizing enzymes in differentiating murine clara cells. Toxicol. Appl. Pharmacol. 2000;168(3):253–267. doi: 10.1006/taap.2000.9020. [DOI] [PubMed] [Google Scholar]
- 136.Raijmakers M.T., Steegers E.A., Peters W.H. Glutathione S-transferases and thiol concentrations in embryonic and early fetal tissues. Hum. Reprod. 2001;16(11):2445–2450. doi: 10.1093/humrep/16.11.2445. [DOI] [PubMed] [Google Scholar]
- 137.Cossar D., Bell J., Strange R., Jones M., Sandison A., Hume R. The alpha and pi isoenzymes of glutathione S-transferase in human fetal lung: in utero ontogeny compared with differentiation in lung organ culture. Biochim. Biophys. Acta. 1990;1037(2):221–226. doi: 10.1016/0167-4838(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 138.Beckett G.J., Howie A.F., Hume R., Matharoo B., Hiley C., Jones P. Human glutathione S-transferases: radioimmunoassay studies on the expression of alpha-, mu- and pi-class isoenzymes in developing lung and kidney. Biochim. Biophys. Acta. 1990;1036(3):176–182. doi: 10.1016/0304-4165(90)90031-q. [DOI] [PubMed] [Google Scholar]
- 139.Pandey G., Pandey O.P., Rogers A.J., Ahsen M.E., Hoffman G.E., Raby B.A. A nasal brush-based classifier of asthma identified by machine learning analysis of nasal RNA sequence data. Sci. Rep. 2018;8(1):8826. doi: 10.1038/s41598-018-27189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vieira Braga F.A., Kar G., Berg M., Carpaij O.A., Polanski K., Simon L.M. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019;25(7):1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 141.Lambrecht B.N., Hammad H. The airway epithelium in asthma. Nat. Med. 2012;18(5):684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 142.Montoro D.T., Haber A.L., Biton M., Vinarsky V., Lin B., Birket S.E. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560(7718):319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Plasschaert L.W., Zilionis R., Choo-Wing R., Savova V., Knehr J., Roma G. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560(7718):377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anttila S., Hirvonen A., Vainio H., Husgafvel-Pursiainen K., Hayes J.D., Ketterer B. Immunohistochemical localization of glutathione S-transferases in human lung. Canc. Res. 1993;53(23):5643–5648. [PubMed] [Google Scholar]
- 145.Cantlay A.M., Smith C.A., Wallace W.A., Yap P.L., Lamb D., Harrison D.J. Heterogeneous expression and polymorphic genotype of glutathione S-transferases in human lung. Thorax. 1994;49(10):1010–1014. doi: 10.1136/thx.49.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dittrich A.M., Meyer H.A., Krokowski M., Quarcoo D., Ahrens B., Kube S.M. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur. Respir. J. 2010;35(5):1148–1154. doi: 10.1183/09031936.00026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harju T., Mazur W., Merikallio H., Soini Y., Kinnula V.L. Glutathione-S-transferases in lung and sputum specimens, effects of smoking and COPD severity. Respir. Res. 2008;9:80. doi: 10.1186/1465-9921-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Howie A.F., Bell D., Hayes P.C., Hayes J.D., Beckett G.J. Glutathione S-transferase isoenzymes in human bronchoalveolar lavage: a possible early marker for the detection of lung cancer. Carcinogenesis. 1990;11(2):295–300. doi: 10.1093/carcin/11.2.295. [DOI] [PubMed] [Google Scholar]
- 149.Schultz E.S., Hallberg J., Bellander T., Bergstrom A., Bottai M., Chiesa F. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am. J. Respir. Crit. Care Med. 2016;193(2):171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 150.Ierodiakonou D., Zanobetti A., Coull B.A., Melly S., Postma D.S., Boezen H.M. Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J. Allergy Clin. Immunol. 2016;137(2):390–399. doi: 10.1016/j.jaci.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Postma D.S., Bush A., van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899–909. doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- 152.Um-Bergstrom P., Hallberg J., Pourbazargan M., Berggren-Brostrom E., Ferrara G., Eriksson M.J. Pulmonary outcomes in adults with a history of Bronchopulmonary Dysplasia differ from patients with asthma. Respir. Res. 2019;20(1):102. doi: 10.1186/s12931-019-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gilliland F.D., Li Y.F., Dubeau L., Berhane K., Avol E., McConnell R. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am. J. Respir. Crit. Care Med. 2002;166(4):457–463. doi: 10.1164/rccm.2112064. [DOI] [PubMed] [Google Scholar]
- 154.Ali Z., Schmidt P., Dodd J., Jeppesen D.L. Bronchopulmonary dysplasia: a review. Arch. Gynecol. Obstet. 2013;288(2):325–333. doi: 10.1007/s00404-013-2753-8. [DOI] [PubMed] [Google Scholar]
- 155.Wang X., Li W., Liu W., Cai B., Cheng T., Gao C. GSTM1 and GSTT1 gene polymorphisms as major risk factors for bronchopulmonary dysplasia in a Chinese Han population. Gene. 2014;533(1):48–51. doi: 10.1016/j.gene.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 156.Manar M.H., Brown M.R., Gauthier T.W., Brown L.A. Association of glutathione-S-transferase-P1 (GST-P1) polymorphisms with bronchopulmonary dysplasia. J. Perinatol. 2004;24(1):30–35. doi: 10.1038/sj.jp.7211020. [DOI] [PubMed] [Google Scholar]
- 157.Zachaki S., Daraki A., Polycarpou E., Stavropoulou C., Manola K.N., Gavrili S. GSTP1 and CYP2B6 genetic polymorphisms and the risk of bronchopulmonary dysplasia in preterm neonates. Am. J. Perinatol. 2017;34(8):729–734. doi: 10.1055/s-0036-1597994. [DOI] [PubMed] [Google Scholar]
- 158.Karagianni P., Rallis D., Fidani L., Porpodi M., Kalinderi K., Tsakalidis C. Glutathion-S-Transferase P1 polymorphisms association with broncopulmonary dysplasia in preterm infants. Hippokratia. 2013;17(4):363–367. [PMC free article] [PubMed] [Google Scholar]
- 159.Sampath V., Garland J.S., Helbling D., Dimmock D., Mulrooney N.P., Simpson P.M. Antioxidant response genes sequence variants and BPD susceptibility in VLBW infants. Pediatr. Res. 2015;77(3):477–483. doi: 10.1038/pr.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murdzoska J., Devadason S.G., Khoo S.K., Landau L.I., Young S., Goldblatt J. In utero smoke exposure and role of maternal and infant glutathione s-transferase genes on airway responsiveness and lung function in infancy. Am. J. Respir. Crit. Care Med. 2010;181(1):64–71. doi: 10.1164/rccm.200812-1887OC. [DOI] [PubMed] [Google Scholar]
- 161.Lee A.G., Le Grand B., Hsu H.L., Chiu Y.M., Brennan K.J., Bose S. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: sex-specific effects. Respir. Res. 2018;19(1):76. doi: 10.1186/s12931-018-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu J., Hankinson J., Kopec-Harding K., Custovic A., Simpson A. Interaction between glutathione S-transferase variants, maternal smoking and childhood wheezing changes with age. Pediatr. Allergy Immunol. 2013;24(5):501–508. doi: 10.1111/pai.12086. [DOI] [PubMed] [Google Scholar]
- 163.Chen X., Abdulhamid I., Woodcroft K. Maternal smoking during pregnancy, polymorphic CYP1A1 and GSTM1, and lung-function measures in urban family children. Environ. Res. 2011;111(8):1215–1221. doi: 10.1016/j.envres.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 164.Kabesch M., Hoefler C., Carr D., Leupold W., Weiland S.K., von Mutius E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax. 2004;59(7):569–573. doi: 10.1136/thx.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu C.C., Ou C.Y., Chang J.C., Hsu T.Y., Kuo H.C., Liu C.A. Gender-dependent effect of GSTM1 genotype on childhood asthma associated with prenatal tobacco smoke exposure. BioMed Res. Int. 2014;2014:769452. doi: 10.1155/2014/769452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rogers A.J., Brasch-Andersen C., Ionita-Laza I., Murphy A., Sharma S., Klanderman B.J. The interaction of glutathione S-transferase M1-null variants with tobacco smoke exposure and the development of childhood asthma. Clin. Exp. Allergy. 2009;39(11):1721–1729. doi: 10.1111/j.1365-2222.2009.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alexander M., Karmaus W., Holloway J.W., Zhang H., Roberts G., Kurukulaaratchy R.J. Effect of GSTM2-5 polymorphisms in relation to tobacco smoke exposures on lung function growth: a birth cohort study. BMC Pulm. Med. 2013;13:56. doi: 10.1186/1471-2466-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Breton C.V., Vora H., Salam M.T., Islam T., Wenten M., Gauderman W.J. Variation in the GST mu locus and tobacco smoke exposure as determinants of childhood lung function. Am. J. Respir. Crit. Care Med. 2009;179(7):601–607. doi: 10.1164/rccm.200809-1384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Korytina G.F., Yanbaeva D.G., Babenkova L.I., Etkina E.I., Victorova T.V. Genetic polymorphisms in the cytochromes P-450 (1A1, 2E1), microsomal epoxide hydrolase and glutathione S-transferase M1, T1, and P1 genes, and their relationship with chronic bronchitis and relapsing pneumonia in children. J. Mol. Med. (Berl.) 2005;83(9):700–710. doi: 10.1007/s00109-005-0660-6. [DOI] [PubMed] [Google Scholar]
- 170.Gehring U., Wijga A.H., Hoek G., Bellander T., Berdel D., Bruske I. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3(12):933–942. doi: 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- 171.Palmer C.N., Doney A.S., Lee S.P., Murrie I., Ismail T., Macgregor D.F. Glutathione S-transferase M1 and P1 genotype, passive smoking, and peak expiratory flow in asthma. Pediatrics. 2006;118(2):710–716. doi: 10.1542/peds.2005-3030. [DOI] [PubMed] [Google Scholar]
- 172.de Jong K., Boezen H.M., Hacken N.H., Postma D.S., Vonk J.M. GST-omega genes interact with environmental tobacco smoke on adult level of lung function. Respir. Res. 2013;14:83. doi: 10.1186/1465-9921-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alexis N.E., Zhou H., Lay J.C., Harris B., Hernandez M.L., Lu T.S. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. J. Allergy Clin. Immunol. 2009;124(6):1222–1228. doi: 10.1016/j.jaci.2009.07.036. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dillon M.A., Harris B., Hernandez M.L., Zou B., Reed W., Bromberg P.A. Enhancement of systemic and sputum granulocyte response to inhaled endotoxin in people with the GSTM1 null genotype. Occup. Environ. Med. 2011;68(10):783–785. doi: 10.1136/oem.2010.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen B.Y., Chen C.H., Chuang Y.C., Kim H., Honda Y., Chiang H.C. Schoolchildren's antioxidation genotypes are susceptible factors for reduced lung function and airway inflammation caused by air pollution. Environ. Res. 2016;149:145–150. doi: 10.1016/j.envres.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 176.Wu W., Peden D.B., McConnell R., Fruin S., Diaz-Sanchez D. Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells. Part. Fibre Toxicol. 2012;9:31. doi: 10.1186/1743-8977-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jiang X.Q., Mei X.D., Feng D. Air pollution and chronic airway diseases: what should people know and do? J. Thorac. Dis. 2016;8(1):E31–E40. doi: 10.3978/j.issn.2072-1439.2015.11.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carey I.M., Atkinson R.W., Kent A.J., van Staa T., Cook D.G., Anderson H.R. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am. J. Respir. Crit. Care Med. 2013;187(11):1226–1233. doi: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peden D.B. Effect of pollution on allergy/immunology. J. Allergy Clin. Immunol. 2018;141(3):878–879. doi: 10.1016/j.jaci.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 180.Burney P., Amaral A.F.S. Air pollution and chronic airway disease: is the evidence always clear? Lancet. 2019;394:2198–2200. doi: 10.1016/S0140-6736(19)32537-1. 10215. [DOI] [PubMed] [Google Scholar]
- 181.Moorman J.E., Akinbami L.J., Bailey C.M., Zahran H.S., King M.E., Johnson C.A. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3(35):1–58. [PubMed] [Google Scholar]
- 182.Lotvall J., Akdis C.A., Bacharier L.B., Bjermer L., Casale T.B., Custovic A. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 183.Peters U., Dixon A.E., Forno E. Obesity and asthma. J. Allergy Clin. Immunol. 2018;141(4):1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dixon A.E., Poynter M.E. Mechanisms of asthma in obesity. Pleiotropic aspects of obesity produce distinct asthma phenotypes. Am. J. Respir. Cell Mol. Biol. 2016;54(5):601–608. doi: 10.1165/rcmb.2016-0017PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sahiner U.M., Birben E., Erzurum S., Sackesen C., Kalayci O. Oxidative stress in asthma. World Allergy Organ J. 2011;4(10):151–158. doi: 10.1097/WOX.0b013e318232389e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fahy J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lambrecht B.N., Hammad H., Fahy J.V. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 188.Centers for Disease C Prevention Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001--2009. MMWR Morb. Mortal. Wkly. Rep. 2011;60(17):547–552. [PubMed] [Google Scholar]
- 189.Hirose K., Iwata A., Tamachi T., Nakajima H. Allergic airway inflammation: key players beyond the Th2 cell pathway. Immunol. Rev. 2017;278(1):145–161. doi: 10.1111/imr.12540. [DOI] [PubMed] [Google Scholar]
- 190.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.WHO . 2008. COPD Predicted to Be Third Leading Cause of Death in 2030.http://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/ Available online. [Google Scholar]
- 192.Singh D., Agusti A., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 193.Agusti A., Hogg J.C. Update on the pathogenesis of chronic obstructive pulmonary disease. N. Engl. J. Med. 2019;381(13):1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 194.Kc R., Shukla S.D., Gautam S.S., Hansbro P.M., O'Toole R.F. The role of environmental exposure to non-cigarette smoke in lung disease. Clin. Transl. Med. 2018;7(1):39. doi: 10.1186/s40169-018-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meiners S., Eickelberg O., Konigshoff M. Hallmarks of the ageing lung. Eur. Respir. J. 2015;45(3):807–827. doi: 10.1183/09031936.00186914. [DOI] [PubMed] [Google Scholar]
- 196.Barnes P.J. Pulmonary diseases and ageing. Subcell. Biochem. 2019;91:45–74. doi: 10.1007/978-981-13-3681-2_3. [DOI] [PubMed] [Google Scholar]
- 197.McDonough J.E., Yuan R., Suzuki M., Seyednejad N., Elliott W.M., Sanchez P.G. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boorsma C.E., Dekkers B.G., van Dijk E.M., Kumawat K., Richardson J., Burgess J.K. Beyond TGFbeta--novel ways to target airway and parenchymal fibrosis. Pulm. Pharmacol. Therapeut. 2014;29(2):166–180. doi: 10.1016/j.pupt.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 199.Sohn S.W., Jung J.W., Lee S.Y., Kang H.R., Park H.W., Min K.U. Expression pattern of GSTP1 and GSTA1 in the pathogenesis of asthma. Exp. Lung Res. 2013;39(4–5):173–181. doi: 10.3109/01902148.2013.789572. [DOI] [PubMed] [Google Scholar]
- 200.Morel F., Rauch C., Coles B., Le Ferrec E., Guillouzo A. The human glutathione transferase alpha locus: genomic organization of the gene cluster and functional characterization of the genetic polymorphism in the hGSTA1 promoter. Pharmacogenetics. 2002;12(4):277–286. doi: 10.1097/00008571-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 201.Polimanti R., Piacentini S., Moscatelli B., Pellicciotti L., Manfellotto D., Fuciarelli M. GSTA1, GSTO1 and GSTO2 gene polymorphisms in Italian asthma patients. Clin. Exp. Pharmacol. Physiol. 2010;37(8):870–872. doi: 10.1111/j.1440-1681.2010.05385.x. [DOI] [PubMed] [Google Scholar]
- 202.Piacentini S., Polimanti R., Iorio A., Cortesi M., Papa F., Rongioletti M. GSTA1*-69C/T and GSTO2*N142D as asthma- and allergy-related risk factors in Italian adult patients. Clin. Exp. Pharmacol. Physiol. 2014;41(3):180–184. doi: 10.1111/1440-1681.12201. [DOI] [PubMed] [Google Scholar]
- 203.Minelli C., Granell R., Newson R., Rose-Zerilli M.J., Torrent M., Ring S.M. Glutathione-S-transferase genes and asthma phenotypes: a Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Int. J. Epidemiol. 2010;39(2):539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hanene C., Jihene L., Jamel A., Kamel H., Agnes H. Association of GST genes polymorphisms with asthma in Tunisian children. Mediat. Inflamm. 2007;2007:19564. doi: 10.1155/2007/19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Islam T., Berhane K., McConnell R., Gauderman W.J., Avol E., Peters J.M. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009;64(3):197–202. doi: 10.1136/thx.2008.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Holla L.I., Stejskalova A., Vasku A. Polymorphisms of the GSTM1 and GSTT1 genes in patients with allergic diseases in the Czech population. Allergy. 2006;61(2):265–267. doi: 10.1111/j.1398-9995.2006.01000.x. [DOI] [PubMed] [Google Scholar]
- 207.Sokulsky L.A., Goggins B., Sherwin S., Eyers F., Kaiko G.E., Board P.G. GSTO1-1 is an upstream suppressor of M2 macrophage skewing and HIF-1alpha-induced eosinophilic airway inflammation. Clin. Exp. Allergy. 2020;50(5):609–624. doi: 10.1111/cea.13582. [DOI] [PubMed] [Google Scholar]
- 208.Schroer K.T., Gibson A.M., Sivaprasad U., Bass S.A., Ericksen M.B., Wills-Karp M. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J. Allergy Clin. Immunol. 2011;128(3):539–548. doi: 10.1016/j.jaci.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou J., Wolf C.R., Henderson C.J., Cai Y., Board P.G., Foster P.S. Glutathione transferase P1: an endogenous inhibitor of allergic responses in a mouse model of asthma. Am. J. Respir. Crit. Care Med. 2008;178(12):1202–1210. doi: 10.1164/rccm.200801-178OC. [DOI] [PubMed] [Google Scholar]
- 210.Lopez-Rodriguez J.C., Manosalva J., Cabrera-Garcia J.D., Escribese M.M., Villalba M., Barber D. Human glutathione-S-transferase pi potentiates the cysteine-protease activity of the Der p 1 allergen from house dust mite through a cysteine redox mechanism. Redox Biol. 2019;26:101256. doi: 10.1016/j.redox.2019.101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mak J.C., Ho S.P., Leung H.C., Cheung A.H., Law B.K., So L.K. Relationship between glutathione S-transferase gene polymorphisms and enzyme activity in Hong Kong Chinese asthmatics. Clin. Exp. Allergy. 2007;37(8):1150–1157. doi: 10.1111/j.1365-2222.2007.02704.x. [DOI] [PubMed] [Google Scholar]
- 212.Reddy P., Naidoo R.N., Robins T.G., Mentz G., London S.J., Li H. GSTM1, GSTP1, and NQO1 polymorphisms and susceptibility to atopy and airway hyperresponsiveness among South African schoolchildren. Lung. 2010;188(5):409–414. doi: 10.1007/s00408-010-9246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee Y.L., Hsiue T.R., Lee Y.C., Lin Y.C., Guo Y.L. The association between glutathione S-transferase P1, M1 polymorphisms and asthma in Taiwanese schoolchildren. Chest. 2005;128(3):1156–1162. doi: 10.1378/chest.128.3.1156. [DOI] [PubMed] [Google Scholar]
- 214.Melen E., Nyberg F., Lindgren C.M., Berglind N., Zucchelli M., Nordling E. Interactions between glutathione S-transferase P1, tumor necrosis factor, and traffic-related air pollution for development of childhood allergic disease. Environ. Health Perspect. 2008;116(8):1077–1084. doi: 10.1289/ehp.11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hwang B.F., Young L.H., Tsai C.H., Tung K.Y., Wang P.C., Su M.W. Fine particle, ozone exposure, and asthma/wheezing: effect modification by glutathione S-transferase P1 polymorphisms. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0052715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nickel R., Haider A., Sengler C., Lau S., Niggemann B., Deichmann K.A. Association study of Glutathione S-transferase P1 (GSTP1) with asthma and bronchial hyper-responsiveness in two German pediatric populations. Pediatr. Allergy Immunol. 2005;16(6):539–541. doi: 10.1111/j.1399-3038.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 217.Fryer A.A., Bianco A., Hepple M., Jones P.W., Strange R.C., Spiteri M.A. Polymorphism at the glutathione S-transferase GSTP1 locus. A new marker for bronchial hyperresponsiveness and asthma. Am. J. Respir. Crit. Care Med. 2000;161(5):1437–1442. doi: 10.1164/ajrccm.161.5.9903006. [DOI] [PubMed] [Google Scholar]
- 218.Hoskins A., Wu P., Reiss S., Dworski R. Glutathione S-transferase P1 Ile105Val polymorphism modulates allergen-induced airway inflammation in human atopic asthmatics in vivo. Clin. Exp. Allergy. 2013;43(5):527–534. doi: 10.1111/cea.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Piacentini S., Polimanti R., Simonelli I., Donno S., Pasqualetti P., Manfellotto D. Glutathione S-transferase polymorphisms, asthma susceptibility and confounding variables: a meta-analysis. Mol. Biol. Rep. 2013;40(4):3299–3313. doi: 10.1007/s11033-012-2405-2. [DOI] [PubMed] [Google Scholar]
- 220.Turner S., Francis B., Wani N., Vijverberg S., Pino-Yanes M., Mukhopadhyay S. Variants in genes coding for glutathione S-transferases and asthma outcomes in children. Pharmacogenomics. 2018;19(8):707–713. doi: 10.2217/pgs-2018-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hackett N.R., Heguy A., Harvey B.G., O'Connor T.P., Luettich K., Flieder D.B. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am. J. Respir. Cell Mol. Biol. 2003;29(3 Pt 1):331–343. doi: 10.1165/rcmb.2002-0321OC. [DOI] [PubMed] [Google Scholar]
- 222.Thum T., Erpenbeck V.J., Moeller J., Hohlfeld J.M., Krug N., Borlak J. Expression of xenobiotic metabolizing enzymes in different lung compartments of smokers and nonsmokers. Environ. Health Perspect. 2006;114(11):1655–1661. doi: 10.1289/ehp.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pastor M.D., Nogal A., Molina-Pinelo S., Melendez R., Salinas A., Gonzalez De la Pena M. Identification of proteomic signatures associated with lung cancer and COPD. J Proteomics. 2013;89:227–237. doi: 10.1016/j.jprot.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 224.Harrison D.J., Cantlay A.M., Rae F., Lamb D., Smith C.A. Frequency of glutathione S-transferase M1 deletion in smokers with emphysema and lung cancer. Hum. Exp. Toxicol. 1997;16(7):356–360. doi: 10.1177/096032719701600703. [DOI] [PubMed] [Google Scholar]
- 225.Tomaki M., Sugiura H., Koarai A., Komaki Y., Akita T., Matsumoto T. Decreased expression of antioxidant enzymes and increased expression of chemokines in COPD lung. Pulm. Pharmacol. Therapeut. 2007;20(5):596–605. doi: 10.1016/j.pupt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 226.Pierrou S., Broberg P., O'Donnell R.A., Pawlowski K., Virtala R., Lindqvist E. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175(6):577–586. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- 227.Yanbaeva D.G., Wouters E.F., Dentener M.A., Spruit M.A., Reynaert N.L. Association of glutathione-S-transferase omega haplotypes with susceptibility to chronic obstructive pulmonary disease. Free Radic. Res. 2009;43(8):738–743. doi: 10.1080/10715760903038440. [DOI] [PubMed] [Google Scholar]
- 228.Ishii T., Matsuse T., Igarashi H., Masuda M., Teramoto S., Ouchi Y. Tobacco smoke reduces viability in human lung fibroblasts: protective effect of glutathione S-transferase P1. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280(6):L1189–L1195. doi: 10.1152/ajplung.2001.280.6.L1189. [DOI] [PubMed] [Google Scholar]
- 229.Lakhdar R., Denden S., Knani J., Leban N., Daimi H., Hassine M. Relationship between glutathione S-transferase P1 polymorphisms and chronic obstructive pulmonary disease in a Tunisian population. Genet. Mol. Res. 2010;9(2):897–907. doi: 10.4238/vol9-2gmr770. [DOI] [PubMed] [Google Scholar]
- 230.Rodriguez F., de la Roza C., Jardi R., Schaper M., Vidal R., Miravitlles M. Glutathione S-transferase P1 and lung function in patients with alpha1-antitrypsin deficiency and COPD. Chest. 2005;127(5):1537–1543. doi: 10.1378/chest.127.5.1537. [DOI] [PubMed] [Google Scholar]
- 231.Yim J.J., Yoo C.G., Lee C.T., Kim Y.W., Han S.K., Shim Y.S. Lack of association between glutathione S-transferase P1 polymorphism and COPD in Koreans. Lung. 2002;180(2):119–125. doi: 10.1007/s004080000086. [DOI] [PubMed] [Google Scholar]
- 232.Ishii T., Matsuse T., Teramoto S., Matsui H., Miyao M., Hosoi T. Glutathione S-transferase P1 (GSTP1) polymorphism in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(8):693–696. doi: 10.1136/thx.54.8.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Calikoglu M., Tamer L., Ates Aras N., Karakas S., Ercan B. The association between polymorphic genotypes of glutathione S-transferases and COPD in the Turkish population. Biochem. Genet. 2006;44(7–8):307–319. doi: 10.1007/s10528-006-9031-4. [DOI] [PubMed] [Google Scholar]
- 234.Smolonska J., Wijmenga C., Postma D.S., Boezen H.M. Meta-analyses on suspected chronic obstructive pulmonary disease genes: a summary of 20 years' research. Am. J. Respir. Crit. Care Med. 2009;180(7):618–631. doi: 10.1164/rccm.200905-0722OC. [DOI] [PubMed] [Google Scholar]
- 235.Smolonska J., Wijmenga C., Postma D.S., Boezen H.M. Erratum: meta-analyses on suspected chronic obstructive pulmonary disease genes: a summary of 20 years' research. Am. J. Respir. Crit. Care Med. 2010;181(7):765. doi: 10.1164/ajrccm.181.7.765. [DOI] [PubMed] [Google Scholar]
- 236.Yan F., Chen C., Jing J., Li W., Shen H., Wang X. Association between polymorphism of glutathione S-transferase P1 and chronic obstructive pulmonary disease: a meta-analysis. Respir. Med. 2010;104(4):473–480. doi: 10.1016/j.rmed.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 237.Kukkonen M.K., Hamalainen S., Kaleva S., Vehmas T., Huuskonen M.S., Oksa P. Genetic polymorphisms of xenobiotic-metabolizing enzymes influence the risk of pulmonary emphysema. Pharmacogenetics Genom. 2011;21(12):876–883. doi: 10.1097/FPC.0b013e32834d597f. [DOI] [PubMed] [Google Scholar]
- 238.Xue H., Su J., Sun K., Xie W., Wang H. Glutathione S-transferase M1 and T1 gene polymorphism and COPD risk in smokers: an updated analysis. Mol. Biol. Rep. 2012;39(4):5033–5042. doi: 10.1007/s11033-011-1300-6. [DOI] [PubMed] [Google Scholar]
- 239.Karaca S., Karaca M., Cesuroglu T., Erge S., Polimanti R. GSTM1, GSTP1, and GSTT1 genetic variability in Turkish and worldwide populations. Am. J. Hum. Biol. 2015;27(3):310–316. doi: 10.1002/ajhb.22671. [DOI] [PubMed] [Google Scholar]
- 240.Hu G., Yao W., Zhou Y., Hu J., Shi Z., Li B. Meta- and pooled analyses of the effect of glutathione S-transferase M1 and T1 deficiency on chronic obstructive pulmonary disease. Int. J. Tubercul. Lung Dis. 2008;12(12):1474–1481. [PubMed] [Google Scholar]
- 241.Imboden M., Downs S.H., Senn O., Matyas G., Brandli O., Russi E.W. Glutathione S-transferase genotypes modify lung function decline in the general population: SAPALDIA cohort study. Respir. Res. 2007;8:2. doi: 10.1186/1465-9921-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stankovic M., Nikolic A., Nagorni-Obradovic L., Petrovic-Stanojevic N., Radojkovic D. Gene-gene interactions between glutathione S-transferase M1 and matrix metalloproteinases 1, 9, and 12 in chronic obstructive pulmonary disease in serbians. COPD. 2017;14(6):581–589. doi: 10.1080/15412555.2017.1369022. [DOI] [PubMed] [Google Scholar]
- 243.Cheng S.L., Yu C.J., Chen C.J., Yang P.C. Genetic polymorphism of epoxide hydrolase and glutathione S-transferase in COPD. Eur. Respir. J. 2004;23(6):818–824. doi: 10.1183/09031936.04.00104904. [DOI] [PubMed] [Google Scholar]
- 244.Zidzik J., Slaba E., Joppa P., Kluchova Z., Dorkova Z., Skyba P. Glutathione S-transferase and microsomal epoxide hydrolase gene polymorphisms and risk of chronic obstructive pulmonary disease in Slovak population. Croat. Med. J. 2008;49(2):182–191. doi: 10.3325/cmj.2008.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lakhdar R., Denden S., Mouhamed M.H., Chalgoum A., Leban N., Knani J. Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic polymorphisms with antioxidative stress markers in chronic obstructive pulmonary disease. Exp. Lung Res. 2011;37(4):195–204. doi: 10.3109/01902148.2010.535093. [DOI] [PubMed] [Google Scholar]
- 246.Hackett T.L. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr. Opin. Allergy Clin. Immunol. 2012;12(1):53–59. doi: 10.1097/ACI.0b013e32834ec6eb. [DOI] [PubMed] [Google Scholar]
- 247.Wang W., Liu F., Wang C., Wang C., Tang Y., Jiang Z. Glutathione S-transferase A1 mediates nicotine-induced lung cancer cell metastasis by promoting epithelial-mesenchymal transition. Exp Ther Med. 2017;14(2):1783–1788. doi: 10.3892/etm.2017.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Terayama M., Yamada K., Hagiwara T., Inazuka F., Sezaki T., Igari T. Glutathione S-transferase omega 2 regulates cell growth and the expression of E-cadherin via post-transcriptional downregulation of beta-catenin in human esophageal squamous cells. Carcinogenesis. 2019;41(7):875–886. doi: 10.1093/carcin/bgz189. [DOI] [PubMed] [Google Scholar]
- 249.Hackett N.R., Butler M.W., Shaykhiev R., Salit J., Omberg L., Rodriguez-Flores J.L. RNA-Seq quantification of the human small airway epithelium transcriptome. BMC Genom. 2012;13:82. doi: 10.1186/1471-2164-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Walters M.S., De B.P., Salit J., Buro-Auriemma L.J., Wilson T., Rogalski A.M. Smoking accelerates aging of the small airway epithelium. Respir. Res. 2014;15:94. doi: 10.1186/s12931-014-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Buro-Auriemma L.J., Salit J., Hackett N.R., Walters M.S., Strulovici-Barel Y., Staudt M.R. Cigarette smoking induces small airway epithelial epigenetic changes with corresponding modulation of gene expression. Hum. Mol. Genet. 2013;22(23):4726–4738. doi: 10.1093/hmg/ddt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Deeb R.S., Walters M.S., Strulovici-Barel Y., Chen Q., Gross S.S., Crystal R.G. Smoking-associated disordering of the airway basal stem/progenitor cell metabotype. Am. J. Respir. Cell Mol. Biol. 2016;54(2):231–240. doi: 10.1165/rcmb.2015-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hayes J.D., Chanas S.A., Henderson C.J., McMahon M., Sun C., Moffat G.J. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000;28(2):33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 254.Chanas S.A., Jiang Q., McMahon M., McWalter G.K., McLellan L.I., Elcombe C.R. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem. J. 2002;365(Pt 2):405–416. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Walsh J., Jenkins R.E., Wong M., Olayanju A., Powell H., Copple I. Identification and quantification of the basal and inducible Nrf2-dependent proteomes in mouse liver: biochemical, pharmacological and toxicological implications. J Proteomics. 2014;108:171–187. doi: 10.1016/j.jprot.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 257.Goven D., Boutten A., Lecon-Malas V., Marchal-Somme J., Amara N., Crestani B. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63(10):916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 258.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114(9):1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sussan T.E., Rangasamy T., Blake D.J., Malhotra D., El-Haddad H., Bedja D. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106(1):250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sussan T.E., Gajghate S., Chatterjee S., Mandke P., McCormick S., Sudini K. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309(1):L27–L36. doi: 10.1152/ajplung.00398.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Aggarwal B.B., Deb L., Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2014;20(1):185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wood L.G., Gibson P.G., Garg M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003;21(1):177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 263.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 264.Nath P., Eynott P., Leung S.Y., Adcock I.M., Bennett B.L., Chung K.F. Potential role of c-Jun NH2-terminal kinase in allergic airway inflammation and remodelling: effects of SP600125. Eur. J. Pharmacol. 2005;506(3):273–283. doi: 10.1016/j.ejphar.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 265.Eurlings I.M., Reynaert N.L., van de Wetering C., Aesif S.W., Mercken E.M., de Cabo R. Involvement of c-jun N-terminal kinase in TNF-alpha-driven remodeling. Am. J. Respir. Cell Mol. Biol. 2017;56(3):393–401. doi: 10.1165/rcmb.2015-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Donovan C., Seow H.J., Royce S.G., Bourke J.E., Vlahos R. Alteration of airway reactivity and reduction of ryanodine receptor expression by cigarette smoke in mice. Am. J. Respir. Cell Mol. Biol. 2015;53(4):471–478. doi: 10.1165/rcmb.2014-0400OC. [DOI] [PubMed] [Google Scholar]
- 267.Stamenkovic M., Radic T., Stefanovic I., Coric V., Sencanic I., Pljesa-Ercegovac M. Glutathione S-transferase omega-2 polymorphism Asn142Asp modifies the risk of age-related cataract in smokers and subjects exposed to ultraviolet irradiation. Clin. Exp. Ophthalmol. 2014;42(3):277–283. doi: 10.1111/ceo.12180. [DOI] [PubMed] [Google Scholar]
- 268.Reddy P.H. Mitochondrial dysfunction and oxidative stress in asthma: implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals. 2011;4(3):429–456. doi: 10.3390/ph4030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Aravamudan B., Thompson M.A., Pabelick C.M., Prakash Y.S. Mitochondria in lung diseases. Expet Rev. Respir. Med. 2013;7(6):631–646. doi: 10.1586/17476348.2013.834252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jiang Z., Knudsen N.H., Wang G., Qiu W., Naing Z.Z.C., Bai Y. Genetic control of fatty acid beta-oxidation in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2017;56(6):738–748. doi: 10.1165/rcmb.2016-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hoffman S.M., Qian X., Nolin J.D., Chapman D.G., Chia S.B., Lahue K.G. Ablation of glutaredoxin-1 modulates house dust mite-induced allergic airways disease in mice. Am. J. Respir. Cell Mol. Biol. 2016;55(3):377–386. doi: 10.1165/rcmb.2015-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kuipers I., Louis R., Manise M., Dentener M.A., Irvin C.G., Janssen-Heininger Y.M. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur. Respir. J. 2013;41(2):469–472. doi: 10.1183/09031936.00115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lee G.B., Brandt E.B., Xiao C., Gibson A.M., Le Cras T.D., Brown L.A. Diesel exhaust particles induce cysteine oxidation and s-glutathionylation in house dust mite induced murine asthma. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kuipers I., Bracke K.R., Brusselle G.G., Aesif S.W., Krijgsman R., Arts I.C. Altered cigarette smoke-induced lung inflammation due to ablation of Grx1. PloS One. 2012;7(6) doi: 10.1371/journal.pone.0038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kuipers I., Bracke K.R., Brusselle G.G., Wouters E.F., Reynaert N.L. Smoke decreases reversible oxidations S-glutathionylation and S-nitrosylation in mice. Free Radic. Res. 2012;46(2):164–173. doi: 10.3109/10715762.2011.647011. [DOI] [PubMed] [Google Scholar]
- 276.Pan Y., Liu L., Li S., Wang K., Ke R., Shi W. Activation of AMPK inhibits TGF-beta1-induced airway smooth muscle cells proliferation and its potential mechanisms. Sci. Rep. 2018;8(1):3624. doi: 10.1038/s41598-018-21812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lee J.S., Park S.J., Cho Y.S., Huh J.W., Oh Y.M., Lee S.D. Role of AMP-activated protein kinase (AMPK) in smoking-induced lung inflammation and emphysema. Tuberc. Respir. Dis. 2015;78(1):8–17. doi: 10.4046/trd.2015.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yang Y., Dong X., Zheng S., Sun J., Ye J., Chen J. GSTpi regulates VE-cadherin stabilization through promoting S-glutathionylation of Src. Redox Biol. 2020;30:101416. doi: 10.1016/j.redox.2019.101416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Inoue D., Yamaya M., Kubo H., Sasaki T., Hosoda M., Numasaki M. Mechanisms of mucin production by rhinovirus infection in cultured human airway epithelial cells. Respir. Physiol. Neurobiol. 2006;154(3):484–499. doi: 10.1016/j.resp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 280.Krymskaya V.P., Goncharova E.A., Ammit A.J., Lim P.N., Goncharov D.A., Eszterhas A. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. Faseb. J. 2005;19(3):428–430. doi: 10.1096/fj.04-2869fje. [DOI] [PubMed] [Google Scholar]
- 281.El-Hashim A.Z., Khajah M.A., Renno W.M., Babyson R.S., Uddin M., Benter I.F. Src-dependent EGFR transactivation regulates lung inflammation via downstream signaling involving ERK1/2, PI3Kdelta/Akt and NFkappaB induction in a murine asthma model. Sci. Rep. 2017;7(1):9919. doi: 10.1038/s41598-017-09349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang J., Boja E.S., Tan W., Tekle E., Fales H.M., English S. Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 2001;276(51):47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 283.Dalle-Donne I., Giustarini D., Rossi R., Colombo R., Milzani A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic. Biol. Med. 2003;34(1):23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- 284.Sakai J., Li J., Subramanian K.K., Mondal S., Bajrami B., Hattori H. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37(6):1037–1049. doi: 10.1016/j.immuni.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Karin M., Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 286.Reynaert N.L., van der Vliet A., Guala A.S., McGovern T., Hristova M., Pantano C. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. U. S. A. 2006;103(35):13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jones J.T., Qian X., van der Velden J.L., Chia S.B., McMillan D.H., Flemer S. Glutathione S-transferase pi modulates NF-kappaB activation and pro-inflammatory responses in lung epithelial cells. Redox Biol. 2016;8:375–382. doi: 10.1016/j.redox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Qanungo S., Starke D.W., Pai H.V., Mieyal J.J., Nieminen A.L. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J. Biol. Chem. 2007;282(25):18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 289.Zhang X., Liu P., Zhang C., Chiewchengchol D., Zhao F., Yu H. Positive regulation of interleukin-1beta bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 2017;20(1):224–235. doi: 10.1016/j.celrep.2017.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Montgomery M.T., Sajuthi S.P., Cho S.H., Everman J.L., Rios C.L., Goldfarbmuren K.C. Genome-wide analysis reveals mucociliary remodeling of the nasal airway epithelium induced by urban PM2.5. Am. J. Respir. Cell Mol. Biol. 2020;63(2):172–184. doi: 10.1165/rcmb.2019-0454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Qian X., Aboushousha R., van de Wetering C., Chia S.B., Amiel E., Schneider R.W. IL-1/inhibitory kappaB kinase epsilon-induced glycolysis augment epithelial effector function and promote allergic airways disease. J. Allergy Clin. Immunol. 2018;142(2):435–450 e10. doi: 10.1016/j.jaci.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.van de Wetering C., Aboushousha R., Manuel A.M., Chia S.B., Erickson C., MacPherson M.B. Pyruvate kinase M2 promotes expression of proinflammatory mediators in house dust mite-induced allergic airways disease. J. Immunol. 2020;204(4):763–774. doi: 10.4049/jimmunol.1901086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mohr S., Hallak H., de Boitte A., Lapetina E.G., Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1999;274(14):9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 294.Nulton-Persson A.C., Starke D.W., Mieyal J.J., Szweda L.I. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42(14):4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 295.Axelsson K., Mannervik B. An essential role of cytosolic thioltransferase in protection of pyruvate kinase from rabbit liver against oxidative inactivation. FEBS Lett. 1983;152(1):114–118. doi: 10.1016/0014-5793(83)80494-3. [DOI] [PubMed] [Google Scholar]
- 296.Butturini E., Darra E., Chiavegato G., Cellini B., Cozzolino F., Monti M. S-Glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation. ACS Chem. Biol. 2014;9(8):1885–1893. doi: 10.1021/cb500407d. [DOI] [PubMed] [Google Scholar]
- 297.Maryam A., Mehmood T., Zhang H., Li Y., Khan M., Ma T. Alantolactone induces apoptosis, promotes STAT3 glutathionylation and enhances chemosensitivity of A549 lung adenocarcinoma cells to doxorubicin via oxidative stress. Sci. Rep. 2017;7(1):6242. doi: 10.1038/s41598-017-06535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen D., Liu J., Rui B., Gao M., Zhao N., Sun S. GSTpi protects against angiotensin II-induced proliferation and migration of vascular smooth muscle cells by preventing signal transducer and activator of transcription 3 activation. Biochim. Biophys. Acta. 2014;1843(2):454–463. doi: 10.1016/j.bbamcr.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 299.Zhou S., Sorokina E.M., Harper S., Li H., Ralat L., Dodia C. Peroxiredoxin 6 homodimerization and heterodimerization with glutathione S-transferase pi are required for its peroxidase but not phospholipase A2 activity. Free Radic. Biol. Med. 2016;94:145–156. doi: 10.1016/j.freeradbiomed.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Woo H.A., Jeong W., Chang T.S., Park K.J., Park S.J., Yang J.S. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J. Biol. Chem. 2005;280(5):3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 301.Ralat L.A., Manevich Y., Fisher A.B., Colman R.F. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45(2):360–372. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- 302.Korfei M., von der Beck D., Henneke I., Markart P., Ruppert C., Mahavadi P. Comparative proteome analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF), non-specific interstitial pneumonia (NSIP) and organ donors. J Proteomics. 2013;85:109–128. doi: 10.1016/j.jprot.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 303.Townsend D.M., Tew K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22(47):7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang J., Ye Z.W., Janssen-Heininger Y., Townsend D.M., Tew K.D. Development of telintra as an inhibitor of glutathione S-transferase P. Handb. Exp. Pharmacol. 2020;264:71–91. doi: 10.1007/164_2020_392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Morgan A.S., Ciaccio P.J., Tew K.D., Kauvar L.M. Isozyme-specific glutathione S-transferase inhibitors potentiate drug sensitivity in cultured human tumor cell lines. Canc. Chemother. Pharmacol. 1996;37(4):363–370. doi: 10.1007/s002800050398. [DOI] [PubMed] [Google Scholar]
- 306.McMillan D.H., van der Velden J.L., Lahue K.G., Qian X., Schneider R.W., Iberg M.S. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase pi. JCI Insight. 2016;1(8) doi: 10.1172/jci.insight.85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tew K.D., Dutta S., Schultz M. Inhibitors of glutathione S-transferases as therapeutic agents. Adv. Drug Deliv. Rev. 1997;26(2–3):91–104. doi: 10.1016/s0169-409x(97)00029-x. [DOI] [PubMed] [Google Scholar]
- 308.Rosario L.A., O'Brien M.L., Henderson C.J., Wolf C.R., Tew K.D. Cellular response to a glutathione S-transferase P1-1 activated prodrug. Mol. Pharmacol. 2000;58(1):167–174. doi: 10.1124/mol.58.1.167. [DOI] [PubMed] [Google Scholar]
- 309.Young MD, Behjati, S. . SoupX removes ambient RNA contamination from droplet based single cell RNA sequencing data. Preprint at 10.1101/303727.%202020.. [DOI] [PMC free article] [PubMed]
- 310.Ju Flanagan, Rossjohn J., Parker M.W., Board P.G., Chelvanayagam G. Mutagenic analysis of conserved arginine residues in and around the novel sulfate binding pocket of the human Theta class glutathione transferase T2-2. Protein Sci. 1999;8(10):2205–2212. doi: 10.1110/ps.8.10.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Board P.G., Anders M.W. Human glutathione transferase zeta. Methods Enzymol. 2005;401:61–77. doi: 10.1016/S0076-6879(05)01004-9. [DOI] [PubMed] [Google Scholar]