Abstract
Oral administration of fluorescein isothiocyanate dextran (FITC-d) has been used as an indicator for intestinal permeability in poultry research for several years. Under healthy conditions, tight junctions in the intestinal wall will not allow the 4-6kDa FITC-d to enter the bloodstream. Detection of FITC-d in serum (1-hour post-oral administration of FITC-d) has proven to be a reliable indicator of leaky gut syndrome (increased intestinal inflammation and disruption of tight junctions). Administration of supplementary phytobiotics in feed, particularly products with high beta-carotene levels or other pigments, has resulted in strong serum background fluorescence, which can render this assay unreliable. To account for this increase in background autofluorescence, the FITC-d assay procedure has been modified to accommodate these particular serum samples by including pre-administration serum collection from each treatment group to remove background fluorescence. The modified FITC-d procedure detailed will allow for analysis of intestinal permeability in pigmented serum.
Key words: leaky gut, FITC-d, pigment, serum, poultry
INTRODUCTION
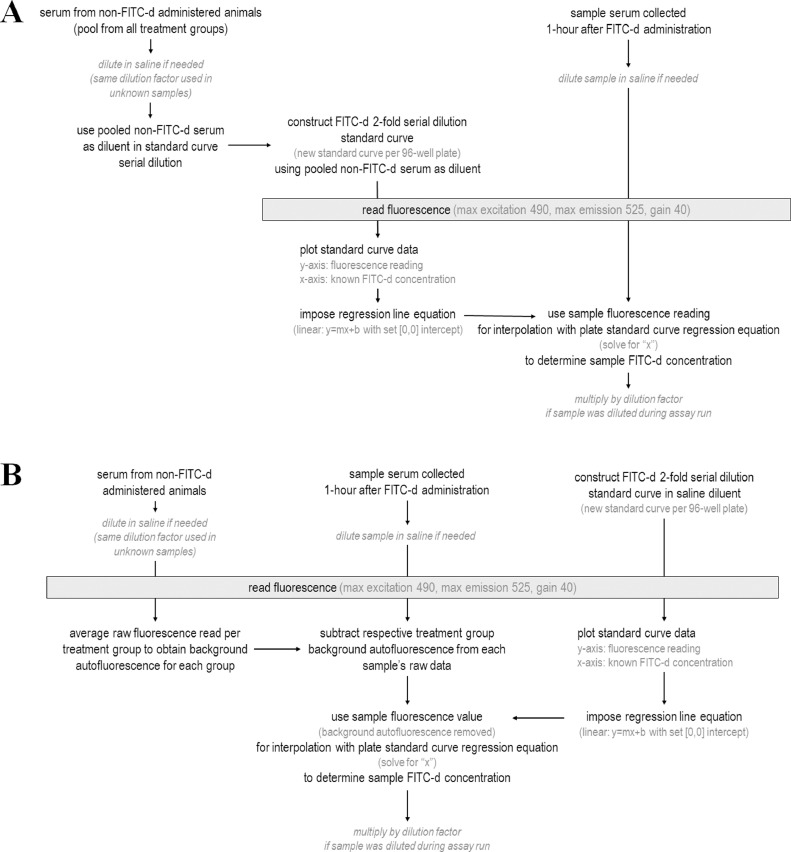
Fluorescein isothiocyanate dextran (FITC-d) has been successfully used as a marker for enteric permeability (Tellez et al., 2015; Barekatain et al., 2019; Hernandez-Patlan et al., 2019; Morales-Mena et al., 2020; Ruff et al., 2020; Ruff et al., 2021). The 4-6kDa size of FITC-d is too large to pass through the intestinal wall unless damage has occurred to the intestinal barrier. This damage can occur from enteric infections, stress responses, chronic inflammation, or even feed components (Tellez et al., 2015; Barekatain et al., 2019; Hernandez-Patlan et al., 2019; Morales-Mena et al., 2020; Ruff et al., 2020; Ruff et al., 2021). When this barrier is impaired, FITC-d in the digesta can translocate into circulation and is detectable in the serum. With increased leaky gut, there is an increase in detectable serum FITC-d. The original serum FITC-d procedure (Kuttappan et al., 2015) is not designed to accommodate serum containing high levels of additional pigmentation, specifically from animals with diets high in natural pigments or beta-carotene products. Serum collected from these animals possess naturally high background autofluorescence, which distorts the fluorescence readings. To accurately detect serum FITC-d levels in these samples, additional controls must be included in the sampling design for these experiments (Figure 1). Modifications to the originally described standard FITC-d protocol (Kuttappan et al., 2015; Baxter et al., 2017) are addressed in this manuscript to accommodate serum samples containing unusually high autofluorescence.
Figure 1.
FITC-d assay work flow for (A) the standard protocol used with normal serum samples (serum pooled from all treatment groups is used as diluent in the standard curve and unknown samples are blanked to the pooled background of all treatments) and (B) the modified protocol for highly autofluorescent serum samples (per treatment background autofluorescence subtracted from each unknown serum sample, respective to treatment group, and standard curve constructed with saline as diluent).
MATERIALS AND METHODS
Reagents
Fluorescein isothiocyanate dextran with size ranging from 4-6kDa is required for this assay (Sigma-Aldrich, St. Louis, MO, USA, cat no. FD4). This FITC-d must be pre-weighed and reconstituted in saline before oral administration to the animal. Additional FITC-d will be used for the construction of the assay standard curve (each assay plate run must possess its own standard curve). Sterile saline is also necessary for use as a diluent (for both the orally-administered FITC-d and in sample preparation of the assay plate).
Equipment
An oral gavage needle (curved blunt needle with a smooth round ball at the end) is required for oral administration of FITC-d. Essential blood collection tools (needle gauge appropriate to vein size, syringes, and tubes for blood collection) are also necessary. A centrifuge will be needed to separate serum from the collected blood samples. A basic 96-well microtiter plate for sample preparation/dilutions is required. A black, flat-bottom 96-well microtiter plate will be used for the final assay read in a plate reader capable of fluorescence quantification (excitation/emission of 495/519 is optimal for FITC-d).
FITC-d Preparation
FITC-d must be reconstituted in sterile saline before use. The FITC-d solution must be administered at 8.32mg per kg of bodyweight by oral gavage. Average bodyweight per treatment can be used to estimate the amount of FITC-d to prepare but is dependent on whether there is high variability in bodyweights within the treatment. Treatment groups may present with vastly different bodyweights, and using the average overall bodyweight of the experiment is not suggested. The FITC-d powder should be mixed shortly before use with saline, 30 minutes before administration, to allow the FITC-d to fully reconstitute. Protect the FITC-d solution from light.
Animal Administration and Sample Collection
In all FITC-d experimental trials, a negative control group (with healthy, intact intestinal barrier) must be included in the design. If possible, a positive control (stressed/leaky enteric barrier) group should also be included. In poultry, a bare minimum of n=20 samples per treatment group must be included to account for biological variations and to maintain statistical power. Administer 8.32mg FITC-d per kg of bodyweight by oral gavage (Baxter et al., 2017). Oral gavage administration is quicker than subsequent blood collection. To ensure a one-hour interval between the administration and blood collection to maintain consistency for all samples, include rest time/wait time intervals between oral gavage administrations. These rest intervals will allow for more accurately timed subsequent blood sampling (for 1-hour post-FITC-d administration). During blood collection, take precautions not to lyse/shear red blood cells when transferring to the sample tube. Protect blood samples from light after collection.
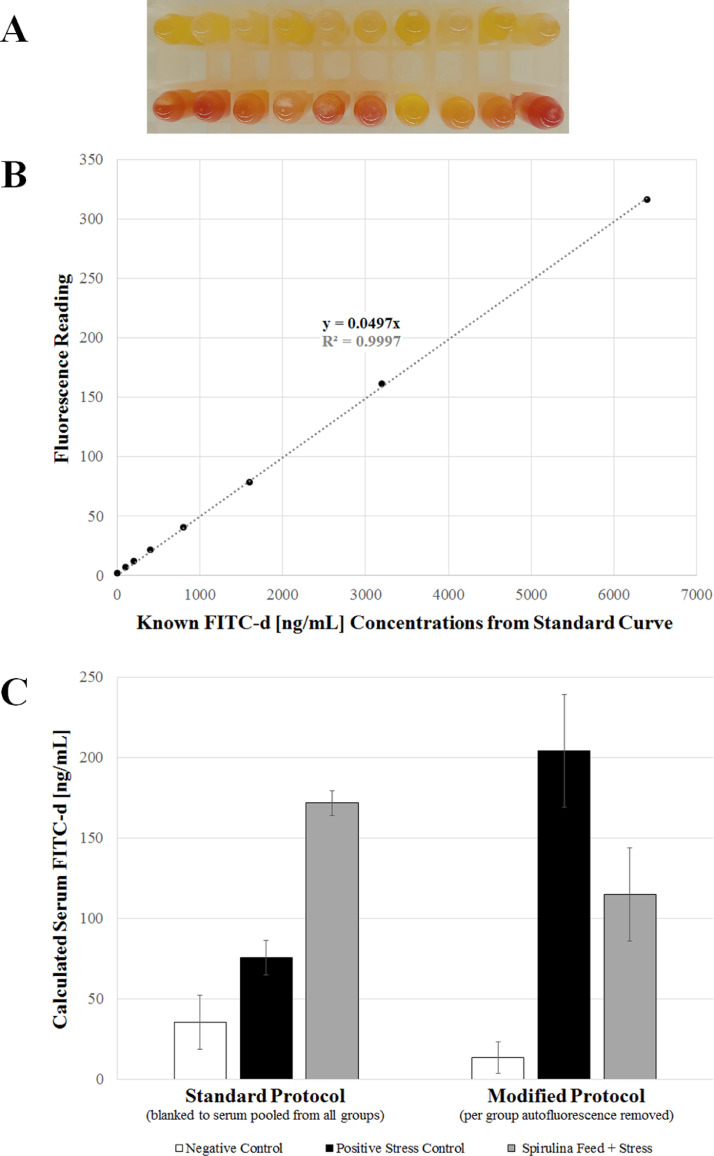
Under normal FITC-d assay conditions (no high pigment levels in the diet), serum utilized as the blank is pooled from a subset of birds from all treatment groups (which have not been administered FITC-d). The blank serum is either used: 1) to construct the standard curve (which uses the blank serum as diluent in construction of the standard curve; and therefore, intrinsically adjusts for background fluorescence when utilizing the standard curve regression equation to calculate FITC-d concentration of the treated samples) or 2) to serve as a separately measured sample (used to subtract the background from the individual FITC-d treated serum samples) before calculation with the standard curve made in saline diluent. In the specific cases in which animals have been fed diets with high concentrations of natural pigments (Figure 2A), hence altering the pigmentation of the serum, it is important to collect blood/serum from additional animals (which have not received FITC-d administration) from every treatment group to use for blanking/subtraction of background autofluorescence. This must be completed in a per treatment group manner, as different dietary inclusions of feed containing natural pigments will alter the background autofluorescence at different degrees.
Figure 2.
Example serum used for comparison between the standard and modified FITC-d procedures. Serum samples were obtained from an experiment with a three treatment diets with ad libitum access to feed and water. Negative (no stress) control diet was a standard corn-soybean meal diet meeting the Aviagen nutrient recommendations (21% crude protein; 201g/kg). Both the positive stress control and Spirulina platensis algae feed treatment groups had a 15% reduction in crude protein (still meeting all amino acid requirements), but the Spirulina treatment group had 50% of available crude protein from soybean meal replaced with a protein equivalent amount of Spirulina algae meal (Pond Tech, Inc., Markham, Ontario, Canada). All groups (Ross-708 line broilers) received the negative control diet from 0-14 days-of-age as a crumble, followed by respective pelleted treatment feed for the remainder of the trial (up to 37 days-of-age). The FITC-d assay was performed and serum samples were collected at termination of the trial. (A) Color difference observed in normal control serum (top row) compared to Spirulina-fed/beta-carotene-containing broiler serum (bottom row). (B) Standard curve with imposed linear regression line and equation. This equation will be used to calculate the ng/mL of FITC-d in the unknown sample by solving for “x”. To calculated this, the sample fluorescence value (with background autofluorescence removed) will be the “y” value, divided by the slope (slope “m” is 0.0497 in this example). Example using this standard curve: if raw sample fluorescence is 11 and treatment group background autofluorescence is 3, (11-3)/0.0497 = 160.97 ng/mL of FITC-d in the sample. (C) Example data of Spirulina-fed broiler serum samples run with the standard protocol (blanked to serum pooled from all treatment groups) compared to the modified protocol (blanked to per treatment background autofluorescence).
Sample Processing
Collected blood samples must be protected from light. Allow whole blood samples to stand at room temperature for 0.5-2 hours to ensure proper red blood cell coagulation before centrifugation. During balancing and sample manipulation, continue to work in minimal light conditions. To separate clotted red blood cells from serum, centrifuge samples at 800-1,000 x g for 20-30 minutes at 4-10°C. Do not allow samples to freeze, as this will lyse red blood cells. After centrifugation, check samples for a clear separation between the pelleted red blood cell clot and the serum supernatant portion. (Serum should not have granular red specks/residual red blood cells in solution.) If clear separation is not observed, repeat the centrifugation step. Under low light conditions, collect serum supernatant and continue to cover from light after sample collection. Samples can be used for FITC-d assay measurement immediately or frozen at -20°C (protected from light).
FITC-d Assay Procedure
Keep collected serum samples covered from light while the dilution plates are prepared. If serum samples have been previously frozen, confirm complete thaw and thorough mixing before use in the assay. Complete all dilutions and standard curves in a microtiter dilution plate at higher volumes than needed (samples will be run at a final volume of 100uL/well in the plate reader). Due to the variable degree of serum autofluorescence observed in dietary experiments containing high natural pigments (if different feed inclusion rates are tested), the standard curve must be prepared in saline as a diluent. The standard curve must consist of a 2-fold serial dilution in saline at 6400, 3200, 1600, 800, 400, 200, 100, and 0 ng final FITC-d/mL. This standard curve begins at an upper detection limit to facilitate ease of pipetting. Each 96-well assay plate run with samples must contain its own standard curve. The serum samples (both the FITC-d treatment serum samples and the non-FITC-d blank serum samples collected from each treatment group) can be run in the assay undiluted (neat) or up to a dilution factor of 5, depending on the volume of sample serum available. Once the FITC-d standard curve and samples are adequately diluted and prepared, transfer 100uL of each well to a black flat-bottom microtiter plate (confirm all samples have been well mixed and no bubbles are introduced during transfer to the black plate). Read the prepared assay plate at 495nm maximum excitation and 519nm maximum emission, gain 40 (Baxter et al., 2017).
Data Analysis
First, the serum sample raw fluorescent readings must have the autofluorescent background removed (Figure 1). To accomplish this, average the fluorescence readings of the non-FITC blank serum samples of each treatment group. This value will serve as the treatment group background that must be subtracted from each individual serum sample raw reading, respective to their treatment group, to account for dietary-induced autofluorescence. After subtraction, this serum sample value will be considered blanked for autofluorescence. The minimum fluorescence reading per sample is “0”; therefore, calculations that yield negative fluorescence values must be set to “0”.
To establish the standard curve, plot the known concentrations of FITC-d on the x-axis (6400, 3200, 1600, 800, 400, 200, 100, and 0 ng FITC-d/mL) and their respective fluorescence readings on the y-axis (Figure 2B). The 2-fold serial dilution FITC-d standard curve should yield a linear trend; and therefore, a linear regression line should be imposed (y=mx+b). Set the equation intercept at (0,0), thus a “0” fluorescence reading is “0” ng/mL FITC-d concentration. This will allow data analysis across multiple assay plates. The produced standard curve regression equation should be used to calculate the FITC-d concentrations of treatment serum samples (solving for “x” FITC-d concentration and “y” being the serum fluorescence value previously calculated). If serum samples were run on the assay at a dilution, multiply by the dilution factor previously used to report the final ng/mL FITC-d in undiluted serum.
RESULTS AND DISCUSSION
FITC-d is an assay based on relative quantification, with results interpreted purely comparative to the control groups. This is due to the variability in inflammation observed in animals raised in different conditions (environmental, nutritional, infectious disease, and handling stresses), which alters the intestinal permeability (Kuttappan et al., 2015; Tellez et al., 2015; Barekatain et al., 2019; Hernandez-Patlan et al., 2019; Morales-Mena et al., 2020; Ruff et al., 2020; Ruff et al., 2021). Age can also be a factor in enteric permeability, as younger animals possess more permeable intestinal barriers than their adult counterparts (Udall et al., 1981). Because of this variation between experimental environments, it is crucial to include a no-treatment/healthy control group for comparison. The negative control group (healthy, no enteric leakage) is expected to measure at the lowest possible serum FITC-d levels compared to the leaky gut positive control or any adversely affecting treatment groups. The specific FITC-d range of the negative control will be dependent on the trial, as these mentioned factors can affect this baseline level. When treatment group-specific serum autofluorescence is anticipated, researchers are encouraged to follow the modified procedure outlined (Figure 1). Use of the overall pooled background serum (mixing pre-FITC-d treatment serum from all treatment groups) from the standard protocol, instead of treatment-specific background used in the modified protocol, alters the calculated FITC-d values and final data interpretation (Figure 2C). If background serum is pooled from all groups and used to subtract from all treatment samples, the pigmented treatment groups will be calculated as overly high FITC-d levels because the pooled background includes non-pigmented serum from other groups, lowing the overall background value used to subtract from the pigmented treatment group. Conversely, the other non-pigmented treatments groups will be calculated as abnormally low FITC-d levels because the pooled background includes the pigmented serum, falsely causing the subtracted background value to be higher than necessary for the non-pigmented treatment group. If it is unknown whether the applied treatment will alter the background autofluorescence levels, it is advised to plan for the additional sample collections to accommodate the modified procedure as a precaution, as these added samples will not alter final data.
The detection limits of this assay are very narrow. Most serum samples will possess raw fluorescence readings ranging from 0 to 15, depending on the dilution used, which corresponds with the lower detection range of the constructed standard curve, typically ranging from 0-200 ng/mL FITC-d. To offset this limitation, it is crucial to include sufficient sample numbers (n per treatment group) to distinguish overall group trends. A more constricted standard curve range (for example, a 2-fold serial dilution starting from 1600ng/mL instead of 6400ng/mL) does not improve the accuracy or precision of the standard curve regression and yields the same level of interpolation (data not shown).
Utilization of the FITC-d procedure as a marker for intestinal permeability is a useful tool that permits detection of leaky gut without requiring euthanasia. In contrast, measurement of bacterial translocation from the gut to peripheral organs necessitates euthanization and organ collection for further processing and culture (Tellez et al., 2015). This process requires additional labor and overnight incubation (possibly 48 hours if initially culturing for enrichment), while the FITC-d procedure facilitates detection within hours of blood collection. The serum collected for FITC-d detection can also be reused for other assays (interferon-gamma for detection of inflammation or superoxide dismutase for the ability to scavenge reactive oxygen species/free radicals) (Ruff et al., 2021). If possible, it is always recommended to evaluate multiple enteric inflammation parameters to improve overall meaningful data collection and interpretation.
The FITC-d method for measuring relative enteric tight-junction disruptions is becoming increasingly popular. With this, all researchers must include appropriate controls and normalize data processing to interpret the results. This procedure is relatively simple, does not require euthanasia, and allows researchers to detect enteric permeability without expensive equipment, high technical training, or dedicated statistical software. This procedure has also proven reliable, showing particular stressors consistently induce leaky gut, even when studies are completed by different researchers (Kuttappan et al., 2015; Barekatain et al., 2019). It is anticipated that this method will become more common in future research.
ACKNOWLEDGMENTS
The serum samples used to generate example data presented in Figure 2 was obtained from research supported in part by Promitec S.A. and funds provided by USDA-NIFA Sustainable Agriculture Systems, Grant No. 2019-69012-29905. Title of Project: Empowering U.S. Broiler Production for Transformation and Sustainability USDA-NIFA (Sustainable Agriculture Systems): No. 2019-69012-29905. Example serum was obtained from an animal trial performed under approved University of Arkansas: Division of Agriculture animal permit #21002.
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that the example serum and data presented in Figure 2 was received from a study supported by Promitec S.A. and USDA-NIFA Sustainable Agriculture System Grant No. 2019-69012-29905. The funders were not involved in the design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
References
- Barekatain R., Chrystal P.V., Howarth G.S., McLaughlan C.J., Gilani S., Nattrass G.S. Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poult. Sci. 2019;98:6761–6771. doi: 10.3382/ps/pez393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017;4:56. doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Adhikari B., Pontin K.P., Latorre J.D., Baxter M.F.A., Hernandex-Velasco X., Merino-Guzman R., Mendez-Albores A., Kwon Y.M., Hargis B.M., Lopez-Arellano R., Arreguin-Nava M.A., Tellez-Isaias G. Evaluation of the antimicrobial and intestinal integrity properties of boric acid in broiler chickens infected with Salmonella enteritidis: proof-of-concept. Res. Vet. Sci. 2019;123:7–13. doi: 10.1016/j.rvsc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Berghman L.R., Vicuna E.A., Latorre J.D., Menconi A., Wolchok J.D., Wolfenden A.D., Faulkner O.B., Tellez G., Hargis B.M., Bielke L.R. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015;94:1220–1226. doi: 10.3382/ps/pev114. [DOI] [PubMed] [Google Scholar]
- Morales-Mena A., Martinez-Gonzalez S., Teague K.D., Graham L.E., Senas-Cuesta R., Vuong C.N., Lester H., Hernandez-Patlan D., Solis-Cruz B., Fuente-Martinez B., Hernandez-Velasco X., Hargis B.M., Tellez-Isaias G. Assessment of fermented soybean meal on Salmonella Typhimurium infection in neonatal turkey poults. Animals. 2020;10:1849. doi: 10.3390/ani10101849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J., Barros T.L., Tellez G., Blankenship J., Lester H., Graham B.D., Selby S.M., Vuong C.N., Dridi S., Greene E.S., Hernandez-Velasco X., Hargis B.M., Tellez-Isaias G. Research Note: evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poult. Sci. 2020;99:1687–1692. doi: 10.1016/j.psj.2019.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J., Tellez G., Forga A.J., Senas-Cuesta R., Vuong C.N., Greene E.S., Hernandez-Velasco X., Uribe A.J., Martinez B.C., Angel-Isaza J.A., Dridi S., Maynard C.J., Owens C.M., Hargis B.M., Tellez-Isaias G. Evaluation of three formulations of essential oils in broiler chickens under cyclic heat stress. Animals. 2021;11:1084. doi: 10.3390/ani11041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez G., Latorre J.D., Kuttappan V.A., Hargis B.M., Hernandez-Velasco X. Rye affects bacterial translocation, intestinal viscosity, microbiota composition and bone mineralization in turkey poults. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall J.N., Pang K., Fritze L., Kleinman R., Walker W.A. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr. Res. 1981;15:241–244. doi: 10.1203/00006450-198103000-00008. [DOI] [PubMed] [Google Scholar]