Abstract
CD34+ cells are multipotent hematopoietic stem cells also known as endothelial progenitor cells and are useful in regenerative medicine. Naturally, these cells are mobilized from the bone marrow into peripheral circulation in response to ischemic tissue injury. CD34+ cells are known for their high proliferative and differentiation capacities that play a crucial role in the repair process of myocardial damage. They have an important paracrine activity in secreting factors to stimulate vasculogenesis, reduce endothelial cells and cardiomyocytes apoptosis, remodel extracellular matrix and activate additional progenitor cells. Once they migrate to the target site, they enhance angiogenesis, neovascularization and tissue regeneration. Several trials have demonstrated the safety and efficacy of CD34+ cell therapy in different settings, such as peripheral limb ischemia, stroke and cardiovascular disease. Herein, we review the potential utility of CD34+ cell transplantation in acute myocardial infarction, refractory angina and ischemic heart failure.
Keywords: Cell therapy, Endothelial progenitor cells, Myocardial ischemia, Refractory angina, Heart failure, Coronary microvascular dysfunction
Core Tip: CD34+ cells are mobilized from the bone marrow into the peripheral circulation in response to ischemic tissue injury. Once they migrate to the target site, they enhance angiogenesis, neovascularization and tissue regeneration. Safety and efficacy of CD34+ cell transplantation has been investigated in order to limit left ventricular dysfunction after acute myocardial infarction, refractory angina and heart failure.
INTRODUCTION
Ischemic heart disease or myocardial ischemia (MI) is a common disorder characterized by an imbalance between myocardial oxygen demand and supply. A wide spectrum of clinical manifestations ranging from chest discomfort to myocardial infarction is attributed to ischemic heart disease[1]. Conceptually, it is related to atherosclerotic coronary artery disease and considered the principal cause of death worldwide[2]. An epicardial coronary stenosis limits blood flow to a specific myocardial area, leading to ischemia, infarction and apoptosis[3]. In line with scientific development, microvascular dysfunction becomes responsible for a major part of MI[4]. It plays a crucial role by impairing the reactivity of coronary microcirculation in response to an increase in myocardial oxygen demand, which is equivalent to the effect of an obstructive plaque[4]. A large variety of therapeutic modalities targeting the pathophysiological patterns, consequences and underlying causes of MI are available.
CD34+ cell implantation has emerged as a promising approach to overcome the main limitations of conventional therapies by combining optimal medical treatment and myocardial revascularization by percutaneous coronary intervention or coronary artery bypass graft. CD34+ cells are multipotent hematopoietic stem cells also known as endothelial progenitor cells and are useful in regenerative medicine for treating ischemic injuries[5]. These cells are easily mobilized from the bone marrow into peripheral circulation and characterized by their ability to promote neoangiogenesis and cardiomyocyte regeneration[6]. Previous published trials have reported the effectiveness of CD34+ cell implantation for treating ischemic vascular disease like ischemic stroke, peripheral limb ischemia and MI[7,8]. Herein, we focus on the utility of CD34+ cell administration in the settings of myocardial infarction, refractory angina and ischemic heart failure.
MECHANISMS OF ACTION OF CD34+ CELLS
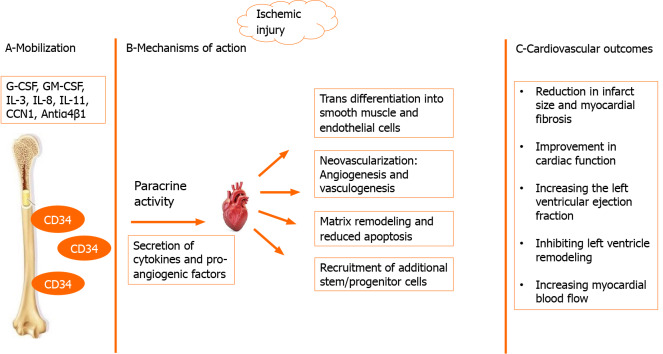
CD34+ cells are released into peripheral blood circulation in response to ischemic tissue injury. High circulating levels of CD34+ cells were detected after myocardial infarction[9]. These cells are known for their high proliferative and differentiation capacities that play a crucial role in the repair process after myocardial damage (Figure 1)[5]. Integrin antibodies, cysteine-rich angiogenic protein 61, stromal cell derived-factor 1 (SDF-1) and granulocyte colony stimulating factor were identified as agents incorporated in CD34+ cell mobilization from the bone marrow to peripheral circulation[10,11]. Then, an interaction between several factors (SDF-1, hepatocyte growth factor, vascular cell adhesion molecule, stem cell factor) and homing receptors such as CXC-chemokine receptor-4 is responsible for CD34+ cells traveling to ischemic tissue[12]. Once CD34+ cells migrate to the target site, they enhance angiogenesis, neovascularization and cardiac regeneration in two ways. First, CD34+ cells differentiate into smooth muscle cells and endothelial cells, which are the main structural components of internal vascular walls that lead to vascular re-endothelialization[13]. Second, they have an important paracrine role in secreting factors to stimulate vasculogenesis, reduce endothelial cell and cardiomyocyte apoptosis, remodel extracellular matrix and activate additional progenitor cells[5,13]. CD34+ cells produce angiogenic cytokines, such as interleukin-8, vascular epithelial growth factor and cytokine like 1[14]. A major part of their proangiogenic mechanism is mediated by producing exosomes (membrane-bound nanovesicles)[15]. These exosomes transfer proangiogenic microRNAs that may amplify the stem cell function and explain the angiogenic and therapeutic benefits associated with CD34+ stem cell therapy[15].
Figure 1.
Role of CD34+ cells in ischemic myocardial repair. A: In response to ischemic injury, several factors like granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, interleukin (IL)-3, IL-8, IL-11, cysteine-rich angiogenic protein 61 (CCN1) and integrin antibodies (Antiα4β1) trigger mobilization of CD34+ cells from the bone marrow into peripheral circulation; B: Mechanisms of action of CD34+ cells on cardiomyocytes via paracrine activity are transdifferentiation into smooth muscle and endothelial cells, neovascularization, matrix remodeling, reduced apoptosis and recruitment of additional stem/progenitor cells; C: The main cardiovascular outcomes of CD34+ cells. G-CSF: Granulocyte colony stimulating factor; GM-CSF: Granulocyte macrophage colony stimulating factor; IL: Interleukin; CCN1: Cysteine-rich angiogenic protein 61.
Limited data from the literature describe a beneficial effect of autologous CD34+ cell therapy on endothelial dependent/independent microvascular dysfunction[16]. Previous trials have reported a significant improvement in coronary flow reserve[17,18]. Lastly, replicative efficiency of endothelial progenitor cells is inversely correlated with age[19]. A decline in CD34+ cell function and in circulating number were associated with aging above 60 years[20,21] and type 2 diabetes mellitus[22,23].
EFFICACY OF CD34+ CELL IMPLANTATION IN ISCHEMIC CARDIOVASCULAR DISEASE
Myocardial infarction
Numerous trials have investigated the efficacy and safety of CD34+ cell therapy in the setting of acute myocardial infarction (AMI) (Table 1). In general, stimulation of angiogenesis and reduction in the infarct size, scar formation and myocardial fibrosis were commonly observed after CD34+ cell injection in the hibernating zone surrounding the infracted myocardial area[24-34]. Inhibition of cardiomyocyte apoptosis and collagen deposition were reported by Kocher et al[24] in an animal model. An improvement in left ventricular function was associated with CD34+ cell transplantation after AMI in human and nonhuman trials[25,28,29,32]. Shintani et al[30] observed a synergistic effect while combining CD34+ cells and vascular epithelial growth factor 2 gene therapies in the management of AMI, which yielded better outcomes. Also, Mackie et al[33] showed that injection of genetically modified CD34+ cells expressing sonic hedgehog protein enhanced angiogenic potency of CD34+ cells in ischemic myocardial tissue. In parallel, the injection of CD34+ cells deficient in microRNA377 following AMI significantly promoted angiogenesis and reduced left ventricle remodeling and cardiac fibrosis when compared to regular cells[34]. It is known that ischemic pre-conditioning (IPC) is beneficial for MI. Subsequently, Kamota et al[35] have demonstrated that this positive outcome was linked to the released cardioprotective factors in the early phase of IPC and to the CD34+ cells mobilization in the late phase of IPC.
Table 1.
Summary of clinical studies of CD34+ cell therapy
|
Disease
|
Ref.
|
Model
|
Delivery route
|
Results
|
| Myocardial infarction | Kocher et al[24] | Animal | Intravenous | (+) Angiogenesis; (-) Cardiomyocyte apoptosis, collagen deposition, scar formation |
| Kawamoto et al[25] | Human | Intramyocardial | (+) Angiogenesis, cardiac function; (-) Myocardial fibrosis | |
| Botta et al[26] | Animal | Intramyocardial | (+) Cardiac function; (+) Cardiac hemodynamics with CD34+ KDR+ subset | |
| Brenner et al[27] | Animal | Intracavitary of LV | (+) LV function | |
| Ott et al[28] | Animal | Intramyocardial | (+) LVEF | |
| Yoshioka et al[29] | Human | Intracardiac | (+) Blood flow; (+) Cardiac function | |
| Shintani et al[30] | Animal | Intramyocardial | (+) Capillary density; (-) Myocardial infarct size | |
| Zhang et al[31] | Animal | Intracoronary | (+) Cardiac repair, therapeutic benefits | |
| Wang et al[32] | Animal | Intramyocardial | (+) Angiogenesis, cardiac function | |
| Mackie et al[33] | Animal | Intramyocardial | (+) Capillary density; (-) Ventricular dilation, infarct size, cardiac function alteration | |
| Joladarashi et al[34] | Animal | Intramyocardial | (+) Angiogenesis; (-) LV remodeling and cardiac fibrosis with CD34 deficient microRNA 377 | |
| Quyyumi et al[38] | Human | Intracoronary | (+) LVEF; (-) Infarct size and MACE | |
| Quyyumi et al[40] | Human | Intracoronary | (+) Infarcted area perfusion | |
| Refractory angina | Losordo et al[43] | Human | Intramyocardial | (+) Exercise tolerance; (-) Angina frequency, 12 mo mortality |
| Henry et al[45] | Human | Intramyocardial | (-) Angina frequency at 2 yr | |
| Povsic et al[46] | Human | Intramyocardial | (-) Angina frequency | |
| Wang et al[47] | Human | Intramyocardial | (+) Myocardial perfusion by PET; (-) Angina frequency | |
| Lee et al[48] | Human | Intracoronary | (+) LVEF; (-) Angina frequency | |
| Johnson et al[49] | Human | Intramyocardial | (-) Mortality, cardiac admissions, hospital visits, health care costs | |
| Heart failure (dilated cardiomyopathy) | Vrtovec et al[50] | Human | Intracoronary | (+) LVEF, 6MWD; (-) NT-proBNP, mortality |
| Lezaic et al[51] | Human | Intracoronary | (+) Myocardial perfusion, LVEF, 6MWD | |
| Bervar et al[53] | Human | Transendocardial | (+) 6MWD, diastolic function; (-) NT-proBNP | |
| Poglajen et al[54] | Human | Transendocardial | (+) 6MWD, LVEF; (-) NT-proBNP | |
| Coronary microvascular dysfunction | Erbs et al[17] | Human | Intracoronary | (+) CFR |
| Schächinger et al[18] | Human | Intracoronary | (+) CFR |
6MWD: 6-min walk distance; CFR: Coronary flow reserve; KDR: Vascular endothelial growth factor receptor 2; LV: Left ventricle; LVEF: Left ventricular ejection fraction; MACE: Major adverse cardiovascular events; NT-proBNP: N-terminal pro brain natriuretic peptide; PET: Positron emission tomography.
Findings from the BONAMI trial showed that a decreased number of CD34+ cells in smokers was negatively correlated to viability recovery measured by single-photon emission computerized tomography at 3 mo post-AMI[36,37]. This suggests that these cells play a significant role after AMI. Thus, the PreSERVE-AMI trial revealed the safety of intracoronary injection of autologous CD34+ cells in revascularized ST-segment elevated myocardial infarction patients with altered left ventricular ejection fraction[38]. Indeed, a reduction in post-AMI major adverse cardiovascular events and an improvement in left ventricular function at 6 mo after cell injection were observed[39]. We noticed that therapeutic results of CD34+ cell implantation were dose-dependent[6,38-41]. Greater improvement was associated with higher doses with a threshold dose of over 10 million cells, particularly in the setting of myocardial infarction[40,41].
Subsequently, it was relevant to develop new delivery systems to allow the administration of higher numbers of CD34+ cells. The main purpose of the EXCELLENT trial (ClinicalTrials.gov Identifier: NCT02669810) was to evaluate the safety, tolerance and efficacy of intramyocardial injections of ProtheraCytes (autologous peripheral blood-CD34+ stem cells after automated ex vivo expansion with the StemXpandâ machine) in patients with AMI and decreased ejection fraction. In this ongoing trial, ProtheraCytes are currently reinjected using the BioCardia Helix biotherapeutic delivery system introduced through the femoral artery and guided towards the infarcted myocardium, thus avoiding surgical access.
Refractory angina
Intramyocardial injection of autologous CD34+ cells for the treatment of patients with refractory angina (Table 1) despite optimal medical therapy and no alternative therapeutic options has been studied considerably. Losordo et al[42] first showed the safety and favorable effectiveness of CD34+ cell therapy in these patients. The major positive effects included decreased frequency of weekly angina episodes and nitroglycerine use and improved Canadian Cardiovascular Society classification, exercise tolerance and quality of life[43,44]. Data from the ACT34-CMI study showed sustained efficacy of a single intramyocardial CD34+ cell injection for up to 2 years, with a significant reduction in deaths and major adverse cardiovascular events compared to those treated by placebo[45].
The incomplete RENEW phase III trial, which enrolled 112 of the 444 planned patients, failed to show a significant difference in total exercise time between the three study groups (CD34+ cells, placebo and conventional therapy) at 3, 6 and 12 mo[46]. However, it did confirm the findings from previous studies concerning the safety and efficacy of intramyocardial CD34+ cell therapy on angina frequency[47]. The efficacy and safety of intracoronary administration of CD34+ cells for refractory angina were evaluated in patients unsuitable for revascularization strategies with diffuse obstructive coronary artery disease[47,48]. A reduction in weekly angina frequency without significant adverse events were observed. A recently published study by Johnson et al[49] showed a significant reduction in mortality, cardiac-related admissions, hospital visits, coronary interventions and health care costs in the 12 mo following intramyocardial administration of CD34+ cells compared to the year before their injection.
Heart failure
CD34+ cell therapy was investigated in ischemic and non-ischemic dilated cardiomyopathy (DCM) (Table 1). Improvement in the 6-min walk test, left ventricular ejection fraction, N-terminal pro brain natriuretic peptide level and resting myocardial perfusion were observed after intracoronary injection of autologous CD34+ cells in patients with non-ischemic DCM and reduced ejection fraction[50-52]. Similar results with improvement in diastolic function were found by Bervar et al[53] after transendocardial CD34+ cell delivery in non-ischemic heart failure patients. Also, a significant decrease in the prevalence of heart failure and total mortality rate without a difference in the prevalence of sudden cardiac death were observed at 5 years after CD34+ cell transplantation therapy[50].
In patients with ischemic cardiomyopathy and left ventricular ejection fraction below 40%, Poglajen et al[54] observed significant amelioration in left ventricular ejection fraction, 6-min walk test and N-terminal pro brain natriuretic peptide levels after transendocardial injection of CD34+ cells into hibernating myocardium, while no change in these parameters was observed after optimal medical treatment. It is noteworthy that greater clinical improvement was associated with higher delivered doses and extended injections[54]. Comparing delivery routes of CD34+ cells in DCM patients, a significantly better response was correlated with transendocardial administration than with intracoronary injections[55]. However, CD34+ cell transplantation was not beneficial in diabetic patients with non-ischemic DCM as shown by a small study sample size[56].
Coronary microvascular dysfunction
Currently, coronary microvascular dysfunction (CMD) is a hot topic in cardiology. It plays a pivotal role in the pathophysiology of myocardial infarction with nonobstructive coronary artery disease, which accounts for 10% of acute coronary syndromes[57]. While therapeutic options are limited, the role of CD34+ cell therapy remains unclear in CMD, and available data are scarce in the literature (Table 1)[16]. Otherwise, reduced circulating levels of CD34+ cells were detected in the presence of coronary endothelial dysfunction even in the absence of atherosclerotic disease[58,59]. REPAIR-AMI and TOPCARE-AMI trials reported potential benefits of intracoronary injection of autologous CD34+ cells on CMD by revealing a normalization of coronary flow reserve in the studied AMI population[17,18]. Additionally, two ongoing trials are investigating the safety and efficacy of CD34+ cell therapy in patients with CMD (CLBS14 trial, ClinicalTrials.gov Identifier: NCT03508609; CD34 trial, ClinicalTrials. gov Identifier: NCT03471611).
CONCLUSION
Regenerative medicine is a promising therapeutic approach for acquired cardiovascular disease, and routine use of stem cell therapy would be an ultimate goal of this area. Several clinical trials support the safety and efficacy of autologous CD34+ cell transplantation in AMI, refractory angina and systolic heart failure. Administration of genetically modified CD34+ sonic hedgehog+ microRNA377- cells amplifies the angiogenic target. Intramyocardial injection is likely the preferred delivery route, and a threshold dose over 10 million cells is desirable at least in the setting of AMI. Future studies investigating the tolerability and efficacy of allogenic CD34+ cells from youthful donors into elderly and diabetic recipients are warranted. Moving beyond clinical trials and translation of preliminary data into clinical practice after establishing a standardized procedure for CD34+ cell transplantation may revolutionize the management and overall prognosis of ischemic cardiovascular disease.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: January 29, 2021
First decision: February 25, 2021
Article in press: April 13, 2021
Specialty type: Transplantation
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao L S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
Contributor Information
Anthony Matta, Department of Cardiology, Institute CARDIOMET, University Hospital of Toulouse, Toulouse 31059, France; Faculty of Medicine, Holy Spirit University of Kaslik, Kaslik 00000, Lebanon.
Vanessa Nader, Department of Cardiology, Institute CARDIOMET, University Hospital of Toulouse, Toulouse 31059, France; Faculty of Pharmacy, Lebanese University, Beirut 961, Lebanon.
Michel Galinier, Department of Cardiology, Institute CARDIOMET, University Hospital of Toulouse, Toulouse 31059, France.
Jerome Roncalli, Department of Cardiology, Institute CARDIOMET, University Hospital of Toulouse, Toulouse 31059, France. roncalli.j@chu-toulouse.fr.
References
- 1.Irwin S. Clinical manifestations and assessment of ischemic heart disease. Phys Ther. 1985;65:1806–1811. doi: 10.1093/ptj/65.12.1806. [DOI] [PubMed] [Google Scholar]
- 2.Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R. Mortality From Ischemic Heart Disease. Circ Cardiovasc Qual Outcomes. 2019;12:e005375. doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezende PC, Ribas FF, Serrano CV Jr, Hueb W. Clinical significance of chronic myocardial ischemia in coronary artery disease patients. J Thorac Dis. 2019;11:1005–1015. doi: 10.21037/jtd.2019.02.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of Ischemic Heart Disease. Circulation. 2018;138:1463–1480. doi: 10.1161/CIRCULATIONAHA.118.031373. [DOI] [PubMed] [Google Scholar]
- 5.Roncalli JG, Tongers J, Renault MA, Losordo DW. Endothelial progenitor cells in regenerative medicine and cancer: a decade of research. Trends Biotechnol. 2008;26:276–283. doi: 10.1016/j.tibtech.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Hénon P. Key Success Factors for Regenerative Medicine in Acquired Heart Diseases. Stem Cell Rev Rep. 2020;16:441–458. doi: 10.1007/s12015-020-09961-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sietsema WK, Kawamoto A, Takagi H, Losordo DW. Autologous CD34+ Cell Therapy for Ischemic Tissue Repair. Circ J. 2019;83:1422–1430. doi: 10.1253/circj.CJ-19-0240. [DOI] [PubMed] [Google Scholar]
- 8.Roncalli J, Tongers J, Renault MA, Losordo DW. Biological approaches to ischemic tissue repair: gene- and cell-based strategies. Expert Rev Cardiovasc Ther. 2008;6:653–668. doi: 10.1586/14779072.6.5.653. [DOI] [PubMed] [Google Scholar]
- 9.Wojakowski W, Tendera M, Michałowska A, Majka M, Kucia M, Maślankiewicz K, Wyderka R, Ochała A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 10.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, Kearney M, Thorne T, Curry C, Eaton L, Heyd L, Dinesh D, Kishore R, Zhu Y, Losordo DW. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood. 2007;110:877–885. doi: 10.1182/blood-2006-07-036202. [DOI] [PubMed] [Google Scholar]
- 12.Theiss HD, David R, Engelmann MG, Barth A, Schotten K, Naebauer M, Reichart B, Steinbeck G, Franz WM. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM) Eur Heart J. 2007;28:1258–1264. doi: 10.1093/eurheartj/ehm011. [DOI] [PubMed] [Google Scholar]
- 13.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010;79:200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneller D, Hofer-Warbinek R, Sturtzel C, Lipnik K, Gencelli B, Seltenhammer M, Wen M, Testori J, Bilban M, Borowski A, Windwarder M, Kapel SS, Besemfelder E, Cejka P, Habertheuer A, Schlechta B, Majdic O, Altmann F, Kocher A, Augustin HG, Luttmann W, Hofer E. Cytokine-Like 1 Is a Novel Proangiogenic Factor Secreted by and Mediating Functions of Endothelial Progenitor Cells. Circ Res. 2019;124:243–255. doi: 10.1161/CIRCRESAHA.118.313645. [DOI] [PubMed] [Google Scholar]
- 15.Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ, Sahoo S. Angiogenic Mechanisms of Human CD34+ Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ Res. 2017;120:1466–1476. doi: 10.1161/CIRCRESAHA.116.310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad M, Corban MT, Henry TD, Dietz AB, Lerman LO, Lerman A. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc Res. 2020;116:1424–1433. doi: 10.1093/cvr/cvaa027. [DOI] [PubMed] [Google Scholar]
- 17.Erbs S, Linke A, Schächinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 18.Schächinger V, Assmus B, Honold J, Lehmann R, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Normalization of coronary blood flow in the infarct-related artery after intracoronary progenitor cell therapy: intracoronary Doppler substudy of the TOPCARE-AMI trial. Clin Res Cardiol. 2006;95:13–22. doi: 10.1007/s00392-006-0314-x. [DOI] [PubMed] [Google Scholar]
- 19.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, Vaccarino V, Waller EK, Quyyumi AA. Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors. Circ Res. 2016;119:801–809. doi: 10.1161/CIRCRESAHA.116.308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozid AM, Jones D, Arnous S, Saunders N, Wragg A, Martin J, Agrawal S, Mathur A. The effects of age, disease state, and granulocyte colony-stimulating factor on progenitor cell count and function in patients undergoing cell therapy for cardiac disease. Stem Cells Dev. 2013;22:216–223. doi: 10.1089/scd.2012.0139. [DOI] [PubMed] [Google Scholar]
- 21.Wu TW, Liu CC, Hung CL, Yen CH, Wu YJ, Wang LY, Yeh HI. Genetic profiling of young and aged endothelial progenitor cells in hypoxia. PLoS One. 2018;13:e0196572. doi: 10.1371/journal.pone.0196572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavromatis K, Ghasemzadeh N, Forghani Z, Hameed S, Waller EK, Quyyumi AA, Taylor W. Patients with diabetes have reduced circulating CD34+ cells after coronary stenting. Arterioscler Thromb Vasc Biol. 2008;28:E77. [Google Scholar]
- 23.Yamashita A, Nishihira K, Matsuura Y, Ito T, Kawahara K, Hatakeyama K, Hashiguchi T, Maruyama I, Yagi H, Matsumoto M, Fujimura Y, Kitamura K, Shibata Y, Asada Y. Paucity of CD34-positive cells and increased expression of high-mobility group box 1 in coronary thrombus with type 2 diabetes mellitus. Atherosclerosis. 2012;224:511–514. doi: 10.1016/j.atherosclerosis.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 25.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 26.Botta R, Gao E, Stassi G, Bonci D, Pelosi E, Zwas D, Patti M, Colonna L, Baiocchi M, Coppola S, Ma X, Condorelli G, Peschle C. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J. 2004;18:1392–1394. doi: 10.1096/fj.03-0879fje. [DOI] [PubMed] [Google Scholar]
- 27.Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U, Heeschen C, Kampen WU, Zeiher AM, Dimmeler S, Henze E. 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med. 2004;45:512–518. [PubMed] [Google Scholar]
- 28.Ott I, Keller U, Knoedler M, Götze KS, Doss K, Fischer P, Urlbauer K, Debus G, von Bubnoff N, Rudelius M, Schömig A, Peschel C, Oostendorp RA. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. FASEB J. 2005;19:992–994. doi: 10.1096/fj.04-3219fje. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, Matsui K, Yamamoto K, Terao K, Shimada K, Ikeda U, Ozawa K, Hanazono Y. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005;23:355–364. doi: 10.1634/stemcells.2004-0200. [DOI] [PubMed] [Google Scholar]
- 30.Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, Wecker A, Gavin M, Ma H, Kearney M, Silver M, Thorne T, Murohara T, Losordo DW. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Ge J, Zhao L, Qian J, Huang Z, Shen L, Sun A, Wang K, Zou Y. Host vascular niche contributes to myocardial repair induced by intracoronary transplantation of bone marrow CD34+ progenitor cells in infarcted swine heart. Stem Cells. 2007;25:1195–1203. doi: 10.1634/stemcells.2006-0605. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, Bankson JA, Shpall E, Willerson JT, Gelovani JG, Yeh ET. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–1911. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joladarashi D, Garikipati VNS, Thandavarayan RA, Verma SK, Mackie AR, Khan M, Gumpert AM, Bhimaraj A, Youker KA, Uribe C, Suresh Babu S, Jeyabal P, Kishore R, Krishnamurthy P. Enhanced Cardiac Regenerative Ability of Stem Cells After Ischemia-Reperfusion Injury: Role of Human CD34+ Cells Deficient in MicroRNA-377. J Am Coll Cardiol. 2015;66:2214–2226. doi: 10.1016/j.jacc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamota T, Li TS, Morikage N, Murakami M, Ohshima M, Kubo M, Kobayashi T, Mikamo A, Ikeda Y, Matsuzaki M, Hamano K. Ischemic pre-conditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 2009;53:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Roncalli J, Mouquet F, Piot C, Trochu JN, Le Corvoisier P, Neuder Y, Le Tourneau T, Agostini D, Gaxotte V, Sportouch C, Galinier M, Crochet D, Teiger E, Richard MJ, Polge AS, Beregi JP, Manrique A, Carrie D, Susen S, Klein B, Parini A, Lamirault G, Croisille P, Rouard H, Bourin P, Nguyen JM, Delasalle B, Vanzetto G, Van Belle E, Lemarchand P. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur Heart J. 2011;32:1748–1757. doi: 10.1093/eurheartj/ehq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamirault G, de Bock E, Sébille V, Delasalle B, Roncalli J, Susen S, Piot C, Trochu JN, Teiger E, Neuder Y, Le Tourneau T, Manrique A, Hardouin JB, Lemarchand P. Sustained quality of life improvement after intracoronary injection of autologous bone marrow cells in the setting of acute myocardial infarction: results from the BONAMI trial. Qual Life Res. 2017;26:121–125. doi: 10.1007/s11136-016-1366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, Toma C, Davidson CJ, Barsness GW, Shavelle DM, Cohen M, Poole J, Moss T, Hyde P, Kanakaraj AM, Druker V, Chung A, Junge C, Preti RA, Smith RL, Mazzo DJ, Pecora A, Losordo DW. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ Res. 2017;120:324–331. doi: 10.1161/CIRCRESAHA.115.308165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 40.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Poole JC, Quyyumi AA. Progenitor Cell Therapy to Treat Acute Myocardial Infarction: The Promise of High-Dose Autologous CD34(+) Bone Marrow Mononuclear Cells. Stem Cells Int. 2013;2013:658480. doi: 10.1155/2013/658480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 43.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA ACT34-CMI Investigators. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benck L, Henry TD. CD34+ Cell Therapy for No-Option Refractory Disabling Angina: Time for FDA Approval? Cardiovasc Revasc Med. 2019;20:177–178. doi: 10.1016/j.carrev.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Henry TD, Schaer GL, Traverse JH, Povsic TJ, Davidson C, Lee JS, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Patel AN, Riedel N, Junge C, Hunt A, Kereiakes DJ, White C, Harrington RA, Schatz RA, Losordo DW ACT. Autologous CD34+ Cell Therapy for Refractory Angina: 2-Year Outcomes From the ACT34-CMI Study. Cell Transplant. 2016;25:1701–1711. doi: 10.3727/096368916X691484. [DOI] [PubMed] [Google Scholar]
- 46.Povsic TJ, Henry TD, Traverse JH, Fortuin FD, Schaer GL, Kereiakes DJ, Schatz RA, Zeiher AM, White CJ, Stewart DJ, Jolicoeur EM, Bass T, Henderson DA, Dignacco P, Gu Z, Al-Khalidi HR, Junge C, Nada A, Hunt AS, Losordo DW RENEW Investigators. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34(+) Cell Administration in Patients With Refractory Angina. JACC Cardiovasc Interv. 2016;9:1576–1585. doi: 10.1016/j.jcin.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Cui J, Peng W, Lu M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology. 2010;117:140–147. doi: 10.1159/000320217. [DOI] [PubMed] [Google Scholar]
- 48.Lee FY, Chen YL, Sung PH, Ma MC, Pei SN, Wu CJ, Yang CH, Fu M, Ko SF, Leu S, Yip HK. Intracoronary Transfusion of Circulation-Derived CD34+ Cells Improves Left Ventricular Function in Patients With End-Stage Diffuse Coronary Artery Disease Unsuitable for Coronary Intervention. Crit Care Med. 2015;43:2117–2132. doi: 10.1097/CCM.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 49.Johnson GL, Henry TD, Povsic TJ, Losordo DW, Garberich RF, Stanberry LI, Strauss CE, Traverse JH. CD34+ cell therapy significantly reduces adverse cardiac events, health care expenditures, and mortality in patients with refractory angina. Stem Cells Transl Med. 2020;9:1147–1152. doi: 10.1002/sctm.20-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 51.Lezaic L, Socan A, Poglajen G, Peitl PK, Sever M, Cukjati M, Cernelc P, Wu JC, Haddad F, Vrtovec B. Intracoronary transplantation of CD34(+) cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2015;21:145–152. doi: 10.1016/j.cardfail.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, Haddad F, Torre-Amione G. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail. 2011;17:272–281. doi: 10.1016/j.cardfail.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Bervar M, Kozelj M, Poglajen G, Sever M, Zemljic G, Frljak S, Cukjati M, Cernelc P, Haddad F, Vrtovec B. Effects of Transendocardial CD34+ Cell Transplantation on Diastolic Parameters in Patients with Nonischemic Dilated Cardiomyopathy. Stem Cells Transl Med. 2017;6:1515–1521. doi: 10.1002/sctm.16-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poglajen G, Sever M, Cukjati M, Cernelc P, Knezevic I, Zemljic G, Haddad F, Wu JC, Vrtovec B. Effects of transendocardial CD34+ cell transplantation in patients with ischemic cardiomyopathy. Circ Cardiovasc Interv. 2014;7:552–559. doi: 10.1161/CIRCINTERVENTIONS.114.001436. [DOI] [PubMed] [Google Scholar]
- 55.Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre-Amione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128:S42–S49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 56.Vrtovec B, Sever M, Jensterle M, Poglajen G, Janez A, Kravos N, Zemljic G, Cukjati M, Cernelc P, Haddad F, Wu JC, Jorde UP. Efficacy of CD34+ Stem Cell Therapy in Nonischemic Dilated Cardiomyopathy Is Absent in Patients With Diabetes but Preserved in Patients With Insulin Resistance. Stem Cells Transl Med. 2016;5:632–638. doi: 10.5966/sctm.2015-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niccoli G, Camici PG. Myocardial infarction with non-obstructive coronary arteries: what is the prognosis? Eur Heart J Suppl. 2020;22:E40–E45. doi: 10.1093/eurheartj/suaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boilson BA, Kiernan TJ, Harbuzariu A, Nelson RE, Lerman A, Simari RD. Circulating CD34+ cell subsets in patients with coronary endothelial dysfunction. Nat Clin Pract Cardiovasc Med. 2008;5:489–496. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hale G, Waldmann H. Use of Ceprate CD34-positive selection system for depletion of T cells in allogeneic transplantation. Bone Marrow Transplant. 1997;20:709–710. doi: 10.1038/sj.bmt.1700956. [DOI] [PubMed] [Google Scholar]