Abstract
D-Allose (D-All), a C-3 epimer of D-glucose (D-Glc), is a naturally rare monosaccharide, which shows anti-proliferative activity against several human cancer cell lines. Unlike conventional anticancer drugs, D-All targets glucose metabolism and is non-toxic to normal cells. Therefore, it has attracted attention as a unique “seed” compound for anticancer agents. However, the anti-proliferative activities of the other rare aldohexoses have not been examined yet. In this study, we evaluated the anti-proliferative activity of rare aldohexoses against human leukemia MOLT-4F and human prostate cancer DU-145 cell lines. We found that D-All and D-idose (D-Ido) at 5 mM inhibited cell proliferation of MOLT-4F cells by 46 % and 60 %, respectively. On the other hand, the rare aldohexoses at 5 mM did not show specific anti-proliferative activity against DU-145 cells. To explore the structure–activity relationship of D-Ido, we evaluated the anti-proliferative activity of D-sorbose (D-Sor), 6-deoxy-D-Ido, and L-xylose (L-Xyl) against MOLT-4F cells and found that D-Sor, 6-deoxy-D-Ido, and L-Xyl showed no inhibitory activity at 5 mM, suggesting that the aldose structure and the C-6 hydroxy group of D-Ido are important for its activity. Cellular glucose uptake assay and western blotting analysis of thioredoxin-interacting protein (TXNIP) expression suggested that the anti-proliferative activity of D-Ido is induced by inhibition of glucose uptake via TXNIP-independent pathway.
Keywords: rare sugar, D-idose, anti-proliferative activity, leukemia
Abbreviations
2DG, 2-deoxy-D-glucose; All, allose; Alt, altrose; Alu, allulose; FBS, fetal bovine serum; Gal, galactose; Glc, glucose; GLUTs, glucose transporters; Gul, gulose; Ido, idose; Man, mannose; Sor, sorbose; Tal, talose; TXNIP, thioredoxin- interacting protein; Xyl, xylose.
INTRODUCTION
Rare sugars are certain monosaccharides and their derivatives that rarely existing in nature. 1) Recently, rare sugars have attracted attention owing to their biological activities. For example, D-allulose (D-Alu, D-psicose), a C-3 epimer of D-fructose, has antihyperglycemic 2) and anti-obesity activity. 3) D-Allose (D-All), a C-3 epimer of D-glucose (D-Glc), has been shown to have plant growth-regulatory, 4) anti-oxidant, 5) and neuroprotective activities. 6) 7) In addition to these activities, D-All also showed anti-proliferative activity against several human cancer cell lines (ovarian, 8) prostate, 9) liver, 10) leukemia, 11) and tongue 12) ) without affecting normal cells. 13) D-All strongly induced the expression of thioredoxin-interacting protein (TXNIP) via the glucose-sensing transcription factor MondoA, which led to biological events such as endocytosis of glucose transporters (GLUTs). 14) 15) 2-Deoxy-D-glucose (2DG) is another anti-proliferative monosaccharide, which is known to be a glycolytic inhibitor. 16) Unlike conventional chemotherapeutic drugs interacting with the DNA or cytoskeleton, D-All and 2DG suppressed cell growth through modulation of carbohydrate metabolism. Therefore, D-All is considered a potential anticancer agent with a novel mechanism of action and fewer side effects than conventional chemotherapeutic drugs. Although the promising biological activities of certain rare sugars such as D-Alu and D-All have been shown, the anti-proliferative activity of other rare sugars had not been investigated yet.
Sato et al . examined the growth inhibitory activity of all aldo- and ketohexoses against Caenorhabditis elegans ( C . elegans ) and found that L-idose (L-Ido, a C-5 epimer of D-glucose) and D-talose (D-Tal, a C-2 epimer of D-galactose and a C-4 epimer of D-mannose) along with D-Alu and D-All exhibited significant activity, which prompted us to evaluate the anti-proliferative effects of the rare sugars other than D-Alu and D-All. 17) 18)
In this study, we evaluated the anti-proliferative activity of thirteen types of rare aldohexoses ( Fig. 1 ) against MOLT-4F human leukemia cell line, and found that D-All and D-idose (D-Ido) showed significant anti-proliferative activity. To the best of our knowledge, this is the first report describing the biological activity of D-Ido. On the other hand, the tested aldohexoses did not show significant anti-proliferative activity against DU-145 human prostate cancer cell line at 5 mM. In addition, we investigated the structure–activity relationship of D-Ido and the results suggested that the aldose structure and a C-6 hydroxy group of D-Ido are important for its anti-proliferative activity. To clarify the anti-proliferative mechanism of D-Ido, we also conducted the 2DG uptake assay and western blotting analysis using anti-TXNIP antibody. These experiments indicated that the activity of D-Ido was induced by inhibition of glucose uptake via TXNIP-independent pathway.
Fig. 1. The structure of rare aldohexoses.
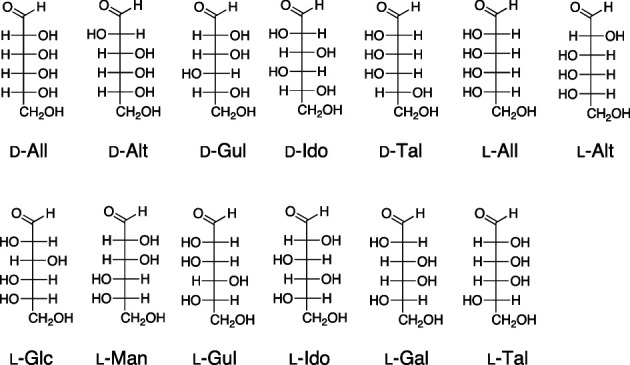
All, allose; Alt, altrose; Gul, gulose; Ido, idose, Tal, talose; Glc, glucose; Man, mannose; Gal, galactose.
RESULTS AND DISCUSSION
Anti-proliferative activity of rare aldohexoses.
There are sixteen stereoisomers of aldohexoses: Eight D-forms and eight L-forms. Among the aldohexoses, only three members, D-Glc, D-Man, and D-Gal, are abundantly found in nature. The other thirteen members are classified as rare sugars. To evaluate the anti-proliferative activity of these rare aldohexoses, at first, we used MOLT-4F human leukemia cell line, which is known to be susceptible to D-All, for performing growth inhibitory assay.
MOLT-4F cells were incubated in the presence of different concentrations of the rare aldohexoses (1, 3, 5, 10, and 20 mM for D-All, D-Alt, D-Gul, D-Tal, L-All, L-Alt, L-Glc, L-Man, L-Gul, L-Gal, and L-Tal, each and 1, 3, and 5 mM each for D-Ido and L-Ido) and the cell viability was measured as previously described. 11) Cell growth was expressed as the relative percentage of viable cells compared to those in the untreated control group ( Fig. 2 ). Because D-Ido and L-Ido were commercially available only as 140 mM aqueous solutions, they could not be tested with a concentration of more than 5 mM.
Fig. 2. Anti-proliferative activities of D-All, D-Alt, D-Gul, D-Tal, L-All, L-Alt, L-Glc, L-Man, L-Gul, L-Gal, and L-Tal at 1–20 mM each and D-Ido and L-Ido at 1–5 mM against the MOLT-4F cell line.
Values are represented as means ± standard deviation ( n = 6 or 21). † p < 0.001, * p < 0.01 (Dunnett’s test).
Among the D-form rare aldohexoses, D-Ido showed significant inhibitory activity at 5 mM, while D-Tal and D-Alt showed activity at 20 mM. However, among the L-forms of aldohexoses, only L-talose showed significant inhibitory activity at 20 mM. Cell growth in the presence of 5 mM D-Ido (60 %) was comparable to that in the 5 mM D-All-treated group (46 %). To the best of our knowledge, this is the first report on the biological activity of D-Ido.
We next examined the anti-proliferative activity of rare aldohexoses against DU-145 human prostate cancer cell line, which is also known to be susceptible to D-All. 9) While MOLT-4F cells was reported to undergo cell cycle arrest upon treatment of D-All, 11) DU-145 cells was reported to undergo apoptosis by 20 or 40 mM of D-All. 9) We evaluated the effect of the 5 or 20 mM of the rare aldohexoses (D-All, D-Alt, D-Gul, D-Tal, L-All, L-Alt, L-Glc, L-Man, L-Gul, L-Gal, L-Tal, D-Ido, and L-Ido) against DU-145 cells. Among 5 mM of the aldohexoses, only L-Ido (77 %) inhibited cell growth to less than 80 % ( Fig. 3 ). On the other hand, to our surprise, at 20 mM, not only D-All but also the rest of the rare aldohexoses, except for D-Ido and L-Ido due to the sample concentration limit, showed low to moderate anti-proliferative activity. This result implies that the anti-proliferative activity of those rare aldohexoses against DU-145 cells are partly caused by physicochemical factors such as osmotic stress rather than biochemical processes specific to type of sugars.
Fig. 3. Anti-proliferative activities of D-All, D-Alt, D-Gul, D-Tal, L-All, L-Alt, L-Glc, L-Man, L-Gul, L-Gal, and L-Tal at 5 and 20 mM each D-Ido and L-Ido at 5 mM against the DU-145 cell line.
Values are represented as means ± standard deviation ( n = 6). † p < 0.001, * p < 0.01 (Dunnett’s test).
In light of these results, we used MOLT-4F cells which seems to be sensitive to D-Ido for the further analysis of the anti-proliferative activity of D-Ido.
Structure–activity relationship study on D-Ido.
Previous studies on D-Ido mainly focused on its characteristic conformational features, and its biological activity has not been investigated. D-Ido exists in water as a mixture of isomers consisting of α-pyranose:β-pyranose:α-furanose:β-furanose at a ratio of 3.4:3.1:1.2:1. In addition, α-D-idopyranose was reported to be relatively unstable owing to its 1,3-diaxial interaction and adopts chair and twisted-boat conformations. 19) Because of its instability, D-Ido can isomerize relatively easily into D-sorbose (D-Sor, Fig. 4 ) in acidic or basic conditions via Lobry de Bruyn–van Ekenstein transformation. 20) Therefore, we evaluated the activity of D-Sor to clarify whether it contributes to the activity of D-Ido.
Fig. 4. Structures of D-Ido, D-Sor, 6-deoxy-D-Ido, and L-Xyl.

While phosphorylation of the hydroxy group at the C-6 position of D-All is crucial for its anti-proliferative activity, it was reported that the phosphorylation of the hydroxy group at the C-6 position of D-Ido did not occur in Ehrlich ascites cells, Jensen sarcoma, or Walker carcinoma. 21) To examine the role of the C-6 hydroxy group of D-Ido on its bioactivity, we evaluated the anti-proliferative activity of 6-deoxy-D-Ido and L-xylose (L-Xyl) that have the same configurations as D-Ido at the C-1 to C-4 positions ( Fig. 4 ).
6-Deoxy-D-Ido was synthesized from D-xylose according to a previously reported procedure with several modifications ( Fig. 5 ). 22) 23) Protecting a hydroxy group at the C-1 position of D-Xyl followed by benzylation of secondary hydroxy groups at position C-2 to C-4 gave 2 . Deprotection of methyl acetal at the C-1 position afforded 3 , which was followed by an addition of a methyl group to C-1 aldehyde group using CH 3 MgBr to give 4 with almost 100% stereoselectivity. 23) Selective Corey–Kim oxidation of a primary hydroxy group of 4 to an aldehyde group gave 5 . Finally, deprotection of benzyl groups at C-2 to C-4 positions with catalytic hydrogenation afforded 1 (α-pyranose:β-pyranose:β-furanose:α-furanose = 7.7:4.0:1.3:1 in D 2 O). The J value of H-1 ( J = 7.0 Hz) of α-pyranose form of 1 was larger than that of D-Ido ( J = 6.0 Hz). This difference might be attributed to the effect of the removal of the hydroxy group at the C-6 position of 1 on its conformation.
Fig. 5. Synthesis of 6-deoxy-D-idose.

(a) (i) H 2 SO 4 -silica, MeOH, 14 h, reflux, (ii) NaH, BnBr, TBAI, DMF, 0 °C to rt, 14 h, 63 %; (b) 1 M H 2 SO 4 , AcOH, 90 °C, 62 %; (c) CH 3 MgBr, Et 2 O, THF, rt to reflux, 2 h, 83 %; (d) N -chlorosuccinimide, Me 2 S, Et 3 N, toluene, −25 °C, 4 h, 13 %; (e) H 2 , Pd(OH) 2 /C, MeOH, rt, 2 h, 88 %.
The anti-proliferative activity of D-Sor, 6-deoxy-D-Ido, and L-Xyl against MOLT-4F cells was evaluated by the same method as above ( Fig. 6 ). D-Sor weakly inhibited the growth of MOLT-4F cells at 20 mM. This result suggests that if D-Ido isomerizes to D-Sor in the assay condition, it would not affect the cell growth. 6-Deoxy-D-Ido and L-Xyl showed little or no activity at 5 mM. These results suggest that both the aldose structure and the hydroxy group at the C-6 position of D-Ido are important for its activity. Although the role of the C-6 hydroxy group of D-Ido is unknown, it might be involved in the interaction with the protein-related sugar metabolism or intracellular signaling.
Fig. 6. Anti-proliferative activities of D-Ido at 1–3 mM, D-Sor and L-Xyl at 1–20 mM, and 6-deoxy-D-Ido at 0.1–5 mM concentration against the MOLT-4F cell line.

Values are means ± standard deviation ( n = 6 or 21). † p < 0.001 (Dunnett’s test).
Analysis of the mechanism of action of D-Ido.
Although D-Ido showed anti-proliferative activity, there was no information on its mechanism of action. Thus, we first examined effect of D-Ido treatment on cellular glucose uptake by using the 2DG Uptake Measurement Kit (Cosmo Bio). The 2DG uptakes of 5 mM D-Ido- and D-All-treated groups were 78 % and 65 % of the untreated group, respectively ( Fig. 7 ), which has some correlation to the suppression of cell growth in the presence of 5 mM of D-Ido or D-All (60 and 46 %, respectively). Therefore, the anti-proliferative activity of D-Ido might be ascribable to the inhibition of cellular glucose uptake and consequent depletion of intracellular glucose and metabolites of glycolytic pathway. D-All was reported to inhibit glucose uptake via induction of TXNIP expression and subsequent downregulation of GLUTs. 11) Therefore, to clarify whether the TXNIP expression is involved in the anti-proliferative activity of D-Ido, we examined the TXNIP expression in MOLT-4F cells treated with D-Ido. Western blotting experiment showed that D-Ido did not induce TXNIP expression at 5 mM ( Fig. 8 ). This result suggests that, unlike D-All, D-Ido inhibited uptake of D-Glc via TXNIP-independent mechanism. Although the specific mechanism of action of D-Ido remains unknown, it is found that other rare sugars such as 2DG 24) and D-arabinose 25) act as glycolytic inhibitors. Thus, D-Ido might act as a glycolytic inhibitor and thereby slow uptake of D-Glc.
Fig. 7. 2DG uptake by MOLT-4F cells treated with D-All and D-Ido.

MOLT-4F cells were treated with D-All (1 and 5 mM) or D-Ido (5 mM) for 48 h, and then washed by KRPH buffer and incubated with 1 mM of 2DG for 20 min. After incubation, cells were collected and sonicated. The 2DG concentration of the cell extract was measured by using 2DG Uptake Measurement Kit (Cosmo Bio). Values are means ± standard deviation ( n = 2). † p < 0.001 vs . Control (Dunnett’s test).
Fig. 8. TXNIP expression induced by D-All and D-Ido.

MOLT-4F cells were incubated with 5 mM of D-All or D-Ido for 48 h, and then the expressions of TXNIP and β-actin proteins were analyzed by western blotting. The experiment was repeated twice and a representative result is shown.
CONCLUSION
We evaluated the anti-proliferative activity of rare aldohexoses against MOLT-4F human leukemia and DU-145 human prostate cancer cell lines and found that D-Ido inhibited growth of MOLT-4F cells by 40 % at 5 mM, which was comparable to the effect of 5 mM of D-All. To the best of our knowledge, this is the first report on the biological activity of D-Ido. To understand the structure–activity relationship of D-Ido, we evaluated the anti-proliferative activity of D-Sor, 6-deoxy-D-Ido, and L-Xyl. D-Sor, 6-deoxy-D-Ido, and L-Xyl did not inhibit the MOLT-4F cell growth. These results suggest that the aldose structure and the C-6 hydroxy group of D-Ido are important for its activity. In addition, we found that D-Ido as well as D-All inhibited cellular glucose uptake through TXNIP independent mechanism. This study paves the way for exploring the biological activity of rare sugars and its derivatives other than well-studied D-All and D-Alu. Screening of rare sugar derivatives and further investigation of their mechanism of action is now underway.
EXPERIMENTAL
General remarks.
The following spectroscopic and analytical instruments were used: 1 H and 13 C NMR, JOEL JNM-ECA 600 (JEOL, Japan; tetramethylsilane (TMS) was used as an internal standard for 1 H and 13 C; HPLC, JASCO PU-4086 Intelligent HPLC pump with a JASCO UV-4075 Intelligent UV/VIS Detector (JASCO, Tokyo, Japan); HPLC was carried out on a YMC-Pack SIL SL12S16-1520WT (YMC, Kyoto, Japan). Wakogel ® C-200 and C-300 (silica gel, Wako Pure Chemical Laboratory, Osaka, Japan) were used for column chromatography. RPMI-1640 medium with L-glutamine, phenol red and HEPES, glucose-free RPMI-1640, Penicillin-Streptomycin, and Cell Counting Kit-8 (DOJINDO) were purchased from Wako Pure Chemical Corporation. Fetal Bovine Serum was purchased from Equitech Bio. Anti-TXNIP antibody (Medical & Biological Laboratories Corporation), anti-β-actin antibody (Wako Pure Chemical Corporation), horseradish peroxidase conjugated anti-mouse IgG (Sigma), and Western Lightning Plus-ECL (Perkin Elmer) were used for Western blotting. All other chemicals and reagents were purchased from chemical companies and used without further purification.
Cell culture.
MOLT-4F human leukemia and DU-145 human prostate cancer cell lines were purchased from RIKEN Cell Bank in Japan (RCB1936, RCB2143, respectively) and grown in RPMI-1640 medium with 10 % heat-inactivated FBS (Fetal Bovine Serum), 100 IU/mL penicillin, and 100 μg/mL streptomycin in a 5 % CO 2 , humidified incubator at 37 °C. For the growth inhibition assay, cells were cultured in a low-glucose medium that was prepared using glucose-free RPMI-1640 medium supplemented with 1 g/L (5 mM) D-glucose and 10 % heat-inactivated FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin.
Growth inhibition assay.
The assay was conducted as previously described by Hirata et al . 11) with modifications. Test compounds were dissolved in H 2 O and the aqueous solution was diluted to each concentration. MOLT-4F or DU-145 cells were seeded in 96-well plates at a density of 5 × 10 4 cells/well in 95 µL of assay medium, and then a 5 µL solution of each aldohexose was added. After 48 h incubation, 10 µL of Cell Counting Kit-8 was added to each well, and the plate was incubated at 37 °C for another 4 h (for MOLT-4F cells) or 1 h (for DU-145 cells). Absorbance was measured for the control wells ( C ) and test wells ( T ) at 450 nm along with the absorbance at time 0 ( C 0 ). The blank value was subtracted from each value. Cell growth (% of control) was expressed as 100[( T − C 0 )/( C − C 0 )] ( T ≥ C 0 ) or 100[( T − C 0 )/ C 0 ] ( T < C 0 ).
Glucose uptake measurement.
MOLT-4F cells were incubated with D-Ido (5 mM) or D-All (1 and 5 mM) for 48 h in a 6-well plate. After 48 h, the medium was removed and cells were incubated with serum-free RPMI-1640 for 6 h and then washed three times with Krebs-Ringer-phosphate-HEPES (KRPH) buffer (1.2 mM KH 2 PO 4 , 1.2 mM MgSO 4 , 1.3 mM CaCl 2 , 118 mM NaCl, 5 mM KCl, 30 mM HEPES, pH 7.5). The cells were further incubated with KRPH buffer containing 2 % bovine serum albumin and 1 mM 2DG for 20 min. Then, cells were washed three times with ice-cold PBS and collected with 10 mM Tris-HCl buffer (pH 8.0). Collected cells were sonicated, heated at 80 °C for 15 min, and centrifuged at 15,000 × G for 20 min at 4 °C. The supernatant was collected and the concentration of 2DG was measured using the 2DG Uptake Measurement Kit (Cosmo Bio) according to the manufacturer’s protocol.
Western blotting.
MOLT-4F cells were seeded in 6-well plates at a density of 2 × 10 5 cells/well and treated with D-Ido or D-All. After 48 h incubation, the cells were collected and washed with PBS. The cells were solubilized in lysis buffer and incubated at 100 °C. The protein concentration of the cell lysate was determined by the Lowry method with bovine serum albumin (BSA) as a standard, and SDS-PAGE was carried out according to the method of Laemmli 26) in slab gels consisting of a 10 % (w/v) acrylamide separating gel and a 3 % (w/v) stacking gel. The resolved proteins were electrophoretically transferred to nitrocellulose membranes. After transfer, the membrane was blocked with 0.5 % skim milk in phosphate buffered saline with Tween 20 (PBST) and incubated with anti-TXNIP antibody diluted 1:1,000 or anti-β-actin antibody diluted 1:5000 for overnight at 4 °C. The membranes were then incubated with horseradish peroxidase-conjugated anti-mouse IgG diluted 1:2,000 for 1 h, and the immunoreactive bands were detected using the chemiluminescent substrate, Western Lightning Plus-ECL.
Synthetic procedures.
Synthesis of (3R,4S,5R)-3,4,5-tris(benzyloxy)-2-methoxytetrahydro-2H-pyran (2).
To a suspension of D-Xyl (1.01 g, 6.73 mmol) in MeOH (13.5 mL) was added H 2 SO 4 -silica and heated under reflux for 14 h. The reaction mixture was filtered, and the filtrate was concentrated in vacuo . The residue was added DMF (20.2 mL) and NaH (1.48 g, 37.0 mmol) at 0 °C under an Ar atmosphere. After 15 min of stirring, the reaction mixture was added tetrabutylammonium iodide (TBAI, 480 mg, 135 mmol) and BnBr (4.4 mL, 37.0 mmol) at rf. After 15 h of stirring, the reaction mixture was cooled to 0 °C and quenched with H 2 O (30 mL). The resulting mixture was extracted with EtOAc (30 mL × 3). The combined organic layers were washed with brine, dried over Na 2 SO 4 , filtered, and concentrated in vacuo . The residue was purified by column chromatography (silica gel, 10 % EtOAc in hexane) to afford 2 (1.84 g, 63 %).
Synthesis of (3R,4S,5R)-3,4,5-tris(benzyloxy)tetrahydro-2H-pyran-2-ol (3).
To a mixture of 2 (1.09 g, 2.52 mmol), acetic acid (AcOH, 9.7 mL) and 1 M H 2 SO 4 (1.3 mL) at 90 °C under an Ar atmosphere. After 1 h of stirring, the reaction was quenched with sat. NaHCO 3 aq. (10 mL) and then the resulting mixture was extracted with EtOAc (15 mL × 3). The combined organic layers were washed with brine, dried over Na 2 SO 4 , filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, 5–30 % EtOAc in hexane) to afford 3 (656.6 mg, 62 %).
Synthesis of (2R,3S,4S,5R)-2,3,4-tris(benzyloxy)hexane-1,5-diol (4).
The 1.0 M CH 3 MgBr solution (3.0 M CH 3 MgBr in Et 2 O 560 µL, Et 2 O 1.12 mL) was cooled to 0 °C under an Ar atmosphere. The solution was added dropwise to the mixture of 3 (41.4 mg, 0.099 mmol) in THF (420 µL) and heated under reflux for 1 h. The reaction was quenched with sat. NaHCO 3 aq (3 mL). and then the resulting mixture was extracted with EtOAc (5 mL × 3). The combined organic layers were washed with brine, dried over Na 2 SO 4 , filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, 40 % EtOAc in hexane) to afford 4 (35.5 mg, 83 %).
Synthesis of (3S,4R,5S,6R)-3,4,5-tris(benzyloxy)-6-methyltetrahydro-2H-pyran-2-ol (5).
A solution of N -chlorosuccinimide (109.2 mg, 0.818 mmol) and dimethyl sulfide (98 µL, 1.13 mmol) in toluene (5.5 mL) was cooled to −25 °C under an Ar atmosphere. After 30 min, the mixture was added dropwise to 4 (291.1 mg, 0.667 mmol) in toluene (1.1 mL) at −25 °C. After 4 h of stirring, the reaction was quenched with triethylamine (Et 3 N, 93 µL, 0.667 mmol) in toluene (1.1 mL) and then the resulting mixture was extracted with EtOAc (10 mL × 3). The combined organic layers were washed with brine, dried over Na 2 SO 4 , filtered, and concentrated in vacuo . The residue was purified by column chromatography (silica gel, 10–30 % EtOAc in hexane) to afford mixture of 6-deoxy-D-idose derivative and 1-deoxy-L-sorbose derivative. The mixture was further purified by HPLC (column: YMC SL12S16-1520WT; solvent: 30 % EtOAc in hexane, flow rate: 8.0 mL, UV detector 254 nm, retention time: 13.5 min) to afford 5 (51.8 mg, 13 %).
Synthesis of 6-deoxy-D-Ido (1).
A solution of 5 (22.2 mg, 0.051 mmol) in MeOH (820 µL) was stirred in the presence of 20 wt% palladium hydroxide on activated carbon (24.5 mg, purchased from Sigma-Aldrich) under hydrogen atmosphere by using an aluminum bag for 2 h. The reaction mixture was filtered and concentrated in vacuo. The filtrate was purified by column chromatography (silica gel, 10 % MeOH in EtOAc) to afford 1 (12.8 mg, 88 %). 1 H NMR (600 MHz, 296 K, D 2 O, 0.0451 M, α-pyranose:β-pyranose:β-furanose:α-furanose = 7.7:4.0:1.3:1) for the α-pyranose, δ 1.25 (3H, d, J = 7.0 Hz, H-6), 3.31 (1H, t, J = 7.8 Hz, H-2), 3.70 (1H, dd, J = 8.5, 5.0 Hz, H-4), 3.67 (1H, t, J = 8.3 Hz, H-3), 4.23 (1H, qd, J = 7.0, 4.8 Hz, H-5), 4.88 (1H, d, J = 7.0 Hz, H-1) ppm; for the β-pyranose (overlapped), δ 1.26 (3H, d, J = 6.7 Hz, H-6), 5.04 (1H, d, J = 1.6 Hz, H-1) ppm; for the β-furanose (overlapped), δ 5.46 (1H, d, J = 4.1 Hz, H-1) ppm; for the α-furanose (overlapped), δ 5.21 (1H, br.s, H-1) ppm. 13 C NMR (150 MHz, 297 K, D 2 O, 0.0451 M) for the α-pyranose, δ 15.5 (C-6), 72.6 (C-3), 74.4 (C-5), 74.7 (C-2), 76.7 (C-4), 95.1 (C-1) ppm; for the β-pyranose, δ 18.5 (C-6), 72.5 (C-3), 72.7 (C-2), 73.0 (C-5), 73.2 (C-4), 95.2 (C-1) ppm; for the β-furanose, δ 21.0 (C-6), 69.1 (C-5), 77.7 (C-3), 78.3 (C-2), 85.1 (C-4), 98.8 (C-1) ppm, for the α-furanose, δ 69.9 (C-5), 79.1 (C-3), 83.7 (C-2), 88.7 (C-4), 104.9 (C-1) ppm.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the Rare Sugar Research grant funded by Kagawa Prefecture, Japan.
References
- 1).Granström T.B., Takata G., Tokuda M., and Izumori K.: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng., 97, 89–94 (2004). [DOI] [PubMed] [Google Scholar]
- 2).Oshima H., Ozaki Y., Kitakubo Y., and Hayakawa S.: Decrease in the D-psicose content of processed foods fortified with a rare sugar. Food Sci. Technol. Res., 20, 415–421 (2014). [Google Scholar]
- 3).Matsuo T. and Izumori K.: Effects of dietary D-Psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem., 70, 2081–2085 (2006). [DOI] [PubMed] [Google Scholar]
- 4).Afach G., Kawanami Y., Kato-Noguchi H., and Izumori K.: Practical production of 6- O -Octanoyl-D-allose and Its biological activity on plant growth. Biosci. Biotechnol. Biochem., 70, 2010–2012 (2006). [DOI] [PubMed] [Google Scholar]
- 5).Sun Y., Hayakawa S., Puangmanee S., and Izumori K.: Chemical properties and antioxidative activity of glycated-lactalbumin with a rare sugar, D-allose, by Maillard reaction. Food Chem., 95, 509–517 (2006). [Google Scholar]
- 6).Nakamura T., Tanaka S., Hirooka K., Hirooka K., Toyoshima T., Kawai N., Tamiya T., Shiraga F., Tokuda M., Keep R.F., Itano T., and Miyamoto O.: Anti-oxidative effects of D-allose, a rare sugar, on ischemia-reperfusion damage following focal cerebral ischemia in rat. Neurosci. Lett., 487, 103–106 (2011). [DOI] [PubMed] [Google Scholar]
- 7).Gao D., Kawai N., Nakamura T., Lu F., Fei Z., and Tamiya T.: Anti-inflammatory effect of D-allose in cerebral ischemia/reperfusion injury in rats. Neurol. Med. Chir., 53, 365–374 (2013). [DOI] [PubMed] [Google Scholar]
- 8).Sui L., Dong Y., Watanabe Y., Yamaguchi F., Hatano N., Izumori K., and Tokuda M.: Growth inhibitory effect of D-allose on human ovarian carcinoma cells in vitro. Anticancer Res., 25, 2639–2644 (2005). [PubMed] [Google Scholar]
- 9).Naha N., Lee H.Y., Jo M.J., Chung B.C., Kim S.H., and Kim M.O.: Rare sugar D-allose induces programmed cell death in hormone refractory prostate cancer cells. Apoptosis, 13, 1121–1134 (2008) [DOI] [PubMed] [Google Scholar]
- 10).Sui L., Dong Y., Watanabe Y., Yamaguchi F., Hatano N., Tsukamoto I., Izumori K., and Tokuda M.: The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. Int. J. Oncol., 27, 907–912 (2005). [PubMed] [Google Scholar]
- 11).Hirata Y., Saito M., Tsukamoto I., Yamaguchi F., Sui L., Kamitori K., Dong Y., Uehara E., Konishi R., Janjua N., and Tokuda M.: Analysis of the inhibitory mechanism of D-allose on MOLT-4F leukemia cell proliferation. J. Biosci. Bioeng., 107, 562–568 (2009). [DOI] [PubMed] [Google Scholar]
- 12).Yamaguchi F., Takata M., Kamitori N., Nonaka M., Dong Y., Sui L., and Tokuda M.: Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27 kip1. Int. J. Oncol., 32, 377–385 (2008). [PubMed] [Google Scholar]
- 13).Iga Y., Nakamichi K., Shirai Y., and Matsuo T.: Acute and sub-chronic toxicity of D-allose in rats. Biosci. Biotechnol. Biochem., 74, 1476–1478 (2010). [DOI] [PubMed] [Google Scholar]
- 14).Billin A.N., Eilers A.L., Coulter K.L., Logan J.S., and Ayer D.E.: MondoA, a novel basic helix-loop-helix–leucine zipper transcriptional activator that constitutes a positive branch of a max-like network. Mol. Cell. Biol., 20, 8845–8854 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Havula E. and Hietakangas V.: Glucose sensing by ChREBP/MondoA–Mlx transcription factors. Semin. Cell. Dev. Biol., 23, 640–647 (2012). [DOI] [PubMed] [Google Scholar]
- 16).Robinson G.L., Dinsdale D., MacFarlane M., and Cain K.: Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene, 31, 4996–5006 (2012). [DOI] [PubMed] [Google Scholar]
- 17).Sakoguchi H., Yoshihara A., Izumori K., and Sato M.: Screening of biologically active monosaccharides: growth inhibitory effects of D-allose, D-talose, and L-idose against the nematode Caenorhabditis elegans. Biosci. Biotechnol. Biochem., 80, 1058–1061 (2016). [DOI] [PubMed] [Google Scholar]
- 18).Sato M., Kurose H., Yamasaki T., and Izumori K.: Potential anthelmintic: D-psicose inhibits motility, growth and reproductive maturity of L1 larvae of Caenorhabditis elegans. J. Nat. Med., 62, 244–246 (2008). [DOI] [PubMed] [Google Scholar]
- 19).Snyder J.R. and Serianni A.S.: D-Idose: A one- and two-dimensional NMR investigation of solution composition and conformation. J. Org. Chem., 51, 2694–2702 (1986). [Google Scholar]
- 20).Vargha L.: Über die substitution von tosyloxy-gruppen durch acetoxy-gruppen in polyoxy-verbindungen. Eine neue Darstellungsweise von l-Idose-derivaten aus D-glucose. Chem. Ber., 87, 1351–1356 (1954). [Google Scholar]
- 21).Lange C.F. and Kohn P.: Hexokinase specificity of some tumor tissue extracts. Cancer. Res., 21, 1055–1061 (1961). [PubMed] [Google Scholar]
- 22).Choi S.H., Mansoorabadi S.O., Liu Y.N., Chien T.C., and Liu H.W.: Analysis of UDP-D-apiose/UDP-D-xylose synthase-catalyzed conversion of UDP-D-apiose phosphonate to UDP-D-xylose phosphonate: implications for a Retroaldol−Aldol mechanism. J. Am. Chem. Soc., 134, 13946–13949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Tsuda Y., Nunozawa T., and Yoshimoto K.: Highly stereoselective grignard reaction of an Aldopyranose: A simple synthesis of 6-deoxy-D-idose from D-xylose. Chem. Pharm. Bull., 28, 3223–3231 (1980). [Google Scholar]
- 24).Robinson G.L., Dinsdale D., and MacFarlane M.: Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene, 31, 4996–5006 (2012). [DOI] [PubMed] [Google Scholar]
- 25).Sakoguchi H., Yoshihara A., Shintani T., Okuma K., Izumori K., and Sato M.: Growth inhibitory effect of D-arabinose against the nematode Caenorhabditis elegans : Discovery of a novel bioactive monosaccharide. Bioorg. Med. Chem. Lett., 26, 726–729 (2016). [DOI] [PubMed] [Google Scholar]
- 26).Laemmli U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685 (1970). [DOI] [PubMed] [Google Scholar]




